Annually, >300,000 brain tumor cases are
diagnosed globally, making them the fifth most prevalent type of
cancer. Meningiomas represent the most prevalent primary benign
tumors of the central nervous system (CNS). Recent figures indicate
that 40.8% of all CNS tumors in the USA are meningiomas,
constituting 56.2% of all benign tumors, whereas gliomas account
for 26.3% of all tumors and 50.9% of all malignant tumors (1). Brain metastases (BM) constitute a
distinct category and are the most prevalent form of brain tumor,
with an incidence ~5-fold greater than that of primary brain tumors
(2). Lung cancers, breast
cancers and melanomas are the most likely to metastasize to the
brain (2). The incidence of
seizures varies significantly among different types of brain
tumors, ranging from 10% to over 80%, contingent upon the tumor
type (3,4). This is a significant side effect
that should not be underestimated due to its potential impact on
the patients' quality of life (QoL). Numerous studies conducted
throughout the years have endeavored to elucidate the
pathophysiological mechanisms of brain tumor-related epilepsy
(BTRE), yielding various degrees of results. It is generally
acknowledged that mutations in the isocitrate dehydrogenase type 1
(IDH1) and type 2 genes contribute to the development of BTRE in
gliomas at the molecular level (5,6).
In general, various mechanisms, including mechanical (compression),
vascular (imbalance in vascularization), chemical (neurotransmitter
dysregulation), and inflammatory processes, have been identified in
the pathophysiology of BTRE (7).
Neurosurgeons frequently prescribe anti-seizure medications (ASMs)
during the perioperative period, particularly post-operatively,
despite the absence of studies demonstrating the advantages of this
practice for seizure-naïve patients. Current research on BTRE has
predominantly focused on gliomas and glioneuronal tumors, while
other tumor forms, including meningiomas and BM, have received far
less attention. The present review examines the pathophysiology of
BTRE, encompassing molecular mechanisms and genetic implications.
Risk factors, the correlation between epileptogenesis and
tumorigenesis, and the influence of ASMs on tumor progression were
also discussed. Finally, some insight was provided into
anti-epileptogenesis, which aims to cure epilepsy. The progress
made so far and the challenges associated with developing novel
therapies are discussed; and the potential solutions that may be
advantageous for epilepsy broadly and BTRE specifically are
delineated.
Inflammation has always played a role in epilepsy,
as evidenced by the existence of febrile seizures. This indicates a
mechanism through which inflammation induces a hyper-excitable
state in brain tissue. Numerous studies have sought to elucidate
this mechanism, and while the findings remain partially
unsatisfactory, the principal pathophysiological agents have been
identified. In neuroinflammation, the microglia and astrocytes
secrete pro-inflammatory cytokines (PICs) such as interleukin 1
beta (IL-1β), tumor necrosis factor alpha (TNF-α) and interleukin 6
(IL-6), among others. The overload of PICs in the CNS leads to the
blood-brain barrier (BBB) breakdown, resulting in further
recruitment of PICs from the systemic circulation (8,9).
This review focuses on the role of IL-1β, TNF-α, toll-like receptor
4 (TLR4) and High-mobility group box 1 (HMGB1) in BTRE
pathophysiology.
Il-1β can induce hyperexcitability via several
mechanisms and possesses various receptors, with IL-1R1 being the
most involved in epileptogenesis. Upon binding of Il-1β to IL-1R1,
ceramide is generated through the activation of neutral
sphingomyelinase (N-SMase). Ceramide stimulates phosphorylation of
the NR2B subunit of N-methyl-D-aspartate receptors (NMDAR), leading
to an increased influx of Ca2+ in neuron cells and,
subsequently, increased excitability (8). This increase in intracellular
calcium (Ca2+) may also stimulate the overproduction of
nitric oxide (NO) through nitric oxide synthase. NO induces
oxidative stress and cell damage, therefore leading to increased
PICs' secretion (10). IL-1β may
also induce epilepsy via the synaptic protein synaptophysin (SYN).
Using rat models, Xiao et al (11) proposed that SYN can regulate
neurotransmitter release by acting on Ca2+, and
discovered that IL-1β increases SYN expression in the hippocampal
neurons via the activation of the PI3K/Akt/mTOR pathway. In another
study on temporal lobe epilepsy (TLE), using hippocampal tissues
from human subjects, researchers demonstrated that the complex
Il-1β/IL-1R1 was responsible for a decrease of up to 30% in
GABA-mediated neurotransmission, with protein kinase C also
contributing to this phenomenon (12).
TNF-α is secreted by microglial cells and astrocytes
under physiological conditions to palliate an eventual decrease of
glutamate, thus maintaining an adequate level of neuronal
excitability (13). TNF-α has
two receptors, TNFR1 and TNFR2, which are considered to have
opposing functions in epilepsy (14). TNFR1 serves as the proconvulsive
receptor, evidenced by neuroinflammation where excessive
TNF-α-TNRF1 binding results in increased glutamate levels through
various mechanisms, such as the upregulation of glutaminase in
microglia and the upregulation of
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors
(AMPAR) (15,16). TNFR1 is also responsible for the
neurotoxicity effects of TNF-α due to its possession of a death
domain, the TNFR-associated death domain (TADD), which is lacking
in TNFR2. TADD facilitates the activation of caspase enzymes
(caspases 8 and 10), resulting in cell death. While studies still
remain ambiguous on the predominant pathway during TNF-α
activation, some discovered that low doses of TNF-α triggered the
TNF-α/TNFR1 pathway, while higher doses were required for
anticonvulsant effects (TNF-α/TNFR2 pathway) (17,18). While TNF-α enhances the
hyperactivity of AMPAR and NMDAR, it conversely induces endocytosis
of GABA receptors, increasing GABA absorption and thus resulting in
hyperexcitability (19-21).
Another inflammatory mediator with a key role in
neuroinflammation would be TLRs. In immunology, TLRs function by
binding to specific molecules known as damage-associated molecular
patterns. One such molecule is the HMGB1, a DNA-binding protein. In
pathological conditions, excess HMGB1 is produced by glial cells
and neurons (among others), a reaction that is amplified by
cytokines. HMGB1 acts by binding to molecules known as pattern
recognition receptors, specifically TLR4 and the receptor for
advanced glycated end-products (RAGE). It is considered that TLR4
has a more significant role in epileptogenesis than RAGE (22). Regardless of its binding to RAGE
or TLR4, HMGB1 ultimately induces the release and activation of
multiple transcription factors (23,24), with nuclear factor kappa B
(NF-κB), being a prominent factor in both pathways, crucial in
inflammatory and immune gene expression. Activated TLR4 can also
enhance calcium influx in neurons through NMDAR, a mechanism that
involves N-SMases (8,25). Moreover, HMGB1 is involved in the
BBB breakdown, either through binding to RAGE or TLR4 (26), and studies reported decreased
seizure activity upon inhibition of the HMGB1-TLR4 pathway
(27). HMGB1 protein can also
bind to Il-1β, thereby activating it. The latter has similar
intracellular domains to TLR4s, and thus is involved in similar
metabolic pathways (8). Il-1β
and TLR4 can enhance the activation of pro-inflammatory genes
through the stimulation of the NF-κB transcription factor. NOD-like
receptor protein 3 is among the several activated genes. The latter
activates caspase-1 via its inflammasome, which mediates
inflammation and is responsible for the production of PICs. The
synthesized PICs can then activate the Il-1β/TLR4 pathway,
resulting in a cycle of sustained inflammation, and increased
seizure risk (28-31).
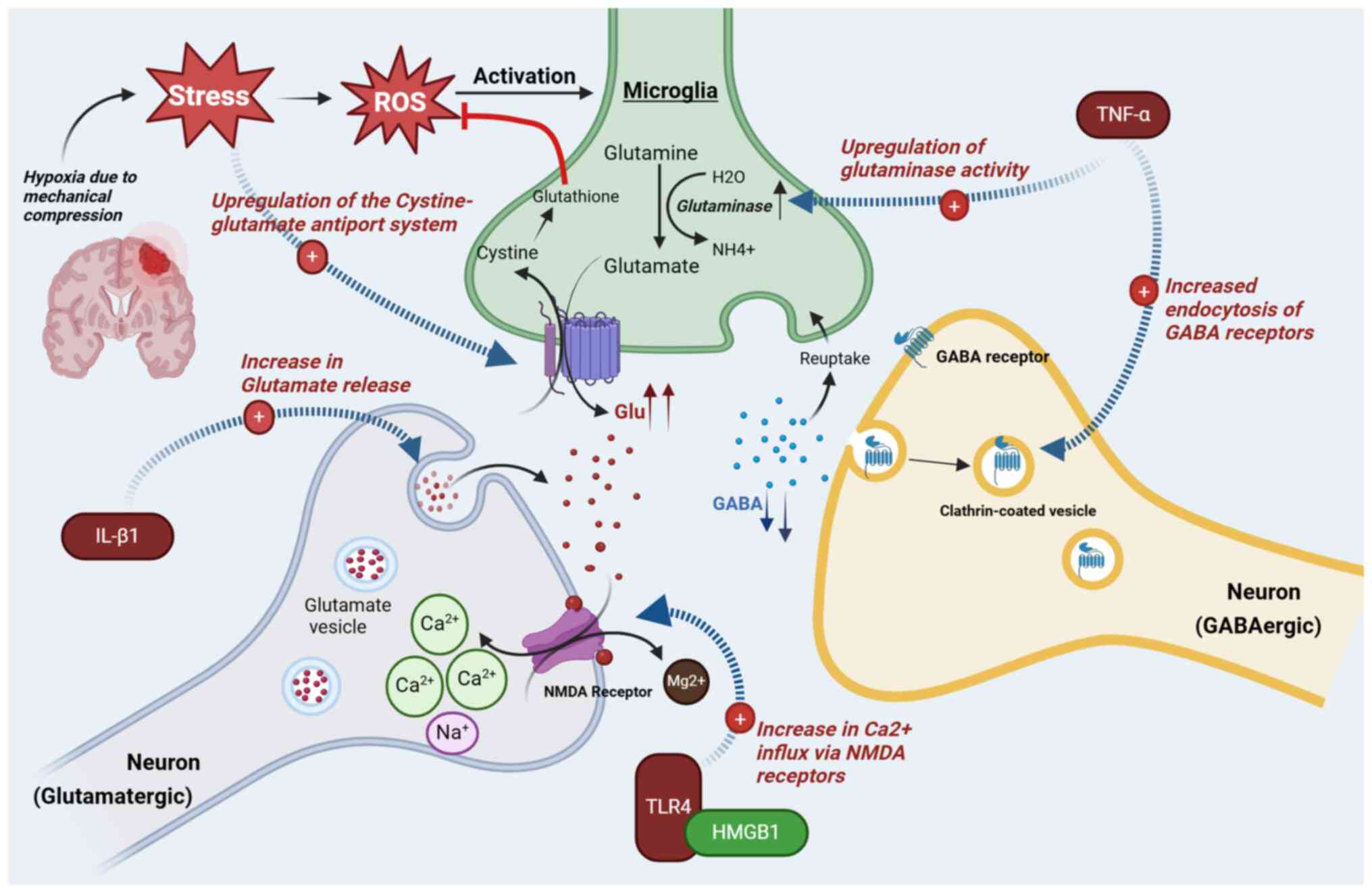
Alternative mechanisms can lead to an excessive
release of glutamate or inadequate levels of GABA. The Xc-Cystine
glutamate antiporter system in the brain imports cystine into the
cell and in exchange, glutamate is released. Glioma cells not only
express Xc-Cystine channels but due to mechanical compression and
oxidative stress on adjacent tissues, there is overexpression of
these channels on normal cells in an attempt to supply adequate
cystine for glutathione synthesis (32). This would ultimately result in an
excessive release of Glu in the synaptic space (Fig. 1). Furthermore, a comparison of
glutamate transport between normal and malignant astrocytes
revealed a significant deficiency of sodium-dependent reuptake
channels, namely excitatory amino acid transporters 1 and 2 (EAAT1
and EAAT2) (33,34). Moreover, research has indicated a
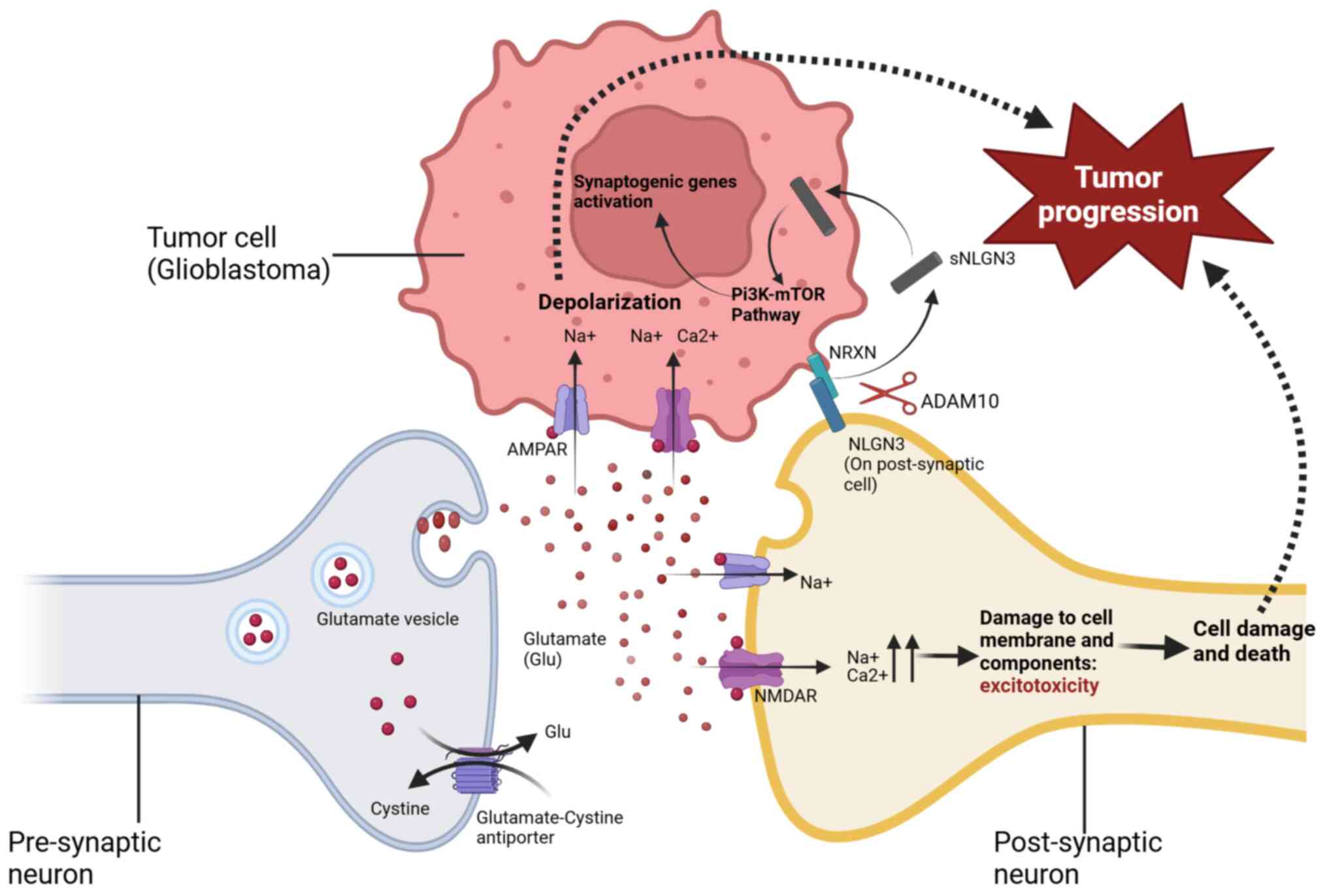
complex mechanism for glutamate transport from glioma or metastatic
tumor cells to neurons, utilizing structures similar to synapses
found in a normally functioning brain (35) (Fig. 2). In another study on gliomas,
glutamate was found to directly down-regulate GABA receptors on
astrocytes, resulting in a loss of GABAergic inhibition (36).
IDH, comprising 3 isoforms, IDH1, IDH2 and IDH3,
plays a crucial role in the Krebs cycle, by facilitating the
conversion of isocitrate to α-ketoglutarate (α-KG). In anaerobic
conditions, lactate dehydrogenase A (LDHA) catalyzes the synthesis
of L-lactate from pyruvate. LDHA levels are increased in most tumor
types, and tumor cells utilize the enzyme to increase glycolysis
and lactate production rates, even under optimal aerobic
conditions. This phenomenon is known as the Warburg effect
(37). Missense mutations in the
IDH1 gene in gliomas results in the production of
2-hydroxyglutarate (2-HG) from α-KG (38). 2-HG is considered to facilitate
seizures by enhancing mTOR activity for metabolic functions such as
the production of LDHA (high levels of LDHA were observed in
IDH1-mutated tissues treated with 2-HG) (39). The proposed mechanism is that
2-HG elevates levels of ribosomal protein S6, which plays a role in
mTOR signaling (39). Other
mechanisms by which 2-HG may induce epilepsy remain contentious. A
study indicated that 2-HG, owing to its structural resemblance to
glutamate, may imitate its function by binding to NMDARs, hence
enhancing ion influx into neuronal cells and increasing seizure
susceptibility (6). The role of
2-HG in epileptogenesis is further substantiated by a condition
known as 2-hydroxyglutaric aciduria, characterized by elevated
levels of 2-HG, with seizures that exacerbate as the disease
progresses being one of the primary symptoms.
The aggressive and anarchic growth pattern of
glioblastomas (GBM) results in genetic mutations that can be
detected even intratumorally (40). Tumor suppressor genes phosphatase
and tensin homolog (PTEN), tumor protein p53 (TP53) and
Neurofibromin 1 (NF1) are affected by these mutations (41,42). It has been suggested that these
changes promote tumorigenesis and subsequent tumor progression,
while also contributing to epileptogenesis (43). In a healthy organism, PTEN
downregulates the PI3K/AKT/mTOR pathway; however, in the context of
GBM, a loss-of-function mutation in PTEN results in the
disinhibition of this pathway, leading to excessive mTOR signaling
(44). The role of PTEN in
epileptogenesis was further suggested when scientists discovered
that the targeted deletion of this gene generated seizures in
animal models (45,46).
The NF1 gene, however, exerts a negative regulatory
effect on the Ras (Ras/MAPK and Ras/PI3K) pathways, which are
additional pathways involved in cell cycle activity. In GBM, a
loss-of-function mutation in the NF1 gene results in the
disinhibition of the Ras/MAPK pathway, which subsequently increases
mTOR signaling (47). A study by
Sabetghadam et al (48)
showed that silencing of the NF1 gene in rats resulted in lowered
seizure thresholds and increased severity.
TP53 (also known as P53) mutations in GBM are
predominantly gain-of-function mutations, producing an altered P53
protein known as mut-P53. The latter stimulates the activation of
receptor tyrosine kinases such as MNNG HOS transforming gene and
EGFR (41), ultimately resulting
in cell proliferation. The significance of P53 in epileptogenesis
remains incompletely elucidated. Increased expression of P53 has
been observed in rats and patients with TLE, particularly in the
hippocampus (49,50). Engel et al (51) observed that following the
triggering of epilepsy in experimental models, P53 levels increased
significantly; this increase subsequently activates apoptotic and
neuronal cell death processes, exacerbating imbalances and
enhancing hyperexcitability. The study also demonstrated that the
inhibition of P53 results in more severe seizures. This contrasts
with the study by Burla et al (52) where P53 inhibition resulted in
decreased seizures and inflammation. Nonetheless, inhibition of P53
has shown neuroprotective effects in a variety of conditions,
including seizure-induced neuronal cell death (53,54). Further research is required to
elucidate the role of P53 in epileptogenesis.
Preoperative risk factors for seizures in brain
tumors include sex, tumor location and size, and the presence of
peritumoral edema. Postoperative seizures are primarily associated
with the extent of resection, the presence of preoperative
seizures, and again, tumor size and location.
The occipital lobe exhibits the lowest propensity
for epilepsy, whereas tumors located in the frontal and temporal
lobes present the greatest seizure risk (60). Similarly, skull base tumors are
less frequently associated with seizures compared with tumors
located nearer to the cortex, such as convexity or
parasagittal/parafalcine tumors.
Nonetheless, certain risk factors vary amongst
different tumor types. IDH mutations in gliomas increase the
incidence of seizures in younger patients, as low-grade gliomas
(LGGs) are more prevalent in this demographic group. Regarding
gliomas, a study revealed that high-grade gliomas associated with
seizures were generally smaller, whereas low-grade gliomas were
typically larger (61). This
contrasts with meningiomas, where an increase in size correlates
with a higher likelihood of seizures (62).
A significant component, other than location and
size, associated with seizures in meningiomas is peritumoral edema.
At a pathophysiological level, peritumoral edema is considered to
contribute to a reduction in seizure threshold due to excess
secretion of substances such as VEGF and glutamate (63). The male sex is linked to an
increased risk of seizures in cases of meningiomas and brain
metastasis (64,65). This is comprehensible, as the
primary cancers that are most prone to spread to the brain are lung
cancers and melanomas (66), and
men are at an increased risk of developing lung cancers due to a
greater prevalence of smoking and high-risk employment. The
meningioma case is interesting as the tumor occurs more frequently
in women than in men, with an approximate 3 to 1 ratio (1).
Brain metastasis exhibits a unique profile with
specific risk factors exclusive to this category. Multiple
metastasis heightens the risk of seizures in BM, as it increases
the probability of a metastasis being located in a susceptible
region. Studies have also shown that patients with melanoma or lung
cancer as their primary tumor had an increased risk of developing
seizures in case of brain invasion (67,68). In the case of melanomas, one
rationale is that they are among the metastatic tumors most prone
to induce intracranial hemorrhage and tumor bleeding, which is a
risk factor for seizures in BM (68,69). Furthermore, a previous study by
Urban et al (70)
examined the impact of immune checkpoint inhibitors (ICIs) on
epileptogenesis associated with brain metastasis. The study
highlighted that new-onset status epilepticus occurred more
frequently in patients receiving ICIs compared with those not
undergoing this treatment (70),
although it is important to note that the majority of patients in
the ICIs group had melanoma as their primary malignancy, while lung
cancer was the predominant pathology in the non-ICIs group.
Additionally, a retrospective analysis of 348 patients with brain
metastasis by Garcia et al (68) identified ICIs as a risk factor
for late postoperative seizures. Overall, further research on this
topic is necessary.
The extent of resection significantly influences the
incidence of postoperative seizures across all tumor types.
Complete resection diminishes the likelihood of postoperative
seizure persistence. ASM prophylaxis has shown no impact on the
risk of post-operative seizure, which may explain the rationale
behind experts' recommendations against its usage in seizure-naïve
patients. New-onset postoperative seizures are relatively rare and
seem to be more closely associated with tumor grade, tumor
recurrence/progression, and IDH1 mutation in glioma cases (71).
It is estimated that 60-90% of patients with
preoperative seizures due to brain tumors attain seizure freedom
post-surgery, with glioneuronal tumors exhibiting the most
favorable epilepsy prognosis, whereas seizures associated with
glioblastomas tend to be more refractory and recurrent (4). Furthermore, seizures at the onset
of disease serve as a favorable prognostic factor for long-term
survival in brain tumors (72-74). Convexity tumors are more prone to
be symptomatic in the early stages due to cortical irritation,
facilitating earlier detection and intervention before progression
to an advanced stage; total resection is also more attainable in
these instances. Plus, IDH mutations are predominant in
slow-growing, low-grade tumors, with a favorable prognosis compared
with the more aggressive, high-grade ones. However, persistent
seizures are a negative prognostic factor, as they are often
indicative of tumor recurrence or progression (74).
Surgery is a mainstay in the management of brain
tumors and influences seizure freedom, if seizures are part of the
disease burden. Gross total resection has shown its efficacy in
seizure control compared with partial resection in LGGs,
meningiomas and BM (77,78). In GBM, the proliferative
characteristics of the tumor may cause the diseased tissue to
extend beyond the boundaries shown on a contrast-enhanced MRI.
Thus, supra-total resection has shown greater efficacy in seizure
control in GBM compared with gross total resection (79). In addition, a study about the
relationship between the extent of resection and its impact on
seizure freedom in temporal lobe LGGs and glioneuronal tumors
revealed that gross total resection combined with hippocampectomy
showed a higher seizure freedom rate compared with gross total
resection alone (80),
potentially suggesting a new strategy for managing BTRE in that
specific region of the brain. Further studies are needed.
Over the years, numerous studies have proven the
efficacy of radiotherapy on seizure control in LGGs (81-83), alongside chemotherapy with
temozolomide (84,85). There appears to be less of a
consensus for glioblastomas, with some studies suggesting
radiotherapy is beneficial, while others reported an increase in
seizures post-radiation (86).
The systematic review by Wu et al (87) also identified seizures as a side
effect of radiotherapy in higher-grade meningiomas (grades II and
III), however with a low incidence. Acute seizures are a known risk
associated with brain irradiation resulting from neuronal and
vascular alterations that may ultimately lead to brain edema and
can occur several months post-radiotherapy (88,89). The impact of radio/chemotherapy
on seizure control in patients with brain tumors remains poorly
researched, maybe accounting for the aforementioned contradictory
findings. Further research is encouraged to evaluate the
risk-benefit ratio of initiating radiotherapy in brain tumor
patients with epilepsy.
The use of ASMs in the context of BTRE poses a
unique challenge. In addition to potential adverse side effects,
the physician should also consider probable interactions with other
anticancer therapies. The ideal medications should consequently be
those that alleviate both of these difficulties. BTRE is
categorized as focal seizures, with or without secondary
generalization (4). Multiple
professional organizations have advised against the administration
of ASMs as a prophylactic treatment in seizure-naïve patients with
brain lesions and have discredited the need to use ASMs for
patients undergoing brain tumor surgery in the absence of seizures
(90). However, it is not
consistently implemented in practice (91,92).
The International League Against Epilepsy
classification of some ASMs is as follows: Levetiracetam (LEV),
Carbamazepine (CBZ), Phenytoin (PHT) and Zonisamide (ZSD) are
classified as level A ASMs. At level B, Valproic Acid (VPA) is
classified; at level C, Gabapentin (GBP), Lamotrigine (LMG),
Oxcarbazepine (OXC), Phenobarbital (PHB), Topiramate (TOP) and
Vigabatrin (VGB) are classified; and at level D, Clonazepam (CPM)
and Primidone (PMD) (93). LEV
has shown superior efficacy and fewer side effects compared with
other ASMs for BTRE, establishing it as the preferable treatment
for monotherapy, in addition to its being a class A anticonvulsant
(94,95). Moreover, LEV has no
pharmacological interactions with other drugs and has minimal
enzymatic activity via cytochrome P450, making it suitable for
poly-medicated patients (96).
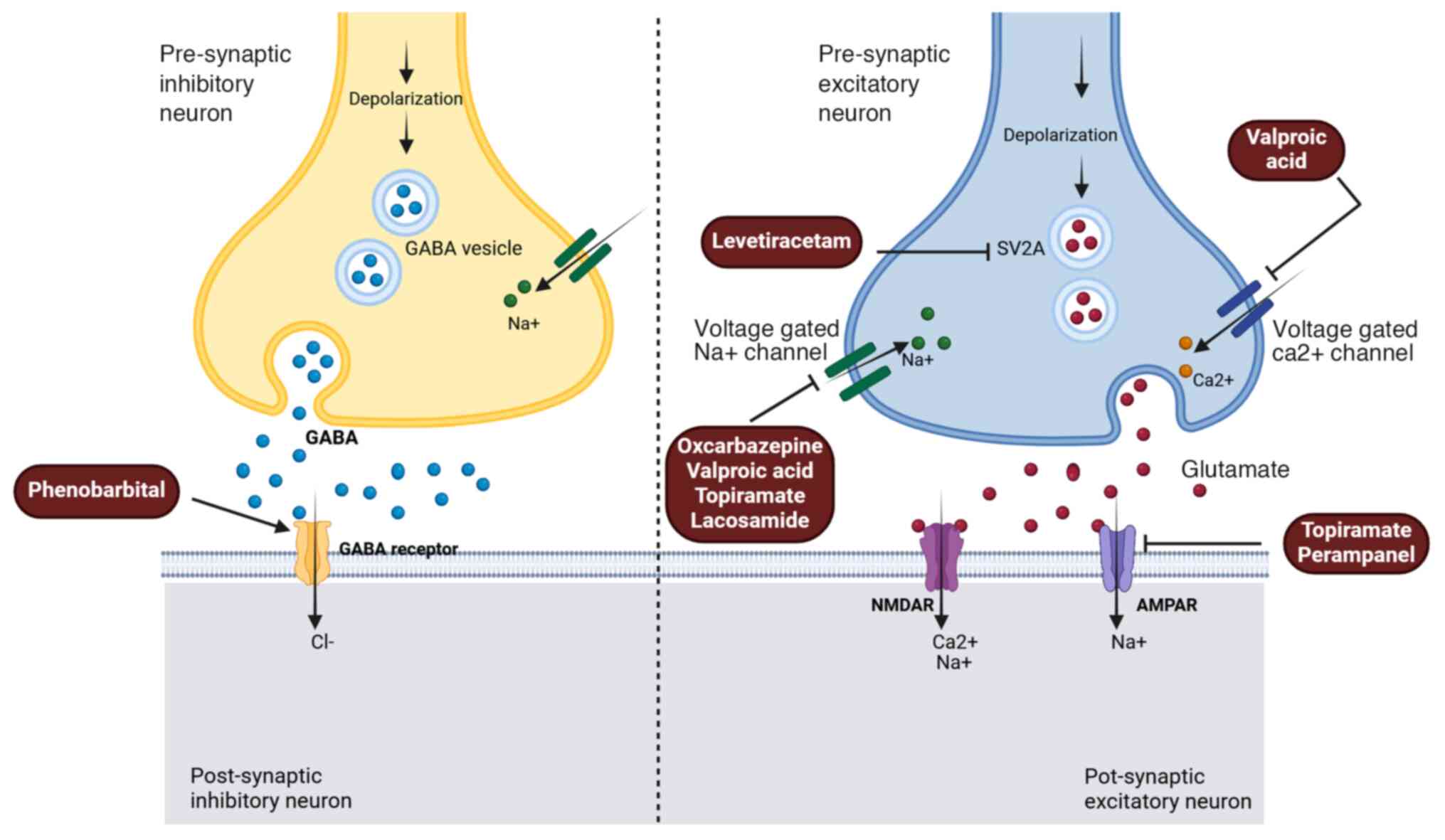
Some commonly used ASMs, their side effects, and their role in the
management of BTRE are summarized in Table I; whereas the mechanism of action
of the ASMs predominantly employed in BTRE instances is
demonstrated in Fig. 3.
Refractory seizures occur in ~15-40% of people with epilepsy due to
brain tumors or other etiologies (97,98). In this case, an adjunctive ASM is
typically implemented rather than altering the overall treatment
regimen. Polytherapy combines drugs with different mechanisms of
action to maximize the results. The combination of LEV and VPA has
shown significant results in cases of drug-resistant seizures
(99). It should be highlighted
that ASM polytherapy should be avoided in patients with BTRE
wherever possible. It can significantly impact the QoL of these
patients. Some studies have in fact shown that patients on multiple
ASMs exhibit a higher prevalence of psychiatric comorbidities and
more frequently compared with those on monotherapy (100,101).
PRN exhibits a noteworthy profile regarding BTRE. In
the United States, it is utilized in monotherapy against
focal-onset seizures, with or without generalized tonic-clonic
seizures. AMPA antagonism is a relatively novel concept in
anti-epilepsy healthcare, with the first ASMs employing this
mechanism emerging in the 2nd generation. The majority of ASMs
available are ion channel blockers or GABA enhancers, and over
time, there is a possibility for drug resistance to occur.
Targeting NMDA receptors has so far produced limited outcomes, as
they facilitate slow and long-lasting synaptic changes, which are
essential for learning and memory processes. As a result,
researchers have shifted their attention to AMPA receptor blockade.
The most ubiquitous AMPA receptor antagonist, PRN, a 3rd generation
ASM, holds significant potential. In clinical trials, it has shown
minimal adverse reactions, with dizziness being the most common
(123,124). Currently, the clinical dosage
of PRN ranges from 2 to 12 mg daily, with an increased likelihood
of adverse side effects at higher doses, while the improvements in
efficacy do not appear to increase proportionally (124). Although PRN is utilized as
monotherapy for epilepsy in a few countries, its efficacy in BTRE
has predominantly been demonstrated when used as an adjunctive ASM.
A recent study reported promising results when using PRN
monotherapy in patients with brain tumors, although the sample size
was limited (118). Future
studies with larger cohorts are needed.
Enzyme-inducing ASMs (EIASMs) include PHT, PHB, CBZ,
PMD, OXC and TOP. VPA functions as an enzyme inhibitor. EIASMs can
shorten the bioavailability of other ASMs in polytherapy; however,
this is often not challenging, as the additional drug compensates
for the diminished efficacy of the others. Yet still, physicians
should bear these interactions in mind when implementing
anti-seizure polytherapy, particularly with ASMs such as LMG that
pose further challenges in clinical use.
The most sensible area for meticulous monitoring of
these interactions is chemotherapy. A systematic review by Bénit
and Vecht (125) estimated that
EIASMs could increase the clearance of chemotherapeutic agents by 2
to 3-fold the normal rate. Glucocorticoids are also a mainstay in
the treatment plan for patients with brain tumor. A study showed
that dexamethasone (DXM) bioavailability was only 33% in
neurosurgical patients on phenytoin, whereas it was 84% in those
not receiving any ASMs (126).
The inverse effect also occurs; DXM as an enzyme inducer can reduce
PHT levels while also increasing it through a protein-binding
mechanism (88). VPA is still
routinely used in BTRE monotherapy and exhibits notable drug
interactions, such as increasing the toxicity of nitrosoureas,
cisplatin and etoposide (103).
Inversely, cisplatin reduces the bioavailability of VPA, possibly
affecting seizure control (127). All these challenges have
resulted in the emergence of new classes of ASMs with minimal
enzymatic activity, encouraging physicians to choose these over
older generations. The chemotherapeutic drug temozolomide also
presents an effective profile in glioma treatment due to its dearth
of pharmacologic interactions with ASMs, as it is not metabolized
by hepatic enzymes.
Patients with brain tumors often present additional
health complications that require extra precautions while using
ASMs. Furthermore, the management of comorbidities may occasionally
benefit from certain anti-seizure medications. Patients with brain
tumors who also experience migraine headaches may benefit from VPA,
ZSD and TOP (128,129). In the mentally disabled, ASM
monotherapy should be prioritized whenever feasible due to the
higher susceptibility of this population to the adverse effects of
ASMs (130). Patients with
behavioral issues should avoid LEV, Brivaracetam, PRN and TOP. VPA
appears to be well tolerated in patients with psychiatric problems
such as anxiety, depression, or psychosis, but LEV should be
avoided in this group (130).
Concerning infectious comorbidities,
anti-tuberculosis drugs Isoniazid and Rifampicin exert opposing
effects on the metabolism of VPA (131). Numerous studies present
conflicting results on the impact of VPA on viral load in patients
with HIV (132-134). Further studies with larger
cohorts are needed to evaluate potential interactions between VPA
and antiretroviral drugs. In patients with HIV, LEV should be
prioritized, with TOP as a second line option (130).
Seizures resulting from brain tumors during
pregnancy are thankfully a rare phenomenon. PHT is a highly
teratogenic ASM, responsible for the Fetal Hydantoin Syndrome; it
should be considered a last-line option of treatment during
pregnancy. Studies suggest that VPA is also responsible for
numerous birth defects. In a large French cohort study by Blotiere
et al (135), they
investigated the risk of developing 23 distinct malformations due
to prenatal exposure to 10 different ASMs; VPA emerged as the most
teratogenic, with TOP also identified as a significant risk factor.
ASMs suitable for BTRE monotherapy, such as LEV, OXC and the
adjunct ASM LMG, were the least likely to cause birth defects
(135). These results
corroborated the findings of a previous study on the same topic
(136). Furthermore, VPA would
also be responsible for developmental and cognitive impairments in
children born to mothers who received this ASM during pregnancy
(137,138).
Some of the mechanisms underlying epileptogenesis
are also involved in tumor growth and progression. Tumor-to-neuron
synapses act bidirectionally; the tumor increases neuronal
excitability by upregulating AMPA receptor activity and glutamate
release, while neurons release a mitogen known as neuroligin-3,
which stimulates tumor growth through the activation of the
PI3K-mTOR pathway (35,139,140). Further evidence of this
mechanism was shown when AMPA receptor antagonist Perampanel (PRN)
successfully slowed down tumor growth (140). Another growth mechanism is
considered to involve NMDA receptors, as blockade of these
receptors was successful in mitigating tumor growth in another
study (141). This then
presupposes that tumor treatment should influence epileptic
activity, and vice-versa. Over the years, studies have emerged
about the potential role of ASMs in improving survivability in
brain tumor patients, to varying degrees of results. The majority
of the studies have focused on LEV and VPA, due to their greater
potential.
LEV has been shown to inhibit O6-Methylguanine-DNA
Methyltransferase (MGMT), a DNA repair enzyme involved in the
proliferation of cancer cells (142). A few retrospective studies
concluded that LEV improved survival in patients undergoing
chemotherapy with temozolomide for glioblastoma (143,144). However, a meta-analysis and a
prospective randomized control trial, both featuring larger sample
sizes and more curated statistical methodologies, found no evidence
supporting the efficacy of LEV as an antineoplastic agent in
patients with GBM (145,146).
The differences in outcome across these studies suggest the
potential influence of additional factors, such as the molecular
profile of the GBM (MGMT methylated vs non-methylated; IDH-mutant
vs. IDH-wild type). This presents a promising avenue for future
research.
The role of VPA as an antineoplastic agent is less
controversial than that of LEV, and it is the most extensively
researched in this context, possibly due to the initial
identification of potential beneficial effects of ASMs on survival
in brain tumors involving this drug (147). Survival rate is increased when
combining VPA with temozolomide for glioblastoma management
(85,148,149). VPA can act as a histone
deacetylase inhibitor (HDACI), particularly at high doses (150,151). HDACIs are a new group of
anticancer agents that induce cell cycle arrest and eventually
apoptosis in cancer cells. Another hypothesis is that VPA, through
its enzyme inhibitory activity, increases the bioavailability of
temozolomide, hence augmenting its chemotherapeutic efficacy
(149).
Other ASMs have displayed antitumor effects, mostly
in a preclinical setting. Salmaggi et al (152) and Lange et al (153) investigated the influence of PRN
on tumor growth in vitro. Both studies concluded that PRN
limits tumor growth by promoting cell apoptosis (152,153). Interestingly, Lange et
al (154) discovered no
advantage in survival improvement regarding the role of PRN on
tumor progression in vivo (154). Brivaracetam and Lacosamide
(LCM) have also demonstrated antineoplastic properties in in
vitro experiments (155).
Additionally, studies indicated that LCM and CBZ exhibit HDAC
inhibition activity (156,157), suggesting a similar mechanism
to VPA in inhibiting tumor growth; this requires further
exploration. Other studies have shown the antiproliferative effects
of LMG and PHT on breast cancers (158,159). Future studies could help
determine if these two ASMs should be prioritized for seizures
resulting from BM related to a breast tumor.
There is no consensus on the timing of
discontinuation of ASMs in patients with BTRE, necessitating
cooperation between the patient and their physician. Factors
influencing ASM withdrawal include patient preference, cognitive or
significant side effects, polypharmacy, and sedation or fatigue.
Notable risk factors for postoperative seizures include a history
of preoperative seizures, tumor progression, incomplete surgical
resection and tumor location, particularly in the temporal, insular
and frontal lobes.
The timing of withdrawal is also important. For
non-tumor-related epilepsy, epileptologists suggest it is safe to
discontinue ASMs after 1 year of seizure freedom; nevertheless,
this choice varies significantly across clinicians (162,163). Research on tumor-associated
epilepsy is limited. In a study by Jiang et al (164) on seizure relapse in glioma
patients, 28 patients experienced relapse, with 20 of them (71.4%)
relapsing within 6 months following the withdrawal of ASMs. All
patients included in the study were on anti-seizure medication for
at least 2 years postoperatively prior to withdrawal (164). In summary, withdrawal remains a
contentious topic with no consensus; it is hoped that future
studies could help guide practitioners in decision-making about
discontinuation of ASMs.
There is ongoing research focused on new targets for
the development of ASMs. While the majority of this research is
centered on primary epilepsy, certain mechanisms described in the
physiopathology section of the present review are relevant to
epilepsy due to causes other than brain tumors. This suggests that
prospective new molecules resulting from these studies may still be
beneficial to patients suffering from BTRE. Some new molecules,
under investigation, and targeting mechanisms outlined in the
pathophysiology section of this review are discussed below. Ongoing
initiatives are being made to cure epilepsy by directly targeting
epileptogenesis to alter the disease process or develop preventive
measures.
Generally, tumor cells utilize more energy than
normal for their metabolism compared with normal cells. With IDH
mutation, glioma cells mostly utilize glycolysis for ATP
production, rather than the Krebs cycle (Warburg effect), and they
upregulate GLUT1 and GLUT3 transporters expression to further
augment energy production (165). 2-deoxy-D-glucose (2DG), a
glycolysis inhibitor, is currently the focus of several preclinical
trials for the treatment of various cancers, including GBM
(166). PET imaging studies
with radiolabeled 2DG have shown increased glucose utilization in
epileptic brain regions during the ictal phase of seizures,
correlating with increased neuronal activity (167). This suggests a possible
anti-seizure effect of 2DG. Both the acute and chronic anti-seizure
effects of 2DG were evidenced in a study by. Stafstrom et al
(168), whilst another study
found that long-term administration of the drug was pro-convulsant
(169). A proposed rationale
for these contradictory results was that 2DG, by blocking glucose
entry into cells, could lead to seizures, as hypoglycemia-induced
seizures are a well-documented phenomenon (169). Additionally, some cardiac side
effects have been reported in preclinical trials involving 2DG
(170), further undermining its
safety profile for clinical application.
Ivosidenib is an IDH1 inhibitor approved for
treating acute myeloid leukemia. Its efficacy as an ASM is
currently confined to a limited number of case reports, with
seizure control being a secondary objective in one instance
(171,172). It is anticipated that these
reports will stimulate further interest in the function of this
molecule as an anti-seizure agent and result in more comprehensive
and definitive research.
HMGB1 overexpression during neuroinflammation leads
to downstream activation of PICs such as TLR4, and other factors
such as receptor for RAGE and NF-κB. It is then stipulated that the
HMGB1/TLR4/RAGE/NF-κB signaling pathway is responsible for the
overexpression of P-glycoprotein (P-gp), a transporter protein
associated with drug-resistant epilepsy (DRE) (173,174). This has led to various studies
on HMGB1 blockage for the treatment of DRE, including the
development of the monoclonal antibody anti-HMGB1 mAb, which has
proved its anti-seizure potential in animal models (175-177). Anti-HMGB1 mAb has also shown
its efficacy in a variety of CNS diseases, some of which may entail
seizures (178).
BBB breakdown secondary to neuroinflammation
positions astrocytes as well as PICs as potential new targets for
the development of new ASMs. VX-765 (Belnacasan), an inhibitor of
interleukin converting enzyme/caspase-1, which subsequently
inhibits IL-1β and HMGB1 downstream, has shown anti-seizure
efficacy (179), and possesses
a favorable clinical profile (180). In other studies, the antagonism
of TLR4 and IL-1R1 with Anakinra, a second-line drug used in the
treatment of rheumatoid arthritis, diminished seizure activity in
animal models (27,181,182), presumably via decreased
expression of NMDA glutamate receptors. Anakinra has mostly been
utilized in the care of febrile infection-related epilepsy syndrome
in humans, yielding positive results (183-185). Most of the evidence derives
from case reports; hence, studies with larger cohorts may be
warranted. Elsewhere, research has shown that the activation of the
ALK5/TGF-β signaling pathway in astrocytes, facilitated by albumin
following BBB breakdown, promotes seizures via excitatory
synaptogenesis (186). The
mechanism involves the overactivity of this pathway, which inhibits
K+ buffering by decreasing Kir 4.1 channel function and
glutamate reuptake abilities of astrocytes (187). The ALK5/TGF-β pathway then
constitutes another target for the development of novel
anti-seizure medications.
Another prospect for inhibition in the fight
against seizures and epilepsy is the complement receptor C5ar1.
Complement activation is a critical occurrence in inflammation, and
the study by Benson et al (188) found that pro-inflammatory
receptor C5ar1 was upregulated in rats following induced status
epilepticus. Utilizing the C5ar1 inhibitor PMX35, they effectively
diminished both the frequency and severity of acute and chronic
seizures (188). The proposed
mechanism posits that the inhibition of C5ar1 results in a
decreased release of PICs, including TNF-α and IL-1β.
The mammalian target of rapamycin (mTOR) plays a
crucial role in several phases of the cell cycle and is primarily
composed of two subunit complexes: mTORC1 and mTORC2. The complex
mTORC1 facilitates the overexpression of AMPA receptors in
vitro (189). mTOR
inhibitors, such as rapamycin, have shown antiepileptic properties
in animal models across multiple studies (190,191). Nonetheless, other studies have
indicated that rapamycin exhibits inconsistency in its efficacy
against seizures (192,193). The extensive range of mTOR
signaling targets in the brain may explain the inconsistent
findings among these studies. Everolimus, a rapamycin derivative,
utilized in the treatment of some cancers, has shown efficacy in
refractory seizures (194,195), and is currently used for the
management of seizures in patients with tuberous sclerosis complex
(196). Other mTOR inhibitors
currently studied for their anti-seizure effects include the
immunosuppressant mycophenolate, novel drugs PQR620 and PQR530, as
well as natural compounds curcumin and resveratrol (197-201). PQR620 and PQR530 showed
enhanced efficacy compared with rapamycin and everolimus for the
management of seizures due to their expedited penetration of brain
tissues (199).
Researchers mostly rely on animal models to
elucidate the pathogenesis of epilepsy and develop new drugs. Given
that BTRE seizures are classified as focal with secondary
generalization, the animal model most suitable for research is the
kindling epilepsy model (202).
However, the predominant models for ASM screenings are the
pentylenetetrazol and maximum electroshock models (acute models).
Experiments with these two models were unable to demonstrate the
antiepileptic characteristics of LEV, characteristics that were
ultimately identified using the kindling model (202). Due to financial constraints,
the kindling model is not widely utilized; yet, to develop new
therapeutic agents targeted specifically at BTRE, it is imperative
to devise new, more economical models that function similarly to
the kindling model.
All ASMs attain their objective by diminishing
neuronal excitability in the brain, and given that this activity is
crucial for normal cerebral functions, most ASMs are associated
with a plethora of adverse side effects. Side effects may result in
non-compliance with treatment, incurring significant costs and
serving as a primary factor for breakthrough of seizures (203,204). The prolonged administration of
these drugs necessitates careful consideration of adverse effects.
For development of new ASMs, the efficacy is consequently at risk
of being jeopardized by safety concerns. The development of agents
that specifically target cell populations or circuits may enhance
efficacy and improve tolerance (205). A previous study concentrated on
this strategy, yielding promising results (206). Furthermore, researchers have
long endeavored to develop therapies that target epileptogenesis,
which, in the context of BTRE, could facilitate the establishment
of preventive care and/or disease-modifying therapies (DMTs). The
objective is to integrate different drugs with different mechanisms
of action (some may not necessarily be ASMs), to simultaneously
target multiple pathways involved in epileptogenesis, and to
delineate pertinent circuits (207). The Search Tool for Interacting
Chemicals is an extensive database that encompasses known and
predicted interactions between chemical molecules and proteins
across numerous species (208),
and is utilized to determine optimal pairings. Studies using this
methodology yielded combinations demonstrating promising outcomes,
including LEV/TOP, LEV/TOP/GBP and LEV/Atorvastatin/Ceftriaxone
(209,210). Notably, none of these combos
succeeded in retaining the neuroprotective effect associated with
the individual administration of some of the drugs (209,210). Nevertheless, further research
would significantly aid in identifying new combinations.
BTRE remains a crucial public health concern, with
its pathophysiological mechanisms not yet fully elucidated.
Neuroinflammation significantly contributes, as do genetic
mutations. Mechanical compression of tumors can induce stress that
results in the release of PICs from glial cells. These PICs not
only alter the electrical activity of the brain through changes in
ion channel traffic and receptor expression modulation, but they
can also trigger the BBB's rupture to amplify inflammation. The
risk of seizure development varies with the type of brain tumor,
with prevalent risk factors encompassing tumor location, size,
histopathology and level of resection.
Currently, no cure for epilepsy exists, and
available treatments are solely symptomatic, aimed at alleviating
seizures. LEV, a modulator of neurotransmitter release through
synaptic vesicle 2A binding, is currently the recommended drug for
BTRE. Other agents are rapidly emerging as monotherapy
alternatives, with PRN receiving special attention. The minimal
drug-drug interactions, selectivity for AMPARs, and antitumor
properties represent an appealing profile. Numerous anti-seizure
drugs are presently undergoing various stages of development, some
with new mechanisms of action and others aimed at restructuring
already existing ASMs (204).
In recent years, research aimed at discovering a
cure for epilepsy has achieved minimal yet significant progress.
Anti-epileptogenesis has recently been a focal center of
discussions on epilepsy therapy. Focused on prevention and disease
modification, the achievement of anti-epileptogenesis by drug
combinations would signal a significant scientific breakthrough.
Especially for BTRE, the prospects are intriguing: Preventive ASM
therapy in a meningioma patient with large peritumoral edema, a
patient with IDH1 mutated glioma, or a melanoma patient with
multiple brain metastasis. However, research on preventive
anti-seizure therapy is impeded by prolonged experiment durations
due to the necessity of monitoring the onset of epilepsy (or its
absence, indicating successful prevention), which may last for
numerous years (207,211). Consequently, most endeavors in
this research area are directed towards disease modification, with
the aspiration of finding a cure. DMT may also mitigate the adverse
side effects associated with ASMs, whose risk escalates with
prolonged usage.
New therapies require evaluation, necessitating the
use of animal models. Efforts have been made to create new models,
and the pursuit of developing more cost-effective and efficient
alternatives aimed at BTRE may be vital for this patient
population. Organizations such as the Epilepsy Therapy Screening
Program (ETSP), a division of the National Institute of
Neurological Disorders and Stroke in the USA, have played a
significant role in this field. The ETSP allows researchers
globally to evaluate new compounds through its diverse array of
animal models tailored for various circumstances (212,213). Animal models developed for
epilepsy prevention and disease modification research have recently
been incorporated into the ETSP catalog (204).
Finally, epileptogenesis and tumorigenesis are
closely interconnected processes, wherein seizures induce neuronal
cell death via excitotoxicity, subsequently leading to the
replacement of dead neurons with tumor cells for tumor
proliferation. It is hoped that future studies will facilitate the
development of a new therapeutic approach that simultaneously slows
down tumor progression and prevents seizures.
Not applicable.
CDD chose the research subject, researched the
literature for relevant articles and wrote the drafts. DOF
performed language and grammar editing. CT and XL revised the
manuscript drafts and restructured the content, and provided access
to tools used to generate the figures in the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
During the preparation of this work, Quillbot
artificial intelligence tool was used to improve the readability
and language of the manuscript or to generate images, and
subsequently, the authors revised and edited the content produced
by the artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82270825).
|
1
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the united states in 2016-2020. Neuro Oncol.
25:IV1–IV99. 2023.PubMed/NCBI
|
|
2
|
Bertolini F, Spallanzani A, Fontana A,
Depenni R and Luppi G: Brain metastases: An overview. CNS Oncol.
4:37–46. 2015.PubMed/NCBI
|
|
3
|
Englot DJ, Magill ST, Han SJ, Chang EF,
Berger MS and McDermott MW: Seizures in supratentorial meningioma:
A systematic review and meta-analysis. J Neurosurg.
124:15522015.PubMed/NCBI
|
|
4
|
Englot DJ, Chang EF and Vecht CJ: Epilepsy
and brain tumors. Handb Clin Neurol. 134:267–285. 2016.PubMed/NCBI
|
|
5
|
Easwaran TP, Lancki N, Henriquez M,
Vortmeyer AO, Barbaro NM, Scholtens DM, Ahmed AU and Dey M:
Molecular classification of gliomas is associated with seizure
control: A retrospective analysis. Neuromolecular. 23:315–326.
2021.
|
|
6
|
Chen H, Judkins J, Thomas C, Wu M, Khoury
L, Benjamin CG, Pacione D, Golfinos JG, Kumthekar P and Ghamsari F:
Mutant IDH1 and seizures in patients with glioma. Neurology.
88:1805–1813. 2017.PubMed/NCBI
|
|
7
|
Seidel S, Wehner T, Miller D, Wellmer J,
Schlegel U and Grönheit W: Brain tumor related epilepsy:
Pathophysiological approaches and rational management of
antiseizure medication. Neurol Res Pract. 4:452022.PubMed/NCBI
|
|
8
|
Soltani Khaboushan A, Yazdanpanah N and
Rezaei N: Neuroinflammation and proinflammatory cytokines in
epileptogenesis. Mol Neurobiol. 59:1724–1743. 2022.PubMed/NCBI
|
|
9
|
Rana A and Musto AE: The role of
inflammation in the development of epilepsy. J Neuroinflammation.
15:1442018.PubMed/NCBI
|
|
10
|
Zhu X, Dong J, Han B, Huang R, Zhang A,
Xia Z, Chang H, Chao J and Yao H: Neuronal nitric oxide synthase
contributes to PTZ kindling epilepsy-induced hippocampal
endoplasmic reticulum stress and oxidative damage. Front Cell
Neurosci. 11:3772017.PubMed/NCBI
|
|
11
|
Xiao Z, Peng J, Wu L, Arafat A and Yin F:
The effect of IL-1β on synaptophysin expression and
electrophysiology of hippocampal neurons through the PI3K/Akt/mTOR
signaling pathway in a rat model of mesial temporal lobe epilepsy.
Neurol Res. 39:640–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roseti C, van Vliet EA, Cifelli P, Ruffolo
G, Baayen JC, Di Castro MA, Bertollini C, Limatola C, Aronica E,
Vezzani A and Palma E: GABAA currents are decreased by IL-1β in
epileptogenic tissue of patients with temporal lobe epilepsy:
Implications for ictogenesis. Neurobiol Dis. 82:311–320. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stellwagen D and Malenka RC: Synaptic
scaling mediated by glial TNF-α. Nature. 440:1054–1059. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balosso S, Ravizza T, Perego C, Peschon J,
Campbell IL, De Simoni MG and Vezzani A: Tumor necrosis factor-α
inhibits seizures in mice via p75 receptors. Ann Neurol.
57:804–812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galic MA, Riazi K and Pittman QJ:
Cytokines and brain excitability. Front Neuroendocrinol.
33:116–125. 2011. View Article : Google Scholar
|
|
16
|
Takeuchi H, Jin S, Wang J, Zhang G,
Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T and Suzumura A: Tumor
necrosis factor-α induces neurotoxicity via glutamate release from
hemichannels of activated microglia in an autocrine manner. J Biol
Chem. 281:21362–21368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuhas Y, Weizman A and Ashkenazi S:
Bidirectional concentration-dependent effects of tumor necrosis
factor alpha in Shigella dysenteriae-related seizures. Infect
Immun. 71:2288–2291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grell M, Wajant H, Zimmermann G and
Scheurich P: The type 1 receptor (CD120a) is the high-affinity
receptor for soluble tumor necrosis factor. Proc Natl Acad Sci USA.
95:570–575. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stellwagen D, Beattie EC, Seo JY and
Malenka RC: Differential regulation of AMPA receptor and GABA
receptor trafficking by tumor necrosis factor-α. J Neurosci.
25:3219–3228. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu CY, He XY, Li XF, Zhang X, Huang ZW, Li
J, Chen M and Duan CZ: Nefiracetam attenuates Pro-inflammatory
cytokines and GABA transporter in specific brain regions of rats
with Post-ischemic seizures. Cell Physiol Biochem. 37:2023–2031.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su J, Yin J, Qin W, Sha S, Xu J and Jiang
C: Role for Pro-inflammatory cytokines in regulating expression of
GABA transporter type 1 and 3 in specific brain regions of kainic
Acid-induced status epilepticus. Neurochem Res. 40:621–627.
2015.PubMed/NCBI
|
|
22
|
Iori V, Maroso M, Rizzi M, Iyer AM,
Vertemara R, Carli M, Agresti A, Antonelli A, Bianchi ME, Aronica
E, et al: Receptor for advanced glycation endproducts is
upregulated in temporal lobe epilepsy and contributes to
experimental seizures. Neurobiol Dis. 58:102–114. 2013.PubMed/NCBI
|
|
23
|
Xie J, Méndez JD, Méndez-Valenzuela V and
Aguilar-Hernández MM: Cellular signalling of the receptor for
advanced glycation end products (RAGE). Cell Signal. 25:2185–2197.
2013.PubMed/NCBI
|
|
24
|
Chang ZL: Important aspects of Toll-like
receptors, ligands and their signaling pathways. Inflamm Res.
59:791–808. 2010.PubMed/NCBI
|
|
25
|
Balosso S, Liu J, Bianchi ME and Vezzani
A: Disulfide-containing high mobility group Box-1 promotes
N-Methyl-d-Aspartate receptor function and excitotoxicity by
activating Toll-like receptor 4-Dependent signaling in hippocampal
neurons. Antioxid Redox Signal. 21:1726–1740. 2014.
|
|
26
|
Festoff BW, Sajja RK, van Dreden P and
Cucullo L: HMGB1 and thrombin mediate the blood-brain barrier
dysfunction acting as biomarkers of neuroinflammation and
progression to neurodegeneration in Alzheimer's disease. J
Neuroinflammation. 13:1942016.PubMed/NCBI
|
|
27
|
Maroso M, Balosso S, Ravizza T, Liu J,
Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi
AA, et al: Toll-like receptor 4 and high-mobility group box-1 are
involved in ictogenesis and can be targeted to reduce seizures. Nat
Med. 16:413–419. 2010.PubMed/NCBI
|
|
28
|
Ulusoy C, Vanlı-Yavuz EN, Şanlı E,
Timirci-Kahraman Ö, Yılmaz V, Bebek N, Küçükali Cİ, Baykan B and
Tüzün E: Peripheral blood expression levels of inflammasome complex
components in two different focal epilepsy syndromes. J
Neuroimmunol. 347:5773432020.PubMed/NCBI
|
|
29
|
Vezzani A, Balosso S and Ravizza T:
Neuroinflammatory pathways as treatment targets and biomarkers in
epilepsy. Nat Rev Neurol. 15:459–472. 2019.PubMed/NCBI
|
|
30
|
Kigerl KA, de Rivero Vaccari JP, Dietrich
WD, Popovich PG and Keane RW: Pattern recognition receptors and
central nervous system repair. Exp Neurol. 258:5–16.
2014.PubMed/NCBI
|
|
31
|
Vezzani A and Baram TZ: New Roles for
interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr.
7:45–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JY, Kanai Y, Chairoungdua A, Cha SH,
Matsuo H, Kim DK, Inatomi J, Sawa H, Ida Y and Endou H: Human
cystine/glutamate transporter: cDNA cloning and upregulation by
oxidative stress in glioma cells. Biochim Biophys Acta.
1512:335–344. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Groot J and Sontheimer H: Glutamate and
the biology of gliomas. Glia. 59:1181–1189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Savaskan NE, Fan Z, Broggini T, Buchfelder
M and Eyüpoglu IY: Neurodegeneration in the brain tumor
microenvironment: Glutamate in the limelight. Curr Neuropharmacol.
13:258–265. 2015. View Article : Google Scholar :
|
|
35
|
Venkatesh HS, Morishita W, Geraghty AC,
Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami
A, Ni L, et al: Electrical and synaptic integration of glioma into
neural circuits. Nature. 573:539–545. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Groot M, Reijneveld JC, Aronica E and
Heimans JJ: Epilepsy in patients with a brain tumour: Focal
epilepsy requires focused treatment. Brain. 135:1002–1016. 2012.
View Article : Google Scholar
|
|
37
|
Kim JW and Dang CV: Cancer's molecular
sweet tooth and the Warburg effect. Cancer Res. 66:8927–8930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mortazavi A, Fayed I, Bachani M, Dowdy T,
Jahanipour J, Khan A, Owotade J, Walbridge S, Inati SK, Steiner J,
et al: IDH-mutated gliomas promote epileptogenesis through
d-2-hydroxyglutarate-dependent mTOR hyperactivation. Neuro Oncol.
24:1423–1435. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szopa W, Burley TA, Kramer-Marek G and
Kaspera W: Diagnostic and therapeutic biomarkers in glioblastoma:
Current status and future perspectives. Biomed Res Int.
2017:80135752017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Dube C, Gibert M Jr, Cruickshanks
N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K, et al:
The p53 pathway in glioblastoma. Cancers (Basel). 10:2972018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smith JS, Tachibana I, Passe SM, Huntley
BK, Borell TJ, Iturria N, O'Fallon JR, Schaefer PL, Scheithauer BW,
James CD, et al: PTEN mutation, EGFR Amplification, and outcome in
patients with anaplastic astrocytoma and glioblastoma multiforme. J
Natl Cancer Inst. 93:1246–1256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu S, Kao HY, Yang T and Wang Y: Early and
Bi-hemispheric seizure onset in a rat glioblastoma Multiforme
model. Neurosci Lett. 766:1363512022. View Article : Google Scholar
|
|
44
|
Venkatesan S, Lamfers MLM, Dirven CMF and
Leenstra S: Genetic biomarkers of drug response for small-molecule
therapeutics targeting the RTK/Ras/PI3K, p53 or Rb pathway in
glioblastoma. CNS Oncol. 5:77–790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Williams MR, De-Spenza T, Li M, Gulledge
AT and Luikart BW: Hyperactivity of newborn pten Knock-out neurons
results from increased excitatory synaptic drive. J Neurosci.
35:943–953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yonan JM, Chen KD, Baram TZ and Steward O:
PTEN deletion in the adult dentate gyrus induces epilepsy.
Neurobiol Dis. 203:1067362024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hills KE, Kostarelos K and Wykes RC:
Converging mechanisms of epileptogenesis and their insight in
glioblastoma. Front Mol Neurosci. 15:9031152022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sabetghadam A, Wu C, Liu J, Zhang L and
Reid AY: Increased epileptogenicity in a mouse model of
neurofibromatosis type 1. Exp Neurol. 331:1133732020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Engel T, Murphy BM, Schindler CK and
Henshall DC: Elevated p53 and lower MDM2 expression in hippocampus
from patients with intractable temporal lobe epilepsy. Epilepsy
Res. 77:151–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morrison RS, Wenzel HJ, Kinoshita Y,
Robbins CA, Donehower LA and Schwartzkroin PA: Loss of the p53
tumor suppressor gene protects neurons from kainate-induced cell
death. J Neurosci. 16:1337–1345. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Engel T, Murphy BM, Hatazaki S,
Jimenez-Mateos EM, Concannon CG, Woods I, Prehn JH and Henshall DC:
Reduced hippocampal damage and epileptic seizures after status
epilepticus in mice lacking proapoptotic Puma. FASEB J. 24:853–861.
2010. View Article : Google Scholar
|
|
52
|
Burla R, La Torre M, Zanetti G,
Bastianelli A, Merigliano C, Del Giudice S, Vercelli A, Di Cunto F,
Boido M, Vernì F and Saggio I: P53-sensitive epileptic behavior and
inflammation in Ft1 hypomorphic mice. Front Genet. 9:5812018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Culmsee C, Zhu X, Yu QS, Chan SL,
Camandola S, Guo Z, Greig NH and Mattson MP: A synthetic inhibitor
of p53 protects neurons against death induced by ischemic and
excitotoxic insults, and amyloid β-peptide. J Neurochem.
77:220–228. 2001.PubMed/NCBI
|
|
54
|
Djebali M, Lerner-Natoli M, Pascale M,
Baille V, Bockaert J and Rondouin G: Molecular events involved in
neuronal death induced in the mouse hippocampus by in-vivo
injection of kainic acid. Brain Res Mol Brain Res. 93:190–198.
2001. View Article : Google Scholar
|
|
55
|
Butt AM and Kalsi A: Inwardly rectifying
potassium channels (Kir) in central nervous system glia: A special
role for Kir4.1 in glial functions. J Cell Mol Med. 10:33–44. 2007.
View Article : Google Scholar
|
|
56
|
Nadella RK, Chellappa A, Subramaniam AG,
More RP, Shetty S, Prakash S, Ratna N, Vandana VP, Purushottam M,
Saini J, et al: Identification and functional characterization of
two novel mutations in KCNJ10 and PI4KB in SeSAME syndrome without
electrolyte imbalance. Hum Genomics. 13:532019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reichold M, Zdebik AA, Lieberer E,
Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton
D, Baukrowitz T, et al: KCNJ10 gene mutations causing EAST syndrome
(epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt
channel function. Proc Natl Acad Sci USA. 107:14490–14495. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Curry RN, Aiba I, Meyer J, Lozzi B, Ko Y,
McDonald MF, Rosenbaum A, Cervantes A, Huang-Hobbs E, Cocito C, et
al: Glioma epileptiform activity and progression are driven by
IGSF3-mediated potassium dysregulation. Neuron. 111:682–695.e9.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
de Curtis M, Uva L, Gnatkovsky V and
Librizzi L: Potassium dynamics and seizures: Why is potassium
ictogenic? Epilepsy Res. 143:50–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Audrey C, Lim KS, Ahmad Zaki R, Narayanan
V, Fong SL and Tan CT: From location to manifestation: A systematic
review and meta-analysis of seizure prevalence in different brain
tumor sites. Brain Disord. 14:1001462024. View Article : Google Scholar
|
|
61
|
Lee JW, Wen PY, Hurwitz S, Black P, Kesari
S, Drappatz J, Golby AJ, Wells WM III, Warfield SK, Kikinis R and
Bromfield EB: Morphological characteristics of brain tumors causing
seizures. Arch Neurol. 67:336–342. 2010.PubMed/NCBI
|
|
62
|
Le VT, Nguyen AM, Pham TA and Nguyen PL:
Tumor-related epilepsy and post-surgical outcomes: Tertiary
hospital experience in Vietnam. Sci Rep. 13:108592023.PubMed/NCBI
|
|
63
|
Elbadry Ahmed R, Tang H, Asemota A, Huang
L, Boling W and Bannout F: Meningioma related
Epilepsy-Pathophysiology, Pre/postoperative seizures predicators
and treatment. Front Oncol. 12:9059762022.
|
|
64
|
Harward SC, Rolston JD and Englot DJ:
Seizures in meningioma. Handb Clin Neurol. 170:187–200.
2020.PubMed/NCBI
|
|
65
|
Asano K, Hasegawa S, Matsuzaka M and
Ohkuma H: Brain tumor-related epilepsy and risk factors for
metastatic brain tumors: Analysis of 601 consecutive cases
providing real-world data. J Neurosurg. 136:76–87. 2021.PubMed/NCBI
|
|
66
|
Sankey EW, Tsvankin V, Grabowski MM, Nayar
G, Batich KA, Risman A, Champion CD, Salama AKS, Goodwin CR and
Fecci PE: Operative and peri-operative considerations in the
management of brain metastasis. Cancer Med. 8:6809–6831.
2019.PubMed/NCBI
|
|
67
|
Rudà R, Mo F and Pellerino A: Epilepsy in
brain metastasis: An emerging entity. Curr Treat Options Neurol.
22:62020.PubMed/NCBI
|
|
68
|
Garcia JH, Morshed RA, Chung J, Millares
Chavez MA, Sudhakar V, Saggi S, Avalos LN, Gallagher A, Young JS,
Daras M, et al: Factors associated with preoperative and
postoperative seizures in patients undergoing resection of brain
metastases. J Neurosurg. 138:19–26. 2023.
|
|
69
|
Wolpert F, Lareida A, Terziev R,
Grossenbacher B, Neidert MC, Roth P, Poryazova R, Imbach LL, Le
Rhun E, Weller M, et al: Risk factors for the development of
epilepsy in patients with brain metastases. Neuro Oncol.
22:718–728. 2020.
|
|
70
|
Urban H, Willems LM, Ronellenfitsch MW,
Rosenow F, Steinbach JP and Strzelczyk A: Increased occurrence of
status epilepticus in patients with brain metastases and checkpoint
inhibition. Oncoimmunology. 9:18515172020.PubMed/NCBI
|
|
71
|
Li L, Li G, Fang S, Zhang K, Huang R, Wang
Y, Zhang C, Li Y, Zhang W, Zhang Z, et al: New-Onset postoperative
seizures in patients with diffuse gliomas: A risk assessment
analysis. Front Neurol. 12:6825352021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mastall M, Wolpert F, Gramatzki D, Imbach
L, Becker D, Schmick A, Hertler C, Roth P, Weller M and Wirsching
HG: Survival of brain tumour patients with epilepsy. Brain.
144:3322–3327. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ge H, Di G, Yan Z, Liu D, Liu Y, Song K,
Yang K, Hu X, Jiang Z, Hu X, et al: Does epilepsy always indicate
worse outcomes? A longitudinal follow-up analysis of 485 glioma
patients. World J Surg Oncol. 20:2972022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Vecht CJ, Kerkhof M and Duran-Pena A:
Seizure prognosis in brain tumors: New insights and Evidence-based
management. Oncologist. 19:751–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Giraldi L, Hansen JV, Wohlfahrt J,
Fugleholm K, Melbye M and Munch TN: Postoperative de novo epilepsy
after craniotomy: A nationwide register-based cohort study. J
Neurol Neurosurg Psychiatry. 93:436–444. 2022. View Article : Google Scholar
|
|
76
|
Abzalova DI, Sinkin MV, Yakovlev AA,
Prirodov AV and Guekht AB: Risk factors for the development of de
novo generalized tonic-clonic epileptic seizures in patients with
supratentorial meningiomas after neurosurgical treatment. Neurosci
Behav Physiol. 54:404–409. 2024.In Russian. View Article : Google Scholar
|
|
77
|
Englot DJ, Berger MS, Barbaro NM and Chang
EF: Predictors of seizure freedom after resection of supratentorial
low-grade gliomas: A review. J Neurosurg. 115:240–244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schneider M, Güresir Á, Borger V, Hamed M,
Rácz A, Vatter H, Güresir E and Schuss P: Preoperative
tumor-associated epilepsy in patients with supratentorial
meningioma: Factors influencing seizure outcome after meningioma
surgery. J Neurosurg. 133:1655–1661. 2020. View Article : Google Scholar
|
|
79
|
Jackson C, Choi J, Khalafallah AM, Price
C, Bettegowda C, Lim M, Gallia G, Weingart J, Brem H and Mukherjee
D: A systematic review and meta-analysis of supratotal versus gross
total resection for glioblastoma. J Neurooncol. 148:419–431. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Englot DJ, Han SJ, Berger MS, Barbaro NM
and Chang EF: Extent of surgical resection predicts seizure freedom
in low-grade temporal lobe brain tumors. Neurosurgery. 70:921–927.
2012. View Article : Google Scholar
|
|
81
|
Koekkoek JAF, Kerkhof M, Dirven L, Heimans
JJ, Reijneveld JC and Taphoorn MJB: Seizure outcome after
radiotherapy and chemotherapy in low-grade glioma patients: A
systematic review. Neuro Oncol. 17:924–934. 2015.PubMed/NCBI
|
|
82
|
Rudá R, Magliola U, Bertero L, Trevisan E,
Bosa C, Mantovani C, Ricardi U, Castiglione A, Monagheddu C and
Soffietti R: Seizure control following radiotherapy in patients
with diffuse gliomas: A retrospective study. Neuro Oncol.
15:1739–1749. 2013.PubMed/NCBI
|
|
83
|
Van Den Bent MJ, Afra D, De Witte O, Ben
Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L,
Piérart M, Mirimanoff R, et al: Long-term efficacy of early versus
delayed radiotherapy for low-grade astrocytoma and
oligodendroglioma in adults: The EORTC 22845 randomised trial.
Lancet. 366:985–990. 2005.PubMed/NCBI
|
|
84
|
Brada M, Viviers L, Abson C, Hines F,
Britton J, Ashley S, Sardell S, Traish D, Gonsalves A, Wilkins P
and Westbury C: Phase II study of primary temozolomide chemotherapy
in patients with WHO grade II gliomas. Ann Oncol. 14:1715–1721.
2003.PubMed/NCBI
|
|
85
|
Sherman JH, Moldovan K, Yeoh HK, Starke
RM, Pouratian N, Shaffrey ME and Schiff D: Impact of temozolomide
chemotherapy on seizure frequency in patients with low-grade
gliomas: Clinical article. J Neurosurg. 114:1617–1621.
2011.PubMed/NCBI
|
|
86
|
Rades D, Witteler J, Trillenberg P,
Olbrich D, Schild SE, Tvilsted S and Kjaer TW: Increasing seizure
activity during radiation treatment for High-grade Gliomas-final
results of a prospective interventional study. In Vivo.
36:2308–2313. 2022.PubMed/NCBI
|
|
87
|
Wu A, Jin MC, Meola A, Wong HN and Chang
SD: Efficacy and toxicity of particle radiotherapy in WHO grade II
and grade III meningiomas: A systematic review. Neurosurg Focus.
46:E122019.PubMed/NCBI
|
|
88
|
Grewal J, Grewal HK and Forman AD:
Seizures and epilepsy in cancer: Etiologies, Evaluation, and
Management. Curr Oncol Rep. 10:63–71. 2008.PubMed/NCBI
|
|
89
|
Smart DD: Radiation toxicity in the
central nervous system: Mechanisms and strategies for injury
reduction. Semin Radiat Oncol. 27:332–339. 2017.PubMed/NCBI
|
|
90
|
Walbert T, Harrison RA, Schiff D, Avila
EK, Chen M, Kandula P, Lee JW, Le Rhun E, Stevens GHJ, Vogelbaum
MA, et al: SNO and EANO practice guideline update: Anticonvulsant
prophylaxis in patients with newly diagnosed brain tumors. Neuro
Oncol. 23:1835–1844. 2021.PubMed/NCBI
|
|
91
|
Van Der Meer PB, Dirven L, Van Den Bent
MJ, Preusser M, Taphoorn MJB, Rudá R and Koekkoek JAF: Prescription
preferences of antiepileptic drugs in brain tumor patients: An
international survey among EANO members. Neurooncol Pract.
9:105–113. 2022.PubMed/NCBI
|
|
92
|
Dewan MC, Thompson RC, Kalkanis SN, Barker
FG and Hadjipanayis CG: Prophylactic antiepileptic drug
administration following brain tumor resection: Results of a recent
AANS/CNS section on tumors survey. J Neurosurg. 126:1772–1778.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Glauser T, Ben-Menachem E, Bourgeois B,
Cnaan A, Guerreiro C, Kälviäinen R, Mattson R, French JA, Perucca E
and Tomson T; ILAE Subcommission on AED Guidelines: Updated ILAE
evidence review of antiepileptic drug efficacy and effectiveness as
initial monotherapy for epileptic seizures and syndromes.
Epilepsia. 54:551–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Maschio M, Dinapoli L, Sperati F, Pace A,
Fabi A, Vidiri A and Muti P: Levetiracetam monotherapy in patients
with brain tumor-related epilepsy: Seizure control, safety, and
quality of life. J Neurooncol. 104:205–214. 2011. View Article : Google Scholar
|
|
95
|
Bähr O, Hermisson M, Rona S, Rieger J,
Nussbaum S, Körtvelyessy P, Franz K, Tatagiba M, Seifert V, Weller
M and Steinbach JP: Intravenous and oral levetiracetam in patients
with a suspected primary brain tumor and symptomatic seizures
undergoing neurosurgery: The HELLO trial. Acta Neurochir (Wien).
154:229–235. 2012. View Article : Google Scholar
|
|
96
|
LaPenna P and Tormoehlen LM: The
pharmacology and toxicology of Third-generation anticonvulsant
drugs. J Med Toxicol. 13:329–342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jo J, Nevel K, Sutyla R, Smolkin M, Lopes
MB and Schiff D: Predictors of early, recurrent, and intractable
seizures in low-grade glioma. Neurooncol Pract. 8:40–47. 2020.
|
|
98
|
Singh SP, Agarwal S and Faulkner M:
Refractory status epilepticus. Ann Indian Acad Neurol. 17(Suppl 1):
S32–S36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Van Breemen MSM, Rijsman RM, Taphoorn MJB,
Walchenbach R, Zwinkels H and Vecht CJ: Efficacy of anti-epileptic
drugs in patients with gliomas and seizures. J Neurol.
256:1519–1526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yadav J, Singh P, Dabla S and Gupta R:
Psychiatric comorbidity and quality of life in patients with
epilepsy on anti-epileptic monotherapy and polytherapy. Tzu Chi Med
J. 34:226–231. 2021. View Article : Google Scholar
|
|
101
|
Khalid B, Waqar Z, Khan S, Ali I, Afzal N,
Irfan A, Malik W, Muhammad Adil M, Saddiqa A, Khalil M and Munawar
Z: Psychiatric implications of anti-seizure medications in
epileptic population. BMC Neurol. 24:1662024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Besag FMC, Vasey MJ and Sen A: Current
evidence for adjunct pyridoxine (vitamin B6) for the treatment of
behavioral adverse effects associated with levetiracetam: A
systematic review. Epilepsy Behav. 140:1090652023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bourg V, Lebrun C, Chichmanian RM, Thomas
P and Frenay M: Nitroso-urea-cisplatin-based chemotherapy
associated with valproate: Increase of haematologic toxicity. Ann
Oncol. 12:217–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Simó M, Velasco R, Graus F, Verger E, Gil
M, Pineda E, Blasco J and Bruna J: Impact of antiepileptic drugs on
thrombocytopenia in glioblastoma patients treated with standard
chemoradiotherapy. J Neurooncol. 108:451–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kerkhof M, Dielemans JCM, Van Breemen MS,
Zwinkels H, Walchenbach R, Taphoorn MJ and Vecht CJ: Effect of
valproic acid on seizure control and on survival in patients with
glioblastoma multiforme. Neuro Oncol. 15:961–967. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sánchez-Villalobos JM, Aledo-Serrano Á,
Villegas-Martínez I, Shaikh MF and Alcaraz M: Epilepsy treatment in
neuro-oncology: A rationale for drug choice in common clinical
scenarios. Front Pharmacol. 13:9912442022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mo F, Meletti S, Belcastro V, Quadri S,
Napolitano M, Bello L, Dainese F, Scarpelli M, Florindo I, Mascia
A, et al: Lacosamide in monotherapy in BTRE (brain tumor-related
epilepsy): Results from an Italian multicenter retrospective study.
J Neurooncol. 157:551–559. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Villanueva V, Saiz-Diaz R, Toledo M, Piera
A, Mauri JA, Rodriguez-Uranga JJ, López-González FJ, Gómez-Ibáñez
A, Garcés M, González de la Aleja J, et al: NEOPLASM study:
Real-life use of lacosamide in patients with brain tumor-related
epilepsy. Epilepsy Behav. 65:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
van Opijnen MP, van der Meer PB, Dirven L,
Fiocco M, Kouwenhoven MCM, van den Bent MJ, Taphoorn MJB and
Koekkoek JAF: The effectiveness of antiepileptic drug treatment in
glioma patients: Lamotrigine versus lacosamide. J Neurooncol.
154:73–81. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wilding J, Van Gaal L, Rissanen A,
Vercruysse F and Fitchet M: A randomized double-blind
placebo-controlled study of the long-term efficacy and safety of
topiramate in the treatment of obese subjects. Int J Obes Relat
Metab Disord. 28:1399–1410. 2004.2004. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu YT, Chen GT, Huang YC, Ho JT, Lee CC,
Tsai CC and Chang CN: Effectiveness of dose-escalated topiramate
monotherapy and add-on therapy in neurosurgery-related epilepsy: A
prospective study. Medicine (Baltimore). 99:e237712020.PubMed/NCBI
|
|
112
|
Maschio M, Dinapoli L, Zarabla A,
Maialetti A, Giannarelli D, Fabi A, Vidiri A and Cantelmi T:
Zonisamide in brain tumor-related epilepsy: An observational pilot
study. Clin Neuropharmacol. 40:113–119. 2017.PubMed/NCBI
|
|
113
|
De A, Rajagopalan M, Sarda A, Das S and
Biswas P: Drug reaction with eosinophilia and systemic symptoms: An
update and review of recent literature. Indian J Dermatol.
63:30–40. 2018.PubMed/NCBI
|
|
114
|
Patocka J, Wu Q, Nepovimova E and Kuca K:
Phenytoin-An anti-seizure drug: Overview of its chemistry,
pharmacology and toxicology. Food Chem Toxicol. 142:1113932020.
|
|
115
|
Mauro AM, Bomprezzi C, Morresi S,
Provinciali L, Formica F, Iacoangeli M and Scerrati M: Prevention
of early postoperative seizures in patients with primary brain
tumors: Preliminary experience with oxcarbazepine. J Neurooncol.
81:279–285. 2007.
|
|
116
|
Maschio M, Dinapoli L, Sperati F, Fabi A,
Pace A, Vidiri A and Muti P: Oxcarbazepine monotherapy in patients
with brain tumor-related epilepsy: Open-label pilot study for
assessing the efficacy, tolerability and impact on quality of life.
J Neurooncol. 106:651–656. 2012.
|
|
117
|
Zoccarato M, Basile AM, Padovan M, Caccese
M, Zagonel V and Lombardi G: Eslicarbazepine in patients with brain
tumor-related epilepsy: A single-center experience. Int J Neurosci.
131:879–884. 2021.
|
|
118
|
Hino U, Tamura R, Kosugi K, Ezaki T,
Karatsu K, Yamamoto K, Tomioka A and Toda M: Optimizing perampanel
monotherapy for surgically resected brain tumors. Mol Clin Oncol.
20:422024.PubMed/NCBI
|
|
119
|
Perry JR and Sawka C: Add-on gabapentin
for refractory seizures in patients with brain tumours. Can J
Neurol Sci. 23:128–131. 1996.PubMed/NCBI
|
|
120
|
Brahmbhatt N, Stupp R, Bushara O, Bachman
E, Schuele SU and Templer JW: Efficacy of clobazam as add-on
therapy in brain tumor-related epilepsy. J Neurooncol. 151:287–293.
2021.PubMed/NCBI
|
|
121
|
Striano S, Striano P, Boccella P, Nocerino
C and Bilo L: Tiagabine in glial tumors. Epilepsy Res. 49:81–85.
2002.PubMed/NCBI
|
|
122
|
Maschio M, Maialetti A, Mocellini C,
Domina E, Pauletto G, Costa C, Mascia A, Romoli M and Giannarelli
D: Effect of brivaracetam on efficacy and tolerability in patients
with brain Tumor-related epilepsy: A retrospective multicenter
study. Front Neurol. 11:8132020.PubMed/NCBI
|
|
123
|
Gao L, Lu Q, Wang Z, Yue W, Wang G, Shao
X, Guo Y, Yi Y, Hong Z, Jiang Y, et al: Efficacy and safety of
perampanel as early add-on therapy in Chinese patients with
focal-onset seizures: A multicenter, open-label, single-arm study.
Front Neurol. 14:12360462023.PubMed/NCBI
|
|
124
|
Lavu A, Aboulatta L, Abou-Setta AM, Aloud
B, Askin N, Rabbani R, Shouman W, Zarychanski R and Eltonsy S:
Efficacy and safety of perampanel in epilepsy: A systematic review
and meta-analysis of randomised controlled trials. Seizure.
102:54–60. 2022.PubMed/NCBI
|
|
125
|
Bénit CP and Vecht CJ: Seizures and
cancer: Drug interactions of anticonvulsants with chemotherapeutic
agents, tyrosine kinase inhibitors and glucocorticoids. Neurooncol
Pract. 3:245–260. 2016.PubMed/NCBI
|
|
126
|
Chalk JB, Ridgeway K, Brophy T, Yelland
JDN and Eadie MJ: Phenytoin impairs the bioavailability of
dexamethasone in neurological and neurosurgical patients. J Neurol
Neurosurg Psychiatry. 47:1087–1090. 1984.PubMed/NCBI
|
|
127
|
Ikeda H, Murakami T, Takano M, Usui T and
Kihira K: Pharmacokinetic interaction on valproic acid and
recurrence of epileptic seizures during chemotherapy in an
epileptic patient. Br J Clin Pharmacol. 59:593–597. 2005.PubMed/NCBI
|
|
128
|
Bagnato F and Good J: The use of
antiepileptics in migraine prophylaxis. Headache. 56:603–615.
2016.PubMed/NCBI
|
|
129
|
Linde M, Mulleners WM, Chronicle EP and
Mccrory DC: Antiepileptics other than gabapentin, pregabalin,
topiramate, and valproate for the prophylaxis of episodic migraine
in adults. Cochrane Database Syst Rev. 2013:CD0106082013.PubMed/NCBI
|
|
130
|
Ruiz-Giménez J, Sánchez-Álvarez JC,
Cañadillas-Hidalgo F and Serrano-Castro PJ: Antiepileptic treatment
in patients with epilepsy and other comorbidities. Seizure.
19:375–382. 2010.PubMed/NCBI
|
|
131
|
Patsalos PN and Perucca E: Clinically
important drug interactions in epilepsy: Interactions between
antiepileptic drugs and other drugs. Lancet Neurol. 2:473–481.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Routy JP, Tremblay CL, Angel JB, Trottier
B, Rouleau D, Baril JG, Harris M, Trottier S, Singer J, Chomont N,
et al: Valproic acid in association with highly active
antiretroviral therapy for reducing systemic HIV-1 reservoirs:
Results from a multicentre randomized clinical study. HIV Med.
13:291–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lehrman G, Hogue IB, Palmer S, Jennings C,
Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW,
et al: Depletion of latent HIV-1 infection in vivo: A
proof-of-concept study. Lancet. 366:5492005. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sagot-Lerolle N, Lamine A, Chaix ML,
Boufassa F, Aboulker JP, Costagliola D, Goujard C, Pallier C,
Delfraissy JF and Lambotte O; ANRS EP39 study: Prolonged valproic
acid treatment does not reduce the size of latent HIV reservoir.
AIDS. 22:1125–1129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Blotière PO, Raguideau F, Weill A, Elefant
E, Perthus I, Goulet V, Rouget F, Zureik M, Coste J and Dray-Spira
R: Risks of 23 specific malformations associated with prenatal
exposure to 10 antiepileptic drugs. Neurology. 93:e167–e180. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jentink J, Loane MA, Dolk H, Barisic I,
Garne E, Morris JK and de Jong-van den Berg LT; EUROCAT
Antiepileptic Study Working Group: Valproic acid monotherapy in
pregnancy and major congenital malformations. N Engl J Med.
362:2185–2193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Meador KJ, Baker GA, Browning N, Cohen MJ,
Bromley RL, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD,
Pennell PB, et al: Fetal antiepileptic drug exposure and cognitive
outcomes at age 6 years (NEAD study): A prospective observational
study. Lancet Neurol. 12:244–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Christensen J, Grnøborg TK, Srøensen MJ,
Schendel D, Parner ET, Pedersen LH and Vestergaard M: Prenatal
valproate exposure and risk of autism spectrum disorders and
childhood autism. JAMA. 309:1696–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Venkatesh HS, Johung TB, Caretti V, Noll
A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS,
et al: Neuronal activity promotes glioma growth through
Neuroligin-3 secretion. Cell. 161:803–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Venkataramani V, Tanev DI, Strahle C,
Studier-Fischer A, Fankhauser L, Kessler T, Körber C, Kardorff M,
Ratliff M, Xie R, et al: Glutamatergic synaptic input to glioma
cells drives brain tumour progression. Nature. 573:532–538. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Stepulak A, Sifringer M, Rzeski W,
Endesfelder S, Gratopp A, Pohl EE, Bittigau P, Felderhoff-Mueser U,
Kaindl AM, Bührer C, et al: NMDA antagonist inhibits the
extracellular signal-regulated kinase pathway and suppresses cancer
growth. Proc Natl Acad Sci USA. 102:15605–15610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Bobustuc GC, Baker CH, Limaye A, Jenkins
WD, Pearl G, Avgeropoulos NG and Konduri SD: Levetiracetam enhances
p53-mediated MGMT inhibition and sensitizes glioblastoma cells to
temozolomide. Neuro Oncol. 12:917–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Roh TH, Moon JH, Park HH, Kim EH, Hong CK,
Kim SH, Kang SG and Chang JH: Association between survival and
levetiracetam use in glioblastoma patients treated with
temozolomide chemoradiotherapy. Sci Rep. 10:107832020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ryu JY, Min KL and Chang MJ: Effect of
anti-epileptic drugs on the survival of patients with glioblastoma
multiforme: A retrospective, single-center study. PLoS One.
14:e02255992019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Chen JS, Clarke R, Haddad AF, Wang EJ,
Lacroix M, Sarkar IN, Zand R, Chen ES and Toms SA: The effect of
levetiracetam treatment on survival in patients with glioblastoma:
A systematic review and meta-analysis. J Neurooncol. 156:257–267.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Happold C, Gorlia T, Chinot O, Gilbert MR,
Nabors LB, Wick W, Pugh SL, Hegi M, Cloughesy T, Roth P, et al:
Does valproic acid or levetiracetam improve survival in
glioblastoma? A pooled analysis of prospective clinical trials in
newly diagnosed glioblastoma. J Clin Oncol. 34:731–739. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Oberndorfer S, Piribauer M, Marosi C,
Lahrmann H, Hitzenberger P and Grisold W: P450 enzyme inducing and
non-enzyme inducing antiepileptics in glioblastoma patients treated
with standard chemotherapy. J Neurooncol. 72:255–260. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Krauze A, Megan M, Theresa CZ, Peter M,
Shih JH, Tofilon PJ, Rowe L, Gilbert M and Camphausen K: The
addition of Valproic acid to concurrent radiation therapy and
temozolomide improves patient outcome: A Correlative analysis of
RTOG 0525, SEER and a Phase II NCI trial. Cancer Stud Ther.
5:310382020.
|
|
149
|
Weller M, Gorlia T, Cairncross JG, van den
Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, Macdonald DR,
Forsyth P, et al: Prolonged survival with valproic acid use in the
EORTC/NCIC temozolomide trial for glioblastoma. Neurology.
77:1156–1164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Michaelis M, Doerr HW and Jr JC: Valproic
acid as Anti-cancer drug. Curr Pharm Des. 13:3378–3393. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Göttlicher M, Minucci S, Zhu P, Krämer OH,
Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG
and Heinzel T: Valproic acid defines a novel class of HDAC
inhibitors inducing differentiation of transformed cells. EMBO J.
20:6969–6978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Salmaggi A, Corno C, Maschio M, Donzelli
S, D'urso A, Perego P and Ciusani E: Synergistic effect of
perampanel and temozolomide in human glioma cell lines. J Pers Med.
11:3902021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lange F, Weßlau K, Porath K, Hörnschemeyer
MF, Bergner C, Krause BJ, Mullins CS, Linnebacher M, Köhling R and
Kirschstein T: AMPA receptor antagonist perampanel affects
glioblastoma cell growth and glutamate release in vitro. PLoS One.
14:e02116442019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Lange F, Hartung J, Liebelt C, Boisserée
J, Resch T, Porath K, Hörnschemeyer MF, Reichart G, Sellmann T,
Neubert V, et al: Perampanel Add-on to standard radiochemotherapy
in vivo promotes neuroprotection in a rodent F98 glioma Model.
Front Neurosci. 14:5982662020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Rizzo A, Donzelli S, Girgenti V, Sacconi
A, Vasco C, Salmaggi A, Blandino G, Maschio M and Ciusani E: In
vitro antineoplastic effects of brivaracetam and lacosamide on
human glioma cells. J Exp Clin Cancer Res. 36:762017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Bang SR, Ambavade SD, Jagdale PG, Adkar
PP, Waghmare AB and Ambavade PD: Lacosamide reduces HDAC levels in
the brain and improves memory: Potential for treatment of
Alzheimer's disease. Pharmacol Biochem Behav. 134:65–69. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Beutler AS, Li S, Nicol R and Walsh MJ:
Carbamazepine is an inhibitor of histone deacetylases. Life Sci.
76:3107–3115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Nelson M, Yang M, Dowle AA, Thomas JR and
Brackenbury WJ: The sodium channel-blocking antiepileptic drug
phenytoin inhibits breast tumour growth and metastasis. Mol Cancer.
14:132015. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pellegrino M, Rizza P, Nigro A, Ceraldi R,
Ricci E, Perrotta I, Aquila S, Lanzino M, Andò S, Morelli C and
Sisci D: FoxO3a mediates the inhibitory effects of the
antiepileptic drug lamotrigine on breast cancer growth. Mol Cancer
Res. 16:923–934. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Koekkoek JAF, Kerkhof M, Dirven L, Heimans
JJ, Postma TJ, Vos MJ, Bromberg JE, van den Bent MJ, Reijneveld JC
and Taphoorn MJ: Withdrawal of antiepileptic drugs in glioma
patients after long-term seizure freedom: Design of a prospective
observational study. BMC Neurol. 14:1572014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Das RR, Artsy E, Hurwitz S, Wen PY, Black
P, Golby A, Dworetzky B and Lee JW: Outcomes after discontinuation
of antiepileptic drugs after surgery in patients with low grade
brain tumors and meningiomas. J Neurooncol. 107:565–570. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Tellez-Zenteno JF, Hernandez-Ronquillo L
and Moien-Afshari F: Discontinuation of antiepileptic drugs after
successful surgery: Who and when? Epileptic Disord. 14:363–370.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Berg AT, Langfitt JT, Spencer SS and
Vickrey BG: Stopping antiepileptic drugs after epilepsy surgery: A
survey of U.S. epilepsy center neurologists. Epilepsy Behav.
10:219–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Jiang H, Deng G, Liu B, Cheng J, Li Y, Tan
Y, Wang J and Chen Q: Analysis of the short-term outcomes and risk
factors of seizure relapse in patients with gliomas after
antiepileptic drugs withdrawal. J Clin Neurosci. 82:20–25. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Han W, Shi J, Cao J, Dong B and Guan W:
Emerging roles and therapeutic interventions of aerobic glycolysis
in glioma. Onco Targets Ther. 13:6937–6955. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Singh R, Gupta V, Kumar A and Singh K:
2-Deoxy-D-Glucose: A novel pharmacological agent for killing
hypoxic tumor cells, oxygen dependence-lowering in covid-19, and
other pharmacological activities. Adv Pharmacol Pharm Sci.
2023:99933862023.PubMed/NCBI
|
|
167
|
Siclari F, Prior JO and Rossetti AO: Ictal
cerebral positron emission tomography (PET) in focal status
epilepticus. Epilepsy Res. 105:356–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Stafstrom CE, Roopra A and Sutula TP:
Seizure suppression via glycolysis inhibition with
2-deoxy-D-glucose (2DG). Epilepsia. 49:97–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Gasior M, Yankura J, Hartman AL, French A
and Rogawski MA: Anticonvulsant and proconvulsant actions of
2-deoxy-d-glucose. Epilepsia. 51:1385–1394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Minor RK, Smith DL Jr, Sossong AM, Kaushik
S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R,
et al: Chronic ingestion of 2-deoxy-D-glucose induces cardiac
vacuolization and increases mortality in rats. Toxicol Appl
Pharmacol. 243:332–339. 2010. View Article : Google Scholar :
|
|
171
|
Tejera D, Kushnirsky M, Gultekin SH, Lu M,
Steelman L and De La Fuente MI: Ivosidenib, an IDH1 inhibitor, in a
patient with recurrent, IDH1-mutant glioblastoma: A case report
from a Phase I study. CNS Oncol. 9:CNS622020. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Vo AH, Ambady P and Spencer D: The IDH1
inhibitor ivosidenib improved seizures in a patient with
drug-resistant epilepsy from IDH1 mutant oligodendroglioma.
Epilepsy Behav Rep. 18:1005262022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Xie Y, Yu N, Chen Y, Zhang K, Ma HY and Di
Q: HMGB1 regulates P-glycoprotein expression in status epilepticus
rat brains via the RAGE/NF-κB signaling pathway. Mol Med Rep.
16:1691–1700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Chen Y, Huang XJ, Yu N, Xie Y, Zhang K,
Wen F, Liu H and Di Q: HMGB1 Contributes to the Expression of
P-Glycoprotein in mouse epileptic brain through Toll-like receptor
4 and receptor for advanced glycation end products. PLoS One.
10:e01409182015. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Fu L, Liu K, Wake H, Teshigawara K,
Yoshino T, Takahashi H, Mori S and Nishibori M: Therapeutic effects
of anti-HMGB1 monoclonal antibody on pilocarpine-induced status
epilepticus in mice. Sci Rep. 7:11792017. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Ravizza T, Terrone G, Salamone A, Frigerio
F, Balosso S, Antoine DJ and Vezzani A: High Mobility Group Box 1
is a novel pathogenic factor and a mechanistic biomarker for
epilepsy. Brain Behav Immun. 72:14–21. 2018. View Article : Google Scholar
|
|
177
|
Paudel YN, Othman I and Shaikh MF:
Anti-high mobility group Box-1 monoclonal antibody attenuates
Seizure-induced cognitive decline by suppressing neuroinflammation
in an adult zebrafish model. Front Pharmacol. 11:6130092021.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Nishibori M, Mori S and Takahashi HK:
Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and
PNS diseases. J Pharmacol Sci. 140:94–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Maroso M, Balosso S, Ravizza T, Iori V,
Wright CI, French J and Vezzani A: Interleukin-1β biosynthesis
inhibition reduces acute seizures and drug resistant chronic
epileptic activity in mice. Neurotherapeutics. 8:304–315. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Kaur H, Kumar B and Medhi B: Antiepileptic
drugs in development pipeline: A recent update. eNeurologicalSci.
4:42–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Bertani I, Iori V, Trusel M, Maroso M,
Foray C, Mantovani S, Tonini R, Vezzani A and Chiesa R: Inhibition
of IL-1β signaling normalizes NMDA-Dependent neurotransmission and
reduces seizure susceptibility in a mouse model of
Creutzfeldt-Jakob disease. J Neurosci. 37:10278–10289.
2017.PubMed/NCBI
|
|
182
|
Semple BD, O'Brien TJ, Gimlin K, Wright
DK, Kim SE, Casillas-Espinosa PM, Webster KM, Petrou S and
Noble-Haeusslein LJ: Interleukin-1 receptor in seizure
susceptibility after traumatic injury to the pediatric brain. J
Neurosci. 37:7864–7877. 2017.PubMed/NCBI
|
|
183
|
Dilena R, Mauri E, Aronica E, Bernasconi
P, Bana C, Cappelletti C, Carrabba G, Ferrero S, Giorda R, Guez S,
et al: Therapeutic effect of Anakinra in the relapsing chronic
phase of febrile infection-related epilepsy syndrome. Epilepsia
Open. 4:344–350. 2019.PubMed/NCBI
|
|
184
|
Kenney-Jung DL, Vezzani A, Kahoud RJ,
LaFrance-Corey RG, Ho ML, Muskardin TW, Wirrell EC, Howe CL and
Payne ET: Febrile infection-related epilepsy syndrome treated with
anakinra. Ann Neurol. 80:939–945. 2016.PubMed/NCBI
|
|
185
|
Dilena R, Mauri E, Aronica E, Bernasconi
P, Bana C, Cappelletti C, Carrabba G, Ferrero S, Giorda R, Guez S,
et al: Therapeutic effect of Anakinra in the relapsing chronic
phase of febrile infection-related epilepsy syndrome. Epilepsia
Open. 4:344–350. 2019.PubMed/NCBI
|
|
186
|
Weissberg I, Wood L, Kamintsky L, Vazquez
O, Milikovsky DZ, Alexander A, Oppenheim H, Ardizzone C, Becker A,
Frigerio F, et al: Albumin induces excitatory synaptogenesis
through astrocytic TGF-β/ALK5 signaling in a model of acquired
epilepsy following blood-brain barrier dysfunction. Neurobiol Dis.
78:115–125. 2015.PubMed/NCBI
|
|
187
|
Sanz P and Garcia-Gimeno MA: Reactive glia
inflammatory signaling pathways and epilepsy. Int J Mol Sci.
21:40962020.PubMed/NCBI
|
|
188
|
Benson MJ, Thomas NK, Talwar S, Hodson MP,
Lynch JW, Woodruff TM and Borges K: A novel anticonvulsant
mechanism via inhibition of complement receptor C5ar1 in murine
epilepsy models. Neurobiol Dis. 76:87–97. 2015.PubMed/NCBI
|
|
189
|
Ryther RCC and Wong M: Mammalian target of
rapamycin (mTOR) inhibition: Potential for antiseizure,
antiepileptogenic, and epileptostatic therapy. Curr Neurol Neurosci
Rep. 12:410–418. 2012.PubMed/NCBI
|
|
190
|
Zeng LH, Xu L, Gutmann DH and Wong M:
Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis
complex. Ann Neurol. 63:444–453. 2008.PubMed/NCBI
|
|
191
|
Sunnen CN, Brewster AL, Lugo JN, Vanegas
F, Turcios E, Mukhi S, Parghi D, D'Arcangelo G and Anderson AE:
Inhibition of the mammalian target of rapamycin blocks epilepsy
progression in NS-Pten conditional knockout mice. Epilepsia.
52:2065–2075. 2011.PubMed/NCBI
|
|
192
|
Gericke B, Brandt C, Theilmann W, Welzel
L, Schidlitzki A, Twele F, Kaczmarek E, Anjum M, Hillmann P and
Löscher W: Selective inhibition of mTORC1/2 or PI3K/mTORC1/2
signaling does not prevent or modify epilepsy in the
intrahippocampal kainate mouse model. Neuropharmacology.
162:1078172020.
|
|
193
|
Buckmaster PS and Lew FH: Rapamycin
suppresses mossy fiber sprouting but not seizure frequency in a
mouse model of temporal lobe epilepsy. J Neurosci. 31:2337–2347.
2011.PubMed/NCBI
|
|
194
|
Overwater IE, Rietman AB, van Eeghen AM
and de Wit MCY: Everolimus for the treatment of refractory seizures
associated with tuberous sclerosis complex (TSC): Current
perspectives. Ther Clin Risk Manag. 15:951–955. 2019.PubMed/NCBI
|
|
195
|
Franz DN, Lawson JA, Yapici Z, Ikeda H,
Polster T, Nabbout R, Curatolo P, de Vries PJ, Dlugos DJ, Voi M, et
al: Everolimus for treatment-refractory seizures in TSC: Extension
of a randomized controlled trial. Neurol Clin Pract. 8:412–420.
2018.PubMed/NCBI
|
|
196
|
Ravizza T, Scheper M, Di Sapia R, Gorter
J, Aronica E and Vezzani A: mTOR and neuroinflammation in epilepsy:
Implications for disease progression and treatment. Nat Rev
Neurosci. 25:334–350. 2024.PubMed/NCBI
|
|
197
|
Mazumder AG, Patial V and Singh D:
Mycophenolate mofetil contributes to downregulation of the
hippocampal interleukin type 2 and 1β mediated PI3K/AKT/mTOR
pathway hyperactivation and attenuates neurobehavioral
comorbidities in a rat model of temporal lobe epilepsy. Brain Behav
Immun. 75:84–93. 2019.
|
|
198
|
Drion CM, Borm LE, Kooijman L, Aronica E,
Wadman WJ, Hartog AF, van Vliet EA and Gorter JA: Effects of
rapamycin and curcumin treatment on the development of epilepsy
after electrically induced status epilepticus in rats. Epilepsia.
57:688–697. 2016.PubMed/NCBI
|
|
199
|
Brandt C, Hillmann P, Noack A, Römermann
K, Öhler LA, Rageot D, Beaufils F, Melone A, Sele AM, Wymann MP, et
al: The novel, catalytic mTORC1/2 inhibitor PQR620 and the
PI3K/mTORC1/2 inhibitor PQR530 effectively cross the blood-brain
barrier and increase seizure threshold in a mouse model of chronic
epilepsy. Neuropharmacology. 140:107–120. 2018.PubMed/NCBI
|
|
200
|
Castro OW, Upadhya D, Kodali M and Shetty
AK: Resveratrol for easing status epilepticus induced brain injury,
inflammation, epileptogenesis, and cognitive and memory
Dysfunction-Are We There Yet? Front Neurol. 8:6032017.PubMed/NCBI
|
|
201
|
Theilmann W, Gericke B, Schidlitzki A,
Muneeb Anjum SM, Borsdorf S, Harries T, Roberds SL, Aguiar DJ,
Brunner D, Leiser SC, et al: Novel brain permeant mTORC1/2
inhibitors are as efficacious as rapamycin or everolimus in mouse
models of acquired partial epilepsy and tuberous sclerosis complex.
Neuropharmacology. 180:1082972020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Wang Y and Chen Z: An update for epilepsy
research and antiepileptic drug development: Toward precise circuit
therapy. Pharmacol Ther. 201:77–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Davis KL, Candrilli SD and Edin HM:
Prevalence and cost of nonadherence with antiepileptic drugs in an
adult managed care population. Epilepsia. 49:446–454. 2008.
View Article : Google Scholar
|
|
204
|
Klein P, Kaminski RM, Koepp M and Löscher
W: New epilepsy therapies in development. Nat Rev Drug Discov.
23:682–708. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Forcelli PA: Seizing control of neuronal
activity: Chemogenetic applications in epilepsy. Epilepsy Curr.
22:303–308. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Hadjiabadi D, Lovett-Barron M, Raikov IG,
Sparks FT, Liao Z, Baraban SC, Leskovec J, Losonczy A, Deisseroth K
and Soltesz I: Maximally selective single cell target for circuit
control in epilepsy models. Neuron. 109:2556–2572.e6. 2021.
View Article : Google Scholar :
|
|
207
|
Löscher W: Drug Combinations for
Antiepileptogenesis. Jasper's Basic Mechanisms Epilepsies.
1402–1418. 2024.
|
|
208
|
Szklarczyk D, Santos A, Von Mering C,
Jensen LJ, Bork P and Kuhn M: STITCH 5: Augmenting Protein-chemical
interaction networks with tissue and affinity data. Nucleic Acids
Res. 44:D380–D384. 2016. View Article : Google Scholar
|
|
209
|
Welzel L, Bergin DH, Schidlitzki A, Twele
F, Johne M, Klein P and Löscher W: Systematic evaluation of
rationally chosen multitargeted drug combinations: A combination of
low doses of levetiracetam, atorvastatin and ceftriaxone exerts
antiepileptogenic effects in a mouse model of acquired epilepsy.
Neurobiol Dis. 149:1052272021. View Article : Google Scholar
|
|
210
|
Schidlitzki A, Bascuñana P, Srivastava PK,
Welzel L, Twele F, Töllner K, Käufer C, Gericke B, Feleke R, Meier
M, et al: Proof-of-concept that network pharmacology is effective
to modify development of acquired temporal lobe epilepsy. Neurobiol
Dis. 134:1046642020. View Article : Google Scholar
|
|
211
|
Galanopoulou AS, Löscher W, Lubbers L,
O'Brien TJ, Staley K, Vezzani A, D'Ambrosio R, White HS, Sontheimer
H, Wolf JA, et al: Antiepileptogenesis and disease modification:
Progress, challenges, and the path forward-Report of the
Preclinical Working Group of the 2018 NINDS-sponsored
antiepileptogenesis and disease modification workshop. Epilepsia
Open. 6:276–296. 2021.PubMed/NCBI
|
|
212
|
Porter RJ and Kupferberg HJ: The
anticonvulsant screening program of the National institute of
neurological disorders and stroke, NIH: History and contributions
to clinical care in the twentieth century and beyond. Neurochem
Res. 42:1889–1893. 2017.PubMed/NCBI
|
|
213
|
Kehne JH, Klein BD, Raeissi S and Sharma
S: The national institute of neurological disorders and stroke
(NINDS) epilepsy therapy screening program (ETSP). Neurochem Res.
42:1894–1903. 2017.PubMed/NCBI
|