|
1
|
Lymperopoulos A, Cora N, Maning J, Brill
AR and Sizova A: Signaling and function of cardiac autonomic
nervous system receptors: Insights from the GPCR signalling
universe. FEBS J. 288:2645–2659. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santos R, Ursu O, Gaulton A, Bento AP,
Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI
and Overington JP: A comprehensive map of molecular drug targets.
Nat Rev Drug Discov. 16:19–34. 2017. View Article : Google Scholar
|
|
3
|
Maeda S, Qu Q, Robertson MJ, Skiniotis G
and Kobilka BK: Structures of the M1 and M2 muscarinic
acetylcholine receptor/G-protein complexes. Science. 364:552–557.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foster DJ: Muscarinic receptors: From
clinic to bench to clinic. Trends Pharmacol Sci. 43:461–463. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saternos HC, Almarghalani DA, Gibson HM,
Meqdad MA, Antypas RB, Lingireddy A and AbouAlaiwi WA: Distribution
and function of the muscarinic receptor subtypes in the
cardiovascular system. Physiol Genomics. 50:1–9. 2018. View Article : Google Scholar
|
|
6
|
Palma JA: Muscarinic control of
cardiovascular function in humans: A review of current clinical
evidence. Clin Auton Res. 34:31–44. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alom F, Miyakawa M, Matsuyama H, Nagano H,
Tanahashi Y and Unno T: Possible antagonistic effects of the TRPC4
channel blocker ML204 on M2 and M3 muscarinic receptors in mouse
ileal and detrusor smooth muscles and atrial myocardium. J Vet Med
Sci. 80:1407–1415. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schoeller C, Hoffmann S, Adolph S,
Regenthal R and Abraham G: Expression of muscarinic acetylcholine
receptors in turkey cardiac chambers. Res Vet Sci. 136:602–608.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pontes CNR, Scalzo S, Jesus ICG, Jesus EF,
Nunes ADC, Mendonça MM, Mendes EP, Colugnati DB, Xavier CH, Pedrino
GR, et al: Angiotensin-(1-7) attenuates the negative inotropic
response to acetylcholine in the heart. Peptides. 158:1708622022.
View Article : Google Scholar : PubMed/NCBI
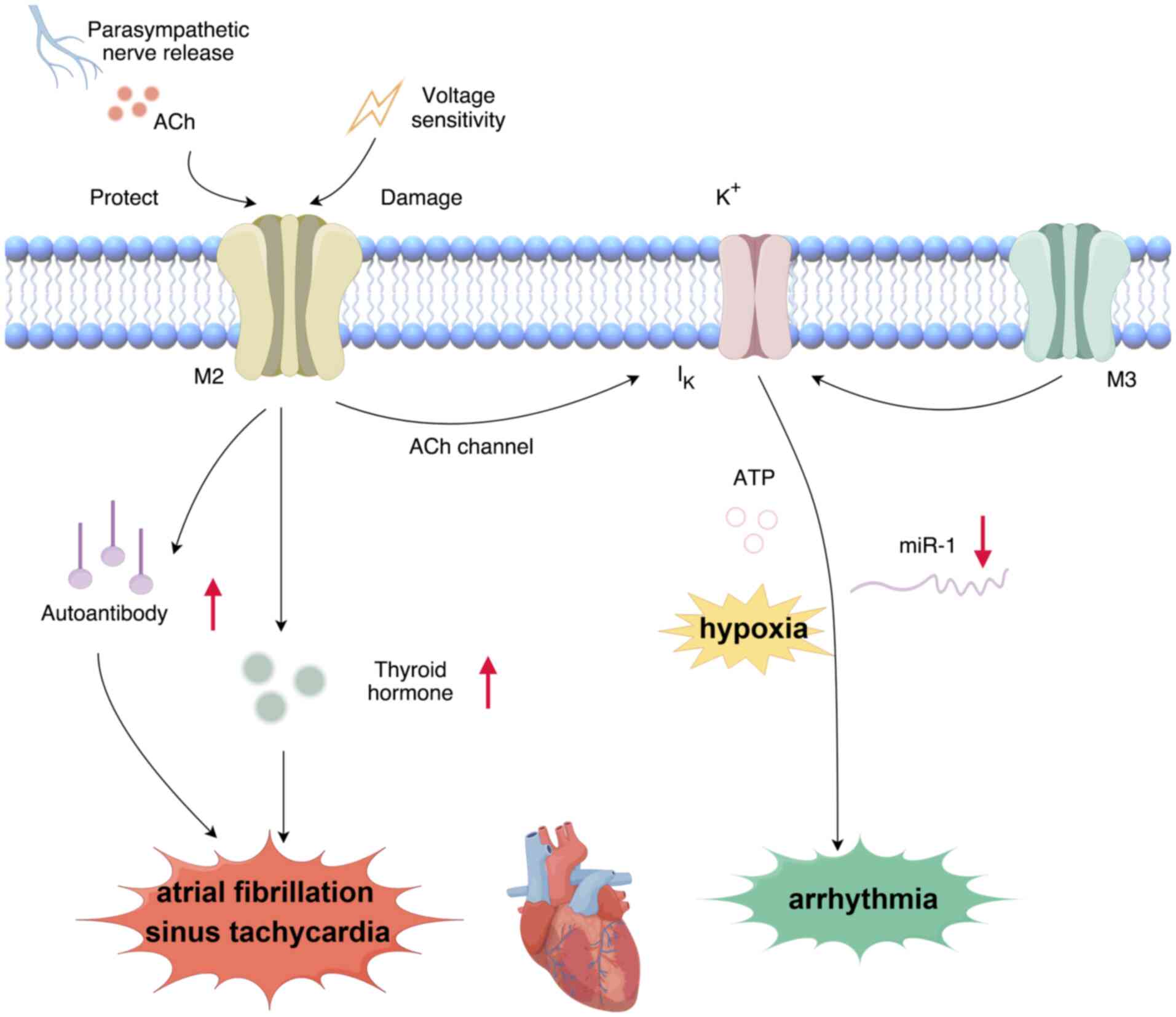
|
|
10
|
Riefolo F, Matera C, Garrido-Charles A,
Gomila AMJ, Sortino R, Agnetta L, Claro E, Masgrau R, Holzgrabe U,
Batlle M, et al: Optical control of cardiac function with a
photoswitchable muscarinic agonist. J Am Chem Soc. 141:7628–7636.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woudstra J, Feenstra RGT, Vink CEM,
Marques KMJ, Boerhout CKM, de Jong EAM, de Waard GA, van de Hoef
TP, Chamuleau SAJ, Eringa EC, et al: Comparison of the diagnostic
yield of intracoronary acetylcholine infusion and acetylcholine
bolus injection protocols during invasive coronary function
testing. Am J Cardiol. 217:49–58. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ibrahim E, Diakonov I, Arunthavarajah D,
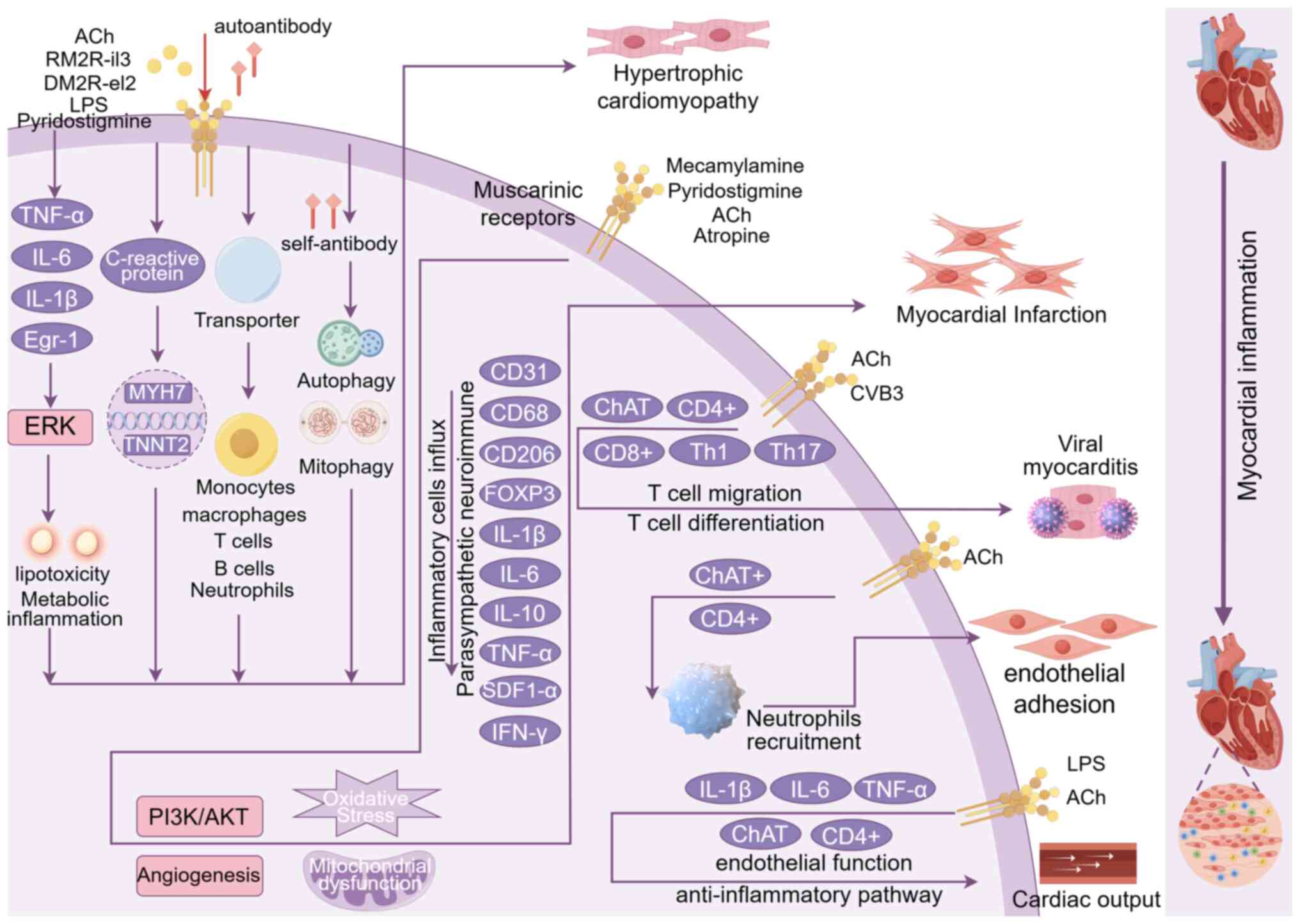
Swift T, Goodwin M, McIlvride S, Nikolova V, Williamson C and
Gorelik J: Bile acids and their respective conjugates elicit
different responses in neonatal cardiomyocytes: Role of Gi protein,
muscarinic receptors and TGR5. Sci Rep. 8:71102018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levay MK, Krobert KA, Vogt A, Ahmad A,
Jungmann A, Neuber C, Pasch S, Hansen A, Müller OJ, Lutz S and
Wieland T: RGS3L allows for an M2 muscarinic
receptor-mediated RhoA-dependent inotropy in cardiomyocytes. Basic
Res Cardiol. 117:82022. View Article : Google Scholar
|
|
14
|
Winger G, Jutkiewicz EM and Woods JH:
Comparison of the muscarinic antagonist effects of scopolamine and
L-687,306. Behav Pharmacol. 31:359–367. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butova X, Myachina T, Simonova R,
Kochurova A, Bozhko Y, Arkhipov M, Solovyova O, Kopylova G,
Shchepkin D and Khokhlova: Peculiarities of the acetylcholine
action on the contractile function of cardiomyocytes from the left
and right atria in rats. Cells. 11:38092022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baine S, Thomas J, Bonilla I, Ivanova M,
Belevych A, Li J, Veeraraghavan R, Radwanski PB, Carnes C and
Gyorke S: Muscarinic-dependent phosphorylation of the cardiac
ryanodine receptor by protein kinase G is mediated by PI3K-AKT-nNOS
signaling. J Biol Chem. 295:11720–11728. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ho HT, Belevych AE, Liu B, Bonilla IM,
Radwański PB, Kubasov IV, Valdivia HH, Schober K, Carnes CA and
Györke S: Muscarinic stimulation facilitates sarcoplasmic reticulum
Ca release by modulating ryanodine receptor 2 phosphorylation
through protein kinase G and Ca/calmodulin-dependent protein kinase
II. Hypertension. 68:1171–1178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cassambai S, Mee CJ, Renshaw D and Hussain
A: Tiotropium bromide, a long acting muscarinic receptor antagonist
triggers intracellular calcium signalling in the heart. Toxicol
Appl Pharmacol. 384:1147782019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dolejší E, Janoušková A and Jakubík J:
Muscarinic receptors in cardioprotection and vascular tone
regulation. Physiological research. 2024. View Article : Google Scholar
|
|
20
|
Perera RK, Fischer TH, Wagner M, Dewenter
M, Vettel C, Bork NI, Maier LS, Conti M, Wess J, El-Armouche A, et
al: Atropine augments cardiac contractility by inhibiting
cAMP-specific phosphodiesterase type 4. Sci Rep. 7:152222017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HY, Choi HR, Lee YJ, Cui HZ, Jin SN,
Cho KW, Kang DG and Lee HS: Accentuation of ursolic acid on
muscarinic receptor-induced ANP secretion in beating rabbit atria.
Life Sci. 94:145–150. 2014. View Article : Google Scholar
|
|
22
|
Kawada T, Sonobe T, Nishikawa T, Hayama Y,
Li M, Zheng C, Uemura K, Akiyama T, Pearson JT and Sugimachi M:
Contribution of afferent pathway to vagal nerve stimulation-induced
myocardial interstitial acetylcholine release in rats. Am J Physiol
Regul Integr Comp Physiol. 319:R517–R525. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bencze M, Boros A, Behuliak M, Vavrinova
A, Vaneckova I and Zicha J: Changes in cardiovascular autonomic
control induced by chronic inhibition of acetylcholinesterase
during pyridostigmine or donepezil treatment of spontaneously
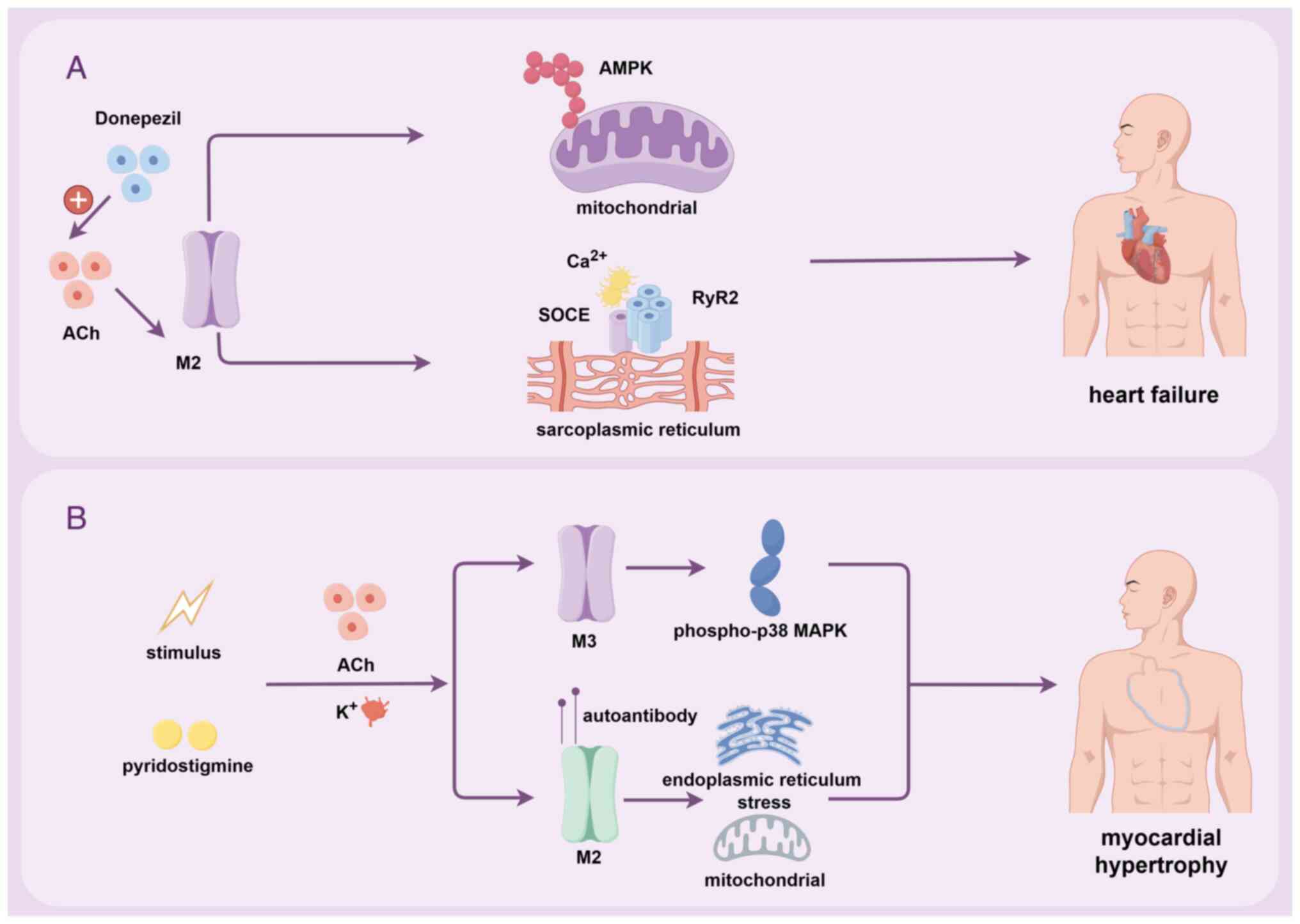
hypertensive rats. Eur J Pharmacol. 971:1765262024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harada N, Ochi K, Yaosaka N, Teraoka H,
Hiraga T, Iwanaga T, Unno T, Komori S, Yamada M and Kitazawa T:
Immunohistochemical and functional studies for M3 muscarinic
receptors and cyclo-oxygenase-2 expressed in the mouse atrium.
Auton Autacoid Pharmacol. 32:41–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stavrakis S, Kem DC, Patterson E, Lozano
P, Huang S, Szabo B, Cunningham MW, Lazzara R and Yu X: Opposing
cardiac effects of autoantibody activation of β-adrenergic and M2
muscarinic receptors in cardiac-related diseases. Int J Cardiol.
148:331–336. 2011. View Article : Google Scholar
|
|
26
|
Camara H, da Silva Junior ED, Garcia AG,
Jurkiewicz A and Rodrigues JQD: Cardiac arrest induced by
muscarinic or adenosine receptors agonists is reversed by DPCPX
through double mechanism. Eur J Pharmacol. 819:9–15. 2018.
View Article : Google Scholar
|
|
27
|
Sassu E, Tumlinson G, Stefanovska D,
Fernández MC, Iaconianni P, Madl J, Brennan TA, Koch M, Cameron BA,
Preissl S, et al: Age-related structural and functional changes of
the intracardiac nervous system. J Mol Cell Cardiol. 187:1–14.
2024. View Article : Google Scholar
|
|
28
|
Poller U, Nedelka G, Radke J, Pönicke K
and Brodde OE: Age-dependent changes in cardiac muscarinic receptor
function in healthy volunteers. J Am Coll Cardiol. 29:187–193.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Jiang Y, Chen J, Dai C, Liu D, Pan
W, Wang L, Fasae MB, Sun L, Wang L and Liu Y: Activation of M3
muscarinic acetylcholine receptors delayed cardiac aging by
inhibiting the caspase-1/IL-1beta signaling pathway. Cell Physiol
Biochem. 49:1208–1216. 2018. View Article : Google Scholar
|
|
30
|
Arnett DK, Blumenthal RS, Albert MA,
Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A,
Lloyd-Jones D, McEvoy JW, et al: 2019 ACC/AHA guideline on the
primary prevention of cardiovascular disease: A report of the
american college of Cardiology/American heart association task
force on clinical practice guidelines. Circulation. 140:e596–e646.
2019.PubMed/NCBI
|
|
31
|
Tompkins JD, Buckley U, Salavatian S,
Shivkumar K and Ardell JL: Vagally-mediated heart block after
myocardial infarction associated with plasticity of epicardial
neurons controlling the atrioventricular node. Front Synaptic
Neurosci. 14:9604582022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh S, Loke YK and Furberg CD: Inhaled
anticholinergics and risk of major adverse cardiovascular events in
patients with chronic obstructive pulmonary disease: A systematic
review and meta-analysis. JAMA. 300:1439–1450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mazzadi AN, Pineau J, Costes N, Le Bars D,
Bonnefoi F, Croisille P, Porcher R and Chevalier P: Muscarinic
receptor upregulation in patients with myocardial infarction: A new
paradigm. Circ Cardiovasc Imaging. 2:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Buchholz B, Kelly J, Munoz M, Bernatené
EA, Méndez Diodati N, González Maglio DH, Dominici FP and Gelpi RJ:
Vagal stimulation mimics preconditioning and postconditioning of
ischemic myocardium in mice by activating different protection
mechanisms. Am J Physiol Heart Circ Physiol. 314:H1289–H1297. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv
F, Liu Y, Zheng W, Shang H, Zhang J, et al: CaMKII is a RIP3
substrate mediating ischemia- and oxidative stress-induced
myocardial necroptosis. Nat Med. 22:175–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lauro FV, Maria LR, Tomas LG, Francisco
DC, Rolando GM, Marcela RN, Virginia MA, Alejandra GE and Yazmin
OA: Design and synthesis of two new steroid derivatives with
biological activity on heart failure via the M2-muscarinic receptor
activation. Steroids. 158:1086202020. View Article : Google Scholar
|
|
37
|
Rinaldi R, Colucci M, Torre I, Ausiello D,
Bonanni A, Basile M, Salzillo C, Sanna T, Liuzzo G, Leone AM, et
al: Predicting the response to acetylcholine in ischemia or
infarction with non-obstructive coronary arteries: The ABCD score.
Atherosclerosis. 391:1175032024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao F, Zheng Y, Cai J, Fan J, Wang J,
Yang J, Cui Q, Xu G, Tang C, Geng B, et al: Catestatin attenuates
endoplasmic reticulum induced cell apoptosis by activation type 2
muscarinic acetylcholine receptor in cardiac ischemia/reperfusion.
Sci Rep. 5:165902015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kakinuma Y, Tsuda M, Okazaki K, Akiyama T,
Arikawa M, Noguchi T and Sato T: Heart-specific overexpression of
choline acetyltransferase gene protects murine heart against
ischemia through hypoxia-inducible factor-1α-related defense
mechanisms. J Am Heart Assoc. 2:e0048872013. View Article : Google Scholar
|
|
40
|
Xue RQ, Zhao M, Wu Q, Yang S, Cui YL, Yu
XJ, Liu J and Zang WJ: Regulation of mitochondrial cristae
remodelling by acetylcholine alleviates palmitate-induced
cardiomyocyte hypertrophy. Free Radic Biol Med. 145:103–117. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Palee S, Apaijai N, Shinlapawittayatorn K,
Chattipakorn SC and Chattipakorn N: Acetylcholine attenuates
hydrogen peroxide-induced intracellular calcium dyshomeostasis
through both muscarinic and nicotinic receptors in cardiomyocytes.
Cell Physiol Biochem. 39:341–349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lv YX, Zhong S, Tang H, Luo B, Chen SJ,
Chen L, Zheng F, Zhang L, Wang L, Li XY, et al: VEGF-A and VEGF-B
coordinate the arteriogenesis to repair the infarcted heart with
vagus nerve stimulation. Cell Physiol Biochem. 48:433–449. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Travieso A, Jeronimo-Baza A, Faria D,
Shabbir A, Mejia-Renteria H and Escaned J: Invasive evaluation of
coronary microvascular dysfunction. J Nucl Cardiol. 29:2474–2486.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alves-Lopes R, Neves KB and Touyz RM:
Muscarinic receptor type-3 in hypertension and
cholinergic-adrenergic crosstalk: Genetic insights and potential
for new antihypertensive targets. Can J Cardiol. 35:555–557. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Khuanjing T, Palee S, Chattipakorn SC and
Chattipakorn N: The effects of acetylcholinesterase inhibitors on
the heart in acute myocardial infarction and heart failure: From
cells to patient reports. Acta Physiol (Oxf). 228:e133962020.
View Article : Google Scholar
|
|
46
|
Shahim B, Xu H, Haugaa K, Zetterberg H,
Jurga J, Religa D and Eriksdotter M: Cholinesterase inhibitors are
associated with reduced mortality in patients with Alzheimer's
disease and previous myocardial infarction. Eur Heart J Cardiovasc
Pharmacother. 10:128–136. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pan Z, Guo Y, Qi H, Fan K, Wang S, Zhao H,
Fan Y, Xie J, Guo F, Hou Y, et al: M3 subtype of muscarinic
acetylcholine receptor promotes cardioprotection via the
suppression of miR-376b-5p. PLoS One. 7:e325712012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao J, Su Y, Zhang Y, Pan Z, Yang L, Chen
X, Liu Y, Lu Y, Du Z and Yang B: Activation of cardiac muscarinic
M3 receptors induces delayed cardioprotection by preserving
phosphorylated connexin43 and up-regulating cyclooxygenase-2
expression. Br J Pharmacol. 159:1217–1225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao L, Chen T, Hang P, Li W, Guo J, Pan
Y, Du J, Zheng Y and Du Z: Choline attenuates cardiac fibrosis by
inhibiting p38MAPK signaling possibly by acting on M3 muscarinic
acetylcholine receptor. Front Pharmacol. 10:13862019. View Article : Google Scholar :
|
|
50
|
Liu H, Hofmann J, Fish I, Schaake B, Eitel
K, Bartuschat A, Kaindl J, Rampp H, Banerjee A, Hübner H, et al:
Structure-guided development of selective M3 muscarinic
acetylcholine receptor antagonists. Proc Natl Acad Sci USA.
115:12046–12050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang YP, Hang PZ, Sun LH, Zhang Y, Zhao
JL, Pan ZW, Ji HR, Wang LA, Bi H and Du ZM: M3 muscarinic
acetylcholine receptor is associated with beta-catenin in
ventricular myocytes during myocardial infarction in the rat. Clin
Exp Pharmacol Physiol. 36:995–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Harvey KL, Hussain A and Maddock HL:
Ipratropium bromide-mediated myocardial injury in in vitro models
of myocardial Ischaemia/reperfusion. Toxicol Sci. 138:457–467.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nuntaphum W, Pongkan W, Wongjaikam S,
Thummasorn S, Tanajak P, Khamseekaew J, Intachai K, Chattipakorn
SC, Chattipakorn N and Shinlapawittayatorn K: Vagus nerve
stimulation exerts cardioprotection against myocardial
ischemia/reperfusion injury predominantly through its efferent
vagal fibers. Basic Res Cardiol. 113:222018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pickard JMJ, Burke N, Davidson SM and
Yellon DM: Intrinsic cardiac ganglia and acetylcholine are
important in the mechanism of ischaemic preconditioning. Basic Res
Cardiol. 112:112017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Khuanjing T, Palee S, Kerdphoo S,
Jaiwongkam T, Anomasiri A, Chattipakorn SC and Chattipakorn N:
Donepezil attenuated cardiac ischemia/reperfusion injury through
balancing mitochondrial dynamics, mitophagy, and autophagy. Transl
Res. 230:82–97. 2021. View Article : Google Scholar
|
|
56
|
Intachai K, Chattipakorn SC, Chattipakorn
N and Shinlapawittayatorn K: Acetylcholine exerts cytoprotection
against hypoxia/reoxygenation-induced apoptosis, autophagy and
mitochondrial impairment through both muscarinic and nicotinic
receptors. Apoptosis. 27:233–245. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu M, Bi X, He X, Yu X, Zhao M and Zang W:
Inhibition of the mitochondrial unfolded protein response by
acetylcholine alleviated hypoxia/reoxygenation-induced apoptosis of
endothelial cells. Cell Cycle. 15:1331–1343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xue RQ, Sun L, Yu XJ, Li DL and Zang WJ:
Vagal nerve stimulation improves mitochondrial dynamics via an M3
receptor/CaMKKbeta/AMPK pathway in isoproterenol-induced myocardial
ischaemia. J Cell Mol Med. 21:58–71. 2017. View Article : Google Scholar
|
|
59
|
Li W, Yu J, Yang Y, Wang J, Liu Y, Wang J,
Hu J, Yuan Y and Du Z: M3 subtype of muscarinic
acetylcholine receptor inhibits cardiac fibrosis via targeting
microRNA-29b/beta-site app cleaving enzyme 1 axis. Cardiovasc Diagn
Ther. 14:143–157. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu JJ, Huang N, Lu Y, Zhao M, Yu XJ, Yang
Y, Yang YH and Zang WJ: Improving vagal activity ameliorates
cardiac fibrosis induced by angiotensin II: in vivo and in vitro.
Sci Rep. 5:2015.
|
|
61
|
Gurses KM, Yalcin MU, Kocyigit D, Kesikli
SA, Canpolat U, Yorgun H, Sahiner ML, Kaya EB, Hazirolan T, Ozer N,
et al: M2-muscarinic acetylcholine receptor autoantibody levels
predict left atrial fibrosis severity in paroxysmal lone atrial
fibrillation patients undergoing cryoablation. Europace.
17:239–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ma G, Wu X, Zeng L, Jin J, Liu X, Zhang J
and Zhang L: Association of autoantibodies against M2-muscarinic
acetylcholine receptor with atrial fibrosis in atrial fibrillation
patients. Cardiol Res Pract. 2019:82718712019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Heijman J, Kirchner D, Kunze F, Chrétien
EM, Michel-Reher MB, Voigt N, Knaut M, Michel MC, Ravens U and
Dobrev D: Muscarinic type-1 receptors contribute to IK,ACh in human
atrial cardiomyocytes and are upregulated in patients with chronic
atrial fibrillation. Int J Cardiol. 255:61–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Garcia-Domingo M, Garcia-Pedraza JA,
Fernandez-Gonzalez JF, Lopez C, Martin ML and Moran A: Fluoxetine
treatment decreases cardiac vagal input and alters the serotonergic
modulation of the parasympathetic outflow in diabetic rats. Int J
Mol Sci. 23:57362022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jungen C, Scherschel K, Eickholt C, Kuklik
P, Klatt N, Bork N, Salzbrunn T, Alken F, Angendohr S, Klene C, et
al: Disruption of cardiac cholinergic neurons enhances
susceptibility to ventricular arrhythmias. Nat Commun. 8:141552017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gergs U, Wackerhagen S, Fuhrmann T,
Schafer I and Neumann J: Further investigations on the influence of
protein phosphatases on the signaling of muscarinic receptors in
the atria of mouse hearts. Naunyn Schmiedebergs Arch Pharmacol.
39:5731–5743. 2024. View Article : Google Scholar
|
|
67
|
Magyar T, Árpádffy-Lovas T, Pászti B, Tóth
N, Szlovák J, Gazdag P, Kohajda Z, Gyökeres A, Györe B, Gurabi Z,
et al: Muscarinic agonists inhibit the ATP-dependent potassium
current and suppress the ventricle-Purkinje action potential
dispersion. Can J Physiol Pharmacol. 99:247–253. 2021. View Article : Google Scholar
|
|
68
|
Voigt N, Friedrich A, Bock M, Wettwer E,
Christ T, Knaut M, Strasser RH, Ravens U and Dobrev D: Differential
phosphorylation-dependent regulation of constitutively active and
muscarinic receptor-activated IK,ACh channels in patients with
chronic atrial fibrillation. Cardiovasc Res. 74:426–437. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Petersen J, Castro L, Bengaard AKP, Pecha
S, Ismaili D, Schulz C, Sahni J, Steenpass A, Meier C,
Reichenspurner H, et al: Muscarinic receptor activation reduces
force and arrhythmias in human atria independent of IK,ACh. J
Cardiovasc Pharmacol. 79:678–686. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Couselo-Seijas M, Lopez-Canoa JN,
Agra-Bermejo RM, Díaz-Rodriguez E, Fernandez AL, Martinez-Cereijo
JM, Durán-Muñoz D, Bravo SB, Velo A, González-Melchor L, et al:
Cholinergic activity regulates the secretome of epicardial adipose
tissue: Association with atrial fibrillation. J Cell Physiol.
234:10512–10522. 2019. View Article : Google Scholar
|
|
71
|
Deng J, Guo Y, Zhang G, Zhang L, Kem D, Yu
X, Jiang H and Li H: M2 muscarinic autoantibodies and
thyroid hormone promote susceptibility to atrial fibrillation and
sinus tachycardia in an autoimmune rabbit model. Exp Physiol.
106:882–890. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Moss R, Sachse FB, Moreno-Galindo EG,
Navarro-Polanco RA, Tristani-Firouzi M and Seemann G: Modeling
effects of voltage dependent properties of the cardiac muscarinic
receptor on human sinus node function. PLoS Comput Biol.
14:e10064382018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Y, Sun L, Pan Z, Bai Y, Wang N, Zhao
J, Xu C, Li Z, Li B, Du Z, et al: Overexpression of M3
muscarinic receptor is a novel strategy for preventing sudden
cardiac death in transgenic mice. Mol Med. 17:1179–1187. 2011.
View Article : Google Scholar
|
|
74
|
Olivas A, Gardner RT, Wang L, Ripplinger
CM, Woodward WR and Habecker BA: Myocardial infarction causes
transient cholinergic transdifferentiation of cardiac sympathetic
nerves via gp130. J Neurosci. 36:479–488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Prado MB Jr and Adiao KJ:
Acetylcholinesterase inhibitors in myasthenic crisis: A systematic
review of observational studies. Neurocrit Care. 35:528–544. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bober SL, Ciriello J and Jones DL: Atrial
arrhythmias and autonomic dysfunction in rats exposed to chronic
intermittent hypoxia. Am J Physiol Heart Circ Physiol.
314:H1160–H1168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cavalcante GL, Brognara F, Oliveira LVC,
Lataro RM, Durand MT, de Oliveira AP, da Nóbrega ACL, Salgado HC
and Sabino JPJ: Benefits of pharmacological and electrical
cholinergic stimulation in hypertension and heart failure. Acta
Physiol (Oxf). 232:e136632021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Baine S, Bonilla I, Belevych A, Stepanov
A, Dorn LE, Terentyeva R, Terentyev D, Accornero F, Carnes CA and
Gyorke S: Pyridostigmine improves cardiac function and rhythmicity
through RyR2 stabilization and inhibition of STIM1-mediated calcium
entry in heart failure. J Cell Mol Med. 25:4637–4648. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fernandez SF and Canty JM Jr: Adrenergic
and cholinergic plasticity in heart failure. Circ Res.
116:1639–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Teixeira VP, Miranda K, Scalzo S,
Rocha-Resende C, Silva MM, Tezini GCSV, Melo MB, Souza-Neto FP,
Silva KSC, Jesus ICG, et al: Increased cholinergic activity under
conditions of low estrogen leads to adverse cardiac remodeling. Am
J Physiol Cell Physiol. 320:C602–C612. 2021. View Article : Google Scholar
|
|
81
|
Ma G, Chen L, Yue Y, Liu X, Wang Y, Shi C,
Song F, Shi W, Lo Y and Zhang L: Impact of autoantibodies against
the M2-muscarinic acetylcholine receptor on clinical outcomes in
peripartum cardiomyopathy patients with standard treatment. BMC
Cardiovasc Disord. 21:6192021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li M, Zheng C, Kawada T, Inagaki M, Uemura
K and Sugimachi M: Intracerebroventricular infusion of donepezil
prevents cardiac remodeling and improves the prognosis of chronic
heart failure rats. J Physiol Sci. 70:112020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Williams AM, Shave RE, Coulson JM, White
H, Rosser-Stanford B and Eves ND: Influence of vagal control on
sex-related differences in left ventricular mechanics and
hemodynamics. Am J Physiol Heart Circ Physiol. 315:H687–H698. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Schultheiss HP, Fairweather D, Caforio
ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A,
Mazzanti A, McMurray J and Priori SG: Dilated cardiomyopathy. Nat
Rev Dis Primers. 5:322019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen X, Bai Y, Sun H, Su Z, Guo J, Sun C
and Du Z: Overexpression of m3 muscarinic receptor suppressed
adverse electrical remodeling in hypertrophic myocardium via
increasing repolarizing K+ currents. Cell Physiol Biochem.
43:915–925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang S, Han HM, Pan ZW, Hang PZ, Sun LH,
Jiang YN, Song HX, Du ZM and Liu Y: Choline inhibits angiotensin
II-induced cardiac hypertrophy by intracellular calcium signal and
p38 MAPK pathway. Naunyn Schmiedebergs Arch Pharmacol. 385:823–831.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ma M, Chen W, Hua Y, Jia H, Song Y and
Wang Y: Aerobic exercise ameliorates cardiac hypertrophy by
regulating mitochondrial quality control and endoplasmic reticulum
stress through M2 AChR. J Cell Physiol. 236:6581–6596.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Matsui S, Fu ML, Hayase M, Katsuda S,
Yamaguchi N, Teraoka K, Kurihara T and Takekoshi N: Active
immunization of combined beta1-adrenoceptor and M2-muscarinic
receptor peptides induces cardiac hypertrophy in rabbits. J Card
Fail. 5:246–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
da Silva Gonçalves Bós D, Van Der Bruggen
CEE, Kurakula K, Sun XQ, Casali KR, Casali AG, Rol N, Szulcek R,
Dos Remedios C, Guignabert C, et al: Contribution of impaired
parasympathetic activity to right ventricular dysfunction and
pulmonary vascular remodeling in pulmonary arterial hypertension.
Circulation. 137:910–924. 2018. View Article : Google Scholar
|
|
90
|
Minassa VS, Aitken AV, Hott SC, de Sousa
GJ, Batista TJ, Gonçalves RCR, Coitinho JB, Paton JFR, Beijamini V,
Bissoli NS and Sampaio KN: Intermittent exposure to chlorpyrifos
results in cardiac hypertrophy and oxidative stress in rats.
Toxicology. 482:1533572022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Duan X, Liu R, Luo XL, Gao XJ, Hu FH, Guo
C, Wang J, Hu XY, Chun YS, Yuan JS, et al: The relationship between
β1-adrenergic and M2-muscarinic receptor autoantibodies and
hypertrophic cardiomyopathy. Exp Physiol. 105:522–530. 2020.
View Article : Google Scholar
|
|
92
|
Ribeiro KC, Campelo RP, Rodrigues DDRF,
Mattos EC, Brandão IT, da Silva CL, Bouskela E, Martinez CG and
Kurtenbach E: Immunization with plasmids encoding M2 acetylcholine
muscarinic receptor epitopes impairs cardiac function in mice and
induces autophagy in the myocardium. Autoimmunity. 51:245–257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bezerra OC, Franca CM, Rocha JA, Neves GA,
Souza PRM, Teixeira Gomes M, Malfitano C, Loleiro TCA, Dourado PM,
Llesuy S, et al: Cholinergic stimulation improves oxidative stress
and inflammation in experimental myocardial infarction. Sci Rep.
7:136872017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Barboza CA, Fukushima AR, Carrozzi N,
Machi JF, Dourado PMM, Mostarda CT, Irigoyen MC, Nathanson L,
Morris M, Caperuto EC and Rodrigues B: Cholinergic stimulation by
pyridostigmine bromide before myocardial infarction prevent cardiac
and autonomic dysfunction. Sci Rep. 9:24812019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Halder N and Lal G: Cholinergic system and
its therapeutic importance in inflammation and autoimmunity. Front
Immunol. 12:6603422021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cox MA, Duncan GS, Lin GHY, Steinberg BE,
Yu LX, Brenner D, Buckler LN, Elia AJ, Wakeham AC, Nieman B, et al:
Choline acetyltransferase-expressing T cells are required to
control chronic viral infection. Science. 363:639–644. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Reardon C, Duncan GS, Brüstle A, Brenner
D, Tusche MW, Olofsson PS, Rosas-Ballina M, Tracey KJ and Mak TW:
Lymphocyte-derived ACh regulates local innate but not adaptive
immunity. Proc Natl Acad Sci USA. 110:1410–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
De-Pu Z, Li-Sha G, Guang-Yi C, Xiaohong G,
Chao X, Cheng Z, Wen-Wu Z, Jia L, Jia-Feng L, Maoping C and
Yue-Chun L: The cholinergic anti-inflammatory pathway ameliorates
acute viral myocarditis in mice by regulating CD4+ T cell
differentiation. Virulence. 9:1364–1376. 2018. View Article : Google Scholar :
|
|
99
|
Wang Y, Liu Y, Li XY, Yao LY, Mbadhi M,
Chen SJ, Lv YX, Bao X, Chen L, Chen SY, et al: Vagus nerve
stimulation-induced stromal cell-derived factor-l alpha
participates in angiogenesis and repair of infarcted hearts. ESC
Heart Fail. 10:3311–3329. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Albano GD, Bonanno A, Moscato M, Anzalone
G, Di Sano C, Riccobono L, Wenzel SE and Profita M: Crosstalk
between mAChRM3 and beta2AR, via acetylcholine PI3/PKC/PBEP1/Raf-1
MEK1/2/ERK1/2 pathway activation, in human bronchial epithelial
cells after Long-term cigarette smoke exposure. Life Sci.
192:99–109. 2018. View Article : Google Scholar
|
|
101
|
Wu Q, Zhao M, Li D, He X and Zang W:
Cholinergic drugs reduce metabolic inflammation and diabetic
myocardial injury by regulating the gut bacterial component
lipopolysaccharide-induced ERK/Egr-1 pathway. FASEB J.
37:e229172023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jiang W, Li D, Han R, Zhang C, Jin WN,
Wood K, Liu Q, Shi FD and Hao J: Acetylcholine-producing NK cells
attenuate CNS inflammation via modulation of infiltrating
monocytes/macrophages. Proc Natl Acad Sci USA. 114:E6202–E6211.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rocha-Resende C, da Silva AM, Prado MAM
and Guatimosim S: Protective and anti-inflammatory effects of
acetylcholine in the heart. Am J Physiol Cell Physiol.
320:C155–C161. 2021. View Article : Google Scholar
|
|
104
|
Plaschke K, Do TQM, Uhle F, Brenner T,
Weigand MA and Kopitz J: Ablation of the right cardiac vagus nerve
reduces acetylcholine content without changing the inflammatory
response during endotoxemia. Int J Mol Sci. 19:4422018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tarnawski L, Shavva VS, Kort EJ, Zhuge Z,
Nilsson I, Gallina AL, Martínez-Enguita D, Heller Sahlgren B,
Weiland M, Caravaca AS, et al: Cholinergic regulation of vascular
endothelial function by human ChAT+ T cells. Proc Natl
Acad Sci USA. 120:e22124761202023. View Article : Google Scholar
|
|
106
|
Suissa S, Dell'Aniello S and Ernst P:
Long-acting bronchodilator initiation in COPD and the risk of
adverse cardiopulmonary events: A population-based comparative
safety study. Chest. 151:60–67. 2017. View Article : Google Scholar
|
|
107
|
Rogliani P, Calzetta L, Matera MG, di
Daniele N, Girolami A, Cazzola M and Ora J: Inhaled therapies and
cardiovascular risk in patients with chronic obstructive pulmonary
disease. Expert Opin Pharmacother. 20:737–750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shin J and Lee JH: Effects of tiotropium
on the risk of coronary heart disease in patients with COPD: A
nationwide cohort study. Sci Rep. 12:166742022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Parkin L, Williams S, Sharples K, Barson
D, Horsburgh S, Jackson R, Wu B and Dummer J: Dual versus single
long-acting bronchodilator use could raise acute coronary syndrome
risk by over 50%: A Population-based nested Case-control study. J
Intern Med. 290:1028–1038. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Arana A, Margulis AV, McQuay LJ, Ziemiecki
R, Bartsch JL, Rothman KJ, Franks B, D'Silva M, Appenteng K,
Varas-Lorenzo C and Perez-Gutthann S: Variation in cardiovascular
risk related to individual antimuscarinic drugs used to treat
overactive bladder: A UK cohort study. Pharmacotherapy. 38:628–637.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Guo F, Wang Y, Wang J, Liu Z, Lai Y, Zhou
Z, Liu Z, Zhou Y, Xu X, Li Z, et al: Choline potects the heart from
doxorubicin-induced cardiotoxicity through vagal activation and
Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2022:47409312022.
View Article : Google Scholar
|
|
112
|
Khuanjing T, Ongnok B, Maneechote C,
Siri-Angkul N, Prathumsap N, Arinno A, Chunchai T, Arunsak B,
Chattipakorn SC and Chattipakorn N: Acetylcholinesterase inhibitor
ameliorates doxorubicin-induced cardiotoxicity through reducing
RIP1-mediated necroptosis. Pharmacol Res. 173:1058822021.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Khalaf HA and El-Mansy AAE: The possible
alleviating effect of saffron on chlorpyrifos experimentally
induced cardiotoxicity: Histological, immunohistochemical and
biochemical study. Acta Histochem. 121:472–483. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Prathumsap N, Ongnok B, Khuanjing T,
Arinno A, Maneechote C, Apaijai N, Chunchai T, Arunsak B, Kerdphoo
S, Janjek S, et al: Vagus nerve stimulation exerts cardioprotection
against doxorubicin-induced cardiotoxicity through inhibition of
programmed cell death pathways. Cell Mol Life Sci. 80:212022.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wu Q, Zhao M, He X, Xue R, Li D, Yu X,
Wang S and Zang W: Acetylcholine reduces palmitate-induced
cardiomyocyte apoptosis by promoting lipid droplet lipolysis and
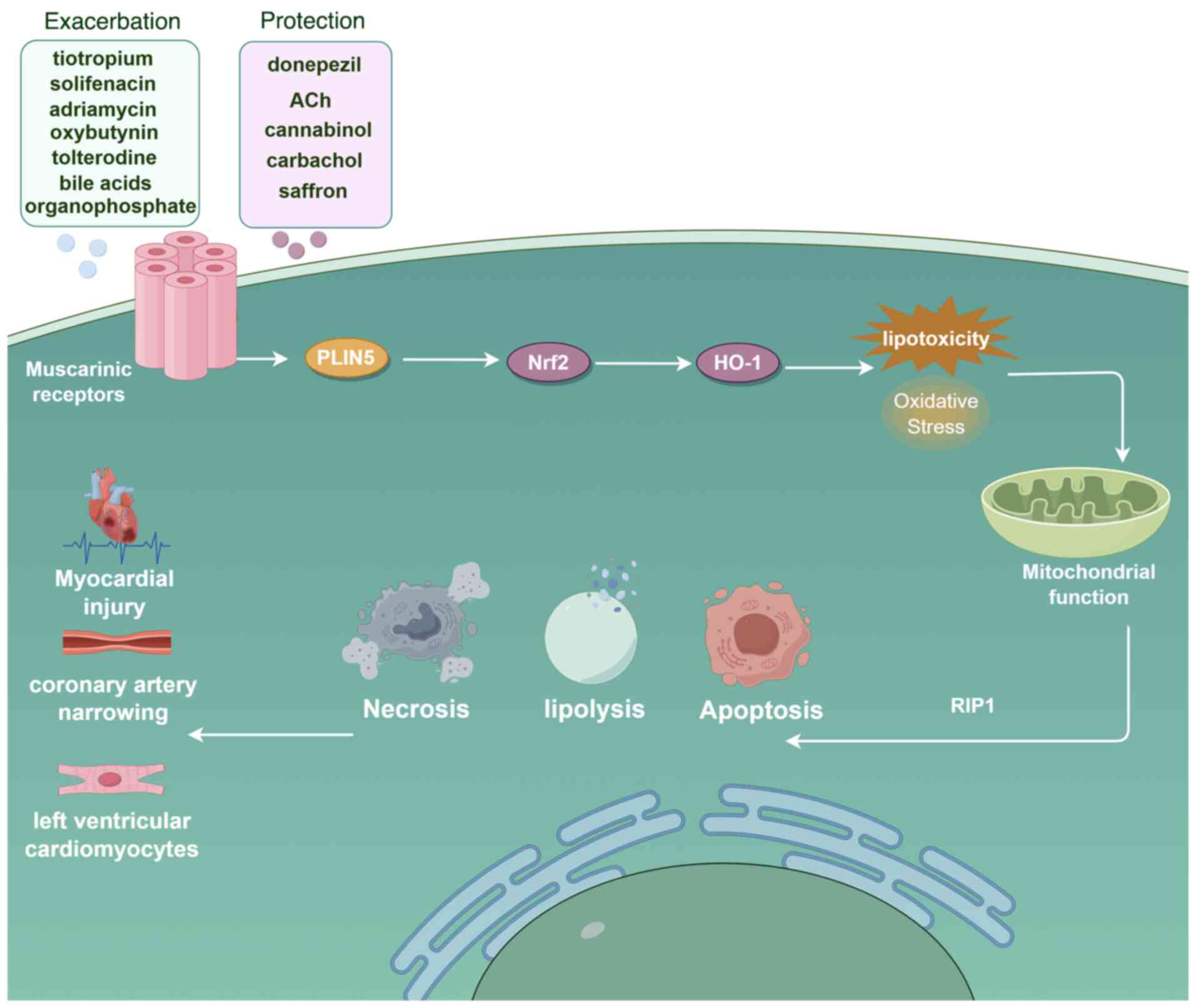
perilipin 5-mediated lipid droplet-mitochondria interaction. Cell
Cycle. 20:1890–1906. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Pedzinska-Betiuk A, Weresa J, Schlicker E,
Harasim-Symbor E, Toczek M, Kasacka I, Gajo B and Malinowska B:
Chronic cannabidiol treatment reduces the carbachol-induced
coronary constriction and left ventricular cardiomyocyte width of
the isolated hypertensive rat heart. Toxicol Appl Pharmacol.
411:1153682021. View Article : Google Scholar
|
|
117
|
Pickett MA, Dush MK and Nascone-Yoder NM:
Acetylcholinesterase plays a non-neuronal, non-esterase role in
organogenesis. Development. 144:2764–2770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wessler I and Kirkpatrick CJ:
Acetylcholine beyond neurons: The non-neuronal cholinergic system
in humans. Br J Pharmacol. 154:1558–1571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Huang D, Zhang L, Liu Y, Wang J, Zhang J,
Baines KJ, Liu G, Hsu AC, Wang F, Chen Z, et al: Activated
non-neuronal cholinergic system correlates with non-type 2
inflammation and exacerbations in severe asthma. Ann Allergy Asthma
Immunol. 133:e64–e72.e4. 2024. View Article : Google Scholar
|
|
120
|
Braczko F, Fischl SR, Reinders J, Lieder
HR and Kleinbongard P: Activation of the nonneuronal cholinergic
cardiac system by hypoxic preconditioning protects isolated adult
cardiomyocytes from hypoxia/reoxygenation injury. Am J Physiol
HeartCirc Physiol. 327:H70–H79. 2024. View Article : Google Scholar
|
|
121
|
Chotirat S, Suriyo T, Hokland M, Hokland
P, Satayavivad J and Auewarakul CU: Cholinergic activation enhances
retinoic acid-induced differentiation in the human NB-4 acute
promyelocytic leukemia cell line. Blood Cells Mol Dis. 59:77–84.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Oikawa S, Kai Y, Mano A, Sugama S,
Mizoguchi N, Tsuda M, Muramoto K and Kakinuma Y: Potentiating a
non-neuronal cardiac cholinergic system reinforces the functional
integrity of the blood brain barrier associated with systemic
anti-inflammatory responses. Brain Behav Immun. 81:122–137. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rocha-Resende C, Weinheimer C, Bajpai G,
Adamo L, Matkovich SJ, Schilling J, Barger PM, Lavine KJ and Mann
DL: Immunomodulatory role of non-neuronal cholinergic signaling in
myocardial injury. JCI Insight. 5:e1289612019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kakinuma Y, Akiyama T and Sato T:
Cholinoceptive and cholinergic properties of cardiomyocytes
involving an amplification mechanism for vagal efferent effects in
sparsely innervated ventricular myocardium. FEBS J. 276:5111–5125.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Oikawa S, Kai Y, Mano A, Ohata H, Nemoto T
and Kakinuma Y: Various regulatory modes for circadian rhythmicity
and sexual dimorphism in the non-neuronal cardiac cholinergic
system. J Cardiovasc Transl Res. 10:411–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Oikawa S, Kai Y, Mano A, Nakamura S and
Kakinuma Y: A novel nitric oxide donor,
S-Nitroso-NPivaloyl-D-Penicillamine, activates a non-neuronal
cardiac cholinergic system to synthesize acetylcholine and augments
cardiac function. Cell Physiol Biochem. 52:922–934. 2019.
View Article : Google Scholar : PubMed/NCBI
|