|
1
|
Shenoy A and Blelloch RH: Regulation of
microRNA function in somatic stem cell proliferation and
differentiation. Nat Rev Mol Cell Biol. 15:565–576. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonas S and Izaurralde E: Towards a
molecular understanding of microRNA-mediated gene silencing. Nat
Rev Genet. 16:421–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swarts DC, Makarova K, Wang Y, Nakanishi
K, Ketting RF, Koonin EV, Patel DJ and Van Der Oost J: The
evolutionary journey of argonaute proteins. Nat Struct Mol Biol.
21:743–753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
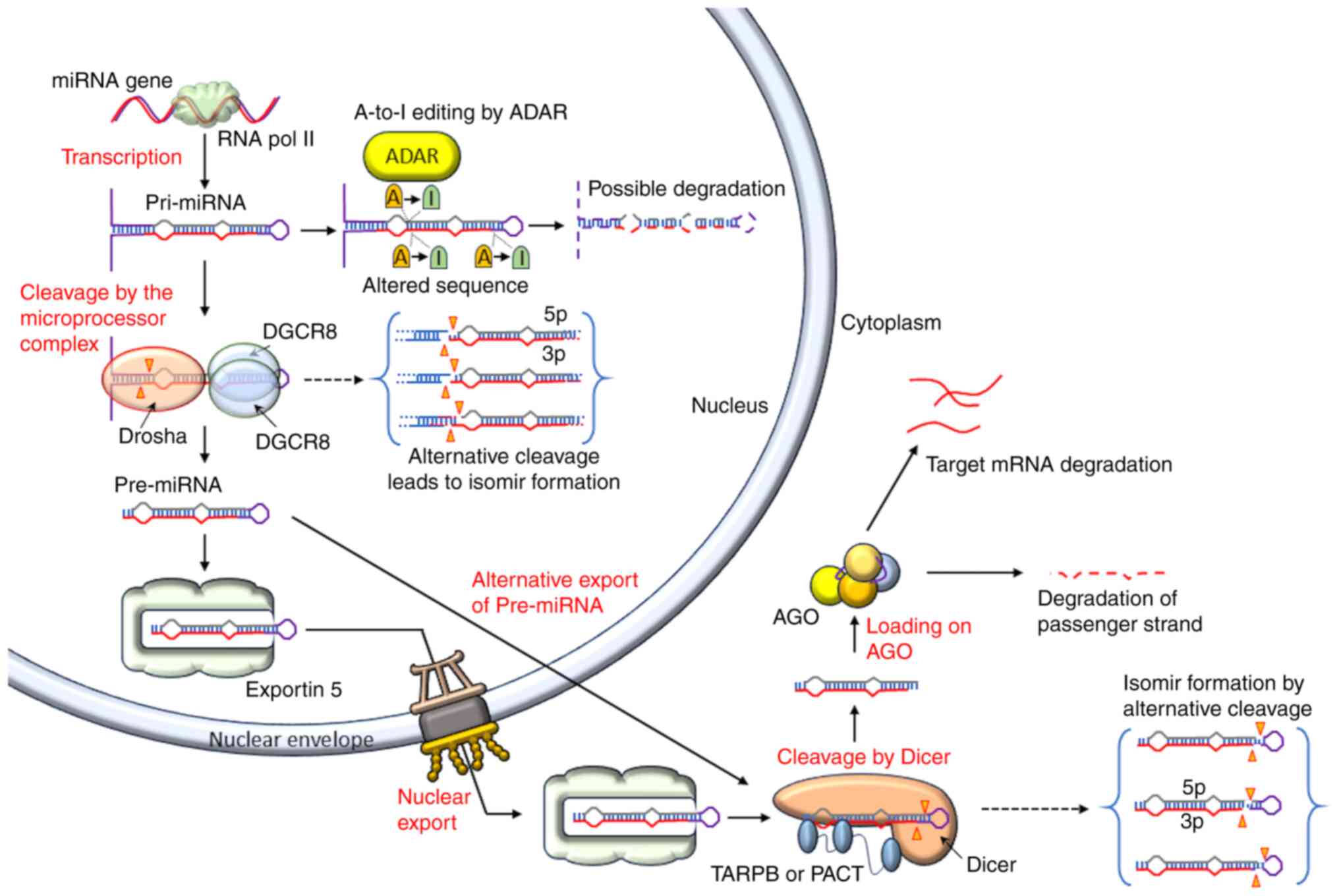
Res. 19:92–105. 2009. View Article : Google Scholar :
|
|
5
|
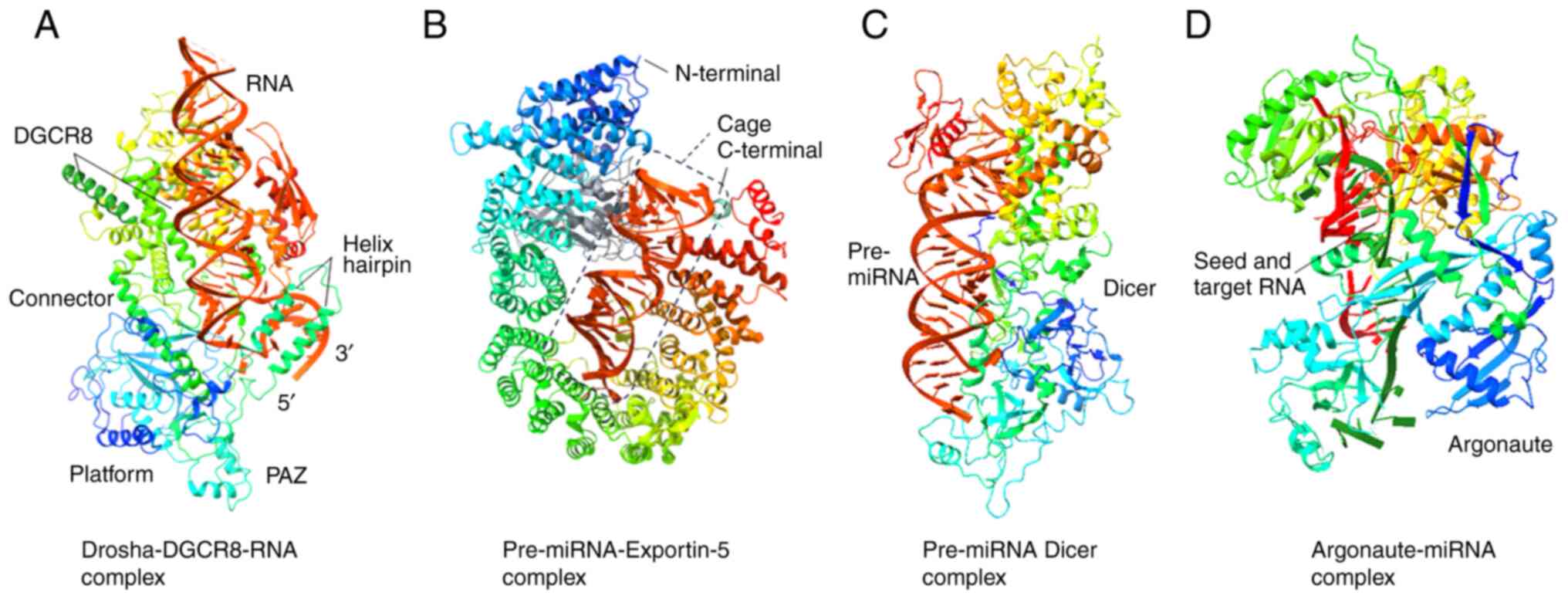
Kozomara A and Griffiths-Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42:D68–D73. 2014. View Article : Google Scholar :
|
|
6
|
Otmani K, Rouas R and Lewalle P: OncomiRs
as noncoding RNAs having functions in cancer: Their role in immune
suppression and clinical implications. Front Immunol.
13:9139512022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali Syeda Z, Langden SSS, Munkhzul C, Lee
M and Song SJ: Regulatory mechanism of MicroRNA expression in
cancer. Int J Mol Sci. 21:17232020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Otmani K and Lewalle P: Tumor suppressor
miRNA in cancer cells and the tumor microenvironment: Mechanism of
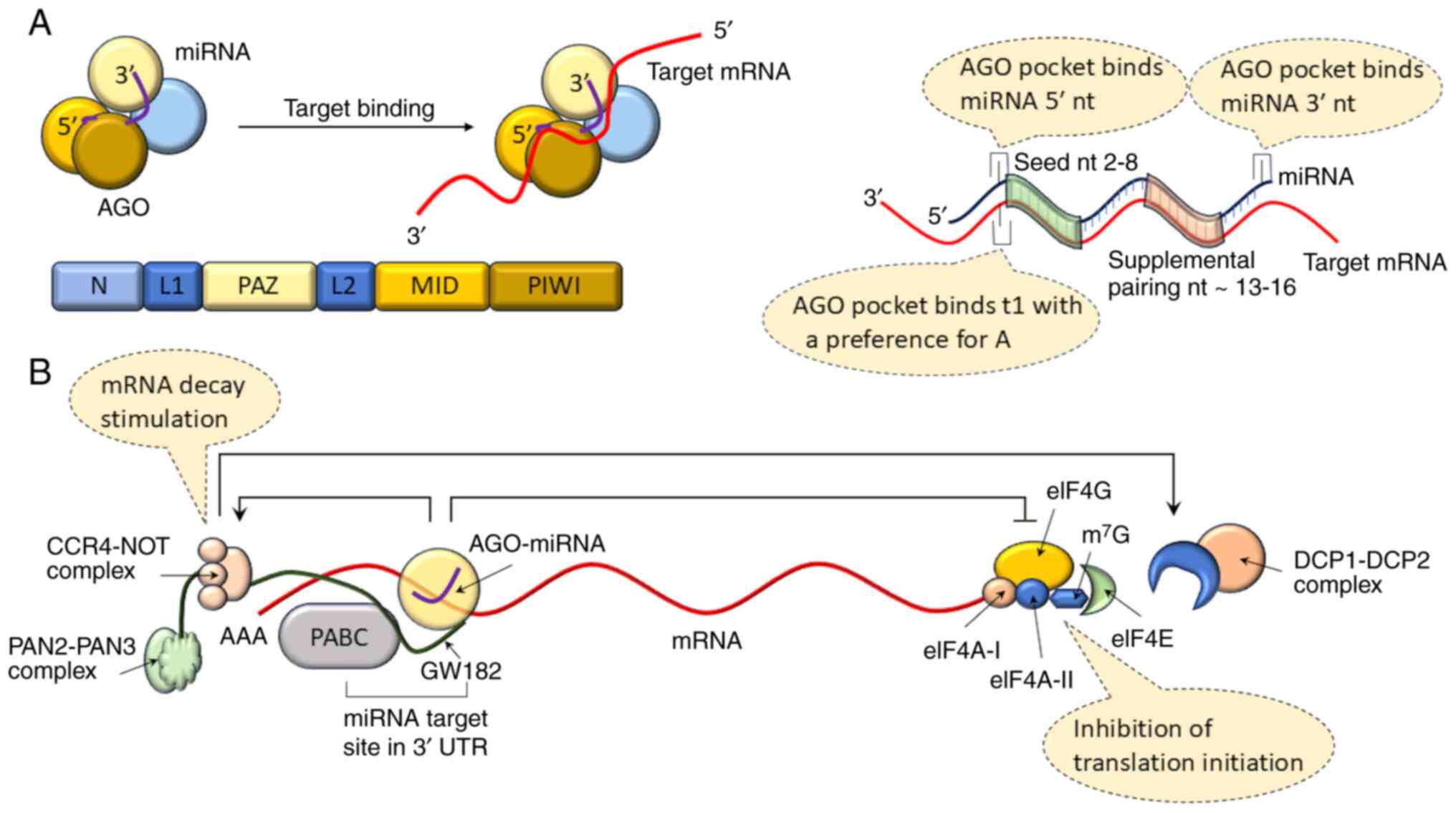
deregulation and clinical implications. Front Oncol. 11:7087652021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yousefnia S and Negahdary M: Role of
miRNAs in cancer: Oncogenic and tumor suppressor miRNAs, Their
regulation and therapeutic applications. Interdisciplinary Cancer
Research. Springer; Cham: pp. 1–27. 2024
|
|
10
|
Siddika T and Heinemann IU: Bringing
MicroRNAs to light: Methods for MicroRNA quantification and
visualization in live cells. Front Bioeng Biotechnol. 8:6195832021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mockly S and Seitz H: Inconsistencies and
limitations of current MicroRNA target identification methods.
Methods Mol Biol. 1970:291–314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koshiol J, Wang E, Zhao Y, Marincola F and
Landi MT: Strengths and limitations of laboratory procedures for
microRNA detection. Cancer Epidemiol Biomarkers Prev. 19:907–911.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allemailem KS, Alsahli MA, Almatroudi A,
Alrumaihi F, Alkhaleefah FK, Rahmani AH and Khan AA: Current
updates of CRISPR/Cas9-mediated genome editing and targeting within
tumor cells: An innovative strategy of cancer management. Cancer
Commun (Lond). 42:1257–1287. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allemailem KS, Almatroudi A, Alrumaihi F,
Alradhi AE, Theyab A, Algahtani M, Alhawas MO, Dobie G, Moawad AA,
Rahmani AH and Khan AA: Current updates of CRISPR/Cas system and
anti-CRISPR proteins: Innovative applications to improve the genome
editing strategies. Int J Nanomedicine. 19:10185–10212. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Chen Y, Dang L, Liu Y, Huang S, Wu
S, Ma P, Jiang H, Li Y, Pan Y, et al: EasyCatch, a convenient,
sensitive and specific CRISPR detection system for cancer gene
mutations. Mol Cancer. 20:1572021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Z, Fang J, Zhou M, Gong Z and Xiang
T: CRISPR-Cas13: A new technology for the rapid detection of
pathogenic microorganisms. Front Microbiol. 13:10113992022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allemailem KS, Alsahli MA, Almatroudi A,
Alrumaihi F, Al Abdulmonem W, Moawad AA, Alwanian WM, Almansour NM,
Rahmani AH and Khan AA: Innovative strategies of reprogramming
immune system cells by targeting CRISPR/Cas9-based genome-editing
tools: A new era of cancer management. Int J Nanomedicine.
18:5531–5559. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koonin EV and Makarova KS: CRISPR-Cas: An
adaptive immunity system in prokaryotes. F1000 Biol Rep. 1:952009.
View Article : Google Scholar
|
|
19
|
Allemailem KS, Almatroudi A, Rahmani AH,
Alrumaihi F, Alradhi AE, Alsubaiyel AM, Algahtani M, Almousa RM,
Mahzari A, Sindi AA, et al: Recent updates of the CRISPR/Cas9
genome editing system: Novel approaches to regulate its
spatiotemporal control by genetic and physicochemical strategies.
Int J Nanomedicine. 19:5335–5363. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abudayyeh OO, Gootenberg JS,
Essletzbichler P, Han S, Joung J, Belanto JJ, Verdine V, Cox DB,
Kellner MJ, Regev A, et al: RNA targeting with CRISPR-Cas13.
Nature. 550:280–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Connell MR: Molecular mechanisms of RNA
targeting by Cas13-containing type VI CRISPR-Cas systems. J Mol
Biol. 431:66–87. 2019. View Article : Google Scholar
|
|
22
|
Ali Z, Mahas A and Mahfouz M: CRISPR/Cas13
as a tool for RNA interference. Trends Plant Sci. 23:374–378. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang LZ, Wang Y, Li SQ, Yao RW, Luan PF,
Wu H, Carmichael GG and Chen LL: Dynamic imaging of RNA in living
cells by CRISPR-Cas13 systems. Mol Cell. 76:981–997.e7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Granados-Riveron JT and Aquino-Jarquin G:
CRISPR-Cas13 precision transcriptome engineering in cancer. Cancer
Res. 78:4107–4113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Wang Q and Wang Y: Direct and
accurate miRNA detection based on CRISPR/Cas13a-triggered
exonuclease-iii-assisted colorimetric assay. J Anal Sci Technol.
15:212024. View Article : Google Scholar
|
|
26
|
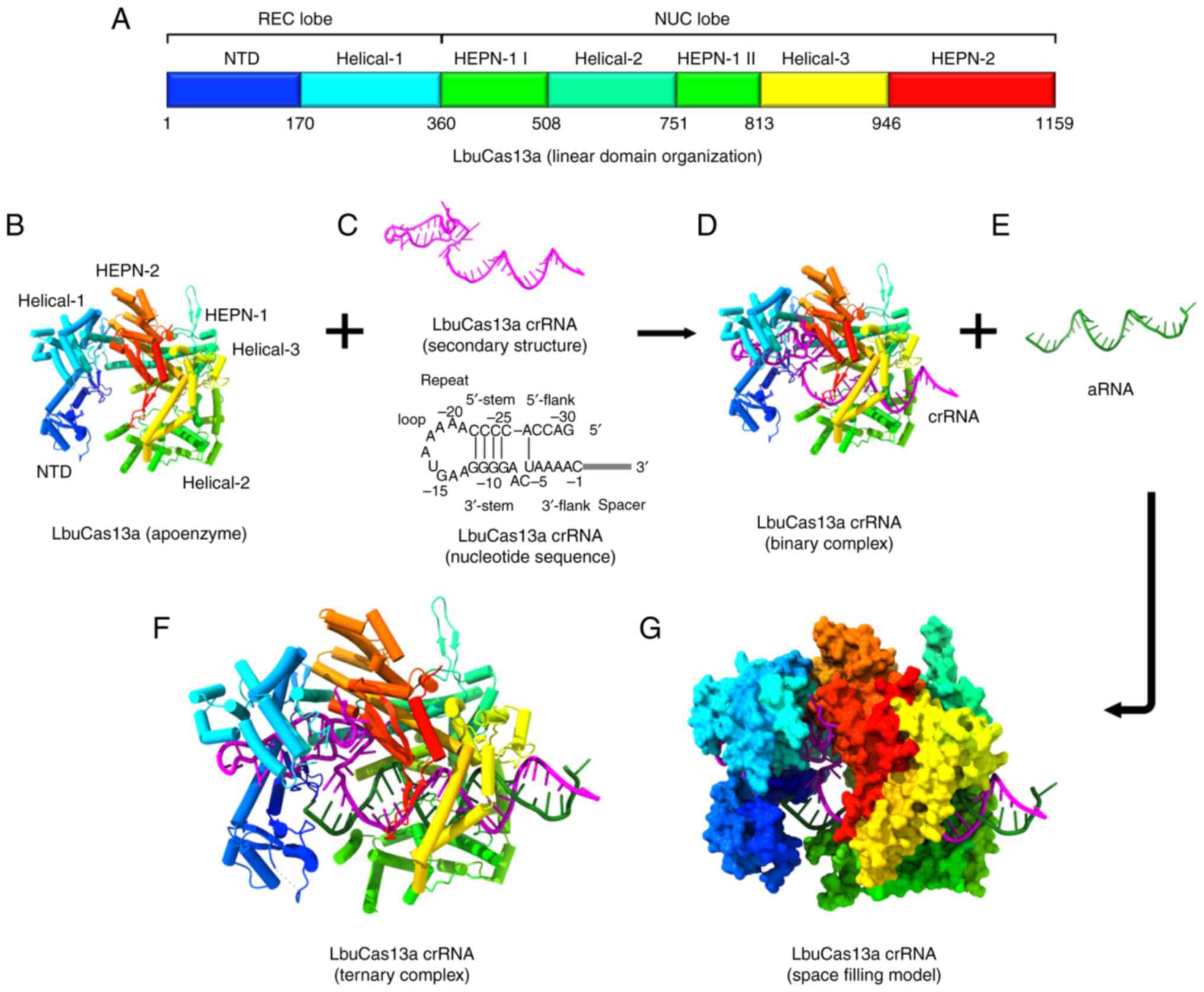
Kim JJ, Hong JS, Kim H, Choi M, Winter U,
Lee H and Im H: CRISPR/Cas13a-assisted amplification-free miRNA
biosensor via dark-field imaging and magnetic gold nanoparticles.
Sens Diagn. 3:1310–1318. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang M, Cai S, Wu Y, Li Q, Wang X, Zhang Y
and Zhou N: A lateral flow assay for miRNA-21 based on
CRISPR/Cas13a and MnO2 nanosheets-mediated recognition
and signal amplification. Anal Bioanal Chem. 416:3401–3413. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leitão AL and Enguita FJ: A structural
view of miRNA biogenesis and function. Noncoding RNA.
8:102022.PubMed/NCBI
|
|
29
|
Shang R, Lee S, Senavirathne G and Lai EC:
microRNAs in action: Biogenesis, function and regulation. Nat Rev
Genet. 24:816–833. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishikura K: A-to-I editing of coding and
non-coding RNAs by ADARs. Nat Rev Mol Cell Biol. 17:83–96. 2016.
View Article : Google Scholar :
|
|
31
|
Denli AM, Tops BBJ, Plasterk RHA, Ketting
RF and Hannon GJ: Processing of primary microRNAs by the
Microprocessor complex. Nature. 432:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gregory RI, Yan KP, Amuthan G, Chendrimada
T, Doratotaj B, Cooch N and Shiekhattar R: The microprocessor
complex mediates the genesis of microRNAs. Nature. 432:235–240.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen TA, Jo MH, Choi YG, Park J, Kwon
SC, Hohng S, Kim VN and Woo JS: Functional anatomy of the human
microprocessor. Cell. 161:1374–1387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwon SC, Nguyen TA, Choi YG, Jo MH, Hohng
S, Kim VN and Woo JS: Structure of human DROSHA. Cell. 164:81–90.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim B, Jeong K and Kim VN: Genome-wide
mapping of DROSHA cleavage sites on primary microRNAs and
noncanonical substrates. Mol Cell. 66:258–269.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neilsen CT, Goodall GJ and Bracken CP:
IsomiRs-the overlooked repertoire in the dynamic microRNAome.
Trends Genet. 28:544–549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nicholson AW: Ribonuclease III mechanisms
of double-stranded RNA cleavage. Wiley Interdiscip Rev RNA.
5:31–48. 2014. View Article : Google Scholar
|
|
41
|
Okada C, Yamashita E, Lee SJ, Shibata S,
Katahira J, Nakagawa A, Yoneda Y and Tsukihara T: A high-resolution
structure of the pre-microRNA nuclear export machinery. Science.
326:1275–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim YK, Kim B and Kim VN: Re-evaluation of
the roles of DROSHA, export in 5, and DICER in microRNA biogenesis.
Proc Natl Acad Sci USA. 113:E1881–E1889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grishok A, Pasquinelli AE, Conte D, Li N,
Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G and Mello CC: Genes
and mechanisms related to RNA interference regulate expression of
the small temporal RNAs that control C. elegans developmental
timing. Cell. 106:23–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsutsumi A, Kawamata T, Izumi N, Seitz H
and Tomari Y: Recognition of the pre-miRNA structure by Drosophila
Dicer-1. Nat Struct Mol Biol. 18:1153–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tian Y, Simanshu DK, Ma JB, Park JE, Heo
I, Kim VN and Patel DJ: A phosphate-binding pocket within the
platform-PAZ-connector helix cassette of human Dicer. Mol Cell.
53:606–616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matranga C, Tomari Y, Shin C, Bartel DP
and Zamore PD: Passenger-strand cleavage facilitates assembly of
siRNA into Ago2-containing RNAi enzyme complexes. Cell.
123:607–620. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zamore PD, Tuschl T, Sharp PA and Bartel
DP: RNAi: Double-stranded RNA directs the ATP-dependent cleavage of
mRNA at 21 to 23 nucleotide intervals. Cell. 101:25–33. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Suzuki HI, Katsura A, Yasuda T, Ueno T,
Mano H, Sugimoto K and Miyazono K: Small-RNA asymmetry is directly
driven by mammalian argonautes. Nat Struct Mol Biol. 22:512–521.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek
SH and Kim VN: MicroRNA genes are transcribed by RNA polymerase II.
EMBO J. 23:4051–4060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cai X, Hagedorn CH and Cullen BR: Human
microRNAs are processed from capped, polyadenylated transcripts
that can also function as mRNAs. RNA. 10:1957–1966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Berezikov E: Evolution of microRNA
diversity and regulation in animals. Nat Rev Genet. 12:846–860.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lim LP, Lau NC, Weinstein EG, Abdelhakim
A, Yekta S, Rhoades MW, Burge CB and Bartel DP: The microRNAs of
Caenorhabditis elegans. Genes Dev. 17:991–1008. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hall TMT: Structure and function of
argonaute proteins. Structure. 13:1403–1408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang J, Cho WC and Zheng Y: Argonaute
proteins: Structural features, functions and emerging roles. J Adv
Res. 24:317–324. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schirle NT and MacRae IJ: The crystal
structure of human argonaute2. Science. 336:1037–1040. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sheu-Gruttadauria J and MacRae IJ:
Structural foundations of RNA silencing by argonaute. J Mol Biol.
429:2619–2639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Diederichs S and Haber DA: Dual role for
argonautes in microRNA processing and posttranscriptional
regulation of microRNA expression. Cell. 131:1097–1108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu J, Carmell MA, Rivas FV, Marsden CG,
Thomson JM, Song JJ, Hammond SM, Joshua-Tor L and Hannon GJ:
Argonaute2 is the catalytic engine of mammalian RNAi. Science.
305:1437–1441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Burroughs AM, Ando Y, de Hoon MJL, Tomaru
Y, Suzuki H, Hayashizaki Y and Daub CO: Deep-sequencing of human
argonaute-associated small RNAs provides insight into miRNA sorting
and reveals Argonaute association with RNA fragments of diverse
origin. RNA Boil. 8:158–177. 2011. View Article : Google Scholar
|
|
64
|
Dueck A, Ziegler C, Eichner A, Berezikov E
and Meister G: microRNAs associated with the different human
argonaute proteins. Nucleic Acids Res. 40:9850–9862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:D146–D152. 2015. View Article : Google Scholar :
|
|
67
|
Gebert LF and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019. View Article : Google Scholar :
|
|
68
|
Schirle NT, Sheu-Gruttadauria J and MacRae
IJ: Structural basis for microRNA targeting. Science. 346:608–613.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schirle NT, Sheu-Gruttadauria J,
Chandradoss SD, Joo C and MacRae IJ: Water-mediated recognition of
t1-adenosine anchors argonaute2 to microRNA targets. Elife.
4:e076462015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yuan YR, Pei Y, Ma JB, Kuryavyi V, Zhadina
M, Meister G, Chen HY, Dauter Z, Tuschl T and Patel DJ: Crystal
structure of A. aeolicus argonaute, a site-specific DNA-guided
endoribonuclease, provides insights into RISC-mediated mRNA
cleavage. Mol Cell. 19:405–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li Z, Li Z, Zhang Y, Zhou L, Xu Q, Li L,
Zeng L, Xue J, Niu H, Zhong J, et al: Mammalian PIWI-piRNA-target
complexes reveal features for broad and efficient target silencing.
Nat Struct Mol Biol. 31:1222–1231. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Braun JE, Huntzinger E and Izaurralde E:
The role of GW182 proteins in miRNA-mediated gene silencing. Adv
Exp Med Biol. 768:147–163. 2013. View Article : Google Scholar
|
|
74
|
Chekulaeva M, Mathys H, Zipprich JT, Attig
J, Colic M, Parker R and Filipowicz W: miRNA repression involves
GW182-mediated recruitment of CCR4-NOT through conserved
W-containing motifs. Nat Struct Mol Biol. 18:1218–1226. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fabian MR, Cieplak MK, Frank F, Morita M,
Green J, Srikumar T, Nagar B, Yamamoto T, Raught B, Duchaine TF and
Sonenberg N: miRNA-mediated deadenylation is orchestrated by GW182
through two conserved motifs that interact with CCR4-NOT. Nat
Struct Mol Biol. 18:1211–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chen CYA, Zheng D, Xia Z and Shyu AB:
Ago-TNRC6 triggers microRNA-mediated decay by promoting two
deadenylation steps. Nat Struct Mol Biol. 16:1160–1166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Braun JE, Truffault V, Boland A,
Huntzinger E, Chang CT, Haas G, Weichenrieder O, Coles M and
Izaurralde E: A direct interaction between DCP1 and XRN1 couples
mRNA decapping to 5′ exonucleolytic degradation. Nat Struct Mol
Biol. 19:1324–1331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mathys H, Basquin J, Ozgur S,
Czarnocki-Cieciura M, Bonneau F, Aartse A, Dziembowski A, Nowotny
M, Conti E and Filipowicz W: Structural and biochemical insights to
the role of the CCR4-NOT complex and DDX6 ATPase in microRNA
repression. Mol Cell. 54:751–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fukaya T, Iwakawa HO and Tomari Y:
MicroRNAs block assembly of eIF4F translation initiation complex in
Drosophila. Mol Cell. 56:67–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fukao A, Mishima Y, Takizawa N, Oka S,
Imataka H, Pelletier J, Sonenberg N, Thoma C and Fujiwara T:
MicroRNAs trigger dissociation of eIF4AI and eIF4AII from target
mRNAs in humans. Mol Cell. 56:79–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nishihara T, Zekri L, Braun JE and
Izaurralde E: miRISC recruits decapping factors to miRNA targets to
enhance their degradation. Nucleic Acids Res. 41:8692–8705. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jungers CF and Djuranovic S: Modulation of
miRISC-mediated gene silencing in eukaryotes. Front Mol Biosci.
9:8329162022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kuzuoğlu-Öztürk D, Bhandari D, Huntzinger
E, Fauser M, Helms S and Izaurralde E: miRISC and the CCR4-NOT
complex silence mRNA targets independently of 43S ribosomal
scanning. EMBO J. 35:1186–1203. 2016. View Article : Google Scholar
|
|
84
|
Elkayam E, Faehnle CR, Morales M, Sun J,
Li H and Joshua-Tor L: Multivalent recruitment of human argonaute
by GW182. Mol Cell. 67:646–658.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Eichhorn SW, Guo H, McGeary SE,
Rodriguez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villén J and
Bartel DP: mRNA destabilization is the dominant effect of mammalian
microRNAs by the time substantial repression ensues. Mol Cell.
56:104–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bhattacharyya SN, Habermacher R, Martine
U, Closs EI and Filipowicz W: Relief of microRNA-mediated
translational repression in human cells subjected to stress. Cell.
125:1111–1124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Uhlmann S, Mannsperger H, Zhang JD, Horvat
EÁ, Schmidt C, Küblbeck M, Henjes F, Ward A, Tschulena U, Zweig K,
et al: Global microRNA level regulation of EGFR-driven cell-cycle
protein network in breast cancer. Mol Syst Biol. 8:5702012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mestdagh P, Boström AK, Impens F, Fredlund
E, Van Peer G, De Antonellis P, Von Stedingk K, Ghesquière B,
Schulte S, Dews M, et al: The miR-17-92 microRNA cluster regulates
multiple components of the TGF-β pathway in neuroblastoma. Mol
Cell. 40:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Saetrom P, Heale BS, Snøve O Jr, Aagaard
L, Alluin J and Rossi JJ: Distance constraints between microRNA
target sites dictate efficacy and cooperativity. Nucleic Acids Res.
35:2333–2342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Broderick JA, Salomon WE, Ryder SP, Aronin
N and Zamore PD: Argonaute protein identity and pairing geometry
determine cooperativity in mammalian RNA silencing. RNA.
17:1858–1869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tsang J, Zhu J and van Oudenaarden A:
MicroRNA-mediated feedback and feedforward loops are recurrent
network motifs in mammals. Mol Cell. 26:753–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Suzuki H, Maruyama R, Yamamoto E and Kai
M: Epigenetic alteration and microRNA dysregulation in cancer.
Front Genet. 4:2582013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Calin GA and Croce CM: MicroRNAs and
chromosomal abnormalities in cancer cells. Oncogene. 25:6202–6210.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et
al: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tagawa H and Seto M: A microRNA cluster as
a target of genomic amplification in malignant lymphoma. Leukemia.
19:2013–2016. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hayashita Y, Osada H, Tatematsu Y, Yamada
H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y and
Takahashi T: A polycistronic microRNA cluster, miR-17-92, is
overexpressed in human lung cancers and enhances cell
proliferation. Cancer Res. 65:9628–9632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mavrakis KJ, Wolfe AL, Oricchio E,
Palomero T, De Keersmaecker K, McJunkin K, Zuber J, James T, Khan
AA, Leslie CS, et al: Genome-wide RNA-mediated interference screen
identifies miR-19 targets in Notch-induced T-cell acute
lymphoblastic leukaemia. Nat Cell Boil. 12:372–379. 2010.
View Article : Google Scholar
|
|
101
|
Zhang L, Huang J, Yang N, Greshock J,
Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR,
et al: microRNAs exhibit high frequency genomic alterations in
human cancer. Proc Natl Acad Sci USA. 103:9136–9141. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wijnhoven BP, Michael MZ and Watson DI:
MicroRNAs and cancer. Br J Surg. 94:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Qin S, Xu J, Yi Y, Jiang S, Jin P, Xia X
and Ma F: Transcription factors and methylation drive prognostic
miRNA dysregulation in hepatocellular carcinoma. Front Oncol.
11:6911152021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sachdeva M, Zhu S, Wu F, Wu H, Walia V,
Kumar S, Elble R, Watabe K and Mo YY: p53 represses c-Myc through
induction of the tumor suppressor miR-145. PProc Natl Acad Sci USA.
106:3207–3212. 2009. View Article : Google Scholar
|
|
106
|
O'Donnell KA, Wentzel EA, Zeller KI, Dang
CV and Mendell JT: c-Myc-regulated microRNAs modulate E2F1
expression. Nature. 435:839–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chang TC, Yu D, Lee YS, Wentzel EA, Arking
DE, West KM, Dang CV, Thomas-Tikhonenko A and Mendell JT:
Widespread microRNA repression by Myc contributes to tumorigenesis.
Nat Genet. 40:43–50. 2008. View Article : Google Scholar
|
|
108
|
Wang B, Hsu SH, Wang X, Kutay H, Bid HK,
Yu J, Ganju RK, Jacob ST, Yuneva M and Ghoshal K: Reciprocal
regulation of microRNA-122 and c-Myc in hepatocellular cancer: Role
of E2F1 and transcription factor dimerization partner 2.
Hepatology. 59:555–566. 2014. View Article : Google Scholar
|
|
109
|
Han H, Sun D, Li W, Shen H, Zhu Y, Li C,
Chen Y, Lu L, Li W, Zhang J, et al: A c-Myc-MicroRNA functional
feedback loop affects hepatocarcinogenesis. Hepatology.
57:2378–2389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
He L, He X, Lim LP, De Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hermeking HJ: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar
|
|
112
|
Xiao J, Lin H, Luo X, Luo X and Wang Z:
miR-605 joins p53 network to form a p53:miR-605:Mdm2 positive
feedback loop in response to stress. EMBO J. 30:524–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang Y, Liao JM, Zeng SX and Lu H: p53
downregulates Down syndrome-associated DYRK1A through miR-1246.
EMBO Rep. 12:811–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yamakuchi M, Lotterman CD, Bao C, Hruban
RH, Karim B, Mendell JT, Huso D and Lowenstein CJ: P53-induced
microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad
Sci USA. 107:6334–6339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Eyholzer M, Schmid S, Schardt JA,
Haefliger S, Mueller BU and Pabst T: Complexity of miR-223
regulation by CEBPA in human AML. Leuk Res. 34:672–676. 2010.
View Article : Google Scholar
|
|
116
|
Hessam S, Sand M, Skrygan M, Gambichler T
and Bechara FG: Inflammation induced changes in the expression
levels of components of the microRNA maturation machinery Drosha,
Dicer, Drosha co-factor DGRC8 and Exportin-5 in inflammatory
lesions of hidradenitis suppurativa patients. J Dermatol Sci.
82:166–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kim J, Park WJ, Jeong KJ, Kang SH, Kwon
SY, Kim S and Park JW: Racial differences in expression levels of
miRNA machinery-related genes, dicer, drosha, DGCR8, and AGO2, in
Asian Korean papillary thyroid carcinoma and comparative validation
using the cancer genome atlas. Int J Genomics. 2017:57897692017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yan M, Huang HY, Wang T, Wan Y, Cui SD,
Liu ZZ and Fan QX: Dysregulated expression of dicer and drosha in
breast cancer. Pathol Oncol Res. 18:343–348. 2012. View Article : Google Scholar
|
|
119
|
Zhang Z, Zhang G, Kong C, Bi J, Gong D, Yu
X, Shi D, Zhan B and Ye P: EIF2C, Dicer, and Drosha are
up-regulated along tumor progression and associated with poor
prognosis in bladder carcinoma. Tumour Biol. 36:5071–5079. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Thomson JM, Newman M, Parker JS,
Morin-Kensicki EM, Wright T and Hammond SM: Extensive
post-transcriptional regulation of microRNAs and its implications
for cancer. Genes Dev. 20:2202–2207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Walz AL, Ooms A, Gadd S, Gerhard DS, Smith
MA, Auvil JMG, Meerzaman D, Chen QR, Hsu CH, Yan C, et al:
Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable
histology Wilms tumors. Cancer Cell. 27:286–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Iliou MS, da Silva-Diz V, Carmona FJ,
Ramalho-Carvalho J, Heyn H, Villanueva A, Muñoz P and Esteller M:
Impaired DICER1 function promotes stemness and metastasis in colon
cancer. Oncogene. 33:4003–4015. 2014. View Article : Google Scholar :
|
|
123
|
Merritt WM, Lin YG, Han LY, Kamat AA,
Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick
AM, et al: Dicer, Drosha, and outcomes in patients with ovarian
cancer. N Engl J Med. 359:2641–2650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Pampalakis G, Diamandis EP, Katsaros D and
Sotiropoulou G: Down-regulation of dicer expression in ovarian
cancer tissues. Clin Biochem. 43:324–327. 2010. View Article : Google Scholar
|
|
125
|
Faggad A, Budczies J, Tchernitsa O,
Darb-Esfahani S, Sehouli J, Müller BM, Wirtz R, Chekerov R,
Weichert W, Sinn B, et al: Prognostic significance of Dicer
expression in ovarian cancer-link to global microRNA changes and
oestrogen receptor expression. J Pathol. 220:382–391. 2010.
View Article : Google Scholar
|
|
126
|
Karube Y, Tanaka H, Osada H, Tomida S,
Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S,
Mitsudomi T and Takahashi T: Reduced expression of Dicer associated
with poor prognosis in lung cancer patients. Cancer Sci.
96:111–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Dome JS and Coppes MJ: Recent advances in
Wilms tumor genetics. Curr Opin Pediatr. 14:5–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang J, Fan XS, Wang CX, Liu B, Li Q and
Zhou XJ: Up-regulation of Ago2 expression in gastric carcinoma. Med
Oncol. 30:6282013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Völler D, Reinders J, Meister G and
Bosserhoff AK: Strong reduction of AGO2 expression in melanoma and
cellular consequences. Br J Cancer. 109:3116–3124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Melo SA, Moutinho C, Ropero S, Calin GA,
Rossi S, Spizzo R, Fernandez AF, Davalos V, Villanueva A, Montoya
G, et al: A genetic defect in exportin-5 traps precursor microRNAs
in the nucleus of cancer cells. Cancer Cell. 18:303–315. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chu R, Mo G, Duan Z, Huang M, Chang J, Li
X and Liu P: miRNAs affect the development of hepatocellular
carcinoma via dysregulation of their biogenesis and expression.
CCell Commun Signal. 12:452014. View Article : Google Scholar
|
|
132
|
Han L, Witmer PD, Casey E, Valle D and
Sukumar S: DNA methylation regulates MicroRNA expression. Cancer
Biol Ther. 6:1290–1294. 2007. View Article : Google Scholar
|
|
133
|
Saito Y and Jones PM: Epigenetic
activation of tumor suppressor microRNAs in human cancer cells.
Cell Cycle. 5:2220–2222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fazi F, Racanicchi S, Zardo G, Starnes LM,
Mancini M, Travaglini L, Diverio D, Ammatuna E, Cimino G, Lo-Coco
F, et al: Epigenetic silencing of the myelopoiesis regulator
microRNA-223 by the AML1/ETO oncoprotein. Cancer Cell. 12:457–466.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Saito Y, Liang G, Egger G, Friedman JM,
Chuang JC, Coetzee GA and Jones PA: Specific activation of
microRNA-127 with downregulation of the proto-oncogene BCL6 by
chromatin-modifying drugs in human cancer cells. Cancer Cell.
9:435–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lujambio A, Calin GA, Villanueva A, Ropero
S, Sánchez-Céspedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso
MS, Faller WJ, et al: A microRNA DNA methylation signature for
human cancer metastasis. Proc Natl Acad Sci USA. 105:13556–13561.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Baharudin R, Rus Bakarurraini NQ, Ismail
I, Lee LH and Ab Mutalib NS: MicroRNA methylome signature and their
functional roles in colorectal cancer diagnosis, prognosis, and
chemoresistance. Int J Mol Sci. 23:72812022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lehmann U, Hasemeier B, Christgen M,
Müller M, Römermann D, Länger F and Kreipe H: Epigenetic
inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J
Pathol. 214:17–24. 2008. View Article : Google Scholar
|
|
139
|
Donzelli S, Mori F, Bellissimo T, Sacconi
A, Casini B, Frixa T, Roscilli G, Aurisicchio L, Facciolo F,
Pompili A, et al: Epigenetic silencing of miR-145-5p contributes to
brain metastasis. Oncotarget. 6:35183–35201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Trimarchi JM and Lees JA: Sibling rivalry
in the E2F family. Nat Rev Mol Cell Biol. 3:11–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Coller HA, Forman JJ and Legesse-Miller A:
'Myc'ed messages': myc induces transcription of E2F1 while
inhibiting its translation via a microRNA polycistron. PLoS Genet.
3:e1462007. View Article : Google Scholar
|
|
143
|
Sylvestre Y, De Guire V, Querido E,
Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G and Chartrand P:
An E2F/miR-20a autoregulatory feedback loop. J Biol Chem.
282:2135–2143. 2007. View Article : Google Scholar
|
|
144
|
Woods K, Thomson JM and Hammond SM: Direct
regulation of an oncogenic micro-RNA cluster by E2F transcription
factors. J Biol Chem. 282:2130–2134. 2007. View Article : Google Scholar
|
|
145
|
He L, Thomson JM, Hemann MT,
Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe
SW, Hannon GJ and Hammond SM: A microRNA polycistron as a potential
human oncogene. Nature. 435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Hatfield SD, Shcherbata HR, Fischer KA,
Nakahara K, Carthew RW and Ruohola-Baker H: Stem cell division is
regulated by the microRNA pathway. Nature. 435:974–978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yu JY, Reynolds SH, Hatfield SD,
Shcherbata HR, Fischer KA, Ward EJ, Long D, Ding Y and
Ruohola-Baker H: Dicer-1-dependent Dacapo suppression acts
downstream of Insulin receptor in regulating cell division of
Drosophila germline stem cells. Development. 136:1497–1507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gillies JK and Lorimer IAJ: Regulation of
p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle. 6:2005–2009.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Galardi S, Mercatelli N, Giorda E,
Massalini S, Frajese GV, Ciafrè SA and Farace MG: miR-221 and
miR-222 expression affects the proliferation potential of human
prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem.
282:23716–23724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
le Sage C, Nagel R, Egan DA, Schrier M,
Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA, et
al: Regulation of the p27(Kip1) tumor suppressor by miR-221 and
miR-222 promotes cancer cell proliferation. EMBO J. 26:3699–3708.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhang C, Kang C, You Y, Pu P, Yang W, Zhao
P, Wang G, Zhang A, Jia Z, Han L and Jiang H: Co-suppression of
miR-221/222 cluster suppresses human glioma cell growth by
targeting p27kip1 in vitro and in vivo. Int J Oncol. 34:1653–1660.
2009.PubMed/NCBI
|
|
152
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Choudhry H and Harris AL: Advances in
hypoxia-inducible factor biology. Cell Metab. 27:281–298. 2018.
View Article : Google Scholar
|
|
154
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tiwari A, Mukherjee B and Dixit M:
MicroRNA key to angiogenesis regulation: MiRNA biology and therapy.
Curr Cancer Drug Targets. 18:266–277. 2018. View Article : Google Scholar
|
|
156
|
Landskroner-Eiger S, Moneke I and Sessa
WC: miRNAs as modulators of angiogenesis. Cold Spring Harb Perspect
Med. 3:a0066432013. View Article : Google Scholar
|
|
157
|
Camps C, Buffa FM, Colella S, Moore J,
Sotiriou C, Sheldon H, Harris AL, Gleadle JM and Ragoussis J:
hsa-miR-210 is induced by hypoxia and is an independent prognostic
factor in breast cancer. Clin Cancer Res. 14:1340–1348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Fasanaro P, D'Alessandra Y, Di Stefano V,
Melchionna R, Romani S, Pompilio G, Capogrossi MC and Martelli F:
MicroRNA-210 modulates endothelial cell response to hypoxia and
inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Lou YL, Guo F, Liu F, Gao FL, Zhang PQ,
Niu X, Guo SC, Yin JH, Wang Y and Deng ZF: miR-210 activates notch
signaling pathway in angiogenesis induced by cerebral ischemia. Mol
Cell Biochem. 370:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ghosh G, Subramanian IV, Adhikari N, Zhang
X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y and
Ramakrishnan S: Hypoxia-induced microRNA-424 expression in human
endothelial cells regulates HIF-α isoforms and promotes
angiogenesis. J Clin Invest. 120:4141–4154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R,
Jiang Y, Kung HF, Lai L and Jiang BH: MiR-21 induced angiogenesis
through AKT and ERK activation and HIF-1α expression. PLoS One.
6:e191392011. View Article : Google Scholar
|
|
162
|
Lei Z, Li BO, Yang Z, Fang H, Zhang GM,
Feng ZH and Huang B: Regulation of HIF-1alpha and VEGF by miR-20b
tunes tumor cells to adapt to the alteration of oxygen
concentration. PLoS One. 4:e76292009. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Cha ST, Chen PS, Johansson G, Chu CY, Wang
MY, Jeng YM, Yu SL, Chen JS, Chang KJ, Jee SH, et al: MicroRNA-519c
suppresses hypoxia-inducible factor-1alpha expression and tumor
angiogenesis. Cancer Res. 70:2675–2685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The E-cadherin and
N-cadherin switch in epithelial-to-mesenchymal transition:
Signaling, therapeutic implications, and challenges. Cells.
8:11182019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Rutnam ZJ, Wight TN and Yang BB: miRNAs
regulate expression and function of extracellular matrix molecules.
Matrix Biol. 32:74–85. 2013. View Article : Google Scholar
|
|
166
|
Raines EW: The extracellular matrix can
regulate vascular cell migration, proliferation, and survival:
Relationships to vascular disease. Int J Exp Pathol. 81:173–182.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zitka O, Kukacka J, Krizkova S, Huska D,
Adam V, Masarik M, Prusa R and Kizek R: Matrix metalloproteinases.
Curr Med Chem. 17:3751–3768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Wu D, Huang P, Wang L, Zhou Y, Pan H and
Qu P: MicroRNA-143 inhibits cell migration and invasion by
targeting matrix metalloproteinase 13 in prostate cancer. Mol Med
Rep. 8:626–630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Osaki M, Takeshita F, Sugimoto Y, Kosaka
N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T,
et al: MicroRNA-143 regulates human osteosarcoma metastasis by
regulating matrix metalloprotease-13 expression. Mol Ther.
19:1123–1130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Liu H, Cao YD, Ye WX and Sun YY: Effect of
microRNA-206 on cytoskeleton remodelling by downregulating Cdc42 in
MDA-MB-231 cells. Tumori. 96:751–755. 2010. View Article : Google Scholar
|
|
172
|
Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J,
Zhao JJ, Mao SS, Zhang GH, Xu XC and Zhang N: miR-340 inhibition of
breast cancer cell migration and invasion through targeting of
oncoprotein c-Met. Cancer. 117:2842–2852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Kong W, Yang H, He L, Zhao JJ, Coppola D,
Dalton WS and Cheng JQ: MicroRNA-155 is regulated by the
transforming growth factor beta/Smad pathway and contributes to
epithelial cell plasticity by targeting RhoA. Mol Cell Biol.
28:6773–6784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Bracken CP, Gregory PA, Kolesnikoff N,
Bert AG, Wang J, Shannon MF and Goodall GJ: A double-negative
feedback loop between ZEB1-SIP1 and the microRNA-200 family
regulates epithelial-mesenchymal transition. Cancer Res.
68:7846–7854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lima RT, Busacca S, Almeida GM, Gaudino G,
Fennell DA and Vasconcelos MH: MicroRNA regulation of core
apoptosis pathways in cancer. Eur J Cancer. 47:163–174. 2011.
View Article : Google Scholar
|
|
178
|
Li C, Hashimi SM, Good DA, Cao S, Duan W,
Plummer PN, Mellick AS and Wei MQ: Apoptosis and microRNA
aberrations in cancer. Clin Exp Pharmacol Physiol. 39:739–146.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Cadwell C and Zambetti GP: The effects of
wild-type p53 tumor suppressor activity and mutant p53
gain-of-function on cell growth. Gene. 277:15–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
O'Brien MA and Kirby R: Apoptosis: A
review of pro-apoptotic and anti-apoptotic pathways and
dysregulation in disease. J Vet Emerg Crit Care (San Antonio).
18:572–585. 2008. View Article : Google Scholar
|
|
181
|
Hermeking H: MicroRNAs in the p53 network:
Micromanagement of tumour suppression. Nat Rev Cancer. 12:613–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Pichiorri F, Suh SS, Rocci A, De Luca L,
Taccioli C, Santhanam R, Zhou W, Benson DM Jr, Hofmainster C, Alder
H, et al: RETRACTED: Downregulation of p53-inducible microRNAs 192,
194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple
myeloma development. Cancer Cell. 18:367–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Bi C and Chng WJ: MicroRNA: Important
player in the pathobiology of multiple myeloma. Biomed Res Int.
2014:5215862014. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Fornari F, Gramantieri L, Giovannini C,
Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM,
Tavolari S, et al: MiR-122/cyclin G1 interaction modulates p53
activity and affects doxorubicin sensitivity of human
hepatocarcinoma cells. Cancer Res. 69:5761–5767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Burns DM, D'Ambrogio A, Nottrott S and
Richter JD: CPEB and two poly(A) polymerases control miR-122
stability and p53 mRNA translation. Nature. 473:105–108. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Hajizadeh M, Hajizadeh F, Ghaffarei S,
Doustvandi MA, Hajizadeh K, Yaghoubi SM, Mohammadnejad F, Khiabani
NA, Mousavi P and Baradaran B: MicroRNAs and their vital role in
apoptosis in hepatocellular carcinoma: miRNA-based diagnostic and
treatment methods. Gene. 888:1478032023. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yan HL, Xue G, Mei Q, Wang YZ, Ding FX,
Liu MF, Lu MH, Tang Y, Yu HY and Sun SH: Repression of the
miR-17-92 cluster by p53 has an important function in
hypoxia-induced apoptosis. EMBO J. 28:2719–2732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Jiang X, Liu Y, Zhang G, Lin S, Wu J, Yan
X, Ma Y and Ma M: Aloe-emodin induces breast tumor cell apoptosis
through upregulation of miR-15a/miR-16-1 that suppresses BCL2. Evid
Based Complement Alternat Med. 2020:51082982020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Sacconi A, Biagioni F, Canu V, Mori F, Di
Benedetto A, Lorenzon L, Ercolani C, Di Agostino S, Cambria AM,
Germoni S, et al: miR-204 targets Bcl-2 expression and enhances
responsiveness of gastric cancer. Cell Death Dis. 3:e4232012.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Li M, Yang Y, Kuang Y, Gan X, Zeng W, Liu
Y and Guan H: miR-365 induces hepatocellular carcinoma cell
apoptosis through targeting Bcl-2. Exp Ther Med. 13:2279–2285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Denoyelle C, Lambert B, Meryet-Figuière M,
Vigneron N, Brotin E, Lecerf C, Abeilard E, Giffard F, Louis MH,
Gauduchon P, et al: miR-491-5p-induced apoptosis in ovarian
carcinoma depends on the direct inhibition of both BCL-XL and EGFR
leading to BIM activation. Cell Death Dis. 5:e14452014. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Burmistrz M, Krakowski K and
Krawczyk-Balska A: RNA-targeting CRISPR-Cas systems and their
applications. Int J Mol Sci. 21:11222020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Donohoue PD, Barrangou R and May AP:
Advances in industrial biotechnology using CRISPR-Cas systems.
Trends Biotechnol. 36:134–146. 2018. View Article : Google Scholar
|
|
194
|
Konermann S, Lotfy P, Brideau NJ, Oki J,
Shokhirev MN and Hsu PD: Transcriptome engineering with
RNA-targeting type VI-D CRISPR effectors. Cell. 173:665–676 e14.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Yan WX, Chong S, Zhang H, Makarova KS,
Koonin EV, Cheng DR and Scott DA: Cas13d is a compact RNA-targeting
type VI CRISPR effector positively modulated by a
WYL-domain-containing accessory protein. Mol Cell. 70:327–339.e5.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Makarova KS, Wolf YI, Iranzo J, Shmakov
SA, Alkhnbashi OS, Brouns SJ, Charpentier E, Cheng D, Haft DH,
Horvath P, et al: Evolutionary classification of CRISPR-Cas
systems: A burst of class 2 and derived variants. Nat Rev
Microbiol. 18:67–83. 2020. View Article : Google Scholar
|
|
197
|
Xu C, Zhou Y, Xiao Q, He B, Geng G, Wang
Z, Cao B, Dong X, Bai W, Wang Y, et al: Programmable RNA editing
with compact CRISPR-Cas13 systems from uncultivated microbes. Nat
Methods. 18:499–506. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Kannan S, Altae-Tran H, Jin X, Madigan VJ,
Oshiro R, Makarova KS, Koonin EV and Zhang F: Compact RNA editors
with small Cas13 proteins. Nat Biotechnol. 40:194–197. 2022.
View Article : Google Scholar :
|
|
199
|
Granados-Riveron JT and Aquino-Jarquin G:
CRISPR/Cas13-based approaches for ultrasensitive and specific
detection of microRNAs. Cells. 10:16552021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Wang H, La Russa M and Qi LS: CRISPR/Cas9
in genome editing and beyond. Annu Rev Biochem. 85:227–264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Liu L, Li X, Ma J, Li Z, You L, Wang J,
Wang M, Zhang X and Wang Y: The molecular architecture for
RNA-guided RNA cleavage by Cas13a. Cell. 170:714–726.e10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Watanabe S, Cui B, Kiga K, Aiba Y, Tan XE,
Sato'o Y, Kawauchi M, Boonsiri T, Thitiananpakorn K, Taki Y, et al:
Composition and diversity of CRISPR-Cas13a systems in the genus
Leptotrichia. Front Microbiol. 10:28382019. View Article : Google Scholar
|
|
203
|
Jain I: CRISPR Cas adaptive immunity in
Leptotrichia shahii type VI-A system. Rutgers University Community
Repository. 1132022.
|
|
204
|
Liu L, Li X, Wang J, Wang M, Chen P, Yin
M, Li J, Sheng G and Wang Y: Two distant catalytic sites are
responsible for C2c2 RNase activities. Cell. 168:121–134.e12. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Abudayyeh OO, Gootenberg JS, Konermann S,
Joung J, Slaymaker IM, Cox DBT, Shmakov S, Makarova KS, Semenova E,
Minakhin L, et al: C2c2 is a single-component programmable
RNA-guided RNA-targeting CRISPR effector. Science. 353:aaf55732016.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Mahas A, Aman R and Mahfouz M:
CRISPR-Cas13d mediates robust RNA virus interference in plants.
Genome Boil. 20:2632019. View Article : Google Scholar
|
|
207
|
Yang L and Chen LL: Enhancing the RNA
engineering toolkit. Science. 358:996–997. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Wessels HH, Méndez-Mancilla A, Guo X,
Legut M, Daniloski Z and Sanjana NE: Massively parallel Cas13
screens reveal principles for guide RNA design. Nat Biotechnol.
38:722–727. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
East-Seletsky A, O'Connell MR, Burstein D,
Knott GJ and Doudna JA: RNA targeting by functionally orthogonal
type VI-A CRISPR-Cas enzymes. Mol Cell. 66:373–383.e3. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Smargon AA, Cox DBT, Pyzocha NK, Zheng K,
Slaymaker IM, Gootenberg JS, Abudayyeh OA, Essletzbichler P,
Shmakov S, Makarova KS, et al: Cas13b is a type VI-B
CRISPR-associated RNA-guided RNase differentially regulated by
accessory proteins Csx27 and Csx28. Mol Cell. 65:618–630. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Cox DBT, Gootenberg JS, Abudayyeh OO,
Franklin B, Kellner MJ, Joung J and Zhang F: RNA editing with
CRISPR-Cas13. Science. 358:1019–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Dong Y, Zhang B, Wei Y, Murashev A, Wang
S, Wu Y, Ma W and Liu T: Development of Cas13a-based therapy for
cancer treatment. Mol Biol Rep. 51:942024. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Bot JF, van der Oost J and Geijsen N: The
double life of CRISPR-Cas13. Curr Opin Biotechnol. 78:1027892022.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Fonfara I, Richter H, Bratovič M, Le Rhun
A and Charpentier E: The CRISPR-associated DNA-cleaving enzyme Cpf1
also processes precursor CRISPR RNA. Nature. 532:517–521. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Palaz F, Kalkan AK, Can Ö, Demir AN,
Tozluyurt A, Özcan A and Ozsoz M: CRISPR-Cas13 system as a
promising and versatile tool for cancer diagnosis, therapy, and
research. ACS Synth Biol. 10:1245–1267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Chen H, Guo R, Li G, Zhang W and Zhang Z:
Comparative analysis of similarity measurements in miRNAs with
applications to miRNA-disease association predictions. BMC
Bioinformatics. 21:1762020. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Liu S, Jiang X, Wan F, Jia S and Si S: A
novel detection of MicroRNA based on homogeneous electrochemical
sensor with enzyme-assisted signal amplification. Talanta.
256:1242632023. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Zhang H, Fan M, Jiang J, Shen Q, Cai C and
Shen J: Sensitive electrochemical biosensor for MicroRNAs based on
duplex-specific nuclease-assisted target recycling followed with
gold nanoparticles and enzymatic signal amplification. Anal Chim
Acta. 1064:33–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Lizardi PM, Huang X, Zhu Z, Bray-Ward P,
Thomas DC and Ward DC: Mutation detection and single-molecule
counting using isothermal rolling-circle amplification. Nat Genet.
19:225–232. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
220
|
de la Torre TZG, Mezger A, Herthnek D,
Johansson C, Svedlindh P, Nilsson M and Strømme M: Detection of
rolling circle amplified DNA molecules using probe-tagged magnetic
nanobeads in a portable AC susceptometer. Biosens Bioelectron.
29:195–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Liu K, Tong H, Li T, Wang X and Chen Y:
Research progress in molecular biology related quantitative methods
of MicroRNA. Am J Transl Res. 12:3198–3211. 2020.PubMed/NCBI
|
|
222
|
Zhou T, Huang M, Lin J, Huang R and Xing
D: High-fidelity CRISPR/Cas13a trans-cleavage-triggered rolling
circle amplified DNAzyme for visual profiling of microRNA. Anal
Chem. 93:2038–2044. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Albada HB, Golub E and Willner I: Rational
design of supramolecular hemin/G-quadruplex-dopamine aptamer
nucleoapzyme systems with superior catalytic performance. Chem Sci.
7:3092–3101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Singh R, Pochampally R, Watabe K, Lu Z and
Mo YY: Exosome-mediated transfer of miR-10b promotes cell invasion
in breast cancer. Mol Cancer. 13:2562014. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Yoo B, Kavishwar A, Ross A, Wang P,
Tabassum DP, Polyak K, Barteneva N, Petkova V, Pantazopoulos P,
Tena A, et al: Combining miR-10b-targeted nanotherapy with low-dose
doxorubicin elicits durable regressions of metastatic breast
cancer. Cancer Res. 75:4407–4415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Ye J, Xu M, Tian X, Cai S and Zeng S:
Research advances in the detection of miRNA. J Pharm Anal.
9:217–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Harrington LB, Burstein D, Chen JS,
Paez-Espino D, Ma E, Witte IP, Cofsky JC, Kyrpides NC, Banfield JF
and Doudna JA: Programmed DNA destruction by miniature CRISPR-Cas14
enzymes. Science. 362:839–842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Li SY, Cheng QX, Liu JK, Nie XQ, Zhao GP
and Wang J: CRISPR-Cas12a has both cis- and trans-cleavage
activities on single-stranded DNA. Cell Res. 28:491–493. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Yuan C, Tian T, Sun J, Hu M, Wang X, Xiong
E, Cheng M, Bao Y, Lin W, Jiang J, et al: Universal and naked-eye
gene detection platform based on the clustered regularly
interspaced short palindromic repeats/Cas12a/13a system. Anal Chem.
92:4029–4037. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Sha Y, Huang R, Huang M, Yue H, Shan Y, Hu
J and Xing D: Cascade CRISPR/cas enables amplification-free
microRNA sensing with fM-sensitivity and single-base-specificity.
Chem Commun (Camb). 57:247–250. 2021. View Article : Google Scholar
|
|
231
|
Chen JS, Ma E, Harrington LB, Da Costa M,
Tian X, Palefsky JM and Doudna JA: CRISPR-Cas12a target binding
unleashes indiscriminate single-stranded DNase activity. Science.
360:436–439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Zhou T, Huang R, Huang M, Shen J, Shan Y
and Xing D: CRISPR/Cas13a powered portable electrochemiluminescence
chip for ultrasensitive and specific MiRNA detection. Adv Sci
(Weinh). 7:19036612020. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Shan Y, Zhou X, Huang R and Xing D:
High-fidelity and rapid quantification of miRNA combining crRNA
programmability and CRISPR/Cas13a trans-cleavage activity. Anal
Chem. 91:5278–5285. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Russo G, Zegar C and Giordano A:
Advantages and limitations of microarray technology in human
cancer. Oncogene. 22:6497–6507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Tian T, Shu B, Jiang Y, Ye M, Liu L, Guo
Z, Han Z, Wang Z and Zhou X: An ultralocalized Cas13a assay enables
universal and nucleic acid amplification-free single-molecule RNA
diagnostics. ACS Nano. 15:1167–1178. 2020. View Article : Google Scholar
|
|
236
|
Aquino-Jarquin G: Recent progress on rapid
SARS-CoV-2/COVID-19 detection by CRISPR-Cas13-based platforms. Drug
Discov Today. 26:2025–2035. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Sheng Y, Zhang T, Zhang S, Johnston M,
Zheng X, Shan Y, Liu T, Huang Z, Qian F, Xie Z, et al: A
CRISPR/Cas13a-powered catalytic electrochemical biosensor for
successive and highly sensitive RNA diagnostics. Biosens
Bioelectron. 178:1130272021. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Bruch R, Baaske J, Chatelle C, Meirich M,
Madlener S, Weber W, Dincer C and Urban GA: CRISPR/Cas13a-powered
electrochemical microfluidic biosensor for nucleic acid
amplification-free miRNA diagnostics. Adv Mater. 31:19053112019.
View Article : Google Scholar
|
|
239
|
Bruch R, Johnston M, Kling A, Mattmüller
T, Baaske J, Partel S, Madlener S, Weber W, Urban GA and Dincer C:
CRISPR-powered electrochemical microfluidic multiplexed biosensor
for target amplification-free miRNA diagnostics. Biosens
Bioelectron. 177:1128872021. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Kimmel DW, LeBlanc G, Meschievitz ME and
Cliffel DE: Electrochemical sensors and biosensors. Anal Chem.
84:685–707. 2012. View Article : Google Scholar :
|
|
241
|
Cui Y, Fan S, Yuan Z, Song M, Hu J, Qian
D, Zhen D, Li J and Zhu B: Ultrasensitive electrochemical assay for
microRNA-21 based on CRISPR/Cas13a-assisted catalytic hairpin
assembly. Talanta. 224:1218782021. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Zou L, Wu Q, Zhou Y, Gong X, Liu X and
Wang F: A DNAzyme-powered cross-catalytic circuit for amplified
intracellular imaging. Chem Commun (Camb). 55:6519–6522. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Park C, Park H, Lee HJ, Lee HS, Park KH,
Choi CH and Na S: Double amplified colorimetric detection of DNA
using gold nanoparticles, enzymes and a catalytic hairpin assembly.
Mikrochim Acta. 186:342018. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Li J, Lei P, Ding S, Zhang Y, Yang J,
Cheng Q and Yan Y: An enzyme-free surface plasmon resonance
biosensor for real-time detecting microRNA based on allosteric
effect of mismatched catalytic hairpin assembly. Biosens
Bioelectron. 77:435–441. 2016. View Article : Google Scholar
|
|
245
|
Zhao RN, Feng Z, Zhao YN, Jia LP, Ma RN,
Zhang W, Shang L, Xue QW and Wang HS: A sensitive electrochemical
aptasensor for Mucin 1 detection based on catalytic hairpin
assembly coupled with PtPdNPs peroxidase-like activity. Talanta.
200:503–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Xu D, Cai Y, Tang L, Han X, Gao F, Cao H,
Qi F and Kapranov P: A CRISPR/Cas13-based approach demonstrates
biological relevance of vlinc class of long non-coding RNAs in
anticancer drug response. Sci Rep. 10:17942020. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Gootenberg JS, Abudayyeh OO, Lee JW,
Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer
NM, Freije CA, et al: Nucleic acid detection with
CRISPR-Cas13a/C2c2. Science. 356:438–442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Kellner MJ, Koob JG, Gootenberg JS,
Abudayyeh OO and Zhang F: SHERLOCK: Nucleic acid detection with
CRISPR nucleases. Nat Protoc. 14:2986–3012. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Makarova KS, Zhang F and Koonin EV:
SnapShot: Class 2 CRISPR-Cas systems. Cell. 168:328–328.e1. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Sheedy P and Medarova Z: The fundamental
role of miR-10b in metastatic cancer. Am J Cancer Res. 8:1674–1688.
2018.PubMed/NCBI
|
|
251
|
Kolenda T, Guglas K, Kopczyńska M,
Sobocińska J, Teresiak A, Bliźniak R and Lamperska K: Good or not
good: Role of miR-18a in cancer biology. Rep Pract Oncol Radiother.
25:808–819. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Feng YH and Tsao CJ: Emerging role of
microRNA-21 in cancer. Biomed Rep. 5:395–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38:532019. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Mahesh G and Biswas R: MicroRNA-155: A
master regulator of inflammation. J Interferon Cytokine Res.
39:321–330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Lam J, van den Bosch M, Wegrzyn J, Parker
J, Ibrahim R, Slowski K, Chang L, Martinez-Høyer S, Condorelli G,
Boldin M, et al: miR-143/145 differentially regulate hematopoietic
stem and progenitor activity through suppression of canonical TGFβ
signaling. Nat Commun. 9:24182018. View Article : Google Scholar
|
|
256
|
Cavallari I, Ciccarese F, Sharova E, Urso
L, Raimondi V, Silic-Benussi M, D'agostino DM and Ciminale V: The
miR-200 family of microRNAs: Fine tuners of epithelial-mesenchymal
transition and circulating cancer biomarkers. Cancers (Basel).
13:58742021. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Zaccagnini G, Maimone B, Fuschi P, Maselli
D, Spinetti G, Gaetano C and Martelli F: Overexpression of miR-210
and its significance in ischemic tissue damage. Sci Rep.
7:95632017. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Torres-Berrío A, Nouel D, Cuesta S, Parise
EM, Restrepo-Lozano JM, Larochelle P, Nestler EJ and Flores C:
MiR-218: A molecular switch and potential biomarker of
susceptibility to stress. Mol Psychiatry. 25:951–964. 2020.
View Article : Google Scholar
|
|
259
|
Abak A, Amini S, Sakhinia E and Abhari A:
MicroRNA-221: Biogenesis, function and signatures in human cancers.
Eur Rev Med Pharmacol Sci. 22:3094–3117. 2018.PubMed/NCBI
|
|
260
|
Ahmad W, Gull B, Baby J and Mustafa F: A
comprehensive analysis of Northern versus liquid hybridization
assays for mRNAs, small RNAs, and miRNAs using a non-radiolabeled
approach. Curr Issues Mol Biol. 43:457–484. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Gaarz A, Debey-Pascher S, Classen S, Eggle
D, Gathof B, Chen J, Fan JB, Voss T, Schultze JL and
Staratschek-Jox A: Bead array-based microrna expression profiling
of peripheral blood and the impact of different RNA isolation
approaches. J Mol Diagn. 12:335–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Mehta N: RT-qPCR made simple: A
comprehensive guide on the methods, advantages, disadvantages, and
everything in between. Undergrad Res Nat Clin Sci Technol J. 6:1–6.
2022.
|
|
263
|
Tan M, Liao C, Liang L, Yi X, Zhou Z and
Wei G: Recent advances in recombinase polymerase amplification:
Principle, advantages, disadvantages and applications. Front Cell
Infect Microbiol. 12:10190712022. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Pervez MT, Hasnain MJU, Abbas SH, Moustafa
MF, Aslam N and Shah SSM: A comprehensive review of performance of
next-generation sequencing platforms. Biomed Res Int.
2022:34578062022. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Li X, Liao L, Jiang B, Yuan R and Xiang Y:
Invader assay-induced catalytic assembly of multi-DNAzyme junctions
for sensitive detection of single nucleotide polymorphisms. Anal
Chim Acta. 1224:3402252022. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Jin Y, Chen Z, Liu X and Zhou X:
Evaluating the microRNA targeting sites by luciferase reporter gene
assay. Methods Mol Biol. 936:117–127. 2013. View Article : Google Scholar :
|
|
267
|
Oh SW, Hwang DW and Lee DS: In vivo
monitoring of microRNA biogenesis using reporter gene imaging.
Theranostics. 3:1004–1011. 2013. View Article : Google Scholar
|
|
268
|
Hwang JY, Kim ST, Kwon J, Lee J, Chun YO,
Han JS and Han HS: Ultrasensitive fluorescence monitoring and in
vivo live imaging of circulating tumor cell-derived miRNAs using
molecular beacon system. ACS Sens. 3:2651–2659. 2018. View Article : Google Scholar : PubMed/NCBI
|