Introduction
Sepsis-induced multiple organ dysfunction syndrome
(MODS) involves dysfunction of two or more organs triggered by a
dysregulated response to infection and is characterized by a series
of physiological, pathological and biochemical abnormalities caused
by infection (1) (Fig. 1). During the early stage of
sepsis, inflammation is activated, leading to the release of
numerous proinflammatory cytokines, such as tumor necrosis factor-α
(TNF-α), interleukin-1 (IL-1) and IL-6, and inflammatory mediators,
such as high-mobility group protein B1 and prostaglandins,
resulting in immune overactivation. Additionally, immune cells,
including T lymphocytes, release various cytokines, accompanied by
redox imbalance. These mechanisms collectively activate endothelial
cells, increasing the release of cytokines, nitric oxide (NO),
platelet-activating factor and coagulation factors, thereby
exacerbating sepsis and advancing to MODS, ultimately resulting in
patient mortality (2) (Fig. 1). Despite extensive research on
the pathogenesis and therapeutic targets of MODS, the incidence and
mortality rates have improved little.
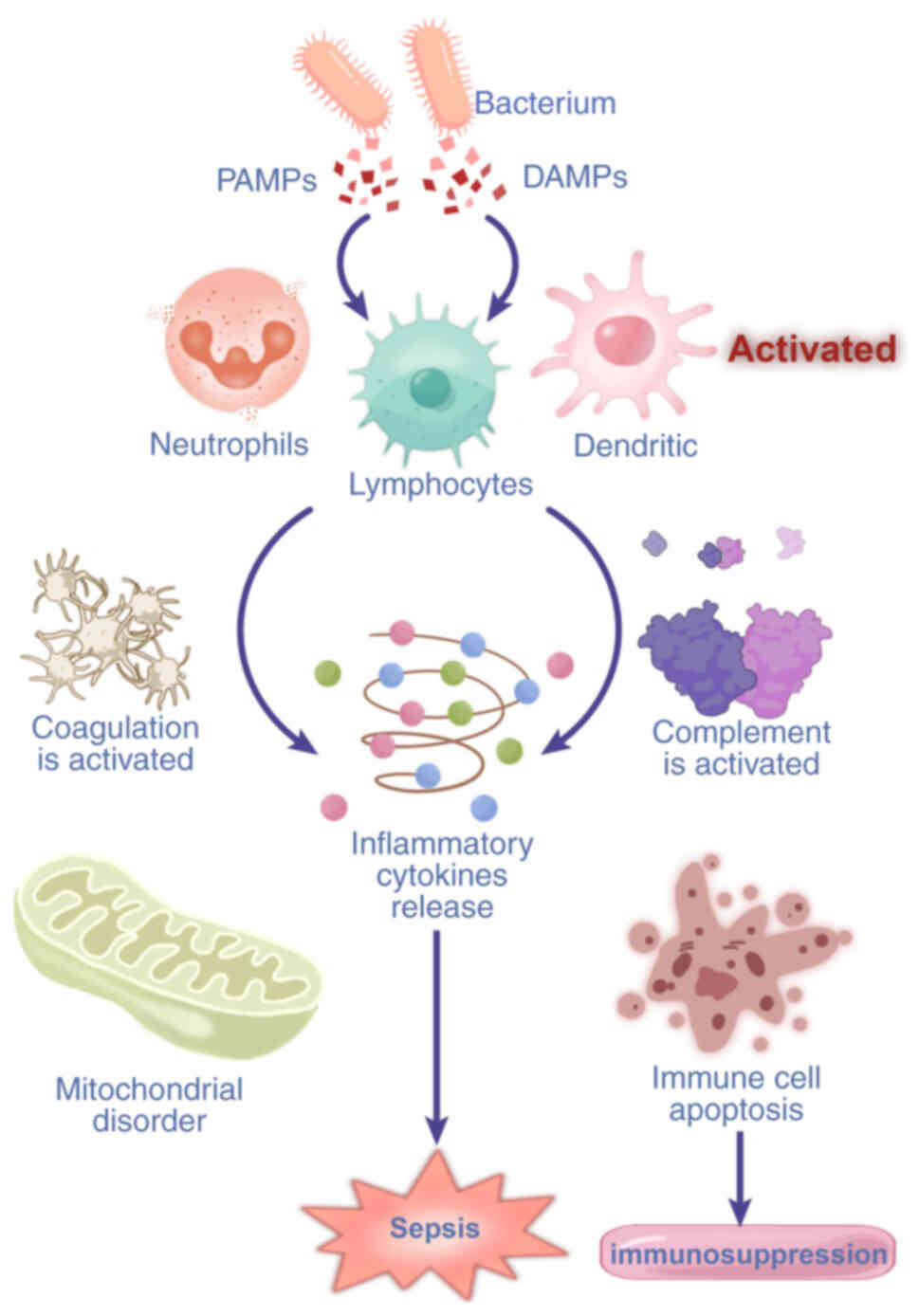
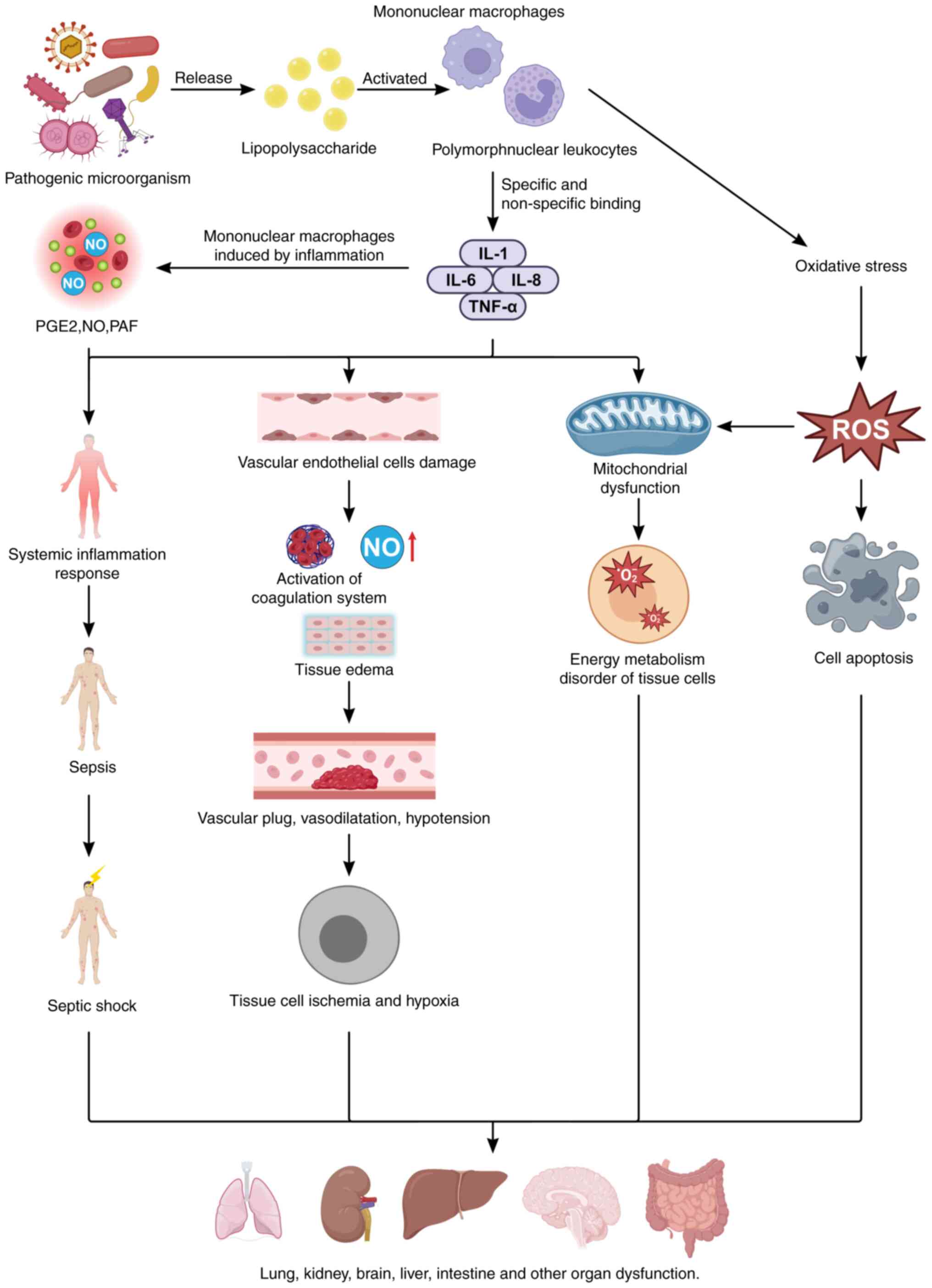 | Figure 1Mechanism of multiple organ
dysfunction caused by sepsis. The endotoxins produced by pathogenic
microorganisms stimulate inflammatory cells, which produce a large
concentration of inflammatory mediators, leading to sepsis, septic
shock and multiple organ dysfunction. Moreover, inflammatory
mediators can lead to mitochondrial dysfunction, an oxidative
stress response, coagulation dysfunction and microvascular blockage
and exacerbate damage to multiple organs, such as the liver, brain
and lungs. PGE2, prostaglandin E2; NO, nitric oxide; PAF,
platelet-activating factor; ROS, reactive oxygen species. |
Dexmedetomidine (DEX), a potent selective
α2-adrenergic receptor agonist, functions as a sympathetic blockade
within the nervous system and provides anxiolytic, sedative,
analgesic and hemodynamic stabilizing effects (3). It induces sedation without causing
respiratory depression, which is significant in clinical treatment.
DEX is commonly used for preanesthetic induction and as a sedative
in intensive care units (ICUs) (3,4).
Previous studies have indicated that DEX has protective effects on
various disease models: i) DEX enhances the 2-year survival of
elderly patients in ICUs and improves cognitive function and
quality of life in 3-year survivors (5); ii) DEX mitigates apoptosis,
necrosis and autophagy in the brain and surrounding tissues in both
in vitro and in vivo models (6); iii) DEX promotes cell survival by
inhibiting apoptosis and protecting against bilirubin-related lung
injury (7); iv) DEX protects
against cerebral ischemia-reperfusion injury by inhibiting
autophagy (7); and v) DEX
shields against sepsis-related organ injury (8-11). The roles of DEX in mitigating
lung, kidney, liver, brain and immune dysfunctions by regulating
signaling pathways related to inflammation, apoptosis, pyroptosis,
autophagy, and ferroptosis in sepsis treatment are summarized in
Fig. 2.
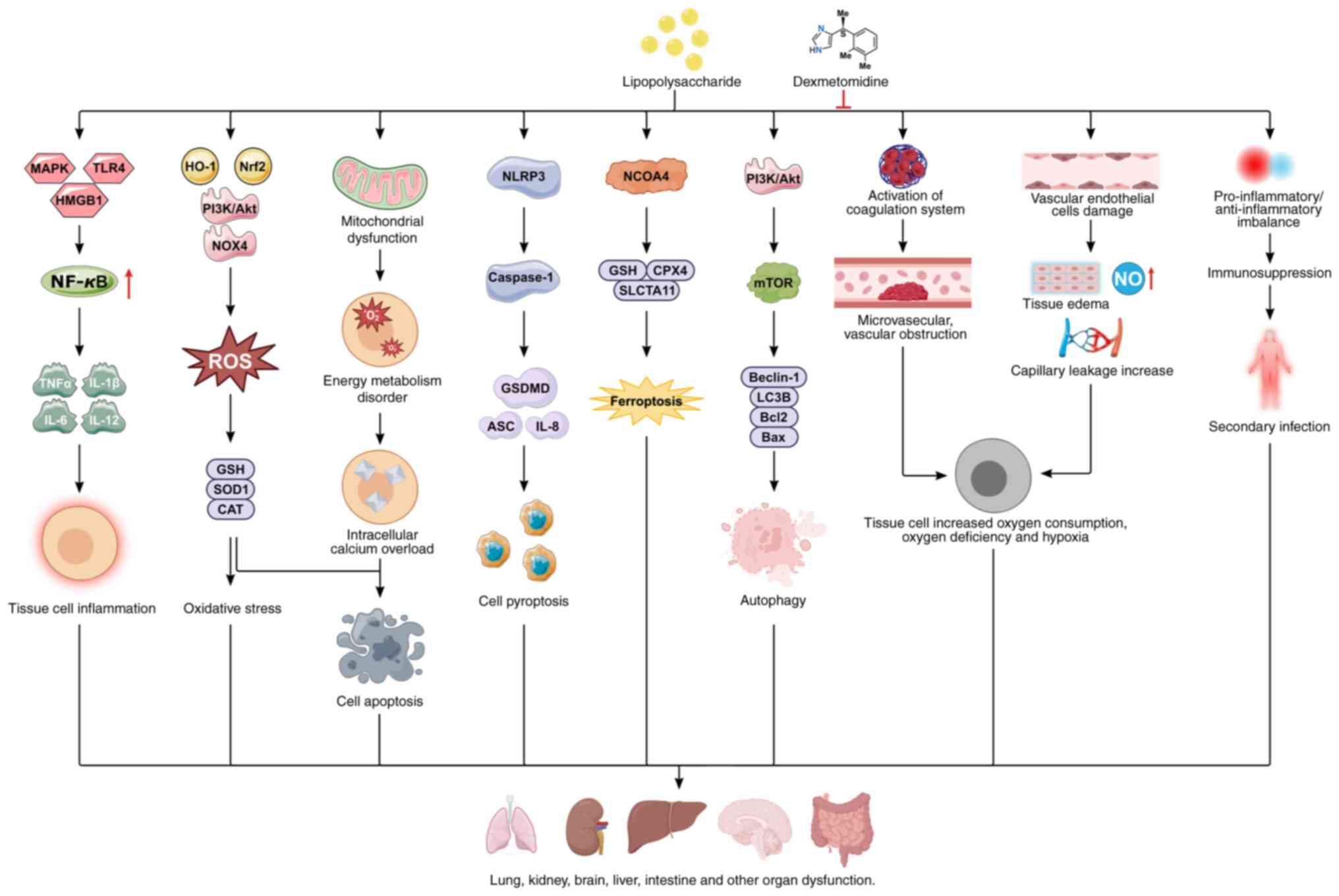 | Figure 2Protective mechanism of
dexmedetomidine against multiorgan dysfunction. LPS regulate tissue
cell inflammation by modulating the MAPK, TLR4, or HMGB1/NF-κB
signaling pathways; regulate tissue cell oxidative stress by
modulating Nrf2/HO-1, P13K/Akt and NOX4 signaling pathways; and
regulate cellular autophagy by modulating the P13K/Akt/mTOR
signaling pathways. In addition, lipolysis can lead to endothelial
damage, abnormal coagulation function and immune imbalance.
Lipopolysaccharides induce multiple organ dysfunction through these
mechanisms; however, dexmedetomidine alleviates
lipopolysaccharide-induced multiple organ dysfunction by regulating
these pathways. LPS, lipopolysaccharide; MAPK, mitogen-activated
protein kinase; TLR4, Toll-like receptor 4; HMGB1, high mobility
group protein box-1; NLRP3, NOD-like receptor family pyrin domain
containing 3; NF-κB, Nuclear factor κB; CAT, catalase; HO-1, heme
oxygenase 1; ASC, apoptosis-associated speck-like protein; NCOA4,
nuclear receptor coactivator 4; GSH, glutathione; GPX4, glutathione
peroxidase 4; ROS, reactive oxygen species; SOD, superoxide
dismutase; GSDMD, gasdermin D. |
Epidemiology of sepsis and multiple organ
dysfunction
Sepsis, a severe disease that threatens human
health, is not only a medical issue but also a public health
problem. A search of the World Health Organization website revealed
that, according to data released in 2020 (12), there are 48.9 million cases and
11 million sepsis-related deaths globally, accounting for nearly
20% of all global deaths. Of these, nearly half of the sepsis cases
occur in children, with an estimated 20 million child cases and 2.9
million deaths among children under the age of 5. The incidence and
mortality rates of sepsis vary by region, with ~85% of sepsis cases
and sepsis-related deaths occurring in low- and middle-income
countries. Sepsis treatment is costly, and in high-income
countries, the average hospital cost for sepsis exceeds $32,000 per
patient (13). The incidence of
sepsis in mainland China is similar to that in developed countries.
However, owing to the large population base and relatively low ICU
bed-to-population ratio, more patients with sepsis are not admitted
to the ICU because of insufficient bed availability. A
population-based epidemiological study of hospitalized sepsis
patients conducted by Zhou et al (14) revealed that only 20% of patients
with severe sepsis and 59% of patients with septic shock were
admitted to the ICU. The burden of sepsis in mainland China may
thus be underestimated. The study also revealed that the
standardized incidence rate of sepsis is 461/10 million, with a
mortality rate of 79/10 million. The high incidence and mortality
of sepsis, as well as the annual medical burden it causes,
highlight the need to improve the diagnosis and treatment of sepsis
and, at the macrolevel, strengthen the management of sepsis
prevention and treatment.
Sepsis-related organ injury or dysfunction is a
common complication of sepsis, and in severe cases, it can lead to
multiple organ dysfunction or failure, resulting in a poor
prognosis (15). According to
the American Society of Critical Care Medicine, the incidence of
MODS in the United States is ~240-300/100,000 people, with ~54% of
ICU-admitted patients developing MODS (16). The pathophysiology of MODS is
complex, with an unclear pathogenesis and rapid disease
progression, and the mortality rate is as high as ~30-40% (14). Studies have reported that the
average mortality rate for individuals with two organ dysfunctions
is 59%; for those with three organ dysfunctions, the average
mortality rate is 75%; and for those with four or more organ
dysfunctions, the average mortality rate is 100% (17). In terms of the frequency of organ
dysfunctions occurring in MODS, the highest incidence is
respiratory dysfunction, followed by gastrointestinal and renal
dysfunction. Among them, renal dysfunction has the highest
mortality rate, with an average of 79%, followed by respiratory
dysfunction at 68%, gastrointestinal dysfunction at 59%, hepatic
dysfunction at 55% and coagulation dysfunction at 44% (18). If severe infection is present,
the mortality rate increases significantly. In recent years, the
mortality rate of MODS has significantly increased with the number
of organ failures and the age of patients, making MODS a major
clinical challenge that urgently needs to be addressed in
medicine.
Pharmacokinetics of DEX
The chemical molecular formula of DEX is C13H16N2,
and its chemical name is
(+)-4-(2,3-dimethylphenyl)-ethyl-1H-imidazole (19). DEX shares a similar chemical
structure with clonidine, including an imidazole ring and a benzene
ring, indicating that both can act as agonists on α-adrenergic
receptors. As a highly selective α2-adrenergic receptor agonist,
DEX has a binding affinity ratio of 1,620:1 for α2-adrenergic
receptors compared with α1 receptors. Its affinity for
α2-adrenergic receptors is 8-fold greater than that of clonidine.
Studies have shown that the pharmacokinetics of DEX are strongly
predictable (20). For example,
during absorption, owing to the first-pass effect, the oral
bioavailability of DEX is extremely low. The bioavailability is
only 16% for sublingual administration, whereas intranasal
administration achieves a bioavailability of 84%. For intramuscular
and subcutaneous injections, the bioavailabilities are 73 and 51%,
respectively. Therefore, intranasal, intravenous and intramuscular
routes of administration are all applicable. However, considering
the storage and usage of DEX, along with its physicochemical
property of being fully soluble in water at a pH of 7.4, clinical
formulations are commonly provided as colorless, clear liquid
hydrochloride injection solutions. Intranasal administration is
particularly beneficial for special populations, such as children
and elderly individuals (21).
DEX is a highly plasma protein-bound drug, with a binding rate of
94% to plasma albumin and α1-glycoprotein. Studies have shown that
the plasma protein binding rate of DEX is not significantly
influenced by sex or age (22).
Additionally, DEX has a shorter half-life than does clonidine. Its
distribution half-life is ~6 min, allowing it to be distributed
rapidly in the body following intravenous injection. The
elimination half-life (t1/2) is ~2 h, with intravenous infusion
lasting up to 24 h. The linear pharmacokinetic range of DEX in the
body is 0.2-0.7 µg/kg·h. It is metabolized primarily in the
liver, with the metabolites being excreted mainly through urine
(95%) and feces (5%) (21).
Pharmacodynamics of DEX
DEX is a potent and highly selective α2-adrenergic
receptor agonist that exerts various effects by stimulating three
subtypes of α2 receptors in the body (α2A, α2B and α2C). For
example, the activation of α2A receptors, which are located
primarily in the locus coeruleus of the brain, can produce effects
such as sedation, analgesia, hypnosis and neuroprotection; the
activation of α2B receptors, which are distributed mainly in the
thalamus, heart, liver, spleen and aorta, can lead to analgesia,
peripheral arterial vasoconstriction, and the suppression of
centrally mediated shivering; and the activation of α2C receptors,
which are found in the heart, spleen and aorta, can result in
cognitive and emotional changes (3). Therefore, by stimulating α2
receptors in these organs and tissues, DEX can exert multiple
biological effects, including sedation, analgesia, hypnosis,
maintenance of normal respiration, and a reduction in organ
damage.
DEX induces a unique sedative response characterized
by a smooth transition from sleep to wakefulness, allowing patients
to communicate and interact when stimulated by external factors. It
mimics the effects of 'natural sleep', enabling patients to remain
in a sleep-like state during surgery while being easily arousable.
DEX has minimal toxic side effects on the body and, regardless of
the dosage, does not cause respiratory depression (23). Sleigh et al (24) suggested that the sedative and
hypnotic effects of DEX are mediated by the activation of pre- and
postsynaptic α2 receptors in the central nucleus of the brainstem
locus coeruleus, which function through endogenous physiological
sleep pathways to exert sedative and hypnotic effects. Clinical
studies have shown that DEX has dose-dependent sedative effects,
with doses of 0.2-0.6 µg/kg·h inducing significant yet
easily reversible sedation. At sufficiently high doses, DEX can
produce deep sedation and even general anesthesia, indicating its
potential role as part of total intravenous anesthesia. Although
the U.S. Food and Drug Administration has approved DEX for
short-term sedation (<24 h), multiple clinical studies have
confirmed that continuous use of DEX for several days can mitigate
adverse effects in patients with ICU (3,23). Ding et al (25) reported that, compared with
traditional sedatives, long-term ICU sedation with DEX reduced
mechanical ventilation time of patients by 22%, shortened hospital
stays by 14%, and did not significantly affect mortality. When the
sedative effects of midazolam, propofol and DEX in mechanically
ventilated ICU sepsis patients were compared, DEX demonstrated
comparable sedation efficacy while better stabilizing blood
pressure and cardiac function. Kawazoe et al (26), in a study of 201
mechanically-ventilated patients with sepsis, reported that the
sedation control rate during mechanical ventilation was
significantly greater in the DEX group than in the non-DEX group.
Furthermore, DEX significantly reduced the levels of inflammatory
markers such as C-reactive protein and procalcitonin, alleviating
systemic inflammatory responses.
Pharmacokinetic study of DEX in special
populations
Inside the child's body
DEX can be administered via intramuscular injection,
intravenous injection, nasal drops, or oral administration inside
the child's body. The use of nasal drops or oral administration can
prevent high peak plasma levels that may cause respiratory and
circulatory depression after intravenous injection. It also helps
avoid the irritation caused by intravenous administration,
rendering it more suitable for children. The nasal mucosa has a
rich distribution of aqueous channels, providing high drug
permeability and rapid absorption. It also bypasses the hepatic
first-pass effect. Research by Miller et al (27) indicates that nasal administration
of DEX for pediatric sedation can provide adequate blood drug
concentrations, with a bioavailability >80%, whereas oral
administration has a bioavailability of only 16% owing to the
first-pass effect (20). Yadav
and Ramdas (28) conducted a
randomized controlled trial of intranasal DEX for preoperative
pediatric anesthesia. The results showed that, compared with
midazolam, it offered more stable hemodynamics, a shorter time to
achieve sedation, and lower anxiety scores. It is an excellent
sedative and anxiolytic and can be safely used in children. DEX is
a drug with a strong binding ability to proteins, with a protein
binding rate of 94%. It is distributed quickly and extensively in
the human body and is also a highly lipophilic drug that easily
crosses the blood-brain barrier (29). Non-compartmental analysis has
shown that the distribution half-life in healthy volunteers is ~6
min (30). Studies have
indicated that in critically ill patients with hypoalbuminemia,
long-term administration of DEX significantly increases its
apparent Vd (31). In pediatric
pharmacokinetics for children <11 years old, the results
revealed that the steady-state Vd (Vss) is negatively correlated
with the age of the subjects (32), indicating that younger children
require a larger initial dose of DEX to reach a certain blood
concentration. DEX is metabolized primarily in the liver, with most
of its metabolites excreted through the kidneys and a small portion
through feces (33,34). Research on the pharmacokinetics
of DEX in neonates and infants shows that the drug clearance (CL)
rate increases with age, particularly within the first 2 weeks
after birth, whereas after 1 month of age, the impact of age on the
CL rate is minimal (33). The
metabolic enzyme system of newborns and infants is not fully
mature, resulting in low CL rates. Research has shown that the
expression and activity of cytochrome P450 enzymes approach adult
levels by the age of 1 (35).
The CL rate of full-term newborns is ~42.2% of that of adults,
reaching 84.5% at 1 year of age (29). Premature infants have even lower
CL rates [0.3 l/(h x kg) than 0.9 l/(h x kg)] and longer
elimination half-lives (7.6 compared with 3.2 h) (33). The CL rate of DEX is only mildly
affected by hypoproteinemia, but since the liver is the primary
metabolic organ, the CL rate decreases as the severity of liver
damage increases (32).
Among the elderly group
With increasing age, the body fat content also
increases in the elderly group (36). For males, from the ages of 20 to
30, the fat content increases from 8% to 20%, whereas for females,
it increases from 30 to 50% (37). First, lipophilic drugs (such as
DEX) have a relatively large volume of distribution (Vd) (38). Second, the plasma protein
concentration decreases with age, which in turn increases the free
fraction of drugs with a high protein binding rate (39), thus increasing the Vd of DEX.
Changes in liver and kidney function in elderly individuals can
affect plasma protein binding rates. Additionally, an increase in
fat content affects organ blood flow and the activity of
mixed-function oxidases (40).
Studies on the population pharmacokinetics of ICU patients
receiving long-term DEX infusion have revealed that age and plasma
protein concentration can influence the pharmacokinetic
characteristics of DEX. In the elderly population, CL decreases,
and the t1/2 increases. Moreover, patients with low plasma ALB
concentrations have an increased Vd and a prolonged t1/2. The CL of
the elderly population, with an average age of 80 years, is 25%
lower than that of those aged 60 years (41). This finding is consistent with
the report by Venn et al (42). CL is related primarily to liver
blood flow and is less affected by the protein binding rate
(43). As various bodily
functions decline in elderly individuals, the CL of DEX decreases,
leading to an extended t1/2. Lin et al (44) conducted a population
pharmacokinetic study on post-operative ICU patients, and the
results revealed that factors such as age and sex had no impact on
their pharmacokinetics. This may be due to the small sample size
and the high variability in the pharmacokinetic parameters of DEX
(45). Moreover, the
aforementioned study did not provide standard errors or confidence
intervals, indicating insufficient model stability and
reliability.
In individuals with liver
dysfunction
Individuals with liver dysfunction, the CL of DEX
decreases, with significant individual variability (46). According to the Child-Pugh
classification, liver dysfunction patients are divided into three
groups: mild (n=5), moderate (n=6), and severe (n=5). Compared with
those in the three control groups, the average CL rates of DEX were
41, 49 and 68% lower in patients with mild, moderate and severe
liver dysfunction, respectively. Compared with those in both the
mild group and the control group, the Vss and t1/2 were
significantly greater in severe liver dysfunction patients
(P<0.05) (47), indicating
that when DEX is used in patients with liver dysfunction, dose
adjustments should be considered.
In individuals with renal
insufficiency
There was no statistically significant difference in
the pharmacokinetic parameters of DEX in patients with renal
insufficiency compared with healthy subjects. A comparative study
was conducted between 6 patients with renal insufficiency
(creatinine CL <30 ml/min) and 6 healthy volunteers, and the
results revealed that there were no statistically significant
differences in maximal plasma/serum concentration, time to maximal
plasma/serum concentration, area-under-the-curve plasma/serum
concentrations, CL, or Vss (48). However, in patients with renal
insufficiency, the elimination-phase concentration was lower, and
elimination was faster, with a 17% decrease at t1/2 (P<0.05). In
patients with renal insufficiency, the protein binding rate of
alfentanil, which has a high plasma protein binding rate, decreases
from 19 to 11% (49). Therefore,
the decrease in the t1/2 of DEX may also be due to the reduced
plasma protein binding rate in patients with renal insufficiency,
leading to an increase in the amount of drug entering the
elimination phase.
Others
There have been few studies on the pharmacokinetics
of DEX in other special populations (including pregnant women,
parturient women and lactating patients). In one case, a pregnant
woman with spinal muscular atrophy who underwent routine cesarean
section had DEX infusion stopped, and 68 min later, the fetus was
delivered. DEX was detected in the umbilical blood, suggesting that
DEX can cross the placental barrier (50). DEX can cause a significant
increase in blood glucose levels in both the mother and the fetus
(51).
Organ protection mechanisms of DEX
In recent years, an increasing number of basic and
clinical studies have shown that DEX may exert organ-protective
effects through anti-inflammatory, antiapoptotic, antioxidative
stress, and autophagy-regulating mechanisms involving the heart,
brain, lungs, kidneys, liver and gastrointestinal tract. Despite
its common effects, the biological mechanisms of DEX vary across
different organs. Studies have shown that perioperative infusion of
DEX can reduce the levels of inflammatory factors such as
interleukin-6 (IL-6), IL-8, and TNF-α, with the effect being more
significant during continuous intraoperative infusion than during
single-dose administration (52). The anti-inflammatory mechanism of
DEX is closely related to the inhibition of the nuclear factor κB
(NF-κB) pathway and Toll-like receptors via α2 receptors (53). The vagus nerve and nicotinic
acetylcholine receptors are key components of the cholinergic
anti-inflammatory pathway. In addition to acting directly on α2
receptors, DEX can activate the cholinergic anti-inflammatory
pathway to exert its anti-inflammatory effects (54,55). DEX also has antiapoptotic effects
on various cell types, potentially through activation of the
α2AR/PI3K/Akt and p38MAPK/ERK signaling pathways (56-58). Additionally, DEX may inhibit
mitochondrial apoptosis by activating the JAK2/STAT3 signaling
pathway via the long non-coding RNA HCP5 (59). However, some studies have shown
that high concentrations of DEX can induce hippocampal neuronal
apoptosis. In vitro experiments suggest that DEX
concentrations ≥100 µmol/l significantly reduce cell
viability and induce neuronal apoptosis (60). In different cell injury models,
such as vascular smooth muscle cells, renal tubular epithelial
cells and hepatocytes, DEX can alleviate oxidative stress by
inhibiting the production of malondialdehyde (MDA) and reactive
oxygen species (ROS) and increasing the activity of superoxide
dismutase (SOD) (61-63). DEX also inhibits the decrease in
the mitochondrial membrane potential and reduces respiratory chain
enzyme complex damage, thereby suppressing the mitochondrial
oxidative stress induced by general anesthesia (64). Autophagy is an adaptive catabolic
process that maintains intracellular homeostasis by engulfing
intracellular substances, including damaged organelles, unfolded
proteins and pathogens. In cases of damage to critical organs such
as the heart, lungs, brain and kidneys, DEX treatment can increase
autophagy levels (65,66). However, studies have also shown
that DEX can suppress excessive autophagy in cardiomyocytes and
neurons to protect organs (67,68). Therefore, under different
pathological conditions in various organs, DEX has distinct
regulatory effects on autophagy.
Mechanism of MODS induced by sepsis
Currently, MODS caused by sepsis is considered to be
associated with factors such as uncontrollable infection, the
systemic inflammatory response, immune paralysis, coagulation
activation, microcirculatory failure and hypoxia (69). Studies have shown that
immune-metabolic reprogramming occurs at various stages of sepsis,
making it a key regulatory factor in the pathogenesis of this
disease (70). Polymorphonuclear
neutrophils (PMNs) are innate immune cells that respond first to
infectious pathogens and play a crucial role in host defense.
During infection, the body reacts rapidly to local inflammatory
stimuli, recruiting numerous PMNs efficiently from the bloodstream
to the site of inflammation. PMNs eliminate pathogens through
phagocytosis, degranulation, respiratory bursts, the generation and
release of neutrophil extracellular traps (NETs), and the
production of inflammatory factors such as TNF-α, IL-1β, IL-6 and
IL-8, thus controlling infection (71). Under normal conditions,
inflammatory responses are tightly regulated and confined to the
infection site. After infection is resolved, inflammation subsides,
and PMNs undergo apoptosis and are cleared. However, during sepsis,
systemic inflammation causes PMN polarization. The chemotaxis and
migration functions of PMNs are impaired, leading to a reduced
number of PMNs at the infection site (source site) and increased
PMN infiltration in distant vital organs (72). Activated PMNs in organs release
proinflammatory cytokines, ROS, lysosomal enzymes, NETs and other
active substances, resulting in cell, tissue and organ damage and
ultimately leading to MODS (73). A previous study has reported that
ROS produced by polarized PMNs during sepsis can damage various
organs, including the heart, kidneys, liver and lungs, through
multiple mechanisms, contributing to the development of MODS
(74). Moreover, activated PMNs
are significant sources of matrix metalloproteinases (MMPs),
particularly MMP-9, which can degrade extracellular matrix proteins
such as elastin and collagen. This degradation can stimulate the
immune system and create a positive feedback loop of tissue
degradation and immune cell infiltration, further exacerbating
organ damage at infiltration sites (75). Substances released by PMNs also
stimulate the production of NO by various cells, leading to blood
pressure reduction and the generation of peroxynitrite, which
causes cellular damage. In sepsis-induced myocardial injury,
peroxynitrite can alter protein structure and function, causing
PMNs to adhere to and accumulate in the endothelium and myocardium,
ultimately resulting in cardiovascular dysfunction (76). Additionally, ROS, NETs and
lysosomal enzymes released by polarized PMNs can act as
damage-associated molecular patterns (DAMPs), causing endothelial
injury and increased vascular permeability. DAMPs can also activate
immune cells, including macrophages, triggering uncontrolled
inflammatory responses. The release of inflammatory factors results
in the recruitment of more PMNs, further stimulating the excessive
production of NETs and ROS and creating a positive feedback loop
that exacerbates organ damage. These harmful substances contribute
to vascular injury and death in organs, eventually leading to organ
failure. During the development of sepsis-induced lung injury,
increased PMN infiltration promotes bacterial translocation in the
lungs, exacerbating ROS production and worsening lung damage
(77). The progression of MODS
in sepsis significantly increases patient mortality, making the
protection of organ function a critical strategy for improving
sepsis outcomes, with the regulation of immune cell function being
a key factor.
Protective mechanism of DEX on important
organs
Current research indicates that DEX has a protective
effect on sepsis-induced multiorgan dysfunction, as follows:
Lung injury
Sepsis often leads to multiple organ injuries, with
lung injury being particularly prevalent, occurring in 83-100% of
cases (78). The
lipopolysaccharide (LPS)-induced inflammatory response and
oxidative stress through the regulation of inflammatory pathways
such as MAPK and TLR4, as well as the oxidative stress pathway
Nrf2/heme oxygenase-1 (HO-1), are the primary causes of lung
injury, and sepsis-induced alveolar cell pyroptosis and ferroptosis
through the regulation of pyroptosis pathways such as the NLRP3
pathway, as well as the ferroptosis pathway SLC7A11/glutathione
peroxidase 4 (GPX4), are also involved in acute lung injury
(Fig. 3) (78). Research has shown that inhibiting
the high mobility group protein box-1 (HMGB1)/myeloid
differentiation factor-88 (MyD88)/NF-κB pathway and NLRP
inflammasome activation induced by cecal ligation and puncture
(CLP) can reduce CLP-induced lung inflammation and alveolar cell
pyroptosis and ameliorate sepsis-induced acute lung injury
(79), and increasing the
activity of the Nrf2/HO-1 pathway can reduce LPS-induced oxidative
stress and inflammation in alveolar cells and ameliorate
sepsis-induced acute lung injury (80,81). Therefore, inhibitors of
sepsis-induced alveolar cell inflammation, oxidative stress,
pyroptosis and ferroptosis may help improve sepsis-induced lung
injury. Wu et al (82)
demonstrated that DEX inhibits the expression of Toll-like receptor
4 (TLR4), a molecule associated with endotoxin recognition, and the
activation of the nuclear transcription factor NF-κB. This
inhibition reduces TNF-α and IL-6 levels in alveolar lavage fluid
and plasma, significantly decreasing the fatality rate in
CLP-induced septic rats within 24 h. Additionally, DEX attenuates
LPS-induced acute lung injury in rats by inhibiting the
phosphatidylinositol 3-kinase (PI3K)/protein kinase B
(Akt)/forkhead box O1 (FoxO1) signaling pathway and mitigating
oxidative stress, mitochondrial dysfunction and apoptosis (83). DEX also reduces oxidative stress
and inflammation in alveolar cells and maintains the balance of
mitochondrial dynamics through the HIF-1α/HO-1 signaling pathway,
thereby ameliorating endotoxin-induced acute lung injury both in
vivo and in vitro (84). Furthermore, DEX inhibits the
secretion of inflammatory mediators such as TNF-α, IL-6, IL-1β and
NO by alveolar cells via the HMGB1-mediated TLR4/NF-κB and
PI3K/Akt/mammalian target of rapamycin (mTOR) pathways, increases
the production of the antioxidant stress markers SOD and
glutathione peroxidase, and alleviates LPS-induced lung injury by
reducing inflammation and oxidative stress (85). A previous study indicated that
DEX maintains endothelial barrier integrity by reducing
angiopoietin 2 (ANG2) levels and increasing endothelial VE-cadherin
in rats with CLP-induced sepsis, thereby mitigating lung
inflammation (86,87). The Ang-tyrosine kinase receptor 2
(Tie2) axis is a well-studied pathway that regulates endothelial
barrier function. Ang1 binds to and activates angiopoietin Tie2
receptors, upregulating VE-cadherin to stabilize the cell barrier.
Conversely, ANG2 competes with Tie2 receptors, antagonizing the
anti-inflammatory effects of Ang1, leading to barrier damage and
endothelial cell activation, which induces inflammation (87). DEX reduces the wet/dry weight
ratio of lung tissue, leukocyte infiltration, plasma ANG2 levels
and ANG2/ANG1 ratios and decreases VE-cadherin phosphorylation
through the ANG1-Tie2-VE-cadherin signaling pathway, thereby
alleviating endothelial barrier damage and lung injury in septic
rats (87). Additionally, DEX
ameliorates sepsis-induced acute lung injury by modulating the
adenosine monophosphate-activated protein kinase (AMPK)/silent
information regulator 1 (SIRT1) pathway (88). These data suggest that DEX
improves sepsis-induced lung injury by inhibiting multiple
proinflammatory and oxidative stress pathways. However, these
studies remain in the animal experimentation stage, and further
research is needed to explore their clinical applications.
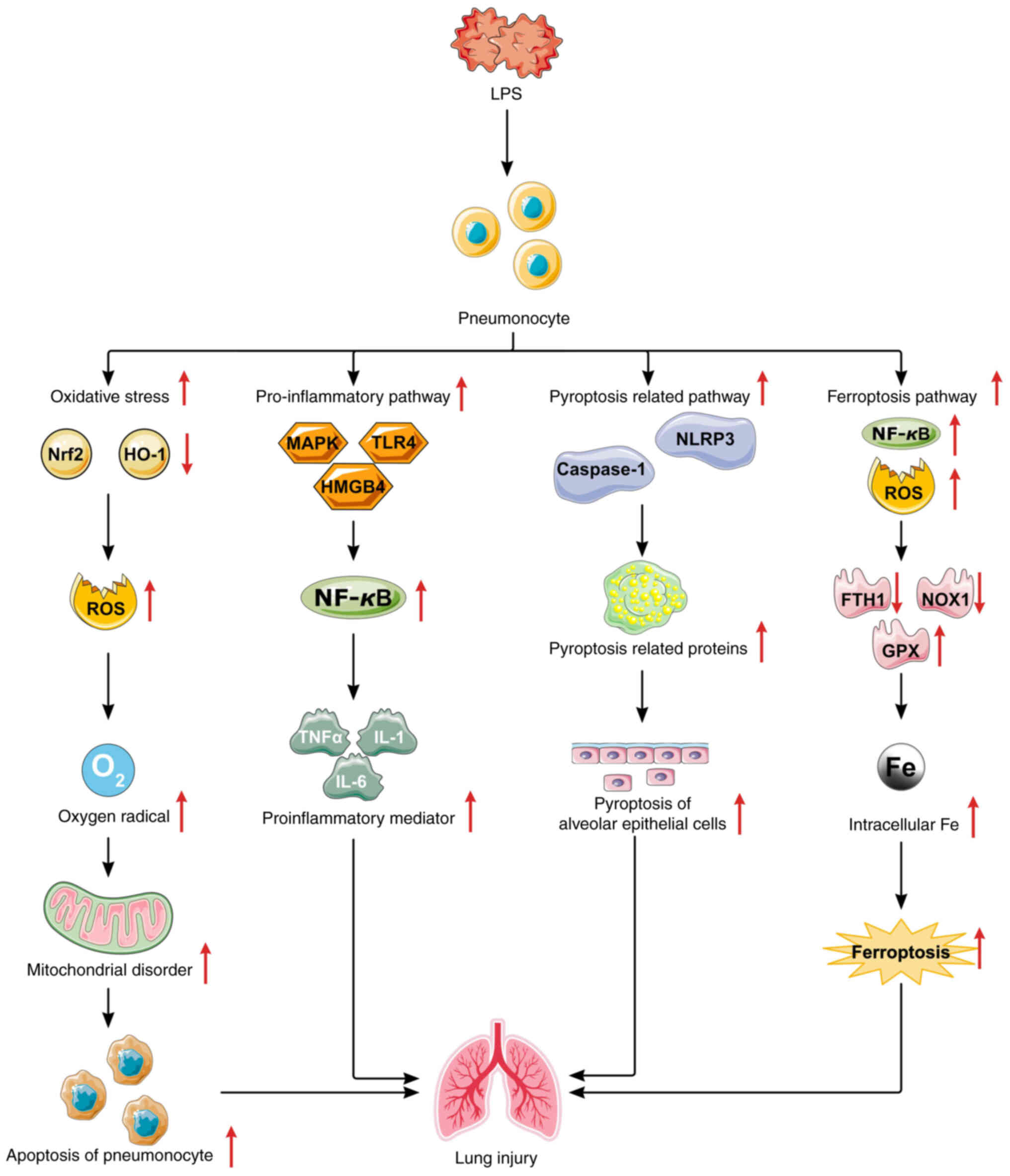 | Figure 3Mechanisms of sepsis-induced lung
injury. LPS regulates oxidative stress and mitochondrial
dysfunction by modulating the Nrf2/HO-1 signaling pathways;
regulates lung inflammation by modulating the MAPK, TLR4 or
HMGB1/NF-κB signaling pathways; regulates lung inflammation by
modulating the MAPK or TLR4 or HMGB1/NF-κB signaling pathways; and
regulates alveolar cell pyroptosis by modulating the
NLRP3/caspase-1 signaling pathway and regulates alveolar cell
ferroptosis by modulating the NF-κB/ROS/GPX4 signaling pathway.
LPS, lipopolysaccharide; Nrf2, nuclear factor erythroid 2-related
factor 2; HO-1, heme oxygenase 1; ROS, reactive oxygen species;
MAPK, mitogen-activated protein kinase; TLR4, Toll-like receptor 4;
HMGB1, high mobility group protein box-1; NLRP3, NOD-like receptor
family pyrin domain containing 3; NF-κB, nuclear factor κB. |
Kidney injury
The kidney is a primary target organ in sepsis
(89). Acute kidney injury (AKI)
manifests as progressively worsening renal function, severe
oliguria and fluid overload, often necessitating renal replacement
therapy. This condition not only increases hospitalization and
treatment costs but also severely impacts patient prognosis. The
pathophysiological process of sepsis-induced AKI remains poorly
understood, and effective prevention and treatment measures are
lacking in clinical settings. Studies have revealed that the
pathogenic mechanisms of sepsis-induced AKI are complex, involving
LPS-induced inflammation, microcirculatory disorders and oxidative
stress by regulating inflammatory pathways, such as TLR4 and MAPK,
as well as oxidative stress-related pathways, such as Nrf2, NADPH
oxidase 4 (NOX4) and P13k/Akt, with inflammation being a pivotal
factor (Fig. 4) (89,90). Research has shown that inhibiting
the activity of the TLR4/MyD88/NF-κB signaling pathway can inhibit
LPS-induced kidney inflammation and improve sepsis-induced AKI in
mice (91) and that decreasing
NLRP3 inflammasome activation can alleviate LPS-induced tubular
cell inflammation and pyroptosis and ameliorate LPS-induced kidney
injury (92). The inhibition of
NOX4 protects against sepsis-induced AKI by reducing the generation
of ROS and the activation of NF-κB signaling, which suppresses
mitochondrial dysfunction, oxidative stress, inflammation and
apoptosis (93). Therefore,
inhibiting inflammation and oxidative stress and improving renal
microcirculation may help alleviate sepsis-induced AKI. Liu et
al (94) investigated the
immunomodulatory effects of DEX on sepsis-related AKI induced by
tail vein injection of LPS in rats at doses of 3, 5, 10 and 20
µg/kg iv. DEX was found to reduce inflammatory cytokine
levels in a dose-dependent manner. The addition of the α2
adrenergic receptor antagonist yohimbine negated the regulatory
effect of DEX on cytokine production, confirming its
anti-inflammatory role as an α2 adrenergic receptor agonist. Jin
et al (95) established a
sepsis-related AKI model in rats via LPS induction. The DEX group
received 80 mg/kg DEX daily starting 4 days before modeling. The
results indicated that DEX downregulates TLR4 protein expression,
inhibits MyD88 and NF-κB activation, and causes expression of
inducible NO synthase (iNOS), thereby suppressing inflammatory
factor production. These findings indicate that DEX may reduce
inflammation by inhibiting the TLR4/MyD88/NF-κB/iNOS signaling
pathway and attenuating LPS-induced NLRP3 inflammasome activation
through the TLR4/NOX4/NF-κB pathway, thereby mitigating oxidative
stress (96). Wang et al
(97) investigated an
inflammatory proximal tubular epithelial cell model and an
LPS-induced sepsis-related AKI model in mice. The results revealed
that DEX significantly increased SOD activity in renal tissue,
decreased the production of the lipid peroxidation product MDA,
inhibited ROS production in renal tubular epithelial cells, and
ameliorated sepsis-induced AKI. Zhao et al (98) pretreated an LPS-induced
sepsis-related AKI model in rats with ip DEX at 30 µg/kg and
discovered that DEX enhanced autophagy through α2 adrenergic
receptors and inhibited the PI3K/Akt/mTOR pathway, leading to the
CL of damaged mitochondria and a reduction in oxidative stress and
apoptosis in LPS-induced AKI. The use of autophagy inhibitors, such
as AT2 receptor-interacting proteins and 3-methyladenine, blocked
the reno-protective effect of DEX, suggesting that regulating
autophagy is crucial in the pathogenesis of LPS-induced AKI. By
modulating renal autophagy, DEX improved oxidative stress and cell
apoptosis. These studies demonstrate the protective effects of DEX
on the kidney through various signaling pathways. However, since
most experiments are based on animal models and the mechanisms of
action have not yet been fully elucidated, further research is
necessary.
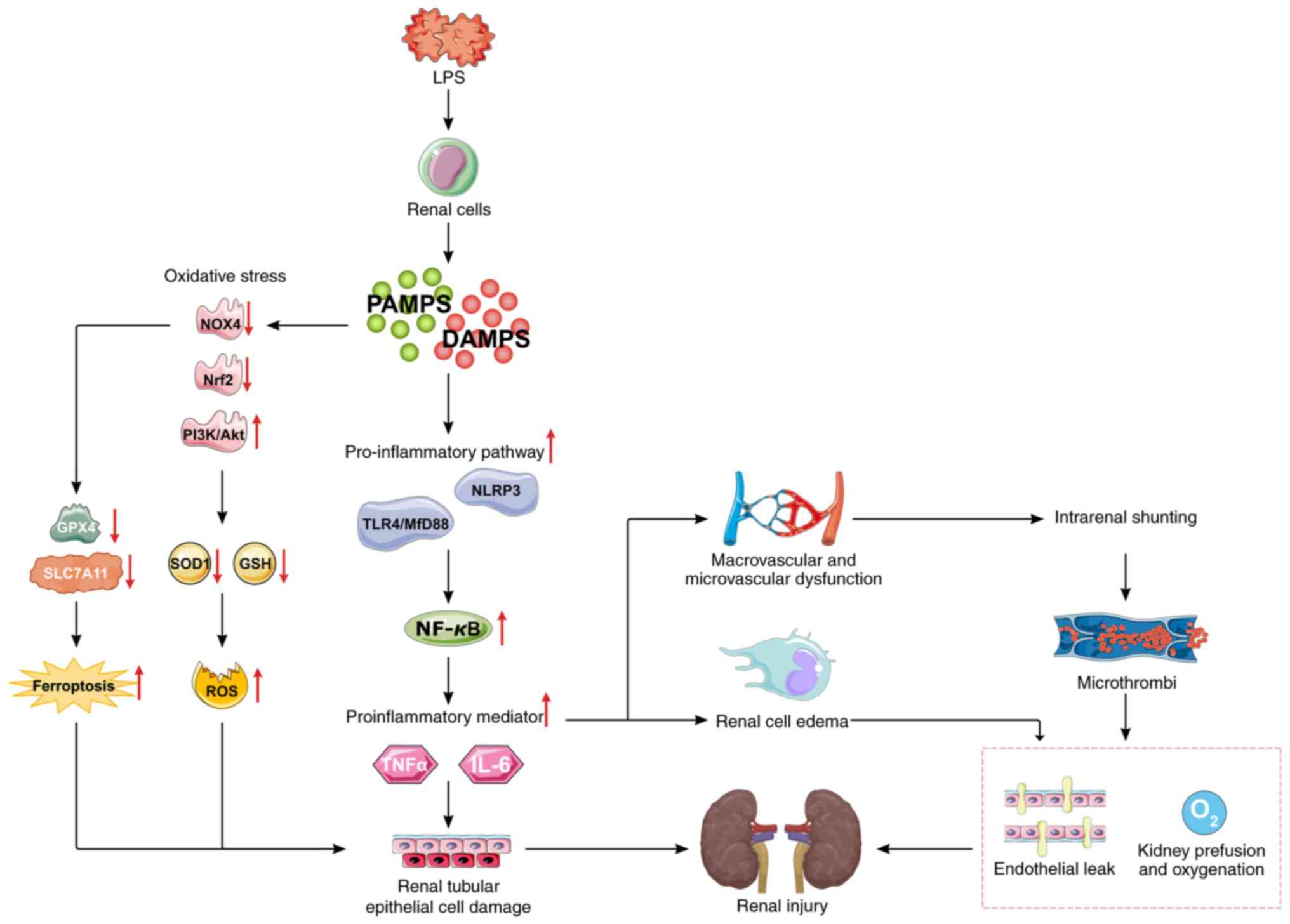 | Figure 4Mechanisms of sepsis-induced kidney
injury. LPS regulate kidney inflammation by modulating the
NLRP3/NF-κB or TLR4/Myd88/NF-κB signaling pathways; regulate
oxidative stress and ferroptosis by modulating the NOX4, Nrf2 or
P13K/Akt signaling pathways; and LPS-induced inflammatory mediators
can lead to renal microcirculation disorders, renal hypoperfusion,
ischemia and hypoxia and exacerbate sepsis-induced kidney injury.
LPS, lipopolysaccharide; PAMPS, pathogen-associated molecular
patterns; DAMPs, damage-associated molecular patterns; NLRP3,
NOD-like receptor family pyrin domain containing 3; TLR4, Toll-like
receptor 4; MyD88, myeloid differentiation factor 88; PI3K,
phosphatidylinositol 3-kinase; Akt, protein kinase B; NOX4, NADPH
oxidase 4; ROS, reactive oxygen species; SOD, superoxide dismutase;
GSH, glutathione; GPX4, glutathione peroxidase 4. |
Liver injury
Endotoxin-induced liver failure is the second
leading cause of multiple organ failure (99). Sepsis-induced hepatic injury
resulting from endotoxin induces inflammation, hepatic blood flow
alterations, microcirculatory disturbances and mitochondrial
dysfunction by regulating inflammatory pathways, such as the MAPK
and TLR4 pathways, and oxidative stress- and ferroptosis-related
pathways, such as the Nrf2/HO-1 pathways (Fig. 5) (99,100). Hepatic sinusoidal cells are the
initial sites of liver injury. The macrophages (KCs) within these
sinusoids are pivotal in the inflammatory process and
endotoxin-mediated hepatic injury (101). High doses of LPS provoked KC
overactivation and reduced phagocytic capacity while increasing
CD14 expression on KC surfaces. The LPS receptor/signaling complex,
which is composed of TLR4, CD14 and MD2, further activates KCs,
releasing a cascade of proinflammatory mediators that exacerbate
endotoxin-induced hepatic damage (102). Thus, inflammation is central to
sepsis-induced liver injury. Suppressing this inflammation may
mitigate acute hepatic injury in sepsis. Studies on septic murine
liver tissue revealed that DEX mitigates portal venous and
sinusoidal congestion, reduces hepatic sinusoidal swelling, and
decreases lymphocyte and neutrophil infiltration in the liver
hilum, effectively curbing hepatic inflammation (103). DEX has been demonstrated to
prevent acute hepatic injury in LPS-induced septic rats by
inhibiting the TLR4/MyD88/NF-KB pathway. Additionally, DEX
modulates caveolin-1, inhibits the TLR4/NLRP3 pathway, and curtails
proinflammatory cytokine release, thus alleviating sepsis-induced
hepatic injury (104,105). Furthermore, autophagy in the
liver is crucial for maintaining cellular energy and nutrient
balance, clearing damaged hepatic proteins, and counteracting
oxidative stress (106).
Research conducted by Yu et al (106) indicated that DEX potentially
enhances autophagy via the SIRT1/AMPK pathway, mitigating
inflammation in CLP-induced liver injury and increasing the
survival rate of model mice by 20% within 24 h. LPS induces liver
damage through apoptosis mediated by ROS. DEX activates the
GSK-3β/MKP-1/Nrf2 pathway via the α2-adrenergic receptor,
diminishing LPS-induced oxidative stress and apoptosis in the rat
liver and thereby ameliorating sepsis-induced liver injury
(107). DEX pretreatment
facilitates M2 macrophage activation through PPARγ/STAT3 signaling,
reducing liver inflammation and alleviating ischemia-reperfusion
injury in mice (108). This
evidence indicates that DEX confers protection against
sepsis-induced liver injury by modulating inflammation, apoptosis,
autophagy, and ferroptosis-related pathways and proteins. However,
these findings are confined to animal research, and clinical
validation is needed.
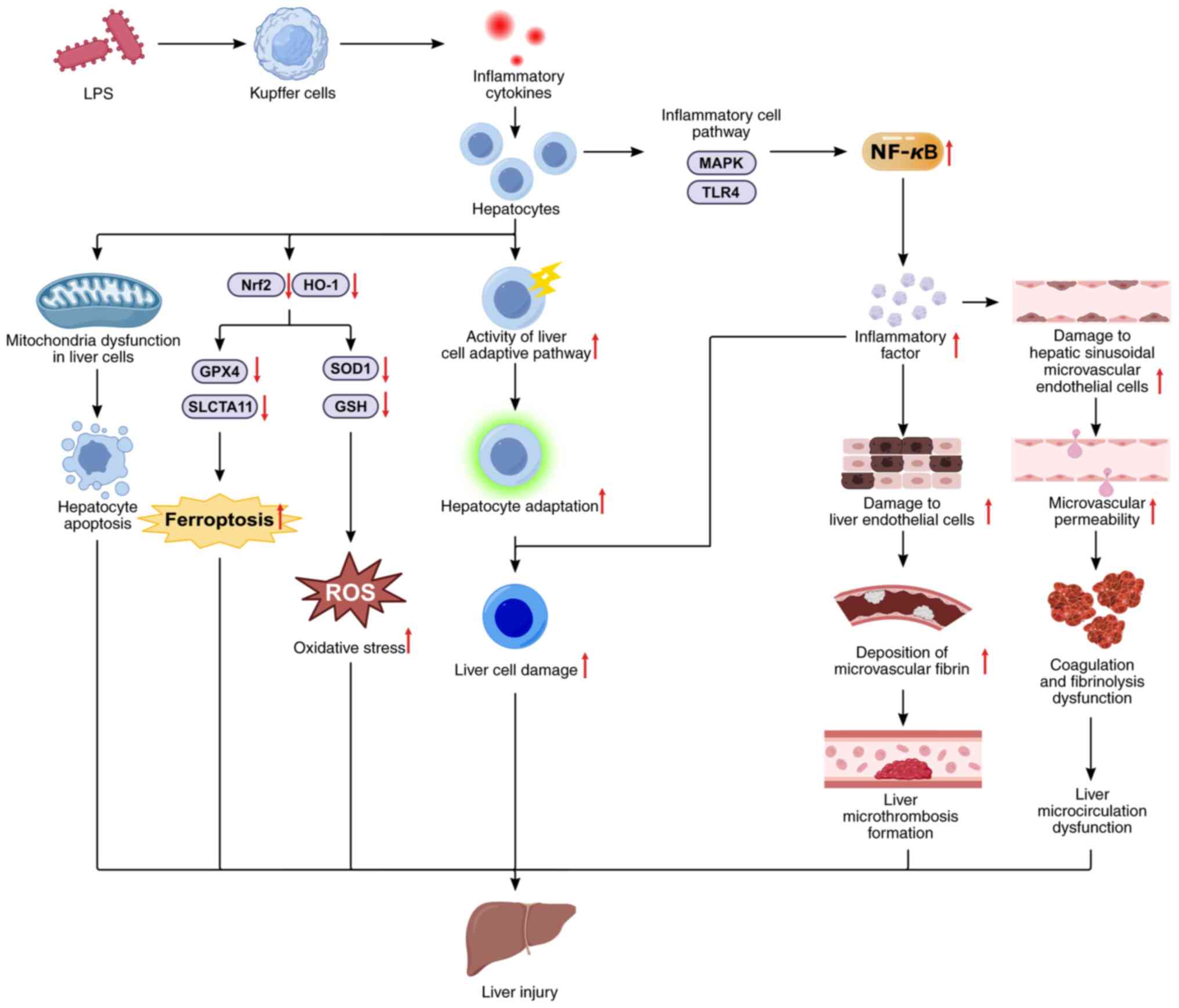 | Figure 5Mechanism of sepsis-induced liver
injury. LPS regulate liver cell inflammation by modulating the
MAPK/NF-κB and TLR4/NF-κB signaling pathways; regulate liver cell
oxidative stress and ferroptosis by modulating the Nrf2/HO-1
signaling pathways; and LPS-induced inflammatory mediators can lead
to endothelial cell damage, microcirculation disorders, adaptive
pathway activity in liver cells, and exacerbated sepsis-induced
liver injury. LPS, lipopolysaccharide; TLR4, Toll-like receptor 4;
MyD88, myeloid differentiation factor 88; NLRP3, NOD-like receptor
family pyrin domain containing 3; PI3K, phosphatidylinositol
3-kinase; Akt, protein kinase B; NOX4, NADPH oxidase 4; PPARγ,
peroxisome proliferator-activated receptor gamma; ROS, reactive
oxygen species; SOD, superoxide dismutase; GSH, glutathione; GPX4,
glutathione peroxidase 4; NF-κB, nuclear factor-kappa B. |
Myocardial injury
Epidemiological studies indicate that the global
mortality rate of sepsis remains high and is gradually increasing
(109). The heart is the organ
most frequently affected during sepsis. It has been revealed that
40-60% of septic patients suffer from myocardial injury, with a
fatality rate of 70-90%, which correlates positively with the
severity of the injury (110).
The extensive inflammation and 'cytokine storm' associated with
sepsis lead to ischemia, oxidative stress, pyroptosis, ferroptosis,
and hypoxic damage to myocardial cells by regulating pathways
related to pyroptosis and inflammation, such as NLRP3 and MAPK, as
well as pathways related to oxidative stress and iron death, such
as Nrf2/HO-1 and NCOA4/GPX4 (Fig.
6), resulting in a significantly higher mortality rate than
that in septic patients without myocardial injury. Long-term
prognosis studies have also shown that survivors of sepsis-induced
myocardial injury experience markedly reduced quality of life
(111). Previous studies have
identified that the activation of Nrf2/HO-1 and the inhibition of
the NLRP3 signaling pathway can inhibit LPS-induced inflammation
and oxidative stress; protect cardiomyocytes against LPS-induced
injury (112); suppress
NLRP3-mediated pyroptosis via the upregulation of the Nrf2/HO-1
signaling pathway in vivo and in vitro; decrease
oxidative stress, inflammatory responses and NLRP3-mediated
pyroptosis; and reduce LPS-induced myocardial injury (113,114). Thus, mitigating sepsis-related
inflammation may alleviate myocardial injury and reduce mortality
(115). In experimental mice
treated with DEX, the incidence of septic shock was lower, and the
plasma levels of the inflammatory factors TNF-a, IL-1β and IL-6
were significantly lower. Additionally, NF-кB binding activity was
downregulated, increasing survival rates (116,117). In LPS-induced septic heart
injury in rats, DEX appears to confer cardioprotective effects by
diminishing the expression of NF-κB p65 and phosphorylated STAT3,
thereby inhibiting the expression of the apoptotic proteins
Caspase-3, Caspase-8, Bax and p53 and the inflammatory factors
IL-6, IL-1β and TNF-α (118).
DEX also inhibits GPX4 expression, upregulates HO-1, and reduces
sepsis-induced cardiomyocyte ferroptosis and myocardial injury in
mice (119). Compared with
pre-surgery, the administration of varying doses of DEX to septic
patients significantly decreased the levels of inflammatory factors
such as IL-1, IL-6 and TNF-α within 24 h post-surgery. Moreover,
high, medium, and low doses of DEX maintain and enhance cardiac
function, significantly mitigating the adverse effects of
inflammatory mediators on the heart (120). Despite substantial progress in
understanding the myocardial protective effects of DEX, the
underlying mechanisms remain incompletely elucidated, necessitating
further research.
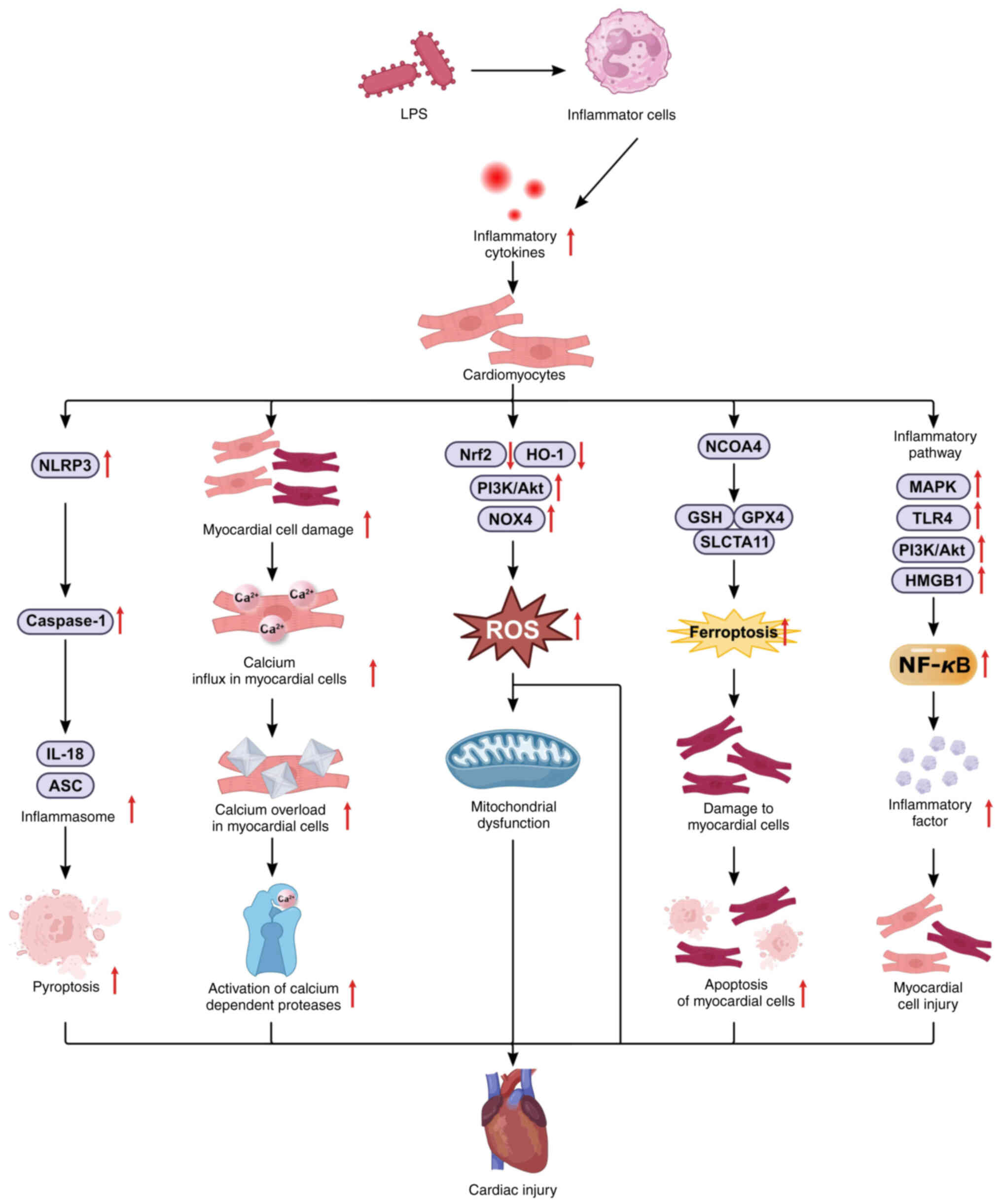 | Figure 6Mechanisms of sepsis-induced
myocardial injury. LPS regulate cardiomyocyte inflammation by
modulating the MAPK/NF-κB, MGB1/TLR4/NF-κB, and P13K/Akt/NF-κB
signaling pathways; regulate liver cell oxidative stress and
ferroptosis by modulating the Nrf2/HO-1 signaling pathways;
regulate cardiomyocyte pyroptosis by modulating the NLRP3/caspase-1
signaling pathways; and regulate cardiomyocyte oxidative stress by
modulating the Nrf2/HO-1, P13K/Akt, and NOX4 signaling pathways. In
addition, LPS-induced inflammatory mediators can lead to calcium
influx, and calcium overload in myocardial cells exacerbates
sepsis-induced cardiac injury. LPS, lipopolysaccharide; TLR4,
Toll-like receptor 4; MyD88, Myeloid differentiation factor 88;
NLRP3, NOD-like receptor family pyrin domain containing 3; PI3K,
phosphatidylinositol 3-kinase; Akt, protein kinase B; NOX4, NADPH
oxidase 4; HO-1, heme oxygenase 1; ASC, apoptosis-associated
speck-like protein; NCOA4, nuclear receptor coactivator 4; GSH,
glutathione; GPX4, glutathione peroxidase 4; MAPK,
mitogen-activated protein kinase; ROS, reactive oxygen species. |
Gastrointestinal injury
Late-stage sepsis often progresses to MODS, a
primary cause of mortality in ICU patients (121). As the body's largest 'bacteria
reservoir' and 'endotoxin reservoir', the gastrointestinal tract is
among the first and most severely impacted organs in the
pathophysiological progression of sepsis (122). Sepsis results in the generation
of substantial amounts of endotoxins and inflammatory mediators
that compromise the gastrointestinal mucosal barrier, increase
mucosal permeability, and promote intestinal bacterial
translocation. Consequently, numerous bacteria and endotoxins
infiltrate the bloodstream via lymphatic and capillary pathways,
instigating a 'secondary infection' and triggering entheogenic
endotoxemia. This can elicit unrestrained inflammatory responses,
exacerbate intestinal damage, create a vicious cycle, and
potentially advance to MODS, resulting in fatality (122,123). Hence, prompt evaluation of
gastrointestinal function and early intervention in sepsis patients
are essential to mitigate damage and improve patient prognosis. As
shown in Table I (124-131), DEX ameliorates
ischemia/reperfusion, LPS and CLP-induced intestinal injury in rats
by suppressing TLR4, p38 MAPK/NF-κB SIRT3-dependent
PINK1/HDAC3/p53, and alpha2AR/caveolin-1/p38MAPK/NF-κB signaling
pathways in intestinal tissue; increasing ZO-1 and occludin
expression; decreasing inflammatory cytokine levels and MLCK
protein expression; and increasing intestinal permeability
resulting from burns with concurrent histological damage. However,
deep sedation with DEX, unlike propofol, significantly suppresses
gastrointestinal motility in endotoxemic mice, an effect that
dissipates after 24 h of sedation (131). These results indicate that
while DEX has protective effects against intestinal barrier
dysfunction, its administration in sepsis and intestinal paralysis
requires careful consideration.
 | Table IStudy on the protective mechanism of
DEX against intestinal injury caused by sepsis. |
Table I
Study on the protective mechanism of
DEX against intestinal injury caused by sepsis.
| First author,
year | Object of
study | Dosage of DEX | Effect | Regulatory
mechanism of the intestine injury | (Refs.) |
|---|
| Chen et al,
2015 | CLP-ICLP-induced
intestinal injury | 5 µg ×
kg−1 × h−1 | Reducing systemic
inflammation and alleviating CLP-induced intestinal tissue
damage | Suppressing TLR4
expression in intestinal tissue, curtailing inflammatory
mediators | (124) |
| Qin et al,
2021 | Burn-induced
intestinal barrier damage | 5 µg ×
kg−1 × h−1 | Decrease in
intestinal permeability and histological damage to the
intestine | Anti-inflammatory
effect and protected tight junction complexes by inhibiting the
MLCK/ p-MLC signaling pathways | (125) |
| Yang et al,
2021 | Intestinal
ischemia/ reperfusion injury | 5 µg ×
kg−1 × h−1 | Reduce intestinal
I/R injury in rats and OGD/R damage in Caco-2 cells | Attributed to
anti-inflammatory effects and inhibition of the TLR4/MyD88/NF-κB
signaling pathway | (126) |
| Ye et al,
2022 | DSS-induced colitis
mice | 5 µg ×
kg−1 × h−1 | Improve weight
loss, shortening of the colon, increased DAI score, histological
abnormalities | Reduces colonic
inflammation and intestinal epithelial cell apoptosis by inhibiting
the p38 MAPK/NF-κB signaling pathway | (127) |
| Zhang et al,
2021 | Rat model of
intestinal I/R injury | 2.5 µg ×
kg−1 × h−1 and 5 µg × kg−1
× h−1 | Protection against
experimentally induced intestinal I/R injury | Suppresses
apoptosis of EGCs through SIRT3-mediated PINK1/HDAC3/p53
pathway | (128) |
| Dong et al,
2022 | Mice model of
intestinal I/R injury | 5
µg·kg−1 × h−1 | Alleviate
intestinal I/R injury | Reverses
I/R-induced bacterial abnormalities | (129) |
| Xu et al,
2021 | Rat model of
intestinal I/R injury | 5 µg ×
kg−1 × h−1 | Protection against
lung injury following intestinal ischemia-reperfusion |
Ca2+-regulating alpha
2AR/Caveolin-1/ p38MAPK/NF-kappa-B axis | (130) |
| Chang et al,
2020 | LPS-induced
intestinal injury in mice | 80 µg ×
kg−1 | Inhibits
gastrointestinal motility disorder | Markedly inhibits
gastric emptying, small intestinal transit, colonic transit,
gastrointestinal transit and the whole gut transit | (131) |
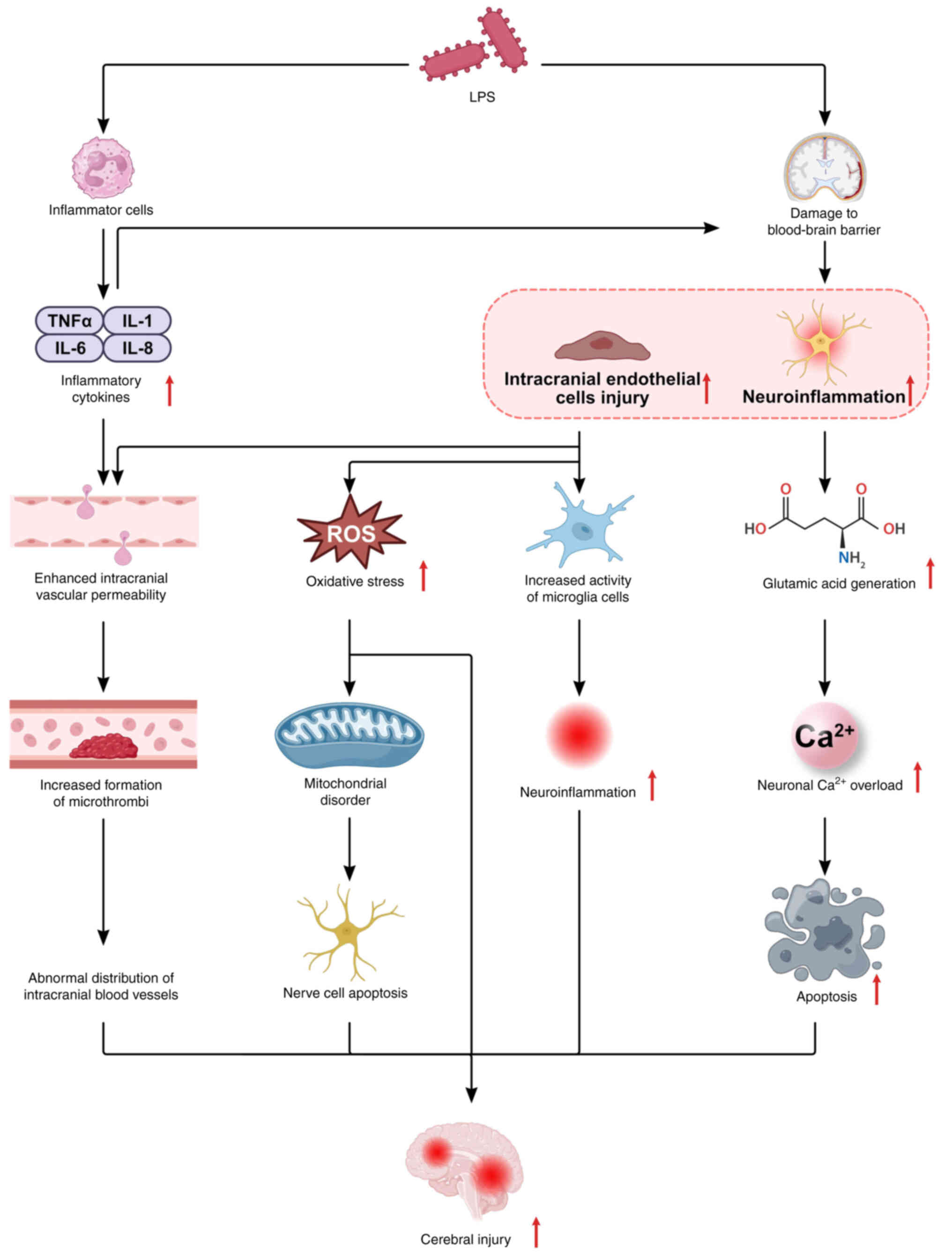
Brain injury
During sepsis, various inflammatory mediators and
endotoxins damage capillary endothelial cells, resulting in
leakage, inflammatory damage and tight junction dysfunction. This
compromises the blood-brain barrier, permitting systemic
inflammation and neurotoxic mediators to infiltrate the brain,
leading to sepsis-associated brain injury (Fig. 7) (132). Following central nervous system
damage, microglia are activated, proliferate rapidly, polarize,
migrate to the injury site, and secrete proinflammatory factors
such as IL-1β and TNF-α (133),
which further degrade the blood-brain barrier, perpetuating
microglial activation in a vicious cycle that exacerbates neuronal
damage (Fig. 7). Mitigation of
microglial activation is crucial for ameliorating sepsis-induced
brain injury. Research indicates that DEX attenuates
neuroinflammation in LPS-stimulated BV2 microglia by upregulating
miR-340 (134) and modulates
neuroinflammation and microglial activation through the
circ-Shank3/miR-140-3p/TLR4 axis (135). Additionally, DEX inhibits the
TLR4/MyD88/NF-κB signaling pathway, reducing the expression of
inflammatory factors, such as TNF-α, thereby protecting against
ischemia-reperfusion injury (126). By inhibiting MAPK and NF-κB
activation, DEX decreases TNF-α and IL-1β levels, shielding brain
tissue from inflammatory damage (136). It also enhances microglial M2
polarization by inhibiting ERK1/2 phosphorylation, exerting
anti-inflammatory effects in BV2 cells (137). Consequently, DEX mitigates
sepsis-induced brain injury through multiple mechanisms. Current
research on the protective mechanisms of DEX against sepsis-induced
injury has focused primarily on animal and cell studies. Extensive
clinical research is necessary to validate these findings and
establish a theoretical foundation for the clinical treatment of
sepsis-induced injury.
Modulating the
proinflammatory/anti-inflammatory imbalance
Sepsis involves hyperinflammation and acquired
immunosuppression (Fig. 8).
Multiple studies (138-140) have indicated immune
dysregulation during sepsis. Initially, infection triggers an
immune response, leading to the extensive production of
inflammatory factors and consequent immune stress, exacerbating
organ injury. As sepsis progresses, there is a decline in
lymphocyte subsets, further intensifying inflammation. This period
is characterized by concurrent immunosuppression and inflammation,
which mutually exacerbate sepsis. Thus, immune regulation is
pivotal in sepsis treatment. Research on DEX, a novel sedative in
sepsis, is expanding, although studies on its impact on immune
dysfunction are limited. Wang et al's meta-analysis
(52) demonstrated that
intraoperative DEX infusion is correlated with i) reduced plasma
levels of epinephrine, norepinephrine and cortisol; ii) increased
NK cells, B cells and CD4 cells; decreased CD8+ T cells;
iii) elevated CD4+CD8+ and Th1:Th2 ratios;
iv) reduced TNF-α and IL-6; and increased IL-10. The Th1/Th2 helper
T-cell 2 (Th2) and helper T-cell 17 Th17/regulatory T-cell (Treg)
ratios reflect immune response dynamics. In patients under surgical
and anesthesia stress, DEX elevates the IFN-γ to IL-4 and IL-17 to
IL-10 ratios, shifting the Th1/Th2 and Th17/Treg cytokine balance
toward Th1 and Th17 in an alcohol-dependent manner and resulting in
immunomodulatory effects (141). DEX effectively increases
CD3+, CD4+ and NK cells and
CD4+/CD8+ levels in patients undergoing
radical mastectomy for breast cancer, maintaining cellular immune
function homeostasis (142).
These studies reveal that DEX possesses anti-inflammatory
properties and enhances cellular immunity, making it suitable for
septic patients with immunosuppression.
Maintaining hemodynamics
DEX inhibits norepinephrine neuron activity in the
locus coeruleus through negative feedback, suppresses sympathetic
nerve excitation, and lowers blood catecholamine levels. This
stabilizes the intraoperative circulatory status and minimizes the
hemodynamic fluctuations induced by surgical trauma and painful
stimulation (143,144). Norepinephrine is the preferred
vasopressor for restoring mean arterial pressure in septic
patients. Refractory septic shock often necessitates higher doses
of norepinephrine to maintain blood pressure. However, in
sepsis-related AKI, norepinephrine-induced blood pressure
restoration exacerbates renal medullary hypoperfusion and hypoxia
(145). DEX enhances
responsiveness to exogenous vasopressors (146). The results of the present study
are shown in Table II (11,117,147-151). DEX significantly reduced
catecholamine requirements, halved the required norepinephrine
dose, prevented further renal medullary hypoperfusion and hypoxia,
and progressively improved creatinine CL, potentially through an
enhanced vascular response to catecholamines and angiotensin II.
Thus, DEX reduces the norepinephrine dosage in septic patients and
is suitable for anesthesia and sedation in severe sepsis and septic
shock patients. Nonetheless, large, randomized trials are needed to
confirm whether DEX consistently reduces the norepinephrine dosage
in septic patients.
 | Table IIStudies on the effects of DEX on
sepsis-induced hemodynamics. |
Table II
Studies on the effects of DEX on
sepsis-induced hemodynamics.
| First author,
year | Object of
study | Dosage of DEX | Effect | Regulatory
mechanism of the hemodynamics | (Refs.) |
|---|
| Morelli et
al, 2019 | Patients with
septic shock | 0.7 µg/kg x
h | Significantly
reducing catecholamine. requirements | Inhibits
norepinephrine neuron activity in the locus coeruleus. | (147) |
| Lankadeva et
al, 2019 | Sheep model of
sepsis-related acute kidney injury | 0.5 µg/kg x
h | Restore mean
arterial pressure to pre-illness levels, halved the required
norepinephrine dose. | Enhanced vascular
response to catecholamines and angiotensin II. | (148) |
| Taniguchi et
al, 2004 | Endotoxemic
rats | 0.5 µg/kg x
h | Improving endotoxin
induced. hypotension | Inhibitory effect
on inflammatory response during endotoxemia. | (117) |
| Yao et al,
2021 | Patients with
septic shock | 0.7 µg/kg x
h | Has a smaller
effect on hemodynamics in patients with septic shock than
midazolam. | Reduce plasma
catecholamine levels in patients with septic shock. | (149) |
| Carnicelli et
al, 2022 | Model of septic
shock pigs | 0.5 µg/kg x
h | Maintain
hemodynamic stability. | Not affecting the
early metabolic, and inflammatory disorders induced by septic
shock. | (11) |
| Moore et al,
2022 | Patients who
underwent rhytidectomy | 0.7 µg/kg x
h | Resulted in a
significant reduction and maintenance of blood pressure from onset
of anesthesia. | Suppresses
sympathetic nerve excitation and lowers blood catecholamine
levels. | (150) |
| Hazrati et
al, 2022 | Patients with hip
fracture surgery. | 0.7 µg/kg x
h | Maintain
hemodynamic stability. | Reduces the amount
of intraoperative bleeding without causing any significant
hemodynamic disturbances. | (151) |
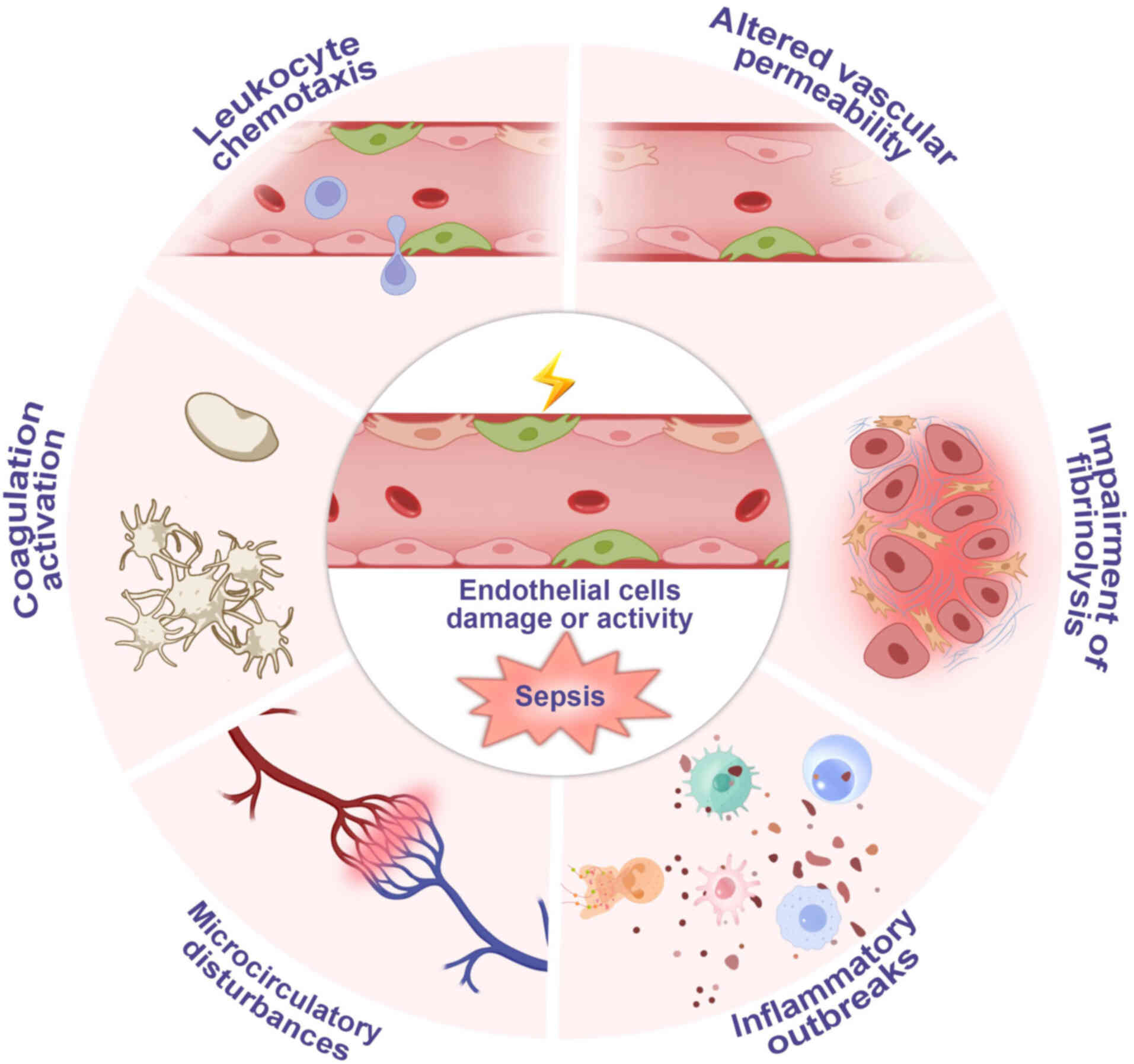
Regulating endothelial cells
During sepsis, the activation and dysfunction of
vascular endothelial cells result in leukocyte chemotaxis,
inflammatory outbreaks, microcirculatory disturbances and altered
vascular permeability (Fig. 9)
(152). Endothelial cell damage
leads to intermittent or complete cessation of capillary blood
flow, reducing capillary density and causing nonuniform organ
perfusion (153). This
endothelial damage-induced microcirculatory disorder results in
tissue hypoxia and abnormal cellular oxygenation despite adequate
organ blood supply, precipitating organ dysfunction (154). The results of the present study
are shown in Table III
(87,155-160). Dex pretreatment effectively
decreased EC inflammation, apoptosis and hypoxia/reoxygenation
injury by inhibiting the DUSP1/MAPK/NF-κB, HMGB1/TLR4/NF-κB, MAPK
and angiopoietin II signaling pathways. These studies indicate that
DEX protects endothelial barrier function; mitigates sepsis-induced
inflammation, microcirculatory disturbances and vascular
permeability; and exerts protective effects on vital organs such as
the brain, lungs, heart and kidneys during sepsis.
 | Table IIIStudy of the protective mechanism of
DEX against sepsis-induced injury to endothelial cells. |
Table III
Study of the protective mechanism of
DEX against sepsis-induced injury to endothelial cells.
| First author,
year | Object of
study | Dosage of Dex | Effect | Regulatory
mechanism of the alleviating endothelial cells injury | (Refs.) |
|---|
| Dai et al,
2023 | Angiotensin
II-induced endothelial cell | 100 ng/ml | Attenuates Ang
II-induced EC dysfunction | Inactivating
HMGB1/TLR4/NF-κB signaling. pathway | (155) |
| Newton et
al, 2002 |
Statin-statin-induced apoptosis of
vascular endothelial cells | 1 µM | Blocks apoptosis of
vascular endothelial cells | Inhibit serum
deprivation, tumor necrosis factor-alpha, oxidants, DNA damage and
mitochondrial disruption. | (156) |
| Chai et al,
2020 | Monocyte-vascular
endothelial cells | 0.1 and 1 nM | Reduce
monocyte-endothelial adherence | Inhibit Cx43
alternation regulating the activation of MAPK signaling
pathways. | (157) |
| Dong et al,
2020 | H/R induced mouse
pulmonary vascular endothelial cells | 0.1 nM | Reduce mouse
pulmonary vascular endothelial cells apoptosis | Increased cell
viability, reduced apoptotic ratio and downregulated expression
levels of Cleaved Caspase-3 and Cleaved Caspase-9 in H/R induced
MPVECs | (158) |
| Han et al,
2022 | LPS induced
MPMVECs | 0.1 nM | Alleviate
LPS-induced MPMVECs injury | Regulate
DUSP1/MAPK/NF-κB axis by inhibiting miR-152-3p | (159) |
| Zhao et al,
2021 |
Hypoxia/reoxygenation-induced brain
endothelial cells | 0.1 nM | Alleviated
hypoxia/reoxygenation-induced brain endothelial cells injury | OGD/alleviated
Hypoxia/reoxygenation-induced inflammatory injury and permeability
in brain endothelial cells mediated by sigma-1 receptor | (160) |
| Zhang et al,
2021 | CLP-induced rat
model of sepsis | 10
µg/kg | Protective effect
against CLP-induced endothelial injury | Decreasing
angiopoietin 2 and increasing vascular endothelial cadherin
levels | (87) |
Reducing postoperative arrhythmias
DEX mitigates postoperative arrhythmias through its
antisympathetic effects. Perioperative administration of DEX [with
or without a loading dose of 0.2-1.5 µg/kg or an
intraoperative maintenance dose of 0.2-1.5 µg/(kg x h)] has
been shown to decrease the incidence of atrial fibrillation
following cardiac surgery (161), although it may increase the
risk of bradycardia (162). A
previous large multicenter randomized controlled trial indicated
that a lower dose of DEX [0.1-0.4 µg/(kg x h)] as a
continuous infusion for 24 h from the induction of anesthesia did
not reduce atrial arrhythmias post-cardiac surgery (163). The efficacy of DEX in managing
postoperative atrial arrhythmias in septic patients remains
controversial and warrants further investigation.
Alleviating brain injury related to
general anesthetics
Neurotoxicity associated with general anesthetic
drugs is a significant concern. Animal studies have demonstrated
that exposure to general anesthetics can induce structural and
functional changes in the developing brain, leading to the
apoptosis of neurons and glial cells (164,165). The results of the present study
are shown in Table IV (166-176). DEX reduced the serum levels of
IL-6, TNF-α, MDA and S100β neuron-specific enolase, whereas SOD
levels significantly increased 24 h post-surgery, decreased the
incidence of postoperative delirium from 23-9%, reduced
postoperative agitation within 3 days, inhibited caspase-3
expression in certain cortical and subcortical regions, and
exhibited notable neuroprotective effects. However, higher doses of
DEX (5 µg/kg) increased mortality rates in rats. Thus, the
use of DEX during anesthesia in septic patients may mitigate brain
injury caused by sepsis and anesthetic drugs.
 | Table IVStudy on the protective mechanism of
DEX against brain injury related to general anesthetics. |
Table IV
Study on the protective mechanism of
DEX against brain injury related to general anesthetics.
| First author,
year | Object of
study | Dosage of DEX | Brain protective
effect | Regulatory
mechanism of the alleviating brain injury | (Refs.) |
|---|
| Luo et al,
2016 | Patients undergoing
craniotomy | 1 µg/kg
before anesthesia induction, maintenance dose of 0.4 µg/kg x
h | Alleviating brain
injury by inhibiting inflammation | Reduces serum
levels of IL-6, TNF-α, malondialdehyde, and S100β neuron-specific
enolase, while superoxide dismutase levels significantly
increased | (166) |
| Sanches et
al, 2022 | Cats subjected to
ovariohysterectomy | 1-5 µg/kg x
h | Alleviating brain
injury by reducing propofol consumption and quicker recovery from
anesthesia | Reduces propofol
consumption and improves cardiorespiratory stability and
intraoperative analgesia, while promoting a improved and quicker
recovery from anesthesia | (167) |
| Liu et al,
2022 | Mice undergoing
Intracerebral hemorrhage | 25-100
µg/kg | Alleviating brain
injury by reducing the neuro-logical damage, brain edema, the
lesion volume and pathological damage | Reducing damage
induced by ferroptosis after ICH by regulating iron metabolism,
amino acid metabolism and lipid peroxidation processes | (168) |
| Perez-Zoghbi et
al, 2017 | sevoflurane-induced
neonatal rats | 1-50
µg/kg | Affording the
highest protection in the thalamus (84% reduction) and lowest in
the hippocampus and cortical areas (50% reduction) | Decreases
sevoflurane anesthesia-induced widespread apoptosis | (169) |
| Goyagi, 2019 | Sevoflurane-induced
neonatal rats | 6.6-50
µg/kg | Improving long-term
cognitive function and ameliorate the neuronal degeneration | Suppresses neuronal
apoptosis | (170) |
| Sun et al,
2021 | Sevoflurane-induced
mice | 10
µg/kg | Improved
sevoflurane-induced cognitive impairment | Inhibits the
sevoflurane-induced Tau phosphorylation via activation of alpha-2
adrenergic receptor | (171) |
| Chai et al,
2024 | Ketamine-induced
mice | 0.1 µM | Improved
ketamine-anesthesia-induced the neurotoxicity (reduced motor
coordination in mice) | Mitigated
ketamine-induced apoptosis in hippocampal neurons. | (172) |
| Lv et al,
2020 | Ethanol-neonatal
mice | | Protects against
EtOH-mediated inhibition of hippocampal neurogenesis | Reverses
EtOH-induced neuroinflammation via repressing microglia activation
and the expression of inflammatory cytokines | (173) |
| Lee et al,
2021 | Sevoflurane-induced
neonatal rats | 2.5-50
µg/kg/h | Reduce the
anesthetic dosage of Sevofluran and improve the neurotoxicity
induced by Sevofluran. | Inhibit programmed
cell death in several developing brain regions. | (174) |
| Fu et al,
2024 | Patients undergoing
craniocerebral surgery | 0.5
µg/kg/h | enhance
postoperative cognitive function | Mitigates oxidative
stress | (175) |
| Shan et al,
2018 | Sevoflurane-induced
rats | 20
µg/kg | Improved
sevoflurane-anesthesia-induced the neurotoxicity (including
apoptosis and axonal injury, long-term learning and memory
dysfunction in the offspring rats) | Inhibits neuronal
apoptosis by activating the BMP/SMAD signaling pathway | (176) |
Improving coagulation disorders
Pathogenic microorganisms invade the body,
triggering immune cells to release proinflammatory mediators that
induce tissue factor expression (177). Concurrently, activated
coagulation factors engage protein-activated receptors on cell
membranes, activating NF-κB and exacerbating inflammation, leading
to systemic inflammatory response syndrome, organ damage and
potentially MODS (178). The
antisympathetic effects of DEX mitigate inflammation, alleviating
the hypercoagulable state. Additionally, DEX promotes vasodilation,
accelerates blood circulation, reduces red blood cell aggregation,
reverses thrombosis, and mitigates organ damage caused by
coagulation. Thus, DEX diminishes sepsis-induced inflammation,
enhances coagulation function in septic patients, dilates blood
vessels, and inhibits the progression of multiple organ failure
induced by sepsis.
Future expectations
Although DEX shows promise in reducing organ
damage, randomized controlled trials on its efficacy and safety in
sepsis are limited. Future research should further assess the
therapeutic potential of DEX in sepsis. At present, the protective
mechanism of DEX against sepsis-induced damage to organs such as
the brain, lungs, liver and heart remains unclear and requires
further research. With further research, new targets and clear
mechanisms of DEX in the treatment of sepsis may be discovered,
expanding its clinical indications beyond perioperative sedatives
and providing evidence-based support for promoting patient recovery
and improving prognosis. Although numerous studies have reported
the organ-protective effects of DEX, numerous aspects of this
effect remain controversial. For example, the clinical evidence on
whether DEX can reduce postoperative atrial fibrillation and
long-term postoperative neurocognitive disorders is not yet unified
and still needs to be validated through multicenter large-scale
randomized controlled trials.
Conclusions
Sepsis-induced MODS is a leading cause of mortality
among critically ill patients and those outside ICUs, with
infections primarily originating from the respiratory, digestive
and urinary systems. Respiratory infections constitute ~50% of
these cases. Sepsis impairs the respiratory and circulatory systems
to varying extents, initially presenting as decreased systemic
vascular resistance and increased cardiac workload. The severity of
sepsis progression is correlated with the characteristics of the
infecting bacteria and the patient's immune status. Effective
treatment of sepsis-induced MODS hinges on early administration of
broad-spectrum antibiotics, stabilization of blood pressure and
organ perfusion, timely surgical intervention at the primary
infection site and restoration of organ function. Despite these
measures, the incidence and mortality rates of sepsis-related MODS
remain high (179).
DEX, a highly selective α2-adrenergic receptor
agonist initially used for surgical sedation in intensive care
units, exhibits anti-inflammatory, antioxidant and antiapoptotic
properties both in vivo and in vitro. Research
indicates that DEX modulates immune dysfunction, enhances the
immune response, lowers cytokine levels, and mitigates organ
inflammation during sepsis. It protects against lung and kidney
injury, reduces astrocyte inflammatory necrosis in the nervous
system, decreases hepatocyte oxidative stress, and inhibits
apoptosis pathways, thereby improving circulatory function and
reducing mortality in septic rats.
Availability of data and materials
Not applicable.
Authors' contributions
MWL conceptualized the study, acquired funding, and
wrote, reviewed and edited the manuscript. SXD curated data. XYZ
conducted investigation and design. QFW provided resources,
analyzed and interpretated the data. SLY performed software
analysis and validation. XL supervised the study and interpretation
of data. NM performed visualization and interpretation of data. All
authors read and approved the final the version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Union Foundation of
Yunnan Provincial Science and Technology Department and Kunming
Medical University (grant no. 202201AY070001-091) and the National
Natural Science Foundation of China (grant no. 81960350).
References
|
1
|
Kumar S, Srivastava VK, Kaushik S, Saxena
J and Jyoti A: Free radicals, mitochondrial dysfunction and
sepsis-induced organ dysfunction: A mechanistic insight. Curr Pharm
Des. 30:161–168. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lelubre C and Vincent JL: Mechanisms and
treatment of organ failure in sepsis. Nat Rev Nephrol. 14:417–427.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keating GM: Dexmedetomidine: A review of
its use for sedation in the intensive care setting. Drugs.
75:1119–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poon WH, Ling RR, Yang IX, Luo H, Kofidis
T, MacLaren G, Tham C, Teoh KLK and Ramanathan K: Dexmedetomidine
for adult cardiac surgery: A systematic review, meta-analysis and
trial sequential analysis. Anesthesia. 78:371–380. 2023. View Article : Google Scholar
|
|
5
|
Zhang DF, Su X, Meng ZT, Li HL, Wang DX,
Xue-Ying Li, Maze M and Ma D: Impact of dexmedetomidine on
long-term outcomes after noncardiac surgery in elderly: 3-Year
follow-up of a randomized controlled trial. Ann Surg. 270:356–363.
2019. View Article : Google Scholar
|
|
6
|
Sun YB, Zhao H, Mu DL, Zhang W, Cui J, Wu
L, Alam A, Wang DX and Ma D: Dexmedetomidine inhibits astrocyte
pyroptosis and subsequently protects the brain in in vitro and in
vivo models of sepsis. Cell Death Dis. 10:1672019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui J, Zhao H, Yi B, Zeng J, Lu K and Ma
D: Dexmedetomidine attenuates bilirubin-induced lung alveolar
epithelial cell death in vitro and in vivo. Crit Care Med.
43:e356–e368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei B, Li J and Zuo Z: Dexmedetomidine
attenuates sepsis-associated inflammation and encephalopathy via
central α2A adrenoceptor. Brain Behav Immun. 91:296–314. 2021.
View Article : Google Scholar
|
|
9
|
Bo JH, Wang JX, Wang XL, Jiao Y, Jiang M,
Chen JL, Hao WY, Chen Q, Li YH, Ma ZL and Zhu GQ: Dexmedetomidine
attenuates lipopolysaccharide-induced sympathetic activation and
sepsis via suppressing superoxide signaling in paraventricular
nucleus. Antioxidants (Basel). 11:23952022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma S, Evans RG, Iguchi N, Tare M,
Parkington HC, Bellomo R, May CN and Lankadeva YR: Sepsis-induced
acute kidney injury: A disease of the microcirculation.
Microcirculation. 26:e124832019. View Article : Google Scholar
|
|
11
|
Carnicelli P, Otsuki DA, Filho AM,
Kahvegian MAP, Ida KK, Auler JOC Jr, Rouby JJ and Fantoni DT:
Effects of dexmedetomidine on hemodynamic, oxygenation,
microcirculation, and inflammatory markers in a porcine model of
sepsis. Acta Cir Bras. 37:e3707032022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990-2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arefian H, Heublein S, Scherag A,
Brunkhorst FM, Younis MZ, Moerer O, Fischer D and Hartmann M:
Hospital-related cost of sepsis: A systematic review. J Infect.
74:107–117. 2017. View Article : Google Scholar
|
|
14
|
Zhou J, Tian H, Du X, Xi X, An Y, Duan M,
Weng L and Du B; for China Critical Care Clinical Trials Group
(CCCCTG): Population-based epidemiology of sepsis in a Subdistrict
of Beijing. Crit Care Med. 45:1168–1176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Lin Y, Zhang Z, Chen Y and Zhong
J: Immune regulation and organ damage link adiponectin to sepsis.
Front Immunol. 15:14448842024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caraballo C and Jaimes F: Organ
dysfunction in sepsis: An ominous trajectory from infection to
death. Yale J Biol Med. 92:629–640. 2019.PubMed/NCBI
|
|
17
|
Killien EY, Zahlan JM, Lad H, Watson RS,
Vavilala MS, Huijsmans RLN and Rivara FP: Epidemiology and outcomes
of multiple organ dysfunction syndrome following pediatric trauma.
J Trauma Acute Care Surg. 93:829–837. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fleiss N and Polin RA: Sequential organ
failure assessment scores to predict outcomes: From adults to
neonates. Curr Opin Pediatr. 35:218–222. 2023. View Article : Google Scholar
|
|
19
|
Nair AS, Saifuddin MS, Naik V and Rayani
BK: Dexmedetomidine in cancer surgeries: Present status and
consequences with its use. Indian J Cancer. 57:234–238. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weerink MAS, Struys MMRF, Hannivoort LN,
Barends CRM, Absalom AR and Colin P: Clinical pharmacokinetics and
pharmacodynamics of dexmedetomidine. Clin Pharmacokinet.
56:893–913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Behera BK, Misra S, Jena SS and Mohanty
CR: The effect of perioperative dexmedetomidine on postoperative
bowel function recovery in adult patients receiving general
anesthesia. Minerva Anestesiol. 88:51–61. 2022. View Article : Google Scholar
|
|
22
|
Benet LZ and Hoener BA: Changes in plasma
protein binding have little clinical relevance. Clin Pharmacol
Ther. 71:115–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gagnon DJ, Riker RR, Glisic EK, Kelner A,
Perrey HM and Fraser GL: Transition from dexmedetomidine to enteral
clonidine for ICU sedation: An observational pilot study.
Pharmacotherapy. 35:251–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sleigh JW, Vacas S, Flexman AM and Talke
PO: Electroencephalographic arousal patterns under dexmedetomidine
sedation. Anesth Analg. 127:951–959. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding J, Chen Y and Gao Y: Effect of
propofol, midazolam and dexmedetomidine on ICU patients with sepsis
and on arterial blood gas. Exp Ther Med. 18:4340–4346.
2019.PubMed/NCBI
|
|
26
|
Kawazoe Y, Miyamoto K, Morimoto T,
Yamamoto T, Fuke A, Hashimoto A, Koami H, Beppu S, Katayama Y, Itoh
M, et al: Effect of dexmedetomidine on mortality and
ventilator-free days in patients requiring mechanical ventilation
with sepsis: A randomized clinical trial. JAMA. 317:1321–1328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller JW, Balyan R, Dong M, Mahmoud M,
Lam JE, Pratap JN, Paquin JR, Li BL, Spaeth JP, Vinks A and Loepke
AW: Does intranasal dexmedetomidine provide adequate plasma
concentrations for sedation in children: A pharmacokinetic study.
Br J Anaesth. 120:1056–1065. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yadav S and Ramdas E: Intranasal
dexmedetomidine versus oral midazolam for premedication in
pediatric anesthesia: A prospective double-blinded randomized
controlled study. Anesth Analg. 123(3S_Suppl): S3552016. View Article : Google Scholar
|
|
29
|
Murphy KS, Golden JC and Tampi RR:
Dexmedetomidine for agitation in dementia: Current data and future
direction. J Am Geriatr Soc. 73:552–557. 2025. View Article : Google Scholar
|
|
30
|
Scheckenbach V and Fideler F: Optimizing
pediatric sedation: Evaluating remimazolam and dexmedetomidine for
safety and efficacy in clinical practice. Paediatr Drugs.
27:181–189. 2025. View Article : Google Scholar :
|
|
31
|
Buckley MS, Smithburger PL, Wong A, Fraser
GL, Reade MC, Klein-Fedyshin M, Ardiles T and Kane-Gill SL:
Dexmedetomidine for facilitating mechanical ventilation extubation
in difficult-to-wean ICU patients: Systematic review and
meta-analysis of clinical trials. J Intensive Care Med. 36:925–936.
2021. View Article : Google Scholar
|
|
32
|
Khan A, Sinha R, Kumar KR, Velpandian T,
Maitra S and Ray BR: Comparison of plasma concentration and
sedative effect of sublingual and intranasal dexmedetomidine in
children: A double-blind randomized controlled study. Acta
Anesthesiol Scand. 69:e145832025. View Article : Google Scholar
|
|
33
|
Portelli K, Kandraju H, Ryu M and Shah PS:
Efficacy and safety of dexmedetomidine for analgesia and sedation
in neonates: A systematic review. J Perinatol. 44:164–172. 2024.
View Article : Google Scholar
|
|
34
|
Lim JY, Ker CJ, Lai NM, Romantsik O,
Fiander M and Tan K: Dexmedetomidine for analgesia and sedation in
newborn infants receiving mechanical ventilation. Cochrane Database
Syst Rev. 5:CD0123612024.PubMed/NCBI
|
|
35
|
Kayki G, Yalcin N, Celik HT and Yigit S:
Dexmedetomidine in neonates: Utilisation trends and safety profile
over time in a neonatal intensive care unit. BMJ Paediatr Open.
9:e0030042025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji SJ, Qian ZH, Hu PY and Chen FY:
Age-dependent changes in skeletal muscle mass and visceral fat area
in a Chinese Population. Curr Med Sci. 43:838–844. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bucław M, Majewska D, Szczerbińska D and
Ligocki M: The influence of age and gender on emu (Dromaius
novaehollandiae) fat. Sci Rep. 10:110822020. View Article : Google Scholar
|
|
38
|
Degrassi I, Leonardi I, Di Profio E,
Montanari C, Zuccotti G and Verduci E: Fat-Soluble vitamins
deficiency in pediatric cholestasis: A scoping review. Nutrients.
15:24912023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang Y, Feng J, Xu J, Dong L, Su W, Li B,
Witwer KW and Zheng L: Associations of age and sex with
characteristics of extracellular vesicles and protein-enriched
fractions of blood plasma. Aging Cell. 24:e143562025. View Article : Google Scholar
|
|
40
|
Peixoto TC, Quitete FT, Teixeira AVS,
Martins BC, Soares RA, Atella GC, Bertasso IM, Lisboa PC, Resende
AC, Mucci DB, et al: Palm and interesterified palm oil-enhanced
brown fat whitening contributes to metabolic dysfunction in
C57BL/6J mice. Nutr Res. 133:94–107. 2025. View Article : Google Scholar
|
|
41
|
Zhao S, Zhou R, Zhong Q and Zhang M:
Effect of age and ICU types on mortality in invasive mechanically
ventilated patients with sepsis receiving dexmedetomidine: A
retrospective cohort study with propensity score matching. Front
Pharmacol. 15:13443272024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Venn RM, Karol MD and Grounds RM:
Pharmacokinetics of dexmedetomidine infusions for sedation of
postoperative patients requiring intensive caret. Br J Anaesth.
88:669–675. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hart CG, Voelz BE, Brockus KE and Lemley
CO: Hepatic steroid inactivating enzymes, hepatic portal blood flow
and corpus luteum blood perfusion in cattle. Reprod Domest Anim.
53:751–758. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin L, Guo X, Zhang MZ, Qu CJ, Sun Y and
Bai J: Pharmacokinetics of dexmedetomidine in Chinese post-surgical
intensive care unit patients. Acta Anesthesiol Scand. 55:359–367.
2011. View Article : Google Scholar
|
|
45
|
Iirola T, Laitio R, Kentala E, Aantaa R,
Kurvinen JP, Scheinin M and Olkkola KT: Highly variable
pharmacokinetics of dexmedetomidine during intensive care: A case
report. J Med Case Rep. 25:732010. View Article : Google Scholar
|
|
46
|
Yang L, Zhu L, Qi B, Zhang Y, Ni C, Zhang
Y, Shi X, Xia Q, Masters J, Ma D and Yu W: Dexmedetomidine use
during orthotopic liver transplantation surgery on early allograft
dysfunction: A randomized controlled trial. Int J Surg.
110:5518–5526. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ustun YB, Koksal E, Turunc E, Komurcu O,
Dost B, Ozsay O and Karabulut K: Early extubation after liver
transplantation: Is dexmedetomidine a good option?: A retrospective
cohort study. Int J Clin Pract. 75:e146292021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu H, An S, Sha T, Wu F, Jin Y, Li L, Zeng
Z, Wu J and Chen Z: Association between dexmedetomidine
administration and outcomes in critically ill patients with
sepsis-associated acute kidney injury. J Clin Anesth.
83:1109602022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Evans RG, Iguchi N, Cochrane AD, Marino B,
Hood SG, Bellomo R, McCall PR, May CN and Lankadeva YR: Renal
hemodynamics and oxygenation during experimental cardiopulmonary
bypass in sheep under total intravenous anesthesia. Am J Physiol
Regul Integr Comp Physiol. 318:R206–R213. 2020. View Article : Google Scholar :
|
|
50
|
Neumann MM, Davio MB, Macknet MR and
Applegate RL II: Dexmedetomidine for awake fiberoptic intubation in
a parturient with spinal muscular atrophy type III for cesarean
delivery. Int J Obstet Anesth. 18:403–407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Aghamiri SM, Samimi AS, Hajian M, Samimi
AM and Oroumieh A: Effect of xylazine, detomidine, medetomidine and
dexmedetomidine during laparoscopic SCNT embryo transfer on
pregnancy rate and some physiological variables in goats. BMC Vet
Res. 18:982022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G
and Ma D: Effects of dexmedetomidine on perioperative stress,
inflammation, and immune function: Systematic review and
meta-analysis. Br J Anaesth. 123:777–794. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen M, Li X and Mu G: Myocardial
protective and anti-inflammatory effects of dexmedetomidine in
patients undergoing cardiovascular surgery with cardiopulmonary
bypass: A systematic review and meta-analysis. J Anesth. 36:5–16.
2022. View Article : Google Scholar
|
|
54
|
Ma J, Chen Q, Li J, Zhao H, Mi E, Chen Y,
Yi B, Ning J, Ma D, Lu K and Gu J: Dexmedetomidine-mediated
prevention of renal ischemia-reperfusion injury depends in part on
cholinergic anti-inflammatory mechanisms. Anesth Analg.
130:1054–1062. 2020. View Article : Google Scholar
|
|
55
|
Han QQ, Li XY and Wang YX: Dexmedetomidine
attenuates lipopolysaccharide-induced inflammation through
macrophageal IL-10 expression following α7 nAchR activation. Int
Immunopharmacol. 109:1089202022. View Article : Google Scholar
|
|
56
|
Li J, Chen Q, He X, Alam A, Ning J, Yi B,
Lu K and Gu J: Dexmedetomidine attenuates lung apoptosis induced by
renal ischemia-reperfusion injury through α2AR/PI3K/Akt pathway. J
Transl Med. 16:782018. View Article : Google Scholar
|
|
57
|
Wang K and Zhu Y: Dexmedetomidine protects
against oxygen-glucose deprivation/reoxygenation injury-induced
apoptosis via the p38 MAPK/ERK signalling pathway. J Int Med Res.
46:675–686. 2018. View Article : Google Scholar :
|
|
58
|
Zhu Z, Ling X, Zhou H, Zhang C and Yan W:
Dexmedetomidine attenuates cellular injury and apoptosis in H9c2
cardiomyocytes by regulating p-38MAPK and endoplasmic reticulum
stress. Drug Des Dev Ther. 14:4231–4243. 2020. View Article : Google Scholar
|
|
59
|
Deng X, Ye F, Zeng L, Luo W, Tu S, Wang X
and Zhang Z: Dexmedetomidine mitigates myocardial
ischemia/reperfusion-induced mitochondrial apoptosis through
targeting lncRNA HCP5. Am J Chin Med. 50:1529–1551. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wei Q, Chen J, Xiao F, Tu Y, Zhong Y and
Xie Y: High-dose dexmedetomidine promotes apoptosis in fetal rat
hippocampal neurons. Drug Des Dev Ther. 15:2433–2444. 2021.
View Article : Google Scholar
|
|
61
|
Zhang Z, Mu X and Zhou X: Dexmedetomidine
alleviates inflammatory response and oxidative stress injury of
vascular smooth muscle cell via α2AR/GSK-3β/MKP-1/NRF2 axis in
intracranial aneurysm. BMC Pharmacol Toxico. 23:812022. View Article : Google Scholar
|
|
62
|
Choi HI, Kim HJ, Park JS, Kim IJ, Bae EH,
Ma SK and Kim SW: PGC-1α attenuates hydrogen peroxide-induced
apoptotic cell death by upregulating Nrf-2 via GSK3β inactivation
mediated by activated p38 in HK-2 cells. Sci Rep. 7:43192017.
View Article : Google Scholar
|
|
63
|
Zhao Y, Kong GY, Pei WM, Zhou B, Zhang QQ
and Pan BB: Dexmedetomidine alleviates hepatic injury via the
inhibition of oxidative stress and activation of the Nrf2/HO-1
signaling pathway. Eur Cytokine Netw. 30:88–97. 2019. View Article : Google Scholar
|
|
64
|
Chen J, Shen N, Duan X and Guo Y: An
investigation of the mechanism of dexmedetomidine in improving
postoperative cognitive dysfunction from the perspectives of
alleviating neuronal mitochondrial membrane oxidative stress and
electrophysiological dysfunction. Exp Ther Med. 15:2037–2043.
2018.PubMed/NCBI
|
|
65
|
Yu T, Liu D, Gao M, Yang P, Zhang M, Song
F, Zhang X and Liu Y: Dexmedetomidine prevents septic myocardial
dysfunction in rats via activation of α7nAChR and PI3K/Akt-mediated
autophagy. Biomed Pharmacother. 120:1092312019. View Article : Google Scholar
|
|
66
|
Lempiäinen J, Finckenberg P, Mervaala EE,
Storvik M, Kaivola J, Lindstedt K, Levijoki J and Mervaala EM:
Dexmedetomidine preconditioning ameliorates kidney
ischemia-reperfusion injury. Pharmacol Res Perspe. 2:e000452014.
View Article : Google Scholar
|
|
67
|
Li Y, Qu M, Xing F, Li H, Cheng D, Xing N
and Zhang W: The protective mechanism of dexmedetomidine in
regulating Atg14L-Beclin1-Vps34 complex against myocardial
ischemia-reperfusion injury. J Cardiovasc Transl Res. 14:1063–1074.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhu Y, Li S, Liu J, Wen Q, Yu J, Yu L and
Xie K: Role of JNK signaling pathway in dexmedetomidine
post-conditioning-induced reduction of the inflammatory response
and autophagy effect of focal cerebral ischemia reperfusion injury
in rats. Inflammation. 42:2181–2191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Qin W, Zhang X, Yang S, Li Y, Yuan J, Yang
L, Li S and Hu W: Risk factors for multiple organ dysfunction
syndrome in severe stroke patients. PLoS One. 11:e01671892016.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
van der Poll T, Shankar-Hari M and
Wiersinga WJ: The immunology of sepsis. Immunity. 54:2450–2464.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gordon S: Phagocytosis: An immunobiologic
process. Immunity. 44:463–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kumar V: Immunometabolism: Another road to
sepsis and its therapeutic targeting. Inflammation. 42:765–788.
2019. View Article : Google Scholar
|
|
73
|
Hoesel LM, Neff TA, Neff SB, Younger JG,
Olle EW, Gao H, Pianko MJ, Bernacki KD, Sarma JV and Ward PA:
Harmful and protective roles of neutrophils in sepsis. Shock.
24:40–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lu J, Liu J and Li A: Roles of neutrophil
reactive oxygen species (ROS) generation in organ function
impairment in sepsis. J Zhejiang Univ Sci B. 23:437–450. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Christoffersson G, Vågesjö E, Vandooren J,
Lidén M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G
and Phillipson M: VEGF-A recruits a proangiogenic MMP-9-delivering
neutrophil subset that induces angiogenesis in transplanted hypoxic
tissue. Blood. 120:4653–4662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bateman RM, Sharpe MD and Ellis CG:
Bench-to-bedside review: Microvascular dysfunction in
sepsis-hemodynamics, oxygen transport, and nitric oxide. Crit Care.
7:359–373. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang Y, Bai C and Wang X: COPD-associated
vascular pathology: A future targeting area. Expert Rev Respir Med.
2:297–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fein AM and Calalang-Colucci MG: Acute
lung injury and acute respiratory distress syndrome in sepsis and
septic shock. Crit Care Clin. 16:289–317. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen G, Hou Y, Li X, Pan R and Zhao D:
Sepsis-induced acute lung injury in young rats is relieved by
calycosin through inactivating the HMGB1/MyD88/NF-κB pathway and
NLRP3 inflammasome. Int Immunopharmacol. 96:1076232021. View Article : Google Scholar
|
|
80
|
Li J, Lu K, Sun F, Tan S, Zhang X, Sheng
W, Hao W, Liu M, Lv W and Han W: Panaxydol attenuates ferroptosis
against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1
pathway. J Transl Med. 19:962021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hou L, Zhang J, Liu Y, Fang H, Liao L,
Wang Z, Yuan J, Wang X, Sun J, Tang B, et al: MitoQ alleviates
LPS-mediated acute lung injury through regulating Nrf2/Drp1
pathway. Free Radic Biol Med. 165:219–228. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F
and Zhou C, Huang L, Li X and Zhou C: Dexmedetomidine inhibits
inflammatory reaction in lung tissues of septic rats by suppressing
TLR4/NF-κB pathway. Mediators Inflamm. 2013:5621542013. View Article : Google Scholar
|
|
83
|
Cui H and Zhang Q: Dexmedetomidine
ameliorates lipopolysaccharide-induced acute lung injury by
inhibiting the PI3K/Akt/FoxO1 signaling pathway. J Anesth.
35:394–404. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fu C, Dai X, Yang Y, Lin M, Cai Y and Cai
S: Dexmedetomidine attenuates lipopolysaccharide-induced acute lung
injury by inhibiting oxidative stress, mitochondrial dysfunction
and apoptosis in rats. Mol Med Rep. 15:131–138. 2017. View Article : Google Scholar :
|
|
85
|
Shi J, Yu T, Song K, Du S, He S, Hu X, Li
X, Li H, Dong S, Zhang Y, et al: Dexmedetomidine ameliorates
endotoxin-induced acute lung injury in vivo and in vitro by
preserving mitochondrial dynamic equilibrium through the
HIF-1a/HO-1 signaling pathway. Redox Biol. 41:1019542021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Meng L, Li L, Lu S, Li K, Su Z, Wang Y,
Fan X, Li X and Zhao G: The protective effect of dexmedetomidine on
LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB
and PI3K/Akt/mTOR pathways. Mol Immunol. 94:7–17. 2018. View Article : Google Scholar
|
|
87
|
Zhang P, Peng J, Ren YQ, Zheng H and Yan
H: Dexmedetomidine protects against endothelial injury in septic
rats induced by cecal ligation and puncture by decreasing
angiopoietin 2 and increasing vascular endothelial cadherin levels.
Exp Ther Med. 21:1112021. View Article : Google Scholar
|
|
88
|
Wang R, Xie Y, Qiu J and Chen J: The
effects of dexmedetomidine in a rat model of sepsis-induced lung
injury are mediated through the adenosine monophosphate-activated
protein kinase (AMPK)/silent information regulator 1 (SIRT1)
pathway. Med Sci Monit. 26:e9192132020.PubMed/NCBI
|
|
89
|
Peerapornratana S, Manrique-Caballero CL,
Gómez H and Kellum JA: Acute kidney injury from sepsis: Current
concepts, epidemiology, pathophysiology, prevention and treatment.
Kidney Int. 96:1083–1099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Manrique-Caballero CL, Del Rio-Pertuz G
and Gomez H: Sepsis-associated acute kidney injury. Crit Care Clin.
37:279–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hu X, Zhou W, Wu S, Wang R, Luan Z, Geng
X, Xu N, Zhang Z, Ruan Z, Wang Z, et al: Tacrolimus alleviates
LPS-induced AKI by inhibiting TLR4/MyD88/NF-κB signalling in mice.
J Cell Mol Med. 26:507–514. 2022. View Article : Google Scholar
|
|
92
|
Huang G, Bao J, Shao X, Zhou W, Wu B, Ni Z
and Wang L: Inhibiting pannexin-1 alleviates sepsis-induced acute
kidney injury via decreasing NLRP3 inflammasome activation and cell
apoptosis. Life Sci. 254:1177912020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li J, Wang L, Wang B, Zhang Z, Jiang L,
Qin Z, Zhao Y and Su B: NOX4 is a potential therapeutic target in
septic acute kidney injury by inhibiting mitochondrial dysfunction
and inflammation. Theranostics. 13:2863–2878. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu W, Yu W, Weng Y, Wang Y and Sheng M:
Dexmedetomidine ameliorates the inflammatory immune response in
rats with acute kidney damage. Exp Ther Med. 14:3602–3608. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jin YH, Li ZT, Chen H, Jiang XQ, Zhang YY
and Wu F: Effect of dexmedetomidine on kidney injury in sepsis rats
through TLR4/MyD88/NF-κB/iNOS signaling pathway. Eur Rev Med
Pharmacol Sci. 23:5020–5025. 2019.PubMed/NCBI
|
|
96
|
Yao Y, Hu X, Feng X, Zhao Y, Song M, Wang
C and Fan H: Dexmedetomidine alleviates lipopolysaccharide-induced
acute kidney injury by inhibiting the NLRP3 inflammasome activation
via regulating the TLR4/NOX4/NF-κB pathway. J Cell Biochem.
120:18509–18523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang Z, Wu J, Hu Z, Luo C, Wang P, Zhang Y
and Li H: Dexmedetomidine alleviates lipopolysaccharide-induced
acute kidney injury by inhibiting p75NTR-mediated oxidative stress
and apoptosis. Oxid Med Cell Longev. 2020:54542102020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao Y, Feng X, Li B, Sha J, Wang C, Yang
T, Cui H and Fan H: Dexmedetomidine protects against
lipopolysaccharide-induced acute kidney injury by enhancing
autophagy through inhibition of the PI3K/AKT/mTOR pathway. Front
Pharmacol. 11:1282020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kim TS and Choi DH: Liver dysfunction in
sepsis. Korean J Gastroenterol. 75:182–187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cai Y, Zhao D, Pan Y, Chen B, Cao Y, Han
S, Lian F, Zhang Y and Yan X: Gallic acid attenuates sepsis-induced
liver injury through C/EBPbeta-dependent MAPK signaling pathway.
Mol Nutr Food Res. 29:e24001232024. View Article : Google Scholar
|
|
101
|
Ou Z, Zhong H, Zhang L, Deng M, Zhang W,
Wang J, Feng H, Gong J, Miao C and Yi Z: Macrophage membrane-coated
nanoparticles alleviate hepatic ischemia-reperfusion injury caused
by orthotopic liver transplantation by neutralizing endotoxin. Int
J Nanomedicine. 15:4125–4138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Song H, Zhang X, Zhai R, Liang H, Song G,
Yuan Y, Xu Y, Yan Y, Qiu L and Sun T: Metformin attenuated
sepsis-associated liver injury and inflammatory response in aged
mice. Bioengineered. 13:4598–4609. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hong KS, Kim NR, Song SH and Hong G:
Cycling of dexmedetomidine may prevent delirium after liver
transplantation. Transplant Proc. 50:1080–1082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tong F, Shen W, Song P, Song J, Hu Y, Liu
F, Meng Z and Liu J: Dexmedetomidine attenuates
lipopolysaccharide-induced acute liver injury in rats by inhibiting
caveolin-1 downstream signaling pathway. Biosci Rep.
41:BSR202042792021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zi SF, Li JH, Liu L, Deng C, Ao X, Chen DD
and Wu SZ: Dexmedetomidine-mediated protection against septic liver
injury depends on TLR4/MyD88/NF-κB signaling downregulation partly
via cholinergic anti-inflammatory mechanisms. Int Immunopharmacol.
76:1058982019. View Article : Google Scholar
|
|
106
|
Yu Q, Zou L, Yuan X, Fang F and Xu F:
Dexmedetomidine protects against septic liver injury by enhancing
autophagy through activation of the AMPK/SIRT1 signaling pathway.
Front Pharmacol. 12:6586772021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sha J, Zhang H, Zhao Y, Feng X, Hu X, Wang
C, Song M and Fan H: Dexmedetomidine attenuates
lipopolysaccharide-induced liver oxidative stress and cell
apoptosis in rats by increasing GSK-3β/MKP-1/Nrf2 pathway activity
via the α2 adrenergic receptor. Toxicol Appl Pharmacol.
364:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhou H, Sun J, Zhong W, Pan X, Liu C,
Cheng F, Wang P and Rao Z: Dexmedetomidine preconditioning
alleviated murine liver ischemia and reperfusion injury by
promoting macrophage M2 activation via PPARγ/STAT3 signaling. Int
Immunopharmacol. 82:1063632020. View Article : Google Scholar
|
|
109
|
Frencken JF, Donker DW, Spitoni C,
Koster-Brouwer ME, Soliman IW, Ong DSY, Horn J, van der Poll T, van
Klei WA, Bonten MJM and Cremer OL: Myocardial injury in patients
with sepsis and its association with long-term outcome. Circ
Cardiovasc Qual Outcomes. 11:e0040402018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Orde SR, Pulido JN, Masaki M, Gillespie S,
Spoon JN, Kane GC and Oh JK: Outcome prediction in sepsis: Speckle
tracking echocardiography based assessment of myocardial function.
Crit Care. 18:R1492014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Maeder M, Fehr T, Rickli H and Ammann P:
Sepsis-associated myocardial dysfunction: Diagnostic and prognostic
impact of cardiac troponins and natriuretic peptides. Chest.
129:1349–1366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sun F, Xu K, Zhou J, Zhang W, Duan G and
Lei M: Allicin protects against LPS-induced cardiomyocyte injury by
activating Nrf2-HO-1 and inhibiting NLRP3 pathways. BMC Cardiovasc
Disord. 23:4102023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang Y, Feng W, Li S, Liu C, Jia L, Wang
P, Li L, Du H and Yu W: Oxycodone attenuates
lipopolysaccharide-induced myocardial injury by inhibiting
inflammation, oxidation and pyroptosis via Nrf2/HO-1 signalling
pathway. Clin Exp Pharmacol Physiol. 51:e139102024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lu C, Liu J, Escames G, Yang Y, Wu X, Liu
Q, Chen J, Song Y, Wang Z, Deng C, et al: PIK3CG regulates NLRP3/
GSDMD-mediated pyroptosis in septic myocardial injury.
Inflammation. 46:2416–2432. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bi CF, Liu J, Yang LS and Zhang JF:
Research progress on the mechanism of sepsis induced myocardial
injury. J Inflamm Res. 15:4275–4290. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Taniguchi T, Kurita A, Kobayashi K,
Yamamoto K and Inaba H: Dose- and time-related effects of
dexmedetomidine on mortality and inflammatory responses to
endotoxin-induced shock in rats. J Anesth. 22:221–228. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Taniguchi T, Kidani Y, Kanakura H,
Takemoto Y and Yamamoto K: Effects of dexmedetomidine on mortality
rate and inflammatory responses to endotoxin-induced shock in rats.
Crit Care Med. 32:1322–1326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kong W, Kang K, Gao Y, Liu H, Meng X, Yang
S, Yu K and Zhao M: Dexmedetomidine alleviates LPS-induced septic
cardiomyopathy via the cholinergic anti-inflammatory pathway in
mice. Am J Transl Res. 9:5040–5047. 2017.PubMed/NCBI
|
|
119
|
Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang
CF, Li Y and Zhang Z: Dexmedetomidine alleviated sepsis-induced
myocardial ferroptosis and septic heart injury. Mol Med Rep.
22:175–184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang T, Mei Q, Dai S, Liu Y and Zhu H:
Use of dexmedetomidine in patients with sepsis: A systematic review
and meta-analysis of randomized-controlled trials. Ann Intensive
Care. 12:812022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Pool R, Gomez H and Kellum JA: Mechanisms
of organ dysfunction in sepsis. Crit Care Clin. 34:63–80. 2018.
View Article : Google Scholar
|
|
122
|
Haussner F, Chakraborty S, Halbgebauer R
and Huber-Lang M: Challenge to the intestinal mucosa during sepsis.
Front Immunol. 10:8912019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rowlands BJ, Soong CV and Gardiner KR: The
gastrointestinal tract as a barrier in sepsis. Br Med Bull.
55:196–211. 1999. View Article : Google Scholar
|
|
124
|
Chen Y, Miao L, Yao Y, Wu W, Wu X, Gong C,
Qiu L and Chen J: Dexmedetomidine ameliorate CLP-induced rat
intestinal injury via inhibition of inflammation. Mediators
Inflamm. 2015:9183612015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Qin C, Jiang Y, Chen X, Bian Y, Wang Y,
Xie K and Yu Y: Dexmedetomidine protects against burn-induced
intestinal barrier injury via the MLCK/p-MLC signalling pathway.
Burns. 47:1576–1585. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yang J, Wu Y, Xu Y, Jia J, Xi W, Deng H
and Tu W: Dexmedetomidine resists intestinal Ischemia-reperfusion
injury by inhibiting TLR4/MyD88/NF-κB signaling. J Surg Res.
260:350–358. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ye X, Xu H and Yuan X: Dexmedetomidine
alleviates intestinal barrier dysfunction and inflammatory response
in mice via suppressing TLR4/MyD88/NF-κB signaling in an
experimental model of ulcerative colitis. Folia Histochem Cytobiol.
60:311–322. 2022. View Article : Google Scholar
|
|
128
|
Zhang Q, Liu XM, Hu Q, Liu ZR, Liu ZY,
Zhang HG, Huang YL, Chen QH, Wang WX and Zhang XK: Dexmedetomidine
inhibits mitochondria damage and apoptosis of enteric glial cells
in experimental intestinal ischemia/reperfusion injury via
SIRT3-dependent PINK1/HDAC3/p53 pathway. J Transl Med. 19:4632021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Dong YH, Hu JJ, Deng F, Chen XD, Li C, Liu
KX and Zhao BC: Use of dexmedetomidine to alleviate intestinal
ischemia-reperfusion injury via intestinal microbiota modulation in
mice. Ann Transl Med. 10:11612022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Xu L, Li T, Chen Q, Liu Z, Chen Y, Hu K
and Zhang X: The α2AR/Caveolin-1/p38MAPK/NF-κB axis explains
dexmedetomidine protection against lung injury following intestinal
ischaemia-reperfusion. J Cell Mol Med. 25:6361–6372. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chang H, Li S, Li Y, Hu H, Cheng B, Miao
J, Gao H, Ma H, Gao Y and Wang Q: Effect of sedation with
dexmedetomidine or propofol on gastrointestinal motility
inlipopolysaccharide-induced endotoxemic mice. BMC Anesthesiol.
20:2272020. View Article : Google Scholar
|
|
132
|
Zhao L, Gao Y, Guo S, Lu X, Yu S, Ge ZZ,
Zhu HD and Li Y: Sepsis-associated encephalopathy: Insight into
injury and pathogenesis. CNS Neurol Disord Drug Targets.
20:112–124. 2021.
|
|
133
|
Subhramanyam CS, Wang C, Hu Q and Dheen
ST: Microglia-mediated neuroinflammation in neurodegenerative
diseases. Semin Cell Dev Biol. 94:112–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bao Y, Zhu Y, He G, Ni H, Liu C, Ma L,
Zhang L and Shi D: Dexmedetomidine attenuates neuroinflammation in
LPS-stimulated BV2 microglia cells through upregulation Of miR-340.
Drug Des Devel Ther. 13:3465–3475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
He G, He Y, Ni H, Wang K, Zhu Y and Bao Y:
Dexmedetomidine attenuates neuroinflammation and microglia
activation in LPS-stimulated BV2 microglia cells through targeting
circ-Shank3/miR-140-3p/TLR4 axis. Eur J Histochem. 67:37662023.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lei S, Lu P, Lu Y, Zheng J, Li W, Wang N,
Zhang H, Li R, Wang K, Wen J, et al: Dexmedetomidine alleviates
neurogenesis damage following neonatal midazolam exposure in rats
through JNK and P38 MAPK pathways. ACS Chem Neurosci. 11:579–591.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Sun Z, Lin Y, Li Y, Ren T, Du G, Wang J,
Jin X and Yang LC: The effect of dexmedetomidine on inflammatory
inhibition and microglial polarization in BV-2 cells. Neurol Res.
40:838–846. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hamers L, Kox M and Pickkers P:
Sepsis-induced immunoparalysis: Mechanisms, markers, and treatment
options. Minerva Anestesiol. 81:426–439. 2015.
|
|
139
|
Martin MD, Badovinac VP and Griffith TS:
CD4 T cell responses and the sepsis-induced immunoparalysis state.
Front Immunol. 11:13642020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Łysenko L, Leśnik P, Nelke K and Gerber H:
Immune disorders in sepsis and their treatment as a significant
problem of modern intensive care. Postepy Hig Med Dosw (Online).
71:703–712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lee JM, Han HJ, Choi WK, Yoo S, Baek S and
Lee J: Immunomodulatory effects of intraoperative dexmedetomidine
on T helper 1, T helper 2, T helper 17 and regulatory T cells
cytokine levels and their balance: A prospective, randomised,
double-blind, dose-response clinical study. BMC Anesthesiol.
18:1642018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yang XH, Bai Q, LvMM, Fu HG, Dong TL and
Zhou Z: Effect of dexmedetomidine on Immune function of patients
undergoing radical mastectomy: A double blind and placebo control
study. Eur Rev Med Pharmacol Sci. 21:1112–1116. 2017.PubMed/NCBI
|
|
143
|
Biccard BM, Goga S and de Beurs J:
Dexmedetomidine and cardiac protection for non-cardiac surgery: A
meta-analysis of randomised controlled trials. Anaesthesia.
63:4–14. 2008. View Article : Google Scholar
|
|
144
|
Farouk I, Hassan MM, Fetouh AM, Elgayed
AEA, Eldin MH and Abdelhamid BM: Analgesic and hemodynamic effects
of intravenous infusion of magnesium sulphate versus
dexmedetomidine in patients undergoing bilateral inguinal hernial
surgeries under spinal anesthesia: A randomized controlled study.
Braz J Anesthesiol. 71:489–497. 2021.PubMed/NCBI
|
|
145
|
Lankadeva YR, Kosaka J, Evans RG, Bailey
SR, Bellomo R and May CN: Intrarenal and urinary oxygenation during
norepinephrine resuscitationin ovine septic acute kidney injury.
Kidney Int. 90:100–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Julien C, Oréa V, Quintin L, Piriou V and
Barrès C: Renal sympathetic nerve activity and vascular reactivity
to phenylephrine after lipopolysaccharide administration in
conscious rats. Physiol Rep. 5:e131392017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Morelli A, Sanfilippo F, Arnemann P,
Hessler M, Kampmeier TG, D'Egidio A, Orecchioni A, Santonocito C,
Frati G, Greco E, et al: The effect of propofol and dexmedetomidine
sedation on norepinephrine requirements in septic shock patients: A
crossover trial. Crit Care Med. 47:e89–e95. 2019. View Article : Google Scholar
|
|
148
|
Lankadeva YR, Ma S, Iguchi N, Evans RG,
Hood SG, Farmer DGS, Bailey SR, Bellomo R and May CN:
Dexmedetomidine reduces norepinephrine requirements and preserves
renal oxygenation and function in ovine septic acute kidney injury.
Kidney Int. 96:1150–1161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yao L, Liu B, He T and Shen F: Comparative
study of dexmedetomidine vs. midazolam on plasma catecholamine
levels and hemodynamics in patients with septic shock. Zhonghua Wei
Zhong Bing Ji Jiu Yi Xue. 33:1193–1197. 2021.In Chinese. PubMed/NCBI
|
|
150
|
Moore ACM, Kachare SD, Barber DA, Barrow L
and O'Daniel TG: Total intravenous anesthesia with dexmedetomidine
for hemodynamic stability and enhanced recovery in facial aesthetic
surgery. Aesthet Surg J. 42:NP602–NP610. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hazrati E, Vosoughi F, Chamanara M and
Teymourian H: Effect of dexmedetomidine infusion during hip
fracture surgery on hemodynamic parameters and blood loss: A
triple-blinded randomized clinical trial. Injury. 53:551–554. 2022.
View Article : Google Scholar
|
|
152
|
Joffre J, Hellman J, Ince C and
Ait-Oufella H: Endothelial responses in sepsis. Am J Respir Crit
Care Med. 202:361–370. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Joffre J and Hellman J: Oxidative stress
and endothelial dysfunction in sepsis and acute inflammation.
Antioxid Redox Signal. 35:1291–1307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Cusack R, Leone M, Rodriguez AH and
Martin-Loeches I: Endothelial Damage And the microcirculation in
critical illness. Biomedicines. 10:31502022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Dai M, Zhu X, Zeng S, Liu Q, Hu R, Huang
L, Wang Y, Deng J and Yu Q: Dexmedetomidine protects cells from
angiotensin II-induced smooth muscle cell phenotype switch and
endothelial cell dysfunction. Cell Cycle. 22:450–463. 2023.
View Article : Google Scholar :
|
|
156
|
Newton CJ, Ran G, Xie YX, Bilko D,
Burgoyne CH, Adams I, Abidia A, McCollum PT and Atkin SL:
Statin-induced apoptosis of vascular endothelial cells is blocked
by dexamethasone. J Endocrinol. 174:7–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Chai Y, Yu R, Liu Y, Wang S, Yuan D and
Chen J: Dexmedetomidine attenuates monocyte-endothelial adherence
via inhibiting connexin43 on vascular endothelial cells. Mediators
Inflamm. 2020:70398542020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Dong W, Yang H, Cheng M, Zhang X, Yin J,
Zeng Z and Huang G: Dexmedetomidine alleviates pulmonary
ischemia-reperfusion injury through modulating the miR-21-5p/Nr4a1
signaling pathway. Acta Biochim Pol. 67:521–529. 2020.PubMed/NCBI
|
|
159
|
Han J, Liu X and Wang L: Dexmedetomidine
protects against acute lung injury in mice via the DUSP1/MAPK/NF-κB
axis by inhibiting miR-152-3p. Pulm Pharmacol Ther. 9:1021312022.
View Article : Google Scholar
|
|
160
|
Zhao Q, Yu S, Ling Y, Hao S and Liu J: The
protective effects of dexmedetomidine against
hypoxia/reoxygenation-induced inflammatory injury and permeability
in brain endothelial cells mediated by sigma-1 receptor. ACS Chem
Neurosci. 12:1940–1947. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhu Z, Zhou H, Ni Y, Wu C, Zhang C and
Ling X: Can dexmedetomidine reduce atrial fibrillation after
cardiac surgery? A systematic review and meta-analysis. Drug Des
Devel Ther. 12:521–531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Liu X, Xie G, Zhang K, Song S, Song F, Jin
Y and Fang X: Dexmedetomidine vs propofol sedation reduces delirium
in patients after cardiac surgery: A meta analysis with trial
sequential analysis of randomized controlled trials. J Crit Care.
38:190–196. 2017. View Article : Google Scholar
|
|
163
|
Turan A, Duncan A, Leung S, Karimi N, Fang
J, Mao G, Hargrave J, Gillinov M, Trombetta C, Ayad S, et al:
Dexmedetomidine for reduction of atrial fibrillation and delirium
after cardiac surgery(DECADE): A randomised placebo-controlled
trial. Lancet. 396:177–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Hu X, Sun Z, Wang W, Xiao G, Yu Q, Chi L
and Liu H: Dexmedetomidine attenuates isoflurane-induced
neuroapoptosis through the miR-137/GSK-3β pathway in the developing
rat hippocampus. Heliyon. 10:e313722024. View Article : Google Scholar
|
|
165
|
Wu Q, Shang Y, Shen T, Liu F and Zhang W:
Biochanin A protects SH-SY5Y cells against isoflurane-induced
neurotoxicity by suppressing oxidative stress and apoptosis.
Neurotoxicology. 86:10–18. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Luo X, Zheng X and Huang H: Protective
effects of dexmedetomidine on brain function of glioma patients
undergoing craniotomy resection and its underlying mechanism. Clin
Neurol Neurosurg. 146:105–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Sanches MC, Bessi WH, Rusch E,
Schaffhausser MB, Cassoli AA, Freitas SH, Gehrcke MI and Carregaro
AB: Cardiopulmonary and propofol-sparing effects of dexmedetomidine
in total intravenous anesthesia in cats undergoing
ovariohysterectomy. J Feline Med Surg. 24:e490–e497. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Liu MJ, Zhao XC, Gong HS, You YQ and Li
JY: Dexmedetomidine prevents hemorrhagic brain injury by reducing
damage induced by ferroptosis in mice. Neurosci Lett.
788:1368422022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Perez-Zoghbi JF, Zhu W, Grafe MR and
Brambrink AM: Dexmedetomidine-mediated neuroprotection against
sevoflurane-induced neurotoxicity extends to several brain regions
in neonatal rats. Br J Anaesth. 119:506–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Goyagi T: Dexmedetomidine reduced
sevoflurane-induced neurodegeneration and long-term memory deficits
in neonatal rats. Int J Dev Neurosci. 75:19–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Sun M, Dong Y, Li M, Zhang Y, Liang F,
Zhang J, Soriano SG and Xie Z: Dexmedetomidine and clonidine
attenuate sevoflurane-induced tau phosphorylation and cognitive
impairment in young mice via α-2 adrenergic receptor. Anesth Analg.
132:878–889. 2021. View Article : Google Scholar :
|
|
172
|
Chai D, Jiang H and Lv X: The impact of
dexmedetomidine on ketamine-induced neurotoxicity and cognitive
impairment in young mice. Int J Dev Neurosci. 84:735–744. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lv K, Yang C, Xiao R, Yang L, Liu T, Zhang
R and Fan X: Dexmedetomidine attenuates ethanol-induced inhibition
of hippocampal neurogenesis in neonatal mice. Toxicol Appl
Pharmacol. 390:1148812020. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Lee JR, Joseph B, Hofacer RD, Upton B, Lee
SY, Ewing L, Zhang B, Danzer SC and Loepke AW: Effect of
dexmedetomidine on sevoflurane-induced neurodegeneration in
neonatal rats. Br J Anaesth. 126:1009–1021. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Fu Y and Jin Z: Effects of dexmedetomidine
on cognitive function, oxidative stress and brain protection in
patients undergoing craniocerebral surgery. Actas Esp Psiquiatr.
52:19–27. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Shan Y, Yang F, Tang Z, Bi C, Sun S, Zhang
Y and Liu H: Dexmedetomidine ameliorates the neurotoxicity of
sevoflurane on the immature brain through the BMP/SMAD signaling
pathway. Front Neurosci. 12:9642018. View Article : Google Scholar
|
|
177
|
Williams B, Zou L, Pittet JF and Chao W:
Sepsis-Induced coagulopathy: A comprehensive narrative review of
pathophysiology, clinical presentation, diagnosis, and management
strategies. Anesth Analg. 138:696–711. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Chen Z, Zhang H, Qu M, Nan K, Cao H, Cata
JP, Chen W and Miao C: Review: The emerging role of neutrophil
extracellular traps in sepsis and sepsis-associated thrombosis.
Front Cell Infect Microbiol. 11:6532282021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Hagel S, Bach F, Brenner T, Bracht H,
Brinkmann A, Annecke T, Hohn A, Weigand M, Michels G, Kluge S, et
al: Effect of therapeutic drug monitoring-based dose optimization
of piperacillin/tazobactam on sepsis-related organ dysfunction in
patients with sepsis: A randomized controlled trial. Intensive Care
Med. 48:311–321. 2022. View Article : Google Scholar : PubMed/NCBI
|