Ovarian cancer (OC) ranks third among all
gynecologic malignancies, is one of the three most diagnosed
malignancies in the female reproductive system and has the highest
mortality rate among gynecologic tumors (1). Due to the insidious nature of its
onset during the early stages, the majority of patients are
diagnosed with advanced-stage OC in the first instance, and at this
stage, the primary treatment methods, such as surgery,
radiotherapy, chemotherapy and immunotherapy, have well-established
side effects, including recurrence, metastasis and chemotherapy
resistance, and thus, OC is characterized by a high mortality rate
(2). As the incidence of OC is
increasing annually, with an increasing number of younger
individuals developing OC (3,4),
the need for methods and biomarkers to allow earlier detection and
more effective treatment options after diagnosis is becoming ever
more pertinent (5-8).
Ferroptosis, distinct from apoptosis, necrosis and
autophagy, is an iron-dependent form of programmed cell death
triggered by lipid peroxide (LPO) accumulation (9). While conventional cancer therapies
usually eliminate tumor cells by inducing cell death to generate
drug resistance, ferroptosis has become an increasingly researched
topic in cancer therapy given its role in regulating cell death
(10-12). Several studies have shown that
ferroptosis is associated with resistance to cancer treatment,
potentially allowing for the reversal of cancer treatment
resistance (13-15). Current research has shown that
neurological disorders, kidney damage, breast cancer, pancreatic
cancer and several other diseases are associated with ferroptosis
(16-19). Among these, ferroptosis is most
closely related to malignant tumors, and tumor cells may be notably
sensitive to ferroptosis (20).
Ferroptosis can regulate the development of OC through different
mechanisms or factors, thereby increasing the sensitivity of OC
cells to ferroptosis-targeting therapeutics, and thus, combatting
resistance to chemotherapy (21,22) and improving the efficacy of
chemotherapeutic drugs in the management of OC (23). Additionally, a related study has
demonstrated the pattern of immune infiltration and associated
genetic features of ferroptosis, which can hopefully be applied to
predict the prognosis of patients with OC (24). The combination of ferroptosis
with chemotherapy, nanotechnology, X-ray therapy and photodynamic
therapy has been demonstrated to improve therapeutic efficacy
(25), providing potential
targets and novel therapeutic directions for ferroptosis in the
treatment of OC.
Several studies have found that ferroptosis and ERS
share a common regulatory pathway and that the two can alter the
development of several diseases by interacting with each other
(33-36). However, to the best of our
knowledge, the link between them has not been investigated in
detail in studies on the pathogenesis of OC or related therapies.
Therefore, the present review discusses the mechanisms of
ferroptosis and ERS in OC and the potential relevance of both to
highlight novel strategies and potential targets for the treatment
of OC.
Ferroptosis is a mode of programmed cell death
characterized by excessive accumulation of iron, lipid
antioxidation and lipid peroxidation (37). Patients with OC frequently
exhibit resistance to chemotherapy, and a study has shown that this
is closely related to ferroptosis (38).
Iron is one of the essential trace elements in the
human body and serves an important role in human growth and
development, energy metabolism, and immune system functions
(39). Abnormalities in iron
metabolism affect redox reactions, gene regulation, enzymatic
reactions and DNA synthesis or repair (40). In the human body, iron has a
complex and elaborate circulatory mechanism to ensure its proper
distribution, utilization and storage to maintain appropriate and
non-toxic cellular iron levels (41). Iron in the body exists in two
forms, Fe2+ and Fe3+, and there are transport
carriers and modes for the different forms of iron. Daily intake of
dietary iron includes heme iron and non-heme iron (42). They arrive in the intestinal
lumen as Fe3+ and are reduced to Fe2+ by
duodenal cytochrome B to be absorbed; non-heme iron in the
intestine is absorbed via divalent metal transporter protein 1
(43). Heme iron is then
transported into the duodenal epithelium via heme carrier protein 1
and is absorbed, internalized, and broken down into Fe2+
and heme oxygenase-1 (HO-1) (44). Thereafter, iron can remain in the
enterocytes or enter the bloodstream from the basolateral membrane
of intestinal epithelial cells via membrane iron transport protein
1, while being oxidized by ferrous oxidase or ceruloplasmin to
produce Fe3+ (45).
Upon entering the bloodstream, plasma transferrin (TF) spreads the
captured Fe3+ throughout the cells of the body and is
used by various organs to synthesize specific iron-containing
components through TF receptor (TFR)-mediated holo-TF endocytosis
(46). For example, the liver
synthesizes hemosiderin, muscle tissue produces myoglobin and the
bone marrow produces red blood cells containing hemoglobin. Cells
promote iron uptake primarily through the TF and TFR systems, and
Fe3+ is reduced to Fe2+ by ferric oxide
reductase, most of which binds to ferritin to form storage iron,
and a small portion enters the cytoplasm to collectively form the
labile iron pool (47). Due to
the instability and high oxidizability of Fe2+, excess
iron ions catalyze the production of reactive oxygen species (ROS),
which promotes lipid peroxidation through the Fenton reaction
(48), thereby causing oxidative
damage to lipid membranes, proteins and DNA, and ultimately leading
to cell death (49). Among the
three main mechanisms of ferroptosis, excessive accumulation of
Fe2+ in the labile iron pool increases the sensitivity
of cells to ferroptosis and is the initial component responsible
for ferroptosis (47).
To the best of our knowledge, the initial signal for
ferroptosis has not yet been identified, but ferroptosis is closely
related to the levels of intracellular free iron content (50). Iron metabolism serves a key role
in the pathophysiology of OC, and the degree of intracellular iron
accumulation serves a decisive role in the course of OC (51). Concurrently, abnormalities in
iron metabolism, especially the acquisition and retention of excess
iron, contribute to tumorigenesis and tumor growth (52,53). Iron accumulation increases the
risk of diseases such as cancer and damage to tissues (54). Therefore, maintaining homeostasis
of intracellular iron ions is critical. According to Basuli et
al (55), OC-initiating
cells show high iron dependence. Increased iron efflux also
decreases the proliferation and invasion of OC-initiating cells,
and conversely increased iron uptake increases OC cell
proliferation and invasion. High-grade serous OC (HGSOC) typically
originates from the fallopian tube and is diagnosed at a later
stage, and it has been observed that the iron content of HGSOC is
higher than that of normal ovarian tissues and low-grade serous OC,
suggesting that there is a strong association between HGSOC and
iron metabolism (56). In
addition, the malignant transformation and metastasis of cancer
cells are closely associated with changes in cellular redox status
(57). The molecular damage
caused by the excessive and harmful ROS catalyzed by free iron is
often referred to as 'oxidative damage', and Bauckman et al
(58) reported that ROS can
transform normal ovarian tissue into cancerous tissue by promoting
the MAPK pathway. In addition, ROS can hydroxylate DNA residues to
generate the highly mutagenic 8-hydroxy-2′-deoxyguanosine, elevated
levels of which have been found to be associated with a poor
prognosis in patients with HGSOC (59,60). Iron polyporphyrin heme binds to
p53, interfering with p53-DNA interactions, leading to nuclear
export and cellular degradation of p53, and increased
susceptibility to HGSOC (61,62). Basuli et al (55) demonstrated that excess iron
simultaneously affects tumor cell proliferation, metabolism and
metastasis. Increasing the expression of ferroportin on cell
membranes (63), decreasing iron
intake (64), or decreasing the
concentration of TF (65) and
TFR in vivo (66) can
inhibit tumor growth. In addition, iron metabolism can contribute
to the development of OC by regulating hypoxia-inducible factor
(HIF). HIF-1α induces OC progression by inhibiting p53 function,
promoting IL-6 expression or being regulated by long non-coding
RNAs (67,68). Iron metabolism is also closely
related to resistance to chemotherapy, and solute carrier family 40
member 1 (SLC40A1), an iron metabolism-related gene, which is the
only known iron-exporting gene (69), serves a crucial role in
transporting iron from the intracellular environment to the
extracellular environment, and thus, the physiological expression
of SLC40A serves a vital role in the regulation of iron
homeostasis. SLC40A1-induced iron overload leads to cisplatin
resistance in OC (70).
Upregulation of SLC40A1 reduces cisplatin resistance by exporting
iron, decreasing the intracellular iron concentration and oxidative
stress. By contrast, an increase in the intracellular iron
concentration and enhanced oxidative stress induced by the
downregulation of SLC40A1 increase cisplatin resistance (70). Thus, modulating iron levels to
influence the redox system may be a potential strategy to reverse
drug resistance in OC therapy.
The iron-dependent nature of OC tumor-initiating
cells also makes them sensitive to drugs that induce ferroptosis,
as well as to iron chelators, providing a potential therapeutic
approach for the management of OC (71). Desferrioxamine (72), a natural iron chelator used in
the treatment of iron overload, has shown promising results in the
treatment of OC. Wang et al (72) assessed the effects of
desferrioxamine on OC cell lines and found that it not only
inhibited OC stem cells but also enhanced cisplatin efficacy,
improved resistance to chemotherapy and increased the length of
survival. There are also drugs that regulate iron metabolism in
several ways and may have effects on other biological processes.
For example, artemisinin is known for its anti-malarial,
anti-inflammatory and antitumor effects, and its compounds
(artesunate) can reduce cell proliferation and induce ROS
production in OC cells (73). In
addition, artesunate can activate the function of lysosomes,
promote the degradation of ferritin and lead to the release of iron
in lysosomes, and thereby mediate cell death (74). Regulators, including iron
uptake-related regulators (75),
iron storage-related regulators (76) and iron transport-related
regulators (77), influence the
pathological process of OC through the regulation of iron
metabolism levels.
Ferroptosis is an iron-dependent non-apoptotic cell
death mechanism caused by iron-dependent membrane lipid
peroxidation and massive accumulation of cellular ROS (78). Membrane lipids serve an important
role in the regulation of cell fate and lipid metabolism, which is
fundamental in determining the fate of ferroptosis (79), and is similarly critical for
ferroptosis execution.
Among various lipids, polyunsaturated fatty acids
(PUFAs) associated with several phospholipids such as
phosphatidylethanolamine and phosphatidylcholine are responsible
for inducing lipid peroxidation in ferroptosis. Phospholipids
containing PUFAs are susceptible to oxidation, but less oxidizable
saturated/monounsaturated fatty acids protect cells from
ferroptosis (80). Thus, the
enzymes and pathways that regulate the metabolism of PUFAs and
monounsaturated fatty acids, as well as the balance of PUFAs and
monounsaturated fatty acids in membrane phospholipids, can
influence cellular sensitivity to ferroptosis (81). The findings of the aforementioned
study provide a novel strategy for lipid peroxidation treatment of
OC ferroptosis. Since the membrane phospholipids of PUFAs that
drive ROS production catalyzed by iron ions interacting with PUFAs
are responsible for triggering lipid peroxidation leading to
cellular ferroptosis, and not free PUFA itself, the enzymes that
bind free PUFA to phospholipids serve a crucial role in ferroptosis
(81). Acyl-CoA synthetase
long-chain family member 4 is an essential component of ferroptosis
execution as shown by microarray analysis of ferroptosis-resistant
cell lines and using a genome-wide CRISPR-based genetic screening
system (82). Under the
catalytic action of Acyl-CoA synthetase long-chain family member 4,
free long-chain fatty acids are converted to acyl-CoA by linking
with CoA. Acyl-CoA inserts into membrane phospholipids and binds to
phosphatidylethanolamine to form PUFA phospholipids catalyzed by
lysophosphatidylcholine acyltransferase 3 (83). Lipid peroxidation, via two
primary mechanisms, non-enzymatic spontaneous oxidation and
enzyme-mediated lipid peroxidation (84), results in the production of
phospholipid hydroperoxides (PLOOHs), and if antioxidants do not
convert PLOOH to phospholipid hydroxides (PLOHs) in a timely
manner, the resulting accumulation of PLOOH leads to extensive
lipid peroxidation and activation of the antioxidant system,
directly inducing damage to cell membranes and ultimately leading
to cell dysfunction and ferroptosis (85). Non-enzymatic lipid peroxidation
(also known as lipid autoxidation) is a free radical-driven chain
reaction. Hydroxyl radicals (OH·) are generated through the
reaction of H2O2 with Fe2+, which
reacts with PUFAs in the plasma membrane in a Fenton reaction to
generate LPO, leading to ferroptosis (86,87). Enhanced accumulation of LPO is
therefore necessary to increase the efficiency of ferroptosis
(88), and OH· is the most
active ROS (89), and thus, it
also serves as an emerging cancer treatment target for the
management of OC via chemodynamic therapy.
H2O2 nano-enzymes developed by Sun et
al (90) using CoNi alloys
encapsulated with nitrogen-doped carbon nanotubes exhibited glucose
oxidase and lactate oxidase activities to effectively disrupt the
antioxidant defense system by catalyzing the formation of OH·,
increasing ROS content in the tumor microenvironment and damaging
the tumor cells, while depleting glutathione (GSH) to induce
ferroptosis of the cells. Liang et al (91) demonstrated that
polydopamine-mediated Michael addition combined with
Fe2+ depletion of GSH enhanced OH· accumulation and
ultimately resulted in the intracellular release of the
chemotherapeutic drug doxorubicin, thus inducing ferroptosis. In
addition, lipid peroxidation is tightly regulated by various
metabolic and signaling pathways, such as the cytochrome P450
oxidoreductase pathway, and iron-containing enzymes, including
lipoxygenase, also contribute to the process of lipid peroxidation
(92). Enzymatic lipid
peroxidation, conversely, is a process of direct oxidation of free
PUFAs into various types of lipid hydroperoxides catalyzed by
lipoxygenase (93,94). Among them, the arachidonic acid
lipoxygenase family regulates lipid peroxidation. It has been shown
that the binding of 5-lipoxygenase to microsomal GSH S-transferase
1 led to a decrease in lipid peroxidation and mediated ferroptosis
in cancer cells (95).
12-lipoxygenase, conversely, has been identified as a key factor in
p53-mediated ferroptosis under conditions of ROS-induced stress
(96), while the expression
levels of 15-lipoxygenase are associated with the spermine/spermine
N1-acetyltransferase 1 gene, a transcriptional target of p53
(97). Zhang et al
(98) demonstrated that
chemotherapeutic drugs for OC induced excessive lipid peroxidation
through ROS and initiated ovarian cell ferroptosis, thus leading to
ovarian cell death. A study (99) has demonstrated that p53 serves an
important role in the ferroptosis process. p53 exhibits a
bidirectional regulatory effect based on specific environmental
conditions. When lipid peroxidation levels are low, p53 inhibits
the occurrence of ferroptosis, promoting cell survival. However,
when excess lipid peroxidation persists, ferroptosis is induced
(99).
In summary, further studies on the effects of
various lipid metabolic pathways on lipid peroxidation and
ferroptosis from the perspective of chemotherapy-induced oxidative
stress and ferroptosis may help control ovarian damage and improve
the quality of life of patients with OC. All of them will provide a
deeper understanding of ferroptosis and therapeutic strategies for
OC.
Oxidative damage occurs due to an imbalance between
the cellular antioxidant system with the production of free
radicals and/or the neutralization or elimination of their
deleterious effects. ROS-mediated lipid peroxidation is a key step
to drive cellular ferroptosis, and inactivation of the antioxidant
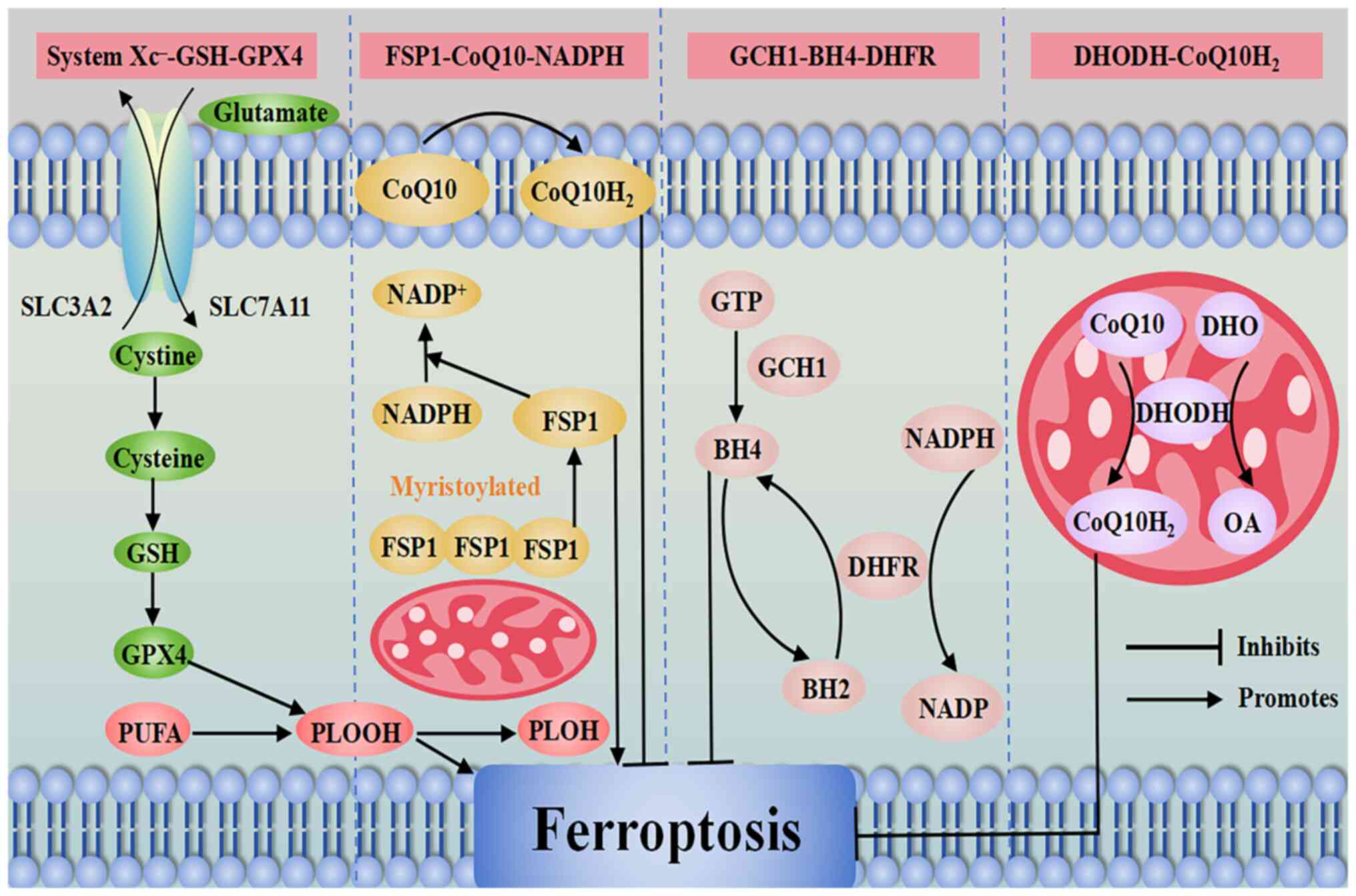
system is the primary cause of ferroptosis (100). At present, it has been shown
that the primary antioxidant systems regulating ferroptosis include
the System Xc−-GSH-GSH peroxidase 4 (GPX4) pathway, the
ferroptosis suppressor protein 1 (FSP1)-coenzyme Q10 (CoQ10)
pathway, the guanosine triphosphate cyclohydrolase 1
(GCH1)-tetrahydrobiopterin (BH4) pathway and the dihydroorotate
dehydrogenase (DHODH)-CoQ10H2 pathway. Among these, the
System Xc−-GSH-GPX4 pathway is the most fundamental
antioxidant system, which serves a key role in protecting cells
from ferroptosis (101). An
overview of the primary antioxidant systems regulating ferroptosis
is shown in Fig. 1.
Therefore, the FSP1-CoQ10-NADPH pathway may
complement and work with the System Xc−-GSH-GPX4 pathway
to inhibit lipid peroxidation in ferroptosis, providing a potential
therapeutic strategy for the treatment of OC.
A previous study has identified the GCH1-BH4-DHFR
pathway as an alternative compensatory mechanism for the System
Xc−-GSH-GPX4 pathway (120). GCH1 is the rate-limiting enzyme
for BH4 biosynthesis, which promotes ferroptosis via the
metabolites BH4 and dihydrobiopterin (BH2). BH4 acts as a free
radical trapping antioxidant that can be recycled by DHFR for redox
cycling, and BH4 has antioxidant degradation effects on
phospholipids containing two PUFA tails and prevents lipid
peroxidation and thus ferroptosis by inhibiting the production of
specific LPOs (120,121).
Through the direct trapping of antioxidant free
radicals and the production of CoQ10 (120), when GCH1 is upregulated, it
promotes BH4 production and abrogates the deleterious effects of
RSL3-induced cellular ferroptosis. Additionally, overexpression of
GCH1 has been shown to decrease the sensitivity of drug-resistant
cancer cells to ferroptosis, which in turn further ameliorated the
progression of ferroptosis in cancer cells through the regulation
of CoQ10 (122).
In addition, relevant studies have shown that BH4 is
involved in the synthesis of dopamine, nitric oxide synthase and
melatonin (123), whereas
exogenous dopamine or melatonin can inhibit ferroptosis (124). Several studies have shown that
nitric oxide can induce or inhibit ferroptosis in tumor cells
depending on environmental conditions (125-127). DHFR reduces BH2 through the
involvement of NADPH, thereby promoting BH4 synthesis. If DHFR is
inhibited, ferroptosis of tumor cells is promoted via the
synergistic action of GPX4 inhibitors (87).
Therefore, the GCH1-BH4-DHFR pathway serves a role
in regulating the balance between oxidative damage and antioxidant
defense during ferroptosis and interacts with the System
Xc−-GSH-GPX4 pathway and the FSP1-CoQ10-NADPH pathway in
a synergistic or complementary manner. Although other specific
mechanisms and potential therapeutic targets remain to be
determined, the identified pathways may serve as potential
therapeutic targets for overcoming resistance to chemotherapy in
OC.
When tumor cells are challenged by intrinsic factors
such as oncogenic activation, altered chromosome numbers or
enhanced secretory capacity, as well as by extrinsic factors such
as hypoxia, nutrient deprivation and acidosis, protein homeostasis
is altered, resulting in the accumulation of unfolded or misfolded
proteins in the lumen of the ER, activating the ERS and the UPR,
thus restoring homeostasis to the cell (32). However, when ERS is prolonged or
the stimulus is too strong, exceeding the tolerance threshold of
the UPR leads to cell death, which in turn causes the development
of cancer (130).
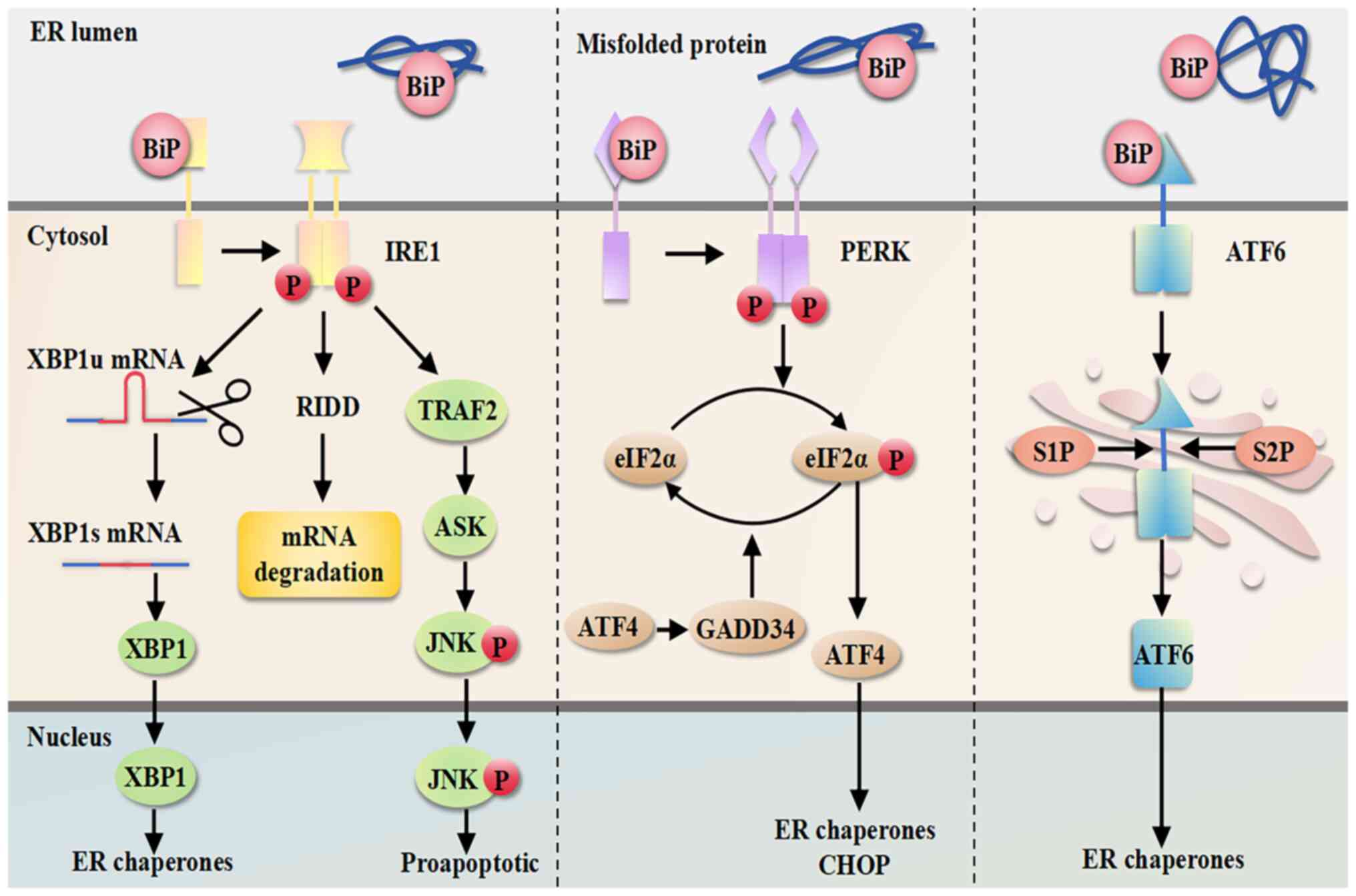
UPR is initiated by three major ERS sensors located
in the ER membrane, including inositol-requiring protein 1α
(IRE1α), protein kinase RNA-like ER kinase (PERK) and activating
transcription factor 6 (ATF6) (131). The ER-resident chaperone
binding immunoglobulin protein (BiP) acts as a master regulator of
the UPR by binding to and inactivating three ERS sensors, IRE1α,
PERK and ATF6, negatively regulating them and keeping them in an
inactive state. When misfolded proteins accumulate in the ER, they
bind to the inhibitory chaperone BiP and sequester it, which
activates the ERS sensors to initiate UPR signaling (132). An overview of the mechanisms of
ERS is shown in Fig. 2.
IRE1 consists of two isoforms, IRE1α and IRE1β;
IRE1β is primarily expressed in the respiratory and
gastrointestinal tracts, whereas IRE1α is more widely expressed
(133). The cytoplasmic tail of
IRE1α has two domains, a serine/threonine kinase structural domain
and a ribonuclease (RNase) structural domain, which work together
(134). The kinase of IRE1α is
activated upon binding to misfolded proteins and undergoes
dimerization/coupling and trans-autophosphorylation, leading to
ectopic activation of the structural domain of RNase (135). Under the catalysis of active
RNase, the intron containing 26 nucleotides is excised from the
mRNA encoding the homeostasis transcription factor X-box protein 1
(XBP1). Then, two mRNA fragments are cleaved by RNA splicing ligase
RNA 2′,3′-cyclic phosphate and 5′-OH ligase, thereby generating the
stable active transcription factor XBP1s (spliced form) (136,137). XBP1s (spliced form) is involved
in the expression of genes encoding ER membrane biogenesis, ER
protein folding, ER-associated degradation and multiple UPR targets
(138). C/EBP homologous
protein (CHOP), a major regulator of ERS-induced apoptosis, is
activated by activating transcription factor 4 (ATF4) through the
PERK-ATF4-CHOP pathway (139).
In addition, IRE1α promotes apoptosis by activating the apoptosis
signal-regulating kinase 1/JNK pathway, and by binding to tumor
necrosis factor receptor-associated factor 2 (140). In addition, regulated
IRE1-dependent decay is a novel UPR-regulated pathway that has been
identified to control cell fate under ERS. Activated RNase can
target other mRNAs and microRNAs (miRs) by regulating this pathway
(141).
PERK is a type I transmembrane protein with
similarities to IRE1, which has an ER lumenal dimerization
structural domain and a cytoplasmic kinase structural domain. The
tubulin dimerization structural domain of PERK has less sequence
similarity to IRE1 but is structurally and functionally similar to
the structural domain of IRE1. The cytoplasmic kinase structural
domain of PERK also undergoes trans-autophosphorylation in response
to ERS, but it differs from IRE1 in that PERK also phosphorylates
the eukaryotic translation initiation factor 2α (eIF2α) at serine
51, and phosphorylated eIF2α inhibits overall translation of
proteins, and thus, reduces the amount of proteins entering the
lumen of the ER (147,148). In addition, phosphorylation of
eIF2α alters the utilization efficiency of the AUG start codon
(149), which results in
preferential translation of the ATF4 mRNA (148).
ATF4 is a transcription factor that activates
downstream UPR target genes, such as the expression of growth
arrest DNA-damage 34 (GADD34), which induces CHOP expression
(148,150). CHOP promotes DNA damage,
inhibits cell proliferation and activates apoptosis by upregulating
the pro-apoptotic members of the B-cell lymphoma-2 family of
proteins (151). Therefore,
ATF4 serves an important role in ER-functional gene expression,
ERS-mediated ROS production and ERS-induced apoptosis. ATF4 can
also regulate the dephosphorylation of eIF2α through GADD34 to form
an inhibitory feedback loop to reverse PERK-mediated translational
decay (152). In addition, PERK
phosphorylates nuclear factor erythroid 2-related factor 2 (Nrf2),
thereby upregulating antioxidants to promote cellular antioxidation
(153). Overall, the PERK-eIF2α
pathway first mediates the promotion of cell survival during ERS
but shifts to the promotion of apoptosis when ERS is sustained and
maintains cellular homeostatic balance by activating ATF4 and NRF2.
Therefore, the PERK pathway is an attractive therapeutic target in
OC therapy.
ATF6 is a type II transmembrane protein with a
carboxy-terminal stress-sensing lumenal structural domain and an
amino-terminal bZip transcription factor structural domain
(154). ATF6 is transported to
the Golgi under conditions of ERS, where it is sequentially
hydrolyzed by serine protease site 1 and metalloprotease site 2
(S2P) proteins to release its amino-terminal transcription factor
structural domain, which, synergistically with XBP1, upregulates
genes involved in protein folding and ER amplification, as well as
genes involved in ER-associated degradation pathway components
(155). It has been shown that
ATF6 expression in OC tumor tissues is higher than that in normal
ovarian tissues (156), and
under irreversible ERS, it downregulates the levels of
anti-apoptotic proteins (157).
In addition, by regulating ATF6, the sensitivity of OC cells to
chemotherapeutic agents can be altered (158). However, the role of ATF6 in
ER-induced cell death and co-targeting of chemotherapeutic agents
to improve survival in OC still needs to be explored further.
With the gradual increase in interest in ferroptosis
and ERS, an increasing number of studies have shown that
ferroptosis and ERS have an important impact on OC and that there
is a close relationship between the two (35,159-161).
ERS also serves a crucial role in the development
and prognosis of OC. Studies have shown that activation of UPR
sensors, and thus, induction of ERS can lead to apoptosis of OC
cells (169), and regulation of
ERS-associated targets influences drug resistance in OC treatment
(170,171), highlighting potentially novel
targets for the management of OC. Zhang et al (172) developed promising medical aids
for the prognostic assessment of patients with epithelial OC by
establishing a risk classifier for differentially expressed genes
associated with ERS. Ma et al (173) utilized nanotechnology to
accurately and durably induce ERS through photodynamic responses,
thus inducing an antitumor effect in a mouse model of OC.
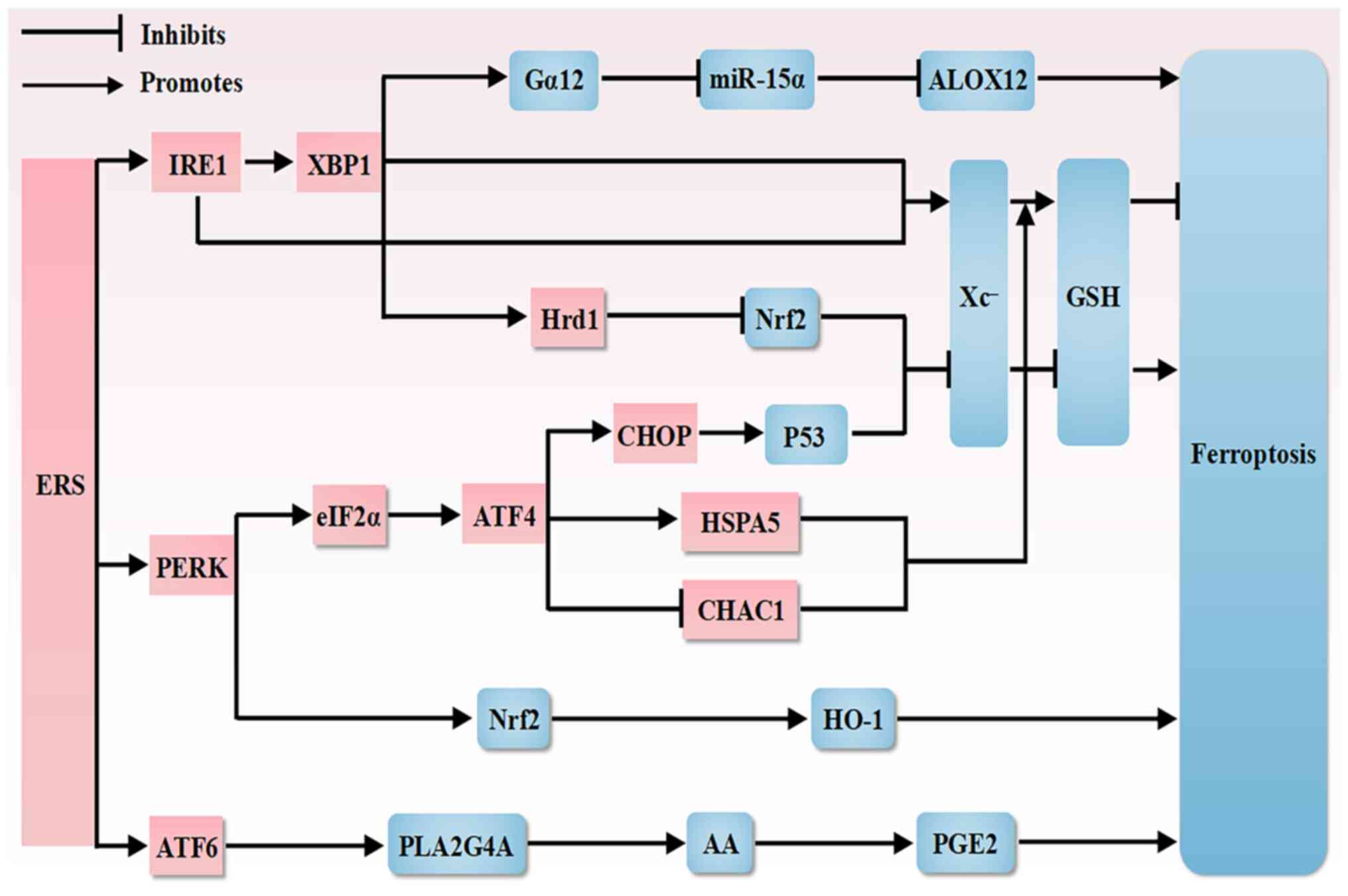
A growing body of research has shown a link between
ferroptosis and ERS, which share common pathways (174-176), and that ROS, a byproduct of
ERS, may exacerbate ferroptosis, while the ER is a key site for
lipid peroxidation during ferroptosis, and conversely ROS produced
during ferroptosis further exacerbate ERS. However, ferroptosis and
ERS have been discussed separately regarding OC, and, to the best
of our knowledge, there are no studies assessing their interactions
in OC. In the present review, the potential therapeutic targets for
OC treatment are examined based on a potential association between
ERS and ferroptosis in OC using the existing literature. An
overview of interactions between ferroptosis and ERS is shown in
Fig. 3 and an overview of
mechanisms associated with ferroptosis and ERS is shown in Table I.
Colorectal cancer is a common digestive malignancy
in which both surgery and chemotherapy exhibit limited therapeutic
efficacy (187). Tagitinin C, a
natural product, induces ERS production, which results in nuclear
translocation of Nrf2 and upregulation of HO-1. HO-1 is a
downstream effector of Nrf2, which leads to an increase in the pool
of unstable iron, and thus, promotes lipid peroxidation. This
increase in the pool of unstable iron together with erastin exerts
synergistic antitumor effects in inducing ferroptosis in colorectal
cancer cells. Thus, tagitinin C has been identified as a novel
ferroptosis inducer and potent sensitizer (188). Furthermore, in a prostate
cancer study, the mediation of arachidonic acid release and
prostaglandin E2 production via ATF6α-phospholipase A2 group IVA
was found to protect prostate cancer cells from ferroptosis
(189). Upregulation of Gα12
through the IRE1α-XBP1 pathway after ERS in hepatocytes thereby
promotes hepatic ferroptosis and exacerbates acute liver injury
through Rho associated coiled-coil containing protein kinase
1-mediated dysregulation of 12-lipoxygenase and miR-15α (190). Furthermore, in a study related
to diabetic nephropathy, ERS downregulated SLC7A11 expression
through the XBP1-E3 ligase-Nrf2 pathway, which decreased GSH
antioxidant levels and enhanced cellular sensitivity to
ferroptosis, thereby inducing ferroptosis, providing insights into
the potential mechanism of delaying epithelial-mesenchymal
transition in renal tubular epithelial cells (35).
In addition, several studies have demonstrated that
herbal components can also improve cellular ferroptosis by
modulating ERS. Esculin, a compound extracted from the cortex of
the willow bark, inhibits the development and progression of colon
cancer by activating the ERS PERK signaling pathway, and inducing
apoptosis and ferroptosis through the Nrf2/HO-1 and eIF2α/CHOP
pathways (191). Tanshinone
IIA, the primary active ingredient of the traditional Chinese
medicine Danshen, has been shown to exert antitumor effects
primarily in the ER-mediated ferroptosis signaling pathway,
downregulating the ferroptosis of tumor cells through the
PERK-ATF4-heat shock 70 kDa protein 5 pathway (192). Acetaminophen overdose is a
major cause of drug-induced liver injury. Salidroside inhibits ER
stress-mediated ferroptosis via the ATF4-cation transport regulator
homolog 1 axis by activating AMPK-SIRT1 signaling, and serves an
important role in alleviating acetaminophen-induced acute liver
injury (193). In a study on
glioma, DHA-induced ERS upregulated ATF4 via PERK, thereby
increasing the expression and activity of GPX4, thus inhibiting
DHA-induced lipid peroxidation and protecting glioma cells from
ferroptosis, highlighting a novel anticancer mechanism in glioma
(194).
It has been shown that ERS and ferroptosis are also
related in terms of tumor angiogenesis. In a glioma-related study,
elevated expression of ATF4, a transcription factor that activates
downstream UPR target genes, promoted angiogenesis by facilitating
tumor shaping of vascular structures in a System
Xc−-dependent manner, and erastin, a ferroptosis
inducer, and RSL3, a GPX4 inhibitor, could reduce ATF4-induced
angiogenesis (197).
Drug development research on natural compounds and
their derivatives is a popular research topic for cancer treatment.
Research on novel drugs also takes into consideration the side
effects and off-target effects, which can negatively affect the
quality of life of patients. Drugs with high specificity and
selectivity need to be developed to minimize off-target effects and
their potential toxicities (198). In OC, reducing off-target
effects during treatment can help improve patient outcomes and
prognosis (199). Studies have
shown that off-target effects are strongly linked to ferroptosis
and ERS. Dahlmanns et al (200) found that adjusting the
mechanism of ferroptosis improved tumor therapy, but that
interfering with the induction of ferroptosis produced an
off-target effect, and thus, a reduction in therapeutic efficacy.
Similarly, it has been shown that ERS may modulate off-target
effects in glioblastoma multiforme (201). Inhibitors of ROS, which are
closely related to ferroptosis and ERS, likewise have some degree
of influence on off-target effects (202). Recent advances in research on
coupled drug delivery systems (203), liposomes (204) and nanotechnology (205) in combination with bioactive
agents for the treatment of a wide range of diseases, including
cancer, may lead to improved efficacy through the localized and
precise delivery of drugs, thereby reducing off-target effects.
In summary, ERS interacts with ferroptosis in
several diseases through various pathways, regulatory proteins and
related factors to regulate disease development, highlighting
potential therapeutic targets and strategies for disease treatment
and prevention. However, the specific mechanisms by which ERS
interacts with ferroptosis in the development, treatment and
prevention of OC remain to be investigated.
Ferroptosis and ERS are both increasingly popular
topics in relation to cancer research, and studies have shown that
ferroptosis and ERS are related to the development and potential
treatment of gynecological tumors (13,20,32,206,207). OC, as the gynecological
malignant tumor with the highest mortality rate, has attracted
attention; however, there is no relevant research on the potential
mechanism of the interaction between ERS and ferroptosis in OC. In
the present review, the pathogenesis of ERS and ferroptosis in OC,
and the common pathways and related links between the two in liver,
lung and colorectal cancer, are discussed, with the aim of
providing novel strategies for the prevention, treatment and
prognosis of OC in the future.
There is also an association between the two, with
ERS inducing ferroptosis by causing Fe2+ accumulation
and lipid peroxidation through related pathways (195), and the ER, which contains more
than half of all the lipid bilayers in any given cell, being the
source of lipids for the majority of the cytosolic membranes, thus
being critical for the initiation of ferroptosis (216). Ferroptosis can maintain ER
homeostasis by signaling and regulating the degree of ERS.
Ferroptosis can also be positively regulated by inducing ERS
through various pathways.
However, ferroptosis and ERS have different and
complex mechanistic pathways, and several novel mechanisms,
pathways and potential targets are gradually being identified,
although it is likely that more remain to be uncovered. For
example, the detailed mechanisms of the four pathways associated
with lipid antioxidation, particularly the GCH1-BH4-DHFR pathway
and the DHODH-CoQ10H2 pathway in OC, still remain to be
explored. Oxidative stress in mitochondria is closely associated
with related processes in ferroptosis and ERS, and the mechanisms
involved in mitochondria are not fully understood at present.
Furthermore, the early onset of OC is insidious, and regarding
diagnostic accuracy, there is still a lack of research on the
difference in iron content between OC tissues of different stages
and normal ovarian tissues (55). Interfering with iron metabolism
during early OC may inhibit the further development of OC (104). The occurrence, progression and
treatment of OC is a complex process, and there is a complex
connection between ERS and ferroptosis. Whether the relevant
pathways present in other diseases have the same role in OC,
whether there are obvious differences in the roles in different
cells at different stages of OC, and whether they have the same
regulatory role in the different stages of OC occurrence,
development, and recurrence remain to be determined. Furthermore,
the prognostic prediction of OC still has major deficiencies and
lacks the support of clinical data. Although current studies on
various diseases have shown that the crosstalk between ferroptosis
and ERS has a related improvement effect on the diseases (34,217-219), there is still a lack of
relevant research regarding mechanisms for the crosstalk between
ERS and ferroptosis in terms of OC.
Based on the clearer understanding of potential
targets of ERS and ferroptosis, the role of the mechanistic
pathways in the process of OC, the impact of the crosstalk between
the two, and how to translate the results of research and
experiments into clinical practice is an important and challenging
aspect. In terms of clinical index detection, the aforementioned
mechanism and modern scientific technologies such as nano-molecular
materials, MRI and X-ray may be combined to detect the pathological
factors of OC before the onset of the disease, and analyze the
efficacy or assess prognosis during and following treatment of the
disease. Assessment of iron metabolism levels during relevant
clinical testing may allow for earlier detection of OC, potentially
improving treatment options and patient outcomes. The direction of
these studies, which await further translation to the clinic, may
serve as a means of assessing patients to improve prevention, and
assess efficacy and recurrence. In terms of drug development, for
the commonly used chemotherapeutic regimens, there are obvious side
effects alongside drug resistance. Thus, additional screening of
safe and effective therapeutic drugs is required. Whether
ferroptosis inhibitors and inducers of ERS can be used clinically
or as references for future drug development remains to be
explored. In addition, several studies (14,80,192,220) have found that traditional
Chinese herbs are effective in regulating the crosstalk between
ferroptosis and ERS-related mechanisms in relation to OC, thus the
dosage and clinical safety of these options should be investigated.
Whether the combination of various drugs, chemotherapeutic
treatments and modern technologies will have a synergistic effect
or inhibit resistance to chemotherapy and side effects also needs
to be tested.
The present review highlights the potential
therapeutic promise of crosstalk between ferroptosis and ERS in OC,
suggesting that the intricate interactions merit deeper
exploration. In addition, several potential treatments have shown
therapeutic potential and efficacy in combination with conventional
chemotherapy for the treatment of OC in preclinical testing, and
future studies are required to determine the detailed molecular
mechanisms of these compounds and to assess their efficacy in the
clinic. To address these unknown challenges, it is necessary to
draw on the results of existing research to help understand the
focus and direction of the research. After clarifying the mechanism
of each target and pathway, it is necessary to screen for important
links or representative factors and more precise biomarkers. For
drug development, a large amount of clinical data is required.
Overall, understanding ferroptosis and ERS, and the
potential crosstalk between them, may provide novel therapeutic
approaches for the management of OC.
Not applicable.
JY wrote and revised the manuscript. YW revised the
manuscript. FL revised the manuscript. YZ and FH conceived the
topic of study. Data authentication is not applicable. All authors
have read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present review was supported by funding from the National
Natural Science Foundation of China (grant no. 82274566).
|
1
|
Jiang C, Shen C, Ni M, Huang L, Hu H, Dai
Q, Zhao H and Zhu Z: Molecular mechanisms of cisplatin resistance
in ovarian cancer. Genes Dis. 11:1010632023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maioru OV, Radoi VE, Coman MC, Hotinceanu
IA, Dan A, Eftenoiu AE, Burtavel LM, Bohiltea LC and Severin EM:
Developments in genetics: better management of ovarian cancer
patients. Int J Mol Sci. 24:159872023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ekmann-Gade AW, Høgdall CK, Seibæk L, Noer
MC, Fagö-Olsen CL and Schnack TH: Incidence, treatment, and
survival trends in older versus younger women with epithelial
ovarian cancer from 2005 to 2018: A nationwide Danish study.
Gynecol Oncol. 164:120–128. 2022. View Article : Google Scholar
|
|
4
|
Mallen A, Todd S, Robertson SE, Kim J,
Sehovic M, Wenham RM, Extermann M and Chon HS: Impact of age,
comorbidity, and treatment characteristics on survival in older
women with advanced high grade epithelial ovarian cancer. Gynecol
Oncol. 161:693–699. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lumish MA, Kohn EC and Tew WP: Top
advances of the year: Ovarian cancer. Cancer. 130:837–845. 2024.
View Article : Google Scholar
|
|
6
|
Wang A, Wang Y, Du C, Yang H, Wang Z, Jin
C and Hamblin MR: Pyroptosis and the tumor immune microenvironment:
A new battlefield in ovarian cancer treatment. Biochim Biophys Acta
Rev Cancer. 1879:1890582024. View Article : Google Scholar
|
|
7
|
Wong RW and Cheung ANY: Predictive and
prognostic biomarkers in female genital tract tumours: An update
highlighting their clinical relevance and practical issues.
Pathology. 56:214–227. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Liu L, Jin X and Yu Y: Efficacy
and safety of mirvetuximab soravtansine in recurrent ovarian cancer
with FRa positive expression: A systematic review and
meta-analysis. Crit Rev Oncol Hematol. 194:1042302024. View Article : Google Scholar
|
|
9
|
Zhang C, Liu X, Jin S, Chen Y and Guo R:
Ferroptosis in cancer therapy: A novel approach to reversing drug
resistance. Mol Cancer. 21:472022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang N, Chen M, Wu M, Liao Y, Xia Q, Cai
Z, He C, Tang Q, Zhou Y, Zhao L, et al: High-adhesion ovarian
cancer cell resistance to ferroptosis: The activation of NRF2/FSP1
pathway by junctional adhesion molecule JAM3. Free Radic Biol Med.
228:1–13. 2025. View Article : Google Scholar
|
|
11
|
Teng K, Ma H, Gai P, Zhao X and Qi G:
SPHK1 enhances olaparib resistance in ovarian cancer through the
NFκB/NRF2/ferroptosis pathway. Cell Death Discov. 11:292025.
View Article : Google Scholar
|
|
12
|
Yao Y, Wang B, Jiang Y, Guo H and Li Y:
The mechanisms crosstalk and therapeutic opportunities between
ferroptosis and ovary diseases. Front Endocrinol (Lausanne).
14:11940892023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kapper C, Oppelt P, Arbeithuber B, Gyunesh
AA, Vilusic I, Stelzl P and Rezk-Füreder M: Targeting ferroptosis
in ovarian cancer: Novel strategies to overcome chemotherapy
resistance. Life Sci. 349:1227202024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo W, Wang W, Lei F, Zheng R, Zhao X, Gu
Y, Yang M, Tong Y and Wang Y: Angelica sinensis polysaccharide
combined with cisplatin reverses cisplatin resistance of ovarian
cancer by inducing ferroptosis via regulating GPX4. Biomed
Pharmacother. 175:1166802024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ni M, Zhou J, Zhu Z, Xu Q, Yin Z, Wang Y,
Zheng Z and Zhao H: Shikonin and cisplatin synergistically overcome
cisplatin resistance of ovarian cancer by inducing ferroptosis via
upregulation of HMOX1 to promote Fe2+ accumulation.
Phytomedicine. 112:1547012023. View Article : Google Scholar
|
|
16
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Liu J, Wu S, Xiao J and Zhang Z:
Ferroptosis: Opening up potential targets for gastric cancer
treatment. Mol Cell Biochem. 479:2863–2874. 2024. View Article : Google Scholar
|
|
18
|
Tang D, Kroemer G and Kang R: Ferroptosis
in hepatocellular carcinoma: From bench to bedside. Hepatology.
80:721–739. 2024. View Article : Google Scholar
|
|
19
|
Qiu X, Bi Q, Wu J, Sun Z and Wang W: Role
of ferroptosis in fibrosis: From mechanism to potential therapy.
Chin Med J (Engl). 137:806–817. 2024. View Article : Google Scholar
|
|
20
|
Hushmandi K, Klionsky DJ, Aref AR, Bonyadi
M, Reiter RJ, Nabavi N, Salimimoghadam S and Saadat SH: Ferroptosis
contributes to the progression of female-specific neoplasms, from
breast cancer to gynecological malignancies in a manner regulated
by non-coding RNAs: Mechanistic implications. Noncoding RNA Res.
9:1159–1177. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Y, Jia C, Liu W, Zhan W, Chen Y, Lu J,
Bao Y, Wang S, Yu C, Zheng L, et al: Sodium citrate targeting
Ca2+/CAMKK2 pathway exhibits anti-tumor activity through
inducing apoptosis and ferroptosis in ovarian cancer. J Adv Res.
65:89–104. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao L, Yang H, Wang Y, Yang S, Jiang Q,
Tan J, Zhao X and Zi D: STUB1 suppresses paclitaxel resistance in
ovarian cancer through mediating HOXB3 ubiquitination to inhibit
PARK7 expression. Commun Biol. 7:14392024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Chen M, Huang D, Li Z, Chen Y, Huang
J, Chen Y, Zhou Z and Yu Z: Inhibition of Selenoprotein I promotes
ferroptosis and reverses resistance to platinum chemotherapy by
impairing Akt phosphorylation in ovarian cancer. MedComm (2020).
5:e700332024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang B, Guo B, Kong H, Yang L, Yan H, Liu
J, Zhou Y, An R and Wang F: Decoding the ferroptosis-related gene
signatures and immune infiltration patterns in ovarian cancer:
Bioinformatic prediction integrated with experimental validation. J
Inflamm Res. 17:10333–10346. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li G, Shi S, Tan J, He L, Liu Q, Fang F,
Fu H, Zhong M, Mai Z, Sun R, et al: Highly efficient synergistic
chemotherapy and magnetic resonance imaging for targeted ovarian
cancer therapy using hyaluronic acid-coated coordination polymer
nanoparticles. Adv Sci (Weinh). 11:e23094642024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hetz C, Chevet E and Oakes SA:
Proteostasis control by the unfolded protein response. Nat Cell
Biol. 17:829–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sims SG, Cisney RN, Lipscomb MM and Meares
GP: The role of endoplasmic reticulum stress in astrocytes. Glia.
70:5–19. 2022. View Article : Google Scholar :
|
|
28
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren J, Bi Y, Sowers JR, Hetz C and Zhang
Y: Endoplasmic reticulum stress and unfolded protein response in
cardiovascular diseases. Nat Rev Cardiol. 18:499–521. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar
|
|
31
|
Song M and Cubillos-Ruiz JR: Endoplasmic
reticulum stress responses in intratumoral immune cells:
Implications for cancer immunotherapy. Trends Immunol. 40:128–141.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan T, Ma X, Guo L and Lu R: Targeting
endoplasmic reticulum stress signaling in ovarian cancer therapy.
Cancer Biol Med. 20:748–764. 2023.PubMed/NCBI
|
|
33
|
Wang L, Ma X, Zhou L, Luo M, Lu Y, Wang Y,
Zheng P, Liu H, Liu X, Liu W and Wei S: Dual-targeting TrxR-EGFR
Alkynyl-Au(I) gefitinib complex induces ferroptosis in
gefitinib-resistant lung cancer via degradation of GPX4. J Med
Chem. 68:5275–5291. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhong P, Li L, Feng X, Teng C, Cai W,
Zheng W, Wei J, Li X, He Y, Chen B, et al: Neuronal ferroptosis and
ferroptosismediated endoplasmic reticulum stress: Implications in
cognitive dysfunction induced by chronic intermittent hypoxia in
mice. Int Immunopharmacol. 138:1125792024. View Article : Google Scholar
|
|
35
|
Liu Z, Nan P, Gong Y, Tian L, Zheng Y and
Wu Z: Endoplasmic reticulum stress-triggered ferroptosis via the
XBP1-Hrd1-Nrf2 pathway induces EMT progression in diabetic
nephropathy. Biomed Pharmacother. 164:1148972023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song W, Zhang W, Yue L and Lin W:
Revealing the effects of endoplasmic reticulum stress on
ferroptosis by two-channel real-time imaging of pH and viscosity.
Anal Chem. 94:6557–6565. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye R, Mao YM, Fei YR, Shang Y, Zhang T,
Zhang ZZ, Liu YL, Li JY, Chen SL and He YB: Targeting ferroptosis
for the treatment of female reproductive system disorders. J Mol
Med (Berl). 103:381–402. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muckenthaler MU, Rivella S, Hentze MW and
Galy B: A red carpet for iron metabolism. Cell. 168:344–361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Galy B, Conrad M and Muckenthaler M:
Mechanisms controlling cellular and systemic iron homeostasis. Nat
Rev Mol Cell Biol. 25:133–155. 2024. View Article : Google Scholar
|
|
41
|
Anderson GJ and Frazer DM: Current
understanding of iron homeostasis. Am J Clin Nutr. 106(Suppl 6):
1559S–1566S. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gulec S, Anderson GJ and Collins JF:
Mechanistic and regulatory aspects of intestinal iron absorption.
Am J Physiol Gastrointest Liver Physiol. 307:G397–G409. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Montalbetti N, Simonin A, Kovacs G and
Hediger MA: Mammalian iron transporters: Families SLC11 and SLC40.
Mol Aspects Med. 34:270–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li H, Wang D, Wu H, Shen H, Lv D, Zhang Y,
Lu H, Yang J, Tang Y and Li M: SLC46A1 contributes to hepatic iron
metabolism by importing heme in hepatocytes. Metabolism.
110:1543062020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mayneris-Perxachs J, Moreno-Navarrete JM
and Fernández Real JM: The role of iron in host-microbiota
crosstalk and its effects on systemic glucose metabolism. Nat Rev
Endocrinol. 18:683–698. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Seyoum Y, Baye K and Humblot C: Iron
homeostasis in host and gut bacteria-a complex interrelationship.
Gut Microbes. 13:1–19. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao H, Tang C, Wang M, Zhao H and Zhu Y:
Ferroptosis as an emerging target in rheumatoid arthritis. Front
Immunol. 14:12608392023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kajarabille N and Latunde-Dada GO:
Programmed cell-death by ferroptosis: Antioxidants as mitigators.
Int J Mol Sci. 20:49682019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang L, Rao J, Liu X, Wang X, Wang C, Fu
S and Xiao J: Attenuation of sepsis-induced acute kidney injury by
exogenous H2S via inhibition of ferroptosis. Molecules.
28:47702023. View Article : Google Scholar
|
|
51
|
Deng M, Tang F, Chang X, Zhang Y, Liu P,
Ji X, Zhang Y, Yang R, Jiang J, He J and Miao J: A targetable
OSGIN1-AMPK-SLC2A3 axis controls the vulnerability of ovarian
cancer to ferroptosis. NPJ Precis Oncol. 9:152025. View Article : Google Scholar
|
|
52
|
Torti SV and Torti FM: Iron and cancer:
more ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Torti SV and Torti FM: Ironing out cancer.
Cancer Res. 71:1511–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Toyokuni S: The origin and future of
oxidative stress pathology: From the recognition of carcinogenesis
as an iron addiction with ferroptosis-resistance to non-thermal
plasma therapy. Pathol Int. 66:245–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Basuli D, Tesfay L, Deng Z, Paul B,
Yamamoto Y, Ning G, Xian W, McKeon F, Lynch M, Crum CP, et al: Iron
addiction: A novel therapeutic target in ovarian cancer. Oncogene.
36:4089–4099. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen Y, Liao X, Jing P, Hu L, Yang Z, Yao
Y, Liao C and Zhang S: Linoleic acid-glucosamine hybrid for
endogenous iron-activated ferroptosis therapy in high-grade serous
ovarian cancer. Mol Pharm. 19:3187–3198. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bauckman K, Haller E, Taran N, Rockfield
S, Ruiz-Rivera A and Nanjundan M: Iron alters cell survival in a
mitochondria-dependent pathway in ovarian cancer cells. Biochem J.
466:401–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xu X, Wang Y, Guo W, Zhou Y, Lv C, Chen X
and Liu K: The significance of the alteration of 8-OHdG in serous
ovarian carcinoma. J Ovarian Res. 6:742013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Valavanidis A, Vlachogianni T and Fiotakis
C: 8-Hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of
oxidative stress and carcinogenesis. J Environ Sci Health C Environ
Carcinog Ecotoxicol Rev. 27:120–139. 2009.PubMed/NCBI
|
|
61
|
Prat J, D'Angelo E and Espinosa I: Ovarian
carcinomas: At least five different diseases with distinct
histological features and molecular genetics. Hum Pathol. 80:11–27.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shen J, Sheng X, Chang Z, Wu Q, Wang S,
Xuan Z, Li D, Wu Y, Shang Y, Kong X, et al: Iron metabolism
regulates p53 signaling through direct heme-p53 interaction and
modulation of p53 localization, stability, and function. Cell Rep.
7:180–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Deng Z, Manz DH, Torti SV and Torti FM:
Effects of ferroportin-mediated iron depletion in cells
representative of different histological subtypes of prostate
cancer. Antioxid Redox Signal. 30:1043–1061. 2019. View Article : Google Scholar :
|
|
64
|
Hann HW, Stahlhut MW and Blumberg BS: Iron
nutrition and tumor growth: Decreased tumor growth in
iron-deficient mice. Cancer Res. 48:4168–4170. 1988.PubMed/NCBI
|
|
65
|
White S, Taetle R, Seligman PA, Rutherford
M and Trowbridge IS: Combinations of anti-transferrin receptor
monoclonal antibodies inhibit human tumor cell growth in vitro and
in vivo: Evidence for synergistic antiproliferative effects. Cancer
Res. 50:6295–6301. 1990.PubMed/NCBI
|
|
66
|
Sandoval-Acuña C, Torrealba N, Tomkova V,
Jadhav SB, Blazkova K, Merta L, Lettlova S, Adamcová MK, Rosel D,
Brábek J, et al: Targeting mitochondrial iron metabolism suppresses
tumor growth and metastasis by inducing mitochondrial dysfunction
and mitophagy. Cancer Res. 81:2289–2303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Moon EJ, Mello SS, Li CG, Chi JT, Thakkar
K, Kirkland JG, Lagory EL, Lee IJ, Diep AN, Miao Y, et al: The HIF
target MAFF promotes tumor invasion and metastasis through IL11 and
STAT3 signaling. Nat Commun. 12:43082021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang X, Du ZW, Xu TM, Wang XJ, Li W, Gao
JL, Li J and Zhu H: HIF-1α is a rational target for future ovarian
cancer therapies. Front Oncol. 11:7851112021. View Article : Google Scholar
|
|
69
|
Benyamin B, Esko T, Ried JS, Radhakrishnan
A, Vermeulen SH, Traglia M, Gögele M, Anderson D, Broer L, Podmore
C, et al: Novel loci affecting iron homeostasis and their effects
in individuals at risk for hemochromatosis. Nat Commun. 5:49262014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu J, Bao L, Zhang Z and Yi X: Nrf2
induces cisplatin resistance via suppressing the iron export
related gene SLC40A1 in ovarian cancer cells. Oncotarget.
8:93502–93515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Szymonik J, Wala K, Górnicki T, Saczko J,
Pencakowski B and Kulbacka J: The impact of iron chelators on the
biology of cancer stem cells. Int J Mol Sci. 23:892021. View Article : Google Scholar
|
|
72
|
Wang L, Li X, Mu Y, Lu C, Tang S, Lu K,
Qiu X, Wei A, Cheng Y and Wei W: The iron chelator desferrioxamine
synergizes with chemotherapy for cancer treatment. J Trace Elem Med
Biol. 56:131–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Greenshields AL, Shepherd TG and Hoskin
DW: Contribution of reactive oxygen species to ovarian cancer cell
growth arrest and killing by the anti-malarial drug artesunate. Mol
Carcinog. 56:75–93. 2017. View Article : Google Scholar
|
|
74
|
Yang ND, Tan SH, Ng S, Shi Y, Zhou J, Tan
KS, Wong WS and Shen HM: Artesunate induces cell death in human
cancer cells via enhancing lysosomal function and lysosomal
degradation of ferritin. J Biol Chem. 289:33425–33441. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cheng Y, Qu W, Li J, Jia B, Song Y, Wang
L, Rui T, Li Q and Luo C: Ferristatin II, an iron uptake inhibitor,
exerts neuroprotection against traumatic brain injury via
suppressing ferroptosis. ACS Chem Neurosci. 13:664–675. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang Y, He F, Hu W, Sun J, Zhao H, Cheng
Y, Tang Z, He J, Wang X, Liu T, et al: Bortezomib elevates
intracellular free Fe2+ by enhancing NCOA4-mediated
ferritinophagy and synergizes with RSL-3 to inhibit multiple
myeloma cells. Ann Hematol. 103:3627–3637. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mortensen MS, Ruiz J and Watts JL:
Polyunsaturated fatty acids drive lipid peroxidation during
ferroptosis. Cells. 12:8042023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu S, Yang X, Zheng S, Chen C, Qi L, Xu X
and Zhang D: Research progress on the use of traditional Chinese
medicine to treat diseases by regulating ferroptosis. Genes Dis.
12:1014512024. View Article : Google Scholar
|
|
81
|
Rodencal J and Dixon SJ: A tale of two
lipids: Lipid unsaturation commands ferroptosis sensitivity.
Proteomics. 23:e21003082023. View Article : Google Scholar
|
|
82
|
Kagan VE, Mao G, Qu F, Angeli JP, Doll S,
Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al: Oxidized
arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem
Biol. 13:81–90. 2017. View Article : Google Scholar
|
|
83
|
Forcina GC and Dixon SJ: GPX4 at the
crossroads of lipid homeostasis and ferroptosis. Proteomics.
19:e18003112019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yin H, Xu L and Porter NA: Free radical
lipid peroxidation: Mechanisms and analysis. Chem Rev.
111:5944–5972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar
|
|
87
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen J, Duan Z, Deng L, Li L, Li Q, Qu J,
Li X and Liu R: Cell membrane-targeting type I/II photodynamic
therapy combination with FSP1 inhibition for ferroptosis-enhanced
photodynamic immunotherapy. Adv Healthc Mater. 13:e23044362024.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang Q, Ji H, Hao Y, Jia D, Ma H, Song C,
Qi H, Li Z and Zhang C: Illumination of hydroxyl radical generated
in cells during ferroptosis, Arabidopsis thaliana, and mice using a
new turn-on near-infrared fluorescence probe. Anal Chem.
96:20189–20196. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sun M, Chen Q, Ren Y, Zhuo Y, Xu S, Rao H,
Wu D, Feng B and Wang Y: CoNiCoNC tumor therapy by two-ways
producing H2O2 ferroptosis. J to aggravate
energy metabolism, chemokinetics, and Colloid Interface Sci.
678:925–937. 2025. View Article : Google Scholar
|
|
91
|
Liang KA, Chih HY, Liu IJ, Yeh NT, Hsu TC,
Chin HY, Tzang BS and Chiang WH: Tumor-targeted delivery of
hyaluronic acid/polydopamine-coated Fe2+-doped
nano-scaled metal-organic frameworks with doxorubicin payload for
glutathione depletion-amplified chemodynamic-chemo cancer therapy.
J Colloid Interface Sci. 677:400–415. 2025. View Article : Google Scholar
|
|
92
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Foret MK, Lincoln R, Do Carmo S, Cuello AC
and Cosa G: Connecting the 'Dots': From free radical lipid
autoxidation to cell pathology and disease. Chem Rev.
120:12757–12787. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stoyanovsky DA, Tyurina YY, Shrivastava I,
Bahar I, Tyurin VA, Protchenko O, Jadhav S, Bolevich SB, Kozlov AV,
Vladimirov YA, et al: Iron catalysis of lipid peroxidation in
ferroptosis: Regulated enzymatic or random free radical reaction?
Free Radic Biol Med. 133:153–161. 2019. View Article : Google Scholar :
|
|
95
|
Kuang F, Liu J, Xie Y, Tang D and Kang R:
MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic
cancer cells. Cell Chem Biol. 28:765–775.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chu B, Kon N, Chen D, Li T, Liu T, Jiang
L, Song S, Tavana O and Gu W: ALOX12 is required for p53-mediated
tumour suppression through a distinct ferroptosis pathway. Nat Cell
Biol. 21:579–591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ou Y, Wang SJ, Li D, Chu B and Gu W:
Activation of SAT1 engages polyamine metabolism with p53-mediated
ferroptotic responses. Proc Natl Acad Sci USA. 113:E6806–E6812.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang S, Liu Q, Chang M, Pan Y, Yahaya BH,
Liu Y and Lin J: Chemotherapy impairs ovarian function through
excessive ROS-induced ferroptosis. Cell Death Dis. 14:3402023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xu R, Wang W and Zhang W: Ferroptosis and
the bidirectional regulatory factor p53. Cell Death Discov.
9:1972023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kuang F, Liu J, Tang D and Kang R:
Oxidative damage and antioxidant defense in ferroptosis. Front Cell
Dev Biol. 8:5865782020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu Y, Lu S, Wu LL, Yang L, Yang L and
Wang J: The diversified role of mitochondria in ferroptosis in
cancer. Cell Death Dis. 14:5192023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lewerenz J, Hewett SJ, Huang Y, Lambros M,
Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M,
et al: The cystine/glutamate antiporter system x(c)(-) in health
and disease: From molecular mechanisms to novel therapeutic
opportunities. Antioxid Redox Signal. 18:522–555. 2013. View Article : Google Scholar :
|
|
103
|
Hu S, Chu Y, Zhou X and Wang X: Recent
advances of ferroptosis in tumor: From biological function to
clinical application. Biomed Pharmacother. 166:1154192023.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li D, Zhang M and Chao H: Significance of
glutathione peroxidase 4 and intracellular iron level in ovarian
cancer cells-'utilization' of ferroptosis mechanism. Inflamm Res.
70:1177–1189. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sun X, Niu X, Chen R, He W, Chen D, Kang R
and Tang D: Metallothionein-1G facilitates sorafenib resistance
through inhibition of ferroptosis. Hepatology. 64:488–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Guan J, Lo M, Dockery P, Mahon S, Karp CM,
Buckley AR, Lam S, Gout PW and Wang YZ: The xc-cystine/glutamate
antiporter as a potential therapeutic target for small-cell lung
cancer: Use of sulfasalazine. Cancer Chemother Pharmacol.
64:463–472. 2009. View Article : Google Scholar
|
|
108
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yuan J, Wang J, Song M, Zhao Y, Shi Y and
Zhao L: Brain-targeting biomimetic disguised manganese dioxide
nanoparticles via hybridization of tumor cell membrane and bacteria
vesicles for synergistic chemotherapy/chemodynamic therapy of
glioma. J Colloid Interface Sci. 676:378–395. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Luo Y, Liu X, Chen Y, Tang Q, He C, Ding
X, Hu J, Cai Z, Li X, Qiao H and Zou Z: Targeting PAX8 sensitizes
ovarian cancer cells to ferroptosis by inhibiting glutathione
synthesis. Apoptosis. 29:1499–1514. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG and
Gao LC: System Xc -/GSH/GPX4 axis: An important
antioxidant system for the ferroptosis in drug-resistant solid
tumor therapy. Front Pharmacol. 13:9102922022. View Article : Google Scholar
|
|
112
|
Okuno S, Sato H, Kuriyama-Matsumura K,
Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T and Bannai
S: Role of cystine transport in intracellular glutathione level and
cisplatin resistance in human ovarian cancer cell lines. Br J
Cancer. 88:951–956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang W, Kryczek I, Dostál L, Lin H, Tan L,
Zhao L, Lu F, Wei S, Maj T, Peng D, et al: Effector T cells
abrogate stroma-mediated chemoresistance in ovarian cancer. Cell.
165:1092–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tesfay L, Paul BT, Konstorum A, Deng Z,
Cox AO, Lee J, Furdui CM, Hegde P, Torti FM and Torti SV:
Stearoyl-CoA desaturase 1 protects ovarian cancer cells from
ferroptotic cell death. Cancer Res. 79:5355–5366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Shimada K, Skouta R, Kaplan A, Yang WS,
Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ and
Stockwell BR: Global survey of cell death mechanisms reveals
metabolic regulation of ferroptosis. Nat Chem Biol. 12:497–503.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yang N, Pan X, Zhou X, Liu Z, Yang J,
Zhang J, Jia Z and Shen Q: Biomimetic nanoarchitectonics with
chitosan nanogels for collaborative induction of ferroptosis and
anticancer immunity for cancer therapy. Adv Healthc Mater.
13:e23027522024. View Article : Google Scholar
|
|
119
|
Liu MR, Shi C, Song QY, Kang MJ, Jiang X,
Liu H and Pei DS: Sorafenib induces ferroptosis by promoting
TRIM54-mediated FSP1 ubiquitination and degradation in
hepatocellular carcinoma. Hepatol Commun. 7:e02462023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ge A, Xiang W, Li Y, Zhao D, Chen J, Daga
P, Dai CC, Yang K, Yan Y, Hao M, et al: Broadening horizons: The
multifaceted role of ferroptosis in breast cancer. Front Immunol.
15:14557412024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang M, Liu J, Yu W, Shao J, Bao Y, Jin M,
Huang Q and Huang G: Gambogenic acid suppresses malignant
progression of non-small cell lung cancer via GCH1-mediated
ferroptosis. Pharmaceuticals (Basel). 18:3742025. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Werner ER, Blau N and Thöny B:
Tetrahydrobiopterin: Biochemistry and pathophysiology. Biochem J.
438:397–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
NaveenKumar SK, Hemshekhar M, Kemparaju K
and Girish KS: Hemin-induced platelet activation and ferroptosis is
mediated through ROS-driven proteasomal activity and inflammasome
activation: Protection by melatonin. Biochim Biophys Acta Mol Basis
Dis. 1865:2303–2316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Homma T, Kobayashi S, Conrad M, Konno H,
Yokoyama C and Fujii J: Nitric oxide protects against ferroptosis
by aborting the lipid peroxidation chain reaction. Nitric Oxide.
115:34–43. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jiang L, Zheng H, Lyu Q, Hayashi S, Sato
K, Sekido Y, Nakamura K, Tanaka H, Ishikawa K, Kajiyama H, et al:
Lysosomal nitric oxide determines transition from autophagy to
ferroptosis after exposure to plasma-activated Ringer's lactate.
Redox Biol. 43:1019892021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Deng G, Li Y, Ma S, Gao Z, Zeng T, Chen L,
Ye H, Yang M, Shi H, Yao X, et al: Caveolin-1 dictates ferroptosis
in the execution of acute immune-mediated hepatic damage by
attenuating nitrogen stress. Free Radic Biol Med. 148:151–161.
2020. View Article : Google Scholar
|
|
128
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Vasan K, Werner M and Chandel NS:
Mitochondrial metabolism as a target for cancer therapy. Cell
Metab. 32:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang Y, Wang K, Jin Y and Sheng X:
Endoplasmic reticulum proteostasis control and gastric cancer.
Cancer Lett. 449:263–271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kopp MC, Larburu N, Durairaj V, Adams CJ
and Ali MMU: UPR proteins IRE1 and PERK switch BiP from chaperone
to ER stress sensor. Nat Struct Mol Biol. 26:1053–1062. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lu Y, Liang FX and Wang X: A synthetic
biology approach identifies the mammalian UPR RNA ligase RtcB. Mol
Cell. 55:758–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Belyy V, Zuazo-Gaztelu I, Alamban A,
Ashkenazi A and Walter P: Endoplasmic reticulum stress activates
human IRE1α through reversible assembly of inactive dimers into
small oligomers. Elife. 11:e743422022. View Article : Google Scholar
|
|
136
|
Grandjean JMD, Madhavan A, Cech L,
Seguinot BO, Paxman RJ, Smith E, Scampavia L, Powers ET, Cooley CB,
Plate L, et al: Pharmacologic IRE1/XBP1s activation confers
targeted ER proteostasis reprogramming. Nat Chem Biol.
16:1052–1061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mahdizadeh SJ, Stier M, Carlesso A, Lamy
A, Thomas M and Eriksson LA: Multiscale in silico study of the
mechanism of activation of the RtcB ligase by the PTP1B
phosphatase. J Chem Inf Model. 64:905–917. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Choy KW, Murugan D and Mustafa MR: Natural
products targeting ER stress pathway for the treatment of
cardiovascular diseases. Pharmacol Res. 132:119–129. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yang J and Yao S: JNK-Bcl-2/Bcl-xL-Bax/Bak
pathway mediates the crosstalk between matrine-induced autophagy
and apoptosis via interplay with beclin 1. Int J Mol Sci.
16:25744–25758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Maurel M, Chevet E, Tavernier J and Gerlo
S: Getting RIDD of RNA: IRE1 in cell fate regulation. Trends
Biochem Sci. 39:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zundell JA, Fukumoto T, Lin J, Fatkhudinov
N, Nacarelli T, Kossenkov AV, Liu Q, Cassel J, Hu CA, Wu S and
Zhang R: Targeting the IRE1α/XBP1 endoplasmic reticulum stress
response pathway in ARID1A-mutant ovarian cancers. Cancer Res.
81:5325–5335. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Song M, Sandoval TA, Chae CS, Chopra S,
Tan C, Rutkowski MR, Raundhal M, Chaurio RA, Payne KK, Konrad C, et
al: IRE1α-XBP1 controls T cell function in ovarian cancer by
regulating mitochondrial activity. Nature. 562:423–428. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Dong YF, Zhang J, Zhou JH, Xiao YL, Pei WJ
and Liu HP: Mitochondrial-associated endoplasmic reticulum membrane
interference in ovarian cancer (Review). Oncol Rep. 52:1122024.
View Article : Google Scholar
|
|
145
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Molecular pathways: Immunosuppressive roles of
IRE1α-XBP1 signaling in dendritic cells of the tumor
microenvironment. Clin Cancer Res. 22:2121–2126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Lin J, Liu H, Fukumoto T, Zundell J, Yan
Q, Tang CA, Wu S, Zhou W, Guo D, Karakashev S, et al: Targeting the
IRE1α/XBP1s pathway suppresses CARM1-expressing ovarian cancer. Nat
Commun. 12:53212021. View Article : Google Scholar
|
|
147
|
Harding HP, Zhang Y and Ron D: Protein
translation and folding are coupled by an
endoplasmic-reticulum-resident kinase. Nature. 397:271–274. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress-induced gene expression in mammalian cells. Mol Cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Jackson RJ, Hellen CUT and Pestova TV: The
mechanism of eukaryotic translation initiation and principles of
its regulation. Nat Rev Mol Cell Biol. 11:113–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Scheuner D, Song B, McEwen E, Liu C,
Laybutt R, Gillespie P, Saunders T, Bonner-Weir S and Kaufman RJ:
Translational control is required for the unfolded protein response
and in vivo glucose homeostasis. Mol Cell. 7:1165–1176. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Giampietri C, Petrungaro S, Conti S,
Facchiano A, Filippini A and Ziparo E: Cancer microenvironment and
endoplasmic reticulum stress response. Mediators Inflamm.
2015:4172812015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Harding HP, Zhang Y, Zeng H, Novoa I, Lu
PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al: An
integrated stress response regulates amino acid metabolism and
resistance to oxidative stress. Mol Cell. 11:619–633. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Cullinan SB, Zhang D, Hannink M, Arvisais
E, Kaufman RJ and Diehl JA: Nrf2 is a direct PERK substrate and
effector of PERK-dependent cell survival. Mol Cell Biol.
23:7198–7209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Dan W, Fan Y, Wang Y, Hou T, Wei Y, Liu B,
Li M, Chen J, Fang Q, Que T, et al: The tumor suppressor
TPD52-governed endoplasmic reticulum stress is modulated by
APCCdc20. Adv Sci (Weinh). 11:e24054412024. View Article : Google Scholar
|
|
155
|
Wiseman RL, Mesgarzadeh JS and Hendershot
LM: Reshaping endoplasmic reticulum quality control through the
unfolded protein response. Mol Cell. 82:1477–1491. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Park SJ, Yoon BH, Kim SK and Kim SY:
GENT2: An updated gene expression database for normal and tumor
tissues. BMC Med Genomics. 12(Suppl 5): S1012019. View Article : Google Scholar
|
|
157
|
Cai Y, Arikkath J, Yang L, Guo ML,
Periyasamy P and Buch S: Interplay of endoplasmic reticulum stress
and autophagy in neurodegenerative disorders. Autophagy.
12:225–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Meng J, Liu K, Shao Y, Feng X, Ji Z, Chang
B, Wang Y, Xu L and Yang G: ID1 confers cancer cell chemoresistance
through STAT3/ATF6-mediated induction of autophagy. Cell Death Dis.
11:1372020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Qiu L, Liu H, Chen S, Wu Y and Yan J:
Ferroptosis contributed to endoplasmic reticulum stress in preterm
birth by targeting LHX1 and IRE-1. Cell Signal. 132:1117772025.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Song T, Li J, Xia Y, Hou S, Zhang X and
Wang Y: 1,25-D3 ameliorates ischemic brain injury by alleviating
endoplasmic reticulum stress and ferroptosis: Involvement of
vitamin D receptor and p53 signaling. Cell Signal. 122:1113312024.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zeng T, Zhou Y, Yu Y, Wang JW, Wu Y, Wang
X, Zhu L, Zhou LM and Wan LH: rmMANF prevents sepsis-associated
lung injury via inhibiting endoplasmic reticulum stressinduced
ferroptosis in mice. Int Immunopharmacol. 114:1096082023.
View Article : Google Scholar
|
|
162
|
Chen Y, Li H, Liu J, Ni J, Deng Q, He H,
Wu P, Wan Y, Seeram NP, Liu C, et al: Cytotoxicity of natural and
synthetic cannabinoids and their synergistic antiproliferative
effects with cisplatin in human ovarian cancer cells. Front
Pharmacol. 15:14961312024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang Z, Liu M, Li GX, Zhang L, Ding KY, Li
SQ, Gao BQ, Chen P, Choe HC, Xia LY, et al: A herbal pair of
Scutellaria barbata D. Don and Scleromitrion diffusum (Willd.) R.J.
Wang induced ferroptosis in ovarian cancer A2780 cells via inducing
heme catabolism and ferritinophagy. J Integr Med. 22:665–682. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Liu Y, Li J, Xu J, Long Y, Wang Y, Liu X,
Hu J, Wei Q, Luo Q, Luo F, et al: m6A-driven NAT10
translation facilitates fatty acid metabolic rewiring to suppress
ferroptosis and promote ovarian tumorigenesis through enhancing
ACOT7 mRNA acetylation. Oncogene. 43:3498–3516. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Furutake Y, Yamaguchi K, Yamanoi K,
Kitamura S, Takamatsu S, Taki M, Ukita M, Hosoe Y, Murakami R,
Abiko K, et al: YAP1 Suppression by ZDHHC7 is associated with
ferroptosis resistance and poor prognosis in ovarian clear cell
carcinoma. Mol Cancer Ther. 23:1652–1665. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Han Y, Fu L, Kong Y, Jiang C, Huang L and
Zhang H: STEAP3 affects ovarian cancer progression by regulating
ferroptosis through the p53/SLC7A11 pathway. Mediators Inflamm.
2024:40485272024. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Nevins S, McLoughlin CD, Oliveros A, Stein
JB, Rashid MA, Hou Y, Jang MH and Lee KB: Nanotechnology approaches
for prevention and treatment of chemotherapy-induced neurotoxicity,
neuropathy, and cardiomyopathy in breast and ovarian cancer
survivors. Small. 20:e23007442024.
|
|
168
|
Luo L, Zhou H, Wang S, Pang M, Zhang J, Hu
Y and You J: The application of nanoparticle-based imaging and
phototherapy for female reproductive organs diseases. Small.
20:e22076942024.
|
|
169
|
Lee J, Jang S, Im J, Han Y, Kim S, Jo H,
Wang W, Cho U, Kim SI, Seol A, et al: Stearoyl-CoA desaturase 1
inhibition induces ER stress-mediated apoptosis in ovarian cancer
cells. J Ovarian Res. 17:732024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Yan T, Ma X, Zhou K, Cao J, Tian Y, Zheng
H, Tong Y, Xie S, Wang Y, Guo L and Lu R: A novel CSN5/CRT
O-GlcNAc/ER stress regulatory axis in platinum resistance of
epithelial ovarian cancer. Int J Biol Sci. 20:1279–1296. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Kim TW and Lee HG: 6-Shogaol overcomes
gefitinib resistance via ER stress in ovarian cancer cells. Int J
Mol Sci. 24:26392023. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Zhang M and Wang Y, Xu S, Huang S, Wu M,
Chen G and Wang Y: Endoplasmic reticulum stress-related
ten-biomarker risk classifier for survival evaluation in epithelial
ovarian cancer and TRPM2: A potential therapeutic target of ovarian
cancer. Int J Mol Sci. 24:140102023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Ma X, Zhou W, Zhang R, Zhang C, Yan J,
Feng J, Rosenholm JM, Shi T, Shen X and Zhang H: Minimally invasive
injection of biomimetic Nano@Microgel for in situ ovarian cancer
treatment through enhanced photodynamic reactions and photothermal
combined therapy. Mater Today Bio. 20:1006632023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zhang J, Guo J, Yang N, Huang Y, Hu T and
Rao C: Endoplasmic reticulum stress-mediated cell death in liver
injury. Cell Death Dis. 13:10512022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Cheng C, Yuan Y, Yuan F and Li X: Acute
kidney injury: Exploring endoplasmic reticulum stress-mediated cell
death. Front Pharmacol. 15:13087332024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Yang J, Xu H, Wu W, Huang H, Zhang C, Tang
W, Tang Q and Bi F: Ferroptosis signaling promotes the release of
misfolded proteins via exosomes to rescue ER stress in
hepatocellular carcinoma. Free Radic Biol Med. 202:110–120. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Li FJ, Abudureyimu M, Zhang ZH, Tao J,
Ceylan AF, Lin J, Yu W, Reiter RJ, Ashrafizadeh M, Guo J and Ren J:
Inhibition of ER stress using tauroursodeoxycholic acid rescues
obesity-evoked cardiac remodeling and contractile anomalies through
regulation of ferroptosis. Chem Biol Interact. 398:1111042024.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Han N, Yang ZY, Xie ZX, Xu HZ, Yu TT, Li
QR, Li LG, Peng XC, Yang XX, Hu J, et al: Dihydroartemisinin
elicits immunogenic death through ferroptosis-triggered ER stress
and DNA damage for lung cancer immunotherapy. Phytomedicine.
112:1546822023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Han Y, Hao G, Han S, Zhu T, Dong Y, Chen
L, Yang X, Li X, Jin H, Liang G, et al: Polydatin ameliorates early
brain injury after subarachnoid hemorrhage through up-regulating
SIRT1 to suppress endoplasmic reticulum stress. Front Pharmacol.
15:14502382024. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Wang S, Xu F, Liu H, Shen Y, Zhang J, Hu L
and Zhu L: Suppressing endoplasmic reticulum stress alleviates
LPS-induced acute lung injury via inhibiting inflammation and
ferroptosis. Inflammation. 47:1067–1082. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Ding H, Xiang Y, Zhu Q, Wu H, Xu T, Huang
Z and Ge H: Endoplasmic reticulum stress-mediated ferroptosis in
granulosa cells contributes to follicular dysfunction of polycystic
ovary syndrome driven by hyperandrogenism. Reprod Biomed Online.
49:1040782024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Jiang D, Guo Y, Wang T, Wang L, Yan Y, Xia
L, Bam R, Yang Z, Lee H, Iwawaki T, et al: IRE1α determines
ferroptosis sensitivity through regulation of glutathione
synthesis. Nat Commun. 15:41142024. View Article : Google Scholar
|
|
183
|
Guan Q, Wang Z, Hu K, Cao J, Dong Y and
Chen Y: Melatonin ameliorates hepatic ferroptosis in NAFLD by
inhibiting ER stress via the MT2/cAMP/PKA/IRE1 signaling pathway.
Int J Biol Sci. 19:3937–3950. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
He Z, Shen P, Feng L, Hao H, He Y, Fan G,
Liu Z, Zhu K, Wang Y, Zhang N, et al: Cadmium induces liver
dysfunction and ferroptosis through the endoplasmic
stress-ferritinophagy axis. Ecotoxicol Environ Saf. 245:1141232022.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhao C, Yu D, He Z, Bao L, Feng L, Chen L,
Liu Z, Hu X, Zhang N, Wang T and Fu Y: Endoplasmic reticulum
stress-mediated autophagy activation is involved in cadmium-induced
ferroptosis of renal tubular epithelial cells. Free Radic Biol Med.
175:236–248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Xu M, Tao J, Yang Y, Tan S, Liu H, Jiang
J, Zheng F and Wu B: Ferroptosis involves in intestinal epithelial
cell death in ulcerative colitis. Cell Death Dis. 11:862020.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Thulasinathan B, Suvilesh KN, Maram S,
Grossmann E, Ghouri Y, Teixeiro EP, Chan J, Kaif JT and Rachagani
S: The impact of gut microbial short-chain fatty acids on
colorectal cancer development and prevention. Gut Microbes.
17:24837802025. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Wei R, Zhao Y, Wang J, Yang X, Li S, Wang
Y, Yang X, Fei J, Hao X, Zhao Y, et al: Tagitinin C induces
ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal
cancer cells. Int J Biol Sci. 17:2703–2717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhao R, Lv Y, Feng T, Zhang R, Ge L, Pan
J, Han B, Song G and Wang L: ATF6α promotes prostate cancer
progression by enhancing PLA2G4A-mediated arachidonic acid
metabolism and protecting tumor cells against ferroptosis.
Prostate. 82:617–629. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Tak J, Kim YS, Kim TH, Park GC, Hwang S
and Kim SG: Gα12 overexpression in hepatocytes by ER
stress exacerbates acute liver injury via ROCK1-mediated miR-15a
and ALOX12 dysregulation. Theranostics. 12:1570–1588. 2022.
View Article : Google Scholar :
|
|
191
|
Ji X, Chen Z, Lin W, Wu Q, Wu Y, Hong Y,
Tong H, Wang C and Zhang Y: Esculin induces endoplasmic reticulum
stress and drives apoptosis and ferroptosis in colorectal cancer
via PERK regulating eIF2α/CHOP and Nrf2/HO-1 cascades. J
Ethnopharmacol. 328:1181392024. View Article : Google Scholar
|
|
192
|
Guo C, Zhao W, Wang W, Yao Z, Chen W and
Feng X: Study on the antitumor mechanism of tanshinone IIA in vivo
and in vitro through the regulation of PERK-ATF4-HSPA5
pathway-mediated ferroptosis. Molecules. 29:15572024. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Xu J, Zhao L, Zhang X, Ying K, Zhou R, Cai
W, Wu X, Jiang H, Xu Q, Miao D, et al: Salidroside ameliorates
acetaminophen-induced acute liver injury through the inhibition of
endoplasmic reticulum stress-mediated ferroptosis by activating the
AMPK/SIRT1 pathway. Ecotoxicol Environ Saf. 262:1153312023.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang
L, Wang D, Xing J, Hou B, Li H, et al: Dihydroartemisinin-induced
unfolded protein response feedback attenuates ferroptosis via
PERK/ATF4/HSPA5 pathway in glioma cells. J Exp Clin Cancer Res.
38:4022019. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kuang H, Sun X, Liu Y, Tang M, Wei Y, Shi
Y, Li R, Xiao G, Kang J, Wang F, et al: Palmitic acid-induced
ferroptosis via CD36 activates ER stress to break calcium-iron
balance in colon cancer cells. FEBS J. 290:3664–3687. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Fu F, Wang W, Wu L, Wang W, Huang Z, Huang
Y, Wu C and Pan X: Inhalable biomineralized liposomes for cyclic
Ca2+-burst-centered endoplasmic reticulum stress
enhanced lung cancer ferroptosis therapy. ACS Nano. 17:5486–5502.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Chen D, Fan Z, Rauh M, Buchfelder M,
Eyupoglu IY and Savaskan N: ATF4 promotes angiogenesis and neuronal
cell death and confers ferroptosis in a xCT-dependent manner.
Oncogene. 36:5593–5608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Li Y, Gao Y, Zhang X, Guo H and Gao H:
Nanoparticles in precision medicine for ovarian cancer: From
chemotherapy to immunotherapy. Int J Pharm. 591:1199862020.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wu M and Zhou S: Harnessing tumor
immunogenomics: Tumor neoantigens in ovarian cancer and beyond.
Biochim Biophys Acta Rev Cancer. 1878:1890172023. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Dahlmanns M, Yakubov E and Dahlmanns JK:
Genetic profiles of ferroptosis in malignant brain tumors and
off-target effects of ferroptosis induction. Front Oncol.
11:7830672021. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
He Y, Su J, Lan B, Gao Y and Zhao J:
Targeting off-target effects: Endoplasmic reticulum stress and
autophagy as effective strategies to enhance temozolomide
treatment. Onco Targets Ther. 12:1857–1865. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Sassetti E, Clausen MH and Laraia L:
Small-molecule inhibitors of reactive oxygen species production. J
Med Chem. 64:5252–5275. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Zhu YS, Wu J and Zhi F: Advances in
conjugate drug delivery system: Opportunities and challenges. Int J
Pharm. 667:1248672024. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Agrawal SS, Baliga V and Londhe VY:
Liposomal formulations: A recent update. Pharmaceutics. 17:362024.
View Article : Google Scholar
|
|
205
|
Liu Z, Xiang C, Zhao X, Aizawa T, Niu R,
Zhao J, Guo F, Li Y, Luo W, Liu W and Gu R: Regulation of dynamic
spatiotemporal inflammation by nanomaterials in spinal cord injury.
J Nanobiotechnology. 22:7672024. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Tang S and Chen L: The recent advancements
of ferroptosis of gynecological cancer. Cancer Cell Int.
24:3512024. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Ying Y, Zhang J, Ren D, Zhao P, Zhang W
and Lu X: ERP29 regulates the proliferation of endometrial
carcinoma via M6A modification. Life Sci. 354:1229762024.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Zhao Y, Lu L, Chen X and Yin Q: Natural
compounds targeting ferroptosis in ovarian cancer: Research
progress and application potential. Pharmacol Res. 215:1077292025.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Jin N, Qian YY, Jiao XF, Wang Z, Li X, Pan
W, Jiang JK, Huang P, Wang SY, Jin P, et al: Niraparib restricts
intraperitoneal metastases of ovarian cancer by eliciting
CD36-dependent ferroptosis. Redox Biol. 80:1035282025. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Miglietta S, Sollazzo M, Gherardi I,
Milioni S, Cavina B, Marchio L, De Luise M, Coada CA, Fiorillo M,
Perrone AM, et al: Mitochondrial chaperonin DNAJC15 promotes
vulnerability to ferroptosis of chemoresistant ovarian cancer
cells. Open Biol. 15:2401512025. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Yin ZY, He SM, Zhang XY, Yu XC, Sheng KX,
Fu T, Jiang YX, Xu L, Hu BX, Zhang JB, et al: Apolipoprotein B100
acts as a tumor suppressor in ovarian cancer via lipid/ER stress
axis-induced blockade of autophagy. Acta Pharmacol Sin. Jan
29–2025.Epub ahead of print. View Article : Google Scholar
|
|
212
|
Li D, Geng D and Wang M: Advances in
natural products modulating autophagy influenced by cellular stress
conditions and their anticancer roles in the treatment of ovarian
cancer. FASEB J. 38:e700752024. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Chan DW, Yung MM, Chan YS, Xuan Y, Yang H,
Xu D, Zhan JB, Chan KK, Ng TB and Ngan HY: MAP30 protein from
Momordica charantia is therapeutic and has synergic activity with
cisplatin against ovarian cancer in vivo by altering metabolism and
inducing ferroptosis. Pharmacol Res. 161:1051572020. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Zhang GH, Kai JY, Chen MM, Ma Q, Zhong AL,
Xie SH, Zheng H, Wang YC, Tong Y, Tian Y, et al: Downregulation of
XBP1 decreases serous ovarian cancer cell viability and enhances
sensitivity to oxidative stress by increasing intracellular ROS
levels. Oncol Lett. 18:4194–4202. 2019.PubMed/NCBI
|
|
215
|
Janczar S, Nautiyal J, Xiao Y, Curry E,
Sun M, Zanini E, Paige AJ and Gabra H: WWOX sensitises ovarian
cancer cells to paclitaxel via modulation of the ER stress
response. Cell Death Dis. 8:e29552017. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Chen Q, Wang Y, Yue T, Wei H, Li S and
Dong B: Fluorescence imaging of intracellular glutathione levels in
the endoplasmic reticulum to reveal the inhibition effect of rutin
on ferroptosis. Anal Chem. January 9–2023.Epub ahead of print.
|
|
217
|
Huang M, Wang Y, Wu X and Li W: Crosstalk
between endoplasmic reticulum stress and ferroptosis in liver
diseases. Front Biosci (Landmark Ed). 29:2212024. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Ao Q, Hu H and Huang Y: Ferroptosis and
endoplasmic reticulum stress in rheumatoid arthritis. Front
Immunol. 15:14388032024. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Li Y, Li M, Feng S, Xu Q, Zhang X, Xiong X
and Gu L: Ferroptosis and endoplasmic reticulum stress in ischemic
stroke. Neural Regen Res. 19:611–618. 2024. View Article : Google Scholar
|
|
220
|
Zhang YG, Yan XF, Liu F, Hao WZ, Cai Y,
Liu Y, Liu LL and Li XJ: Astragalus polysaccharides induces
ferroptosis in ovarian adenocarcinoma cells through
Nrf2/SLC7A11/GPX4 signaling pathway. Zhongguo Zhong Yao Za Zhi.
49:6459–6467. 2024.In Chinese.
|