Ion channels are a major class of membrane proteins
that are sensitive to a variety of stimuli, including changes in
membrane potential, ligand binding, temperature fluctuations and
mechanical forces (1). These
stimuli trigger conformational changes in the protein, leading to
the formation of gated ion-permeable pores in biological membranes,
which facilitate the passive diffusion of ions along their
respective electrochemical gradients both across the cell membrane
and between intracellular compartments. Ion channels contribute to
the regulation of various processes, such as the maintenance of
cellular osmotic pressure and membrane potential, motility (through
interactions with the cytoskeleton), invasion, signal transduction,
transcriptional activity and cell cycle progression, all of which
can lead to tumor progression and metastasis (2). Their central role in intracellular
and intercellular signaling also facilitates the coupling of
extracellular events to cellular responses (3).
Over the past decade, interest in the study of ion
channels in digestive disorders has increased. The literature
suggests that dysregulated ion channel activity plays a pathogenic
role in various digestive diseases, including cancer. For instance,
dysfunction of ion channels and transporter proteins can lead to
gastrointestinal mucosal damage, such as disruption of the
bicarbonate and mucosal layers (4-7),
loss of epithelial cells (8,9),
atrophy of glandular mucosa (10), loss of tight junction (TJ)
proteins (11-13), imbalances in the gut flora
(14) and changes in mucosal
blood flow (15). These
alterations contribute to gastrointestinal mucosal diseases,
including gastritis, ulcers, inflammatory bowel disease and
gastrointestinal cancers (16).
Ion channels and transporter proteins have been
reported to be crucial for maintaining both the exocrine and
endocrine functions of the pancreas. They facilitate enzymatic
reactions necessary for the digestion of fats and proteins in the
intestines, while also facilitating the secretion of
bicarbonate-rich fluids (17,18). Pancreatic islet β-cells express a
variety of ion channels located in lipid membranes or subcellular
organelles, which are collectively involved in glucose-dependent
insulin secretion. Consequently, dysfunction of these ion channels
and transporter proteins can result in pancreatic injury,
potentially leading to conditions such as pancreatitis, diabetes
mellitus, pancreatic cystic fibrosis (CF) and an increased risk of
pancreatic cancer (19,20).
In addition, the role of ion channels and
transporter proteins in liver diseases has garnered considerable
attention. For instance, dysfunction of various calcium channels
can disrupt intraand extracellular calcium homeostasis, leading to
endoplasmic reticulum stress, mitochondrial dysfunction and the
inhibition of autophagy in hepatocytes, all of which contribute to
the development of nonalcoholic fatty liver disease (NAFLD)
(21). Ion channels intersect
with multiple signaling pathways, promoting hepatocellular
carcinoma (HCC) cell invasion and metastasis by modulating gene
expression, regulating cell proliferation and triggering
epithelial-mesenchymal transition (22). In addition, ion channels and
transporter proteins also regulate the growth and invasion of
esophageal cancer (EC) (23-25).
As mentioned above, ion channels and transporter
proteins play critical roles in regulating pathophysiological
processes in various organs of the digestive system. While numerous
studies have extensively investigated the role of cation channels,
particularly calcium channels (26-32), sodium channels (33-36) and potassium channels (37-39), anion channels have received
comparatively less attention. Anion channels (40,41) have been identified as important
molecules with aberrant expression, activity and localization in
various pathological conditions, including cardiovascular diseases,
neurological disorders, metabolic diseases and cancer.
As the primary anion in the human body,
intracellular chloride concentrations are dynamically regulated
(42) and play a crucial role in
maintaining intracellular and osmotic homeostasis, primarily
through transmembrane transport and ion channels (43). Chloride channels have been
implicated in a variety of pathological diseases (44). These channels and transport
proteins are located both in the plasma membrane and intracellular
membranes, with functions ranging from ion homeostasis and cell
volume regulation to transepithelial transport and electrical
excitability regulation (45).
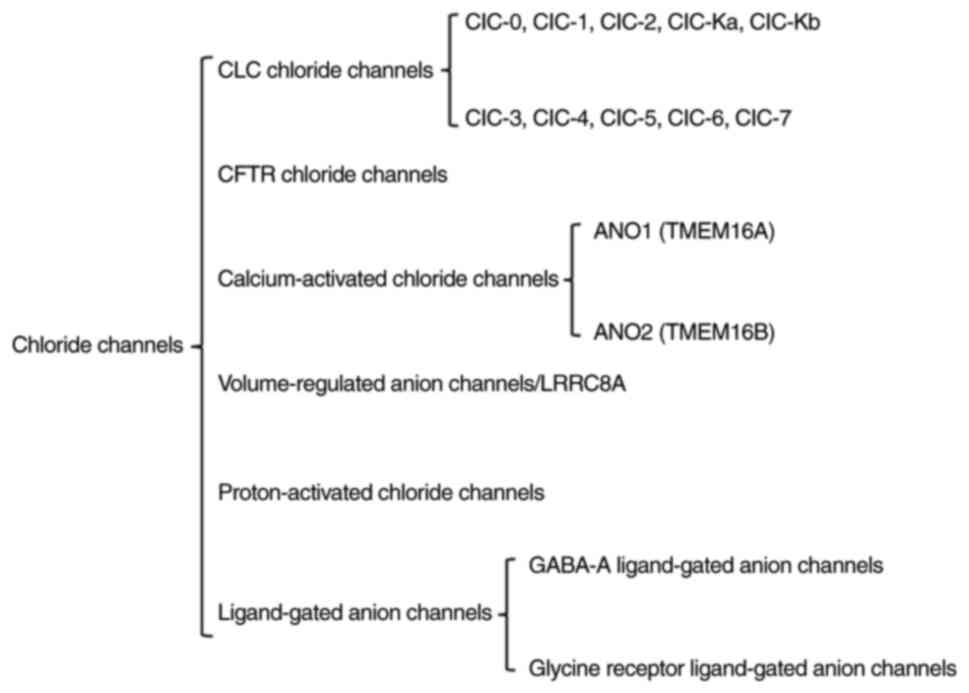
Chloride ions are regulated by chloride channels for their intra-
and extracellular distribution and transport. Chloride channels are
currently classified into several groups (Fig. 1), including the chloride channel
(ClC) family, CF transmembrane conductance regulator (CFTR),
calcium-activated chloride channels (CaCCs), volume-regulated anion
channels (VRACs), proton-activated chloride channels (PACs)
(46) and ligand-gated anion
channels (47). Chloride
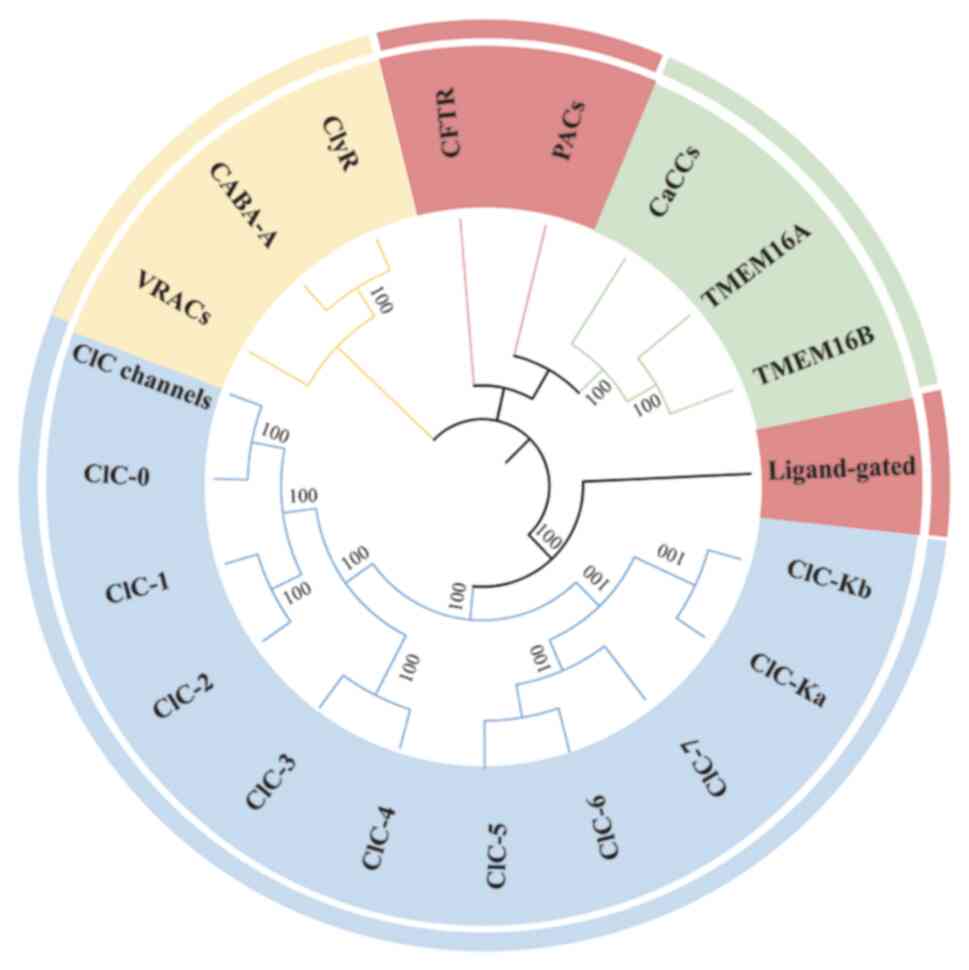
channels have undergone significant functional differentiation and
diversification during evolution and are distributed from
prokaryotes to higher vertebrates (Fig. 2). The ClC family is one of the
evolutionarily oldest classes of chloride channels and is
widespread in prokaryotes and eukaryotes (48). During evolution, the ClC family
differentiated into voltage-gated chloride channels and
chloride/proton exchangers. In vertebrates, the ClC family has
further differentiated into several isoforms (e.g., ClC-1 to -7),
which are involved in the regulation of cell membrane potential or
chloride homeostasis of intracellular organelles (49). Among CaCCs, anoctamin (ANO)1 has
been extensively studied. ANO1 [transmembrane protein (TMEM)16A]
belongs to the anoctamin family and is found in both invertebrates
and vertebrates (50). ANO1 may
have initially evolved as a lipid scramblase, and subsequently
gained calcium-activated chloride channel function. In mammals,
ANO1 is expressed in a variety of tissues and is involved in
secretion, smooth muscle contraction and signaling (51). CFTR belongs to the ATP-binding
cassette (ABC) superfamily of transporter proteins and is a
vertebrate-specific chloride channel (52). CFTR has evolved to acquire a
unique regulatory domain (R-domain) that allows it to be activated
by cAMP-dependent phosphorylation (53). PACs/TMEM206 belongs to a family
of transmembrane proteins, TMEM, that are highly conserved in
vertebrates. No direct homologs have been identified in
invertebrates, but functionally similar channels may exist in some
lower organisms. In mammals, PACs are expressed in a variety of
tissues, particularly in the brain, lungs and kidneys, and may be
associated with pathological processes related to acidosis. PACs
may have evolved as an adaptive mechanism for cells to cope with
acidic environments, and are involved in maintaining intracellular
pH homeostasis (54).
Ligand-gated anion channels belong to the Cys-loop ligand-gated ion
channel superfamily and share a common ancestor with related
channels in invertebrates, such as glutamate-gated chloride
channels (55). In vertebrates,
gamma-aminobutyric acid type A receptor (GABA-A) and glycine
receptor (GlyR) mediate inhibitory neurotransmission (56) (Fig. 2). Of note, in a study
investigating chloride channels in an in vivo porcine model
of esophageal acid damage, ClC-2 was found to localize to the basal
epithelium of the porcine esophageal mucosa and esophageal
submucosal glands. Chloride intracellular channel protein 1
recruited phosphatidylinositol 4-phosphate 5-kinases to lipid
membranes, which, in turn, regulate cell-matrix adhesion and
membrane protrusion, contributing to tumor aggressiveness,
metastasis and a poor prognosis (57).
To date, there has been no comprehensive review of
the role of chloride ion channels in digestive system diseases.
Therefore, the aim of this review was to compile and summarize the
physiopathological roles of chloride channels and transporter
proteins in digestive system diseases, with the goal of providing a
solid theoretical foundation for future research and offering new
perspectives for the treatment of these diseases.
The ClC channel exists as a dimer, with each monomer
forming a separate ion-conducting pore, and each monomer contains
18 alpha helices, some of which form ion-conducting pores (49). The ClC family comprises ClC-0,
ClC-1, ClC-2, ClC-Ka, ClC-Kb, ClC-3, ClC-4, ClC-5, ClC-6 and ClC-7.
On the basis of their distribution, ClC-0, ClC-1, ClC-2, ClC-Ka and
ClC-Kb are plasma membrane channels, whereas the others are
intracellular membrane channels. Functionally, ClC-1, ClC-2, ClC-Ka
and ClC-Kb are voltage-gated chloride channels, whereas the
remaining members act as Cl−/H+ exchangers.
The ClC family is involved in several key functions, including
volume regulation, ion dynamic homeostasis, transepithelial
transport and the regulation of electrical excitability. ClC-2, a
voltage-gated chloride channel composed of 907 amino acids with a
molecular weight of 99 kDa, features a two-pore homodimeric
structure (58,59). Its activity is regulated by
changes in the extracellular pH, displaying a biphasic response.
ClC-2 is activated by moderate acidification but abruptly shuts
down when the pH drops below ~7.
ClC-2 mRNA levels are significantly elevated in
patients with nonalcoholic steatohepatitis and mice lacking ClC-2
exhibit a beneficial metabolic phenotype, suggesting that reducing
or knocking down ClC-2 may be a potential strategy for treating
NAFLD and further research revealed that ClC-2 downregulated the
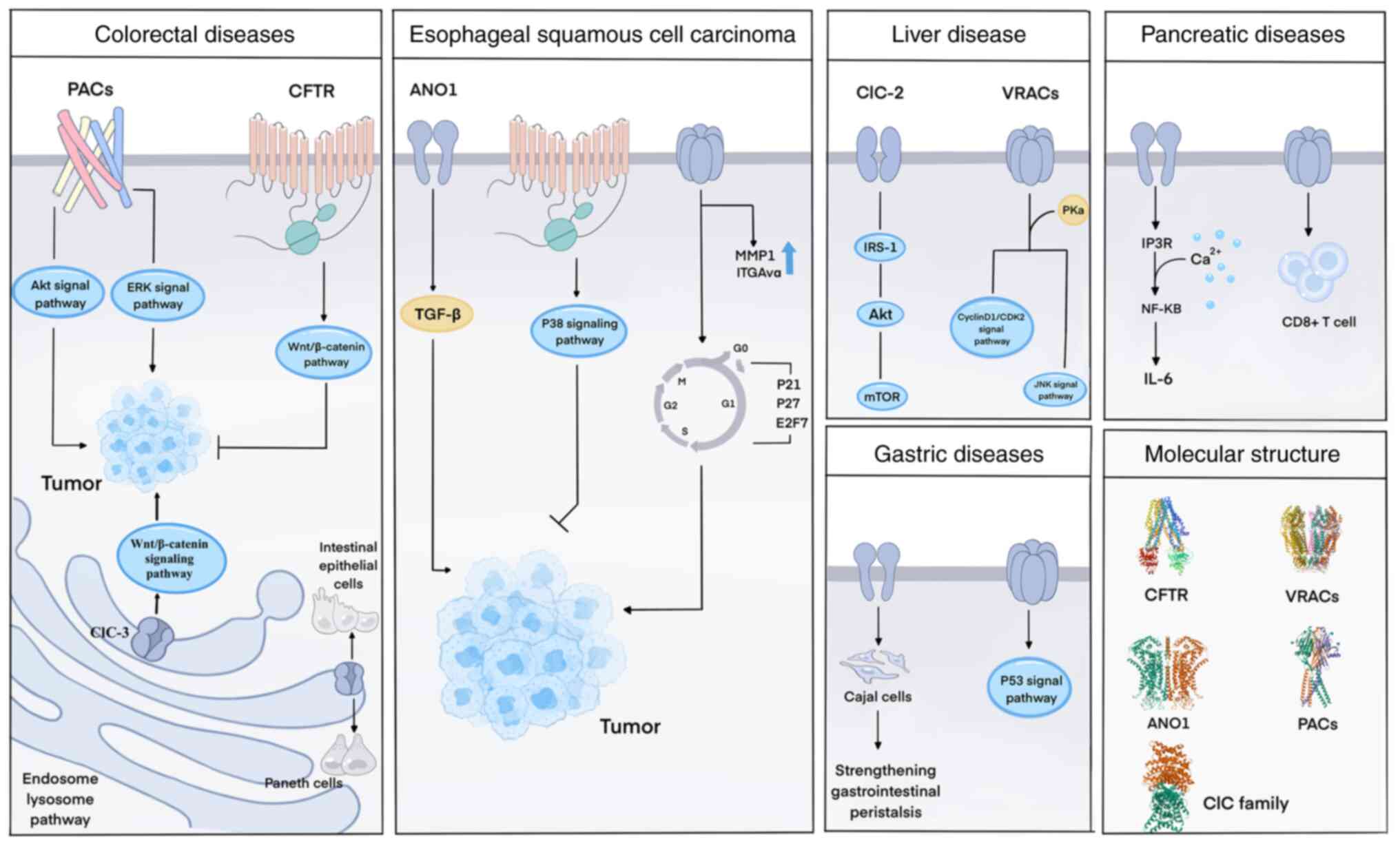
activation of IRS-1/Akt/mTOR signaling (60) (Fig. 3).
Chloride channels also play a critical role in
intestinal diseases. The intestinal barrier, which is essential for
nutrient absorption and preventing the entry of harmful substances,
consists of epithelial cells connected by apical junction
complexes, comprising TJs and adherens junctions (AJs) (61,62). In a ClC-2 knockout mouse model,
the absence of ClC-2 delayed recovery of intestinal barrier
function after ischemic injury, possibly because ClC-2 anchors the
TJ structure following injury (63). Additionally, disruption of AJs
affected colon homeostasis and differentiation, promoting
tumorigenicity through the regulation of β-catenin signaling
(64). Furthermore, genetic
inactivation variants in the ClC-Ka and ClC-Kb genes have been
associated with renal salt-losing nephropathies, with or without
deafness, including the rare Bartter's syndrome (BS) (65), highlighting the crucial role of
the ClC-Ka and ClC-Kb genes in renal and inner ear chloride
processing (66); however, to
the best of our knowledge, no studies to date have explored the
role of these chloride channels in digestive diseases.
ClC-3 plays an important role in cancer regulation
as a volume-regulated anion channel that also controls the cell
cycle and is overexpressed in various cancers. ClC-3 can promote
the development and metastasis of colorectal cancer (CRC) through
the Wnt/β-catenin signaling pathway (67) (Fig. 3). In a study on prognostic
biomarkers in gastric cancer (GC), high expression of ClC-3 was
associated with deeper tumor invasion and increased lymph node
metastasis, with overexpression of ClC-3 being regulated by x-ray
repair cross complementing 5 (68). ClC-3 is also significantly
overexpressed in HCC, and its upregulation is associated with tumor
size and overall prognosis, suggesting that ClC-3 expression may
serve as a predictor of tumor size and overall prognosis in HCC
(69). Furthermore, ClC-3 has
been reported to be expressed in intestinal tissues and to be
involved in the regulation of intestinal inflammation (70). ClC-3 deficiency may exacerbate
inflammatory bowel disease (IBD) by promoting the apoptosis of
intestinal epithelial cells and causing Paneth cell deficiency
(Fig. 3), indicating that
modulating ClC-3 expression could be a novel therapeutic strategy
for treating IBD (71).
ClC-3 to ClC-7 are located primarily in
intracellular organelles, particularly along the endosome-lysosome
pathway, and are distributed differentially across compartments
(43,72-74). ClC-4 localizes to various
endosomal clusters, whereas ClC-5 is predominantly expressed in the
kidney, where it localizes to early endosomes and participates in
endocytic uptake in the proximal tubule. Both ClC-3 and ClC-4 are
widely expressed and found in various endosomal populations. ClC-6
is expressed primarily in neurons and localizes to late endosomes,
whereas ClC-7, along with its β-subunit osteopetrosis-associated
transmembrane protein 1, localizes to lysosomes in all cell types
and is also found at the ruffled borders of bone-resorbing
osteoclasts. Dysfunction of ClC-3, ClC-4, ClC-6 and ClC-7 has been
linked to intellectual disability, epilepsy, lysosomal storage
disorders and neurodegeneration, respectively (75-77). However, to the best of our
knowledge, their roles in digestive disorders have not yet been
reported.
Mutations in the CFTR gene lead to CF due to CFTR
dysfunction. This gene encodes an anion channel that mediates the
transport of chloride (Cl−) and bicarbonate
(HCO3−) across epithelial surfaces, which is
crucial for osmotic homeostasis and the maintenance of normal
electrolyte transport in the lungs, upper respiratory tract,
pancreas, liver, gallbladder, intestine and other exocrine glands
on the apical surface of epithelial cells (80). While the CFTR protein is located
primarily in the plasma membrane, increasing evidence suggests its
presence in intracellular organelles such as endosomes, lysosomes,
phagosomes and mitochondria. Dysfunction of CFTR not only impairs
ion transport across epithelial tissues but also disrupts the
normal functioning of these organelles (81).
Following CFTR dysfunction, bicarbonate ion
transport is inhibited, disrupting the pH balance of the esophageal
epithelium. This imbalance can result in cellular stress, DNA
damage and increased susceptibility to carcinogenesis. In
esophageal squamous cell carcinoma (ESCC), overexpression of CFTR
was found to activate the p38 signaling pathway, which inhibits
cell proliferation, migration and invasion; induces apoptosis; and
is associated with a favorable patient prognosis (82). In esophageal cancer,
gamma-aminobutyric acid receptor subunit pi(GABRP) promoted CFTR
expression, and CFTR knockdown significantly counteracted the
inhibitory effects of GABRP overexpression on EC cell
proliferation, migration and invasion. These findings suggest that
GABRP overexpression inhibits EC progression by increasing CFTR
expression (83). Therefore,
CFTR may act as a mediator and/or biomarker for ESCC (82). In addition, CFTR has been shown
to inhibit the growth and migration of ESCC by downregulating NF-κB
protein expression (23). CFTR
modulators improve gastroesophageal reflux disease symptoms in
patients with advanced CF (84).
One of the specific reasons for this is that CFTR modulators
improve gastroduodenal pH (85),
in addition, CFTR is expressed in esophageal tissues and CFTR
modulators may have a direct effect on esophageal motility and
gastric emptying (82).
Pancreatitis is a common cause of hospitalization
for digestive diseases, with high morbidity and mortality rates
(86). It is a complex
inflammatory disease of the acinar and ductal epithelium that is
often caused by the premature activation of digestive enzymes
(87). There is substantial
evidence that CFTR plays a critical role as an anion transporter in
maintaining normal pancreatic exocrine function. Dysfunction of
CFTR triggers pancreatic disorders, including impaired insulin
regulation, pancreatic ductal obstruction, acute and chronic
inflammation and carcinogenesis (88,89). However, the specific
pathophysiological mechanisms remain to be fully understood.
In the pancreas, digestive enzymes play a well-known
role; however, electrolytes are equally important as carriers,
helping to transport enzymes to the small intestine, neutralize
gastric acid and increase the duodenal pH to the optimal level for
enzyme activity (90). In
particular, bicarbonate (HCO3−) secretion is
essential, as it regulates the pH of the ductal epithelium,
increases mucin secretion (which acts as an antimicrobial) and
prevents the premature activation of pancreatic enzymes (91). CFTR is a key player in
HCO3− secretion in pancreatic fluid. This
secretion involves the coordinated action of ion-transporting
proteins located on the basolateral and luminal membranes of
pancreatic ductal cells (PDCs). HCO3− can
accumulate via electrogenic sodium-bicarbonate cotransporter
1 (NBCe1), the solute carrier family 4 member 4 (SLC4A4)
cotransporter protein, which is produced intracellularly through
the carbonic anhydrase-catalyzed reaction of CO2 and
water. It is then secreted into the ductal lumen via the
coordinated action of CFTR and SLC26A6 (91,92). CFTR not only functions in
conjunction with SLC26A6 but its R domain also binds to the spread
through air spaces domain of SLC26A6, further regulating
HCO3− secretion (93,94). CFTR channels directly mediate
epithelial HCO3− transport and selectively
secrete HCO3− when intracellular
Cl− levels are low and HCO3−
levels are relatively high (95). The dynamic regulation of CFTR
anion conductance is thought to be mediated by With No Lysine
Kinase (WNK) (18). WNK1, for
example, has been shown to activate oxidative stress-responsive
kinase 1 and STE20/SPS1-related proline/alanine-rich kinase (SPAK)
at low extracellular Cl− concentrations, significantly
increasing HCO3− secretion in
CFTR-transfected 293 cells and guinea pig PDCs (96). However, WNK1 and WNK4 can also
inhibit CFTR by reducing its level at the cell surface, thus
inhibiting CFTR-mediated anion efflux (97). This inhibition is counteracted by
the binding of the inositol 1,4,5-trisphosphate (IP3) receptor,
with released IP3 antagonizing the WNK/SPAK pathway (98). IRBIT, through its PDZ domain,
binds to CFTR and NBCe1 at the apical and basolateral membranes,
recruiting protein phosphatase 1 to counteract SPAK-mediated
phosphorylation of CFTR and NBCe1 (99).
In addition, patients with CF are at increased risk
of developing various types of cancer, particularly
gastrointestinal cancers (104). A study involving 1,468
heterozygous germline carriers of the CFTR F508del mutation
revealed an increased risk of GC in this population. Another report
showed that serum CFTR levels in patients with GC correlated with
the expression of the tumor biomarker CA199 (105). A study by Yamada et al
(106) further highlighted that
patients with CF have a significantly increased risk of
gastrointestinal cancers, including small bowel cancer, and
recommended the development of individualized screening protocols
for site-specific gastrointestinal cancers in this population.
CFTR is expressed throughout the intestine, with a
decreasing gradient of expression from the proximal (duodenum) to
the distal (ileum) small intestine. In both the small and large
intestines, CFTR is most strongly expressed at the base of the
crypts and can influence intestinal stem cell function. Studies
have demonstrated that CFTR is expressed by intestinal stem cells
(107). The transmembrane
domains of CFTR form water channels that allow Cl− and
HCO3− ions to be secreted from epithelial
cells into the intestinal lumen, particularly within the crypts,
while simultaneously increasing water secretion in the same
direction. This regulates the water homeostasis and pH of the
intestine (108). CFTR also
influences the composition of the intestinal flora (109,110), the maintenance of the
epithelial barrier (111,112), and the balance of innate and
adaptive immune responses (113,114). Clinical manifestations of
gastrointestinal issues in patients with CF, such as inflammation,
chronic abdominal pain and complications such as Crohn's disease
and gastroesophageal reflux disease (115,116), are associated with
dysregulation of these processes, increasing the risk of cancer. A
20-year epidemiologic study reported that patients with CF have an
elevated risk of gastrointestinal cancers with age, particularly a
sixfold increase in the risk of Colorectal Cancer (CRC), the most
common gastrointestinal malignancy in patients with CF (117).
CFTR deficiency has also been linked to CRC in the
general population, with disease-free survival being lower in
patients with CRC with reduced CFTR expression in the tumor
(118). CFTR-depleted CRC cell
lines exhibit enhanced oncogenic features, including increased
colony formation, migration and invasion (119). Increased β-catenin activity,
associated with increased proliferation, has been observed in CFTR
knockout crypt cells and organoids. Although studies on CRC without
CF are limited, Palma et al (120) reported that CFTR may play a
nontumor suppressive role in CRC initiation and progression by
enhancing a set of genes associated with cancer stemness in
patients without CFTR mutations. Mechanistic studies have shown
that CFTR knockdown or transient inhibition by CFTR (inh)-172 leads
to an increase in the intracellular pH of leucine-rich
repeat-containing g-protein coupled receptor 5+ stem cells,
promoting the binding of dishevelled segment polarity protein 2 (a
member of the Wnt/β-catenin signaling pathway) to plasma membrane
phospholipids and thereby enhancing Wnt/β-catenin signaling
(121).
CFTR expression is downregulated in CRC, potentially
due to promoter methylation. Overexpression of CFTR has been shown
to inhibit CRC tumor growth by suppressing cell proliferation,
migration and invasion. Furthermore, CFTR promoter methylation is
significantly correlated with lymph node metastasis, suggesting
that CFTR may serve as a potential marker for lymph node metastasis
in patients with CRC (122).
CaCCs are chloride channels that can be
synergistically activated by intracellular calcium and voltage.
ANO1/TMEM16A contains 8 transmembrane structural domains (TM1-TM8),
of which TM3-TM7 form the ion-conducting pore. The calcium binding
site is located on the cytoplasmic side and contains multiple
acidic residues responsible for calcium ion binding and channel
activation. ANO1 exists as a dimer, with each monomer having a
separate ion-conducting pore (123,124). CaCCs are expressed in both
invertebrates and mammals, suggesting that they play crucial
physiological roles, including regulating epithelial Cl−
secretion, neuronal and cardiomyocyte excitability, smooth muscle
contraction and injury perception (125). The main CaCCs identified so far
are ANO1, also known as TMEM16A, and ANO2, also known as TMEM16B
(126). ANO1 is widely
expressed in various cancers, where it controls cancer cell
proliferation, survival and migration (127). It also regulates the secretory
function of epithelial cells (128) and the electrical pacing
activity of interstitial cells of Cajal in gastrointestinal smooth
muscle (129). By contrast,
ANO2 (TMEM16B), although functioning as a CaCC, is expressed
primarily in olfactory sensory neurons (130), hippocampal pyramidal neurons
(131) and thalamocortical
neurons in the brain (132).
In research on acute pancreatitis (AP), IL-6 was
found to promote TMEM16A (ANO1) expression via IL-6R/STAT3
signaling activation, and overexpression of TMEM16A further
increased IL-6 secretion through the inositol 1,4,5-triphosphate
receptor/Ca2+/NF-κB signaling pathway (143). Therefore, inhibiting TMEM16A
could represent a novel therapeutic strategy for treating AP. In
addition, studies have identified ANO1 as a prognostic factor after
radical resection of pancreatic carcinoma, where ANO1 may induce an
immunosuppressive tumor microenvironment in a paracrine manner
(144), suggesting that ANO1
could be a potential therapeutic target for pancreatic cancer.
Ion channels have been extensively studied in liver
diseases in recent years, but chloride channels, including TMEM16A
(ANO1), have been less frequently explored. Studies have shown that
TMEM16A expression is elevated in the liver tissues of both mice
and patients with NAFLD. Hepatocyte-specific knockdown of TMEM16A
in mice improved hepatic glucose metabolism disorders and
steatosis. TMEM16A in hepatocytes interacts with vesicle-associated
membrane protein 3 (VAMP3), inducing its degradation and inhibiting
the formation of VAMP3/synaptobrevin complexes, such as
VAMP3/synaptosome-associated protein 23 and VAMP3/syntaxin 4. This
interaction disrupts the translocation of hepatic glucose
transporter protein 2 (GLUT2), leading to impaired glucose uptake.
Notably, VAMP3 overexpression counteracts the inhibitory effect of
TMEM16A on GLUT2 translocation and helps reduce lipid deposition,
insulin resistance and inflammation (145). These findings suggest that
inhibiting hepatic TMEM16A or disrupting the TMEM16A/VAMP3
interaction could offer new therapeutic strategies for NAFLD. Guo
et al (146) reported
that the TMEM16A-glutathione peroxidase 4 (GPX4) interaction and
GPX4 ubiquitination are essential for TMEM16A-regulated hepatic
ischemia/reperfusion injury, suggesting that blockade of the
TMEM16A-GPX4 interaction or inhibition of TMEM16A in hepatocytes
may represent promising therapeutic strategies for acute liver
injury. Kondo et al (147) reported that downregulation of
TMEM16A expression in cirrhotic portal hypertension-associated
portal vein smooth muscle cells reduces Ca2+-activated
Cl− channel activity; it is mediated by the increase in
angiotensin II in cirrhosis. In addition, while ANO1 plays a
regulatory role in various cancers, its role in HCC has been less
studied. TMEM16A is overexpressed in HCC, and the inhibition of
TMEM16A suppresses the MAPK signaling pathway (Fig. 3), reducing HCC growth. These
findings indicate that TMEM16A may serve as a promising therapeutic
target for HCC (148).
VRACs, also known as volume-sensitive outwardly
rectifying channels, regulate vertebrate cell volume by mediating
the extrusion of chloride ions and organic osmolytes. VRACs are
composed of a heterodimer of leucine-rich repeat-containing 8
(LRRC8) proteins, where LRRC8A (also known as SWELL1) serves as an
essential subunit. LRRC8A binds to any of its homologs, such as
LRRC8B-E, to form the hexameric VRACs complex, which functions as
an anion-selective channel. VRACs are activated either by cellular
swelling or by reactive oxygen species, a process dependent on
intracellular ATP (149-151).
VRACs play a crucial role in various physiological processes,
including cell volume regulation, proliferation, migration and
apoptosis (152).
LRRC8A has been shown to influence the growth of GC
cells, and high expression of LRRC8A was identified as an
independent prognostic factor for 5-year survival in patients with
GC on the basis of multivariate analysis of clinical samples.
Further research revealed that knockdown of LRRC8A inhibited cell
proliferation and migration, enhanced apoptosis and affected the
expression of genes associated with the p53 signaling pathway,
including JNK, p53, p21, Bcl-2 and FAS (154).
Pancreatic adenocarcinoma (PAAD) is a highly
malignant tumor of the digestive system with increasing morbidity
and mortality rates. LRRC8A has been shown to influence the
prognosis of PAAD and to be associated with cell proliferation,
migration, drug resistance and immune infiltration. Notably, LRRC8A
plays a role in the progression and prognosis of patients with PAAD
by regulating the immune infiltration of CD8+ T cells
(155).
SWELL1, an integral component of the VRACs, is
highly expressed in HCC tissues and is associated with poor
prognosis. An in vitro study showed that the overexpression
of SWELL1 significantly promoted cell proliferation and migration
while inhibiting apoptosis. Mechanistically, SWELL1 interacts with
protein kinase Cα, activates the cyclin D1/cyclin-dependent kinase
2 pathway to induce cell growth, and regulates cell migration
through the JNK pathway (156).
LRRC8A was found to be elevated in 60% of CRC
patient tissues, and patients with high LRRC8A expression had
shorter survival times, suggesting that LRRC8A may serve as a novel
prognostic biomarker for CRC. Elevated expression of LRRC8A has
been linked to enhanced cancer cell growth and metastasis (157), whereas downregulation of VRACs
has been shown to inhibit colon cancer cell proliferation (158). Furthermore, one study reported
that LRRC8A contributed to oxaliplatin resistance in colon cancer
cells (159). Of note,
exosomes, which are crucial mediators of intercellular
communication in the tumor microenvironment and accelerate colon
cancer progression, contain LRRC8A. This protein, which is one of
the components of exosomes released from colon cancer HCT116 cells,
is responsible for exosome production and is involved in volume
regulation (160).
Research has reported elevated TMEM206 expression in
CRC tissues compared with adjacent noncancer tissues. TMEM206
promotes CRC cell proliferation, invasion and migration by
increasing the levels of phosphorylated AKT and downstream
signaling components, as well as the level of phosphorylated
extracellular signal-regulated kinase (ERK), facilitating
interactions between the AKT and ERK pathways (163). Bioinformatics analysis revealed
that TMEM206 expression is significantly elevated in CRC, breast
cancer, HCC and lymphoma tissues compared with corresponding normal
tissues, identifying TMEM206 as a potential prognostic biomarker
for HCC (164).
Ligand-gated chloride channels, such as GABA-A and
glycine receptor channels, consist of five subunits that form the
central ionotropic pore. Each subunit contains a characteristic
Cys-loop structural domain involved in ligand binding and channel
gating (165). They primarily
play roles in the development and function of the nervous system
(166). Channelopathies
involving these channels have been linked to neurological disorders
such as Parkinson's disease, Huntington's disease and Alzheimer's
disease (167-169). However, to the best of our
knowledge, no studies have explored the role of these channels in
digestive disorders.
Ion channels and transport proteins play crucial
roles in regulating pathophysiological processes across various
organs of the digestive system. In recent years, sodium, calcium
and potassium ion channels have been extensively studied, whereas
chloride channels have received comparatively less attention.
Chloride, the most abundant anion in the body, is regulated
primarily by chloride channels or transporters. Existing studies on
chloride channels have demonstrated their significant role in the
development of numerous digestive system diseases (Table I), including esophagitis,
esophageal cancer, gastrointestinal dyskinesia, gastritis, GC,
colonic diverticulitis, diverticulosis, congenital megacolon, colon
cancer, pancreatitis, pancreatic cancer, hepatic lipid metabolism
disorders and HCC. Although the involvement of chloride channels in
the digestive system has been explored, the underlying
physiological and pathological mechanisms within various organs
have not been thoroughly investigated. Most research to date has
focused on verifying the high or low expression of chloride
channels in digestive diseases or their involvement in relevant
signaling pathways, whereas fewer studies have delved into deeper
mechanisms such as transcriptional regulation or posttranslational
modifications. Therefore, further investigations into chloride
channels constitute an important and promising research area that
remains to be fully explored.
Not applicable.
YH wrote the original manuscript and performed the
literature review. BT was responsible for the conceptualization and
review of the study and provided supervision. Both authors have
read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors thank Professor Hui Dong (Department of
Medicine, School of Medicine, University of California, San Diego
University, San Diego, USA) for providing guidance in the writing
of the paper.
The authors gratefully acknowledge financial support from the
National Natural Science Foundation of China (grant no. 81960507),
the Basic Research Projects of Science and Technology Department of
Guizhou Province (grant code, Qian Ke He-zk[2022]-646) and the 2023
Graduate Research Fund Program B (grant no. ZYK248).
|
1
|
Nguyen HX and Bursac N: Ion channel
engineering for modulation and de novo generation of electrical
excitability. Curr Opin Biotechnol. 58:100–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capatina AL, Lagos D and Brackenbury WJ:
Targeting ion channels for cancer treatment: Current progress and
future challenges. Rev Physiol Biochem Pharmacol. 183:1–43.
2022.
|
|
3
|
Bulk E, Todesca LM and Schwab A: Ion
channels in lung cancer. Rev Physiol Biochem Pharmacol. 181:57–79.
2021. View Article : Google Scholar
|
|
4
|
Xiao F, Yu Q, Li J, Johansson ME, Singh
AK, Xia W, Riederer B, Engelhardt R, Montrose M, Soleimani M, et
al: Slc26a3 deficiency is associated with loss of colonic HCO3 (-)
secretion, absence of a firm mucus layer and barrier impairment in
mice. Acta Physiol (Oxf). 211:161–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zahn A, Moehle C, Langmann T, Ehehalt R,
Autschbach F, Stremmel W and Schmitz G: Aquaporin-8 expression is
reduced in ileum and induced in colon of patients with ulcerative
colitis. World J Gastroenterol. 13:1687–1695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu H, Zhang B, Li J, Wang C, Chen H and
Ghishan FK: Impaired mucin synthesis and bicarbonate secretion in
the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver
Physiol. 303:G335–G343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh AK, Sjöblom M, Zheng W, Krabbenhöft
A, Riederer B, Rausch B, Manns MP, Soleimani M and Seidler U: CFTR
and its key role in in vivo resting and luminal acid-induced
duodenal HCO3-secretion. Acta Physiol (Oxf). 193:357–365. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schultheis PJ, Clarke LL, Meneton P,
Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller
ML and Shull GE: Targeted disruption of the murine Na+/H+ exchanger
isoform 2 gene causes reduced viability of gastric parietal cells
and loss of net acid secretion. J Clin Invest. 101:1243–1253. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang A, Li J, Zhao Y, Johansson ME, Xu H
and Ghishan FK: Loss of NHE8 expression impairs intestinal mucosal
integrity. Am J Physiol Gastrointest Liver Physiol. 309:G855–G864.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boivin GP, Schultheis PJ, Shull GE and
Stemmermann GN: Variant form of diffuse corporal gastritis in NHE2
knockout mice. Comp Med. 50:511–515. 2000.PubMed/NCBI
|
|
11
|
Ding X, Li D, Li M, Wang H, He Q, Wang Y,
Yu H, Tian D and Yu Q: SLC26A3 (DRA) prevents TNF-alpha-induced
barrier dysfunction and dextran sulfate sodium-induced acute
colitis. Lab Invest. 98:462–476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Xu Y, Chen Z, Xu Z and Xu H:
Knockdown of aquaporin 3 is involved in intestinal barrier
integrity impairment. FEBS Lett. 585:3113–3119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moeser AJ, Nighot PK, Ryan KA, Simpson JE,
Clarke LL and Blikslager AT: Mice lacking the Na+/H+ exchanger 2
have impaired recovery of intestinal barrier function. Am J Physiol
Gastrointest Liver Physiol. 295:G791–G797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar A, Priyamvada S, Ge Y, Jayawardena
D, Singhal M, Anbazhagan AN, Chatterjee I, Dayal A, Patel M, Zadeh
K, et al: A novel role of SLC26A3 in the maintenance of intestinal
epithelial barrier integrity. Gastroenterology. 160:1240–1255.e3.
2021. View Article : Google Scholar
|
|
15
|
Doi K, Nagao T, Kawakubo K, Ibayashi S,
Aoyagi K, Yano Y, Yamamoto C, Kanamoto K, Iida M, Sadoshima S and
Fujishima M: Calcitonin gene-related peptide affords gastric
mucosal protection by activating potassium channel in Wistar rat.
Gastroenterology. 114:71–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng Z, Zhao Y, Ma Z, Zhang M, Wang H, Yi
Z, Tuo B, Li T and Liu X: Pathophysiological role of ion channels
and transporters in gastrointestinal mucosal diseases. Cell Mol
Life Sci. 78:8109–8125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pallagi P, Hegyi P and Rakonczay Z Jr: The
physiology and pathophysiology of pancreatic ductal secretion: The
background for clinicians. Pancreas. 44:1211–1233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishiguro H, Yamamoto A, Nakakuki M, Yi L,
Ishiguro M, Yamaguchi M, Kondo S and Mochimaru Y: Physiology and
pathophysiology of bicarbonate secretion by pancreatic duct
epithelium. Nagoya J Med Sci. 74:1–18. 2012.PubMed/NCBI
|
|
19
|
Novak I, Haanes KA and Wang J: Acid-base
transport in pancreas-new challenges. Front Physiol. 4:3802013.
View Article : Google Scholar
|
|
20
|
Schnipper J, Dhennin-Duthille I, Ahidouch
A and Ouadid-Ahidouch H: Ion channel signature in healthy pancreas
and pancreatic ductal adenocarcinoma. Front Pharmacol.
11:5689932020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Zhang L, Zheng L and Tuo B: Role
of Ca(2+) channels in non-alcoholic fatty liver disease and their
implications for therapeutic strategies (Review). Int J Mol Med.
50:1132022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stock C: How dysregulated ion channels and
transporters take a hand in esophageal, liver, and colorectal
cancer. Rev Physiol Biochem Pharmacol. 181:129–222. 2021.
View Article : Google Scholar
|
|
23
|
Li W, Wang C, Peng X, Zhang H, Huang H and
Liu H: CFTR inhibits the invasion and growth of esophageal cancer
cells by inhibiting the expression of NF-κB. Cell Biol Int.
42:1680–1687. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldman A, Chen H, Khan MR, Roesly H, Hill
KA, Shahidullah M, Mandal A, Delamere NA and Dvorak K: The
Na+/H+ exchanger controls deoxycholic
acid-induced apoptosis by a H+-activated,
Na+-dependent ionic shift in esophageal cells. PLoS One.
6:e238352011. View Article : Google Scholar
|
|
25
|
Boult J, Roberts K, Brookes MJ, Hughes S,
Bury JP, Cross SS, Anderson GJ, Spychal R, Iqbal T and Tselepis C:
Overexpression of cellular iron import proteins is associated with
malignant progression of esophageal adenocarcinoma. Clin Cancer
Res. 14:379–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao N, Yang F, Chen S, Wan H, Zhao X and
Dong H: The role of TRPV1 ion channels in the suppression of
gastric cancer development. Exp Clin Cancer Res. 39:2062020.
View Article : Google Scholar
|
|
27
|
Chang Y, Roy S and Pan Z: Store-operated
calcium channels as drug target in gastroesophageal cancers. Front
Pharmacol. 12:6687302021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Csekő K, Pécsi D, Kajtár B, Hegedűs I,
Bollenbach A, Tsikas D, Szabó IL, Szabó S and Helyes Z:
Upregulation of the TRPA1 Ion channel in the gastric mucosa after
iodoacetamide-induced gastritis in rats: A potential new
therapeutic target. Int J Mol Sci. 21:55912020. View Article : Google Scholar
|
|
29
|
Capurro MI, Greenfield LK, Prashar A, Xia
S, Abdullah M, Wong H, Zhong XZ, Bertaux-Skeirik N, Chakrabarti J,
Siddiqui I, et al: VacA generates a protective intracellular
reservoir for Helicobacter pylori that is eliminated by activation
of the lysosomal calcium channel TRPML1. Nat Microbiol.
4:1411–1423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Zhang Y, Jiang Y, Dou X, Li S,
Chai H, Qian Q and Wang M: Upregulated SOCC and IP3R calcium
channels and subsequent elevated cytoplasmic calcium signaling
promote nonalcoholic fatty liver disease by inhibiting autophagy.
Mol Cell Biochem. 476:3163–3175. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Swain SM, Romac JM, Shahid RA, Pandol SJ,
Liedtke W, Vigna SR and Liddle RA: TRPV4 channel opening mediates
pressure-induced pancreatitis initiated by Piezo1 activation. J
Clin Invest. 130:2527–2541. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin R, Bao X, Wang H, Zhu S, Liu Z, Chen
Q, Ai K and Shi B: TRPM2 promotes pancreatic cancer by PKC/MAPK
pathway. Cell Death Dis. 12:5852021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Han J, Guo G, Sun Y, Zhang T, Zhao
M, Xu Y, Cui Y, Liu Y and Zhang J: Voltage-gated sodium channels β3
subunit promotes tumorigenesis in hepatocellular carcinoma by
facilitating p53 degradation. FEBS Lett. 594:497–508. 2020.
View Article : Google Scholar
|
|
34
|
Nattramilarasu PK, Bücker R, Lobo de Sá
FD, Fromm A, Nagel O, Lee IM, Butkevych E, Mousavi S, Genger C,
Kløve S, et al: Campylobacter concisus impairs sodium absorption in
colonic epithelium via ENaC dysfunction and claudin-8 disruption.
Int J Mol Sci. 21:3732020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao F, Wang D, Liu X, Wu YH, Wang HT and
Sun SL: Sodium channel 1 subunit alpha SCNN1A exerts oncogenic
function in pancreatic cancer via accelerating cellular growth and
metastasis. Arch Biochem Biophys. 727:1093232022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Li C, Peng M, Wang L, Zhao D, Wu
T, Yi D, Hou Y and Wu G: N-Acetylcysteine improves intestinal
function and attenuates intestinal autophagy in piglets challenged
with β-conglycinin. Sci Rep. 11:12612021. View Article : Google Scholar
|
|
37
|
Goswami S: Interplay of potassium channel,
gastric parietal cell and proton pump in gastrointestinal
physiology, pathology and pharmacology. Minerva Gastroenterol
(Torino). 68:289–305. 2022.
|
|
38
|
Patel SH, Edwards MJ and Ahmad SA:
Intracellular ion channels in pancreas cancer. Cell Physiol
Biochem. 53:44–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schroeder BC, Waldegger S, Fehr S, Bleich
M, Warth R, Greger R and Jentsch TJ: A constitutively open
potassium channel formed by KCNQ1 and KCNE3. Nature. 403:196–199.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ponnalagu D and Singh H: Anion channels of
mitochondria. Handb Exp Pharmacol. 240:71–101. 2017. View Article : Google Scholar
|
|
41
|
Gururaja Rao S, Ponnalagu D, Patel NJ and
Singh H: Three decades of chloride intracellular channel proteins:
From organelle to organ physiology. Curr Protoc Pharmacol.
80:11.21.1–11.21.17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kouyoumdzian NM, Kim G, Rudi MJ, Rukavina
Mikusic NL, Fernández BE and Choi MR: Clues and new evidences in
arterial hypertension: Unmasking the role of the chloride anion.
Pflugers Arch. 474:155–176. 2022. View Article : Google Scholar
|
|
43
|
Jentsch TJ and Pusch M: CLC chloride
channels and transporters: Structure, function, physiology, and
disease. Physiol Rev. 98:1493–1590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi J, Shi S, Yuan G, Jia Q, Shi S, Zhu X,
Zhou Y, Chen T and Hu Y: Bibliometric analysis of chloride channel
research (2004-2019). Channels (Austin). 14:393–402. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jentsch TJ, Stein V, Weinreich F and
Zdebik AA: Molecular structure and physiological function of
chloride channels. Physiol Rev. 82:503–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peng F, Wu Y, Dong X and Huang P:
Proton-activated chloride channel: Physiology and disease. Front
Biosci (Landmark Ed). 28:112023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Patil VM and Gupta SP: Studies on chloride
channels and their modulators. Curr Top Med Chem. 16:1862–1876.
2016. View Article : Google Scholar
|
|
48
|
Accardi A and Miller C: Secondary active
transport mediated by a prokaryotic homologue of ClC Cl- hannels.
Nature. 427:803–807. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jentsch TJ: CLC chloride channels and
transporters: From genes to protein structure, pathology and
physiology. Crit Rev Biochem Mol Biol. 43:3–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang YD, Cho H, Koo JY, Tak MH, Cho Y,
Shim WS, Park SP, Lee J, Lee B, Kim BM, et al: TMEM16A confers
receptor-activated calcium-dependent chloride conductance. Nature.
455:1210–1215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Caputo A, Caci E, Ferrera L, Pedemonte N,
Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O and
Galietta LJ: TMEM16A, a membrane protein associated with
calcium-dependent chloride channel activity. Science. 322:590–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gadsby DC, Vergani P and Csanády L: The
ABC protein turned chloride channel whose failure causes cystic
fibrosis. Nature. 440:477–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Riordan JR, Rommens JM, Kerem B, Alon N,
Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et
al: Identification of the cystic fibrosis gene: Cloning and
characterization of complementary DNA. Science. 245:1066–1073.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ullrich F, Blin S, Lazarow K, Daubitz T,
von Kries JP and Jentsch TJ: Identification of TMEM206 proteins as
pore of PAORAC/ASOR acid-sensitive chloride channels. Elife.
8:e491872019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Olsen RW and Sieghart W: International
Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A)
receptors: Classification on the basis of subunit composition,
pharmacology, and function. Update. Pharmacol Rev. 60:243–260.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lynch JW: Molecular structure and function
of the glycine receptor chloride channel. Physiol Rev.
84:1051–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Peng JM, Lin SH, Yu MC and Hsieh SY: CLIC1
recruits PIP5K1A/C to induce cell-matrix adhesions for tumor
metastasis. J Clin Invest. 131:e1335252021. View Article : Google Scholar :
|
|
58
|
Bi MM, Hong S, Zhou HY, Wang HW, Wang LN
and Zheng YJ: Chloride channelopathies of ClC-2. Int J Mol Sci.
15:218–249. 2013. View Article : Google Scholar
|
|
59
|
Okada Y, Okada T, Sato-Numata K, Islam MR,
Ando-Akatsuka Y, Numata T, Kubo M, Shimizu T, Kurbannazarova RS,
Marunaka Y and Sabirov RZ: Cell volume-activated and
volume-correlated anion channels in mammalian cells: Their
biophysical, molecular, and pharmacological properties. Pharmacol
Rev. 71:49–88. 2019. View Article : Google Scholar
|
|
60
|
Fu D, Cui H and Zhang Y: Lack of ClC-2
alleviates high fat diet-induced insulin resistance and
non-alcoholic fatty liver disease. Cell Physiol Biochem.
45:2187–2198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Blikslager AT, Moeser AJ, Gookin JL, Jones
SL and Odle J: Restoration of barrier function in injured
intestinal mucosa. Physiol Rev. 87:545–564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gehren AS, Rocha MR, de Souza WF and
Morgado-Díaz JA: Alterations of the apical junctional complex and
actin cytoskeleton and their role in colorectal cancer progression.
Tissue Barriers. 3:e10176882015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nighot PK, Moeser AJ, Ryan KA, Ghashghaei
T and Blikslager AT: ClC-2 is required for rapid restoration of
epithelial tight junctions in ischemic-injured murine jejunum. Exp
Cell Res. 315:110–118. 2009. View Article : Google Scholar
|
|
64
|
Jin Y, Ibrahim D, Magness ST and
Blikslager AT: Knockout of ClC-2 reveals critical functions of
adherens junctions in colonic homeostasis and tumorigenicity. Am J
Physiol Gastrointest Liver Physiol. 315:G966–G979. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mrad FCC, Soares SBM, de Menezes Silva
LAW, Dos Anjos Menezes PV and Simões-E-Silva AC: Bartter's
syndrome: Clinical findings, genetic causes and therapeutic
approach. World J Pediatr. 17:31–39. 2021. View Article : Google Scholar
|
|
66
|
Andrini O, Eladari D and Picard N: ClC-K
kidney chloride channels: From structure to pathology. Handb Exp
Pharmacol. 283:35–58. 2024. View Article : Google Scholar
|
|
67
|
Mu H, Mu L and Gao J: Suppression of CLC-3
reduces the proliferation, invasion and migration of colorectal
cancer through Wnt/β-catenin signaling pathway. Biochem Biophys Res
Commun. 533:1240–1246. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gu Z, Li Y, Yang X, Yu M, Chen Z, Zhao C,
Chen L and Wang L: Overexpression of CLC-3 is regulated by XRCC5
and is a poor prognostic biomarker for gastric cancer. J Hematol
Oncol. 11:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cheng W, Zheng S, Li L, Zhou Q, Zhu H, Hu
J and Luo H: Chloride channel 3 (CIC-3) predicts the tumor size in
hepatocarcinoma. Acta Histochem. 121:284–288. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang LY, Li YJ, Li PP, Li HC and Ma P:
Aggravated intestinal apoptosis by ClC-3 deletion is lethal to mice
endotoxemia. Cell Biol Int. 42:1445–1453. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Huang LY, He Q, Liang SJ, Su YX, Xiong LX,
Wu QQ, Wu QY, Tao J, Wang JP, Tang YB, et al: ClC-3 chloride
channel/antiporter defect contributes to inflammatory bowel disease
in humans and mice. Gut. 63:1587–1595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Stauber T, Weinert S and Jentsch TJ: Cell
biology and physiology of CLC chloride channels and transporters.
Compr Physiol. 2:1701–1744. 2012. View Article : Google Scholar
|
|
73
|
Stauber T and Jentsch TJ: Chloride in
vesicular trafficking and function. Annu Rev Physiol. 75:453–477.
2013. View Article : Google Scholar
|
|
74
|
Wartosch L, Fuhrmann JC, Schweizer M,
Stauber T and Jentsch TJ: Lysosomal degradation of endocytosed
proteins depends on the chloride transport protein ClC-7. FASEB J.
23:4056–4068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nicoli ER, Weston MR, Hackbarth M,
Becerril A, Larson A, Zein WM, Baker PR II, Burke JD, Dorward H,
Davids M, et al: Lysosomal storage and albinism due to effects of a
de novo CLCN7 variant on lysosomal acidification. Am J Hum Genet.
104:1127–1138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Polovitskaya MM, Barbini C, Martinelli D,
Harms FL, Cole FS, Calligari P, Bocchinfuso G, Stella L, Ciolfi A,
Niceta M, et al: A recurrent gain-of-function mutation in CLCN6,
encoding the ClC-6 Cl(-)/H(+)-exchanger, causes early-onset
neurodegeneration. Am J Hum Genet. 107:1062–1077. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bose S, He H and Stauber T:
Neurodegeneration upon dysfunction of endosomal/lysosomal CLC
chloride transporters. Front Cell Dev Biol. 9:6392312021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Locher KP: Mechanistic diversity in
ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol.
23:487–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Csanády L, Vergani P and Gadsby DC:
Structure, gating, and regulation of the CFTR anion channel.
Physiol Rev. 99:707–738. 2019. View Article : Google Scholar
|
|
80
|
Fonseca C, Bicker J, Alves G, Falcão A and
Fortuna A: Cystic fibrosis: Physiopathology and the latest
pharmacological treatments. Pharmacol Res. 162:1052672020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lukasiak A and Zajac M: The distribution
and role of the CFTR protein in the intracellular compartments.
Membranes (Basel). 11:8042021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Matsumoto Y, Shiozaki A, Kosuga T, Kudou
M, Shimizu H, Arita T, Konishi H, Komatsu S, Kubota T, Fujiwara H,
et al: Expression and role of CFTR in human esophageal squamous
cell carcinoma. Ann Surg Oncol. 28:6424–6436. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang J, Liu X, Zeng L and Hu Y: GABRP
inhibits the progression of oesophageal cancer by regulating CFTR:
Integrating bioinformatics analysis and experimental validation.
Int J Exp Pathol. 105:118–132. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shakir S, Echevarria C, Doe S, Brodlie M,
Ward C and Bourke SJ: Elexacaftor-tezacaftor-ivacaftor improve
gastro-oesophageal reflux and sinonasal symptoms in advanced cystic
fibrosis. J Cyst Fibros. 21:807–810. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rowe SM, Heltshe SL, Gonska T, Donaldson
SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van
Dalfsen JM, et al: Clinical mechanism of the cystic fibrosis
transmembrane conductance regulator potentiator ivacaftor in
G551D-mediated cystic fibrosis. Am J Respir Crit Care Med.
190:175–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Petrov MS and Yadav D: Global epidemiology
and holistic prevention of pancreatitis. Nat Rev Gastroenterol
Hepatol. 16:175–184. 2019. View Article : Google Scholar :
|
|
87
|
Mayerle J, Sendler M, Hegyi E, Beyer G,
Lerch MM and Sahin-Tóth M: Genetics, cell biology, and
pathophysiology of pancreatitis. Gastroenterology.
156:1951–1968.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hou Y, Guan X, Yang Z and Li C: Emerging
role of cystic fibrosis transmembrane conductance regulator-an
epithelial chloride channel in gastrointestinal cancers. World J
Gastrointest Oncol. 8:282–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fűr G, Bálint ER, Orján EM, Balla Z,
Kormányos ES, Czira B, Szűcs A, Kovács DP, Pallagi P, Maléth J, et
al: Mislocalization of CFTR expression in acute pancreatitis and
the beneficial effect of VX-661 + VX-770 treatment on disease
severity. J Physiol. 599:4955–4971. 2021. View Article : Google Scholar
|
|
90
|
Kim Y, Jun I, Shin DH, Yoon JG, Piao H,
Jung J, Park HW, Cheng MH, Bahar I, Whitcomb DC and Lee MG:
Regulation of CFTR bicarbonate channel activity by WNK1:
Implications for pancreatitis and CFTR-Related disorders. Cell Mol
Gastroenterol Hepatol. 9:79–103. 2020. View Article : Google Scholar
|
|
91
|
Lee MG, Ohana E, Park HW, Yang D and
Muallem S: Molecular mechanism of pancreatic and salivary gland
fluid and HCO3 secretion. Physiol Rev. 92:39–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fong P: CFTR-SLC26 transporter
interactions in epithelia. Biophys Rev. 4:107–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH,
Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ and Muallem S:
Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell
Biol. 6:343–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Berg P, Svendsen SL, Sorensen MV, Larsen
CK, Andersen JF, Jensen-Fangel S, Jeppesen M, Schreiber R, Cabrita
I, Kunzelmann K and Leipziger J: Impaired renal HCO(3)(-) excretion
in cystic fibrosis. J Am Soc Nephrol. 31:1711–1727. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ishiguro H, Steward MC, Naruse S, Ko SB,
Goto H, Case RM, Kondo T and Yamamoto A: CFTR functions as a
bicarbonate channel in pancreatic duct cells. J Gen Physiol.
133:315–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Park HW, Nam JH, Kim JY, Namkung W, Yoon
JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH and Lee MG:
Dynamic regulation of CFTR bicarbonate permeability by [Cl-]i and
its role in pancreatic bicarbonate secretion. Gastroenterology.
139:620–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang CL, Liu X, Paliege A, Zhu X, Bachmann
S, Dawson DC and Ellison DH: WNK1 and WNK4 modulate CFTR activity.
Biochem Biophys Res Commun. 353:535–540. 2007. View Article : Google Scholar
|
|
98
|
Yang D, Li Q, So I, Huang CL, Ando H,
Mizutani A, Seki G, Mikoshiba K, Thomas PJ and Muallem S: IRBIT
governs epithelial secretion in mice by antagonizing the WNK/SPAK
kinase pathway. J Clin Invest. 121:956–965. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shirakabe K, Priori G, Yamada H, Ando H,
Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G and Mikoshiba K:
IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein,
specifically binds to and activates pancreas-type
Na+/HCO3-cotransporter 1 (pNBC1). Proc Natl Acad Sci USA.
103:9542–9547. 2006. View Article : Google Scholar
|
|
100
|
Park S, Shcheynikov N, Hong JH, Zheng C,
Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, et al:
Irbit mediates synergy between ca(2+) and cAMP signaling pathways
during epithelial transport in mice. Gastroenterology. 145:232–241.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pankonien I, Quaresma MC, Rodrigues CS and
Amaral MD: CFTR, cell junctions and the cytoskeleton. Int J Mol
Sci. 23:2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wen G, Jin H, Deng S, Xu J, Liu X, Xie R
and Tuo B: Effects of helicobacter pylori infection on the
expressions and functional activities of human duodenal mucosal
bicarbonate transport proteins. Helicobacter. 21:536–547. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Reyes EA, Castillo-Azofeifa D, Rispal J,
Wald T, Zwick RK, Palikuqi B, Mujukian A, Rabizadeh S, Gupta AR,
Gardner JM, et al: Epithelial TNF controls cell differentiation and
CFTR activity to maintain intestinal mucin homeostasis. J Clin
Invest. 133:e1635912023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bhattacharya R, Blankenheim Z, Scott PM
and Cormier RT: CFTR and gastrointestinal cancers: An update. J
Pers Med. 12:8682022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu H, Wu W, Liu Y, Zhang C and Zhou Z:
Predictive value of cystic fibrosis transmembrane conductance
regulator (CFTR) in the diagnosis of gastric cancer. Clinical and
investigative medicine. Clin Invest Med. 37:E226–E232. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yamada A, Komaki Y, Komaki F, Micic D,
Zullow S and Sakuraba A: Risk of gastrointestinal cancers in
patients with cystic fibrosis: A systematic review and
meta-analysis. Lancet Oncol. 19:758–767. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Jakab RL, Collaco AM and Ameen NA:
Physiological relevance of cell-specific distribution patterns of
CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the
intestine. Am J Physiol Gastrointest Liver Physiol. 300:G82–G98.
2011. View Article : Google Scholar :
|
|
108
|
Tse CM, Yin J, Singh V, Sarker R, Lin R,
Verkman AS, Turner JR and Donowitz M: cAMP stimulates SLC26A3
activity in human colon by a CFTR-dependent mechanism that does not
require CFTR activity. Cell Mol Gastroenterol Hepatol. 7:641–653.
2019. View Article : Google Scholar :
|
|
109
|
Vernocchi P, Del Chierico F, Russo A, Majo
F, Rossitto M, Valerio M, Casadei L, La Storia A, De Filippis F,
Rizzo C, et al: Gut microbiota signatures in cystic fibrosis: Loss
of host CFTR function drives the microbiota enterophenotype. PLoS
One. 13:e02081712018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Burke DG, Fouhy F, Harrison MJ, Rea MC,
Cotter PD, O'Sullivan O, Stanton C, Hill C, Shanahan F, Plant BJ
and Ross RP: The altered gut microbiota in adults with cystic
fibrosis. BMC Microbiol. 17:582017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
De Lisle RC: Disrupted tight junctions in
the small intestine of cystic fibrosis mice. Cell Tissue Res.
355:131–142. 2014. View Article : Google Scholar :
|
|
112
|
Broadbent D, Ahmadzai MM, Kammala AK, Yang
C, Occhiuto C, Das R and Subramanian H: Roles of NHERF family of
PDZ-binding proteins in regulating GPCR functions. Adv Immunol.
136:353–385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Meeker SM, Mears KS, Sangwan N,
Brittnacher MJ, Weiss EJ, Treuting PM, Tolley N, Pope CE, Hager KR,
Vo AT, et al: CFTR dysregulation drives active selection of the gut
microbiome. PLoS Pathog. 16:e10082512020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mulcahy EM, Cooley MA, McGuire H, Asad S,
Fazekas de St Groth B, Beggs SA and Roddam LF: Widespread
alterations in the peripheral blood innate immune cell profile in
cystic fibrosis reflect lung pathology. Immunol Cell Biol.
97:416–426. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Trigo Salado C, Leo Carnerero E and de la
Cruz Ramírez MD: Crohn's disease and cystic fibrosis: There is
still a lot to learn. Rev Esp Enferm Dig. 110:835–836. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gabel ME, Galante GJ and Freedman SD:
Gastrointestinal and hepatobiliary disease in cystic fibrosis.
Semin Respir Crit Care Med. 40:825–841. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Maisonneuve P, Marshall BC, Knapp EA and
Lowenfels AB: Cancer risk in cystic fibrosis: A 20-year nationwide
study from the United States. J Natl Cancer Inst. 105:122–129.
2013. View Article : Google Scholar
|
|
118
|
Than BL, Linnekamp JF, Starr TK,
Largaespada DA, Rod A, Zhang Y, Bruner V, Abrahante J, Schumann A,
Luczak T, et al: CFTR is a tumor suppressor gene in murine and
human intestinal cancer. Oncogene. 35:4179–4187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sun TT, Wang Y, Cheng H, Xiao HZ, Xiang
JJ, Zhang JT, Yu SB, Martin TA, Ye L, Tsang LL, et al: Disrupted
interaction between CFTR and AF-6/afadin aggravates malignant
phenotypes of colon cancer. Biochim Biophys Acta. 1843:618–628.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Palma AG, Soares Machado M, Lira MC, Rosa
F, Rubio MF, Marino G, Kotsias BA and Costas MA: Functional
relationship between CFTR and RAC3 expression for maintaining
cancer cell stemness in human colorectal cancer. Cell Oncol
(Dordr). 44:627–641. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Strubberg AM, Liu J, Walker NM, Stefanski
CD, MacLeod RJ, Magness ST and Clarke LL: Cftr modulates
Wnt/β-catenin signaling and stem cell proliferation in murine
intestine. Cell Mol Gastroenterol Hepatol. 5:253–271. 2017.
View Article : Google Scholar
|
|
122
|
Liu C, Song C, Li J and Sun Q: CFTR
functions as a tumor suppressor and is regulated by DNA methylation
in colorectal cancer. Cancer Manag Res. 12:4261–4270. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Paulino C, Neldner Y, Lam AK, Kalienkova
V, Brunner JD, Schenck S and Dutzler R: Structural basis for anion
conduction in the calcium-activated chloride channel TMEM16A.
Elife. 6:e262322017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dang S, Feng S, Tien J, Peters CJ, Bulkley
D, Lolicato M, Zhao J, Zuberbühler K, Ye W, Qi L, et al: Cryo-EM
structures of the TMEM16A calcium-activated chloride channel.
Nature. 552:426–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ji Q, Guo S, Wang X, Pang C, Zhan Y, Chen
Y and An H: Recent advances in TMEM16A: Structure, function, and
disease. J Cell Physiol. 234:7856–7873. 2019. View Article : Google Scholar
|
|
126
|
Liu Y, Liu Z and Wang K: The
Ca(2+)-activated chloride channel ANO1/TMEM16A: An emerging
therapeutic target for epithelium-originated diseases? Acta Pharm
Sin B. 11:1412–1433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Crottès D and Jan LY: The multifaceted
role of TMEM16A in cancer. Cell Calcium. 82:1020502019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jang Y and Oh U: Anoctamin 1 in secretory
epithelia. Cell Calcium. 55:355–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ji Q, Shi S, Guo S, Zhan Y, Zhang H, Chen
Y and An H: Activation of TMEM16A by natural product canthaxanthin
promotes gastrointestinal contraction. FASEB J. 34:13430–13444.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Stephan AB, Shum EY, Hirsh S, Cygnar KD,
Reisert J and Zhao H: ANO2 is the cilial calcium-activated chloride
channel that may mediate olfactory amplification. Proc Natl Acad
Sci USA. 106:11776–11781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Huang WC, Xiao S, Huang F, Harfe BD, Jan
YN and Jan LY: Calcium-activated chloride channels (CaCCs) regulate
action potential and synaptic response in hippocampal neurons.
Neuron. 74:179–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ha GE, Lee J, Kwak H, Song K, Kwon J, Jung
SY, Hong J, Chang GE, Hwang EM, Shin HS, et al: The
Ca(2+)-activated chloride channel anoctamin-2 mediates
spike-frequency adaptation and regulates sensory transmission in
thalamocortical neurons. Nat Commun. 7:137912016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Vanoni S, Zeng C, Marella S, Uddin J, Wu
D, Arora K, Ptaschinski C, Que J, Noah T, Waggoner L, et al:
Identification of anoctamin 1 (ANO1) as a key driver of esophageal
epithelial proliferation in eosinophilic esophagitis. J Allergy
Clin Immunol. 145:239–254.e2. 2020. View Article : Google Scholar :
|
|
134
|
Yu Y, Cao J, Wu W, Zhu Q, Tang Y, Zhu C,
Dai J, Li Z, Wang J, Xue L, et al: Genome-wide copy number
variation analysis identified ANO1 as a novel oncogene and
prognostic biomarker in esophageal squamous cell cancer.
Carcinogenesis. 40:1198–1208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Le SC, Jia Z, Chen J and Yang H: Molecular
basis of PIP(2)-dependent regulation of the Ca(2+)-activated
chloride channel TMEM16A. Nat Commun. 10:37692019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Fairfield CJ, Drake TM, Pius R, Bretherick
AD, Campbell A, Clark DW, Fallowfield JA, Hayward C, Henderson NC,
Iakovliev A, et al: Genome-wide analysis identifies
gallstone-susceptibility loci including genes regulating
gastrointestinal motility. Hepatology. 75:1081–1094. 2022.
View Article : Google Scholar
|
|
137
|
Camilleri M, Sandler RS and Peery AF:
Etiopathogenetic mechanisms in diverticular disease of the colon.
Cell Mol Gastroenterol Hepatol. 9:15–32. 2020. View Article : Google Scholar
|
|
138
|
Schafmayer C, Harrison JW, Buch S, Lange
C, Reichert MC, Hofer P, Cossais F, Kupcinskas J, von Schönfels W,
Schniewind B, et al: Genome-wide association analysis of
diverticular disease points towards neuromuscular, connective
tissue and epithelial pathomechanisms. Gut. 68:854–865. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zeng X, Pan D, Wu H, Chen H, Yuan W, Zhou
J, Shen Z and Chen S: Transcriptional activation of ANO1 promotes
gastric cancer progression. Biochem Biophys Res Commun.
512:131–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xie R, Liu L, Lu X and Hu Y: LncRNA
OIP5-AS1 facilitates gastric cancer cell growth by targeting the
miR-422a/ANO1 axis. Acta Biochim Biophys Sin (Shanghai).
52:430–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lu G, Shi W and Zheng H: Inhibition of
STAT6/Anoctamin-1 activation suppresses proliferation and invasion
of gastric cancer cells. Cancer Biother Radiopharm. 33:3–7.
2018.PubMed/NCBI
|
|
142
|
Yan Y, Ding X, Han C, Gao J, Liu Z, Liu Y
and Wang K: Involvement of TMEM16A/ANO1 upregulation in the
oncogenesis of colorectal cancer. Biochim Biophys Acta Mol Basis
Dis. 1868:1663702022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wang Q, Bai L, Luo S, Wang T, Yang F, Xia
J, Wang H, Ma K, Liu M, Wu S, et al: TMEM16A Ca(2+)-activated Cl(-)
channel inhibition ameliorates acute pancreatitis via the IP(3)
R/Ca(2+)/NFκB/IL-6 signaling pathway. J Adv Res. 23:25–35. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhang G, Shu Z, Yu J, Li J, Yi P, Wu B,
Deng D, Yan S, Li Y, Ren D, et al: High ANO1 expression is a
prognostic factor and correlated with an immunosuppressive tumor
microenvironment in pancreatic cancer. Front Immunol.
15:13412092024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Guo JW, Liu X, Zhang TT, Lin XC, Hong Y,
Yu J, Wu QY, Zhang FR, Wu QQ, Shang JY, et al: Hepatocyte TMEM16A
deletion retards NAFLD progression by ameliorating hepatic glucose
metabolic disorder. Adv Sci (Weinh). 7:19036572020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Guo J, Song Z, Yu J, Li C, Jin C, Duan W,
Liu X, Liu Y, Huang S, Tuo Y, et al: Hepatocyte-specific TMEM16A
deficiency alleviates hepatic ischemia/reperfusion injury via
suppressing GPX4-mediated ferroptosis. Cell Death Dis. 13:10722022.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Kondo R, Furukawa N, Deguchi A, Kawata N,
Suzuki Y, Imaizumi Y and Yamamura H: Downregulation of
Ca(2+)-activated Cl(-) channel TMEM16A mediated by angiotensin II
in cirrhotic portal hypertensive mice. Front Pharmacol.
13:8313112022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Deng L, Yang J, Chen H, Ma B, Pan K, Su C,
Xu F and Zhang J: Knockdown of TMEM16A suppressed MAPK and
inhibited cell proliferation and migration in hepatocellular
carcinoma. Onco Targets Ther. 9:325–333. 2016.PubMed/NCBI
|
|
149
|
König B and Stauber T: Biophysics and
structure-function relationships of LRRC8-formed volume-regulated
anion channels. Biophys J. 116:1185–1193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Sawicka M and Dutzler R: Regulators of
cell volume: The structural and functional properties of anion
channels of the LRRC8 family. Curr Opin Struct Biol. 74:1023822022.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Okada Y, Sabirov RZ, Sato-Numata K and
Numata T: Cell death induction and protection by activation of
ubiquitously expressed anion/cation channels. Part 1: Roles of
VSOR/VRAC in cell volume regulation, release of double-edged
signals and apoptotic/necrotic cell death. Front Cell Dev Biol.
8:6140402021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chen L, König B, Liu T, Pervaiz S,
Razzaque YS and Stauber T: More than just a pressure relief valve:
Physiological roles of volume-regulated LRRC8 anion channels. Biol
Chem. 400:1481–1496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Konishi T, Shiozaki A, Kosuga T, Kudou M,
Shoda K, Arita T, Konishi H, Komatsu S, Kubota T, Fujiwara H, et
al: LRRC8A expression influences growth of esophageal squamous cell
carcinoma. Am J Pathol. 189:1973–1985. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kurashima K, Shiozaki A, Kudou M, Shimizu
H, Arita T, Kosuga T, Konishi H, Komatsu S, Kubota T, Fujiwara H,
et al: LRRC8A influences the growth of gastric cancer cells via the
p53 signaling pathway. Gastric Cancer. 24:1063–1075. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Xu R, Hu Y, Xie Q, Zhang C, Zhao Y, Zhang
H, Shi H, Wang X and Shi C: LRRC8A is a promising prognostic
biomarker and therapeutic target for pancreatic adenocarcinoma.
Cancers (Basel). 14:55262022. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lu P, Ding Q, Li X, Ji X, Li L, Fan Y, Xia
Y, Tian D and Liu M: SWELL1 promotes cell growth and metastasis of
hepatocellular carcinoma in vitro and in vivo. EBioMedicine.
48:100–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zhang H, Deng Z, Zhang D, Li H, Zhang L,
Niu J, Zuo W, Fu R, Fan L, Ye JH and She J: High expression of
leucine-rich repeat-containing 8A is indicative of a worse outcome
of colon cancer patients by enhancing cancer cell growth and
metastasis. Oncol Rep. 40:1275–1286. 2018.PubMed/NCBI
|
|
158
|
Fujii T, Shimizu T, Yamamoto S, Funayama
K, Fujita K, Tabuchi Y, Ikari A, Takeshima H and Sakai H: Crosstalk
between Na(+),K(+)-ATPase and a volume-regulated anion channel in
membrane microdomains of human cancer cells. Biochim Biophys Acta
Mol Basis Dis. 1864:3792–3804. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang H, Jing Z, Liu R, Shada Y, Shria S,
Cui S, Ren Y, Wei Y, Li L and Peng S: LRRC8A promotes the initial
development of oxaliplatin resistance in colon cancer cells.
Heliyon. 9:e168722023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhang H, Cui S, Jing Z, Fu G, Liu R, Zhao
W, Xu L, Yu L, Bai Y, Lv C, et al: LRRC8A is responsible for
exosome biogenesis and volume regulation in colon cancer cells.
Biochem J. 480:701–713. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang HY, Shimizu T, Numata T and Okada Y:
Role of acid-sensitive outwardly rectifying anion channels in
acidosis-induced cell death in human epithelial cells. Pflugers
Arch. 454:223–233. 2007. View Article : Google Scholar
|
|
162
|
Osei-Owusu J, Kots E, Ruan Z, Mihaljević
L, Chen KH, Tamhaney A, Ye X, Lü W, Weinstein H and Qiu Z:
Molecular determinants of pH sensing in the proton-activated
chloride channel. Proc Natl Acad Sci USA. 119:e22007271192022.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhao J, Zhu D, Zhang X, Zhang Y, Zhou J
and Dong M: TMEM206 promotes the malignancy of colorectal cancer
cells by interacting with AKT and extracellular signal-regulated
kinase signaling pathways. J Cell Physiol. 234:10888–10898. 2019.
View Article : Google Scholar
|
|
164
|
Zhang L, Liu SY, Yang X, Wang YQ and Cheng
YX: TMEM206 is a potential prognostic marker of hepatocellular
carcinoma. Oncol Lett. 20:1742020.PubMed/NCBI
|
|
165
|
Huang X, Chen H, Michelsen K, Schneider S
and Shaffer PL: Crystal structure of human glycine receptor-α3
bound to antagonist strychnine. Nature. 526:277–280. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Clar DT and Maani CV: Physiology, Ligand
Gated Chloride Channel. StatPearls. StatPearls Publishing; Treasure
Island, FL: 2024
|
|
167
|
Sanjari Moghaddam H, Zare-Shahabadi A,
Rahmani F and Rezaei N: Neurotransmission systems in Parkinson's
disease. Rev Neurosci. 28:509–536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hsu YT, Chang YG and Chern Y: Insights
into GABA(A)ergic system alteration in Huntington's disease. Open
Biol. 8:1801652018. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Govindpani K, Calvo-Flores Guzmán B,
Vinnakota C, Waldvogel HJ, Faull RL and Kwakowsky A: Towards a
better understanding of GABAergic remodeling in Alzheimer's
disease. Int J Mol Sci. 18:18132017. View Article : Google Scholar : PubMed/NCBI
|