Introduction
The prevalence of age-related hearing loss (ARHL) is
rising, with over two-thirds of adults aged ≥60 and older
experiencing clinically significant hearing impairment worldwide
(1). ARHL may cause progressive
sensorineural hearing loss, impaired hearing sensitivity, and
language discrimination (2),
which may lead to late-life depression and cognitive decline
(3). ARHL is linked to multiple
risk factors, including genetic, noise and ototoxic drugs (4), making it a complex disorder that
results from cumulative effects. Previous evidence has revealed
that the predominant reason for ARHL is sensory inner and outer
hair cell damage, which contradicts the long-accepted theory that
impairment of the stria vascularis is the primary reason (5). Human autopsy specimens demonstrate
that the degree of inner-ear sensory cell loss is positively
associated with severity of hearing loss, indicating the importance
of understanding the mechanism underlying cell loss since hair
cells cannot regenerate (5).
Because of the slow and uncontrollable nature of the
normal aging process, D-galactose (D-gal) is widely used to induce
rapid aging in ARHL studies (6-8).
D-gal is a reducing sugar that undergoes oxidation by galactose
oxidase to aldehydes and hydrogen peroxide while present in
excessive amounts, resulting in oxidative damage (9). Auditory dysfunction and
corresponding pathological impairments in D-gal-treated rats and
mice are similar to those observed in natural aging (10,11), making D-gal a viable approach to
mimic aging in vitro and in vivo.
MicroRNAs (miRNAs or miRs) are non-coding RNAs,
typically ~22 nucleotides in length, serving key regulatory roles
in various biological processes. They can bind to mRNA targets to
degrade mRNA or prevent mRNA translation into protein (12). Thus, miRNAs exert a negative
regulatory effect on the expression of genes or proteins and are
involved in cellular processes, including development,
differentiation, proliferation, autophagy and apoptosis. A miRNA
triad (composed of miR-96, miR-182, and miR-183) was initially
identified in zebrafish hair cells of sensory epithelia using in
situ hybridization in 2005 (13). Numerous miRNAs, such as miR-96,
-182 and -183, have been found to be highly expressed in the
cochlea (14) and are considered
as future therapeutic interventions for hearing loss, primarily
ARHL (15). The present study
aimed to identify key miRNAs that contribute to ARHL and the
underlying mechanisms and regulatory pathways in vitro and
in vivo.
Materials and methods
Animals and D-gal aging model
A total of 16 C57BL/6 mice (male; age, ~16 weeks;
weight, 26-30 g) were obtained from Shanghai SLAC Laboratory Animal
Co., Ltd. The mice were housed at 23°C with 60% humidity with a
12/12-h light/dark cycle and free access to food and water. After
adapting to the environment for seven days, mice were randomly
divided into a control and an aging group (both n=8). D-gal (cat.
no. G0625, Sigma-Aldrich; Merck KGaA) was dissolved in sodium
carboxymethyl cellulose solution (CMC-Na; Sigma-Aldrich). Mice in
the aging group received 150 mg/kg D-gal once daily
(intraperitoneal injection) for six weeks. The control group was
injected with CMC-Na without D-gal following the same schedule.
Animal experiments were approved by the Ethics Committee for Animal
Research (approval no. 2020-NZR-037, People's Hospital of Ningxia
Hui Autonomous Region, Yinchuan, China) and performed in accordance
with the Guide for the Care and Use of Laboratory Animals prepared
by the National Academy of Sciences and published by the National
Institutes of Health (16).
All mice were intraperitoneally injected with 30
mg/kg pentobarbital sodium and sacrificed by cervical dislocation.
Death was verified by cessation of respiration and heartbeat. The
cochleae were then collected. Fine forceps were used under a
stereomicroscope to remove the stria vascularis and tectorial
membranes. The apical organ of the Corti was used for further
procedures. If any animal reached the predefined humane endpoints
[loss of >20% of body weight; signs of pian and stress
(piloerection, hunched posture, dehydration, sunken or closed eyes
and self-isolation)], they were humanely euthanized. Animal health
and behaviors were monitored daily. To avoid repeated damage to the
same site, abdominal areas (left or right side) were alternated
during daily injections, while avoiding proximity to internal
organs.
Cell culture
House Ear Institute-Organ of Corti 1 (HEI-OC1) cells
(cat. no. CVCL_D899, American Type Culture Collection) were
cultured at 33°C, 10% CO2 in high-glucose DMEM
containing 10% FBS (both Gibco; Thermo Fisher Scientific, Inc.)
without antibiotics. D-gal was applied at a concentration of 5, 10
and 20 mg/ml for 72 h at 33°C. For miR inhibitor transfection,
HEI-OC1 cells were seeded in six-well plates at a density of
5x105 cells/well and allowed to reach 60-70% confluency
before transfection. Cells were transfected with 10 nM miR-34a
inhibitor (cat. no. MH19474, Thermo Fisher Scientific, Inc.) using
Lipofectamine 3000 Transfection Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. Briefly,
Lipofectamine 3000 (2.5 µl/well) was diluted in Opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.), and miR inhibitor (10 nM)
was mixed in a separate tube with Opti-MEM. The two solutions were
combined and incubated at room temperature for 15 min before being
added to the cells. Cells were incubated for 6 h at 33°C, after
which the medium was replaced with fresh high-glucose DMEM with 10%
FBS, followed by incubation for 24 h before subsequent treatments.
For Mdivi-1 pretreatment, cells were pretreated with 10 µM
Mdivi-1 (cat. no. S7162, Selleck Chemicals) for 1 h at 33°C before
the addition of D-gal (20 mg/ml, 72 h). Control groups were treated
with DMSO (0.1%) as a vehicle control.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from cells and tissues was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
and cDNA was synthesized with PrimeScript RT Master Mix (cat. no.
RR036B; Takara Biotechnology, Ltd.), according to the
manufacturers' protocols. The cDNA underwent qPCR with Power UP
SYBR Green Master Mix (cat. no. A25742, Thermo Fisher Scientific,
Inc.) on an Analytik Jena qTOWER (Analytik Jena GmbH). The
amplification conditions for qPCR were as follow: 50°C for 2 min
and 95°C for 5 min, followed by 40 cycles at 95°C for 15 sec and
60°C for 1 min, with a melt curve stage of 95°C for 15 sec, 60°C
for 1 min and 95°C for 15 sec. The relative mRNA expression levels
of all genes were normalized to β-actin or U6 and were calculated
using the 2-ΔΔCq method (17).
The primers (Table I) were
synthesized by Shanghai GenePharma Co., Ltd.
 | Table IList of primer sequences. |
Table I
List of primer sequences.
| Gene | Forward primer (5′
→ 3′) | Reverse primer (5′
→ 3′) |
|---|
| mmu-miR-140-5p |
CAGTGGTTTTACCCTATGGTAG |
GTGCAGGGTCCGAGGT |
| mmu-miR-141-3p |
TAACACTGTCTGGTAAAGATGG |
GTGCAGGGTCCGAGGT |
| mmu-miR-15a-5p |
TAGCAGCACATAATGGTTTGUG |
GTGCAGGGTCCGAGGT |
| mmu-miR-34a-5p |
TGGCAGTGTCTTAGCTGGTTGT |
GTGCAGGGTCCGAGGT |
|
mmu-miR-130a-3p |
CAGTGCAATGTTAAAAGGGCAT |
GTGCAGGGTCCGAGGT |
|
mmu-miR-148a-3p |
TCAGTGCATCACAGAACTTTGT |
GTGCAGGGTCCGAGGT |
| mmu-miR-17-5p |
CAAAGTGCTTACAGTGCAGGTAG |
GTGCAGGGTCCGAGGT |
| mmu-miR-574-5p |
TGAGTGTGTGTGTGTGTGTGTGA |
GTGCAGGGTCCGAGGT |
| mmu-miR-446a |
GCTGTTAATGCTAATCGTGATT |
GTGCAGGGTCCGAGGT |
| mmu-miR-22-3p |
AACTGCCTGGTCCAACCTCTAAG |
GTGCAGGGTCCGAGGT |
| mmu-miR-455-3p |
GCGACAATGAAGAATTGCCCG |
GTGCAGGGTCCGAGGT |
|
mmu-miR-148a-3p |
TCAGTGCATCACAGAACTTTGT |
GTGCAGGGTCCGAGGT |
| mmu-miR-27a-3p |
ACTGTTGCTGCAGGGTCTTAGC |
GTGCAGGGTCCGAGGT |
| mmu-miR-364-3p |
GCGAAATGGTTCTGGTAGTCTGCT |
GTGCAGGGTCCGAGGT |
| mmu-miR-362-5p |
AGGCTGGGAAGGAGTGGTTGGA |
GTGCAGGGTCCGAGGT |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| TFAM |
GCCTGGATCTCTGCAGAACT |
GTTGCTTTTTTCCACTCCCTG |
| β-actin |
GGCTGTATTCCCCTCCATCG |
CCAGTTGGTAACAATGCCATGT |
Intracellular reactive oxygen species
(ROS) detection
Intracellular ROS levels were detected by DCFH-DA
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocols. FACS Calibur system (BD Biosciences) with
FlowJo (FlowJo 7.6.1; BD Biosciences) was used to measure
fluorescence intensity. The experiment was repeated ≥3 times.
Apoptosis analysis
Annexin V-FITC kit (Beyotime Institute of
Biotechnology) was used for apoptosis analysis according to the
manufacturer's instructions. The cells were collected by
centrifugation (1,000 g, 5 min, 4°C) and resuspended. FACS Calibur
system (BD Biosciences) with FlowJo software (FlowJo 7.6.1; BD
Biosciences) was used to measure fluorescence intensity. Cells that
were Annexin V-positive and PI-negative were identified as
apoptotic. The experiment was repeated ≥3 times.
Western blot analysis
Total protein from cells and tissue was extracted
using RIPA lysis buffer (Beyotime Institute of Biotechnology) and
the protein concentration was quantified using the BCA Protein
Assay kit. The samples (30 µg/lane) were subjected to 10%
SDS-PAGE and transferred to poly-vinylidene difluoride membranes.
Following blocking with 5% bovine serum albumin (Gibco; Thermo
Fisher Scientific, Inc.) in TBST (0.1%) for 30 min, they were
incubated with the following primary antibodies (1:1,000) in 4°C
overnight: Rabbit anti-superoxide dismutase type 1 (SOD1; cat. no.
A22594; ABclonal Biotech Co., Ltd.), anti-NLRP3 (A24294, ABclonal
Biotech Co., Ltd.), anti-Apoptosis-associated speck-like protein
containing a CARD (ASC, cat. no. A22046; ABclonal Biotech Co.,
Ltd.), anti-caspase1 (cat. no. A23429, ABclonal Biotech Co., Ltd.),
anti-pro-caspase1 (cat. no. A21085, ABclonal Biotech Co., Ltd.),
anti-dynamin-related protein 1 (DRP1; cat. no. A21968, ABclonal
Biotech Co., Ltd.), anti-phosphorylated DRP1 (Ser616; cat. no.
4494, Cell Signaling Technology, Inc.), anti-TFAM (cat. no. A13552,
ABclonal Biotech Co., Ltd.) and anti-β-actin (cat. no. AC026,
ABclonal Biotech Co., Ltd.). After that, the membranes were
incubated with HRP-conjugated secondary antibodies (cat. no. AS014,
ABclonal Biotech Co., Ltd. 1:1,000 diluted in TBST) in room
temperature for 1 h and were then washed with TBST for three times.
The relative protein expression levels were normalized to β-actin.
The signals were detected using the ECL Immobilon Western Chemilum
HRP Substrate (cat. no. WBKLS0500; Merck Millipore) and an
ultra-high sensitivity chemiluminescence imaging system (Bio-Rad
Laboratories, Inc.) and quantified using ImageJ 1.48v software
(National Institutes of Health).
Cell counting kit (CCK)-8 assay
HEI-OC1 cells were seeded into 96-well plates at a
density of 5,000 cells/well and cultured for 24 h. After three
washes with PBS, 10 µl CCK-8) reagent (C0005, TargetMol) was
added to each well, and the cells were then incubated at 37°C for
another 1 h. The absorbance was measured at an optical density of
450 nm using a microplate reader (Thermo Fisher Scientific,
Inc.).
Mitochondrial membrane potential
(MMP)
MMP was detected using MMP assay kit with JC-1 (cat.
no. C2003S, Beyotime Institute of Biotechnology) following the
manufacturer's instructions. Cells were viewed under an inverted
light microscope (Olympus Corporation; cat. no. X51). Fluorescence
intensity was analyzed using ImageJ 1.48v software (National
Institutes of Health).
Dual-luciferase reporter assay
The dual-luciferase reporter assay was performed to
evaluate the activity of wild-type (WT) and mutant (MUT) TFAM 3′UTR
in HEI-OC1 cells under mimic NC or miR-43a mimic treatment. The
3′UTR of mouse TFAM was cloned into the pGL3-Basic luciferase
reporter vector (Promega Corporation). To create the mutant
construct, the miR-43a binding site in the TFAM 3′UTR was mutated
using a site-directed mutagenesis kit (QuickChange Site-Directed
Mutagenesis Kit, Agilent). HEI-OC1 cells were cultured in DMEM
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 33°C in 5% CO2. Cells were seeded in 24-well plates
at approximately 70-80% confluence. Transfections were performed
using Lipofectamine 3,000 (Invitrogen) according to the
manufacturer's protocol. The following groups were transfected:
pGL3-TFAM-WT + mimic NC, pGL3-TFAM-WT + miR-43a mimic,
pGL3-TFAM-MUT + mimic NC, pGL3-TFAM-MUT + miR-43a mimic. Each well
was co-transfected with 0.5 µg of the luciferase reporter
plasmid and 0.05 µg of Renilla luciferase plasmid (pRL-TK,
Promega) as an internal control. After 24-48 h of transfection,
cells were lysed using Passive Lysis Buffer (Promega). Firefly and
Renilla luciferase activities were measured using the
Dual-Luciferase Reporter Assay System (Promega) following the
manufacturer's instructions. Luminescence was detected using a
microplate reader.
Immunofluorescence staining
The tissue and cells were collected and embedded in
paraffin resin before sectioning at a thickness of 5 µm
using a microtome. For antigen retrieval, sections were heated to
95°C for 15 min in citrate buffer (pH 6.0), followed by washing in
xylene and rehydration through a descending alcohol series (100,
95, 85, 70%) into distilled water. After fixation in 4%
paraformaldehyde for 30 min at room temperature, samples were
blocked with 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) at 33°C for 30 min and incubated overnight at 4°C
with primary antibodies: anti-phosphorylated DRP1 (Ser616; cat. no.
3455, Cell Signaling Technology, Inc.), anti-myosin VIIA (Myo7a,
cat. no. 3402, Cell Signaling Technology, Inc.), anti-TFAM (cat.
no. 8076, Cell Signaling Technology, Inc.), and anti-gasdermin D
(GSDMD, Thermo Fisher Scientific, Inc.). Primary antibodies were
diluted at 1:200 in blocking buffer. The tissue and cells were then
incubated with a fluorochrome-conjugated secondary antibody (1:500
dilution) for 1 h at room temperature. Samples were visualized
under a fluorescence microscope (Olympus Corporation; cat. no. X51)
at 40x magnification. Image analysis and fluorescence intensity
quantification were performed using ImageJ software (version 1.48v;
National Institutes of Health).
Statistical analysis
All data are presented as the mean ± SEM and each
experiment was performed in triplicate. Statistical analyses were
performed using R (version R-3.4.3). Unpaired Student's t-test was
used to compare two groups. One-way analysis of variance followed
by Tukey's post hoc test was used to compare >2 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-34a is upregulated in D-gal-induced
HEI-OC1 cells and cochleae of C57BL/6 mice
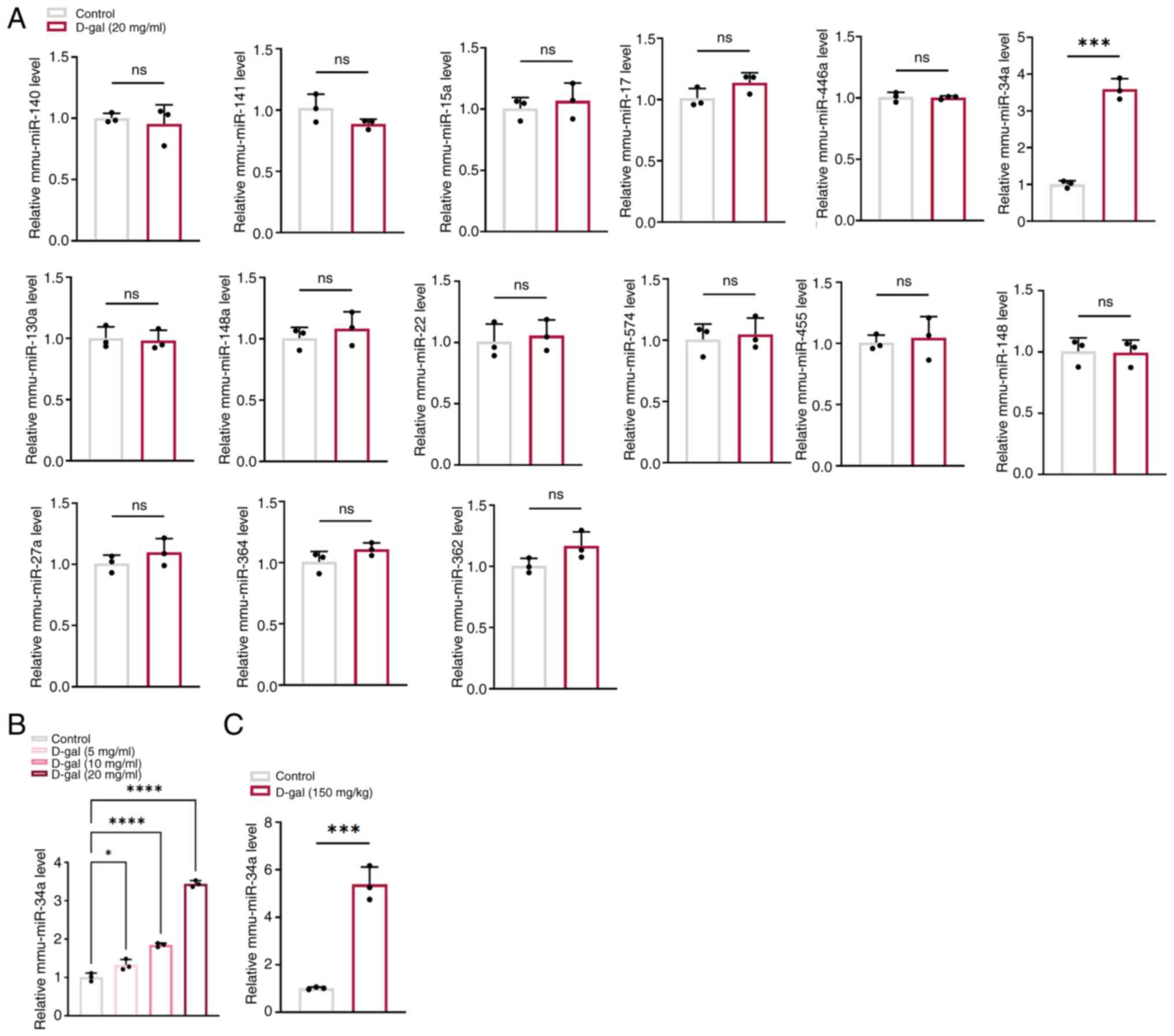
A total of 15 miRNAs including miR-140, miR-141,
miR-15a, miR-34a, miR-130a, miR-148a, miR-17, miR-574, miR-446a,
miR-22, miR-455, miR-148a, miR-27a, miR-364 and miR-362 were
selected from our previous study (18) to identify the key miRNAs involved
in D-gal-induced aging. Among these, miR-34a was the only miRNA
that was significantly increased in D-gal-induced HEI-OC1 cells
compared with controls (Fig. 1A)
and the expression of miR-34a was positively associated with
concentration of D-gal (Fig.
1B), suggesting that miR-34a may play a key role in
D-gal-induced aging in HEI-OC1 cells. This was confirmed in
cochleae of D-gal-induced aging C57BL/6 mice as miR-34a
significantly increased in D-gal treated mice compared with the
control (Fig. 1C).
D-gal induces apoptosis and pyroptosis in
HEI-OC1 cells
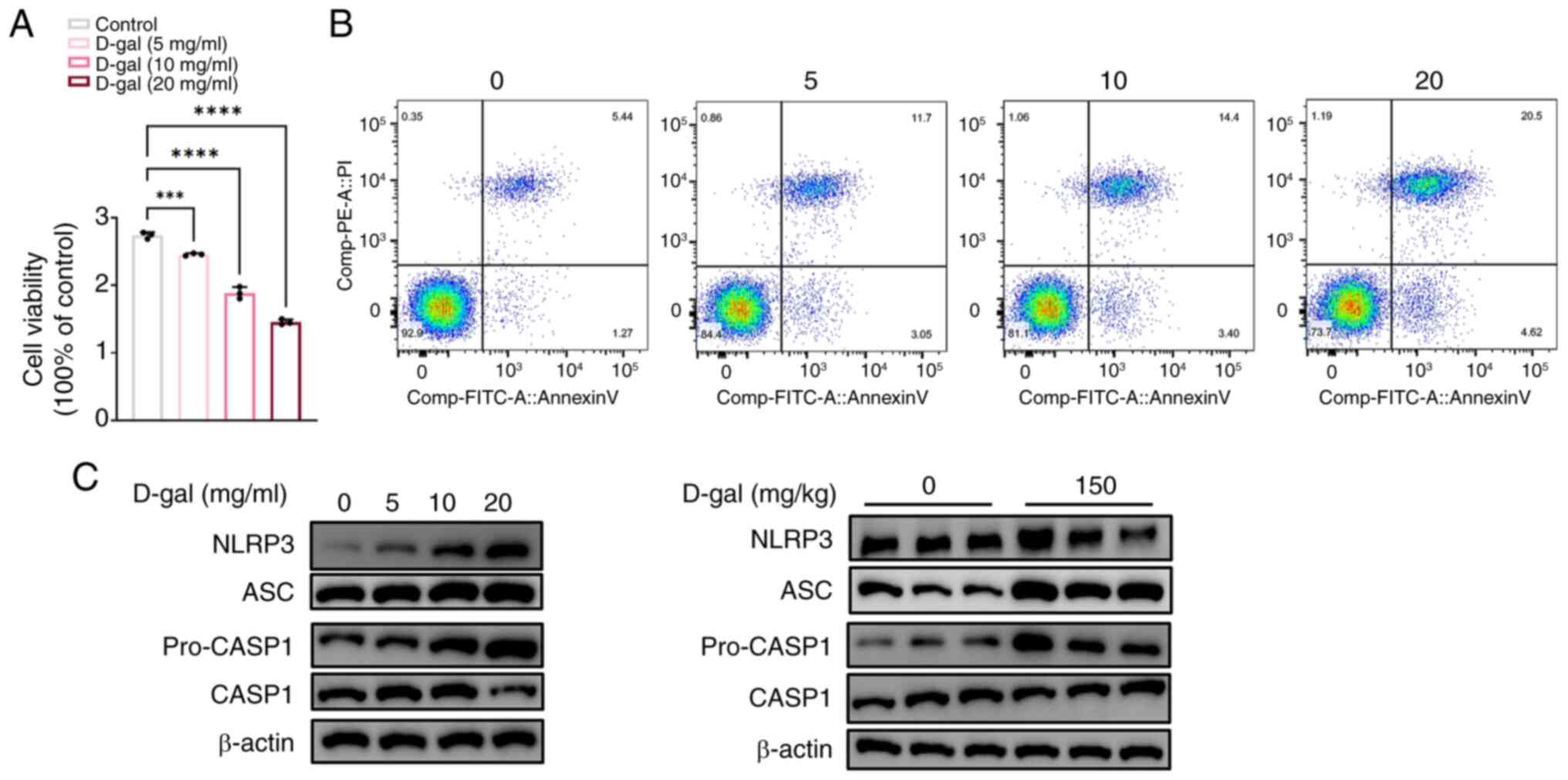
The effect of D-gal was assessed using the Cell
Counting Kit-8 assay (Fig. 2A).
HEI-OC1 cell viability decreased in a dose-dependent manner,
consistent with the flow cytometry results (Fig. 2B). Annexin V-positive staining
increased with increasing D-gal concentration, indicating increased
apoptotic cells (Fig. 2B).
Compared with the control groups, the expression of four key
pyroptosis-related proteins, pro-caspase1, caspase1, ASC and NLRP3,
significantly increased in HEI-OC1 cells after exposure D-gal for
72 h (Fig. 2C) in a
dose-dependent manner. These results indicate that D-gal induces
apoptosis and pyroptosis in HEI-OC1 cells. The upregulation of
pyroptosis-associated proteins was confirmed in the aging cochleae
of D-gal-induced C57BL/6 mice (Fig.
2C).
D-gal induces oxidative damage and
mitochondrial dysfunction in HEI-OC1 cells
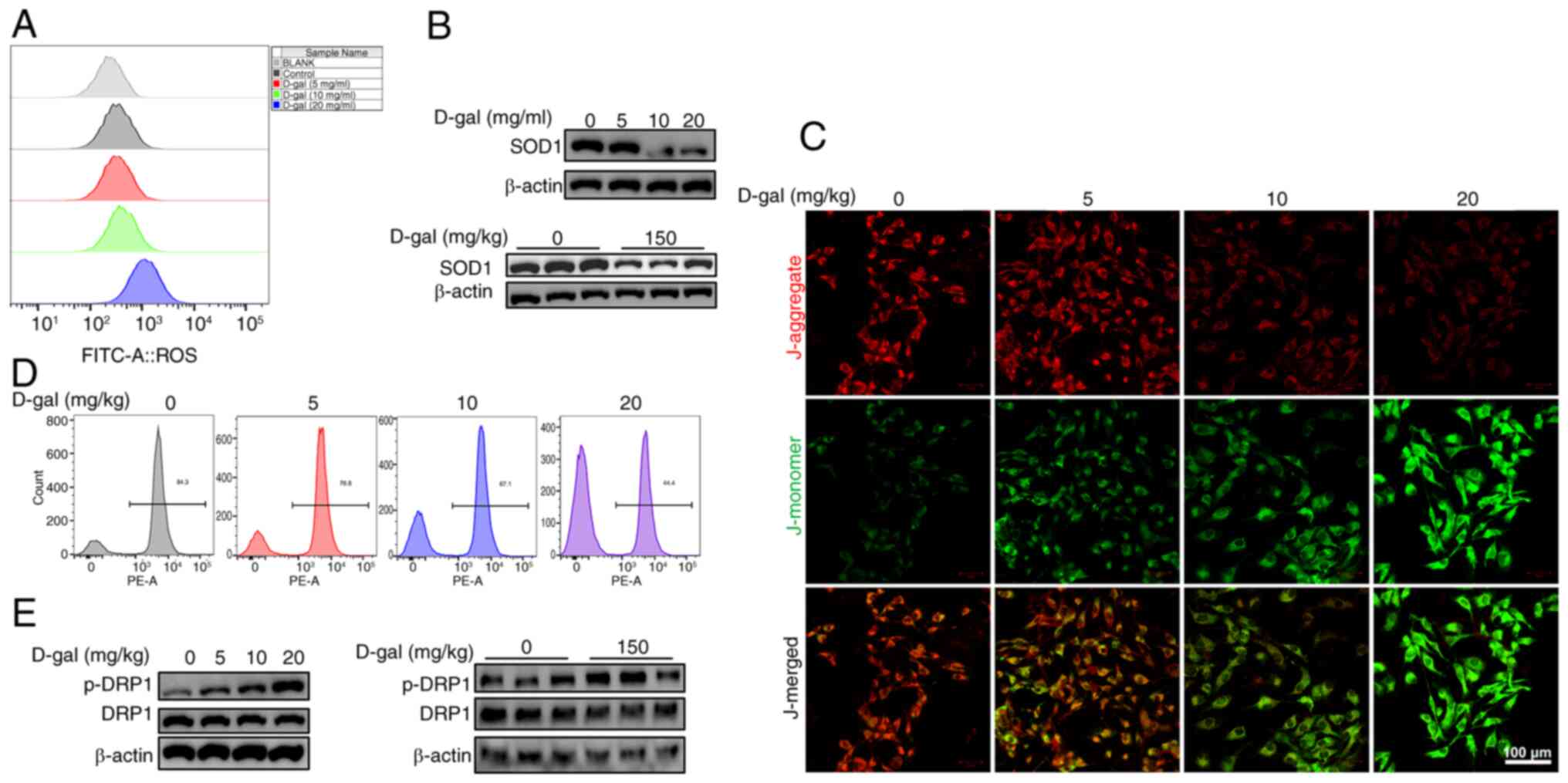
Similar to what is hypothesized to occur in ARHL,
the mechanism underlying D-gal-induced and natural aging is
oxidative stress resulting from excessive ROS production and
imbalance between excessive ROS and the antioxidant system
(19,20). ROS levels in HEI-OC1 cells
following 5, 10, and 20 mg/ml D-gal exposure for 72 h was assessed
by DCFH-DA staining and the expression of the antioxidant enzyme
SOD1, an enzyme that protects the cells from excessive ROS
(21), was detected using
western blot analysis. Fluorescence intensity of ROS notably
increased with increasing D-gal concentration (Fig. 3A), accompanied by a decrease in
SOD1 levels (Fig. 3B),
suggesting enhanced ROS production and insufficient antioxidant
system after D-gal exposure. As mitochondria are the primary source
of ROS and are susceptible to oxidative damage (22), the present study investigated
whether mitochondrial function was compromised following exposure
to D-gal. Mitochondrial integrity and bioenergetic function were
assessed by MMP analysis using immunofluorescence staining and flow
cytometry. Immunofluorescence staining revealed a notable increase
in the number of green-stained cells (indicating damaged
mitochondria), while the number of red-stained cells (representing
healthy mitochondria) notably decreased (Fig. 3C). This was consistent with flow
cytometry results, where nearly half of the mitochondria in HEI-OC1
cells exposed to 20 mg/ml D-gal were damaged compared with the
control (Fig. 3D), indicating
disrupted mitochondrial dynamics in D-gal-induced HEI-OC1 cells.
DRP1 is a key component of mitochondrial fission, representing the
normal function of mitochondria, and a downstream protein of
miR-34a (23,24). Western blot analysis (Fig. 3E) demonstrated a notable increase
in DRP1 phosphorylation, indicating activation in D-gal-induced
HEI-OC1 cells and the cochleae of C57BL/6 mice, implying excessive
mitochondrial fission and dysfunction.
miR-34a inhibitor improves apoptosis and
pyroptosis induced by D-gal in HEI-OC1 cells via inhibiting
mitochondrial dysfunction
To investigate the role of miR-34a and mitochondrial
dysfunction in D-gal-induced aging process, HEI-OC1 cells were
either transfected with a miR inhibitor (to inhibit miR-34a) or
pretreated with Mdivi-1 (to inhibit DRP1). miR inhibitor and
Mdivi-1 significantly reversed oxidative damage, mitochondrial
dysfunction, apoptosis and pyroptosis in D-gal-induced HEI-OC1
cells (Fig. 4), indicating
miR-34a is a key element in promoting apoptosis and pyroptosis
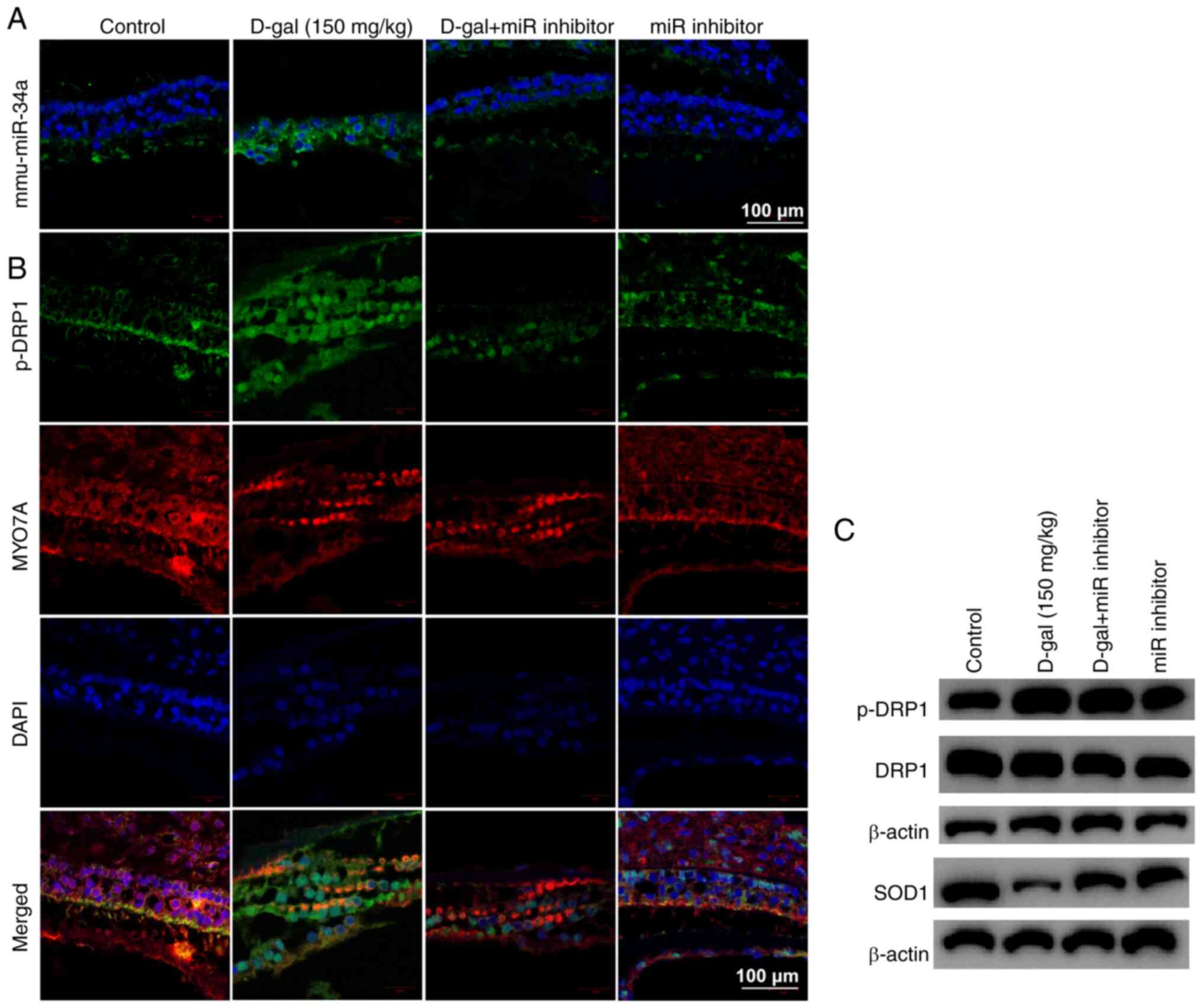
through mitochondrial dysfunction. In mouse cochleae, miR inhibitor
decreased miR-34a expression (Fig.
5A) and DRP1 phosphorylation and increased SOD1 (Fig. 5B and C). Immunostaining (Fig. 5B) confirmed that there was an
overlap between cells expressing phosphorylated DRP1 and hair
cell-specific marker Myo7A. Taken together, these data validated
the role of miR-34a in D-gal-induced aging process in
vivo.
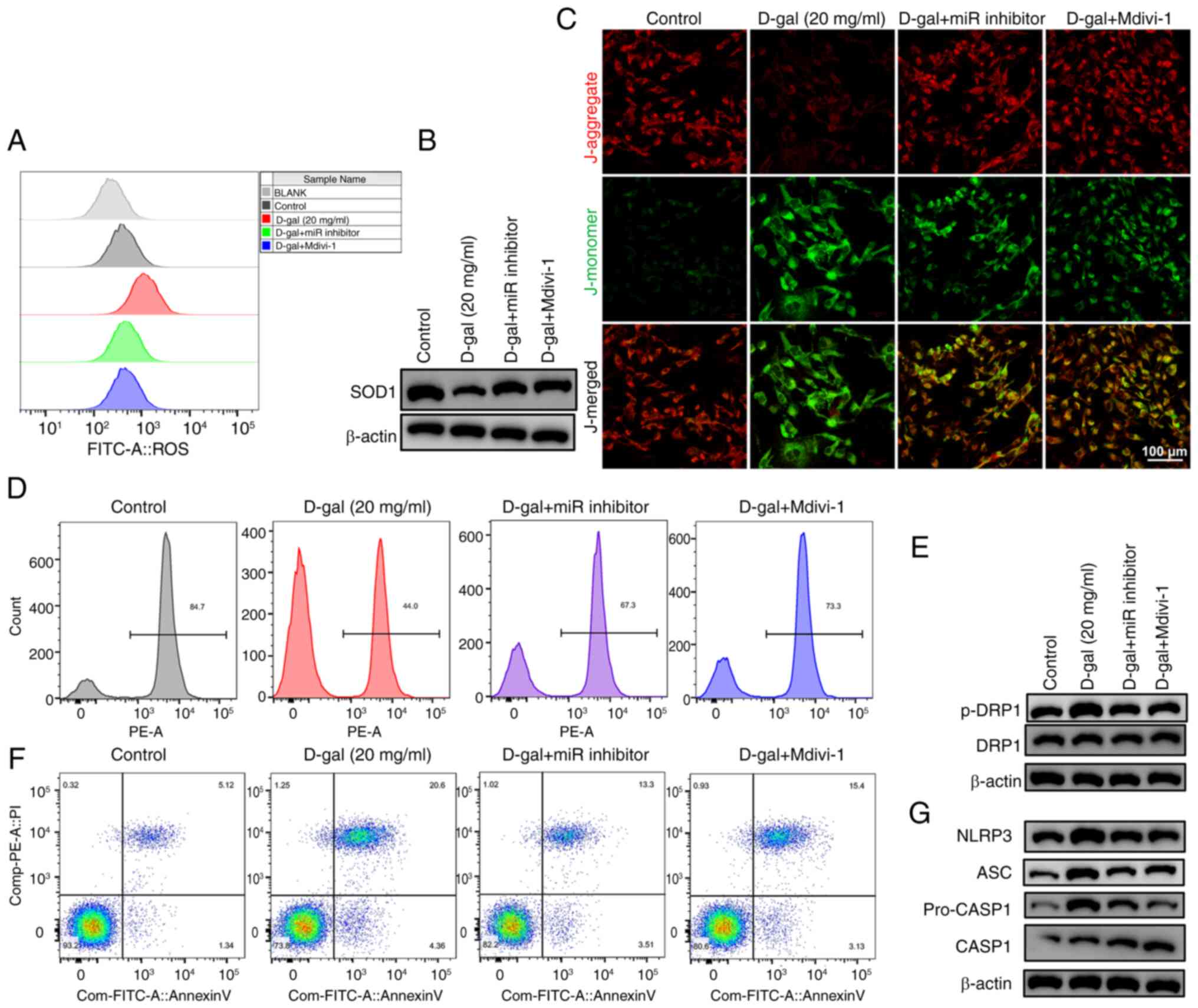 | Figure 4Effect of miR inhibitor and Mdivi-1
on mitochondrial dysfunction, apoptosis and pyroptosis in
D-gal-induced HEI-OC1 cells. (A) ROS production. (B) Western blot
analysis of SOD1 expression. (C) Immunostaining of (D)
mitochondrial membrane potential. Scale bar, 50 µm. (E)
Western blot analysis of DRP1 phosphorylation. (F) Flow cytometry
results of Annexin V/PI double-stained HEI-OC1 cells. (G) Western
blot analysis of pyroptosis-related proteins in HEI-OC1 cells. miR,
microRNA; D-gal, D-galactose; HEI-OC1, House Ear Institute-Organ of
Corti 1; ROS, reactive oxygen species; SOD, superoxide dismutase;
DRP, dynamin-related protein 1; p-, phosphorylated; ASC,
apoptosis-associated speck-like protein containing a CARD; CASP,
caspase. |
miR-34a regulates aging process via
inhibiting TFAM in D-gal-induced HEI-OC1 cells and cochleae of
C57BL/6 mice
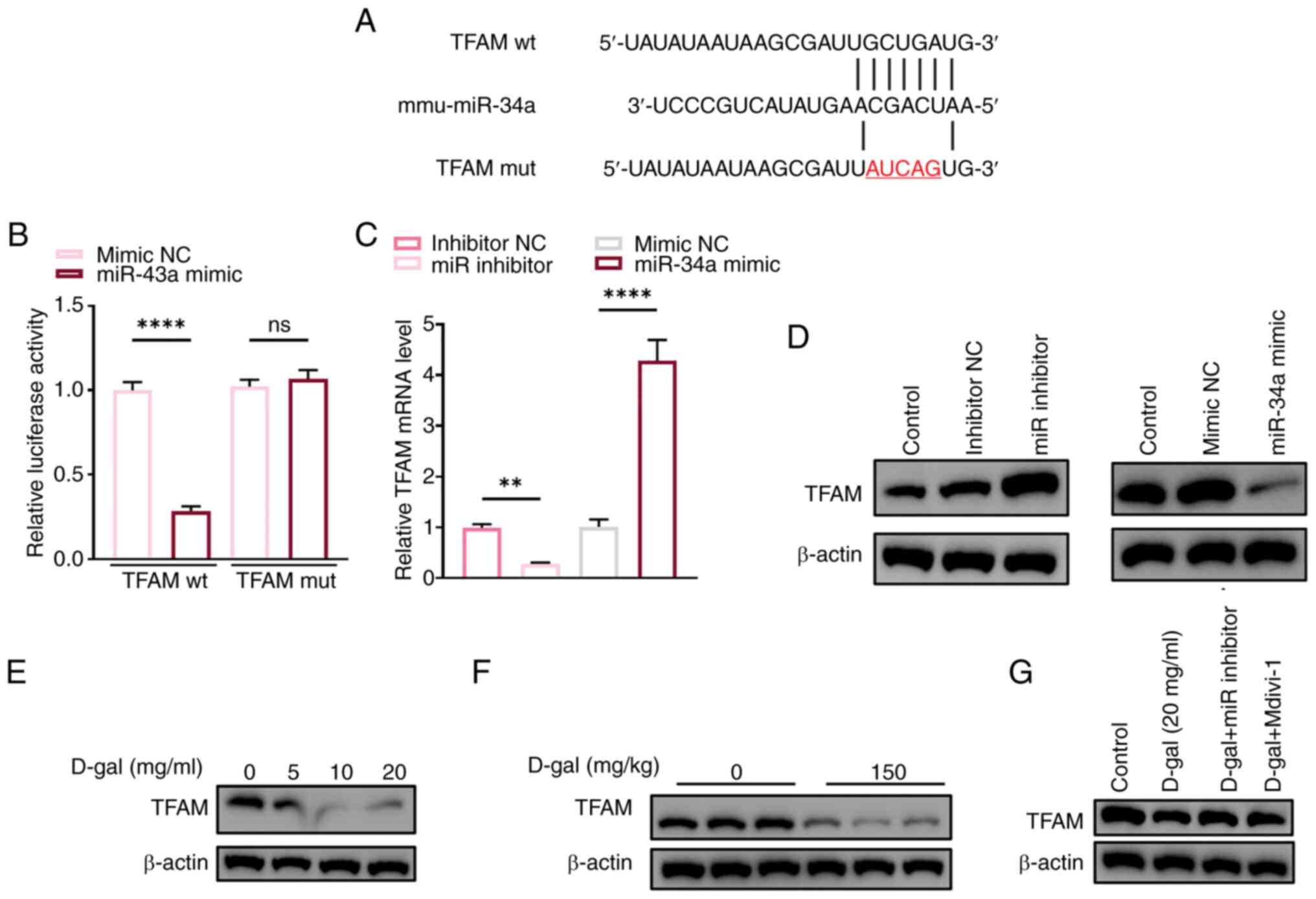
Based on several studies (25,26), it was hypothesized that TFAM
might be the target gene of miR-34a based on putative target
sequences of TFAM localized in the 3′ untranslated region (Fig. 6A). A dual luciferase reporter
assay provided direct evidence for the interaction between miR-34a
and TFAM (Fig. 6B). Western blot
analysis revealed that miR-34a inhibited protein expression of TFAM
(Fig. 6D) while transcription of
TFAM was promoted (Fig. 6C).
Expression of TFAM exhibited a notable decrease after exposure to
D-gal (Fig. 6E and F).
Furthermore, the decrease was reversed by miR 34a inhibitor,
indicating the downstream inhibitory effect of miR-34a on TFAM.
TFAM was silenced to verify its involvement in
mitochondrial dysfunction, apoptosis and pyroptosis induced by
D-gal in HEI-OC1 cells. The protective effect of miR-inhibitor was
notably reduced by the interference of TFAM in D-gal-induced
HEI-OC1 cells (Fig. 7):
Oxidative stress and mitochondrial damage, proportion of annexin
V-positive D-gal-induced HEI-OC1 cells and expression levels of
pyroptosis-associated NLRP3, ASC, caspase1 and p-caspase1 notably
increased, indicating TFAM is a key component of the aging process
induced by D-gal. Furthermore, there was colocalization of TFAM
with GSDMD in vitro and in vivo (Fig. 7G and H), suggesting TFAM may
serve a key role in D-gal-induced pyroptosis as GSDMD is crucial
for pyroptosis.
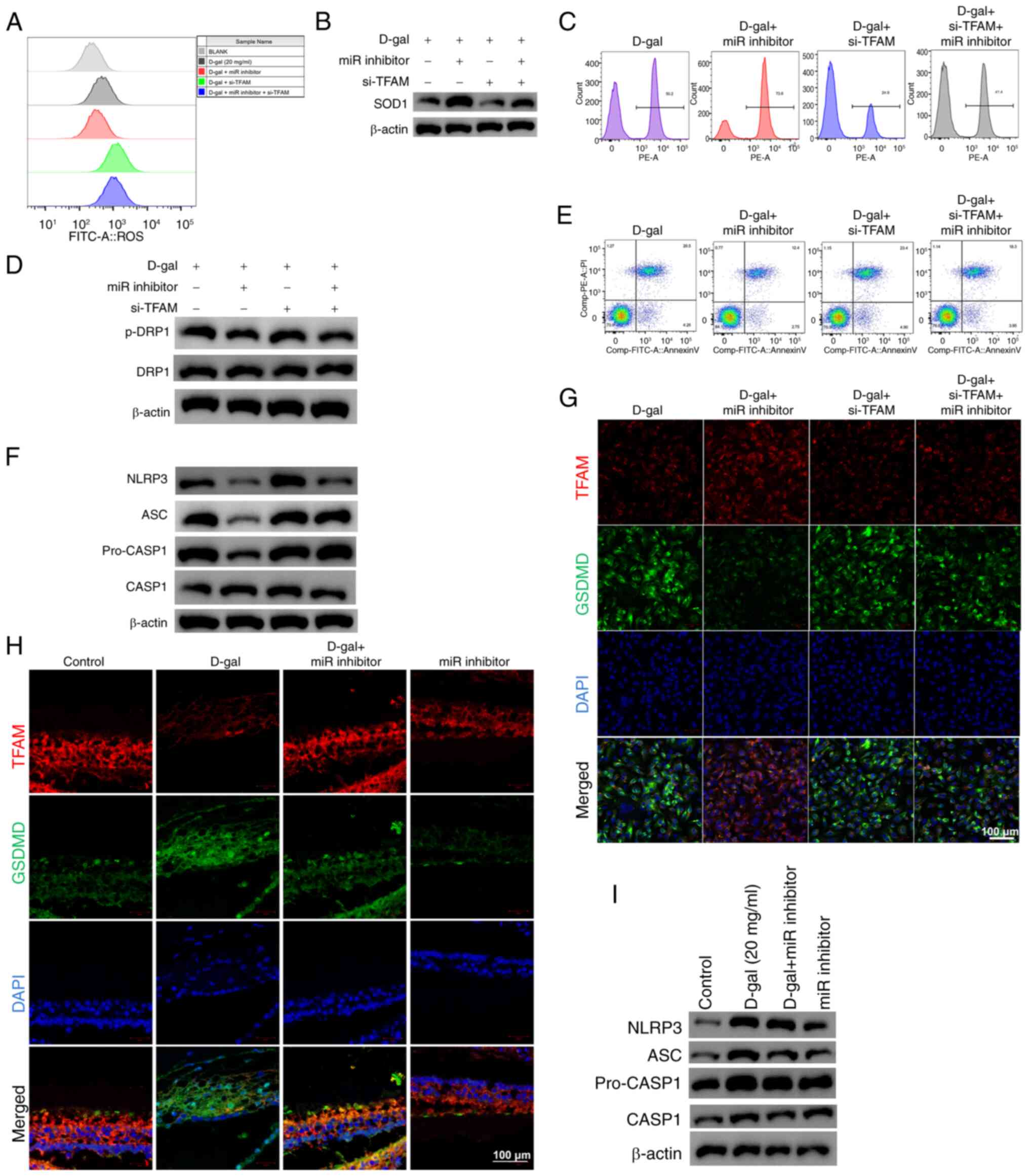 | Figure 7Effect of TFAM on D-gal-induced
mitochondrial dysfunction, apoptosis and pyroptosis in HEI-OC1
cells. (A) ROS production. (B) Western blot analysis of SOD1
expression. (C) Mitochondrial membrane potential. (D) Western blot
analysis of DRP1 phosphorylation. (E) Effect of TFAM and miR-34a on
early apoptosis. (F) Western blot analysis of pyroptosis-associated
proteins. Representative TFAM (red), GSDMD (green), and DAPI
(blue)-staining of (G) HEI-OC1 cells (scale bar, 50 µm) and
(H) D-gal-induced cochleae. n=8. Scale bar, 20 µm. (I)
Western blot analysis of pyroptosis-associated proteins in
D-gal-induced cochleae (n=3). TFAM, mitochondrial transcription
factor A; D-gal, D-galactose; HEI-OC1, House Ear Institute-Organ of
Corti 1; ROS, reactive oxygen species; SOD, superoxide dismutase;
DRP, dynamin-related protein 1; miR, microRNA; GSDMD, gasdermin D;
si, small interfering; p-, phosphorylated; CASP, caspase; ASC,
Apoptosis-associated speck-like protein containing a CARD. |
Discussion
D-gal is used to accelerate aging in mammalian
cochlea and investigate the underlying mechanism of ARHL (11). As ARHL is currently irreversible,
progressive and untreatable (4),
the present study aimed to elucidate the key components underlying
its molecular pathogenesis to identify potential treatment targets.
miRNAs have recently gained increasing interest (27-29) in hearing loss due to their
potential involvement in auditory development, cochlear homeostasis
maintenance and pathological processes of hearing loss. The present
study investigated the role of miR-34a in ARHL using D-gal-induced
aging in HEI-OC1 cells and C57BL/6 mice and suggested that miR-34a
may serve as a potential therapeutic target for ARHL.
Cochlear hair cell loss is the leading cause of
sensory deficit in ARHL (5). By
applying D-gal to HEI-OC1 cells and C57BL/6 mice, cell loss,
apoptosis and mitochondrial dysfunction were induced, similar to
other studies (7,11). Oxidative stress/mitochondrial
dysfunction-induced apoptosis are hypothesized to be the main
contributors to hair cell death. Inflammation may also contribute
to ARHL (2,4). With aging, patients with ARHL
present a gradual increase in systemic inflammation with a decrease
in hearing thresholds (30).
This suggests inflammation serves a vital role in ARHL progression
and pyroptosis, a programmed cell death triggered by inflammatory
cystathionases, may be involved in cochlear hair cell loss during
aging (31). Here,
pyroptosis-associated NLRP3, ASC, caspase1 and pro-caspase1 were
upregulated in both D-gal-induced in vitro and in
vivo aging models, suggesting that pyroptosis contributes to
aging cochlear hair cell loss and apoptosis. This is in line with
recent studies (31,32), which found pyroptosis to be a key
element in ARHL.
miR-34a has recently been found to serve as a key
regulator of different types of hearing loss (28,31,33). Apart from modulating autophagy
and contributing to cochlear hair cell apoptosis in ARHL (34,35), miR-34a participates in
cisplatin-induced ototoxicity via mediating mitophagy (36). In the present study, miR-34a was
identified in a miRNA screen based on five ARHL databases in our
previous study (18). This was
consistent with previous studies that have revealed miR-34a as a
vital regulator of age-dependent tissue changes and a cell
senescence inducer (37-39). miR-34a increases with age in
several organs such as heart and blood vessels and serves a key
role in age-associated functional impairment. By applying the
miR-inhibitor, mitochondrial dysfunction, apoptosis and pyroptosis
were reversed in vitro and in vivo, suggesting that
miR-34a served as a key modulator of the aging process.
Apoptosis and pyroptosis were notably decreased when
cells were pretreated with Mdivi-1, a DRP1 inhibitor. Since DRP1
represents the normal function of mitochondria, this indicates that
miR-34a modulates apoptosis and pyroptosis via mitochondrial
dysfunction. miR-34a and mitochondrial apoptosis are associated;
miR-34a was first described as a p53-induced tumor suppressor miRNA
that controls apoptosis and senescence of tumor cells (40) and directly targets antioxidative
genes (41). miR-34a inhibits
sirtuin 3 expression, aggravates pyroptosis (42,43) and is considered to be associated
with inflammation (44).
However, there are cross-talk pathways between mitochondrial
apoptosis and pyroptosis (45,46), but these interactions require
further elucidation.
TFAM is a key mitochondrial DNA (mtDNA) packaging
protein whose disruption may lead to mtDNA depletion and
mitochondrial dysfunction (47),
which can lead to aging (48,49). TFAM deficiency has been
recognized as an accelerator of senescence (50,51), but not yet in ARHL. The present
study demonstrated that miR-34a inhibited TFAM to regulate
apoptosis and pyroptosis in D-gal-induced aging HEI-OC1 cells and
cochleae. The present results demonstrated a negative association
between miR-34a and its target TFAM in D-gal-induced aging HEI-OC1
cells and cochleae, suggesting miR-34a exerts pro-death effects by
targeting TFAM, thus inhibiting its antioxidative functions. TFAM
expression was found to be decreased and to serve a protective role
in noise-exposed (52) and
cisplatin-induced (53) cochleae
but increased and damages cells in D-gal-induced cochlea (54) and auditory cortex (6). This may be because the
concentration of D-gal in the aforementioned experiments was
relatively high (500 mg/kg). However, miR-34a may lose its
inhibitory effect on TFAM and TFAM may serve a pro-death role with
high concentrations of D-gal. The present study observed
colocalization of TFAM and the pyroptotic protein GSDMD in
vitro and in vivo, suggesting that TFAM may serve a key
role in pyroptosis, but more detailed research including RNA
pull-down assay to verify the binding, is needed.
In conclusion, miR-34a is a key regulator of
apoptosis and pyroptosis in D-gal-induced aging HEI-OC1 cells and
cochleae of C57BL/6 mice via inhibition of TFAM, thus promoting
mitochondrial dysfunction. The present results improve
understanding of miR-34a-mediated cochlear hair cell loss in ARHL
development and suggest miR-34a as a promising therapeutic target
for ARHL treatment.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW performed experiments. YW and GW wrote the
manuscript. MY and BD performed experiments. GW constructed
figures. WL designed the methodology. BD and GW analyzed data. XY
and XL conceived the study. XL and GW edited the manuscript. YW and
XL confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by animal care and
the Ethics Committee for Animal Research (2020-NZR-037, People's
Hospital of Ningxia Hui Autonomous Region, Yinch.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Ningxia Natural Science
Foundation (grant no. 2021AAC03297) and Ningxia Hui Autonomous
Region Key Research and Development Program Project (grant no.
2022BEG03163).
References
|
1
|
World Health Organization: World Report on
Hearing. World Health Organization; Geneva: 2021
|
|
2
|
Gates GA and Mills JH: Presbycusis.
Lancet. 366:1111–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rutherford BR, Brewster K, Golub JS, Kim
AH and Roose SP: Sensation and psychiatry: Linking age-related
hearing loss to late-life depression and cognitive decline. Am J
Psychiatry. 175:215–224. 2018. View Article : Google Scholar :
|
|
4
|
Bowl MR and Dawson SJ: Age-related hearing
loss. Cold Spring Harb Perspect Med. 9:a0332172019. View Article : Google Scholar
|
|
5
|
Wu PZ, O'Malley JT, de Gruttola V and
Liberman MC: Age-related hearing loss is dominated by damage to
inner ear sensory cells, not the cellular battery that powers them.
J Neurosci. 40:6357–6366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong Y, Hu Y, Peng W, Sun Y, Yang Y, Zhao
X, Huang X, Zhang H and Kong W: Age-related decline of the
cytochrome c oxidase subunit expression in the auditory cortex of
the mimetic aging rat model associated with the common deletion.
Hear Res. 294:40–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du Z, Yang Y, Hu Y, Sun Y, Zhang S, Peng
W, Zhong Y, Huang X and Kong W: A long-term high-fat diet increases
oxidative stress, mitochondrial damage and apoptosis in the inner
ear of D-galactose-induced aging rats. Hear Res. 287:15–24. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu J, Wang Y, Liu P, Li Q, Sun Y and Kong
W: Mitochondrial DNA common deletion increases susceptibility to
noise-induced hearing loss in a mimetic aging rat model. Biochem
Biophys Res Commun. 453:515–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parameshwaran K, Irwin MH, Steliou K and
Pinkert CA: D-galactose effectiveness in modeling aging and
therapeutic antioxidant treatment in mice. Rejuvenation Res.
13:729–735. 2010. View Article : Google Scholar
|
|
10
|
Chen B, Zhong Y, Peng W, Sun Y and Kong
WJ: Age-related changes in the central auditory system: Comparison
of D-galactose-induced aging rats and naturally aging rats. Brain
Res. 1344:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He ZH, Li M, Fang QJ, Liao FL, Zou SY, Wu
X, Sun HY, Zhao XY, Hu YJ, Xu XX, et al: FOXG1 promotes aging inner
ear hair cell survival through activation of the autophagy pathway.
Autophagy. 17:4341–4362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics biogenesis,
mechanism and function. Cell. 116:281–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wienholds E, Kloosterman WP, Miska E,
Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen
S and Plasterk RH: MicroRNA expression in zebrafish embryonic
development. Science. 309:310–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudnicki A and Avraham KB: microRNAs: The
art of silencing in the ear. EMBO Mol Med. 4:849–859. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rudnicki A, Isakov O, Ushakov K, Shivatzki
S, Weiss I, Friedman LM, Shomron N and Avraham KB: Next-generation
sequencing of small RNAs from inner ear sensory epithelium
identifies microRNAs and defines regulatory pathways. BMC Genomics.
15:4842014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. The National
Academies Press; Washington, DC: pp. 2462011
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Yang X, Wang G, Liu W, Zhang J, Deng B, Li
X and Wang L: Key genes and potential drugs in age-related hearing
loss: Transcriptome analysis of cochlear hair cells in old mice.
Cell Mol Biol (Noisy-le-grand). 69:67–74. 2023. View Article : Google Scholar
|
|
19
|
Fujimoto C and Yamasoba T: Oxidative
stresses and mitochondrial dysfunction in age-related hearing loss.
Oxid Med Cell Longev. 2014:5828492014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu Y, Liu Y and Tao J: Progress of
clinical evaluation for vascular aging in humans. J Transl Int Med.
9:17–23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fridovich I: Superoxide anion radical
(O2-.), superoxide dismutases, and related matters. J Biol Chem.
272:18515–18517. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kowaltowski AJ, de Souza-Pinto NC,
Castilho RF and Vercesi AE: Mitochondria and reactive oxygen
species. Free Radic Biol Med. 47:333–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kornfeld OS, Qvit N, Haileselassie B,
Shamloo M, Bernardi P and Mochly-Rosen D: Interaction of
mitochondrial fission factor with dynamin related protein 1 governs
physiological mitochondrial function in vivo. Sci Rep. 8:140342018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen KH, Dasgupta A, Lin J, Potus F,
Bonnet S, Iremonger J, Fu J, Mewburn J, Wu D, Dunham-Snary K, et
al: Epigenetic dysregulation of the dynamin-related protein 1
binding partners MiD49 and MiD51 increases mitotic mitochondrial
fission and promotes pulmonary arterial hypertension: Mechanistic
and therapeutic implications. Circulation. 138:287–304. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thounaojam MC, Jadeja RN, Warren M, Powell
FL, Raju R, Gutsaeva D, Khurana S, Martin PM and Bartoli M:
MicroRNA-34a (miR-34a) mediates retinal endothelial cell premature
senescence through mitochondrial dysfunction and loss of
antioxidant activities. Antioxidants (Basel). 8:3282019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan X, Zhou S, Zheng M, Deng X, Yi Y and
Huang T: MiR-199a-3p enhances breast cancer cell sensitivity to
cisplatin by downregulating TFAM (TFAM). Biomed Pharmacother.
88:507–514. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding L and Wang J: MiR-106a facilitates
the sensorineural hearing loss induced by oxidative stress by
targeting connexin-43. Bioengineered. 13:14080–14093. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nunez DA and Guo RC: Acquired
sensorineural hearing loss, oxidative stress, and microRNAs. Neural
Regen Res. 20:2513–2519. 2025.
|
|
29
|
Zhang J, Sun W, Kuang S, Gan Q, Li H, Ma
H, Yang G, Guo J, Tang Y and Yuan W: miR-130b-3p involved in the
pathogenesis of age-related hearing loss via targeting PPARγ and
autophagy. Hear Res. 449:1090292024. View Article : Google Scholar
|
|
30
|
Verschuur CA, Dowell A, Syddall HE, Ntani
G, Simmonds SJ, Baylis D, Gale CR, Walsh B, Cooper C, Lord JM and
Sayer AA: Markers of inflammatory status are associated with
hearing threshold in older people: Findings from the hertfordshire
ageing study. Age Ageing. 41:92–97. 2012. View Article : Google Scholar
|
|
31
|
Yang X, Wu Y, Zhang M, Zhang L, Zhao T,
Qian W, Zhu M, Wang X, Zhang Q, Sun J and Dong L: Piceatannol
protects against age-related hearing loss by inhibiting cellular
pyroptosis and inflammation through regulated Caspase11-GSDMD
pathway. Biomed Pharmacother. 163:1147042023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang A, Pan Y, Wang H, Ding R, Zou T, Guo
D, Shen Y, Ji P, Huang W, Wen Q, et al: Excessive processing and
acetylation of OPA1 aggravate age-related hearing loss via the
dysregulation of mitochondrial dynamics. Aging Cell. 23:e140912024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Safabakhsh S, Wijesinghe P, Nunez M and
Nunez DA: The role of hypoxia-associated miRNAs in acquired
sensorineural hearing loss. Front Cell Neurosci. 16:9166962022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiong H, Pang J, Min X, Ye Y, Lai L and
Zheng Y: miR-34a/ATG9A/TFEB signaling modulates autophagy in
cochlear hair cells and correlates with age-related hearing loss.
Neuroscience. 491:98–109. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pang J, Xiong H, Lin P, Lai L, Yang H, Liu
Y, Huang Q, Chen S, Ye Y, Sun Y and Zheng Y: Activation of miR-34a
impairs autophagic flux and promotes cochlear cell death via
repressing ATG9A: Implications for age-related hearing loss. Cell
Death Dis. 8:e30792017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Lin H, Kang W, Huang L, Gong S,
Zhang T, Huang X, He F, Ye Y, Tang Y, et al: miR-34a/DRP-1-mediated
mitophagy participated in cisplatin-induced ototoxicity via
increasing oxidative stress. BMC Pharmacol Toxicol. 24:162023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boon RA, Iekushi K, Lechner S, Seeger T,
Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, et
al: MicroRNA-34a regulates cardiac ageing and function. Nature.
495:107–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ito T, Yagi S and Yamakuchi M:
MicroRNA-34a regulation of endothelial senescence. Biochem Biophys
Res Commun. 398:735–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang J, Chen D, He Y, Meléndez A, Feng Z,
Hong Q, Bai X, Li Q, Cai G, Wang J and Chen X: MiR-34 modulates
Caenorhabditis elegans lifespan via repressing the autophagy gene
atg9. Age (Dordr). 35:11–22. 2013. View Article : Google Scholar
|
|
40
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar
|
|
41
|
Bai XY, Ma Y, Ding R, Fu B, Shi S and Chen
XM: miR-335 and miR-34a Promote renal senescence by suppressing
mitochondrial antioxidative enzymes. J Am Soc Nephrol.
22:1252–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong Z, Gao Y, Zhou J, Wang F, Zhang P,
Hu S, Wu H, Lou H, Chi J, Lin H and Guo H: Inhibiting mir-34a-5p
regulates doxorubicin-induced autophagy disorder and alleviates
myocardial pyroptosis by targeting Sirt3-AMPK pathway. Biomed
Pharmacother. 168:1156v2023. View Article : Google Scholar
|
|
43
|
Chen S, Ding R, Hu Z, Yin X, Xiao F, Zhang
W, Yan S and Lv C: MicroRNA-34a inhibition alleviates lung injury
in cecal ligation and puncture induced septic mice. Front Immunol.
11:18292020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li C, Qu L, Farragher C, Vella A and Zhou
B: MicroRNA regulated macrophage activation in obesity. J Transl
Int Med. 7:46–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bao H and Peng A: The green tea
polyphenol(-)-epigallocate-chin-3-gallate and its beneficial roles
in chronic kidney disease. J Transl Int Med. 4:99–103. 2016.
View Article : Google Scholar
|
|
46
|
Li Q, Shi N, Cai C, Zhang M, He J, Tan Y
and Fu W: The role of mitochondria in pyroptosis. Front Cell Dev
Biol. 8:6307712021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Campbell CT, Kolesar JE and Kaufman BA:
Mitochondrial transcription factor A regulates mitochondrial
transcription initiation, DNA packaging, and genome copy number.
Biochim Biophys Acta. 1819:921–929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu M, Ding Q, Lin Z, Chen X, Chen S and
Zhu Y: New insights of epigenetics in vascular and cellular
senescence. J Transl Int Med. 9:239–248. 2021. View Article : Google Scholar
|
|
49
|
Wang P, Zhang N, Wu B, Wu S, Zhang Y and
Sun Y: The role of mitochondria in vascular calcification. J Transl
Int Med. 8:80–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Desdín-Micó G, Soto-Heredero G, Aranda JF,
Oller J, Carrasco E, Gabandé-Rodríguez E, Blanco EM, Alfranca A,
Cussó L, Desco M, et al: T cells with dysfunctional mitochondria
induce multimorbidity and premature senescence. Science.
368:1371–1376. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao M, Liu S, Wang C, Wang Y, Wan M, Liu
F, Gong M, Yuan Y, Chen Y, Cheng J, et al: Mesenchymal stem
cell-derived extracellular vesicles attenuate mitochondrial damage
and inflammation by stabilizing mitochondrial DNA. ACS Nano.
15:1519–1538. 2021. View Article : Google Scholar
|
|
52
|
Chen JW, Ma PW, Yuan H, Wang WL, Lu PH,
Ding XR, Lun YQ, Yang Q and Lu LJ: mito-TEMPO attenuates oxidative
stress and mitochondrial dysfunction in noise-induced hearing loss
via maintaining TFAM-mtDNA interaction and mitochondrial
biogenesis. Front Cell Neurosci. 16:8037182022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nong H, Song X, Li Y, Xu Y, Wang F, Wang
Y, Zhang J, Chen C and Li J: AdipoRon reduces cisplatin-induced
ototoxicity in hair cells:possible relation to the regulation of
mitochondrial biogenesis. Neurosci Lett. 819:1375772024. View Article : Google Scholar
|
|
54
|
Zhong Y, Hu YJ, Chen B, Peng W, Sun Y,
Yang Y, Zhao XY, Fan GR, Huang X and Kong WJ: Mitochondrial
transcription factor A overexpression and base excision repair
deficiency in the inner ear of rats with D-galactose-induced aging.
FEBS J. 278:2500–2510. 2011. View Article : Google Scholar : PubMed/NCBI
|