Stroke poses a threat to the elderly, being the
second leading cause of death and the third leading cause of
disability worldwide (1). Among
all types of stroke, ischemic stroke (IS) accounts for the highest
percentage of strokes, ~85% (2).
IS is caused by a lack of blood supply due to the occlusion of
cerebral arteries, and the lack of oxygen and glucose contributes
to brain tissue damage (3).
Focal ischemia due to IS is an inevitable consequence of cellular
damage in the infarcted area, and reperfusion of the infarcted area
would further aggravate the infarct size (4). At present, the only viable
treatment for cerebral ischemia is thrombolysis; however, the rapid
reperfusion caused by thrombolysis could lead to further damage to
brain tissue, which is known as cerebral ischemia/reperfusion
injury (CIRI) (5). At present,
tissue plasminogen activator is the only drug approved by the Food
and Drug Administration for the treatment of IS, but its scope of
application is narrow, accounting for only 10% of patients with
stroke (6,7). The high prevalence of IS and the
scarcity of available clinical drugs make it particularly important
to explore potential protective drugs for neurological recovery.
Although the pathophysiological process of IS is complex,
scientists have devoted themselves to exploring potential
protective drugs for IS over the past decades with remarkable
success, and a considerable number of potential protective drugs
have been identified for IS treatment (8,9).
Cell death in the IS infarcted area is either
regulated by specific cellular genes or molecules, also known as
programmed cell death (PCD), or it follows an unregulated pathway,
also known as accidental cell death (4). PCD is controllable, but accidental
cell death is uncontrollable. PCD was first proposed in 1965, and
it follows strict signaling cascades and regulation by molecularly
defined effector mechanisms (10). After the occurrence of IS, PCD of
neurons, microglia, astrocytes and other cells in brain tissues
markedly promotes the progression of CIRI, severely affecting the
prognosis and functional recovery of patients (11,12). Alleviating brain injury by
intervening in IS-induced PCD has shown positive results in
previous studies (13,14). At present, at least five types of
PCD, including apoptosis, autophagy, necroptosis, pyroptosis and
ferroptosis, have been identified by scientists to be associated
with IS-induced injury, based on the timing of appearance (15).
Intervention in IS-induced PCD has shown positive
effects in protecting against IS-induced brain injury in recent
studies (31,32). The present study is a systematic
review of the specific molecular mechanisms of IS-induced PCD and
the potential protective drugs that have been available to
attenuate IS-induced injury by interfering in IS-induced PCD in the
last decade. The present review is beneficial for scientists to
understand the molecular mechanisms of IS-induced injury and helps
provide ideas for the development of targeted pharmacotherapy for
IS.
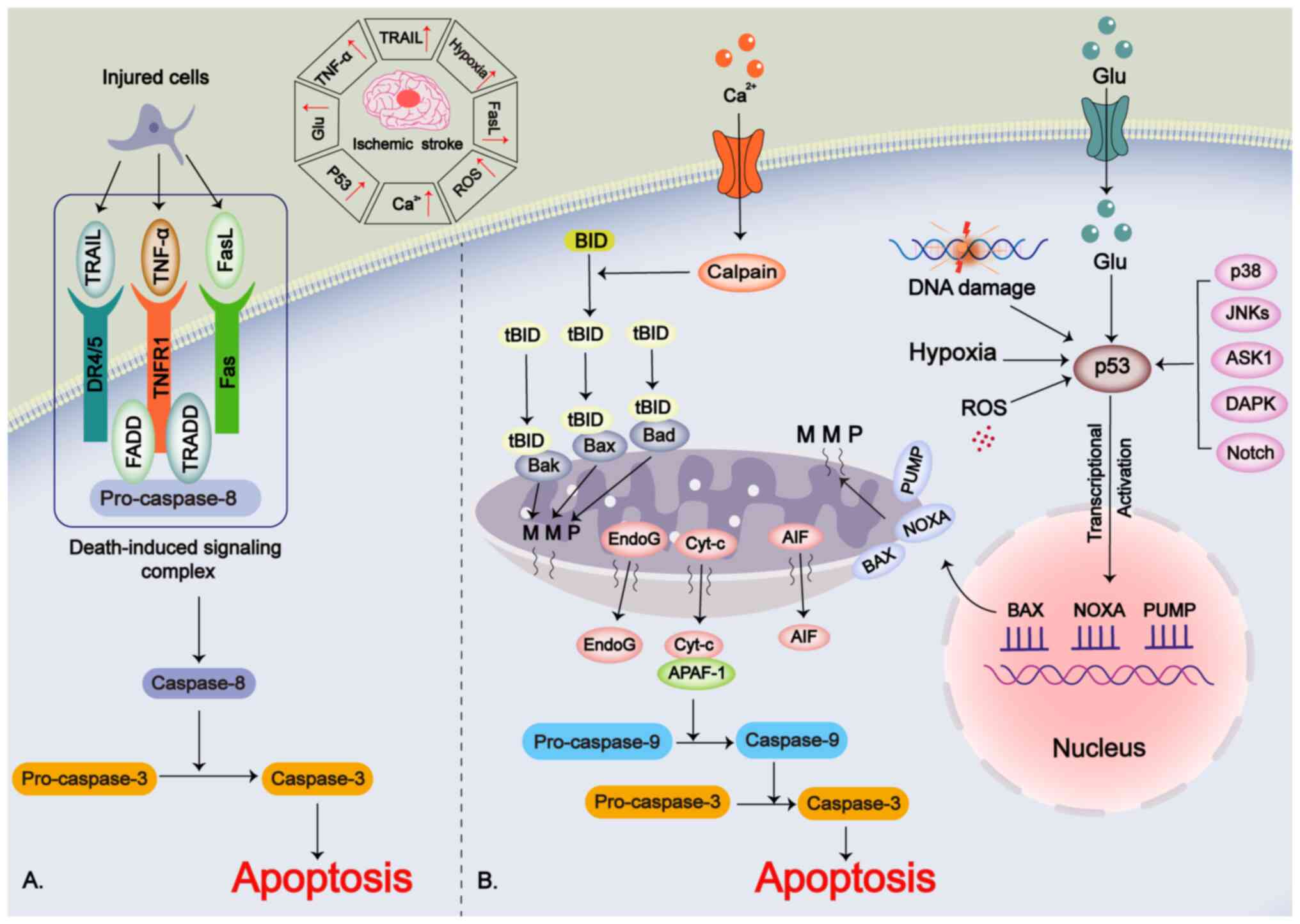
Apoptosis, the first identified form of PCD, serves
a critical role in the pathophysiology of IS. It is induced via
three main pathways: i) The receptor-mediated extrinsic apoptotic
pathway; ii) the mitochondria-mediated intrinsic apoptotic pathway;
and iii) the p53-mediated intrinsic apoptotic pathway (33-35).
In the extrinsic pathway, ligands binding to 'death
receptors' on the cell membrane is the main trigger of this
apoptotic pathway (36). The
ligands include TNF-α, TNF-related apoptosis-inducing ligand
(TRAIL) and FasL, and the 'death receptors' include
apoptosis-related factors (Fas/CD95), tumor necrosis factor
receptor 1 (TNFR1) and TRAIL receptors (including DR4 and DR5)
(37,38). The aforementioned ligands bind to
the 'death receptor' and form a death-induced signaling complex
with the caspase-8 precursor, which promotes the maturation of
caspase-8 (39). The mature
caspase-8 promotes the shearing of its downstream target caspase-3,
which in turn promotes apoptosis (40). During the pathological process of
IS, TNF-α released by injured neurons or glial cells will then
mediate apoptosis through this extrinsic pathway (41,42).
The intrinsic pathway is primarily a cellular
suicide mechanism triggered by intracellular signals (43). Internal signals for intrinsic
apoptosis in IS include DNA damage, hypoxia and reactive oxygen
species (ROS) (43). Disruption
of calcium homeostasis allows calcium to enter the cell, activating
calpain, which cleaves the BH3-interacting domain (BID) into its
truncated (t) form, tBID (44).
tBID interacts with pro-apoptotic proteins such as Bax, Bak and Bad
in the mitochondrial membrane, causing mitochondrial membrane
permeabilization (MMP) (45).
This permeabilization leads to the release of cytochrome c,
apoptosis-inducing factor (AIF), endonuclease G and other
mitochondrial components (46).
Cytochrome c binds to apoptotic protease activating factor 1,
promoting the activation of caspase-9, which in turn enhances the
pro-apoptotic function of caspase-3 (47,48).
The p53 protein, an oncogene and transcription
factor, is associated with IS-induced neuronal damage and is
upregulated during the pathological process of IS (49,50). Factors such as glutamate
excitotoxicity, ROS, hypoxia and DNA damage can induce p53
upregulation (51,52). Additionally, upstream proteins
such as JNKs, p38, death-associated protein kinase, apoptosis
signal-regulated kinase-1 and Notch regulate p53 (35,53). p53 promotes the transcription of
pro-apoptotic genes, including Bax, p53-upregulated modulator of
apoptosis and NADPH oxidase activator (33). This promotion leads to MMP and
the subsequent release of cytochrome c, activating the
mitochondria-mediated intrinsic apoptotic pathway (52) (Fig. 1).
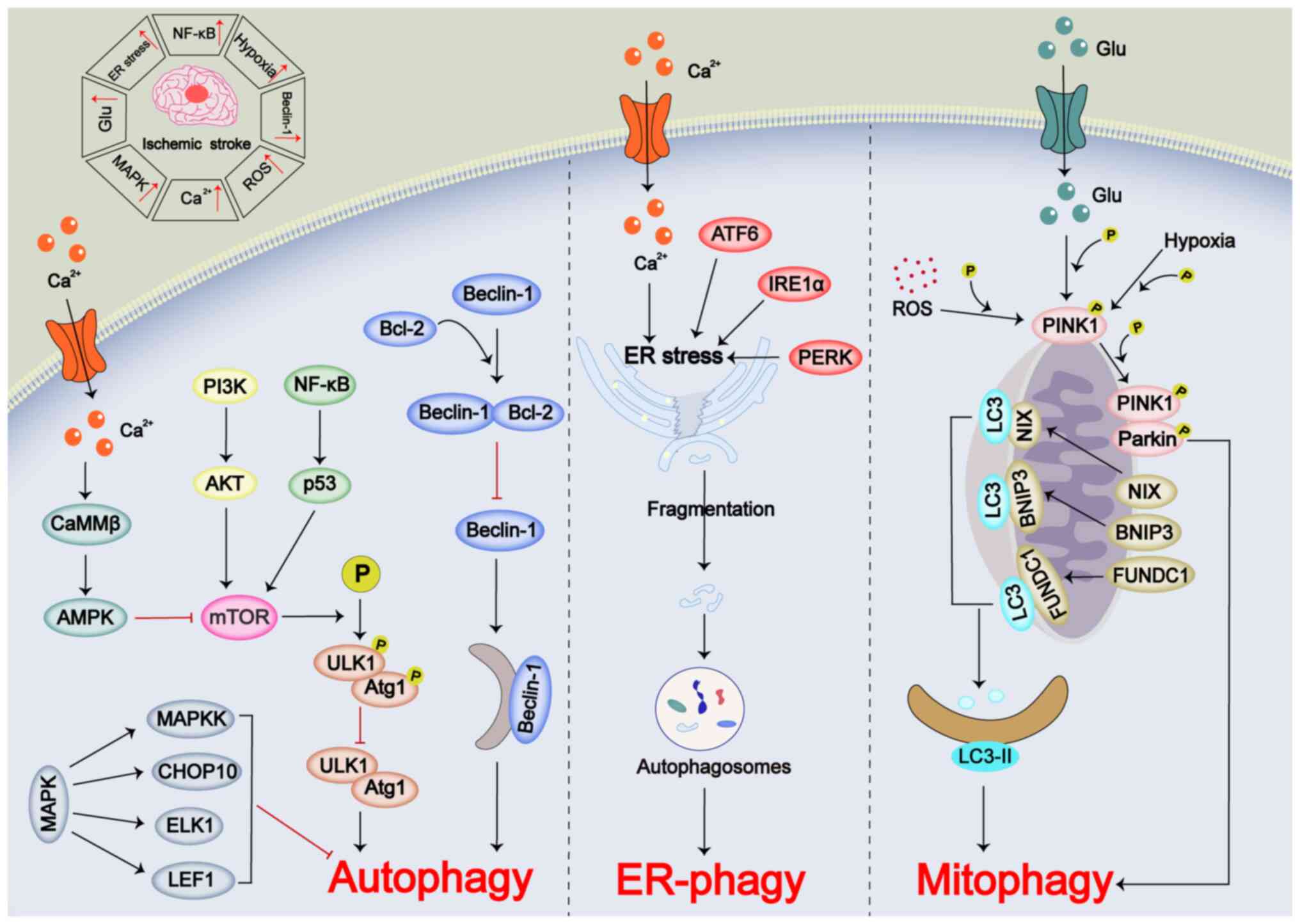
Autophagy is a highly coordinated process that
sequesters misfolded or mutated proteins, aged organelles and other
damaged cellular components into double-membrane vesicles known as
autophagosomes. These autophagosomes then fuse with lysosomes,
leading to the degradation of their contents (54). Under normal conditions, autophagy
maintains a dynamic balance within the cell; however, pathological
changes can disrupt this homeostasis (55). Autophagy can be categorized into
selective and non-selective types based on the presence of target
substrates (56,57). Selective autophagy encompasses
various processes, including mitochondrial autophagy (mitophagy),
endoplasmic reticulum (ER) autophagy (ER-phagy), lipid droplet
autophagy, peroxisomal autophagy and ribosomal autophagy (57,58). In the pathophysiology of IS,
increased excitotoxicity, mitochondrial dysfunction, ER stress and
ROS are conditions that induce autophagy (59). However, autophagy serves a dual
role in IS-induced injury. While moderate autophagy can help
mitigate damage, inadequate, defective or excessive autophagy may
worsen the injury (58). The
activation of autophagy in IS is regulated by various intracellular
signaling pathways (56). These
include non-selective autophagy mediated by mTOR and MAPK pathways,
as well as the Beclin-1/Bcl-2 signaling pathway (56). In addition, selective forms of
autophagy such as Parkin-dependent mitophagy, LC3-mediated
mitophagy and ER stress-mediated ER-phagy also serve important
roles (58).
mTOR is a conserved serine/threonine protein kinase
that serves a crucial role in the initiation of autophagy by
inhibiting autophagy through the phosphorylation of the
autophagy-related 1/UNC-51-like kinase 1 (ULK1) protease complex
(60,61). In the pathological process of IS,
the PI3K/AKT signaling pathway regulates mTOR, and its inhibition
markedly suppresses mTOR activity (62). Additionally, the NF-κB-dependent
p53 signaling pathway has been shown to inhibit autophagy via mTOR
during IS (63). Ca2+
disruption in IS can activate 5'-AMP-activated protein kinase
(AMPK) via Ca2+/calmodulin-dependent protein kinase
kinase β, leading to mTOR inhibition and autophagy activation
(64-66). The MAPK family, including JNK,
ERK and p38MAPK, serves a role in autophagy regulation. Notably,
activation of the MAPK pathway in early IS stages can inhibit
autophagy through MAPK kinase, C/EBP homologous protein 10, ETS
transcription factor ELK1 and lymphoid enhancer-binding factor 1,
while promoting neuronal survival (67-70).
Beclin-1, a protein containing a BH3 domain, is a
key regulator of autophagy and is involved in autophagosome
membrane formation (71).
Beclin-1 expression increases in IS, promoting autophagy (72). Beclin-1 forms a complex with
Bcl-2, which inhibits Beclin-1-induced autophagy (73,74). Although the mechanism of complex
dissociation during ischemia remains unclear, it has been observed
that autophagy increased during ischemia-reperfusion injury (IRI)
via a Beclin-1-dependent but AMPK-independent pathway (75).
Mitochondria serve a vital role in regulating
intracellular homeostasis, ATP production and Ca2+
balance (76). Mitophagy helps
remove damaged mitochondria, promoting mitochondrial renewal
(77). In IS, mitophagy is
primarily mediated by the PTEN-induced putative kinase 1
(PINK1)/Parkin pathway, with ROS, hypoxia and excitatory amino
acids promoting PINK1 activation, which phosphorylates Parkin on
the outer mitochondrial membrane (78). Additionally, LC3 can mediate
mitophagy through mitochondrial receptors such as Nip3-like protein
X, Bcl-2/adenovirus E1B 19 kDa protein-interacting protein 3
(BNIP3) and FUN14 domain containing 1, which interact with LC3 to
induce LC3-II expression and promote mitophagy (79).
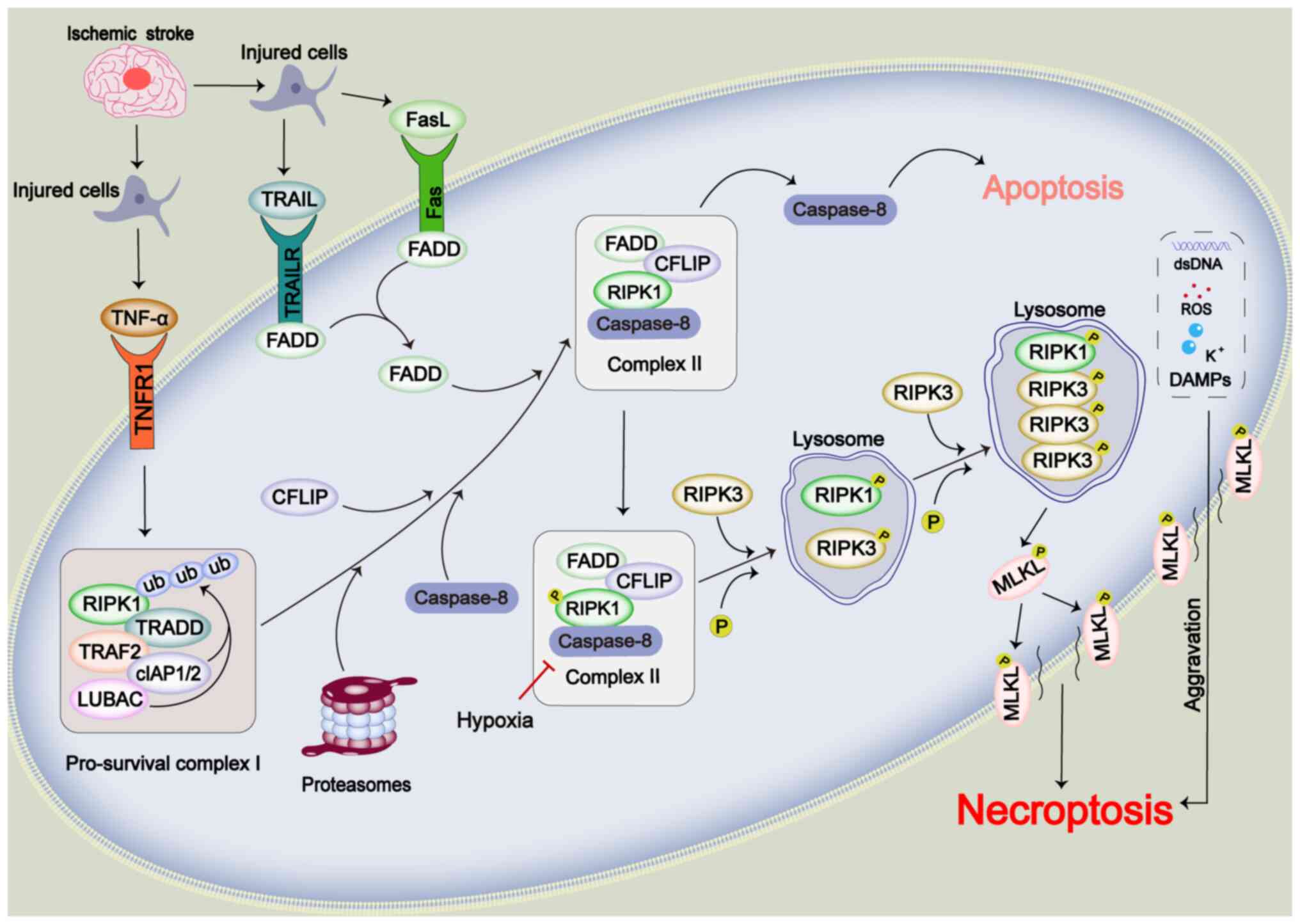
Necroptosis exhibits distinct features compared with
those of traditional apoptosis. Morphologically, necroptosis
resembles unregulated necrosis, characterized by early loss of
cytosolic membrane integrity, increased cell volume and swelling of
organelles. By contrast, apoptosis is marked by cell shrinkage,
plasma membrane blistering, and condensation and fragmentation of
the nucleus and organelles (84). Mechanistically, necroptosis is
caspase-independent and is regulated by receptor interacting
serine/threonine kinase 1 (RIPK1) and receptor interacting
serine/threonine kinase 3 (RIPK3), along with mixed lineage kinase
domain-like protein (MLKL) (22).
In the pathological context of IS, TNF-α serves as a
key initiator of necroptosis (85). Following cerebral ischemia, TNF-α
binds to its receptor, TNFR1 (85). This interaction recruits
components to form pro-survival complex I, which includes the
TNFRSF1A associated via death domain, RIPK1, TNF receptor
associated factor 2, the ubiquitin E3 ligase linear ubiquitin chain
assembly complex and cellular inhibitor of apoptosis protein ½
(86). Within complex I, RIPK1
undergoes polyubiquitination, leading to its disassembly into
complex II through proteasomal action (86). Under normal conditions, complex
II mediates apoptosis via the caspase-8 pathway. However, hypoxia
induced by cerebral ischemia inhibits caspase-8 and activates
complex II (87,88). Upon activation, RIPK3 binds to
RIPK1, forming a complex where both proteins are phosphorylated and
activated (89). The
oligomerization and subsequent phosphorylation of RIPK3 in this
complex trigger the phosphorylation and activation of MLKL, which
mediates necroptosis and promotes the release of DAMPs (90). The leakage of DAMPs can further
exacerbate necroptosis and inflammation (90) (Fig. 3).
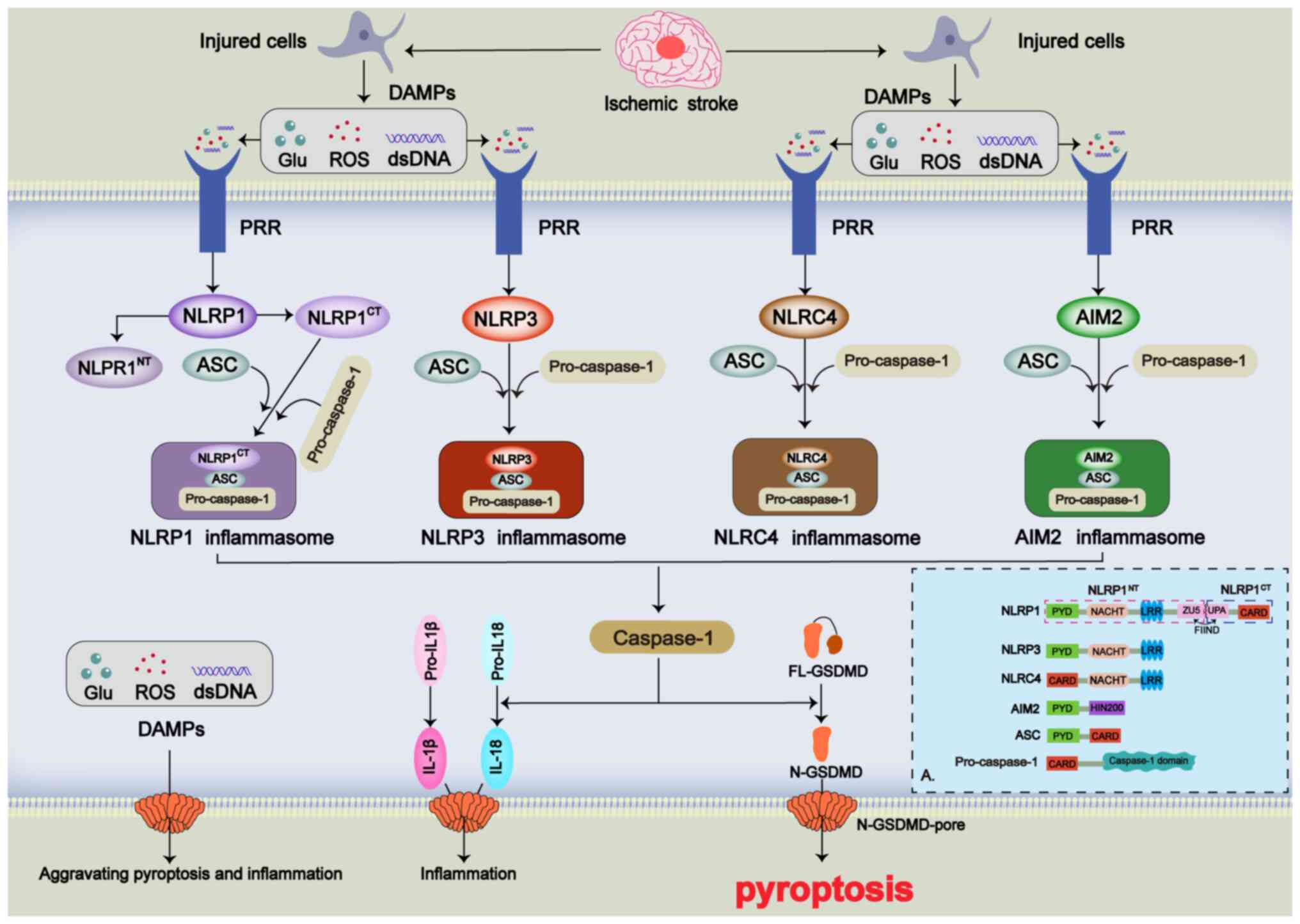
The gasdermin (GSDM) family has been recognized as a
key regulator of pyroptosis (91). This family comprises five protein
molecules (GSDMA, GSDMB, GSDMC, GSDMD and GSDME), all of which can
mediate pyroptosis (91). The
primary mechanism by which the GSDM family induces pyroptosis
involves forming pores in the cell membrane. This leads to changes
in intraand extracellular osmotic pressure, ultimately causing cell
rupture and the release of intracellular DAMPs, which promote
inflammation (92). Among these
proteins, GSDMD is considered to be the most prominent executor of
pyroptosis (14). GSDMD consists
of two conserved structural domains, the N-terminal functional
domain (N-GSDMD) and the C-terminal autoinhibitory domain
(C-GSDMD), linked by a ring structure (93). Under normal conditions, GSDMD is
non-toxic to cells and does not mediate pyroptosis. GSDMD only
promotes pyroptosis when cleaved into N-GSDMD by caspase-1
(94). Caspase-1 also cleaves
the inflammatory precursors pro-IL-18 and pro-IL-1β; once N-GSDMD
opens non-ion channels in the cell membrane, it facilitates the
release of these inflammatory factors, triggering a cascade of
inflammatory responses (95).
Additionally, caspases-4, -5 and -11, as well as -3 and -8, can
cleave GSDMD into N- and C-GSDMD; however, their impact on
pyroptosis is less pronounced (96-98).
In the pathophysiological process of IS, pyroptosis
acts as a proinflammatory inducer primarily occurring in the
ischemic penumbral region of the brain (99). The initiation of pyroptosis in
this area is mainly due to DAMPs released from dying cells,
including excitotoxins, double-stranded DNA and TNF-α (100). Pattern recognition receptors
(PRRs) on the cell membrane, including NOD-like receptors (NLRs)
and toll-like receptors (TLRs), recognize these DAMPs (100). Pyroptosis in IS is primarily
triggered by the activation of intracellular inflammasomes by DAMPs
(4). Inflammasomes are
multiprotein complexes composed of three main components: i)
Sensors; ii) adapters; and iii) effectors (101). In IS, the sensors involved in
pyroptosis include NLRs [NLR family pyrin domain containing
(NLRP)1, NLRP3 and NLR family CARD domain containing 4 (NLRC4)] and
absent in melanoma 2 (AIM2) (102,103). NLRP1 features a pyrin domain
(PYD), NACHT domain, leucine-rich repeat (LRR) domain, function to
find (FIIND) domain and caspase recruitment domain (CARD). The
FIIND domain of NLRP1 cleaves into ZO-1 and Unc5-like domain 5 and
ubiquitin protease associated domain, splitting NLRP1 into N- and
C-terminal fragments, with the latter participating in the
formation of the NLRP1 inflammasome (104). NLRP3 comprises PYD, NACHT and
LRR domains (105), while NLRC4
includes CARD, NACHT and LRR domains (106). AIM2 consists of PYD and
hematopoietic interferon-inducible nuclear protein with a 200-amino
acid repeat domains (103).
Under pathological conditions, the adapter protein,
apoptosis-associated speck-like protein containing CARD and PYD
domains, interacts with the effector caspase-1, which is primarily
responsible for cleaving GSDMD (14,101). When PRRs on the cell membrane
recognize signals from external DAMPs such as K+, ROS
and double-stranded DNA, they promote the maturation of caspase-1.
This, in turn, cleaves GSDMD into N-GSDMD and facilitates the
maturation of IL-18 and IL-1β, ultimately inducing cellular
pyroptosis and inflammatory cascades (107) (Fig. 4).
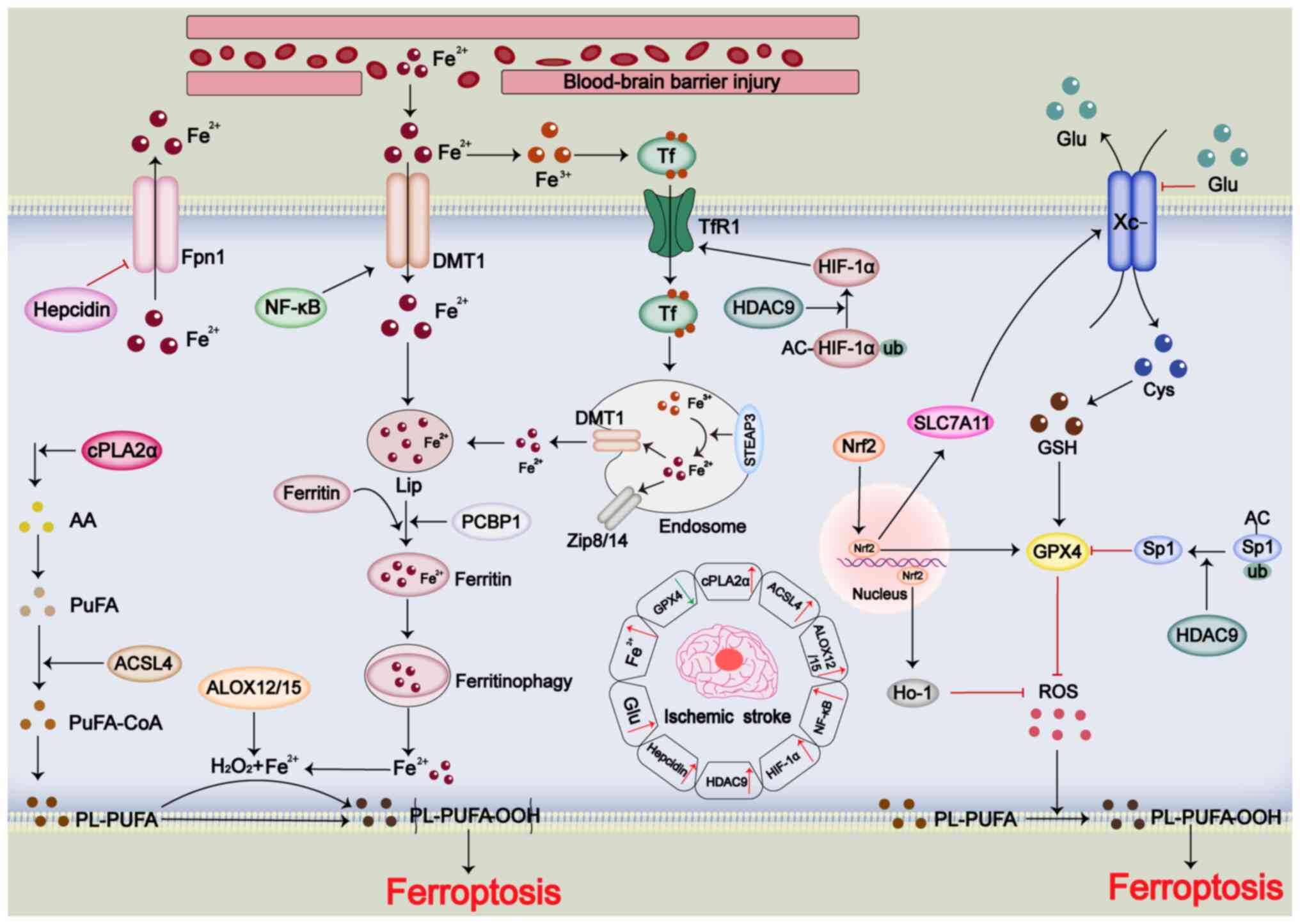
In the past decade, there has been an increase in
research on ferroptosis, revealing its links to various diseases,
including cancer and IRI neurodegeneration (29). Ferroptosis is driven by lipid
peroxidation and abnormal iron metabolism (108). Mechanisms that confer
resistance to ferroptosis include the glutathione (GSH)-glutathione
peroxidase 4 (GPX4), ferroptosis suppressor protein 1-reduced
coenzyme Q10 (CoQH2), dihydroorotate dehydrogenase-CoQH2 and GTP
cyclohydrolase 1-tetrahydrobiopterin pathways (109). Previous studies have identified
ferroptosis as a potential therapeutic target for IS (110,111). During IS, lipid peroxidation
products accumulate and iron levels rise, while GPX4 expression
decreases, and acyl-CoA synthetase long-chain family member 4
(ACSL4), cyclooxygenase-2 and ROS levels are increased (112). This suggests that ferroptosis
may be a potential therapeutic target for IS.
Lipid peroxidation involves the production of
hydroperoxides from PUFAs through enzymatic and iron-mediated
reactions (113-115). Cytosolic phospholipase A2
(cPLA2α) is upregulated during IS, and cPLA2α promotes lipid
peroxidation of arachidonic acid and ROS generation (116,117). ACSL4 is also upregulated in IS
and promotes lipid peroxidation (118). In addition, arachidonic acid
lipoxygenase 12/15, which promotes lipid peroxidation, is
upregulated in IS (119,120).
Overaccumulation of iron is a key feature of
ferroptosis, with ferric ions promoting intracellular ROS
production and membrane lipid peroxidation through iron-dependent
reactions that decompose H2O2 to ·OH
(29). In vivo, divalent
iron is oxidized to trivalent iron, which binds to transferrin and
enters cells via transferrin receptor 1 (TfR1) (121). Inside endosomes, trivalent iron
is reduced to divalent iron by six-transmembrane epithelial antigen
of the prostate 3 (STEAP3), and then transported into the cytoplasm
via divalent metal transporter 1 (DMT1) or zinc transporter 8/14
(121-123). Iron ions are taken up into the
cell and form the labile iron pool (LIP) (123). Divalent iron forms a LIP and is
packaged into ferritin by poly (rC)-binding protein 1 (123). Even before the concept of
ferroptosis emerged, abnormal accumulation of iron was observed in
brain tissues during IS (124,125). Injury to the blood-brain
barrier facilitates iron entry from the blood into the brain
tissue, promoting ferroptosis (114). In IS, NF-κB activation
increases DMT1 expression, enhancing intracellular iron uptake
(126,127). Hypoxia-inducible factor-1α
(HIF-1α) is upregulated in IS, promoting TfR1 expression and
contributing to iron overload (128,129). Ferritin regulates ferroptosis,
with increased ferritin attenuating CIRI, while decreased ferritin
exacerbates CIRI (130-132). Histone deacetylase 9 (HDAC9) is
expressed at high levels in IS and induces TfR1 expression through
HIF-1α modification (133).
Nuclear receptor coactivator 4 (NCOA4) exacerbates IS-induced
ferroptosis by promoting ferritinophagy, releasing iron ions
(134). Additionally, during
IS, hepcidin is upregulated, inhibiting divalent iron transport
(135,136).
In IS-induced ferroptosis, lipid peroxidation and
iron accumulation coincide with inhibition of the intracellular
anti-ferroptosis system (137).
The solute carrier family 7 member 11 (SLC7A11)/GSH/GPX4 system is
crucial for ferroptosis resistance, and its expression is
downregulated during IS, indicating the role of the
SLC7A11/GSH/GPX4 system in mediating IRI (137-139). High extracellular glutamate
concentrations inhibit the Xc-system, reducing GPX4 production and
promoting ferroptosis (140).
High HDAC9 expression downregulates GPX4 through specificity
protein 1 modification (133).
Conversely, nuclear factor erythroid 2-related factor 2 (Nrf2)
activation enhances SLC7A11, GPX4 and heme oxygenase 1 (HO-1)
expression, inhibiting ROS production and attenuating IS-induced
ferroptosis (141) (Fig. 5).
The treatment of IS can vary widely. In addition to
thrombolytic therapy, strategies aimed at enhancing neurological
recovery post-thrombolysis and various prophylactic measures can
help mitigate IS-induced injury (140). However, no existing treatment
guarantees complete protection against such injuries (142). Consequently, investigating
potential protective drugs holds promise for improving IS treatment
outcomes. Over the past few decades, advances have been made in
exploring potential protective drugs for IS (4), and the present review summarizes
the specific mechanisms of various forms of PCD induced by IS,
highlighting potential protective drugs targeting different PCD
pathways investigated over the last decade.
Apoptosis is the earliest recognized form of PCD,
and numerous studies have demonstrated that it serves a crucial
role in cellular damage within brain tissues during the
pathophysiological process of IS (13,143). Over the past decade, several
potential drugs that inhibit IS-induced apoptosis have been
identified (Table I).
Caspase-3 is the primary executor of apoptosis
during IS-induced injury, and inhibiting caspase-3 can effectively
reduce apoptosis (144). Chen
et al (145)
demonstrated that memantine, a non-competitive N-methyl-D-aspartate
receptor antagonist, inhibited apoptosis by blocking the
calpain/caspase-3 signaling pathway, thereby mitigating neuronal
damage induced by middle cerebral artery occlusion/reperfusion
(MCAO/R) in rats. Zhang et al (146) found that Pien-Tze-Huang
inhibited apoptosis by reducing the levels of cytochrome c, Bax,
p53, and cleaved caspase-3 and -9 after IS, leading to decreased
injury in rat MCAO/R models. Additionally, Nie et al
(147) reported that the Tanhuo
formula also reduced neuronal apoptosis after IS by inhibiting the
caspase-3 pathway. In terms of the extrinsic pathways of apoptosis,
Mei et al (148) showed
that Shuan-Tong-Ling inhibited apoptosis mediated by the extrinsic
pathway by lowering the levels of TNF-α and IL-1β following IS.
Shuan-Tong-Ling also inhibited intrinsic pathway-mediated apoptosis
by upregulating sirtuin 1 (SIRT1) and Bcl-2, while downregulating
p53 and Bax expression (148).
Wang et al (149)
demonstrated that Comp. B, a novel bicoumarin derivative,
attenuated mitochondria-mediated apoptosis by decreasing the Bax/
Bcl-2 ratio, thus reducing middle cerebral artery occlusion
(MCAO)-induced brain tissue injury in mice. Raghavan and Shah
(150) found that Withania
somnifera, also known as 'rutabaga', markedly mitigated
apoptosis induced by permanent MCAO in mice through inhibition of
the poly(ADP-ribose) polymerase 1-AIF pathway. Peng et al
(151) showed that artemisinin
could reduce IS-induced apoptosis by activating the ERK1/2/cAMP
response element binding protein (CREB)/ BCL-2 signaling pathway,
where CREB promoted Bcl-2 expression to prevent apoptosis.
Similarly, rolipram, a phosphodiesterase-4 inhibitor, was found to
diminish transient middle cerebral artery occlusion-induced
apoptosis in rats by activating the cAMP/CREB signaling pathway, as
reported by Hu et al (152).
While investigating therapeutic drugs for IS,
researchers have identified that targeting various intracellular
signaling mechanisms can enhance resistance to IS-induced
apoptosis, particularly through the PI3K/AKT pathway, which serves
a critical role in cell survival (153). Hafeez et al (154) demonstrated that ethanol
protected against IRI in rats by inhibiting protein kinase C δ
(PKC-δ) and enhancing Akt expression. Fan et al (155) showed that S-oxiracetam
mitigated MCAO/R-induced brain damage and apoptosis via α7
nicotinic acetylcholine receptor activation of the PI3K/AKT/GSK3β
pathway. Xu et al (156)
found that Xiaoyao San reduced oxygen glucose
deprivation/reoxygenation (OGD/R)-induced apoptosis in PC12 cells
via the PI3K/AKT pathway. Furthermore, Wang et al (157) reported that total flavonoids of
Chuju activated the PI3K/AKT pathway to reduce IS-induced
apoptosis. Zhang et al (158) suggested that kaempferol may
exert anti-apoptotic effects in IS via the brain-derived
neurotrophic factor-tropomyosin receptor kinase B-PI3K/AKT pathway.
Luo et al (159) showed
that D-allose inhibited IS-induced apoptosis and neuroinflammation
by blocking the galectin-3/toll like receptor 4 (TLR4)/PI3K/AKT
pathway. Qi et al (160)
confirmed that Chuanzhitongluo reduced IS-induced apoptosis via
PI3K/AKT activation. Lastly, Xu et al (161) indicated that Brassaiopsis
glomerulata mitigated MCAO/R and oxygen-glucose
deprivation-induced apoptosis by activating the PI3K/AKT/mTOR
pathway. These findings emphasize the role of PI3K/AKT signaling in
reducing IS-induced apoptosis.
Interfering with cell-intrinsic signals is crucial
for reducing IS-induced apoptosis (13). Zheng et al (171) found that carvedilol protected
against OGD/R-induced PC12 cell injury by inhibiting activating
transcription factor 3 (ATF3), thereby mitigating
mitochondria-mediated apoptosis. Xu et al (172) demonstrated that YiQiFuMai
alleviated IS-induced neuronal apoptosis by preventing
mitochondrial dysfunction and overfission mediated by
PKC-δ/dynamin-related protein 1. Qiu et al (173) showed that Apelin-36 decreased
cerebral I/R-induced infarction and apoptosis by reducing ER
stress. Wu et al (174)
indicated that Apelin-13 mitigated neuronal apoptosis via G protein
α inhibitory subunit/G protein αq subunit-casein kinase 2
signaling. Li et al (175) reported that Xuesaitong promoted
recovery in MCAO/R mice by downregulating the STAT3 pathway, thus
reducing neuronal apoptosis and enhancing M2 microglial
polarization. Li et al (176) found that γ-glutamylcysteine
alleviated OGD/R-induced neuronal apoptosis by activating PERK and
IRE1α. Joshi et al (177) showed that tideglusib reduced
IS-induced apoptosis and neuroinflammation by inhibiting
phosphorylated GSK-3β S9. Ding et al (178) reported that candesartan
inhibited IS-induced apoptosis via the free fatty acid receptor
1/integrin subunit α4 axis. Li et al (179) demonstrated that darutoside or
oridonin reduced IS-induced neuronal apoptosis by inhibiting RIPK3.
Zhang et al (180)
showed that myricetin attenuated apoptosis via the MAPK-ERK
pathway.
Autophagy serves a dual role in IS pathology, with
both excessive and insufficient autophagy exacerbating injury
(181). Studies have indicated
that modulating autophagy after IS can effectively reduce
IS-induced damage (61,181). The present review summarizes
potential protective drugs that attenuate IS-induced injury by
targeting autophagy disruption (Table II).
Excessive autophagy can worsen IS injury, and some
drugs exert protective effects by inhibiting autophagy (61). mTOR is a key regulator of
autophagy, and targeting it can alleviate autophagy disorders
associated with IS (61). Jiang
et al (182) showed that
vitexin reduced MCAO/R-induced brain injury by inhibiting autophagy
through the mTOR/Ulk1 pathway. Tang et al (183) found that exogenous netrin-1
protected against IS injury by inhibiting autophagy via the
PI3K/mTOR pathway. Zhang et al (184) reported that the dichloromethane
fraction (DF) of Piper nigrum and P. longum reduced
IS damage by activating the AKT-mTOR signaling pathway. Other
mechanisms also help inhibit excessive autophagy. Liu et al
(185) suggested that activin A
reduced IS injury by inhibiting cyclic GMP-AMP synthase-stimulator
of interferon genes-mediated autophagy. Lv et al (186) showed that phenothiazines
mitigated IS injury by reducing ER stress-mediated autophagy via
the PERK-eukaryotic initiation factor-2α pathway. Yuan et al
(187) reported that DF
inhibited the expression of autophagy-related proteins such as LC3
and Beclin1 to attenuate IS-induced autophagy. Zhu et al
(188) found that the protein
tyrosine phosphatase 1B inhibitor sc-222227 regulated ER
stress-induced autophagy in microglia, reducing IS injury. Wang
et al (189)
demonstrated that medioresinol inhibited autophagy after IS via the
PPARα/glutamic-oxaloacetic transaminase 1 pathway, protecting
endothelial cells. Xia et al (190) revealed that extracellular
vesicles from induced pluripotent stem cell-derived mesenchymal
stem cells inhibited autophagy and promoted angiogenesis via STAT3
activation. Zhang et al (191) showed that hydroxysafflor yellow
A reduced IS-induced autophagy by inhibiting HIF-1, BNIP3 and
Notch1.
A lack of autophagy hampers the removal of damaged
organelles, harmful proteins and nucleic acids, exacerbating
IS-induced cellular damage (192). The AMPK pathway has been widely
studied due to its role in developing protective drugs that enhance
autophagy and mitigate IS injury (193,194). Li et al (193) demonstrated that stilbene
glycoside protected against IS injury by promoting mitophagy and
inhibiting apoptosis through the SIRT3/AMPK pathway. Ao et
al (194) showed that the
newly synthesized cyclovirobuxine D analog, JLX-001, enhanced
autophagy and reduced IS damage via the AMPK/ULK1 signaling
pathway. Li et al (195)
found that ginaton attenuated MCAO-induced brain injury in rats by
activating autophagy via the AMPK pathway. Additionally, Zhou et
al (196) reported that
oxymatrine promoted autophagy and reduced IS-induced damage by
activating SIRT1.
Necroptosis differs from ordinary necrosis
primarily due to its regulatory role in intracellular signaling,
with the RIPK3/MLKL pathway being central to IS-induced necroptosis
(197). Deng et al
(198) first highlighted the
protective effect of inhibiting necroptosis in IS, showing that the
necrosis inhibitor necrostatin-1 mitigated IS injury by blocking
the RIPK3/MLKL signaling pathway. Subsequently, Zhang et al
(199) made significant
contributions to identifying protective drugs against IS-induced
necroptosis. The authors found that ligustroflavone inhibited
necroptosis by targeting the RIPK1/RIPK3/MLKL pathway, providing
protective effects in IS (199). Additionally, caspofungin was
shown to inhibit necroptosis by upregulating Pellino3 and
ubiquitinating RIPK1, thereby reducing IS-induced damage (200). Telaprevir also mitigated
IS-induced brain injury by inhibiting necroptosis through the
RIPK1/RIPK3/MLKL pathway (201)
(Table III).
Pyroptosis is a key type of PCD that triggers
inflammatory responses, notably contributing to IS-induced injury
(202). Studies have identified
protective drugs that inhibit pyroptosis as promising treatments
for IS (Table III).
The TLR4/NF-κB signaling pathway and inflammasomes
are central to mediating pyroptosis in IS (203). Wang et al (203) showed that Taohong Siwu
decoction reduced MCAO/R-induced pyroptosis and inflammation by
inhibiting the HMGB1/TLR4/NF-κB and MAPK pathways. Curcumin
mitigated IS-induced pyroptosis via the NF-κB/NLRP3 pathway
(204). Li et al
(205) found that indobufen or
aspirin, combined with clopidogrel or ticagrelor, reduced
pyroptosis via the NF-κB/NLRP3 pathway. Ge et al (206) reported that exogenous
recombinant C-X3-C motif chemokine ligand 1 inhibited
NLRP3-mediated pyroptosis. Hu et al (207) demonstrated that edaravone
dexborneol inhibited the NF-κB/NLRP3/GSDMD pathway. Long et
al (208) found that
ginsenoside Rg1 reduced pyroptosis by inhibiting the chemokine like
factor pathway. Zhou et al (209) showed that tetrahedral framework
nucleic acids blocked the TLR2-MyD88-NF-κB pathway, mitigating
brain injury. Wang et al (210) reported that artemisinin reduced
pyroptosis by inhibiting the ROS/thioredoxin interacting
protein/NLRP3/caspase-1 pathway. Furthermore, Alattar et al
(211) found that quercetin, by
activating Nrf2, attenuated MCAO-induced pyroptosis via the
Nrf2/HO-1 signaling pathway.
Ferroptosis, a novel type of PCD, serves a notable
regulatory role in IS-induced injury (5). There has been increased attention
on the development of ferroptosis-targeted drugs, with researchers
actively exploring potential protective agents to inhibit
IS-induced ferroptosis, yielding promising results (Table IV).
Iron-dependent lipid peroxidation is central to
ferroptosis, and managing iron metabolism can reduce IS-induced
ferroptosis (212). Yu et
al (212) showed that
melatonin inhibited ferritinophagy by disrupting the NCOA4-ferritin
heavy chain 1 interaction. Rosmarinic acid liposomes also regulated
iron metabolism by inhibiting TfR1, as demonstrated by Jia et
al (213).
Excess lipid peroxidation exacerbates ferroptosis,
but lipid-regulating drugs offer protection (214). Sun et al (214) found that ecdysterone mitigated
ferroptosis by inhibiting ACSL4. Sun et al (215) reported that melatonin targeted
the ACSL4/cytochrome P450 family 1 subfamily B member 1 pathway to
protect against IS-induced ferroptosis. Jin et al (216) indicated that astragaloside IV
reduced ferroptosis by upregulating ATF3 and enhancing m6A
methylation of ACSL4.
Phospholipid peroxidation is critical in
ferroptosis, and enhancing antioxidants such as Nrf2, SLC7A11 and
GPX4 can counteract phospholipid peroxidation (217). The Danlou tablet boosted
SLC7A11 and GPX4 expression to inhibit ferroptosis (218). Angong Niuhuang Wan activated
the PPARγ/AKT/GPX4 pathway for similar effects (219). Danhong injection activated the
SATB homeobox 1/SLC7A11/HO-1 pathway to mitigate ferroptosis
(220). Caffeic acid and
quercetin enhanced Nrf2 signaling, as shown by Li et al
(221,222) and Peng et al (221,222). Mi et al (223) revealed that kellerin could
attenuate IS-induced ferroptosis by promoting AKT-regulated Nrf2
transcriptional activity. Neutral polysaccharides from Gastrodia
elata activated the Nrf2/HO-1 pathway to reduce ferroptosis
according to Zhang et al (224). Wu et al (225) indicated that 15,
16-dihydrotanshinone I inhibited ferroptosis by activating Nrf2.
Duan et al (226)
revealed that the small molecule compound N6022 was able to
attenuate IS-induced microglial ferroptosis by promoting the
nuclear translocation of Nrf2 and inhibiting the interaction of
S-nitrosoglutathione reductase with glutathione S-transferase pi 1,
which in turn attenuated IS-induced injury. Ozone also activated
the Nrf2/SLC7A11/GPX4 pathway to protect against IS-induced injury
(227). Xiao et al
(228) showed that edaravone
dexborneol activated the Nrf2/HO-1/GPX4 pathway to inhibit
ferroptosis. In addition, activation of the PI3K/AKT signaling
pathway confers resistance to IS-induced ferroptosis (229). Hu et al (229) showed that ginsenoside Rd
attenuated IS-induced ferroptosis in vascular endothelial cells by
activating neuregulin 1/erb-b2 receptor tyrosine kinase
4/PI3K/Akt/mTOR signaling. Li et al (230) showed that voacangine attenuated
IS-induced ferroptosis by activating the PI3K-Akt-FoxO pathway.
IS is a notable neurological condition that can
lead to disability and even death in the elderly, and is
characterized by a complex pathogenesis and limited availability of
effective clinical therapies (231). During IS, cerebral blood flow
is either restricted or interrupted, resulting in an ischemic
state. This condition, along with the reperfusion process that
follows thrombolysis, activates various forms of PCD, including
apoptosis, autophagy, necroptosis, pyroptosis and ferroptosis
(4). PCD represents a
fundamental physiological response of cells under pathological
conditions, with complex molecular mechanisms and significant
biological implications. Numerous studies have demonstrated that
various PCD pathways are closely linked to the pathophysiological
processes of IS (14,232,233). To enhance the understanding of
the molecular mechanisms underlying IS-induced injury and to assist
in the development of therapeutic strategies, the present review
categorizes the different PCDs triggered by IS and systematically
summarizes their potential molecular mechanisms, along with the
targeted therapeutic agents that may mitigate these effects.
Apoptosis serves a critical role in the
pathological processes of IS-induced injury. Research indicates
that, in addition to inhibiting classical intrinsic and extrinsic
apoptotic pathways, targeted therapies that activate the PI3K/AKT
pathway, inhibit the NF-κB pathway and modulate other intracellular
signals can provide protective effects against IS-induced
apoptosis. Both excessive and insufficient autophagy markedly
contribute to IS injury; however, the disruption of autophagic flux
during the pathology of IS in both spatial and temporal context
requires further investigation to optimize the timing of targeted
pharmacotherapy. Necroptosis, distinct from traditional necrosis
and apoptosis, has fewer targeted drugs developed for its
inhibition in IS, but notable effects in reducing IS-induced
necroptosis have been observed by targeting the classical
necroptosis pathway (RIPK1/RIPK3/MLKL). The inflammatory cascade
triggered by pyroptosis exacerbates IS-induced injury, with the
TLR4/NF-κB pathway and inflammasomes serving as key mediators.
Targeted therapies that inhibit these pathways have shown promise
in alleviating IS-induced damage. The role of ferroptosis in IS
pathology has garnered increasing attention. Agents that modulate
intracellular iron and lipid metabolism, as well as peroxidation
levels, exhibit inhibitory effects on IS-induced ferroptosis.
However, the key cytokines involved in ferroptosis and ferroptosis
resistance remain to be fully elucidated, indicating a valuable
avenue for future research. Additionally, a novel form of PCD,
termed cuprotosis, has been defined by Tsvetkov et al
(234) in 2022. Cuprotosis
occurs when excessive copper ion accumulation leads to the abnormal
aggregation of thioctylated proteins, causing a proteotoxic stress
response (234). At present,
there are no reports confirming the presence of cuprotosis in IS,
making it an intriguing area for future exploration to assess its
potential benefits in mitigating IS-induced injury.
The present review provides an overview of
potential targeted pharmacotherapies for IS. Despite the
exploration of numerous potential drugs, few have been translated
into clinical treatments for IS. In our opinion, this is primarily
due to several limitations in elucidating the mechanisms of PCD in
IS and in developing effective therapeutic strategies. These
limitations include the following: i) Limitations of experimental
models: Most current IS studies are based on cell and animal
models, which fail to fully replicate the complex pathological
environment of human IS. For example, the cerebrovascular structure
and immune response in mice markedly differ from those in humans,
potentially affecting the translational applicability of
PCD-targeted therapies. ii) Temporal specificity: The role of PCD
in IS may vary notably at different stages of the disease. For
instance, enhanced autophagy in the early phase of IS may exert
neuroprotective effects, whereas excessive autophagy in the later
stages may exacerbate ischemic brain injury. Therefore, therapeutic
strategies targeting PCD should take its temporal dynamics into
account to optimize intervention timing. iii) Spatial specificity:
The impact of PCD on IS pathology may differ among various cell
types. In IS, PCD in neurons or vascular endothelial cells may
directly worsen the cerebral injury (11,233,235). In the chronic phase, persistent
inflammation mediated by M1-type microglia or A1-type astrocytes
may further aggravate IS-induced damage, whereas M2-type microglia
and A2-type astrocytes may contribute to neuroprotection and
functional recovery (236,237). At present, the precise roles of
different PCD types in various time windows and cell populations
during IS remain incompletely understood, posing a marked challenge
to the development of precise intervention strategies. iv)
Complexity of interactions: Different PCD pathways may exhibit
crosstalk and mutual regulation. For example, a form of cell death
referred to as PANoptosis, which involves pyroptosis, apoptosis and
necroptosis, may arise, where several different PCD types influence
each other through oxidative stress or mitochondrial dysfunction
(238,239). However, current research
predominantly focuses on individual PCD types, with limited
exploration of their interactions within the pathology of IS.
To address these challenges, future research should
focus on the following: i) Developing in vitro and in
vivo models that better mimic the human pathological
environment; ii) elucidating the roles of PCD in different phases
of IS and across various cell types; iii) exploring multi-target
combinational interventions for PCD regulation; and iv)
accelerating the clinical translation of PCD-targeted therapies.
These efforts will provide critical theoretical and practical
guidance to improve IS treatment strategies.
The present review article followed a systematic
literature review approach. A search for relevant articles
published since 2014 was conducted using the key words 'apoptosis
and ischemic stroke', 'autophagy and ischemic stroke', 'necroptosis
and ischemic stroke', 'pyroptosis and ischemic stroke' and
'ferroptosis and ischemic stroke' in the PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of
Science (https://clarivate.com.cn/solutions/web-of-science/)
databases.
The following inclusion criteria were applied: i)
Studies focusing on the mechanisms of PCD in IS; and ii)
experimental studies on protective drugs that mitigate IS-induced
damage through PCD pathways. Experimental studies or reviews
unrelated to PCD or IS were excluded.
Not applicable.
WLD and LXY were involved in conceptualization.
WLD, LHG, AG, XJW and YYD were involved in the literature search,
data collection and writing. PZ, LXY, QL and BGZ reviewed and
edited the manuscript. Data authentication is not applicable. All
authors have read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82373124 and 81872163), the Shandong
Provincial Natural Science Foundation (grant no. ZR2023MH073), the
Zhejiang Provincial Medical and Health Science and Technology
Program (grant no. 2025KY1652), and the research startup fund of
Shaoxing People's Hospital (grant nos. YJ202401 and YJ202402).
|
1
|
Katan M and Luft A: Global burden of
stroke. Semin Neurol. 38:208–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tao T, Liu M, Chen M, Wang C, Xu T, Jiang
Y, Guo Y and Zhang JH: Natural medicine in neuroprotection for
ischemic stroke: Challenges and prospective. Pharmacol Ther.
216:1076952020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Datta A, Sarmah D, Mounica L, Kaur H,
Kesharwani R, Verma G, Veeresh P, Kotian V, Kalia K, Borah A, et
al: Cell death pathways in ischemic stroke and targeted
pharmacotherapy. Transl Stroke Res. 11:1185–1202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J, Tuo QZ and Lei P: Iron,
ferroptosis, and ischemic stroke. J Neurochem. 165:487–520. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adibhatla RM and Hatcher JF: Tissue
plasminogen activator (tPA) and matrix metalloproteinases in the
pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord
Drug Targets. 7:243–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mosconi MG and Paciaroni M: Treatments in
ischemic stroke: Current and future. Eur Neurol. 85:349–366. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chai Z, Zheng J and Shen J: Mechanism of
ferroptosis regulating ischemic stroke and pharmacologically
inhibiting ferroptosis in treatment of ischemic stroke. CNS
Neurosci Ther. 30:e148652024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou Z and Brenner JS: Developing targeted
antioxidant nanomedicines for ischemic penumbra: Novel strategies
in treating brain ischemia-reperfusion injury. Redox Biol.
73:1031852024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lockshin RA and Williams CM: programmed
cell death-I. Cytology of degeneration in the intersegmental
muscles of the pernyi silkmoth. J Insect Physiol. 11:123–133. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Zhang X, Chen X and Wei Y:
Neuronal injuries in cerebral infarction and ischemic stroke: From
mechanisms to treatment (Review). Int J Mol Med. 49:152022.
View Article : Google Scholar :
|
|
12
|
Qin C, Yang S, Chu YH, Zhang H, Pang XW,
Chen L, Zhou LQ, Chen M, Tian DS and Wang W: Signaling pathways
involved in ischemic stroke: molecular mechanisms and therapeutic
interventions. Signal Transduct Target Ther. 7:2152022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong Z, Guo J, Liu B, Guo Y, Cheng C,
Jiang Y, Liang N, Hu M, Song T, Yang L, et al: Mechanisms of immune
response and cell death in ischemic stroke and their regulation by
natural compounds. Front Immunol. 14:12878572024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long J, Sun Y, Liu S, Yang S, Chen C,
Zhang Z, Chu S, Yang Y, Pei G, Lin M, et al: Targeting pyroptosis
as a preventive and therapeutic approach for stroke. Cell Death
Discov. 9:1552023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Newton K, Strasser A, Kayagaki N and Dixit
VM: Cell death. Cell. 187:235–256. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nano M and Montell DJ: Apoptotic
signaling: Beyond cell death. Semin Cell Dev Biol. 156:22–34. 2024.
View Article : Google Scholar
|
|
19
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao S, Li X, Wang J and Wang H: The role
of the effects of autophagy on NLRP3 inflammasome in inflammatory
nervous system diseases. Front Cell Dev Biol. 9:6574782021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edinger AL and Thompson CB: Death by
design: Apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Almagro MC and Vucic D: Necroptosis:
Pathway diversity and characteristics. Semin Cell Dev Biol.
39:56–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Festjens N, Vanden Berghe T and
Vandenabeele P: Necrosis, a well-orchestrated form of cell demise:
Signalling cascades, important mediators and concomitant immune
response. Biochim Biophys Acta. 1757:1371–1387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chaudhary GR, Yadav PK, Yadav AK, Tiwari
M, Gupta A, Sharma A, Sahu K, Pandey AN, Pandey AK and Chaube SK:
Necrosis and necroptosis in germ cell depletion from mammalian
ovary. J Cell Physiol. 234:8019–8027. 2019. View Article : Google Scholar
|
|
25
|
Zychlinsky A, Prevost MC and Sansonetti
PJ: Shigella flexneri induces apoptosis in infected macrophages.
Nature. 358:167–169. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu P, Zhang X, Liu N, Tang L, Peng C and
Chen X: Pyroptosis: Mechanisms and diseases. Signal Transduct
Target Ther. 6:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dixon SJ and Olzmann JA: The cell biology
of ferroptosis. Nat Rev Mol Cell Biol. 25:424–442. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Xu P, Hong Y, Xie Y, Peng M, Sun R,
Guo H, Zhang X, Zhu W, Wang J and Liu X: Lipocalin-2-mediated
astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3
inflammasome activation in cerebral ischemia/reperfusion injury. J
Neuroinflammation. 20:1482023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai D, Fraunfelder M, Fujise K and Chen
SY: ADAR1 exacerbates ischemic brain injury via astrocyte-mediated
neuron apoptosis. Redox Biol. 67:1029032023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Culmsee C and Mattson MP: p53 in neuronal
apoptosis. Biochem Biophys Res Commun. 331:761–777. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Radak D, Katsiki N, Resanovic I, Jovanovic
A, Sudar-Milovanovic E, Zafirovic S, Mousad SA and Isenovic ER:
Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc
Pharmacol. 15:115–122. 2017. View Article : Google Scholar
|
|
35
|
Cregan SP, Arbour NA, Maclaurin JG,
Callaghan SM, Fortin A, Cheung EC, Guberman DS, Park DS and Slack
RS: p53 activation domain 1 is essential for PUMA upregulation and
p53-mediated neuronal cell death. J Neurosci. 24:10003–10012. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakka VP, Gusain A, Mehta SL and Raghubir
R: Molecular mechanisms of apoptosis in cerebral ischemia: Multiple
neuroprotective opportunities. Mol Neurobiol. 37:7–38. 2008.
View Article : Google Scholar
|
|
38
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Love S: Apoptosis and brain ischaemia.
Prog Neuropsychopharmacol Biol Psychiatry. 27:267–282. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Velier JJ, Ellison JA, Kikly KK, Spera PA,
Barone FC and Feuerstein GZ: Caspase-8 and caspase-3 are expressed
by different populations of cortical neurons undergoing delayed
cell death after focal stroke in the rat. J Neurosci. 19:5932–5941.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rupalla K, Allegrini PR, Sauer D and
Wiessner C: Time course of microglia activation and apoptosis in
various brain regions after permanent focal cerebral ischemia in
mice. Acta Neuropathol. 96:172–178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Botchkina GI, Geimonen E, Bilof ML,
Villarreal O and Tracey KJ: Loss of NF-kappaB activity during
cerebral ischemia and TNF cytotoxicity. Mol Med. 5:372–381. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sarmah D, Kaur H, Saraf J, Vats K,
Pravalika K, Wanve M, Kalia K, Borah A, Kumar A, Wang X, et al:
Mitochondrial dysfunction in stroke: implications of stem cell
therapy. Transl Stroke Res. 2018.Epub ahead of print. PubMed/NCBI
|
|
44
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar
|
|
45
|
Harada H and Grant S: Apoptosis
regulators. Rev Clin Exp Hematol. 7:117–138. 2003.
|
|
46
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sugawara T, Fujimura M, Noshita N, Kim GW,
Saito A, Hayashi T, Narasimhan P, Maier CM and Chan PH: Neuronal
death/survival signaling pathways in cerebral ischemia. NeuroRx.
1:17–25. 2004. View Article : Google Scholar
|
|
48
|
Polster BM and Fiskum G: Mitochondrial
mechanisms of neural cell apoptosis. J Neurochem. 90:1281–1289.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhai D, Chin K, Wang M and Liu F:
Disruption of the nuclear p53-GAPDH complex protects against
ischemia-induced neuronal damage. Mol Brain. 7:202014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao J, Liu J, Li Y, Liu J, Wang H, Chai M,
Dong Y, Zhang Z, Su G and Wang M: Targeting p53 for
neuroinflammation: New therapeutic strategies in ischemic stroke. J
Neurosci Res. 101:1393–1408. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Morrison RS, Kinoshita Y, Johnson MD, Guo
W and Garden GA: p53-dependent cell death signaling in neurons.
Neurochem Res. 28:15–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar
|
|
53
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang J, Wu D and Wang H: Hydrogen sulfide
plays an important protective role by influencing autophagy in
diseases. Physiol Res. 68:335–345. 2019.PubMed/NCBI
|
|
55
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guan R, Zou W, Dai X, Yu X, Liu H, Chen Q
and Teng W: Mitophagy, a potential therapeutic target for stroke. J
Biomed Sci. 25:872018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Scrivo A, Bourdenx M, Pampliega O and
Cuervo AM: Selective autophagy as a potential therapeutic target
for neurodegenerative disorders. Lancet Neurol. 17:802–815. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang X, Fang Y, Huang Q, Xu P, Lenahan C,
Lu J, Zheng J, Dong X, Shao A and Zhang J: An updated review of
autophagy in ischemic stroke: From mechanisms to therapies. Exp
Neurol. 340:1136842021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Solenski NJ, diPierro CG, Trimmer PA, Kwan
AL and Helm GA: Ultrastructural changes of neuronal mitochondria
after transient and permanent cerebral ischemia. Stroke.
33:816–824. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Y, Wang B, Liu Y, Guo Y, Lu H and Liu
X: Inhibition of PI3K/Akt/mTOR signaling by NDRG2 contributes to
neuronal apoptosis and autophagy in ischemic stroke. J Stroke
Cerebrovasc Dis. 32:1069842023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cui DR, Wang L, Jiang W, Qi AH, Zhou QH
and Zhang XL: Propofol prevents cerebral ischemia-triggered
autophagy activation and cell death in the rat hippocampus through
the NF-κB/p53 signaling pathway. Neuroscience. 246:117–132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bootman MD, Chehab T, Bultynck G, Parys JB
and Rietdorf K: The regulation of autophagy by calcium signals: Do
we have a consensus? Cell Calcium. 70:32–46. 2018. View Article : Google Scholar
|
|
65
|
Li L, Li L, Zhou X, Yu Y, Li Z, Zuo D and
Wu Y: Silver nanoparticles induce protective autophagy via
Ca(2+)/CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains.
Nanotoxicology. 13:369–391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shi W, Xu D, Gu J, Xue C, Yang B, Fu L,
Song S, Liu D, Zhou W, Lv J, et al: Saikosaponin-d inhibits
proliferation by up-regulating autophagy via the CaMKKβ-AMPK-mTOR
pathway in ADPKD cells. Mol Cell Biochem. 449:219–226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Krishna M and Narang H: The complexity of
mitogen-activated protein kinases (MAPKs) made simple. Cell Mol
Life Sci. 65:3525–3544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li H, Zhou S, Wu L, Liu K, Zhang Y, Ma G
and Wang L: The role of p38MAPK signal pathway in the
neuroprotective mechanism of limb postconditioning against rat
cerebral ischemia/reperfusion injury. J Neurol Sci. 357:270–275.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Song YQ, Zou HL, Zhao YJ, Yu LQ, Tan ZX
and Kong R: Activation of p38-mitogen-activated protein kinase
contributes to ischemia reperfusion in rat brain. Genet Mol Res.
15:gmr.150384922016. View Article : Google Scholar
|
|
70
|
Ferrer I, Friguls B, Dalfó E and Planas
AM: Early modifications in the expression of mitogen-activated
protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and
p38, and their phosphorylated substrates following focal cerebral
ischemia. Acta Neuropathol. 105:425–437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Oberstein A, Jeffrey PD and Shi Y: Crystal
structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a
novel BH3-only protein. J Biol Chem. 282:13123–13132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xu F, Gu JH and Qin ZH: Neuronal autophagy
in cerebral ischemia. Neurosci Bull. 28:658–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen W, Sun Y, Liu K and Sun X: Autophagy:
A double-edged sword for neuronal survival after cerebral ischemia.
Neural Regen Res. 9:1210–1216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Marquez RT and Xu L: Bcl-2:Beclin 1
complex: Multiple, mechanisms regulating autophagy/apoptosis toggle
switch. Am J Cancer Res. 2:214–221. 2012.PubMed/NCBI
|
|
75
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ham PB III and Raju R: Mitochondrial
function in hypoxic ischemic injury and influence of aging. Prog
Neurobiol. 157:92–116. 2017. View Article : Google Scholar
|
|
77
|
Park J, Lee SB, Lee S, Kim Y, Song S, Kim
S, Bae E, Kim J, Shong M, Kim JM and Chung J: Mitochondrial
dysfunction in Drosophila PINK1 mutants is complemented by parkin.
Nature. 441:1157–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu K, Sun Y, Gu Z, Shi N, Zhang T and Sun
X: Mitophagy in ischaemia/reperfusion induced cerebral injury.
Neurochem Res. 38:1295–1300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Choe SC, Hamacher-Brady A and Brady NR:
Autophagy capacity and sub-mitochondrial heterogeneity shape
Bnip3-induced mitophagy regulation of apoptosis. Cell Commun
Signal. 13:372015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jang W and Haucke V: ER remodeling via
lipid metabolism. Trends Cell Biol. 34:942–954. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gubas A and Dikic I: ER remodeling via
ER-phagy. Mol Cell. 82:1492–1500. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Garcia-Huerta P, Troncoso-Escudero P,
Jerez C, Hetz C and Vidal RL: The intersection between growth
factors autophagy and ER stress: A new target to treat
neurodegenerative diseases? Brain Res. 1649(Pt B): 173–180. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yin Y, Sun G, Li E, Kiselyov K and Sun D:
ER stress and impaired autophagy flux in neuronal degeneration and
brain injury. Ageing Res Rev. 34:3–14. 2017. View Article : Google Scholar :
|
|
84
|
Jun-Long H, Yi L, Bao-Lian Z, Jia-Si L,
Ning Z, Zhou-Heng Y, Xue-Jun S and Wen-Wu L: Necroptosis signaling
pathways in stroke: From mechanisms to therapies. Curr
Neuropharmacol. 16:1327–1339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Holler N, Zaru R, Micheau O, Thome M,
Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp
J: Fas triggers an alternative, caspase-8-independent cell death
pathway using the kinase RIP as effector molecule. Nat Immunol.
1:489–495. 2000. View
Article : Google Scholar
|
|
86
|
Moriwaki K and Chan FK: RIP3: A molecular
switch for necrosis and inflammation. Genes Dev. 27:1640–1649.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Vieira M, Fernandes J, Carreto L,
Anuncibay-Soto B, Santos M, Han J, Fernández-López A, Duarte CB,
Carvalho AL and Santos AE: Ischemic insults induce necroptotic cell
death in hippocampal neurons through the up-regulation of
endogenous RIP3. Neurobiol Dis. 68:26–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sun L, Wang H, Wang Z, He S, Chen S, Liao
D, Wang L, Yan J, Liu W, Lei X and Wang X: Mixed lineage kinase
domain-like protein mediates necrosis signaling downstream of RIP3
kinase. Cell. 148:213–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Weber K, Roelandt R, Bruggeman I, Estornes
Y and Vandenabeele P: Nuclear RIPK3 and MLKL contribute to
cytosolic necrosome formation and necroptosis. Commun Biol.
1:62018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Feng Y, Li M, Yangzhong X, Zhang X, Zu A,
Hou Y, Li L and Sun S: Pyroptosis in inflammation-related
respiratory disease. J Physiol Biochem. 78:721–737. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xia S, Hollingsworth LR IV and Wu H:
Mechanism and regulation of gasdermin-mediated cell death. Cold
Spring Harb Perspect Biol. 12:a0364002020. View Article : Google Scholar
|
|
93
|
Liu X, Xia S, Zhang Z, Wu H and Lieberman
J: Channelling inflammation: Gasdermins in physiology and disease.
Nat Rev Drug Discov. 20:384–405. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang K, Sun Q, Zhong X, Zeng M, Zeng H,
Shi X, Li Z, Wang Y, Zhao Q, Shao F and Ding J: Structural
mechanism for GSDMD targeting by autoprocessed caspases in
pyroptosis. Cell. 180:941–955.e20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fritsch M, Günther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu
X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, et al: Gasdermin E
suppresses tumour growth by activating anti-tumour immunity.
Nature. 579:415–420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Dong Z, Pan K, Pan J, Peng Q and Wang Y:
The possibility and molecular mechanisms of cell pyroptosis after
cerebral ischemia. Neurosci Bull. 34:1131–1136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kono H, Kimura Y and Latz E: Inflammasome
activation in response to dead cells and their metabolites. Curr
Opin Immunol. 30:91–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Puleo MG, Miceli S, Di Chiara T, Pizzo GM,
Della Corte V, Simonetta I, Pinto A and Tuttolomondo A: Molecular
mechanisms of inflammasome in ischemic stroke pathogenesis.
Pharmaceuticals (Basel). 15:11682022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fu R, Zhao L, Guo Y, Qin X, Xu W, Cheng X,
Zhang Y and Xu S: AIM2 inflammasome: A potential therapeutic target
in ischemic stroke. Clin Immunol. 259:1098812024. View Article : Google Scholar
|
|
104
|
Mitchell PS, Sandstrom A and Vance RE: The
NLRP1 inflammasome: New mechanistic insights and unresolved
mysteries. Curr Opin Immunol. 60:37–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Duan WL, Wang XJ, Ma YP, Sheng ZM, Dong H,
Zhang LY, Zhang BG and He MT: Therapeutic strategies targeting the
NLRP3-mediated inflammatory response and pyroptosis in cerebral
ischemia/reperfusion injury (Review). Mol Med Rep. 29:462024.
View Article : Google Scholar
|
|
106
|
Duncan JA and Canna SW: The NLRC4
inflammasome. Immunol Rev. 281:115–123. 2018. View Article : Google Scholar :
|
|
107
|
Man SM and Kanneganti TD: Regulation of
inflammasome activation. Immunol Rev. 265:6–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hu X, Bao Y, Li M, Zhang W and Chen C: The
role of ferroptosis and its mechanism in ischemic stroke. Exp
Neurol. 372:1146302024. View Article : Google Scholar
|
|
109
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen J, Yang L, Geng L, He J, Chen L, Sun
Q, Zhao J and Wang X: Inhibition of Acyl-CoA Synthetase long-chain
family member 4 facilitates neurological recovery after stroke by
regulation ferroptosis. Front Cell Neurosci. 15:6323542021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang L, Bai XY, Sun KY, Li X, Zhang ZQ,
Liu YD, Xiang Y and Liu XL: A new perspective in the treatment of
ischemic stroke: Ferroptosis. Neurochem Res. 49:815–833. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li X, Ma N, Xu J, Zhang Y, Yang P, Su X,
Xing Y, An N, Yang F, Zhang G, et al: Targeting ferroptosis:
Pathological mechanism and treatment of ischemia-reperfusion
injury. Oxid Med Cell Longev. 2021:15879222021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Das UN: Saturated fatty acids, MUFAs and
PUFAs regulate ferroptosis. Cell Chem Biol. 26:309–311. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bu ZQ, Yu HY, Wang J, He X, Cui YR, Feng
JC and Feng J: Emerging role of ferroptosis in the pathogenesis of
ischemic stroke: A new therapeutic target? ASN Neuro.
13:175909142110375052021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zou Y, Li H, Graham ET, Deik AA, Eaton JK,
Wang W, Sandoval-Gomez G, Clish CB, Doench JG and Schreiber SL:
Cytochrome P450 oxidoreductase contributes to phospholipid
peroxidation in ferroptosis. Nat Chem Biol. 16:302–309. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Muralikrishna Adibhatla R and Hatcher JF:
Phospholipase A2, reactive oxygen species, and lipid peroxidation
in cerebral ischemia. Free Radic Biol Med. 40:376–387. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu X: Changes and significance of serum
CXCL-16, GDF-15, PLA-2 levels in patients with cerebral infarction.
Am J Transl Res. 13:5617–5622. 2021.PubMed/NCBI
|
|
118
|
Tuo QZ, Liu Y, Xiang Z, Yan HF, Zou T, Shu
Y, Ding XL, Zou JJ, Xu S, Tang F, et al: Thrombin induces
ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion.
Signal Transduct Target Ther. 7:592022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cheng G, Zhao W, Xin Y, Huang G and Liu Y,
Li Z, Zhan M, Li Y, Lu L, van Leyen K and Liu Y: Effects of ML351
and tissue plasminogen activator combination therapy in a rat model
of focal embolic stroke. J Neurochem. 157:586–598. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yigitkanli K, Zheng Y, Pekcec A, Lo EH and
van Leyen K: Increased 12/15-lipoxygenase leads to widespread brain
injury following global cerebral ischemia. Transl Stroke Res.
8:194–202. 2017. View Article : Google Scholar :
|
|
121
|
Ohgami RS, Campagna DR, Greer EL,
Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE
and Fleming MD: Identification of a ferrireductase required for
efficient transferrin-dependent iron uptake in erythroid cells. Nat
Genet. 37:1264–1269. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ji C and Kosman DJ: Molecular mechanisms
of nontransferrin-bound and transferring-bound iron uptake in
primary hippocampal neurons. J Neurochem. 133:668–683. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Philpott CC, Patel SJ and Protchenko O:
Management versus miscues in the cytosolic labile iron pool: The
varied functions of iron chaperones. Biochim Biophys Acta Mol Cell
Res. 1867:1188302020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dietrich RB and Bradley WG Jr: Iron
accumulation in the basal ganglia following severe ischemic-anoxic
insults in children. Radiology. 168:203–206. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kondo Y, Ogawa N, Asanuma M, Ota Z and
Mori A: Regional differences in late-onset iron deposition,
ferritin, transferrin, astrocyte proliferation, and microglial
activation after transient forebrain ischemia in rat brain. J Cereb
Blood Flow Metab. 15:216–226. 1955. View Article : Google Scholar
|
|
126
|
DeGregorio-Rocasolano N, Martí-Sistac O,
Ponce J, Castelló-Ruiz M, Millán M, Guirao V, García-Yébenes I,
Salom JB, Ramos-Cabrer P, Alborch E, et al: Iron-loaded transferrin
(Tf) is detrimental whereas iron-free Tf confers protection against
brain ischemia by modifying blood Tf saturation and subsequent
neuronal damage. Redox Biol. 15:143–158. 2018. View Article : Google Scholar :
|
|
127
|
Ingrassia R, Lanzillotta A, Sarnico I,
Benarese M, Blasi F, Borgese L, Bilo F, Depero L, Chiarugi A, Spano
PF and Pizzi M: 1B/(-)IRE DMT1 expression during brain ischemia
contributes to cell death mediated by NF-κB/RelA acetylation at
Lys310. PLoS One. 7:e380192012. View Article : Google Scholar
|
|
128
|
Hirayama Y and Koizumi S:
Hypoxia-independent mechanisms of HIF-1α expression in astrocytes
after ischemic preconditioning. Glia. 65:523–530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yang L, Wang D, Wang XT, Lu YP and Zhu L:
The roles of hypoxia-inducible Factor-1 and iron regulatory protein
1 in iron uptake induced by acute hypoxia. Biochem Biophys Res
Commun. 507:128–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Chen W, Jiang L, Hu Y, Tang N, Liang N, Li
XF, Chen YW, Qin H and Wu L: Ferritin reduction is essential for
cerebral ischemia-induced hippocampal neuronal death through
p53/SLC7A11-mediated ferroptosis. Brain Res. 1752:1472162021.
View Article : Google Scholar
|
|
131
|
Wu Q, Wu WS, Su L, Zheng X, Wu WY,
Santambrogio P, Gou YJ, Hao Q, Wang PN, Li YR, et al: Mitochondrial
ferritin is a hypoxia-inducible factor 1α-inducible gene that
protects from hypoxia-induced cell death in brain. Antioxid Redox
Signal. 30:198–212. 2019. View Article : Google Scholar
|
|
132
|
Wang P, Cui Y, Ren Q, Yan B, Zhao Y, Yu P,
Gao G, Shi H, Chang S and Chang YZ: Mitochondrial ferritin
attenuates cerebral ischaemia/reperfusion injury by inhibiting
ferroptosis. Cell Death Dis. 12:4472021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sanguigno L, Guida N, Anzilotti S, Cuomo
O, Mascolo L, Serani A, Brancaccio P, Pennacchio G, Licastro E,
Pignataro G, et al: Stroke by inducing HDAC9-dependent
deacetylation of HIF-1 and Sp1, promotes TfR1 transcription and
GPX4 reduction, thus determining ferroptotic neuronal death. Int J
Biol Sci. 19:2695–2710. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao
Z, Zhao P, Miao Z, Zhao L, et al: Nuclear receptor coactivator
4-mediated ferritinophagy contributes to cerebral ischemia-induced
ferroptosis in ischemic stroke. Pharmacol Res. 174:1059332021.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Słomka A, Świtońska M and Żekanowska E:
Hepcidin levels are increased in patients with acute ischemic
stroke: Preliminary report. J Stroke Cerebrovasc Dis. 24:1570–1576.
2015. View Article : Google Scholar
|
|
136
|
Ding H, Yan CZ, Shi H, Zhao YS, Chang SY,
Yu P, Wu WS, Zhao CY, Chang YZ and Duan XL: Hepcidin is involved in
iron regulation in the ischemic brain. PLoS One. 6:e253242011.
View Article : Google Scholar :
|
|
137
|
Zhu K, Zhu X, Liu S, Yu J, Wu S and Hei M:
Glycyrrhizin attenuates hypoxic-ischemic brain damage by inhibiting
ferroptosis and neuroinflammation in neonatal rats via the
HMGB1/GPX4 pathway. Oxid Med Cell Longev. 2022:84385282022.
View Article : Google Scholar :
|
|
138
|
Shi Y, Han L, Zhang X, Xie L, Pan P and
Chen F: Selenium alleviates cerebral ischemia/reperfusion injury by
regulating oxidative stress, mitochondrial fusion and ferroptosis.
Neurochem Res. 47:2992–3002. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Guan X, Li Z, Zhu S, Cheng M, Ju Y, Ren L,
Yang G and Min D: Galangin attenuated cerebral ischemia-reperfusion
injury by inhibition of ferroptosis through activating the
SLC7A11/GPX4 axis in gerbils. Life Sci. 264:1186602021. View Article : Google Scholar
|
|
140
|
Zhang Y, Lu X, Tai B, Li W and Li T:
Ferroptosis and its multifaceted roles in cerebral stroke. Front
Cell Neurosci. 15:6153722021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Fu C, Wu Y, Liu S, Luo C, Lu Y, Liu M,
Wang L, Zhang Y and Liu X: Rehmannioside A improves cognitive
impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2
and SLC7A11/GPX4 signaling pathway after ischemia. J
Ethnopharmacol. 289:1150212022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Haas S, Weidner N and Winkler J: Adult
stem cell therapy in stroke. Curr Opin Neurol. 18:59–64. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tuo QZ, Zhang ST and Lei P: Mechanisms of
neuronal cell death in ischemic stroke and their therapeutic
implications. Med Res Rev. 42:259–305. 2022. View Article : Google Scholar
|
|
144
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Chen B, Wang G, Li W, Liu W, Lin R, Tao J,
Jiang M, Chen L and Wang Y: Memantine attenuates cell apoptosis by
suppressing the calpain-caspase-3 pathway in an experimental model
of ischemic stroke. Exp Cell Res. 351:163–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang X, Zhang Y, Tang S, Yu L, Zhao Y,
Ren Q, Huang X, Xu W, Huang M and Peng J: Pien-Tze-Huang protects
cerebral ischemic injury by inhibiting neuronal apoptosis in acute
ischemic stroke rats. J Ethnopharmacol. 219:117–125. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Nie Y, Wen L, Li H, Song J, Wang N, Huang
L, Gao L and Qu M: Tanhuo formula inhibits astrocyte activation and
apoptosis in acute ischemic stroke. Front Pharmacol. 13:8592442022.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mei ZG, Tan LJ, Wang JF, Li XL, Huang WF
and Zhou HJ: Fermented Chinese formula Shuan-Tong-Ling attenuates
ischemic stroke by inhibiting inflammation and apoptosis. Neural
Regen Res. 12:425–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang J, Zhang W, Ma B, Zhang H, Fan Z, Li
M and Li X: A novel biscoumarin derivative dephosphorylates ERK and
alleviates apoptosis induced by mitochondrial oxidative damage in
ischemic stroke mice. Life Sci. 264:1184992021. View Article : Google Scholar
|
|
150
|
Raghavan A and Shah ZA: Withania somnifera
improves ischemic stroke outcomes by attenuating PARP1-AIF-mediated
caspase-independent apoptosis. Mol Neurobiol. 52:1093–1105. 2015.
View Article : Google Scholar
|
|
151
|
Peng T, Li S, Liu L, Yang C, Farhan M,
Chen L, Su Q and Zheng W: Artemisinin attenuated ischemic stroke
induced cell apoptosis through activation of ERK1/2/CREB/BCL-2
signaling pathway in vitro and in vivo. Int J Biol Sci.
18:4578–4594. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Hu S, Cao Q, Xu P, Ji W, Wang G and Zhang
Y: Rolipram stimulates angiogenesis and attenuates neuronal
apoptosis through the cAMP/cAMP-responsive element binding protein
pathway following ischemic stroke in rats. Exp Ther Med.
11:1005–1010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu T, Wang W, Li X, Chen Y, Mu F, Wen A,
Liu M and Ding Y: Advances of phytotherapy in ischemic stroke
targeting PI3K/Akt signaling. Phytother Res. 37:5509–5528. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hafeez A, Elmadhoun O, Peng C, Ding JY,
Geng X, Guthikonda M and Ding Y: Reduced apoptosis by ethanol and
its association with PKC-δ and Akt signaling in ischemic stroke.
Aging Dis. 5:366–372. 2014.PubMed/NCBI
|
|
155
|
Fan W, Li X, Huang L, He S, Xie Z, Fu Y,
Fang W and Li Y: S-oxiracetam ameliorates ischemic stroke induced
neuronal apoptosis through up-regulating α7 nAChR and
PI3K/Akt/GSK3β signal pathway in rats. Neurochem Int. 115:50–60.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Xu Y, Chen W, Chen Z, Huang M, Yang F and
Zhang Y: Mechanism of action of xiaoyao san in treatment of
ischemic stroke is related to anti-apoptosis and activation of
PI3K/Akt pathway. Drug Des Devel Ther. 15:753–767. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wang C, Chen H, Jiang HH, Mao BB and Yu H:
Total flavonoids of chuju decrease oxidative stress and cell
apoptosis in ischemic stroke rats: Network and experimental
analyses. Front Neurosci. 15:7724012021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zhang SS, Liu M, Liu DN, Shang YF, Du GH
and Wang YH: Network pharmacology analysis and experimental
validation of kaempferol in the treatment of ischemic stroke by
inhibiting apoptosis and regulating neuroinflammation involving
neutrophils. Int J Mol Sci. 23:126942022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Luo Y, Cheng J, Fu Y, Zhang M, Gou M, Li
J, Li X, Bai J, Zhou Y, Zhang L and Gao D: D-allose Inhibits
TLR4/PI3K/AKT signaling to attenuate neuroinflammation and neuronal
apoptosis by inhibiting Gal-3 following ischemic stroke. Biol
Proced Online. 25:302023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Qi J, Han B, Wang Z, Jing L, Tian X and
Sun J: Chuanzhitongluo inhibits neuronal apoptosis in mice with
acute ischemic stroke by regulating the PI3K/AKT signaling pathway.
Neuroscience. 537:21–31. 2024. View Article : Google Scholar
|
|
161
|
Xu Z, Li Y, Pi P, Yi Y, Tang H, Zhang Z,
Xiong H, Lei B, Shi Y, Li J and Sun Z: B. glomerulata promotes
neuroprotection against ischemic stroke by inhibiting apoptosis
through the activation of PI3K/AKT/mTOR pathway. Phytomedicine.
132:1558172024. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Meffert MK and Baltimore D: Physiological
functions for brain NF-kappaB. Trends Neurosci. 28:37–43. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Baltimore D: Discovering NF-kappaB. Cold
Spring Harb Perspect Biol. 1:a0000262009. View Article : Google Scholar
|
|
164
|
Zhang Q, Lenardo MJ and Baltimore D: 30
Years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Li R, Zhou Y, Zhang S, Li J, Zheng Y and
Fan X: The natural (poly)phenols as modulators of microglia
polarization via TLR4/NF-κB pathway exert anti-inflammatory
activity in ischemic stroke. Eur J Pharmacol. 914:1746602022.
View Article : Google Scholar
|
|
166
|
Yang CH, Yen TL, Hsu CY, Thomas PA, Sheu
JR and Jayakumar T: Multi-targeting andrographolide, a novel NF-κB
inhibitor, as a potential therapeutic agent for stroke. Int J Mol
Sci. 18:16382017. View Article : Google Scholar
|
|
167
|
Zhou H, Yang WS, Li Y, Ren T, Peng L, Guo
H, Liu JF, Zhou Y, Zhao Y, Yang LC and Jin X: Oleoylethanolamide
attenuates apoptosis by inhibiting the TLR4/NF-κB and ERK1/2
signaling pathways in mice with acute ischemic stroke. Naunyn
Schmiedebergs Arch Pharmacol. 390:77–84. 2017. View Article : Google Scholar
|
|
168
|
Yan L and Zhu T: Effects of rosuvastatin
on neuronal apoptosis in cerebral ischemic stroke rats via
Sirt1/NF-kappa B signaling pathway. Eur Rev Med Pharmacol Sci.
23:5449–5455. 2019.PubMed/NCBI
|
|
169
|
Li R, Si M, Jia HY, Ma Z, Li XW, Li XY,
Dai XR, Gong P and Luo SY: Anfibatide alleviates inflammation and
apoptosis via inhibiting NF-kappaB/NLRP3 axis in ischemic stroke.
Eur J Pharmacol. 926:1750322022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Wang Y, Wu H, Han Z, Sheng H, Wu Y and
Wang Y, Guo X, Zhu Y, Li X and Wang Y: Guhong injection promotes
post-stroke functional recovery via attenuating cortical
inflammation and apoptosis in subacute stage of ischemic stroke.
Phytomedicine. 99:1540342022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Zheng Z, Hou F, He G, Jiang F, Bao X and
Tong M: Carvedilol reduces the neuronal apoptosis after ischemic
stroke by modulating activator of transcription 3 expression in
vitro. Dev Neurosci. 45:94–104. 2023. View Article : Google Scholar
|
|
172
|
Xu Y, Wang Y, Wang G, Ye X, Zhang J, Cao
G, Zhao Y, Gao Z, Zhang Y, Yu B and Kou J: YiQiFuMai powder
injection protects against ischemic stroke via inhibiting neuronal
apoptosis and PKCδ/Drp1-mediated excessive mitochondrial fission.
Oxid Med Cell Longev. 2017:18320932017. View Article : Google Scholar
|
|
173
|
Qiu J, Wang X, Wu F, Wan L, Cheng B, Wu Y
and Bai B: Low dose of apelin-36 attenuates ER stress-associated
apoptosis in rats with ischemic stroke. Front Neurol. 8:5562017.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wu F, Qiu J, Fan Y, Zhang Q, Cheng B, Wu Y
and Bai B: Apelin-13 attenuates ER stress-mediated neuronal
apoptosis by activating Gα(i)/Gα(q)-CK2 signaling in ischemic
stroke. Exp Neurol. 302:136–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Li F, Zhao H, Han Z, Wang R, Tao Z, Fan Z,
Zhang S, Li G, Chen Z and Luo Y: Xuesaitong may protect against
ischemic stroke by modulating microglial phenotypes and inhibiting
neuronal cell apoptosis via the STAT3 signaling pathway. CNS Neurol
Disord Drug Targets. 18:115–123. 2019. View Article : Google Scholar
|
|
176
|
Li HQ, Xia SN, Xu SY, Liu PY, Gu Y, Bao
XY, Xu Y and Cao X: γ-glutamylcysteine alleviates ischemic
stroke-induced neuronal apoptosis by inhibiting ROS-mediated
endoplasmic reticulum stress. Oxid Med Cell Longev.
2021:29610792021. View Article : Google Scholar
|
|
177
|
Joshi B, Singh D, Wasan H, Sharma U and
Reeta KH: Tideglusib ameliorates ischemia/reperfusion damage by
inhibiting GSK-3β and apoptosis in rat model of ischemic stroke. J
Stroke Cerebrovasc Dis. 31:1063492022. View Article : Google Scholar
|
|
178
|
Ding Y, Lang Y, Zhang H, Li Y, Liu X and
Li M: Candesartan reduces neuronal apoptosis caused by ischemic
stroke via regulating the FFAR1/ITGA4 pathway. Mediators Inflamm.
2022:23565072022. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li L, Song JJ, Zhang MX, Zhang HW, Zhu HY,
Guo W, Pan CL, Liu X, Xu L and Zhang ZY: Oridonin ameliorates
caspase-9-mediated brain neuronal apoptosis in mouse with ischemic
stroke by inhibiting RIPK3-mediated mitophagy. Acta Pharmacol Sin.
44:726–740. 2023. View Article : Google Scholar :
|
|
180
|
Zhang L, Zhou T, Ji Q, He L, Lan Y, Ding
L, Li L and Wang Z: Myricetin improves apoptosis after ischemic
stroke via inhibiting MAPK-ERK pathway. Mol Biol Rep. 50:2545–2557.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Shi GS, Qin QL, Huang C, Li ZR, Wang ZH,
Wang YY, He XY and Zhao XM: The pathological mechanism of neuronal
autophagy-lysosome dysfunction after ischemic stroke. Cell Mol
Neurobiol. 43:3251–3263. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Jiang J, Dai J and Cui H: Vitexin reverses
the autophagy dysfunction to attenuate MCAO-induced cerebral
ischemic stroke via mTOR/Ulk1 pathway. Biomed Pharmacother.
99:583–590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Tang T, Gao D, Yang X, Hua X, Li S and Sun
H: Exogenous netrin-1 inhibits autophagy of ischemic brain tissues
and hypoxic neurons via PI3K/mTOR pathway in ischemic stroke. J
Stroke Cerebrovasc Dis. 28:1338–1345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zhang Y, He Q, Yang M, Hua S, Ma Q, Guo L,
Wu X, Zhang C, Fu X and Liu J: Dichloromethane extraction from
Piper nigrum L. and P. longum L. to mitigate ischemic stroke by
activating the AKT/mTOR signaling pathway to suppress autophagy.
Brain Res. 1749:1470472020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Liu M, Li Y, Han S, Wang H and Li J:
Activin A alleviates neuronal injury through inhibiting
cGAS-STING-mediated autophagy in mice with ischemic stroke. J Cereb
Blood Flow Metab. 43:736–748. 2023. View Article : Google Scholar :
|
|
186
|
Lv S, Geng X, Yun HJ and Ding Y:
Phenothiazines reduced autophagy in ischemic stroke through
endoplasmic reticulum (ER) stress-associated PERK-eIF2α pathway.
Exp Neurol. 369:1145242023. View Article : Google Scholar
|
|
187
|
Yuan Q, Ren H, Lu J, Yang M, Xie Z, Ma B,
Ma L, Fu X, Liu J and Zhang Y: Effects of dichloromethane
extraction from Piper nigrum L. and P. longum L. on the expression
of autophagy-related proteins in ischemic stroke. J Chem Neuroanat.
127:1022012023. View Article : Google Scholar
|
|
188
|
Zhu Y, Yu J, Gong J, Shen J, Ye D, Cheng
D, Xie Z, Zeng J, Xu K, Shen J, et al: PTP1B inhibitor alleviates
deleterious microglial activation and neuronal injury after
ischemic stroke by modulating the ER stress-autophagy axis via PERK
signaling in microglia. Aging (Albany NY). 13:3405–3427. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wang Y, Guan X, Gao CL, Ruan W, Zhao S,
Kai G, Li F and Pang T: Medioresinol as a novel PGC-1α activator
prevents pyroptosis of endothelial cells in ischemic stroke through
PPARα-GOT1 axis. Pharmacol Res. 169:1056402021. View Article : Google Scholar
|
|
190
|
Xia Y, Ling X, Hu G, Zhu Q, Zhang J, Li Q,
Zhao B, Wang Y and Deng Z: Small extracellular vesicles secreted by
human iPSC-derived MSC enhance angiogenesis through inhibiting
STAT3-dependent autophagy in ischemic stroke. Stem Cell Res Ther.
11:3132020. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhang Y, Liu Y, Cui Q, Fu Z, Yu H, Liu A,
Liu J, Qin X, Ge S and Zhang G: Hydroxysafflor yellow A Alleviates
ischemic stroke in rats via HIF-1[Formula: See text], BNIP3, and
Notch1-mediated inhibition of autophagy. Am J Chin Med. 50:799–815.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Nabavi SF, Sureda A, Sanches-Silva A,
Pandima Devi K, Ahmed T, Shahid M, Sobarzo-Sánchez E, Dacrema M,
Daglia M, Braidy N, et al: Novel therapeutic strategies for stroke:
The role of autophagy. Crit Rev Clin Lab Sci. 56:182–199. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Li Y, Hu K, Liang M, Yan Q, Huang M, Jin
L, Chen Y, Yang X and Li X: Stilbene glycoside upregulates
SIRT3/AMPK to promotes neuronal mitochondrial autophagy and inhibit
apoptosis in ischemic stroke. Adv Clin Exp Med. 30:139–146. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Ao LY, Li WT, Zhou L, Yan YY, Ye AQ, Liang
BW, Shen WY, Zhu X and Li YM: Therapeutic effects of JLX-001 on
ischemic stroke by inducing autophagy via AMPK-ULK1 signaling
pathway in rats. Brain Res Bull. 153:162–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Li X, Zhang D, Bai Y, Xiao J, Jiao H and
He R: Ginaton improves neurological function in ischemic stroke
rats via inducing autophagy and maintaining mitochondrial
homeostasis. Neuropsychiatr Dis Treat. 15:1813–1822. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Zhou S, Qiao B, Chu X and Kong Q:
Oxymatrine attenuates cognitive deficits through SIRT1-mediated
autophagy in ischemic stroke. J Neuroimmunol. 323:136–142. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Zhang X, Li H, Zhao Y, Zhao T, Wang Z and
Tang Q: Neuronal injury after ischemic stroke: Mechanisms of
crosstalk involving necroptosis. J Mol Neurosci. 75:152025.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Deng XX, Li SS and Sun FY: Necrostatin-1
prevents necroptosis in brains after ischemic stroke via inhibition
of RIPK1-mediated RIPK3/MLKL signaling. Aging Dis. 10:807–817.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Zhang YY, Liu WN, Li YQ, Zhang XJ, Yang J,
Luo XJ and Peng J: Ligustroflavone reduces necroptosis in rat brain
after ischemic stroke through targeting RIPK1/RIPK3/MLKL pathway.
Naunyn Schmiedebergs Arch Pharmacol. 392:1085–1095. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zhang YY, Tian J, Peng ZM, Liu B, Peng YW,
Zhang XJ, Hu ZY, Luo XJ and Peng J: Caspofungin suppresses brain
cell necroptosis in ischemic stroke rats via up-regulation of
pellino3. Cardiovasc Drugs Ther. 37:9–23. 2023. View Article : Google Scholar
|
|
201
|
Zhang YY, Peng JJ, Chen D, Liu HQ, Yao BF,
Peng J and Luo XJ: Telaprevir improves memory and cognition in mice
suffering ischemic stroke via targeting MALT1-mediated calcium
overload and necroptosis. ACS Chem Neurosci. 14:3113–3124. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Li L, Shi C, Dong F, Xu G, Lei M and Zhang
F: Targeting pyroptosis to treat ischemic stroke: From molecular
pathways to treatment strategy. Int Immunopharmacol.
133:1121682024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Wang M, Liu Z, Hu S, Duan X, Zhang Y, Peng
C, Peng D and Han L: Taohong siwu decoction ameliorates ischemic
stroke injury via suppressing pyroptosis. Front Pharmacol.
11:5904532020. View Article : Google Scholar
|
|
204
|
Ran Y, Su W, Gao F, Ding Z, Yang S, Ye L,
Chen X, Tian G, Xi J and Liu Z: Curcumin ameliorates white matter
injury after ischemic stroke by inhibiting microglia/macrophage
pyroptosis through NF-κB suppression and NLRP3 inflammasome
inhibition. Oxid Med Cell Longev. 2021:15521272021. View Article : Google Scholar
|
|
205
|
Li F, Xu D, Hou K, Gou X, Lv N, Fang W and
Li Y: Pretreatment of indobufen and aspirin and their combinations
with clopidogrel or ticagrelor alleviates inflammasome mediated
pyroptosis via inhibiting NF-κB/NLRP3 pathway in ischemic stroke. J
Neuroimmune Pharmacol. 16:835–853. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Ge Y, Wang L, Wang C, Chen J, Dai M, Yao S
and Lin Y: CX3CL1 inhibits NLRP3 inflammasome-induced microglial
pyroptosis and improves neuronal function in mice with
experimentally-induced ischemic stroke. Life Sci. 300:1205642022.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Hu R, Liang J, Ding L, Zhang W, Liu X,
Song B and Xu Y: Edaravone dexborneol provides neuroprotective
benefits by suppressing NLRP3 inflammasome-induced microglial
pyroptosis in experimental ischemic stroke. Int Immunopharmacol.
113(Pt A): 1093152022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Long J, Sun Y, Liu S, Chen C, Yan Q, Lin
Y, Zhang Z, Chu S, Yang Y, Yang S, et al: Ginsenoside Rg1 treats
ischemic stroke by regulating CKLF1/CCR5 axis-induced neuronal cell
pyroptosis. Phytomedicine. 123:1552382024. View Article : Google Scholar
|
|
209
|
Zhou M, Zhang T, Zhang B, Zhang X, Gao S,
Zhang T, Li S, Cai X and Lin Y: A DNA nanostructure-based
neuroprotectant against neuronal apoptosis via inhibiting toll-like
receptor 2 signaling pathway in acute ischemic stroke. ACS Nano.
16:1456–1470. 2022. View Article : Google Scholar
|
|
210
|
Wang Y, Yuan H, Shen D, Liu S, Kong W,
Zheng K, Yang J and Ge L: Artemisinin attenuated ischemic stroke
induced pyroptosis by inhibiting ROS/TXNIP/NLRP3/Caspase-1
signaling pathway. Biomed Pharmacother. 177:1168942024. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Alattar A, Alshaman R, Althobaiti YS,
Soliman GM, Ali HS, Khubrni WS, Koh PO, Rehman NU and Shah FA:
Quercetin alleviated inflammasome-mediated pyroptosis and modulated
the mTOR/P70S6/P6/eIF4E/4EBP1 pathway in ischemic stroke.
Pharmaceuticals (Basel). 16:11822023. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Yu X, Wang S, Wang X, Li Y and Dai Z:
Melatonin improves stroke by inhibiting autophagy-dependent
ferroptosis mediated by NCOA4 binding to FTH1. Exp Neurol.
379:1148682024. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Jia CL, Gou Y, Gao Y, Pei X, Jin X, Li BL,
Zhang Z, He Y, Ji ES and Zhao Y: Rosmarinic acid liposomes suppress
ferroptosis in ischemic brain via inhibition of TfR1 in BMECs.
Phytomedicine. 132:1558352024. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Sun J, Zhao K, Zhang W, Guo C and Liu H:
Ecdysterone improves oxidative damage induced by acute ischemic
stroke via inhibiting ferroptosis in neurons through ACSL4. J
Ethnopharmacol. 331:1182042024. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Sun Y, Jin H, He J, Lai J, Lin H and Liu
X: Melatonin alleviates ischemic stroke by inhibiting ferroptosis
through the CYP1B1/ACSL4 pathway. Environ Toxicol. 39:2623–2633.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Jin Z, Gao W, Guo F, Liao S, Hu M, Yu T,
Yu S and Shi Q: Astragaloside IV alleviates neuronal ferroptosis in
ischemic stroke by regulating fat mass and
obesity-associated-N6-methyladenosine-acyl-CoA synthetase
long-chain family member 4 axis. J Neurochem. 166:328–345. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Kajarabille N and Latunde-Dada GO:
Programmed cell-death by ferroptosis: Antioxidants as mitigators.
Int J Mol Sci. 20:49682019. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Liu C, Liu E, Li Z, Li W, Jin J, Sui H,
Chen G, Sun Z and Xi H: Danlou tablet attenuates ischemic stroke
injury and blood-brain barrier damage by inhibiting ferroptosis. J
Ethnopharmacol. 322:1176572024. View Article : Google Scholar
|
|
219
|
Bai X, Zheng E, Tong L, Liu Y, Li X and
Yang H, Jiang J, Chang Z and Yang H: Angong Niuhuang Wan inhibit
ferroptosis on ischemic and hemorrhagic stroke by activating
PPARγ/AKT/GPX4 pathway. J Ethnopharmacol. 321:1174382024.
View Article : Google Scholar
|
|
220
|
Zhan S, Liang J, Lin H, Cai J, Yang X, Wu
H, Wei J, Wang S and Xian M: SATB1/SLC7A11/HO-1 axis ameliorates
ferroptosis in neuron cells after ischemic stroke by danhong
injection. Mol Neurobiol. 60:413–427. 2023. View Article : Google Scholar
|
|
221
|
Li XN, Shang NY, Kang YY, Sheng N, Lan JQ,
Tang JS, Wu L, Zhang JL and Peng Y: Caffeic acid alleviates
cerebral ischemic injury in rats by resisting ferroptosis via Nrf2
signaling pathway. Acta Pharmacol Sin. 45:248–267. 2024. View Article : Google Scholar :
|
|
222
|
Peng C, Ai Q, Zhao F, Li H, Sun Y, Tang K,
Yang Y, Chen N and Liu F: Quercetin attenuates cerebral ischemic
injury by inhibiting ferroptosis via Nrf2/HO-1 signaling pathway.
Eur J Pharmacol. 963:1762642024. View Article : Google Scholar
|
|
223
|
Mi Y, Wang Y, Liu Y, Dang W, Xu L, Tan S,
Liu L, Chen G, Liu Y, Li N and Hou Y: Kellerin alleviates cerebral
ischemic injury by inhibiting ferroptosis via targeting
Akt-mediated transcriptional activation of Nrf2. Phytomedicine.
128:1554062024. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Zhang Y, Ye P, Zhu H, Gu L, Li Y, Feng S,
Zeng Z, Chen Q, Zhou B and Xiong X: Neutral polysaccharide from
Gastrodia elata alleviates cerebral ischemia-reperfusion injury by
inhibiting ferroptosis-mediated neuroinflammation via the NRF2/HO-1
signaling pathway. CNS Neurosci Ther. 30:e144562024. View Article : Google Scholar
|
|
225
|
Wu C, Duan F, Yang R, Dai Y, Chen X and Li
S: 15, 16-Dihydrotanshinone I protects against ischemic stroke by
inhibiting ferroptosis via the activation of nuclear factor
erythroid 2-related factor 2. Phytomedicine. 114:1547902023.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Duan WL, Ma YP, Wang XJ, Ma CS, Han B,
Sheng ZM, Dong H, Zhang LY, Li PA, Zhang BG and He MT: N6022
attenuates cerebral ischemia/reperfusion injury-induced microglia
ferroptosis by promoting Nrf2 nuclear translocation and inhibiting
the GSNOR/GSTP1 axis. Eur J Pharmacol. 972:1765532024. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Zhu F, Ding S, Liu Y, Wang X and Wu Z:
Ozone-mediated cerebral protection: Unraveling the mechanism
through ferroptosis and the NRF2/SLC7A11/GPX4 signaling pathway. J
Chem Neuroanat. 136:1023872024. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Xiao P, Huang H, Zhao H, Liu R, Sun Z, Liu
Y, Chen N and Zhang Z: Edaravone dexborneol protects against
cerebral ischemia/reperfusion-induced blood-brain barrier damage by
inhibiting ferroptosis via activation of nrf-2/HO-1/GPX4 signaling.
Free Radic Biol Med. 217:116–125. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Hu S, Fei Y, Jin C, Yao J, Ding H, Wang J
and Liu C: Ginsenoside Rd enhances blood-brain barrier integrity
after cerebral ischemia/reperfusion by alleviating endothelial
cells ferroptosis via activation of N RG1/ ErbB4 -mediated PI3K /A
kt /mTOR signaling pathway. Neuropharmacology. 251:1099292024.
View Article : Google Scholar
|
|
230
|
Li Y, Sun Y, Wang J, Wang X and Yang W:
Voacangine protects hippocampal neuronal cells against
oxygen-glucose deprivation/reoxygenation-caused oxidative stress
and ferroptosis by activating the PI3K-Akt-FoxO signaling. J Appl
Toxicol. 44:1246–1256. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Hilkens NA, Casolla B, Leung TW and de
Leeuw FE: Stroke. Lancet. 403:2820–2836. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Yuan J and Ofengeim D: A guide to cell
death pathways. Nat Rev Mol Cell Biol. 25:379–395. 2024. View Article : Google Scholar
|
|
233
|
Tian X, Li X, Pan M, Yang LZ, Li Y and
Fang W: Progress of ferroptosis in ischemic stroke and therapeutic
targets. Cell Mol Neurobiol. 44:252024. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Candelario-Jalil E, Dijkhuizen RM and
Magnus T: Neuroinflammation, stroke, blood-brain barrier
dysfunction, and imaging modalities. Stroke. 53:1473–1486. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Liang Z, Lou Y, Hao Y, Li H, Feng J and
Liu S: The relationship of astrocytes and microglia with different
stages of ischemic stroke. Curr Neuropharmacol. 21:2465–2480. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen
M, Bosco DB, Wu LJ and Tian DS: Dual functions of microglia in
ischemic stroke. Neurosci Bull. 35:921–933. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Sun X, Yang Y, Meng X, Li J, Liu X and Liu
H: PANoptosis: Mechanisms, biology, and role in disease. Immunol
Rev. 321:246–262. 2024. View Article : Google Scholar
|
|
239
|
Tian HY, Lei YX, Zhou JT, Liu LJ, Yang T,
Zhou Y, Ge JW, Xu C and Mei ZG: Insight into interplay between
PANoptosis and autophagy: novel therapeutics in ischemic stroke.
Front Mol Neurosci. 17:14820152024. View Article : Google Scholar
|