Osteoporosis is a progressive metabolic bone
disorder defined by diminished bone mineral density (BMD) and
microarchitectural deterioration of osseous tissue (1). A previous report indicated that the
prevalence of osteoporosis among middle-aged and elderly
individuals in China is ~33.49%, broken down as 20.73% in men and
38.05% in women (2).
Osteoporosis primarily manifests as pain and skeletal deformities,
with fractures constituting the most severe complication that
imposes substantial burdens on patients and significantly
compromises their quality of life (3). Osteoporotic fractures predominantly
comprise vertebral compression fractures and hip fragility
fractures. Notably, hip fractures represent >40% of these cases,
while the refracture rate following vertebroplasty for vertebral
compression fractures approaches 20% (4,5).
Skeletal homeostasis is dynamically regulated through coupled bone
remodeling processes, mediated by osteoblastic bone formation and
osteoclastic bone resorption within specialized bone multicellular
units (6). The balanced
interplay between osteoblastic and osteoclastic activities
maintains physiological bone turnover while preventing pathological
alterations in bone mass. However, age-related endocrine changes,
particularly estrogen deficiency in postmenopausal women, coupled
with metabolic dysregulation such as chronic hyperglycemia and
adiposity, disrupt this equilibrium. This imbalance leads to
predominant osteoclastic resorption over osteoblastic formation,
resulting in progressive bone mass depletion and eventual
osteoporotic deterioration (7).
Owing to the exclusion of BMD assessments from routine physical
examinations and insufficient awareness of bone health maintenance,
patients frequently present with clinically detectable reductions
in BMD only upon the manifestation of severe complications.
Consequently, implementing proactive diagnostic measures and
preventative strategies becomes imperative for populations with
elevated osteopathic risks.
Vitamins represent a category of essential
micronutrients that must be acquired through dietary sources to
support fundamental physiological processes. These micronutrients
serve critical functions in human growth, metabolic regulation and
developmental processes (8,9).
Although vitamins neither constitute structural components of
bodily tissues nor serve as energy substrates, they mediate the
regulation of biochemical metabolism through enzymatic cofactor
activities (10). Vitamins play
crucial roles in maintaining skeletal integrity and facilitating
bone repair mechanisms (11).
Clinical evidence demonstrates that vitamin deficiencies constitute
significant risk factors for osteoporosis development (12). Vitamin D plays a pivotal role in
skeletal mineralization through the regulation of calcium
homeostasis; it facilitates intestinal calcium absorption by
inducing the expression of calcium-binding proteins and enhancing
the bioavailability of calcium salts within the gastrointestinal
tract (13). Clinical evidence
also supports the therapeutic efficacy of vitamin B supplementation
in enhancing BMD (14).
Additionally, vitamins C and E demonstrate therapeutic potential in
ameliorating oxidative stress-mediated skeletal deterioration,
effectively mitigating osteoporotic bone loss in clinical
populations (15). Furthermore,
Vitamin K has been established as a critical biochemical indicator
for assessing osteoporotic vertebral fracture risk (16). This evidence collectively
establishes that vitamins play integral roles in the diagnostic
evaluation, preventive strategies and therapeutic management of
osteoporosis. The present review systematically examines the
regulatory functions of vitamins in bone metabolism while
elucidating the pathophysiological mechanisms underlying
osteoporosis associated with vitamin homeostasis dysregulation.
Vitamin A plays a critical role in regulating
cellular differentiation and metabolic homeostasis, and is
predominantly stored in hepatic tissues of animal-derived food
sources (17). Vitamin A and its
provitamin precursors exhibit osteoprotective effects by
stimulating osteoblastic activity while suppressing osteoclastic
differentiation through retinoic acid receptor-mediated
transcriptional regulation (18). Vitamin A exerts a dose-dependent
influence on bone metabolism, where moderate short-term intake
enhances bone formation, while chronic excessive accumulation
demonstrates catabolic effects on skeletal integrity (19). Recent investigation has revealed
stage-dependent effects of vitamin A during osteogenesis, promoting
osteoblast differentiation while impairing matrix mineralization
(18). Retinoid receptors
participate in the modulatory effects of vitamin A in bone
metabolism (16). Vitamin A
deficiency induces significant developmental abnormalities
characterized by impaired skeletal morphogenesis and aberrant
cortical bone remodeling (20).
Lind et al (21)
demonstrated that supraphysiological vitamin A intake induces
aberrant vascular endothelial growth factor overexpression, thereby
compromising bone marrow microvascular integrity and predisposing
to spontaneous fracture development (21). Emerging evidence suggests that
vitamin A demonstrates therapeutic potential in ameliorating
mechanical loading-induced skeletal dysregulation (22). Retinol and retinoic acid
represent the principal bioactive forms of vitamin A. A significant
U-shaped relationship exists between plasma retinol concentrations
and BMD (23). Moderate
enhancement of retinol signaling promotes osteogenesis while
inhibiting osteoclast genesis (24). Crosstalk exists between the
Wnt/β-catenin signaling pathway and retinoic acid signaling
pathways (25). In
postmenopausal women, elevated serum retinol levels exhibit
significant associations with accelerated bone loss and heightened
risk of osteoporotic fractures (26). Excessive vitamin A leads to
accumulation of its metabolite retinoic acid, which accelerates
receptor activator of nuclear factor κB ligand (RANKL)-dependent
osteoclast differentiation and subsequent bone mass reduction
(27,28) (Table I). In animal experiments,
retinoic acid was found to markedly decrease bone calcium and
phosphorus by promoting bone resorption and collagen metabolism
(29,30). All-trans retinoic acid further
inhibits osteogenic activity through suppression of the canonical
Wnt/β-catenin signaling pathways (31). Carotenes demonstrate beneficial
effects on bone formation. Epidemiological studies have established
that increased β-carotene intake enhances BMD and reduces the risk
of fractures (32,33). The B vitamin complex exerts
essential regulatory functions in skeletal metabolic homeostasis
(34). Osteoporosis and hip
fracture incidence have both been associated with inadequate
dietary intake of B vitamins (35). Current research on the role of
vitamin B1 in osteoporosis pathogenesis remains limited.
Functioning as a coenzyme, vitamin B1 facilitates glycolytic
metabolism in bone cells. Osteoblasts primarily rely on aerobic
glycolysis for energy production, whereas osteoclasts predominantly
utilize oxidative phosphorylation as their main energy-generating
pathway (36). Vitamin B1
modulates osteoblastic glucose metabolism to regulate osteogenic
processes. Preliminary evidence suggests its potential to inhibit
RANKL-induced osteoclast differentiation, thereby mitigating
osteoporosis progression (37).
Vitamin B2 exerts beneficial effects on bone homeostasis. A
clinical investigation has demonstrated that dietary vitamin B2
supplementation correlates with reduced osteoporosis incidence
(38). Vitamin B2 promotes
osteogenic differentiation and vascular angiogenesis, thereby
enhancing bone mass accrual (39). Vitamin B2 treatment significantly
upregulates the expression levels of Runt-related transcription
factor 2 (Runx2) and β-catenin (40). Elevated vitamin B6 intake is
associated with increased BMD at the hip and spine (41), while diminished serum vitamin B6
concentrations demonstrate a significant association with an
elevated risk (42).
Bioactivated vitamin B6 enhances in vivo osteointegration
processes (43), while vitamin
B6 deficiency impedes fracture repair by impairing callus
maturation (44). Vitamin B9
supplementation demonstrates beneficial effects on BMD (14). Vitamin B9 enhances osteogenic
differentiation by inducing the expression of Runx2 and alkaline
phosphatase (ALP) (45). Vitamin
B9 activates the canonical Wnt/β-catenin signaling pathway during
osteogenic differentiation (46)
(Table I). Adults with
suboptimal vitamin B12 status exhibit compromised skeletal
integrity and diminished bone health outcomes (47). Vitamin B12 deficiency is
associated with diminished total skeletal mass and elevated
fracture susceptibility (48,49). Additionally, deficiency of the
vitamin B family leads to high homocysteine concentrations, which
could promote bone resorption and increase the fracture risk
(50). Hyperhomocysteinemia
potentiates osteoclastogenesis through enhanced catalytic activity
of tartrate-resistant acid phosphatase (TRAP) and cathepsin K
(CTSK) (51). Homocysteine
exerts inhibitory effects on osteoblastic proliferation and
mineralization, thereby compromising bone formation processes
(52).
Vitamin C, scientifically termed ascorbic acid,
critically catalyzes collagen biosynthesis within osseous matrices
(53). Vitamin C in combination
with β-glycerophosphate and dexamethasone constitutes a standard
osteogenic differentiation induction protocol (54). Clinical investigations have
established a positive correlation between serum vitamin C
concentrations and BMD, with enhanced dietary vitamin C intake
demonstrating favorable associations with improved bone mass
parameters (55). Increased
vitamin C intake is associated with elevated BMD at the hip and
lumbar spine (56), and a diet
high in vitamin C demonstrates a significant reduction in hip
fracture risk (57). Vitamin C
actively contributes to the regenerative processes following
osseous defect formation (58).
Vitamin C and its derivatives demonstrate significant osteogenic
enhancement (59). Vitamin C
administration upregulates the expression of BMP2, Runx2,
Osteocalcin and Collagen I during osteogenic differentiation
processes (60). Vitamin C also
facilitates proline hydroxylase activity to catalyze collagen
biosynthesis and post-translational maturation, thereby supporting
osteocalcin production and coordinating bone tissue remodeling
processes (61). Vitamin C
metabolism is mechanistically linked to the biosynthesis of
procollagen type I N-terminal propeptide (PINP) (62). Additionally, vitamin C
facilitates calcified nodule formation during biomineralization and
activates extracellular matrix deposition processes (63). Vitamin C downregulates the
expression of TRAP and CTSK, effectively inhibiting osteoclast
differentiation (60) (Table I).
Vitamin D serves as a critical biomarker for bone
formation, and its deficiency directly contributes to osteoporosis
development (64). Vitamin D is
closely related to bone metabolism and its main function is to
regulate the absorption of calcium and phosphorus, which are
involved in neo-osteogenesis (65). Vitamin D primarily enhances bone
tissue calcification by regulating plasma calcium and phosphorus
levels, and promoting their deposition as bone salts (66); it facilitates Ca2+
transport across osteoblast membranes into osseous tissue (67). Vitamin D further stimulates
osteogenic signaling pathways through upregulation of SMAD1-3 and 5
and β-catenin expression (68).
Vitamin D stimulation significantly upregulates the expression of
osteogenic biomarkers, including Runx2 and collagen type I
(69). The vitamin D receptor
(VDR) critically regulates skeletal metabolic homeostasis (70). VDR activation enhances ALP
enzymatic activity and stimulates osteocalcin biosynthesis in
osteoblasts (71) (Table I). Vitamin D further induces VDR
activation within osteoblasts, demonstrating the capacity to
suppress osteoclastic activity (72). VDR-targeted genetic interventions
demonstrate therapeutic efficacy in bone metabolic regulation,
establishing innovative pharmacological strategies for osteoporosis
management (73). Additionally,
Vitamin D exerts dual therapeutic effects by restoring trabecular
microarchitecture and augmenting musculoskeletal function, thereby
enhancing skeletal integrity and physical performance in
osteoporotic populations (74).
Vitamin D, as a fundamental supplement for skeletal health, is
extensively employed in osteoporosis clinical management alongside
calcium. A longitudinal investigation involving over 20,000
participants demonstrated that sustained bolus vitamin D
administration maintained fracture risk neutrality, affirming its
safety in both preventive and therapeutic osteoporosis applications
(75). Moreover, vitamin D
deficiency serves as a principal etiological determinant in the
pathogenesis of pediatric rickets (76). Vitamin D deficiency precipitates
dysregulated calcium-phosphorus homeostasis and impairs both
metaphyseal mineralization and skeletal matrix maturation. Enhanced
vitamin D supplementation during pregnancy and the neonatal period
effectively prevents the development of rickets (77).
Vitamin E and its derivatives demonstrate beneficial
effects on bone metabolism (78). Vitamin E participates in the
maintenance of bone homeostasis by modulating osteocyte-derived
bone-related peptides (79)
Diminished serum concentrations predispose to the development of
hip fractures (80). Vitamin E
stimulates osteoblast proliferation through upregulation of cyclin
D1 and c-myc expression, while concurrently enhancing osteogenic
differentiation via activation of the canonical Wnt/β-catenin
signaling pathway (81).
Osteogenic markers, including BMP-2, Osterix, Collagen type I and
Osteocalcin, are upregulated to induce osteogenesis (82,83). Vitamin E preserves plasma
membrane integrity in osteocytes subjected to mechanical loading by
mitigating membrane disruptions (84). Vitamin E exhibits protective
effects on mineralized surfaces, effectively preventing bone
erosion (85). Meanwhile, it
also significantly suppresses osteoclast-specific biomarker
expression and attenuates osteoclastic resorptive activity
(86). Vitamin E reduces serum
levels of type I collagen C-terminal telopeptide, thereby
suppressing bone resorption activity (87). Vitamin E deficiency activates
RANKL-mediated osteoclast genesis in multinucleated precursor cells
(88) (Table I).
Vitamin K constitutes a critically underrecognized
modulator of skeletal homeostasis (89). Genetic polymorphisms in vitamin K
regulatory enzymes constitute significant genetic determinants for
osteoporosis risk stratification (90). Vitamin K deficiency significantly
accelerates bone loss progression (91). Increased vitamin K intake reduces
osteoporosis risk and prevents fractures (92). Both osteocalcin and matrix Gla
protein (MGP) are vitamin K-dependent proteins (93). Vitamin K1 is essential for the
biosynthesis of osteocalcin and MGP (94). Periostin, Protein S and growth
arrest-specific 6 protein constitute vitamin K1-dependent proteins
essential for skeletal homeostasis (95). Vitamin K2 demonstrates beneficial
regulatory effects on skeletal homeostasis through dual mechanisms
of stimulating osteoblastic activity and suppressing osteoclastic
differentiation (96). Vitamin
K2 has been applied as a supplement for the prevention and
treatment of osteoporosis (97).
Transcriptomic profiling demonstrated vitamin K2-mediated
upregulation of Runx2 and osteocalcin through coordinated
activation of the Bcl-6/STAT transcriptional axis and the
IL-6/JAK/STAT signaling cascade (98) (Table I). The intestinal microbiota and
metabolites regulate bone metabolism (99). Integrative analysis of 16S rRNA
sequencing data with serum bone metabolic parameters revealed
Bacteroides-mediated modulation of vitamin K2 homeostasis
and skeletal remodeling processes (100). Vitamin K2 enhances osteogenic
differentiation and matrix mineralization through autophagy pathway
activation in osteoblasts (101). Vitamin K in combination with
vitamin D and calcium may constitute a more efficacious supplement
regimen for osteoporosis prophylaxis and therapeutic management
(102,103). However, notably, vitamin K
antagonist oral antibiotics demonstrate diminished BMD and elevated
fracture susceptibility through coherent pharmacological mechanisms
(104).
Vitamins, as essential micronutrients, demonstrate
significant associations with osteopenia in postmenopausal women
(105). Reduced vitamin intake
exacerbates postmenopausal osteoporotic deterioration (106). Vitamin A serves as a critical
supplement for postmenopausal women, effectively enhancing
health-related quality of life (107). Serum carotenoid concentrations
demonstrate significant associations with estrogen
receptor-positive malignancy risk (108). However, elevated circulating
vitamin A concentrations constitute a significant risk factor for
osteoporosis (26). Chronic
supplementation significantly elevates fracture incidence (109). Vitamin B9 and vitamin B12 are
the primary B vitamins associated with bone metabolism in
postmenopausal women. Epidemiological evidence indicates that
vitamin B9 and B12 deficiencies are associated with an increased
incidence of asymptomatic osteoporotic vertebral fractures
(110). Vitamin B9 demonstrates
beneficial regulatory effects through upregulation of osteocalcin
biosynthesis and β-cross-linked C-telopeptide expression (111). Vitamin B12 serves as a
protective factor against osteoporosis in postmenopausal women
(112). Vitamin C stimulates
gonadal secretory activity and enhances circulating estrogen levels
(113); it primarily mediates
the enhancement of endothelial function in postmenopausal
physiological states (114,115). Serum vitamin C concentrations
demonstrated an inverse association with the pathogenesis of
postmenopausal osteoporosis (116). Plasma vitamin C deficiency
serves as a significant predictive biomarker for hip BMD (117). Vitamin C-enriched dietary
regimens ameliorate the dysfunctional crosstalk among skeletal,
muscular and adipose tissues secondary to estrogen deficiency
through multi-tissue regulatory mechanisms (118). Vitamin D is essential for
female reproductive physiology, with its receptor (VDR) mediating
therapeutic benefits within the genitourinary system (119). Vitamin D deficiency is
associated with premature menopause onset and diminished
reproductive lifespan in women (120). Serum 25-hydroxyvitamin D
[25(OH)D] levels are associated with the healthy index (lower
levels of glycohemoglobin, glucose and higher levels of HDL) of
postmenopausal women (121).
Serum 25(OH D concentrations demonstrated significant correlations
with health status indices in postmenopausal populations (122). Vitamin D is critical for
maintaining BMD and reducing fracture risk in postmenopausal women
(123), serving as a pivotal
bone turnover marker that enhances bone quality and mechanical
strength (124). Postmenopausal
women with combined vitamin D insufficiency and elevated
circulating retinol concentrations demonstrate significantly
heightened susceptibility to osteoporotic progression (125). Serum vitamin D metabolites
serve as critical diagnostic biomarkers for predicting the onset of
postmenopausal osteoporosis (126). Therapeutically, vitamin D
supplementation is employed to prevent estrogen deficiency-induced
bone loss (127). Vitamin D
combined with calcium supplementation is more effective for
improving the process of bone remodeling (128). Vitamin D upregulates irisin
expression to synergistically augment musculoskeletal function and
BMD (129). Vitamin E,
scientifically termed tocopherol, demonstrates the capacity to
stimulate estrogen biosynthesis and secretory processes (130). Vitamin E has been incorporated
into hormone replacement regimens, serving as an adjuvant to
mitigate climacteric symptomatology (131). Oral gavage of vitamin E
significantly enhances bone mineral content and restores trabecular
microarchitecture in ovariectomized (OVX) rat models (132). The principal therapeutic
mechanism involves sclerostin downregulation, which enhances
osteogenic activity through modulation of the RANKL/osteoprotegerin
equilibrium (133). Meanwhile,
vitamin E suppresses estrogen deficiency-driven monocyte/lymphocyte
proliferation, thereby attenuating inflammation-mediated osteoclast
differentiation (134). The
antioxidative capacity of vitamin E attenuates oxidative
stress-mediated bone resorption, thereby ameliorating osteopenic
progression in postmenopausal women (135). Vitamin K demonstrates
beneficial effects on bone ultrastructure enhancement (136); it plays a pivotal role in
promoting osteogenic differentiation and facilitating matrix
mineralization during postmenopausal bone remodeling. Oral vitamin
K administration upregulates the expression of Runx2 and BMP2 while
downregulating nuclear factor of activated T-cells cytoplasmic 1
(NFATC1) expression (137).
Vitamin K derivatives also inhibited the expression of NFATc1 and
CTSK to reverse bone resorption in a study with OVX mice (92). Vitamin K derivatives inhibited
NFATc1 and CTSK expression, effectively reversing bone resorption
in OVX mice (138). Vitamin K1
serves as a critical diagnostic and therapeutic biomarker for
postmenopausal osteoporosis management (139). Elevated circulating vitamin K1
concentrations positively correlate with enhanced BMD in
postmenopausal populations (140). Vitamin K2 supplementation may
contribute to the maintenance and improvement of lumbar BMD by
enhancing osteocalcin biosynthesis (141,142) (Table II). In summary, vitamins
primarily counteract bone loss through direct interaction with
estrogen receptors to modulate estrogen secretion or ameliorate
pathological alterations induced by estrogen deficiency. Early
assessment of serum vitamin levels facilitates the prevention of
BMD reduction in postmenopausal women. Targeted vitamin
supplementation further refines intervention strategies and
enhances therapeutic outcomes for postmenopausal osteoporosis.
Vitamins serve as critical factors in the
synergistic regulation of blood glucose homeostasis. B-group
vitamins function as coenzymes in glucose metabolism pathways, with
vitamin B1 deficiency specifically impairing glucose oxidation,
thereby inducing pyruvate accumulation and compromising cellular
energy supply (143). Notably,
vitamin B1 insufficiency is highly prevalent in diabetic
populations (144). Serum
vitamin B1 concentrations demonstrate a significant positive
correlation with diabetes mellitus progression (145). Vitamin B1 supplementation
demonstrates therapeutic efficacy in ameliorating osteoporosis
associated with diabetes mellitus (146). Vitamin B12 deficiency is
prevalent among diabetic patients, particularly following metformin
administration (147). Vitamin
B12 supplementation may mitigate the risk of
hyperglycemia-associated fragility fractures (148). Vitamin C exerts indirect
regulatory effects on blood glucose homeostasis. Notably, vitamin C
has been shown to inhibit the formation of advanced glycation end
products (149). Conversely,
vitamin C has been demonstrated to promote insulin secretion,
enhance the degree to which insulin promotes glucose breakdown and
attenuate insulin resistance (150). Low serum vitamin C levels have
been established as a significant contributing factor to reduced
BMD in diabetic patients (55).
Vitamin D plays a pivotal role in blood glucose homeostasis and the
modulation of glycemic indicators (151). Serum 25(OH)D3 concentrations
exhibit a progressive decline associated with diabetes mellitus
progression (152). Vitamin D
deficiency has been mechanistically linked to insulin resistance,
potentiating the risk of diabetic osteoporosis through impaired
bone metabolic efficiency (153). VDR activation has been shown to
mitigate Wnt signaling pathway suppression and stimulate the
upregulation of osteogenic marker proteins, thereby counteracting
diabetes-associated bone resorption (154). Vitamin D-induced osteogenic
effects on osteoblasts are mediated through forkhead box protein O1
(FoxO1) signaling inhibition (155,156). Vitamin E demonstrates
therapeutic potential in modulating glycemic regulation (78). Vitamin E supplementation enhances
glucose homeostasis through improved glucose-insulin regulatory
mechanisms, while ameliorating hyperglycemia-associated bone
resorption by upregulating PINP synthesis to stimulate osteogenic
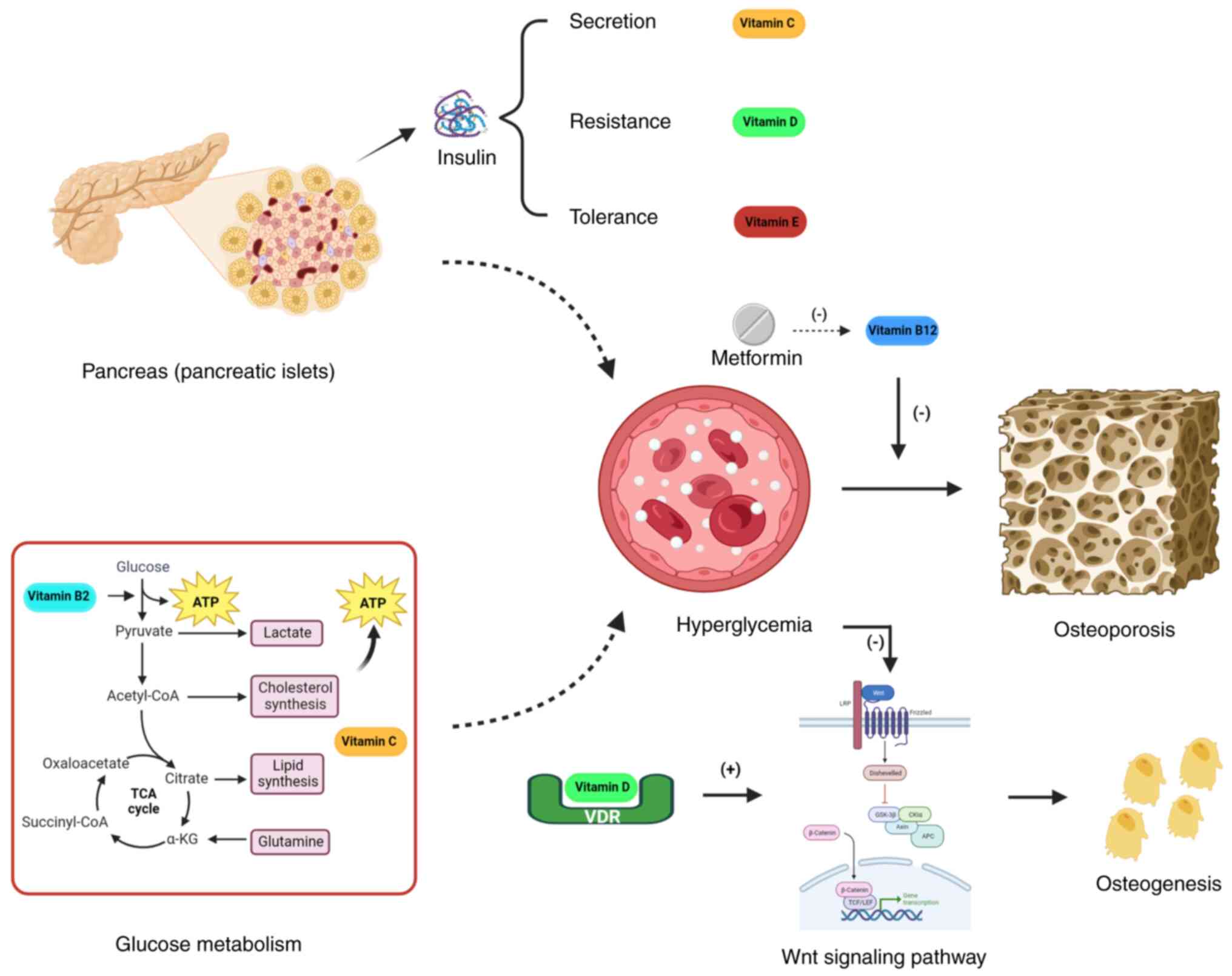
activity (157) (Fig. 1). Vitamin K2 upregulates SIRT1
expression to inhibit hyperglycemia-induced mesenchymal stem cell
(MSC) ferroptosis (158). The
synergistic administration of vitamins K2 and D3 represents a
promising therapeutic strategy for the management of diabetic
osteoporosis (159). In
conclusion, vitamins primarily ameliorate bone loss in diabetic
populations through their involvement in glucose metabolism and
modulation of insulin secretory dynamics and bioactivity.
Optimal-dose vitamin supplementation demonstrates dual efficacy in
maintaining glycemic stability and attenuating hyperglycemia-driven
osteoclastic resorption.
In obese populations, vitamin A deficiency exhibits
a stronger association with pathological bone deterioration and
microarchitectural degradation (18). Obesity demonstrates a significant
inverse association with serum retinol concentrations (160). Vitamin A enhances lipid
oxidative metabolism and promotes adipose tissue catabolism
(161). Retinol-binding protein
4 serves as a critical adipokine mediating the regulatory effects
of vitamin A on lipid metabolism, while exhibiting an inverse
correlation with serum osteocalcin concentrations (162,163). The vitamin B group plays
integral roles in lipid metabolic processes. Specifically, vitamin
B2 orchestrates adipose tissue biosynthesis, catabolism and
secretory regulation. Vitamin B2-enriched dietary regimens
effectively reduce adipose accumulation while preventing BMD loss
(157). Vitamin B9 activates
the AMPK signaling pathway to attenuate high fat diet (HFD)-induced
osteoporosis by increasing the expression of nuclear factor
erythroid 2-related factor 2 (Nrf2) and antioxidant enzymes
(164). Meanwhile, serum
vitamin B12 concentrations exhibited a significant inverse
association with adiposity indices (165). Vitamin C demonstrates
inhibitory effects on triglyceride deposition and adipose tissue
hypertrophy (166), and
modulates MSC differentiation trajectories, preferentially steering
lineage commitment toward osteogenesis while suppressing adipogenic
propensity (167). Clinical
investigations have established that dietary vitamin C sufficiency
exerts preventive effects against the development of
obesity-related osteoporosis (118). Comparative analysis stratified
by body mass index revealed that obese individuals demonstrated a
significant reduction in serum 25-hydroxyvitamin D concentrations
relative to non-obese controls (168). Obese individuals exhibit
impaired vitamin D metabolism that adversely impacts bone formation
(169). In the osteoporotic
population, serum 25(OH D3 levels (the predominant circulating form
of vitamin D) demonstrate a significant inverse association with
triglyceride concentrations, while exhibiting positive correlations
with apolipoprotein A1, lipoprotein (a) and high-density
lipoprotein cholesterol levels (170). Vitamin E inhibits the synthesis
of cholesterol to treat hyperlipidemia (171). Vitamin E exerts inhibitory
effects on hepatic cholesterol biosynthesis, thereby ameliorating
hyperlipidemic conditions through modulation of key lipid metabolic
pathways (172). Vitamin K
demonstrates regulatory effects on adipose tissue metabolism
(173). The supplementation of
vitamin K in HFD rats was shown to prevent bone loss through dual
mechanisms, namely, enhancing osteocalcin-mediated bone formation
and suppressing RANKL-induced osteoclastic resorption (174). The combined administration of
vitamin K2 and D3 synergistically enhanced the expression of
osteogenic transcription factors, specifically osteocalcin, osterix
and Runx2, in diet-induced obese murine models (159) (Table III). Overall, vitamins serve as
key regulatory factors in lipid metabolism homeostasis. Adequate
vitamin intake prevents adipocyte hypertrophy by optimizing lipid
turnover rates and attenuating hyperlipidemia-associated
comorbidities. Implementation of a vitamin-enriched dietary regimen
synergized with structured physical activity constitutes an
evidence-based intervention for improving metabolic parameters and
preserving BMD in obese populations.
Oxidative stress has been identified as a
pathophysiological hallmark in clinically vulnerable cohorts
predisposed to osteoporosis, functioning as a critical underlying
mechanism driving accelerated BMD decline (7). Vitamins A, C and E are primary
regulators of the redox balance. Vitamin A elevates thioredoxin
reductase expression to counteract nitric oxide-induced oxidative
damage and augments glutathione peroxidase activity to reduce free
radical accumulation (175).
Meanwhile, vitamin A effectively attenuates lipid peroxidation
chain reactions and suppresses malondialdehyde production in lipid
metabolic processes (176).
Vitamin A intake enhances systemic antioxidant capacity through
dual pathways: Attenuating plasma sulfhydryl (SH) group depletion
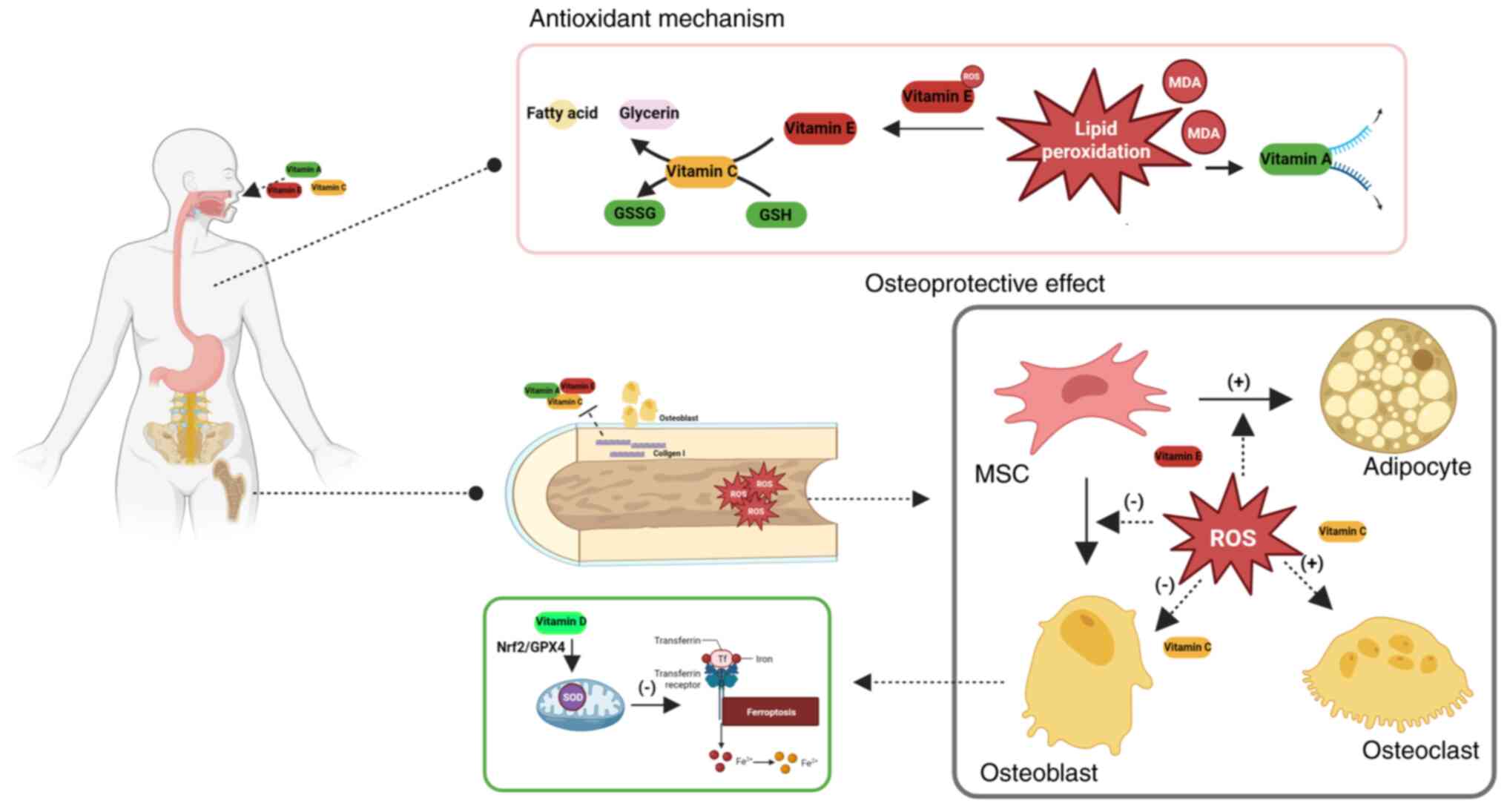
while mitigating oxidative protein modifications (177). Vitamin C exerts antioxidant
effects through two primary mechanisms: Protecting SH groups from
oxidation, and serving as an electron donor to regenerate reduced
glutathione (GSH) from its oxidized form (GSSG) (178). Vitamin E suppresses lipid
peroxidation chain reactions, thereby attenuating oxidative
stress-induced cellular damage. Concurrently, it facilitates the
transfer of membrane-bound lipid peroxides to vitamin C for
subsequent enzymatic reduction into non-toxic fatty acids and
glycerol derivatives (15,179). Vitamin E significantly enhances
systemic antioxidant defense by elevating total antioxidant
capacity in serum through potentiation of radical-scavenging
enzymatic systems (180).
Antioxidant vitamins demonstrate significant
therapeutic potential in enhancing BMD through attenuation of
oxidative stress-mediated bone resorption and promotion of
osteoblastic activity (181).
Epidemiological evidence substantiates that dietary vitamins A, C
and E confer significant risk reduction for osteoporosis through
synergistic mitigation of oxidative stress, while serving as
essential modulators of bone remodeling homeostasis (182). The composite dietary
antioxidant index, encompassing vitamins A, C and E, demonstrates a
significant inverse correlation with BMD during osteoporotic
progression (106). Oral
antioxidant vitamins demonstrate therapeutic benefits in
attenuating age-related physiological decline and ameliorating
oxidative stress-associated bone deterioration through
comprehensive modulation of redox homeostasis (183). Meanwhile, these antioxidant
vitamins, especially vitamin E, can notably decrease fracture
occurrence at any site (184).
Vitamins C and E enhance collagen synthesis, osteogenic
differentiation and matrix mineralization through their antioxidant
properties (185). Vitamin C
demonstrates therapeutic efficacy in counteracting oxidative
skeletal damage resulting from diverse pathological stressors
(186,187). Vitamin C treatment effectively
reduces stress-induced osteoblast death and inhibits
RANKL/macrophage colony-stimulating factor-mediated osteoclast
differentiation under oxidative conditions (188). Sodium-dependent vitamin C
co-transporters orchestrate the homeostatic regulation of redox
balance in osseous tissue through dual mechanisms: Modulating
transcriptional activation of antioxidant enzyme systems, while
coordinating osteogenic differentiation processes (189,190). Vitamin E exerts bone-protective
effects during osseous metabolism through its antioxidant
properties, which mitigate oxidative damage to bone tissue
components and maintain redox homeostasis (191). Vitamin E modulates the redox
balance to suppress adipogenic differentiation in bone marrow MSCs,
thereby preserving their osteogenic potential under oxidative
conditions (192). Vitamin E
protects osteoblasts from reactive oxygen species (ROS)-induced
oxidative stress by elevating the GSH redox ratio (GSSH/GSSG),
thereby enhancing cellular antioxidant defenses in bone-forming
cells (193). Moreover, vitamin
E restores mitochondrial function to suppress ROS-mediated
osteoclastogenesis (86).
Multiple vitamins modulate redox processes to regulate bone
metabolic equilibrium. Activated vitamin D enhances mitochondrial
ultrastructure and functional restoration, thereby optimizing redox
homeostasis in osseous tissue (194). Vitamin D upregulates
antioxidant enzyme expression to alleviate oxidative stress,
thereby preventing osteoblast apoptosis through enhanced cellular
redox homeostasis (195). VDR
activation suppresses oxidative damage-induced osteoblast
ferroptosis through Nrf2/glutathione peroxidase 4 pathway
stimulation (196) (Fig. 2). Pantothenic acid orchestrates
dual cytoprotective mechanisms through FoxO1/2 and Nrf2 signaling
pathway activation, attenuating osteoclastogenesis via suppression
of intracellular ROS overproduction (197). Vitamin K2 functions as an
antioxidant in bone tissue engineering, effectively reducing
ROS-induced damage to enhance bone repair processes (198). Vitamin K2 derivatives
demonstrate inhibitory effects on oxidative stress-mediated
osteoblast apoptosis (199).
Inflammation is an important pathogenic factor in
the development of osteoporosis (200). Vitamin C demonstrates
anti-inflammatory efficacy in OVX rats by attenuating
TNF-α-mediated inflammatory infiltration in osseous tissues
(201). Vitamin D deficiency
induces systemic metabolic derangements and endocrine axis
imbalance, culminating in elevated secretion of pro-inflammatory
cytokines IL-6 and TNF-α (122). Vitamin D deficiency is also
associated with an elevated erythrocyte sedimentation rate, C
reactive protein (CRP) level and IL-6 level, which has a negative
effect on BMD by decreasing osteocalcin (202). Vitamin D deficiency is
associated with elevated levels of inflammatory markers
(erythrocyte sedimentation rate (ESR), CRP and IL-6), which
collectively contribute to reduced BMD via inhibition of
osteocalcin production (203,204). Vitamin E supplementation
attenuates inflammatory responses through downregulation of IL-1,
IL-6 and CRP, effectively inhibiting osteoporotic progression
(205). Vitamin K2 suppresses
the secretion of pro-inflammatory cytokines, specifically IL-1α,
IL-1β and TNF-α (206). Vitamin
K2 suppresses both the activation and proliferation of circulating
T cells, thereby enhancing bone mass through immunoregulatory
mechanisms (207). Vitamin A
exerts detrimental effects on bone metabolism through
pro-inflammatory pathways. Specifically, retinoic acid suppresses
the osteogenic differentiation of MSCs by stimulating the
NF-κB/NLRP3 inflammasome axis and triggering IL-1β release
(208). Downregulation of IL-6
secretion correlates with reduced expression of ALP and
osteocalcin, critical biomarkers of osteogenic differentiation
(209).
Amidst the global demographic aging trend and the
proliferation of sedentary lifestyle patterns, contemporary
healthcare systems face escalating demands for enhanced
quality-of-life metrics and optimized management of chronic
degenerative diseases. Osteoporosis, a prevalent bone metabolic
disorder, is pathologically manifested as progressive deterioration
of bone microarchitecture with concomitant declines in mineral
density (210). Current
diagnostic paradigms rely on dual-energy X-ray absorptiometry (DXA)
as the clinical gold standard, while suffering from inherent
limitations, including cost-prohibitive screening protocols and
suboptimal diagnostic accuracy in heterogeneous populations due to
physiological confounding factors (211). Meanwhile, current osteoporosis
treatment primarily employs drugs that directly stimulate
osteogenesis and suppress osteoclastogenesis, yet these therapeutic
approaches demonstrate constrained clinical efficacy and notable
adverse effects (212). This
context necessitates the development of novel diagnostic
methodologies and therapeutic regimens to address contemporary
clinical requirements in osteoporosis management.
Vitamins constitute indispensable micronutrients
critical for human physiological homeostasis. These compounds
orchestrate fundamental biological processes encompassing substrate
metabolism, bioenergetic flux and homeostatic governance (213). Emerging evidence has elucidated
their regulatory roles in bone metabolism through pleiotropic
mechanisms (214,215). The present review
systematically delineates vitamin-mediated physiological networks
and elucidates their differential modulatory effects across
heterogeneous bone tissue compartments. In the diagnostic paradigm,
serum vitamin D quantification combined with bone turnover marker
profiling augments the clinical utility of DXA through
comprehensive evaluation of osteoporotic status (216). Research has also demonstrated
the potential of other vitamins in the prediction of the risk of
osteoporosis. Vitamin K and its derivatives have been confirmed to
decrease in serum concentration earlier than vitamin D during the
development of osteoporosis, suggesting that their measurement
might detect the trend of bone loss earlier (126). Vitamin E demonstrates
predictive value for BMD, although its clinical utility is
confounded by demographic variables, including age, sex and
ethnicity (217). Quantitative
analysis of multiple vitamins in the blood is a potential method
for predicting bone mass and evaluating bone mass based on
different conditions. Vitamins are also effective substances for
preventing and treating osteoporosis. In addition to the dual
effects of vitamin A, other related vitamins showed positive
effects on bone homeostasis. Vitamin D-calcium co-supplementation
is recognized as the foundational therapeutic adjuvant in
osteoporosis management (218).
Moreover, the present review demonstrates that vitamins could
increase the expression of osteogenic biomarkers and promote the
mineralization of the bone matrix. Inhibition of bone resorption
could also be enhanced after vitamin intake. Deeply exploring the
role of vitamins in the development of osteoporosis contributes to
optimizing diagnosis and treatment plans.
Vitamin disturbance is common in high-risk
populations for osteoporosis. In postmenopausal women, vitamins
participate in the regulation of reproductive function. Vitamins
influence the secretion of estrogen, and estrogen performs
biological functions through vitamin receptors. Vitamins are also
important mediators involved in the action of estrogen in bone
metabolism, and are associated with the development of
osteoporosis. Vitamins A and D could also predict the risk of
osteoporotic fractures in postmenopausal women. In diabetic
patients, vitamin supplementation is beneficial for improving blood
glucose and preventing complications. Vitamins not only regulate
glucose metabolism processes, but also increase sensitivity and
reduce resistance to insulin. Conventional treatments for diabetes,
such as oral metformin, could lead to deficiencies in certain
vitamins. Therefore, vitamins are essential in the maintenance and
treatment of diabetes mellitus. In obesity groups, vitamins mainly
affect blood lipid concentration by regulating the synthesis and
decomposition of adipose tissue. On the one hand, vitamins induced
osteogenic differentiation of MSCs rather than adipogenesis. On the
other hand, reasonable lipid composition and content maintain the
balance of bone formation and resorption. Additionally, the
pathological changes can be improved with vitamin treatment in
osteoporosis. Vitamins increase the expression of antioxidant
enzymes and activate the antioxidant pathways to relieve the
oxidative stress induced by the primary changes in osteoporosis.
Decreasing the production of ROS and repairing the function of
mitochondria contribute to attenuating osteoblast injury and
inhibiting osteoclast activity. The redox balance is also positive
for the maintenance of bone homeostasis. Improving the inflammatory
response is another mechanism of vitamins in the treatment of
osteoporosis. Vitamins inhibit the secretion of inflammatory
cytokines and deactivate inflammasomes, which relieves the
inhibition of osteogenesis. Circular RNA enrichment analysis has
also indicated that vitamin digestion and absorption are associated
with the development of osteoporosis (219,220). Based on large sample sizes of
clinical trial data, it can be concluded that targeted delivery of
vitamin molecules, such as loading nanoparticles, contributes to
improving the diagnosis and treatment efficiency of osteoporosis in
high-risk populations (221-223).
The present review systematically delineates the
pleiotropic regulatory roles of vitamins in bone metabolism through
their functional interplay with osseous homeostasis and skeletal
remodeling dynamics. Blood vitamin content is significantly
associated with the development of osteoporosis. The combination of
multiple vitamins helps predict the risk of osteoporosis and bone
loss under different primary etiologies. The positive effect of
vitamins on bone formation determines the potential for
osteoporosis treatment. The improvement of oxidative stress and
inflammation in high-risk populations for osteoporosis also
indicates the systemic modulation of vitamins. Specific vitamin
supplements will effectively prevent and improve bone loss.
Developing vitamin guidelines for people with osteoporosis is a
research topic of considerable interest.
Not applicable.
MJ was responsible for data curation, literature
search methodology, use of the software for literature analysis and
classification, and writing the original draft. GL was responsible
for literature search methodology, validation of the literature and
writing the original draft. KY was responsible for literature
organization, use of the software for literature analysis,
validation of the literature, and reviewing and editing the
manuscript. LT was responsible for funding acquisition, project
administration, and reviewing and editing the manuscript. All
authors have read and approved the manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by project funding from the
National Science Fund for Distinguished Young Scholars (grant no.
32200943), and Shenyang Young and Middle-Aged Innovative Talents
Project (grant no. RC210171).
|
1
|
Atkinson EG, Adaway M, Horan DJ, Korff C,
Klunk A, Orr AL, Ratz K, Bellido T, Plotkin LI, Robling AG and
Bidwell JP: Conditional loss of Nmp4 in mesenchymal stem progenitor
cells enhances PTH-induced bone formation. J Bone Miner Res.
38:70–85. 2023. View Article : Google Scholar
|
|
2
|
Wang J, Shu B, Tang DZ, Li CG, Xie XW,
Jiang LJ, Jiang XB, Chen BL, Lin XC, Wei X, et al: The prevalence
of osteoporosis in China, a community based cohort study of
osteoporosis. Front Public Health. 11:10840052023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kobayashi H, Ito N, Nakai Y, Katoh H,
Okajima K, Zhang L, Tsuda Y and Tanaka S: Patterns of symptoms and
insufficiency fractures in patients with tumour-induced
osteomalacia. Bone Joint J. 105-B:568–574. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Z, Xing D, Dong Z, Wei J, Zhang L,
Gao S and Li W: Epidemiological investigation of 387 individuals
over 65 years old with osteoporotic fractures. Altern Ther Health
Med. 29:207–211. 2023.PubMed/NCBI
|
|
5
|
Ju G and Liu X: A nomogram prediction
model for refracture in elderly patients with osteoporotic
vertebral compression fractures after percutaneous vertebroplasty.
Eur Spine J. 32:3919–3926. 2023. View Article : Google Scholar
|
|
6
|
Deosthale P, Balanta-Melo J, Creecy A, Liu
C, Marcial A, Morales L, Cridlin J, Robertson S, Okpara C, Sanchez
DJ, et al: Fragile X Messenger Ribonucleoprotein 1 (FMR1), a novel
inhibitor of osteoblast/osteocyte differentiation, regulates bone
formation, mass, and strength in young and aged male and female
mice. Bone Res. 11:252023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Li H, Li J, Hu J, Yang K and Tao
L: Oxidative stress: A common pathological state in a high-risk
population for osteoporosis. Biomed Pharmacother. 163:1148342023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agliardi C, Guerini FR, Bolognesi E,
Zanzottera M and Clerici M: VDR gene single nucleotide
polymorphisms and autoimmunity: A narrative review. Biology
(Basel). 12:9162023.
|
|
9
|
Talib WH, Ahmed Jum'AH DA, Attallah ZS,
Jallad MS, Al Kury LT, Hadi RW and Mahmod AI: Role of vitamins A,
C, D, E in cancer prevention and therapy: Therapeutic potentials
and mechanisms of action. Front Nutr. 10:12818792024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prieto-de Lima TS, Rojas-Jimenez K and
Vaglio C: Strategy for optimizing vitamin B12 production in
pseudomonas putida KT2440 using metabolic modeling. Metabolites.
14:6362024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rizzi F, Panniello A, Comparelli R,
Arduino I, Fanizza E, Iacobazzi RM, Perrone MG, Striccoli M, Curri
ML, Scilimati A, et al: Luminescent alendronic acid-conjugated
micellar nanostructures for potential application in the
bone-targeted delivery of cholecalciferol. Molecules. 29:23672024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parhiala M, Ukkonen M, Sand J and
Laukkarinen J: Osteoporosis and sarcopenia are common and
insufficiently diagnosed among chronic pancreatitis patients. BMC
Gastroenterol. 23:1242023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirby BJ, Ma Y, Martin HM, Buckle Favaro
KL, Karaplis AC and Kovacs CS: Upregulation of calcitriol during
pregnancy and skeletal recovery after lactation do not require
parathyroid hormone. J Bone Miner Res. 28:1987–2000. 2013.
View Article : Google Scholar
|
|
14
|
Rondanelli M, Tartara A, Fossari F,
Vecchio V, Faliva MA, Naso M, Perna S, Nichetti M and Peroni G:
Adequate intake and supplementation of B vitamins, in particular
folic acid, can play a protective role in bone health. Curr Aging
Sci. 15:110–120. 2022. View Article : Google Scholar
|
|
15
|
Yang K, Cao F, Xue Y, Tao L and Zhu Y:
Three classes of antioxidant defense systems and the development of
postmenopausal osteoporosis. Front Physiol. 13:8402932022.
View Article : Google Scholar :
|
|
16
|
Salma, Ahmad SS, Karim S, Ibrahim IM,
Alkreathy HM, Alsieni M and Khan MA: Effect of vitamin K on bone
mineral density and fracture risk in adults: Systematic review and
meta-analysis. Biomedicines. 10:10482022. View Article : Google Scholar
|
|
17
|
Chen G, Weiskirchen S and Weiskirchen R:
Vitamin A: Too good to be bad? Front Pharmacol. 14:11863362023.
View Article : Google Scholar :
|
|
18
|
Yee MMF, Chin KY, Ima-Nirwana S and Wong
SK: Vitamin A and bone health: A review on current evidence.
Molecules. 26:17572021. View Article : Google Scholar :
|
|
19
|
Tanumihardjo SA, Gannon BM, Kaliwile C,
Chileshe J and Binkley NC: Restricting vitamin A intake increases
bone formation in Zambian children with high liver stores of
vitamin. Arch Osteoporos. 14:722019. View Article : Google Scholar
|
|
20
|
Baybutt RC, Standard JT, Dim D, Quinn T,
Hamdan H, Lin D, Kunz K, Bomstein ZS, Estorge BJ, Herndon B, et al:
Cod liver oil, but not retinoic acid, treatment restores bone
thickness in a vitamin A-deficient rat. Nutrients. 14:4862022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lind T, Lugano R, Gustafson AM, Norgård M,
van Haeringen A, Dimberg A, Melhus H, Robertson SP and Andersson G:
Bones in human CYP26B1 deficiency and rats with hypervitaminosis A
phenocopy Vegfa overexpression. Bone Rep. 9:27–36. 2018. View Article : Google Scholar
|
|
22
|
Lionikaite V, Henning P, Drevinge C, Shah
FA, Palmquist A, Wikström P, Windahl SH and Lerner UH: Vitamin A
decreases the anabolic bone response to mechanical loading by
suppressing bone formation. FASEB J. 33:5237–5247. 2019. View Article : Google Scholar
|
|
23
|
Zhang X, Huang J, Zhou Y, Hong Z, Lin X,
Chen S, Ye Y and Zhang Z: Vitamin A nutritional status is a key
determinant of bone mass in children. Nutrients. 14:46942022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fraher D, Mann RJ, Dubuisson MJ, Ellis MK,
Yu T, Walder K, Ward AC, Winkler C and Gibert Y: The
endocannabinoid system and retinoic acid signaling combine to
influence bone growth. Mol Cell Endocrinol. 529:1112672021.
View Article : Google Scholar :
|
|
25
|
Zhang S, Chen X, Hu Y, Wu J, Cao Q, Chen S
and Gao Y: All-trans retinoic acid modulates Wnt3A-induced
osteogenic differentiation of mesenchymal stem cells via activating
the PI3K/AKT/GSK3β signalling pathway. Mol Cell Endocrinol.
422:243–253. 2016. View Article : Google Scholar
|
|
26
|
Navarro-Valverde C, Caballero-Villarraso
J, Mata-Granados JM, Casado-Díaz A, Sosa-Henríquez M, Malouf-Sierra
J, Nogués-Solán X, Rodríguez-Mañas L, Cortés-Gil X,
Delgadillo-Duarte J and Quesada-Gómez JM: High serum retinol as a
relevant contributor to low bone mineral density in postmenopausal
osteoporotic women. Calcif Tissue Int. 102:651–656. 2018.
View Article : Google Scholar
|
|
27
|
Zhuang Y, Sun X, Liu B, Hou H and Sun Y:
Effects of rambutan peel (nepheliumlappaceum) PhenolicExtract on
RANKL-induced differentiation of RAW264.7 cells into osteoclasts
and retinoic acid-induced osteoporosis in rats. Nutrients.
12:8832020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Bi W, Liu Y, Cheng J, Sun W, Wu G
and Xu X: The antagonist of retinoic acid receptor α, ER-50891
antagonizes the inhibitive effect of all-trans retinoic acid and
rescues bone morphogenetic protein 2-induced osteoblastogenic
differentiation. Drug Des Devel Ther. 14:297–308. 2020. View Article : Google Scholar :
|
|
29
|
Zhao S, Niu F, Xu CY, Liu Y, Ye L, Bi GB,
Chen L, Tian G and Nie TH: Diosgenin prevents bone loss on retinoic
acid-induced osteoporosis in rats. Ir J Med Sci. 185:581–587. 2016.
View Article : Google Scholar
|
|
30
|
Liu RH, Kang X, Xu LP, Nian HL, Yang XW,
Shi HT and Wang XJ: Effects of the combined extracts of Herba
Epimedii and Fructus Ligustri Lucidi on bone mineral content and
bone turnover in osteoporotic rats. BMC Complement Altern Med.
15:1122015. View Article : Google Scholar
|
|
31
|
Krutzen CLJM, Roa LA, Bloemen M and Von
den Hoff JW: Excess vitamin a might contribute to submucous
clefting by inhibiting WNT-mediated bone formation. Orthod
Craniofac Res. 26:132–139. 2023. View Article : Google Scholar
|
|
32
|
Charkos TG, Liu Y, Oumer KS, Vuong AM and
Yang S: Effects of β-carotene intake on the risk of fracture: A
Bayesian meta-analysis. BMC Musculoskelet Disord. 21:7112020.
View Article : Google Scholar
|
|
33
|
Gao SS and Zhao Y: The effects of
β-carotene on osteoporosis: A systematic review and meta-analysis
of observational studies. Osteoporos Int. 34:627–639. 2023.
View Article : Google Scholar
|
|
34
|
Ahn TK, Kim JO, An HJ, Park HS, Choi UY,
Sohn S, Kim KT, Kim NK and Han IB: 3′-UTR polymorphisms of vitamin
B-related genes are associated with osteoporosis and osteoporotic
vertebral compression fractures (OVCFs) in postmenopausal women.
Genes (Basel). 11:6122020. View Article : Google Scholar
|
|
35
|
Dai Z and Koh WP: B-vitamins and bone
health-a review of the current evidence. Nutrients. 7:3322–3346.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Jiao G, You Y, Li X, Liu J, Sun
Z, Li Q, Dai Z, Ma J, Zhou H, et al: Arginine methylation of PPP1CA
by CARM1 regulates glucose metabolism and affects osteogenic
differentiation and osteoclastic differentiation. Clin Transl Med.
13:e13692023. View Article : Google Scholar
|
|
37
|
Ma Q, Liang M, Wang Y, Ding N, Wu Y, Duan
L, Yu T, Lu Y, Xu J, Kang F and Dou C: Non-coenzyme role of vitamin
B1 in RANKL-induced osteoclastogenesis and ovariectomy induced
osteoporosis. J Cell Biochem. 121:3526–3536. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wan M, Wu H, Wang X, Gu Y, Meng G, Zhang
Q, Liu L, Zhang J, Sun S, Jia Q, et al: There is a significantly
inverse relationship between dietary riboflavin intake and
prevalence of osteoporosis in women but not in men: Results from
the TCLSIH cohort study. Front Nutr. 10:11120282023. View Article : Google Scholar
|
|
39
|
Guo M and Zhang J: Vitamin B2
prevents glucocorticoid-caused damage of blood vessels in
osteonecrosis of the femoral head. Biomed Res Int.
2022:40061842022. View Article : Google Scholar
|
|
40
|
Chaves Neto AH, Yano CL, Paredes-Gamero
EJ, Machado D, Justo GZ, Peppelenbosch MP and Ferreira CV:
Riboflavin and photoproducts in MC3T3-E1 differentiation. Toxicol
In Vitro. 24:1911–1919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Welan R: Effect of vitamin B6 on
osteoporosis fracture. J Bone Metab. 30:141–147. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Chen L, Zhang Y, Li CG, Zhang H,
Wang Q, Qi X, Qiao L, Da WW, Cui XJ, et al: Association between
serum vitamin B6 concentration and risk of osteoporosis
in the middle-aged and older people in China: A cross-sectional
study. BMJ Open. 9:e0281292019. View Article : Google Scholar
|
|
43
|
Lee JS, Kim K, Park JP, Cho SW and Lee H:
Role of pyridoxal 5′-phosphate at the titanium implant interface in
vivo: Increased hemophilicity, inactive platelet adhesion, and
osteointegration. Adv Healthc Mater. 6:16009622017. View Article : Google Scholar
|
|
44
|
Dodds RA, Catterall A, Bitensky L and
Chayen J: Abnormalities in fracture healing induced by vitamin
B6-deficiency in rats. Bone. 7:489–495. 1986. View Article : Google Scholar
|
|
45
|
Santos C, Gomes P, Duarte JA, Almeida MM,
Costa MEV and Fernandes MH: Development of hydroxyapatite
nanoparticles loaded with folic acid to induce osteoblastic
differentiation. Int J Pharm. 516:185–195. 2017. View Article : Google Scholar
|
|
46
|
Georgiou KR, Nadhanan RR, Fan CM and Xian
CJ: Methotrexate-induced bone marrow adiposity is mitigated by
folinic acid supplementation through the regulation of
Wnt/β-catenin signalling. J Cell Physiol. 230:648–656. 2015.
View Article : Google Scholar
|
|
47
|
Clements M, Heffernan M, Ward M, Hoey L,
Doherty LC, Hack Mendes R, Clarke MM, Hughes CF, Love I, Murphy S,
et al: A 2-year randomized controlled trial with low-dose B-vitamin
supplementation shows benefits on bone mineral density in adults
with lower B12 status. J Bone Miner Res. 37:2443–2455. 2022.
View Article : Google Scholar
|
|
48
|
Hussain SIB, AlKhenizan A, Mahmoud A and
Qashlaq H: The correlation between vitamin B12 and folate levels
and bone mineral density among the Saudi population in a primary
care setting. J Family Med Prim Care. 12:1063–1068. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ates Bulut E, Soysal P, Aydin AE, Dokuzlar
O, Kocyigit SE and Isik AT: Vitamin B12 deficiency might be related
to sarcopenia in older adults. Exp Gerontol. 95:136–140. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stone KL, Lui LY, Christen WG, Troen AM,
Bauer DC, Kado D, Schambach C, Cummings SR and Manson JE: Effect of
combination folic acid, vitamin B6, and vitamin
B12 fracture risk in women: A randomized, controlled
trial. J supplementation on Bone Miner Res. 32:2331–2338. 2017.
View Article : Google Scholar
|
|
51
|
Herrmann M, Schmidt J, Umanskaya N,
Colaianni G, Al Marrawi F, Widmann T, Zallone A, Wildemann B and
Herrmann W: Stimulation of osteoclast activity by low B-vitamin
concentrations. Bone. 41:584–591. 2007. View Article : Google Scholar
|
|
52
|
Su S, Zhang D, Liu J, Zhao H, Tang X, Che
H, Wang Q, Ren W and Zhen D: Folate ameliorates
homocysteine-induced osteoblast dysfunction by reducing endoplasmic
reticulum stress-activated PERK/ATF-4/CHOP pathway in MC3T3-E1
cells. J Bone Miner Metab. 40:422–433. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rondanelli M, Peroni G, Fossari F, Vecchio
V, Faliva MA, Naso M, Perna S, Di Paolo E, Riva A, Petrangolini G,
et al: Evidence of a positive link between consumption and
supplementation of ascorbic acid and bone mineral density.
Nutrients. 13:10122021. View Article : Google Scholar
|
|
54
|
Yang K, Cao F, Qiu S, Jiang W, Tao L and
Zhu Y: Metformin promotes differentiation and attenuates
H2O2-induced oxidative damage of osteoblasts
via the PI3K/AKT/Nrf2/HO-1 pathway. Front Pharmacol. 13:8298302022.
View Article : Google Scholar
|
|
55
|
Jain SK, McLean WE, Stevens CM and Dhawan
R: The positive association of plasma levels of vitamin C and
inverse association of VCAM-1 and total adiponectin with bone
mineral density in subjects with diabetes. Nutrients. 14:38932022.
View Article : Google Scholar
|
|
56
|
Malmir H, Shab-Bidar S and Djafarian K:
Vitamin C intake in relation to bone mineral density and risk of
hip fracture and osteoporosis: A systematic review and
meta-analysis of observational studies. Br J Nutr. 119:847–858.
2018. View Article : Google Scholar
|
|
57
|
Zeng LF, Luo MH, Liang GH, Yang WY, Xiao
X, Wei X, Yu J, Guo D, Chen HY, Pan JK, et al: Can dietary intake
of vitamin C-oriented foods reduce the risk of osteoporosis,
fracture, and BMD loss? Systematic review with meta-analyses of
recent studies. Front Endocrinol (Lausanne). 10:8442020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sarkar N, Morton H and Bose S: Effects of
vitamin C on osteoblast proliferation and osteosarcoma inhibition
using plasma coated hydroxyapatite on titanium implants. Surf Coat
Technol. 394:1257932020. View Article : Google Scholar
|
|
59
|
Xie Y, Bao Z, Wang Z, Du D, Chen G, Liu C,
Wang H, Feng N, Xiao X, Wang S, et al: Magnesium ascorbyl phosphate
promotes bone formation via CaMKII signaling. J Bone Miner Res.
38:1015–1031. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Choi HK, Kim GJ, Yoo HS, Song DH, Chung
KH, Lee KJ, Koo YT and An JH: Vitamin C activates
osteoblastogenesis and inhibits osteoclastogenesis via
Wnt/β-catenin/ATF4 signaling pathways. Nutrients. 11:5062019.
View Article : Google Scholar
|
|
61
|
Smith-Cortinez N, Fagundes RR, Gomez V,
Kong D, de Waart DR, Heegsma J, Sydor S, Olinga P, de Meijer VE,
Taylor CT, et al: Collagen release by human hepatic stellate cells
requires vitamin C and is efficiently blocked by hydroxylase
inhibition. FASEB J. 35:e212192021. View Article : Google Scholar
|
|
62
|
Bellissimo MP, Roberts JL, Jones DP, Liu
KH, Taibl KR, Uppal K, Weitzmann MN, Pacifici R, Drissi H, Ziegler
TR and Alvarez JA: Metabolomic associations with serum bone
turnover markers. Nutrients. 12:31612020. View Article : Google Scholar
|
|
63
|
Okajima LS, Martinez EF, Pinheiro IF,
Fonseca Silva AS and Demasi APD: Effect of sodium ascorbyl
phosphate on osteoblast viability and differentiation. J
Periodontal Res. 55:660–666. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu G, Li W, Zhang L, Zhou C and Cong R:
The role of vitamin D on rotator cuff tear with osteoporosis. Front
Endocrinol (Lausanne). 13:10178352022. View Article : Google Scholar
|
|
65
|
Ryan BA, McGregor NE, Kirby BJ, Al-Tilissi
A, Poulton IJ, Sims NA and Kovacs CS: Calcitriol-dependent and
-independent regulation of intestinal calcium absorption,
osteoblast function, and skeletal mineralization during lactation
and recovery in mice. J Bone Miner Res. 37:2483–2497. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Beckett E: More than bone health: The many
roles for vitamin D. Nutrients. 12:23882020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Imai K, Neuman MW, Kawase T and Saito S:
Calcium in osteoblast-enriched bone cells. Bone. 13:217–223. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Al Saedi A, Myers DE, Stupka N and Duque
G: 1,25(OH)2D3 ameliorates palmitate-induced
lipotoxicity in human primary osteoblasts leading to improved
viability and function. Bone. 141:1156722020. View Article : Google Scholar
|
|
69
|
Posa F, Di Benedetto A, Colaianni G,
Cavalcanti-Adam EA, Brunetti G, Porro C, Trotta T, Grano M and Mori
G: Vitamin D effects on osteoblastic differentiation of mesenchymal
stem cells from dental tissues. Stem Cells Int. 2016:91508192016.
View Article : Google Scholar :
|
|
70
|
Kanemoto Y, Iwaki M, Sawada T, Nojiri K,
Kurokawa T, Tsutsumi R, Nagasawa K and Kato S: Advances in the
administration of vitamin D analogues to support bone health and
treat chronic diseases. J Bone Metab. 30:219–229. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mansell JP, Tanatani A and Kagechika H: An
N-cyanoamide derivative of lithocholic acid co-operates with
lysophosphatidic acid to promote human osteoblast (MG63)
differentiation. Biomolecules. 13:11132023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nakamichi Y, Liu Z, Mori T, He Z, Yasuda
H, Takahashi N and Udagawa N: The vitamin D receptor in
osteoblastic cells but not secreted parathyroid hormone is crucial
for soft tissue calcification induced by the proresorptive activity
of 1,25(OH)2D3. J Steroid Biochem Mol Biol.
232:1063512023. View Article : Google Scholar
|
|
73
|
Mondockova V, Kovacova V, Zemanova N,
Babikova M, Martiniakova M, Galbavy D and Omelka R: Vitamin D
receptor gene polymorphisms affect osteoporosis-related traits and
response to antiresorptive therapy. Genes (Basel). 14:1932023.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Haeri NS, Perera S and Greenspan SL: The
association of vitamin D with bone microarchitecture, muscle
strength, and mobility performance in older women in long-term
care. Bone. 176:1168672023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Waterhouse M, Ebeling PR, McLeod DSA,
English D, Romero BD, Baxter C, Armstrong BK, Hartel G, Kimlin M,
O'Connell RL, et al: The effect of monthly vitamin D
supplementation on fractures: a tertiary outcome from the
population-based, double-blind, randomised, placebo-controlled
D-health trial. Lancet Diabetes Endocrinol. 11:324–332. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gallagher JC and Rosen CJ: Vitamin D: 100
Years of discoveries, yet controversy continues. Lancet Diabetes
Endocrinol. 11:362–374. 2023. View Article : Google Scholar
|
|
77
|
Mansur JL, Oliveri B, Giacoia E, Fusaro D
and Costanzo PR: Vitamin D: Before, during and after pregnancy:
Effect on neonates and children. Nutrients. 14:19002022. View Article : Google Scholar
|
|
78
|
Zainal Z, Khaza'ai H, Kutty Radhakrishnan
A and Chang SK: Therapeutic potential of palm oil vitamin E-derived
tocotrienols in inflammation and chronic diseases: Evidence from
preclinical and clinical studies. Food Res Int. 156:1111752022.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wong SK, Chin KY and Ima-Nirwana S: The
effects of tocotrienol on bone peptides in a rat model of
osteoporosis induced by metabolic syndrome: The possible
communication between bone cells. Int J Environ Res Public Health.
16:33132019. View Article : Google Scholar :
|
|
80
|
Holvik K, Gjesdal CG, Tell GS, Grimnes G,
Schei B, Apalset EM, Samuelsen SO, Blomhoff R, Michaëlsson K and
Meyer HE: Low serum concentrations of alpha-tocopherol are
associated with increased risk of hip fracture. A NOREPOS study.
Osteoporos Int. 25:2545–2554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xu W, Li Y, Feng R, He P and Zhang Y:
γ-Tocotrienol induced the proliferation and differentiation of
MC3T3-E1 cells through the stimulation of the Wnt/β-catenin
signaling pathway. Food Funct. 13:398–410. 2022. View Article : Google Scholar
|
|
82
|
Wan Hasan WN, Chin KY, Abd Ghafar N and
Soelaiman IN: Annatto-derived tocotrienol promotes mineralization
of MC3T3-E1 cells by enhancing BMP-2 protein expression via
inhibiting RhoA activation and HMG-CoA reductase gene expression.
Drug Des Devel Ther. 14:969–976. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wan Hasan WN, Abd Ghafar N, Chin KY and
Ima-Nirwana S: Annatto-derived tocotrienol stimulates osteogenic
activity in preosteoblastic MC3T3-E1 cells: A temporal sequential
study. Drug Des Devel Ther. 12:1715–1726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hagan ML, Bahraini A, Pierce JL, Bass SM,
Yu K, Elsayed R, Elsalanty M, Johnson MH, McNeil A, McNeil PL and
McGee-Lawrence ME: Inhibition of osteocyte membrane repair activity
via dietary vitamin E deprivation impairs osteocyte survival.
Calcif Tissue Int. 104:224–234. 2019. View Article : Google Scholar :
|
|
85
|
Mohamad NV, Soelaiman IN and Chin KY:
Effects of tocotrienol from Bixa orellana (annatto) on bone
histomorphometry in a male osteoporosis model induced by buserelin.
Biomed Pharmacother. 103:453–462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mohamad Hazir NS, Yahaya NHM, Zawawi MSF,
Damanhuri HA, Mohamed N and Alias E: Changes in metabolism and
mitochondrial bioenergetics during polyethylene-induced
osteoclastogenesis. Int J Mol Sci. 23:83312022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Vallibhakara SAO, Nakpalat K,
Sophonsritsuk A, Tantitham C and Vallibhakara O: Effect of vitamin
E supplement on bone turnover markers in postmenopausal osteopenic
women: A double-blind, randomized, placebo-controlled trial.
Nutrients. 13:42262021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fujiwara Y, Ko Y, Sonoda M, Ichi I and
Ishikawa T: Effects of vitamin E and dietary conditions on the
differentiation and maturation of osteoclast. J Nutr Sci Vitaminol
(Tokyo). 68:73–77. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Piscaer I, Janssen R, Franssen FME,
Schurgers LJ and Wouters EFM: The pleiotropic role of vitamin K in
multimorbidity of chronic obstructive pulmonary disease. J Clin
Med. 12:12612023. View Article : Google Scholar :
|
|
90
|
He J, Xie H, Yan C, Sun Y, Xu Z and Zhang
X: Genetic variation in VKORC1 and risk for osteoporosis. Arch Med
Res. 52:211–216. 2021. View Article : Google Scholar
|
|
91
|
Urano A, Hotta M, Ohwada R and Araki M:
Vitamin K deficiency evaluated by serum levels of undercarboxylated
osteocalcin in patients with anorexia nervosa with bone loss. Clin
Nutr. 34:443–448. 2015. View Article : Google Scholar
|
|
92
|
Lee AS, Sung MJ, Son SJ, Han AR, Hong SM
and Lee SH: Effect of menaquinone-4 on receptor activator of
nuclear factor κB ligand-induced osteoclast differentiation and
ovariectomy-induced bone loss. J Med Food. 26:128–134. 2023.
|
|
93
|
Popa DS, Bigman G and Rusu ME: The role of
vitamin K in humans: Implication in aging and age-associated
diseases. Antioxidants (Basel). 10:5662021. View Article : Google Scholar
|
|
94
|
Alonso N, Meinitzer A, Fritz-Petrin E,
Enko D and Herrmann M: Role of vitamin K in bone and muscle
metabolism. Calcif Tissue Int. 112:178–196. 2023. View Article : Google Scholar
|
|
95
|
Stock M and Schett G: Vitamin K-dependent
proteins in skeletal development and disease. Int J Mol Sci.
22:93282021. View Article : Google Scholar
|
|
96
|
Wang J, Shao L, Wu X, Liu C, Ni S, Dai T,
Liu H and Zhao H: Electrospun sandwich mesh structures loaded with
naringenin and vitamin K2 polycaprolactone/gelatin
nanofibers synergistically promote bone regeneration. Mater Today
Bio. 23:1007942023. View Article : Google Scholar
|
|
97
|
Giordani C, Matacchione G, Giuliani A,
Valli D, Scarpa ES, Antonelli A, Sabbatinelli J, Giacchetti G,
Sabatelli S, Olivieri F and Rippo MR: Pro-osteogenic and
anti-inflammatory synergistic effect of orthosilicic acid, vitamin
K2, curcumin, polydatin and quercetin combination in young and
senescent bone marrow-derived mesenchymal stromal cells. Int J Mol
Sci. 24:88202023. View Article : Google Scholar :
|
|
98
|
Wang H, Li L, Zhang N and Ma Y: Vitamin K2
improves osteogenic differentiation by inhibiting STAT1 via the
Bcl-6 and IL-6/JAK in C3H10 T1/2 clone 8 cells. Nutrients.
14:29342022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Castaneda M, Strong JM, Alabi DA and
Hernandez CJ: The gut microbiome and bone strength. Curr Osteoporos
Rep. 18:677–683. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ozaki D, Kubota R, Maeno T, Abdelhakim M
and Hitosugi N: Association between gut microbiota, bone
metabolism, and fracture risk in postmenopausal Japanese women.
Osteoporos Int. 32:145–156. 2021. View Article : Google Scholar
|
|
101
|
Li W, Zhang S, Liu J, Liu Y and Liang Q:
Vitamin K2 stimulates MC3T3-E1 osteoblast differentiation and
mineralization through autophagy induction. Mol Med Rep.
19:3676–3684. 2019.
|
|
102
|
AlHajri L, Ayoub A, Ahmed H and AlMulla M:
Effect of vitamin K2 alone or in combination on various bone
turnover markers amongst postmenopausal females. J Bone Metab.
28:11–26. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Capozzi A, Scambia G and Lello S: Calcium,
vitamin D, vitamin K2, and magnesium supplementation and skeletal
health. Maturitas. 140:55–63. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Marietta M, Coluccio V, Boriani G and
Luppi M: Effects of anti-vitamin k oral anticoagulants on bone and
cardiovascular health. Eur J Intern Med. 79:1–11. 2020. View Article : Google Scholar
|
|
105
|
Grili PPDF, Vidigal CV, Cruz GFD,
Albergaria BH, Marques-Rocha JL, Pereira TSS and Guandalini VR:
Nutrient patterns and risk of osteopenia in postmenopausal women.
Nutrients. 15:16702023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang R, Ni Z, Wei M, Cui Y, Zhou H, Di D
and Wang Q: Composite dietary antioxidant intake and osteoporosis
likelihood in premenopausal and postmenopausal women: A
population-based study in the United States. Menopause. 30:529–538.
2023.
|
|
107
|
Nguyen HD: Interactions between nutrient
intake and comorbidities for quality of life in premenopausal and
postmenopausal women. Menopause. 29:1285–1295. 2022. View Article : Google Scholar
|
|
108
|
Bakker MF, Peeters PH, Klaasen VM,
Bueno-de-Mesquita HB, Jansen EH, Ros MM, Travier N, Olsen A,
Tjønneland A, Overvad K, et al: Plasma carotenoids, vitamin C,
tocopherols, and retinol and the risk of breast cancer in the
European prospective investigation into cancer and nutrition
cohort. Am J Clin Nutr. 103:454–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Feskanich D, Singh V, Willett WC and
Colditz GA: Vitamin A intake and hip fractures among postmenopausal
women. JAMA. 287:47–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
El Maghraoui A, Ghozlani I, Mounach A,
Rezqi A, Oumghar K, Achemlal L, Bezza A and Ouzzif Z: Homocysteine,
folate, and vitamin B12 levels and vertebral fracture risk in
postmenopausal women. J Clin Densitom. 15:328–333. 2012. View Article : Google Scholar
|
|
111
|
Salari P, Abdollahi M, Heshmat R, Meybodi
HA and Razi F: Effect of folic acid on bone metabolism: A
randomized double blind clinical trial in postmenopausal
osteoporotic women. Daru. 22:622014. View Article : Google Scholar
|
|
112
|
Zhang H, Tao X and Wu J: Association of
homocysteine, vitamin B12, and folate with bone mineral density in
postmenopausal women: a meta-analysis. Arch Gynecol Obstet.
289:1003–1009. 2014. View Article : Google Scholar
|
|
113
|
Murray AA, Molinek MD, Baker SJ, Kojima
FN, Smith MF, Hillier SG and Spears N: Role of ascorbic acid in
promoting follicle integrity and survival in intact mouse ovarian
follicles in vitro. Reproduction. 121:89–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
McSorley PT, Young IS, Bell PM, Fee JP and
McCance DR: Vitamin C improves endothelial function in healthy
estrogen-deficient postmenopausal women. Climacteric. 6:238–247.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Moreau KL, Hildreth KL, Klawitter J,
Blatchford P and Kohrt WM: Decline in endothelial function across
the menopause transition in healthy women is related to decreased
estradiol and increased oxidative stress. Geroscience.
42:1699–1714. 2020. View Article : Google Scholar
|
|
116
|
Sugiura M, Nakamura M, Ogawa K, Ikoma Y
and Yano M: High vitamin C intake with high serum β-cryptoxanthin
associated with lower risk for osteoporosis in post-menopausal
Japanese female subjects: Mikkabi cohort study. J Nutr Sci
Vitaminol (Tokyo). 62:185–191. 2016. View Article : Google Scholar
|
|
117
|
Mangano KM, Noel SE, Dawson-Hughes B and
Tucker KL: Sufficient plasma vitamin C is related to greater bone
mineral density among postmenopausal women from the boston puerto
rican health study. J Nutr. 151:3764–3772. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bae YJ: Fruit intake and osteosarcopenic
obesity in Korean postmenopausal women aged 50-64 years. Maturitas.
134:41–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hassanein MM, Huri HZ, Abduelkarem AR and
Baig K: Therapeutic effects of vitamin D on vaginal, sexual, and
urological functions in postmenopausal women. Nutrients.
15:38042023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Alinia T, Sabour S, Hashemipour M,
Hovsepian S, Pour HR and Jahanfar S: Relationship between vitamin D
levels and age of menopause and reproductive lifespan: Analysis
based on the National health and nutrition examination survey
(NHANES) 2001-2018. Eur J Obstet Gynecol Reprod Biol. 289:183–189.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Shi JW, Wu JN, Zhu XY, Zhou WH, Yang JY
and Li MQ: Association of serum 25-hydroxyvitamin D levels with
all-cause and cause-specific mortality among postmenopausal
females: Results from NHANES. J Transl Med. 21:6292023. View Article : Google Scholar
|
|
122
|
Begga A, Mehaoudi RI, Ghozlani A, Azzoug S
and Soltani Y: The risk of metabolic syndrome is associated with
vitamin D and inflammatory status in premenopausal and
postmenopausal Algerian women. Ir J Med Sci. 193:615–626. 2024.
View Article : Google Scholar
|
|
123
|
Anagnostis P, Livadas S, Goulis DG, Bretz
S, Ceausu I, Durmusoglu F, Erkkola R, Fistonic I, Gambacciani M,
Geukes M, et al: EMAS position statement: Vitamin D and menopausal
health. Maturitas. 169:2–9. 2023. View Article : Google Scholar
|
|
124
|
Chan CY, Subramaniam S, Mohamed N,
Muhammad N, Ramli FF, Ima-Nirwana S and Chin KY: Circulating
biomarkers related to osteocyte and calcium homeostasis between
postmenopausal women with and without osteoporosis. Endocr Metab
Immune Disord Drug Targets. 21:2273–2280. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Mata-Granados JM, Cuenca-Acevedo JR, Luque
de Castro MD, Holick MF and Quesada-Gómez JM: Vitamin D
insufficiency together with high serum levels of vitamin A
increases the risk for osteoporosis in postmenopausal women. Arch
Osteoporos. 8:1242013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li C, Liang C, Kong Z, Su Y, Ren W, Dong
H, Wu Y, Yang N, Liu R, Wu J and Zheng Y: Determination of vitamin
K1, MK-4, MK-7, and D levels in human serum of postmenopausal
osteoporosis women based on high stability LC-MS/MS: MK-7 may be a
new marker of bone metabolism. Ann Nutr Metab. 79:334–342. 2023.
View Article : Google Scholar
|
|
127
|
Jeong C, Ha J, Yoo JI, Lee YK, Kim JH, Ha
YC, Min YK, Byun DW, Baek KH and Chung HY: Effects of
bazedoxifene/vitamin D combination therapy on serum vitamin D
levels and bone turnover markers in postmenopausal women with
osteopenia: A randomized controlled trial. J Bone Metab.
30:189–199. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Reis AR, Santos RKF, Dos Santos CB, Santos
BDC, de Carvalho GB, Brandão-Lima PN, de Oliveira E Silva AM and
Pires LV: Supplementation of vitamin D isolated or
calcium-associated with bone remodeling and fracture risk in
postmenopausal women without osteoporosis: A systematic review of
randomized clinical trials. Nutrition. 116:1121512023. View Article : Google Scholar
|
|
129
|
Dell Aquila LP, Morales A, Moreira P,
Cendoroglo MS, Elias RM and Dalboni MA: Prospective effects of
cholecalciferol supplementation on irisin levels in sedentary
postmenopausal women: A pilot study. J Clin Transl Endocrinol.
34:1003242023.PubMed/NCBI
|
|
130
|
Samare-Najaf M, Zal F and Safari S:
Primary and secondary markers of doxorubicin-induced female
infertility and the alleviative properties of quercetin and vitamin
E in a rat model. Reprod Toxicol. 96:316–326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Feduniw S, Korczyńska L, Górski K,
Zgliczyńska M, Bączkowska M, Byrczak M, Kociuba J, Ali M and
Ciebiera M: The effect of vitamin E supplementation in
postmenopausal women-A systematic review. Nutrients. 15:1602022.
View Article : Google Scholar
|
|
132
|
Ibrahim N, Mohd Noor H, Shuid AN, Mohamad
S, Abdul Malik MM, Jayusman PA, Shuid AN and Naina Mohamed I:
Osteoprotective effects in postmenopausal osteoporosis rat model:
Oral tocotrienol vs intraosseous injection of tocotrienol-poly
lactic-co-glycolic acid combination. Front Pharmacol.
12:7067472021. View Article : Google Scholar
|
|
133
|
Mohamad NV, Ima-Nirwana S and Chin KY:
Self-emulsified annatto tocotrienol improves bone histomorphometric
parameters in a rat model of oestrogen deficiency through
suppression of skeletal sclerostin level and RANKL/OPG ratio. Int J
Med Sci. 18:3665–3673. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Johnson SA, Feresin RG, Soung do Y, Elam
ML and Arjmandi BH: Vitamin E suppresses ex vivo osteoclastogenesis
in ovariectomized rats. Food Funct. 7:1628–1633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shen CL, Yang S, Tomison MD, Romero AW,
Felton CK and Mo H: Tocotrienol supplementation suppressed bone
resorption and oxidative stress in postmenopausal osteopenic women:
A 12-week randomized double-blinded placebo-controlled trial.
Osteoporos Int. 29:881–891. 2018. View Article : Google Scholar
|
|
136
|
Rangel LBA, de Siqueira D, Soares ODR,
Santana HS, Miguel EC, da Cunha M, Oliveira ALA, Pedrosa DF,
Resgala LCR, Neto HAR, et al: Vitamin K supplementation modulates
bone metabolism and ultra-structure of ovariectomized mice. Cell
Physiol Biochem. 51:356–374. 2018. View Article : Google Scholar
|
|
137
|
Wang H, Zhang N, Li L, Yang P and Ma Y:
Menaquinone 4 reduces bone loss in ovariectomized mice through dual
regulation of bone remodeling. Nutrients. 13:25702021. View Article : Google Scholar :
|
|
138
|
Wasilewski GB, Vervloet MG and Schurgers
LJ: the bone-vasculature axis: Calcium supplementation and the role
of vitamin K. Front Cardiovasc Med. 6:62019. View Article : Google Scholar
|
|
139
|
Jaghsi S, Hammoud T and Haddad S: Relation
between circulating vitamin K1 and osteoporosis in the lumbar spine
in syrian post-menopausal women. Open Rheumatol J. 12:1–9. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Moore AE, Kim E, Dulnoan D, Dolan AL,
Voong K, Ahmad I, Gorska R, Harrington DJ and Hampson G: Serum
vitamin K1 (phylloquinone) is associated with fracture
risk and hip strength in post-menopausal osteoporosis: A
cross-sectional study. Bone. 141:1156302020. View Article : Google Scholar
|
|
141
|
Ma ML, Ma ZJ, He YL, Sun H, Yang B, Ruan
BJ, Zhan WD, Li SX, Dong H and Wang YX: Efficacy of vitamin K2 in
the prevention and treatment of postmenopausal osteoporosis: A
systematic review and meta-analysis of randomized controlled
trials. Front Public Health. 10:9796492022. View Article : Google Scholar :
|
|
142
|
Zhou M, Han S, Zhang W and Wu D: Efficacy
and safety of vitamin K2 for postmenopausal women with osteoporosis
at a long-term follow-up: Meta-analysis and systematic review. J
Bone Miner Metab. 40:763–772. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Pan X, Ren Z, Liang W, Dong X, Li J, Wang
L, Bhatia M, Pan LL and Sun J: Thiamine deficiency aggravates
experimental colitis in mice by promoting glycolytic reprogramming
in macrophages. Br J Pharmacol. 182:1897–1911. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Biegstraaten M, Mengel E, Maródi L,
Petakov M, Niederau C, Giraldo P, Hughes D, Mrsic M, Mehta A,
Hollak CEM and van Schaik IN: Peripheral neuropathy in adult type 1
Gaucher disease: A 2-year prospective observational study. Brain.
133:2909–2919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Al-Daghri NM, Alharbi M, Wani K,
Abd-Alrahman SH, Sheshah E and Alokail MS: Biochemical changes
correlated with blood thiamine and its phosphate esters levels in
patients with diabetes type 1 (DMT1). Int J Clin Exp Pathol.
8:13483–13488. 2015.
|
|
146
|
Al-Attas O, Al-Daghri N, Alokail M,
Abd-Alrahman S, Vinodson B and Sabico S: Metabolic benefits of
six-month thiamine supplementation in patients with and without
diabetes mellitus type 2. Clin Med Insights Endocrinol Diabetes.
7:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Al Quran T, Khader A, Allan H, Al-Momani
R, Aqel HT, Alsaleh M and Bataineh Z: Prevalence of vitamin B12
deficiency in type 2 diabetic patients taking metformin, a
cross-sectional study in primary healthcare. Front Endocrinol
(Lausanne). 14:12267982023. View Article : Google Scholar :
|
|
148
|
Ekeuku SO, Chong PN, Chan HK, Mohamed N,
Froemming GRA and Okechukwu PN: Spirulina supplementation improves
bone structural strength and stiffness in streptozocin-induced
diabetic rats. J Tradit Complement Med. 12:225–234. 2021.
View Article : Google Scholar
|
|
149
|
Toraman A, Arabaci T, Aytekin Z, Albayrak
M and Bayir Y: Effects of vitamin C local application on
ligature-induced periodontitis in diabetic rats. J Appl Oral Sci.
28:e202004442020. View Article : Google Scholar :
|
|
150
|
Mason SA, Della Gatta PA, Snow RJ, Russell
AP and Wadley GD: Ascorbic acid supplementation improves skeletal
muscle oxidative stress and insulin sensitivity in people with type
2 diabetes: Findings of a randomized controlled study. Free Radic
Biol Med. 93:227–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hao L, Lu A, Gao H, Niu J, Prabahar K,
Seraj SS and Pan Y: The effects of vitamin D on markers of glucose
and obesity in postmenopausal women: A meta-analysis of randomized
controlled trials. Clin Ther. 45:913–920. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ji Y, Geng N, Niu Y, Zhao H, Fei W, Chen S
and Ren LP: Relationship between geriatric nutritional risk index
and osteoporosis in type 2 diabetes in Northern China. BMC Endocr
Disord. 22:3082022. View Article : Google Scholar :
|
|
153
|
Zhang J, Li Y, Lai D, Lu D, Lan Z, Kang J,
Xu Y and Cai S: Vitamin D status is negatively related to insulin
resistance and bone turnover in Chinese non-osteoporosis patients
with type 2 diabetes: A retrospective cross-section research. Front
Public Health. 9:7271322022. View Article : Google Scholar :
|
|
154
|
Luo W, Jiang Y, Yi Z, Wu Y, Gong P and
Xiong Y: 1α,25-Dihydroxyvitamin D3 promotes osteogenesis
by down-regulating FGF23 in diabetic mice. J Cell Mol Med.
25:4148–4156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Jiang Y, Luo W, Wang B, Yi Z, Gong P and
Xiong Y: 1α,25-Dihydroxyvitamin D3 ameliorates diabetes-induced
bone loss by attenuating FoxO1-mediated autophagy. J Biol Chem.
296:1002872021. View Article : Google Scholar
|
|
156
|
Xiong Y, Zhang Y, Guo Y, Yuan Y, Guo Q,
Gong P and Wu Y: 1α,25-Dihydroxyvitamin D3 increases
implant osseointegration in diabetic mice partly through FoxO1
inactivation in osteoblasts. Biochem Biophys Res Commun.
494:626–633. 2017. View Article : Google Scholar
|
|
157
|
Shen CL, Kaur G, Wanders D, Sharma S,
Tomison MD, Ramalingam L, Chung E, Moustaid-Moussa N, Mo H and
Dufour JM: Annatto-extracted tocotrienols improve glucose
homeostasis and bone properties in high-fat diet-induced type 2
diabetic mice by decreasing the inflammatory response. Sci Rep.
8:113772018. View Article : Google Scholar :
|
|
158
|
Jin C, Tan K, Yao Z, Lin BH, Zhang DP,
Chen WK, Mao SM, Zhang W, Chen L, Lin Z, et al: A novel
anti-osteoporosis mechanism of VK2: Interfering with ferroptosis
via AMPK/SIRT1 pathway in type 2 diabetic osteoporosis. J Agric
Food Chem. 71:2745–2761. 2023. View Article : Google Scholar
|
|
159
|
Poon CCW, Li RWS, Seto SW, Kong SK, Ho HP,
Hoi MPM, Lee SMY, Ngai SM, Chan SW, Leung GPH and Kwan YW: In vitro
vitamin K(2) and 1α,25-dihydroxyvitamin D(3) combination enhances
osteoblasts anabolism of diabetic mice. Eur J Pharmacol. 767:30–40.
2015. View Article : Google Scholar
|
|
160
|
Carioca AAF, Verde SMM, Luzia LA, Rondó
PHC, Latorre MRDO, Ellery THP and Damasceno NRT: Association of
oxidative stress biomarkers with adiposity and clinical staging in
women with breast cancer. Eur J Clin Nutr. 69:1256–1261. 2015.
View Article : Google Scholar
|
|
161
|
Fenzl A, Kulterer OC, Spirk K, Mitulović
G, Marculescu R, Bilban M, Baumgartner-Parzer S, Kautzky-Willer A,
Kenner L, Plutzky J, et al: Intact vitamin A transport is critical
for cold-mediated adipose tissue browning and thermogenesis. Mol
Metab. 42:1010882020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Daneshzad E, Farsad-Naeimi A, Heshmati J,
Mirzaei K, Maghbooli Z and Keshavarz SA: The association between
dietary antioxidants and adipokines level among obese women.
Diabetes Metab Syndr. 13:1369–1373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Högström M, Nordström A and Nordström P:
Retinol, retinol-binding protein 4, abdominal fat mass, peak bone
mineral density, and markers of bone metabolism in men: The
northern osteoporosis and obesity (NO2) study. Eur J Endocrinol.
158:765–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
He H, Zhang Y, Sun Y, Zhang Y, Xu J, Yang
Y and Chen J: Folic acid attenuates high-fat diet-induced
osteoporosis through the AMPK signaling pathway. Front Cell Dev
Biol. 9:7918802022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Fu L, Wang Y and Hu YQ: Causal effects of
B vitamins and homocysteine on obesity and musculoskeletal
diseases: A Mendelian randomization study. Front Nutr.
9:10481222022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Jeon S, Lee J, Shin Y and Yoon M: Ascorbic
acid reduces insulin resistance and pancreatic steatosis by
regulating adipocyte hypertrophy in obese ovariectomized mice. Can
J Physiol Pharmacol. 101:294–303. 2023. View Article : Google Scholar
|
|
167
|
Rahman F, Bordignon B, Culerrier R,
Peiretti F, Spicuglia S, Djabali M, Landrier JF and Fontes M:
Ascorbic acid drives the differentiation of mesoderm-derived
embryonic stem cells. Involvement of p38 MAPK/CREB and SVCT2
transporter. Mol Nutr Food Res. 61:16005062017. View Article : Google Scholar
|
|
168
|
Alzahrani BA, Badri ZA, Aljuhani JA,
Alshamrani RM, Ahmed ME and Jari Alshumrani M: A comparison of
magnesium levels in obese versus normal-weight children. Cureus.
15:e440532023.PubMed/NCBI
|
|
169
|
Li J, Gao Y, Yu T, Lange JK, LeBoff MS,
Gorska A, Luu S, Zhou S and Glowacki J: Obesity and leptin
influence vitamin D metabolism and action in human marrow stromal
cells. J Steroid Biochem Mol Biol. 198:1055642020. View Article : Google Scholar
|
|
170
|
Xu SM, Lu K, Yang XF, Ye YW, Xu MZ, Shi Q,
Gong YQ and Li C: Association of 25-hydroxyvitamin D levels with
lipid profiles in osteoporosis patients: A retrospective
cross-sectional study. J Orthop Surg Res. 18:5972023. View Article : Google Scholar
|
|
171
|
Chin KY, Pang KL and Soelaiman IN:
Tocotrienol and its role in chronic diseases. Adv Exp Med Biol.
928:97–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ima-Nirwana S and Suhaniza S: Effects of
tocopherols and tocotrienols on body composition and bone calcium
content in adrenalectomized rats replaced with dexamethasone. J Med
Food. 7:45–51. 2004. View Article : Google Scholar
|
|
173
|
Ilich JZ: Osteosarcopenic adiposity
syndrome update and the role of associated minerals and vitamins.
Proc Nutr Soc. 80:344–355. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Kim M, Na W and Sohn C: Vitamin K1
(phylloquinone) and K2 (menaquinone-4) supplementation improves
bone formation in a high-fat diet-induced obese mice. J Clin
Biochem Nutr. 53:108–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Shi HY, Yan SM, Guo YM, Zhang BQ, Guo XY
and Shi BL: Vitamin A pretreatment protects NO-induced bovine
mammary epithelial cells from oxidative stress by modulating Nrf2
and NF-κB signaling pathways. J Anim Sci. 96:1305–1316. 2018.
View Article : Google Scholar :
|
|
176
|
Jiang WD, Zhang L, Feng L, Wu P, Liu Y,
Kuang SY, Li SW, Tang L, Mi HF, Zhang L and Zhou XQ: New insight on
the immune modulation and physical barrier protection caused by
vitamin A in fish gills infected with flavobacterium columnare.
Front Immunol. 13:8334552022. View Article : Google Scholar :
|
|
177
|
Behr GA, Schnorr CE and Moreira JCF:
Increased blood oxidative stress in experimental menopause rat
model: The effects of vitamin A low-dose supplementation upon
antioxidant status in bilateral ovariectomized rats. Fundam Clin
Pharmacol. 26:235–249. 2012. View Article : Google Scholar
|
|
178
|
Xu LL, Zhao B, Sun SL, Yu SF, Wang YM, Ji
R, Yang ZT, Ma L, Yao Y, Chen Y, et al: High-dose vitamin C
alleviates pancreatic injury via the NRF2/NQO1/HO-1 pathway in a
rat model of severe acute pancreatitis. Ann Transl Med. 8:8522020.
View Article : Google Scholar :
|
|
179
|
Ulas M and Cay M: 17β-Estradiol and
vitamin E modulates oxidative stress-induced kidney toxicity in
diabetic ovariectomized rat. Biol Trace Elem Res. 144:821–831.
2011. View Article : Google Scholar
|
|
180
|
Farshbaf-Khalili A, Ostadrahimi A,
Mirghafourvand M, Ataei-Almanghadim K, Dousti S and Iranshahi AM:
Clinical efficacy of curcumin and vitamin E on
inflammatory-oxidative stress biomarkers and primary symptoms of
menopause in healthy postmenopausal women: A triple-blind
randomized controlled trial. J Nutr Metab. 2022:63397152022.
View Article : Google Scholar :
|
|
181
|
Liu J, Tang Y, Peng B, Tian C and Geng B:
Bone mineral density is associated with composite dietary
antioxidant index among US adults: Results from NHANES. Osteoporos
Int. 34:2101–2110. 2023. View Article : Google Scholar
|
|
182
|
Zhou Q, Chen X, Chen Q and Hao L:
Independent and combined associations of dietary antioxidant intake
with bone mineral density and risk of osteoporosis among elderly
population in United States. J Orthop Sci. 29:1064–1072. 2024.
View Article : Google Scholar
|
|
183
|
Arnold M, Rajagukguk YV and
Gramza-Michałowska A: Functional food for elderly high in
antioxidant and chicken eggshell calcium to reduce the risk of
osteoporosis-A narrative review. Foods. 10:6562021. View Article : Google Scholar
|
|
184
|
Zhou P, Shao R, Wang H, Miao J and Wang X:
Dietary vitamin A, C, and E intake and subsequent fracture risk at
various sites: A meta-analysis of prospective cohort studies.
Medicine (Baltimore). 99:e208412020. View Article : Google Scholar
|
|
185
|
Zinnuroglu M, Dincel AS, Kosova F, Sepici
V and Karatas GK: Prospective evaluation of free radicals and
antioxidant activity following 6-month risedronate treatment in
patients with postmenopausal osteoporosis. Rheumatol Int.
32:875–880. 2012. View Article : Google Scholar
|
|
186
|
Scioli MG, Coniglione F, Greggi C,
Evangelista L, Fiorelli E, Savino L, Ferlosio A, Piccirilli E,
Gasbarra E, Iundusi R, et al: Ascorbic acid reduces
Ropivacaine-induced myotoxicity in cultured human osteoporotic
skeletal muscle cells. BMC Musculoskelet Disord. 24:5762023.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Deyhim F, Strong K, Deyhim N, Vandyousefi
S, Stamatikos A and Faraji B: Vitamin C reverses bone loss in an
osteopenic rat model of osteoporosis. Int J Vitam Nutr Res.
88:58–64. 2018. View Article : Google Scholar
|
|
188
|
Nojiri H, Saita Y, Morikawa D, Kobayashi
K, Tsuda C, Miyazaki T, Saito M, Marumo K, Yonezawa I, Kaneko K, et
al: Cytoplasmic superoxide causes bone fragility owing to
low-turnover osteoporosis and impaired collagen cross-linking. J
Bone Miner Res. 26:2682–2694. 2011. View Article : Google Scholar
|
|
189
|
Sangani R, Naime M, Zakhary I, Ahmad S,
Chutkan N, Zhu A, Ha Y, Hamrick M, Isales C, Elsalanty M, et al:
Regulation of vitamin C transporter in the type 1 diabetic mouse
bone and bone marrow. Exp Mol Pathol. 95:298–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Fulzele S, Chothe P, Sangani R, Chutkan N,
Hamrick M, Bhattacharyya M, Prasad PD, Zakhary I, Bowser M, Isales
C and Ganapathy VC: Sodium-dependent vitamin C transporter SVCT2:
Expression and function in bone marrow stromal cells and in
osteogenesis. Stem Cell Res. 10:36–47. 2013. View Article : Google Scholar
|
|
191
|
Massaccesi L, Ragone V, Papini N, Goi G,
Corsi Romanelli MM and Galliera E: Effects of vitamin E-stabilized
ultra high molecular weight polyethylene on oxidative stress
response and osteoimmunological response in human osteoblast. Front
Endocrinol (Lausanne). 10:2032019. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Casado-Díaz A, Túnez-Fiñana I,
Mata-Granados JM, Ruiz-Méndez MV, Dorado G, Romero-Sánchez MC,
Navarro-Valverde C and Quesada-Gómez JM: Serum from postmenopausal
women treated with a by-product of olive-oil extraction process
stimulates osteoblastogenesis and inhibits adipogenesis in human
mesenchymal stem-cells (MSC). Exp Gerontol. 90:71–78. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Casati L, Pagani F, Limonta P, Vanetti C,
Stancari G and Sibilia V: Beneficial effects of δ-tocotrienol
against oxidative stress in osteoblastic cells: Studies on the
mechanisms of action. Eur J Nutr. 59:1975–1987. 2020. View Article : Google Scholar
|
|
194
|
Quigley M, Rieger S, Capobianco E, Wang Z,
Zhao H, Hewison M and Lisse TS: Vitamin D modulation of
mitochondrial oxidative metabolism and mTOR enforces stress
adaptations and anticancer responses. JBMR Plus. 6:e105722021.
View Article : Google Scholar
|
|
195
|
Yüksek V, Dede S, Çetin S, Usta A and
Taşpınar M: Vitamin D may assist the UPR against sodium
fluoride-induced damage by reducing RIPK1, ATG5, BECN1, oxidative
stress and increasing caspase-3 in the osteoblast MC3T3-E1 cell
line. J Trace Elem Med Biol. 80:1272932023. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Xu P, Lin B, Deng X, Huang K, Zhang Y and
Wang N: VDR activation attenuates osteoblastic ferroptosis and
senescence by stimulating the Nrf2/GPX4 pathway in age-related
osteoporosis. Free Radic Biol Med. 193:720–735. 2022. View Article : Google Scholar
|
|
197
|
Ma Q, Liang M, Tang X, Luo F and Dou C:
Vitamin B5 inhibit RANKL induced osteoclastogenesis and ovariectomy
induced osteoporosis by scavenging ROS generation. Am J Transl Res.
11:5008–5018. 2019.
|
|
198
|
Bădilă AE, Rădulescu DM, Ilie A, Niculescu
AG, Grumezescu AM and Rădulescu AR: Bone regeneration and oxidative
stress: An updated overview. Antioxidants (Basel). 11:3182022.
View Article : Google Scholar
|
|
199
|
Cui Y, Zhang W, Yang P, Zhu S, Luo S and
Li M: Menaquinone-4 prevents medication-related osteonecrosis of
the jaw through the SIRT1 signaling-mediated inhibition of cellular
metabolic stresses-induced osteoblast apoptosis. Free Radic Biol
Med. 206:33–49. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Torres HM, Arnold KM, Oviedo M, Westendorf
JJ and Weaver SR: Inflammatory processes affecting bone health and
repair. Curr Osteoporos Rep. 21:842–853. 2023. View Article : Google Scholar
|
|
201
|
Moazen M, Mazloom Z, Tanideh N,
Dabbaghmanesh MH, Rahmdel S, Azarpira N and Fararouei M:
Osteoprotective effects of kefir fortified with omega-3 and vitamin
C in ovariectomized rats. Int J Vitam Nutr Res. 93:200–209. 2023.
View Article : Google Scholar
|
|
202
|
Shevchuk S, Marynych L, Malovana T and
Denyshchych L: Vitamin D level in patients with systemic lupus
erythematosus: Its relationship to disease course and bone mineral
density. Lupus Sci Med. 10:e0009682023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Shen CL, Mo H, Dunn DM and Watkins BA:
Tocotrienol Supplementation led to higher serum levels of
lysophospholipids but lower acylcarnitines in postmenopausal women:
A randomized double-blinded placebo-controlled clinical trial.
Front Nutr. 8:7667112021. View Article : Google Scholar
|
|
204
|
Liang G, Kow ASF, Tham CL, Ho YC and Lee
MT: Ameliorative effect of tocotrienols on
perimenopausal-associated osteoporosis-A review. Antioxidants
(Basel). 11:21792022. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Nazrun Shuid A, Das S and Mohamed IN:
Therapeutic effect of vitamin E in preventing bone loss: An
evidence-based review. Int J Vitam Nutr Res. 89:357–370. 2019.
View Article : Google Scholar
|
|
206
|
Jadhav N, Ajgaonkar S, Saha P, Gurav P,
Pandey A, Basudkar V, Gada Y, Panda S, Jadhav S, Mehta D and Nair
S: Molecular pathways and roles for vitamin K2-7 as a
health-beneficial nutraceutical: Challenges and opportunities.
Front Pharmacol. 13:8969202022. View Article : Google Scholar
|
|
207
|
Myneni VD and Mezey E: Immunomodulatory
effect of vitamin K2: Implications for bone health. Oral Dis.
24:67–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Guo L, Zhang Y, Liu H, Cheng Q, Yang S and
Yang D: All-trans retinoic acid inhibits the osteogenesis of
periodontal ligament stem cells by promoting IL-1β production via
NF-κB signaling. Int Immunopharmacol. 108:1087572022. View Article : Google Scholar
|
|
209
|
Ahmed N, Sammons J, Khokher MA and Hassan
HT: Retinoic acid suppresses interleukin 6 production in normal
human osteoblasts. Cytokine. 12:289–293. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Yang K, Wang X, Zhang C, Liu D and Tao L:
Metformin improves HPRT1-targeted purine metabolism and repairs
NR4A1-mediated autophagic flux by modulating FoxO1
nucleocytoplasmic shuttling to treat postmenopausal osteoporosis.
Cell Death Dis. 15:7952024. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Li Y, Hou B, Zhang Y, Wang Y, Chu Y and Li
X: In vivo quantitative assessment of proximal femoral cortical
bone microstructure using double-echo ultrashort echo time magnetic
resonance imaging. Quant Imaging Med Surg. 14:9323–9334. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
McClung MR: Sequential and long-term
therapy for osteoporosis. Curr Osteoporos Rep. 23:152025.
View Article : Google Scholar
|
|
213
|
Li P, Zhu Z, Wang Y, Zhang X, Yang C, Zhu
Y, Zhou Z, Chao Y, Long Y, Gao Y, et al: Substrate transport and
drug interaction of human thiamine transporters SLC19A2/A3. Nat
Commun. 15:109242024. View Article : Google Scholar
|
|
214
|
Mydlárová Blaščáková M, Lőrinczová Z,
Anderková L, Czerwińska-Ledwig O, Mikulová Ľ, Hrušovská H,
Jędrzejkiewicz B and Piotrowska A: Relationship between vitamin D
Receptor gene bsmi polymorphism and 25-hydroxyvitamin D total
levels in slovak postmenopausal women with reduced bone mineral
density. Genes (Basel). 16:3372025. View Article : Google Scholar
|
|
215
|
Wu Z, Ye Q, Zhang S, Hu LP, Wang XQ, Yao
LL, Zhu L, Xiao SY, Duan ZH, Zhang XL, et al: Vitamin K-dependent
gamma-carboxyglutamic acid protein 1 promotes pancreatic ductal
adenocarcinoma progression through stabilizing oncoprotein KRAS and
tyrosine kinase receptor EGFR. Clin Transl Med. 15:e701912025.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Rupp T, Butscheidt S, Vettorazzi E, Oheim
R, Barvencik F, Amling M and Rolvien T: High FGF23 levels are
associated with impaired trabecular bone microarchitecture in
patients with osteoporosis. Osteoporos Int. 30:1655–1662. 2019.
View Article : Google Scholar
|
|
217
|
Cui A, Xiao P, Fan Z, Zeng Y, Wang H and
Zhuang Y: Associations between vitamin E status and bone mineral
density in children and adolescents aged 8-19 years: Evidence based
on NHANES 2005-2006, 2017-2018. PLoS One. 18:e02831272023.
View Article : Google Scholar
|
|
218
|
Orhadje E, Berg K, Hauser B and Ralston
SH: Clinical features, incidence and treatment outcome in
pregnancy-associated osteoporosis: A single-centre experience over
two decades. Calcif Tissue Int. 113:591–596. 2023. View Article : Google Scholar :
|
|
219
|
Li H, Wang C, Jin Y, Cai Y, Sun H and Liu
M: The integrative analysis of competitive endogenous RNA
regulatory networks in osteoporosis. Sci Rep. 12:95492022.
View Article : Google Scholar :
|
|
220
|
Li S, Liu F, Zheng K, Wang W, Qiu E, Pei
Y, Wang S, Zhang J and Zhang X: CircDOCK1 promotes the
tumorigenesis and cisplatin resistance of osteogenic sarcoma via
the miR-339-3p/IGF1R axis. Mol Cancer. 20:1612021. View Article : Google Scholar
|
|
221
|
Guan Y, Zhang W, Mao Y and Li S:
Nanoparticles and bone microenvironment: A comprehensive review for
malignant bone tumor diagnosis and treatment. Mol Cancer.
23:2462024. View Article : Google Scholar :
|
|
222
|
Zhu X and Li S: Nanomaterials in tumor
immunotherapy: New strategies and challenges. Mol Cancer.
22:942023. View Article : Google Scholar
|
|
223
|
Chen Z, Yue Z, Yang K and Li S:
Nanomaterials: Small particles show huge possibilities for cancer
immunotherapy. J Nanobiotechnology. 20:4842022. View Article : Google Scholar : PubMed/NCBI
|