Introduction
Stroke represents a critical neurological emergency
characterized by sudden cessation of brain perfusion, leading to
rapid-onset neuronal degeneration (1,2).
Clinically, it manifests in two distinct forms: Ischemic stroke
(cerebral infarction due to thrombotic or embolic vessel
obstruction) and hemorrhagic stroke (parenchymal or subarachnoid
bleeding from cerebrovascular rupture). Established risk factors
include hypertension, smoking, advanced age, diabetes, obesity,
dyslipidemia, atrial fibrillation and genetic predisposition
(3). As a major global public
health burden, stroke affects ~15 million individuals annually
(4). It ranks as the second
leading cause of mortality worldwide, responsible for 11.8% of
total mortalities (5-8). Stroke is also a major cause of
long-term disabilities, including paralysis, speech difficulties
and memory loss (9,10). While stroke is more common in
men, women are more likely to succumb to it (11). Additionally, the risk factors for
stroke may vary depending on ethnicity, with African-Americans and
Hispanic-Americans having a greater incidence of stroke than
White-Americans (5). Currently,
stroke prevention and treatment options are limited, and there is
an ongoing search for more effective drugs.
Interleukin (IL)-27, a member of the IL-12 cytokine
family (12), is a pleiotropic
immunomodulator that has demonstrated context-dependent dual
functionality, exhibiting both pro-inflammatory and
anti-inflammatory properties (13). Emerging evidence has revealed its
neuroprotective potential in neurological disorders, including
multiple sclerosis and Alzheimer's disease models, where IL-27
attenuates neuroinflammation and ameliorates neuronal injury
(14,15). Notably, preclinical studies on
both ischemic and hemorrhagic stroke have consistently highlighted
IL-27's critical involvement in neuroimmune regulation and
cerebroprotection (16,17). Supporting these observations,
Martha et al (18)
proposed IL-27 as a potential biomarker for predicting post-stroke
infarct volume and cerebral edema formation in patients with acute
ischemic stroke. Collectively, these findings position IL-27 as a
key regulator in maintaining central nervous system (CNS) immune
homeostasis, with dual capabilities in neurodegeneration prevention
and neural tissue repair.
Despite the demonstrated therapeutic potential of
IL-27 in stroke treatment, substantial challenges remain for
translating these findings into clinical practice. Research in this
field must address two fundamental questions: i) How to ensure
consistent therapeutic effects and ii) how to identify optimal
delivery approaches. The present review i) summarizes the current
knowledge on stroke-related inflammatory pathways; ii) introduces
IL-27's immunoregulatory functions; and iii) summarizes recent
progress in IL-27-related stroke research. Furthermore, the
promising application of engineered exosomes as delivery systems
for IL-27 is explored, and both the obstacles and future
perspectives for developing IL-27 into an effective stroke therapy
are discussed.
Stroke and inflammation
Ischemic and hemorrhagic stroke
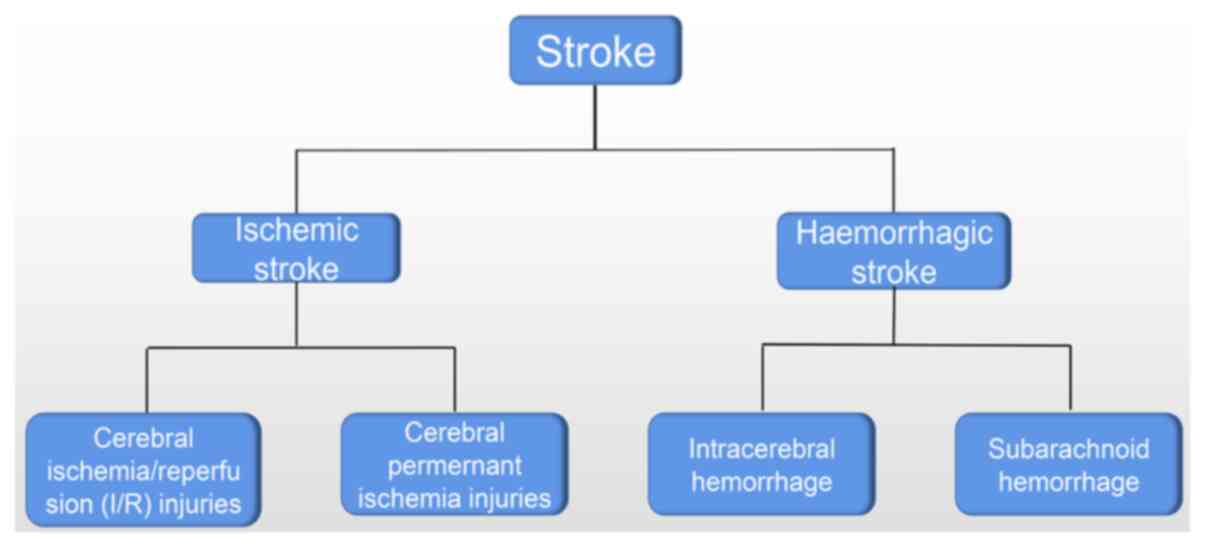
Ischemic and hemorrhagic stroke are the two primary
types of stroke, each with distinct pathogenesis and clinical
presentations (Fig. 1). From a
pathophysiological perspective, ischemic stroke occurs when
cerebral blood flow is compromised due to arterial occlusion or
stenosis, resulting in cerebral ischemia (10). By contrast, hemorrhagic stroke
develops from cerebrovascular rupture that causes intracranial
bleeding (1). These stroke
subtypes also exhibit distinct temporal progression patterns:
Ischemic stroke typically manifests with progressive symptom onset,
while hemorrhagic stroke presents abruptly. Neuroimaging serves as
the diagnostic cornerstone for differentiating between these two
cerebrovascular events. Ischemic stroke typically involves areas of
cerebral infarction, indicating the presence of tissue damage due
to inadequate blood flow. By contrast, patients with hemorrhagic
stroke exhibit cerebral hemorrhage or hematoma, highlighting the
presence of bleeding within the brain. Complications also vary
between the two types of stroke. Hemorrhagic stroke can give rise
to complications such as increased intracranial pressure, cerebral
edema and rebleeding. These complications are relatively rare in
patients with ischemic stroke (19,20).
There are several commonalities between ischemic
stroke and hemorrhagic stroke (21). First, both types of stroke
present with similar symptoms, such as the sudden onset of a
headache, impaired consciousness, limb paralysis and speech
impairment. These symptoms serve as key indicators of neurological
deficits. Second, both stroke types share several strategies for
prevention and treatment. In the acute phase, anticoagulation
therapy is often employed to prevent further clot formation or
bleeding. Additionally, controlling blood pressure and blood
glucose levels is crucial for managing the aftermath of both types
of stroke. Rehabilitation, including physical and speech therapy,
is also a common approach to aid in the recovery of motor skills
and language abilities. These shared pathophysiological features
underscore the necessity for an integrated, multidisciplinary
strategy encompassing prevention, acute intervention and long-term
rehabilitation in managing both ischemic and hemorrhagic stroke
(19,20).
Commonalities and differences in
inflammation among patients with stroke
Both types of stroke trigger an inflammatory
response as part of the body's natural defense mechanism. When a
stroke occurs, the body responds by sending immune cells to the
damaged area to clear out the damaged tissue and begin the healing
process (22-24). Inflammation is a natural response
to this injury, but it can cause further damage to brain tissue
through the release of harmful chemicals and enzymes. These
pathophysiological processes may elevate thrombotic risk and induce
vasoconstriction, thereby compromising cerebral perfusion.
Furthermore, inflammatory cascades promote fibrotic scar formation
(25), which impedes post-stroke
neuroplasticity and functional recovery. While inflammation
modulation constitutes a critical therapeutic target in stroke
management, the inflammatory profiles exhibit both shared and
distinct characteristics between ischemic and hemorrhagic stroke
subtypes.
Both stroke subtypes share a common inflammatory
cascade characterized by early activation of resident immune cells
(microglia and astrocytes), which secrete proinflammatory
mediators, including cytokines and chemokines (26). These signaling molecules recruit
circulating leukocytes to the injury site, notably neutrophils,
monocytes and various T cell subsets (27). Neutrophils exacerbate tissue
damage through the release of reactive oxygen species and
proteolytic enzymes (28).
Monocytes subsequently differentiate into macrophage populations
with distinct functional phenotypes (29).
The inflammatory responses in different stroke
subtypes diverge in their primary origins. In ischemic stroke,
inflammation predominantly stems from the ischemic core, where
hypoperfusion-induced tissue necrosis releases damage-associated
molecular patterns that activate the innate immune system (30). By contrast, in hemorrhagic
stroke, blood in the brain parenchyma acts as an irritant and
directly triggers inflammation (23). Second, the duration of
inflammation is not the same. In ischemic stroke, the inflammatory
response occurs in two phases: An acute phase, which starts
immediately after the onset of ischemia, and a delayed or secondary
phase, which occurs hours to days later. In hemorrhagic stroke, the
inflammatory response is more acute and immediate, as bleeding into
the brain tissue rapidly triggers the release of inflammatory
molecules. Third, inflammatory cells have different activation
patterns. Ischemic stroke is characterized by infiltrating immune
cells, such as neutrophils and monocytes, into ischemic brain
tissue (24). Hemorrhagic stroke
elicits a distinct inflammatory response characterized by
substantial erythrocyte extravasation and hemoglobin release. These
blood components drive neuroinflammation through two primary
mechanisms: Iron-mediated oxidative stress and direct activation of
resident and infiltrating immune cells (31).
Macrophage polarization
Macrophages play a pivotal role in orchestrating
inflammatory responses (32),
with their functional polarization between classically activated
(M1) and alternatively activated (M2) phenotypes being crucial for
immune homeostasis (33,34). M1 macrophages, characterized by
their proinflammatory properties, mediate inflammatory initiation
and perpetuation through the secretion of cytokines, including
TNF-α, IL-1β and IL-6. These cells perform essential immunological
functions such as microbial phagocytosis, pathogen elimination and
cellular debris clearance. Current therapeutic strategies for
stroke emphasize modulating excessive M1-mediated neuroinflammation
to prevent secondary neurological damage, reflecting the
established clinical consensus.
M2 macrophages represent an alternatively activated
phenotype that mediates tissue regeneration and inflammatory
resolution. These immunoregulatory cells secrete anti-inflammatory
cytokines, including IL-10 and TGF-β, while promoting key
reparative processes such as extracellular matrix remodeling,
neovascularization and efferocytosis. Their polarization is
primarily driven by IL-4 and IL-13 secreted from T helper (Th)2
lymphocytes, eosinophils and basophils (35). Emerging evidence has demonstrated
that M2 polarization significantly attenuates cerebral infarct
volume and enhances neurological recovery in stroke models
(36). Therapeutic induction of
M2 phenotype can be accomplished through multiple strategies,
including pharmacological interventions (statins and minocycline)
or adoptive transfer of M2-polarized macrophages (37-41). Given their neuroprotective and
reparative properties, targeted modulation of macrophage
polarization toward the M2 phenotype offers considerable
therapeutic potential for stroke treatment.
The aforementioned description illustrates the
characterization of inflammation in both types of stroke. Although
inflammation in the acute phase causes cellular damage, it is also
crucial for cellular debris removal. Modulating the duration of the
M1 and M2 phases is crucial to prevent prolonged damage and to
promote nerve repair and regeneration.
IL-27 and inflammation
IL-27 and its receptor
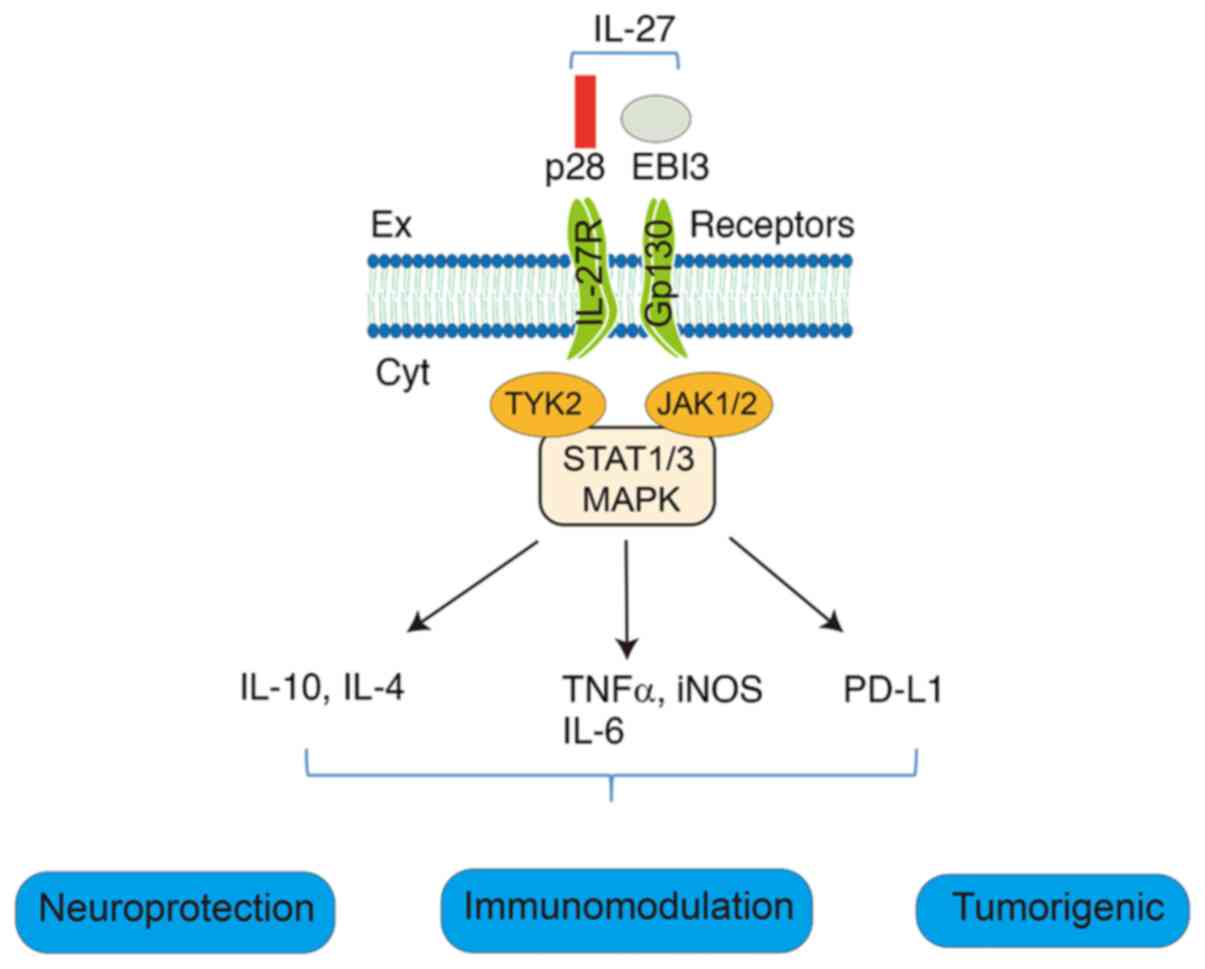
IL-27 is a heterodimeric cytokine composed of p28
and EBI3 subunits that belongs to the IL-6/IL-12 superfamily
(42). This immunoregulatory
cytokine serves as a crucial modulator of both the adaptive and
innate immune responses. Produced primarily by activated
antigen-presenting cells, particularly macrophages and dendritic
cells, IL-27 exerts pleiotropic effects on multiple lymphocyte
populations, including natural killer (NK), B and T cells (43,44).
The IL-27 receptor is a heterodimeric complex
composed of two distinct subunits: Glycoprotein 130 (gp130) and
IL-27 receptor α (IL-27Rα) (45-47). IL-27Rα serves as the
ligand-specific binding component, while gp130 functions as a
shared signal-transducing subunit utilized by multiple cytokines
(46). The IL-27Rα subunit
demonstrates high-affinity binding to IL-27, initiating downstream
signaling upon ligand engagement. Following IL-27 binding, IL-27Rα
recruits gp130 to form a functional receptor complex. This
activated receptor triggers multiple intracellular signaling
cascades, most notably the PI3K-AKT, MAPK and JAK-STAT pathways,
ultimately regulating diverse biological processes, including
immune responses and inflammatory reactions (48-50) (Fig. 2).
IL-27 and immunomodulation
IL-27 has context-dependent immunomodulatory
properties, exhibiting both pro-inflammatory and anti-inflammatory
effects based on the specific immune microenvironment (51,52). The present study focused
particularly on IL-27's regulatory effects on macrophage
polarization. In vitro analyses have demonstrated that IL-27
treatment upregulates M1 macrophage markers, including nitric oxide
synthase 2 and IFN-γ (53).
Notably, in a murine inflammatory bowel disease model, while
colonic epithelial cells and intact crypts showed no response to
IL-27, tissue-resident macrophages displayed significant reactivity
(53). Mechanistic studies have
revealed that IL-27 enhances macrophage antiviral activity via
phosphorylated STAT1 activation (54,55). In a separate acute lung
injury-sepsis model, adipose-derived mesenchymal stem cell (ADMSC)
exosomes attenuated inflammation and its sequelae, an effect that
was paradoxically reversed by recombinant IL-27 administration
(56). Further in vitro
experiments showed that ADMSC-derived exosomes suppressed
LPS-induced IL-27 secretion from macrophages (56,57). Notably, a comparative cellular
study revealed species-specific responses: IL-27 exerted minimal
effects on murine macrophages but significantly modulated human
macrophages through STAT1/TLR pathway activation, resulting in
decreased IL-10 production and pro-inflammatory activation. This
response was rapidly terminated by LPS via p38-mediated inhibition
of IL-27 signaling (57).
Beyond its role in promoting M1 polarization, IL-27
is capable of downregulating characteristic M2 markers (IL-10 and
arginase-1) while inhibiting the secretion of anti-inflammatory
cytokines (IL-13 and IL-4) (57-60). However, these observations
primarily stem from single-factor experimental systems. In
physiological contexts, macrophage polarization is dynamically
regulated through complex interactions among multiple mediators. A
previous study by Rückerl et al (61) revealed that IL-4, IL-10 and IL-27
collectively orchestrated macrophage activation via sequential
upregulation of WSX-1 and IL-4Rα receptors on alternatively
activated macrophages.
Notably, IL-27 exhibits distinct immunomodulatory
properties in inflammatory microenvironments following tissue
injury. In a murine peritonitis model, IL-27 administration
significantly reduced peritoneal fluid concentrations of the
chemokines MCP-1/CCL2, KC/CXCL1 and MIP-1α/CCL3, while
simultaneously inhibiting neutrophil mobilization from bone marrow,
ultimately attenuating innate immune-mediated inflammation
(62). In a previous study on a
combined alcohol-burn injury model, gut-specific IL-27
downregulation could be therapeutically reversed, with exogenous
IL-27 promoting intestinal epithelial proliferation, suppressing
proinflammatory cytokines and enhancing IL-10 production, thus
collectively restoring intestinal barrier homeostasis (63). Further mechanistic insights
emerged from a rat spondyloarthritis study, where recombinant IL-27
ameliorated disease progression by selectively inhibiting
IL-17/TNF-producing CD4+ T cells (64). This finding was corroborated by
cellular coculture experiments showing IL-27's dual capacity to
suppress Th17 responses (reducing IL-17 production) while promoting
regulatory responses (enhancing IL-10 secretion) in conventional
dendritic cell-CD4+ T cell interactions. These
collective findings highlight the therapeutic potential of IL-27 as
a multifunctional immunomodulator capable of resolving inflammation
across diverse pathological contexts.
IL-27 generally exhibits pleiotropic effects on T
cell subsets, promoting CD8+ T and Th1 cell activation,
while facilitating the differentiation of type 1 regulatory and
follicular helper T cells. Conversely, it suppresses the functional
responses of regulatory T (Treg), Th17, Th9 and Th2 cell
populations (44,65-70). However, existing literature
presents some contradictory findings regarding these
immunomodulatory effects (71-73). The precise mechanisms underlying
IL-27-mediated T cell regulation in post-stroke conditions remain
to be fully elucidated, as T cell differentiation dynamics are
influenced by microenvironmental cues. This knowledge gap
highlights the need for further mechanistic investigations to
elucidate the context-dependent actions of IL-27 in stroke
pathophysiology.
IL-27 and stroke
Post-stroke neuroinflammatory responses trigger
significant upregulation of IL-27 expression in the brain
parenchyma, as demonstrated in multiple experimental stroke models
(74-76). Clinical evidence further supports
the diagnostic potential of IL-27, with serum IL-27α levels showing
promise as a predictive biomarker for ischemic stroke occurrence
(77). Notably, in patients
undergoing emergency endovascular treatment for large vessel
occlusion, circulating IL-27 levels were found to correlate with
both infarct volume and development of post-ischemic cerebral edema
(18). These findings
collectively suggest that IL-27 may serve as both a
pathophysiological mediator and clinically relevant biomarker in
cerebrovascular events.
While existing studies have established that brain
injury induces IL-27 upregulation, conclusive evidence for its
neuroprotective effects remained elusive until recent preclinical
investigations. Emerging data from animal models strongly support
IL-27's therapeutic potential in stroke management. In cerebral
ischemia-reperfusion models, IL-27 administration demonstrated
neuroprotection by activating the gp130/STAT3 signaling pathway to
attenuate neuronal apoptosis (16) and modulating cytokine balance
through downregulation of proinflammatory mediators (TNF-α, IL-1β
and MCP-1), while upregulating anti-inflammatory factors (IL-10 and
TGF-β). Notably, in rodent models of hemorrhagic stroke, elevated
IL-27 levels were observed in both CNS and peripheral circulation
following brain injury (17).
Mechanistic studies revealed that IL-27 exerted its beneficial
effects through regulating bone marrow neutrophil maturation,
suppressing the production of proinflammatory and cytotoxic
substances, and promoting the expression of iron-chelating
molecules critical for hematoma resolution (17). These multifaceted actions
collectively contribute to reduced cerebral edema, enhanced
hematoma clearance and improved neurological outcomes (16,17).
Beyond its implications in stroke, emerging evidence
underscores IL-27's neuroprotective and regenerative capacities
across various neurological disorders. In multiple sclerosis, IL-27
exhibits dual anti-inflammatory and immunomodulatory effects by
suppressing Th17 cell differentiation while promoting Treg cell
development, positioning it as both a neuroprotective agent and
therapeutic candidate (78,79). Conversely, reduced IL-27 levels
in patients with Parkinson's disease are associated with
neuroinflammation and cognitive decline, although its precise
pathogenic role requires further investigation (80). Experimental studies on autoimmune
encephalomyelitis have revealed that intrathecal delivery of
IL-27-expressing lentiviral vectors attenuates neuroinflammation
through two distinct mechanisms: i) Suppressing GM-CSF production
in CD4+ T cells; and ii) upregulating PD-L1 expression
in both CNS-resident and infiltrating myeloid cells (81,82). Retinal degeneration models have
further demonstrated IL-27's protective effects, showing increased
arginase-1+ neuroprotective microglia in the
photoreceptor layer alongside elevated IL-27 levels, which
collectively enhance photoreceptor survival (83). Additional findings have indicated
that IL-27 reduces photoreceptor apoptosis while decreasing retinal
concentrations of proinflammatory mediators (IL-12, IL-18 and
CCL22) (84). Furthermore,
vascular regeneration plays a crucial role in poststroke recovery.
Notably, IL-27 promotes the endothelial differentiation of cardiac
stem cells (85), suggesting
potential angiogenic properties. However, direct evidence for
IL-27-mediated cerebrovascular regeneration post-stroke remains to
be established. Collectively, these studies highlight IL-27's
multifaceted role in stroke pathophysiology, encompassing
immunomodulation, neuroprotection and tissue repair. Future
research should focus on elucidating the molecular mechanisms
underlying these diverse functions of IL-27 in order to fully
exploit its therapeutic potential in stroke management.
Recent preclinical studies have established the
therapeutic potential of IL-27 in stroke management. Luo et
al (16) demonstrated that
intraperitoneal administration of recombinant mouse IL-27 (10
µg/kg/day), initiated within 30 min post-middle cerebral
artery occlusion (MCAO) and continued daily for 7 days,
significantly attenuated ischemia-reperfusion injury. Similarly,
Zhao et al (17) reported
that intravenous IL-27 treatment (50 ng/kg) initiated 30 min after
intracerebral hemorrhage (ICH), followed by subcutaneous
administration for 6 consecutive days, improved functional outcomes
in ICH models. Emerging evidence has suggested that IL-27 exhibits
synergistic effects when combined with other therapeutic
modalities. Particularly promising are combination therapies with
immune checkpoint inhibitors (anti-PD-1/PD-L1), vaccine-based
approaches and vitamin D supplementation (86-88). These findings suggest that
combinatorial treatment strategies incorporating IL-27 may
represent a novel therapeutic paradigm for stroke. Future clinical
translation should focus on optimizing these combination protocols
to maximize neuroprotection while minimizing potential side
effects.
IL-27 and PANoptosis
PANoptosis is a newly identified mode of programmed
cell death that activates a series of cellular death processes,
such as pyroptosis, apoptosis and necroptosis, initiating potent
inflammatory responses after ischemic stroke (89,90). Prior studies demonstrated that
activating the TLR4/NF-ĸB signaling pathway induces multiple
pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6
(91,92). These pro-inflammatory cytokines
trigger cell death by binding to their respective receptors and
activating downstream signal transduction (93). Caspase-8 is a PANoptosome
component scaffold that regulates the three cell death pathways
(pyroptosis, apoptosis and necroptosis) (94). Earlier reports have indicated
that the binding of TNF-α to TNFR1 activates caspase-8 and triggers
cell death (95). As
aforementioned, IL-27 can attenuate inflammation after stroke.
Thus, based on the TLR4/NF-ĸB/TNF-α pathway, future studies focused
on inflammation-PANoptosis are needed to investigate how IL-27
improves post-stroke.
IL-27 and mitophagy
Ischemic stroke is usually accompanied by
neuroinflammation, which is related to unsuitable activation of
Nod-like receptor protein 3 (NLRP3) (96). Research has demonstrated that
reactive oxygen species (ROS) play a key role in exacerbating the
expression of NLRP3 inflammasome, which triggers an inflammatory
response that can promote neuronal damage post-stroke.
Mitochondrial autophagy, termed mitophagy, involves removing
disrupted mitochondria and maintaining cellular homeostasis
(97). Upon ischemic injury,
mitochondria dysfunction leads to the production of plenty amounts
of ROS and reduced clearance. As several studies have reported,
obstruction of the mitophagy process results in the insufficient
degrading of damaged mitochondria and facilitates the onset of
neuroinflammation (98-101). Recent studies found that
PTEN-induced kinase 1 and Parkin regulate mitophagy, which is
important in attenuating inflammation by cleaning damaged
mitochondria (98,99). Sufficient mitophagy will decrease
the amounts of pro-inflammatory cytokines, which are the crucial
mediators of neuroinflammation after cerebral ischemia (100,101). The previous findings indicated
that IL-27 effectively inhibits the inflammatory response by
modulating the ROS/NF-ĸB/NLRP3 axis in lung carcinogenesis
(102). However, IL-27 mediates
mitophagy and inhibits inflammation following cerebral ischemia
remains uncovered.
Blood-brain barrier (BBB) and engineered
exosome delivery strategies
The therapeutic application of IL-27 faces marked
limitations due to the BBB, a highly selective vascular interface
that protects the CNS. This specialized structure, composed of
endothelial tight junctions, astrocytic end-feet and pericytes,
forms a formidable neurovascular unit that actively excludes
neurotoxic compounds and pathogens, regulates nutrient and
metabolite transport, and restricts the penetration of ~98% of
small-molecule drugs (103).
While essential for CNS homeostasis, the BBB's restrictive
properties substantially impair drug delivery to brain parenchyma,
particularly for protein-based therapeutics such as IL-27,
ultimately compromising their potential clinical efficacy in
neurological disorders. Various approaches have been proposed to
overcome the challenge of BBB penetration for protein drugs,
including brain-targeted delivery systems, transporter inhibitors,
brain penetration enhancement technologies and gene therapy
(104,105). However, these solutions have
their own limitations and associated risks. Consequently, further
research and exploration are warranted to address the issue of BBB
penetration by IL-27.
Exosomes, which are nanoscale extracellular vesicles
(measuring 30-150 nm) actively secreted by cells, serve as pivotal
mediators of intercellular communication and participate in diverse
pathophysiological processes (33). In stroke therapeutics,
exosome-based drug delivery has emerged as a particularly promising
strategy, demonstrating substantial translational potential in
preclinical studies (106-108). Notably, stem cell-derived
exosomes have shown remarkable therapeutic efficacy across multiple
stroke subtypes through several synergistic mechanisms, including
immunomodulation of both innate and adaptive immune responses,
reduction of neuroinflammation and oxidative stress, inhibition of
apoptotic pathways, secretion of neurotrophic factors, and
promotion of angiogenesis and neurogenesis (109-112). These multifaceted actions
collectively contribute to neural repair and functional recovery
following cerebrovascular injury.
Beyond their inherent therapeutic properties,
exosomes have garnered notable attention as natural nanoscale drug
delivery vehicles. Engineered exosomes are particularly promising
for CNS-targeted therapy due to their following properties: i)
Overcoming the BBB through receptor-mediated transcytosis; ii)
enhancing drug accumulation at lesion sites via active targeting;
and iii) reducing off-target effects through their biocompatible
nature. This targeted delivery approach markedly improves the
therapeutic index by maximizing local drug concentrations while
minimizing systemic exposure and associated toxicity. Notably,
immune cell-derived exosomes (particularly those from macrophages,
neutrophils and NK cells) exhibit ideal characteristics for
brain-targeted delivery, since their membrane proteins retain
native chemotactic properties that facilitate precise homing to
inflammatory lesions, efficient BBB penetration and selective
cellular uptake. These intrinsic targeting capabilities make immune
cell exosomes superior candidates for neurological applications
compared to synthetic delivery systems (113-116).
Recent advances in exosome engineering have focused
on precision targeting through surface protein modification to
enhance therapeutic delivery. In a murine model of ischemic stroke,
cyclo(RGDyK) peptide-functionalized exosomes derived from bone
marrow mesenchymal stem cells significantly improved the targeted
delivery of curcumin to ischemic regions, thus potentiating its
anti-inflammatory and anti-apoptotic effects (117). Similarly, AS1411
aptamer-conjugated macrophage-derived exosomes have demonstrated
enhanced BBB penetration capability, markedly improving the
precision and efficacy of sonodynamic therapy in glioblastoma
treatment (118). Emerging
targeting strategies have further expanded the repertoire of
exosome-based CNS delivery systems, including neuropilin-1-targeted
peptides for vascular targeting, Angiopep-2 peptides for
low-density lipoprotein receptor-related protein-1-mediated
transport, T7 peptide-modified chimeric antigen receptors for
selective neuronal uptake, transferrin receptor-targeting
approaches for receptor-mediated transcytosis and ANG-TRP-PK1
peptide sequences for enhanced parenchymal penetration. These
engineering approaches collectively represent remarkable progress
in overcoming the BBB limitation for neurological therapeutics
(115,119-122).
As IL-27 is a protein, it can also be transported to
the site of injury via engineered exosomes (123). This approach allows synergistic
harnessing of the therapeutic potential of both exosomes and IL-27.
Although exosome delivery holds great promise in the realm of
stroke treatment, its practical application remains primarily
confined to laboratory investigations and clinical trials. Further
research endeavors are imperative to ascertain the safety, efficacy
and optimal methodologies for its implementation.
Exosomes, nanoparticles and cells: Pros
and cons in delivery
Exosomes, nanoparticles and engineered cells stand
out as the main approaches for targeted brain delivery, each with
strengths and weaknesses (124). Nanoparticles excel with their
potential for large-scale manufacturing and customizable surface
modifications, enabling the fine-tuning of size and shape to
enhance drug delivery efficacy. They also offer precise control
over the release kinetics of medications, accommodating a broad
spectrum of drugs, from hydrophilic to lipophilic. However,
nanoparticle-based therapies face several limitations, including
rapid clearance by the immune system, potential immunogenicity and
toxicity, and difficulties in achieving tissue- or cell-specific
targeting (125). Consequently,
regulatory oversight for nanomedicine is stringent, underscoring
the need for meticulous safety and efficacy assessments.
Engineered cells represent a highly promising drug
delivery platform, particularly induced pluripotent stem cells and
other stem cell types such as totipotent stem cells, unipotent stem
cells (126). These modified
cells function as sophisticated therapeutic systems, capable of
serving as 'living drug factories' that provide sustained
therapeutic molecule production while responding to specific
microenvironmental cues for controlled drug release. However, this
approach has its challenges. The intricacies of cellular
manipulation and genetic engineering can be daunting and engender
ethical considerations. The host's physiological environment may
compromise the cells' survival, proliferation and efficacy
post-implantation, and there is the ever-present risk of triggering
immune reactions or being eliminated by the immune system (127). Thus, despite their vast
potential, the path to clinical realization for engineered cells is
fraught with considerable complexity.
Beyond the aforementioned methods, exosomes stand
out as a potent candidate for drug delivery. These engineered
cellular systems exhibit several therapeutic advantages, including
precise tissue targeting capability, low systemic toxicity
profiles, diminished immunogenic potential and the capacity to
overcome critical biological barriers such as the BBB (128). Additionally, they can transport
various bioactive molecules, including proteins, lipids, RNA and
DNA. The innate targeting abilities of exosomes, particularly those
originating from specific cells, can be significantly amplified
through surface modifications, employing techniques of genetic
engineering or chemical approaches. However, this delivery method
has its challenges. The intricate process of exosome isolation and
purification demands considerable resources and sophistication, and
their capacity to carry drugs is modest compared with that of other
delivery systems (129).
Additionally, the state of the donor cells can influence the
biogenesis and secretion of exosomes. These complexities highlight
the challenges that must be addressed to fully realize the clinical
potential of exosomes.
From a translational perspective, while nanoparticle
and cellular therapies have reached relative maturity, exosomes
exhibit superior safety profiles and targeting precision.
Consequently, exosomes may be considered as the optimal delivery
vehicle for IL-27. The following section will focus on the
strategic implementation of exosome-based delivery systems.
Challenges and limitations of using IL-27 as
a drug
Cost
Despite the numerous benefits of IL-27 as a
protein-based therapeutic agent, it is crucial to acknowledge its
associated limitations. Foremost among these is the substantial
cost associated with protein-based medications, including
production, transportation and preservation expenses.
Safety concerns and toxicities
IL-27 is considered to be a cytokine, and, in
previous research, it did not cause significant toxicity or tissue
damage (130). The therapeutic
potential of IL-27 is further supported by its demonstrated low
toxicity in animal models, which may be attributed to its limited
induction of IFN in vivo (131). While IL-27 itself exhibits
excellent tolerability with minimal toxicity, recombinant protein
formulations may potentially trigger immune responses, leading to
allergic reactions and impaired immune tolerance. Recent studies
have explored adeno-associated virus (AAV) vectors for IL-27
delivery, demonstrating several advantages (86,132). First, AAV-IL-27 effectively
suppresses tumor cell proliferation while maintaining low toxicity.
Second, this approach significantly reduces Tregs without inducing
autoimmunity. Third, AAV-IL-27 can be administered either locally
or systemically while retaining its biological activity (86,87). These findings suggest that
optimizing AAV vector delivery systems and incorporating chemical
modifications to reduce immunogenicity may enhance both safety and
efficacy, thereby expanding therapeutic applications.
Stability
Protein-based therapeutics are characterized by
their relatively short plasma half-life and susceptibility to
enzymatic degradation in vivo, often requiring repeated
administration to maintain clinically effective concentrations.
Therefore, it is imperative to explore methodologies that can
prolong the half-life of protein drugs and attenuate their release
rate. Presently, PEGylation stands out as the predominant technique
by enhancing the stability of protein drugs and extending their
circulation period via augmenting their additional volume (133). Another viable approach involves
modifying the Fc region of protein drugs to extend their half-life
within the body (134).
Furthermore, refining the structure of protein drugs through
alterations in their amino acid sequence or the incorporation of
stabilizers can increase their stability. These strategies can be
implemented individually or in conjunction, thereby enhancing
therapeutic effectiveness and diminishing the need for frequent
dosing.
Tumorigenic and antitumor properties
The multifaceted nature of IL-27 in immunomodulation
has led to controversy over its tumor-suppressive and
tumor-promoting activities (135). The majority of previous studies
support that IL-27 exhibits antitumor effects by inducing
tumor-specific Th1 and cytotoxic T lymphocyte responses, and
directly impeding tumor cell proliferation, survival, invasion and
angiogenesis (87,88). However, contradictory findings
indicate that IL-27 upregulates the transcription factor nuclear
factor IL-3 regulated, which synergizes with T-bet to enhance Tim-3
expression. Previous studies have demonstrated that IL-27 signaling
is essential to generate Tim-3+ exhausted T cells and to
facilitate tumor progression. These observations suggest that
IL-27-mediated induction of immune checkpoint molecules, including
PD-L1 and Tim-3, may promote tumor immune evasion (136,137). Notably, the recent development
of SRF388, an IL-27-targeting monoclonal antibody, represents a
remarkable therapeutic advance. By blocking IL-27 receptor
engagement, SRF388 enhances antitumor immunity within the tumor
microenvironment (138). SRF388
has progressed to phase II clinical trials, indicating a promising
avenue for therapeutic intervention. These advancements underscore
the need of comprehensively assessing the application scenarios and
contraindications of IL-27 before considering its utilization as a
therapeutic agent for stroke treatment.
Pro-inflammatory and anti-inflammatory
properties
IL-27 exhibits a dual role in immune regulation
(139). Initially characterized
as a pro-inflammatory cytokine (140,141), IL-27 exhibits canonical
inflammatory features, including: i) Signal transduction primarily
through the JAK/STAT and MAPK/ERK pathways (141,142); ii) production during effector
immune responses (137,143); iii) structural similarity to
other pro-inflammatory cytokines (IL-35, IL-23, IL-12 and IL-6)
(144,145); and iv) capacity to promote T
lymphocyte proliferation and activate NK cells (146). The pro-inflammatory mechanism
involves sequential events. Binding to the WSX-1 receptor subunit
induces rapid STAT3 phosphorylation within 30 min (144), subsequently driving the
production of CXCL-10, which is a key mediator of monocyte-derived
pro-inflammatory cytokine release (135,143). Conversely, IL-27 also possesses
well-documented anti-inflammatory properties that are
context-dependent, including the suppression of pro-inflammatory
cytokines (TNF-α, IFN-γ, IL-17 and IL-21) and the concurrent
upregulation of immunoregulatory IL-10 production (135,139). While current stroke research
predominantly focuses on IL-27's anti-inflammatory effects,
emerging evidence have revealed that neutrophils recruited during
the pro-inflammatory phase may paradoxically contribute to
neuroprotection and axonal regeneration (17,147). These findings highlight the
need for further investigation into the complex, biphasic
mechanisms of IL-27 in post-stroke recovery.
Limitations of animal models of ischemic
stroke
Human stroke comprises ~85% ischemic and ~10%
hemorrhagic cases (148).
Current preclinical research utilizes several animal models to
study ischemic stroke pathogenesis. Global ischemia models,
typically induced by permanent vertebral artery ligation and/or
transient bilateral common carotid artery occlusion, present
several limitations, including technically challenging surgical
procedures, frequent seizure induction and lower clinical relevance
compared to that of focal ischemia models (149).
Focal ischemia models, particularly MCAO, are
superior in replicating human stroke pathophysiology by showing a
distinct ischemic penumbra analogous to human stroke, large
reproducible infarct volumes and realistic ischemia-reperfusion
injury dynamics. However, MCAO models exhibit notable differences
from human stroke, including frequent hypothalamic damage (which is
rare in humans), inability to simulate thrombolytic reperfusion
hemodynamics and infarct volumes (21-45% of ipsilateral hemisphere)
that model malignant infarction (39% in humans) rather than typical
stroke (4-14% hemisphere involvement) (149,150).
The photothrombotic stroke model offers distinct
advantages for investigating cellular and molecular mechanisms of
neurodegeneration, neuroprotection and neuroregeneration. However,
this model presents several limitations when compared to human
ischemic stroke pathology, including pronounced edema formation,
minimal penumbral region and inability to replicate
ischemia-reperfusion dynamics (149).
The thromboembolic model represents another approach
to studying transient focal cerebral ischemia. This technique
involves arterial occlusion through either exogenous thrombin
administration or injection of macrospheres/microspheres into the
internal carotid artery. While clinically relevant to human stroke
mechanisms, this model suffers from poor reproducibility in terms
of infarct location, size and ischemic duration (149).
Collectively, these experimental models contribute
valuable insights into stroke pathophysiology. However, the
translational gap remains notable, with numerous therapeutics
demonstrating efficacy in animal models but failing to show
comparable benefits in human clinical trials. This discrepancy
likely derives from two key factors: i) Oversimplified
pathophysiology of current animal models compared to the complexity
of human stroke and ii) inadequate preclinical evaluation methods
that poorly predict clinical outcomes. Addressing these limitations
through improved model systems and more rigorous translational
paradigms represents a critical need in stroke research.
Potential therapeutic mechanisms
The recently recognized heterodimeric member of the
IL-6/IL-12 family of cytokines (151), IL-27, exhibits potent antitumor
effects in multiple tumor models and independence from toxicity in
preclinical trials (130).
Emerging evidence suggests that IL-6 can stimulate neurogenesis and
influence neural differentiation under stress conditions (152). Similarly, IL-27 may potentially
play a similar role in post-stroke neurogenesis. Current research
indicates that neuroinflammation and iron dyshomeostasis contribute
to neuronal death in various CNS disorders (153). Due to its established
anti-inflammatory properties, IL-27 may modulate the expression of
key iron regulatory proteins (hepcidin, ferroportin-1 and divalent
metal transporter 1), thereby reducing iron accumulation and
preventing ferroptosis in neural cells. In the context of ischemic
stroke pathogenesis, cerebrovascular occlusion or stenosis
represents the primary pathological event. Notably, while IL-27 has
been shown to promote tumor cell proliferation, survival and
angiogenesis (51), these same
properties could be therapeutically harnessed to enhance cerebral
angiogenesis and improve perfusion in ischemic brain regions. This
dual functionality positions IL-27 as a promising candidate for
developing novel stroke treatment strategies targeting both
neuroprotection and vascular repair.
The exact time window for using IL-27 as a
therapeutic agent remains contradictory. A study indicated that
IL-27 was injected within 30 min after the MCAO operation,
resulting in IL-27 ameliorating the neurological function, reducing
neuron death, upregulating anti-inflammatory factors and
downregulating pro-inflammatory factors (16). Similarly, the IL-27 treatment was
initiated 30 min after the hemorrhagic stroke, leading to improved
neurological outcomes (17).
Paradoxically, Furukawa et al (154) showed that mice lacking IL-27
showed reduced infarction area and suppressed inflammatory
cytokines in the acute stage of intracerebral ischemia.
Additionally, Li et al (155) reported recovery after stroke is
associated with axonal sprouting in the cortex adjacent to the
infarct. The gene expression level of IL-27 was upregulated in
sprouting neurons from the peri-infarct cortex after 7 days of
stroke (155). Li et al
(155) also demonstrated that
growth differentiation factor 10 (GDF10) was induced in sprouting
neurons during the initiation phase of axonal sprouting, 7 days
after stroke. Mice received a stroke in the forelimb motor cortex,
followed 7 days later by administration of hydrogel of GDF10, which
releases GDF10 over 2-3 weeks, stimulates axonal outgrowth, and
improves functional recovery (156). Thus, IL-27 potentially enhances
axonal sprouting and triggers neuronal growth programs at the
subacute phase. In summary, future studies are needed to explore
the accurate timing of IL-27 administration after stroke in
depth.
Conclusions
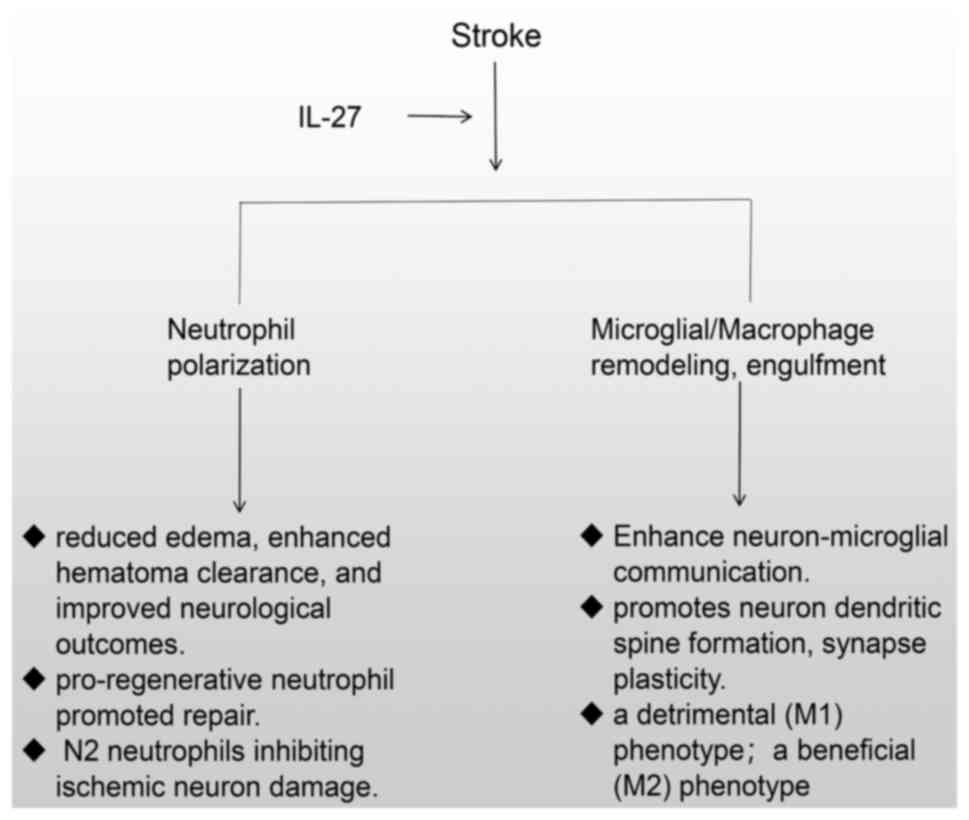
In conclusion, IL-27 upregulation following stroke
demonstrates neuroprotective potential through dual mechanisms of
inflammation suppression (via M2 polarization) and enhanced tissue
regeneration (Fig. 3). While
engineered exosomes offer promising targeted delivery capabilities,
clinical translation faces several challenges, as follows: i)
Dosage precision is critical, as the neurogenic and proliferative
effects of IL-27 may pose oncogenic risks; ii) temporal specificity
is required, since acute-phase pro-inflammatory cytokine release
(IL-6, IL-1β and TNF-α) exacerbates injury, whereas
subacute/chronic phase M1-to-M2 transition promotes angiogenesis
and neuroprotection; and iii) mechanistic uncertainties persist
regarding IL-27-mediated regulation of PANoptosis and mitophagy
post-stroke. Future research should prioritize comprehensive safety
profiling and development of clinically viable delivery systems to
optimize therapeutic outcomes.
Availability of data and materials
Not applicable.
Authors' contributions
WLiu, ZZo, ZZh and HY wrote the original draft, and
reviewed and edited the manuscript. WLi, GY and JZ reviewed and
edited the manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This work is supported by the Natural Science Foundation of
Guangxi, China (grant nos. 2021JJA141110, 2024JJH140496,
2023JJA140122 and 2020GXNSFAA29704), the Science and Technology
Plan of Jiangxi Provincial Health Commission (grant no.
SKJP220226304), the Ganzhou City Joint Plan Project of 'Science and
Technology National Regional Medical Center' (grant nos.
2022-YB1413 and GZ2024YLJ052), the Ganzhou Municipal Health
Commission Municipal Research Program Project (grant no.
G2WJV020V402072), the Guilin Innovation Platform and Talent Project
(grant no. 20210218-6), the Natural Science Foundation of China
(grant no. 82060268), Shenzhen Science and Technology Innovation
Commission (grant no. JCYJ20220530150412026), and the Shenzhen
Postdoctoral Research Grant (grant no. 50820191286).
References
|
1
|
Yamada H, Kase Y, Okano Y, Kim D, Goto M,
Takahashi S, Okano H and Toda M: Subarachnoid hemorrhage triggers
neuroinflammation of the entire cerebral cortex, leading to
neuronal cell death. Inflamm Regen. 42:612022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raskob GE, Angchaisuksiri P, Blanco AN,
Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV,
McCumber M, et al: Thrombosis: A major contributor to global
disease burden. Arterioscler Thromb Vasc Biol. 34:2363–2371. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boehme AK, Esenwa C and Elkind MSV: Stroke
risk factors, genetics, and prevention. Circ Res. 120:472–495.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feigin VL, Abajobir AA, Abate KH,
Abd-Allah F, Abdulle AM, Abera SF, Abyu GY, Ahmed MB, Aichour AN,
Aichour I, et al: Global, regional, and national burden of
neurological disorders during 1990-2015: A systematic analysis for
the global burden of disease study 2015. Lancet Neurol. 16:877–897.
2017. View Article : Google Scholar
|
|
5
|
Aldayel AY, Alharbi MM, Shadid AM and
Zevallos JC: The association between race/ethnicity and the
prevalence of stroke among United States adults in 2015: A
secondary analysis study using behavioural risk factor surveillance
system (BRFSS). Electron Physician. 9:5871–5876. 2017. View Article : Google Scholar
|
|
6
|
Katan M and Luft A: Global burden of
stroke. Semin Neurol. 38:208–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Virani SS, Alonso A, Aparicio HJ, Benjamin
EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng
S, Delling FN, et al: Heart disease and stroke statistics-2021
update: A report from the american heart association. Circulation.
143:e254–e743. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsao CW, Aday AW, Almarzooq ZI, Alonso A,
Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP,
Commodore-Mensah Y, et al: Heart disease and stroke statistics-2022
update: A report from the American heart association. Circulation.
145:e153–e639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Li X and Wang H: Application of
action observation therapy in stroke rehabilitation: A systematic
review. Brain Behav. 13:e31572023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campbell BCV and Khatri P: Stroke. Lancet.
396:129–142. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Virani SS, Alonso A, Benjamin EJ,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Delling FN, et al: Heart disease and stroke
statistics-2020 update: A report from the American heart
association. Circulation. 141:e139–e596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun L, He C, Nair L, Yeung J and Egwuagu
CE: Interleukin 12 (IL-12) family cytokines: Role in immune
pathogenesis and treatment of CNS autoimmune disease. Cytokine.
75:249–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwasaki Y, Fujio K, Okamura T and Yamamoto
K: Interleukin-27 in T cell immunity. Int J Mol Sci. 16:2851–2863.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nortey AN, Garces KN and Hackam AS:
Exploring the role of interleukin-27 as a regulator of neuronal
survival in central nervous system diseases. Neural Regen Res.
17:2149–2152. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sénécal V, Deblois G, Beauseigle D,
Schneider R, Brandenburg J, Newcombe J, Moore CS, Prat A, Antel J
and Arbour N: Production of IL-27 in multiple sclerosis lesions by
astrocytes and myeloid cells: Modulation of local immune responses.
Glia. 64:553–569. 2016. View Article : Google Scholar
|
|
16
|
Luo C, Li B, Chen L, Zhao L and Wei Y:
IL-27 protects the brain from ischemia-reperfusion injury via the
gp130/STAT3 signaling pathway. J Mol Neurosci. 71:1838–1848. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao X, Ting S, Liu C, Sun G, Kruzel M,
Roy-O'Reilly M and Aronowski J: Neutrophil polarization by IL-27 as
a therapeutic target for intracerebral hemorrhage. Nat Commun.
8:6022017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martha SR, Cheng Q, Fraser JF, Gong L,
Collier LA, Davis SM, Lukins D, Alhajeri A, Grupke S and
Pennypacker KR: Expression of cytokines and chemokines as
predictors of stroke outcomes in acute ischemic stroke. Front
Neurol. 10:13912020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Montaño A, Hanley DF and Hemphill JC III:
Hemorrhagic stroke. Handb Clin Neurol. 176:229–248. 2021.
View Article : Google Scholar
|
|
20
|
Feske SK: Ischemic stroke. Am J Med.
134:1457–1464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hilkens NA, Casolla B, Leung TW and de
Leeuw FE: Stroke. Lancet. 403:2820–2836. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng F, Qiu J, Chen H, Shi X, Yin M, Zhu M
and Yang G: Dietary supplementation with N-3 polyunsaturated fatty
acid-enriched fish oil promotes wound healing after ultraviolet
B-induced sunburn in mice. Food Sci Nutr. 9:3693–3700. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohashi SN, DeLong JH, Kozberg MG,
Mazur-Hart DJ, van Veluw SJ, Alkayed NJ and Sansing LH: Role of
inflammatory processes in hemorrhagic stroke. Stroke. 54:605–619.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Couch C, Mallah K, Borucki DM, Bonilha HS
and Tomlinson S: State of the science in inflammation and stroke
recovery: A systematic review. Ann Phys Rehabil Med. 65:1015462022.
View Article : Google Scholar :
|
|
25
|
Yang G, Chen H, Chen Q, Qiu J, Qahar M,
Fan Z, Chu W, Tredget EE and Wu Y: Injury-induced interleukin-1
alpha promotes Lgr5 hair follicle stem cells de novo regeneration
and proliferation via regulating regenerative microenvironment in
mice. Inflamm Regen. 43:142023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Z, Lou Y, Hao Y, Li H, Feng J and
Liu S: The relationship of astrocytes and microglia with different
stages of ischemic stroke. Curr Neuropharmacol. 21:2465–2480. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jayaraj RL, Azimullah S, Beiram R, Jalal
FY and Rosenberg GA: Neuroinflammation: Friend and foe for ischemic
stroke. J Neuroinflammation. 16:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Orellana-Urzúa S, Rojas I, Líbano L and
Rodrigo R: Pathophysiology of ischemic stroke: Role of oxidative
stress. Curr Pharm Des. 26:4246–4260. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu M, Zhu M, Wu X, Xu M, Fan K, Wang J,
Zhang L, Yin M, Wu J, Zhu Z and Yang G: Porcine acellular dermal
matrix increases fat survival rate after fat grafting in nude mice.
Aesthetic Plast Surg. 45:2426–2436. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton
MT and Shi FD: Global brain inflammation in stroke. Lancet Neurol.
18:1058–1066. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yip S and Sastry BR: Effects of hemoglobin
and its breakdown products on synaptic transmission in rat
hippocampal CA1 neurons. Brain Res. 864:1–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hammer A, Yang G, Friedrich J, Kovacs A,
Lee DH, Grave K, Jörg S, Alenina N, Grosch J, Winkler J, et al:
Role of the receptor Mas in macrophage-mediated inflammation in
vivo. Proc Natl Acad Sci USA. 113:14109–14114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang G, Waheed S, Wang C, Shekh M, Li Z
and Wu J: Exosomes and their bioengineering strategies in the
cutaneous wound healing and related complications: Current
knowledge and future perspectives. Int J Biol Sci. 19:1430–1454.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang G, Tan L, Yao H, Xiong Z, Wu J and
Huang X: Long-term effects of severe burns on the kidneys: Research
advances and potential therapeutic approaches. J Inflamm Res.
16:1905–1921. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai
M, Ji X, Leak RK, Gao Y, Chen J and Hu M: Interleukin-4 is
essential for microglia/macrophage M2 polarization and long-term
recovery after cerebral ischemia. Stroke. 47:498–504. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Salayandia VM, Thompson JF, Yang
LY, Estrada EY and Yang Y: Attenuation of acute stroke injury in
rat brain by minocycline promotes blood-brain barrier remodeling
and alternative microglia/macrophage activation during recovery. J
Neuroinflammation. 12:262015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ge R, Tornero D, Hirota M, Monni E,
Laterza C, Lindvall O and Kokaia Z: Choroid plexus-cerebrospinal
fluid route for monocyte-derived macrophages after stroke. J
Neuroinflammation. 14:1532017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Amantea D and Bagetta G: Drug repurposing
for immune modulation in acute ischemic stroke. Curr Opin
Pharmacol. 26:124–130. 2016. View Article : Google Scholar
|
|
40
|
Zi L, Zhou W, Xu J, Li J, Li N, Xu J, You
C, Wang C and Tian M: Rosuvastatin nanomicelles target
neuroinflammation and improve neurological deficit in a mouse model
of intracerebral hemorrhage. Int J Nanomedicine. 16:2933–2947.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chernykh ER, Shevela EY, Starostina NM,
Morozov SA, Davydova MN, Menyaeva EV and Ostanin AA: Safety and
therapeutic potential of M2-macrophages in stroke treatment. Cell
Transplant. 25:1461–1471. 2016. View Article : Google Scholar
|
|
42
|
Jin Y, Fyfe PK, Gardner S, Wilmes S,
Bubeck D and Moraga I: Structural insights into the assembly and
activation of the IL-27 signaling complex. EMBO Rep. 23:e554502022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida H and Hunter CA: The immunobiology
of interleukin-27. Annu Rev Immunol. 33:417–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Q and Liu J: Regulation and immune
function of IL-27. Adv Exp Med Biol. 941:191–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schneider R, Yaneva T, Beauseigle D,
El-Khoury L and Arbour N: IL-27 increases the proliferation and
effector functions of human naïve CD8+ T lymphocytes and promotes
their development into Tc1 cells. Eur J Immunol. 41:47–59. 2011.
View Article : Google Scholar
|
|
46
|
Hibi M, Murakami M, Saito M, Hirano T,
Taga T and Kishimoto T: Molecular cloning and expression of an IL-6
signal transducer, gp130. Cell. 63:1149–1157. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pflanz S, Hibbert L, Mattson J, Rosales R,
Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R
and Kastelein RA: WSX-1 and glycoprotein 130 constitute a
signal-transducing receptor for IL-27. J Immunol. 172:2225–2231.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wan X, Zhang Y, Tang H, Li M, Jiang T, He
J, Bao C, Wang J, Song Y, Xiao P, et al: IL-27 signaling negatively
regulates FcεRI-mediated mast cell activation and allergic
response. J Leukoc Biol. 112:411–424. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han L, Chen Z, Yu K, Yan J, Li T, Ba X,
Lin W, Huang Y, Shen P, Huang Y, et al: Interleukin 27 signaling in
rheumatoid arthritis patients: Good or evil? Front Immunol.
12:7872522022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Valdés-López JF, Fernandez GJ and
Urcuqui-Inchima S: Synergistic effects of toll-like receptor 1/2
and toll-like receptor 3 signaling triggering interleukin 27 gene
expression in chikungunya virus-infected macrophages. Front Cell
Dev Biol. 10:8121102022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Beizavi Z, Zohouri M, Asadipour M and
Ghaderi A: IL-27, a pleiotropic cytokine for fine-tuning the immune
response in cancer. Int Rev Immunol. 40:319–329. 2021. View Article : Google Scholar
|
|
52
|
Frangieh M, McHenry A, Phillips R, Ye C,
Bernier A, Laffel L, Elyaman W and Bradshaw EM: IL-27: An
endogenous constitutive repressor of human monocytes. Clin Immunol.
217:1084982020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Andrews C, McLean MH, Hixon JA, Pontejo
SM, Starr T, Malo C, Cam M, Ridnour L, Hickman H, Steele-Mortimer
O, et al: IL-27 induces an IFN-like signature in murine macrophages
which in turn modulate colonic epithelium. Front Immunol.
14:10218242023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Frank AC, Zhang X, Katsounas A, Bharucha
JP, Kottilil S and Imamichi T: Interleukin-27, an anti-HIV-1
cytokine, inhibits replication of hepatitis C virus. J Interferon
Cytokine Res. 30:427–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Imamichi T, Yang J, Huang DW, Brann TW,
Fullmer BA, Adelsberger JW, Lempicki RA, Baseler MW and Lane HC:
IL-27, a novel anti-HIV cytokine, activates multiple
interferon-inducible genes in macrophages. AIDS. 22:39–45. 2008.
View Article : Google Scholar
|
|
56
|
Wang X, Liu D, Zhang X, Yang L, Xia Z and
Zhang Q: Exosomes from adipose-derived mesenchymal stem cells
alleviate sepsis-induced lung injury in mice by inhibiting the
secretion of IL-27 in macrophages. Cell Death Discov. 8:182022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kalliolias GD and Ivashkiv LB: IL-27
activates human monocytes via STAT1 and suppresses IL-10 production
but the inflammatory functions of IL-27 are abrogated by TLRs and
p38. J Immunol. 180:6325–6333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin H, Lin D, Xiong XS, Dai XX and Lin T:
Expression and regulation of interleukin-9 in chronic
rhinosinusitis. Am J Rhinol Allergy. 29:e18–e23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dong Z, Lu X, Yang Y, Zhang T, Li Y, Chai
Y, Lei W, Li C, Ai L and Tai W: IL-27 alleviates the
bleomycin-induced pulmonary fibrosis by regulating the Th17 cell
differentiation. BMC Pulm Med. 15:132015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jirmo AC, Daluege K, Happle C, Albrecht M,
Dittrich AM, Busse M, Habener A, Skuljec J and Hansen G: IL-27 is
essential for suppression of experimental allergic asthma by the
TLR7/8 agonist R848 (resiquimod). J Immunol. 197:4219–4227. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rückerl D, Heßmann M, Yoshimoto T, Ehlers
S and Hölscher C: Alternatively activated macrophages express the
IL-27 receptor alpha chain WSX-1. Immunobiology. 211:427–436. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Watzlawick R, Kenngott EE, Liu FD, Schwab
JM and Hamann A: Anti-inflammatory effects of IL-27 in
zymosan-induced peritonitis: Inhibition of neutrophil recruitment
partially explained by impaired mobilization from bone marrow and
reduced chemokine levels. PLoS One. 10:e01376512015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Luck ME, Li X, Herrnreiter CJ, Cannon AR
and Choudhry MA: IL-27 promotes intestinal barrier integrity
following ethanol intoxication and burn injury. ImmunoHorizons.
6:600–613. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jouhault Q, Cherqaoui B, Jobart-Malfait A,
Glatigny S, Lauraine M, Hulot A, Morelle G, Hagege B, Ermoza K, El
Marjou A, et al: Interleukin 27 is a novel cytokine with
anti-inflammatory effects against spondyloarthritis through the
suppression of Th17 responses. Front Immunol. 13:10724202023.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zou X, Zhang Y, Wang S, Wang X, Yang W and
Li Y: Attenuate ICOSL and IL-27 in Aire-overexpressing DC2.4 cells
suppress TFH cell differentiation. Immunobiology. 226:1521472021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pan L, Wang J, Liu J, Guo L and Yang S:
Deficiency in the frequency and function of Tr1 cells in IgAV and
the possible role of IL-27. Rheumatology (Oxford). 60:3432–3442.
2021. View Article : Google Scholar
|
|
67
|
Gerhardt L, Hong MMY, Yousefi Y, Figueredo
R and Maleki Vareki S: IL-12 and IL-27 promote CD39 expression on
CD8+ T cells and differentially regulate the CD39+CD8+ T cell
phenotype. J Immunol. 210:1598–1606. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mei Y, Ran Y, Liu Z, Zhou Y, He J, Yin N
and Qi H: IL-27 mediates Th1 cells infiltration in fetal membranes
in preterm labor. Reprod Sci. 29:1764–1775. 2022. View Article : Google Scholar
|
|
69
|
Cox JH, Kljavin NM, Ramamoorthi N, Diehl
L, Batten M and Ghilardi N: IL-27 promotes T cell-dependent colitis
through multiple mechanisms. J Exp Med. 208:115–123. 2011.
View Article : Google Scholar :
|
|
70
|
Chen X, Deng R, Chi W, Hua X, Lu F, Bian
F, Gao N, Li Z, Pflugfelder SC, de Paiva CS and Li DQ: IL-27
signaling deficiency develops Th17-enhanced Th2-dominant
inflammation in murine allergic conjunctivitis model. Allergy.
74:910–921. 2019. View Article : Google Scholar
|
|
71
|
Gong H, Ma S, Chen J, Yang B, Liu S, Liu
X, Han J, Wu X, Lei L, Yin Z, et al: Dendritic cell-derived IL-27
p28 regulates T cell program in pathogenicity and alleviates acute
graft-versus-host disease. Signal Transduc Target Ther. 7:3192022.
View Article : Google Scholar
|
|
72
|
Park YJ, Ryu H, Choi G, Kim BS, Hwang ES,
Kim HS and Chung Y: IL-27 confers a protumorigenic activity of
regulatory T cells via CD39. Proc Natl Acad Sci USA. 116:3106–3111.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Le HT, Keslar K, Nguyen QT, Blazar BR,
Hamilton BK and Min B: Interleukin-27 enforces regulatory T cell
functions to prevent graft-versus-host disease. Front Immunol.
11:1812020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xia C, Li XQ, Zhou ZH and Chen HS:
Identification of cytokines for early prediction of malignant
middle cerebral artery infarction. Int J Neurosci. 127:86–91. 2017.
View Article : Google Scholar
|
|
75
|
Zhou Z, Zhang J, Li X, Xia C, Han Y and
Chen H: Protein microarray analysis identifies key cytokines
associated with malignant middle cerebral artery infarction. Brain
Behav. 7:e007462017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lowe DW, Hollis BW, Wagner CL, Bass T,
Kaufman DA, Horgan MJ, Givelichian LM, Sankaran K, Yager JY,
Katikaneni LD, et al: Vitamin D insufficiency in neonatal
hypoxic-ischemic encephalopathy. Pediatr Res. 82:55–62. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lind L, Siegbahn A, Lindahl B, Stenemo M,
Sundström J and Ärnlöv J: Discovery of new risk markers for
ischemic stroke using a novel targeted proteomics chip. Stroke.
46:3340–3347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sweeney CM, Lonergan R, Basdeo SA,
Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy
N, Mills KHG and Fletcher JM: IL-27 mediates the response to IFN-β
therapy in multiple sclerosis patients by inhibiting Th17 cells.
Brain Behav Immun. 25:1170–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Meka RR, Venkatesha SH, Dudics S, Acharya
B and Moudgil KD: IL-27-induced modulation of autoimmunity and its
therapeutic potential. Autoimmun Rev. 14:1131–1141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kouchaki E, Kakhaki RD, Tamtaji OR,
Dadgostar E, Behnam M, Nikoueinejad H and Akbari H: Increased serum
levels of TNF-α and decreased serum levels of IL-27 in patients
with Parkinson disease and their correlation with disease severity.
Clin Neurol Neurosurg. 166:76–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Casella G, Finardi A, Descamps H, Colombo
F, Maiorino C, Ruffini F, Patrone M, Degano M, Martino G, Muzio L,
et al: IL-27, but not IL-35, inhibits neuroinflammation through
modulating GM-CSF expression. Sci Rep. 7:165472017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Casella G, Rasouli J, Thome R, Descamps
HC, Vattikonda A, Ishikawa L, Boehm A, Hwang D, Zhang WF, Xiao D,
et al: Interferon-γ/Interleukin-27 axis induces programmed death
ligand 1 expression in monocyte-derived dendritic cells and
restores immune tolerance in central nervous system autoimmunity.
Front Immunol. 11:5767522020. View Article : Google Scholar
|
|
83
|
Garces K, Carmy T, Illiano P, Brambilla R
and Hackam AS: Increased neuroprotective microglia and
photoreceptor survival in the retina from a peptide inhibitor of
myeloid differentiation factor 88 (MyD88). J Mol Neurosci.
70:968–980. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nortey A, Garces K, Carmy-Bennun T and
Hackam AS: The cytokine IL-27 reduces inflammation and protects
photoreceptors in a mouse model of retinal degeneration. J
Neuroinflammation. 19:2162022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tanaka T, Obana M, Mohri T, Ebara M, Otani
Y, Maeda M and Fujio Y: Interleukin-27 induces the endothelial
differentiation in Sca-1+ cardiac resident stem cells. Cytokine.
75:365–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hu A, Ding M, Zhu J, Liu JQ, Pan X,
Ghoshal K and Bai XF: Intra-tumoral delivery of IL-27 using
adeno-associated virus stimulates anti-tumor immunity and enhances
the efficacy of immunotherapy. Front Cell Dev Biol. 8:2102020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu JQ, Zhu J, Hu A, Zhang A, Yang C, Yu
J, Ghoshal K, Basu S and Bai XF: Is AAV-delivered IL-27 a potential
immunotherapeutic for cancer. Am J Cancer Res. 10:3565–3574.
2020.
|
|
88
|
Yoshimoto T, Chiba Y, Furusawa JI, Xu M,
Tsunoda R, Higuchi K and Mizoguchi I: Potential clinical
application of interleukin-27 as an antitumor agent. Cancer Sci.
106:1103–1110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tian HY, Lei YX, Zhou JT, Liu LJ, Yang T,
Zhou Y, Ge JW, Xu C and Mei ZG: Insight into interplay between
PANoptosis and autophagy: novel therapeutics in ischemic stroke.
Front Mol Neurosci. 17:14820152025. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wan H, Ban X, He Y, Yang Y, Hu X, Shang L,
Wan X, Zhang Q and Xiong K: Voltage-dependent anion channel 1
oligomerization regulates PANoptosis in retinal
ischemia-reperfusion injury. Neural Regen Res. Jan 13–2025.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tajalli-Nezhad S, Karimian M, Beyer C,
Atlasi MA and Azami Tameh AA: The regulatory role of Toll-like
receptors after ischemic stroke: Neurosteroids as TLR modulators
with the focus on TLR2/4. Cell Mol Life Sci. 76:523–537. 2019.
View Article : Google Scholar
|
|
92
|
Wu Y, Li W and Zhou C, Lu F, Gao T, Liu Y,
Cao J, Zhang Y, Zhang Y and Zhou C: Ketamine inhibits
lipopolysaccharide-induced astrocytes activation by suppressing
TLR4/NF-ĸB pathway. Cell Physiol Biochem. 30:609–617. 2012.
View Article : Google Scholar
|
|
93
|
Peltzer N and Walczak H: Cell death and
inflammation-a vital but dangerous liaison. Trends Immunol.
40:387–402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fritsch M, Günther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mandal R, Barrón JC, Kostova I, Becker S
and Strebhardt K: Caspase-8: The double-edged sword. Biochim
Biophys Acta Rev Cancer. 1873:1883572020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shi Y, Liu Q, Chen W, Wang R, Wang L, Liu
ZQ, Duan XC, Zhang Y, Shen A, Peng D, et al: Protection of Taohong
Siwu Decoction on PC12 cells injured by oxygen glucose
deprivation/reperfusion via mitophagy-NLRP3 inflammasome pathway in
vitro. J Ethnopharmacol. 301:1157842023. View Article : Google Scholar
|
|
97
|
Yang YD, Li ZX, Hu XM, Wan H, Zhang Q,
Xiao R and Xiong K: Insight into crosstalk between mitophagy and
apoptosis/necroptosis: mechanisms and clinical applications in
ischemic stroke. Curr Med Sci. 42:237–248. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang H, Ye J, Peng Y, Ma W, Chen H, Sun H,
Feng Z, He W, Li G, Chu S, et al: CKLF induces microglial
activation via triggering defective mitophagy and mitochondrial
dysfunction. Autophagy. 20:590–613. 2024. View Article : Google Scholar :
|
|
99
|
Zhao J, Qiu YK, Xie YX, Li XY, Li YB, Wu
B, Wang YW, Tian XY, Lv YL, Zhang LH, et al: Imbalance of
mitochondrial quality control regulated by STING and PINK1 affects
cyfluthrin-induced neuroinflammation. Sci Total Environ.
946:1743132024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li F, Yifei W and Zheng K: Microglial
mitophagy integrates the microbiota-gut-brain axis to restrain
neuroinflammation during neurotropic herpesvirus infection.
Autophagy. 19:734–736. 2023. View Article : Google Scholar :
|
|
101
|
Zhou X, Wang J, Yu L, Qiao G, Qin D,
Yuen-Kwan Law B, Ren F, Wu J and Wu A: Mitophagy and cGAS-STING
crosstalk in neuroinflammation. Acta Pharm Sin B. 14:3327–3361.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Majumder D, Sarkar C, Debnath R, Tribedi P
and Maiti D: Mechanistic insight into the synergism of IL-27 and
IL-28B in regulation of benzo(a)pyrene-induced lung carcinogenesis
associated ROS/NF-κB/NLRP3 crosstalk. Chem Biol Interact.
354:1098072022. View Article : Google Scholar
|
|
103
|
Persidsky Y, Ramirez SH, Haorah J and
Kanmogne GD: Blood-brain barrier: Structural components and
function under physiologic and pathologic conditions. J Neuroimmune
Pharmacol. 1:223–236. 2006. View Article : Google Scholar
|
|
104
|
Pardridge WM: A historical review of brain
drug delivery. Pharmaceutics. 14:12832022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dong X: Current strategies for brain drug
delivery. Theranostics. 8:1481–1493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Venkat P, Chopp M and Chen J: Cell-based
and exosome therapy in diabetic stroke. Stem Cells Transl Med.
7:451–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen J and Chopp M: Exosome therapy for
stroke. Stroke. 49:1083–1090. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Giovannelli L, Bari E, Jommi C, Tartara F,
Armocida D, Garbossa D, Cofano F, Torre ML and Segale L:
Mesenchymal stem cell secretome and extracellular vesicles for
neurodegenerative diseases: Risk-benefit profile and next steps for
the market access. Bioact Mater. 29:16–35. 2023.PubMed/NCBI
|
|
109
|
Yang H, Tu Z, Yang D, Hu M, Zhou L, Li Q,
Yu B and Hou S: Exosomes from hypoxic pre-treated ADSCs attenuate
acute ischemic stroke-induced brain injury via delivery of
circ-Rps5 and promote M2 microglia/macrophage polarization.
Neurosci Lett. 769:1363892022. View Article : Google Scholar
|
|
110
|
Wang C, Börger V, Mohamud Yusuf A, Tertel
T, Stambouli O, Murke F, Freund N, Kleinschnitz C, Herz J, Gunzer
M, et al: Postischemic neuroprotection associated with
anti-inflammatory effects by mesenchymal stromal cell-derived small
extracellular vesicles in aged mice. Stroke. 53:e14–e18. 2022.
View Article : Google Scholar :
|
|
111
|
Zhou L, Liang J and Xiong T: Research
progress of mesenchymal stem cell-derived exosomes on inflammatory
response after ischemic stroke. Zhejiang Da Xue Xue Bao Yi Xue Ban.
51:500–506. 2022.
|
|
112
|
Hirsch Y, Geraghty JR, Reiter CR, Katz EA,
Little CF, Tobin MK and Testai FD: Unpacking the role of
extracellular vesicles in ischemic and hemorrhagic stroke:
Pathophysiology and therapeutic implications. Transl Stroke Res.
14:146–159. 2023. View Article : Google Scholar
|
|
113
|
Huang Z, Guo L, Huang L, Shi Y, Liang J
and Zhao L: Baicalin-loaded macrophage-derived exosomes ameliorate
ischemic brain injury via the antioxidative pathway. Mater Sci Eng
C Mater Biol Appl. 126:1121232021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li F, Zhao L, Shi Y and Liang J:
Edaravone-loaded macrophage-derived exosomes enhance
neuroprotection in the rat permanent middle cerebral artery
occlusion model of stroke. Mol Pharm. 17:3192–3201. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tao B, Du R, Zhang X, Jia B, Gao Y, Zhao Y
and Liu Y: Engineering CAR-NK cell derived exosome disguised
nano-bombs for enhanced HER2 positive breast cancer brain
metastasis therapy. J Control Release. 363:692–706. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang J, Tang W, Yang M, Yin Y, Li H, Hu F,
Tang L, Ma X, Zhang Y and Wang Y: Inflammatory tumor
microenvironment responsive neutrophil exosomes-based drug delivery
system for targeted glioma therapy. Biomaterials. 273:1207842021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi
C, Huang NP, Xiao ZD, Lu ZH, Tannous BA and Gao J: Surface
functionalized exosomes as targeted drug delivery vehicles for
cerebral ischemia therapy. Biomaterials. 150:137–149. 2018.
View Article : Google Scholar
|
|
118
|
Wu T, Liu Y, Cao Y and Liu Z: Engineering
macrophage exosome disguised biodegradable nanoplatform for
enhanced sonodynamic therapy of glioblastoma. Adv Mater.
34:21103642022. View Article : Google Scholar
|
|
119
|
Liu J, Sun Y, Zeng X, Liu Y, Liu C, Zhou
Y, Liu Y, Sun G and Guo M: Engineering and characterization of an
artificial drug-carrying vesicles nanoplatform for enhanced
specifically targeted therapy of glioblastoma. Adv Mater.
35:23036602023. View Article : Google Scholar
|
|
120
|
Jia G, Han Y, An Y, Ding Y, He C, Wang X
and Tang Q: NRP-1 targeted and cargo-loaded exosomes facilitate
simultaneous imaging and therapy of glioma in vitro and in vivo.
Biomaterials. 178:302–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Li B, Chen X, Qiu W, Zhao R, Duan J, Zhang
S, Pan Z, Zhao S, Guo Q, Qi Y, et al: Synchronous disintegration of
ferroptosis defense axis via engineered exosome-conjugated magnetic
nanoparticles for glioblastoma therapy. Adv Sci (Weinh).
9:21054512022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang R, Wang X, Zhao H, Li N, Li J, Zhang
H and Di L: Targeted delivery of hybrid nanovesicles for enhanced
brain penetration to achieve synergistic therapy of glioma. J
Control Release. 365:331–347. 2024. View Article : Google Scholar
|
|
123
|
Kang M, Yadav MK, Mbanefo EC, Yu CR and
Egwuagu CE: IL-27-containing exosomes secreted by innate B-1a cells
suppress and ameliorate uveitis. Front Immunol. 14:10711622023.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ha D, Yang N and Nadithe V: Exosomes as
therapeutic drug carriers and delivery vehicles across biological
membranes: Current perspectives and future challenges. Acta Pharm
Sin B. 6:287–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Alavian F and Shams N: Oral and
intra-nasal administration of nanoparticles in the cerebral
ischemia treatment in animal experiments: Considering its
advantages and disadvantages. Curr Clin Pharmacol. 15:20–29.
2020.
|
|
126
|
Jang SF, Liu WH, Song WS, Chiang KL, Ma
HI, Kao CL and Chen MT: Nanomedicine-based neuroprotective
strategies in patient specific-iPSC and personalized medicine. Int
J Mol Sci. 15:3904–3925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Otsuka R, Wada H, Murata T and Seino KI:
Immune reaction and regulation in transplantation based on
pluripotent stem cell technology. Inflamm Regen. 40:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y
and Zheng Y: Engineered exosomes: Desirable target-tracking
characteristics for cerebrovascular and neurodegenerative disease
therapies. Theranostics. 11:8926–8944. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chen J, Li P, Zhang T, Xu Z, Huang X, Wang
R and Du L: Review on strategies and technologies for exosome
isolation and purification. Front Bioeng Biotechnol. 9:8119712022.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Murugaiyan G and Saha B: IL-27 in tumor
immunity and immunotherapy. Trends Mol Med. 19:108–116. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Oniki S, Nagai H, Horikawa T, Furukawa J,
Belladonna ML, Yoshimoto T, Hara I and Nishigori C: Interleukin-23
and interleukin-27 exert quite different antitumor and vaccine
effects on poorly immunogenic melanoma. Cancer Res. 66:6395–6404.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhu J, Liu JQ, Shi M, Cheng X, Ding M,
Zhang JC, Davis JP, Varikuti S, Satoskar AR, Lu L, et al: IL-27
gene therapy induces depletion of Tregs and enhances the efficacy
of cancer immunotherapy. JCI Insight. 3:e987452018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Jevševar S, Kunstelj M and Porekar VG:
PEGylation of therapeutic proteins. Biotechnol J. 5:113–128. 2010.
View Article : Google Scholar
|
|
134
|
Levin D, Golding B, Strome SE and Sauna
ZE: Fc fusion as a platform technology: Potential for modulating
immunogenicity. Trends Biotechnol. 33:27–34. 2015. View Article : Google Scholar
|
|
135
|
Fabbi M, Carbotti G and Ferrini S: Dual
roles of IL-27 in cancer biology and immunotherapy. Mediators
Inflamm. 2017:39580692017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gonin J, Carlotti A, Dietrich C, Audebourg
A, Radenen-Bussière B, Caignard A, Avril MF, Vacher-Lavenu MC,
Larousserie F and Devergne O: Expression of IL-27 by tumor cells in
invasive cutaneous and metastatic melanomas [corrected]. PLoS One.
8:e756942013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kourko O, Seaver K, Odoardi N, Basta S and
Gee K: IL-27, IL-30, and IL-35: A cytokine triumvirate in cancer.
Front Oncol. 9:9692019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Składanowska K, Bloch Y, Strand J, White
KF, Hua J, Aldridge D, Welin M, Logan DT, Soete A and Merceron R:
Structural basis of activation and antagonism of receptor signaling
mediated by interleukin-27. Cell Rep. 41:1114902022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Xu WD, Wang DC, Zhao M and Huang AF: An
updated advancement of bifunctional IL-27 in inflammatory
autoimmune diseases. Front Immunol. 15:13663772024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Chen Q, Ghilardi N, Wang H, Baker T, Xie
MH, Gurney A, Grewal IS and de Sauvage FJ: Development of Th1-type
immune responses requires the type I cytokine receptor TCCR.
Nature. 407:916–920. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lucas S, Ghilardi N, Li J and de Sauvage
FJ: IL-27 regulates IL-12 responsiveness of naive CD4+ T cells
through Stat1-dependent and -independent mechanisms. Proc Natl Acad
Sci USA. 100:15047–15052. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Huang D, Ran Y, Liu Z, He J, Yin N and Qi
H: IL-27 mediates pro-inflammatory effects via the ERK signaling
pathway during preterm labor. Front Immunol. 12:7092292021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Hunter CA and Kastelein R: Interleukin-27:
Balancing protective and pathological immunity. Immunity.
37:960–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Petes C, Mariani MK, Yang Y, Grandvaux N
and Gee K: Interleukin (IL)-6 inhibits IL-27- and IL-30-mediated
inflammatory responses in human monocytes. Front Immunol.
9:2562018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Olson BM, Sullivan JA and Burlingham WJ:
Interleukin 35: A key mediator of suppression and the propagation
of infectious tolerance. Front Immunol. 4:3152013. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Takeda A, Hamano S, Yamanaka A, Hanada T,
Ishibashi T, Mak TW, Yoshimura A and Yoshida H: Cutting edge: Role
of IL-27/WSX-1 signaling for induction of T-bet through activation
of STAT1 during initial Th1 commitment. J Immunol. 170:4886–4890.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sas AR, Carbajal KS, Jerome AD, Menon R,
Yoon C, Kalinski AL, Giger RJ and Segal BM: A new neutrophil subset
promotes CNS neuron survival and axon regeneration. Nat Immunol.
21:1496–1505. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Martin SS, Aday AW, Almarzooq ZI, Anderson
CAM, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ
and Boehme AK: 2024 Heart disease and stroke statistics: A report
of US and global data from the American heart association.
Circulation. 149:e347–e913. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Uzdensky AB: Photothrombotic stroke as a
model of ischemic stroke. Transl Stroke Res. 9:437–451. 2018.
View Article : Google Scholar
|
|
150
|
Fluri F, Schuhmann MK and Kleinschnitz C:
Animal models of ischemic stroke and their application in clinical
research. Drug Des Devel Ther. 9:3445–3454. 2015.PubMed/NCBI
|
|
151
|
Rose-John S: Interleukin-6 family
cytokines. Cold Spring Harb Perspect Biol. 10:a0284152018.
View Article : Google Scholar
|
|
152
|
Bongartz H, Seiß EA, Bock J and Schaper F:
Glucocorticoids attenuate interleukin-6-induced c-Fos and Egr1
expression and impair neuritogenesis in PC12 cells. J Neurochem.
157:532–549. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Urrutia P, Aguirre P, Esparza A, Tapia V,
Mena NP, Arredondo M, González-Billault C and Núñez MT:
Inflammation alters the expression of DMT1, FPN1 and hepcidin, and
it causes iron accumulation in central nervous system cells. J
Neurochem. 126:541–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Furukawa T, Miyake Y, Ito H, Ogata A,
Maeyama H, Nakahara Y, Yoshioka F, Masuoka J, Yoshida H and Abe T:
Interleukin-27 deletion has neuroprotective effects in the acute
ischemic stage of cerebral infarction. Biochem Biophys Res Commun.
755:1515812025. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Li S, Overman JJ, Katsman D, Kozlov SV,
Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH and
Carmichael ST: An age-related sprouting transcriptome provides
molecular control of axonal sprouting after stroke. Nat Neurosci.
13:1496–1504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Li S, Nie EH, Yin Y, Benowitz LI, Tung S,
Vinters HV, Bahjat FR, Stenzel-Poore MP, Kawaguchi R, Coppola G and
Carmichael ST: GDF10 is a signal for axonal sprouting and
functional recovery after stroke. Nat Neurosci. 18:1737–1745. 2015.
View Article : Google Scholar : PubMed/NCBI
|