Introduction
Lung cancer is the most newly diagnosed cancer
worldwide, comprising 12.4% (2.48 million cases) of cases and
causing 18.7% (1.82 million deaths) of cancer-associated
mortalities, and it is now the leading concern among males, with an
incidence rate of 15.3% (1.57 million cases), surpassing prostate
cancer in 2022 (1). In addition,
domestic cancer statistics in South Korea demonstrate the highest
fatality rates, with crude mortality rates of 36.8 per 100,000
individuals (18,902 deaths) in 2021, aligning with international
trends (2). While lung cancer is
traditionally classified into non-small cell lung cancer and small
cell lung cancer, recent advancements such as next-generation
sequencing (NGS) have revealed a significant heterogeneity within
the disease. This has led to the identification of various
pathological subtypes, highlighting the complexity of lung cancer
and underscoring the need for tailored treatment strategies for
individual patients (3-5).
Well-known risk factors for lung cancer include
genetic polymorphisms, tobacco smoking, diet, alcohol consumption,
chronic inflammation, exposure to ionizing radiation and
occupational exposure to substances such as asbestos, chromium
compounds, silica and diesel exhaust (6). Similar to most types of cancer,
lung cancer is recognized to result from the interplay of various
multifactorial causes. Among these factors, the microbiome has also
emerged as a topic of growing interest in research (7). In the past, it was believed that
the lung was sterile; however, recent research has uncovered a
range of commensal microbiomes, including fungi, bacteria and
viruses, all of which contribute to homeostasis (8,9).
Numerous studies have investigated the association between these
microbiomes and various lung diseases, including cancer (10). Although dysbiosis is not
exclusively associated with cancer development, it has been found
to correlate with innate immunity, suggesting potential therapeutic
implications (11-13). Therefore, the present review aims
to summarize the research on the influence of both human and
environmental microbiomes on the occurrence and progression of lung
cancer, as well as their potential clinical implications.
Association between the microbiome and lung
cancer
Human microbiome
Multiple studies have highlighted significant
associations between lung cancer and the human microbiome using
fecal, sputum, tissue and saliva samples, as summarized in Table I. The lung microbiota is shaped
by interactions with the gut and oral microbiome, as well as
external exposures (14). In
patients with lung cancer, Streptococcus was consistently found at
higher levels in sputum, tissue and saliva compared to healthy
individuals (15-17), while its abundance was
significantly reduced in fecal samples (18). Conversely,
Faecalibacterium, which is typically abundant in feces, was
found in higher levels in fecal samples from patients with lung
cancer but reduced in their saliva (17-19). Other pro-inflammatory bacteria,
such as Ruminococcus and Klebsiella, were also
elevated in fecal samples of patients with lung cancer compared to
healthy controls (18,20). By contrast, beneficial genera
such as Bifidobacterium and Bacteroides were observed
in greater abundance in fecal samples from healthy individuals
(19,21,22).
 | Table IClinical studies on microbiome genera
alteration in lung cancer compared with healthy individuals and
other lung diseases using 16S ribosomal RNA method. |
Table I
Clinical studies on microbiome genera
alteration in lung cancer compared with healthy individuals and
other lung diseases using 16S ribosomal RNA method.
| Sample | Microbiome
| (Refs.) |
|---|
| Increase | Decrease |
|---|
| Feces | Haemophilus,
Faecalibacterium | Neisseria,
Fusobacterium, Treponema, Rothia, Burkholderia, Filifactor,
Dialister, Mycoplasma, Catonella, Anaerovorax, Acholeplasma,
Bacteroides, Peptococcus, Megamonas, Bradyrhizobium, TG5 | (19) |
| Eubacterium,
Ruminococcus, Faecalibacterium | Streptococcus,
Enterococcus, Roseburia | (18) |
| Klebsiella,
Streptococcus |
Haemophilus | (20) |
|
Enterococcus |
Bifidobacterium | (21) |
|
Lachnospira | (124) |
|
Ruminococcus |
Faecalibacterium, Streptococcus,
Bifidobacterium, Veillonella | (22) |
| Sputum | Parabacteroides,
Eggerthella, Weissella | Haemophilus,
Dialister, Burkholderia, WAL_1855D, Neisseria, Bulleidia | (19) |
| Granulicatella,
Abiotrophia, Streptococcus, | Sphinogomonas,
Leptotrichia | (15) |
| Tissue |
Streptococcus |
Staphylococcus | (16) |
| Corynebacterium,
Halomonas, Lachnoanaerobaculum, | (125) |
| Acidovorax | (126) |
| Saliva | Veillonella,
Streptococcus | Fusobacterium,
Prevotella, Bacteroides, Faecalibacterium | (17) |
In addition to comparisons with healthy controls,
studies have also evaluated differences in microbiota composition
between patients with lung cancer and those with benign pulmonary
conditions or other cancer types. For instance, higher levels of
Veillonella, Megasphaera, Atopobium and
Selenomonas were reported in patients with lung cancer
compared to individuals with benign lung lesions (23). Similarly, Haemophilus
levels showed significant variability between lung cancer and
patients with esophageal squamous cell carcinoma (20). These findings suggest that the
lung microbiota may interact with bacteria originating from the
oral cavity or pharynx, further emphasizing the concept of a
gut-lung axis (14).
Despite these advances, variability in study
outcomes remains a challenge, often attributed to small sample
sizes, diverse cancer subtypes, and differences in patient
characteristics, such as age, sex and medical history. Furthermore,
discrepancies arise from variations in sequencing approaches and
taxonomic databases. For example, targeting different 16S ribosomal
RNA regions (such as V1-V3 vs. V3-V4) and using different
platforms, such as Illumina MiSeq and Roche 454, yield differing
results. Additionally, inconsistencies across databases [such as
SILVA (https://www.arb-silva.de/), Ribosomal
Database Project (RDP interface is no longer available), Greengenes
(https://greengenes2.ucsd.edu/) and
National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/)] contribute to the
variability (24-26). Nonetheless, several studies have
consistently identified Streptococcus and
Faecalibacterium as key genera associated with lung cancer,
with the former being particularly prominent (14,17).
Dysbiosis, characterized by a shift in the
microbiome that favors harmful over beneficial bacteria, plays a
central role in cancer progression. It promotes inflammation,
alters metabolic pathways and dysregulates immune responses, as
observed in studies of colorectal cancer (14,27,28). In the lung, similar mechanisms
are suspected but remain underexplored. Emerging evidence suggests
that dysbiosis of the lung microbiota may facilitate lung cancer
progression through bacterial metabolite release and activation of
inflammatory pathways (29).
Moreover, the gut-lung axis, influenced by gut microbiota such as
Lactobacillus reuteri and Clostridium, shapes immune
responses in the lungs, highlighting its potential role in lung
cancer pathogenesis (30,31).
Despite these findings, large-scale studies are needed to validate
microbial biomarkers for lung cancer, which could pave the way for
novel diagnostic and therapeutic strategies.
Environmental microbiome
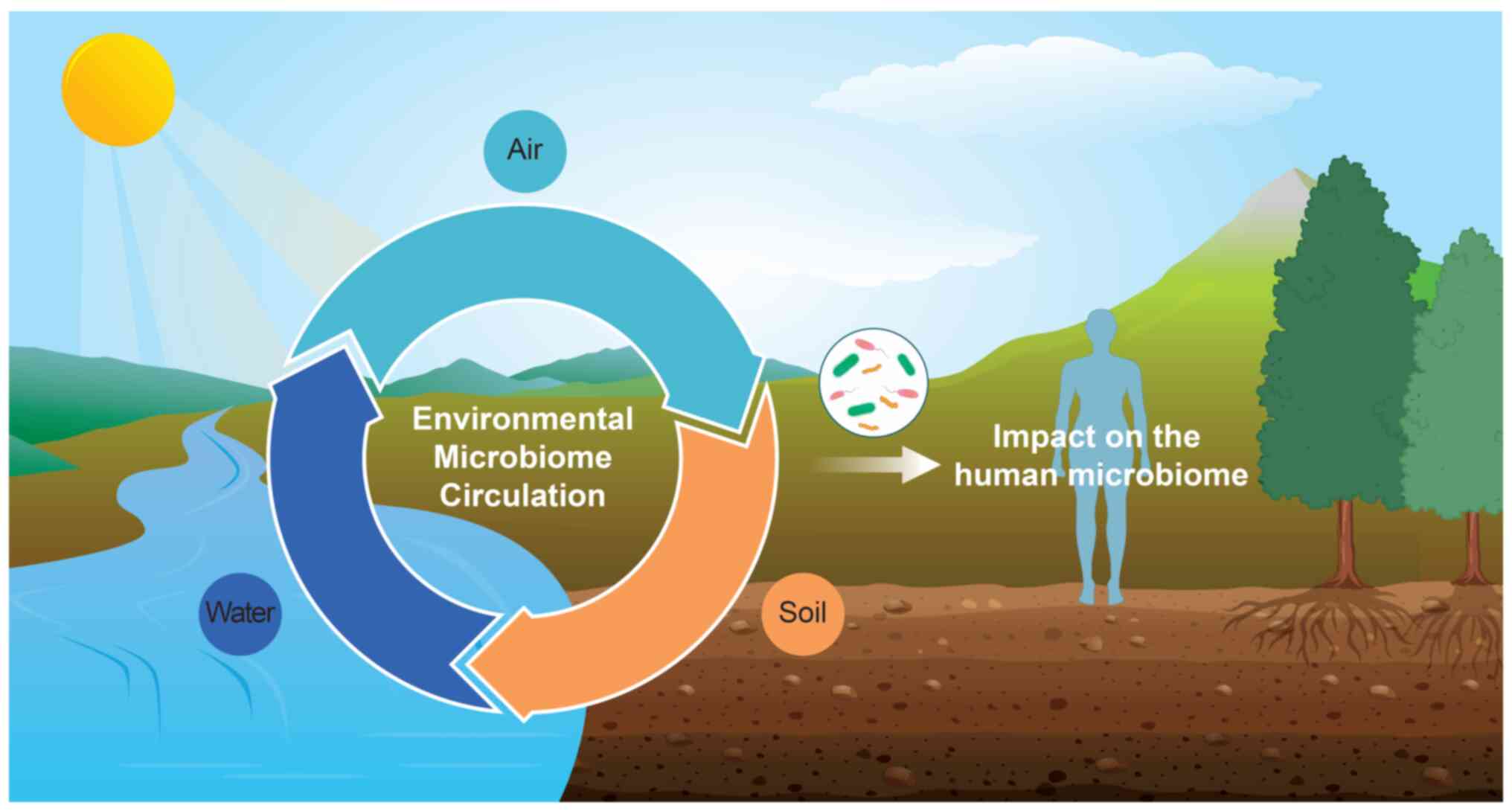
A previous study investigated the relationship
between environmental microorganisms and human health (Fig. 1). Humans are exposed to
environmental microorganisms through the air, food, soil and water,
which circulate and interact, influencing various ecological
systems (32). These microbes
can infiltrate the body via respiration, skin contact and
ingestion, integrating into the complex interactions of these
ecosystems. Subsequently, environmental microorganisms that have
penetrated the body can directly impact human health by introducing
pathogenic bacteria or indirectly by altering the human microbiome
(33,34).
In particular, pulmonary diseases are related to
exposure to airborne bacteria. Previous studies have identified
some prevalent airborne bacteria, revealing that their composition
varies depending on the characteristics of the outdoor
environments. In outdoor air, the dominant genera varied according
to meteorological conditions (32). In the indoor air of an office,
the most dominant genera were Methylobacterium,
Enterobacteriaceae_unidentified genus,
Exiguobacteirum and Bacteroides (32). Also, Shin et al (35) reported that Micrococcus,
Paracoccus, Staphylococcus and Enhydrobacter were the common genera
in indoor air of childcare facilities.
Several studies have elucidated that airborne
microbes are associated with lung diseases (36-40). Exposure to airborne microbes has
been implicated in developing and exacerbating lung diseases, such
as asthma and chronic obstructive pulmonary disease (COPD)
(36). For example,
Pseudomonas aeruginosa is common in patients with cystic
fibrosis and COPD (37). It is
known that exposure to bioaerosols, such as allergens, toxins and
pro-inflammatory agents, induces airway inflammation, leading to
respiratory symptoms (38).
Asthma and bioaerosol exposure have been found to reduce lung
function while increasing pulmonary inflammation (39). This series of lung function
decline, increased inflammation and dysregulation can contribute to
the development of lung cancer. Additionally, a study reported a
relationship between exposure to bioaerosols and the development of
specific cancers, including pancreatic, liver and lung cancer
(40). In summary, alterations
in the pulmonary micro-environment and functions, which may
contribute to the development of lung cancer, are increasingly
acknowledged; however, research into the definitive impact of the
microbiome on its pathogenesis remains limited.
Microbial extracellular vesicles as key
communication materials between the microbiome and lung cancer
Exposure to microbial extracellular
vesicles
Extracellular vehicles (EVs) are cell-to-cell
communication materials enclosed in a lipid bilayer containing
proteins, lipopolysaccharides (LPS) and nucleic acids, ranging from
20 to 200 nm in diameter. Microbial EVs (MEVs), found in all
bacteria, are known as outer membrane vesicles (OMVs) in
Gram-negative bacteria and membrane vesicles (MVs) in Gram-positive
bacteria (41,42). Gut commensal microbes secrete
MEVs, which penetrate the mucosal barrier and circulate throughout
the body, reaching organs such as the lung, liver and skeletal
muscle after oral administration (43). Additionally, dietary habits
influence the microbiome and MEV composition, impacting human
health and disease risk (44-46). For example, the microbial
diversity in the feces of individuals on habitual Western diets was
decreased compared with plant-based diets (44). Nanosized particles, including
MEVs, are absorbed through inhalation and spread to various organs.
These particles can accumulate in deep lung tissue, potentially
affecting lung function over time (47).
Epidemiological studies have linked MEVs in indoor
dust with chronic pulmonary diseases. A clinical study found that
63.6% of children with asthma had IgG1 sensitization to MEVs in
indoor dust, suggesting a role in chronic lung diseases (48). Higher levels of anti-dust EV IgG
antibodies were observed in patients with asthma, COPD and lung
cancer compared to healthy controls (49,50). In summary, while research is in
its early stages, MEVs may pose a significant risk for lung
disease, including cancer.
Pathogenesis of microbial extracellular
vesicles in the development of lung diseases
As the EV membrane is embedded with surface ligands
that interact with receptors on target cells, EVs can attach to and
modify the physiological state of recipient cells (51,52). Furthermore, MEVs have recently
been shown to be involved in the development of a wide variety of
diseases, including cancer (24,53).
MEVs in beds were found to be mainly derived from
pathogenic bacteria, such as Pseudomonas,
Acinetobacter, Enterobacter and Staphylococcus
(50). The prolonged exposure to
MEVs in inhaled indoor dust induces significant airway
inflammation, leading to severe asthma-like responses as well as
emphysema. The induction of emphysema is of particular concern, as
it is known to be a major factor in the development of irreversible
airway obstruction (48). The
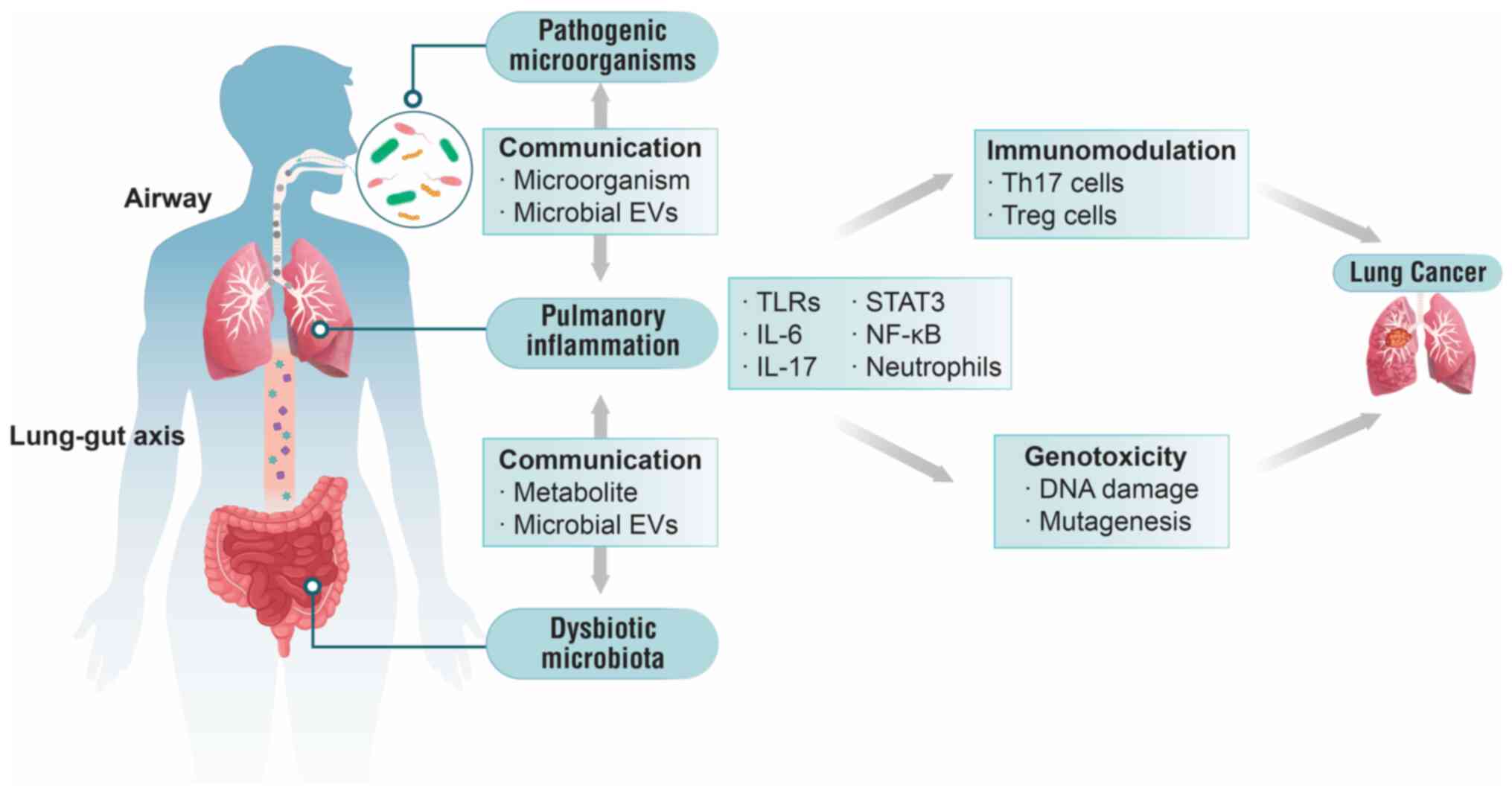
exposure to MEVs during respiration and dysbiosis of gut microbiota
constitute two primary pathophysiological mechanisms-the airway and
gut-lung axis-contributing to disease development (Fig. 2).
When the parent cell is an extracellular
gram-negative bacterium, OMVs induce T helper (Th)17 responses,
leading to neutrophilic inflammation via the release of IL-17. This
inflammation often causes airway hyperreactivity, fibrosis and
conditions such as asthma and COPD, which may elevate the risk of
lung cancer (54). A previous
study has shown that OMVs from Escherichia coli trigger
IL-17A-dependent neutrophilic inflammation and emphysema in mice,
accompanied by elastase upregulation (55). Intraperitoneal injection of E.
coli EVs induces lung dysfunction and mortality (56). Similarly, P. aeruginosa
EVs exacerbate pulmonary inflammation through Toll-like receptor
(TLR)2 and TLR4 activation, elevating in the chemokines (CXCL1 and
C-C motif ligand 2) and the cytokines (IL-1β, TNF-α, IL-6 and
IFN-γ), alongside neutrophil and macrophage infiltration (37). Moreover, indoor dust, including
various bacterial components, has been associated with both Th1 and
Th17 responses, leading to the induction of neutrophilic pulmonary
inflammation (48,57). By contrast, MVs from
intracellular Gram-positive bacteria primarily induce Th1
polarization via IFN-γ, leading to mononuclear inflammation and
alveolar elastase production, which may cause emphysema (54). Although research on MVs has not
been as extensive as on OMVs, a previous study has revealed
immunological responses to some common MVs in the airway. For
example, Repeated airway exposure to Staphylococcus aureus
EVs triggers both Th1 and Th17 responses, increasing neutrophilic
inflammation through TLR2 (58).
These results suggest that the pathogenicity of MEVs is strongly
related to lung diseases. Understanding these immunological
pathways is crucial for advancing pulmonary health research.
As aforementioned, numerous immune responses are
triggered by MEVs, and it is evident that the mechanisms of these
responses in the airways vary according to the Gram type of the
bacteria. For example, common airway OMVs, such as those derived
from P. aeruginosa, E. coli and Acinetobacter
baumannii, increase IL-6 levels and neutrophilic activity
(37,56,59). Meanwhile, MVs, such as those
derived from S. aureus and Faecalibacterium prausnitzii,
have been reported to commonly increase IFN-γ levels (58,60).
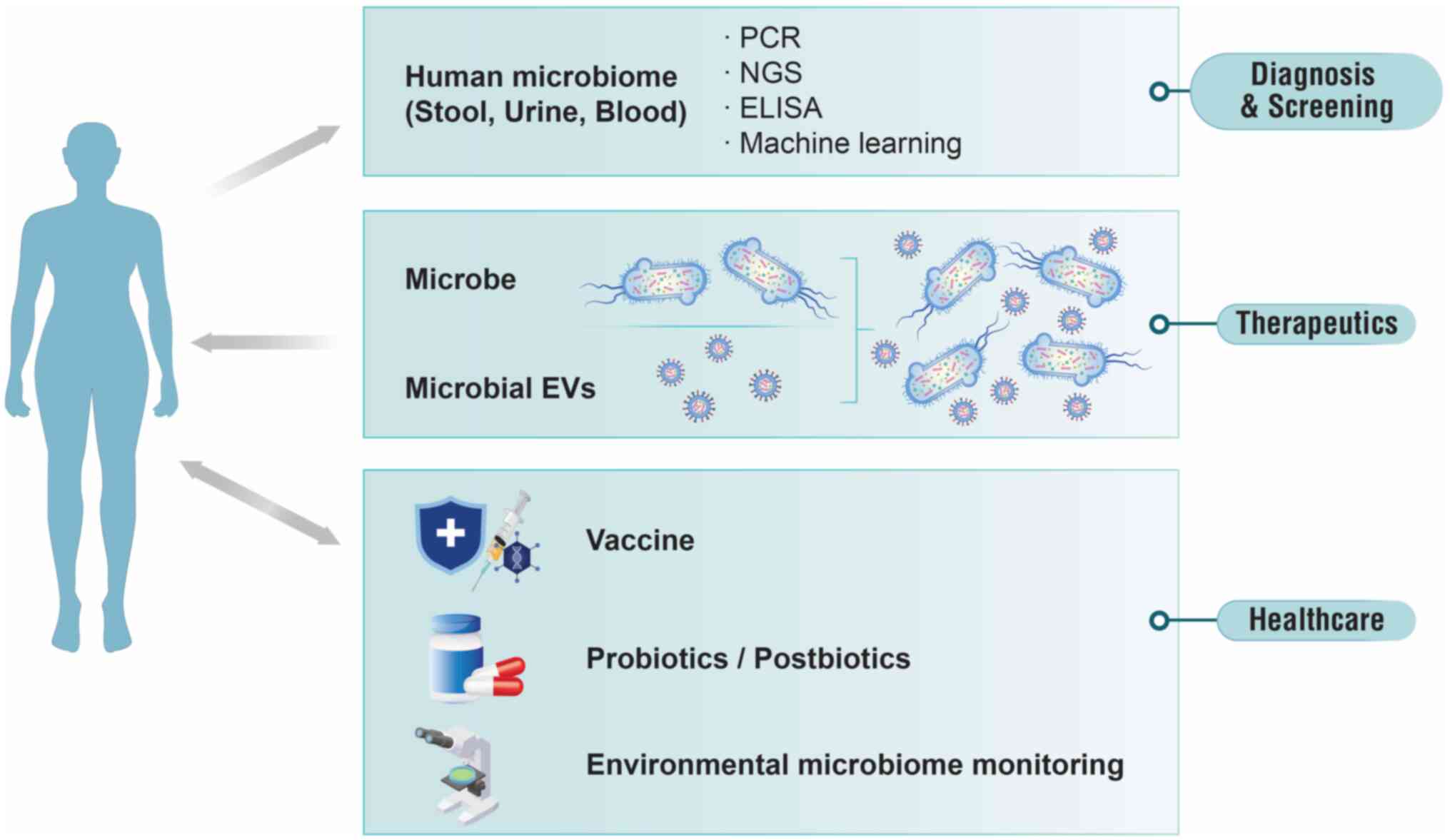
Clinical implications of the microbiome for
lung cancer
Recently, interest in the relationship between the
microbiome and human health and efforts toward clinical application
have increased. Previous studies have suggested that the microbiome
holds valuable information, demonstrating its potential as a
material or biomarker for diagnosis, therapeutics and healthcare
(Fig. 3) (24,61,62). After MEVs circulate throughout
the body, they are excreted via feces, urine and exhaled air in
their intact forms, unlike live microbes, which are restricted to
the mucosal lumen or skin surface (43). Certain MEVs act as etiological
agents of various diseases, while some MEVs have a protective role
in disease pathogenesis. Therefore, circulating MEVs in our body
provides us with noteworthy information for health and disease
status (24).
Diagnostic potential of microbiome
Risk assessment, early diagnosis, treatment response
prediction and disease monitoring are crucial for reducing
mortality and enhancing quality of life in patients with cancer
(63,64). There is a growing trend in
developing diagnostic or screening technologies that utilize the
microbiome-based quantitative polymerase chain reaction (qPCR),
NGS, machine learning and enzyme-linked immunosorbent assay
(Fig. 3). Most studies utilize
fecal samples, which contain sufficient microbiomes for analysis,
and therefore, research on microbiome-based diagnostics primarily
targets gastrointestinal diseases. Previous studies on colorectal
cancer diagnosis showed that a metagenomics algorithm achieved an
area under the curve (AUC) of 0.89 (65), while qPCR demonstrated higher
accuracy with an AUC of 0.93 (66). Additionally, this approach has
been applied to lung cancer diagnostics using feces samples, with
models using Enterococcus, Streptococcus and
Klebsiella achieving an AUC of 0.96, a
Haemophilus-specific model showing an AUC of 0.75 (20), and a model based on 13 OTU
biomarkers demonstrating an AUC of 0.976 (22).
Recent studies have been conducted using diverse
human samples, including urine, blood, saliva, bronchoalveolar
lavage fluid (BALF) and sputum, with a focus on specific diseases,
to development of diagnostic or screening technology based on
microbiomes. For instance, to distinguish between benign lung
disease and lung cancer using BALF, Kim et al (67) developed a prediction model based
on unclassified_SAR202_clade (phylum Chloroflexi), achieving
an AUC of 0.98, while a model using Veillonella and
Megasphaera showed an AUC of 0.89 (23).
MEVs, rather than live microorganisms, are emerging
as precise biomarkers for disease diagnostics using artificial
intelligence (AI)-based analysis (61). McDowell et al (68) developed machine learning models
using MEV metagenomes from serum, achieving AUCs of 0.93 for COPD,
0.99 for asthma and 0.94 for lung cancer. Antibodies against MEVs
have also shown diagnostic potential, with IgG against MEVs derived
from S. aureus, Acinetobacter baumannii,
Enterobacter cloacae and P. aeruginosa, which are
predominant in indoor dust, achieving AUCs of 0.78 for asthma, 0.79
for COPD and 0.81 for lung cancer (50).
MEV-based diagnostics have broad utility, with AUC
of 0.95 for colorectal cancer using feces (69), 0.93 for brain tumor using blood
(45), 0.87 for hepatocellular
carcinoma using blood (70),
0.82 for gastric cancer using urine (71) and 1.00 for pancreatic cancer
using blood (72). Furthermore,
diagnostic models based on MEVs incorporating additional markers
demonstrate improved performance. Combining MEV data with
additional markers, such as metabolomics or tumor markers,
significantly enhances diagnostic accuracy across various cancers
(69,73). Thus, MEV-based diagnostic
technologies, including composition assessment and immunoassays,
can provide information on exposure to etiological agents (50). Additionally, MEVs derived from
various samples can assist in the diagnosis of lung diseases.
Therapeutic potential of the microbiome
Live biotherapeutic products
Commensal bacteria are essential to human health,
with growing recognition that humans are holobionts or
supra-organisms. This means that the combined metabolic
capabilities of both eukaryotic and prokaryotic components surpass
those of each component alone (24). The U.S. Food and Drug
Administration (FDA) has announced a new category called live
biotherapeutic products (LBPs). The FDA has identified LBPs as
biological products containing live organisms such as bacteria,
which are used for disease prevention, treatment or cures, but are
not vaccines (74). LBPs are
administered in sufficient quantities to provide health benefits to
the host (24,75).
Several studies have demonstrated the efficacy of
LBP monotherapy. For examples, Lactococcus lactis inhibited
lung cell proliferation (76,77). Other studies have shown that
β-glucan, derived from Saccharomyces cerevisiae, can modulate
immune responses and inhibit cancer cell viability in the lung
cancer microenvironment (78,79). Short-chain fatty acids such as
butyrate, propionate and acetate, when delivered from the gut to
the lung, induce apoptosis in lung cancer cells (80). Although the exact mechanisms of
LBPs remain unclear, immune system regulation and pathogen
attachment interference are possible explanations.
LBPs can also be applied with regular treatments,
such as conventional chemotherapy and immunotherapy, and have
enhanced tumor suppression. Kotzampassi et al (81) found that the intake of
Lactobacillus and Bifidobacterium reduced
postoperative complications. In addition, Wada et al
(82), demonstrated that the
intake of Bifidobacterium breve during the chemotherapy
period reduced the incidence of fever and decreased the need for
intravenous antibiotics, thereby facilitating more effective
therapy. Furthermore, combining Lactobacillus with cisplatin
has been shown to reduce tumor size and increase immune responses
in lung cancer models (83).
Microbial extracellular vesicles-based
therapy as new-generation therapeutics
Recently, there has been a growing demand for
developing new therapeutic targets distinct from conventional ones,
suggesting the use of MEVs to address unmet medical needs as
next-generation therapeutics. While the potential of using
mammalian EVs for therapeutic purposes has been widely discussed,
MEVs have yet to receive much attention thus far (84). Nevertheless, several studies have
reported the beneficial effects of MEVs as therapeutic agents. EV
derived from Lactobacillus paracasei significantly affects
colorectal homeostasis in inflammation-mediated pathogenesis by
attenuating LPS-induced inflammation in the intestine by activating
endoplasmic reticulum stress (85). EVs derived from Lactococcus
lactis can modulate airway inflammation by promoting a shift in
immune responses from Th2 to Th1 by stimulating dendritic cells to
produce IL-12, which offers a possible advantage for managing
allergic asthma (86).
Conversely, Micrococcus luteus-derived EVs alleviate
neutrophilic airway inflammation by reducing IL-1β and IL-17 levels
in BALF and inhibiting group 3 innate lymphoid cells activation
through upregulation of microRNA (miRNA) in airway epithelial
cells, proposing them as a potential therapeutic for unresolved
neutrophilic asthma (87).
Additionally, Lactobacillus plantarum-derived EVs have been
suggested to treat atopic dermatitis, decreasing skin inflammation
and epidermal thickness (88).
EVs derived from Bifidobacterium longum have been shown to
reduce the occurrence of diarrhea, which can be a symptom of food
allergy, by inducing mast cell apoptosis without affecting T
cell-mediated immune responses (89). Therefore, therapeutic strategies
can utilize beneficial MEVs as potential immunomodulators while
suppressing harmful MEVs by inhibiting their production or function
(90). In addition, postbiotics
represent a new modality for next-generation therapy to complement
current cancer treatment, including those for lung cancer, such as
small molecules, proteins, monoclonal antibodies and cell-based
therapeutics (24).
Therefore, LBPs and MEVs-derived treatments can be
used independently or as adjuncts to chemotherapy or immunotherapy,
potentially becoming a major component of lung cancer treatment in
the near future.
Healthcare potential of the microbiome
It has been demonstrated that the human microbiome
is strongly associated with health. Therefore, it can be utilized
within healthcare systems for: i) Vaccines; ii) supplements such as
probiotics and postbiotics; and iii) monitoring systems.
Vaccine
Cancer vaccines primarily target tumor-specific
antigens but often lack sufficient efficacy. Researchers are
exploring the potential of combining probiotics with cancer
vaccines to enhance their effectiveness. Plasmodium, the
malaria parasite, shows promise as an adjuvant for cancer vaccines,
particularly in combination with DNA vaccines (91). Additionally, MEVs can deliver
genetic materials of vaccine components into target cells,
potentially improving vaccine efficacy (92,93). MEVs, with their bilayered lipids,
primarily containing LPS and outer membrane lipids, could also
potentially be used to deliver beneficial proteins, miRNAs and act
as adjuvants in vaccine development (62,94). Due to the variety of glycolipids
and glycoproteins in their composition, MEVs can introduce
biological activity into cells, making them suitable as drug
delivery vehicles for cyclic nucleotides, enzymes and antitumor
drugs (95-97). Recently, nano and micro
materials, such as virus-like particles and liposomal vesicles, are
also being explored for vaccine delivery (98,99). Kim et al (100) demonstrated the successful
modification of OMVs as multifunctional vaccine delivery vehicles
to enhance immune responses against cancer cells. This approach
aims to boost the immune response against cancer cells. Ongoing
research is investigating these combinations' potential to improve
cancer treatment outcomes.
Supplements such as probiotics and
postbiotics
Probiotics, or LBPs, are live microorganisms that,
when consumed in adequate amounts, provide beneficial effects to
the host and are widely used in clinical practice. Numerous studies
suggest that microbiome intake plays a role in cancer prevention
(77,101-112), initially evidenced by Goldin
and Gorbach (100) in 1980,
which showed that Lactobacillus acidophilus supplementation
reduced intestinal cancer incidence in rat models. Subsequent
studies, particularly in vitro research, have demonstrated
that probiotics can reduce cell proliferation, induce cell cycle
arrest and trigger apoptosis (102-106). Strains such as Lactobacillus
plantarum, Lactobacillus rhamnosus and
Bifidobacterium polyfermenticus have been shown to reduce
tumor incidence and progression in animal models (107-111). However, most research focuses
on gastrointestinal cancers, with limited studies on lung cancer.
Preclinical data on mice suggest that Lactococcus lactis can
inhibit cancer cell proliferation and proinflammatory cytokine
production, showing promise for lung cancer prevention (77,112). Despite limited research on the
use of probiotics for lung cancer, the findings mentioned are
promising, and future studies are expected to yield further
positive outcomes.
Postbiotics, including MEVs, are soluble factors
released by microbes or after microbial lysis that provide
physiological benefits (113).
MEVs are emerging as key postbiotics in precision medicine,
facilitating intercellular communication through proteins and small
molecules enclosed in a lipid bilayer (114). Cell-to-cell communication is
tightly regulated, and its disruption prompts disease advancement.
Soluble factors include proteins and small molecules, and
cell-to-cell communication is performed by MEVs, which are packages
of information from microbial cells enclosed by a cell membrane.
Moreover, recent scientific evidence has shown that certain MEVs as
postbiotics have protective effects against disease development or
progression (62,85,115,116).
Therefore, we propose that the intake of probiotics
and postbiotics holds significant potential in the prevention of
lung cancer, offering a hopeful avenue for future research and
preventive measures.
Monitoring system
A healthcare monitoring system can be employed to
track human health biomarkers by analyzing the airborne microbiome
(34). Considering the
significant association between human health and air pollution,
which includes particulate matter, bioaerosols and gaseous
substances, the vigilant monitoring of atmospheric pollutants can
contribute to disease prevention. In numerous countries, bioaerosol
regulation has been implemented through measurements based on
culturing techniques (117).
Culture-based analysis can directly observe bacteria in the air and
yield colony-forming units. However, this method faces several
limitations: i) It can only measure bacteria counted >1% of the
total in a solid medium agar plate (118); ii) it is restricted to
analyzing specific bacterial species; and iii) it is incapable of
analyzing unculturable bacterial material such as MEVs and dead
bacteria. For these reasons, numerous studies are underway to
enable real-time on-site monitoring of bioaerosols and biomarkers
related to human health. Cho et al (119) developed the bioaerosol
monitoring system based on ATP extracted from E. coli and
demonstrated that this system can continuously monitor with high
sensitivity in real-time. Additionally, a previous study has
utilized reverse transcription-PCR to detect airborne bacteria
(120). Furthermore, to
facilitate the precise and rapid detection of bioaerosols, droplet
digital PCR (ddPCR) has been employed extensively in various
studies for pathogen diagnosis, mutation detection and transgenic
research (120-122). For instance, airborne
Mycobacterium tuberculosis was detected using ddPCR
(123); however, this method
currently cannot detect airborne bacteria in real-time on-site,
indicating that the technology requires further improvement.
Through such monitoring systems, lung cancer
surveillance can be enhanced. Furthermore, by observing microbiomes
associated with lung cancer risk, these systems have the potential
to utilize these microbiomes as biomarkers for the disease.
Conclusion
The present review explored the potential of
microbiomes and MEVs as innovative tools in precision medicine for
lung cancer. Disease patterns are linked to cellular aging and
elevated reactive oxygen species, contributing to conditions such
as inflammation, immune diseases and cancer. There is a growing
shift toward promoting health through advanced diagnostics, safer
therapeutics and prevention-focused healthcare systems. To support
this shift, advancements in biological data analysis, including
metagenomics and AI technologies such as machine learning, are
essential for disease prediction and personalized therapies. While
significant research on the microbiome exists, understanding the
interactions between microbiota and host, particularly microbial
products, remains limited. A deeper understanding of these
interactions is key to developing beneficial microbial products.
MEVs, unlike LBPs, can penetrate cells and target distant organs,
offering significant advantages as diagnostic biomarkers and
therapeutic candidates. We propose MEVs as next-generation
technologies for lung cancer, capable of replacing current
biologics such as proteins, antibodies and genes.
Future research on MEVs is expected to enhance our
understanding of their role in lung cancer and foster precision
medicine approaches, including diagnostics and therapies utilizing
MEVs from beneficial microorganisms.
Availability of data and materials
Not applicable.
Authors' contributions
JYJ and JHS conducted the literature research,
developed the methodology, generated the figures, and wrote the
original draft. HJR, JJC, HY, and DMH contributed to the literature
research and edited the manuscript. JY conceptualized the study,
acquired funding, conducted the investigation, managed the project,
supervised the research, visualized the results, and reviewed and
edited the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This research was supported by Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Education (grant no. RS-2023-00244833).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park EH, Jung KW, Park NJ, Kang MJ, Yun
EH, Kim HJ, Kim JE, Kong HJ, Im JS and Seo HG; Community of
Population-Based Regional Cancer Registries: Cancer statistics in
Korea: Incidence, Mortality, survival, and prevalence in 2021.
Cancer Res Treat. 56:357–371. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zito Marino F, Bianco R, Accardo M, Ronchi
A, Cozzolino I, Morgillo F, Rossi G and Franco R: Molecular
heterogeneity in lung cancer: from mechanisms of origin to clinical
implications. Int J Med Sci. 16:981–989. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Sousa VML and Carvalho L: Heterogeneity
in lung cancer. Pathobiology. 85:96–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim ZF and Ma PC: Emerging insights of
tumor heterogeneity and drug resistance mechanisms in lung cancer
targeted therapy. J Hematol Oncol. 12:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malhotra J, Malvezzi M, Negri E, La
Vecchia C and Boffetta P: Risk factors for lung cancer worldwide.
Eur Respir J. 48:889–902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahman MM, Islam MR, Shohag S, Ahasan MT,
Sarkar N, Khan H, Hasan AM, Cavalu S and Rauf A: Microbiome in
cancer: Role in carcinogenesis and impact in therapeutic
strategies. Biomed Pharmacother. 149:1128982022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dickson RP, Erb-Downward JR, Freeman CM,
McCloskey L, Beck JM, Huffnagle GB and Curtis JL: Spatial variation
in the healthy human lung microbiome and the adapted Island model
of lung biogeography. Ann Am Thorac Soc. 12:821–830. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu G, Gail MH, Consonni D, Carugno M,
Humphrys M, Pesatori AC, Caporaso NE, Goedert JJ, Ravel J and Landi
MT: Characterizing human lung tissue microbiota and its
relationship to epidemiological and clinical features. Genome Biol.
17:1632016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yagi K, Huffnagle GB, Lukacs NW and Asai
N: The lung microbiome during health and disease. Int J Mol Sci.
22:108722021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruan R, Deng X, Dong X, Wang Q, Lv X and
Si C: Microbiota emergencies in the diagnosis of lung diseases: A
meta-analysis. Front Cell Infect Microbiol. 11:7096342021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang HJ, Choi JY, Kim K, Yong SH, Kim YW,
Kim SY, Kim EY, Jung JY, Kang YA, Park MS, et al: Relationship of
the lung microbiome with PD-L1 expression and immunotherapy
response in lung cancer. Respir Res. 22:3222021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang D, Xing Y, Song X and Qian Y: The
impact of lung microbiota dysbiosis on inflammation. Immunology.
159:156–166. 2020. View Article : Google Scholar :
|
|
14
|
Xu N, Wang L, Li C, Ding C, Li C, Fan W,
Cheng C and Gu B: Microbiota dysbiosis in lung cancer: Evidence of
association and potential mechanisms. Transl Lung Cancer Res.
9:1554–1568. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosgood HD III, Sapkota AR, Rothman N,
Rohan T, Hu W, Xu J, Vermeulen R, He X, White JR, Wu G, et al: The
potential role of lung microbiota in lung cancer attributed to
household coal burning exposures. Environ Mol Mutagen. 55:643–651.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu HX, Tao LL, Zhang J, Zhu YG, Zheng Y,
Liu D, Zhou M, Ke H, Shi MM and Qu JM: Difference of lower airway
microbiome in bilateral protected specimen brush between lung
cancer patients with unilateral lobar masses and control subjects.
Int J Cancer. 142:769–778. 2018. View Article : Google Scholar
|
|
17
|
Zhang W, Luo J, Dong X, Zhao S, Hao Y,
Peng C, Shi H, Zhou Y, Shan L, Sun Q, et al: Salivary microbial
dysbiosis is associated with systemic inflammatory markers and
predicted oral metabolites in non-small cell lung cancer patients.
J Cancer. 10:1651–1662. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Zhou H, Xu S, Liu D, Cheng Y, Gao
B, Li X and Chen J: The gut microbiome can be used to predict the
gastrointestinal response and efficacy of lung cancer patients
undergoing chemotherapy. Ann Palliat Med. 9:4211–4227. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu H, Gao NL, Tong F, Wang J, Li H, Zhang
R, Ma H, Yang N, Zhang Y, Wang Y, et al: Alterations of the human
lung and gut microbiomes in non-small cell lung carcinomas and
distant metastasis. Microbiol Spectr. 9:e00802212021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen W, Tang D, Deng Y, Li H, Wang T, Wan
P and Liu R: Association of gut microbiomes with lung and
esophageal cancer: A pilot study. World J Microbiol Biotechnol.
37:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuang H, Cheng L, Wang Y, Zhang YK, Zhao
MF, Liang GD, Zhang MC, Li YG, Zhao JB, Gao YN, et al: Dysbiosis of
the gut microbiome in lung cancer. Front Cell Infect Microbiol.
9:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Y, Fang Z, Xue Y, Zhang J, Zhu J,
Gao R, Yao S, Ye Y, Wang S, Lin C, et al: Specific gut microbiome
signature predicts the early-stage lung cancer. Gut Microbes.
11:1030–1042. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SH, Sung JY, Yong D, Chun J, Kim SY,
Song JH, Chung KS, Kim EY, Jung JY, Kang YA, et al:
Characterization of microbiome in bronchoalveolar lavage fluid of
patients with lung cancer comparing with benign mass like lesions.
Lung Cancer. 102:89–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Shin TS, Kim JS, Jee YK and Kim
YK: A new horizon of precision medicine: Combination of the
microbiome and extracellular vesicles. Exp Mol Med. 54:466–482.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castelino M, Eyre S, Moat J, Fox G, Martin
P, Ho P, Upton M and Barton A: Optimisation of methods for
bacterial skin microbiome investigation: Primer selection and
comparison of the 454 versus MiSeq platform. BMC Microbiol.
17:232017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balvočiūtė M and Huson DH: SILVA, RDP,
Greengenes, NCBI and OTT-how do these taxonomies compare? BMC
Genomics. 18(Suppl 2): S1142017. View Article : Google Scholar
|
|
27
|
Rajagopala SV, Vashee S, Oldfield LM,
Suzuki Y, Venter JC, Telenti A and Nelson KE: The human microbiome
and cancer. Cancer Prev Res (Phila). 10:226–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Russo E, Taddei A, Ringressi MN, Ricci F
and Amedei A: The interplay between the microbiome and the adaptive
immune response in cancer development. Therap Adv Gastroenterol.
9:594–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi Y, Park H, Park HS and Kim YK:
Extracellular vesicles, a key mediator to link environmental
microbiota to airway immunity. Allergy Asthma Immunol Res.
9:101–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karimi K, Inman MD, Bienenstock J and
Forsythe P: Lactobacillus reuteri-induced regulatory T cells
protect against an allergic airway response in mice. Am J Respir
Crit Care Med. 179:186–193. 2009. View Article : Google Scholar
|
|
31
|
Atarashi K, Tanoue T, Shima T, Imaoka A,
Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al:
Induction of colonic regulatory T cells by indigenous Clostridium
species. Science. 331:337–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang J, Seo JH, Jee YK, Kim YK and Sohn
JR: Composition analysis of airborne microbiota in outdoor and
indoor based on dust separated by micro-sized and nano-sized.
Aerosol Air Qual Res. 23:2102312023. View Article : Google Scholar
|
|
33
|
Panthee B, Gyawali S, Panthee P and
Techato K: Environmental and human microbiome for health. Life
(Basel). 12:4562022.PubMed/NCBI
|
|
34
|
Mbareche H, Morawska L and Duchaine C: On
the interpretation of bioaerosol exposure measurements and impacts
on health. J Air Waste Manag Assoc. 69:789–804. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin SK, Kim J, Ha SM, Oh HS, Chun J, Sohn
J and Yi H: Metagenomic insights into the bioaerosols in the indoor
and outdoor environments of childcare facilities. PLoS One.
10:e01269602015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wolf M and Lai PS: Indoor microbial
exposures and chronic lung disease: From microbial toxins to the
microbiome. Clin Chest Med. 41:777–796. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park KS, Lee J, Jang SC, Kim SR, Jang MH,
Lötvall J, Kim YK and Gho YS: Pulmonary inflammation induced by
bacteria-free outer membrane vesicles from Pseudomonas aeruginosa.
Am J Respir Cell Mol Biol. 49:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim KH, Kabir E and Jahan SA: Airborne
bioaerosols and their impact on human health. J Environ Sci
(China). 67:23–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baldacci S, Maio S, Cerrai S, Sarno G,
Baïz N, Simoni M, Annesi-Maesano I and Viegi G; HEALS Study:
Allergy and asthma: Effects of the exposure to particulate matter
and biological allergens. Respir Med. 109:1089–1104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hayleeyesus SF, Ejeso A and Derseh FA:
Quantitative assessment of bio-aerosols contamination in indoor air
of University dormitory rooms. Int J Health Sci (Qassim).
9:249–256. 2015.PubMed/NCBI
|
|
41
|
Lee EY, Bang JY, Park GW, Choi DS, Kang
JS, Kim HJ, Park KS, Lee JO, Kim YK, Kwon KH, et al: Global
proteomic profiling of native outer membrane vesicles derived from
Escherichia coli. Proteomics. 7:3143–3153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee EY, Choi DY, Kim DK, Kim JW, Park JO,
Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP and Gho YS:
Gram-positive bacteria produce membrane vesicles: Proteomics-based
characterization of Staphylococcus aureus-derived membrane
vesicles. Proteomics. 9:5425–5436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choi Y, Kwon Y, Kim DK, Jeon J, Jang SC,
Wang T, Ban M, Kim MH, Jeon SG, Kim MS, et al: Gut microbe-derived
extracellular vesicles induce insulin resistance, thereby impairing
glucose metabolism in skeletal muscle. Sci Rep. 5:158782015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Conlon MA and Bird AR: The impact of diet
and lifestyle on gut microbiota and human health. Nutrients.
7:17–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang J, Moon HE, Park HW, McDowell A, Shin
TS, Jee YK, Kym S, Paek SH and Kim YK: Brain tumor diagnostic model
and dietary effect based on extracellular vesicle microbiome data
in serum. Exp Mol Med. 52:1602–1613. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang J, McDowell A, Kim EK, Seo H, Yum K,
Lee WH, Jee YK and Kim YK: Consumption of a Leuconostoc
holzapfelii-enriched synbiotic beverage alters the composition of
the microbiota and microbial extracellular vesicles. Exp Mol Med.
51:1–11. 2019.
|
|
47
|
Son T, Cho YJ, Lee H, Cho MY, Goh B, Kim
HM, Hoa PTN, Cho SH, Park YJ, Park HS and Hong KS: Monitoring in
vivo behavior of size-dependent fluorescent particles as a model
fine dust. J Nanobiotechnology. 20:2272022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY,
Park J, Jee YK, Zhu Z, Koh YY, Gho YS and Kim YK: Extracellular
vesicles, especially derived from Gram-negative bacteria, in indoor
dust induce neutrophilic pulmonary inflammation associated with
both Th1 and Th17 cell responses. Clin Exp Allergy. 43:443–454.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim YS, Choi JP, Kim MH, Park HK, Yang S,
Kim YS, Kim TB, Cho YS, Oh YM, Jee YK, et al: IgG sensitization to
extracellular vesicles in indoor dust is closely associated with
the prevalence of non-eosinophilic asthma, COPD, and lung cancer.
Allergy Asthma Immunol Res. 8:198–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang J, Hong G, Kim YS, Seo H, Kim S,
McDowell A, Lee WH, Kim YS, Oh YM, Cho YS, et al: Lung disease
diagnostic model through IgG sensitization to microbial
extracellular vesicles. Allergy Asthma Immunol Res. 12:669–683.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Ann Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar
|
|
53
|
Mishra S, Tejesvi MV, Hekkala J, Turunen
J, Kandikanti N, Kaisanlahti A, Suokas M, Leppä S, Vihinen P,
Kuitunen H, et al: Gut microbiome-derived bacterial extracellular
vesicles in patients with solid tumours. J Adv Res. 68:375–386.
2025. View Article : Google Scholar :
|
|
54
|
Yang J, Kim EK, Park HJ, McDowell A and
Kim YK: The impact of bacteria-derived ultrafine dust particles on
pulmonary diseases. Exp Mol Med. 52:338–347. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim YS, Lee WH, Choi EJ, Choi JP, Heo YJ,
Gho YS, Jee YK, Oh YM and Kim YK: Extracellular vesicles derived
from gram-negative bacteria, such as Escherichia coli, induce
emphysema mainly via IL-17A-mediated neutrophilic inflammation. J
Immunol. 194:3361–3368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park KS, Choi KH, Kim YS, Hong BS, Kim OY,
Kim JH, Yoon CM, Koh GY, Kim YK and Gho YS: Outer membrane vesicles
derived from Escherichia coli induce systemic inflammatory response
syndrome. PLoS One. 5:e113342010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lundin JI and Checkoway H: Endotoxin and
cancer. Environ Health Perspect. 117:1344–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim MR, Hong SW, Choi EB, Lee WH, Kim YS,
Jeon SG, Jang MH, Gho YS and Kim YK: Staphylococcus aureus-derived
extracellular vesicles induce neutrophilic pulmonary inflammation
via both Th1 and Th17 cell responses. Allergy. 67:1271–1281. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jun SH, Lee JH, Kim BR, Kim SI, Park TI,
Lee JC and Lee YC: Acinetobacter baumannii outer membrane vesicles
elicit a potent innate immune response via membrane proteins. PLoS
One. 8:e717512013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jafari B, Khavari Nejad RA, Vaziri F and
Siadat SD: Evaluation of the effects of extracellular vesicles
derived from Faecalibacterium prausnitzii on lung cancer cell line.
Biologia. 74:889–898. 2019. View Article : Google Scholar
|
|
61
|
Yang J: Insight into the potential of
algorithms using AI technology as in vitro diagnostics utilizing
microbial extracellular vesicles. Mol Cell Probes. 78:1019922024.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang J, Kim EK, McDowell A and Kim YK:
Microbe-derived extracellular vesicles as a smart drug delivery
system. Transl Clin Pharmacol. 26:103–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Whitaker K: Earlier diagnosis: The
importance of cancer symptoms. Lancet Oncol. 21:6–8. 2020.
View Article : Google Scholar
|
|
64
|
Seo JH, Lee JW and Cho D: The market trend
analysis and prospects of cancer molecular diagnostics kits.
Biomater Res. 22:22018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang J, McDowell A, Kim EK, Seo H, Lee WH,
Moon CM, Kym SM, Lee DH, Park YS, Jee YK and Kim YK: Development of
a colorectal cancer diagnostic model and dietary risk assessment
through gut microbiome analysis. Exp Mol Med. 51:1–15. 2019.
|
|
66
|
Yang J, Li D, Yang Z, Dai W, Feng X, Liu
Y, Jiang Y, Li P, Li Y, Tang B, et al: Establishing high-accuracy
biomarkers for colorectal cancer by comparing fecal microbiomes in
patients with healthy families. Gut Microbes. 11:918–929. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim G, Park C, Yoon YK, Park D, Lee JE,
Lee D, Sun P, Park S, Yun C, Kang DH and Chung C: Prediction of
lung cancer using novel biomarkers based on microbiome profiling of
bronchoalveolar lavage fluid. Sci Rep. 14:16912024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
McDowell A, Kang J, Yang J, Jung J, Oh YM,
Kym SM, Shin TS, Kim TB, Jee YK and Kim YK: Machine-learning
algorithms for asthma, COPD, and lung cancer risk assessment using
circulating microbial extracellular vesicle data and their
application to assess dietary effects. Exp Mol Med. 54:1586–1595.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim DJ, Yang J, Seo H, Lee WH, Ho Lee D,
Kym S, Park YS, Kim JG, Jang IJ, Kim YK and Cho JY: Colorectal
cancer diagnostic model utilizing metagenomic and metabolomic data
of stool microbial extracellular vesicles. Sci Rep. 10:28602020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cho EJ, Leem S, Kim SA, Yang J, Lee YB,
Kim SS, Cheong JY, Cho SW, Kim JW, Kim SM, et al: Circulating
microbiota-based metagenomic signature for detection of
hepatocellular carcinoma. Sci Rep. 9:75362019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Park JY, Kang CS, Seo HC, Shin JC, Kym SM,
Park YS, Shin TS, Kim JG and Kim YK: Bacteria-derived extracellular
vesicles in urine as a novel biomarker for gastric cancer:
Integration of liquid biopsy and metagenome analysis. Cancers
(Basel). 13:46872021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim JR, Han K, Han Y, Kang N, Shin TS,
Park HJ, Kim H, Kwon W, Lee S, Kim YK, et al: Microbiome markers of
pancreatic cancer based on bacteria-derived extracellular vesicles
acquired from blood samples: A retrospective propensity score
matching analysis. Biology (Basel). 10:2192021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kim SI, Kang N, Leem S, Yang J, Jo H, Lee
M, Kim HS, Dhanasekaran DN, Kim YK, Park T and Song YS: Metagenomic
analysis of serum microbe-derived extracellular vesicles and
diagnostic models to differentiate ovarian cancer and benign
ovarian tumor. Cancers (Basel). 12:13092020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ağagündüz D, Gençer Bingöl F, Çelik E,
Cemali Ö, Özenir Ç, Özoğul F and Capasso R: Recent developments in
the probiotics as live biotherapeutic products (LBPs) as modulators
of gut brain axis related neurological conditions. J Transl Med.
20:4602022. View Article : Google Scholar :
|
|
75
|
Cordaillat-Simmons M, Rouanet A and Pot B:
Live biotherapeutic products: The importance of a defined
regulatory framework. Exp Mol Med. 52:1397–1406. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee NK, Han KJ, Son SH, Eom SJ, Lee SK and
Paik HD: Multifunctional effect of probiotic Lactococcus lactis
KC24 isolated from kimchi. LWT Food Sci Technol. 64:1036–1041.
2015. View Article : Google Scholar
|
|
77
|
Han KJ, Lee NK, Park H and Paik HD:
Anticancer and anti-inflammatory activity of probiotic Lactococcus
lactis NK34. J Microbiol Biotechnol. 25:1697–1701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Peymaeei F, Sadeghi F, Safari E, Khorrami
S, Falahati M, Roudbar Mohammadi S and Roudbary M: Candida albicans
beta-glucan induce anti-cancer activity of mesenchymal stem cells
against lung cancer cell line: An in-vitro experimental study.
Asian Pac J Cancer Prev. 21:837–843. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Albeituni SH, Ding C, Liu M, Hu X, Luo F,
Kloecker G, Bousamra M II, Zhang HG and Yan J: Yeast-derived
particulate β-glucan treatment subverts the suppression of
myeloid-derived suppressor cells (MDSC) by inducing
polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation
to APC in cancer. J Immunol. 196:2167–2180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim K, Kwon O, Ryu TY, Jung CR, Kim J, Min
JK, Kim DS, Son MY and Cho HS: Propionate of a microbiota
metabolite induces cell apoptosis and cell cycle arrest in lung
cancer. Mol Med Rep. 20:1569–1574. 2019.PubMed/NCBI
|
|
81
|
Kotzampassi K, Stavrou G, Damoraki G,
Georgitsi M, Basdanis G, Tsaousi G and Giamarellos-Bourboulis EJ: A
four-probiotics regimen reduces postoperative complications after
colorectal surgery: A randomized, double-blind, placebo-controlled
study. World J Surg. 39:2776–2783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wada M, Nagata S, Saito M, Shimizu T,
Yamashiro Y, Matsuki T, Asahara T and Nomoto K: Effects of the
enteral administration of Bifidobacterium breve on patients
undergoing chemotherapy for pediatric malignancies. Support Care
Cancer. 18:751–759. 2010. View Article : Google Scholar
|
|
83
|
Gui QF, Lu HF, Zhang CX, Xu ZR and Yang
YH: Well-balanced commensal microbiota contributes to anti-cancer
response in a lung cancer mouse model. Genet Mol Res. 14:5642–5651.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhou X, Xie F, Wang L, Zhang L, Zhang S,
Fang M and Zhou F: The function and clinical application of
extracellular vesicles in innate immune regulation. Cell Mol
Immunol. 17:323–334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Choi JH, Moon CM, Shin TS, Kim EK,
McDowell A, Jo MK, Joo YH, Kim SE, Jung HK, Shim KN, et al:
Lactobacillus paracasei-derived extracellular vesicles attenuate
the intestinal inflammatory response by augmenting the endoplasmic
reticulum stress pathway. Exp Mol Med. 52:423–437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee DH, Park HK, Lee HR, Sohn H, Sim S,
Park HJ, Shin YS, Kim YK, Choi Y and Park HS: Immunoregulatory
effects of Lactococcus lactis-derived extracellular vesicles in
allergic asthma. Clin Transl Allergy. 12:e121382022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sim S, Lee DH, Kim KS, Park HJ, Kim YK,
Choi Y and Park HS: Micrococcus luteus-derived extracellular
vesicles attenuate neutrophilic asthma by regulating miRNAs in
airway epithelial cells. Exp Mol Med. 55:196–204. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim MH, Choi SJ, Choi HI, Choi JP, Park
HK, Kim EK, Kim MJ, Moon BS, Min TK, Rho M, et al: Lactobacillus
plantarum-derived extracellular vesicles protect atopic dermatitis
induced by Staphylococcus aureus-derived extracellular vesicles.
Allergy Asthma Immunol Res. 10:516–532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kim JH, Jeun EJ, Hong CP, Kim SH, Jang MS,
Lee EJ, Moon SJ, Yun CH, Im SH, Jeong SG, et al: Extracellular
vesicle-derived protein from Bifidobacterium longum alleviates food
allergy through mast cell suppression. J Allergy Clin Immunol.
137:507–516.e8. 2016. View Article : Google Scholar
|
|
90
|
Chen J, Zhang H, Wang S, Du Y, Wei B, Wu Q
and Wang H: Inhibitors of bacterial extracellular vesicles. Front
Microbiol. 13:8350582022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Guo H, Zhao L, Zhu J, Chen P, Wang H,
Jiang M, Liu X, Sun H, Zhao W, Zheng Z, et al: Microbes in lung
cancer initiation, treatment, and outcome: Boon or bane? Semin
Cancer Biol. 86:1190–1206. 2022. View Article : Google Scholar
|
|
92
|
Kim SI, Ha JY, Choi SY, Hong SH and Lee
HJ: Use of bacterial extracellular vesicles for gene delivery to
host cells. Biomolecules. 12:11712022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu H, Zhang Q, Wang S, Weng W, Jing Y and
Su J: Bacterial extracellular vesicles as bioactive nanocarriers
for drug delivery: Advances and perspectives. Bioact Mater.
14:169–181. 2021.
|
|
94
|
Lee EY, Choi DS, Kim KP and Gho YS:
Proteomics in gram-negative bacterial outer membrane vesicles. Mass
Spectrom Rev. 27:535–555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Papahadjopoulos D, Poste G, Schaeffer BE
and Vail WJ: Membrane fusion and molecular segregation in
phospholipid vesicles. Biochim Biophys Acta. 352:10–28. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Papahadjopoulos D, Mayhew E, Poste G,
Smith S and Vail WJ: Incorporation of lipid vesicles by mammalian
cells provides a potential method for modifying cell behaviour.
Nature. 252:163–166. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Poste G and Papahadjopoulos D: Lipid
vesicles as carriers for introducing materials into cultured cells:
Influence of vesicle lipid composition on mechanism(s) of vesicle
incorporation into cells. Proc Natl Acad Sci USA. 73:1603–1607.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Casal JI, Rueda P and Hurtado A:
Parvovirus-like particles as vaccine vectors. Methods. 19:174–186.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Parmar MM, Edwards K and Madden TD:
Incorporation of bacterial membrane proteins into liposomes:
Factors influencing protein reconstitution. Biochim Biophys Acta.
1421:77–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kim SH, Kim KS, Lee SR, Kim E, Kim MS, Lee
EY, Gho YS, Kim JW, Bishop RE and Chang KT: Structural
modifications of outer membrane vesicles to refine them as vaccine
delivery vehicles. Biochim Biophys Acta. 1788:2150–2159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Goldin BR and Gorbach SL: Effect of
Lactobacillus acidophilus dietary supplements on
1,2-dimethylhydrazine dihydrochloride-induced intestinal cancer in
rats. J Natl Cancer Inst. 64:263–265. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ghoneum M and Gimzewski J: Apoptotic
effect of a novel kefir product, PFT, on multidrug-resistant
myeloid leukemia cells via a hole-piercing mechanism. Int J Oncol.
44:830–837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Thirabunyanon M and Hongwittayakorn P:
Potential probiotic lactic acid bacteria of human origin induce
antiproliferation of colon cancer cells via synergic actions in
adhesion to cancer cells and short-chain fatty acid bioproduction.
Appl Biochem Biotechnol. 169:511–525. 2013. View Article : Google Scholar
|
|
104
|
Orlando A, Refolo MG, Messa C, Amati L,
Lavermicocca P, Guerra V and Russo F: Antiproliferative and
proapoptotic effects of viable or heat-killed Lactobacillus
paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric
and DLD-1 colon cell lines. Nutr Cancer. 64:1103–1111. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Baldwin C, Millette M, Oth D, Ruiz MT,
Luquet FM and Lacroix M: Probiotic Lactobacillus acidophilus and L.
casei mix sensitize colorectal tumoral cells to
5-fluorouracil-induced apoptosis. Nutr Cancer. 62:371–378. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kim Y, Lee D, Kim D, Cho J, Yang J, Chung
M, Kim K and Ha N: Inhibition of proliferation in colon cancer cell
lines and harmful enzyme activity of colon bacteria by
Bifidobacterium adolescentis SPM0212. Arch Pharm Res. 31:468–473.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Park E, Jeon GI, Park JS and Paik HD: A
probiotic strain of Bacillus polyfermenticus reduces DMH induced
precancerous lesions in F344 male rat. Biol Pharm Bull. 30:569–574.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ma EL, Choi YJ, Choi J, Pothoulakis C,
Rhee SH and Im E: The anticancer effect of probiotic Bacillus
polyfermenticus on human colon cancer cells is mediated through
ErbB2 and ErbB3 inhibition. Int J Cancer. 127:780–790. 2010.
View Article : Google Scholar
|
|
109
|
Gamallat Y, Meyiah A, Kuugbee ED, Hago AM,
Chiwala G, Awadasseid A, Bamba D, Zhang X, Shang X, Luo F and Xin
Y: Lactobacillus rhamnosus induced epithelial cell apoptosis,
ameliorates inflammation and prevents colon cancer development in
an animal model. Biomed Pharmacother. 83:536–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hu J, Wang C, Ye L, Yang W, Huang H, Meng
F, Shi S and Ding Z: Anti-tumour immune effect of oral
administration of Lactobacillus plantarum to CT26 tumour-bearing
mice. J Biosci. 40:269–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Walia S, Kamal R, Dhawan DK and Kanwar SS:
Chemoprevention by probiotics during 1,2-dimethylhydrazine-induced
colon carcinogenesis in rats. Dig Dis Sci. 63:900–909. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jacouton E, Torres Maravilla E, Boucard
AS, Pouderous N, Pessoa Vilela AP, Naas I, Chain F, Azevedo V,
Langella P and Bermúdez-Humarán LG: Anti-tumoral effects of
recombinant Lactococcus lactis strain secreting IL-17A cytokine.
Front Microbiol. 9:33552019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Aguilar-Toalá JE, Garcia-Varela R, Garcia
HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B and
Hernández-Mendoza A: Postbiotics: An evolving term within the
functional foods field. Trends Food Sci Technol. 75:105–114. 2018.
View Article : Google Scholar
|
|
114
|
Fafián-Labora JA and O'Loghlen A:
Classical and nonclassical intercellular communication in
senescence and ageing. Trends Cell Biol. 30:628–639. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu Y, Defourny KAY, Smid EJ and Abee T:
Gram-positive bacterial extracellular vesicles and their impact on
health and disease. Front Microbiol. 9:15022018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS,
Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, et al: Extracellular
vesicles derived from gut microbiota, especially Akkermansia
muciniphila, protect the progression of dextran sulfate
sodium-induced colitis. PLoS One. 8:e765202013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kim YJ, Lee BG, Shim JE, Lee H, Park JH
and Yeo MK: Airborne bacteria in institutional and commercial
buildings in Korea: Characterization with 16S rRNA gene sequencing
and association with environmental conditions. Aerosol Sci Technol.
58:1281–1292. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fahlgren C, Hagström Å, Nilsson D and
Zweifel UL: Annual variations in the diversity, viability, and
origin of airborne bacteria. Appl Environ Microbiol. 76:3015–3025.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cho YS, Kim HR, Ko HS, Jeong SB, Chan Kim
B and Jung JH: Continuous surveillance of bioaerosols on-site using
an automated bioaerosol-monitoring system. ACS Sens. 5:395–403.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gerdes L, Iwobi A, Busch U and Pecoraro S:
Optimization of digital droplet polymerase chain reaction for
quantification of genetically modified organisms. Biomol Detect
Quantif. 7:9–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Biron VL, Kostiuk M, Isaac A, Puttagunta
L, O'Connell DA, Harris J, Côté DW and Seikaly H: Detection of
human papillomavirus type 16 in oropharyngeal squamous cell
carcinoma using droplet digital polymerase chain reaction. Cancer.
122:1544–1551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Brambati C, Galbiati S, Xue E, Toffalori
C, Crucitti L, Greco R, Sala E, Crippa A, Chiesa L, Soriani N, et
al: Droplet digital polymerase chain reaction for DNMT3A and IDH1/2
mutations to improve early detection of acute myeloid leukemia
relapse after allogeneic hematopoietic stem cell transplantation.
Haematologica. 101:e157–e161. 2016. View Article : Google Scholar :
|
|
123
|
Patterson B, Morrow C, Singh V, Moosa A,
Gqada M, Woodward J, Mizrahi V, Bryden W, Call C, Patel S, et al:
Detection of Mycobacterium tuberculosis bacilli in bio-aerosols
from untreated TB patients. Gates Open Res. 1:112018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Lu X, Xiong L, Zheng X, Yu Q, Xiao Y and
Xie Y: Structure of gut microbiota and characteristics of fecal
metabolites in patients with lung cancer. Front Cell Infect
Microbiol. 13:11703262023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Najafi S, Abedini F, Azimzadeh Jamalkandi
S, Shariati P, Ahmadi A and Gholami Fesharaki M: The composition of
lung microbiome in lung cancer: A systematic review and
meta-analysis. BMC Microbiol. 21:3152021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Greathouse KL, White JR, Vargas AJ,
Bliskovsky VV, Beck JA, von Muhlinen N, Polley EC, Bowman ED, Khan
MA, Robles AI, et al: Interaction between the microbiome and TP53
in human lung cancer. Genome Biol. 19:1232018. View Article : Google Scholar : PubMed/NCBI
|