Introduction
Coronary heart disease (CHD) is a globally prevalent
cardiovascular disorder with a complex pathogenesis associated with
multiple risk factors, including high-level low-density lipoprotein
cholesterol, smoking, chronic kidney disease, diabetes and
hypertension (1). The primary
pathological feature of CHD is coronary atherosclerosis, which can
lead to myocardial ischemia, hypoxia and necrosis (2,3).
Inflammation assumes a pivotal position in the development of CHD,
with atherosclerosis essentially being a chronic inflammatory
disease. Inflammatory factors contribute to atherosclerotic plaque
formation, rupture and the progression of CHD through processes
such as vascular endothelial injury and thrombosis (4,5).
While the role of inflammation in CHD is
well-established, emerging evidence has shown that amino acid
metabolic disorders can also have a profound impact on the
inflammatory response in CHD. Such metabolic disorders can initiate
a cascade of events, including the dysregulation of the
tricarboxylic acid cycle (TCA), which is integral to the oxidative
phosphorylation processes in cardiac myocytes (6). Dysregulation of the TCA cycle can
lead to mitochondrial dysfunction, subsequently inducing oxidative
stress and promoting an inflammatory state. Furthermore, amino acid
metabolic disorders can alter the plaque microenvironment through
immunometabolic reprogramming and oxidative stress, thereby
exacerbating inflammation in CHD.
In atherosclerotic plaques, M1-type macrophages
secrete pro-inflammatory cytokines such as interleukin-1β (IL-1β),
IL-6 and tumor necrosis factor-α (TNF-α). This secretion promotes
the progression of the inflammatory response, increasing plaque
instability and rupture risk (7). Furthermore, oxidized low-density
lipoprotein (ox-LDL) not only promotes the polarization of M1-type
macrophages but also inhibits the anti-inflammatory and
tissue-repair functions of M2-type macrophages. This disruption of
the M1/M2 balance leads to an uncontrolled inflammatory response,
which aggravates atherosclerosis (8). These mechanisms illustrate a
vicious cycle of CHD inflammation through immunometabolic
reprogramming.
During atherosclerosis, various subsets of T
lymphocytes play distinct roles. Type 1 T-helper (Th) cells can
secrete pro-inflammatory cytokines such as interferon-γ (IFN-γ),
which drives macrophage activation and intensifies plaque
inflammation (9). Th17 cells
produce IL-17, which aggravates endothelial damage and increases
plaque instability (10). By
contrast, regulatory T cells (Tregs) secrete anti-inflammatory
factors such as IL-10 and TGF-β, suppressing excessive immune
responses and delaying the progression of atherosclerosis (11). In addition, in acute coronary
syndrome, neutrophils release toxic substances such as elastase and
myeloperoxidase, and form neutrophil extracellular traps, further
intensifying inflammation and thrombosis (12,13). Natural killer (NK) cells and B
lymphocytes also participate in the regulation of the plaque
microenvironment through their respective unique mechanisms
(14-16).
Exposure to harmful stimuli disrupts the balance
between oxidants and antioxidants, leading to excessive reactive
oxygen species (ROS) production. This imbalance, exacerbated by
amino acid metabolic issues, weakens the antioxidant defense,
causing ROS accumulation. ROS oxidize molecules like LDL, which
macrophages absorb, forming foam cells and releasing inflammatory
mediators. Excessive ROS damage endothelial cell membranes,
activate the inflammation pathway through molecules like nuclear
factor-κB (NF-κB) and increase pro-inflammatory factors like IL-6
and TNF-α. Thus, oxidative stress from amino acid metabolism
disruption harms endothelial cells and enhances inflammation
(17).
Importantly, the 'metabolism-inflammation'
perspective holds great promise for identifying novel therapeutic
targets and diagnostic markers for CHD. By re-evaluating CHD
through this lens, it is possible to lay a theoretical groundwork
for the development of more effective treatment strategies and
early-stage diagnostic tools, which are crucial for clinical
practice. This perspective not only uncovers additional factors
driving CHD development but also offers fresh insights into the
intricate interplay between metabolism and inflammation in CHD, an
aspect that has been hitherto under-emphasized.
Despite the increasing attention paid to the
regulatory role of amino acid metabolism in the inflammatory
response associated with CHD, the precise mechanisms and
comprehensive functions involved in disease progression remain
insufficiently explored. This review seeks to synthesize current
research advancements concerning the interplay between amino acid
metabolism and the CHD inflammatory response. It aims to
systematically elucidate the underlying molecular mechanisms,
evaluate the implications for clinical diagnosis and therapeutic
applications, and thereby offer novel perspectives and directions
for future research in this domain.
Overview of amino acid metabolism
Amino acids, the building blocks of proteins, are
central to metabolic pathways and also participate in immune
regulation and the inflammatory response. Amino acids such as
arginine, glutamate, branched-chain amino acids (BCAAs) and
tryptophan, can regulate the inflammatory cascade through their
metabolites and signaling pathways, influencing the onset and
development of CHD.
Arginine
Arginine, a notable non-essential amino acid, plays
a vital role in the cardiovascular system, mainly via its
involvement in the nitric oxide (NO) synthesis pathway. The
synthesis of NO is mediated by the NO synthase (NOS) enzyme family,
which includes endothelial NOS (eNOS), neuronal NOS and inducible
NOS (iNOS). Notably, NO can also be synthesized through
non-enzymatic pathways, particularly under hypoxic conditions
(18). NO is essential for
modulating immune cell activity, reducing inflammatory responses
and enhancing vascular endothelial function (19).
Importantly, eNOS is constitutively expressed in
vascular endothelial cells, producing low levels of NO to maintain
basal vascular tone. The competition between arginase and eNOS for
their common substrate L-arginine impairs NO-mediated vasodilation
(20). Specifically, arginase
utilizes L-arginine to produce urea and ornithine, limiting the
production of NO (21).
Disruption of arginine metabolism can result in increased levels of
asymmetric dimethylarginine, which serves as a competitive
inhibitor of eNOS. This inhibition reduces the production of
antioxidant NO and increases the generation of superoxide anions
(O2−) (22).
Conversely, iNOS is upregulated in response to inflammatory
stimuli, generating substantial quantities of NO to facilitate
immune defense and inflammatory processes (23,24).
NO's mechanisms in vascular endothelial
cells
Under normal physiological conditions, eNOS uses
L-arginine to produce NO. NO, a potent vasodilator, reduces
intracellular calcium, leading to the relaxation of vascular smooth
muscle and vessel dilation. Furthermore, it exhibits
anti-atherosclerotic properties by suppressing platelet
aggregation, smooth muscle cell proliferation and leukocyte
adhesion, and increasing vascular permeability and inflammation
(25). In addition, NO decreases
arterial stiffness (26),
thereby maintaining vascular homeostasis.
From the perspective of vascular endothelial cells,
NO serves as a crucial mediator in maintaining vascular health
through various mechanisms. NO, released by endothelial cells,
diffuses into vascular smooth muscle cells, where it inhibits the
activation of the TGFβR1 and its downstream Smad signaling pathway.
Such inhibition leads to a reduced expression of inflammatory
factors and genes related to calcification (27). Additionally, omega-3
polyunsaturated fatty acids, such as eicosapentaenoic acid, enhance
the activity of eNOS by activating the PI3K/Akt signaling pathway,
thereby increasing NO production and reducing oxidative stress.
This leads to improved endothelial function and mitigates the
proinflammatory response induced by IL-6 (28).
Conversely, insufficient NO impairs the
endothelium's vasodilatory, antithrombotic and anti-inflammatory
functions, resulting in endothelial dysfunction (29,30). Endothelial dysfunction activates
NF-κB, which upregulates adhesion molecules like intercellular
adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1
(31). These molecules attract
leukocytes to adhere to the endothelium. Subsequently, chemokines
help leukocytes infiltrate the vessel wall. Activated leukocytes
release TNF-α and IL-1β, fueling local inflammation. This cascade
promotes atherosclerosis and CHD, worsening plaque development and
instability.
Factors influencing NO generation and
function
Numerous factors exert a significant influence on
the synthesis and functionality of NO, with certain factors having
a more pronounced impact on the pathogenesis of CHD. Oxidative
stress is highly important in this process. On one hand, oxidative
stress can reduce the production of NO and interfere with the NO
signaling pathway by oxidatively modifying NO target proteins
(32). On the other hand, a
large number of ROS generated by oxidative stress, such as
superoxide anions and peroxynitrites, can rapidly react with NO,
greatly reducing the bioavailability of NO and promoting the
occurrence and development of CHD. For instance, angiotensin II
activates the angiotensin II receptor type 1/NADPH oxidase pathway,
resulting in substantial generation of ROS. These ROS oxidize the
co-factor tetrahydrobiopterin (BH4) of endothelial eNOS, rendering
eNOS inactive and causing eNOS uncoupling, which ultimately results
in an imbalance between NO and ROS (33). Conversely, an adequate supply of
BH4 can restore eNOS functionality, enhance NO production and
inhibit ROS generation, thus alleviating inflammation and
senescence in endothelial cells (34). Furthermore, the orphan nuclear
receptor 77 can bind to the promoter region of eNOS, enhancing NO
production. Concurrently, it upregulates the expression of
antioxidant enzymes, mitigating endothelial damage caused by
oxidative stress (35). By
contrast, the inhibition of sirtuin (Sirt)3 activates the NF-κB
signaling pathway, leading to worsening vascular inflammation,
reduced NO production, increased ROS levels and decreased levels of
L-arginine, further deteriorating vascular lesions (36).
In the context of macrophage polarization, NOS
serves as a marker for M1 polarization of macrophages, while
arginase-1 (ARG1) is a marker for M2 polarization (37). Regarding the different isoforms
of ARG, ARG2 has a distinct intracellular localization compared
with ARG1. ARG2 is mainly restricted to mitochondria and exhibits a
lower affinity for L-arginine than NOS (38). ARG2 can regulate NO production
and vascular endothelial function by modulating eNOS activity
(39). The regulation of
inflammatory cytokines and immune cells by arginine and NO is
presented in Fig. 1.
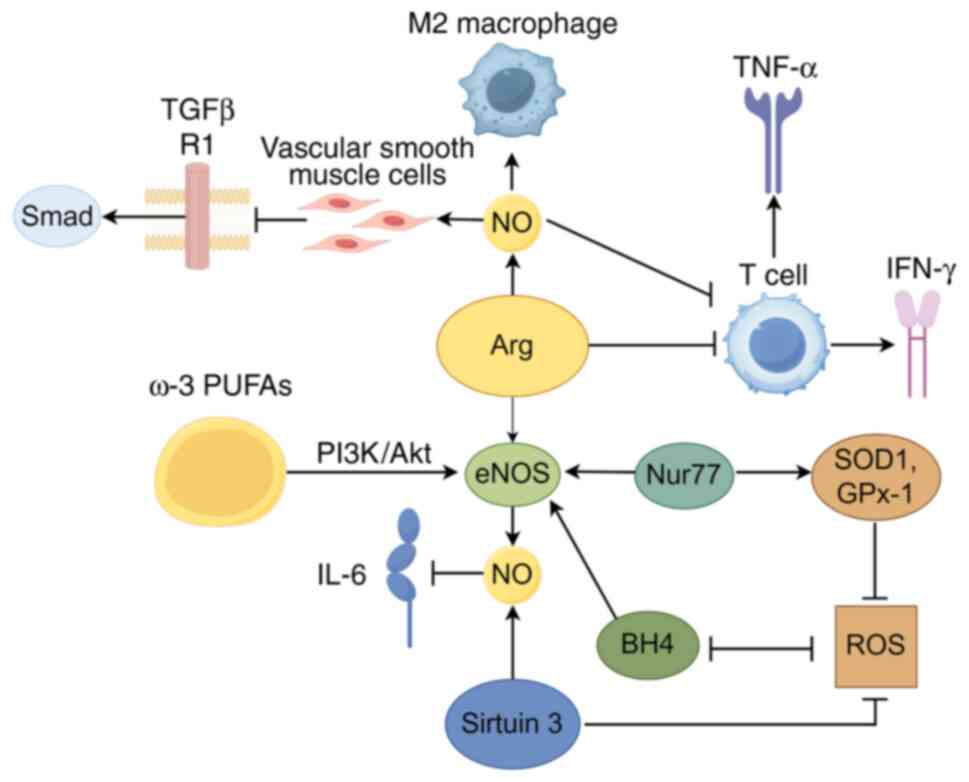 | Figure 1Regulation of inflammatory cytokines
and immune cells by Arg and NO (figure generated with Figdraw). NO,
nitric oxide; eNOS, endothelial NO synthase; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ; ω-3 PUFAs, omega-3 polyunsaturated
fatty acids; Nur77, orphan nuclear receptor 77; SOD1, superoxide
dismutase 1; GPx-1, glutathione peroxidase 1; BH4,
tetrahydrobiopterin; ROS, reactive oxygen species; Arg,
Arginine. |
Clinically, NO and related factors like BH4 could be
potential biomarkers for CHD diagnosis. Monitoring their levels
helps assess disease status. For treatment, they offer drug
targets, and understanding them aids in optimizing existing drugs
and personalized therapy.
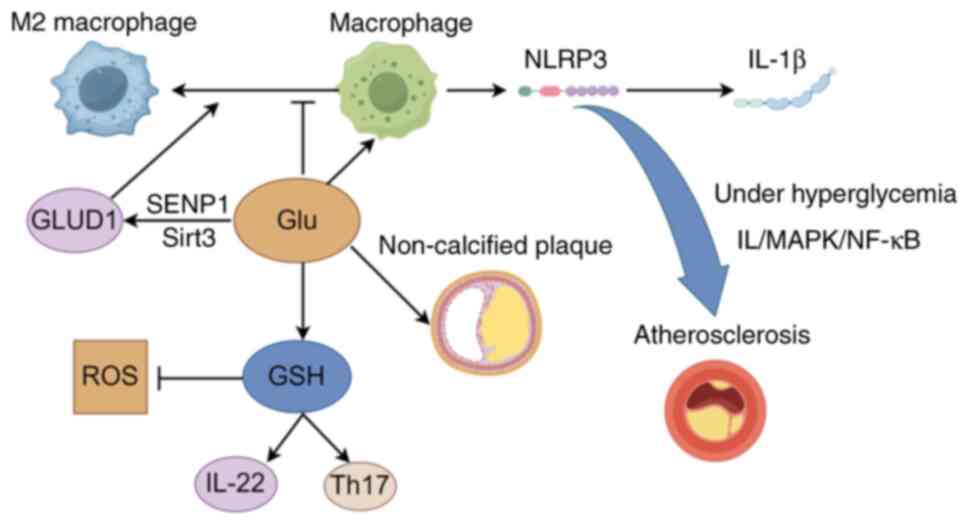
Glutamate
Research has provided critical insights into the
relationship between glutamate and cardiovascular diseases (CVDs).
A comprehensive study conducted by Ottosson et al (40), which included 6,865 Swedish
participants, demonstrated that glutamate serves as an independent
predictor of aortic stiffness. Similarly, the investigation by
Vernon et al (41)
involving 1,002 patients with CHD identified a significant positive
correlation between glutamate levels and the presence of
non-calcified plaques. Taken together, these findings imply that
fluctuations in glutamate levels may play a role in the initiation
and progression of CVDs.
Mechanisms of glutamate metabolism
dysregulation affecting cardiovascular health
The dysregulation of glutamate metabolism has
valuable implications for cardiovascular health through various
mechanisms. Notably, it can induce oxidative stress in endothelial
cells, initiating inflammatory responses (42). Research conducted by Wang et
al (43), which utilized
liquid chromatography-mass spectrometry (LC-MS) technology,
demonstrated a marked increase in serum glutamate levels in
patients with atherosclerosis, further validating the involvement
of glutamate metabolic disorders in the pathogenesis of
atherosclerosis. Glutathione (GSH), a tripeptide composed of
glutamate, cysteine and glycine, acts as a crucial intracellular
antioxidant. It plays an essential role in scavenging free radicals
and maintaining cellular redox homeostasis, mitigating the
progression of atherosclerosis (44).
Roles of glutamate and related
metabolites in cardiovascular events
Beyond their direct influence on cardiovascular
health via metabolic pathways, glutamate and its related
metabolites are integral to specific cardiovascular events and
immune regulation. Empirical evidence indicates that glutamine
enhances left ventricular systolic function post-myocardial
infarction, with notable alterations observed in glutamine
metabolism-related metabolites within macrophages following such
events (45). Furthermore, a
significant inverse correlation exists between the
glutamine/glutamate ratio and the coronary artery disease (CAD)
phenotype, suggesting that this ratio could serve as a potential
biomarker for CAD assessment (46). These findings are particularly
noteworthy, as they not only elucidate the protective mechanisms of
glutamine in myocardial infarction but also propose a potential
biomarker for the early diagnosis and monitoring of CAD, which
could transform the management of this widespread cardiovascular
condition.
Functions of glutamate, glutamine and
glutathione in immune regulation
In the realm of immune regulation, glutamine, GSH
and glutamate are also important. GSH is essential for maintaining
the immune functionality of Th17 cells by facilitating
mitochondrial signal transduction and the synthesis of IL-22
protein in these cells (47).
Simultaneously, glutamine plays a pivotal role in the activation
and proliferation of CD8+ T cells, as well as in the secretion of
cytokines such as IFN-γ, underscoring its significance in
modulating cellular immune responses (48). Dysregulation of glutamate
metabolism leads to multiple pro-inflammatory responses. Firstly,
it increases ROS levels. ROS activates the IκB kinase (IKK)
complex, which phosphorylates and degrades IκBα. This liberates
NF-κB, enabling its translocation into the nucleus to activate
inflammation-related genes (49). Furthermore, overactivation of
glutamate receptors, such as NMDA receptors, raises intracellular
Ca2+ levels. Ca2+ then activates protein
kinase C, which in turn activates the IKK complex. This leads to
IκBα phosphorylation and degradation, ultimately activating the
NF-κB pathway (50). In
macrophages, glutamate activates the NF-κB signaling pathway, which
upregulates genes related to NOD-like receptor protein 3 (NLRP3)
activation. Subsequently, the NLRP3 complex assembles and proceeds
to convert pro-IL-1β into its active form, IL-1β, intensifying the
inflammatory response (51).
Under high-glucose conditions, NLRP3 expression is upregulated,
deteriorating inflammation through the IL/MAPK/NF-κB pathway and
accelerating the progression of atherosclerosis (52). The roles of glutamine, GSH and
glutamate in immune regulation highlight their potential as
therapeutic targets for modulating immune responses in CVDs,
indicating promising avenues for future research and treatment. The
regulation of inflammatory cytokines and immune cells by glutamate
and GSH is shown in Fig. 2.
In clinical diagnosis, the glutamine/glutamate ratio
can be a biomarker. A lower ratio indicates a higher risk of CAD,
enabling early detection. On the other hand, regarding therapeutic
interventions, glutamine supplementation may exert cardioprotective
effects after myocardial infarction. Drugs regulating glutamate
metabolism to control macrophage-mediated inflammation could also
be developed, aiming to improve CVD outcomes.
Role of branched-chain amino acids in
CHD
Association with atherosclerosis
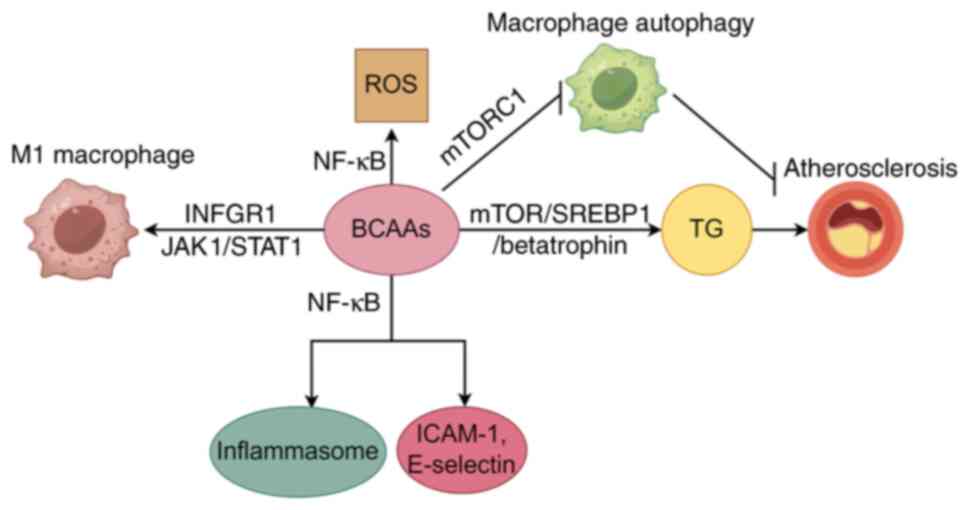
BCAAs, a subset of essential amino acids
distinguished by their aliphatic side-chain structures, primarily
comprise leucine, isoleucine and valine. Considering that carotid
intima-media thickness (CIMT) is a critical marker of early
atherosclerotic lesions, the positive correlation between elevated
levels of BCAAs and increased CIMT suggests a potential role for
BCAAs in the early development of atherosclerosis (53). Epidemiological evidence further
substantiates the independent association between elevated levels
of BCAAs and the incidence of CHD, as well as its prevalent
traditional risk factors, including insulin resistance,
dyslipidemia and endothelial dysfunction. This association
significantly heightens an individual's risk of developing CHD and
stroke (54,55). Recent research has focused on the
potential significance of the ratio of BCAAs to other compounds in
the context of CHD. Notably, the ratio of total BCAAs to glycine
appears to exert a more substantial influence on CHD than
individual indicators. This observation suggests that this ratio
may be of considerable importance in the assessment of CHD risk
(56).
Recent studies have elucidated the impact of BCAAs
on myocardial metabolism, highlighting their potential to disrupt
normal triglyceride metabolism via the activation of the
mTOR/SREBP-1/betatrophin pathway, a process contingent upon
p38-MAPK (57). This
hyperactivation of the lipid synthesis pathway results in
dysregulated triglyceride metabolism and subsequent myocardial
glycolipid metabolic disorders, thereby importantly contributing to
the pathological progression of CHD (58). In addition, BCAAs have been shown
to enhance macrophage activation and the secretion of
pro-inflammatory cytokines, such as IL-6 and TNF-α, through the
mTOR/NF-κB signaling pathway, thus promoting inflammatory processes
(59-61).
Impact on signaling pathways and
inflammatory response
In the pathogenesis of CVDs, the metabolism of BCAAs
and their associated signaling pathways play a critical role. The
complex mechanisms of interaction among these pathways have become
a focal point of contemporary scientific investigation.
Increased levels of BCAAs can trigger a series of
adverse physiological reactions. On one hand, they activate the
IKK, leading to the phosphorylation and degradation of IκB, and
subsequently releasing the NF-κB. After NF-κB translocates into the
nucleus, it promotes the transcription of inflammatory genes such
as ICAM-1 and E-selectin, which not only disrupts mitochondrial
function but also leads to metabolic disorders (62). Furthermore, increased BCAA
concentrations can activate macrophages with a pro-inflammatory
phenotype, triggering a cascade of inflammatory responses (63). The accumulation of BCAAs also
activates the NF-κB signaling pathway in blood cells, resulting in
the release of inflammatory mediators that facilitate the adhesion
of inflammatory cells. This sequence of events enhances the
production of ROS, ultimately leading to endothelial dysfunction
(61). On the other hand, BCAA
transaminase acts on all three BCAAs, converting them into
branched-chain keto acids (BCKAs) and glutamate. BCKAs can promote
the production of ROS due to their ability to cause mitochondrial
respiratory dysfunction, indicating that the BCKA-ROS axis may
contribute to the development of heart diseases (64). In addition, BCAAs can induce the
formation and secretion of disulfide-group-modified high-mobility
group box 1 protein (HMGB1) in macrophages through the
mitochondrial-nuclear H2O2 signaling pathway,
which activate pro-inflammatory macrophages and trigger
inflammation via the HMGB1/Toll-like receptor 4 (TLR4)/NF-κB
pathway, promoting the progression of atherosclerosis (63). In addition, the accumulation of
BCAAs can over-activate mTOR, which not only leads to a phenotypic
transformation of vascular smooth muscle cells, causing
mitochondrial ROS damage and triggering vascular inflammation
(65), but also mediates the
upregulation of IL-6 and TNF-α. Elevated IL-6 levels can disrupt
the normal function of the cardiovascular system by promoting
chronic inflammation and inducing endothelial cell activation,
leading to the recruitment of immune cells and the release of
additional pro-inflammatory factors (66). TNF-α, on the other hand, can
damage cardiomyocytes, promote the apoptosis of endothelial cells
and contribute to the development of cardiac fibrosis (67,68).
Leucine and valine positively influence the
AKT/forkhead box O1 signaling pathway in hepatic and adipose
tissues by inhibiting gluconeogenesis and promoting glucose
homeostasis. This modulation aids in stabilizing blood glucose
levels, enhancing insulin sensitivity and reducing inflammatory
processes (69). These
physiological effects are particularly significant in the context
of CHD, as they mitigate risk factors and offer a potential
therapeutic target for both prevention and treatment. In addition,
the MAPK signaling pathway plays a crucial role in the inflammatory
response associated with amino acid metabolism. In neonatal
patients with CHD, elevated leucine levels may suppress the WD
repeat containing planar cell polarity effector/MAPK axis,
resulting in increased endomucin expression and subsequently
impairing the normal function of cardiac microvascular endothelial
cells (70). The regulation of
inflammatory cytokines and immune cells by BCAAs is exhibited in
Fig. 3.
Clinical significance and dietary
considerations of BCAAs in CHD
In the context of clinical relevance, BCAAs have
emerged as key players in multiple aspects related to
cardiovascular health. Plasma levels of BCAAs are valuable
biomarkers for cardiac-metabolic complications following orthotopic
liver transplantation (71).
High intakes of BCAAs, including isoleucine, leucine and valine,
are independently associated with an increased risk of the
progression of coronary artery calcification (72). This indicates that the amount of
BCAAs in the diet can directly impact CVD progression. In terms of
dietary impacts, BCAAs from different sources show distinct effects
on cardiometabolic health. BCAAs from poultry, whole grains and
nuts, soy, as well as vegetables and fruits may be beneficial for
cardiometabolic health, while those from red meat, processed meat,
fish and refined grains are detrimental (73). Such dietary differences in BCAA
sources highlight the importance of dietary choices in modulating
CVD risk. Furthermore, 3-mercaptopyruvate sulfurtransferase-derived
mitochondrial H2S may play a regulatory role in BCAA
catabolism and mediate critical cardiovascular protection in heart
failure (74). This reveals an
additional layer of complexity in the regulation of BCAA metabolism
and its connection to cardiovascular protection. Dietary
supplementation with BCAAs can alleviate atherosclerosis induced by
high lipoproteins in apolipoprotein (Apo)E−/− mice. The
mechanisms involve improving dyslipidemia and inflammation,
modulating the gut microbiota and promoting bile acid excretion
(75).
In clinical diagnosis, integrating BCAA-related
markers with traditional biomarkers could potentially enhance the
early detection of CHD. Regarding treatment, small-scale human
trials have investigated BCAA-targeted therapies. However,
large-scale, long-term clinical trials are urgently needed to
validate the efficacy and safety of such interventions. These
findings add to the existing knowledge about BCAAs, which in turn
help us better understand the implications of the current
study.
The complex roles of tryptophan
metabolism in CHD
In contrast to the mechanisms of BCAAs in CVDs,
tryptophan and its metabolites exhibit complex roles within this
context. Recent evidence suggests an inverse relationship between
tryptophan levels and CVD risk, indicating that higher tryptophan
levels are associated with a decreased risk of CVD. However,
tryptophan metabolites, such as kynurenine and serotonin, are
positively correlated with an increased risk of CVD (76). Elevated kynurenine levels are
remarkably associated with insulin resistance and β-cell
dysfunction, potentially contributing to the pathogenesis of
diabetes through mechanisms involving inflammatory responses and
oxidative stress, influencing the pathological progression of CHD
(77).
Pro-inflammatory effects mediated by
tryptophan metabolites
Regarding the regulation of the inflammatory
response, tryptophan metabolites influence the process by
activating the aryl hydrocarbon receptor (AhR), which significantly
impacts the stability of atherosclerotic plaques, endothelial
function and the myocardial inflammatory microenvironment (78). Upon activation, the AhR signaling
pathway promotes the activation of inflammatory cells and the
substantial release of inflammatory mediators, intensifying the
inflammatory response within the cardiovascular system (79). Specifically, activated AhR
translocates to the nucleus and binds to specific DNA sequences,
leading to the upregulation of genes encoding pro-inflammatory
cytokines such as TNF-α and IL-6, as well as chemokines like C-X-C
motif chemokine ligand (CXCL)8. These cytokines and chemokines
subsequently recruit and activate immune cells, perpetuating the
inflammatory cascade in cardiovascular tissues. Efferocytosis
enhances tryptophan metabolism and indoleamine 2,3-dioxygenase 1
(IDO1) activity, facilitating the kynurenine-mediated catabolic
process through the production of IL-10. This mechanism may
elucidate why macrophage IDO1 deficiency hinders the regression of
atherosclerosis (80). However,
the role of tryptophan metabolites in inflammation regulation is
not one-sided. Intriguingly, certain tryptophan metabolites operate
in an opposing manner, acting as guardians against inflammation
rather than its instigators (81).
Anti-inflammatory tryptophan metabolites
and their clinical potential
It is important to note that not all tryptophan
metabolites exhibit pro-inflammatory effects. For instance,
5-methoxytryptophan (5-MTP), an endogenous tryptophan metabolite,
has been shown to possess anti-inflammatory properties and to
inhibit cell proliferation and migration. 5-MTP may serve as a
valuable marker for assessing the long-term prognosis of patients
with acute myocardial infarction following percutaneous coronary
intervention (82). Another
tryptophan-derived metabolite, indole-3-acetic acid (IAA), inhibits
the TLR4/MyD88/NF-κB signaling pathway in M1 macrophages, promotes
M2 macrophage polarization and restores the balance of M1/M2
polarization, thereby reducing aortic inflammation (83). The anti-inflammatory and
anti-proliferative properties of 5-MTP and IAA, along with their
potential utility in evaluating the long-term prognosis of patients
with acute myocardial infarction post-percutaneous coronary
intervention, make them highly promising as biomarkers and
prospective therapeutic targets. Further research into their
underlying mechanisms and clinical applications may reveal novel
strategies for the management of CHD.
Tryptophan metabolism and
cardiovascular-related conditions
Coronary thrombosis has been associated with
elevated levels of circulating l-tryptophan, which is associated
with gut microbiota dysbiosis (84). Reduced circulating tryptophan
levels are associated with an increased risk of CVD events
(85). The metabolism of
L-tryptophan is related to lower limb ischemia and thrombosis
(86). Taurine stimulates
tryptophan metabolism, reducing the inflammatory response and
oxidative stress in the paraventricular nucleus through gut-brain
communication, thereby decreasing sympathetic nerve activity and
blood pressure in hypertensive rats (87).
The tryptophan metabolite indole-3-propionic acid
can alleviate the occurrence and severity of aortic dissection (AD)
in mice (88). Although this
finding is mainly related to AD, inflammation is involved in the
pathogenesis of various diseases, and there may be potential
connections between the role of tryptophan metabolites in AD and
CVDs. Further research in this area may provide new insights into
the relationship between tryptophan metabolism and CVD.
The anti-inflammatory and other properties of
tryptophan-related substances and their metabolites further enhance
the understanding of the intricate relationship between tryptophan
metabolism and CVDs. In clinical diagnosis, the levels of
tryptophan and key metabolites, such as kynurenine, may serve as
novel biomarkers for CHD. Monitoring the kynurenine-to-tryptophan
ratio may be particularly useful in assessing disease progression,
particularly in patients with diabetes, where tryptophan metabolism
is often disrupted. Regarding treatment, drugs that target
tryptophan metabolism pathways hold promise. For instance,
inhibitors of IDO1 could potentially reduce inflammation.
Additionally, supplementing with anti-inflammatory metabolites like
5-MTP and IAA is an area worth exploring. However, more research is
needed to determine optimal dosages, effective delivery methods and
long-term safety for these potential therapeutic approaches.
Relationship between amino acid metabolism
and the immune system
In the analysis of the roles of various amino acids
in the inflammation associated with CHD, it is imperative to
understand the interplay between amino acid metabolism and the
immune system, which is integral to the pathogenesis of CHD.
Furthermore, amino acid metabolism has the potential to modulate
immune cell functions, thereby influencing the inflammatory
response in the context of CHD.
Amino acid metabolism serves a dual function:
Supplying energy and substrates essential for intracellular protein
synthesis, while also directly modulating immune cell functions and
activities via its metabolites. This progression is critical in the
inflammatory response associated with CHD. The functional states of
T cells, B cells and macrophages-key constituents of the immune
system-are intricately connected to amino acid metabolism,
collectively impacting the onset and progression of
atherosclerosis.
T-cell activation and function dependence
on amino acid metabolism
The activation and normal functioning of T-cells are
critically dependent on amino acid metabolism. Extensive research
has demonstrated that diminished arginine bioavailability
significantly affects T-cell metabolism and function. This
metabolic alteration results in enhanced activation and
differentiation of T-cells, culminating in the excessive production
of pro-inflammatory cytokines, such as IFN-γ and TNF-α. These
inflammatory mediators contribute to endothelial dysfunction, foam
cell formation and plaque progression, thereby exacerbating the
pathogenesis of atherosclerosis (89,90). Arginine is a critical substrate
for NOS in T-cells, where NO typically functions as a negative
regulator of T-cell activation. In the absence of adequate NO,
T-cells are more susceptible to overactivation, disrupting the
immune response balance. This imbalance results in the
overproduction of pro-inflammatory cytokines, which facilitate the
recruitment and activation of additional immune cells, further
amplifying the inflammatory process in atherosclerosis. In studies
related to non-small cell lung cancer, it has been observed that a
reduction in amino acid metabolism, along with the inactivation of
the mTOR signaling pathway, can lead to the exhaustion of
CD8+ T cells, thereby diminishing the body's immune
defense capabilities (91).
Given the close association between immune regulation and CHD, it
is imperative to conduct in-depth investigations to ascertain
whether a similar phenomenon occurs in the pathogenesis and
progression of CHD.
Beyond the well-established link between T-cell
activation and amino acid metabolism, it is crucial to explore the
distinct roles of various T-cell subsets in conditions related to
CHD. Notably, individuals with premature coronary artery disease
(PCAD) exhibit significantly enhanced cytotoxic and proliferative
capabilities mediated by T-cells, underscoring their pivotal role
in the early stages of disease progression (92). By contrast, Tregs demonstrate a
protective effect in patients with CAD and type 2 diabetes
mellitus, with Treg levels showing an inverse correlation with the
severity of coronary artery lesions (93). A risk-prediction model
incorporating peripheral blood IL-6 levels and Treg percentages
presents a novel method for the early detection of CHD risk in
patients with primary Sjögren's syndrome (94). These findings underscore the
potential of targeting Tregs as a therapeutic strategy for CHD.
Amino acid metabolism and B-cell function
in CHD
In recent years, the role of B-cells in the
pathogenesis and progression of CHD has attracted increasing
scholarly attention. Amino acid metabolism is pivotal in regulating
the metabolic reprogramming and functional activities of B-cells, a
process that is of significant importance for the immune response
and the modulation of inflammation within the body.
Glutamine, an essential energy substrate for B-cell
activation, is vital for maintaining B-cell survival and proper
function by supporting mitochondrial integrity and protein
synthesis (95,96). The metabolism of glutamine
inhibits glycogen synthase kinase 3 activity by activating
mammalian target of rapamycin complex 1 (mTORC1), thereby enhancing
the expression of IL-10 in B-cells (97). Additionally, tryptophan
metabolism primarily produces AhR ligands via the kynurenine,
serotonin and indole-3-pyruvate pathways. Activation of AhR can
modulate B-cell polarization and promote the secretion of IL-10 by
B-cells (98,99). Collectively, these findings
underscore the influence of amino acid metabolism on B-cell
functionality, with potential implications for the inflammatory
response and the pathogenesis of CHD.
In individuals diagnosed with CHD, the functional
states of B-cell subsets are intricately associated with the
severity of the disease. Circulating CD11c+ B-cells
include various subsets, notably age-related B-cells and
IgD−CD27− double-negative B-cells, which
demonstrate a positive correlation with CHD severity (100). Among patients with low-severity
CAD, there is an observed increase in the negative regulators of
B-cell receptor signaling mediated by CD72, which may potentially
slow disease progression by maintaining B-cell homeostasis.
Furthermore, elevated levels of B-cell-activating factor (BAFF) are
linked to the severity of CAD and acute myocardial infarction
(101), suggesting that BAFF
could serve as a viable biomarker for disease diagnosis and
assessment. The p62 protein promotes the proliferation of B1B cells
through the regulation of the NF-κB signaling pathway and exhibits
a degree of resistance to CHD (102).
Macrophages play a dual role in
atherosclerosis
Macrophages play a dual role in vascular homeostasis
and the pathogenesis of atherosclerosis. They contribute to
vascular homeostasis through the clearance of ox-LDL. However, they
also secrete pro-inflammatory cytokines, such as TNF-α and IL-6,
which exacerbate inflammatory responses and facilitate
atherosclerosis progression (103).
Leucine is known to activate the mTORC1 signaling
pathway, thereby inhibiting macrophage autophagy and leading to
macrophage accumulation on the endothelial surface of blood
vessels, which accelerates atherosclerosis development (104). Furthermore, BCAAs promote
macrophage polarization towards the pro-inflammatory M1 phenotype
through the INFGR1/JAK1/STAT1 signaling pathway, thereby enhancing
inflammatory responses and insulin resistance (105). By contrast, glutamate
metabolism enhances the activity of glutamate dehydrogenase 1 via
the sentrin-specific protease 1-Sirt3 axis, inducing macrophage
polarization towards the anti-inflammatory M2 phenotype (106). In addition, small extracellular
vesicles found in the pericardial fluid of patients with CHD
influence macrophage phenotypes, resulting in the upregulation of
pro-inflammatory markers such as CD86 and inflammatory cytokines
including IL-1β and TNF-α, thereby augmenting the inflammatory
response (107).
Given the pivotal role of macrophages in the
pathogenesis of CVDs, a variety of targeted therapeutic strategies
have been developed. These strategies frequently achieve their
therapeutic effects by modulating amino acid metabolism or related
signaling pathways. For instance, cannabidiol has been demonstrated
to regulate the TLR4/MyD88/NF-κB signaling pathway, effectively
inhibiting the polarization of macrophages towards the
pro-inflammatory M1 phenotype and exhibiting important
anti-atherosclerotic properties (108). Furthermore, the alkaloid SZ-A,
derived from mulberry twigs, enhances endothelial cell function and
mitigates inflammatory responses by reducing CXCL-10 secretion from
M1 macrophages, offering a novel therapeutic approach for
atherosclerosis treatment (109). In addition, the
mitochondria-targeted ROS scavenger MitoTEMPO and the lon peptidase
1, mitochondrial inhibitor bortezomib have been shown to restore
mitochondrial homeostasis in foam cells and reduce oxidative
stress-induced damage (110).
Furthermore, the long non-coding (lnc)RNA lnc-MRGPRF-6:1 has been
identified as a promoter of ox-LDL-induced macrophage ferroptosis
through the inhibition of GSH peroxidase 4, thereby influencing the
progression of atherosclerosis (111).
In recent years, chimeric antigen receptor
macrophage (CAR-M) therapy has shown considerable promise in the
treatment of CVDs. Fibroblast activation protein CAR-Ms have been
demonstrated to alleviate myocardial fibrosis and maintain cardiac
function, presenting a novel therapeutic strategy for myocardial
ischemia-reperfusion injury and other CVDs characterized by a
fibrotic phenotype (112).
Furthermore, the expression of circulating RNA ARCN1 in macrophages
plays a pivotal role in the pathogenesis of atherosclerosis by
regulating HuR-mediated ubiquitin-specific protease 31 mRNA
stability and NF-κB activation (113). Glutamine, as a crucial
metabolic substrate, not only inhibits the excessive production of
ROS and the activity of matrix metalloproteinases in vascular
smooth muscle cells but also suppresses the activation of M1
macrophages and the apoptosis of vascular smooth muscle cells
(114). This effectively
safeguards the cardiovascular system against inflammation and
damage.
These intervention strategies underscore the pivotal
role of macrophages in atherosclerosis and provide novel insights
for therapies targeting amino acid metabolism and its associated
signaling pathways.
In summary, amino acid metabolism exerts a
substantial influence on the inflammatory response and progression
of atherosclerosis by intricately modulating the functions of T
cells, B cells and macrophages. Extensive research into these
mechanisms is anticipated to establish a more robust theoretical
foundation for the prevention, diagnosis and treatment of CVDs,
facilitate the development of innovative intervention strategies
and potentially lead to remarkable advancements in the prevention
and management of cardiovascular conditions. The specific effects
of arginine, glutamic acid, BCAAs and tryptophan on inflammation,
immune cells and CHD outcomes are detailed in Table I.
 | Table IComparison of the effects of
different amino acids on inflammation, immune cells and outcomes of
CHD. |
Table I
Comparison of the effects of
different amino acids on inflammation, immune cells and outcomes of
CHD.
| Amino acids | Impact on
inflammation | Effect on immune
cells | Impact on the
outcomes of CHD |
|---|
| Arginine | Reduces NO
production during metabolic disorders and promotes inflammation;
iNOS is upregulated under inflammatory stimulation. | Influences the
activation of T cells; when there is a deficiency of arginine, T
cells are over-activated and the amount of pro-inflammatory factors
increases. | Supplementation
with L-arginine can reduce plaques and vascular inflammation;
arginine methylation regulates vascular calcification; high
arginine levels are associated with CHD. |
| Glutamate | Metabolic disorders
trigger oxidative stress and inflammatory responses, activating the
NF-κB pathway. | Glutathione
maintains the function of Th17 cells; glutamine promotes the
function of CD8+ T cells; metabolic disorders interfere with the
function of immune cells. | The level of
glutamate is associated with cardiovascular diseases; gluta mine
has a cardioprotective effect and the ratio of glutamine to
glutamic acid can be used to evaluate CHD. |
| BCAAs | An increase in the
level activates the inflammation-related pathways, promotes the
polarization of macrophages towards the M1 phenotype and releases
pro-inflammatory factors. | Leucine inhibits
macrophage. autophagy; BCAAs promote the polarization of
macrophages towards the M1 phenotype; glutamine inhibits the
activation of M1 macrophages | It is positively
correlated with CIMT and affects the incidence risk of CHD; high
intake is related to the progression of coronary artery
calcification and different food sources have different effects;
BCAA metabolic defects affect cardiac function. |
| Tryptophan | The level of
tryptophan is negatively correlated with the risk of CHD, while
some of its metabolites, such as kynurenine, are positively
correlated. Some metabolites activate the AhR and exacerbate
inflammation. | Tryptophan
metabolites regulate the polarization of B cells and the secretion
of IL-10; tryptophan metabolism in macrophages affects
inflammation. | Circulating
tryptophan levels are associated with CHD events; 5-MTP and IAA
have anti-inflammatory properties and can be used to evaluate the
prognosis. |
Inflammatory markers and risk assessment of
CHD
The interplay between amino acid metabolism and the
immune system exerts a profound influence on the inflammatory state
in CHD. This section will concentrate on the inflammatory markers
intricately linked to the pathogenesis and progression of CHD.
These markers not only facilitate the assessment of CHD risk but
also elucidate the underlying inflammatory mechanisms.
Inflammation emerges as a central theme in the
pathogenesis and progression of CHD, serving as a critical
determinant of disease advancement and prognosis. Within this
complex pathophysiological framework, a wide array of inflammatory
cytokines and markers are involved, each playing an indispensable
role and notably impacting the developmental trajectory of CHD.
Notably, C-reactive protein (CRP), IL-6 and TNF-α have been widely
recognized as significant risk factors for CHD (115).
High-sensitivity (hs)-CRP
hs-CRP is a vital biomarker for assessing systemic
inflammation and predicting the risk of CHD (116). Together with the plasma
atherogenic index (AIP), hs-CRP and AIP act as independent risk
factors for PCAD. The simultaneous evaluation of these biomarkers
improves the precision of predicting PCAD occurrence and the
severity of arterial lesions. Furthermore, their levels demonstrate
a significant positive correlation with the severity of coronary
artery lesions (117). In
patients with chronic coronary syndrome, persistent low-grade
inflammation remarkably influences disease prognosis, with hs-CRP
serving as an effective marker for assessing this impact (118). Furthermore, elevated hs-CRP
levels are strongly associated with an increased risk of abdominal
aortic aneurysm, particularly among smokers and the elderly
(119), underscoring the
importance of hs-CRP in CVD risk assessment.
Arginine intake exhibits a negative correlation with
elevated CRP levels (>3.0 mg/l). However, L-arginine
supplementation does not exert a notable impact on CRP levels
(120). Conversely, glutamine
supplementation has been shown to reduce hs-CRP levels (121). In addition, increased intake of
BCAAs is associated with a decrease in inflammation, as indicated
by reduced hs-CRP levels (122). Within the context of metabolic
syndrome, a correlation exists between the kynurenine/tryptophan
ratio and hs-CRP levels (123).
Further comprehensive research is warranted to elucidate the
specific regulatory mechanisms involved.
CRP primarily exerts its effects through binding to
Fcγ receptors on immune cells, initiating downstream signaling
pathways, particularly the NF-κB pathway (124). This activation results in the
production of pro-inflammatory cytokines and enhanced immune cell
activation. In the context of CHD, these processes contribute to
the recruitment of inflammatory cells to the arterial wall,
accelerate the progression of atherosclerotic plaques, and increase
the risk of plaque rupture and subsequent cardiovascular events
(125).
The interleukin family
IL-6, a pivotal pro-inflammatory cytokine, exhibits
a positive correlation with an elevated risk of cardiovascular
mortality and the incidence of major adverse cardiovascular events
(66). In a study conducted on
patients with CAD, researchers found that IL-6 concentrations in
blood samples obtained from the left anterior descending artery
distal to the stenosis were higher compared to those from the
healthy left internal thoracic artery (P<0.01) (126). Mechanistic studies have
demonstrated that IL-6 activates the JAK1/STAT3 signaling pathway,
leading to the upregulation of divalent metal transporter 1
expression. This upregulation results in a substantial accumulation
of intracellular Fe2+, causing tissue damage (127). Furthermore, IL-6-induced
phosphorylation of STAT3 at Tyr705 promotes the degradation of
mitofusin 2 through an unconventional mitochondrial localization
mechanism. This signaling cascade accelerates the senescence of
vascular smooth muscle cells and the onset of mitochondrial
dysfunction, thereby remarkably advancing the progression of CHD
(128). IL-1β promotes the
transcytosis of LDL via a specific pathway reliant on the LDL
receptor and Rab27a, contributing to the early stages of
atherosclerosis formation (129).
In patients with ST-segment elevation myocardial
infarction, IL-22 levels are markedly lower compared to those in
healthy control subjects (130). Importantly, resveratrol has
been shown to modulate bone marrow-derived dendritic cells,
subsequently affecting the secretion levels of IL-22 and IL-10,
thereby influencing the progression of atherosclerosis (131).
IL-33 enhances cardio-endothelial angiogenesis
post-myocardial infarction by activating the AKT/eNOS/NO signaling
pathway. This mechanism is significant for cardiac repair and
functional recovery, playing a pivotal role during the myocardial
injury repair phase in CHD (132).
In conclusion, the interleukin family members
demonstrate diverse and interrelated roles in the pathogenesis of
CHD. Specifically, IL-6-induced inflammation may facilitate an
environment conducive to IL-1β-mediated initiation of
atherosclerotic plaque formation. Simultaneously, the regulatory
functions of IL-22 and IL-33, characterized by anti-inflammatory
and cardio-reparative effects, respectively, may counteract the
pro-inflammatory actions of IL-6 and IL-1β at various stages of the
disease. A detailed investigation of these complex interactions is
crucial for achieving a more comprehensive understanding of the
inflammatory mechanisms underlying CHD.
IFN-γ
IFN-γ is crucial for exacerbating inflammation and
advancing the progression of atherosclerotic plaques following
anti-programmed death-1 therapy (133). Concurrently, M1 macrophages
possess the ability to activate NK T cells to secrete IFN-γ, thus
accelerating the development of early-stage atherosclerosis
(134). Spatial transcriptomics
analyses have revealed that within the intimal and medial regions
of atherosclerotic plaques, there is a marked increase in the
expression of IFN-γ and major histocompatibility complex (MHC)
class II molecules, accompanied by the presence of pro-inflammatory
and pro-thrombotic signaling pathways (135).
The increased expression of IFN-γ and MHC class II
molecules, along with the activation of pro-inflammatory and
pro-thrombotic signaling pathways, is intricately associated with
the vulnerability of atherosclerotic plaques. This escalation in
inflammation and alteration of immune-related molecular expressions
can undermine the structural integrity of the plaque's fibrous cap,
making it more prone to rupture. When rupture occurs, the plaque
exposes underlying pro-thrombotic substances, leading to platelet
aggregation and thrombus formation, which are key processes in the
pathogenesis of acute coronary syndromes, such as myocardial
infarction and unstable angina.
Monocyte chemoattractant protein-1
(MCP-1)
An elevation in MCP-1 levels has been shown to be
positively correlated with an increased risk of PCAD (136). MCP-1 facilitates the
chemotactic migration of monocytes beneath the vascular intima,
thereby promoting the aggregation and activation of inflammatory
cells, which in turn accelerates the formation and progression of
atherosclerotic plaques.
Furthermore, MCP-1 engages in complex interactions
with other inflammatory mediators within the context of CHD. For
instance, increased levels of IL-6 can upregulate MCP-1 expression
in endothelial cells and macrophages, establishing a positive
feedback loop that amplifies the inflammatory response (137). Similarly, CRP may affect the
production and function of MCP-1, highlighting the intricate
network of inflammatory mediators involved in the pathogenesis of
CHD (138).
Inflammatory markers and cytokines, through a
complex network of signaling pathways and mechanisms, are
profoundly involved throughout the continuum of CHD.
Clinical research and technological
applications
In the preceding sections, the intricate
interconnections between amino acid metabolism, inflammation and
the immune system within the context of CHD were examined. It is
now imperative to investigate how these insights can be applied to
clinical research and technological innovations. This field of
study seeks to devise effective strategies for the diagnosis,
treatment and prevention of CHD, grounded in our comprehension of
amino acid-related mechanisms.
Insights from animal experimentation
Animal studies are fundamental in elucidating the
pathological connections between amino acid metabolism and CHD. For
instance, supplementation with L-arginine has been demonstrated to
reduce plaque formation and vascular inflammation in Sirt3
endothelial cell knockout mice (36). In cholesterol-fed rabbits,
supplementation with L-aspartate and L-glutamate has been shown to
inhibit the thickening of the aortic intima and the development of
liver injury (139). Mice with
defects in BCAA metabolism exhibit cardiac dysfunction and
remodeling post-myocardial infarction, which is closely associated
with inflammation (140).
Restricting isoleucine intake in young mice has been found to
improve metabolic health, resulting in weight loss, improved blood
glucose control and an extension of lifespan (141). The specific knockout of IDO in
intestinal epithelial cells leads to the formation of larger
atherosclerotic plaques in mice fed a high-fat and high-cholesterol
diet (142). Additionally,
indole-3-acrylic acid, through activation of the AhR, inhibits the
TGF-β/Smad pathway, thereby improving endothelial-mesenchymal
transition in atherosclerotic mice (143).
Findings of human studies
In the domain of human studies, research has
revealed complex relationships between various amino acids and
cardiovascular-related conditions. Arginine methylation has been
shown to regulate vascular calcification through multiple signaling
pathways, including NF-κB, WNT, AKT/PI3K, TGF-β/bone morphogenetic
protein/SMAD and IL-6/STAT3 (144). Lower levels of homoarginine
have been identified as an independent predictor of a high
atherosclerotic burden in patients with ST-elevation myocardial
infarction (145). A reduction
in glutamate levels is correlated with an increase in macrophages
and a pro-inflammatory phenotype in unstable plaques (146). The deletion of glutaminase 2
raises the glutamine/glutamate ratio, accelerating atherosclerotic
lesion progression (147). The
research conducted by Ruiz-Canela et al (148) has proved a significant
correlation between plasma levels of BCAAs and the risk of CVDs,
but the limitations of observational studies, such as confounding
factors related to diet and physical activity, affect the
robustness of this relationship. Laferrère et al (149) reported decreased BCAA, CRP and
IL-6 levels after gastric bypass surgery, yet the small sample size
and the physiological changes induced by surgery limit the
generalizability of these findings. In adolescent patients with
type 2 diabetes, the lipoprotein insulin resistance index and
elevated levels of BCAAs are associated with suboptimal blood
glucose regulation and early onset of atherosclerosis (150). In addition, a high intake of
BCAAs is independently correlated with the progression of coronary
artery calcification (72),
while reduced circulating levels of tryptophan are linked to an
increased risk of CVD (85).
Collectively, these varied findings concerning different amino
acids underscore the complex relationship between amino acid
metabolism and atherosclerosis. This suggests that targeting amino
acid metabolism could serve as a potential therapeutic approach to
reduce inflammation in patients with CHD.
Metabolomics applications
Metabolomics, an advanced and rapidly developing
technical field, provides highly effective methodologies for
in-depth analysis of the complex interaction mechanisms between
amino acid metabolism and CHD. Numerous studies have employed
metabolomics techniques, yielding a range of valuable findings in
this area.
Regarding the relationship between amino acid
metabolism and CHD, several studies have evidenced the crucial
roles of specific amino acids. Research shows that
Huang-lian-Jie-du decoction can reduce oxidative stress by
upregulating arginine biosynthesis, suggesting a novel CHD
prevention and treatment target (151). Additionally, metabolomics-based
analyses reveal a positive correlation between aortic stiffness and
the levels of glutamate and cystine (40). At the plaque level, the
concentration of glutamate within the plaque significantly
influences its vulnerability. Metabolic pathway analysis
demonstrates that D-glutamine and D-glutamate metabolism, as well
as tryptophan metabolism, are involved in the regulation of plaque
vulnerability (152).
In the realm of technical methodologies, the
non-targeted metabolomics approach utilizing LC-MS offers robust
analytical capabilities, enabling a comprehensive and systematic
examination of metabolite profiles within biological samples.
Employing this technique, researchers have discovered a negative
correlation between arteriosclerosis markers and metabolites
associated with BCAAs, as well as indicators of energy metabolism
and oxidative stress (153).
This finding provides novel insights into the pathogenesis of
atherosclerosis. Furthermore, the distinctive features of the amino
acid metabolome hold promise as potential biomarkers for accurately
differentiating between myocardial infarction-induced mortality and
asphyxia-induced mortality in murine models (154). This advancement paves the way
for the early and precise diagnosis of CHD-related conditions and
facilitates the scientific evaluation of the disease.
The integration of nuclear magnetic resonance
spectroscopy with metabolomics has enhanced the precision of CAD
diagnosis. This synergistic approach facilitates dynamic and
real-time disease monitoring while furnishing clinicians with more
comprehensive and reliable data, thereby enabling the optimization
of treatment strategies (155).
Consequently, it offers promising prospects for the personalized
treatment of patients with CHD.
Machine learning contributions
Machine learning techniques, renowned for their
robust data analysis capabilities, have brought new breakthroughs
to the diagnosis of CHD. For instance, a machine learning model
developed by combining immunoglobulin light chains with clinical
data has demonstrated high accuracy and sensitivity in CHD
diagnosis (156), potentially
enhancing the early diagnosis rate of the condition. Due to its
non-invasive nature and rapid analytical capabilities, this model
holds substantial promise for application in large-scale screening
endeavors. Furthermore, machine learning has been applied to
analyze key metabolites associated with atherosclerotic CVD
(157) and to investigate
diagnostic and immune markers (158,159). Although a direct connection
with amino acid metabolism has not yet been established, these
metabolites and markers may contain amino acid-related information,
offering potential insights for further in-depth research on the
role of amino acids in CHD.
Microfluidic technology advancements
Microfluidic technology offers a unique experimental
platform for the research of CHD, particularly in exploring the
macrophage-mediated cardiac immune mechanisms. The advancement of
microfluidic co-culture models allows for an in-depth exploration
of macrophage functions within the cardiac immune process, thereby
providing novel insights for the development of therapeutic
strategies for CHD (160).
In addition, microfluidic technology facilitates the
study of arterial thrombosis formation by constructing artificial
blood vessels and accurately simulating hemodynamics (161). The 3-dimensional microfluidic
atherosclerotic model demonstrates stability under perfusion
conditions and enables real-time observation of immune cell
behavior, thus serving as a valuable tool for atherosclerosis
research (162). Additionally,
the microfluidic model allows for systematic and reproducible
investigation of the effects of arrhythmic blood flow on the
mechanobiology of vascular endothelium (163). In spite of these microfluidic
models being available for research, challenges such as scalability
must be thoroughly addressed to achieve wider applications.
In summary, research on amino acid metabolism and
its role in CHD has enhanced the diagnosis, treatment and
prevention of this condition. It is imperative to further explore
the intricate mechanisms of amino acid metabolism and its specific
pathways in the pathogenesis of CHD. The development of innovative
diagnostic and therapeutic strategies, utilizing metabolomics and
artificial intelligence, should be actively pursued to broaden the
scope for the prevention and management of CVDs.
Clinical applications and future
directions
Building on the knowledge from clinical research and
technological advancements, this chapter focuses on the practical
clinical applications of modulating amino acid metabolism in CHD
and the future directions of this research field. These aspects are
crucial for translating scientific findings into effective patient
care and for advancing the understanding of CHD.
In the context of CVD risk reduction, the regulation
of amino acid metabolism has gained prominence as an important
research focus, primarily involving two main strategies: Dietary
intervention and pharmacotherapy. The primary aim of these
interventions is to enhance cardiovascular health by precisely
modulating amino acid metabolism.
Dietary intervention
Dietary intervention constitutes a fundamental
strategy for regulating amino acid metabolism, with adjustments in
protein intake and composition exerting substantial effects. For
instance, the accumulation of glycated human serum albumin around
cardiac cells can lead to the dysregulation of Nrf-2, exacerbating
oxidative stress. L-arginine, at a low concentration of 20 mM, has
been shown to upregulate Nrf-2 expression, promote its nuclear
translocation and enhance the antioxidant capacity of
cardiomyocytes (164). In an
experimental rat model of isoproterenol-induced myocardial
infarction, supplementation with L-arginine at a dosage of 50
mg/kg/day significantly mitigated the pathophysiological risks
associated with myocardial infarction (165). In a C57BL/6J mouse model,
glutamine supplementation at 1 g/kg has been found to alleviate
atherosclerosis by downregulating O-GlcNAcylation, glycolysis,
oxidative stress and pro-inflammatory pathways (166). Furthermore, leucine, glutamate
and glutamine remarkably influence the atherogenicity of
macrophages through modulating cellular triglyceride metabolism
(167). In the context of
BCAA-enriched diets, each 100 g of diet supplemented with BCAAs
contains additional quantities of 0.56 g of L-leucine, 0.40 g of
L-isoleucine and 0.40 g of valine. This dietary intervention has
been demonstrated to be advantageous in ApoE−/− mice, as
it leads to reductions in serum cholesterol and low-density
lipoprotein cholesterol levels, decreases macrophage infiltration
and suppresses the systemic inflammatory response. Specifically, it
lowers serum levels of inflammatory markers such as MCP-1, TNF-α,
IL-1β and IL-6, thus alleviating atherosclerosis (75). Furthermore, in young individuals
with subclinical atherosclerosis, a high intake of BCAAs (≥18.5
mg/day) is of considerable importance for primary prevention
dietary adjustments (72).
In addition, dietary composition impacts tryptophan
metabolism. Research has shown that a high-fat diet substantially
enhances the activity of IDO1 in the intestine. This increase leads
to an elevated conversion of tryptophan into kynurenine, a notable
reduction in the production of indole-type metabolites, and an
exacerbation of atherosclerosis (142). Another study discovered that
intraperitoneal administration of kynurenine (100 mg/kg) regulated
the interaction between cullin 4B and the AhR. This interaction
forms an E3 ubiquitin ligase complex that binds to RUNX family
transcription factor 2, promoting its ubiquitination and subsequent
proteasomal degradation, thereby reducing arterial calcification
(168). Separately, the
tryptophan metabolite indole-3-propionic acid, derived from the
intestinal microbiota, influences the miR-142-5p/ATP-binding
cassette transporter A1/reverse cholesterol transport axis in
macrophages, affecting the progression of atherosclerotic plaques
in ApoE−/− mice (169). Collectively, these findings
highlight the complex role of tryptophan metabolism in
atherosclerosis and propose that targeting this metabolic pathway
offers a novel strategy for atherosclerosis intervention.
The close association between hepatic fat
accumulation and the increased susceptibility to CHD is a
particular concern (170).
Metabolic-dysfunction-associated fatty liver disease (MAFLD) has
been identified as an independent risk factor for adverse
cardiovascular outcomes in patients with CHD, even when LDL-C
<1.8 mmol/l (171). Research
indicates that moderate to high protein intake is correlated with a
1.45-fold increase in the risk of developing MAFLD (172), which subsequently heightens the
risk of CHD and myocardial infarction (173).
Therefore, dietary interventions aimed at
prevention and treatment of CVD should emphasize a balanced intake
of specific amino acids. For instance, adequate consumption of
L-arginine-rich foods may provide cardioprotective effects.
Regulating fat intake to affect tryptophan metabolism and
monitoring protein consumption to reduce MAFLD-related
cardiovascular risks are essential strategies. These dietary
modifications should be based on a comprehensive understanding of
nutritional biochemistry and its implications for cardiovascular
health.
Drug therapy
Pharmacological interventions represent a potent
strategy for modulating amino acid metabolism, providing
sophisticated approaches for the treatment of CVDs. Nanoliposomes
encapsulating L-arginine and cerium-zirconium oxide nanoparticles
have demonstrated the ability to scavenge ROS, inhibit cholesterol
absorption and promote the phenotypic transformation of
macrophages. These actions confer antioxidant and anti-inflammatory
effects that mitigate the progression of atherosclerosis (174). Significant progress has been
achieved in the research domain of NO donors. In hypoxic
microenvironments, the enzyme nitroreductase is highly expressed
and facilitates the reduction of the nitro group in N6. This
reduction initiates intramolecular electron transfer, thereby
enhancing the electronegativity of the relatively stable NO donor
moiety within the molecule. Consequently, N6 exhibits an increased
affinity for binding to the positively charged active site of the
catalyst cytochrome P450, which ultimately results in the release
of NO. Its effectiveness in preventing and treating myocardial
hypoxic injury exceeds that of the conventional drug isosorbide
mononitrate (175).
Furthermore, the curcumin-encapsulated NO peptide-conjugated
hydrogel offers an innovative approach to cardiovascular
protection. This hydrogel is designed to release NO in response to
β-galactosidase stimulation while simultaneously facilitating the
gradual release of curcumin through hydrogel hydrolysis. Such a
distinctive characteristic equips it with the capacity to
effectively protect vascular endothelial cells from oxidative
stress-induced damage (176).
Recent advancements in research have established a reliable
theoretical framework for the development of novel NO donor drugs,
offering renewed hope for the treatment of CVDs. Although the
investigation of BCAAs and tryptophan in the context of CVDs has
gradually attracted attention, there remains a paucity of
pharmaceutical studies focused on the treatment of CHD using these
two amino acids. Future research should aim to further elucidate
their mechanisms of action and develop relevant therapeutic
drugs.
It is important to acknowledge that not all
pharmacological agents confer benefits to the cardiovascular
system. For instance, the antibacterial compound triclocarban (TCC)
has been shown to exert adverse effects. TCC disrupts amino acid
metabolism in cardiac organoids and induces oxidative stress
responses in human umbilical vein endothelial cells. Specifically,
it upregulates the expression of iNOS, resulting in increased
protein nitrosylation, which impairs endothelial cell function and
heightens the risk of CVDs (177). These findings support the
importance of vigilance in clinical medication safety and the
management of environmental chemical exposures. They emphasize the
necessity for increased awareness and monitoring of potential
cardiovascular risks associated with substances like TCC in both
clinical and environmental settings.
In contrast, colchicine demonstrates a beneficial
effect on cardiovascular protection by mitigating the release of
inflammatory mediators, including IL-1 receptor antagonist, IL-18,
IL-6 and hs-CRP, through the inhibition of NLRP3 inflammasome
activation. Additionally, colchicine reduces the release of
myeloperoxidase and elastase during neutrophil activation (178). These combined effects
substantially diminish vascular inflammation, enhance the stability
of coronary plaques in patients with acute coronary syndrome (ACS),
effectively lower the risk of cardiovascular events and offer a
valuable therapeutic option for the management of patients with
ACS.
Conclusion and prospects
Current consensus
Amino acid metabolism is closely associated with
inflammation in CHD. Metabolic disorders of amino acids such as
arginine, glutamate, BCAAs and tryptophan can affect immune cell
function, inflammatory mediators and vascular endothelial function
through mechanisms of immunometabolic reprogramming and oxidative
stress, thus contributing to the pathogenesis of CHD. Inflammatory
biomarkers, such as hs-CRP and interleukins, serve as indicators
for assessing disease risk. Advances in clinical research and
technological applications have introduced novel perspectives to
related studies, while dietary interventions and pharmacological
treatments exhibit promising potential.
Remaining challenges
Current research predominantly addresses common
amino acids and traditional pathways, resulting in a limited
understanding of the roles of rare amino acids and the interplay
between amino acid metabolism and non-coding RNAs. Furthermore,
dietary and pharmacological interventions lack validation through
large-scale clinical trials and the translation of research
findings into precision medicine continues to encounter significant
challenges. For instance, factors such as interference with dietary
patterns (104) and metabolic
variability among individuals (179) contribute to these ongoing
difficulties.
Future directions
Advanced technologies offer the capability to
accurately evaluate amino acid metabolic status, while machine
learning can be employed to extract valuable information. It is
imperative to enhance clinical trials to validate the efficacy of
interventions and to formulate personalized treatment plans. In
addition, a comprehensive examination of the interactions between
amino acid metabolism and other variables may unveil novel
strategies for the prevention and management of CHD, thereby
facilitating the transition towards precision medicine.
Availability of data and materials
Not applicable.
Authors' contributions
YZ designed the study and analyzed the literature.
RS drafted the manuscript. All authors contributed toward data
analysis, drafting and revision of the manuscript, and have read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Młynarska E, Czarnik W, Fularski P, Hajdys
J, Majchrowicz G, Stabrawa M, Rysz J and Franczyk B: From
atherosclerotic plaque to myocardial infarction-the leading cause
of coronary artery occlusion. Int J Mol Sci. 25:72952024.
View Article : Google Scholar
|
|
2
|
Li J, Huang P, Cheng W and Niu Q:
Stilbene-based derivatives as potential inhibitors of
trimethylamine (TMA)-lyase affect gut microbiota in coronary heart
disease. Food Sci Nutr. 11:93–100. 2022. View Article : Google Scholar
|
|
3
|
Zhou P, Zhao XN, Ma YY, Tang TJ, Wang SS,
Wang L and Huang JL: Virtual screening analysis of natural
flavonoids as trimethylamine (TMA)-lyase inhibitors for coronary
heart disease. J Food Biochem. 46:e143762022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madaudo C, Coppola G, Parlati ALM and
Corrado E: Discovering inflammation in atherosclerosis: Insights
from pathogenic pathways to clinical practice. Int J Mol Sci.
25:60162024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cimmino G, Muscoli S, De Rosa S, Cesaro A,
Perrone MA, Selvaggio S, Selvaggio G, Aimo A, Pedrinelli R, Mercuro
G, et al: Evolving concepts in the pathophysiology of
atherosclerosis: From endothelial dysfunction to thrombus formation
through multiple shades of inflammation. J Cardiovasc Med
(Hagerstown). 24(Suppl 2): e156–e167. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv J, Pan C, Cai Y, Han X, Wang C, Ma J,
Pang J, Xu F, Wu S, Kou T, et al: Plasma metabolomics reveals the
shared and distinct metabolic disturbances associated with
cardiovascular events in coronary artery disease. Nat Commun.
15:57292024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang J, Zhong J, Liu H, Li W, Chen
M, Xu L, Zhang W, Zhang Z, Wei Z, et al: LRG1 promotes
atherosclerosis by inducing macrophage M1-like polarization. Proc
Natl Acad Sci USA. 121:e24058451212024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Xia W, Zhou J, Lv J, Dai Q, Li X,
Tian Q, Liu X, Du X, Tu R and Liu S: Oxidized low-density
lipoprotein induces M2-type differentiation of macrophages to
promote the protracted progression of atherosclerotic inflammation
in high-fat diet-fed ApoE -/- mice. Cell Mol Biol (Noisy-le-grand).
69:235–248. 2023. View Article : Google Scholar
|
|
9
|
Chen J, Xiang X, Nie L, Guo X, Zhang F,
Wen C, Xia Y and Mao L: The emerging role of Th1 cells in
atherosclerosis and its implications for therapy. Front Immunol.
13:10796682023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Li W, Zhao T, Zou Y, Deng T, Yang
Z, Yuan Z, Ma L, Yu R, Wang T and Yu C: Interleukin-17-producing
CD4+ T cells promote inflammatory response and foster
disease progression in hyperlipidemic patients and atherosclerotic
mice. Front Cardiovasc Med. 8:6677682021. View Article : Google Scholar
|
|
11
|
Lotfy H and Moaaz M and Moaaz M: The novel
role of IL-37 to enhance the anti-inflammatory response of
regulatory T cells in patients with peripheral atherosclerosis.
Vascular. 28:629–642. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Wei S, Wu X, Li Y and Han X:
Neutrophil extracellular traps in acute coronary syndrome. J
Inflamm (Lond). 20:172023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klopf J, Brostjan C, Eilenberg W and
Neumayer C: Neutrophil extracellular traps and their implications
in cardiovascular and inflammatory disease. Int J Mol Sci.
22:5592021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumrić M, Kurir TT, Borovac JA and Božić
J: The role of natural killer (NK) cells in acute coronary
syndrome: A comprehensive review. Biomolecules. 10:15142020.
View Article : Google Scholar
|
|
15
|
Backteman K, Ernerudh J and Jonasson L:
Natural killer (NK) cell deficit in coronary artery disease: No
aberrations in phenotype but sustained reduction of NK cells is
associated with low-grade inflammation. Clin Exp Immunol.
175:104–112. 2014. View Article : Google Scholar :
|
|
16
|
Ma J, Wang X, Jia Y, Tan F, Yuan X and Du
J: The roles of B cells in cardiovascular diseases. Mol Immunol.
171:36–46. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solanki K, Bezsonov E, Orekhov A, Parihar
SP, Vaja S, White FA, Obukhov AG and Baig MS: Effect of reactive
oxygen, nitrogen, and sulfur species on signaling pathways in
atherosclerosis. Vascul Pharmacol. 154:1072822024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carlstrom M, Weitzberg E and Lundberg JO:
Nitric oxide signaling and regulation in the cardiovascular system:
Recent advances. Pharmacol Rev. 76:1038–1062. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andrabi SM, Sharma NS, Karan A, Shahriar
SMS, Cordon B, Ma B and Xie J: Nitric oxide: Physiological
functions, delivery, and biomedical applications. Adv Sci (Weinh).
10:e23032592023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thengchaisri N, Kuo L and Hein TW:
H2O2 mediates VEGF- and flow-induced
dilations of coronary arterioles in early type 1 diabetes: Role of
vascular arginase and PI3K-linked eNOS uncoupling. Int J Mol Sci.
24:4892022. View Article : Google Scholar
|
|
21
|
Alzayadneh EM, Shatanawi A, Caldwell RW
and Caldwell RB: Methylglyoxal-modified albumin effects on
endothelial arginase enzyme and vascular function. Cells.
12:7952023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan M, Singh I and Won J: Asymmetric
dimethylarginine-induced oxidative damage leads to cerebrovascular
dysfunction. Neural Regen Res. 16:1793–1794. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu X, Lu H, Gao M, Li P, He Y, He Y, Luo
X, Rao X and Liu W: Nitric oxide in the cardio-cerebrovascular
system: Source, regulation and application. Nitric Oxide.
152:48–57. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Le Thi P, Tran DL, Park KM, Lee S, Oh DH
and Park KD: Biocatalytic nitric oxide generating hydrogels with
enhanced anti-inflammatory, cell migration, and angiogenic
capabilities for wound healing applications. J Mater Chem B.
12:1538–1549. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Incalza MA, D'Oria R, Natalicchio A,
Perrini S, Laviola L and Giorgino F: Oxidative stress and reactive
oxygen species in endothelial dysfunction associated with
cardiovascular and metabolic diseases. Vascul Pharmacol. 100:1–19.
2018. View Article : Google Scholar
|
|
26
|
Son WH, Jeong WM, Park IY and Ha MS:
Enhancing inflammatory factors, nitric oxide, and arterial
stiffness through aquatic walking for amelioration and disease
prevention: Targeting in obese elderly women. Mediators Inflamm.
2024:55209872024. View Article : Google Scholar
|
|
27
|
Cui X, Zhang L, Lin L, Hu Y, Zhang M, Sun
B, Zhang Z, Lu M, Guan X, Hao J, et al: Notoginsenoside
R1-Protocatechuic aldehyde reduces vascular inflammation and
calcification through increasing the release of nitric oxide to
inhibit TGFbetaR1-YAP/TAZ pathway in vascular smooth muscle cells.
Int Immunopharmacol. 143:1135742024. View Article : Google Scholar
|
|
28
|
Sherratt SCR, Libby P, Dawoud H, Bhatt DL
and Mason RP: Eicosapentaenoic acid improves endothelial nitric
oxide bioavailability via changes in protein expression during
inflammation. J Am Heart Assoc. 13:e0340762024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vanhoutte PM: Nitric oxide: From good to
bad. Ann Vasc Dis. 11:41–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gryko A, Głowińska-Olszewska B, Pludowska
K, Smithson WH, Owłasiuk A, Żelazowska-Rutkowska B, Wojtkielewicz
K, Milewski R and Chlabicz S: Significant differences in parameters
of glucose metabolism in children of hypertensive and normotensive
parents. Pediatr Endocrinol Diabetes Metab. 23:14–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Theofilis P, Sagris M, Oikonomou E,
Antonopoulos AS, Siasos G, Tsioufis C and Tousoulis D: Inflammatory
mechanisms contributing to endothelial dysfunction. Biomedicines.
9:7812021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Batty M, Bennett MR and Yu E: The role of
oxidative stress in atherosclerosis. Cells. 11:38432022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janaszak-Jasiecka A, Płoska A, Wierońska
JM, Dobrucki LW and Kalinowski L: Endothelial dysfunction due to
eNOS uncoupling: Molecular mechanisms as potential therapeutic
targets. Cell Mol Biol Lett. 28:212023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hernandez-Navarro I, Botana L, Diez-Mata
J, Tesoro L, Jimenez-Guirado B, Gonzalez-Cucharero C, Alcharani N,
Zamorano JL, Saura M and Zaragoza C: Replicative endothelial cell
senescence may lead to endothelial dysfunction by increasing the
BH2/BH4 ratio induced by oxidative stress, reducing BH4
availability, and decreasing the expression of eNOS. Int J Mol Sci.
25:98902024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu L, Jang S, Zhu J, Qin Q, Sun L and Sun
J: Nur77 mitigates endothelial dysfunction through activation of
both nitric oxide production and anti-oxidant pathways. Redox Biol.
70:1030562024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao X, Wu VWY, Han Y, Hong H, Wu Y, Kong
APS, Lui KO and Tian XY: Role of argininosuccinate synthase
1-dependent L-arginine biosynthesis in the protective effect of
endothelial sirtuin 3 against atherosclerosis. Adv Sci (Weinh).
11:e23072562024. View Article : Google Scholar
|
|
37
|
Zhang M, Wu Z, Salas SS, Aguilar MM,
Trillos-Almanza MC, Buist-Homan M and Moshage H: Arginase 1
expression is increased during hepatic stellate cell activation and
facilitates collagen synthesis. J Cell Biochem. 124:808–817. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marzęta-Assas P, Jacenik D and Zasłona Z:
Pathophysiology of arginases in cancer and efforts in their
pharmacological inhibition. Int J Mol Sci. 25:97822024. View Article : Google Scholar
|
|
39
|
Lim HK, Lim HK, Ryoo S, Benjo A, Shuleri
K, Miriel V, Baraban E, Camara A, Soucy K, Nyhan D, et al:
Mitochondrial arginase II constrains endothelial NOS-3 activity. Am
J Physiol Heart Circ Physiol. 293:H3317–H3324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ottosson F, Engström G, Orho-Melander M,
Melander O, Nilsson PM and Johansson M: Plasma metabolome predicts
aortic stiffness and future risk of coronary artery disease and
mortality after 23 years of follow-up in the general population. J
Am Heart Assoc. 13:e0334422024. View Article : Google Scholar
|
|
41
|
Vernon ST, Tang O, Kim T, Chan AS, Kott
KA, Park J, Hansen T, Koay YC, Grieve SM, O'Sullivan JF, et al:
Metabolic signatures in coronary artery disease: Results from the
BioHEART-CT study. Cells. 10:9802021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Z, Ajam A, Huang J, Yeh YS and Razani
B: Glutamine-glutamate imbalance in the pathogenesis of
cardiovascular disease. Nat Cardiovasc Res. 3:1377–1379. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, Yang R, Zhang W, Wang S, Mu H, Li
H, Dong J, Chen W, Yu X and Ji F: Serum glutamate and
glutamine-to-glutamate ratio are associated with coronary
angiography defined coronary artery disease. Nutr Metab Cardiovasc
Dis. 32:186–194. 2022. View Article : Google Scholar
|
|
44
|
Rom O, Liu Y, Finney AC, Ghrayeb A, Zhao
Y, Shukha Y, Wang L, Rajanayake KK, Das S, Rashdan NA, et al:
Induction of glutathione biosynthesis by glycine-based treatment
mitigates atherosclerosis. Redox Biol. 52:1023132022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mouton AJ, Aitken NM, Morato JG, O'Quinn
KR, do Carmo JM, da Silva AA, Omoto ACM, Li X, Wang Z,
Schrimpe-Rutledge AC, et al: Glutamine metabolism improves left
ventricular function but not macrophage-mediated inflammation
following myocardial infarction. Am J Physiol Cell Physiol.
327:C571–C586. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Prechtl L, Carrard J, Gallart-Ayala H,
Borreggine R, Teav T, Königstein K, Wagner J, Knaier R, Infanger D,
Streese L, et al: Circulating amino acid signature features urea
cycle alterations associated with coronary artery disease. Sci Rep.
14:258482024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bonetti L, Horkova V, Grusdat M, Longworth
J, Guerra L, Kurniawan H, Franchina DG, Soriano-Baguet L, Binsfeld
C, Verschueren C, et al: A Th17 cell-intrinsic
glutathione/mitochondrial-IL-22 axis protects against intestinal
inflammation. Cell Metab. 36:1726–1744.e10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bopp L, Martinez ML, Schumacher C, Seitz
R, Arana MH, Klapproth H, Lukas D, Oh JH, Neumayer D, Lackmann JW,
et al: Glutamine promotes human CD8+ T cells and
counteracts imiquimod-induced T cell hyporesponsiveness. iScience.
27:1097672024. View Article : Google Scholar
|
|
49
|
Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M,
Zeng C, Zhou T and Zhang J: NF-κB in biology and targeted therapy:
New insights and translational implications. Signal Transduct
Target Ther. 9:532024. View Article : Google Scholar
|
|
50
|
Haroon E, Miller AH and Sanacora G:
Inflammation, glutamate, and glia: A trio of trouble in mood
disorders. Neuropsychopharmacology. 42:193–215. 2017. View Article : Google Scholar
|
|
51
|
Yan M, Li X, Sun C, Tan J, Liu Y, Li M, Qi
Z, He J, Wang D and Wu L: Sodium butyrate attenuates AGEs-induced
oxidative stress and inflammation by inhibiting autophagy and
affecting cellular metabolism in THP-1 cells. Molecules.
27:87152022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun H, Ma X, Ma H, Li S, Xia Y, Yao L,
Wang Y, Pang X, Zhong J, Yao G, et al: High glucose levels
accelerate atherosclerosis via NLRP3-IL/MAPK/NF-κB-related
inflammation pathways. Biochem Biophys Res Commun. 704:1497022024.
View Article : Google Scholar
|
|
53
|
Zhang Y, Peng K, Liu J, Chen X, Wang T, Li
M, Chen Y, Xu Y, Lu J, Bi Y, et al: Carotid intima-media thickness
and plagues are associated with indicators of peripheral artery
diseases in patients with diabetes. Diabetes Res Clin Pract.
144:245–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tanase DM, Valasciuc E, Costea CF,
Scripcariu DV, Ouatu A, Hurjui LL, Tarniceriu CC, Floria DE,
Ciocoiu M, Baroi LG and Floria M: Duality of branched-chain amino
acids in chronic cardiovascular disease: Potential biomarkers
versus active pathophysiological promoters. Nutrients. 16:19722024.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fine KS, Wilkins JT and Sawicki KT:
Circulating branched chain amino acids and cardiometabolic disease.
J Am Heart Assoc. 13:e0316172024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dziedzic M, Jozefczuk E, Guzik TJ and
Siedlinski M: Interplay between plasma glycine and branched-chain
amino acids contributes to the development of hypertension and
coronary heart disease. Hypertension. 81:1320–1331. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang J, Liu Z, Ni Y, Yu Y, Guo F, Lu Y,
Wang X, Hao H, Li S, Wei P, et al: Branched-chain amino acids
promote occurrence and development of cardiovascular disease
dependent on triglyceride metabolism via activation of the
mTOR/SREBP-1/betatrophin pathway. Mol Cell Endocrinol.
584:1121642024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Z, Wang Y and Sun H: The role of
branched-chain amino acids and their metabolism in cardiovascular
diseases. J Cardiovasc Transl Res. 17:85–90. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gan Z, Guo Y, Zhao M, Ye Y, Liao Y, Liu B,
Yin J, Zhou X, Yan Y, Yin Y and Ren W: Excitatory amino acid
transporter supports inflammatory macrophage responses. Sci Bull
(Beijing). 69:2405–2419. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Deng X, Tang C, Fang T, Li T, Li X, Liu Y,
Zhang X, Sun B, Sun H and Chen L: Disruption of branched-chain
amino acid homeostasis promotes the progression of DKD via
enhancing inflammation and fibrosis-associated
epithelial-mesenchymal transition. Metabolism. 162:1560372025.
View Article : Google Scholar
|
|
61
|
Zhenyukh O, Civantos E, Ruiz-Ortega M,
Sánchez MS, Vázquez C, Peiró C, Egido J and Mas S: High
concentration of branched-chain amino acids promotes oxidative
stress, inflammation and migration of human peripheral blood
mononuclear cells via mTORC1 activation. Free Radic Biol Med.
104:165–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ye Z, Wang S, Zhang C and Zhao Y:
Coordinated modulation of energy metabolism and inflammation by
branched-chain amino acids and fatty acids. Front Endocrinol
(Lausanne). 11:6172020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao S, Zhou L, Wang Q, Cao JH, Chen Y,
Wang W, Zhu BD, Wei ZH, Li R, Li CY, et al: Elevated branched-chain
amino acid promotes atherosclerosis progression by enhancing
mitochondrial-to-nuclear H2O2-disulfide HMGB1
in macrophages. Redox Biol. 62:1026962023. View Article : Google Scholar
|
|
64
|
Sun H, Olson KC, Gao C, Prosdocimo DA,
Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, et al: Catabolic
defect of branched-chain amino acids promotes heart failure.
Circulation. 133:2038–2049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu L, Huang T, Zhao J, Zhou Z, Cao Z, Chi
Y, Meng S, Huang Y, Xu Y, Xia L, et al: Branched-chain amino acid
catabolic defect in vascular smooth muscle cells drives thoracic
aortic dissection via mTOR hyperactivation. Free Radic Biol Med.
210:25–41. 2024. View Article : Google Scholar
|
|
66
|
Mehta NN, deGoma E and Shapiro MD: IL-6
and cardiovascular risk: A narrative review. Curr Atheroscler Rep.
27:122024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang W, Xiong Y, Li X and Yang Y: Cardiac
fibrosis: Cellular effectors, molecular pathways, and exosomal
roles. Front Cardiovasc Med. 8:7152582021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen ZW, Qian JY, Ma JY, Chang SF, Yun H,
Jin H, Sun AJ, Zou YZ and Ge JB: TNF-α-induced cardiomyocyte
apoptosis contributes to cardiac dysfunction after coronary
microembolization in mini-pigs. J Cell Mol Med. 18:1953–1963. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhou X, Zhang J, Shen J, Cheng B, Bi C and
Ma Q: Branched-chain amino acid modulation of lipid metabolism,
gluconeogenesis, and inflammation in a finishing pig model:
Targeting leucine and valine. Food Funct. 14:10119–10134. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hong W, You G, Luo Z, Zhang M and Chen J:
High gestational leucine level dampens WDPCP/MAPK signaling to
impair the EMT and migration of cardiac microvascular endothelial
cells in congenital heart defects. Pulm Circ. 14:e700132024.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bohler M, van den Berg EH, Almanza MCT,
Connelly MA, Bakker SJL, de Meijer VE, Dullaart RPF and Blokzijl H;
TransplantLines Investigators: Branched chain amino acids are
associated with metabolic complications in liver transplant
recipients. Clin Biochem. 102:26–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hao QY, Weng J, Zeng TT, Zeng YH, Guo JB,
Li SC, Chen YR, Yang PZ, Gao JW and Li ZH: Dietary branched-chain
amino acids intake and coronary artery calcium progression:
Insights from the coronary artery risk development in young adults
(CARDIA) study. Eur J Nutr. 64:1312025. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rao S, Zhang Y, Xie S, Cao H, Zhang Z and
Yang W: Dietary intake of branched-chain amino acids (BCAAs), serum
BCAAs, and cardiometabolic risk markers among community-dwelling
adults. Eur J Nutr. 63:1835–1845. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Z, Xia H, Sharp TE III, LaPenna KB,
Elrod JW, Casin KM, Liu K, Calvert JW, Chau VQ, Salloum FN, et al:
Mitochondrial H2S regulates BCAA catabolism in heart
failure. Circ Res. 131:222–235. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li Z, Zhang R, Mu H, Zhang W, Zeng J, Li
H, Wang S, Zhao X, Chen W, Dong J and Yang R: Oral administration
of branched-chain amino acids attenuates atherosclerosis by
inhibiting the inflammatory response and regulating the gut
microbiota in ApoE-deficient mice. Nutrients. 14:50652022.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Teunis CJ, Stroes ESG, Boekholdt SM,
Wareham NJ, Murphy AJ, Nieuwdorp M, Hazen SL and Hanssen NMJ:
Tryptophan metabolites and incident cardiovascular disease: The
EPIC-Norfolk prospective population study. Atherosclerosis.
387:1173442023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Luo Z, Liu Y, Wang X, Fan F, Yang Z and
Luo D: Exploring tryptophan metabolism: The transition from
disturbed balance to diagnostic and therapeutic potential in
metabolic diseases. Biochem Pharmacol. 230:1165542024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Grishanova AY and Perepechaeva ML:
Kynurenic acid/AhR signaling at the junction of inflammation and
cardiovascular diseases. Int J Mol Sci. 25:69332024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li K, Li K, He Y, Liang S, Shui X and Lei
W: Aryl hydrocarbon receptor: A bridge linking immuno-inflammation
and metabolism in atherosclerosis. Biochem Pharmacol.
216:1157442023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sukka SR, Ampomah PB, Darville LNF, Ngai
D, Wang X, Kuriakose G, Xiao Y, Shi J, Koomen JM, McCusker RH and
Tabas I: Efferocytosis drives a tryptophan metabolism pathway in
macrophages to promote tissue resolution. Nat Metab. 6:1736–1755.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Paeslack N, Mimmler M, Becker S, Gao Z,
Khuu MP, Mann A, Malinarich F, Regen T and Reinhardt C:
Microbiota-derived tryptophan metabolites in vascular inflammation
and cardiovascular disease. Amino Acids. 54:1339–1356. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang K, Wen XQ, Zhang W, Wang JX, Liang
Y, Li WQ, Wang YH, Liang MM, Jing AR, Ma J, et al: Predictive value
of 5-methoxytryptophan on long-term clinical outcome after PCI in
patients with acute myocardial infarction-a prospective cohort
study. J Cardiovasc Transl Res. 17:1036–1047. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu W, Wang J, Yang H, Li C, Lan W, Chen T
and Tang Y: The metabolite indole-3-acetic acid of bacteroides
ovatus improves atherosclerosis by restoring the polarisation
balance of M1/M2 macrophages and inhibiting inflammation. Adv Sci
(Weinh). 12:e24130102025. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu H, Feng S, Tang M, Tian R and Zhang S:
Gut commensal bacteroides thetaiotaomicron promote atherothrombosis
via regulating L-tryptophan metabolism. Rev Cardiovasc Med.
25:3952024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang J, Jiang X, Pang B, Li D, Kang L,
Zhou T, Wang B, Zheng L, Zhou CM and Zhang L: Association between
tryptophan concentrations and the risk of developing cardiovascular
diseases: A systematic review and meta-analysis. Nutr Metab (Lond).
21:822024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li L, Xiao C, Liu H, Chen S, Tang Y, Zhou
H, Jiang G and Tian J: A circular network of coregulated
L-threonine and L-tryptophan metabolism dictates acute lower limb
ischemic injury. Int J Med Sci. 21:2402–2413. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Su Q, Pan XF, Li HB, Xiong LX, Bai J, Wang
XM, Qu XY, Zhang NR, Zou GQ, Shen Y, et al: Taurine supplementation
alleviates blood pressure via gut-brain communication in
spontaneously hypertensive rats. Biomedicines. 12:27112024.
View Article : Google Scholar
|
|
88
|
Wang Q, Lv H, Ainiwan M, Yesitayi G,
Abudesimu A, Siti D, Aizitiaili A and Ma X: Untargeted metabolomics
identifies indole-3-propionic acid to relieve Ang II-induced
endothelial dysfunction in aortic dissection. Mol Cell Biochem.
479:1767–1786. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sterpetti AV: Inflammatory cytokines and
atherosclerotic plaque progression. therapeutic implications. Curr
Atheroscler Rep. 22:752020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Poznyak AV, Bharadwaj D, Prasad G, Grechko
AV, Sazonova MA and Orekhov AN: Anti-inflammatory therapy for
atherosclerosis: Focusing on cytokines. Int J Mol Sci. 22:70612021.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li G, Wen Z and Xiong S:
Microenvironmental β-TrCP negates amino acid transport to trigger
CD8+ T cell exhaustion in human non-small cell lung
cancer. Cell Rep. 44:1151282025. View Article : Google Scholar
|
|
92
|
Chen S, Li Z, Li H, Zeng X, Yuan H and Li
Y: RNA sequencing of whole blood in premature coronary artery
disease: Identification of novel biomarkers and involvement of T
cell imbalance. J Cardiovasc Transl Res. 17:638–647. 2024.
View Article : Google Scholar
|
|
93
|
Liu R, Bao J, Tang Y, Xu D, Shen L and Qin
H: Changes in Treg cells and cytokines in the peripheral blood of
patients with coronary artery disease combined with type 2 diabetes
mellitus. Heart Lung. 69:147–154. 2025. View Article : Google Scholar
|
|
94
|
Wang X, Huang L, Hu B, Yang B, Wei R, Rong
S and Li B: Establishment and evaluation of a risk prediction model
for coronary heart disease in primary Sjögren's syndrome based on
peripheral blood IL-6 and Treg percentages. Front Immunol.
15:14403702024. View Article : Google Scholar
|
|
95
|
Johnstone JC, Yazicioglu YF and Clarke AJ:
Fuelling B cells: Dynamic regulation of B cell metabolism. Curr
Opin Immunol. 91:1024842024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hu T, Liu CH, Lei M, Zeng Q, Li L, Tang H
and Zhang N: Metabolic regulation of the immune system in health
and diseases: Mechanisms and interventions. Signal Transduct Target
Ther. 9:2682024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu JQ, Geng XR, Hu TY, Mo LH, Luo XQ, Qiu
SY, Liu DB, Liu ZG, Shao JB, Liu ZQ and Yang PC: Glutaminolysis is
required in maintaining immune regulatory functions in B cells.
Mucosal Immunol. 15:268–278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Seo SK and Kwon B: Immune regulation
through tryptophan metabolism. Exp Mol Med. 55:1371–1379. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Riaz F, Pan F and Wei P: Aryl hydrocarbon
receptor: The master regulator of immune responses in allergic
diseases. Front Immunol. 13:10575552022. View Article : Google Scholar
|
|
100
|
Pattarabanjird T, Srikakulapu P,
Ransegnola B, Marshall MA, Ghosheh Y, Gulati R, Durant C, Drago F,
Taylor AM, Ley K and McNamara CA: Single-cell profiling of CD11c+ B
cells in atherosclerosis. Front Immunol. 14:12966682024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen Z, Wang Z, Cui Y, Xie H, Yi L, Zhu Z,
Ni J, Du R, Wang X, Zhu J, et al: Serum BAFF level is associated
with the presence and severity of coronary artery disease and acute
myocardial infarction. BMC Cardiovasc Disord. 24:4712024.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pattarabanjird T, Marshall M, Upadhye A,
Srikakulapu P, Garmey JC, Haider A, Taylor AM, Lutgens E and
McNamara CA: B-1b cells possess unique bHLH-driven P62-dependent
self-renewal and atheroprotection. Circ Res. 130:981–993. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bobryshev YV, Ivanova EA, Chistiakov DA,
Nikiforov NG and Orekhov AN: Macrophages and their role in
atherosclerosis: Pathophysiology and transcriptome analysis. Biomed
Res Int. 2016:95824302016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang X, Kapoor D, Jeong SJ, Fappi A,
Stitham J, Shabrish V, Sergin I, Yousif E, Rodriguez-Velez A, Yeh
YS, et al: Identification of a leucine-mediated threshold effect
governing macrophage mTOR signalling and cardiovascular risk. Nat
Metab. 6:359–377. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Huang H, Chen H, Yao Y and Lou X:
Branched-chain amino acids supplementation induces insulin
resistance and pro-inflammatory macrophage polarization via
INFGR1/JAK1/STAT1 signal pathway. Mol Med. 30:1492024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhou W, Hu G, He J, Wang T, Zuo Y, Cao Y,
Zheng Q, Tu J, Ma J, Cai R, et al: SENP1-Sirt3 signaling promotes
α-ketoglutarate production during M2 macrophage polarization. Cell
Rep. 39:1106602022. View Article : Google Scholar
|
|
107
|
Ben-Aicha S, Anwar M, Vilahur G, Martino
F, Kyriazis PG, de Winter N, Punjabi PP, Angelini GD, Sattler S and
Emanueli C: Small extracellular vesicles in the pericardium
modulate macrophage immunophenotype in coronary artery disease.
JACC Basic Transl Sci. 9:1057–1072. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Quan YZ, Ma A, Ren CQ, An YP, Qiao PS, Gao
C, Zhang YK, Li XW, Lin SM, Li NN, et al: Ganoderic acids alleviate
atherosclerosis by inhibiting macrophage M1 polarization via
TLR4/MyD88/NF-κB signaling pathway. Atherosclerosis.
391:1174782024. View Article : Google Scholar
|
|
109
|
Peng D, Zhuge F, Wang M, Zhang B, Zhuang
Z, Zhou R, Zhang Y, Li J, Yu Z and Shi J: Morus alba L. (Sangzhi)
alkaloids mitigate atherosclerosis by regulating M1/M2 macrophage
polarization. Phytomedicine. 128:1555262024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Peng X, Sun B, Tang C, Shi C, Xie X, Wang
X, Jiang D, Li S, Jia Y, Wang Y, et al: HMOX1-LDHB interaction
promotes ferroptosis by inducing mitochondrial dysfunction in foamy
macrophages during advanced atherosclerosis. Dev Cell.
60:1070–1086.e8. 2025. View Article : Google Scholar
|
|
111
|
You Z, Ye X, Jiang M, Gu N and Liang C:
lnc-MRGPRF-6:1 promotes ox-LDL-induced macrophage ferroptosis via
suppressing GPX4. Mediators Inflamm. 2023:55132452023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang J, Du H, Xie W, Bi J, Zhang H, Liu X,
Wang Y, Zhang S, Lei A, He C, Yuan H, et al: CAR-macrophage therapy
alleviates myocardial ischemia-reperfusion injury. Circ Res.
135:1161–1174. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pan Z, Lv J, Zhao L, Xing K, Ye R, Zhang
Y, Chen S, Yang P, Yu H, Lin Y, et al: CircARCN1 aggravates
atherosclerosis by regulating HuR-mediated USP31 mRNA in
macrophages. Cardiovasc Res. 120:1531–1549. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang J, Da X, Chen Y, Yuan A and Pu J:
Glutamine protects against mouse abdominal aortic aneurysm through
modulating VSMC apoptosis and M1 macrophage activation. Int J Med
Sci. 21:1414–1427. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Grira N, Lahidheb D, Lamine O, Ayoub M,
Wassaifi S, Aouni Z, Fehri W and Mazigh C: The association of IL-6,
TNFα and CRP gene polymorphisms with coronary artery disease in a
tunisian population: A case-control study. Biochem Genet.
59:751–766. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Attiq A, Afzal S, Ahmad W and Kandeel M:
Hegemony of inflammation in atherosclerosis and coronary artery
disease. Eur J Pharmacol. 966:1763382024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Song Y, Wang M, Li Y and Lian Y: The
evaluation value of atherogenic index of plasma and
high-sensitivity C-reactive protein for the degree of coronary
artery lesion in premature coronary artery disease. BMC Cardiovasc
Disord. 24:4102024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Iwata H, Miyauchi K, Naito R Iimuro S,
Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, et al:
Significance of persistent inflammation in patients with chronic
coronary syndrome: Insights from the REAL-CAD study. JACC Adv.
3:1009962024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Håland AB, Mattsson E, Videm V, Albrektsen
G and Nyrønning LÅ: Elevated high sensitivity C reactive protein
and risk of abdominal aortic aneurysm: A prospective population
based study in the norwegian HUNT study. Eur J Vasc Endovasc Surg.
69:733–741. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Nazarian B, Fazeli Moghadam E, Asbaghi O,
Zeinali Khosroshahi M, Choghakhori R and Abbasnezhad A: Effect of
l-arginine supplementation on C-reactive protein and other
inflammatory biomarkers: A systematic review and meta-analysis of
randomized controlled trials. Complement Ther Med. 47:1022262019.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Nemati A, Alipanah-Moghadam R, Molazadeh L
and Naghizadeh Baghi A: The effect of glutamine supplementation on
oxidative stress and matrix metalloproteinase 2 and 9 after
exhaustive exercise. Drug Des Devel Ther. 13:4215–4223. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jennings A, MacGregor A, Pallister T,
Spector T and Cassidy A: Associations between branched chain amino
acid intake and biomarkers of adiposity and cardiometabolic health
independent of genetic factors: A twin study. Int J Cardiol.
223:992–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mallmann NH, Lima ES and Lalwani P:
Dysregulation of tryptophan catabolism in metabolic syndrome. Metab
Syndr Relat Disord. 16:135–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kim ES, Kim SY, Koh M, Lee HM, Kim K, Jung
J, Kim HS, Moon WK, Hwang S and Moon A: C-reactive protein binds to
integrin α2 and Fcγ receptor I, leading to breast cell adhesion and
breast cancer progression. Oncogene. 37:28–38. 2018. View Article : Google Scholar
|
|
125
|
Amin MN, Siddiqui SA, Ibrahim M, Hakim ML,
Ahammed MS, Kabir A and Sultana F: Inflammatory cytokines in the
pathogenesis of cardiovascular disease and cancer. SAGE Open Med.
8:20503121209657522020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Salica A, Cammisotto V, Scaffa R, Folino
G, De Paulis R, Carnevale R, Benedetto U, Saade W, Marullo A,
Sciarretta S, et al: Different oxidative stress and inflammation
patterns of diseased left anterior descending coronary artery
versus internal thoracic artery. Antioxidants (Basel). 13:11802024.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Xie Q, Wang J, Li R, Liu H, Zhong Y, Xu Q,
Ge Y, Li C, Sun L and Zhu J: IL-6 signaling accelerates iron
overload by upregulating DMT1 in endothelial cells to promote
aortic dissection. Int J Biol Sci. 20:4222–4237. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fang C, Du L, Gao S, Chen Y, Chen Z, Wu Z,
Li L, Li J, Zeng X, Li M, et al: Association between premature
vascular smooth muscle cells senescence and vascular inflammation
in Takayasu's arteritis. Ann Rheum Dis. 83:1522–1535. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Jang E, Ho TWW, Brumell JH, Lefebvre F,
Wang C and Lee WL: IL-1β induces LDL transcytosis by a novel
pathway involving LDLR and Rab27a. Arterioscler Thromb Vasc Biol.
44:2053–2068. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Correia AF, de Oliveira CGC, de Oliveira
DC Jr, Pereira MC, Carvalho FA, Martins ECC and de Oliveira DC:
Circulating interleukin-22 in patients with acute myocardial
infarction undergoing primary percutaneous coronary intervention. J
Clin Med. 13:49712024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang L, Wang H, Wang Z, Xu J, Wang M,
Wang W, He Q, Yu Y, Yuan D, Bu G, et al: Resveratrol promotes
cholesterol efflux from dendritic cells and controls costimulation
and T-cell activation in high-fat and lipopolysaccharide-driven
atherosclerotic mice. Front Cardiovasc Med. 11:14508982024.
View Article : Google Scholar
|
|
132
|
Yu J, Li Y, Hu J and Wang Y:
Interleukin-33 induces angiogenesis after myocardial infarction via
AKT/eNOS signaling pathway. Int Immunopharmacol. 143:1134332024.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lou L, Detering L, Luehmann H, Amrute JM,
Sultan D, Ma P, Li A, Lahad D, Bredemeyer A, Zhang X, et al:
Visualizing immune checkpoint inhibitors derived inflammation in
atherosclerosis. Circ Res. 135:990–1003. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wang Y, Zou Y, Jiang Q, Li W, Chai X, Zhao
T, Liu S, Yuan Z, Yu C and Wang T: Ox-LDL-induced CD80+ macrophages
expand pro-atherosclerotic NKT cells via CD1d in atherosclerotic
mice and hyperlipidemic patients. Am J Physiol Cell Physiol.
326:C1563–C1572. 2024. View Article : Google Scholar
|
|
135
|
Gastanadui MG, Margaroli C, Litovsky S,
Richter RP, Wang D, Xing D, Wells JM, Gaggar A, Nanda V, Patel RP
and Payne GA: Spatial transcriptomic approach to understanding
coronary atherosclerotic plaque stability. Arterioscler Thromb Vasc
Biol. 44:e264–e276. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Posadas-Sánchez R, Velázquez-Sánchez F,
Reyes-Barrera J, Cardoso-Saldaña G, Velázquez-Argueta F,
Antonio-Villa NE, Fragoso JM and Vargas-Alarcón G: MCP-1 rs1024611
polymorphism MCP-1 concentrations and premature coronary artery
disease: Results of the genetics of atherosclerotic disease (GEA)
Mexican study. Biomedicines. 12:12922024. View Article : Google Scholar
|
|
137
|
Ma X, Gao HJ, Ge HZ, Zhang Q and Bu BT:
Interleukin-6 trans-signalling regulates monocyte chemoattractant
protein-1 production in immune-mediated necrotizing myopathy.
Rheumatology (Oxford). 64:849–859. 2025. View Article : Google Scholar
|
|
138
|
Sproston NR and Ashworth JJ: Role of
C-reactive protein at sites of inflammation and infection. Front
Immunol. 9:7542018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yanni AE, Agrogiannis G, Nomikos T,
Fragopoulou E, Pantopoulou A, Antonopoulou S and Perrea D: Oral
supplementation with L-aspartate and L-glutamate inhibits
atherogenesis and fatty liver disease in cholesterol-fed rabbit.
Amino Acids. 38:1323–1331. 2010. View Article : Google Scholar
|
|
140
|
Wang W, Zhang F, Xia Y, Zhao S, Yan W,
Wang H, Lee Y, Li C, Zhang L, Lian K, et al: Defective branched
chain amino acid catabolism contributes to cardiac dysfunction and
remodeling following myocardial infarction. Am J Physiol Heart Circ
Physiol. 311:H1160–H1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Green CL, Trautman ME, Chaiyakul K, Jain
R, Alam YH, Babygirija R, Pak HH, Sonsalla MM, Calubag MF, Yeh CY,
et al: Dietary restriction of isoleucine increases healthspan and
lifespan of genetically heterogeneous mice. Cell Metab.
35:1976–1995.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chajadine M, Laurans L, Radecke T,
Mouttoulingam N, Al-Rifai R, Bacquer E, Delaroque C, Rytter H,
Bredon M, Knosp C, et al: Harnessing intestinal tryptophan
catabolism to relieve atherosclerosis in mice. Nat Commun.
15:63902024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Luo Z, Yang L, Zhu T, Fan F, Wang X, Liu
Y, Zhan H, Luo D and Guo J: Aucubin ameliorates atherosclerosis by
modulating tryptophan metabolism and inhibiting
endothelial-mesenchymal transitions via gut microbiota regulation.
Phytomedicine. 135:1561222024. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen C, Ding Y, Huang Q, Zhang C, Zhao Z,
Zhou H, Li D and Zhou G: Relationship between arginine methylation
and vascular calcification. Cell Signal. 119:1111892024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Bingöl G, Huraıbat A, Ayduk Gövdeli E, Ser
ÖS, Ünlü S, Çelik M, Bulut L, Özden Ö, Özmen E and Kılıçkesmez K:
Effect of homoarginine on coronary artery complexity and
atherosclerotic burden in patients with STEMI. J Clin Med.
14:15012025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Jin H, Zhang C, Nagenborg J, Juhasz P,
Ruder AV, Sikkink CJJM, Mees BME, Waring O, Sluimer JC, Neumann D,
et al: Genome-scale metabolic network of human carotid plaque
reveals the pivotal role of glutamine/glutamate metabolism in
macrophage modulating plaque inflammation and vulnerability.
Cardiovasc Diabetol. 23:2402024. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Murcy F, Borowczyk C, Gourion-Arsiquaud S,
Torrino S, Ouahrouche N, Barouillet T, Dussaud S, Couralet M,
Vaillant N, Merlin J, et al: GLS2 links glutamine metabolism and
atherosclerosis by remodeling artery walls. Nat Cardiovasc Res.
3:1454–1467. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Ruiz-Canela M, Toledo E, Clish CB, Hruby
A, Liang L, Salas-Salvadó J, Razquin C, Corella D, Estruch R, Ros
E, et al: Plasma branched-chain amino acids and incident
cardiovascular disease in the PREDIMED trial. Clin Chem.
62:582–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Laferrère B, Reilly D, Arias S, Swerdlow
N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner
BR, et al: Differential metabolic impact of gastric bypass surgery
versus dietary intervention in obese diabetic subjects despite
identical weight loss. Sci Transl Med. 3:80re22011. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Katz LEL, Gidding SS, Otvos JD, Drews KL,
Bacha F, Willi S, Marcovina S, McKay S and Weinstock RS; TODAY
Study Group: Atherogenic lipoproteins associate with loss of
glycemic control in youth-onset type 2 diabetes: Results from the
TODAY study. J Clin Lipidol. Feb 6–2025.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yang X, Chi C, Li W, Zhang Y, Yang S, Xu R
and Liu R: Metabolomics and lipidomics combined with serum
pharmacochemistry uncover the potential mechanism of
Huang-Lian-Jie-Du decoction alleviates atherosclerosis in ApoE(-/-)
mice. J Ethnopharmacol. 324:1177482024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Botello-Marabotto M, Plana E,
Martinez-Bisbal MC, Medina P, Bernardos A, Martínez-Máñez R and
Miralles M: Metabolomic study for the identification of symptomatic
carotid plaque biomarkers. Talanta. 284:1272112025. View Article : Google Scholar
|
|
153
|
du Toit WL, Kruger R, Gafane-Matemane LF,
Schutte AE, Louw R and Mels CMC: Markers of arterial stiffness and
urinary metabolomics in young adults with early cardiovascular
risk: The African-PREDICT study. Metabolomics. 19:282023.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wang SJ, Liu BR, Zhang F, Su XR, Li YP,
Yang CT, Zhang ZH and Cong B: The amino acid metabolomics signature
of differentiating myocardial infarction from strangulation death
in mice models. Sci Rep. 13:149992023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Mei Z, Xu L, Huang Q, Lin C, Yu M, Shali
S, Wu H, Lu Y, Wu R, Wang Z, et al: Metabonomic biomarkers of
plaque burden and instability in patients with coronary
atherosclerotic disease after moderate lipid-lowering therapy. J Am
Heart Assoc. 13:e0369062024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ren W, Zhang Z, Wang Y, Wang J, Li L, Shi
L, Zhai T and Huang J: Coronary health index based on
immunoglobulin light chains to assess coronary heart disease risk
with machine learning: A diagnostic trial. J Transl Med. 23:222025.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Santana E, Ibrahimi E, Ntalianis E,
Cauwenberghs N and Kuznetsova T: Integrating metabolomics domain
knowledge with explainable machine learning in atherosclerotic
cardiovascular disease classification. Int J Mol Sci. 25:129052024.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Ke Y, Yue J, He J and Liu G: Integrating
machine learning algorithms and single-cell analysis to identify
gut microbiota-related macrophage biomarkers in atherosclerotic
plaques. Front Cell Infect Microbiol. 14:13957162024. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Chen H, Wu B, Guan K, Chen L, Chai K, Ying
M, Li D and Zhao W: Identification of lipid metabolism related
immune markers in atherosclerosis through machine learning and
experimental analysis. Front Immunol. 16:15491502025. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Gao Z, Du Z, Hou Y, Hua K, Tu P, Ai X and
Jiang Y: A microfluidic coculture model for mapping signaling
perturbations and precise drug screening against
macrophage-mediated dynamic myocardial injury. Acta Pharm Sin B.
14:5393–5406. 2024. View Article : Google Scholar
|
|
161
|
Lin J, Chen S, Zhang C, Liao J, Chen Y,
Deng S, Mao Z, Zhang T, Tian N, Song Y and Zeng T: Recent advances
in microfluidic technology of arterial thrombosis investigations.
Platelets. 35:23167432024. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Maringanti R, van Dijk CGM, Meijer EM,
Brandt MM, Li M, Tiggeloven VPC, Krebber MM, Chrifi I, Duncker DJ,
Verhaar MC and Cheng C: Atherosclerosis on a chip: A 3-dimensional
microfluidic model of early arterial events in human plaques.
Arterioscler Thromb Vasc Biol. 44:2453–2472. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Lai A, Hawke A, Mohammed M, Thurgood P,
Concilia G, Peter K, Khoshmanesh K and Baratchi S: A microfluidic
model to study the effects of arrhythmic flows on endothelial
cells. Lab Chip. 24:2347–2357. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Thakur MR, Nachane SS and Tupe RS:
Alleviation of albumin glycation-induced diabetic cardiomyopathy by
L-arginine: Insights into Nrf-2 signaling. Int J Biol Macromol.
264:1304782024. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Kaya S and Yalcin T: In an experimental
myocardial infarction model, L-arginine pre-intervention may exert
cardioprotective effects by regulating OTULIN levels and
mitochondrial dynamics. Cell Stress Chaperones. 28:811–820. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhang H, Wang C, Sun H, Zhou T, Ma C, Han
X, Zhang T and Xia J: Glutamine supplementation alleviated aortic
atherosclerosis in mice model and in vitro. Proteomics.
24:e23001792024. View Article : Google Scholar
|
|
167
|
Grajeda-Iglesias C and Aviram M: Specific
amino acids affect cardiovascular diseases and atherogenesis via
protection against macrophage foam cell formation: Review article.
Rambam Maimonides Med J. 9:e00222018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ouyang L, Yu C, Xie Z, Su X, Xu Z, Song P,
Li J, Huang H, Ding Y and Zou MH: Indoleamine 2,3-dioxygenase 1
deletion-mediated kynurenine insufficiency in vascular smooth
muscle cells exacerbates arterial calcification. Circulation.
145:1784–1798. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Xue H, Chen X, Yu C, Deng Y, Zhang Y, Chen
S, Chen X, Chen K, Yang Y and Ling W: Gut microbially produced
indole-3-propionic acid inhibits atherosclerosis by promoting
reverse cholesterol transport and its deficiency is causally
related to atherosclerotic cardiovascular disease. Circ Res.
131:404–420. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Miao Y, Wang Y, Yan P, Li Y, Chen Z, Tong
N and Wan Q: Association between the fatty liver index (FLI) and
incident coronary heart disease: Insights from a cohort study on
the Chinese population. Front Endocrinol (Lausanne).
15:13678532024. View Article : Google Scholar
|
|
171
|
Song J, Liu Y, Liu Y, Liu Y, Zhou Q, Chen
J, Meng X, Wang W and Tang YD: MAFLD as a predictor of adverse
cardiovascular events among CHD patients with LDL-C<1.8 mmol/l.
Nutr Metab Cardiovasc Dis. 35:1037982025. View Article : Google Scholar
|
|
172
|
Liao Y, Chen Q, Liu L, Huang H, Sun J, Bai
X, Jin C, Li H, Sun F, Xiao X, et al: Amino acid is a major carbon
source for hepatic lipogenesis. Cell Metab. 36:2437–2448.e8. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Katsiki N, Kolovou G and Vrablik M:
Metabolic dysfunction associated-steatotic liver disease (MASLD)
and cardiovascular risk: Embrace all facets of the disease. Curr
Cardiol Rep. 27:192025. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Peng Y, Feng W, Huang H, Chen Y and Yang
S: Macrophagetargeting Antisenescence nanomedicine enables in-Situ
NO induction for Gaseous and antioxidative atherosclerosis
intervention. Bioact Mater. 48:294–312. 2025.PubMed/NCBI
|
|
175
|
Yang W, Zhou W and Gou S: Hypoxia
activated nitric oxide donor compounds for the prevention and
treatment of myocardial hypoxia-induced injury. J Med Chem.
68:491–505. 2025. View Article : Google Scholar
|
|
176
|
Fu C, Li Q, Li M, Zhang J, Zhou F, Li Z,
He D, Hu X, Ning X, Guo W, et al: An integrated arterial remodeling
hydrogel for preventing restenosis after angioplasty. Adv Sci
(Weinh). 11:e23070632024. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Yang N, Chen J, Zhu Y, Shan W, Cao Z, Fu
Y, Cao H, Li Y, Xiang Y, Ding S, et al: Human cardiac organoid
model reveals antibacterial triclocarban promotes myocardial
hypertrophy by interfering with endothelial cell metabolism. Sci
Bull (Beijing). 70:342–346. 2025. View Article : Google Scholar
|
|
178
|
Yu M, Yang Y, Dong SL, Zhao C, Yang F,
Yuan YF, Liao YH, He SL, Liu K, Wei F, et al: Effect of colchicine
on coronary plaque stability in acute coronary syndrome as assessed
by optical coherence tomography: The COLOCT randomized clinical
trial. Circulation. 150:981–993. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zaric BL, Radovanovic JN, Gluvic Z,
Stewart AJ, Essack M, Motwalli O, Gojobori T and Isenovic ER:
Atherosclerosis linked to aberrant amino acid metabolism and
immunosuppressive amino acid catabolizing enzymes. Front Immunol.
11:5517582020. View Article : Google Scholar : PubMed/NCBI
|