Introduction
Schizophrenia affects ~1% of the global population,
typically emerging in late adolescence or early adulthood and
significantly impairing cognitive and emotional functions (1). It is characterized by positive
symptoms such as hallucinations and delusions, negative symptoms
including reduced emotional expression and social withdrawal and
cognitive impairments affecting attention, memory and executive
functions (2). Neuroimaging
studies demonstrate substantial brain alterations in patients,
including reduced gray matter in the medial temporal and prefrontal
regions, pronounced ventricular enlargement, extensive cortical
thinning and white matter abnormalities (3,4).
Emerging evidence highlights blood-brain barrier (BBB) dysfunction
as a critical factor in the pathophysiology of schizophrenia,
potentially triggering neuroinflammatory cascades, aberrant
neurotransmission and structural remodeling that contribute to
symptom onset and progression (5,6).
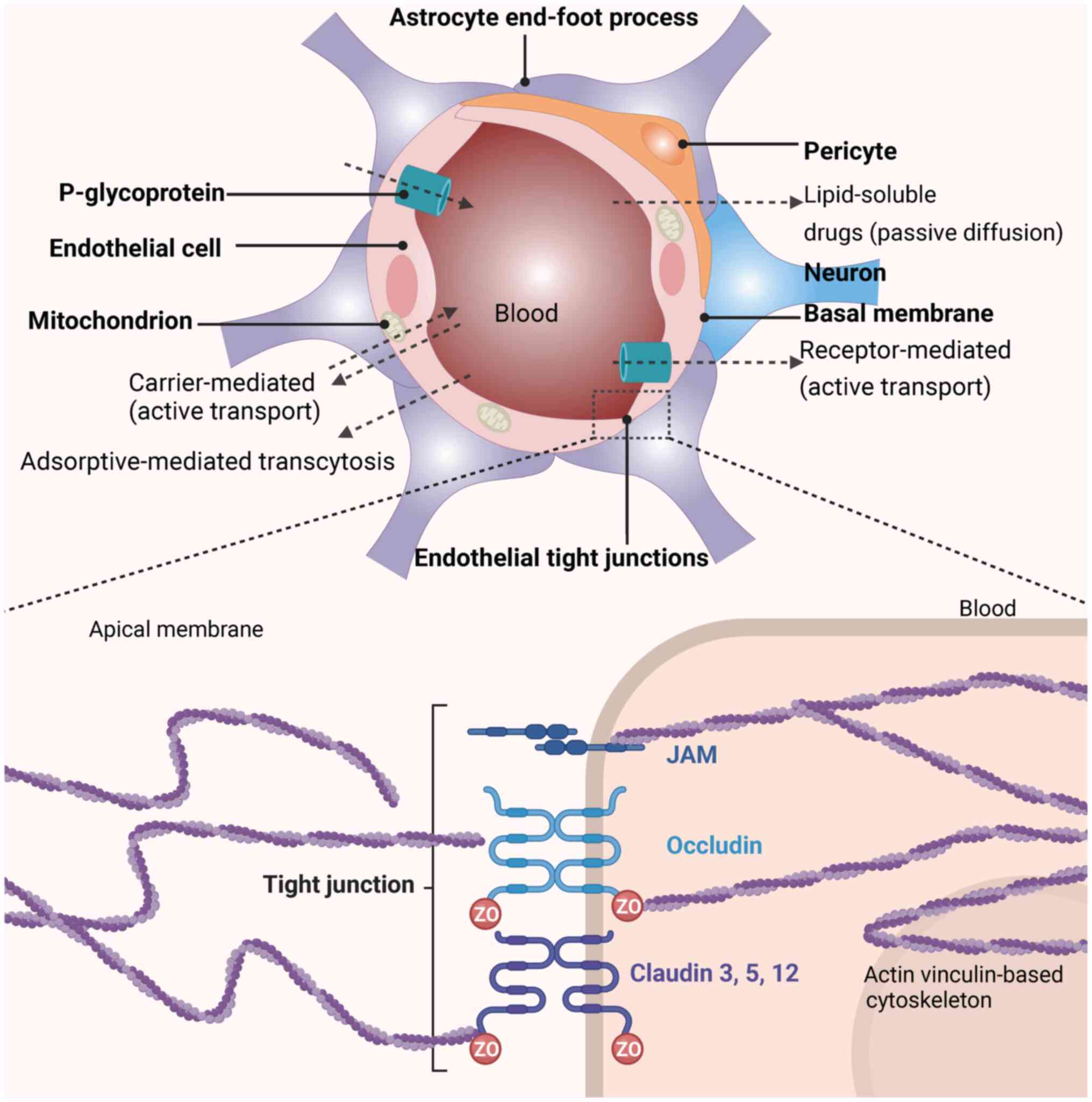
The BBB, a dynamic interface composed of tightly
joined endothelial cells, pericytes and astrocytes (Fig. 1) regulates the molecular exchange
between the bloodstream and the central nervous system (CNS). The
selective permeability of the BBB depends on tight junction
proteins such as claudin-5 (CLDN5) and occludin (OCLN), limited
endothelial pinocytosis and efflux transporters (7,8).
Beyond its barrier function, the BBB serves an active role in
modulating neuroimmune signaling and maintaining neuronal
homeostasis, making its integrity critical for brain health
(9).
In schizophrenia, BBB disruption has emerged as a
key pathological feature. Elevated cerebrospinal fluid (CSF)
albumin levels and altered expression of tight junction proteins,
particularly hippocampal claudin-5 deficits, indicate compromised
barrier selectivity (10,11).
Dysregulation of permeability regulators, including zonulin and
matrix metalloproteinase 9 (MMP9), further suggests active BBB
breakdown (12). Such
disruptions allow the infiltration of neurotoxic substances,
inflammatory cytokines and potentially pathogenic autoantibodies
into the brain parenchyma. These infiltrating molecules may
initiate or exacerbate pathological processes in schizophrenia,
including synaptic dysfunction, glial activation and oxidative
stress, all consistently observed in patients with schizophrenia
(13). Notably, evidence
suggests that BBB leakage may precede the clinical onset of
schizophrenia, indicating its potential role as an initiating
factor rather than merely a secondary consequence of disease
pathology (14).
The present review synthesized current evidence
linking BBB dysfunction to the pathophysiology of schizophrenia,
emphasizing structural and molecular alterations in the BBB, their
contribution to neuroinflammation and neurodegeneration, and the
implications for therapeutic strategies targeting barrier
integrity.
Schizophrenia overview
Introduction to schizophrenia
Schizophrenia is a debilitating neuropsychiatric
disorder that profoundly alters mental processes, including
thought, perception and emotional regulation (15). Schizophrenia typically manifests
in late adolescence or early adulthood, with rare diagnoses in
childhood or later stages of life. Although the prevalence is
similar across men and women, the progression and severity of
symptoms exhibit notable differences; men generally experience more
severe symptoms at an earlier age, while women often present with
more depressive symptoms and a later onset (16,17).
Clinically, schizophrenia is categorized into three
primary symptom types: Positive, negative and cognitive (1). Positive symptoms include
hallucinations (commonly auditory), delusions and disorganized
speech, which represent distortions or exaggerations of normal
perceptions and beliefs. For instance, patients may hear
non-existent voices or believe they are being persecuted without
basis (18). Negative symptoms
are characterized by reduced emotional expression, infrequent
speech and a lack of motivation, often resulting in withdrawal and
inactivity (19). Cognitive
symptoms involve impairments in executive functions, such as
processing complex information, maintaining focus and managing
working memory, skills essential for decision-making and daily
functioning (2). The Positive
and Negative Syndrome Scale (PANSS) is key for assessing
schizophrenia, evaluating both positive and negative symptoms
(20). This scale serves a vital
role in diagnosis, treatment guidance and symptom monitoring
(21). Neuroimaging studies have
highlighted both structural and functional brain abnormalities in
schizophrenia (22). Structural
imaging demonstrates decreased gray matter volume and cortical
thinning, especially in regions such as the hippocampus, amygdala,
thalamus and frontal cortex, areas critical for memory, emotion
regulation, sensory gating and decision-making (22). Functional imaging demonstrates
altered activation patterns during cognitive tasks and atypical
resting-state brain activity (23). Furthermore, diffusion tensor
imaging has identified white matter tract abnormalities, suggesting
disrupted connectivity between brain regions (24,25).
Genetics strongly influence the development of
schizophrenia, with heritability estimates ranging from 60-85%
(26). The disorder is
polygenic, involving complex interactions across multiple genetic
loci that increase susceptibility. Genome-wide association studies
(GWAS) have pinpointed several genomic regions linked to
schizophrenia, highlighting the significance of synaptic
organization and neurotransmission in its pathogenesis (27). Environmental factors also serve a
substantial role in the onset of schizophrenia. Prenatal and
perinatal exposures to infections or malnutrition, psychosocial
stress, trauma, substance use during adolescence, urban living and
migration all contribute to the schizophrenia development. These
factors emphasize the developmental vulnerability of the brain to
environmental stressors (28).
Together, these findings illustrate the multifaceted nature of
schizophrenia, underscoring the need for an integrated approach
that considers both biological and environmental influences in its
management and treatment.
Pathophysiology of schizophrenia
The pathophysiology of schizophrenia is
multifaceted, involving genetic factors, neurodevelopmental
disruptions, neurotransmitter imbalances and neuroimmune
alterations (Table I) (15,28-56). Each of these components
contributes to the complexity of schizophrenia, emphasizing the
need for comprehensive therapeutic approaches that target these
diverse mechanisms.
 | Table IPathophysiological factors in
mechanisms of schizophrenia. |
Table I
Pathophysiological factors in
mechanisms of schizophrenia.
| Mechanism | Related
factors | Results | (Refs.) |
|---|
|
Neurodevelopment | NRG1 gene
variations | Altered neuronal
development and synaptic plasticity; disturbed cognitive
function. | (29) |
| C4-A gene
activation | Pruned and lost
synapses | (30,31) |
| Microglial
overactivity | Disturbed synaptic
maintenance and stability | (32) |
| Mutation or
dysfunction of DISC1 gene | Compromised
neurogenesis; destabilized neuronal networks. | (33,34) |
| Malnutrition | Compromised
neurogenesis; disrupted fetal brain development. | (35,36) |
| Early-life
stress | Compromised
neurogenesis; destabilized neuronal networks. | (37-39) |
| Neurotransmitter
dysregulation | COMT gene
variations | Disturbed
neurotransmitter regulation | (40) |
| Variations in MAO-B
gene | Disturbed
astrocytic enzyme levels; affected neurotransmitter dynamics. | (41,42) |
| Increased dopamine
receptor sensitivity | Elevated dopamine
activity; destabilized neuronal networks. | (38,43,44) |
| Increased dopamine
synthesis and release | Elevated dopamine
activity. | (43,45) |
| NMDAR
dysfunction | Dysregulated
glutamate; imbalanced neurotransmitters. | (45-47) |
| Norepinephrine
dysregulation | Disrupted synaptic
transmission; distorted stress response mechanisms. | (48) |
| Neuroimmune
interactions | MHC genes | Dysregulated
immunity; exacerbated neuroinflammation. | (49,50) |
| Elevated cytokines
(TNF-α, IL-4, IL-6, IgM, PON1) | Exacerbated
neuroinflammation; disturbed neurotransmitter function. | (51,52) |
| Prenatal infections
(influenza, Toxoplasma gondii) | Activated maternal
immune responses. | (53,54) |
| p75 neurotrophin
receptor | Compromised
neuroprotection; neurotoxicity. | (55) |
Neurodevelopmental factors
Schizophrenia is increasingly recognized as a
neurodevelopmental disorder, characterized by disruptions during
critical stages of brain maturation that significantly influence
its onset and progression. These disruptions are often compounded
by genetic vulnerabilities and environmental factors such as
prenatal exposure to toxins or infections (31). The neurodevelopmental hypothesis
asserts that key developmental phases, particularly early gestation
and adolescence, involve crucial processes such as cell
proliferation and synaptic pruning, which are essential for normal
cognitive and emotional development (32).
During adolescence, synaptic pruning serves a vital
role in enhancing neuronal network efficiency by eliminating
redundant synapses; however, in schizophrenia, this process may
become excessive. For instance, the immune response of the brain
which is mediated by microglia that clear pathogens and prune
synapses, may become overactive. The activation of the complement
system, particularly the complement C4-A (C4-A) gene during late
adolescence, has been implicated in driving the excessive synaptic
pruning observed in schizophrenia (33). C4-A is essential for synaptic
pruning, marking synapses for removal by microglia, thereby
establishing a direct genetic-neuropathological connection to the
disorder. This excessive pruning leads to significant synaptic
loss, disrupting the balance between excitatory and inhibitory
signals in the brain (34,56).
Key genes such as neuregulin-1 and
disrupted-in-schizophrenia-1 (DISC1) also serve pivotal roles in
brain development and are functionally interconnected (35,36). For example, DISC1 influences
neurogenesis in the dentate gyrus and is involved in synaptic
transmission and astrocyte development (57). Furthermore, environmental factors
such as prenatal infections, malnutrition and early-life stress
exacerbate genetic predispositions, increasing the complexity and
susceptibility of schizophrenia (39). This pathological synaptic loss
significantly affects gray matter volume in critical brain regions,
such as the prefrontal cortex, which is essential for higher
cognitive functions (38,44).
Neurotransmitter dysregulation
The initial model of schizophrenia attributed
positive symptoms, such as hallucinations and delusions, to
hyperdopaminergic activity in the mesolimbic pathway, while
negative symptoms, such as social withdrawal and apathy, were
linked to dopaminergic hypoactivity in the mesocortical pathway
(58). Consistently, elevated
dopamine synthesis and release capacity have been observed in the
striatum of individuals with schizophrenia (59). Recent research, however, presents
a more nuanced perspective, emphasizing that the timing, specific
sites of dopamine release and receptor sensitivities are critical
factors in understanding the variability of symptoms among patients
(60-62). This approach moves beyond
dopamine dysregulation, offering further insights into the
neurochemical dynamics underlying the disorder (46).
There is increasing recognition of the complex
interactions between dopamine and other neurotransmitter systems,
particularly glutamate, in schizophrenia (46,56). Glutamate, the primary excitatory
neurotransmitter, has been implicated in both cognitive deficits
and negative symptoms, underscoring its pivotal role in the
pathology of schizophrenia (45,47). Variations in glutamate levels
across brain regions highlight the potential role of
region-specific glutamatergic dysfunction in the development and
symptom heterogeneity of schizophrenia (47). Dopaminergic activity in the
midbrain is heavily influenced by glutamatergic inputs from the
frontal cortex. This interaction forms a complex neural circuit,
where cortical pyramidal glutamatergic neurons activate GABAergic
interneurons, which, in turn, inhibit further glutamate release
from cortical neurons projecting to midbrain dopamine neurons. This
regulatory mechanism helps control the excitatory inputs received
by dopamine-producing neurons (50,63). γ-aminobutyric acid (GABA), the
principal inhibitory neurotransmitter in the CNS, is critical for
maintaining neural circuit balance. In schizophrenia, disrupted
N-methyl-D-aspartate receptor (NMDAR) activity on GABA interneurons
impairs inhibitory control, contributing to the cognitive deficits
associated with the disorder (59). Imbalances in this system can lead
to either increased or decreased dopaminergic output, significantly
affecting the pathophysiology of schizophrenia (64). These disturbances highlight the
need to elucidate the complex interplay between neurotransmitter
systems, as this may reveal novel therapeutic targets, such as
glutamatergic modulators or GABAergic enhancers, offering more
effective interventions for schizophrenia.
Neuroimmune interactions
The etiology of schizophrenia is increasingly viewed
in terms of neuroimmune interactions, highlighting the complex
interplay between genetic predispositions, environmental triggers
and immune system dysregulation (65,66). This perspective shifts the
traditional understanding of neuroinflammation from a consequence
to a potential contributing factor in schizophrenia development
(67).
The major histocompatibility complex on chromosome
6, essential for immune regulation, is associated with
schizophrenia (68,69). Dysregulation of genes within this
locus, related to innate immunity, may induce a pro-inflammatory
state in the CNS, exacerbating synaptic and neurotransmitter
disturbances (27,49). Increased levels of inflammatory
markers in both blood and CSF have been consistently observed in
patients with schizophrenia (70-72). Specifically, IL-6 levels are
significantly higher in the CSF of patients with schizophrenia
compared with that of healthy controls (73). IL-6 levels remain elevated in
both acute and chronic phases of psychosis, reinforcing the complex
relationship between neuroinflammatory processes and schizophrenia
pathology (71,74). This inflammation, marked by
increased levels of cytokines such as IL-6, influences
neurotransmitter systems, including dopaminergic and glutamatergic
pathways, which may exacerbate schizophrenia symptoms (51,75). Furthermore, increased IL-6 levels
correlate with cognitive decline and greater severity of both
positive and negative symptoms. Elevated C-reactive protein, a
systemic inflammation marker, has also been associated with
worsened cognitive function during acute psychosis (76). The entry of pro-inflammatory
cytokines and neurotoxic compounds into the CNS triggers an
inflammatory cascade linked to reactive oxygen species (ROS)
production. While ROS are normally byproducts of cellular
metabolism, excessive production leads to substantial cellular
damage. This inflammatory response, in conjunction with elevated
ROS, activates microglia and astrocytes, critical immune cells in
the brain, further promoting cytokine release and ROS production.
The resulting cycle of increased oxidative stress damages cellular
components, including membranes, proteins and DNA, contributing to
neuronal injury and dysfunction (77,78).
Additionally, maternal immune activation during
pregnancy due to infections such as influenza or Toxoplasma
gondii can disrupt immune regulation in the developing fetus.
The associated increase in cytokines such as IL-6 may impair normal
brain development, elevating the risk of schizophrenia in offspring
(79). This emerging
understanding underscores the significant role immune system
interactions serve in shaping the neuropsychiatric outcomes of
individuals predisposed to schizophrenia.
Treatment of schizophrenia
Antipsychotics remain the primary treatment for
schizophrenia, targeting dopamine receptors to alleviate core
symptoms such as delusions and hallucinations. These medications
are broadly classified into two categories: Typical
(first-generation) and atypical (second-generation) antipsychotics.
Typical antipsychotics, such as haloperidol and chlorpromazine,
exert their effects mainly through dopamine D2 receptor antagonism
and are often associated with significant adverse effects,
including extrapyramidal symptoms (EPS), tardive dyskinesia and
neuroleptic malignant syndrome (80,81). By contrast, atypical
antipsychotics, including risperidone, olanzapine, quetiapine and
aripiprazole, target both dopamine D2 and serotonin 5-HT2A
receptors. While these agents carry a reduced risk of motor side
effects, they are more frequently linked to metabolic
complications, such as weight gain, insulin resistance and
increased cardiovascular risk (82,83). Current treatment guidelines, such
as those from the American Psychiatric Association and the National
Institute for Health and Care Excellence, recommend
second-generation antipsychotics as first-line therapy, with drug
selection tailored to individual symptom profiles, side effect
tolerance and comorbid conditions. Despite their efficacy, ~33% of
patients with schizophrenia exhibit resistance to standard
antipsychotic treatments (84,85).
Targeting the BBB has demonstrated new therapeutic
avenues for treating schizophrenia. Although no FDA-approved drugs
currently regulate tight junction proteins, existing
anti-inflammatory therapies have demonstrated promise in improving
schizophrenia symptoms. Cyclooxygenase inhibitors, minocycline,
neurosteroids, N-acetylcysteine (NAC), statins and estrogens have
all shown consistent benefits (86). Minocycline inhibits microglial
activation and TNF-α production, indirectly stabilizing BBB
integrity (87). NAC boosts
glutathione levels and inhibits IL-6 and IL-1β, reducing oxidative
stress (88). A 6-month course
of NAC at 2 g/day has been shown to reduce overall symptom severity
significantly (PANSS effect size=0.45) (89).
Among novel therapies, lumateperone
(Caplyta®) has shown potential as a treatment option.
Lumateperone modulates dopaminergic, serotonergic and glutamatergic
neurotransmission, while its anti-inflammatory properties reduce
cytokines such as IL-1β, IL-6 and TNF-α. By restoring BBB
integrity, lumateperone improves barrier function and mitigates
inflammation triggered by immune challenges or stress (90). Clinical trials, including
ITI-007-301 and ITI-007-005, have shown significant efficacy in
managing schizophrenia and bipolar depression symptoms (91). The favorable side effect profile
of lumateperone, lower rates of EPS and minimal metabolic impact,
makes it an attractive treatment option. Lumateperone also reduced
relapse risk by 63% over 26 weeks (hazard ratio=0.37), with relapse
rates of 16.4% compared with 38.6% for placebo. The drug
demonstrated a favorable safety profile, with low akathisia rates
(2.1 vs. 6.9% placebo), minimal sedation [24%; number needed to
harm (NNH)=8] and few treatment discontinuations. Serious adverse
events, such as seizures (0.2%) and orthostatic hypotension (0.3%),
were rare (92). However,
variability in clinical trial outcomes underscores the need for
further studies to further understand the long-term benefits and
limitations, particularly in diverse patient subgroups (93,94).
BBB
Structure and function
The BBB serves a critical role in maintaining CNS
homeostasis by regulating the exchange of substances between the
blood and the brain. Comprised of endothelial cells lining the
capillaries of the brain, the BBB is specifically structured to
protect the brain from potential harm while facilitating the
efficient transport of essential nutrients into the environment of
the brain (95).
The BBB is reinforced by endothelial cells connected
by tight junctions formed by transmembrane proteins, including OCLN
and CLDNs, particularly CLDN5 (96). These proteins are essential for
maintaining the selective permeability of the barrier, permitting
the passage of essential nutrients while blocking harmful
substances. Scaffolding proteins such as zonula occludens-1 (ZO-1)
and junctional adhesion molecules (JAMs) provide structural support
and facilitate cellular signaling critical for regulating barrier
function and integrity. Surrounding the endothelial cells, the
basal lamina offers additional reinforcement, incorporating
proteins such as collagen type IV, laminin and fibronectin
(95). Collagen type IV forms a
scaffold supporting endothelial cells and pericytes, laminin
enhances cell adhesion and influences differentiation and
migration, while fibronectin facilitates cell adhesion and
modulates growth and migration (97). These interactions are pivotal for
the organization and stability of the BBB (98). Embedded in this extracellular
matrix, pericytes regulate blood flow and contribute to BBB
integrity, serving a pivotal role in processes such as angiogenesis
and barrier repair. Astrocytic end-feet closely envelop the
endothelium, augmenting tight junction functionality and supporting
the overall operations of the barrier. This intricate structure
ensures that the BBB effectively controls the entry and exit of
substances, maintaining the protected environment of the brain
(98).
The tight junctions of the BBB limit the diffusion
of hydrophilic molecules, allowing only selective substances to
penetrate. This selective permeability is essential for preventing
harmful substances from entering the brain while enabling the
passage of necessary nutrients. Nutrient transport across the BBB
is mediated by specialized transporter proteins, such as GLUT1 for
glucose and LAT1 for amino acids, which are essential for supplying
neuronal cells with the substrates required for energy production
and neurotransmitter synthesis (99). In addition to nutrient transport,
the BBB employs efflux transporters such as P-glycoprotein (P-gp)
to actively expel potentially harmful substances, such as
neurotoxins and excess neurotransmitters, that may have crossed
into the brain. This protective mechanism helps maintain chemical
stability and safeguards the brain against toxic threats, ensuring
optimal function (100,101). Transport across the BBB occurs
via several mechanisms: Passive diffusion for small lipophilic
molecules, carrier-mediated transport for essential nutrients,
receptor-mediated transcytosis for larger molecules and efflux
pumps for removing potentially harmful substances (7). Furthermore, the BBB serves a
critical role in maintaining ion balance, preventing electrolyte
disturbances that could disrupt neuronal function. It also
dynamically adjusts its permeability in response to both
physiological and pathological stimuli, such as inflammation, to
meet the needs of the CNS (3).
Evidence of BBB dysfunction in
schizophrenia
Clinical studies and case reports (76,102) provide compelling evidence of
BBB abnormalities in individuals diagnosed with schizophrenia,
suggesting that BBB dysfunction may serve a significant role in the
pathophysiology of schizophrenia (Table II) (13,18,71,103-111). A meta-analysis demonstrated
that ~16% of patients with first-episode psychosis exhibit
increased BBB permeability (18), indicating that BBB dysfunction
may manifest early in the illness and potentially serve as a
biomarker. However, it is essential to consider potential
confounding factors such as medication use, concurrent medical
conditions and lifestyle factors, all of which may impact BBB
integrity (112-114). Furthermore, the heterogeneity
among patients with schizophrenia, including variations in
symptomatology, disease progression and treatment response,
presents challenges in generalizing these findings across the
broader patient population (115). Future studies should address
these confounders and explore subgroup analyses to refine the
current understanding of BBB dysfunction in specific cohorts.
 | Table IIBBB-associated molecules in
schizophrenia. |
Table II
BBB-associated molecules in
schizophrenia.
| Marker | Normal
function | Change observed in
schizophrenia | Implication | (Refs.) |
|---|
| Q-Alb | Maintains blood
environment | Elevated in
CSF | Increased BBB
permeability | (103) |
| Total protein | - | Elevated in
CSF | Increased BBB
permeability | (18,71) |
| S100B | Supports astrocyte
function and CNS homeostasis | Elevated in
blood | Increased CNS
leakage due to compromised BBB | (104) |
| MMP9 | Regulates
extracellular matrix integrity and tight junction stability in the
BBB | Elevated in
blood | Compromised
BBB | (105,106) |
| Claudin-5 | Key component in
maintaining tight junctions that seal the BBB | Decreased in
postmortem hippocampus | Damaged BBB | (13,107) |
| Zonulin | Regulates tight
junctions and intestinal barrier function, extrapolated to BBB | Elevated in
blood | Damaged BBB | (108-110) |
| Soluble
P-selectin | Serves a role in
vascular health and regulating inflammation | Elevated in
blood | May facilitate
worsening of neuroinflammation, cognitive deficits and psychotic
symptoms | (111) |
Biochemical indicators
BBB dysfunction in schizophrenia is evident in both
the CSF and systemic biomarkers. For example, studies have shown
that ~29.4% of patients with schizophrenia display elevated
CSF/serum albumin quotient (Q-Alb) levels, indicating impaired
barrier selectivity that allows blood-derived proteins to
infiltrate the CNS (103,116). In addition to increased Q-Alb,
elevated total protein levels in CSF further underscore the extent
of BBB disruption, reflecting a loss of selective permeability.
This indicates that the BBB is no longer effectively restricting
the passage of large plasma-derived proteins, allowing non-specific
leakage into the central nervous system (18,71).
Serum biomarkers also support the evidence of BBB
compromise. S100 calcium binding protein B (S100B), an
astrocyte-derived protein, leaks into the bloodstream when the BBB
is disrupted, correlating with neuroinflammatory activity (104). Similarly, elevated MMP9 levels
in schizophrenia contribute to the degradation of tight junction
proteins and extracellular matrix components, perpetuating BBB
leakage and neurovascular remodeling (105,106). Increased zonulin levels, a
regulator of paracellular permeability, in the serum of patients
with schizophrenia further indicate compromised BBB integrity
(108).
Neuroimaging insights
Neuroimaging studies, particularly dynamic
contrast-enhanced magnetic resonance imaging (DCE-MRI), have
notably advanced the current understanding of BBB dysfunction in
schizophrenia (14,23,102). These studies demonstrate
substantial BBB leakage in patients at their first episode of
psychosis, suggesting that such disruptions may be foundational to
disease onset rather than secondary manifestations and could
potentially serve as early biomarkers (117). DCE-MRI findings demonstrate
that increased BBB permeability, quantified by the volume transfer
constant (Ktrans), correlates with increased symptom severity and
longer disease duration (118).
Advancements in positron emission tomography (PET)
imaging have also enhanced the current understanding by enabling
non-invasive assessments of BBB permeability using specialized
radiotracers. Advancements in positron emission tomography (PET)
imaging have also enhanced the current understanding by enabling
non-invasive assessments of BBB permeability using specialized
radiotracers. In schizophrenia, PET studies utilizing tracers such
as [¹¹C]-verapamil and [¹¹C]-L-dopa have demonstrated
region-specific reductions in P-glycoprotein function and altered
dopamine synthesis capacity, particularly in the striatum and
prefrontal cortex (10,119). Additionally, functional MRI
(fMRI) can assess cerebral blood flow (CBF) and neuronal activity,
offering insights into how BBB dysfunction affects the clinical
symptoms of schizophrenia (12,120).
Evidence of genetic and developmental
correlation
Genetic and developmental factors serve a pivotal
role in the pathogenesis of BBB dysfunction in schizophrenia
(12). The genetic basis of BBB
disruption is exemplified by 22q11.2 deletion syndrome (22qDS), a
disorder caused by a deletion of a small DNA segment on chromosome
22, which not only elevates schizophrenia risk but also impairs key
genes essential for BBB integrity (120). Notably, CLDN5, encoding
claudin-5, a protein critical for maintaining BBB tight junctions,
is significantly affected in this context and implicated in the
pathophysiology of schizophrenia (107). Induced pluripotent stem cell
models derived from patients with 22qDS exhibit a 'leaky' BBB
phenotype, marked by disorganized claudin-5 and enhanced
permeability (120,121).
Additionally, GWAS have identified loci linked to
both schizophrenia susceptibility and vascular health, highlighting
genes such as slit guidance ligand (SLIT)1, SLIT3 and roundabout
guidance receptor 1, which regulate endothelial signaling and
angiogenesis (13,122). The Frizzled Class Receptor 1
(FZD1) gene, integral to the Wnt signaling pathway that governs
endothelial function and BBB integrity, shows a notable independent
genetic association with schizophrenia, as identified in GWAS and
supported by transcriptomic analyses of patient-derived brain
tissue (123). In particular,
reduced FZD1 expression has been observed in the prefrontal cortex
of individuals with schizophrenia, suggesting impaired Wnt
signaling may contribute to BBB dysfunction and heightened
neuroinflammatory susceptibility (124).
These findings underscore the direct impact of
genetic predispositions on BBB integrity in schizophrenia,
increasing susceptibility to environmental factors and potentially
accelerating disease progression.
Mechanisms linking BBB dysfunction to
schizophrenia
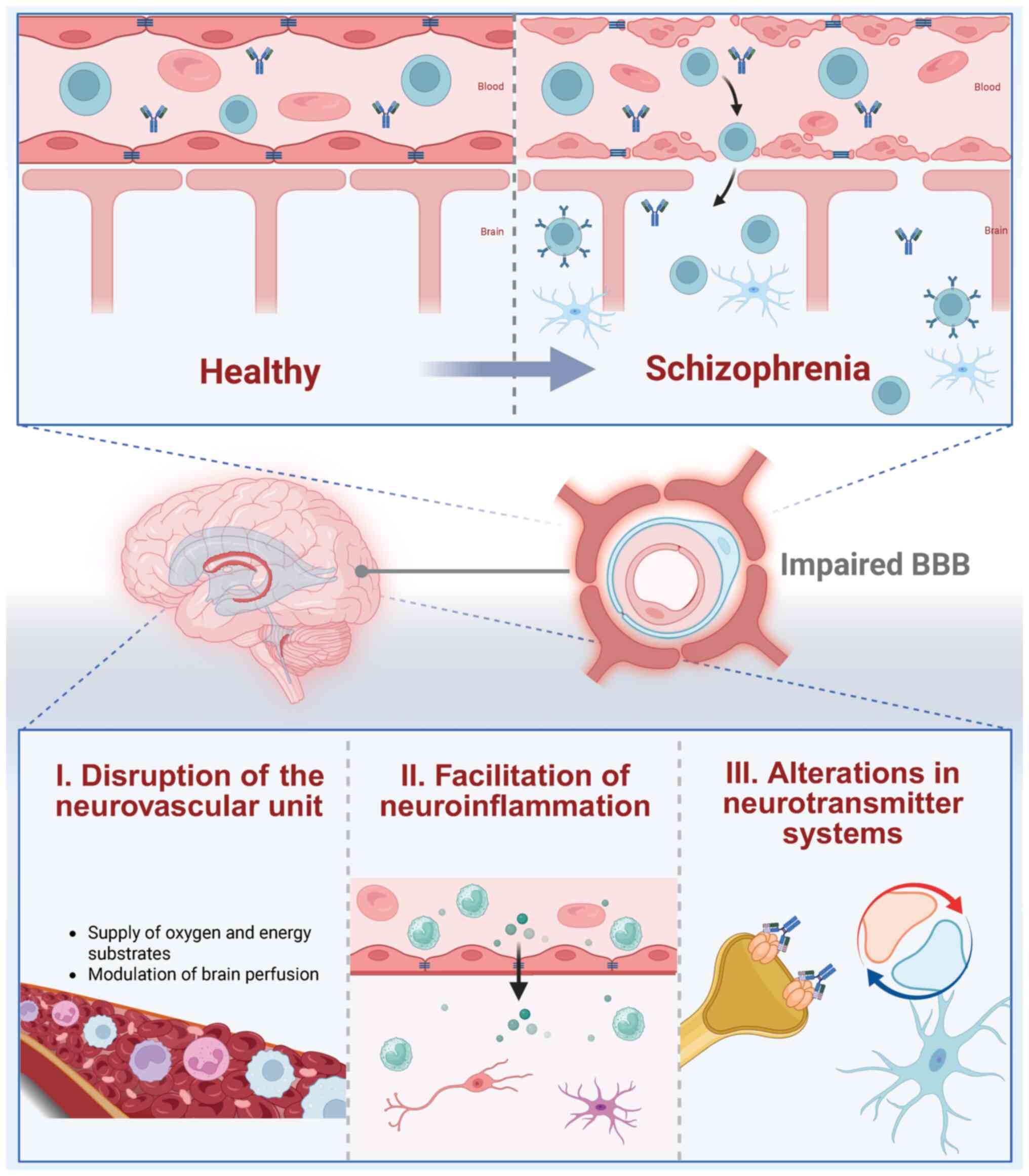
The breakdown of the BBB in schizophrenia
contributes to disease progression through multiple mechanisms
(Fig. 2), facilitating the entry
of inflammatory mediators and immune cells into the CNS. This
disruption exacerbates neuroinflammation, further affecting key
neurotransmitter systems and CBF, which are critical for the
development of cognitive and psychotic symptoms (10,14).
Dysregulating CBF
CBF quantifies the volume of blood flow through
brain tissue over time, increasing in response to neuronal activity
to ensure adequate oxygen and glucose supply for metabolic demands
(125). However, in
schizophrenia, reduced CBF impairs this process, exacerbating
neuronal damage and cognitive deficits, including auditory verbal
hallucinations (AVHs) (126). A
failure in neurovascular coupling has been identified, correlating
with specific symptoms such as AVHs (127), as well as a broader reduction
in CBF associated with cognitive decline and dementia progression
(128).
The BBB is integral in regulating CBF and
maintaining brain health by stabilizing the microenvironment and
facilitating the clearance of metabolic waste (40,129). BBB dysfunction disrupts CBF
regulation, leading to the accumulation of neurotoxic substances
and the amplification of neuroinflammatory processes that impair
synaptic function (130). This
relationship underscores the critical role of BBB integrity in
mitigating the progression and symptom severity of schizophrenia
(131).
Neuroinflammation promotion
In schizophrenia, BBB disruption permits the entry
of peripheral immune components into the CNS, exacerbating deficits
in synaptic pruning and neurotransmitter imbalances central to the
pathology of schizophrenia (10).
Elevated levels of soluble P-selectin (sP-selectin)
and soluble intercellular adhesion molecule in patients indicate a
pro-inflammatory state that promotes immune cell migration across
the compromised BBB (111).
This infiltration activates glial cells, including microglia and
astrocytes, which release pro-inflammatory cytokines such as IL-6
and TNF-α (132), exacerbating
cognitive and psychotic symptoms by disrupting neurotransmitter
systems, particularly glutamatergic and dopaminergic pathways
(73,132). Additionally, inflammation can
further disrupt GABAergic signaling, disrupting the delicate
balance between excitatory (glutamatergic) and inhibitory
(GABAergic) neurotransmission, essential for cognitive function
(10,133). Inflammation related to BBB
dysfunction may also affect serotonin transporter activity and
receptor function, influencing these symptoms (10). Furthermore, the neuroinflammatory
process may damage the BBB further, creating a harmful feedback
loop that exacerbates the symptoms and progression of schizophrenia
(134,135). The increased vulnerability of
the brain to immune mediators following BBB compromise highlights
the need for longitudinal studies on BBB function in individuals at
high risk for psychosis to further understand and address these
complex interactions (133,136).
Alteration of neurotransmitter
systems
BBB disruption in schizophrenia leads to
neurochemical imbalances, significantly impacting neurotransmitter
systems essential for CNS homeostasis, and contributing to a wide
range of symptoms including hallucinations, delusions, social
withdrawal, apathy and cognitive deficits. Hammer et al
(137) suggested that although
NMDAR autoantibody prevalence is similar between patients with
schizophrenia and controls, these autoantibodies correlate with
more severe neurological and affective symptoms in patients
exhibiting markers of BBB disruption, such as a history of head
trauma or the presence of the apolipoprotein E4 allele. Animal
models further support these findings by demonstrating that the
pathogenic effects of neuronal autoantibodies manifest only when
BBB integrity is compromised (10). In one study, mice passively
infused with human-derived anti-NMDAR IgG displayed no behavioral
abnormalities under normal BBB conditions; however, when BBB
permeability was transiently increased, via systemic
lipopolysaccharide injection or mannitol-induced osmotic opening,
mice developed anxiety-like behaviors, memory impairments and
reduced exploratory activity (138). Molecular analyses revealed
decreased synaptic NMDAR density and altered glutamatergic
signaling in the hippocampus. These findings highlight that
circulating autoantibodies require BBB disruption to access
neuronal targets and exert neurotoxic effects, underscoring the
pivotal role of BBB integrity in modulating autoimmune
contributions to schizophrenia-like phenotypes.
The BBB also regulates the transport of essential
nutrients and precursors required for neurotransmitter synthesis.
For instance, dopamine synthesis depends on the availability of
tyrosine, an amino acid that must cross the BBB. When BBB
permeability is compromised, the transport of key precursors such
as tyrosine is hindered, resulting in reduced dopamine synthesis
and potentially affecting other neurotransmitters (9,46,139,140). Increased BBB permeability
allows neurotoxic substances and bioactive molecules, such as
glutamate, norepinephrine, epinephrine and glycine, to infiltrate
the brain, disrupting neurotransmitter balance. This dysregulation
further impairs neuronal communication, intensifying cognitive
dysfunction, emotional instability and psychotic symptoms in
schizophrenia (141).
Limitations and future directions
Limitations and novel techniques of
current studies
Research on BBB permeability in schizophrenia has
become a pivotal area of investigation, providing insight on the
neurobiological mechanisms underlying of the disorder. Given the
heterogeneity of schizophrenia, with distinct subtypes potentially
exhibiting different pathophysiological mechanisms, variability in
BBB dysfunction may arise and should be considered a potential
source of bias (142).
Demographic factors such as age, sex and
comorbidities such as depression may also influence BBB
permeability. Furthermore, the impact of medications, particularly
antipsychotics, on BBB integrity represents a significant
confounding factor. For instance, clozapine may stabilize BBB
function in certain patients, complicating causal interpretations
(143). Environmental factors,
including stress and trauma, commonly experienced by individuals
with psychosis, can further impact BBB function (144).
From a methodological perspective, imaging
techniques used to assess BBB permeability, such as DCE-MRI and
ASL, could undergo further rigorous evaluation in improve
reliability and standardization across participants. The
generalizability of current findings is constrained by relatively
small sample sizes (schizophrenia, n=29; controls, n=18) (14). Larger and more diverse cohorts
are essential to determine the applicability of these findings to a
broader schizophrenia population. Furthermore, inconsistencies
across studies may arise from heterogeneity in imaging parameters,
contrast agent administration protocols, region-of-interest
definitions and permeability quantification models. For instance,
while some DCE-MRI studies have reported increased BBB leakage in
regions such as the hippocampus and temporal cortex, others have
not detected significant changes, likely due to variations in
acquisition timing, contrast dosage or differences in permeability
metrics (e.g., Ktrans vs. Vp) (14). ASL, although non-invasive and
contrast-free, measures cerebral perfusion rather than direct
barrier permeability, making it difficult to disentangle BBB
disruption from secondary vascular changes (129). Addressing these gaps will
deepen the current understanding of BBB permeability changes in
schizophrenia and guide future research directions.
Recent technological advancements have provided new
avenues for molecular, physiological, neurophysiological and
genomic insights. Dynamic in vitro BBB models, microfluidic
BBB and BBB-on-a-chip platforms offer sophisticated systems for
studying BBB permeability (145-147). Additionally, new cell culture
scaffolds containing essential anchoring or adhesion molecules
allow more precise control over cell differentiation, interactions
and responses (148).
Patient-derived brain organoids have emerged as
invaluable models for investigating BBB dysfunction in
schizophrenia. Organoids derived from patients with schizophrenia
exhibit a 30-50% increase in paracellular permeability and display
elongated microvascular networks, associated with the dysregulated
expression of tight junction proteins CLDN5 and OCLN. Differential
gene expression analysis of endothelial cells isolated from
schizophrenia patient-derived brain organoids reveals significant
enrichment of genes related to angiogenesis, vascular regulation
and inflammatory responses when compared with controls (149,150).
Using primary cultures derived from human cells
could mitigate species-specific differences; however, their
availability is limited due to ethical considerations. The
olfactory system, closely associated with significant olfactory
deficits in patients with schizophrenia, has become a key model in
schizophrenia research (151,152). The olfactory epithelium (OE)
offers a 'unique' window into the brain, providing an opportunity
to assess various hypotheses related to the neurodevelopmental
models of schizophrenia (153).
Recent single-cell RNA sequencing of cultured nasal turbinate cells
derived from patients with schizophrenia has shown that these cells
closely resemble neural progenitors and mesenchymal cells found in
the olfactory neuroepithelium and the developing brain (154). The OE presents a minimally
invasive source of neurons and precursor cells, sharing
developmental origins with CNS cells, facilitating the study of
gene-genomic interactions and neurophysiological processes relevant
to both schizophrenia and BBB pathology (155).
These innovations enable researchers to further
simulate the complex interactions underlying BBB dysfunction and
explore potential therapeutic interventions for conditions such as
schizophrenia.
Biomarkers and advanced imaging
techniques
Current research on BBB dysfunction in schizophrenia
highlights the critical role of biomarkers and imaging techniques
in understanding and diagnosing the disorder. However, the reliable
assessment of BBB integrity remains challenging due to the indirect
nature of numerous existing biomarkers and imaging methods. For
instance, plasma S100β levels have been shown to correlate with
negative symptoms and cognitive deficits in schizophrenia (156). Meta-analyses further indicate a
positive association between S100β levels, positive symptoms and
disease progression, suggesting that BBB permeability may increase
as the disease advances (157).
However, it remains unclear whether elevated S100β directly
reflects increased BBB permeability or merely signifies enhanced
glial cell production/secretion or degeneration.
The integration of blood-based biomarkers with
advanced neuroimaging techniques has potential for more
comprehensive and patient-specific diagnostic approach. For
example, while DCE-MRI Ktrans values provide valuable insights into
BBB dysfunction, they lack the molecular specificity needed to
fully elucidate the mechanisms underlying BBB impairment (14). To overcome this limitation,
combining DCE-MRI with PET imaging could enable molecular-level
visualization. PET could employ radiolabeled probes targeting
specific BBB markers such as CLDN5 or OCLN, which are vital for
tight junction formation and permeability regulation. However,
these PET tracers are still in early developmental stages, and
conclusive evidence supporting their clinical application for BBB
visualization, particularly in schizophrenia, remains lacking
(158,159).
By integrating these biomarkers with advanced
imaging techniques, diagnostic accuracy can be significantly
improved, enabling longitudinal tracking of BBB dysfunction. This
combined approach would facilitate more targeted, personalized
diagnostics, enhancing the monitoring of disease progression and
therapeutic responses in individual patients with
schizophrenia.
BBB-targeted therapies in
schizophrenia
Future directions regarding
BBB-targeted therapies
The development of drugs targeting BBB tight
junctions represents an emerging and promising avenue for
schizophrenia treatment. Preclinical studies have shown that
restoration of the endothelial glycocalyx can reduce oxidative
stress by up to 40% and enhance the expression of tight junction
proteins (160). Combination
strategies, such as pairing BBB stabilizers (e.g., mucin-domain
glycoproteins) with conventional antipsychotics, may not only
improve therapeutic efficacy but also allow dose reduction and
minimize systemic side effects (161).
Molecular modulators that reinforce tight junction
architecture, including CLDNs and OCLNs, are central to BBB
stabilization. Experimental evidence indicates that specific
peptides can promote the assembly of these proteins and restore
barrier integrity (162).
Epalrestat, an aldose reductase inhibitor with an established
safety profile, has been shown to improve BBB function and enhance
neurobehavioral outcomes in animal models (163). Gene therapy approaches, such as
adeno-associated virus-mediated overexpression of mucin
biosynthesis enzymes (e.g., C1galt1), have successfully
reversed BBB dysfunction and cognitive deficits in preclinical
models (164). In addition,
small molecules targeting frizzled receptors have demonstrated the
ability to upregulate CLDN5 and OCLN, further supporting their
potential in preserving tight junction integrity (165).
Innovative dual-action strategies are also gaining
attention. For instance, co-administration of the LRP1-targeting
peptide KS-487 with the VIPR2 antagonist KS-133 has been shown to
enhance BBB penetration, reduce neuroinflammation and improve
cognitive performance in murine models, reflected by a ~30%
increase in novel object recognition (166). Such approaches, which
simultaneously target psychiatric symptoms and BBB dysfunction,
exemplify a new generation of therapeutics that extend beyond
neurotransmitter modulation. Ultimately, integrating BBB
stabilization strategies with standard pharmacological treatments
may lead to more effective, biologically grounded interventions for
schizophrenia and related neuropsychiatric disorders (85,160,161).
Impact of improving drug efficacy and
side effects
BBB variability serves a critical role in
influencing the therapeutic efficacy and side effects of
antipsychotic drugs. P-gp, a key efflux transporter at the BBB,
directly regulates the CNS concentration of these medications
(167). P-gp actively exports
numerous antipsychotics from the brain, creating variability in
patient responses and increasing the risk of side effects due to
suboptimal drug levels in the brain (167). Genetic polymorphisms or drug
interactions can alter P-gp activity (168). Among the most studied variants,
the ABCB1 C3435T single nucleotide polymorphism has been associated
with reduced P-gp expression and activity. Individuals carrying the
T allele often exhibit higher plasma and CNS concentrations of P-gp
substrates, including antipsychotics such as olanzapine and
risperidone (169). This
increased CNS exposure has been linked to both enhanced therapeutic
effects and a higher incidence of adverse reactions, such as
sedation and extrapyramidal symptoms. Furthermore, pharmacokinetic
interactions involving P-gp inhibitors, such as verapamil and
ketoconazole, can also lead to elevated brain-to-plasma
concentration ratios of co-administered P-gp substrates in both
preclinical and clinical settings, raising concerns about potential
neurotoxicity and altered efficacy (170). Chronic exposure to
antipsychotics in patients with compromised BBB function may also
lead to cumulative side effects or long-term neurovascular changes
(171,172). Ongoing research suggests that
prolonged medication use can deteriorate neurovascular integrity,
potentially worsening BBB dysfunction and exacerbating psychiatric
symptoms (133). Addressing the
increased activity of P-gp at the BBB, a significant contributor to
pharmacoresistance, is a key focus of current research, which
serves to advance treatment models and improve outcomes for
patients with schizophrenia (173). Various strategies have been
developed to enhance drug delivery to the CNS, such as intranasal
administration, incorporation of nanomaterials, RNA interference
and extracellular vesicles (174-177). Advanced delivery platforms,
including ligand-conjugated nanoparticles targeting receptors such
as LRP1, offer the potential for more efficient BBB crossing and
improved therapeutic outcomes (178). Combining anti-inflammatory
agents, such as minocycline, with nanoparticle-based delivery
systems presents a promising approach to stabilizing BBB function
and alleviating schizophrenia symptoms (166). However, most of these methods
assume a functional BBB in patients, often overlooking the dynamic
nature of the BBB and the potential dysfunction in psychiatric
populations.
Patients with impaired P-gp functionality may
experience higher drug concentrations in the brain, potentially
exacerbating side effects such as EPS due to enhanced dopamine
receptor blockade (179,180).
By contrast, robust P-gp activity could lower brain drug levels,
potentially reducing efficacy and decreasing side effect risks.
This dynamic is supported by research using Mdr1a/b knockout mice,
which lack functional P-gp and show increased brain concentrations
of drugs such as risperidone (167), highlighting critical role of
P-gp in modulating CNS drug access. Emerging strategies aim to
optimize BBB penetration without provoking unwanted side effects.
For instance, the novel TAAR1 agonist compound 50B demonstrates
favorable BBB permeability and does not induce EPS, suggesting that
strategic drug design can enhance efficacy while minimizing adverse
effects (181). Furthermore,
individual variability in BBB integrity underscores the importance
of personalized treatment approaches. While some patients maintain
intact BBB function, others exhibit significant permeability
changes, necessitating tailored therapeutic strategies that account
for individual differences in BBB dysfunction.
Conclusion
The present review synthesized key findings on the
role of BBB dysfunction in schizophrenia, emphasizing its
association with the neuropathological features of schizophrenia.
Notable insights include increased BBB permeability, reduced
expression of tight junction proteins, and elevated inflammatory
markers. These alterations not only exacerbate neuroinflammation
but also disrupt neurotransmitter systems, thereby influencing
symptom severity and treatment response (100). Thus, maintaining BBB integrity
is critical for the comprehensive management of schizophrenia,
underscoring its importance in both disease strategy and patient
care.
Future research focused on the BBB could
revolutionize diagnostic and therapeutic approaches in
schizophrenia by facilitating early detection and improving
treatment outcomes (4).
Non-invasive imaging techniques, such as DCE-MRI, and biomarker
discovery could enable earlier identification of BBB dysfunction,
leading to timely interventions (23,182). Furthermore, integrating DCE-MRI
with PET tracers may enhance diagnostic accuracy, facilitating the
detection of early BBB changes. Additionally, the development of
BBB-targeted therapies, including pharmacological agents that
promote BBB repair and nanoparticle-based drug delivery systems,
holds the potential to significantly improve treatment efficacy by
enhancing drug penetration into the brain (75,183). However, challenges remain,
particularly regarding the safe delivery of therapeutics across the
BBB and minimizing potential toxicity to non-target brain regions.
Personalized medicine approaches, incorporating genetic profiling
related to BBB integrity, could enable the development of tailored
treatment plans that address both symptom management and the
underlying neurobiological mechanisms of schizophrenia (184). Monitoring biomarkers of BBB
integrity, such as changes in the levels of tight junction proteins
and inflammatory markers, could offer valuable insights into the
effectiveness of treatment and inform the refinement of disease
management strategies (185).
Tailored treatment strategies, informed by genetic factors
affecting BBB function, may further enhance antipsychotic efficacy
by targeting both the symptoms and the underlying neurobiological
mechanisms of the disorder (133,186). Thus, future research into
diagnostic tools such as DCE-MRI and BBB-targeting therapies have
potential to improve the management and treatment of
schizophrenia.
Availability of data and materials
Not applicable.
Authors' contributions
SL contributed to the writing and editing of this
review. CL collected the data. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Hany M, Rehman B, Rizvi A and Chapman J:
Schizophrenia. StatPearls. StatPearls Publishing; Treasure Island,
FL: 2024
|
|
2
|
Jauhar S, Johnstone M and McKenna PJ:
Schizophrenia. Lancet. 399:473–486. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keshavan MS, Collin G, Guimond S, Kelly S,
Prasad KM and Lizano P: Neuroimaging in schizophrenia. Neuroimaging
Clin N Am. 30:73–83. 2020. View Article : Google Scholar :
|
|
4
|
DeLisi LE, Szulc KU, Bertisch HC, Majcher
M and Brown K: Understanding structural brain changes in
schizophrenia. Dialogues Clin Neurosci. 8:71–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldwaser EL, Swanson RL II, Arroyo EJ,
Venkataraman V, Kosciuk MC, Nagele RG, Hong LE and Acharya NK: A
preliminary report: The hippocampus and surrounding temporal cortex
of patients with schizophrenia have impaired blood-brain barrier.
Front Hum Neurosci. 16:8369802022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldwaser EL, Wang DJJ, Adhikari BM,
Chiappelli J, Shao X, Yu J, Lu T, Chen S, Marshall W, Yuen A, et
al: Evidence of neurovascular water exchange and endothelial
vascular dysfunction in schizophrenia: An exploratory study.
Schizophr Bull. 49:1325–1335. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardridge WM: Drug transport across the
blood-brain barrier. J Cereb Blood Flow Metab. 32:1959–1972. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daneman R and Prat A: The blood-brain
barrier. Cold Spring Harb Perspect Biol. 7:a0204122015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knox EG, Aburto MR, Clarke G, Cryan JF and
O'Driscoll CM: The blood-brain barrier in aging and
neurodegeneration. Mol Psychiatry. 27:2659–2673. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Najjar S, Pahlajani S, De Sanctis V, Stern
JNH, Najjar A and Chong D: Neurovascular unit dysfunction and
blood-brain barrier hyperpermeability contribute to schizophrenia
neurobiology: A theoretical integration of clinical and
experimental evidence. Front Psychiatry. 8:832017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Puvogel S, Palma V and Sommer IEC: Brain
vasculature disturbance in schizophrenia. Curr Opin Psychiatry.
35:146–156. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greene C, Kealy J, Humphries MM, Gong Y,
Hou J, Hudson N, Cassidy LM, Martiniano R, Shashi V, Hooper SR, et
al: Dose-dependent expression of claudin-5 is a modifying factor in
schizophrenia. Mol Psychiatry. 23:2156–2166. 2018. View Article : Google Scholar :
|
|
13
|
Greene C, Hanley N and Campbell M:
Blood-brain barrier associated tight junction disruption is a
hallmark feature of major psychiatric disorders. Transl Psychiatry.
10:3732020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moussiopoulou J, Yakimov V, Roell L,
Rauchmann BS, Toth H, Melcher J, Jäger I, Lutz I, Kallweit MS,
Papazov B, et al: Higher blood-brain barrier leakage in
schizophrenia-spectrum disorders: A comparative dynamic
contrast-enhanced magnetic resonance imaging study with healthy
controls. Brain Behav Immun. 128:256–265. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murray AJ, Rogers JC, Katshu MZUH, Liddle
PF and Upthegrove R: Oxidative stress and the pathophysiology and
symptom profile of schizophrenia spectrum disorders. Front
Psychiatry. 12:7034522021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su G, Chen Y, Li X and Shao JW: Virus
versus host: Influenza A virus circumvents the immune responses.
Front Microbiol. 15:13945102024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giordano GM, Bucci P, Mucci A, Pezzella P
and Galderisi S: Gender differences in clinical and psychosocial
features among persons with schizophrenia: A mini review. Front
Psychiatry. 12:7891792021. View Article : Google Scholar
|
|
18
|
Maurus I, Wagner S, Campana M, Roell L,
Strauss J, Fernando P, Muenz S, Eichhorn P, Schmitt A, Karch S, et
al: The relationship between blood-brain barrier dysfunction and
neurocognitive impairments in first-episode psychosis: Findings
from a retrospective chart analysis. BJPsych Open. 9:e602023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCutcheon RA, Reis Marques T and Howes
OD: schizophrenia-an overview. JAMA Psychiatry. 77:201–210. 2020.
View Article : Google Scholar
|
|
20
|
Kay SR, Fiszbein A and Opler LA: The
positive and negative syndrome scale (PANSS) for schizophrenia.
Schizophr Bull. 13:261–276. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim K, Peh OH, Yang Z, Rekhi G, Rapisarda
A, See YM, Rashid NAA, Ang MS, Lee SA, Sim K, et al: Large-scale
evaluation of the positive and negative syndrome scale (PANSS)
symptom architecture in schizophrenia. Asian J Psychiatr.
62:1027322021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karlsgodt KH, Sun D and Cannon TD:
Structural and functional brain abnormalities in schizophrenia.
Curr Dir Psychol Sci. 19:226–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dabiri M, Dehghani Firouzabadi F, Yang K,
Barker PB, Lee RR and Yousem DM: Neuroimaging in schizophrenia: A
review article. Front Neurosci. 16:10428142022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tønnesen S, Kaufmann T, Doan NT, Alnæs D,
Córdova-Palomera A, Meer DV, Rokicki J, Moberget T, Gurholt TP,
Haukvik UK, et al: White matter aberrations and age-related
trajectories in patients with schizophrenia and bipolar disorder
revealed by diffusion tensor imaging. Sci Rep. 8:141292018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kubicki M, McCarley R, Westin CF, Park HJ,
Maier S, Kikinis R, Jolesz FA and Shenton ME: A review of diffusion
tensor imaging studies in schizophrenia. J Psychiatr Res. 41:15–30.
2007. View Article : Google Scholar
|
|
26
|
Trifu SC, Kohn B, Vlasie A and Patrichi
BE: Genetics of schizophrenia (review). Exp Ther Med. 20:3462–3468.
2020.PubMed/NCBI
|
|
27
|
V'Kovski P, Kratzel A, Steiner S, Stalder
H and Thiel V: Coronavirus biology and replication: Implications
for SARS-CoV-2. Nat Rev Microbiol. 19:155–170. 2021. View Article : Google Scholar
|
|
28
|
Georgiades A, Almuqrin A, Rubinic P,
Mouhitzadeh K, Tognin S and Mechelli A: Psychosocial stress,
interpersonal sensitivity, and social withdrawal in clinical high
risk for psychosis: A systematic review. Schizophrenia (Heidelb).
9:382023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nisha Aji K, Lalang N, Ramos-Jiménez C,
Rahimian R, Mechawar N, Turecki G, Chartrand D, Boileau I, Meyer
JH, Rusjan PM and Mizrahi R: Evidence of altered monoamine oxidase
B, an astroglia marker, in early psychosis and high-risk state. Mol
Psychiatry. 30:2049–2058. 2025. View Article : Google Scholar
|
|
30
|
Schmitt A, Falkai P and Papiol S:
Neurodevelopmental disturbances in schizophrenia: Evidence from
genetic and environmental factors. J Neural Transm (Vienna).
130:195–205. 2023. View Article : Google Scholar
|
|
31
|
Kotsiri I, Resta P, Spyrantis A,
Panotopoulos C, Chaniotis D, Beloukas A and Magiorkinis E: Viral
infections and schizophrenia: A comprehensive review. Viruses.
15:13452023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Selemon LD and Zecevic N: Schizophrenia: A
tale of two critical periods for prefrontal cortical development.
Transl Psychiatry. 5:e6232015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sekar A, Bialas AR, de Rivera H, Davis A,
Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V,
et al: Schizophrenia risk from complex variation of complement
component 4. Nature. 530:177–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Uliana DL, Zhu X, Gomes FV and Grace AA:
Using animal models for the studies of schizophrenia and
depression: The value of translational models for treatment and
prevention. Front Behav Neurosci. 16:9353202022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mei L and Xiong WC: Neuregulin 1 in neural
development, synaptic plasticity and schizophrenia. Nat Rev
Neurosci. 9:437–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soares DC, Carlyle BC, Bradshaw NJ and
Porteous DJ: DISC1: Structure, function, and therapeutic potential
for major mental illness. ACS Chem Neurosci. 2:609–632. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen L, Xu J, Zhu L, Xu P, Chang L, Han Y
and Wu Q: Disrupted in schizophrenia 1 reverse ectopic migration of
neural precursors in mouse hilus after pilocarpine-induced status
epilepticus. Mol Neurobiol. 60:6689–6703. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Breitmeyer R, Vogel S, Heider J, Hartmann
SM, Wüst R, Keller AL, Binner A, Fitzgerald JC, Fallgatter AJ and
Volkmer H: Regulation of synaptic connectivity in schizophrenia
spectrum by mutual neuron-microglia interaction. Commun Biol.
6:4722023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McGrath J, Saari K, Hakko H, Jokelainen J,
Jones P, Järvelin MR, Chant D and Isohanni M: Vitamin D
supplementation during the first year of life and risk of
schizophrenia: A Finnish birth cohort study. Schizophr Res.
67:237–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Duan L, Li S, Chen D, Shi Y, Zhou X and
Feng Y: Causality between autoimmune diseases and schizophrenia: A
bidirectional Mendelian randomization study. BMC Psychiatry.
24:8172024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bondrescu M, Dehelean L, Farcas SS, Papava
I, Nicoras V, Podaru CA, Sava M, Bilavu ES, Putnoky S and Andreescu
NI: Cognitive impairments related to COMT and neuregulin 1
phenotypes as transdiagnostic markers in schizophrenia spectrum
patients. J Clin Med. 13:64052024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bergen SE, Fanous AH, Walsh D, O'Neill FA
and Kendler KS: Polymorphisms in SLC6A4, PAH, GABRB3, and MAOB and
modification of psychotic disorder features. Schizophr Res.
109:94–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang AC and Tsai SJ: New targets for
schizophrenia treatment beyond the dopamine hypothesis. Int J Mol
Sci. 18:16892017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luvsannyam E, Jain MS, Pormento MKL,
Siddiqui H, Balagtas ARA, Emuze BO and Poprawski T: Neurobiology of
schizophrenia: A comprehensive review. Cureus.
14:e239592022.PubMed/NCBI
|
|
45
|
Wu Q, Huang J and Wu R: Drugs based on
NMDAR hypofunction hypothesis in schizophrenia. Front Neurosci.
15:6410472021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McCutcheon RA, Krystal JH and Howes OD:
Dopamine and glutamate in schizophrenia: Biology, symptoms and
treatment. World Psychiatry. 19:15–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kruse AO and Bustillo JR: Glutamatergic
dysfunction in schizophrenia. Transl Psychiatry. 12:5002022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jain R, Chepke C, Davis LL, McIntyre RS
and Raskind MA: Dysregulation of noradrenergic activity: Its role
in conceptualizing and treating major depressive disorder,
schizophrenia, agitation in Alzheimer's disease, and posttraumatic
stress disorder. J Clin Psychiatry. 85:plunaro2417ah2024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Murphy CE, Walker AK and Weickert CS:
Neuroinflammation in schizophrenia: The role of nuclear factor
kappa B. Transl Psychiatry. 11:5282021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hughes H, Brady LJ and Schoonover KE:
GABAergic dysfunction in postmortem dorsolateral prefrontal cortex:
Implications for cognitive deficits in schizophrenia and affective
disorders. Front Cell Neurosci. 18:14408342024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Müller N, Weidinger E, Leitner B and
Schwarz MJ: The role of inflammation in schizophrenia. Front
Neurosci. 9:3722015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Borovcanin MM, Jovanovic I, Radosavljevic
G, Pantic J, Minic Janicijevic S, Arsenijevic N and Lukic ML:
Interleukin-6 in schizophrenia-is there a therapeutic relevance?
Front Psychiatry. 8:2212017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brown AS: Exposure to prenatal infection
and risk of schizophrenia. Front Psychiatry. 2:632011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jenkins TA: Perinatal complications and
schizophrenia: Involvement of the immune system. Front Neurosci.
7:1102013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chandra J: The potential role of the p75
receptor in schizophrenia: Neuroimmunomodulation and making life or
death decisions. Brain Behav Immun Health. 38:1007962024.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hu W, MacDonald ML, Elswick DE and Sweet
RA: The glutamate hypothesis of schizophrenia: Evidence from human
brain tissue studies. Ann N Y Acad Sci. 1338:38–57. 2015.
View Article : Google Scholar :
|
|
57
|
Chen L, Zhu L, Xu J, Xu P, Han Y, Chang L
and Wu Q: Disrupted in schizophrenia 1 regulates ectopic
neurogenesis in the mouse hilus after pilocarpine-induced status
epilepticus. Neuroscience. 494:69–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Winship IR, Dursun SM, Baker GB, Balista
PA, Kandratavicius L, Maia-de-Oliveira JP, Hallak J and Howland JG:
An overview of animal models related to schizophrenia. Can J
Psychiatry. 64:5–17. 2019. View Article : Google Scholar :
|
|
59
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Deserno L, Schlagenhauf F and Heinz A:
Striatal dopamine, reward, and decision making in schizophrenia.
Dialogues Clin Neurosci. 18:77–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Eisenberg DP, Yankowitz L, Ianni AM,
Rubinstein DY, Kohn PD, Hegarty CE, Gregory MD, Apud JA and Berman
KF: Presynaptic dopamine synthesis capacity in schizophrenia and
striatal blood flow change during antipsychotic treatment and
medication-free conditions. Neuropsychopharmacology. 42:2232–2241.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bulumulla C, Walpita D, Iyer N, Eddison M,
Patel R, Alcor D, Ackerman D and Beyene AG: Synaptic
specializations at dopamine release sites orchestrate efficient and
precise neuromodulatory signaling. bioRxiv: 2024.09.16.613338.
2024.
|
|
63
|
Jahangir M, Zhou JS, Lang B and Wang XP:
GABAergic system dysfunction and challenges in schizophrenia
research. Front Cell Dev Biol. 9:6638542021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cohen SM, Tsien RW, Goff DC and Halassa
MM: The impact of NMDA receptor hypofunction on GABAergic neurons
in the pathophysiology of schizophrenia. Schizophr Res. 167:98–107.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Khandaker GM, Cousins L, Deakin J, Lennox
BR, Yolken R and Jones PB: Inflammation and immunity in
schizophrenia: Implications for pathophysiology and treatment.
Lancet Psychiatry. 2:258–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Müller N and Schwarz MJ: Immune system and
Schizophrenia. Curr Immunol Rev. 6:213–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vallée A: Neuroinflammation in
schizophrenia: The key role of the WNT/β-catenin pathway. Int J Mol
Sci. 23:28102022. View Article : Google Scholar
|
|
68
|
Ebrahimi M, Teymouri K, Chen CC, Mohiuddin
AG, Pouget JG, Goncalves VF, Tiwari AK, Zai CC and Kennedy JL:
Association study of the complement component C4 gene and suicide
risk in schizophrenia. Schizophrenia (Heidelb). 10:142024.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mokhtari R and Lachman HM: The major
histocompatibility complex (MHC) in schizophrenia: A review. J Clin
Cell Immunol. 7:4792016. View Article : Google Scholar
|
|
70
|
Jeppesen R, Orlovska-Waast S, Sørensen NV,
Christensen RHB and Benros ME: Cerebrospinal fluid and blood
biomarkers of neuroinflammation and blood-brain barrier in
psychotic disorders and individually matched healthy controls.
Schizophr Bull. 48:1206–1216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Orlovska-Waast S, Köhler-Forsberg O, Brix
SW, Nordentoft M, Kondziella D, Krogh J and Benros ME:
Cerebrospinal fluid markers of inflammation and infections in
schizophrenia and affective disorders: A systematic review and
meta-analysis. Mol Psychiatry. 24:869–887. 2019. View Article : Google Scholar :
|
|
72
|
Chaves C, Dursun SM, Tusconi M and Hallak
JEC: Neuroinflammation and schizophrenia-is there a link? Front
Psychiatry. 15:13569752024. View Article : Google Scholar
|
|
73
|
Garver DL, Tamas RL and Holcomb JA:
Elevated interleukin-6 in the cerebrospinal fluid of a previously
delineated schizophrenia subtype. Neuropsychopharmacology.
28:1515–1520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhilyaeva TV, Rukavishnikov GV, Manakova
EA and Mazo GE: Serum interleukin-6 in schizophrenia: Associations
with clinical and sociodemographic characteristics. Consort
Psychiatr. 4:5–16. 2023.
|
|
75
|
Fond G, Lançon C, Korchia T, Auquier P and
Boyer L: The role of inflammation in the treatment of
schizophrenia. Front Psychiatry. 11:1602020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Miller BJ, Buckley P, Seabolt W, Mellor A
and Kirkpatrick B: Meta-analysis of cytokine alterations in
schizophrenia: Clinical status and antipsychotic effects. Biol
Psychiatry. 70:663–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Y, Li YJ and Zhu ZQ: To re-examine the
intersection of microglial activation and neuroinflammation in
neurodegenerative diseases from the perspective of pyroptosis.
Front Aging Neurosci. 15:12842142023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Salim S: Oxidative stress and the central
nervous system. J Pharmacol Exp Ther. 360:201–205. 2017. View Article : Google Scholar :
|
|
79
|
Choudhury Z and Lennox B: Maternal immune
activation and schizophrenia-evidence for an immune priming
disorder. Front Psychiatry. 12:5857422021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Siafis S, Wu H, Wang D, Burschinski A,
Nomura N, Takeuchi H, Schneider-Thoma J, Davis JM and Leucht S:
Antipsychotic dose, dopamine D2 receptor occupancy and
extrapyramidal side-effects: A systematic review and dose-response
meta-analysis. Mol Psychiatry. 28:3267–3277. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kapur S, Zipursky R, Jones C, Remington G
and Houle S: Relationship between dopamine D(2) occupancy, clinical
response, and side effects: A double-blind PET study of
first-episode schizophrenia. Am J Psychiatry. 157:514–520. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Grinchii D and Dremencov E: Mechanism of
action of atypical antipsychotic drugs in mood disorders. Int J Mol
Sci. 21:95322020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Richtand NM, Welge JA, Logue AD, Keck PE
Jr, Strakowski SM and McNamara RK: Dopamine and serotonin receptor
binding and antipsychotic efficacy. Neuropsychopharmacology.
32:1715–1726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mørup MF, Kymes SM and Oudin Åström D:
PLoS One. 15:e02341212020. View Article : Google Scholar
|
|
85
|
Potkin SG, Kane JM, Correll CU,
Lindenmayer JP, Agid O, Marder SR, Olfson M and Howes OD: The
neurobiology of treatment-resistant schizophrenia: Paths to
antipsychotic resistance and a roadmap for future research. NPJ
Schizophr. 6:12020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Müller N, Strassnig M, Schwarz MJ,
Ulmschneider M and Riedel M: COX-2 inhibitors as adjunctive therapy
in schizophrenia. Expert Opin Investig Drugs. 13:1033–1044. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Deakin B, Suckling J, Barnes TRE, Byrne K,
Chaudhry IB, Dazzan P, Drake RJ, Giordano A, Husain N, Jones PB, et
al: The benefit of minocycline on negative symptoms of
schizophrenia in patients with recent-onset psychosis (BeneMin): A
randomised, double-blind, placebo-controlled trial. Lancet
Psychiatry. 5:885–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tenório MCDS, Graciliano NG, Moura FA,
Oliveira ACM and Goulart MOF: N-acetylcysteine (NAC): Impacts on
human health. Antioxidants (Basel). 10:9672021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ghaderi A, Bussu A, Tsang C and Jafarnejad
S: Effect of N-acetylcysteine on positive and negative syndrome
scale associated with schizophrenia: A meta-analysis. Rev Clin Med.
7:134–144. 2020.
|
|
90
|
Titulaer J, Radhe O, Danielsson K, Dutheil
S, Marcus MM, Jardemark K, Svensson TH, Snyder GL, Ericson M, Davis
RE and Konradsson-Geuken Å: Lumateperone-mediated effects on
prefrontal glutamatergic receptor-mediated neurotransmission: A
dopamine D1 receptor dependent mechanism. Eur
Neuropsychopharmacol. 62:22–35. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Edinoff A, Wu N, deBoisblanc C, Feltner
CO, Norder M, Tzoneva V, Kaye AM, Cornett EM, Kaye AD, Viswanath O
and Urits I: Lumateperone for the treatment of schizophrenia.
Psychopharmacol Bull. 50:32–59. 2020.PubMed/NCBI
|
|
92
|
Correll CU, Davis RE, Weingart M, Saillard
J, O'Gorman C, Kane JM, Lieberman JA, Tamminga CA, Mates S and
Vanover KE: Efficacy and safety of lumateperone for treatment of
schizophrenia: A randomized clinical trial. JAMA Psychiatry.
77:3492020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Krasavin M, Peshkov AA, Lukin A, Komarova
K, Vinogradova L, Smirnova D, Kanov EV, Kuvarzin SR, Murtazina RZ,
Efimova EV, et al: Discovery and in vivo efficacy of trace
amine-associated receptor 1 (TAAR1) agonist
4-(2-Aminoethyl)-N-(3,5-dimethylphenyl)piperidine-1-carboxamide
hydrochloride (AP163) for the treatment of psychotic disorders. Int
J Mol Sci. 23:115792022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Achtyes ED, Hopkins SC, Dedic N, Dworak H,
Zeni C and Koblan K: Ulotaront: Review of preliminary evidence for
the efficacy and safety of a TAAR1 agonist in schizophrenia. Eur
Arch Psychiatry Clin Neurosci. 273:1543–1556. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Alajangi HK, Kaur M, Sharma A, Rana S,
Thakur S, Chatterjee M, Singla N, Jaiswal PK, Singh G and Barnwal
RP: Blood-brain barrier: Emerging trends on transport models and
new-age strategies for therapeutics intervention against
neurological disorders. Mol Brain. 15:492022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Greene C, Hanley N and Campbell M:
Claudin-5: Gatekeeper of neurological function. Fluids Barriers
CNS. 16:32019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zapata-Acevedo JF, García-Pérez V,
Cabezas-Pérez R, Losada-Barragán M, Vargas-Sánchez K and
González-Reyes RE: Laminin as a biomarker of blood-brain barrier
disruption under neuroinflammation: A systematic review. Int J Mol
Sci. 23:67882022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Baeten KM and Akassoglou K: Extracellular
matrix and matrix receptors in blood-brain barrier formation and
stroke. Dev Neurobiol. 71:1018–1039. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Serlin Y, Shelef I, Knyazer B and Friedman
A: Anatomy and physiology of the blood-brain barrier. Semin Cell
Dev Biol. 38:2–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kadry H, Noorani B and Cucullo L: A
blood-brain barrier overview on structure, function, impairment,
and biomarkers of integrity. Fluids Barriers CNS. 17:692020.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Löscher W and Potschka H: Blood-brain
barrier active efflux transporters: ATP-binding cassette gene
family. NeuroRx. 2:86–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang F, Zhang J, Wang X, Han M, Fei Y and
Wang J: Blood-brain barrier disruption in schizophrenia: Insights,
mechanisms, and future directions. Int J Mol Sci. 26:8732025.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Oviedo-Salcedo T, Wagner E, Campana M,
Gagsteiger A, Strube W, Eichhorn P, Louiset ML, Luykx J, de Witte
LD, Kahn RS, et al: Cerebrospinal fluid abnormalities in first- and
multi-episode schizophrenia-spectrum disorders: Impact of clinical
and demographical variables. Transl Psychiatry. 11:6212021.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Koh SXT and Lee JKW: S100B as a marker for
brain damage and blood-brain barrier disruption following exercise.
Sports Med. 44:369–385. 2014. View Article : Google Scholar
|
|
105
|
Rempe RG, Hartz AMS and Bauer B: Matrix
metalloproteinases in the brain and blood-brain barrier: Versatile
breakers and makers. J Cereb Blood Flow Metab. 36:1481–1507. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Turner RJ and Sharp FR: Implications of
MMP9 for blood brain barrier disruption and hemorrhagic
transformation following ischemic stroke. Front Cell Neurosci.
10:562016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Omidinia E, Mashayekhi Mazar F, Shahamati
P, Kianmehr A and Shahbaz Mohammadi H: Polymorphism of the CLDN5
gene and schizophrenia in an iranian population. Iran J Public
Health. 43:79–83. 2014.PubMed/NCBI
|
|
108
|
Usta A, Kılıç F, Demirdaş A, Işık Ü, Doğuç
DK and Bozkurt M: Serum zonulin and claudin-5 levels in patients
with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 271:767–773.
2021. View Article : Google Scholar
|
|
109
|
Zengil S and Laloğlu E: Evaluation of
serum zonulin and occludin levels in bipolar disorder. Psychiatry
Investig. 20:382–389. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Fasano A: All disease begins in the
(leaky) gut: Role of zonulin-mediated gut permeability in the
pathogenesis of some chronic inflammatory diseases. F1000Res.
9:F1000 Faculty Rev-69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Puvogel S, Alsema A, Kracht L, Webster MJ,
Weickert CS, Sommer IEC and Eggen BJL: Single-nucleus RNA
sequencing of midbrain blood-brain barrier cells in schizophrenia
reveals subtle transcriptional changes with overall preservation of
cellular proportions and phenotypes. Mol Psychiatry. 27:4731–4740.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kornhuber J, Wiltfang J, Riederer P and
Bleich S: Neuroleptic drugs in the human brain: Clinical impact of
persistence and region-specific distribution. Eur Arch Psychiatry
Clin Neurosci. 256:274–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kalaria RN, Akinyemi R and Ihara M: Stroke
injury, cognitive impairment and vascular dementia. Biochim Biophys
Acta. 1862:915–925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kanbay M, Ozbek L, Guldan M, Abdel-Rahman
SM, Sisman U, Mallamaci F and Zoccali C: Nutrition, cognition and
chronic kidney disease: A comprehensive review of interactions and
interventions. Eur J Clin Invest. 55:e700452025. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Case M, Stauffer VL, Ascher-Svanum H,
Conley R, Kapur S, Kane JM, Kollack-Walker S, Jacob J and Kinon BJ:
The heterogeneity of antipsychotic response in the treatment of
schizophrenia. Psychol Med. 41:1291–1300. 2011. View Article : Google Scholar :
|
|
116
|
Kirch DG, Alexander RC, Suddath RL,
Papadopoulos NM, Kaufmann CA, Daniel DG and Wyatt RJ: Blood-CSF
barrier permeability and central nervous system immunoglobulin G in
schizophrenia. J Neural Transm Gen Sect. 89:219–2132. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Rowsthorn E, Pham W, Nazem-Zadeh MR, Law
M, Pase MP and Harding IH: Imaging the neurovascular unit in health
and neurodegeneration: A scoping review of interdependencies
between MRI measures. Fluids Barriers CNS. 20:972023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cheng Y, Wang T, Zhang T, Yi S, Zhao S, Li
N, Yang Y, Zhang F, Xu L, Shan B, et al: Increased blood-brain
barrier permeability of the thalamus correlated with symptom
severity and brain volume alterations in patients with
schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging.
7:1025–1034. 2022.PubMed/NCBI
|
|
119
|
Chung KJ, Abdelhafez YG, Spencer BA, Jones
T, Tran Q, Nardo L, Chen MS Jr, Sarkar S, Medici V, Lyo V, et al:
Quantitative PET imaging and modeling of molecular blood-brain
barrier permeability. medRxiv [Preprint]: 2024.07.26.24311027.
2024.
|
|
120
|
Crockett AM, Ryan SK, Vásquez AH, Canning
C, Kanyuch N, Kebir H, Ceja G, Gesualdi J, Zackai E,
McDonald-McGinn D, et al: Disruption of the blood-brain barrier in
22q11.2 deletion syndrome. Brain. 144:1351–1360. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hashimoto Y, Greene C, Munnich A and
Campbell M: The CLDN5 gene at the blood-brain barrier in health and
disease. Fluids Barriers CNS. 20:222023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Casas BS, Arancibia-Altamirano D,
Acevedo-La Rosa F, Garrido-Jara D, Maksaev V, Pérez-Monje D and
Palma V: It takes two to tango: Widening our understanding of the
onset of schizophrenia from a neuro-angiogenic perspective. Front
Cell Dev Biol. 10:9467062022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Laksitorini MD, Yathindranath V, Xiong W,
Hombach-Klonisch S and Miller DW: Modulation of Wnt/β-catenin
signaling promotes blood-brain barrier phenotype in cultured brain
endothelial cells. Sci Rep. 9:197182019. View Article : Google Scholar
|
|
124
|
Liu X, Low SK, Atkins JR, Wu JQ, Reay WR,
Cairns HM, Green MJ, Schall U, Jablensky A, Mowry B, et al: Wnt
receptor gene FZD1 was associated with schizophrenia in genome-wide
SNP analysis of the Australian schizophrenia research bank cohort.
Aust N Z J Psychiatry. 54:902–908. 2020. View Article : Google Scholar
|
|
125
|
Fusaro A, Zecchin B, Giussani E, Palumbo
E, Agüero-García M, Bachofen C, Bálint Á, Banihashem F, Banyard AC,
Beerens N, et al: High pathogenic avian influenza A(H5) viruses of
clade 2.3.4.4b in Europe-why trends of virus evolution are more
difficult to predict. Virus Evol. 10:veae0272024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yu X, Ji C and Shao A: Neurovascular unit
dysfunction and neurodegenerative disorders. Front Neurosci.
14:3342020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhao FY, Fu QQ, Spencer SJ, Kennedy GA,
Conduit R, Zhang WJ and Zheng Z: Acupuncture: A promising approach
for comorbid depression and insomnia in perimenopause. Nat Sci
Sleep. 13:1823–1863. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen J, Xue K, Yang M, Wang K, Xu Y, Wen
B, Cheng J, Han S and Wei Y: Altered coupling of cerebral blood
flow and functional connectivity strength in first-episode
schizophrenia patients with auditory verbal hallucinations. Front
Neurosci. 16:8210782022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Sukumar N, Sabesan P, Anazodo U and
Palaniyappan L: Neurovascular uncoupling in schizophrenia: A
bimodal meta-analysis of brain perfusion and glucose metabolism.
Front Psychiatry. 11:7542020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Iadecola C: The neurovascular unit coming
of age: A journey through neurovascular coupling in health and
disease. Neuron. 96:17–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Matrisciano F: Functional nutrition as
integrated intervention for in- and outpatient with schizophrenia.
Curr Neuropharmacol. 21:2409–2423. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Shebl N: Neuroinflammation and microglial
activation in schizophrenia: An overview. Handbook of
Neurodegenerative Disorders. Mohamed E: Springer Nature Singapore;
Singapore: pp. 1–16. 2023
|
|
133
|
Stanca S, Rossetti M, Bokulic Panichi L
and Bongioanni P: The cellular dysfunction of the brain-blood
barrier from endothelial cells to astrocytes: The pathway towards
neurotransmitter impairment in schizophrenia. Int J Mol Sci.
25:12502024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bitanihirwe BK and Woo TU: Oxidative
stress in schizophrenia: An integrated approach. Neurosci Biobehav
Rev. 35:878–893. 2011. View Article : Google Scholar :
|
|
135
|
Cuenod M, Steullet P, Cabungcal JH, Dwir
D, Khadimallah I, Klauser P, Conus P and Do KQ: Caught in vicious
circles: A perspective on dynamic feed-forward loops driving
oxidative stress in schizophrenia. Mol Psychiatry. 27:1886–1897.
2022. View Article : Google Scholar :
|
|
136
|
Zang X, Chen S, Zhu J, Ma J and Zhai Y:
The emerging role of central and peripheral immune systems in
neurodegenerative diseases. Front Aging Neurosci. 14:8721342022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hammer C, Zerche M, Schneider A, Begemann
M, Nave KA and Ehrenreich H: Apolipoprotein E4 carrier status plus
circulating anti-NMDAR1 autoantibodies: Association with
schizoaffective disorder. Mol Psychiatry. 19:1054–1056. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ladépêche L, Planagumà J, Thakur S, Suárez
I, Hara M, Borbely JS, Sandoval A, Laparra-Cuervo L, Dalmau J and
Lakadamyali M: NMDA receptor autoantibodies in autoimmune
encephalitis cause a subunit-specific nanoscale redistribution of
NMDA receptors. Cell Rep. 23:3759–3768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hawkins RA, Mans AM, Hibbard LS, Davis DW
and Biebuyck JF: Regional transport of some essential nutrients
across the blood-brain barrier in normal and diseased states. Ann N
Y Acad Sci. 529:40–49. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Srivastav S, Cui X, Varela RB, Kesby JP
and Eyles D: Increasing dopamine synthesis in nigrostriatal
circuits increases phasic dopamine release and alters dorsal
striatal connectivity: Implications for schizophrenia.
Schizophrenia (Heidelb). 9:692023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pollak TA, Drndarski S, Stone JM, David
AS, McGuire P and Abbott NJ: The blood-brain barrier in psychosis.
Lancet Psychiatry. 5:79–92. 2018. View Article : Google Scholar
|
|
142
|
Fang K, Wen B, Niu L, Wan B and Zhang W:
Higher brain structural heterogeneity in schizophrenia. Front
Psychiatry. 13:10173992022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Gammon D, Cheng C, Volkovinskaia A, Baker
GB and Dursun SM: Clozapine: Why is it so uniquely effective in the
treatment of a range of neuropsychiatric disorders? Biomolecules.
11:10302021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Brown AS: The environment and
susceptibility to schizophrenia. Prog Neurobiol. 93:23–58. 2011.
View Article : Google Scholar
|
|
145
|
Wang YI, Abaci HE and Shuler ML:
Microfluidic blood-brain barrier model provides in vivo-like
barrier properties for drug permeability screening. Biotechnol
Bioeng. 114:184–194. 2017. View Article : Google Scholar
|
|
146
|
Brown TD, Nowak M, Bayles AV,
Prabhakarpandian B, Karande P, Lahann J, Helgeson ME and Mitragotri
S: A microfluidic model of human brain (μHuB) for assessment of
blood brain barrier. Bioeng Transl Med. 4:e101262019. View Article : Google Scholar
|
|
147
|
Kawakita S, Mandal K, Mou L, Mecwan MM,
Zhu Y, Li S, Sharma S, Hernandez AL, Nguyen HT, Maity S, et al:
Organ-On-A-chip models of the blood-brain barrier: Recent advances
and future prospects. Small. 18:e22014012022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Chen X, Liu C, Muok L, Zeng C and Li Y:
Dynamic 3D On-chip BBB model design, development, and applications
in neurological diseases. Cells. 10:31832021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Stankovic I, Notaras M, Wolujewicz P, Lu
T, Lis R, Ross ME and Colak D: Schizophrenia endothelial cells
exhibit higher permeability and altered angiogenesis patterns in
patient-derived organoids. Transl Psychiatry. 14:532024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dao L, You Z, Lu L, Xu T, Sarkar AK, Zhu
H, Liu M, Calandrelli R, Yoshida G, Lin P, et al: Modeling
blood-brain barrier formation and cerebral cavernous malformations
in human PSC-derived organoids. Cell Stem Cell. 31:818–833.e11.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kiparizoska S and Ikuta T: Disrupted
olfactory integration in schizophrenia: Functional connectivity
study. Int J Neuropsychopharmacol. 20:740–746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Moberg PJ, Agrin R, Gur RE, Gur RC,
Turetsky BI and Doty RL: Olfactory dysfunction in schizophrenia: A
qualitative and quantitative review. Neuropsychopharmacology.
21:325–340. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Cascella NG, Takaki M, Lin S and Sawa A:
Neurodevelopmental involvement in schizophrenia: The olfactory
epithelium as an alternative model for research. J Neurochem.
102:587–594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Tung VSK, Mathews F, Boruk M, Suppa G,
Foronjy R, Pato MT, Pato CN, Knowles JA and Evgrafov OV: Cultured
mesenchymal cells from nasal turbinate as a cellular model of the
neurodevelopmental component of schizophrenia etiology. Int J Mol
Sci. 24:153392023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yang K, Hasegawa Y, Bhattarai JP, Hua J,
Dower M, Etyemez S, Prasad N, Duvall L, Paez A, Smith A, et al:
Inflammation-related pathology in the olfactory epithelium: Its
impact on the olfactory system in psychotic disorders. Mol
Psychiatry. 29:1453–1464. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Deng H, Kahlon RS, Mohite S, Amin PA,
Zunta-Soares G, Colpo GD, Stertz L, Fries GR, Walss-Bass C, Soares
JC and Okusaga OO: Elevated plasma S100B, psychotic symptoms, and
cognition in schizophrenia. Psychiatr Q. 89:53–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Langeh U and Singh S: Targeting S100B
protein as a surrogate biomarker and its role in various
neurological disorders. Curr Neuropharmacol. 19:265–277. 2021.
View Article : Google Scholar :
|
|
158
|
Chen L, Sutharsan R, Lee JL, Cruz E,
Asnicar B, Palliyaguru T, Wasielewska JM, Gaudin A, Song J,
Leinenga G and Götz J: Claudin-5 binder enhances focused
ultrasound-mediated opening in an in vitro blood-brain barrier
model. Theranostics. 12:1952–1970. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yuan S, Liu KJ and Qi Z: Occludin
regulation of blood-brain barrier and potential therapeutic target
in ischemic stroke. Brain Circ. 6:152–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Dancy C, Heintzelman KE and Katt ME: The
glycocalyx: The importance of sugar coating the blood-brain
barrier. Int J Mol Sci. 25:84042024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zorkina Y, Abramova O, Ushakova V,
Morozova A, Zubkov E, Valikhov M, Melnikov P, Majouga A and
Chekhonin V: Nano carrier drug delivery systems for the treatment
of neuropsychiatric disorders: Advantages and limitations.
Molecules. 25:52942020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Hashimoto Y and Campbell M: Tight junction
modulation at the blood-brain barrier: Current and future
perspectives. Biochim Biophys Acta Biomembr. 1862:1832982020.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhang T, Wu J, Yao X, Zhang Y, Wang Y, Han
Y, Wu Y, Xu Z, Lan J, Han S, et al: The aldose reductase inhibitor
epalrestat maintains blood-brain barrier integrity by enhancing
endothelial cell function during cerebral ischemia. Mol Neurobiol.
60:3741–3757. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Shi SM, Suh RJ, Shon DJ, Garcia FJ, Buff
JK, Atkins M, Li L, Lu N, Sun B, Luo J, et al: Glycocalyx
dysregulation impairs blood-brain barrier in ageing and disease.
Nature. 639:985–994. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ding J, Lee SJ, Vlahos L, Yuki K, Rada CC,
van Unen V, Vuppalapaty M, Chen H, Sura A, McCormick AK, et al:
Therapeutic blood-brain barrier modulation and stroke treatment by
a bioengineered FZD4-selective WNT surrogate in mice.
Nat Commun. 14:29472023. View Article : Google Scholar
|
|
166
|
Sakamoto K, Iwata S, Jin Z, Chen L,
Miyaoka T, Yamada M, Katahira K, Yokoyama R, Ono A, Asano S, et al:
Cyclic peptides KS-133 and KS-487 multifunctionalized nanoparticles
enable efficient brain targeting for treating schizophrenia. JACS
Au. 4:2811–2817. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Pacchioni AM, Gabriele A, Donovan JL,
DeVane CL and See RE: P-glycoprotein inhibition potentiates the
behavioural and neurochemical actions of risperidone in rats. Int J
Neuropsychopharmacol. 13:1067–1077. 2010. View Article : Google Scholar
|
|
168
|
Brzozowska NI, de Tonnerre EJ, Li KM, Wang
XS, Boucher AA, Callaghan PD, Kuligowski M, Wong A and Arnold JC:
The differential binding of antipsychotic drugs to the ABC
transporter P-glycoprotein predicts cannabinoid-antipsychotic drug
interactions. Neuropsychopharmacology. 42:2222–2231. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Lin YC, Ellingrod VL, Bishop JR and Miller
DD: The relationship between P-glycoprotein (PGP) polymorphisms and
response to olanzapine treatment in schizophrenia. Ther Drug Monit.
28:668–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Miyama T, Takanaga H, Matsuo H, Yamano K,
Yamamoto K, Iga T, Naito M, Tsuruo T, Ishizuka H, Kawahara Y and
Sawada Y: P-glycoprotein-mediated transport of itraconazole across
the blood-brain barrier. Antimicrob Agents Chemother. 42:1738–1744.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Qosa H, Miller DS, Pasinelli P and Trotti
D: Regulation of ABC efflux transporters at blood-brain barrier in
health and neurological disorders. Brain Res. 1628:298–316. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Al Shuhaimi L, Henman M, McCallion P,
McCarron M and O'Dwyer M: The adverse effects of long-term exposure
to anticholinergics among people with intellectual disabilities: A
scoping review. HRB Open Res. 5:632022. View Article : Google Scholar
|
|
173
|
Peng A, Chai J, Wu H, Bai B, Yang H, He W
and Zhao Y: New therapeutic targets and drugs for schizophrenia
beyond dopamine D2 receptor antagonists. Neuropsychiatr Dis Treat.
20:607–620. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Dhuria SV, Hanson LR and Frey WH II:
Intranasal delivery to the central nervous system: Mechanisms and
experimental considerations. J Pharm Sci. 99:1654–1673. 2010.
View Article : Google Scholar
|
|
175
|
Teleanu DM, Negut I, Grumezescu V,
Grumezescu AM and Teleanu RI: Nanomaterials for drug delivery to
the central nervous system. Nanomaterials (Basel). 9:3712019.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Harper SQ: Progress and challenges in RNA
interference therapy for Huntington disease. Arch Neurol.
66:933–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Loch-Neckel G, Matos AT, Vaz AR and Brites
D: Challenges in the development of drug delivery systems based on
small extracellular vesicles for therapy of brain diseases. Front
Pharmacol. 13:8397902022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Alkhalifa AE, Al-Ghraiybah NF, Odum J,
Shunnarah JG, Austin N and Kaddoumi A: Blood-brain barrier
breakdown in Alzheimer's disease: Mechanisms and targeted
strategies. Int J Mol Sci. 24:162882023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Moons T, de Roo M, Claes S and Dom G:
Relationship between P-glycoprotein and second-generation
antipsychotics. Pharmacogenomics. 12:1193–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Hardy RE, Chung I, Yu Y, Loh SHY, Morone
N, Soleilhavoup C, Travaglio M, Serreli R, Panman L, Cain K, et al:
The antipsychotic medications aripiprazole, brexpiprazole and
cariprazine are off-target respiratory chain complex I inhibitors.
Biol Direct. 18:432023. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Sykes DA, Moore H, Stott L, Holliday N,
Javitch JA, Lane JR and Charlton SJ: Extrapyramidal side effects of
antipsychotics are linked to their association kinetics at dopamine
D2 receptors. Nat Commun. 8:7632017. View Article : Google Scholar
|
|
182
|
Elschot EP, Backes WH, Postma AA, van
Oostenbrugge RJ, Staals J, Rouhl RPW and Jansen JFA: A
comprehensive view on MRI techniques for imaging blood-brain
barrier integrity. Invest Radiol. 56:10–19. 2021. View Article : Google Scholar
|
|
183
|
Tenchov R, Bird R, Curtze AE and Zhou Q:
Lipid nanoparticles-rom liposomes to mRNA vaccine delivery, a
landscape of research diversity and advancement. ACS Nano.
15:16982–17015. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Huntley MA, Bien-Ly N, Daneman R and Watts
RJ: Dissecting gene expression at the blood-brain barrier. Front
Neurosci. 8:3552014. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Futtrup J, Margolinsky R, Benros ME, Moos
T, Routhe LJ, Rungby J and Krogh J: Blood-brain barrier pathology
in patients with severe mental disorders: A systematic review and
meta-analysis of biomarkers in case-control studies. Brain Behav
Immun Health. 6:1001022020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Xinchen Y, Jing T and Jiaoqiong G:
Lipid-based nanoparticles via nose-to-brain delivery: A mini
review. Front Cell Dev Biol. 11:12144502023. View Article : Google Scholar : PubMed/NCBI
|