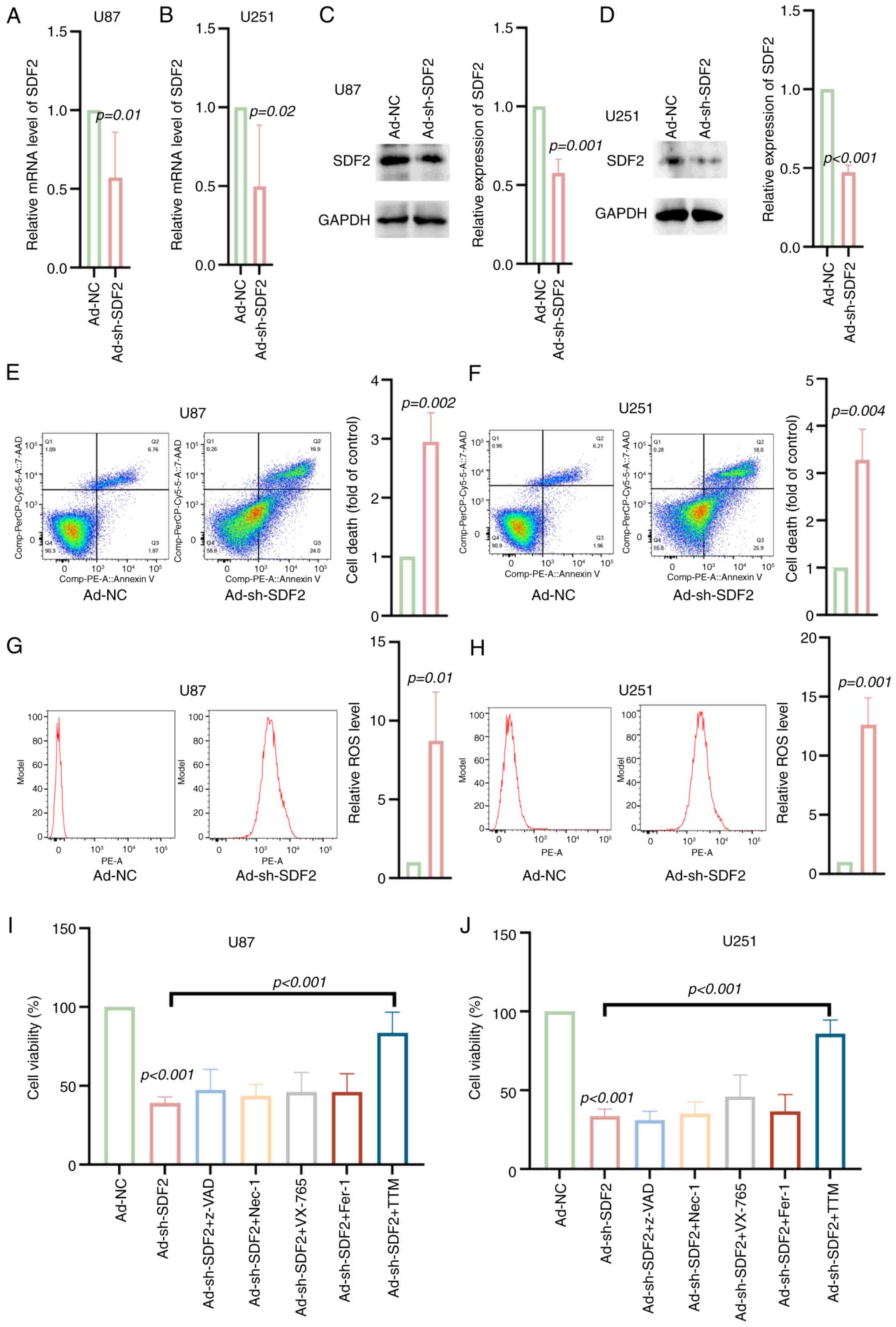
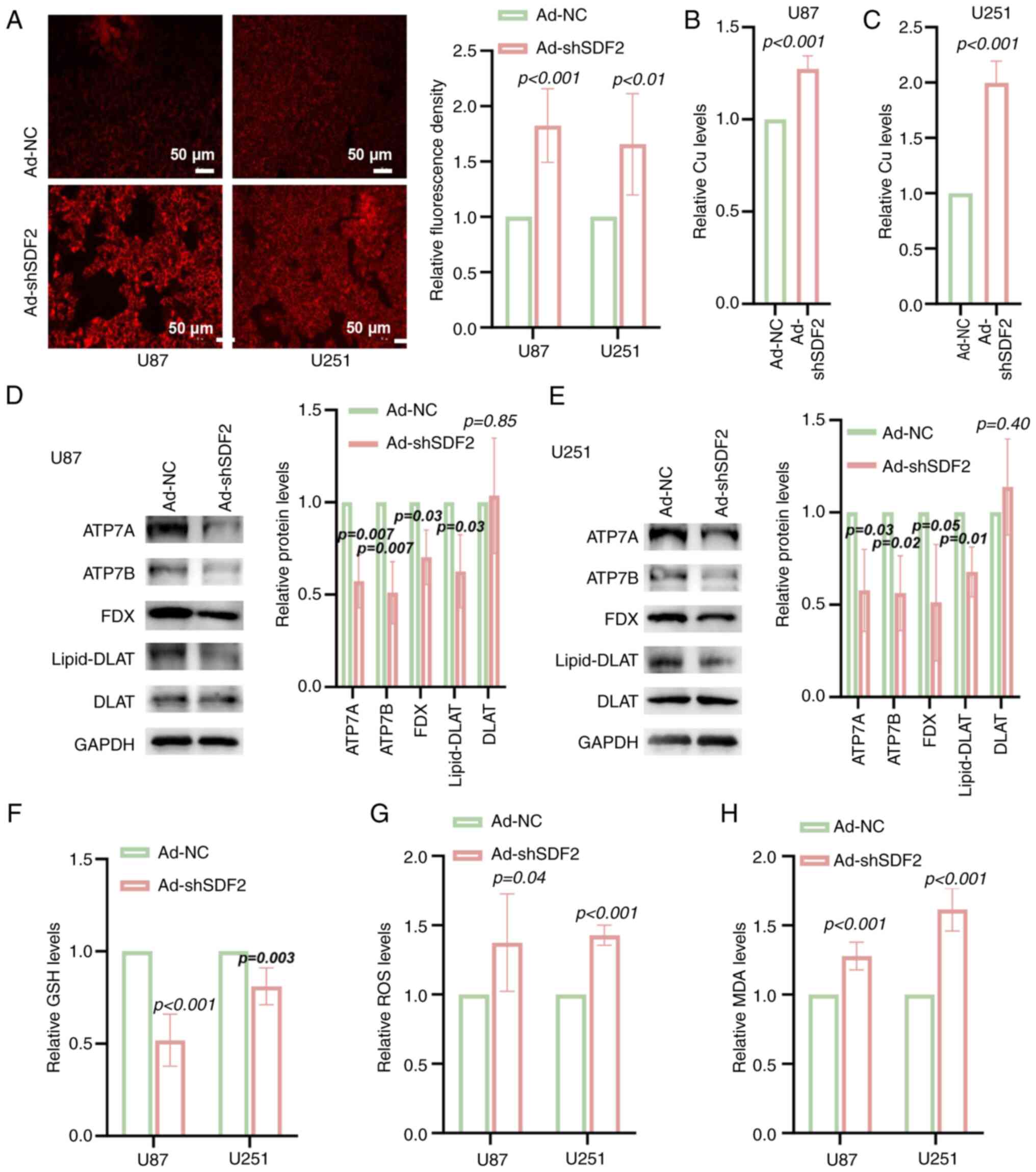
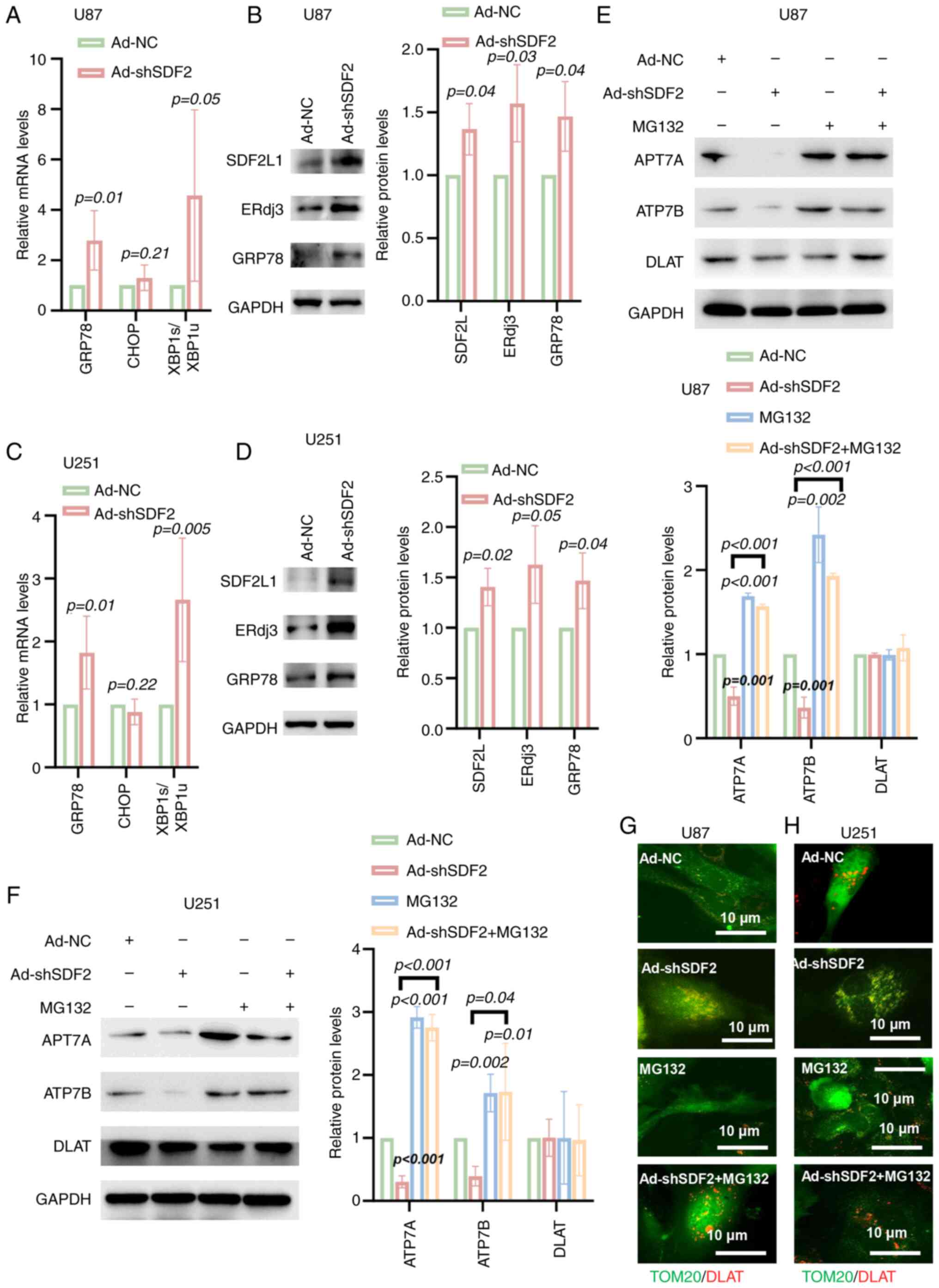
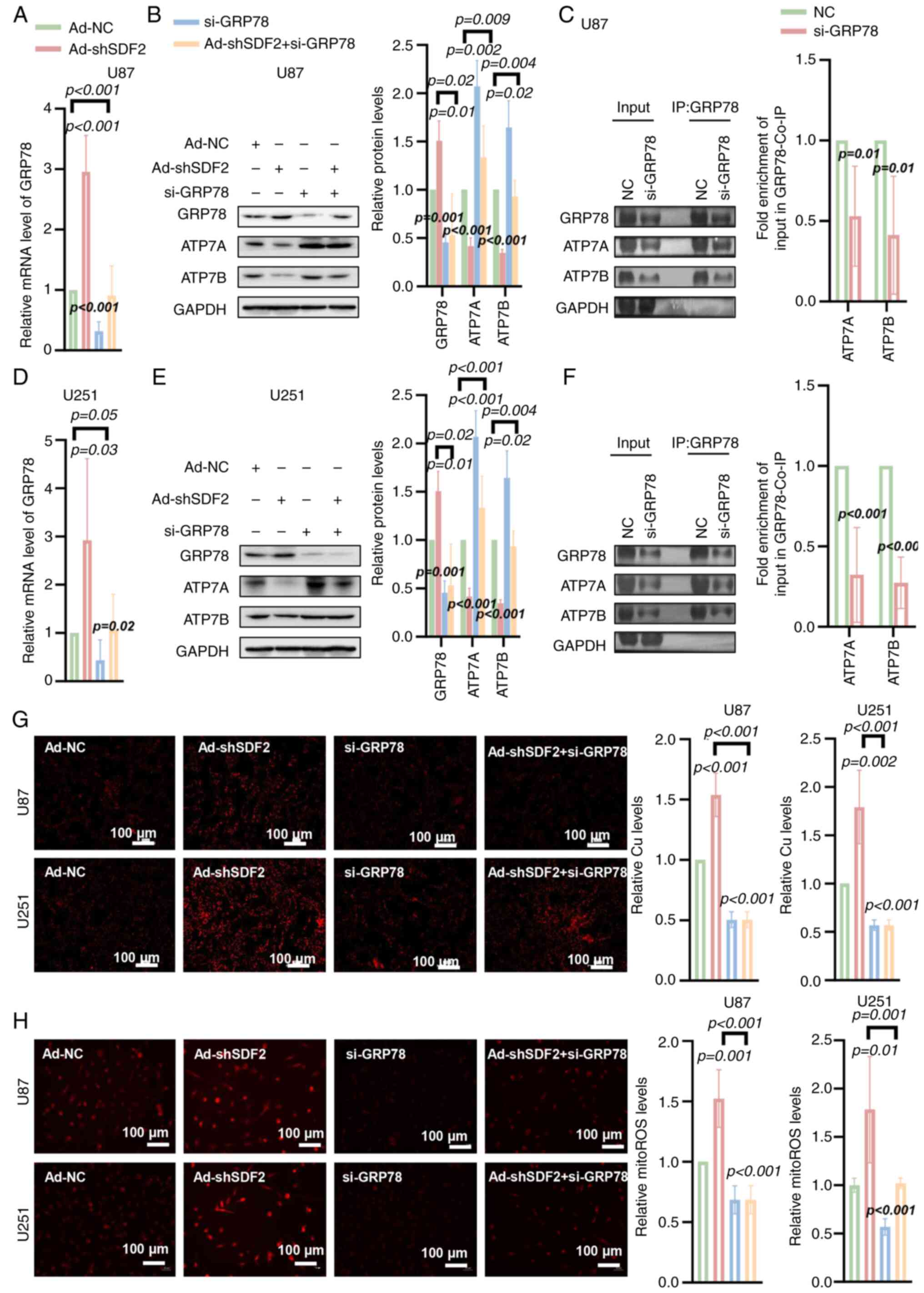
|
1
|
Wang LM, Englander ZK, Miller ML and Bruce
JN: Malignant glioma. Adv Exp Med Biol. 1405:1–30. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weller M, Wen PY, Chang SM, Dirven L, Lim
M, Monje M and Reifenberger G: Glioma. Nat Rev Dis Primers.
10:332024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakar MH and Wood MD: Updates in pediatric
glioma pathology. Surg Pathol Clin. 13:801–816. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei R, Zhou J, Bui B and Liu X: Glioma
actively orchestrate a self-advantageous extracellular matrix to
promote recurrence and progression. BMC Cancer. 24:9742024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krshnan L, van de Weijer ML and Carvalho
P: Endoplasmic reticulum-associated protein degradation. Cold
Spring Harb Perspect Biol. 14:a0412472022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Z, Torres M, Sha H, Halbrook CJ, Van
den Bergh F, Reinert RB, Yamada T, Wang S, Luo Y, Hunter AH, et al:
Endoplasmic reticulum-associated degradation regulates
mitochondrial dynamics in brown adipocytes. Science. 368:54–60.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turk SM, Indovina CJ, Miller JM, Overton
DL, Runnebohm AM, Orchard CJ, Tragesser-Tiña ME, Gosser SK, Doss
EM, Richards KA, et al: Lipid biosynthesis perturbation impairs
endoplasmic reticulum-associated degradation. J Biol Chem.
299:1049392023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stevenson J, Huang EY and Olzmann JA:
Endoplasmic reticulum-associated degradation and lipid homeostasis.
Annu Rev Nutr. 36:511–542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Y and Fang D: Endoplasmic
reticulum-associated degradation and beyond: The multitasking roles
for HRD1 in immune regulation and autoimmunity. J Autoimmun.
109:1024232020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun S, Shi G, Sha H, Ji Y, Han X, Shu X,
Ma H, Inoue T, Gao B, Kim H, et al: IRE1α is an endogenous
substrate of endoplasmic-reticulum-associated degradation. Nat Cell
Biol. 17:1546–1555. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang MJ, Shi M, Yu Y, Ou R, Ge RS and
Duan P: Curcuminoid PBPD induces cuproptosis and endoplasmic
reticulum stress in cervical cancer via the Notch1/RBP-J/NRF2/FDX1
pathway. Mol Carcinog. 63:1449–1466. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu Y, Wang H, Xue W, Zhu L, Fu C, Zhang W,
Mu G, Xia Y, Wei K and Wang J: Endoplasmic reticulum stress related
super-enhancers suppress cuproptosis via glycolysis reprogramming
in lung adenocarcinoma. Cell Death Dis. 16:3162025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lorenzon-Ojea AR, Yung HW, Burton GJ and
Bevilacqua E: The potential contribution of stromal cell-derived
factor 2 (SDF2) in endoplasmic reticulum stress response in severe
preeclampsia and labor-onset. Biochim Biophys Acta Mol Basis Dis.
1866:1653862020. View Article : Google Scholar
|
|
14
|
Hanafusa K, Wada I and Hosokawa N:
SDF2-like protein 1 (SDF2L1) regulates the endoplasmic reticulum
localization and chaperone activity of ERdj3 protein. J Biol Chem.
294:19335–19348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujimori T, Suno R, Iemura SI, Natsume T,
Wada I and Hosokawa N: Endoplasmic reticulum proteins SDF2 and
SDF2L1 act as components of the BiP chaperone cycle to prevent
protein aggregation. Genes Cells. 22:684–698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
DiVita Dean B, Wildes T, Dean J, Yegorov
O, Yang C, Shin D, Francis C, Figg JW, Sebastian M, Font LF, et al:
Immunotherapy reverses glioma-driven dysfunction of immune system
homeostasis. J Immunother Cancer. 11:e0048052023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Xiang Z, Chen L, Deng X, Liu H
and Peng X: PSMA2 promotes glioma proliferation and migration via
EMT. Pathol Res Pract. 256:1552782024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zamler DB, Shingu T, Kahn LM, Huntoon K,
Kassab C, Ott M, Tomczak K, Liu J, Li Y, Lai I, et al: Immune
landscape of a genetically engineered murine model of glioma
compared with human glioma. JCI Insight. 7:e1489902022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong W, Martin TA, Sanders AJ, Jiang A,
Sun P and Jiang WG: Location, function and role of stromal
cell-derived factors and possible implications in cancer (Review).
Int J Mol Med. 47:435–443. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Zheng M, Du S, Wang P, Zhang T,
Zhang X and Zu G: Clinicopathological and prognostic significance
of stromal cell derived factor 2 in the patients with gastric
cancer. BMC Gastroenterol. 24:3252024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slika H, Mansour H, Wehbe N, Nasser SA,
Iratni R, Nasrallah G, Shaito A, Ghaddar T, Kobeissy F and Eid AH:
Therapeutic potential of flavonoids in cancer: ROS-mediated
mechanisms. Biomed Pharmacother. 146:1124422022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jelic MD, Mandic AD, Maricic SM and
Srdjenovic BU: Oxidative stress and its role in cancer. J Cancer
Res Ther. 17:22–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Li S, Guo Y, Zhao C, Chen Y, Ning
W, Yang J and Zhang H: Cuproptosis-related gene SLC31A1 expression
correlates with the prognosis and tumor immune microenvironment in
glioma. Funct Integr Genomics. 23:2792023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang B, Xie L, Liu J, Liu A and He M:
Construction and validation of a cuproptosis-related prognostic
model for glioblastoma. Front Immunol. 14:10829742023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo F and Snapp EL: ERdj3 regulates BiP
occupancy in living cells. J Cell Sci. 126:1429–1439.
2013.PubMed/NCBI
|
|
27
|
Lorenzon-Ojea AR, Guzzo CR, Kapidzic M,
Fisher SJ and Bevilacqua E: Stromal cell-derived factor 2: A novel
protein that interferes in endoplasmic reticulum stress pathway in
human placental cells. Biol Reprod. 95:412016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ibrahim IM, Abdelmalek DH and Elfiky AA:
GRP78: A cell's response to stress. Life Sci. 226:156–163. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cesaratto F, Sasset L, Myers MP, Re A,
Petris G and Burrone OR: BiP/GRP78 mediates ERAD targeting of
proteins produced by membrane-bound ribosomes stalled at the
STOP-Codon. J Mol Biol. 431:123–141. 2019. View Article : Google Scholar
|
|
30
|
Hu F, Huang J, Bing T, Mou W, Li D, Zhang
H, Chen Y, Jin Q, Yu Y and Yang Z: Stimulus-responsive copper
complex nanoparticles induce cuproptosis for augmented cancer
immunotherapy. Adv Sci (Weinh). 11:e23093882024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi RQ, Chen YF, Cheng J, Song JW, Chen YH,
Wang SY, Liu Y, Yan KX, Liu XY, Li J and Zhong JC: Elabela
alleviates cuproptosis and vascular calcification in
vitaminD3-overloaded mice via regulation of the PPAR-γ/FDX1
signaling. Mol Med. 30:2232024. View Article : Google Scholar
|
|
32
|
Xiao Y, Yin J, Liu P, Zhang X, Lin Y and
Guo J: Triptolide-induced cuproptosis is a novel antitumor strategy
for the treatment of cervical cancer. Cell Mol Biol Lett.
29:1132024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai X, Ito S, Noi K, Inoue M, Ushioda R,
Kato Y, Nagata K and Inaba K: Mechanistic characterization of
disulfide bond reduction of an ERAD substrate mediated by
cooperation between ERdj5 and BiP. J Biol Chem. 299:1052742023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akinyemi AO, Simpson KE, Oyelere SF, Nur
M, Ngule CM, Owoyemi BCD, Ayarick VA, Oyelami FF, Obaleye O, Esoe
DP, et al: Unveiling the dark side of glucose-regulated protein 78
(GRP78) in cancers and other human pathology: A systematic review.
Mol Med. 29:1122023. View Article : Google Scholar : PubMed/NCBI
|