Introduction
Ischemic brain injury (IBI) is a leading cause of
disability and death worldwide. In China, it has been categorized
as one of the important causes of disease-related deaths (1). The condition is primarily caused by
the narrowing or blockage of blood vessels, which leads to
insufficient blood and oxygen supply to the brain tissue; this, in
turn, causes necrosis or apoptosis of neurons in the brain tissue,
resulting in irreversible neurological damage. A number of patients
who survive may experience long-term neurological sequelae, such as
cerebral palsy, paraplegia and other lifelong disabilities.
Currently, clinical treatment for IBI entails symptomatic support,
cerebrovascular circulation improvement, neuroprotective medication
or interventional therapy, such as endovascular stenting and
percutaneous transluminal angioplasty. However, the administration
of therapy is impeded by the blood-brain barrier, and recurrence of
the intervention is possible, with incomplete repair of the
infarcted area (2,3). Mesenchymal stem cells (MSCs) are
adult stem cells with the capacity for self-renewal and
multidirectional differentiation, and under certain conditions they
can be differentiated into various neural cells across the
embryonic layer (4,5). Research has shown that MSCs possess
the ability to repair tissue damage when locally transplanted,
intravenously or intra-arterially administered after an injury
(6,7). Furthermore, MSCs have been shown to
possess the capacity to survive, migrate, integrate and
differentiate into neuronal cells within living organisms. The
utilization of MSCs to address neurological disorders through the
replacement of defective or damaged neural tissues represents a
promising therapeutic strategy that circumvents various challenges.
These challenges include the scarcity of adult neural stem cells
(NSCs), the difficulties involved in their isolation and culture,
and the ethical and immunological problems that are associated with
them (8).
Notably, obstacles to the clinical application of
MSCs persist. MSCs necessitate hundreds of millions of cells in
vitro, and although MSCs can be harvested from a range of
organs, the number of isolated cells is inadequate (9) and long-term expansion in
vitro is required to obtain enough stem cells. However, as the
number of passages increase, MSCs display senescence
characteristics, including changes in cell morphology, reduced
proliferation and diminished directed differentiation ability
(10-12). The loss of stemness in MSCs
during in vitro culture, their low survival rates following
transplantation, and compromised self-renewal and differentiation
capacities collectively pose major obstacles to the clinical
translation of MSC-based therapies. Therefore, enhancing the
regulatory mechanisms that sustain MSC proliferation, migration and
differentiation in vivo is critical for optimizing MSC
transplantation efficacy in treating neurological disorders and
promoting the recovery of neurological function.
The neural differentiation of adult stem cells is
regulated by various factors, with epigenetic mechanisms serving a
crucial role in precisely controlling gene expression (13). Polycomb family proteins are
notable epigenetic regulators of adult stem cells, comprising
mainly of two protein complexes: PRC1 and PRC2. B lymphoma Mo-MLV
insertion region 1 homolog (Bmi1), discovered in 1991, is localized
on human chromosome 10p12.2 locus, and consists of nine exons and
nine introns, mainly encoding poly sparse histones (components of
PRC1-like complexes) (14). PRC1
complexes containing Bmi1 proteins can act through chromatin
remodeling and histone modifications to cause epigenetic
alterations (15). Numerous
studies have confirmed that Bmi1 is crucial for neurogenesis in
vivo (16). Notably,
knocking down the Bmi1 gene has been shown to disrupt the stable
development of NSCs, hamper NSC proliferation and leads to an
increase in glial cells (17).
Previous studies (18,19) demonstrated that, through Bmi1
knockdown experiments, Bmi1 serves a crucial role in the
proliferation and survival of cortical bone-derived stem cells.
Previous studies have demonstrated that Bmi1 can
regulate the expansion capacity of mammary epithelial cells and
fibroblasts in vitro and in vivo through the Wnt
signaling pathway (20). The Wnt
signaling pathway is one of the major signaling pathways that
regulate cell proliferation and differentiation, including the
classical Wnt/β-catenin pathway and the non-classical Wnt pathway.
Our prior research has shown that the Wnt/β-catenin signaling
pathway notably impacts the neural differentiation process of MSCs
(21,22). The non-classical Wnt pathway is
less explored and more complex than the classical Wnt/β-catenin
pathway. The planar cell polarity (PCP) signaling pathway is a
specific type of non-classical Wnt pathway that controls cell
motility and cellular differentiation; this pathway triggers
signaling cascades via cytoplasmic cytoskeletal proteins, RhoA,
Rac-activated c-Jun amino-terminal kinases and Rho-associated
kinases (23). Wnt3a is the
primary ligand responsible for Wnt signaling pathways, as research
has indicated that when Wnt3a binds to the cell membrane receptor,
it extracellularly activates both the Wnt/β-catenin signaling
pathway and the nonclassical Wnt signaling pathway (24). RhoA is present in the cell as a
bound form in the signaling pathway and serves as a downstream
element of the PCP pathway. RhoA, along with its regulatory
proteins, has crucial roles in neural differentiation (25). Nonetheless, it remains unclear
whether Bmi1 has the ability to regulate neural differentiation in
MSCs through the Wnt3a-RhoA pathway.
The present study therefore investigated the role of
Bmi1 in regulating the Wnt3a-RhoA signaling pathway and promoting
neural differentiation of MSCs in vitro. In addition, a
middle cerebral artery occlusion (MCAO) model was established in
vivo to evaluate the therapeutic potential of MSC
transplantation in IBI. To assess this, recombinant Wnt3a cytokine
was administered to upregulate the Wnt3a-RhoA pathway, whereas the
small-molecule inhibitor PTC209 was utilized to suppress Bmi1
expression.
Materials and methods
Animals
In the present study, male Sprague-Dawley (SD) rats
(weight, 80-120 g; age, 2-4 weeks) were used for MSC isolation and
culture. One rat was required for each T25 cell culture flask of
primary MSCs collected, and 33 male SD rats were used for MSC
isolation. The MCAO model was established using 45 male SD rats
(weight, 260-300 g; age, 6-8 weeks). All rats were provided by
Zhejiang Chinese Medical University Animal Center (Laboratory
Animal Certificate: SCXK 2018-0006; Hangzhou, China). The rats were
maintained at a temperature of 20±2°C, a humidity of 55±5%, under a
12-h light/dark cycle, and were given free access to food and
water. All animal experiments were approved by the Animal Ethical
and Welfare Committee of Zhejiang Chinese Medical University
(approval no. 20210524-05, review no. IACUC-202108-05). All SD rats
in all experiments were euthanized by intravenous injection of an
overdose of pentobarbital (100 mg/kg), which was confirmed by
observing their vital signs 10 min after administration.
Chemicals
Wnt3a recombinant cytokine (cat. no. 96-315-20-10)
was purchased from PeproTech; Thermo Fisher Scientific, Inc. and
the small molecule inhibitor PTC209 (cat. n. S7372) was purchased
from Selleck Chemicals. Before use, Wnt3a powder was dissolved in
sterile water, and PTC209 powder was dissolved in dimethyl
sulfoxide (DMSO). A quantity of 10 mg PTC209 powder was dissolved
in 1.14 ml DMSO to create a 2 mmol/l master mix.
Isolation and culture of rat MSCs
MSCs were obtained from rat bone marrow by flushing
the contents of the tibia and femur with phosphate-buffered saline
(PBS) in a sterile environment, and were then transferred to a
centrifuge tube to be centrifuged at 10,000 × g for 5 min at room
temperature. After centrifugation, the supernatant was discarded,
and the cells were resuspended in MSC medium prepared with
DMEM/F-12 (cat. no. TBD10565; Tianjin Haoyang Biological Products
Technology Co., Ltd.) containing 10% fetal bovine serum (FBS; cat.
no. 10091-148; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin solution, and were inoculated in T25 cell
culture flasks at a temperature of 37°C in a 5% CO2
incubator. Cell morphology was observed under an inverted
fluorescence microscope (ECLIPSE TE2000-U; Nikon Corporation).
Cell proliferation assay
MSCs were cultured in 96-well plates with varying
concentrations of recombinant cytokine Wnt3a (0, 10, 50 and 100
ng/ml) and a small molecule inhibitor PTC209 (0, 1, 2.5, 5 and 10
μmol/l). Each group was subjected to a 24-h stimulation
period within a 37°C incubator, comprising five replicate wells at
varying concentrations. Subsequently, the cells were exposed to 5
mg/ml MTT for 4 h, the blue-violet formazan crystals were dissolved
in DMSO and the absorbance was measured at 450 nm using an
enzyme-linked instrument. The MTT kit was purchased from Abcam
(cat. no. ab211091).
In vitro induction of neural
differentiation in MSCs
The 3rd-5th generation MSCs in logarithmic growth
phase were collected and inoculated in two 6-well plates at a cell
density of 1×105 cells/ml. The cells were randomly
divided into four groups (n=3 wells each): Normal control (NC)
group, β-mercaptoethanol (BME) group, Wnt3a group and PTC209 group.
The NC group comprised normal cultured cells without induction of
neural differentiation; the BME group was pre-induced with neural
differentiation pre-induction medium for 24 h, followed by
induction with neural differentiation medium for 3-6 h. The Wnt3a
group and the PTC209 group were treated with neural differentiation
medium containing 10 ng/ml Wnt3a and 1 μmol/ml PTC209,
respectively, and the incubation steps were the same as that of the
BME group. The neural differentiation pre-induction medium
consisted of DMEM/F-12 supplemented with 20% FBS, 1 mmol/l BME and
1% penicillin-streptomycin solution. The neural differentiation
induction medium was prepared by adding 5 mmol/l BME and 1%
penicillin-streptomycin solution to DMEM/F-12.
Immunofluorescence staining of MSCs
The culture medium in the cell plates was aspirated
and discarded, and the cells were washed with PBS and fixed with 4%
paraformaldehyde at room temperature for 10 min. The cells were
washed again with PBS and cell membranes were ruptured with a
permeabilizing solution of 0.5% TritonX-100 (cat. no. V900502;
MilliporeSigma) at room temperature for 10 min. After further
washing with PBS, the cells were blocked in PBS containing 2% BSA
(cat. no. 9048-46-8; Shanghai Macklin Biochemical Co., Ltd.) and
0.1% Tween-20 (cat. no. 9005-64-5; Shanghai Macklin Biochemical
Co., Ltd.) for 1 h at room temperature. The appropriate primary
antibody dilutions were then added and incubated overnight at 4°C.
Subsequently, the primary antibody was removed, the cells were
washed three times with PBS (8 min each) and the corresponding
secondary antibody dilution was added and incubated in the dark for
1 h at room temperature. Both primary and secondary antibodies were
prepared at a ratio of 1:200. Finally, DAPI staining solution was
added in the dark, and the cells were observed and images were
captured under an inverted phase contrast fluorescence microscope
to detect neuronal cell markers. The intermediate filament protein
nestin is a common specific marker for NSCs, which is mainly
expressed in the cytoplasm; NeuN is a neuron-specific nuclear
protein; Olig2 is an oligodendrocyte-specific marker; glial
fibrillary acidic protein (GFAP) is primarily found in astrocytes
within the central nervous system and is a commonly used cellular
marker. The primary antibodies anti-GFAP (cat. no. ab7260),
anti-NeuN (cat. no. ab177487), anti-nestin (cat. no. ab254048) and
anti-Olig2 (cat. no. ab254043), and the Goat Anti-Rabbit IgG
H&L (Alexa Fluor® 488) secondary antibody (cat. no.
ab150077) were obtained from Abcam. The antibodies were diluted
with 5% BSA solution according to the instructions.
Modeling of rat MCAO
The rats were deprived of food for 12 h prior to
surgery and administered an intraperitoneal injection of 3% sodium
pentobarbital (45 mg/kg, provided by the Animal Experiment Center
of Zhejiang University of Traditional Chinese Medicine) for
anesthesia. The rat MCAO model was created using the modified Zea
Longa wire bolus method. The rats were immobilized in the supine
position, and an incision was made in the midline of the neck. The
right common carotid artery (CCA), external carotid artery (ECA)
and internal carotid artery (ICA) were sequentially isolated, and
threads (4-0 sutures) were placed distal and proximal to the CCA
and at the ECA. The ICA was temporarily clamped with a
microarterial clip, and then the CCA and ECA were ligated
proximally. Subsequently, a small opening was cut with scissors 4
mm from the bifurcation of the CCA, the prepared suture was
inserted into the ICA, and the suture was tied securely with a thin
wire wrapped around the distal end of the CCA when the marked point
on the suture reached the bifurcation. The wound was covered with a
saline-soaked cotton ball to keep it moist, and after 1.5 h of
embolization, the suture was removed, the vessels were ligated, and
the wound was sutured and disinfected. Rats were scored according
to the Longa 5-point scale (26)
on day 3 after modeling, and modeling was considered successful in
rats with ≥1 point.
CM-Dil labeling of MSCs
The 3rd-5th generation MSCs in logarithmic growth
phase were collected, and after discarding the original medium,
they were rinsed twice with PBS. Staining solution was prepared by
adding the labeling solution to the medium in a ratio of 1 ml MSC
medium: 1 μl CM-Dil labeling solution (cat. no. C7000;
Invitrogen; Thermo Fisher Scientific, Inc.). The cells were
incubated in a CO2 incubator at 37°C for 10-20 min after
the addition of the staining solution. At the end of the
incubation, the cells were rinsed twice with PBS and then incubated
again at 4°C for 15 min to avoid staining of the nuclei. The MSCs
that had been labeled with CM-Dil were cultured for a further 24 h,
and were then observed and images were captured under an inverted
fluorescence microscope, which was used to detect the survival of
MSCs in brain tissues after subsequent cell transplantation.
Brain stereotactic transplantation of
MSCs and animal grouping
On day 3 after modeling, the rats were anesthetized
by inhalation of 4% isoflurane through a mask, fixed into a brain
stereotaxic apparatus, sterilized and a 10 μl suspension of
MSCs (107 cells/ml) was injected into the injection
sites of the hippocampus at a rate of 1 μl/min. The rats
were observed for infection and mortality.
A total of 45 SD rats were randomly assigned to five
groups: Sham, MCAO, MSCs, Wnt3a-MSCs and PTC209-MSCs (n=9
rats/group). The Sham and MCAO groups were transplanted with 10
μl PBS on the postoperative day 3, the MSCs group was
transplanted with MSCs on postoperative day 3, the Wnt3a-MSCs group
was transplanted with 10 ng/ml Wnt3a-treated MSCs on day 3 after
modeling, and the PTC209-MSCs group was transplanted with 1
μmol/l PTC209-treated MSCs on postoperative day 3. MSCs in
each group were pretreated for 48 h.
Neurological function tests
The neurological injury severity in rats was
assessed using the Neurological Severity Score (NSS) on days 1, 3,
5 and 7 after MSC transplantation. The rat tail was lifted 30 cm
off the ground and the forelimbs were observed. Rats with both
forelimbs symmetrically extended to the ground, with the left
shoulder internally rotated and the left forelimb internally
retracted were considered normal and rated 4 points, otherwise they
were rated 0 points. In addition, the rat was placed on a smooth
floor and resistance to movement was examined by pushing the left
(or right) shoulder to the opposite side, respectively. Rats with
resistance that was clearly symmetrical on both sides were
considered normal (0 points); when the resistance was decreased,
this was rated 1-3 points according to the degree of decrease.
Furthermore, the two forelimbs of the rats were placed on a metal
net and the tension of the two forelimbs was observed. When the
tension of the two forelimbs was obviously symmetrical, this was
considered normal (0 points); when the muscle tension of the left
forelimb decreased, this was rated 1-3 points according to the
degree of decrease. Based on the aforementioned scores out of 10,
the higher the score, the more severe the behavioral disorder.
Grip assays were also performed on days 1, 3, 5 and
7 after transplantation of MSCs. In order to measure grip force,
the grip force measuring instrument was placed on a horizontal
table and levelled so that the grip force plate was in a horizontal
direction. The rat was then gently placed on the grip plate and the
tail was pulled back horizontally to record the maximum grip force
of the rat.
Triphenyltetrazolium chloride (TTC)
staining
On day 7 after MSC transplantation, intact brain
tissues were obtained from three randomly selected rats in each
group after euthanasia.; the animals were euthanized as
aforementioned. These tissues were placed in a pre-cooled tank
(4°C), rapidly frozen at −20°C for 20 min, and 2-mm coronal
sections were cut and placed in 2% TTC dye at 37°C away from light
for 15 min. After staining, images were captured, and were
processed using the Image-Pro Plus 6.0 image analysis system
software (Media Cybernetics, Inc.) to calculate the infarction rate
of the rat brain tissue.
Hematoxylin and eosin (H&E)
staining
On day 14 after the transplantation of MSCs, three
rats in each group were randomly selected for the removal of intact
brain tissues, which were subjected to H&E staining to observe
the pathological changes in the CA1 area of the hippocampus in each
group. The animals were euthanized as aforementioned. Brain tissues
were fixed with 4% paraformaldehyde at 4°C for 24 h, embedded in
paraffin and were cut into 5-10 μm slices on a sectioning
machine at 4°C. Tissue sections were deparaffinized with xylene two
times (10 min each), hydrated with a descending series of ethanol
concentrations, washed with water and distilled water, and stained
with hematoxylin for 5 min. Subsequently, the sections were washed
with water, incubated with 1% hydrochloric acid and 70% ethanol for
3 sec, washed with water for 10 min and stained with 1% eosin for 1
min. Finally, the sections were washed with water, dehydrated in an
ascending series of ethanol concentrations, permeabilized twice
with xylene (1 min each) and sealed with neutral gum. Finally, the
sections were observed and images were captured under a light
microscope.
Immunofluorescence staining
On day 14 after transplantation of MSCs, three brain
tissue sections were randomly collected from each group. Rat brain
tissues were fixed in 4% paraformaldehyde for 8 h at room
temperature., dehydrated in 30% sucrose solution, and embedded in
OCT embedding agent, before being frozen at −80°C. Subsequently,
the tissues were sectioned into 5-10 μm slices.
Subsequently, 3% H2O2 was added dropwise to
the sections and incubated at 37°C for 10 min to block endogenous
peroxidase, after which, the sections were rinsed with distilled
water three times (5 min each). The sections were then placed in
boiling sodium citrate buffer solution and reacted at 92-98°C for
20 min for antigen retrieval. After cooling, the slices were washed
three times with PBS solution (2 min each). Immunofluorescence was
then performed as aforementioned for MSCs, using the same
antibodies.
Western blotting
The MSCs of each group, as well as the ischemic
penumbra tissues and hippocampal tissues on the damaged side of the
rat brain of each group, were lysed with protein lysis solution
(cat. no. 89900; Thermo Fisher Scientific, Inc.) on ice. The
supernatant was collected after centrifugation at 10,000 × g for 15
min at 4°C after lysis. Following the extraction of total proteins
from the cells and tissues, the protein concentration was detected
according to the instructions in the BCA kit (cat. no.AR0146;
Boster Biological Technology). Depending on the molecular weight of
the protein, a 10 or 12% separation gel was prepared, and 20
μg proteins underwent SDS-PAGE. Proteins were then
transferred to polyvinylidene fluoride membranes, which were
blocked with a ready-to-use 5% skimmed milk powder sealer. The
membranes were then incubated at 4°C overnight with the following
primary antibodies: Anti-Bmi1 (cat. no. ab38295), anti-RhoA (cat.
no. ab187027), anti-Wnt3a (cat. no. ab219412) (all from Abcam) and
anti-β-actin (cat. no. BK7018; Bioker), anti-GFAP (cat. no.
ab7260), anti-NeuN (cat. no. ab177487) and anti-nestin (cat. no.
ab254048) (all from Abcam). Primary antibodies were diluted and
prepared in 5% BSA solution at a ratio of 1:1,000 according to the
antibody instructions. Subsequently, the membranes were incubated
for 2 h at room temperature with a Goat Anti-Rabbit IgG H&L
(Alexa Fluor® 488) secondary antibody (cat. no.
ab150077; Abcam), which was diluted and prepared according to the
antibody instructions. Images of membranes were captured using an
ultrasensitive chemiluminescent substrate kit (cat. no. AR1111;
Boster Biological Technology) utilizing a chemiluminescent imaging
system (cat. no. 6000; Clinx Science Instruments Co., Ltd.) was
used to capture images of the membranes, which were subsequently
analyzed via ImageJ v1.8.0 analysis software (National Institutes
of Health) for semi-quantification.
Statistical analysis
All experiments were repeated more than three times.
The experimental data were statistically analyzed using SPSS 25.0
(IBM Corp.). Experimental data are presented as the mean ± standard
deviation and bar graphs were constructed using GraphPad Prism 9
(Dotmatics). A χ2 test for variance was performed on the
experimental data to assess distribution of data. One-way analysis
of variance was used to compare the differences between groups and
the data were analyzed by Tukey's post-hoc test for multiple
comparisons between groups. The analysis of NSS scores was
performed using the Kruskal-Wallis test followed by Dunn's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
MSC isolation culture and proliferative
activity
Primary MSCs were cultured in T25 cell culture
flasks and allowed to adhere to the wall and aggregate for 3 days.
By day 7, the cells had formed colonies and were vigorously
proliferating and dividing. Under a microscope, the cells were
found to be either polygonal or irregularly shuttle-shaped
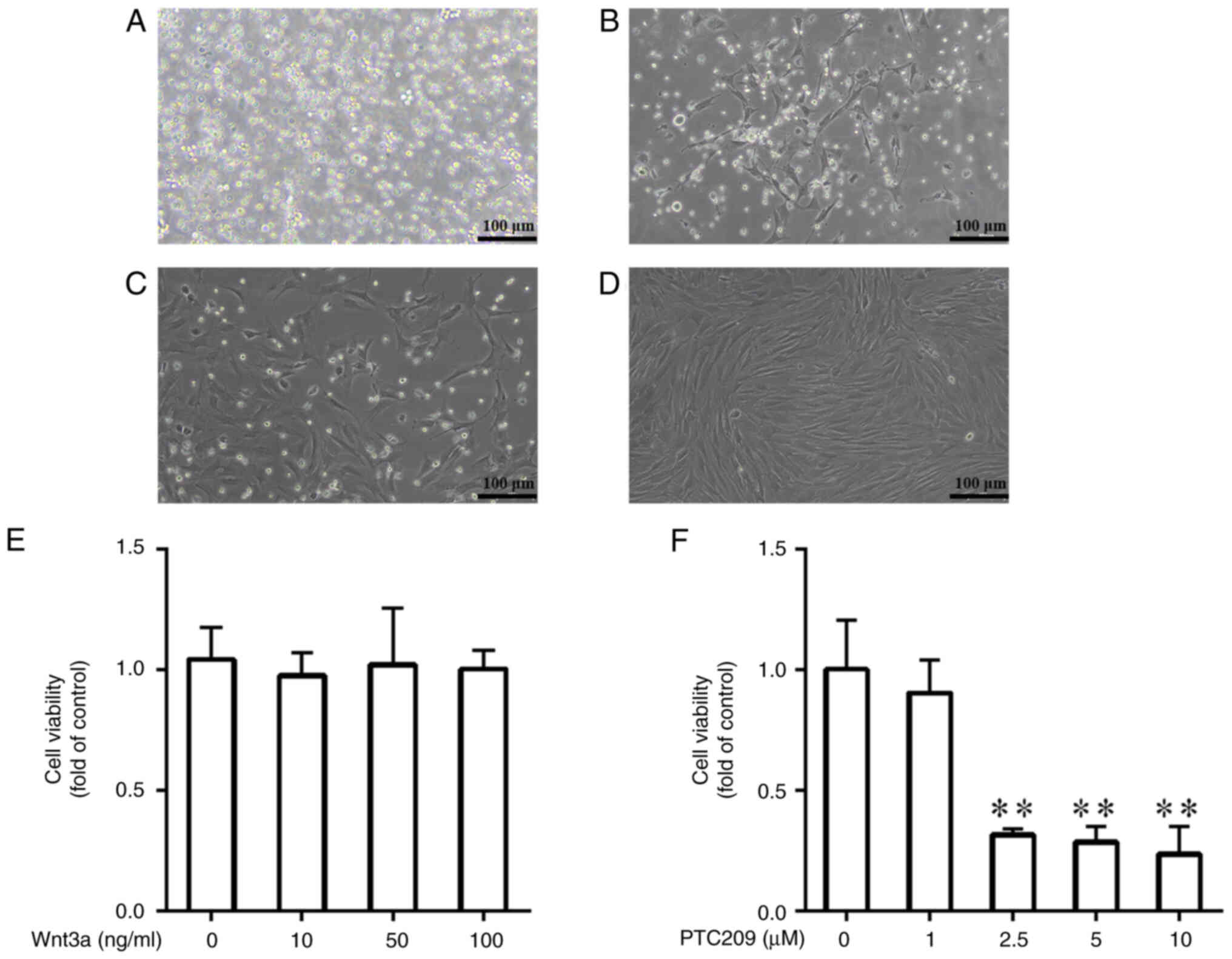
(Fig. 1A-C). When the primary
cultured MSCs reached 80-90% fusion, passaging culture was
performed. Subsequently, MSCs experienced an increase in
proliferative activity, as well as a continuous improvement in
purity. Furthermore, their cell morphology gradually changed from
an irregular polygonal shape to a shuttle or spindle shape
(Fig. 1D). Different
concentrations of Wnt3a (0, 10, 50 and 100 ng/ml) and Bmi1 small
molecule inhibitor PTC209 (0, 1, 2.5, 5 and 10 μmol/l) were
utilized to treat MSCs for 24 h, and the impact of both on cell
viability was measured through MTT analysis. The results indicated
that the viability of MSCs was normal in all groups of
Wnt3a-treated cells, and there was no significant difference in the
proliferative activity of MSCs between the different concentration
groups, thus indicating that Wnt3a treatment did not affect the
activity of MSCs (Fig. 1E). By
contrast, in the cell groups treated with PTC209, that 1
μmol/l PTC209 had no significant effect on cell viability,
whereas 2.5, 5 and 10 μmol/l PTC209 had a toxic effect on
cells (Fig. 1F). Combining the
findings of previous studies (27,28) and the present experimental
results, 10 ng/ml Wnt3a and 1 μmol/l PTC209 were selected
for subsequent experiments in the current study.
Wnt3a promotes and PTC209 inhibits
differentiation of MSCs toward neurons
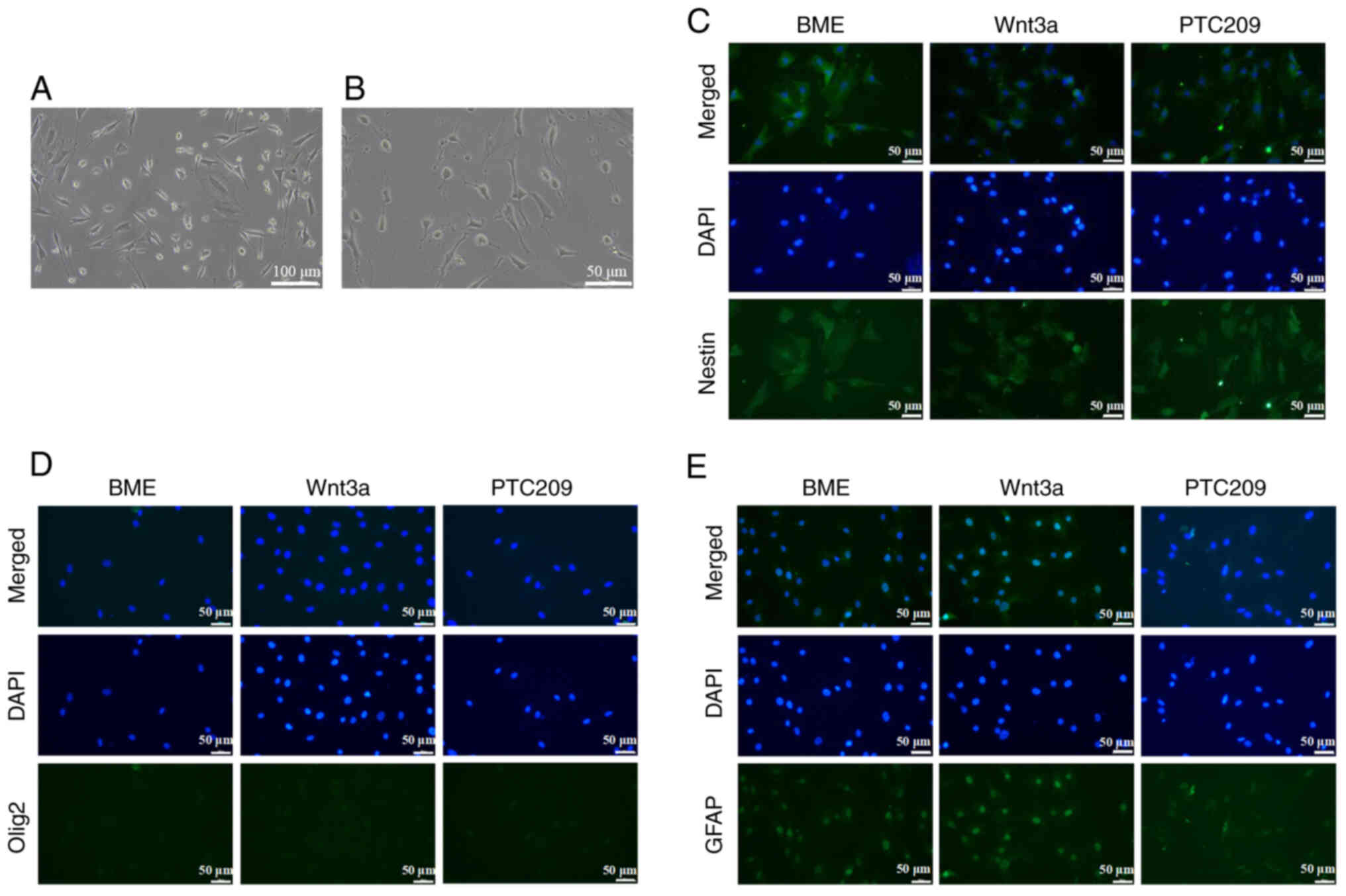
Compared with untreated MSCs, after 3 h of neural
differentiation, the cytoplasm of MSCs contracted, longer synapses
were formed, tiny branches appeared to converge into a meshwork,
the cells grew well and a neuronal cell-like appearance was
presented (Fig. 2A and B). MSCs
induced by neural differentiation for 3 h could therefore be used
for subsequent experiments. The BME, Wnt3a and PTC209 groups
demonstrated specific green fluorescence of Nestin, indicating
Nestin positive expression (Fig.
2C). In addition, the immunofluorescence results indicated the
absence of marked green fluorescence in both the nucleus and
cytoplasm following Olig2 staining; double-color staining of the
nucleus and cytoplasm was not observed under a microscope, thus
establishing that the expression of Olig2 was negative in all cell
groups (Fig. 2D). Furthermore,
after inducing neural differentiation of MSCs in vitro, the
cytoplasm and nucleus emitted green fluorescence following GFAP
staining; GFAP-positive cells could be clearly seen under the
microscope in the BME and Wnt3a groups, whereas GFAP-positive cells
were almost unobservable in the PTC209 group (Fig. 2E).
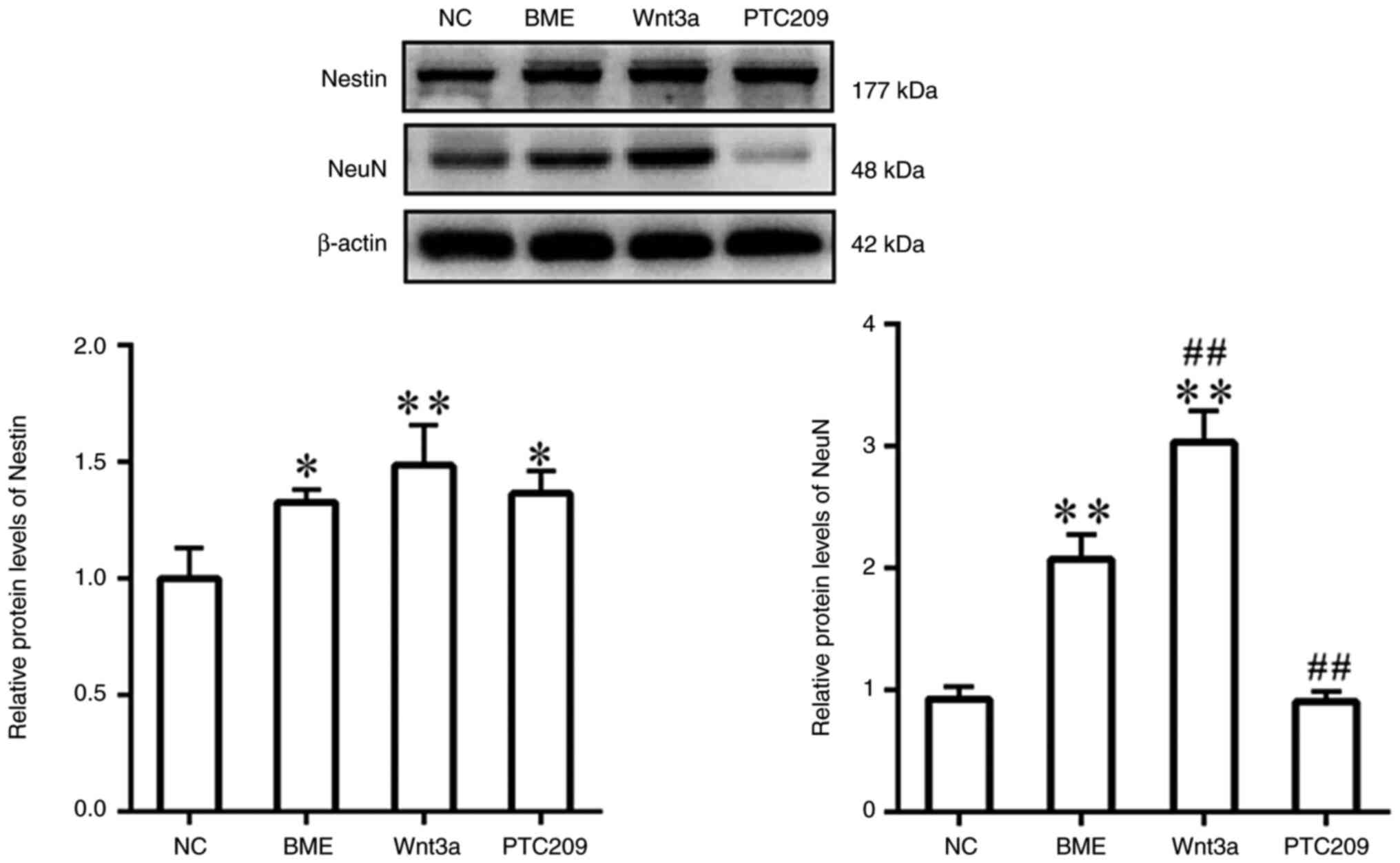
As determined by western blotting, the protein
expression levels of Nestin were enhanced in the BME, Wnt3a and
PTC209 groups compared with those in the NC group; however, no
significant difference emerged between the three groups (Fig. 3). In addition, the protein levels
of NeuN were significantly increased in the BME group compared with
those in the NC group, and were significantly higher in the Wnt3a
group compared with in the BME group. By contrast, NeuN protein
expression levels were significantly lower in the PTC209 group
compared with those in the BME group.
These findings indicated that BME stimulated MSCs to
differentiate into NSCs in vitro, but not towards downstream
oligodendrocyte lineage cells. Furthermore, Wnt3a treatment
enhanced the differentiation of MSCs towards neurons, whereas
PTC209 treatment impeded the differentiation of MSCs into
astrocytes and neurons.
Bmi1 regulates the protein expression of
Wnt3a and RhoA in vitro
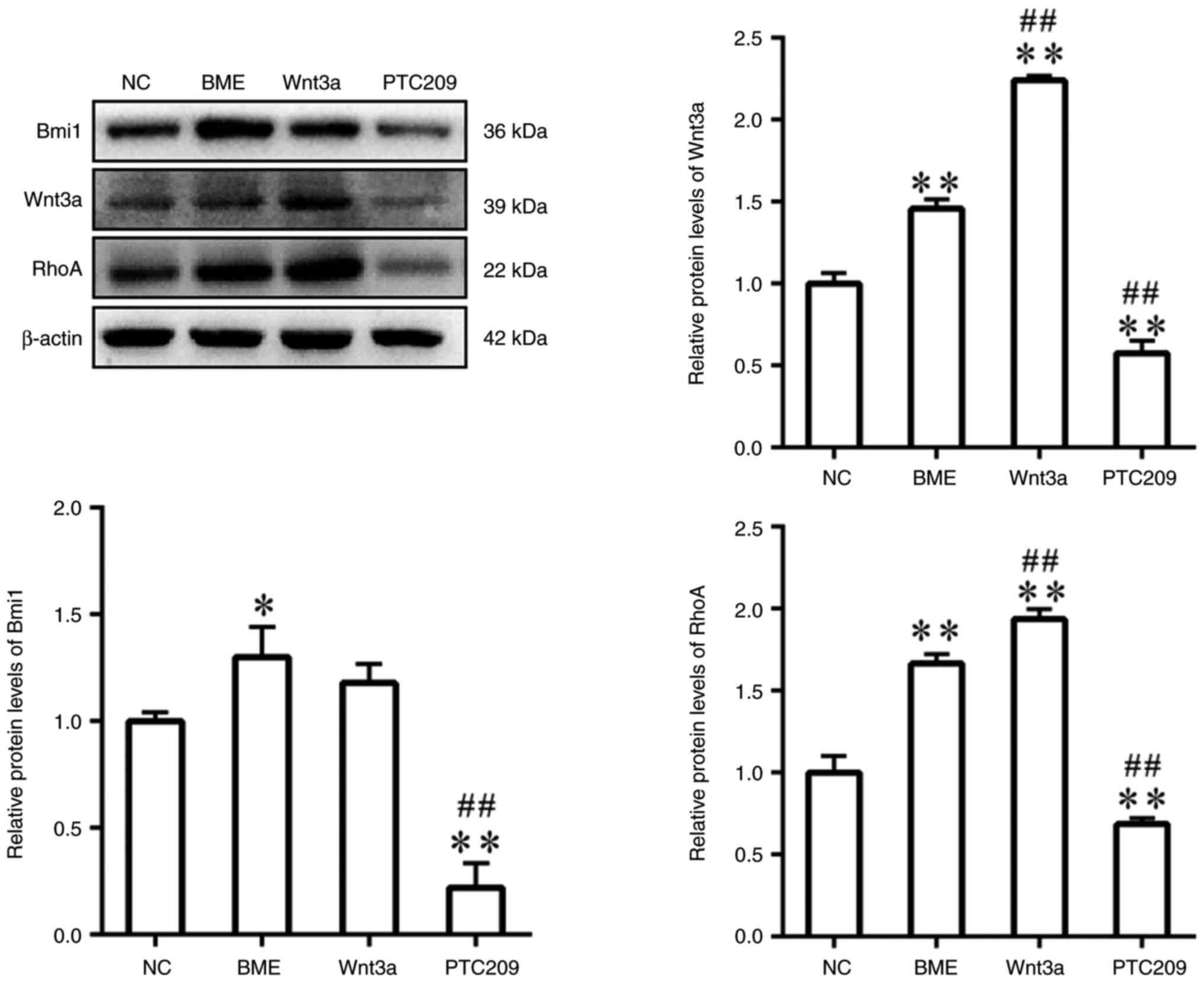
The protein expression levels of Bmi1, Wnt3a and
RhoA were significantly increased in the BME group compared with
those in the NC group (Fig. 4).
In comparison with the BME group, the protein expression levels of
Wnt3a and RhoA were increased in the Wnt3a group; however, there
was no significant difference in Bmi1 protein expression. By
contrast, the protein expression levels of Bmi1, Wnt3a and RhoA
were significantly decreased in the PTC209 group compared with
those in the BME group. These findings indicated that Bmi1 may be
implicated in the neural differentiation process of MSCs by
controlling the expression of Wnt3a and RhoA.
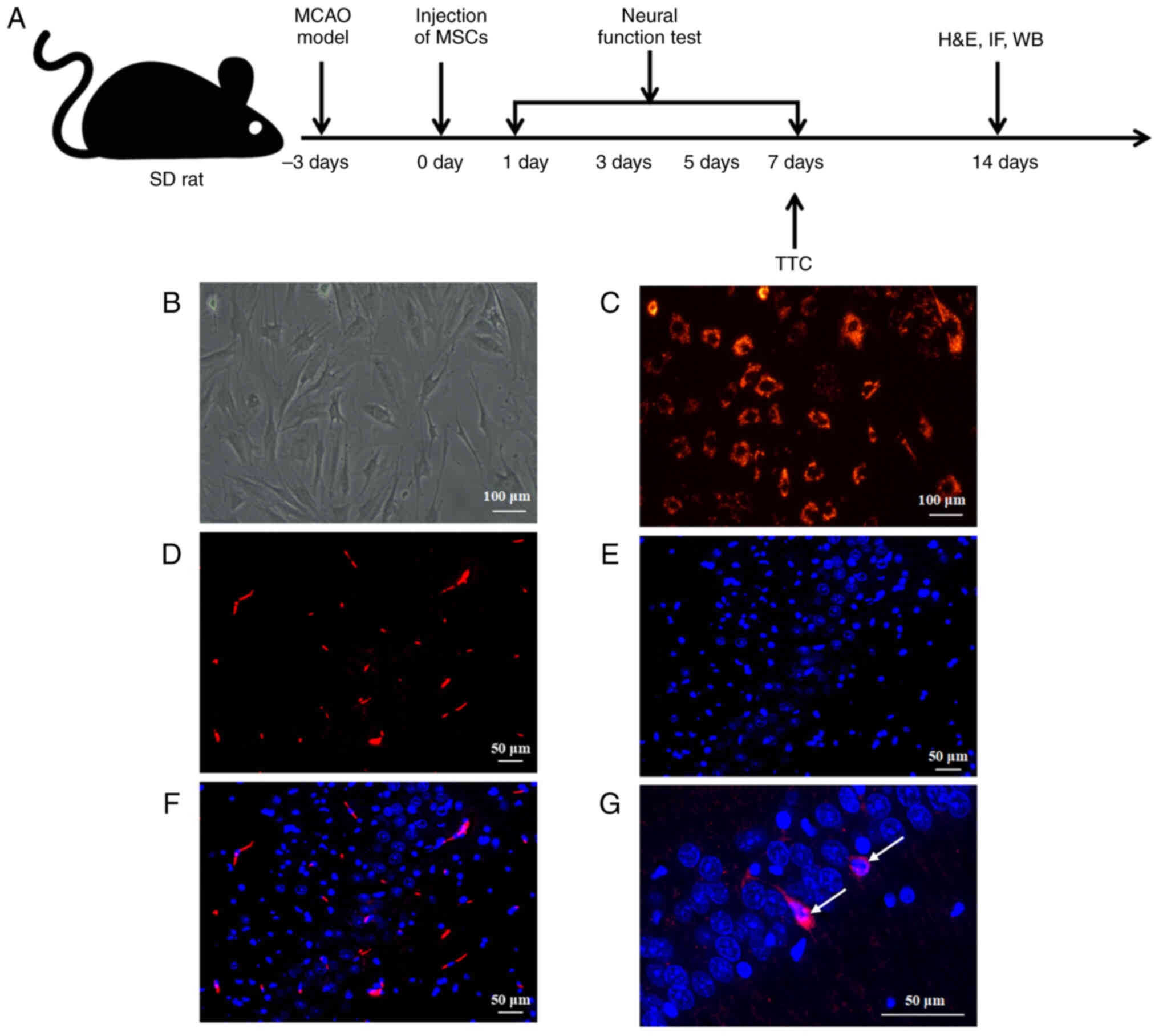
Engraftment and survival of MSCs in
ischemically injured brain tissue
In the present study, SD rats were subjected to the
experiment shown in Fig. 5A.
When cultured in vitro to the third generation, MSCs
displayed spindle-shaped morphology with high homogeneity (Fig. 5B), and CM-Dil-labeled MSCs
exhibited strong red fluorescence with an uncolored nucleus and
high labeling efficiency (Fig.
5C). A total of 7 days after MSCs transplantation the rats were
sacrificed and their brain tissues were analyzed using fluorescence
detection. The transplanted cells were visibly labeled with red
fluorescence after CM-Dil labeling (Fig. 5D), and blue cell fluorescence was
observed following DAPI staining (Fig. 5E). The cells that displayed
co-localization of red and blue fluorescence were considered MSCs
that had been implanted in the brain tissue (Fig. 5F and G). These findings indicated
that the MSCs were successfully transplanted into the brain tissues
of rats that had IBI and were viable.
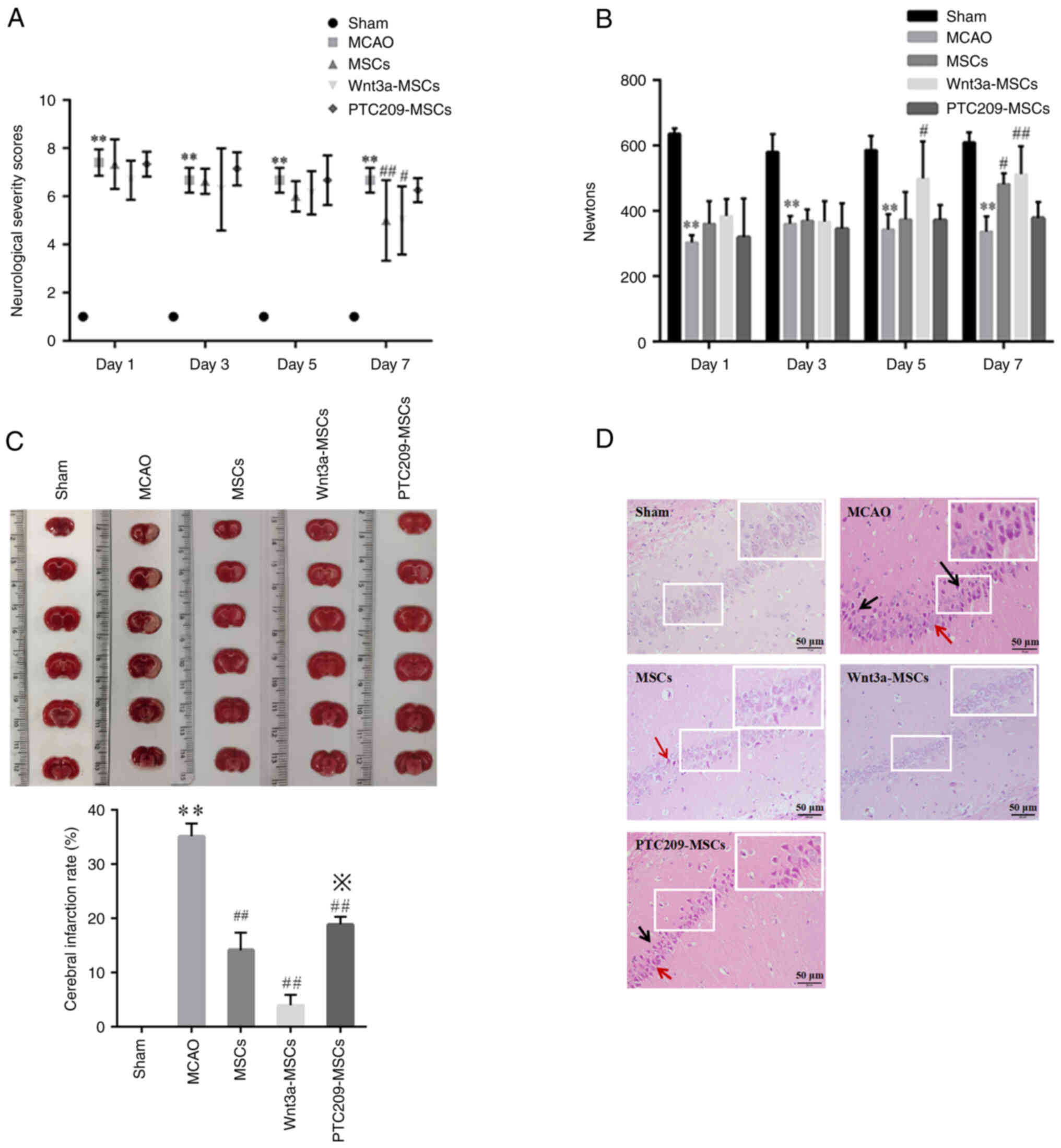
Bmi1 downregulation inhibits repair of
IBI by MSC transplantation
The NSS scores demonstrated that rats in the Sham
group obtained a score of 1 on days 1, 3, 5 and 7, and displayed no
observable behavioral abnormalities (Fig. 6A). Conversely, rats in the MCAO
group exhibited substantial behavioral deficits, including left
shoulder internal rotation, decreased unilateral resistance to
countermovement, and grip strength reduction on the left side due
to IBI. There were no significant differences between the three
groups receiving MSC transplantation on days 1, 3 and 5 when
compared with the MCAO group; however, on day 7, rats in the MSC
and Wnt3a-MSC groups had lower scores compared with in the MCAO
group, whereas the PTC209-MSC group showed no significant change
compared with the MCAO group. Furthermore, the results suggested
that rats in the MCAO group had significantly weaker grasping
strength than those in the Sham group on days 1, 3, 5 and 7
(Fig. 6B). On day 7, the MSCs
group showed an improvement in grasping strength compared with the
MCAO group. Furthermore, the Wnt3a-MSCs group exhibited an
improvement in grasp strength on days 5 and 7, whereas the
PTC209-MSCs group did not show any significant improvement at all
time points. The TTC staining results indicated uniformly symmetric
brain tissue coloring without infarction in the Sham group rats,
whereas different degrees of infarction conditions were observed in
the brain tissues of the MCAO group and the three MSC
transplantation groups (Fig.
6C). The infarction rate in brain tissue was significantly
decreased in all three groups receiving MSC transplantation
compared with that in the MCAO group. Moreover, the Wnt3a-MSCs
group had a lower infarction rate than the MSCs group, whereas the
PTC209-MSCs group had an increased infarction rate compared with
that in the MSCs group. After performing H&E staining on brain
tissue sections, a normal and clear cell structure was observed in
the Sham group; by contrast, the MCAO group exhibited more deeply
stained, degenerated and atrophied neuronal cells, as well as a
markedly enlarged tissue gap (Fig.
6D). The three groups that underwent MSC transplantation
exhibited fewer cellular wrinkles and necrotic areas compared with
the MCAO group, with varying degrees of improvement in tissue
damage. However, among these three groups, there were still more
wrinkled and deeply stained cells in the PTC209-MSCs group. This
suggests that Wnt3a may enhance the neural repair function of MSCs
and PTC209 could attenuate the neural repair function of MSCs.
All of the aforementioned findings indicated that
the MCAO model rats developed notable behavioral disturbances
following ischemic brain damage, whereas neurological function and
localized brain infarction symptoms improved following
transplantation of MSCs into MCAO model rats. Treatment of MSCs
with Wnt3a further improved infarction symptoms in damaged brain
tissues, whereas downregulation of Bmi1 using PTC209 hindered the
reduction of the cerebral infarction rate, thus suggesting that
Bmi1 serves a role in repairing IBI through MSC
transplantation.
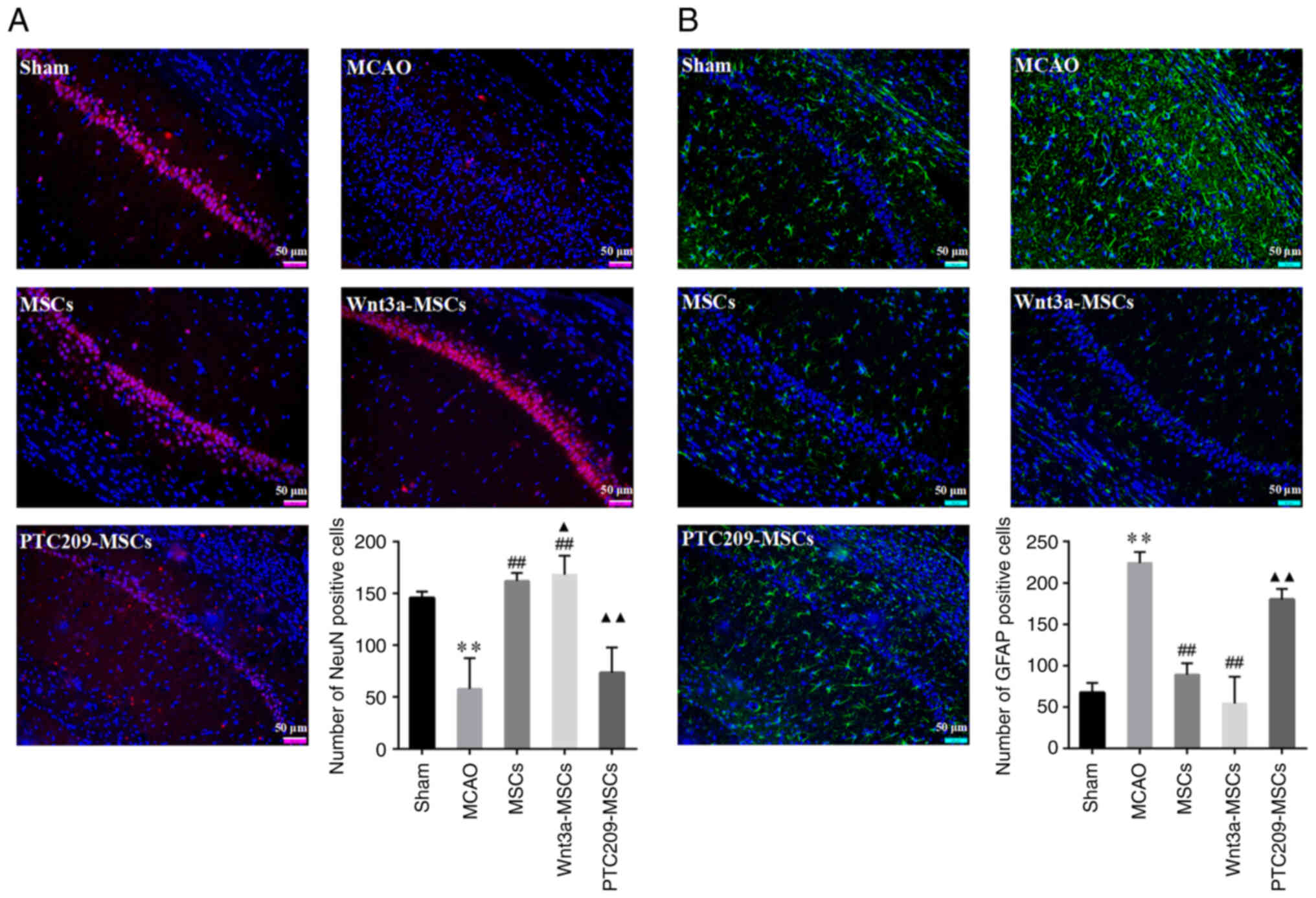
Downregulation of Bmi1 inhibits recovery
of neurological function in MCAO rats
Sections of the rat hippocampal CA1 area were
stained with red fluorescence for NeuN-positive cells and green
fluorescence for GFAP-positive cells. The NeuN immunofluorescence
results indicated a significant decrease in the number of
NeuN-positive cells in the MCAO group as compared with in the Sham
group (Fig. 7A). By contrast,
the MSCs and Wnt3a-MSCs groups exhibited a significant increase in
NeuN-positive cells compared with those in the MCAO group, whereas
the PTC209-MSCs group showed no significant difference. When
compared with the MSCs group, the Wnt3a-MSCs group showed a
significant increase in NeuN staining while the PTC209-MSCs group
showed a significant decrease. By contrast, the GFAP
immunofluorescence results revealed a notable increase in
GFAP-positive cells in the MCAO group when compared with the Sham
group (Fig. 7B). Furthermore,
the MSCs and Wnt3a-MSCs groups exhibited a significant reduction in
the number of GFAP-positive cells compared with in the MCAO group,
whereas there was no statistically significant difference in GFAP
staining between the PTC209-MSCs group and the MCAO group. The
number of GFAP-positive cells was significantly increased in the
PTC209-MSCs group compared with the MSCs group.
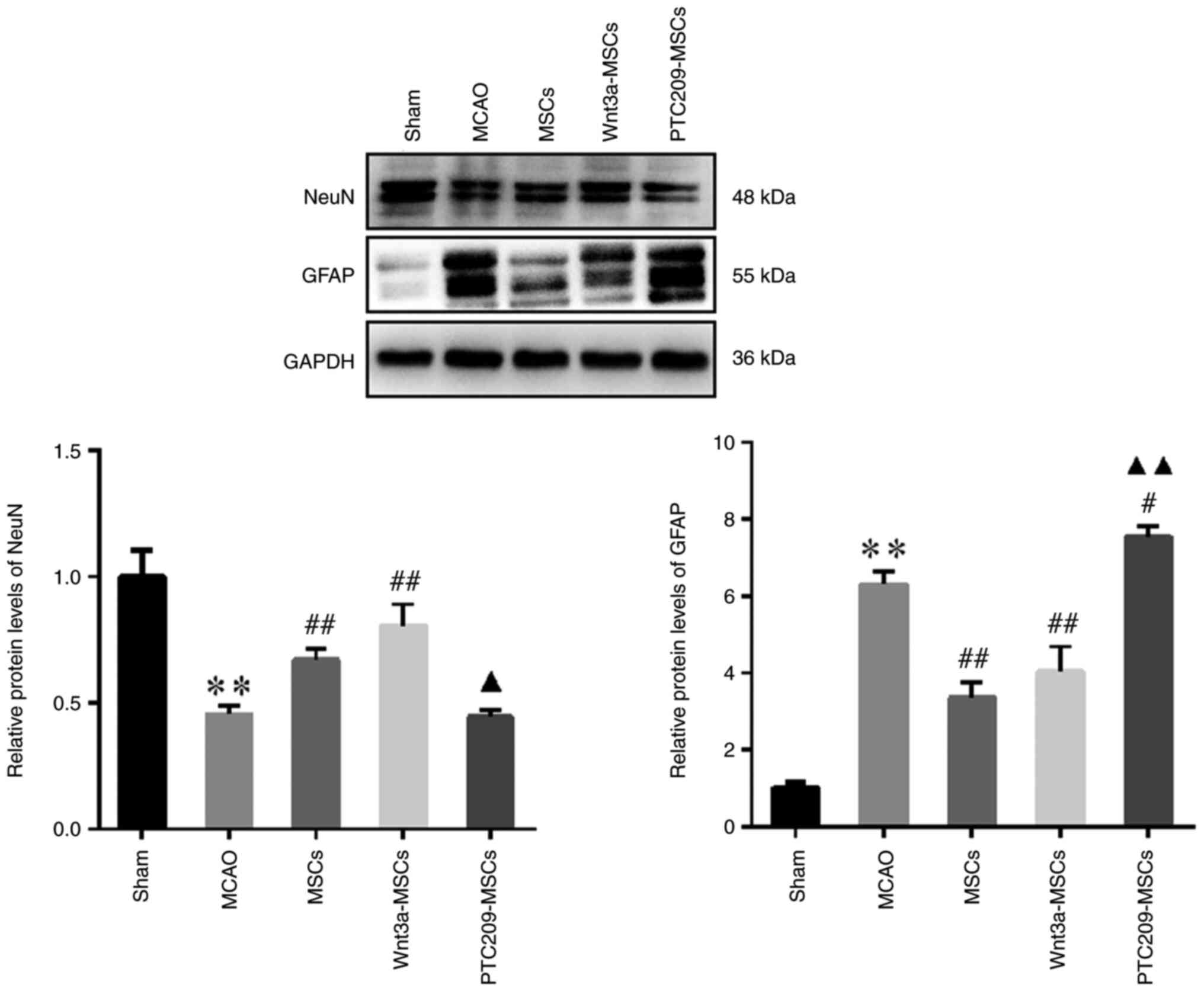
The results of western blotting indicated that in
the MCAO group, NeuN protein expression was lower and GFAP
expression was higher compared with that in the Sham group
(Fig. 8). Conversely, in the
MSCs and Wnt3a-MSCs groups, NeuN protein expression was higher
whereas GFAP protein expression was lower than that in the MCAO
group. Notably, neither NeuN or GFAP expression was significantly
altered in the Wnt3a-MSCs group compared with in the MSCs group.
NeuN expression was decreased and GFAP expression was elevated in
the PTC209-MSCs group compared with the MSCs group.
The present findings suggested that Wnt3a may
promote an increase in the number of neurons in the CA1 region of
the rat hippocampus and enhance the neural repair function of MSCs,
whereas downregulation of Bmi1 could inhibit the increased neuronal
number and the decrease of astrocyte number in the CA1 region of
the hippocampus of MSC-transplanted rats, and may thus suppress
neural repair.
Discussion
IBIs are associated with high morbidity, disability
and mortality due to their complex pathogenesis, with MCAO being a
prevalent cause. Cerebral ischemia results in restored blood supply
after a certain period of time; however, brain function is not
restored and is accompanied by severe delayed neuronal apoptosis
and necrosis, leading to brain dysfunction. Notably, stem cell
therapy utilizing MSCs has become a promising approach for treating
neurological disorders (29).
However, the altered homeostasis of the internal brain tissue
environment in IBI adversely affects the survival, proliferation
and effective differentiation rate of transplanted stem cells;
these factors notably impede the efficacy of MSC transplantation
(30,31). To address this issue, the present
study showed that Wnt3a can promote MSC neural differentiation by
upregulating the Wnt3a-RhoA signaling pathway. Investigations have
shown that Wnt3a treatment or Wnt3a overexpression in MSCs
activates both classical and non-classical Wnt signaling pathways
(32). Several cytokines and
chemokines, such as stromal cell-derived factor-1α, a CXC-type
chemokine produced by bone marrow stromal cells, can induce the
chemotactic migration of MSCs toward the injured part of the nerve
and contribute to the repair of structure and function at the site
of injury (33). In our previous
study, it was found that upregulation of the Wnt3a-RhoA signaling
pathway using Wnt3a could promote the migration of MSCs in
vitro (27). Therefore, 10
ng/ml Wnt3a was used in the present study.
MSCs have the potential to differentiate into neural
cells, offering a promising option for treating IBI. The present
study confirmed that BME induced the differentiation of MSCs to the
neuronal lineage, as indicated by positive staining of Nestin, a
marker for tumor stem cells, NSCs and astrocytes and GFAP, an
astrocyte surface marker.
Cerebral ischemia instigates neuronal necrosis,
apoptosis and progressive neuronal depletion, particularly in the
CA1 section of the hippocampus, culminating in notable debilitation
in cognitive ability, spatial perception and behavior (34). The CA1 region of the hippocampus
in the brain is particularly vulnerable to IBI, and the neuronal
cells within this area are susceptible to necrosis and apoptosis
following such an injury (35).
This region of the hippocampus is comprised of dense, large
neurons, and these vertebral neurons are the principal output
neurons of the hippocampus (36), which have the capacity to
selectively transmit different behavioral information to various
target areas (37).
Consequently, the CA1 region of the hippocampus was selected as the
primary assessment target in the present study. In the present
study, rats that underwent MCAO showed notable behavioral deficits
after IBI episodes. CM-Dil-labeled cells were visible in the
hippocampal region of rat brain tissue sections after MSCs
transplantation, and the NSS and grasping ability of rats gradually
improved. Transplanting MSCs promoted neurogenesis, which aligns
with the results indicating that MSCs have the ability to nourish
cells, promote cell proliferation and differentiation, inhibit cell
death and regulate homeostasis in the injured area (38-41). The current study indicated that
upregulation of the Wnt3a-RhoA signaling pathway in MSCs led to an
increase in the number of neurons in the CA1 region of the rat
hippocampus by immunofluorescence staining and H&E staining,
thus improving the neural repair ability of MSCs.
The Bmi1 protein belongs to the multi-comb
inhibitory complex 1 family, and contributes to various biological
processes, including embryonic development, organogenesis,
tumorigenesis, and stem cell stabilization and differentiation
(42). The results of the
current study demonstrated an increase in Bmi1 protein expression
during the induction of neural differentiation of MSCs in
vitro, suggesting its potential involvement in this process.
However, the effects of Bmi1 on the neural differentiation of MSCs
and the underlying mechanism remain unclear. Notably, Bmi1 acts as
an oncogene, and its anomalous expression is associated with the
progression and drug resistance of various types of cancer,
including bladder cancer and B-cell lymphoma (43,44). Therefore, the present study
implemented targeted small molecule inhibition of Bmi1 to
specifically restrict Bmi1 levels, enabling the investigation of
its association with and function in stem cell self-renewal and
differentiation. The Bmi1-selective inhibitor PTC209 was initially
screened by Kreso et al (45) through small-molecule gene
expression modulation technology. As demonstrated in a preceding
study, a concentration of 1 μM of the small molecule
compound PTC-209 was shown to inhibit the expression of Bmi1 during
the process of induced pluripotent stem cell differentiation
(46). The findings of the
present study demonstrated that 1 μmol/l PTC209 exerted no
effect on the proliferation of MSCs. Furthermore, the inhibition of
Bmi1 expression by PTC209 impeded the differentiation of MSCs
towards astrocytes and neurons during BME-induced neural
differentiation of MSCs in vitro.
NeuN is a neuronal protein the expression of which
directly reflects the maturity of neurons, whereas GFAP acts as a
marker for astrocyte activation. GFAP upregulation leads to a low
percentage of NeuN protein phosphorylation and the formation of
glial scarring, which hinders axon regeneration and thus negatively
affects the repair of neural tissue structures. Changes in the
expression of these two proteins may indicate the extent of nerve
cell injury caused by cerebral ischemia (47). In the current study, the quantity
of neurons and astrocytes were analyzed in brain tissues following
MSC transplantation. The outcomes revealed that downregulation of
Bmi1 by PTC209 inhibited the increase in neuron number and the
decrease in astrocytes in the CA1 region of the hippocampus of
MSC-transplanted rats and attenuated the frontal neural repair
ability of MSCs. It may be hypothesized that the upregulation of
GFAP resulting from reduced Bmi1 expression following IBI in rats
may hinder CA1 hippocampal neuron repair and compromise the neural
repair capacity of MSCs. It is important to note that the
experimental results differed slightly due to the different
experimental conditions employed in the present study between in
vitro and in vivo experiments. For example, the results
of in vitro experiments demonstrated that after inducing
neural differentiation of MSCs, GFAP-positive cells could be
observed under a microscope in the BME and Wnt3a groups, whereas
GFAP-positive cells were almost unobservable in the PTC209 group.
Conversely, the in vivo experiments demonstrated that the
MCAO group exhibited significantly higher levels of GFAP-positive
cells compared with the Sham group, whereas the MSCs and Wnt3a-MSCs
groups exhibited a decrease in GFAP-positive cells compared with
the MCAO group. A comprehensive review of the literature on GFAP
revealed its expression in the intermediate filaments of the
astrocyte lineage, thus serving as a marker for astrocytes.
Astrocytes can form physical barriers to isolate damaged tissues,
regulate blood flow after ischemia, promote the blood-brain
barrier, support myelin formation and provide mechanical strength;
however, it is important to note that prolonged upregulation of
GFAP and proliferation of astrocytes can result in neurological
damage, as well as the inhibition of the proliferation of mature
brain neurons and protrusion extension (48-50). It is possible that the results of
the in vitro and in vivo experiments are not
completely uniform because of the different time and conditions for
inducing neural differentiation in vitro and nerve injury
in vivo.
Numerous studies have demonstrated that Bmi1 can
regulate Wnt signaling in various types of cells (51-54). To further explore the
relationship between Bmi1 and the Wnt3a-RhoA signaling pathway, the
present study examined the effects of Wnt3a-induced upregulation of
the Wnt3a-RhoA signaling pathway and PTC209-induced inhibition of
Bmi1 on the expression of proteins related to Bmi1 and the
Wnt3a-RhoA signaling pathway. No significant difference in Bmi1
protein expression was observed after upregulating Wnt3a-RhoA in
MSCs; however, downregulation of Bmi1 in MSCs resulted in a
significant decrease in the protein expression levels of both Wnt3a
and RhoA. These findings suggested that Bmi1 might serve a role in
the neural differentiation process of MSCs by regulating the
expression of Wnt3a and RhoA.
In summary, the repair of IBI by MSC transplantation
is a multifaceted process that is affected by numerous factors. The
utilization of MSCs in the replacement of damaged or functionally
deficient neural tissue for the treatment of IBI represents a
highly promising therapeutic modality. It has been reported that
Bmi1 is essential for the maintenance of stemness in a variety of
stem cells (55,56); however, studies on Bmi1 in MSCs
are still scarce. The present study aimed to explore the mechanism
of Bmi1 and the Wnt3a-RhoA signaling pathway on the in vitro
neural differentiation of MSCs and on in vivo IBI repair.
Through the findings of the in vivo and in vitro
experiments, it was indicated that Bmi1 may regulate neural
differentiation of MSCs through the Wnt3a-RhoA signaling pathway to
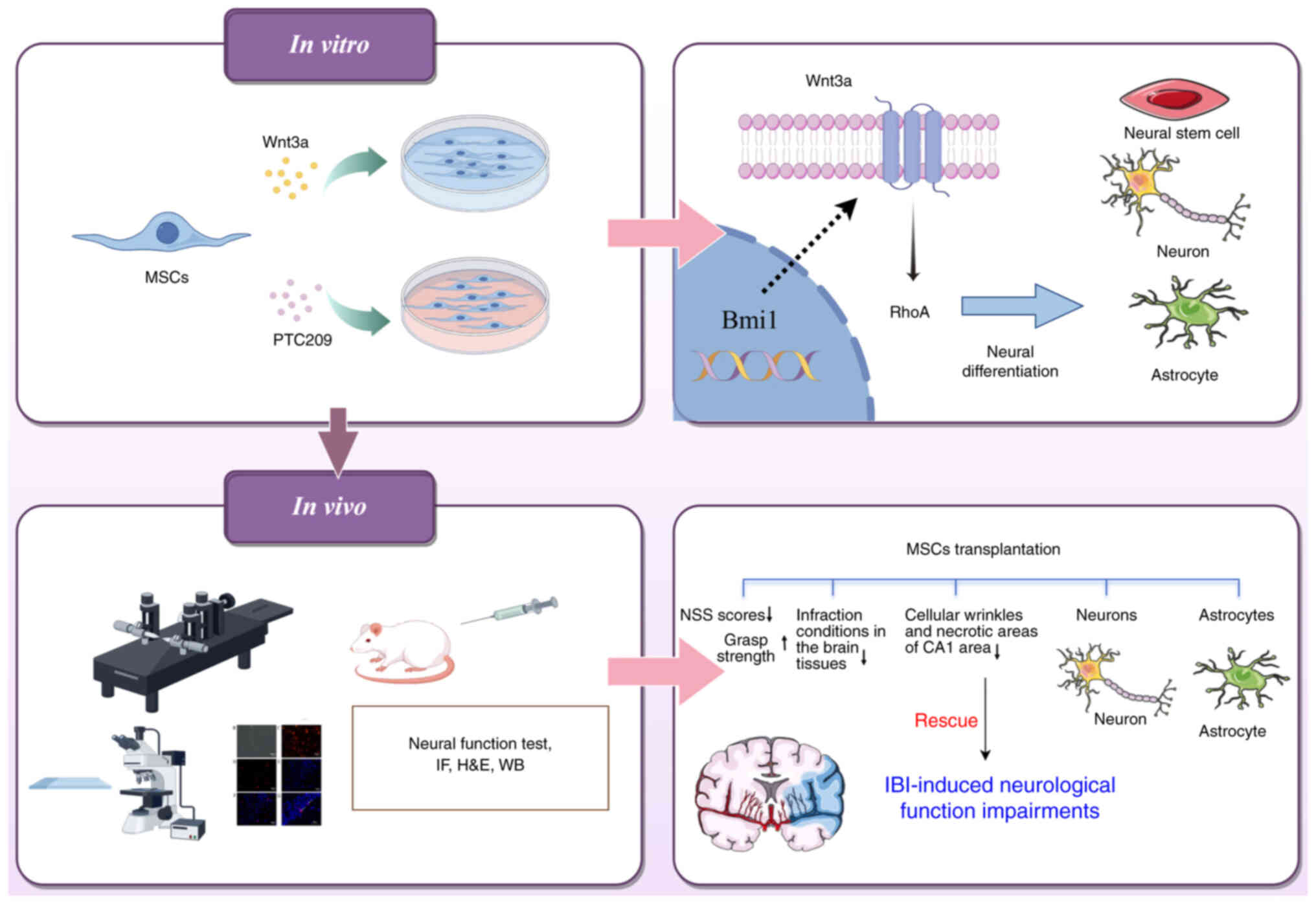
repair ischemic brain injury in rats (Fig. 9). In the current study, the role
of Bmi1 in the repair of IBI by MSC transplantation was
investigated. With the continuous development of translational and
neuroregenerative medicine, these findings may provide novel
insights and targets for the treatment of IBI. Furthermore, Bmi1
could serve as a new therapeutic target for the treatment of
neurological diseases. Nevertheless, the present study is not
without limitations. Firstly, only the Wnt3a and RhoA components of
the Wnt signaling pathway were analyzed. Secondly, the antagonistic
effect of Wnt3a, an agonist of Wnt3a-RhoA, on PTC209, a Bmi1
inhibitor, requires further investigation. It is evident that
further exploration is required to gain a more comprehensive
understanding of the subject; this necessitates the conduction of
additional in-depth studies. Finally, following extensive
consultation of the literature, normal MSC culture conditions were
employed in the in vitro experiments to explore the
mechanism. The paucity of studies based on ischemic cell conditions
was noted and it was acknowledged that this area should be a focus
of future research.
In conclusion, the results of the present study
provide conclusive evidence that suggests Bmi1 may serve a crucial
role in the process of neural differentiation of MSCs by regulating
the expression of Wnt3a and RhoA. Furthermore, the findings
indicated that elevating the Wnt3a-RhoA signaling pathway in MSCs
may foster the restoration of neural function in rats with brain
injuries. Conversely, downregulation of Bmi1 in MSCs may impede the
recovery of neural function in brain-damaged rats.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KC performed all relevant experiments and prepared
the drafts. HZ performed the in vitro experiments. JZ and YZ
performed the in vivo experiments. XD prepared the drafts,
revised the manuscript, and analyzed and interpreted the data. QY
and LZ designed and directed the project. All authors confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethical and Welfare Committee of Zhejiang Chinese Medical
University (approval no. 20210524-05; review no.
IACUC-202108-05).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
MSC
|
mesenchymal stem cell
|
|
Bmi1
|
B lymphoma Mo-MLV insertion region 1
homolog
|
|
IBI
|
ischemic brain injury
|
|
BME
|
β-mercaptoethanol
|
|
NSC
|
neural stem cell
|
|
PCP
|
planar cell polarity
|
|
SDF-1α
|
stromal cell-derived factor-1α
|
|
FBS
|
fetal bovine serum
|
|
DMSO
|
dimethyl sulfoxide
|
|
TTC
|
triphenyltetrazolium chloride
|
|
NSS
|
Neurological Severity Score
|
|
MCAO
|
middle cerebral artery occlusion
|
|
H&E
|
hematoxylin and eosin
|
Acknowledgements
Not applicable.
Funding
This work was supported by the Research Project of Zhejiang
Chinese Medical University (grant no. 2022JKZKTS21) and the
Research Project on Laboratory Work in Universities of Zhejiang
Province (grant no. YB202268).
References
|
1
|
Global burden of 369 diseases and injuries
in 204 countries and territories, 1990-2019: A systematic analysis
for the Global Burden of Disease Study 2019. Lancet. 396:1204–1222.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McIntyre CW and Goldsmith DJ: Ischemic
brain injury in hemodialysis patients: Which is more dangerous,
hypertension or intradialytic hypotension? Kidney Int.
87:1109–1115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X and Jia X: Neuroprotection of stem
cells against ischemic brain injury: From bench to clinic. Transl
Stroke Res. 15:691–713. 2024. View Article : Google Scholar :
|
|
4
|
Sakai S and Shichita T: Inflammation and
neural repair after ischemic brain injury. Neurochem Int.
130:1043162019. View Article : Google Scholar
|
|
5
|
Nie X, Leng X, Miao Z, Fisher M and Liu L:
Clinically ineffective reperfusion after endovascular therapy in
acute ischemic stroke. Stroke. 54:873–881. 2023. View Article : Google Scholar
|
|
6
|
Guo Y, Peng Y, Zeng H and Chen G: Progress
in mesenchymal stem cell therapy for ischemic stroke. Stem Cells
Int. 2021:99235662021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chrostek MR, Fellows EG, Crane AT, Grande
AW and Low WC: Efficacy of stem Cell-based therapies for stroke.
Brain Res. 1722:1463622019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Sun T and Jiang C: Recent advances
in nanomedicines for the treatment of ischemic stroke. Acta Pharm
Sin B. 11:1767–1788. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leveque X, Hochane M, Geraldo F, Dumont S,
Gratas C, Oliver L, Gaignier C, Trichet V, Layrolle P, Heymann D,
et al: Low-dose pesticide mixture induces accelerated mesenchymal
stem cell aging in vitro. Stem Cells. 37:1083–1094. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Z, Xie Y, Yu L, Li Y and Wang Y:
Hepatocyte growth factor (HGF) and stem cell factor (SCF)
maintained the stemness of human bone marrow mesenchymal stem cells
(hBMSCs) during long-term expansion by preserving mitochondrial
function via the PI3K/AKT, ERK1/2, and STAT3 signaling pathways.
Stem Cell Res Ther. 11:3292020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Xiong N, Liu Y and Gan L:
Biomimetic cell-adhesive ligand-functionalized peptide composite
hydrogels maintain stemness of human amniotic mesenchymal stem
cells. Regen Biomater. 8:rbaa0572021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou T, Yang Y, Chen Q and Xie L:
Glutamine metabolism is essential for stemness of bone marrow
mesenchymal stem cells and bone homeostasis. Stem Cells Int.
2019:89289342019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Morree A and Rando TA: Regulation of
adult stem cell quiescence and its functions in the maintenance of
tissue integrity. Nat Rev Mol Cell Biol. 24:334–354. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sahasrabuddhe AA: BMI1: A biomarker of
hematologic malignancies. Biomark Cancer. 8:65–75. 2016.PubMed/NCBI
|
|
15
|
Yang J, Xue J, Hu W, Zhang L, Xu R, Wu S,
Wang J, Ma J, Wei J, Wang Y, et al: Human embryonic stem
cell-derived mesenchymal stem cell secretome reverts silica-induced
airway epithelial cell injury by regulating Bmi1 signaling. Environ
Toxicol. 38:2084–2099. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng X, Wang Q, Xie Z and Li J: The
elevated level of IL-1α in the bone marrow of aged mice leads to
MSC senescence partly by down-regulating Bmi-1. Exp Gerontol.
148:1113132021. View Article : Google Scholar
|
|
17
|
Mich JK, Signer RA, Nakada D, Pineda A,
Burgess RJ, Vue TY, Johnson JE and Morrison SJ: Prospective
identification of functionally distinct stem cells and
neurosphere-initiating cells in adult mouse forebrain. Elife.
3:e026692014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kraus L, Bryan C, Wagner M, Kino T,
Gunchenko M, Jalal W, Khan M and Mohsin S: Bmi1 augments
proliferation and survival of cortical Bone-derived stem cells
after injury through novel epigenetic signaling via histone 3
Regulation. Int J Mol Sci. 22:78132021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Huang J, Wang F, Ding H, Cui Y,
Yang Y, Xu J, Luo H, Gao Y, Pan L, et al: BMI1 regulates multiple
myeloma-associated macrophage's pro-myeloma functions. Cell Death
Dis. 12:4952021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russell JO and Monga SP: Wnt/β-Catenin
signaling in liver development, homeostasis, and pathobiology. Annu
Rev Pathol. 13:351–378. 2018. View Article : Google Scholar
|
|
21
|
Yu Q, Liu L, Duan Y, Wang Y, Xuan X, Zhou
L and Liu W: Wnt/β-catenin signaling regulates neuronal
differentiation of mesenchymal stem cells. Biochem Biophys Res
Commun. 439:297–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schunk SJ, Floege J, Fliser D and Speer T:
WNT-β-catenin signalling-a versatile player in kidney injury and
repair. Nat Rev Nephrol. 17:172–184. 2021. View Article : Google Scholar
|
|
23
|
Torban E and Sokol SY: Planar cell
polarity pathway in kidney development, function and disease. Nat
Rev Nephrol. 17:369–385. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bengoa-Vergniory N, Gorroño-Etxebarria I,
González-Salazar I and Kypta RM: A switch from canonical to
noncanonical Wnt signaling mediates early differentiation of human
neural stem cells. Stem Cells. 32:3196–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Govek EE, Newey SE and Van Aelst L: The
role of the Rho GTPases in neuronal development. Genes Dev.
19:1–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Li HQ, Lu L, Fu DL, Liu AJ, Li JH
and Zheng GQ: Ginsenoside Rg1 provides neuroprotection against
blood brain barrier disruption and neurological injury in a rat
model of cerebral ischemia/reperfusion through downregulation of
aquaporin 4 expression. Phytomedicine. 21:998–1003. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao P, Yu Q, Zhu L, Li J, Zhou X, Wu L,
Cai Y, Shen H and Zhou L: Wnt/PCP pathway regulates the migration
and neural differentiation of mesenchymal stem cells in vitro.
Folia Histochem Cytobiol. 60:44–54. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zaghloul RA, Elsherbiny NM, Kenawy HI,
El-Karef A, Eissa LA and El-Shishtawy MM: Hepatoprotective effect
of hesperidin in hepatocellular carcinoma: Involvement of Wnt
signaling pathways. Life Sci. 185:114–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu D, Ye Y, Xu L, Yuan W and Zhang Q:
Icariin and mesenchymal stem cells synergistically promote
angiogenesis and neurogenesis after cerebral ischemia via PI3K and
ERK1/2 pathways. Biomed Pharmacother. 108:663–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldman SA: Stem and Progenitor Cell-based
therapy of the central nervous system: Hopes, hype, and wishful
thinking. Cell Stem Cell. 18:174–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q, Guo Y, Chen F, Liu J and Jin P:
Stromal cell-derived factor-1 promotes human adipose tissue-derived
stem cell survival and chronic wound healing. Exp Ther Med.
12:45–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng Z, Feng G, Hu X, Yang L, Yang X and
Jin Q: SDF Factor-1α promotes the migration, proliferation, and
osteogenic differentiation of mouse bone marrow mesenchymal stem
cells through the Wnt/β-catenin pathway. Stem Cells Dev.
30:106–117. 2021. View Article : Google Scholar
|
|
33
|
Marquez-Curtis LA and Janowska-Wieczorek
A: Enhancing the migration ability of mesenchymal stromal cells by
targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013:5610982013.
View Article : Google Scholar
|
|
34
|
Liu P, Xu J, Chen Y, Xu Q, Zhang W, Hu B,
Li A and Zhu Q: Electrophysiological signatures in global cerebral
ischemia: Neuroprotection via chemogenetic inhibition of CA1
pyramidal neurons in rats. J Am Heart Assoc. 13:e0361462024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu HY, Cui Y, Li Q, Wang D, Li H, Yang L,
Wang DJ and Zhou JW: LAMP-2A ablation in hippocampal CA1 astrocytes
confers cerebroprotection and ameliorates neuronal injury after
global brain ischemia. Brain Pathol. 33:e131142023. View Article : Google Scholar
|
|
36
|
Lalkovičová M, Bonová P, Burda J and
Danielisová V: Effect of bradykinin postconditioning on ischemic
and toxic brain damage. Neurochem Res. 40:1728–1738. 2015.
View Article : Google Scholar
|
|
37
|
Mao M, Xu Y, Zhang XY, Yang L, An XB, Qu
Y, Chai YN, Wang YR, Li TT and Ai J: MicroRNA-195 prevents
hippocampal microglial/macrophage polarization towards the M1
phenotype induced by chronic brain hypoperfusion through regulating
CX3CL1/CX3CR1 signaling. J Neuroinflammation. 17:2442020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang CY, Liang MZ, Wu CC, Huang PY, Chen
HI, Yet SF, Tsai JW, Kao CF and Chen L: WNT3A promotes neuronal
regeneration upon traumatic brain injury. Int J Mol Sci.
21:14632020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He M, Shi X, Yang M, Yang T, Li T and Chen
J: Mesenchymal stem cells-derived IL-6 activates AMPK/mTOR
signaling to inhibit the proliferation of reactive astrocytes
induced by hypoxic-ischemic brain damage. Exp Neurol. 311:15–32.
2019. View Article : Google Scholar
|
|
40
|
Pourheydar B, Soleimani Asl S, Azimzadeh
M, Rezaei Moghadam A, Marzban A and Mehdizadeh M: Neuroprotective
effects of bone marrow mesenchymal stem cells on bilateral common
carotid arteries occlusion model of cerebral ischemia in rat. Behav
Neurol. 2016:29647122016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xin H, Katakowski M, Wang F, Qian JY, Liu
XS, Ali MM, Buller B, Zhang ZG and Chopp M: MicroRNA cluster
miR-17-92 cluster in exosomes enhance neuroplasticity and
functional recovery after stroke in rats. Stroke. 48:747–753. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park IK, Qian D, Kiel M, Becker MW,
Pihalja M, Weissman IL, Morrison SJ and Clarke MF: Bmi-1 is
required for maintenance of adult self-renewing haematopoietic stem
cells. Nature. 423:635–642. 2003. View Article : Google Scholar
|
|
43
|
Wen T, Zhang X, Gao Y, Tian H, Fan L and
Yang P: SOX4-BMI1 axis promotes non-small cell lung cancer
progression and facilitates angiogenesis by suppressing ZNF24. Cell
Death Dis. 15:6982024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao Y, Yang W, Zheng K, Chen J and Jin X:
The role of BMI1 in endometrial cancer and other cancers. Gene.
856:1471292023. View Article : Google Scholar
|
|
45
|
Kreso A, van Galen P, Pedley NM,
Lima-Fernandes E, Frelin C, Davis T, Cao L, Baiazitov R, Du W,
Sydorenko N, et al: Self-renewal as a therapeutic target in human
colorectal cancer. Nat Med. 20:29–36. 2014. View Article : Google Scholar
|
|
46
|
Shan W, Zhou L, Liu L, Lin D and Yu Q:
Polycomb group protein Bmi1 is required for the neuronal
differentiation of mouse induced pluripotent stem cells. Exp Ther
Med. 21:6192021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Okoh OS, Akintunde JK, Akamo AJ and Akpan
U: Thymoquinone inhibits Neuroinflammatory mediators and
vasoconstriction injury via NF-κB dependent NeuN/GFAP/Ki-67 in
hypertensive Dams and F1 male pups on exposure to a mixture of
Bisphenol-A analogues. Toxicol Appl Pharmacol. 494:1171622025.
View Article : Google Scholar
|
|
48
|
Li M, Li Z, Yao Y, Jin WN, Wood K, Liu Q,
Shi FD and Hao J: Astrocyte-derived interleukin-15 exacerbates
ischemic brain injury via propagation of cellular immunity. Proc
Natl Acad Sci USA. 114:E396–E405. 2017.
|
|
49
|
Duan CL, Liu CW, Shen SW, Yu Z, Mo JL,
Chen XH and Sun FY: Striatal astrocytes transdifferentiate into
functional mature neurons following ischemic brain injury. Glia.
63:1660–1670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sullivan SM, Sullivan RK, Miller SM,
Ireland Z, Björkman ST, Pow DV and Colditz PB: Phosphorylation of
GFAP is associated with injury in the neonatal pig hypoxic-ischemic
brain. Neurochem Res. 282:29414–29423. 2012.
|
|
51
|
Hosoya A, Takebe H, Seki-Kishimoto Y,
Noguchi Y, Ninomiya T, Yukita A, Yoshiba N, Washio A, Iijima M,
Morotomi T, et al: Polycomb protein Bmi1 promotes odontoblast
differentiation by accelerating Wnt and BMP signaling pathways.
Histochem Cell Biol. 163:112024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu J, Shen C, Lin M, Chen X, Dai X, Li Z,
Wu Y, Fu Y, Lv J, Huang X, et al: BMI1 promotes spermatogonial stem
cell maintenance by epigenetically repressing Wnt10b/β-catenin
signaling. Int J Biol Sci. 18:2807–2820. 2022. View Article : Google Scholar :
|
|
53
|
Chen MH, Fu LS, Zhang F, Yang Y and Wu XZ:
LncAY controls BMI1 expression and activates BMI1/Wnt/β-catenin
signaling axis in hepatocellular carcinoma. Life Sci.
280:1197482021. View Article : Google Scholar
|
|
54
|
Yu H, Gao R, Chen S, Liu X, Wang Q, Cai W,
Vemula S, Fahey AC, Henley D, Kobayashi M, et al: Bmi1 regulates
wnt signaling in hematopoietic stem and progenitor cells. Stem Cell
Rev Rep. 17:2304–2313. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bartucci M, Hussein MS, Huselid E,
Flaherty K, Patrizii M, Laddha SV, Kui C, Bigos RA, Gilleran JA, El
Ansary MMS, et al: Synthesis and characterization of novel BMI1
Inhibitors Targeting Cellular Self-renewal in hepatocellular
carcinoma. Target Oncol. 12:449–462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu D, Wan X, Huang H, Chen X, Liang W,
Zhao F, Lin T, Han J and Xie W: Knockdown of Bmi1 inhibits the
stemness properties and tumorigenicity of human bladder cancer stem
cell-like side population cells. Oncol Rep. 31:727–736. 2014.
View Article : Google Scholar
|