Introduction
The maintenance of redox homeostasis is dependent
upon the levels of pro-oxidant active molecules and antioxidants
(1). The disruption of this
equilibrium plays a crucial role in the development and progression
of numerous diseases (2). The
accumulation of pro-oxidative reactive factors, including reactive
oxygen species (ROS), reactive nitrogen species (RNS) and reactive
lipids (RLS), leads to the oxidation of DNA, proteins and lipids,
altering their structure, activity and physical properties, and
resulting in oxidative stress and cellular dysfunction (3). ROS are among the most ubiquitous
oxidants in cells and are capable of reacting with numerous
substances to form superoxide (4). The reaction between ROS and
polyunsaturated fatty acids (PUFAs) in plasma, cell membranes and
organelle membranes is critical, leading to the production of lipid
peroxides (5). RLS produced by
lipid peroxidation, such as 4-hydroxynonenal (4-HNE) and
malondialdehyde (MDA), are closely associated with the onset and
progression of numerous diseases, including cancer, diabetes,
neurodegenerative disorders, cardiovascular diseases and
ischemia/reperfusion (I/R) injury. Redox homeostasis therefore
plays a critical role in the pathogenesis of multiple diseases.
The aryl hydrocarbon receptor (AhR) is a
transcription factor intricately linked to redox homeostasis in a
variety of inflammatory diseases (6). AhR is activated by a diverse array
of exogenous and endogenous ligands and transcriptional regulates
downstream target genes. This process consequently modulates the
oxidative and antioxidant balance and a variety of cellular
functions. AhR target genes, including the NAD(P)H dehydrogenase
[quinone] 1 (NQO1), glutathione S-transferase (GST),
UDP-glucuronosyltransferase 1-1 (UGT1A1), CYP1A1 and CYP1B1 genes,
play a crucial role in regulating cellular redox homeostasis
(7). Moreover, AhR plays a
crucial role in the metabolism of exogenous substances and drugs,
iron and heme metabolism, glutathione synthesis, and lipid
metabolism (8,9). The dysregulation of AhR is observed
in numerous disease processes, highlighting its essential role in
cell survival, particularly under conditions of redox homeostasis
imbalance.
AhR plays a multifaceted role in maintaining cell
survival, and both AhR agonists and inhibitors have been shown to
modulate cell survival or death, particularly in the context of
tumor therapy (10-14). In 2003, Dixon et al
(15) identified a novel form of
cell death termed ferroptosis, characterized as an iron-dependent
process triggered by lipid peroxidation. This pathway was initially
discovered through the high-throughput screening of anti-neoplastic
drugs (15). The
ferroptosis-inducing drug, erastin, induces ferroptosis through the
inhibition of the cystine/glutamate antiporter system xC-/xCT,
which is essential for the synthesis of reduced glutathione,
ultimately leading to the accumulation of toxic lipid peroxides
(16). Transcriptome analysis
conducted by Kwon et al (17) on pan-cancer cell lines revealed
that the activity of transcription factors, including Nuclear
factor erythroid 2-related factor 2 (NFE2L2/NRF2) and AhR, serves
as a critical marker of erastin resistance. AhR plays a
context-dependent role in modulating ferroptosis sensitivity across
tumor cell lines (18,19). For instance, the siRNA-mediated
knockdown of AhR increases erastin sensitivity in
erastin-insensitive A549 cells, while reducing sensitivity in
erastin-sensitive Calu1 cells, highlighting its dual role in
ferroptosis resistance (17).
Supporting this, Kou et al (19) and Peng et al (20) demonstrated that AhR expression
levels were closely associated with erastin sensitivity in both
A549 and BEAS-2B (human normal bronchial epithelial cells). Their
research further identified the solute carrier family 7 member 11
(SLC7A11) antioxidant system as a key downstream effector of AhR
(19,20). Despite these advancements, the
precise molecular mechanisms underlying the involvement of AhR in
ferroptosis remain to be fully elucidated.
AhR plays a pivotal role in modulating disease
progression through the regulation of lipid peroxidation and
ferroptosis across diverse pathological contexts (17,18). Beyond its established functions
in tumors, AhR activation has been implicated in other diseases.
For example, in I/R injury (liver, myocardium and kidneys), it
exacerbates tissue damage by promoting lipid peroxidation and
ferroptosis (21-24); AhR-mediated lipid peroxidation
and ferroptosis have been implicated in neurodegenerative
disorders, such as Alzheimer's disease (25); in intestinal inflammation, AhR
induces ferroptosis in intestinal intraepithelial lymphocytes
(natural TCRαβ+ CD8αα+ T-cells and
TCRγδ+ CD8αα+ T-cells, as well as induced
TCRαβ+ CD4+ and TCRαβ+
CD8αβ+ T-cells) (26); and in asthma pathogenesis, AhR
modulates ferroptosis in lung epithelial cells (BEAS-2B) (27). Notably, AhR exhibits
context-dependent roles in these processes. In I/R injury, AhR
activation uniformly aggravates tissue damage. By contrast, its
role in tumors is more intricate, displaying dual effects that vary
by cell type and ligand specificity. While the AhR-ferroptosis axis
has been extensively studied in tumors and I/R injury, its
implications in inflammatory bowel disease, neurodegenerative
diseases and lung diseases remain underexplored, representing
critical gaps in current research.
Early studies have identified several key mechanisms
underlying the context-dependent roles of AhR in response to
diverse ligands or cellular environments (28). The differential effects of AhR
activation may be attributed to the following factors: i)
Ligand-specific receptor conformation. Distinct ligands, such as
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and the tryptophan
metabolite, 6-formylindolo[3,2-b]carbazole (FICZ), induce unique
conformational changes in AhR, affecting its interaction with the
exenobiotic response element (XRE), the core DNA sequence
regulating gene transcription in the AhR signaling pathway. ii)
Ligand affinity and chemical properties: The binding stability and
nuclear retention of AhR are determined by ligand characteristics
(e.g., hydrophobicity, molecular size). High-affinity ligands
prolong nuclear localization and sustained signaling, while
low-affinity ligands (e.g., plant-derived metabolites) typically
trigger transient activation. iii) Cofactor recruitment
specificity: The AhR-AhR nuclear translocator (ARNT) complex
recruits ligand-dependent cofactors, such as inflammation-related
cofactors (e.g., NF-κB and STAT), metabolic regulators (e.g.,
glypican 1α and sterol regulatory element-binding protein 1), to
direct the downstream responses. iv) Cell type and microenvironment
dependency: Tissue-specific expression of cofactors results in the
activation of different genes by the same ligand in different
cells. For example, AhR may preferentially bind to IL-6 or IL-22
promoters in immune cells, while promoting CYP450 transcriptione in
hepatocytes. Furthermore, ligands may remodel the chromatin
accessibility of AhR, and at the same time, change the epigenetic
state through metabolites. v) Species/tissues-specific AhR
isoforms: AhR subtype expression (e.g., AhR1 and AhR2) induces
differential responses across tissues or species.
Given that AhR target genes critically regulate
redox homeostasis, its role in lipid peroxidation and ferroptosis
varies by ligand context and disease setting, suggesting that
targeted AhR modulation may have potential for use in various
pathological conditions (Table
I). Ferroptosis has emerged as a crucial pathological mechanism
in diverse diseases, particularly in cancer and I/R injury, where
its initiation is intricately linked to disruptions in redox
homeostasis and excessive lipid peroxidation (29). This review specifically examines
the therapeutic implications of targeting AhR-mediated lipid
peroxidation and ferroptosis in these two clinically significant
conditions. The present review focuses on the fundamental
association between lipid peroxidation and ferroptosis, summarizing
and critically analyzing the role and potential mechanisms of AhR
in these processes. The present review aimed to enhance the
understanding of AhR signaling in diseases pathogenesis and to
explore novel therapeutic avenues targeting this pathway for
disease intervention.
 | Table ISummary of AhR ligands and
antagonists regulating lipid peroxidation and ferroptosis
pathways. |
Table I
Summary of AhR ligands and
antagonists regulating lipid peroxidation and ferroptosis
pathways.
| Ligands or
antagonists | Promotes/inhibits
the AhR signaling pathway | Cell line/animal
model | Target gene and
signaling pathway | Influence of
ferroptosis and lipid peroxidation | (Refs.) |
|---|
| CH223191 | Inhibits | Mesenchymal stem
cells (MSCS) in hepatic ischemia-reperfusion injury models | STAT3-HO-1/COX-2
pathway | Inhibits | (67) |
| CH223191 | Inhibits | Hepatocytes of the
mouse model of Metabolic Disaster-associated steatohepatitis
(MASH) | Pten/Akt/β-catenin
pathway | Inhibits | (24) |
| CH223191 | Inhibits | Primary murine
renal proximal tubular epithelial cells (RPTECs) | The cytochrome P450
superfamily | Inhibits | (21) |
| TCDD | Promotes | Prostate cancer
(LNCap, 22Rv1) | NR4A1/SCD1
axis | Promotes | (18) |
| IDA | Promotes | Colorectal cancer
cell lines and mouse models of colorectal cancer (HT29) | ALDH1A3-FSP1
axis | Inhibits | (91) |
| indirubin | Promotes | Mouse embryonic
fibroblast (MEF) cell line induced by cysteine deprivation for
ferroptosis | ATF4-SLC7A11
axis | Inhibits | (84) |
| SR1 | Inhibits | Mouse embryonic
fibroblast (MEF) cell line induced by cysteine deprivation for
ferroptosis | ATF4-SLC7A11
axis | Promotes | (84) |
| DIM | Inhibits | Non-small cell lung
cancer cell lines (A549, H1299) | NRF2-GPX4 axis | Promotes | (112) |
| I3P | Promotes | BEAS-2B | SLC7A11 axis | Inhibits | (20) |
| Benzopyrene | Promotes | Airway epithelial
cells (AEC) of the mouse asthma model | cPLA2-ACSL4
axis | Promotes | (27) |
| Kyn | Promotes | Human lung cancer
cell lines A549 and H1299 and mouse lung cancer cell lines | NRF2 pathway | Inhibits | (76) |
| Kyn | Promotes | Glioblastoma
multiforme (GBM) cell line | FTO-SLC7A11
axis | Inhibits | (108) |
| NBP | Inhibits | In vitro
ferroptosis models (HT22 cells, hippocampal sections and primary
neurons) and in vivo controlled cortical shock mouse
models | CYP1B1-ROS
axis | Inhibits | (53) |
| IS | Promotes | MC3T3-E1 cell line
and mouse models of chronic kidney disease | SLC7A11/GPX4
axis | Promotes | (81) |
| ILA | Promotes | Cardiotoxic (DIC)
mice and DOX-treated H9C2 cells | NRF2 pathway | Inhibits | (107) |
| YH439 | Promotes | Mouse hepatic
stellate cells (mHSCs) | Mrp1 | Promotes | (111) |
| Ethanol extract of
Hibiscus (HS) calyx | Inhibits | Human keratinocytes
HaCaT cells | FTH1, GPX4 and
SLC7A11 pathway | Promotes | (113) |
Association between lipid peroxidation and
ferroptosis
Lipid peroxidation is an ubiquitous phenomenon
observed in a wide array of disease conditions and serves as a
critical triggering factor for ferroptosis. This process typically
manifests as a free radical chain reaction, characterized by an
overabundance of reactive species, such as ROS, RNS and RLS, along
with the excessive accumulation of labile iron pools in cells
(30). The primary event in
lipid peroxidation involves the generation of labile hydroperoxides
(LOOH) through the interaction of ROS with membrane PUFAs. LOOH
compounds are highly susceptible to decomposition, leading to the
formation of metabolically toxic by-products, MDA and 4-HNE, which
are closely associated with iron-induced cell death (31). Furthermore, ferrous iron plays a
crucial role as an initiator of ferroptosis (32). Ferrous iron can catalyze the
conversion of hydrogen peroxide into hydroxyl radicals, which
subsequently react with PUFAs to produce lipid peroxides, leading
to cellular membrane damage and the onset of ferroptosis.
Lipid peroxides and their degradation products are
controlled by intracellular antioxidant mechanisms (33). It has been found that the defense
systems against ferroptosis primarily consist of glutathione
(GSH)-dependent and GSH-independent antioxidant pathways. Reduced
glutathione serves as a substrate for glutathione peroxidase 4
(GPX4), which catalyzes the reduction of lipid peroxides to
non-toxic alcohol forms, thereby detoxifying these harmful
compounds. Ferroptosis suppressor protein 1 (FSP1) utilizes NADPH
to generate reduced coenzyme Q10, thereby shielding the cell
membrane from damage induced by lipid peroxides. These two
independent antioxidant defense systems exert stringent control
over lipid peroxidation levels. Erastin and icFSP1 are specific
inhibitors of both ferroptosis defense systems and have been used
in the treatment of various diseases (34,35).
The induction of lipid peroxidation-mediated
ferroptosis is critically influenced by three pivotal factors: The
generation of reactive species, the concentration of the
intracellular labile iron pool and the modulation of the
antioxidant defense system (Fig.
1). These pathways are governed by multiple factors, many of
which are transcriptionally regulated by AhR. For instance,
stearoyl-CoA desaturase 1 regulates the metabolism of unsaturated
fatty acids and reduces the oxidative vulnerability of PUFAs,
thereby promoting lipid peroxide formation (36). Heme oxygenase 1 (HMOX1) regulates
heme catabolism, releasing free iron that upregulates the levels of
labile iron pools within cells (37). Cyclooxygenase-2 (COX-2)
facilitates ROS production via multiple metabolic pathways,
inducing lipid peroxidation and ferroptosis (38). The SLC7A1/GPX4 system, a
GSH-dependent antioxidant mechanism, maintains intracellular redox
homeostasis by promoting the synthesis of reduced GSH (39). FSP1, an antioxidant system
independent of GSH, inhibits lipid peroxidation and ferroptosis by
consuming NADPH and promoting the reduction of ubiquinone Q10
(34). NFE2L2/NRF2 is a master
regulator of the cellular antioxidant response, promoting the
transcription of numerous downstream antioxidant-related genes
through binding to their antioxidant response elements (ARE)
(40). CYP1B1, a member of the
cytochrome P450 enzyme family, mediates metabolic processes that
generate ROS, contributing to lipid peroxidation and ferroptosis
(41). Other members of the
cytochrome P450 enzyme family, including CYP1A1 and CYP1A2, have
also been implicated in lipid peroxidation, although to the best of
our knowledge, no studies to date have demonstrated a direct link
between these enzymes and ferroptosis. Notably, the key factors
regulating lipid peroxidation and ferroptosis are either target
genes of AhR or are regulated by AhR target genes. This suggests
that AhR may serve as a crucial regulatory node for lipid
peroxidation and ferroptosis. Investigating the mechanisms by which
AhR modulates ferroptosis and identifying the potential targets of
AhR in this process holds significant promise for the development
of novel therapeutic agents and the treatment of associated
diseases.
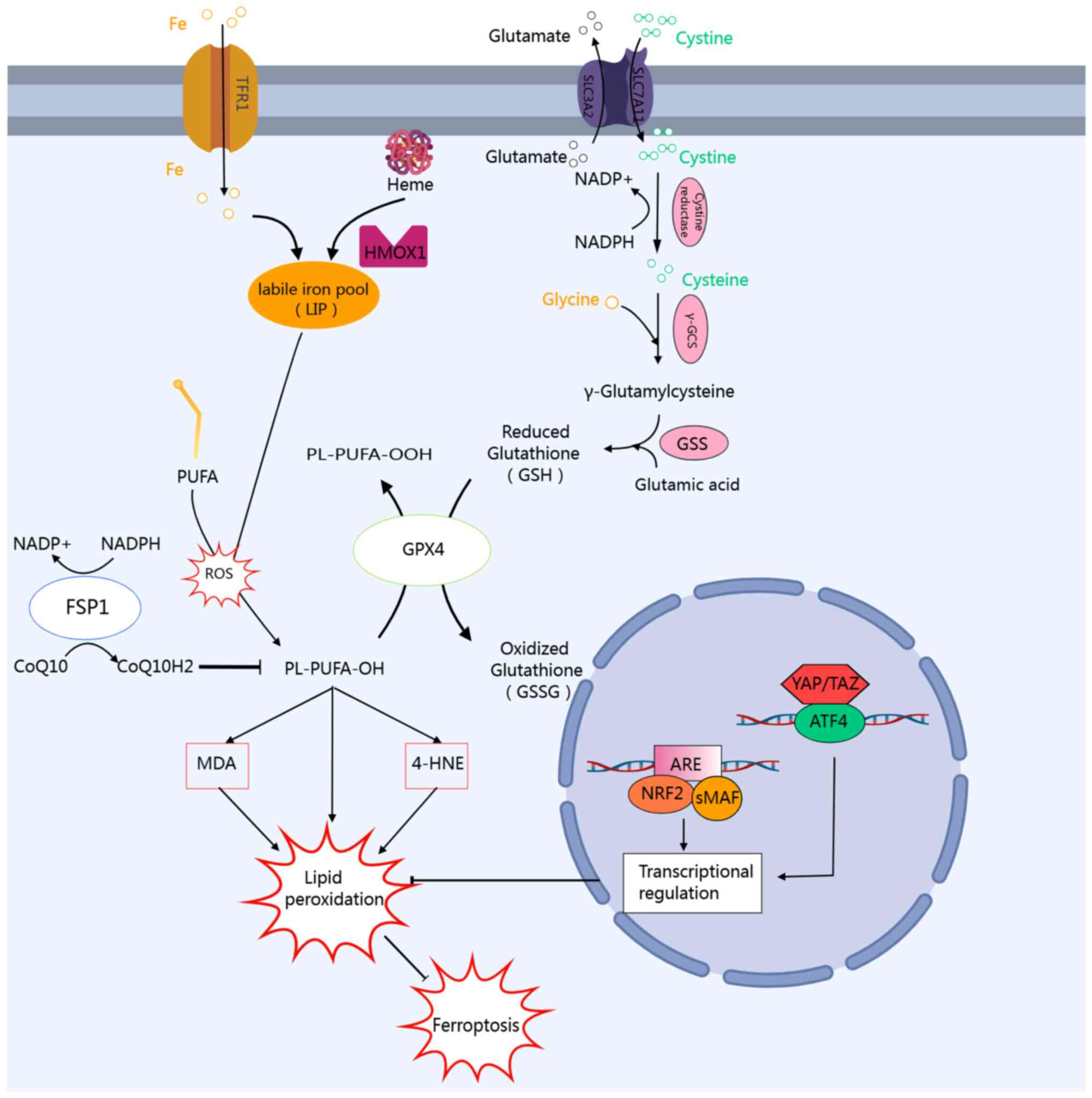 | Figure 1Molecular mechanisms and key
regulators of lipid peroxidation and ferroptosis. The initiation of
ferroptosis is primarily driven by the accumulation of labile iron
pools within cells, which originates from two main sources: Iron
uptake mediated by transferrin receptor 1 and free iron release
during heme metabolism catalyzed by heme oxygenase 1. In normal
cells, free iron, polyunsaturated fatty acids, and ROS generate
lipid hydroperoxides via the Fenton reaction. This process is
predominantly modulated by GSH-dependent and non-GSH-dependent
intracellular antioxidant systems. The GSH-dependent system
facilitates the uptake of cystine, an essential precursor for
reduced glutathione, through the SLC7A11/SLC3A2 isodimer, and
subsequently consumes reduced GSH via GPX to detoxify lipid
peroxides. FSP1, also known as AIFM2 or AMID, represents a recently
identified core inhibitor of ferroptosis. It regulates the redox
cycle of coenzyme Q10 through pathways independent of GSH and GPX4,
thereby forming a secondary defense mechanism against lipid
peroxidation. ATF4 and NRF2 function as the core transcription
factors enabling cells to respond to oxidative stress and metabolic
imbalance. Both ATF4 and NRF2 inhibit lipid peroxidation and confer
resistance to ferroptosis by regulating distinct target gene
networks. ROS, reactive oxygen species; GSH, glutathione; SLC7A11,
solute carrier family 7 member 11; GPX, glutathione peroxidase;
FSP1, ferroptosis suppressor protein 1; ATF4, activating
transcription factor 4; NRF2, nuclear factor erythroid 2-related
factor 2; CoQ10, coenzyme Q10; NADP+, nicotinamide
adenine dinucleotide phosphate; PUFA, polyunsaturated fatty acid;
MDA, malondialdehyde; 4-HNE, 4-hydroxynonenal; ARE, antioxidant
response element. |
AhR-mediated regulation of lipid
peroxidation and ferroptosis
AhR orchestrates lipid peroxidation and ferroptosis
through diverse molecular mechanisms, thereby critically
influencing the pathogenesis of multiple diseases. The AhR-mediated
regulation of lipoferroptosis includes the following: The
regulation of the gene expression levels of pro-oxidant and
antioxidant enzymes; the regulation of iron, heme and arachidonic
acid metabolism; and the regulation of the ferroptosis defense
system and synthesis of antioxidant compounds. Notably, AhR
activation plays a dual role in lipid peroxidation-ferroptosis
axis, with distinct regulatory effects depending on the specific
ligands (42). This mechanistic
plasticity, evidenced by ChIP-seq and microarray analyses across
different cell lineages and developmental stage (43-46), positions AhR as both a potential
therapeutic target and a challenge for precise pharmacological
intervention.
AhR mediates lipid peroxidation and
ferroptosis by regulating the transcription of pro-oxidant and
antioxidant enzymes AhR activation by pro-oxidative ligands induces
pro-oxidative enzymes expression, mediating lipid peroxidation and
ferroptosis
AhR is a ligand-dependent transcription factor that
can mediate both protective and pathological responses. Initially
identified due to its involvement in the toxicity of dioxins such
as TCDD, AhR predominantly resides in the cytoplasm in an inactive
state, complexed with chaperone proteins, including HSP90, AIP and
p23. Upon binding to TCDD, AhR translocates to the nucleus,
dimerizes with ARNT, and binds to XRE in target gene enhancer
regions (47). This classical
AhR/ARNT pathway upregulates the expression of CYP1 family members,
such as CYP1A1, CYP1A2 and CYP1B1 (48). These enzymes represent the most
extensively studied target genes of the AhR/ARNT complex of the
activation of the classic pathway of AhR. CYP450 enzymes, including
CYP1, CYP2 and CYP3, catalyze metabolic reactions that facilitate
the production of ROS (49). The
upregulation of CYP1A1 and CYP1B1 promote lipid peroxidation and
trigger ferroptosis, and both TCDD and benzo(a)pyrene promote lipid
peroxidation via this pathway (50), while the AhR antagonist,
CH223191, reverses this effect (51). Similarly, decabromodiphenyl ether
(BDE-209) induces lipid peroxidation via the AhR-CYP1A1-ROS pathway
(52).
Notably, AhR activation by natural ligands, such as
dietary indole and sulforaphane, as well as other low-affinity AhR
ligands, supports intestinal homeostasis and immune regulation.
However, in chronic inflammatory bowel disease, dysregulated AhR
signaling, driven by gut microbiota imbalances, exacerbates
oxidative stress, lipid peroxidation and iron deficiency in
intestinal epithelial lymphocytes, and this can potentially be
mitigated by specific AhR inhibitors (26). Moreover, in neurological and
hepatic contexts, butylphthalide mitigates traumatic brain
injury-induced ferroptosis by inhibiting the AhR-CYP1B1-ROS pathway
(53), while CYP1B1 drives
ferroptosis in hepatocellular and cholangiocarcinoma (41). Collectively, these findings
underscore the pivotal role of AhR in modulating lipid peroxidation
and ferroptosis through pro-oxidant enzyme regulation.
Antioxidant AhR ligands induce
antioxidant enzyme expression to regulate lipid peroxidation and
ferroptosis
While pro-oxidative ligands, such as TCDD and
benzo(a) pyrene induce lipid peroxidation via AhR activation,
emerging evidence highlights the dual role of AhR in redox
regulation. Natural exogenous AhR agonists, such as extracts from
artichoke (Cynara scolymus), soybean, fig tree and
Houttuynia cordata, mitigate ROS production, oxidative
stress and lipid peroxidation by regulation of antioxidant enzyme
target genes, such as NQO1, GST and UGT1A1 genes by AhR (54-56) (Fig. 2). Furthermore, the key
antioxidant stress transcription factor NRF2 serves as a direct
target for AhR signaling transduction. Notably, its promoter region
containing multiple XRE (57),
antioxidant enzymes such as NQO1, GST and UGT1A1 are also direct
target genes of NRF2. This indicates that AhR regulates the NRF2
gene and thereby promotes the transcription of antioxidant enzymes.
In summary, the activation or inhibition of AhR classic signaling
using appropriate AhR ligands under various disease conditions can
influence lipid peroxidation and ferroptosis levels by regulating
the synthesis of pro-oxidant and antioxidant enzymes.
![Oxidative and antioxidant AhR ligands
regulate lipid peroxidation and ferroptosis via an
oxidative-antioxidant bidirectional regulatory network. Exogenous
pro-oxidative AhR ligands, such as the toxins TCDD and
benzo[a]pyrene, bind to AhR, forming the AhR-ARNT complex that
translocates into the nucleus. This complex subsequently binds to
the XRE, initiating the transcription of various progenitor
enzymes, including CYP1A1, CYP1B1, CYP1A2, COX-2 and NOX, then
mediating ROS production, which in turn induces lipid peroxidation
and ferroptosis. Conversely, natural AhR ligands derived from plant
sources, such as snake tail grass, soybean, fig tree, and fish tail
grass, activate the transcription of antioxidant enzymes (e.g.,
NQO1, GST and UGT1A1) through AhR signaling. This activation
suppresses ROS production, thereby mitigating lipid peroxidation
and inhibiting ferroptosis. AhR, aryl hydrocarbon receptor; TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin; ARNT, AhR nuclear
translocator; NOX, nitrogen oxide; ROS, reactive oxygen species;
NQO1, NAD(P) H dehydrogenase [quinone] 1; GST, glutathione
S-transferase; UGT1A1, UDP-glucuronosyltransferase 1-1; HMOX1, heme
oxygenase 1; IDO1, indoleamine 2,3-dioxygenase 1; SLC7A11, solute
carrier family 7 member 11; COX-2, cyclooxygenase-2; ALDH1A3,
aldehyde dehydrogenase 1 family, member A3.](/article_images/ijmm/56/4/ijmm-56-04-05597-g01.jpg) | Figure 2Oxidative and antioxidant AhR ligands
regulate lipid peroxidation and ferroptosis via an
oxidative-antioxidant bidirectional regulatory network. Exogenous
pro-oxidative AhR ligands, such as the toxins TCDD and
benzo[a]pyrene, bind to AhR, forming the AhR-ARNT complex that
translocates into the nucleus. This complex subsequently binds to
the XRE, initiating the transcription of various progenitor
enzymes, including CYP1A1, CYP1B1, CYP1A2, COX-2 and NOX, then
mediating ROS production, which in turn induces lipid peroxidation
and ferroptosis. Conversely, natural AhR ligands derived from plant
sources, such as snake tail grass, soybean, fig tree, and fish tail
grass, activate the transcription of antioxidant enzymes (e.g.,
NQO1, GST and UGT1A1) through AhR signaling. This activation
suppresses ROS production, thereby mitigating lipid peroxidation
and inhibiting ferroptosis. AhR, aryl hydrocarbon receptor; TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin; ARNT, AhR nuclear
translocator; NOX, nitrogen oxide; ROS, reactive oxygen species;
NQO1, NAD(P) H dehydrogenase [quinone] 1; GST, glutathione
S-transferase; UGT1A1, UDP-glucuronosyltransferase 1-1; HMOX1, heme
oxygenase 1; IDO1, indoleamine 2,3-dioxygenase 1; SLC7A11, solute
carrier family 7 member 11; COX-2, cyclooxygenase-2; ALDH1A3,
aldehyde dehydrogenase 1 family, member A3. |
AhR regulates lipid peroxidation and
ferroptosis by modulating a range of metabolic pathways
Targeted lipid peroxidation and
ferroptosis affect the pathogenesis of I/R injury
I/R injury represents a condition wherein tissue
cells, following a period of ischemia, experience reperfusion,
leading to an accelerated exacerbation of tissue damage. This
pathological process, observed in multiple organs and diseases,
results in massive ROS production in I/R injury (58). During I/R injury, various
endogenous ligands of AhR, such as the tryptophan metabolite
kynurenine (Kyn), are generated to sustain AhR activation, leading
to CYP450-mediated ROS overproduction and exacerbating oxidative
stress (59). The restoration of
blood flow and subsequent reoxygenation during reperfusion
frequently result in reperfusion injury, a condition increasingly
recognized as being mediated by ferroptosis (60). Previous studies have indicated
that ferroptosis is more likely to occur during the reperfusion
phase rather than the ischemic phase in I/R injury (61). The endogenous mechanisms
responsible for scavenging ROS are compromised following I/R,
leading to ineffective ROS neutralization. I/R injury is
characterized by a series of intracellular events, including
excessive ROS production during reperfusion, lipid peroxidation and
elevated intracellular iron levels (62). Lipid peroxidation and oxidative
damage induced by ROS can result in cellular damage and death,
which aligns with the hallmarks of ferroptosis. Ferroptosis
inhibitors, such as Fer-1 and deferoxamine (DFO), can effectively
prevent or mitigate the progression of I/R injury when administered
prior to or during the I/R event. DFO is applied in coronary artery
bypass grafting procedures. The intravenous administration of DFO
has been shown to protect the myocardium from reperfusion injury
and mitigate lipid peroxidation (63).
Targeting AhR-mediated arachidonic
acid and heme metabolism to mitigate I/R injury
Emerging evidence highlights AhR as a crucial
regulator in I/R injury affecting the liver, kidneys and myocardium
(21-23,64). During the early phase of I/R
injury, oxidative stress stimulates the production of oxyindole, a
compound that has been demonstrated to be an effective AhR agonist
and significantly activates AhR (65). Moreover, under the oxidative
stress conditions associated with I/R injury, there is an increased
presence of endogenous pro-oxidative ligands for AhR (66). These multiple pro-oxidative
endogenous ligands may constitute a critical component in the
pathophysiology of I/R injury. In a previous study, in two distinct
mouse models of liver I/R injury, both the conventional ferroptosis
inhibitor DFO and the AhR antagonist CH223191 were found to be
effective in reversing ferroptosis and lipid peroxidation.
Moreover, CH223191 demonstrated superior efficacy compared with DFO
(67), suggesting that AhR
activation is closely related to I/R injury. HMOX1 and COX-2 are
pivotal enzymes in heme and arachidonic acid metabolism,
respectively. Transcriptomic analysis revealed that the
STAT3-HMOX1/COX-2 signaling pathway was inhibited following the
administration of CH223191 (67). The activation of HMOX1
facilitates the release of free iron ions from heme, consequently
elevating intracellular free iron levels (68). COX-2 also catalyzes the
production of ROS, leading to oxidative stress and lipid
peroxidation (38). Both HMOX1
and COX-2 genes play crucial roles in the induction of ferroptosis.
Previous studies have demonstrated that AhR signaling exerts direct
or indirect effects on the HMOX1/COX-2 signaling pathway via
multiple mechanisms (69-72),
and the AhR signaling pathway is intricately associated with the
metabolism of arachidonic acid and heme (73).
A previous study demonstrated that targeting
ferroptosis is a promising therapeutic approach to mitigate renal
tubular cell injury in acute tubular necrosis induced by I/R
(74). The administration of the
AhR antagonist, CH223191, or the ferroptosis inhibitor, α-col,
prior to the onset of reperfusion and reoxygenation injury has the
potential to prevent the production of ROS, reduce lipid
peroxidation and mitigate cellular iron toxicity. The underlying
mechanism may involve the transcriptional regulation of AhR through
indoleamine 2,3-dioxygenase (IDO)-mediated Kyn metabolism (21,66). The inhibition of AhR signaling
has been shown to mitigate reperfusion injury in a rat model of
cardiac I/R (23). Arachidonate
12/15-lipoxygenase-mediated arachidonic acid metabolism
significantly contributes to myocardial I/R injury (75). Therefore, arachidonic acid
metabolism mediated by AhR signaling may be a potential target for
alleviating myocardial I/R injury.
These studies demonstrate that AhR plays a crucial
role in I/R injury across various parenchymal organs. The
underlying mechanisms may be associated with the metabolism of
hemin and arachidonic acid. Oxidative stress induced by I/R injury
results in the activation of AhR through endogenous pro-oxidative
ligands, such as Kyn and oxyindole. IDO, as a key enzyme in
tryptophan metabolism, can produce many endogenous ligands of AhR.
IDO also serves as the target gene of AhR. Due to this particular
cyclic activation characteristic, it will easily cause the
continuous activation of AhR (76). Moreover, studies have shown that
IDO plays a key role in I/R injury. The pharmacological inhibition
of IDO by using 1-MT (IDO inhibitor) limits the production of Kyn,
inhibits the activation of AhR, and subsequently the production of
ROS. This effect can also be achieved by using the AhR antagonist,
CH223191 (66,77). This indicates that it is of
utmost importance to reduce the production of ROS induced by
arachidonic acid and heme metabolism mediated by the continuous
activation of AhR through targeting this circular pathway. The
overproduction of these pro-oxidant ligands during I/R injury may
contribute to continuous activation of AhR signaling. This
activation subsequently mediates oxidative stress and lipid
peroxidation, indicating that targeting AhR signaling may be a
promising therapeutic strategy for mitigating reperfusion
injury.
AhR affects lipid peroxidation and
ferroptosis by regulating the levels of antioxidant active
substances and ferroptosis defense system
In chronic kidney disease (CKD), AhR
activation by indoxyl sulfate (IS) suppresses the antioxidant
system via SLC7A11, and subsequently promotes lipid peroxidation
and induces ferroptosis
SLC7A11 is a critical component of the
cystine/glutamate antiporter, which is crucial for glutathione
synthesis, and it exerts a significant negative regulatory
influence on ferroptosis (78).
The SLC7A11-GPX4-GSH-cysteine axis serves as the pivotal hub in the
ferroptosis cascade (79). In
patients with CKD, the accumulation of IS, a metabolite of
tryptophan, as a result of impaired renal excretion, can result in
various health-related complications (80). In a previous study, in a mouse
model of CKD, IS induced ferroptosis in MC3T3-E1 cells by
activating the AhR signaling pathway and mediating the inhibition
of the SLC7A11-GPX4 antioxidant signaling pathway. This process
inhibited the osteogenic differentiation of MC3T3-E1 cells and
contributed to alveolar bone resorption in mice with CKD via
ferroptosis (81). The
inhibition of AhR activity can mitigate the osteotoxic effects
induced by IS. However, the exact mechanisms through which AhR
influences SLC7A11-GPX4 signaling to trigger ferroptosis in the
context of IS activation remain elusive. A plausible explanation
for this phenomenon is the interaction between AhR signaling and
HIF-1α signaling. In patients with CKD, HIF-1α signaling is
upregulated as a result of tissue hypoxia, which is caused by
diminished erythropoietin synthesis (82). Research has demonstrated that the
activation of HIF-1α signaling leads to the increased expression of
SLC7A11 (83,84). AhR competes with HIF-1α for
binding to ARNT, thereby competitively inhibiting the HIF-1α
signaling pathway. This inhibition of SLC7A11 expression, mediated
by the HIF-1α signaling pathway, may promote lipid peroxidation and
ferroptosis.
Indole-3-pyruvate (I3P)-activated AhR
upregulates SLC7A11 to suppress lipid peroxidation and ferroptosis
in both BEAS-2B and non-small cell lung cancer (NSCLC) cells
In contrast to the previously described induction of
ferroptosis by IS through the activation of the AhR-mediated
inhibition of the SLC7A11-GPX4 antioxidant signaling pathway, the
knockdown of AhR influences lipid peroxidation levels in both
BEAS-2B and NSCLC cells. AhR affects the transcriptional regulation
of SLC7A11. Notably, the pharmacological inhibition and genetic
knockdown of AhR in BEAS-2B cells markedly increases
erastin-induced ferroptosis. In PC9 cells, the promoter region of
SLC7A11 harbors an AhR binding site. Furthermore, a comprehensive
database search and analysis using JASPAR indicated that the
promoter region of SLC7A11 contains specific XREs. The study also
highlighted the role of I3P, an endogenous AhR agonist ligand and a
tryptophan analog, in the development of a novel AhR receptor. The
AhR-SLC7A11-GSH-GPX4 axis protects cells against ferroptosis via an
AhR-dependent mechanism (19,20). These findings demonstrate that
the activation of AhR signaling directly modulates the
transcriptional expression of the antioxidant defense system,
SLC7A11, thereby conferring resistance to ferroptosis in both
BEAS-2B and NSCLC cells.
The AhR signaling pathway indirectly
activates the SLC7A11 antioxidant system via activating
transcription factor 4 (ATF4), thereby enhancing ferroptosis
resistance in tumors
In addition to its direct influence on the
transcriptional expression of SLC7A11, AhR signaling also
indirectly transcriptionally regulates SLC7A11 by detecting
lysosomal cysteine levels and inducing the adaptive expression of
ATF4. ATF4 is crucial for cells adapting to adverse conditions of
amino acid deprivation and mediates a variety of cellular
phenotypic changes (85). The
study by Swanda et al (86) revealed that lysosomal cysteine
deficiency, sensed through the Kyn pathway of AhR, leads to
adaptive ATF4 expression, which subsequently enhances SLC7A11
transcription and renders cells less susceptible to ferroptosis.
The synthetic mRNA reagent, CysRx, was designed to convert
cytosolic cysteine into lysosomal cysteine, thereby sensitizing
tumor cells to ferroptosis by downregulating ATF4 expression. The
AhR inhibitor stemregenin (SR1) was used to induce ferroptosis in
cysteine-deficient cells, while the AhR activator indirubin fully
rescued these cells from ferroptosis (86). Notably, this pathway regulates
the transcriptional activity of ATF4 in the nucleus independently
of the conventional integrated stress response and oxidative stress
effects. These findings demonstrate that, apart from modulating
cellular sensitivity to ferroptosis through the regulation of
lysosomal cysteine levels, manipulating the AhR signaling pathway
to induce ferroptosis in cancer cells is a promising therapeutic
target.
Intestinal microbiota metabolites
activate AhR to confer ferroptosis resistance in intestinal tumors
through non-GSH antioxidant pathways
Gut commensal microbiota metabolites serve as a
significant source of AhR agonists (87). FICZ, Kyn, indirubin, indole and
I3P are endogenous ligands of AhR. Metabolites produced by the gut
microbiota can be categorized into two main groups:
Immunosuppressive metabolites, including short-chain fatty acids,
tryptophan metabolites and bile acid metabolites; inflammatory
metabolites, such as those activating the stimulator of interferon
genes pathway and lipopolysaccharides (88). Immunosuppressive metabolites
derived from the gut microbiota are inclined to inhibit ferroptosis
(89), whereas inflammatory
metabolites facilitate ferroptosis (90). Studies have shown that the
anaerobic gut bacterium Streptococcus gastro metabolizes
tryptophan to produce trans-3-indoleacrylic acid (IDA), which, upon
activating AhR, regulates the expression of aldehyde dehydrogenase
1 family, member A3 (ALDH1A3) by binding with a high affinity
region approximately 100 base pairs upstream of the ALDH1A3
transcription start site. This regulation occurs through
ARNT-mediated nuclear translocation, indicating that when AhR is
activated by IDA, it is recruited to the ALDH1A3 promoter (91). ALDH1A3, an aldehyde dehydrogenase
which uses retinaldehyde as its substrate, is predominantly
overexpressed in tumor cells (92), potentially due to the elevated
levels of redox metabolism observed in tumor cells. ALDH1A3 plays a
crucial role in the production of NADPH, which serves as the
primary electron donor essential for reduction reactions and
activates the classical FSP1, thereby inhibiting ferroptosis
(34). This indicates that the
intestinal microbiota metabolite IDA can enhance FSP1-mediated
synthesis of reduced coenzyme Q10 via the AhR-ALDH1A3 pathway,
leading to NADPH production, ferroptosis inhibition, and ultimately
promoting the progression of colorectal cancer (91).
Previously, transcriptomic analysis revealed a
significant association between AhR levels and erastin sensitivity
(17). Given the influence of
AhR activity on the sensitivity of various tumor cell lines to
erastin, and the role of erastin as a specific inhibitor of
SLC7A11, it was hypothesized that the bidirectional effect of AhR
signaling on SLC7A11 may be attributed to context-dependent
variations in AhR function. Investigating the association between
AhR and erastin across diverse tumor cell lines and precisely
modulating AhR activity to influence erastin-induced ferroptosis
sensitivity is an area worth further exploration. Pharmacologically
regulating AhR activity in conjunction with erastin to eradicate
tumor cells and delay tumor progression represents a promising
therapeutic strategy.
Potential applications of targeting
AhR-mediated lipid peroxidation and ferroptosis in cancer and I/R
injury therapy
Pharmacological modulation by inducing or inhibiting
ferroptosis is a key potential approach for addressing
drug-resistant cancers, ischemic organ damage, and a range of other
diseases characterized by extensive lipid peroxidation (29). Previous studies have indicated
that drug-resistant cancer cells, particularly those exhibiting a
mesenchymal phenotype and propensity for metastasis, are highly
vulnerable to ferroptosis (17,93). AhR modulates ferroptosis via
multiple pathways, functioning as a promoter in specific conditions
such as I/R injury and certain tumors (Fig. 3), while serving as a suppressor
in bronchial epithelial cells and other tumor types (Fig. 4). This dual regulatory role of
AhR in ferroptosis reflects its complex function. However, the role
of AhR as either a promoter or inhibitor of ferroptosis in numerous
diseases remains underexplored. A more comprehensive understanding
of the regulatory mechanisms of AhR in ferroptosis could pave the
way for novel therapeutic approaches and may provide profound
insight into the fundamental mechanisms underlying ferroptosis.
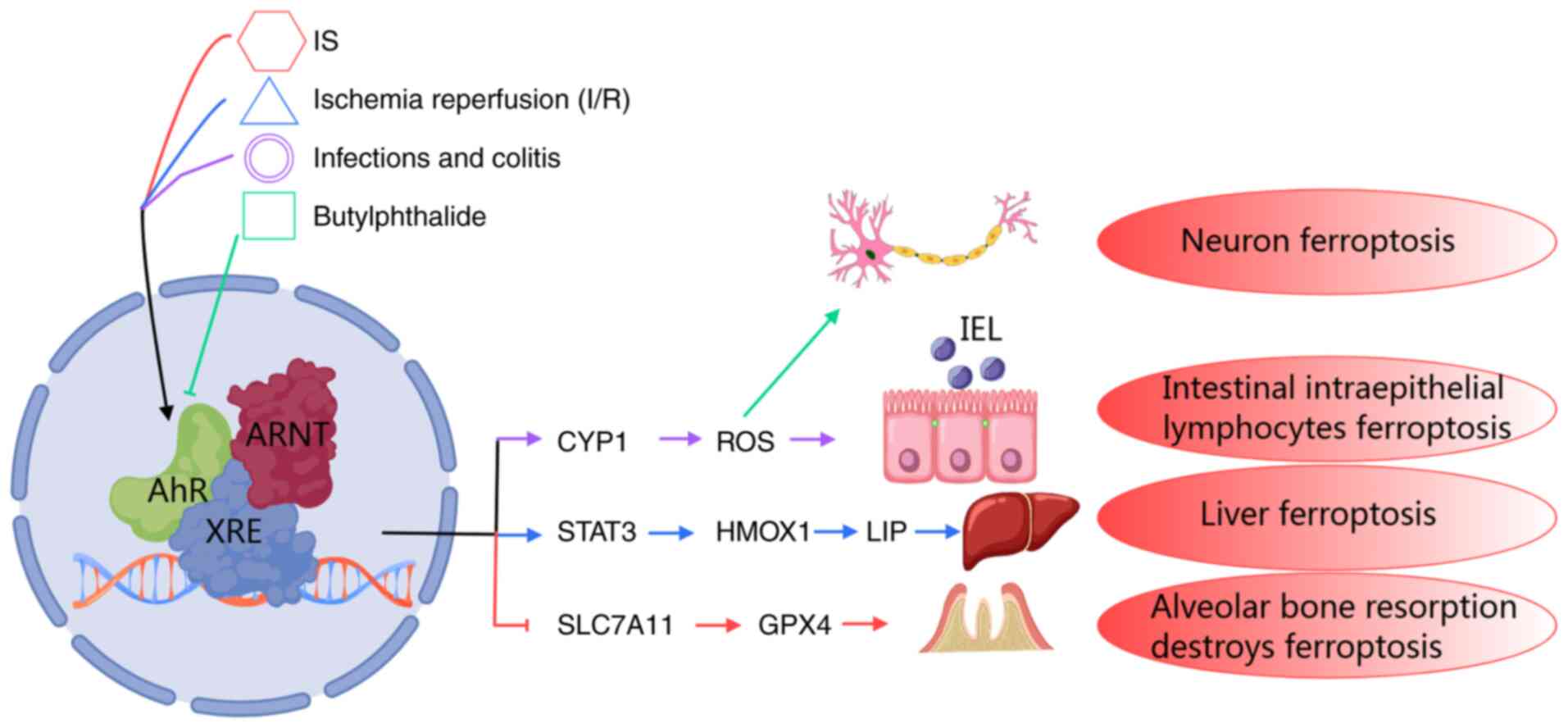 | Figure 3The mechanism of AhR mediating lipid
peroxidation-ferroptosis via CYP1, SLC7A11/GPX4 axis, or HMOX1 heme
metabolism by different ligands in different disease states. AhR
modulates the expression of diverse target genes across various
contexts, consequently influences ferroptosis in distinct disease
processes. IS triggers ferroptosis in MC3T3-E1 cells by activating
the SLC7A11/GPX4 signaling pathway via AhR. In renal tubular
epithelial cells and liver I/R injury, suppression of the AhR
signaling pathway mitigates the extent of damage. Under the
condition of oxidative stress of intestinal epithelial lymphocytes,
inhibition of AhR signaling can alleviate intestinal epithelial
lymphocytes ferroptosis by reducing ROS production. Butylphthalide
exerts an inhibit effect on neuronal ferroptosis in traumatic brain
injury by inhibiting the AhR-CYP1A-ROS axis. AhR, aryl hydrocarbon
receptor; SLC7A11, solute carrier family 7 member 11; GPX,
glutathione peroxidase; HMOX1, heme oxygenase 1; I/R,
ischemia/reperfusion; ROS, reactive oxygen species. |
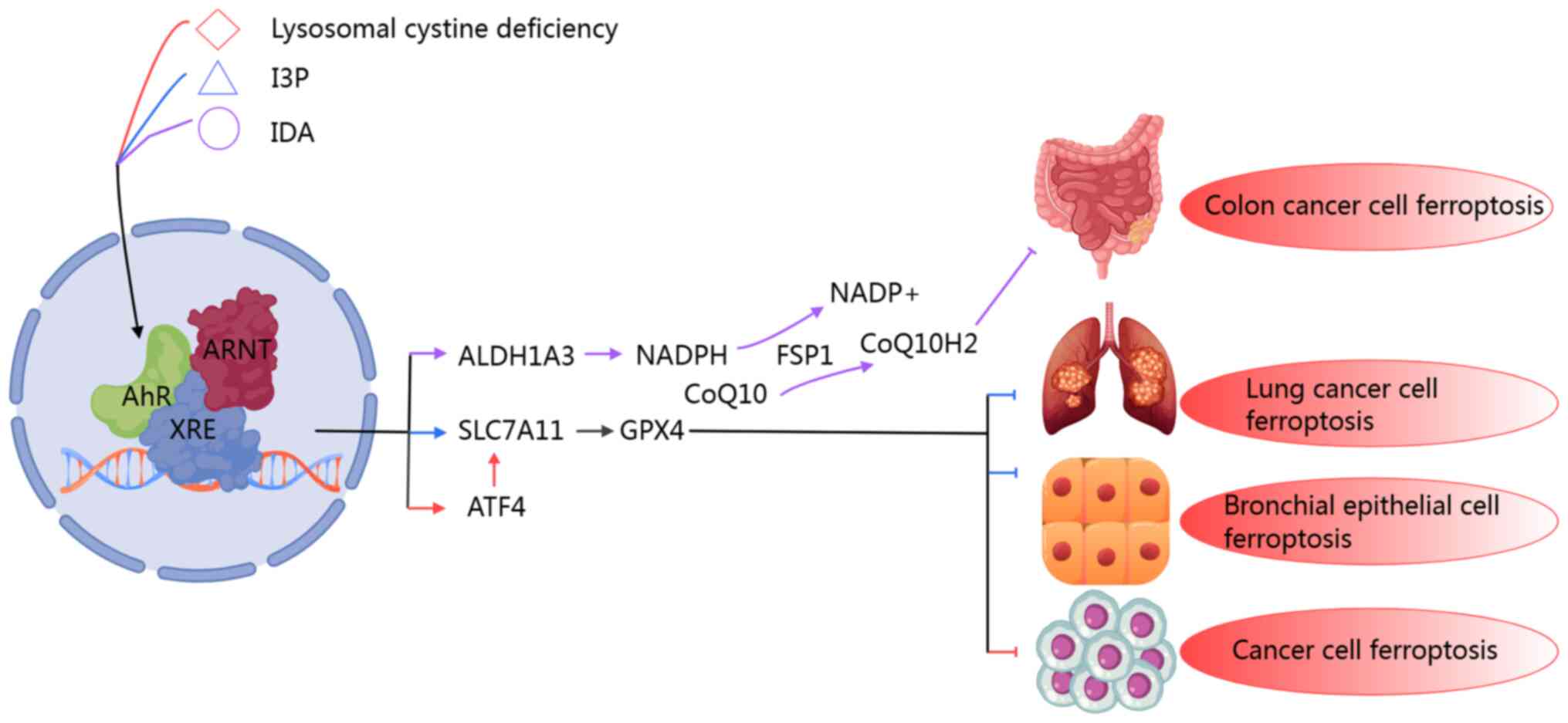 | Figure 4The mechanism of AhR mediating lipid
peroxidation-ferroptosis through the SLC7A11/GPX4 axis or the
ALDH1A3/FSP1 axis under different ligand binding or cytosolic
lysosomal cystine deficiency states. AhR modulates the expression
of diverse target genes across different contexts, thereby
influencing ferroptosis in various disease states. Specifically,
lysosomal cystine deficiency triggers ATF4 expression via the AhR
signaling pathway, leading to the activation of the SLC7A11/GPX4
antioxidant system, which in turn inhibits ferroptosis in tumors.
I3P inhibits ferroptosis by activating the AhR-mediated
SLC7A11/GPX4 axis antioxidant system in bronchial epithelial cells
and non-small cell lung cancer. In colorectal cancer, intestinal
metabolites regulate the IDA-AhR-ALDH1A3 pathway and affect the
level of NADPH to promote the production of FSP1-mediated reduced
ubiquinone, thereby inhibiting tumor cell ferroptosis and promoting
tumor progression. AhR, aryl hydrocarbon receptor; SLC7A11, solute
carrier family 7 member 11; ALDH1A3, aldehyde dehydrogenase 1
family, member A3; FSP1, ferroptosis suppressor protein 1; GPX,
glutathione peroxidase; IDA, trans-3-indoleacrylic acid; ATF4,
activating transcription factor 4; CoQ10, coenzyme Q10;
NADP+, nicotinamide adenine dinucleotide phosphate. |
Potential to mitigate hepatic, renal and
myocardial I/R injury by targeting the AhR-mediated metabolism of
arachidonic acid and heme
In models of I/R injury, ferroptosis is
predominantly observed during the reperfusion phase, rather than
the ischemic phase (94).
Consistent with these observations, AhR is reactivated during the
reperfusion phase of I/R injury, leading to an increase in
intracellular ROS production and lipid peroxidation (21). Following I/R, the antioxidant
system is significantly impaired, leading to the inefficient
scavenging of ROS. The reperfusion of ischemic tissue results in an
overproduction of ROS, which mediates I/R injury. Evidence suggests
that I/R injury is associated with multiple cellular events,
including excessive ROS production (62), lipid peroxidation, and increased
intracellular iron concentrations (95). Lipid peroxidation and the
generation of ROS contribute to cellular damage and mortality
(21). These phenomena align
with the characteristics of iron-dependent ferroptosis, and studies
in both experimental models and clinical settings have demonstrated
that iron chelators can mitigate I/R injury by inhibiting the
initiation and progression of ferroptosis (21,63,66).
DFO, a widely used non-toxic iron chelator, has been
shown to suppress lipid peroxidation-induced ferroptosis across
diverse conditions. Beyond its role in inhibiting AhR, as detailed
in the preceding section, the AhR antagonist, CH223191, has proven
effective in reducing I/R injury in multiple organ systems,
including the liver, kidneys and myocardium, as evidenced in
various animal models (21,66,77). Previous studies have demonstrated
that AhR is closely related to arachidonic acid metabolism, and
various AhR ligands can regulate arachidonic acid metabolism in
endothelial cells, hepatocytes, and cardiomyocytes (96-98). Moreover, the COX-2 pathway,
lipoxygenase (LOX) pathway and CYP450 pathway in arachidonic acid
metabolism play key roles in I/R injury (99) and are potential targets for the
AhR-mediated relief of I/R injury. Additionally, heme metabolism is
closely related to AhR, such as in I/R injury, atherosclerosis, and
liver poisoning, and AhR can affect their occurrence and
development through iron and heme metabolism (72,100). As a transcriptional regulator,
AhR may also modulate ferroptosis via multiple potential pathways.
AhR may alleviate I/R injury through ROS production mediated by
CYP450 and COX-2 arachidonic acid metabolism, iron/heme metabolism
mediated by STAT3/6 and HMOX1 and Kyn metabolism mediated by AhR.
Notably, some studies have demonstrated that the application of
CH223191 in hepatic I/R injury is more effective than DFO in
alleviating hepatocyte I/R injury and ferroptosis (21,66,67). These findings suggest the
feasibility of targeting the AhR signaling pathway with specific
inhibitors to ameliorate I/R injury in parenchymal organs.
Inhibiting tumor progression by targeting
the AhR-mediated CYP450/ROS axis, ferroptosis antioxidant defense
system, heme metabolism and epithelial-mesenchymal transition
(EMT)
Ferroptosis exerts a dual effect on tumor biology;
targeting ferroptosis is a promising direct strategy for cancer
cell eradication, while ferroptosis simultaneously contributes to
tumor progression and immune response suppression (101). Similarly, AhR exhibits
intricate roles in tumorigenesis and cancer therapy, exhibiting
both pro-tumor and anti-tumor effects (102). The activation of AhR is also
closely associated with tumor immunity (103), and these effects are contingent
upon the specific tumor type and the involved ligand (18,91). In ferroptosis in cancer cells,
AhR exhibits various effects, potentially associated with varying
activation levels of the downstream transcription of AhR in
distinct cell lines. For example, it was demonstrated that the
activation level of CYP1A1 in the H358 lung cancer cell line was
markedly higher compared with that in the Calu1 cell line. The
disparity in the downstream transcriptional response of AhR to the
same ligand across different tumor cell lines is likely a critical
factor of the differential roles of AhR in ferroptosis within these
cells (17). The mechanisms by
which AhR inhibits tumor ferroptosis include the modulation of the
SLC7A11/GPX4 axis and the enhancement of reduced NADPH
production.
It has been shown that AhR-mediated CYP450
influences the extent of ferroptosis in neurons following traumatic
brain injury (53). The
stabilization of CYP1B1 protein may also play a crucial role in
regulating ferroptosis in hepatocellular carcinoma and
cholangiocarcinoma (41).
However, to the best of our knowledge, there is currently no direct
evidence demonstrating that AhR promotes ferroptosis in tumor cells
via the transcriptional regulation of CYP450. Moreover, AhR
activation in certain tumor cells has been hypothesized to be
associated with a metastatic mesenchymal state, characterized by
both metastatic and immunosuppressive properties (104). Mesenchyma-like tumor cells
exhibit heightened sensitivity to ferroptosis inducers, which
aligns with the dual role of ferroptosis in tumor biology. The AhR
signaling pathway is intricately linked to heme and iron metabolism
mediated by HMOX1. Previous research has demonstrated that tumor
cells overexpressing AhR exhibit elevated basal levels of HMOX1
compared with wild-type cells (59), and a high HMOX1-mediated increase
in the free iron pool level is a key mediator for the triggering of
ferroptosis. A reasonable explanation for the close association
between AhR and heme metabolism is that AhR signaling may
indirectly mediate heme metabolism through the NRF2 signaling
pathway. CYP450, EMT and heme metabolism may serve as key
mechanisms underlying AhR-mediated ferroptosis in tumor cells.
The majority of drugs known to induce ferroptosis,
such as sorafenib, sulfadiazine, FIN and 3-phenylquinazolinone, are
systemic inhibitors of xCT, GPX4 and FSP1, which are regulated by
AhR (19,81,88). Therefore, the modulation of AhR
levels is anticipated to substantially enhance the efficacy of
ferroptosis-inducing drugs. Both AhR gene silencing and stimulation
with the exogenous ligand TCDD can induce ferroptosis and increase
the sensitivity of tumor cells to erastin (17,18). Consequently, the strategic use of
efficient AhR ligands, either alone or in combination with
ferroptosis inducers, is a viable strategy for triggering
ferroptosis in tumor cells.
The AhR agonists and inhibitors currently used in in
cancer therapy include aminoflavone, ITE and SR1 (10,11,14,105). These compounds exhibit notable
tumor-suppressive effects. The antitumor mechanism of ITE involves
restoring tumor cell sensitivity to sorafenib. The antitumor
effects of SR1 and aminoflavonone are partially mediated by ROS.
These mechanisms may be facilitated through various potential
pathways, such as those involving AhR-mediated CYP450, SLC7A11 and
EMT. The application of these highly potent AhR ligands to modulate
AhR signaling in conjunction with ferroptosis-inducing drugs is
anticipated to yield enhanced antitumor efficacy.
Synopsis
AhR modulation combined with ferroptosis
inducers triggers lipid peroxidation and ferroptosis in tumor
cells
AhR-mediated lipid peroxidation-ferroptosis plays a
critical role in various diseases, particularly in tumors and I/R
injury. Silencing the AhR gene in NSCLC A549 cells was previously
shown to enhance the antitumor efficacy of erastin (17). Another study elucidated the
underlying mechanism, revealing that the activation of AhR in A549
cells mediates the transcription of SLC7A11, a well-established
target of the antitumor activity of erastin (20). It is notable that the lung cancer
cell line, A549, with the silencing of the AhR gene, promotes the
antitumor effect of erastin (19). Conversely, in the lung cancer
cell line, Calu1, the silencing of the AhR gene inhibited the
antitumor effect of erastin (17). The underlying mechanisms for
these discrepancies may resemble those observed in immune cells,
where AhR preferentially binds to the IL-6 or IL-22 promoter. In
hepatocytes, AhR is more inclined to promote the transcription of
the CYP450 enzyme system. These divergent outcomes may be
attributed to variations in AhR reactivity towards target genes
across different cell lines and within different states of the same
cell line. Such differences may involve multiple factors, including
unsaturated fatty acid anabolism, EMT, heme/iron metabolism,
transcriptional regulation of oxidative antioxidant enzymes and
GSH-dependent/independent ferroptosis defense mechanisms. Moreover,
the application of specific AhR agonists and inhibitors, including
aminoflavone, ITE, SR1 and CH223191, in conjunction with
ferroptosis inducers, is anticipated to augment the antitumor
efficacy against targeted tumor cells.
Targeting AhR signaling via specific AhR
ligands to control lipid peroxidation and ferroptosis in oxidative
stress-driven diseases
While AhR plays a dual role in mediating ferroptosis
in cancer cells, it mainly acts as a pro-oxidant to promote
ferroptosis induced by lipid peroxidation in disease models, such
as I/R injury, CKD and colitis. This phenomenon may be attributed
to the pro-oxidative nature of the predominant AhR ligands in these
contexts, including oxyindole, IS, TCDD and dioxins. Conversely,
the antioxidant AhR ligand I3P maintains redox homeostasis by
activating the AhR-mediated GSH-dependent ferroptosis defense
system in BEAS-2B cells (20).
I3P also exhibits antioxidant properties across various disease
types (106). Similarly, ILA,
another antioxidant AhR agonist ligand, has also been shown to
exert an inhibitory effect on ferroptosis (107). These observations suggest that
AhR-mediated lipid peroxidation-ferroptosis in non-neoplastic
conditions may primarily depend on the specific ligand involved.
During I/R injury, oxidative stress activates the tryptophan
metabolic pathway, generating numerous endogenous AhR ligands, such
as Kyn and FICZ. Kyn has been shown to promote tumor progression by
inhibiting ferroptosis in an AhR-dependent manner in glioblastoma
multiforme and lung cancer A549 cell lines (20,108). However, Kyn also demonstrates
context-dependent effects: It induces ferroptosis in natural killer
cells within the gastric cancer microenvironment independently of
AhR, while inhibiting ferroptosis in Hela cells also via an
AhR-independent mechanism (89,109). Notably, the key enzyme IDO
produced by Kyn is the target gene of AhR, indicating that AhR is a
central molecule mediating oxidative stress and affecting
ferroptosis pathways (76). This
highlights the complex and multifaceted association between AhR and
ferroptosis, requiring the consideration of multiple factors.
Targeting AhR signaling with specific ligands presents a potential
therapeutic strategy across various diseases. A comprehensive
ligand-AhR-disease database could guide this approach.
Therapeutic potential of novel AhR
ligands: Mitigating toxicity through natural derivatives and
engineered synthetics
Numerous ligands have demonstrated antitumor
efficacy in cellular experiments or therapeutic effects in animal
models. However, well-known AhR ligands, such as TCDD and
benzo[a]pyrene may induce adverse effects. At present, a number of
low-toxicity or non-toxic AhR ligands are under investigation and
development, including indoles and flavonoids derived from natural
plant metabolites, which are characterized by their favorable
safety profiles. For instance, indole-3-carbinol exhibits both low
toxicity and anti-cancer properties. As previously mentioned, a
number of tryptophan derivatives/metabolites serve as endogenous
AhR ligands (87). Recent
findings indicate their potential in treating various inflammatory
bowel diseases, although excessive levels may concurrently promote
intestinal tumor progression (91).
Given the potent toxicity of traditional AhR ligands
such as TCDD and the potential limitations of low-affinity natural
ligands, synthesis AhR ligands represent a promising avenue.
Compounds such as 6,2′,4′-trimethoxyflavone (TMF) retain AhR
activation capabilities, but eliminate the chlorine atoms present
in TCDD, reducing abnormal signaling. TMF also mimics structural
features of endogenous ligands, such as the indole/carbazole
scaffold of tryptophan metabolites, to enhance compatibility and
incorporates formyl groups to facilitate metabolism and prevent
long-term cellular accumulation. TMF has been shown to exert
antitumor effects in liver cancer models (110). Similarly, YH439, a synthetic
low-toxicity AhR ligand, demonstrated efficacy in alleviating
hepatic fibrosis (111).
3,3′-diindolylmethane, a low-toxicity phytochemical from
cruciferous vegetables, exhibits anti-hepatocellular carcinoma
properties and can induce ferroptosis in hepatocytes (112). These findings underscore the
substantial potential of both synthetic AhR ligands and natural
plant-derived alternatives (113). Systematic investigations to
discover or synthesize clinically applicable AhR ligands are highly
promising. Furthermore, in diseases such as intestinal disorders,
modulating the gut microbiota to regulate the production of
endogenous AhR ligands provides an alternative therapeutic strategy
worthy of attention.
In conclusion, the present review underscores the
pivotal role of AhR in mediating disease onset and progression
through ferroptosis across various diseases. Pharmacological
inhibition of AhR signaling, particularly in I/R injury, can
mitigate tissue damage. The pharmacological modulation of AhR
signaling in specific tumor cell lines, either through activation
or inhibition, can induce lipid peroxidation-ferroptosis directly
or in combination with ferroptosis inducers, which provides a
biological foundation for a promising therapeutic strategy against
tumor progression (Fig. 5).
Currently, research on AhR-regulated lipid peroxidation and
ferroptosis in diseases is limited, with the majority of studies
focusing on I/R injury in tumors or various organs. Exploring the
role of AhR in lipid peroxidation and ferroptosis across different
diseases and tissues, and implementing targeted regulation using
natural low-toxicity or synthetic AhR ligands, holds substantial
clinical potential.
![Pharmacological targeting of AhR to
modulate ferroptosis in cancer and I/R injury. AhR ligands regulate
lipid peroxidation and ferroptosis through different molecular
axes, demonstrating environment-dependent therapeutic potential. In
parenchymal organs, such as the heart, liver and kidneys, AhR
antagonists CH223191 and SR1 alleviate I/R injury by inhibiting the
iron-stimulating pathway: CH223191 downregulates CYP1A-driven ROS
amplification and COX-2-mediated inflammatory signals, while SR1
inhibits HMOX1-dependent iron release and IDO1-induced oxidative
stress, jointly maintaining the redox balance of cells. In tumors,
AhR is pharmacologically regulated through AhR ligands, such as
aminoflavone, ITE, SR1 and CH223191, thereby influencing key
molecules in various lipid peroxidation and ferroptosis processes,
and further inducing ferroptosis in tumor cells or enhancing their
sensitivity to erastin. This is expected to eliminate tumor cells,
alleviate tumor progression and enhance the anti-tumor efficacy of
iron deposition inducers. AhR, aryl hydrocarbon receptor; HMOX1,
heme oxygenase 1; IDO1, indoleamine 2,3-dioxygenase 1; ROS,
reactive oxygen species; COX-2, cyclooxygenase-2; TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin; NQO1, NAD(P)H dehydrogenase
[quinone] 1; GST, glutathione S-transferase; UGT1A1,
UDP-glucuronosyltransferase 1-1.](/article_images/ijmm/56/4/ijmm-56-04-05597-g04.jpg) | Figure 5Pharmacological targeting of AhR to
modulate ferroptosis in cancer and I/R injury. AhR ligands regulate
lipid peroxidation and ferroptosis through different molecular
axes, demonstrating environment-dependent therapeutic potential. In
parenchymal organs, such as the heart, liver and kidneys, AhR
antagonists CH223191 and SR1 alleviate I/R injury by inhibiting the
iron-stimulating pathway: CH223191 downregulates CYP1A-driven ROS
amplification and COX-2-mediated inflammatory signals, while SR1
inhibits HMOX1-dependent iron release and IDO1-induced oxidative
stress, jointly maintaining the redox balance of cells. In tumors,
AhR is pharmacologically regulated through AhR ligands, such as
aminoflavone, ITE, SR1 and CH223191, thereby influencing key
molecules in various lipid peroxidation and ferroptosis processes,
and further inducing ferroptosis in tumor cells or enhancing their
sensitivity to erastin. This is expected to eliminate tumor cells,
alleviate tumor progression and enhance the anti-tumor efficacy of
iron deposition inducers. AhR, aryl hydrocarbon receptor; HMOX1,
heme oxygenase 1; IDO1, indoleamine 2,3-dioxygenase 1; ROS,
reactive oxygen species; COX-2, cyclooxygenase-2; TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin; NQO1, NAD(P)H dehydrogenase
[quinone] 1; GST, glutathione S-transferase; UGT1A1,
UDP-glucuronosyltransferase 1-1. |
Conclusion and future perspectives
Current research demonstrates that AhR target genes
regulate numerous key genes and mediators involved in ferroptosis,
encompassing cellular antioxidant systems, oxidative/antioxidant
enzymes, heme/iron metabolism and the arachidonic acid pathway.
Notably, AhR can regulate the SLC7A11-GPX4 axis, a pivotal
suppressor of ferroptosis. Moreover, AhR modulates ferroptosis
through the GSH-independent FSP1 pathway. Consequently, targeting
AhR represents a promising therapeutic strategy for diseases driven
by lipid peroxidation and ferroptosis, such as cancer and I/R
injury.
Availability of data and materials
Not applicable.
Authors' contributions
ZL, WL and XS designed the study. ZL completed the
first draft of this manuscript. ZL, MH, SH and YZ collected the
data from the literature and revised the manuscript. XS, WL and ZL
designed the figures. YZ, WL and XS obtained funding. All authors
have read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the high-level talents
scientific research start-up funds of the Affliated Hospital of
Guangdong Medical University (grant no. GCC2022023) and the
Zhanjiang Science and Technology Project (grant no.
2023A20709).
References
|
1
|
Ferreira MJ, Rodrigues TA, Pedrosa AG,
Gales L, Salvador A, Francisco T and Azevedo JE: The mammalian
peroxisomal membrane is permeable to both GSH and GSSG-Implications
for intraperoxisomal redox homeostasis. Redox Biol. 63:1027642023.
View Article : Google Scholar
|
|
2
|
Fujiki Y, Abe Y, Imoto Y, Tanaka AJ,
Okumoto K, Honsho M, Tamura S, Miyata N, Yamashita T, Chung WK and
Kuroiwa T: Recent insights into peroxisome biogenesis and
associated diseases. J Cell Sci. 133:jcs2369432020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agmon E, Solon J, Bassereau P and
Stockwell BR: Modeling the effects of lipid peroxidation during
ferroptosis on membrane properties. Sci Rep. 8:51552018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jambunathan N: Determination and detection
of reactive oxygen species (ROS), lipid peroxidation, and
electrolyte leakage in plants. Methods Mol Biol. 639:292–298.
2010.PubMed/NCBI
|
|
5
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grishanova AY and Perepechaeva ML: Aryl
hydrocarbon receptor in oxidative stress as a double agent and its
biological and therapeutic significance. Int J Mol Sci.
23:67192022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sondermann NC, Faßbender S, Hartung F,
Hätälä AM, Rolfes KM, Vogel CFA and Haarmann-Stemmann T: Functions
of the aryl hydrocarbon receptor (AHR) beyond the canonical
AHR/ARNT signaling pathway. Biochem Pharmacol. 208:1153712023.
View Article : Google Scholar :
|
|
8
|
Hu DG, Marri S, McKinnon RA, Mackenzie PI
and Meech R: Deregulation of the genes that are involved in drug
absorption, distribution, metabolism, and excretion in
hepatocellular carcinoma. J Pharmacol Exp Ther. 368:363–381. 2019.
View Article : Google Scholar
|
|
9
|
Martins M, Santos JM, Diniz MS, Ferreira
AM, Costa MH and Costa PM: Effects of carcinogenic versus
non-carcinogenic AHR-active PAHs and their mixtures: Lessons from
ecological relevance. Environ Res. 138:101–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brantley E, Callero MA, Berardi DE,
Campbell P, Rowland L, Zylstra D, Amis L, Yee M, Simian M, Todaro
L, et al: AhR ligand Aminoflavone inhibits α6-integrin expression
and breast cancer sphere-initiating capacity. Cancer Lett.
376:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McLean L, Soto U, Agama K, Francis J,
Jimenez R, Sowers L and Brantley E: Aminoflavone induces oxidative
DNA damage and reactive oxidative species-mediated apoptosis in
breast cancer cells. Int J Cancer. 122:1665–1674. 2008. View Article : Google Scholar
|
|
12
|
Elson DJ, Nguyen BD, Korjeff NA, Wilferd
SF, Sanvicens VP, Jang HS, Bernales S, Chakravarty S, Belmar S,
Ureta G, et al: Suppression of Ah Receptor (AhR) increases the
aggressiveness of TNBC cells and 11-Cl-BBQ-activated AhR inhibits
their growth. Biochem Pharmacol. 215:1157062023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kober C, Roewe J, Schmees N, Roese L,
Roehn U, Bader B, Stoeckigt D, Prinz F, Gorjánácz M, Roider HG, et
al: Targeting the aryl hydrocarbon receptor (AhR) with BAY 2416964:
A selective small molecule inhibitor for cancer immunotherapy. J
Immunother Cancer. 11:e0074952023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, He B, Chen E, Lu J, Wang J, Cao H
and Li L: The aryl hydrocarbon receptor ligand ITE inhibits cell
proliferation and migration and enhances sensitivity to
drug-resistance in hepatocellular carcinoma. J Cell Physiol.
236:178–192. 2021. View Article : Google Scholar
|
|
15
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M,
Ding HF, Zhang J, Wang H, Chen X and Yan C: ATF3 promotes
erastin-induced ferroptosis by suppressing system Xc. Cell Death
Differ. 27:662–675. 2020. View Article : Google Scholar
|
|
17
|
Kwon OS, Kwon EJ, Kong HJ, Choi JY, Kim
YJ, Lee EW, Kim W, Lee H and Cha HJ: Systematic identification of a
nuclear receptor-enriched predictive signature for erastin-induced
ferroptosis. Redox Biol. 37:1017192020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Yao Y, Gong G, He T, Ma C and Yu
J: The potential role of AhR/NR4A1 in androgen-dependent prostate
cancer: Focus on TCDD-induced ferroptosis. Biochem Cell Biol.
103:1–11. 2025. View Article : Google Scholar
|
|
19
|
Kou Z, Tran F, Colon T, Shteynfeld Y, Noh
S, Chen F, Choi BH and Dai W: AhR signaling modulates ferroptosis
by regulating SLC7A11 expression. Toxicol Appl Pharmacol.
486:1169362024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng Y, Ouyang L, Zhou Y, Lai W, Chen Y,
Wang Z, Yan B, Zhang Z, Zhou Y, Peng X, et al: AhR promotes the
development of non-small cell lung cancer by inducing
SLC7A11-dependent antioxidant function. J Cancer. 14:821–834. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eleftheriadis T, Pissas G, Filippidis G,
Liakopoulos V and Stefanidis I: Reoxygenation induces reactive
oxygen species production and ferroptosis in renal tubular
epithelial ; cells by activating aryl hydrocarbon
receptor. Mol Med Rep. 23:412021.
|
|
22
|
Kwon JI, Heo H, Chae YJ, Min J, Lee DW,
Kim ST, Choi MY, Sung YS, Kim KW, Choi Y, et al: Is aryl
hydrocarbon receptor antagonism after ischemia effective in
alleviating acute hepatic ischemia-reperfusion injury in rats?
Heliyon. 9:e155962023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Wang B, Yuan S, Xue K, Zhang J and
Xu A: Blocking the Aryl hydrocarbon receptor alleviates myocardial
ischemia/reperfusion injury in rats. Curr Med Sci. 42:966–973.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma C, Han L, Zhao W, Chen F, Huang R, Pang
CH, Zhu Z and Pan G: Targeting AhR suppresses hepatocyte
ferroptosis in MASH by regulating the Pten/Akt/β catenin axis.
Biochem Pharmacol. 232:1167112025. View Article : Google Scholar
|
|
25
|
Qian C, Yang C, Lu M, Bao J, Shen H, Deng
B, Li S, Li W, Zhang M and Cao C: Activating AhR alleviates
cognitive deficits of Alzheimer's disease model mice by
upregulating endogenous Aβ catabolic enzyme Neprilysin.
Theranostics. 11:8797–8812. 2021. View Article : Google Scholar :
|
|
26
|
Panda SK, Peng V, Sudan R, Ulezko Antonova
A, Di Luccia B, Ohara TE, Fachi JL, Grajales-Reyes GE, Jaeger N,
Trsan T, et al: Repression of the aryl-hydrocarbon receptor
prevents oxidative stress and ferroptosis of intestinal
intraepithelial lymphocytes. Immunity. 56:797–812.e4. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Zhang C, Pan H, Gao X, Wang X, Xiao
W, Yan S, Gao Y, Fu J and Zhou Y: Indeno[1,2,3-cd]pyrene enhances
the sensitivity of airway epithelial cells to ferroptosis and
aggravates asthma. Chemosphere. 363:1428852024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avilla MN, Malecki KMC, Hahn ME, Wilson RH
and Bradfield CA: The Ah receptor: Adaptive metabolism, ligand
diversity, and the xenokine model. Chem Res Toxicol. 33:860–879.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi
AA and Lei P: Ferroptosis: Mechanisms and links with diseases.
Signal Transduct Target Ther. 6:492021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar
|
|
31
|
Ramana KV, Srivastava S and Singhal SS:
Lipid peroxidation products in human health and disease 2016. Oxid
Med Cell Longev. 2017:21632852017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Y, Ran L, Yang Y, Gao X, Peng M, Liu S,
Sun L, Wan J, Wang Y, Yang K, et al: Deferasirox alleviates
DSS-induced ulcerative colitis in mice by inhibiting ferroptosis
and improving intestinal microbiota. Life Sci. 314:1213122023.
View Article : Google Scholar
|
|
33
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2022. View Article : Google Scholar
|
|
34
|
Nakamura T, Hipp C, Santos Dias Mourão A,
Borggräfe J, Aldrovandi M, Henkelmann B, Wanninger J, Mishima E,
Lytton E, Emler D, et al: Phase separation of FSP1 promotes
ferroptosis. Nature. 619:371–377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Tan H, Daniels JD, Zandkarimi F,
Liu H, Brown LM, Uchida K, O'Connor OA and Stockwell BR: Imidazole
ketone erastin induces ferroptosis and slows tumor growth in a
mouse lymphoma model. Cell Chem Biol. 26:623–633.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xuan Y, Wang H, Yung MM, Chen F, Chan WS,
Chan YS, Tsui SK, Ngan HY, Chan KK and Chan DW: SCD1/FADS2 fatty
acid desaturases equipoise lipid metabolic activity and
redox-driven ferroptosis in ascites-derived ovarian cancer cells.
Theranostics. 12:3534–3552. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu D, Hu Q, Wang Y, Jin M, Tao Z and Wan
J: Identification of HMOX1 as a critical ferroptosis-related gene
in atherosclerosis. Front Cardiovasc Med. 9:8336422022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adham AN, Abdelfatah S, Naqishbandi AM,
Mahmoud N and Efferth T: Cytotoxicity of apigenin toward multiple
myeloma cell lines and suppression of iNOS and COX-2 expression in
STAT1-transfected HEK293 cells. Phytomedicine. 80:1533712021.
View Article : Google Scholar
|
|
39
|
He F, Zhang P, Liu J, Wang R, Kaufman RJ,
Yaden BC and Karin M: ATF4 suppresses hepatocarcinogenesis by
inducing SLC7A11 (xCT) to block stress-related ferroptosis. J
Hepatol. 79:362–377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu C, Zhang C, Wu H, Zhao Z, Wang Z,
Zhang X, Yang J, Yu W, Lian Z, Gao M and Zhou L: The
AKR1C1-CYP1B1-cAMP signaling axis controls tumorigenicity and
ferroptosis susceptibility of extrahepatic cholangiocarcinoma. Cell
Death Differ. 32:506–520. 2025. View Article : Google Scholar
|
|
42
|
Apetoh L, Quintana FJ, Pot C, Joller N,
Xiao S, Kumar D, Burns EJ, Sherr DH and Weiner HL: The aryl
hydrocarbon receptor interacts with c-Maf to promote the
differentiation of type 1 regulatory T cells induced by IL-27. Nat
Immunol. 11:854–861. 2011. View Article : Google Scholar
|
|
43
|
Adachi J, Mori Y, Matsui S and Matsuda T:
Comparison of gene expression patterns between
2,3,7,8-tetrachlorodibenzo-p-dioxin and a natural arylhydrocarbon
receptor ligand, indirubin. Toxicol Sci. 80:161–169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murray IA, Morales JL, Flaveny CA,
DiNatale BC, Chiaro C, Gowdahalli K, Amin S and Perdew GH: Evidence
for ligand-mediated selective modulation of Aryl hydrocarbon
receptor activity. Mol Pharmacol. 77:247–254. 2010. View Article : Google Scholar :
|
|
45
|
Lo R and Matthews J: High-resolution
genome-wide mapping of AHR and ARNT binding sites by ChIP-Seq.
Toxicol Sci. 130:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hao N, Lee KL, Furness SGB, Bosdotter C,
Poellinger L and Whitelaw ML: Xenobiotics and loss of cell adhesion
drive distinct transcriptional outcomes by aryl hydrocarbon
receptor signaling. Mol Pharmacol. 82:1082–1093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen G and Shin JA: AhR/Arnt:XRE
interaction: Turning false negatives into true positives in the
modified yeast one-hybrid assay. Anal Biochem. 382:101–106. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vogel CFA, Van Winkle LS, Esser C and
Haarmann-Stemmann T: The aryl hydrocarbon receptor as a target of
environmental stressors-implications for pollution mediated stress
and inflammatory responses. Redox Biol. 34:1015302020. View Article : Google Scholar
|
|
49
|
Lim HJ, Jang WB, Rethineswaran VK, Choi J,
Lee EJ, Park S, Jeong Y, Ha JS, Yun J, Choi YJ, et al:
StemRegenin-1 attenuates endothelial progenitor cell senescence by
regulating the AhR pathway-mediated CYP1A1 and ROS generation.
Cells. 12:20052023. View Article : Google Scholar :
|
|
50
|
Shertzer HG, Clay CD, Genter MB, Chames
MC, Schneider SN, Oakley GG, Nebert DW and Dalton TP:
Uncoupling-mediated generation of reactive oxygen by halogenated
aromatic hydrocarbons in mouse liver microsomes. Free Radic Biol
Med. 36:618–631. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mi P, Li N, Ai K, Li L and Yuan D:
AhR-mediated lipid peroxidation contributes to TCDD-induced cardiac
defects in zebrafish. Chemosphere. 317:1379422023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan J, Sun X, Che S, Zhang L, Ruan Z, Li
X and Yang J: AhR-mediated CYP1A1 and ROS overexpression are
involved in hepatotoxicity of decabromodiphenyl ether (BDE-209).
Toxicol Lett. 352:26–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yan L, Gu L, Lv X, Ni Z, Qian W, Chen Z,
Yang S, Zhuge Q, Yuan L and Ni H: Butylphthalide mitigates
traumatic brain injury by activating anti-ferroptotic AHR-CYP1B1
pathway. J Ethnopharmacol. 337:1187582025. View Article : Google Scholar
|
|
54
|
Nakahara T, Mitoma C, Hashimoto-Hachiya A,
Takahara M, Tsuji G, Uchi H, Yan X, Hachisuka J, Chiba T, Esaki H,
et al: Antioxidant opuntia ficus-indica extract activates AHR-NRF2
signaling and upregulates filaggrin and loricrin expression in
human keratinocytes. J Med Food. 18:1143–1149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takei K, Mitoma C, Hashimoto-Hachiya A,
Uchi H, Takahara M, Tsuji G, Kido-Nakahara M, Nakahara T and Furue
M: Antioxidant soybean tar Glyteer rescues T-helper-mediated
downregulation of filaggrin expression via aryl hydrocarbon
receptor. J Dermatol. 42:171–180. 2015. View Article : Google Scholar :
|
|
56
|
Takei K, Hashimoto-Hachiya A, Takahara M,
Tsuji G, Nakahara T and Furue M: Cynaropicrin attenuates
UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol
Lett. 234:74–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Miao W, Hu L, Scrivens PJ and Batist G:
Transcriptional regulation of NF-E2 p45-related factor (NRF2)
expression by the aryl hydrocarbon receptor-xenobiotic response
element signaling pathway: Direct cross-talk between phase I and II
drug-metabolizing enzymes. J Biol Chem. 280:20340–20348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang L, Yin X, Chen YH, Chen Y, Jiang W,
Zheng H, Huang FQ, Liu B, Zhou W, Qi LW and Li J: Proteomic
analysis reveals ginsenoside Rb1 attenuates myocardial
ischemia/reperfusion injury through inhibiting ROS production from
mitochondrial complex I. Theranostics. 11:1703–1720. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kou Z, Tran F and Dai W: Heavy metals,
oxidative stress, and the role of AhR signaling. Toxicol Appl
Pharmacol. 482:1167692024. View Article : Google Scholar :
|
|
60
|
Li X, Ma N, Xu J, Zhang Y, Yang P, Su X,
Xing Y, An N, Yang F, Zhang G, et al: Targeting ferroptosis:
pathological mechanism and treatment of ischemia-reperfusion
injury. Oxid Med Cell Longev. 2021:15879222021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang X, Shen T, Lian J, Deng K, Qu C, Li
E, Li G, Ren Y, Wang Z, Jiang Z, et al: Resveratrol reduces
ROS-induced ferroptosis by activating SIRT3 and compensating the
GSH/GPX4 pathway. Mol Med. 29:1372023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yan HF, Tuo QZ, Yin QZ and Lei P: The
pathological role of ferroptosis in ischemia/reperfusion-related
injury. Zool Res. 41:220–230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Paraskevaidis IA, Iliodromitis EK,
Vlahakos D, Tsiapras DP, Nikolaidis A, Marathias A, Michalis A and
Kremastinos DT: Deferoxamine infusion during coronary artery bypass
grafting ameliorates lipid peroxidation and protects the myocardium
against reperfusion injury: Immediate and long-term significance.
Eur Heart J. 26:263–270. 2005. View Article : Google Scholar
|
|
64
|
Wang B and Xu A: Aryl hydrocarbon receptor
pathway participates in myocardial ischemia reperfusion injury by
regulating mitochondrial apoptosis. Med Hypotheses. 123:2–5. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kou Z, Yang R, Lee E, Cuddapah S, Choi BH
and Dai W: Oxidative stress modulates expression of immune
checkpoint genes via activation of AhR signaling. Toxicol Appl
Pharmacol. 457:1163142022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Eleftheriadis T, Pissas G, Golfinopoulos
S, Liakopoulos V and Stefanidis I: Role of indoleamine
2,3-dioxygenase in ischemia-reperfusion injury of renal tubular
epithelial cells. Mol Med Rep. 23:4722021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Han L, Ma C, Wu Z, Xu H, Li H and Pan G:
AhR-STAT3-HO-1/COX-2 signalling pathway may restrict ferroptosis
and improve hMSC accumulation and efficacy in mouse liver. Br J
Pharmacol. 181:125–141. 2024. View Article : Google Scholar
|
|
68
|
Zheng C, Zhang B, Li Y, Liu K, Wei W,
Liang S, Guo H, Ma K, Liu Y, Wang J and Liu L: Donafenib and GSK-J4
synergistically induce ferroptosis in liver cancer by upregulating
HMOX1 expression. Adv Sci (Weinh). 10:e22067982023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wong PS, Li W, Vogel CF and Matsumura F:
Characterization of MCF mammary epithelial cells overexpressing the
Arylhydrocarbon receptor (AhR). BMC Cancer. 9:2342009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhu P, Zhou K, Lu S, Bai Y, Qi R and Zhang
S: Modulation of aryl hydrocarbon receptor inhibits esophageal
squamous cell carcinoma progression by repressing COX2/PGE2/STAT3
axis. J Cell Commun Signal. 14:175–192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lo R and Matthews J: The aryl hydrocarbon
receptor and estrogen receptor alpha differentially modulate
nuclear factor erythroid-2-related factor 2 transactivation in
MCF-7 breast cancer cells. Toxicol Appl Pharmacol. 270:139–148.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fader KA, Nault R, Kirby MP, Markous G,
Matthews J and Zacharewski TR: Convergence of hepcidin deficiency,
systemic iron overloading, heme accumulation, and REV-ERBα/β
activation in aryl hydrocarbon receptor-elicited hepatotoxicity.
Toxicol Appl Pharmacol. 321:1–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Davies R, Clothier B, Robinson SW, Edwards
RE, Greaves P, Luo J, Gant TW, Chernova T and Smith AG: Essential
role of the AH receptor in the dysfunction of heme metabolism
induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem Res Toxicol.
21:330–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao Z, Wu J, Xu H, Zhou C, Han B, Zhu H,
Hu Z, Ma Z, Ming Z, Yao Y, et al: XJB-5-131 inhibited ferroptosis
in tubular epithelial cells after ischemia-reperfusion injury. Cell
Death Dis. 11:6292020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cai W, Liu L, Shi X, Liu Y, Wang J, Fang
X, Chen Z, Ai D, Zhu Y and Zhang X: Alox15/15-HpETE aggravates
myocardial ischemia-reperfusion injury by promoting cardiomyocyte
ferroptosis. Circulation. 147:1444–1460. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhan J, Chen Y, Liu Y, Chen Y, Li Z, Li X,
He Z, Meng F, Qian X, Yang L and Yang Q: IDO1-mediated AhR
activation up-regulates pentose phosphate pathway via NRF2 to
inhibit ferroptosis in lung cancer. Biochem Pharmacol.
236:1169132025. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu J, Sun X, Qin F, Wang X, Chen Q and Yan
R: Protective effects of salvianolic acid B on intestinal
ischemia/reperfusion injury in rats by regulating the
AhR/IL-22/STAT6 axis. J Recept Signal Transduct Res. 43:73–82.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shen L, Zhang J, Zheng Z, Yang F, Liu S,
Wu Y, Chen Y, Xu T, Mao S, Yan Y, et al: PHGDH inhibits ferroptosis
and promotes malignant progression by upregulating SLC7A11 in
bladder cancer. Int J Biol Sci. 18:5459–5474. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Conrad M and Pratt DA: The chemical basis
of ferroptosis. Nat Chem Biol. 15:1137–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lin CJ, Liu HL, Pan CF, Chuang CK,
Jayakumar T, Wang TJ, Chen HH and Wu CJ: Indoxyl sulfate predicts
cardiovascular disease and renal function deterioration in advanced
chronic kidney disease. Arch Med Res. 43:451–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen H, Zhou Y, Liu Y, Zhou W, Xu L, Shang
D, Ni J and Song Z: Indoxyl sulfate exacerbates alveolar bone loss
in chronic kidney disease through ferroptosis. Oral Dis.
31:264–277. 2025. View Article : Google Scholar
|
|
82
|
Curran CS and Kopp JB: The complexity of
nicotinamide adenine dinucleotide (NAD), hypoxic, and aryl
hydrocarbon receptor cell signaling in chronic kidney disease. J
Transl Med. 21:7062023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yuan S, Wei C, Liu G, Zhang L, Li J, Li L,
Cai S and Fang L: Sorafenib attenuates liver fibrosis by triggering
hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell
Prolif. 55:e131582022. View Article : Google Scholar
|
|
84
|
Fan Y, Ma L, Fang X, Du S, Mauck J, Loor
JJ, Sun X, Jia H, Xu C and Xu Q: Role of
hypoxia-inducible-factor-1α (HIF-1α) in ferroptosis of adipose
tissue during ketosis. J Dairy Sci. 107:10611–10627. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jin HO, Hong SE, Kim JY, Jang SK and Park
IC: Amino acid deprivation induces AKT activation by inducing
GCN2/ATF4/REDD1 axis. Cell Death Dis. 12:11272021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Swanda RV, Ji Q, Wu X, Yan J, Dong L, Mao
Y, Uematsu S, Dong Y and Qian SB: Lysosomal cystine governs
ferroptosis sensitivity in cancer via cysteine stress response. Mol
Cell. 83:3347–3359.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao C, Bao L, Qiu M, Feng L, Chen L, Liu
Z, Duan S, Zhao Y, Wu K, Zhang N, et al: Dietary
tryptophan-mediated Aryl hydrocarbon receptor activation by the gut
microbiota alleviates escherichia coli-induced endometritis in
mice. Microbiol Spectr. 10:e00811222022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang J, Zhu N, Su X, Gao Y and Yang R:
Gut-microbiota-derived metabolites maintain gut and systemic immune
homeostasis. Cells. 12:7932023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fiore A, Zeitler L, Russier M, Groß A,
Hiller MK, Parker JL, Stier L, Köcher T, Newstead S and Murray PJ:
Kynurenine importation by SLC7A11 propagates anti-ferroptotic
signaling. Mol Cell. 82:920–932.e7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cui Y, Zhang Z, Zhou X, Zhao Z, Zhao R, Xu
X, Kong X, Ren J, Yao X, Wen Q, et al: Microglia and macrophage
exhibit attenuated inflammatory response and ferroptosis resistance
after RSL3 stimulation via increasing Nrf2 expression. J
Neuroinflammation. 18:2492021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cui W, Guo M, Liu D, Xiao P, Yang C, Huang
H, Liang C, Yang Y, Fu X, Zhang Y, et al: Gut microbial metabolite
facilitates colorectal cancer development via ferroptosis
inhibition. Nat Cell Biol. 26:124–137. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Marcato P, Dean CA, Liu RZ, Coyle KM,
Bydoun M, Wallace M, Clements D, Turner C, Mathenge EG, Gujar SA,
et al: Aldehyde dehydrogenase 1A3 influences breast cancer
progression via differential retinoic acid signaling. Mol Oncol.
9:17–31. 2015. View Article : Google Scholar
|
|
93
|
Schwab A, Rao Z, Zhang J, Gollowitzer A,
Siebenkäs K, Bindel N, D'Avanzo E, Van Roey R, Hajjaj Y, Özel E, et
al: Zeb1 mediates EMT/plasticity-associated ferroptosis sensitivity
in cancer cells by regulating lipogenic enzyme expression and
phospholipid composition. Nat Cell Biol. 26:1470–1481. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tang LJ, Luo XJ, Tu H, Chen H, Xiong XM,
Li NS and Peng J: Ferroptosis occurs in phase of reperfusion but
not ischemia in rat heart following ischemia or
ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol.
394:401–410. 2021. View Article : Google Scholar
|
|
95
|
Scindia Y, Leeds J and Swaminathan S: Iron
homeostasis in healthy kidney and its role in acute kidney injury.
Semin Nephrol. 39:76–84. 2019. View Article : Google Scholar
|
|
96
|
Doskey CM, Fader KA, Nault R, Lydic T,
Matthews J, Potter D, Sharratt B, Williams K and Zacharewski T:
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters hepatic
polyunsaturated fatty acid metabolism and eicosanoid biosynthesis
in female Sprague-Dawley rats. Toxicol Appl Pharmacol.
398:1150342020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Aboutabl ME, Zordoky BNM and El-Kadi AOS:
3-Methylcholanthrene and benzo(a)pyrene modulate cardiac cytochrome
P450 gene expression and arachidonic acid metabolism in male
Sprague Dawley rats. Br J Pharmacol. 158:1808–1819. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sroczyńska K, Totoń-Żurańska J, Czepiel J,
Zając-Grabiec A, Jurczyszyn A, Wołkow P, Librowski T and
Gdula-Argasińska J: Therapeutic role of eicosapentaenoic and
arachidonic acid in benzo(a) pyrene-induced toxicity in HUVEC
endothelial cells. Life Sci. 293:1203452022. View Article : Google Scholar
|
|
99
|
Zhang C, He M, Ni L, He K, Su K, Deng Y,
Li Y and Xia H: The role of arachidonic acid metabolism in
myocardial ischemia-reperfusion injury. Cell Biochem Biophys.
78:255–265. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bock KW and Köhle C: Contributions of the
Ah receptor to bilirubin homeostasis and its antioxidative and
atheroprotective functions. Biol Chem. 391:645–653. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kim R, Hashimoto A, Markosyan N, Tyurin
VA, Tyurina YY, Kar G, Fu S, Sehgal M, Garcia-Gerique L, Kossenkov
A, et al: Ferroptosis of tumor neutrophils causes immune
suppression in cancer. Nature. 612:338–346. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Paris A, Tardif N, Galibert MD and Corre
S: AhR and cancer: From gene profiling to targeted therapy. Int J
Mol Sci. 22:7522021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hezaveh K, Shinde RS, Klötgen A, Halaby
MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, et
al: Tryptophan-derived microbial metabolites activate the aryl
hydrocarbon receptor in tumor-associated macrophages to suppress
anti-tumor immunity. Immunity. 55:324–340.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Duan Z, Li Y and Li L: Promoting
epithelial-to-mesenchymal transition by D-kynurenine via activating
aryl hydrocarbon receptor. Mol Cell Biochem. 448:165–173. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li HM, Li X, Xia R, Zhang X, Jin TZ and
Zhang HS: PHGDH knockdown increases sensitivity to SR1, an aryl
hydrocarbon receptor antagonist, in colorectal cancer by activating
the autophagy pathway. FEBS J. 291:1780–1794. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zeitler L, Fiore A, Meyer C, Russier M,
Zanella G, Suppmann S, Gargaro M, Sidhu SS, Seshagiri S, Ohnmacht
C, et al: Anti-ferroptotic mechanism of IL4i1-mediated amino acid
metabolism. Elife. 10:e648062021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lian J, Lin H, Zhong Z, Song Y, Shao X,
Zhou J, Xu L, Sun Z, Yang Y, Chi J, et al: Indole-3-lactic acid
inhibits doxorubicin-induced ferroptosis through activating Aryl
hydrocarbon receptor/Nrf2 signalling pathway. J Cell Mol Med.
29:e703582025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tian Q, Dan G, Wang X, Zhu J, Chen C, Tang
D, Wang Z, Chen D, Lei S, Yang C, et al: IDO1 inhibits ferroptosis
by regulating FTO-mediated m6A methylation and SLC7A11 mRNA
stability during glioblastoma progression. Cell Death Discov.
11:222025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cui JX, Xu XH, He T, Liu JJ, Xie TY, Tian
W and Liu JY: L-kynurenine induces NK cell loss in gastric cancer
microenvironment via promoting ferroptosis. J Exp Clin Cancer Res.
42:522023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yu L, Shang D, Lang L, Liu Y, Yu M, Song
D, Jia S, Han S, Li C, Liu J, et al: TMF, a natural
dihydroflavonoid isolated from Scutellaria javanica Jungh,
stimulates anticancer activity of s180 cancer-bearing mice, induces
apoptosis, inhibits invasion and migration on HepG-2 cells. J
Ethnopharmacol. 263:1130722020. View Article : Google Scholar
|
|
111
|
Liu S: Aryl hydrocarbon receptor
alleviates hepatic fibrosis by inducing hepatic stellate cell
ferroptosis. J Cell Mol Med. 28:e702782024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Guo L, Zhang J and Li Y, Gao Y, Huang J,
Liu M, Li J, Chai W and Li Y: 3,3′-Diindolylmethane induces
ferroptosis and inhibits proliferation in non-small-cell lung
cancer through the AHR/NRF2/GPX4 axis. Discov Oncol. 16:3442025.
View Article : Google Scholar
|
|
113
|
Prasanth MI, Malar DS, Verma K,
Prasansuklab A and Tencomnao T: Hibiscus sabdariffa calyx extract
protects human keratinocyte cells from fluoranthene-induced
ferroptosis via the repression of aryl hydrocarbon receptor.
Ecotoxicol Environ Saf. 291:1178712025. View Article : Google Scholar
|
















![Oxidative and antioxidant AhR ligands
regulate lipid peroxidation and ferroptosis via an
oxidative-antioxidant bidirectional regulatory network. Exogenous
pro-oxidative AhR ligands, such as the toxins TCDD and
benzo[a]pyrene, bind to AhR, forming the AhR-ARNT complex that
translocates into the nucleus. This complex subsequently binds to
the XRE, initiating the transcription of various progenitor
enzymes, including CYP1A1, CYP1B1, CYP1A2, COX-2 and NOX, then
mediating ROS production, which in turn induces lipid peroxidation
and ferroptosis. Conversely, natural AhR ligands derived from plant
sources, such as snake tail grass, soybean, fig tree, and fish tail
grass, activate the transcription of antioxidant enzymes (e.g.,
NQO1, GST and UGT1A1) through AhR signaling. This activation
suppresses ROS production, thereby mitigating lipid peroxidation
and inhibiting ferroptosis. AhR, aryl hydrocarbon receptor; TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin; ARNT, AhR nuclear
translocator; NOX, nitrogen oxide; ROS, reactive oxygen species;
NQO1, NAD(P) H dehydrogenase [quinone] 1; GST, glutathione
S-transferase; UGT1A1, UDP-glucuronosyltransferase 1-1; HMOX1, heme
oxygenase 1; IDO1, indoleamine 2,3-dioxygenase 1; SLC7A11, solute
carrier family 7 member 11; COX-2, cyclooxygenase-2; ALDH1A3,
aldehyde dehydrogenase 1 family, member A3.](/article_images/ijmm/56/4/ijmm-56-04-05597-g01.jpg)


![Pharmacological targeting of AhR to
modulate ferroptosis in cancer and I/R injury. AhR ligands regulate
lipid peroxidation and ferroptosis through different molecular
axes, demonstrating environment-dependent therapeutic potential. In
parenchymal organs, such as the heart, liver and kidneys, AhR
antagonists CH223191 and SR1 alleviate I/R injury by inhibiting the
iron-stimulating pathway: CH223191 downregulates CYP1A-driven ROS
amplification and COX-2-mediated inflammatory signals, while SR1
inhibits HMOX1-dependent iron release and IDO1-induced oxidative
stress, jointly maintaining the redox balance of cells. In tumors,
AhR is pharmacologically regulated through AhR ligands, such as
aminoflavone, ITE, SR1 and CH223191, thereby influencing key
molecules in various lipid peroxidation and ferroptosis processes,
and further inducing ferroptosis in tumor cells or enhancing their
sensitivity to erastin. This is expected to eliminate tumor cells,
alleviate tumor progression and enhance the anti-tumor efficacy of
iron deposition inducers. AhR, aryl hydrocarbon receptor; HMOX1,
heme oxygenase 1; IDO1, indoleamine 2,3-dioxygenase 1; ROS,
reactive oxygen species; COX-2, cyclooxygenase-2; TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin; NQO1, NAD(P)H dehydrogenase
[quinone] 1; GST, glutathione S-transferase; UGT1A1,
UDP-glucuronosyltransferase 1-1.](/article_images/ijmm/56/4/ijmm-56-04-05597-g04.jpg)