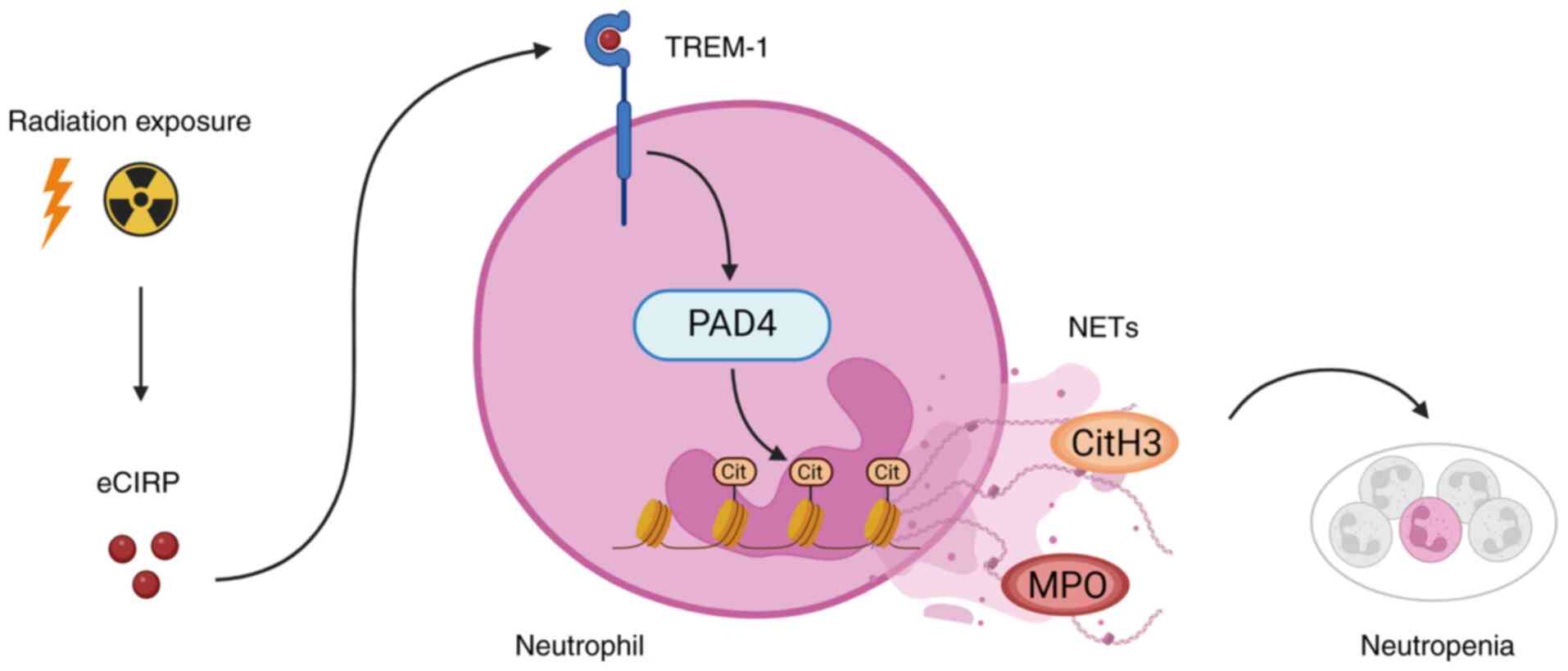
|
1
|
Harada KH, Soleman SR, Ang JSM and
Trzcinski AP: Conflict-related environmental damages on health:
Lessons learned from the past wars and ongoing Russian invasion of
Ukraine. Environ Health Prev Med. 27:352022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hasegawa A, Tanigawa K, Ohtsuru A, Yabe H,
Maeda M, Shigemura J, Ohira T, Tominaga T, Akashi M, Hirohashi N,
et al: Health effects of radiation and other health problems in the
aftermath of nuclear accidents, with an emphasis on Fukushima.
Lancet. 386:479–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirohashi N, Shime N and Fujii T: Beyond
the unthinkable: Are we prepared for rare disasters? Anaesth Crit
Care Pain Med. 42:1012662023.PubMed/NCBI
|
|
4
|
Baranov A, Gale RP, Guskova A, Piatkin E,
Selidovkin G, Muravyova L, Champlin RE, Danilova N, Yevseeva L and
Petrosyan L: Bone marrow transplantation after the Chernobyl
nuclear accident. N Engl J Med. 321:205–212. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macià I, Garau M, Lucas Calduch A and
López EC: Radiobiology of the acute radiation syndrome. Rep Pract
Oncol Radiother. 16:123–130. 2011. View Article : Google Scholar
|
|
6
|
McCart EA, Lee YH, Jha J, Mungunsukh O,
Rittase WB, Summers TA Jr, Muir J and Day RM: Delayed captopril
administration mitigates hematopoietic injury in a murine model of
total body irradiation. Sci Rep. 9:21982019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong K, Chang PY, Fielden M, Downey AM,
Bunin D, Bakke J, Gahagen J, Iyer L, Doshi S, Wierzbicki W and
Authier S: Pharmacodynamics of romiplostim alone and in combination
with pegfilgrastim on acute radiation-induced thrombocytopenia and
neutropenia in Non-human primates. Int J Radiat Biol. 96:155–166.
2020. View Article : Google Scholar
|
|
8
|
Tanigawa K: Case review of severe acute
radiation syndrome from whole body exposure: Concepts of
radiation-induced multi-organ dysfunction and failure. J Radiat
Res. 62:i15–i20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams JP and McBride WH: After the bomb
drops: A new look at Radiation-induced multiple organ dysfunction
syndrome (MODS). Int J Radiat Biol. 87:851–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim YJ, Jeong J, Park K, Sohn KY, Yoon SY
and Kim JW: Mitigation of hematopoietic syndrome of acute radiation
syndrome by 1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) is
associated with regulation of systemic inflammation in a murine
model of Total-Body Irradiation. Radiat Res. 196:55–65. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerassy-Vainberg S, Blatt A, Danin-Poleg
Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O,
Dheer R, et al: Radiation induces proinflammatory dysbiosis:
Transmission of inflammatory susceptibility by host cytokine
induction. Gut. 67:97–107. 2018. View Article : Google Scholar
|
|
12
|
English J, Dhanikonda S, Tanaka KE, Koba
W, Eichenbaum G, Yang WL and Guha C: Thrombopoietin mimetic reduces
mouse lung inflammation and fibrosis after radiation by attenuating
activated endothelial phenotypes. JCI Insight. 9:e1813302024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaue D, Micewicz ED, Ratikan JA, Xie MW,
Cheng G and McBride WH: Radiation and inflammation. Semin Radiat
Oncol. 25:4–10. 2015. View Article : Google Scholar :
|
|
14
|
Dainiak N and Albanese J: Medical
management of acute radiation syndrome. J Radiol Prot. 42:2022.
View Article : Google Scholar
|
|
15
|
Thiam HR, Wong SL, Wagner DD and Waterman
CM: Cellular mechanisms of NETosis. Annu Rev Cell Dev Biol.
36:191–218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christophorou MA, Castelo-Branco G,
Halley-Stott RP, Oliveira CS, Loos R, Radzisheuskaya A, Mowen KA,
Bertone P, Silva JC, Zernicka-Goetz M, et al: Citrullination
regulates pluripotency and histone H1 binding to chromatin. Nature.
507:104–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiam HR, Wong SL, Qiu R, Kittisopikul M,
Vahabikashi A, Goldman AE, Goldman RD, Wagner DD and Waterman CM:
NETosis proceeds by cytoskeleton and endomembrane disassembly and
PAD4-mediated chromatin decondensation and nuclear envelope
rupture. Proc Natl Acad Sci USA. 117:7326–7337. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chapman EA, Lyon M, Simpson D, Mason D,
Beynon RJ, Moots RJ and Wright HL: Caught in a trap? Proteomic
analysis of neutrophil extracellular traps in rheumatoid arthritis
and systemic lupus erythematosus. Front Immunol. 10:4232019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Kim SJ, Lei Y, Wang S, Wang H,
Huang H, Zhang H and Tsung A: Neutrophil extracellular traps in
homeostasis and disease. Signal Transduct Target Ther. 9:2352024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Silva CMS, Wanderley CWS, Veras FP, Sonego
F, Nascimento DC, Goncalves AV, Martins TV, Colon DF, Borges VF,
Brauer VS, et al: Gasdermin D inhibition prevents multiple organ
dysfunction during sepsis by blocking NET formation. Blood.
138:2702–2713. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan C, Aziz M and Wang P: The vitals of
NETs. J Leukoc Biol. 110:797–808. 2021. View Article : Google Scholar
|
|
22
|
Stephenson HN, Herzig A and Zychlinsky A:
Beyond the grave: When is cell death critical for immunity to
infection? Curr Opin Immunol. 38:59–66. 2016. View Article : Google Scholar
|
|
23
|
Lawrence SM, Corriden R and Nizet V: How
neutrophils meet their end. Trends Immunol. 41:531–544. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shinde-Jadhav S, Mansure JJ, Rayes RF,
Marcq G, Ayoub M, Skowronski R, Kool R, Bourdeau F, Brimo F, Spicer
J and Kassouf W: Role of neutrophil extracellular traps in
radiation resistance of invasive bladder cancer. Nat Commun.
12:27762021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aziz M, Brenner M and Wang P:
Extracellular CIRP (eCIRP) and inflammation. J Leukoc Biol.
106:133–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou M, Aziz M, Li J, Jha A, Ma G, Murao A
and Wang P: BMAL2 promotes eCIRP-induced macrophage endotoxin
tolerance. Front Immunol. 15:14266822024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murao A, Aziz M, Wang H, Brenner M and
Wang P: Release mechanisms of major DAMPs. Apoptosis. 26:152–162.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiang X, Yang WL, Wu R, Zhou M, Jacob A,
Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al: Cold-inducible
RNA-binding protein (CIRP) triggers inflammatory responses in
hemorrhagic shock and sepsis. Nat Med. 19:1489–1495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hollis R, Li J, Lee Y, Jin H, Zhou M, Nofi
CP, Sfakianos M, Coppa G, Aziz M and Wang P: A novel opsonic
extracellular cirp inhibitor Mop3 alleviates gut
Ischemia/reperfusion injury. Shock. 63:101–109. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaga S, Aziz M, Murao A, Brenner M and
Wang P: DAMPs and radiation injury. Front Immunol. 15:13539902024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carrasco K, Boufenzer A, Jolly L, Le
Cordier H, Wang G, Heck AJ, Cerwenka A, Vinolo E, Nazabal A,
Kriznik A, et al: TREM-1 multimerization is essential for its
activation on monocytes and neutrophils. Cell Mol Immunol.
16:460–472. 2019. View Article : Google Scholar :
|
|
32
|
Siskind S, Brenner M and Wang P: TREM-1
modulation strategies for sepsis. Front Immunol. 13:9073872022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Borjas T, Jacob A, Yen H, Patel V, Coppa
GF, Aziz M and Wang P: Inhibition of the Interaction of TREM-1 and
eCIRP attenuates inflammation and improves survival in hepatic
Ischemia/reperfusion. Shock. 57:246–255. 2022. View Article : Google Scholar :
|
|
34
|
Denning NL, Aziz M, Diao L, Prince JM and
Wang P: Targeting the eCIRP/TREM-1 interaction with a small
molecule inhibitor improves cardiac dysfunction in neonatal sepsis.
Mol Med. 26:1212020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murao A, Arif A, Brenner M, Denning NL,
Jin H, Takizawa S, Nicastro B, Wang P and Aziz M: Extracellular
CIRP and TREM-1 axis promotes ICAM-1-Rho-mediated NETosis in
sepsis. FASEB J. 34:9771–9786. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaga S, Murao A, Ma G, Brenner M, Aziz M
and Wang P: Radiation upregulates macrophage TREM-1 expression to
exacerbate injury in mice. Front Immunol. 14:11512502023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Denning NL, Aziz M, Murao A, Gurien SD,
Ochani M, Prince JM and Wang P: Extracellular CIRP as an endogenous
TREM-1 ligand to fuel inflammation in sepsis. JCI Insight.
5:e1341722020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Knops K, Boldt S, Wolkenhauer O and
Kriehuber R: Gene expression in low- and high-dose-irradiated human
peripheral blood lymphocytes: Possible applications for
biodosimetry. Radiat Res. 178:304–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida K, Misumi M, Hamasaki K, Kyoizumi
S, Satoh Y, Tsuruyama T, Uchimura A and Kusunoki Y: High-dose
radiation preferentially induces the clonal expansion of
hematopoietic progenitor cells over mature T and B cells in mouse
bone marrow. Stem Cell Reports. 20:1024232025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Albrecht H, Durbin-Johnson B, Yunis R,
Kalanetra KM, Wu S, Chen R, Stevenson TR and Rocke DM:
Transcriptional response of ex vivo human skin to ionizing
radiation: Comparison between low- and high-dose effects. Radiat
Res. 177:69–83. 2012. View Article : Google Scholar
|
|
41
|
Ode Y, Aziz M and Wang P: CIRP increases
ICAM-1+ phenotype of neutrophils exhibiting elevated iNOS and NETs
in sepsis. J Leukoc Biol. 103:693–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using Real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Dainiak N: Medical management of acute
radiation syndrome and associated infections in a High-casualty
incident. J Radiat Res. 59(Suppl_2): ii54–ii64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teijeira A, Garasa S, Ochoa MC,
Sanchez-Gregorio S, Gomis G, Luri-Rey C, Martinez-Monge R, Pinci B,
Valencia K, Palencia B, et al: Low-dose ionizing gamma-radiation
elicits the extrusion of neutrophil extracellular traps. Clin
Cancer Res. 30:4131–4142. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arzumanyan G, Mamatkulov K, Arynbek Y,
Zakrytnaya D, Jevremovic A and Vorobjeva N: Radiation from UV-A to
red light induces ROS-Dependent release of neutrophil extracellular
traps. Int J Mol Sci. 24:57702023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zawrotniak M, Bartnicka D and Rapala-Kozik
M: UVA and UVB radiation induce the formation of neutrophil
extracellular traps by human polymorphonuclear cells. J Photochem
Photobiol B. 196:1115112019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yipp BG, Petri B, Salina D, Jenne CN,
Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert
HC, et al: Infection-induced NETosis is a dynamic process involving
neutrophil multitasking in vivo. Nat Med. 18:1386–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rossaint J, Herter JM, Van Aken H, Napirei
M, Doring Y, Weber C, Soehnlein O and Zarbock A: Synchronized
integrin engagement and chemokine activation is crucial in
neutrophil extracellular Trap-mediated sterile inflammation. Blood.
123:2573–2584. 2014. View Article : Google Scholar
|
|
49
|
Aleyd E, van Hout MW, Ganzevles SH, Hoeben
KA, Everts V, Bakema JE and van Egmond M: IgA enhances NETosis and
release of neutrophil extracellular traps by polymorphonuclear
cells via Fcα receptor I. J Immunol. 192:2374–2383. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Keshari RS, Jyoti A, Dubey M, Kothari N,
Kohli M, Bogra J, Barthwal MK and Dikshit M: Cytokines induced
neutrophil extracellular traps formation: Implication for the
inflammatory disease condition. PLoS One. 7:e481112012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamaga S, Murao A, Zhou M, Aziz M, Brenner
M and Wang P: Radiation-induced eCIRP impairs macrophage bacterial
phagocytosis. J Leukoc Biol. 116:1072–1079. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Arts RJ, Joosten LA, van der Meer JW and
Netea MG: TREM-1: Intracellular signaling pathways and interaction
with pattern recognition receptors. J Leukoc Biol. 93:209–215.
2013. View Article : Google Scholar
|
|
53
|
Parker H, Dragunow M, Hampton MB, Kettle
AJ and Winterbourn CC: Requirements for NADPH oxidase and
myeloperoxidase in neutrophil extracellular trap formation differ
depending on the stimulus. J Leukoc Biol. 92:841–849. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kenny EF, Herzig A, Kruger R, Muth A,
Mondal S, Thompson PR, Brinkmann V, Bernuth HV and Zychlinsky A:
Diverse stimuli engage different neutrophil extracellular trap
pathways. Elife. 6:e244372017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Baruah S, Murthy S, Keck K, Galvan I,
Prichard A, Allen LH, Farrelly M and Klesney-Tait J: TREM-1
regulates neutrophil chemotaxis by promoting NOX-dependent
superoxide production. J Leukoc Biol. 105:1195–1207. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cassatt DR, Winters TA and PrabhuDas M:
Immune dysfunction from radiation exposure. Radiat Res.
200:389–395. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun S, Duan Z, Wang X, Chu C, Yang C, Chen
F, Wang D, Wang C, Li Q and Ding W: Neutrophil extracellular traps
impair intestinal barrier functions in sepsis by regulating
TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis.
12:6062021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao J, Zhen N, Zhou Q, Lou J, Cui W,
Zhang G and Tian B: NETs promote inflammatory injury by activating
cGAS-STING pathway in acute lung injury. Int J Mol Sci.
24:51252023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hawez A, Taha D, Algaber A, Madhi R,
Rahman M and Thorlacius H: MiR-155 regulates neutrophil
extracellular trap formation and lung injury in abdominal sepsis. J
Leukoc Biol. 111:391–400. 2022. View Article : Google Scholar
|
|
60
|
Gao X, Hao S, Yan H, Ding W, Li K and Li
J: Neutrophil extracellular traps contribute to the intestine
damage in endotoxemic rats. J Surg Res. 195:211–218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luo L, Zhang S, Wang Y, Rahman M, Syk I,
Zhang E and Thorlacius H: Proinflammatory role of neutrophil
extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol
Physiol. 307:L586–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chu C, Wang X, Chen F, Yang C, Shi L, Xu
W, Wang K, Liu B, Wang C, Sun D, et al: Neutrophil extracellular
traps aggravate intestinal epithelial necroptosis in
ischaemia-reperfusion by regulating TLR4/RIPK3/FUNDC1-required
mitophagy. Cell Prolif. 57:e135382024. View Article : Google Scholar
|
|
63
|
Zhan Y, Ling Y, Deng Q, Qiu Y, Shen J, Lai
H, Chen Z, Huang C, Liang L, Li X, et al: HMGB1-Mediated neutrophil
extracellular trap formation exacerbates intestinal
Ischemia/Reperfusion-Induced acute lung injury. J Immunol.
208:968–978. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jansen MP, Emal D, Teske GJ, Dessing MC,
Florquin S and Roelofs JJ: Release of extracellular DNA influences
renal ischemia reperfusion injury by platelet activation and
formation of neutrophil extracellular traps. Kidney Int.
91:352–364. 2017. View Article : Google Scholar
|
|
65
|
Ye D, Yao J, Du W, Chen C, Yang Y, Yan K,
Li J, Xu Y, Zang S, Zhang Y, et al: Neutrophil extracellular traps
mediate acute liver failure in regulation of miR-223/Neutrophil
elastase signaling in mice. Cell Mol Gastroenterol Hepatol.
14:587–607. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Allam R, Scherbaum CR, Darisipudi MN,
Mulay SR, Hagele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu
M, Schwarzenberger C, et al: Histones from dying renal cells
aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol.
23:1375–1388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Silk E, Zhao H, Weng H and Ma D: The role
of extracellular histone in organ injury. Cell Death Dis.
8:e28122017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Manchanda K, Kolarova H, Kerkenpass C,
Mollenhauer M, Vitecek J, Rudolph V, Kubala L, Baldus S, Adam M and
Klinke A: MPO (Myeloperoxidase) reduces endothelial glycocalyx
thickness dependent on its cationic charge. Arterioscler Thromb
Vasc Biol. 38:1859–1867. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ushakumari CJ, Zhou QL, Wang YH, Na S,
Rigor MC, Zhou CY, Kroll MK, Lin BD and Jiang ZY: Neutrophil
elastase increases vascular permeability and leukocyte
transmigration in cultured endothelial cells and obese mice. Cells.
11:22882022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen R, Kang R and Tang D: The mechanism
of HMGB1 secretion and release. Exp Mol Med. 54:91–102. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zheng L, Zhu Q, Xu C, Li M, Li H, Yi PQ,
Xu FF, Cao L and Chen JY: Glycyrrhizin mitigates radiation-induced
acute lung injury by inhibiting the HMGB1/TLR4 signalling pathway.
J Cell Mol Med. 24:214–226. 2020. View Article : Google Scholar
|
|
72
|
Denning NL, Aziz M, Ochani M, Prince JM
and Wang P: Inhibition of a triggering receptor expressed on
myeloid cells-1 (TREM-1) with an extracellular cold-inducible
RNA-binding protein (eCIRP)-derived peptide protects mice from
intestinal ischemia-reperfusion injury. Surgery. 168:478–485. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Francois B, Lambden S, Fivez T, Gibot S,
Derive M, Grouin JM, Salcedo-Magguilli M, Lemarie J, De Schryver N,
Jalkanen V, et al: Prospective evaluation of the efficacy, safety,
and optimal biomarker enrichment strategy for nangibotide, a TREM-1
inhibitor, in patients with septic shock (ASTONISH): A
double-blind, randomised, controlled, phase 2b trial. Lancet Respir
Med. 11:894–904. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Francois B, Levy M, Ferrer R, Laterre PF
and Angus DC: A mechanism-based prognostic enrichment strategy for
the development of the TREM-1 inhibitor nangibotide in septic
shock. Intensive Care Med. 51:965–967. 2025. View Article : Google Scholar : PubMed/NCBI
|















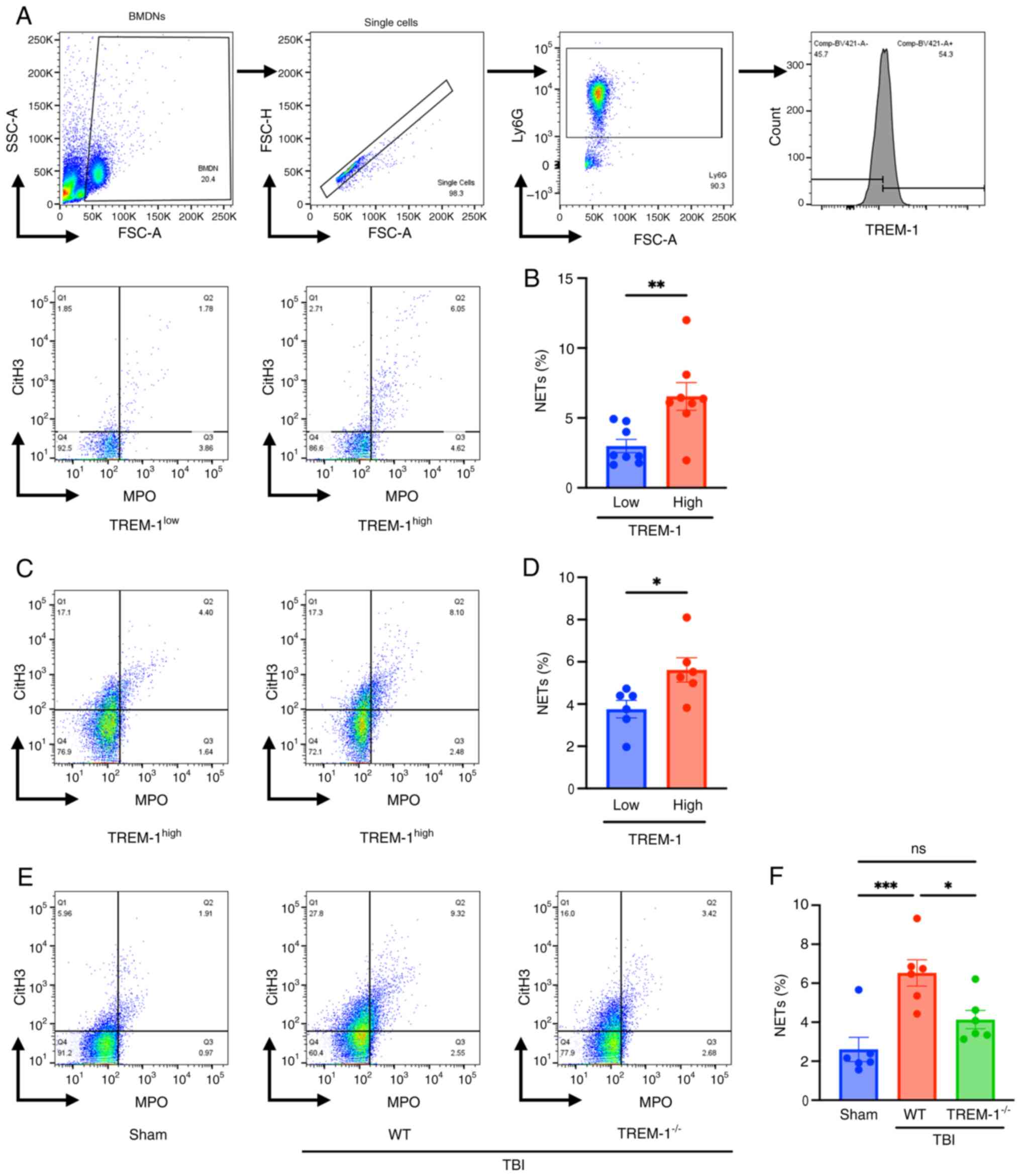
![Exposure to ionizing radiation
induces NETs. (A) BMDNs were exposed to 5, 10 and 15 Gy radiation.
NETs were assessed via flow cytometry 24 h after irradiation. (B)
The frequencies of NET positive neutrophils are shown. Groups were
compared using a one-way ANOVA and Tukey's multiple comparison
test. (C) Representative images of BMDNs that were exposed to 10 Gy
radiation. NET formation was assessed by fluorescence microscopy
using SYTOX Green 24 h after irradiation. White arrows indicate the
NET structures. Scale bar, 200 μm (Bright field and SYTOX
Green); scale bar, 100 μm [SYTOX Green (enlarged)]. (D)
Wild-type adult mice were exposed to 10 Gy TBI. Bone marrow cells
were isolated 24 h after TBI and NETs were assessed using flow
cytometry. (E) The frequencies of NET positive neutrophils. Groups
were compared using an unpaired two-tailed Student's t-test. Data
are expressed as the mean ± SEM (n=6/group). *P<0.05,
**P<0.01 and ****P<0.0001. ns, not
significant; cont, control; NET, neutrophil extracellular traps;
BMDNs, bone marrow-derived neutrophils; TBI, total body
irradiation.](/article_images/ijmm/56/4/ijmm-56-04-05598-g00.jpg)