Introduction
The threat of radiation exposure from nuclear
terrorism and warfare, amplified by ongoing global conflicts and
events such as the occupation of Ukraine's Zaporizhzhia nuclear
power plant in 2022 (1), the
Fukushima Daiichi nuclear disaster in Japan in 2011 (2,3)
and the Chernobyl disaster in Ukraine in 1986 (4), has become a significant concern.
Exposure to high-dose radiation causes acute radiation syndrome
(ARS), characterized by various clinical complications, including
hematopoietic, gastrointestinal and neurovascular syndrome
(5). Damage to radiosensitive
hematopoietic stem and progenitor cells leads to anemia,
neutropenia and thrombocytopenia (6). Neutrophils are crucial to host
defense against pathogens. Following radiation exposure,
neutropenia increases susceptibility to life-threatening infections
and risk of mortality (7).
Furthermore, severe ARS typically includes multiple organ
dysfunction (8,9). Radiation-induced inflammation has
been shown to contribute to organ dysfunction in several systems,
including hematopoiesis, the gastrointestinal tract and lungs, and
is associated with an increased risk of mortality (5,10-13). Medical management of ARS focuses
on the targeted treatment of the subsyndromes. Treatment of
hematopoietic syndrome involves the administration of granulocyte
colony stimulating factor, granulocyte macrophage stimulating
factor, erythropoiesis-stimulating factor and platelet-stimulating
thrombopoietin mimetic (14).
Hematopoietic stem cell transplantation may be considered in cases
of persistent aplasia (8).
Management of gastrointestinal syndrome is currently limited to
symptomatic treatment, including antiemetics, antidiarrheals and
antibiotics (5,8). Neurovascular syndrome is primarily
addressed with supportive care (14).
Neutrophil extracellular traps (NETs) are critical
for host defense against infection, trapping and eliminating
pathogens such as bacteria, fungi and viruses (15). NET formation is initiated by
peptidyl arginine deiminase 4 (PAD4)-mediated citrullination of
histones, leading to decondensation of chromatin (16). Expanded chromatin is released
into the cytoplasm, causing the rupture of the plasma membrane and
the release of NETs, which form web-like structures (17). Released NETs include histones,
myeloperoxidase (MPO) and other antimicrobial proteins such as
neutrophil elastase (NE) (18).
Conversely, excessive NETs can exacerbate inflammation and
contribute to organ dysfunction in sepsis (19-21). Furthermore, NETosis is a form of
regulated cell death of neutrophils, which can potentially
contribute to neutropenia (22,23). NET formation is also implicated
in sterile inflammatory conditions such as autoimmune disorders,
vasculitis and thrombosis (19).
However, the impact of high-dose ionizing radiation on NET
formation in healthy tissue and the underlying mechanisms involved
remains largely unexplored. Current research on radiation-induced
NETs primarily focuses in the context of cancer radiotherapy
(24).
Cold-inducible RNA-binding protein (CIRP) is an 18
kDa RNA chaperone that regulates the translation of stress response
genes (25,26). Extracellular CIRP (eCIRP), which
is released actively from stressed cells and passively from dying
cells (27), acts as a
damage-associated molecular pattern (DAMP). eCIRP exacerbates
inflammation and worsens survival in conditions such as sepsis,
hemorrhagic shock, ischemia/reperfusion (I/R) injury and radiation
injury (28-30). Triggering receptor expressed on
myeloid cells-1 (TREM-1) is an immune receptor expressed on the
cell surface, primarily on neutrophils and macrophages, that
initiates intracellular signaling cascades via phosphorylation of
DNAX-activation protein 12 (DAP12) and spleen tyrosine kinase (Syk)
(31). TREM-1 is known to induce
pro-inflammatory responses and amplify the severity of inflammatory
diseases (32,33). Our previous studies have
identified eCIRP as a novel ligand for TREM-1 and eCIRP-mediated
activation of TREM-1 as a key trigger for NET formation in sepsis
(34,35). Notably, it was demonstrated that
radiation-induced eCIRP upregulates TREM-1 surface expression on
macrophages, and that TREM-1 deficiency improves survival after
total body irradiation (TBI) (36). The present study aimed to
investigate NET formation after exposure to high-dose ionizing
radiation and the role of eCIRP and TREM-1 in radiation-induced NET
formation.
Materials and methods
Experimental animals
A total of 84 adult C57BL/6 wild-type (WT) male mice
(age, 8-12 weeks; weight, 20-25 g) were purchased from Jackson
Laboratory. CIRP knockout mice (CIRP−/−) were obtained
from Professor Jun Fujita (Kyoto University; Kyoto, Japan). TREM-1
knockout mice (TREM-1−/−;
Trem1tm1(KOMP)Vlcg) were generated by the
trans-National Institutes of Health Knockout Mouse Project (KOMP)
and obtained from the KOMP repository (University of California)
(37). Six each of
CIRP−/− and TREM-1−/− mice were used for the
assessment of NET formation in vivo by flow cytometry. Mice
were maintained in a temperature-controlled room ranging between 20
and 26°C, with a humidity level of 30-70%, under a 12-h light/dark
cycle and were fed a standard rodent diet and water ad
libitum. All animal experiments were performed in accordance
with the guidelines for using experimental animals by the National
Institutes of Health. All study procedures were approved by
Institutional Animal Care and Use Committees of the Feinstein
Institutes for Medical Research (approval no. 2023-007; Manhasset,
NY, USA).
TBI and isolation of bone marrow
cells
A total of 54 WT mice were randomly assigned to
experimental and control groups, with 24 sham and 30 TBI mice. Mice
were exposed to a single dose of 10 Gy TBI at a dose rate of 1
Gy/min (320 kV, 12.5 mA and 50 cm source-to-skin distance) using an
X-Rad320 X-ray irradiation system (Precision X-Ray, Inc.). The 10
Gy model is considered to be high-dose irradiation in mice based on
prior studies (38-40). Mice were restrained and placed in
a fitted container without shielding during irradiation. The
container was continuously rotated for even exposure. Mice were
returned to their cages after completion. Sham (control) mice were
not irradiated. After 24 h of TBI, mice were euthanized by
exsanguination under isoflurane anesthesia (induction, 3%;
maintenance, 2%). The femurs and tibias were then dissected for
analysis. The bone marrow was flushed with PBS supplemented with 2%
FBS (Gibco; Thermo Fisher Scientific, Inc), and suspensions of
cells were filtered through a 70-μm cell strainer. Red blood
cells in the bone marrow were lysed with RBC lysis buffer
(Invitrogen; Thermo Fisher Scientific, Inc.). Bone marrow cells
were collected by centrifugation at 300 × g and suspended with FACS
buffer (PBS containing 2% FBS). The humane criteria established for
the present study were as follows: minimal or no response to
stimuli, hunched or recumbent posture, grimace score of =2, body
condition score ≤2, weight loss of ≥20% or bleeding. All animals
fulfilling ≥2 humane criteria were subjected to euthanasia.
Isolation and irradiation of bone
marrow-derived neutrophils (BMDNs)
A total of 30 naïve WT mice were euthanized by
CO2 asphyxiation at a fill rate of 50% displacement of
the chamber volume per min followed by cervical dislocation, and
the femurs and tibias were subsequently dissected. The bone marrow
was flushed with ice-cold PBS supplemented with 2% FBS, and
suspensions of cells were filtered through a 70-μm cell
strainer and placed on ice. BMDNs were purified by negative
selection using the EasySep mouse neutrophil enrichment kit
(Stemcell Technologies, Inc.) according to the manufacturer's
instructions and cultured in complete RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) on a cell culture plate at 37°C in
5% CO2 for in vitro experiments. BMDNs were
either exposed to radiation at a dose rate of 3 Gy/min using the
X-ray Precision X-Rad320 (Precision X-Ray, Inc.) or not irradiated
(control). After irradiation, cells were incubated at 37°C in 5%
CO2. For the experiment of additional stimulation of
eCIRP, BMDNs were treated with 1 μg/ml eCIRP, prepared
in-house using a bacterial expression system (28), immediately after irradiation for
24 h at 37°C in 5% CO2.
Assessment of NETs and TREM-1 expression
on neutrophils by flow cytometry
The purity of the sorted neutrophils was assessed by
labeling the cells with APC-rat anti-mouse Ly6G antibody (cat. no.
127614; BioLegend, Inc.) using a LSRFortessa™ flow cytometer (BD
Biosciences). NETs were assessed using flow cytometry for both
in vivo and in vitro experiments (35). Single-cell suspensions of
neutrophils after irradiation were blocked with TruStain FcX™ PLUS
(anti-mouse CD16/32) antibody (cat. no. 156604; BioLegend, Inc.)
for 15 min at 4°C and surface-stained with APC-Ly6G, FITC anti-MPO
(cat. no. ab90812; Abcam) and rabbit anti-histone H3 (citrulline
R2+ R8+ R17) antibodies (cat. no. ab5103; Abcam) for 30 min at 4°C,
followed by staining with PE-donkey anti-rabbit IgG antibody (cat.
no. 406421, BioLegend, Inc.) as a secondary antibody for 30 min at
4°C. For the assessment of TREM-1 expression, neutrophils were also
stained with BV421-rat anti-mouse TREM-1 antibody (cat. no. 747899;
BD Biosciences) or BV 421-rat IgG2a κ isotype control (cat. no.
562602; BD Biosciences) for 30 min at 4°C. Unstained cells were
used to establish control voltage setting and single-color
compensation was performed using UltraComp eBeads™ (Invitrogen;
Thermo Fisher Scientific, Inc.). Data were obtained using the
LSRFortessa flow cytometer (BD Biosciences) and analyzed using the
FlowJo software (version 10.10.0; BD Biosciences). The population
of MPO and citrullinated H3 double-positive neutrophils were
identified as NET-forming neutrophils. Mean fluorescence intensity
(MFI) was used to evaluate the levels of TREM-1 expression.
Assessment of NETs via fluorescence
microscopy
NET formation by BMDNs after radiation exposure was
assessed by fluorescence microscopy in vitro (41). At 24 h after irradiation, BMDNs
were incubated with 250 nM SYTOX Green (cat. no. S7020; Invitrogen;
Thermo Fisher Scientific, Inc.) for 30 min at 37°C in 5%
CO2. The cells were visualized as live-cell images
without fixation using fluorescence microscopy (EVOS FL Auto
Imaging System; Thermo Fisher Scientific, Inc.).
Western blotting
PAD4 protein expression levels were assessed by
western blotting. BMDNs were collected by centrifugation at 300 × g
at 20 h after irradiation and lysed in RIPA lysis buffer [10 mM
Tris-buffered saline (TBS; pH 7.5), 1% Triton X-100, 1 mM EDTA, 1
mM EGTA, 2 mM sodium orthovanadate and 0.2 mM phenylmethylsulfonyl
fluoride] containing protease inhibitors (Pierce; Thermo Fisher
Scientific, Inc.). Protein concentrations in the cell lysates were
measured using DC protein assay kit (Bio-Rad Laboratories, Inc.).
The cell lysates of BMDNs (20 μg/lane) were dissolved in 4X
SDS sample buffer and resolved on NuPAGE 4-12% Bis-Tris gels
(Invitrogen; Thermo Fisher Scientific, Inc.), after which, they
were transferred to nitrocellulose membranes (Invitrogen; Thermo
Fisher Scientific, Inc.). After blocking with 0.1% casein in TBS
for 1 h at room temperature, each membrane was incubated overnight
at 4°C with primary antibodies of rabbit anti-PAD4 antibody
(1:1,000; cat. no. 17373-1-AP; Proteintech Group, Inc.) and mouse
anti-β-actin antibody (1:5,000; cat. no. A5441; MilliporeSigma).
The membranes were washed with TBS and then incubated with the
corresponding fluorescent secondary antibodies for 1 h at room
temperature. The secondary antibodies IRDye 800CW goat anti-rabbit
IgG (1:5,000; cat. no. 926-32211; LI-COR Biosciences) and IRDye
680RD goat anti-mouse IgG (1:5,000; cat. no. 926-68070; LI-COR
Biosciences) were used. Detection was performed using the Odyssey
Clx (LI-COR Biosciences) imaging system and quantification with the
Image Studio software (version 5.2; LI-COR Biosciences).
Reverse transcription-quantitative
PCR
BMDNs were collected by centrifugation at 300 × g at
4°C for 10 min a total of 4 h after irradiation. Illustra™ RNAspin
Mini RNA Isolation kit (Cytiva) was utilized to extract total RNA
according to the manufacturer's instructions. Subsequently, 1
μg RNA was reverse-transcribed into complementary DNA
(cDNA). Briefly, first-strand cDNA was synthesized using oligo(dT)
primers (Invitrogen; Thermo Fisher Scientific, Inc.) via incubation
at 70°C for 10 min, followed by cooling to 4°C. RT was performed by
adding a reaction mixture containing PCR buffer with
MgCl2 (Applied Biosystems; Thermo Fisher Scientific,
Inc.), dNTP mix (Invitrogen; Thermo Fisher Scientific, Inc.), RNase
inhibitor (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
and by incubation at 42°C for 60 min, heating to 95°C for 5 min and
cooling to 4°C. PCR was performed using a final volume of 20
μl containing forward and reverse primers (final
concentration, 0.06 mM each), cDNA and SYBR Green master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using a Step
One Plus real-time PCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following program was run: 95°C for 10 min
as an initial denaturation step, followed by 40 cycles of
denaturation at 95°C for 15 sec, and annealing and extension at
60°C for 1 min. Mouse β-actin mRNA served as a reference gene for
normalization. Relative gene expression levels were calculated
using the comparative 2−ΔΔCq method (42). Relative expression levels of mRNA
were represented as fold-change relative to the control group. The
primer sequences used were as follows: PAD4 forward (F),
5′-GCAGGACATGTCTCCAATGA-3′ and reverse (R),
5′-AGCTCCAGGCAATACGAGAA-3′; TREM-1 F, 5′-CTACAACCCGATCCCTACCC-3′
and R, 5′-AAACCAGGCTCTTGCTGAGA-3′; and β-actin F,
5′-CGTGAAAAGATGACCCAGATCA-3′ and R,
5′-TGGTACGACCAGAGGCATACAG-3′.
Statistical analysis
Data represented in the figures are expressed as
mean and SEM. An unpaired two-tailed Student's t-test was used for
comparisons of two groups and one-way analysis of variance followed
by Tukey's multiple comparisons post hoc test were used for
comparisons of multiple groups. Data were analyzed using the
GraphPad Prism software (version 10.3.1; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Exposure to ionizing radiation induces
NET formation
To determine whether exposure to ionizing radiation
induces NETs, BMDNs were exposed to 5 to 15 Gy radiation and NET
formation was assessed 24 h after irradiation via flow cytometry.
Citrullinated histone H3 and MPO double-positive cells were
considered as neutrophils with NETs. The number of NET-forming
neutrophils were significantly increased following 5 to 10 Gy
irradiation in a dose-dependent manner and reached a plateau at 10
Gy (Fig. 1A and B). NET
formation was further confirmed as extracellular DNA using
microscopy with SYTOX Green labelling, a nucleic acid stain that
fluoresces green when bound to DNA. Notably, SYTOX Green does not
cross intact membranes but penetrates compromised membranes. The
number of neutrophils with expanded nuclear area stained by SYTOX
Green were increased after irradiation compared with that of the
control cells, and a number of irradiated neutrophils showed long
stretches of extracellular DNA, which indicated NET formation
(Fig. 1C). NET formation was
also evaluated in vivo. Bone marrow from irradiated WT mice
24 h after 10 Gy TBI were isolated and NETs evaluated using flow
cytometry. Consistent with the in vitro experiments,
NET-forming neutrophils were significantly increased after TBI
compared with that of sham mice (Fig. 1D and E). Thus, these results
demonstrated that exposure to ionizing radiation induced NET
formation.
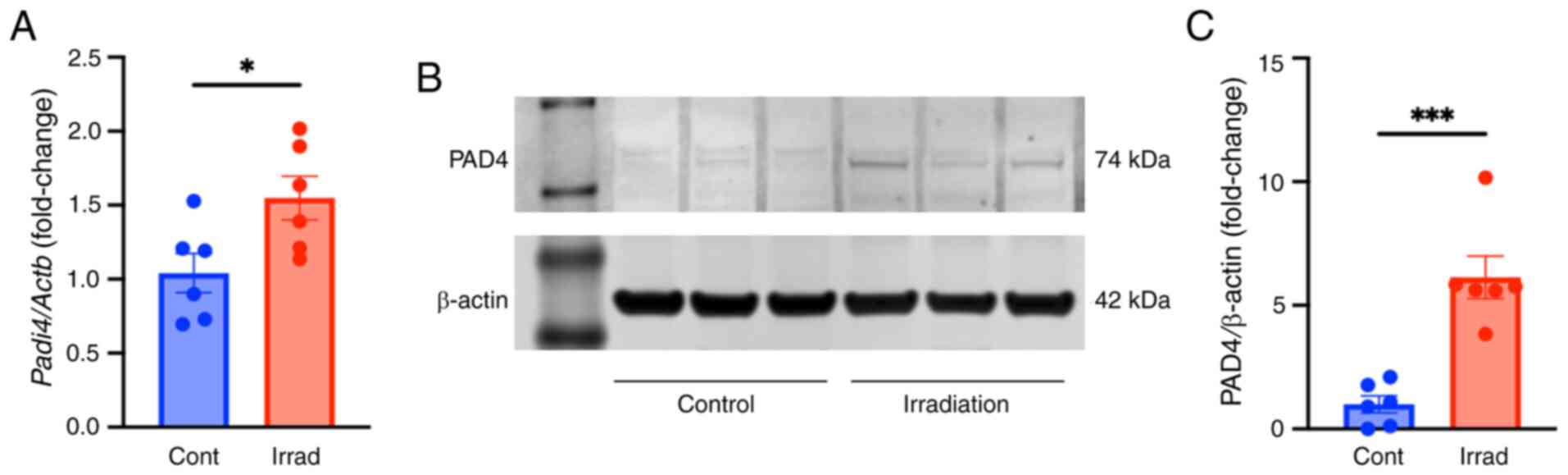
Radiation upregulates PAD4 expression
levels in neutrophils
PAD4 catalyzes histone citrullination, leading to
chromatin decondensation and NETosis (15). PAD4 mRNA and protein expression
levels in BMDNs after irradiation were investigated. BMDNs were
exposed to 10 Gy radiation and mRNA and protein expression levels
of PAD4 were evaluated 4 or 20 h after irradiation, respectively.
The mRNA levels of PAD4 were significantly upregulated after
irradiation compared with that of control cells (Fig. 2A). Similarly, PAD4 protein
expression levels were also significantly upregulated after
irradiation compared with that of control cells (Fig. 2B and C). These findings further
evidenced the induction of NET formation after irradiation.
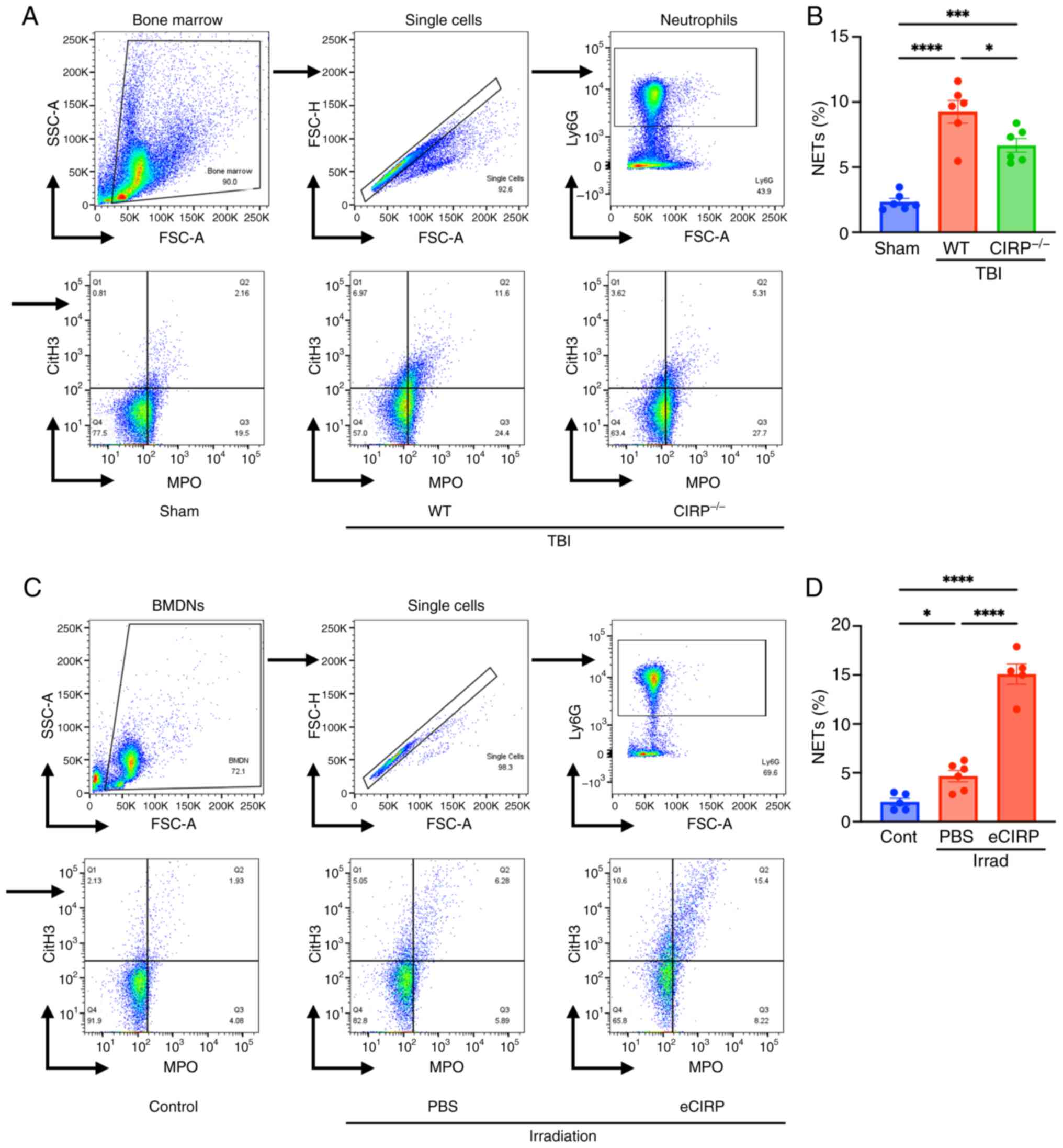
eCIRP induces NET formation after
irradiation
To determine the role of eCIRP on radiation-induced
NET formation, NET formation in bone marrow isolated from WT and
CIRP−/− mice 24 h after 10 Gy TBI was assessed.
NET-forming neutrophils were significantly increased in WT TBI mice
compared with that in sham mice. CIRP knockout ameliorated NET
formation compared with that of WT TBI mice (Fig. 3A and B). Additional effects of
eCIRP on radiation-induced NETs were assessed as a gain-of-function
experiment. The addition of eCIRP to BMDNs in culture significantly
increased NET formation after irradiation (Fig. 3C and D). These loss- and
gain-of-functional experiments indicated that eCIRP promotes
radiation-induced NET formation.
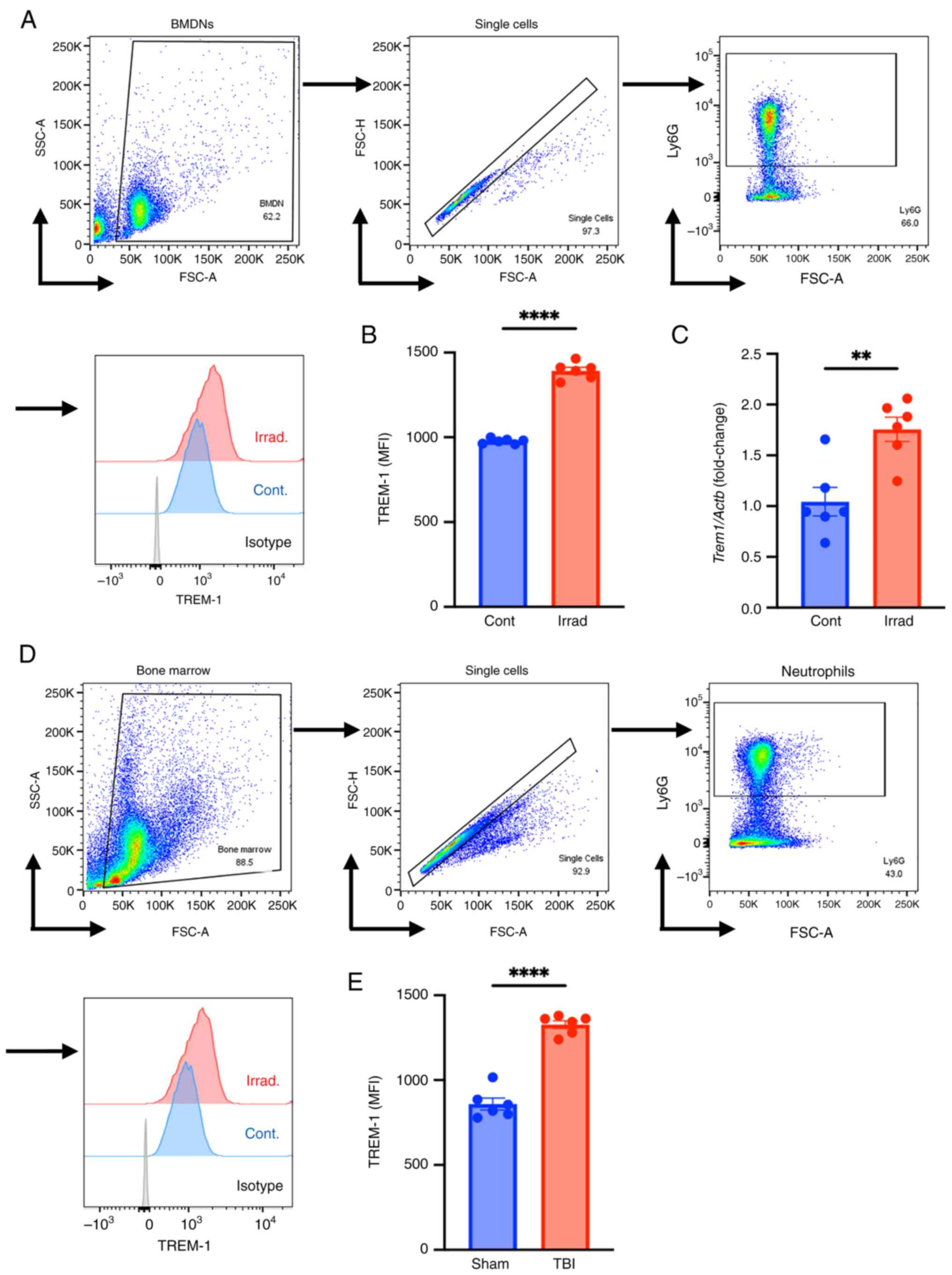
Radiation upregulates neutrophil
TREM-1
TREM-1 has been identified as a key receptor of
eCIRP and has been shown to initiate NETosis (35). Therefore, the effect of radiation
on TREM-1 expression on BMDNs after irradiation was investigated.
BMDNs isolated from WT mice were exposed to 10 Gy irradiation and
TREM-1 cell surface protein expression levels were evaluated 24 h
after irradiation using flow cytometry. Exposure to ionizing
radiation significantly upregulated TREM-1 expression levels in
BMDNs compared with that in control cells (Fig. 4A and B). A total of 4 h after
irradiation, the mRNA level of TREM-1 was significantly upregulated
compared with that of control cells (Fig. 4C). TREM-1 expression levels in WT
mice were also significantly upregulated after TBI compared with
those in the sham group (Fig. 4D and
E). Thus, radiation exposure increases mRNA and surface protein
expression of TREM-1 in neutrophils.
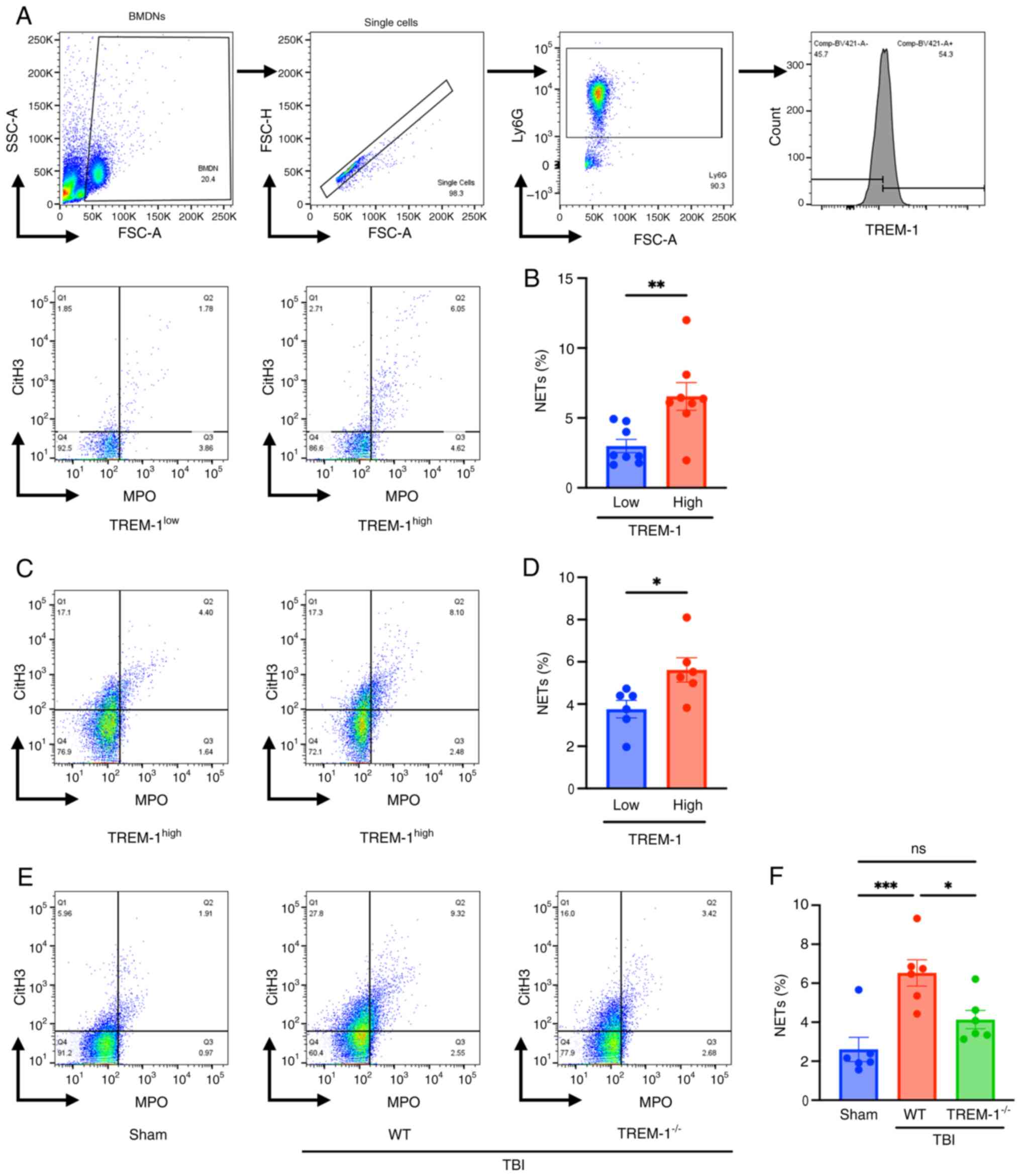
TREM-1 positively regulates
radiation-induced NETs
Next, the association between radiation-induced NETs
and the intensity of TREM-1 expression was investigated in BMDNs
in vitro. Irradiated BMDNs were classified into two
populations, neutrophils with high and low TREM-1 expression based
on the median TREM-1 expression as the cut-off value, and NET
formation was evaluated (Fig.
5A). There was a positive association between the intensity of
TREM-1 expression and the number of NET-forming neutrophils
(Fig. 5B). The association in
the bone marrow neutrophils isolated from WT TBI mice was
determined; neutrophils with high TREM-1 expression demonstrated a
significant increase in NETs after irradiation compared with that
in the low TREM-1 expression group (Fig. 5C and D). The effect of TREM-1
knockout on NET formation after irradiation was evaluated. Bone
marrow cells were isolated from irradiated WT and
TREM-1−/− mice 24 h after 10 Gy TBI and NET formation
assessed via flow cytometry. NET-forming neutrophils were
significantly increased in WT TBI mice compared with that in sham
mice, whereas NET-forming neutrophils were significantly decreased
in TREM-1−/− TBI mice compared with that in WT TBI mice
(Fig. 5E and F). These data
indicated that TREM-1 was associated with NET formation after
exposure to ionizing radiation.
Discussion
Clinical experiences from nuclear accidents and war
have shown that ARS exhibits a variety of clinical manifestations
with lethal consequences (8).
The initial treatment of patients exposed to high doses of
radiation focuses on hematopoietic dysfunction. Bacterial
translocation due to gastrointestinal injury, combined with
neutropenia, can lead to severe infections and sepsis (43). In addition, uncontrolled
inflammatory responses worsen multi-organ dysfunction in severe ARS
(8,9). NETosis is a form of regulated cell
death that could contribute to the initiation and duration of
neutropenia (23). Furthermore,
excessive NET formation has been shown to induce pro-inflammatory
responses and exacerbate organ damage (19). The present study demonstrated
that exposure to 5-15 Gy of ionizing radiation induces NET
formation, and identified the eCIRP/TREM-1 pathway as the mechanism
that mediates NET formation after radiation exposure (Fig. 6). Therefore, focusing on
radiation-induced NET formation by targeting eCIRP/TREM-1 axis can
provide novel therapeutic strategies for acute neutropenia in the
future.
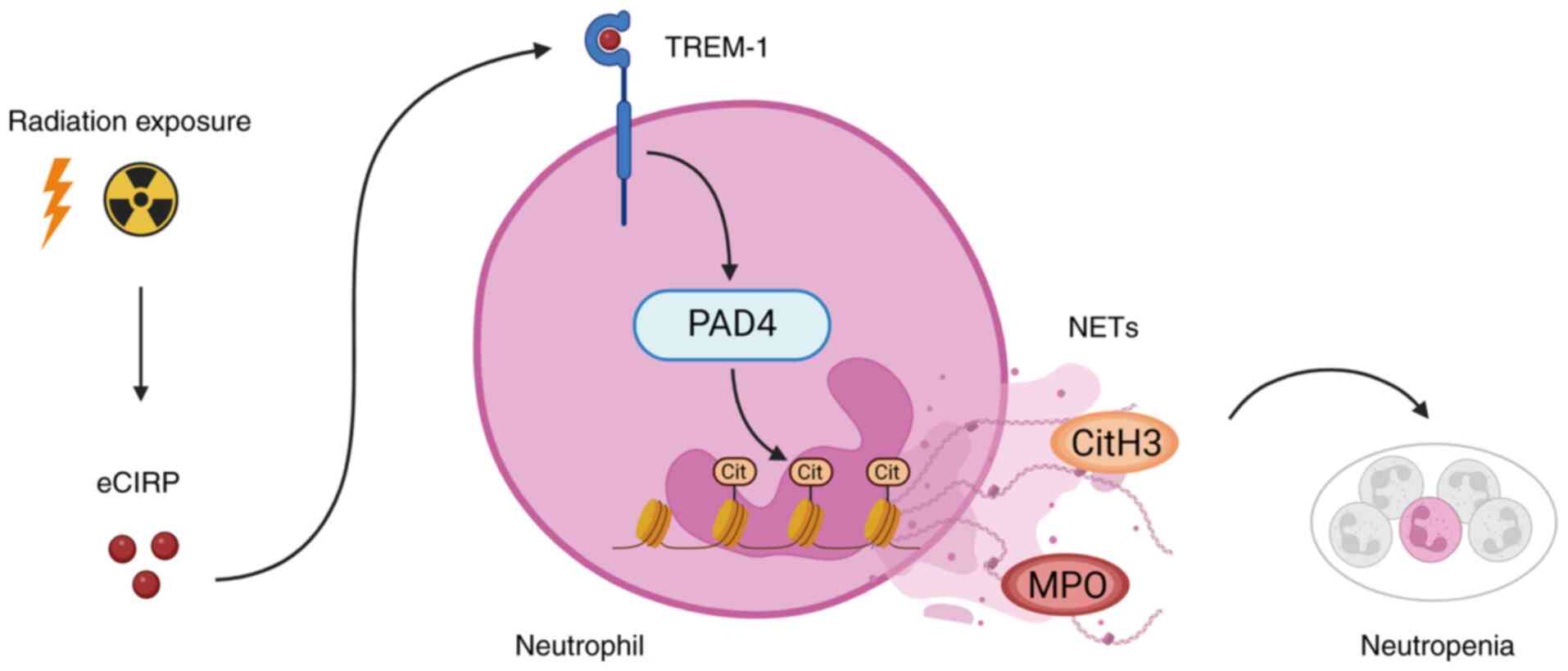 | Figure 6Exposure to ionizing radiation
induces NETs via eCIRP/TREM-1 axis. Radiation-induced eCIRP
upregulates and activates TREM-1 on neutrophils, resulting in
increased expression of PAD4 and NET formation, causing
neutropenia. NETs, neutrophil extracellular traps; PAD4, peptidyl
arginine deiminase 4; eCIRP, extracellular cold-inducible
RNA-binding protein; TREM-1, triggering receptor expressed on
myeloid cells-1; MPO, myeloperoxidase; cit, citrulline; CitH3,
citrullinated H3. The schema was created in BioRender. Murao, A.
(2025) https://BioRender.com/qhaiby0 |
To the best of our knowledge, only two studies of
ionizing radiation-induced NETosis and NET formation have been
conducted, and they were confined to the context of tumor
microenvironment (24,44). Radiation therapy targeting cancer
tissues has been shown to activate peritumoral neutrophils and
induce NET formation, which promotes angiogenesis, enhances tumor
cell adhesion and increases the risk of radiation resistance and
metastasis (19). Teijeira et
al (44) recently showed
that exposure of neutrophils isolated from healthy human blood to
low-dose γ-radiation (0.5-1 Gy) induces NET formation. It was also
shown that oxidative stress, NADPH oxidase activity and IL-8
triggers NET formation (44).
Additionally, ultraviolet irradiation has been reported to induce
NET formation via oxidative stress (45,46). However, the formation of NETs
induced by high-dose ionizing radiation and the underlying
mechanisms have not yet been studied. The present study first
demonstrated radiation-induced NET formation by the quantitative
evaluation of NETs using flow cytometry, along with direct
observation through fluorescence microscopy. The increased mRNA and
protein expression levels of PAD4 upon exposure to ionizing
radiation further supports the radiation-induced NET formation.
Subsequently, the mechanisms that regulate radiation-induced NET
formation were investigated. Several types of receptors, such as
Toll-like receptor (TLR) 2, TLR4, G protein-coupled receptor and Fc
receptors, have been reported to be involved in NET formation
(47-50). Neutrophils can be activated via
these receptors by pathogens, lipopolysaccharide, cytokines and
DAMPs to induce NET formation (15). Our previous studies reported
TREM-1 as a key receptor responsible for NET formation in a sepsis
model (35) and demonstrated the
strong binding affinity between eCIRP and TREM-1 using surface
plasmon resonance (37). The
interaction of eCIRP with TREM-1 on mouse macrophages using a
fluorescence resonance energy transfer assay was also demonstrated
(37). In addition, our previous
studies have shown that exposure to high-dose radiation induces
release of eCIRP in mice (36,51). The present study demonstrated
that the eCIRP/TREM-1 axis serves a critical role in NET induction
after radiation exposure. Currently, it has not yet been
established how TREM-1 activation promotes NET formation. However,
in a sepsis model, eCIRP-mediated activation of TREM-1 induces the
surface expression of ICAM-1, leading to NET formation via Rho
activation (35). Furthermore,
eCIRP activates the downstream signaling pathway of TREM-1, as
evaluated by DAP12 and Syk phosphorylation (35). Syk activation mediates the
phosphorylation of phospholipase Cγ (PLCγ) (52). Activated PLCγ degrades
phosphatidylinositol 4,5-biphosphate in the plasma membrane to
inositol 1,4,5-triophosphate, triggering the release of calcium
from endoplasmic reticulum (52). Elevated intracellular calcium
levels induce PAD4 activation and extracellular calcium chelation
has been shown to inhibit NETosis induced by IL-8 and
phorbol-12-myristate-13-acetate (53,54). Furthermore, activation of Syk
following TREM-1 stimulation has been shown to induce reactive
oxygen species (ROS) production via the PIK3/AKT pathway (55). ROS are also critical in NETosis
signaling, as demonstrated by the inhibition of NETosis upon
treatment of neutrophils with ROS scavengers (53,54). A previous study has reported that
low-dose γ-radiation induces NET formation depending on oxidative
stress and ROS inhibition significantly attenuates
radiation-induced NETosis (44).
Since TREM-1 is associated with ROS production, the aforementioned
findings with low-dose irradiation could potentially also be
mediated, at least in part, via the TREM-1 pathway. Nevertheless,
whether the induction of NETs triggered by the eCIRP/TREM-1 axis is
concurrent after low-dose irradiation may require further
investigation.
Bone marrow damage from radiation exposure leads to
less recruitment of polymorphonuclear leukocytes into the systemic
circulation (56). Furthermore,
the half-life of circulating neutrophils is short, ranging from 18
to 19 h. (23). Consequently,
induction of neutrophil cell death after radiation exposure
inevitably exacerbates neutropenia, resulting in high
susceptibility to infection (7).
Furthermore, organ dysfunction caused by NETs has been demonstrated
in a variety of diseases, including sepsis, I/R injury, and renal
and hepatic injuries (57-65). In sepsis and I/R injury, NETs
have been shown to cause systemic inflammation and epithelial
barrier disruption, resulting in intestinal and lung injury
(57-63). Inflammation and organ damage by
NETs have also been demonstrated in liver and renal injuries
(64,65). Histones, a key component of NETs,
have been shown to induce inflammatory responses via the
TLR2/TLR4-MyD88 signaling or the NLPR3 inflammasome pathway
(66). Histones also bind to
phospholipids in the plasma membrane of epithelial and endothelial
tissues, altering their permeability and causing cell death and
tissue damage (67). NET
components such as MPO and NE impair junctional integrity and
promote vascular permeability (68,69). MPO has been shown to bind to the
endothelial glycocalyx via heparan sulfate side chains, disrupting
the endothelial glycocalyx structure and causing endothelial injury
(68). Considering that
excessive inflammatory responses accompanied by disruption of
vascular homeostasis contribute to multi-organ dysfunction after
severe ARS, radiation-induced NET formation may potentially be an
exacerbating factor in radiation injury.
The detrimental role of DAMPs has been addressed in
a number of inflammatory conditions such as sepsis, hemorrhage and
I/R injury (27). Although
evidence for DAMPs in radiation injury is limited, targeting DAMPs
presents a promising potential strategy for mitigating
radiation-induced organ damage (30). High mobility group box 1 (HMGB1)
is a nuclear protein, which can be translocated to cytoplasm in
response to cellular stress and act as a DAMP (70). Glycyrrhizin, an HMGB1 inhibitor,
suppresses the HMGB1/TLR4 signaling, including NF-κB, JNK and
ERJ1/2, and reduces the production of inflammatory cytokines,
resulting in mitigating radiation-induced lung injury (71). Our previous study has shown that
pharmacological inhibition of eCIRP/TREM-1 binding using an
eCIRP-derived decoy peptide improves systemic inflammation, tissue
injury and survival in sepsis and intestinal I/R injury (37,72). Additionally, the TREM-1 inhibitor
LP17 was shown to reduce eCIRP-induced NET formation (35). Furthermore, another TREM-1
inhibitor LR12 is currently in clinical trials for sepsis (73,74). In radiation injury, knockout of
TREM-1 improves survival after TBI, and that treatment with a
monoclonal neutralizing antibody against eCIRP improves macrophage
phagocytic function following high-dose radiation exposure
(36,51). The present study provides novel
insights into the role of eCIRP and TREM-1 in radiation-induced
immune dysfunction.
Several limitations of the present study existed.
First, the impact of radiation-induced NETosis on neutropenia was
not assessed. Studies evaluating the impact of NETosis inhibition
on neutropenia and susceptibility to infection may provide valuable
insights into the role of NETosis in radiation injury. Second,
whether the formation of NETs contributes to the progression of
organ injuries after radiation exposure was not investigated;
elucidating the possible involvement of NETs in radiation-induced
organ injuries may be important for the development of therapeutic
strategies. Third, NET formation in the tissues by
histopathological evaluation was not directly observed. Detection
of NETs in the tissues including bone marrow and intestines may
clarify the relevance of radiation-induced NET formation and organ
injuries.
In conclusion, the present study demonstrated that
exposure to ionizing radiation induces NET formation. NETosis
resulted in neutrophil death to exacerbate neutropenia, which
contributed to increased susceptibility to infection. The
eCIRP/TREM-1 axis served a key role in radiation-induced NET
formation. Therefore, targeting the eCIRP/TREM-1 axis to regulate
NETs may potentially offer a novel therapeutic strategy for
alleviating neutropenia and preventing infection after radiation
exposure in the future.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MA, MB and PW conceived the study. SY performed the
experiments. SY and AM analyzed the data and drafted the
manuscript. MA, MB and PW reviewed and revised the manuscript. MB
and PW confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All study procedures were approved by Institutional
Animal Care and Use Committees of the Feinstein Institutes for
Medical Research (approval no. 2023-007; Manhasset, NY, USA).
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
Abbreviations:
|
ARS
|
acute radiation syndrome
|
|
BMDNs
|
bone marrow-derived neutrophils
|
|
CIRP
|
cold-inducible RNA-binding protein
|
|
DAMP
|
damage-associated molecular
pattern
|
|
eCIRP
|
extracellular CIRP
|
|
HMGB1
|
high mobility group box 1
|
|
IL
|
interleukin
|
|
I/R
|
ischemia/reperfusion
|
|
MFI
|
mean fluorescence intensity
|
|
MPO
|
myeloperoxidase
|
|
NE
|
neutrophil elastase
|
|
NETs
|
neutrophil extracellular traps
|
|
PAD4
|
peptidyl arginine deiminase 4
|
|
ROS
|
reactive oxygen species
|
|
Syk
|
spleen tyrosine kinase
|
|
TBI
|
total body irradiation
|
|
TLR
|
toll-like receptor
|
|
TREM-1
|
triggering receptor expressed on
myeloid cells-1
|
Acknowledgements
The authors thank Dr Yongchan Lee and Dr Dmitriy
Lapin of the Center for Immunology and Inflammation at the
Feinstein Institutes for Medical Research (Manhasset, NY, USA) for
their technical support and thoughtful discussion.
Funding
The present study was supported by the National Institutes of
Health (grant nos. U01AI170018, U01AI133655, U01AI186997 and
R35GM118337).
References
|
1
|
Harada KH, Soleman SR, Ang JSM and
Trzcinski AP: Conflict-related environmental damages on health:
Lessons learned from the past wars and ongoing Russian invasion of
Ukraine. Environ Health Prev Med. 27:352022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hasegawa A, Tanigawa K, Ohtsuru A, Yabe H,
Maeda M, Shigemura J, Ohira T, Tominaga T, Akashi M, Hirohashi N,
et al: Health effects of radiation and other health problems in the
aftermath of nuclear accidents, with an emphasis on Fukushima.
Lancet. 386:479–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirohashi N, Shime N and Fujii T: Beyond
the unthinkable: Are we prepared for rare disasters? Anaesth Crit
Care Pain Med. 42:1012662023.PubMed/NCBI
|
|
4
|
Baranov A, Gale RP, Guskova A, Piatkin E,
Selidovkin G, Muravyova L, Champlin RE, Danilova N, Yevseeva L and
Petrosyan L: Bone marrow transplantation after the Chernobyl
nuclear accident. N Engl J Med. 321:205–212. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macià I, Garau M, Lucas Calduch A and
López EC: Radiobiology of the acute radiation syndrome. Rep Pract
Oncol Radiother. 16:123–130. 2011. View Article : Google Scholar
|
|
6
|
McCart EA, Lee YH, Jha J, Mungunsukh O,
Rittase WB, Summers TA Jr, Muir J and Day RM: Delayed captopril
administration mitigates hematopoietic injury in a murine model of
total body irradiation. Sci Rep. 9:21982019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong K, Chang PY, Fielden M, Downey AM,
Bunin D, Bakke J, Gahagen J, Iyer L, Doshi S, Wierzbicki W and
Authier S: Pharmacodynamics of romiplostim alone and in combination
with pegfilgrastim on acute radiation-induced thrombocytopenia and
neutropenia in Non-human primates. Int J Radiat Biol. 96:155–166.
2020. View Article : Google Scholar
|
|
8
|
Tanigawa K: Case review of severe acute
radiation syndrome from whole body exposure: Concepts of
radiation-induced multi-organ dysfunction and failure. J Radiat
Res. 62:i15–i20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams JP and McBride WH: After the bomb
drops: A new look at Radiation-induced multiple organ dysfunction
syndrome (MODS). Int J Radiat Biol. 87:851–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim YJ, Jeong J, Park K, Sohn KY, Yoon SY
and Kim JW: Mitigation of hematopoietic syndrome of acute radiation
syndrome by 1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) is
associated with regulation of systemic inflammation in a murine
model of Total-Body Irradiation. Radiat Res. 196:55–65. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerassy-Vainberg S, Blatt A, Danin-Poleg
Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O,
Dheer R, et al: Radiation induces proinflammatory dysbiosis:
Transmission of inflammatory susceptibility by host cytokine
induction. Gut. 67:97–107. 2018. View Article : Google Scholar
|
|
12
|
English J, Dhanikonda S, Tanaka KE, Koba
W, Eichenbaum G, Yang WL and Guha C: Thrombopoietin mimetic reduces
mouse lung inflammation and fibrosis after radiation by attenuating
activated endothelial phenotypes. JCI Insight. 9:e1813302024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaue D, Micewicz ED, Ratikan JA, Xie MW,
Cheng G and McBride WH: Radiation and inflammation. Semin Radiat
Oncol. 25:4–10. 2015. View Article : Google Scholar :
|
|
14
|
Dainiak N and Albanese J: Medical
management of acute radiation syndrome. J Radiol Prot. 42:2022.
View Article : Google Scholar
|
|
15
|
Thiam HR, Wong SL, Wagner DD and Waterman
CM: Cellular mechanisms of NETosis. Annu Rev Cell Dev Biol.
36:191–218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christophorou MA, Castelo-Branco G,
Halley-Stott RP, Oliveira CS, Loos R, Radzisheuskaya A, Mowen KA,
Bertone P, Silva JC, Zernicka-Goetz M, et al: Citrullination
regulates pluripotency and histone H1 binding to chromatin. Nature.
507:104–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiam HR, Wong SL, Qiu R, Kittisopikul M,
Vahabikashi A, Goldman AE, Goldman RD, Wagner DD and Waterman CM:
NETosis proceeds by cytoskeleton and endomembrane disassembly and
PAD4-mediated chromatin decondensation and nuclear envelope
rupture. Proc Natl Acad Sci USA. 117:7326–7337. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chapman EA, Lyon M, Simpson D, Mason D,
Beynon RJ, Moots RJ and Wright HL: Caught in a trap? Proteomic
analysis of neutrophil extracellular traps in rheumatoid arthritis
and systemic lupus erythematosus. Front Immunol. 10:4232019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Kim SJ, Lei Y, Wang S, Wang H,
Huang H, Zhang H and Tsung A: Neutrophil extracellular traps in
homeostasis and disease. Signal Transduct Target Ther. 9:2352024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Silva CMS, Wanderley CWS, Veras FP, Sonego
F, Nascimento DC, Goncalves AV, Martins TV, Colon DF, Borges VF,
Brauer VS, et al: Gasdermin D inhibition prevents multiple organ
dysfunction during sepsis by blocking NET formation. Blood.
138:2702–2713. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan C, Aziz M and Wang P: The vitals of
NETs. J Leukoc Biol. 110:797–808. 2021. View Article : Google Scholar
|
|
22
|
Stephenson HN, Herzig A and Zychlinsky A:
Beyond the grave: When is cell death critical for immunity to
infection? Curr Opin Immunol. 38:59–66. 2016. View Article : Google Scholar
|
|
23
|
Lawrence SM, Corriden R and Nizet V: How
neutrophils meet their end. Trends Immunol. 41:531–544. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shinde-Jadhav S, Mansure JJ, Rayes RF,
Marcq G, Ayoub M, Skowronski R, Kool R, Bourdeau F, Brimo F, Spicer
J and Kassouf W: Role of neutrophil extracellular traps in
radiation resistance of invasive bladder cancer. Nat Commun.
12:27762021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aziz M, Brenner M and Wang P:
Extracellular CIRP (eCIRP) and inflammation. J Leukoc Biol.
106:133–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou M, Aziz M, Li J, Jha A, Ma G, Murao A
and Wang P: BMAL2 promotes eCIRP-induced macrophage endotoxin
tolerance. Front Immunol. 15:14266822024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murao A, Aziz M, Wang H, Brenner M and
Wang P: Release mechanisms of major DAMPs. Apoptosis. 26:152–162.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiang X, Yang WL, Wu R, Zhou M, Jacob A,
Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al: Cold-inducible
RNA-binding protein (CIRP) triggers inflammatory responses in
hemorrhagic shock and sepsis. Nat Med. 19:1489–1495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hollis R, Li J, Lee Y, Jin H, Zhou M, Nofi
CP, Sfakianos M, Coppa G, Aziz M and Wang P: A novel opsonic
extracellular cirp inhibitor Mop3 alleviates gut
Ischemia/reperfusion injury. Shock. 63:101–109. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaga S, Aziz M, Murao A, Brenner M and
Wang P: DAMPs and radiation injury. Front Immunol. 15:13539902024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carrasco K, Boufenzer A, Jolly L, Le
Cordier H, Wang G, Heck AJ, Cerwenka A, Vinolo E, Nazabal A,
Kriznik A, et al: TREM-1 multimerization is essential for its
activation on monocytes and neutrophils. Cell Mol Immunol.
16:460–472. 2019. View Article : Google Scholar :
|
|
32
|
Siskind S, Brenner M and Wang P: TREM-1
modulation strategies for sepsis. Front Immunol. 13:9073872022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Borjas T, Jacob A, Yen H, Patel V, Coppa
GF, Aziz M and Wang P: Inhibition of the Interaction of TREM-1 and
eCIRP attenuates inflammation and improves survival in hepatic
Ischemia/reperfusion. Shock. 57:246–255. 2022. View Article : Google Scholar :
|
|
34
|
Denning NL, Aziz M, Diao L, Prince JM and
Wang P: Targeting the eCIRP/TREM-1 interaction with a small
molecule inhibitor improves cardiac dysfunction in neonatal sepsis.
Mol Med. 26:1212020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murao A, Arif A, Brenner M, Denning NL,
Jin H, Takizawa S, Nicastro B, Wang P and Aziz M: Extracellular
CIRP and TREM-1 axis promotes ICAM-1-Rho-mediated NETosis in
sepsis. FASEB J. 34:9771–9786. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaga S, Murao A, Ma G, Brenner M, Aziz M
and Wang P: Radiation upregulates macrophage TREM-1 expression to
exacerbate injury in mice. Front Immunol. 14:11512502023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Denning NL, Aziz M, Murao A, Gurien SD,
Ochani M, Prince JM and Wang P: Extracellular CIRP as an endogenous
TREM-1 ligand to fuel inflammation in sepsis. JCI Insight.
5:e1341722020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Knops K, Boldt S, Wolkenhauer O and
Kriehuber R: Gene expression in low- and high-dose-irradiated human
peripheral blood lymphocytes: Possible applications for
biodosimetry. Radiat Res. 178:304–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida K, Misumi M, Hamasaki K, Kyoizumi
S, Satoh Y, Tsuruyama T, Uchimura A and Kusunoki Y: High-dose
radiation preferentially induces the clonal expansion of
hematopoietic progenitor cells over mature T and B cells in mouse
bone marrow. Stem Cell Reports. 20:1024232025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Albrecht H, Durbin-Johnson B, Yunis R,
Kalanetra KM, Wu S, Chen R, Stevenson TR and Rocke DM:
Transcriptional response of ex vivo human skin to ionizing
radiation: Comparison between low- and high-dose effects. Radiat
Res. 177:69–83. 2012. View Article : Google Scholar
|
|
41
|
Ode Y, Aziz M and Wang P: CIRP increases
ICAM-1+ phenotype of neutrophils exhibiting elevated iNOS and NETs
in sepsis. J Leukoc Biol. 103:693–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using Real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Dainiak N: Medical management of acute
radiation syndrome and associated infections in a High-casualty
incident. J Radiat Res. 59(Suppl_2): ii54–ii64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teijeira A, Garasa S, Ochoa MC,
Sanchez-Gregorio S, Gomis G, Luri-Rey C, Martinez-Monge R, Pinci B,
Valencia K, Palencia B, et al: Low-dose ionizing gamma-radiation
elicits the extrusion of neutrophil extracellular traps. Clin
Cancer Res. 30:4131–4142. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arzumanyan G, Mamatkulov K, Arynbek Y,
Zakrytnaya D, Jevremovic A and Vorobjeva N: Radiation from UV-A to
red light induces ROS-Dependent release of neutrophil extracellular
traps. Int J Mol Sci. 24:57702023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zawrotniak M, Bartnicka D and Rapala-Kozik
M: UVA and UVB radiation induce the formation of neutrophil
extracellular traps by human polymorphonuclear cells. J Photochem
Photobiol B. 196:1115112019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yipp BG, Petri B, Salina D, Jenne CN,
Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert
HC, et al: Infection-induced NETosis is a dynamic process involving
neutrophil multitasking in vivo. Nat Med. 18:1386–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rossaint J, Herter JM, Van Aken H, Napirei
M, Doring Y, Weber C, Soehnlein O and Zarbock A: Synchronized
integrin engagement and chemokine activation is crucial in
neutrophil extracellular Trap-mediated sterile inflammation. Blood.
123:2573–2584. 2014. View Article : Google Scholar
|
|
49
|
Aleyd E, van Hout MW, Ganzevles SH, Hoeben
KA, Everts V, Bakema JE and van Egmond M: IgA enhances NETosis and
release of neutrophil extracellular traps by polymorphonuclear
cells via Fcα receptor I. J Immunol. 192:2374–2383. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Keshari RS, Jyoti A, Dubey M, Kothari N,
Kohli M, Bogra J, Barthwal MK and Dikshit M: Cytokines induced
neutrophil extracellular traps formation: Implication for the
inflammatory disease condition. PLoS One. 7:e481112012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamaga S, Murao A, Zhou M, Aziz M, Brenner
M and Wang P: Radiation-induced eCIRP impairs macrophage bacterial
phagocytosis. J Leukoc Biol. 116:1072–1079. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Arts RJ, Joosten LA, van der Meer JW and
Netea MG: TREM-1: Intracellular signaling pathways and interaction
with pattern recognition receptors. J Leukoc Biol. 93:209–215.
2013. View Article : Google Scholar
|
|
53
|
Parker H, Dragunow M, Hampton MB, Kettle
AJ and Winterbourn CC: Requirements for NADPH oxidase and
myeloperoxidase in neutrophil extracellular trap formation differ
depending on the stimulus. J Leukoc Biol. 92:841–849. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kenny EF, Herzig A, Kruger R, Muth A,
Mondal S, Thompson PR, Brinkmann V, Bernuth HV and Zychlinsky A:
Diverse stimuli engage different neutrophil extracellular trap
pathways. Elife. 6:e244372017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Baruah S, Murthy S, Keck K, Galvan I,
Prichard A, Allen LH, Farrelly M and Klesney-Tait J: TREM-1
regulates neutrophil chemotaxis by promoting NOX-dependent
superoxide production. J Leukoc Biol. 105:1195–1207. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cassatt DR, Winters TA and PrabhuDas M:
Immune dysfunction from radiation exposure. Radiat Res.
200:389–395. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun S, Duan Z, Wang X, Chu C, Yang C, Chen
F, Wang D, Wang C, Li Q and Ding W: Neutrophil extracellular traps
impair intestinal barrier functions in sepsis by regulating
TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis.
12:6062021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao J, Zhen N, Zhou Q, Lou J, Cui W,
Zhang G and Tian B: NETs promote inflammatory injury by activating
cGAS-STING pathway in acute lung injury. Int J Mol Sci.
24:51252023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hawez A, Taha D, Algaber A, Madhi R,
Rahman M and Thorlacius H: MiR-155 regulates neutrophil
extracellular trap formation and lung injury in abdominal sepsis. J
Leukoc Biol. 111:391–400. 2022. View Article : Google Scholar
|
|
60
|
Gao X, Hao S, Yan H, Ding W, Li K and Li
J: Neutrophil extracellular traps contribute to the intestine
damage in endotoxemic rats. J Surg Res. 195:211–218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luo L, Zhang S, Wang Y, Rahman M, Syk I,
Zhang E and Thorlacius H: Proinflammatory role of neutrophil
extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol
Physiol. 307:L586–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chu C, Wang X, Chen F, Yang C, Shi L, Xu
W, Wang K, Liu B, Wang C, Sun D, et al: Neutrophil extracellular
traps aggravate intestinal epithelial necroptosis in
ischaemia-reperfusion by regulating TLR4/RIPK3/FUNDC1-required
mitophagy. Cell Prolif. 57:e135382024. View Article : Google Scholar
|
|
63
|
Zhan Y, Ling Y, Deng Q, Qiu Y, Shen J, Lai
H, Chen Z, Huang C, Liang L, Li X, et al: HMGB1-Mediated neutrophil
extracellular trap formation exacerbates intestinal
Ischemia/Reperfusion-Induced acute lung injury. J Immunol.
208:968–978. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jansen MP, Emal D, Teske GJ, Dessing MC,
Florquin S and Roelofs JJ: Release of extracellular DNA influences
renal ischemia reperfusion injury by platelet activation and
formation of neutrophil extracellular traps. Kidney Int.
91:352–364. 2017. View Article : Google Scholar
|
|
65
|
Ye D, Yao J, Du W, Chen C, Yang Y, Yan K,
Li J, Xu Y, Zang S, Zhang Y, et al: Neutrophil extracellular traps
mediate acute liver failure in regulation of miR-223/Neutrophil
elastase signaling in mice. Cell Mol Gastroenterol Hepatol.
14:587–607. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Allam R, Scherbaum CR, Darisipudi MN,
Mulay SR, Hagele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu
M, Schwarzenberger C, et al: Histones from dying renal cells
aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol.
23:1375–1388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Silk E, Zhao H, Weng H and Ma D: The role
of extracellular histone in organ injury. Cell Death Dis.
8:e28122017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Manchanda K, Kolarova H, Kerkenpass C,
Mollenhauer M, Vitecek J, Rudolph V, Kubala L, Baldus S, Adam M and
Klinke A: MPO (Myeloperoxidase) reduces endothelial glycocalyx
thickness dependent on its cationic charge. Arterioscler Thromb
Vasc Biol. 38:1859–1867. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ushakumari CJ, Zhou QL, Wang YH, Na S,
Rigor MC, Zhou CY, Kroll MK, Lin BD and Jiang ZY: Neutrophil
elastase increases vascular permeability and leukocyte
transmigration in cultured endothelial cells and obese mice. Cells.
11:22882022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen R, Kang R and Tang D: The mechanism
of HMGB1 secretion and release. Exp Mol Med. 54:91–102. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zheng L, Zhu Q, Xu C, Li M, Li H, Yi PQ,
Xu FF, Cao L and Chen JY: Glycyrrhizin mitigates radiation-induced
acute lung injury by inhibiting the HMGB1/TLR4 signalling pathway.
J Cell Mol Med. 24:214–226. 2020. View Article : Google Scholar
|
|
72
|
Denning NL, Aziz M, Ochani M, Prince JM
and Wang P: Inhibition of a triggering receptor expressed on
myeloid cells-1 (TREM-1) with an extracellular cold-inducible
RNA-binding protein (eCIRP)-derived peptide protects mice from
intestinal ischemia-reperfusion injury. Surgery. 168:478–485. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Francois B, Lambden S, Fivez T, Gibot S,
Derive M, Grouin JM, Salcedo-Magguilli M, Lemarie J, De Schryver N,
Jalkanen V, et al: Prospective evaluation of the efficacy, safety,
and optimal biomarker enrichment strategy for nangibotide, a TREM-1
inhibitor, in patients with septic shock (ASTONISH): A
double-blind, randomised, controlled, phase 2b trial. Lancet Respir
Med. 11:894–904. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Francois B, Levy M, Ferrer R, Laterre PF
and Angus DC: A mechanism-based prognostic enrichment strategy for
the development of the TREM-1 inhibitor nangibotide in septic
shock. Intensive Care Med. 51:965–967. 2025. View Article : Google Scholar : PubMed/NCBI
|















![Exposure to ionizing radiation
induces NETs. (A) BMDNs were exposed to 5, 10 and 15 Gy radiation.
NETs were assessed via flow cytometry 24 h after irradiation. (B)
The frequencies of NET positive neutrophils are shown. Groups were
compared using a one-way ANOVA and Tukey's multiple comparison
test. (C) Representative images of BMDNs that were exposed to 10 Gy
radiation. NET formation was assessed by fluorescence microscopy
using SYTOX Green 24 h after irradiation. White arrows indicate the
NET structures. Scale bar, 200 μm (Bright field and SYTOX
Green); scale bar, 100 μm [SYTOX Green (enlarged)]. (D)
Wild-type adult mice were exposed to 10 Gy TBI. Bone marrow cells
were isolated 24 h after TBI and NETs were assessed using flow
cytometry. (E) The frequencies of NET positive neutrophils. Groups
were compared using an unpaired two-tailed Student's t-test. Data
are expressed as the mean ± SEM (n=6/group). *P<0.05,
**P<0.01 and ****P<0.0001. ns, not
significant; cont, control; NET, neutrophil extracellular traps;
BMDNs, bone marrow-derived neutrophils; TBI, total body
irradiation.](/article_images/ijmm/56/4/ijmm-56-04-05598-g00.jpg)