Cardiovascular diseases (CVDs), the leading cause of
death worldwide, claims ~18 million lives each year and has high
morbidity rates (1-4). Atherosclerosis is the main
pathologic mechanism of most CVDs (5). These chronic plaques in vessel,
composed mainly of cholesterol, fat, calcium and blood cells,
gradually harden over time, leading to arterial stenosis and
thereby restricting the flow of oxygen-rich blood to various parts
of the body (6). Atherosclerosis
has been found to be the central mechanism of this pathological
process and the leading cause of CVDs worldwide (7).
In recent years, atherosclerosis has been recognized
as a chronic inflammatory disease and leads to arterial stenosis
and plaque formation due to the accumulation of lipids and
inflammatory cells within the arterial wall (8-11). The role of adaptive immunity in
atherosclerosis has increasingly attracted attention (12). The adaptive immune system
participates in the development of atherosclerosis through various
cellular and molecular mechanisms, including the interactions of T
cells (13), B cells (14), antigen-presenting cells (15) and cytokines (16). Inflammation plays a key role in
the progression of atherosclerosis, and adaptive immune responses
influence plaque formation and stability by modulating the activity
of inflammatory cells and the secretion of cytokines. These studies
further confirm the key role of inflammation and immunity in
atherosclerosis and provide a theoretical basis for
immune-modulating therapies.
Epigenetics focuses on heritable changes in gene
expression that are not derived from alterations in the nucleotide
sequence, such as DNA or RNA methylation modifications, as well as
post-translational modifications of proteins (17). Lactylation, an emerging
post-translational modification, has garnered widespread attention
in biological and medical research (18-20). Lactylation plays a crucial role
in various biological processes, including the regulation of
macrophage function, modulation of glycolytic activity and the
promotion of tumorigenesis (21). As a key regulatory factor in
numerous biological processes and disease mechanisms, lactylation
modification has become a hot topic in molecular biology studies
(22,23).
Although the potential role of lactylation
modification in CVD has been demonstrated (24), its role in regulating immunity in
the context of atherosclerosis remains largely elusive. During the
pathological process of atherosclerosis, the accumulation of
lactate in local tissues obviously impacts this process. Therefore,
vast research efforts have explored the interaction between
lactylation modifications and adaptive immunity within the
atherosclerotic microenvironment. This review aims to clarify the
role of lactylation in immunity in the context of atherosclerosis
and summarize the driving mechanisms. This article not only
explores the roles of lactate and lactylation in atherosclerosis
but also systematically analyzes their impacts on various
components of the immune system, such as macrophages, T cells and B
cells. To the best of our knowledge, this multidimensional
perspective is relatively rare in previous reviews (12,25-27), as most studies have focused
solely on a single cell type or mechanism. By closely integrating
the metabolic characteristics of lactate with the regulation of
immune responses, the article highlights the crucial role of
metabolic reprogramming in atherosclerosis. This integrative
approach provides a more comprehensive framework for understanding
the pathogenesis of atherosclerosis. These innovative aspects not
only enrich our understanding of the pathogenic mechanisms of
atherosclerosis but also offer new directions for future
therapeutic strategies.
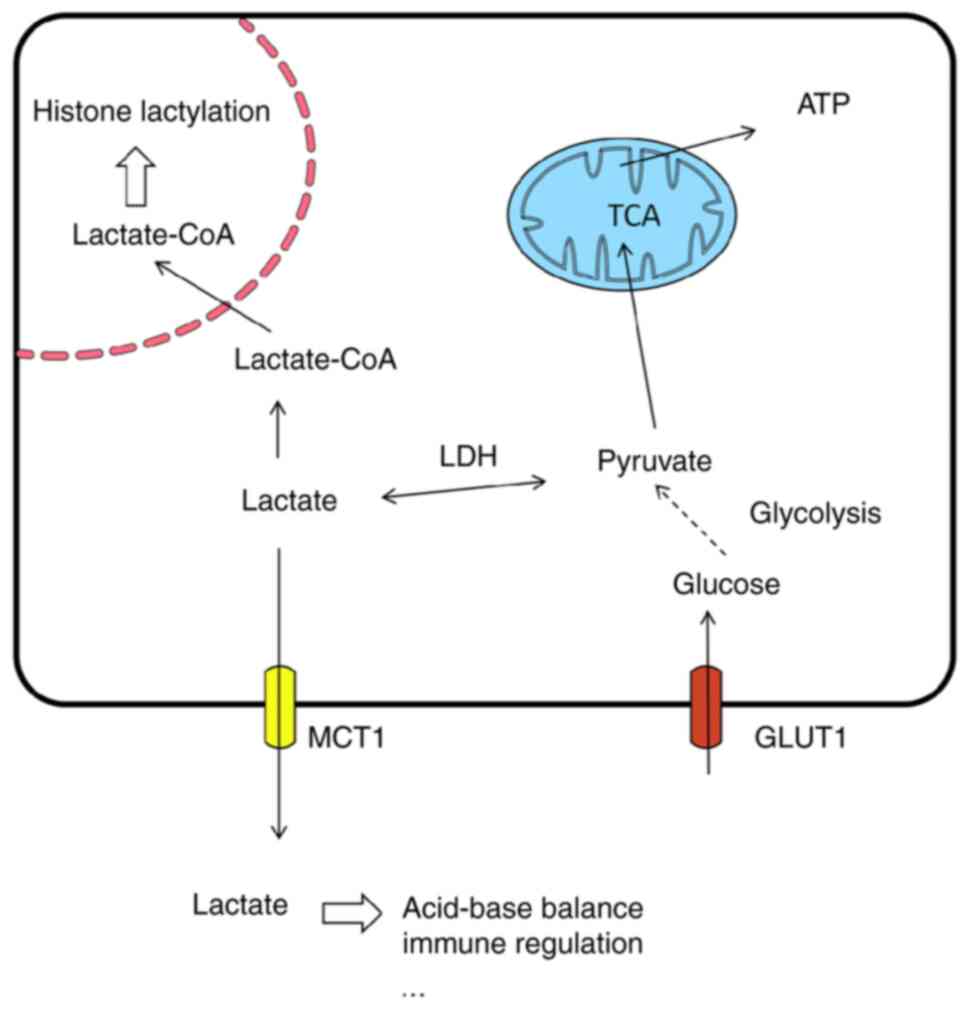
Lactate exists in two isomeric forms: D-lactate and
L-lactate, with L-lactate being the most common form in living
organisms (28). Lactate
transport mainly relies on monocarboxylate transporters (MCTs)
(29,30). In organisms, glucose is
metabolized to lactate via the glycolytic pathway under a short
supply of oxygen. Although only 2 ATP molecules are generated per
glucose molecule during this process, the energy output is
relatively low and the rapid production of lactate quickly provides
energy under hypoxic conditions to maintain normal cellular
functions (31). As an acidic
substance, lactate plays an important role in the acid-base balance
of the body. Furthermore, tumor cells often exhibit aerobic
glycolysis, producing large amounts of lactate even in the presence
of oxygen (32,33). This metabolic phenomenon is known
as the 'Warburg effect' and the accumulation of lactate facilitates
the growth and invasion of tumor cells (34,35).
Under normal physiological conditions, the
production and clearance of lactate are in a dynamic equilibrium.
It has been found that lactate is a metabolic byproduct of
glycolysis and an important signaling molecule (36-39). The metabolic pathways of lactate
vary with the cell type and environment. Lactate can be produced
through anaerobic glycolysis, leading to its accumulation, or it
can be oxidized to acetyl-coenzyme A (CoA) and enter the
tricarboxylic acid cycle (TCA) cycle during aerobic respiration
(40). Additionally, lactate can
be generated from pyruvate via the Warburg effect (41). These distinct metabolic fates
highlight the versatile roles of lactate in different physiological
contexts. For instance, in the immune system, lactate modulates the
activity of immune cells, inhibiting the overactivation of certain
immune cells while promoting the proliferation and differentiation
of others (42-44). In addition, lactate is involved
in various physiological and pathological processes as an energy
source and a signaling molecule and participate in epigenetic
modifications (e.g., lactylation) (19).
In the heart, lactate is a byproduct of glycolysis
and a key energy source for cardiomyocytes. Under normal
physiological conditions, lactate oxidation provides >50% of the
energy demands of cardiomyocytes. Even at rest, the proportion of
energy supplied by lactate oxidation can reach 10%, and during
exercise, this proportion can exceed 50% (45-47). Lactate enters the mitochondria
through the lactate oxidation complex, is converted into pyruvate,
and then enters the TCA cycle to ultimately generate ATP.
Furthermore, lactate activates a series of transcriptional
networks, affecting the expression and subcellular localization of
metabolism-related proteins in cardiomyocytes, such as
hexokinase-2, pyruvate kinase M2 (PKM2) and lactate dehydrogenase
A/B, thereby regulating metabolic homeostasis in cardiomyocytes
(48-52). Lactate and pyruvate can also
scavenge free radicals, protecting cardiomyocytes from oxidative
stress damage (53-55). A cross-sectional study involving
1,496 participants reported that blood lactate levels were
independently associated with carotid atherosclerosis, suggesting
that lactate is involved in atherosclerosis development (56). During the progression of
atherosclerosis, lactic acid accumulates in the microenvironment of
atherosclerotic plaques as an end product of glycolysis and leads
to local acidification. This acidic microenvironment facilitates
the binding of oxidized low-density lipoprotein (oxLDL) to aortic
proteoglycans, triggers local immune responses and recruits immune
cells, thereby exacerbating inflammatory reactions (57). Furthermore, lactic acid
stabilizes hypoxia-inducible factor 1α (HIF-1α) and N-myc
downstream regulated gene 3. This further promotes the expression
of pro-angiogenic factors such as vascular endothelial growth
factor and forms a positive feedback loop that promotes
atherosclerosis development (58) (Fig. 1).
Lactate participates in cellular metabolism to
provide energy, serves as a donor for lactylation modification and
contribute to the regulation of the local tissue
microenvironment.
Atherosclerosis is a chronic inflammatory disease
triggered by lipid metabolism disorders and characterized by the
formation of atherosclerotic plaques beneath the intima of large
and medium-sized arteries (5,11,12). During the progression of
atherosclerosis, the glycolysis levels of cells within the plaque
significantly increase, leading to excessive production and release
of lactic acid, which in turn acidifies the extracellular
environment (59-61). This metabolic change further
influences the development of atherosclerosis through multiple
mechanisms.
There is a close and complex interrelationship
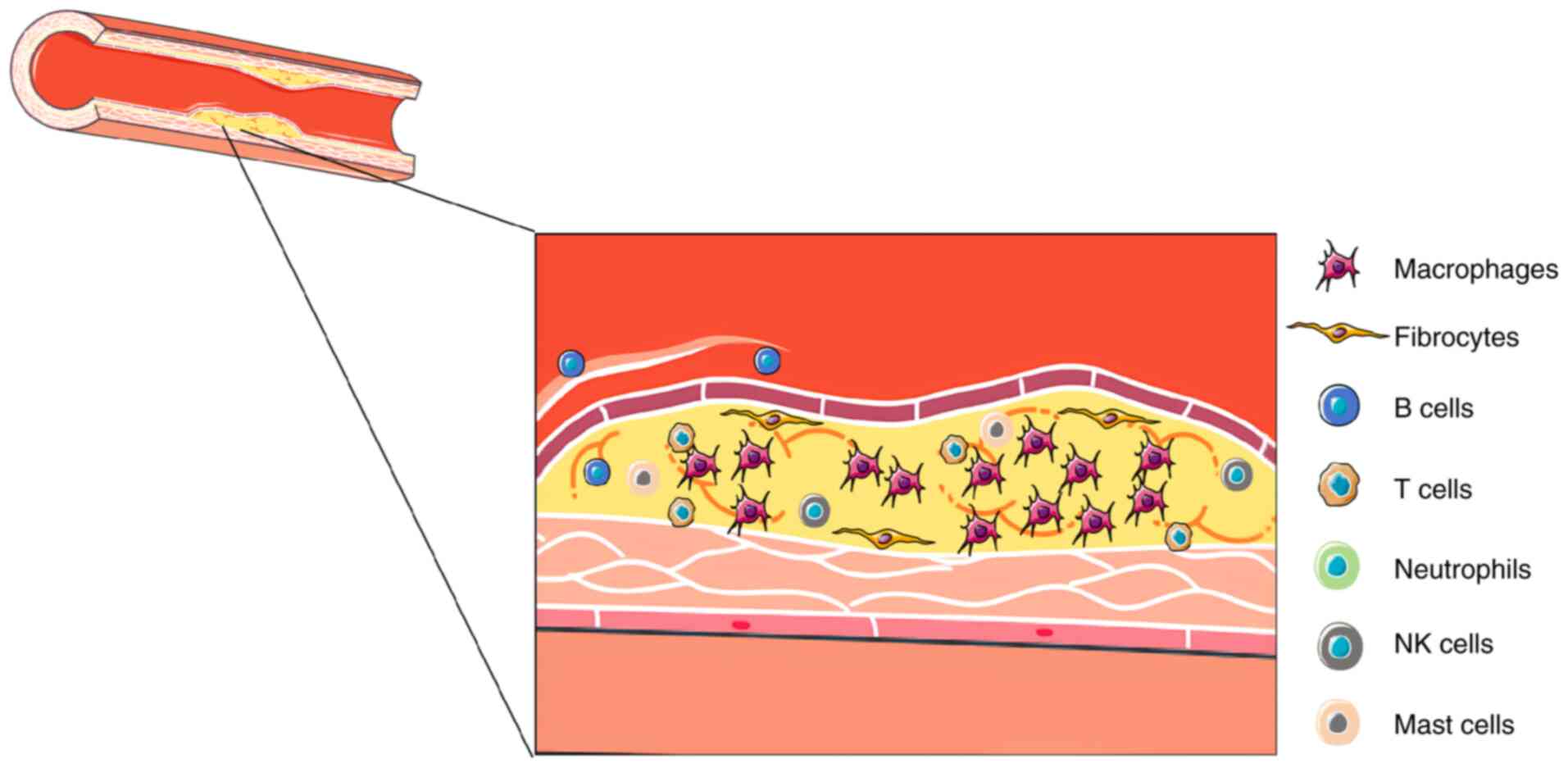
between atherosclerosis and the immune system (25,62,63). As a chronic inflammatory disease,
the inflammatory process in atherosclerosis is typically initiated
by the deposition of oxLDL and damage to endothelial cells. After
endothelial injury, cytokines and chemokines are released as
inflammatory mediators and then attract monocytes and other
inflammatory cells into the vascular wall, thereby initiating the
inflammatory response (Fig. 2)
(64). The immune system plays a
critical role in atherosclerosis through the interplay of innate
and adaptive immune responses, immune cell metabolism and cytokine
networks, driving inflammation and plaque progression.
The involvement of various immune cells plays a
crucial role in atherosclerosis. Macrophages engulf oxLDL to form
foam cells that accumulate within the vascular wall and serve as
the foundation for plaque formation (65-67). Lipidomics and transcriptomics
analyses have shown that foam cells themselves do not possess
pro-inflammatory properties. However, the increased oxidative
stress in the arterial wall and the accumulation of modified
lipoproteins reprogram the metabolic processes of macrophages,
thereby triggering inflammatory responses that promote
atherosclerosis (68,69). After cell death, lipid-overloaded
macrophages are usually phagocytized by other macrophages (70). However, when phagocytic cells
become overwhelmed, they release pro-inflammatory components. The
retention of macrophages in the arterial wall is promoted by
adhesion molecules (e.g., vascular cell adhesion molecule 1) and
neuroguidance factors (such as netrin 1), while high-density
lipoprotein facilitates the egress of macrophages from plaques
through a C-C motif chemokine receptor (CCR)7-mediated mechanism
(71-74). Macrophages in atherosclerotic
plaques exhibit pro-inflammatory or anti-inflammatory
characteristics but do not follow the classical M1/M2
classification (25,75). Single-cell studies have
identified at least five distinct macrophage subpopulations in the
mouse aorta, including inflammatory macrophages, type I
interferon-inducible cells, foam cells with high expression of
triggering receptor expressed on myeloid cells 2, aortic
intima-resident macrophages and embryonic-derived resident
macrophages (68,69,76,77). These subpopulations play diverse
roles in atherosclerosis. These cells exert both pro-inflammatory
and anti-inflammatory effects during disease progression and their
functions are regulated by multiple factors, such as cholesterol
metabolism, oxidative stress and cell-to-cell interactions. Efforts
should be invested to further characterize the functions of these
macrophage subpopulations and explore their specific mechanisms of
action in atherosclerosis.
Adaptive immunity is a key modulator of
atherosclerosis. T cells play a complex role in atherosclerosis and
different subsets influence disease progression through numerous
mechanisms. Type 1 T-helper (Th1) cells are the most prominent
CD4+T-cell subset in atherosclerotic plaques, promoting
lesion development and plaque instability by secreting IFNγ and
expressing T-bet (78,79). The mechanisms include inducing
the uptake of oxLDL, foam cell formation, polarization of
macrophages to a pro-inflammatory phenotype and proliferation of
vascular smooth muscle cells (13,80,81).
The role of Th17 cells in atherosclerosis remains
controversial. In mouse models, the number of T cells expressing
IL-17 is positively correlated with atherosclerosis. Depletion of
Th17 cells inhibits the production of pro-inflammatory cytokines
and chemokines, as well as leukocyte infiltration and plaque
formation (92). In addition to
its pro-inflammatory effects, IL-17A has a plaque-stabilizing role
in atherosclerosis (93).
Neutrophils are mainly associated with events
following plaque rupture or erosion, releasing various enzymes and
cytokines that participate in the inflammatory response and tissue
repair process (105-107). NK cells modulate the activity
of other immune cells by releasing cytokines and cytotoxic
granules, thereby influencing the immune response within
atherosclerotic plaques (108).
Mast cells, primarily located in the adventitia of arteries,
release histamine, enzymes and cytokines, degrade the extracellular
matrix, promote the further recruitment of inflammatory cells and
enhance local inflammation, increasing the complexity and potential
rupture risk of atherosclerotic plaques (109,110) (Table I).
During the progression of atherosclerosis, the
persistent inflammatory response significantly impacts the immune
system. Chronic inflammation can lead to overactivation or
dysfunction of the immune system, thus increasing the risk of
autoimmune diseases. Furthermore, vascular changes caused by
atherosclerosis can affect the transport and distribution of immune
cells, thereby influencing the normal function of the immune system
(111-113). Considering the close
relationship between atherosclerosis and the immune system, immune
regulation strategies have potential in treating atherosclerosis.
By modulating the function of immune cells, inhibiting the
production of pro-inflammatory cytokines or enhancing the effects
of anti-inflammatory cytokines, it is possible to reduce the
inflammatory response, stabilize atherosclerotic plaques and
thereby reduce the risk of cardiovascular events, offering new
potential therapeutic options for atherosclerosis.
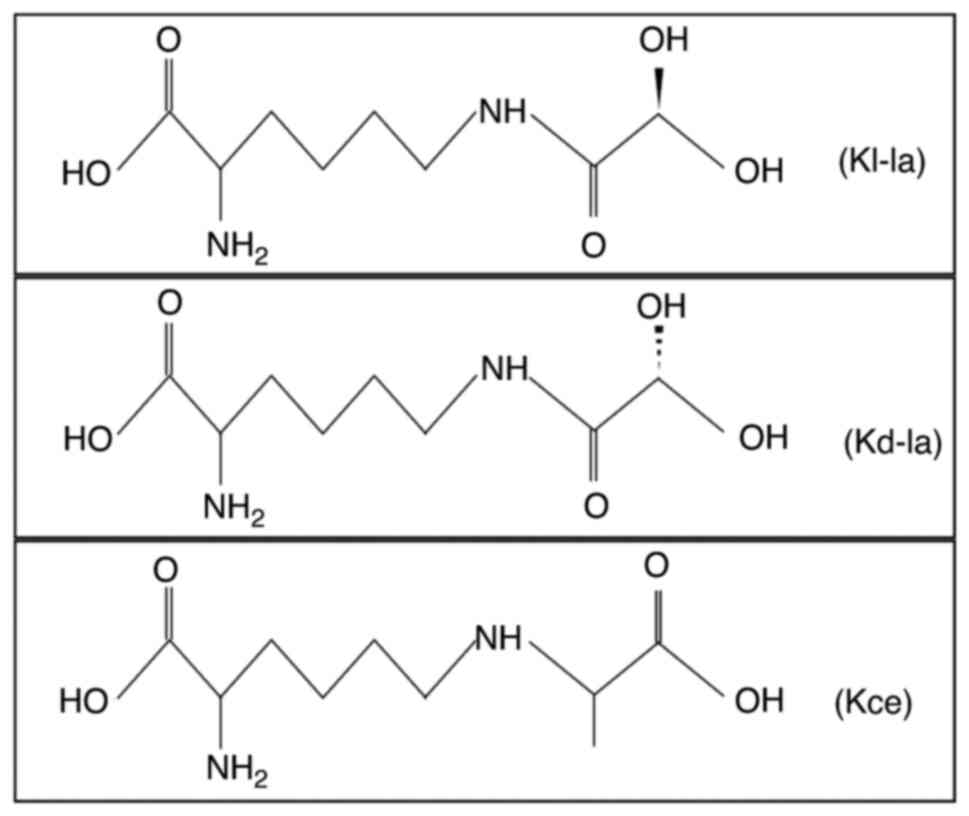
Lactylation is an epigenetic modification that
involves post-translational modifications of both histone and
non-histone proteins and plays a key role in the regulation of gene
transcription and protein function (114). There are three isomers
generated in this process: Lysine L-lactylation (Kl-la),
N-ε-(carboxyethyl) lysine and D-lactyl lysine. It has been shown
that Kl-la is the primary form of lactylation within cells and
significantly contributes to glycolysis and the Warburg effect
(115). Lactylation tends to
occur at nucleophilic sites, such as amino groups
(-NH2), interacting with corresponding functional
groups, and this high level of selectivity allows lactylation to
precisely target specific proteins, causing significant changes in
their properties and functions (114) (Fig. 3).
Induced by the accumulation of lactate, lactylation
modifies proteins and alters the spatial conformation of histones,
thereby affecting gene transcription and the regulation of gene
expression. Similarly, it affects the function of non-histone
proteins by altering their spatial conformation. Lactylation
involves a series of enzyme-catalyzed, reversible chemical
reactions, rather than spontaneous chemical activity (116). The 'writers' of lactylation
refer to enzymes or proteins capable of catalyzing lactylation
reactions, transferring lactoyl groups to target proteins after
lactate is converted into lactoyl-CoA. The known lactylation
'writers' proteins mainly include histone acetyltransferases
Sirtuin (SIRT)1 (117), P300
(117), lysine
acetyltransferase 2A (KAT2A) (118), Tat-interactive protein 60 kDa
(119,120) and KAT8 (121,122). 'Erasers' include enzymes or
proteins that remove or erase lactoyl groups through hydrolysis,
restoring the target molecule to its original state, with histone
deacetylases (123) and SIRTs
(124,125) being known 'erasers' that remove
lactylation modifications. In biology, 'readers' are defined as
proteins or domains capable of recognizing and interacting with
lactoyl groups. Although no specific lactylation readers have been
discovered, this may be related to the recent discovery of
lactylation and an incomplete understanding of its underlying
molecular mechanisms. Lactylation, a post-translational
modification first reported in 2019 by Professor Yingming Zhao's
group at the University of Chicago, has a shorter research history
compared to classical modifications like acetylation and
phosphorylation (19). Although
the identification of specific 'reader' proteins for lactylation is
still in progress, their functional roles have been confirmed
through various studies. For instance, histone H3K18 lactylation
influences macrophage polarization and tumor microenvironment
regulation (126), lactylation
of cyclic GMP-AMP synthase inhibits its immune activity, and
lactylation in NK cells causes mitochondrial dysfunction (127). The lack of identified 'readers'
does not diminish lactylation's biological significance but rather
highlights the field's rapid development. Current evidence
underscores its crucial roles in gene regulation, immune responses
and disease. Future technological advancements and in-depth
research are expected to uncover lactylation-specific 'readers',
further clarifying its molecular mechanisms. As research
progresses, more information about lactylation readers will be
revealed in the future, leading to a more comprehensive
understanding of the molecular mechanisms of lactylation.
Lactylation is an important epigenetic modification
mechanism that plays a key role in various physiological and
pathological processes by affecting protein stability, enzyme
activity, protein-protein interactions, protein distribution,
structure and function (128).
Specifically, lactylation involves a series of complex
physiological activities, including somatic cell reprogramming
(129,130), embryonic development (131-133), neural excitation (134), osteoblast differentiation
(135,136), pyroptosis (137,138), macroautophagy (139), decidualization (140), homologous recombination
(119,141), cuproptosis (142), myogenesis (143) and oxidative phosphorylation
(52,144). In addition, lactylation is
closely related to the occurrence and development of various
diseases, including but not limited to malignant tumors (22,119), inflammation-related diseases
(145-147), fibrosis-related diseases
(148,149), Alzheimer's disease (150), heart disease (60,117,151), insulin resistance (152), nonalcoholic fatty liver disease
(153), cerebral infarction
(154,155), viral infections (156,157) and endometriosis (158).
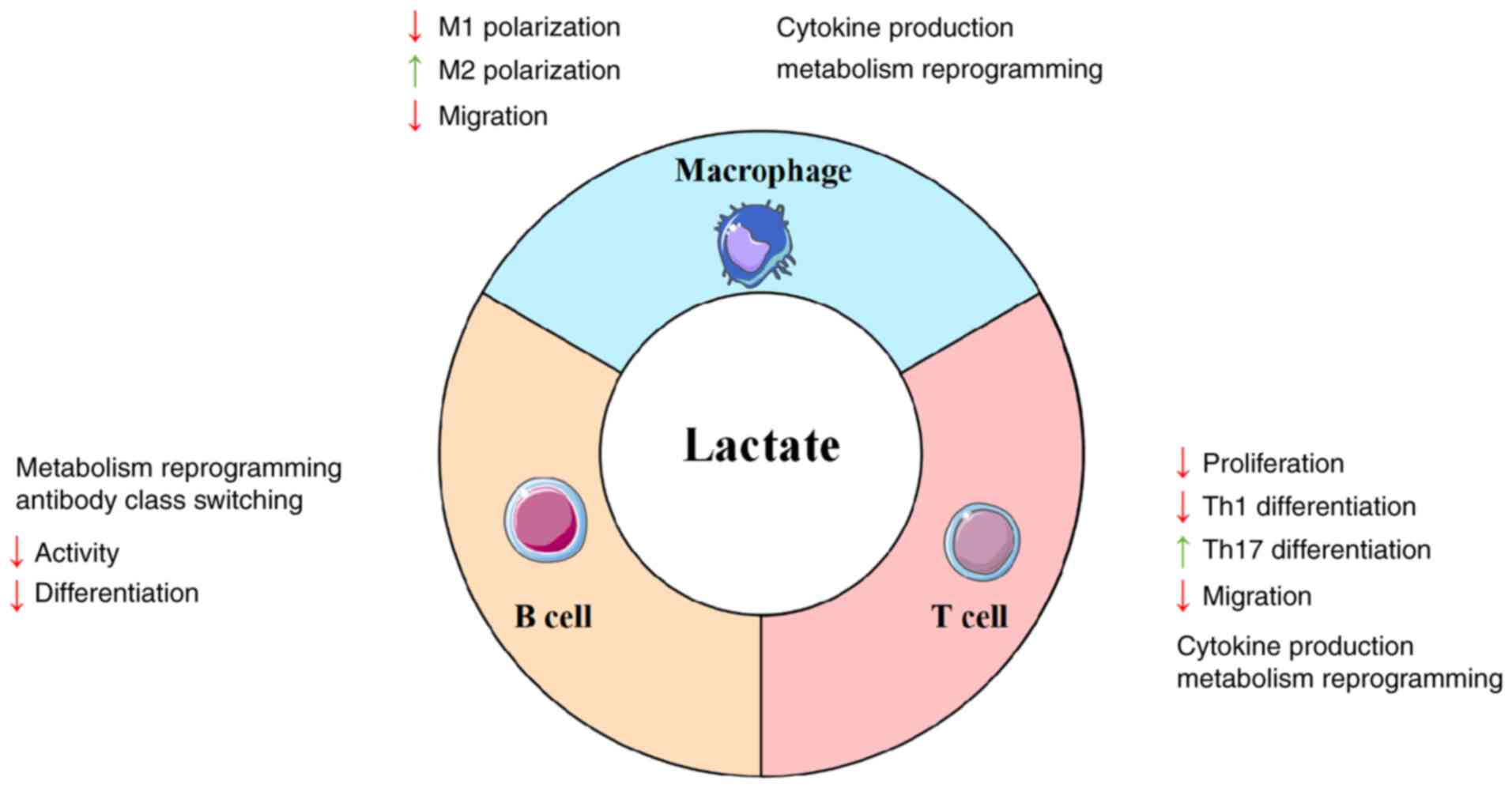
In atherosclerotic plaques, high concentrations of
lactate induce metabolic reprogramming in macrophages, shifting
their metabolism from glycolysis to oxidative phosphorylation
(159-161). This metabolic remodeling
significantly impacts the functional status of macrophages: The
accumulation of lactate inhibits mitochondrial function, reduces
ATP production and consequently diminishes the activity and
functional performance of macrophages (111,162,163). Furthermore, lactate induces the
polarization of macrophages towards the M2 phenotype while
inhibiting M1 polarization, thereby promoting their
anti-inflammatory and immunosuppressive functions (164,165). Besides, lactate attenuates
inflammatory responses by reducing the phosphorylation levels of
NF-κB, thereby suppressing the transcription of
inflammation-related genes (166).
More critically, lactate can induce histone
lactylation, a modification that regulates macrophage metabolism
and phenotypic transformation by acting on both histones (such as
H3K18la) and non-histone proteins (such as PKM2) (167-169). For instance, H3K18la
modification activates the expression of M2-related genes and
drives the transformation of M1 macrophages towards the M2
phenotype. Macrophage activation is one of the key features of
atherosclerosis, typically accompanied by a shift in core
metabolism from oxidative phosphorylation to glycolysis (19). It has been found that histone
lactylation mediated by MCT4, which is associated with lactate
efflux, is closely related to atherosclerosis. In the absence of
MCT4, histone lactylation at lysine 18 of histone H3 activates the
transcription of anti-inflammatory and TCA cycle genes, thereby
initiating local repair and homeostasis (170). This finding indicates that
lactylation plays an important role in regulating macrophage
function and the progression of atherosclerosis.
Lactate is a byproduct of glycolysis and usually
accumulates in large amounts in the tumor microenvironment,
creating an acidic ecological niche. In the acute inflammatory
phase, lactate primarily exerts anti-inflammatory effects by
activating the G protein-coupled receptor 81 receptor, thereby
inhibiting the production of inflammatory mediators and alleviating
the inflammatory response (171,172). However, the acidic environment
caused by lactate accumulation exacerbates the persistence of
inflammation and promotes tissue damage in the chronic inflammatory
phase. Nevertheless, lactate also induces the polarization of
immune cells and modulates metabolic pathways to facilitate the
resolution of inflammation and exert certain anti-inflammatory
effects (173,174).
The activation and differentiation of T cells are
accompanied by metabolic reprogramming, one of the metabolic
hallmarks being aerobic glycolysis, which refers to the conversion
of glucose to lactate in the presence of oxygen (175). T cells undergo metabolic
reprogramming in different states of differentiation (176). For instance, effector T cells
primarily rely on glycolysis, whereas regulatory T cells and memory
T cells mainly depend on oxidative phosphorylation (177,178). T cells sense lactate through
the expression of specific transporters, which leads to the
inhibition of their migratory capacity. This 'stop signal' for
migration depends on the interference of lactate with intracellular
metabolic pathways, particularly glycolysis. Lactate promotes the
differentiation of CD4+ T cells into the IL-17+ subset while
reducing the cytotoxic capacity of CD8+ T cells. These phenomena
lead to the formation of ectopic lymphoid structures at sites of
inflammation and the production of autoantibodies (43,179). Lactate triggers the nuclear
translocation of the PKM2 via an active transmembrane influx
mediated by the sodium-coupled monocarboxylate transporter 2 (also
known as solute carrier family 5 member 12). Within the nucleus,
PKM2 forms a complex with phosphorylated signal transducer and
activator of transcription 3, synergistically enhancing the
transcriptional activity of the IL-17 gene, thereby markedly
promoting IL-17 secretion by Th17 cells (180-182). The activity of Tregs relies on
the metabolic reprogramming from glycolysis to oxidative
phosphorylation, a process strictly regulated by the transcription
factor forkhead box P3. Lactate promotes the accumulation of Tregs
at inflammatory sites and activates the HIF-1α-dependent CCL20/CCR6
signaling axis, facilitating Treg migration to the lesion area. In
addition, Tregs utilize lactate as a substrate for the TCA cycle,
further enhancing their immunosuppressive function (164,183,184). Nevertheless, in chronic
inflammatory responses, the persistent accumulation of lactate
causes local tissue damage and drives the progression of chronic
inflammation, which significantly exacerbates the pathological
impact on atherosclerotic lesions and accelerates disease
progression (173,179). Lactate plays a complex dual
role in inflammation and immune responses. It exerts
anti-inflammatory effects by regulating metabolism and immune cell
functions, but it exacerbates tissue damage and disease progression
in chronic inflammation.
Lactylation promotes Th17 differentiation and
participates in the development of autoimmune diseases and
inflammatory responses by regulating site-specific modifications of
key proteins such as IKAROS family zinc finger 1, which directly
modulates the expression of Th17-related genes (185). In the tumor microenvironment,
lactate regulates the generation of Treg cells through lactylation
modification at lysine 72 of the MOESIN protein. This process
enhances the interaction between MOESIN and transforming growth
factor-β receptor I, activates the downstream SMAD3 signaling
pathway and significantly improves the stability and function of
Treg cells (186). This
discovery provides an important reference for understanding the
regulatory mechanisms of lactate accumulation on Treg cells in the
context of atherosclerotic tissue environments.
Lactate significantly inhibits the proliferation
capacity and antibody production of B cells by reducing the
extracellular pH (159,187). Additionally, lactate modulates
the metabolic pathways of B cells, particularly by enhancing the
glycolytic process, which provides rapid energy supply to support
their proliferation and differentiation. At the same time, lactate
suppresses oxidative phosphorylation and results in significant
alterations in the metabolic state of B cells (28,188). At the molecular level, lactate
promotes the growth and metabolic activities of B cells by
activating the mammalian target of rapamycin signaling pathway
(189,190). Under hypoxic conditions,
lactate enhances the adaptability of B cells to low oxygen
environments by stabilizing HIF-1α (191-193). Furthermore, lactate activates
the NF-κB signaling pathway, promoting the secretion of
pro-inflammatory cytokines by B cells, thereby participating in the
regulation of inflammatory responses (194,195). These findings demonstrate that
lactate plays a multifaceted role in the functional regulation of B
cells, influencing metabolic reprogramming, signaling pathway
activation and environmental adaptability, among other aspects.
Lactylation modulates the antibody class switch recombination (CSR)
in B cells by influencing metabolic pathways. For instance, the
MCT1-mediated lactate transport and pyruvate metabolism regulates
the acetylation modification of histone H3K27, thereby affecting
the transcriptional efficiency of activation-induced cytidine
deaminase, which ultimately impacts the CSR in B cells (196) (Fig. 4).
Lactate and its mediated lactylation modifications
play a complex dual role in atherosclerosis by regulating metabolic
reprogramming, functional polarization and epigenetic modifications
of macrophages, T cells and B cells.
In summary, this review comprehensively analyzes the
multifaceted roles of lactate and lactylation in shaping the immune
system within the context of atherosclerosis. As a chronic
inflammatory disease, atherosclerosis is characterized by the
complex interplay between lipid metabolism, immune cell
infiltration and the local microenvironment. The results highlight
the critical roles of lactate and lactylation in modulating immune
cell functions, metabolic reprogramming and epigenetic regulation,
thereby influencing the progression and stability of
atherosclerotic plaques.
The involvement of lactate and lactylation in the
regulation of macrophage polarization, T cell differentiation and B
cell metabolism underscores their potential as therapeutic targets.
Specifically, lactate-induced metabolic reprogramming and
lactylation modifications have been shown to significantly impact
the phenotypes and functions of immune cells and contribute to
pro-inflammatory and anti-inflammatory responses. These insights
provide a deeper understanding of the immune metabolic mechanisms
underlying atherosclerosis and pave the way for novel therapeutic
strategies targeting lactate metabolism and lactylation
pathways.
However, despite the progress made in elucidating
the roles of lactate and lactylation in atherosclerosis, several
gaps remain in our knowledge. The complex microenvironment of
atherosclerotic plaques, characterized by hypoxia, nutrient
deprivation and acidosis, is likely to influence the behavior of
immune cells and the efficacy of potential treatments. Yet, the
precise mechanisms through which these microenvironmental factors
interact with lactate and lactylation to modulate immune responses
are still not fully understood. Additionally, the diverse metabolic
profiles and functional states of immune cells within plaques
suggest that a more detailed characterization of immune cell
subsets and their specific roles is needed.
Furthermore, the dynamic changes in the
atherosclerotic microenvironment over time, from plaque initiation
to rupture, add another layer of complexity to the study of immune
regulation. The impact of these temporal changes on lactate
production, lactylation modifications and immune cell function
remains elusive. Furthermore, the potential compensatory mechanisms
and feedback loops involving other metabolic pathways and
epigenetic modifications in response to lactate accumulation and
lactylation need further investigation.
In conclusion, although significant advancements
have been made in understanding the roles of lactate and
lactylation in atherosclerosis, the intricate interplay between the
microenvironment, metabolic changes and immune regulation remains
an area of considerable research need. Future studies should focus
on unraveling the detailed mechanisms through which the
atherosclerotic microenvironment influences lactate metabolism and
lactylation, as well as their downstream effects on immune cell
function. These will enhance our understanding of the
pathophysiology of atherosclerosis and provide new avenues for
developing targeted therapies aimed at modulating immune responses
and improving clinical outcomes.
Not applicable.
YX was involved in the conceptualization of the
review, data curation, investigation, methodology and
writing-original draft. JZ was responsible for data curation and
project administration. JW contributed to the conceptualization,
data curation and methodology. HH was involved in
conceptualization, data curation, investigation, methodology and
writing-original draft, writing-review & editing. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Luengo-Fernandez R, Walli-Attaei M, Gray
A, Torbica A, Maggioni AP, Huculeci R, Bairami F, Aboyans V, Timmis
AD, Vardas P and Leal J: Economic burden of cardiovascular diseases
in the European Union: A population-based cost study. Eur Heart J.
44:4752–4767. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weintraub WS: High costs of cardiovascular
disease in the European Union. Eur Heart J. 44:4768–4770. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldsborough E III, Osuji N and Blaha MJ:
Assessment of cardiovascular disease risk: A 2022 update.
Endocrinol Metab Clin North Am. 51:483–509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raleigh V and Colombo F: Cardiovascular
disease should be a priority for health systems globally. BMJ.
382:e0765762023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47(Suppl 8): C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan J and Watanabe T: Atherosclerosis:
Known and unknown. Pathol Int. 72:151–160. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Libby P: The changing landscape of
atherosclerosis. Nature. 592:524–533. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rocha VZ and Libby P: Obesity,
inflammation, and atherosclerosis. Nat Rev Cardiol. 6:399–409.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shoaran M and Maffia P: Tackling
inflammation in atherosclerosis. Nat Rev Cardiol. 21:4422024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bäck M, Yurdagul A Jr, Tabas I, Öörni K
and Kovanen PT: Inflammation and its resolution in atherosclerosis:
Mediators and therapeutic opportunities. Nat Rev Cardiol.
16:389–406. 2019.PubMed/NCBI
|
|
11
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther. 7:1312022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolf D and Ley K: Immunity and
inflammation in atherosclerosis. Circ Res. 124:315–327. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saigusa R, Winkels H and Ley K: T cell
subsets and functions in atherosclerosis. Nat Rev Cardiol.
17:387–401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srikakulapu P and McNamara CA: B cells and
atherosclerosis. Am J Physiol Heart Circ Physiol. 312:H1060–H1067.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taghavie-Moghadam PL, Butcher MJ and
Galkina EV: The dynamic lives of macrophage and dendritic cell
subsets in atherosclerosis. Ann N Y Acad Sci. 1319:19–37. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tedgui A and Mallat Z: Cytokines in
atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev.
86:515–581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kzhyshkowska J, Shen J and Larionova I:
Targeting of TAMs: Can we be more clever than cancer cells? Cell
Mol Immunol. 21:1376–1409. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun P, Ma L and Lu Z: Lactylation: Linking
the Warburg effect to DNA damage repair. Cell Metab. 36:1637–1639.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li F, Si W, Xia L, Yin D, Wei T, Tao M,
Cui X, Yang J, Hong T and Wei R: Positive feedback regulation
between glycolysis and histone lactylation drives oncogenesis in
pancreatic ductal adenocarcinoma. Mol Cancer. 23:902024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Z, Zheng Y and Gao Q: Lysine
lactylation in the regulation of tumor biology. Trends Endocrinol
Metab. 35:720–731. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Sun L, Gao P and Hu H: Lactylation
in cancer: Current understanding and challenges. Cancer Cell.
42:1803–1807. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu W, Guo S, Sun J, Zhao Y and Liu C:
Lactate and lactylation in cardiovascular diseases: Current
progress and future perspectives. Metabolism. 158:1559572024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy P, Orecchioni M and Ley K: How the
immune system shapes atherosclerosis: Roles of innate and adaptive
immunity. Nat Rev Immunol. 22:251–265. 2022. View Article : Google Scholar
|
|
26
|
Ouyang J, Wang H and Huang J: The role of
lactate in cardiovascular diseases. Cell Commun Signal. 21:3172023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Cai P, Tang X, Wu Y, Zhang Y and
Rong X: Lactylation modification in cardiometabolic disorders:
Function and mechanism. Metabolites. 14:2172024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonen A: Lactate transporters (MCT
proteins) in heart and skeletal muscles. Med Sci Sports Exerc.
32:778–789. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh M, Afonso J, Sharma D, Gupta R and
Kumar V, Rani R, Baltazar F and Kumar V: Targeting monocarboxylate
transporters (MCTs) in cancer: How close are we to the clinics?
Semin Cancer Biol. 90:1–14. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Certo M, Llibre A, Lee W and Mauro C:
Understanding lactate sensing and signalling. Trends Endocrinol
Metab. 33:722–735. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Apostolova P and Pearce EL: Lactic acid
and lactate: Revisiting the physiological roles in the tumor
microenvironment. Trends Immunol. 43:969–977. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goenka A, Khan F, Verma B, Sinha P, Dmello
CC, Jogalekar MP, Gangadaran P and Ahn BC: Tumor microenvironment
signaling and therapeutics in cancer progression. Cancer Commun
(Lond). 43:525–561. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao M, Yao D, Wu L, Luo C, Wang Z, Zhang
J and Liu B: Targeting the Warburg effect: A revisited perspective
from molecular mechanisms to traditional and innovative therapeutic
strategies in cancer. Acta Pharm Sin B. 14:953–1008. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y and Patti GJ: The Warburg effect: A
signature of mitochondrial overload. Trends Cell Biol.
33:1014–1020. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Magistretti PJ and Allaman I: Lactate in
the brain: From metabolic end-product to signalling molecule. Nat
Rev Neurosci. 19:235–249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J,
Chang Y, Chen Y, Lu Y, Zeng H, et al: Lactate is a natural
suppressor of RLR signaling by targeting MAVS. Cell.
178:176–189.e15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brown TP and Ganapathy V: Lactate/GPR81
signaling and proton motive force in cancer: Role in angiogenesis,
immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther.
206:1074512020. View Article : Google Scholar
|
|
39
|
Li H, Liu C, Li R, Zhou L, Ran Y, Yang Q,
Huang H, Lu H, Song H, Yang B, et al: AARS1 and AARS2 sense
L-lactate to regulate cGAS as global lysine lactyltransferases.
Nature. 634:1229–1237. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee WD, Weilandt DR, Liang L, MacArthur
MR, Jaiswal N, Ong O, Mann CG, Chu Q, Hunter CJ, Ryseck RP, et al:
Lactate homeostasis is maintained through regulation of glycolysis
and lipolysis. Cell Metab. 37:758–771.e8. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feron O: Pyruvate into lactate and back:
from the Warburg effect to symbiotic energy fuel exchange in cancer
cells. Radiother Oncol. 92:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang K, Zhang Y and Chen ZN: Metabolic
interaction: Tumor-derived lactate inhibiting CD8+ T
cell cytotoxicity in a novel route. Signal Transduct Target Ther.
8:522023. View Article : Google Scholar
|
|
43
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang
J, Wilhelm J, Li S, Song J, Li W, et al: Lactate increases stemness
of CD8+ T cells to augment anti-tumor immunity. Nat
Commun. 13:49812022. View Article : Google Scholar
|
|
45
|
Mohazzab-H KM, Kaminski PM and Wolin MS:
Lactate and PO2 modulate superoxide anion production in bovine
cardiac myocytes: Potential role of NADH oxidase. Circulation.
96:614–620. 1997. View Article : Google Scholar
|
|
46
|
Gaspar JA, Doss MX, Hengstler JG, Cadenas
C, Hescheler J and Sachinidis A: Unique metabolic features of stem
cells, cardiomyocytes, and their progenitors. Circ Res.
114:1346–1360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X, Wu H, Liu Y, Liu L, Houser SR and
Wang WE: Metabolic reprogramming: A byproduct or a driver of
cardiomyocyte proliferation? Circulation. 149:1598–1610. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chung S, Arrell DK, Faustino RS, Terzic A
and Dzeja PP: Glycolytic network restructuring integral to the
energetics of embryonic stem cell cardiac differentiation. J Mol
Cell Cardiol. 48:725–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dziegala M, Kobak KA, Kasztura M, Bania J,
Josiak K, Banasiak W, Ponikowski P and Jankowska EA: Iron depletion
affects genes encoding mitochondrial electron transport chain and
genes of non-oxidative metabolism, pyruvate kinase and lactate
dehydrogenase, in primary human cardiac myocytes cultured upon
mechanical stretch. Cells. 7:1752018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Zhao L, Chen Z, Lin Y, Yu P and Mao
L: Continuous electrochemical monitoring of extracellular lactate
production from neonatal rat cardiomyocytes following myocardial
hypoxia. Anal Chem. 84:5285–5291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hammond GL, Nadal-Ginard B, Talner NS and
Markert CL: Myocardial LDH isozyme distribution in the ischemic and
hypoxic heart. Circulation. 53:637–643. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pan RY, He L, Zhang J, Liu X, Liao Y, Gao
J, Liao Y, Yan Y, Li Q, Zhou X, et al: Positive feedback regulation
of microglial glucose metabolism by histone H4 lysine 12
lactylation in Alzheimer's disease. Cell Metab. 34:634–648.e6.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu M, Yu W, Fang Y, Zhou H, Liang Y,
Huang C, Liu H and Zhao G: Pyruvate and lactate based hydrogel film
inhibits UV radiation-induced skin inflammation and oxidative
stress. Int J Pharm. 634:1226972023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang YF, Wang G, Ding L, Bai ZR, Leng Y,
Tian JW, Zhang JZ, Li YQ, Ahmad, Qin YH, et al: Lactate-upregulated
NADPH-dependent NOX4 expression via HCAR1/PI3K pathway contributes
to ROS-induced osteoarthritis chondrocyte damage. Redox Biol.
67:1028672023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Groussard C, Morel I, Chevanne M, Monnier
M, Cillard J and Delamarche A: Free radical scavenging and
antioxidant effects of lactate ion: An in vitro study. J Appl
Physiol (1985). 89:169–175. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shantha GPS, Wasserman B, Astor BC, Coresh
J, Brancati F, Sharrett AR and Young JH: Association of blood
lactate with carotid atherosclerosis: The atherosclerosis risk in
communities (ARIC) carotid MRI study. Atherosclerosis. 228:249–255.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Perrotta P, Van der Veken B, Van Der Veken
P, Pintelon I, Roosens L, Adriaenssens E, Timmerman V, Guns PJ, De
Meyer GRY and Martinet W: Partial Inhibition of glycolysis reduces
atherogenesis independent of intraplaque neovascularization in
mice. Arterioscler Thromb Vasc Biol. 40:1168–1181. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sneck M, Kovanen PT and Oörni K: Decrease
in pH strongly enhances binding of native, proteolyzed, lipolyzed,
and oxidized low density lipoprotein particles to human aortic
proteoglycans. J Biol Chem. 280:37449–37454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li L, Wang M, Ma Q, Ye J and Sun G: Role
of glycolysis in the development of atherosclerosis. Am J Physiol
Cell Physiol. 323:C617–C629. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li X, Chen M, Chen X, He X, Li X, Wei H,
Tan Y, Min J, Azam T, Xue M, et al: TRAP1 drives smooth muscle cell
senescence and promotes atherosclerosis via HDAC3-primed histone H4
lysine 12 lactylation. Eur Heart J. 45:4219–4235. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou LJ, Lin WZ, Meng XQ, Zhu H, Liu T, Du
LJ, Bai XB, Chen BY, Liu Y, Xu Y, et al: Periodontitis exacerbates
atherosclerosis through Fusobacterium nucleatum-promoted hepatic
glycolysis and lipogenesis. Cardiovasc Res. 119:1706–1717. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nilsson J and Hansson GK: Vaccination
strategies and immune modulation of atherosclerosis. Circ Res.
126:1281–1296. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xue S, Su Z and Liu D: Immunometabolism
and immune response regulate macrophage function in
atherosclerosis. Ageing Res Rev. 90:1019932023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim KW, Ivanov S and Williams JW: Monocyte
recruitment, specification, and function in atherosclerosis. Cells.
10:152020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang B, Tang X, Yao L, Wang Y, Chen Z, Li
M, Wu N, Wu D, Dai X, Jiang H and Ai D: Disruption of USP9X in
macrophages promotes foam cell formation and atherosclerosis. J
Clin Invest. 132:e1542172022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tabas I and Bornfeldt KE: Macrophage
phenotype and function in different stages of atherosclerosis. Circ
Res. 118:653–667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Luo Y, Duan H, Qian Y, Feng L, Wu Z, Wang
F, Feng J, Yang D, Qin Z and Yan X: Macrophagic CD146 promotes foam
cell formation and retention during atherosclerosis. Cell Res.
27:352–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kim K, Shim D, Lee JS, Zaitsev K, Williams
JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, et al:
Transcriptome analysis reveals nonfoamy rather than foamy plaque
macrophages are proinflammatory in atherosclerotic murine models.
Circ Res. 123:1127–1142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Spann NJ, Garmire LX, McDonald JG, Myers
DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D,
et al: Regulated accumulation of desmosterol integrates macrophage
lipid metabolism and inflammatory responses. Cell. 151:138–152.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kojima Y, Weissman IL and Leeper NJ: The
role of efferocytosis in atherosclerosis. Circulation. 135:476–489.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wanschel A, Seibert T, Hewing B,
Ramkhelawon B, Ray TD, van Gils JM, Rayner KJ, Feig JE, O'Brien ER,
Fisher EA, et al: Neuroimmune guidance cue Semaphorin 3E is
expressed in atherosclerotic plaques and regulates macrophage
retention. Arterioscler Thromb Vasc Biol. 33:886–893. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
van Gils JM, Derby MC, Fernandes LR,
Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL,
Alvarez-Leite JI, et al: The neuroimmune guidance cue netrin-1
promotes atherosclerosis by inhibiting the emigration of
macrophages from plaques. Nat Immunol. 13:136–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Trogan E, Feig JE, Dogan S, Rothblat GH,
Angeli V, Tacke F, Randolph GJ and Fisher EA: Gene expression
changes in foam cells and the role of chemokine receptor CCR7
during atherosclerosis regression in ApoE-deficient mice. Proc Natl
Acad Sci USA. 103:3781–3786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Feig JE, Parathath S, Rong JX, Mick SL,
Vengrenyuk Y, Grauer L, Young SG and Fisher EA: Reversal of
hyperlipidemia with a genetic switch favorably affects the content
and inflammatory state of macrophages in atherosclerotic plaques.
Circulation. 123:989–998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Colin S, Chinetti-Gbaguidi G and Staels B:
Macrophage phenotypes in atherosclerosis. Immunol Rev. 262:153–166.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zernecke A, Winkels H, Cochain C, Williams
JW, Wolf D, Soehnlein O, Robbins CS, Monaco C, Park I, McNamara CA,
et al: Meta-analysis of leukocyte diversity in atherosclerotic
mouse aortas. Circ Res. 127:402–426. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Williams JW, Zaitsev K, Kim KW, Ivanov S,
Saunders BT, Schrank PR, Kim K, Elvington A, Kim SH, Tucker CG, et
al: Limited proliferation capacity of aortic intima resident
macrophages requires monocyte recruitment for atherosclerotic
plaque progression. Nat Immunol. 21:1194–1204. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ait-Oufella H, Taleb S, Mallat Z and
Tedgui A: Recent advances on the role of cytokines in
atherosclerosis. Arterioscler Thromb Vasc Biol. 31:969–979. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ley K, Laudanna C, Cybulsky MI and
Nourshargh S: Getting to the site of inflammation: The leukocyte
adhesion cascade updated. Nat Rev Immunol. 7:678–689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ait-Oufella H, Sage AP, Mallat Z and
Tedgui A: Adaptive (T and B cells) immunity and control by
dendritic cells in atherosclerosis. Circ Res. 114:1640–1660. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chen J, Xiang X, Nie L, Guo X, Zhang F,
Wen C, Xia Y and Mao L: The emerging role of Th1 cells in
atherosclerosis and its implications for therapy. Front Immunol.
13:10796682023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kuan R, Agrawal DK and Thankam FG: Treg
cells in atherosclerosis. Mol Biol Rep. 48:4897–4910. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shao Y, Yang WY, Saaoud F, Drummer C IV,
Sun Y, Xu K, Lu Y, Shan H, Shevach EM, Jiang X, et al: IL-35
promotes CD4+Foxp3+ Tregs and inhibits atherosclerosis via
maintaining CCR5-amplified Treg-suppressive mechanisms. JCI
Insight. 6:e1525112021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Klingenberg R, Gerdes N, Badeau RM,
Gisterå A, Strodthoff D, Ketelhuth DFJ, Lundberg AM, Rudling M,
Nilsson SK, Olivecrona G, et al: Depletion of FOXP3+ regulatory T
cells promotes hypercholesterolemia and atherosclerosis. J Clin
Invest. 123:1323–1334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sharma M, Schlegel MP, Afonso MS, Brown
EJ, Rahman K, Weinstock A, Sansbury BE, Corr EM, van Solingen C,
Koelwyn GJ, et al: Regulatory T cells license macrophage
pro-resolving functions during atherosclerosis regression. Circ
Res. 127:335–353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fernández-Gallego N, Castillo-González R,
Méndez-Barbero N, López-Sanz C, Obeso D, Villaseñor A, Escribese
MM, López-Melgar B, Salamanca J, Benedicto-Buendía A, et al: The
impact of type 2 immunity and allergic diseases in atherosclerosis.
Allergy. 77:3249–3266. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Vinson A, Curran JE, Johnson MP, Dyer TD,
Moses EK, Blangero J, Cox LA, Rogers J, Havill LM, Vandeberg JL and
Mahaney MC: Genetical genomics of Th1 and Th2 immune response in a
baboon model of atherosclerosis risk factors. Atherosclerosis.
217:387–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Weinstock A, Rahman K, Yaacov O, Nishi H,
Menon P, Nikain CA, Garabedian ML, Pena S, Akbar N, Sansbury BE, et
al: Wnt signaling enhances macrophage responses to IL-4 and
promotes resolution of atherosclerosis. Elife. 10:e679322021.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Engelbertsen D, Andersson L, Ljungcrantz
I, Wigren M, Hedblad B, Nilsson J and Björkbacka H: T-helper 2
immunity is associated with reduced risk of myocardial infarction
and stroke. Arterioscler Thromb Vasc Biol. 33:637–644. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Knutsson A, Björkbacka H, Dunér P,
Engström G, Binder CJ, Nilsson AH and Nilsson J: Associations of
interleukin-5 with plaque development and cardiovascular events.
JACC Basic Transl Sci. 4:891–902. 2019. View Article : Google Scholar
|
|
91
|
Cardilo-Reis L, Gruber S, Schreier SM,
Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H,
Soehnlein O and Binder CJ: Interleukin-13 protects from
atherosclerosis and modulates plaque composition by skewing the
macrophage phenotype. EMBO Mol Med. 4:1072–1086. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang
P, Guo C, Wang Q, Wang X, Ma C, et al: A critical function of Th17
proinflammatory cells in the development of atherosclerotic plaque
in mice. J Immunol. 185:5820–5827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gisterå A, Robertson AK, Andersson J,
Ketelhuth DFJ, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO,
Flavell RA and Hansson GK: Transforming growth factor-β signaling
in T cells promotes stabilization of atherosclerotic plaques
through an interleukin-17-dependent pathway. Sci Transl Med.
5:196ra1002013. View Article : Google Scholar
|
|
94
|
Lu H and Daugherty A: Regulatory B cells,
interleukin-10, and atherosclerosis. Curr Opin Lipidol. 26:470–471.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bu T, Li Z, Hou Y, Sun W, Zhang R, Zhao L,
Wei M, Yang G and Yuan L: Exosome-mediated delivery of
inflammation-responsive Il-10 mRNA for controlled atherosclerosis
treatment. Theranostics. 11:9988–10000. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Guo S, Mao X and Liu J: Multi-faceted
roles of C1q/TNF-related proteins family in atherosclerosis. Front
Immunol. 14:12534332023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fanola CL, Morrow DA, Cannon CP, Jarolim
P, Lukas MA, Bode C, Hochman JS, Goodrich EL, Braunwald E and
O'Donoghue ML: Interleukin-6 and the risk of adverse outcomes in
patients after an acute coronary syndrome: Observations from the
SOLID-TIMI 52 (stabilization of plaque using
darapladib-thrombolysis in myocardial infarction 52) trial. J Am
Heart Assoc. 6:e0056372017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Schieffer B, Schieffer E, Hilfiker-Kleiner
D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W
and Drexler H: Expression of angiotensin II and interleukin 6 in
human coronary atherosclerotic plaques: Potential implications for
inflammation and plaque instability. Circulation. 101:1372–1378.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Rosenfeld SM, Perry HM, Gonen A, Prohaska
TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM,
Tsimikas S, et al: B-1b cells secrete atheroprotective IgM and
attenuate atherosclerosis. Circ Res. 117:e28–e39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ravandi A, Boekholdt SM, Mallat Z, Talmud
PJ, Kastelein JJP, Wareham NJ, Miller ER, Benessiano J, Tedgui A,
Witztum JL, et al: Relationship of IgG and IgM autoantibodies and
immune complexes to oxidized LDL with markers of oxidation and
inflammation and cardiovascular events: Results from the
EPIC-norfolk study. J Lipid Res. 52:1829–1836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lappalainen J, Lindstedt KA, Oksjoki R and
Kovanen PT: OxLDL-IgG immune complexes induce expression and
secretion of proatherogenic cytokines by cultured human mast cells.
Atherosclerosis. 214:357–363. 2011. View Article : Google Scholar
|
|
102
|
Tew JG, El Shikh ME, El Sayed RM and
Schenkein HA: Dendritic cells, antibodies reactive with oxLDL, and
inflammation. J Dent Res. 91:8–16. 2012. View Article : Google Scholar :
|
|
103
|
Yang H, Chen J, Liu S, Xue Y, Li Z, Wang
T, Jiao L, An Q, Liu B, Wang J and Zhao H: Exosomes from
IgE-stimulated mast cells aggravate asthma-mediated atherosclerosis
through circRNA CDR1as-mediated endothelial cell dysfunction in
mice. Arterioscler Thromb Vasc Biol. 44:e99–e115. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang X, Li J, Luo S, Wang M, Huang Q,
Deng Z, de Febbo C, Daoui A, Liew PX, Sukhova GK, et al: IgE
contributes to atherosclerosis and obesity by affecting macrophage
polarization, macrophage protein network, and foam cell formation.
Arterioscler Thromb Vasc Biol. 40:597–610. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Silvestre-Roig C, Braster Q, Ortega-Gomez
A and Soehnlein O: Neutrophils as regulators of cardiovascular
inflammation. Nat Rev Cardiol. 17:327–340. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Franck G: Role of mechanical stress and
neutrophils in the pathogenesis of plaque erosion. Atherosclerosis.
318:60–69. 2021. View Article : Google Scholar
|
|
107
|
Ionita MG, van den Borne P, Catanzariti
LM, Moll FL, de Vries JPPM, Pasterkamp G, Vink A and de Kleijn DPV:
High neutrophil numbers in human carotid atherosclerotic plaques
are associated with characteristics of rupture-prone lesions.
Arterioscler Thromb Vasc Biol. 30:1842–1848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Palano MT, Cucchiara M, Gallazzi M, Riccio
F, Mortara L, Gensini GF, Spinetti G, Ambrosio G and Bruno A: When
a friend becomes your enemy: Natural killer cells in
atherosclerosis and atherosclerosis-associated risk factors. Front
Immunol. 12:7981552021. View Article : Google Scholar
|
|
109
|
Kovanen PT and Bot I: Mast cells in
atherosclerotic cardiovascular disease-activators and actions. Eur
J Pharmacol. 816:37–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Spinas E, Kritas SK, Saggini A, Mobili A,
Caraffa A, Antinolfi P, Pantalone A, Tei M, Speziali A, Saggini R
and Conti P: Role of mast cells in atherosclerosis: A classical
inflammatory disease. Int J Immunopathol Pharmacol. 27:517–521.
2014. View Article : Google Scholar
|
|
111
|
Lin A, Miano JM, Fisher EA and Misra A:
Chronic inflammation and vascular cell plasticity in
atherosclerosis. Nat Cardiovasc Res. 3:1408–1423. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Moriya J: Critical roles of inflammation
in atherosclerosis. J Cardiol. 73:22–27. 2019. View Article : Google Scholar
|
|
113
|
Song B, Bie Y, Feng H, Xie B, Liu M and
Zhao F: Inflammatory factors driving atherosclerotic plaque
progression new insights. J Transl Int Med. 10:36–47. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jing F, Zhang J, Zhang H and Li T:
Unlocking the multifaceted molecular functions and diverse disease
implications of lactylation. Biol Rev Camb Philos Soc. 100:172–189.
2025. View Article : Google Scholar
|
|
115
|
Zhang D, Gao J, Zhu Z, Mao Q, Xu Z, Singh
PK, Rimayi CC, Moreno-Yruela C, Xu S, Li G, et al: Lysine
L-lactylation is the dominant lactylation isomer induced by
glycolysis. Nat Chem Biol. 21:91–99. 2025. View Article : Google Scholar
|
|
116
|
Hu XT, Wu XF, Xu JY and Xu X:
Lactate-mediated lactylation in human health and diseases: Progress
and remaining challenges. J Adv Res. S2090-1232(24)00529-02024.Epub
ahead of print. PubMed/NCBI
|
|
117
|
Zhang N, Zhang Y, Xu J, Wang P, Wu B, Lu
S, Lu X, You S, Huang X, Li M, et al: α-myosin heavy chain
lactylation maintains sarcomeric structure and function and
alleviates the development of heart failure. Cell Res. 33:679–698.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhu R, Ye X, Lu X, Xiao L, Yuan M, Zhao H,
Guo D, Meng Y, Han H, Luo S, et al: ACSS2 acts as a lactyl-CoA
synthetase and couples KAT2A to function as a lactyltransferase for
histone lactylation and tumor immune evasion. Cell Metab.
37:361–376.e7. 2025. View Article : Google Scholar
|
|
119
|
Chen H, Li Y, Li H, Chen X, Fu H, Mao D,
Chen W, Lan L, Wang C, Hu K, et al: NBS1 lactylation is required
for efficient DNA repair and chemotherapy resistance. Nature.
631:663–669. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jia M, Yue X, Sun W, Zhou Q, Chang C, Gong
W, Feng J, Li X, Zhan R, Mo K, et al: ULK1-mediated metabolic
reprogramming regulates Vps34 lipid kinase activity by its
lactylation. Sci Adv. 9:eadg49932023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Xie B, Zhang M, Li J, Cui J, Zhang P, Liu
F, Wu Y, Deng W, Ma J, Li X, et al: KAT8-catalyzed lactylation
promotes eEF1A2-mediated protein synthesis and colorectal
carcinogenesis. Proc Natl Acad Sci USA. 121:e23141281212024.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zou Y, Cao M, Tao L, Wu S, Zhou H, Zhang
Y, Chen Y, Ge Y, Ju Z and Luo S: Lactate triggers KAT8-mediated
LTBP1 lactylation at lysine 752 to promote skin rejuvenation by
inducing collagen synthesis in fibroblasts. Int J Biol Macromol.
277:1344822024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Moreno-Yruela C, Zhang D, Wei W, Bæk M,
Liu W, Gao J, Danková D, Nielsen AL, Bolding JE, Yang L, et al:
Class I histone deacetylases (HDAC1-3) are histone lysine
delactylases. Sci Adv. 8:eabi66962022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jin J, Bai L, Wang D, Ding W, Cao Z, Yan
P, Li Y, Xi L, Wang Y, Zheng X, et al: SIRT3-dependent
delactylation of cyclin E2 prevents hepatocellular carcinoma
growth. EMBO Rep. 24:e560522023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Fan Z, Liu Z, Zhang N, Wei W, Cheng K, Sun
H and Hao Q: Identification of SIRT3 as an eraser of H4K16la.
iScience. 26:1077572023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li XM, Yang Y, Jiang FQ, Hu G, Wan S, Yan
WY, He XS, Xiao F, Yang XM, Guo X, et al: Histone lactylation
inhibits RARγ expression in macrophages to promote colorectal
tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling.
Cell Rep. 43:1136882024. View Article : Google Scholar
|
|
127
|
Dai J, Huang YJ, He X, Zhao M, Wang X, Liu
ZS, Xue W, Cai H, Zhan XY, Huang SY, et al: Acetylation blocks cGAS
activity and inhibits self-DNA-induced autoimmunity. Cell.
176:1447–1460.e14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang T, Ye Z, Li Z, Jing DS, Fan GX, Liu
MQ, Zhuo QF, Ji SR, Yu XJ, Xu XW and Qin Y: Lactate-induced protein
lactylation: A bridge between epigenetics and metabolic
reprogramming in cancer. Cell Prolif. 56:e134782023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tian Q and Zhou LQ: Lactate activates
germline and cleavage embryo genes in mouse embryonic stem cells.
Cells. 11:5482022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li L, Chen K, Wang T, Wu Y, Xing G, Chen
M, Hao Z, Zhang C, Zhang J, Ma B, et al: Glis1 facilitates
induction of pluripotency via an epigenome-metabolome-epigenome
signalling cascade. Nat Metab. 2:882–892. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yang W, Wang P, Cao P, Wang S, Yang Y, Su
H and Nashun B: Hypoxic in vitro culture reduces histone
lactylation and impairs pre-implantation embryonic development in
mice. Epigenetics Chromatin. 14:572021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang D, Zheng H, Lu W, Tian X, Sun Y and
Peng H: Histone lactylation is involved in mouse oocyte maturation
and embryo development. Int J Mol Sci. 25:48212024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Merkuri F, Rothstein M and Simoes-Costa M:
Histone lactylation couples cellular metabolism with developmental
gene regulatory networks. Nat Commun. 15:902024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hagihara H, Shoji H, Otabi H, Toyoda A,
Katoh K, Namihira M and Miyakawa T: Protein lactylation induced by
neural excitation. Cell Rep. 37:1098202021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ma W, Jia K, Cheng H, Xu H, Li Z, Zhang H,
Xie H, Sun H, Yi L, Chen Z, et al: Orphan nuclear receptor NR4A3
promotes vascular calcification via histone lactylation. Circ Res.
134:1427–1447. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wu J, Hu M, Jiang H, Ma J, Xie C, Zhang Z,
Zhou X, Zhao J, Tao Z, Meng Y, et al: Endothelial cell-derived
lactate triggers bone mesenchymal stem cell histone lactylation to
attenuate osteoporosis. Adv Sci (Weinh). 10:e23013002023.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Li Q, Zhang F, Wang H, Tong Y, Fu Y, Wu K,
Li J, Wang C, Wang Z, Jia Y, et al: NEDD4 lactylation promotes APAP
induced liver injury through caspase11 dependent non-canonical
pyroptosis. Int J Biol Sci. 20:1413–1435. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
You X, Xie Y, Tan Q, Zhou C, Gu P, Zhang
Y, Yang S, Yin H, Shang B, Yao Y, et al: Glycolytic reprogramming
governs crystalline silica-induced pyroptosis and inflammation
through promoting lactylation modification. Ecotoxicol Environ Saf.
283:1169522024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sun W, Jia M, Feng Y and Cheng X: Lactate
is a bridge linking glycolysis and autophagy through lactylation.
Autophagy. 19:3240–3241. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zhao W, Wang Y, Liu J, Yang Q, Zhang S, Hu
X, Shi Z, Zhang Z, Tian J, Chu D and An L: Progesterone activates
the histone lactylation-Hif1α-glycolysis feedback loop to promote
decidualization. Endocrinology. 165:bqad1692023. View Article : Google Scholar
|
|
141
|
Chen Y, Wu J, Zhai L, Zhang T, Yin H, Gao
H, Zhao F, Wang Z, Yang X, Jin M, et al: Metabolic regulation of
homologous recombination repair by MRE11 lactylation. Cell.
187:294–311.e21. 2024. View Article : Google Scholar
|
|
142
|
Sun L, Zhang Y, Yang B, Sun S, Zhang P,
Luo Z, Feng T, Cui Z, Zhu T, Li Y, et al: Lactylation of METTL16
promotes cuproptosis via m6A-modification on FDX1 mRNA
in gastric cancer. Nat Commun. 14:65232023. View Article : Google Scholar
|
|
143
|
Dai W, Wu G, Liu K, Chen Q, Tao J, Liu H
and Shen M: Lactate promotes myogenesis via activating H3K9
lactylation-dependent up-regulation of Neu2 expression. J Cachexia
Sarcopenia Muscle. 14:2851–2865. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Mao Y, Zhang J, Zhou Q, He X, Zheng Z, Wei
Y, Zhou K, Lin Y, Yu H, Zhang H, et al: Hypoxia induces
mitochondrial protein lactylation to limit oxidative
phosphorylation. Cell Res. 34:13–30. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wu J, Lv Y, Hao P, Zhang Z, Zheng Y, Chen
E and Fan Y: Immunological profile of lactylation-related genes in
Crohn's disease: A comprehensive analysis based on bulk and
single-cell RNA sequencing data. J Transl Med. 22:3002024.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang Y, Gao Y, Wang Y, Jiang Y, Xiang Y,
Wang X, Wang Z, Ding Y, Chen H, Rui B, et al: RBM25 is required to
restrain inflammation via ACLY RNA splicing-dependent metabolism
rewiring. Cell Mol Immunol. 21:1231–1250. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sun Z, Gao Z, Xiang M, Feng Y, Wang J, Xu
J, Wang Y and Liang J: Comprehensive analysis of lactate-related
gene profiles and immune characteristics in lupus nephritis. Front
Immunol. 15:13290092024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Rho H, Terry AR, Chronis C and Hay N:
Hexokinase 2-mediated gene expression via histone lactylation is
required for hepatic stellate cell activation and liver fibrosis.
Cell Metab. 35:1406–1423.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wang Y, Li H, Jiang S, Fu D, Lu X, Lu M,
Li Y, Luo D, Wu K, Xu Y, et al: The glycolytic enzyme PFKFB3 drives
kidney fibrosis through promoting histone lactylation-mediated
NF-κB family activation. Kidney Int. 106:226–240. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wei L, Yang X, Wang J, Wang Z, Wang Q,
Ding Y and Yu A: H3K18 lactylation of senescent microglia
potentiates brain aging and Alzheimer's disease through the NFκB
signaling pathway. J Neuroinflammation. 20:2082023. View Article : Google Scholar
|
|
151
|
Huang Y, Wang C, Zhou T, Xie F, Liu Z, Xu
H, Liu M, Wang S, Li L, Chi Q, et al: Lumican promotes calcific
aortic valve disease through H3 histone lactylation. Eur Heart J.
45:3871–3885. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Maschari D, Saxena G, Law TD, Walsh E,
Campbell MC and Consitt LA: Lactate-induced lactylation in skeletal
muscle is associated with insulin resistance in humans. Front
Physiol. 13:9513902022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Gao R, Li Y, Xu Z, Zhang F, Xu J, Hu Y,
Yin J, Yang K, Sun L, Wang Q, et al: Mitochondrial pyruvate carrier
1 regulates fatty acid synthase lactylation and mediates treatment
of nonalcoholic fatty liver disease. Hepatology. 78:1800–1815.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Si WY, Yang CL, Wei SL, Du T, Li LK, Dong
J, Zhou Y, Li H, Zhang P, Liu QJ, et al: Therapeutic potential of
microglial SMEK1 in regulating H3K9 lactylation in cerebral
ischemia-reperfusion. Commun Biol. 7:17012024. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Liao Z, Chen B, Yang T, Zhang W and Mei Z:
Lactylation modification in cardio-cerebral diseases: A
state-of-the-art review. Ageing Res Rev. 104:1026312025. View Article : Google Scholar
|
|
156
|
Li W, Zhou J, Gu Y, Chen Y, Huang Y, Yang
J, Zhu X, Zhao K, Yan Q, Zhao Z, et al: Lactylation of RNA
m6A demethylase ALKBH5 promotes innate immune response
to DNA herpesviruses and mpox virus. Proc Natl Acad Sci USA.
121:e24091321212024. View Article : Google Scholar
|
|
157
|
Yan Q, Zhou J, Gu Y, Huang W, Ruan M,
Zhang H, Wang T, Wei P, Chen G, Li W and Lu C: Lactylation of NAT10
promotes N4-acetylcytidine modification on
tRNASer-CGA-1-1 to boost oncogenic DNA virus KSHV
reactivation. Cell Death Differ. 31:1362–1374. 2024. View Article : Google Scholar :
|
|
158
|
Wang Z, Mao Y, Wang Z, Li S, Hong Z, Zhou
R, Xu S, Xiong Y and Zhang Y: Histone lactylation-mediated
overexpression of RASD2 promotes endometriosis progression via
upregulating the SUMOylation of CTPS1. Am J Physiol Cell Physiol.
328:C500–C513. 2025. View Article : Google Scholar
|
|
159
|
Ye L, Jiang Y and Zhang M: Crosstalk
between glucose metabolism, lactate production and immune response
modulation. Cytokine Growth Factor Rev. 68:81–92. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kes MMG, Van den Bossche J, Griffioen AW
and Huijbers EJM: Oncometabolites lactate and succinate drive
pro-angiogenic macrophage response in tumors. Biochim Biophys Acta
Rev Cancer. 1874:1884272020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhou HC, Xin-Yan Yan, Yu WW, Liang XQ, Du
XY, Liu ZC, Long JP, Zhao GH and Liu HB: Lactic acid in macrophage
polarization: The significant role in inflammation and cancer. Int
Rev Immunol. 41:4–18. 2022. View Article : Google Scholar
|
|
162
|
Cai X, Ng CP, Jones O, Fung TS, Ryu KW, Li
D and Thompson CB: Lactate activates the mitochondrial electron
transport chain independently of its metabolism. Mol Cell.
83:3904–3920.e7. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Adam C, Paolini L, Gueguen N, Mabilleau G,
Preisser L, Blanchard S, Pignon P, Manero F, Le Mao M, Morel A, et
al: Acetoacetate protects macrophages from lactic acidosis-induced
mitochondrial dysfunction by metabolic reprograming. Nat Commun.
12:71152021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Noe JT, Rendon BE, Geller AE, Conroy LR,
Morrissey SM, Young LEA, Bruntz RC, Kim EJ, Wise-Mitchell A,
Barbosa de Souza Rizzo M, et al: Lactate supports a
metabolic-epigenetic link in macrophage polarization. Sci Adv.
7:eabi86022021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ajam-Hosseini M, Heydari R, Rasouli M,
Akhoondi F, Asadi Hanjani N, Bekeschus S and Doroudian M: Lactic
acid in macrophage polarization: A factor in carcinogenesis and a
promising target for cancer therapy. Biochem Pharmacol.
222:1160982024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Yang K, Xu J, Fan M, Tu F, Wang X, Ha T,
Williams DL and Li C: Lactate suppresses macrophage
pro-inflammatory response to LPS stimulation by inhibition of YAP
and NF-κB activation via GPR81-mediated signaling. Front Immunol.
11:5879132020. View Article : Google Scholar
|
|
167
|
Wang J, Yang P, Yu T, Gao M, Liu D, Zhang
J, Lu C, Chen X, Zhang X and Liu Y: Lactylation of PKM2 suppresses
inflammatory metabolic adaptation in pro-inflammatory macrophages.
Int J Biol Sci. 18:6210–6225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Dichtl S, Lindenthal L, Zeitler L, Behnke
K, Schlösser D, Strobl B, Scheller J, El Kasmi KC and Murray PJ:
Lactate and IL6 define separable paths of inflammatory metabolic
adaptation. Sci Adv. 7:eabg35052021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Irizarry-Caro RA, McDaniel MM, Overcast
GR, Jain VG, Troutman TD and Pasare C: TLR signaling adapter BCAP
regulates inflammatory to reparatory macrophage transition by
promoting histone lactylation. Proc Natl Acad Sci USA.
117:30628–30638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang Y, Jiang H, Dong M, Min J, He X, Tan
Y, Liu F, Chen M, Chen X, Yin Q, et al: Macrophage MCT4 inhibition
activates reparative genes and protects from atherosclerosis by
histone H3 lysine 18 lactylation. Cell Rep. 43:1141802024.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Hoque R, Farooq A, Ghani A, Gorelick F and
Mehal WZ: Lactate reduces liver and pancreatic injury in Toll-like
receptor- and inflammasome-mediated inflammation via GPR81-mediated
suppression of innate immunity. Gastroenterology. 146:1763–1774.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Fang Y, Li Z, Yang L, Li W, Wang Y, Kong
Z, Miao J, Chen Y, Bian Y and Zeng L: Emerging roles of lactate in
acute and chronic inflammation. Cell Commun Signal. 22:2762024.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Pucino V, Certo M, Bulusu V, Cucchi D,
Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et
al: Lactate buildup at the site of chronic inflammation promotes
disease by inducing CD4+ T cell metabolic rewiring. Cell
Metab. 30:1055–1074.e8. 2019. View Article : Google Scholar
|
|
174
|
Subudhi I, Konieczny P, Prystupa A,
Castillo RL, Sze-Tu E, Xing Y, Rosenblum D, Reznikov I, Sidhu I,
Loomis C, et al: Metabolic coordination between skin epithelium and
type 17 immunity sustains chronic skin inflammation. Immunity.
57:1665–1680.e7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ma S, Ming Y, Wu J and Cui G: Cellular
metabolism regulates the differentiation and function of T-cell
subsets. Cell Mol Immunol. 21:419–435. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Zhang YT, Xing ML, Fang HH, Li WD, Wu L
and Chen ZP: Effects of lactate on metabolism and differentiation
of CD4+T cells. Mol Immunol. 154:96–107. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Cao J, Liao S, Zeng F, Liao Q, Luo G and
Zhou Y: Effects of altered glycolysis levels on CD8+ T
cell activation and function. Cell Death Dis. 14:4072023.
View Article : Google Scholar
|
|
178
|
Almeida L, Dhillon-LaBrooy A, Carriche G,
Berod L and Sparwasser T: CD4+ T-cell differentiation
and function: Unifying glycolysis, fatty acid oxidation, polyamines
NAD mitochondria. J Allergy Clin Immunol. 148:16–32. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Haas R, Smith J, Rocher-Ros V, Nadkarni S,
Montero-Melendez T, D'Acquisto F, Bland EJ, Bombardieri M, Pitzalis
C, Perretti M, et al: Lactate regulates metabolic and
pro-inflammatory circuits in control of T cell migration and
effector functions. PLoS Biol. 13:e10022022015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Bechara R, McGeachy MJ and Gaffen SL: The
metabolism-modulating activity of IL-17 signaling in health and
disease. J Exp Med. 218:e202021912021. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Chang CH, Curtis JD, Maggi LB Jr, Faubert
B, Villarino AV, O'Sullivan D, Huang SCC, van der Windt GJW, Blagih
J, Qiu J, et al: Posttranscriptional control of T cell effector
function by aerobic glycolysis. Cell. 153:1239–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Yin Y, Choi SC, Xu Z, Zeumer L, Kanda N,
Croker BP and Morel L: Glucose oxidation is critical for CD4+ T
cell activation in a mouse model of systemic lupus erythematosus. J
Immunol. 196:80–90. 2016. View Article : Google Scholar
|
|
183
|
Watson MJ, Vignali PDA, Mullett SJ,
Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV,
Rittenhouse NL, DePeaux K, Whetstone RD, et al: Metabolic support
of tumour-infiltrating regulatory T cells by lactic acid. Nature.
591:645–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Tay C, Tanaka A and Sakaguchi S:
Tumor-infiltrating regulatory T cells as targets of cancer
immunotherapy. Cancer Cell. 41:450–465. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Fan W, Wang X, Zeng S, Li N, Wang G, Li R,
He S, Li W, Huang J, Li X, et al: Global lactylome reveals
lactylation-dependent mechanisms underlying TH17
differentiation in experimental autoimmune uveitis. Sci Adv.
9:eadh46552023. View Article : Google Scholar
|
|
186
|
Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X,
Shao Q, Zhou B, Zhou H, Wei S, et al: Tumor metabolite lactate
promotes tumorigenesis by modulating MOESIN lactylation and
enhancing TGF-beta signaling in regulatory T cells. Cell Rep.
39:1109862022. View Article : Google Scholar
|
|
187
|
Sharma R, Smolkin RM, Chowdhury P,
Fernandez KC, Kim Y, Cols M, Alread W, Yen WF, Hu W, Wang ZM, et
al: Distinct metabolic requirements regulate B cell activation and
germinal center responses. Nat Immunol. 24:1358–1369. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Martinis E, Tonon S, Colamatteo A, La Cava
A, Matarese G and Pucillo CEM: B cell immunometabolism in health
and disease. Nat Immunol. 26:366–377. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Lee SC, Marzec M, Liu X, Wehrli S,
Kantekure K, Ragunath PN, Nelson DS, Delikatny EJ, Glickson JD and
Wasik MA: Decreased lactate concentration and glycolytic enzyme
expression reflect inhibition of mTOR signal transduction pathway
in B-cell lymphoma. NMR Biomed. 26:106–114. 2013. View Article : Google Scholar
|
|
190
|
Yao Y, Zhu J, Qin S, Zhou Z, Zeng Q, Long
R, Mao Z, Dong X, Zhao R, Zhang R, et al: Resveratrol induces
autophagy impeding BAFF-stimulated B-cell proliferation and
survival by inhibiting the Akt/mTOR pathway. Biochem Pharmacol.
202:1151392022. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Lee P, Chandel NS and Simon MC: Cellular
adaptation to hypoxia through hypoxia inducible factors and beyond.
Nat Rev Mol Cell Biol. 21:268–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Meng X, Asadi-Asadabad S, Cao S, Song R,
Lin Z, Safhi M, Qin Y, Tcheumi Tactoum E, Taudte V, Ekici A, et al:
Metabolic rewiring controlled by HIF-1α tunes IgA-producing B-cell
differentiation and intestinal inflammation. Cell Mol Immunol.
22:54–67. 2025. View Article : Google Scholar
|
|
193
|
Lee DC, Sohn HA, Park ZY, Oh S, Kang YK,
Lee K, Kang M, Jang YJ, Yang SJ, Hong YK, et al: A lactate-induced
response to hypoxia. Cell. 161:595–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Sig Transduct Target Ther. 2:170232017.
View Article : Google Scholar
|
|
195
|
Ma N, Wang L, Meng M, Wang Y, Huo R, Chang
G and Shen X: D-sodium lactate promotes the activation of NF-κB
signaling pathway induced by lipopolysaccharide via histone
lactylation in bovine mammary epithelial cells. Microb Pathog.
199:1071982025. View Article : Google Scholar
|
|
196
|
Chi W, Kang N, Sheng L, Liu S, Tao L, Cao
X, Liu Y, Zhu C, Zhang Y, Wu B, et al: MCT1-governed pyruvate
metabolism is essential for antibody class-switch recombination
through H3K27 acetylation. Nat Commun. 15:1632024. View Article : Google Scholar : PubMed/NCBI
|