Introduction
Under physiological conditions, the immune system of
the gastric mucosa interacts with gastric epithelial cells, immune
cells and signaling molecules to form a protective barrier
(1). Innate and adaptive
immunity maintain the homeostasis of the gastric mucosal immune
microenvironment (2-8), whereas a disruption of the balance
of the immune microenvironment results in gastric mucosal diseases,
such as autoimmune gastritis and gastric neoplasia (5,8,9).
Chronic and persistent inflammatory microenvironment stimulation
can lead to cellular differentiation disorders and tumor-related
lesions; for example, the occurrence and progression of spasmolytic
polypeptide-expressing metaplasia (SPEM) (9-11).
Pyroptosis is a type of inflammatory programmed cell
death (12,13), which can regulate the balance
between innate and adaptive immunity by identifying injury factors,
and participating in the maturation and release of inflammatory
factors (12,14-16). As an important target of immune
regulation, pyroptosis is involved in almost all processes related
to the resistance of the gastric mucosa to microbial infections and
endogenous damage. In addition, pyroptosis manipulates immune
remodeling in the gastric mucosa by releasing inflammatory factors,
further affecting the repair process and cell differentiation
direction (17-21). The present review summarizes the
role of pyroptosis in gastric mucosal diseases, including focal and
diffuse gastric mucosal injury. The aim of the current review is to
provide a novel theoretical basis for the optimization of
prevention strategies and treatment plans for current gastric
mucosal diseases, proposing a new perspective for disease
management (Fig. 1).
Functional role of pyroptosis
Pyroptosis is an inflammatory form of programmed
cell death characterized by plasma membrane pore formation and the
release of inflammatory contents (12,22-24).
Triggering of pyroptosis
Inflammasomes, which include pattern recognition
receptors (PRRs), apoptosis-associated speck-like protein
containing a CARD (ASC) proteins and caspase-1, recognize damage
signals such as damage-associated molecular patterns or
pathogen-associated molecular patterns (PAMPs) (16,25,26). PRRs are representative of immune
receptors involved in innate immunity, and the mutual recognition
and interaction between PRRs and PAMPs are key to initiating innate
immune responses (27). In cases
of microbial infection or tissue damage, PRRs rapidly activate
innate immunity and inflammasomes, promote pyroptosis and recruit
inflammatory cells to guide the initiation of adaptive immune
responses (28).
Activation of pyroptosis
Classical caspase-1-dependent activation occurs as
follows: PRR signals activate caspase-1 via ASC, and activated
caspase-1 cleaves gasdermin (GSDM) D causing membrane rupture and
activation of IL-1β/IL-18, thus amplifying inflammation (29). Non-classical activation occurs as
follows: Human caspase-4/5 or murine caspase-11 directly recognize
cytosolic lipopolysaccharide, cleave GSDMD and may indirectly
activate caspase-1, exacerbating inflammation (30).
Effector phase of pyroptosis
Cell rupture releases inflammatory mediators [such
as IL-1β, IL-18, IL-33 and high mobility group protein B1 (HMGB1)],
which recruit and activate immune cells [such as dendritic cells
(DCs) and macrophages] to promote antigen presentation and initiate
adaptive immunity (16,24,21-33).
Therefore, pyroptosis acts as a bridge between
innate and adaptive immunity, clearing infected/damaged cells and
remodeling the immune microenvironment for antimicrobial defense
and tissue repair (34,35). However, excessive inflammation
exacerbates damage, remodels the microenvironment, and can promote
autoimmune diseases or tumorigenesis (6,9,12,14). Research regarding
pyroptosis-mediated microenvironment remodeling is crucial for
understanding disease mechanisms and developing therapeutic
strategies (34,36-38).
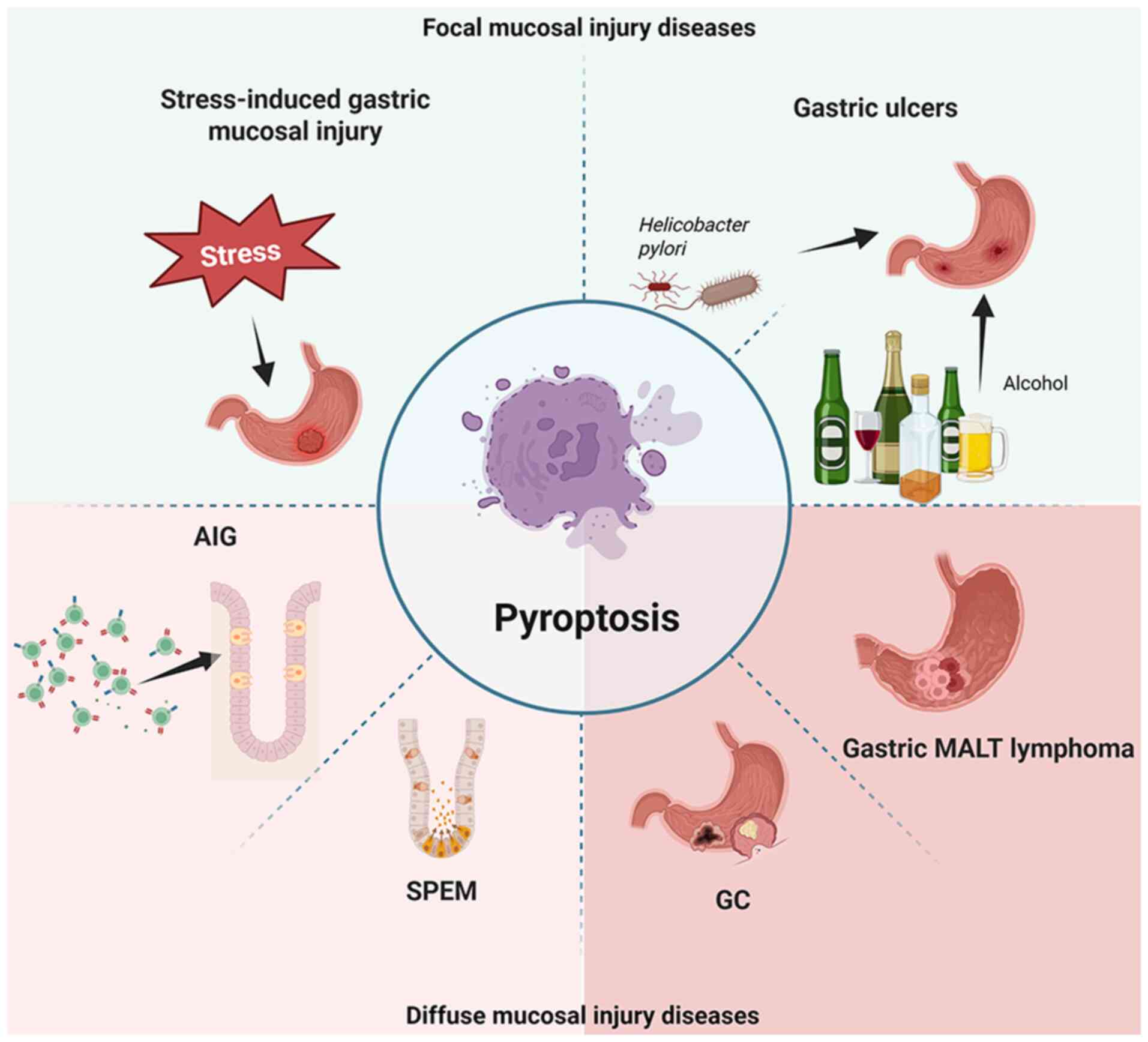
Pyroptosis in gastric mucosal diseases
Gastric mucosal diseases can be divided into focal
mucosal injury and diffuse mucosal injury (2,39). Focal mucosal injury is a
repairable injury that does not alter cell differentiation patterns
(4,40). When injury and chronic
inflammation persist, cells undergo changes in differentiation
patterns and develop diffuse mucosal injury (4,41). Notably, damage to the gastric
mucosa (via factors such as stress, Helicobacter pylori
infection and alcohol) is recognized by inflammasomes, and
pyroptosis activates the rapid response of the innate immune
response, initiates adaptive immunity, reshapes the immune
microenvironment, affects cell differentiation, and participates in
the occurrence and development of various gastric mucosal
diseases.
Role of pyroptosis in focal mucosal
injury diseases
Pyroptosis may serve a bidirectional
regulatory role in stress-induced gastric mucosal injury
Stress-induced gastric mucosal injury refers to
ulcers or erosions that occur due to strong and persistent stress
stimuli, such as trauma, shock, burns or surgery, causing an
imbalance between the protective and injury mechanisms of the
gastric mucosa (42,43). Stress-induced gastric mucosal
injury is a serious complication of physical and mental stress
caused by factors such as neuroendocrine imbalance (44), disruption of the gastric mucosal
protective barrier (45) and
enhancement of gastric mucosal injury factors (46).
Stress activates pro-IL-18 in the adrenal cortex via
the adrenocorticotropic hormone/superoxide-mediated caspase-1
activation pathway, converting it into mature IL-18 for release
into the bloodstream (17).
IL-18 has been reported to mediate water immersion restraint stress
(WIRS)-induced gastric injury in an animal model by increasing
gastric histidine decarboxylase activity and histamine production,
and synergizes with sympathetic overexcitation to exacerbate
gastric hemorrhage following WIRS (47).
Local reactive oxygen species (ROS) serve as
initiating factors in stress-induced gastric mucosal injury
(48). ROS promote gastrokine 2
expression, activate NF-κB, increase nucleotide-binding domain and
leucine-rich repeat protein 3 (NLRP3) activation and ultimately
exacerbate WIRS-induced inflammation (49). This process triggers
neutrophil-dominated inflammation, in which elevated NLRP3/IL-1β
levels are positively associated with neutrophil infiltration and
ulcer severity (49). Zhang et
al (50) demonstrated that
cold-induced stress increases IL-1β/IL-18 expression, whereas the
caspase-1 inhibitor AC-YVAD-CMK protects the gastric mucosa by
suppressing the NLRP3 inflammasome, thereby mitigating inflammation
and pyroptosis. However, Higashimori et al (51) reported opposing conclusions.
NLRP3/IL-1β levels in the gastric mucosa were revealed to be
transiently increased at 0.5 h post-stress (returning to baseline
by 3 h) without a concurrent elevation in IL-18. The NLRP3
inflammasome-derived IL-1β exerted gastric protective effects by
activating NF-κB, inducing the cyclooxygenase-2/prostaglandin E2
(PGE2) axis, and upregulating PGE2 production. The notable
discrepancy with the observations of Zhang et al (50) of elevated IL-1β/IL-18 at 8 h
post-stress may stem from distinct observational timepoints (0.5-3
h vs. 8 h). Therefore, a phase-dependent bidirectional regulatory
role for the NLRP3 inflammasome may be hypothesized in
stress-induced gastric injury: In the early phase (≤3 h), IL-1β
secretion may mediate mucosal protection, whereas in the late phase
(≥8 h), IL-18 release could contribute to mucosal damage. The
dynamic equilibrium between IL-1β and IL-18 dictates pathological
progression.
Collectively, pyroptosis-associated mediators serve
key regulatory roles, although their precise mechanisms require
further elucidation to establish novel therapeutic targets.
Role of pyroptosis in gastric
ulcers
The occurrence of gastric ulcers is the
comprehensive result of one or more invasive and damaging factors.
When the action of mucosal injury factors exceeds that of mucosal
protective factors, causing an imbalance between resistance to
injury and self-repair ability, the injury can penetrate the muscle
layer of the mucosa, leading to the occurrence of gastric ulcers
(52). The present review
discusses the role of pyroptosis in gastric ulcers from two
perspectives: Mucosal injury and mucosal protection.
Pyroptosis serves an important
proinflammatory role in the formation of gastric ulcers
In gastric ulcers caused by Helicobacter
pylori infection, macrophages simultaneously activate the
classical caspase-1-dependent pyroptosis pathway and the
non-classical pyroptosis pathway mediated by caspase-11 (a direct
murine homolog of human caspase-4), activating the NLRP3
inflammasome, triggering pyroptosis, and promoting the maturation
and secretion of IL-1β (18,53,54). IL-1β can inhibit gastric acid
secretion and contraction of the stomach muscles, and further drive
the expression of adhesion molecules on immune cells; regulate
neutrophil recruitment, macrophage activation, circulating monocyte
infiltration and T-cell expansion; amplify inflammatory responses;
and reshape the microenvironment of gastric mucosal tissue
(55).
In ethanol-induced gastric mucosal injury, the
expression of NLRP3 and GSDMD has been shown to be increased in
gastric tissue. Further research revealed that ethanol can directly
induce pyroptosis in GES-1 cells, leading to the activation of
caspase-1, and the release of IL-1β and IL-18, causing tissue
inflammatory damage (19).
Moreover, ethanol can activate NF-κB p65 and the NLRP3
inflammasome, leading to an increase in HMGB1 expression in the
gastric mucosa (56). HMGB1 can
stimulate cytokine production through receptor for advanced
glycation end products or Toll-like receptor 4, trigger
inflammatory responses, attract immune cells to the site of injury
and further shape the immune microenvironment (57-60). The increased expression of HMGB1
in gastric ulcers can accelerate and maintain inflammatory damage,
delay tissue healing and increase the risk of tumor formation
(60). The inhibition of the
HMGB1/NLRP3/NF-κB pathway results in notable anti-inflammatory and
anti-ulcer effects in ethanol-induced gastric ulcers (55). In addition, the caspase-1
inhibitor AC-YVAD-CMK can protect mice from ethanol-induced acute
gastric injury by reducing pyroptosis and the inflammatory
response, further confirming the important proinflammatory role of
pyroptosis in ethanol-induced acute gastric ulcers (50).
Furthermore, research regarding treatment strategies
for gastric ulcers has shown that various drugs, such as
rabeprazole (61), fucoidan
(62), ALDH2 (63), saxagliptin (64), C-phycocyanin (56) and irbesartan (65), inhibit the activation of NLRP3
and pyroptosis, reduce IL-1β release, notably alleviate
inflammation and promote ulcer healing. Therefore, in the complex
pathological process of gastric ulcers, pyroptosis has been
identified as a key proinflammatory factor, and exploring
pyroptosis as a potential therapeutic target may provide new
effective avenues for the treatment of gastric ulcers.
Pyroptosis may lead to an increase in
the release of the gastric mucosal protective factor PGE2, which is
beneficial for promoting wound repair
PGE2 is an important mucosal protective factor for
gastric ulcers (66). PGE2 can
inhibit caspase-11-driven pyroptosis in macrophages, limiting the
activation of typical and atypical inflammasomes, and effectively
blocking the inflammatory cascade (54). Notably, a recent study (67) confirmed the protective role of
pyroptosis in epithelial injury repair from a new perspective. This
previous study used a system (denoted Pyro-1) that was shown to
induce macrophage pyroptosis without the release of IL-1β or IL-1α,
and reported that Pyro-1 supernatant can promote the migration of
primary fibroblasts and macrophages, facilitate faster wound
closure in vitro and improve tissue repair in vivo.
The presence of oxidized lipids and metabolites in the supernatant
of Pyro-1 was identified through lipidomics and metabolomics. The
mechanism of action of Pyro-1 was shown to involve the synthesis of
PGE2 during the late stage of pyroptosis and PGE2 release through
pores opened by GSDMD during pyroptosis to promote tissue
regeneration. These findings suggest that the occurrence of
pyroptosis may balance tissue damage and regeneration, and that
pyroptotic secretion may promote wound repair. Although it is
unknown whether pyroptosis also participates in tissue repair
through the same mechanism in gastric ulcer injury repair, these
results highlight the 'double-edged sword' of pyroptosis in immune
microenvironment regulation. Therefore, extensive research is
needed to further clarify whether pyroptosis serves a dual role in
promoting inflammation and repair in gastric mucosal injury and
repair.
Overall, pyroptosis has an important regulatory role
in damage to and repair of the gastric mucosa in gastric ulcers by
reshaping the gastric mucosal tissue microenvironment. Elucidating
the specific related mechanisms of pyroptosis may enrich the
understanding of gastric mucosal injury and repair, and provide new
targets for the treatment of gastric ulcers.
Functional role of pyroptosis in
diffuse mucosal injury diseases
Diffuse gastric mucosal injury alters cell
differentiation patterns, and is typically associated with gastric
tumors and precancerous lesions (4,40,41). Pyroptosis affects immune
remodeling of the gastric mucosa by releasing inflammatory factors,
further affecting cell differentiation, determining cell fate and
causing diffuse damage to the gastric mucosa, leading to
disease.
Role of pyroptosis in autoimmune
gastritis (AIG) (Fig. 2)
AIG is type of chronic progressive inflammation
mediated by organ-specific immunity, which characterized by
anti-parietal cell antibodies that target the
H+/K+ ATPases of parietal cells, leading to
CD4+ T cell-mediated parietal cell death and progressive
atrophy of the secretory gland (68). AIG can lead to hypergastrinemia,
resulting in enhanced lymphocyte proliferation and an increased
risk of type 1 gastric neuroendocrine tumor development (69). In addition, immune cell-cytokine
interactions further disrupt chief/mucous neck cell
differentiation, triggering cellular phenotype transformation,
SPEM, intestinal metaplasia and intestinal-type gastric
carcinogenesis (70). Therefore,
AIG is considered the basis for the occurrence of diffuse damage to
the gastric mucosa.
The type 1 immune response, which is characterized
by T helper (Th)1 CD4+ cells and the appearance of
interferon-γ (IFN-γ), is a key driving factor in the pathology of
AIG (71,72). This pathological cascade is
initiates when CD4+ T cells specifically recognize
H+/K+ ATPase (73,74), activating the NLRP3
inflammasome/ROS pathway (75)
to induce pyroptosis and exacerbate gastric inflammation (76,77). Pyroptosis further fuels disease
progression by regulating CD4+ T-cell differentiation.
Complement-driven assembly of the NLRP3 inflammasome in
CD4+ T cells triggers pyroptosis-dependent IL-1β
secretion, which promotes Th1 differentiation and IFN-γ production
via autocrine signaling (78).
Upon binding with IFN-γ receptor on gastric epithelial cells, IFN-γ
not only directly induces epithelial death but also upregulates
GSDMB expression to accelerate pyroptotic cell death (79), collectively reshaping the gastric
mucosal immune microenvironment.
Additionally, CD4+ T cells differentiate
into Th17 cells (79) that
secrete IL-17 to recognize H+/K+-ATPase and
activate the NLRP3 inflammasome/ROS pathway, driving parietal cell
death and AIG pathogenesis (80-82). Notably the Th17 immune response
is regulated by the nucleotide-binding domain and leucine-rich
repeat containing family CARD domain-containing protein (NLRC)4
inflammasome (83,84). Several studies (84-88) have confirmed that mutations in
NLRC4 and NLR family apoptosis inhibitory proteins are involved in
the occurrence of various autoinflammatory diseases in humans by
affecting pyroptosis and inflammatory responses. Therefore, it may
be hypothesized that NLRC4-mediated pyroptosis further regulates
the immune microenvironment of the gastric mucosa by modulating the
Th17 immune response, and may thus be a novel target for the
treatment of AIG.
In summary, pyroptosis critically regulates
CD4+ T cells in the pathogenesis of AIG, although its
precise mechanisms require further validation. Elucidating
pyroptosis-mediated remodeling of the gastric mucosal immune
microenvironment is essential for discovering novel therapeutic
targets.
Pyroptosis is involved in regulating
SPEM (Fig. 3)
SPEM cells express mucin 6 and trefoil factor 2, and
have a morphology similar to that of deep pyloric gland cells or
duodenal Brunner gland cells. SPEM cells can be seen as a repair
lineage, and under continuous stimulation by chronic inflammation,
SPEM can develop into intestinal metaplasia, which is an important
precancerous lesion in gastric cancer (GC) (40,41,89).
Recent research has suggested that the absence of
parietal cells is the initiating factor for the occurrence of SPEM
(90). Parietal cells are
regulated by gene associated with retinoid-IFN-induced mortality
19, which activates the NLRP3 inflammasome through the ROS/nuclear
factor (erythroid-derived 2)-like 2/heme oxygenase-1/NF-κB axis,
initiating the classical pyroptosis pathway (20). After pyroptosis in parietal
cells, intracellular activated GSDMD mediates the transport of the
inflammatory factor IL-33 from the nucleus to the cytoplasm under
the synergistic effect of Ca2+ (91). The cell membrane of parietal
cells ruptures, and IL-33 in the cytoplasm is passively released
into the extracellular space (92,93). The release of IL-33 promotes the
secretion of Th2 cytokines (mainly IL-13) and the polarization of
M2 macrophages through the IL-33/ST2 signaling pathway, reshapes
the immune microenvironment of the gastric mucosal region,
initiates epithelial cell reprogramming and promotes the occurrence
of SPEM (94,95).
In the absence of parietal cells, the immune
microenvironment is reshaped, and epithelial cells are
reprogrammed, leading to the transdifferentiation of mature host
cells, mucinous neck cells or isthmus stem cells into SPEM cells.
SRY-box transcription factor 9 (Sox9) is the main regulatory factor
for the differentiation of mucinous neck cells during gastric
development (89) and is also an
essential mediator of chief cell recruitment to promote SPEM after
acute gastric injury. Under inflammatory conditions, macrophage
Sox9 directly targets the transcription of S100A9 in the nucleus.
Macrophage S100A9 is involved in regulating the inflammatory
response and pyroptosis driven by macrophage NIMA-related kinase
7/NLRP3 (96). However, the role
of pyroptosis in the process of gastric mucosal epithelial cell
differentiation remains unclear, and its specific regulatory role
requires extensive research. In summary, exploring the mechanisms
by which pyroptosis reshapes the immune microenvironment and
initiates epithelial cell reprogramming can help identify
therapeutic targets for SPEM, and prevent the development of more
severe phenotypes of gastric mucosal damage.
Role of pyroptosis in the development of
gastric neoplasia
Accurately locating and identifying
the cell types that undergo pyroptosis will help achieve precise
treatment of GC
GC is a common malignant tumor of the digestive
system. A number of reviews have summarized pyroptosis as an
inflammatory cell death mechanism that reshapes the tumor
microenvironment, and affects the occurrence and development of GC.
However, due to the high heterogeneity of GC, the role of
pyroptosis among different cells in tumor tissue is not consistent,
and pyroptosis also dynamically changes as a tumor progresses,
making targeted interventions for GC more challenging (97-100).
As an example, the pyroptosis inflammasome NLRP3
serves a dual role in the pathological and physiological processes
of GC. First, H. pylori infection and its virulence factor
CagA promote the upregulation of the NLRP3 inflammasome in GC by
enhancing the microRNA (miR)-1290/NKD1/NLRP3 axis (21). NLRP3 upregulation not only drives
cancer cell pyroptosis, invasion, migration (via inflammasome
activation) and malignant progression, but also induces M2
macrophage polarization through the IL-6/IL-10/JAK2/STAT3 signaling
pathway, and modulates the infiltration of CD4+ T cells
and M2 macrophages within the tumor microenvironment (21). NLRP3 upregulation ultimately
accelerates GC progression and is a predictor of poor prognosis
(21). In addition, miR-22 has
been confirmed to directly target and suppress NLRP3 expression,
inhibiting its pro-tumorigenic effects. H. pylori infection
suppresses miR-22, leading to NLRP3 upregulation, disruption of
gastric mucosal homeostasis and initiation of carcinogenesis
(38). By inhibiting NLRP3
activity, the carcinogenic potential of NLRP3 has been shown to be
effectively suppressed in in vitro experiments and animal
models, further confirming the core role of NLRP3 in the
progression of GC (101).
However, the functional complexity of NLRP3 is not
limited to its carcinogenic potential. The occurrence of pyroptosis
may increase the sensitivity of GC to chemotherapy. Specifically,
activation strategies targeting lengsin (a crystalline lens protein
highly expressed in cancer stem cells that is associated with
malignant progression in patients with GC) can induce pyroptosis in
GC stem cells, thereby restoring the responsiveness of GC to
chemotherapy drugs (102). In
addition, low-dose diosbulbin-B activates the endogenous programmed
death ligand 1/NLRP3 signaling pathway in tumors, triggering
pyroptosis and notably increasing the sensitivity of GC cells to
cisplatin chemotherapy (103).
Furthermore, West et al (104) suggested that NLRP3 has no
notable prognostic value in predicting patient survival outcomes
and that it does not serve a dominant role in the inflammatory
environment-driven development of gastric tumors. These findings
challenge the traditional understanding of the comprehensive role
of NLRP3 in promoting cancer, emphasizing the diversity and
complexity of pyroptosis functions under specific pathological
conditions. Therefore, only by mechanistically elucidating the
bidirectional regulation of pyroptosis and its critical molecular
switches can human trials of pyroptosis inhibitors be rationally
guided, ultimately achieving the precise therapeutic goal of
selectively inducing pyroptosis in target cells, enhancing
treatment sensitivity while circumventing tumor-promoting
microenvironments.
However, a relative lack of precise identification
and related evidence for the phenomenon of pyroptosis in specific
single-cell populations in GC tissues remains. How different cell
subpopulations in the GC microenvironment trigger complex
interactions between cells through pyroptotic mechanisms and
further reshape the specific mechanisms of the tumor
microenvironment remains unclear; this directly leads to notable
differences in the conclusions of the aforementioned studies.
Therefore, the scientific validity and effectiveness of solely
adopting strategies to inhibit or promote pyroptosis as a cancer
treatment method are questionable. Furthermore, considering that
the role of pyroptosis is regulated by complex factors, such as the
tumor microenvironment and cell type, its biological effects
exhibit marked bidirectionality and can affect the development
trajectory and treatment response of tumors through diverse
mechanisms. This complexity requires the use of a more
comprehensive and detailed strategy when designing experiments. In
summary, future research regarding pyroptosis in GC should focus on
specific analyses of cell types and occurrence periods, in order to
achieve a comprehensive evaluation and precise regulation of the
effects of pyroptosis. The findings of these studies may provide a
solid theoretical basis for optimizing treatment strategies for
patients with cancer, and novel ideas for improving patient
prognosis and survival status.
Pyroptosis serves a role in the immune
regulation of gastric mucosa-associated lymphoid tissue lymphoma
(MALT lymphoma)
Gastric MALT lymphoma is one of the most common
types of non-Hodgkin's lymphoma (105). The proliferation and
differentiation of gastric mucosal-associated lymphoma cells are
regulated by antigen-specific intratumoral T cells (via
CD40-mediated signaling, Th2-type cytokines, chemokines,
costimulatory molecules and FOXP3+ regulatory T cells)
and their communication with B cells (106).
There is a strong causal relationship between MALT
lymphoma and chronic H. pylori-associated gastritis
(107,108). NLRC5 expression is upregulated
in the macrophages and gastric tissues of mice and humans after
H. pylori infection (109). Mice with bone marrow-specific
deletion of NLPC5 develop precursor B-cell lesions of MALT lymphoma
3 months after H. pylori infection (109). The absence of NLRC5 inhibits
GSDMD cleavage and the activation of IL-1β and caspase-3, leading
to the upregulation of IFNγ expression and the activation of
lipopolysaccharide-induced proinflammatory responses in macrophages
(110), as well as increased
IL-1β and CD40 (111).
Anti-CD40L antibodies can prevent gastric B-cell damage in
NLRC5-deficient mice, reduce the number of DCs, and CD8+
and FOXP3+ T cells, and decrease B-cell lymphoma gene
expression in the stomach (109,111). Therefore, the involvement of
NLRC5 in pyroptosis affects antigen-specific T cells and their
communication with B cells in tumors, and further regulates the
occurrence of MALT lymphoma. However, further research and
exploration regarding the specific intercellular dialog and
mechanisms involved are needed.
Conclusion
As scientific research progresses, the understanding
of the mechanisms by which pyroptosis contributes to gastric
mucosal injury and repair has become increasingly refined. However,
key questions remain unanswered. In stress-induced injury,
elucidating the phase transition mechanism whereby the NLRP3
inflammasome shifts from early protection to late damage requires
the definition of precise neural signaling controls. Furthermore,
gastric ulcer healing hinges on cellular sources of pyroptosis,
inflammatory mediator (such as IL-1β and IL-18) and
microenvironmental integration, particularly regarding how PGE2
spatiotemporally coordinates anti-inflammatory and pro-repair
functions. For SPEM, the molecular mechanisms underlying
GSDMD-mediated IL-33 release (especially Ca2+-dependent
regulation) and whether additional factors from pyroptotic parietal
cells directly drive mucous neck/stem cell reprogramming must be
resolved. Furthermore, it remains unknown as to whether pyroptosis
or its effectors directly modulate key transcription factors to
initiate SPEM, or whether targeting pyroptosis can halt/reverse
SPEM progression. In heterogeneous GC microenvironments,
identifying which cellular subpopulations undergo pyroptosis at
specific stages (with cell type-dependent triggers) and defining
their stage-dependent roles (early tumor suppression vs. late
metastasis) are essential for developing strategies for selectively
inducing cancer cell pyroptosis to enhance chemosensitization while
avoiding tumor-promoting microenvironments. These focused efforts
will provide more precise targets for elucidating disease
pathogenesis, and hold promise for facilitating innovative and
highly effective interventions for gastric mucosal disorders.
Availability of data and materials
Not applicable.
Authors' contributions
XL and TL conceived and designed the paper. BJ
drafted the manuscript. ZM and BT edited and revised the
manuscript. SL, SY, KM and ST participated in the literature search
and analysis of the data to be included in the review. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
PRRs
|
pattern recognition receptors
|
|
ASC
|
apoptosis associated speck-like
protein containing a CARD
|
|
PAMP
|
pathogen-associated molecular
pattern
|
|
GSDM
|
gasdermin
|
|
HMGB1
|
high mobility group protein B1
|
|
DCs
|
dendritic cells
|
|
WIRS
|
water immersion restraint stress
|
|
ROS
|
reactive oxygen species
|
|
NLRP3
|
nucleotide-binding domain and
leucine-rich repeat protein 3
|
|
PGE2
|
prostaglandin E2
|
|
AIG
|
autoimmune gastritis
|
|
IFN-γ
|
interferon-γ
|
|
NLRC
|
nucleotide-binding domain and
leucine-rich repeat containing family CARD domain-containing
protein 4
|
|
SPEM
|
spasmolytic polypeptide-expressing
metaplasia
|
|
GC
|
gastric cancer
|
|
MALT lymphoma
|
mucosa-associated lymphoid tissue
lymphoma
|
Acknowledgements
The authors are grateful to Ms. Guorong Wen,
Professor Hai Jin and Professor Jiaxing An (Department of
Gastroenterology, Digestive Disease Hospital, Affiliated Hospital
of Zunyi Medical University, Zunyi, China), who provided
suggestions for the article.
Funding
The present review was funded by the National Natural Science
Foundation of China (National Science Foundation of China) (grant
nos. 82070536, 82073087, 82470540, 32460215 and 82160505), the
Guizhou Province International Science and Technology Cooperation
(Gastroenterology) Base [grant no. Qian Ke He Platform Talents-HZJD
(2021) 001], the Major Project of Guizhou Province Basic Research
Program [grant no. Qian Ke He basic research-ZK (2023) major
project 059], the Guizhou Province Science Plan Program [grant no.
Qian Ke He Foundation-ZK (2021) General 461], the Guizhou Province
High-level Innovative Talent Selection and Training Plan (100
level) [grant no. Qian Ke He Platform Talents-GCC (2023) 043] and
the Guizhou Innovative Talent Team on Ion Channels and Malignant
Tumors of Epithelial Origin [grant no. Qian Ke He Platform
Talents-CXTD (2023) 001].
References
|
1
|
Liu S, Deng Z, Zhu J, Ma Z, Tuo B, Li T
and Liu X: Gastric immune homeostasis imbalance: An important
factor in the development of gastric mucosal diseases. Biomed
Pharmacother. 161:1143382023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mills JC and Shivdasani RA: Gastric
epithelial stem cells. Gastroenterology. 140:412–424. 2011.
View Article : Google Scholar
|
|
3
|
Goldenring JR and Nam KT: Oxyntic atrophy,
metaplasia, and gastric cancer. Prog Mol Biol Transl Sci.
96:117–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldenring JR and Mills JC: Cellular
plasticity, reprogramming, and regeneration: metaplasia in the
stomach and beyond. Gastroenterology. 162:415–430. 2022. View Article : Google Scholar
|
|
5
|
Zhou CB and Fang JY: The role of
pyroptosis in gastrointestinal cancer and immune responses to
intestinal microbial infection. Biochim Biophys Acta Rev Cancer.
1872:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang W, Niu L, Zhao X, Duan L, Wang X, Li
Y, Chen J, Zhou W, Zhang Y, Fan D and Hong L: Pyroptosis impacts
the prognosis and treatment response in gastric cancer via immune
system modulation. Am J Cancer Res. 12:1511–1534. 2022.PubMed/NCBI
|
|
7
|
Villarroel-Espindola F, Ejsmentewicz T,
Gonzalez-Stegmaier R, Jorquera RA and Salinas E: Intersections
between innate immune response and gastric cancer development.
World J Gastroenterol. 29:2222–2240. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gobert AP and Wilson KT: Induction and
regulation of the innate immune response in Helicobacter pylori
infection. Cell Mol Gastroenterol Hepatol. 13:1347–1363. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiao Y, Yan Z and Yang A: The roles of
innate lymphoid cells in the gastric mucosal immunology and
oncogenesis of gastric cancer. Int J Mol Sci. 24:66522023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ianiro G, Molina-Infante J and Gasbarrini
A: Gastric microbiota. Helicobacter. 20(Suppl 1): S68–S71. 2015.
View Article : Google Scholar
|
|
11
|
Goldenring JR: Pyloric metaplasia,
pseudopyloric metaplasia, ulcer-associated cell lineage and
spasmolytic polypeptide-expressing metaplasia: Reparative lineages
in the gastrointestinal mucosa. J Pathol. 245:132–137. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barnett KC, Li S, Liang K and Ting JPY: A
360° view of the inflammasome: Mechanisms of activation, cell
death, and diseases. Cell. 186:2288–2312. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Guo J and Bi L: Role of the NLRP3
inflammasome in autoimmune diseases. Biomed Pharmacother.
130:1105422020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mridha AR, Wree A, Robertson AAB, Yeh MM,
Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou
GN, et al: NLRP3 inflammasome blockade reduces liver inflammation
and fibrosis in experimental NASH in mice. J Hepatol. 66:1037–1046.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kayagaki N, Kornfeld OS, Lee BL, Stowe IB,
O'Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, et al: NINJ1
mediates plasma membrane rupture during lytic cell death. Nature.
591:131–136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu J and Wu H: Structural mechanisms of
NLRP3 inflammasome assembly and activation. Annu Rev Immunol.
41:301–316. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sekiyama A, Ueda H, Kashiwamura SI,
Sekiyama R, Takeda M, Rokutan K and Okamura H: A stress-induced,
superoxide-mediated caspase-1 activation pathway causes plasma
IL-18 upregulation. Immunity. 22:669–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tran LS, Ying L, D'Costa K, Wray-McCann G,
Kerr G, Le L, Allison CC, Ferrand J, Chaudhry H, Emery J, et al:
NOD1 mediates interleukin-18 processing in epithelial cells
responding to Helicobacter pylori infection in mice. Nat Commun.
14:38042023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li G, Zhu L, Cao Z, Wang J, Zhou F, Wang
X, Li X and Nie G: A new participant in the pathogenesis of
alcoholic gastritis: Pyroptosis. Cell Physiol Biochem. 49:406–418.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng X, Yang M, Ye T, Feng J, Xu X, Yang
H, Wang X, Bao L, Li R, Xue B, et al: Mitochondrial GRIM-19 loss in
parietal cells promotes spasmolytic polypeptide-expressing
metaplasia through NLR family pyrin domain-containing 3
(NLRP3)-mediated IL-33 activation via a reactive oxygen species
(ROS)-NRF2-Heme oxygenase-1(HO-1)-NF-кB axis. Free Radic Biol Med.
202:46–61. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wan C, Wang P, Xu Y, Zhu Y, Chen H, Cao X
and Gu Y: Mechanism and role of H. pylori CagA-induced NLRP3
inflammasome in gastric cancer immune cell infiltration. Sci Rep.
15:143352025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ketelut-Carneiro N and Fitzgerald KA:
Apoptosis, pyroptosis, and necroptosis-Oh My! The many ways a cell
can die. J Mol Biol. 434:1673782022. View Article : Google Scholar
|
|
24
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Opdenbosch N, Gurung P, Vande Walle L,
Fossoul A, Kanneganti TD and Lamkanfi M: Activation of the NLRP1b
inflammasome independently of ASC-mediated caspase-1
autoproteolysis and speck formation. Nat Commun. 5:32092014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Man SM, Hopkins LJ, Nugent E, Cox S, Glück
IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP and Bryant CE:
Inflammasome activation causes dual recruitment of NLRC4 and NLRP3
to the same macromolecular complex. Proc Natl Acad Sci USA.
111:7403–7408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berry R and Call ME: Modular activating
receptors in innate and adaptive immunity. Biochemistry.
56:1383–1402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Que X, Zheng S, Song Q, Pei H and Zhang P:
Fantastic voyage: The journey of NLRP3 inflammasome activation.
Genes Dis. 11:819–829. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rathinam VAK and Fitzgerald KA:
Inflammasome complexes: Emerging mechanisms and effector functions.
Cell. 165:792–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viganò E and Mortellaro A: Caspase-11: The
driving factor for noncanonical inflammasomes. Eur J Immunol.
43:2240–2245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pérez-Figueroa E, Torres J, Sánchez-Zauco
N, Contreras-Ramos A, Alvarez-Arellano L and Maldonado-Bernal C:
Activation of NLRP3 inflammasome in human neutrophils by
Helicobacter pylori infection. Innate Immun. 22:103–112. 2016.
View Article : Google Scholar
|
|
32
|
Kumar S and Dhiman M: Inflammasome
activation and regulation during Helicobacter pylori pathogenesis.
Microb Pathog. 125:468–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Liu Z, Wang C, Yang R, Rathkey JK,
Pinkard OW, Shi W, Chen Y, Dubyak GR, Abbott DW and Xiao TS:
Mechanism of gasdermin D recognition by inflammatory caspases and
their inhibition by a gasdermin D-derived peptide inhibitor. Proc
Natl Acad Sci USA. 115:6792–6797. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fink SL and Cookson BT: Pyroptosis and
host cell death responses during Salmonella infection. Cell
Microbiol. 9:2562–2570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Man SM: Inflammasomes in the
gastrointestinal tract: Infection, cancer and gut microbiota
homeostasis. Nat Rev Gastroenterol Hepatol. 15:721–737. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toldo S, Mezzaroma E, Buckley LF, Potere
N, Di Nisio M, Biondi-Zoccai G, Van Tassell BW and Abbate A:
Targeting the NLRP3 inflammasome in cardiovascular diseases.
Pharmacol Ther. 236:1080532022. View Article : Google Scholar :
|
|
37
|
Wen J, Xuan B, Liu Y, Wang L, He L, Meng
X, Zhou T and Wang Y: NLRP3 inflammasome-induced pyroptosis in
digestive system tumors. Front Immunol. 14:10746062023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li S, Liang X, Ma L, Shen L, Li T, Zheng
L, Sun A, Shang W, Chen C, Zhao W and Jia J: MiR-22 sustains NLRP3
expression and attenuates H. pylori-induced gastric carcinogenesis.
Oncogene. 37:884–896. 2018. View Article : Google Scholar
|
|
39
|
Zhao Y, Deng Z, Ma Z, Zhang M, Wang H, Tuo
B, Li T and Liu X: Expression alteration and dysfunction of ion
channels/transporters in the parietal cells induces gastric
diffused mucosal injury. Biomed Pharmacother. 148:1126602022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, Ma Z, Deng Z, Yi Z, Tuo B, Li T and
Liu X: Role of spasmolytic polypeptide-expressing metaplasia in
gastric mucosal diseases. Am J Cancer Res. 13:1667–1681.
2023.PubMed/NCBI
|
|
41
|
Meyer AR and Goldenring JR: Injury,
repair, inflammation and metaplasia in the stomach. J Physiol.
596:3861–3867. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brzozowski T, Zwirska-Korczala K, Konturek
PC, Konturek SJ, Sliwowski Z, Pawlik M, Kwiecien S, Drozdowicz D,
Mazurkiewicz-Janik M, Bielanski W and Pawlik WW: Role of circadian
rhythm and endogenous melatonin in pathogenesis of acute gastric
bleeding erosions induced by stress. J Physiol Pharmacol. 58(Suppl
6): S53–S64. 2007.
|
|
43
|
Nithiwathanapong C, Reungrongrat S and
Ukarapol N: Prevalence and risk factors of stress-induced
gastrointestinal bleeding in critically ill children. World J
Gastroenterol. 11:6839–6842. 2005. View Article : Google Scholar
|
|
44
|
Kromin AA and Zenina OI: Hypothalamic
control of the myoelectric activity of the gastric antrum in
rabbits during acute emotional stress. Eksp Klin Gastroenterol.
71-76:1652007.In Russian.
|
|
45
|
Bregonzio C, Armando I, Ando H, Jezova M,
Baiardi G and Saavedra JM: Anti-inflammatory effects of angiotensin
II AT1 receptor antagonism prevent stress-induced gastric injury.
Am J Physiol Gastrointest Liver Physiol. 285:G414–G423. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Olaleye SB, Adaramoye OA, Erigbali PP and
Adeniyi OS: Lead exposure increases oxidative stress in the gastric
mucosa of HCl/ethanol-exposed rats. World J Gastroenterol.
13:5121–5126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seino H, Ueda H, Kokai M, Tsuji NM,
Kashiwamura S, Morita Y and Okamura H: IL-18 mediates the formation
of stress-induced, histamine-dependent gastric lesions. Am J
Physiol Gastrointest Liver Physiol. 292:G262–G267. 2007. View Article : Google Scholar
|
|
48
|
Das D, Bandyopadhyay D, Bhattacharjee M
and Banerjee RK: Hydroxyl radical is the major causative factor in
stress-induced gastric ulceration. Free Radic Biol Med. 23:8–18.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Z, Xue H, Dong Y, Hu J, Jiang T, Shi
L and Du J: Inhibition of GKN2 attenuates acute gastric lesions
through the NLRP3 inflammasome. Adv Wound Care (New Rochelle).
9:219–232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang F, Wang L, Wang JJ, Luo PF, Wang XT
and Xia ZF: The caspase-1 inhibitor AC-YVAD-CMK attenuates acute
gastric injury in mice: Involvement of silencing NLRP3 inflammasome
activities. Sci Rep. 6:241662016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Higashimori A, Watanabe T, Nadatani Y,
Nakata A, Otani K, Hosomi S, Tanaka F, Kamata N, Taira K, Nagami Y,
et al: Role of nucleotide binding oligomerization domain-like
receptor protein 3 inflammasome in stress-induced gastric injury. J
Gastroenterol Hepatol. 36:740–750. 2021. View Article : Google Scholar
|
|
52
|
Bojanowicz K, Zubowski A, Durasiewicz Z
and Szeszenia N: Gastric or duodenal ulcer location and the more
important internal and environmental factors. Przegl Lek.
28:457–460. 1971.In Polish.
|
|
53
|
Yu Q, Shi H, Ding Z, Wang Z, Yao H and Lin
R: The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome
activation in Helicobacter pylori-associated gastritis by
regulating ROS and autophagy. Cell Commun Signal. 21:12023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zaslona Z, Flis E, Nulty C, Kearney J,
Fitzgerald R, Douglas AR, McNamara D, Smith S, O'Neill LAJ and
Creagh EM: Caspase-4: A therapeutic target for peptic ulcer
disease. Immunohorizons. 4:627–633. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yuan XY, Zhang Y, Zhao X, Chen A and Liu
P: IL-1β, an important cytokine affecting Helicobacter
pylori-mediated gastric carcinogenesis. Microb Pathog.
174:1059332023. View Article : Google Scholar
|
|
56
|
Alzokaky AA, Abdelkader EM, El-Dessouki
AM, Khaleel SA and Raslan NA: C-phycocyanin protects against
ethanol-induced gastric ulcers in rats: Role of HMGB1/NLRP3/NF-κB
pathway. Basic Clin Pharmacol Toxicol. 127:265–277. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang H, Wang H and Andersson U: Targeting
inflammation Driven by HMGB1. Front Immunol. 11:4842020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
El-Gendy ZA, Taher RF, Elgamal AM, Serag
A, Hassan A, Jaleel GAA, Farag MA and Elshamy AI: Metabolites
profiling and bioassays reveal bassia indica ethanol extract
protective effect against stomach ulcers development via
HMGB1/TLR-4/NF-κB pathway. Antioxidants (Basel). 12:12632023.
View Article : Google Scholar
|
|
60
|
Elbaz EM, Abdel Rahman AAS, El-Gazar AA
and Ali BM: Protective effect of dimethyl fumarate against
ethanol-provoked gastric ulcers in rats via regulation of
HMGB1/TLR4/NF-κB, and PPARγ/SIRT1/Nrf2 pathways: Involvement of
miR-34a-5p. Arch Biochem Biophys. 759:1101032024. View Article : Google Scholar
|
|
61
|
Xie J, Fan L, Xiong L, Chen P, Wang H,
Chen H, Zhao J, Xu Z, Geng L, Xu W and Gong S: Rabeprazole inhibits
inflammatory reaction by inhibition of cell pyroptosis in gastric
epithelial cells. BMC Pharmacol Toxicol. 22:442021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Selim HM, Negm WA, Hawwal MF, Hussein IA,
Elekhnawy E, Ulber R and Zayed A: Fucoidan mitigates gastric ulcer
injury through managing inflammation, oxidative stress, and
NLRP3-mediated pyroptosis. Int Immunopharmacol. 120:1103352023.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Y, Yuan Z, Chai J, Zhu D, Miao X,
Zhou J and Gu X: ALDH2 ameliorates ethanol-induced gastric ulcer
through suppressing NLPR3 inflammasome activation and ferroptosis.
Arch Biochem Biophys. 743:1096212023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Arab HH, Ashour AM, Gad AM, Mahmoud AM and
Kabel AM: Activation of AMPK/mTOR-driven autophagy and inhibition
of NLRP3 inflammasome by saxagliptin ameliorate ethanol-induced
gastric mucosal damage. Life Sci. 280:1197432021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Arab HH, Eid AH, El-Sheikh AAK, Arafa EA
and Ashour AM: Irbesartan reprofiling for the amelioration of
ethanol-induced gastric mucosal injury in rats: Role of
inflammation, apoptosis, and autophagy. Life Sci. 308:1209392022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Suleyman H, Albayrak A, Bilici M, Cadirci
E and Halici Z: Different mechanisms in formation and prevention of
indomethacin-induced gastric ulcers. Inflammation. 33:224–234.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mehrotra P, Maschalidi S, Boeckaerts L,
Maueröder C, Tixeira R, Pinney J, Burgoa Cardás J, Sukhov V, Incik
Y, Anderson CJ, et al: Oxylipins and metabolites from pyroptotic
cells act as promoters of tissue repair. Nature. 631:207–215. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Soykan İ, Er RE, Baykara Y and Kalkan C:
Unraveling the mysteries of autoimmune gastritis. Turk J
Gastroenterol. 36:135–144. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jove A, Lin C, Hwang JH, Balasubramanian
V, Fernandez-Becker NQ and Huang RJ: Serum gastrin levels are
associated with prevalent neuroendocrine tumors in autoimmune
metaplastic atrophic gastritis. Am J Gastroenterol. 120:1140–1143.
2025. View Article : Google Scholar
|
|
70
|
Huang J, Fang M, Wu C and Qiao Z:
Autoimmune atrophic gastritis complicated with oxyntic gland
adenoma and low-grade intraepithelial neoplasia. Asian J Surg. Sep
12–2024.Epub ahead of print.
|
|
71
|
Park JY, Lam-Himlin D and Vemulapalli R:
Review of autoimmune metaplastic atrophic gastritis. Gastrointest
Endosc. 77:284–292. 2013. View Article : Google Scholar
|
|
72
|
Neumann WL, Coss E, Rugge M and Genta RM:
Autoimmune atrophic gastritis-pathogenesis, pathology and
management. Nat Rev Gastroenterol Hepatol. 10:529–541. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
D'Elios MM, Bergman MP, Azzurri A, Amedei
A, Benagiano M, De Pont JJ, Cianchi F, Vandenbroucke-Grauls CM,
Romagnani S, Appelmelk BJ and Del Prete G: H(+),K(+)-atpase (proton
pump) is the target autoantigen of Th1-type cytotoxic T cells in
autoimmune gastritis. Gastroenterology. 120:377–386. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Toh BH, Sentry JW and Alderuccio F: The
causative H+/K+ ATPase antigen in the pathogenesis of autoimmune
gastritis. Immunol Today. 21:348–354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hu Z and Chai J: Structural mechanisms in
NLR inflammasome assembly and signaling. Curr Top Microbiol
Immunol. 397:23–42. 2016.PubMed/NCBI
|
|
76
|
van Driel IR, Baxter AG, Laurie KL, Zwar
TD, La Gruta NL, Judd LM, Scarff KL, Silveira PA and Gleeson PA:
Immunopathogenesis, loss of T cell tolerance and genetics of
autoimmune gastritis. Autoimmun Rev. 1:290–297. 2002. View Article : Google Scholar
|
|
77
|
Benítez J, Marra R, Reyes J and Calvete O:
A genetic origin for acid-base imbalance triggers the mitochondrial
damage that explains the autoimmune response and drives to gastric
neuroendocrine tumours. Gastric Cancer. 23:52–63. 2020. View Article : Google Scholar
|
|
78
|
Arbore G, West EE, Spolski R, Robertson
AAB, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O'Neill
LA, et al: T helper 1 immunity requires complement-driven NLRP3
inflammasome activity in CD4+ T cells. Science.
352:aad12102016. View Article : Google Scholar
|
|
79
|
Lee GR: The balance of Th17 versus Treg
cells in autoimmunity. Int J Mol Sci. 19:7302018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lei L, Sun J, Han J, Jiang X, Wang Z and
Chen L: Interleukin-17 induces pyroptosis in osteoblasts through
the NLRP3 inflammasome pathway in vitro. Int Immunopharmacol.
96:1077812021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Stummvoll GH, DiPaolo RJ, Huter EN,
Davidson TS, Glass D, Ward JM and Shevach EM: Th1, Th2, and Th17
effector T cell-induced autoimmune gastritis differs in
pathological pattern and in susceptibility to suppression by
regulatory T cells. J Immunol. 181:1908–1916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Feng WQ, Zhang YC, Xu ZQ, Yu SY, Huo JT,
Tuersun A, Zheng MH, Zhao JK, Zong YP and Lu AG: IL-17A-mediated
mitochondrial dysfunction induces pyroptosis in colorectal cancer
cells and promotes CD8+ T-cell tumour infiltration. J
Transl Med. 21:3352023. View Article : Google Scholar
|
|
83
|
Faure E, Mear JB, Faure K, Normand S,
Couturier-Maillard A, Grandjean T, Balloy V, Ryffel B, Dessein R,
Chignard M, et al: Pseudomonas aeruginosa type-3 secretion system
dampens host defense by exploiting the NLRC4-coupled inflammasome.
Am J Respir Crit Care Med. 189:799–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kitamura A, Sasaki Y, Abe T, Kano H and
Yasutomo K: An inherited mutation in NLRC4 causes autoinflammation
in human and mice. J Exp Med. 211:2385–2396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kay C, Wang R, Kirkby M and Man SM:
Molecular mechanisms activating the NAIP-NLRC4 inflammasome:
Implications in infectious disease, autoinflammation, and cancer.
Immunol Rev. 297:67–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Triantafilou K, Ward CJK, Czubala M,
Ferris RG, Koppe E, Haffner C, Piguet V, Patel VK, Amrine-Madsen H,
Modis LK, et al: Differential recognition of HIV-stimulated IL-1β
and IL-18 secretion through NLR and NAIP signalling in
monocyte-derived macrophages. PLoS Pathog. 17:e10094172021.
View Article : Google Scholar
|
|
87
|
Zhao Y and Shao F: The NAIP-NLRC4
inflammasome in innate immune detection of bacterial flagellin and
type III secretion apparatus. Immunol Rev. 265:85–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Romberg N, Al Moussawi K, Nelson-Williams
C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK,
Huttner AJ, et al: Mutation of NLRC4 causes a syndrome of
enterocolitis and autoinflammation. Nat Genet. 46:1135–1139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Willet SG, Thanintorn N, McNeill H, Huh
SH, Ornitz DM, Huh WJ, Hoft SG, DiPaolo RJ and Mills JC: SOX9
governs gastric mucous neck cell identity and is required for
injury-induced metaplasia. Cell Mol Gastroenterol Hepatol.
16:325–339. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Petersen CP, Weis VG, Nam KT, Sousa JF,
Fingleton B and Goldenring JR: Macrophages promote progression of
spasmolytic polypeptide-expressing metaplasia after acute loss of
parietal cells. Gastroenterology. 146:1727–1738.e8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen W, Chen S, Yan C, Zhang Y, Zhang R,
Chen M, Zhong S, Fan W, Zhu S, Zhang D, et al: Allergen
protease-activated stress granule assembly and gasdermin D
fragmentation control interleukin-33 secretion. Nat Immunol.
23:1021–1030. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kita H: Gasdermin D pores for IL-33
release. Nat Immunol. 23:989–991. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chauvin C, Retnakumar SV and Bayry J:
Gasdermin D as a cellular switch to orientate immune responses via
IL-33 or IL-1β. Cell Mol Immunol. 20:8–10. 2023. View Article : Google Scholar
|
|
94
|
Bernink JH, Germar K and Spits H: The role
of ILC2 in pathology of type 2 inflammatory diseases. Curr Opin
Immunol. 31:115–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Meyer AR, Engevik AC, Madorsky T, Belmont
E, Stier MT, Norlander AE, Pilkinton MA, McDonnell WJ, Weis JA,
Jang B, et al: Group 2 innate lymphoid cells coordinate damage
response in the stomach. Gastroenterology. 159:2077–2091.e8. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sheng M, Weng Y, Cao Y, Zhang C, Lin Y and
Yu W: Caspase 6/NR4A1/SOX9 signaling axis regulates hepatic
inflammation and pyroptosis in ischemia-stressed fatty liver. Cell
Death Discov. 9:1062023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Qiang R, Li Y, Dai X and Lv W: NLRP3
inflammasome in digestive diseases: From mechanism to therapy.
Front Immunol. 13:9781902022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shadab A, Mahjoor M, Abbasi-Kolli M,
Afkhami H, Moeinian P and Safdarian AR: Divergent functions of
NLRP3 inflammasomes in cancer: A review. Cell Commun Signal.
21:2322023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Si Y, Liu L and Fan Z: Mechanisms and
effects of NLRP3 in digestive cancers. Cell Death Discov.
10:102024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu W, Peng J, Xiao M, Cai Y, Peng B,
Zhang W, Li J, Kang F, Hong Q, Liang Q, et al: The implication of
pyroptosis in cancer immunology: Current advances and prospects.
Genes Dis. 10:2339–2350. 2023. View Article : Google Scholar :
|
|
101
|
Zhang X, Li C, Chen D, He X, Zhao Y, Bao
L, Wang Q, Zhou J and Xie Y: H. pylori CagA activates the NLRP3
inflammasome to promote gastric cancer cell migration and invasion.
Inflamm Res. 71:141–155. 2022. View Article : Google Scholar
|
|
102
|
Li YT, Tan XY, Ma LX, Li HH, Zhang SH,
Zeng CM, Huang LN, Xiong JX and Fu L: Targeting LGSN restores
sensitivity to chemotherapy in gastric cancer stem cells by
triggering pyroptosis. Cell Death Dis. 14:5452023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li C, Qiu J and Xue Y: Low-dose
Diosbulbin-B (DB) activates tumor-intrinsic PD-L1/NLRP3 signaling
pathway mediated pyroptotic cell death to increase
cisplatin-sensitivity in gastric cancer (GC). Cell Biosci.
11:382021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
West AJ, Deswaerte V, West AC, Gearing LJ,
Tan P and Jenkins BJ: Inflammasome-associated gastric tumorigenesis
is independent of the NLRP3 pattern recognition receptor. Front
Oncol. 12:8303502022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Raderer M, Kiesewetter B and Ferreri AJ:
Clinicopathologic characteristics and treatment of marginal zone
lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA
Cancer J Clin. 66:153–171. 2016.PubMed/NCBI
|
|
106
|
Kuo SH, Wu MS, Yeh KH, Lin CW, Hsu PN,
Chen LT and Cheng AL: Novel insights of lymphomagenesis of
Helicobacter pylori-dependent gastric mucosa-associated lymphoid
tissue lymphoma. Cancers (Basel). 11:5472019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kiesewetter B and Raderer M:
Immunomodulatory treatment for mucosa-associated lymphoid tissue
lymphoma (MALT lymphoma). Hematol Oncol. 38:417–424. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Della Bella C, Soluri MF, Puccio S,
Benagiano M, Grassi A, Bitetti J, Cianchi F, Sblattero D, Peano C
and D'Elios MM: The Helicobacter pylori CagY protein drives gastric
Th1 and Th17 inflammation and B cell proliferation in gastric MALT
lymphoma. Int J Mol Sci. 22:94592021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chonwerawong M, Ferrand J, Chaudhry HM,
Higgins C, Tran LS, Lim SS, Walker MM, Bhathal PS, Dev A, Moore GT,
et al: Innate immune molecule NLRC5 protects mice from
helicobacter-induced formation of gastric lymphoid tissue.
Gastroenterology. 159:169–182.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Deng Y, Fu Y, Sheng L, Hu Y, Su L, Luo J,
Yan C and Chi W: The regulatory NOD-like receptor NLRC5 promotes
ganglion cell death in ischemic retinopathy by inducing microglial
pyroptosis. Front Cell Dev Biol. 9:6696962021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ying L, Liu P, Ding Z, Wray-McCann G,
Emery J, Colon N, Le LH, Tran LS, Xu P, Yu L, et al: Anti-CD40L
therapy prevents the formation of precursor lesions to gastric
B-cell MALT lymphoma in a mouse model. J Pathol. 259:402–414. 2023.
View Article : Google Scholar : PubMed/NCBI
|