Introduction
Functional constipation (FC) is a common,
multifactorial, nonorganic disease characterized by infrequent,
difficult, or incomplete bowel movements (1). FC considerably impairs the quality
of life of patients and imposes a substantial burden on global
healthcare systems (2,3). Epidemiological studies have
estimated that the global prevalence of FC ranges from 14-20%
(4,5). Despite its widespread occurrence,
the underlying pathophysiological mechanisms of FC remain
incompletely understood, which further complicates its effective
clinical management and development of appropriate treatment
strategies.
Recent advances in gastrointestinal (GI) research
have identified the crucial role of enterochromaffin (EC) cells in
the pathophysiology of FC (6).
EC cells respond to various environmental and endogenous stimuli,
including microbial metabolites, inflammatory factors, mechanical
distension and stress-related hormones, by releasing serotonin
[5-hydroxytryptamine (5-HT)] (7-10). Of 5-HT in the body, >90% is
synthesized from tryptophan through the catalytic activity of the
enzyme tryptophan hydroxylase-1 (TPH-1) in EC cells (11,12). Once synthesized, 5-HT is released
into the extracellular space, where it binds to various serotonin
receptors to regulate intestinal functions, including motility,
secretion and sensory perception (13-15). The activity of 5-HT is terminated
predominantly through its reuptake by serotonin transporter (SERT)
(16). Aberrant 5-HT release
from EC cells is associated with dysregulated intestinal motility
and abnormal gut sensation, which are the key contributors to the
pathophysiology of FC (17,18). Notably, therapeutic agents
targeting 5-HT receptors have shown substantial efficacy in
alleviating constipation-related symptoms (19).
Piezo ion channels, including Piezo1 and Piezo2, are
critical mechanosensors in various tissues and play a crucial role
in the functional regulation of mechanosensitive cells such as EC
cells (20,21). Following various mechanical
stimuli, Piezo ion channels open and convert mechanical forces into
cellular signals through calcium influx (22). This process regulates various
cellular activities, including cell proliferation, differentiation,
secretion, metabolism and signaling (23-25). Although Piezo1 and Piezo2 belong
to the same Piezo family, they exhibit distinct distribution
patterns in the digestive system, respond differently to mechanical
signals and regulate target organs uniquely (26,27). These differences suggest that
Piezo1 and Piezo2 may have complementary roles in regulating GI
function.
Current research has revealed that both Piezo1 and
Piezo2 individually promote the release of 5-HT from EC cells, thus
highlighting their key roles in GI function (21,28). However, it remains unclear
whether Piezo1 or Piezo2 independently promote the
pathophysiological changes associated with FC through this
mechanism. Moreover, the specific contributions of Piezo1 and
Piezo2 to 5-HT release and whether they interact cooperatively or
independently in EC cells remain to be elucidated. Hence, the
present study aimed to investigate the distinct and combined
effects of Piezo1 and Piezo2 on the regulation of 5-HT release from
EC cells and their involvement in the pathophysiology of FC.
Materials and methods
Mice
Male C57BL/6 mice 6-8 weeks old and weighing 20±2 g
(SPF grade) were obtained from Chengdu Dashuo Experimental Animal
Co., Ltd. [cat. no. SCXK (Chuan) 2020-0030 and cat. no. SCXK
(Chuan) 2024-0031]. A total of 54 mice were used in the present
study. Animals were acclimatized in an environment-controlled room
with a temperature of 20±2°C, humidity of 50±5% and a 12-h
light/dark cycle. Food and water were provided ad libitum
throughout the experiment. All animal experiments were conducted in
accordance with ethical guidelines and were approved by the Animal
Experiment Ethics Committee of Chengdu University of Traditional
Chinese Medicine (approval no. 2024012).
Adeno-associated virus construction and
transduction
Technical support for the adeno-associated virus
(AAV) vector construction, packaging and purification was provided
by Shandong Weizhen Biosciences Co., Ltd.
AAV vectors were constructed using the pAV-U6-shRNA
plasmids, designed to specifically target the Piezo1 and Piezo2
genes. The plasmids were constructed with three different short
hairpin (sh)RNA sequences (Table
SI) targeting Piezo1 or Piezo2, each inserted into the vector
under the control of the U6 promoter. The plasmids were then
amplified in bacterial cultures with appropriate antibiotic
selection (Amp; 100 μg/ml) and extracted using standard
plasmid purification techniques. Following plasmid extraction, the
AAV vectors were packaged in 293 cells (Shandong Weizhen
Biosciences Co., Ltd.) using a third-generation packaging system
with a triple plasmid transfection system, according to the
manufacturer's instructions. For each transfection, 10 μg of
transfer plasmid, 6 μg of packaging plasmid, and 3 μg
of envelope plasmid were used, at a ratio of 10:6:3. Viral
particles were harvested at 72 h post-transfection. The packaged
AAV particles were subsequently purified and titers were determined
using reverse transcription-quantitative (RT-q) PCR.
Two weeks prior to model induction, AAV transduction
was performed on colonic epithelial cells via enema, following the
protocol described below. Mice were fasted for 24 h before the
procedure and anesthetized using isoflurane (2% for induction and
1-1.5% for maintenance). A stainless steel round-tip needle was
gently inserted approximately 2.5 cm into the colon via the anus.
Subsequently, 300 μl of 20 mM N-acetylcysteine solution was
slowly infused into the colon to cleanse the area, with the
solution retained for 30 min. After re-anesthesia, 600 μl of
AAV (total dose: 5×1010 vg per mouse) was administered
into the colon via the same route. To facilitate uniform
distribution of the viral vector along the colonic mucosa, several
standardized procedures were employed: i) deep catheter insertion,
ii) a relatively large infusion volume (600 μl), iii)
temporary anal closure and iv) inversion of the mice into a
vertical position for at least one min. After full recovery from
anesthesia, mice were returned to their cages with free access to
food and water. The time interval between AAV transduction and
subsequent experimentation (model induction) was 2 weeks.
Animal model
A loperamide-induced mouse model was used to
establish functional constipation, given its well-established
reliability and reproducibility in gastrointestinal research. This
model closely mimics the core pathophysiological features of human
slow-transit constipation, including delayed colonic transit,
decreased fecal water content and altered serotonergic signaling.
Its technical simplicity and consistency also make it suitable for
evaluating gut motility and related molecular mechanisms in
experimental settings (29).
Male mice in the FC group were administered
loperamide (10 mg/kg) twice daily by gavage for 14 days, while
control mice received an equivalent volume of physiological saline
by gavage. After one week of adaptive feeding, 54 mice were divided
randomly into 6 groups: Control group, FC group, Control AAV group,
AAV Piezo1-knockdown (KD) group, AAV Piezo2-KD group and AAV
Piezo1/2-KD group. At the end of the modeling period, the
defecation status of the mice was recorded over a 6-h period
according to previously published methods (30), including the number of fecal
pellets, the Bristol stool form scale score (BSFS) (31) and the fecal moisture content.
Fecal pellets were collected immediately after
expulsion and placed in sealed 2-ml tubes to avoid evaporation. The
fecal pellets were weighed (wet weight, in mg), dried at 60°C
overnight, and weighed again (dry weight, in mg). Fecal water
content was calculated using the equation: water content (%)=100×
(wet weight-dry weight)/wet weight. Additionally, the total fecal
output, BSFS and wet weight were assessed every 2 h.
At the end of the experiments, all mice were
sacrificed by cervical dislocation under deep isoflurane
anesthesia, in accordance with institutional ethical
guidelines.
Assessment of functional GI motility
To measure total gastrointestinal transit time
(TGITT), overnight-fasted mice were orally gavaged with 0.2 ml of
Evans blue solution (Beijing Solarbio Science & Technology Co.,
Ltd.). TGITT was assessed as the time from gavage until the
appearance of the first blue fecal pellet. Colonic transit time was
measured by recording the time from gavage to the expulsion of the
first blue fecal pellet.
To assess the small intestinal propulsive rate and
gastric emptying rate, mice were sacrificed 20 min after oral
gavage with the Evans blue solution. The gastric emptying rate was
calculated by the total stomach weight and the net stomach weight,
while the intestinal propulsion rate was calculated based on the
distance the blue solution travels in the small intestine. The
abdominal cavity was opened using sterile forceps and surgical
scissors, and the stomach was quickly removed after ligating the
gastric cardia and pylorus. The stomach was placed on filter paper,
opened along the greater curvature, and rinsed with
phosphate-buffered saline (PBS) at pH 7.4 (HyClone; Cytiva). Excess
tissue fluids and PBS were absorbed with filter paper. The net
weight of the stomachs was recorded after washing away the
contents. Simultaneously, the small intestine was quickly removed
and spread gently onto a piece of white paper to measure its total
length and the distance traveled by the Evans blue solution. The
small intestinal propulsive rate and gastric emptying rates were
calculated using the following formulas:
Small intestinal propulsive rate (%)=(distance
traveled by Evans blue/total length of the small intestine) ×100.
Gastric emptying rate (%)=[1‑(total stomach weight‑net stomach
weight)/total stomach weight] ×100.
Colonic sensitivity assessment
Behavioral responses to Colorectal Distension (CRD)
were assessed by measuring the Abdominal Withdrawal Reflex (AWR)
using a semi-quantitative score in conscious animals. Mice were
initially anesthetized with isoflurane and a lubricated latex
balloon attached to polyethylene tubing, connected to a 1 ml
syringe, was inserted into the rectum and descending colon through
the anus. The tubing was secured to the tail to keep the balloon in
place. After allowing the mice to recover from anesthesia for 30
min, AWR measurements were performed. Observers, blinded to the
experimental conditions, visually assessed the mice's responses to
graded CRD volumes (0.25, 0.35, 0.5 and 0.65 ml) and assigned AWR
scores based on the following criteria: 0, no behavioral response;
1, immobility during CRD with occasional head clinching at stimulus
onset; 2, mild contraction of the abdominal muscles without
abdominal lifting; 3, strong contraction of the abdominal muscles
and lifting of the abdomen off the platform; 4, arching of the body
with lifting of the pelvic structures and scrotum.
Cell culture
QGP-1 cells were purchased from Procell Life Science
& Technology Co., Ltd. (cat. no. CL-0977). The cells were
divided into the following five groups: Negative control (NC),
vector control (VC), LV-Piezo1 KD (Piezo1 knockdown), LV-Piezo2 KD
(Piezo2 knockdown) and LV-Piezo1/2 KD (combined Piezo1 and Piezo2
knockdown) groups. Cells were cultured in OPTI-MEM medium (Gibco;
Thermo Fisher Scientific, Inc.; cat. no. 51985-034) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.; cat. no. 16000-044) and penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.; cat. no. P1400-100).
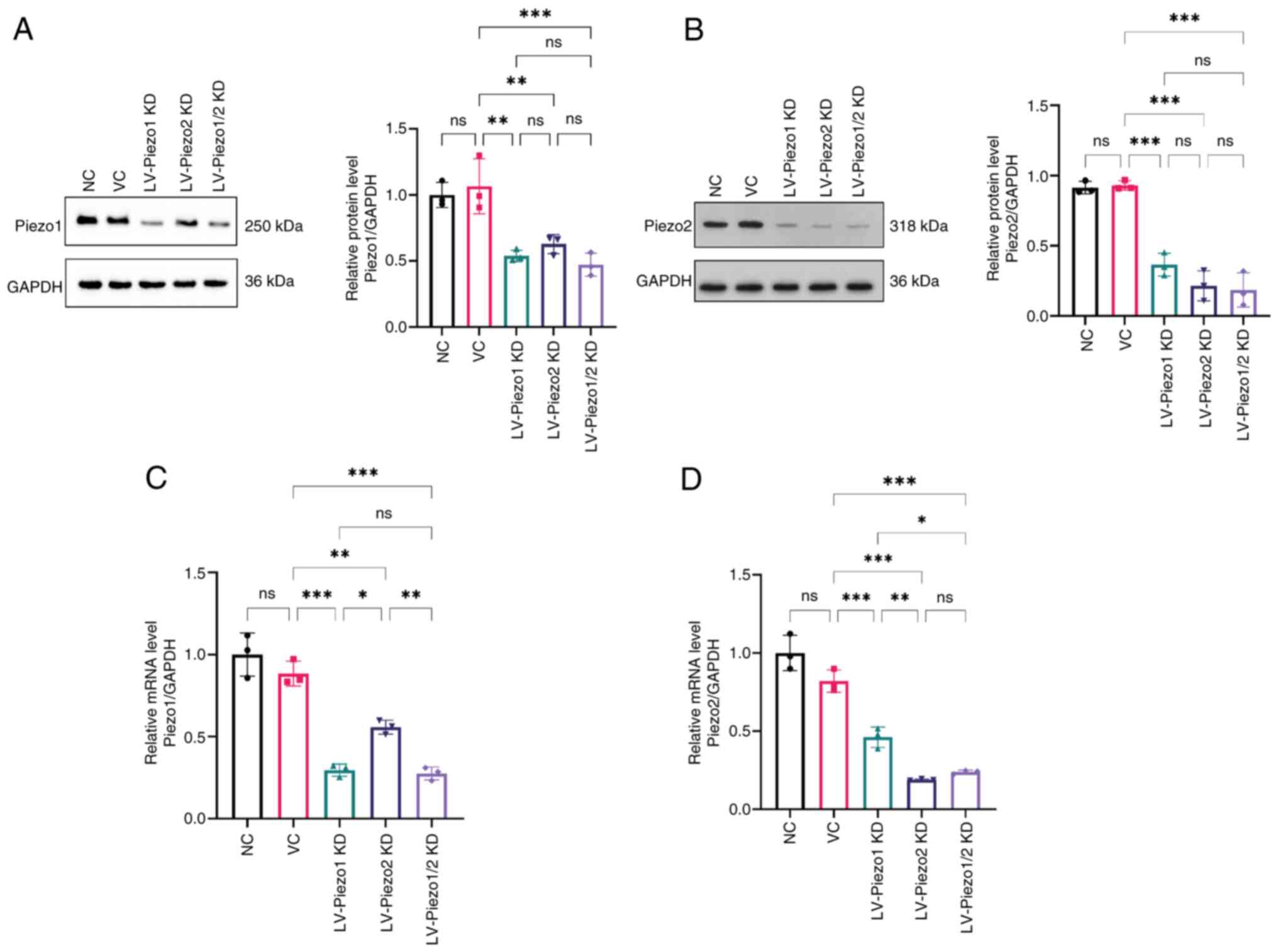
Lentivirus construction and cell
infection
RNA interference (RNAi) construction and lentivirus
particle collection were conducted according to previously
described methods (32).
Lentiviral vectors were packaged in 293T cells (Shanghai GeneChem
Co., Ltd., China), using a third-generation packaging system. For
each transfection, 20 μg of transfer plasmid, 15 μg
of packaging plasmid, and 10 μg of envelope plasmid were
used, at a ratio of 4:3:2. Viral particles were harvested at 72 h
post-transfection. QGP-1 cells were infected with lentivirus
harboring RNAi targeting Piezo1 (LV-RNAi-Piezo1), Piezo2
(LV-RNAi-Piezo2) and a control (LV-RNAi-NC) at a multiplicity of
infection of 10 for 72 h. The efficiency of interference was
examined by RT-qPCR and western blotting. Cells were then used for
subsequent experiments 72 h post-infection (transient transduction,
no antibiotic selection), including functional assays and imaging
studies. The target sequences used to design the RNAi constructs
are listed in Table SI.
Histological examination of colonic
tissue
Distal colon tissues were fixed in 4%
paraformaldehyde at 4°C for 24 h, processed in an automatic tissue
processor and embedded in paraffin. Sections of 4 μm
thickness were cut using a rotary microtome. Deparaffinization was
performed by immersing the sections in xylene (2×30 min), followed
by rehydration in 100% ethanol (2×5 min), 95% ethanol (5 min), 85%
ethanol (5 min) and 75% ethanol (5 min). The sections were rinsed
for 5 min, stained with hematoxylin at 22-25°C for 5-10 min,
followed by differentiation in 1% hydrochloric acid alcohol for 3
sec and bluing in alkaline water. Eosin staining was performed at
22-25°C for 3 min, followed by dehydration in graded ethanol,
clearing in xylene and mounting with neutral resin.
Immunofluorescence staining
Immunofluorescence staining was performed to detect
the co-localization and expression levels of Piezo1, Piezo2 and EC
cells in distal colon. Tissues were fixed in 4% paraformaldehyde at
4°C for 24 h, processed and embedded in paraffin. Sections of 3
μm thickness were washed with PBS (cat. no. ZLI-9062;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) three
times at 22-25°C for 5 min each and blocked with 3% bovine serum
albumin (cat. no. GC305010; Wuhan Servicebio Technology Co., Ltd.)
at 22-25°C for 30 min. Primary antibodies used were anti-Piezo1
(cat. no. DF12083; Affinity Biosciences, Ltd.; 1:100), anti-Piezo2
(cat. no. NBP1-78624SS; Novus Biologicals; 1:200),
anti-Chromogranin A (cat. no. 10529-1-AP; Proteintech Group, Inc.;
1:200) and anti-5-HT (cat. no. 20080; ImmunoStar Inc.; 1:100), the
latter two of which were used to identify EC cells. Sections were
incubated with primary antibodies overnight at 4°C, washed with
PBS, and then incubated with HRP-conjugated goat anti-rabbit
secondary antibodies (cat. no. GB23303; Wuhan Servicebio Technology
Co., Ltd.; 1:100) or CY5-conjugated goat anti-rabbit secondary
antibodies (cat. no. GB27303; Wuhan Servicebio Technology Co.,
Ltd.; 1:100) for 50 min at 22-25°C. TSA reagents CY3-Tyramide (cat.
no. G1223; Wuhan Servicebio Technology Co., Ltd.; 1:500) and
FITC-Tyramide (cat. no. G1222; Wuhan Servicebio Technology Co.,
Ltd.; 1:500) were used for signal amplification for 10 min at
22-25°C in the dark followed by PBS washes. DAPI (cat. no. G1012;
Wuhan Servicebio Technology Co., Ltd.) was used for nuclear
staining for 10 min at 22-25°C in the dark followed by PBS washes.
DAPI and fluorescent signals were observed under a fluorescence
microscope at ×200 and ×400 magnifications.
Negative controls were included by omitting the
primary antibodies and replacing them with equal volumes of PBS.
These control sections were processed in parallel with experimental
samples, including incubation with the same secondary antibodies
and DAPI. No fluorescent signals were observed under these
conditions, confirming the specificity of the staining.
The analyses were carried out under an Olympus VS200
digital slide scanning system (Olympus Corporation) and images were
analyzed with ImageJ software (version 1.54f; National Institutes
of Health) to assess the co-localization and quantify the positive
expression of Piezo1, Piezo2 and EC (ChgA+) cells.
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde at 4°C for
24 h, processed and embedded in paraffin. Antigen retrieval was
performed by heating sections in citrate buffer (pH 6.0, cat. no.
GA2307051; Wuhan Servicebio Technology Co., Ltd.) using a microwave
for 20 min. After cooling to 22-25°C, distal colon sections were
washed three times with PBS (cat. no. G0002-2L; Wuhan Servicebio
Technology Co., Ltd.) for 5 min each. Endogenous peroxidase
activity was quenched by incubation with 3% hydrogen peroxide (cat.
no. G1204; Wuhan Servicebio Technology Co., Ltd.) at 22-25°C in the
dark for 25 min, followed by three PBS washes. Nonspecific binding
was blocked with 3% bovine serum albumin (cat. no. GC305010; Wuhan
Servicebio Technology Co., Ltd.) for 20 min at 22-25°C. Sections
were incubated overnight at 4°C in a humidified chamber with the
following primary antibodies: Anti-5-HT3 (cat. no.
120185; Zen Bioscience; 1:100) and anti-SERT (cat. no. 19559-1-AP;
Proteintech Group; 1:400) diluted in PBS. After PBS washes,
sections were incubated with HRP-conjugated goat anti-rabbit
secondary antibody (cat. no. GB23303; Wuhan Servicebio Technology
Co., Ltd., 1:100) for 30 min at 37°C. Immunoreactivity was
visualized using 3,3′-diaminobenzidine (DAB) substrate (cat. no.
ZLI-9018; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.),
with color development monitored under a light microscope and
stopped by rinsing in distilled water. Sections were counterstained
with hematoxylin (cat. no. LM10N13; J&K Scientific) for 3 min
at 22-25°C, blued in running water, dehydrated, cleared in xylene
and mounted with neutral resin. IHC images for 5-HT3 and
SERT were acquired at ×400 magnification using a digital trinocular
microscope imaging system (cat. no. BA400Digital; Motic China Group
Co., Ltd.), including both the mucosal and muscular layers of the
distal colon. For each mouse, three non-overlapping fields were
selected and analyzed using ImageJ software (version 1.54f;
National Institutes of Health). Positive immunoreactivity was
quantified as the percentage of DAB-stained area relative to the
total tissue area (positive area fraction). Color deconvolution and
thresholding were standardized across all images to ensure
consistent measurement.
Co-immunoprecipitation (Co-IP)
Colonic tissues were lysed in IP lysis buffer (cat.
no. G2038; Wuhan Servicebio Technology Co., Ltd.) supplemented with
PMSF (cat. no. G2008; Wuhan Servicebio Technology Co., Ltd.) and
phosphatase inhibitor cocktail (cat. no. G2007; Wuhan Servicebio
Technology Co., Ltd.). Lysates were centrifuged at 14,000 × g for
10 min at 4°C and supernatants were collected. Protein
concentrations were determined using a BCA Protein Assay kit (cat.
no. BL521A; Biosharp Life Sciences). For each IP reaction, 400
μl of lysate was used. Protein A magnetic beads (cat. no.
HY-K0203, MedChemExpress) were pre-washed three times with
binding/wash buffer provided in the kit and incubated with 10
μg of anti-Piezo1 antibody (rabbit polyclonal; cat. no.
15939-1-AP; Proteintech Group, Inc.) at 4°C for 2 h to form
antibody-bead complexes. Then, 400 μl of lysate was added to
the complexes and incubated for an additional 2 h at 4°C with
rotation. After four washes with binding/wash buffer, beads were
collected magnetically at each wash step. Bound proteins were
eluted by boiling the beads in SDS-PAGE loading buffer (cat. no.
BL502B; Biosharp Life Sciences) at 95°C for 5 min. Eluted proteins
were resolved on 7% SDS-PAGE gels, transferred to PVDF membranes
(cat. no. IPVH00010; MilliporeSigma) and probed with anti-Piezo2
monoclonal antibody (mouse; cat. no. HA723342; HuaAn Biotechnology)
at 1:1,000. After washing, membranes were incubated with
HRP-conjugated anti-mouse secondary antibody (cat. no. GB23301;
Wuhan Servicebio Technology Co., Ltd.) at 1:10,000 for 1 h at
22-25°C. Signal was detected using ECL reagents (cat. no. BL520B;
Biosharp Life Sciences) and imaged using a ChemiScope 6100 system
(Clinx). No epitope tag was used.
Western blotting
To measure the expression levels of Piezo1 and
Piezo2 proteins in distal colon, proteins were extracted using RIPA
lysis buffer (cat. no. P0013; Beyotime Institute of Biotechnology),
supplemented with protease and phosphatase inhibitor cocktails,
fractionated by SDS-PAGE (10% gels; 30 μg total protein per
lane) and transferred onto a PVDF membrane. The membrane was
blocked with 5% skimmed milk for 30 min at room temperature.
Primary antibodies were applied overnight at 4°C: anti-Piezo1 (cat.
no. DF12083; Affinity Biosciences; 1:1,000), anti-Piezo2 (cat. no.
NBP1-78624, Novusbio, 1:1,000) and anti-β-actin (cat. no. AC026;
ABclonal Biotech Co., Ltd.; 1:50,000)/anti-GAPDH (cat. no. ab9485;
Abcam; 1:2,500). The membrane was then washed three times with TBST
(0.1% Tween-20) and incubated with HRP-conjugated secondary
antibodies (cat. no. S0001; Affinity Biosciences; 1:5,000) for 2 h
at 22-25°C. After washing three times with TBST (0.1% Tween-20),
immunoblots were developed using enhanced chemiluminescence
substrate (cat. no. 17046; Chengdu Zen-Bioscience Co., Ltd.) and
imaged with a UVP imaging system (Ultra-Violet Products Ltd.).
Densitometric analysis of protein bands was performed using Gel-Pro
Analyzer (version 4; Media Cybernetics, Inc.).
Detection of ERK and protein kinase C
(PKC) pathway proteins
Mouse colonic tissues were rinsed with ice-cold PBS
to remove residual blood and then homogenized in RIPA lysis buffer
(cat. no. P0013; Beyotime Institute of Biotechnology) supplemented
with 1Xprotease inhibitor cocktail (cat. no. BL612A; Biosharp Life
Sciences) and 1 mM phenylmethanesulfonyl fluoride (PMSF; cat. no.
BL507A; Biosharp Life Sciences). Homogenization was performed using
a tissue grinder (Model KZ-III-Fl; Wuhan Servicebio Technology Co.,
Ltd.) for 2-5 min until tissues were completely homogenized. Tissue
lysates were incubated on ice for 30 min, vortexing every 10 min,
followed by centrifugation at 15,805 × g for 10 min at 4°C. Protein
concentrations (30 μg per lane) were determined using a BCA
protein assay kit (cat. no. BL521C; Biosharp Life Sciences).
Protein samples were mixed with loading buffer (cat. no. BL502A;
Biosharp Life Sciences), denatured at 95°C for 10 min, and stored
at −80°C until use. Protein separation was performed by SDS-PAGE
(10% gels) at a constant voltage of 100 V. The proteins were then
transferred onto PVDF membranes (Immobilon-PSQ; MilliporeSigma) at
200 mA for 1-2 h. Membranes were blocked with 5% non-fat milk
diluted in TBST buffer (0.1% Tween-20) for 2 h at 22-25°C and then
incubated overnight at 4°C with primary antibodies: anti-ERK1/2
(cat. no. A4782; ABclonal Biotech Co., Ltd.; 1:2,000),
anti-phosphorylated (p-)ERK1/2 (cat. no. 28733-1-AP; Proteintech
Group, Inc.; 1:2,000), anti-PKC (cat. no. ET1608-15; HuaAn
Biotechnology; 1:5,000), and anti-p-PKC (cat. no. ET1702-17; HuaAn
Biotechnology; 1:2,000). After washing with TBST buffer (0.1%
Tween-20) three times, membranes were incubated with HRP-conjugated
secondary antibody (cat. no. AS014; ABclonal Biotech Co., Ltd.;
1:8,000) for 2 h at 22-25°C. Protein bands were visualized using
enhanced chemiluminescence substrate (cat. no. BL520B; Biosharp
Life Sciences) and imaged using the Tanon 5200 Multi
Chemiluminescent Imaging System (Tanon Science and Technology Co.,
Ltd.). Densitometric analysis of protein bands was performed using
Gel-Pro Analyzer (version 4; Media Cybernetics, Inc.).
Due to antibody compatibility and differences in
molecular weight, target proteins and internal controls were
detected on separate membranes. However, all blots were prepared
from the same set of samples and processed in parallel under
identical electrophoresis, transfer and exposure conditions.
RNA isolation, cDNA synthesis and
RT-qPCR
Total RNA was extracted from distal colon tissue
using the Molpure Cell/Tissue Total RNA kit (Shanghai Yeasen
Biotechnology Co., Ltd.). Genomic DNA was removed with a gDNA
Eraser kit. cDNA was synthesized from RNA using the PrimeScript RT
reagent kit (Takara Bio, Inc.). RT-qPCR was performed with TB Green
Premix Ex Taq II (Takara Bio, Inc.) on a QuantStudio 3 Real-Time
PCR System (Thermo Fisher Scientific, Inc.). Thermocycling
conditions were as follows: an initial denaturation at 95°C for 30
sec, followed by 45 cycles of denaturation at 95°C for 5 sec,
annealing at 55°C for 30 sec and extension at 72°C for 30 sec,
during which fluorescence signals were acquired. All primer
sequences used in the present study are listed in Table SII. Relative mRNA expression
levels of Piezo1, Piezo2 and SERT were calculated using the
2-ΔΔCq method (33),
with β-actin as the internal control. Data analysis was performed
using QuantStudio Design & Analysis Software (version 1.6;
Thermo Fisher Scientific, Inc.). All RNA extraction, cDNA
synthesis, and RT-qPCR procedures were carried out according to the
manufacturers' protocols.
Calcium ion imaging
The intracellular Ca2+ concentration was
measured using the Ca2+ fluorescent probe Fluo-4 AM
(cat. no. S1061S; Beyotime Institute of Biotechnology). Cells were
incubated with 2.5 μmol/l Fluo-4 AM diluted in HBSS solution
for 40 min in an incubator. After incubation, cells were washed
three times with HBSS solution. HBSS solution was added to cover
the cells and they were incubated at 37°C for 25 min. The HBSS
solution was then discarded. Cells were excited at 488 nm using an
inverted fluorescence microscope. Images were analyzed using ImageJ
software (version 1.54f; National Institutes of Health).
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of 5-HT in the serum and colon,
vasoactive intestinal peptide (VIP) and substance P (SP) levels in
the serum were examined using ELISA kits (cat. nos. ZC-37715,
ZC-38836 and ZC-37822; Shanghai Zhuocai Biotechnology Co., Ltd.)
according to the manufacturer's instructions. Colonic calcium ion
concentrations and tryptophan hydroxylase-1 (TPH-1) were determined
using a colorimetric calcium assay kit (cat. no. C004-2-1; Nanjing
Jiancheng Bioengineering Inc.; cat. no. ZC-57264; Shanghai Zhuocai
Biotechnology Co., Ltd.) following the manufacturer's protocol.
Statistical analysis
Statistical analyses were conducted using GraphPad
Prism version 9.0 (Dotmatics). Data normality was assessed using
the Shapiro-Wilk test and Levene's test was used to verify
homogeneity of variances. For group comparisons, unpaired Student's
t-tests were used for two-group comparisons and one-way ANOVA
followed by Tukey correction was applied for multiple group
comparisons. Non-parametric tests, including the Mann-Whitney U
test and Kruskal-Wallis test with Dunn's post hoc analysis, were
applied to analyze data not conforming to normal distribution.
Effect sizes and 95% confidence intervals were calculated where
applicable. P<0.05 was considered to indicate a statistically
significant difference.
Results
Attenuated co-localization and expression
of Piezo1 and Piezo2 in enterochromaffin cells of FC mice
To investigate the expression of Piezo1 and Piezo2
within EC cells, immunofluorescence staining was performed in
control and FC mouse distal colons. ChgA was primarily localized at
the crypt base, exhibiting a distribution pattern similar to that
of 5-HT. (Figs. 1A and S1A). In control tissues, both Piezo1
and Piezo2 were clearly expressed in the colonic epithelium and
showed substantial co-localization with ChgA+ cells
(Fig. 1A). In FC mice,
fluorescence signals for Piezo1, Piezo2 and ChgA were visibly
reduced and the overlap among these markers appeared diminished.
Quantification confirmed a significant decrease in the proportion
of Piezo1+ ChgA+ cells, Piezo2+
ChgA+ cells and triple-positive cells among total
ChgA+ cells in FC mice compared with controls (Fig. 1B-D; P<0.01, P<0.05 and
P<0.05, respectively), suggesting that Piezo expression in EC
cells is downregulated under constipated conditions. Western blot
and RT-qPCR analyses confirmed these findings, showing decreased
protein and mRNA levels for both Piezo1 and Piezo2 in the FC group
(Fig. 1E-G; P<0.05,
P<0.01). Collectively, these results indicated that Piezos
expression and Piezo1/2-EC, Piezo1-Piezo2 colocalization are
significantly attenuated in FC mice.
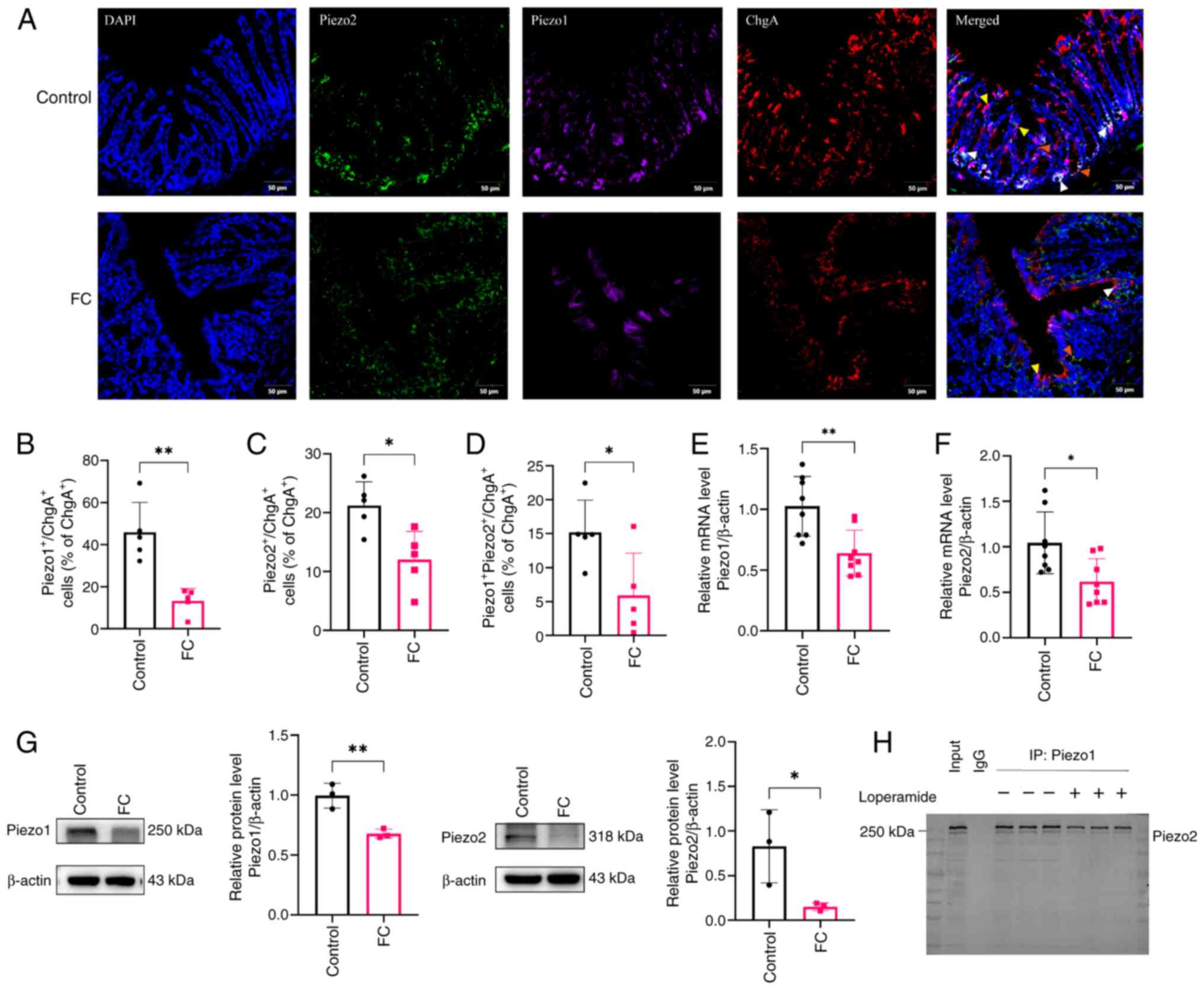 | Figure 1Colocalization and expression of
Piezo1 and Piezo2 in EC cells of FC Mice. (A) Immunofluorescence
staining of Piezo1, Piezo2 and EC cells in colonic sections from
each group. DAPI (blue) indicates nuclei, Piezo2 (green), Piezo1
(purple) and EC cell (red). Scale bar, 50 μm (magnification,
×200). Yellow arrows indicate co-localization of Piezo1 and ChgA;
orange arrows indicate co-localization of Piezo2 and ChgA; white
arrows indicate triple co-localization of Piezo1, Piezo2 and ChgA.
Percentage of (B) Piezo1+ChgA+, (C)
Piezo2+ChgA+ and (D)
Piezo1+Piezo2+ChgA+ cells as a
percentage of total ChgA+ cells in control and FC
groups; n=5 mice per group. (E) Quantification of Piezo1 and (F)
Piezo2 mRNA levels relative to β-actin in the control and FC group;
n=8 mice per group. (G) Western blot analysis of Piezo1 and Piezo2
protein expression in the control and FC groups; n=3 mice per group
Target proteins and internal controls were detected on separate
membranes processed in parallel. The vertical line indicates
membrane separation. (H) Co-immunoprecipitation of Piezo1 and
Piezo2 in colonic tissues from control and FC model mice, with
Piezo1 immunoprecipitated and Piezo2 detected by western blotting.
Data are presented as mean ± SD and were analyzed using an unpaired
two-tailed Student's t-test. Statistical significance indicated as
*P<0.05, **P<0.01. EC, enterochromaffin
cell; FC, functional constipation. |
To determine whether Piezo1 and Piezo2 physically
interact under physiological and pathological conditions, Co-IP was
performed using colonic tissue lysates from control and FC model
mice. Piezo1 was immunoprecipitated with a specific antibody and
western blotting was performed to detect Piezo2. As shown in
Fig. 1H, Piezo2 was strongly
detected in the immunoprecipitated fraction of control samples,
confirming a specific interaction between Piezo1 and Piezo2.
Notably, the intensity of the Piezo2 signal was markedly reduced in
the FC group, suggesting that the pathological condition impairs
the association between these two mechanosensitive ion channels. No
signal was observed in the IgG control, validating the specificity
of the assay.
To elucidate the roles of Piezo1 and Piezo2 in FC
mice, AAV-mediated knockdown of each ion channel individually and
in combination was employed (Fig.
2A). To examine the tissue distribution of AAV transduction,
GFP fluorescence in colonic sections following rectal enema
administration of AAV vectors was evaluated. GFP signals were
mainly detectable in the mucosal layer, whereas comparatively less
fluorescence was observed in the muscularis layer (Fig. S1B). In alignment with this
distribution pattern, quantitative analysis of Piezo1+
and Piezo2+ cells showed significant decreases in the
mucosa of knockdown groups compared with controls, whereas no
obvious changes were observed in the muscular layer (Fig. S2). These results suggested that
AAV-mediated gene modulation was predominantly associated with the
mucosal region under the current delivery protocol. RT-qPCR
confirmed specific and effective knockdown: Piezo1-KD significantly
reduced Piezo1 mRNA (P<0.001), Piezo2-KD markedly decreased
Piezo2 mRNA (P<0.01) and dual KD further diminished both mRNAs
(Fig. 2B and C).
Immunofluorescence-based quantification showed that the proportion
of Piezo1+ ChgA+ cells among total
ChgA+ cells was significantly decreased in the Piezo1-KD
and Piezo1/2-KD groups (P<0.05, P<0.01), whereas
Piezo2+ ChgA+ cells were markedly reduced in
both the Piezo2-KD and Piezo1/2-KD groups (P<0.01) (Fig. 2D-F). Furthermore, the proportion
of triple-positive cells (Piezo1+ Piezo2+
ChgA+) was markedly reduced and fell below detectable
levels in all knockdown groups (P<0.01) (Fig. 2G), suggesting a near-complete
loss of co-localized expression of Piezo1 and Piezo2 in EC cells
upon knockdown.
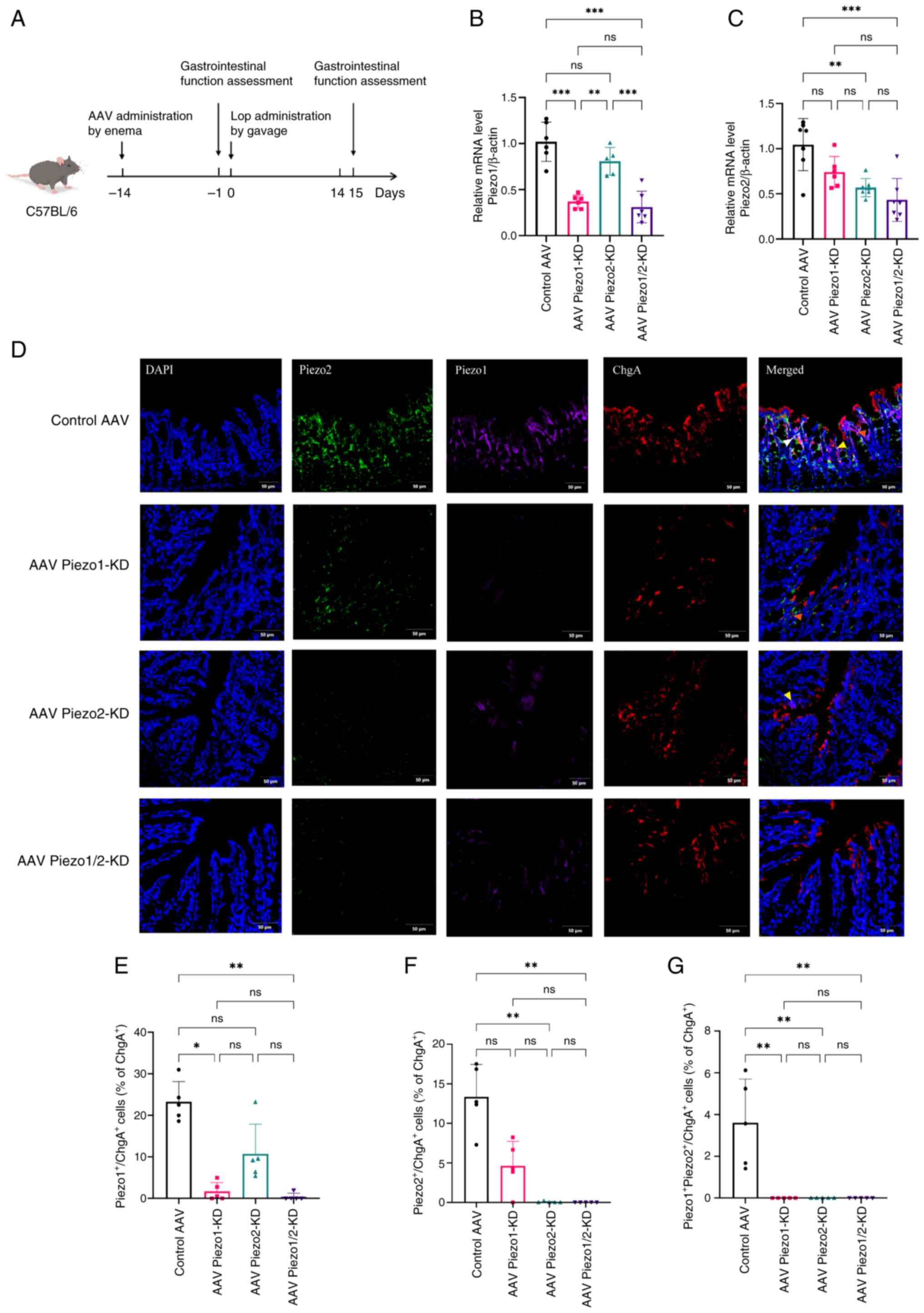 | Figure 2Piezo1/Piezo2 knockdown attenuates
expression of Piezos in EC cells of FC mice. (A) Schematic of the
experimental timeline for AAV-mediated knockdown. RT-qPCR analysis
of (B) Piezo1 and (C) Piezo2 mRNA levels in the control AAV, AAV
Piezo1-KD, AAV Piezo2-KD and AAV Piezo1/2-KD groups; n=5-7 mice per
group. Data analyzed by one-way ANOVA with Tukey's post hoc test.
(D) Immunofluorescence staining of Piezo1, Piezo2 and EC cells in
colonic sections from each group. DAPI (blue) indicates nuclei,
Piezo2 (green), Piezo1 (purple) and EC cell (red). Scale bar, 50
μm (magnification, ×200). Yellow arrows indicate
co-localization of Piezo1 and ChgA; orange arrows indicate
co-localization of Piezo2 and ChgA; white arrows indicate triple
co-localization of Piezo1, Piezo2 and ChgA. Percentage of (E)
Piezo1+ChgA+, (F)
Piezo2+ChgA+ and (G)
Piezo1+Piezo2+ChgA+ cells as a
percentage of total ChgA+ cells in control and knockdown
groups; n=5 mice per group. Data represent mean ± SD and were
analyzed using Kruskal-Wallis test. Statistical significance is
indicated as *P<0.05, **P<0.01,
***P<0.001. ns, not significant; EC, enterochromaffin
cell; FC, functional constipation; AAV, adeno-associated virus; KD,
knockdown. |
These findings indicated that knockdown of Piezo1 or
Piezo2 effectively reduced their respective mRNAs. Furthermore,
dual knockdown markedly diminished the population of
Piezo1+ Piezo2+ ChgA+ cells to
undetectable levels, indicating that Piezo1 and Piezo2 are
co-expressed in a subset of EC cells.
Piezo1 and Piezo2 knockdown exacerbates
impaired gastrointestinal motility and colonic sensitivity in FC
mice
The FC group displayed significantly reduced total
fecal output, lower BSFS scores, decreased stool water content
(Fig. S3) and prolonged TGITT
compared with the control group (all P<0.001), collectively
indicating impaired overall gastrointestinal motility.
Specifically, significant delays in small intestinal transit
(P<0.01) and gastric emptying rates (P<0.001) were observed,
further confirming the reduction in gut motility. Regarding colonic
sensitivity, the FC group required a higher gas volume to reach an
AWR score of 3 (P<0.001) and showed significantly decreased AWR
scores at gas volumes of 0.35, 0.50 and 0.65 ml (all P<0.05),
indicating visceral hyposensitivity (Fig. 3A-E). Histological analysis
revealed normal colonic architecture in the control and FC,
characterized by well-organized crypt structures with no mucosal
damage or inflammatory infiltration (Figs. 3F and S4A). Serum levels of VIP and SP were
assessed as indirect biomarkers of altered gut contractile
function, given their well-established roles in regulating
intestinal smooth muscle activity (34,35). As shown in Fig. 3G and H, VIP levels were
significantly elevated and SP levels were significantly reduced in
the FC group compared with the control group (P<0.01).
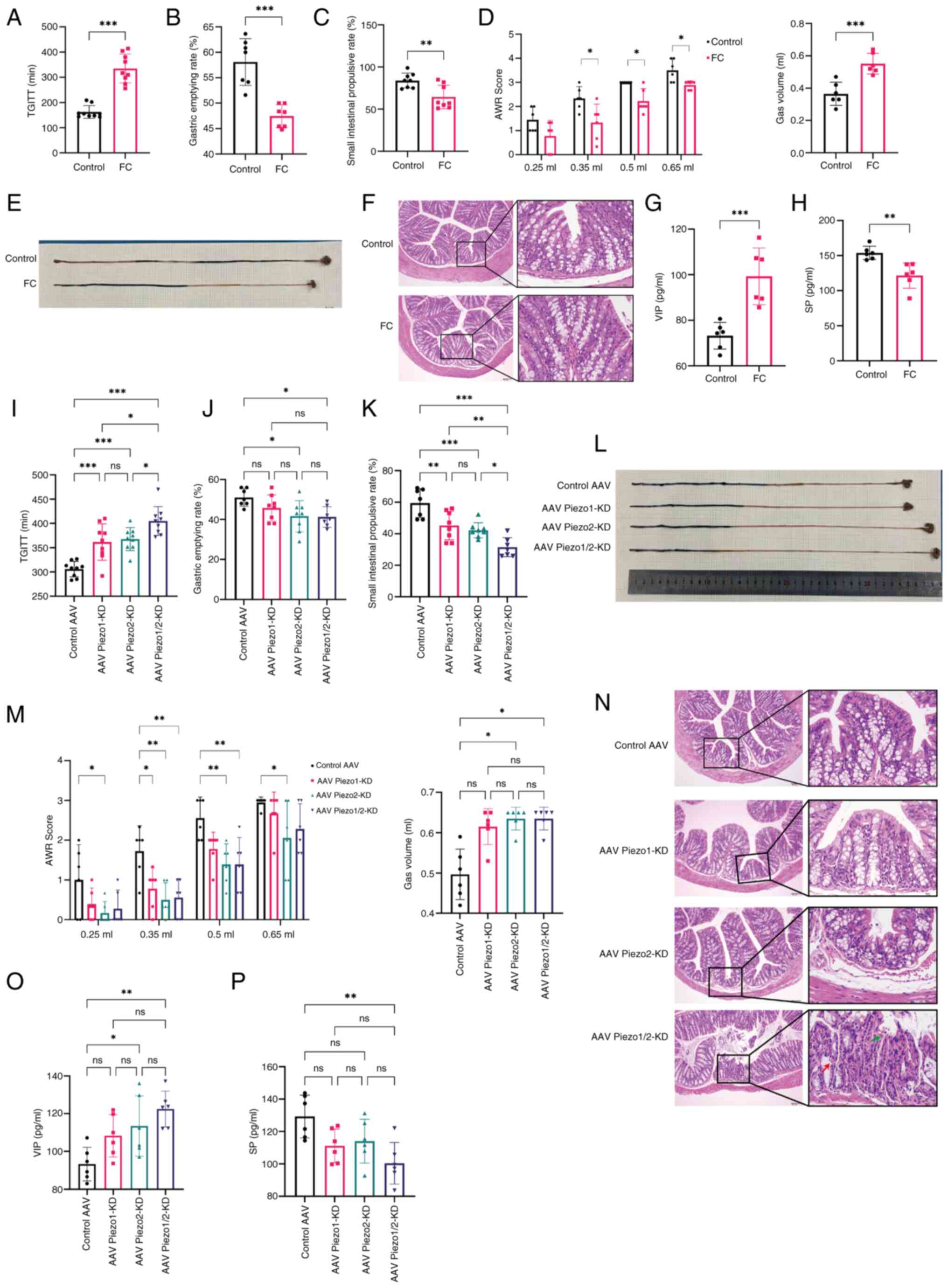 | Figure 3Effects of Piezo1 and Piezo2
knockdown on gastrointestinal motility and colonic sensitivity in
FC Mice. (A-H) General effects of the FC model compared with
control mice. Data were analyzed using an unpaired two-tailed
Student's t-test. (I-P) Effects of individual or simultaneous
Piezo1 and Piezo2 knockdown on gastrointestinal motility and
sensitivity in FC mice. Data were analyzed by one-way ANOVA with
Tukey's post hoc test or Kruskal-Wallis test. (A and I) TGITT, (B
and J) Gastric emptying rate, (C, K, E and L) Small intestinal
transit rate, (D and M) Gas volume required to reach an AWR score
of 3 and AWR scores at gas volumes of 0.25, 0.35, 0.50 and 0.65 ml.
(F and N) Histological analysis of colonic tissue in each group;
n=3 mice per group. Red arrows highlight regions of glandular
necrosis with structural disorganization and green arrows indicate
nuclear pyknosis and cytoplasmic dissolution. Scale bar, 100
μm (left, magnification, ×100) and 10 μm (right,
magnification, ×400). (G and O) Serum levels of VIP in each group,
(H and P) Serum levels of SP in each group. Data were analyzed
using an unpaired two-tailed Student's t-test or one-way ANOVA with
Tukey's post hoc test. n=6-9 mice per group. Data represent mean ±
SD. Statistical significance is indicated as *P<0.05,
**P<0.01, ***P<0.001. FC, functional
constipation; TGITT, total gastrointestinal transit time; AWR,
abdominal withdrawal reflex VIP, vasoactive intestinal peptide; SP,
substance P. |
To investigate the functional involvement of Piezo1
and Piezo2 in gastrointestinal motility and sensitivity, Piezo1 or
Piezo2 were independently knocked down in FC mice and their effects
on gastrointestinal parameters assessed. Piezo1 and Piezo2
knockdown both led to significantly reduced total fecal output,
delayed TGITT and increased small intestinal transit rate compared
with the control AAV group (all P<0.01) (Figs. S3 and 3I, K and L). Specifically, Piezo1
knockdown decreased fecal water content, while Piezo2 knockdown
reduced gastric emptying rate (both P<0.05) (Figs. S3 and 3J). In terms of colonic sensitivity,
Piezo2 knockdown required a higher gas volume to achieve an AWR
score of 3 compared with the control group, indicating reduced
sensitivity (P<0.05). The Piezo2 knockdown group displayed
significantly elevated AWR scores at each gas volume. (P<0.05),
while Piezo1 knockdown showed increased AWR scores only at 0.35 ml
(P<0.01) (Fig. 3M). These
findings suggested that both Piezo1 and Piezo2 contribute to the
regulation of gastrointestinal motility, jointly influencing total
fecal output and transit times. Furthermore, Piezo1 modulates fecal
water content, while Piezo2 plays a role in gastric emptying rate
and colonic sensitivity.
To determine whether Piezo1 and Piezo2 exert
cooperative effects on gastrointestinal function, a dual knockdown
of both proteins was conducted. Simultaneous knockdown of Piezo1
and Piezo2 significantly impaired gut motility and reduced colonic
sensitivity compared with the control group. Notably, dual
knockdown further prolonged TGITT and increased small intestinal
transit rate compared with the individual knockdown of either
Piezo1 or Piezo2 (both P<0.05; Fig. 3I, K and M). Additionally, dual
knockdown significantly reduced total fecal output compared with
Piezo1 knockdown alone (P<0.05) and decreased fecal water
content compared with Piezo2 knockdown alone (P<0.05) (Fig. S3). These findings indicated that
Piezo1 and Piezo2 work in cooperation to maintain optimal
gastrointestinal motility and sensitivity, jointly influencing
fecal output and transit time, with Piezo1 predominantly affecting
fecal water content and colonic sensitivity, while Piezo2 primarily
modulates gastric emptying. This cooperation underscores the
complex regulatory mechanisms involving Piezo channels in
gastrointestinal homeostasis and suggests potential therapeutic
targets for disorders related to impaired gut motility and
sensitivity.
In the Piezo1 or Piezo2 knockdown groups, mild crypt
disorganization was observed compared with the control vector
group. The dual Piezo1/2 knockdown group exhibited pronounced
mucosal atrophy and marked crypt architectural disarray, suggesting
aggravated structural damage (Fig.
3N). Quantitative analysis further confirmed that colonic crypt
depth was significantly reduced in the dual knockdown group
compared with both the control vector group (P<0.001) and the
single knockdown groups (P<0.01 and P<0.05, respectively)
(Fig. S4B).
The Piezo1 knockdown group showed no significant
changes in SP or VIP levels relative to the control vector group,
indicating minimal effect of Piezo1 knockdown alone. By contrast,
the Piezo2 knockdown group exhibited a significant increase in VIP
levels compared with the control vector group (P<0.05), while SP
levels remained unchanged. Notably, in the dual Piezo1/2 knockdown
group, SP levels were significantly reduced (P<0.01) and VIP
levels were further elevated (P<0.01) compared with the control
vector group (Fig. 3O and P).
These results indicated that changes in neuropeptide expression
became more pronounced under combined Piezo1 and Piezo2 knockdown
compared with single-gene knockdown.
Piezo1 and Piezo2 knockdown reduces
colonic 5-HT synthesis, release and increases serotonin transporter
(SERT) in FC mice
To examine the effect of Piezo1 and Piezo2 knockdown
on 5-HT synthesis, release and SERT expression in the colons of FC
mice, we performed immunohistochemical analysis, ELISA and RT-qPCR
for related genes. Immunohistochemical staining showed a
significant reduction in the percentage of 5-hydroxytryptamine
receptor 3 (5-HT3)-positive and a significant increase
in the percentage of SERT-positive areas in the FC group compared
with controls (P<0.01, P<0.001), indicating impaired
serotonergic function in FC mice (Fig. 4A). Consistent with these
findings, serum 5-HT levels were also significantly lower in the FC
group compared with the control (P<0.05; Fig. 4B), while colonic TPH-1 levels, a
key enzyme in 5-HT synthesis, were significantly reduced as well
(P<0.001; Fig. 4C).
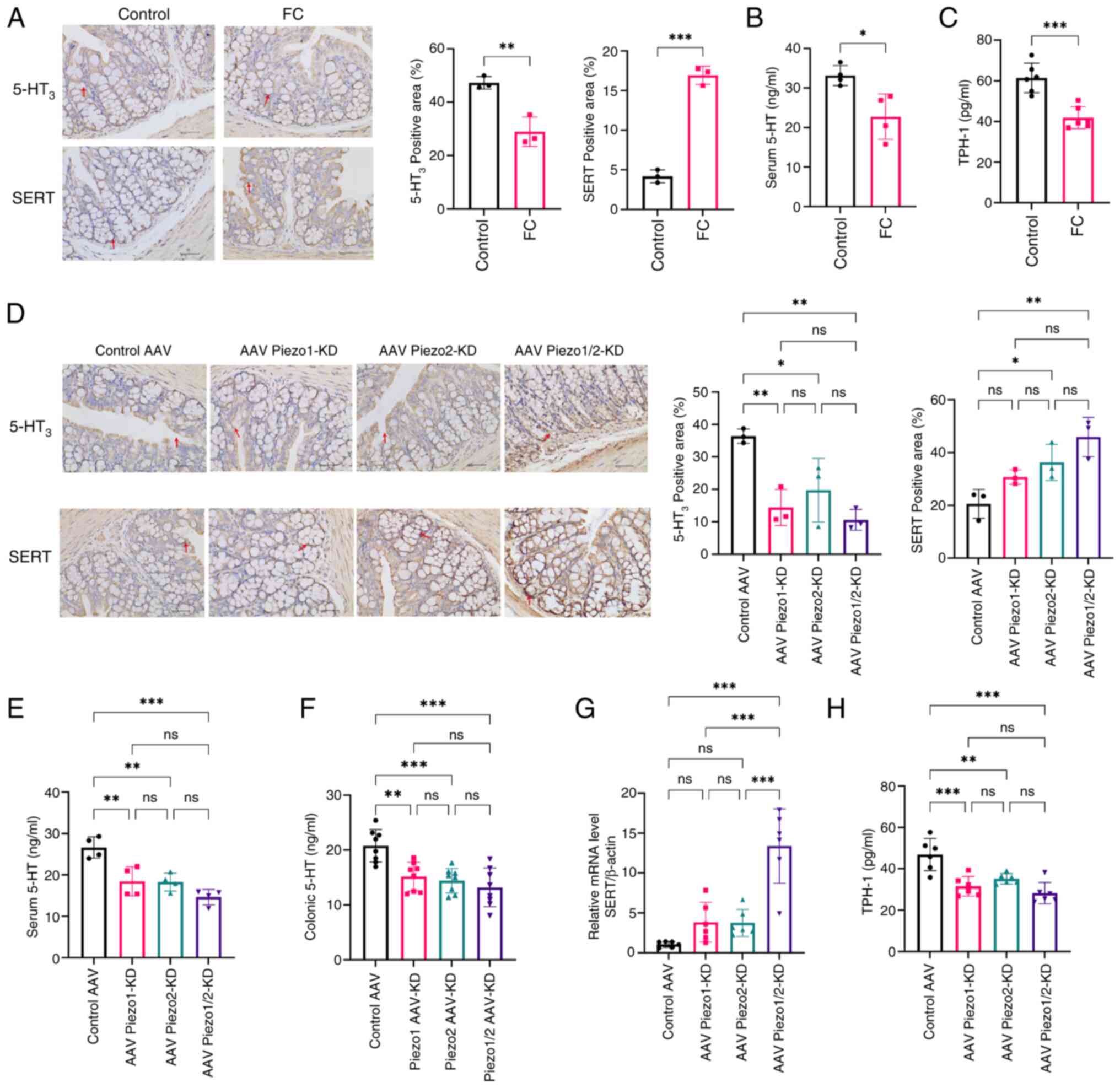 | Figure 4Effects of Piezo1 and Piezo2
knockdown on 5-HT synthesis, release and SERT expression in FC
Mice. (A) Representative immunohistochemical staining of
5-HT3 and SERT in colonic sections from control and FC
mice. Scale bar, 40 μm (magnification, ×400). Quantification
of (B) serum 5-HT and (C) colonic TPH-1 levels in control and FC
mice. Data were analyzed using an unpaired two-tailed Student's
t-test; n=4-6 mice per group. (D) Immunohistochemical staining of
5-HT3 and SERT in colonic sections from control AAV, AAV
Piezo1-KD, AAV Piezo2-KD and AAV Piezo1/2-KD groups. n=3 mice per
group. Scale bar, 40 μm (magnification, ×400). (E and F)
Serum and colonic 5-HT levels in each group, comparing knockdown
groups to the control AAV group. (G and H) Relative mRNA levels of
SERT and colonic TPH-1 levels in each group, comparing knockdown
groups to the control AAV. Data were analyzed by one-way ANOVA with
Tukey's post hoc test. n=4-6 mice per group. Data are presented as
mean ± SD, with statistical significance indicated as
*P<0.05, **P<0.01,
***P<0.001. 5-HT, 5-hydroxytryptamine; SERT,
serotonin transporter; FC, functional constipation; TPH-1,
tryptophan hydroxylase-1; AAV, adeno-associated virus; KD,
knockdown. |
In the knockdown groups (Fig. 4D), 5-HT3 and SERT
expression levels varied based on the specific knockdown of Piezo1,
Piezo2, or both. Quantitative analysis showed that the
5-HT3-positive area was significantly lower in the
Piezo1-KD, Piezo2-KD and combined Piezo1/2-KD groups compared with
the control AAV group (all P<0.05), while no significant
differences were observed between the knockdown groups. Similarly,
SERT-positive area was significantly increased in Piezo2-KD and
Piezo1/2-KD compared with the control AAV group (both P<0.05),
with no significant differences between the individual knockdown
and combined knockdown groups.
As serum 5-HT levels reflect both free and
platelet-stored serotonin and may not accurately represent local
biosynthesis or signaling activity, serotonergic activity in
colonic tissues was further evaluated by measuring tissue 5-HT
levels using ELISA. As shown in Fig.
4E and F, both serum and colonic 5-HT levels were significantly
reduced in all knockdown groups compared with the control AAV group
(P<0.01), with no significant differences between the knockdown
groups. The mRNA levels of SERT were significantly increased only
in combined Piezo1/2-KD group, compared with both the control AAV
group and the individual knockdown groups (Fig. 4G, P<0.001). Furthermore, TPH-1
was reduced in all knockdown groups (Fig. 4H, P<0.01), suggesting that
Piezo1 and Piezo2 are involved in maintaining 5-HT synthesis and
secretion in the colonic tissue of FC mice.
Additionally, calcium levels and ERK/PKC
phosphorylation in colonic tissue were assessed to explore the
downstream signaling events that may be involved in Piezo-regulated
5-HT synthesis (Fig. S5).
Colonic Ca2+ levels were significantly reduced in FC
mice and further decreased in the Piezo1 and Piezo1/2 knockdown
groups, with the lowest levels observed in the double knockdown
group. Western blotting showed that p-ERK/total ERK and p-PKC/total
PKC ratios were decreased in FC mice (Fig. S5C and D, P<0.001) and further
suppressed in Piezo knockdown groups (Fig. S5E and F, P<0.001, P<0.01,
P<0.05). Notably, the reduction in p-PKC/total PKC was more
pronounced in the Piezo1/2-KD group compared with either single
knockdown group (P<0.05). By contrast, total ERK1/2 and PKC
remained unchanged.
These results collectively indicated that knockdown
of Piezo1 and Piezo2 disrupted the synthesis and release of 5-HT in
the colon, potentially via ERK/PKC signaling pathways associated
with altered calcium levels.
Piezo1/2 expression of Piezo1 and Piezo2
knockdown in EC-like cells
To more directly investigate how Piezo channels
affect 5-HT release from EC cells, in vitro experiments were
conducted. Lentivirus-mediated knockdown targeting each protein was
used (Fig. 5A-D). In the
LV-Piezo1 KD group, Piezo1 protein and mRNA levels were
significantly reduced compared with the control (P<0.01 and
P<0.001), confirming successful knockdown. Similarly, Piezo2
protein and mRNA levels were significantly reduced in the LV-Piezo2
KD group (P<0.001).
Notably, Piezo2 expression was also significantly
reduced in the LV-Piezo1 KD group and Piezo1 expression was reduced
in the LV-Piezo2 KD group (P<0.001 and P<0.01), suggesting a
regulatory relationship where knockdown of one Piezo protein
affects the expression of the other. In the combined LV-Piezo1/2 KD
group, Piezo1 and Piezo2 levels were comparable to those in their
respective single knockdown groups, indicating that simultaneous
knockdown is as effective as individual knockdowns in reducing each
protein's expression.
These findings confirmed the efficacy of
lentivirus-mediated knockdown for both Piezo1 and Piezo2 and
suggest a potential interaction between Piezo1 and Piezo2
expression in EC-like cells.
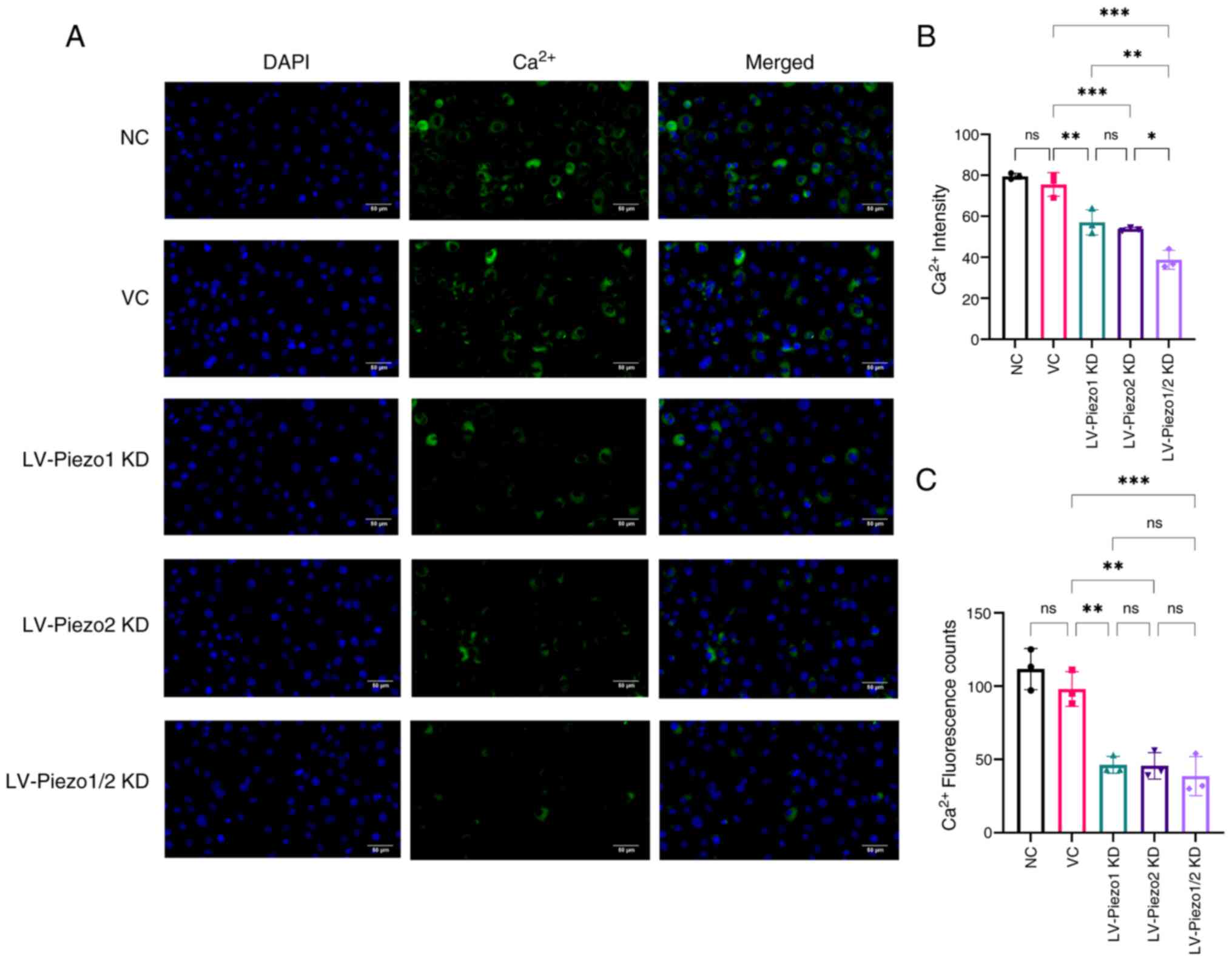
Calcium imaging of Piezo1 and Piezo2
knockdown in EC-like cells
Calcium ions play a crucial role in various cellular
processes and Piezo channels are known to be mechanosensitive ion
channels that regulate calcium influx in response to mechanical
stimuli in cells. To evaluate the effect of Piezo protein knockdown
on channel activity, intracellular calcium concentration in EC
cells was measured via immunofluorescence staining (Fig. 6A-C). The results demonstrated no
significant decrease in total fluorescence intensity in the VC
group compared with the NC group.
In EC-like cells with Piezo1 knockdown, a
significant decrease in average fluorescence intensity and cell
counts (P<0.01) was observed compared with the VC group.
Similarly, Piezo2 knockdown resulted in a marked reduction in these
parameters (P<0.01). These results suggested that knockdown of
either Piezo1 or Piezo2 significantly reduces intracellular calcium
concentration.
Notably, the simultaneous knockdown of both Piezo1
and Piezo2 led to an even more pronounced decrease in average
fluorescence intensity compared with either Piezo1 or Piezo2 single
knockdown groups (P<0.05). This indicated a cooperative effect,
where the combined reduction of Piezo1 and Piezo2 resulted in a
significantly greater decrease in intracellular calcium levels than
the reduction observed with individual gene knockdowns.
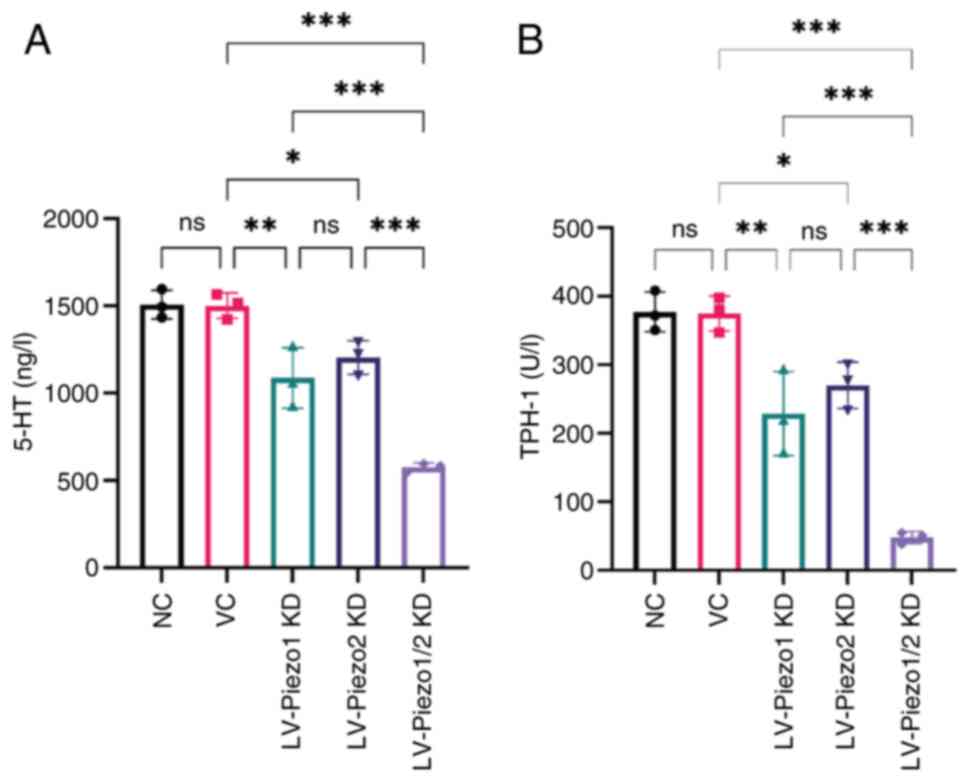
Piezo1 and Piezo2 knockdown downregulates 5-HT and
TPH-1 expression in EC-like cells. To further elucidate the effect
of Piezo1 and Piezo2 on 5-HT synthesis and release from EC-like
cells, lentiviral-mediated knockdown of Piezo1 and Piezo2 in these
cells were employed. The findings showed no significant difference
in 5-HT levels between the VC group and NC group. However,
knockdown of either Piezo1 or Piezo2 alone resulted in a marked
decrease in both 5-HT and TPH-1 expression relative to the VC group
(P<0.05). Notably, there was no significant difference in both
5-HT and TPH-1 levels between the Piezo1 and Piezo2 knockdown
groups, suggesting that both proteins play similar roles in
regulating 5-HT synthesis and release. Dual knockdown of Piezo1 and
Piezo2 led to a further reduction in 5-HT and TPH-1 expression
compared with individual knockdowns (P<0.001; Fig. 7A and B). These results suggested
that Piezo1 and Piezo2 have a cooperative effect on the regulation
of 5-HT and TPH-1 expression.
Discussion
Piezo channels are large trimeric mechanosensitive
ion channels that transduce physical forces such as luminal
distension, shear stress and peristaltic pressure into
intracellular calcium signals, thereby regulating key
gastrointestinal functions (36-38). They are known to influence
serotonin secretion, gut motility and epithelial dynamics and have
been implicated in both physiological and pathological processes
including intestinal inflammation and barrier dysfunction (39-41). The present study provided both
in vivo and in vitro evidence suggesting that Piezo1
and Piezo2 may act in a coordinated manner in EC cells. Their
knockdown was associated with reduced intracellular calcium levels
and diminished 5-HT production, along with impaired intestinal
transit and reduced visceral sensitivity. These findings suggested
that Piezo1 and Piezo2 contributed to gut homeostasis through
EC-mediated serotonergic signaling and that their dysregulation may
be involved in the pathogenesis of FC. Accordingly, targeting Piezo
channels may offer a promising therapeutic strategy for restoring
serotonin balance and improving GI motility in FC.
Piezo1 and Piezo2 are distributed across various
cell types in the gastrointestinal tract, reflecting their
involvement in multiple aspects of gut physiology. Piezo1 has been
reported in epithelial cells, endothelial cells, goblet cells and
smooth muscle cells (21,42,43).
In the enteric nervous system, it is also expressed in cholinergic
neurons along the small intestine, cecum and colon, suggesting a
role in efferent neuromuscular regulation. Piezo2, by contrast, has
a more selective distribution; primarily found in intrinsic primary
afferent neurons and enteroendocrine cells such as ECs (44), supporting its function in
mechanosensory signal input. These distinct patterns suggest that
Piezo1 and Piezo2 may coordinate to regulate gut motility and
sensitivity through both epithelial and neural pathways. In
addition to their individual roles, Piezo channels may functionally
interact with other mechanosensitive channels. Piezo1 activation
facilitates TRPV4 opening via PLA2 signaling, suggesting a
synergistic effect in amplifying mechanical responses (26,45), whereas TRPV1 activation inhibits
Piezo activity by depleting membrane phosphoinositides, indicating
an antagonistic interaction (46). Moreover, Piezo2 and TRPV1 also
co-localize in colonic sensory neurons and contribute to mechanical
hypersensitivity (47), implying
broader crosstalk beyond epithelial pathways. The present study
focused specifically on the expression and function of Piezo1 and
Piezo2 in EC cells under both physiological and pathological
conditions. While previous scRNA-seq studies have not reported
Piezo1 expression in ECs (48),
the findings of the present study supported its presence in the
colonic epithelium. It was observed that Piezo1 protein
co-localized with EC markers by immunofluorescence and Piezo1 mRNA
expression in colonic tissue confirmed by RT-qPCR. These
discrepancies may be attributed to methodological differences, as
Piezo1 expression might be relatively low or confined to a small
subset of ECs, making it difficult to detect in single-cell
transcriptomic datasets (49).
Discrepancies between transcriptomic profiling and experimental
validation have also been reported for Piezo2. Although undetected
in certain scRNA-seq studies beyond the distal colon, Piezo2
expression in the small intestine and EC cells has been confirmed
by RT-PCR and immunohistochemistry (50). To the best of the authors'
knowledge, this is the first in vivo demonstration of Piezo1
expression in colonic EC cells, providing new evidence that both
Piezo1 and Piezo2 may participate in epithelial mechanotransduction
and serotonin-mediated gut regulation.
The present study observed a significant reduction
in Piezo1 and Piezo2 expression in EC cells of FC mice, suggesting
that impaired epithelial mechanotransduction contributes to the
pathophysiology of functional constipation. In vitro,
knockdown of either Piezo1 or Piezo2 reduced the expression of the
other channel, indicating a potential regulatory association,
although this effect was not seen in vivo, probably due to
differences in cellular context or compensatory mechanisms. Despite
their shared mechanosensory roles, Piezo1 and Piezo2 exhibited
distinct physiological effects. Piezo2 knockdown selectively
impaired gastric emptying and colonic sensitivity, consistent with
its involvement in 5-HT-mediated visceral afferent signaling and
intersegmental reflex regulation (41). By contrast, Piezo1 knockdown
mainly reduced fecal water content, possibly reflecting its role in
epithelial ion transport and contractile regulation (51-53). These results suggested a
functional divergence: Piezo2 is more associated with sensory
signaling, while Piezo1 contributes to fluid homeostasis and
motility. Dual knockdown led to exacerbated gastrointestinal
dysfunction, with delayed total transit, reduced fecal hydration
and structural abnormalities. Although knockdown was confined to
the colon, changes in gastric and small intestinal transit were
observed, probably mediated by gut-brain-gut reflexes initiated by
altered colonic 5-HT signaling. While colonic transit time was not
directly assessed, the significant delay in total gastrointestinal
transit, accompanied by reduced fecal output and water content,
strongly suggested impaired colonic transport. Previous studies
have directly demonstrated the critical role of Piezo channels in
colonic propulsion: Piezo2 deletion in sensory neurons
significantly delays colonic transit (41) and enteric Piezo1 ablation impairs
colonic propulsion via disruption of neuroimmune homeostasis
(44).
In the present study, serum SP levels were
significantly decreased only following simultaneous knockdown of
Piezo1 and Piezo2, whereas VIP levels increased with Piezo2
knockdown and were further elevated in the dual knockdown group.
These results suggested that disruption of both Piezo channels may
be required to perturb specific neuropeptide regulatory pathways. A
similar cooperative interaction between Piezo1 and Piezo2 has been
reported in other mechanotransduction contexts, such as bladder
urothelium (54), supporting the
plausibility of such cooperative regulation in the gut. Given the
established roles of SP in promoting smooth muscle contraction and
visceral sensitivity and VIP in facilitating smooth muscle
relaxation and intestinal secretion (55-57), these neuropeptide changes are
consistent with impaired motility and dysregulated autonomic
signaling in FC.
EC cells, the main source of 5-HT in the gut, serve
as mechanosensors that convert physical stimuli into chemical
signals (58). Previous studies
have established Piezo2 as a key transducer of mechanical forces in
EC cells, promoting calcium influx and stimulating 5-HT synthesis
and release via TPH-1 activation (21,59). By contrast, the role of Piezo1 in
EC cells has remained unclear. Prior evidence largely limited to
epithelial responses under inflammatory or immune conditions
(28), with no direct data
confirming its expression or function in EC cells. The present
study filled this gap by providing the first direct in vivo
and in vitro evidence that both Piezo1 and Piezo2 are
expressed in colonic EC cells and act in a coordinated manner to
regulate 5-HT signaling. Notably, genetic knockdown of either
channel impaired calcium responses and reduced 5-HT synthesis and
secretion, but dual knockdown led to more pronounced suppression of
calcium levels, TPH-1 expression and 5-HT production, suggesting a
cooperative interaction between Piezo1 and Piezo2 in regulating EC
cell mechanotransduction. While previous pharmacological studies
using GsMTx4 supported a role for mechanosensitive channels in 5-HT
release, they lacked specificity between Piezo isoforms (59). To overcome this limitation, our
subtype-specific knockdown under both physiological and
pathological conditions enabled a more precise dissection of the
roles of Piezo1 and Piezo2 in regulating serotonergic signaling.
Specifically, Piezo knockdown led to a reduction in 5-HT synthesis
and downregulation of SERT expression, indicating disrupted
serotonergic signaling. Given that serotonin exerts its effects
through multiple receptor subtypes (such as 5-HT3 and
5-HT4) to coordinate intestinal motility and visceral
sensitivity, these disruptions may contribute to the observed
dysmotility and hyposensitivity in functional constipation.
Moreover, the present study examined the phosphorylation status of
ERK1/2 and PKC. ERK1/2 and PKC are both well-established
calcium-sensitive kinases that have been reported in previous
studies to be activated by Piezo1-mediated Ca2+ influx
under mechanical or chemical stimulation (23,60). The present study showed that
Piezo1 or Piezo2 knockdown significantly reduced ERK1/2 and PKC
phosphorylation in colonic tissues, suggesting that Piezo channels
may regulate serotonergic output via calcium-dependent kinase
pathways. Nonetheless, without CaMKII data, the downstream
signaling cascade remains incompletely characterized and additional
studies are needed to dissect the specific contributions of
distinct calcium-dependent kinases.
Current therapies for functional constipation, such
as the 5-HT4 receptor agonist prucalopride, offer
symptomatic relief by enhancing colonic motility through enteric
neuronal activation (61).
However, their clinical efficacy remains modest, with a substantial
proportion of patients exhibiting limited response (62). In addition, prucalopride is
associated with systemic adverse effects (63), including headache, abdominal
pain, nausea and diarrhea, which may lead to treatment
discontinuation in some cases. More importantly, these agents act
downstream of the serotonergic pathway and do not target upstream
mechanisms such as impaired serotonin synthesis or release from
enterochromaffin cells, which may underlie persistent symptoms in
certain patients. By contrast, Piezo channels, serve as
mechanosensitive regulators that directly influence both 5-HT
production and secretion. Their rapid activation kinetics,
cell-type-specific expression and dual roles in modulating
gastrointestinal motility and visceral sensation support their
potential as more physiologically relevant and precisely targeted
therapeutic candidates (64).
These features make Piezo-targeted strategies a promising
complement or alternative to existing serotonergic therapies,
particularly in patients who fail to respond adequately to current
treatment.
Although the present study revealed the
complementary roles of Piezo1 and Piezo2 in regulating the dynamics
of 5-HT release and in maintaining GI homeostasis, it has some
limitations. First, only the loperamide-induced constipation model
was used. While this model reliably reproduces core features of
functional constipation, including delayed transit and serotonergic
dysregulation, it does not fully capture the multifactorial nature
of the disease. Complementary models, such as those induced by
low-fiber diet or aging, may enhance translational relevance in
future studies. Second, the QGP-1 cell line, though widely adopted
as an EC cell model (65), is
derived from pancreatic endocrine tumors and may not fully reflect
the characteristics of native colonic EC cells. Primary EC cells or
intestinal organoids could offer greater physiological relevance.
Third, while calcium imaging showed reduced intracellular calcium
levels after Piezo knockdown, direct mechanical stimulation was not
applied. This limits the ability to directly confirm
mechanosensitive properties. Incorporating real-time mechanical
stimulation would help validate Piezo channel function more
conclusively. Finally, although AAV-mediated knockdown via enema
allows for efficient gene delivery to the colonic epithelium, it
does not offer cell-type specificity. Therefore, off-target effects
on other epithelial cell types cannot be entirely ruled out. Future
work employing EC cell-specific promoters or conditional knockout
models will help to refine the cellular specificity of
Piezo-related mechanisms.
To visually summarize the mechanistic insights
derived from our findings, a schematic diagram is presented in
Fig. 8. It illustrates how
downregulation of Piezo1 and Piezo2 in EC cells reduces
Ca2+ influx and 5-HT synthesis, disrupts serotonergic
and neuropeptide signaling (increased VIP, decreased SP and
enhanced SERT) and ultimately leads to impaired intestinal motility
characteristic of functional constipation.
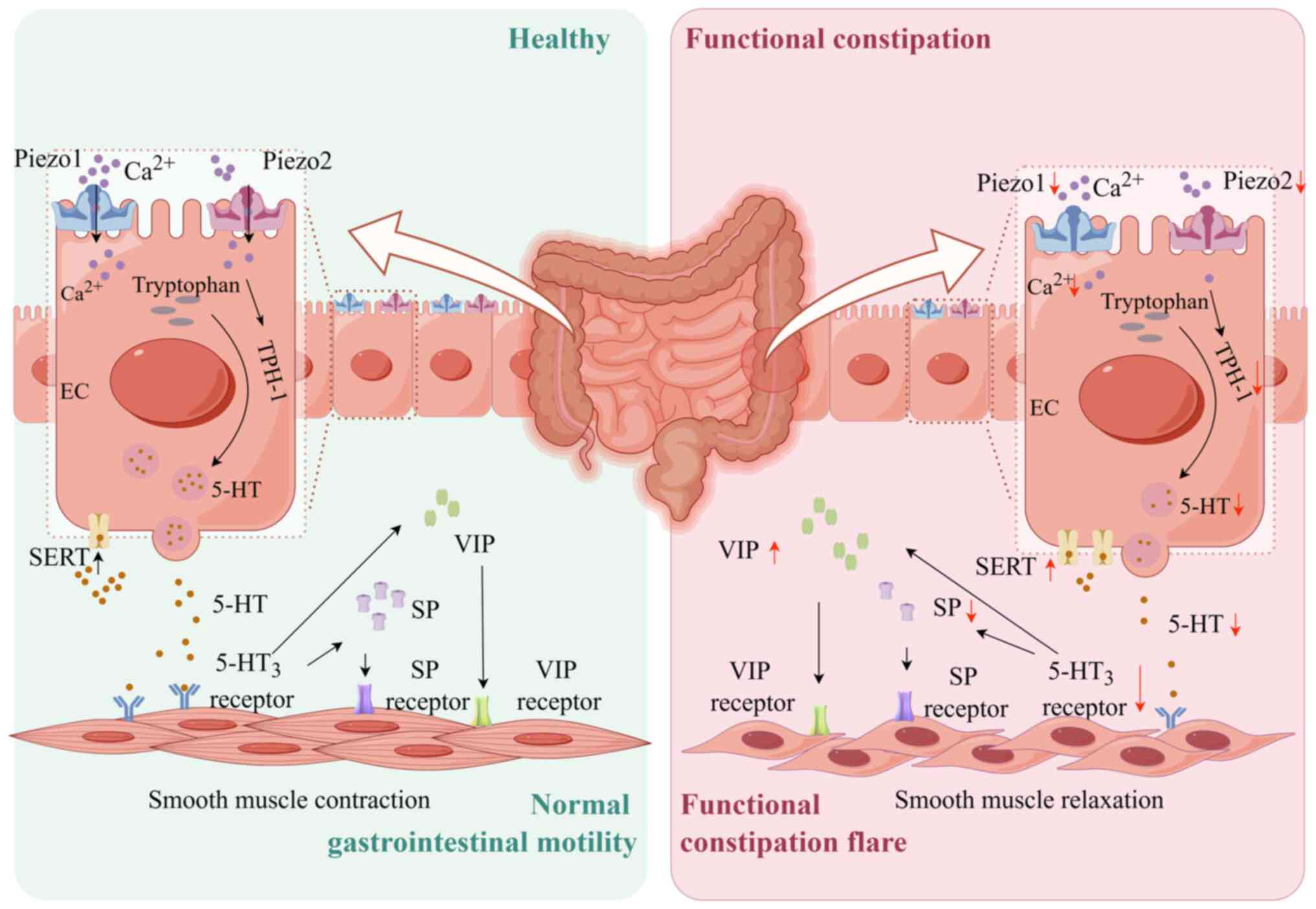 | Figure 8Mechanism diagram of the study. In
functional constipation, Piezo1 and Piezo2 expression is
downregulated, reducing Ca2+ influx and impairing 5-HT
synthesis. Consequently, diminished 5-HT release reduces activation
of 5-HT3 receptors, SP levels decrease and VIP levels
increase, leading to smooth muscle relaxation. Additionally,
heightened SERT activity, exacerbating the deficiency in 5-HT
signaling. These disruptions collectively induce pathological
alterations in intestinal structure and impaired motility, leading
to functional constipation symptoms. (created by figdraw.com). 5-HT, 5-hydroxytryptamine; SP,
substance P; VIP, vasoactive intestinal peptide; SERT, serotonin
transporter; EC, enterochromaffin cell. |
In conclusion, the present study revealed that
Piezo1 and Piezo2 play critical and cooperative roles in
maintaining GI homeostasis and regulating 5-HT signaling in the
context of FC. In vivo, knockdown of Piezo1 or Piezo2
reduced 5-HT synthesis and release, leading to impaired intestinal
motility, while in vitro experiments demonstrated that both
channels contribute to calcium influx and 5-HT secretion. Moreover,
Piezo knockdown suppressed ERK and PKC phosphorylation, indicating
their involvement in calcium-activated downstream signaling
pathways. These results suggested that Piezo1 and Piezo2 may
represent promising therapeutic targets in intestinal motility
disorders such as FC. Future research should investigate the
underlying molecular mechanisms of the interactions between Piezo1
and Piezo2 and evaluate their therapeutic potential in clinical
settings.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL was responsible for conceptualization, funding
acquisition, writing, reviewing and editing. JYe was responsible
for methodology and project administration. XY and PM were
responsible for writing the original draft, investigation, formal
analysis and data curation. WW, WZ, YL and YH were responsible for
investigation and formal analysis. JYao, QZ and WZ were responsible
for visualization and validation. XY and JYao confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with ethical guidelines and were approved by the Animal Experiment
Ethics Committee of Chengdu University of Traditional Chinese
Medicine (approval no. 2024012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
5-HT
|
5-hydroxytryptamine
|
|
EC
|
enterochromaffin cell
|
|
FC
|
functional constipation
|
|
AAV
|
adeno-associated virus
|
|
TGITT
|
total gastrointestinal transit
time
|
|
BSFS
|
Bristol stool form scale score
|
|
GI
|
gastrointestinal
|
|
CRD
|
colon-rectal distension
|
|
AWR
|
abdominal withdrawal reflex
|
|
KD
|
knockdown
|
|
RNAi
|
RNA interference
|
|
SERT
|
serotonin transporter
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
VIP
|
vasoactive intestinal peptide
|
|
SP
|
substance P
|
|
TPH-1
|
tryptophan hydroxylase-1
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82274652).
References
|
1
|
Black CJ, Drossman DA, Talley NJ, Ruddy J
and Ford AC: Functional gastrointestinal disorders: Advances in
understanding and management. Lancet. 396:1664–1674. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma C, Congly SE, Novak KL, Belletrutti PJ,
Raman M, Woo M, Andrews CN and Nasser Y: Epidemiologic burden and
treatment of chronic symptomatic functional bowel disorders in the
United States: A nationwide analysis. Gastroenterology.
160:88–98.e4. 2021. View Article : Google Scholar
|
|
3
|
Rao SSC, Rattanakovit K and Patcharatrakul
T: Diagnosis and management of chronic constipation in adults. Nat
Rev Gastroenterol Hepatol. 13:295–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barberio B, Judge C, Savarino EV and Ford
AC: Global prevalence of functional constipation according to the
Rome criteria: A systematic review and meta-analysis. Lancet
Gastroenterol Hepatol. 6:638–648. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palsson OS, Whitehead W, Törnblom H,
Sperber AD and Simren M: Prevalence of Rome IV functional bowel
disorders among adults in the United States, Canada, and the United
Kingdom. Gastroenterology. 158:1262–1273.e3. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossen ND, Touhara KK, Castro J,
Harrington AM, Caraballo SG, Deng F, Li Y, Brierley SM and Julius
D: Population imaging of enterochromaffin cell activity reveals
regulation by somatostatin. Proc Natl Acad Sci USA.
122:e25015251222025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bellono NW, Bayrer JR, Leitch DB, Castro
J, Zhang C, O'Donnell TA, Brierley SM, Ingraham HA and Julius D:
Enterochromaffin cells are gut chemosensors that couple to sensory
neural pathways. Cell. 170:185–198.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z, Luo J, Li J, Kim G, Stewart A,
Urban JF Jr, Huang Y, Chen S, Wu LG, Chesler A, et al:
Interleukin-33 promotes serotonin release from enterochromaffin
cells for intestinal homeostasis. Immunity. 54:151–163.e6. 2021.
View Article : Google Scholar :
|
|
9
|
Kieslich B, Weiße RH, Brendler J, Ricken
A, Schöneberg T and Sträter N: The dimerized pentraxin-like domain
of the adhesion G protein-coupled receptor 112 (ADGRG4) suggests
function in sensing mechanical forces. J Biol Chem. 299:1053562023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bayrer JR, Castro J, Venkataraman A,
Touhara KK, Rossen ND, Morrie RD, Maddern J, Hendry A, Braverman
KN, Garcia-Caraballo S, et al: Gut enterochromaffin cells drive
visceral pain and anxiety. Nature. 616:137–142. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strege PR, Knutson K, Eggers SJ, Li JH,
Wang F, Linden D, Szurszewski JH, Milescu L, Leiter AB, Farrugia G
and Beyder A: Sodium channel NaV1.3 is important for
enterochromaffin cell excitability and serotonin release. Sci Rep.
7:156502017. View Article : Google Scholar
|
|
12
|
Leiser SF, Miller H, Rossner R, Fletcher
M, Leonard A, Primitivo M, Rintala N, Ramos FJ, Miller DL and
Kaeberlein M: Cell nonautonomous activation of flavin-containing
monooxygenase promotes longevity and health span. Science.
350:1375–1378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raouf Z, Steinway SN, Scheese D, Lopez CM,
Duess JW, Tsuboi K, Sampah M, Klerk D, El Baassiri M, Moore H, et
al: Colitis-induced small intestinal hypomotility is dependent on
enteroendocrine cell loss in mice. Cell Mol Gastroenterol Hepatol.
18:53–70. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhattarai Y, Williams BB, Battaglioli EJ,
Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK,
Zachos NC, et al: Gut microbiota-produced tryptamine activates an
epithelial G-protein-coupled receptor to increase colonic
secretion. Cell Host Microbe. 23:775–785.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Zhao L, Zhang S and Zhu C:
Modified Liu-Jun-Zi decoction alleviates visceral hypersensitivity
in functional dyspepsia by regulating EC cell-5HT3r signaling in
duodenum. J Ethnopharmacol. 250:1124682020. View Article : Google Scholar
|
|
16
|
Nygaard A, Zachariassen LG, Larsen KS,
Kristensen AS and Loland CJ: Fluorescent non-canonical amino acid
provides insight into the human serotonin transporter. Nat Commun.
15:92672024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei L, Singh R, Ha SE, Martin AM, Jones
LA, Jin B, Jorgensen BG, Zogg H, Chervo T, Gottfried-Blackmore A,
et al: Serotonin deficiency is associated with delayed gastric
emptying. Gastroenterology. 160:2451–2466.e19. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cremon C, Carini G, Wang B, Vasina V,
Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De
Ponti F, et al: Intestinal serotonin release, sensory neuron
activation, and abdominal pain in irritable bowel syndrome. Am J
Gastroenterol. 106:1290–1298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin B, Ha SE, Wei L, Singh R, Zogg H,
Clemmensen B, Heredia DJ, Gould TW, Sanders KM and Ro S: Colonic
motility is improved by the activation of 5-HT2B
receptors on interstitial cells of cajal in diabetic mice.
Gastroenterology. 161:608–622.e7. 2021. View Article : Google Scholar
|
|
20
|
Nan N, Gong MX, Wang Q, Li MJ, Xu R, Ma Z,
Wang SH, Zhao H and Xu YS: Wuzhuyu decoction relieves hyperalgesia
by regulating central and peripheral 5-HT in chronic migraine model
rats. Phytomedicine. 96:1539052022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alcaino C, Knutson KR, Treichel AJ, Yildiz
G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia
G and Beyder A: A population of gut epithelial enterochromaffin
cells is mechanosensitive and requires Piezo2 to convert force into
serotonin release. Proc Natl Acad Sci USA. 115:E7632–E7641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kefauver JM, Ward AB and Patapoutian A:
Discoveries in structure and physiology of mechanically activated
ion channels. Nature. 587:567–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gudipaty SA, Lindblom J, Loftus PD, Redd
MJ, Edes K, Davey CF, Krishnegowda V and Rosenblatt J: Mechanical
stretch triggers rapid epithelial cell division through Piezo1.
Nature. 543:118–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baghdadi MB, Houtekamer RM, Perrin L,
Rao-Bhatia A, Whelen M, Decker L, Bergert M, Pérez-Gonzàlez C,
Bouras R, Gropplero G, et al: PIEZO-dependent mechanosensing is
essential for intestinal stem cell fate decision and maintenance.
Science. 386:eadj76152024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma S, Dubin AE, Zhang Y, Mousavi SAR, Wang
Y, Coombs AM, Loud M, Andolfo I and Patapoutian A: A role of PIEZO1
in iron metabolism in mice and humans. Cell. 184:969–982.e13. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Swain SM and Liddle RA: Mechanosensing
Piezo channels in gastrointestinal disorders. J Clin Invest.
133:e1719552023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He H, Zhou J, Xu X, Zhou P, Zhong H and
Liu M: Piezo channels in the intestinal tract. Front Physiol.
15:13563172024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugisawa E, Takayama Y, Takemura N, Kondo
T, Hatakeyama S, Kumagai Y, Sunagawa M, Tominaga M and Maruyama K:
RNA sensing by gut Piezo1 is essential for systemic serotonin
synthesis. Cell. 182:609–624.e21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma T, Li Y, Yang N, Wang H, Shi X, Liu Y,
Jin H, Kwok LY, Sun Z and Zhang H: Efficacy of a postbiotic and its
components in promoting colonic transit and alleviating chronic
constipation in humans and mice. Cell Rep Med. 6:1020932025.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu MM, Guo Y, Chen Y, Zhang W, Wang L and
Li Y: Electro-acupuncture promotes gut motility and alleviates
functional constipation by regulating gut microbiota and increasing
butyric acid generation in mice. J Integr Med. 21:397–406. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lewis SJ and Heaton KW: Stool form scale
as a useful guide to intestinal transit time. Scand J
Gastroenterol. 32:920–924. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Segura MM, Mangion M, Gaillet B and
Garnier A: New developments in lentiviral vector design, production
and purification. Expert Opin Biol Ther. 13:987–1011. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Li J, Zheng H, Liu J, Ding J, Guo Q and
Zhang N: Effects of functional red pine seed direct-drinking oil on
constipation and intestinal barrier function in mice. Antioxidants
(Basel). 14:142024. View Article : Google Scholar
|
|
35
|
Yin J, Liang Y, Wang D, Yan Z, Yin H, Wu D
and Su Q: Naringenin induces laxative effects by upregulating the
expression levels of c-Kit and SCF, as well as those of aquaporin 3
in mice with loperamide-induced constipation. Int J Mol Med.
41:649–658. 2018.
|
|
36
|
Wu J, Lewis AH and Grandl J: Touch,
tension, and transduction-the function and regulation of Piezo ion
channels. Trends Biochem Sci. 42:57–71. 2017. View Article : Google Scholar
|
|
37
|
Yang X, Lin C, Chen X, Li S, Li X and Xiao
B: Structure deformation and curvature sensing of PIEZO1 in lipid
membranes. Nature. 604:377–383. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Passini FS, Jaeger PK, Saab AS, Hanlon S,
Chittim NA, Arlt MJ, Ferrari KD, Haenni D, Caprara S, Bollhalder M,
et al: Shear-stress sensing by PIEZO1 regulates tendon stiffness in
rodents and influences jumping performance in humans. Nat Biomed
Eng. 5:1457–1471. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang Y, Mo H, Yang J, Gao L, Tao T, Shu
Q, Guo W, Zhao Y, Lyu J, Wang Q, et al: Mechano-regulation of GLP-1
production by Piezo1 in intestinal L cells. Elife. 13:RP978542024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Fang F, Xiong Y, Wu J, Li X, Li G,
Bai T, Hou X and Song J: Reprogrammed fecal and mucosa-associated
intestinal microbiota and weakened mucus layer in intestinal goblet
cell-specific Piezo1-deficient mice. Front Cell Infect Microbiol.
12:10353862022. View Article : Google Scholar
|
|
41
|
Servin-Vences MR, Lam RM, Koolen A, Wang
Y, Saade DN, Loud M, Kacmaz H, Frausto S, Zhang Y, Beyder A, et al:
PIEZO2 in somatosensory neurons controls gastrointestinal transit.
Cell. 186:3386–3399. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu Y, Xiong Y, Liu Y, Li G, Bai T, Zheng
G, Hou X and Song J: Activation of goblet cell Piezo1 alleviates
mucus barrier damage in mice exposed to WAS by inhibiting H3K9me3
modification. Cell Biosci. 13:72023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang JE, Buechler MB, Gressier E, Turley
SJ and Carroll MC: Mechanosensing by Peyer's patch stroma regulates
lymphocyte migration and mucosal antibody responses. Nat Immunol.
20:1506–1516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie Z, Rose L, Feng J, Zhao Y, Lu Y, Kane
H, Hibberd TJ, Hu X, Wang Z, Zang K, et al: Enteric neuronal Piezo1
maintains mechanical and immunological homeostasis by sensing
force. Cell. 188:2417–2432.e19. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Swain SM, Romac JMJ, Shahid RA, Pandol SJ,
Liedtke W, Vigna SR and Liddle RA: TRPV4 channel opening mediates
pressure-induced pancreatitis initiated by Piezo1 activation. J
Clin Invest. 130:2527–2541. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Borbiro I, Badheka D and Rohacs T:
Activation of TRPV1 channels inhibits mechanosensitive Piezo
channel activity by depleting membrane phosphoinositides. Sci
Signal. 8:ra152015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xie Z, Feng J, Hibberd TJ, Chen BN, Zhao
Y, Zang K, Hu X, Yang X, Chen L, Brookes SJ, et al: Piezo2 channels
expressed by colon-innervating TRPV1-lineage neurons mediate
visceral mechanical hypersensitivity. Neuron. 111:526–538.e4. 2023.
View Article : Google Scholar :
|
|
48
|
Song Y, Fothergill LJ, Lee KS, Liu BY, Koo
A, Perelis M, Diwakarla S, Callaghan B, Huang J, Wykosky J, et al:
Stratification of enterochromaffin cells by single-cell expression
analysis. Elife. 12:RP905962025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kharchenko PV, Silberstein L and Scadden
DT: Bayesian approach to single-cell differential expression
analysis. Nat Methods. 11:740–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang F, Knutson K, Alcaino C, Linden DR,
Gibbons SJ, Kashyap P, Grover M, Oeckler R, Gottlieb PA, Li HJ, et
al: Mechanosensitive ion channel Piezo2 is important for
enterochromaffin cell response to mechanical forces. J Physiol.
595:79–91. 2017. View Article : Google Scholar :
|
|
51
|
Qian W, Hadi T, Silvestro M, Ma X, Rivera
CF, Bajpai A, Li R, Zhang Z, Qu H, Tellaoui RS, et al:
Microskeletal stiffness promotes aortic aneurysm by sustaining
pathological vascular smooth muscle cell mechanosensation via
Piezo1. Nat Commun. 13:5122022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bi Y, Li H, Diao M, Liu Q, Huang L, Tao Y,
Wan Y and Lin X: Piezo1 overexpression in the uterus contributes to
myometrium contraction and inflammation-associated preterm birth. J
Transl Med. 22:11402024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luo M, Gu R, Wang C, Guo J, Zhang X, Ni K,
Liu L, Pan Y, Li J and Deng L: High stretch associated with
mechanical ventilation promotes piezo1-mediated migration of airway
smooth muscle cells. Int J Mol Sci. 25:17482024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dalghi MG, Ruiz WG, Clayton DR,
Montalbetti N, Daugherty SL, Beckel JM, Carattino MD and Apodaca G:
Functional roles for PIEZO1 and PIEZO2 in urothelial
mechanotransduction and lower urinary tract interoception. JCI
Insight. 6:e1529842021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bai X, De Palma G, Boschetti E, Nishiharo
Y, Lu J, Shimbori C, Costanzini A, Saqib Z, Kraimi N, Sidani S, et
al: Vasoactive intestinal polypeptide plays a key role in the
microbial-neuroimmune control of intestinal motility. Cell Mol
Gastroenterol Hepatol. 17:383–398. 2024. View Article : Google Scholar :
|
|
56
|
Talbot J, Hahn P, Kroehling L, Nguyen H,
Li D and Littman DR: Feeding-dependent VIP neuron-ILC3 circuit
regulates the intestinal barrier. Nature. 579:575–580. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yin Y, Zhong L, Wang JW, Zhao XY, Zhao WJ
and Kuang HX: Tong Xie Yao Fang relieves irritable bowel syndrome
in rats via mechanisms involving regulation of 5-hydroxytryptamine
and substance P. World J Gastroenterol. 21:4536–4546. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chin A, Svejda B, Gustafsson BI, Granlund
AB, Sandvik AK, Timberlake A, Sumpio B, Pfragner R, Modlin IM and
Kidd M: The role of mechanical forces and adenosine in the
regulation of intestinal enterochromaffin cell serotonin secretion.
Am J Physiol Gastrointest Liver Physiol. 302:G397–G405. 2012.
View Article : Google Scholar :
|
|
59
|
Zhu Z, Chen X, Chen S, Hu C, Guo R, Wu Y,
Liu Z, Shu X and Jiang M: Examination of the mechanism of Piezo ion
channel in 5-HT synthesis in the enterochromaffin cell and its
association with gut motility. Front Endocrinol (Lausanne).
14:11935562023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Caulier A, Jankovsky N, Demont Y,
Ouled-Haddou H, Demagny J, Guitton C, Merlusca L, Lebon D, Vong P,
Aubry A, et al: PIEZO1 activation delays erythroid differentiation
of normal and hereditary xerocytosis-derived human progenitor
cells. Haematologica. 105:610–622. 2020. View Article : Google Scholar :
|
|
61
|
Garnock-Jones KP: Prucalopride: A review
in chronic idiopathic constipation. Drugs. 76:99–110. 2016.
View Article : Google Scholar
|
|
62
|
Mugie SM, Korczowski B, Bodi P, Green A,
Kerstens R, Ausma J, Ruth M, Levine A and Benninga MA: Prucalopride
is no more effective than placebo for children with functional
constipation. Gastroenterology. 147:1285–1295.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chang L, Chey WD, Imdad A, Almario CV,
Bharucha AE, Diem S, Greer KB, Hanson B, Harris LA, Ko C, et al:
American gastroenterological association-american college of
gastroenterology clinical practice guideline: Pharmacological
management of chronic idiopathic constipation. Gastroenterology.
164:1086–1106. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu S, Yang X, Chen X, Zhang X, Jiang J,
Yuan J, Liu W, Wang L, Zhou H, Wu K, et al: An intermediate open
structure reveals the gating transition of the mechanically
activated PIEZO1 channel. Neuron. 113:590–604.e6. 2025. View Article : Google Scholar
|
|
65
|
Doihara H, Nozawa K, Kojima R,
Kawabata-Shoda E, Yokoyama T and Ito H: QGP-1 cells release 5-HT
via TRPA1 activation; a model of human enterochromaffin cells. Mol
Cell Biochem. 331:239–245. 2009. View Article : Google Scholar : PubMed/NCBI
|