Introduction
Metabolic dysfunction-associated steatotic liver
disease (MASLD) has emerged as a global public health concern and
is characterized by the accumulation of fat in the liver in the
context of metabolic dysfunction. The pathophysiology of MASLD is
complex, with oxidative stress and insulin resistance (IR) playing
central roles in its development and progression (1). The increasing prevalence of MASLD
is closely linked to increasing rates of obesity, type 2 diabetes,
and other metabolic disorders, making it a significant health issue
worldwide. In the search for effective management strategies,
intermittent fasting (IF), also known as time-restricted feeding,
has garnered attention as a potential therapeutic approach. IF
involves alternating between periods of normal eating and fasting,
with variations such as alternate-day fasting and periodic fasting
(2). The relatively simple
structure of IF has contributed to its widespread popularity as a
dietary regimen among the general population. Studies have
suggested that IF may be beneficial in alleviating liver fat
accumulation by increasing insulin sensitivity, reducing fat
deposition, and modulating inflammatory responses, which are
crucial factors in the pathogenesis of MASLD (3). However, while IF shows promise in
managing MASLD, it has some challenges. Certain individuals,
particularly those with hypoglycemia, diabetes, or gastrointestinal
disorders, may face health risks associated with IF (4). Furthermore, although short-term IF
has demonstrated beneficial effects on liver fat reduction and
liver enzyme levels in MASLD, the long-term impact remains
inadequately studied. Some research has indicated that the
sustainability of IF may be limited, especially after periods of
refeeding, when hepatic steatosis may relapse (5). Therefore, while IF presents a
potential strategy for MASLD management, the effects of IF are
likely to vary depending on individual health conditions and
dietary habits.
Studies have shown that peroxisome
proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)
plays a critical role in the onset and progression of IR (6). In the liver, PGC-1α activates key
enzymes involved in gluconeogenesis, promoting hepatic glucose
production, which results in increased hepatic glucose output
(7). Unrestrained hepatic
glucose output is a hallmark of IR. In addition, mitochondrial
dysfunction is implicated in the development of MASH (8). Mitochondrial ultrastructural damage
has been observed in the livers of patients with non-alcoholic
steatohepatitis, with a decrease in the quantity and activity of
respiratory chain complexes and impaired ATP synthesis (8). PGC-1α influences mitochondrial
function by enhancing mitochondrial respiration, a process in which
PGC-1α strongly induces the mRNA expression of nuclear respiratory
factor (NRF)-1 and NRF-2α (9).
NRF-1 and NRF-2α are key regulators of genes involved in the
mitochondrial respiratory chain, including cytochrome c oxidase IV
(COX IV), β-ATP synthase, and mitochondrial transcription factor A
(mtTFA) (9). Furthermore,
dysregulated lipid metabolism generates large amounts of reactive
oxygen species (ROS), disturbing the dynamic balance between
pro-oxidants and antioxidants, which leads to oxidative stress and
lipid peroxidation (7,10,11). PGC-1α is involved in hepatic
lipid metabolism and plays a crucial role in reducing hepatic fat
accumulation (12). Recent
studies have highlighted the important role of the PPARα nuclear
receptor transcription factor in the transcriptional regulation of
intracellular lipid metabolism (12,13). PPARα controls the transcriptional
activity of genes such as long-chain acyl-CoA dehydrogenase,
medium-chain acyl-CoA dehydrogenase and carnitine
palmitoyltransferase-1. PGC-1α synergistically stimulates PPARα to
increase the transcriptional activity of fatty acid oxidases
(13). Overall, PGC-1α plays a
protective role in the mechanisms currently recognized in MASLD,
such as IR, mitochondrial damage, and lipid metabolism
dysregulation, by increasing insulin sensitivity, promoting
mitochondrial respiration, and facilitating fatty acid β-oxidation,
thus alleviating hepatic triglyceride accumulation.
Heterophyllin B (HP-B) is a cyclopeptide compound
derived from plants that has shown significant progress in research
on blood glucose regulation and anti-inflammatory effects in recent
years (14-16). HP-B interacts with the
glucagon-like peptide-1 receptor (GLP-1R), mimicking the actions of
GLP-1 analogs, promoting insulin secretion, and improving insulin
sensitivity, thereby modulating metabolic disturbances (14). These actions are particularly
relevant in the context of MASLD, the pathogenesis of which is
closely linked to IR, fat deposition, oxidative stress and chronic
inflammation (17). Given the
potential of HP-B to regulate metabolic disturbances and reduce
oxidative stress, it was hypothesized that the combination of HP-B
with IF may have a synergistic effect on MASLD. More importantly,
the integration of a natural GLP-1R activator with a dietary
intervention strategy may provide a more effective and tolerable
therapeutic option for patients with MASLD, particularly those
unable to adhere to strict fasting protocols. By elucidating the
molecular mechanisms underlying this combinatorial approach, the
present findings may contribute to the optimization of MASLD
treatment strategies and advance the development of personalized
metabolic therapies.
Materials and methods
Cell culture
HepG2 and Huh-7 liver cancer cell lines were
purchased from Procell Life Science & Technology Co., Ltd. STR
profiling was used for authentication. The cells were cultured in
DMEM (MedChemExpress) supplemented with 10% fetal bovine serum
(FBS; HyClone; Cytiva) and 1% penicillin-streptomycin (Beijing
Solarbio Science & Technology Co., Ltd.) in an incubator at
37°C with 5% CO2. The cells were divided into the
following groups: Control (Con) group, in which the cells were
treated with vehicle (0.1% DMSO in PBS) for 24 h; OA/PA model
group, in which HepG2 and Huh-7 cells were treated with 750
μM oleic acid + 750 μM palmitic acid (2:1 ratio) for
24 h (18), followed by
treatment with vehicle (0.1% DMSO in PBS) for 24 h; OA/PA + HP-B
group, in which the cells were treated with OA/PA for 24 h,
followed by treatment with 50 μM HP-B (prepared from 10 mM
DMSO stock diluted in PBS) for 24 h; OA/PA + Fasting group, in
which the cells were treated with OA/PA for 24 h, followed by
culture in low-glucose DMEM + 2% FBS + vehicle (0.1% DMSO) for 24
h; and OA/PA + Fasting + HP-B group, in which the cells were
treated with OA/PA for 24 h, followed by cultured in fasting medium
(low-glucose DMEM + 2% FBS) containing 50 μM HP-B for 24
h.
Cell counting kit-8 (CCK-8) assay
HepG2 and Huh-7 liver cancer cells were seeded at a
density of 5×103 cells/well in 96-well plates and
allowed to adhere overnight at 37°C. Following treatment with 5,
10, 25, 50, 75, 100, or 200 μM HP-B for 24 h, cell viability
was then determined according to the instructions of the CCK-8
assay kit (Beijing Solarbio Science & Technology Co., Ltd.). In
brief, 10 μl CCk-8 solution was added to each well
containing 100 μl culture medium and incubated at 37°C for 2
h in a humidified incubator with 5% CO2. Optical density
was measured at 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.).
Oil Red O staining
Following treatment of the HepG2 and Huh-7 liver
cancer cells, the media was removed, and the cells were washed
twice with PBS. The cells in each well were fixed for 10 min at
room temperature with 4% paraformaldehyde (Beijing Solarbio Science
& Technology Co., Ltd.). The cells were subsequently stained
with 0.5% Oil Red O solution at room temperature for 10 min. After
staining, the excess dye was removed by washing with 60%
isopropanol, followed by three washes with distilled water (5 min
each wash) to prevent non-specific binding of the dye. Under an
inverted microscope (Olympus Corporation), the cells were examined,
and images of representative fields were acquired.
Determination of total cholesterol (TC)
and triglyceride (TG) levels
Following the treatment of HepG2 and Huh-7 liver
cancer cells, the supernatants were collected. For the liver tissue
samples, a homogenizer was used to homogenize the liver tissue. The
samples were subsequently centrifuged for 10 min at 4°C and 12,000
× g, after which the supernatants were collected. The TG and TC
levels were measured by TG (cat. no. A110-1-1) and mouse total
cholesterol ELISA kit (cat. no. A111-1-1; both from Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
protocols.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following the manufacturer's instructions, TRIzol
reagent (Beijing Solarbio Science & Technology Co., Ltd.) was
used to extract total RNA from HepG2 and Huh-7 human liver cancer
cells. The concentration and purity (2.0> OD260/280 >1.8) of
the RNA were determined using a Multiskan SkyHigh nanodrop (Thermo
Fisher Scientific, Inc.). Following the instructions supplied by
the PrimeScript RT reagent kit (Takara Bio, Inc.), 1 μg of
total RNA was used for reverse transcription to synthesize cDNA.
qPCR was then performed using SYBR Green qPCR Master Mix (Takara
Bio, Inc.). The qPCR program was as follows: Pre-denaturation at
95°C for 5 min; and 35 cycles of denaturation at 95°C for 10 sec
and annealing/extension at 60°C for 30 sec. Relative quantification
analysis was performed using the 2−ΔΔCq method with
GAPDH used as the reference gene, and the relative expression
levels of the target genes were calculated and expressed as
2−ΔΔCq (19). The
primers used in the present study were listed as follows: SREBP1
forward, 5'-TGCTTAGCCTCCTGACCTGA-3' and reverse,
5'-GAGGCCCTAAGGGTTGACAC-3'; FAS forward, 5'-CAGTGTACACGTCTGGACCC-3'
and reverse, 5'-AATTGTGGGAGGCTGAGAGC-3'; CD36 forward,
5'-ACATGCTAGCCACTGATCATTTT-3' and reverse,
5'-ACAACTTTGGCACAAGTGCTTT-3'; GLP-1R forward,
5'-TTGGGTGGTGCAACCTTTCT-3' and reverse, 5'-GATGCTGGAGTTGGTCTGCT;
PGC1α forward, 5'-ATTGCCTTCATGCCGTGGTA-3' and reverse,
5'-GCAAGGGCTCAACTAATCGC-3'; and GAPDH forward,
5'-AATGGGCAGCCGTTAGGAAA-3' and reverse,
5'-GCGCCCAATACGACCAAATC-3'.
Detection of ROS levels
HepG2 and Huh-7 liver cancer cells were plated at a
density of 3×105 cells/well in 6-well plates. After
overnight incubation, the medium was discarded, and the cells were
gently washed twice with PBS. Then, 2 ml of 10 μM DCFH-DA
(Beijing Solarbio Science & Technology Co., Ltd.) working
solution was added to each well and incubated at 37°C in the dark
for 30 min. After incubation, the DCFH-DA working solution was
discarded, and the cells were gently washed twice with PBS. The
medium was replaced with serum-free medium. Fluorescent images were
acquired using a fluorescence microscope (Olympus Corporation).
Detection of mitochondrial ROS (mitoROS)
levels
HepG2 and Huh-7 liver cancer cells were seeded in
6-well plates at a density of 3×105 cells/well.
Following an overnight incubation period, the medium was discarded,
and the cells were gently washed twice with PBS. Then, 2 ml of 5
μM MitoSOX Red (Beijing Solarbio Science & Technology
Co., Ltd.) working solution was added to each well, followed by
incubation at 37°C in the dark for 30 min. Following incubation,
the cells were gently washed twice with PBS, and the medium was
replaced with 2 ml of serum-free media after the MitoSOX Red
working solution was removed. Fluorescent images were acquired
using a fluorescence microscope (Olympus Corporation).
Detection of the mitochondrial membrane
potential (MMP)
HepG2 and Huh-7 liver cancer cells were seeded in
6-well plates at a density of 3×105 cells/well.
Following an overnight incubation period, the medium was discarded,
and the cells were gently washed twice with PBS. Next, 2 ml of 5
μg/ml JC-1 working solution (Beijing Solarbio Science &
Technology Co., Ltd.) was added to each well, followed by
incubation for 20 min at 37°C in the dark. After incubation, the
JC-1 working solution was discarded, and the medium was replaced
with serum-free medium. The fluorescent JC-1 signals in the cells
were detected using a fluorescence microscope (Olympus
Corporation).
Western blot analysis
The HepG2 and Huh-7 liver cancer cells or liver
tissues were collected and lysed using a RIPA lysis solution
(Beijing Solarbio Science & Technology Co., Ltd.) on ice for 30
min. The samples were subsequently centrifuged for 10 min at 4°C
and 12,000 × g. The protein concentration was measured using a BCA
kit (Beijing Solarbio Science & Technology Co., Ltd.). Protein
samples (20 μg/lane) were separated by 12% SDS-PAGE. After
being transferred, the PVDF membrane was blocked for 1 h at room
temperature in 5% non-fat milk. After blocking, the membrane was
washed three times with TBST (0.1% Tween-20, 5 min each wash). The
membrane was incubated overnight at 4°C with specific primary
antibodies: anti-PGC1α (cat. no. ab176328; 1:1,000), anti-GLP1R
(cat. no. ab218532; 1:1,000) and anti-GAPDH (cat. no. ab8245;
1:5,000; all purchased from Abcam). The membrane was then incubated
at room temperature for 1 h in antibody dilution buffer containing
HRP-conjugated secondary antibodies (SE205; cat. no. K21001M-HRP;
Beijing Solarbio Science & Technology Co., Ltd.). The membrane
was then washed three times with TBST (10 min each wash). The
proteins in the membrane were detected via ECL reagent (Beijing
Solarbio Science & Technology Co., Ltd.). ImageJ version 2
(National Institutes of Health) was used to assess the band
intensity, and GAPDH was used as a loading control.
Establishment of animal model
In this experiment, a total of 45 8-week-old male
C57BL/6J mice [weight, 20-22 g; SiPeiFu, (Beijing) Biotechnology
Co., Ltd.] were randomly assigned to 9 experimental groups, with 5
mice per group. The first phase of the experiment included the
following groups: i) control group (Con), in which the mice were
fed a standard chow diet with daily oral vehicle (10% DMSO/corn
oil, 200 μl) for 8 weeks; ii) HFD group, in which the mice
were fed a high-fat diet (HFD; 45% fat, D12451, Research Diets,
Inc.) for 8 weeks to induce MASLD and then subsequently fed a daily
oral vehicle (10% DMSO/corn oil, 200 μl) for 8 weeks; iii)
HFD + HP-B group, in which the mice were fed a HFD for 8 weeks,
followed by oral administration of HP-B (20 mg/kg/day; total volume
of 200 μl) for 8 weeks (20); iv) HFD + IF group, in which the
mice were fed a HFD for 8 weeks, followed by IF for 8 weeks (two
24-h fasts/week: Wed 9AM→Thu 9AM and Fri 9AM→Sat 9AM) with daily
oral administration of vehicle (10% DMSO/corn oil, 200 μl);
and v) HFD + IF + HP-B group, in which the mice were fed a HFD for
8 weeks, followed by combined HP-B administration (20 mg/kg/day)
and IF for 8 weeks.
In the second phase (initiated after 8 weeks of HFD
induction), the mice received a single tail vein injection of
adenovirus (Day 0), followed by 8 weeks of intervention. To ensure
sustained GLP-1R knockdown, Ad-sh-GLP-1R [5'-AGT
GTCTGAAGCCAACAAGGA-3', designed against the mouse GLP-1R mRNA
(NM_021332)] and an adenoviral vector expressing a non-targeting
scrambled shRNA sequence (Ad-NC, 5'-TTCTCCGAACGTGTCACGT-3') were
readministered at Week 4. The second phases consisted of the
following groups: i) HFD + Ad-NC group, in which the mice were
injected with Ad-NC (100 μl, 1011 pfu/ml) on Day
0 and Week 4, followed by daily oral administration of vehicle (10%
DMSO/corn oil, 200 μl) for 8 weeks; ii) HFD + IF + HP-B +
Ad-NC group, in which the mice were injected with Ad-NC on Day 0
and Week 4, followed by continued IF + HP-B treatment (20
mg/kg/day) for 8 weeks; iii) HFD + sh-GLP-1R group, in which the
mice were injected with Ad-sh-GLP-1R (100 μl,
1011 pfu/ml) on Day 0 and Week 4, followed by daily oral
administration of vehicle for 8 weeks; and iv) HFD + IF + HP-B +
Ad-GLP-1R group, in which the mice were injected with Ad-shPGC1α on
Day 0 and Week 4, followed by IF + HP-B treatment for 8 weeks.
The mice were housed under standard laboratory
conditions with free access to water at a temperature of 24°C and a
humidity of 45%, with a 12/12-h light/dark cycle. Anesthesia was
induced with 3-5% isoflurane, and deep anesthesia was confirmed by
the absence of a response to pain stimuli; 2% isoflurane was used
to maintain anesthesia during the procedures. At the end of the
experimental period, all mice were euthanized under deep anesthesia
with isoflurane (5% in oxygen), followed by cervical dislocation to
ensure death. Death was confirmed by the cessation of heartbeat and
respiratory movement, as well as the loss of reflexes.
In the first phase of the experiment, all the mice
were sacrificed at the end of the 8-week HFD treatment, and blood
and liver tissue samples were collected for analysis. For the IF
group all the mice were sacrificed within 48 h after the last
fasting cycle. The mice from all the groups were sacrificed at the
end of the experiment (after 8 weeks). Blood samples were collected
from the heart, and liver samples were obtained. In addition, liver
weights were recorded. The body weights were measured regularly,
and the liver index (liver weight/body weight) was calculated.
Additionally, liver histology, serum marker assessment of liver
function, and gene/protein expression analysis were performed to
assess the effects of the treatments. The present study was
conducted in strict accordance with ethical guidelines for animal
research and all applicable national regulations. The experimental
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of Beijing University of Aeronautics and
Astronautics (approval no. 2023-KY-064-1; Beijing, China). All
procedures were designed to minimize animal suffering, and humane
care was provided throughout the study. In addition, all the animal
experiments complied with the ARRIVE guidelines and the NIH Guide
for the Care and Use of Laboratory Animals.
Insulin tolerance test (ITT)
ITT was conducted by first administering an
intraperitoneal (IP) injection of insulin at a dose of 1 U/kg body
weight to induce insulin action. Blood samples were then collected
from the tail tip at 0, 15-, 30-, 45-, 60- and 90-min
post-injection to measure blood glucose levels using a portable
glucometer.
Glucose tolerance test (GTT)
Prior to the GTT, the mice were fasted for 6 h (with
free access to water) to standardize the baseline blood glucose
levels. The baseline blood glucose value was measured at 0 min
(pre-injection) via tail-tip blood sampling. A sterile glucose
solution (20% w/v) was then administered via IP injection at a dose
of 2 g/kg body weight. Blood samples were subsequently collected
from the tail tip at 15-, 30-, 45, 60- and 120-min post-glucose
loading, and blood glucose levels were analyzed at each time point
using a portable glucometer.
H&E staining
The liver tissues were collected and fixed for 24 h
in a 4% paraformaldehyde solution (Beijing Solarbio Science &
Technology Co., Ltd.). The fixed liver tissues were then
dehydrated, embedded in paraffin and sectioned into 6-μm
thick sections. The sections were sequentially immersed in xylene
and a gradient of ethanol solutions for deparaffinization and
rehydration. The sections were then washed with tap water after
being stained for 5 min in a solution of hematoxylin (Beijing
Solarbio Science & Technology Co., Ltd.). The sections were
then stained with an eosin solution (Beijing Solarbio Science &
Technology Co., Ltd.) for 1 min and rinsed with tap water. After
staining, the sections were dehydrated by sequential immersion in
70% ethanol (2 min), 80% ethanol (2 min), 95% ethanol (2 min), 100%
ethanol (twice, 2 min each), and xylene (twice, 5 min each).
Finally, the dehydrated sections were mounted with neutral balsam
and covered with coverslips. The stained sections were observed
under a light microscope (Olympus Corporation).
Determination of aspartate
aminotransferase (AST) and alanine aminotransferase (ALT)
levels
The serum levels of AST and ALT were measured in
accordance with the guidelines provided by the AST Assay Kit (cat.
no. C010-2-1) and ALT activity assay kit (cat. no. C009-2-1; both
from Nanjing Jiancheng Bioengineering Institute). The absorbance
was measured at 510 nm using a microplate reader (Thermo Fisher
Scientific, Inc.).
Detection of malondialdehyde (MDA)
levels
MDA levels in liver tissues were measured using MDA
assay kit (cat. no. A003-1-2; Nanjing Jiancheng Bioengineering
Institute) following the manufacturer's instructions.
Small interfering (siRNA)
transfection
HepG2 and Huh-7 liver cancer cells were plated at a
density of 3×105 cells/well in 6-well plates and
cultured overnight. For siRNA transfection, 100 μl of
serum-free culture medium was first added to sterile centrifuge
tubes. Subsequently, 12 μl of a specific siRNA targeting
GLP-1R (si-GLP-1R, 5'-UAUUGGAAAACAAUUAUGCUU-3') or negative control
(NC, 5'-UUCUCCGAACGUGUCACGUAA-3') was added to achieve a final
concentration of 20 nM siRNA. In another tube, 12 μl of
HiPerFect Transfection Reagent (Qiagen China Co., Ltd.) was added
to 100 μl of serum-free culture medium. The contents of
these two tubes were gently mixed and incubated at room temperature
for 20 min to form the transfection complex. Next, the mixture was
added to the wells containing the cells at 37°C for 6 h. The
transfection mixture was then removed, and fresh DMEM containing
10% FBS was added, followed by incubation for 24 h.
Statistical analysis
The data are presented as the mean ± standard
deviations (SDs). Two-tailed unpaired Student's t-tests were used
to compare two groups, and one-way analysis of variance followed by
Tukey's post hoc test was used to compare three or more groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HP-B and fasting work together to
counteract OA/PA-induced lipid accumulation in liver cells
CCK-8 analysis revealed that treatment with 10, 25
and 50 μM HP-B did not affect the viability of HepG2 or
Huh-7 liver cancer cells, whereas treatment with 75, 100 and 200
μM HP-B reduced the viability of both cell types (Fig. 1A and B). Therefore, 50 μM
HP-B was used in subsequent experiments. Oil Red O staining
revealed that OA/PA treatment markedly increased lipid accumulation
in both cell types (Fig. 1C and
D). However, HP-B treatment effectively attenuated
OA/PA-induced lipid accumulation. Similarly, in HepG2 and Huh-7
liver cancer cells, fasting alone restored OA/PA-induced lipid
accumulation, but HP-B treatment in addition to fasting
significantly decreased lipid accumulation (Fig. 1C and D). After OA/PA treatment,
quantitative analysis revealed that HepG2 and Huh-7 liver cancer
cells had higher TC and TG levels than the control cells (Fig. 1E-H). Moreover, the increased TC
and TG levels caused by OA/PA were reversed by HP-B treatment and
fasting, and the combined treatment had a greater effect (Fig. 1E-H). Additionally, the mRNA
levels of genes associated with lipid production and absorption
(SREBP1, FAS and CD36) in HepG2 and Huh-7 liver cancer cells were
quantitatively examined. OA/PA treatment significantly increased
the mRNA levels of these genes compared with the control group
(Fig. 1I and J). However, the
OA/PA-induced increase in the mRNA levels of these genes was
reduced by HP-B treatment and fasting, and the combination
treatment had a more significant effect (Fig. 1I and J). In conclusion,
OA/PA-induced lipid accumulation and related gene upregulation in
HepG2 and Huh-7 liver cancer cells are successfully reversed by
both HP-B treatment and fasting, with their combined use showing
increased efficiency.
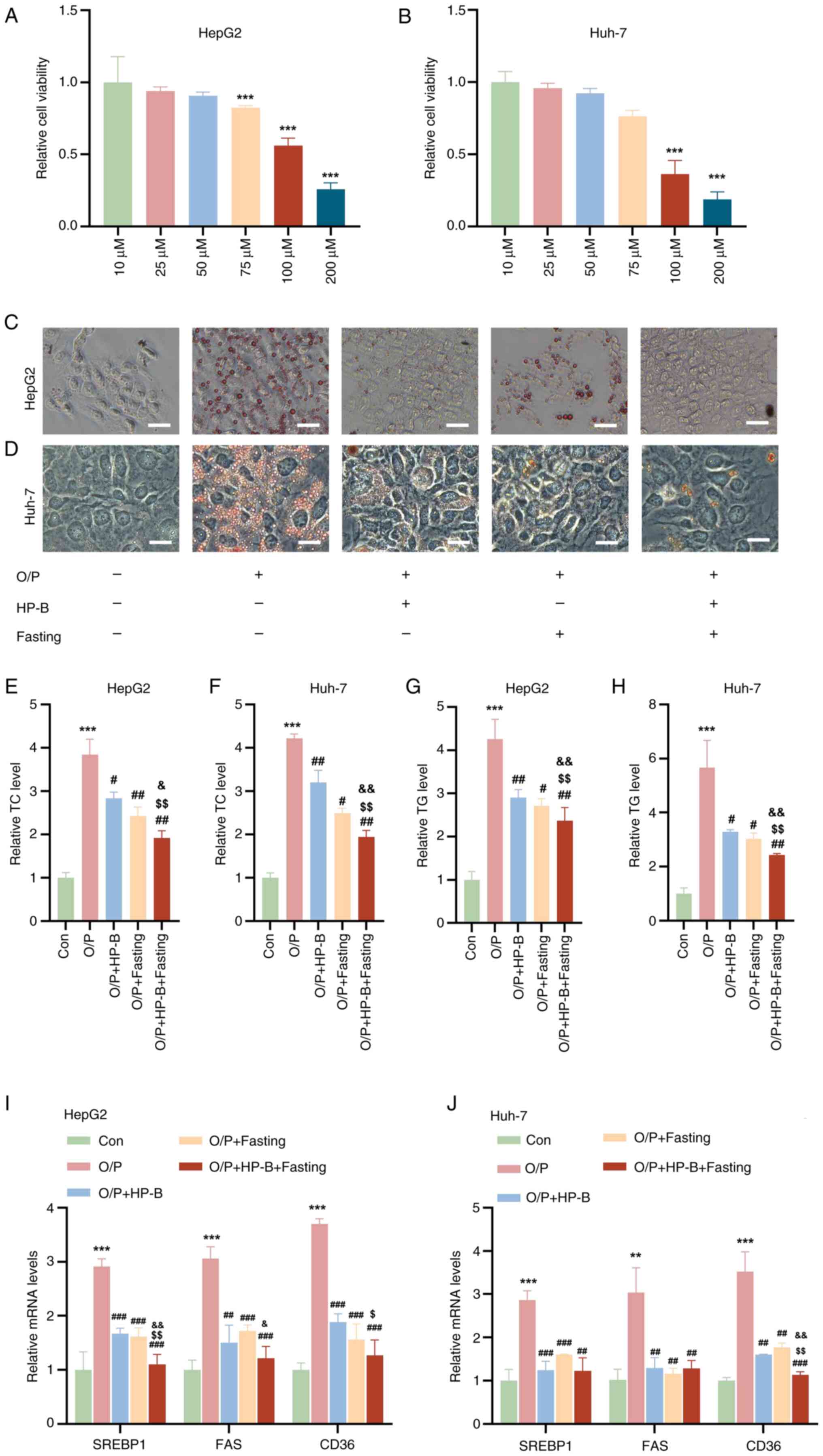 | Figure 1Effects of HP-B treatment and fasting
on OA/PA-induced lipid accumulation and gene expression in HepG2
and Huh-7 liver cancer cells. (A and B) Cell Counting Kit-8
analysis revealed that 10, 25 and 50 μM HP-B did not
significantly alter cell viability, whereas 75, 100 and 200
μM HP-B reduced cell viability in HepG2 and Huh-7 cells. (C
and D) Oil Red O staining of HepG2 and Huh-7 liver cancer cells
treated with OA/PA revealed significant lipid accumulation compared
with the Con group. Both HP-B treatment and fasting reversed
OA/PA-induced lipid accumulation, and the combined HP-B and fasting
treatment further reduced lipid accumulation (Scale bar, 10
μm). (E-H) Quantitative analysis of TC and TG levels in
HepG2 and Huh-7 liver cancer cells. (I and J) Quantitative analysis
of the mRNA levels of lipid synthesis- and uptake-related genes
(SREBP1, FAS and CD36) in HepG2 and Huh-7 liver cancer cells.
**P<0.01 and ***P<0.001 vs. Con;
#P<0.05, ##P<0.01 and
###P<0.001 vs. OA/PA; $P<0.05 and
$$P<0.01 vs. OA/PA + HP-B; &P<0.05
and &&P<0.01 vs. OA/PA + Fasting. HP-B,
heterophyllin B; OA/PA, oleic acid/palmitic acid; TC, total
cholesterol; TG, triglycerides. |
Combined HP-B and fasting treatment
reverses the OA/PA-induced decrease in the MMP
Assessment of the ROS levels in HepG2 and Huh-7
liver cancer cells revealed that OA/PA treatment increased the ROS
levels compared with those in the Con group. However, HP-B
treatment and fasting substantially reduced this increase in ROS
levels in both the HepG2 and Huh-7 liver cancer cells (Fig. 2A-D). Notably, the combined
treatment of HP-B and fasting further reduced the ROS levels
(Fig. 2A-D). Compared with the
controls, OA/PA treatment significantly increased the levels of
mitoROS in HepG2 and Huh-7 liver cancer cells. Both HP-B treatment
and fasting reduced the increase in mitoROS levels caused by OA/PA,
and the combined treatment resulted in a more significant reduction
(Fig. 2E-H). Additionally, OA/PA
treatment significantly decreased the MMP in HepG2 and Huh-7 liver
cancer cells. Both HP-B treatment and fasting reversed the
OA/PA-induced reduction in the MMP, and the combined treatment
further ameliorated this decrease (Fig. 2I and J).
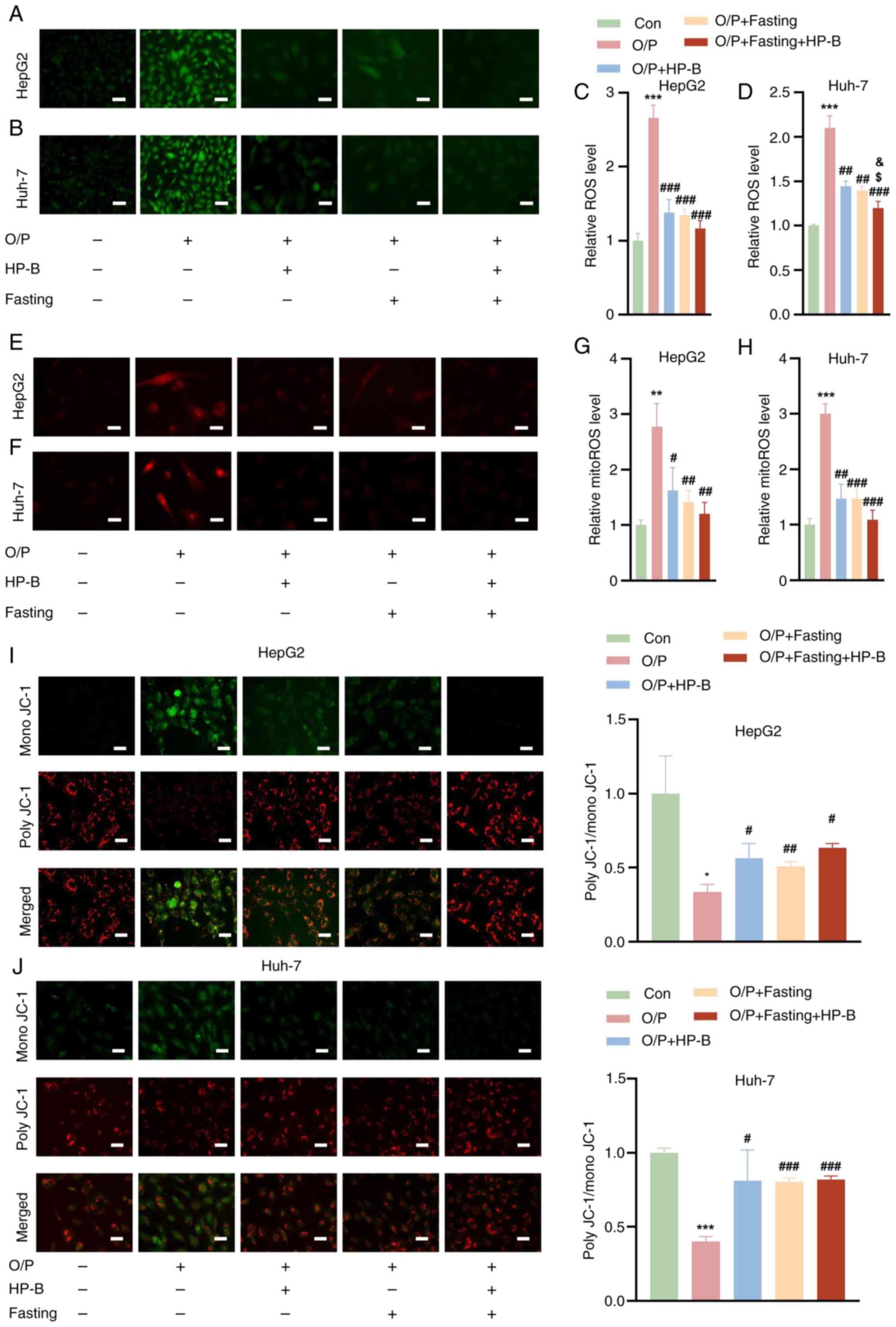 | Figure 2Effects of HP-B treatment and fasting
on OA/PA-induced changes in ROS, mitoROS, and the MMP in HepG2 and
Huh-7 liver cancer cells. (A and B) Representative images of the
ROS levels in (A) HepG2 and (B) Huh-7 cells are shown. (C and D)
Quantitative analysis of ROS levels in (C) HepG2 and (D) Huh-7
cells. (E and F) Representative images and quantification of
mitoROS levels in (E) HepG2 and (F) Huh-7 cells are shown. (G and
H) Quantitative analysis of mitoROS levels in (G) HepG2 and (H)
Huh-7 cells. (I and J) Representative images and quantification of
the MMP in (I) HepG2 and (J) Huh-7 cells are shown. Scale bar, 10
μm. *P<0.05, **P<0.01 and
***P<0.001 vs. Con; #P<0.05,
##P<0.01 and ###P<0.001 vs. OA/PA;
$P<0.05 vs. OA/PA + HP-B; &P<0.05
vs. OA/PA + Fasting. HP-B, heterophyllin B; OA/PA, oleic
acid/palmitic acid; ROS, reactive oxygen species; mitoROS,
mitochondrial ROS; MMP, mitochondrial membrane potential. |
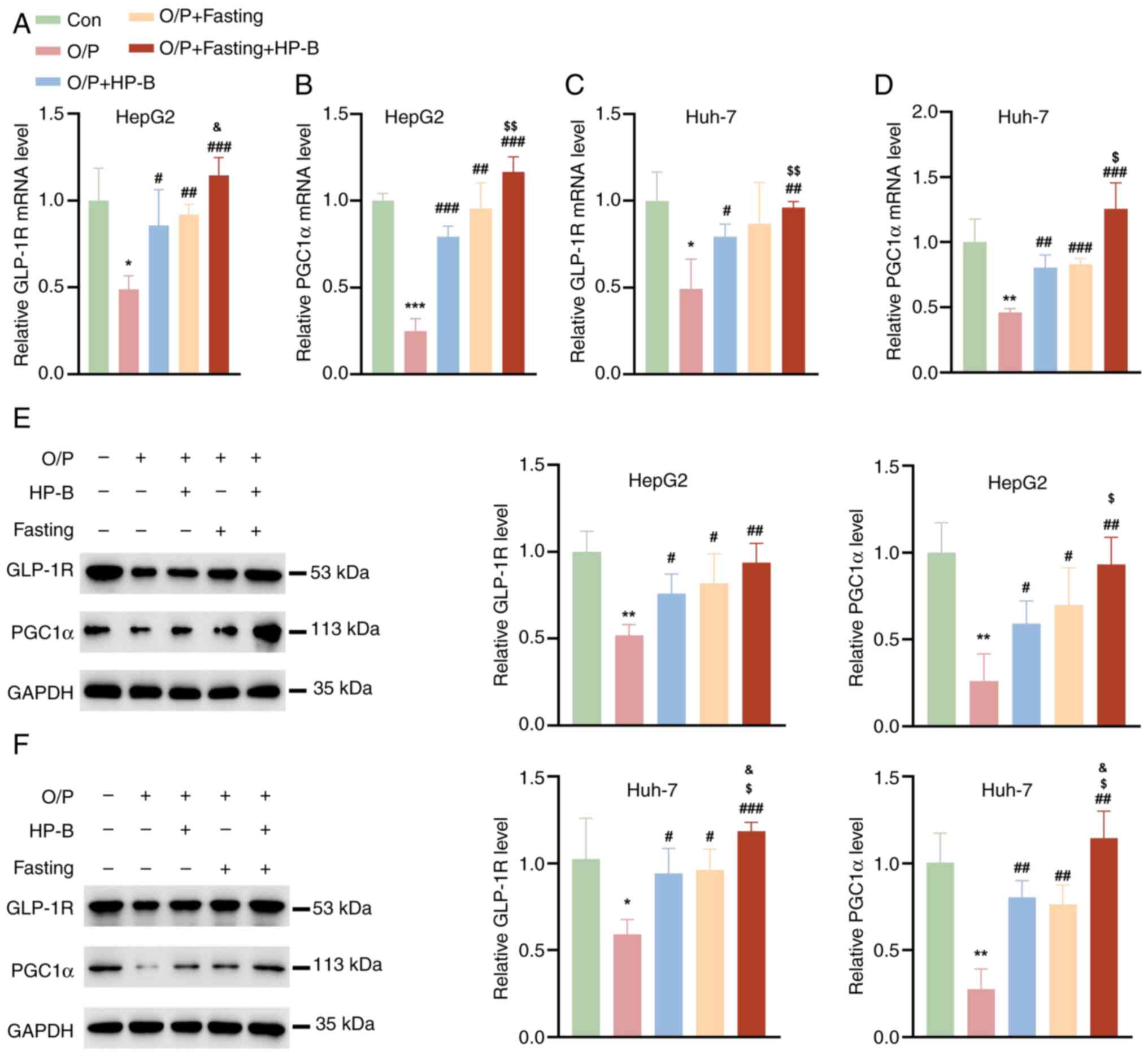
Combined HP-B and fasting treatment
reverses the OA/PA-induced decrease in GLP-1R and PGC1α
To evaluate the effects of HP-B and fasting on the
expression of GLP-1R and PGC1α, the mRNA levels of GLP-1R and PGC1α
were examined in HepG2 and Huh-7 liver cancer cells. Compared with
the Con group, OA/PA significantly decreased the mRNA levels of
GLP-1R and PGC1α, whereas HP-B treatment and fasting reversed the
OA/PA-induced reduction in the mRNA levels of GLP-1R and PGC1α
(Fig. 3A-D). More importantly,
the combination of HP-B treatment and fasting further increased the
mRNA levels of GLP-1R and PGC1α (Fig. 3A-D). Evaluation of the GLP-1R and
PGC1α protein expression levels in HepG2 and Huh-7 liver cancer
cells revealed that OA/PA treatment significantly decreased the
protein expression of GLP-1R and PGC1α (Fig. 3E and F). However, HP-B treatment
or fasting reversed the OA/PA-induced decrease in the protein
expression of GLP-1R and PGC1α, and the combined treatment of HP-B
and fasting was more effective (Fig.
3E and F).
Combined HP-B and fasting treatment
reverses lipid accumulation in the livers of HFD-fed mice
Compared with the Con group, the HFD group presented
significantly impaired glucose tolerance and decreased insulin
sensitivity, indicating HFD-induced IR (Fig. 4A and B). Both interventions
improved glucose metabolism: Fasting monotherapy reduced GTT area
under the curve (AUC) by 19.96 (vs. HFD: 96.84-76.88). HP-B
monotherapy reduced GTT AUC by 32.69 (vs. HFD: 96.84-64.15).
Similarly, ITT AUC decreased by 13.10 with fasting and 9.47 with
HP-B alone (vs. HFD: 40.84) (Fig.
4B). Critically, combination therapy reduced GTT AUC by 61.91
(vs. HFD: 96.84-34.93) and decreased ITT AUC by 26.81 (vs. HFD:
40.84-14.03). This exceeded the sum of individual effects,
specifically, GTT: 61.91> (32.69+19.96)=52.65, and ITT:
26.81> (9.47+13.10)=22.57, demonstrating synergistic reversal of
IR (Fig. 4A and B). Furthermore,
the combined HP-B and fasting treatment had a more pronounced
effect, as evidenced by enhanced glucose clearance in the GTT and
greater improvement in insulin sensitivity in the ITT, which
suggested a synergistic effect of HP-B and fasting in mitigating IR
and metabolic dysfunction (Fig. 4A
and B). Compared with those of the control group, the livers of
HFD-fed mice presented a substantial increase in lipid accumulation
(Fig. 4C and D). HP-B treatment
or fasting reduced hepatic lipid accumulation, and the combined
HP-B and fasting further reduced lipid levels (Fig. 4C and D). Compared with those in
the control group, the mice in the HFD group had significantly
greater liver weights, body weights and liver indices (Fig. 4E-G). Fasting effectively reduced
the liver weight, body weight and liver index in HFD-fed mice,
whereas HP-B treatment specifically reduced the liver weight and
liver index (Fig. 4E-G). Hepatic
steatosis was profoundly induced by HFD, with TG content surging to
64.74±7.23 mg/g wet weight vs. 5.127±0.62 mg/g in controls
(Fig. 4H). Monotherapies
partially attenuated this accumulation: HP-B alone reduced TG by
21.65 mg/g (HFD + HP-B: 43.09±1.54 mg/g), while fasting alone
reduced TG by 15.30 mg/g (HFD + Fasting: 49.44±6.113 mg/g).
Critically, the combination therapy achieved a TG reduction of
45.79 mg/g (HFD + Fasting + HP-B: 18.95±3.70 mg/g), significantly
exceeding the additive effect of monotherapies (21.65+15.30=36.95
mg/g) (Fig. 4H). This
enhancement beyond additivity demonstrates synergistic amelioration
of hepatic lipidosis. Additionally, the HFD group presented
significantly elevated levels of AST and ALT compared with those in
the control group. The HP-B and fasting treatments clearly reduced
these indices (Fig. 4I and J).
The protein expression levels of GLP-1R and PGC1α in the livers of
HFD-fed mice were significantly lower than those in the control
group (Fig. 4K). HP-B treatment
or fasting increased the expression of these proteins, and the
combined treatment exerted a more significant effect on PGC1α
protein expression (Fig. 4K).
These findings suggested that the combination of HP-B treatment and
fasting may exert a synergistic effect on hepatic lipid metabolism
and overall liver function.
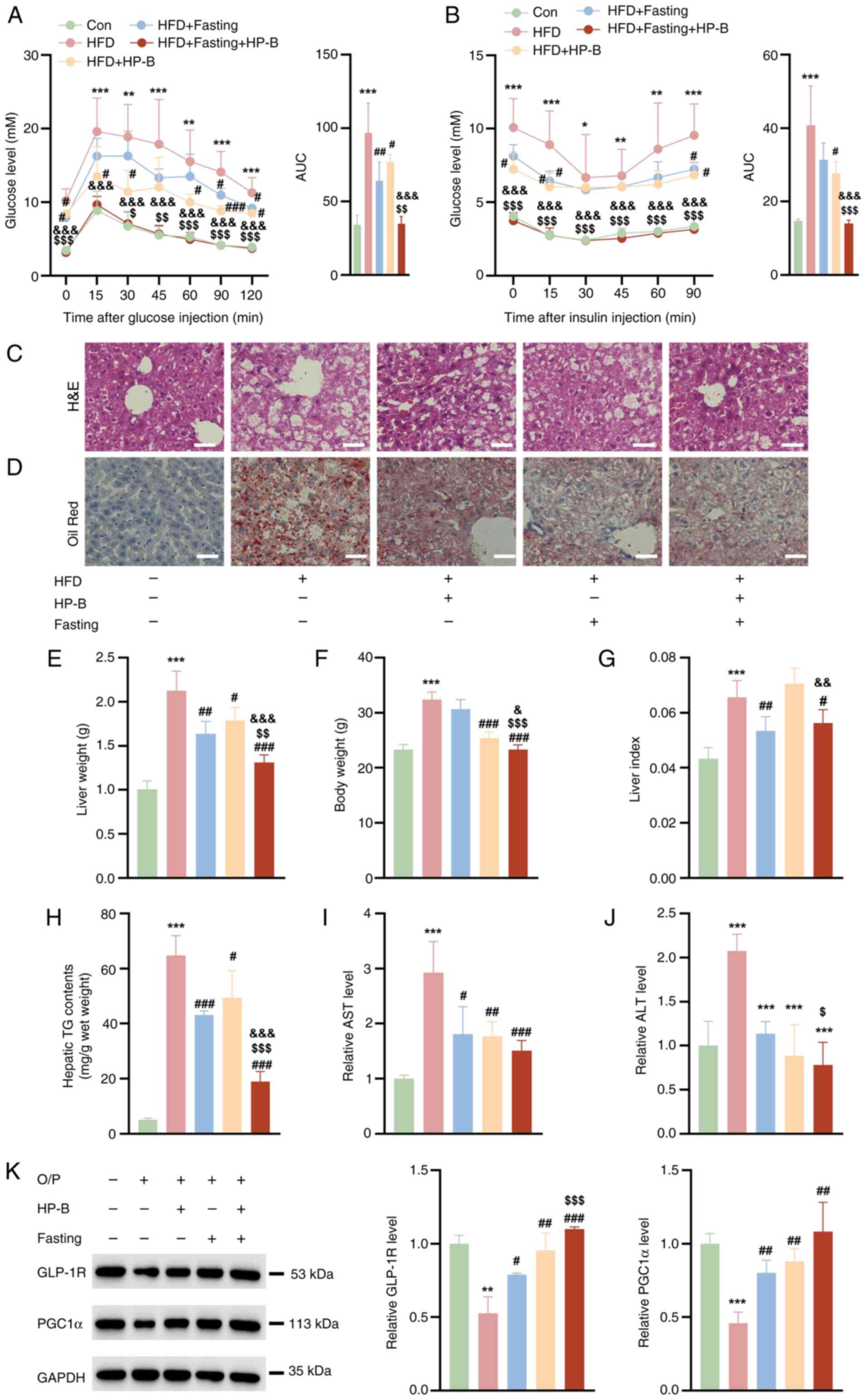 | Figure 4Effects of HP-B treatment and fasting
on lipid accumulation and related parameters in the livers of
HFD-fed mice. (A) Glucose tolerance test and AUC quantification.
(B) Insulin tolerance test and AUC quantification. (C and D)
Representative H&E and Oil Red O images showing that HP-B
treatment or fasting treatment reduced liver lipid accumulation to
some extent, whereas the combined HP-B and fasting treatment
further significantly reduced lipid accumulation (Scale bar, 25
μm). (E-G) HP-B treatment or fasting reduced the liver
weight, body weight and liver index. The combined treatment
resulted in a more pronounced reduction in liver weight and body
weight, but the liver index did not obviously change. (H-J) HP-B
treatment and fasting lowered these levels, and the combined
treatment resulted in a more significant reduction in TG and ALT
levels than HP-B treatment alone, as well as a more significant
reduction in TG levels than fasting alone. (K) HP-B treatment or
fasting increased the protein levels of GLP-1R and PGC1α, and the
combined treatment further increased PGC1α protein expression
compared with HP-B treatment alone. *P<0.05,
**P<0.01 and ***P<0.001 vs. Con;
#P<0.05, ##P<0.01 and
###P<0.001 vs. HFD; $P<0.05,
$$P<0.01 and $$$P<0.001 vs. HFD + HP-B;
&P<0.05, &&P<0.01 and
&&&P<0.001 vs. HFD + Fasting. HP-B,
heterophyllin B; HFD, high-fat diet; AUC, area under the curve; TG,
triglycerides; ALT, alanine aminotransferase; AST aspartate
aminotransferase; GLP-1R, glucagon-like peptide-1 receptor; PGC1α,
peroxisome proliferator-activated receptor gamma coactivator
1-alpha. |
GLP-1R silencing reverses OA/PA-induced
lipid accumulation in HepG2 and Huh-7 liver cancer cells
si-GLP-1R or NC were then transfected into HepG2 and
Huh-7 cells which were not combined with any other treatments.
RT-qPCR analysis showed that the mRNA level of GLP-1R was decreased
in HepG2 and Huh-7 cells compared with those of NC (Fig. 5A and C). To examine whether the
combination of HP-B treatment and fasting ameliorates OA/PA-induced
lipid accumulation in hepatocytes via GLP-1R, siRNAs specifically
targeting SLP-1R were used. Compared with the Con group, si-GLP-1R
significantly reduced GLP-1R expression in HepG2 and Huh-7 liver
cancer cells (Fig. 5B and D).
Moreover, GLP-1R silencing reduced downstream PGC1α protein
expression (Fig. 5B and D).
GLP-1R silencing significantly decreased the protein level of
GLP-1R and suppressed PGC1α expression in HepG2 and Huh-7 liver
cancer cells pretreated with OA/PA (Fig. 5B and D). Oil red O staining
revealed that while OA/PA-induced lipid accumulation was mitigated
by fasting and HP-B treatments, this ameliorative effect was
prevented by PGC1α silencing (Fig.
5E). Furthermore, in HepG2 and Huh-7 liver cancer cells, HP-B
treatment and fasting decreased the TG and TC levels, but GLP-1R
silencing further increased the TG and TC levels in these cells
(Fig. 5F-I). Hence, GLP-1R
silencing restores OA/PA-induced lipid accumulation, suggesting a
possible mechanism by which hepatocyte lipid metabolism is improved
by a combination of HP-B treatment and fasting via GLP-1R.
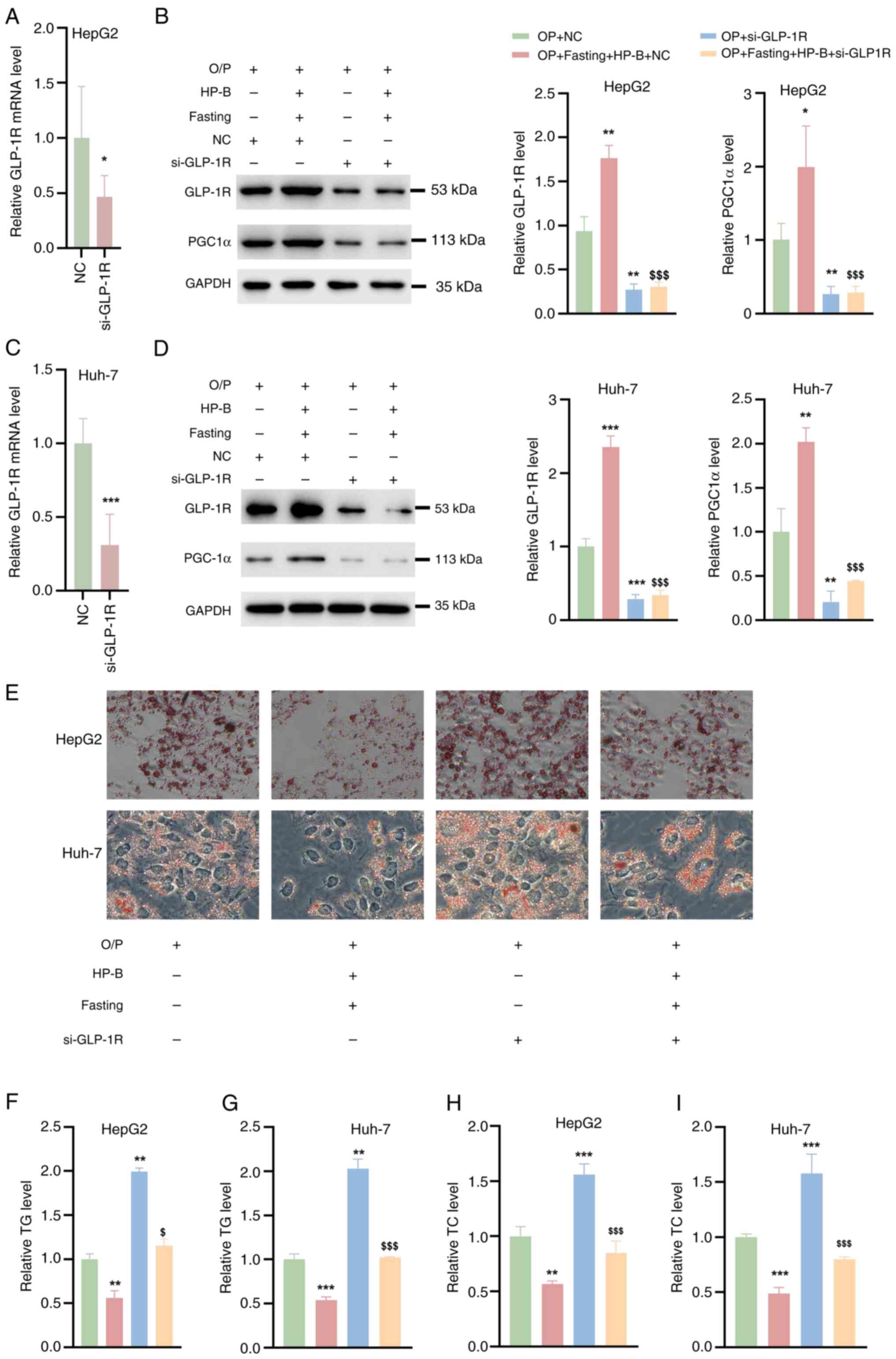 | Figure 5Effects of GLP-1R silencing on
OA/PA-induced lipid accumulation in HepG2 and Huh-7 liver cancer
cells. (A and C) Reverse transcription-quantitative PCR analysis
showed that the mRNA levels of GLP-1R in HepG2 and Huh-7 cells were
reduced after siGLP-1R transfection. (B and D) Western blot
analysis revealed that siGLP-1R silenced GLP-1R expression in OA/PA
pre-treated HepG2 and Huh-7 cells and reversed the OA/PA-induced
increase in PGC1α protein levels. (E) Oil Red O staining
demonstrated that fasting and HP-B treatment reduced lipid
accumulation induced by OA/PA, but this effect was blocked by
GLP-1R silencing. (F-I) GLP-1R silencing further elevated (F and G)
TG and (H and I) TC levels in HepG2 and Huh-7 cells, counteracting
the reductions achieved by fasting and HP-B treatment.
*P<0.05, **P<0.01 and
***P<0.001 vs. OA/PA; $P<0.05 and
$$$P<0.01 vs. OA/PA + HP-B + Fasting. HP-B,
heterophyllin B; GLP-1R, glucagon-like peptide-1 receptor; OA/PA,
oleic acid/palmitic acid; si-, small interfering; TG,
triglycerides; TC, total cholesterol; NC, negative control. |
GLP-1R knockdown reverses the
hepatoprotective effects of fasting and HP-B treatment in HFD-fed
mice
As shown in Fig.
6A, GLP-1R was effectively silenced in the livers of HFD +
Ad-NC and HFD + Fasting + HP-B + Ad-NC mice. GLP-1R silencing
abolished the beneficial effects of HP-B treatment and fasting on
glucose metabolism, as evidenced by impaired GTT and reduced ITT,
with curves approaching those of the HFD group (Fig. 6B and C). Knocking down GLP-1R
abolished the protective benefits of HP-B and fasting in HFD-fed
mice. Compared with HFD + Ad-NC mice alone, Ad-short hairpin
(sh)GLP-1R exacerbated lipid accumulation in the livers of HFD-fed
mice (Fig. 6D and E). Silencing
GLP-1R prevented the combined effect of HP-B treatment and fasting
from reducing lipid droplet deposition, even in HFD-fed animals
subjected to these interventions (Fig. 6D and E). While GLP-1R knockdown
did not significantly affect the body weight of HFD-fed mice, it
increased their liver weight (Fig.
6F and G). Similarly, compared with those in the fasting and
HP-B treatment groups, the liver weights of the GLP-1R-deficient
mice were greater, but their body weights did not appreciably
change (Fig. 6F and G). Compared
with the HFD + Ad-NC group, the hepatic indices of HFD-fed mice
after fasting and HP-B treatment were lower, but these effects were
reversed when GLP-1R was silenced (Fig. 6H). Compared with that in the HFD
+ Ad-NC group, the mouse liver TG content was lower after GLP-1R
knockdown, but this effect was minimized with fasting and HP-B
treatment after silencing GLP-1R (Fig. 6I). Additionally, GLP-1R silencing
reversed the reductions in ALT and AST levels induced by fasting
and HP-B treatment (Fig. 6J and
K). Compared with those in the HFD + Ad-NC group, the MDA and
ROS levels in the livers of HFD-fed mice were lower after fasting
and HP-B treatment (Fig. 6L and
M). Conversely, Ad-shGLP-1R injections into the tail vein of
HFD-fed mice resulted in elevated levels of MDA and ROS in their
livers (Fig. 6L and M). These
findings indicated that GLP-1R is essential for the metabolic
improvements mediated by HP-B treatment and fasting.
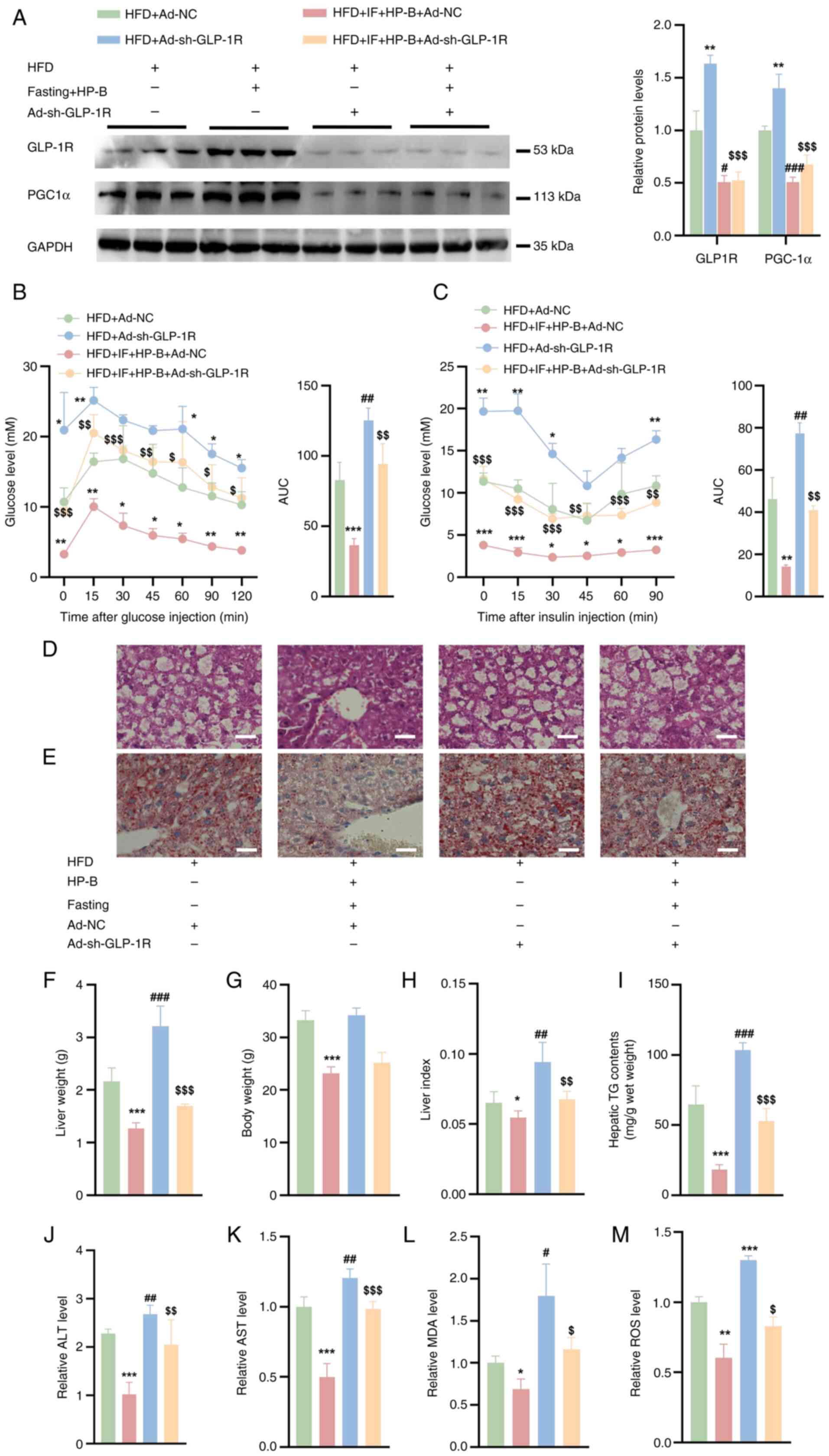 | Figure 6GLP-1R knockdown impacts fasting and
HP-B treatment in HFD + Ad-NC mice. (A) Western blot analysis
showed that the expression of GLP-1R was decreased in the livers of
HFD mice. (B and C) Glucose tolerance test and insulin tolerance
test. (D and E) H&E staining and Oil Red O staining of liver
sections from HFD-fed mice (Scale bar, 25 μm). (F and G)
Liver weights and body weights of HFD-fed mice. (H) Compared with
the oil/control treatment, fasting and HP-B treatment reduced the
liver index, whereas GLP-1R knockdown reversed this effect. (I)
GLP-1R knockdown reduced the TG content compared with that in
HFD-fed mice, and this effect was counteracted by fasting and HP-B
treatment. (J and K) Fasting and HP-B treatment decreased the ALT
and AST levels, and these reductions were reversed by GLP-1R
knockdown. (L and M) Fasting and HP-B treatment reduced the MDA and
ROS levels, whereas Ad-shGLP-1R injection increased these levels.
*P<0.05, **P<0.01 and
***P<0.001 vs. HFD + Ad-NC; #P<0.05,
##P<0.01, ###P<0.001 vs. HFD +
Ad-sh-GLP-1R; $P<0.05, $$P<0.01 and
$$$P<0.001 vs. HFD + HP-B + IF. GLP-1R, glucagon-like
peptide-1 receptor; HP-B, heterophyllin B; HFD, high-fat diet; NC,
negative control; TG, triglycerides; ALT, alanine aminotransferase;
AST aspartate aminotransferase; MDA, malondialdehyde; ROS, reactive
oxygen species; sh-, short hairpin. |
Discussion
Previous studies have demonstrated that IF
effectively improves MASLD by modulating metabolism, reducing fat
accumulation, and mitigating inflammatory responses (5,21). However, prolonged fasting may
lead to potential side effects, such as hypoglycemia and
nutritional deficiencies, and the long-term efficacy remains to be
further validated (22).
Therefore, the inclusion of natural compounds with hepatoprotective
properties, such as HP-B, may enhance the therapeutic effects of IF
while mitigating its adverse outcomes. HP-B has significant
anti-inflammatory, antioxidant and immunomodulatory activities, and
antitumor effects, making it a promising candidate for improving
liver function and providing additional benefits in the management
of MASLD (14-16). Further investigation into the
synergistic effects of HP-B treatment combined with IF is warranted
to optimize treatment strategies for MASLD.
In the context of metabolic disease therapeutics,
GLP-1R agonists have emerged as key pharmacologic agents because of
their multifaceted benefits in glycemic control, weight reduction
and cardiovascular risk mitigation (23,24). These strategies offer distinct
advantages as follows: i) multitarget synergy enables greater
weight loss and comprehensive metabolic restoration, and ii)
potential to delay or reverse multiorgan pathologies such as MASLD.
Nevertheless, co-agonists present several challenges. Tolerability
remains a concern, as effective doses in animal models frequently
induce gastrointestinal adverse events (for example, nausea and
vomiting) in humans, which are particularly problematic for
GLP-1-based therapies. Additionally, the energy expenditure
mechanisms mediated via sympathetic nervous system activation and
brown adipose tissue thermogenesis are robust in rodents but often
fail to translate clinically (23,24). Further complexities include
intricate mechanism-of-action profiles, difficulties in optimizing
receptor activation ratios, and undefined cardiovascular safety
risks.
Previous research on HP-B has focused primarily on
its anti-inflammatory, antioxidant and immunomodulatory activities,
with applications largely confined to tumor suppression,
neurodegenerative diseases and cognitive disorders (16,20,25). However, the potential therapeutic
value of HP-B in MASLD has received limited attention. The present
study provides several novel contributions. First, the present
study provides the first experimental evidence supporting the
synergistic efficacy of HP-B treatment combined with IF in
alleviating MASLD-related metabolic disturbances. Second, the
present findings revealed a previously uncharacterized mechanism by
which HP-B exerts its metabolic benefits. Specifically,
GLP-1R-knockdown models confirmed the central role of the
GLP-1R/PGC1α signaling axis in mediating the protective effects of
HP-B. These findings significantly broaden the pharmacological
profile of HP-B and suggest a new conceptual and therapeutic
framework for MASLD intervention.
Previous studies have shown that the activation of
GLP-1R regulates lipid metabolism and mitochondrial homeostasis via
the AMPK/PGC1α signaling axis (26,27). For example, in a spinal cord
injury model, the exendin9-39 GLP-1R antagonist and the SR18292
PGC1α inhibitor have been used to functionally validate the
GLP-1R/AMPK/PGC1α signaling pathway (26). Similarly, in obesity-related
chronic kidney disease models, the liraglutide GLP-1R agonist
reduces renal lipid deposition and restores mitochondrial function
through the Sirt1/AMPK/PGC1α axis (27). These studies collectively suggest
that AMPK may act as a critical intermediary linking GLP-1R
activation to PGC1α-mediated metabolic protection. To further
validate this hypothesis in the context of HP-B and IF, the authors
plan to employ pharmacologic inhibitors of AMPK (for example,
Compound C) and generate conditional AMPK knockout models. These
strategies will help elucidate the downstream signaling network of
GLP-1R and clarify the mechanistic basis of HP-B-mediated metabolic
regulation.
To ensure the reproducibility and physiological
relevance of the present results, a well-structured and widely
accepted 5:2 IF protocol was adopted. Prior studies have shown that
this regimen activates glucocorticoid (GC) signaling, which
contributes to metabolic reprogramming by upregulating Pck1
expression and enhancing fatty acid oxidation and gluconeogenesis
(4). Importantly, this GC
activation is considered an adaptive physiological response rather
than a marker of stress pathology. In support of this notion, 5:2
IF has been reported to have minimal effects on anxiety-like
behaviors or adult hippocampal neurogenesis in mice (28), suggesting that it is behaviorally
and neurologically well tolerated. Although the present study did
not include direct measurements of serum corticosterone levels at
fasting endpoints due to logistical limitations, the authors plan
to incorporate such assessments in future experiments to
systematically evaluate stress-related responses and control for
their potential confounding impact.
Despite these promising findings, the present study
has certain limitations. Although reproducible, the OA/PA-induced
in vitro model of hepatic lipid accumulation may not fully
capture the complexity of MASLD pathogenesis. In future studies, it
is intended to incorporate additional proinflammatory stimuli, such
as TNF-α or lipopolysaccharide, to better mimic the inflammatory
milieu of the disease and increase the robustness of the findings.
Although GLP-1R knockdown significantly attenuated the beneficial
effects of HP-B treatment and IF, it did not completely abolish
them, which suggests the existence of GLP-1R-independent pathways,
potentially involving direct AMPK activation or the modulation of
oxidative stress responses. Thus, further investigation is
warranted. In addition, the present study did not assess the
pharmacokinetic properties of HP-B, such as its bioavailability,
half-life and tissue distribution. These parameters are crucial for
understanding the in vivo efficacy and safety profile of
HP-B. Without specific data, it is challenging to predict its
behavior in biological systems accurately. Future studies should
aim to characterize the pharmacokinetic parameters of HP-B to
better understand its therapeutic potential and optimize dosing
regimens. Moreover, while our data demonstrate functional
interaction between HP-B and the GLP-1R pathway, it is acknowledged
that direct molecular binding remains uncharacterized. To address
this mechanistic gap, molecular docking or receptor binding assays
will be carried out as future directions.
In summary, the present study elucidates the
mechanism by which HP-B treatment improves lipid metabolism,
protects mitochondrial function, and alleviates oxidative stress
through activation of the GLP-1R/PGC1α axis. The combination of
HP-B treatment and IF has significant synergistic effects and
offers a low-toxicity, mechanistically grounded intervention
strategy for the treatment of MASLD.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KL performed the experiments and wrote the
manuscript. LD and LX performed the experiments and interpreted the
data. SY supervised the project, interpreted the data, and wrote
the manuscript. KL and SY confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The experimental protocol was reviewed and approved
by the Institutional Animal Care and Use Committee (IACUC) of
Beijing University of Aeronautics and Astronautics (approval no.
2023-KY-064-1; Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing University of
Chemical Technology-China-Japan Friendship Hospital Biomedical
Transformation Engineering Research Center Joint Fund Project
(grant no. XK2022-07).
References
|
1
|
Marjot T, Tomlinson JW, Hodson L and Ray
DW: Timing of energy intake and the therapeutic potential of
intermittent fasting and time-restricted eating in NAFLD. Gut.
72:1607–1619. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vasim I, Majeed CN and DeBoer MD:
Intermittent fasting and metabolic health. Nutrients. 14:6312022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patikorn C, Roubal K, Veettil SK, Chandran
V, Pham T, Lee YY, Giovannucci EL, Varady KA and Chaiyakunapruk N:
Intermittent fasting and obesity-related health outcomes: An
umbrella review of meta-analyses of randomized clinical trials.
JAMA Netw Open. 4:e21395582021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gallage S, Ali A, Barragan Avila JE,
Seymen N, Ramadori P, Joerke V, Zizmare L, Aicher D, Gopalsamy IK,
Fong W, et al: A 5:2 intermittent fasting regimen ameliorates NASH
and fibrosis and blunts HCC development via hepatic PPARα and PCK1.
Cell Metab. 36:1371–1393.e7. 2024. View Article : Google Scholar
|
|
5
|
Ezpeleta M, Gabel K, Cienfuegos S, Kalam
F, Lin S, Pavlou V, Song Z, Haus JM, Koppe S, Alexandria SJ, et al:
Effect of alternate day fasting combined with aerobic exercise on
non-alcoholic fatty liver disease: A randomized controlled trial.
Cell Metab. 35:56–70.e3. 2023. View Article : Google Scholar :
|
|
6
|
Wang X, Wang J, Ying C, Xing Y, Su X and
Men K: Fenofibrate alleviates NAFLD by enhancing the PPARα/PGC-1α
signaling pathway coupling mitochondrial function. BMC Pharmacol
Toxicol. 25:72024. View Article : Google Scholar
|
|
7
|
Wu L, Mo W, Feng J, Li J, Yu Q, Li S,
Zhang J, Chen K, Ji J, Dai W, et al: Astaxanthin attenuates hepatic
damage and mitochondrial dysfunction in non-alcoholic fatty liver
disease by up-regulating the FGF21/PGC-1α pathway. Br J Pharmacol.
177:3760–3777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quan Y, Shou D, Yang S, Cheng J, Li Y,
Huang C, Chen H and Zhou Y: Mdivi1 ameliorates mitochondrial
dysfunction in non-alcoholic steatohepatitis by inhibiting JNK/MFF
signaling. J Gastroenterol Hepatol. 38:2215–2227. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng D, Zhang M, Zheng Y, Wang M, Gao Y,
Wang X, Liu X, Lv W, Zeng X, Belosludtsev KN, et al:
α-Ketoglutarate prevents hyperlipidemia-induced fatty liver
mitochondrial dysfunction and oxidative stress by activating the
AMPK-pgc-1α/Nrf2 pathway. Redox Biol. 74:1032302024. View Article : Google Scholar
|
|
10
|
Zhao Q, Liu J, Deng H, Ma R, Liao JY,
Liang H, Hu J, Li J, Guo Z, Cai J, et al: Targeting
mitochondria-located circRNA SCAR alleviates NASH via reducing mROS
output. Cell. 183:76–93.e22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Xu Q, Cao Y, Zhang C, Chen S,
Zhang Y and Liang T: Luteolin ameliorates hepatic steatosis and
enhances mitochondrial biogenesis via AMPK/PGC-1α pathway in
western diet-fed mice. J Nutr Sci Vitaminol (Tokyo). 69:259–267.
2023. View Article : Google Scholar
|
|
12
|
Yang Y, Qiu W, Xiao J, Sun J, Ren X and
Jiang L: Dihydromyricetin ameliorates hepatic steatosis and insulin
resistance via AMPK/PGC-1α and PPARα-mediated autophagy pathway. J
Transl Med. 22:3092024. View Article : Google Scholar
|
|
13
|
Meng D, Yin G, Chen S, Zhang X, Yu W, Wang
L, Liu H, Jiang W, Sun Y and Zhang F: Diosgenin attenuates
nonalcoholic hepatic steatosis through the hepatic SIRT1/PGC-1α
pathway. Eur J Pharmacol. 977:1767372024. View Article : Google Scholar
|
|
14
|
Liao HJ and Tzen JTC: Investigating
potential GLP-1 receptor agonists in cyclopeptides from
Pseudostellaria heterophylla, Linum usitatissimum, and Drymaria
diandra, and peptides derived from heterophyllin B for the
treatment of type 2 diabetes: An in silico study. Metabolites.
12:5492022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Z, Zhang C, Li X, Ma Z, Ge Y, Qian Z
and Song C: Heterophyllin B, a cyclopeptide from Pseudostellaria
heterophylla, enhances cognitive function via neurite outgrowth and
synaptic plasticity. Phytother Res. 35:5318–5329. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng J, Feng X, Zhou L, He C, Li H, Xia J,
Ge Y, Zhao Y, Song C, Chen L and Yang Z: Heterophyllin B, a
cyclopeptide from Pseudostellaria heterophylla, improves memory via
immunomodulation and neurite regeneration in i.c.v.Aβ-induced mice.
Food Res Int. 158:1115762022. View Article : Google Scholar
|
|
17
|
Hutchison AL, Tavaglione F, Romeo S and
Charlton M: Endocrine aspects of metabolic dysfunction-associated
steatotic liver disease (MASLD): Beyond insulin resistance. J
Hepatol. 79:1524–1541. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia M, Wu Z, Wang J, Buist-Homan M and
Moshage H: The coumarin-derivative esculetin protects against
lipotoxicity in primary rat hepatocytes via attenuating
JNK-mediated oxidative stress and attenuates free fatty
acid-induced lipid accumulation. Antioxidants (Basel). 12:19222023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Zhang H, Wang W, Hu X, Wang Z, Lou J, Cui
P, Zhao X, Wang Y, Chen X and Lu S: Heterophyllin B enhances
transcription factor EB-mediated autophagy and alleviates
pyroptosis and oxidative stress after spinal cord injury. Int J
Biol Sci. 20:5415–5435. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holmer M, Lindqvist C, Petersson S,
Moshtaghi-Svensson J, Tillander V, Brismar TB, Hagström H and Stål
P: Treatment of NAFLD with intermittent calorie restriction or
low-carb high-fat diet-a randomised controlled trial. JHEP Rep.
3:1002562021. View Article : Google Scholar
|
|
22
|
Mao T, Sun Y, Xu X and He K: Overview and
prospect of NAFLD: Significant roles of nutrients and dietary
patterns in its progression or prevention. Hepatol Commun.
7:e02342023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baggio LL and Drucker DJ: Glucagon-like
peptide-1 receptor co-agonists for treating metabolic disease. Mol
Metab. 46:1010902021. View Article : Google Scholar :
|
|
24
|
Boland ML, Laker RC, Mather K, Nawrocki A,
Oldham S, Boland BB, Lewis H, Conway J, Naylor J, Guionaud S, et
al: Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR
dual-agonist Cotadutide via modulating mitochondrial function and
lipogenesis. Nat Metab. 2:413–431. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei Y, Yin L, Zhang J, Tang J, Yu X, Wu Z
and Gao Y: Heterophyllin B inhibits the malignant phenotypes of
gastric cancer cells via CXCR4. Hum Cell. 36:676–688. 2023.
View Article : Google Scholar
|
|
26
|
Han W, Li Y, Cheng J, Zhang J, Chen D,
Fang M, Xiang G, Wu Y, Zhang H, Xu K, et al: Sitagliptin improves
functional recovery via GLP-1R-induced anti-apoptosis and
facilitation of axonal regeneration after spinal cord injury. J
Cell Mol Med. 24:8687–8702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amorim M, Martins B and Fernandes R:
Immune fingerprint in diabetes: Ocular surface and retinal
inflammation. Int J Mol Sci. 24:98212023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roberts LD, Hornsby AK, Thomas A, Sassi M,
Kinzett A, Hsiao N, David BR, Good M, Wells T and Davies JS: The
5:2 diet does not increase adult hippocampal neurogenesis or
enhance spatial memory in mice. EMBO Rep. 24:e572692023. View Article : Google Scholar : PubMed/NCBI
|