Preterm birth (PTB) is a global maternal and
neonatal health problem, with ~15 million infants born preterm each
year, 11% of all live births (1). PTB contributes to 15% of under-five
childhood mortality and 35% of neonatal mortality worldwide
(2). It is associated with a
range of serious complications, including bronchopulmonary
dysplasia, respiratory distress syndrome, intraventricular
hemorrhage, neurodevelopmental impairment, and necrotizing
enterocolitis (2), which rank
among the leading direct causes of mortality in children under
five. Roughly 40-45% of PTB cases occur spontaneously, 25-30%
result from preterm premature rupture of the membranes (PPROM), and
the remaining 30-35% are due to medically indicated or elective
early deliveries (3).
Intra-amniotic infection is estimated to underlie ~35% of
spontaneous PTB and 50% of PPROM cases (3,4).
Studies have isolated specific bacterial species from the amniotic
fluid of PTB patients and confirmed that these organisms are common
vaginal colonizers, supporting ascending vaginal infection as the
primary route of microbial invasion into the amniotic cavity
(4,5).
Vaginal microbiota (VMB) plays a critical role in
maintaining maternal and fetal health (6). For example, Tabatabaei et al
(7) reported that
Lactobacillus spp. may reduce the risk of PTB, whereas
community state types (CSTs) associated with bacterial vaginosis
(BV) may increase PTB risk. Moreover, a
Lactobacillus-depleted CST, accompanied by elevated
abundances of Gardnerella or Mycoplasma, is linked to
higher PTB incidence (8).
Compared with early gestation, the VMB becomes increasingly stable
and Lactobacillus-dominated in late pregnancy (9,10), which may represent an
evolutionary mechanism to ensure successful pregnancy. By contrast,
VMBs dominated by other genera, particularly those associated with
BV, are generally considered suboptimal and correlate with greater
adverse outcomes (11,12).
PubMed and Web of Science were searched from
database inception through 31 May 2025. Medical Subject Headings
and free text terms 'preterm birth,' 'vaginal microbiota,'
'microbiota,' and 'probiotic' were combined in the search
strategy.
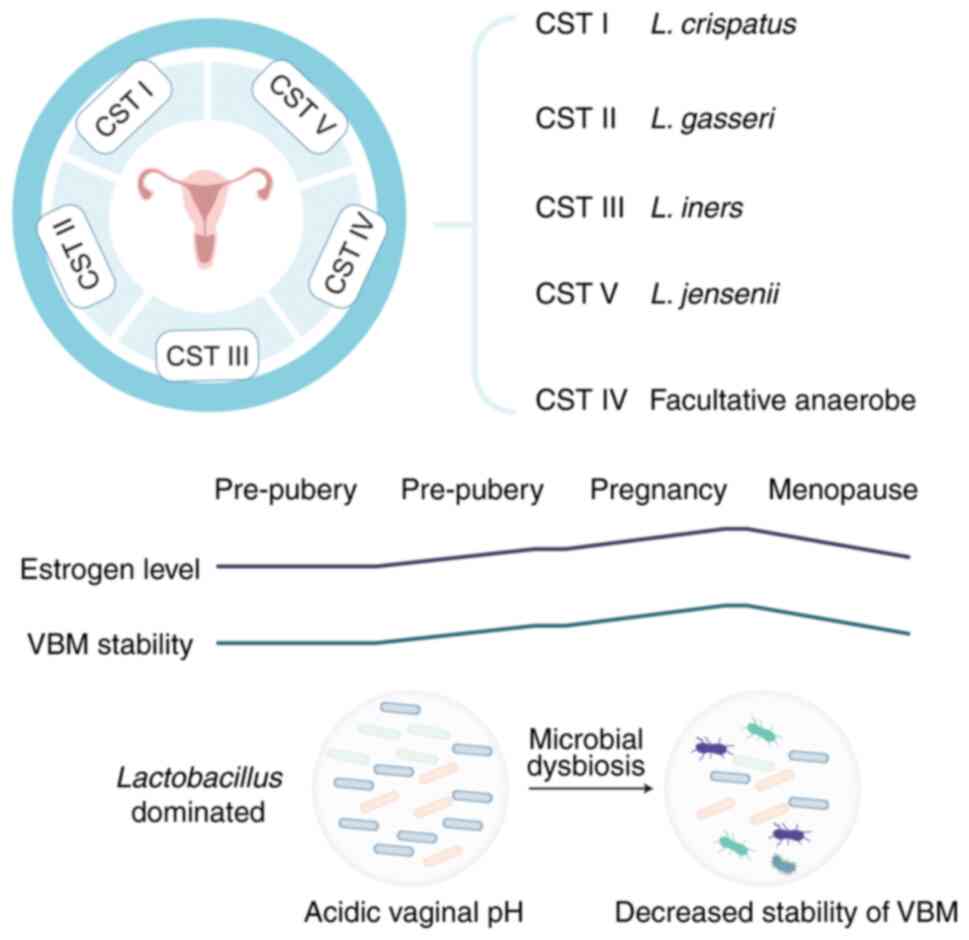
Throughout a woman's life span, both intrinsic and
extrinsic factors modulate VMB composition. Prior to menarche, low
estrogen levels correlate with a high-diversity microbiota
comprising aerobic, anaerobic and enteric bacteria (20). Although some women maintain VMB
stability across the menstrual cycle, the endocrine withdrawal of
estrogen and progesterone during menses, alongside endometrial
shedding and vaginal bleeding, induces transient shifts marked by
decreased Lactobacillus abundance that typically rebounds in
the follicular phase (21).
During pregnancy, hormonal stability, markedly elevated estrogen
concentrations and the absence of menses favor a
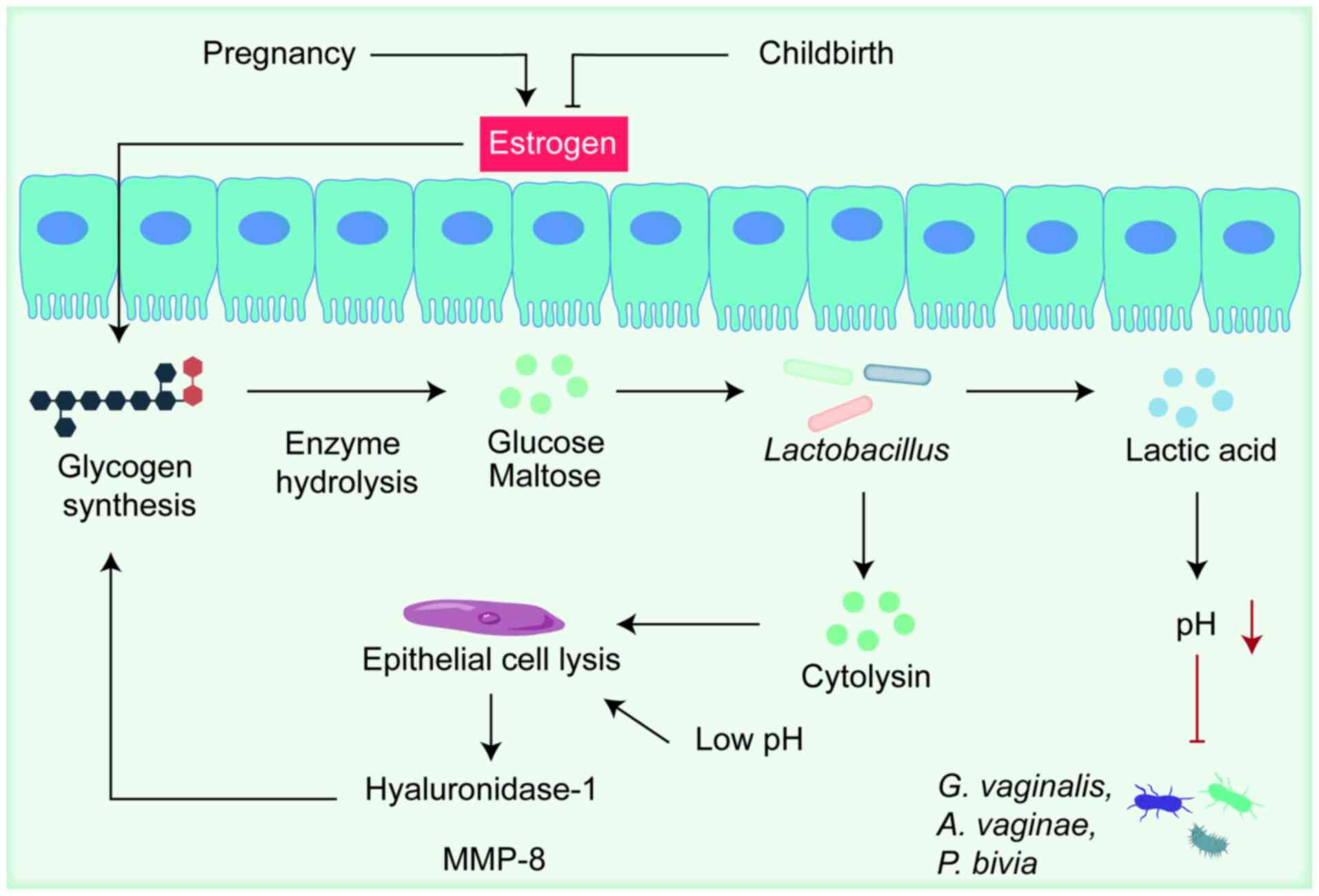
Lactobacillus dominated (LDOM) VMB (22). Estrogen is the principal host
factor sustaining the VMB, promoting epithelial thickening and
intracellular glycogen synthesis (23). Glycogen is hydrolyzed by host
α-amylase into glucose and maltose, substrates fermented by
Lactobacillus spp. into lactic acid, thereby reducing local
pH (24). Moreover, various
vaginal bacteria encode amylase-like enzymes capable of glycogen
metabolism (25,26). Glycogen may also originate from
epithelial-cell lysis induced by high lactate concentrations and
cytolysins secreted by Lactobacilli, a process potentially
mediated by hyaluronidase-1 and matrix metalloproteinase (MMP)-8
(27). With the onset of puberty
and rising estrogen, vaginal pH acidifies and meta-analyses have
demonstrated enhanced VMB stability and Lactobacillus
predominance (28,29). Similarly, cyclical and
pregnancy-related fluctuations in VMB stability parallel estrogen
levels; elevated estrogen is consistently associated with increased
VMB stability, which is beneficial for vaginal health. Conversely,
decreased estrogen post-pregnancy and in menopause is linked to
reduced VMB stability; however, estrogen therapy in postmenopausal
women restores a pronounced LDOM state (30,31). Notably, estrogen-containing
contraceptives increase Lactobacillus abundance in
reproductive-aged women and decrease BV incidence (32,33), further supporting a causal role
for estrogen in VMB regulation.
During pregnancy, the VMB is characterized by
decreased richness and diversity, with Lactobacillus spp.
emerging as the dominant genus. This maintenance of microbial
homeostasis is closely linked to rising estrogen levels (as shown
in Fig. 2), which not only
stimulate glycogen synthesis but also promote increased lactic acid
production by Lactobacillus. As gestation advances into the
late trimester, Lactobacillus proliferation is often
accompanied by a marked decline in dysbiosis-associated taxa such
as G. vaginalis, A. vaginae and P. bivia
(34). Studies show that during
pregnancy, vaginal microbiota communities dominated by
Lactobacillus usually remain stable or slowly change into
other community types still dominated by Lactobacillus
(8,35,36). Communities showing dysbiosis in
mid-gestation often become dominated by Lactobacillus. When
Gardnerella vaginalis dominates, its communities are
relatively unstable and usually shift to a Lactobacillus
iners community (35,36).
These dynamic shifts align with the overall trend toward an
optimized vaginal microenvironment during pregnancy. As white women
generally possess higher baseline Lactobacillus levels prior
to conception (36), black women
demonstrate a more pronounced transition toward
Lactobacillus dominance and enhanced VMB stability
throughout pregnancy (35,36), suggesting a stronger modulatory
effect of gestation on the VMB in this population. At parturition,
the precipitous decline in estrogen precipitates a significant
destabilization of the VMB (37,38), leading to community
restructuring: Lactobacillus proportions decrease while
anaerobic genera such as Peptoniphilus, Prevotella
and Anaerococcus proliferate; taxa whose overgrowth is often
linked to adverse outcomes (8,37). Moreover, short interpregnancy
intervals (<1 year) have been shown to markedly increase the
risk of PTB and other obstetric complications (39-41).
Abnormal VMB is recognized as a potential risk
factor for PTB. Clinical studies show that bacteria isolated from
the placenta and fetal membranes of preterm deliveries closely
match those in the vagina, supporting the idea that ascending
vaginal colonization by pathogens frequently leads to
intra-amniotic infection and PTB (71) and can be reproduced in various
animal models of ascending infection (72,73). Romero et al (74) compared the VMB of 18 women who
delivered preterm with 70 term controls and found no significant
differences in bacterial taxa, relative abundances, or CST
distributions when stratified by gestational age. A Peruvian cohort
likewise reported no association between vaginal CST and PTB
(5). These discrepancies may
reflect differences in maternal ethnicity, geographic location, and
gestational age at sampling. Fettweis et al (35) profiled the VMB and cytokine
milieu of 45 African-American women with PTB vs. 90 term-matched
controls. Multi-omics analyses showed markedly lower levels of
L. crispatus and higher abundances of BVAB1, Sneathia
amnii, TM7-H1 and a subset of Prevotella in the PTB
group. Term deliveries were more likely to harbor L.
crispatus and exhibit reduced prevalence of A. vaginae
and G. vaginalis. A small study of nulliparous
African-American women noted a nonsignificant trend toward reduced
diversity in spontaneous PTB (75), and another black cohort found
that although specific Lactobacillus abundances were not
linked to PTB risk, lower overall diversity associated with PTB
among African-American women (76). In a UK study, L. iners
dominance at 16 weeks was markedly associated with cervical
shortening and PTB before 34 weeks, whereas L. crispatus
dominance strongly predicted term birth; high-diversity communities
were not linked to cervical shortening or PTB, but
Lactobacillus depletion was relatively uncommon in that
cohort (77). The role of L.
iners remains ambiguous, as it appears in both healthy and
dysbiotic VMBs. Unlike the exclusionary L. crispatus, L.
iners has a reduced genome lacking key metabolic functions
(78), suggesting that L.
iners has evolved to tolerate other vaginal microbes, including
pathogens, and is often found in transitional microbiota states.
Consequently, L. iners may be unable to prevent pathogen
overgrowth during pregnancy, indirectly fostering high-risk VMB
profiles. Srinivasan et al (79) reported that high levels of L.
iners can be detected in women who are BV positive or BV
negative and across a broad range of vaginal pH values. Although
this phenomenon has been extensively documented under BV conditions
(80), it does not provide
direct evidence that the species is pathogenic during dysbiosis. Of
women ~50% are thought to harbor L. iners (23), so its persistent detection during
BV is unsurprising. Another study showed that the detection rate of
L. iners is similar among women with normal, intermediate
and BV Nugent scores (81);
however, relative abundance may be more decisive and further work
is required to quantify both relative and absolute levels under
diverse conditions. Shipitsyna et al (82) observed a decline in L.
iners during active BV and therefore suggested that it is
unlikely to be a primary pathogen; by contrast, other investigators
argue that communities dominated by L. iners are more prone
to shift toward dysbiosis, whereas those dominated by L.
crispatus appear more stable (83). A semi-quantitative qPCR study
that sampled Belgian women (predominantly Caucasian) throughout the
menstrual cycle found that during active BV, menstruation and after
intercourse, the previously dominant L. crispatus sharply
decreased, while L. iners became predominant, often
accompanied by a concomitant rise in Gardnerella vaginalis
(84), indicating greater
adaptability of L. iners to fluctuations in the vaginal
environment such as BV. Macklaim et al (85) further demonstrated that genes
involved in mannose and maltose uptake and glycogen degradation are
markedly upregulated in L. iners under BV. Additional
studies show that both the abundance and gene expression of L.
iners vary with the phase of the menstrual cycle (86); it is also possible that host
physiological changes first trigger BV and that L. iners
subsequently adapts. Clarifying this temporal sequence will be
critical for determining whether, and to what extent, L.
iners is harmful to the host.
Multiple studies have demonstrated significant
population specific differences in vaginal microbiome composition
and their associations with PTB risk. DiGiulio et al
(91) identified ethnicity as a
strong determinant of VMB composition and PTB risk. In a
predominantly white cohort, CSTs characterized by
Lactobacillus depletion, especially those enriched for
Gardnerella or Ureaplasma, were associated with
shorter gestational duration and higher PTB risk. A subsequent
comparison of Californian white and Alabaman black women revealed
more frequent Lactobacillus depletion and Gardnerella
enrichment among black women, yet these features predicted PTB only
in white women; in both groups, L. crispatus dominance
conferred protection against early delivery (92). Hyman et al (93) reported greater bacterial
diversity among white women who delivered preterm, while overall
species diversity was higher in African-American women. A more
recent investigation comparing black and non-black American women
confirmed that Lactobacillus depletion was more prevalent in
black women but increased PTB risk only in white women; it also
identified distinct taxa markedly associated with spontaneous PTB,
with stronger effects in the black cohort (94). The protective role of L.
crispatus has also been validated in European, Middle Eastern
and Asian populations, where L. iners dominance associated
with higher PTB risk and L. crispatus dominance with term
delivery (87). Sun et al
(95) showed in stratified
analyses that African-American women have increased abundance of
Lactobacillus iners and decreased abundance of
Lactobacillus crispatus and that lower levels of L.
crispatus are associated with increased risk of spontaneous
PTB. Another study of Asian and Myanmar/Karen minority women
combined microbiome profiling with cytokine analysis and found that
signatures composed of specific taxa and inflammatory cytokines
predict PTB more accurately than single markers, thus providing a
framework for biomarker development in resource limited settings
(96). Fettweis et al
(35) employed multi omics
approaches to identify a marked reduction in L. crispatus
and increases in pathogenic taxa such as BVAB1 and Sneathia
amnii alongside elevated levels of pro inflammatory cytokines
in cases of PTB, highlighting these features as candidate
biomarkers across populations. These findings indicate that
intervention strategies should prioritize probiotic strains that
enhance or maintain protective Lactobacilli colonization in
specific populations and that biomarker discovery should integrate
microbial and host inflammatory multi omics signatures to achieve
precise cross population applicability.
Elevated diversity within vaginal microbial
communities and the presence of bacterial vaginosis-associated
taxa, including Gardnerella vaginalis, Atopobium
vaginae and representatives of the Veillonellaceae family,
demonstrate a robust association with heightened risk of PTB prior
to 34 gestational weeks (7). A
study (97) indicates that one
pathway by which a diverse VMB elevates PTB risk is through
premature activation of parturition-associated inflammatory
cascades. In one early clinical investigation, women undergoing
cervical cerclage with braided sutures exhibited persistent VMB
dysbiosis and local inflammation, accompanied by ultrasound
evidence of premature cervical remodeling (97). A UK multicenter retrospective
analysis further showed that braided-suture cerclage was associated
with a threefold increase in intra-amniotic fetal death and a
twofold rise in PTB, whereas monofilament cerclage had no
significant impact on the VMB, cytokine concentrations or cervical
anatomy. These findings suggest that microbiota-triggered
inflammation may act directly on the cervix as pregnancy advances
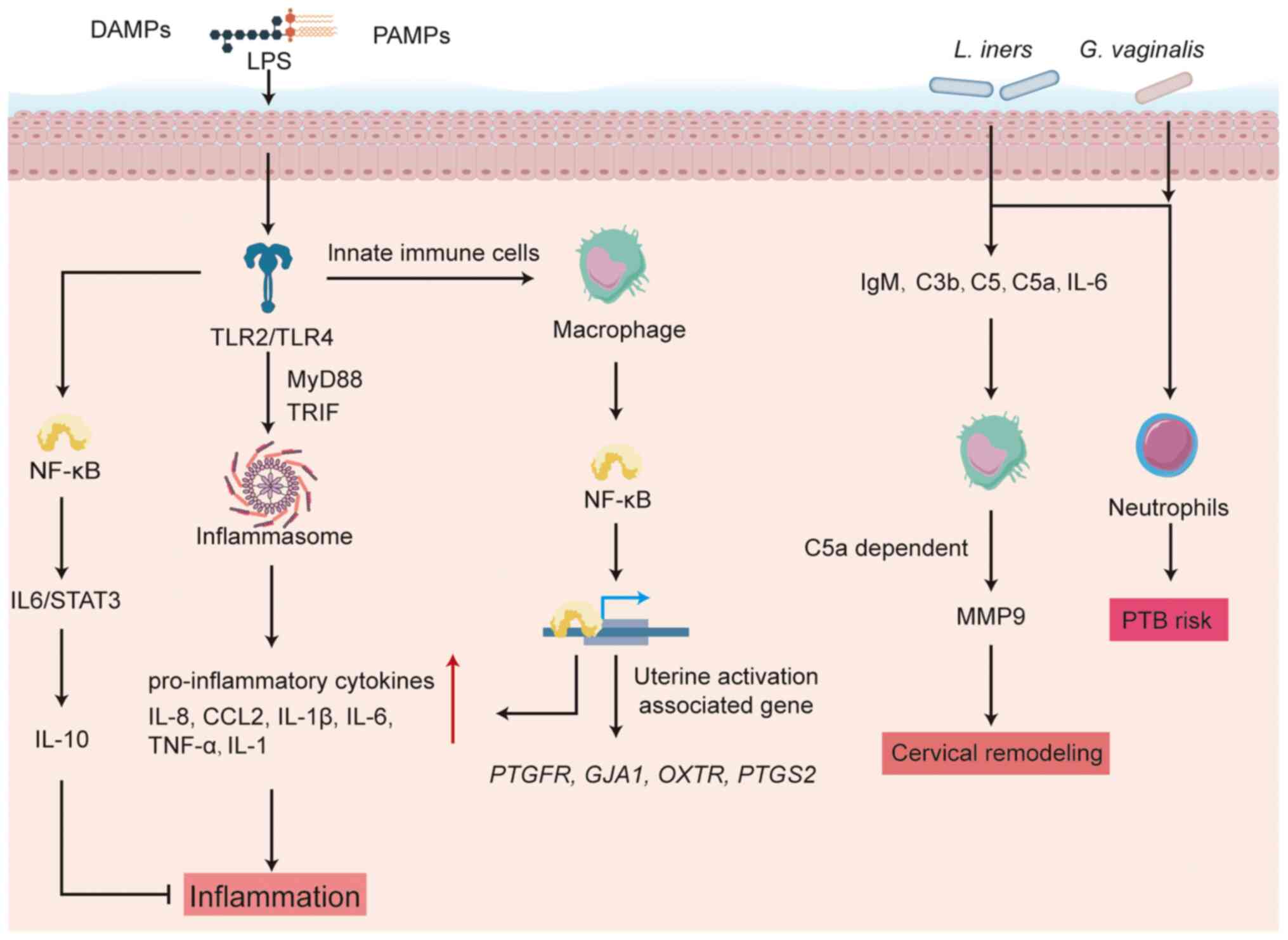
(14). Activation of TLRs is
regarded as a critical initiating event for inflammation in both
term and preterm labor (PTL). TLRs are evolutionarily conserved
pattern recognition receptors that orchestrate innate immune
responses by sensing pathogen associated molecular patterns (PAMPs)
and damage associated molecular patterns (DAMPs), then triggering
downstream signaling to induce cytokine and chemokine release (as
illustrated in Fig. 3) (98). Among TLR family members, the
functional role of TLR4 is best characterized. TLR4 binds
lipopolysaccharide (LPS) and other PAMP/DAMP ligands and its
activation state in the uterus correlates with both term and PTL.
In mice, TLR4 knockout delays parturition and markedly reduces
neonatal survival (99).
Pharmacologic blockade of TLR4 signaling with the antagonist
(+)-naloxone inhibits Escherichia coli induced inflammatory
cascades and prevents PTB (100). More recently, decidual
endothelial cell specific TLR4 expression has been shown to be
essential: Mice lacking endothelial TLR4 resist LPS-induced PTB,
indicating a non-immune-cell locus of the action of TLR4 (101). Clinically, maternal TLR4
single-nucleotide polymorphisms are markedly associated with
extreme preterm delivery (<32 weeks) (102). TLR4 and its co-receptor CD14
signal via both MyD88-dependent and TRIF-dependent pathways to
regulate distinct inflammatory gene programs. MyD88-deficient mice
are completely protected against E. coli induced PTB,
underscoring the dominant role of the MyD88 axis in preterm
parturition (103). Other TLRs,
such as TLR2, also contribute to labor timing (104,105), and fetal TLR4/TLR2
polymorphisms further modulate PTB risk (106,107).
TLR activation can subsequently drive inflammasome
assembly, sustaining secretion of IL-8, CCL2, IL-1β and IL-6 by
placental and decidual tissues (108). These mediators recruit immune
cells and induce prostaglandin and MMP production, collectively
promoting cervical ripening and uterine contractility. Amniotic
cavity macrophage infiltration constitutes a critical
pathophysiological mechanism for spontaneous labor initiation,
functionally mediated through NF-κB-driven transcriptional
activation of uterine activation-associated gene networks (such as
PTGFR, GJA1, OXTR and PTGS2) and
pro-inflammatory cytokines TNFα, IL-1β, IL-6 and IL-8 (109). Thus, pattern-recognition
receptors and downstream effectors orchestrate both physiological
and pathological labor initiation.
Various approaches are used to induce PTL and
parturition in mice, including immunological, hormonal and
environmental triggers. Immunomodulators activate or amplify
physiological inflammatory pathways that drive labor onset,
recapitulating clinical features of infection and
inflammation-associated parturition. The most common murine PTL
models involve intrauterine or intraperitoneal administration of
LPS or heat-killed whole Escherichia coli cells (110). Although LPS is widely employed
to induce PTB via pro-inflammatory responses in mice (72), its role in the human female
reproductive tract remains unclear. Clinical studies (35,96,111) report elevated concentrations of
pro-inflammatory cytokines and chemokines, such as IL-1β, IL-6,
IL-8, IL-10, IL-18, granulocyte-macrophage colony-stimulating
factor (GM-CSF), macrophage inflammatory protein-1β (MIP-1β) and
eosinophil chemotactic factor, in the amniotic fluid,
cervicovaginal fluid and plasma of women who subsequently
experienced spontaneous PTB. Moreover, cervical levels of IL-1β,
IL-6, IL-8, IL-10, eotaxin, MIP-1β and GM-CSF are associated with
VMB composition (112). Animal
models demonstrate a central role for macrophages in both term and
PTL. Macrophage depletion via anti-F4/80 antibody markedly reduces
susceptibility to LPS-induced PTB in mice (113). Mechanistically, macrophages
contribute to PTB pathogenesis by secreting TNF-α, IL-1, IL-6, IL-8
and by regulating expression of contraction-associated genes such
as MMPs (109). Specifically,
IL-1 is critical in inflammatory PTB; exogenous IL-1 induces PTL in
mice, whereas blockade of the IL-1 receptor inhibits labor
initiation (114), probably
through activation of the NF-κB signaling pathway (115). Research indicates that IL-6
deficient mice exhibit delays parturition and resistance to
LPS-induced PTB (116).
Dominance of L. iners is associated with elevated levels of
immunoglobulin M, complement components C3b, C5, C5a, and IL-6,
collectively leading to cervical shortening, reduced endometrial
receptivity, decreased implantation rates and increased PTB risk
(117,118). Complement activation also plays
a pivotal role: Clinical data show raised terminal complement
fragments C3a, C4a and C5a in women undergoing infection-associated
spontaneous PTB (113). In
mouse models, C5a receptor (C5aR)-deficient animals are protected
from both LPS- and RU486-induced PTB; this protection involves
aberrant complement deposition in the cervical epithelium and
C5a-dependent macrophage MMP-9 release that drives cervical
remodeling (119). IL-10 has a
protective effect against PTB. IL-10 knockout or neutralization
markedly increases susceptibility to PTB in mice (120). IL-10 deficiency leads to
downregulation of inflammatory cytokine gene expression in uterine
and placental tissues, suggesting its role in tempering excessive
inflammation during labor. Decidual endothelial cell TLR4 signaling
at term may activate the IL-6/STAT3 axis via NF-κB, promoting
perivascular stromal cell production of IL-10 (101). This mechanism probably
maintains immune homeostasis under inflammatory conditions, a
balance that is disrupted in PTB. Neutrophils, which contribute to
cervical remodeling, may infiltrate the myometrium and facilitate
cervical shortening during labor, thereby increasing PTB risk
(121,122). Limited data suggest that
neutrophil numbers in cervicovaginal fluid decline as gestation
advances (123). Notably, women
with a low-diversity VMB (CST III, dominated by L. iners)
often exhibit elevated vaginal neutrophil counts during pregnancy
(123). One study reported a
positive association between G. vaginalis (CST IV) abundance
and neutrophil levels in samples from women who subsequently
delivered preterm (123).
In vaginal epithelial-cell models, both D-lactic
and L-lactic acid, alone or in combination with a spectrum of
VMB-associated metabolites, induce the anti-inflammatory cytokine
interleukin-1 receptor antagonist (IL-1RA) while suppressing
pro-inflammatory mediators such as IL-6, IL-8, TNF-α, RANTES and
macrophage inflammatory protein-3α (130,131). Organotypic models of the female
reproductive-tract epithelium confirm that L-lactic acid
upregulates IL-1RA and downregulates IL-8 (130). Although certain in vitro
studies have reported pro-inflammatory responses to specific
Lactobacillus strains (132,133), these strains are not dominant
members of the VMB. In vivo experiments and
cervical-epithelial models both demonstrate that lactate production
by Lactobacillus spp. and the resulting acidic vaginal pH
reinforce epithelial-barrier integrity, thereby inhibiting
colonization by anaerobes and pathogens (134,135). Thus, dominance of protective
lactobacilli is associated with reduced risk of suboptimal
vaginal health. However, excessive lactobacilli
proliferation can give rise to cytolytic vaginosis, a condition
marked by epithelial-cell lysis, dissolution and shedding
attributed to overproduction of lactic acid (136). Although cytolytic vaginosis is
less prevalent than BV, its occurrence underscores the clinical
importance of quantitatively assessing the VMB.
Beyond the presence or absence of key taxa, the VMB
influences immune responses and pregnancy outcomes via production
of SCFAs. While SCFAs enhance intestinal-barrier integrity
(137), their effects in the
vaginal milieu appear deleterious. SCFAs are organic fatty acids
typically containing fewer than six carbon atoms; common examples
include acetate, propionate and butyrate (138). Elevated levels of SCFAs, such
as propionate, succinate, acetate and butyrate, are associated with
increased concentrations of IL-1β, IL-2, IL-6, IL-8, IL-10, TNF-α,
IFN-γ and RANTES (139). For
instance, in early gestation, elevated acetate is associated with
PTB under conditions of weakened L. crispatus dominance
(139,140). High SCFA concentrations also
inhibit expression of neutrophil gelatinase-associated lipocalin,
an innate-antimicrobial factor, potentially facilitating overgrowth
of BV-associated communities and further contributing to PTB
(141). In BV settings, high
concentrations of acetate (100 mM) and butyrate (20 mM) selectively
activate TLR1/2/3 in cervicovaginal epithelial cells, resulting in
increased TNF-α secretion and concomitant suppression of IL-6,
RANTES and IP-10 production (131).
Metabolomic analyses of PTB patients reveal
upregulation of glycerophospholipid-metabolism-related metabolites.
Glycerophospholipids are fundamental membrane constituents and
precursors for bioactive lipids such as arachidonic acid (AA) and
lysobisphosphatidic acid (LPA). These bioactive lipids are
generated by phospholipase A2 (PLA2) and, in the case of AA,
further converted by cyclooxygenase-2 (COX-2) into prostaglandins
(PGs) (142,143). LPA3, a G protein-coupled
receptor expressed in uterine epithelium, modulates COX-2 activity
and PG levels (144).
Collectively, these molecules serve as key regulators of
inflammation. Increased phosphoinositol, a glycerophospholipid
metabolite, depletes glycerophospholipid pools and downstream
mediators (LPA and PG), potentially impairing implantation;
additionally, elevated phosphoinositol promotes Ca2+
influx and uterine contractility, which may precipitate premature
labor (145).
Enhanced primary bile-acid and steroid-hormone
metabolism pathways have also been observed in PTB cohorts,
consistent with findings by Menon et al (146) and Lizewska et al
(147). Bile acids, synthesized
from cholesterol in the liver, are modified by the gut microbiota
and facilitate dietary-fat absorption (148). Pregnancy-related hormonal
changes (such as cholestasis) can disrupt bile acid clearance,
leading to elevated circulating levels that induce fetal stress
(148). Steroid hormones, also
derived from cholesterol, govern development, osmoregulation,
metabolism and stress responses. Maturation of the fetal
hypothalamic-pituitary-adrenal axis and increased fetal adrenal
production of C19 steroids (dehydroepiandrosterone sulfate) and
cortisol are pivotal for labor initiation, whereas placental
progesterone maintains uterine quiescence during gestation
(149). Perturbations in
steroid synthesis, metabolism or transport may likewise trigger
labor onset.
The VMB can be modulated by various interventions,
including probiotics, prebiotics, antibiotics and pH modulators
(153). When treating
infectious diseases related to BV, the most commonly used and
fastest-acting method is antibiotic therapy. Although antibiotics
are effective against infection, they also disrupt the overall
microbial balance, including beneficial lactobacilli, leading to
high recurrence rates (154)
and increased risk of spontaneous PTB (12). As an alternative or adjunct to
antibiotics, several approaches have been proposed to restore a
Lactobacillus-dominated vaginal community. Oral probiotics,
leveraging cross-talk between the gut and vaginal microbiomes, are
considered a promising method. Probiotics are live, beneficial
strains capable of colonizing the vagina and improving its
microbial composition, such as Lactobacillus crispatus,
L. jensenii and L. gasseri (12,155). These probiotics can be
administered orally or vaginally, in formulations including
capsules, suppositories or creams (156,157). Most intervention studies have
focused on using Lactobacilli to alleviate BV and aerobic
vaginitis, both of which are dysbiotic states associated with
increased risk of spontaneous PTB. Results have been mixed: some
studies report efficacy, while others find no significant effect.
Probiotic studies are summarized in Table I. For example, one study
(158) showed that oral
capsules containing Lactobacillus rhamnosus GR-1 and L.
fermentum RC-14 reduced vaginal yeast and E. coli
counts, increased lactobacilli levels and restored an asymptomatic
BV microbiota to a healthy Lactobacilli-dominated state. Ho
et al's (159) study of
group B Streptococcus (GBS)-positive pregnant women at 35-37
weeks demonstrated that daily oral GR-1 + RC-14 until delivery
markedly lowered GBS positivity compared with placebo. In 40
BV-afflicted black women, GR-1 + RC-14 achieved higher cure rates
than metronidazole (160).
Maternal oral supplementation with Lactobacillus rhamnosus
GR-1 and Lactobacillus fermentum RC-14 probiotic capsules
markedly reduced vaginal microbial α diversity and species
richness, while concurrently suppressing Gardnerella
vaginalis colonization. The probiotic cohort exhibited
enrichment of Lactobacillus crispatus, Lactobacillus
iners and the gut-associated species Prevotella copri,
taxa positively associated with VMB stability (157). Other studies have shown that
probiotic intervention reduces expression of inflammatory factors,
a known risk factor for PTB. In a double-blind trial of 159
volunteers, vaginal tablets containing Lactobacillus brevis
CD2, L. salivarius subsp. salicinius and L.
plantarum achieved clinical cure in nearly 80% of BV patients,
with 32% restoring a normal VMB (161). Post-treatment vaginal lavage
fluids showed significant decreases in IL-1β and IL-6, whereas the
control group showed no change in local pro-inflammatory cytokines
(161). Bayar et al
(155) reported that
administering LACTIN-V to high-risk pregnant women markedly reduced
early PTB incidence. The protective effect of LACTIN-V may be
attributed to its inhibition of pro-inflammatory responses to
uropathogens. However, in a randomized, double-blind trial, Husain
et al (162)
demonstrated that daily oral administration of a probiotic
preparation comprising Lactobacillus rhamnosus GR-1 and
Lactobacillus reuteri RC-14 from early pregnancy did not
modify the vaginal microbiota of pregnant women nor prevent
bacterial vaginosis. In a similar study, Gille et al
observed that eight weeks of daily oral administration of L.
rhamnosus GR-1 or L. reuteri RC-14, compared with
placebo, yielded no significant difference in the incidence of BV
as assessed by Nugent score before and after intervention (163). These divergent findings may
reflect limitations in study design. In particular, the proportion
of women with abnormal vaginal microbiota in Gille et al's
(163) intervention arm was
substantially lower than that reported in general populations,
warranting validation in larger and more representative cohorts.
Moreover, an unexpectedly high loss to follow up may have reduced
the statistical power to detect intergroup differences. The two
trials employed oral delivery, which may not ensure sufficient
vaginal colonization or confer protection against spontaneous PTB.
Consequently, the investigators recommend that future research
prioritize the identification of probiotic strains and delivery
modalities capable of beneficially modulating the vaginal
microbiota during pregnancy before assessing their efficacy in
preventing PTB (162). VMB
transplantation (VMT) is another strategy under consideration. A
recent case series reported successful engraftment of healthy
vaginal communities into patients with refractory BV, with four out
of five cases achieving sustained remission for up to 21 months
(164). Although limited by
sample size, these findings underscore the need to assess the
long-term efficacy of both probiotic and VMT approaches in pregnant
populations.
PTB remains a major global health challenge, with
35-50% of spontaneous PTB and PPROM cases attributed to ascending
vaginal infections. A growing body of evidence indicates that
dynamic shifts in the VMB critically influence pregnancy outcomes.
Vaginal microbiota dominated by Lactobacillus species,
particularly L. crispatus, confer protective effects through
three synergistic mechanisms: Lactic acid-driven pH homeostasis,
bacteriocin-mediated pathogen suppression, and immunoregulatory
pathways that attenuate proinflammatory cytokine signaling,
including IL-6, IL-8, and TNF-α. By contrast, high-diversity,
Lactobacillus-depleted (CST IV) vaginal microbiota enriched
with opportunistic pathogens such as Gardnerella vaginalis,
Atopobium vaginae, and Prevotella spp. demonstrate a
strong association with increased PTB risk. These pathogens
compromise epithelial integrity through biofilm formation, protease
secretion and production of SCFAs, exacerbating local inflammation
and activating TLR/NF-κB signaling cascades.
The field continues to confront several persistent
challenges despite recent advancements. While observational studies
have reproducibly linked vaginal microbial dysbiosis to PTB risk,
establishing definitive causal relationships requires longitudinal
investigations capable of tracing microbial community trajectories
across pregnancy trimesters. Such studies would clarify whether
specific pathogen colonization patterns precede cervical remodeling
events and identify predictive microbial signatures. A parallel
knowledge gap exists in understanding functional differences
between Lactobacillus species, particularly the clinically
relevant dichotomy between L. crispatus' protective mucosal
interactions and L. iners' ambiguous ecological role, which
demands comparative multi-omic analyses spanning genomic, proteomic
and metabolomic dimensions. Current microbiome profiling techniques
have limitations; for example, 16S rRNA sequencing lacks resolution
at the species level and does not capture functional information.
Future studies should integrate metagenomic and multi-omics
approaches to achieve a more comprehensive characterization of both
microbial communities and their metabolite profiles. Current
therapeutic development efforts face dual limitations:
heterogeneous probiotic formulations lacking strain-specific
rationale and clinical trials inadequately powered to detect
ethnic-geographic variations in microbial-host interactions.
Progress necessitates standardized intervention protocols paired
with mechanistic studies decoding how microbial metabolites
interface with cervical tissue immune networks at single-cell
resolution. Addressing these interconnected biological and
methodological complexities could enable microbiota-informed risk
stratification systems and next-generation therapeutic strategies
tailored to individual microbial ecologies.
Although the present review was based on a
comprehensive search and synthesis of PubMed and Web of Science
from inception to May 2025, it still has several limitations.
First, by restricting its analysis to English-language
publications, it may have overlooked important studies in other
languages. Second, the included studies vary in their definitions
of bacterial vaginosis, sampling time points and sequencing
platforms, making direct comparisons difficult. Third, as a
narrative review, it did not conduct a formal risk of bias
assessment or quantitative meta-analysis, so it cannot rule out the
influence of publication bias. Future research would benefit from
standardized protocols, broader inclusion criteria, and multicenter
collaboration to validate and build upon these findings.
Not applicable.
DC, FG and KW reviewed literature and wrote the
manuscript. NL and QS collected data and prepared figures. Data
authentication is not applicable. All authors reviewed and approved
the final manuscript.
Not applicable.
Not applicable.
Not applicable.
The present review was supported by the Science and Technology
Development Program of Jinan Municipal Health Commission (grant no.
2024203001).
|
1
|
Ohuma EO, Moller AB, Bradley E, Chakwera
S, Hussain-Alkhateeb L, Lewin A, Okwaraji YB, Mahanani WR,
Johansson EW, Lavin T, et al: National, regional, and global
estimates of preterm birth in 2020, with trends from 2010: A
systematic analysis. Lancet. 402:1261–1271. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saigal S and Doyle LW: An overview of
mortality and sequelae of preterm birth from infancy to adulthood.
Lancet. 371:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldenberg RL, Culhane JF, Iams JD and
Romero R: Epidemiology and causes of preterm birth. Lancet.
371:75–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romero R, Gomez-Lopez N, Winters AD, Jung
E, Shaman M, Bieda J, Panaitescu B, Pacora P, Erez O, Greenberg JM,
et al: Evidence that intra-amniotic infections are often the result
of an ascending invasion-a molecular microbiological study. J
Perinat Med. 47:915–931. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witkin SS: The vaginal microbiome, vaginal
anti-microbial defence mechanisms and the clinical challenge of
reducing infection-related preterm birth. BJOG. 122:213–218. 2015.
View Article : Google Scholar
|
|
6
|
Fox C and Eichelberger K: Maternal
microbiome and pregnancy outcomes. Fertil Steril. 104:1358–1363.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tabatabaei N, Eren AM, Barreiro LB, Yotova
V, Dumaine A, Allard C and Fraser WD: Vaginal microbiome in early
pregnancy and subsequent risk of spontaneous preterm birth: A
case-control study. BJOG. 126:349–358. 2019. View Article : Google Scholar
|
|
8
|
Liu L, Yin T, Zhang X, Sun L and Yin Y:
Temporal and spatial variation of the human placental microbiota
during pregnancy. Am J Reprod Immunol. 92:e700232024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Conde-Agudelo A, Papageorghiou AT, Kennedy
SH and Villar J: Novel biomarkers for the prediction of the
spontaneous preterm birth phenotype: A systematic review and
meta-analysis. BJOG. 118:1042–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meertens LJE, van Montfort P, Scheepers
HCJ, van Kuijk SMJ, Aardenburg R, Langenveld J, van Dooren IMA,
Zwaan IM, Spaanderman MEA and Smits LJM: Prediction models for the
risk of spontaneous preterm birth based on maternal
characteristics: A systematic review and independent external
validation. Acta Obstet Gynecol Scand. 97:907–920. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hickey RJ, Zhou X, Pierson JD, Ravel J and
Forney LJ: Understanding vaginal microbiome complexity from an
ecological perspective. Transl Res. 160:267–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Happel AU, Kullin B, Gamieldien H, Wentzel
N, Zauchenberger CZ, Jaspan HB, Dabee S, Barnabas SL, Jaumdally SZ,
Dietrich J, et al: Exploring potential of vaginal Lactobacillus
isolates from South African women for enhancing treatment for
bacterial vaginosis. PLoS Pathog. 16:e10085592020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannella L, Grelloni C, Quintili D,
Fiorelli A, Montironi R, Alia S, Delli Carpini G, Di Giuseppe J,
Vignini A and Ciavattini A: Microbiome changes in pregnancy
disorders. Antioxidants (Basel). 12:4632023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bayar E, Bennett PR, Chan D, Sykes L and
MacIntyre DA: The pregnancy microbiome and preterm birth. Semin
Immunopathol. 42:487–499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bachmann NL, Rockett RJ, Timms VJ and
Sintchenko V: Advances in clinical sample preparation for
identification and characterization of bacterial pathogens using
metagenomics. Front Public Health. 6:3632018. View Article : Google Scholar
|
|
16
|
Sroka-Oleksiak A, Gosiewski T, Pabian W,
Gurgul A, Kapusta P, Ludwig-Słomczyńska AH, Wołkow PP and
Brzychczy-Włoch M: Next-generation sequencing as a tool to detect
vaginal microbiota disturbances during pregnancy. Microorganisms.
8:18132020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linhares IM, Summers PR, Larsen B, Giraldo
PC and Witkin SS: Contemporary perspectives on vaginal pH and
lactobacilli. Am J Obstet Gynecol. 204:120.e1–e5. 2011. View Article : Google Scholar
|
|
18
|
Ravel J, Gajer P, Abdo Z, Schneider GM,
Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO,
et al: Vaginal microbiome of reproductive-age women. Proc Natl Acad
Sci USA. 108(Suppl 1): S4680–S4687. 2011. View Article : Google Scholar
|
|
19
|
Blostein F, Gelaye B, Sanchez SE, Williams
MA and Foxman B: Vaginal microbiome diversity and preterm birth:
Results of a nested case-control study in Peru. Ann Epidemiol.
41:28–34. 2020. View Article : Google Scholar
|
|
20
|
Stennett CA, Dyer TV, He X, Robinson CK,
Ravel J, Ghanem KG and Brotman RM: A cross-sectional pilot study of
birth mode and vaginal microbiota in reproductive-age women. PLoS
One. 15:e02285742020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaban B, Links MG, Jayaprakash TP, Wagner
EC, Bourque DK, Lohn Z, Albert AY, van Schalkwyk J, Reid G,
Hemmingsen SM, et al: Characterization of the vaginal microbiota of
healthy Canadian women through the menstrual cycle. Microbiome.
2:232014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roy EJ and Mackay R: The concentration of
oestrogens in blood during pregnancy. J Obstet Gynaecol Br Emp.
69:13–17. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma B, Forney LJ and Ravel J: Vaginal
microbiome: Rethinking health and disease. Annu Rev Microbiol.
66:371–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van de Wijgert J and Verwijs MC:
Lactobacilli-containing vaginal probiotics to cure or prevent
bacterial or fungal vaginal dysbiosis: A systematic review and
recommendations for future trial designs. BJOG. 127:287–299. 2020.
View Article : Google Scholar
|
|
25
|
Bhandari P, Tingley JP, Palmer DRJ, Abbott
DW and Hill JE: Characterization of an α-Glucosidase enzyme
conserved in gardnerella spp. isolated from the human vaginal
microbiome. J Bacteriol. 203:e00213212021. View Article : Google Scholar
|
|
26
|
Rosca AS, Castro J, Sousa LGV and Cerca N:
Gardnerella and vaginal health: The truth is out there. FEMS
Microbiol Rev. 44:73–105. 2020. View Article : Google Scholar
|
|
27
|
Tachedjian G, Aldunate M, Bradshaw CS and
Cone RA: The role of lactic acid production by probiotic
Lactobacillus species in vaginal health. Res Microbiol.
168:782–792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaur H, Merchant M, Haque MM and Mande SS:
Crosstalk between female gonadal hormones and vaginal microbiota
across various phases of women's gynecological lifecycle. Front
Microbiol. 11:5512020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anderson DJ, Marathe J and Pudney J: The
structure of the human vaginal stratum corneum and its role in
immune defense. Am J Reprod Immunol. 71:618–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen J, Song N, Williams CJ, Brown CJ, Yan
Z, Xu C and Forney LJ: Effects of low dose estrogen therapy on the
vaginal microbiomes of women with atrophic vaginitis. Sci Rep.
6:243802016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cauci S, Driussi S, De Santo D,
Penacchioni P, Iannicelli T, Lanzafame P, De Seta F, Quadrifoglio
F, de Aloysio D and Guaschino S: Prevalence of bacterial vaginosis
and vaginal flora changes in peri- and postmenopausal women. J Clin
Microbiol. 40:2147–2152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brooks JP, Edwards DJ, Blithe DL, Fettweis
JM, Serrano MG, Sheth NU, Strauss JF III, Buck GA and Jefferson KK:
Effects of combined oral contraceptives, depot medroxyprogesterone
acetate and the levonorgestrel-releasing intrauterine system on the
vaginal microbiome. Contraception. 95:405–413. 2017. View Article : Google Scholar
|
|
33
|
Bradshaw CS, Vodstrcil LA, Hocking JS, Law
M, Pirotta M, Garland SM, De Guingand D, Morton AN and Fairley CK:
Recurrence of bacterial vaginosis is significantly associated with
posttreatment sexual activities and hormonal contraceptive use.
Clin Infect Dis. 56:777–786. 2013. View Article : Google Scholar
|
|
34
|
Garcia-Garcia RM, Arias-Álvarez M,
Jordán-Rodríguez D, Rebollar PG, Lorenzo PL, Herranz C and
Rodríguez JM: Female reproduction and the microbiota in mammals:
Where are we? Theriogenology. 194:144–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fettweis JM, Serrano MG, Brooks JP,
Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L,
Glascock AL, et al: The vaginal microbiome and preterm birth. Nat
Med. 25:1012–1021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serrano MG, Parikh HI, Brooks JP, Edwards
DJ, Arodz TJ, Edupuganti L, Huang B, Girerd PH, Bokhari YA, Bradley
SP, et al: Racioethnic diversity in the dynamics of the vaginal
microbiome during pregnancy. Nat Med. 25:1001–1011. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
MacIntyre DA, Chandiramani M, Lee YS,
Kindinger L, Smith A, Angelopoulos N, Lehne B, Arulkumaran S, Brown
R, Teoh TG, et al: The vaginal microbiome during pregnancy and the
postpartum period in a European population. Sci Rep. 5:89882015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Zhai Q, Wang J, Ma X, Xing B, Fan
H, Gao Z, Zhao F and Liu W: Variation of the vaginal microbiome
during and after pregnancy in Chinese women. Genomics Proteomics
Bioinformatics. 20:322–333. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shachar BZ, Mayo JA, Lyell DJ, Baer RJ,
Jeliffe-Pawlowski LL, Stevenson DK and Shaw GM: Interpregnancy
interval after live birth or pregnancy termination and estimated
risk of preterm birth: A retrospective cohort study. BJOG.
123:2009–2017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McKinney D, House M, Chen A, Muglia L and
DeFranco E: The influence of interpregnancy interval on infant
mortality. Am J Obstet Gynecol. 216:316.e1–316.e9. 2017. View Article : Google Scholar
|
|
41
|
Kangatharan C, Labram S and Bhattacharya
S: Interpregnancy interval following miscarriage and adverse
pregnancy outcomes: Systematic review and meta-analysis. Hum Reprod
Update. 23:221–231. 2017.
|
|
42
|
Bobitt JR and Ledger WJ: Unrecognized
amnionitis and prematurity: A preliminary report. J Reprod Med.
19:8–12. 1977.PubMed/NCBI
|
|
43
|
Andrews WW, Hauth JC, Goldenberg RL, Gomez
R, Romero R and Cassell GH: Amniotic fluid interleukin-6:
Correlation with upper genital tract microbial colonization and
gestational age in women delivered after spontaneous labor versus
indicated delivery. Am J Obstet Gynecol. 173:606–612. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andrews WW, Goldenberg RL, Hauth JC,
Cliver SP, Conner M and Goepfert AR: Endometrial microbial
colonization and plasma cell endometritis after spontaneous or
indicated preterm versus term delivery. Am J Obstet Gynecol.
193:739–745. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hillier SL, Krohn MA, Cassen E, Easterling
TR, Rabe LK and Eschenbach DA: The role of bacterial vaginosis and
vaginal bacteria in amniotic fluid infection in women in preterm
labor with intact fetal membranes. Clin Infect Dis. 20(Suppl 2):
S276–S278. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leitich H and Kiss H: Asymptomatic
bacterial vaginosis and intermediate flora as risk factors for
adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol.
21:375–390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hay PE, Lamont RF, Taylor-Robinson D,
Morgan DJ, Ison C and Pearson J: Abnormal bacterial colonisation of
the genital tract and subsequent preterm delivery and late
miscarriage. BMJ. 308:295–298. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leitich H, Bodner-Adler B, Brunbauer M,
Kaider A, Egarter C and Husslein P: Bacterial vaginosis as a risk
factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol.
189:139–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Carey JC, Klebanoff MA, Hauth JC, Hillier
SL, Thom EA, Ernest JM, Heine RP, Nugent RP, Fischer ML, Leveno KJ,
et al: Metronidazole to prevent preterm delivery in pregnant women
with asymptomatic bacterial vaginosis. National institute of child
health and human development network of maternal-fetal medicine
units. N Engl J Med. 342:534–540. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Klebanoff MA, Carey JC, Hauth JC, Hillier
SL, Nugent RP, Thom EA, Ernest JM, Heine RP, Wapner RJ, Trout W, et
al: Failure of metronidazole to prevent preterm delivery among
pregnant women with asymptomatic Trichomonas vaginalis infection. N
Engl J Med. 345:487–493. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brocklehurst P, Hannah M and McDonald H:
Interventions for treating bacterial vaginosis in pregnancy.
Cochrane Database Syst Rev. Jan 20–2000.Epub ahead of print.
PubMed/NCBI
|
|
52
|
Guise JM, Mahon SM, Aickin M, Helfand M,
Peipert JF and Westhoff C: Screening for bacterial vaginosis in
pregnancy. Am J Prev Med. 20(Suppl 3): S62–S72. 2001. View Article : Google Scholar
|
|
53
|
Koumans EH, Markowitz LE and Hogan V; CDC
BV Working Group: Indications for therapy and treatment
recommendations for bacterial vaginosis in nonpregnant and pregnant
women: A synthesis of data. Clin Infect Dis. 35(Suppl 2):
S152–S172. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
54
|
Petrova MI, van den Broek M, Balzarini J,
Vanderleyden J and Lebeer S: Vaginal microbiota and its role in HIV
transmission and infection. FEMS Microbiol Rev. 37:762–792. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Peebles K, Velloza J, Balkus JE,
McClelland RS and Barnabas RV: High global burden and costs of
bacterial vaginosis: A systematic review and meta-analysis. Sex
Transm Dis. 46:304–311. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Onderdonk AB, Delaney ML and Fichorova RN:
The human microbiome during bacterial vaginosis. Clin Microbiol
Rev. 29:223–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang
Y, Li L, Nelson KE, Xia Y and Xiang C: Molecular analysis of the
diversity of vaginal microbiota associated with bacterial
vaginosis. BMC Genomics. 11:4882010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Muzny CA and Schwebke JR: Gardnerella
vaginalis: Still a prime suspect in the pathogenesis of bacterial
vaginosis. Curr Infect Dis Rep. 15:130–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Garcia EM, Serrano MG, Edupuganti L,
Edwards DJ, Buck GA and Jefferson KK: Sequence comparison of
vaginolysin from different gardnerella species. Pathogens.
10:862021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vaneechoutte M, Guschin A, Van Simaey L,
Gansemans Y, Van Nieuwerburgh F and Cools P: Emended description of
Gardnerella vaginalis and description of Gardnerella leopoldii sp.
nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp.
nov., with delineation of 13 genomic species within the genus
Gardnerella. Int J Syst Evol Microbiol. 69:679–687. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ahmed A, Earl J, Retchless A, Hillier SL,
Rabe LK, Cherpes TL, Powell E, Janto B, Eutsey R, Hiller NL, et al:
Comparative genomic analyses of 17 clinical isolates of Gardnerella
vaginalis provide evidence of multiple genetically isolated clades
consistent with subspeciation into genovars. J Bacteriol.
194:3922–3937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Balashov SV, Mordechai E, Adelson ME and
Gygax SE: Identification, quantification and subtyping of
Gardnerella vaginalis in noncultured clinical vaginal samples by
quantitative PCR. J Med Microbiol. 63:162–175. 2014. View Article : Google Scholar
|
|
63
|
Georgijević A, Cjukić-Ivancević S and
Bujko M: Bacterial vaginosis. Epidemiology and risk factors. Srp
Arh Celok Lek. 128:29–33. 2000.In Serbian.
|
|
64
|
van de Wijgert JH, Borgdorff H, Verhelst
R, Crucitti T, Francis S, Verstraelen H and Jespers V: The vaginal
microbiota: what have we learned after a decade of molecular
characterization? PLoS One. 9:e1059982014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
France MT, Fu L, Rutt L, Yang H, Humphrys
MS, Narina S, Gajer PM, Ma B, Forney LJ and Ravel J: Insight into
the ecology of vaginal bacteria through integrative analyses of
metagenomic and metatranscriptomic data. Genome Biol. 23:662022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Garcia EM, Kraskauskiene V, Koblinski JE
and Jefferson KK: Interaction of gardnerella vaginalis and
vaginolysin with the apical versus basolateral face of a
three-dimensional model of vaginal epithelium. Infect Immun.
87:e00646–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ragaliauskas T, Plečkaitytė M, Jankunec M,
Labanauskas L, Baranauskiene L and Valincius G: Inerolysin and
vaginolysin, the cytolysins implicated in vaginal dysbiosis,
differently impair molecular integrity of phospholipid membranes.
Sci Rep. 9:106062019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jung HS, Ehlers MM, Lombaard H,
Redelinghuys MJ and Kock MM: Etiology of bacterial vaginosis and
polymicrobial biofilm formation. Crit Rev Microbiol. 43:651–667.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Swidsinski A, Dörffel Y, Loening-Baucke V,
Schilling J and Mendling W: Response of Gardnerella vaginalis
biofilm to 5 days of moxifloxacin treatment. FEMS Immunol Med
Microbiol. 61:41–46. 2011. View Article : Google Scholar
|
|
70
|
Castro J, Machado D and Cerca N: Unveiling
the role of Gardnerella vaginalis in polymicrobial Bacterial
Vaginosis biofilms: The impact of other vaginal pathogens living as
neighbors. ISME J. 13:1306–1317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jones HE, Harris KA, Azizia M, Bank L,
Carpenter B, Hartley JC, Klein N and Peebles D: Differing
prevalence and diversity of bacterial species in fetal membranes
from very preterm and term labor. PLoS One. 4:e82052009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Suff N, Karda R, Diaz JA, Ng J, Baruteau
J, Perocheau D, Tangney M, Taylor PW, Peebles D, Buckley SMK and
Waddington SN: Ascending vaginal infection using bioluminescent
bacteria evokes intrauterine inflammation, preterm birth, and
neonatal brain injury in pregnant mice. Am J Pathol. 188:2164–2176.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Randis TM, Gelber SE, Hooven TA, Abellar
RG, Akabas LH, Lewis EL, Walker LB, Byland LM, Nizet V and Ratner
AJ: Group B Streptococcus β-hemolysin/cytolysin breaches
maternal-fetal barriers to cause preterm birth and intrauterine
fetal demise in vivo. J Infect Dis. 210:265–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Romero R, Hassan SS, Gajer P, Tarca AL,
Fadrosh DW, Bieda J, Chaemsaithong P, Miranda J, Chaiworapongsa T
and Ravel J: The vaginal microbiota of pregnant women who
subsequently have spontaneous preterm labor and delivery and those
with a normal delivery at term. Microbiome. 2:182014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nelson DB, Shin H, Wu J and
Dominguez-Bello MG: The gestational vaginal microbiome and
spontaneous preterm birth among Nulliparous African American Women.
Am J Perinatol. 33:887–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Stout MJ, Zhou Y, Wylie KM, Tarr PI,
Macones GA and Tuuli MG: Early pregnancy vaginal microbiome trends
and preterm birth. Am J Obstet Gynecol. 217:356.e1–356.e18. 2017.
View Article : Google Scholar
|
|
77
|
Kindinger LM, Bennett PR, Lee YS, Marchesi
JR, Smith A, Cacciatore S, Holmes E, Nicholson JK, Teoh TG and
MacIntyre DA: The interaction between vaginal microbiota, cervical
length, and vaginal progesterone treatment for preterm birth risk.
Microbiome. 5:62017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Petrova MI, Reid G, Vaneechoutte M and
Lebeer S: Lactobacillus iners: Friend or Foe? Trends Microbiol.
25:182–191. 2017. View Article : Google Scholar
|
|
79
|
Srinivasan S, Hoffman NG, Morgan MT,
Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R,
Marrazzo JM and Fredricks DN: Bacterial communities in women with
bacterial vaginosis: High resolution phylogenetic analyses reveal
relationships of microbiota to clinical criteria. PLoS One.
7:e378182012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Santiago GL, Cools P, Verstraelen H, Trog
M, Missine G, El Aila N, Verhelst R, Tency I, Claeys G, Temmerman M
and Vaneechoutte M: Longitudinal study of the dynamics of vaginal
microflora during two consecutive menstrual cycles. PLoS One.
6:e281802011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tamrakar R, Yamada T, Furuta I, Cho K,
Morikawa M, Yamada H, Sakuragi N and Minakami H: Association
between Lactobacillus species and bacterial vaginosis-related
bacteria, and bacterial vaginosis scores in pregnant Japanese
women. BMC Infect Dis. 7:1282007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shipitsyna E, Roos A, Datcu R, Hallén A,
Fredlund H, Jensen JS, Engstrand L and Unemo M: Composition of the
vaginal microbiota in women of reproductive age-sensitive and
specific molecular diagnosis of bacterial vaginosis is possible?
PLoS One. 8:e606702013. View Article : Google Scholar
|
|
83
|
Verstraelen H, Verhelst R, Claeys G, De
Backer E, Temmerman M and Vaneechoutte M: Longitudinal analysis of
the vaginal microflora in pregnancy suggests that L. crispatus
promotes the stability of the normal vaginal microflora and that L.
gasseri and/or L. iners are more conducive to the occurrence of
abnormal vaginal microflora. BMC Microbiol. 9:1162009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Santiago GL, Tency I, Verstraelen H,
Verhelst R, Trog M, Temmerman M, Vancoillie L, Decat E, Cools P and
Vaneechoutte M: Longitudinal qPCR study of the dynamics of L.
crispatus, L. iners, A. vaginae, (sialidase positive) G. vaginalis,
and P. bivia in the vagina. PLoS One. 7:e452812012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Macklaim JM, Fernandes AD, Di Bella JM,
Hammond JA, Reid G and Gloor GB: Comparative meta-RNA-seq of the
vaginal microbiota and differential expression by Lactobacillus
iners in health and dysbiosis. Microbiome. 1:122013. View Article : Google Scholar
|
|
86
|
Gajer P, Brotman RM, Bai G, Sakamoto J,
Schütte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, et al:
Temporal dynamics of the human vaginal microbiota. Sci Transl Med.
4:132ra522012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Petricevic L, Domig KJ, Nierscher FJ,
Sandhofer MJ, Fidesser M, Krondorfer I, Husslein P, Kneifel W and
Kiss H: Characterisation of the vaginal Lactobacillus microbiota
associated with preterm delivery. Sci Rep. 4:51362014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Baldwin EA, Walther-Antonio M, MacLean AM,
Gohl DM, Beckman KB, Chen J, White B, Creedon DJ and Chia N:
Persistent microbial dysbiosis in preterm premature rupture of
membranes from onset until delivery. PeerJ. 3:e13982015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Brown RG, Marchesi JR, Lee YS, Smith A,
Lehne B, Kindinger LM, Terzidou V, Holmes E, Nicholson JK, Bennett
PR and MacIntyre DA: Vaginal dysbiosis increases risk of preterm
fetal membrane rupture, neonatal sepsis and is exacerbated by
erythromycin. BMC Med. 16:92018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Brown RG, Al-Memar M, Marchesi JR, Lee YS,
Smith A, Chan D, Lewis H, Kindinger L, Terzidou V, Bourne T, et al:
Establishment of vaginal microbiota composition in early pregnancy
and its association with subsequent preterm prelabor rupture of the
fetal membranes. Transl Res. 207:30–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
DiGiulio DB, Callahan BJ, McMurdie PJ,
Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DS, Wong RJ,
Shaw G, et al: Temporal and spatial variation of the human
microbiota during pregnancy. Proc Natl Acad Sci USA.
112:11060–11065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Callahan BJ, DiGiulio DB, Goltsman DSA,
Sun CL, Costello EK, Jeganathan P, Biggio JR, Wong RJ, Druzin ML,
Shaw GM, et al: Replication and refinement of a vaginal microbial
signature of preterm birth in two racially distinct cohorts of US
women. Proc Natl Acad Sci USA. 114:9966–9971. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hyman RW, Fukushima M, Jiang H, Fung E,
Rand L, Johnson B, Vo KC, Caughey AB, Hilton JF, Davis RW and
Giudice LC: Diversity of the vaginal microbiome correlates with
preterm birth. Reprod Sci. 21:32–40. 2014. View Article : Google Scholar :
|
|
94
|
Elovitz MA, Gajer P, Riis V, Brown AG,
Humphrys MS, Holm JB and Ravel J: Cervicovaginal microbiota and
local immune response modulate the risk of spontaneous preterm
delivery. Nat Commun. 10:13052019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sun S, Serrano MG, Fettweis JM, Basta P,
Rosen E, Ludwig K, Sorgen AA, Blakley IC, Wu MC, Dole N, et al:
Race, the vaginal microbiome, and spontaneous preterm birth.
mSystems. 7:e00017222022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kumar M, Murugesan S, Singh P, Saadaoui M,
Elhag DA, Terranegra A, Kabeer BSA, Marr AK, Kino T, Brummaier T,
et al: Vaginal microbiota and cytokine levels predict preterm
delivery in Asian women. Front Cell Infect Microbiol.
11:6396652021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kindinger LM, MacIntyre DA, Lee YS,
Marchesi JR, Smith A, McDonald JA, Terzidou V, Cook JR, Lees C,
Israfil-Bayli F, et al: Relationship between vaginal microbial
dysbiosis, inflammation, and pregnancy outcomes in cervical
cerclage. Sci Transl Med. 8:350ra1022016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Green ES and Arck PC: Pathogenesis of
preterm birth: Bidirectional inflammation in mother and fetus.
Semin Immunopathol. 42:413–429. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wahid HH, Dorian CL, Chin PY, Hutchinson
MR, Rice KC, Olson DM, Moldenhauer LM and Robertson SA: Toll-Like
receptor 4 is an essential upstream regulator of on-time
parturition and perinatal viability in mice. Endocrinology.
156:3828–3841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chin PY, Dorian CL, Hutchinson MR, Olson
DM, Rice KC, Moldenhauer LM and Robertson SA: Novel Toll-like
receptor-4 antagonist (+)-naloxone protects mice from
inflammation-induced preterm birth. Sci Rep. 6:361122016.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Deng W, Yuan J, Cha J, Sun X, Bartos A,
Yagita H, Hirota Y and Dey SK: Endothelial cells in the decidual
bed are potential therapeutic targets for preterm birth prevention.
Cell Rep. 27:1755–1768.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liassides C, Papadopoulos A, Siristatidis
C, Damoraki G, Liassidou A, Chrelias C, Kassanos D and
Giamarellos-Bourboulis EJ: Single nucleotide polymorphisms of
Toll-like receptor-4 and of autophagy-related gene 16 like-1 gene
for predisposition of premature delivery: A prospective study.
Medicine (Baltimore). 98:e173132019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Filipovich Y, Lu SJ, Akira S and Hirsch E:
The adaptor protein MyD88 is essential for E coli-induced preterm
delivery in mice. Am J Obstet Gynecol. 200:93.e1–8. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Montalbano AP, Hawgood S and Mendelson CR:
Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2
manifest delayed parturition and decreased expression of
inflammatory and contractile genes. Endocrinology. 154:483–498.
2013. View Article : Google Scholar :
|
|
105
|
Patni S, Wynen LP, Seager AL, Morgan G,
White JO and Thornton CA: Expression and activity of Toll-like
receptors 1-9 in the human term placenta and changes associated
with labor at term. Biol Reprod. 80:243–248. 2009. View Article : Google Scholar
|
|
106
|
Krediet TG, Wiertsema SP, Vossers MJ,
Hoeks SB, Fleer A, Ruven HJ and Rijkers GT: Toll-like receptor 2
polymorphism is associated with preterm birth. Pediatr Res.
62:474–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lorenz E, Hallman M, Marttila R, Haataja R
and Schwartz DA: Association between the Asp299Gly polymorphisms in
the Toll-like receptor 4 and premature births in the Finnish
population. Pediatr Res. 52:373–376. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Romero R, Xu Y, Plazyo O, Chaemsaithong P,
Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, et al:
A role for the inflammasome in spontaneous labor at term. Am J
Reprod Immunol. 79:e124402018. View Article : Google Scholar
|
|
109
|
Lindström TM and Bennett PR: The role of
nuclear factor kappa B in human labour. Reproduction. 130:569–581.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
McCarthy R, Martin-Fairey C, Sojka DK,
Herzog ED, Jungheim ES, Stout MJ, Fay JC, Mahendroo M, Reese J,
Herington JL, et al: Mouse models of preterm birth: Suggested
assessment and reporting guidelines. Biol Reprod. 99:922–937.
2018.PubMed/NCBI
|
|
111
|
Zhu B, Tao Z, Edupuganti L, Serrano MG and
Buck GA: Roles of the microbiota of the female reproductive tract
in gynecological and reproductive health. Microbiol Mol Biol Rev.
86:e00181212022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yudin MH, Landers DV, Meyn L and Hillier
SL: Clinical and cervical cytokine response to treatment with oral
or vaginal metronidazole for bacterial vaginosis during pregnancy:
A randomized trial. Obstet Gynecol. 102:527–534. 2003.PubMed/NCBI
|
|
113
|
Soto E, Romero R, Richani K, Yoon BH,
Chaiworapongsa T, Vaisbuch E, Mittal P, Erez O, Gotsch F, Mazor M
and Kusanovic JP: Evidence for complement activation in the
amniotic fluid of women with spontaneous preterm labor and
intra-amniotic infection. J Matern Fetal Neonatal Med. 22:983–992.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Romero R and Tartakovsky B: The natural
interleukin-1 receptor antagonist prevents interleukin-1-induced
preterm delivery in mice. Am J Obstet Gynecol. 167:1041–1045. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nadeau-Vallée M, Quiniou C, Palacios J,
Hou X, Erfani A, Madaan A, Sanchez M, Leimert K, Boudreault A,
Duhamel F, et al: Novel noncompetitive IL-1 receptor-biased ligand
prevents infection- and inflammation-induced preterm birth. J
Immunol. 195:3402–3415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Robertson SA, Christiaens I, Dorian CL,
Zaragoza DB, Care AS, Banks AM and Olson DM: Interleukin-6 is an
essential determinant of on-time parturition in the mouse.
Endocrinology. 151:3996–4006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Di Simone N, Santamaria Ortiz A, Specchia
M, Tersigni C, Villa P, Gasbarrini A, Scambia G and D'Ippolito S:
Recent insights on the maternal microbiota: Impact on pregnancy
outcomes. Front Immunol. 11:5282022020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chan D, Bennett PR, Lee YS, Kundu S, Teoh
TG, Adan M, Ahmed S, Brown RG, David AL, Lewis HV, et al:
Microbial-driven preterm labour involves crosstalk between the
innate and adaptive immune response. Nat Commun. 13:9752022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Gonzalez JM, Franzke CW, Yang F, Romero R
and Girardi G: Complement activation triggers metalloproteinases
release inducing cervical remodeling and preterm birth in mice. Am
J Pathol. 179:838–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Robertson SA, Skinner RJ and Care AS:
Essential role for IL-10 in resistance to
lipopolysaccharide-induced preterm labor in mice. J Immunol.
177:4888–4896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Osman I, Young A, Ledingham MA, Thomson
AJ, Jordan F, Greer IA and Norman JE: Leukocyte density and
pro-inflammatory cytokine expression in human fetal membranes,
decidua, cervix and myometrium before and during labour at term.
Mol Hum Reprod. 9:41–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Rinaldi SF, Catalano RD, Wade J, Rossi AG
and Norman JE: Decidual neutrophil infiltration is not required for
preterm birth in a mouse model of infection-induced preterm labor.
J Immunol. 192:2315–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mohd Zaki A, Hadingham A, Flaviani F,
Haque Y, Mi JD, Finucane D, Dalla Valle G, Mason AJ, Saqi M,
Gibbons DL and Tribe RM: Neutrophils dominate the cervical immune
cell population in pregnancy and their transcriptome correlates
with the microbial vaginal environment. Front Microbiol.
13:9044512022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cezar-de-Mello PFT, Ryan S and Fichorova
RN: The microRNA cargo of human vaginal extracellular vesicles
differentiates parasitic and pathobiont infections from
colonization by homeostatic bacteria. Microorganisms. 11:5512023.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zen M, Canova M, Campana C, Bettio S,
Nalotto L, Rampudda M, Ramonda R, Iaccarino L and Doria A: The
kaleidoscope of glucorticoid effects on immune system. Autoimmun
Rev. 10:305–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Busillo JM, Azzam KM and Cidlowski JA:
Glucocorticoids sensitize the innate immune system through
regulation of the NLRP3 inflammasome. J Biol Chem. 286:38703–38713.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hermoso MA, Matsuguchi T, Smoak K and
Cidlowski JA: Glucocorticoids and tumor necrosis factor alpha
cooperatively regulate toll-like receptor 2 gene expression. Mol
Cell Biol. 24:4743–4756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Baschant U and Tuckermann J: The role of
the glucocorticoid receptor in inflammation and immunity. J Steroid
Biochem Mol Biol. 120:69–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Franchimont D: Overview of the actions of
glucocorticoids on the immune response: A good model to
characterize new pathways of immunosuppression for new treatment
strategies. Ann NY Acad Sci. 1024:124–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hearps AC, Tyssen D, Srbinovski D, Bayigga
L, Diaz DJD, Aldunate M, Cone RA, Gugasyan R, Anderson DJ and
Tachedjian G: Vaginal lactic acid elicits an anti-inflammatory
response from human cervicovaginal epithelial cells and inhibits
production of pro-inflammatory mediators associated with HIV
acquisition. Mucosal Immunol. 10:1480–1490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Delgado-Diaz DJ, Tyssen D, Hayward JA,
Gugasyan R, Hearps AC and Tachedjian G: Distinct immune responses
elicited from cervicovaginal epithelial cells by lactic acid and
short chain fatty acids associated with optimal and non-optimal
vaginal microbiota. Front Cell Infect Microbiol. 9:4462020.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Galdeano CM and Perdigón G: The probiotic
bacterium Lactobacillus casei induces activation of the gut mucosal
immune system through innate immunity. Clin Vaccine Immunol.
13:219–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Rocha-Ramírez LM, Pérez-Solano RA,
Castañón-Alonso SL, Moreno Guerrero SS, Ramírez Pacheco A, García
Garibay M and Eslava C: Probiotic lactobacillus strains stimulate
the inflammatory response and activate human macrophages. J Immunol
Res. 2017:46074912017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Anton L, Sierra LJ, DeVine A, Barila G,
Heiser L, Brown AG and Elovitz MA: Common cervicovaginal microbial
supernatants alter cervical epithelial function: Mechanisms by
which lactobacillus crispatus contributes to cervical health. Front
Microbiol. 9:21812018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Dizzell S, Nazli A, Reid G and Kaushic C:
Protective effect of probiotic bacteria and estrogen in preventing
HIV-1-mediated impairment of epithelial barrier integrity in female
genital tract. Cells. 8:11202019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Qi W, Li H, Wang C, Li H, Zhang B, Dong M,
Fan A, Han C and Xue F: Recent advances in presentation, diagnosis
and treatment for mixed vaginitis. Front Cell Infect Microbiol.
11:7597952021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Dong M, Dong Y, Bai J, Li H, Ma X, Li B,
Wang C, Li H, Qi W, Wang Y, et al: Interactions between microbiota
and cervical epithelial, immune, and mucus barrier. Front Cell
Infect Microbiol. 13:11245912023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Schönfeld P and Wojtczak L: Short- and
medium-chain fatty acids in energy metabolism: The cellular
perspective. J Lipid Res. 57:943–954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Aldunate M, Srbinovski D, Hearps AC,
Latham CF, Ramsland PA, Gugasyan R, Cone RA and Tachedjian G:
Antimicrobial and immune modulatory effects of lactic acid and
short chain fatty acids produced by vaginal microbiota associated
with eubiosis and bacterial vaginosis. Front Physiol. 6:1642015.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Amabebe E and Anumba DOC: The vaginal
microenvironment: The physiologic role of lactobacilli. Front Med
(Lausanne). 5:1812018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Beghini J, Giraldo PC, Linhares IM, Ledger
WJ and Witkin SS: Neutrophil gelatinase-associated lipocalin
concentration in vaginal fluid: relation to bacterial vaginosis and
vulvovaginal candidiasis. Reprod Sci. 22:964–968. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Stafford GP, Parker JL, Amabebe E, Kistler
J, Reynolds S, Stern V, Paley M and Anumba DOC: spontaneous preterm
birth is associated with differential expression of vaginal
metabolites by lactobacilli-dominated microflora. Front Physiol.
8:6152017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Amabebe E and Anumba DOC: A combination of
cervicovaginal fluid glutamate, acetate and D-Lactate identified
asymptomatic low-risk women destined to deliver preterm: A
prospective cohort study. Reprod Sci. 29:915–922. 2022. View Article : Google Scholar
|
|
144
|
Ghartey J, Bastek JA, Brown AG, Anglim L
and Elovitz MA: Women with preterm birth have a distinct
cervicovaginal metabolome. Am J Obstet Gynecol. 212:776.e1–776.e12.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Srinivasan S, Morgan MT, Fiedler TL,
Djukovic D, Hoffman NG, Raftery D, Marrazzo JM and Fredricks DN:
Metabolic signatures of bacterial vaginosis. mBio. 6:e002042015.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Menon R, Jones J, Gunst PR, Kacerovsky M,
Fortunato SJ, Saade GR and Basraon S: Amniotic fluid metabolomic
analysis in spontaneous preterm birth. Reprod Sci. 21:791–803.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Lizewska B, Teul J, Kuc P, Lemancewicz A,
Charkiewicz K, Goscik J, Kacerovsky M, Menon R, Miltyk W and
Laudanski P: Maternal plasma metabolomic profiles in spontaneous
preterm birth: Preliminary results. Mediators Inflamm.
2018:93628202018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Smith DD and Rood KM: Intrahepatic
cholestasis of pregnancy. Clin Obstet Gynecol. 63:134–151. 2020.
View Article : Google Scholar
|
|
149
|
Tuckey RC: Progesterone synthesis by the
human placenta. Placenta. 26:273–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wilks M, Wiggins R, Whiley A, Hennessy E,
Warwick S, Porter H, Corfield A and Millar M: Identification and
H(2) O(2) production of vaginal lactobacilli from pregnant women at
high risk of preterm birth and relation with outcome. J Clin
Microbiol. 42:713–717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
O'Hanlon DE, Moench TR and Cone RA: In
vaginal fluid, bacteria associated with bacterial vaginosis can be
suppressed with lactic acid but not hydrogen peroxide. BMC Infect
Dis. 11:2002011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mitchell C, Fredricks D, Agnew K and Hitti
J: Hydrogen peroxide-producing lactobacilli are associated with
lower levels of vaginal interleukin-1β, independent of bacterial
vaginosis. Sex Transm Dis. 42:358–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Hemmerling A, Harrison W, Schroeder A,
Park J, Korn A, Shiboski S, Foster-Rosales A and Cohen CR: Phase 2a
study assessing colonization efficiency, safety, and acceptability
of Lactobacillus crispatus CTV-05 in women with bacterial
vaginosis. Sex Transm Dis. 37:745–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Mayer BT, Srinivasan S, Fiedler TL,
Marrazzo JM, Fredricks DN and Schiffer JT: Rapid and profound
shifts in the vaginal microbiota following antibiotic treatment for
bacterial vaginosis. J Infect Dis. 212:793–802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Bayar E, MacIntyre DA, Sykes L, Mountain
K, Parks TP, Lee PP and Bennett PR: Safety, tolerability, and
acceptability of Lactobacillus crispatus CTV-05 (LACTIN-V) in
pregnant women at high-risk of preterm birth. Benef Microbes.
14:45–56. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Tomusiak A, Strus M, Heczko PB, Adamski P,
Stefański G, Mikołajczyk-Cichońska A and Suda-Szczurek M: Efficacy
and safety of a vaginal medicinal product containing three strains
of probiotic bacteria: A multicenter, randomized, double-blind, and
placebo-controlled trial. Drug Des Devel Ther. 9:5345–5354. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Vasundhara D, Raju VN, Hemalatha R, Nagpal
R and Kumar M: Vaginal & gut microbiota diversity in pregnant
women with bacterial vaginosis & effect of oral probiotics: An
exploratory study. Indian J Med Res. 153:492–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Reid G, Charbonneau D, Erb J, Kochanowski
B, Beuerman D, Poehner R and Bruce AW: Oral use of Lactobacillus
rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal
flora: Randomized, placebo-controlled trial in 64 healthy women.
FEMS Immunol Med Microbiol. 35:131–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ho M, Chang YY, Chang WC, Lin HC, Wang MH,
Lin WC and Chiu TH: Oral Lactobacillus rhamnosus GR-1 and
Lactobacillus reuteri RC-14 to reduce Group B Streptococcus
colonization in pregnant women: A randomized controlled trial.
Taiwan J Obstet Gynecol. 55:515–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Anukam KC, Osazuwa E, Osemene GI,
Ehigiagbe F, Bruce AW and Reid G: Clinical study comparing
probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal
gel to treat symptomatic bacterial vaginosis. Microbes Infect.
8:2772–2776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Hemalatha R, Mastromarino P, Ramalaxmi BA,
Balakrishna NV and Sesikeran B: Effectiveness of vaginal tablets
containing lactobacilli versus pH tablets on vaginal health and
inflammatory cytokines: A randomized, double-blind study. Eur J
Clin Microbiol Infect Dis. 31:3097–3105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Husain S, Allotey J, Drymoussi Z, Wilks M,
Fernandez-Felix BM, Whiley A, Dodds J, Thangaratinam S, McCourt C,
Prosdocimi EM, et al: Effects of oral probiotic supplements on
vaginal microbiota during pregnancy: A randomised, double-blind,
placebo-controlled trial with microbiome analysis. BJOG.
127:275–284. 2020. View Article : Google Scholar
|
|
163
|
Gille C, Böer B, Marschal M, Urschitz MS,
Heinecke V, Hund V, Speidel S, Tarnow I, Mylonas I, Franz A, et al:
Effect of probiotics on vaginal health in pregnancy. EFFPRO, a
randomized controlled trial. Am J Obstet Gynecol.
215:608.e1–608.e7. 2016. View Article : Google Scholar
|
|
164
|
Govinden G, Parker JL, Naylor KL, Frey AM,
Anumba DOC and Stafford GP: Inhibition of sialidase activity and
cellular invasion by the bacterial vaginosis pathogen Gardnerella
vaginalis. Arch Microbiol. 200:1129–1133. 2018. View Article : Google Scholar : PubMed/NCBI
|