Introduction
Obesity, defined by an excessive accumulation of
body fat resulting from chronic nutrient overconsumption, has
become a significant global public health issue, not only due to
its high prevalence, but also due to its associated comorbidities
and the resulting disruption of adipocyte-immune cell interactions
within adipose tissue (1,2).
As of 2016, ~650 million adults worldwide were classified as obese,
with projections estimating that this number could reach four
billion by the year 2035. In many developed nations, it is
anticipated that nearly one-third of individuals will be affected
in the coming years (3).
Obesity profoundly affects multiple organ systems
through mechanisms involving oxidative stress, inflammation and
metabolic dysregulation (4,5).
Excessive nutrient intake leads to adipose tissue expansion and
ectopic lipid accumulation, particularly in the liver, where it
contributes to metabolic dysfunction-associated steatotic liver
disease, characterized by mitochondrial dysfunction and the
increased production of reactive oxygen species (ROS) (6-10). Oxidative stress promotes
hepatocellular injury and inflammation, thereby advancing disease
progression (11,12). In skeletal muscle, obesity
promotes the accumulation of intermuscular and intramyocellular
lipids, which impairs muscle regeneration and increases the risk of
atrophy and sarcopenia through mechanisms of lipotoxicity (13,14). The brain is not spared; obesity
leads to hypothalamic inflammation and gliosis, disrupting appetite
regulation and energy homeostasis by impairing neuronal integrity
in this critical region (15).
Moreover, obesity-induced chronic low-grade
inflammation, mediated by adipose tissue macrophages and cytokines,
exacerbates oxidative damage and disrupts metabolic homeostasis.
These interconnected processes underlie the pathogenesis of
metabolic syndrome, increasing cardiovascular risk and organ
dysfunction. Thus, oxidative stress is a central mediator linking
obesity to multisystemic metabolic disturbances (4,16,17).
Despite extensive research into the metabolic and
cardiovascular complications of obesity, its effects on the
reproductive system, particularly at the molecular and cellular
levels, have received comparatively less attention. Recent evidence
underscores that obesity profoundly disrupts reproductive health in
both males and females, impairing gametogenesis, hormonal balance,
fertilization, and subsequent embryonic and postnatal development
(16,18,19). The mechanisms of these effects
are multifactorial and include oxidative stress, altered
steroidogenesis, disrupted endocrine signaling along the
hypothalamic-pituitary-gonadal axis, mitochondrial dysfunction,
epigenetic modifications and chronic inflammation (5,20-23). In females, obesity is associated
with impaired oocyte quality, abnormal follicular environments,
ovulatory dysfunction, and reduced implantation and live birth
rates (18). In males, excess
adiposity is associated with reduced sperm quality, altered hormone
profiles, and increased sperm DNA damage, with evidence of
intergenerational transmission of metabolic dysfunction mediated by
epigenetic changes (24,25). Furthermore, obesity during
pregnancy increases the risk of gestational complications,
adversely affects placental function, and programs offspring for
metabolic and reproductive disorders later in life (26). However, the field lacks
comprehensive mechanistic frameworks that distinguish
obesity-specific effects from those mediated by associated
metabolic disorders or nutritional factors.
Despite these insights, a comprehensive review that
synthesizes currently available knowledge on the mechanisms through
which obesity affects the reproductive system from fertilization
through post-uterine development remains lacking, at least to the
best of our knowledge. The present review thus aimed to fill this
gap by providing an integrative overview of the molecular
mechanisms by which obesity impairs reproductive function, focusing
on oxidative stress and its downstream effects at each stage, from
gamete quality and fertilization to embryonic development and
postnatal outcomes. By elucidating these pathways, it is hoped that
the present review will inform future research and clinical
strategies to mitigate the reproductive and developmental
consequences of obesity.
Obesity as a driver of testicular
inflammation
The male reproductive tract consists of multiple
organs, including the testes, epididymis and prostate gland, which
collectively enable sperm development and androgen production
(27). Within the testes,
spermatogenesis takes place inside seminiferous tubules lined by
Sertoli cells. These cells support sperm maturation, uphold
structural integrity, and establish the blood-testis barrier to
protect developing germ cells from toxins and immune responses
(28). Spermatogenesis
progresses from spermatogonia through several developmental stages
tightly regulated by testosterone and follicle-stimulating hormone
(29-31). To study male reproduction,
rodents that share comparable reproductive features and are
frequently employed in reproductive studies (32,33) are used.
Obesity may compromise both sperm development and
the blood-testis barrier through systemic inflammation, although
exact pathways involved remain unclear. The present comprehensive
overview discusses the complex association between obesity,
particularly the accumulation of excess fatty acids and sugar, and
signaling pathways involved in testicular development, as well as
sperm maturation, motility, morphology and DNA integrity.
Diet-induced obesity and its impact on
testicular function and hormonal regulation
Research utilizing high-fat diet (HFD) models to
induce obesity has revealed significant effects of obesity on
testicular function. A previous study which was performed as early
as 2001 demonstrated that HFD-induced obesity increased the weight
of the ventral prostate in Sprague-Dawley rats (34). It was the first-ever study to
demonstrate a direct effect of obesity caused by saturated fat
consumption on the reproductive tract, laying the groundwork for
future research into how excess fat intake induced by obesity
affects testicular function. Another notable study on Wistar rats
aimed to evaluate the adaptive responses in the testes of adult
rats treated with a non-toxic dose of the environmental chemical
dichlorodiphenyldichloroethylene (DDE), either alone or in
association with a HFD (35).
That study concluded that HFD and DDE induce cellular stress,
leading to antioxidant impairment, apoptosis and decreased levels
of the androgen receptor (AR) and serum testosterone, all of which
are associated with tissue damage. However, notable cellular
proliferation was observed, considered an adaptive response to
counterbalance tissue damage. Critical gaps exist in understanding
how HFD-induced obesity specifically affects key regulatory
pathways. Notably, a subsequent study examined the effect of both a
HFD and exercise on the 'controller' of gonadal development, the
KISS-1/G/G protein-coupled receptor 54 (GPR54) system, in the
testes of growing rats (36).
The increase of KISS-1/GPR54 during the testicular growing period,
as measured using RT-qPCR and western blot analysis, was abolished
by HFD feeding (36). Notably,
that study also revealed that the regular testicular growing
pattern, as well as normal KISS-1/GPR54 expression, can be restored
through moderate-intensity aerobic exercise at 60-70%
VO2 max (36). An
interesting finding was reported in the study by Ma et al
(37) regarding how zinc
improves semen parameters in HFD-fed male rats by regulating the
expression of long non-coding RNA (lncRNA) in testicular
tissue.
Diet-induced reproductive dysfunction in
males
While studies above focused on long-term HFD
feeding, which induces obesity, the study by Falvo et al
(38) examined the effects of
short-term HFD feeding. They proved that HFD implementation as
short as 5 weeks can reduce steroidogenesis, increase the apoptosis
of spermatogenic cells, alter spermatogenesis with reduced protein
levels of meiotic and post-meiotic markers, and notably compromise
blood-testis barrier integrity through sirtuin 1 (SIRT1)/nuclear
factor erythroid 2-related factor 2 (NRF2)/mitogen-activated
protein kinase (MAPK) signaling pathways (38). Moreover, a high-fat, vitamin
D-deficient diet in Sprague-Dawley rats has been shown to reduce
sperm motility, mitochondrial function and fertilizing capacity
(39).
Obesity, characterized by the excess accumulation of
free fatty acids and glucose in blood and cells, is known to
elevate ROS production (40).
Multiple animal studies have confirmed that, for instance, HFD
triggers the accumulation of ROS in sperm and testicular tissue,
thereby leading to reproductive impairment. For example, a study
using an animal model of HFD demonstrated that HFD-fed mice
displayed a decline in the levels of antioxidant enzymes, such as
glutathione peroxidase (GPX), catalase and superoxide dismutase
(SOD), in testicular and epididymal tissues compared to mice fed a
control diet (41). In line with
this finding, mature sperm in HFD-fed mice exhibited higher ROS
levels and decreased levels of GPX1 protein, resulting in impaired
mitochondrial structural integrity and reduced mitochondrial
membrane potential, as well as decreased ATP production. Notably,
considering the significant impairment of mitochondrial function,
sperm motility was found to be reduced. Additionally, the same
conclusions were found to be true for obese males in the scope of
the study (41).
Another study examined the obesity-related increase
in intestinal permeability, which allows the passage of intestinal
bacteria into the circulation, known as metabolic endotoxemia (ME)
(42). That study on 37
infertile males revealed an association between obesity-related ME,
measured as serum zonulin, and impaired sperm DNA integrity. The
association was found to be persistent even after adjusting for
confounding factors, such as age and duration of abstinence.
However, the exact molecular mechanisms involved were not discussed
(42). The key findings of the
study by Bakos et al (43) on C57/Bl6 male mice fed a HFD were
that obesity significantly decreased the percentage of motile
spermatozoa (36 vs. 44% in controls) and that this reduction in
motility was associated with altered energy metabolism and
increased redox imbalance in sperm cells. The study by Han et
al (44) reached the same
conclusions by observing abnormal testicular structures on a larger
scale and detecting excessive oxidative stress, as well as enhanced
apoptosis and impaired glucose metabolism markers, on a molecular
scale.
The impact of obesity on the male reproductive
system has been observed from another perspective through the use
of high-sugar mouse models, thereby mimicking metabolic syndrome
(MetS). The key finding of the study by Rahali et al
(45) was that MetS induces
severe testicular toxicity in male rats, as measured by BAX
downregulation, BCL2 upregulation and a decreased
testosterone level, which was associated with the downregulation of
cytochrome P450 family (CYP)11A1, CYP17A1 and 17β HSD
testicular markers. In another study, hypercholesterolemic rabbits,
fed a diet supplemented with 0.05% cholesterol, concluded that
excess cholesterol leads to a reduced semen volume and sperm
motility, as well as changes in sperm morphology (head and
flagellum) (46). The additional
impact of obesity on the human and rodent male reproductive systems
is presented in Table I.
 | Table IEffects of obesity on the human and
rodent male reproductive systems. |
Table I
Effects of obesity on the human and
rodent male reproductive systems.
| Model | Treatment | Effect | (Refs.) |
|---|
| Sprague-Dawley
rats | 16-week HFD | ↓ Testosterone
synthesis
↑ Spermatogenic cell apoptosis | (57) |
| Ldlr−/−
Leiden | 24-week HFD + BA
administration | ↑ Plasma leptin
abnormal testosterone levels aberrant ITO
↑ Level of tubules in stages VII/VIII | (58) |
| Rats with
STz-NA-induced diabetes | HFD until 12 weeks
of age | ↓ Epididymal sperm
motility
↓ Histone-protamine transition
↓ Plasma testosterone
↓ Sperm count | (59) |
| Wistar rats | 4-week HFD | ↓ Epididymal sperm
maturationas
↓ Testosterone levels
↓ Sperm viability
↓ Capacitation | (60) |
| C57BL/6 mice | 8-week HFD + diet
reversal | disrupted
testicular BTB integrity
↑ levels of oxidative stress abnormal expression of BTB-related
proteins | (61) |
| C57Bl/6 mice
AdipoR1 KO mice Akita mice | 28-week HFD
(C57Bl/6) and standard chow (AdipoR1 KO, Akita) | ↓ Sperm count,
motility fertilizing ability and AdipoR1 gene and protein
(in HFD)
↑ Pro-apoptotic genes and poteins (in HFD)
↓ Testes weight, sperm count, motility, and fertilizing ability,
phosphorylation of AMPK (in AdipoR1 KO)
↑ Pro-apoptotic proteins, CASP6 activity and pathologically
apoptotic seminiferous tubules (in AdipoR1 KO) | (62) |
| C57BL/6J mice | 10-week HFD +
exercise | ↓ LEP-JAK-STAT
pathway
↓ Expression of SF-1, StAR, CYP11A1
↓ Sserum testosterone-to-estradiol ratio, sperm quality effect can
be reversed by exercise | (63) |
| Wistar rats | 16-week HFD | ↓ Male fertility,
fertilization, cell adhesion genes
↑ Obesity, lipid metabolism-related, immune response-related and
CYP genes
↑ Aromatase activity | (64) |
| Wistar rats | 38-week
hyperglycidic diet | 15 Proteins with
differential abundance | (65) |
On the other hand, the relation of obesity to
testicular cancer (TC) appears to be heterogeneous. For instance,
tentative evidence of an inverse association between body mass
index (BMI) and testicular germ-cell tumors (TGCT) was provided in
a previous meta-analysis (47).
Additionally, a Norwegian study found that the risk of developing
TC decreased with adult BMI (48). On the other hand, a high BMI was
more prevalent in TGCT cases than in the control group, according
to Dieckmann et al (49).
In a study on Canadian males, it was demonstrated that a high
dairy, particularly cheese, intake was associated with an elevated
risk of developing TC (50).
Considering that body fat composition serves as a more effective
indicator of obesity than BMI, in another study, computed
tomography was used to determine visceral adipose tissue (VAT),
subcutaneous adipose tissue (SAT) and skeletal muscle area ratios,
as well as TC stage (51). That
study determined a statistically significant moderate positive
association between testicular tumor stage and the VAT/SAT ratio.
While this topic requires further assessment, an interesting
finding was reported in the study by Puri et al (52), who stated that the connection
between TC and obesity was bidirectional. More specifically, it
appears that TC survivors are at an increased risk of metabolic
syndrome and obesity development.
While several studies have assessed the impact of
obesity on testicular tissue and sperm, only a small number of
studies have examined the potential for reversing its effects. A
recent study claimed that micronutrient-based antioxidant
intervention, containing added folate, vitamin B6, choline, betaine
and zinc in rats can ameliorate the effects of a HFD on testicular
tissue (53). Briefly, at 30.5
weeks of post-weaning HFD, sperm was harvested and analyzed for
sperm count and 8-hydroxy-2'-deoxyguanosine (8-OHdG) content and
testes were analyzed for 8-OhdG, malondialdehyde, folate and
glutathione content, the activity of SOD, catalase and GPX,
susceptibility index of pro-oxidative damage and mRNA the
expression of Nrf2, NFκB-p65, interleukin
(IL)-6 and IL-10, and Tnfa. Notably,
micronutrient-based antioxidant intervention in HFD significantly
mitigated HFD-induced sperm and testicular oxidative damage by
enhancing testicular antioxidant capacity through the regulation of
the parameters above (53).
Subsequently, Suleiman et al (54) demonstrated the therapeutic
potential of bee bread, the outcome of the fermentation from the
combination of pollen, digestive enzymes found in saliva and
nectar, on obesity-induced testicular-derived oxidative stress,
inflammation and apoptosis in rats fed a HFD (54). They demonstrated that
administering bee bread in combination with a HFD diminished the
detrimental effects of a HFD, including a decrease in antioxidant
enzymes and proliferating cell nuclear antigen immunostaining, as
well as an increase in inflammation and apoptosis markers (e.g.,
cleaved caspase-3) in the testes (54). Additional research has
demonstrated the ameliorative effects of Myrtus communis L.
extract on HFD-induced damage to the testes in rats, through the
inhibition of oxidative stress (55). In another study, to assess
whether physical exercise can effectively ameliorate
obesity-induced abnormalities in male fertility, the sperm
parameters (motility, concentration, the ability to undergo
capacitation and the acrosome reaction) of HFD-fed mice that
underwent physical labor were evaluated (56). That study provided evidence that
the adverse effects of obesity can be mitigated through physical
exercise. Additionally, that study identified several small
non-coding RNAs, particularly microRNAs (miRNAs/miRs; miR-6538,
miR-129-1, miR-7b, miR-196a-1, miR-872, miR-21a, miR-143 and
miR-200a), in germ cells that may play a crucial role in the
effects of obesity and physical exercise on spermatozoa (56).
In summary, data unequivocally suggest that
excessive fat and sugar intake exert disruptive effects on all
aspects of male reproduction, including sperm maturation,
mitochondrial structure and function, oxidative stress levels, and
sperm motility in both humans and rodent obesity models. However,
several reports claim that normal testicular function can be
restored through physical exercise and therapeutic
intervention.
Metabolic syndrome: Molecular pathways
linking MetS to male infertility
MetS is characterized by a group of conditions,
including insulin resistance, dyslipidemia, hypertension and
chronic low-grade inflammation. Beyond obesity alone, MetS exerts
significant detrimental effects on male fertility. Insulin
resistance disrupts insulin signaling pathways that are critical
for testosterone biosynthesis in Leydig cells. This leads to
hypogonadism and decreased support for spermatogenesis (66). Dyslipidemia promotes lipid
peroxidation in sperm membranes, compromising motility and
acrosomal function. Moreover, hypertension-induced endothelial
dysfunction reduces testicular perfusion, exacerbating oxidative
stress and hypoxia (67).
Chronic inflammation, driven by cytokines, such as TNF-α and IL-6
secreted from adipose tissue, disrupts the blood-testis barrier.
This occurs through the activation of the MAPK and NF-κB signaling
pathways, enabling immune cell infiltration. Subsequently, germ
cell apoptosis also becomes increased (66,67).
Metabolic syndrome also alters adipokine profiles.
Elevated leptin (hyperleptinemia) and reduced adiponectin levels
negatively affect the hypothalamic-pituitary-gonadal axis. This
impairment further decreases testosterone production and
spermatogenesis (68). Emerging
evidence also implicates epigenetic modifications in sperm from
males with MetS, including aberrant DNA methylation and histone
acetylation patterns, which may contribute to the transgenerational
transmission of metabolic and reproductive dysfunction (66). Collectively, these metabolic and
molecular alterations underscore the critical role of MetS in male
infertility and highlight the need for integrated clinical
approaches targeting metabolic health to improve reproductive
outcomes.
Obesity as a driver of ovarian dysfunction
and female reproductive impairment
Located within the pelvis and supported by
ligaments, the female reproductive system consists of internal
organs (the ovaries, fallopian tubes, uterus, cervix and vagina),
as well as external genitalia (69,70). The ovaries lie adjacent to the
uterus and fimbriae and act as the site of oocyte development and
maturation (70). Apart from
producing eggs, they release estrogen and progesterone to regulate
the menstrual cycle and pregnancy. The fallopian tubes carry the
oocyte to the uterus and typically serve as the site of
fertilization; these tubes are segmented into fimbria,
infundibulum, ampulla, and isthmus (71). The muscular uterus supports
implantation and fetal growth (72).
Obesity has been proven to have marked effect on
female reproduction; it has been reported that obesity promotes
lipid accumulation and morphological changes across the uterus,
ovary and oviduct, with increased NF-κB and MAPK signaling
(73). Additionally, obesity
alters the immune cell profile in reproductive tissues, as detailed
by St-Germain et al (74).
The present comprehensive overview discusses the
mechanistic links between obesity, particularly the accumulation of
excess fatty acids and glucose, and the disruption of signaling
pathways in ovarian folliculogenesis, steroidogenesis and oocyte
quality.
Metabolic stress and follicular
development disruption induced by a high-fat diet
A pivotal study from 2014 demonstrated that
progressive obesity in female mice altered ovarian steroidogenic
enzymes and inflammatory pathways, with NF-κB signaling elevated by
12 weeks of age and STAR protein levels reduced by 50% after 24
weeks of exposure to a HFD (75). That foundational study revealed
temporal shifts in CYP11A1 and CYP19A1 expression,
impairing estrogen synthesis and follicular survival (75). Building on this, a study in 2015
demonstrated that hyperglycemic conditions during oocyte maturation
increase ROS by 40% and reduce developmental competence in antral
follicle-derived oocytes, despite normal growth during earlier
follicular stages (76). A
landmark study in 2016 established that a HFD directly reduces
primordial follicle reserves by 32% in mice through ovarian
macrophage infiltration and systemic inflammation, independent of
obesity phenotype, while compromising long-term fertility outcomes
(77).
Lipotoxicity, oxidative stress, and
mitochondrial dysfunction in ovarian cells
Exposure to a HFD induces lipotoxic stress in
ovarian cells, disrupting the follicular microenvironment and
compromising oocyte developmental competence. Wu et al
(78) demonstrated that HFD-fed
mice exhibited lipid accumulation in cumulus-oocyte complexes,
leading to oxidative stress (elevated ROS levels) and endoplasmic
reticulum stress markers (BiP/GRP78), which impaired fertilization
rates by 35% compared to the controls. Single-cell RNA sequencing
by Zhu et al (79)
revealed that HFD altered mitochondrial respiration pathways in
oocytes, resulting in the downregulation of Ppargc1a (a key
regulator of mitochondrial biogenesis) and disrupted metabolic
coupling between granulosa cells and oocytes, which led to delayed
embryo cleavage and reduced blastocyst formation. These defects
were associated with aberrant lipid metabolism in granulosa cells,
including the upregulation of fatty acid-binding protein 4 and the
dysregulation of cholesterol homeostasis (79).
Interventional studies have highlighted potential
therapeutic strategies. Choi et al (80) demonstrated that
high-molecular-weight chitosan supplementation in HFD-fed mice
reduced ovarian lipid droplets by 40% and restored ovulation rates
through improved insulin sensitivity and AMPK activation.
Similarly, Morimoto et al (81) identified HFD-induced glycolytic
disruptions in granulosa cells, characterized by reduced Hk2
and Pfkfb3 expression, which impairs pyruvate provision to
oocytes, a critical energy substrate for maturation. This metabolic
uncoupling was exacerbated in aged obese mice, where oocytes
displayed 50% lower ATP levels and increased aneuploidy rates.
These findings underscore that HFD-induced lipotoxicity in ovarian
cells disrupts structural and functional integrity at multiple
levels, from follicular communication to embryonic viability, with
redox imbalance and mitochondrial dysfunction as central mechanisms
(81).
Hormonal imbalance, inflammation and
fertility outcomes in obese females
Recent evidence suggests that HFD-induced oxidative
stress disrupts the ovarian microenvironment by impairing oocyte
mitochondrial function, thereby accelerating follicular atresia
through ROS-mediated DNA damage (82). These effects are compounded by
hypothalamic-pituitary-ovarian axis dysregulation, in which obese
women exhibit a 25% lower luteinizing hormone (LH) pulse amplitude
and elevated insulin levels that disrupt gonadotropin signaling
(83). Notably, interventions
show promise: A study published in 2024 identified that
antioxidant-rich diets containing phytonutrients and organosulfur
compounds can mitigate 68% of HFD-induced ovulation irregularities
by restoring hormonal balance (82). Concurrently, lifestyle
modifications combining Mediterranean-style diets with
moderate-intensity exercise (45-60% VO2max) improve
pregnancy rates in obese women by enhancing insulin sensitivity and
reducing inflammatory cytokines (84).
Studies on animals have demonstrated that female
rodents fed a HFD exhibit reduced ovarian antioxidant enzyme
activity, resulting in oocyte mitochondrial dysfunction. This
oxidative damage disrupts ATP production and induces lipid
peroxidation in granulosa cells, impairing follicular development.
Studies on humans corroborate these findings, demonstrating
elevated ROS levels in the follicular fluid of obese women
undergoing fertility treatments, which are associated with reduced
oocyte maturation rates and embryo quality (85-87).
The inflammatory milieu in obesity further
exacerbates redox imbalance through adipose-derived cytokines, such
as TNF-α and IL-6, which activate signaling pathways promoting
granulosa cell apoptosis (87).
A previous systematic review demonstrated that obese women with
elevated chemerin levels faced a 2.3-fold higher risk of
anovulatory cycles. Structural analyses of ovarian tissue from
HFD-fed mice exhibit a reduction in primordial follicle reserves
and stromal lipid accumulation, paralleled by downregulation of
enzymes essential for estrogen synthesis. Additionally,
dysregulated adipokines, such as leptin and adiponectin, alter GnRH
pulsatility, reducing LH and FSH secretion and further compromising
ovulation. This hormonal imbalance contributes to menstrual
irregularities, anovulation, and infertility (87). Moreover, obesity-related
alterations in adipokine levels exacerbate insulin resistance and
promote systemic inflammation, further disrupting ovarian function
and impairing folliculogenesis (88). A summary of molecular mechanisms
caused by obesity in both females and males is illustrated in
Fig. 1.
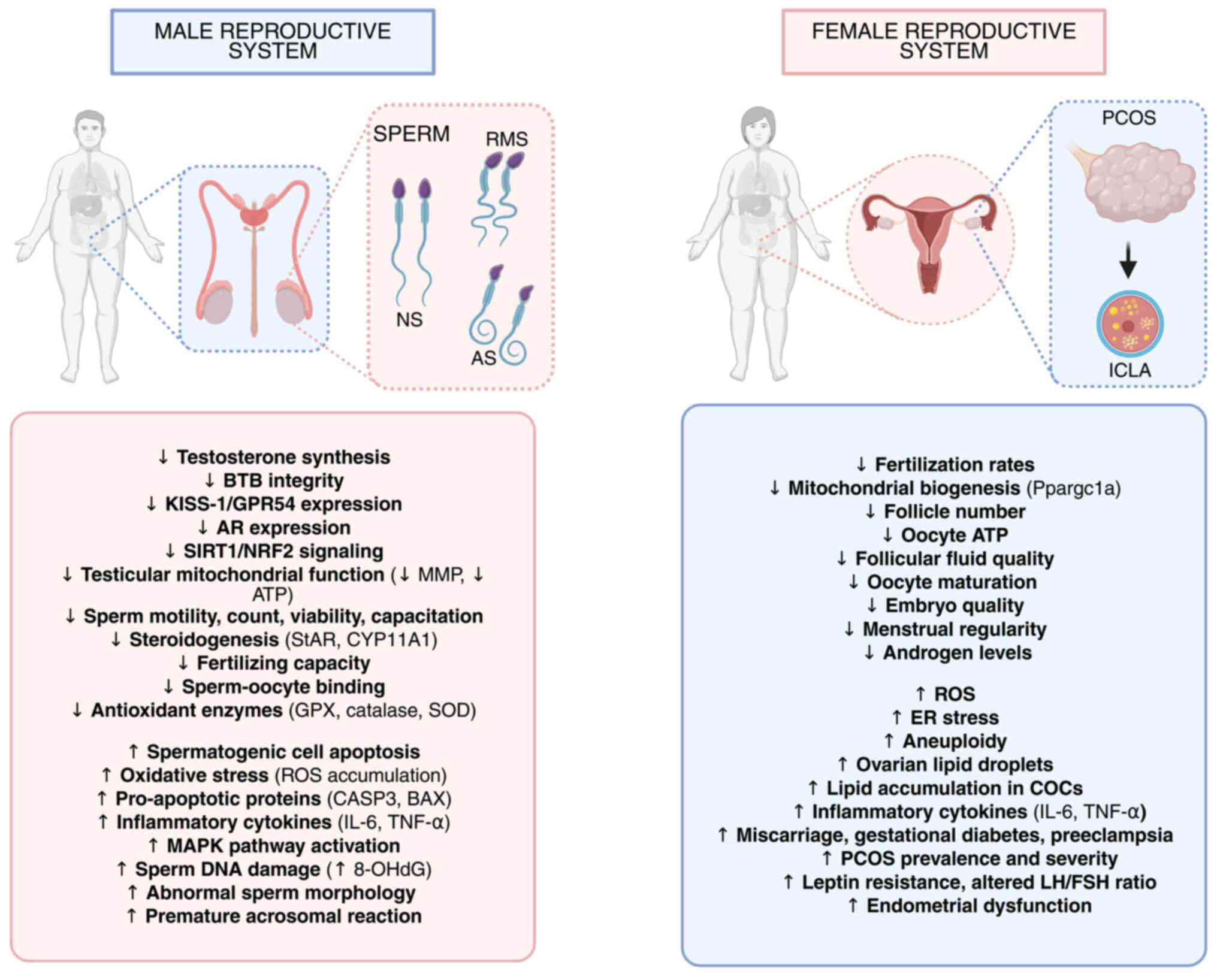 | Figure 1Schematic illustration of the effects
of obesity on male and female reproductive systems. On the left
panel, male obesity is proven to have a significant impact on the
development of normal sperm, thereby causing reduced motility sperm
and atypical sperm production. On the right panel, female obesity
is proven to cause altered ovarian morphology and increased
cytoplasmic lipid accumulation in oocytes. Further effects of
obesity in both female and male reproductive system physiology and
development are indicated as a downward-looking arrow (decrease)
and an upward-looking arrow (increase). NS, normal sperm; RMS,
reduced motility sperm; atypical sperm; ILCA, increased cytoplasmic
lipid accumulation; BTB, blood-testis barrier; KISS-1, KiSS-1
metastasis suppressor; GPR54, G protein-coupled receptor 54; AR,
androgen receptor; SIRT1, sirtuin 1; NRF2, nuclear factor erythroid
2-related factor 2; MMP, mitochondrial membrane potential; ATP,
adenosine triphosphate; StAR, steroidogenic acute regulatory
protein; CYP11A1, cytochrome P450 family 11 subfamily A member 1;
GPX, glutathione peroxidase; SOD, superoxide dismutase; ROS,
reactive oxygen species; CASP3, caspase 3; BAX, BCL2-associated X
protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha;
MAPK, mitogen-activated protein kinase; 8-OHdG,
8-hydroxy-2'-deoxyguanosine; PCOS, polycystic ovary syndrome; ICLA,
in vitro cultured large antral follicle; Ppargc1a,
peroxisome proliferator-activated receptor gamma coactivator
1-alpha; ER, endoplasmic reticulum; COCs, cumulus-oocyte complexes;
LH, luteinizing hormone; FSH, follicle-stimulating hormone. |
Epidemiological research has reported that women
with obesity have a 2.7-fold increased risk of infertility and a
25-37% higher risk of miscarriage compared to women of normal
weight (89). Obesity also
worsens the clinical and metabolic manifestations of polycystic
ovary syndrome (PCOS), a leading cause of female infertility, by
promoting hyperinsulinemia and hyperandrogenism, which impair
follicular development and ovulatory function. Additionally,
obesity negatively affects assisted reproductive technology (ART),
reducing pregnancy and live birth rates, and increasing the
likelihood of ART failure (90,91). The additional impact of obesity
on the human and rodent female reproductive systems is presented in
Table II.
 | Table IIEffects of obesity on the human and
rodent female reproductive systems. |
Table II
Effects of obesity on the human and
rodent female reproductive systems.
| Model |
Treatment/intervention | Effect | (Refs.) |
|---|
| CBA mice | 4-week HFD | Lipid accumulation
in COCs
↑ ROS,
↑ ER stress
↓ Fertilization rates (35%) | (78) |
| C57BL/6 mice | Single-cell RNA-seq
+ HFD | ↓ Ppargc1a
(mitochondrial biogenesis) impaired glucolipid metabolism delayed
embryo cleavage
↓ Follicle number | (96) |
| B6C3F1 mice | 4-week HFD +
WSC | ↓ Ovarian lipid
droplets (40%) restored ovulation via AMPK activation | (80) |
| C57BL/6J mice | HFD + aged
cohorts | ↓ Granulosa cell
Hk2/Pfkfb3
↓ Oocyte ATP (50%)
↑ Aneuploidy | (60) |
| Women
(clinical) | Obesity (BMI
≥30) | ↓ Follicular fluid
quality
↑ ROS
↓ Oocyte maturation
↓ Embryo quality | (78) |
| C57BL/6J mice | Maternal HFD
(preconception) | Altered
mitochondrial membrane potential (↓ΔΨm) in
oocytes/zygotes
↑ ROS | (97) |
| Women (PCOS) | Obesity | ↓ Menstrual
regularity
↑ Androgen levels
Suppressed ovulation
Effect can be reversed by metformin | (98) |
Apart from the physiological impact on the uterus
and ovaries, obesity increases the risk of developing endometrial
cancer, which can be decreased through weight loss by recruiting
protective immune cell types to the endometrium (92). Additionally, obesity influences
DNA methylation in pre-symptomatic endometrial epithelial cells,
and the persistent dysregulation of DNA methylation in obese women
may contribute to the development of endometrial cancer (93).
Weight loss through lifestyle modifications,
pharmacological intervention, or bariatric surgery has been shown
to improve menstrual regularity, reduce androgen levels, enhance
ovulation and increase spontaneous pregnancy rates in obese women.
These improvements underscore the importance of addressing obesity
as a modifiable risk factor to restore reproductive potential
(94,95).
In summary, substantial evidence suggests that the
excessive consumption of fats and sugars has a detrimental effect
on various aspects of female reproductive health. This includes
ovarian folliculogenesis, steroid hormone production, oxidative
balance and oocyte quality. These disruptions are consistently
observed in both human studies and animal models of obesity,
emphasizing the significant effects of metabolic dysregulation on
female fertility. Recent research suggests that lifestyle changes,
such as moderate-intensity exercise and targeted therapeutic
interventions like antioxidant supplementation, may help reduce
these harmful effects and restore normal ovarian function. These
findings highlight the importance of comprehensive strategies to
address obesity-related reproductive dysfunction in women.
Fertilization capacity as an indicator of
metabolic health
Placental development and physiology. Fertilization
is initiated when a sperm penetrates the zona pellucida of an egg,
forming a zygote. This process requires viable gametes, accurate
sperm transport, capacitation, hyperactivation, acrosome reaction
and membrane fusion (99-104).
Precise pH, calcium signaling, hormones, enzymatic activity and
molecular recognition all coordinate fertilization (104-106).
Initial connections between obesity and
fertilization emerged through the introduction and development of
in vitro fertilization (IVF). In 2011, researchers noted
that both PCOS and maternal obesity affected oocyte size in
IVF/intracytoplasmic sperm injection cycles, thereby establishing
the direct impact of metabolic health on gamete quality (100). Considering that the
mitochondria play vital roles in oocyte function, it is evident
that mitochondrial functionality is a direct indicator of oocyte
quality, which in turn affects fertilization potential and
development into viable offspring (107). In this context, a previous
retrospective study explored the effects of overweight and obesity
on the IVF outcomes of poor ovarian responders (PORs) (108). A total of 188 PORs undergoing
IVF cycles were stratified into three groups (normal weight PORs,
overweight PORs and obese PORs), where it was concluded that obese
PORs had decreased fertilization and clinical pregnancy rates. In
another study, the proteomic assessment of follicular fluid from
women undergoing IVF using liquid chromatography-mass spectrometry
analysis found 25 proteins to be significantly altered in the
follicular fluid from women with obesity (109).
Molecular and cellular mechanisms linking
obesity to impaired fertilization
From a molecular perspective, obesity impairs sperm
function. HFD-fed male mice demonstrate reduced sperm-oocyte
binding ability, indicating impaired sperm recognition (43). In another study, HFD exposure was
shown to decrease testicular estradiol, the E2-to-testosterone
ratio and the levels of critical fatty acids, such as
docosahexaenoic acid, leading to premature acrosomal exocytosis and
diminished fertilization capacity (110). In the context of fatty
acid-related sperm modulation, Cooray et al (111) extrapolated that polyunsaturated
FAs (PUFAs), more specifically ω-3 unsaturated fatty acids, have
the highest potency in modulating sperm ion channels to increase
sperm motility, which might indicate higher fertilization potential
(111). In another study, to
identify a molecular marker for reduced fertilization rates caused
by obesity, screening for changes in gene expression in the male
reproductive tract revealed a decreased cysteine-rich secretory
protein 4 (CRISP4) expression in the testis and epididymis of obese
mice (112). The importance of
CRISPR was confirmed when the same study successfully improved the
fertilization rate through CRISPR treatment of sperm from HFD mice
prior to in vitro fertilization. Another potential player in
obesity-related fertilization issues appears to be an inflammatory
marker named lipocalin 2, which remains to be explored in the
reproductive tracts of both females and males (113). Research on other species
supports these findings: Bulls on high-gain diets produce semen
with reduced blastocyst formation capacity following IVF, linked to
increased necrosis and acrosome damage (114). Similarly, rats on high-fat,
vitamin D-deficient diets have been shown to exhibit decreased
sperm motility and mitochondrial dysfunction, impairing fertilizing
ability (39). In females,
obesity-associated implantation failure is associated with an
altered uterine DNA methylation and a reduced Dnmt gene
expression (115).
By contrast, an intergenerational murine model
combining regular chow with a high-energy supplement indicated that
an overweight status was associated with higher ovulation rates and
the increased development of fertilized oocytes, although embryo
quality was poor (113,116).
Dependence of in utero development on
parental obesity
Impact of maternal obesity on placental function and
fetal organ development. The placenta is an essential organ for
normal in utero development in mammals, functioning as the
critical exchange site for nutrients, gases, and waste products
between mother and fetus (117). This transitory organ also
serves endocrine functions by producing hormones necessary for
maintaining pregnancy and regulating fetal growth. Considering the
established connection with the maternal blood supply, it is no
wonder that maternal obesity influences the in utero
development of fetal organ systems through the placenta (118).
The placenta itself is affected by maternal obesity,
with its effects being observable through changes in placental
morphology, metabolism, inflammation, oxidative state, endothelial
function and altered angiogenesis (119,120). Moreover, a previous study
concluded that human placentas exhibit sex-specific adaptive
changes in response to maternal obesity (121). Placental development and
function in a mouse model of diet-induced obesity were proven to be
impaired through the altered transcriptome of placenta progenitor
cells in the preimplantation (trophectoderm) and early
post-implantation (ectoplacental cone) placenta precursors
(121). Crucially, the
differential expression of major upstream transcriptional
regulators in developmental pathways, such as Wingless-related
integration site (Wnt) signaling, p38 MAPK, ERK and Toll-like
receptor 2 signaling, was found to be impaired (121). In line with this, uterine
natural killer cells, which play essential roles in coordinating
uterine angiogenesis and promoting maternal tolerance to fetal
tissue, were found to display an altered repertoire of natural
killer receptors (inhibitory KIR2DL1 and activating KIR2DS1
receptors), thereby impacting early developmental processes
(122). Similarly, a study
using a cohort of obese or lean women found that obesity leads to a
significant reduction in natural killer cell numbers, accompanied
by impaired uterine artery remodeling, potentially influencing
further fetal development (123). A murine study found that
obesity alters the mouse endometrial transcriptome, potentially
affecting the endometrium's ability to function during pregnancy
(124).
To assess the impact of placental and endometrial
changes identified in the aforementioned studies on fetal
development, several studies were conducted. Ford et al
(125) followed fetal
pancreatic development in a sheep model of obesity. They noted
that, although all organs were heavier in fetuses from obesogenic
(OB) ewes, only the pancreatic weight increased as a percentage of
fetal weight (125). This was
accompanied by 50% more insulin-positive cells per unit of
pancreatic area in fetuses from OB ewes, as well as the notion that
obesity accelerates fetal pancreatic beta-cell, but not alpha-cell,
development (122).
The muscular system has also been found to be
affected by maternal obesity and exposure to excessive
glucocorticoids (126).
Specifically, glucocorticoid exposure in embryonic and fetal
developmental stages has emerged as a cause of cardiovascular
disease and muscle atrophy in adulthood. Previous studies even
mention obesity-related epigenetic dysregulation in Prader-Willi
syndrome and the agouti gene (127,128). An interesting study examining
gut intestinal structure and placental vascularization found that
diet-induced obesity decreased the maternal intestinal levels of
short-chain fatty acids and their receptors, impaired gut barrier
integrity, and was associated with fetal intestinal inflammation
(129). Moreover, maternal
obesity during pregnancy has been shown to upregulate the insulin
signaling genes, IRS2, PIK3CB and SREBP1c, in
skeletal muscle and perirenal fat, thereby favoring insulin
sensitivity (130).
Notably, the results of a study performed on baboon
fetuses of obese baboon mothers who consumed a high-sugar, HFD
during pregnancy demonstrated that the fetuses developed a fatty
liver along with significant metabolic disruption in the liver
(131). Most notably, fetal
livers exhibited the dysregulation of the tricarboxylic acid cycle,
proteasome, oxidative phosphorylation, glycolysis and the
Wnt/β-catenin signaling pathway, one of the most critical
developmental signaling pathways, accompanied by marked lipid
accumulation (131). Another
study on baboons proved how maternal obesity affects fetal liver
androgen signaling through reduced CYP2b6 and CYP3A activity in
conjunction with decreased nuclear AR-45 expression. Notably, the
effect was proven to be sex-specific, affecting only males
(132).
Several studies have provided evidence of maternal
obesity-related impairment of kidney development (133,134). For instance, Zhou et al
(133) found a decreased number
of peroxisomes and an increase in inflammasomes, resulting in
pyroptosis and apoptosis in the fetal kidney of obese mothers.
Additionally, the Gomeroi Gaaynggal study discovered that children
born to obese mothers had a reduced kidney size relative to
estimated fetal weight, suggesting a degree of glomerular
hyperfiltration in utero (134). The study by Tain et al
(135) demonstrated that
maternal high-fructose intake causes programmed hypertension in
offspring through changes in the renal transcriptome.
Epigenetic and molecular mechanisms
linking maternal obesity to long-term offspring health
Emerging evidence also highlights the disruption of
fetal oocyte development by maternal obesity. The recent stud by
Tang et al (136)
demonstrated that obesity induced meiotic defects in fetal oocytes,
including synapsis abnormalities and increased aneuploidy rates,
alongside the epigenetic dysregulation of DNA methylation and
histone modifications at critical developmental genes, such as
Stra8 and Sycp3. These alterations were linked to
oxidative stress and impaired DNA repair mechanisms, suggesting a
direct intergenerational transmission of metabolic risks to
reproductive health (136).
Currently, there is limited research available
investigating whether maternal or paternal obesity directly
contributes to the occurrence of chromosomal abnormalities in
offspring, highlighting a significant gap in understanding the
parental origins of such genetic risks.
A previous study on obesity has brought epigenetic
mechanisms, including DNA methylation, histone modifications and
miRNAs into focus during pregnancy as a significant indicator of
disease susceptibility in later stages of human life (137). To combat the effects of obesity
on in utero development, the assessment of alternate-day
fasting (ADF) and time-restricted feeding (TRF) in obese pregnant
mice found contrasting effects on placental function and fetal
development. TRF was proven to be superior to ADF, as it enhanced
placental nutrient transport and fetal development by reducing
endoplasmic reticulum stress and inflammatory responses (138).
There is no doubt that parental obesity is a
critical indicator of impaired fetal development. The current
findings are summarized in Fig.
2. However, there is still room to further explore the
molecular effects of either paternal or maternal obesity on
specific organic systems.
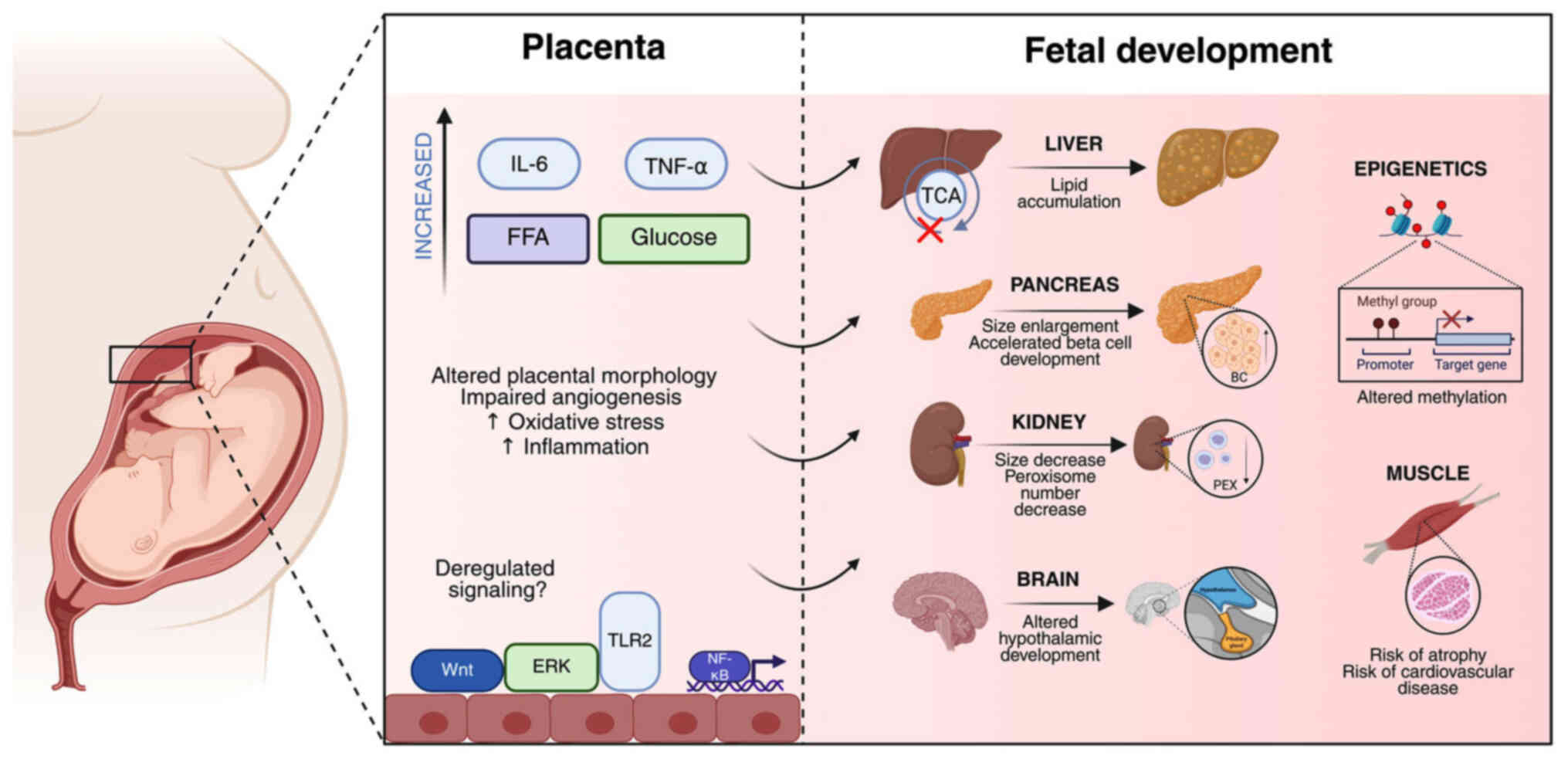 | Figure 2Schematic illustration of the
mechanistic pathways by which maternal obesity influences fetal
development via placental alterations. On the left panel, maternal
obesity is associated with increased circulating levels of
pro-inflammatory cytokines (IL-6, TNF-α), FFAs and glucose. Apart
from the increase in pro-inflammatory markers, altered placental
morphology, impaired angiogenesis, and increased oxidative stress
can also be observed. Importantly, deregulated placental signaling
pathways, including Wnt, ERK, and TLR2, as well as the activation
of NF-κB, collectively disrupt placental function. On the right
panel, these placental changes adversely impact multiple fetal
organ system development processes. The fetal liver exhibits lipid
accumulation and impaired TCA cycle activity. The pancreas shows
enlargement and accelerated BC development. The kidney exhibits a
reduced size and a decreased number of peroxisomes. The brain is
affected through altered hypothalamic and hippocampal development.
In addition, epigenetic modifications, such as altered methylation
patterns, which may program long-term disease susceptibility, are
caused by parental obesity. Fetal muscle is shown to have an
increased risk of atrophy and predisposition to cardiovascular
disease. Together, these pathways underscore the multifaceted
effects of maternal obesity on in utero development and
future offspring health. IL-6, interleukin-6; TNF-α, tumor necrosis
factor-alpha; FFA, free fatty acids; TCA, tricarboxylic acid; Wnt,
wingless-related integration site; ERK, extracellular
signal-regulated kinase; TLR2, Toll-like receptor 2; NF-κB, nuclear
factor κ-light-chain-enhancer of activated B cells; BC, beta cell;
PEX, peroxisome. |
Obesity and offspring
Research and mechanisms linking parental obesity to
offspring health. The association between maternal or paternal
obesity and health outcomes of the offspring has emerged as a
critical area of research over the past several decades.
In the early 90s, research focused on the outcomes
of pregnancy. For instance, a previous study in 1990 reported that
the perinatal mortality rate increased from 37 to 121 per 1,000 in
obese women, indicating that even since then, it was proven that
obese women were more likely to undergo premature labor with a
negative outcome (139).
Nevertheless, mechanistic insight as to what causes specific
pregnancy outcomes and further offspring development did not lack
behind. A study on amniotic fluid at 32 to 38 weeks of gestation
revealed that insulin levels at that time account for a
longitudinal correlation with relative obesity in children at the
age of 6 (140). That study
suggested that premature and excessive exposure to insulin during
gestation may predispose to obesity in childhood. In line withthis
finding, another study in 2005 provided evidence that children who
are large for gestational age at birth and exposed to an
intrauterine environment of either diabetes or maternal obesity are
at an increased risk of developing metabolic syndrome (141).
Recent studies have focused on the signaling
pathways involved in the effects of maternal obesity on the
offspring. The study by Moeckli et al (142) discovered that pathways involved
in metabolism (e.g., liver-related genes such as Egfr, Vegfb, Wnt2
and Pparg), the innate immune system, the clotting cascade and the
cell cycle were consistently dysregulated in the offspring of obese
mothers. Notably, female offspring exhibited higher non-alcoholic
liver disease prevalence, while males exhibited increased fibrosis,
indicating the different effects of the obese mother based on
offspring sex (142).
Furthermore, a review article provided a detailed explanation of
how maternal obesity affects hypothalamic development and later
function (143). Accordingly,
another review article in addressed the emerging role of the
hypothalamus in metabolic regulation in offspring of mothers with
maternal obesity (144). The
analysis of data from two French birth cohorts, EDEN (encompassing
all gestational ages) and EPIPAGE-2 (preterm children born between
24 and 34 weeks of gestation), revealed the impact of maternal
obesity on the cognitive development of children. In both cohorts,
pre-pregnancy obesity was associated with lower verbal intelligence
quotient (IQ) domains, resulting in lower IQ scores at 5 years
(145).
Sertorio et al (146) provided further knowledge on the
topic of the health implications of parental high-fat, high-sugar
diet intake on male offspring. Their study conducted on rats
evaluated multiple markers, including serum levels of testosterone
and FSH, testicular gene expression of steroidogenic enzymes,
epigenetic and inflammatory markers, as well as daily sperm
production, sperm transit time and sperm morphology (146). Of note, several factors were
influenced only by one parent: Maternal diet affected cytokine
production, testicular epigenetic parameters, inflammatory
response, oxidative balance and daily sperm production, while serum
testosterone levels were affected by the paternal diet (146). Furthermore, another study found
that cognitive deficits and impaired sphingolipid metabolism were
induced in offspring through both the maternal and paternal
lineages (147).
Epigenetic, cognitive and metabolic
impacts of parental obesity
Notably, a subsequent study of methylation profiles
of genome-wide CpG sites in blood samples from a pediatric
longitudinal cohort revealed the influence of the weight of mothers
on the methylome of offspring (148). Briefly, abundant DNA
methylation changes during child development from birth to 6 months
were detected in addition to DNA methylation biomarkers that could
discriminate children born to mothers who suffered from obesity or
obesity with gestational diabetes (148). Furthermore, another study aimed
to elucidate the effects of maternal obesity on offspring in
Kawasaki disease-like vasculitis and the underlying mechanisms
(149). The data of that study
demonstrated that maternal obesity led to more severe vasculitis
and induces altered cardiac structure in the offspring, and also
promoted the expression of pro-inflammatory cytokines through the
activation of the NF-κB signaling pathway (149). Maternal obesity during
pregnancy was recently connected to reduced hippocampal
ephrin-A3/EphA4 signaling in adult mouse offspring, thereby
impairing synaptic plasticity and gliovascular unit integrity, with
microglial activation indicating neuroinflammation (150). In 2024, Nelson and Friedman
(151) summarized the immense
impact of a maternal Western-style diet on the programming of the
fetal immune system.
Conversely, paternal obesity has been shown to have
a significant effect on the cognitive functions of offspring in
murine models. The underlying mechanism involves sperm-derived
methylation of the brain-derived neurotrophic factor (BDNF) gene
promoter, which disrupts BDNF/tyrosine receptor kinase B signaling
(132). Another study
demonstrated that offspring fertility appeared to be influenced by
paternal obesity, indicating that paternal obesity can span
multiple generations (152).
Evidence from that study demonstrated a transgenerational increase
in methyltransferase 3, N6-adenosine-methyltransferase complex
catalytic subunit and Wilms tumor protein N6-methyladenosine levels
in the testes of offspring mice, indicating an epigenetic
mechanism. Previous studies have addressed the significant impact
of paternal obesity on offspring metabolism (153,154). Notably, female offspring of an
obese father display impaired gluconeogenesis clues caused by
sperm-transmitted methylation of the insulin-like growth factor
(IGF)2/H19 imprinting control region in the liver of offspring,
inducing enhanced gluconeogenesis and an elevated expression of key
gluconeogenesis enzyme phosphoenolpyruvate carboxykinase (154). Another mechanism of
transgenerational metabolic dysfunction induction was found to be
sperm H3K27me3-dependent epigenetic regulation of glucose
metabolism and apoptosis (155). Of note, only mild, transient
metabolic changes were observed in the early life of offspring in a
sperm chromatin accessibility study, indicating that the
developmental compensatory mechanisms of the offspring can
counteract epigenetic inheritance (156). To establish the role of
paternal caloric restriction (CR) preconception, researchers
discovered that the offspring display attenuated AMPK/SIRT1 pathway
(associated with inflammation and oxidative stress), indicating
beneficial effects of paternal CR (157). Furthermore, another
case-control study found that the risk of congenital urogenital
anomalies increased with paternal BMI during the preconception
period (158). Parental obesity
is associated with decreased mitochondrial functional capacity, as
evidenced by lower intact cell respiration, oxidative
phosphorylation and electron transport system capacity (159).
Effects of maternal obesity on breast
milk composition and infant development
In addition to gamete-related influences, maternal
obesity has been found to affect postnatal development through
alterations in breast milk composition. Its normal composition
includes macronutrients such as lactose, fats and proteins, as well
as micronutrients, immune modulatory proteins (such as
immunoglobulins and cytokines), growth factors, vitamins and a
diverse microbiota (160). A
mother's milk, as the primary source of micro- and macronutrients,
as well as bioactive molecules, plays a central role in the early
stages of infant development. A previous study examined the
scientific evidence on how maternal obesity alters breast milk
composition and its potential effects on infant growth and
development (161).
As demonstrated in a previous study, maternal
obesity appears to significantly alter the composition of breast
milk, particularly the lipid profile. Obese women display increased
breast milk fat and caloric content from foremilk to hindmilk, with
the average milk caloric value being 11% greater (162). In addition, protein content
also appears to be affected. Similarly, the same group of authors
investigated the association between maternal BMI, serum lipids and
insulin, as well as the fat and calorie content of breast milk,
from foremilk to hindmilk (163). They concluded that, among all
women, maternal serum triglycerides, insulin and the homeostatic
model assessment for insulin resistance were significantly
associated with the foremilk triglyceride concentration, suggesting
that maternal serum triglyceride and insulin action contribute to
human milk fat content. Maternal obesity significantly alters the
hormonal composition of milk, as assessed by Enstad et al
(164). The most significant
alteration was observed in n-6:n-3 PUFA and leptin concentrations,
which were later confirmed to be associated with accelerated weight
gain in infancy (164).
However, unlike the study by Enstad et al (164), a Chinese study found that the
emaciated group had a significantly lower level of leptin in breast
milk (165). Of note,
correlation analysis showed that the level of ghrelin in breast
milk was positively correlated with the Z score of current body
weight (165). Another
case-control study aimed to compare the levels of leptin, ghrelin,
adiponectin and IGF-1 in pre-feed and post-feed breast milk from
mothers with obesity and normal weight (166). The pre-feed breast milk of
mothers with obesity had significantly higher levels of ghrelin
than that of mothers with a normal weight. At the same time,
adiponectin, an insulin-sensitizing hormone, appeared to be reduced
in the post-fed milk of mothers with obesity compared to mothers
with normal weight (166).
Obesity appears to alter the expression of leptin, adiponectin, and
miRNA, which typically decrease over time during lactation in
normal-weight mothers (167).
Additionally, miRNAs (miR-103, miR-17, miR-181a, miR-222, miR-let7c
and miR-146b) are negatively associated with infant BMI only in
normal-weight mothers (167).
Other components of human milk, named human milk
oligosaccharides (HMOs), which serve as a source of energy for
commensal intestinal microorganisms, have been studied in the
context of obesity. These complex carbohydrates, unique to human
milk, have been studied in a research project involving Mexican
women (168). Specifically, six
HMOs (LNFPI, 2'-FL, LNT, LNnT, 3'-SL and 6'-SL) were found to be
significantly lower in overweight women compared to those of normal
weight. One review article even mentions that maternal lifestyle
affects the microbial composition of breast milk (169). Most notably, it appears that
milk obtained from obese mothers contains lower microbial
diversity, which may result in reduced amounts of
Bifidobacteria in infants (169).
An overwhelming body of evidence suggests that
maternal obesity, as well as other metabolic disorders such as
diabetes, is the leading cause of divergent offspring development.
However, paternal obesity has become a focus of research due to the
emerging evidence of sperm-transmitted epigenetic changes that have
a significant effect on offspring.
Current controversies and unresolved
debates
Despite notable advances made in the understanding
of the association between obesity and reproductive health,
fundamental gaps and critical controversies remain that limit the
ability of researchers to develop effective therapeutic
interventions. A major ongoing challenge is to disentangle the
direct effects of obesity itself from those attributable to dietary
components, metabolic syndrome, and associated comorbidities. While
a number of experimental models induce obesity using high-fat or
high-sugar diets, the independent contributions of excess adiposity
vs. specific nutritional factors on reproductive dysfunction have
not yet been fully clarified. Molecular pathways, such as oxidative
stress, chronic inflammation and epigenetic modifications
potentially mediate these distinct influences; however, these
require rigorous investigations using carefully controlled
experimental designs that isolate obesity from dietary variables
(Fig. 3).
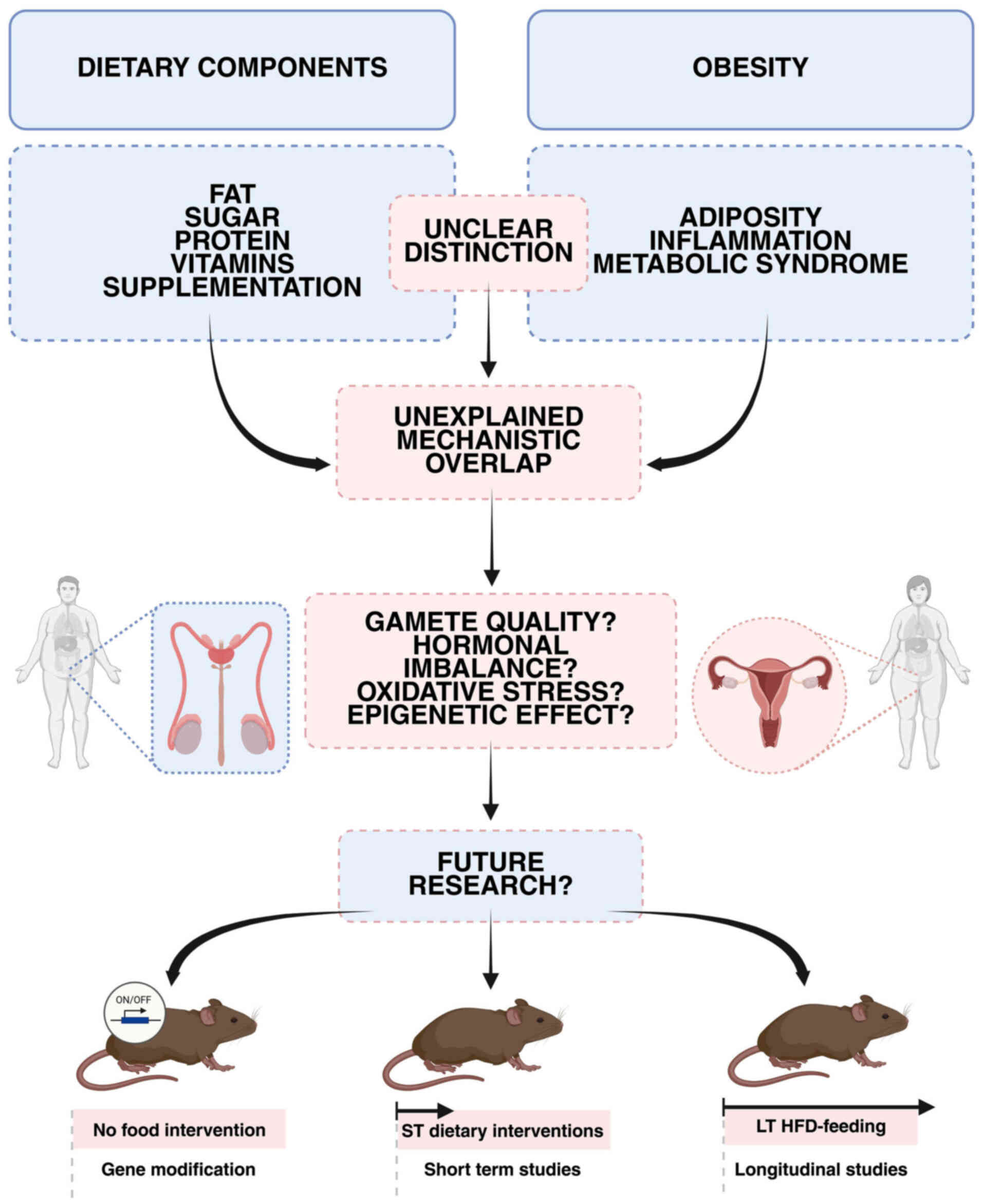 | Figure 3Schematic illustration of the
unresolved distinction between dietary components and obesity
effects on reproductive mechanisms. On the left panel, major
dietary components, including fat, sugar, protein, vitamins and
supplementation, are shown as potential direct influencers on
reproductive health. On the right panel, obesity-related factors,
including adiposity, inflammation, and metabolic syndrome, are
depicted as established contributors to reproductive dysfunction.
However, it remains unclear which reproductive mechanisms are
affected explicitly by dietary factors alone and which are driven
by obesity itself, resulting in an unexplained mechanistic overlap.
Therefore, distinct mechanisms of either diet or obesity on
reproductive parameters such as gamete quality, hormonal imbalance,
oxidative stress, and epigenetic effects remain to be elucidated.
To distinguish the dietary impact from obesity's impact on
reproduction, three approaches can be proposed: murine models of
obesity caused by gene manipulation without dietary interventions
(obesity's impact); short-term special diet feeding (impact of
dietary components without causing obesity); long-term special diet
feeding (obesity's impact). ST, short-term; LT, long-term; HFD,
high-fat diet. |
The direct effects of metabolism on gametes,
fertilization and offspring health are incompletely understood. New
evidence indicates that parental obesity alters DNA methylation,
histone marks and non-coding RNA profiles in germ cells,
potentially transmitting metabolic risks across generations.
However, it remains controversial whether these epigenetic changes
are causally linked to unfavorable reproductive outcomes or
represent biomarkers for broader metabolic dysregulation. The
relative contributions of maternal and paternal obesity to
programming the development and disease susceptibility of offspring
are also not yet clear.
The intrauterine microenvironment, a crucial factor
for fetal development and long-term health, remains an
under-researched, yet therapeutically promising area. A
comprehensive characterization of the effects of obesity on uterine
physiology, including pH, oxygenation, immune cell populations and
molecular signaling networks is required. Cutting-edge
technologies, such as single-cell RNA sequencing, spatial
transcriptomics and metabolomics provide opportunities to map the
molecular landscape of the obese reproductive tract with
unprecedented resolution.
Another area of debate concerns the reversibility
and treatment of obesity-induced reproductive impairments. Although
preclinical studies have shown promise for antioxidant therapy,
pharmacological agents and lifestyle interventions, including
exercise and food modification, further research is required to
confirm their effectiveness and durability across a range of human
populations. Identifying precise molecular targets amenable to
therapeutic modulation remains an urgent priority to translate
mechanistic findings into clinical applications.
Key signaling pathways, including insulin/IGF
signaling, AMPK, mTOR and inflammatory mediators, appear to be
differentially regulated in obesity, affecting gametogenesis,
steroidogenesis and embryo development. A more in-depthy
understanding of their crosstalk, as well as the roles of
epigenetic regulators and non-coding RNAs, will be critical for
developing personalized interventions. To address these
controversies and gaps, further research is required to integrate
sophisticated molecular approaches with longitudinal cohort studies
and experimentally rigorous models. Longitudinal tracking of
metabolic and reproductive changes will clarify temporal
relationships and causal mechanisms. Ultimately, this integrated
effort is essential to improve diagnostic precision, guide the
development of targeted interventions and optimize reproductive
outcomes, thereby advancing clinical care and enhancing
multigenerational health.
Conclusions
The present comprehensive review indicates that
obesity exerts profound and multifaceted effects on reproductive
health through complex molecular mechanisms that remain
incompletely understood. While substantial progress has been made
in documenting obesity-reproduction associations, the field now
requires a fundamental shift toward mechanistic investigations that
can guide therapeutic development.
The available evidence clearly demonstrates that
obesity affects reproductive function through multiple pathways,
including oxidative stress, chronic inflammation, hormonal
disruption and epigenetic modifications. However, critical gaps
remain in the understanding of the relative importance of these
mechanisms, their temporal associations and their therapeutic
targetability. The distinction between obesity-specific effects and
those attributable to dietary factors or metabolic syndrome is a
particularly important area that requires systematic
investigation.
The identification of specific molecular pathways,
such as mitochondrial dysfunction, inflammatory signaling and
epigenetic modifications, provides a foundation for developing
targeted interventions. However, the translation of these
mechanistic insights into effective clinical therapies requires
substantial additional research, including well-designed clinical
trials with mechanistic endpoints.
Future research is warranted to prioritize
mechanistic studies that can distinguish reversible from
irreversible changes in obesity-induced reproductive dysfunction,
identify specific therapeutic targets, and develop personalized
interventions based on individual metabolic and reproductive
profiles. Only through such systematic, mechanistic investigation
can we hope to develop effective strategies for preventing and
treating obesity-induced reproductive dysfunction.
The integration of advanced molecular techniques
with carefully designed clinical studies offers unprecedented
opportunities to understand and address the reproductive
consequences of obesity. This represents both a scientific
challenge and a clinical imperative, given the global prevalence of
obesity and its impact on reproductive health across
generations.
Availability of data and materials
Not applicable.
Authors' contributions
NP, MKr, MKu, KV and JB conceptualized the study.
NP and MKr were involved in the literature search. NP, MKr, KV, DR
and JB were involved in verifying the accuracy of the data
extracted from the literature. NP, MKr, MKu, KV, DR and JB were
involved in the writing and preparation of the original draft of
the manuscript, and in the writing, reviewing and editing of the
manuscript. NP and MKr were involved in the preparation of the
figures. KV and JB supervised the study. JB was involved in project
administration. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ADF
|
alternate-day fasting
|
|
AR
|
androgen receptor
|
|
ART
|
assisted reproductive technology
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
BMI
|
body mass index
|
|
CR
|
caloric restriction
|
|
CRISP4
|
cysteine-rich secretory protein 4
|
|
DDE
|
dichlorodiphenyldichloroethylene
|
|
GPR54
|
G protein-coupled receptor 54
|
|
GPX
|
glutathione peroxidase
|
|
HFD
|
high-fat diet
|
|
HMOs
|
human milk oligosaccharides
|
|
IGF
|
insulin-like growth factor
|
|
IVF
|
in vitro fertilization
|
|
LH
|
luteinizing hormone
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
ME
|
metabolic endotoxemia
|
|
NRF2
|
nuclear factor erythroid 2-related
factor 2
|
|
OB
|
obesogenic
|
|
PCOS
|
polycystic ovary syndrome
|
|
PORs
|
poor ovarian responders
|
|
ROS
|
reactive oxygen species
|
|
SAT
|
subcutaneous adipose tissue
|
|
SIRT1
|
sirtuin 1
|
|
SOD
|
superoxide dismutase
|
|
TC
|
testicular cancer
|
|
TGCT
|
testicular germ-cell tumor
|
|
TRF
|
time-restricted feeding
|
|
VAT
|
visceral adipose tissue
|
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Aida XMU, Ivan TV and Juan G JR: Adipose
tissue immunometabolism: unveiling the intersection of metabolic
and immune regulation. Rev Invest Clin. 76:65–79. 2024.
|
|
2
|
Puljiz Z, Kumric M, Vrdoljak J, Martinovic
D, Ticinovic Kurir T, Krnic MO, Urlic H, Puljiz Z, Zucko J, Dumanic
P, et al: Obesity, gut microbiota, and metabolome: From
pathophysiology to nutritional interventions. Nutrients.
15:22362023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallo G, Desideri G and Savoia C: Update
on obesity and cardiovascular risk: From pathophysiology to
clinical management. Nutrients. 16:27812024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furukawa S, Fujita T, Shimabukuro M, Iwaki
M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and
Shimomura I: Increased oxidative stress in obesity and its impact
on metabolic syndrome. J Clin Invest. 114:1752–1761. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gregor MF and Hotamisligil GS:
Inflammatory mechanisms in obesity. Annu Rev Immunol. 29:415–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White U: Adipose tissue expansion in
obesity, health, and disease. Front Cell Dev Biol. 11:11888442023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Virtue S and Vidal-Puig A: Adipose tissue
expandability, lipotoxicity and the metabolic syndrome-an
allostatic perspective. Biochim Biophys Acta. 1801:338–349. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fabbrini E, Sullivan S and Klein S:
Obesity and nonalcoholic fatty liver disease: Biochemical,
metabolic, and clinical implications. Hepatology. 51:679–689. 2010.
View Article : Google Scholar
|
|
9
|
Palacios-Marin I, Serra D,
Jimenez-Chillarón J, Herrero L and Todorčević M: Adipose tissue
dynamics: Cellular and lipid turnover in health and disease.
Nutrients. 15:39682023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pessayre D and Fromenty B: NASH: A
mitochondrial disease. J Hepatol. 42:928–940. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramachandran A and Jaeschke H: Oxidative
stress and acute hepatic injury. Curr Opin Toxicol. 7:17–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allameh A, Niayesh-Mehr R, Aliarab A,
Sebastiani G and Pantopoulos K: Oxidative stress in liver
pathophysiology and disease. Antioxidants. 12:16532023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goodpaster BH, He J, Watkins S and Kelley
DE: Skeletal muscle lipid content and insulin resistance: Evidence
for a paradox in endurance-trained athletes. J Clin Endocrinol
Metab. 86:5755–5761. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akhmedov D and Berdeaux R: The effects of
obesity on skeletal muscle regeneration. Front Physiol. 4:3712013.
View Article : Google Scholar
|
|
15
|
Thaler JP, Yi CX, Schur EA, Guyenet SJ,
Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, et
al: Obesity is associated with hypothalamic injury in rodents and
humans. J Clin Invest. 122:153–162. 2012. View Article : Google Scholar :
|
|
16
|
Lumeng CN, Bodzin JL and Saltiel AR:
Obesity induces a phenotypic switch in adipose tissue macrophage
polarization. J Clin Invest. 117:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Broughton DE and Moley KH: Obesity and
female infertility: Potential mediators of obesity's impact. Fertil
Steril. 107:840–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal A, Mulgund A, Hamada A and Chyatte
MR: A unique view on male infertility around the globe. Reprod Biol
Endocrinol. 13:372015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu X, Xu J, Song B, Zhu R, Liu J, Liu YF
and Ma YJ: The role of epigenetics in women's reproductive health:
The impact of environmental factors. Front Endocrinol (Lausanne).
15:13997572024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cecchino GN, Seli E, Alves da Motta EL and
García-Velasco JA: The role of mitochondrial activity in female
fertility and assisted reproductive technologies: Overview and
current insights. Reprod Biomed Online. 36:686–697. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pasquali R, Vicennati V, Cacciari M and
Pagotto U: The hypothalamic-pituitary-adrenal axis activity in
obesity and the metabolic syndrome. Ann N Y Acad Sci. 1083:111–128.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agarwal A, Gupta S and Sharma RK: Role of
oxidative stress in female reproduction. Reprod Biol Endocrinol.
3:282005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soubry A: Epigenetic inheritance and
evolution: A paternal perspective on dietary influences. Prog
Biophys Mol Biol. 118:79–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
MacDonald AA, Herbison GP, Showell M and
Farquhar CM: The impact of body mass index on semen parameters and
reproductive hormones in human males: A systematic review with
meta-analysis. Hum Reprod Update. 16:293–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Catalano PM and Shankar K: Obesity and
pregnancy: Mechanisms of short term and long term adverse
consequences for mother and child. BMJ. 356:j12017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stevens A and Lowe J: Chapter 16: Male
reproductive system. Human Histology. 3rd edition. Elsevier-Health
Sciences Division; pp. 327–343. 2025
|
|
28
|
Cheng CY and Mruk DD: The blood-testis
barrier and its implications for male contraception. Pharmacol Rev.
64:16–64. 2012. View Article : Google Scholar :
|
|
29
|
Griswold MD: Spermatogenesis: The
commitment to meiosis. Physiol Rev. 96:1–17. 2016. View Article : Google Scholar :
|
|
30
|
Oduwole OO, Peltoketo H and Huhtaniemi IT:
Role of follicle-stimulating hormone in spermatogenesis. Front
Endocrinol (Lausanne). 9:7632018. View Article : Google Scholar
|
|
31
|
Naamneh Elzenaty R, du Toit T and Flück
CE: Basics of androgen synthesis and action. Best Pract Res Clin
Endocrinol Metab. 36:1016652022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnson L, Thompson DL Jr and Varner DD:
Role of Sertoli cell number and function on regulation of
spermatogenesis. Anim Reprod Sci. 105:23–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Griswold MD: The central role of Sertoli
cells in spermatogenesis. Semin Cell Dev Biol. 9:411–416. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai X, Haleem R, Oram S, Cyriac J, Jiang
F, Grayhack JT, Kozlowski JM and Wang Z: High fat diet increases
the weight of rat ventral prostate. Prostate. 49:1–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Migliaccio V, Sica R, Scudiero R,
Simoniello P, Putti R and Lionetti L: Physiological adaptation to
simultaneous chronic exposure to high-fat diet and
dichlorodipheniletylhene (DDE) in wistar rat testis. Cells.
8:4432019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng J, Xu R, Li Y, Zhou Q, Song G, Deng Y
and Yan Y: The effect of high-fat diet and exercise on KISS-1/GPR54
expression in testis of growing rats. Nutr Metab (Lond). 18:12021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma J, Bi J, Sun B, Li H, Li Y and Wang S:
Zinc improves semen parameters in high-fat diet-induced male rats
by regulating the expression of LncRNA in testis tissue. Biol Trace
Elem Res. 201:4793–4805. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Falvo S, Minucci S, Santillo A, Senese R,
Chieffi Baccari G and Venditti M: A short-term high-fat diet alters
rat testicular activity and blood-testis barrier integrity through
the SIRT1/NRF2/MAPKs signaling pathways. Front Endocrinol
(Lausanne). 14:12740352023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Merino O, Sánchez R, Gregorio MB, Sampaio
F and Risopatrón J: Effect of high-fat and vitamin D deficient diet
on rat sperm quality and fertility. Theriogenology. 125:6–11. 2019.
View Article : Google Scholar
|
|
40
|
Krizanac M, Mass Sanchez PB, Weiskirchen R
and Asimakopoulos A: A scoping review on lipocalin-2 and its role
in non-alcoholic steatohepatitis and hepatocellular carcinoma. Int
J Mol Sci. 22:28652021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jing J, Peng Y, Fan W, Han S, Peng Q, Xue
C, Qin X, Liu Y and Ding Z: Obesity-induced oxidative stress and
mitochondrial dysfunction negatively affect sperm quality. FEBS
Open Bio. 13:763–778. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pearce KL, Hill A and Tremellen KP:
Obesity related metabolic endotoxemia is associated with oxidative
stress and impaired sperm DNA integrity. Basic Clin Androl.
29:62019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bakos HW, Mitchell M, Setchell BP and Lane
M: The effect of paternal diet-induced obesity on sperm function
and fertilization in a mouse model. Int J Androl. 34(5 Pt 1):
402–410. 2011. View Article : Google Scholar
|
|
44
|
Han J, Zhao C, Guo H, Liu T, Li Y, Qi Y,
Deussing JM, Zhang Y, Tan J, Han H and Ma X: Obesity induces male
mice infertility via oxidative stress, apoptosis, and glycolysis.
Reproduction. 166:27–36. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rahali D, Dallagi Y, Hupkens E, Veegh G,
Mc Entee K, Asmi ME, El Fazaa S and El Golli N: Spermatogenesis and
steroidogenesis disruption in a model of metabolic syndrome rats.
Arch Physiol Biochem. 129:222–232. 2023. View Article : Google Scholar
|
|
46
|
Saez Lancellotti TE, Boarelli PV, Monclus
MA, Cabrillana ME, Clementi MA, Espínola LS, Cid Barría JL,
Vincenti AE, Santi AG and Fornés MW: Hypercholesterolemia impaired
sperm functionality in rabbits. PLoS One. 5:e134572010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lerro CC, McGlynn KA and Cook MB: A
systematic review and meta-analysis of the relationship between
body size and testicular cancer. Br J Cancer. 103:1467–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bjørge T, Tretli S, Lie AK and Engeland A:
The impact of height and body mass index on the risk of testicular
cancer in 600,000 Norwegian men. Cancer Causes Control. 17:983–987.
2006.PubMed/NCBI
|
|
49
|
Dieckmann KP, Hartmann JT, Classen J,
Diederichs M and Pichlmeier U: Is increased body mass index
associated with the incidence of testicular germ cell cancer? J
Cancer Res Clin Oncol. 135:731–738. 2009. View Article : Google Scholar
|
|
50
|
Garner MJ, Birkett NJ, Johnson KC,
Shatenstein B, Ghadirian P and Krewski D; Canadian Cancer
Registries Epidemiology Research Group: Dietary risk factors for
testicular carcinoma. Int J Cancer. 106:934–941. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gür Özcan SG, Erkan M, Baralı D and Erkan
A: The importance of body fat composition evaluated by computed
tomography and its prognostic significance in patients with
testicular cancer. Istanb Med J. 26:37–41. 2025. View Article : Google Scholar
|
|
52
|
Puri D, Riviere P, Meagher M, Morgan K,
Nelson T, Yuen K, Pandit K, Yodkhunnatham N, Taylor J, Herchenhorn
D, et al: Metabolic syndrome among testicular cancer survivors:
Long-term follow-up of the veterans affairs health system. Cancer
Med. 14:e708582025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Billah MM, Khatiwada S, Lecomte V, Morris
MJ and Maloney CA: Ameliorating high-fat diet-induced sperm and
testicular oxidative damage by micronutrient-based antioxidant
intervention in rats. Eur J Nutr. 61:3741–3753. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Suleiman JB, Mohamed M, Abu Bakar AB,
Zakaria Z, Othman ZA and Nna VU: Therapeutic effects of bee bread
on obesity-induced testicular-derived oxidative stress,
inflammation, and apoptosis in high-fat diet obese rat model.
Antioxidants (Basel). 11:2552022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tüfek NH, Yahyazadeh A and Altunkaynak BZ:
Protective effect of indole-3-carbinol on testis of a high fat diet
induced obesity. Biotech Histochem. 98:1–12. 2023. View Article : Google Scholar
|
|
56
|
Lin T, Zhang S, Zhou Y, Wu L, Liu X and
Huang H: Small RNA perspective of physical exercise-related
improvement of male reproductive dysfunction due to obesity. Front
Endocrinol (Lausanne). 13:10384492022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Y, Liu L, Wang B, Xiong J, Li Q, Wang J
and Chen D: Impairment of reproductive function in a male rat model
of non-alcoholic fatty liver disease and beneficial effect of N-3
fatty acid supplementation. Toxicol Lett. 222:224–232. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Komninos D, Ramos L, van der Heijden GW,
Morrison MC, Kleemann R, van Herwaarden AE, Kiliaan AJ and
Arnoldussen IAC: High fat diet-induced obesity prolongs critical
stages of the spermatogenic cycle in a Ldlr-/-.Leiden mouse model.
Sci Rep. 12:4302022. View Article : Google Scholar
|
|
59
|
Ahangarpour A, Oroojan AA, Khorsandi L,
Arzani G and Afshari G: Effects of betulinic acid on the male
reproductive system of a streptozotocin-nicotinamide-induced
diabetic mouse model. World J Mens Health. 34:209–216. 2016.
View Article : Google Scholar
|
|
60
|
Ruiz-Valderrama L, Mendoza-Sánchez JE,
Rodríguez-Tobón E, Arrieta-Cruz I, González-Márquez H,
Salame-Méndez PA, Tarragó-Castellanos R, Cortés-Barberena E,
Rodríguez-Tobón A and Arenas-Ríos E: High-fat diets disturb rat
epididymal sperm maturation. Int J Mol Sci. 26:18502025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang W, Tian Z, Qi X, Chen P, Yang Q,
Guan Q, Ye J and Yu C: Switching from high-fat diet to normal diet
ameliorate BTB integrity and improve fertility potential in obese
male mice. Sci Rep. 13:141522023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kobori T, Iwabu M, Okada-Iwabu M, Ohuchi
N, Kikuchi A, Yamauchi N, Kadowaki T, Yamauchi T and Kasuga M:
Decreased AdipoR1 signaling and its implications for
obesity-induced male infertility. Sci Rep. 14:57012024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yi X, Gao H, Chen D, Tang D, Huang W, Li
T, Ma T and Chang B: Effects of obesity and exercise on testicular
leptin signal transduction and testosterone biosynthesis in male
mice. Am J Physiol Regul Integr Comp Physiol. 312:R501–R510. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
El-Shehawi AM, El-Shazly S, Ahmed M,
Alkafafy M, Sayed S, Farouk S, Alotaibi SS and Elseehy MM:
Transcriptome analysis of testis from HFD-induced obese rats
(Rattus norvigicus) indicated predisposition for male infertility.
Int J Mol Sci. 21:64932020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Carvalho MG, Silva KM, Aristizabal VHV,
Ortiz PEO, Paranzini CS, Melchert A, Amaro JL and Souza FF: Effects
of obesity and diabetes on sperm cell proteomics in rats. J
Proteome Res. 20:2628–2642. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lotti F, Marchiani S, Corona G and Maggi
M: Metabolic syndrome and reproduction. Int J Mol Sci. 22:19882021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Salvio G, Ciarloni A, Cutini M, Delli Muti
N, Finocchi F, Perrone M, Rossi S and Balercia G: Metabolic
syndrome and male fertility: Beyond heart consequences of a complex
cardiometabolic endocrinopathy. Int J Mol Sci. 23:54972022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Service CA, Puri D, Al Azzawi S, Hsieh TC
and Patel DP: The impact of obesity and metabolic health on male
fertility: A systematic review. Fertil Steril. 120:1098–1111. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ford C: An overview of the female
reproductive system. Br J Nurs. 32:420–426. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rosner J, Samardzic T and Sarao MS:
Physiology, female reproduction. StatPearls (Internet). StatPearls
Publishing; Treasure Island, FL: 2025
|
|
71
|
Bukovsky A, Svetlikova M and Caudle MR:
Oogenesis in cultures derived from adult human ovaries. Reprod Biol
Endocrinol. 3:172005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hanuman S, Pande G and Nune M: Current
status and challenges in uterine myometrial tissue engineering.
Bioengineered. 14:22518472023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gao X, Li Y, Ma Z, Jing J, Zhang Z, Liu Y
and Ding Z: Obesity induces morphological and functional changes in
female reproductive system through increases in NF-κB and MAPK
signaling in mice. Reprod Biol Endocrinol. 19:1482021. View Article : Google Scholar
|
|
74
|
St-Germain LE, Castellana B, Baltayeva J
and Beristain AG: Maternal obesity and the uterine immune cell
landscape: The shaping role of inflammation. Int J Mol Sci.
21:37762020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nteeba J, Ganesan S and Keating AF:
Progressive obesity alters ovarian folliculogenesis with impacts on
pro-inflammatory and steroidogenic signaling in female mice. Biol
Reprod. 91:862014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tasaki H, Munakata Y, Arai S, Murakami S,
Kuwayama T and Iwata H: The effect of high glucose concentration on
the quality of oocytes derived from different growth stages of
follicles. J Mamm Ova Res. 32:41–48. 2015. View Article : Google Scholar
|
|
77
|
Skaznik-Wikiel ME, Swindle DC, Allshouse
AA, Polotsky AJ and McManaman JL: High-fat diet causes subfertility
and compromised ovarian function independent of obesity in mice.
Biol Reprod. 94:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wu LL, Dunning KR, Yang X, Russell DL,
Lane M, Norman RJ and Robker RL: High-fat diet causes lipotoxicity
responses in cumulus-oocyte complexes and decreased fertilization
rates. Endocrinology. 151:5438–5445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhu Q, Li F, Wang H, Wang X, Xiang Y, Ding
H, Wu H, Xu C, Weng L, Cai J, et al: Single-cell RNA sequencing
reveals the effects of high-fat diet on oocyte and early embryo
development in female mice. Reprod Biol Endocrinol. 22:1052024.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Choi HG, Kim JK, Kwak DH, Cho JR, Kim JY,
Kim BJ, Jung KY, Choi BK, Shin MK and Choo YK: Effects of high
molecular weight water-soluble chitosan on in vitro fertilization
and ovulation in mice fed a high-fat diet. Arch Pharm Res.
25:178–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Morimoto A, Rose RD, Smith KM, Dinh DT,
Umehara T, Winstanley YE, Shibahara H, Russell DL and Robker RL:
Granulosa cell metabolism at ovulation correlates with oocyte
competence and is disrupted by obesity and aging. Hum Reprod.
39:2053–2066. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Di Berardino C, Barceviciute U, Camerano
Spelta Rapini C, Peserico A, Capacchietti G, Bernabò N, Russo V,
Gatta V, Konstantinidou F, Donato M and Barboni B: High-fat
diet-negative impact on female fertility: From mechanisms to
protective actions of antioxidant matrices. Front Nutr.
11:14154552024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yong W, Wang J, Leng Y, Li L and Wang H:
Role of obesity in female reproduction. Int J Med Sci. 20:366–375.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gitsi E, Livadas S and Argyrakopoulou G:
Nutritional and exercise interventions to improve conception in
women suffering from obesity and distinct nosological entities.
Front Endocrinol (Lausanne). 15:14265422024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rakic D, Joksimovic Jovic J, Jakovljevic
V, Zivkovic V, Nikolic M, Sretenovic J, Nikolic M, Jovic N, Bicanin
Ilic M, Arsenijevic P, et al: High fat diet exaggerate metabolic
and reproductive PCOS features by promoting oxidative stress: An
improved EV model in rats. Medicina (Kaunas). 59:11042023.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mirseyyed SF, Zavareh S, Nasiri M and
Hashemi-Moghaddam H: An experimental study on the oxidative status
and inflammatory levels of a rat model of polycystic ovary syndrome
induced by letrozole and a new high-fat diet. Int J Fertil Steril.
18:45–53. 2023.PubMed/NCBI
|
|
87
|
Zheng L, Yang L, Guo Z, Yao N, Zhang S and
Pu P: Obesity and its impact on female reproductive health:
Unraveling the connections. Front Endocrinol (Lausanne).
14:13265462024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Heng N, Zhu H, Talukder AK and Zhao S:
Obesity and oxidative stress: Implications for female fertility.
Anim Res One Health. 2:377–399. 2024. View Article : Google Scholar
|
|
89
|
Dağ ZÖ and Dilbaz B: Impact of obesity on
infertility in women. J Turk Ger Gynecol Assoc. 16:111–117. 2015.
View Article : Google Scholar
|
|
90
|
Sam S: Obesity and polycystic ovary
syndrome. Obes Manag. 3:69–73. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barber TM, Hanson P, Weickert MO and
Franks S: Obesity and polycystic ovary syndrome: Implications for
pathogenesis and novel management strategies. Clin Med Insights
Reprod Health. 13:11795581198740422019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Naqvi A, MacKintosh ML, Derbyshire AE,
Tsakiroglou AM, Walker TDJ, McVey RJ, Bolton J, Fergie M, Bagley S,
Ashton G, et al: The impact of obesity and bariatric surgery on the
immune microenvironment of the endometrium. Int J Obes (Lond).
46:605–612. 2022. View Article : Google Scholar
|
|
93
|
Nagashima M, Miwa N, Hirasawa H, Katagiri
Y, Takamatsu K and Morita M: Genome-wide DNA methylation analysis
in obese women predicts an epigenetic signature for future
endometrial cancer. Sci Rep. 9:64692019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sim KA, Partridge SR and Sainsbury A: Does
weight loss in overweight or obese women improve fertility
treatment outcomes? A systematic review. Obes Rev. 15:839–850.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pettigrew R and Hamilton-Fairley D:
Obesity and female reproductive function. Br Med Bull. 53:341–358.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhu Y, Luo M, Bai X, Li J, Nie P, Li B and
Luo P: SS-31: A mitochondria-targeting peptide, ameliorates kidney
disease. Oxid Med Cell Longev. 2022:12955092022. View Article : Google Scholar
|
|
97
|
Igosheva N, Abramov AY, Poston L, Eckert
JJ, Fleming TP, Duchen MR and McConnell J: Maternal diet-induced
obesity alters mitochondrial activity and redox status in mouse
oocytes and zygotes. PLoS One. 5:e100742010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Legro RS, Arslanian SA, Ehrmann DA, Hoeger
KM, Murad MH, Pasquali R and Welt CK; Endocrine Society: Diagnosis
and treatment of polycystic ovary syndrome: An Endocrine Society
clinical practice guideline. J Clin Endocrinol Metab. 98:4565–4592.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Okabe M: The cell biology of mammalian
fertilization. Development. 140:4471–4479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Marquard KL, Stephens SM, Jungheim ES,
Ratts VS, Odem RR, Lanzendorf S and Moley KH: Polycystic ovary
syndrome and maternal obesity affect oocyte size in in vitro
fertilization/intracytoplasmic sperm injection cycles. Fertil
Steril. 95:2146–2149. 2149.e12011. View Article : Google Scholar
|
|
101
|
Martín-Hidalgo D, Solar-Málaga S,
González-Fernández L, Zamorano J, García-Marín LJ and Bragado MJ:
The compound YK 3-237 promotes pig sperm capacitation-related
events. Vet Res Commun. 48:773–786. 2024. View Article : Google Scholar :
|
|
102
|
Trebichalská Z and Holubcová Z: Perfect
date-the review of current research into molecular bases of
mammalian fertilization. J Assist Reprod Genet. 37:243–256. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Delgado-Bermúdez A, Yeste M, Bonet S and
Pinart E: Physiological role of potassium channels in mammalian
germ cell differentiation, maturation, and capacitation. Andrology.
13:184–201. 2025.In Italian. View Article : Google Scholar :
|
|
104
|
Aldana A, Carneiro J, Martínez-Mekler G
and Darszon A: Discrete dynamic model of the mammalian sperm
acrosome reaction: the influence of acrosomal pH and physiological
heterogeneity. Front Physiol. 12:6827902021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hafez ESE, Goff L and Hafez B: Mammalian
fertilization, IVF, ICSI: Physiological/molecular parameters,
clinical application. Arch Androl. 50:69–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kwon WS, Rahman MS and Pang MG: Diagnosis
and prognosis of male infertility in mammal: The focusing of
tyrosine phosphorylation and phosphotyrosine proteins. J Proteome
Res. 13:4505–4517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Schatten H, Sun QY and Prather R: The
impact of mitochondrial function/dysfunction on IVF and new
treatment possibilities for infertility. Reprod Biol Endocrinol.
12:1112014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Vural F, Vural B and Çakıroğlu Y: The role
of overweight and obesity in in vitro fertilization outcomes of
poor ovarian responders. BioMed Res Int. 2015:7815432015.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Schon SB, Yang K, Schindler R, Jiang L,
Neff LM, Seeley RJ and Marsh EE: Obesity-related alterations in
protein expression in human follicular fluid from women undergoing
in vitro fertilization. F S Sci. 3:331–339. 2022.PubMed/NCBI
|
|
110
|
Bunay J, Gallardo LM, Torres-Fuentes JL,
Aguirre-Arias MV, Orellana R, Sepúlveda N and Moreno RD: A decrease
of docosahexaenoic acid in testes of mice fed a high-fat diet is
associated with impaired sperm acrosome reaction and fertility.
Asian J Androl. 23:306–313. 2021. View Article : Google Scholar :
|
|
111
|
Cooray A, Kim JH, Chae MR, Lee S and Lee
KP: Perspectives on potential fatty acid modulations of motility
associated human sperm ion channels. Int J Mol Sci. 23:37182022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Borges BC, Garcia-Galiano D, da Silveira
Cruz-Machado S, Han X, Gavrilina GB, Saunders TL, Auchus RJ,
Hammoud SS, Smith GD and Elias CF: Obesity-induced infertility in
male mice is associated with disruption of Crisp4 expression and
sperm fertilization capacity. Endocrinology. 158:2930–2943. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Krizanac M, Mass Sanchez PB, Weiskirchen R
and Schröder SK: Overview of the expression patterns and roles of
Lipocalin 2 in the reproductive system. Front Endocrinol
(Lausanne). 15:13656022024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Seekford ZK, Davis DB, Dickson MJ, Melo
Gonçlaves L, Burato S, Holton MP, Gordon J, Pohler KG, Cliff Lamb
G, Pringle TD, et al: Bulls fed a high-gain diet decrease
blastocyst formation after in vitro fertilization. Reproduction.
166:149–159. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bozdemir N, Kablan T, Altintas MO, Sukur
G, Cinar O and Uysal F: Altered DNA methylation and Dnmt expression
in obese uterus may cause implantation failure. J Mol Histol.
55:427–436. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fabian D, Kubandová-Babeľová J, Kšiňanová
M, Waczulíková I, Fabianová K and Koppel J: Overweight and
fertility: What we can learn from an intergenerational mouse
obesity model. Int J Environ Res Public Health. 19:79182022.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Gude NM, Roberts CT, Kalionis B and King
RG: Growth and function of the normal human placenta. Thromb Res.
114:397–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Roberts KA, Riley SC, Reynolds RM, Barr S,
Evans M, Statham A, Hor K, Jabbour HN, Norman JE and Denison FC:
Placental structure and inflammation in pregnancies associated with
obesity. Placenta. 32:247–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Amabebe E, Ikumi N, Pillay K, Matjila M
and Anumba DOC: Maternal obesity-related placental dysfunction:
From peri-conception to late gestation. Placenta Reprod Med.
2:92023. View Article : Google Scholar
|
|
120
|
Louwen F, Kreis NN, Ritter A and Yuan J:
Maternal obesity and placental function: Impaired maternal-fetal
axis. Arch Gynecol Obstet. 309:2279–2288. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Stuart TJ, O'Neill K, Condon D, Sasson I,
Sen P, Xia Y and Simmons RA: Diet-induced obesity alters the
maternal metabolome and early placenta transcriptome and decreases
placenta vascularity in the mouse. Biol Reprod. 98:795–809. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Castellana B, Perdu S, Kim Y, Chan K, Atif
J, Marziali M and Beristain AG: Maternal obesity alters uterine NK
activity through a functional KIR2DL1/S1 imbalance. Immunol Cell
Biol. 96:805–819. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Perdu S, Castellana B, Kim Y, Chan K,
DeLuca L and Beristain AG: Maternal obesity drives functional
alterations in uterine NK cells. JCI Insight. 1:e855602016.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wilson MR, Skalski H, Reske JJ, Wegener M,
Adams M, Hostetter G, Hoffmann HM, Bernard JJ, Bae-Jump VL,
Teixeira JM and Chandler RL: Obesity alters the mouse endometrial
transcriptome in a cell context-dependent manner. Reprod Biol
Endocrinol. 20:1632022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ford SP, Zhang L, Zhu M, Miller MM, Smith
DT, Hess BW, Moss GE, Nathanielsz PW and Nijland MJ: Maternal
obesity accelerates fetal pancreatic β-cell but not alpha-cell
development in sheep: Prenatal consequences. Am J Physiol Regul
Integr Comp Physiol. 297:R835–R843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Liu Y, Ding Q and Guo W: Life course
impact of glucocorticoids during pregnancy on muscle development
and function. Front Anim Sci. 2:7889302021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sahoo T, del Gaudio D, German JR, Shinawi
M, Peters SU, Person RE, Garnica A, Cheung SW and Beaudet AL:
Prader-Willi phenotype caused by paternal deficiency for the
HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 40:719–721.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fowden AL and Forhead AJ: Glucocorticoids
as regulatory signals during intrauterine development. Exp Physiol.
100:1477–1487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Gohir W, Kennedy KM, Wallace JG, Saoi M,
Bellissimo CJ, Britz-McKibbin P, Petrik JJ, Surette MG and Sloboda
DM: High-fat diet intake modulates maternal intestinal adaptations
to pregnancy and results in placental hypoxia, as well as altered
fetal gut barrier proteins and immune markers. J Physiol.
597:3029–3051. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
George G, Draycott SAV, Muir R, Clifford
B, Elmes MJ and Langley-Evans SC: Exposure to maternal obesity
during suckling outweighs in utero exposure in programming for
post-weaning adiposity and insulin resistance in rats. Sci Rep.
9:101342019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Puppala S, Li C, Glenn JP, Saxena R,
Gawrieh S, Quinn A, Palarczyk J, Dick EJ Jr, Nathanielsz PW and Cox
LA: Primate fetal hepatic responses to maternal obesity: Epigenetic
signalling pathways and lipid accumulation. J Physiol.
596:5823–5837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Meakin AS, Nathanielsz PW, Li C, Clifton
VL, Wiese MD and Morrison JL: Maternal obesity impacts fetal liver
androgen signalling in a sex-specific manner. Life Sci.
337:1223442024. View Article : Google Scholar
|
|
133
|
Zhou P, Guan H, Guo Y, Zhu L and Liu X:
Maternal high-fat diet programs renal peroxisomes and activates
NLRP3 inflammasome-mediated pyroptosis in the rat fetus. J Inflamm
Res. 14:5095–5110. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lee YQ, Lumbers ER, Oldmeadow C, Collins
CE, Johnson V, Keogh L, Sutherland K, Gordon A, Smith R, Rae KM and
Pringle KG: The relationship between maternal adiposity during
pregnancy and fetal kidney development and kidney function in
infants: The Gomeroi gaaynggal study. Physiol Rep. 7:e142272019.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tain YL, Chan JY and Hsu CN: Maternal
fructose intake affects transcriptome changes and programmed
hypertension in offspring in later life. Nutrients. 8:7572016.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tang S, Wu H, Chen Q, Tang T, Li J, An H,
Zhu S, Han L, Sun H, Ge J, et al: Maternal obesity induces the
meiotic defects and epigenetic alterations during fetal oocyte
development. Adv Sci (Weinh). 11:e23091842024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Şanlı E and Kabaran S: Maternal obesity,
maternal overnutrition and fetal programming: Effects of epigenetic
mechanisms on the development of metabolic disorders. Curr
Genomics. 20:419–427. 2019. View Article : Google Scholar
|
|
138
|
Liu S, Hua L, Mo X, Lei B, Zhang R, Zhou
S, Jiang X, Fang Z, Feng B, Che L, et al: Comparative impact of
alternate-day fasting and time-restricted feeding on placental
function and fetal development in maternal obesity. Nutrients.
17:252024. View Article : Google Scholar
|
|
139
|
Naeye RL: Maternal body weight and
pregnancy outcome. Am J Clin Nutr. 52:273–279. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Metzger BE, Silverman BL, Freinkel N,
Dooley SL, Ogata ES and Green OC: Amniotic fluid insulin
concentration as a predictor of obesity. Arch Dis Child. 65(10 Spec
No): 1050–1052. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Boney CM, Verma A, Tucker R and Vohr BR:
Metabolic syndrome in childhood: Association with birth weight,
maternal obesity, and gestational diabetes mellitus. Pediatrics.
115:e290–e296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Moeckli B, Delaune V, Prados J, Tihy M,
Peloso A, Oldani G, Delmi T, Slits F, Gex Q, Rubbia-Brandt L, et
al: Impact of maternal obesity on liver disease in the offspring: A
comprehensive transcriptomic analysis and confirmation of results
in a murine model. Biomedicines. 10:2942022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Furigo IC and Dearden L: Mechanisms
mediating the impact of maternal obesity on offspring hypothalamic
development and later function. Front Endocrinol (Lausanne).
13:10789552022. View Article : Google Scholar
|
|
144
|
Zhang J, Li S, Luo X and Zhang C: Emerging
role of hypothalamus in the metabolic regulation in the offspring
of maternal obesity. Front Nutr. 10:10946162023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Dow C, Lorthe E, Bernard JY, Galera C,
Marchand-Martin L, Tafflet M, Ancel PY, Charles MA and Heude B:
Maternal prepregnancy obesity and offspring intelligence quotient
at 5 years: A multicohort analysis. Paediatr Perinat Epidemiol.
39:162–174. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Sertorio MN, César H, de Souza EA,
Mennitti LV, Santamarina AB, De Souza Mesquita LM, Jucá A,
Casagrande BP, Estadella D, Aguiar O Jr and Pisani LP: Parental
high-fat high-sugar diet intake programming inflammatory and
oxidative parameters of reproductive health in male offspring.
Front Cell Dev Biol. 10:8671272022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Santillán JAG, Mezo-González CE, Gourdel
M, Croyal M and Bolaños-Jiménez F: Diet-induced obesity in the rat
impairs sphingolipid metabolism in the brain and this metabolic
dysfunction is transmitted to the offspring via both the maternal
and the paternal lineage. J Neurochem. 169:e163072025. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Alba-Linares JJ, Pérez RF, Tejedor JR,
Bastante-Rodríguez D, Ponce F, Carbonell NG, Zafra RG, Fernández
AF, Fraga MF and Lurbe E: Maternal obesity and gestational diabetes
reprogram the methylome of offspring beyond birth by inducing
epigenetic signatures in metabolic and developmental pathways.
Cardiovasc Diabetol. 22:442023. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zheng Y, Wang W, Huo Y and Gui Y: Maternal
obesity and kawasaki disease-like vasculitis: A new perspective on
cardiovascular injury and inflammatory response in offspring male
mice. Nutrients. 15:38232023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Shiadeh SMJ, Goretta F, Svedin P, Jansson
T, Mallard C and Ardalan M: Long-term impact of maternal obesity on
the gliovascular unit and ephrin signaling in the hippocampus of
adult offspring. J Neuroinflammation. 21:392024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Nelson BN and Friedman JE: Developmental
programming of the fetal immune system by maternal western-style
diet: Mechanisms and implications for disease pathways in the
offspring. Int J Mol Sci. 25:59512024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Xiong YW, Zhu HL, Zhang J, Geng H, Tan LL,
Zheng XM, Li H, Fan LL, Wang XR, Zhang XD, et al: Multigenerational
paternal obesity enhances the susceptibility to male subfertility
in offspring via Wt1 N6-methyladenosine modification. Nat Commun.
15:13532024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Billah MM, Khatiwada S, Morris MJ and
Maloney CA: Effects of paternal overnutrition and interventions on
future generations. Int J Obes (Lond). 46:901–917. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wu HY, Cheng Y, Jin LY, Zhou Y, Pang HY,
Zhu H, Yan CC, Yan YS, Yu JE, Sheng JZ and Huang HF: Paternal
obesity impairs hepatic gluconeogenesis of offspring by altering
Igf2/H19 DNA methylation. Mol Cell Endocrinol. 529:1112642021.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Shi Y, Li W, Yu X, Zhao Y, Zhu D, Song Y,
Zhao Z, Gu Y, Wei B, Li L, et al: Paternal obesity-induced H3K27me3
elevation leads to MANF-mediated transgenerational metabolic
dysfunction in female offspring. Adv Sci (Weinh). 12:e24159562025.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Tahiri I, Llana SR, Fos-Domènech J,
Milà-Guash M, Toledo M, Haddad-Tóvolli R, Claret M and Obri A:
Paternal obesity induces changes in sperm chromatin accessibility
and has a mild effect on offspring metabolic health. Heliyon.
10:e340432024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Anuradha R, Srinivas M, Satyavani M,
Suresh K, Muralidhar MN and Rajender Rao K: Preconceptional
paternal caloric restriction of high-fat diet-induced obesity in
Wistar rats dysregulates the metabolism of their offspring via
AMPK/SIRT1 pathway. Lipids Health Dis. 23:1742024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Achkar ME, Atieh O, Ghadban C, Awad T,
Ghadban E, Grandjean V, Yarkiner Z, Raad G and Khalife MF:
Preconceptional paternal obesity may increase the risk of
congenital urogenital anomalies in offspring: A case-control study.
Andrology. 13:45–54. 2025. View Article : Google Scholar
|
|
159
|
Jevtovic F, Claiborne A, Biagioni EM,
Collier DN, DeVente JE, Mouro S, Kaneko-Tarui T, O-Tierney-Ginn PF,
Goodyear LJ, Houmard JA, et al: Paternal obesity decreases infant
MSC mitochondrial functional capacity. Am J Physiol Endocrinol
Metab. 327:E441–E448. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ballard O and Morrow AL: Human milk
composition: Nutrients and bioactive factors. Pediatr Clin North
Am. 60:49–74. 2013. View Article : Google Scholar :
|
|
161
|
Froń A and Orczyk-Pawiłowicz M:
Understanding the immunological quality of breast milk in maternal
overweight and obesity. Nutrients. 15:50162023. View Article : Google Scholar
|
|
162
|
Ross MG, Coca KP, Rocha ACL, Camargo BTS,
de Castro LS, Horta BL and Desai M: Composition of breast milk in
women with obesity. J Clin Med. 13:69472024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ross MG, Kavasery MP, Cervantes MK, Han G,
Horta B, Coca KP, Costa SO and Desai M: High-fat, high-calorie
breast milk in women with overweight or obesity and its association
with maternal serum insulin concentration and triglycerides levels.
Children (Basel). 11:1412024.PubMed/NCBI
|
|
164
|
Enstad S, Cheema S, Thomas R, Fichorova
RN, Martin CR, O'Tierney-Ginn P, Wagner CL and Sen S: The impact of
maternal obesity and breast milk inflammation on developmental
programming of infant growth. Eur J Clin Nutr. 75:180–188. 2021.
View Article : Google Scholar :
|
|
165
|
Huang LL, Yang F and Xiong F: Association
of leptin, adiponectin, and ghrelin in breast milk with the growth
of infants with exclusive breastfeeding. Zhongguo Dang Dai Er Ke Za
Zhi. 20:91–96. 2018.In Chinese. PubMed/NCBI
|
|
166
|
Tekin Guler T, Koc N, Kara Uzun A and
Fisunoglu M: The association of pre-pregnancy BMI on leptin,
ghrelin, adiponectin and insulin-like growth factor-1 in breast
milk: A case-control study. Br J Nutr. 127:1675–1681. 2022.
View Article : Google Scholar
|
|
167
|
Zamanillo R, Sánchez J, Serra F and Palou
A: Breast milk supply of microrna associated with leptin and
adiponectin is affected by maternal overweight/obesity and
influences infancy BMI. Nutrients. 11:25892019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Urrutia-Baca VH, Gutiérrez-Uribe JA,
Ramos-Parra PA, Domínguez-Uscanga A, Rodriguez-Gutierrez NA,
Chavez-Caraza KL, Martinez-Cano I, Padilla-Garza AS,
Ruiz-Villarreal EG, Espiricueta-Candelaria F and Chuck-Hernández C:
Exploring the impact of maternal factors and dietary habits on
human milk oligosaccharide composition in early breastfeeding among
Mexican women. Sci Rep. 14:146852024. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Garcia-Mantrana I and Collado MC: Obesity
and overweight: Impact on maternal and milk microbiome and their
role for infant health and nutrition. Mol Nutr Food Res.
60:1865–1875. 2016. View Article : Google Scholar : PubMed/NCBI
|

















