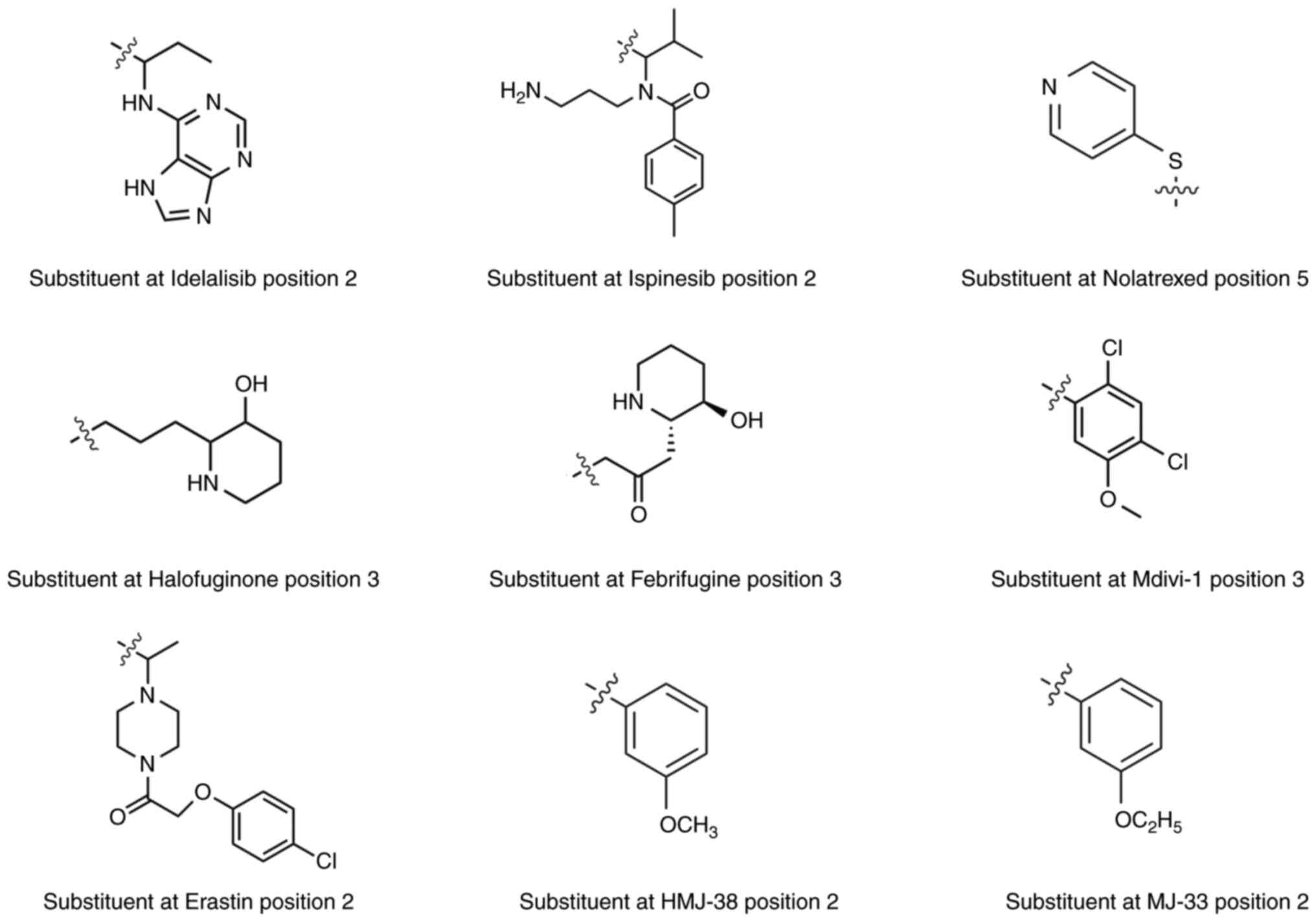
|
1
|
Tiwary BK, Pradhan K, Nanda AK and
Chakraborty R: Implication of quinazoline-4(3H)-ones in medicinal
chemistry: A brief review. J Chem Biol Ther. 1:1042016.
|
|
2
|
Rakesh KP, Manukumar HM and Gowda DC:
Schiff's bases of quinazolinone derivatives: Synthesis and SAR
studies of a novel series of potential anti-inflammatory and
antioxidants. Bioorg Med Chem Lett. 25:1072–1077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sulthana MT, Chitra K, Alagarsamy V,
Saravanan G and Solomon VR: Anti-HIV and antibacterial activities
of novel 2-(3-Substituted-4-oxo-3, 4-dihydroquinazolin-2-yl)-2,
3-dihydrophthalazine-1,4-diones. Russ J Bioorg Chem. 47:112–121.
2021. View Article : Google Scholar
|
|
4
|
Salfi R, Hakim F, Bhikshapathi D and Khan
A: Anticancer evaluation of novel quinazolinone acetamides:
Synthesis and characterization. Anticancer Agents Med Chem.
22:926–932. 2022. View Article : Google Scholar
|
|
5
|
Gatadi S, Lakshmi TV and Nanduri S:
4(3H)-Quinazolinone derivatives: Promising antibacterial drug
leads. Eur J Med Chem. 170:157–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zayed MF: Medicinal Chemistry of
quinazolines as analgesic and anti-inflammatory agents.
ChemEngineering. 6:942022. View Article : Google Scholar
|
|
7
|
Mhetre UV, Haval NB, Bondle GM, Rathod SS,
Choudhari PB, Kumari J, Sriram D and Haval KP: Design, synthesis
and molecular docking study of novel triazole-quinazolinone hybrids
as antimalarial and antitubercular agents. Bioorg Med Chem Lett.
108:1298002024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yaduwanshi PS, Singh S, Sahapuriya P,
Dubey P, Thakur J and Yadav S: Synthesis of some noval qunazolinone
derivatives for their anticonvulsant activity. Orient J Chem.
40:369–373. 2024. View Article : Google Scholar
|
|
9
|
Khalifa MM, Sakr HM, Ibrahim A, Mansour AM
and Ayyad RR: Design and synthesis of new benzylidene-quinazolinone
hybrids as potential anti-diabetic agents: In vitro α-glucosidase
inhibition, and docking studies. J Mol Struct. 1250:1317682022.
View Article : Google Scholar
|
|
10
|
Soliman AM, Karam HM, Mekkawy MH and
Ghorab MM: Antioxidant activity of novel quinazolinones bearing
sulfonamide: Potential radiomodulatory effects on liver tissues via
NF-κB/PON1 pathway. Eur J Med Chem. 197:1123332020. View Article : Google Scholar
|
|
11
|
Osman EO, Emam SH, Sonousi A, Kandil MM,
Abdou AM and Hassan RA: Design, synthesis, anticancer, and
antibacterial evaluation of some quinazolinone-based derivatives as
DHFR inhibitors. Drug Dev Res. 84:888–906. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El-Karim SS, Syam YM, El Kerdawy AM and
Abdel-Mohsen HT: Rational design and synthesis of novel
quinazolinone N-acetohydrazides as type II multi-kinase inhibitors
and potential anticancer agents. Bioorg Chem. 142:1069202024.
View Article : Google Scholar
|
|
13
|
Kabir E and Uzzaman M: A review on
biological and medicinal impact of heterocyclic compounds. Results
Chem. 4:1006062022. View Article : Google Scholar
|
|
14
|
Almulla AF: A review: Biological
importance of heterocyclic compounds. Der Pharma Chemica.
9:141–147. 2017.
|
|
15
|
Arora P, Arora V, Lamba HS and Wadhwa D:
Importance of heterocyclic chemistry:A review. Int J Pharm Sci Res.
3:2947–2954. 2012.
|
|
16
|
Kumar B, Babu NJ and Chowhan RL:
Sustainable synthesis of highly diastereoselective &
fluorescent active spirooxindoles catalyzed by copper oxide
nanoparticle immobilized on microcrystalline cellulose. Appl
Organomet Chem. 36: View Article : Google Scholar : 2022. View Article : Google Scholar
|
|
17
|
Khan I, Ibrar A, Abbas N and Saeed A:
Recent advances in the structural library of functionalized
quinazoline and quinazolinone scaffolds: Synthetic approaches and
multifarious applications. Eur J Med Chem. 76:193–244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borah B, Swain S, Patat M and Chowhan LR:
Recent advances and prospects in the organocatalytic synthesis of
quinazolinones. Front Chem. 10:9910262022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faisal M and Saeed A: Chemical insights
into the synthetic chemistry of quinazolines: Recent advances.
Front Chem. 8:5947172021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alsibaee AM, Al-Yousef HM and Al-Salem HS:
Quinazolinones, the winning horse in drug discovery. Molecules.
28:9782023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma PC, Kaur G, Pahwa R, Sharma A and
Rajak H: Quinazolinone analogs as potential therapeutic agents.
Curr Med Chem. 18:4786–4812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khandelwal P, Wadhwani BD, Rao RS, Mali D,
Vyas P, Kumar T and Nair R: Exploring the pharmacological and
chemical aspects of pyrrolo-quinazoline derivatives in Adhatoda
vasica. Heliyon. 10:e257272024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Padmanabhan D, Manimekalai R,
Senthil-Nathan S, Suganthi M and Palanisamy S: Biosynthesis,
therapeutic characteristics, origin and strategies to improve the
yield of vasicine in plants. Vegetos. 186:2025.
|
|
24
|
Zhang Y, Du W, Zhu D, Li M, Qu L, Rao G,
Lin Y, Tong X, Sun Y and Huang F: Vasicine alleviates
2,4-dinitrochlorobenzene-induced atopic dermatitis and passive
cutaneous anaphylaxis in BALB/c mice. Clin Immunol. 244:1091022022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ali SK, Hamed AR, Soltan MM, El-Halawany
AM, Hegazy UM and Hussein AA: Kinetics and molecular docking of
vasicine from Adhatoda vasica: An acetylcholinesterase inhibitor
for Alzheimer's disease. S Afr J Bot. 104:118–124. 2016. View Article : Google Scholar
|
|
26
|
Srinivasarao D, Jayarraj IA, Jayraaj R and
Prabha ML: A study on antioxidant and anti-inflammatory activity of
vasicine against lung damage in rats. Indian J Allergy Asthma
Immunol. 20:1–7. 2006.
|
|
27
|
Eguchi S: Quinazoline alkaloids and
related chemistry. Bioactive Heterocycles I. 6:113–156. 2006.
View Article : Google Scholar
|
|
28
|
Ghosh P, Ganguly B and Das S: C-H
functionalization of quinazolinones by transition metal catalysis.
Org Biomol Chem. 18:4497–4518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rakesh KP, Darshini N, Shubhavathi T and
Mallesha N: Biological applications of quinazolinone analogues: A
review. Org Med Chem Int J. 2:41–45. 2017.
|
|
30
|
Kalogirou AS, Kourtellaris A and Koutentis
PA: Synthesis of 2-Cyanoquinazolin-4-ones from
3',5'-Dichloro-1H-spiro
(quinazoline-2,4'-[1,2,6]thiadiazin)-4(3H)-ones. ChemistrySelect.
5:1884–1889. 2020. View Article : Google Scholar
|
|
31
|
Zeng R, Huang C, Wang J, Zhong Y, Fang Q,
Xiao S, Nie X, Chen S and Peng D: Synthesis, crystal structure, and
antifungal activity of quinazolinone derivatives. Crystals.
13:12542023. View Article : Google Scholar
|
|
32
|
Ghoneim MM, Abdelgawad MA, Elkanzi NAA,
Parambi DGT, Alsalahat I, Farouk A and Bakr RB: A literature review
on pharmacological aspects, docking studies, and synthetic
approaches of quinazoline and quinazolinone derivatives. Arch Pharm
(Weinheim). 357:e24000572024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haneen DSA, Abdalha AA, Alkhatib MM, Kamal
M, Youssef ASA, Abou-Elmagd WSI and Samir SS: Synthesis,
comprehensive in silico studies, and cytotoxicity evaluation of
novel quinazolinone derivatives as potential anticancer agents. Sci
Rep. 15:236972025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borik RM and Hussein MA: A novel
quinazoline-4-one derivatives as a promising cytokine inhibitors:
Synthesis, molecular docking, and structure-activity relationship.
Curr Pharm Biotechnol. 23:1179–1203. 2022. View Article : Google Scholar
|
|
35
|
Samotrueva MA, Starikova AA, Bashkina OA,
Tsibizova AA, Borisov AV, Merezhkina DV, Tyurenkov IN and Ozerov
AA: Biochemical basis of the antimicrobial activity of
quinazolinone derivatives in the light of insights into the
features of the chemical structure and ways of binding to target
molecules. A review. Dokl Chem. 510:107–129. 2023. View Article : Google Scholar
|
|
36
|
Mhaske SB and Argade NP: The chemistry of
recently isolated naturally occurring quinazolinone alkaloids.
Tetrahedron. 62:9787–9826. 2006. View Article : Google Scholar
|
|
37
|
Mahato AK, Srivastava B and Shanthi CN:
Chemistry structure activity relationship and biological activity
of quinazoline-4(3H)-one derivatives. Inventi Rapid: MedChem.
1:0976–7541. 2011.
|
|
38
|
Asif M: Chemical characteristics,
synthetic methods, and biological potential of quinazoline and
quinazolinone derivatives. Int J Med Chem. 2014:1–27. 2014.
|
|
39
|
Garofalo A, Goossens L, Baldeyrou B,
Lemoine A, Ravez S, Six P, David-Cordonnier MH, Bonte JP, Depreux
P, Lansiaux A and Goossens JF: Design, synthesis, and DNA-binding
of N-Alkyl(anilino)quinazoline derivatives. J Med Chem.
53:8089–8103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
El-Malah A, Malebari AM, Khayyat AN,
Mohammad KA, Gineinah MM and Mahmoud Z: Design, synthesis, and
antiproliferative activities of novel
substitutedhydrazone/triazolo-linked quinazoline derivatives. J Mol
Struct. 1306:1378222024. View Article : Google Scholar
|
|
41
|
Haghighijoo Z, Firuzi O, Hemmateenejad B,
Emami S, Edraki N and Miri R: Synthesis and biological evaluation
of quinazolinone-based hydrazones with potential use in Alzheimer's
disease. Bioorg Chem. 74:126–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Khan I, Ibrar A, Ahmed W and Saeed A:
Synthetic approaches, functionalization and therapeutic potential
of quinazoline and quinazolinone skeletons: The advances continue.
Eur J Med Chem. 90:124–169. 2015. View Article : Google Scholar
|
|
43
|
Kaur J, Kaur S, Muskan, Kaur N, Kumar V
and Anand A: Unveiling the therapeutic potential of quinazolinone
derivatives in cancer treatment: A comprehensive exploration.
ChemistrySelect. 9:e2024013662024. View Article : Google Scholar
|
|
44
|
Haider K, Das S, Joseph A and Yar MS: An
appraisal of anticancer activity with structure-activity
relationship of quinazoline and quinazolinone analogues through
EGFR and VEGFR inhibition: A review. Drug Dev Res. 83:859–890.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reddy MM and Sivaramakrishna A: Remarkably
flexible quinazolinones-synthesis and biological applications. J
Heterocycl Chem. 57:942–954. 2019. View Article : Google Scholar
|
|
46
|
He D, Wang M, Zhao S, Shu Y, Zeng H, Xiao
C, Lu C and Liu Y: Pharmaceutical prospects of naturally occurring
quinazolinone and its derivatives. Fitoterapia. 119:136–149. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shankar GM, Alex VV, Nisthul AA, Bava SV,
Sundaram S, Retnakumari AP, Chittalakkottu S and Anto RJ:
Pre-clinical evidences for the efficacy of tryptanthrin as a potent
suppressor of skin cancer. Cell Prolif. 53:e127102020. View Article : Google Scholar
|
|
48
|
Li H, Fu G and Zhong W: Natural
quinazolinones: From a treasure house to promising anticancer
leads. Eur J Med Chem. 245:1149152023. View Article : Google Scholar
|
|
49
|
Kushwaha N, Sahu A, Mishra J, Soni A and
Dorwal D: An insight on the prospect of quinazoline and
quinazolinone derivatives as anti-tubercular agents. Curr Org
Synth. 20:838–869. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jaiswal S, Verma K, Srivastva A, Arya N,
Dwivedi J and Sharma S: Green synthetic and pharmacological
developments in the hybrid quinazolinone moiety: An updated review.
Curr Top Med Chem. 25:493–532. 2025. View Article : Google Scholar
|
|
51
|
Wu C, Wang Y, Yang F, Shi W, Wang Z, He L,
He Y and Shen J: Synthesis and biological evaluation of
five-atom-linker-based arylpiperazine derivatives with an atypical
antipsychotic profile. ChemMedChem. 14:2042–2051. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wei M, Chai WM, Wang R, Yang Q, Deng Z and
Peng Y: Quinazolinone derivatives: Synthesis and comparison of
inhibitory mechanisms on alpha-glucosidase. Bioorg Med Chem.
25:1303–1308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Obeng E: Apoptosis (programmed cell death)
and its signals-A review. Braz J Biol. 81:1133–1143. 2021.
View Article : Google Scholar
|
|
54
|
Nair P, Lu M, Petersen S and Ashkenazi A:
Apoptosis initiation through the cell-extrinsic pathway. Methods
Enzymol. 544:99–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lossi L: The concept of intrinsic versus
extrinsic apoptosis. Biochem J. 479:357–384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hongmei Z: Extrinsic and intrinsic
apoptosis signal pathway review. Apoptosis and Medicine. 2012.
View Article : Google Scholar
|
|
57
|
Ashkenazi A: Targeting the extrinsic
apoptosis pathway in cancer. Cytokine Growth Factor Rev.
19:325–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liang Y, Zheng Y, Yang J, Ke J and Cheng
K: Design, synthesis and bioactivity evaluation of a series of
quinazolinone derivatives as potent PI3Kγ antagonist. Bioorg Med
Chem. 84:1172612023. View Article : Google Scholar
|
|
59
|
Kim YS, Cheon MG, Boggu PR, Koh SY, Park
GM, Kim G, Park SH, Park SL, Lee CW, Kim JW and Jung YH: Synthesis
and biological evaluation of novel purinyl quinazolinone
derivatives as PI3Kδ-specific inhibitors for the treatment of
hematologic malignancies. Bioorg Med Chem. 45:1163122021.
View Article : Google Scholar
|
|
60
|
Wani ZA, Guru SK, Rao AVS, Sharma S,
Mahajan G, Behl A, Kumar A, Sharma PR, Kamal A, Bhushan S and
Mondhe DM: A novel quinazolinone chalcone derivative induces
mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR
signaling pathway in human colon cancer HCT-116 cells. Food Chem
Toxicol. 87:1–11. 2016. View Article : Google Scholar
|
|
61
|
Madbouly EA, Lashine ESM, Al-Karmalawy AA,
Sebaiy MM, Pratsinis H, Kletsas D and Metwally K: Design and
synthesis of novel quinazolinone-chalcone hybrids as potential
apoptotic candidates targeting caspase-3 and PARP-1:In vitro,
molecular docking, and SAR studies. New J Chem. 46:22013–22029.
2022. View Article : Google Scholar
|
|
62
|
Xie J, Yang MR, Hu X, Hong ZS, Bai YY,
Sheng J, Tian Y and Shi CY: Moringa oleifera Lam. Isothiocyanate
quinazolinone derivatives inhibit U251 glioma cell proliferation
through cell cycle regulation and apoptosis induction. Int J Mol
Sci. 24:113762023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qiu J, Zhou Q, Zhang Y, Guan M, Li X, Zou
Y, Huang X, Zhao Y, Chen W and Gu X: Discovery of novel
quinazolinone derivatives as potential anti-HBV and anti-HCC
agents. Eur J Med Chem. 205:1125812020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
El-Shafey HW, Gomaa RM, El-Messery SM and
Goda FE: Synthetic approaches, anticancer potential, HSP90
inhibition, multitarget evaluation, molecular modeling and
apoptosis mechanistic study of thioquinazolinone skeleton:
Promising antibreast cancer agent. Bioorg Chem. 101:1039872020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hour MJ, Tsai FJ, Lai IL, Tsao JW, Chiang
JH, Chiu YJ, Lu HF, Juan YN, Yang JS and Tsai SC: Efficacy of
HMJ-38, a new quinazolinone analogue, against the
gemcitabine-resistant MIA-PaCa-2 pancreatic cancer cells.
Biomedicine (Taipei). 13:20–31. 2023. View Article : Google Scholar
|
|
66
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pope LE and Dixon SJ: Regulation of
ferroptosis by lipid metabolism. Trends Cell Biol. 33:1077–1087.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dixon SJ and Olzmann JA: The cell biology
of ferroptosis. Nat Rev Mol Cell Biol. 25:424–442. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang M, Guo M, Gao Y, Wu C, Pan X and
Huang Z: Mechanisms and therapeutic targets of ferroptosis:
Implications for nanomedicine design. J Pharm Anal. 14:1009602024.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tang D and Kroemer G: Ferroptosis. Curr
Biol. 30:R1292–R1297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yuan H, Li X, Zhang X, Kang R and Tang D:
Identification of ACSL4 as a biomarker and contributor of
ferroptosis. Biochem Biophys Res Commun. 478:1338–1343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liang X, Long L, Guan F, Xu Z and Huang H:
Research status and potential applications of circRNAs affecting
colorectal cancer by regulating ferroptosis. Life Sci.
352:1228702024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang Y, Luo M, Zhang K, Zhang J, Gao T,
Connell DO, Yao F, Mu C, Cai B, Shang Y and Chen W: Nedd4
ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in
melanoma. Nat Commun. 11:4332020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sun Y, Deng R and Zhang C: Erastin induces
apoptotic and ferroptotic cell death by inducing ROS accumulation
by causing mitochondrial dysfunction in gastric cancer cell HGC-27.
Mol Med Rep. 22:2826–2832. 2020.PubMed/NCBI
|
|
78
|
Li Y, Zeng X, Lu D, Yin M, Shan M and Gao
Y: Erastin induces ferroptosis via ferroportin-mediated iron
accumulation in endometriosis. Hum Reprod. 36:951–964. 2021.
View Article : Google Scholar
|
|
79
|
Huang C, Yang M, Deng J, Li P, Su W and
Jiang R: Upregulation and activation of p53 by erastin-induced
reactive oxygen species contribute to cytotoxic and cytostatic
effects in A549 lung cancer cells. Oncol Rep. 40:2363–2370.
2018.PubMed/NCBI
|
|
80
|
Badgley MA, Kremer DM, Maurer HC,
DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J,
Firl CEM, et al: Cysteine depletion induces pancreatic tumor
ferroptosis in mice. Science. 368:85–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jiang Y and Sun M: SLC7A11: The Achilles
heel of tumor? Front Immunol. 15:14388072024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar
|
|
83
|
Zhao X, Wang T, Shang F, Yan J, Jiang M,
Zou X, Li G, Song Z and Huang J: Coumarin-Quinazolinone based
photosensitizers: Mitochondria and endoplasmic reticulum targeting
for enhanced phototherapy via different cell death pathways. Eur J
Med Chem. 280:1169902024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xing Z, Yan J, Miao Y, Ruan Y, Yao H, Zhou
Y, Tang Y, Li G, Song Z, Peng Y and Huang J: Endoplasmic
reticulum-targeting quinazolinone-based lipophilic probe for
specific photoinduced ferroptosis and its induced lipid dynamic
regulation. J Med Chem. 67:1900–1913. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Huang Y, Xiong K, Wang A, Wang Z, Cui Q,
Xie H, Yang T, Fan X, Jiang W, Tan X and Huang Q: Cold stress
causes liver damage by inducing ferroptosis through the p38
MAPK/Drp1 pathway. Cryobiology. 113:1045632023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li X, Ran Q, He X, Peng D, Xiong A, Jiang
M, Zhang L, Wang J, Bai L, Liu S, et al: HO-1 upregulation promotes
mitophagy-dependent ferroptosis in PM2.5-exposed hippocampal
neurons. Ecotoxicol Environ Saf. 277:1163142024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nakamura T, Hipp C, Dias Mourão A,
Borggräfe J, Aldrovandi M, Henkelmann B, Wanninger J, Mishima E,
Lytton E, Emler D, et al: Phase separation of FSP1 promotes
ferroptosis. Nature. 619:371–377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu Z, Zhu Y, Liu W, Balasubramanian B, Xu
X, Yao J and Lei X: Ferroptosis in liver disease: Natural active
compounds and therapeutic implications. Antioxidants (Basel).
13:3522024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Balushi KA, Hadhrami AA, Balushi HA,
Lawati AA and Das S: Tebentafusp as a promising drug for the
treatment of uveal melanoma. Curr Drug Targets. 25:149–157. 2024.
View Article : Google Scholar
|
|
90
|
Hu S, Sechi M, Singh PK, Dai L, McCann S,
Sun D, Ljungman M and Neamati N: A novel redox modulator induces a
GPX4-mediated cell death that is dependent on iron and reactive
oxygen species. Eur J Med Chem. 63:9838–9855. 2020. View Article : Google Scholar
|
|
91
|
Cheng Z: The FoxO-autophagy axis in health
and disease. Trends Endocrinol Metab. 30:658–671. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Buzun K, Gornowicz A, Lesyk R, Bielawski K
and Bielawska A: Autophagy modulators in cancer therapy. Int J Mol
Sci. 22:58042021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang B and Liu L: Autophagy is a
double-edged sword in the therapy of colorectal cancer. Oncol Lett.
21:3782021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gump JM and Thorburn A: Autophagy and
apoptosis: What is the connection? Trends Cell Biol. 21:387–392.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kumar S, Guru SK, Pathania AS, Mupparapu
N, Kumar A, Malik F, Bharate SB, Ahmed QN, Vishwakarma RA and
Bhushan S: A novel quinazolinone derivative induces cytochrome c
interdependent apoptosis and autophagy in human leukemia MOLT-4
cells. Toxicol Rep. 1:1013–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sharma R, Chatterjee E, Mathew J, Sharma
S, Rao NV, Pan CH, Lee SB, Dhingra A, Grewal AS, Liou JP, et al:
Accommodation of ring C expanded deoxyvasicinone in the HDAC
inhibitory pharmacophore culminates into a tractable anti-lung
cancer agent and pH-responsive nanocarrier. Eur J Med Chem.
240:1146022022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xia X, Wang L, Zhang X, Wang S, Lei L,
Cheng L, Xu Y, Sun Y, Hang B, Zhang G, et al: Halofuginone-induced
autophagy suppresses the migration and invasion of MCF-7 cells via
regulation of STMN1 and p53. J Cell Biochem. 119:4009–4020. 2018.
View Article : Google Scholar
|
|
99
|
Ha HA, Chiang JH, Tsai FJ, Bau DT, Juan
YN, Lo YH, Hour MJ and Yang JS: Novel quinazolinone MJ-33 induces
AKT/mTOR-mediated autophagy-associated apoptosis in 5FU-resistant
colorectal cancer cells. Oncol Rep. 45:680–692. 2020. View Article : Google Scholar
|
|
100
|
ElZahabi HSA, Nafie MS, Osman D, Elghazawy
NH, Soliman DH, El-Helby AAH and Arafa RK: Design, synthesis and
evaluation of new quinazolin-4-one derivatives as apoptotic
enhancers and autophagy inhibitors with potent antitumor activity.
Eur J Med Chem. 222:1136092021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Roger L, Tomas F and Gire V: Mechanisms
and regulation of cellular senescence. Int J Mol Sci. 22:131732021.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Huang W, Hickson LJ, Eirin A, Kirkland JL
and Lerman LO: Cellular senescence: The good, the bad and the
unknown. Nat Rev Nephrol. 18:611–627. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
de Magalhães JP: Cellular senescence in
normal physiology. Science. 384:1300–1301. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Schmitt CA, Wang B and Demaria M:
Senescence and cancer-role and therapeutic opportunities. Nat Rev
Clin Oncol. 19:619–636. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang L, Lankhorst L and Bernards R:
Exploiting senescence for the treatment of cancer. Nat Rev Cancer.
22:340–355. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kamal A, Sultana F, Ramaiah MJ, Srikanth
YVV, Viswanath A, Bharathi EV, Nayak R, Pushpavalli SNCVL, Srinivas
C and Pal-Bhadra M: 3-Diarylethyne quinazolinones: A new class of
senescence inducers. Med Chem Comm. 4:575–581. 2013. View Article : Google Scholar
|
|
107
|
Venkatesh R, Ramaiah MJ, Gaikwad HK,
Janardhan S, Bantu R, Nagarapu L, Sastry GN, Ganesh AR and Bhadra
M: Luotonin-A based quinazolinones cause apoptosis and senescence
via HDAC inhibition and activation of tumor suppressor proteins in
HeLa cells. Eur J Med Chem. 94:87–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Proskuryakov SY and Gabai VL: Mechanisms
of tumor cell necrosis. Curr Pharm Des. 16:56–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Szabó C: Mechanisms of cell necrosis. Crit
Care Med. 33:530–534. 2005. View Article : Google Scholar
|
|
110
|
Loveless R, Bloomquist R and Teng Y:
Pyroptosis at the forefront of anticancer immunity. J Exp Clin
Cancer Res. 40:2642021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T,
Huang J, Wang F, Zhou F and Zhang L: Role of pyroptosis in
inflammation and cancer. Cell Mol Immunol. 19:971–992. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Shams LM, Minaei-Tehrani D, Gholipour H
and Nohehkhan M: Effects of quinazolinones on Balb/C mice embryonic
livers. Indian J Exp Biol. 49:183–190. 2011.PubMed/NCBI
|
|
113
|
Piamsiri C, Maneechote C, Jinawong K,
Arunsak B, Chunchai T, Nawara W, Kerdphoo S, Chattipakorn SC and
Chattipakorn N: Chronic mitochondrial dynamic-targeted therapy
alleviates left ventricular dysfunction by reducing multiple
programmed cell death in post-myocardial infarction rats. Eur J
Pharmacol. 977:1767362024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li L, Mu Z, Liu P, Wang Y, Yang F and Han
X: Mdivi-1 alleviates atopic dermatitis through the inhibition of
NLRP3 inflammasome. Exp Dermatol. 30:1734–1744. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tanriover C, Copur S, Ucku D, Cakir AB,
Hasbal NB, Soler MJ and Kanbay M: The mitochondrion: A promising
target for kidney disease. Pharmaceutics. 15:5702023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu R, Wang SC, Li M, Ma XH, Jia XN, Bu Y,
Sun L and Yu KJ: An inhibitor of DRP1 (Mdivi-1) alleviates
LPS-induced septic AKI by inhibiting NLRP3 inflammasome activation.
Biomed Res Int. 2020:1–11. 2020.
|
|
117
|
Qian W, Wang J, Roginskaya V, McDermott
LA, Edwards RP, Stolz DB, Llambi F, Green DR and Van Houten B:
Novel combination of mitochondrial division inhibitor 1 (mdivi-1)
and platinum agents produces synergistic pro-apoptotic effect in
drug resistant tumor cells. Oncotarget. 5:4180–4194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tusskorn O, Khunluck T, Prawan A,
Senggunprai L and Kukongviriyapan V: Mitochondrial division
inhibitor-1 potentiates cisplatin-induced apoptosis via the
mitochondrial death pathway in cholangiocarcinoma cells. Biomed
Pharmacother. 111:109–118. 2019. View Article : Google Scholar
|
|
119
|
Vargo JW, Walker SN, Gopal SR, Deshmukh
AR, McDermott BM Jr, Alagramam KN and Stepanyan R: Inhibition of
mitochondrial division attenuates cisplatin-induced toxicity in the
neuromast hair cells. Front Cell Neurosci. 11:3932017. View Article : Google Scholar
|
|
120
|
Lai KC, Chia YT, Yih LH, Lu YL, Chang ST,
Hong ZX, Chen TL and Hour MJ: Antitumor effects of the novel
quinazolinone Holu-12: Induction of mitotic arrest and apoptosis in
human oral squamous cell carcinoma CAL27 cells. Anticancer Res.
41:259–268. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Pathania D, Kuang Y, Sechi M and Neamati
N: Mechanisms underlying the cytotoxicity of a novel
quinazolinedione-based redox modulator, QD232, in pancreatic cancer
cells. Br J Pharmacol. 172:50–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou J, Ji M, Yao H, Cao R, Zhao H, Wang
X, Chen X and Xu B: Discovery of quinazoline-2,4(1H,3H)-dione
derivatives as novel PARP-1/2 inhibitors: Design, synthesis and
their antitumor activity. Org Biomol Chem. 16:3189–3202. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhou Q, Ji M, Zhou J, Jin J, Xue N, Chen
J, Xu B and Chen X: Poly (ADP-ribose) polymerases inhibitor,
Zj6413, as a potential therapeutic agent against breast cancer.
Biochem Pharmacol. 107:29–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Giannini G, Battistuzzi G, Vesci L,
Milazzo FM, De Paolis F, Barbarino M, Guglielmi MB, Carollo V,
Gallo G, Artali R and Dallavalle S: Novel PARP-1 inhibitors based
on a 2-propanoyl-3H-quinazolin-4-one scaffold. Bioorg Med Chem
Lett. 24:462–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Ma Y, Yang J, Wei X, Pei Y, Ye J, Li X, Si
G, Tian J, Dong Y and Liu G: Nonpeptidic quinazolinone derivatives
as dual nucleotide-binding oligomerization domain-like receptor 1/2
antagonists for adjuvant cancer chemotherapy. Eur J Med Chem.
207:1127232020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Smolewski P and Rydygier D: Efficacy and
safety of idelalisib for the treatment of indolent B-cell
malignancies. Expert Opin Pharmacother. 21:1915–1926. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Miller BW, Przepiorka D, de Claro RA, Lee
K, Nie L, Simpson N, Gudi R, Saber H, Shord S, Bullock J, et al:
FDA approval: Idelalisib monotherapy for the treatment of patients
with follicular lymphoma and small lymphocytic Lymphoma. Clin
Cancer Res. 21:1525–1529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Merli M, Passamonti F and Arcaini L: The
double significance of idelalisib immune-related toxicity. Leuk
Lymphoma. 62:2815–2817. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu K, Li D, Zheng W, Shi M, Chen Y, Tang
M, Yang T, Zhao M, Deng D, Zhang C, et al: Discovery, optimization,
and evaluation of quinazolinone derivatives with novel linkers as
orally efficacious phosphoinositide-3-kinase delta inhibitors for
treatment of inflammatory diseases. J Med Chem. 64:8951–8970. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yu M, Chen J, Xu Z, Yang B, He Q, Luo P,
Yan H and Yang X: Development and safety of PI3K inhibitors in
cancer. Arch Toxicol. 97:635–650. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ramanathan S, Jin F, Sharma S and Kearney
BP: Clinical pharmacokinetic and pharmacodynamic profile of
idelalisib. Clin Pharmacokinet. 55:33–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wiese W, Barczuk J, Racinska O, Siwecka N,
Rozpedek-Kaminska W, Slupianek A, Sierpinski R and Majsterek I:
PI3K/Akt/mTOR signaling pathway in blood malignancies-new
therapeutic possibilities. Cancers (Basel). 15:52972023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhu J, Wang P, Shehu AI, Lu J, Bi H and Ma
X: Identification of novel pathways in idelalisib metabolism and
bioactivation. Chem Res Toxicol. 31:548–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Graf SA and Gopal AK: Idelalisib for the
treatment of non-Hodgkin lymphoma. Expert Opin Pharmacother.
17:265–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lin X, Zhang Y, Huang H, Zhuang W and Wu
L: Post-marketing safety concern of PI3K inhibitors in the cancer
therapies: An 8-year disproportionality analysis from the FDA
adverse event reporting system. Expert Opin Drug Saf. 24:1–12.
2024.
|
|
136
|
Hus I, Puła B and Robak T: PI3K inhibitors
for the treatment of chronic lymphocytic leukemia: Current status
and future perspectives. Cancers (Basel). 14:15712022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zirlik K and Veelken H: Idelalisib. Recent
Results Cancer Res. 212:243–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Scheffold A, Jebaraj BMC, Tausch E,
Bloehdorn J, Ghia P, Yahiaoui A, Dolnik A, Blätte TJ, Bullinger L,
Dheenadayalan RP, et al: IGF1R as druggable target mediating PI3K-δ
inhibitor resistance in a murine model of chronic lymphocytic
leukemia. Blood. 134:534–547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Park GB, Chung YH, Jeong JY and Kim D: A
p110δ-specific inhibitor combined with bortezomib blocks drug
resistance properties of EBV-related B cell origin cancer cells via
regulation of NF-κB. Int J Oncol. 50:1711–1720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Alossaimi MA, Riadi Y, Alnuwaybit GN, Md
S, Alkreathy HM, Elekhnawy E, Geesi MH, Alqahtani SM and Afzal O:
Design, synthesis, molecular docking, and in vitro studies of
2-mercaptoquinazolin-4(3H)-ones as potential anti-breast cancer
agents. Saudi Pharm J. 32:1019712024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Škubník J, Jurášek M, Ruml T and Rimpelová
S: Mitotic poisons in research and medicine. Molecules.
25:46322020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Shahin R and Aljamal S: Kinesin spindle
protein inhibitors in cancer: From high throughput screening to
novel therapeutic strategies. Future Sci OA. 8:FSO7782022.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Lee CW, Bélanger K, Rao SC, Petrella TM,
Tozer RG, Wood L, Savage KJ, Eisenhauer EA, Synold TW, Wainman N
and Seymour L: A phase II study of ispinesib (SB-715992) in
patients with metastatic or recurrent malignant melanoma: A
national cancer institute of Canada clinical trials group trial.
Invest New Drugs. 26:249–255. 2008. View Article : Google Scholar
|
|
144
|
Purcell JW, Davis J, Reddy M, Martin S,
Samayoa K, Vo H, Thomsen K, Bean P, Kuo WL, Ziyad S, et al:
Activity of the kinesin spindle protein inhibitor ispinesib
(SB-715992) in models of breast cancer. Clin Cancer Res.
16:566–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Lee RT, Beekman KE, Hussain M, Davis NB,
Clark JI, Thomas SP, Nichols KF and Stadler WM: A University of
Chicago consortium phase II trial of SB-715992 in advanced renal
cell cancer. Clin Genitourin Cancer. 6:21–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Murase Y, Ono H, Ogawa K, Yoshioka R,
Ishikawa Y, Ueda H, Akahoshi K, Ban D, Kudo A, Tanaka S and Tanabe
M: Inhibitor library screening identifies ispinesib as a new
potential chemotherapeutic agent for pancreatic cancers. Cancer
Sci. 112:4641–4654. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gomez HL, Philco M, Pimentel P, Kiyan M,
Monsalvo ML, Conlan MG, Saikali KG, Chen MM, Seroogy JJ, Wolff AA
and Escandon RD: Phase I dose-escalation and pharmacokinetic study
of ispinesib, a kinesin spindle protein inhibitor, administered on
days 1 and 15 of a 28-day schedule in patients with no prior
treatment for advanced breast cancer. Anticancer Drugs. 23:335–341.
2012. View Article : Google Scholar
|
|
148
|
Kenchappa RS, Dovas A, Argenziano MG,
Meyer CT, Stopfer LE, Banu MA, Pereira B, Griffith J, Mohammad A,
Talele S, et al: Activation of STAT3 through combined SRC and EGFR
signaling drives resistance to a mitotic kinesin inhibitor in
glioblastoma. Cell Rep. 39:1109912022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Ansbro MR, Shukla S, Ambudkar SV, Yuspa SH
and Li L: Screening compounds with a novel high-throughput
ABCB1-mediated efflux assay identifies drugs with known therapeutic
targets at risk for multidrug resistance interference. PLoS One.
8:e603342013. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Purcell JW, Davis J, Reddy M, Martin S,
Samayoa K, Vo H, Thomsen K, Bean P, Kuo WL, Ziyad S, et al:
Activity of the kinesin spindle protein inhibitor ispinesib
(SB-715992) in models of breast cancer. Clin Cancer Res.
16:566–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Gish RG, Porta C, Lazar L, Ruff P, Feld R,
Croitoru A, Feun L, Jeziorski K, Leighton J, Gallo J and Kennealey
GT: Phase III randomized controlled trial comparing the survival of
patients with unresectable hepatocellular carcinoma treated with
nolatrexed or doxorubicin. J Clin Oncol. 25:3069–3075. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lord R, Suddle A and Ross PJ: Emerging
strategies in the treatment of advanced hepatocellular carcinoma:
The role of targeted therapies. Int J Clin Pract. 65:182–188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Gish RG, Porta C, Lazar L, Ruff P, Feld R,
Croitoru A, Feun L, Jeziorski K, Leighton J, Knox J and Kennealey
GT: Phase III randomized controlled trial comparing the survival of
patients with unresectable hepatocellular carcinoma treated with
nolatrexed or doxorubicin. J Clin Oncol. 25:3069–3075. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Giovannetti E, Backus HH, Wouters D,
Ferreira CG, van Houten VM, Brakenhoff RH, Poupon MF, Azzarello A,
Pinedo HM and Peters GJ: Changes in the status of p53 affect drug
sensitivity to thymidylate synthase (TS) inhibitors by altering TS
levels. Br J Cancer. 96:769–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Van Triest B, Pinedo HM, Giaccone G and
Peters GJ: Downstream molecular determinants of response to
5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann
Oncol. 11:385–391. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Hasan RU, Mian SS, Arfi S, Begum B, Verma
S, Ahmad R, Hussain I and Asif M: Study of pharmacologically active
drugs containing quinazoline pharmacophore: A brief overview. J Adv
Zool. 45:1166–1184. 2024.
|
|
157
|
Han Y, Liu S, Zhu J, Liu P, Meng Z, Li Y,
Li S, Fan F, Zhang M and Liu H: Experimental study on the
inhibitory effect of Halofuginone on NSCLC. European Journal of
Pharmacology. 2024.
|
|
158
|
Chen Y, Liu W and Wang P, Hou H, Liu N,
Gong L, Wang Y, Ji K, Zhao L and Wang P: Halofuginone inhibits
radiotherapy-induced epithelial mesenchymal transition in lung
cancer. Oncotarget. 7:71341–71352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zuo R, Guo X, Song X, Gao X, Zhang J,
Jiang S, Adam V, Kuca K, Wu W and Guo D: New uses of halofuginone
to treat cancer. J Pharm Anal. 15:1010802024. View Article : Google Scholar
|
|
160
|
Mi L, Liu J, Zhang Y, Su A, Tang M, Xing
Z, He T, Wei T, Li Z and Wu W: The EPRS-ATF4-COLI pathway axis is a
potential target for anaplastic thyroid carcinoma therapy.
Phytomedicine. 129:1556702024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhao H, Li R, Chen Y, Yang X and Shang Z:
Stromal nicotinamide N-methyltransferase orchestrates the crosstalk
between fibroblasts and tumour cells in oral squamous cell
carcinoma: Evidence from patient-derived assembled organoids.
Oncogene. 42:1166–1180. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Panda H, Suzuki M, Naito M, Saito R, Wen
H, Baird L, Uruno A, Miyata K and Yamamoto M: Halofuginone micelle
nanoparticles eradicate Nrf2-activated lung adenocarcinoma without
systemic toxicity. Free Radic Biol Med. 187:92–104. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Li Y, Liu P, Liu S, Zhu J, Han Y, Jiang Z,
Tang D, Meng Z, Li S, Zhang M, et al: Halofuginone targets
Serine/Glycine synthesis to reverse epidermal growth factor
receptor tyrosine Kinase inhibitor resistance in lung
adenocarcinoma. Phytomedicine. 143:1567882025. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zhang L, Yue G, Lu Y and Tang J: BRCA1 as
a target for attenuating paclitaxel resistance by Halofuginone
treatment in basal-like breast cancer. J Funct Foods.
118:1062452024. View Article : Google Scholar
|
|
165
|
Wang C, Zhu JB, Yan YY, Zhang W, Gong XJ,
Wang X and Wang XL: Halofuginone inhibits tumorigenic progression
of 5-FU-resistant human colorectal cancer HCT-15/FU cells by
targeting miR-132-3p in vitro. Oncol Lett. 20:3852020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhu S, Wang J, Chandrashekar G, Smith E,
Liu X and Zhang Y: Synthesis and evaluation of 4-quinazolinone
compounds as potential antimalarial agents. Eur J Med Chem.
45:3864–3869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zuo R, Zhang J, Song X, Hu S, Gao X, Wang
J, Ji H, Ji C, Peng L, Si H, et al: Encapsulating halofuginone
hydrobromide in TPGS polymeric micelles enhances efficacy against
triple-negative breast cancer cells. Int J Nanomedicine.
16:1587–1600. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chen J, Fan S, Guo J, Yang J, Pan L and
Xia Y: Discovery of anticancer function of Febrifugine: Inhibition
of cell proliferation, induction of apoptosis and suppression
steroid synthesis in bladder cancer cells. Toxicol Appl Pharmacol.
484:1168782024. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Lin F, Wang R, Du J, Wen C, Wang X, Jin Y
and Shao L: Pharmacokinetics and bioavailability of febrifugine in
rat plasma determined by UPLC-MS/MS. Acta Chromatogr. 37:
View Article : Google Scholar : 2024.
|
|
170
|
Chen J, Fan S, Guo J, Yang J, Pan L and
Xia Y: Discovery of anticancer function of Febrifugine: Inhibition
of cell proliferation, induction of apoptosis and suppression
steroid synthesis in bladder cancer cells. Toxicol Appl Pharmacol.
484:1168782024. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Zhang J, Xu HX, Zhu JQ, Dou YX, Xian YF
and Lin ZX: Natural Nrf2 inhibitors: A review of their potential
for cancer treatment. Int J Biol Sci. 19:3029–3041. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Zhu J, Liu S, Liu P, Zhai K and Liu H:
Traditional medicine meets modern science: Halofuginone's role in
combating autoimmune diseases. J Nat Med. 79:1017–1029. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Hasan RU, Mian SS, Arfi S, Begum B, Verma
S, Ahmad R, Hussain I and Asif M: Study of pharmacologically active
drugs containing quinazoline pharmacophore: A brief overview. J Adv
Zool. 45:1166–1184. 2024.
|
|
174
|
Haider K, Das S, Joseph A and Yar MS: An
appraisal of anticancer activity with structure-activity
relationship of quinazoline and quinazolinone analogues through
EGFR and VEGFR inhibition: A review. Drug Dev Res. 83:859–890.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Jiang X, Yu J, Zhou Z, Kongsted J, Song Y,
Pannecouque C, De Clercq E, Kang D, Poongavanam V, Liu X and Zhan
P: Molecular design opportunities presented by solvent-exposed
regions of target proteins. Med Res Rev. 39:2194–2238. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
El-Sayed NNE, Al-Otaibi TM, Barakat A,
Almarhoon ZM, Hassan MZ, Al-Zaben MI, Krayem N, Masand VH and Bacha
AB: Synthesis and biological evaluation of some New
3-Aryl-2-thioxo-2,3-dihydroquinazolin-4(1H)-ones and
3-Aryl-2-(benzylthio)quinazolin-4(3H)-ones as antioxidants; COX-2,
LDHA, α-Glucosidase and α-amylase inhibitors; and anti-colon
carcinoma and apoptosis-inducing agents. Pharmaceuticals (Basel).
16:13922023. View Article : Google Scholar
|
|
177
|
Kurogi Y, Inoue Y, Tsutsumi K, Nakamura S,
Nagao K, Yoshitsugu H and Tsuda Y: Synthesis and hypolipidemic
activities of novel 2-[4-[diethoxyphosphoryl)methyl]phenyl]
quinazolines and 4(3H)-quinazolinones. J Med Chem. 39:1433–1437.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Huestis MP, Dela Cruz D, DiPasquale AG,
Durk MR, Eigenbrot C, Gibbons P, Gobbi A, Hunsaker TL, La H, Leung
DH, et al: Targeting KRAS mutant cancers via combination treatment:
Discovery of a 5-Fluoro-4-(3H)-quinazolinone Aryl urea pan-RAF
kinase inhibitor. J Med Chem. 64:3940–3955. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Rezaeinasab R, Jafari E and Khodarahmi G:
Quinazolinone-based hybrids with diverse biological activities: A
mini-review. J Res Med Sci. 27:682022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Lv L, Maimaitiming M, Xia S, Yang J, Zhang
T, Wang Y, Li X, Pinchuk I, Wang P, Wang CY and Liu Z: MR2938
relieves DSS-induced colitis in mice through inhibiting NF-κB
signaling and improving epithelial barrier. Mar Life Sci Technol.
18: View Article : Google Scholar : 2025.
|
|
181
|
Upadhyay R, Tandel P and Patel AB:
Halogen-based quinazolin-4(3H)-one derivatives as MCF-7 breast
cancer inhibitors: Current developments and structure-activity
relationship. Arch Pharm (Weinheim). 358:e24007402024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Wahan SK, Sharma B and Chawla PA:
Medicinal perspective of quinazolinone derivatives: Recent
developments and structure-activity relationship studies. Curr Top
Med Chem. 59:239–257. 2021.
|
|
183
|
Kumar P, Tomar V, Joshi RK and Nemiwal M:
Nanocatalyzed synthetic approach for quinazoline and quinazolinone
derivatives: A review (2015-present). Synth Commun. 52:795–826.
2022. View Article : Google Scholar
|
|
184
|
Chen Y, Sun SN, Chen XH, Chen ML, Lin JM,
Niu Q, Li SL, Liu J and Lan YQ: Predesign of covalent-organic
frameworks for efficient photocatalytic dehydrogenative
cross-coupling reaction. Adv Mater. 37:e24136382025. View Article : Google Scholar
|
|
185
|
Huang FP, Qin WJ, Pan XY, Yang K, Wang K
and Teng QH: Visible-Light-Induced chemodivergent synthesis of
tetracyclic quinazolinones and 3-iminoisoindoliones via the
substrate control strategy. J Org Chem. 89:4395–4405. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Kavitha K, Srinivasan N and Harbabu Y:
Review of quinazolinone scaffold as anticancer agents. World J
Pharm Res. 7:434–454. 2018.
|
|
187
|
Wang Q, Pan Y, Luo H, Zhang Y, Gao F, Wang
J and Zheng J: Novel approaches for the solid-phase synthesis of
dihydroquinazoline-2(1H)-one derivatives and biological evaluation
as potential anticancer agents. Molecules. 27:85772022. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Aljohani AKB, Almadani SA, Alsulaimany M,
Aljuhani N, Samman WA, Al-Shareef AH, Alghamdi R, Tayeb SM, Alharbi
HY, Aljohani MS, et al: Nano-carrier, design, synthesis, in silico
ADMET, anti-proliferative assessments and docking of
[1,2,4]triazolo[4,3-a]quinoxalines as Topo-II inhibitors and DNA
intercalators. Naunyn Schmiedebergs Arch Pharmacol. 11: View Article : Google Scholar : 2025.
|
|
189
|
Udayasree N, Haridasyam RB, Palabindela R,
Krishna TM and Narsimha S: One-pot synthesis, anticancer, EGFR and
caspases assays of novel fused
[1,2,3]triazolo-pyrrolo[2,1-b]quinazolinones. J Mol Struct.
1320:1395702025. View Article : Google Scholar
|
|
190
|
Zhu MS, Zhang G, Xu YJ, Sun R and Ge JF:
Conjugated structures based on quinazolinones and their application
in fluorescent labeling. Org Biomol Chem. 21:1992–2000. 2023.
View Article : Google Scholar : PubMed/NCBI
|















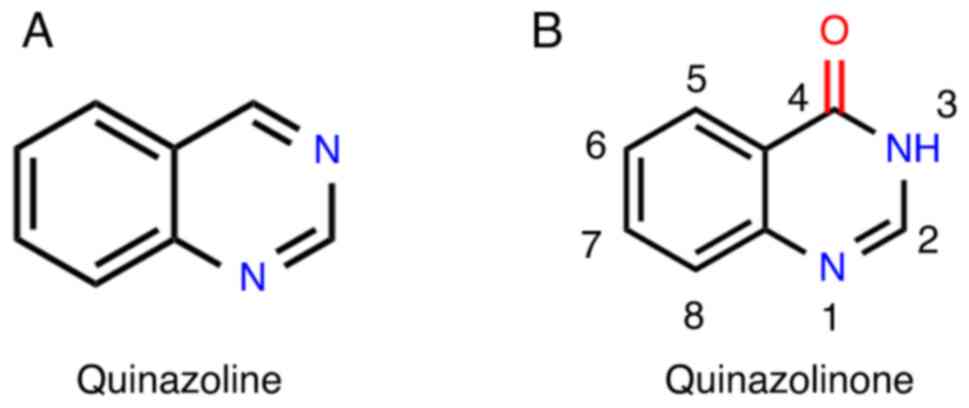
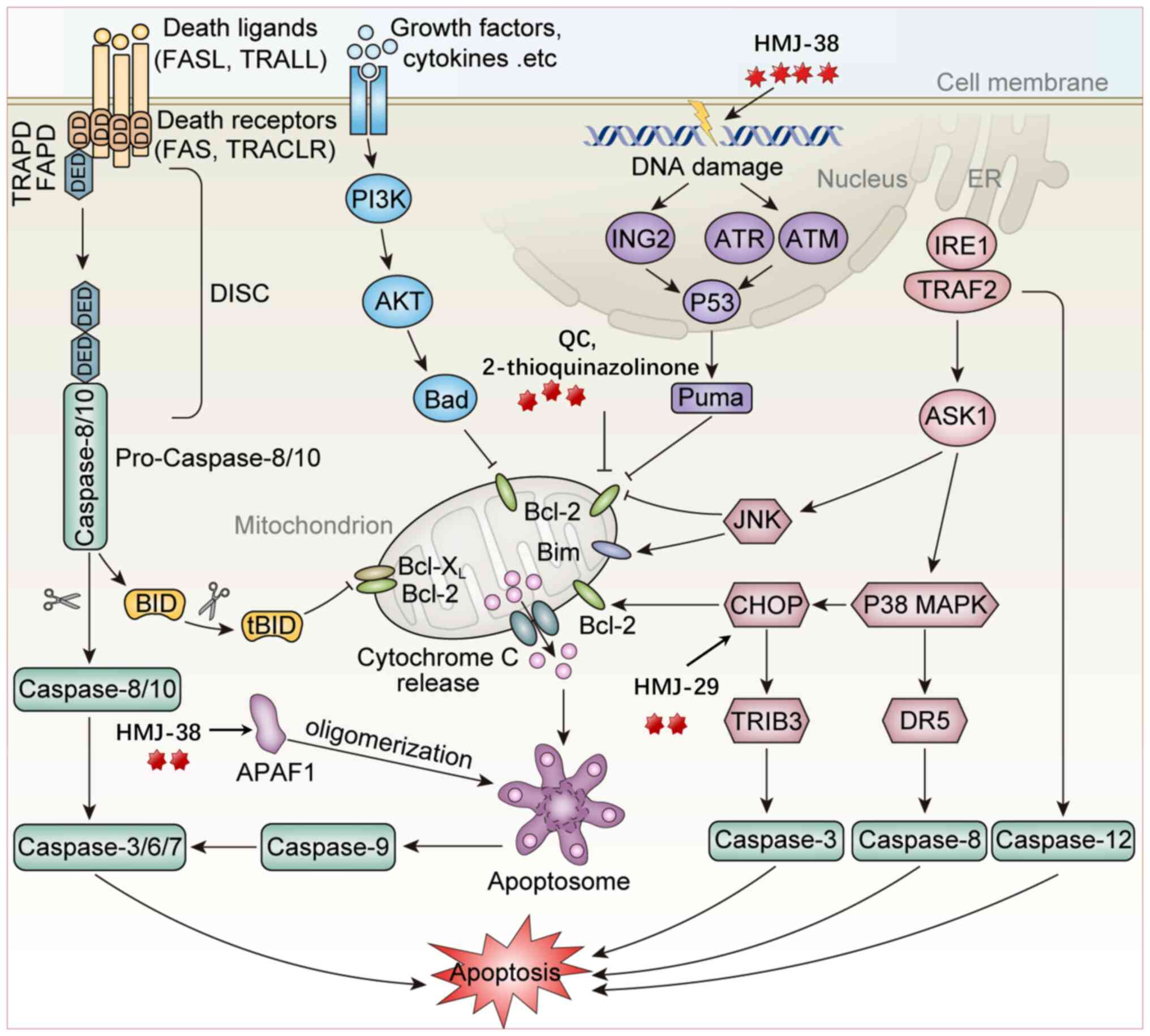
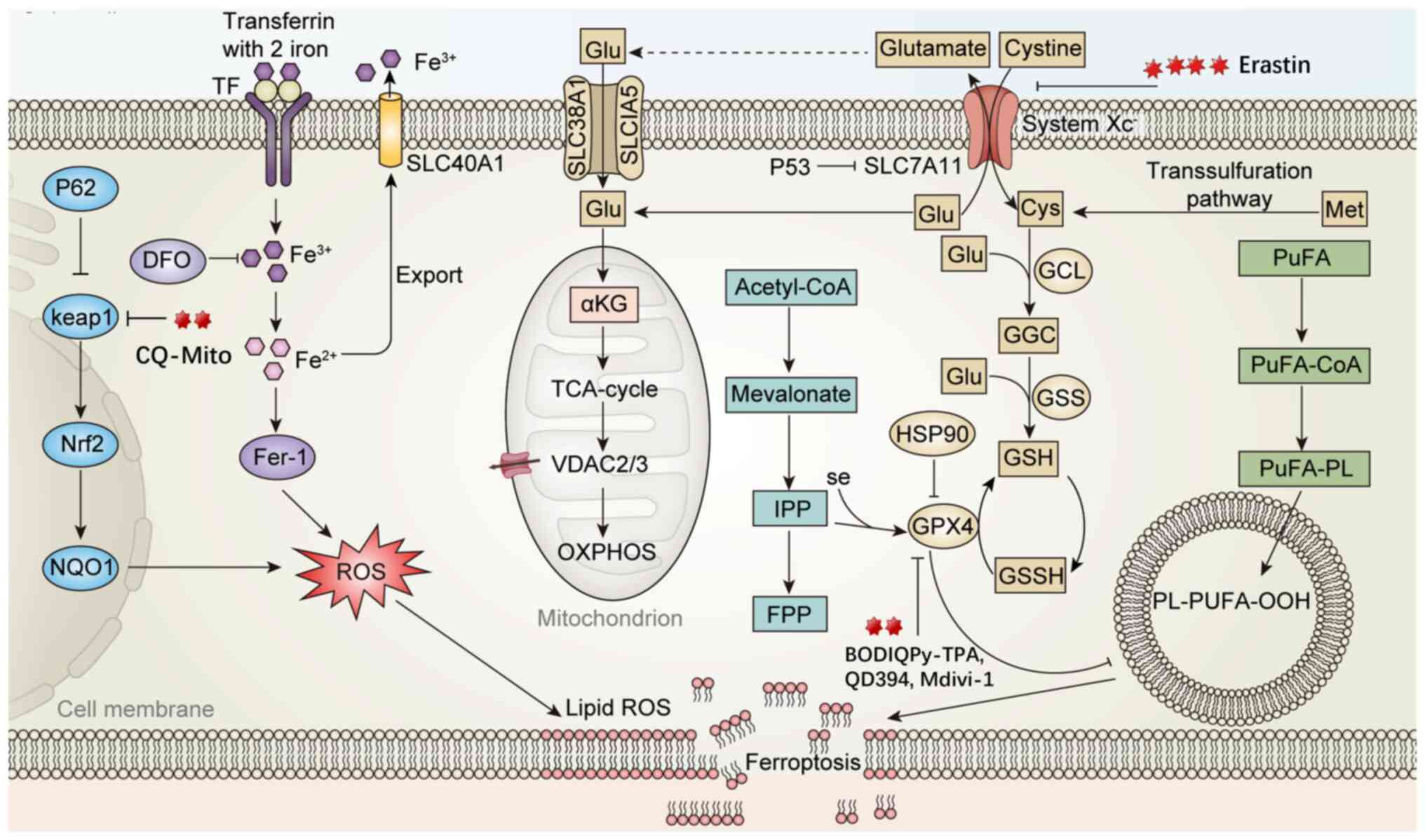
![Mechanism of action of Quinazolinone
compounds in pyroptosis, autophagy and cellular senescence
pathways. i) In the senescence pathway, telomere shortening and
limited telomerase function activate the p53/p21/p16 pathway,
causing G1 phase arrest and cellular senescence
3-(2-(hydroxymethyl) phenyl)-2-methylquinazolin-4(3H)-ones
upregulate the expression of TRF1, POT1 and p53/p21/p16, and
inhibit telomerase, inducing cells to enter a senescence
phenotype]. ii) In the pyroptosis pathway, NLRP3 assembles with ASC
and procaspase-1 to form the inflammasome, activating caspase-1,
which mediates the maturation of IL-1β and IL-18, and induces
pyroptosis through GSDMD, forming membrane pores. Quinazolinone
derivatives can inhibit this process by blocking NLRP3 inflammasome
activation and the release of inflammatory factors (Mdivi-1
inhibits NLRP3 inflammasome activation, reduces the activation of
NLRP3, ASC, Caspase-1 and the level of GSDMD-NT, and decreases the
release of IL-1β and IL-18). iii) In the autophagy pathway, ATG
family proteins mediate phagophore nucleation and extension. LC3 is
modified by ATG4 and ATG7, transforming into LC3-II, which promotes
autophagosome formation and fusion with lysosomes for substrate
degradation (DQQ and HF promote LC3-II generation, enhance
ATG5-ATG12 complex formation and promote autophagy. MJ-33 initiates
autophagy by activating ATG proteins during vesicle nucleation but
reduces the LC3/LC3-II ratio and increases p62 levels, inhibiting
autophagy). NLRP3, NOD-like receptor thermal protein domain
associated protein 3; ASC, apoptosis-associated speck-like protein
containing a CARD; GSDMD, gasdermin D; ATG, autophagy related gene;
LC3, microtubule-associated-proteinlight-chain-3; NIX, NIP3-like
protein X; TIN2, Terf1 interacting nuclear factor 2; RAP1,
Ras-proximate-1; TRF1, telomeric repeat binding factor 2; POT1,
protection of telomeres 1.](/article_images/ijmm/56/6/ijmm-56-06-05646-g03.jpg)