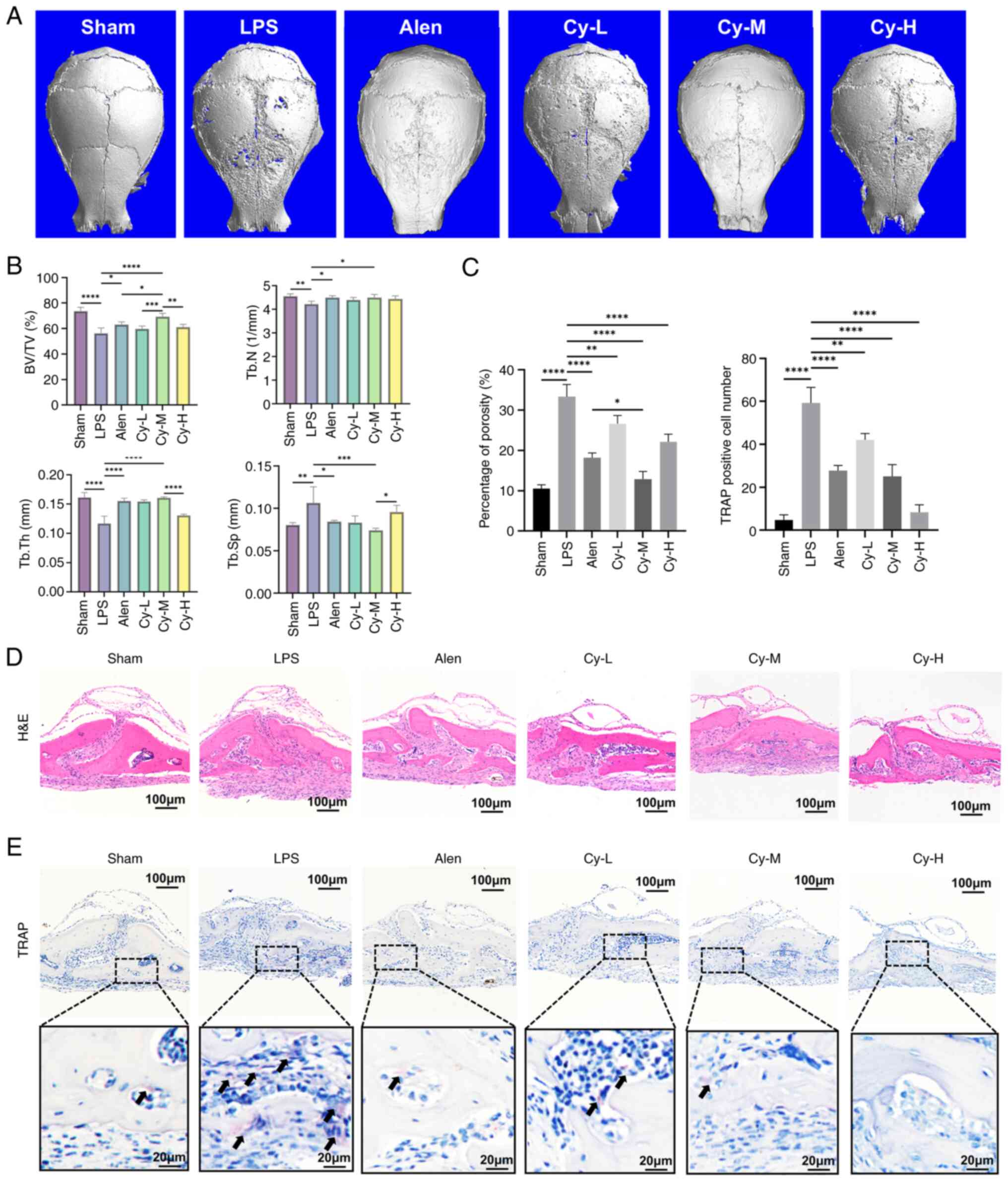
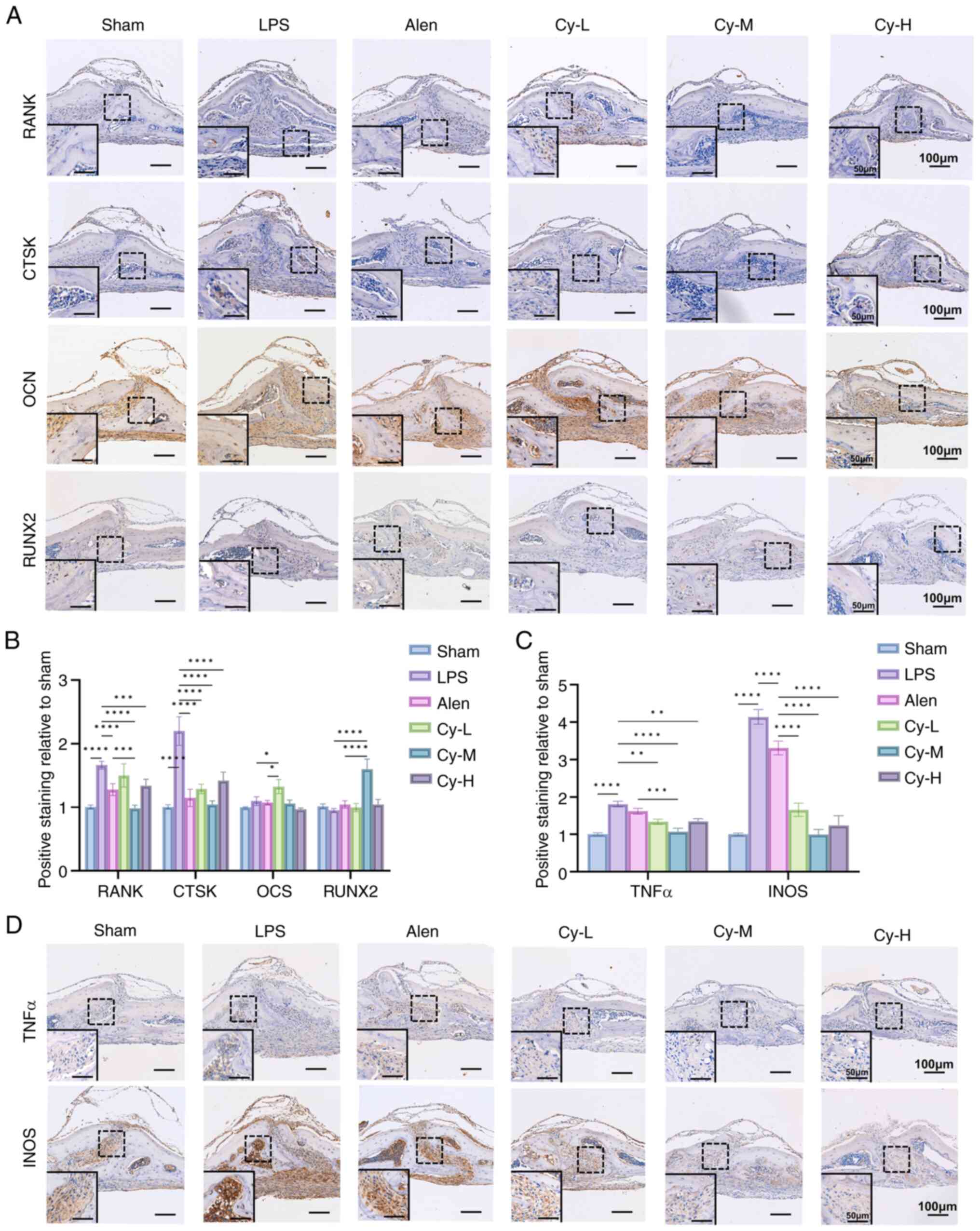
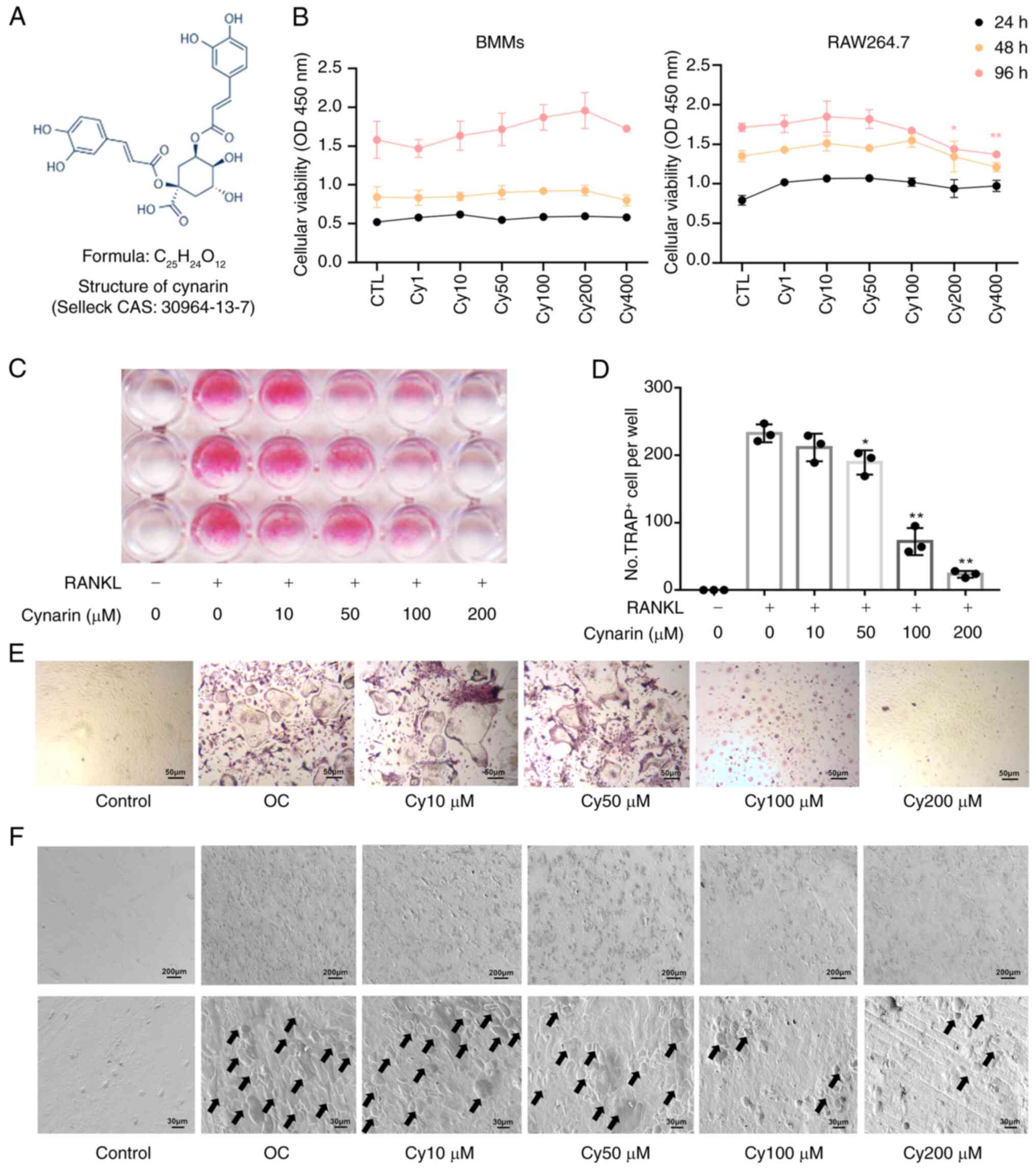
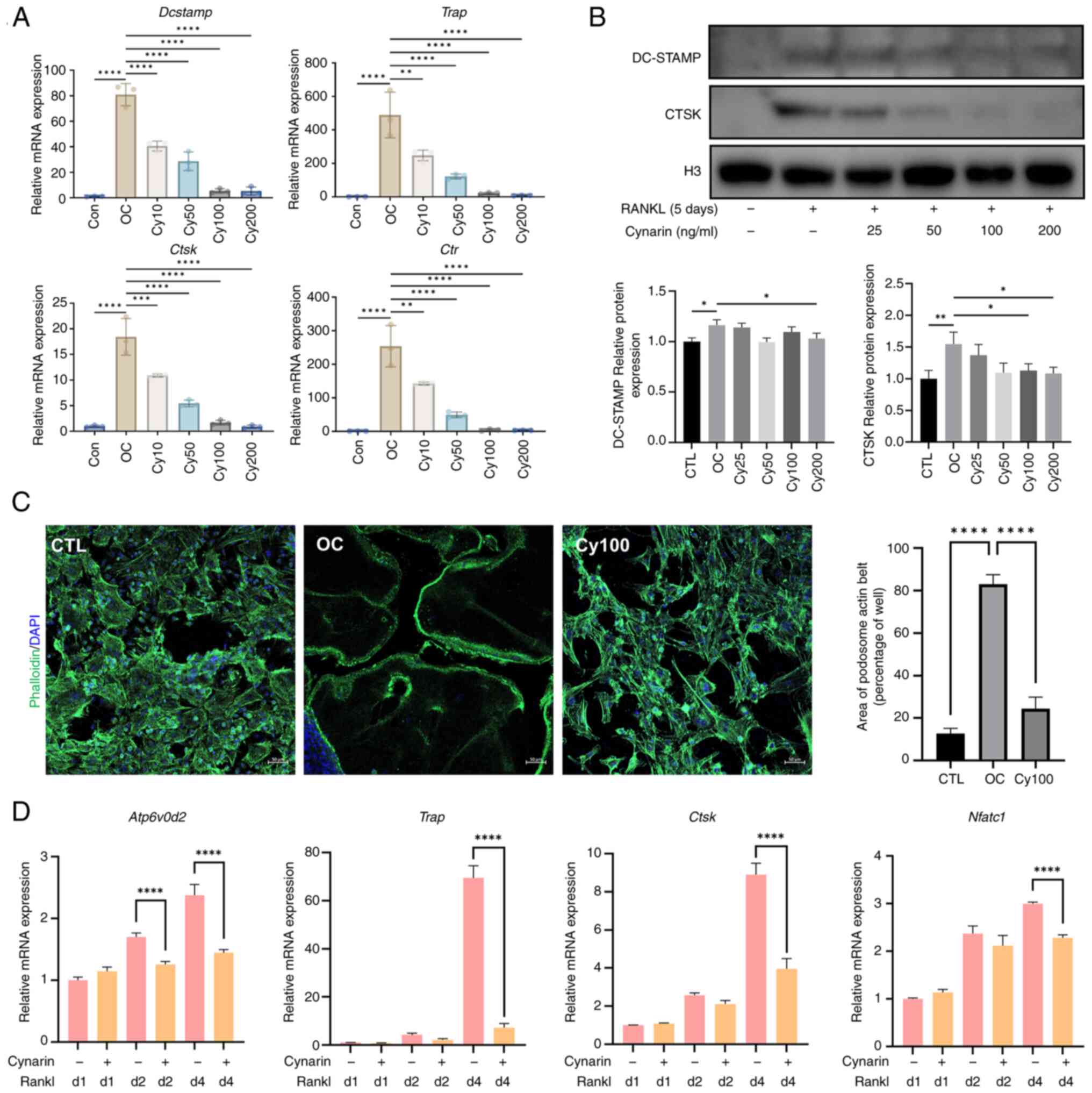
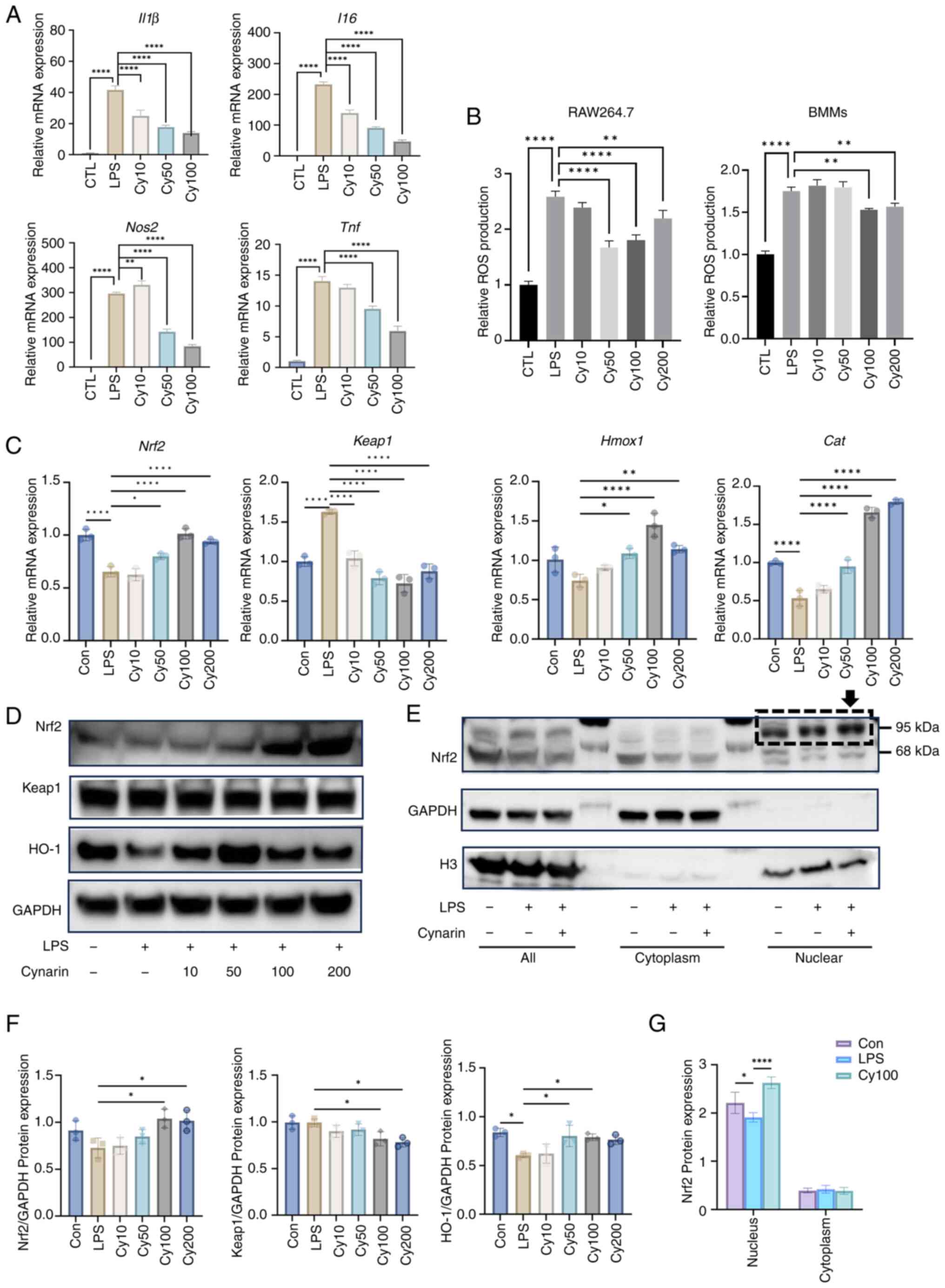
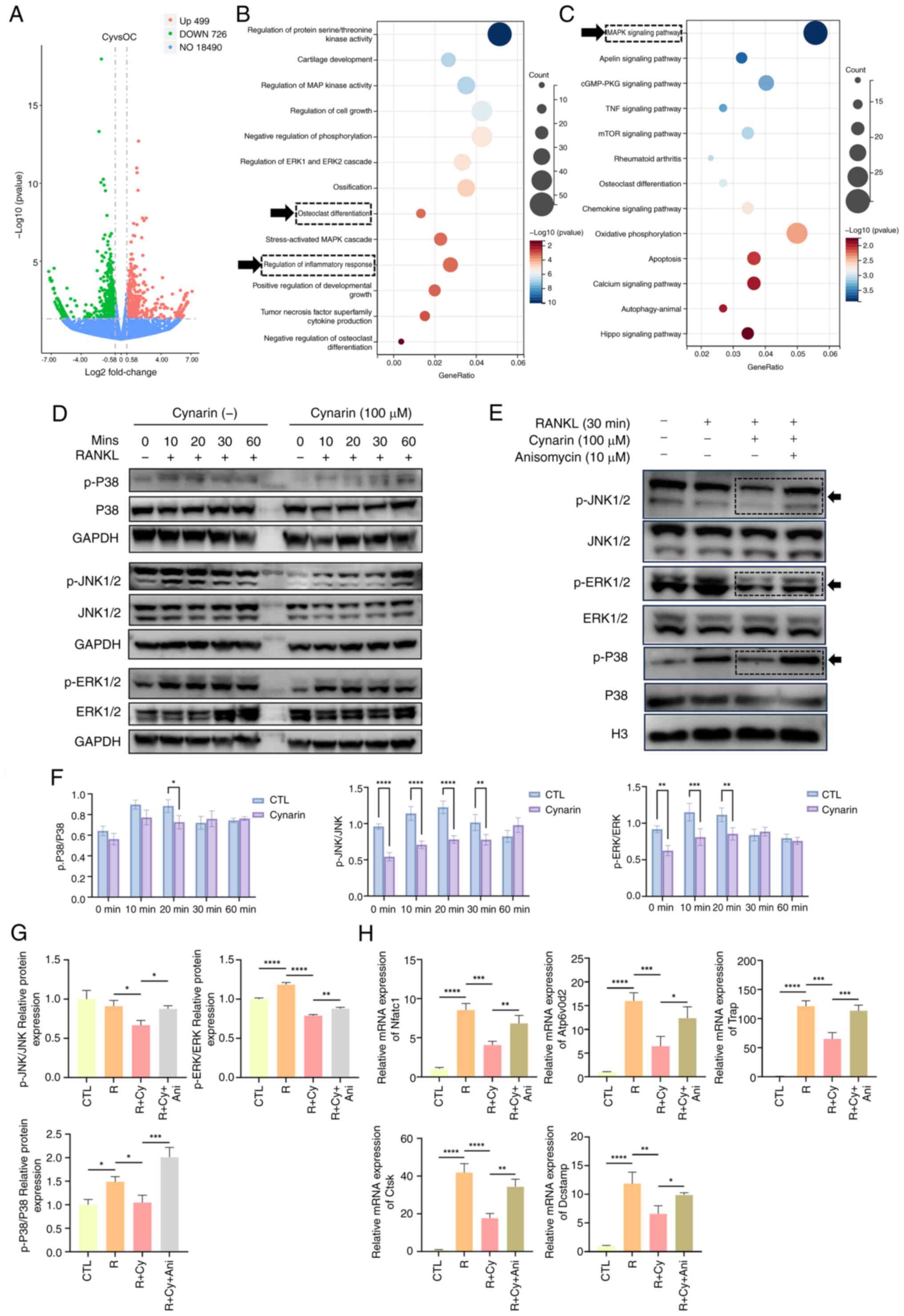
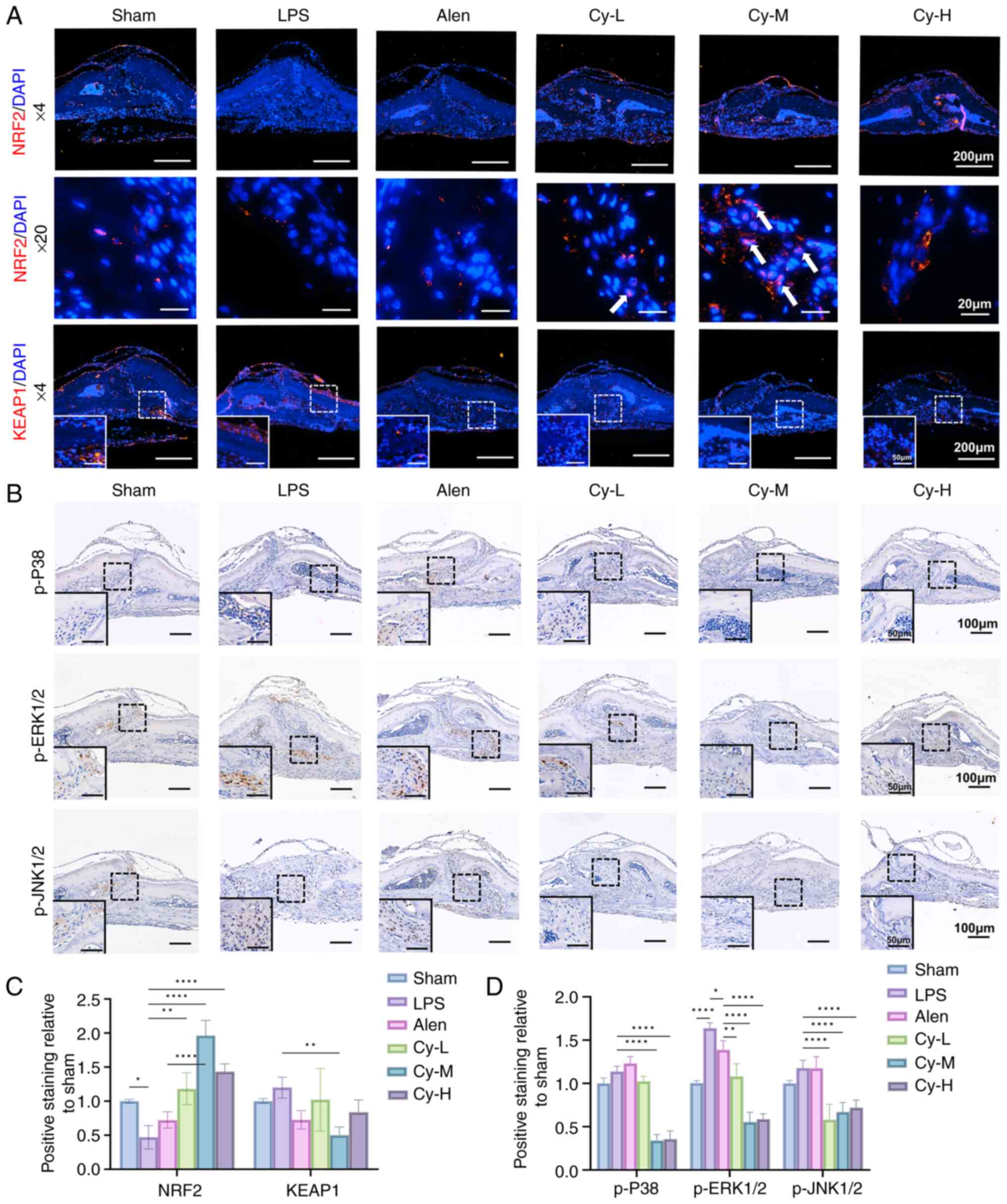
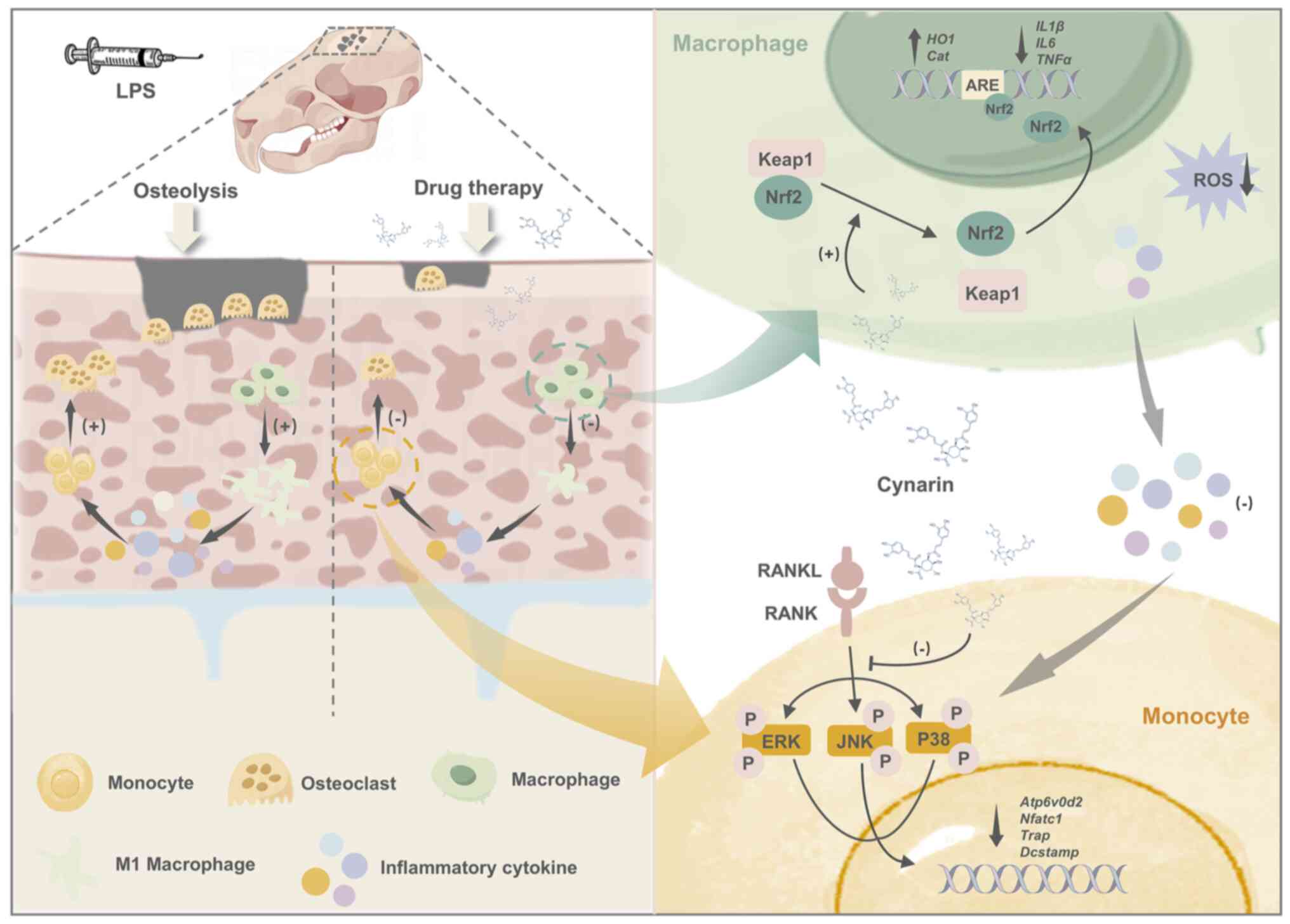
|
1
|
Nakano S, Inoue K, Xu C, Deng Z,
Syrovatkina V, Vitone G, Zhao L, Huang XY and Zhao B: G-protein
Gα13 functions as a cytoskeletal and mitochondrial
regulator to restrain osteoclast function. Sci Rep. 9:42362019.
View Article : Google Scholar
|
|
2
|
Udagawa N, Koide M, Nakamura M, Nakamichi
Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C
and Tsuda E: Osteoclast differentiation by RANKL and OPG signaling
pathways. J Bone Miner Metab. 39:19–26. 2021. View Article : Google Scholar
|
|
3
|
Park-Min KH, Lim E, Lee MJ, Park SH,
Giannopoulou E, Yarilina A, van der Meulen M, Zhao B, Smithers N,
Witherington J, et al: Inhibition of osteoclastogenesis and
inflammatory bone resorption by targeting BET proteins and
epigenetic regulation. Nat Commun. 5:54182014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyce BF, Li J, Yao Z and Xing L: Nuclear
factor-kappa B regulation of osteoclastogenesis and
osteoblastogenesis. Endocrinol Metab (Seoul). 38:504–521. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M,
Zeng C, Zhou T and Zhang J: NF-κB in biology and targeted therapy:
New insights and translational implications. Signal Transduct
Target Ther. 9:532024. View Article : Google Scholar
|
|
6
|
Liu L, Geng H, Mei C and Chen L:
Zoledronic acid enhanced the antitumor effect of cisplatin on
orthotopic osteosarcoma by ROS-PI3K/AKT signaling and attenuated
osteolysis. Oxid Med Cell Longev. 2021:66615342021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Place DE, Malireddi RKS, Kim J, Vogel P,
Yamamoto M and Kanneganti TD: Osteoclast fusion and bone loss are
restricted by interferon inducible guanylate binding proteins. Nat
Commun. 12:4962021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cauley JA, Barbour KE, Harrison SL,
Cloonan YK, Danielson ME, Ensrud KE, Fink HA, Orwoll ES and
Boudreau R: Inflammatory markers and the risk of hip and vertebral
fractures in men: The osteoporotic fractures in men (MrOS). J Bone
Miner Res. 31:2129–2138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Domazetovic V, Marcucci G, Iantomasi T,
Brandi ML and Vincenzini MT: Oxidative stress in bone remodeling:
Role of antioxidants. Clin Cases Miner Bone Metab. 14:209–216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreau MF, Guillet C, Massin P, Chevalier
S, Gascan H, Baslé MF and Chappard D: Comparative effects of five
bisphosphonates on apoptosis of macrophage cells in vitro. Biochem
Pharmacol. 73:718–723. 2007. View Article : Google Scholar
|
|
11
|
Kimachi K, Kajiya H, Nakayama S, Ikebe T
and Okabe K: Zoledronic acid inhibits RANK expression and migration
of osteoclast precursors during osteoclastogenesis. Naunyn
Schmiedebergs Arch Pharmacol. 383:297–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakaguchi O, Kokuryo S, Tsurushima H,
Tanaka J, Habu M, Uehara M, Nishihara T and Tominaga K:
Lipopolysaccharide aggravates bisphosphonate-induced osteonecrosis
in rats. Int J Oral Maxillofac Surg. 44:528–534. 2015. View Article : Google Scholar
|
|
13
|
Cohen SB, Dore RK, Lane NE, Ory PA,
Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W and Newmark
R; Denosumab Rheumatoid Arthritis Study Group: Denosumab treatment
effects on structural damage, bone mineral density, and bone
turnover in rheumatoid arthritis: A twelve-month, multicenter,
randomized, double-blind, placebo-controlled, phase II clinical
trial. Arthritis Rheum. 58:1299–1309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adzet T, Camarasa J and Laguna JC:
Hepatoprotective activity of polyphenolic compounds from Cynara
scolymus against CCl4 toxicity in isolated rat hepatocytes. J Nat
Prod. 50:612–617. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Topal M, Gocer H, Topal F, Kalin P, Köse
LP, Gülçin İ, Çakmak KC, Küçük M, Durmaz L, Gören AC and Alwasel
SH: Antioxidant, antiradical, and anticholinergic properties of
cynarin purified from the Illyrian thistle (Onopordum illyricum
L.). J Enzyme Inhib Med Chem. 31:266–275. 2016. View Article : Google Scholar
|
|
16
|
Wu C, Chen S, Liu Y, Kong B, Yan W, Jiang
T, Tian H, Liu Z, Shi Q, Wang Y, et al: Cynarin suppresses gouty
arthritis induced by monosodium urate crystals. Bioengineered.
13:11782–11793. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Tang S, Zhang C and Li Y: Cynarin
ameliorates dextran sulfate sodium-induced acute colitis in mice
through the STAT3/NF-κB pathway. Immunopharmacol Immunotoxicol.
46:107–116. 2024. View Article : Google Scholar
|
|
18
|
Rucci N, Zallone A and Teti A: Isolation
and generation of osteoclasts. Methods Mol Biol. 1914:3–19. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao Y, Xie X, Sun G, Yu S, Ma M, Chao R,
Wan T, Xu W, Chen X, Sun L and Zhang S: Multifunctional prosthesis
surface: modification of titanium with cinnamaldehyde-loaded
hierarchical titanium dioxide nanotubes. Adv Healthc Mater.
13:e23033742024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Chen X, Li C, Cao X, Jia X, Chen X, Wang
Z, Xu W, Dai F and Zhang S: Mitochondria-targeted supramolecular
coordination container encapsulated with exogenous itaconate for
synergistic therapy of joint inflammation. Theranostics.
12:3251–3272. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Chen X, Zhou Z, Mao Y, Wang Y, Ma
Z, Xu W, Qin A and Zhang S: Nirogacestat suppresses RANKL-Induced
osteoclast formation in vitro and attenuates LPS-Induced bone
resorption in vivo. Exp Cell Res. 382:1114702019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Chen X, Zhou Z, Qin A, Wang Y, Fan
B, Xu W and Zhang S: LY411575, a potent γ-secretase inhibitor,
suppresses osteoclastogenesis in vitro and LPS-induced calvarial
osteolysis in vivo. J Cell Physiol. 234:20944–20956. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwarz EM, Benz EB, Lu AP, Goater JJ,
Mollano AV, Rosier RN, Puzas JE and Okeefe RJ: Quantitative
small-animal surrogate to evaluate drug efficacy in preventing wear
debris-induced osteolysis. J Orthop Res. 18:849–855. 2000.
View Article : Google Scholar
|
|
25
|
Tsutsumi R, Hock C, Bechtold CD, Proulx
ST, Bukata SV, Ito H, Awad HA, Nakamura T, O'Keefe RJ and Schwarz
EM: Differential effects of biologic versus bisphosphonate
inhibition of wear debris-induced osteolysis assessed by
longitudinal micro-CT. J Orthop Res. 26:1340–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inchingolo AD, Inchingolo AM, Malcangi G,
Avantario P, Azzollini D, Buongiorno S, Viapiano F, Campanelli M,
Ciocia AM, De Leonardis N, et al: Effects of resveratrol, curcumin
and quercetin supplementation on bone metabolism-a systematic
review. Nutrients. 14:35192022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu L, Guo Q, Yang J and Ni B: Tumor
necrosis factor alpha promotes osteoclast formation via PI3K/Akt
pathway-mediated blimp1 expression upregulation. J Cell Biochem.
118:1308–1315. 2017. View Article : Google Scholar
|
|
28
|
Lin TH, Tamaki Y, Pajarinen J, Waters HA,
Woo DK, Yao Z and Goodman SB: Chronic inflammation in
biomaterial-induced periprosthetic osteolysis: NF-κB as a
therapeutic target. Acta Biomater. 10:1–10. 2014. View Article : Google Scholar
|
|
29
|
Sakai E and Tsukuba T: Transcriptomic
characterization reveals mitochondrial involvement in
Nrf2/Keap1-mediated osteoclastogenesis. Antioxidants (Basel).
13:15752024. View Article : Google Scholar
|
|
30
|
Dong Y, Kang H, Peng R, Liu Z, Liao F, Hu
SA, Ding W, Wang P, Yang P, Zhu M, et al: A clinical-stage Nrf2
activator suppresses osteoclast differentiation via the
iron-ornithine axis. Cell Metab. 36:1679–1695.e6. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baird L and Dinkova-Kostova AT: The
cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol.
85:241–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tao H, Ge G, Liang X, Zhang W, Sun H, Li M
and Geng D: ROS signaling cascades: Dual regulations for osteoclast
and osteoblast. Acta Biochim Biophys Sin (Shanghai). 52:1055–1062.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joo JH, Huh JE, Lee JH, Park DR, Lee Y,
Lee SG, Choi S, Lee HJ, Song SW, Jeong Y, et al: A novel pyrazole
derivative protects from ovariectomy-induced osteoporosis through
the inhibition of NADPH oxidase. Sci Rep. 6:223892016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Chen Y, Mao Y, Dai P, Sun X, Zhang
X, Cheng H, Wang Y, Banda I, Wu G, et al: Curcumin protects
osteoblasts from oxidative stress-induced dysfunction via
GSK3β-Nrf2 signaling pathway. Front Bioeng Biotechnol. 8:6252020.
View Article : Google Scholar
|
|
35
|
Saha S, Buttari B, Panieri E, Profumo E
and Saso L: An overview of Nrf2 signaling pathway and its role in
inflammation. Molecules. 25:54742020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu H, Liu T, Jia Y, Li J, Jiang L, Hu C,
Wang X and Sheng J: (-)-Epigallocatechin-3-gallate inhibits
osteoclastogenesis by blocking RANKL-RANK interaction and
suppressing NF-κB and MAPK signaling pathways. Int Immunopharmacol.
95:1074642021. View Article : Google Scholar
|
|
37
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morrison DK: MAP kinase pathways. Cold
Spring Harb Perspect Biol. 4:a0112542012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Y, Li J, Zhang Z, Li Q, Tian Y, Wang
S, Shi C and Sun H: Echinococcus granulosus promotes MAPK
pathway-mediated osteoclast differentiation by inhibiting Nrf2 in
osseous echinococcosis. Vet Res. 56:812025. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thummuri D, Naidu VGM and Chaudhari P:
Carnosic acid attenuates RANKL-induced oxidative stress and
osteoclastogenesis via induction of Nrf2 and suppression of NF-κB
and MAPK signalling. J Mol Med (Berl). 95:1065–1076. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Antonino M, Nicolò M, Jerome Renee L,
Federico M, Chiara V, Stefano S, Maria S, Salvatore C, Antonio B,
Calvo-Henriquez C, et al: Single-nucleotide polymorphism in chronic
rhinosinusitis: A systematic review. Clin Otolaryngol. 47:14–23.
2022. View Article : Google Scholar
|