Programmed cell death (PCD) is a tightly regulated
biological process influenced by various external stimuli and
intracellular conditions. PCD encompasses several distinct forms of
cell death, including apoptosis, pyroptosis, necroptosis,
ferroptosis and autophagy, each mediated through specific genetic
and molecular mechanisms (1).
These forms of cell death are essential for embryonic development,
the maintenance of tissue homeostasis and the clearance of
intracellular pathogens, and are regarded as fundamental processes
for preserving physiological stability (1,2).
PCD pathways can be categorized into lytic and non-lytic forms of
cell death. Lytic cell death, such as pyroptosis and necroptosis,
involves membrane potential loss and cellular swelling, resulted in
disrupted cell integrity and ultimately leading to cell rupture,
releasing a large amount of inflammatory mediators (2). Comparatively, non-lytic forms of
PCD, such as apoptosis, involve the coordinated breakdown of dying
cells into smaller fragments for the isolation of cellular
contents, which limits the release of cytokines and
damage-associated molecular patterns (DAMPs), typically in a silent
manner without triggering a pronounced immune response (1).
PANoptosis is a recently identified form of PCD that
is initiated by innate immune signaling, and integrates key
features of pyroptosis, apoptosis and necroptosis. Notably, its
biological effects cannot be fully explained by any of these
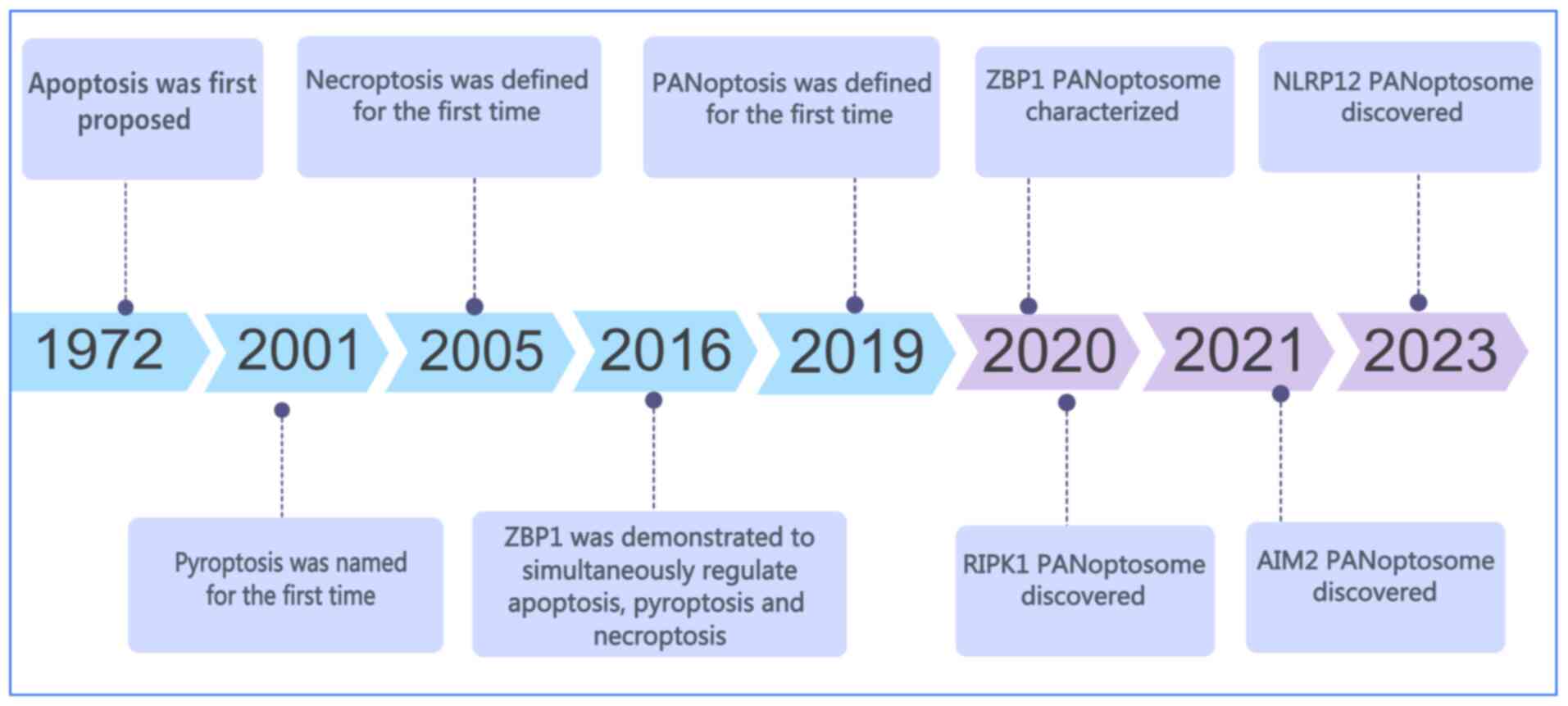
pathways alone. The concept of PANoptosis was first introduced by
Malireddi et al in 2019 (3) (Fig.
1). Mechanistically, PANoptosis is mediated through the
assembly of a multiprotein complex known as the PANoptosome, which
incorporates essential components from the pyroptotic, apoptotic
and necroptotic pathways. This complex enables cross-talk and
coordinated activation among the three pathways, resulting in a
unified mode of cell death (4,5).
Due to the regulatory role of the PANoptosome, inhibition of a
single form of cell death is insufficient to prevent PANoptosis.
Instead, effective blockade requires targeting components within
the PANoptosome complex.
Emerging evidence has revealed that PANoptosis
contributes to the pathogenesis of several kidney diseases,
including both acute and chronic forms (6-12). Although the role of PANoptosis
has been extensively studied in cancer, infections and inflammatory
conditions, its function in renal pathology remains insufficiently
characterized. The present review summarizes the molecular
mechanisms underlying PANoptosis activation and explores its
potential involvement in acute kidney injury (AKI), chronic kidney
disease (CKD), diabetic kidney disease (DKD), renal cell carcinoma
(RCC) and drug-induced kidney injury, aiming to provide a
theoretical foundation for PANoptosis-targeted therapeutic
strategies in kidney disease.
A comprehensive search for studies published up to
May 2025 was performed on platforms including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar
(https://scholar.google.com/), Web of
Science (https://webofscience.com/WOS),
ScienceDirect (https://www.sciencedirect.com/) and CNKI (https://www.cnki.net/). The search adopted a
combination of subject terms and free terms, and was appropriately
adjusted according to the rules of the different databases. The key
terms included 'Kidney disease', 'Kidney failure', 'Renal
insufficiency', 'Renal failure', 'Nephropathy', 'Renal disease' and
'PANoptosome' or 'PANoptosis'. Notably, low-quality articles and
papers with incomplete information were excluded. The main
inclusion criteria for the literature were original research or
comprehensive reports. The research content clearly involved the
mechanism or therapeutic significance of PANoptosis in kidney
diseases, and the research subjects included humans and mammals.
The screening was independently conducted by two researchers, and
any disputes arising during the process were resolved through
mutual discussion or arbitration by a third-party senior
researcher.
Apoptosis, pyroptosis and necroptosis represent the
three primary forms of PCD, each characterized by distinct
initiators, signaling mediators and execution mechanisms. Among
them, apoptosis is the most extensively studied and was first
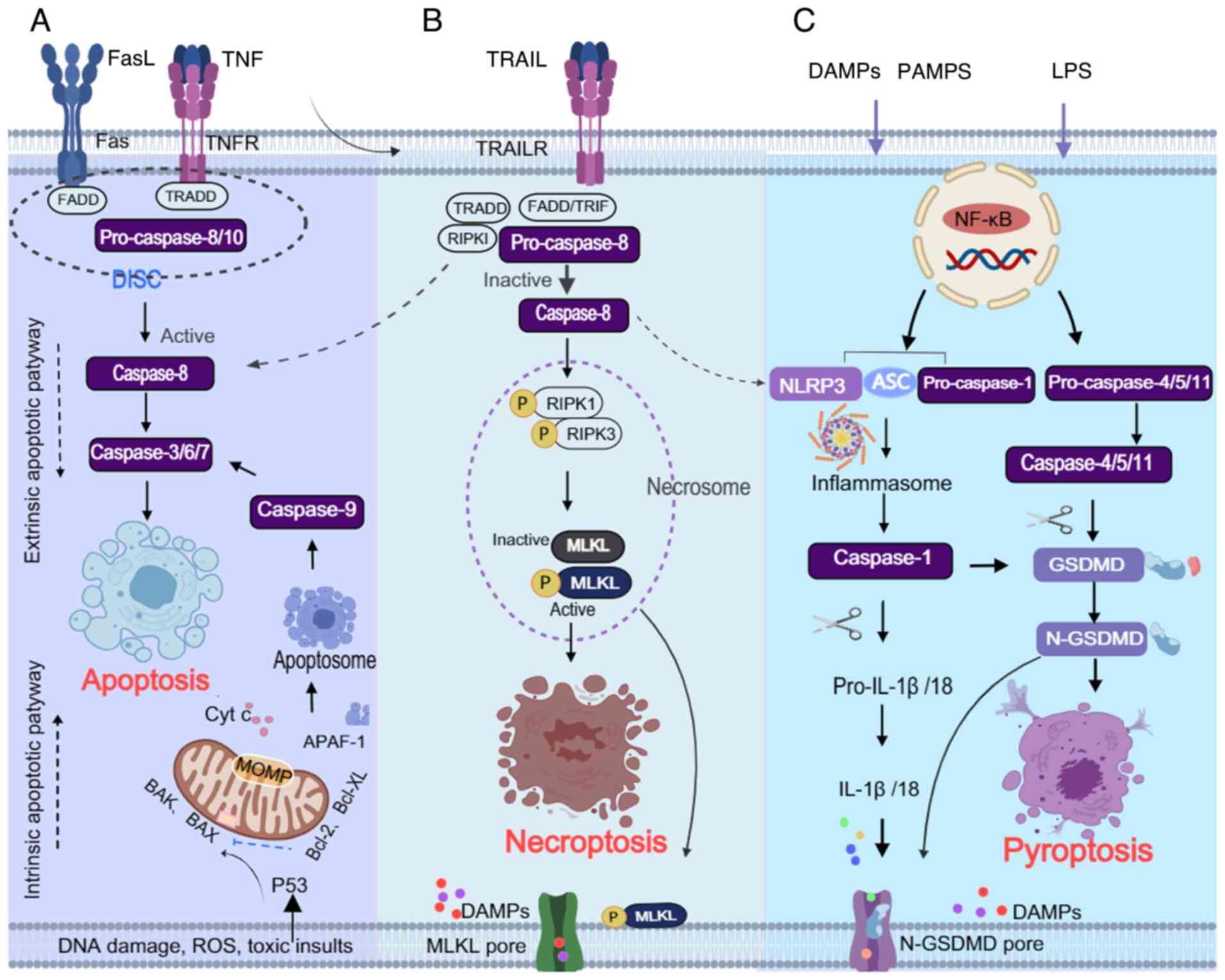
described by Kerr et al in 1972 (13) (Fig. 1). Apoptosis depends on the
involvement of caspases. Morphologically, apoptosis is defined by
characteristic features including cell shrinkage, cytoplasmic
condensation, membrane blebbing and nuclear chromatin condensation
(13,14). Apoptosis occurs through two main
pathways: The intrinsic (mitochondrial) pathway and the extrinsic
(death receptor-mediated) pathway (Fig. 2A). The intrinsic pathway is
activated in response to intracellular stressors such as DNA
damage, oxidative stress or toxic insults. In this context, key
stress-sensing proteins, such as the tumor suppressor protein 53
(P53), serve a central regulatory role by transcriptionally
activating pro-apoptotic members of the Bcl-2 family, such as BAK
and BAX, while concurrently suppressing anti-apoptotic proteins,
such as Bcl-2 and Bcl-XL (15-17). Activated BAK and BAX oligomerize
on the mitochondrial outer membrane, resulting in mitochondrial
outer membrane permeabilization and the release of cytochrome
c (Cyt c) into the cytosol (18). Cyt c subsequently binds to
apoptotic protease-activating factor 1, promoting the formation of
the apoptosome complex, which leads to the activation of caspase-9
(19,20). Caspase-9 then cleaves and
activates downstream effector caspases, including caspase-3 and
caspase-7, ultimately executing the apoptotic process (21). The extrinsic pathway is initiated
by the binding of death ligands to their corresponding receptors.
Fas and tumor necrosis factor receptor (TNFR) recruit adaptor
proteins, such as TNFR-associated death domain protein (TRADD) and
Fas-associated death domain protein (FADD), forming the
death-inducing signaling complex, which activates caspase-8 and
caspase-10 (22-24). Active caspase-8/10 cleaves and
activates the effector caspases, including caspase-3, caspase-7 and
caspase-6, leading to apoptosis (25,26). Caspase-8 also cleaves BID, which
activates the intrinsic pathway and further promotes apoptosis
(27).
Pyroptosis is an inflammation-associated form of PCD
that occurs through the classical or non-classical pathways. While
this process shares some morphological features with apoptosis,
such as nuclear condensation and DNA fragmentation, its hallmark
characteristics include pore formation in the plasma membrane, cell
swelling, membrane rupture and the release of pro-inflammatory
intracellular contents; these events trigger a robust inflammatory
response (49-51). Upon exposure to toxic stimuli,
intracellular and extracellular danger signals induce the formation
of inflammasome complexes in the cytoplasm through the classical
caspase-1-dependent pathway or the non-classical
caspase-4/5/11-dependent pathway; these inflammasome complexes are
composed of Nod-like receptor family pyrin domain containing
(NLRP)3/NLRP1, apoptosis-related speck-like protein (ASC) and
pro-caspase-1 (Fig. 2C).
Inflammasomes promote the production of caspase-1, and the
maturation and secretion of IL-1β and IL-18, and induce cleavage of
the pyroptosis executioner protein gasdermin D (GSDMD). The
N-terminal fragment of GSDMD forms pores in the membrane,
initiating pyroptosis (51-54).
For a long time, different cell death modalities
were examined in isolation due to their regulation by distinct
molecular mechanisms. However, the identification of PANoptosis has
revealed that these pathways are interconnected at multiple levels
and can be activated simultaneously or sequentially within the same
cell. Rather than acting independently, these forms of cell death
can switch from one to another under specific conditions,
highlighting their close association and mutual regulation
(3,4).
Among the key mediators linking these pathways,
caspases have a central role. Caspase-8, classically recognized as
the initiator enzyme of the extrinsic apoptotic pathway, promotes
the cleavage of BID to tBID, which subsequently activates BAX and
BAK, and initiates apoptotic signaling. However, increasing
evidence (22,55-60) has indicated that caspase-8 also
participates in necroptosis and pyroptosis (Fig. 2B and C); it can trigger
necroptosis mediated by RIPK3 and MLKL, and also contributes to
pyroptotic regulation (55-58). Caspase-8 forms an oligomeric
complex with RIPK1 and FADD, which facilitates the activation of
downstream effector caspases-3/7 and promotes apoptosis (59,60). Additionally, caspase-8 can
directly cleave RIPK1 and RIPK3, thereby suppressing necroptosis.
When caspase-8 activity is inhibited, this suppression is lifted,
allowing RIPK3 activation and promoting necroptotic cell death
(61,62). RIPK1 also contributes to
FADD/caspase-8-dependent apoptosis. Upon TNFR1 engagement, the
TNFR1 signaling complex recruits TRADD, which subsequently recruits
FADD. FADD then binds pro-caspase-8, facilitates its dimerization
and activation, and initiates apoptosis (63). Dillon et al (64) demonstrated through both in
vitro and in vivo studies that RIPK1 can regulate
FADD/caspase-8-mediated apoptosis as well as RIPK3/MLKL-induced
necroptosis, thereby preventing perinatal mortality in mice
(Fig. 2A and B). Caspase-8 also
has a regulatory role in pyroptosis by facilitating activation of
the NLRP3 inflammasome, promoting ASC complex formation and
caspase-1 activation, which in turn induces IL-1β secretion and
pyroptosis (55,62). In the context of Yersinia
infection, inhibition or deletion of transforming growth
factor-β-activated kinase 1 (TAK1) has been shown to enhance
caspase-8-mediated GSDMD cleavage, thereby favoring pyroptosis
while reducing apoptosis (65)
(Fig. 2A and C). Furthermore,
caspase-8 can act on other GSDM family members, such as GSDMC,
which is specifically cleaved under TNFα stimulation to generate
the N-terminal domain of GSDMC, leading to pore formation in the
plasma membrane and the induction of pyroptosis (66,67). During influenza virus infection,
caspase-8 activates caspase-3, which cleaves GSDMD into an inactive
form, thereby reducing IL-1β and IL-18 release, and suppressing
pyroptosis (68). These findings
collectively indicate that caspase-8 is a pivotal regulator linking
apoptosis, necroptosis and pyroptosis.
Caspase-3, a key executioner of apoptosis, also
contributes to pyroptosis. Upon activation, it cleaves GSDME,
generating the GSDME-N fragment that induces pyroptotic membrane
disruption (69,70). Similarly, caspase-1, the terminal
effector of pyroptosis, has been shown to mediate apoptosis in the
absence of GSDMD by activating the BID/caspase-9/caspase-3 axis
(71,72).
In addition to caspases, multiple signaling
complexes and inflammasomes contribute to the cross-talk among
these cell death pathways. NLRP3, the most extensively studied
inflammasome, serves a critical role in pyroptosis by promoting
inflammatory responses. Potassium efflux, a known activator of
NLRP3, can result from MLKL-mediated membrane disruption during
necroptosis, which subsequently leads to caspase-1 activation and
IL-1β maturation (73-75) (Fig. 2B and C). Moreover, upregulation
of RIPK3 has been shown to trigger the RIPK3/MLKL/NLRP3/caspase-1
signaling cascade, further supporting the interconnected nature of
these pathways (76).
Collectively, these findings indicate that
apoptosis, pyroptosis and necroptosis constitute an interconnected
network rather than three independent cell death pathways, with key
regulators, such as caspase-8, exerting multiple functions,
including promoting apoptosis through activation of caspase-3/7,
suppressing necroptosis by cleaving RIPK1 and RIPK3, and
participating in NLRP3 inflammasome-dependent pyroptosis.
Similarly, RIPK1 is involved in apoptosis and pyroptosis. These
pathways are not isolated but rather interdependent, with key
molecules functioning as receptors for initiating and recognizing
death signals, as adapters for recruiting downstream mediators, and
as effectors that execute the final cell death program.
The concept of PANoptosis emerged from studies
investigating the molecular overlap among pyroptosis, apoptosis and
necroptosis. Increasing evidence has suggested that these three
forms of PCD can occur simultaneously and are interconnected
through a complex molecular network, rather than acting as isolated
pathways (77-80), leading to the recognition of
PANoptosis as a distinct, coordinated form of cell death.
PANoptosis is a newly characterized PCD pathway regulated by innate
immune responses and mediated through the assembly of a
multiprotein complex known as the PANoptosome. It is initiated by
innate immune sensors and executed via caspases and RIPKs. The
PANoptosome integrates components from the pyroptotic, apoptotic
and necroptotic machinery, enabling their coordinated activation.
Regulation of the PANoptosome allows simultaneous control of all
three death pathways, while also providing a molecular scaffold
that facilitates interaction among key signaling molecules, thereby
driving PANoptosis (81,82).
Activation of PANoptosis involves upstream receptors
and signaling cascades that lead to the formation of the
PANoptosome. These complexes serve as platforms for cross-talk
among the three PCD pathways and are essential for their
integration (83). The
PANoptosome typically comprises three categories of components
(5,8,11,80,83): i) Pattern recognition receptors
or sensors that detect pathogen-associated molecular patterns
(PAMPs) and DAMPs, including Z-DNA binding protein 1 (ZBP1),
NLRP12, AIM2, RIPK1, pyrin and NLRP3; ii) adaptor proteins such as
ASC and FADD; and iii) catalytic effectors, including RIPK1, RIPK3,
caspase-1 and caspase-8. The composition of the PANoptosome is
context-dependent and varies according to the nature of the
stimulus or pathological condition. In general, the sensors
recognize PAMPs or DAMP,s and initiate PANoptosome assembly through
homotypic or heterotypic interactions among adaptor domains. This
assembly provides a structural platform for the convergence of
apoptotic, pyroptotic and necroptotic effectors. The resulting
complex enables the coordinated activation of caspases and RIPKs,
thereby inducing pyroptosis, apoptosis and necroptosis in a
synchronized manner (80,83).
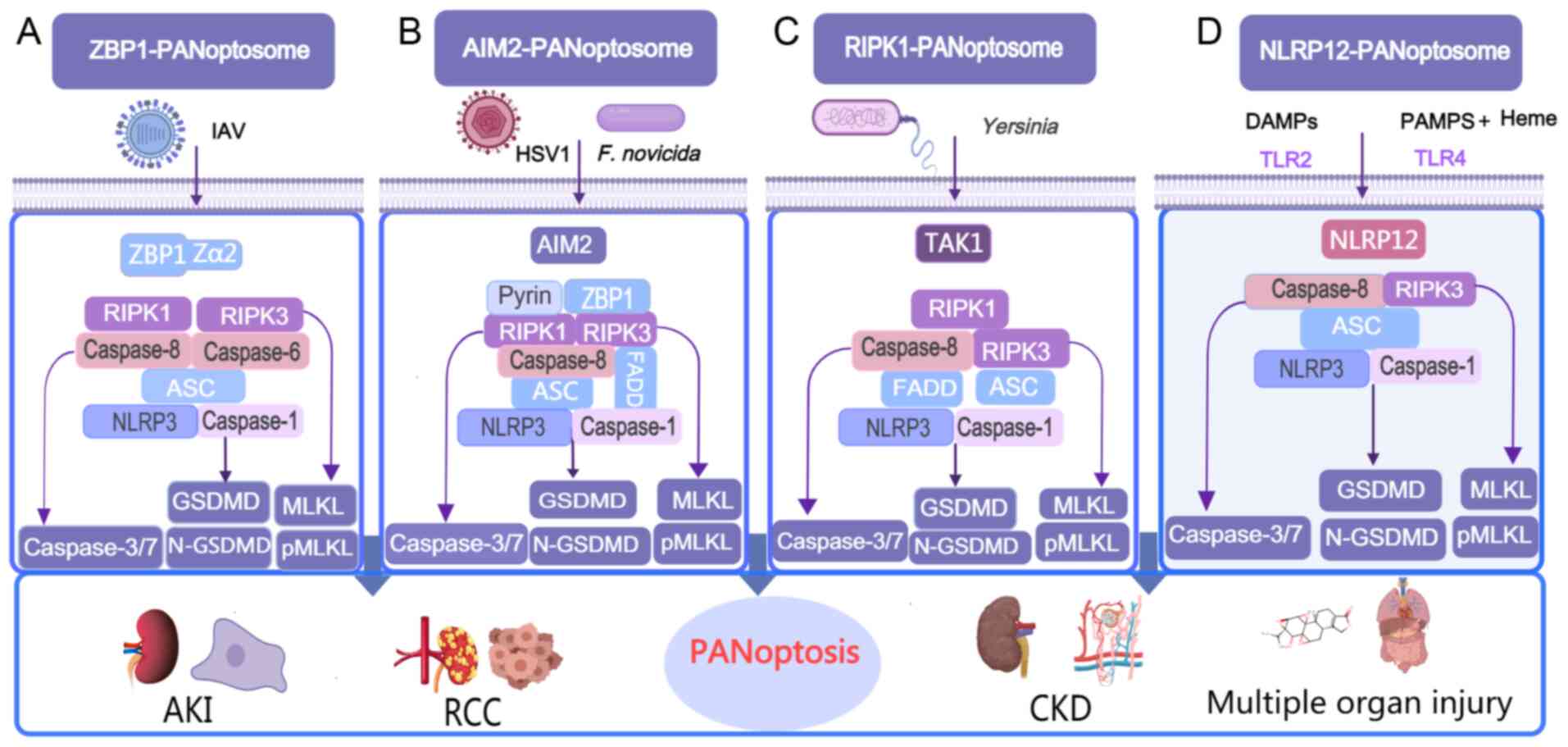
AIM2 is an inflammasome sensor belonging to the
PYHIN protein family that recognizes cytoplasmic double-stranded
DNA (dsDNA). Structurally, AIM2 contains an N-terminal pyrin domain
and a C-terminal HIN200 domain, and is primarily localized in the
cytoplasm (96-98). Upon detection of pathogen-derived
dsDNA, AIM2 initiates the formation of the AIM2-PANoptosome
complex. This assembly activates the inflammasome pathway, leading
to the secretion of IL-1β and IL-18, and inducing pyroptosis
(99,100). In bone marrow-derived
macrophage models infected with herpes simplex virus 1 and
Francisella novicida, AIM2 has been shown to regulate other
innate immune sensors, including pyrin and ZBP1. Together with ASC,
caspase-1, caspase-8, RIPK3, RIPK1, NLRP3 and FADD, these
components assemble to form the AIM2-PANoptosome, driving
PANoptosis (82) (Fig. 3B). During infection, AIM2
deficiency markedly reduces inflammation and cell death, whereas
the absence of pyrin or ZBP1 produces a partial reduction in these
responses. These observations suggest that AIM2 functions as an
upstream regulator controlling the assembly and activation of the
AIM2-PANoptosome (82).
RIPK1 is another RHIM-containing protein that serves
a central role in inflammation, cell death and the regulation of
PANoptosis. It can activate necroptosis by promoting the
phosphorylation of RIPK3, which interacts with MLKL to induce cell
death through the RIPK3/MLKL axis (40,89,101). Dillon et al (64) reported that RIPK1 also
contributes to FADD/caspase-8-mediated apoptosis and
RIPK3-dependent necroptosis. TAK1 serves as a key upstream
regulator of RIPK1, controlling the formation and activation of
PANoptosomes (3,102,103). Under normal physiological
conditions, TAK1 inhibits TNF-mediated autocrine signaling by
suppressing TNF production and RIPK1 phosphorylation, thereby
preventing PANoptosis activation (28,79). In Yersinia-infected cells,
TAK1 inhibition or knockout promotes the assembly of the
RIPK1-PANoptosome, which includes RIPK1, caspase-8, caspase-1,
FADD, NLRP3, ASC and RIPK3 (Fig.
3C). This complex activates FADD-Caspase-8-mediated apoptosis,
RIPK3-MLKL-dependent necroptosis, and NLRP3 inflammasome-mediated
pyroptosis (79,87). Moreover, in the absence of TAK1,
microbial signals can activate PANoptosis through the RIPK3/MLKL
axis independent of RIPK1 kinase activity (3). Using models specifically lacking
TAK1, studies have demonstrated that inhibition of RIPK1 kinase
activity in TAK1-deficient bone marrow-derived macrophages can
suppress PANoptosis and inflammation, infection and myeloid
proliferation (77,79,104,105).
NLRP12 is a member of the NLR family and contains an
N-terminal pyrin domain, a central nucleotide-binding domain and a
C-terminal leucine-rich repeat region. It functions as an innate
immune sensor that modulates inflammatory signaling in response to
infection and cellular stress (106). It has been demonstrated that
heme, in conjunction with PAMPs, activates the Toll-like receptor
2/4 signaling axis, which induces the expression of NLRP12
(88). This induction promotes
the assembly of a PANoptosome complex composed of NLRP12, NLRP3,
ASC, caspase-1, caspase-8 and RIPK3, collectively referred to as
the NLRP12-PANoptosome (Fig.
3D). Activation of this complex leads to PANoptosis,
characterized by caspase-1, caspase-8 and caspase-3 activation,
MLKL phosphorylation, and cleavage of GSDMD or GSDME. A functional
study further revealed that NLRP12 deficiency confers protection
against heme-induced AKI and mortality in mice, underscoring its
critical role in disease pathogenesis (88). This finding suggests that NLRP12
and associated PANoptotic components may represent promising
therapeutic targets for hemolytic and inflammation-related
disorders (88).
AKI is a clinical syndrome characterized by a sudden
decline in renal function, and is associated with a high incidence
and poor clinical outcomes (107,108). The incidence of AKI affects
~20% of hospitalized patients and exceeds 50% among those in
intensive care units, making it a major contributor to in-hospital
mortality (109). Emerging
evidence (8,110-112) has indicated that PANoptosis
serves an important role in various AKI models, including those
induced by sepsis, ischemia/reperfusion (I/R) injury and exposure
to nephrotoxic agents, such as chemicals and certain traditional
Chinese medicines.
Cell death and inflammation are central events in
the pathogenesis of AKI, with I/R injury being a primary cause.
Previous studies have demonstrated that apoptosis, pyroptosis and
necroptosis coexist in models of middle cerebral artery occlusion,
oxygen-glucose deprivation/recovery and retinal I/R injury
(113,114). Another study, by collecting
data from animal and cell experiments, reported that PANoptosis is
observed in ischemic brain injury (115). These findings suggest that
PANoptosis may represent a promising therapeutic target for
diseases associated with I/R injury. In I/R-induced AKI, renal
tubular epithelial cells experience endoplasmic reticulum stress,
oxidative stress and inflammation due to hypoxia, followed by
reperfusion, leading to acute tubular cell loss. Numerous animal
studies and analyses of kidney biopsies from transplant recipients
have demonstrated that I/R induces apoptosis in tubular epithelial
cells (116-118). Although apoptosis was initially
regarded as the principal mechanism of AKI, further studies have
shown that multiple cell death pathways are involved, and
inhibiting apoptosis alone is insufficient to prevent renal injury
(119). In I/R-induced AKI
models, NLRP3 activates caspase-1, leading to GSDMD cleavage and
the release of the pro-inflammatory cytokines IL-1β and IL-18,
thereby inducing pyroptosis in renal tubular epithelial cells
(120-122). Pyroptosis has also been
observed in AKI models caused by cisplatin and sepsis (123). Linkermann et al
(124,125) reported increased expression of
RIPK1 and RIPK3 in I/R-induced AKI, and demonstrated that treatment
with the necroptosis inhibitor necrostatin-1 (Nec-1) markedly
improved renal function. Similarly, the RIPK1 inhibitor Compound-71
has been shown to alleviate cisplatin-induced AKI by suppressing
necroptosis (126). In calcium
oxalate-induced AKI, ZBP1 activation of the RIPK3/MLKL axis
mediates necroptosis, whereas ZBP1 knockout can reduce necroptotic
activity and tubular injury (127). These findings underscore the
importance of simultaneously targeting apoptosis, pyroptosis and
necroptosis to protect against AKI-associated kidney damage.
While previous studies have often focused on
individual PCD pathways, the discovery of PANoptosis has shifted
attention to the integrated role of multiple death mechanisms in
AKI. In a mouse model of sepsis-induced AKI established by cecal
ligation and puncture, increased AIM2 expression in kidney tissue
has been shown to be associated with concurrent activation of
apoptosis, pyroptosis, necroptosis and inflammasome signaling,
supporting the presence of PANoptosis (110). In vitro, PANoptosis has
been observed in lipopolysaccharide (LPS)-treated human proximal
tubular epithelial HK-2 cells, and AIM2 silencing may reverse this
effect, suggesting that AIM2 mediates sepsis-induced renal injury
through regulation of PANoptosis (110). NLRP3, a key component of the
four types of PANoptosome, has also been shown to be involved in
I/R-induced AKI. The clinical agent 3,4-methylenedioxy-β-nit
rostyrene has been report to reduce apoptosis, necroptosis and
pyroptosis in a renal I/R injury model by targeting NLRP3, thereby
alleviating tissue damage (8).
Furthermore, an aqueous extract of Achyranthes aspera can
ameliorate cisplatin-induced AKI by inhibiting DNA damage,
oxidative stress, inflammation and PANoptosis (111). In sepsis-induced AKI mouse
models, elevated levels of PANoptosis-related molecules, including
RIPK1, MLKL, caspase-3/7 and FADD, have been observed in renal
tissue, along with marked upregulation of ZBP1, particularly in the
renal interstitium, highlighting the essential role of PANoptosis
in the pathogenesis of sepsis-induced AKI (112) (Table I).
PANoptosis has been increasingly linked to
tumorigenesis and cancer progression (12). The boundaries between different
forms of PCD have become progressively less distinct, and
therapeutic strategies targeting a single form of cell death often
show limited efficacy (105).
The discovery of PANoptosis not only broadens the understanding of
cell death pathways but also offers novel approaches for cancer
therapy. RCC, characterized by the proliferation of malignant
epithelial cells, relies on strategies that effectively inhibit or
eliminate these cells. Inducing PANoptosis, which simultaneously
activates apoptosis, pyroptosis and necroptosis, holds potential
therapeutic value for enhancing cancer cell clearance, and
suppressing tumor growth and metastasis. PANoptosis has been
investigated in several types of cancer, including colorectal
cancer, breast cancer and low-grade glioma (12,128,129).
Several studies have explored the association
between PANoptosis and cancer prognosis using data from The Cancer
Genome Atlas. One study reported that high expression of
PANoptosis-related genes, such as AIM2, caspase-3, caspase-4 and
TNFRSF10, was significantly associated with poor prognosis in KIRC
(132). Conversely, in skin
cutaneous melanoma, higher expression of genes including ZBP1,
NLRP1, caspase-8 and GSDMD was shown to be associated with better
survival outcomes (132).
Further analysis has identified PANoptosis-related prognostic
clusters, revealing underlying genetic mutations, distinct immune
cell populations and activated oncogenic pathways that may account
for differences in patient survival across tumor types (7). These findings highlight the
heterogeneous nature of PANoptosis gene expression and suggest it
may exert different roles depending on the cancer context.
Another study applied PANoptosis-related
differentially expressed genes to predict prognosis in patients
with KIRC. The high-risk PANoptosis group demonstrated an
immunosuppressive tumor microenvironment, characterized by reduced
infiltration of antitumor CD4+ T cells and natural
killer cells, and elevated levels of immunosuppressive M2
macrophages and regulatory T cells. These findings suggest that
PANoptosis may contribute to KIRC progression and influence immune
regulation within the tumor microenvironment (9). In a separate study, Wang et
al (133) developed a ccRCC
prognostic risk model based on three specific PANoptosis-related
microRNAs, revealing that patients in the high-risk group exhibited
poorer outcomes and greater tumor malignancy. This group also
showed enrichment of immune-related pathways and heightened immune
activity, implying potential responsiveness to immunotherapy and
chemotherapy. By contrast, the low-risk group demonstrated
metabolic reprogramming, with increased fatty acid and amino acid
metabolism. Furthermore, PANoptosis-related long non-coding RNAs
have been associated with clinical outcomes, immune cell
infiltration, immunosuppressive states, oncogenic signaling
pathways and therapeutic responses in ccRCC. Single-cell sequencing
identified LINC00944 as a potential biomarker linked to T-cell
infiltration and PCD regulation, supporting its prognostic
relevance (134). A recent
study used bioinformatics analysis to characterize PANoptosis in
the three RCC subtypes (KIRC, KIRP and KICH) and proposed a novel
scoring system, the PANoptosis-Immunity Index (PANII). The PANII
effectively reflected the immune microenvironment status across RCC
subtypes and predicted response to immunotherapy. Functional
validation revealed that knockout of the PYCARD gene significantly
inhibited proliferation and invasion of KIRC cells in vitro,
confirming its role in promoting tumor progression (135) (Table I).
CKD has emerged as a growing global public health
concern, which markedly impairs patient quality of life. Over the
past three decades, the global mortality rate associated with CKD
has reached 41.5%, with ~1.2 million deaths reported in 2017. By
2040, CKD is projected to become the fifth leading cause of death
worldwide (136,137). CKD is a progressive and
long-term condition with diverse etiologies; however, renal
fibrosis represents the common pathological hallmark driving
progression to end-stage renal disease. The fibrotic process in CKD
typically begins with the gradual loss of epithelial cells,
including podocytes and tubular cells, as well as endothelial
cells, which is followed by progressive glomerular sclerosis,
collapse of peritubular capillaries and extensive
tubulointerstitial fibrosis. The development of renal fibrosis is
tightly associated with sustained inflammation and various forms of
cell death (138-140). Regardless of the initial cause
of kidney injury, nephrons are continuously lost, and fibrotic
lesions advance until end-stage renal disease becomes inevitable.
Therefore, targeting cell death pathways represents a potential
therapeutic strategy to prevent or slow CKD progression by limiting
the loss of functional kidney cells and suppressing inflammation.
Notably, apoptosis, necroptosis and pyroptosis have been recognized
as central pathogenic events in CKD of different etiologies. A
large number of previous studies have confirmed that the
pathological mechanisms of CKD and DKD are related to renal cell
apoptosis (141-144), necroptosis (138,145-147) and pyroptosis (148,149).
Recent studies have revealed that three death modes
occur simultaneously in CKD and DKD, which is different from the
single death mode of PCD. Zhang et al (150) employed bioinformatics
approaches to investigate the relationship between PANoptosis and
CKD, identifying two central genes, FOS and PTGS2, as potential
mediators. These genes have previously been associated with tissue
inflammation, cell death and renal fibrosis, suggesting that
PANoptosis may contribute to the onset and progression of CKD
through their regulation (151-154). In a mouse model of post-burn
CKD, elevated levels of caspase-1, caspase-3 and IL-1β have been
observed in renal tissue, and apoptosis, pyroptosis and necroptosis
were demonstrated to be concurrently activated (155). Sustained inflammation following
burn injury led to caspase pathway activation, promoting
PANoptosis, and aggravating renal injury and CKD progression.
Treatment with the anti-inflammatory agent dexamethasone was able
to suppress multiple forms of cell death and alleviate renal damage
in this model (155) (Table I).
DKD, a major cause of end-stage kidney failure, is
marked by progressive podocyte loss. Using single-cell RNA
sequencing data, Lv et al (156) reported that TRAIL and death
receptor 5 (DR5) were highly expressed in the podocytes of patients
with DKD. These findings were further validated in renal biopsy
samples. In vitro and in vivo experiments
demonstrated that TRAIL induced the expression of
PANoptosis-related markers, including caspase-1, caspase-3,
phosphorylated (p)-MLKL and p-RIPK1, via DR5 in DKD podocytes. In
addition, the simultaneous activation of apoptosis, pyroptosis and
necroptosis exacerbated proteinuria and renal injury in mouse
models of DKD. These effects were mitigated by TRAIL inhibition or
gene knockout, indicating that the TRAIL/DR5 axis mediates podocyte
PANoptosis injury in DKD (156)
(Table I).
Collectively, these findings suggest that
PANoptosis may serve as a promising therapeutic target in CKD and
DKD. However, given the complex and heterogeneous nature of CKD
pathogenesis, current research on PANoptosis in this context
remains limited. Further investigation is needed to elucidate the
mechanistic roles and therapeutic implications of PANoptosis across
diverse CKD etiologies.
Recent studies have increasingly focused on the
role of PANoptosis in drug-induced renal injury (6,10,11,157,158). One investigation revealed that
the herbicide atrazine, known for its nephrotoxic effects, can
downregulate Sam50 expression in the kidney, resulting in
mitochondrial DNA (mtDNA) instability and the release of mtDNA into
the cytoplasm. This mtDNA release activates the stimulator of IFN
genes (STING) pathway, which subsequently upregulates inflammatory
cytokines and key PANoptosis-associated molecules, including AIM2,
ZBP1 and pyrin. These molecular events drive STING-dependent
PANoptosis in renal tissue, ultimately leading to renal failure
(10).
Triptolide-induced multi-organ damage has also been
linked to a systemic inflammatory response and the activation of
various lytic cell death pathways. Notably, the cell death
triggered by triptolide cannot be mitigated by the inhibition of a
single pathway. It has been shown that triptolide simultaneously
activates markers of pyroptosis, apoptosis and necroptosis in
macrophages. Immunofluorescence and immunoprecipitation assays have
confirmed the colocalization and aggregation of ASC, RIPK3 and
caspase-8, promoting the formation of the PANoptosome and inducing
PANoptosis, which contributes to both nephrotoxicity and
hepatotoxicity. Given that renal tubular epithelial cells and
podocytes possess the molecular machinery required for all three
forms of cell death, the authors of this previous study
hypothesized that PANoptosis likely occurs in these renal cell
types as well (157). In
aristolochic acid nephropathy (AAN), administration of aristolochic
acid I (AAI) has been shown to induce elevations in serum urea
nitrogen and creatinine levels, and to induce PANoptosis in renal
tubular epithelial cells. Both in vitro and in vivo
experiments have demonstrated that inhibition of PANoptosis may
alleviate AAI-induced renal injury, supporting its role in AAN
pathogenesis (158). The
anticancer agent CBL0137, either alone or in combination with LPS,
has been shown to activate systemic inflammation and PANoptosis
signaling. In mice, this treatment induced PANoptosis in
macrophages and resulted in multi-organ injury, including kidney
damage; mechanistically, CBL0137 promoted the formation of Z-DNA in
macrophage nuclei, thereby activating ZBP1. By contrast, knockout
of ZBP1 significantly reduced cell death induced by CBL0137
combined with LPS, highlighting the essential role of ZBP1 as a
core component of the PANoptosome in mediating PANoptosis (11). In a mouse model sensitized to
trichloroethylene, PANoptosis was observed in renal endothelial
cells, and the pro-inflammatory cytokines TNFα and IFNγ appeared to
act synergistically in driving this process. Inhibition of
peripheral TNFα and IFNγ attenuated PANoptosis, preserved
endothelial function and improved renal outcomes (6).
Beyond drug-induced renal injury, PANoptosis has
also been implicated in renal damage resulting from non-chemical
stressors. A recent study reported multi-organ injury, including
damage to the kidneys and liver, following heatstroke. It was shown
that ZBP1 mediates a PANoptosis-like cell death pathway during
heatstroke, characterized by concurrent activation of apoptosis,
pyroptosis and necroptosis. By contrast, deletion of ZBP1 abrogated
this cell death process, and reversed the renal and hepatic damage
induced by heatstroke (159)
(Table I).
Loss of parenchymal cells, together with
proliferation or recruitment of maladaptive cells, disrupts
cellular homeostasis and drives kidney injury and fibrosis
(160,161). Modulating cell death programs
may therefore represent an important approach to limiting renal
damage across kidney diseases. Since PANoptosis encompasses
multiple PCD patterns simultaneously, targeting and inhibiting
these pathways in parallel may enhance renal protection. Although
drugs directly targeting PANoptosis have not yet entered clinical
trials, a number of compounds that act on its key node molecules
are already in the clinical development stage for other indications
(162-166), which provides a unique
opportunity for the rapid initiation of clinical research on kidney
diseases.
Molecules that act as central hubs in PANoptosis,
such as ZBP1 and AIM2, can concurrently trigger pyroptosis,
apoptosis and necroptosis in response to microbial or endogenous
cues. Their inhibition or loss impairs activation of these effector
modules and reduces cell death (82,167,168). Notably, these hubs have been
implicated in AKI, RCC, drug-induced nephrotoxicity and other renal
pathologies, highlighting their broad relevance. At present,
specific inhibitors for ZBP1 and AIM2 are scarce, and traditional
treatment strategies mainly focus on the inhibition of their
downstream factors. However, some new technologies are attempting
to precisely degrade ZBP1. For example, a recent study on the
covalent recognition-based PROTAC molecule has confirmed that it
can cause upregulation of ZBP1 expression in H1N1 infection models,
inhibit the downstream necroptosis pathway, reduce the
phosphorylation levels of RIPK3 and MLKL, and simultaneously lower
the expression levels of IL-18, IL-6, IL-1β, TNF-α and IFN-β,
effectively alleviating inflammation (169). In tumor research, it has been
shown that the ZBP1 agonist CBL0137 can induce potent PANoptosis
and antitumor immunity in mouse models of ZBP1-deficient melanoma
(170). Conversely,
necrosulfonamide, a small molecule inhibitor targeting the ZBP1
RHIM domain, can be used to alleviate therapeutic side effects
(such as intestinal damage) (171). Recently, 4-sulfonic calixarenes
have been described as being able to block the binding of dsDNA to
the HIN200 domain of AIM2 and to inhibit AIM2 in vivo,
further blocking the release of the downstream factors caspase-1,
IL-1β and GSDMD (172). Further
work revealed that these compounds are structurally similar to the
clinically approved drug suramin, which can also inhibit AIM2
(172).
Therapeutic targeting of PANoptosome components is
also considered promising. For example, the NLRP3 inhibitor MCC950
has been shown to attenuate kidney injury in models of AKI
(173-175), CDK (176,177), DKD (178,179) and RCC (180). It has shown good safety and
efficacy both in vivo and in vitro. Another NLRP3
inhibitor, OLT1177 (dapansutrile), has been proven to effectively
reduce inflammatory indicators and has been shown to possess a good
safety profile in a phase II trial of acute gout attacks (181). This also indicates prospects
for the treatment of kidney diseases driven by metabolic stress,
such as DKD. Z-VAD-FMK, a broad-spectrum caspase inhibitor widely
used to suppress apoptosis (182), and Nec-1, a necroptosis
inhibitor targeting RIPK1, have both demonstrated protective
effects in various kidney diseases (182,183), but have not yet entered
clinical trials. GSK2982772 is another RIPK1 inhibitor that has
been tested for safety and efficacy in phase II clinical trials for
psoriasis (165,166), rheumatoid arthritis (162) and ulcerative colitis (163). Given that RIPK1 is a key hub of
PANoptosis in multiple nephropathy models, reusing it for the
treatment of inflammatory nephropathy (such as lupus nephritis) is
an attractive clinically available strategy. Caspase-8 functions as
a key regulator across pathways, simultaneously influencing
apoptosis, necroptosis and pyroptosis, thereby determining the cell
death mode following activation signals (62). A recent study reported that
inhibition of the caspase-8/caspase-3/NLRP3/GSDME-mediated pathway
alleviated pyroptosis and rescued renal tubular cell injury induced
by uric acid (184). Another
investigation revealed that TP53INP2, an autophagy-related protein,
can inhibit RCC progression by regulating the caspase-8 apoptotic
pathway (185). Another caspase
inhibitor (emricasan) has been shown in a clinical study on
non-alcoholic steatohepatitis to significantly reduce ALT levels
and the activation of caspase-3/7 in patients with non-alcoholic
fatty liver disease. Although the results of this study have not
been clinically translated, it provides a foundation for subsequent
research on specific caspase inhibitors (such as those targeting
caspase-8) (164). RIPK3 is
also another important component of PANoptosomes. A novel inhibitor
of RIPK3, Uh15-38, has been proven to effectively block necroptosis
of alveolar epithelial cells caused by IAV infection in mouse
models by inhibiting MLKL, and the levels of IL-1β and IL-18. In
addition, it can reduce lung inflammation and mortality without
affecting virus clearance or immune response (186). Its efficacy is consistent with
other pre-clinical type I kinase inhibitors and comparable to that
of currently Food and Drug Administration-approved drugs, such as
sorafenib and dasatinib (186).
In addition, sorafenib and edaravone have been shown to attenuate
renal fibrosis induced by unilateral ureteral obstruction through
inhibition of oxidative stress, inflammation and the RIPK3/MLKL
pathway (187). Collectively,
these findings suggest that targeting hub molecules and components
of PANoptosis, including ZBP1, AIM2, RIPK1, NLRP3 and caspase
family members, represents a promising therapeutic option for
kidney diseases.
However, the interplay among apoptosis, pyroptosis
and necroptosis complicates therapeutic development. These pathways
often share molecular mediators and defense mechanisms, and they
may coexist within the same kidney disease, acting either
simultaneously or sequentially across different cell types.
Furthermore, inhibition of one pathway may be compensated for by
activation of others, resulting in limited therapeutic efficacy
with single-target approaches. Therefore, identifying strategies
that can simultaneously block multiple forms of cell death remains
a key focus for future research.
Current evidence has indicated that PANoptosis may
serve as a novel and promising therapeutic target in kidney
diseases. Unlike interventions aimed at suppressing a single form
of PCD, modulation of PANoptosis offers the potential to block
multiple cell death pathways concurrently, thereby providing
greater therapeutic benefit. This integrated approach represents a
new direction for the prevention of kidney injury and the
attenuation of disease progression; however, important challenges
remain. Although key molecules involved in PANoptosis and their
roles in distinct death pathways have been identified, the precise
mechanisms by which these factors cooperate within the PANoptosome,
and the hierarchy of control governing its assembly and signaling,
are not yet fully understood. The dynamic and context-dependent
composition of the PANoptosome, shaped by trigger signals, cell
type and microenvironment, further complicates efforts to define
universal assembly patterns. In vivo, notable technical
barriers also exist in capturing transient, large multiprotein
complexes and their intricate interaction networks. Additionally,
the essential components, recruitment rules, sequential assembly
mechanisms, and possible molecular switches or master regulators of
PANoptosomes remain to be established. Finally, reports of
PANoptosis in kidney diseases beyond AKI (8,110-112), CKD (150,155), DKD (156) and RCC (7,9,132-135) are limited (188). Its roles in hypertensive
nephropathy, nephrotic syndrome, IgA nephropathy, lupus nephritis
and polycystic kidney disease remain largely unexplored. These
knowledge gaps highlight urgent priorities for future
investigation.
Current research has revealed the mechanistic
complexity of PANoptosis, and its involvement in the onset,
progression and potential treatment of AKI, RCC, CKD, DKD and
drug-induced renal injury. The activation and composition of the
PANoptosome are highly variable, reflecting the context-dependent
nature of PANoptosis and offering deeper insight into its
regulatory role in cell death. As a result, PANoptosis has emerged
as a key process closely associated with the development of various
kidney diseases and presents a promising avenue for identifying
novel therapeutic targets. Nevertheless, the role of PANoptosis in
disease is not unidirectional; its effects on disease progression
and treatment can be dual in nature, which is particularly evident
in renal malignancies, where PANoptosis may exert both pro-tumor
and antitumor effects. Furthermore, the dominant cell death
pathways and the composition of PANoptotic complexes may vary
depending on disease etiology or pathological stage. In AKI and
CKD, greater attention should be directed toward cell death
occurring within the renal parenchyma, whereas in RCC, further
investigation is required to elucidate how the tumor immune
microenvironment shapes PANoptosis. These observations underscore
the need for continued in-depth research to elucidate its
regulatory mechanisms and disease-specific outcomes. Although
recent advances have expanded the understanding, the clinical
application of PANoptosis in kidney disease remains in its early
stages, and numerous questions remain unanswered. For example,
while it is known that the composition of the PANoptosome varies
with different stimuli or disease states, the mechanisms underlying
this variability remain unclear. Additionally, although specific
sensors are required to initiate PANoptosis in response to
pathogens or damage-associated signals, the precise identity and
disease-specific roles of these sensors remain to be fully defined.
Therefore, several future research directions merit attention.
First, it is essential to discover and validate reliable biomarkers
that can facilitate the precise selection of therapeutic strategies
targeting PANoptosis. Second, kidney cell-specific mechanisms
should be elucidated, as tubular epithelial cells, podocytes and
endothelial cells may assemble PANoptosomes with distinct
components and functional outputs, which is crucial for therapeutic
specificity. Third, a detailed characterization of the assembly and
regulatory mechanisms of renal PANoptosomes is needed to support
the identification of small-molecule targets. Fourth, across
diverse renal diseases, the validation of hub molecules and the
identification of regulators of PANoptosomes will provide a
foundation for the development of selective inhibitors.
Since its initial conceptualization in 2019, the
concept of PANoptosis has rapidly advanced as a field, offering a
transformative framework to understand the intricate interplay
between cell death and inflammation in kidney diseases. Unlike
strategies that focus on blocking a single cell death pathway,
targeting the integrated process of PANoptosis holds promise for
achieving more effective protection of kidney tissue, attenuation
of inflammation and improved clinical outcomes. Despite ongoing
challenges, continued investigation into kidney-specific mechanisms
of PANoptosis, together with the verification of therapeutic
targets, the development of efficient and safe pharmacological
agents, and the incorporation of precise biomarkers, is expected to
drive major advances. Thus, targeted modulation of PANoptosis may
emerge as a breakthrough approach in nephrology, providing novel
therapeutic opportunities for currently refractory kidney
diseases.
Not applicable.
LJ wrote the original manuscript. YL and LZ revised
the manuscript. ZF and MW searched the literature, and made
substantial contributions to conception and design. ZM edited the
manuscript. ML revised and supervised the manuscript. Data
authentication is not applicable. All authors contributed to the
article, and read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work is funded by the National Nature Science Foundation of
China (grant no. 82274482).
|
1
|
Bedoui S, Herold MJ and Strasser A:
Emerging connectivity of programmed cell death pathways and its
physiological implications. Nat Rev Mol Cell Biol. 21:678–695.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Newton K, Strasser A, Kayagaki N and Dixit
VM: Cell death. Cell. 187:235–256. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malireddi RKS, Kesavardhana S and
Kanneganti TD: ZBP1 and TAK1: Master regulators of NLRP3
inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis).
Front Cell Infect Microbiol. 9:4062019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pandian N and Kanneganti TD: PANoptosis: A
unique innate immune inflammatory cell death modality. J Immunol.
209:1625–1633. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Pandian N, Han JH, Sundaram B, Lee
S, Karki R, Guy CS and Kanneganti TD: Single cell analysis of
PANoptosome cell death complexes through an expansion microscopy
method. Cell Mol Life Sci. 79:5312022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie H, Liang B, Zhu Q, Wang L, Li H, Qin
Z, Zhang J, Liu Z and Wu Y: The role of PANoptosis in renal
vascular endothelial cells: Implications for
trichloroethylene-induced kidney injury. Ecotoxicol Environ Saf.
278:1164332024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mall R and Kanneganti TD: Comparative
analysis identifies genetic and molecular factors associated with
prognostic clusters of PANoptosis in glioma, kidney and melanoma
cancer. Sci Rep. 13:209622023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uysal E, Dokur M, Kucukdurmaz F, Altınay
S, Polat S, Batcıoglu K, Sezgın E, Sapmaz Erçakallı T, Yaylalı A,
Yılmaztekin Y, et al: Targeting the PANoptosome with 3,4-meth
ylenedioxy-β-nitrostyrene, reduces PANoptosis and protects the
kidney against renal ischemia-reperfusion injury. J Invest Surg.
35:1824–1835. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Z, Wang J, Dao C, Zhu M, Li Y, Liu
F, Zhao Y, Li J, Yang Y and Pan Z: Utilizing a novel model of
PANoptosis-related genes for enhanced prognosis and immune status
prediction in kidney renal clear cell carcinoma. Apoptosis.
29:681–692. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi BJ, Wang CC, Li XW, Xu YR, Ma XY, Jian
PA, Talukder M, Li XN and Li JL: Lycopene protects against
atrazine-induced kidney STING-dependent panoptosis through
stabilizing mtDNA via interaction with Sam50/PHB1. J Agric Food
Chem. 72:14956–14966. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YP, Zhou ZY, Yan L, You YP, Ke HY, Yuan
T, Yang HY, Xu R, Xu LH, Ouyang DY, et al: Inflammatory cell death
PANoptosis is induced by the anti-cancer curaxin CBL0137 via
eliciting the assembly of ZBP1-associated PANoptosome. Inflamm Res.
73:597–617. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karki R, Sharma BR, Lee E, Banoth B,
Malireddi RKS, Samir P, Tuladhar S, Mummareddy H, Burton AR, Vogel
P and Kanneganti TD: Interferon regulatory factor 1 regulates
PANoptosis to prevent colorectal cancer. JCI Insight.
5:e1367202020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kroemer G: The pharmacology of T cell
apoptosis. Adv Immunol. 58:211–296. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar
|
|
16
|
Li PF, Dietz R and von Harsdorf R: p53
regulates mitochondrial membrane potential through reactive oxygen
species and induces cytochrome c-independent apoptosis blocked by
Bcl-2. EMBO J. 18:6027–6036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar
|
|
18
|
Kalkavan H and Green DR: MOMP, cell
suicide as a BCL-2 family business. Cell Death Differ. 25:46–55.
2018. View Article : Google Scholar
|
|
19
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hengartner MO and Horvitz HR: The ins and
outs of programmed cell death during C. elegans development. Philos
Trans R Soc Lond B Biol Sci. 345:243–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fox JL, Hughes MA, Meng X, Sarnowska NA,
Powley IR, Jukes-Jones R, Dinsdale D, Ragan TJ, Fairall L, Schwabe
JWR, et al: Cryo-EM structural analysis of FADD:Caspase-8 complexes
defines the catalytic dimer architecture for co-ordinated control
of cell fate. Nat Commun. 12:8192021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu TM, Li Y, Lu A, Li Z, Vajjhala PR, Cruz
AC, Srivastava DB, DiMaio F, Penczek PA, Siegel RM, et al: Cryo-EM
structure of caspase-8 tandem DED filament reveals assembly and
regulation mechanisms of the death-inducing signaling complex. Mol
Cell. 64:236–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feoktistova M, Geserick P, Kellert B,
Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Häcker G
and Leverkus M: cIAPs block Ripoptosome formation, a RIP1/caspase-8
containing intracellular cell death complex differentially
regulated by cFLIP isoforms. Mol Cell. 43:449–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seol DW, Li J, Seol MH, Park SY, Talanian
RV and Billiar TR: Signaling events triggered by tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL): Caspase-8 is
required for TRAIL-induced apoptosis. Cancer Res. 61:1138–1143.
2001.PubMed/NCBI
|
|
28
|
Degterev A, Huang Z, Boyce M, Li Y, Jagtap
P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA and Yuan J:
Chemical inhibitor of nonapoptotic cell death with therapeutic
potential for ischemic brain injury. Nat Chem Biol. 1:112–119.
2005. View Article : Google Scholar
|
|
29
|
Khoury MK, Gupta K, Franco SR and Liu B:
Necroptosis in the pathophysiology of disease. Am J Pathol.
190:272–285. 2020. View Article : Google Scholar :
|
|
30
|
Weinlich R, Oberst A, Beere HM and Green
DR: Necroptosis in development, inflammation and disease. Nat Rev
Mol Cell Biol. 18:127–136. 2017. View Article : Google Scholar
|
|
31
|
Murphy JM: The killer pseudokinase mixed
lineage kinase domain-like protein (MLKL). Cold Spring Harb
Perspect Biol. 12:a0363762020. View Article : Google Scholar
|
|
32
|
Petrie EJ, Sandow JJ, Lehmann WIL, Liang
LY, Coursier D, Young SN, Kersten WJA, Fitzgibbon C, Samson AL,
Jacobsen AV, et al: Viral MLKL homologs subvert necroptotic cell
death by sequestering cellular RIPK3. Cell Rep. 28:3309–3319.e5.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holler N, Zaru R, Micheau O, Thome M,
Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp
J: Fas triggers an alternative, caspase-8-independent cell death
pathway using the kinase RIP as effector molecule. Nat Immunol.
1:489–495. 2000. View
Article : Google Scholar
|
|
34
|
He S, Liang Y, Shao F and Wang X:
Toll-like receptors activate programmed necrosis in macrophages
through a receptor-interacting kinase-3-mediated pathway. Proc Natl
Acad Sci USA. 108:20054–20059. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaiser WJ, Sridharan H, Huang C, Mandal P,
Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J and Mocarski ES:
Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J
Biol Chem. 288:31268–31279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang T, Wang Y, Inuzuka H and Wei W:
Necroptosis pathways in tumorigenesis. Semin Cancer Biol. 86:32–40.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho YS, Challa S, Moquin D, Genga R, Ray
TD, Guildford M and Chan FK: Phosphorylation-driven assembly of the
RIP1-RIP3 complex regulates programmed necrosis and virus-induced
inflammation. Cell. 137:1112–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vanden Berghe T, Kaiser WJ, Bertrand MJ
and Vandenabeele P: Molecular crosstalk between apoptosis,
necroptosis, and survival signaling. Mol Cell Oncol. 2:e9750932015.
View Article : Google Scholar
|
|
39
|
Wong WW, Gentle IE, Nachbur U, Anderton H,
Vaux DL and Silke J: RIPK1 is not essential for TNFR1-induced
activation of NF-kappaB. Cell Death Differ. 17:482–487. 2010.
View Article : Google Scholar
|
|
40
|
Wang H, Sun L, Su L, Rizo J, Liu L, Wang
LF, Wang FS and Wang X: Mixed lineage kinase domain-like protein
MLKL causes necrotic membrane disruption upon phosphorylation by
RIP3. Mol Cell. 54:133–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai Z, Jitkaew S, Zhao J, Chiang HC,
Choksi S, Liu J, Ward Y, Wu LG and Liu ZG: Plasma membrane
translocation of trimerized MLKL protein is required for
TNF-induced necroptosis. Nat Cell Biol. 16:55–65. 2014. View Article : Google Scholar
|
|
42
|
Robinson N, McComb S, Mulligan R, Dudani
R, Krishnan L and Sad S: Type I interferon induces necroptosis in
macrophages during infection with Salmonella enterica serovar
Typhimurium. Nat Immunol. 13:954–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McComb S, Cessford E, Alturki NA, Joseph
J, Shutinoski B, Startek JB, Gamero AM, Mossman KL and Sad S:
Type-I interferon signaling through ISGF3 complex is required for
sustained Rip3 activation and necroptosis in macrophages. Proc Natl
Acad Sci USA. 111:E3206–E3213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia B, Fang S, Chen X, Hu H, Chen P, Wang
H and Gao Z: MLKL forms cation channels. Cell Res. 26:517–528.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Negroni A, Colantoni E, Cucchiara S and
Stronati L: Necroptosis in intestinal inflammation and cancer: New
concepts and therapeutic perspectives. Biomolecules. 10:14312020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woo Y, Lee HJ, Jung YM and Jung YJ:
Regulated necrotic cell death in alternative tumor therapeutic
strategies. Cells. 9:27092020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zychlinsky A, Prevost MC and Sansonetti
PJ: Shigella flexneri induces apoptosis in infected macrophages.
Nature. 358:167–169. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hilbi H, Moss JE, Hersh D, Chen Y, Arondel
J, Banerjee S, Flavell RA, Yuan J, Sansonetti PJ and Zychlinsky A:
Shigella-induced apoptosis is dependent on caspase-1 which binds to
IpaB. J Biol Chem. 273:32895–32900. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wallach D, Kang TB, Dillon CP and Green
DR: Programmed necrosis in inflammation: Toward identification of
the effector molecules. Science. 352:aaf21542016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Atabaki R, Khaleghzadeh-Ahangar H,
Esmaeili N and Mohseni-Moghaddam P: Role of pyroptosis, a
pro-inflammatory programmed cell death, in epilepsy. Cell Mol
Neurobiol. 43:1049–1059. 2023. View Article : Google Scholar
|
|
53
|
Bergsbaken T, Fink SL and Cookson BT:
Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol.
7:99–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rathinam VAK, Vanaja SK, Waggoner L,
Sokolovska A, Becker C, Stuart LM, Leong JM and Fitzgerald KA: TRIF
licenses caspase-11-dependent NLRP3 inflammasome activation by
gram-negative bacteria. Cell. 150:606–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fritsch M, Günther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gurung P, Anand PK, Malireddi RK, Vande
Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR,
Lamkanfi M and Kanneganti TD: FADD and caspase-8 mediate priming
and activation of the canonical and noncanonical Nlrp3
inflammasomes. J Immunol. 192:1835–1846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Man SM, Tourlomousis P, Hopkins L, Monie
TP, Fitzgerald KA and Bryant CE: Salmonella infection induces
recruitment of caspase-8 to the inflammasome to modulate IL-1β
production. J Immunol. 191:5239–5246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Van Opdenbosch N, Van Gorp H, Verdonckt M,
Saavedra PHV, de Vasconcelos NM, Gonçalves A, Vande Walle L, Demon
D, Matusiak M, Van Hauwermeiren F, et al: Caspase-1 engagement and
TLR-induced c-FLIP expression suppress ASC/caspase-8-dependent
apoptosis by inflammasome sensors NLRP1b and NLRC4. Cell Rep.
21:3427–3444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park MY, Ha SE, Vetrivel P, Kim HH,
Bhosale PB, Abusaliya A and Kim GS: Differences of key proteins
between apoptosis and necroptosis. Biomed Res Int.
2021:34201682021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Newton K, Wickliffe KE, Dugger DL,
Maltzman A, Roose-Girma M, Dohse M, Kőműves L, Webster JD and Dixit
VM: Cleavage of RIPK1 by caspase-8 is crucial for limiting
apoptosis and necroptosis. Nature. 574:428–431. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: An ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Schwarzer R, Laurien L and Pasparakis M:
New insights into the regulation of apoptosis, necroptosis, and
pyroptosis by receptor interacting protein kinase 1 and caspase-8.
Curr Opin Cell Biol. 63:186–193. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hsu H, Shu HB, Pan MG and Goeddel DV:
TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF
receptor 1 signal transduction pathways. Cell. 84:299–308. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dillon CP, Weinlich R, Rodriguez DA,
Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F,
Gong YN, et al: RIPK1 blocks early postnatal lethality mediated by
caspase-8 and RIPK3. Cell. 157:1189–1202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Orning P, Weng D, Starheim K, Ratner D,
Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, et al: Pathogen
blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin
D and cell death. Science. 362:1064–1069. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sarhan J, Liu BC, Muendlein HI, Li P,
Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR and
Poltorak A: Caspase-8 induces cleavage of gasdermin D to elicit
pyroptosis during Yersinia infection. Proc Natl Acad Sci USA.
115:E10888–E10897. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu
JM, Nie L, Chen Y, Wang YC, Liu C, et al: PD-L1-mediated gasdermin
C expression switches apoptosis to pyroptosis in cancer cells and
facilitates tumour necrosis. Nat Cell Biol. 22:1264–1275. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Y, Karki R, Zheng M, Kancharana B,
Lee S, Kesavardhana S, Hansen BS, Pruett-Miller SM and Kanneganti
TD: Cutting edge: Caspase-8 is a linchpin in caspase-3 and
gasdermin D activation to control cell death, cytokine release, and
host defense during influenza A virus infection. J Immunol.
207:2411–2416. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rogers C, Fernandes-Alnemri T, Mayes L,
Alnemri D, Cingolani G and Alnemri ES: Cleavage of DFNA5 by
caspase-3 during apoptosis mediates progression to secondary
necrotic/pyroptotic cell death. Nat Commun. 8:141282017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tsuchiya K, Nakajima S, Hosojima S, Thi
Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y,
Miura M, et al: Caspase-1 initiates apoptosis in the absence of
gasdermin D. Nat Commun. 10:20912019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lamkanfi M, Kanneganti TD, Van Damme P,
Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P,
Gevaert K and Núñez G: Targeted peptidecentric proteomics reveals
caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell
Proteomics. 7:2350–2363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Conos SA, Chen KW, De Nardo D, Hara H,
Whitehead L, Núñez G, Masters SL, Murphy JM, Schroder K, Vaux DL,
et al: Active MLKL triggers the NLRP3 inflammasome in a
cell-intrinsic manner. Proc Natl Acad Sci USA. 114:E961–E969. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gutierrez KD, Davis MA, Daniels BP, Olsen
TM, Ralli-Jain P, Tait SW, Gale M Jr and Oberst A: MLKL activation
triggers NLRP3-mediated processing and release of IL-1β
independently of gasdermin-D. J Immunol. 198:2156–2164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Muñoz-Planillo R, Kuffa P, Martínez-Colón
G, Smith BL, Rajendiran TM and Núñez G: K+ efflux is the
common trigger of NLRP3 inflammasome activation by bacterial toxins
and particulate matter. Immunity. 38:1142–1153. 2013. View Article : Google Scholar
|
|
76
|
Lawlor KE, Khan N, Mildenhall A, Gerlic M,
Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL,
et al: RIPK3 promotes cell death and NLRP3 inflammasome activation
in the absence of MLKL. Nat Commun. 6:62822015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Malireddi RKS, Gurung P, Mavuluri J,
Dasari TK, Klco JM, Chi H and Kanneganti TD: TAK1 restricts
spontaneous NLRP3 activation and cell death to control myeloid
proliferation. J Exp Med. 215:1023–1034. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zheng M, Karki R, Vogel P and Kanneganti
TD: Caspase-6 is a key regulator of innate immunity, inflammasome
activation, and host defense. Cell. 181:674–687.e13. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Malireddi RKS, Gurung P, Kesavardhana S,
Samir P, Burton A, Mummareddy H, Vogel P, Pelletier S, Burgula S
and Kanneganti TD: Innate immune priming in the absence of TAK1
drives RIPK1 kinase activity-independent pyroptosis, apoptosis,
necroptosis, and inflammatory disease. J Exp Med.
217:jem.201916442020. View Article : Google Scholar
|
|
80
|
Christgen S, Tweedell RE and Kanneganti
TD: Programming inflammatory cell death for therapy. Pharmacol
Ther. 232:1080102022. View Article : Google Scholar :
|
|
81
|
Tweedell RE and Kanneganti TD: Advances in
inflammasome research: Recent breakthroughs and future hurdles.
Trends Mol Med. 26:969–971. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lee S, Karki R, Wang Y, Nguyen LN,
Kalathur RC and Kanneganti TD: AIM2 forms a complex with pyrin and
ZBP1 to drive PANoptosis and host defence. Nature. 597:415–419.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shi C, Cao P, Wang Y, Zhang Q, Zhang D,
Wang Y, Wang L and Gong Z: PANoptosis: A cell death characterized
by pyroptosis, apoptosis, and necroptosis. J Inflamm Res.
16:1523–1532. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Banoth B, Tuladhar S, Karki R, Sharma BR,
Briard B, Kesavardhana S, Burton A and Kanneganti TD: ZBP1 promotes
fungi-induced inflammasome activation and pyroptosis, apoptosis,
and necroptosis (PANoptosis). J Biol Chem. 295:18276–18283. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kesavardhana S, Malireddi RKS, Burton AR,
Porter SN, Vogel P, Pruett-Miller SM and Kanneganti TD: The Zα2
domain of ZBP1 is a molecular switch regulating influenza-induced
PANoptosis and perinatal lethality during development. J Biol Chem.
295:8325–8330. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Karki R, Lee S, Mall R, Pandian N, Wang Y,
Sharma BR, Malireddi RS, Yang D, Trifkovic S, Steele JA, et al:
ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine
storm disrupt IFN therapeutic efficacy during coronavirus
infection. Sci Immunol. 7:eabo62942022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Malireddi RKS, Kesavardhana S, Karki R,
Kancharana B, Burton AR and Kanneganti TD: RIPK1 distinctly
regulates Yersinia-induced inflammatory cell death, PANoptosis.
Immunohorizons. 4:789–796. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sundaram B, Pandian N, Mall R, Wang Y,
Sarkar R, Kim HJ, Malireddi RKS, Karki R, Janke LJ, Vogel P and
Kanneganti TD: NLRP12-PANoptosome activates PANoptosis and
pathology in response to heme and PAMPs. Cell. 186:2783–2801.e20.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kuriakose T, Man SM, Malireddi RK, Karki
R, Kesavardhana S, Place DE, Neale G, Vogel P and Kanneganti TD:
ZBP1/DAI is an innate sensor of influenza virus triggering the
NLRP3 inflammasome and programmed cell death pathways. Sci Immunol.
1:aag20452016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rebsamen M, Heinz LX, Meylan E, Michallet
MC, Schroder K, Hofmann K, Vazquez J, Benedict CA and Tschopp J:
DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction
motifs to activate NF-kappaB. EMBO Rep. 10:916–922. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Upton JW, Kaiser WJ and Mocarski ES:
DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced
programmed necrosis that is targeted by murine cytomegalovirus
vIRA. Cell Host Microbe. 11:290–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kuriakose T and Kanneganti TD: ZBP1:
Innate sensor regulating cell death and inflammation. Trends
Immunol. 39:123–134. 2018. View Article : Google Scholar :
|
|
93
|
Thapa RJ, Ingram JP, Ragan KB, Nogusa S,
Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner
VJ, et al: DAI senses influenza A virus genomic RNA and activates
RIPK3-dependent cell death. Cell Host Microbe. 20:674–681. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zheng M and Kanneganti TD: Newly
identified function of caspase-6 in ZBP1-mediated innate immune
responses, NLRP3 inflammasome activation, PANoptosis, and host
defense. J Cell Immunol. 2:341–347. 2020.
|
|
95
|
Momota M, Lelliott P, Kubo A, Kusakabe T,
Kobiyama K, Kuroda E, Imai Y, Akira S, Coban C and Ishii KJ: ZBP1
governs the inflammasome-independent IL-1α and neutrophil
inflammation that play a dual role in anti-influenza virus
immunity. Int Immunol. 32:203–212. 2020. View Article : Google Scholar
|
|
96
|
Hornung V, Ablasser A, Charrel-Dennis M,
Bauernfeind F, Horvath G, Caffrey DR, Latz E and Fitzgerald KA:
AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating
inflammasome with ASC. Nature. 458:514–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bürckstümmer T, Baumann C, Blüml S, Dixit
E, Dürnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett
KL and Superti-Furga G: An orthogonal proteomic-genomic screen
identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome.
Nat Immunol. 10:266–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fernandes-Alnemri T, Yu JW, Datta P, Wu J
and Alnemri ES: AIM2 activates the inflammasome and cell death in
response to cytoplasmic DNA. Nature. 458:509–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Fernandes-Alnemri T, Yu JW, Juliana C,
Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott
E, et al: The AIM2 inflammasome is critical for innate immunity to
Francisella tularensis. Nat Immunol. 11:385–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rathinam VA, Jiang Z, Waggoner SN, Sharma
S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et
al: The AIM2 inflammasome is essential for host defense against
cytosolic bacteria and DNA viruses. Nat Immunol. 11:395–402. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kist M, Kőműves LG, Goncharov T, Dugger
DL, Yu C, Roose-Girma M, Newton K, Webster JD and Vucic D: Impaired
RIPK1 ubiquitination sensitizes mice to TNF toxicity and
inflammatory cell death. Cell Death Differ. 28:985–1000. 2021.
View Article : Google Scholar :
|
|
103
|
Mihaly SR, Ninomiya-Tsuji J and Morioka S:
TAK1 control of cell death. Cell Death Differ. 21:1667–1676. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sanjo H, Nakayama J, Yoshizawa T, Fehling
HJ, Akira S and Taki S: Cutting edge: TAK1 safeguards macrophages
against proinflammatory cell death. J Immunol. 203:783–788. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Malireddi RKS, Tweedell RE and Kanneganti
TD: PANoptosis components, regulation, and implications. Aging
(Albany NY). 12:11163–11164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kanneganti TD: Intracellular innate immune
receptors: Life inside the cell. Immunol Rev. 297:5–12. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ostermann M, Basu RK and Mehta RL: Acute
kidney injury. Intensive Care Med. 49:219–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ostermann M, Lumlertgul N, Jeong R, See E,
Joannidis M and James M: Acute kidney injury. Lancet. 405:241–256.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wu C, Zhang Y, Nie S, Hong D, Zhu J, Chen
Z, Liu B, Liu H, Yang Q, Li H, et al: Predicting in-hospital
outcomes of patients with acute kidney injury. Nat Commun.
14:37392023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wei S, Wu L, Xiang Z, Yang X, Pei D, Jiang
L and Du Z: EIF2AK2 protein targeted activation of AIM2-mediated
PANoptosis promotes sepsis-induced acute kidney injury. Ren Fail.
46:24036492024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lin SY, Chang CL, Liou KT, Kao YK, Wang
YH, Chang CC, Kuo TBJ, Huang HT, Yang CCH, Liaw CC and Shen YC: The
protective role of Achyranthes aspera extract against
cisplatininduced nephrotoxicity by alleviating oxidative stress,
inflammation, and PANoptosis. J Ethnopharmacol. 319:1170972024.
View Article : Google Scholar
|
|
112
|
You R: Mining biomarkers of acute kidney
injury in sepsis and preliminary exploration of the
PANoptosis-related mechanism. PhD dissertation. Peking Union
Medical College; 2023
|
|
113
|
Yan WT, Zhao WJ, Hu XM, Ban XX, Ning WY,
Wan H, Zhang Q and Xiong K: PANoptosis-like cell death in
ischemia/reperfusion injury of retinal neurons. Neural Regen Res.
18:357–363. 2023.
|
|
114
|
Wan H, Ban X, He Y, Yang Y, Hu X, Shang L,
Wan X, Zhang Q and Xiong K: Voltage-dependent anion channel 1
oligomerization regulates PANoptosis in retinal
ischemia-reperfusion injury. Neural Regen Res. 21:1652–1664. 2026.
View Article : Google Scholar
|
|
115
|
Yan WT, Yang YD, Hu XM, Ning WY, Liao LS,
Lu S, Zhao WJ, Zhang Q and Xiong K: Do pyroptosis, apoptosis, and
necroptosis (PANoptosis) exist in cerebral ischemia? Evidence from
cell and rodent studies. Neural Regen Res. 17:1761–1768. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hamar P, Song E, Kökény G, Chen A, Ouyang
N and Lieberman J: Small interfering RNA targeting Fas protects
mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci
USA. 101:14883–14888. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zheng X, Zang G, Jiang J, He W, Johnston
NJ, Ling H, Chen R, Zhang X, Liu Y, Haig A, et al: Attenuating
ischemia-reperfusion injury in kidney transplantation by perfusing
donor organs with siRNA cocktail solution. Transplantation.
100:743–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Basile DP, Liapis H and Hammerman MR:
Expression of bcl-2 and bax in regenerating rat renal tubules
following ischemic injury. Am J Physiol. 272:F640–F647.
1997.PubMed/NCBI
|
|
119
|
Thomas K, Zondler L, Ludwig N, Kardell M,
Lüneburg C, Henke K, Mersmann S, Margraf A, Spieker T, Tekath T, et
al: Glutamine prevents acute kidney injury by modulating oxidative
stress and apoptosis in tubular epithelial cells. JCI Insight.
7:e1631612022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang B, Wang J, Qiao J, Zhang Q, Liu Q,
Tan Y, Wang Q, Sun W, Feng W, Li Z, et al: Circ DENND4C inhibits
pyroptosis and alleviates ischemia-reperfusion acute kidney injury
by exosomes secreted from human urine-derived stem cells. Chem Biol
Interact. 391:1109222024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tonnus W, Maremonti F, Belavgeni A, Latk
M, Kusunoki Y, Brucker A, von Mässenhausen A, Meyer C, Locke S,
Gembardt F, et al: Gasdermin D-deficient mice are hypersensitive to
acute kidney injury. Cell Death Dis. 13:7922022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Miao N, Yin F, Xie H, Wang Y, Xu Y, Shen
Y, Xu D, Yin J, Wang B, Zhou Z, et al: The cleavage of gasdermin D
by caspase-11 promotes tubular epithelial cell pyroptosis and
urinary IL-18 excretion in acute kidney injury. Kidney Int.
96:1105–1120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li T, Sun H, Li Y, Su L, Jiang J, Liu Y,
Jiang N, Huang R, Zhang J and Peng Z: Downregulation of macrophage
migration inhibitory factor attenuates NLRP3 inflammasome mediated
pyroptosis in sepsis-induced AKI. Cell Death Discov. 8:612022.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Linkermann A and Green DR: Necroptosis. N
Engl J Med. 370:455–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Linkermann A, Bräsen JH, Himmerkus N, Liu
S, Huber TB, Kunzendorf U and Krautwald S: Rip1
(receptor-interacting protein kinase 1) mediates necroptosis and
contributes to renal ischemia/reperfusion injury. Kidney Int.
81:751–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang JN, Liu MM, Wang F, Wei B, Yang Q,
Cai YT, Chen X, Liu XQ, Jiang L, Li C, et al: RIPK1 inhibitor
Cpd-71 attenuates renal dysfunction in cisplatin-treated mice via
attenuating necroptosis, inflammation and oxidative stress. Clin
Sci (Lond). 133:1609–1627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chen C, Xie J, Chen Z, Ye K, Wu C, Dai X,
Yuan Y, Lin Y, Wang Y, Chen H, et al: Role of Z-DNA binding protein
1 sensing mitochondrial Z-DNA and triggering necroptosis in
oxalate-induced acute kidney injury. J Am Soc Nephrol. 36:361–377.
2025. View Article : Google Scholar
|
|
128
|
He P, Ma Y, Wu Y, Zhou Q and Du H:
Exploring PANoptosis in breast cancer based on scRNA-seq and
bulk-seq. Front Endocrinol (Lausanne). 14:11649302023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chen G, He Z, Jiang W, Li L, Luo B, Wang X
and Zheng X: Construction of a machine learning-based artificial
neural network for discriminating PANoptosis related subgroups to
predict prognosis in low-grade gliomas. Sci Rep. 12:221192022.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Liu W, Liu Y, Chen S, Hui J and He S:
AURKB promotes immunogenicity and immune infiltration in clear cell
renal cell carcinoma. Discov Oncol. 15:2862024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Choueiri TK and Motzer RJ: Systemic
therapy for metastatic renal-cell carcinoma. N Engl J Med.
376:354–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Mall R, Bynigeri RR, Karki R, Malireddi
RKS, Sharma BR and Kanneganti TD: Pancancer transcriptomic
profiling identifies key PANoptosis markers as therapeutic targets
for oncology. NAR Cancer. 4:zcac0332022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wang Y, Zhou J, Zhang N, Zhu Y, Zhong Y,
Wang Z, Jin H and Wang X: A novel defined PANoptosis-related miRNA
signature for predicting the prognosis and immune characteristics
in clear cell renal cell carcinoma: A miRNA signature for the
prognosis of ccRCC. Int J Mol Sci. 24:93922023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Liu W, Qu C and Wang X: Comprehensive
analysis of the role of immune-related PANoptosis lncRNA model in
renal clear cell carcinoma based on RNA transcriptome and
single-cell sequencing. Oncol Res. 31:543–567. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Xu Y, Hua J, Que H, Zeng T, Li Q, Deng J
and Xie J: Identification of PANoptosis-related signature reveals
immune infiltration characteristics and immunotherapy responses for
renal cell carcinoma. BMC Cancer. 24:2922024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
GBD Chronic Kidney Disease Collaboration:
Global, regional, and national burden of chronic kidney disease,
1990-2017: A systematic analysis for the global burden of disease
study 2017. Lancet. 395:709–733. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Foreman KJ, Marquez N, Dolgert A, Fukutaki
K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW,
et al: Forecasting life expectancy, years of life lost, and
all-cause and cause-specific mortality for 250 causes of death:
Reference and alternative scenarios for 2016-40 for 195 countries
and territories. Lancet. 392:2052–2090. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lin Z, Chen A, Cui H, Shang R, Su T, Li X,
Wang K, Yang J, Gao K, Lv J, et al: Renal tubular epithelial cell
necroptosis promotes tubulointerstitial fibrosis in patients with
chronic kidney disease. FASEB J. 36:e226252022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhu Y, Cui H, Xia Y and Gan H:
RIPK3-mediated necroptosis and apoptosis contributes to renal
tubular cell progressive loss and chronic kidney disease
progression in rats. PLoS One. 11:e01567292016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Pang Q, Wang P, Pan Y, Dong X, Zhou T,
Song X and Zhang A: Irisin protects against vascular calcification
by activating autophagy and inhibiting NLRP3-mediated vascular
smooth muscle cell pyroptosis in chronic kidney disease. Cell Death
Dis. 13:2832022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Xia Z, Wei Z, Li X, Liu Y, Gu X, Huang S,
Zhang X and Wang W: C/EBPα aggravates renal fibrosis in CKD through
the NOX4-ROS-apoptosis pathway in tubular epithelial cells. Biochim
Biophys Acta Mol Basis Dis. 1870:1670392024. View Article : Google Scholar
|
|
142
|
Liu X, Liu Z, Wang C, Miao J, Zhou S, Ren
Q, Jia N, Zhou L and Liu Y: Kidney tubular epithelial cells control
interstitial fibroblast fate by releasing TNFAIP8-encapsulated
exosomes. Cell Death Dis. 14:6722023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Huang F, Wang Q, Guo F, Zhao Y, Ji L, An
T, Song Y, Liu Y, He Y and Qin G: FoxO1-mediated inhibition of
STAT1 alleviates tubulointerstitial fibrosis and tubule apoptosis
in diabetic kidney disease. EBioMedicine. 48:491–504. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cao Y, Chen Z, Hu J, Feng J, Zhu Z, Fan Y,
Lin Q and Ding G: Mfn2 regulates high glucose-induced MAMs
dysfunction and apoptosis in podocytes via PERK pathway. Front Cell
Dev Biol. 9:7692132021. View Article : Google Scholar
|
|
145
|
Wang Y, Yu L, Li Y, Cha S, Shi L, Wang J,
Ge F, Huang C, Huang H, Tu Y, et al: Supplemented gegen qinlian
decoction formula attenuates podocyte mitochondrial fission and
renal fibrosis in diabetic kidney disease by inhibiting
TNF-α-mediated necroptosis, compared with empagliflozin. J
Ethnopharmacol. 334:1185722024. View Article : Google Scholar
|
|
146
|
Yu Q, Chen Y, Zhao Y, Huang S, Xin X,
Jiang L, Wang H, Wu W, Qu L, Xiang C, et al: Nephropathy is
aggravated by fatty acids in diabetic kidney disease through
tubular epithelial cell necroptosis and is alleviated by an RIPK-1
inhibitor. Kidney Dis (Basel). 9:408–423. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Shi Y, Chen X, Huang C and Pollock C:
RIPK3: A new player in renal fibrosis. Front Cell Dev Biol.
8:5022020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Hu B, Ma K, Wang W, Han Z, Chi M, Nasser
MI and Liu C: Research progress of pyroptosis in renal diseases.
Curr Med Chem. 31:6656–6671. 2024. View Article : Google Scholar
|
|
149
|
Liu P, Zhang Z and Li Y: Relevance of the
pyroptosis-related inflammasome pathway in the pathogenesis of
diabetic kidney disease. Front Immunol. 12:6034162021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang WT, Ge HW, Wei Y, Gao JL, Tian F and
Zhou EC: Molecular characterization of PANoptosis-related genes in
chronic kidney disease. PLoS One. 19:e03126962024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Huang SC, Zhang L, Sung YS, Chen CL,
Krausz T, Dickson BC, Kao YC, Agaram NP, Fletcher CD and Antonescu
CR: Frequent FOS gene rearrangements in epithelioid hemangioma: A
molecular study of 58 cases with morphologic reappraisal. Am J Surg
Pathol. 39:1313–1321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Durchdewald M, Angel P and Hess J: The
transcription factor Fos: A Janus-type regulator in health and
disease. Histol Histopathol. 24:1451–1461. 2009.PubMed/NCBI
|
|
153
|
Huang YS, Lo CH, Tsai PH, Hou YC, Chang
YT, Guo CY, Hsieh HY, Lu KC, Shih HM and Wu CC: Downregulation of
AANAT by c-Fos in tubular epithelial cells with membranous
nephropathy. Biochem Biophys Res Commun. 584:32–38. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wang F, Wang S, Wang J, Huang K, Chen G,
Peng Y, Liu C and Tao Y: Pharmacological mechanisms of Fuzheng
Huayu formula for Aristolochic acid I-induced kidney fibrosis
through network pharmacology. Front Pharmacol. 13:10568652022.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yang G, Wang M, Qahar M, He J, Lai Z, Li
S, He D, Yan X, Xiong Z, Xiong Z and Le TH: Post-burns persistent
inflammation leads to kidney PANoptosis with caspases pathway
activation. Heliyon. 11:e414852024. View Article : Google Scholar
|
|
156
|
Lv Z, Hu J, Su H, Yu Q, Lang Y, Yang M,
Fan X, Liu Y, Liu B, Zhao Y, et al: TRAIL induces podocyte
PANoptosis via death receptor 5 in diabetic kidney disease. Kidney
Int. 107:317–331. 2025. View Article : Google Scholar
|
|
157
|
Zhang HR, Li YP, Shi ZJ, Liang QQ, Chen
SY, You YP, Yuan T, Xu R, Xu LH, Ouyang DY, et al: Triptolide
induces PANoptosis in macrophages and causes organ injury in mice.
Apoptosis. 28:1646–1665. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Xu C, Wang Q, Du C, Chen L, Zhou Z, Zhang
Z, Cai N, Li J, Huang C and Ma T: Histone deacetylase-mediated
silencing of PSTPIP2 expression contributes to aristolochic acid
nephropathy-induced PANoptosis. Br J Pharmacol. 181:1452–1473.
2024. View Article : Google Scholar
|
|
159
|
Yuan F, Cai J, Wu J, Tang Y, Zhao K, Liang
F, Li F, Yang X, He Z, Billiar TR, et al: Z-DNA binding protein 1
promotes heatstroke-induced cell death. Science. 376:609–615. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Sanz AB, Sanchez-Niño MD, Ramos AM and
Ortiz A: Regulated cell death pathways in kidney disease. Nat Rev
Nephrol. 19:281–299. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Ruiz-Ortega M, Rayego-Mateos S, Lamas S,
Ortiz A and Rodrigues-Diez RR: Targeting the progression of chronic
kidney disease. Nat Rev Nephrol. 16:269–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Weisel K, Berger S, Thorn K, Taylor PC,
Peterfy C, Siddall H, Tompson D, Wang S, Quattrocchi E, Burriss SW,
et al: A randomized, placebo-controlled experimental medicine study
of RIPK1 inhibitor GSK2982772 in patients with moderate to severe
rheumatoid arthritis. Arthritis Res Ther. 23:852021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Weisel K, Scott N, Berger S, Wang S, Brown
K, Powell M, Broer M, Watts C, Tompson DJ, Burriss SW, et al: A
randomised, placebo-controlled study of RIPK1 inhibitor GSK2982772
in patients with active ulcerative colitis. BMJ Open Gastroenterol.
8:e0006802021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Shiffman M, Freilich B, Vuppalanchi R,
Watt K, Chan JL, Spada A, Hagerty DT and Schiff E: Randomised
clinical trial: emricasan versus placebo significantly decreases
ALT and caspase 3/7 activation in subjects with non-alcoholic fatty
liver disease. Aliment Pharmacol Ther. 49:64–73. 2019. View Article : Google Scholar
|
|
165
|
Ludbrook VJ, Budd DC, Thorn K, Tompson D,
Votta BJ, Walker L, Lee A, Chen X, Peppercorn A and Loo WJ:
Inhibition of receptor-interacting protein kinase 1 in chronic
plaque psoriasis: A multicenter, randomized, double-blind,
placebo-controlled study. Dermatol Ther (Heidelb). 14:489–504.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Weisel K, Berger S, Papp K, Maari C,
Krueger JG, Scott N, Tompson D, Wang S, Simeoni M, Bertin J and
Peter Tak P: Response to inhibition of receptor-interacting protein
kinase 1 (RIPK1) in active plaque psoriasis: A randomized
placebo-controlled study. Clin Pharmacol Ther. 108:808–816. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Karki R, Sundaram B, Sharma BR, Lee S,
Malireddi RKS, Nguyen LN, Christgen S, Zheng M, Wang Y, Samir P, et
al: ADAR1 restricts ZBP1-mediated immune response and PANoptosis to
promote tumorigenesis. Cell Rep. 37:1098582021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Oh S, Lee J, Oh J, Yu G, Ryu H, Kim D and
Lee S: Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation
and assembly drive PANoptosis. Cell Mol Immunol. 20:1513–1526.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Huang R, Hu Y, Wang YF, Zhang S, Wang ZG,
Pang DW and Liu SL: Targeted degradation of ZBP1 with covalent
PROTACs for anti-inflammatory treatment of infections. Angew Chem
Int Ed Engl. 64:e2024235242025. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang T, Yin C, Fedorov A, Qiao L, Bao H,
Beknazarov N, Wang S, Gautam A, Williams RM, Crawford JC, et al:
ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven
necroptosis. Nature. 606:594–602. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Yang W, Tao K, Wang Y, Huang Y, Duan C,
Wang T, Li C, Zhang P, Yin Y, Gao J and Li R: Necrosulfonamide
ameliorates intestinal inflammation via inhibiting GSDMD-medicated
pyroptosis and MLKL-mediated necroptosis. Biochem Pharmacol.
206:1153382022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Green JP, El-Sharkawy LY, Roth S, Zhu J,
Cao J, Leach AG, Liesz A, Freeman S and Brough D: Discovery of an
inhibitor of DNA-driven inflammation that preferentially targets
the AIM2 inflammasome. iScience. 26:1067582023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lin Q, Li S, Jiang N, Jin H, Shao X, Zhu
X, Wu J, Zhang M, Zhang Z, Shen J, et al: Inhibiting NLRP3
inflammasome attenuates apoptosis in contrast-induced acute kidney
injury through the upregulation of HIF1A and BNIP3-mediated
mitophagy. Autophagy. 17:2975–2990. 2021. View Article : Google Scholar :
|
|
174
|
Li C, Yang H, Wu Y, Zhou M, Luo H, Yuan P
and Shen F: Carnosol alleviates cisplatin-induced acute kidney
injury by regulating apoptosis and pyroptosis. Cell Biol Int.
49:101–117. 2025. View Article : Google Scholar
|
|
175
|
Chen L, Fang H, Li X, Yu P, Guan Y, Xiao
C, Deng Z, Hei Z, Chen C and Luo C: Connexin32 gap junction
channels deliver miR155-3p to mediate pyroptosis in renal
ischemia-reperfusion injury. Cell Commun Signal. 22:1212024.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Li J, Lin Q, Shao X, Li S, Zhu X, Wu J,
Mou S, Gu L, Wang Q, Zhang M, et al: HIF1α-BNIP3-mediated mitophagy
protects against renal fibrosis by decreasing ROS and inhibiting
activation of the NLRP3 inflammasome. Cell Death Dis. 14:2002023.
View Article : Google Scholar
|
|
177
|
Ji Y, Hua H, Jia Z, Zhang A and Ding G:
Therapy targeted to the NLRP3 inflammasome in chronic kidney
disease. Kidney Dis (Basel). 10:369–383. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Østergaard JA, Jha JC, Sharma A, Dai A,
Choi JSY, de Haan JB, Cooper ME and Jandeleit-Dahm K: Adverse renal
effects of NLRP3 inflammasome inhibition by MCC950 in an
interventional model of diabetic kidney disease. Clin Sci (Lond).
136:167–180. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Wu M, Yang Z, Zhang C and Shi Y, Han W,
Song S, Mu L, Du C and Shi Y: Inhibition of NLRP3 inflammasome
ameliorates podocyte damage by suppressing lipid accumulation in
diabetic nephropathy. Metabolism. 118:1547482021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Chen YM, Tang BX, Chen WY and Zhao MS:
Ursolic acid inhibits the invasiveness of A498 cells via NLRP3
inflammasome activation. Oncol Lett. 20:1702020. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Klück V, Jansen TLTA, Janssen M,
Comarniceanu A, Efdé M, Tengesdal IW, Schraa K, Cleophas MCP,
Scribner CL, Skouras DB, et al: Dapansutrile, an oral selective
NLRP3 inflammasome inhibitor, for treatment of gout flares: An
open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet
Rheumatol. 2:e270–e280. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Yin P, Jia J, Li J, Song Y, Zhang Y and
Chen F: ABT-737, a Bcl-2 selective inhibitor, and chloroquine
synergistically kill renal cancer cells. Oncol Res. 24:65–72. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Sun H, Wang W, Che Y and Jiang X: Fungal
secondary metabolites rasfonin induces autophagy, apoptosis and
necroptosis in renal cancer cell line. Mycology. 7:81–87. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Ding Z, Zhao J, Wang X, Li W, Chen C, Yong
C, Zhu Y, Tian F, Liu L, Yu M, et al: Total extract of Abelmoschus
manihot L. alleviates uric acid-induced renal tubular epithelial
injury via inhibition of caspase-8/caspase-3/NLRP3/GSDME signaling.
Front Pharmacol. 13:9079802022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Li X, Hu D, Li Y, Luo Y, Liang B, Yu K,
Xiong W and Zuo D: Overexpression of TP53INP2 promotes apoptosis in
clear cell renal cell cancer via caspase-8/TRAF6 signaling pathway.
J Immunol Res. 2022:12604232022.PubMed/NCBI
|
|
186
|
Gautam A, Boyd DF, Nikhar S, Zhang T,
Siokas I, Van de Velde LA, Gaevert J, Meliopoulos V, Thapa B,
Rodriguez DA, et al: Necroptosis blockade prevents lung injury in
severe influenza. Nature. 628:835–843. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Abou Taha MA, Ali FEM, Saleh IG and Akool
ES: Sorafenib and edaravone protect against renal fibrosis induced
by unilateral ureteral obstruction via inhibition of oxidative
stress, inflammation, and RIPK-3/MLKL pathway. Naunyn Schmiedebergs
Arch Pharmacol. 397:8961–8977. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Hou Y, Feng Q, Wei C, Cao F, Liu D, Pan S,
Shi Y, Liu Z and Liu F: Emerging role of PANoptosis in kidney
diseases: Molecular mechanisms and therapeutic opportunities.
Apoptosis. 30:579–596. 2025. View Article : Google Scholar : PubMed/NCBI
|