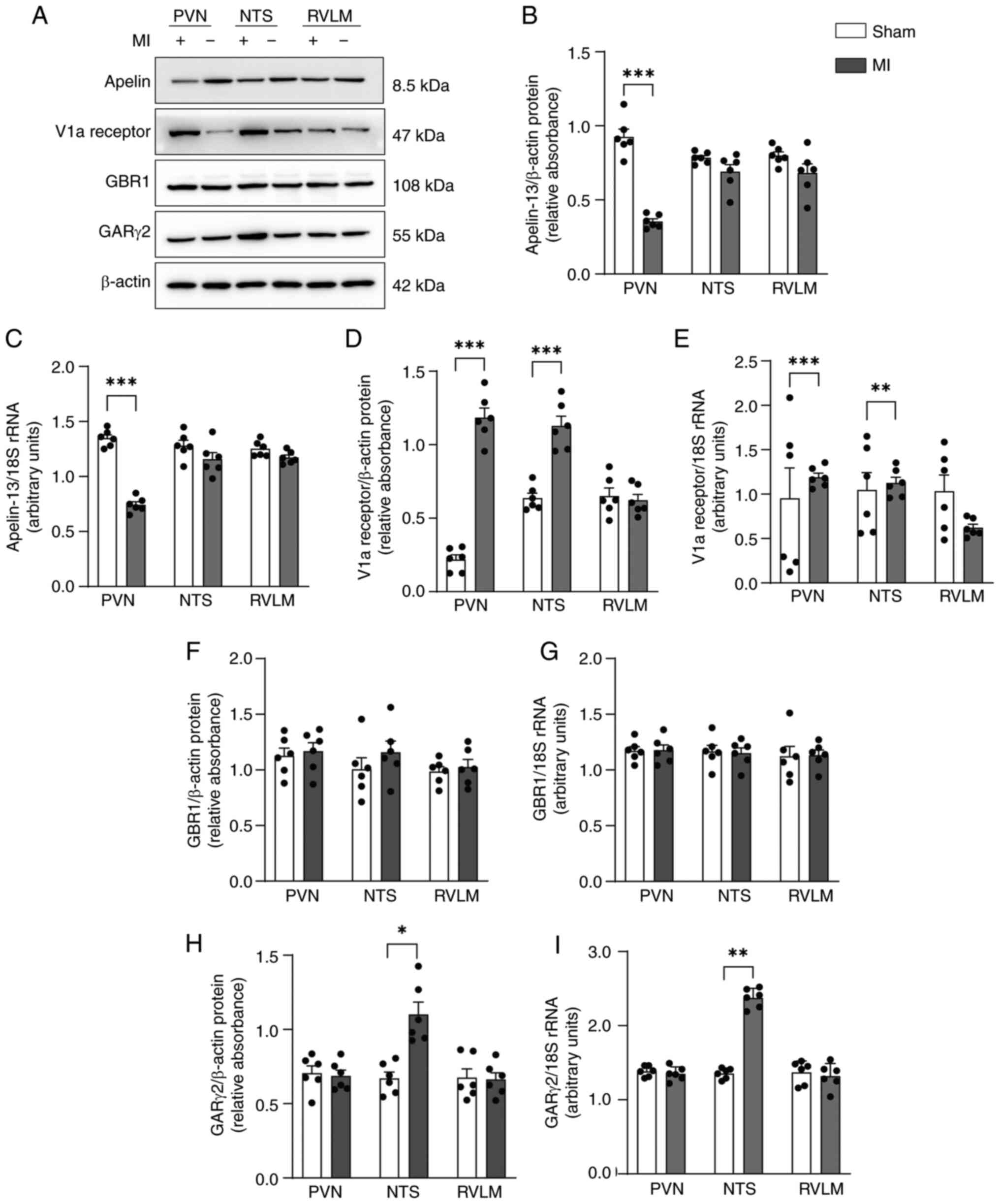
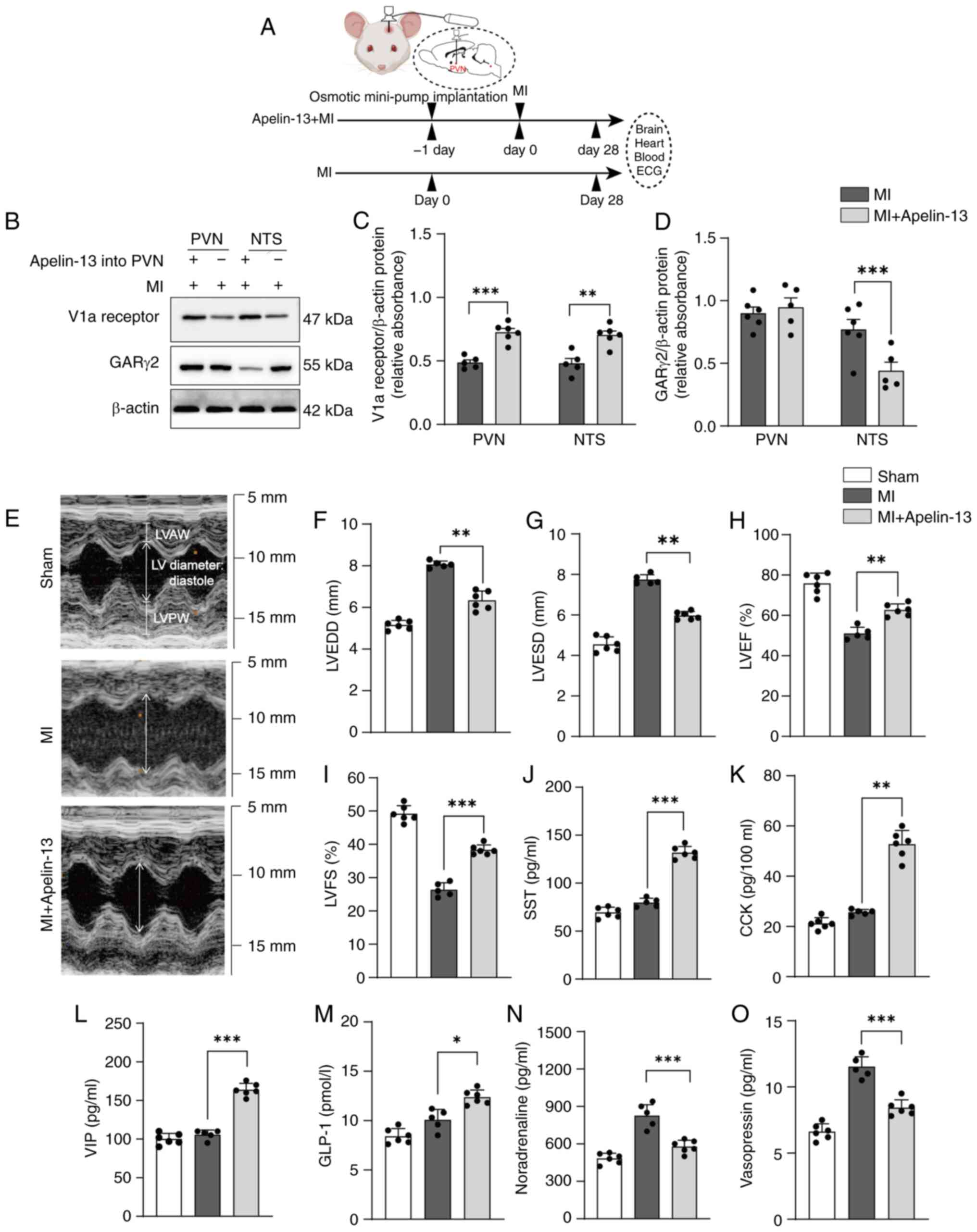
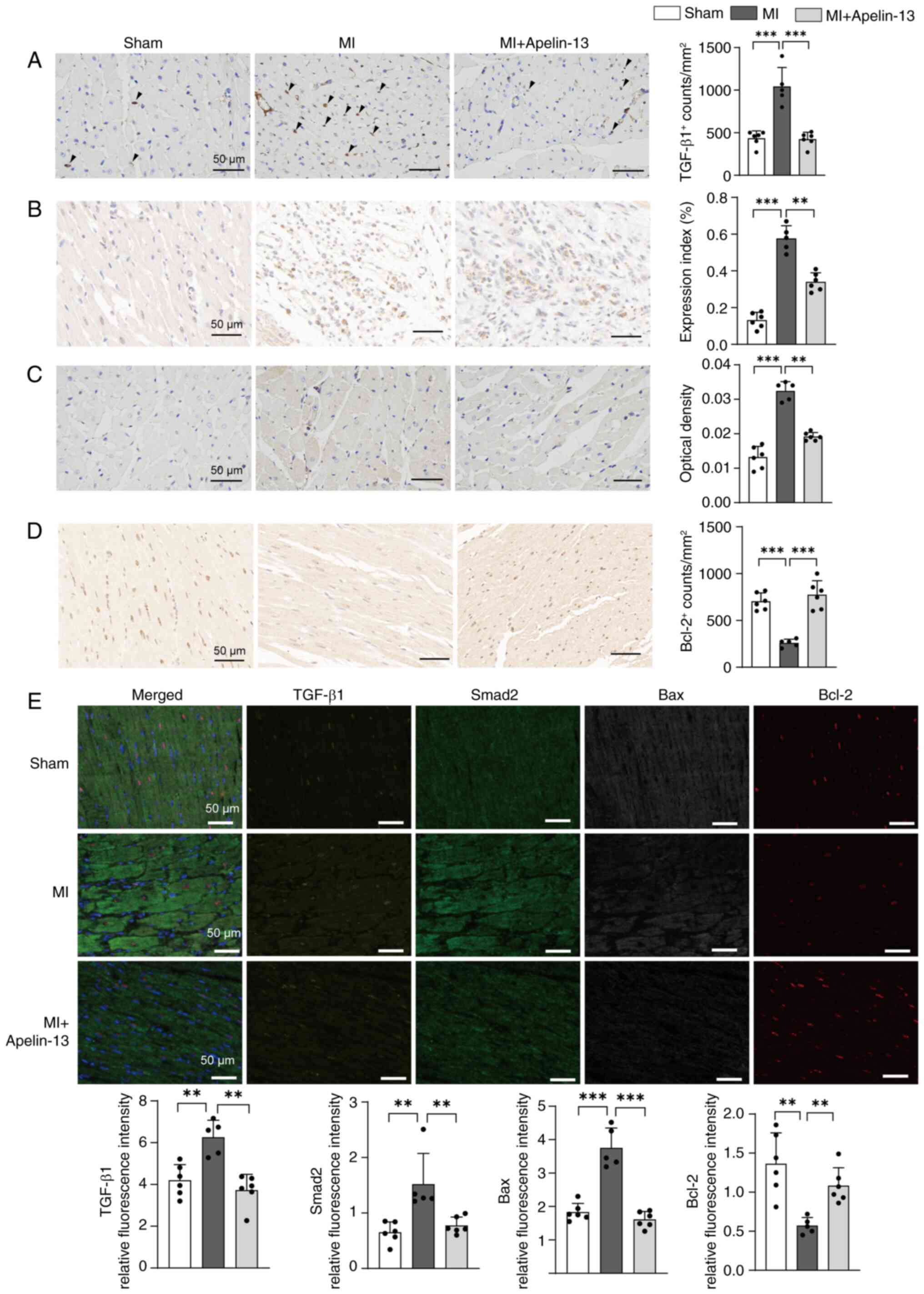
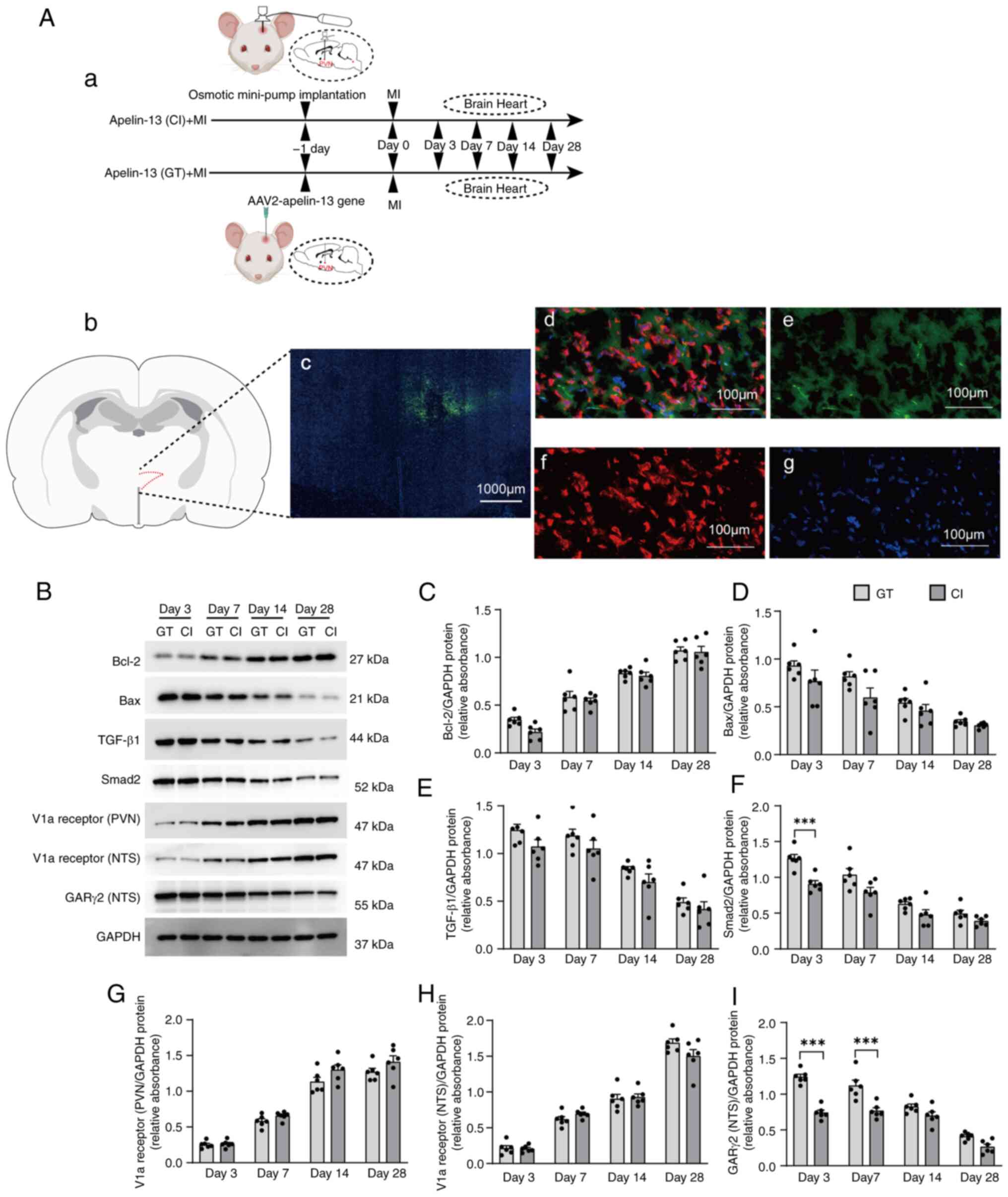
|
1
|
Chen Z, Venkat P, Seyfried D, Chopp M, Yan
T and Chen J: Brain-heart interaction: Cardiac complications after
stroke. Circ Res. 121:451–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simats A, Sager H and Liesz A: Heart brain
axis in health and disease: Role of innate and adaptive immunity.
Cardiovasc Res. 120:2325–2335. 2025. View Article : Google Scholar
|
|
3
|
Zhao B, Li T, Fan Z, Yang Y, Shu J, Yang
X, Wang X, Luo T, Tang J, Xiong D, et al: Heart-brain connections:
Phenotypic and genetic insights from magnetic resonance images.
Science. 380:abn65982023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herman JP and Tasker JG: Paraventricular
hypothalamic mechanisms of chronic stress adaptation. Front
Endocrinol (Lausanne). 7:1372016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benarroch EE: The central autonomic
network: Functional organization, dysfunction, and perspective.
Mayo Clin Proc. 68:988–1001. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Infanger DW, Cao X, Butler SD, Burmeister
MA, Zhou Y, Stupinski JA, Sharma RV and Davisson RL: Silencing nox4
in the paraventricular nucleus improves myocardial
infarction-induced cardiac dysfunction by attenuating
sympathoexcitation and peri-infarct apoptosis. Circ Res.
106:1763–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee DK, Cheng R, Nguyen T, Fan T,
Kariyawasam AP, Liu Y, Osmond DH, George SR and O'Dowd BF:
Characterization of apelin, the ligand for the APJ receptor. J
Neurochem. 74:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang N, Li T, Cheng J, Tuo Q and Shen J:
Role of apelin/APJ system in hypothalamic-pituitary axis. Clin Chim
Acta. 499:149–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv SY, Chen WD and Wang YD: The apelin/APJ
system in psychosis and neuropathy. Front Pharmacol. 11:3202020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masaki T, Yasuda T and Yoshimatsu H:
Apelin-13 microinjection into the paraventricular nucleus increased
sympathetic nerve activity innervating brown adipose tissue in
rats. Brain Res Bull. 87:540–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji M, Wang Q, Zhao Y, Shi L, Zhou Z and Li
Y: Targeting hypertension: superoxide anions are involved in apelin
induced long-term high blood pressure and sympathetic activity in
the paraventricular nucleus. Curr Neurovasc Res. 16:455–464. 2019.
View Article : Google Scholar
|
|
13
|
Zhang Q, Yao F, Raizada MK, O'Rourke ST
and Sun C: Apelin gene transfer into the rostral ventrolateral
medulla induces chronic blood pressure elevation in normotensive
rats. Circ Res. 104:1421–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Mota N, Reaux-Le Goazigo A, El Messari
S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F and
Llorens-Cortes C: Apelin, a potent diuretic neuropeptide
counteracting vasopressin actions through inhibition of vasopressin
neuron activity and vasopressin release. Proc Natl Acad Sci USA.
101:10464–10469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griffiths PR, Lolait SJ, Harris LE, Paton
JFR and O'Carroll AM: Vasopressin V1a receptors mediate the
hypertensive effects of [Pyr1]apelin-13 in the rat rostral
ventrolateral medulla. J Physiol. 595:3303–3318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Czarzasta K, Cudnoch-Jedrzejewska A,
Szczepanska-Sadowska, Fus L, Puchalska L, Gondek A, Dobruch J,
Gomolka R, Wrzesien R, Zera T, et al: The role of apelin in central
cardiovascular regulation in rats with post-infarct heart failure
maintained on a normal fat or high fat diet. Clin Exp Pharmacol
Physiol. 43:983–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuriyama K, Hirouchi M and Kimura H:
Neurochemical and molecular pharmacological aspects of the GABA(B)
receptor. Neurochem Res. 25:1233–1239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Liu Q, Xuan C, Guo L, Shi R, Zhang
Q, O'Rourke ST, Liu K and Sun C: GABAB receptor gene transfer into
the nucleus tractus solitarii induces chronic blood pressure
elevation in normotensive rats. Circ J. 77:2558–2566. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dampney RA: Functional organization of
central pathways regulating the cardiovascular system. Physiol Rev.
74:323–364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korpal AK, Han SY, Schwenke DO and Brown
CH: A switch from GABA inhibition to excitation of vasopressin
neurons exacerbates the development angiotensin II-dependent
hypertension. J Neuroendocrinol. Dec 9–2017.Epub ahead of print.
PubMed/NCBI
|
|
21
|
Klok MD, Jakobsdottir S and Drent ML: The
role of leptin and ghrelin in the regulation of food intake and
body weight in humans: A review. Obes Rev. 8:21–34. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gorky J and Schwaber J: Conceptualization
of a parasympathetic endocrine system. Front Neurosci. 13:10082019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Watson C and Paxinos G: The rat brain in
stereotaxic coordinates. 5th ed. Amsterdam: Elsevier; 2004, pp.
51–57
|
|
25
|
Zhao Y, Li Y, Li Z, Xu B, Chen P and Yang
X: Superoxide anions modulate the performance of apelin in the
paraventricular nucleus on sympathetic activity and blood pressure
in spontaneously hypertensive rats. Peptides. 121:1700512019.
View Article : Google Scholar
|
|
26
|
Kc P, Balan KV, Tjoe SS, Martin RJ,
Lamanna JC, Haxhiu MA and Dick TE: Increased vasopressin
transmission from the paraventricular nucleus to the rostral
medulla augments cardiorespiratory outflow in chronic intermittent
hypoxia-conditioned rats. J Physiol. 588:725–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benarroch EE: Paraventricular nucleus,
stress response, and cardiovascular disease. Clin Auton Res.
15:254–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Havakuk O, King KS, Grazette L, Yoon AJ,
Fong M, Bregman N, Elkayam U and Kloner RA: Heart failure-induced
brain injury. J Am Coll Cardiol. 69:1609–1616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silvani A, Calandra-Buonaura G, Dampney RA
and Cortelli P: Brain-heart interactions: Physiology and clinical
implications. Philos Trans A Math Phys Eng Sci.
374:201501812016.PubMed/NCBI
|
|
30
|
Coote JH: Myths and realities of the
cardiac vagus. J Physiol. 591:4073–4085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JJ and Hibbs RE: Direct structural
insights into GABAA receptor pharmacology. Trends Biochem Sci.
46:502–517. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Komnenov D, Quaal H and Rossi NF: V1a and
V1b vasopressin receptors within the paraventricular nucleus
contribute to hypertension in male rats exposed to chronic mild
unpredictable stress. Am J Physiol Regul Integr Comp Physiol.
320:R213–R225. 2021. View Article : Google Scholar
|
|
33
|
Dalzell JR, Rocchiccioli JP, Weir RA,
Jackson CE, Padmanabhan N, Gardner RS, Petrie MC and McMurray JJ:
The emerging potential of the apelin-APJ system in heart failure. J
Card Fail. 21:489–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vörös I, Sághy É, Pohóczky K, Makkos A,
Onódi Z, Brenner GB, Baranyai T, Ágg B, Váradi B, Kemény Á, et al:
Somatostatin and its receptors in myocardial ischemia/reperfusion
injury and cardioprotection. Front Pharmacol. 12:6636552021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han Z, Bi S, Xu Y, Dong X, Mei L, Lin H
and Li X: Cholecystokinin expression in the development of
myocardial hypertrophy. Scanning. 2021:82315592021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simms-Williams N, Treves N, Yin H, Lu S,
Yu O, Pradhan R, Renoux C, Suissa S and Azoulay L: Effect of
combination treatment with glucagon-like peptide-1 receptor
agonists and sodium-glucose cotransporter-2 inhibitors on incidence
of cardiovascular and serious renal events: Population based cohort
study. BMJ. 385:e0782422024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duggan KA, Hodge G, Chen J and Hunter T:
Vasoactive intestinal peptide infusion reverses existing myocardial
fibrosis in the rat. Eur J Pharmacol. 862:1726292019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sarker M, Chowdhury N, Bristy AT, Emran T,
Karim R, Ahmed R, Shaki MM, Sharkar SM, Sayedur Rahman GM and Reza
HM: Astaxanthin protects fludrocortisone acetate-induced cardiac
injury by attenuating oxidative stress, fibrosis, and inflammation
through TGF-β/Smad signaling pathway. Biomed Pharmacother.
181:1177032024. View Article : Google Scholar
|
|
39
|
Liu H, Yu L, Yang L and Green MS:
Vasoplegic syndrome: An update on perioperative considerations. J
Clin Anesth. 40:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu Q, Yu Q, Luo H, Liu Z, Ma X, Wang H and
Cheng Z: Protective effects of wogonin in the treatment of central
nervous system and degenerative diseases. Brain Res Bull.
221:1112022025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Y, Donovan M, Jia X and Wang Z: The
ventromedial hypothalamic circuitry and male alloparental behaviour
in a socially monogamous rodent species. Eur J Neurosci.
50:3689–3701. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Loveland JL and Fernald RD: Differential
activation of vasotocin neurons in contexts that elicit aggression
and courtship. Behav Brain Res. 317:188–203. 2017. View Article : Google Scholar
|
|
43
|
Yan R, Liu Q, Zhang D, Li K, Li Y, Nie Y,
Zhang Y, Li P, Mao S and Li H: Inhibition of spreading
depolarizations by targeting GABAA receptors and voltage-gated
sodium channels improves neurological deficits in rats with
traumatic brain injury. Br J Pharmacol. Jun 24–2025.Epub ahead of
print. View Article : Google Scholar
|
|
44
|
Greenwood M, Gillard BT, Murphy D and
Greenwood MP: Dimerization of hub protein DYNLL1 and bZIP
transcription factor CREB3L1 enhances transcriptional activation of
CREB3L1 target genes like arginine vasopressin. Peptides.
179:1712692024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Szczepanska-Sadowska E, Czarzasta K,
Bogacki-Rychlik W and Kowara M: The interaction of vasopressin with
hormones of the hypothalamo-pituitary-adrenal axis: The
significance for therapeutic strategies in cardiovascular and
metabolic diseases. Int J Mol Sci. 25:73942024. View Article : Google Scholar : PubMed/NCBI
|