Introduction
Brain-heart crosstalk is now referred to as
neurocardiology, which addresses the effects of brain injury on the
heart or the effects of cardiac injury on the brain (1). There is increasing experimental and
clinical evidence not only suggesting a causal link from the heart
to the brain but also indicating the presence of a bidirectional
relationship, highlighting the powerful influence of the brain on
the heart (2).
Hypothalamus-pituitary-adrenal (HPA) axis activation
plays a pivotal role in communication between the brain and heart,
leading to cardiac dysfunction (3). As the primary integrator of this
axis, the paraventricular nucleus (PVN) of the hypothalamus acts as
the primary integrator that modulates HPA axis activity, which can
increase blood pressure, alter heart rate and promote inflammation
in the cardiovascular system (4). The PVN is also a master controller
of the autonomic nervous system, which provides specialized
innervation to all autonomic relay centers (5), projecting to the rostral
ventrolateral medulla (RVLM) and spinal cord to regulate
sympathetic outflow (6).
Notably, a reduction in PVN activity promotes the improved recovery
of cardiac function after myocardial infarction (6).
Apelin, containing 77 amino acids, has been isolated
from bovine stomach extracts and its effects are mediated via
ligands for the orphan G-protein-coupled (APJ) receptor (7,8).
Apelin-13 is the main isoform that activates the APJ receptor. As a
novel regulator of activity, the apelin/APJ system is involved in
neurohumoral regulation of the HPA (9) and emerging evidence indicates that
apelin-13 and the APJ receptor are widely expressed in microglia
and neurons of the central nervous system (CNS) (10). Microinjection of apelin-13 into
the PVN increases c-Fos-like immunoreactivity in the PVN after 1.5
h (11), while chronic infusion
of apelin-13 in the PVN induces long-term hypertension and
sympathetic activation mediated by superoxide anions (12). Based on previous data, apelin-13
may affect the cardiovascular system via the vasopressinergic
system in the PVN and supraoptic nucleus of the hypothalamus
(13-15). A reduction in APJ mRNA expression
has been observed in the hypothalamus in postinfarct heart failure,
especially in presynaptic neurons of the PVN, which could attenuate
the pressor response to intracerebroventricularly infused apelin-13
(16).
γ-Aminobutyric acid (GABA) is a well-known neuronal
transmitter that exerts inhibitory effects on the brain via the
GABAA receptor (GAR) and GABAB receptor
(GBR), which have been defined on the basis of pharmacological and
physiological research (17).
According to our previous study, the GABAergic system in the
medullary nucleus tractus solitarii (NTS) contributes to the
central resetting of blood pressure and the development of
hypertension (18). NTS is
reciprocally connected with the pontine parabrachial and
Kölliker-Fuse nuclei to relay visceral afferent information to
other central autonomic network structures (19). Baroreceptor activation inhibits
vasopressin secretion, which might be regulated by the activation
of GABAergic projections to vasopressinergic neurons and the local
administration of a GAR antagonist inhibits vasopressinergic
neurons during the onset of hypertension (20). Thus, the apelin/APJ system,
vasopressinergic system and GABAergic system are vital pathways of
the CNS involved in the neural control of the cardiovascular system
in the hypothalamus, but they remain poorly understood in relation
to myocardial injury.
The parasympathetic endocrine system (PES) comprises
circulating peptides released from secretory cells that are
markedly modulated by vagal projections from the dorsal motor
nucleus of the vagus nerve (21). A total of four peptides present
in the circulatory system, isomatostatin (SST), cholecystokinin
(CCK), glucagon-like peptide 1 (GLP-1) and vasoactive intestinal
peptide (VIP), perform sympathetic antagonism to balance
sympathetic activation-induced pupillary dilation and increase the
rate and contractility of the heart (22).
The present study aimed to assess the effects of
apelin-13 in the PVN on the cardiac function of rats with
myocardial infarction (MI). The involved mechanisms, including
myocardial ischemia-related apoptotic and inflammatory pathway
markers in the heart and neuropeptides in the serum, were also
explored.
Materials and methods
All animal procedures were conducted in accordance
with the institutional guidelines of The First Hospital of Jilin
University and were approved by the Institutional Animal Care and
Use Committee (approval no. 2020-0128). All studies were approved
locally and conformed to the guidelines from Directive 2010/63/EU
of the European Parliament on the protection of animals used for
scientific purposes. The male Sprague-Dawley rats were purchased
from Charles River. A total of 246 male rats were used in the
present study (250-300 g; age, 8 weeks). The rats were maintained
under a 12-h light/dark cycle at a temperature of 20±2°C and a
relative humidity of 55±5%. Food and water were provided ad
libitum. The rat studies were performed under isoflurane
anesthesia (2.0-2.5% isoflurane, 100% oxygen at 2 l/min) in
spontaneous breathing. To perform histology studies and blood
tests, sodium pentobarbital (40-60 mg/kg) was intraperitoneally
injected to anesthetize the rats. The present study typically used
an initial dose of 40 mg/kg and occasionally administered
additional doses based on the condition of the rat. The rats were
then sacrificed by exsanguination while still under anesthesia.
Explanations of each reagent and buffer are presented in Appendix S1, Tables SI and SII.
Myocardial infarction (MI)
Male SD rats at 8 weeks of age were randomly
selected for MI modelling via left anterior descending (LAD) artery
ligation. Anesthesia was induced and maintained in the SD rats with
isoflurane (1-2% oxygen). Following anesthesia, the animals were
intubated and ventilated with a 16-gauge intravenous catheter and
placed in a supine position on a temperature control pad.
Left-sided thoracotomy was performed via a small incision between
the third and fourth intercostal spaces. The incision was expanded
by a blunt-ended retractor such that the lungs were not retracted.
The pericardial sac surrounding the heart was cut open to access
the heart. The heart was not exteriorized. Using a tapered
atraumatic needle, a 5-0 silk Prolene suture ligature was passed
underneath the LAD and tied with three knots. Visible blanching and
cyanosis of the anterior wall of the left ventricle and swelling of
the left atrium were indicative of successful ligation. Rats
subjected to cardiac exposure without coronary ligation served as
the sham-operated control group.
Western blot analysis
Micropunched NTS or PVN tissue from the brain
sections of the rats was treated with 1 ml lysis buffer,
homogenized for 15 sec, boiled for 3 min, ultrasonicated and
centrifuged. The supernatants were stored in a −80°C freezer.
Vasopressin 1a (V1a) receptor protein levels in
neuronal cultures were assessed using western blot analysis.
Cultures were washed with ice-cold PBS, scraped into lysis buffer
containing 8 μl/ml inhibitor cocktail and centrifuged at
10,000 × g for 10 min at 4°C. The supernatant was saved for the
protein assay.
Murine heart lysates were prepared by digesting
tissues in lysis buffer with protease and phosphatase inhibitors
and PMSF using a Qiagen Tissue Lyser (Qiagen GmbH; 50 rpm; 8 min;
4°C), followed by rocking for 1 h at 4°C, sonication on ice using
20 kHz in a pulse mode (3 sec on/5 sec off) for 10 cycles and
centrifugation at 12,000 × g for 20 min at 4°C, with supernatants
stored at −80°C.
The protein concentration was determined with a BCA
protein concentration assay kit. For each sample, 20 μg
protein was separated on a 10% SDS-PAGE gel and transferred to
nitrocellulose membranes (100 V, 2 h). Membranes were blocked in
PBS-T containing 10% milk and 1% bovine serum albumin (BSA) for 3
h, then incubated overnight at 4°C with primary antibodies
(anti-apelin polyclonal antibody, anti-GAR γ2 antibody,
anti-GABAB receptor1 antibody, anti-V1a receptor
antibody, anti-TGF-β1 antibody, anti-Smad2 antibody, anti-Bax
antibody and anti-Bcl-2 antibody; Table SI). After washing (15 min PBS-T
with 0.1% Tween followed by four 5-min PBS-T washes), the membranes
were incubated for 2 h at room temperature in horseradish
peroxidase-conjugated anti-rabbit or anti-mouse antibodies diluted
1:5,000. Immunoreactivity was detected by enhanced
chemiluminescence (Beyotime Institute of Biotechnology) and the
protein bands were analyzed with ImageJ (National Institutes of
Health; v.1.53q).
Reverse transcription-quantitative (RT-q)
PCR
The present study used RT-qPCR to detect changes in
the expression of apelin-13, V1a receptor, GBR1 and GARγ2 in the
PVN and NTS of the rats and in the neuronal cultures. The isolation
of total RNA from PVN and NTS tissue and neuronal cultures was
performed with a RNeasy Mini Kit (cat. no. 74104; Qiagen GmbH) in
accordance with the manufacturer's instructions. RNA purity and
concentration were determined spectrophotometrically (Nanodrop
2000c; Thermo Fisher Scientific, Inc.). For each RT-qPCR, 2
μg of total RNA was converted into cDNA with reverse
transcriptase. Genomic DNA was eliminated by DNase I. Real-time
PCRs were performed in a 10 μl total reaction volume using
96-well plates with the QuantiTect SYBR Green PCR Kit (cat. no.
208054; Qiagen GmbH) and the relative difference was expressed as
the fold change compared with control values, calculated using the
comparative cycle method (23).
The RT-qPCR system (ABI 7500; Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the following protocol: Initial denaturation
at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and
60°C for 34 sec. All steps adhered to the manufacturers' protocols
and each condition was assayed in three technical replicates. Data
were normalized to 18S rRNA. The sequences of the primers are
presented in Table SIII.
Brain nucleus microinjection
The rats were anaesthetized with a mixture of oxygen
and isoflurane (2-2.5%) via a nose cone. They were placed in a
stereotaxic frame (DW-2000, Chengdu Techman Co., Ltd.). The skin
overlying the midline of the skull was incised and a small hole was
drilled bilaterally on the dorsal surface of the cranium according
to the nucleus coordinates (24), including the PVN (bregma -1.92
mm, interaural 7.08 mm, midline 0.5, 5.8 mm ventral to the dura
mater) and NTS (bregma -13.68 mm, interaural -4.68 mm, midline 0.5,
8.0 mm ventral to the dura mater). A microsyringe was positioned in
the nucleus and controlled by a microsyringe pump controller.
Apelin gene transfer: The AAV2-apelin-13 virus or
AAV2-GFP (5×1012 GC/ml in 50 nl) was microinjected
unilaterally (right) into the PVN to induce endogenous apelin
expression.
Osmotic mini-pump implantation: A 28-gauge lower end
of a stainless-steel cannula (Alzet Brain Infusion Kit 2; Durect
Corporation) was implanted into the right NTS or PVN according to
the nucleus location aforementioned and then fixed on the skull
with dental cement. The upper end was connected to an osmotic
mini-pump for chronic continuous intranuclear infusion at 0.11
μl/h for 4 weeks. The pumps were filled with apelin-13 (100
μM; 100 μl) (25,13), a GAR agonist (muscimol; 100
μM, 100 μl) (18),
or a V1a receptor antagonist (SR49059; 200 μM, 100
μl) (26) and placed
subcutaneously on the backs of the rats.
Echocardiography
Echocardiography was performed under inhaled
anesthesia with isoflurane (1-2% oxygen) using a 14-MHz transducer
at a depth of 1 cm for two-dimensional imaging. M-mode images were
taken at the mid-papillary muscle level and were used for
measurements.
Blood sample tests
Blood collected in a serum separation tube was
allowed to clot at room temperature for 30 min before being
centrifuged at 1,000 × g for 15 min. The serum from each subject
was either used immediately for analysis or divided into aliquots
and stored at −20°C for future use. The levels of somatostatin,
cholecystokinin, glucagon-like peptide-1 and vasoactive intestinal
peptide in the serum were quantified using enzyme-linked
immunosorbent assay (ELISA) kits: Somatostatin (cat. no.
NBP2-80271; Novus Biologicals; Bio-Techne), cholecystokinin (cat.
no. EIAR-CCK-1; RayBiotech, Inc.), glucagon-like peptide-1 (cat.
no. E-EL-R3007-96T; Wuhan Elabscience Biotechnology Co., Ltd. Wuhan
Elabscience Biotechnology Co., Ltd.), and vasoactive intestinal
peptide (cat. no. NBP2-82466; Novus Biologicals; Bio-Techne). A
commercial arg8-Vasopressin ELISA kit (cat. no.
ADI-900-017A; Enzo Life Sciences) and a noradrenaline competitive
ELISA kit (cat. no. EEL010; Thermo Fisher Scientific, Inc.) were
used for the quantitative measurement of plasma vasopressin and
noradrenaline levels.
Cardiac immunohistochemistry and
multiplex fluorescent immunohistochemistry staining
To assess the alterations in TGF-β1, Smad2, Bax and
Bcl-2 expression in cardiac tissues, the heart samples were fixed
in 4% paraformaldehyde at 4°C for 24 h and embedded in paraffin.
Briefly, tissues were dehydrated in graded ethanol (70-100%),
cleared in xylene, infiltrated with paraffin wax at 60°C and
embedded in molds for solidification. All steps were performed with
agitation. To perform immunohistochemical staining, samples were
sectioned at 4-5 μm thickness. Following deparaffinization
and rehydration, antigen retrieval was conducted using citrate
buffer (pH 6.0) at 95°C for 20 min. After blocking endogenous
peroxidase activity with 3% H2O2 and
nonspecific binding sites with normal serum (cat. no. abs933;
Absin), sections were incubated with primary antibodies (TGF-β1,
Smad2, Bax and Bcl-2) overnight at 4°C. HRP-conjugated secondary
antibodies were then applied, followed by DAB development (room
temperature; 10 min) and hematoxylin counterstaining (room
temperature; 5 min). Finally, sections were dehydrated, cleared in
xylene and mounted for microscopic examination. The multiplex
fluorescent immunohistochemistry staining was performed on
4-μm paraffin-embedded cardiac sections using sequential
tyramide signal amplification. Following deparaffinization and
initial antigen retrieval in citrate buffer (pH 6.0), sections were
blocked and incubated with the first primary antibody at 4°C 16 h,
followed by HRP-conjugated secondary antibody and TSA570
development. Following microwave stripping in citrate buffer, the
cycle was repeated for Bax, SMAD2 and Bcl-2, with sodium azide
treatment between each round to inactivate residual HRP activity.
Nuclei were counterstained with DAPI (room temperature; 5 min) and
slides were mounted with anti-fade medium. Multispectral imaging
was performed using a Vectra system (AKOYA Vectra Polaris; AKOYA
Inform 2.6; Akoya Biosciences, Inc.), with spectral unmixing to
separate fluorophore signals.
Rat apelin-13 gene adeno-associated virus
type 2 (AAV2) production
The rat apelin-13 gene was synthesized by Wuhan
Beisai Model Biotechnology Co., Ltd. The CMV promoter partial
sequence and the mCherry partial sequence were connected to both
ends of the gene (Appendix S1).
The virus concentration was 5×1012 GC/ml. The
information concerning the apelin-13 gene from NCBI is described in
Appendix S1.
Primary neuron culture
Primary neuron cultures were prepared from brain of
1-day-old neonatal rats. The neonatal rats were euthanized via an
intraperitoneal injection of sodium pentobarbital at a dose of 150
mg/kg and washed with 70% ethanol. Brain tissue (from 5-7 rats) was
mechanically dissociated in Solution D by pipetting 10 times. Cells
were further dissociated with 0.25% trypsin and DNase I (496 U/ml),
resuspended in DMEM containing 10% PDHS and plated onto
poly-L-lysine-precoated (for at least 4 h) Nunc plastic tissue
culture dishes. Prior to plating, the cell suspension was diluted
to the required number of cells/unit volume (3×106
cells/2 ml) of medium per 35-mm dish. The cells were grown in a
humidified atmosphere with 95% O2 and 5% CO2
for 3 days, after which the culture medium was replaced with fresh
DMEM containing cytosine arabinoside (ARC; 1 μM) and 10%
PDHS. After 3 days, the ARC was removed and replaced with normal
medium for an additional 5-9 days before use.
Immunocytochemistry of neuronal
cultures
Neuronal cultures from newborn Sprague-Dawley rats
were washed briefly with Dulbecco's PBS three times and then fixed
with PBS containing 0.1% Tween 20 (PBS/Tween) and 4% formaldehyde
solution for 15 min at 4°C. After three brief PBS-Tween washes,
cells were blocked with 10% goat serum in PBS-Tween at 37°C for 30
min. The blocking solution was completely removed without
additional washing. Primary antibodies (rabbit anti-rat APJ or V1a
antibody) diluted in 1 ml PBS-Tween were added and incubated at 4°C
overnight. Following three 3-min PBS-Tween washes, neurons were
incubated with appropriate secondary antibodies for 1 h (Alexa
Fluor 488-conjugated goat anti-rabbit IgG; cat. no. 4412S; CST;
1:1,000) and washed three times (3 min each) with PBS-Tween. Nuclei
were stained with DAPI at room temperature for 5 min, washed four
times thoroughly, then mounted with anti-fade medium under glass
coverslips.
Masson's trichrome staining
To evaluate cardiac morphological changes and the
extent of cardiac fibrosis, heart tissues were fixed in 4%
paraformaldehyde for 24 h and embedded in paraffin. Cardiac
fibrosis was determined using Masson's trichrome staining of
4%-PFA-fixed, paraffin-embedded, 4-μm-thick heart
cross-sections. Briefly, dewaxed sections are treated with
potassium dichromate overnight, rinsed, then stained with Ponceau S
(5-10 min). After washing, slides are treated with phosphomolybdic
acid (1-3 min), directly stained with aniline blue (30 sec-2 min),
and differentiated in 1% acetic acid. Images were captured by light
microscopy for analysis. The fibrotic fraction was obtained by
calculating the ratio of blue (fibrotic) to total myocardial area
using ImageJ (v.1.53q; National Institutes of Health).
TUNEL staining
For apoptosis and α-actin co-staining,
formalin-fixed, paraffin-embedded heart sections were first
deparaffinized. Antigen retrieval was performed using the steamer
method in citric acid antigen repair buffer (pH 6.0), followed by
blocking with 3% goat serum at room temperature for 30 min. The
sections were incubated with terminal deoxyribonucleotidyl
transferase (TdT) mixed with deoxyuridine triphosphate (dUTP; 1:10)
at 4°C overnight. The following day, the sections were washed with
PBS three times and then nuclei were stained with DAPI for 10 min
at room temperature. After three washes with PBS, the sections were
mounted with an anti-fade mounting medium.
Triphenyltetrazolium chloride (TTC)
staining
After anaesthetizing the rats, heart tissue was
collected immediately and cold PBS was used to perfuse the left
ventricle within 5 min. The heart was then stored at −20°C for
20-30 min. Each heart was sectioned into five 2-mm transverse
slices, which were stained with preheated TTC solution for 15-30
min at 37°C. Next, the sections were fixed in 4% formaldehyde (room
temperature; 24 h) and images of the TTC-stained sections were
captured. The cardiac infarction area was pale and normal cardiac
tissue was red. The infarct size was quantified as the total
infarct area divided by the area at risk using ImageJ ((v.1.53q;
National Institutes of Health).
Statistical analysis
The number of samples (n) used in each experiment is
indicated in the legends or shown in the figures and indicates
biological replicates. The results are presented as the mean ±
standard deviation (SD) or standard error of the mean (SEM). The
normality of the data was assessed using the Shapiro-Wilk test. For
datasets with a normal distribution, statistical analyses were
performed via an unpaired two-tailed t test or one-way analysis of
variance (ANOVA) with Tukey's multiple-comparisons test and
Dunnett's multiple-comparisons test. Two groups were statistically
compared via the unpaired Mann-Whitney U test if the datasets did
not pass the normality test. Statistical analyses were performed
using PRISM (GraphPad Software Inc.; Dotmatics; version 10).
Post-hoc power analysis was conducted for all key statistical
comparisons using the observed effect sizes and variances via the
WebPower platform (webpower.psychstat.org). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of apelin-13, the V1a receptor
and the GARγ2 receptor in MI model rats
On day 28 following MI, apelin-13 expression was
markedly lower at both the protein and mRNA levels in the PVN but
not in the NTS or RVLM in MI model rats compared with sham rats
(Fig. 1A-C). V1a receptor
expression was increased in the PVN and NTS but not in the RVLM at
both the protein and mRNA levels (Fig. 1A, D and E). GARγ2 was higher in
the NTS but not in the PVN or RVLM in MI model rats compared with
sham control rats (Fig. 1A, H and
I). By contrast, GBR1 expression was not markedly affected
(Fig. 1A, F and G). These
findings indicated that apelin-13 expression was downregulated in
the PVN of MI rats and suggested that both V1a receptors and GARγ2
may play a role in central nervous system regulation of MI within
the PVN and nucleus NTS.
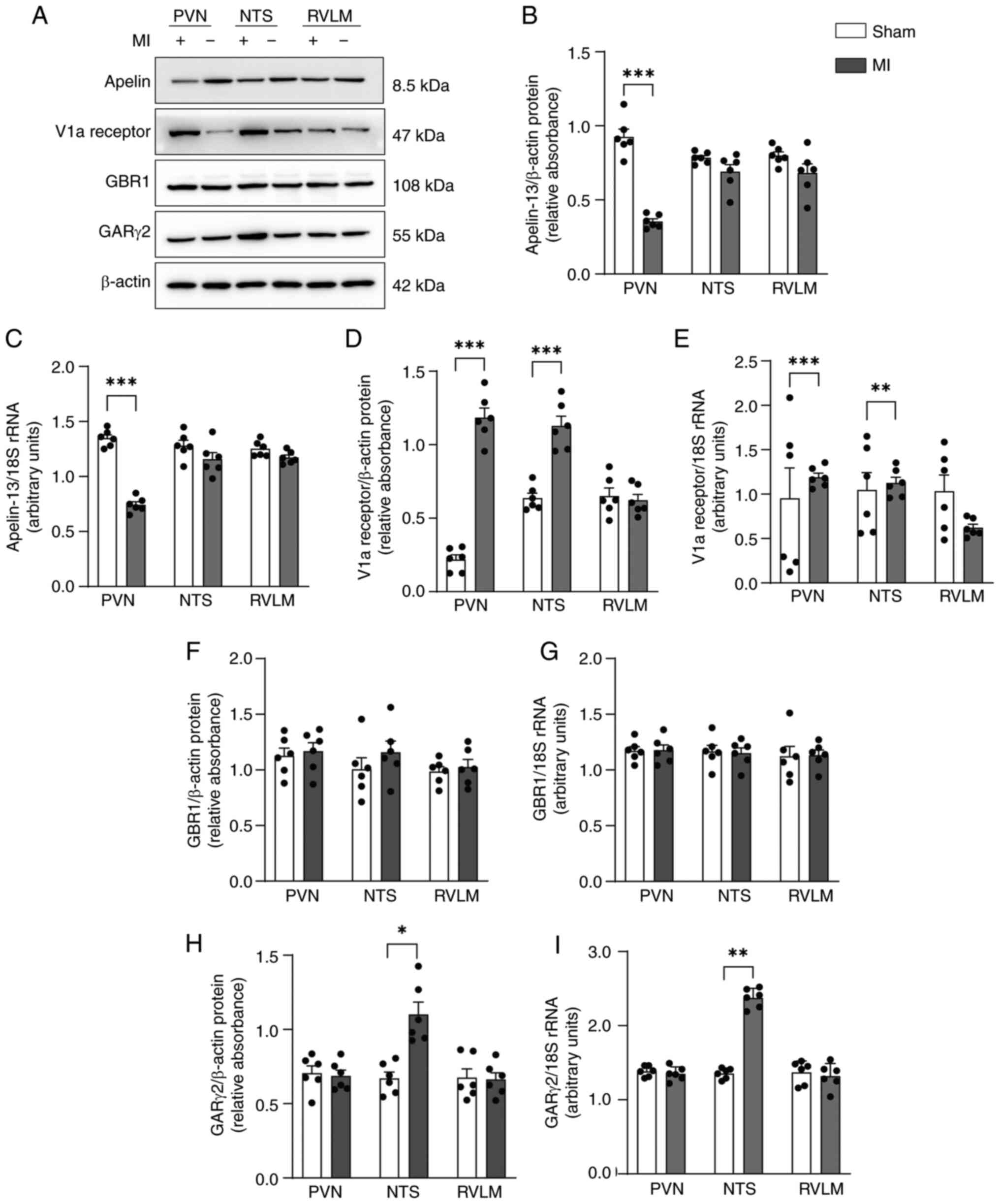 | Figure 1Expression of apelin-13, the V1a
receptor and the GARγ2 receptor in MI model rats. (A)
Representative western blots of apelin-13, the V1a receptor, the
GBR1 and the GARγ2 in the PVN, NTS and RVLM from MI model rats and
sham-operated control rats. (B) Quantification of apelin-13 protein
levels. (C) mRNA expression levels of apelin-13. (D) Quantification
of V1a receptor protein levels. (E) mRNA expression levels of the
V1a receptor. (F) Quantification of GBR1 protein levels. (G) mRNA
expression levels of GBR1. (H) Quantification of GARγ2 protein
levels. (I) mRNA expression levels of GARγ2. Normality was tested
using the Shapiro-Wilk test. The data (n=6 rats per group; 3
technical replicates) in B-I are shown as mean ± SEM and were
analyzed using an unpaired two-tailed t test.
*P<0.05; **P<0.01;
***P<0.001. Post-hoc power exceeded 80% for all
comparisons. V1a, Vasopressin 1a; GAR, GABAA receptor;
MI, myocardial infarction; GBR1, GABAB receptor1; PVN,
paraventricular nucleus; NTS, nucleus tractus solitarii; RVLM,
rostral ventrolateral medulla. |
Effects of apelin-13 microinjection into
the PVN on cardiac function in MI model rats
The present study investigated the effects of
continuous exogenous apelin-13 infusion into the PVN on MI rats
using osmotic minipump implantation. MI models were established one
day after implanting an osmotic mini-pump with apelin-13 into the
PVN (Fig. 2A). Continuous
apelin-13 infusion into the PVN of MI rats increased V1a receptor
expression in the PVN and NTS but decreased GARγ2 expression in the
NTS (Fig. 2B-D).
Echocardiography on day 28 after MI revealed improved cardiac
function in the rats that received continuous apelin-13 infusion
into the PVN (Fig. 2E).
Specifically, these rats presented a greater lower left ventricular
end-diastolic diameter (LVEDD; 6.34±0.45 vs. 8.06±0.16 mm,
P<0.01; Fig. 2F), lower left
ventricular end-systolic diameter (LVESD; 5.97±0.20 mm vs.
7.74±0.24 mm, P<0.001; Fig.
2G), left ventricular ejection fraction (LVEF; 62.7±3.0% vs.
51.0±3.2%, P<0.01; Fig. 2H),
left ventricular fraction shortening (LVFS; 38.3±1.5% vs.
26.4±2.1%, P<0.001; Fig. 2I)
compared with the MI group. Compared with MI model rats, MI model
rats that received apelin-13 continuous infusion into the PVN
presented higher serum levels of SST (131.7±6.6 vs. 79.8±4.5 pg/ml;
P<0.001), CCK (52.7±5.6 vs. 25.6±1.1 pg/100 ml; P<0.001), VIP
(163.8±8.3 vs. 105.6±6.3 pg/ml; P<0.001) and GLP-1 (12.4±0.7 vs.
10.0±1.1 pg/ml; P<0.05; Fig.
2J-M), while showing markedly lower noradrenaline levels
(578.3±51.5 vs. 825.0±89.4 pg/ml, P<0.001; Fig. 2N) and vasopressin levels (8.4±0.6
vs. 11.5±0.8 pg/ml, P<0.001; Fig.
2O). These findings suggested apelin-13 in the PVN improved
post-MI cardiac function by activating both the vasopressinergic
signaling and parasympathetic neuroendocrine pathways.
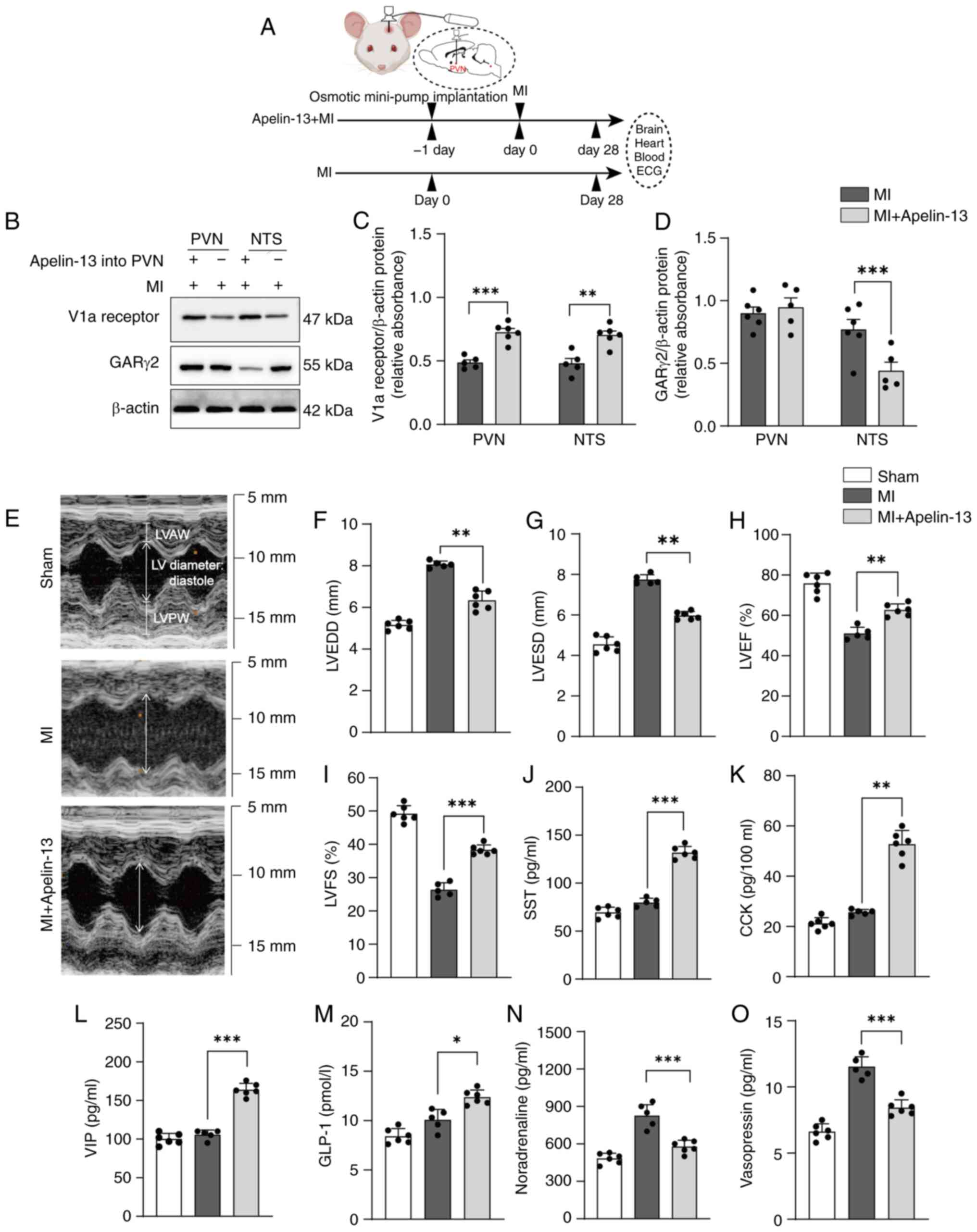 | Figure 2Effects of apelin-13 microinjection
into the PVN on cardiac function in MI model rats. (A) MI models
were established one day after implanting an osmotic mini-pump
delivering apelin-13 into the PVN. Cardiac function was assessed
and tissue samples were collected 28 days after mini-pump
implantation. (B) Representative western blots for the V1a receptor
and GARγ2 in the PVN and NTS from MI model rats and MI model rats
continuously microinjected with apelin-13 into the PVN for 28 days.
(C) Quantification of V1a receptor protein levels. (D)
Quantification of GARγ2 receptor protein levels. (E) Representative
ultrasound images (M-mode) performed on days 28 after MI and
sham-operated control. Echocardiographic results for (F) LVEDD, (G)
LVESD, (H) LVEF and (I) LVFS (n=6 in the sham-operated control
group; n=5 in the MI group, one rat succumbed at 20 days; n=6 in
the MI + apelin group). Plasma levels of (J) SST, (K) CCK, (L) VIP,
(M) GLP-1, (N) noradrenaline and (O) vasopressin (n=6 in the
sham-operated control group; n=5 in the MI group, one rat succumbed
at 20 days; n=6 in the MI + apelin group). Normality was tested
using the Shapiro-Wilk test. The data in C-D show mean ± SEM; F-O
show mean ± SD. Statistical significance was determined by one-way
ANOVA, followed by Tukey's multiple-comparisons test.
*P<0.05; **P<0.01;
***P<0.001. Post-hoc power exceeded 80% for all
comparisons. PVN, paraventricular nucleus; MI, myocardial
infarction; V1a, Vasopressin 1a; GAR, GABAA receptor;
NTS, nucleus tractus solitarii; LVEDD, left ventricular
end-diastolic diameter; LVESD, lower left ventricular end-systolic
diameter; LVEF, left ventricular ejection fraction; LVFS, left
ventricular fraction shortening; SST, isomatostatin; CCK,
cholecystokinin; VIP, vasoactive intestinal peptide; GLP-1,
glucagon-like peptide 1. |
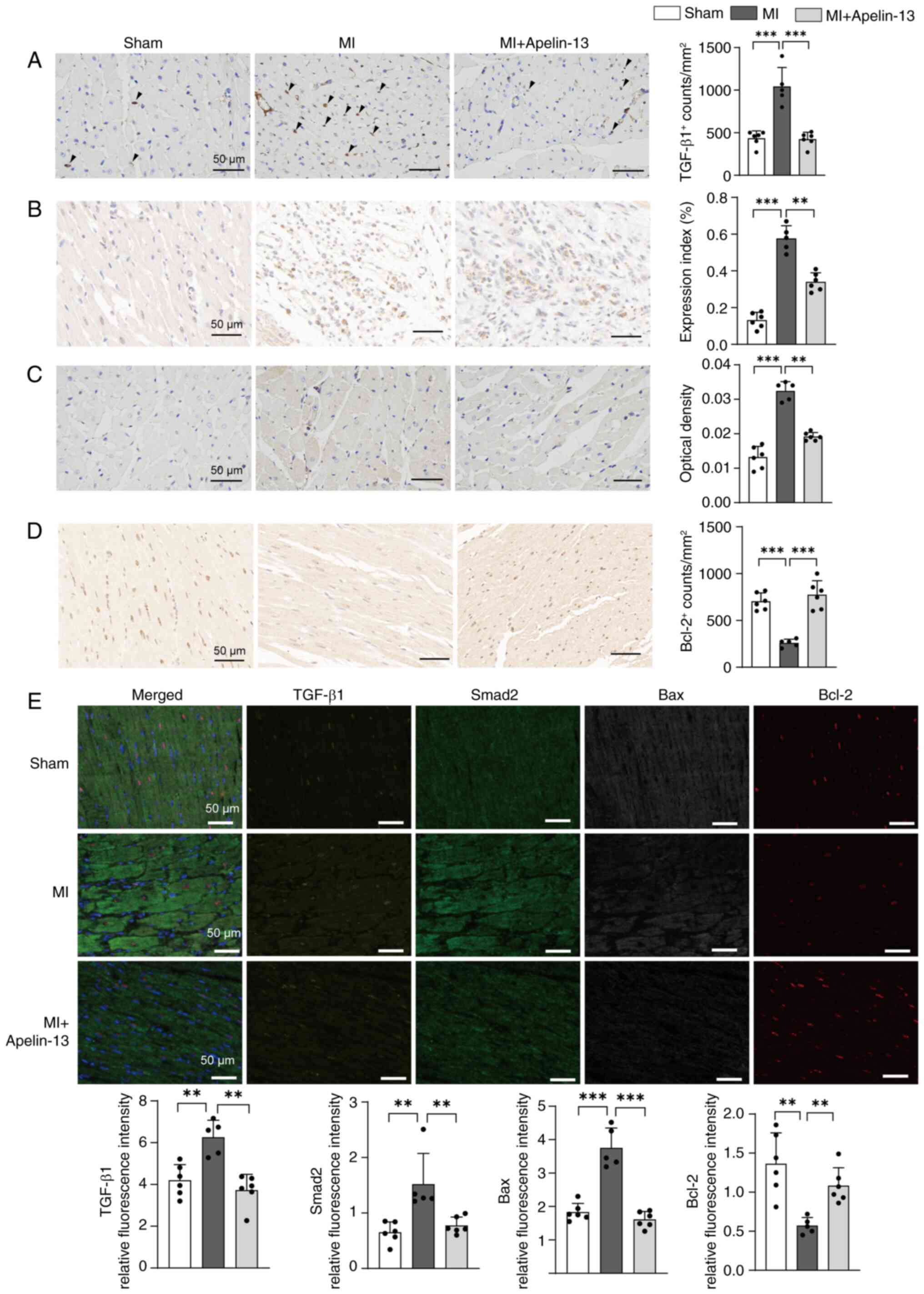
Effects of continuous apelin-13 infusion
into the PVN on myocardial ischemia-associated apoptotic and
inflammatory protein expression in MI rats
To investigate the cardioprotective mechanisms of
PVN apelin-13 infusion, myocardial expression of
ischemia-associated apoptotic and inflammatory proteins was
analyzed by immunohistochemistry and multiplex fluorescent
immunohistochemistry staining in heart tissues. Results from dual
staining consistently demonstrated the involvement of both
TGF-β/Smad and Bax/Bcl-2 signaling pathway in apelin-13-induced
cardioprotection within the PVN. Post-MI rats showed a significant
increase in TGF-β1 and Smad2 expression in heart tissue, which
contributed to ventricular remodeling. PVN infusion of apelin-13
effectively suppressed these increases compared to untreated MI
rats (Fig. 3A, B and E).
Compared to sham-operated controls, post-MI rats exhibited markedly
elevated Bax and reduced Bcl-2 expression in the myocardium.
Notably, PVN administration of apelin-13 effectively reversed these
alterations by downregulating Bax while upregulating Bcl-2
(Fig. 3C-E).
Comparison of AAV2-apelin-13 gene
transfer vs. apelin-13 infusion in PVN effects on myocardial
ischemia-related apoptotic and inflammatory proteins in MI model
rats
MI models were established one day after implanting
osmotic mini-pumps with apelin-13 or performing AAV2-apelin-13 gene
transfer into PVN. Heart and brain tissues were collected at 3-,
7-, 14- and 28-days post-MI (Fig.
4Aa). First, successful microinjection of AAV2-apelin-13 gene
into the PVN and its subsequent overexpression was confirmed
(Fig. 4Ab-g). Subsequently, the
effects of AAV2-apelin-13 gene transfer compared with continuous
apelin-13 infusion in the PVN were compared. AAV2-apelin-13 gene
transfer and continuous exogenous apelin-13 infusion into the PVN
of MI model rats both induced time-dependent upregulation of Bcl-2
and downregulation of Bax in cardiac tissue (Fig. 4B-D). Concurrently, the expression
of TGF-β1 and Smad2 progressively decreased over time (Fig. 4B, E and F). Correspondingly, V1a
receptor expression in the PVN and NTS were elevated, while GARγ2
expression in the NTS declined gradually (Fig. 4B and G-I). At the same selected
time points, there were no significant differences in the
aforementioned protein expression between AAV2-apelin-13 gene
transfer and continuous exogenous apelin-13 infusion. Therefore,
subsequent studies will employ a combined approach using
AAV2-mediated apelin-13 gene transfer specifically targeting the
PVN, coupled with concurrent implantation of osmotic mini-pumps to
deliver various antagonists to either the PVN or NTS for
comprehensive study.
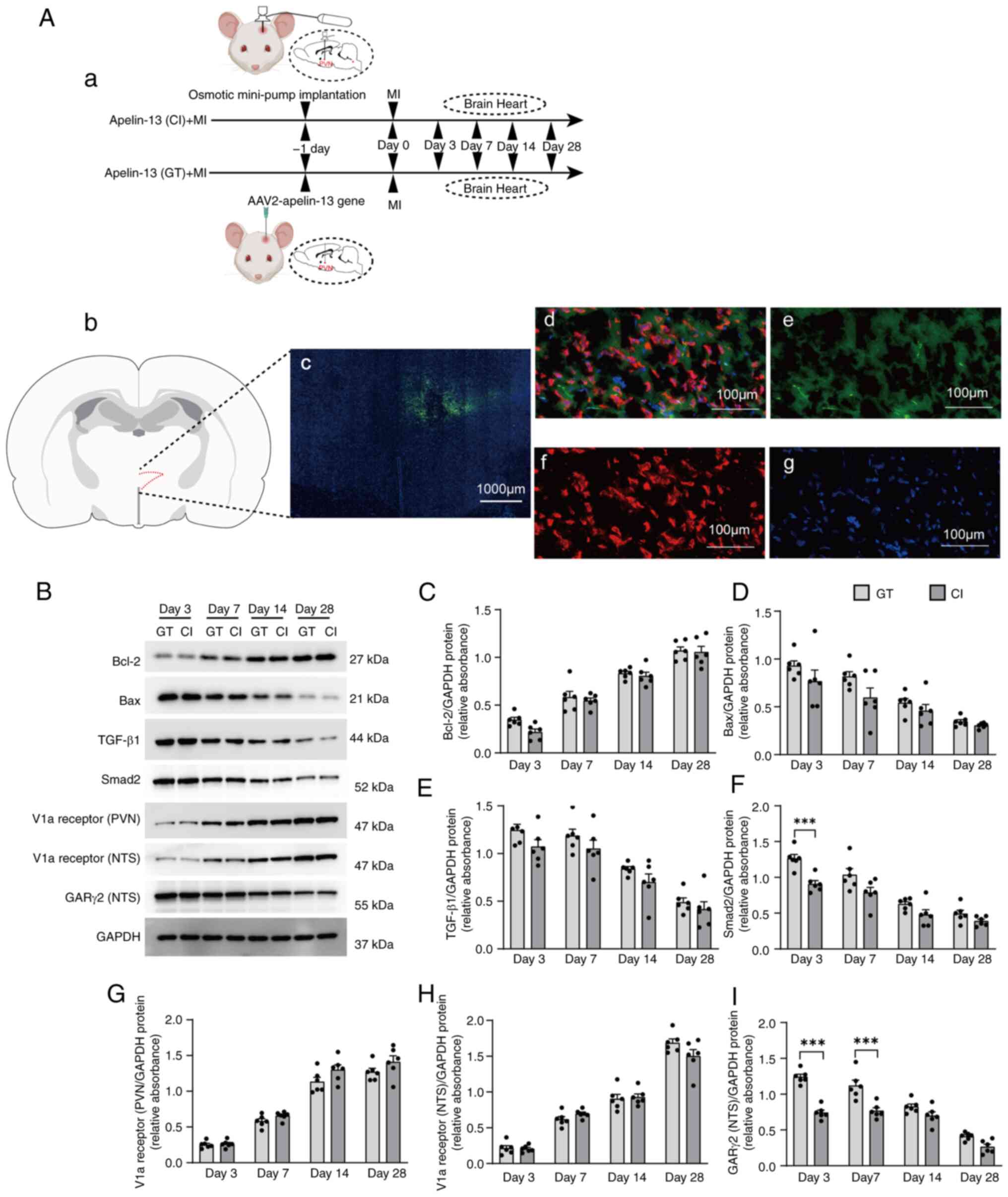 | Figure 4Comparison of effects between
apelin-13 gene transfer and continuous infusion approaches. (Aa)
After implanting apelin-13 osmotic mini-pumps or delivering
AAV2-apelin-13 to the PVN, MI was induced and heart and brain
tissues collected at 3-, 7-, 14- and 28-days post-MI (n=6 in each
group). Location of the stained PVN brain sections shown in (b) and
(c), based on the rat brain atlas of C. Watson and G. Paxinos
(24). (c) Fluorescence
micrograph (magnification, ×10) demonstrating the localization of
apelin-13 within the PVN. 3v is the third ventricle. (d) Overlap of
(e), (f) and (g), showing that the green fluorescence is neuronally
located. (e) Fluorescence micrograph (magnification, ×40)
indicating the localization of apelin-13 in PVN cells immunostained
with an anti-apelin antibody (green). (f) Same field of cells as in
panel (e), immunostained with anti-NeuN antibodies (red). (g) DAPI
staining of the same field of cells in panel (e). (B) Molecular
pathways regulating apoptotic and inflammatory pathways were
assessed through western blotting of the expression of Bax, Bcl-2,
TGF-β1 and Smad2 over time in MI model rats and the expression of
the V1a receptor and GARγ2 induced by apelin-13 overexpression in
the PVN. (C and I) Quantification of the fold change in target gene
expression vs. GAPDH expression. The data (n=6 rats per group, 3
technical replicates) in (C-I) are shown as mean ± SEM and were
analyzed via an unpaired two-tailed t test. *P<0.05;
**P<0.01; ***P<0.001. Post-hoc power
exceeded 80% for all key comparisons. PVN, paraventricular nucleus;
MI, myocardial infarction; GT, gene transfer; CI, continuous
infusion. |
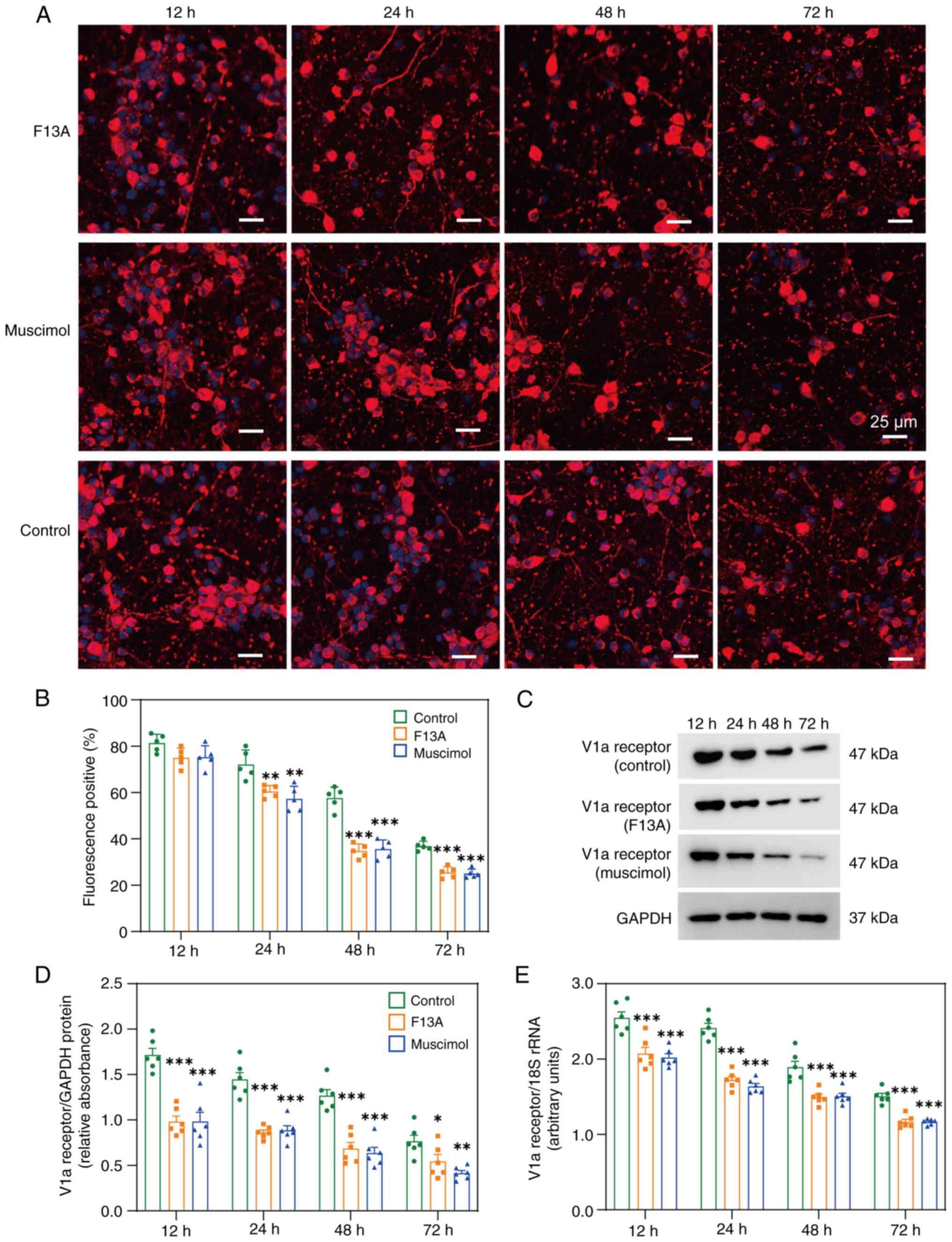
Effects of the APJ receptor and the GARγ2
receptor on the V1a receptor in primary cultured neurons
Prior to proceeding to further animal studies, it
was assessed whether the APJ receptor and GARγ2 modulated V1a
receptor expression in primary neuronal cultures.
Immunofluorescence staining of primary culture neurons (Fig. 5A and B) revealed a time-dependent
decrease in V1a receptor expression at both the protein (Fig. 5C and D) and mRNA levels (Fig. 5E) after treatment with either the
APJ receptor antagonist F13A or the GAR agonist muscimol.
Therefore, based on these observations, it was concluded that both
APJ receptor antagonist and GAR agonist muscimol markedly altered
V1a receptor expression, although the underlying mechanisms require
further investigation.
The effect of apelin-13 overexpression
(AAV2-apelin-13 gene transfer) in the PVN on V1a receptor-mediated
improvement in the cardiac function in MI model rats
To investigate PVN V1a receptor-mediated
cardioprotection, three interventions were employed: i) PVN
AAV2-apelin-13 with PVN osmotic minipump delivering V1a antagonist
SR49059; ii) PVN AAV2-apelin-13 with ipsilateral NTS minipump
(SR49059); and iii) PVN AAV2-apelin-13 with ipsilateral NTS
minipump containing the GAR agonist muscimol. The MI model was
constructed one day after the transfer of the apelin-13 gene into
PVN and the implantation of mini-pump (Fig. 6A). Prior to formal experiments,
the AAV2-GFP group was compared with the sham-operated group and no
significant difference was observed in relevant protein expression.
Therefore, in this experiment, only the sham-operated group was
used as controls (Fig. S1). The
overexpression of apelin-13 in the PVN of the MI model rats
markedly improved cardiac function, including the LVEF (79.1±1.8%
vs. 66.2±2.2%; P<0.05; Fig.
6B), LVFS (45.0±1.9% vs. 32.8±1.3%; P<0.05; Fig. 6C), LVEDD (7.14±0.45 vs. 8.34±0.36
mm; P<0.05; Fig. 6D) and
LVESD (5.01±0.17 vs. 6.12±0.23 mm; P<0.05) (Fig. 6E). These effects were attenuated
by continuous PVN/NTS microinjection of the V1a antagonist SR49059
and continuous NTS microinjection of the GAR agonist muscimol
(Fig. 6B-E). Compared with the
continuous microinjection of SR49059 into the PVN in MI model rats
overexpressing apelin-13, the continuous microinjection of SR49059
or muscimol into the NTS had fewer attenuating effects (Fig. 6B-E). The protective effects of
apelin-13 overexpression were evident in Masson staining (Fig. 6F), TUNEL assay (Fig. 6G) and TTC staining (Fig. 6H) following microinjection of
SR49059 into the PVN or NTS, or muscimol into the NTS. Bar graphs
of the Masson, TUNEL and TTC staining results indicated that
apelin-13 overexpression in the PVN markedly decreased fibrosis
(29.0±3.4% vs. 56.0±3.4%; P<0.05; Fig. 6I), the mean fluorescence
intensity (27.27±2.81 vs. 55.34±3.32 AU; P<0.05; Fig. 6J) and infarction area (24.4±4.1%
vs. 46.8±4.9%; P<0.05; Fig.
6K). Compared with the microinjection of SR49059 into the PVN,
the microinjection of SR49059 or muscimol into the NTS led to less
fibrosis, a lower mean fluorescence intensity and a reduced
infarction area (Fig. 6I-K).
Compared with the sham group, the MI rats with apelin-13
overexpression in the PVN had a similar MAP (94.9±2.7 vs. 97.1±4.1
mmHg; n=7; P>0.05) and HR (383.4±9.1 vs. 395.4±10.7 bpm; n=7;
P>0.05; Fig. 6L and M) at 28
days after MI modelling. Continuous infusion of SR49059 into the
PVN/NTS and muscimol into the NTS differentially affected the
cardioprotective effects of apelin-13 in the PVN. The increase in
V1a receptor expression in the PVN caused by AAV2-apelin-13 gene
overexpression in the PVN was attenuated by the continuous
microinjection of the V1a receptor antagonist SR49059 into the NTS
but not into the PVN or the microinjection of the GAR antagonist
muscimol into the NTS (Fig. 7A and
B). In the NTS, increased V1a receptor expression caused by
AAV2-apelin-13 gene overexpression in the PVN was not attenuated by
the continuous microinjection of the V1a receptor antagonist
SR49059 into the PVN or NTS or by the GAR antagonist muscimol into
the NTS (Fig. 7A and C). The
decrease in GARγ2 expression in the NTS caused by AAV2-mediated
apelin-13 overexpression in the PVN was markedly attenuated by the
continuous microinjection of the V1a receptor antagonist SR49059 in
the NTS but not by the microinjection of SR49059 in the PVN or
muscimol in the NTS (Fig. 7A and
D). From the aforementioned results, it was found that the
expression of both V1a receptors and GAR2 in the NTS was regulated
by V1a receptors in the PVN. The apelin-13 gene
overexpression-induced increase in Bcl-2 (Fig. 7E and F) and decrease in Bax
(Fig. 7E and G), TGF-β1
(Fig. 7E and H) and Smad2
(Fig. 7E and I) were attenuated
by the microinjection of SR49059 into the PVN but not into the NTS
or by the microinjection of muscimol into the NTS. However, in the
serum, the increased levels of SST (Fig. 7J), CCK (Fig. 7K), VIP (Fig. 7L) and GLP-1 (Fig. 7M) induced by apelin-13
overexpression in the PVN of the MI model rats were attenuated by
the microinjection of SR49059 in the PVN or NTS and muscimol in the
NTS. In contrast, the decreased level of noradrenaline (Fig. 7N) and vasopressin (Fig. 7O) increased after the
microinjection of SR49059 in the PVN or NTS and muscimol in the
NTS. Together, these findings demonstrated that the V1a receptor in
the PVN/NTS and GARγ2 in NTS contribute to apelin-13-mediated
cardioprotection in MI models through vasopressinergic signaling
and PES.
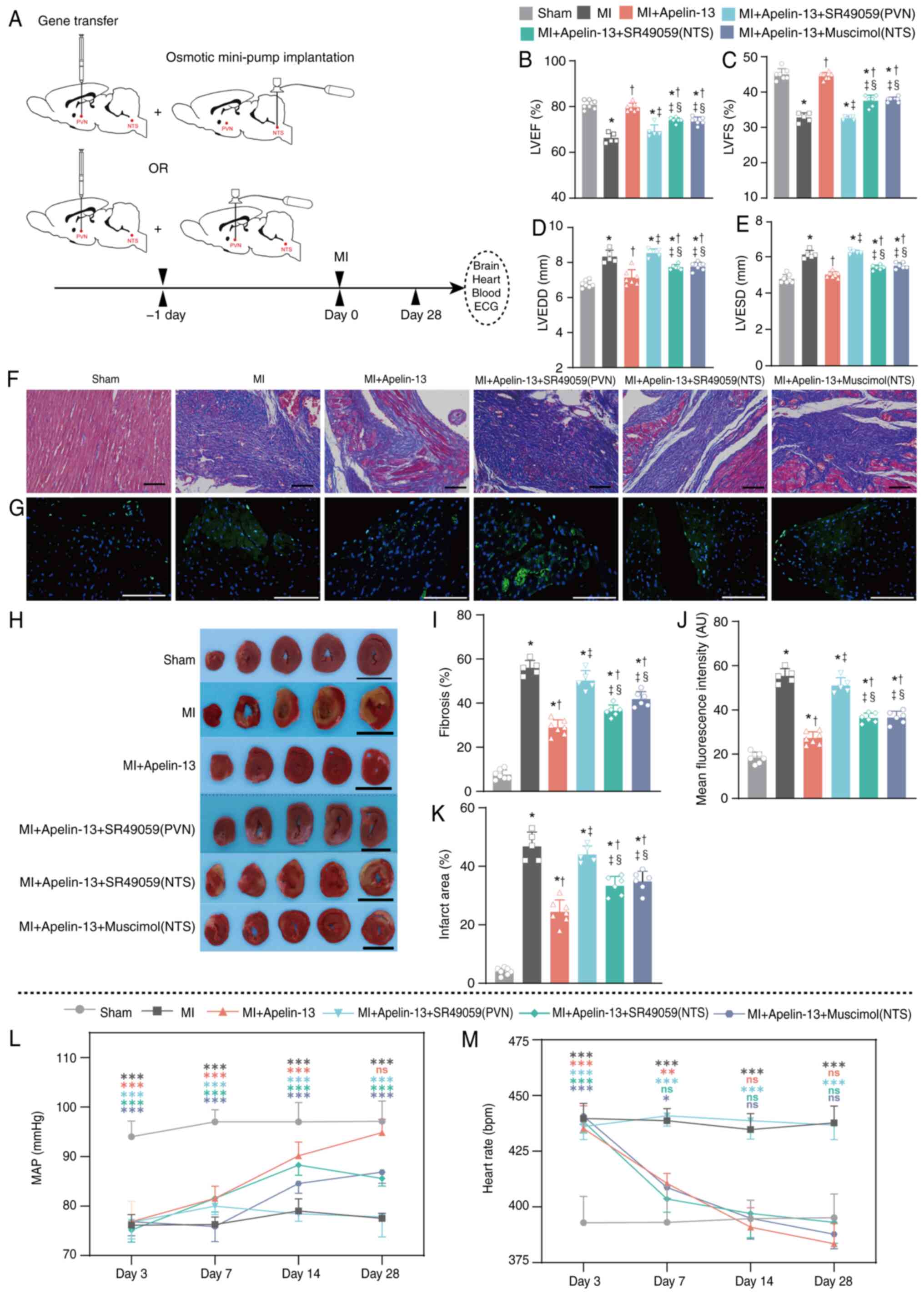 | Figure 6Effect of apelin-13 overexpression
(AAV2-apelin-13 gene transfer) in the PVN on V1a receptor-mediated
improvements in cardiac function and cardiac morphology in MI model
rats. (A) The MI model was constructed one day following the
transfer of the apelin-13 gene into PVN and the implantation of
mini-pump (7 rats in each group). Echocardiographic results for (B)
LVEF, (C) LVSF, (D) LVEDD and (E) LVESD. (F) Representative
Masson's trichrome staining and (I) quantification. (G)
Representative TUNEL staining and (J) quantification of TUNEL
staining. (H) Representative TTC staining and (K) quantification.
The black scale bar in F is 100 μm; the white scale bar in G
is 50 μm; and the black scale bar in I is 1 cm. At 28 days
after MI modelling, the sham-operated control group had 7 rats, the
MI group had 5 rats (2 rats succumbed), the MI + apelin-13 group
had 7 rats, the MI + apelin-13 + SR49059 (PVN) group had 5 rats (2
rats succumbed), the MI + apelin-13 + SR49059 (NTS) group had 6
rats (1 rat succumbed) and the MI + apelin-13 + muscimol (NTS)
group had 6 rats (1 rat succumbed). Normality was tested using the
Shapiro-Wilk test. The data in B-E and H-M are shown as means ± SDs
and were analyzed using one-way ANOVA, followed by Tukey's
multiple-comparisons test. In B-E and H-K, *P<0.05
vs. sham-operated control group; †P<0.05 vs. MI
group; ‡P<0.05 vs. MI + apelin-13 group;
§P<0.05 vs. MI + apelin-13 + SR49059(PVN);
#P<0.05 vs. MI + apelin-13 + SR49059 (NTS). The (L)
MAP and (M) heart rate were recorded in a conscious state at days
3, 7, 14 and 28 after MI modelling. *P<0.05;
**P<0.01; ***P<0.001; and ns, not
significant, vs. the sham-operated control group. Post-hoc power
exceeded 80% for all key comparisons. PVN, paraventricular nucleus;
V1a, Vasopressin 1a; MI, myocardial infarction; ECG,
Echocardiographic; LVEF, left ventricular ejection fraction; LVFS,
left ventricular fraction shortening; LVEDD, left ventricular
end-diastolic diameter; LVESD, lower left ventricular end-systolic
diameter; NTS, nucleus tractus solitarii; MAP, mean arterial
pressure. |
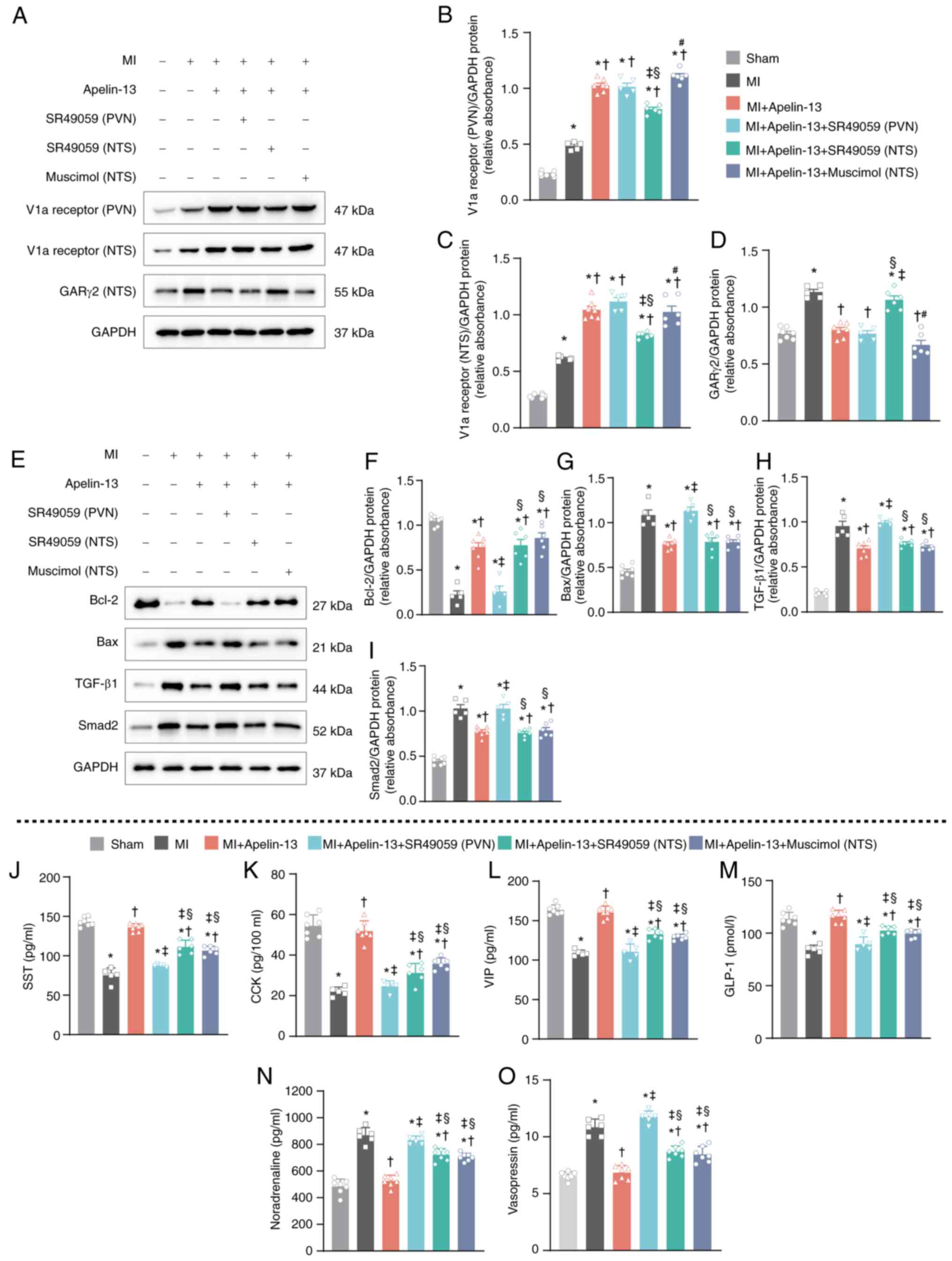 | Figure 7Mechanisms of the effect of apelin-13
overexpression (AAV2-apelin-13 gene transfer) in the PVN on V1a
receptor-mediated improvement in cardiac function in MI model rats.
(A) Representative western blot image of V1a receptor expression in
the PVN or NTS and GARγ2 expression in the NTS. (B-D)
Quantification of V1a receptor expression. (E) Representative
western blotting to assess Bcl-2, Bax, TGF-β1 and Smad2 protein
levels in the heart and (F-I) quantification of the protein levels.
Plasma levels of (J) SST, (K) CCK, (L) VIP and (M) GLP-1 (n=7 in
the sham-operated control group; n=5 in the MI group, 2 rats
succumbed; n=7 in the MI + apelin-13 group; n=5 in the MI +
apelin-13 + SR49059 (PVN) group, 2 rats succumbed; n=6 in the MI +
apelin-13 + SR49059 (NTS) group, 1 rat succumbed; n=6 in the MI +
apelin-13 + muscimol (NTS) group, 1 rat succumbed). Representative
plasma levels of (N) noradrenaline and (O) vasopressin (n=7 in the
sham-operated control group; n=6 in the MI group, 1 rat succumbed;
n=7 in the MI + apelin-13 group; n=6 in the MI + apelin-13 +
SR49059 (PVN) group, 1 rat succumbed; n=6 in the MI + apelin-13 +
SR49059 (NTS) group, 1 rat succumbed; n=7 in the MI + apelin-13 +
muscimol (NTS) group). Normality was tested using the Shapiro-Wilk
test. The data in B-D and F-I show mean ± SEM; data in J-O are
shown as mean ± SD and were analyzed via one-way ANOVA, followed by
Tukey's multiple-comparisons test. In B-D and F-O,
*P<0.05 vs. sham-operated control group;
†P<0.05 vs. MI group; ‡P<0.05 vs. MI +
apelin-13 group; §P<0.05 vs. MI + apelin-13 + SR49059
(PVN); #P<0.05 vs. MI + apelin-13 + SR49059 (NTS).
Post-hoc power exceeded 80% for all key comparisons. PVN,
paraventricular nucleus; V1a, Vasopressin 1a; MI, myocardial
infarction; NTS, nucleus tractus solitarii; GAR, GABAA
receptor; SST, isomatostatin; CCK, cholecystokinin; VIP, vasoactive
intestinal peptide; GLP-1, glucagon-like peptide 1. |
Discussion
The present study provided novel evidence of the
neural regulation of cardiac functions via apelin-13 in the PVN;
apelin-13 upregulated the expression of the V1a receptor in the PVN
and NTS and downregulated the expression of GARγ2 in the NTS.
Effects of a V1a receptor antagonist and GARγ2 agonist further
demonstrated that apelin-13 in the PVN is a novel factor involved
in the centrally mediated neural control of myocardial injury,
including in MI rat models. Furthermore, both myocardial TGF-β/Smad
signaling and the Bax/Bcl-2 apoptotic balance contribute to the
central-mediated cardioprotective effects of apelin-13 in PVN.
First, in MI model rats, apelin-13 expression was
decreased in the PVN but not in the NTS or RVLM. The PVN is a
critical central regulator of autonomic and humoral responses; it
receives afferent inputs from visceral receptors and circulating
hormones and then sends out projections to key cardiovascular
brainstem centers and spinal cord preganglionic neurons, thereby
modulating parasympathetic and sympathetic activity (27). The PVN is also involved in the
treatment of MI-induced heart failure via the inhibition of oxidant
signaling (6). Thus, it was
hypothesized that there were more pathways involved in the
mediation of cardiac function by apelin-13 in the PVN. Notably, V1a
receptor expression in the PVN and NTS increased following
apelin-13 microinjection into the PVN of MI model rats, whereas
GARγ2 expression in the NTS decreased after apelin-13
microinjection; these findings were further confirmed in MI models
with apelin-13 gene transmission. In the RVLM, the pressor effect
evoked by the bilateral microinjection of apelin-13 as a modulating
neurotransmitter in normotensive rats via the V1a receptor affects
vascular tone independent of GAR, whereas the presence of
presynaptic V1a receptors affects vasopressin release from the
PVN-RVLM projecting fibers (15). The observations in the present
study suggested that synaptic V1a receptors act as communicators
between the PVN and NTS for apelin-13-mediated nerve connections
and GARγ2 expression in the NTS.
These findings indicated that myocardial injury
induced the downregulation of apelin-13 expression in the PVN and
that upregulating apelin-13 expression in the PVN improved cardiac
function. Thus, it was hypothesized that the apelin/APJ system is
involved in the PVN-NTS axis for the regulation of cardiac
function, a novel concept of heart-brain interactions. The heart
and the brain are linked by multiple feedback signals and the
concept of cardio-cerebral syndrome in heart failure has been built
on the bidirectional interactions of failing heart and neuronal
signals (28). A previous review
indicated that the mechanisms of brain-heart interactions include
the physiological effects of sympathetic and parasympathetic nerve
activities, central pathways regulating autonomic outflow, reflex
control of autonomic outflow and the integrative regulation of
autonomic outflow to the heart (29). More specifically, the sympathetic
and parasympathetic branches of the autonomic nervous system
directly control the heart and parasympathetic postganglionic
fibers innervate the atrial and ventricular myocardium by releasing
acetylcholine and VIP (30).
Consistently, in the present study, as a critical mediator of
synaptic inhibition in the brain (31), the expression of the γ2 subunit
of GAR in the NTS decreased after apelin-13 overexpression in the
PVN, which indirectly elevated parasympathetic efferent
excitability to activate the PES.
The V1a receptor is a transmembrane protein that
belongs to the G protein-coupled receptor superfamily. The combined
activation of V1a receptors by vasopressin in the PVN has been
shown to increase renal sympathetic nerve activity (32). Subsequent to MI, within the PVN,
a notable elevation in the expression level of the V1a receptor was
observed. This augmented expression subsequently led to a
significant enhancement in the modulation of the autonomic
cardiovascular system, thereby playing a crucial role in the
intricate physiological adjustments that occur in response to the
myocardial damage. In NTS, V1a receptor can influence the
transmission and integration of baroreceptor reflex signals, which
play a crucial role in regulating blood pressure and heart rate.
Dysfunction of the V1a receptor-mediated modulation in the NTS may
contribute to abnormal blood pressure regulation and heart rate
variability after MI. The present study showed that apelin-13
overexpression in the PVN of MI model rats further elevated the
increased V1a receptor expression in the PVN and NTS, indicating
its involvement in apelin-13-mediated cardiac function.
The apelin/APJ system plays several important roles
in the neurohormonal regulation of heart function, including
vasodilatory effects, positive inotropic effects, fluid balance
regulation, antiapoptotic effects and interactions with other
neurohormonal systems (25,33). The present study revealed that
the improved cardiac function mediated by apelin/APJ in the PVN
involves PES activation by apelin-13 overexpression, including an
increase in the expression of four effectors, namely, SST, CCK,
GLP-1 and VIP. SST has been shown to exert a direct
cardiocytoprotective effect against simulated cardiomyocyte injury
via SST receptor 1 and SST receptor 2 in cardiomyocytes and
vascular endothelial cells (34). CCK, GLP-1 and VIP are involved in
cardiovascular regulation via different mechanisms in the
circulation (35-37). Thus, it was hypothesized that the
PES plays an important role in the heart-brain circuit. While the
pro-fibrotic and remodeling role of TGF-β/Smad signaling in the
heart has been well established (38), the histomorphometry and molecular
analyses of the present study demonstrated that apelin-13 in the
PVN mediates cardioprotection by modulating PES, ultimately
downregulating the TGF-β/Smad signaling pathway, thereby
attenuating cardiac fibrosis and remodeling. Meanwhile, the
regulation of myocardial Bax/Bcl-2 expression was also involved in
the central cardioprotective effects mediated by apelin-13 in the
PVN.
The present study provided novel evidence that
PVN-derived apelin-13 modulates cardiac function through dual
mechanisms: Upregulating V1a receptor expression in both PVN and
NTS nuclei and downregulating GARγ2 expression specifically in the
NTS. Mechanistically, PVN apelin-13 overexpression improved cardiac
performance via V1a receptors (PVN/NTS) and GARγ2 (NTS)-dependent
pathways. This regulation involves both parasympathetic signaling
and myocardial ischemia-associated pro-apoptotic/pro-inflammatory
cascades, establishing the first evidence for central neural
control of cardiovascular pathogenesis (Fig. 8).
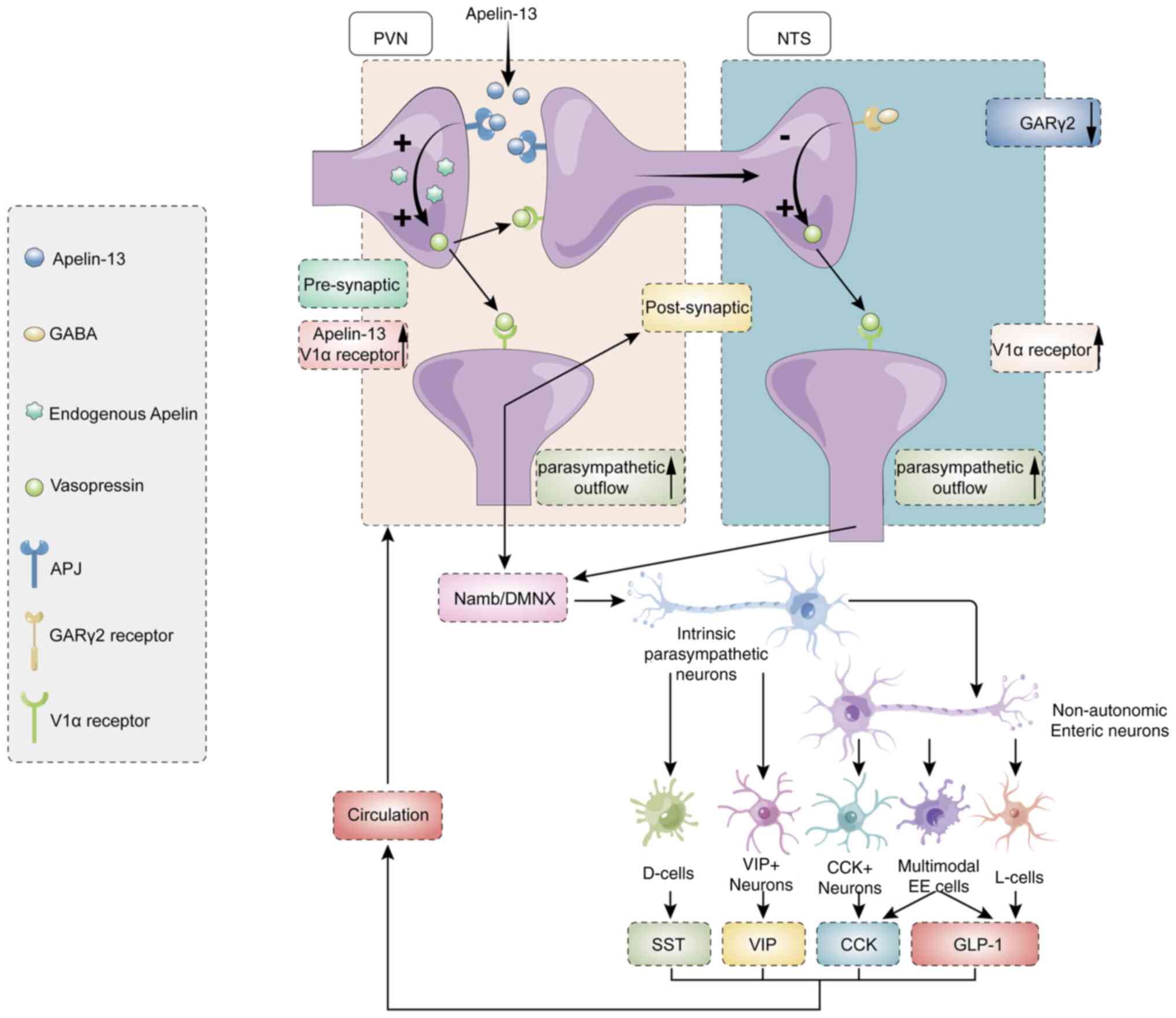 | Figure 8Graphical model summarizing the
hypothesized pathway. Overexpression of apelin-13 in the PVN
promotes upregulation of V1a receptors in both the PVN and NTS,
while downregulating GARγ2 in the NTS. These neural modifications
collectively enhance parasympathetic outflow. Furthermore,
paraventricular endocrine system secretes circulating neurohumoral
factors that systemically mediate cardioprotection. PVN,
paraventricular nucleus; V1a, Vasopressin 1a; MI, myocardial
infarction; NTS, nucleus tractus solitarii; GAR, GABAA
receptor; SST, isomatostatin; CCK, cholecystokinin; GLP-1,
glucagon-like peptide 1; VIP, vasoactive intestinal peptide. |
Despite these meaningful findings, the present study
had certain limitations. Although it found novel evidence of neural
control of cardiomyocyte injury, the mechanism of interaction
between the PVN and NTS is not fully clear, except for direct
exposure to the neurotransmitter apelin-13 and vasopressin.
Importantly, the V1a receptor and GARγ2 in the NTS are indirectly
regulated by the apelin/APJ system in the NTS, but postsynaptic GAR
currents should be considered for modulating receptor expression.
Therefore, other unclear mechanisms are involved in receptor
interactions between the PVN and NTS. To address the limitation of
lacking electrophysiological evidence, future work will directly
record from PVN neurons that project to the NTS to determine how
their firing activity changes following apelin-13 administration.
Due to the technical challenges in precisely isolating the PVN and
NTS from neonatal rats, primary neuronal culture studies cannot
fully reflect the regulatory effects of APJ and GARγ2 on V1a
receptor expression in these nuclei. Moreover, the interaction
mechanisms between these receptors within specific nuclei require
further investigation. The mechanism by which GARγ2 regulates V1a
receptor expression remains unclear. Candidate transcription
factors potentially mediating this regulation include nuclear
factor-κB (NF-κB) (39,40), early growth response protein 1
(EGR1) (41,42) and cAMP response element-binding
protein (CREB) (43,44). Subsequent studies should further
investigate this mechanism through integrated approaches including
electrophysiology and single-cell RNA-sequencing. Since drugs may
diffuse from injection sites and transgene expression may spread
beyond target areas, other hypothalamic regions might unexpectedly
participate in cardiac regulation. Vasopressin present in the
cerebrospinal fluid (CSF) can interact with the HPA axis. This
interaction can enhance the release of adrenocorticotropic hormone
from the anterior pituitary gland to modulate cardiac function
(45). However, the levels of
vasopressin in CSF were unfortunately not assessed. Finally, four
peptides that act as neurotransmitters are released through a
complex network of gut sensing and vagal mechanisms. Other
neuropeptide levels could also be affected by apelin-13 in the PVN;
however, these levels were not detected or explored in the present
study. The present study selected male rats to minimize sex hormone
variability and ensure precision in stereotaxic brain nucleus
targeting during injections and minipump implantation.
Consequently, findings may not generalize to females; subsequent
studies will further validate results in ovariectomized and intact
female models.
In conclusion, apelin-13 in the PVN performs neural
control for cardiac function via the V1a receptor in the PVN and
NTS and GARγ2 in the NTS, offering novel evidence of brain-heart
interactions. The present study provided evidence for improving
cardiac function through the CNS in the future.
Supplementary Data
Availability of data and materials
The data generated in the present study may be found
in the Sequence Read Archive under at the following URL: https://www.ncbi.nlm.nih.gov/gene?cmd=Search&doptcmdl=EntrezGene&term=58812,
https://www.ncbi.nlm.nih.gov/gene/2550, https://www.ncbi.nlm.nih.gov/gene/552
and https://www.ncbi.nlm.nih.gov/gene/2566.
Authors' contributions
WY, DW and XZ conducted the experiments. CX and WY
designed the experiments and analyzed the data. DW and CX drafted
the manuscript and all authors edited the manuscript. WY and DW
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82070361).
References
|
1
|
Chen Z, Venkat P, Seyfried D, Chopp M, Yan
T and Chen J: Brain-heart interaction: Cardiac complications after
stroke. Circ Res. 121:451–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simats A, Sager H and Liesz A: Heart brain
axis in health and disease: Role of innate and adaptive immunity.
Cardiovasc Res. 120:2325–2335. 2025. View Article : Google Scholar
|
|
3
|
Zhao B, Li T, Fan Z, Yang Y, Shu J, Yang
X, Wang X, Luo T, Tang J, Xiong D, et al: Heart-brain connections:
Phenotypic and genetic insights from magnetic resonance images.
Science. 380:abn65982023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herman JP and Tasker JG: Paraventricular
hypothalamic mechanisms of chronic stress adaptation. Front
Endocrinol (Lausanne). 7:1372016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benarroch EE: The central autonomic
network: Functional organization, dysfunction, and perspective.
Mayo Clin Proc. 68:988–1001. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Infanger DW, Cao X, Butler SD, Burmeister
MA, Zhou Y, Stupinski JA, Sharma RV and Davisson RL: Silencing nox4
in the paraventricular nucleus improves myocardial
infarction-induced cardiac dysfunction by attenuating
sympathoexcitation and peri-infarct apoptosis. Circ Res.
106:1763–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee DK, Cheng R, Nguyen T, Fan T,
Kariyawasam AP, Liu Y, Osmond DH, George SR and O'Dowd BF:
Characterization of apelin, the ligand for the APJ receptor. J
Neurochem. 74:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang N, Li T, Cheng J, Tuo Q and Shen J:
Role of apelin/APJ system in hypothalamic-pituitary axis. Clin Chim
Acta. 499:149–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv SY, Chen WD and Wang YD: The apelin/APJ
system in psychosis and neuropathy. Front Pharmacol. 11:3202020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masaki T, Yasuda T and Yoshimatsu H:
Apelin-13 microinjection into the paraventricular nucleus increased
sympathetic nerve activity innervating brown adipose tissue in
rats. Brain Res Bull. 87:540–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji M, Wang Q, Zhao Y, Shi L, Zhou Z and Li
Y: Targeting hypertension: superoxide anions are involved in apelin
induced long-term high blood pressure and sympathetic activity in
the paraventricular nucleus. Curr Neurovasc Res. 16:455–464. 2019.
View Article : Google Scholar
|
|
13
|
Zhang Q, Yao F, Raizada MK, O'Rourke ST
and Sun C: Apelin gene transfer into the rostral ventrolateral
medulla induces chronic blood pressure elevation in normotensive
rats. Circ Res. 104:1421–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Mota N, Reaux-Le Goazigo A, El Messari
S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F and
Llorens-Cortes C: Apelin, a potent diuretic neuropeptide
counteracting vasopressin actions through inhibition of vasopressin
neuron activity and vasopressin release. Proc Natl Acad Sci USA.
101:10464–10469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griffiths PR, Lolait SJ, Harris LE, Paton
JFR and O'Carroll AM: Vasopressin V1a receptors mediate the
hypertensive effects of [Pyr1]apelin-13 in the rat rostral
ventrolateral medulla. J Physiol. 595:3303–3318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Czarzasta K, Cudnoch-Jedrzejewska A,
Szczepanska-Sadowska, Fus L, Puchalska L, Gondek A, Dobruch J,
Gomolka R, Wrzesien R, Zera T, et al: The role of apelin in central
cardiovascular regulation in rats with post-infarct heart failure
maintained on a normal fat or high fat diet. Clin Exp Pharmacol
Physiol. 43:983–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuriyama K, Hirouchi M and Kimura H:
Neurochemical and molecular pharmacological aspects of the GABA(B)
receptor. Neurochem Res. 25:1233–1239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Liu Q, Xuan C, Guo L, Shi R, Zhang
Q, O'Rourke ST, Liu K and Sun C: GABAB receptor gene transfer into
the nucleus tractus solitarii induces chronic blood pressure
elevation in normotensive rats. Circ J. 77:2558–2566. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dampney RA: Functional organization of
central pathways regulating the cardiovascular system. Physiol Rev.
74:323–364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korpal AK, Han SY, Schwenke DO and Brown
CH: A switch from GABA inhibition to excitation of vasopressin
neurons exacerbates the development angiotensin II-dependent
hypertension. J Neuroendocrinol. Dec 9–2017.Epub ahead of print.
PubMed/NCBI
|
|
21
|
Klok MD, Jakobsdottir S and Drent ML: The
role of leptin and ghrelin in the regulation of food intake and
body weight in humans: A review. Obes Rev. 8:21–34. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gorky J and Schwaber J: Conceptualization
of a parasympathetic endocrine system. Front Neurosci. 13:10082019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Watson C and Paxinos G: The rat brain in
stereotaxic coordinates. 5th ed. Amsterdam: Elsevier; 2004, pp.
51–57
|
|
25
|
Zhao Y, Li Y, Li Z, Xu B, Chen P and Yang
X: Superoxide anions modulate the performance of apelin in the
paraventricular nucleus on sympathetic activity and blood pressure
in spontaneously hypertensive rats. Peptides. 121:1700512019.
View Article : Google Scholar
|
|
26
|
Kc P, Balan KV, Tjoe SS, Martin RJ,
Lamanna JC, Haxhiu MA and Dick TE: Increased vasopressin
transmission from the paraventricular nucleus to the rostral
medulla augments cardiorespiratory outflow in chronic intermittent
hypoxia-conditioned rats. J Physiol. 588:725–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benarroch EE: Paraventricular nucleus,
stress response, and cardiovascular disease. Clin Auton Res.
15:254–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Havakuk O, King KS, Grazette L, Yoon AJ,
Fong M, Bregman N, Elkayam U and Kloner RA: Heart failure-induced
brain injury. J Am Coll Cardiol. 69:1609–1616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silvani A, Calandra-Buonaura G, Dampney RA
and Cortelli P: Brain-heart interactions: Physiology and clinical
implications. Philos Trans A Math Phys Eng Sci.
374:201501812016.PubMed/NCBI
|
|
30
|
Coote JH: Myths and realities of the
cardiac vagus. J Physiol. 591:4073–4085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JJ and Hibbs RE: Direct structural
insights into GABAA receptor pharmacology. Trends Biochem Sci.
46:502–517. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Komnenov D, Quaal H and Rossi NF: V1a and
V1b vasopressin receptors within the paraventricular nucleus
contribute to hypertension in male rats exposed to chronic mild
unpredictable stress. Am J Physiol Regul Integr Comp Physiol.
320:R213–R225. 2021. View Article : Google Scholar
|
|
33
|
Dalzell JR, Rocchiccioli JP, Weir RA,
Jackson CE, Padmanabhan N, Gardner RS, Petrie MC and McMurray JJ:
The emerging potential of the apelin-APJ system in heart failure. J
Card Fail. 21:489–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vörös I, Sághy É, Pohóczky K, Makkos A,
Onódi Z, Brenner GB, Baranyai T, Ágg B, Váradi B, Kemény Á, et al:
Somatostatin and its receptors in myocardial ischemia/reperfusion
injury and cardioprotection. Front Pharmacol. 12:6636552021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han Z, Bi S, Xu Y, Dong X, Mei L, Lin H
and Li X: Cholecystokinin expression in the development of
myocardial hypertrophy. Scanning. 2021:82315592021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simms-Williams N, Treves N, Yin H, Lu S,
Yu O, Pradhan R, Renoux C, Suissa S and Azoulay L: Effect of
combination treatment with glucagon-like peptide-1 receptor
agonists and sodium-glucose cotransporter-2 inhibitors on incidence
of cardiovascular and serious renal events: Population based cohort
study. BMJ. 385:e0782422024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duggan KA, Hodge G, Chen J and Hunter T:
Vasoactive intestinal peptide infusion reverses existing myocardial
fibrosis in the rat. Eur J Pharmacol. 862:1726292019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sarker M, Chowdhury N, Bristy AT, Emran T,
Karim R, Ahmed R, Shaki MM, Sharkar SM, Sayedur Rahman GM and Reza
HM: Astaxanthin protects fludrocortisone acetate-induced cardiac
injury by attenuating oxidative stress, fibrosis, and inflammation
through TGF-β/Smad signaling pathway. Biomed Pharmacother.
181:1177032024. View Article : Google Scholar
|
|
39
|
Liu H, Yu L, Yang L and Green MS:
Vasoplegic syndrome: An update on perioperative considerations. J
Clin Anesth. 40:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu Q, Yu Q, Luo H, Liu Z, Ma X, Wang H and
Cheng Z: Protective effects of wogonin in the treatment of central
nervous system and degenerative diseases. Brain Res Bull.
221:1112022025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Y, Donovan M, Jia X and Wang Z: The
ventromedial hypothalamic circuitry and male alloparental behaviour
in a socially monogamous rodent species. Eur J Neurosci.
50:3689–3701. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Loveland JL and Fernald RD: Differential
activation of vasotocin neurons in contexts that elicit aggression
and courtship. Behav Brain Res. 317:188–203. 2017. View Article : Google Scholar
|
|
43
|
Yan R, Liu Q, Zhang D, Li K, Li Y, Nie Y,
Zhang Y, Li P, Mao S and Li H: Inhibition of spreading
depolarizations by targeting GABAA receptors and voltage-gated
sodium channels improves neurological deficits in rats with
traumatic brain injury. Br J Pharmacol. Jun 24–2025.Epub ahead of
print. View Article : Google Scholar
|
|
44
|
Greenwood M, Gillard BT, Murphy D and
Greenwood MP: Dimerization of hub protein DYNLL1 and bZIP
transcription factor CREB3L1 enhances transcriptional activation of
CREB3L1 target genes like arginine vasopressin. Peptides.
179:1712692024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Szczepanska-Sadowska E, Czarzasta K,
Bogacki-Rychlik W and Kowara M: The interaction of vasopressin with
hormones of the hypothalamo-pituitary-adrenal axis: The
significance for therapeutic strategies in cardiovascular and
metabolic diseases. Int J Mol Sci. 25:73942024. View Article : Google Scholar : PubMed/NCBI
|