Introduction
Mitochondria and endoplasmic reticulum (ER) engage
in intricate physical and biochemical interactions within
specialized subcellular domains, the mitochondria-associated
membranes (MAM), where their intermembrane distance is finely tuned
to 10-20 nm (1,2). ER-mitochondria juxtaposition at MAM
is mediated by tethering proteins including inositol trisphosphate
receptor (IP3R), voltage-dependent anion channel (VDAC), Mitofusin
2 (Mfn2), vesicle-associated membrane protein-associated protein B
(VAPB) and protein tyrosine phosphatase-interacting protein-51
(PTPIP51) (3,4). IP3R-VDAC and VAPB-PTPIP51
complexes, as well as Mfn2 and Sigma-1R, regulate ER-mitochondria
Ca2+ flux (5-9). MAM are also enriched in lipid
biosynthesis enzymes, namely those involved in lipid droplets (LDs)
biogenesis (10,11). By regulating Ca2+ and
lipid exchange, MAM govern pivotal cellular events, including
mitochondrial bioenergetics and dynamics, redox balance, as well as
ER stress and innate immune responses, namely NOD-like receptor
family pyrin domain-containing 3 (NLRP3) inflammasome activation
(4,12-14). Dynamic and responsive to cellular
cues, MAM are vital platforms for intra-cellular homeostasis
(15) and their dysfunction is
implicated in various pathologies, including neurodegenerative
diseases, metabolic and cardiovascular disorders and cancer, some
of which are comorbidities of bipolar disorder (BD) (2,16-18).
BD is a chronic mental illness characterized by mood
swings between mania and depression, affecting 1-2% of the world's
population (19). Lifelong
treatment is required (20),
however, current drugs are limited and have notable side effects
(21). BD diagnosis is
complicated by the lack of specific biomarkers, emphasizing the
need for a deeper understanding of its pathophysiology (20,22). Despite its neurobiology remaining
unclear (20), anatomical and
neuropathological examinations of the brains of patients with BD
revealed abnormalities in the cellular resilience of neurons and
glial cells towards stress (23). Disruptions in mitochondrial
function and Ca2+ signaling, oxidative stress, apoptosis
and perturbation of ER stress and immune responses are consistently
reported in BD (23-27). Since these events are regulated
by MAM (18,25), which play a pivotal role in
coordinating cellular responses to stress, it was hypothesized that
MAM impairment may underlie the diminished cellular resilience
observed in BD. To test this, structural and functional changes
resulting from ER-mitochondria miscommunication were assessed in
dermal fibroblasts and monocytes from early-stage patients with BD
compared with matched healthy controls.
Given the challenges of live brain tissue sampling,
alternative in vitro models have emerged in BD research,
using both non-neuronal cells (such as fibroblasts) and neuronal
cells [such as olfactory neuronal epithelium and induced
pluripotent stem cells (iPSCs) differentiated in a neuronal
phenotype]. Several studies emphasize the occurrence of peripheral
tissue alterations in various brain diseases (28-30). Moreover, patient-derived skin
fibroblasts are a relevant BD cellular model due to: i) Sharing
ontogenetic origin with the central nervous system (CNS) and
expressing similar receptors and signaling pathways as neurons
(31,32); ii) expressing genes implicated in
psychiatric disorders (33);
iii) easy accessibility through skin biopsies, with efficient
establishment and maintenance in culture (32); iv) external factors (such as
medication) disappearing after five passages (34); v) displaying molecular
similarities with CNS cells, reflecting findings observed in brain
tissue. Notably, the skin-brain axis is increasingly recognized as
a dynamic interaction where changes in the skin not only mirror
brain conditions but can also influence brain function. This
bidirectional communication is rooted in the shared ectodermal
origin of epidermal and neural tissues, with overlapping genetic,
environmental and molecular risk factors shaping both systems
(35). In the last decade, the
skin-brain axis has been presented as a new perspective for
understanding several diseases, including neurodevelopmental
diseases such as autism spectrum disorder (35), neurodegenerative diseases such as
Alzheimer's disease (AD) (36)
and, more recently, behavioral changes such as depression and
anxiety (37).
The present study, by investigating structural and
functional alterations of MAM in dermal fibroblasts from patients
with BD compared with matched healthy controls, identified
increased ER-mitochondria juxtaposition at MAM in BD cells, which
was associated with enhanced lipid transfer and formation of LDs,
decreased ER-mitochondria Ca2+ exchange, as well as
oxidative stress, ER stress-induced UPR and basal NLRP3
inflammasome activation. The present study revealed MAM as a
potential pathophysiological mechanism driving impaired cellular
resilience in BD and the skin-brain axis as a novel framework for
studying the molecular mechanisms underlying BD
pathophysiology.
Materials and methods
Culture of primary fibroblasts and
monocytes
Dermal fibroblasts and peripheral blood (monocytes)
were obtained from five male subjects diagnosed with early BD
[stage 2 (38)] during euthymia
and five age-(average age of 26±4.6) and gender-matched healthy
controls, aged between 18-35 years old (Table SI). Participants were assessed
regarding a DSM-5 BD criteria (39) through a validated diagnostic
interview (40). This study was
approved by the Ethics Committee of the Centro Hospitalar e
Universitário de Coimbra (now ULS de Coimbra), reference 150/CES,
July 3, 2018, and the samples were taken following written informed
consent also approved by the same ethics committee (30). Dermal fibroblasts isolated from
skin biopsies were maintained in HAM's F10 medium (cat. no.
31550-023; Thermo Fisher Scientific, Inc.) supplemented with 10%
(v/v) heat-inactivated fetal bovine serum (FBS), 1% (v/v)
antibiotic solution (10,000 U/ml penicillin, 10,000 μ/ml
streptomycin) and 1% (v/v) L-Glutamine and AmnioMAX™-II Complete
Medium (cat. no. 11269-016; Thermo Fisher Scientific, Inc.) in a
proportion of 1:5. Cells were cultured in 75 cm2 flasks
and maintained in a humidified 5% CO2-95% air atmosphere
at 37°C, with the medium changed every 3 days. Cultures were
passaged by trypsinization when cells reached 80-90% confluence.
Fibroblasts with 6-16 passages were used for assays (41). Primary monocytes were isolated
and cultured as previously described (42,43). Briefly, collected peripheral
blood was carefully layered onto Ficoll-Paque Plus, followed by
centrifugation to separate mononuclear cells. Monocytes
(CD14+ fraction) were then isolated using a magnetic
separation system with the Human Monocyte Isolation kit II
(Miltenyi Biotec), following the manufacturer's instructions.
Primary monocytes were cultured in glutamine-free RPMI 1640 Medium
(cat. no. 21870084 Gibco, Thermo Fisher Scientific, Inc.),
supplemented with HEPES, 10% (v/v) heat-inactivated FBS, 100 U/ml
penicillin, 100 μg/ml streptomycin, 2 mM glutamax, 1 mM
sodium pyruvate, and 0.1 mM non-essentials amino acids. Cells were
maintained at 37°C in a humidified 5% CO2 incubator.
Live cell imaging of ER-tracker and
Mitotracker colocalization
Fibroblasts seeded for 48 h at a density of
0.1×105 cells/cm2 on 18 mm coverslips were
loaded for 30 min at 37°C with 0.1 μM Mitotracker Green
(cat. no. m7514; Invitrogen; Thermo Fisher Scientific, Inc.) and
0.5 μM ER-tracker Red (cat. no. E34250 Thermo Fisher
Scientific, Inc.) in Krebs medium (140 mM NaCl, 5 mM KCl, 1.2 mM
Na2HPO4, 1 mM MgCl2, 9.6 mM
glucose, 20 mM Hepes, 1 mM CaCl2, pH 7.4). Nuclei were
stained with 15 μg/ml Hoechst 33342 for 5 min at room
temperature (RT). Images were obtained using a Zeiss LSM 710
confocal microscope with plan-Apochromat 40×/1.4 objective (Zeiss
AG). Colocalization of Mitotracker with ER-tracker was measured by
using the Mander's coefficient of colocalization with the ImageJ
v2.14 (National Institutes of Health) JACoP plugin (44). Mitochondrial area was calculated
using the same plugin in Mitotracker Green images.
Confocal microscopy analysis of IP3R-VDAC
colocalization
Fibroblasts were cultured for 48 h at a density of
0.1×105 cells/cm2, at 37°C, in 12-well plates
with 18 mm coverslips. Immunocytochemistry was performed as
previously described (41).
Briefly, cells were fixed with 4% (w/v) paraformaldehyde, pH 7.4,
in PBS for 15 min. After permeabilization with 0.1% (v/v) Triton
X-100 for 5 min, fibroblasts were blocked for 1 h with 3% (w/v) BSA
prepared in 0.2% (v/v) Tween 20. Afterwards, cells were incubated
overnight at 4°C with mouse anti-VDAC1 (1:250; cat. no. ab14734;
Abcam) and rabbit anti-IP3R (1:1,000; cat. no. ab5804; Abcam)
primary antibodies. Cells were then incubated with the secondary
antibodies Alexa Fluor 594 goat anti-rabbit (1:200; cat. no.
A11012; Invitrogen; Thermo Fisher Scientific, Inc.) and Alexa Fluor
488 goat anti-mouse (1:200; cat. no. A11001; Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h, at RT. Proximity ligation assay
(PLA), using the Duolink reagents (cat. no. DUO92002/4-100RXN;
MilliporeSigma), was used following the manufacturer's
instructions. Cells were fixed with 4% (w/v) paraformaldehyde, pH
7.4, in PBS for 15 min. Permeabilization was performed using 0.1%
(v/v) Triton X-100 in PBS for 5 min, and blocking was done with 3%
(w/v) BSA prepared in 0.2% (v/v) Tween 20, for 1 h, at RT. After
permeabilization, fibroblasts were incubated overnight at 4°C with
the mouse anti-VDAC1 (1:250; cat. no. ab14734; Abcam) and rabbit
anti-IP3R (1:1,000; cat. no. ab5804; Abcam) primary antibodies,
prepared in blocking solution. Cells were then incubated with plus
and minus PLA probes (1:5) for 1 h at RT. The coverslips were
incubated with ligase (1:40) for 30 min at RT, followed by
incubation with polymerase (1:80) for 1 h and 20 min at RT. In both
techniques, nuclei were stained with Hoechst 33342 (15
μg/ml) for 5 min at RT, and the coverslips were mounted
using Aqua-Poly/Mount mounting medium (cat. no. 18606 Polysciences
Inc.). Images were obtained using a Zeiss LSM 710 confocal
microscope with plan-Apochromat 40×/1.4 objective (Zeiss AG).
Colocalization of VDAC1 with the IP3R was measured with Mander's
coefficient of colocalization through ImageJ v2.14 (National
Institutes of Health) JACoP plugin (44). The particle analysis function of
ImageJ was used to quantify the number of PLA red fluorescent spots
per cell.
Transmission electron microscopy
(TEM)
Fibroblasts cultured for 48 h in a 100 mm cell
culture dish were fixed with 2.5% (w/v) glutaraldehyde in 0.1 M
sodium cacodylate buffer (pH 7.2) for 2 h, at RT. Post-fixation was
performed using 1% (w/v) osmium tetroxide for 90 min. After rinsing
in buffer and distilled water, 1% (w/v) aqueous uranyl acetate was
added and incubated for 1 h, at RT, in the dark for contrast
enhancement. Samples were embedded in 2% (w/v) molten agar,
dehydrated in a graded ethanol series (30-100%) and then
impregnated and embedded in Epoxy resin. Ultrathin sections (70 nm)
were mounted on copper grids and stained with 0.2% (w/v) lead
citrate for 7 min. Observations were carried out on a FEI-Tecnai
G2 Spirit BioTwin electron microscope (Thermo Fisher
Scientific, Inc.) at 100 kV. ImageJ v2.14 (National Institutes of
Health) was used to measure the ER-mitochondria distance in
fibroblasts.
Western blot analysis of MAM components,
Ca2+ channels and ER stress markers
Skin fibroblasts were lysed on ice with an ice-cold
lysis RIPA buffer [250 mM NaCl, 50 mM Tris base, 1% (v/v) Nonidet
P-40, 0.5% (v/v) sodium deoxycholate (DOC), 0.1% (v/v) Sodium
Dodecyl Sulfate (SDS), pH 8.0] freshly supplemented with 2 mM DTT,
1% (v/v) protease inhibitor cocktail (cat. no. P2714;
MilliporeSigma), and PhosSTOP™ (cat. no. 4906837001;
MilliporeSigma). After 20 min on ice, nuclei and insoluble cell
debris were removed by centrifugation at 18,000 × g for 10 min at
4°C, and the supernatants were collected and stored at −20°C until
further use. The total protein amount of each sample was quantified
using the Pierce bicinchoninic acid (BCA) assay kit (cat. no.
23225; Thermo Fisher Scientific, Inc.). Then, thirty micrograms of
protein from total cell lysates were separated by electrophoresis
in 10-12.5% SDS polyacrylamide gels (SDS/PAGE) and transferred to
PVDF membranes (cat. no. ipvh00010; MilliporeSigma). After blocking
with 5% (w/v) BSA during 1 h, at RT, membranes were incubated
overnight, at 4°C with the following primary antibodies: mouse
anti-VDAC (1:1,000; cat. no. sc-390996; Santa Cruz Biotechnology,
Inc.), rabbit anti-MCU (1:1,000; cat. no. 14997S; Cell Signaling
Technology, Inc.), rabbit anti-STIM1 (1:1,000; cat. no. 11565-1-AP;
Proteintech Group, Inc.), mouse anti-GRP78 (1:1,000; cat. no.
610978 BD Biosciences), rabbit anti-ATF4 (1:1,000; cat. no. 11815S;
Cell Signaling Technology, Inc.), rabbit anti-ATF6 (1:1,000; cat.
no. ab37149; Abcam), mouse anti-Mfn2 (1:1,000; cat. no. sc-100560;
Santa Cruz Biotechnology, Inc.), goat anti-Sigma-1R (1:1,000; cat.
no. sc-22948; Santa Cruz Biotechnology, Inc.), rabbit anti-VAPB
(1:1,000; cat. no. 14477-1-AP; Proteintech Group, Inc.), and rabbit
anti-PTPIP51 (1:1,000; cat. no. 20641-1-AP; Proteintech Group,
Inc.). Then membranes were incubated for 1 h, at RT, with
HRP-conjugated goat anti-mouse IgG (1:20,000; cat. no. 31432;
Thermo Fisher Scientific, Inc.), goat anti-rabbit IgG (1:20,000;
cat. no. 31462; Thermo Fisher Scientific, Inc.) or rabbit anti-goat
IgG (1:20,000; cat. no. 31402; Thermo Fisher Scientific, Inc.). The
protein immunoreactive bands were visualized by chemiluminescence
with the Pierce ECL Western Blotting Substrate (cat. no. 32106;
Thermo Fisher Scientific, Inc.) in a Chemidoc Imaging System
(Bio-Rad Laboratories, Inc.). The monoclonal anti-β-actin antibody
(1:10,000; cat. no. A5441; MilliporeSigma) was used for protein
loading control. The optical density of the bands was quantified
with the Image Lab Software (Bio-Rad Laboratories, Inc.) (41). Stripping followed by reprobing
with another antibody to label proteins of different molecular
weight was performed in some membranes, as indicated in the figure
legends.
Fluorescence microscopy quantification of
lipid droplets and TOM-20
Fibroblasts cultured for 48 h at a density of
0.4×104 cells/cm2 in μ-slide 8 well
plates (cat. no. 80806 Ibidi) were fixed with 4% (w/v)
paraformaldehyde, pH 7.4 for 15 min, permeabilized with 0.1% (v/v)
Triton X-100 for 5 min and blocked with 3% (w/v) BSA prepared in
0.05% (w/v) saponin for 1 h, at RT. Cells were then incubated
overnight at 4°C with the rabbit anti-translocase of outer
mitochondrial membrane 20 (TOM20; 1:500; cat. no. sc-11415; Santa
Cruz Biotechnology, Inc.) primary antibody and then with Alexa
Fluor 488 goat anti-rabbit secondary antibody (1:200; cat. no.
A11008; Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h, at RT.
After loading with the fluorescent probe LipidTOX red (1:200; cat.
no. H34476; Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h,
nuclei were stained with Hoechst 33342 (15 μg/ml) for 5 min
at RT and confocal images were obtained using a Zeiss LSM 710
confocal microscope (Zeiss AG) with a plan-Apochromat 40×/1.4
objective. The number of red spots (LDs)/cell was quantified using
the Imaris Microscopy Image Analysis Software Spot function v10.2
(Oxford Instruments).
Single cell calcium imaging (SCCI)
Fibroblasts seeded for 48 h on 18 mm coverslips at a
density of 0.1×105 cells/cm2 were loaded for
30 min at 37°C with 4 μM Fluo-4/AM (cat. no. F14201;
Invitrogen; Thermo Fisher Scientific, Inc.) or 10 μM
Rhod-2/AM (cat. no. R1244; Invitrogen; Thermo Fisher Scientific,
Inc.), both prepared in solution A (135 mM NaCl, 5 mM KCl, 1 mM
MgCl2, 1 mM MgSO4, 20 mM HEPES, 0.4 mM
KH2PO4, 5.5 mM glucose and 1 mM
CaCl2, pH 7.4). After incubation, cells were kept in
solution B (135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM
MgSO4, 20 mM HEPES, 0.4 mM KH2PO4,
5.5 mM glucose and 0.5 mM EGTA, pH 7.4), at 37°C. Basal
fluorescence was recorded every 20 sec for 2 min and upon stimuli
with 100 μM histamine (cat. no. H7125; MilliporeSigma) the
fluorescence was recorded every 1 sec for at least 4 min. In a
Ca2+-free medium, store-operated Ca2+ entry
(SOCE) was analyzed upon induction of ER Ca2+ depletion
with 1 μM thapsigargin (cat. no. T9033; MilliporeSigma)
followed by the addition of 2 mM Ca2+ to promote the
uptake of Ca2+ from the extracellular medium (45). Images were acquired using a Zeiss
Cell Observer Spinning Disk microscope with plan-Apochromat 20×/0.8
objective (Zeiss AG).
Analysis of oxidative stress
Fibroblasts seeded for 48 h at a density of
0.4×104 cells/cm2 in μ-slide 8 well
ibidi plates (cat. no. 80806 Ibidi) were loaded with 5 μM
CellROX green (cat. no. C10444; Invitrogen; Thermo Fisher
Scientific, Inc.) in Krebs medium for 30 min, at 37°C. Live cell
imaging was performed during 3 min using a Zeiss Cell Observer Z1
microscope with a plan-Apochromat 20×/0.8 objective (Zeiss AG).
Fibroblasts cultured for 48 h at a density of 0.1×105
cells/cm2 were incubated with 10 μM MitoPY (cat.
no. SML0734; MilliporeSigma) in Krebs medium for 30 min at 37°C.
Basal fluorescence values were measured for 5 min in a fluorimeter
SpectraMax Gemini EM microplate reader set to 503 nm excitation and
540 nm emission wavelengths. Values were normalized to the protein
amount. An enzymatic superoxide dismutase (SOD) assay kit (cat. no.
19160-1KT-F; MilliporeSigma) was used to determine the activity of
the mitochondrial isoform of SOD, the superoxide dismutase 2 (SOD2)
in primary fibroblasts derived from patients with BD and healthy
subjects, according to the manufacturer's instructions. For this
purpose and as previously described (42), fibroblasts were lysed on RIPA
buffer [250 mM NaCl, 50 mM Tris base, 1% (v/v) Nonidet P-40, 0.5%
(v/v) sodium deoxycholate (DOC), 0.1% (v/v) Sodium Dodecyl Sulfate
(SDS), pH 8.0], without supplements. The total activity of SOD and
particular the activity of SOD2 were differentiated by the
inclusion of 2 mM potassium cyanide (KCN), which selectively
inhibits cytosolic isoform of SOD antioxidant enzyme, the
superoxide dismutase 1 (SOD1). The plate was then incubated at 37°C
for 20 min, and the absorbance was read in a spectrophotometer
Spectramax plus 384 microplate reader (BioTek Instruments) set to
450 nm. Values were normalized to the protein amount determined by
the BCA method.
Reverse transcription-quantitative (RT-q)
PCR quantification of pro-inflammatory mediators
Fibroblasts were plated at a density of
0.5×105 cells/cm2 and stabilized for 24 h.
RNA was extracted with the NZYol reagent (cat. no. MB18501;
Nzytech), and its concentration was measured using a NanoDrop 2000c
Spectrophotometer (Thermo Fisher Scientific, Inc.). Samples were
stored at −80°C for subsequent use. Briefly, the total RNA (2
μg per sample) was reverse transcribed into cDNA using the
NZY First-Strand cDNA Synthesis kit (cat. no. MB12502; Nzytech).
Then, RT-qPCR were performed, in triplicate for each sample, using
the NZYSpeedy qPCR Green Master Mix Kit (cat. no. MB22403;
Nzytech), in the CFX Connect RT-PCR Detection System (Bio-Rad
Laboratories, Inc.), as previously described (46). Briefly, samples were denatured at
95°C for 3 min. 40 cycles were run for 10 sec at 95°C for
denaturation, 30 sec at 55°C for annealing and 30 sec at 72°C for
elongation. After amplification, a threshold was set for each gene,
and Cq values were calculated for all the samples (47). Gene expression changes were
analyzed using the CFX Maestro 1.1 system software (Bio-Rad
Laboratories, Inc.). The primer sequences were designed using the
Beacon Designer software version 7.7 (Premier Biosoft
International), and were thoroughly tested. The forward (F) and
reverse (R) primers used were indicated in Table SII (Eurofins Scientific). Hprt-1
was used as a house-keeping gene to normalize the results of the
genes of interest.
Colorimetric determination of caspase-1
activity
After 24 h of stabilization, the culture medium of
plated fibroblasts at a density of 0.5×105
cells/cm2 was replaced by FBS-free HAM's F10 medium and
cells were exposed to 5 μg/ml lipopolysaccharide (LPS) for
32 h or were kept in FBS-free HAM's F10 medium. Fibroblasts were
then lysed in lyses buffer (25 mM HEPES, 1 mM EDTA, 1 mM EGTA, 2 mM
MgCl2, pH 7.5) through consecutive series of
freezing/thawing in liquid N2. Proteins (100
μg/ml) were incubated with 4 mM caspase-1 substrate (cat.
no. SCP0066; MilliporeSigma) in buffer [25 mM HEPES, 10% (w/v)
sucrose, pH 7.5] freshly supplemented with 0.1% (w/v) CHAPS and 10
mM DTT. After 2 h of incubation at 37°C, absorbance was measured at
405 nm in a standard Synergy HT Multi Detection Microplate
Reader.
ELISA measurement of secreted IL-1β
levels
Fibroblasts and monocytes were seeded at a density
of 0.5×105 cells/cm2 and 0.12×106
cells/well, respectively. Culture medium was then replaced by
FBS-free medium and cells were exposed to LPS at a concentration of
5 μg/ml (fibroblasts) or 1 μg/ml (monocytes) for 32 h
or kept in FBS-free medium. After concentrating fibroblast
supernatants with concentration columns (cat. no. VS0192; Sartorius
AG), IL-1β secretion was quantified by ELISA kit (cat. no. 437004;
BioLegend, Inc.) following manufacturer's instructions (42,43). Absorbance values were measured in
a standard Synergy HT Multi Detection Microplate Reader (BioTek
Instruments) set to 450 nm wavelength. IL-1β levels were expressed
as pg/ml in the case of fibroblasts and as values normalized to the
baseline values in the case of monocytes. For both cells, the
results were also presented as fold increase calculated by the
difference between the LPS-treated and baseline values.
Data analysis
Results were expressed as mean ± standard error of
the mean. Normality was assessed by the D'Agostino and Pearson and
Shapiro-Wilk tests. Statistical analysis was performed by using the
parametric unpaired Student's t-test or the Mann-Whitney
non-parametric test. A two-way ANOVA test followed by Tukey's or
Sidak's post-hoc test was used for multiple comparisons with two
factors. Statistical analysis was performed using the GraphPad
Prism software 9.0 (Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
Tight ER-mitochondria juxtaposition in BD
fibroblasts
To investigate alterations in ER-mitochondria
apposition in fibroblasts derived from patients with BD and healthy
controls, the colocalization between the ER (ER-Tracker) and
mitochondria (Mitotracker) was assessed by confocal microscopy
(Fig. 1A). BD fibroblasts
exhibited increased ER-mitochondria colocalization compared with
controls (Fig. 1B). This
observation was corroborated by results demonstrating higher
proximity between IP3R (ER marker) and VDAC (mitochondria marker;
Fig. 1C and D), as well as by a
rise in the number of PLA dots, which results from enhanced
IP3R-VDAC contacts points, in BD fibroblasts compared with controls
(Fig. 1E and F), strongly
suggesting increased ER-mitochondria contacts in this BD cellular
model. Furthermore, the contact sites between the ER and
mitochondria (≤80 nm) were quantified by TEM (Fig. 1J). A significant reduction in the
physical distance between both organelles was identified in BD
fibroblasts compared with controls (Fig. 1K). The distribution of those
contacts clear demonstrate that BD fibroblasts exhibit a markedly
higher percentage of close contacts ≤20 nm (MAM) and a lower
percentage of long contacts >20 nm, in comparison with control
cells (Fig. 1L). Together, the
findings obtained using different microscopy approaches suggested
increased ER-mitochondria close contacts sites (MAM) in fibroblasts
derived from patients with BD. Notably, these alterations are
independent of changes in the number and area of mitochondria
(Fig. 1G-I). To investigate
whether MAM upregulation in fibroblasts derived from patients with
BD is associated with changes in ER-mitochondria tethers, the
protein levels of key MAM tethering complexes, specifically Mfn2
(Fig. 2A and B), VDAC (Fig. 2C and D), VAPB (Fig. 2G and H) and PTPIP51 (Fig. 2K and L), were assessed by western
blotting. A significant rise in VDAC levels (Fig. 2C and D) and a qualitative, though
not statistically significant, increase in PTPIP51 content
(Fig. 2K and L) was observed in
BD fibroblasts compared with control cells.
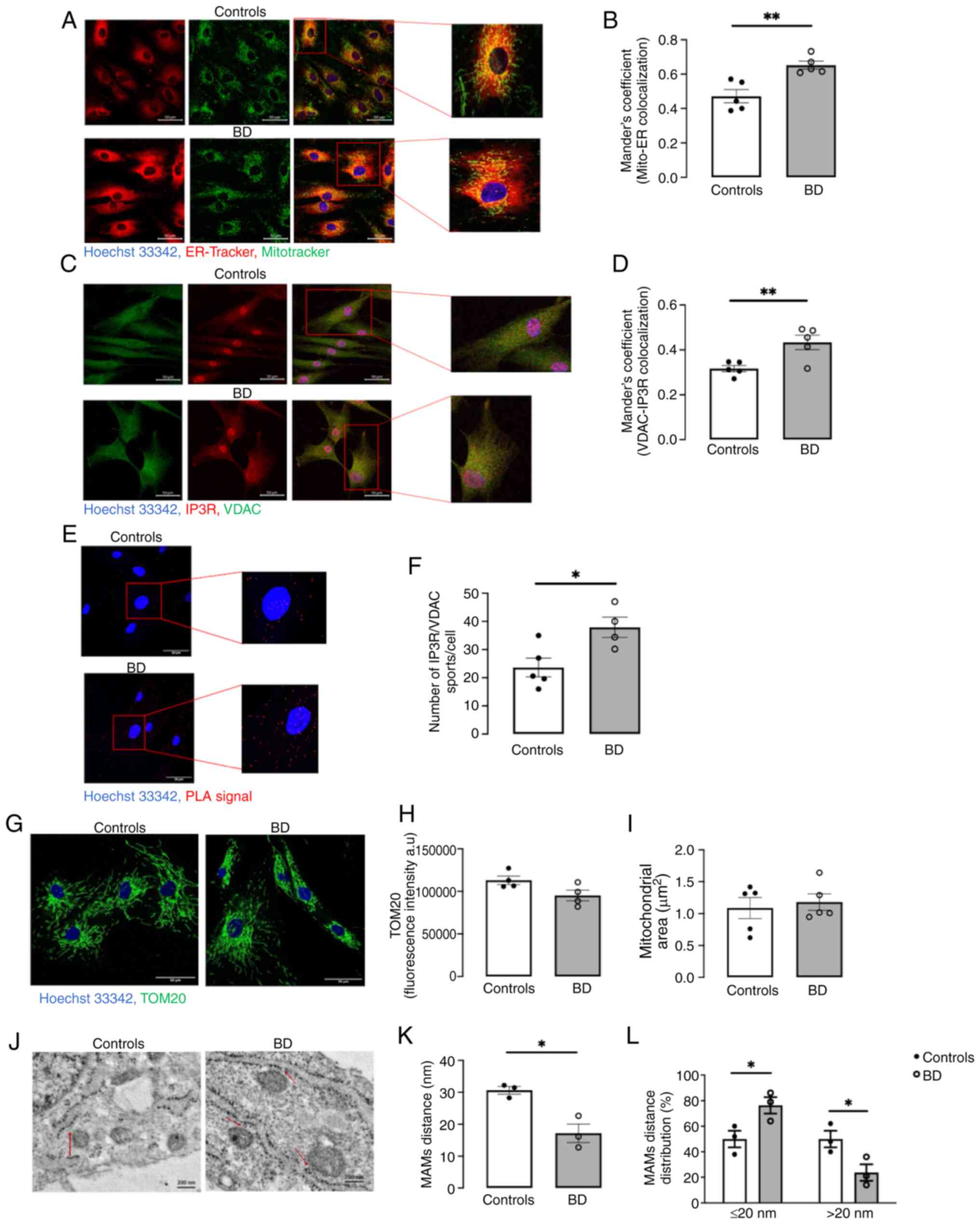 | Figure 1ER-mitochondria contacts in primary
fibroblasts derived from patients with BD and healthy controls.
Colocalization of Mitotracker and ER-Tracker fluorescent probes was
evaluated by live cell imaging. (A) Representative confocal
microscopy images of Mitotracker (green) and ER-Tracker (red)
immunoreactivity and nuclei labelling with Hoechst 33342 (blue;
scale bar, 50 μm). (B) The mitochondrial fraction
(Mitotracker) that colocalizes with the ER (ER-Tracker) was
measured with the Mander's coefficient of colocalization using the
ImageJ software. Colocalization of the IP3R (ER marker) and the
VDAC (mitochondrial marker) was evaluated by immunocytochemistry.
(C) Representative confocal microscopy images of VDAC (green) and
IP3R (red) immunoreactivity and nuclei labelling with Hoechst 33342
(blue; scale bar, 50 μm). (D) The mitochondrial fraction
(VDAC) that colocalizes with the ER (IP3R) was measured with the
Mander's coefficient of colocalization (ImageJ software). Data
represent the means ± standard error of the mean of results
obtained by the analysis of five images from five participants per
group. The number of IP3R/VDAC dots per cell was determined by the
PLA. (E) Representative confocal microscopy images of PLA signal
IP3R/VDAC dots (red) and nuclei labelling with Hoechst 33342 (blue;
scale bar, 50 μm). (F) PLA fluorescent quantification was
performed using the Particle Analysis function of ImageJ software.
Data represent the means ± standard error of the mean of results
obtained by the analysis of five images from five controls and four
patients. The mitochondrial network was analyzed by
immunocytochemistry. (G) Representative confocal microscopy images
of TOM20 immunoreactivity (green) and Hoechst 33342-stained nuclei
(blue; scale bar, 50 μm). (H) TOM20 staining was analyzed
using the ImageJ software. Data represent the means ± standard
error of the mean of results obtained by the analysis of five
images collected from four participants per group. (I) The
mitochondrial area was quantified in the confocal images of
Mitotracker (Fig. 1A) using a
macro designed in the ImageJ software. Statistical significance of
differences between experimental groups was determined by unpaired
Student's t-test. The ER-mitochondria contacts were evaluated by
transmission electron microscopy. (J) Representative images of the
ER-mitochondria contacts (arrow; scale bar, 200 nm). (K)
ER-mitochondria distance was measured using the ImageJ software.
(L) Distribution of the ER-mitochondria contacts: close contacts
(≤20 nm; MAM) and large contacts (>20 nm). Data represent the
means ± standard error of the mean of results obtained by the
analysis of at least five images collected from three participants
per group. Statistical significance between groups was determined
using the unpaired Student's t-test *P<0.05,
**P<0.01; and the comparison of close (≤20 nm) and
large contacts (>20 nm) between the two groups was obtained
using the two-way ANOVA test, followed by the Sidak's post hoc
test: *P<0.05. ER, endoplasmic reticulum; BD, bipolar
disorder; IP3R, inositol trisphosphate receptor; VDAC,
voltage-dependent anion channel; PLA, proximity ligation assay;
TOM20, translocase of outer mitochondrial membrane 20; MAM,
mitochondria-associated membranes. |
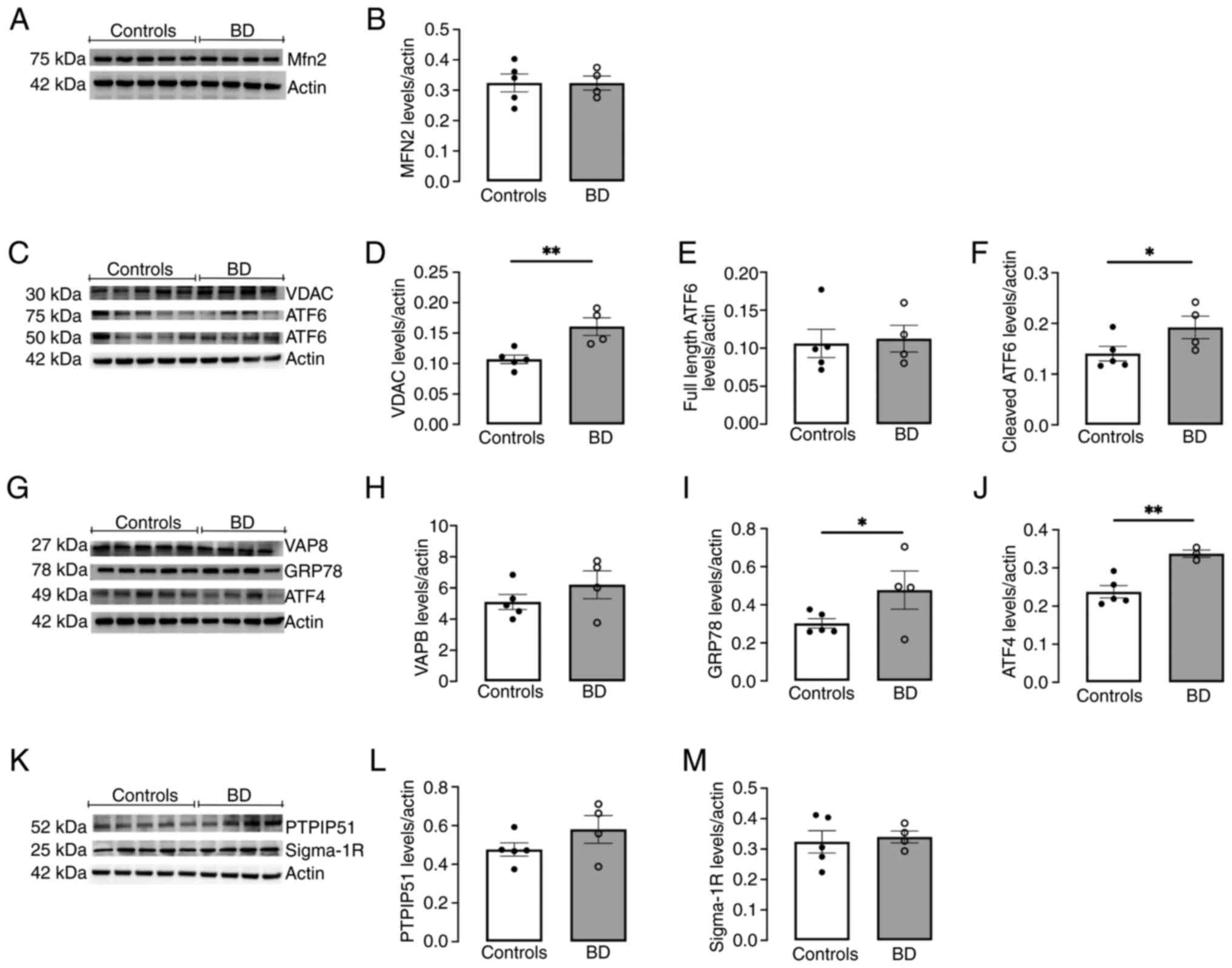 | Figure 2Levels of components of
ER-mitochondria contacts (Mfn2, VDAC, VAPB, PTPIP51 and Sigma-1R)
and ER stress-induced UPR markers (GRP78, ATF4 and ATF6) in primary
fibroblasts derived from patients with BD and healthy controls.
Protein levels of (A and B) Mfn2, (C and D) VDAC, (C, E and F)
ATF6, (G and H) VAPB, (G and I) GRP78, (G and J) ATF4, (K and L)
PTPIP51 and (K and M) Sigma-1R, were quantified by western blotting
in total cellular extracts obtained from primary fibroblasts
derived from patients with BD and healthy controls. β-actin was
used to control protein loading and to normalize the levels of the
proteins of interest. Data represent the means ± standard error of
the mean of results obtained in samples from five healthy controls
and four patients with BD. Statistical differences between
fibroblasts from patients with BD and healthy controls were
analyzed using the unpaired Student's t-test:
*P<0.05, **P<0.01. (A) The Mfn2
antibody was incubated in the first western blotting. VDAC and ATF6
antibodies were both incubated in the second western blotting. (C)
The ATF6 antibody, which recognizes both full-length (75 kDa) and
cleaved protein (50 kDa), was incubated on the VDAC western
blotting membrane, after a stripping procedure. (G) The VAPB, GRP78
and ATF4 antibodies were labelled on the third western blotting.
Upon incubation with the VAPB antibody, the western blotting
membrane was then incubated with ATF4 and GRP78, after a stripping.
(K) PTPIP51 and Sigma-1R antibodies were both incubated in the
fourth western blotting. The Sigma-1R antibody was incubated on the
PTPIP51 western blotting membrane, after a stripping. ER,
endoplasmic reticulum; UPR, unfolded protein response; BD, bipolar
disorder; MAM, mitochondria-associated membranes; Mfn2, Mitofusin
2; VDAC, voltage-dependent anion channel; VAPB, vesicle-associated
membrane protein associated protein B; PTPIP51, protein tyrosine
phosphatase-interacting protein-51; GRP78, glucose-regulated
protein 78; ATF4. activating transcription factor 4;
ATF6-activating transcription factor 6. |
Altered lipid metabolism and calcium
homeostasis in BD fibroblasts
Given the pivotal role of MAM in the transfer of
Ca2+ and lipids between the ER and mitochondria, the
present study then evaluated MAM function in control and BD
fibroblasts, by examining lipid metabolism and Ca2+
fluxes (4,12-14).
The content of LDs was assessed as an indicator of
the efficacy of ER-mitochondria lipid transfer and, therefore, as a
measure of MAM function (10).
The quantity of LDs was evaluated by using lipidTOX, a fluorescent
probe with high affinity for LDs (Fig. 3A). Quantitative analysis
demonstrated a notable enhancement in the number of LDs per cell in
BD fibroblasts compared with control cells (Fig. 3B), suggesting a correlation
between upregulated MAM and enhanced lipid metabolism in BD.
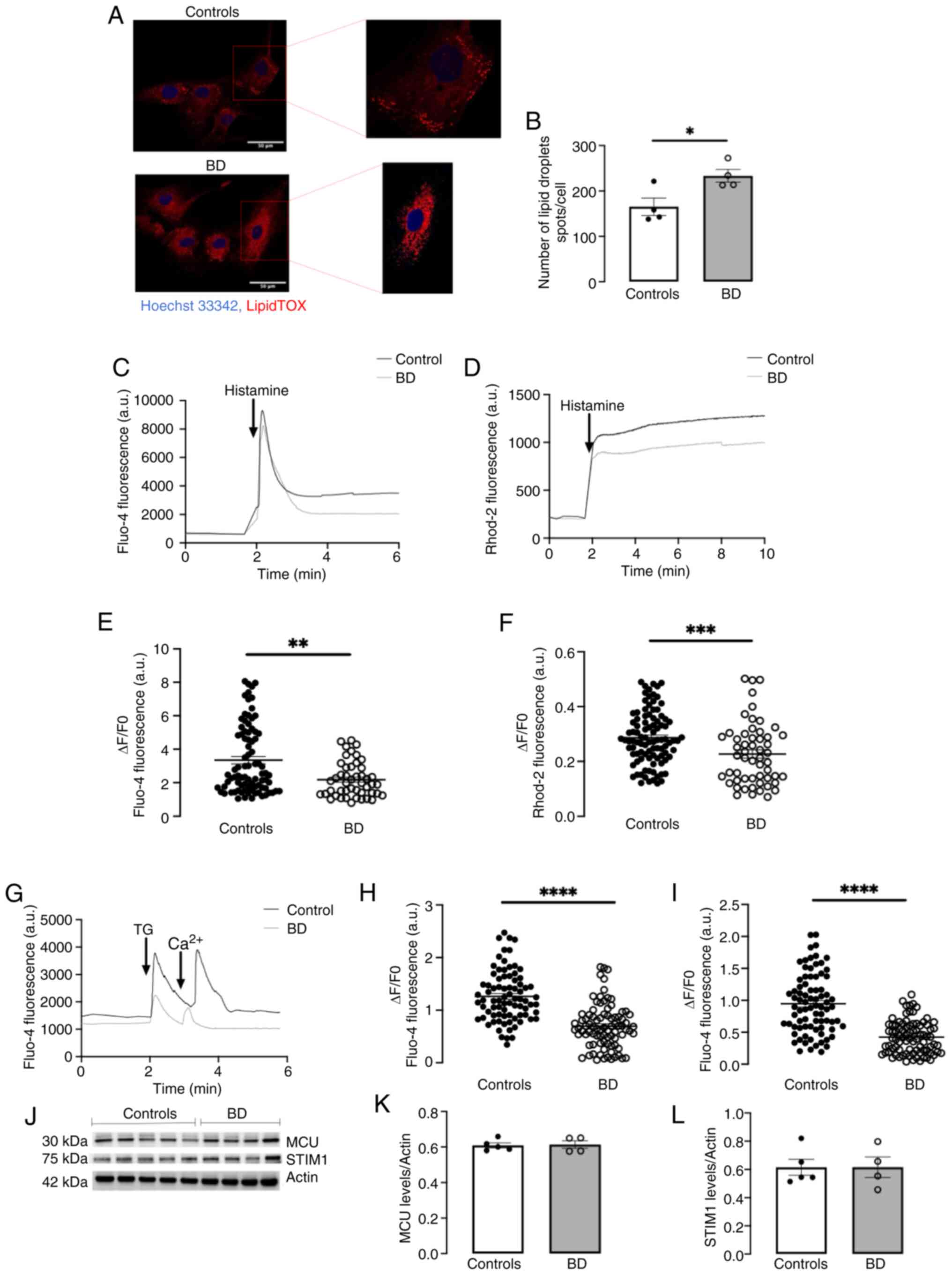 | Figure 3Lipid droplets accumulation and
Ca2+ dynamics in primary fibroblasts derived from
patients with BD and healthy controls. The number of lipid droplets
per cell was determined using the LipidTOX fluorescent probe. (A)
Representative confocal microscopy images of lipid droplets spots
(red) and nuclei labelling with Hoechst 33342 (blue; scale bar, 50
μm). (B) Quantification of LipidTOX fluorescent was
performed using the Spot function of Imaris Microscopy Image
Analysis Software. Data represent the means ± standard error of the
mean of results obtained by the analysis of at least five images
collected from four participants per group. IP3R-mediated ER
Ca2+ efflux and its influx into mitochondria were
measured by SCCI with the fluorescent probes (C) Fluo-4 and (D)
Rhod-2, respectively, after treatment with histamine (100
μM). (E and F) The SCCI results were baseline-corrected and
normalized to calculate the normalized fluorescent calcium signals.
Data were expressed as ΔF/F0, where ΔF=F-F0. F represents the
highest post-stimulus value and F0 the mean baseline level. Data
represent the means ± standard error of the mean of results
obtained from 15-30 cells from each of the four participants per
group. (G) SOCE was evaluated by measuring Ca2+
oscillations with Fluo-4 after thapsigargin (1 μM,
TG)-induced ER Ca2+ depletion in Ca2+-free
medium and after addition of 2 mM CaCl2 to the
extracellular medium. (H and I) The SCCI results were
baseline-corrected and normalized to calculate the normalized
fluorescent calcium signals. Data were expressed as ΔF/F0, where
ΔF=F-F0. F represents the highest post-stimulus value and F0 the
mean baseline level. Data represent the means ± standard error of
the mean of results obtained from 15-30 cells from each of the four
participants per group. (J and K) MCU and (J and L) STIM1 protein
levels were evaluated in total cellular extracts obtained from
control and BD fibroblasts through western blotting. β-actin was
used to control protein loading and to normalize the levels of the
proteins of interest. Data represent the means ± standard error of
the mean of results obtained in samples from five healthy controls
and four patients with BD. The STIM1 antibody was incubated on the
Sigma-1R western blotting membrane, after a stripping. Statistical
difference between groups was evaluated with unpaired Student's
t-test (B, D, H, I and L) or with Mann-Whitney non-parametric test
(F and K): *P<0.05; **P<0.01;
***P<0.001; ****P<0.0001. BD, bipolar
disorder; IP3R, inositol trisphosphate receptor; ER, endoplasmic
reticulum; SCCI, single cell calcium imaging; STIM1, stromal
interaction molecule 1; MCU, mitochondrial Ca2+
uniporter protein. |
The IP3R-mediated ER-mitochondria Ca2+
transfer was determined by using Rhod-2AM, a mitochondrial
Ca2+ indicator. Notably, BD fibroblasts stimulated with
the IP3R agonist histamine exhibited a significant reduction in
mitochondrial Ca2+ uptake compared with controls
(Fig. 3D and F), despite the
decreased ER-mitochondria distance in BD cells. The data showed
that these differences were not due to downregulation of the
mitochondrial calcium uniport (MCU) at IMM, which aligns with the
ER IP3R and VDAC at outer mitochondrial membrane (OMM) to
facilitate the transfer of Ca2+ into mitochondria
(48), since similar protein
levels were detected in both control and BD cells (Fig. 3J and K). Furthermore, they were
not associated with alterations in Sigma-1R, a modulator of
IP3R-mediated ER-mitochondria Ca2+ transfer, since its
protein levels were not affected in BD (Fig. 2K and M). The alterations in the
ER-mitochondria Ca2+ transfer observed between control
and BD cells might instead arise from compromised IP3R-mediated ER
Ca2+ release, since the cytosolic Ca2+ levels
measured with Fluo-4/AM in histamine-stimulated BD fibroblasts were
lower compared with those of control cells (Fig. 3C and E). This diminished release
observed in BD cells can result from a deficient replenishment of
Ca2+ in the ER lumen by SOCE-mediated extracellular
Ca2+ influx, which in turn can modulate mitochondrial
Ca2+ uptake (49,50). To evaluate SOCE, fibroblasts were
loaded with Fluo-4/AM in Ca2+-free medium and stimulated
with thapsigargin, an inhibitor of the Sarco/endoplasmic reticulum
Ca2+-ATPase (SERCA) pump, to assess ER Ca2+
release, followed by the addition of extracellular Ca2+
to investigate SOCE-mediated Ca2+ entry. The analysis of
ER Ca2+ content in thapsigargin-stimulated cells
revealed the depletion of ER Ca2+ and impaired
SOCE-dependent Ca2+ influx in BD fibroblasts compared
with controls (Fig. 3G-I). These
observations suggested that the reduced ER-mitochondria
Ca2+ transfer in BD cells resulted from the depletion of
ER Ca2+ stores caused by impaired Ca2+ influx
from the extracellular space through the SOCE pathway, which fails
to replenish the ER. Notably, the BD-associated SOCE impairment is
not due to alterations in the protein levels of stromal interaction
molecule 1 (STIM1), a key component of the SOCE machinery (Fig. 3J and L).
Overall, the results showed that the proximity
between the ER and mitochondria at MAM in BD cells was intimately
linked to increased lipid exchange and biogenesis of LDs, but not
with stimulation of the ER-mitochondria Ca2+
transfer.
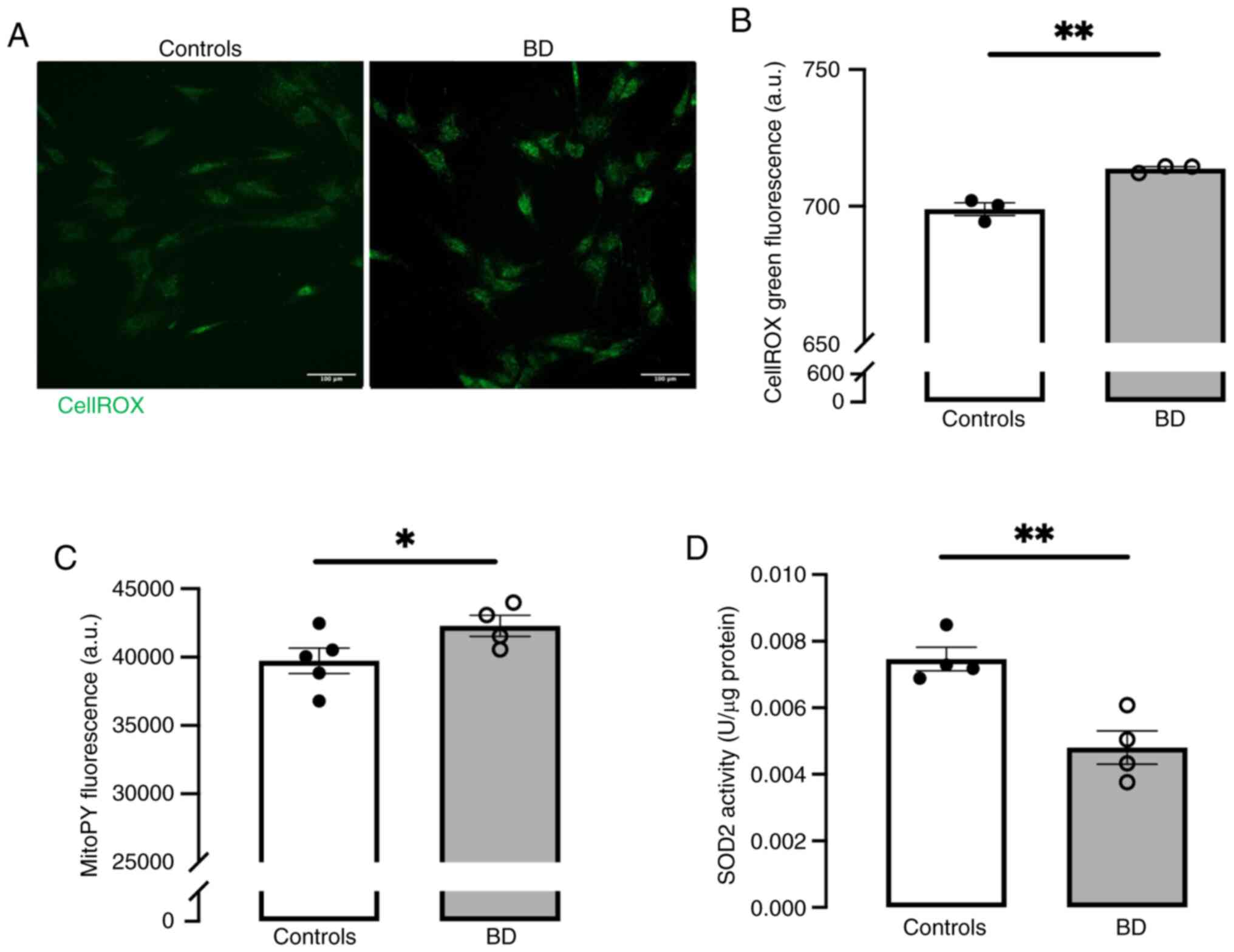
Impaired redox status in BD
fibroblasts
MAM play a crucial role in cellular redox status,
maintaining a dynamic equilibrium between reactive oxygen species
(ROS) production and antioxidant defenses (51). To assess oxidative stress in
fibroblasts derived from controls and patients with BD, ROS levels
were measured using CellROX and the mitochondrial-specific MitoPY
probes. A significant increase in ROS levels was observed (Fig. 4A and B) that are, at least in
part, generated in mitochondria (Fig. 4C), probably due to the inhibition
of SOD2 mitochondrial antioxidant enzyme (Fig. 4D).
ER stress-induced unfolded protein
response (UPR) markers in BD fibroblasts
The ER-mitochondria communication at MAM
coordinates cellular stress responses, particularly the UPR
(52). A notable upregulation in
the protein levels of several UPR markers, namely GPR78 (Fig. 2G and I), activating transcription
factor 4 (ATF4) (Fig. 2G and J)
and fragmented activating transcription factor 6 (ATF6) (Fig. 2C and F), was found in BD cells
compared with controls, indicating ER stress induction in these
patients.
Sterile inflammation in fibroblasts and
monocytes derived from patients with BD
MAM provide a physical platform for NLRP3
inflammasome assembly by facilitating the communication among its
components: NLRP3, caspase-1 and ASC (53,54). Activation of the NLRP3
inflammasome induces a signaling cascade culminating in the release
of pro-inflammatory mediators such as interleukin-1 beta (IL-1β)
(14,55). This cytosolic complex is
activated by MAM-derived signals, namely danger signals released by
dysfunctional mitochondria including ROS (13,14). Given the upregulation of MAM and
significant ROS accumulation in BD fibroblasts, it is hypothesized
that NLRP3 is activated in these cells playing a role in
maintaining a potent chronic inflammatory environment (56).
Increased mRNA levels of NLRP3 and pro-IL-1β
assessed by RT-qPCR (Fig. 5A and
B), coupled with enhanced caspase-1 activity (Fig. 5F), in fibroblasts from patients
with BD compared with controls, suggested NLRP3 inflammasome
activation and sterile inflammation in BD. The upregulation of
other pro-inflammatory cytokines, including TNFα, IL-6 and IL-8
detected in BD fibroblasts (Fig.
5C-E), provided strong evidence for a BD-associated
inflammatory state. Then, NLRP3 inflammasome activation was
evaluated under LPS-induced inflammation-prone conditions, by
quantifying secreted IL-1β levels, the classical readout of NLRP3
activation. A notable rise in IL-1β secretion was observed in
control fibroblasts treated with LPS for 32 h compared with
untreated control cells, which was not observed in BD cells
(Fig. 5I). This distinct pattern
of response to LPS was reinforced upon examining the stimulated
release of IL-1β, with a statistically significant decrease
observed in BD fibroblasts (Fig.
5J). Comparable outcomes were found in fibroblasts from
patients with BD and healthy subjects exposed to LPS for 12 h (data
not shown). Moreover, a marked increase in caspase-1 activity was
identified in LPS-exposed control fibroblasts, a response not
observed in treated BD fibroblasts (Fig. 5G). Comparing the fold increase of
caspase-1 in LPS-treated controls and BD fibroblasts reveals a
substantial reduction in the later (Fig. 5H). Lastly, IL-1β secretion was
quantified in primary monocytes isolated from the peripheral blood
of both patients with BD and healthy controls, exposed to LPS for
32 h. Similarly, these innate immune cells also exhibited a
deficient NLRP3 inflammasome activation when exposed to LPS
(Fig. 5K and L).
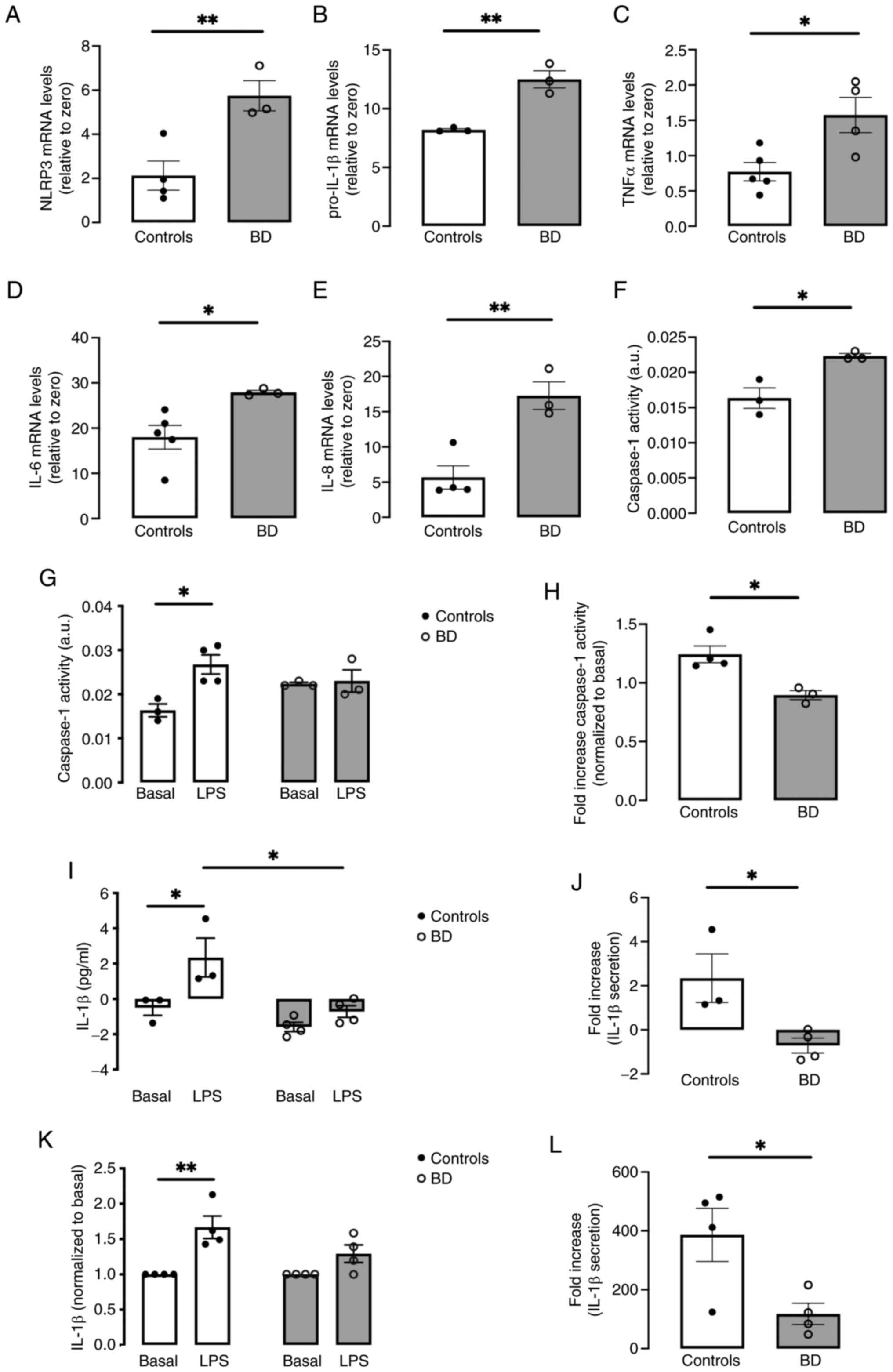 | Figure 5NLRP3 inflammasome activity and
inflammatory status in primary fibroblasts and monocytes derived
from patients with BD and healthy controls. Basal mRNA levels of
(A) NLRP3 and pro-inflammatory cytokines (B) pro-IL-1β, (C) TNFα,
(D) IL-6 and (E) IL-8 were evaluated by reverse
transcription-quantitative PCR in fibroblasts from patients with BD
and healthy controls. (F) Baseline levels of caspase-1 activity in
control and BD fibroblasts. (G) Caspase-1 activity was analyzed in
total extracts from control and BD fibroblasts treated in the
absence or presence of 5 μg/ml LPS for 32 h. (H) Fold
increase of caspase-1 activity was calculated by normalizing the
levels of caspase-1 activity in control and BD fibroblasts treated
with LPS to the baseline values. Data represent the means ±
standard error of the mean of results obtained in cells from 3-5
participants per group. (I) IL-1β levels in supernatants from
control and BD fibroblasts incubated in the presence or absence of
5 μg/ml LPS for 32 h, were quantified using an ELISA kit an
expressed as pg/ml. (J) Fold increase of IL-1β secretion was
calculated by the difference between LPS-treated and basal values.
Data represent the means ± standard error of the mean of results
obtained in cells from three controls and four patients with BD.
(K) IL-1β levels in supernatants from primary monocytes derived
from healthy controls and patients with BD incubated in the
presence or absence of 1 μg/ml LPS for 32 h, were quantified
by ELISA. Results were normalized to the basal levels and represent
the means ± standard error of the mean of results obtained in cells
from four participants per group. (L) IL-1β secretion in monocytes
was also calculated as the fold increase observed upon LPS
treatment. Data represent means ± standard error of the mean of
results obtained in cells from four participants per group.
Statistical significance of differences between groups was
determined by the unpaired Student's t-test (A-F, H, J and L):
*P>0.05; **P>0.01; significance between
both basal and LPS-treated cells within each experimental group and
between the two groups were obtained using the two-way ANOVA test,
followed by the Tukey's post hoc test (G, I and K):
*P>0.05; **P>0.01. NLRP3, NOD-like
receptor family pyrin domain-containing 3; BD, bipolar disorder;
LPS, lipopolysaccharide. |
Together, these findings illustrated that, under
basal conditions, patients with BD manifest a pro-inflammatory
state characterized by the upregulation of pro-inflammatory
cytokines. However, the response of the NLRP3 inflammasome to an
inflammatory stimulus is compromised in BD-derived cells,
indicating that these individuals are less responsive to
inflammation-prone conditions.
Discussion
The disruption of ER-mitochondria crosstalk at MAM
has been linked to the onset and progression of various human
pathologies, including cancer, neurodegenerative disorders, and
metabolic and cardiovascular diseases (2,16-18), some of which are comorbidities of
BD. MAM play a crucial role in essential biological functions,
namely lipid metabolism, calcium homeostasis, mitochondrial
bioenergetics and dynamics, redox balance and control over ER
stress and inflammatory responses (15,52). These MAM-related cellular events
are disrupted in BD (25),
suggesting a link between MAM alterations and BD pathophysiology.
Hence, the present study addressed the role of MAM in BD by using
cell-based models derived from early-stage patients with BD and
matched healthy controls.
Results from several microscopy techniques
demonstrated increased ER-mitochondria colocalization and higher
percentage of close ER-mitochondria contacts (MAM) in BD
fibroblasts compared with controls, together with increased levels
of the OMM protein VDAC and a qualitative trend towards increased
PTPIP51 levels, suggesting alterations in ER-mitochondria tethering
in BD. These alterations could be further clarified if the
isolation of MAM was feasible in primary fibroblasts since
variations in MAM components are more accurately discerned in
isolated MAM fractions than total cell extracts (57). To the best of the authors'
knowledge, only one previous study has investigated MAM in BD.
Based on PLA findings, this study reported a reduction in these
contacts in iPSCs-derived neurons from patients with BD compared
with controls (58). In the
present study, data from PLA analysis were corroborated by other
fluorescence microscopy approaches and by TEM analysis, the gold
standard technique to measure the distance between these organelles
and, thus, to properly evaluate MAM. The discrepancies between the
present study and that of Kathuria et al (58) might arise from variability in
cohorts in terms of age, gender and disease stage, which are
determinant factors that can influence outcomes (59). Indeed, this study included only
early-stage male patients with BD (BD stage 2) aged between 18-35
years, whereas the previous study included both male and female
patients with BD aged between 18-65 years-old, meaning that
patients at different disease stages were probably enrolled in the
study.
The close ER-mitochondria juxtaposition at MAM
facilitates the efficient transfer of both Ca2+ and
lipids between these organelles. Notably, a decrease of
ER-mitochondria Ca2+ transfer was observed in BD cells
in conditions of MAM upregulation, which might arise from
compromised IP3R-mediated ER Ca2+ release. The present
study data strongly suggested that compromised ER-mitochondria
Ca2+ transfer in BD fibroblasts was likely to result
from the inability of SOCE-mediated extracellular Ca2+
influx to replenish ER Ca2+ stores, leading to
Ca2+ depletion that will affect the uptake of
Ca2+ by mitochondria (49,60). ROS generation in BD cells might
be the cause of SOCE impairment, as SOCE is modulated in a
redox-dependent manner (61). In
agreement, the downregulation of SOCE was recently reported in
iPSCs-derived neurons obtained from patients with BD (62). The hypothesis that BD
pathophysiology is at least partly caused by aberrant
Ca2+ homeostasis is supported by alterations in genes
encoding to Ca2+ channels such as calcium voltage-gated
channel subunit α1 C (CACNA1C), identified as greater risk for BD
(63).
The similar protein levels of the ER-resident
Sigma-1R suggested that the differences between BD and control
cells do not arise from alterations in this well-known modulator of
the IP3R-mediated ER Ca2+ release. However, Sigma-1R
activation leading to an antagonistic effect of IP3R in BD
fibroblasts cannot be ruled out. Previous reports indicate that
Sigma-1R can function in an agonist- or antagonist-sensitive
manner, by modulating the interaction between ankyrin B and IP3R3,
which in turn affects IP3R-VDAC coupling, thus directly interfering
with Ca2+ handling (64). Indeed, genetic variations in
SIGMAR1 have been identified in patients with BD (65). Another viable hypothesis is that
the deficient transfer of Ca2+ between the ER and
mitochondria reported in this study may be associated with the
mitochondrial dysfunction previously described by our research team
in BD fibroblasts (41).
Unchanged MCU protein levels ruled out that inhibition of
mitochondrial Ca2+ uptake at IMM contributes to
attenuate the ER-mitochondria Ca2+ transfer in BD cells.
However, the hypothesis that MCU activity is inhibited in these
cells in the absence of changes in protein content cannot be
discarded.
To the best of the authors' knowledge, the present
study was pioneering in investigating LDs in BD. Its data showing
higher number of LDs in BD fibroblasts indicated disrupted lipid
metabolism in this pathology, which agrees with previous studies
highlighting significant changes in the lipidome of patients with
BD (66-69). The increase of LDs in BD cells is
expected to arise from heightened lipid transfer at MAM, a crucial
place for LDs biogenesis (70).
This positive correlation between upregulated MAM and increased LDs
content was previously documented in AD (10).
LDs have recently emerged as critical players in
cellular stress responses (71,72). Disruptions in LDs turnover can
instigate ER stress, which in turn can contribute to the
accumulation of LDs in several types of cells (73). Accordingly, LDs accumulation in
BD fibroblasts coincides with ER stress induction, as demonstrated
by increased protein levels of ER stress-induced UPR markers such
as ATF4 and ATF6, mediators of PERK and ATF6 UPR branches,
respectively, as well as glucose-regulated protein 78 (GRP78), the
major ER-resident chaperone. The involvement of ER stress in BD
pathophysiology is supported by studies reporting higher GRP78
levels in peripheral blood cells from these patients, as well as by
the modulation of this ER stress marker by several mood stabilizers
(74,75).
The UPR activation under chronic ER stress
conditions markedly enhances the expression of pro-inflammatory
cytokines. Specifically, the PERK arm of UPR stimulates IL-6
production, while the ATF-6 branch upregulates IL-1β, IL-6 and TNFα
cytokines (76). Notably, the
activation of both PERK and ATF6 UPR arms in the fibroblasts of
patients with BD occurs concomitantly with increased mRNA levels of
pro-IL-1β, TNFα, IL-6 and IL-8. The present study underscored that
BD fibroblasts display a baseline pro-inflammatory profile,
probably resulting from chronic ER stress. This observation agreed
with previous studies consistently reporting a rise of
pro-inflammatory cytokines in BD such as TNFα, IL-6 and IL-8 and
lower concentrations of anti-inflammatory cytokines, in both manic
and depressive BD states (77).
Moreover, higher IL-1β levels have been detected in the
cerebrospinal fluid of patients with BD, not only during periods of
remission following mania but also during euthymic states (78). BD-associated pro-inflammatory
status is also further supported by previous findings showing
altered numbers of circulating immune cells in peripheral blood
collected from those patients, including monocytes, leukocytes,
neutrophils and lymphocytes (42,79,80). Of note, the neuroanatomical white
matter brain changes described in patients with BD have been
closely linked to immune alterations (81). Additionally, the clinical
evidence that these patients exhibit a high prevalence of chronic
comorbidities, such as metabolic and autoimmune diseases further
strengthens the role of inflammation in BD pathophysiology
(82). Evidence from
meta-analysis reveals that the incidence of BD is markedly
increased in patients with autoimmune diseases such as psoriasis
(83). Similarly, psoriasis is
often accompanied by psychiatric comorbidities including anxiety
and depression, sleep disorders and suicidal behavior (37). This bidirectional interaction
highlights the intricate connection between skin diseases and
psychiatric conditions, reinforcing the hypothesis of the
skin-brain axis in BD. Recently, inflammation has been identified
as a key mediator underlying the interaction between psoriasis and
mental health commodities. T cells and their secreted inflammatory
cytokines play pivotal roles in the pathogenesis of both psoriasis
and psychiatric disorders by interacting with the
hypothalamic-pituitary-adrenal axis, exacerbating immune
dysregulation and disease progression (37). Notably, a basal pro-inflammatory
status is evident in patients with BD, as shown by elevated levels
of pro-inflammatory cytokines in fibroblasts derived from them.
This inflammatory profile observed in fibroblasts from patients
with BD might suggest that dysregulated immune responses and
chronic inflammation are key players in disease development.
Moreover, these findings may reflect the neuroinflammatory
environment described in the brains of patients with BD (84), further supporting the skin-brain
axis as a critical route for understanding this complex
neuropsychiatric condition.
The activation of both IRE1a and PERK UPR pathways
induces the expression of pro-IL-1β and NLRP3 via NF-kB, promoting
the NLRP3 inflammasome activation and subsequent IL-1β release
(85). Notably, mitochondrial
ROS, which were found increased in BD fibroblasts, primarily due to
the inhibition of SOD2 mitochondrial antioxidant enzyme, have been
recognized as classical triggers for NLRP3 inflammasome activation
(14). In agreement, oxidative
stress has been implicated in BD pathophysiology (86). Furthermore, variations in genes
encoding antioxidant enzymes such as SOD2 and glutathione
peroxidase 3 have been reported in patients with BD (87). For instance, the polymorphism
rs4880 in SOD2, identified as a BD risk factor, results in reduced
activity of this enzyme (88).
Notably, NLRP3 inflammasome assembly takes place at
MAM platforms (13). Therefore,
the upregulation of MAM in fibroblasts from patients with BD can be
responsible for NLRP3 inflammasome activation, as evidenced by
increased mRNA levels of NLRP3 and pro-IL-1β, along with enhanced
caspase-1 activity, compared with controls. The pivotal role of
NLRP3 inflammasome in fostering the BD-associated pro-inflammatory
state has already been suggested by the upregulation of IL-1β,
NLRP3, ASC and caspase-1 in post-mortem frontal cortex of patients
with BD (26). The present study
disclosed that NLRP3 inflammasome activation under LPS-induced
inflammation-prone conditions was markedly lower in BD cells
compared with controls. Although fibroblasts possess the capability
to modulate inflammatory responses, their cytokines secretion is
relatively modest compared with immune cells (89,90), therefore, this study also
explored NLRP3 inflammasome activation in LPS-treated primary
monocytes isolated from both patients with BD and controls, and
similar results were obtained. Together, the present study
suggested a diminished responsiveness of cells from patients with
BD to inflammation-prone stressful conditions. The lower resilience
exhibited by BD cells can be attributed to the pre-activated state
of NLRP3 inflammasome under non-stimulated conditions. This
hypothesis is strengthened by our previous findings, showing that
NLRP3 inflammasome activation occurs in BD monocytes upon ER stress
induction, even in the absence of LPS priming (42). Accordingly, ATP treatment in
fibroblasts from patients with systemic sclerosis, which are in
pre-activated state, also induces IL-6 release in the absence of
LPS priming (91). Lastly, the
reduced cellular resilience due to compromised NLRP3 inflammasome
activity in BD may contribute to explain the high prevalence of
infectious diseases in these patients (92).
Fibroblasts and blood cells from individuals with
diagnosis of multiple brain diseases, including neuropsychiatric
conditions such as BD, have long been considered as valuable
patient-derived cell models, markedly contributing to increasing
our knowledge about the pathophysiology of brain disorders.
Although the findings of this study obtained in fibroblasts and
monocytes derived from patients with BD and control individuals
corroborate its conclusions, some limitations have been identified:
i) the sample size was relatively small; however, the statistically
significant differences observed between control and BD cells
support the present study as a pioneering proof-of-concept
demonstration of the role of MAM in BD; ii) the present study
focused exclusively on early-stage patients with BD and did not
include an assessment of later-stage cases, which should be
addressed in future studies; and iii) validation using neurons
differentiated from iPSCs could further strengthen the evidence of
MAM impairment in BD.
The comprehensive structural and functional
analysis conducted on cells obtained from early-stage patients with
BD and healthy controls markedly contributes to unravelling the
pivotal role of MAM in BD pathophysiology (Fig. 6). The increased ER-mitochondria
juxtaposition at MAM observed in BD cells correlated with enhanced
lipid transfer and formation of LDs, which occurs concomitantly
with decreased ER-mitochondria Ca2+ transfer that is
associated with impaired SOCE activity and a subsequent inability
to refill ER Ca2+ stores. The dysfunction of MAM may
underlie the previously reported mitochondrial respiratory
impairment and ATP depletion in BD cells (41), as well as the induction of
MAM-associated events identified in the present study, including
oxidative stress, ER stress-induced UPR and basal NLRP3
inflammasome activation.
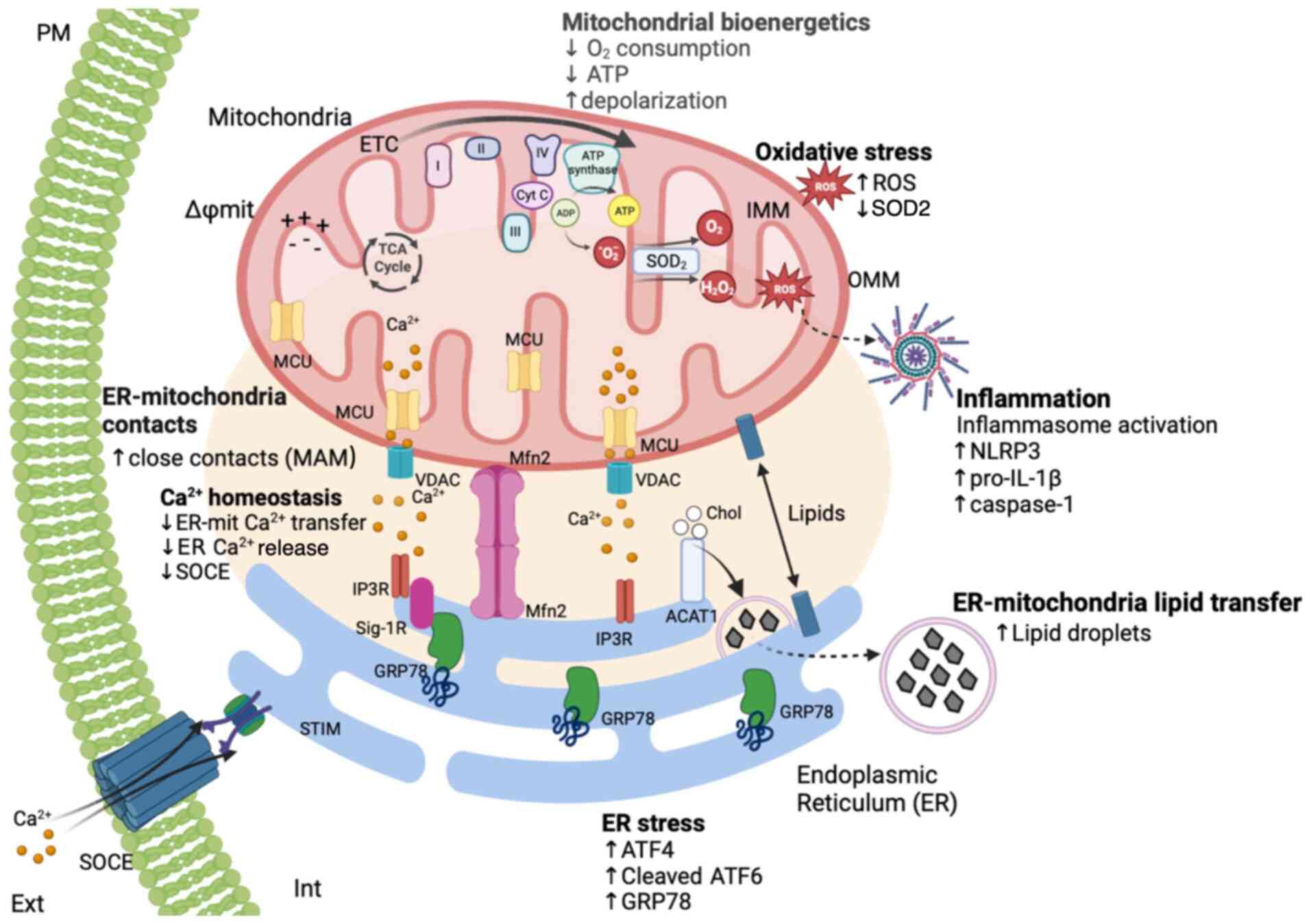 | Figure 6The MAM hypothesis for BD.
Fibroblasts derived from patients diagnosed with BD exhibit
increased ER-mitochondria apposition and markedly higher percentage
of close ER-mitochondria contacts sites (MAM) in comparison with
healthy controls, in particular those <20 nm. This structural
alteration in the ER-mitochondria juxtaposition directly affects
the main functions of MAM, which are the inter-organelle transfer
of Ca2+ and lipids. As a consequence, ER-mitochondria
lipids transfer is enhanced increasing the formation of lipid
droplets while Ca2+ transfer is inhibited.
Concomitantly, BD fibroblasts exhibit lower SOCE and higher
oxidative stress levels due to the inhibition of SOD2 mitochondrial
antioxidant enzyme and accumulation of ROS in the cytosol and in
the mitochondria. In addition to these alterations in
Ca2+ and redox homeostasis as well as in lipid
metabolism, BD-specific changes in MAM are also associated with
induction of ER stress and sterile inflammation. A basal
pro-inflammatory status in BD is supported by the upregulation of
pro-inflammatory cytokines, which seems to be implicated in the
diminished susceptibility of both fibroblasts and monocytes derived
from patients with BD to inflammatory stimuli, as shown by the
lower activation of the NLRP3 inflammasome in response to LPS. PM,
plasma membrane; MAM, mitochondria-associated membranes; BD,
bipolar disorder; MCU, mitochondrial calcium uniport; Cyt C,
cytochrome c; TCA cycle, tricarboxylic acid cycle; ADP,
adenosine diphosphate; ATP, adenosine triphosphate; O2,
superoxide anion; O2, oxygen;
H2O2, hydrogen peroxide; SOD2, superoxide
dismutase 2; ROS, reactive oxygen species; VDAC, voltage-dependent
anion channels; Mfn2, mitofusin 2; IP3R, inositol trisphosphate
receptor; Sig-1R, sigma-1 receptor; ACAT1, Acetyl-CoA
acetyltransferase 1; CE, cholesterol ester; SOCE, store-operated
calcium entry; STIM1, stromal interaction molecule 1; UPR, unfolded
protein response; ER, endoplasmic reticulum; NLRP3, NOD-like
receptor family pyrin domain-containing 3; BD, bipolar disorder;
LPS, lipopolysaccharide. |
Although further research is needed, the present
study suggested that the skin-brain axis may provide a novel route
for understanding the pathophysiology of BD, with inflammation
potentially being a central mediator between these interconnected
systems.
In conclusion, MAM impairment was underscored as a
key pathophysiological mechanism in early BD stages suggesting that
these subcellular structures should be further exploring to search
novel therapeutic targets.
Supplementary Data
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article.
Authors' contributions
ACP: Data curation, formal analysis, investigation,
writing-original draft. APM, RR, MC, TF, LSC: Data curation, formal
analysis, investigation. MB, JBDM, AM: Resources, methodology. NM:
Resources, methodology, funding acquisition. CC, MTC: Supervision,
writing-review and editing. CFP: Conceptualization, supervision,
project administration, funding acquisition, writing-review and
editing. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Centro Hospitalar e Universitário de Coimbra (now ULS de
Coimbra), reference 150/CES, July 3, 2018 and both the skin
biopsies and the peripheral blood samples were taken following
informed consent also approved by the same ethics committee
(30).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AD
|
Alzheimer's disease
|
|
ASC
|
apoptosis-associated speck-like
protein containing a C-terminal caspase-activation-and-recruitment
(CARD) domain
|
|
ATF4
|
activating transcription factor 4
|
|
ATF6
|
activating transcription factor 6
|
|
BD
|
bipolar disorder
|
|
Ca2+
|
calcium
|
|
CNS
|
central nervous system
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
IMM
|
inner mitochondria membrane
|
|
iPSCs
|
induced pluripotent stem cells
|
|
IP3R
|
inositol trisphosphate receptor
|
|
LDs
|
Lipid droplets
|
|
LPS
|
lipopolysaccharide
|
|
MAM
|
mitochondria-associated membranes
|
|
MCU
|
mitochondrial Ca2+
uniporter protein
|
|
Mfn2
|
Mitofusin 2
|
|
SOD2
|
superoxide dismutase 2
|
|
NLRP3
|
NOD-like receptor family pyrin
domain-containing 3
|
|
OMM
|
outer mitochondrial membrane
|
|
PLA
|
proximity ligation assay
|
|
PTPIP51
|
protein tyrosine
phosphatase-interacting protein-51
|
|
RT-PCR
|
reverse transcriptase-polymerase
chain reactions
|
|
ROS
|
reactive oxygen species
|
|
SCCI
|
single cell calcium imaging
|
|
SOCE
|
store-operated Ca2+
entry
|
|
SOD
|
superoxide dismutase
|
|
STIM1
|
stromal interaction molecule 1
|
|
TEM
|
transmission electron microscopy
|
|
TOM-20
|
Translocase of Outer Mitochondrial
Membrane 20
|
|
ULS
|
Unidade Local de Saúde de Coimbra
|
|
UPR
|
unfolded protein response
|
|
VAPB
|
vesicle-associated membrane protein
associated protein B
|
|
VDAC
|
voltage-dependent anion channel
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the European Regional
Development Fund (ERDF), through the Centro 2020 Regional
Operational Programme under grant no. CENTRO-01-0145-FEDER-000012
(HealthyAging2020) and through the COMPETE 2020-Operational
Programme for Competitiveness and Internationalization and
Portuguese national funds via FCT-Fundação para a Ciência e a
Tecnologia, under grant nos. PTDC/MED-NEU/28214/2017,
PTDC/MED-FAR/29369/2017 and UIDB/04539/2020, UIDP/04539/2020 and
LA/P/0058/2020. AP is a PhD fellow from FCT (grant no.
SFRH/BD/148653/2019). RR is Post-Doctoral Researcher (grant no. DL
57/2016/CP1448/CT0012).
References
|
1
|
Csordás G, Renken C, Várnai P, Walter L,
Weaver D, Buttle KF, Balla T, Mannella CA and Hajnóczky G:
Structural and functional features and significance of the physical
linkage between ER and mitochondria. J Cell Biol. 174:915–921.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Filadi R, Theurey P and Pizzo P: The
endoplasmic reticulum-mitochondria coupling in health and disease:
Molecules, functions and significance. Cell Calcium. 62:1–15. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Wen Y, Dong J, Cao C and Yuan S:
Systematic In-depth proteomic analysis of Mitochondria-associated
endoplasmic reticulum membranes in mouse and human testes.
Proteomics. 18:e17004782018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rowland AA and Voeltz GK: Endoplasmic
reticulum-mitochondria contacts: Function of the junction. Nat Rev
Mol Cell Biol. 13:607–625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Brito OM and Scorrano L: Mitofusin-2
regulates mitochondrial and endoplasmic reticulum morphology and
tethering: The role of Ras. Mitochondrion. 9:222–226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Casellas-D Iaz S, Larramona-Arcas R, Riqu
E-Pujol G, Tena-Morraja P, Müller-Sánchez C, Segarra-Mondejar M,
Gavaldà-Navarro A, Villarroya F, Reina M, Martínez-Estrada OM and
Soriano FX: Mfn2 localization in the ER is necessary for its
bioenergetic function and neuritic development. EMBO Rep.
22:e519542021. View Article : Google Scholar
|
|
7
|
Yang S, Zhou R, Zhang C, He S and Su Z:
Mitochondria-associated endoplasmic reticulum membranes in the
pathogenesis of type 2 diabetes mellitus. Front Cell Dev Biol.
8:5715542020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gómez-Suaga P, Pérez-Nievas BG, Glennon
EB, Lau DHW, Paillusson S, Mórotz GM, Calì T, Pizzo P, Noble W and
Miller CCJ: The VAPB-PTPIP51 endoplasmic reticulum-mitochondria
tethering proteins are present in neuronal synapses and regulate
synaptic activity. Acta Neuropathol Commun. 7:352019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu H, Guan N, Ren YL, Wei QJ, Tao YH, Yang
GS, Liu XY, Bu DF, Zhang Y and Zhu SN: IP3R-Grp75-VDAC1-MCU calcium
regulation axis antagonists protect podocytes from apoptosis and
decrease proteinuria in an Adriamycin nephropathy rat model. BMC
Nephrol. 19:1402018. View Article : Google Scholar :
|
|
10
|
Area-Gomez E, Del Carmen Lara Castillo M,
Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J,
Umeda M, Bird TD, Sturley SL and Schon EA: Upregulated function of
mitochondria-associated ER membranes in Alzheimer disease. EMBO J.
31:4106–4123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raturi A and Simmen T: Where the
endoplasmic reticulum and the mitochondrion tie the knot: The
mitochondria-associated membrane (MAM). Biochimica et Biophysica
Acta (BBA)-Mol Cell Res. 1833:213–224. 2013. View Article : Google Scholar
|
|
12
|
van Vliet AR, Verfaillie T and Agostinis
P: New functions of mitochondria associated membranes in cellular
signaling. Biochim Biophys Acta. 1843:2253–2262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar
|
|
14
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Missiroli S, Patergnani S, Caroccia N,
Pedriali G, Perrone M, Previati M, Wieckowski MR and Giorgi C:
Mitochondria-associated membranes (MAMs) and inflammation. Cell
Death Dis. 9:3292018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Filadi R, Greotti E and Pizzo P:
Highlighting the endoplasmic reticulum-mitochondria connection:
Focus on Mitofusin 2. Pharmacol Res. 128:42–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Area-Gomez E, de Groof A, Bonilla E,
Montesinos J, Tanji K, Boldogh I, Pon L and Schon EA: A key role
for MAM in mediating mitochondrial dysfunction in Alzheimer
disease. Cell Death Dis. 9:3352018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Resende R, Fernandes T, Pereira AC,
Marques AP and Pereira CF: Endoplasmic Reticulum-mitochondria
contacts modulate reactive oxygen Species-mediated signaling and
oxidative stress in brain disorders: The key role of Sigma-1
receptor. Antioxid Redox Signal. 37:758–780. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elsayed OH, Ercis M, Pahwa M and Singh B:
Treatment-resistant bipolar depression: Therapeutic trends,
challenges and future directions. Neuropsychiatr Dis Treat.
18:2927–2943. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Zhang Z, Lin S, Zhou H and Xu G:
Cognitive impairment mechanism in patients with bipolar disorder.
Neuropsychiatr Dis Treat. 19:361–366. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sampogna G, Janiri D, Albert U, Caraci F,
Martinotti G, Serafini G, Tortorella A, Zuddas A, Sani G and
Fiorillo A: Why lithium should be used in patients with bipolar
disorder? A scoping review and an expert opinion paper. Expert Rev
Neurother. 22:923–934. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu X, Yu C, Dong T, Yang Z, Fang Y and
Jiang Z: Biomarkers and detection methods of bipolar disorder.
Biosens Bioelectron. 220:1148422023. View Article : Google Scholar
|
|
23
|
Pereira AC, Resende R, Morais S, Madeira N
and Fragão Pereira C: The ups and downs of cellular stress: The
'MAM hypothesis' for Bipolar disorder pathophysiology.
International J Clin Neurosciences Mental Health. S042017.
View Article : Google Scholar
|
|
24
|
Pereira AC, Oliveira J, Silva S, Madeira
N, Pereira CMF and Cruz MT: Inflammation in bipolar disorder (BD):
Identification of new therapeutic targets. Pharmacol Res.
163:1053252021. View Article : Google Scholar
|
|
25
|
Resende R, Fernandes T, Pereira AC, De
Pascale J, Marques AP, Oliveira P, Morais S, Santos V, Madeira N,
Pereira CF and Moreira PI: Mitochondria, endoplasmic reticulum and
innate immune dysfunction in mood disorders: Do
Mitochondria-associated Membranes (MAMs) play a role? Biochim
Biophys Acta Mol Basis Dis. 1866:1657522020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HK, Andreazza AC, Elmi N, Chen W and
Young LT: Nod-like receptor pyrin containing 3 (NLRP3) in the
post-mortem frontal cortex from patients with bipolar disorder: A
potential mediator between mitochondria and immune-activation. J
Psychiatr Res. 72:43–50. 2016. View Article : Google Scholar
|
|
27
|
Viswanath B, Jose SP, Squassina A,
Thirthalli J, Purushottam M, Mukherjee O, Vladimirov V, Patrinos
GP, Del Zompo M and Jain S: Cellular models to study bipolar
disorder: A systematic review. J Affect Disord. 184:36–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnston JA, Cowburn RF, Norgren S,
Wiehager B, Venizelos N, Winblad B, Vigo-Pelfrey C, Schenk D,
Lannfelt L and O'Neill C: Increased β-amyloid release and levels of
amyloid precursor protein (APP) in fibroblast cell lines from
family members with the Swedish Alzheimer's disease APP670/671
mutation. FEBS Lett. 354:274–278. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoepken HH, Gispert S, Azizov M,
Klinkenberg M, Ricciardi F, Kurz A, Morales-Gordo B, Bonin M, Riess
O, Gasser T, et al: Parkinson patient fibroblasts show increased
alpha-synuclein expression. Exp Neurol. 212:307–313. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Onofre I, Mendonça N, Lopes S, Nobre R, de
Melo JB, Carreira IM, Januário C, Gonçalves AF and de Almeida LP:
Fibroblasts of machado joseph disease patients reveal autophagy
impairment. Sci Rep. 6:282202016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oliveira NC, Russo FB and Beltrão-Braga
PCB: Differentiation of peripheral sensory neurons from iPSCs
derived from stem cells from human exfoliated deciduous teeth
(SHED). Front Cell Dev Biol. 11:12035032023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kálmán S, Garbett KA, Janka Z and Mirnics
K: Human dermal fibroblasts in psychiatry research. Neuroscience.
320:105–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Etemadikhah M, Niazi A, Wetterberg L and
Feuk L: Transcriptome analysis of fibroblasts from schizophrenia
patients reveals differential expression of schizophrenia-related
genes. Sci Rep. 10:6302020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Akin D, Manier DH, Sanders-Bush E and
Shelton RC: Decreased serotonin 5-HT2A receptor-stimulated
phosphoinositide signalling in fibroblasts from melancholic
deppresed patients. Neuropsychopharmacology. 29:2081–2087. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jameson C, Boulton KA, Silove N, Nanan R
and Guastella AJ: Ectodermal origins of the skin-brain axis: A
novel model for the developing brain, inflammation, and
neurodevelopmental conditions. Mol Psychiatry. 28:108–117. 2023.
View Article : Google Scholar :
|
|
36
|
Kim HS, Jung H, Park YH, Heo SH, Kim S and
Moon M: Skin-brain axis in Alzheimer's disease-Pathologic,
diagnostic, and therapeutic implications: A hypothetical review.
Aging Dis. 16:901–916. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang J, Zhang S, Wu Q, Chen P, Dai Y, Long
J, Wu Y and Lin Y: T cell-mediated skin-brain axis: Bridging the
gap between psoriasis and psychiatric comorbidities. J Autoimmun.
144:1031762024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berk M, Post R, Ratheesh A, Gliddon E,
Singh A, Vieta E, Carvalho AF, Ashton MM, Berk L, Cotton SM, et al:
Staging in bipolar disorder: From theoretical framework to clinical
utility. World Psychiatry. 16:236–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
American Psychiatric Association:
Diagnostic and Statistical Manual of Mental Disorders. Diagnostic
and Statistical Manual of Mental Disorders. 2013.
|
|
40
|
Martins MJ, Palmeira L, Xavier A, Castilho
P, Macedo A, Pereira AT, Pinto AM, Carreiras D and Barreto-Carvalho
C: The clinical interview for psychotic disorders (CIPD):
Preliminary results on interrater agreement, reliability and
qualitative feedback. Psychiatry Res. 272:723–729. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marques AP, Resende R, Silva DF, Batista
M, Pereira D, Wildenberg B, Morais S, Macedo A, Pais C, Melo JB, et
al: Mitochondrial alterations in fibroblasts of early stage bipolar
disorder patients. Biomedicines. 9:5222021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pereira AC, De Pascale J, Resende R,
Cardoso S, Ferreira I, Neves BM, Carrascal MA, Zuzarte M, Madeira
N, Morais S, et al: ER-mitochondria communication is involved in
NLRP3 inflammasome activation under stress conditions in the innate
immune system. Cell Mol Life Sci. 79:2132022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pereira AC, Madeira N, Morais S, Macedo A,
Cruz MT and Pereira CMF: Mitochondria fusion upon SERCA inhibition
prevents activation of the NLRP3 inflammasome in human monocytes.
Cells. 11:4332022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bolte S and Cordelières FP: A guided tour
into subcellular colocalization analysis in light microscopy. J
Microsc. 224:213–232. 2006. View Article : Google Scholar
|
|
45
|
Tiscione SA, Vivas O, Ginsburg KS, Bers
DM, Ory DS, Santana LF, Dixon RE and Dickson EJ: Disease-associated
mutations in Niemann-Pick type C1 alter ER calcium signaling and
neuronal plasticity. J Cell Biol. 218:4141–4156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moreira P, Sousa FJ, Matos P, Brites GS,
Gonçalves MJ, Cavaleiro C, Figueirinha A, Salgueiro L, Batista MT,
Branco PC, et al: Chemical composition and effect against skin
alterations of bioactive extracts obtained by the hydrodistillation
of eucalyptus globulus leaves. Pharmaceutics. 14:5612022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
48
|
Hayashi A, Kasahara T, Kametani M, Toyota
T, Yoshikawa T and Kato T: Aberrant endoplasmic reticulum stress
response in lymphoblastoid cells from patients with bipolar
disorder. Int J Neuropsychopharmacol. 12:33–43. 2009. View Article : Google Scholar
|
|
49
|
Seegren PV, Harper LR, Downs TK, Zhao XY,
Viswanathan SB, Stremska ME, Olson RJ, Kennedy J, Ewald SE, Kumar P
and Desai BN: Reduced mitochondrial calcium uptake in macrophages
is a major driver of inflammaging. Nat Aging. 3:796–812. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nan J, Li J, Lin Y, Saif Ur Rahman M, Li Z
and Zhu L: The interplay between mitochondria and store-operated
Ca2+ entry: Emerging insights into cardiac diseases. J Cell Mol
Med. 25:9496–9512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao J, Li J, Li G and Chen M: The role of
mitochondria-associated membranes mediated ROS on NLRP3
inflammasome in cardiovascular diseases. Front Cardiovasc Med.
9:10595762022. View Article : Google Scholar :
|
|
52
|
van Vliet AR and Agostinis P:
Mitochondria-associated membranes and ER stress. Curr Top Microbiol
Immunol. 414:73–102. 2018.
|
|
53
|
Guo H, Callaway JB and Ting JPY:
Inflammasomes: Mechanism of action, role in disease, and
therapeutics. Nat Med. 21:677–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Walsh JG, Muruve DA and Power C:
Inflammasomes in the CNS. Nat Rev Neurosci. 15:84–97. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Davidson S, Coles M, Thomas T, Kollias G,
Ludewig B, Turley S, Brenner M and Buckley CD: Fibroblasts as
immune regulators in infection, inflammation and cancer. Nat Rev
Immunol. 21:704–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fernandes T, Resende R, Silva DF, Marques
AP, Santos AE, Cardoso SM, Domingues MR, Moreira PI and Pereira CF:
Structural and functional alterations in mitochondria-associated
membranes (MAMs) and in mitochondria activate stress response
mechanisms in an in vitro model of Alzheimer's disease.
Biomedicines. 9:8812021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kathuria A, Lopez-Lengowski K, Vater M,
McPhie D, Cohen BM and Karmacharya R: Transcriptome analysis and
functional characterization of cerebral organoids in bipolar
disorder. Genome Med. 12:342020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rich-Edwards JW, Kaiser UB, Chen GL,
Manson JAE and Goldstein JM: Sex and gender differences research
design for basic, clinical, and population studies: Essentials for
investigators. Endocr Rev. 39:424–439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Trebak M and Putney JW Jr: ORAI Calcium
channels. Physiology. 32:332–342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nunes P and Demaurex N: Redox regulation
of store-operated Ca2+ entry. Antioxid Redox Signal. 21:915–932.
2014. View Article : Google Scholar :
|
|
62
|
Hewitt T, Alural B, Tilak M, Wang J, Becke
N, Chartley E, Perreault M, Haggarty SJ, Sheridan SD, Perlis RH, et
al: Bipolar disorder-iPSC derived neural progenitor cells exhibit
dysregulation of store-operated Ca2+ entry and accelerated
differentiation. Mol Psychiatry. 28:5237–5250. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nurnberger JI Jr, Koller DL, Jung J,
Edenberg HJ, Foroud T, Guella I, Vawter MP and Kelsoe JR;
Psychiatric Genomics Consortium Bipolar Group: Identification of
pathways for bipolar disorder: A meta-analysis. JAMA Psychiatry.
71:657–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Weng TY, Tsai SYA and Su TP: Roles of
sigma-1 receptors on mitochondrial functions relevant to
neurodegenerative diseases. J Biomed Sci. 24:742017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mandelli L, Wang SM, Han C, Lee SJ, Patkar
AA, Masand PS, Pae CU and Serretti A: The impact of a single
nucleotide polymorphism in SIGMAR1 on depressive symptoms in major
depressive disorder and bipolar disorder. Adv Ther. 34:713–724.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Scola G, Versace A, Metherel AH,
Monsalve-Castro LA, Phillips ML, Bazinet RP and Andreazza AC:
Alterations in peripheral fatty acid composition in bipolar and
unipolar depression. J Affect Disord. 233:86–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Knowles EEM, Meikle PJ, Huynh K, Göring
HH, Olvera RL, Mathias SR, Duggirala R, Almasy L, Blangero J,
Curran JE and Glahn DC: Serum phosphatidylinositol as a biomarker
for bipolar disorder liability. Bipolar Disord. 19:107–115. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ribeiro HC, Klassen A, Pedrini M, Carvalho
MS, Rizzo LB, Noto MN, Zeni-Graiff M, Sethi S, Fonseca FAH, Tasic
L, et al: A preliminary study of bipolar disorder type I by mass
spectrometry-based serum lipidomics. Psychiatry Res. 258:268–273.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Guo L, Zhang T, Li R, Cui ZQ, Du J, Yang
JB, Xue F, Chen YH, Tan QR and Peng ZW: Alterations in the plasma
lipidome of adult women with bipolar disorder: A mass
spectrometry-based lipidomics research. Front Psychiatry.
13:8027102022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhu Y, Chen CY, Li J, Cheng JX, Jang M and
Kim KH: In vitro exploration of ACAT contributions to lipid droplet
formation during adipogenesis. J Lipid Res. 59:820–829. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Henne WM, Reese ML and Goodman JM: The
assembly of lipid droplets and their roles in challenged cells.
EMBO J. 37:e989472018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Olzmann JA and Carvalho P: Dynamics and
functions of lipid droplets. Nat Rev Mol Cell Biol. 20:137–155.
2019. View Article : Google Scholar :
|
|
73
|
Jarc E and Petan T: Lipid droplets and the
management of cellular stress. Yale J Biol Med. 92:435–452.
2019.PubMed/NCBI
|
|
74
|
Bengesser SA, Fuchs R, Lackner N, Birner
A, Reininghaus B, Meier-Allard N, Stracke A, Kapfhammer HP,
Reininghaus EZ and Wallner-Liebmann S: Endoplasmic reticulum stress
and bipolar disorder-Almost forgotten therapeutic drug targets in
the unfolded protein response pathway revisited. CNS Neurol Disord
Drug Targets. 15:403–413. 2016. View Article : Google Scholar
|
|
75
|
Bengesser SA, Reininghaus EZ, Dalkner N,
Birner A, Hohenberger H, Queissner R, Fellendorf F, Platzer M, Pilz
R, Hamm C, et al: Endoplasmic reticulum stress in bipolar disorder?
-BiP and CHOP gene expression- and XBP1 splicing analysis in
peripheral blood. Psychoneuroendocrinology. 95:13–119. 2018.
View Article : Google Scholar
|
|
76
|
Thoudam T, Jeon JH, Ha CM and Lee IK: Role
of mitochondria-associated endoplasmic reticulum membrane in
inflammation-mediated metabolic diseases. Mediators Inflamm.
2016:18514202016. View Article : Google Scholar
|
|
77
|
Sayana P, Colpo GD, Simões LR, Giridharan
VV, Teixeira AL, Quevedo J and Barichello T: A systemic review of
evidence for the role of inflammatory biomarkers in bipolar
patients. J Psychiatr Res. 92:160–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Söderlund J, Olsson S, Samuelsson M,
Walther-Jallow L, Johansson C, Erhardt S, Landén M and Engberg G:
Elevation of cerebrospinal fluid interleukin-1β in bipolar
disorder. J Psychiatry Neurosci. 36:114–118. 2011. View Article : Google Scholar
|
|
79
|
Munkholm K, Jacoby AS, Lenskjold T,
Bruunsgaard H, Vinberg M and Kessing LV: Leukocytes in peripheral
blood in patients with bipolar disorder-Trait and state alterations
and association with levels of cytokines and C-reactive protein.
Psychiatry Res. 261:383–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Barbosa IG, Rocha NP, Assis F, Vieira ELM,
Soares JC, Bauer ME and Teixeira AL: Monocyte and lymphocyte
activation in bipolar disorder: A new piece in the puzzle of immune
dysfunction in mood disorders. Int J Neuropsychopharmacol.
18:pyu0212014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Magioncalda P, Martino M, Tardito S,
Sterlini B, Conio B, Marozzi V, Adavastro G, Capobianco L, Russo D,
Parodi A, et al: White matter microstructure alterations correlate
with terminally differentiated CD8+ effector T cell depletion in
the peripheral blood in mania: Combined DTI and immunological
investigation in the different phases of bipolar disorder. Brain
Behav Immun. 73:192–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
SayuriYamagata A, Brietzke E, Rosenblat
JD, Kakar R and McIntyre RS: Medical comorbidity in bipolar
disorder: The link with metabolic-inflammatory systems. J Affect
Disord. 211:99–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen M, Jiang Q and Zhang L: The
prevalence of bipolar disorder in autoimmune disease: A systematic
review and meta-analysis. Ann Palliat Med. 10:350–361. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lima DD, Cyrino LAR, Ferreira GK, Magro
DDD, Calegari CR, Cabral H, Cavichioli N, Ramos SA, Ullmann OM,
Mayer Y, et al: Neuroinflammation and neuroprogression produced by
oxidative stress in euthymic bipolar patients with different onset
disease times. Sci Rep. 12:167422022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou Y, Tong Z, Jiang S, Zheng W, Zhao J
and Zhou X: The roles of endoplasmic reticulum in NLRP3
inflammasome activation. Cells. 9:12192020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kotzaeroglou A and Tsamesidis I: The role
of equilibrium between free radicals and antioxidants in depression
and bipolar disorder. Medicines (Basel). 9:572022.PubMed/NCBI
|
|
87
|
Zou Y, Kennedy KG, Grigorian A, Fiksenbaum
L, Freeman N, Zai CC, Kennedy JL, MacIntosh BJ and Goldstein BI:
Antioxidative defense genes and brain structure in youth bipolar
disorder. Int J Neuropsychopharmacol. 25:89–98. 2022. View Article : Google Scholar :
|
|
88
|
Gallegos-Arreola MP, Ramírez-Patiño R,
Sánchez-López JY, Zúñiga-González GM, Figuera LE, Delgado-Saucedo
JI, Gómez-Meda BC, Rosales-Reynoso MA, Puebla-Pérez AM,
Lemus-Varela ML, et al: SOD2 Gene Variants (rs4880 and rs5746136)
and Their Association with Breast Cancer Risk. Curr Issues Mol
Biol. 44:5221–5233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
van Linthout S, Miteva K and Tschöpe C:
Crosstalk between fibroblasts and inflammatory cells. Cardiovasc
Res. 102:258–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sandanger Ø, Ranheim T, Vinge LE, Bliksøen
M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G,
Christensen G, et al: The NLRP3 inflammasome is up-regulated in
cardiac fibroblasts and mediates myocardial ischaemia-reperfusion
injury. Cardiovasc Res. 99:164–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
lo Monaco A, Gulinelli S, Castellino G,
Solini A, Ferrari D, La Corte R, Trotta F and Di Virgilio F:
Increased sensitivity to extracellular ATP of fibroblasts from
patients affected by systemic sclerosis. Ann Rheum Dis.
66:1124–1125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rosenblat JD and McIntyre RS: Bipolar
disorder and immune dysfunction: Epidemiological findings, proposed
pathophysiology and clinical implications. Brain Sci. 7:1442017.
View Article : Google Scholar : PubMed/NCBI
|