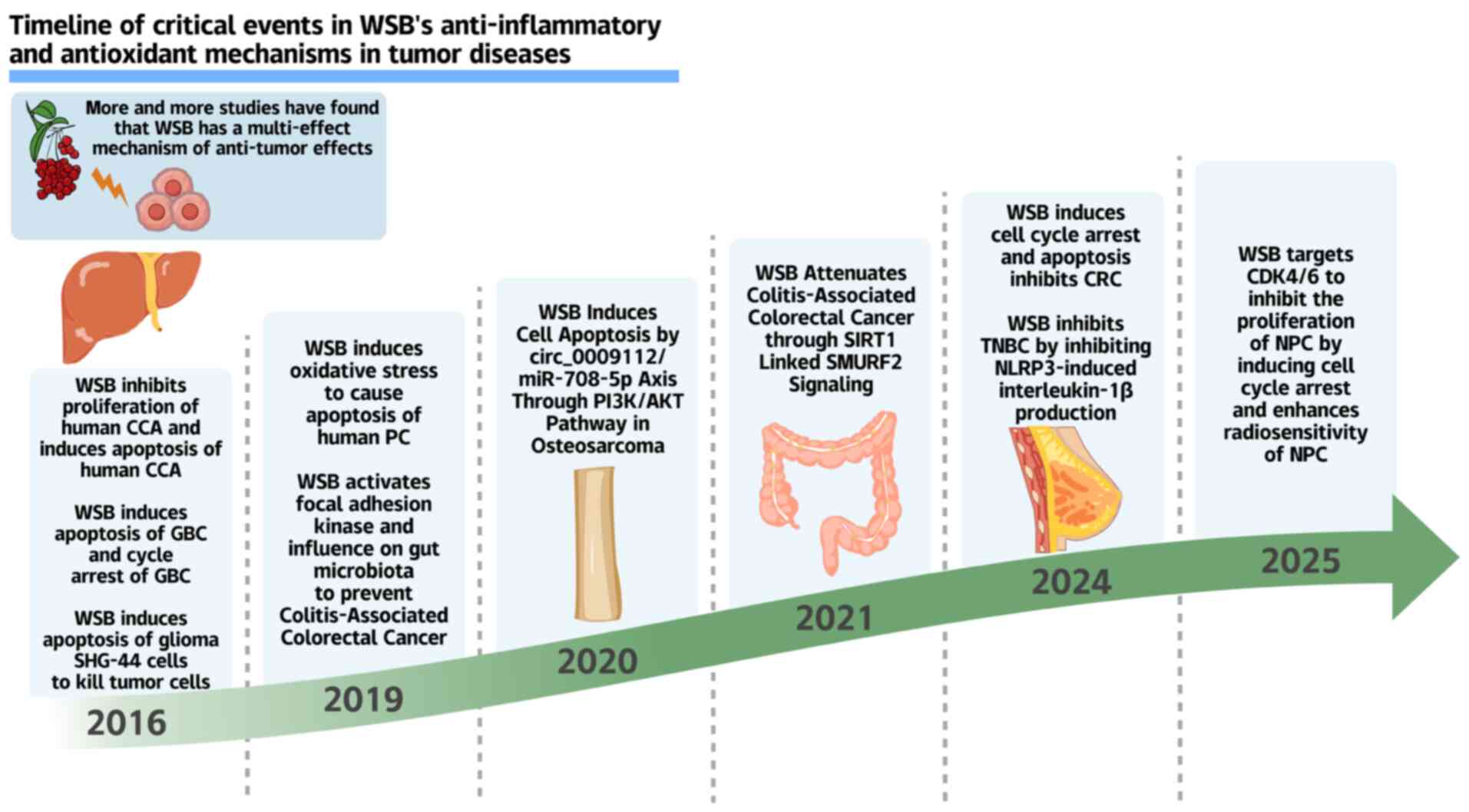
The global cancer burden has reached alarming
proportions, with the International Agency for Research on Cancer
(IARC) reporting >20 million new cases and 9.7 million
fatalities in 2022, a figure projected to surge to 35 million by
2050, creating an urgent need for innovative therapies that balance
efficacy with safety (1). This
escalating crisis has renewed interest in Traditional Chinese
Medicine (TCM) as a source of complementary agents to counter the
limitations of conventional treatments, such as toxicity and
resistance (2,3), with Schisandra chinensis (Wu
Wei Zi) emerging as a pivotal candidate. Its berries, called 'five
flavors' (sour, sweet, bitter, pungent, and salty), are classified
in the Chinese Pharmacopoeia (2020) as warm and sour-sweet
(5), targeting lung/heart/kidney
meridians for chronic cough and liver disorders (4-6).
Critically, modern research has validated its systemic
bioactivities: organ protection via Nrf2/NF-κB modulation to
alleviate oxidative stress and inflammation (7,8),
neuro-hepatic shielding through antifibrotic repair (9-11), and immune reprogramming via
macrophage/Th1-Th2 regulation (12,13) (Fig. 1).
Among its bioactive lignans, Wuweizisu B
(Schisandrin B, WSB) stands out as the premier anticancer agent
(6,14-17) (Fig. 2), boasting exceptional
bioavailability and tissue distribution (14,18) to orchestrate pleiotropic effects
from hepatoprotection to proliferation control (19-22). Its structural analogs (such as
schisandrin A/C and gomisins) synergistically combat cancer through
direct tumor suppression (apoptosis/cell cycle arrest), metastasis
blockade, microenvironment remodeling (immune activation), and
chemosensitization (MDR reversal via STAT3/P-gp/survivin
modulation) (4,17,23-27). Notably, WSB's ability to reverse
MDR, a major driver of chemotherapy failure, positions it as a key
ally against refractory cancers (28-30).
The present review therefore deciphered WSB's
anticancer potential through molecular mechanisms, organ-specific
efficacy, and clinical translation strategies as a dual
chemosensitizer-cytoprotective agent, bridging traditional wisdom
with contemporary pharmacology to chart a roadmap for integrated
oncology (Figs. 3-6).
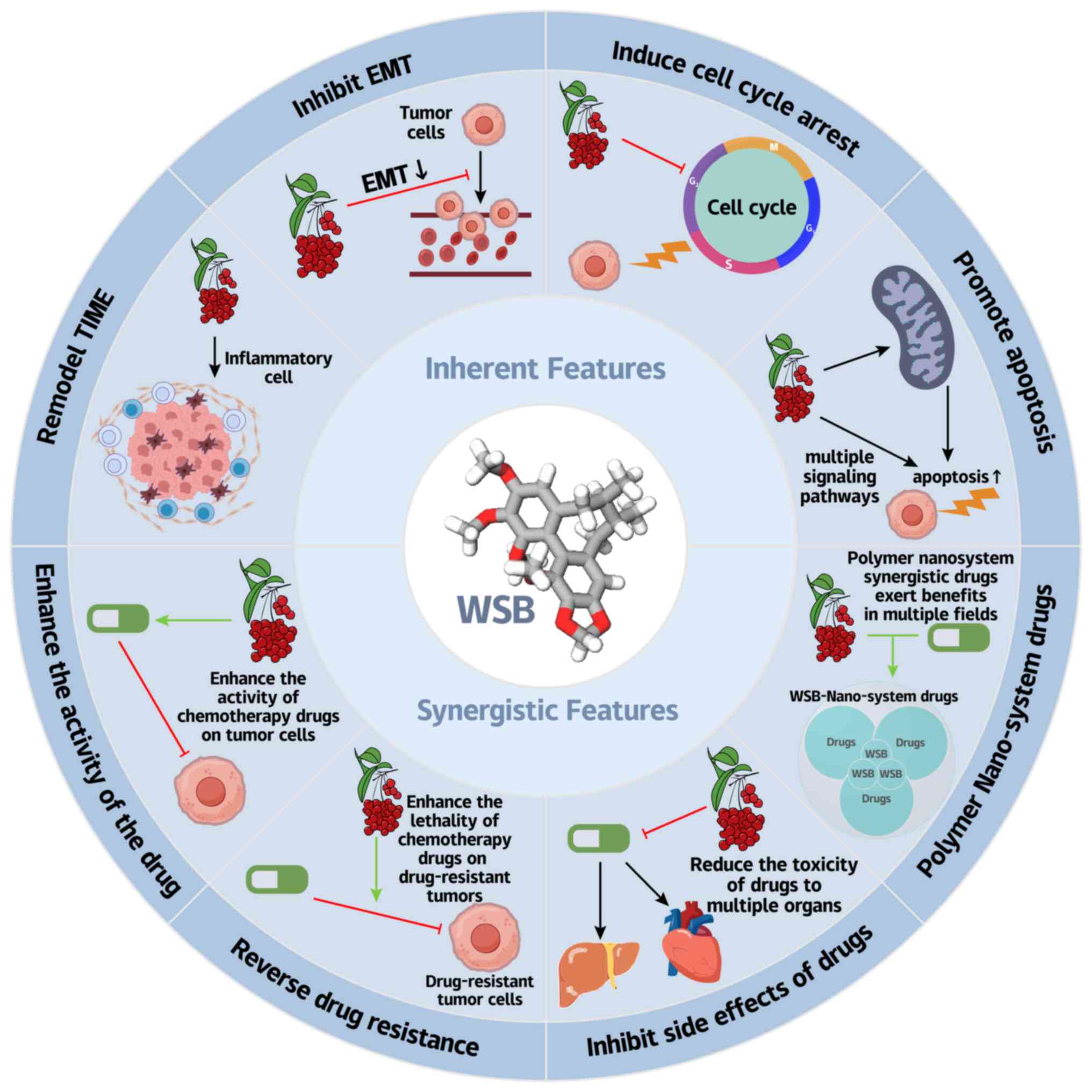
As a prototypical multitarget natural compound, WSB
exhibits remarkable therapeutic potential across a spectrum of
gastrointestinal malignancies through its unique ability to
simultaneously modulate oncogenic signaling networks, metabolic
reprogramming, and tumor microenvironment remodeling. This section
systematically elucidates the organ-specific anticancer mechanisms
of WSB along the digestive tract, supported by compelling
preclinical evidence highlighting it as a promising candidate for
integrative oncology approaches (Fig. 3).
GC remains a significant global health burden, with
the IARC reporting ~968,000 new cases and 660,000 deaths worldwide
in 2022, ranking fifth in incidence and mortality among all cancers
(1,31). Chemotherapy and immunotherapy,
have spurred interest in TCM as a complementary approach for GC
treatment (32). WSB exhibits
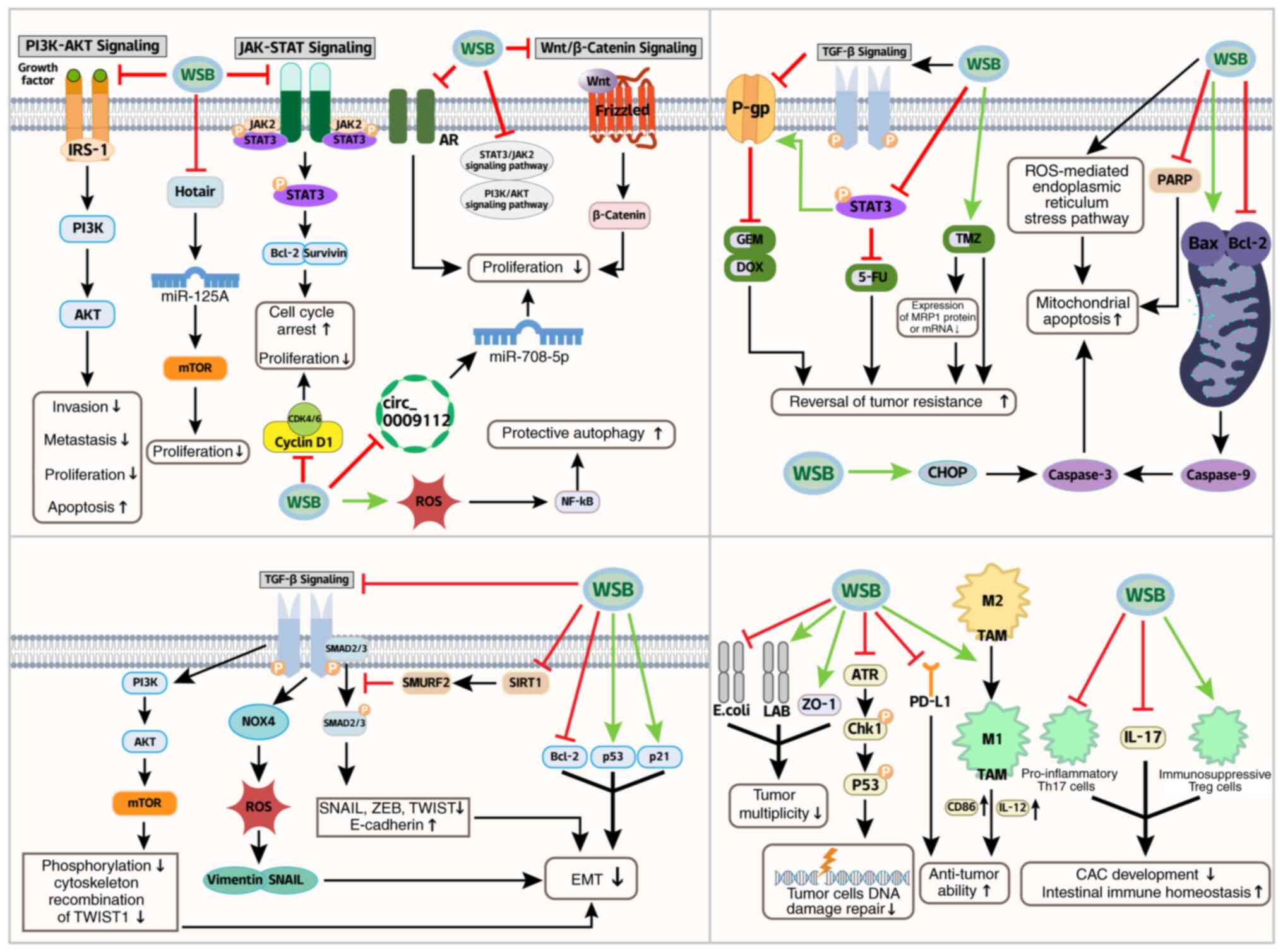
potent antitumor effects in GC. WSB exerts its antitumor effects
through a coordinated molecular cascade: by effectively inhibiting
STAT3 phosphorylation, WSB inactivates this oncogenic transcription
factor, thereby blocking downstream survival genes (Bcl-2 and
Survivin) and metastatic drivers (33) (Fig. 3; Table I). Crucially, suppression of
STAT3 expression directly represses Cyclin D1 transcription
(34) (Fig. 3), leading to significant
downregulation of the expression of Cyclin D1 mRNA, the core
regulator of the G1/S transition, which induces cell
cycle arrest at the G0/G1 phase in SGC-7901
cells. Notably, the optimal inhibitory effect was observed at a
concentration of 50 mg/l after 48 h of treatment (35) (Fig. 3; Table I). However, this therapeutic
cascade is concentration dependent: while 50 mg/l optimally
balanced these effects, higher doses (100 mg/l) paradoxically
activated protective autophagy via reactive oxygen species
(ROS)/NF-κB feedback, potentially compromising treatment efficacy
(36) (Fig. 3).
Despite these advances, the precise molecular
mechanisms underlying the anti-GC effects of WSB remain unclear.
Furthermore, studies indicate that the effective in vitro
concentration of WSB (50 mg/l) far exceeds the achievable peak
plasma concentration in humans (2.1 μM) (37). Existing research is based solely
on cell and mouse experiments and lacks tumor microenvironment
simulation, which may lead to overestimation of clinical efficacy.
Future studies should focus on elucidating these pathways to
facilitate the clinical translation of WSB-based therapies.
CRC ranks as the third most prevalent malignancy
globally, with GLOBOCAN 2022 reporting 1.9 million new cases and
930,000 mortalities annually (38). Projections indicate that this
burden will increase to 3.2 million cases by 2040, underscoring the
urgent need for safer therapies. While current regimens combining
5-Fluorouracil (5-FU)-based chemotherapy with surgery/radiotherapy
remain standard, ≥50% of patients experience grade 3-4 toxicity,
including myelosuppression, gastrointestinal injury, and
neurotoxicity (39,40). These limitations highlight the
imperative for novel agents such as WSB that synergize with
conventional therapies while mitigating adverse effects.
Critically, TGF-β drives metastatic plasticity by
dynamically regulating both EMT and its reverse process,
mesenchymal-epithelial transition (MET). TGF-β induces EMT through
the activation of the suppressor of mothers against decapentaplegic
(SMAD) and non-SMAD pathways, leading to the suppression of
epithelial genes (such as CDH1) and the upregulation of mesenchymal
markers (such as VIM and FN1), thereby enabling cancer cell
migration and invasion (45,48) (Fig. 3). Conversely, at metastatic
sites, reduced TGF-β signaling promotes MET through SMAD7-mediated
suppression of the EMT transcription factors SNAIL/ZEB and
reactivation of the miR-200 family, which collectively restore
epithelial phenotypes to facilitate metastatic colonization
(49,50) (Fig. 3). This bidirectional regulation
of cellular plasticity highlights the central role of TGF-β in
coordinating both the dissemination and secondary outgrowth of
metastatic cancer cells.
Similar to its mechanism in colorectal cancer, WSB
also exhibits inhibitory effects on TGF-β-induced EMT in lung
cancer (LC) and breast cancer (BC). In LC, the WSB downregulates
the expression of proteins such as TGF-β1 and Bcl-2 while
upregulating p53 and p21 expression (51) (Fig. 3; Table I). These regulatory effects
reduce the suppression of epithelial genes and the upregulation of
mesenchymal markers. In BC, WSB blocks TGF-β-promoted ROS
production and increases cell motility by inhibiting NADPH oxidase
4, thereby reducing the metastasis of BC cells (52) (Fig. 3). Collectively, these findings
underscore WSB's conserved capacity to target the TGF-β-mediated
EMT across diverse malignancies, with context-dependent molecular
nuances tailored to tissue-specific pathways. Such cross-organ
inhibitory activity reinforces the WSB as a promising candidate for
developing broad-spectrum antimetastatic therapeutics, in cancers
characterized by dysregulated TGF-β-EMT signaling axes.
In colitis-associated cancer models, WSB
demonstrates dual chemopreventive effects: FAK-dependent tight
junction stabilization (increased ZO-1 expression) and microbiota
remodeling (increased Lactobacillus abundance with reduced
Escherichia coli colonization), reducing tumor multiplicity
by 75% (53) (Fig. 3; Table I). Additionally, compared with
that in the general population, the incidence of colitis-associated
cancer (CAC) induced by ulcerative colitis (UC) is markedly greater
(54,55). Moreover, the pathogenesis of UC
is closely associated with environmental factors, genetic
predisposition, the gut microbiota, and immune dysregulation
(56,57). WSB markedly inhibits the
differentiation of the proinflammatory Th17 cells and IL-17
secretion while promoting the generation of immunosuppressive Treg
cells, thereby preventing CAC development and maintaining
intestinal immune homeostasis (21) (Fig. 3).
Building upon the multitarget mechanism of WSB in
coordinately regulating the microbiota-barrier-immunity axis,
through the FAK-dependent tight junction restoration and the
microbiota remodeling to suppress the carcinogenic microenvironment
coupled with immune reprogramming to block the inflammation-cancer
transition from UC to CRC, its integrated preventive and
therapeutic potential offers novel strategies for high-risk UC
patients. Although preclinical data are promising, clinical
translation of WSB still faces challenges in terms of
pharmacokinetic optimization and validation of target biomarkers
such as SIRT1/SMURF2. Current exploratory trials are focusing on
combination therapies of WSB with immune checkpoint inhibitors (TCM
+ immunotherapy), which may overcome chemoresistance through
synergistic effects and unlock new therapeutic dimensions for
high-risk CAC patients. Furthermore, integration with targeted
delivery systems could enhance its translational value, enabling
the transition from the multi-mechanism intervention to precision
medicine (58).
Nonalcoholic fatty liver disease (NAFLD) has emerged
as a significant global health challenge, affecting 25-30% of
adults worldwide; the progressive of NAFLD, nonalcoholic
steatohepatitis, occurs in 10-20% of cases and often progresses to
cirrhosis and HCC (59-61). In the absence of FDA-approved
therapies for NAFLD/nonalcoholic steatohepatitis, WSB, a bioactive
lignan derived from Schisandra chinensis, has shown promise
as a multi-target therapeutic agent capable of modulating metabolic
homeostasis, inflammatory responses, and fibrotic processes.
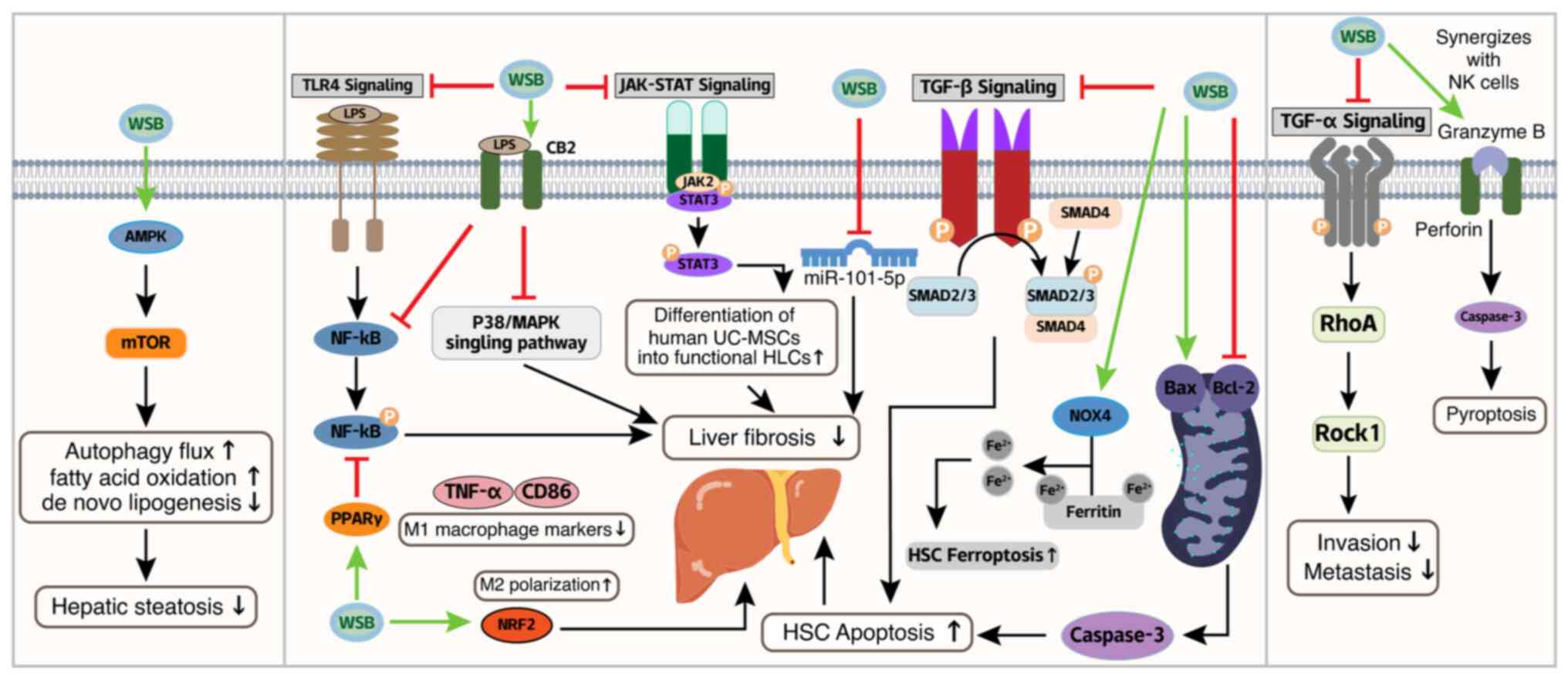
Mechanistically, WSB ameliorates hepatic steatosis by activating
the AMPK/mechanistic target of mTOR pathway, which enhances
autophagic flux, promotes fatty acid oxidation, and suppresses de
novo lipogenesis (62-64) (Fig. 4).
Notably, hepatic steatosis is often the initial step
in NAFLD progression, and unresolved steatosis can drive the
development of liver fibrosis, a critical pathological feature that
accelerates disease severity. Liver fibrosis is primarily driven by
the activation of hepatic stellate cells (HSCs), and WSB targets
these activated HSCs through multiple coordinated pathways to exert
anti-fibrotic effects. It induces mitochondrial-dependent apoptosis
in HSCs by modulating the Bax/Bcl-2 ratio and activating caspase-3,
while simultaneously inhibiting TGF-β1-induced HSC activation
(65) (Fig. 4). WSB promotes ferroptosis in
HSCs via NCOA4-mediated ferritinophagy, suggesting a novel approach
for fibrosis treatment (66)
(Fig. 4). Cannabinoid receptor 2
has been identified as a key target in liver fibrosis, and WSB
alleviates CCl4-induced fibrosis by inhibiting the NF-κB
and p38 mitogen-activated protein kinase (MAPK) signaling pathways
in Kupffer cells, thereby reducing inflammatory cytokine release
(67-69) (Fig. 4). Further studies revealed that
WSB directly targets miR-101-5p, downregulating the activity of the
TGF-β signaling pathway to inhibit fibrosis (70-72) (Fig. 4).
As well as targeting HSCs and Kupffer cells, WSB
modulates macrophage polarization to further alleviate liver
inflammation and fibrosis, although this regulation is context- and
dose-dependent, reflecting its adaptability to diverse
microenvironments. WSB inhibits peroxisome proliferator-activated
receptor γ (PPARγ)-mediated NF-κB activation to suppress M1
macrophage markers (CD86/TNF-α) in liver fibrosis models (73) (Fig. 4), under oxidative stress, it
promotes M2 polarization via the Nrf2 pathway (74) (Fig. 4), while in tumor
microenvironments, high-dose WSB may increase immunosuppression
through STAT3 (75) (Fig. 4). These seemingly contradictory
results probably reflect the WSB's multitarget regulatory effects
on macrophage plasticity rather than genuine mechanistic
conflicts.
Moreover, the therapeutic potential of WSB in liver
disease extends to regenerative strategies: It enhances the
differentiation of human umbilical cord mesenchymal stem cells into
functional hepatocyte-like cells by activating the JAK2/STAT3
pathway, which could complement its antifibrotic effects by
promoting liver repair (76)
(Fig. 4). However, compared with
those of other differentiation methods, the efficiency and
functional maturity of WSB-induced HLCs require thorough
assessment. More importantly, the direct application of WSB in
vivo to modulate the fate of the endogenous or the transplanted
MSCs requires a careful evaluation of pharmacokinetics, targeted
delivery, potential off-target effects on other cell types, and
long-term safety.
As NAFLD progresses to advanced stages, cirrhosis
can predispose patients to HCC, a leading cause of cancer-related
mortality globally, with limited therapeutic options for
advanced-stage patients (77).
WSB demonstrates significant anti-HCC activity through mechanisms
that align with its earlier roles in metabolic regulation and
inflammation while also targeting tumor-specific pathways. It
synergizes with NK cells to induce pyroptosis in HepG2 cells via
perforin-granzyme B-caspase 3-GSDME, highlighting its role in
immune microenvironment modulation (24) (Fig. 4; Table I). The Ras homolog family member
α/ρ-associated protein kinase 1 (RhoA/ROCK1) pathway, which is
involved in HCC cell proliferation and metastasis, is effectively
suppressed by WSB, leading to inhibited migration and invasion of
Huh-7 cells both in vitro and in vivo (78-80) (Fig. 4; Table I). These findings underscore the
potential of WSB as a multifaceted therapeutic agent for treating
HCC that targets both tumor cells and the immune system. However,
the narrow therapeutic window (IC50 ~266.4 μM in
HepG2 cells vs. significant toxicity in normal cells at >200
μM) poses a major translational challenge (81). In addition, achieving effective
antitumor concentrations in vivo without unacceptable
toxicity may require advanced delivery strategies or synergistic
combinations with standard therapies.
In summary, while WSB shows preclinical promise for
NAFLD, fibrosis, and HCC via multiple target mechanisms, its
translational challenges are significant. The narrow therapeutic
window and context-dependent immune modulation complicate clinical
application. Key gaps include defining the in vivo
therapeutic window, developing toxicity mitigation strategies (such
as targeted delivery), and obtaining human pharmacokinetic/safety
data, necessitating rigorous clinical trials.
CCA, a highly aggressive malignancy originating from
the biliary epithelium with frequent extension to the ampulla of
Vater (82), represents the
second most common primary liver cancer and remains notoriously
resistant to conventional chemotherapy, contributing to its dismal
5-year survival rate, which is <20% for advanced cases (83). Preclinical studies have
demonstrated that WSB exerts multimodal anti-CCA activity through
i) potent induction of mitochondrial apoptosis, as evidenced by an
increased Bax/Bcl-2 ratio and significant activation of caspase-3/9
and PARP cleavage and ii) effective G0/G1
cell cycle arrest via downregulation of Cyclin D1 and CDK4/6
inhibition at clinically achievable concentrations (84) (Fig. 3; Table I). These findings demonstrate the
potential of WSB as a promising therapeutic candidate, particularly
given its dual-targeting ability against both proliferative
signaling and apoptotic resistance pathways, two hallmark features
of CCA pathogenesis that contribute to clinical
chemoresistance.
GBC, one of the most aggressive malignancies of the
biliary system, is associated with a dismal clinical prognosis.
Studies by Pehlivanoglu et al (85) have indicated that only 5% of
invasive GBC patients are diagnosed at the early T1b stage, with a
5-year survival rate of up to 92%; however, most patients are
diagnosed at the locally advanced or metastatic stage, resulting in
an overall 5-year survival rate of <10%. Furthermore, Brindley
et al (86) reported that
the objective response rate to current chemotherapy regimens (such
as gemcitabine plus cisplatin) for biliary tract cancers, including
GBC, is only ~20%, highlighting the urgency to explore novel
therapeutic agents (86)
(Table I). As an active
component derived from the traditional Chinese herb, WSB has
recently demonstrated multitargeted antitumor potential in GBC
treatment, with its mechanisms of action supported by multiple
studies (84,87,88). In terms of apoptosis induction,
Yang et al (84) reported
that WSB can upregulate the proapoptotic protein Bax and
downregulate the antiapoptotic protein Bcl-2, disrupt mitochondrial
membrane potential, promote the release of cytochrome c,
activate the caspase-9/3 cascade, and cleave PARP, thereby inducing
apoptosis in cholangiocarcinoma cells (Fig. 3; Table I). Similarly, studies on BC have
confirmed that WSB can enhance mitochondrial apoptotic signaling
through a ROS-mediated endoplasmic reticulum stress pathway (such
as upregulating CHOP and GPR78), suggesting a common mechanism of
apoptotic regulation across different tumor types (52) (Fig. 3). With respect to cell cycle
regulation, Hui et al (87) reported that the overexpression of
Cyclin D1 in GBC is closely associated with poor prognosis. WSB can
markedly inhibit the activity of the Cyclin D1/CDK4/6 complex while
upregulating the expression of the CDK inhibitor p21, thereby
blocking the phosphorylation of the retinoblastoma protein (Rb) and
causing cell arrest in the G0/G1 phase
(Fig. 3; Table I). This mechanism is highly
consistent with the action pathway of CDK4/6 inhibitors observed in
BC, further validating its rationality (88) (Table I).
Notably, the antitumor potential of WSB is not
limited to GBC. Studies on its mechanisms in various other tumors,
such as BC, have shown commonalities in regulating apoptosis, the
cell cycle, and metastasis, providing a basis for its potential as
a broad-spectrum antitumor candidate. In terms of clinical
translation, combined with the current application of targeted
drugs such as FGFR and IDH1 inhibitors in biliary tract cancers
(89), WSB is expected to be
used in combination with existing targeted immunotherapies, further
enhancing the therapeutic efficacy of GBC through synergistic
effects on multiple pathways (90,91).
NPC represents a distinct epithelial malignancy with
a unique geographical distribution and strong etiological
association with EBV infection (92). While radiotherapy demonstrates
excellent efficacy in early-stage disease (achieving 90% cure
rates), the majority of patients present with locally advanced or
metastatic disease at diagnosis because of the tumor's insidious
growth pattern and early metastatic propensity (93). This clinical reality highlights
the urgent need for novel therapeutic strategies that can overcome
the limitations of current treatments, which are often associated
with significant toxicity and suboptimal outcomes in advanced cases
(94).
The mechanism of action of WSB in NPC is reflected
mainly in two aspects: First, WSB inhibits cell proliferation by
targeting CDK4/6. It can directly bind to CDK4/6 and downregulate
the expression of CDK4/6 in NPC cells (such as HONE-1 and CNE-1
cells) and although it upregulates Cyclin D1, it inhibits the
formation of complexes between Cyclin D1 and CDK4/6, thereby
hindering Rb phosphorylation and inducing cell cycle arrest at the
G1 phase. Notably, it has no significant effect on
normal nasopharyngeal epithelial cells. Second, it enhances
radiosensitivity by promoting radiotherapy-induced DNA
double-strand breaks [at 4 h after radiotherapy, the number of
phosphorylated histone H2A variant X (γ-H2AX) foci in the
combination group was markedly greater than that in the
radiotherapy alone group: for example, 21.4 vs. 13.2 foci/nucleus
in HONE-1 cells] and delaying damage repair (at 12 h after
radiotherapy, the number of γ-H2AX foci was still greater: For
example, 7.6 vs. 3.9 foci/nucleus in HONE-1 cells) (95) (Fig. 3; Table I). In addition, WSB can induce
NPC cells to arrest at the G1 phase, which is more
sensitive to radiotherapy, and prevent them from entering the S
phase (radiation-resistant phase), thereby enhancing the killing
effect of radiotherapy on tumor cells.
Notably, this mechanism of action of WSB is highly
consistent with its performance in other malignant tumors. For
instance, in LC studies, WSB also blocks the cell cycle at the
G0/G1 phase by downregulating the expression
of CDK4/6 and Cyclin D1 (96)
(Fig. 3; Table I). Similarly, in gallbladder
cancer (GBC), a correlation between CDK4 downregulation and
G1 phase arrest has been observed, suggesting that its
conservation of cell cycle regulation may make it a potential
therapeutic agent across tumor types (97). More importantly, compared with
clinically used CDK4/6 inhibitors such as palbociclib, WSB is
safer. Although palbociclib can enhance the radiosensitivity of NPC
by inhibiting DNA damage repair, it is often accompanied by adverse
reactions such as neutropenia and peripheral neuropathy (98). By contrast, WSB at a
concentration of 40 μM exerts no significant effect on the
proliferation or cell cycle of normal nasopharyngeal epithelial
cells (NP69) and has even been confirmed to exert protective
effects on tissues such as the liver and myocardium in animal
models, which supports its low toxicity advantage in clinical
applications (65,99,100).
From the perspective of the molecular mechanisms of
radioresistance, the radioresistance of NPC cells is often
associated with efficient DNA double-strand break repair capacity
(101) (Table I). The repair-inhibiting effect
of WSB, as reflected by the delay in γ-H2AX foci, may be related to
the regulation of the ATM and ATR kinases pathway (102) (Table I). Existing studies have shown
that WSB can inhibit ATR protein kinase activity to interfere with
the DNA damage response, and this mechanism may further explain its
radiosensitizing effect in NPC. In addition, the synergistic effect
of WSB and radiotherapy in 3D organoid models (markedly reduced
activity of HONE-1 organoids) is more consistent with the in
vivo tumor microenvironment, verifying its effectiveness under
complex physiological conditions and providing key evidence for
translation from basic research to clinical practice. These
characteristics make WSB not only promising for improving the
radiotherapy effect of locally advanced NPC, but also potentially
applicable in combination with existing targeted drugs (such as
EBV-related signaling inhibitors) to construct multitarget
therapeutic regimens, thereby offering new ideas for overcoming the
therapeutic bottlenecks of NPC.
LC is the most commonly diagnosed malignancy
globally, with recent epidemiological data revealing ~2.5 million
new cases each year, 12.4% of all cancer diagnoses worldwide and
1.8 million annual fatalities, accounting for 18.7% of total cancer
mortality (1). While the advent
of molecularly targeted therapies and immunotherapies has
revolutionized treatment paradigms, the prognosis for advanced
non-small cell lung cancer (NSCLC) patients remains poor, with
5-year survival rates persistently <20% (103). This therapeutic impasse stems
primarily from two key challenges: The development of drug
resistance mechanisms and dose-limiting treatment toxicity.
At the molecular level, the WSB strongly inhibits
the ATR protein kinase activity, effectively compromising the
G2/M checkpoint integrity following DNA damage through
the suppression of critical phosphorylation events in p53 and
checkpoint kinase 1 (CHK1) (102) (Fig. 3; Table I). This distinctive mechanism of
action potentiates the efficacy of conventional DNA-damaging
anticancer therapies, presenting a novel strategic approach for
targeting ATR-dependent DNA repair mechanisms in LC treatment.
In the A549 non-small cell LC cell line, WSB
demonstrates dual cell cycle regulatory effects, inducing
G0/G1 phase arrest while simultaneously
promoting caspase-dependent apoptotic cell death. Notably, it
suppresses TGF-β1-induced EMT in A549 cells through epigenetic
silencing of ZEB1 and restoration of E-cadherin expression
(104), a key mechanism given
that EMT drives LC metastasis by disrupting epithelial adhesion via
downregulated the E-cadherin and upregulated mesenchymal markers
(Fig. 3), enabling invasive
potential, thereby equipping WSB with the capacity to inhibit
metastatic progression through suppression of the EMT pathway.
These findings collectively position WSB as a
multifaceted therapeutic candidate capable of targeting both
primary tumor growth and metastatic dissemination. The anti-cancer
mechanisms of WSB require further elucidation, particularly
regarding its dose-response relationship/optimal therapeutic
window. Systematic pharmacokinetic/pharmacodynamic studies are
needed to establish clinically effective and safe dosing
regimens.
WSB demonstrates significant antitumor activity in
PC through a multipronged mechanism that addresses both disease
progression and therapeutic resistance (15,105,106). In the context of standard
androgen deprivation therapies, which aim to block AR signaling,
which is crucial for PC cell growth (107), WSB effectively suppresses AR
signaling (108) (Fig. 3; Table I). This not only complements the
action of existing therapies but also enhances sensitivity to them,
potentially overcoming the resistance that often develops over
time. For instance, drugs such as gonadotropin-releasing hormone
agonists/antagonists and anti-androgen drugs target AR signaling,
and the modulation of this pathway by WSB could enhance the
efficacy of these drugs.
Of particular clinical relevance, WSB maintains its
efficacy against chemotherapy-resistant phenotypes. In
radiotherapy, which aims to kill cancer cells by damaging their
DNA, the ability of WSB to modulate cellular processes may enhance
the radiosensitivity of cancer cells (95) (Table I). Overall, these findings
underscore the promise of WSB as a multitarget therapeutic agent
for PC management, with potential applications in combination with
existing endocrine, chemotherapy, and radiotherapy approaches.
Gliomas, which originate from brain glial cells,
represent the most prevalent primary intracranial tumors in adults
(40-60% of central nervous system tumors) (112). WSB exerts potent antitumor
effects on glioblastoma through the induction of mitochondrial
apoptosis (via Bax/Bcl-2 modulation and caspase-3 cleavage) and
G0/G1 cell cycle arrest (through
CDK4/6-Cyclin D1 suppression) in SHG-44 and U87MG cells (113,114) (Fig. 3; Table I). RNA sequencing analyses
reveals that WSB inhibits the HOTAIR/miR-125a/mTOR pathway,
reducing glioma cell migration by 55-70% (115) (Fig. 3).
Synergy with standard therapies enhances efficacy:
Temozolomide combination therapy overcomes resistance by
suppressing O6-methylguanine-DNA methyltransferase activity and
blocking the repair of O6-methylguanine lesions. This leads to
unrepaired DNA damage, persistent double-strand breaks, and
enhanced apoptotic signaling through DNA damage response pathway
activation (116,117) (Fig. 3; Table I). Similarly, rapamycin
synergizes with WSB through dual epigenetic and mTOR-targeted
inhibition, WSB indirectly suppresses mTOR expression via
downregulation of HOTAIR expression and upregulation of miR-125a-5p
expression, whereas rapamycin directly inhibits mTOR activity.
Preclinically, WSB monotherapy achieves 68% tumor growth
inhibition, which increases to 85% when WSB is combined with
rapamycin (115,118) (Figs. 3 and 5; Table
I).
These findings position WSB as a multifaceted
therapeutic agent against glioblastoma, leveraging intrinsic
pro-apoptotic and cell cycle regulatory properties while
synergizing with conventional and targeted (rapamycin) therapies to
overcome key resistance mechanisms. Future clinical translation
should prioritize optimizing delivery strategies to penetrate the
blood-brain barrier and validating predictive biomarkers (such as
HOTAIR/MGMT expression) for patient stratification in combination
regimens.
In BC, WSB overcomes chemoresistance through
multimodal targeting: i) Chemosensitization: STAT3 is often
overactivated, which contributes to the upregulation of P-gp, a
major efflux pump responsible for pumping chemotherapeutic drugs
out of cancer cells, leading to drug resistance (119,120). WSB acts by inhibiting STAT3
signaling. As reported in studies on other cancer types, such as
neuroblastoma, the activation of STAT3 can protect cells from
apoptosis and is associated with drug resistance (121) (Fig. 3). By blocking the STAT3
phosphorylation and nuclear translocation, WSB effectively
downregulates the expression of P-gp in MCF-7/ADR cells. This
downregulation reverses the efflux of doxorubicin (DOX), thereby
increasing the intracellular concentration of DOX. As a result, the
IC50 of DOX in MCF-7/ADR cells is markedly reduced from
12.3 to 2.8 μM, enhancing the sensitivity of cancer cells to
chemotherapeutic agents (122)
(Fig. 3; Table I); ii) by blocking the
TGF-β/NOX4/ROS signaling, WSB inhibits vimentin and Snail
expression, reducing metastatic potential in 4T1-Luc models (lung
nodules ↓65%) (52,104,123) (Fig. 3); and iii) WSB polarizes
tumor-associated macrophages (TAMs) toward the antitumor M1
phenotype, increasing the expression of the M1 marker CD86 and
upregulating M1-characteristic IL-12. In triple-negative breast
cancer (TNBC) cells, WSB suppresses programmed cell death ligand 1
(PD-L1) and high levels of PD-L1 bind to T-cell PD-1, inhibiting
T-cell activation and cytotoxicity. By reducing PD-L1 expression,
WSB enhances T-cell recognition and the killing of cancer cells,
strengthening antitumor immune responses in the BC microenvironment
(23,124) (Fig. 3; Table I). Preclinical studies have
demonstrated that WSB monotherapy achieves 58% tumor growth
inhibition in estrogen receptor (ER) + BC xenografts, synergizing
with tamoxifen to achieve 82% suppression (Fig. 5).
Building on its dual anti-inflammatory and
chemosensitizing actions, WSB warrants clinical prioritization in
endometritis and TNBC, conditions with unmet therapeutic needs.
Synergistic strategies (such as PD-1 inhibition) could amplify
STAT3/PD-L1 blockade, while nanotechnology may overcome
bioavailability barriers. The mechanistic exploration of
AR-mitochondrial crosstalk in orchitis could redefine the
management of male infertility. Cross-disciplinary innovation will
catalyze WSB's transition to transformative therapies.
Osteosarcoma, the most prevalent malignant bone
tumor in adolescents, has a poor prognosis, especially in
metastatic cases (125-127) (Fig. 3). WSB inhibits osteosarcoma
progression by targeting circ_0009112/miR-708-5p and by suppressing
migration and invasion via the PI3K/AKT pathway while inducing
apoptosis (128,129) (Fig. 3; Table I). It also blocks Wnt/β-catenin
and PI3K/AKT signaling, arresting the cell cycle at the
G1 phase, inhibiting proliferation, and reducing lung
metastasis in preclinical models without significant toxicity
(63). These findings highlight
WSB's potential as a therapeutic candidate for osteosarcoma.
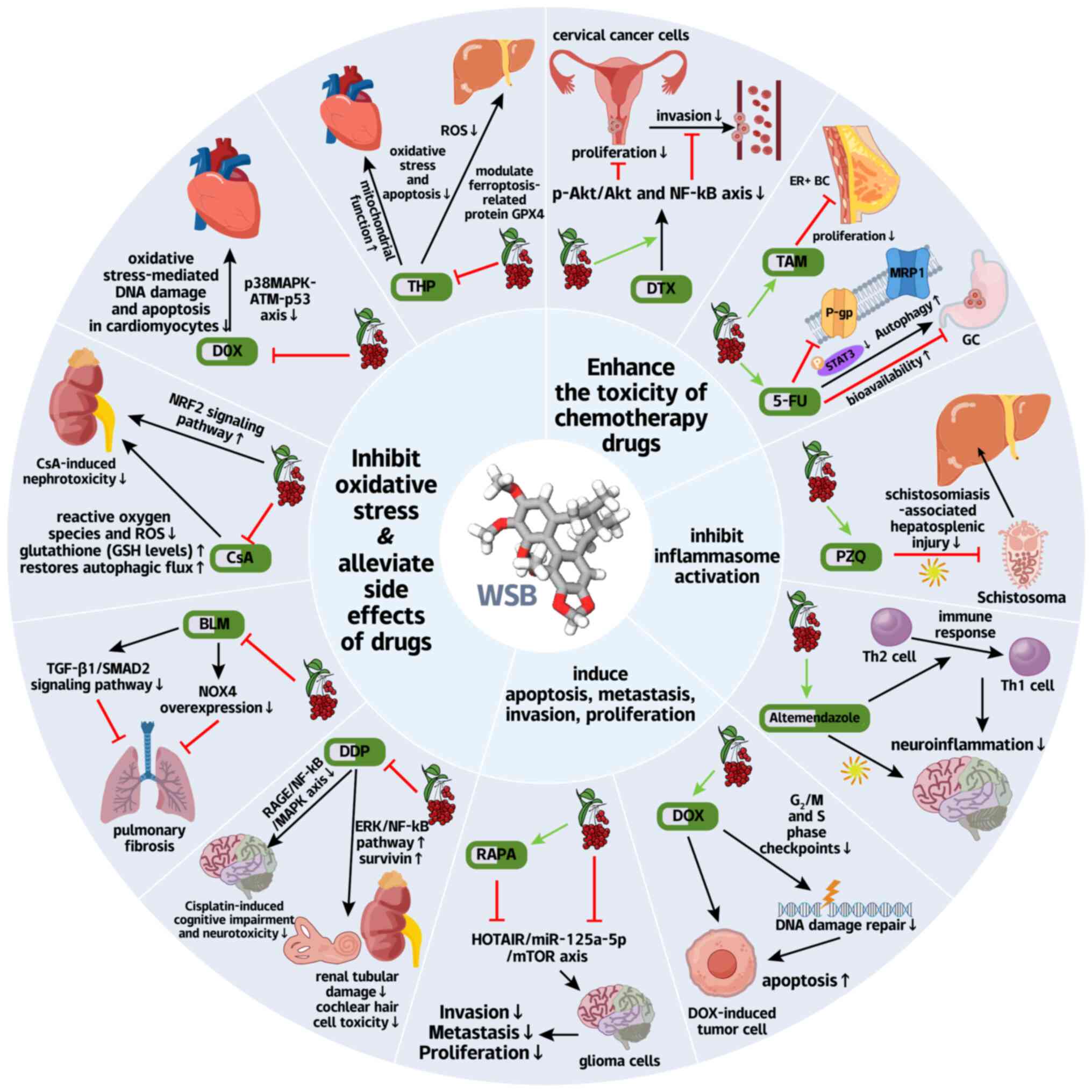
As a pleiotropic bioactive compound, WSB enhances
chemotherapy efficacy while mitigating adverse effects through
multitarget modulation of oxidative stress, inflammation, and cell
death pathways. Its integration with advanced drug delivery systems
further improves pharmacokinetics, offering a paradigm for
low-toxicity precision oncology (Fig. 5).
Notably, the capacity of WSB to modulate MDR,
enhance chemosensitivity, particularly well-characterized in BC,
extends to a coordinated protective role against
chemotherapy-induced organ damage, creating a synergistic
therapeutic profile. In BC, WSB reverses DOX resistance by
downregulating the P-gp via STAT3 inhibition (122) (Fig. 5; Table II), while simultaneously
counteracting DOX-induced cardiotoxicity through p38MAPK-ATM-p53
axis regulation (130,131) (Fig. 5; Table II), thus amplifying anti-tumor
efficacy while mitigating cardiac injury. This dual action aligns
with its ability to sensitize ER+ BC to tamoxifen (achieving 82%
tumor suppression in combination) (132) (Fig. 5), demonstrating that WSB's
MDR-reversing effects are paired with tissue-specific toxicity
attenuation.
Furthermore, in cisplatin-based regimens, where
drug resistance and systemic toxicity often limit efficacy, WSB not
only enhances chemosensitivity by overcoming efflux mechanisms but
also protects normal tissues: it upregulates survivin via ERK/NF-κB
to shield renal tubules and cochlear hair cells (133,134) (Table II) and inhibits the
RAGE/NF-κB/MAPK pathways to prevent neurotoxicity (135) (Fig. 5; Table II). Similarly, for thiotepa, the
pro-oxidant activity of WSB in tumor cells (driving apoptosis) is
balanced by its suppression of excessive ROS in normal tissues,
preserving hepatic and cardiac function via GPX4-mediated
ferroptosis inhibition (136-138) (Fig. 5; Table II).
Beyond specific chemotherapeutics, WSB synergizes
with supportive agents to mitigate broader complications: In
combination with glycyrrhizic acid, it suppresses TGF-β1/SMAD2
signaling and NOX4 overexpression in bleomycin-induced pulmonary
fibrosis (139) (Fig. 5), a common side effect of
alkylating agents; in cyclosporine A-induced nephrotoxicity, it
activates Nrf2 to reduce ROS, elevate glutathione, and restore
autophagy (140) (Fig. 5), thus protecting renal function
during immunosuppressive chemotherapy regimens; and in
schistosomiasis, it enhances praziquantel efficacy while
alleviating hepatosplenic injury (141,142) (Fig. 5), a model for reducing parasitic
coinfection complications during chemotherapy.
Collectively, these findings underscore the unique
therapeutic duality of WSB in cancer chemotherapy: It not only
enhances antitumor efficacy by reversing MDR and sensitizing
malignancies (as exemplified in BC) but also safeguards normal
tissues through targeted mechanisms, mitigating the systemic
toxicity that often limits treatment tolerance. This multifaceted
profile positions WSB as a promising adjuvant in chemotherapeutic
regimens, addressing the longstanding challenge of balancing
efficacy and safety across diverse cancer types and treatment
modalities.
WSB potentiates chemotherapy through synergistic
pathway inhibition: When combined with docetaxel (DTX), WSB
downregulates phosphorylated (p-)AKT/AKT and NF-κB expression,
ultimately inhibiting cervical cancer proliferation and invasion
(143) (Fig. 5; Table II). In GC, WSB and apatinib
induce G0/G1 arrest and suppress
MMP-9-mediated metastasis (144,145) (Fig. 5; Table II). With the DOX, WSB disrupts
DNA repair by abrogating G2/M checkpoints and amplifying
apoptosis (146,147) (Table II). Moreover, Additional studies
have demonstrated that WSB can also increase the sensitivity of
glioblastoma cells (GBM U87 and A172) to the antitumor drug
temozolomide by downregulating the expression of MRP1 protein or
mRNA (116,148) (Fig. 3; Table II).
However, when WSB is co-administered with P-gp
substrate chemotherapeutic agents such as paclitaxel and
doxorubicin, it promotes the drug efflux, thereby reducing
intracellular drug accumulation in tumor cells (81,149) (Figs. 3 and 5; Table
II). Additionally, WSB downregulates organic anion transporting
polypeptide 1B1 (OATP1B1) expression, which may impair the hepatic
uptake and subsequent activation metabolism of OATP-dependent drugs
such as irinotecan (150).
Furthermore, WSB exhibits complex pharmacokinetic interactions with
FK506; by competing for CYP3A-mediated metabolism, WSB accelerates
its own clearance while simultaneously inhibiting FK506 metabolism
(149) (Table II). These findings necessitate
the preclinical-to-clinical translation via the pharmacogenomic
screening: tumors with low P-gp/MRP1 and high OATP1B1 could
prioritize WSB combinations (such as DTX/WSB in cervical cancer)
while avoiding coadministration with P-gp substrates in
ABCB1-overexpressing tumors.
In summary, WSB acts as a double-edged sword in
combination chemotherapy: On one hand, it enhances the antitumor
efficacy of drugs such as docetaxel and apatinib by inhibiting key
signaling pathways (p-AKT/NF-κB) and disrupting DNA repair
mechanisms. On the other hand, the WSB-induced upregulation of
P-gp/MRP efflux pumps and suppression of OATP1B1 may antagonize the
therapeutic effects of paclitaxel, doxorubicin, and irinotecan.
Additionally, it engages in complex pharmacokinetic competition
with CYP3A substrate drugs (such as tacrolimus). Therefore,
clinical combination strategies must be precisely designed on the
basis of drug transport and metabolic profiles to mitigate
interaction risks and maximize synergistic benefits.
MDR, driven by drug efflux, cancer stem cells and
the tumor microenvironment (TME), remains a critical clinical
hurdle (151).
Preclinical studies have demonstrated that WSB
effectively overcomes RAS-mediated resistance to EGFR inhibitors in
colorectal cancer (152)
(Fig. 6; Tables I and II). Resistance to EGFR-targeted
therapy in colorectal cancer primarily arises from tumor-intrinsic
and microenvironment-driven adaptive mechanisms: RAS/RAF mutations
(such as KRAS in 40% and BRAF V600E in 8-10% of cases) lead to
constitutive activation of downstream signaling, while bypass
pathways such as the ERBB2/MET/IGF-1R amplification or
overexpression maintain survival signals through feedback loops
(153-156). Additionally, metabolic
reprogramming (enhanced glycolysis and dysregulated lipid
synthesis) and tumor microenvironment factors (such as HGF/TGF-β
secretion by cancer-associated fibroblasts) further contribute to
drug resistance (157-159). To counteract these resistance
mechanisms, WSB synergizes with panitumumab by activating
caspase-3-dependent apoptosis and suppressing Bcl-2 expression,
thereby reversing drug resistance. This mechanism could translate
to clinical trials testing WSB + panitumumab in RAS-mutant CRC,
with Bcl-2 as a predictive biomarker (Fig. 6).
In combination with 5-FU, WSB downregulates P-gp
and MRP1, reverses MDR, and triggers autophagic death via STAT3
inhibition (33,160) (Figs. 3 and 5; Table
II). This mechanism is supported by extensive evidence that ABC
transporters (such as P-gp/ABCB1 and MRP1/ABCC1) mediate drug
efflux in CRC (161-164), and TCM compounds (such as
evodiamine and neferine) restore chemosensitivity by suppressing
these transporters (165,166). Such data support the potential
of WSB as a chemosensitizer in neoadjuvant settings, where reducing
the ABC transporter activity could increase 5-FU response
rates.
These findings confirm the potential of WSB as an
ideal chemosensitizer and provide a new strategy for the
comprehensive treatment of colorectal cancer.
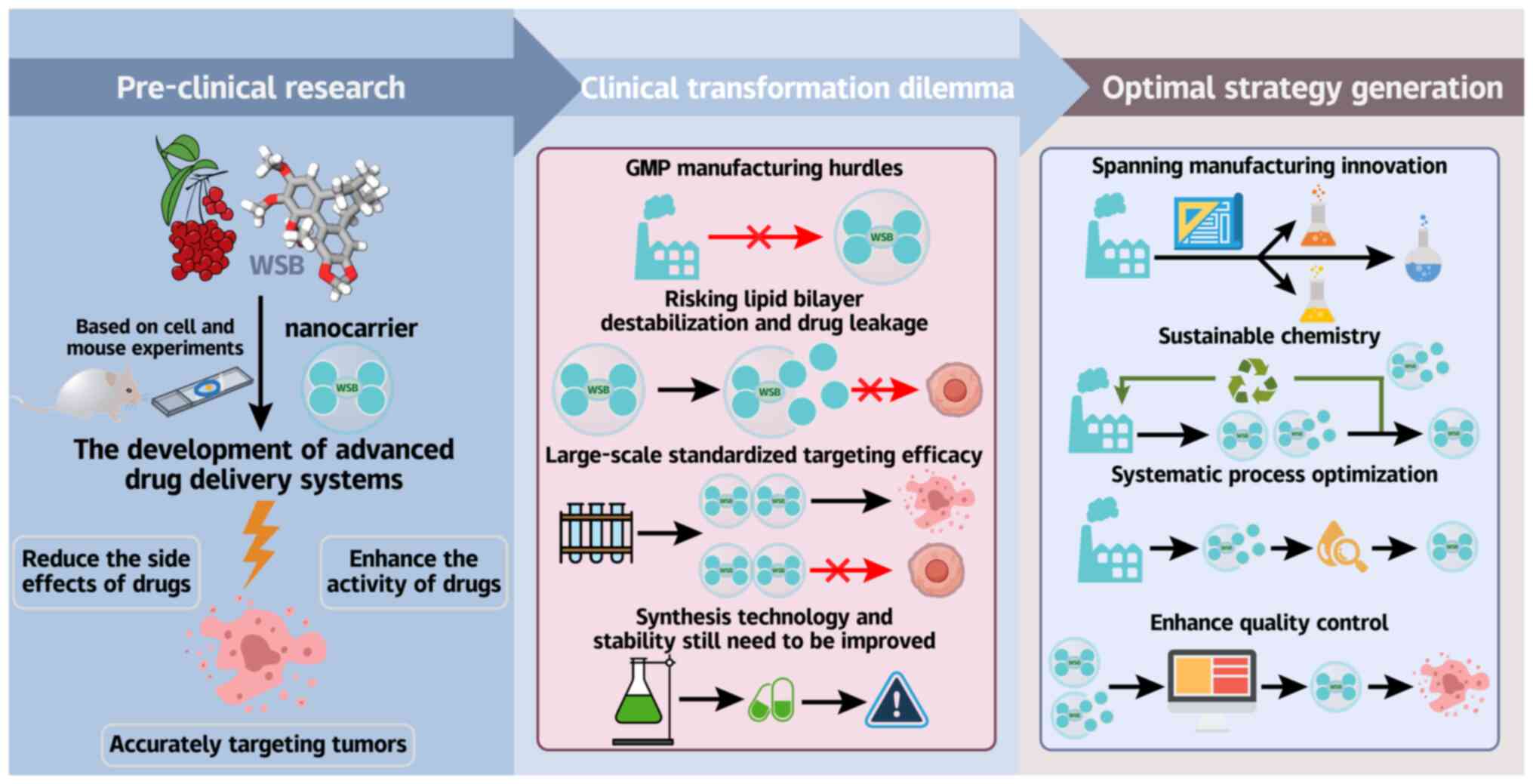
WSB has been established as a promising and
clinically feasible anticancer agent (167). The core advantages of these
systems include the following: i) Passive tumor targeting
leveraging the enhanced permeability and retention effect (168); ii) enhanced active targeting
via ligand modification (169);
and iii) precise drug release enabled by the design of
pH/enzyme-sensitive carriers (170). Clinically, this is evidenced by
successful nanoscale antitumor formulations, such as the polymeric
micelle Genexol®-PM (loaded with paclitaxel), approved
in South Korea for the treatment of metastatic BC and NSCLC.
Compared with conventional paclitaxel, Genexol®-PM
markedly improved the objective response rate (ORR: 56 vs. 27%) and
reduced the incidence of neutropenia (171) (Fig. 6). Similarly, the liposomal
formulation Lipusu® (paclitaxel for injection) is
approved in China for the treatment of ovarian cancer and BC. Its
combination regimen with cisplatin effectively treats inoperable
NSCLC patients (172) (Fig. 6).
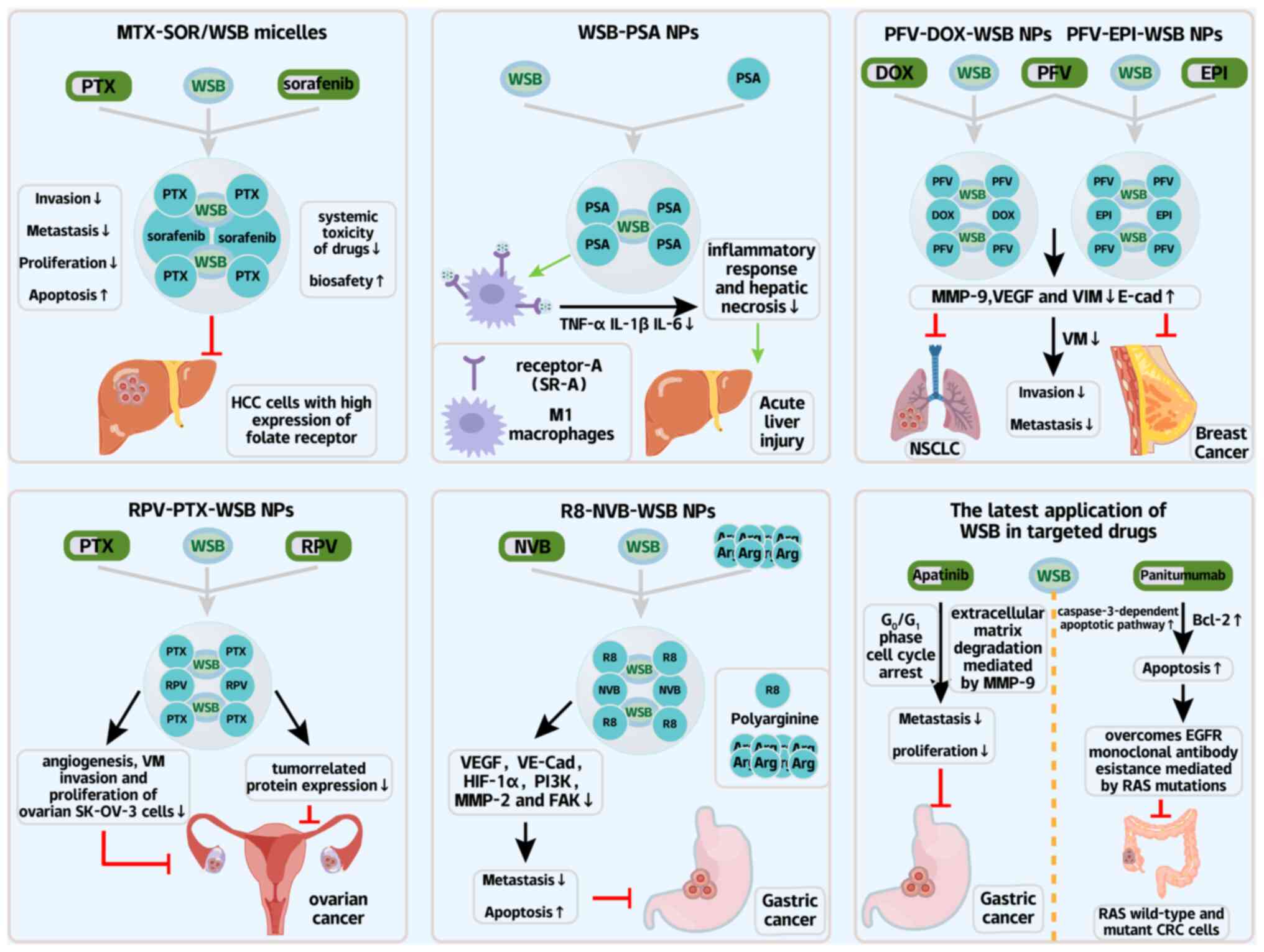
Stimulus-responsive nanotechnology have yielded the
pH-sensitive formulations in which methotrexate-modified
sorafenib/WSB micelles (methotrexate-SOR/WSB) demonstrate
remarkable tumor specificity, dissociating in the acidic tumor
microenvironment to simultaneously inhibit HCC progression and
reduce systemic toxicity (173)
(Fig. 6; Table II). With the respect to
inflammatory modulation, albumin-based nanocarriers engineered with
palmitic acid modifications (PSA systems) achieve targeted WSB
delivery to M1 macrophages, effectively suppressing NF-κB-mediated
inflammation in liver injury models while improving survival by 50%
(75 vs. 25% in controls) (174)
(Fig. 6; Table II).
Combinatorial approaches use polymeric nanosystems
that coencapsulate the WSB with doxorubicin. These factors leverage
the pH-dependent release kinetics to enhance mitochondrial
targeting in BC stem cells, demonstrating the dual benefits of
overcoming chemoresistance while preserving cardiac function
(175,176) (Fig. 6; Table II). The field has further
advanced through peptide-directed delivery platforms, including the
PFV-modified liposomes that exploit tumor-specific integrin αvβ3
overexpression to disrupt metastatic vasculogenic mimicry networks
(177) (Fig. 6; Table II), and RPV-conjugated systems
that the achieve tumor penetration while suppressing the
angiogenesis through VE-cadherin/MMP-9 regulation (178,179) (Fig. 6). Particularly innovative are
charge-mediated R8-modified vinorelbine/WSB liposomes that
concurrently inhibit EMT and prevent the treatment-limiting
myelosuppression (180,181) (Fig. 6; Table II).
Despite these compelling preclinical results,
clinical translation faces the significant hurdles associated with
Good Manufacturing Practice manufacturing. For PFV-liposomes,
extrusion-based preparation results in shear sensitivity during
scale-up, risking lipid bilayer destabilization and drug leakage
(177). The PSA platform
requires strict control of palmitic acid conjugation stoichiometry
to maintain targeting efficacy, a critical quality attribute that
is challenging to standardize in large batches (182,183). Organic solvent residues
necessitate complex purification (174), increasing production costs.
Analytical complexity is heightened in codelivery systems, where
simultaneous quantification of hydrophilic/hydrophobic drugs
requires specialized methods (177).
To bridge this translational gap, integrated
solutions are essential. These include the adoption of continuous
manufacturing approaches such as microfluidic mixing to replace
batchwise liposome extrusion (184,185), coupled with the greener
synthesis methods such as supercritical CO2-based
nanoparticle production to eliminate toxic chlorinated solvents
(186,187). The implementation of QbD
principles is essential for optimizing critical conjugation
parameters (such as reaction time and pH control) in PSA
nanoparticle fabrication, whereas advanced process analytical
technology (PAT) systems should be employed for real-time
monitoring of critical quality attributes throughout production
(188). These synergistic
advancements, spanning manufacturing innovation, sustainable
chemistry, systematic process optimization and enhanced quality
control, collectively address the key technical and regulatory
hurdles impeding clinical translation (Fig. 7).
While WSB nanocarriers exemplify the TCM-modern
oncology integration, industrial viability hinges on resolving the
material, process, and regulatory gaps, a challenge demanding
cross-disciplinary collaboration.
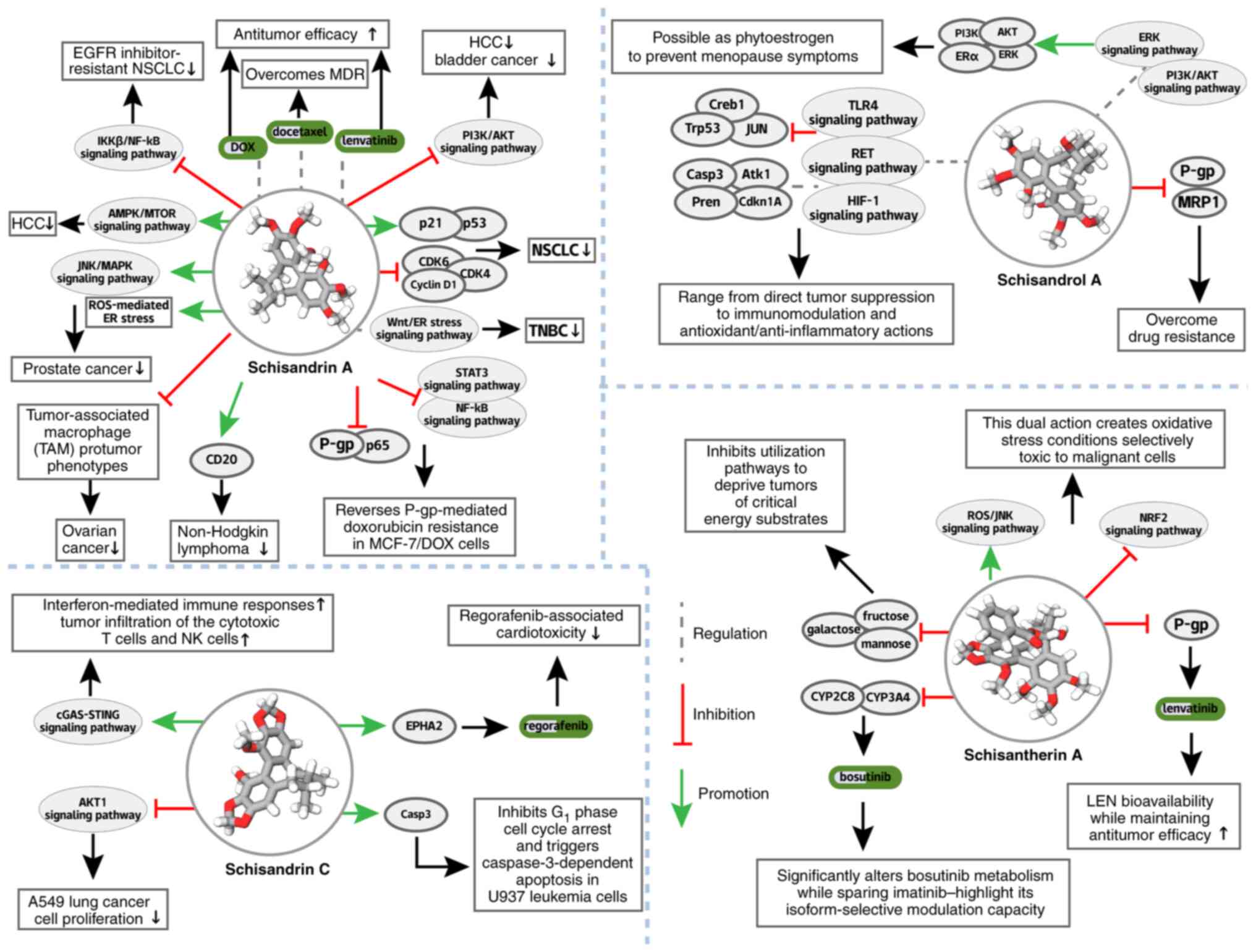
Further studies have demonstrated the efficacy of
Sch A in bladder cancer through inhibition of the ALOX5-mediated
activity of the PI3K/AKT signaling pathway (206) (Fig. 8; Table III). In HCC, Sch A acts via
AMPK/mTOR-dependent mitochondrial ferroptosis (207) (Fig. 8; Table III) and in PC, it activates
ROS-mediated ER stress and the JNK/MAPK signaling pathway (208) (Fig. 8; Table III). Emerging evidence also
suggests its activity against non-Hodgkin lymphoma through CD20
inhibition (209) (Fig. 8; Table III).
In addition, Sch A reverses P-gp-mediated
doxorubicin resistance via the P-gp/NF-κB/STAT3 inhibition
(210) (Fig. 8), complementing the WSB's
STAT3-dependent P-gp downregulation by disrupting
transporter-substrate interactions. It restores gefitinib
sensitivity in EGFR-resistant NSCLC (211) (Fig. 8), synergizing with Lenvatinib
(LEN) (212) (Fig. 8), expanding WSB's
chemosensitization scope to the EGFR-TKI resistance.
Innovative drug delivery platforms have augmented
the therapeutic potential of Sch A. Ultrasound-targeted microbubble
destruction with Span-PEG carriers enhances Sch A uptake in
Walker-256 hepatoma cells, inhibiting the PI3K/AKT/mTOR pathway
(213) (Fig. 8). A microemulsion system loaded
with docetaxel and Sch A overcomes esophageal cancer MDR (211) (Fig. 8), whereas long-circulating
liposomes loaded with Sch A/doxorubicin (SchA-DOX-Lip) enhance
apoptosis induction and tumor targeting in HCC (214) (Fig. 8). These advancement strategies
are directly applicable to WSB, leveraging structural similarity
for formulation optimization. Together, their complementary
mechanisms and shared delivery solutions support lignan-based
combination therapies, maximizing clinical utility.
In toxicity mitigation, SC counteracts
regorafenib-induced hepatotoxicity and cardiotoxicity via EphA2
Ser897 phosphorylation and EPHA2 restoration (217,218), complementing WSB's DOX
cardioprotection (p38MAPK-ATM-p53 axis) (130,131) (Fig. 8), with structural similarity
enabling tissue-specific cytoprotection (liver/heart vs. WSB's
cardiac focus).
SC's dual capacity supports combination strategies:
Coadministration with regorafenib to reduce toxicity while
enhancing efficacy. Formulation advances (such as targeted
delivery), adaptable from WSB's nanotechnology research, could
optimize tissue-specific distribution, maximizing its integrative
potential.
Pharmacokinetically, the STA modulates drug
metabolism to enhance efficacy: it increases the cyclophosphamide
exposure (227), selectively
inhibits CYP3A4/CYP2C8 (altering bosutinib but not imatinib
metabolism) (228) (Fig. 8) and, most notably, increases LEN
bioavailability in patients with HCC via intestinal P-gp
suppression (229,230) (Fig. 8).
SAL, a dibenzocyclooctadiene lignan that shares
structural homology with WSB, exhibits multitargeted activity
across liver diseases, from NAFLD to HCC, complementing the hepatic
effects of WSB through overlapping yet distinct mechanisms
(231,232).
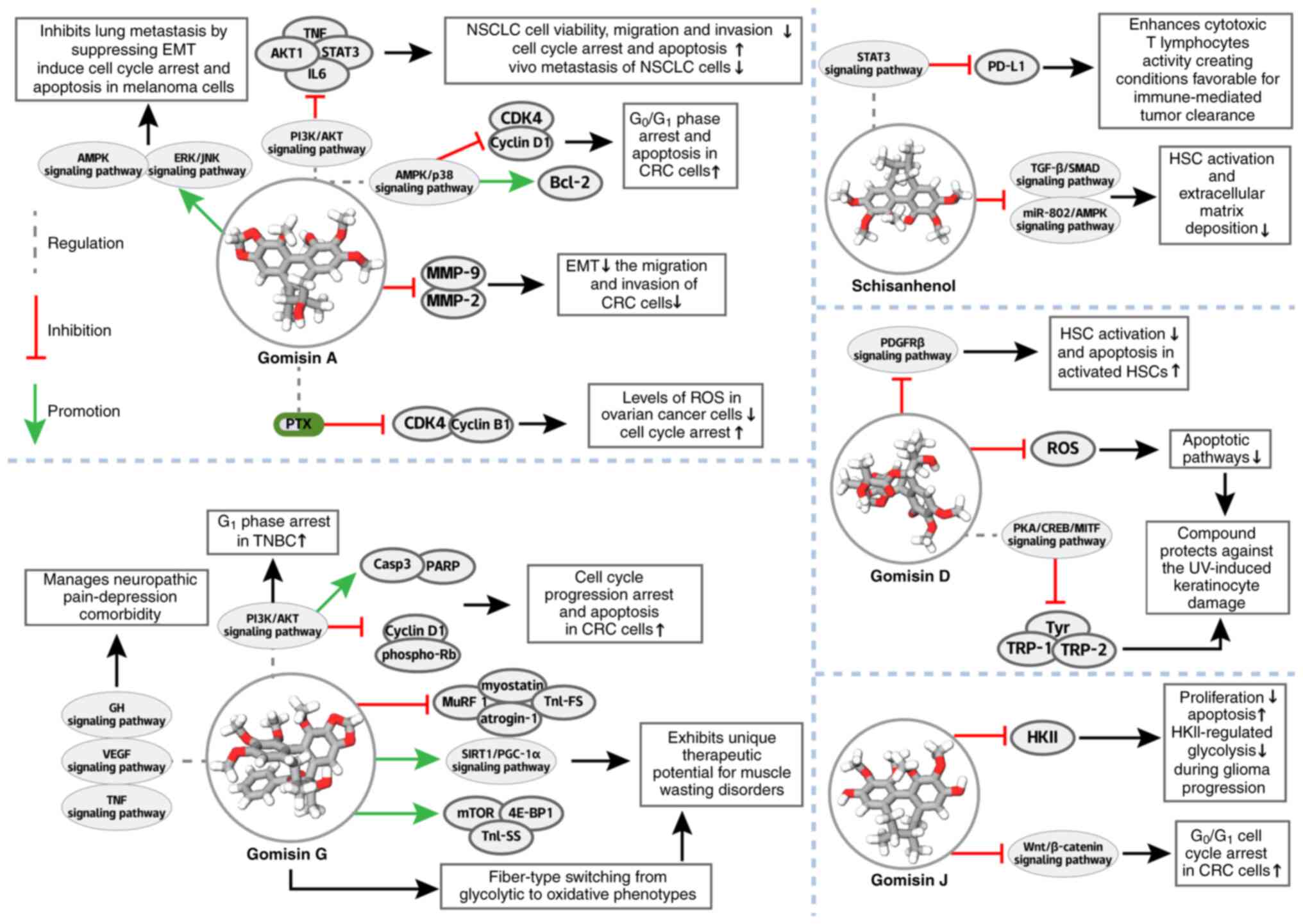
In HCC, SAL targets STAT3 to inhibit proliferation
and downregulate PD-L1, enhancing CTL activity (235) (Fig. 9) and complementing anti-HCC
mechanisms of WSB [such as RhoA/ROCK1 inhibition (78-80) (Fig. 9)] by increasing the degree of
immunomodulation and WSB minimally affects liver cancer. This
convergence of metabolic, fibrotic, and immunologic regulation
positions SAL as a bridge between WSB's hepatic protection and
novel immunotherapeutic strategies in HCC.
Clinically, the ability of SAL to span
NAFLD-fibrosis-HCC progression, paired with the broader anticancer
spectrum of WSB, supports the use of combination strategies.
Formulation advances, which are adaptable to WSB research, could
optimize the pharmacokinetics of both compounds, maximizing their
synergistic potential in liver disease management.
Translational efforts should focus on optimizing
bioavailability (exploiting WSB's formulation advances),
identifying biomarkers and developing combinations, capitalizing on
the structural overlap of GA with WSB to design coordinated
regimens that maximize pathway coverage across NSCLC, osteosarcoma,
CRC and melanoma.
In hepatic fibrosis, GD selectively disrupts the
PDGF-BB/PDGFRβ cascade to suppress HSC activation and induce
apoptosis in activated HSCs (241) (Fig. 9), complementing the anti-fibrotic
effects of WSB (such as TGF-β/SMAD inhibition) by targeting PDGFRβ,
a pathway that WSB does not directly engage in, thereby broadening
lignan-mediated control of fibrosis in CCl4-induced
models. Beyond the liver, GD protects against UV-induced
keratinocyte damage via the ROS scavenging and the apoptotic
pathway inhibition while suppressing melanogenesis through the
PKA/CREB/MITF axis inhibition (242) (Fig. 9), extending the ROS-modulating
properties (shared with WSB) of the lignan class to dermatological
contexts, with structural similarity enabling both to regulate
oxidative stress, but in tissue-specific ways (hepatic vs.
cutaneous).
Translational efforts should focus on optimizing
targeted delivery systems (adaptable from formulation research on
WSB), exploring synergies with existing
antifibrotics/dermatological agents and investigating preventive
potential in high-risk populations, leveraging the structural
overlap of GD with WSB to design combination strategies that
maximize dual efficacy in hepatic and dermatological
pathologies.
In oncology, GG inhibits PI3K-AKT signaling to
induce apoptosis (via PARP/caspase-3 cleavage) and suppresses the
cell cycle (Cyclin D1/phospho-Rb downregulation) in colorectal
cancer and triggers G1 arrest in TNBC through inhibition
of the AKT activity (243,244) (Fig. 9; Table III), mirroring the ability of
WSB to target PI3K/AKT in various types of cancer but with enhanced
specificity for inhibition of the activity of PI3K, creating a
complementary strategy with respect to the broader inhibition of
WSB. As well as tumors, GG counteracts muscle wasting via myostatin
modulation and the SIRT1/PGC-1α-mediated mitochondrial
biogenesis (245) (Fig. 9) and alleviates neuropathic
pain-depression comorbidity through TNF/VEGF/GH regulation
(246) (Fig. 9), extending the multitarget
potential (shared with WSB) of the lignan class to nononcologic
contexts, leveraging structural similarity to drive pleiotropic
effects.
Translational efforts should focus on WSB progress:
Optimizing structure-activity relationships (to increase target
specificity), improving pharmacokinetics via advances in
formulation (adaptable from WSB delivery research) and identifying
biomarkers for stratification. The ability of GG to complement WSB
in oncology while expanding into other therapeutic areas
underscores the versatility of the lignan class, rooted in their
conserved dibenzocyclooctadiene scaffold.
GJ demonstrates significant potential as an
anticancer agent, exhibiting efficacy across multiple cancer types
through simultaneous targeting of diverse oncogenic pathways. In BC
models, GJ uniquely induces both classical apoptosis and
necroptosis, showing particular efficacy against
apoptosis-resistant populations that drive treatment failure and
recurrence (247). This dual
cell death mechanism addresses a critical clinical challenge in
managing aggressive BC subtypes.
The anticancer activity of GJ extends to CRC, where
it suppresses the Wnt/β-catenin pathway (a key driver of colorectal
carcinogenesis) and induces G0/G1 cell cycle
arrest (248) (Fig. 9; Table III). These coordinated actions
on oncogenic signaling and cell cycle regulation underlie its
potent antitumor effects in CRC. In glioma models, GJ adopts a
multifaceted approach: triggering mitochondrial apoptosis while
disrupting tumor metabolism via the HKII-mediated glycolysis
inhibition (249) (Fig. 9; Table III), simultaneously targeting
cell survival and energy production pathways essential for tumor
growth.
The multi-target activity of GJ its and capacity to
overcome treatment resistance position it as a promising
therapeutic candidate, necessitating further development of
optimized formulations, predictive biomarkers, and rational
combination strategies for clinical translation.
Not applicable.
LZ, YL, CL, and YT conceived and supervised this
work. LZ, YL, ZC, JM, ZH, and XS organized the literature, wrote
the manuscript and created charts. LZ and YL provided funding. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was financially supported by the Social
Science and Technology Research Major Project of Zhongshan (grant
no. 2021B3011) and the Guangdong Medical University Undergraduate
Innovation and Entrepreneurship Training Program (grant nos.
GDMUCX2024017, GDMU2023355 and 202510571017).
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Liu Y, Fang C, Luo J, Gong C, Wang L and
Zhu S: Traditional Chinese medicine for cancer treatment. Am J Chin
Med. 52:583–604. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Miao J, Liu C, Tao J, Zhou S, Song
X, Zou Y, Huang Y and Zhong L: Kushenol O Regulates GALNT7/NF-κB
axis-Mediated Macrophage M2 Polarization and Efferocytosis in
papillary thyroid carcinoma. Phytomedicine. 138:1563732025.
View Article : Google Scholar
|
|
4
|
Szopa A, Ekiert R and Ekiert H: Current
knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese
magnolia vine) as a medicinal plant species: A review on the
bioactive components, pharmacological properties, analytical and
biotechnological studies. Phytochem Rev. 16:195–218. 2017.
|
|
5
|
Yang K, Qiu J, Huang Z, Yu Z, Wang W, Hu H
and You Y: A comprehensive review of ethnopharmacology,
phytochemistry, pharmacology, and pharmacokinetics of Schisandra
chinensis (Turcz.) Baill. and Schisandra sphenanthera Rehd. et
Wils. J Ethnopharmacol. 284:1147592022. View Article : Google Scholar
|
|
6
|
Olas B: Cardioprotective potential of
berries of Schisandra chinensis Turcz. (Baill.), their components
and food products. Nutrients. 15:5922023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shan Y, Jiang B, Yu J, Wang J, Wang X, Li
H, Wang C, Chen J and Sun J: Protective Effect of Schisandra
chinensis polysaccharides against the immunological liver injury in
mice based on Nrf2/ARE and TLR4/NF-κB signaling pathway. J Med
Food. 22:885–895. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan T, Mao Q, Zhang X, Wu B, Bi K, He B
and Jia Y: Schisandra chinensis protects against dopaminergic
neuronal oxidative stress, neuroinflammation and apoptosis via the
BDNF/Nrf2/NF-κB pathway in 6-OHDA-induced Parkinson's disease mice.
Food Funct. 12:4079–4091. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu G, Lv X, Feng Y, Li H, Chen C, Lin H,
Li H, Wang C, Chen J and Sun J: Study on the effect of active
components of Schisandra chinensis on liver injury and its
mechanisms in mice based on network pharmacology. Eur J Pharmacol.
910:1744422021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan R, Tao X, Liang S, Pan Y, He L, Sun
J, Wenbo J, Li X, Chen J and Wang C: Protective effect of acidic
polysaccharide from Schisandra chinensis on acute ethanol-induced
liver injury through reducing CYP2E1-dependent oxidative stress.
Biomed Pharmacother. 99:537–542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang M, Xu L and Yang H: Schisandra
chinensis Fructus and its active ingredients as promising resources
for the treatment of neurological diseases. Int J Mol Sci.
19:19702018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu Q, Zhang W, Liu K, Huang F, Su J, Deng
L, He J, Lin Q and Luo L: Schisandrin A ameliorates airway
inflammation in model of asthma by attenuating Th2 response. Eur J
Pharmacol. 953:1758502023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee K, Ahn JH, Lee KT, Jang DS and Choi
JH: Deoxyschizandrin, Isolated from Schisandra berries, induces
cell cycle arrest in ovarian cancer cells and inhibits the
protumoural activation of tumour-associated macrophages. Nutrients.
10:912018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee PK, Co VA, Yang Y, Wan MLY, El-Nezami
H and Zhao D: Bioavailability and interactions of schisandrin B
with 5-fluorouracil in a xenograft mouse model of colorectal
cancer. Food Chem. 463:1413712025. View Article : Google Scholar
|
|
15
|
Nasser MI, Han T, Adlat S, Tian Y and
Jiang N: Inhibitory effects of Schisandrin B on human prostate
cancer cells. Oncol Rep. 41:677–685. 2019.
|
|
16
|
Sowndhararajan K, Deepa P, Kim M, Park SJ
and Kim S: An overview of neuroprotective and cognitive enhancement
properties of lignans from Schisandra chinensis. Biomed
Pharmacother. 97:958–968. 2018. View Article : Google Scholar
|
|
17
|
Tan S, Zheng Z, Liu T, Yao X, Yu M and Ji
Y: Schisandrin B induced ROS-mediated autophagy and Th1/Th2
imbalance via selenoproteins in Hepa1-6 cells. Front Immunol.
13:8570692022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan SY, Shao Q, Fan XH, Li Z and Cheng
YY: Tissue distribution and excretion of herbal components after
intravenous administration of a Chinese medicine (Shengmai
injection) in rat. Arch Pharm Res. Apr 19–2014.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
Guo J, Shen Y, Lin X, Chen H and Liu J:
Schisandrin B promotes T(H)1 cell differentiation by targeting
STAT1. Int Immunopharmacol. 101:1082132021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nasser MI, Zhu S, Chen C, Zhao M, Huang H
and Zhu P: A comprehensive review on Schisandrin B and its
biological properties. Oxid Med Cell Longev. 2020:21727402020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Z, Guo M, Song G, Gao J, Zhang Y,
Jing Z, Liu T and Dong C: Schisandrin B inhibits Th1/Th17
differentiation and promotes regulatory T cell expansion in mouse
lymphocytes. Int Immunopharmacol. 35:257–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kortesoja M, Karhu E, Olafsdottir ES,
Freysdottir J and Hanski L: Impact of dibenzocyclooctadiene lignans
from Schisandra chinensis on the redox status and activation of
human innate immune system cells. Free Radic Biol Med. 131:309–317.
2019. View Article : Google Scholar
|
|
22
|
Chang CM, Liang TR and Lam HYP: The use of
Schisandrin B to combat Triple-negative breast cancers by
inhibiting NLRP3-Induced interleukin-1β production. Biomolecules.
14:742024. View Article : Google Scholar
|
|
23
|
Song A, Ding T, Wei N, Yang J, Ma M, Zheng
S and Jin H: Schisandrin B induces HepG2 cells pyroptosis by
activating NK cells mediated anti-tumor immunity. Toxicol Appl
Pharmacol. 472:1165742023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie Z, Yang T, Zhou C, Xue Z, Wang J and
Lu F: Integrative bioinformatics analysis and experimental study of
NLRP12 reveal its prognostic value and potential functions in
ovarian cancer. Mol Carcinog. 64:383–398. 2025. View Article : Google Scholar
|
|
25
|
Chen X, Liu C, Deng J, Xia T, Zhang X, Xue
S, Song MK and Olatunji OJ: Schisandrin B ameliorates
adjuvant-induced arthritis in rats via modulation of inflammatory
mediators, oxidative stress, and HIF-1α/VEGF pathway. J Pharm
Pharmacol. 76:681–690. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Chen H, Tian J and Chen S:
Antioxidant and anti-proliferative activities of five compounds
from Schisandra chinensis fruit. Industrial Crops Products.
50:690–693. 2013. View Article : Google Scholar
|
|
27
|
Chen T, Xiao Z, Liu X, Wang T, Wang Y, Ye
F, Su J, Yao X, Xiong L and Yang DH: Natural products for combating
multidrug resistance in cancer. Pharmacol Res. 202:1070992024.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krishnan SR and Bebawy M: Circulating
biosignatures in multiple myeloma and their role in multidrug
resistance. Mol Cancer. 22:792023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z and Yin P: Tumor microenvironment
diversity and plasticity in cancer multidrug resistance. Biochim
Biophys Acta Rev Cancer. 1878:1889972023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen YC, Malfertheiner P, Yu HT, Kuo CL,
Chang YY, Meng FT, Wu YX, Hsiao JL, Chen MJ, Lin KP, et al: Global
prevalence of helicobacter pylori infection and incidence of
gastric cancer between 1980 and 2022. Gastroenterology.
166:605–619. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan J, Khan SU, Yan J, Lu J, Yang C and
Tong Q: Baicalin enhances the efficacy of 5-Fluorouracil in gastric
cancer by promoting ROS-mediated ferroptosis. Biomed Pharmacother.
164:1149862023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He L, Chen H, Qi Q, Wu N, Wang Y, Chen M,
Feng Q, Dong B, Jin R and Jiang L: Schisandrin B suppresses gastric
cancer cell growth and enhances the efficacy of chemotherapy drug
5-FU in vitro and in vivo. Eur J Pharmacol. 920:1748232022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang F, Wang Z, Yuan J, Wei X, Tian R and
Niu R: RNAi-mediated silencing of Anxa2 inhibits breast cancer cell
proliferation by downregulating cyclin D1 in STAT3-dependent
pathway. Breast Cancer Res Treat. 153:263–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu XN, Zhang CY, Jin XD, Li YZ, Zheng XZ
and Li L: Inhibitory effect of schisandrin B on gastric cancer
cells in vitro. World J Gastroenterol. 13:6506–6511. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li M, Tang Q, Li S, Yang X, Zhang Y, Tang
X, Huang P and Yin D: Inhibition of autophagy enhances the
anticancer effect of Schisandrin B on head and neck squamous cell
carcinoma. J Biochem Mol Toxicol. 38:e235852024. View Article : Google Scholar
|
|
36
|
Zhang F, Zhai J, Weng N, Gao J, Yin J and
Chen W: A comprehensive review of the main lignan components of
Schisandra chinensis (North Wu Wei Zi) and Schisandra
sphenanthera (South Wu Wei Zi) and the Lignan-induced drug-drug
interactions based on the inhibition of cytochrome P450 and
P-glycoprotein activities. Front Pharmacol. 13:8160362022.
|
|
37
|
Morgan E, Arnold M, Gini A, Lorenzoni V,
Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N and Bray F:
Global burden of colorectal cancer in 2020 and 2040: Incidence and
mortality estimates from GLOBOCAN. Gut. 72:338–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vodenkova S, Buchler T, Cervena K,
Veskrnova V, Vodicka P and Vymetalkova V: 5-fluorouracil and other
fluoropyrimidines in colorectal cancer: Past, present and future.
Pharmacol Ther. 206:1074472020. View Article : Google Scholar
|
|
39
|
Yoshino T, Cervantes A, Bando H,
Martinelli E, Oki E, Xu RH, Mulansari NA, Govind Babu K, Lee MA and
Tan CK: Pan-Asian adapted ESMO Clinical Practice Guidelines for the
diagnosis, treatment and follow-up of patients with metastatic
colorectal cancer. ESMO Open. 8:1015582023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Co VA, El-Nezami H, Liu Y, Twum B, Dey P,
Cox PA, Joseph S, Agbodjan-Dossou R, Sabzichi M, Draheim R and Wan
MLY: Schisandrin B suppresses colon cancer growth by inducing cell
cycle arrest and apoptosis: Molecular mechanism and therapeutic
potential. ACS Pharmacol Transl Sci. 7:863–877. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pu Z, Zhang W, Wang M, Xu M, Xie H and
Zhao J: Schisandrin B attenuates Colitis-associated colorectal
cancer through SIRT1 linked SMURF2 signaling. Am J Chin Med.
49:1773–1789. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheifetz S, Hernandez H, Laiho M, ten
Dijke P, Iwata KK and Massagué J: Distinct transforming growth
factor-beta (TGF-beta) receptor subsets as determinants of cellular
responsiveness to three TGF-beta isoforms. J Biol Chem.
265:20533–20538. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong X, Zhao B, Iacob RE, Zhu J, Koksal
AC, Lu C, Engen JR and Springer TA: Force interacts with
macromolecular structure in activation of TGF-β. Nature. 542:55–59.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of Epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vincent T, Neve EP, Johnson JR, Kukalev A,
Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL,
et al: A SNAIL1-SMAD3/4 transcriptional repressor complex promotes
TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol.
11:943–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xue G, Restuccia DF, Lan Q, Hynx D,
Dirnhofer S, Hess D, Rüegg C and Hemmings BA: Akt/PKB-mediated
phosphorylation of Twist1 promotes tumor metastasis via mediating
cross-talk between PI3K/Akt and TGF-β signaling axes. Cancer
Discov. 2:248–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin T, Zeng L, Liu Y, DeFea K, Schwartz
MA, Chien S and Shyy JY: Rho-ROCK-LIMK-cofilin pathway regulates
shear stress activation of sterol regulatory element binding
proteins. Circ Res. 92:1296–1304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu J, Lei R, Zhuang X, Li X, Li G, Lev S,
Segura MF, Zhang X and Hu G: MicroRNA-182 targets SMAD7 to
potentiate TGFβ-induced epithelial-mesenchymal transition and
metastasis of cancer cells. Nat Commun. 7:138842016. View Article : Google Scholar
|
|
49
|
Tian XJ, Zhang H and Xing J: Coupled
reversible and irreversible bistable switches underlying
TGFβ-induced epithelial to mesenchymal transition. Biophys J.
105:1079–1089. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Z, Zhang B, Liu K, Ding Z and Hu X:
Schisandrin B attenuates cancer invasion and metastasis via
inhibiting epithelial-mesenchymal transition. PLoS One.
7:e404802012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang B, Liu Z and Hu X: Inhibiting cancer
metastasis via targeting NAPDH oxidase 4. Biochem Pharmacol.
86:253–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li J, Lu Y, Wang D, Quan F, Chen X, Sun R,
Zhao S, Yang Z, Tao W, Ding D, et al: Schisandrin B prevents
ulcerative colitis and colitis-associated-cancer by activating
focal adhesion kinase and influence on gut microbiota in an in vivo
and in vitro model. Eur J Pharmacol. 854:9–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun R, Zhang Y, Zhao X, Tang T, Cao Y,
Yang L, Tian Y, Zhang Z, Zhang P and Xu F: Temporal and spatial
metabolic shifts revealing the transition from ulcerative colitis
to colitis-associated colorectal cancer. Adv Sci (Weinh).
12:e24125512025. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang J, Mengli Y, Zhang T, Song X, Ying
S, Shen Z and Yu C: Deficiency in epithelium RAD50 aggravates UC
via IL-6-Mediated JAK1/2-STAT3 signaling and promotes development
of Colitis-associated cancer in mice. J Crohns Colitis.
19:jjae1342025. View Article : Google Scholar
|
|
55
|
Porter RJ, Kalla R and Ho GT: Ulcerative
colitis: Recent advances in the understanding of disease
pathogenesis. F1000Res. 9:F1000Faculty Rev-294. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tatiya-Aphiradee N, Chatuphonprasert W and
Jarukamjorn K: Immune response and inflammatory pathway of
ulcerative colitis. J Basic Clin Physiol Pharmacol. 30:1–10. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo G, Tan Z, Liu Y, Shi F and She J: The
therapeutic potential of stem Cell-derived exosomes in the
ulcerative colitis and colorectal cancer. Stem Cell Res Ther.
13:1382022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo X, Yin X, Liu Z and Wang J:
Non-alcoholic fatty liver disease (NAFLD) pathogenesis and natural
products for prevention and treatment. Int J Mol Sci. 23:154892022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Asaoka Y, Terai S, Sakaida I and Nishina
H: The expanding role of fish models in understanding non-alcoholic
fatty liver disease. Dis Model Mech. 6:905–914. 2013.PubMed/NCBI
|
|
60
|
Sheka AC, Adeyi O, Thompson J, Hameed B,
Crawford PA and Ikramuddin S: Nonalcoholic steatohepatitis: A
review. JAMA. 323:1175–1183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Leong PK and Ko KM: Schisandrin B: A
Double-edged sword in nonalcoholic fatty liver disease. Oxid Med
Cell Longev. 2016:61716582016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yan LS, Zhang SF, Luo G, Cheng BC, Zhang
C, Wang YW, Qiu XY, Zhou XH, Wang QG, Song XL, et al: Schisandrin B
mitigates hepatic steatosis and promotes fatty acid oxidation by
inducing autophagy through AMPK/mTOR signaling pathway. Metabolism.
131:1552002022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ma R, Zhan Y, Zhang Y, Wu L, Wang X and
Guo M: Schisandrin B ameliorates non-alcoholic liver disease
through anti-inflammation activation in diabetic mice. Drug Dev
Res. 83:735–744. 2022.
|
|
64
|
Li Z, Zhao L, Xia Y, Chen J, Hua M and Sun
Y: Schisandrin B attenuates hepatic stellate cell activation and
promotes apoptosis to protect against liver fibrosis. Molecules.
26:68822021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ma M, Wei N, Yang J, Ding T, Song A, Chen
L, Zheng S and Jin H: Schisandrin B promotes senescence of
activated hepatic stellate cell via NCOA4-mediated ferritinophagy.
Pharm Biol. 61:621–629. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Trojnar E, Erdelyi K, Matyas C, Zhao S,
Paloczi J, Mukhopadhyay P, Varga ZV, Hasko G and Pacher P:
Cannabinoid-2 receptor activation ameliorates hepatorenal syndrome.
Free Radic Biol Med. 152:540–550. 2020. View Article : Google Scholar
|
|
67
|
Wang HQ, Wan Z, Zhang Q, Su T, Yu D, Wang
F, Zhang C, Li W, Xu D and Zhang H: Schisandrin B targets
cannabinoid 2 receptor in Kupffer cell to ameliorate CCl4-induced
liver fibrosis by suppressing NF-κB and p38 MAPK pathway.
Phytomedicine. 98:1539602022. View Article : Google Scholar
|
|
68
|
Yang YY, Hsieh SL, Lee PC, Yeh YC, Lee KC,
Hsieh YC, Wang YW, Lee TY, Huang YH, Chan CC and Lin HC: Long-term
cannabinoid type 2 receptor agonist therapy decreases bacterial
translocation in rats with cirrhosis and ascites. J Hepatol.
61:1004–1013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lei Y, Wang QL, Shen L, Tao YY and Liu CH:
MicroRNA-101 suppresses liver fibrosis by downregulating
PI3K/Akt/mTOR signaling pathway. Clin Res Hepatol Gastroenterol.
43:575–584. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cuiqiong W, Chao X, Xinling F and Yinyan
J: Schisandrin B suppresses liver fibrosis in rats by targeting
miR-101-5p through the TGF-β signaling pathway. Artif Cells Nanomed
Biotechnol. 48:473–478. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Meroni M, Longo M, Erconi V, Valenti L,
Gatti S, Fracanzani AL and Dongiovanni P: mir-101-3p downregulation
promotes fibrogenesis by facilitating hepatic stellate cell
transdifferentiation during insulin resistance. Nutrients.
11:25972019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen Q, Bao L, Lv L, Xie F, Zhou X, Zhang
H and Zhang G: Schisandrin B regulates macrophage polarization and
alleviates liver fibrosis via activation of PPARγ. Ann Transl Med.
9:15002021. View Article : Google Scholar
|
|
73
|
Leong PK, Wong HS, Chen J, Chan WM, Leung
HY and Ko KM: Differential Action between Schisandrin A and
Schisandrin B in eliciting an Anti-inflammatory Action: The
depletion of reduced glutathione and the induction of an
antioxidant response. PLoS One. 11:e01558792016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ma Z, Xu G, Shen Y, Hu S, Lin X, Zhou J,
Zhao W, Liu J, Wang J and Guo J: Schisandrin B-mediated TH17 cell
differentiation attenuates bowel inflammation. Pharmacol Res.
166:1054592021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chen Q, Zhang H, Cao Y, Li Y, Sun S, Zhang
J and Zhang G: Schisandrin B attenuates CCl4-induced liver fibrosis
in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling
pathways. Drug Des Devel Ther. 11:2179–2191. 2017. View Article : Google Scholar :
|
|
76
|
Jin M, Yi X, Zhu X, Hu W, Wang S, Chen Q,
Yang W, Li Y, Li S, Peng Q, et al: Schisandrin B promotes hepatic
differentiation from human umbilical cord mesenchymal stem cells.
iScience. 27:1089122024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar :
|
|
78
|
Yang H and Wu T: Schisandrin B inhibits
tumor progression of hepatocellular carcinoma by targeting the
RhoA/ROCK1 pathway. J Gastrointest Oncol. 14:533–543. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang JG, Zhou HM, Zhang X, Mu W, Hu JN,
Liu GL and Li Q: Hypoxic induction of vasculogenic mimicry in
hepatocellular carcinoma: Role of HIF-1 α, RhoA/ROCK and Rac1/PAK
signaling. BMC Cancer. 20:322020. View Article : Google Scholar
|
|
80
|
He JL, Zhou ZW, Yin JJ, He CQ, Zhou SF and
Yu Y: Schisandra chinensis regulates drug metabolizing enzymes and
drug transporters via activation of Nrf2-mediated signaling
pathway. Drug Des Devel Ther. 9:127–146. 2015.PubMed/NCBI
|
|
81
|
Benson AB, D'Angelica MI, Abrams T, Abbott
DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, et al:
NCCN Guidelines® Insights: Biliary tract cancers,
version 2.2023. J Natl Compr Canc Netw. 21:694–704. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sarcognato S, Sacchi D, Fassan M, Fabris
L, Cadamuro M, Zanus G, Cataldo I, Capelli P, Baciorri F,
Cacciatore M and Guido M: Cholangiocarcinoma. Pathologica.
113:158–169. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang X, Wang S, Mu Y and Zheng Y:
Schisandrin B inhibits cell proliferation and induces apoptosis in
human cholangiocarcinoma cells. Oncol Rep. 36:1799–1806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pehlivanoglu B, Akkas G, Memis B, Basturk
O, Reid MD, Saka B, Dursun N, Bagci P, Balci S, Sarmiento J, et al:
Reappraisal of T1b gallbladder cancer (GBC): Clinicopathologic
analysis of 473 in situ and invasive GBCs and critical review of
the literature highlights its rarity, and that it has a very good
prognosis. Virchows Arch. 482:311–323. 2023. View Article : Google Scholar
|
|
85
|
Brindley PJ, Bachini M, Ilyas SI, Khan SA,
Loukas A, Sirica AE, The BT, Wongkham S and Gores GJ:
Cholangiocarcinoma. Nat Rev Dis Primers. 7:652021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hui AM, Li X, Shi YZ, Takayama T, Torzilli
G and Makuuchi M: Cyclin D1 overexpression is a critical event in
gallbladder carcinogenesis and independently predicts decreased
survival for patients with gallbladder carcinoma. Clin Cancer Res.
6:4272–4277. 2000.PubMed/NCBI
|
|
87
|
Cai Z, Wang J, Li Y, Shi Q, Jin L, Li S,
Zhu M, Wang Q, Wong LL, Yang W, et al: Overexpressed Cyclin D1 and
CDK4 proteins are responsible for the resistance to CDK4/6
inhibitor in breast cancer that can be reversed by PI3K/mTOR
inhibitors. Sci China Life Sci. 66:94–109. 2023. View Article : Google Scholar
|
|
88
|
Vogel A, Segatto O, Stenzinger A and
Saborowski A: FGFR2 Inhibition in Cholangiocarcinoma. Annu Rev Med.
74:293–306. 2023. View Article : Google Scholar
|
|
89
|
Banik K, Khatoon E, Harsha C, Rana V,
Parama D, Thakur KK, Bishayee A and Kunnumakkara AB: Wogonin and
its analogs for the prevention and treatment of cancer: A
systematic review. Phytother Res. 36:1854–1883. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao C, Zhang J, Yang ZY, Shi LQ, Liu SL,
Pan LJ, Dong P, Zhang Y, Xiang SS, Shu YJ and Mei JW: Ponicidin
inhibited gallbladder cancer proliferation and metastasis by
decreasing MAGEB2 expression through FOXO4. Phytomedicine.
114:1547852023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tsang CM, Lui VWY, Bruce JP, Pugh TJ and
Lo KW: Translational genomics of nasopharyngeal cancer. Semin
Cancer Biol. 61:84–100. 2020. View Article : Google Scholar
|
|
92
|
Pan JJ, Ng WT, Zong JF, Chan LL,
O'Sullivan B, Lin SJ, Sze HC, Chen YB, Choi HC, Guo QJ, et al:
Proposal for the 8th edition of the AJCC/UICC staging system for
nasopharyngeal cancer in the era of intensity-modulated
radiotherapy. Cancer. 122:546–558. 2016. View Article : Google Scholar
|
|
93
|
Hong S, Zhang Y, Yu G, Peng P, Peng J, Jia
J, Wu X, Huang Y, Yang Y, Lin Q, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin as First-Line therapy for
recurrent or metastatic nasopharyngeal carcinoma: Final overall
survival analysis of GEM20110714 Phase III study. J Clin Oncol.
39:3273–3282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fang Y, Lv X, Li G, Wang P, Zhang L, Wang
R, Jia L and Liang S: Schisandrin B targets CDK4/6 to suppress
proliferation and enhance radiosensitivity in nasopharyngeal
carcinoma by inducing cell cycle arrest. Sci Rep. 15:84522025.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lv XJ, Zhao LJ, Hao YQ, Su ZZ, Li JY, Du
YW and Zhang J: Schisandrin B inhibits the proliferation of human
lung adenocarcinoma A549 cells by inducing cycle arrest and
apoptosis. Int J Clin Exp Med. 8:6926–6936. 2015.PubMed/NCBI
|
|
96
|
Xiang SS, Wang XA, Li HF, Shu YJ, Bao RF,
Zhang F, Cao Y, Ye YY, Weng H, Wu WG, et al: Schisandrin B induces
apoptosis and cell cycle arrest of gallbladder cancer cells.
Molecules. 19:13235–13250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xie X, Zheng W, Chen T, Lin W, Liao Z, Liu
J and Ding Y: CDK4/6 inhibitor palbociclib amplifies the
radiosensitivity to nasopharyngeal carcinoma cells via mediating
apoptosis and suppressing DNA damage repair. Onco Targets Ther.
12:11107–11117. 2019. View Article : Google Scholar
|
|
98
|
Luo W, Lin K, Hua J, Han J, Zhang Q, Chen
L, Khan ZA, Wu G, Wang Y and Liang G: Schisandrin B attenuates
diabetic cardiomyopathy by targeting MyD88 and inhibiting
MyD88-Dependent inflammation. Adv Sci (Weinh). 9:e22025902022.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xu C, Deng Y, Man J, Wang H, Che T, Ding L
and Yang L: Unveiling the renoprotective mechanisms of Schisandrin
B in ischemia-reperfusion injury through transcriptomic and
pharmacological analysis. Drug Des Devel Ther. 18:4241–4256. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Huang W, Zhang L, Yang M, Wu X, Wang X,
Huang W, Yuan L, Pan H, Wang Y and Wang Z: Cancer-associated
fibroblasts promote the survival of irradiated nasopharyngeal
carcinoma cells via the NF-κB pathway. J Exp Clin Cancer Res.
40:872021. View Article : Google Scholar
|
|
101
|
Nishida H, Tatewaki N, Nakajima Y, Magara
T, Ko KM, Hamamori Y and Konishi T: Inhibition of ATR protein
kinase activity by schisandrin B in DNA damage response. Nucleic
Acids Res. 37:5678–5689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Meyer ML, Fitzgerald BG, Paz-Ares L,
Cappuzzo F, Jänne PA, Peters S and Hirsch FR: New promises and
challenges in the treatment of advanced non-small-cell lung cancer.
Lancet. 404:803–822. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhuang W, Li Z, Dong X, Zhao N, Liu Y,
Wang C and Chen J: Schisandrin B inhibits TGF-β1-induced
epithelial-mesenchymal transition in human A549 cells through
epigenetic silencing of ZEB1. Exp Lung Res. 45:157–166. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Desai K, McManus JM and Sharifi N:
Hormonal therapy for prostate cancer. Endocr Rev. 42:354–373. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ho Y and Dehm SM: Androgen receptor
rearrangement and splicing variants in resistance to endocrine
therapies in prostate cancer. Endocrinology. 158:1533–1542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dai C, Heemers H and Sharifi N: Androgen
signaling in prostate cancer. Cold Spring Harb Perspect Med.
7:a0304522017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang BY, Yang R, Zhu WQ, Zhu CL, Chen LX,
Zhao YS, Zhang Y, Wang YQ, Jiang DZ and Tang B: Schisandrin B
alleviates testicular inflammation and Sertoli cell apoptosis via
AR-JNK pathway. Sci Rep. 14:184182024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang Q, Liu J, Duan H, Li R, Peng W and
Wu C: Activation of Nrf2/HO-1 signaling: An important molecular
mechanism of herbal medicine in the treatment of atherosclerosis
via the protection of vascular endothelial cells from oxidative
stress. J Adv Res. 34:43–63. 2021. View Article : Google Scholar
|
|
109
|
Zafar MK and Eoff RL: Translesion DNA
synthesis in cancer: Molecular mechanisms and therapeutic
opportunities. Chem Res Toxicol. 30:1942–1955. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wu Y, Li ZC, Yao LQ, Li M and Tang M:
Schisandrin B alleviates acute oxidative stress via modulation of
the Nrf2/Keap1-mediated antioxidant pathway. Appl Physiol Nutr
Metab. 44:1–6. 2019. View Article : Google Scholar
|
|
111
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the united states in 2015-2019. Neuro Oncol.
24:v1–v95. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Qi L, Yu HQ, Li YQ, Jin H, Zhao DH and Xu
Y: Schidandrin B kills tumor cells by initiating apoptosis in
glioma SHG-44 cells. Chin J Integr Med. Aug 2–2016.Epub ahead of
print. View Article : Google Scholar
|
|
113
|
Li Q, Lu XH, Wang CD, Cai L, Lu JL, Wu JS,
Zhuge QC, Zheng WM and Su ZP: Antiproliferative and
apoptosis-inducing activity of schisandrin B against human glioma
cells. Cancer Cell Int. 15:122015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jiang Y, Zhang Q, Bao J, Du C, Wang J,
Tong Q and Liu C: Schisandrin B suppresses glioma cell metastasis
mediated by inhibition of mTOR/MMP-9 signal pathway. Biomed
Pharmacother. 74:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang Z, Wang Y, Chen J, Tan Q, Xie C, Li
C, Zhan W and Wang M: Silencing of histone deacetylase 2 suppresses
malignancy for proliferation, migration, and invasion of
glioblastoma cells and enhances temozolomide sensitivity. Cancer
Chemother Pharmacol. 78:1289–1296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tomar MS, Kumar A, Srivastava C and
Shrivastava A: Elucidating the mechanisms of Temozolomide
resistance in gliomas and the strategies to overcome the
resistance. Biochim Biophys Acta Rev Cancer. 1876:1886162021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jiang Y, Zhang Q, Bao J, Du C, Wang J,
Tong Q and Liu C: Schisandrin B inhibits the proliferation and
invasion of glioma cells by regulating the HOTAIR-micoRNA-125a-mTOR
pathway. Neuroreport. 28:93–100. 2017. View Article : Google Scholar
|
|
118
|
Kostka L, Sivák L, Šubr V, Kovářová J,
Šírová M, Říhová B, Sedlacek R, Etrych T and Kovář M: Simultaneous
delivery of doxorubicin and protease inhibitor derivative to solid
tumors via Star-shaped polymer nanomedicines overcomes P-gp- and
STat3-mediated chemoresistance. Biomacromolecules. 23:2522–2535.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen XY, Wu ZX, Wang JQ, Teng QX, Tang H,
Liu Q, Chen ZS and Chen W: Multidrug resistance transporters P-gp
and BCRP limit the efficacy of ATR inhibitor ceralasertib in cancer
cells. Front Pharmacol. 15:14006992024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Rebbaa A, Chou PM and Mirkin BL: Factors
secreted by human neuroblastoma mediated doxorubicin resistance by
activating STAT3 and inhibiting apoptosis. Mol Med. 7:393–400.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang S, Wang A, Shao M, Lin L, Li P and
Wang Y: Schisandrin B reverses doxorubicin resistance through
inhibiting P-glycoprotein and promoting proteasome-mediated
degradation of survivin. Sci Rep. 7:84192017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Boudreau HE, Casterline BW, Rada B,
Korzeniowska A and Leto TL: Nox4 involvement in TGF-beta and
SMAD3-driven induction of the epithelial-to-mesenchymal transition
and migration of breast epithelial cells. Free Radic Biol Med.
53:1489–1499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Dai X, Yin C, Guo G, Zhang Y, Zhao C, Qian
J, Wang O, Zhang X and Liang G: Schisandrin B exhibits potent
anticancer activity in triple negative breast cancer by inhibiting
STAT3. Toxicol Appl Pharmacol. 358:110–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Beird HC, Bielack SS, Flanagan AM, Gill J,
Heymann D, Janeway KA, Livingston JA, Roberts RD, Strauss SJ and
Gorlick R: Osteosarcoma. Nat Rev Dis Primers. 8:772022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sayles LC, Breese MR, Koehne AL, Leung SG,
Lee AG, Liu HY, Spillinger A, Shah AT, Tanasa B, Straessler K, et
al: Genome-informed targeted therapy for osteosarcoma. Cancer
Discov. 9:46–63. 2019. View Article : Google Scholar
|
|
126
|
Bacci G, Rocca M, Salone M, Balladelli A,
Ferrari S, Palmerini E, Forni C and Briccoli A: High grade
osteosarcoma of the extremities with lung metastases at
presentation: Treatment with neoadjuvant chemotherapy and
simultaneous resection of primary and metastatic lesions. J Surg
Oncol. 98:415–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T and
Zhang L: Analyzing the interactions of mRNAs and ncRNAs to predict
competing endogenous RNA networks in osteosarcoma Chemo-resistance.
Mol Ther. 27:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang B, Wang X, Tong X and Zhang Y:
Schisandrin B inhibits cell viability and migration, and induces
cell apoptosis by circ_0009112/miR-708-5p axis through PI3K/AKT
pathway in osteosarcoma. Front Genet. 11:5886702020. View Article : Google Scholar
|
|
129
|
Thandavarayan RA, Giridharan VV, Arumugam
S, Suzuki K, Ko KM, Krishnamurthy P, Watanabe K and Konishi T:
Schisandrin B prevents doxorubicin induced cardiac dysfunction by
modulation of DNA damage, oxidative stress and inflammation through
inhibition of MAPK/p53 signaling. PLoS One. 10:e01192142015.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li L, Pan Q, Han W, Liu Z, Li L and Hu X:
Schisandrin B prevents doxorubicin-induced cardiotoxicity via
enhancing glutathione redox cycling. Clin Cancer Res. 13:6753–6760.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG); Davies C, Godwin J, Gray R, Clarke M,
Cutter D, Darby S, McGale P, Pan HC, Taylor C, et al: Relevance of
breast cancer hormone receptors and other factors to the efficacy
of adjuvant tamoxifen: Patient-level meta-analysis of randomised
trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Li Y, Liu Z, Chen J, Wang R, An X, Tian C,
Yang H and Zha D: Schisandrin B protect inner hair cells from
cisplatin by inhibiting celluar oxidative stress and apoptosis.
Toxicol In Vitro. 99:1058522024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Mei H, Zhao L, Li W, Zheng Z, Tang D, Lu X
and He Y: Inhibition of ferroptosis protects House Ear
Institute-Organ of Corti 1 cells and cochlear hair cells from
cisplatin-induced ototoxicity. J Cell Mol Med. 24:12065–12081.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Santos N, Ferreira RS and Santos ACD:
Overview of Cisplatin-induced neurotoxicity and ototoxicity, and
the protective agents. Food Chem Toxicol. 136:1110792020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shi H, Yan Y, Yang H, Pu P and Tang H:
Schisandrin B diet inhibits oxidative stress to reduce ferroptosis
and lipid peroxidation to prevent Pirarubicin-induced
hepatotoxicity. Biomed Res Int. 2022:56235552022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Shi H, Tang H, Ai W, Zeng Q, Yang H, Zhu
F, Wei Y, Feng R, Wen L, Pu P and He Q: Schisandrin B antagonizes
cardiotoxicity induced by pirarubicin by inhibiting mitochondrial
permeability transition pore (mPTP) opening and decreasing
cardiomyocyte apoptosis. Front Pharmacol. 12:7338052021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tang H, Zhao J, Feng R, Pu P and Wen L:
Reducing oxidative stress may be important for treating
pirarubicin-induced cardiotoxicity with schisandrin B. Exp Ther
Med. 23:682022. View Article : Google Scholar
|
|
138
|
Zhang D, Liu B, Cao B, Wei F, Yu X, Li GF,
Chen H, Wei LQ and Wang PL: Synergistic protection of Schizandrin B
and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis
by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4. Int
Immunopharmacol. 48:67–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Lai Q, Luo Z, Wu C, Lai S, Wei H, Li T,
Wang Q and Yu Y: Attenuation of cyclosporine A induced
nephrotoxicity by schisandrin B through suppression of oxidative
stress, apoptosis and autophagy. Int Immunopharmacol. 52:15–23.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lam HYP, Cheng PC and Peng SY: Resolution
of systemic complications in Schistosoma mansoni-infected mice by
concomitant treatment with praziquantel and Schisandrin B. Int J
Parasitol. 52:275–284. 2022. View Article : Google Scholar
|
|
141
|
Lam HYP, Liang TR and Peng SY:
Ameliorative effects of Schisandrin B on Schistosoma
Mansoni-induced hepatic fibrosis in vivo. PLoS Negl Trop Dis.
15:e00095542021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yan C, Gao L, Qiu X and Deng C:
Schisandrin B synergizes docetaxel-induced restriction of growth
and invasion of cervical cancer cells in vitro and in vivo. Ann
Transl Med. 8:11572020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Huang H: Matrix Metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:32492018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Li YJ, Liu HT, Xue CJ, Xing XQ, Dong ST,
Wang LS, Ding CY, Meng L and Dong ZJ: The synergistic anti-tumor
effect of schisandrin B and apatinib. J Asian Nat Prod Res.
22:839–849. 2020. View Article : Google Scholar
|
|
145
|
Chiu PY, Chen N, Leong PK, Leung HY and Ko
KM: Schisandrin B elicits a glutathione antioxidant response and
protects against apoptosis via the redox-sensitive ERK/Nrf2 pathway
in H9c2 cells. Mol Cell Biochem. 350:237–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Xu Y, Liu Z, Sun J, Pan Q, Sun F, Yan Z
and Hu X: Schisandrin B prevents doxorubicin-induced chronic
cardiotoxicity and enhances its anticancer activity in vivo. PLoS
One. 6:e283352011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Nooreen Z, Gupta VK, Singh K, Wal A, Rai
AK and Tandon S: An updated insight on Phyto-therapeutics and their
novel approaches in the management of brain cancer. Curr Top Med
Chem. Jul 7–2025.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Qin XL, Chen X, Zhong GP, Fan XM, Wang Y,
Xue XP, Wang Y, Huang M and Bi HC: Effect of Tacrolimus on the
pharmacokinetics of bioactive lignans of Wuzhi tablet (Schisandra
sphenanthera extract) and the potential roles of CYP3A and P-gp.
Phytomedicine. 21:766–772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhan T, Yao N, Wu L, Lu Y, Liu M, Liu F,
Xiong Y and Xia C: The major effective components in Shengmai
Formula interact with sodium taurocholate co-transporting
polypeptide. Phytomedicine. 59:1529162019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wei JR, Lu MY, Wei TH, Fleishman JS, Yu H,
Chen XL, Kong XT, Sun SL, Li NG, Yang Y and Ni HW: Overcoming
cancer therapy resistance: From drug innovation to therapeutics.
Drug Resist Updat. 81:1012292025. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Sana-Eldine AO, Abdelgawad HM, Kotb NS and
Shehata NI: The potential effect of Schisandrin-B combination with
panitumumab in wild-type and mutant colorectal cancer cell lines:
Role of apoptosis and autophagy. J Biochem Mol Toxicol.
37:e233242023. View Article : Google Scholar
|
|
152
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD,
Robitaille S, et al: K-ras mutations and benefit from cetuximab in
advanced colorectal cancer. N Engl J Med. 359:1757–1765. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
De Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, et al: Cetuximab plus irinotecan, fluorouracil, and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Loupakis F, Pollina L, Stasi I, Ruzzo A,
Scartozzi M, Santini D, Masi G, Graziano F, Cremolini C, Rulli E,
et al: PTEN expression and KRAS mutations on primary tumors and
metastases in the prediction of benefit from cetuximab plus
irinotecan for patients with metastatic colorectal cancer. J Clin
Oncol. 27:2622–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Bardelli A, Corso S, Bertotti A, Hobor S,
Valtorta E, Siravegna G, Sartore-Bianchi A, Scala E, Cassingena A,
Zecchin D, et al: Amplification of the MET receptor drives
resistance to anti-EGFR therapies in colorectal cancer. Cancer
Discov. 3:658–673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kavuri SM, Jain N, Galimi F, Cottino F,
Leto SM, Migliardi G, Searleman AC, Shen W, Monsey J, Trusolino L,
et al: HER2 activating mutations are targets for colorectal cancer
treatment. Cancer Discov. 5:832–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Maddalena F, Condelli V, Matassa DS,
Pacelli C, Scrima R, Lettini G, Li Bergolis V, Pietrafesa M, Crispo
F, Piscazzi A, et al: TRAP1 enhances Warburg metabolism through
modulation of PFK1 expression/activity and favors resistance to
EGFR inhibitors in human colorectal carcinomas. Mol Oncol.
14:3030–3047. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Yue Y, Zhang Q, Wang X and Sun Z: STAT3
regulates 5-Fu resistance in human colorectal cancer cells by
promoting Mcl-1-dependent cytoprotective autophagy. Cancer Sci.
114:2293–2305. PubMed/NCBI
|
|
160
|
Wang Y, Wang Y, Qin Z, Cai S, Yu L, Hu H
and Zeng S: The role of non-coding RNAs in ABC transporters
regulation and their clinical implications of multidrug resistance
in cancer. Expert Opin Drug Metab Toxicol. 17:291–306. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Hsu HH, Chen MC, Baskaran R, Lin YM, Day
CH, Lin YJ, Tu CC, Vijaya Padma V, Kuo WW and Huang CY: Oxaliplatin
resistance in colorectal cancer cells is mediated via activation of
ABCG2 to alleviate ER stress induced apoptosis. J Cell Physiol.
233:5458–5467. 2018. View Article : Google Scholar
|
|
162
|
Theile D and Wizgall P: Acquired
ABC-transporter overexpression in cancer cells: Transcriptional
induction or Darwinian selection? Naunyn Schmiedebergs Arch
Pharmacol. 394:1621–1632. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhu Y, Huang S, Chen S, Chen J, Wang Z,
Wang Y and Zheng H: SOX2 promotes chemoresistance, cancer stem
cells properties, and epithelial-mesenchymal transition by
β-catenin and Beclin1/autophagy signaling in colorectal cancer.
Cell Death Dis. 12:4492021. View Article : Google Scholar
|
|
164
|
Kadioglu O, Law BYK, Mok SWF, Xu SW,
Efferth T and Wong VKW: Mode of action analyses of neferine, a
bisbenzylisoquinoline alkaloid of lotus (Nelumbo nucifera) against
Multidrug-resistant tumor cells. Front Pharmacol. 8:2382017.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sui H, Zhou LH, Zhang YL, Huang JP, Liu X,
Ji Q, Fu XL, Wen HT, Chen ZS, Deng WL, et al: Evodiamine suppresses
ABCG2 mediated drug resistance by inhibiting p50/p65 NF-κB pathway
in colorectal cancer. J Cell Biochem. 117:1471–1481. 2016.
View Article : Google Scholar
|
|
166
|
Song B, Shuang L, Zhang S, Tong C, Chen Q,
Li Y, Hao M, Niu W and Jin CH: Research progress of nano drug
delivery systems in the anti-tumor treatment of traditional Chinese
medicine monomers. PeerJ. 13:e193322025. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wu J: The enhanced permeability and
retention (EPR) Effect: The significance of the concept and methods
to enhance its application. J Pers Med. 11:7712021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hristova-Panusheva K, Xenodochidis C,
Georgieva M and Krasteva N: Nanoparticle-mediated drug delivery
systems for precision targeting in oncology. Pharmaceuticals
(Basel). 17:6772024. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Sun T and Jiang C: Stimuli-responsive drug
delivery systems triggered by intracellular or subcellular
microenvironments. Adv Drug Deliv Rev. 196:1147732023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Gupta A, Costa AP, Xu X and Burgess DJ:
Continuous processing of paclitaxel polymeric micelles. Int J
Pharm. 607:1209462021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Kara G, Calin GA and Ozpolat B: RNAi-based
therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv
Rev. 182:1141132022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Yan YH, Kong L, Lu YB, Li SY, Yan AW, Song
YW, Huang ZH and Zhu HN: Inhibition of hepatocellular carcinoma
progression by methotrexate-modified pH-sensitive sorafenib and
schisandrin B micelles. Biomed Mater. Dec 13–2024.Epud ahead of
print. PubMed/NCBI
|
|
173
|
Zhang R, Luo S, Zhao T, Wu M, Huang L,
Zhang L, Huang Y, Gao H, Sun X, Gong T and Zhang Z: Scavenger
receptor A-mediated nanoparticles target M1 macrophages for acute
liver injury. Asian J Pharm Sci. 18:1008132023.PubMed/NCBI
|
|
174
|
Di Stefano M, Galati S, Piazza L, Granchi
C, Mancini S, Fratini F, Macchia M, Poli G and Tuccinardi T:
VenomPred 2.0: A novel in silico platform for an extended and human
interpretable toxicological profiling of small molecules. J Chem
Inf Model. 64:2275–2289. 2024. View Article : Google Scholar :
|
|
175
|
Cai FY, Yao XM, Jing M, Kong L, Liu JJ, Fu
M, Liu XZ, Zhang L, He SY, Li XT and Ju RJ: Enhanced antitumour
efficacy of functionalized doxorubicin plus schisandrin B
co-delivery liposomes via inhibiting epithelial-mesenchymal
transition. J Liposome Res. 31:113–129. 2021. View Article : Google Scholar
|
|
176
|
Jing M, Bi XJ, Yao XM, Cai F, Liu JJ, Fu
M, Kong L, Liu XZ, Zhang L and He SY: Enhanced antitumor efficacy
using epirubicin and schisandrin B co-delivery liposomes modified
with PFV via inhibiting tumor metastasis. Drug Dev Ind Pharm.
46:621–634. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Zhang L, Kong L, He SY, Liu XZ, Liu Y,
Zang J, Ju RJ and Li XT: The anti-ovarian cancer effect of RPV
modified paclitaxel plus schisandra B liposomes in SK-OV-3 cells
and tumor-bearing mice. Life Sci. 285:1200132021. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Kong L, Cai FY, Yao XM, Jing M, Fu M, Liu
JJ, He SY, Zhang L, Liu XZ, Ju RJ and Li XT: RPV-modified
epirubicin and dioscin co-delivery liposomes suppress non-small
cell lung cancer growth by limiting nutrition supply. Cancer Sci.
111:621–636. 2020. View Article : Google Scholar :
|
|
179
|
Chen RJ and Menezes RG: Vinca Alkaloid
Toxicity. StatPearls. Disclosure: Ritesh Menezes Declares no
Relevant Financial Relationships with Ineligible Companies.
StatPearls Publishing LLC; Treasure Island, FL: 2025
|
|
180
|
Li XY, Shi LX, Yao XM, Jing M, Li QQ, Wang
YL and Li QS: Functional vinorelbine plus schisandrin B liposomes
destroying tumor metastasis in treatment of gastric cancer. Drug
Dev Ind Pharm. 47:100–112. 2021. View Article : Google Scholar
|
|
181
|
Gong T, Tan T, Zhang P, Li H, Deng C,
Huang Y, Gong T and Zhang Z: Palmitic acid-modified bovine serum
albumin nanoparticles target scavenger receptor-A on activated
macrophages to treat rheumatoid arthritis. Biomaterials.
258:1202962020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Feng J, Xiang L, Fang C, Tan Y, Li Y, Gong
T, Wu Q, Gong T and Zhang Z: Dual-targeting of tumor cells and
Tumor-associated macrophages by palmitic acid modified albumin
nanoparticles for antitumor and antimetastasis therapy. ACS Appl
Mater Interfaces. 14:14887–14902. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Castro R, Aisenbrey E and Hui L: The role
of formulation approaches in presenting targeting ligands on lipid
nanoparticles. Nanomedicine (Lond). 18:589–597. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Piunti C and Cimetta E: Microfluidic
approaches for producing Lipid-based nanoparticles for drug
delivery applications. Biophys Rev (Melville). 4:0313042023.
View Article : Google Scholar
|
|
185
|
Sheth P, Sandhu H, Singhal D, Malick W,
Shah N and Kislalioglu MS: Nanoparticles in the pharmaceutical
industry and the use of supercritical fluid technologies for
nanoparticle production. Curr Drug Deliv. 9:269–284. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Sun Z, Liu Z, Han B, Miao S, Miao Z and An
G: Decoration carbon nanotubes with Pd and Ru nanocrystals via an
inorganic reaction route in supercritical carbon dioxide-methanol
solution. J Colloid Interface Sci. 304:323–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Birla D, Khandale N, Bashir B, ShahbazAlam
M, Vishwas S, Gupta G, Dureja H, Kumbhar PS, Disouza J, Patravale
V, et al: Application of quality by design in optimization of
nanoformulations: Principle, perspectives and practices. Drug Deliv
Transl Res. 15:798–830. 2025. View Article : Google Scholar
|
|
188
|
Rybnikář M, Malaník M, Šmejkal K,
Švajdlenka E, Shpet P, Babica P, Dall'Acqua S, Smištík O, Jurček O
and Treml J: Dibenzocyclooctadiene Lignans from Schisandra
chinensis with Anti-Inflammatory effects. Int J Mol Sci.
25:34652024. View Article : Google Scholar
|
|
189
|
Song L, Yao L, Zhang L, Piao Z and Lu Y:
Schizandrol A protects against Aβ1-42-induced autophagy via
activation of PI3K/AKT/mTOR pathway in SH-SY5Y cells and primary
hippocampal neurons. Naunyn Schmiedebergs Arch Pharmacol.
393:1739–1752. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Arken N: Schizandrol A reverses multidrug
resistance in resistant chronic myeloid leukemia cells K562/A02.
Cell Mol Biol (Noisy-le-Grand). 65:78–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Han SJ, Jun J, Eyun SI, Lee CG, Jeon J and
Pan CH: Schisandrol A suppresses catabolic factor expression by
blocking NF-κB signaling in osteoarthritis. Pharmaceuticals
(Basel). 14:412021.
|
|
192
|
Zhou X, Zhao S, Liu T, Yao L, Zhao M, Ye
X, Zhang X, Guo Q, Tu P and Zeng K: Schisandrol A protects
AGEs-induced neuronal cells death by allosterically targeting
ATP6V0d1 subunit of V-ATPase. Acta Pharm Sin B. 12:3843–3860. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Fong WF, Wan CK, Zhu GY, Chattopadhyay A,
Dey S, Zhao Z and Shen XL: Schisandrol A from Schisandra chinensis
reverses P-glycoprotein-mediated multidrug resistance by affecting
Pgp-substrate complexes. Planta Med. 73:212–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Li L, Pan Q, Sun M, Lu Q and Hu X:
Dibenzocyclooctadiene lignans: A class of novel inhibitors of
multidrug resistance-associated protein 1. Life Sci. 80:741–748.
2007. View Article : Google Scholar
|
|
195
|
Xu G, Feng Y, Li H, Chen C, Li H, Wang C,
Chen J and Sun J: Molecular mechanism of the regulatory effect of
schisandrol a on the immune function of mice based on a
transcription factor regulatory network. Front Pharmacol.
12:7853532021. View Article : Google Scholar
|
|
196
|
Lee D, Kim YM, Chin YW and Kang KS:
Schisandrol A exhibits estrogenic activity via estrogen receptor
α-Dependent signaling pathway in estrogen Receptor-positive breast
cancer Cells. Pharmaceutics. 13:10822021. View Article : Google Scholar
|
|
197
|
Guo X, Lei M, Ma G, Ouyang C, Yang X, Liu
C, Chen Q and Liu X: Schisandrin A alleviates spatial learning and
memory impairment in diabetic rats by inhibiting inflammatory
response and through modulation of the PI3K/AKT pathway. Mol
Neurobiol. 61:2514–2529. 2024. View Article : Google Scholar
|
|
198
|
Hassanein EHM, Althagafy HS, Baraka MA,
Abd-Alhameed EK, Ibrahim IM, Abd El-Maksoud MS, Mohamed NM and Ross
SA: The promising antioxidant effects of lignans: Nrf2 activation
comes into view. Naunyn Schmiedebergs Arch Pharmacol.
397:6439–6458. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
He D, Pei X, Liu B, Li J, Dong J, Efferth
T and Ma P: Lignan contents of Schisandra chinensis (Turcz.) Baill.
from different origins-A new model for evaluating the content of
prominent components of Chinese herbs. Phytomedicine.
128:1553612024. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Li B, Zhou W, Zhang J, Wang N, Yang X and
Ge X: Schisandrin a ameliorates cardiac injury and dysfunction
induced by hemorrhagic shock via activating the Nrf2 signaling
pathway. Am J Chin Med. 52:2453–2468. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Wang P, Lan Q, Huang Q, Zhang R, Zhang S,
Yang L, Song Y, Wang T, Ma G, Liu X, et al: Schisandrin A
attenuates diabetic nephropathy via EGFR/AKT/GSK3β signaling
pathway based on network pharmacology and experimental validation.
Biology (Basel). 13:5972024.
|
|
202
|
Xiao Y, Zhou H, Cui Y, Zhu X, Li S, Yu C,
Jiang N, Liu L and Liu F: Schisandrin A enhances pathogens
resistance by targeting a conserved p38 MAPK pathway. Int
Immunopharmacol. 128:1114722024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Xu X, Rajamanicham V, Xu S, Liu Z, Yan T,
Liang G, Guo G, Zhou H and Wang Y: Schisandrin A inhibits triple
negative breast cancer cells by regulating Wnt/ER stress signaling
pathway. Biomed Pharmacother. 115:1089222019. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Zhu L, Wang Y, Lv W, Wu X, Sheng H, He C
and Hu J: Schizandrin A can inhibit non-small cell lung cancer cell
proliferation by inducing cell cycle arrest, apoptosis and
autophagy. Int J Mol Med. 48:2142021. View Article : Google Scholar :
|
|
205
|
Chi B, Sun Y, Zhao J and Guo Y:
Deoxyschizandrin inhibits the proliferation, migration, and
invasion of bladder cancer cells through ALOX5 regulating PI3K-AKT
signaling pathway. J Immunol Res. 2022:30798232022. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
He LW, Lin CJ, Zhuang LJ, Sun YH, Li YC
and Ye ZY: Targeting hepatocellular carcinoma: Schisandrin A
triggers mitochondrial disruption and ferroptosis. Chem Biol Drug
Des. 104:e700102024. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Peng CW, Ma PL and Dai HT: Schizandrin A
promotes apoptosis in prostate cancer by inducing ROS-mediated
endoplasmic reticulum stress and JNK MAPK signaling activation.
Pathol Res Pract. 269:1558892025. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Ding Y, Guo N, Jiang Y, Liu S, Zhou T, Bai
H, Lv Y, Han S and He L: Establishment of cluster of
differentiation 20 immobilized cell membrane chromatography for the
screening of active antitumor components in traditional Chinese
medicine. J Chromatogr A. 1721:4648452024. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Zhang ZL, Jiang QC and Wang SR:
Schisandrin A reverses doxorubicin-resistant human breast cancer
cell line by the inhibition of P65 and Stat3 phosphorylation.
Breast Cancer. 25:233–242. 2018. View Article : Google Scholar
|
|
210
|
Xian H, Feng W and Zhang J: Schizandrin A
enhances the efficacy of gefitinib by suppressing IKKβ/NF-κB
signaling in non-small cell lung cancer. Eur J Pharmacol.
855:10–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Zheng A, Yang D, Pan C, He Q, Zhu X, Xiang
X and Ji P: Modeling the complexity of drug-drug interactions: A
physiologically-based pharmacokinetic study of Lenvatinib with
Schisantherin A/Schisandrin A. Eur J Pharm Sci. 196:1067572024.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Wang X, Wang F, Dong P and Zhou L: The
therapeutic effect of ultrasound targeted destruction of
schisandrin A contrast microbubbles on liver cancer and its
mechanism. Radiol Oncol. 58:221–233. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Xu SY, Wang WW, Qu ZH, Zhang XK, Chen M,
Zhang XY, Xing NN, Su H, Wang XY, Cui MY, et al: Long-circulating
doxorubicin and Schizandrin A liposome with Drug-resistant liver
cancer activity: In vitro and in vivo evaluation. J Liposome Res.
33:338–352. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Kim JS and Yi HK: Schisandrin C enhances
mitochondrial biogenesis and autophagy in C2C12 skeletal muscle
cells: Potential involvement of anti-oxidative mechanisms. Naunyn
Schmiedebergs Arch Pharmacol. 391:197–206. 2018. View Article : Google Scholar
|
|
215
|
Takanche JS, Lee YH, Kim JS, Kim JE, Han
SH, Lee SW and Yi HK: Anti-inflammatory and antioxidant properties
of Schisandrin C promote mitochondrial biogenesis in human dental
pulp cells. Int Endod J. 51:438–447. 2018. View Article : Google Scholar
|
|
216
|
Yan H, Wu W, Hu Y, Li J, Xu J, Chen X, Xu
Z, Yang X, Yang B, He Q and Luo P: Regorafenib inhibits EphA2
phosphorylation and leads to liver damage via the ERK/MDM2/p53
axis. Nat Commun. 14:27562023. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Xu B, Liu N, Zhou T, Chen J, Jiang L, Wu
W, Fu H, Chen X, Yan H, Yang X, et al: Schisandrin C prevents
regorafenib-induced cardiotoxicity by recovering EPHA2 expression
in cardiomyocytes. Toxicol Sci. 202:220–235. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Park C, Choi YW, Hyun SK, Kwon HJ, Hwang
HJ, Kim GY, Choi BT, Kim BW, Choi IW, Moon SK, et al: Induction of
G1 arrest and apoptosis by schisandrin C isolated from Schizandra
chinensis Baill in human leukemia U937 cells. Int J Mol Med.
24:495–502. 2009.PubMed/NCBI
|
|
219
|
Wang Z, Xie S, Li L, Liu Z and Zhou W:
Schisandrin C inhibits AKT1-regulated cell proliferation in A549
cells. Int Immunopharmacol. 142:1131102024. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Samson N and Ablasser A: The cGAS-STING
pathway and cancer. Nat Cancer. 3:1452–1463. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Yang H, Zhan X, Zhao J, Shi W, Liu T, Wei
Z, Li H, Hou X, Mu W, Chen Y, et al: Schisandrin C enhances type I
IFN response activation to reduce tumor growth and sensitize
chemotherapy through antitumor immunity. Front Pharmacol.
15:13695632024. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Wu Z, Zhao X, Li R, Wen X, Xiu Y, Long M,
Li J, Huang X, Wen J, Dong X, et al: The combination of Schisandrin
C and Luteolin synergistically attenuates hepatitis B virus
infection via repressing HBV replication and promoting cGAS-STING
pathway activation in macrophages. Chin Med. 19:482024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Liu HW, Yu XZ, Padula D, Pescitelli G, Lin
ZW, Wang F, Ding K, Lei M and Gao JM: Lignans from Schisandra
sphenathera Rehd. et Wils. and semisynthetic schisantherin A
analogues: Absolute configuration, and their estrogenic and
Anti-proliferative activity. Eur J Med Chem. 59:265–273. 2013.
View Article : Google Scholar
|
|
224
|
Wang Z, Yu K, Hu Y, Su F, Gao Z, Hu T,
Yang Y, Cao X and Qian F: Schisantherin A induces cell apoptosis
through ROS/JNK signaling pathway in human gastric cancer cells.
Biochem Pharmacol. 173:1136732020. View Article : Google Scholar
|
|
225
|
Feng F, Pan L, Wu J, Liu M, He L, Yang L
and Zhou W: Schisantherin A inhibits cell proliferation by
regulating glucose metabolism pathway in hepatocellular carcinoma.
Front Pharmacol. 13:10194862022. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Chen L, Ji N, Zhang M and Chen W: The
influence of wuzhi capsule on the pharmacokinetics of
cyclophosphamide. Recent Pat Anticancer Drug Discov. 17:195–203.
2022. View Article : Google Scholar
|
|
227
|
Adiwidjaja J, Boddy AV and McLachlan AJ:
Potential for pharmacokinetic interactions between Schisandra
sphenanthera and bosutinib, but not imatinib: In vitro metabolism
study combined with a physiologically-based pharmacokinetic
modelling approach. Br J Clin Pharmacol. 86:2080–2094. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G and Jassem J: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Cui Y, Ma Y, Li Y, Song H and Dong Z:
Influence of schisantherin A on the pharmacokinetics of lenvatinib
in rats and its potential mechanism. J Gastrointest Oncol.
13:802–811. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Sobstyl E, Szopa A, Dziurka M, Ekiert H,
Nikolaichuk H and Choma IM: Schisandra rubriflora fruit and leaves
as promising new materials of high biological potential: Lignan
profiling and Effect-directed analysis. Molecules. 27:21162022.
View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Deng L, Cheng S, Li J, Xu X, Hao X, Fan Y
and Mu S: Synthesis and biological evaluation of novel schisanhenol
derivatives as potential hepatoprotective agents. Eur J Med Chem.
227:1139192022. View Article : Google Scholar
|
|
232
|
Li B, Xiao Q, Zhao H, Zhang J, Yang C, Zou
Y, Zhang B, Liu J, Sun H and Liu H: Schisanhenol ameliorates
Non-alcoholic fatty liver disease via inhibiting miR-802 activation
of AMPK-mediated modulation of hepatic lipid metabolism. Acta Pharm
Sin B. 14:3949–3963. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
He X, Chen J, Mu Y, Zhang H, Chen G, Liu P
and Liu W: The effects of inhibiting the activation of hepatic
stellate cells by lignan components from the fruits of Schisandra
chinensis and the mechanism of schisanhenol. J Nat Med. 74:513–524.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Zhang Z, Zhong Y, Han X, Hu X, Wang Y,
Huang L, Li S, Li Z, Wang C, Li H, et al: Schisanhenol inhibits the
proliferation of hepatocellular carcinoma cells by targeting
programmed cell Death-ligand 1 via the STAT3 pathways. Anticancer
Agents Med Chem. 25:697–710. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Jeong M, Kim HM, Kim HJ, Choi JH and Jang
DS: Kudsuphilactone B, a nortriterpenoid isolated from Schisandra
chinensis fruit, induces caspase-dependent apoptosis in human
ovarian cancer A2780 cells. Arch Pharm Res. 40:500–508. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Liu M, Yang K and Qiu H: Exploring the
effect of gomisin A on non-small cell lung cancer with network
pharmacology, molecular docking, in vitro and in vivo assays. Chem
Biol Drug Des. 104:e700142024. View Article : Google Scholar
|
|
237
|
Wang T, Liu J, Huang X, Zhang C, Shangguan
M, Chen J, Wu S, Chen M, Yang Z and Zhao S: Gomisin A enhances the
antitumor effect of paclitaxel by suppressing oxidative stress in
ovarian cancer. Oncol Rep. 48:2022022. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Kee JY, Han YH, Mun JG, Park SH, Jeon HD
and Hong SH: Gomisin A suppresses colorectal lung metastasis by
inducing AMPK/p38-mediated apoptosis and decreasing metastatic
abilities of colorectal cancer cells. Front Pharmacol. 9:9862018.
View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Han YH, Mun JG, Jeon HD, Park J, Kee JY
and Hong SH: Gomisin A ameliorates metastatic melanoma by
inhibiting AMPK and ERK/JNK-mediated cell survival and metastatic
phenotypes. Phytomedicine. 68:1531472020. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Wang R, Liu F, Chen P, Li S, Gu Y, Wang L,
Chen C and Yuan Y: Gomisin D alleviates liver fibrosis through
targeting PDGFRβ in hepatic stellate cells. Int J Biol Macromol.
235:1236392023. View Article : Google Scholar
|
|
241
|
Jeon JS, Kang HM, Park SY and Choi YW:
Comparative study of the photo-protective and anti-melanogenic
properties of gomisin D, J and O. Mol Med Rep. 25:82022. View Article : Google Scholar
|
|
242
|
Maharjan S, Park BK, Lee SI, Lim Y, Lee K,
Lee Y and Kwon HJ: Gomisin G Suppresses the Growth of colon cancer
cells by attenuation of AKT phosphorylation and arrest of cell
cycle progression. Biomol Ther (Seoul). 27:210–215. 2019.
View Article : Google Scholar
|
|
243
|
Maharjan S, Park BK, Lee SI, Lim Y, Lee K
and Kwon HJ: Gomisin G inhibits the growth of triple-Negative
breast cancer cells by suppressing AKT phosphorylation and
decreasing cyclin D1. Biomol Ther (Seoul). 26:322–327. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Yeon M, Choi H, Chun KH, Lee JH and Jun
HS: Gomisin G improves muscle strength by enhancing mitochondrial
biogenesis and function in disuse muscle atrophic mice. Biomed
Pharmacother. 153:1134062022. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Mokhtari T and El-Meghawry El-Kenawy A:
Molecular mechanisms of Schisandra chinensis in treating
depression-neuropathic pain comorbidity by network pharmacology and
molecular docking analysis. Neuroscience. 555:92–105. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Jung S, Moon HI, Kim S, Quynh NTN, Yu J,
Sandag Z, Le DT, Lee H, Lee H and Lee MS: Anticancer activity of
gomisin J from Schisandra chinensis fruit. Oncol Rep. 41:711–717.
2019.
|
|
247
|
Kang K, Lee KM, Yoo JH, Lee HJ, Kim CY and
Nho CW: Dibenzocyclooctadiene lignans, gomisins J and N inhibit the
Wnt/β-catenin signaling pathway in HCT116 cells. Biochem Biophys
Res Commun. 428:285–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Li R and Yang W: Gomisin J inhibits the
glioma progression by inducing apoptosis and reducing
HKII-regulated glycolysis. Biochem Biophys Res Commun. 529:15–22.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
He XR, Han SY, Li XH, Zheng WX, Pang LN,
Jiang ST and Li PP: Chinese medicine Bu-Fei decoction attenuates
epithelial-mesenchymal transition of Non-small cell lung cancer via
inhibition of transforming growth factor β1 signaling pathway in
vitro and in vivo. J Ethnopharmacol. 204:45–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Garcia-Aguilar J, Patil S, Gollub MJ, Kim
JK, Yuval JB, Thompson HM, Verheij FS, Omer DM, Lee M, Dunne RF, et
al: Organ preservation in patients with rectal adenocarcinoma
treated with total neoadjuvant therapy. J Clin Oncol. 40:2546–2556.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Schaff LR and Mellinghoff IK: Glioblastoma
and other primary brain malignancies in adults: A review. JAMA.
329:574–587. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Moris D, Palta M, Kim C, Allen PJ, Morse
MA and Lidsky ME: Advances in the treatment of intrahepatic
cholangiocarcinoma: An overview of the current and future
therapeutic landscape for clinicians. CA Cancer J Clin. 73:198–222.
2023.
|
|
253
|
Kim SM, Vetrivel P, Ha SE, Kim HH, Kim JA
and Kim GS: Apigetrin induces extrinsic apoptosis, autophagy and
G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS
human gastric cancer cell. J Nutr Biochem. 83:1084272020.
View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Wu YF, Cao MF, Gao YP, Chen F, Wang T,
Zumbika EP and Qian KX: Down-modulation of heat shock protein 70
and up-modulation of Caspase-3 during schisandrin B-induced
apoptosis in human hepatoma SMMC-7721 cells. World J Gastroenterol.
10:2944–2948. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Raychaudhuri R, Lin DW and Montgomery RB:
Prostate cancer: A review. JAMA. 333:1433–1446. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Wang Y, Chen J, Huang Y, Yang S, Tan T,
Wang N, Zhang J, Ye C, Wei M, Luo J and Luo X: Schisandrin B
suppresses osteosarcoma lung metastasis in vivo by inhibiting the
activation of the Wnt/β-catenin and PI3K/Akt signaling pathways.
Oncol Rep. 47:502022. View Article : Google Scholar
|
|
257
|
Maharjan S, Park BK, Lee SI, Lim Y, Lee K
and Kwon HJ: Erratum to 'Gomisin G Inhibits the Growth of
Triple-Negative Breast Cancer Cells by Suppressing AKT
Phosphorylation and Decreasing Cyclin D1' [Biomol.Ther. 26 (2018)
322-327]. Biomol Ther (Seoul). 26:5202018. View Article : Google Scholar
|