Since its discovery in the early 20th-century, the
biological function of carnosine has increasingly garnered interest
(1). One of the most significant
functions of carnosine is its antioxidant effect (2). Oxidative stress serves as a common
pathological basis for numerous diseases (3). By scavenging free radicals,
chelating metal ions and enhancing endogenous defenses, carnosine
reduces oxidative stress (4). It
further inhibits lipid peroxidation, protein oxidation and DNA
damage, thereby preserving cellular integrity (4-7).
Furthermore, the anti-glycation properties of carnosine offer novel
insights into the prevention and treatment of diabetes mellitus and
its complications by inhibiting the formation of advanced glycation
end products (AGEs) (8,9). The accumulation of AGE is closely
associated with diabetes, cardiovascular disease and aging;
carnosine competes with sugars to bind proteins to reduce AGEs
formation by inhibiting glycosylation processes (10). In terms of neuroprotection,
carnosine demonstrates potential therapeutic value for
neurodegenerative diseases such as Alzheimer's disease (AD) and
Parkinson's disease (PD) (11).
AD is characterized by β-amyloid protein (Aβ) deposition along with
neuronal loss. Studies have shown that carnosine can inhibit Aβ
aggregation and toxicity while reducing oxidative damage and
inflammation in neurons, thus delaying AD progression (12,13). PD is a neurodegenerative disorder
characterized by the loss of dopaminergic neurons. Evidence
indicates that carnosine may protect these neurons from damage
induced by oxidative stress and inflammatory responses;
consequently, it has the potential to delay the progression of PD
(14-16). Carnosine exerts anti-inflammatory
and immunomodulatory effects by suppressing pro-inflammatory
mediators and regulating immune cell activity, supporting its
potential in chronic inflammatory and autoimmune diseases (17-20). Research demonstrates that
carnosine can inhibit the secretion of tumor necrosis factor-α
(TNF-α) and interleukin-6 (IL-6), among other inflammatory
mediators, thereby reducing inflammation (21-24). Furthermore, carnosine also plays
a role in regulating immune cell functions such as those of
macrophages and T cells, enhancing the body's overall
anti-inflammatory capacity (25).
Although carnosine has shown a wide range of
potential applications in disease therapy, it still faces several
challenges for clinical use. First, carnosine has low
bioavailability in vivo and is readily degraded by enzymes,
which affects its efficacy (28). Studies have explored various
strategies to address this. Nanotechnology-based delivery systems,
such as liposomes and polymeric nanoparticles, have been developed
to protect carnosine from rapid hydrolysis by carnosinase and to
facilitate targeted delivery to specific tissues. For instance,
liposomal encapsulation has been shown to enhance the stability of
carnosine (29). In addition,
derivatization and conjugation strategies (such as cyclodextrins
and trehalose) have been employed to enhance the stability of
carnosine against carnosinase-mediated degradation as well as its
pharmacological activity (30).
Secondly, the efficacy of carnosine is dose-dependent, and high
doses may result in certain side effects (31,32). In addition, the current clinical
research data on carnosine remain insufficient and there is a lack
of large-scale clinical trials on this drug. Therefore, it is
necessary to improve the bioavailability and stability of carnosine
through chemical modification or nanotechnology and develop novel
carnosine-based drugs. Similarly, when combined with precision
medicine, a personalized carnosine treatment program may be
feasible to improve efficacy and reduce side effects. In addition,
conducting large-scale clinical trials to verify the safety and
effectiveness of carnosine in disease treatment is also an
important direction for future research. By systematically
reviewing the multiple biological functions of carnosine and its
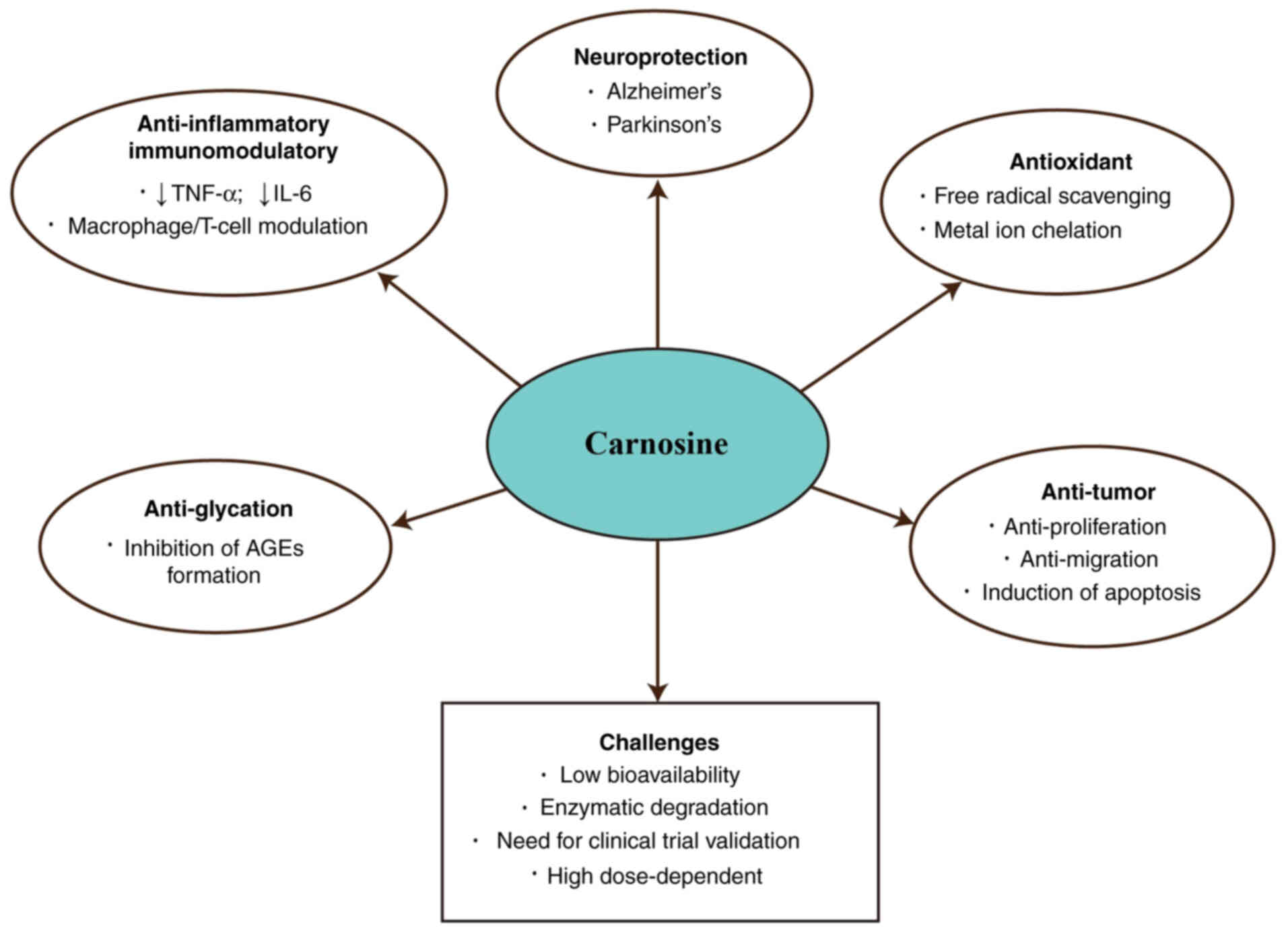
application in the treatment of diseases (Fig. 1), this review aims to provide
comprehensive theoretical support and practical guidance for
researchers in related fields and promote the wider application of
carnosine in the medical field.
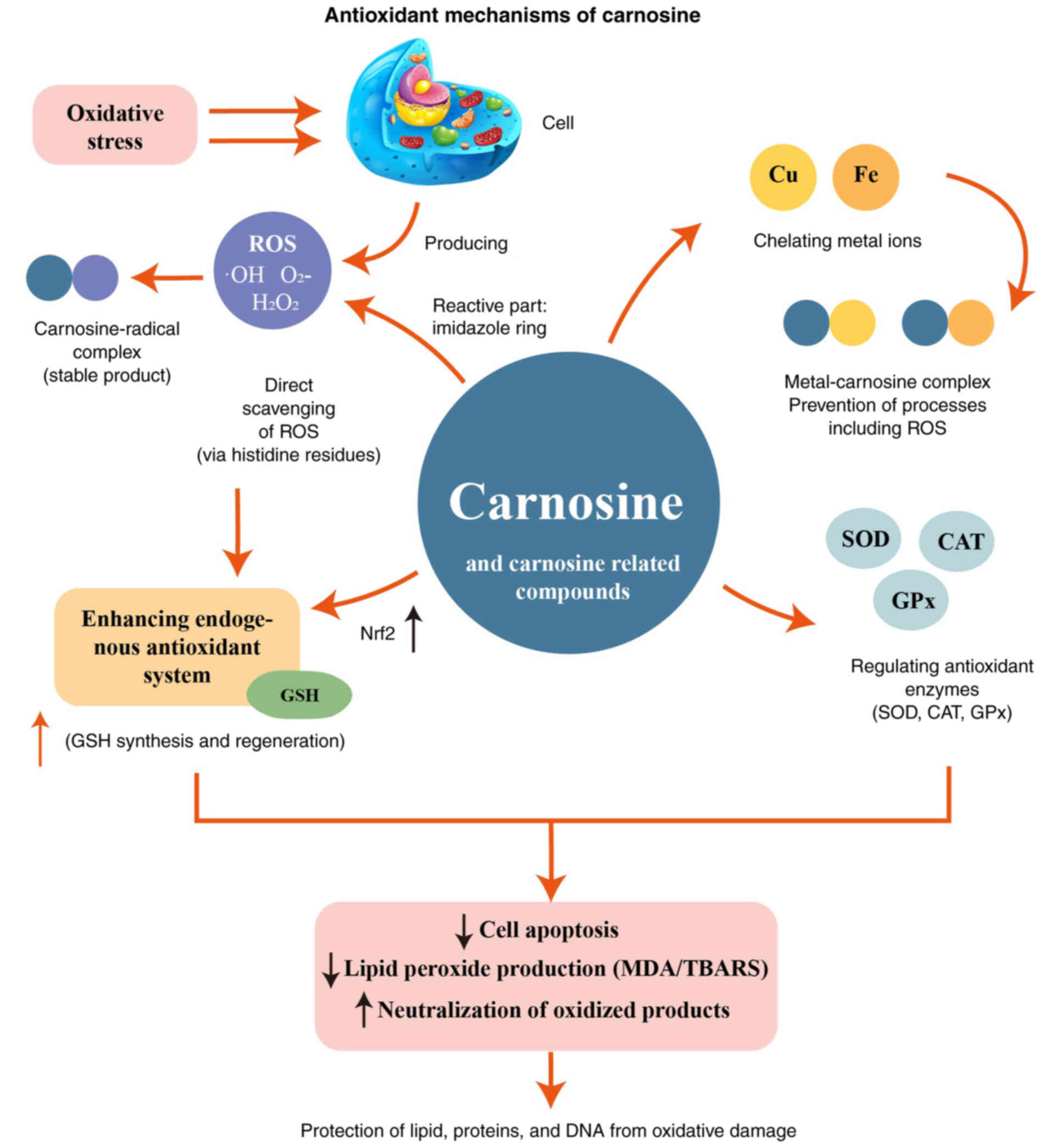
Carnosine is a powerful antioxidant capable of
scavenging free radicals [such as reactive oxygen species] through
a variety of mechanisms to protect cells from oxidative stress
(33). Oxidative stress is a
common pathological basis for a variety of diseases such as
neurodegenerative diseases, cardiovascular diseases and cancer, and
the antioxidant effects of carnosine provide a scientific basis for
its application in these diseases (34-37). Studies have shown that carnosine
can inhibit lipid peroxidation, protein oxidation and DNA damage,
thereby protecting the structural and functional integrity of cells
(4,38). The antioxidant effect of
carnosine is primarily achieved through several mechanisms
(Fig. 2): i) Direct removal of
free radicals: Carnosine can react directly with free radicals to
neutralize their activity, thereby reducing the damage of free
radicals to cells (12,14,39). Free radicals are the primary
mediators of oxidative stress, capable of attacking cell membranes,
proteins and DNA, leading to the destruction of cell structure and
function. Carnosine, through its histidine residues in its
molecular structure, can effectively trap and neutralize free
radicals, thereby protecting cells from oxidative damage. ii)
Chelating metal ions: Carnosine can bind to metal ions (such as
copper and iron) and inhibit the oxidation reaction catalyzed by
metal ions (40-43). Metal ions play an important role
in oxidative stress, which can catalyze the formation of free
radicals and the oxidation reaction. Carnosine can form stable
complexes with metal ions through carboxyl and amino groups in its
molecular structure, thus inhibiting the oxidation reaction
catalyzed by metal ions and reducing the generation of free
radicals. iii) Enhancing the endogenous antioxidant system:
Carnosine can enhance the levels of endogenous antioxidants such as
glutathione (GSH) in the cell, thereby improving the antioxidant
capacity of the cell (24,44). GSH is one of the most important
antioxidants in the cell, which can directly remove free radicals
and participate in the regulation of redox reactions. Carnosine
enhances the function of the intracellular antioxidant system by
promoting the synthesis and regeneration of GSH, thereby improving
the resistance of cells to oxidative stress.
In addition, carnosine can further enhance the
antioxidant capacity of cells by regulating the activity of
antioxidant enzymes such as superoxide dismutase, catalase and
glutathione peroxidase (GPx). These antioxidant enzymes can
catalyze the breakdown and removal of free radicals, thereby
protecting cells from oxidative damage. By regulating the activity
of these enzymes, carnosine can effectively reduce the damage to
cells caused by oxidative stress (45,46).
Carnosine has been shown to play a significant role
in the field of anti-glycation, effectively inhibiting the
non-enzymatic reaction of proteins and sugars (glycation reaction),
thereby reducing the formation of AGEs (8). Glycosylation is a non-enzymatic
reaction between proteins, lipids or nucleic acids and reducing
sugars, and the accumulation of AGEs in the body is closely
associated with a variety of diseases, such as diabetes,
cardiovascular disease and age-related diseases (47-50). The anti-glycation effect of
carnosine is of great significance in delaying aging and preventing
diabetic complications (such as vasculopathy and neuropathy), and
its mechanism primarily includes the following aspects: i)
Inhibition of glycosylation: Carnosine can compete with sugars to
bind proteins, thereby inhibiting the occurrence of glycosylation.
At the heart of the glycosylation reaction is the reduction of
sugars (such as glucose) with free amino groups in proteins (such
as lysine and arginine residues) to form unstable Schiff bases,
which, in turn, rearrange into stable AGEs. Carnosine, through the
amino and carboxyl groups in its molecular structure, can compete
with sugars to bind the free amino group of proteins, blocking the
initial step of the glycosylation reaction, thereby reducing the
production of AGEs (8,51). ii) Elimination of AGE precursors:
Carnosine can eliminate the precursor substances of AGEs, such as
α-dicarbonyl compounds (such as methylglyoxal and glyoxal), thereby
reducing the formation of AGEs. α-dicarbonyl compounds are
important intermediates in glycation reactions, which are highly
reactive and can rapidly bind to proteins to form AGEs. Carnosine
blocks the AGE formation pathways by binding to these highly active
intermediates and neutralizing their reactivity (52). iii) Protection of protein
function: Carnosine can protect the structure and function of
proteins and reduce the damage of the glycosylation reaction to
protein function. Glycosylation can lead to protein cross-linking,
structural changes and loss of function, which can affect the
normal physiological function of cells (53,54). By binding to proteins, carnosine
stabilizes their conformation and prevents protein denaturation
caused by glycosylation. In addition, carnosine can also repair
certain proteins that have been glycated, restoring their function.
iv) Regulation of AGE receptor signaling pathway: Carnosine can
also reduce the toxic effects of AGEs on cells by regulating the
signaling pathway of AGE receptors (RAGEs). AGEs induce
inflammation and oxidative stress-related signaling pathways by
binding to RAGE, leading to cell damage. Carnosine can inhibit the
binding of AGEs and RAGE, and block the activation of downstream
signaling pathways, thus reducing the damage of AGEs to cells
(55).
The anti-glycation effect of carnosine is
particularly important in the prevention and treatment of diabetes
and its complications. Long-term hyperglycemia in diabetic patients
accelerates the glycosylation reaction, leading to the accumulation
of AGEs in blood vessels, nerves and kidneys, which can lead to
diabetic complications (such as diabetic nephropathy, retinopathy
and neuropathy). Carnosine can effectively delay the progression of
diabetic complications by inhibiting the glycation reaction and
clearing the precursors of AGEs. In addition, the anti-glycation
effect of carnosine is also of great value in the field of
anti-aging. The accumulation of AGEs is one of the important signs
of aging and is closely related to age-related diseases such as
skin aging, arteriosclerosis and cognitive decline. By reducing the
formation of AGEs, carnosine can delay the aging process and
improve age-related functional decline.
Carnosine exerts neuroprotective effects in
neurodegenerative diseases (such as AD and PD) by targeting
multiple pathological processes, including oxidative stress,
inflammation, neurotransmitter imbalance and abnormal protein
aggregation (56,57). The primary mechanisms of the
neuroprotective effect of carnosine are as follows: i) Antioxidant
effect: Carnosine can clear free radicals in neurons and reduce the
damage of neurons caused by oxidative stress (58-61). In addition, carnosine can chelate
metal ions (such as iron and copper), inhibiting oxidation
reactions catalyzed by metal ions, thereby further reducing
oxidative stress (27,40). ii) Anti-inflammatory effect:
Carnosine can inhibit the inflammatory response of neurons (such as
the NF-κB pathway) and reduce the damage caused by inflammatory
factors (such as TNF-α, IL-1β and IL-6) to neurons (62). In addition, carnosine can also
regulate the activity of microglia and astrocytes, inhibit their
overactivation and further relieve neuroinflammation (63-65). iii) Regulation of
neurotransmitters: Carnosine can regulate the release and
metabolism of neurotransmitters, thereby protecting the function of
neurons (66). Imbalances in
neurotransmitters such as dopamine, glutamate and γ-aminobutyric
acid are important pathological features of neurodegenerative
diseases. For instance, in PD, loss of dopaminergic neurons leads
to decreased dopamine levels, and in AD, the excitotoxicity of
glutamate exacerbates neuronal damage (67). Carnosine regulates the synthesis,
release and degradation of neurotransmitters and maintains the
balance of neurotransmitters, thereby protecting the function of
neurons (68). In addition,
carnosine can also inhibit the excitatory toxicity of glutamate and
reduce the damage caused by the overexcitation of neurons (69). iv) Inhibition of abnormal protein
aggregation: Carnosine can inhibit the aggregation of abnormal
proteins in neurodegenerative diseases, such as Aβ in AD and in PD
(70,71). The aggregation of these abnormal
proteins can form toxic aggregates, which result in neuronal
dysfunction and death. Carnosine protects neurons by binding to
these abnormal proteins, inhibiting their aggregation and toxicity.
v) Promotion of the expression of neurotrophic factors: Carnosine
can promote the expression of neurotrophic factors (such as
brain-derived neurotrophic factor) and enhance the survival and
regeneration ability of neurons (72). Neurotrophic factors play an
important role in the growth, differentiation and repair of
neurons, and carnosine can further play a neuroprotective role by
upregulating their expression.
Carnosine has been shown to play a significant role
in anti-inflammatory and immune regulation. It can regulate the
function of the immune system through several mechanisms and
enhance the anti-inflammatory capacity of the body, so it has
important value in the treatment of inflammation-related diseases.
Inflammation is the body's defense response to injury or infection,
but an excessive inflammatory response can lead to tissue damage
and disease. Carnosine exerts its anti-inflammatory and
immunomodulatory effects by inhibiting the release of inflammatory
factors, regulating the function of immune cells and protecting
tissues from inflammatory damage. The primary mechanisms of
carnosine's anti-inflammatory and immunomodulatory effects are as
follows: i) Inhibition of inflammatory factors: Carnosine can
inhibit the release of inflammatory factors (such as TNF-α, IL-6
and IL-1β), thereby reducing the inflammatory response (21). Inflammatory factors are key
molecules mediating the inflammatory response and their excessive
release can lead to tissue damage and disease progression.
Carnosine reduces the transcription and release of inflammatory
factors by inhibiting the activation of inflammatory signaling
pathways, such as the NF-κB pathway (73). For instance, in chronic
inflammatory diseases such as rheumatoid arthritis and inflammatory
bowel disease, carnosine can significantly reduce the levels of
TNF-α and IL-6, thereby reducing inflammatory symptoms (73). ii) Regulation of immune cells:
Carnosine can regulate the function of immune cells (such as
macrophages, T cells and neutrophils) and enhance the body's
anti-inflammatory ability. Macrophages are key cells in the
inflammatory response, releasing a large number of inflammatory
factors. Carnosine reduces the inflammatory response by regulating
the polarization of macrophages and promoting their transformation
from pro-inflammatory (M1 type) to anti-inflammatory (M2 type)
(58,74). In addition, carnosine can
regulate the differentiation and function of T cells, inhibit the
activity of pro-inflammatory type 17 T-helper cells, and enhance
the function of regulatory T cells, thereby maintaining immune
balance (75,76). iii) Protection of tissue:
Carnosine can protect the tissue from the damage of the
inflammatory response to maintain the normal function of the
tissue. Inflammatory response can lead to tissue oxidative stress,
cell apoptosis and fibrosis. Carnosine, through its antioxidant
effect, clears free radicals in inflammatory sites and reduces
tissue damage caused by oxidative stress (77). In addition, carnosine can inhibit
inflammation-mediated apoptosis and protect the survival of tissue
cells (65). For instance, in
inflammatory diseases such as myocarditis and hepatitis, carnosine
can significantly reduce tissue damage and improve organ function
(78,79). iv) Regulation of inflammatory
mediators: Carnosine can regulate the production of inflammatory
mediators [such as nitric oxide (NO) and prostaglandin], thereby
reducing inflammatory response (80,81). NO is an important mediator in
inflammatory response and its overproduction can lead to tissue
damage. Carnosine reduces the production of NO by inhibiting the
expression of inducible NO synthase, thereby alleviating
inflammatory damage (72). In
addition, carnosine can also inhibit the activity of
cyclooxygenase-2, reduce the production of prostaglandins and
further alleviate the inflammatory response (82). v) Promotion of tissue repair:
Carnosine can promote tissue repair after inflammation and
accelerate the healing of injured tissue. The attenuation of
inflammatory response and tissue repair are important steps in the
treatment of inflammatory diseases. Carnosine enhances tissue
regeneration by promoting cell proliferation and migration. For
instance, in models of skin lesions and ulcers, carnosine
significantly accelerated wound healing and reduced scarring
(17).
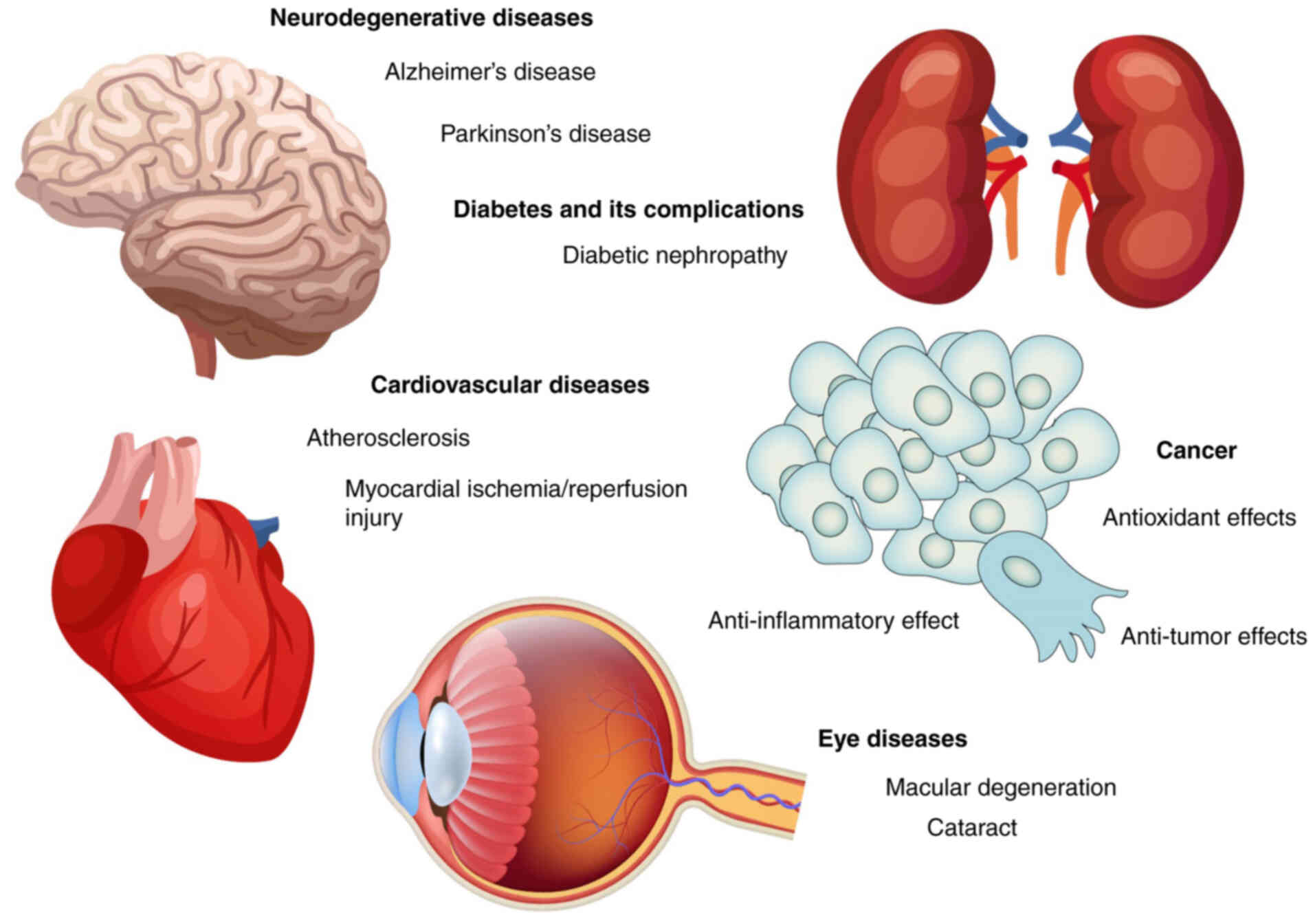
Studies have demonstrated that carnosine exhibits
significant protective effects across multiple disease systems
(Fig. 3). In neurodegenerative
disorders such as AD and PD, carnosine is implicated in mitigating
oxidative stress and protein aggregation. Its role in diabetes,
particularly in preventing complications like diabetic nephropathy,
has also been recognized. Furthermore, carnosine contributes to
cardiovascular health by alleviating conditions like
atherosclerosis and myocardial ischemia-reperfusion injury. In
oncology, its antioxidant, anti-inflammatory and anti-tumor
properties provide a promising adjunct in cancer therapy.
Additionally, carnosine shows potential in protecting against
age-related eye diseases including macular degeneration and
cataract. These diverse applications highlight carnosine's
multifunctional nature and establish a strong rationale for
exploring its clinical relevance in greater depth.
AD is a common neurodegenerative disease
characterized by Aβ deposition and loss of neurons, leading to
progressive decline in cognitive function (83). Studies have shown that carnosine
plays an important role in the treatment of AD and can delay the
progression of the disease through multiple mechanisms. First,
carnosine can bind to Aβ, inhibiting its aggregation and toxicity,
thereby reducing the damage caused by Aβ to neurons (84). Abnormal aggregation of Aβ is one
of the core pathological features of AD and its toxicity can lead
to neuronal dysfunction and death. Secondly, carnosine, through its
powerful antioxidant effect, can clear free radicals in neurons and
reduce the damage of neurons caused by oxidative stress (63). Oxidative stress plays a key role
in the pathogenesis of AD, where excess free radicals attack the
lipid membranes, proteins and DNA of neurons, leading to the
destruction of cell structure and function. In addition, carnosine
can also inhibit the inflammatory response of neurons and reduce
the release of inflammatory factors (such as TNF-α and IL-6), thus
reducing inflammatory damage to neurons (28). Neuroinflammation is another
important pathological feature of AD, and excessive release of
inflammatory factors aggravates neuronal damage and cognitive
decline.
PD is a neurodegenerative disease characterized by
loss of dopaminergic neurons, characterized by motor dysfunction
and cognitive decline (85).
Studies have shown that carnosine plays an important role in the
treatment of PD and can delay the progression of the disease
through a variety of mechanisms. Firstly, carnosine, through its
powerful antioxidant effect, can clear free radicals in
dopaminergic neurons and reduce the damage of neurons caused by
oxidative stress (86).
Oxidative stress is one of the core pathological mechanisms of PD.
Free radicals generated during dopamine metabolism attack neurons,
leading to the destruction of cell structure and function.
Secondly, carnosine can inhibit the inflammatory response of
dopaminergic neurons and reduce the release of inflammatory factors
(such as TNF-α and IL-6), thereby reducing inflammatory damage to
neurons (87). Neuroinflammation
plays an important role in the pathogenesis of PD, and excessive
release of inflammatory factors can aggravate neuronal injury and
motor dysfunction. In addition, carnosine can regulate the release
and metabolism of dopamine and maintain the balance of
neurotransmitters, protecting the function of dopaminergic neurons.
The imbalance of dopamine metabolism is an important pathological
feature of PD. Experimental studies report that carnosine
ameliorates motor deficits in PD models, associated with increased
dopamine or dopamine metabolite levels (88).
Diabetes is a metabolic disease characterized by
high blood sugar, and long-term high blood sugar can lead to a
variety of complications, including vascular disease, and
neuropathy and kidney disease (89). Studies have shown that carnosine
plays an important role in the treatment of diabetes and can delay
the progression of complications through multiple mechanisms.
Firstly, carnosine, through its anti-glycation effect, can inhibit
the non-enzymatic reaction between proteins and sugars and reduce
the formation of AGEs (90). The
accumulation of AGEs is a core pathological mechanism of diabetic
complications, which can lead to vascular sclerosis, neurological
dysfunction and glomerular injury. Secondly, carnosine, through its
powerful antioxidant effect, can clear free radicals and reduce the
damage of blood vessels and nerves caused by oxidative stress
(77). Oxidative stress plays a
key role in the pathogenesis of diabetes complications, as excess
free radicals attack vascular endothelial cells and nerve cells,
leading to their dysfunction and structural destruction. In
addition, carnosine can directly protect blood vessels and nerves
from the damage of hyperglycemia and maintain their physiological
function. For instance, carnosine inhibits hyperglycemia-induced
apoptosis of vascular endothelial cells and neurofibrosis, and thus
delays the progression of diabetic complications (91).
Diabetic nephropathy is one of the major
complications of diabetes, characterized by glomerular sclerosis
and progressive decline in kidney function, which may eventually
lead to end-stage renal disease (92). Studies have shown that carnosine
plays an important role in the treatment of diabetic nephropathy
and can delay the progression of the disease through multiple
mechanisms. First, carnosine can clear free radicals in the
glomeruli and reduces damage caused by oxidative stress to the
glomeruli (93). Oxidative
stress is one of the core pathological mechanisms of diabetic
nephropathy. Free radicals induced by hyperglycemia attack
glomerular cells, leading to the destruction of their structure and
function. Secondly, carnosine can inhibit the inflammatory response
of the glomeruli and reduce the release of inflammatory factors
(such as TNF-α and IL-6), and thus reduces inflammatory damage to
the glomeruli. Chronic inflammation plays an important role in the
pathogenesis of diabetic nephropathy. Excessive release of
inflammatory factors may aggravate glomerular sclerosis and renal
function decline (94,95). In addition, carnosine can
directly protect the structure and function of the glomeruli,
inhibit apoptosis and fibrosis induced by hyperglycemia, and delay
the deterioration of renal function (96,97). For instance, carnosine can reduce
the thickening of the glomerular basement membrane and the
expansion of the mesangial matrix, maintaining glomerular
filtration function (98).
Atherosclerosis is a type of cardiovascular disease
characterized by lipid deposition and chronic inflammatory response
in blood vessel walls, and is the primary pathological basis of
cardiovascular and cerebrovascular events such as myocardial
infarction and stroke (99).
Studies have shown that carnosine plays an important role in the
treatment of atherosclerosis and can delay the progression of the
disease through multiple mechanisms. First, carnosine, through its
antioxidant effect, clears free radicals in the blood vessel wall
and reduces oxidative stress damage to vascular endothelial cells
(100). Oxidative stress is one
of the core pathological mechanisms of atherosclerosis. Oxidative
modification of low-density lipoprotein promotes the formation of
foam cells and lipid deposition in blood vessel walls. Secondly,
carnosine can inhibit the inflammatory response of the blood vessel
wall and reduce the release of inflammatory factors, thereby
reducing the damage of inflammation to the blood vessel wall
(74). Chronic inflammation
plays a key role in the pathogenesis of atherosclerosis, with
excessive release of inflammatory factors exacerbating vascular
endothelial dysfunction and plaque formation. In addition,
carnosine can directly protect the structure and function of the
blood vessel wall, inhibit the abnormal proliferation and migration
of vascular smooth muscle cells and thus maintain the normal
physiological function of blood vessels (101).
Myocardial ischemia-reperfusion injury is one of the
primary complications in the treatment of myocardial infarction.
Its pathological process involves several complex mechanisms such
as oxidative stress, inflammation and apoptosis, which ultimately
lead to the dysfunction and death of cardiomyocytes (102). Studies have shown that
carnosine plays an important role in the treatment of myocardial
ischemia-reperfusion injury and can alleviate myocardial injury
through multiple mechanisms. Firstly, carnosine, through its
antioxidant effect, clears free radicals in cardiomyocytes and
reduces oxidative stress damage to cardiomyocytes (103-105). During ischemia-reperfusion, a
large number of free radicals are produced, which attack the lipid
membrane, protein and DNA of cardiomyocytes, destroying cell
structure and function. Secondly, carnosine can inhibit the
inflammatory response of cardiomyocytes and reduce the release of
inflammatory factors, thus reducing inflammatory damage to
cardiomyocytes. Inflammatory response plays a key role in
ischemia-reperfusion injury. Excessive release of inflammatory
factors can aggravate apoptosis and necrosis of cardiomyocytes. In
addition, carnosine can directly protect the structure and function
of cardiomyocytes, inhibit apoptosis and mitochondrial dysfunction,
and thus maintain the systolic and diastolic functions of
myocardium (7).
The occurrence and development of cancer are closely
related to oxidative stress and inflammatory response, which not
only promote the proliferation and metastasis of cancer cells, but
also lead to damage to normal tissues (106). Research has shown that
carnosine plays an important role in cancer treatment, delaying
cancer progression and reducing treatment side effects caused by
treatments through multiple mechanisms. First, carnosine, through
its antioxidant effect, can clear free radicals in cancer cells and
reduce oxidative stress damage to cancer cells (107). Oxidative stress plays a key
role in the development and progression of cancer, and excess free
radicals can damage DNA, leading to genetic mutations and
carcinogenesis. Secondly, carnosine can inhibit the inflammatory
response of cancer cells and reduce the release of inflammatory
factors, thus reducing the promoting effect of inflammation on
cancer cells (108). Chronic
inflammation is an important driver of cancer, and excessive
release of inflammatory factors will promote the formation of the
tumour microenvironment and invasion of cancer cells. In addition,
carnosine protects normal cells from damage caused by oxidative
stress and inflammatory responses, reducing side effects caused by
cancer treatments such as radiation and chemotherapy. For instance,
carnosine reduces the oxidative damage to normal tissues caused by
radiotherapy and the toxicity of chemotherapy drugs to healthy
cells (109,110).
Studies have shown that carnosine exerts anti-tumor
effects in preclinical settings, including in vitro and
animal models, where it inhibits the proliferation and migration of
cancer cells and induces apoptosis through various mechanisms.
However, its application in cancer therapy has not yet entered
routine clinical practice, and current evidence mainly derives from
experimental studies. To date, only limited clinical investigations
have been reported, and further trials are required to clarify its
translational potential. First, carnosine inhibits the
proliferation of cancer cells and slow the growth of tumours
(2). The mechanism involves
regulating the expression of cell cycle-related proteins, such as
inhibiting Cyclin D1 and upregulating cyclin suppressor proteins
(such as p21), and thus blocking the proliferation cycle of cancer
cells (111). Secondly,
carnosine can inhibit the migration of cancer cells and reduce
metastasis of tumours (112).
Carnosine reduces the invasion and migration of cancer cells by
inhibiting the activity of matrix metalloproteinases and
downregulating molecules associated with epithelial to mesenchymal
transition, such as N-cadherin and vimentin (113,114). In addition, carnosine can
induce apoptosis of cancer cells and reduce tumor volume (115). The mechanisms include
activation of mitochondria-dependent apoptotic pathways,
upregulation of pro-apoptotic proteins (such as Bax) and
downregulation of anti-apoptotic proteins (such as Bcl-2), thus
promoting the programmed death of cancer cells (116).
Cataract is an ocular condition characterized by the
opacity of the lens, and its occurrence is closely related to
oxidative stress and glycation reactions, which eventually lead to
vision loss and even blindness (117). Studies have shown that
carnosine plays an important role in the treatment of cataracts and
can delay the progression of the disease through multiple
mechanisms. First, carnosine, through its antioxidant effect,
clears free radicals in the lens and reduces oxidative stress
damage to lens proteins and lipids (118). Oxidative stress is one of the
core pathological mechanisms of cataracts, and excessive free
radicals attack the lens cells, leading to protein denaturation and
lipid peroxidation. Secondly, carnosine can inhibit the glycation
reaction of the lens and reduce the formation of AGEs (119). The accumulation of AGEs can
lead to cross-linking and structural changes in lens proteins,
which can lead to opacity. In addition, carnosine can directly
protect the structure and function of the lens, maintaining its
transparency and refractive properties. For instance, carnosine
stabilizes the conformation of lens proteins, preventing their
denaturation and aggregation (120).
Macular degeneration is an ocular disease
characterized by damage to the macular area of the retina (121). The pathological process
involves multiple mechanisms such as oxidative stress, inflammatory
response and vascular abnormalities, which ultimately lead to loss
of central vision. Studies have shown that carnosine plays an
important role in the treatment of macular degeneration and can
delay the progression of the disease through multiple mechanisms.
Firstly, carnosine, through its antioxidant effect, clears free
radicals in the retina and reduces the damage of retinal pigment
epithelial cells and photoreceptor cells caused by oxidative stress
(122,123). Oxidative stress is one of the
core pathological mechanisms of macular degeneration, in which
excess free radicals attack retinal cells, leading to their
dysfunction and death. Secondly, carnosine inhibits inflammatory
responses in the retina and reduces the release of inflammatory
factors, thus reducing inflammatory damage to the retina. Chronic
inflammation plays a key role in the pathogenesis of macular
degeneration, with excessive release of inflammatory factors
exacerbating retinal cell apoptosis and vascular abnormalities. In
addition, carnosine can directly protect the structure and function
of the retina, inhibit the degeneration of retinal pigment
epithelial cells and the loss of photoreceptor cells, and maintain
the normal physiological function of the retina (124,125).
To provide a balanced and comprehensive overview,
the clinical studies investigating carnosine and its derivatives
across different disease contexts are summarized in Table I (90,126-155). At present, existing clinical
studies on carnosine can mainly be categorized into three groups
based on the form of administration: i) Carnosine alone; ii)
complexes containing carnosine; and iii) carnosine as an adjunct
therapy. Most studies are randomized controlled trials,
predominantly small-scale in design. The administered dosage ranges
from 400 mg to 4 g, with an observation period of 2-3 months. The
therapeutic effects vary across different diseases, as detailed in
Table I. The table includes both
positive and negative findings, thus addressing not only the
reported therapeutic benefits but also inconclusive or
contradictory outcomes. There are certain limitations in the
research on carnosine: First, certain studies report contradictory
results. Second, several studies lack endpoints with direct
clinical relevance. Additionally, carnosine was often administered
in combination formulations, making it difficult to isolate its
individual effects. Finally, larger, well-designed clinical trials
are required to confirm its efficacy across different disease
contexts. Overall, the current clinical evidence remains limited in
scale and rigor, underscoring the need for larger, well-controlled
trials to establish the therapeutic value and safety profile of
carnosine in human disease.
Preclinical studies have explored a range of
carnosine dosages across various disease models, typically between
50-500 mg/kg body weight in rodents, depending on the route of
administration and experimental design. However, in certain cases,
the dosage can be as low as 10 mg/kg, or even reach ≥1,000 mg/kg
(156,157). In neurodegenerative models,
oral or intraperitoneal doses of 100-250 mg/kg have shown
significant antioxidative and anti-aggregation effects (158,159). In cardiovascular models, the
effective treatment doses vary. A dose of 250 mg/kg significantly
reduces oxidative stress in the myocardium of mice with
isoproterenol-induced myocardial infarction, whereas a dose of 10
mg/kg is used to modulate the circadian resetting of clock genes in
the rat heart (157,160). In clinical trials, carnosine is
currently administered at doses ranging from 500 mg to 2 g/day. In
a 14-week study on prediabetes and type 2 diabetes for glycemic
control, oral supplementation with 2 g/day of carnosine
significantly reduced blood glucose levels at 90 and 120 min during
an oral glucose tolerance test, as well as the total area under the
glucose curve (126). In
patients with major depressive disorder receiving 400 mg of
L-carnosine twice daily, symptoms showed significant improvement
compared to the control group (149).
The majority of studies suggest that oral carnosine
is safe within the commonly used dose range (0.1-2 g) (161). However, certain studies
reported a higher incidence of adverse events compared to placebo
(30 vs. 14%) (152). Despite
this, the adverse effects were generally mild and manageable,
indicating that carnosine supplementation is overall
well-tolerated.
As a naturally occurring dipeptide, carnosine has
shown wide potential for the treatment of diseases due to its
multiple biological functions (such as antioxidant, anti-glycation,
neuroprotective, anti-inflammatory and immunomodulatory). Its
advantage lies in its multi-target mechanism of action, which can
simultaneously act on multiple pathological links such as free
radicals, inflammatory factors and glycation reaction, thus playing
a comprehensive protective role in a variety of diseases. For
instance, in neurodegenerative diseases, carnosine is not only able
to clear free radicals from neurons and reduce oxidative stress,
but also inhibits inflammatory responses and regulates
neurotransmitters, thereby delaying disease progression. In
diabetes and its complications, carnosine reduces the formation of
AGEs by inhibiting the glycation reaction and antioxidant effects,
and protects blood vessel and nerve function. In addition,
carnosine has also demonstrated significant therapeutic effects in
cardiovascular diseases, cancer and ocular diseases, showing its
broad therapeutic prospects. Another significant advantage of
carnosine is its high safety profile. As an endogenous substance,
carnosine has a clear metabolic pathway in the body, fewer side
effects and is suitable for long-term use (162). This gives carnosine a unique
advantage in the treatment of chronic diseases such as diabetes, AD
and atherosclerosis.
However, although carnosine has shown notable
potential in the treatment of diseases, its practical application
still faces certain challenges and limitations. First, the low
bioavailability of carnosine is one of the major factors limiting
its clinical application (30).
Carnosine is easily degraded by enzymes (such as carnosinase) in
the body, resulting in a short half-life and difficulty in reaching
effective concentrations in target tissues. Secondly, the efficacy
of carnosine is dose-dependent, and high doses may result in
certain side effects, such as gastrointestinal discomfort or
metabolic burden. In addition, the current clinical data on
carnosine remain insufficient; most studies are still in the basic
experimental stage and there is a lack of translational or
large-scale, multi-center clinical trial data (Table I). Thus, carnosine faces certain
scientific and normative hurdles before it can be adopted for
clinical use.
Despite these challenges, the future prospects of
carnosine in disease treatment remain promising. First, the
bioavailability and stability of carnosine can be improved through
pharmacochemical modification or nanotechnology. For instance,
combining carnosine with carrier molecules or developing
sustained-release formulations can extend its action time in
vivo and increase the drug concentration in target tissues
(29). Second, combining the
concept of precision medicine and developing a personalized
carnosine treatment program can further improve the treatment
effect and reduce side effects. For instance, genetic testing and
metabolomics analysis are used to screen out patient populations
that are sensitive to carnosine therapy and optimize dosage and
administration. In addition, carnosine combined with other drugs or
therapies also has broad prospects. For instance, in cancer
treatment, carnosine can be used in combination with
chemotherapeutic drugs or immunotherapy to reduce the side effects
of treatment through its antioxidant and anti-inflammatory effects
while enhancing anti-tumor effects. In neurodegenerative diseases,
carnosine can be used in combination with neurotrophic factors or
anti-inflammatory drugs to play a synergistic therapeutic role.
In the future, large-scale clinical trials are key
for establishing the clinical application of carnosine. Through
multi-center, randomized controlled clinical trials, the safety and
efficacy of carnosine in different diseases can be systematically
evaluated to provide a scientific basis for its clinical
application. In addition, further research on the mechanism of
carnosine and exploring its novel targets and novel means in the
treatment of diseases will also open up new directions for its
application. For instance, studying the role of carnosine in
epigenetic regulation, mitochondrial function repair and immune
microenvironment regulation may provide new ideas for its
application in complex diseases such as cancer and autoimmune
diseases.
As a natural dipeptide with multiple biological
functions, carnosine has shown wide application potential in the
treatment of diseases. In the present review, the research progress
on carnosine for neurodegenerative diseases, diabetes and its
complications, cardiovascular diseases, several types of cancer and
ophthalmic diseases was systematically reviewed, highlighting its
unique mechanism of action in antioxidant, anti-glycation,
neuroprotective, anti-inflammatory and immunomodulatory aspects.
Combining basic experiments and clinical studies, carnosine not
only provides novel ideas for the treatment of several complex
diseases, but also lays the foundation for its application in
precision medicine and personalized therapy. However, despite
significant advances in carnosine research, issues such as its
bioavailability, dose optimization and long-term safety in clinical
applications still need to be further explored. In the future,
carnosine may play a greater role in disease treatment through
pharmacochemical modification, nanotechnology and combined
application with other therapeutic means. Similarly, large-scale,
multicenter clinical trials will be a key step in moving carnosine
from the laboratory to the clinic. Overall, carnosine research will
not only provide a novel strategy for disease treatment, but may
also serve as a model for the development and application of
natural active substances.
Not applicable.
CF conceived the present review. CF and YH designed
the review. YH, DX and LX wrote the manuscript, conducted the
literature investigation and interpreted the related literature,
and prepared the figures. YH, ZN and DX critically analyzed the key
knowledge in the present review. LX, CS and DX edited and revised
the manuscript. Data authentication is not applicable. All authors
have read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was funded by National Key R&D Program of China
(grant nos. 2023YFC2508601, 2023YFC2508604 and 2023YFC2508605),
Tongji University Medicine-X Interdisciplinary Research Initiative
(grant no. 2025-0554-ZD-08), Shanghai Hospital Development Center
Foundation (grant nos. SHDC22025208 and SHDC12024125), Clinical
Research Foundation of Shanghai Pulmonary Hospital (grant no.
LYRC202401), The Innovation Team Project of the Faculty of Chinese
Medicine Science, Guangxi University of Chinese Medicine (grant
nos. 2023CX001 and 2024ZZA004) and the Project for Enhancing Young
and Middle-aged Teacher's Research Basis Ability in Colleges of
Guangxi (grant no. 2025KY1124).
|
1
|
Kwiatkowski S, Kiersztan A and Drozak J:
Biosynthesis of carnosine and related dipeptides in vertebrates.
Curr Protein Pept Sci. 19:771–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maugeri S, Sibbitts J, Privitera A,
Cardaci V, Di Pietro L, Leggio L, Iraci N, Lunte SM and Caruso G:
The anti-cancer activity of the naturally occurring dipeptide
carnosine: Potential for breast cancer. Cells. 12:25922023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayes JD, Dinkova-Kostova AT and Tew KD:
Oxidative stress in cancer. Cancer Cell. 38:167–197. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng J, Zhong YF, Wu YP, Luo Z, Sun YM,
Wang GE, Kurihara H, Li YF and He RR: Carnosine attenuates
cyclophosphamide-induced bone marrow suppression by reducing
oxidative DNA damage. Redox Biol. 14:1–6. 2018. View Article : Google Scholar
|
|
5
|
Babizhayev MA, Seguin MC, Gueyne J,
Evstigneeva RP, Ageyeva EA and Zheltukhina GA: L-carnosine
(beta-alanyl-L-histidine) and carcinine (beta-alanylhistamine) act
as natural antioxidants with hydroxyl-radical-scavenging and
lipid-peroxidase activities. Biochem J. 304:509–516. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Posa DK, Miller J, Hoetker D, Ramage MI,
Gao H, Zhao J, Doelling B, Bhatnagar A, Wigmore SJ, Skipworth RJE
and Baba SP: Skeletal muscle analysis of cancer patients reveals a
potential role for carnosine in muscle wasting. J Cachexia
Sarcopenia Muscle. 14:1802–1814. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao J, Posa DK, Kumar V, Hoetker D, Kumar
A, Ganesan S, Riggs DW, Bhatnagar A, Wempe MF and Baba SP:
Carnosine protects cardiac myocytes against lipid peroxidation
products. Amino Acids. 51:123–138. 2019. View Article : Google Scholar :
|
|
8
|
Freund MA, Chen B and Decker EA: The
inhibition of advanced glycation end products by carnosine and
other natural dipeptides to reduce diabetic and age-related
complications. Compr Rev Food Sci Food Saf. 17:1367–1378. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moulahoum H, Sanli S, Timur S and
Zihnioglu F: Potential effect of carnosine encapsulated niosomes in
bovine serum albumin modifications. Int J Biol Macromol.
137:583–591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boldyrev AA, Aldini G and Derave W:
Physiology and pathophysiology of carnosine. Physiol Rev.
93:1803–1845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bell SM, Hariharan R, Laud P, Majid A and
Courten B: Carnosine supplementation improves delayed recall: A
systematic review and meta-analysis. Alzheimer's Dement.
19:e0710042023. View Article : Google Scholar
|
|
12
|
Caruso G, Benatti C, Musso N, Fresta CG,
Fidilio A, Spampinato G, Brunello N, Bucolo C, Drago F, Lunte SM,
et al: Carnosine protects macrophages against the toxicity of
Aβ1-42 oligomers by decreasing oxidative stress. Biomedicines.
9:4772021. View Article : Google Scholar
|
|
13
|
Caruso G, Godos J, Castellano S, Micek A,
Murabito P, Galvano F, Ferri R, Grosso G and Caraci F: The
therapeutic potential of carnosine/anserine supplementation against
cognitive decline: A systematic review with meta-analysis.
Biomedicines. 9:2532021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cardaci V, Di Pietro L, Zupan MC, Sibbitts
J, Privitera A, Lunte SM, Caraci F, Hartley MD and Caruso G:
Characterizing oxidative stress induced by Aβ oligomers and the
protective role of carnosine in primary mixed glia cultures. Free
Radic Biol Med. 229:213–224. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corona C, Frazzini V, Silvestri E,
Lattanzio R, La Sorda R, Piantelli M, Canzoniero LMT, Ciavardelli
D, Rizzarelli E and Sensi SL: P2-452: Effects of dietary
supplementation of carnosine on mitochondrial dysfuncti2on, amyloid
pathology, and cognitive deficits in 3xTg-AD mice. PLoS One.
6:e179712011. View Article : Google Scholar
|
|
16
|
Costa R, Speretta E, Saraiva MJ, Crowther
DC and Cardoso I: P2-453: Testing the therapeutic potential of
doxycycline in a drosophila melanogaster model of Alzheimer's
disease. Alzheimer's Dement. 7:S4582011. View Article : Google Scholar
|
|
17
|
Keykhaee M, Rahimifard M, Najafi A, Baeeri
M, Abdollahi M, Mottaghitalab F, Farokhi M and Khoobi M:
Alginate/gum arabic-based biomimetic hydrogel enriched with
immobilized nerve growth factor and carnosine improves diabetic
wound regeneration. Carbohydr Polym. 321:1211792023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu J, Lv D, Lin W, Mao Y, Xia Y, Feng L,
Zhao T, Mao X, Shu F and Guo H: Chronic exposure to liquid crystal
monomer EBCN at environmentally relevant concentrations induces
testicular dysfunction via the gut-testis axis. J Hazard Mater.
486:1370332025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu T, Xu X, Liu Y, Wang X, Wu S, Qiu Z,
Liu X, Pan X, Gu C, Wang S, et al: Multi-omics signatures reveal
genomic and functional heterogeneity of Cutibacterium acnes in
normal and diseased skin. Cell Host Microbe. 32:1129–1146.e8. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lei S, Zhang Z, Wang J, Yu X, Jiang J,
Wang Y, Fu S, Shi J, Tang G and Wang S: Carnosine-copper
chelator-modified small-diameter vascular grafts for the promotion
of anticoagulation and endothelial regeneration. Chem Eng J.
493:1524682024. View Article : Google Scholar
|
|
21
|
Calabrese V, Scuto M, Salinaro AT,
Dionisio G, Modafferi S, Ontario ML, Greco V, Sciuto S, Schmitt CP,
Calabrese EJ and Peters V: Hydrogen sulfide and carnosine:
Modulation of oxidative stress and inflammation in kidney and brain
axis. Antioxidants (Basel). 9:13032020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang J, Wang T, Yu D, Fang X, Fan H and
Liu Q, Yi G, Yi X and Liu Q: l-Homocarnosine attenuates
inflammation in cerebral ischemia-reperfusion injury through
inhibition of nod-like receptor protein 3 inflammasome. Int J Biol
Macromol. 118:357–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YT, Hsu CC, Lin MH, Liu KS and Yin MC:
Histidine and carnosine delay diabetic deterioration in mice and
protect human low density lipoprotein against oxidation and
glycation. Eur J Pharmacol. 513:145–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsai SJ, Kuo WW, Liu WH and Yin MC:
Antioxidative and anti-inflammatory protection from carnosine in
the striatum of MPTP-treated mice. J Agric Food Chem.
58:11510–11516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Swietach P, Jäättelä M, Pillon-Thomas S
and Boedtkjer E: Carnosine facilitates lysosomal release of
inhibitors of T cell surveillance. Cell Metab. 36:461–462. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
No authors listed. Carnosine helps cancer
cells to evade immune surveillance by regulating intracellular pH.
Nat Immunol. 25:399–400. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garofalo M, Iovine B, Kuryk L, Capasso C,
Hirvinen M, Vitale A, Bevilacqua MA and Cerullo V: 622. Oncolytic
adenoviruses loaded with active drugs as a novel drug delivery
system for cancer therapy. Mol Ther. 23(Suppl 1): S2472015.
View Article : Google Scholar
|
|
28
|
Caruso G, Caraci F and Jolivet RB: Pivotal
role of carnosine in the modulation of brain cells activity:
Multimodal mechanism of action and therapeutic potential in
neurodegenerative disorders. Prog Neurobiol. 175:35–53. 2019.
View Article : Google Scholar
|
|
29
|
Russo S, Privitera A, Greco G, Di Pietro
L, Cardaci V, Carota G, Sarpietro MG and Caruso G: Development and
in vitro characterization of new carnosine-loaded liposomal
formulations. J Liposome Res. 35:117–124. 2025. View Article : Google Scholar
|
|
30
|
Bonaccorso A, Privitera A, Grasso M,
Salamone S, Carbone C, Pignatello R, Musumeci T, Caraci F and
Caruso G: The therapeutic potential of novel carnosine
formulations: Perspectives for drug development. Pharmaceuticals
(Basel). 16:7782023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jain S, Kim ES, Kim D, Burrows D, De
Felice M, Kim M, Baek SH, Ali A, Redgrave J, Doeppner TR, et al:
Comparative cerebroprotective potential of d- and l-carnosine
following ischemic stroke in mice. Int J Mol Sci. 21:30532020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim ES, Kim D, Nyberg S, Poma A, Cecchin
D, Jain SA, Kim KA, Shin YJ, Kim EH, Kim M, et al: LRP-1
functionalized polymersomes enhance the efficacy of carnosine in
experimental stroke. Sci Rep. 10:6992020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma H, Zhao J, Meng H, Hu D, Zhou Y, Zhang
X, Wang C, Li J, Yuan J and Wei Y: Carnosine-modified fullerene as
a highly enhanced ROS scavenger for mitigating acute oxidative
stress. ACS Appl Mater Interfaces. 12:16104–16113. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dash UC, Bhol NK, Swain SK, Samal RR,
Nayak PK, Raina V, Panda SK, Kerry RG, Duttaroy AK and Jena AB:
Oxidative stress and inflammation in the pathogenesis of
neurological disorders: Mechanisms and implications. Acta Pharm Sin
B. 15:15–34. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kunsch C and Medford RM: Oxidative stress
as a regulator of gene expression in the vasculature. Circ Res.
85:753–766. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sack MN, Fyhrquist FY, Saijonmaa OJ,
Fuster V and Kovacic JC: Basic biology of oxidative stress and the
cardiovascular system: Part 1 of a 3-part series. J Am Coll
Cardiol. 70:196–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan Z and Spaulding HR: Extracellular
superoxide dismutase, a molecular transducer of health benefits of
exercise. Redox Biol. 32:1015082020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ansari FA, Ali SN and Mahmood R:
P-28-Protective effect of carnosine on sodium nitrite-induced
oxidative stress and DNA damage in rat intestine. Free Radic Biol
Med. 96(Suppl 1): S442016. View Article : Google Scholar
|
|
39
|
Husain N and Mahmood R: P
013-Copper(II)-induced cytotoxicity and oxidative stress in human
blood cells and its attenuation by carnosine. Free Radic Biol Med.
108(Suppl 1): S212017. View Article : Google Scholar
|
|
40
|
Di Giulio T, Barca A, Verri T, De Gennaro
M, Giancane G, Giancane E and Mazzotta C: Molecular imprinting
based on metal-ion mediated recognition: Electrosynthesis of
artificial receptors for the selective detection of peptides. Sens
Actuators B Chem. 383:1335892023. View Article : Google Scholar
|
|
41
|
Jukić I, Kolobarić N, Stupin A, Matić A,
Kozina N, Mihaljević Z, Mihalj M, Šušnjara P, Stupin M, Ćurić ŽB,
et al: Carnosine, small but mighty-prospect of use as functional
ingredient for functional food formulation. Antioxidants (Basel).
10:10372021. View Article : Google Scholar
|
|
42
|
Pasternack RF and Kustin K: The reactions
of L-carnosine with metal ions. Copper(II). J Am Chem Soc.
90:2295–2299. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Velez S, Nair NG and Reddy VP: Transition
metal ion binding studies of carnosine and histidine: Biologically
relevant antioxidants. Colloids Surf B Biointerfaces. 66:291–294.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Berdaweel I, Mahoney J, Alowaisi A, Berns
K and Anderson E: Oral carnosine supplementation restores insulin
sensitivity but not glucose tolerance in mice with glutathione
peroxidase-4 deficiency. Physiology. 38:S57959842023. View Article : Google Scholar
|
|
45
|
Kopec W, Jamroz D, Wiliczkiewicz A, Biazik
E, Pudlo A, Korzeniowska M, Hikawczuk T and Skiba T: Antioxidative
characteristics of chicken breast meat and blood after diet
supplementation with carnosine, L-histidine, and β-alanine.
Antioxidants (Basel). 9:10932020. View Article : Google Scholar
|
|
46
|
Zhao J, Shi L and Zhang LR:
Neuroprotective effect of carnosine against salsolinol-induced
Parkinson's disease. Exp Ther Med. 14:664–670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chatham JC and Patel RP: Protein
glycosylation in cardiovascular health and disease. Nat Rev
Cardiol. 21:525–544. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu YT, Che Y, Qiu HL, Feng YZ, Deng JY,
Yuan Y and Tang QZ: ADP-ribosylation: An emerging direction for
disease treatment. Ageing Res Rev. 94:1021762024. View Article : Google Scholar
|
|
49
|
Ng BG and Freeze HH: Perspectives on
glycosylation and its congenital disorders. Trends Genet.
34:466–476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Trujillo MN and Galligan JJ: Reconsidering
the role of protein glycation in disease. Nat Chem Biol.
19:922–927. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hipkiss AR, Brownson C and Carrier MJ:
Carnosine, the anti-ageing, anti-oxidant dipeptide, may react with
protein carbonyl groups. Mech Ageing Dev. 122:1431–1445. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Menini S, Iacobini C, Fantauzzi CB and
Pugliese G: L-carnosine and its derivatives as new therapeutic
agents for the prevention and treatment of vascular complications
of diabetes. Curr Med Chem. 27:1744–1763. 2020. View Article : Google Scholar
|
|
53
|
Aiello G, Rescigno F, Meloni M, Zoanni B,
Aldini G, Carini M and D'Amato A: The effect of carnosine on
UVA-induced changes in intracellular signaling of human skin
fibroblast spheroids. Antioxidants (Basel). 12:3002023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lavilla C Jr, Billacura MP, Hanna K,
Boocock DJ, Coveney C, Miles AK, Foulds GA, Murphy A, Tan A,
Jackisch L, et al: Carnosine protects stimulus-secretion coupling
through prevention of protein carbonyl adduction events in cells
under metabolic stress. Free Radic Biol Med. 175:65–79. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li X, Yang K, Gao S, Zhao J, Liu G, Chen
Y, Lin H, Zhao W, Hu Z and Xu N: Carnosine stimulates
macrophage-mediated clearance of senescent skin cells through
activation of the AKT2 signaling pathway by CD36 and RAGE. Front
Pharmacol. 11:5938322020. View Article : Google Scholar :
|
|
56
|
Boldyrev AA: Carnosine and free-radical
defence mechanisms. Trends Neurosci. 17:4681994. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liew WC, Sun S, Tang M, Sudarsono N and Li
C: 50553 Combination of retinol, carnosine, and soothing
ingredients delivers anti-aging benefits with antioxidative and
anti-glycation protection. J Am Acad Dermatol. 91(Suppl 1):
AB1622024. View Article : Google Scholar
|
|
58
|
Caruso G, Fresta CG, Fidilio A, O'Donnell
F, Musso N, Lazzarino G, Grasso M, Amorini AM, Tascedda F, Bucolo
C, et al: Carnosine decreases PMA-induced oxidative stress and
inflammation in murine macrophages. Antioxidants (Basel).
8:2812019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fresta CG, Hogard ML, Caruso G, Melo Costa
EE, Lazzarino G and Lunte SM: Monitoring carnosine uptake by RAW
264.7 macrophage cells using microchip electrophoresis with
fluorescence detection. Anal Methods. 9:402–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Husain N and Mahmood R: Mitigation of
Cu(II)-induced damage in human blood cells by carnosine: An in
vitro study. Toxicol In Vitro. 68:1049562020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Miceli V, Pampalone M, Frazziano G, Grasso
G, Rizzarelli E, Ricordi C, Casu A, Iannolo G and Conaldi PG:
Carnosine protects pancreatic beta cells and islets against
oxidative stress damage. Mol Cell Endocrinol. 474:105–118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ooi TC, Chan KM and Sharif R: Zinc
carnosine inhibits lipopolysaccharide-induced inflammatory
mediators by suppressing NF-κb activation in raw 264.7 macrophages,
independent of the MAPKs signaling pathway. Biol Trace Elem Res.
172:458–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Caruso G, Fresta CG, Musso N, Giambirtone
M, Grasso M, Spampinato SF, Merlo S, Drago F, Lazzarino G, Sortino
MA, et al: Carnosine prevents Aβ-induced oxidative stress and
inflammation in microglial cells: A key role of TGF-β1. Cells.
8:642019. View Article : Google Scholar
|
|
64
|
Diniz F, Parmeggiani B, Brandão G,
Ferreira BK, Teixeira MF, Streck EL, Olivera-Bravo S, Barbeito LH,
Schuck PF, de Melo Reis RA and Ferreira GC: Dual effect of
carnosine on ROS formation in rat cultured cortical astrocytes. Mol
Neurobiol. 61:4908–4922. 2024. View Article : Google Scholar
|
|
65
|
Shen J, Xu J, Wen Y, Tang Z, Li J and Sun
J: Carnosine ameliorates postoperative cognitive dysfunction of
aged rats by limiting astrocytes pyroptosis. Neurotherapeutics.
21:e003592024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Trombley PQ, Horning MS and Blakemore LJ:
Carnosine modulates zinc and copper effects on amino acid receptors
and synaptic transmission. Neuroreport. 9:3503–3507. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Andersen JV, Schousboe A and Verkhratsky
A: Astrocyte energy and neurotransmitter metabolism in Alzheimer's
disease: Integration of the glutamate/GABA-glutamine cycle. Prog
Neurobiol. 217:1023312022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chern H, Caruso G, Desaire H and Jarosova
R: Carnosine mitigates cognitive impairment and dopamine release in
an okadaic acid-induced zebrafish model with Alzheimer's
disease-like symptoms. ACS Chem Neurosci. 16:790–801. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shen Y, He P, Fan YY, Zhang JX, Yan HJ, Hu
WW, Ohtsu H and Chen Z: Carnosine protects against permanent
cerebral ischemia in histidine decarboxylase knockout mice by
reducing glutamate excitotoxicity. Free Radic Biol Med. 48:727–735.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Banerjee S, Mukherjee B, Poddar MK and
Dunbar GL: Carnosine improves aging-induced cognitive impairment
and brain regional neurodegeneration in relation to the
neuropathological alterations in the secondary structure of amyloid
beta (Aβ). J Neurochem. 158:710–723. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Brown JM, Baker LS, Seroogy KB and Genter
MB: Intranasal carnosine mitigates α-synuclein pathology and motor
dysfunction in the Thy1-aSyn mouse model of Parkinson's disease.
ACS Chem Neurosci. 12:2347–2359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Naletova I, Greco V, Sciuto S, Attanasio F
and Rizzarelli E: Ionophore ability of carnosine and its trehalose
conjugate assists copper signal in triggering brain-derived
neurotrophic factor and vascular endothelial growth factor
activation in vitro. Int J Mol Sci. 22:135042021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Odashima M, Otaka M, Jin M, Wada I,
Horikawa Y, Matsuhashi T, Ohba R, Hatakeyama N, Oyake J and
Watanabe S: Zinc L-carnosine protects colonic mucosal injury
through induction of heat shock protein 72 and suppression of
NF-kappaB activation. Life Sci. 79:2245–2250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fresta CG, Fidilio A, Lazzarino G, Musso
N, Grasso M, Merlo S, Amorini AM, Bucolo C, Tavazzi B, Lazzarino G,
et al: Modulation of pro-oxidant and pro-inflammatory activities of
M1 macrophages by the natural dipeptide carnosine. Int J Mol Sci.
21:7762020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Trushina EN, Riger NA, Mustafina OK,
Timonin AN, Aksenov IV, Guseva GV and Tutelyan VA: Effect of
carnosine and α-lipoic acid on hepatocyte apoptosis and the
cytokine profile in induced fatty liver disease in Wistar rats.
Vopr Pitan. 89:6–16. 2020.In Russian.
|
|
76
|
Meng C, Zhi X, Li C, Li C, Chen Z, Qiu X,
Ding C, Ma L, Lu H, Chen D, et al: Graphene oxides decorated with
carnosine as an adjuvant to modulate innate immune and improve
adaptive immunity in vivo. ACS Nano. 10:2203–2213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Aldini G, de Courten B, Regazzoni L,
Gilardoni E, Ferrario G, Baron G, Altomare A, D'Amato A, Vistoli G
and Carini M: Understanding the antioxidant and carbonyl
sequestering activity of carnosine: direct and indirect mechanisms.
Free Radic Res. 55:321–330. 2021. View Article : Google Scholar
|
|
78
|
Jamshidzadeh A, Heidari R, Latifpour Z,
Ommati MM, Abdoli N, Mousavi S, Azarpira N, Zarei A, Zarei M, Asadi
B, et al: Carnosine ameliorates liver fibrosis and hyperammonemia
in cirrhotic rats. Clin Res Hepatol Gastroenterol. 41:424–434.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Creighton JV, de Souza Gonçalves L,
Artioli GG, Tan D, Elliott-Sale KJ, Turner MD, Doig CL and Sale C:
Physiological roles of carnosine in myocardial function and health.
Adv Nutr. 13:1914–1929. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Caruso G, Fresta CG, Martinez-Becerra F,
Antonio L, Johnson RT, de Campos RPS, Siegel JM, Wijesinghe MB,
Lazzarino G and Lunte SM: Carnosine modulates nitric oxide in
stimulated murine RAW 264.7 macrophages. Mol Cell Biochem.
431:197–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Park IG, Jin SH, An S, Ki MW, Park WS, Kim
HJ, Na Y and Noh M: Carnosine and retinol synergistically inhibit
UVB-induced PGE2 synthesis in human keratinocytes
through the up-regulation of hyaluronan synthase 2. Biomol Ther
(Seoul). 32:635–639. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Impellizzeri D, Siracusa R, Cordaro M,
Peritore AF, Gugliandolo E, D'amico R, Fusco R, Crupi R, Rizzarelli
E, Cuzzocrea S, et al: Protective effect of a new hyaluronic
acid-carnosine conjugate on the modulation of the inflammatory
response in mice subjected to collagen-induced arthritis. Biomed
Pharmacother. 125:1100232020. View Article : Google Scholar
|
|
83
|
Scheltens P, De Strooper B, Kivipelto M,
Holstege H, Chételat G, Teunissen CE, Cummings J and van der Flier
WM: Alzheimer's disease. Lancet. 397:1577–1590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang H, Dong X and Sun Y:
Carnosine-LVFFARK-NH2 conjugate: A moderate chelator but
potent inhibitor of Cu2+-mediated amyloid β-protein
aggregation. ACS Chem Neurosci. 9:2689–2700. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kalia LV and Lang AE: Parkinson's disease.
Lancet. 386:896–912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kulikova O, Troshev D, Berezhnoy D,
Stvolinsky S, Timoshina Y, Abaimov D, Muzychuk O, Latanov A and
Fedorova T: Neuroprotective efficacy of a nanomicellar complex of
carnosine and lipoic acid in a rat model of rotenone-induced
Parkinson's disease. Antioxidants (Basel). 12:12152023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kubota M, Kobayashi N, Sugizaki T, Shimoda
M, Kawahara M and Tanaka KI: Carnosine suppresses neuronal cell
death and inflammation induced by 6-hydroxydopamine in an in vitro
model of Parkinson's disease. PLoS One. 15:e02404482020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kulikova OI, Berezhnoy DS, Stvolinsky SL,
Lopachev AV, Orlova VS and Fedorova TN: Neuroprotective effect of
the carnosine-α-lipoic acid nanomicellar complex in a model of
early-stage Parkinson's disease. Regul Toxicol Pharmacol.
95:254–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cole JB and Florez JC: Genetics of
diabetes mellitus and diabetes complications. Nat Rev Nephrol.
16:377–390. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Houjeghani S, Kheirouri S, Faraji E and
Jafarabadi MA: l-Carnosine supplementation attenuated fasting
glucose, triglycerides, advanced glycation end products, and tumor
necrosis factor-α levels in patients with type 2 diabetes: a
double-blind placebo-controlled randomized clinical trial. Nutr
Res. 49:96–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shi Y and Zhang CJ: The effects of
carnosine on high glucose-induced apoptosis of human umbilical vein
endothelial cells. Adv Mat Res. 345:365–369. 2011.
|
|
92
|
Kanwar YS, Sun L, Xie P, Liu FY and Chen
S: A glimpse of various pathogenetic mechanisms of diabetic
nephropathy. Annu Rev Pathol. 6:395–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yay A, Akkuş D, Yapıslar H, Balcıoglu E,
Sonmez MF and Ozdamar S: Antioxidant effect of carnosine treatment
on renal oxidative stress in streptozotocin-induced diabetic rats.
Biotech Histochem. 89:552–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Peters V, Yard B and Schmitt CP: Carnosine
and diabetic nephropathy. Curr Med Chem. 27:1801–1812. 2020.
View Article : Google Scholar
|
|
95
|
Albrecht T, Schilperoort M, Zhang S, Braun
JD, Qiu J, Rodriguez A, Pastene DO, Krämer BK, Köppel H, Baelde H,
et al: Carnosine attenuates the development of both type 2 diabetes
and diabetic nephropathy in BTBR ob/ob mice. Sci Rep. 7:444922017.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhu W, Li YY, Zeng HX, Liu XQ, Sun YT,
Jiang L, Xia LL and Wu YG: Carnosine alleviates podocyte injury in
diabetic nephropathy by targeting caspase-1-mediated pyroptosis.
Int Immunopharmacol. 101:1082362021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang S, Li Y, Liu X, Guo S, Jiang L,
Huang Y and Wu Y: Carnosine alleviates kidney tubular epithelial
injury by targeting NRF2 mediated ferroptosis in diabetic
nephropathy. Amino Acids. 55:1141–1155. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu XQ, Jiang L, Lei L, Nie ZY, Zhu W,
Wang S, Zeng HX, Zhang SQ, Zhang Q, Yard B and Wu YG: Carnosine
alleviates diabetic nephropathy by targeting GNMT, a key enzyme
mediating renal inflammation and fibrosis. Clin Sci (Lond).
134:3175–3193. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47(Suppl 8): C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Feehan J, Hariharan R, Buckenham T,
Handley C, Bhatnagar A, Baba SP and de Courten B: Carnosine as a
potential therapeutic for the management of peripheral vascular
disease. Nutr Metab Cardiovasc Dis. 32:2289–2296. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hwang B, Song JH, Park SL, Kim JT, Kim WJ
and Moon SK: Carnosine impedes PDGF-stimulated proliferation and
migration of vascular smooth muscle cells in vitro and sprout
outgrowth ex vivo. Nutrients. 12:26972020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023. View Article : Google Scholar
|
|
103
|
Lee JW, Miyawaki H, Bobst EV, Hester JD,
Ashraf M and Bobst AM: Improved functional recovery of ischemic rat
hearts due to singlet oxygen scavengers histidine and carnosine. J
Mol Cell Cardiol. 31:113–121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Toufektsian MC, Morel S, Tanguy S, Jeunet
A, de Leiris J and Boucher F: Involvement of reactive oxygen
species in cardiac preconditioning in rats. Antioxid Redox Signal.
5:115–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhao J, Conklin DJ, Guo Y, Zhang X, Obal
D, Guo L, Jagatheesan G, Katragadda K, He L, Yin X, et al:
Cardiospecific overexpression of ATPGD1 (carnosine synthase)
increases histidine dipeptide levels and prevents myocardial
ischemia reperfusion injury. J Am Heart Assoc. 9:e0152222020.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar
|
|
107
|
Turner MD, Sale C, Garner AC and Hipkiss
AR: Anti-cancer actions of carnosine and the restoration of normal
cellular homeostasis. Biochim Biophys Acta Mol Cell Res.
1868:1191172021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ooi TC, Chan KM and Sharif R: Antioxidant,
anti-inflammatory, and genomic stability enhancement effects of
zinc l-carnosine: A potential cancer chemopreventive agent? Nutr
Cancer. 69:201–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Guney Y, Turkcu UO, Hicsonmez A, Andrieu
MN, Guney HZ, Bilgihan A and Kurtman C: Carnosine may reduce lung
injury caused by radiation therapy. Med Hypotheses. 66:957–959.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yanase K, Funaguchi N, Iihara H, Yamada M,
Kaito D, Endo J, Ito F, Ohno Y, Tanaka H, Itoh Y, et al: Prevention
of radiation esophagitis by polaprezinc (zinc L-carnosine) in
patients with non-small cell lung cancer who received
chemoradiotherapy. Int J Clin Exp Med. 8:16215–16222.
2015.PubMed/NCBI
|
|
111
|
Gaafar PME, El-Salamouni NS, Farid RM,
Hazzah HA, Helmy MW and Abdallah OY: Pegylated liquisomes: A novel
combined passive targeting nanoplatform of L-carnosine for breast
cancer. Int J Pharm. 602:1206662021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Aggarwal N, Singh G, Panda HS and Panda
JJ: Unravelling the potential of L-carnosine analog-based
nano-assemblies as pH-responsive therapeutics in treating glioma:
An in vitro perspective. J Mater Chem B. 12:10665–10681. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chuang CH and Hu ML: L-carnosine inhibits
metastasis of SK-Hep-1 cells by inhibition of matrix
metaoproteinase-9 expression and induction of an antimetastatic
gene, nm23-H1. Nutr Cancer. 60:526–533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wu CC, Lai PY, Hsieh S, Cheng CC and Hsieh
SL: Suppression of carnosine on adhesion and extravasation of human
colorectal cancer cells. Anticancer Res. 39:6135–6144. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lei L, Nan B, Yang F, Xu L, Guan G, Xu J,
Yue R, Wang Y, Huan S, Yin X, et al: Zinc-Carnosine metallodrug
network as dual metabolism inhibitor overcoming metabolic
reprogramming for efficient cancer therapy. Nano Lett.
23:2659–2668. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lee J, Park JR, Lee H, Jang S, Ryu SM, Kim
H, Kim D, Jang A and Yang SR: L-carnosine induces apoptosis/cell
cycle arrest via suppression of NF-κB/STAT1 pathway in HCT116
colorectal cancer cells. In Vitro Cell Dev Biol Anim. 54:505–512.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Asbell PA, Dualan I, Mindel J, Brocks D,
Ahmad M and Epstein S: Age-related cataract. Lancet. 365:599–609.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Dubois VD and Bastawrous A:
N-acetylcarnosine (NAC) drops for age-related cataract. Cochrane
Database Syst Rev. 2:CD0094932017.PubMed/NCBI
|
|
119
|
Babizhayev MA, Guiotto A and Kasus-Jacobi
A: N-Acetylcarnosine and histidyl-hydrazide are potent agents for
multitargeted ophthalmic therapy of senile cataracts and diabetic
ocular complications. J Drug Target. 17:36–63. 2009. View Article : Google Scholar
|
|
120
|
Liao JH, Lin IL, Huang KF, Kuo PT, Wu SH
and Wu TH: Carnosine ameliorates lens protein turbidity formations
by inhibiting calpain proteolysis and ultraviolet C-induced
degradation. J Agric Food Chem. 62:5932–5938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Guymer RH and Campbell TG: Age-related
macular degeneration. Lancet. 401:1459–1472. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Caruso G, Fresta CG, Fidilio A, Lazzara F,
Musso N, Cardaci V, Drago F, Caraci F and Bucolo C: Carnosine
counteracts the molecular alterations Aβ oligomers-induced in human
retinal pigment epithelial cells. Molecules. 28:33242023.
View Article : Google Scholar
|
|
123
|
Kim HG, Heo H, Sung MS and Park SW:
Carnosine decreases retinal ganglion cell death in a mouse model of
optic nerve crushing. Neurosci Lett. 711:1344312019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li S, Li H, Bennewitz K, Poschet G,
Buettner M, Hausser I, Szendroedi J, Nawroth PP and Kroll J:
Combined loss of glyoxalase 1 and aldehyde dehydrogenase 3a1
amplifies dicarbonyl stress, impairs proteasome activity resulting
in hyperglycemia and activated retinal angiogenesis. Metabolism.
165:1561492025. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Pfister F, Riedl E, Wang Q, vom Hagen F,
Deinzer M, Harmsen MC, Molema G, Yard B, Feng Y and Hammes HP: Oral
carnosine supplementation prevents vascular damage in experimental
diabetic retinopathy. Cell Physiol Biochem. 28:125–136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hariharan R, Cameron J, Menon K, Mesinovic
J, Jansons P, Scott D, Lu ZX, de Courten M, Feehan J and de Courten
B: Carnosine supplementation improves glucose control in adults
with pre-diabetes and type 2 diabetes: A randomised controlled
trial. Nutr Metab Cardiovasc Dis. 34:485–496. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
de Courten B, Jakubova M, de Courten MP,
Kukurova IJ, Vallova S, Krumpolec P, Valkovic L, Kurdiova T, Garzon
D, Barbaresi S, et al: Effects of carnosine supplementation on
glucose metabolism: Pilot clinical trial. Obesity (Silver Spring).
24:1027–1034. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Saadati S, de Courten M, Deceneux C,
Plebanski M, Scott D, Mesinovic J, Jansons P, Aldini G, Cameron J,
Feehan J, et al: Carnosine supplementation Has No effect on
inflammatory markers in adults with prediabetes and type 2
diabetes: A randomised controlled trial. Nutrients. 16:39002024.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Siriwattanasit N, Satirapoj B and
Supasyndh O: Effect of Oral carnosine supplementation on urinary
TGF-β in diabetic nephropathy: A randomized controlled trial. BMC
Nephrol. 22:2362021. View Article : Google Scholar
|
|
130
|
Saadati S, Jansons P, Scott D, de Courten
M, Mousa A, Feehan J, Mesinovic J and de Courten B: The effect of
carnosine supplementation on musculoskeletal health in adults with
prediabetes and type 2 diabetes: A secondary analysis of a
randomized controlled trial. Nutrients. 16:43282024. View Article : Google Scholar :
|
|
131
|
Saadati S, Cameron J, Menon K, Hodge A, Lu
ZX, de Courten M, Feehan J and de Courten B: Carnosine did not
affect vascular and metabolic outcomes in patients with prediabetes
and type 2 diabetes: A 14-week randomized controlled trial.
Nutrients. 15:48352023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Baye E, Ukropec J, de Courten MPJ, Mousa
A, Kurdiova T, Johnson J, Wilson K, Plebanski M, Aldini G,
Ukropcova B and de Courten B: Carnosine supplementation improves
serum resistin concentrations in overweight or obese otherwise
healthy adults: A pilot randomized trial. Nutrients. 10:12582018.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Baye E, Ukropec J, de Courten MP, Vallova
S, Krumpolec P, Kurdiova T, Aldini G, Ukropcova B and de Courten B:
Effect of carnosine supplementation on the plasma lipidome in
overweight and obese adults: A pilot randomised controlled trial.
Sci Rep. 7:174582017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Abraham DA, Narasimhan U, Mahalingam VT,
Krishnan M, Ganesan RM, Goh KW, Tan CS, Ming LC and Ardianto C:
Estimation of plasma concentration of L-carnosine and its
correlation with core symptoms of autism spectrum disorder
children: A pilot clinical trial. Front Biosci (Landmark Ed).
29:3652024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Mehrazad-Saber Z, Kheirouri S and Noorazar
SG: Effects of l-carnosine supplementation on sleep disorders and
disease severity in autistic children: A randomized, controlled
clinical trial. Basic Clin Pharmacol Toxicol. 123:72–77. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
O'Toole TE, Amraotkar AR, Gao H, Sears CG,
Rai SN, Basner M and Bhatnagar A: Carnosine supplementation
improves cognitive outcomes in younger participants of the NEAT
trial. Neurotherapeutics. 22:e005412025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zanini D, Jezdimirovic T, Stajer V,
Ostojic J, Maksimovic N and Ostojic SM: Dietary supplementation
with L-carnosine improves patient-reported outcomes, autonomic
nervous system performance, and brain metabolism in 3 adult
patients with multiple sclerosis. Nutr Res. 84:63–69. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Tharoor H, Maran S, Chandan AK, Pari M,
Rao S and Durairaj J: Cognitive and negative symptoms in
schizophrenia with L-Carnosine adjuvant therapy-A randomized
double-blind placebo-controlled study. Pharmacol Res Perspect.
11:e010742023. View Article : Google Scholar
|
|
139
|
Slowinska-Lisowska M, Zembron-Lacny A,
Rynkiewicz M, Rynkiewicz T and Kopec W: Influence of l-carnosine on
pro-antioxidant status in elite kayakers and canoeists. Acta
Physiol Hung. 101:461–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yehia R, Saleh S, El Abhar H, Saad AS and
Schaalan M: L-Carnosine protects against Oxaliplatin-induced
peripheral neuropathy in colorectal cancer patients: A perspective
on targeting Nrf-2 and NF-κB pathways. Toxicol Appl Pharmacol.
365:41–50. 2019. View Article : Google Scholar
|
|
141
|
Rondanelli M, Gasparri C, Cavioni A,
Sivieri C, Barrile GC, Mansueto F and Perna S: A Patented dietary
supplement (hydroxy-methyl-butyrate, carnosine, magnesium,
butyrate, lactoferrin) is a promising therapeutic target for
age-related sarcopenia through the regulation of gut permeability:
A randomized controlled trial. Nutrients. 16:13692024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Masuoka N, Yoshimine C, Hori M, Tanaka M,
Asada T, Abe K and Hisatsune T: Effects of anserine/carnosine
supplementation on mild cognitive impairment with APOE4. Nutrients.
11:16262019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Saldi S, Perrucci E, Fulcheri CPL,
Mariucci C, Chierchini S, Ingrosso G, Falcinelli L, Podlesko AM,
Merluzzi M, Bini V and Aristei C: Zinc-L-carnosine prevented
dysphagia in breast cancer patients undergoing adjuvant
radiotherapy: Results of a phase III randomized trial. Breast J.
26:1882–1884. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Itagaki M, Saruta M, Saijo H, Mitobe J,
Arihiro S, Matsuoka M, Kato T, Ikegami M and Tajiri H: Efficacy of
zinc-carnosine chelate compound, Polaprezinc, enemas in patients
with ulcerative colitis. Scand J Gastroenterol. 49:164–172. 2014.
View Article : Google Scholar
|
|
145
|
Sakae K, Agata T, Kamide R and Yanagisawa
H: Effects of L-carnosine and its zinc complex (Polaprezinc) on
pressure ulcer healing. Nutr Clin Pract. 28:609–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Mutolo MG, Albanese G, Rusciano D and
Pescosolido N: Oral administration of forskolin, homotaurine,
carnosine, and folic acid in patients with primary open angle
glaucoma: Changes in intraocular pressure, pattern
electroretinogram amplitude, and foveal sensitivity. J Ocul
Pharmacol Ther. 32:178–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Elbarbary NS, Ismail EAR, El-Naggar AR,
Hamouda MH and El-Hamamsy M: The effect of 12 weeks carnosine
supplementation on renal functional integrity and oxidative stress
in pediatric patients with diabetic nephropathy: A randomized
placebo-controlled trial. Pediatr Diabetes. 19:470–477. 2018.
View Article : Google Scholar
|
|
148
|
Lombardi C, Carubelli V, Lazzarini V,
Vizzardi E, Bordonali T, Ciccarese C, Castrini AI, Dei Cas A,
Nodari S and Metra M: Effects of oral administration of
orodispersible levo-carnosine on quality of life and exercise
performance in patients with chronic heart failure. Nutrition.
31:72–78. 2015. View Article : Google Scholar
|
|
149
|
Araminia B, Shalbafan M, Mortezaei A,
Shirazi E, Ghaffari S, Sahebolzamani E, Mortazavi SH, Shariati B,
Ardebili ME, Aqamolaei A, et al: L-Carnosine combination therapy
for major depressive disorder: A randomized, double-blind,
placebo-controlled trial. J Affect Disord. 267:131–136. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Arabzadeh S, Shahhossenie M, Mesgarpour B,
Rezaei F, Shalbafan MR, Ghiasi Z and Akhondzadeh S: L-carnosine as
an adjuvant to fluvoxamine in treatment of obsessive compulsive
disorder: A randomized double-blind study. Hum Psychopharmacol.
32:2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Ghajar A, Khoaie-Ardakani MR, Shahmoradi
Z, Alavi AR, Afarideh M, Shalbafan MR, Ghazizadeh-Hashemi M and
Akhondzadeh S: L-carnosine as an add-on to risperidone for
treatment of negative symptoms in patients with stable
schizophrenia: A double-blind, randomized placebo-controlled trial.
Psychiatry Res. 262:94–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chengappa KN, Turkin SR, DeSanti S, Bowie
CR, Brar JS, Schlicht PJ, Murphy SL, Hetrick ML, Bilder R and Fleet
D: A preliminary, randomized, double-blind, placebo-controlled
trial of L-carnosine to improve cognition in schizophrenia.
Schizophr Res. 142:145–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ghajar A, Aghajan-Nashtaei F, Afarideh M,
Mohammadi MR and Akhondzadeh S: l-Carnosine as adjunctive therapy
in children and adolescents with attention-deficit/hyperactivity
disorder: a randomized, double-blind, placebo-controlled clinical
trial. J Child Adolesc Psychopharmacol. 28:331–338. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hajizadeh-Zaker R, Ghajar A, Mesgarpour B,
Afarideh M, Mohammadi MR and Akhondzadeh S: l-Carnosine as an
adjunctive therapy to risperidone in children with autistic
disorder: A randomized, double-blind, placebo-controlled trial. J
Child Adolesc Psychopharmacol. 28:74–81. 2018. View Article : Google Scholar
|
|
155
|
Ann Abraham D, Narasimhan U, Christy S and
Muhasaparur Ganesan R: Effect of L-Carnosine as adjunctive therapy
in the management of children with autism spectrum disorder: A
randomized controlled study. Amino Acids. 52:1521–1528. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Hu X, Fukui Y, Feng T, Bian Z, Yu H,
Morihara R, Hu X, Bian Y, Sun H, Takemoto M, et al: Neuroprotective
effects of carnosine in a mice stroke model concerning oxidative
stress and inflammatory response. J Neurol Sci. 447:1206082023.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wu T, Tao Y, Tsang F, Abe K, Xu L, Jiang
Q, Xu L, Fu H and Fu Z: The effect of L-carnosine on the circadian
resetting of clock genes in the heart of rats. Mol Biol Rep.
42:87–94. 2015. View Article : Google Scholar
|
|
158
|
Dai Z, Lu XY, Zhu WL, Liu XQ, Li BY, Song
L, Liu HF, Cai WW, Deng YX, Xu TT, et al: Carnosine ameliorates
age-related dementia via improving mitochondrial dysfunction in
SAMP8 mice. Food Funct. 11:2489–2497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Afshin-Majd S, Khalili M, Roghani M,
Mehranmehr N and Baluchnejadmojarad T: Carnosine exerts
neuroprotective effect against 6-hydroxydopamine toxicity in
hemiparkinsonian rat. Mol Neurobiol. 51:1064–1070. 2015. View Article : Google Scholar
|
|
160
|
Evran B, Karpuzoğlu H, Develi S, Kalaz EB,
Soluk-Tekkeşin M, Olgaç V, Doğru-Abbasoğlu S and Uysal M: Effects
of carnosine on prooxidant-antioxidant status in heart tissue,
plasma and erythrocytes of rats with isoproterenol-induced
myocardial infarction. Pharmacol Rep. 66:81–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Holeček M: Side effects of amino acid
supplements. Physiol Res. 71:29–45. 2022. View Article : Google Scholar
|
|
162
|
Bae ON, Serfozo K, Baek SH, Lee KY,
Dorrance A, Rumbeiha W, Fitzgerald SD, Farooq MU, Naravelta B,
Bhatt A and Majid A: Safety and efficacy evaluation of carnosine,
an endogenous neuroprotective agent for ischemic stroke. Stroke.
44:205–212. 2013. View Article : Google Scholar :
|