Cholesterol is an essential component of mammalian
cell membranes and is indispensable for maintaining normal cellular
functions (1). The synthesis of
cholesterol is maintained through a dynamic homeostatic process,
with intracellular cholesterol levels being precisely regulated by
a sophisticated feedback system involving synthesis, uptake,
efflux, transport, esterification and enzymatic conversion
(2). Cancer cells frequently
exhibit an elevated demand for cholesterol to facilitate their
growth (3). Specifically, they
augment the uptake of exogenous cholesterol and lipoproteins and
reprogram cholesterol metabolism by regulating genes related to
cholesterol synthesis, efflux and intake to increase cholesterol
influx and decrease efflux (4,5).
Additionally, tumors can elevate cholesterol levels within the
tumor microenvironment (TME) by stimulating cholesterol efflux from
monocytes and macrophages via intercellular signaling (6). Furthermore, cholesterol serves as a
precursor for biologically active metabolites, such as oxysterols
(7), steroid hormones (8) and lipid rafts (LRs) (9), which collectively promote cancer
cell proliferation, angiogenesis, metastasis and other
pro-tumorigenic processes (10).
A previous study has shown that the crosstalk
between cholesterol metabolism and the TME contributes to
tumorigenesis and progression (11). Moreover, oncogenic signaling
pathways (12), ferroptosis
(13), autophagy (14), epithelial-mesenchymal transition
(EMT) (15) and the immune
response (16) are modulated
through cholesterol metabolism. Current evidence indicates that
targeting cholesterol metabolism, either alone or in combination
with other strategies, to enhance cancer treatment efficacy has
proven to be a viable antitumor approach. Notably, interventions
targeting cholesterol metabolism enzymes [such as HMG-CoA reductase
(HMGCR) (17), sterol regulatory
element binding proteins (SREBPs) (18), squalene epoxidase (SQLE)
(19) and acetyl-CoA
acetyltransferase 1 (ACAT1) (20)] or transporters [ATP-binding
cassette transporter A1 (ABCA1) (21), ATP-binding cassette transporter
G1 (ABCG1) (22) and low-density
lipoprotein receptor (LDLR) (23)] have shown considerable anticancer
potential.
However, the association between cholesterol and
cancer progression is currently controversial. Several
epidemiological studies have shown that hypercholesterolemia and
high cholesterol diets are associated with an increased risk of
developing hepatocellular carcinoma (HCC) (24), colorectal cancer (CRC) (25), prostate cancer (PC) (26) and other cancer types (27). Consistently, statins and
proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors,
which effectively lower serum cholesterol levels by targeting
cholesterol metabolism genes, have been found to suppress cancer
progression in certain settings (28). By contrast, other large cohort
studies report that serum total cholesterol (TC) levels are either
inversely correlated with cancer risk or show no significant
association (29,30). For instance, a large-scale
prospective Korean cohort study demonstrated that higher TC levels
were negatively associated with mortality rates in HCC and gastric
cancer (GC) (31). The role of
dietary cholesterol in carcinogenesis is similarly debated. While
high-cholesterol diets have been shown to accelerate HCC and CRC
development (32), a recent
study showed a significant positive association between higher
daily dietary cholesterol intake and ovarian cancer (OV) risk,
whereas abnormal lipid levels were not associated with the risk of
OV (33). These conflicting
epidemiological data underscore the complexity of the role of
cholesterol in cancer and highlight the need for further
mechanistic and population-level studies to clarify the
relationship between serum cholesterol levels and cancer risk.
In the present review, the latest progress in the
interaction between cholesterol and cancer progression is
summarized, focusing on the functional roles of cholesterol and its
derivatives within cancer cells and their reciprocal regulation
within the TME. The current therapeutic strategies targeting
molecules such as HMGCR, SREBPs and SQLE are also discussed,
offering new perspectives for anticancer drug development.
Cholesterol is an essential lipid molecule and a
critical component for maintaining normal functions in the human
body (1,34). Cholesterol fulfills multiple
vital roles, including maintaining membrane integrity, facilitating
cell signaling cell membrane structure, mediating cell
communication, enhancing immunity and serving as a precursor for
synthesizing steroids, sex hormones, vitamin D, bile salts and
oxysterols (35-37). Cholesterol metabolites also hold
significant value. In the liver, cholesterol is converted into bile
acids, which are essential for the emulsification and absorption of
dietary lipids and represent the primary route for eliminating
excess cholesterol from the body (38,39). Moreover, cholesterol serves as
the precursor for all steroid hormones (such as cortisol,
aldosterone, estrogen and testosterone). In the skin, under
ultraviolet radiation, cholesterol is transformed into a vitamin D
precursor, which is subsequently activated to regulate calcium and
phosphate homeostasis (40-42). Thus, in healthy individuals, the
homeostasis of cholesterol metabolism is indispensable for cellular
activity, digestion function, endocrine regulation and skeletal
health.
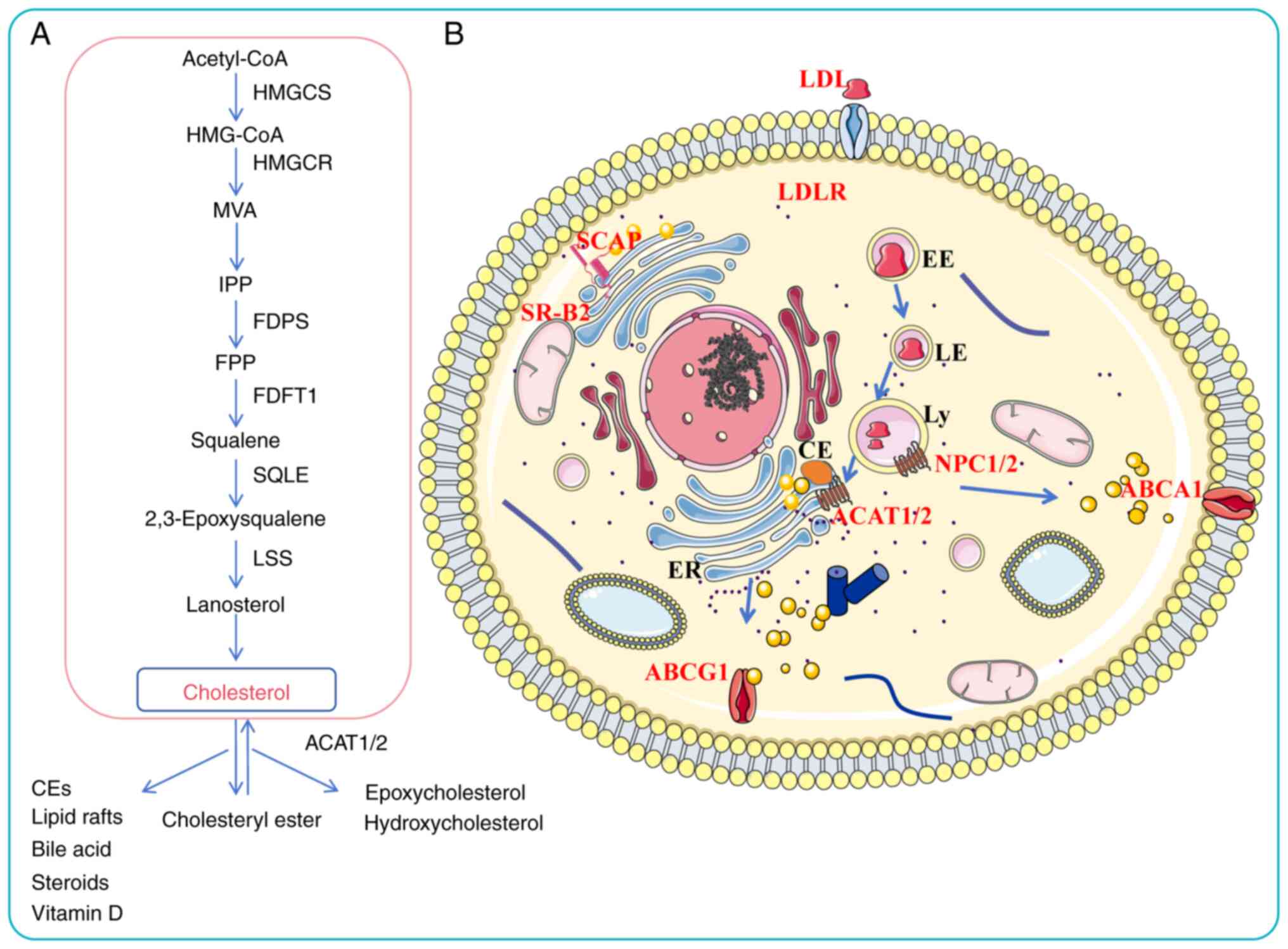
The body acquires cholesterol through two primary
pathways: Endogenous synthesis (70-80%) and exogenous dietary
intake (20-30%) (43). Dietary
cholesterol is absorbed by Niemann-Pick-C1 like-1 protein (NPC1L1)
on the intestinal epithelial cell membrane and subsequently
esterified by ACAT1/2, enabling its uptake by the liver in the form
of chylomicrons (44). The liver
esterifies cholesterol synthesized internally and absorbed from
food, assembling it with apolipoproteins and other components into
very low-density lipoproteins (VLDLs), which are subsequently
secreted into the bloodstream. These VLDLs are metabolized into
low-density lipoproteins (LDLs), which are taken up by peripheral
tissues via LDLRs. Excess cholesterol in peripheral tissues is
delivered back to the liver via high-density lipoprotein
cholesterol (HDL-C)-mediated reverse cholesterol transport, where
it can be recycled or converted to bile acids for excretion
(1,45). Intracellular cholesterol levels
are regulated through receptor-mediated endocytosis of LDL-C and
HDL-C, as well as through de novo synthesis in the
endoplasmic reticulum (ER) via the mevalonate (MVA) pathway, which
includes squalene biosynthesis and modification procedures
(34). Key regulatory enzymes in
this pathway are HMGCR and SQLE, which catalyze the conversion of
HMG-CoA to MVA and squalene to 2,3-epoxysqualene, respectively.
Subsequent steps involve enzymes such as farnesyl diphosphate
synthase (FDPS) and geranylgeranyl pyrophosphate synthase (GGPPs),
which are crucial for the biosynthesis of farnesyl pyrophosphate
(FPP) and geranylgeranyl pyrophosphate (GGPP). Under the action of
squalene synthase and squalene epoxidase, FPP is converted to
squalene and eventually to cholesterol (46).
Cholesterol homeostasis is transcriptionally
regulated by SREBPs. When intracellular cholesterol levels are
high, SREBPs are retained in the ER through interaction with the
SREBP cleavage-activating protein/insulin-induced gene-1 (INSIG-1)
complex. When the cholesterol level of the endoplasmic membrane
decreases, SREBPs are transported to the Golgi apparatus. Following
proteolytic processing, their transcriptionally active N-terminal
domains are released (47).
Excess intracellular cholesterol is either esterified by ACAT1 and
stored in lipid droplets or refluxed from cells via transporters
such as ABCA1 and ABCG1 (48).
The dysregulation of cholesterol metabolic enzymes is implicated in
various pathologies, including familial hypercholesterolemia
(49), atherosclerosis (35) and Alzheimer's disease (50). In summary, cholesterol levels in
normal cells are tightly controlled through a balance of synthesis,
uptake, efflux, transport and esterification. A thorough
understanding of these regulatory mechanisms is essential for
elucidating the pathophysiology of cholesterol-related disorders
(Fig. 1).
During growth and invasion, cancer cells require a
continuous supply of cholesterol to sustain biosynthetic processes
and cellular functions. Consequently, abnormal activation of
cholesterol metabolism genes is commonly observed across multiple
types of cancer (51).
Cholesterol metabolism-related genes are upregulated, cholesterol
influx is increased and cholesterol efflux is decreased. HMGCR is
the primary rate-limiting enzyme in cholesterol biosynthesis, and
its high expression in malignant tumors promotes tumor progression.
For instance, HMGCR induces immunosuppression in OV by activating
X-box binding protein 1 and programmed death-ligand 1 (PD-L1),
correlating with poor prognosis (52). In PC, stromal HMGCR upregulation
induced by coculture with malignant cells promotes tumor growth
(53). HCC is an aggressive
human cancer with increasing incidence worldwide (54). Research has revealed that HMGCR
promotes tumor growth in a mouse model of primary liver cancer by
enhancing cholesterol synthesis and activating the PDZ-binding
motif/TEA domain transcription factors 2/anillin/kinesin family
member 23 pathway (55).
Additionally, HMGCR overexpression augments the growth and
migration of GC cells (56),
breast cancer (BC) cells (57)
and glioblastoma (GBM) cells (58).
SQLE, as the second rate-limiting enzyme downstream
of HMGCR, is considered an oncogene that promotes carcinogenic
signaling (68). It has been
reported that SQLE could enhance the PI3K/AKT signaling pathway to
promote distant metastasis in head and neck squamous cell carcinoma
(69). In CRC, SQLE induces EMT
by triggering the Wnt/β-catenin pathway, thereby promoting tumor
metastasis (70). Moreover,
SQLE, which is abnormally expressed in liver cancer models, induces
immune suppression by promoting cholesterol accumulation in the TME
and inhibiting CD8+ T cell function (71). Research has also shown that SQLE
contributes to the resistance of BC cells to apoptosis by enhancing
glycolysis (72). SQLE
expression is specifically elevated in HCC and is strongly
associated with poor clinical outcomes (73). SQLE significantly augments HCC
growth in both in vitro and in vivo models, linked to
the activation of serine-threonine kinase receptor associated
protein-dependent transforming growth factor-β (TGF-β)/SMAD
signaling pathways (74).
Farnesyl-diphosphate farnesyltransferase (FDFT1),
another key enzyme in the cholesterol pathway, is dysregulated in
various tumor types and represents a potential biomarker and
therapeutic target (75). FDFT1
is highly expressed in HCC tissues and is correlated with poor
patient prognosis (76,77). In HCC, FDFT1 suppressed
fructose-1,6-bisphosphate aldolase B expression, thereby releasing
its inhibitory control over the AKT signaling pathway and
activating the PI3K/AKT pathway, ultimately promoting tumor growth
and metastasis (76). Prognosis
analysis showed that FDFT1, as a potential prognostic marker, was
associated with shorter survival in patients with CRC and may
partially contribute to the formation of a suppressive immune
microenvironment (78). This
indicates that the tumor-promoting effect of FDFT1 is dependent on
the TME (79). Notably, another
study has revealed a unique role of FDFT1 in CRC. This study found
that under fasting conditions, SREBP2 upregulates FDFT1 expression,
while elevated FDFT1 expression negatively regulates the
AKT/mTOR/hypoxia-inducible factor-1α (HIF-1α) signaling pathway,
thereby inhibiting glycolysis and proliferation in CRC cells
(80). This suggests that FDFT1
may exert tumor-suppressive effects under specific metabolic
conditions, such as fasting, indicating the complexity of its
functional role.
ACAT1 is a multifunctional metabolic enzyme that
plays a complex and sometimes seemingly contradictory role in
various cancer types; it can suppress tumors under specific
conditions while simultaneously promoting tumor progression through
different mechanisms (81). In
HCC, ACAT1-mediated acetylation of glycerophosphate
O-acyltransferase (GNPAT) can stabilize GNPAT protein levels
by antagonizing tripartite motif containing 21-catalyzed
ubiquitination, ultimately promoting xenograft tumor growth
(82). In bladder cancer (BLCA),
ACAT1 promotes BLCA cell proliferation and invasion by activating
the AKT/GSK3β/c-Myc signaling pathway (83). Additionally, a recent study has
identified ACAT1 as a key factor in reducing the sensitivity of GBM
to ferroptosis, with its mechanism of action dependent on the
regulation of the iron efflux protein, solute carrier family 40
member 1 (84). However, under
the influence of exogenous proinflammatory factors interleukin
(IL)-12 and IL-18 proteins, ACAT1 is phosphorylated at the S60 site
and translocates from mitochondria to the nucleus. Within the
nucleus, it exerts its acetyltransferase activity, specifically
acetylated the K146 site of the NF-κB family protein p50, thereby
releasing its transcriptional repression on multiple immunochemical
genes and natural killer (NK) cell activation ligands, thereby
powerfully recruiting and activating NK cells. This enhances their
tumor-killing capacity and inhibits tumor growth (85).
NPC1L1 is the primary protein responsible for the
absorption of exogenous cholesterol. NPC1L1 is highly expressed in
CRC and serves as an independent prognostic factor that is
significantly correlated with pathological staging (86,87). An in vitro study revealed
that targeting NPC1L1 with ezetimibe blocks the AKT/mTOR signaling
pathway in HCC and suppresses cancer cell activity (88).
Collectively, these findings underscore that
cholesterol metabolism is notably reprogrammed in cancer cells,
with multiple enzymes in the biosynthetic pathway actively
contributing to tumor development and progression.
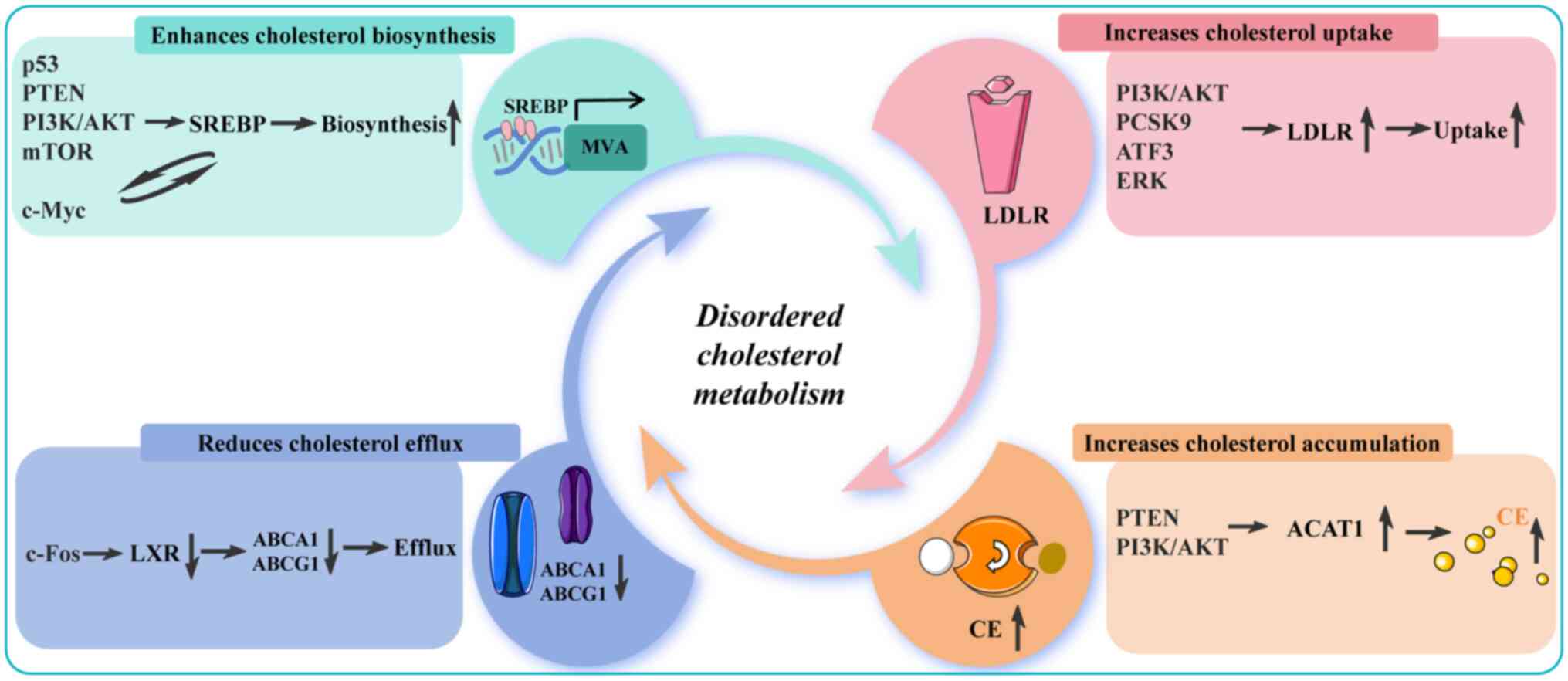
Numerous studies have demonstrated that aberrantly
activated genes or signaling pathways can promote cancer cell
proliferation and suppress apoptosis through the regulation of
cholesterol metabolism-related genes (89-93). For instance, the tumor suppressor
p53 can inhibit the MVA pathway, and its deficiency or mutation
releases multiple constraints on tumor growth (94). The p53 protein directly
suppresses the transcription of SQLE and SREBP genes by binding to
their promoters, thereby downregulating the MVA pathway in CRC,
HCC, OV and BC (95). By
contrast, upregulated or unmutated p53 transcriptionally induces
ABCA1 gene expression to block SREBP2 activation and inhibit the
MVA pathway (96,97). In recent years, the function of
the tumor suppressor phosphatase and tensin homolog protein (PTEN)
in metabolic regulation has attracted significant attention
(98). As a phosphatase, PTEN
dephosphorylates phosphatidylinositol-3,4,5-trisphosphate, thereby
directly inhibiting the oncogenic PI3K/AKT/mTOR signaling pathway
(99). PTEN suppression in
endocrine therapy-resistant BC cells leads to increased SQLE
expression and a corresponding sensitization to the inhibition of
cholesterol synthesis (100).
In pancreatic ductal adenocarcinoma (PDAC), PTEN deficiency
enhances the protein stability of SQLE by activating the
PI3K/AKT/GSK3β-mediated proteasome pathway (101). Activated AKT phosphorylates the
cytoplasmic phosphorylating rate-limiting enzyme
phosphoenolpyruvate carboxy kinase 1, which is translocated to the
ER and acts as a protein kinase that phosphorylates the INSIG
protein and disrupts the interaction between the INSIG protein and
the SREBP shear-activating proteins, which in turn activates the
transcription of SREBPs as well as downstream genes related to
lipid synthesis and uptake and promotes the progression of HCC
(102). AKT regulates SREBP
activation by activating mTOR1; it promotes the protein expression
of nuclear SREBP2 through a dual mechanism: First, by
phosphorylating and inhibiting its nuclear translocation inhibitor,
lipin1; second, by regulating cholesterol transport from lysosomes
to the ER (103). Furthermore,
suppression of ciliary function activates the Wnt/β-catenin
pathway, which synergistically promotes transcription of MVA
pathway genes by directly interacting with SREBP2 (104).
Liver X receptors (LXRs) are nuclear receptors and
members of the nuclear receptor family that regulate intracellular
lipid homeostasis. Activated by high intracellular cholesterol,
LXRs function as cholesterol sensors (105). c-Fos promotes alterations in
cholesterol metabolism by suppressing the transcriptional activity
of LXRα, leading to the accumulation of toxic sterols and bile
acids, thereby promoting hepatocellular carcinogenesis (106). High expression of anoctamin-1
contributes to in vivo metastasis of primary esophageal
squamous cell carcinoma by inhibiting LXR signaling, leading to
cholesterol accumulation and decreased cholesterol hydroxylation
via downregulation of the expression of the cholesterol hydroxylase
cytochrome P450 family 27 subfamily A member 1 (107).
LDLR is the key molecule that maintains cholesterol
balance in the body, releasing cholesterol into cells for
utilization while simultaneously lowering cholesterol levels in the
blood (108). LDLR-mediated
cholesterol uptake plays a contributory role in cancer (109). A recent study has demonstrated
that leukocyte immunoglobulin-like receptor B1 directly binds to
the LDLR protein to form a complex, thereby enhancing LDLR-mediated
cholesterol uptake and conferring resistance to ferroptosis in
multiple myeloma (MM) cells (110).
Apolipoprotein B (APOB) is an essential structural
component of VLDL and LDL; its C-terminal region contains an
LDLR-binding domain that specifically recognizes LDLRs on
hepatocyte surfaces, mediating LDL uptake and clearance to maintain
plasma cholesterol homeostasis (111). APOB is classified into two
primary subtypes: i) ApoB-100, which is synthesized in the liver
and serves as the major structural protein of VLDL,
intermediate-density lipoprotein (IDL), and LDL; and ii) ApoB-48,
which is synthesized in the small intestine, constitutes the core
component of chylomicrons and is primarily responsible for
transporting dietary lipids (112). APOB is closely associated with
metastasis in CRC and liver cancer. Compared with the control
group, silencing of APOB in HCC cells has been shown to increase
the relative rate of cell proliferation (113). A recent nested case-control
study revealed that genetically predicted APOB levels are
associated with a 31% reduction in HCC risk [95% confidence
interval (CI), 19-42%] and each 0.1 g/l increase in circulating
APOB levels was linked to an 11% decrease in HCC risk (95% CI,
8-14%) (114). Another
prospective cohort study from Sweden also found that elevated
circulating levels of APOB increased the risk of developing CRC
(115).
PCSK9 is a key regulator of LDLR protein levels,
binding to LDLRs on the cell surface and directing them to
lysosomes for degradation (116). In the TME, tumor cell-derived
PCSK9 disrupts T-cell receptor signaling by binding to LDLR on the
membrane of CD8+ T cells and directing LDLR to lysosomal
degradation. This impaired CD8+ T cell activation,
proliferation and effector function, ultimately leads to lymphoma
metastasis in mice (117). In
lung adenocarcinoma (LUAD), the long non-coding RNA EMX2OS
competitively binds to microRNA-1185-5p to regulate the LDLR,
thereby promoting lung cancer cell proliferation and invasion
(118). In addition, MEK/ERK
signaling increases intracellular cholesterol uptake by
upregulating LDLR expression, leading to HCC metastasis (119).
Similarly, dysregulation of cholesterol metabolism
can activate gene expression. Poly (ADP-ribose) polymerase 1
(PARP1) is a multifunctional human ADP-ribosyl transferase
(120). In addition to
maintaining genomic integrity, PARP1 participates in
transcriptional regulation (121). A recent study revealed that
long-term high cholesterol promotes OV progression by activating
focal adhesion kinase (FAK)/collagen type V alpha 1 chain (COL5A1)
signaling through the upregulation of PARP1 expression (122). Several cholesterol derivatives
and metabolites, including 7-ketocholesterol (123), 15α-hydroxycholesterol (124), 25-hydroxycholesterol (25-HC)
(125), 17-β-estradiol
(126) and vitamin D (127), have been shown to induce PARP1
expression.
Cholesterol is a key structural component of LRs. In
PC cells, elevated serum cholesterol levels can increase
cholesterol content within tumor cell liposomes, thereby activating
the LR-mediated Src/PI3K/AKT signaling pathway (128,129). Mitochondrial cholesterol also
exhibits physiological activity and promotes tumor cell
proliferation and metastasis. For instance, a recent study revealed
that the interaction between cytotoxin-associated protein A and
cytochrome P450scc (CYP11A1) promotes mitochondrial cholesterol
accumulation. This accumulated cholesterol then activates
autophagy, thereby enhancing GC cell proliferation and suppressing
apoptosis (130). The c-Myc
proto-oncogene encodes a family of transcription factors and is one
of the most commonly activated oncogenes in human tumors (131). Yang et al (132) found that c-Myc protein directly
promotes SQLE transcription, thereby increasing cholesterol
production and promoting tumor cell growth. Similarly, activated
c-Myc protein has also been observed to enhance HMGCR
transcriptional expression in T-cell acute lymphoblastic leukemia
(133). Notably, upregulated
SREBP1 protein directly interacts with SREBP1 binding elements in
the c-Myc promoter region in PC to induce c-Myc activation to drive
tumor stemness and metastasis (134). These studies illustrate a
reciprocal regulatory relationship between cholesterol metabolism
and genes, highlighting their critical roles in tumor cell
proliferation and invasion (Fig.
2).
A large body of preclinical evidence has underscored
the critical and multifaceted roles of cholesterol metabolism in
cancer progression (135-138). Targeting key nodes of this
pathway, such as inhibition of the MVA pathway, has emerged as a
promising antitumor strategy (1,139). At present, statins, which act
as inhibitors of HMGCR, and PCSK9 inhibitors have emerged as the
predominant agents targeting cholesterol metabolism, extensively
employed in both basic and clinical research (140,141). The anticancer function of
statins has attracted significant attention. Existing evidence
indicates that statins can promote tumor cell proliferation,
differentiation and apoptosis (76,142,143). A clinical study demonstrated
that initiating statin therapy within 1 year of diagnosis results
in a 58% relative improvement in BC-specific survival and a 30%
relative improvement in OS (144). Similarly, simvastatin (SIM)
blocks the isoprenylation of Rab5 GTPase and its mediated endosomal
maturation in antigen-presenting cells by depleting GGPP through
inhibition of the MVA pathway, ultimately enhancing antitumor
immunity by boosting antigen presentation and T cell activation
(145). Bisphosphonates (BPs)
are another widely studied inhibitor of the MVA pathway, which
inhibits FDPS activity and prevents the conversion of isopentenyl
pyrophosphate (IPP) to FPP (47,146,147). Bisphosphonate therapy has been
reported to improve the prognosis of patients with bona fide MM, PC
and BC (148). Moreover, BPs
can reduce bone and visceral metastases in women with BC (149,150). Previous studies suggest that
SQLE upregulation is closely associated with tumor progression. For
instance, upregulated SQLE promotes HCC cell invasion by activating
ERK (151) or AKT/mTOR
signaling (152). However,
targeting SQLE with terbinafine can delay tumor progression in
endometrial cancer (ECa) (153), PC (154), CRC (155), HCC (156) and BC (157). Ezetimibe is an FDA-approved
drug that acts on NPC1L1 to inhibit the progression of CRC
(86), BC (158) and PC (159) by inhibiting intestinal
cholesterol absorption. Intracellular cholesterol accumulation
triggers LXRs and excess cholesterol is excreted via ABCA1 or ABCG1
(160). The LXR agonists
RGX-104 (161), LXR 623
(162) and T0901317 (163) increase ABCA1 expression in a
variety of tumor cells, decrease intracellular cholesterol levels
and effectively inhibit the growth of mouse xenograft tumors.
Additionally, T0901317 can reduce myeloid-derived suppressor cell
infiltration through the LXR/APOE pathway, thereby lifting immune
suppression, enhancing T cell function and ultimately improving
overall immune therapy response (164).
The NPC1 protein, which regulates endolysosomal
cholesterol transport, has recently emerged as a therapeutic
target. NPC1 represents a convergence point for cholesterol derived
from LDL, HDL and VLDL, allowing simultaneous targeting of multiple
cholesterol sources (170). A
previous study has shown that targeted inhibition of NPC1
downregulates mTORC1 signaling and reduces autophagy and tumor
growth (171). Moreover,
lowering NPC1 expression enhances the therapeutic efficacy of
cisplatin or trastuzumab in NSCLC (172), GC (173) and PC (174), leading to improved patient
prognosis. These findings highlight the clinical potential of
combining cholesterol metabolism inhibitors with existing
anticancer agents to overcome therapy resistance. Cancer therapies
targeting cholesterol metabolism are summarized in Table I.
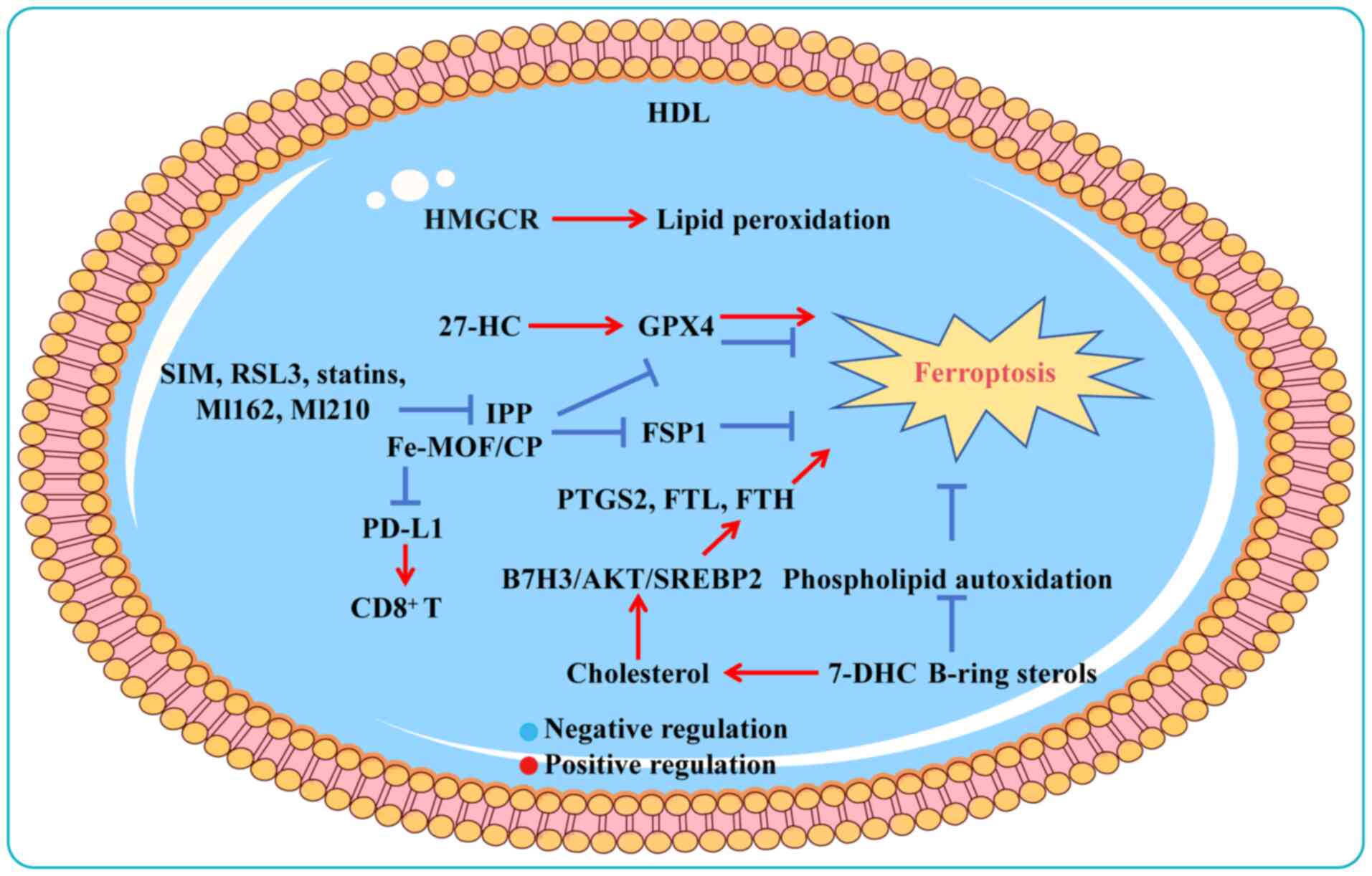
Ferroptosis, a regulated form of cell death driven
by iron-dependent lipid peroxidation, represents a promising
therapeutic avenue in oncology due to its involvement in tumor
progression and treatment resistance (175). Glutathione peroxidase 4 (GPX4)
and solute carrier family 7 member 11 are central to regulating
ferroptosis (176). Elevated
cholesterol levels in cancer cells have been shown to suppress
ferroptosis and impair immune responses (177). Recent studies have shown that
7-dehydrocholesterol (7-DHC) and B-ring sterols act as
free-radical-trapping antioxidants to protect mitochondrial
membranes from phospholipid autoxidation, thereby resisting
ferroptosis (178,179). While most cancer cells succumb
under stress, surviving populations often accumulate high
cholesterol levels to evade ferroptosis death (84,180,181). Chronic exposure to
27-hydroxycholesterol (27-HC), an abundant metabolite of
circulating cholesterol, causes sustained expression of GPX4 and
significantly increases the tumorigenic and metastatic capacity of
BC cells (13). These findings
highlight that aberrant cholesterol metabolism can influence cancer
pathogenesis by activating ferroptosis. In cancer cells, increased
uptake via the upregulation of lipoprotein receptors and increased
synthesis rates through the hyperactive MVA pathway results in
cholesterol accumulation, fostering cellular proliferation, tumor
metastasis and immune suppression (182-184). Studies have revealed that GPX4
relies on the output of the MVA pathway to hinder lipid
peroxidation production and ferroptosis sensitivity in HCC cells
(185,186). Exogenous cholesterol
supplementation activates the B7H3-mediated AKT/SREBP2 signaling
pathway to promote ferroptosis resistance in CRC cells as well as
xenograft tumor formation in mice (187).
Elevated cholesterol levels reduce membrane fluidity
and promote LR formation, thereby inhibiting the diffusion of lipid
peroxidation substrates and suppressing ferroptosis (188-190). A novel nanozyme composed of an
iron metal-organic framework (Fe-MOF) and nanoparticles loaded with
cholesterol oxidase and PEGylation (CP) for integrated ferroptosis
and immunotherapy, depletes cholesterol and disrupts LR integrity,
downregulates GPX4 and ferroptosis suppressor protein 1 (FSP1) and
further promotes ferroptosis. Concurrently, Fe-MOF/CP augments
immunogenic cell death, reduces programmed death-ligand 1
expression and revitalizes exhausted CD8+ T cells
(191). Similarly, in
cholesterol-addicted lymphoma cells, targeting SCARB1 via HDL
nanoparticles to reduce cholesterol uptake not only eliminates GPX4
expression but also triggers a compensatory response that enhances
cholesterol biosynthesis, ultimately leading to ferroptosis
(189). GPX4, a core regulator
of ferroptosis, is modulated by IPP, a product of the MVA pathway
and an intermediate in cholesterol synthesis. Thus, regulating the
upstream synthesis pathways of IPP using inhibitors (such as FIN56,
RSL3, statins, ML162 and ML210) induces ferroptosis by suppressing
GPX4 (a protein responsible for preventing lipid peroxide
formation); whereas FIN56 and withaferin can trigger GPX4
degradation (192). Squalene
and HMGCR are thought to exert anti-ferroptosis effects on cancer
cells (180,193). For example, SIM can inhibit the
expression of HMGCR to downregulate the MVA pathway, GPX4 and FSP1,
thereby inducing triple-negative BC cell ferroptosis (194,195). Conversely, SQLE exerts
anti-ferroptosis effects, whereas exogenous cholesterol
hydroperoxide induces dose-dependent cell death (196,197). These findings highlight the
therapeutic potential of targeting cholesterol biosynthesis to
sensitize cancer cells to ferroptosis (Fig. 3).
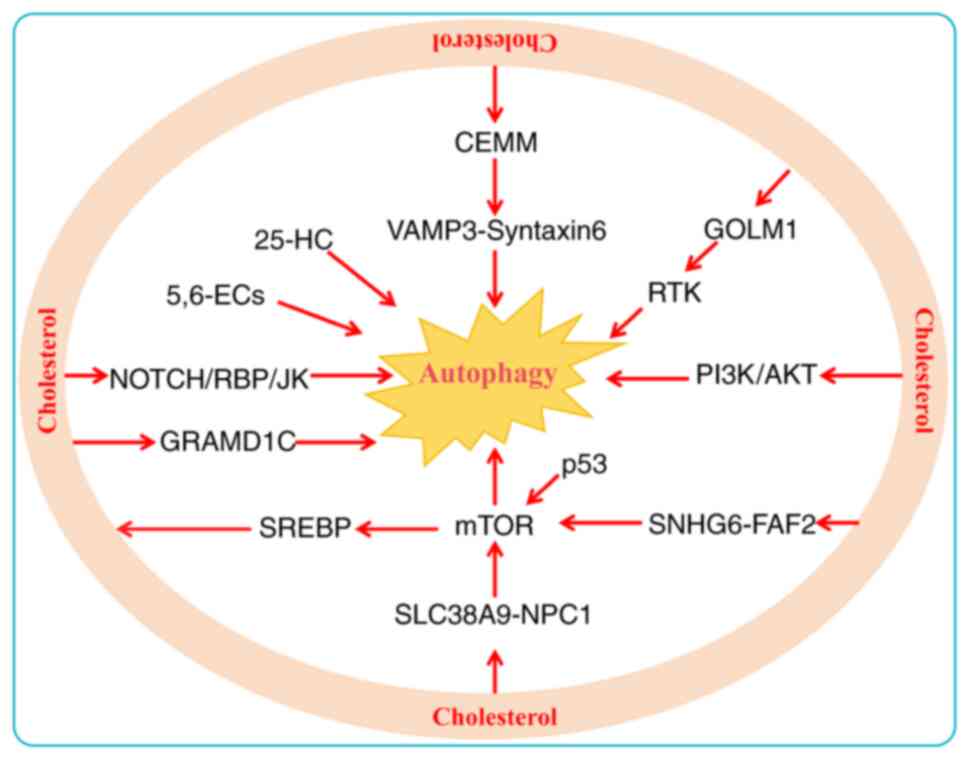
In cancer, autophagy exhibits a context-dependent
dual role, capable of both suppressing and promoting tumorigenesis
(198). During the early
stages, autophagy prevents tumor initiation by eliminating
oncogenic proteins, toxic misfolded aggregates and damaged
organelles (199). However, in
the later stages of tumorigenesis, autophagy functions as a dynamic
degradation and recycling system that can enhance cancer
invasiveness by promoting metastasis, thereby sustaining tumor
survival and growth (200).
Autophagy involves a variety of signaling pathways, such as the
mitogen-activated protein kinase (MAPK), mTOR, AKT, HIF-1α and p53
pathways (201). Lysosomal
cholesterol activates mTORC1 through the SLC38A9/NPC1 signaling
axis, inducing autophagy that promotes tumor metastasis and
invasion (202). Similarly,
another study revealed that cholesterol also induces autophagy in
HCC cells by activating mTORC1 via the SNHG6/FAF2/mTOR axis,
increasing the incidence of HCC driven by a high cholesterol diet
(203). In MM, researchers have
reported that cholesterol accumulation mediates autophagy
activation via the PI3K/AKT pathway (204). Conversely, phosphorylated AKT
levels as well as rapamycin signaling are markedly suppressed after
the use of statins by lowering intracellular cholesterol levels
(205). The mTOR, AKT and p53
pathways also modulate SREBP activity, which in turn regulates
cholesterol synthesis (96,206). Among these, the AKT and mTOR
pathways are the most frequently studied de novo cholesterol
synthesis pathways in cancer cells (207). mTOR serves as the core molecule
mediating the regulatory function of AKT over SREBPs. mTOR operates
through a dual mechanism: on the one hand, it is activated by AKT
(206); on the other, it drives
cholesterol biosynthesis by enhancing SREBP2 activity and
inhibiting its degradation (208). Whether cholesterol-induced
autophagy initiates positive feedback regulation of these pathways
warrants further investigation.
Emerging evidence indicates that cholesterol
enrichment within organelles facilitates the recruitment of
autophagy-initiation proteins and enhances autophagosome formation,
contributing to chemoresistance (209,210). The cholesterol transporter
protein GRAM domain containing 1B can coordinate cellular processes
by mediating cholesterol distribution between organelles, thereby
inhibiting autophagosome formation and reducing mitochondrial
bioenergetic metabolism (211).
Furthermore, cholesterol-rich membrane microstructure domain
(CEMM)-mediated sequestration of the vesicle-associated membrane
protein 3/syntaxin-6 complex inhibits autophagosome fusion.
Conversely, CEMM deficiency promotes autophagosome formation and
confers doxorubicin resistance in BC (212). Similarly, cholesterol inhibits
the autophagic degradation of receptor tyrosine kinase in a Golgi
membrane protein 1-dependent manner to promote HCC metastasis
(213). Cholesterol
accumulation in lysosomes induces autophagy initiation and enhances
carboplatin resistance in OV cells by activating the
NOTCH/DNA-binding protein recombination signal binding protein-Jκ
signaling pathway (214). These
results emphasize that cholesterol is an important regulator of
signaling pathways in cancer.
Cholesterol metabolites also play a key role in
activating autophagy. High 25-HC levels promote Kras-driven PDAC
progression. Mechanistically, 25-HC promotes autophagy, leading to
the downregulation of MHC-I and a reduction in CD8+
T-cell infiltration into tumors (215). BC is one of the most common
female cancer types in the world, with estrogen receptor-positive
BC being the most common subtype (216). The accumulation of
cholesterol-5,6-epoxide (5,6-EC) metabolites enhances resistance to
tamoxifen through the activation of autophagy to potentiate
antiestrogen binding site activity (217,218). Unraveling the molecular
interactions within the regulatory networks of cholesterol
metabolism and autophagy provides core guidance for identifying
novel anti-cancer targets and formulating targeted strategies
(Fig. 4).
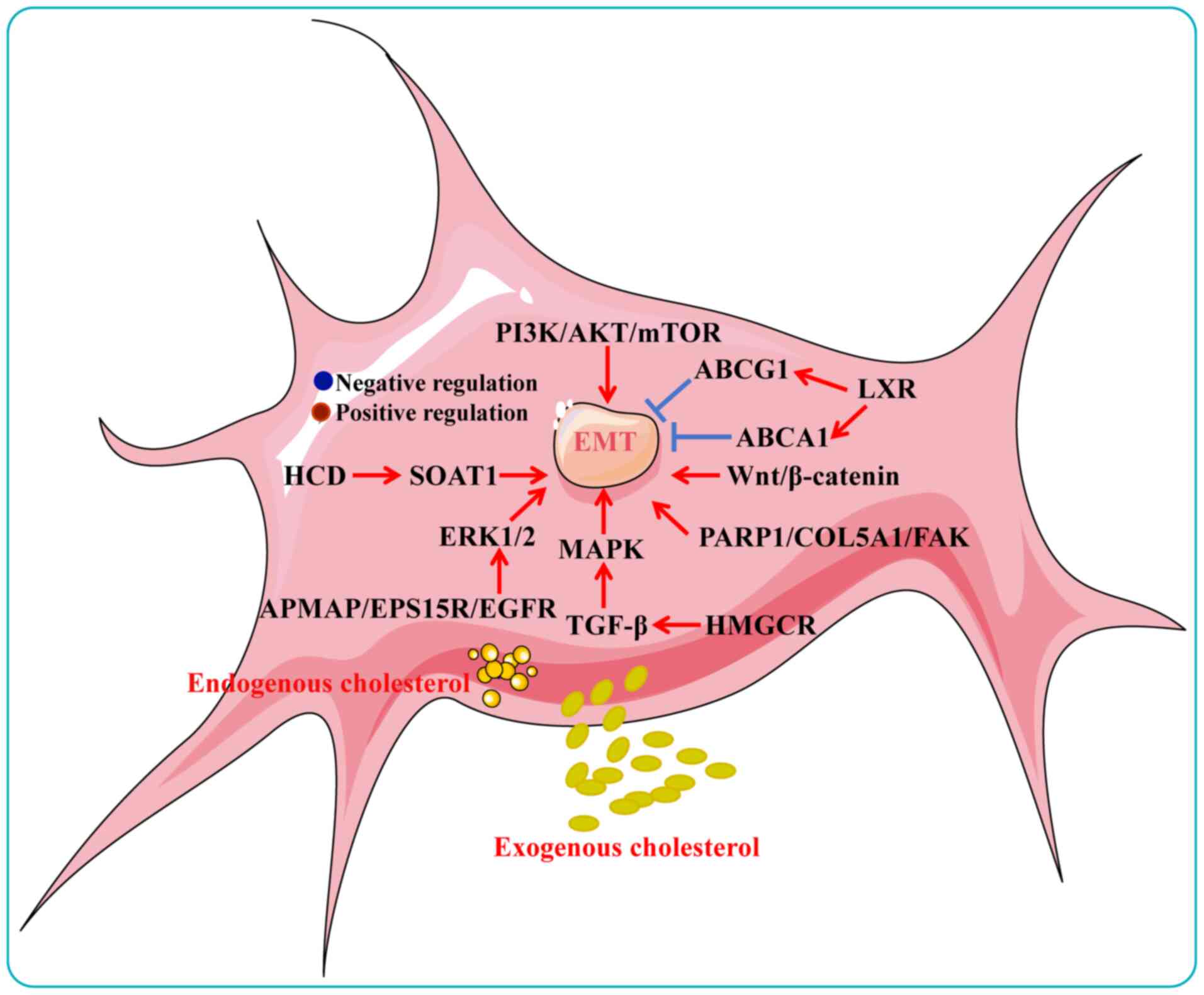
EMT is a key driver of tumor growth and metastasis,
promoting a malignant phenotype by conferring enhanced metastatic
capacity and resistance to therapeutic interventions. Tumor EMT
states have also been found to exhibit high plasticity during
transitions between epithelial and mesenchymal phenotypes (219). The progression of EMT is
governed by the precise coordination of a core set of transcription
factors, such as Snail1/2, zinc finger enhancer-binding protein 1
and Twist1, whose expression levels directly determine the degree
of EMT activation and the transformation of cellular phenotypes
(220). Cholesterol increases
the risk of EMT in cancer. A high-cholesterol diet promotes lung
metastasis of HCC in mice by activating sterol
O-acyltransferase 1 (SOAT1)-mediated EMT. Mechanistically,
SOAT1 increases cholesterol accumulation and esterification,
thereby driving EMT and HCC progression (93). Treatment with 20 μmol/l
cholesterol was found to promote in vivo graft tumor growth
and distal metastasis in CRC by inducing EMT (19). Cholesterol has been demonstrated
to regulate several classical signaling pathways that activate EMT.
Cholesterol (10 μmol/l) induces adipocyte
membrane-associated protein binding to EGFR substrate 15-associated
protein, activates ERK1/2 and facilitates the EMT process in PC
cells (221). The integrity of
LRs is essential for the formation of the TGF-β receptor (TβR)
I/TβRII/TGF-β1 complex, and LR disruption impedes canonical TGF-β
signaling. Cholesterol within LRs promotes EMT, migration and
invasion in BC cells via TGF-β-mediated MAPK activation (222). High cholesterol also promotes
proliferation and migration in BC and PDAC cells through activation
of Wnt/β-catenin signaling (223,224), and similarly induces EMT in
melanoma via the MAPK pathway (225). Notably, our latest study
revealed that long-term treatment of OV cells with high cholesterol
concentrations (>20 μmol/l) significantly induces the
expression of cellular EMT transcription factors and mesenchymal
cell marker proteins. Mechanistically, cholesterol promotes
invasion of orthotopic ovarian tumors into the liver and kidney in
mice through activation of the PARP1/COL5A1/FAK/EMT axis (122). These studies collectively
indicated that cholesterol plays a key role in promoting EMT.
Cholesterol-related genes also have key roles in
EMT-induced metastasis and drug resistance. Upregulation of ABCA1
in CRC facilitates EMT induction and enhances the migratory and
invasive abilities by modulating caveolin-1 stability (226). High ABCG1 expression enhances
cisplatin resistance in LUAD by activating Slug-mediated EMT
(227). Dysregulation of
cholesterol metabolism not only promotes the development of HCC but
also drives tumors to exhibit highly invasive characteristics,
including intrahepatic spread and early extrahepatic metastasis
(76,228,229). A recent study has shown that
high expression of 7-DHC reductase is correlated with poor
prognosis in patients with BLCA and promotes metastasis in mice via
PI3K/AKT/mTOR-mediated EMT activation (230). 27-HC, the most abundant
oxysterol, increases the risk of BC progression by promoting EMT
and oncogenic transformation of BC stem cells through LXR
activation (15). Accumulation
of apolipoprotein E leads to cholesterol enrichment in HCC cells,
stimulating PI3K/AKT signaling, inducing EMT and increasing
sorafenib resistance and invasiveness (231). Aberrant HMGCR expression
enhances statin resistance in therapy-resistant PC cells and
augments TGF-β-induced EMT in lung and ovarian epithelial
carcinomas (232-234). Additionally, HMGCR augments
TGF/β-induced EMT progression in epithelial cell carcinomas (lung
and ovarian) (235).
Collectively, this scientific evidence highlights the important
role played by cholesterol in promoting EMT, providing a new means
of targeting cholesterol metabolism to combat tumor progression
(Fig. 5). Cholesterol-activated
EMT markers and transcription factors in different cancer types are
summarized in Table II.
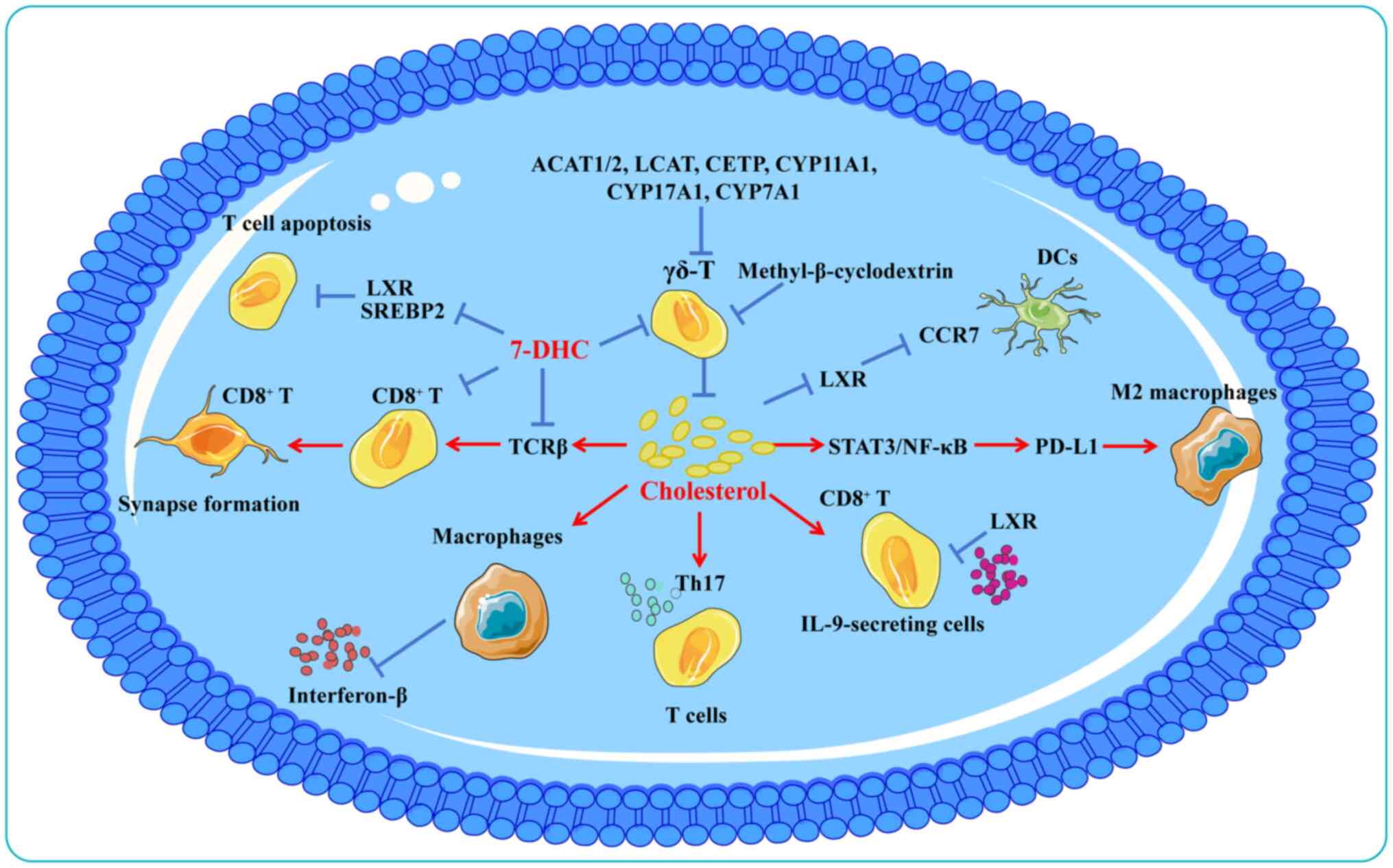
Recent studies have demonstrated that modulation of
systemic cholesterol metabolism could improve responses to
immunotherapy (16,136,236). Cholesterol depletion is a key
metabolic basis for tumor-associated macrophages (TAMs) acquiring
an immunosuppressive phenotype (6). In patients with CRC, cholesterol
metabolism promotes macrophage polarization towards a pro-tumor
phenotype and is associated with poorer prognosis (237). Notably, replenishing
cholesterol or adding 7-DHC to mouse macrophages was shown to
inhibit interferon-β production (238). In a murine melanoma model,
cholesterol accumulation in the TME induced functional exhaustion
of CD8+ T cells (239). Cholesterol metabolism exhibits
different effects on different T cell subsets. Cholesterol enhances
receptor signaling and immune synapse formation in CD8+
T cells by binding to T cell receptor β (240). Another in vivo study
demonstrated that inhibition of ACAT1 by knockdown or
pharmacological inhibition can inhibit intracellular cholesterol
esterification in T cells and enhance the proliferative and
effector capacity of CD8+ T cells, whereas
CD4+ T cells are unaffected (241). In C57BL/6J mice, genes involved
in cholesterol esterification (ACAT1/2, lecithin cholesterol
acyltransferase and cholesterol ester transfer protein) and
utilization (CYP11A1, CYP17A1 and CYP7A1) are upregulated in γδ T
cells compared with αβ T cells (242). Moreover, cholesterol depletion
using methyl-β-cyclodextrin reduces the activation phenotype of γδ
T cells (243). However, the
impact of hypercholesterolemia or lipid-lowering treatments on T
cell signaling in human patients remains unclear.
Cholesterol has also been identified as a key
immunomodulatory factor, whose accumulation promotes tumor immune
escape. In the TME, cholesterol attenuates the efficacy of PD-L1
blockade by activating the signal transducer and activator of
transcription 3/nuclear factor κB pathway, driving macrophages
toward an M2-like phenotype that accelerates CRC progression
(244). Additionally,
cholesterol upregulates PD-L1 expression on cancer cells,
facilitating immune evasion (245,246). Hyaluronic acid oligomers
secreted by tumor cells increase cholesterol efflux from TAMs,
promoting M2 polarization and tumor progression (6). Although IL-9-secreting
CD8+ T cells (Tc9 cells) exhibit potent antitumor
activity, cholesterol suppresses IL-9 expression and impairs Tc9
cell function through LXR activation (247). In microsatellite-stable CRC,
tumor cells secrete distal cholesterol precursors that polarize T
cells into Th17 cells, promoting tumor growth (248).
Cholesterol derivatives similarly modulate immune
responses. 27-HC promotes the development of the BC pre-metastatic
microenvironment by attracting polymorphonuclear neutrophils and
γδ-T cells at metastatic sites and depleting CD8+ T
cells (249). Furthermore,
27-HC induces T cell cholesterol deficiency by inhibiting SREBP2
and activating LXR, leading to autophagy-mediated T cell apoptosis
(14). In HCC, oxidized sterols
activate LXRα in dendritic cells (DCs), downregulating CC chemokine
receptor 7 expression and impairing DC migration to lymphoid
organs, thereby suppressing antitumor immunity (250).
In summary, the conflicting results of cholesterol
in modulating immunotherapy suggest that a deeper understanding of
the regulatory mechanisms of cholesterol metabolism in the
interactions between tumor cells and immune cells in the TME will
contribute to the development of new cancer immunotherapy
strategies (Fig. 6). Targeting
cholesterol metabolism in the tumor immune microenvironment
represents a promising approach to enhance antitumor immunity.
Although extensive basic and preclinical studies
have demonstrated the involvement of cholesterol in cancer
development and the potential of targeting its metabolism, the
relationship between serum cholesterol levels and tumor risk
remains controversial, with epidemiological studies reporting
conflicting findings (3).
Several large-scale studies have indicated a positive association
between elevated serum cholesterol and cancer risk. For example,
two prospective cohort studies provided evidence that higher
cholesterol levels were positively associated with the risk of
high-grade PC (251,252). Another study revealed an
association between the use of statins to lower serum cholesterol
levels and the risk of developing or dying from 13 tumor types,
including HCC, MM, BC, ECa and CRC (253). A 10 mg/dl increase in
cholesterol was correlated with a 9% increase in PC recurrence
(128). In OV, elevated
preoperative LDL-C was significantly associated with a poorer OS,
whereas high HDL-C was correlated with improved progression-free
survival (254). Patients with
advanced-stage OV are typically accompanied by the presence of
malignant ascites (MAs) and high cholesterol levels in these MAs
(255). Moreover, high
cholesterol levels promote drug resistance in OV cells by
increasing the expression of multidrug resistance protein 1
(256). Dietary cholesterol and
tumor risk also deserve attention. According to population-based
cohort studies, tumorigenesis is closely related to dietary
structure (257), and changes
in dietary structure effectively prevent tumorigenesis and
progression (258,259). Increased dietary cholesterol
intake is positively associated with the risk of BC (260). Dietary cholesterol is a risk
factor for the development of ECa, with a 35% increased risk of
tumor development for every 150 mg/kcal of cholesterol consumed
(261). Esophageal cancer (EC)
ranks as the ninth most common cancer worldwide, with genetic
factors, dietary factors and environmental risk factors potentially
influencing its progression (262). A meta-analysis that
investigated the association between high-cholesterol diets and EC
revealed that dietary cholesterol intake may increase the risk of
EC (summarized odds ratio, 1.424; 95% CI, 1.191-1.704) (263). Pancreatic cancer (PCa) risk is
associated with elevated serum cholesterol levels, which are
partially influenced by diet (264). A study involving 258 patients
with PCa and 551 controls demonstrated that a plant-based
cholesterol-lowering diet is associated with a reduced risk of PCa
(265). In addition, higher
cumulative dietary cholesterol intake is positively associated with
the risk of GC (266), HCC
(267) and OV (267). The incidence of OV is
significantly lower by 40% in individuals consuming a
low-cholesterol diet than those on a conventional diet (268). However, studies on dietary
cholesterol and lung cancer risk have consistently shown no
correlation (115,269,270).
Conversely, other epidemiological studies reported
no clear association or even an inverse relationship between
cholesterol and cancer. For instance, a large prospective cohort
study from South Korea found that the association between serum TC
levels and cancer was dependent on the cancer type (251). Further comparison across cancer
types revealed that elevated serum TC levels showed a significant
positive association with the risk of PC and CRC in men, as well as
BC in women. Conversely, there was a significant negative
association with the overall risk of HCC and GC in the general
population. Moreover, a population-based cohort study has suggested
that serum TC levels may be negatively associated with the risk of
OV and that circulating lipid levels are not strongly correlated
with the risk of OV (271).
These studies showed that the positive correlation and negative
correlation coexisted, indicating that the association was cancer
type specific. Similarly, our latest study showed that higher daily
dietary cholesterol intake was significantly positively associated
with the risk of OV, whereas abnormal lipid levels were not
associated with the risk of OV (33).
These conflicting epidemiological results suggest
that the role of cholesterol in cancer development remains
uncertain, warranting further investigation into its underlying
mechanisms. Key issues include the reliability of dietary study
data, the limitations of animal models in simulating human disease
states and the potentially opposing effects of serum cholesterol
across different tumor stages. The current epidemiological evidence
regarding cholesterol and cancer risk is indeed contradictory,
stemming from multiple confounding factors and methodological
challenges. The primary concern is reverse causality: Actively
growing tumors consume large amounts of cholesterol to build
membrane structures and synthesize signaling molecules, potentially
leading to reduced serum cholesterol levels. Thus, observed low
cholesterol levels may result from undetected tumors rather than
cause them. This is particularly notable in studies of malignancies
such as liver and stomach cancer, potentially completely reversing
the apparent 'protective association' (272,273). Cancer type specificity further
complicates this relationship as metabolic dependencies vary
significantly across tumor types: Hormone-sensitive cancers such as
PC and OV may rely more heavily on cholesterol as a steroid hormone
precursor, showing positive correlations; whereas other tumor types
may exhibit no clear association or even negative correlations
(274,275). Methodological limitations also
warrant attention: Dietary cholesterol studies rely on
self-reporting, introducing recall bias; single serum cholesterol
measurements may fail to reflect long-term exposure levels; and
statin use confounds the association between serum cholesterol and
cancer risk. Most critically, correlation does not imply causation.
Observational studies reveal statistical associations but cannot
establish biological mechanisms. These conflicting findings suggest
systemic cholesterol levels may be unreliable cancer risk
indicators, while targeted interventions affecting intracellular
cholesterol metabolism may hold greater therapeutic promise than
systemic cholesterol reduction. Recently, we suggested that low
levels of cholesterol promote tumor progression, that high
cholesterol may inhibit tumor cell growth and that chronically high
levels of cholesterol ultimately screen for more proliferative and
invasive tumor cells (122).
Evidence from these studies suggests that the dysregulation of
cholesterol homeostasis is an important factor in cancer
development, but its true link to tumor progression remains
unclear. Studies are needed to link population epidemiological
data, molecular mechanisms and clinical data more broadly to more
effectively unravel the processes by which cholesterol affects
cancer. The associations of serum cholesterol and dietary
cholesterol with the risk of cancer development are summarized in
Table III.
The present review describes the role of
cholesterol in cancer progression from a multidimensional
perspective. First, how cholesterol activates oncogenes and
oncogenic signals was discussed. Then, the molecular mechanisms
regulated by cholesterol in cancer were summarized. Finally, the
ongoing controversies regarding cholesterol levels and cancer risk
within epidemiological studies were addressed. Although substantial
evidence supports a definitive role for cholesterol metabolism in
tumorigenesis and progression, several critical questions remain
unresolved. For example, whether the specific roles of cholesterol
metabolites or derivatives are consistent, what the causal
relationship is between abnormal cholesterol metabolism and
tumorigenesis and how cholesterol metabolism influences other
metabolic processes such as glucose metabolism. Notably, studies on
the heterogeneity of cholesterol metabolism (such single-cell
metabolomics) and comparative analyses across cancer types (such as
BC vs. liver cancer) are fewer and deserve focused attention. This
complexity suggests that not only do we need to modulate from a
single cholesterol metabolic pathway but also interfere with the
activation of oncogenes or oncogenic signals and that a combination
of different drugs or inhibitors may be a more effective antitumor
regimen.
In conclusion, dysregulation of cholesterol
metabolism is increasingly recognized as a critical facilitator of
cancer development. While population-based epidemiological findings
have been inconsistent, both basic and clinical studies indicate
that targeting cholesterol metabolism can significantly inhibit
tumor progression. These insights provide a robust foundation for
the development of cholesterol-focused therapeutics and offer
promising new strategies for cancer prevention and treatment.
Not applicable.
ZH was responsible for conceptualization, writing
the original draft and visualization. LZ and SG helped to revise
the manuscript and collect literature. GX and XY were responsible
for supervision, writing, reviewing and editing. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This review was funded by the Research Fund of Sichuan Academy
of Medical Sciences and Sichuan Provincial People's Hospital (grant
no. 24QNPY025), in part by the Natural Science Foundation of
Sichuan Province (grant no. 2025YFHZ0308).
|
1
|
Luo J, Yang H and Song BL: Mechanisms and
regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol.
21:225–245. 2020. View Article : Google Scholar
|
|
2
|
Ouimet M, Barrett TJ and Fisher EA: HDL
and reverse cholesterol transport. Circ Res. 124:1505–1518. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuzu OF, Noory MA and Robertson GP: The
role of cholesterol in cancer. Cancer Res. 76:2063–2070. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao D and Liu H: Dysregulated cholesterol
regulatory genes in hepatocellular carcinoma. Eur J Med Res.
28:5802023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geng F and Guo D: SREBF1/SREBP-1
concurrently regulates lipid synthesis and lipophagy to maintain
lipid homeostasis and tumor growth. Autophagy. 20:1183–1185. 2024.
View Article : Google Scholar :
|
|
6
|
Goossens P, Rodriguez-Vita J, Etzerodt A,
Masse M, Rastoin O, Gouirand V, Ulas T, Papantonopoulou O, Van Eck
M, Auphan-Anezin N, et al: Membrane cholesterol efflux drives
tumor-associated macrophage reprogramming and tumor progression.
Cell Metab. 29:1376–1389.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spann NJ and Glass CK: Sterols and
oxysterols in immune cell function. Nat Immunol. 14:893–900. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller WL and Auchus RJ: The molecular
biology, biochemistry, and physiology of human steroidogenesis and
its disorders. Endocr Rev. 32:81–151. 2011. View Article : Google Scholar
|
|
9
|
Simons K and Toomre D: Lipid rafts and
signal transduction. Nat Rev Mol Cell Biol. 1:31–39. 2000.
View Article : Google Scholar
|
|
10
|
Riscal R, Skuli N and Simon MC: Even
cancer cells watch their cholesterol! Mol Cell. 76:220–231. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma X, Xiao L, Liu L, Ye L, Su P, Bi E,
Wang Q, Yang M, Qian J and Yi Q: CD36-mediated ferroptosis dampens
intratumoral CD8+ T cell effector function and impairs
their antitumor ability. Cell Metab. 33:1001–1012.e5. 2021.
View Article : Google Scholar
|
|
12
|
Gabitova-Cornell L, Surumbayeva A, Peri S,
Franco-Barraza J, Restifo D, Weitz N, Ogier C, Goldman AR, Hartman
TR, Francescone R, et al: Cholesterol pathway inhibition induces
TGF-β signaling to promote basal differentiation in pancreatic
cancer. Cancer Cell. 38:567–583.e11. 2020. View Article : Google Scholar
|
|
13
|
Liu W, Chakraborty B, Safi R, Kazmin D,
Chang CY and McDonnell DP: Dysregulated cholesterol homeostasis
results in resistance to ferroptosis increasing tumorigenicity and
metastasis in cancer. Nat Commun. 12:51032021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan C, Zheng L, Jiang S, Yang H, Guo J,
Jiang LY, Li T, Zhang H, Bai Y, Lou Y, et al: Exhaustion-associated
cholesterol deficiency dampens the cytotoxic arm of antitumor
immunity. Cancer Cell. 41:1276–1293.e11. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo M, Bao L, Chen Y, Xue Y, Wang Y, Zhang
B, Wang C, Corley CD, McDonald JG, Kumar A, et al: ZMYND8 is a
master regulator of 27-hydroxycholesterol that promotes
tumorigenicity of breast cancer stem cells. Sci Adv.
8:eabn52952022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu C, Qiao W, Li X, Ning ZK, Liu J,
Dalangood S, Li H, Yu X, Zong Z, Wen Z and Gui J: Tumor-secreted
FGF21 acts as an immune suppressor by rewiring cholesterol
metabolism of CD8+T cells. Cell Metab. 36:630–647.e8.
2024. View Article : Google Scholar
|
|
17
|
Yarmolinsky J, Bull CJ, Vincent EE,
Robinson J, Walther A, Smith GD, Lewis SJ, Relton CL and Martin RM:
Association between genetically proxied inhibition of HMG-CoA
reductase and epithelial ovarian cancer. JAMA. 323:646–655. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawamura S, Matsushita Y, Kurosaki S,
Tange M, Fujiwara N, Hayata Y, Hayakawa Y, Suzuki N, Hata M, Tsuboi
M, et al: Inhibiting SCAP/SREBP exacerbates liver injury and
carcinogenesis in murine nonalcoholic steatohepatitis. J Clin
Invest. 132:e1518952022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Wang Y, Liu D, Wong CC, Coker OO,
Zhang X, Liu C, Zhou Y, Liu Y, Kang W, et al: Squalene epoxidase
drives cancer cell proliferation and promotes gut dysbiosis to
accelerate colorectal carcinogenesis. Gut. 71:2253–2265. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan J, Lin R, Xia S, Chen D, Elf SE, Liu
S, Pan Y, Xu H, Qian Z, Wang M, et al: Tetrameric Acetyl-CoA
acetyltransferase 1 is important for tumor growth. Mol Cell.
64:859–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viaud M, Abdel-Wahab O, Gall J, Ivanov S,
Guinamard R, Sore S, Merlin J, Ayrault M, Guilbaud E, Jacquel A, et
al: ABCA1 exerts tumor-suppressor function in myeloproliferative
neoplasms. Cell Rep. 30:3397–3410.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roundhill EA, Jabri S and Burchill SA:
ABCG1 and Pgp identify drug resistant, self-renewing osteosarcoma
cells. Cancer Lett. 453:142–157. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li
C, Wang Y, Wang Z, Si S, Pan H, et al: Berberine is a novel
cholesterol-lowering drug working through a unique mechanism
distinct from statins. Nat Med. 10:1344–1351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ioannou GN, Morrow OB, Connole ML and Lee
SP: Association between dietary nutrient composition and the
incidence of cirrhosis or liver cancer in the United States
population. Hepatology. 50:175–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu R, Shen J, Song Y, Lu J, Liu Y, Cao Y,
Wang Z and Zhang J: Exploration of the application potential of
serum multi-biomarker model in colorectal cancer screening. Sci
Rep. 14:101272024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jamnagerwalla J, Howard LE, Allott EH,
Vidal AC, Moreira DM, Castro-Santamaria R, Andriole GL, Freeman MR
and Freedland SJ: Serum cholesterol and risk of high-grade prostate
cancer: Results from the REDUCE study. Prostate Cancer Prostatic
Dis. 21:252–259. 2018. View Article : Google Scholar :
|
|
27
|
Zhao B, Gan L, Graubard BI, Männistö S,
Albanes D and Huang J: Associations of dietary cholesterol, serum
cholesterol, and egg consumption with overall and cause-specific
mortality: Systematic review and updated meta-analysis.
Circulation. 145:1506–1520. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Demierre MF, Higgins PD, Gruber SB, Hawk E
and Lippman SM: Statins and cancer prevention. Nat Rev Cancer.
5:930–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siemianowicz K, Gminski J, Stajszczyk M,
Wojakowski W, Goss M, Machalski M, Telega A, Brulinski K and
Magiera-Molendowska H: Serum total cholesterol and triglycerides
levels in patients with lung cancer. Int J Mol Med. 5:201–205.
2000.PubMed/NCBI
|
|
30
|
Zhou P, Li B, Liu B, Chen T and Xiao J:
Prognostic role of serum total cholesterol and high-density
lipoprotein cholesterol in cancer survivors: A systematic review
and meta-analysis. Clin Chim Acta. 477:94–104. 2018. View Article : Google Scholar
|
|
31
|
Oh MJ, Han K, Kim B, Lim JH, Kim B, Kim SG
and Cho SJ: Risk of gastric cancer in relation with serum
cholesterol profiles: A nationwide population-based cohort study.
Medicine (Baltimore). 102:e362602023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Coker OO, Chu ES, Fu K, Lau HCH,
Wang YX, Chan AWH, Wei H, Yang X, Sung JJY and Yu J: Dietary
cholesterol drives fatty liver-associated liver cancer by
modulating gut microbiota and metabolites. Gut. 70:761–774. 2021.
View Article : Google Scholar
|
|
33
|
Zhang X, Ding HM, Deng LF, Chen GC, Li J,
He ZY, Fu L, Li JF, Jiang F, Zhang ZL and Li BY: Dietary fats and
serum lipids in relation to the risk of ovarian cancer: A
meta-analysis of observational studies. Front Nutr. 10:11539862023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ikonen E and Olkkonen VM: Intracellular
cholesterol trafficking. Cold Spring Harb Perspect Biol.
15:a0414042023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
von Eckardstein A, Nordestgaard BG,
Remaley AT and Catapano AL: High-density lipoprotein revisited:
Biological functions and clinical relevance. Eur Heart J.
44:1394–1407. 2023. View Article : Google Scholar :
|
|
36
|
Dang EV and Reboldi A: Cholesterol sensing
and metabolic adaptation in tissue immunity. Trends Immunol.
45:861–870. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Griffiths WJ and Wang Y: Cholesterol
metabolism: From lipidomics to immunology. J Lipid Res.
63:1001652022. View Article : Google Scholar :
|
|
38
|
Long T, Debler EW and Li X: Structural
enzymology of cholesterol biosynthesis and storage. Curr Opin
Struct Biol. 74:1023692022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schade DS, Shey L and Eaton RP:
Cholesterol review: A metabolically important molecule. Endocr
Pract. 26:1514–1523. 2020. View Article : Google Scholar
|
|
40
|
Halimi H and Farjadian S: Cholesterol: An
important actor on the cancer immune scene. Front Immunol.
13:10575462022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goicoechea L, Conde de la Rosa L, Torres
S, García-Ruiz C and Fernández-Checa JC: Mitochondrial cholesterol:
Metabolism and impact on redox biology and disease. Redox Biol.
61:1026432023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng Y, Heybrock S, Neculai D and Saftig
P: Cholesterol handling in lysosomes and beyond. Trends Cell Biol.
30:452–466. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feingold KR: Lipid and lipoprotein
metabolism. Endocrinol Metab Clin North Am. 51:437–458. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chandel NS: Lipid metabolism. Cold Spring
Harb Perspect Biol. 13:a0405762021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xiao X, Kennelly JP, Ferrari A, Clifford
BL, Whang E, Gao Y, Qian K, Sandhu J, Jarrett KE, Brearley-Sholto
MC, et al: Hepatic nonvesicular cholesterol transport is critical
for systemic lipid homeostasis. Nat Metab. 5:165–181. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kennelly JP and Tontonoz P: Cholesterol
transport to the endoplasmic reticulum. Cold Spring Harb Perspect
Biol. 15:a0412632023. View Article : Google Scholar
|
|
47
|
Faulkner RA, Yang Y, Tsien J, Qin T and
DeBose-Boyd RA: Direct binding to sterols accelerates endoplasmic
reticulum-associated degradation of HMG CoA reductase. Proc Natl
Acad Sci USA. 121:e23188221212024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen L, Zhao ZW, Zeng PH, Zhou YJ and Yin
WJ: Molecular mechanisms for ABCA1-mediated cholesterol efflux.
Cell Cycle. 21:1121–1139. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu X, Chen F, Jia L, Long A, Peng Y, Li X,
Huang J, Wei X, Fang X, Gao Z, et al: A gut-derived hormone
regulates cholesterol metabolism. Cell. 187:1685–1700.e18. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saher G: Cholesterol metabolism in aging
and age-related disorders. Annu Rev Neurosci. 46:59–78. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu H, Zhou S, Tang Q, Xia H and Bi F:
Cholesterol metabolism: New functions and therapeutic approaches in
cancer. Biochim Biophys Acta Rev Cancer. 1874:1883942020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mittal S, Nenwani M, Pulikkal Kadamberi I,
Kumar S, Animasahun O, George J, Tsaih SW, Gupta P, Singh M,
Geethadevi A, et al: eIF4E enriched extracellular vesicles induce
immunosuppressive macrophages through HMGCR-mediated metabolic
rewiring. Adv Sci (Weinh). e063072025.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ashida S, Kawada C and Inoue K: Stromal
regulation of prostate cancer cell growth by mevalonate pathway
enzymes HMGCS1 and HMGCR. Oncol Lett. 14:6533–6542. 2017.PubMed/NCBI
|
|
54
|
Martin OP, Wallace MS, Oetheimer C, Patel
HB, Butler MD, Wong LP, Huang P, Elbaz J, Costentin C, Salloum S,
et al: Single-cell atlas of human liver and blood immune cells
across fatty liver disease stages reveals distinct signatures
linked to liver dysfunction and fibrogenesis. Nat Immunol.
26:1596–1611. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saito Y, Yin D, Kubota N, Wang X, Filliol
A, Remotti H, Nair A, Fazlollahi L, Hoshida Y, Tabas I, et al: A
therapeutically targetable TAZ-TEAD2 pathway drives the growth of
hepatocellular carcinoma via ANLN and KIF23. Gastroenterology.
164:1279–1292. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li Z, Liu C, Wang M, Wei R, Li R, Huang K,
Liang H, Li G and Zhao L: Cholesterol confers resistance to
Apatinib-mediated ferroptosis in gastric cancer. Cell Biosci.
15:952025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li MX, Hu S, Lei HH, Yuan M, Li X, Hou WK,
Huang XJ, Xiao BW, Yu TX, Zhang XH, et al: Tumor-derived
miR-9-5p-loaded EVs regulate cholesterol homeostasis to promote
breast cancer liver metastasis in mice. Nat Commun. 15:105392024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xue X, He Z, Liu F, Wang Q, Chen Z, Lin L,
Chen D, Yuan Y, Huang Z and Wang Y: Taurochenodeoxycholic acid
suppresses the progression of glioblastoma via HMGCS1/HMGCR/GPX4
signaling pathway in vitro and in vivo. Cancer Cell Int.
25:1602025. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakagawa H: Lipogenesis and MASLD:
Re-thinking the role of SREBPs. Arch Toxicol. 99:2299–2312. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li Y, Wu S, Zhao X, Hao S, Li F, Wang Y,
Liu B, Zhang D, Wang Y and Zhou H: Key events in cancer:
Dysregulation of SREBPs. Front Pharmacol. 14:11307472023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Geng F, Zhong Y, Su H, Lefai E, Magaki S,
Cloughesy TF, Yong WH, Chakravarti A and Guo D: SREBP-1 upregulates
lipophagy to maintain cholesterol homeostasis in brain tumor cells.
Cell Rep. 42:1127902023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen Y, Deng X, Li Y, Han Y, Peng Y, Wu W,
Wang X, Ma J, Hu E, Zhou X, et al: Comprehensive molecular
classification predicted microenvironment profiles and therapy
response for HCC. Hepatology. 80:536–551. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou J, Qu G, Zhang G, Wu Z, Liu J, Yang
D, Li J, Chang M, Zeng H, Hu J, et al: Glycerol kinase 5 confers
gefitinib resistance through SREBP1/SCD1 signaling pathway. J Exp
Clin Cancer Res. 38:962019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Su F and Koeberle A: Regulation and
targeting of SREBP-1 in hepatocellular carcinoma. Cancer Metastasis
Rev. 43:673–708. 2024. View Article : Google Scholar :
|
|
65
|
Chen F, Li H, Wang Y, Tang X, Lin K, Li Q,
Meng C, Shi W, Leo J, Liang X, et al: CHD1 loss reprograms
SREBP2-driven cholesterol synthesis to fuel androgen-responsive
growth and castration resistance in SPOP-mutated prostate tumors.
Nat Cancer. 6:854–873. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao W, Guo X, Sun L, Gai J, Cao Y and
Zhang S: PKMYT1 knockdown inhibits cholesterol biosynthesis and
promotes the drug sensitivity of triple-negative breast cancer
cells to atorvastatin. PeerJ. 12:e177492024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu R, Li N, Huang W, Yang Y, Zang R, Song
H, Shi J, Zhu S and Liu Q: Melittin suppresses ovarian cancer
growth by regulating SREBP1-mediated lipid metabolism.
Phytomedicine. 137:1563672025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wen J, Zhang X, Wong CC, Zhang Y, Pan Y,
Zhou Y, Cheung AH, Liu Y, Ji F, Kang X, et al: Targeting squalene
epoxidase restores anti-PD-1 efficacy in metabolic
dysfunction-associated steatohepatitis-induced hepatocellular
carcinoma. Gut. 73:2023–2036. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li J, Yang T, Wang Q, Li Y, Wu H, Zhang M,
Qi H, Zhang H and Li J: Upregulation of SQLE contributes to poor
survival in head and neck squamous cell carcinoma. Int J Biol Sci.
18:3576–3591. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Q, Zhang Y, Li H, Gao H, Zhou Y, Luo
D, Shan Z, Yang Y, Weng J, Li Q, et al: Squalene epoxidase promotes
the chemoresistance of colorectal cancer via
(S)-2,3-epoxysqualene-activated NF-κB. Cell Commun Signal.
22:2782024. View Article : Google Scholar
|
|
71
|
Wu J, Hu W, Yang W, Long Y, Chen K, Li F,
Ma X and Li X: Knockdown of SQLE promotes CD8+ T cell infiltration
in the tumor microenvironment. Cell Signal. 114:1109832024.
View Article : Google Scholar
|
|
72
|
Xu M, Pan G, Zhang Q, Huang J, Wu Y and
Ashan Y: FOXM1 boosts glycolysis by upregulating SQLE to inhibit
anoikis in breast cancer cells. J Cancer Res Clin Oncol.
151:1622025. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shen T, Lu Y and Zhang Q: High squalene
epoxidase in tumors predicts worse survival in patients with
hepatocellular carcinoma: Integrated bioinformatic analysis on
NAFLD and HCC. Cancer Control. 27:10732748209146632020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Z, Wu W, Jiao H, Chen Y, Ji X, Cao
J, Yin F and Yin W: Squalene epoxidase promotes hepatocellular
carcinoma development by activating STRAP transcription and
TGF-β/SMAD signalling. Br J Pharmacol. 180:1562–1581. 2023.
View Article : Google Scholar
|
|
75
|
Kanmalar M, Abdul Sani SF, Kamri NINB,
Said NABM, Jamil AHBA, Kuppusamy S, Mun KS and Bradley DA: Raman
spectroscopy biochemical characterisation of bladder cancer
cisplatin resistance regulated by FDFT1: A review. Cell Mol Biol
Lett. 27:92022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cai D, Zhong GC, Dai X, Zhao Z, Chen M, Hu
J, Wu Z, Cheng L, Li S and Gong J: Targeting FDFT1 reduces
cholesterol and bile acid production and delays hepatocellular
carcinoma progression through the HNF4A/ALDOB/AKT1 axis. Adv Sci
(Weinh). 12:e24117192025. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wu Z, Duan W, Xiong Y, Liu J, Wen X, Zhao
F, Xiang D, Wang J, Kasim V and Wu S: NeuroD1 drives a KAT2A-FDFT1
signaling axis to promote cholesterol biosynthesis and
hepatocellular carcinoma progression via histone H3K27 acetylation.
Oncogene. Sep 1–2025.Epub ahead of print.
|
|
78
|
Yang C, Huang S, Cao F and Zheng Y: A
lipid metabolism-related genes prognosis biomarker associated with
the tumor immune microenvironment in colorectal carcinoma. BMC
Cancer. 21:11822021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang HL, Zhao R, Wang D, Mohd Sapudin SN,
Yahaya BH, Harun MSR, Zhang ZW, Song ZJ, Liu YT, Doblin S and Lu P:
Candida albicans and colorectal cancer: A paradoxical role revealed
through metabolite profiling and prognostic modeling. World J Clin
Oncol. 16:1041822025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Weng ML, Chen WK, Chen XY, Lu H, Sun ZR,
Yu Q, Sun PF, Xu YJ, Zhu MM, Jiang N, et al: Fasting inhibits
aerobic glycolysis and proliferation in colorectal cancer via the
Fdft1-mediated AKT/mTOR/HIF1α pathway suppression. Nat Commun.
11:18692020. View Article : Google Scholar
|
|
81
|
Goudarzi A: The recent insights into the
function of ACAT1: A possible anti-cancer therapeutic target. Life
Sci. 232:1165922019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gu L, Zhu Y, Lin X, Tan X, Lu B and Li Y:
Stabilization of FASN by ACAT1-mediated GNPAT acetylation promotes
lipid metabolism and hepatocarcinogenesis. Oncogene. 39:2437–2449.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang T, Wang G, Shan D, Fang Y, Zhou F, Yu
M, Ju L, Li G, Xiang W, Qian K, et al: ACAT1 promotes proliferation
and metastasis of bladder cancer via AKT/GSK3β/c-Myc signaling
pathway. J Cancer. 15:3297–3312. 2024. View Article : Google Scholar :
|
|
84
|
Sun S, Qi G, Chen H, He D, Ma D, Bie Y, Xu
L, Feng B, Pang Q, Guo H and Zhang R: Ferroptosis sensitization in
glioma: Exploring the regulatory mechanism of SOAT1 and its
therapeutic implications. Cell Death Dis. 14:7542023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wei C, Liao K, Chen HJ, Xiao ZX, Meng Q,
Liu ZK, Lu YX, Sheng H, Mo HY, Wu QN, et al: Nuclear mitochondrial
acetyl-CoA acetyltransferase 1 orchestrates natural killer
cell-dependent antitumor immunity in colorectal cancer. Signal
Transduct Target Ther. 10:1382025. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kwon RJ, Park EJ, Lee SY, Lee Y, Hwang C,
Kim C and Cho YH: Expression and prognostic significance of
Niemann-Pick C1-Like 1 in colorectal cancer: A retrospective cohort
study. Lipids Health Dis. 20:1042021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yin W, Ao Y, Jia Q, Zhang C, Yuan L, Liu
S, Xiao W, Luo G, Shi X, Xin C, et al: Integrated singlecell and
bulk RNA-seq analysis identifies a prognostic signature related to
inflammation in colorectal cancer. Sci Rep. 15:8742025. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yin Y, Wu C, Zhou Y, Zhang M, Mai S, Chen
M and Wang HY: Ezetimibe INDUCES PARAPTOSIS THROUGH NIEMANN-PICK
C1-like 1 inhibition of mammalian-target-of-rapamycin signaling in
hepatocellular carcinoma cells. Genes (Basel). 15:42023. View Article : Google Scholar
|
|
89
|
Liu X, Lv M, Zhang W and Zhan Q:
Dysregulation of cholesterol metabolism in cancer progression.
Oncogene. 42:3289–3302. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Guo XJ, Zhu BB, Li J, Guo P, Niu YB, Shi
JL, Yokoyama W, Huang QS and Shao DY: Cholesterol metabolism in
tumor immunity: Mechanisms and therapeutic opportunities for
cancer. Biochem Pharmacol. 234:1168022025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pan Z, Wang K, Wang X, Jia Z, Yang Y, Duan
Y, Huang L, Wu ZX, Zhang JY and Ding X: Cholesterol promotes
EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1
signaling-mediated ERRα re-expression. Mol Cancer. 21:772022.
View Article : Google Scholar
|
|
92
|
Rademaker G, Hernandez GA, Seo Y, Dahal S,
Miller-Phillips L, Li AL, Peng XL, Luan C, Qiu L, Liegeois MA, et
al: PCSK9 drives sterol-dependent metastatic organ choice in
pancreatic cancer. Nature. 643:1381–1390. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fu R, Xue W, Liang J, Li X, Zheng J, Wang
L, Zhang M and Meng J: SOAT1 regulates cholesterol metabolism to
induce EMT in hepatocellular carcinoma. Cell Death Dis. 15:3252024.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Alvarado-Ortiz E, de la Cruz-López KG,
Becerril-Rico J, Sarabia-Sánchez MA, Ortiz-Sánchez E and
García-Carrancá A: Mutant p53 gain-of-function: Role in cancer
development, progression, and therapeutic approaches. Front Cell
Dev Biol. 8:6076702021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Freed-Pastor WA, Mizuno H, Zhao X,
Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li
W, Polotskaia A, et al: Mutant p53 disrupts mammary tissue
architecture via the mevalonate pathway. Cell. 148:244–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Moon SH, Huang CH, Houlihan SL, Regunath
K, Freed-Pastor WA, Morris JP IV, Tschaharganeh DF, Kastenhuber ER,
Barsotti AM, Culp-Hill R, et al: p53 represses the mevalonate
pathway to mediate tumor suppression. Cell. 176:564–580.e19. 2019.
View Article : Google Scholar :
|
|
97
|
Zhang Y, Mohibi S, Vasilatis DM, Chen M,
Zhang J and Chen X: Ferredoxin reductase and p53 are necessary for
lipid homeostasis and tumor suppression through the ABCA1-SREBP
pathway. Oncogene. 41:1718–1726. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fan S, Guo J, Nie H, Xiong H and Xia Y:
Aberrant energy metabolism in tumors and potential therapeutic
targets. Genes Chromosomes Cancer. 63:e700082024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Da Cruz Paula A, Zhu Y, Brown DN, Issa
Bhaloo S, Pareja F, Hoang TJ, Green H, Basili T, Dopeso H, Selenica
P, et al: Evolution and co-occurrence of PI3K pathway gene
mutations in endometrial carcinoma molecular subtypes at the
single-cell level. Clin Cancer Res. August 20–2025.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kaysudu I, Gungul TB, Atici S, Yilmaz S,
Bayram E, Guven G, Cizmecioglu NT, Sahin O, Yesiloz G, Haznedaroglu
BZ and Cizmecioglu O: Cholesterol biogenesis is a PTEN-dependent
actionable node for the treatment of endocrine therapy-refractory
cancers. Cancer Sci. 114:4365–4375. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fitzgerald TL, Lertpiriyapong K, Cocco L,
Martelli AM, Libra M, Candido S, Montalto G, Cervello M, Steelman
L, Abrams SL and McCubrey JA: Roles of EGFR and KRAS and their
downstream signaling pathways in pancreatic cancer and pancreatic
cancer stem cells. Adv Biol Regul. 59:65–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Aylon Y and Oren M: The Hippo pathway, p53
and cholesterol. Cell Cycle. 15:2248–2255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Eid W, Dauner K, Courtney KC, Gagnon A,
Parks RJ, Sorisky A and Zha X: mTORC1 activates SREBP-2 by
suppressing cholesterol trafficking to lysosomes in mammalian
cells. Proc Natl Acad Sci USA. 114:7999–8004. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Deng YZ, Cai Z, Shi S, Jiang H, Shang YR,
Ma N, Wang JJ, Guan DX, Chen TW, Rong YF, et al: Cilia loss
sensitizes cells to transformation by activating the mevalonate
pathway. J Exp Med. 215:177–195. 2018. View Article : Google Scholar :
|
|
105
|
Das S, Parigi SM, Luo X, Fransson J, Kern
BC, Okhovat A, Diaz OE, Sorini C, Czarnewski P, Webb AT, et al:
Liver X receptor unlinks intestinal regeneration and tumorigenesis.
Nature. 637:1198–1206. 2025. View Article : Google Scholar :
|
|
106
|
Bakiri L, Hamacher R, Graña O,
Guío-Carrión A, Campos-Olivas R, Martinez L, Dienes HP, Thomsen MK,
Hasenfuss SC and Wagner EF: Liver carcinogenesis by FOS-dependent
inflammation and cholesterol dysregulation. J Exp Med.
214:1387–1409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Deng CM, Zhang GG, Liu QW, Xu JJ, Liu ZC,
Yang J, Xu TY, Li ZG, Zhang F and Li B: ANO1 reprograms cholesterol
metabolism and the tumor microenvironment to promote cancer
metastasis. Cancer Res. 83:1851–1865. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhou YX, Wei J, Deng G, Hu A, Sun PY, Zhao
X, Song BL and Luo J: Delivery of low-density lipoprotein from
endocytic carriers to mitochondria supports steroidogenesis. Nat
Cell Biol. 25:937–949. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
He W, Wang M, Zhang X, Wang Y, Zhao D, Li
W, Lei F, Peng M, Zhang Z, Yuan Y and Huang Z: Estrogen induces
LCAT to maintain cholesterol homeostasis and suppress
hepatocellular carcinoma development. Cancer Res. 84:2417–2431.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Xian M, Wang Q, Xiao L, Zhong L, Xiong W,
Ye L, Su P, Zhang C, Li Y, Orlowski RZ, et al: Leukocyte
immunoglobulin-like receptor B1 (LILRB1) protects human multiple
myeloma cells from ferroptosis by maintaining cholesterol
homeostasis. Nat Commun. 15:57672024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
De Oliveira-Gomes D, Joshi PH, Peterson
ED, Rohatgi A, Khera A and Navar AM: Apolipoprotein B: Bridging the
gap between evidence and clinical practice. Circulation. 150:62–79.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gilliland T, Dron JS, Selvaraj MS, Trinder
M, Paruchuri K, Urbut SM, Haidermota S, Bernardo R, Uddin MM,
Honigberg MC, et al: Genetic architecture and clinical outcomes of
combined lipid disturbances. Circ Res. 135:265–276. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lee G, Jeong YS, Kim DW, Kwak MJ, Koh J,
Joo EW, Lee JS, Kah S, Sim YE and Yim SY: Clinical significance of
APOB inactivation in hepatocellular carcinoma. Exp Mol Med.
50:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Liu Z, Yuan H, Wang Y, Li K, Suo C, Jin L,
Ding C and Chen X: Proteogenomic analysis identifies a causal
association between plasma apolipoprotein B levels and liver cancer
risk. J Proteome Res. 23:4055–4066. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Borgquist S, Butt T, Almgren P, Shiffman
D, Stocks T, Orho-Melander M, Manjer J and Melander O:
Apolipoproteins, lipids and risk of cancer. Int J Cancer.
138:2648–2656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Guan Y, Liu X, Yang Z, Zhu X, Liu M, Du M,
Pan X and Wang Y: PCSK9 promotes LDLR degradation by preventing
SNX17-mediated LDLR recycling. Circulation. 151:1512–1526. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yuan J, Cai T, Zheng X, Ren Y, Qi J, Lu X,
Chen H, Lin H, Chen Z, Liu M, et al: Potentiating CD8+ T
cell antitumor activity by inhibiting PCSK9 to promote
LDLR-mediated TCR recycling and signaling. Protein Cell.
12:240–260. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ma L, Zhang L, Li L and Zhao L: The
function of lncRNA EMX2OS/miR-653-5p and its regulatory mechanism
in lung adenocarcinoma. Open Med (Wars). 18:202306862023.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen Z, Chen L, Sun B, Liu D, He Y, Qi L,
Li G, Han Z, Zhan L, Zhang S, et al: LDLR inhibition promotes
hepatocellular carcinoma proliferation and metastasis by elevating
intracellular cholesterol synthesis through the MEK/ERK signaling
pathway. Mol Metab. 51:1012302021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhu T, Tan JZA, Zhang L, Huang H, Das SS,
Cheng F, Padmanabhan P, Jones MJK, Lee M, Lee A, et al: FTO
suppresses DNA repair by inhibiting PARP1. Nat Commun. 16:29252025.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Alemasova EE and Lavrik OI:
Poly(ADP-ribosyl)ation by PARP1: Reaction mechanism and regulatory
proteins. Nucleic Acids Res. 47:3811–3827. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
He Z, Gong S, Zhang X, Li J, Xue J, Zeng
Q, Nie J, Zhang Z, Ding H, Pei H and Li B: Activated
PARP1/FAK/COL5A1 signaling facilitates the tumorigenesis of
cholesterol-resistant ovarian cancer cells through promoting EMT.
Cell Signal. 124:1114192024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Diestel A, Aktas O, Hackel D, Hake I,
Meier S, Raine CS, Nitsch R, Zipp F and Ullrich O: Activation of
microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown
products during neuroinflammation: A link between demyelination and
neuronal damage. J Exp Med. 198:1729–1740. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Farez MF, Quintana FJ, Gandhi R, Izquierdo
G, Lucas M and Weiner HL: Toll-like receptor 2 and poly(ADP-ribose)
polymerase 1 promote central nervous system neuroinflammation in
progressive EAE. Nat Immunol. 10:958–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lee DH, Nam YJ, Lee MS, Sohn DS and Lee
CS: Rotundarpene attenuates cholesterol oxidation product-induced
apoptosis by suppressing the mitochondrial pathway and the
caspase-8- and bid-dependent pathways. Eur J Pharmacol. 749:39–48.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Batnasan E, Wang R, Wen J, Ke Y, Li X,
Bohio AA, Zeng X, Huo H, Han L, Boldogh I and Ba X: 17-Beta
estradiol inhibits oxidative stress-induced accumulation of AIF
into nucleolus and PARP1-dependent cell death via estrogen receptor
alpha. Toxicol Lett. 232:1–9. 2015. View Article : Google Scholar
|
|
127
|
Wang D, Li Y, Wang N, Luo G, Wang J, Luo
C, Yu W and Hao L: 1α,25-Dihydroxyvitamin D3 prevents
renal oxidative damage via the PARP1/SIRT1/NOX4 pathway in Zucker
diabetic fatty rats. Am J Physiol Endocrinol Metab. 318:E343–E356.
2020. View Article : Google Scholar
|
|
128
|
Allott EH, Howard LE, Cooperberg MR, Kane
CJ, Aronson WJ, Terris MK, Amling CL and Freedland SJ: Serum lipid
profile and risk of prostate cancer recurrence: Results from the
SEARCH database. Cancer Epidemiol Biomarkers Prev. 23:2349–2356.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xu R, Song J, Ruze R, Chen Y, Yin X, Wang
C and Zhao Y: SQLE promotes pancreatic cancer growth by attenuating
ER stress and activating lipid rafts-regulated Src/PI3K/Akt
signaling pathway. Cell Death Dis. 14:4972023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang Z, Huang H, Chen Z, Yan M, Lu C, Xu
Z and Li Z: Helicobacter pylori promotes gastric cancer through
CagA-mediated mitochondrial cholesterol accumulation by targeting
CYP11A1 redistribution. Int J Biol Sci. 20:4007–4028. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Dhanasekaran R, Deutzmann A,
Mahauad-Fernandez WD, Hansen AS, Gouw AM and Felsher DW: The MYC
oncogene-the grand orchestrator of cancer growth and immune
evasion. Nat Rev Clin Oncol. 19:23–36. 2022. View Article : Google Scholar
|
|
132
|
Yang F, Kou J, Liu Z, Li W and Du W: MYC
enhances cholesterol biosynthesis and supports cell proliferation
through SQLE. Front Cell Dev Biol. 9:6558892021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Tan SH, Tan TK, Yokomori R, Liao M, Huang
XZ, Yeoh AEJ and Sanda T: TAL1 hijacks MYCN enhancer that induces
MYCN expression and dependence on mevalonate pathway in T-cell
acute lymphoblastic leukemia. Leukemia. 37:1969–1981. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wu Y, Chen K and Liu X, Huang L, Zhao D,
Li L, Gao M, Pei D, Wang C and Liu X: Srebp-1 interacts with c-Myc
to enhance somatic cell reprogramming. Stem Cells. 34:83–92. 2016.
View Article : Google Scholar
|
|
135
|
Zhou X, Wang G, Tian C, Du L, Prochownik
EV and Li Y: Inhibition of DUSP18 impairs cholesterol biosynthesis
and promotes anti-tumor immunity in colorectal cancer. Nat Commun.
15:58512024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Liu F, Chen W, Zhang Z, Zeng W, Hu H, Ning
S, Liao Z, Liu Y, Zhang H, Fu Q, et al: Targeting Aurora kinase B
regulates cholesterol metabolism and enhances chemoimmunotherapy in
cholangiocarcinoma. Gut. July 31–2025.Epub ahead of print.
View Article : Google Scholar
|
|
137
|
Gui L, Chen K, Yan J, Chen P, Gao WQ and
Ma B: Targeting the mevalonate pathway potentiates NUAK1
inhibition-induced immunogenic cell death and antitumor immunity.
Cell Rep Med. 6:1019132025. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Singh PK and Mehla K: LXR
signaling-mediated cholesterol metabolism reprogramming regulates
cancer cell metastasis. Cancer Res. 83:1759–1761. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Mullen PJ, Yu R, Longo J, Archer MC and
Penn LZ: The interplay between cell signalling and the mevalonate
pathway in cancer. Nat Rev Cancer. 16:718–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Seidah NG and Prat A: The multifaceted
biology of PCSK9. Endocr Rev. 43:558–582. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Collins R, Reith C, Emberson J, Armitage
J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets
D, et al: Interpretation of the evidence for the efficacy and
safety of statin therapy. Lancet. 388:2532–2561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Xie W, Peng M, Liu Y, Zhang B, Yi L and
Long Y: Simvastatin induces pyroptosis via ROS/caspase-1/GSDMD
pathway in colon cancer. Cell Commun Signal. 21:3292023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Choi J, Nguyen VH, Przybyszewski E, Song
J, Carroll A, Michta M, Almazan E, Simon TG and Chung RT: Statin
use and risk of hepatocellular carcinoma and liver fibrosis in
chronic liver disease. JAMA Intern Med. 185:522–530. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Nowakowska MK, Lei X, Thompson MT,
Shaitelman SF, Wehner MR, Woodward WA, Giordano SH and Nead KT:
Association of statin use with clinical outcomes in patients with
triple-negative breast cancer. Cancer. 127:4142–4150. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Hemmati S, Saeidikia Z, Seradj H and
Mohagheghzadeh A: Immunomodulatory peptides as vaccine adjuvants
and antimicrobial agents. Pharmaceuticals (Basel). 17:2012024.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pospiech M, Owens SE, Miller DJ,
Austin-Muttitt K, Mullins JGL, Cronin JG, Allemann RK and Sheldon
IM: Bisphosphonate inhibitors of squalene synthase protect cells
against cholesterol-dependent cytolysins. FASEB J. 35:e216402021.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Toyota Y, Yoshioka H, Sagimori I,
Hashimoto Y and Ohgane K: Bisphosphonate esters interact with
HMG-CoA reductase membrane domain to induce its degradation. Bioorg
Med Chem. 28:1155762020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Van Acker HH, Anguille S, Willemen Y,
Smits EL and Van Tendeloo VF: Bisphosphonates for cancer treatment:
Mechanisms of action and lessons from clinical trials. Pharmacol
Ther. 158:24–40. 2016. View Article : Google Scholar
|
|
149
|
Yoneda T, Michigami T, Yi B, Williams PJ,
Niewolna M and Hiraga T: Actions of bisphosphonate on bone
metastasis in animal models of breast carcinoma. Cancer.
88:2979–2988. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
D'Oronzo S, Gregory W, Nicholson S, Chong
YK, Brown J and Coleman R: Natural history of stage II/III breast
cancer, bone metastasis and the impact of adjuvant zoledronate on
distribution of recurrences. J Bone Oncol. 28:1003672021.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Liu D, Wong CC, Fu L, Chen H, Zhao L, Li
C, Zhou Y, Zhang Y, Xu W, Yang Y, et al: Squalene epoxidase drives
NAFLD-induced hepatocellular carcinoma and is a pharmaceutical
target. Sci Transl Med. 10:eaap98402018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang Y, Ma X, Xu E, Huang Z, Yang C, Zhu
K, Dong Y and Zhang C: Identifying squalene epoxidase as a
metabolic vulnerability in high-risk osteosarcoma using an
artificial intelligence-derived prognostic index. Clin Transl Med.
14:e15862024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ma L, Huang W, Liang X, Bai G, Wang X,
Jiang H, Xin Y, Hu L, Chen X and Liu C: Inhibition of squalene
epoxidase linking with PI3K/AKT signaling pathway suppresses
endometrial cancer. Cancer Sci. 114:3595–3607. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kalogirou C, Linxweiler J, Schmucker P,
Snaebjornsson MT, Schmitz W, Wach S, Krebs M, Hartmann E, Puhr M,
Müller A, et al: MiR-205-driven downregulation of cholesterol
biosynthesis through SQLE-inhibition identifies therapeutic
vulnerability in aggressive prostate cancer. Nat Commun.
12:50662021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hu LP, Huang W, Wang X, Xu C, Qin WT, Li
D, Tian G, Li Q, Zhou Y, Chen S, et al: Terbinafine prevents
colorectal cancer growth by inducing dNTP starvation and reducing
immune suppression. Mol Ther. 30:3284–3299. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhang EB, Zhang X, Wang K, Zhang F, Chen
TW, Ma N, Ni QZ, Wang YK, Zheng QW, Cao HJ, et al: Antifungal agent
terbinafine restrains tumor growth in preclinical models of
hepatocellular carcinoma via AMPK-mTOR axis. Oncogene.
40:5302–5313. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
He Q, Kong L, Shi W, Ma D, Liu K, Yang S,
Xin Q, Jiang C and Wu J: Ezetimibe inhibits triple-negative breast
cancer proliferation and promotes cell cycle arrest by targeting
the PDGFR/AKT pathway. Heliyon. 9:e213432023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wang Y, You S, Su S, Yeon A, Lo EM, Kim S,
Mohler JL, Freeman MR and Kim HL: Cholesterol-lowering intervention
decreases mTOR complex 2 signaling and enhances antitumor immunity.
Clin Cancer Res. 28:414–424. 2022. View Article : Google Scholar :
|
|
160
|
Bovenga F, Sabbà C and Moschetta A:
Uncoupling nuclear receptor LXR and cholesterol metabolism in
cancer. Cell Metab. 21:517–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Liang H and Shen X: LXR activation
radiosensitizes non-small cell lung cancer by restricting
myeloid-derived suppressor cells. Biochem Biophys Res Commun.
528:330–335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Wan W, Hou Y, Wang K, Cheng Y, Pu X and Ye
X: The LXR-623-induced long non-coding RNA LINC01125 suppresses the
proliferation of breast cancer cells via PTEN/AKT/p53 signaling
pathway. Cell Death Dis. 10:2482019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Munir MT, Ponce C, Santos JM, Sufian HB,
Al-Harrasi A, Gollahon LS, Hussain F and Rahman SM: VD3
and LXR agonist (T0901317) combination demonstrated greater potency
in inhibiting cholesterol accumulation and inducing apoptosis via
ABCA1-CHOP-BCL-2 cascade in MCF-7 breast cancer cells. Mol Biol
Rep. 47:7771–7782. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Tavazoie MF, Pollack I, Tanqueco R,
Ostendorf BN, Reis BS, Gonsalves FC, Kurth I, Andreu-Agullo C,
Derbyshire ML, Posada J, et al: LXR/ApoE activation restricts
innate immune suppression in cancer. Cell. 172:825–840.e18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Gronich N and Rennert G: Beyond
aspirin-cancer prevention with statins, metformin and
bisphosphonates. Nat Rev Clin Oncol. 10:625–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Kamal A, Boerner J, Assad H, Chen W and
Simon MS: The effect of statins on markers of breast cancer
proliferation and apoptosis in women with in situ or early-stage
invasive breast cancer. Int J Mol Sci. 25:95872024. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Graf H, Jüngst C, Straub G, Dogan S,
Hoffmann RT, Jakobs T, Reiser M, Waggershauser T, Helmberger T,
Walter A, et al: Chemoembolization combined with pravastatin
improves survival in patients with hepatocellular carcinoma.
Digestion. 78:34–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Kim Y, Kim TW, Han SW, Ahn JB, Kim ST, Lee
J, Park JO, Park YS, Lim HY and Kang WK: A single arm, phase II
study of simvastatin plus XELOX and bevacizumab as first-line
chemotherapy in metastatic colorectal cancer patients. Cancer Res
Treat. 51:1128–1134. 2019. View Article : Google Scholar :
|
|
169
|
Jian-Yu E, Graber JM, Lu SE, Lin Y, Lu-Yao
G and Tan XL: Effect of metformin and statin use on survival in
pancreatic cancer patients: A systematic literature review and
meta-analysis. Curr Med Chem. 25:2595–2607. 2018. View Article : Google Scholar
|
|
170
|
Nguyen MKL, Jose J, Wahba M, Bernaus-Esqué
M, Hoy AJ, Enrich C, Rentero C and Grewal T: Linking late endosomal
cholesterol with cancer progression and anticancer drug resistance.
Int J Mol Sci. 23:72062022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Du X, Zhang Y, Jo SR, Liu X, Qi Y, Osborne
B, Byrne FL, Smith GC, Turner N, Hoehn KL, et al: Akt activation
increases cellular cholesterol by promoting the proteasomal
degradation of Niemann-Pick C1. Biochem J. 471:243–253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Chen K, Zhang X, Sun G, Fang Z, Liao L,
Zhong Y, Huang F, Dong M and Luo S: Focusing on the abnormal events
of NPC1, NPC2, and NPC1L1 in pan-cancer and further constructing
LUAD and KICH prediction models. J Proteome Res. 23:449–464. 2024.
View Article : Google Scholar
|
|
173
|
Liang B, Wu Q, Wang Y, Shi Y, Sun F, Huang
Q, Li G, Liu Y, Zhang S, Xu X, et al: Cdc42-driven endosomal
cholesterol transport promotes collateral resistance in
HER2-positive gastric cancer. Cancer Lett. 587:2167022024.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Lin MH, Chao TC, Lee CC, Tung CJ, Yeh CY
and Hong JH: Measurement-based Monte Carlo dose calculation system
for IMRT pretreatment and on-line transit dose verifications. Med
Phys. 36:1167–1175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Li Y, Ran Q, Duan Q, Jin J, Wang Y, Yu L,
Wang C, Zhu Z, Chen X, Weng L, et al: 7-Dehydrocholesterol dictates
ferroptosis sensitivity. Nature. 626:411–418. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Song YH, Lei HX, Yu D, Zhu H, Hao MZ, Cui
RH, Meng XS, Sheng XH and Zhang L: Endogenous chemicals guard
health through inhibiting ferroptotic cell death. Biofactors.
50:266–293. 2024. View Article : Google Scholar
|
|
180
|
Lee J and Roh JL: Cholesterol-ferroptosis
nexus: Unveiling novel cancer therapeutic avenues. Cancer Lett.
597:2170462024. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Mao X, Xiong J, Cai M, Wang C, He Q, Wang
B, Chen J, Xiao Z, Wang B, Han S and Zhang Y: SCARB1 links
cholesterol metabolism-mediated ferroptosis inhibition to
radioresistance in tumor cells. J Adv Res. January 18–2025.Epub
ahead of print. View Article : Google Scholar
|
|
182
|
Garcia-Bermudez J, Baudrier L, Bayraktar
EC, Shen Y, La K, Guarecuco R, Yucel B, Fiore D, Tavora B,
Freinkman E, et al: Squalene accumulation in cholesterol
auxotrophic lymphomas prevents oxidative cell death. Nature.
567:118–122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Wang Y, Calvert AE, Cardenas H, Rink JS,
Nahotko D, Qiang W, Ndukwe CE, Chen F, Keathley R, Zhang Y, et al:
Nanoparticle targeting in chemo-resistant ovarian cancer reveals
dual axis of therapeutic vulnerability involving cholesterol uptake
and cell redox balance. Adv Sci (Weinh). 11:e23052122024.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Riscal R, Bull CJ, Mesaros C, Finan JM,
Carens M, Ho ES, Xu JP, Godfrey J, Brennan P, Johansson M, et al:
Cholesterol auxotrophy as a targetable vulnerability in clear cell
renal cell carcinoma. Cancer Discovery. 11:3106–3125. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Huang B, Song BL and Xu C: Cholesterol
metabolism in cancer: Mechanisms and therapeutic opportunities. Nat
Metab. 2:132–141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Yuan J, Lv T, Yang J, Wu Z, Yan L, Yang J
and Shi Y: HDLBP-stabilized lncFAL inhibits ferroptosis
vulnerability by diminishing Trim69-dependent FSP1 degradation in
hepatocellular carcinoma. Redox Biol. 58:1025462022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Jin H, Zhu M, Zhang D, Liu X, Guo Y, Xia
L, Chen Y, Chen Y, Xu R, Liu C, et al: B7H3 increases ferroptosis
resistance by inhibiting cholesterol metabolism in colorectal
cancer. Cancer Sci. 114:4225–4236. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Noguchi N, Saito Y and Niki E: Lipid
peroxidation, ferroptosis, and antioxidants. Free Radic Biol Med.
237:228–238. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zhao X, Lian X, Xie J and Liu G:
Accumulated cholesterol protects tumours from elevated lipid
peroxidation in the microenvironment. Redox Biol. 62:1026782023.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Li Y, Li Z, Ran Q and Wang P: Sterols in
ferroptosis: From molecular mechanisms to therapeutic strategies.
Trends Mol Med. 31:36–49. 2025. View Article : Google Scholar
|
|
191
|
Bai T, Xue P, Shao S, Yan S and Zeng X:
Cholesterol depletion-enhanced ferroptosis and immunotherapy via
engineered nanozyme. Adv Sci (Weinh). 11:e24058262024. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Vitalakumar D, Sharma A and Flora SJS:
Ferroptosis: A potential therapeutic target for neurodegenerative
diseases. J Biochem Mol Toxicol. 35:e228302021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Shimada K, Skouta R, Kaplan A, Yang WS,
Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ and
Stockwell BR: Global survey of cell death mechanisms reveals
metabolic regulation of ferroptosis. Nat Chem Biol. 12:497–503.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Yao X, Xie R, Cao Y, Tang J, Men Y, Peng H
and Yang W: Simvastatin induced ferroptosis for triple-negative
breast cancer therapy. J Nanobiotechnology. 19:3112021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Tang WJ, Xu D, Liang MX, Wo GQ, Chen WQ,
Tang JH and Zhang W: Pitavastatin induces autophagy-dependent
ferroptosis in MDA-MB-231 cells via the mevalonate pathway.
Heliyon. 10:e270842024. View Article : Google Scholar
|
|
196
|
Zhang R, Zhang L, Fan S and Wang L, Wang B
and Wang L: Squalene monooxygenase (SQLE) protects ovarian cancer
cells from ferroptosis. Sci Rep. 14:226462024. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Mao X, Wang L, Chen Z, Huang H, Chen J, Su
J, Li Z, Shen G, Ren Y, Li Z, et al: SCD1 promotes the stemness of
gastric cancer stem cells by inhibiting ferroptosis through the
SQLE/cholesterol/mTOR signalling pathway. Int J Biol Macromol.
275:1336982024. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Vargas JNS, Hamasaki M, Kawabata T, Youle
RJ and Yoshimori T: The mechanisms and roles of selective autophagy
in mammals. Nat Rev Mol Cell Biol. 24:167–185. 2023. View Article : Google Scholar
|
|
200
|
Yamamoto H, Zhang S and Mizushima N:
Autophagy genes in biology and disease. Nat Rev Genet. 24:382–400.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Rybstein MD, Bravo-San Pedro JM, Kroemer G
and Galluzzi L: The autophagic network and cancer. Nat Cell Biol.
20:243–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Castellano BM, Thelen AM, Moldavski O,
Feltes M, van der Welle RE, Mydock-McGrane L, Jiang X, van Eijkeren
RJ, Davis OB, Louie SM, et al: Lysosomal cholesterol activates
mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science.
355:1306–1311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Liu F, Tian T, Zhang Z, Xie S, Yang J, Zhu
L, Wang W, Shi C, Sang L, Guo K, et al: Long non-coding RNA SNHG6
couples cholesterol sensing with mTORC1 activation in
hepatocellular carcinoma. Nat Metab. 4:1022–1040. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Podar K, Tai YT, Cole CE, Hideshima T,
Sattler M, Hamblin A, Mitsiades N, Schlossman RL, Davies FE, Morgan
GJ, et al: Essential role of caveolae in interleukin-6- and
insulin-like growth factor I-triggered Akt-1-mediated survival of
multiple myeloma cells. J Biol Chem. 278:5794–5801. 2003.
View Article : Google Scholar
|
|
205
|
Vilimanovich U, Bosnjak M, Bogdanovic A,
Markovic I, Isakovic A, Kravic-Stevovic T, Mircic A, Trajkovic V
and Bumbasirevic V: Statin-mediated inhibition of cholesterol
synthesis induces cytoprotective autophagy in human leukemic cells.
Eur J Pharmacol. 765:415–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Yi J, Zhu J, Wu J, Thompson CB and Jiang
X: Oncogenic activation of PI3K-AKT-mTOR signaling suppresses
ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA.
117:31189–31197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Guo Y, Zhao M, Bo T, Ma S, Yuan Z, Chen W,
He Z, Hou X, Liu J, Zhang Z, et al: Blocking FSH inhibits hepatic
cholesterol biosynthesis and reduces serum cholesterol. Cell Res.
29:151–166. 2019. View Article : Google Scholar :
|
|
208
|
Jun I, Choi YJ, Kim BR, Lee HK, Seo KY and
Kim TI: Activation of the mTOR pathway enhances
PPARγ/SREBP-mediated lipid synthesis in human meibomian gland
epithelial cells. Sci Rep. 14:281182024. View Article : Google Scholar
|
|
209
|
Hsu JL, Leu WJ, Zhong NS and Guh JH:
Autophagic activation and decrease of plasma membrane cholesterol
contribute to anticancer activities in non-small cell lung cancer.
Molecules. 26:59672021. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Zhong Y, Geng F, Mazik L, Yin X, Becker
AP, Mohammed S, Su H, Xing E, Kou Y, Chiang CY, et al:
Combinatorial targeting of glutamine metabolism and lysosomal-based
lipid metabolism effectively suppresses glioblastoma. Cell Rep Med.
5:1017062024. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Ng MYW, Charsou C, Lapao A, Singh S,
Trachsel-Moncho L, Schultz SW, Nakken S, Munson MJ and Simonsen A:
The cholesterol transport protein GRAMD1C regulates autophagy
initiation and mitochondrial bioenergetics. Nat Commun.
13:62832022. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Shi Y, Ye Z, Lu G, Yang N, Zhang J, Wang
L, Cui J, Del Pozo MA, Wu Y, Xia D and Shen HM:
Cholesterol-enriched membrane micro-domaindeficiency induces
doxorubicin resistancevia promoting autophagy in breast cancer. Mol
Ther Oncolytics. 23:311–329. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Shao WQ, Zhu WW, Luo MJ, Fan MH, Li Q,
Wang SH, Lin ZF, Zhao J, Zheng Y, Dong QZ, et al: Cholesterol
suppresses GOLM1-dependent selective autophagy of RTKs in
hepatocellular carcinoma. Cell Rep. 39:1107122022. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Yang J, Peng S and Zhang K: ARL4C
depletion suppresses the resistance of ovarian cancer to
carboplatin by disrupting cholesterol transport and autophagy via
notch-RBP-Jκ-H3K4Me3-OSBPL5. Hum Exp Toxicol.
41:96032712211350642022. View Article : Google Scholar
|
|
215
|
McBrearty N, Cho C, Chen J, Zahedi F, Peck
AR, Radaelli E, Assenmacher CA, Pavlak C, Devine A, Yu P, et al:
Tumor-suppressive and immune-stimulating roles of cholesterol
25-hydroxylase in pancreatic cancer cells. Mol Cancer Res.
21:228–239. 2023. View Article : Google Scholar :
|
|
216
|
Liu C, Sun L, Niu N, Hou P, Chen G, Wang
H, Zhang Z, Jiang X, Xu Q, Zhao Y, et al: Molecular classification
of hormone receptor-positive/HER2-positive breast cancer reveals
potential neoadjuvant therapeutic strategies. Signal Transduct
Target Ther. 10:972025. View Article : Google Scholar
|
|
217
|
Leignadier J, Dalenc F, Poirot M and
Silvente-Poirot S: Improving the efficacy of hormone therapy in
breast cancer: The role of cholesterol metabolism in SERM-mediated
autophagy, cell differentiation and death. Biochem Pharmacol.
144:18–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
de Medina P, Paillasse MR, Ségala G,
Khallouki F, Brillouet S, Dalenc F, Courbon F, Record M, Poirot M
and Silvente-Poirot S: Importance of cholesterol and oxysterols
metabolism in the pharmacology of tamoxifen and other AEBS ligands.
Chem Phys Lipids. 164:432–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Jiang S, Wang X, Song D, Liu X, Gu Y, Xu
Z, Wang X, Zhang X, Ye Q, Tong Z, et al: Cholesterol induces
epithelial-to-mesenchymal transition of prostate cancer cells by
suppressing degradation of EGFR through APMAP. Cancer Res.
79:3063–3075. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Shapira KE, Ehrlich M and Henis YI:
Cholesterol depletion enhances TGF-β Smad signaling by increasing
c-Jun expression through a PKR-dependent mechanism. Mol Biol Cell.
29:2494–2507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Zheng S, Lin J, Pang Z, Zhang H, Wang Y,
Ma L, Zhang H, Zhang X, Chen M, Zhang X, et al: Aberrant
cholesterol metabolism and Wnt/β-catenin signaling coalesce via
Frizzled5 in supporting cancer growth. Adv Sci (Weinh).
9:e22007502022. View Article : Google Scholar
|
|
224
|
Ehmsen S and Ditzel HJ: Signaling pathways
essential for triple-negative breast cancer stem-like cells. Stem
Cells. 39:133–143. 2021. View Article : Google Scholar
|
|
225
|
Wang XD, Kim C, Zhang Y, Rindhe S, Cobb MH
and Yu Y: Cholesterol regulates the tumor adaptive resistance to
MAPK pathway inhibition. J Proteome Res. 20:5379–5391. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Aguirre-Portolés C, Feliu J, Reglero G and
Ramírez de Molina A: ABCA1 overexpression worsens colorectal cancer
prognosis by facilitating tumour growth and caveolin-1-dependent
invasiveness, and these effects can be ameliorated using the BET
inhibitor apabetalone. Mol Oncol. 12:1735–1752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Zhan J, Wang P, Li S, Song J, He H, Wang
Y, Liu Z, Wang F, Bai H, Fang W, et al: HOXB13 networking with
ABCG1/EZH2/Slug mediates metastasis and confers resistance to
cisplatin in lung adenocarcinoma patients. Theranostics.
9:2084–2099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Bu L, Zhang Z, Chen J, Fan Y, Guo J, Su Y,
Wang H, Zhang X, Wu X, Jiang Q, et al: High-fat diet promotes liver
tumorigenesis via palmitoylation and activation of AKT. Gut.
73:1156–1168. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Dong J, Kong L, Wang S, Xia M, Zhang Y, Wu
J, Yang F, Zuo S and Wei J: Oncolytic adenovirus encoding
apolipoprotein A1 suppresses metastasis of triple-negative breast
cancer in mice. J Exp Clin Cancer Res. 43:1022024. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Li Y, Zhou Y, Huang M, Wang Z, Liu D, Liu
J, Fu X, Yang S, Shan S, Yang L, et al: DHCR7 promotes
tumorigenesis via activating PI3K/AKT/mTOR signalling pathway in
bladder cancer. Cell Signal. 102:1105532023. View Article : Google Scholar
|
|
231
|
Wan S, He QY, Yang Y, Liu F, Zhang X, Guo
X, Niu H, Wang Y, Liu YX, Ye WL, et al: SPARC stabilizes ApoE to
induce cholesterol-dependent invasion and sorafenib resistance in
hepatocellular carcinoma. Cancer Res. 84:1872–1888. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Tashiro J, Sugiura A, Warita T, Irie N,
Dwi Cahyadi D, Ishikawa T and Warita K: CYP11A1 silencing
suppresses HMGCR expression via cholesterol accumulation and
sensitizes CRPC cell line DU-145 to atorvastatin. J Pharmacol Sci.
153:104–112. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Gómez-López S, Alhendi ASN, Przybilla MJ,
Bordeu I, Whiteman ZE, Butler T, Rouhani MJ, Kalinke L, Uddin I,
Otter KEJ, et al: Aberrant basal cell clonal dynamics shape early
lung carcinogenesis. Science. 388:eads91452025. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Lampropoulou DI, Papadimitriou M,
Papadimitriou C, Filippou D, Kourlaba G, Aravantinos G and Gazouli
M: The role of EMT-related lncRNAs in ovarian cancer. Int J Mol
Sci. 24:100792023. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Warita K, Ishikawa T, Sugiura A, Tashiro
J, Shimakura H, Hosaka YZ, Ohta KI, Warita T and Oltvai ZN:
Concomitant attenuation of HMGCR expression and activity enhances
the growth inhibitory effect of atorvastatin on TGF-β-treated
epithelial cancer cells. Sci Rep. 11:127632021. View Article : Google Scholar
|
|
236
|
Xiao J, Wang S, Chen L, Ding X, Dang Y,
Han M, Zheng Y, Shen H, Wu S, Wang M, et al: 25-Hydroxycholesterol
regulates lysosome AMP kinase activation and metabolic
reprogramming to educate immunosuppressive macrophages. Immunity.
57:1087–1104.e7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Sun Z, Xu Y, Shao B, Dang P, Hu S, Sun H,
Chen C, Wang C, Liu J, Liu Y and Hu J: Exosomal circPOLQ promotes
macrophage M2 polarization via activating IL-10/STAT3 axis in a
colorectal cancer model. J Immunother Cancer. 12:e0084912024.
View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Ma Z, Guo L, Pan M, Jiang C, Liu D, Gao Y,
Bai J, Jiang P and Liu X: Inhibition of pseudorabies virus
replication via upregulated interferon response by targeting
7-dehydrocholesterol reductase. Vet Microbiol. 290:1100002024.
View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Ma X, Bi E, Lu Y, Su P, Huang C, Liu L,
Wang Q, Yang M, Kalady MF, Qian J, et al: Cholesterol induces
CD8+ T cell exhaustion in the tumor microenvironment.
Cell Metab. 30:143–156.e5. 2019. View Article : Google Scholar
|
|
240
|
Yang W, Bai Y, Xiong Y, Zhang J, Chen S,
Zheng X, Meng X, Li L, Wang J, Xu C, et al: Potentiating the
antitumour response of CD8(+) T cells by modulating cholesterol
metabolism. Nature. 531:651–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Sugi T, Katoh Y, Ikeda T, Seta D, Iwata T,
Nishio H, Sugawara M, Kato D, Katoh K, Kawana K, et al: SCD1
inhibition enhances the effector functions of CD8+ T
cells via ACAT1-dependent reduction of esterified cholesterol.
Cancer Sci. 115:48–58. 2024. View Article : Google Scholar
|
|
242
|
Xu H, Exner BG, Cramer DE, Tanner MK,
Mueller YM and Ildstad ST: CD8(+), alphabeta-TCR(+), and
gammadelta-TCR(+) cells in the recipient hematopoietic environment
mediate resistance to engraftment of allogeneic donor bone marrow.
J Immunol. 168:1636–1643. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Lü HZ, Zhu AY, Chen Y, Tang J and Li BQ:
Formation and aggregation of lipid rafts in γδ T cells following
stimulation with Mycobacterium tuberculosis antigens. Tohoku J Exp
Med. 223:193–198. 2011. View Article : Google Scholar
|
|
244
|
Júnior RFA, Lira GA, Schomann T,
Cavalcante RS, Vilar NF, de Paula RCM, Gomes RF, Chung CK,
Jorquera-Cordero C, Vepris O, et al: Retinoic acid-loaded PLGA
nanocarriers targeting cell cholesterol potentialize the antitumour
effect of PD-L1 antibody by preventing epithelial-mesenchymal
transition mediated by M2-TAM in colorectal cancer. Transl Oncol.
31:1016472023. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Wang Q, Cao Y, Shen L, Xiao T, Cao R, Wei
S, Tang M, Du L, Wu H, Wu B, et al: Regulation of PD-L1 through
direct binding of cholesterol to CRAC motifs. Sci Adv.
8:eabq47222022. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Sun S, Ma J, Zuo T, Shi J, Sun L, Meng C,
Shu W, Yang Z, Yao H and Zhang Z: Inhibition of PCSK9: A promising
enhancer for Anti-PD-1/PD-L1 immunotherapy. Research (Wash D C).
7:04882024.PubMed/NCBI
|
|
247
|
Ma X, Bi E, Huang C, Lu Y, Xue G, Guo X,
Wang A, Yang M, Qian J, Dong C and Yi Q: Cholesterol negatively
regulates IL-9-producing CD8+ T cell differentiation and
antitumor activity. J Exp Med. 215:1555–1569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Bai Y, Li T, Wang Q, You W, Yang H, Xu X,
Li Z, Zhang Y, Yan C, Yang L, et al: Shaping immune landscape of
colorectal cancer by cholesterol metabolites. EMBO Mol Med.
16:334–360. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Baek AE, Yu YA, He S, Wardell SE, Chang
CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, et al:
The cholesterol metabolite 27 hydroxycholesterol facilitates breast
cancer metastasis through its actions on immune cells. Nat Commun.
8:8642017. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Bruckner M, Dickel D, Singer E and Legler
DF: Converse regulation of CCR7-driven human dendritic cell
migration by prostaglandin E2 and liver X receptor
activation. Eur J Immunol. 42:2949–2958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Kitahara CM, Berrington de González A,
Freedman ND, Huxley R, Mok Y, Jee SH and Samet JM: Total
cholesterol and cancer risk in a large prospective study in Korea.
J Clin Oncol. 29:1592–1598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Mondul AM, Clipp SL, Helzlsouer KJ and
Platz EA: Association between plasma total cholesterol
concentration and incident prostate cancer in the CLUE II cohort.
Cancer Causes Control. 21:61–68. 2010.
|
|
253
|
Nielsen SF, Nordestgaard BG and Bojesen
SE: Statin use and reduced cancer-related mortality. N Engl J Med.
367:1792–1802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Lin Q, Liu W, Xu S and Sun L: Associations
of preoperative serum high-density lipoprotein cholesterol and
low-density lipoprotein cholesterol levels with the prognosis of
ovarian cancer. Arch Gynecol Obstet. 305:683–691. 2022. View Article : Google Scholar
|
|
255
|
Penet MF, Krishnamachary B, Wildes FB,
Mironchik Y, Hung CF, Wu TC and Bhujwalla ZM: Ascites volumes and
the ovarian cancer microenvironment. Front Oncol. 8:5952018.
View Article : Google Scholar
|
|
256
|
Lu L, Katsaros D, Wiley A, Rigault de la
Longrais IA, Puopolo M and Yu H: Expression of MDR1 in epithelial
ovarian cancer and its association with disease progression. Oncol
Res. 16:395–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Mentella MC, Scaldaferri F, Ricci C,
Gasbarrini A and Miggiano GAD: Cancer and mediterranean diet: A
review. Nutrients. 11:20592019. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Mittelman SD: The role of diet in cancer
prevention and chemotherapy efficacy. Annu Rev Nutr. 40:273–297.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Nencioni A, Caffa I, Cortellino S and
Longo VD: Fasting and cancer: molecular mechanisms and clinical
application. Nat Rev Cancer. 18:707–719. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Li C, Yang L, Zhang D and Jiang W:
Systematic review and meta-analysis suggest that dietary
cholesterol intake increases risk of breast cancer. Nutr Res.
36:627–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Bandera EV, Kushi LH, Moore DF, Gifkins DM
and McCullough ML: Dietary lipids and endometrial cancer: The
current epidemiologic evidence. Cancer Causes Control. 18:687–703.
2007.PubMed/NCBI
|
|
262
|
Yang H, Wang F, Hallemeier CL, Lerut T and
Fu J: Oesophageal cancer. Lancet. 404:1991–2005. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Jin Y, Yang T, Li D and Ding W: Effect of
dietary cholesterol intake on the risk of esophageal cancer: A
meta-analysis. J Int Med Res. 47:4059–4068. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Wang QL, Khil J, Hong S, Lee DH, Ha KH,
Keum N, Kim HC and Giovannucci EL: Temporal association of total
serum cholesterol and pancreatic cancer incidence. Nutrients.
14:49382022. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Di Maso M, Augustin LSA, Jenkins DJA,
Crispo A, Toffolutti F, Negri E, La Vecchia C, Ferraroni M and
Polesel J: Adherence to a cholesterol-lowering diet and the risk of
pancreatic cancer: A case-control study. Nutrients. 16:25082024.
View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Hu J, La Vecchia C, de Groh M, Negri E,
Morrison H and Mery L; Canadian Cancer Registries Epidemiology
Research Group: Dietary cholesterol intake and cancer. Ann Oncol.
23:491–500. 2012. View Article : Google Scholar
|
|
267
|
Rice MS, Poole EM, Willett WC and Tworoger
SS: Adult dietary fat intake and ovarian cancer risk. Int J Cancer.
146:2756–2772. 2020. View Article : Google Scholar :
|
|
268
|
Chlebowski RT, Anderson GL, Manson JE,
Prentice RL, Aragaki AK, Snetselaar L, Beresford SAA, Kuller LH,
Johnson K, Lane D, et al: Low-fat dietary pattern and cancer
mortality in the women's health initiative (WHI) randomized
controlled trial. JNCI Cancer Spectr. 2:pky0652019. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Lin X, Liu L, Fu Y, Gao J, He Y, Wu Y and
Lian X: Dietary cholesterol intake and risk of lung cancer: A
meta-analysis. Nutrients. 10:1852018. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Wu Y, Zheng W, Sellers TA, Kushi LH,
Bostick RM and Potter JD: Dietary cholesterol, fat, and lung cancer
incidence among older women: The Iowa women's health study (United
States). Cancer Causes Control. 5:395–400. 1994.PubMed/NCBI
|
|
271
|
Habis M, Wroblewski K, Bradaric M, Ismail
N, Yamada SD, Litchfield L, Lengyel E and Romero IL: Statin therapy
is associated with improved survival in patients with
non-serous-papillary epithelial ovarian cancer: A retrospective
cohort analysis. PLoS One. 9:e1045212014. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Iyengar NM, Gucalp A, Dannenberg AJ and
Hudis CA: Obesity and cancer mechanisms: Tumor microenvironment and
inflammation. J Clin Oncol. 34:4270–4276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Yan P and Zhao D: Association between
serum total cholesterol and the development of gastric cancer: A
two-way two-sample Mendelian randomization study. Medicine
(Baltimore). 103:e389002024. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Gruenbacher G and Thurnher M: Mevalonate
metabolism in immuno-oncology. Front Immunol. 8:17142017.
View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Madan B, Virshup DM, Nes WD and Leaver DJ:
Unearthing the Janus-face cholesterogenesis pathways in cancer.
Biochem Pharmacol. 196:1146112022. View Article : Google Scholar
|
|
276
|
Murto MO, Simolin N, Arponen O, Siltari A,
Artama M, Visvanathan K, Jukkola A and Murtola TJ: Statin use,
cholesterol level, and mortality among females with breast cancer.
JAMA Netw Open. 6:e23438612023. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Kansal V, Burnham AJ, Kinney BLC, Saba NF,
Paulos C, Lesinski GB, Buchwald ZS and Schmitt NC: Statin drugs
enhance responses to immune checkpoint blockade in head and neck
cancer models. J Immunother Cancer. 11:e0059402023. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Zhang Y, Wu K, Chan AT, Meyerhardt JA and
Giovannucci EL: Long-term statin use, total cholesterol level, and
risk of colorectal cancer: A prospective cohort study. Am J
Gastroenterol. 117:158–166. 2022. View Article : Google Scholar :
|
|
279
|
Morote J, Celma A, Planas J, Placer J, de
Torres I, Olivan M, Carles J, Reventós J and Doll A: Role of serum
cholesterol and statin use in the risk of prostate cancer detection
and tumor aggressiveness. Int J Mol Sci. 15:13615–13623. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Hillis AL, Martin TD, Manchester HE,
Högström J, Zhang N, Lecky E, Kozlova N, Lee J, Persky NS, Root DE,
et al: Targeting cholesterol biosynthesis with statins synergizes
with AKT inhibitors in triple-negative breast cancer. Cancer Res.
84:3250–3266. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Trotta F, Avena P, Chimento A, Rago V, De
Luca A, Sculco S, Nocito MC, Malivindi R, Fallo F, Pezzani R, et
al: Statins reduce intratumor cholesterol affecting adrenocortical
cancer growth. Mol Cancer Ther. 19:1909–1921. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Qiu W, Su W, Xu J, Liang M, Ma X, Xue P,
Kang Y, Sun ZJ and Xu Z: Immunomodulatory-photodynamic
nanostimulators for invoking pyroptosis to augment tumor
immunotherapy. Adv Healthc Mater. 11:e22012332022. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Nguyen TTT, Ishida CT, Shang E, Shu C,
Torrini C, Zhang Y, Bianchetti E, Sanchez-Quintero MJ, Kleiner G,
Quinzii CM, et al: Activation of LXRβ inhibits tumor respiration
and is synthetically lethal with Bcl-xL inhibition. EMBO Mol Med.
11:e107692019. View Article : Google Scholar
|
|
284
|
Nguyen TTT, Ishida CT, Shang E, Shu C,
Bianchetti E, Karpel-Massler G and Siegelin MD: Activation of LXR
receptors and inhibition of TRAP1 causes synthetic lethality in
solid tumors. Cancers (Basel). 11:7882019. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Cai J, Ye Z, Hu Y, Ye L, Gao L, Wang Y,
Sun Q, Tong S, Zhang S, Wu L, et al: Fatostatin induces ferroptosis
through inhibition of the AKT/mTORC1/GPX4 signaling pathway in
glioblastoma. Cell Death Dis. 14:2112023. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Gao S, Shi Z, Li X, Li W, Wang Y, Liu Z
and Jiang J: Fatostatin suppresses growth and enhances apoptosis by
blocking SREBP-regulated metabolic pathways in endometrial
carcinoma. Oncol Rep. 39:1919–1929. 2018.PubMed/NCBI
|
|
287
|
Lee HJ, Li J, Vickman RE, Li J, Liu R,
Durkes AC, Elzey BD, Yue S, Liu X, Ratliff TL and Cheng JX:
Cholesterol esterification inhibition suppresses prostate cancer
metastasis by impairing the Wnt/β-catenin pathway. Mol Cancer Res.
16:974–985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Zhang Y, Li L, Chu F, Wu H, Xiao X, Ye J
and Li K: Itraconazole inhibits tumor growth via CEBPB-mediated
glycolysis in colorectal cancer. Cancer Sci. 115:1154–1169. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Aftab BT, Dobromilskaya I, Liu JO and
Rudin CM: Itraconazole inhibits angiogenesis and tumor growth in
non-small cell lung cancer. Cancer Res. 71:6764–6772. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Wang S, Link F, Han M, Chaudhary R,
Asimakopoulos A, Liebe R, Yao Y, Hammad S, Dropmann A, Krizanac M,
et al: The interplay of TGF-β1 and cholesterol orchestrating
hepatocyte cell fate, EMT, and signals for HSC activation. Cell Mol
Gastroenterol Hepatol. 17:567–587. 2024. View Article : Google Scholar
|
|
291
|
Wang Y, Zhou X, Lei Y, Chu Y, Yu X, Tong
Q, Zhu T, Yu H, Fang S, Li G, et al: NNMT contributes to high
metastasis of triple negative breast cancer by enhancing
PP2A/MEK/ERK/c-Jun/ABCA1 pathway mediated membrane fluidity. Cancer
Lett. 547:2158842022. View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Brindisi M, Frattaruolo L, Fiorillo M,
Dolce V, Sotgia F, Lisanti MP and Cappello AR: New insights into
cholesterol-mediated ERRα activation in breast cancer progression
and pro-tumoral microenvironment orchestration. FEBS J.
290:1481–1501. 2023. View Article : Google Scholar
|
|
293
|
Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J
and Huang Y: Targeting lipid metabolism to overcome EMT-associated
drug resistance via integrin β3/FAK pathway and tumor-associated
macrophage repolarization using legumain-activatable delivery.
Theranostics. 9:265–278. 2019. View Article : Google Scholar :
|
|
294
|
Gao CQ, Chu ZZ, Zhang D, Xiao Y, Zhou XY,
Wu JR, Yuan H, Jiang YC, Chen D, Zhang JC, et al: Serine/threonine
kinase TBK1 promotes cholangiocarcinoma progression via direct
regulation of β-catenin. Oncogene. 42:1492–1507. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Wen Q, Xie X, Chen C, Wen B, Liu Y, Zhou
J, Lin X, Jin H and Shi K: Lipid reprogramming induced by the
NNMT-ABCA1 axis enhanced membrane fluidity to promote endometrial
cancer progression. Aging (Albany NY). 15:11860–11874. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Dong G, Huang X, Jiang S, Ni L, Ma L, Zhu
C and Chen S: SCAP mediated GDF15-induced invasion and EMT of
esophageal cancer. Front Oncol. 10:5647852020. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Chen W, Zhang Q, Dai X, Chen X, Zhang C,
Bai R, Chen Y, Zhang K, Duan X, Qiao Y, et al: PGC-1α promotes
colorectal carcinoma metastasis through regulating ABCA1
transcription. Oncogene. 42:2456–2470. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
298
|
Qin WH, Yang ZS, Li M, Chen Y, Zhao XF,
Qin YY, Song JQ, Wang BB, Yuan B, Cui XL, et al: High serum levels
of cholesterol increase antitumor functions of nature killer Cells
and reduce growth of liver tumors in mice. Gastroenterology.
158:1713–1727. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
Esposito G, Augustin LSA, Jenkins DJA,
Ferraroni M, Parazzini F, Crispo A, Dal Maso L, Negri E, La Vecchia
C, Polesel J and Di Maso M: Adherence to a cholesterol-lowering
diet and the risk of female hormone-related cancers: An analysis
from a case-control study network. BJOG. 132:1791–1801. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Furberg AS, Veierød MB, Wilsgaard T,
Bernstein L and Thune I: Serum high-density lipoprotein
cholesterol, metabolic profile, and breast cancer risk. J Natl
Cancer Inst. 96:1152–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Di Maso M, Dal Maso L, Augustin LSA, Puppo
A, Falcini F, Stocco C, Mattioli V, Serraino D and Polesel J:
Adherence to the mediterranean diet and mortality after breast
cancer. Nutrients. 12:36492020. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Maddineni G, Xie JJ, Brahmbhatt B and
Mutha P: Diet and carcinogenesis of gastric cancer. Curr Opin
Gastroenterol. 38:588–591. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Bidoli E, La Vecchia C, Montella M, Maso
LD, Conti E, Negri E, Scarabelli C, Carbone A, Decarli A and
Franceschi S: Nutrient intake and ovarian cancer: An Italian
case-control study. Cancer Causes Control. 13:255–261.
2002.PubMed/NCBI
|
|
304
|
Quan L, Liu Y, Cui W, Wang X, Zhang W,
Wang Z, Guo C, Lu C, Hu F and Chen X: The associations between
serum high-density lipoprotein cholesterol levels and malignant
behavior in pancreatic neuroendocrine neoplasms. Lipids Health Dis.
21:582022. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
de Martino M, Leitner CV, Seemann C,
Hofbauer SL, Lucca I, Haitel A, Shariat SF and Klatte T:
Preoperative serum cholesterol is an independent prognostic factor
for patients with renal cell carcinoma (RCC). BJU Int. 115:397–404.
2015. View Article : Google Scholar
|
|
306
|
Li L, Yu Z, Ren J and Niu T: Low
cholesterol levels are associated with increasing risk of plasma
cell neoplasm: A UK biobank cohort study. Cancer Med.
12:20964–20975. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Yang Z, Tang H, Lu S, Sun X and Rao B:
Relationship between serum lipid level and colorectal cancer: A
systemic review and meta-analysis. BMJ Open. 12:e0523732022.
View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Yuan F, Wen W, Jia G, Long J, Shu XO and
Zheng W: Serum lipid profiles and cholesterol-lowering medication
use in relation to subsequent risk of colorectal cancer in the UK
biobank cohort. Cancer Epidemiol Biomarkers Prev. 32:524–530. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Karayama M, Inui N, Inoue Y, Yoshimura K,
Mori K, Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, et
al: Increased serum cholesterol and long-chain fatty acid levels
are associated with the efficacy of nivolumab in patients with
non-small cell lung cancer. Cancer Immunol Immunother. 71:203–217.
2022. View Article : Google Scholar :
|
|
310
|
Narii N, Zha L, Komatsu M, Kitamura T,
Sobue T and Ogawa T: Cholesterol and breast cancer risk: A cohort
study using health insurance claims and health checkup databases.
Breast Cancer Res Treat. 199:315–322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Yang Z, Zhang D, Sima X, Fu Y, Zeng H, Hu
Z, Hou J, Pan Y, Zhang Y, Zhou Z, et al: Levels of pretreatment
serum lipids predict responses to PD-1 inhibitor treatment in
advanced intrahepatic cholangiocarcinoma. Int Immunopharmacol.
115:1096872023. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Huang F, Li S, Wang X, Wang C, Pan X, Chen
X, Zhang W and Hong J: Serum lipids concentration on prognosis of
high-grade glioma. Cancer Causes Control. 34:801–811.
2023.PubMed/NCBI
|
|
313
|
Min J, Wu Y, Huang S, Li Y, Lv X, Tang R,
Zhao H and Wang J: Serum cholesterol level as a predictive
biomarker for prognosis of neuroblastoma. BMC Pediatr. 24:2052024.
View Article : Google Scholar : PubMed/NCBI
|
|
314
|
Zhang C, Li Y, Wang Y, Hu S, Liu Y, Liang
X, Chen ZJ, Zhang Y and Zhao H: Genetic associations of metabolic
factors and therapeutic drug targets with polycystic ovary
syndrome. J Adv Res. 75:581–590. 2025. View Article : Google Scholar
|
|
315
|
Qian L, Qian B, Xu J, Yang J, Wu G, Zhao
Y, Liu Q, Yuan Z, Fan Y and Li H: Clinical relevance of serum
lipids in the carcinogenesis of oral squamous cell carcinoma. BMC
Oral Health. 23:2002023. View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Leeper H, Viall A, Ruaux C and Bracha S:
Preliminary evaluation of serum total cholesterol concentrations in
dogs with osteosarcoma. J Small Anim Pract. 58:562–569. 2017.
View Article : Google Scholar : PubMed/NCBI
|