Non-alcoholic fatty liver disease (NAFLD) was
proposed by Ludwig in 1986. It refers to a fatty liver disease
characterized by excessive fat deposition in hepatocytes (with a
fat content of ≥5%), excluding alcohol and other factors related to
chronic liver diseases (1-4).
In 2020, the Asian Pacific Association for the Study of the Liver
proposed the term 'metabolic dysfunction-associated fatty liver
disease (MAFLD)', emphasizing the role of metabolic disorders in
the progression of fatty liver disease (5). In 2023, an international panel of
experts reached a consensus and the European Association for the
Study of the Liver, from an etiological perspective, renamed it as
'metabolic dysfunction-associated steatotic liver disease (MASLD)'
(6,7). This change highlights the critical
role of metabolic abnormalities in the occurrence and development
of the disease (8). While
avoiding 'stigmatization', this concept also provides explanations
for the coexistence of MASLD and alcoholic-associated liver disease
(9,10).
The etiology of MASLD is complex, with its core
being the interplay between metabolic dysfunction (such as insulin
resistance and dyslipidemia) and multifactorial elements (including
genetics, environment and lifestyle), rather than being attributed
to a single factor. Researchers have established various animal
models of MASLD using diverse methodologies to investigate its
pathophysiological characteristics. Currently, common
interventional drugs mainly include: Farnesoid X receptor agonists,
peroxisome proliferator-activated receptor (PPAR)α/γ/δ agonists,
GLP-1 agonists and fibroblast growth factor 19/21 analogs (11). For individuals with a body mass
index >35, bariatric surgery is primarily recommended. In the
treatment of MASLD, although chemical drugs have advanced rapidly,
their efficacy remains limited with notable adverse effects. By
contrast, traditional Chinese medicine (TCM) not only enables
comprehensive regulation targeting the multifactorial pathogenesis
of MASLD, but also strives to improve the quality of life of
patients, thereby opening up new possibilities for the management
of MASLD (12).
In recent years, influenced by unhealthy lifestyles
and dietary habits, the prevalence of MASLD has been increasing
annually. The global incidence rate has reached 25-35%, with a
prevalence of 29.2% in China (13). It is projected that the number of
MASLD patients in China will reach 315 million by 2030, imposing a
heavy economic burden on society (14). Even mild fatty liver disease
increases the risk of death by 71% and this risk is positively
associated with the severity of the disease (14). Currently, while the incidence of
viral liver diseases is on the decline, the incidence of MASLD is
rising, making early intervention for MASLD an urgent priority. The
present study analyzed the pathogenesis, experimental models and
therapeutic approaches of MASLD, aiming to provide novel scientific
insights and strategies for future research, clinical treatment and
drug development.
To compile the research progress on the
pathogenesis, model and treatment of MASLD as comprehensively as
possible, the present study searched for 'pathogenesis', 'model',
'treatment' and 'traditional Chinese medicine' in existing
scientific databases. The references related to MASLD were obtained
from both online and offline databases, spanning the period from
1980-2025 with a total of 385 references. Online databases included
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://www.webofscience.com/), Elsevier
(https://www.elsever.com/), Sci-Hub (https://sci-hub.st/), Wiley (https://www.wiley.com/), SpringerLink (https://link.springer.com/), Google Scholar
(https://scholar.google.com/), EMBASE
(https://www.embase.com/), Cochrane Library and
China National Knowledge Infrastructure (CNKI) (https://www.cnki.net/). Other references were obtained
from ancient Chinese books, pharmacopoeias and other articles. The
present authors used BioGDP (https://biogdp.com/) as well as Adobe Illustrator
(Adobe Systems, Inc.) for graphing.
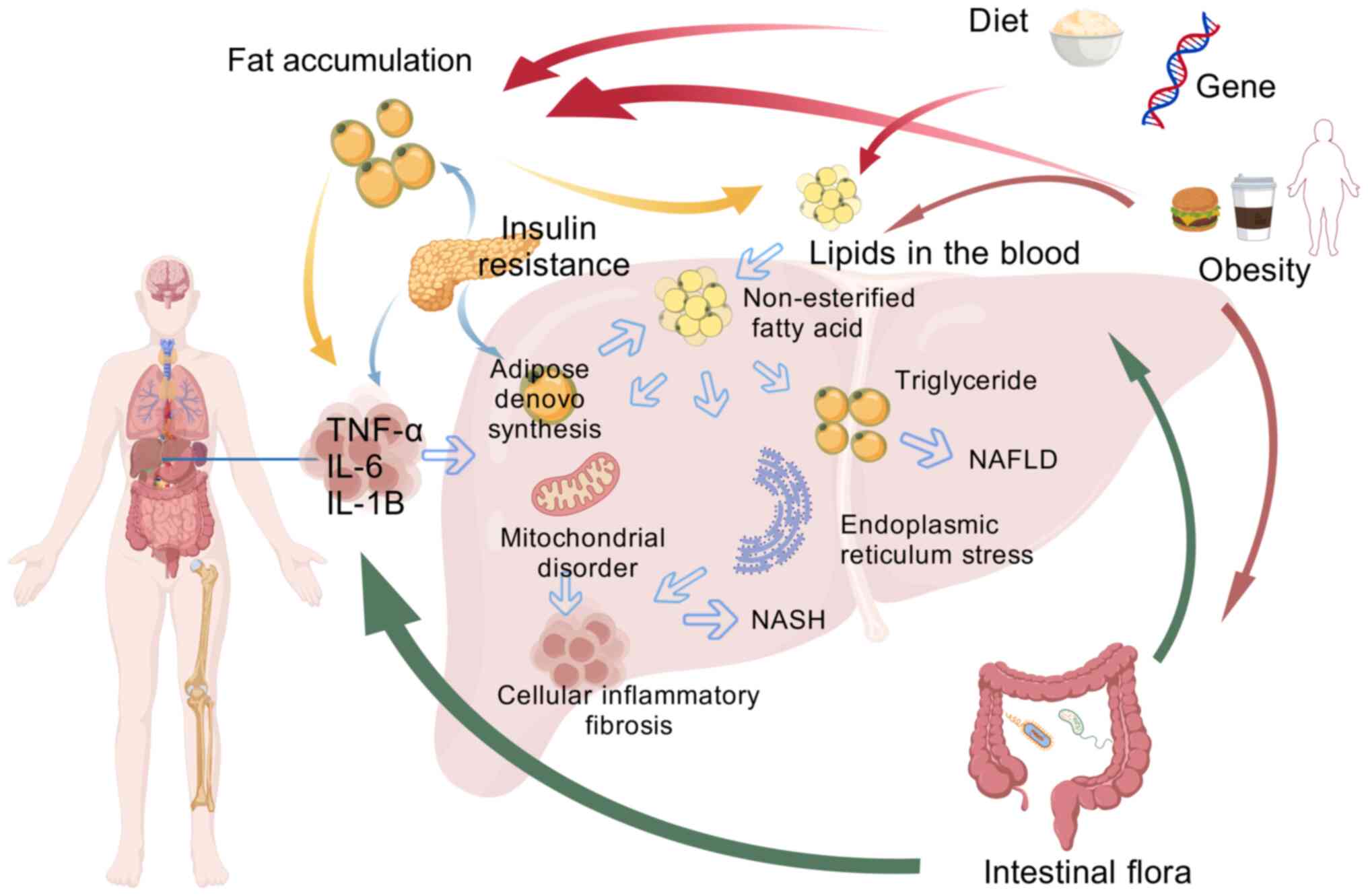
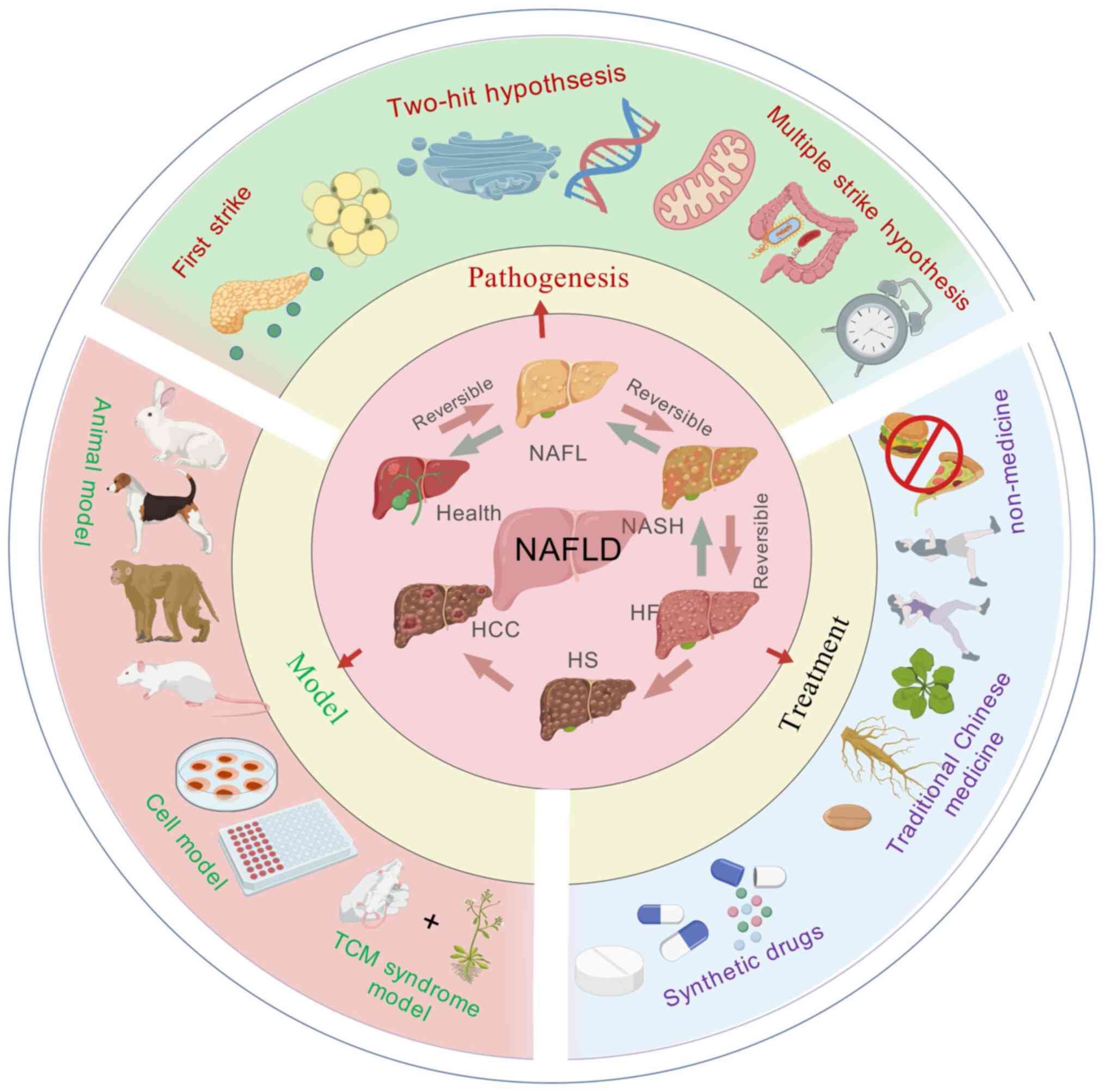
The pathogenesis of MASLD remains unclear due to its
complexity and diversity. As the main metabolic organ, the liver's
metabolism is closely connected with multiple organs and systems in
the human body. There are intricate links between these organs and
systems. Some consider that the hypothesis of 'multiple parallel
strikes' could be an improved explanation of the causes of MASLD
development and these strikes included oxidative stress,
endoplasmic reticulum (ER) stress, lipid metabolism disorders,
abnormal adipokines and cytokines production and mitochondrial
dysfunction (15). One study
found that mitochondrial dysfunction might be a central factor in
the development of MASLD and other factors that cause disease
progression also involve mitochondrial dysfunction (16). The pathogenesis of MASLD is now
being investigated by combining different pathogenic pathways for
an improved understanding of metabolic diseases.
The 'first strike' mainly refers to insulin
resistance (IR) caused by obesity and type 2 diabetes mellitus
(T2DM) and hepatic steatosis caused by excessive accumulation of
triglyceride (TG) and cholesterol in the liver parenchymal cells in
the form of lipid droplets. The 'Two-hit' hypothesis includes
inflammatory cytokines, lipid peroxidation, mitochondrial
dysfunction and oxidative stress (17), of which oxidative stress is the
primary driver (18). This
hypothesis posits that with steatosis, the liver undergoes ER
stress, oxidative stress and mitochondrial dysfunction, leading to
an increase in oxidative metabolites, hepatocellular damage and
secretion of a large number of inflammatory factors, which
ultimately leads to further deterioration into liver disease, liver
fibrosis, cirrhosis, inflammatory necrosis and other reactions
(19,20). In the early stages of MASLD,
classical activation of macrophage M1 in the liver can promote
further inflammation and IR, as well as steatosis (21). Among them, inflammatory cytokines
such as interleukin (IL)-6, IL-8, tumor necrosis factor-α (TNF-α),
IL-1β and cyclooxygenase-2 contribute to the development of chronic
inflammatory diseases (22).
IL-6 has been shown to have both anti-inflammatory and
pro-inflammatory effects, but its role in MASLD is controversial.
IL-10, produced primarily by monocytes and B cells, is an
anti-inflammatory factor with immunomodulatory properties (22). Other inflammatory cells of the
innate immune system, such as natural killer T cell and natural
killer cells, also play the important roles in the pathogenesis of
MASLD and MASH (21). However,
the factors that induce MASLD in clinical practice are diverse and
as research has deepened, the two-hit hypothesis has already shown
certain limitations and cannot fully explain the pathogenesis of
MASLD. Recently, the 'three-strike' and 'multiple-strike'
hypotheses have been proposed, which include gut microbiota toxins,
IR, cytokines and inflammation, oxidative stress due to
mitochondrial dysfunction, lipid peroxidation, ER stress, intrinsic
immune regulation, circadian rhythm disruption and immune system
development. Imbalances, circadian patterns and genetics are all
parallel and mutually reinforcing factors that contribute to the
onset and development of MASLD (23,24) (Fig. 1).
The role of liver sinusoidal endothelial cell (LSEC)
in the development and progression of MASLD is being gradually
recognized. LSEC is the most predominant liver nonparenchymal cell
(NPC), accounting for 15-20% of the total number of hepatocytes but
only 3% of the total volume of the liver (25). The biggest difference between
LSECs and other vascular endothelial cells is the presence of
window pores and the lack of a basement membrane. LSECs are
considered the most permeable endothelial cells in mammals
(25). Window pores are small
pores with diameters ranging from 50-200 nm, accounting for 2-20%
of the endothelial surface area (26). MASLD manifests as NAFL in the
early stage, which is not accompanied, or only accompanied by mild,
inflammation. LSEC acts as the first line of defense in the
initiation of MASLD (27),
exerting anti-inflammatory effects, preventing the over-activation
of the hepatic immune system and maintaining the microenvironmental
homeostasis of hepatic sinusoids. It has been shown that the unique
window pore structure of LSECs in a physiological state serves an
important role in maintaining the stability of the liver, which
manifests pro-vasodilatory, strong endocytosis, anti-inflammatory
and anti-fibrotic effects (28).
This window pore regulates the act in the endothelial cell skeleton
of LSECs through the form of calcium-calmodulin-actin complex to
adjust and control the size of the window pore on the endothelial
cells of LSEC; if the size, number, and permeability of window
pores in LSECs could not be effectively improved, they would
disappear in the course of time. From the initial manifestation of
fat accumulation to inflammatory symptoms, as well as hepatic
fibrosis and in severe cases, cirrhosis, LSEC regulates blood flow
through the secretion of two substances, nitric oxide (NO) and
endothelin-1 (ET-1) (27). NO
promotes vasodilatation and ET-1 induces vasoconstriction. Reduced
NO bioavailability induces LSEC capillarization characterized by
loss of the LSEC window pore and basement membrane formation and
leads to an imbalance in its secretion of vasoactive substances,
which contributes to MASLD progression. With the progression of
MASLD, LSEC undergoes capillarization and hepatic sinusoidal
endothelial dysfunction, which gradually shifts from an
anti-inflammatory to a pro-inflammatory phenotype and promotes the
progression of inflammation, fibrosis and angiogenesis. Hepatic
sinusoidal endothelial dysfunction precedes the development of
hepatic inflammation or fibrosis. It is a major feature and early
event in MASLD pathogenesis (29).
IR refers to the phenomenon of reduced sensitivity
of tissues and organs to insulin, which is the initiation and
central link in the progression of MASLD (30). It has been found that the degree
of IR in MASLD patients is positively associated with the severity
of the disease (31). IR acts on
target organs mainly the liver, skeletal muscle and adipose tissue;
the body's sensitivity to insulin is reduced, so that the
biological effect of insulin on target organs is reduced and
glucose uptake and utilization are reduced, thus failing to
maintain blood glucose stability. Compensatory overproduction of
pancreatic islets and eventual development of hyperinsulinemia,
result in elevated hepatic synthesis and accumulation of total
cholesterol (TC) and elevated low-density lipoprotein (LDL) levels,
leading to inactivation of the enzyme LDL lipase, which is critical
for cholesterol clearance. Large amounts of TG cannot be cleared
and IR-induced hyperinsulinemia inhibits apolipoprotein B100
synthesis and reduces very low-density lipoprotein (VLDL) related
lipid output from hepatocytes (32). In addition, IR leads to
decreasing lipid metabolism, increasing lipolysis and hepatic
uptake of large amounts of free fatty acid (FFA). By contrast,
fatty acid β-oxidation is inhibited by hyperinsulinemia and large
amounts of FFA are deposited in the liver, exacerbating
hepatocellular steatosis (33).
Excessive deposition of lipids further exacerbates IR and they are
mutually influencing and reinforcing processes (34). MASLD also leads to lower levels
of adiponectin (ADPN), a protein released from adipocytes. ADPN
aggravates steatosis in hepatocytes (35) and plays a role in regulating
glucose and lipid metabolism, as well as exhibiting
anti-inflammatory and anti-insulin resistance effects (36). In addition, Shah and Fonseca
(37) found that iron overload
could reduce insulin sensitivity and IR, which are closely related
to the occurrence of MASLD. The main reason is that iron can
activate the nuclear factor-kappa B (NF-κB) signaling pathway.
Activation of this pathway causes hepatocytes to produce an
inflammatory response, which accelerates the formation of MASLD.
Although iron is an essential trace element in the human body, a
study found that iron overload is closely related to the occurrence
of MASLD (37). Iron overload
can generate reactive oxygen species (ROS) and cause oxidative
stress. Iron overload can also affect lipid metabolism and insulin
signaling, which can accelerate the progression of MASLD (38). Using hepatic puncture biopsy,
Nelson et al (39)
discovered that 34.5% of 849 patients with MASLD had intrahepatic
iron deposition, which suggested that iron deposition was a risk
factor for the progression of MASLD patients to advanced liver
diseases.
Adipokines are small-molecule proteins secreted by
tissues to regulate adipocytes, mainly including leptin (LP) and
ADPN. A study (40) found that
IR is closely related to the secretion of LP, resistin and ADPN.
ADPN is an adipokine that affects hepatic fat and glucose
metabolism, which interacts with adiponectin receptor1 and
adiponectin receptor 2. Adiponectin receptor 2 is mainly expressed
in liver tissues. Clinical studies have shown that the serum level
of ADPN in patients with MASLD is low and negatively is associated
with the severity of fatty liver (40). In addition, serum lipocalin
levels are reduced in MASLD patients and are associated with the
degree of hepatic necroinflammation, leading to the suggestion that
hypo-lipocalinemia will lead to the progression of MASLD, which may
be related to the fact that ADPN acts on the sterol regulatory
element-binding protein-1C to inhibit lipolysis of fats (41). Studies have shown a close
relationship between LP and the development of MASLD and that LP
can lead to the activation of hepatic LP and can lead to the
activation of hepatic stellate cells in MASLD and promote the
development of MASLD to liver fibrosis (41). Moreover, LP is also associated
with IR; when the feedback regulation mechanism between pancreatic
islets-adipocytes is damaged, the sensitivity of insulin to LP
decreases. It makes the fatty acid content of hepatocytes rise,
which promotes the increase in the synthesis of TGs in the
hepatocytes, leading to the formation of MASLD (42). Wang et al (43) found that the amount of ADPN
expression can be used to predict the occurrence of MASLD to a
certain extent. Resistin is a prepeptide adipocytokine containing a
108-amino-acid segment, named after its anti-insulin effect. In
another study, RES was found to be highly expressed in adipose
tissue and serum of both high-fat diet-induced obese rats and
transgenic obese rats (44).
Activation of resistin can induce the release of inflammatory
factors and promote the development of liver fibrosis (45).
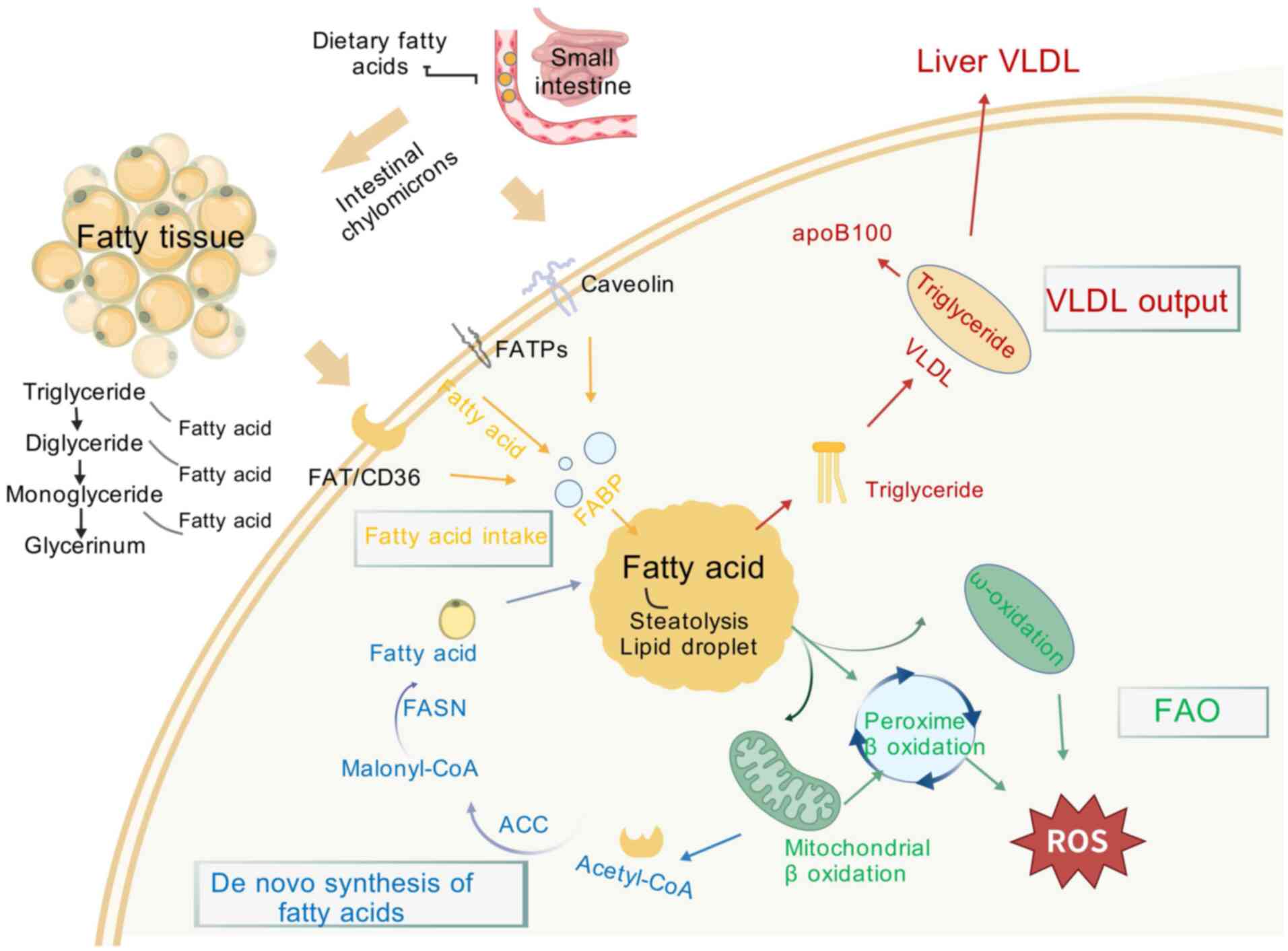
Lipotoxicity refers to lipid metabolism disorders
leading to an increase in FFA, excessive FFA makes pancreatic islet
β-cells dysfunctional, inducing them to produce a large amount of
NO, causing a series of cytotoxic injuries. Markedly increased
plasma FFA level in MASLD patients is the main reason for increased
IR in the body. In addition, increasing plasma FFA content leads to
fatty acid oxidation overload in hepatocytes, induces mitochondrial
damage and generates large amounts of ROS, causing oxidative
stress, ER stress and inflammatory response, which advances the
process of disease progression. The mechanisms of hepatic fatty
acid metabolism on MASLD are shown in Fig. 2.
The normal number of hepatocytes is controlled by
hepatocyte apoptosis, which maintains the liver at a normal size
and plays an important role in the development of the liver and the
maintenance of internal homeostasis, it is the defender of
hepatocytes against infections, tumors and autoimmune reactions;
under pathological conditions apoptosis of hepatocytes is the
central link in the basis of injury and other liver diseases.
Lipotoxicity induces apoptosis in hepatocytes, called
lipoapoptosis. It has been demonstrated that FFA-treated
hepatocytes show an increase in the expression of pro-apoptotic
proteins and apoptosis regulators upregulated by tumor suppressor
genes, a process that is accompanied by a decrease in the
expression of the anti-apoptotic protein B-cell lymphoma-2. The
aforementioned process initiates the mitochondria-induced apoptosis
pathway, which activates caspase-3,6,7 leading to apoptosis
(46). Hepatocyte apoptosis is
closely related to the pathogenesis of MASLD (47).
Mitochondria are an important site for hepatic FFA
oxidation. When FFA and related metabolites in the liver increase
in excess, it causes mitochondrial microstructure swelling and
mitochondrial dysfunction, resulting in impaired β-oxidation,
altering the permeability of the ER membrane, inducing the
production of a large number of ROS. ATP generation is reduced,
causing oxidative stress and mitochondrial damage (48). In the meantime, ROS generated by
oxidative stress in the mitochondrial membrane react with
unsaturated fatty acids of mitochondrial membrane phospholipids,
nucleic acids and other macromolecules to cause lipid peroxidation
and the products of lipid peroxidation produce endogenous ROS and
O2−, which can damage mitochondrial DNA structure and
further diminish the anti-oxidant effect of mitochondria (49). In addition, ROS generated by
oxidative stress in hepatocytes can trigger an inflammatory
response. This response induces neutrophil infiltration and
activates Kupffer cells (KCs) to secrete ROS and TNF-α. These
events create a vicious cycle of oxidative stress, leading to VLDL
deposition in the liver and the development of MASLD. Further
hepatocyte necrosis may occur due to other cytokines, such as
transforming growth factor-β (TGF-β), IL-6 and C-reactive protein
(49,50).
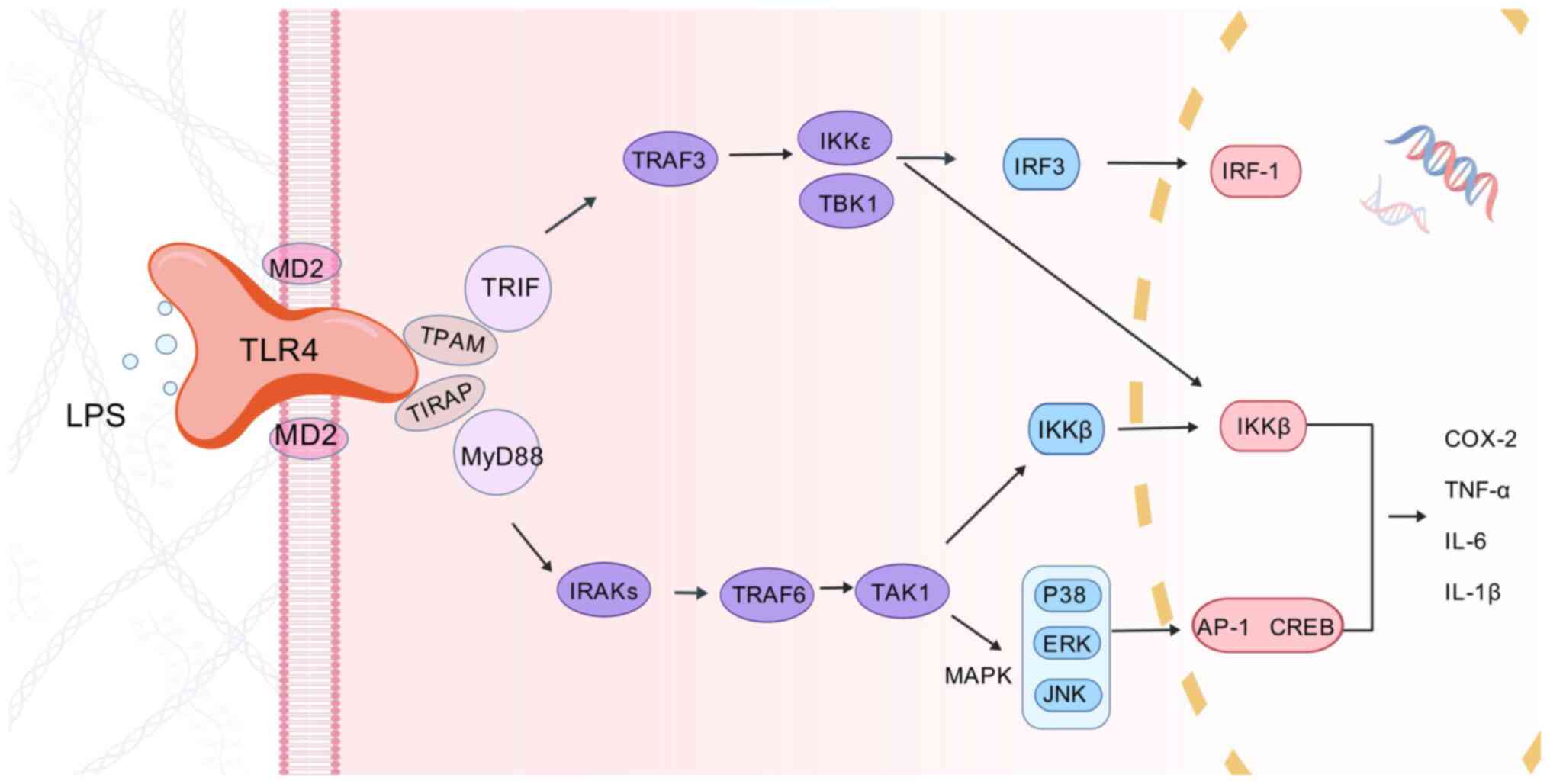
Persistent inflammation is the main driver of MASLD
progression to MASH and fibrosis and activation of toll-like
receptors (TLRs) is thought to be a key factor in triggering
hepatic steatotic inflammation. The liver, as a central immune
organ, possesses the largest number of resident macrophages, known
as KCs. KCs account for 35% of the liver's NPC and 80-90% of all
tissue macrophages in the body. KCs are considered to be the first
immune cells to come into contact with intestinal or hepatic
autoimmune reactive substances and are rich in the expression of
TLRs (51). Accumulation of
lipids in hepatocytes will result in lipotoxicity, which will lead
to hepatocyte damage or death. Damaged or dead hepatocytes release
damage-associated pattern molecules, including mitochondrial DNA
(mtDNA) and high mobility group protein-1 (HMGB1) (52). Among them, mtDNA can directly
activate recombinant TLR9 in KCs, triggering an inflammatory
cascade (53). Recombinant
toll-like receptor 4 (TLR4) (Fig.
3) is another TLR family member upregulated in MASLD and
lipopolysaccharide (LPS), ROS, HMGB1 and various damage-associated
molecular patterns can bind to TLR4 in KCs to promote TNF-α, IL-1β,
IL-6 and interferon-γ (IFN-γ) production of various
pro-inflammatory cytokines, thereby contributing to hepatic
inflammation and the progression of MASLD (54). In addition to triggering the
inflammatory response through lipotoxicity that causes hepatocyte
injury or death, excess FFA in the liver can also exacerbate the
inflammatory response through direct or indirect activation of
TLR4. Circulating FFA, especially saturated fatty acid (SFA), acts
as non-microbial TLR4 agonists and triggers inflammatory responses.
SFA is thought to have a similar recognition pathway to LPS, a
natural ligand of TLR4, in order to activate the TLR4 signaling
pathway, thereby triggering downstream inflammatory pathways
(55). Furthermore, to activate
TLR4, SFA is also thought to activate recombinant TLR2 to promote
the development of inflammatory responses (56). It has been shown that SFA can
activate TLR by interacting with TLR co-receptors, such as cluster
of differentiation36 (CD36) or LDL receptors, to promote
inflammation (57). In addition,
FFA can interact with various other cytokines, such as hepatocyte
nuclear factor 4-α (HNF-4α), leading to overall changes in
signaling pathways that regulate metabolism and stress (58). Lipotoxicity can also further
induce ER stress, impair autophagy and promote aseptic inflammatory
responses, thereby exacerbating hepatocyte injury and death
(59,60). Cholesterol synthesis is markedly
increased in patients with MASLD, suggesting that cholesterol may
also be one of the important driving forces in its development
(61). Cholesterol not only
promotes de novo lipid synthesis but also induces lipid
peroxidation (62). Moreover, it
has been found that increased dietary cholesterol intake leads to
hepatic inflammation and oxidative stress in mice, suggesting that
cholesterol probably plays a contributory role in the disease
progression of MASLD (63).
Lipid accumulation in hepatocytes can affect
mitochondrial function, ER membrane fluidity and calcium ion
homeostasis. It has been found that thioesterase superfamily member
2 (THEM2) promotes the uptake of saturated fatty acids by ER
phospholipid membranes, reduces ER membrane fluidity and affects
the activity of ER Ca2+associated ATPase. The
dysregulation of Ca2+ signaling can cause protein
misfolding and secretion (64).
Non-esterified fatty acids can expand the membrane structure of the
endoplasmic reticulum-mitochondria association. Changes in the
membrane structure of the ER allow Ca2+ to flow into the
cytoplasm or mitochondria, which in turn alters the open channels
of the mitochondria and cytochrome c is released into the
cytoplasm. Ca2+ entering the cytoplasm can activate the
Ca2+ dependent protein kinase, which together with
cytochrome c induces the onset of apoptosis (65). ROS generated by mitochondria can
enter the ER and trigger the unfolded protein response (UPR), which
can activate three types of ER transmembrane protein receptors:
Protein kinase RNA-like endoplasmic reticulum kinase (PERK),
activating transcription factor-6α (ATF-6α) and inosital-requiring
enzyme-1α (IRE1α), which mediate three signaling pathways. The
three transmembrane proteins mediate three signaling pathways,
which can maintain the stability of ER protein synthesis to a
certain extent in the early stage of steatosis, but under the
continuous stimulation of lipids and ROS, the three transmembrane
proteins mediate the signaling pathways that can activate cellular
inflammation and apoptosis, accelerating the progression of hepatic
steatosis (66).
In the process of hepatocellular steatosis, one of
the hallmarks of MASLD is the appearance of the UPR in hepatocytes,
which can inhibit mitochondrial lipid oxidation through the
UPR-induced ATF-6α pathway and promote fatty acid synthesis via the
PERK, ATF-6α and IRE1α signaling pathways, which together promote
intracellular lipid accumulation (67,68). Hepatocyte lipid accumulation and
ER stress form a positive feedback loop that continuously
exacerbates hepatocyte steatosis. The occurrence of chronic
inflammation in hepatocytes is also one of the main features of
metabolic disorders. The inflammatory response can activate the UPR
through three major signaling pathways (PERK, ATF-6α and IRE1α).
The UPR can also interact with NF-κB and N-terminal
kinase/activator protein 1-regulated pro-inflammatory pathways to
accelerate disease progression (66,69).
The intestinal flora is a symbiotic group of
microorganisms that act synergistically with the host. Under normal
circumstances, the intestinal flora of the human body is in a state
of equilibrium. Imbalances in the intestinal flora can be caused by
environmental factors, immune levels, bile secretion, changes in
gastric acidity and alkalinity and impaired intestinal peristalsis.
In recent years, it has been found that dysbiosis is one of the
causative factors of MASLD (70). The intestinal flora mainly
influences the liver through the 'gut-liver axis'. Normally, there
is a balance between the barrier function of the intestine and the
detoxification capacity of the liver and trace amounts of
intestinal bacteria cross the mucosa and enter the venous
bloodstream and are cleared by the liver (71). When intestinal flora dysbiosis
occurs, metabolic disorders will occur, the permeability of the
intestinal tract will increase and a large amount of intestinal
bacterial metabolites, bacterial components and other harmful
substances will enter the liver through the portal vein via the
intestinal-hepatic axis and through the enterohepatic circulation
reach the liver and bind to the TLR4 of hepatocytes and exacerbate
the hepatic inflammatory response, oxidative stress and lipid
accumulation, which further stimulate the inflammatory response.
Intestinal microorganisms change their phenotype and virulence by
sensing the various adverse signals generated during intestinal
stress, shifting from a commensal to a pathogenic mode and causing
damage to the organism (72).
Ley et al (73) found
that changes in the composition of the gut microbial community
associated with obesity as well as IR were observed after the
adoption of two different dietary patterns. IR, an important
feature of MASLD, can be improved after antibiotic treatment
(74). A study conducted by Le
et al (75) demonstrated
that when gut microbes from successfully modeled MASLD mice were
transplanted into normal control mice, the normal control mice
developed MASLD and this study strongly confirmed that the
development of MASLD is associated with the development of MASLD
and its effects on the gut microbial community. This study also
confirms the correlation between the development of MASLD and gut
microbes. A Chinese cohort study reported that
high-alcohol-producing Klebsiella Pneumoniae (HiAlc-Kpn), which
produces large amounts of alcohol, was detected in 61% of MASLD
patients. In order to investigate the relationship between HiAlc
bacteria and fatty liver disease, they fed specific pathogen free
(SPF) mice with HiAlc-Kpn. It is noteworthy that HiAlc-Kpn feeding
induced chronic hepatic steatosis. They investigated the
relationship between intestinal flora imbalance and severe MASLD
lesions (MASH and fibrosis) and showed that an increase in the
number of Ruminococcus was positively associated with an
aggravation of the degree of fibrosis (76). In addition, LPS is an important
component of the cell wall of Gram-negative bacilli. It is
recognized by pattern receptors and mediates the LPS-CD14-TLR4
signaling pathway, which activates intrahepatic KCs. These KCs
release large amounts of cytokines, such as IL-6, CD68 and TNF-α.
LPS also causes inflammation and metabolic disorders in
vivo, including increased fat burning, elevated circulating
free FFA and TG and deposition of FFA in the liver. This process
may contribute to inflammation and trigger IR, which can ultimately
lead to MASLD. The deposition of FFA in the liver may also promote
inflammation, leading to IR, which in turn triggers the development
of MASLD (77). Dysbiosis may
also contribute to MASH through other mechanisms, such as ethanol
production and interference with choline metabolism (22). Overall, intestinal flora and its
harmful metabolites (including EtOH, SFA, polyamines and
H2S) may be drivers of liver injury (76).
BA is synthesized in the liver and the main raw
material for synthesis is cholesterol. BA regulates glucose and
lipid metabolism. BA, along with its downstream receptors farnesoid
X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5),
plays an important role in lipid metabolism in the liver (78). BA also regulates the cholesterol
metabolic pathway, allowing cholesterol to be eliminated as a
water-soluble product and impaired regulation can lead to an
inflammatory response that can exacerbate the development of MASLD
(79). In a MASLD mouse model,
Jiang et al (80) found
that the administration of antibiotics to mice on a high-fat diet
reduced triacylglycerol accumulation in the liver. Activation of
TGR5 by BA in brown adipose tissue and muscle increases energy
expenditure and reduces diet-induced obesity (81). Activated TGR5 can downregulate
the expression level of inflammatory factors, promote energy
expenditure in adipose and muscle tissues and regulate the body's
immunity, which has a positive effect on improving MASLD (82). Watanabe et al (83) found in an animal experiment that
cholesterol and TG levels were markedly elevated in mice lacking
FXR in vivo. FXR has a positive effect on lipid regulation
and also reduces the generation of inflammatory responses; FXR
plays a key role in the regulation of BA and regulates lipid
metabolism. In addition, BA can affect the growth of bacteria and
is a bacteriostatic substance. Conversely, intestinal flora can
also regulate BA, intestinal flora and BA metabolism affect each
other. However, the effects of different flora are inconsistent.
For example, Aspergillus and Anaplastic bacilli in
the intestine will reduce the synthesis of BA and aggravate
inflammatory reactions, while Actinobacteria will increase
the synthesis of BA, reduce the inflammatory response and reduce
the damage of hepatocytes (84).
The normal metabolism of BA plays a crucial role in maintaining the
balance of intestinal flora. Gut microorganisms can also accelerate
the metabolism of primary BA and produce secondary BA, increasing
BA types (85). LPS produced by
intestinal flora can stimulate NF-κB to recruit inflammatory
factors and increase the level of inflammation in the body. These
studies suggest that influencing the gut flora by modulating the
signaling pathways between BA and their controlled receptors is a
promising new therapeutic approach for the treatment of MASLD
(86).
Phenotypic changes in LSEC are one of the key events
in the progression of MASLD in humans, suggesting that the
progression of MASLD inflammation, hepatic fibrosis and impaired
hepatic lipid metabolism may affect the expression of Fc γ receptor
IIb (FcγR IIb) and macrophage function in LSEC (87). Autophagy is an intracellular
process that maintains homeostasis in vivo by forming
double-membrane autophagosomes that encapsulate to-be-degraded
material and bind to lysosomes to self-digest excessive or
defective organelles. Autophagy can be categorized into three
types: Macroautophagy, microautophagy and molecular
chaperone-mediated autophagy, of which the most common is
macroautophagy. Autophagy is related to MASLD and macroautophagy is
the main type of autophagy that regulates MASLD. Liver autophagy is
defective and autophagy level is reduced in MASLD patients and
autophagy shows different functions at different stages of MASLD
(87). In the early stage of
MASLD, autophagy inhibits apoptosis (87), while in the late stage, autophagy
is pro-apoptotic, which also indicates that autophagy is involved
in the whole stage of MASLD development (87). Recombinant autophagy-related
protein 7 (Atg7), a core member of the anti-thymocyte globulin
(ATG) gene family, is responsible for driving the classical
degradation and the major stages of autophagy, which is a key
component of the autophagy-associated gene family. Abnormal
expression of Atg7, a core member of the ATG gene family, can drive
the main stage of classical degradation of autophagy, resulting in
defective autophagy in cells. In Atg7 knockout mice, hepatic
autophagy activity was markedly reduced and intracellular lipid
accumulation was increased, leading to the formation of MASLD
(88). Phosphatidylinositol-3
kinase (PI3K) is a key regulator of the activation of the mammalian
target of rapamycin (mTOR), which is considered an important
regulator upstream of the ATG gene. The PI3K inhibitor
3-methyladenine blocked the formation of autophagosomes, increased
the lipid content of hepatocytes and promoted the formation of
MASLD (89).
Inflammatory response induced by immune system
dysfunction has been found to be one of the hallmarks of MASH and a
progressive form of MASLD (90).
Under normal conditions, the liver has immune defense and
immunoregulatory functions and when the intensity of the immune
system's response to autoantigens exceeds the limits of immune
regulation and affects its physiological functions, it will cause
inflammatory damage to its tissues or organs, leading to the
occurrence of autoimmune diseases. The liver has a large number of
immune cells involved in the immune response and it has been found
that macrophages are the largest proportion of immune cells
(80-90%) in the human liver and they are closely related to the
development and severity of MASLD (91). In patients with MASLD, macrophage
infiltration occurs around the portal vein and is observed at an
early stage before evidence of inflammation appears. In addition,
macrophages are capable of producing a variety of inflammatory
factors, such as NF-κB, TNF-α, IL-2, IL-6 and IL-8 and activating
nuclear transcription factors like NF-κB, which also play an
important role in the progression of MASLD (92). NF-κB, as a nuclear
transcriptional regulator of inflammatory genes, which is
activated, enters the nucleus and promotes the transcription and
expression of inflammatory factors, releasing a large number of
inflammatory factors. After entering the liver, NF-κB leads to
oxidative stress in hepatocytes, activates the expression of
pro-apoptotic proteins in mitochondria, promotes hepatocyte
apoptosis and exacerbates the progression of MASLD (93). TNF-α is one of the important
regulators mediating hepatocyte injury and is also a bridge between
inflammation and metabolism that plays a key role in the
development of MASLD. TNF-α regulates the inflammatory signaling
pathway and exacerbates the inflammatory response in MASLD through
pathways such as NF-κB and p38 mitogen-activated kinase (94). IL-2 can promote the proliferation
of activated B cells and is also a growth factor for all T cell
subpopulations and can positively feedback regulate the production
of cytokines such as TNF-α, leading to a further increase in IL-2
in MASLD livers (95). IL-6 can
inhibit the activity of lipoprotein esterase, which can reduce the
ability to catabolize lipids and promote the formation of hepatic
lipids. IL-8 can cause inflammatory cells to accumulate in the
liver and cause inflammation and lipid accumulation in hepatocytes
can also stimulate the inflammation of MASLD hepatocytes. The
accumulation of lipids in hepatocytes can also stimulate KCs to
release TNF-α, which further promotes the production of IL-8 and
participates in the inflammatory response of hepatocytes, leading
to the aggravation of hepatocyte injury (96).
Genetic biomarkers for MASLD include DNA sequence
variants, such as single nucleotide polymorphism (SNP) and the most
studied SNPs in MASLD are rs738409 and rs58542926, which are
located in patatin-like phospholipase domain containing protein 3
(PNPLA3) and transmembrane 6 superfamily member 2 (TM6SF2),
respectively. The risk of developing MASLD has been found to be
influenced by single nucleotide gene polymorphisms. For example,
SNPs in the PNPLA3 gene and mutations in the PNPLA3 gene are
associated with MASLD. PAPLA3 is one of the members of the
patatin-like phospholipase family that have been identified as
being closely associated with the development of MASLD (97,98), PNPLA3 was found to be expressed
mainly in the liver and adipocytes, with non-specific TG lipase and
acylglycerol transacylase activities involved in TG hydrolysis in
hepatocytes. Mutations in PNPLA3 antagonize normal proteasomal
degradation and increase the risk of MASLD and its severity. TM6SF2
is a multiple transmembrane protein that is involved in lipid
transfer, VLDL secretion and TC synthesis. For the rs58542926
variant of the TM6SF2 gene (E167K), a mutation in the TM6SF2 allele
results in decreased VLDL secretion and fatty acid retention in the
liver, leading to hepatic steatosis and progression of MASLD
(99). Hydroxysteroid (17-β)
dehydrogenase 13 (HSD17B13) is a gene encoding the hepatic lipid
droplet protein 17β-hydroxysteroid dehydrogenase type 13 and it has
been found that HSD17B13 expression is markedly upregulated in
patients and mice with MASLD, suggesting that HSD17B13 usually
produces a product that promotes hepatocyte injury (100). Other SNP genes associated with
MASLD include neuromucin and glucokinase regulatory protein. In
addition, the level of immunity and metabolic rate of an individual
are genetically related.
MicroRNAs are a class of RNA regulators, ~22
nucleotides in length and a growing number of studies on
diet-induced and genetic (ob/ob) models of obese rodents and
patients with severe MASLD suggest a potential role for miRNAs in
MASLD pathogenesis and IR (101). A number of miRNAs have been
identified, including miR-122 and miR-21 and their expression
levels in both peripheral blood and liver have been associated with
the development of MASLD (102). One study found that serum
miR-122 was upregulated 7.2-fold in patients with MASH compared
with healthy subjects and 3.1-fold compared with patients with
simple steatosis. This suggests that serum miR-122 is an
extra-hepatic feature of MASH. Moreover, in a mouse model without
elevated alanine aminotransferase (ALT), the elevated serum miR-122
levels were positively associated with the severity of MASLD
(103). It has been observed
that the expression level of miR-21 in the liver of diet-induced
MASH mice progressively decreased with disease progression,
compared with the control group (103). It has been found that miR-27a
attenuates neoplastic liver lipogenesis and obesity-induced MASLD
by inhibiting the fatty acid synthase (FAS) gene and stearoyl
coenzyme A desaturase-1 in the liver. Histological analyses also
showed reduced lipid accumulation in the livers of mice with
hepatic miR-27a overexpression, suggesting reduced hepatic
steatosis; furthermore, trichrome staining of miR-27a overexpressed
livers showed markedly reduced fibrosis and lower MASLD activity
scores, reflecting improved development of MASLD (104). miR-155 is one of the miRNAs
that can regulate KCs, which are involved in inflammatory processes
that control innate and adaptive immunity in alcoholic and MASLD
diseases (103). Szabo and Csak
(105) proposed that miR-155
was a major regulator of inflammation and mice lacking miR-155 on
methionine choline deficiency (MCD) with attenuated steatosis but
no change in serum ALT or inflammation indicative of liver damage
after diet-induced steatohepatitis. In another MASH model, however,
miR-155 deficiency resulted in enhanced hepatic steatosis (105). A study has found miR-16 to be
elevated in MASLD patients with simple steatosis and others have
found that serum miR-16 is elevated in MASH patients and is
associated with the stage of fibrosis (106). It has been observed that the
expression of miR-197, miR-146b, miR-181d and miR-99a is markedly
decreased in MASLD patients. Additionally, the hepatic levels of
miR-301a-3p and miR-34a-5p increase monotonically from simple
steatosis to MASH to cirrhosis. Conversely, miR-375 levels decrease
monotonically during this progression (107). Some MASLD-related miRNAs, such
as miR-149, had elevated expression levels in both FA-treated human
hepatocellular carcinomas cells and MASLD animal models (102).
Some studies have found a large number of circadian
rhythmically expressed genes in the liver, which are involved in
maintaining the metabolic balance of the body. MASLD is closely
related to daily lifestyle. Irregular lifestyle may cause liver
overload, including lack of sleep and little exercise. In addition,
long-term intake of high-fat and high-protein foods, omitting
breakfast, adding meals before bedtime and other bad dietary habits
are also risk factors for MASLD (110). Sleep deprivation leads to an
increased risk of morbidity and mortality (111). Epidemiologic studies have shown
that sleep deprivation leads to altered glucose homeostasis, IR,
weight gain, obesity, metabolic syndrome and DM, all of which are
associated with MASLD. In experimental studies, it has been found
that sleep disorders may induce MASLD through pro-inflammatory
markers such as TNF-α, IL-1β and IL-6. In addition, sleep
deprivation increases growth hormone-releasing peptide levels and
decreases LEP levels, which increases appetite and further
contributes to obesity and chronic insomnia activates the
hypothalamo-pituitary-adrenal axis, which increases stress
hormones, exacerbates IR and promotes the development of MASLD
(112,113). Another study found that
increased mRNA expression in hepatic biological clock genes, a
decrease in levels of key metabolism-regulating enzymes, hepatic
inflammation and steatosis can occur, which is associated with
glucose and fatty acid metabolism (111). In the case of liver-specific
knockout of BMAL1, which results in the loss of key metabolic gene
oscillations in the liver and subsequently exacerbates oxidative
damage to hepatocytes and induces IR. The glucocorticoid rhythm
plays a key role in coordinating glucose, lipid and protein
metabolism and it is an entrainment signal for the systemic
circadian rhythm through the hypothalamic suprachiasmatic nucleus,
the peripheral clock in the liver and adipose tissue. The kidney is
regulated by the autonomic nervous system and rhythmic entrainment
signals. It has been noted that the oscillation of the cellular
redox state, independently of the biological clock transcriptional
feedback loop and in the metabolic process, may control the
circadian rhythm (114).
Circadian rhythm disruption will lead to cellular dysfunction,
which in turn affects the metabolic function of the liver (115). It induces metabolic dysfunction
and the occurrence of obesity, fatty liver, metabolic syndrome and
other conditions.
Social stress is closely related to emotional and
physical health and one of the factors that can trigger the
development of MASLD is excessive stress (116). Some studies suggest that
emotional problems such as anxiety and depression may have an
effect on the progression of chronic liver diseases, including
MASLD (117). A study by
Youssef et al (118)
confirmed the association of depression and anxiety with the
severity of histologic features of MASLD by examining 567 patients,
demonstrating that depression was markedly dose-dependent with more
severe hepatocellular ballooning and that patients with subclinical
depression were 2.1 times more likely to develop more severe
hepatocellular ballooning than patients without depressive
symptoms.
Studies have shown that hyperinsulinemia, lipid and
lipoprotein metabolism changes caused by high-fat diet (HFD),
high-carbohydrate diet, or high-fat and high-carbohydrate diets may
promote the occurrence and development of MASLD (119). Yu et al (120) explored the relationship between
dietary choline and MASLD, choline deficiency stimulated hepatic
fat accumulation and increased dietary choline intake in
normal-weight Chinese women associated with a reduced risk of
MASLD. Soft drinks and meat intake were markedly associated with
the increased risks of MASLD (121,122). High salt intake may affect
MASLD by increasing plasma levels of triglyceride (TG) and it
intake also stimulates endogenous fructose production. Lanaspa
et al (123) found that
a high salt diet activated the aldose reductase pathway in the
liver, leading to endogenous fructose production, which induced LEP
resistance with the development of the metabolic syndrome and
MASLD. In a study based on a northern Chinese population, the
correlation between salt intake and MASLD in DM patients was
analyzed by Spearman analysis, which showed a positive correlation
between salt intake and the incidence of MASLD, suggesting that the
likelihood of MASLD in DM patients increases with increasing salt
intake (124). A variety of
mechanisms, including the anti-oxidant, anti-inflammatory and
anti-fibrotic effects of coffee, may be related to the protective
effects of coffee against MASLD (125). Studies have shown that caffeine
may stimulate the hepatic autophagy-lysosomal pathway and induce
fatty acid oxidation (126).
However, how caffeine activates autophagic flux is still unclear
(125). Active smoking has also
been associated with a high risk of MASLD and a study has
demonstrated the association of smoking history with advanced liver
fibrosis in patients with MASLD (127). A cross-sectional study of 2,691
Chinese men found that ex-smokers and heavy smokers (≥40
cigarettes/day) had a higher prevalence of MASLD than never-smokers
and some studies have reported that smoking is an independent risk
factor for MASLD, which may exacerbate MASLD (128,129).
Commonly used laboratory animals include rats, mice
and rabbits. Among them, rats and mice are the most widely used.
Rats are represented by Wistar and Sprague Dawley (SD). There are
more diverse mouse strains, with C57BL/6J being the most common.
The present study summarized rat, mouse, rabbit, monkey, chicken,
hamster and guinea pig models. The models summarized include
diet-induced models, drug-induced models, special models and
spontaneous models. Diet-induced models include HFD, MCD,
choline-deficient amino acid-defined (CDAA) and atherogenic diet.
It has the advantages of simple operation, low cost, repeatability
and low mortality, but the disadvantage is that it is
time-consuming. Drug-induced models usually use drugs such as
streptozotocin (STZ), carbon tetrachloride (CCl4), LPS
and tetracycline. Their advantages are short modeling time and
economy, while its disadvantages are that the drugs have high
toxicity. Special models mainly use gene knockout methods to make
model animals prone to fatty liver. Compared with other models, the
modeling time of spontaneous formation of fatty liver in special
models is short, but it has requirements for the variety of animals
and high cost. Spontaneous models mainly include monkeys. Monkeys
are similar to humans in genes and can develop MASLD in old age
without the need for a high-fat diet or drug induction. The
modeling method of the nutritional model is to feed laboratory
animals with high-sugar, high-fat and high-calorie diets or to
create an MCD. The drug-induced model aims to build the model by
administering drugs such as CCl4, tetracycline,
polychlorinated biphenyl 118, so that the drugs exert their effects
or even cause poisoning. The special strain model is to select
certain diseases that can spontaneously form fatty liver,
hyperlipidemia and other diseases closely related to MASLD or can
spontaneously present symptoms relates to MASLD. The aforementioned
modeling methods can be used independently and the pathological
characteristics or disease phenotypes of the model can be
reasonably selected according to different experimental
requirements, they can also be used in combination to reduce the
modeling time and improve the success rate of the model. The
pathogenesis of MASLD is complex and a single animal model cannot
fully mimic human MASLD. The ideal animal experimental model is a
composite model formed by combining gene mutation or specific
target gene modification with diet and drug/toxin induction. The
phenotype of such a MASLD animal model is closer to that of human
MASLD and the experimental results are more applicable to
humans.
Rats are of moderate size and have strong fertility
and blood collection abilities. Currently, commonly used rat models
for constructing MASLD diseases in laboratories include Wistar
rats, SD rats and Zucker rats (Table
I).
It is found that 93% of genomic regions of mice are
arranged in an order similar to that of human beings and they are
characterized by inexpensive feeding, rapid reproduction, easy
modeling and small inter-individual differences that facilitate the
observation of parallel experiments. MASLD mouse models can
simulate different pathogenic factors and the development of MASLD
at each stage of the disease, guide the search for the pathogenesis
of MASLD and its potential therapeutic targets and also be used for
the screening and evaluation of MASLD drugs, which are closely
related to the research of MASLD. Mouse models have been induced by
high-fat and high-sugar diets, subcutaneous injections of
CCl4 and gastrointestinal nutritional solutions, with
high-fat diets being the main modality. Mice commonly used in
current experimental studies include C57BL/6J mice, Kunming mice
and gene-deficient mice (Table
II).
In addition to rodents, other animals are commonly
used to establish MASLD models, including rabbits, monkeys and
chickens (Table III). These
models are helpful for studying the disease progression and
providing crucial evidence for exploring the pathogenesis of MASLD
and evaluating the efficacy of drugs (Table IV).
At present, animal models are the most commonly
used in the research of MASLD. However, animal models have some
unfavorable factors, such as large individual differences, long
model-building cycles, difficult control of experimental conditions
and differences between animals and humans. By contrast, cell
models are superior in overcoming the aforementioned factors.
According to experimental requirements, cell viability can be
maintained all the time, which is close to the process of human
diseases and the cellular mechanisms of diseases can be studied in
a targeted manner. Therefore, establishing a cell model of MASLD
in vitro has important theoretical significance and broad
application value for studying its pathogenesis and disease
development and further preventing and treating MASLD.
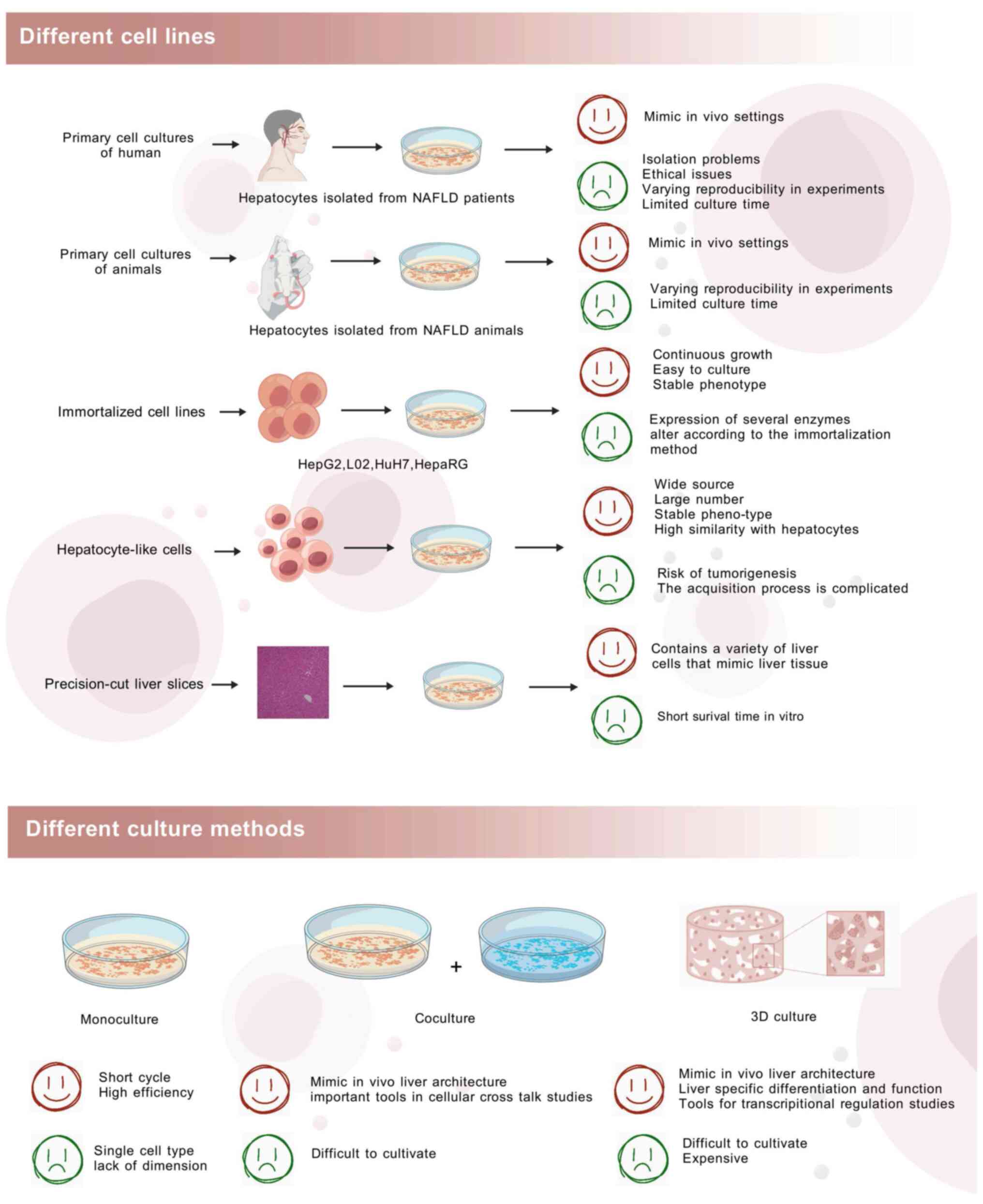
Existing model cells can be classified into human
primary hepatocytes, animal primary hepatocytes, human hepatocytes,
hepatocyte-like cells (HLCs), induced pluripotent stem cells
(iPSCs) and liver slices. The culture forms can be divided into
monoculture, co-culture and three-dimensional culture. Advantages
and disadvantages of different cell lines and different culture
methods were shown in Fig.
4.
Monoculture refers to the adherent culture of one
type of cell, which is the most basic culture method. Compared with
co-culture, monoculture has the advantages of simple operation,
unlimited subculture and short model-building time, which is
conducive to high-throughput drug screening experiments. Therefore,
it is widely used in the research of fatty liver. Human hepatocyte
cell lines, primary human hepatocytes (PHH), animal primary
hepatocytes and HLC can be used for induction culture. The
excessive deposition of extracellular matrix (ECM) leads to the
occurrence of fibrosis (194),
which is a major cause of liver function impairment. The limitation
of monoculture is that it cannot explore the cell-ECM interaction
and cannot well mimic the phenotype of liver fibrosis. The
three-dimensional liver fibrosis cell model mentioned below can
solve this problem and can also well simulate the human ECM
structure in vitro (195).
PHH is generally extracted from surgically resected
tissues and separated from the tissues by two-step collagenase
perfusion or magnetic cell separation techniques (196), which can well mimic the
physiological situation of the human liver. However, due to its
rapid dedifferentiation in vitro and the need to obtain
ethical permission for specimen acquisition, these reasons limit
the use of primary hepatocytes. The improved medium supplemented
with various chemicals solves the problem of limited traditional
primary hepatocyte culture time. The PHH cultured with this medium
can be comparable to PHH spheroids for at least 4 weeks and has the
potential to simulate steatosis disease models (197).
Due to individual differences among different
donors and the influence of various factors in the cell separation
process, the experimental results may be unstable and have poor
reproducibility. In addition, the culture conditions of human
primary hepatocytes are relatively harsh, they can only be cultured
in the short term and cannot be subcultured indefinitely, which is
not conducive to the progress of the experiment. The scarcity of
human liver samples and ethical issues also hinder the wide
application of human primary hepatocytes.
Animal primary hepatocytes are often derived from
the livers of rodents and the hepatocytes are isolated and
extracted by perfusion or non-perfusion methods. The non-perfusion
method mechanically separates and digests with collagenase or
trypsin on small pieces of liver tissue. The non-perfusion method
is simple to operate but often has the problem of incomplete
digestion, while the perfusion method can greatly improve the
viability of the isolated hepatocytes, with a survival rate as high
as 80% (198). The two-step
collagenase perfusion method is the standard procedure for
isolating primary hepatocytes. The improved standard procedure can
show higher cell viability (85-95%) through multi-parameter
perfusion control (199).
Animal primary cells solve the ethical and quantitative restriction
problems of human primary hepatocytes. However, they are affected
by different animal sources and batches, have poor reproducibility,
are relatively sensitive and have harsh cultural conditions.
HLC can be derived from embryonic stem cells,
induced pluripotent stem cells, mesenchymal stem cells, endodermal
cells and hepatic progenitor cells. Hepatocyte nuclear and
epidermal growth factors are often used to promote cell-directed
differentiation. Oncostatin M (OSM) helps to promote the maturation
of fetal hepatocytes and OSM is often added during the maturation
stage to promote complete cell differentiation. Although the
subsequent culture time of HLC is longer than that of traditional
PHH, its induction time is long (2-4 weeks) and the cost is high
(200). There is also no
unified standard for the types and ratios of cytokines and
nutrients during the differentiation process and these factors
limit the application prospects of HLC.
Induced pluripotent stem cells can differentiate
into various somatic cells without causing immune rejection and
ethical issues. The HLCs differentiated from human iPSCs can be
comparable to primary hepatocytes in terms of morphological and
functional characteristics and have the advantages of wide sources,
large cultivation quantities and stable phenotypes, making up for
the deficiencies of primary hepatocytes (204). However, the induced pluripotent
hepatocyte technology still has problems, such as low efficiency of
inducing cell transformation, possible gene mutations during the
induction process and tumorigenic risks, which limit its wide
application.
The co-culture model can make up for the defects of
the monoculture model. Introducing a second type of cell into the
model increases the interaction between cells and can offer an
improved simulation of the physiological functions of the liver.
The advantages of the co-culture model are that it compensates for
the shortcomings of the monoculture model, has simple culture
conditions and enables high-throughput experiments. However, there
are not a great number of existing co-culture model types and there
is great potential for future development. Chen and Ma (205) established a co-culture model of
HepG2 cells and THP-1 macrophages. Firstly, the macrophages adhered
to a sterile glass slide and then the slide was placed into a
6-well plate inoculated with HepG2 cells. A mixed fatty acid with a
concentration of 1 mmol/l (the ratio of PA:OA was 1:2) was added
and an MASLD model was established after 24-h induction. Giraudi
et al (206) co-cultured
HuH7 and human hepatic stellate cells. After 24-h induction with
fatty acids, the intracellular lipid accumulation increased and the
expression of α-smooth muscle actin increased, indicating that the
hepatic stellate cells were activated and the cells showed the
characteristics of steatosis and fibrosis. In another study, human
primary hepatocytes, hepatic stellate cells and KCs were
co-cultured. The cells were stimulated with fatty acids, glucose,
insulin and inflammatory cytokines to simulate MASH. In this model,
the de novo lipogenesis in the cells was enhanced and the
cells showed symptoms of oxidative stress, inflammation, fibrosis
and activation of hepatic stellate cells (207). The co-culture of human primary
hepatocytes and endothelial cells can support hepatocytes to
maintain their phenotypic morphology, improve their specific
functions and form capillary-like structures, which is more
conducive to simulate the in vivo environment and studying
the pathological mechanisms of fatty liver disease (208).
In the living liver tissue, there is substance
transport and signal transduction between hepatocytes and the ECM
and the ECM also plays a role in supporting the three-dimensional
structure (209). When human
primary hepatocytes are cultured in the traditional two-dimensional
system for a long time, morphological changes occur due to
epithelial-mesenchymal transition, resulting in the loss of
hepatocyte polarity and related liver functions (210). The construction of a
three-dimensional model is more complicated. For the
three-dimensional model of PHH, KCs and hepatic stellate cells
co-cultured with the help of a three-dimensional microphysiological
system, at least two weeks of induction with FFA is required
(211). The co-culture mode
with multiple hepatocytes can more accurately simulate the liver
microenvironment, but it requires more stringent culture
conditions. In the case of few or no precedents, researchers are
required to preliminarily explore the culture medium suitable for
the survival and proliferation of multiple cells, at least
considering the effects of pH value, osmotic pressure and gas
environment. Three-dimensional culture is divided into
three-dimensional monoculture and three-dimensional co-culture.
The three-dimensional co-culture model can be used
to study more complex fatty liver phenotypes, such as inflammation
and liver fibrosis. In the three-dimensional co-culture model,
human hepatocytes are cultured together with non-parenchymal cells,
which can simulate the interactions between various types of cells
in the liver in three-dimensional space. The advantage of the
three-dimensional co-culture model is that it can maintain
important metabolic functions for a long time and induce fatty
liver pathological phenotypes under relevant conditions.
Hepatic spheroids are spheres formed by the
self-aggregation of hepatocytes cultured in suspension without a
substrate-promoting cell attachment. The size of the spheroids can
be controlled by changing the number of starting cells and this
controllability provides convenience for standardized measurement.
The cell sources for hepatic spheroid culture can be primary
hepatocytes, hepatocyte cell lines and induced pluripotent stem
cells. Single-cell and multi-cell cultures can both form hepatic
spheroids. Compared with the planar culture of cells, the
three-dimensional structure presents superior characteristics of
the liver. HepG2 cell spheroids are more sensitive to hepatotoxic
substances. Compared with HepG2 cells cultured in a monolayer, the
half-maximal effective concentration values of a number of drugs
are markedly reduced (213).
Hepatic spheroids cultured from PHH can survive up to 7 weeks
(214) and can achieve 100%
specificity and 69% sensitivity in distinguishing hepatotoxic
substances (215). Compared
with hepatic spheroids derived from single-cell culture, multi-cell
co-culture spheroids may have more development potential. Hepatic
spheroids constructed by co-culturing PHH and NPC perform
outstandingly in glycogen storage (216) and albumin synthesis, indicating
that co-culture hepatic spheroids can offer an improved imitation
of the structure and function of the human liver (217).
Organoids have a self-renewing stem cell population
and can exhibit some organ characteristics, which contain single or
multiple cell types. It has been reported that organoids from adult
hepatocytes can be cultured for up to 2.5 months (218). PHH and pluripotent stem cells
can both simulate the adult liver. For the induction of pluripotent
stem cells, a three-dimensional model can be constructed after
monolayer culture induction to maturity or directly cultured in
three dimensions at the undifferentiated stage (219). The induction of MASLD requires
the addition of FFA to the culture medium. Liver organoids in this
hepatotoxic environment for a long time show lipid droplet
formation and TG accumulation and the upregulation of the
expressions of genes related to lipid metabolism, inflammatory
cytokines and fibrosis markers, showing the typical biochemical
characteristics of MASLD progression (220). In addition, liver organoids can
also be derived from liver cancer cells to simulate the cancer
microenvironment, playing an important role in the field of
anti-cancer research.
Liver-on-a-chip technology is used to simulate the
minimum functional unit of liver tissue, mainly constructed with
the assistance of computer aided design software. When designing a
microfluidic chip, factors such as flow rate, design size and
aspect ratio need to be considered. In a three-dimensional culture
system, microfluidic devices can reproduce the characteristics of
the multi-cell microenvironment by controlling various parameters
(221) and conducting cell
culture and sample secretions. Although liver-on-a-chip technology
is costly and the chip manufacturing process is complex, it can
mimic the complex in vivo liver microenvironment, precisely
control chemical gradients and manipulate parameters such as time,
so it has broad development prospects in the research field of
cell-matrix and cell-cell interactions.
3D-bioprinting technology polymerizes various
biological materials through inkjet, extrusion, laser
polymerization, or digital light processing. In the field of liver
model construction, a vascularized hepatic lobule model containing
hepatocytes and endothelial cells has been developed through
extrusion technology. Compared with a simple mixture of hepatocytes
and endothelial cells, this model has increased albumin and urea
secretion and a superior imitation of liver functions (222). With the development of
3D-bioprinting technology, the optimization of 'ink' has also been
put on the agenda. Type I collagen is the main component of the
ECM. When collagen I is mixed with thiolated hyaluronic acid in
different ratios, it was found that the ratio of 3:1 can offer an
improved maintenance of biological activity (223). The bioprinting method for MASLD
needs to be further explored. One possible idea is to culture
monolayers of steatotic hepatocytes as 'ink' to print
three-dimensional structures or to induce lipotoxic substances
after printing a liver model (224,225).
The chronic stress method uses one or several
stress factors to change the emotional state of experimental
animals (226). Although this
method has a good effect, it takes a long time and only one
emotional stimulus is applied to the rats for a long time, which is
likely to cause the rats to adapt to the stimulus, resulting in
insignificant emotional changes. To improve the errors caused by
this method, unpredictable stress is now mostly used in the
experiment, which can minimize errors and prevent rats from
adapting to the stimulus. Sun et al (227) used fixed-time foot electroshock
combined with noise stimulation to increase the diversity of
chronic stress methods.
The chronic restraint method is a way to restrict
the activities of experimental animals by placing them in restraint
devices, thus triggering emotional changes in the experimental
animals. Some scholars have established the MASLD model by using
long-term chronic restraint stress. They mainly applied restraint
stress to rats by using plastic restrainers. To prevent the rats
from breaking free, they also carried out punctures multiple times
to make the restrainers fit closely with the rats. The rats were
restrained for 6 h every day and this lasted for 9 weeks (228-231).
Since MASLD is a chronic disease, according to the
theory that chronic diseases often lead to deficiency and blood
stasis, patients with this disease usually present with various
syndromes of deficiency and blood stasis, among which the syndrome
of blood stasis is the most common. Patients with this syndrome
show symptoms such as masses in the right hypochondrium, loss of
appetite, abdominal distension, weakness, loose stools, dull
complexion and a pale and dark tongue. Liu et al (232) prepared the MASLD model of blood
stasis syndrome by feeding rats with a high-fat and HFFD and
intraperitoneally injecting 30 mg/kg STZ. The results showed that
the rats' body weight firstly increased then decreased. Their fur
was yellowish, dull, wilting and lacking luster. Their mental state
was poor, they were inactive and easily startled and their claws
were dull and had a low temperature. Moreover, the levels of ALT,
AST, TC, TG and LDL-C in rats were increased and there was
inflammatory infiltration in the cytoplasm of hepatocytes.
Researchers have prepared the MASLD model of blood stasis type by
using high-fat feed combined with leg-binding stimulation and
reagent intervention methods. After 8 weeks, the rats showed
manifestations such as dull hair and irritability, an increased
liver index, diffuse fatty hepatocytes, varying degrees of
macrovesicular and microvesicular steatosis and occasionally
inflammatory cell infiltration. Meanwhile, they also had
pathological manifestations consistent with MASLD (233).
In the process of treating MASLD, since the changes
in liver tissue of most patients are at the stage of simple
steatosis, the priority of treatment should be to solve the problem
of overweight and improve the patient's IR. The secondary aim is to
avoid 'additional blows' leading to MASH and acute liver failure
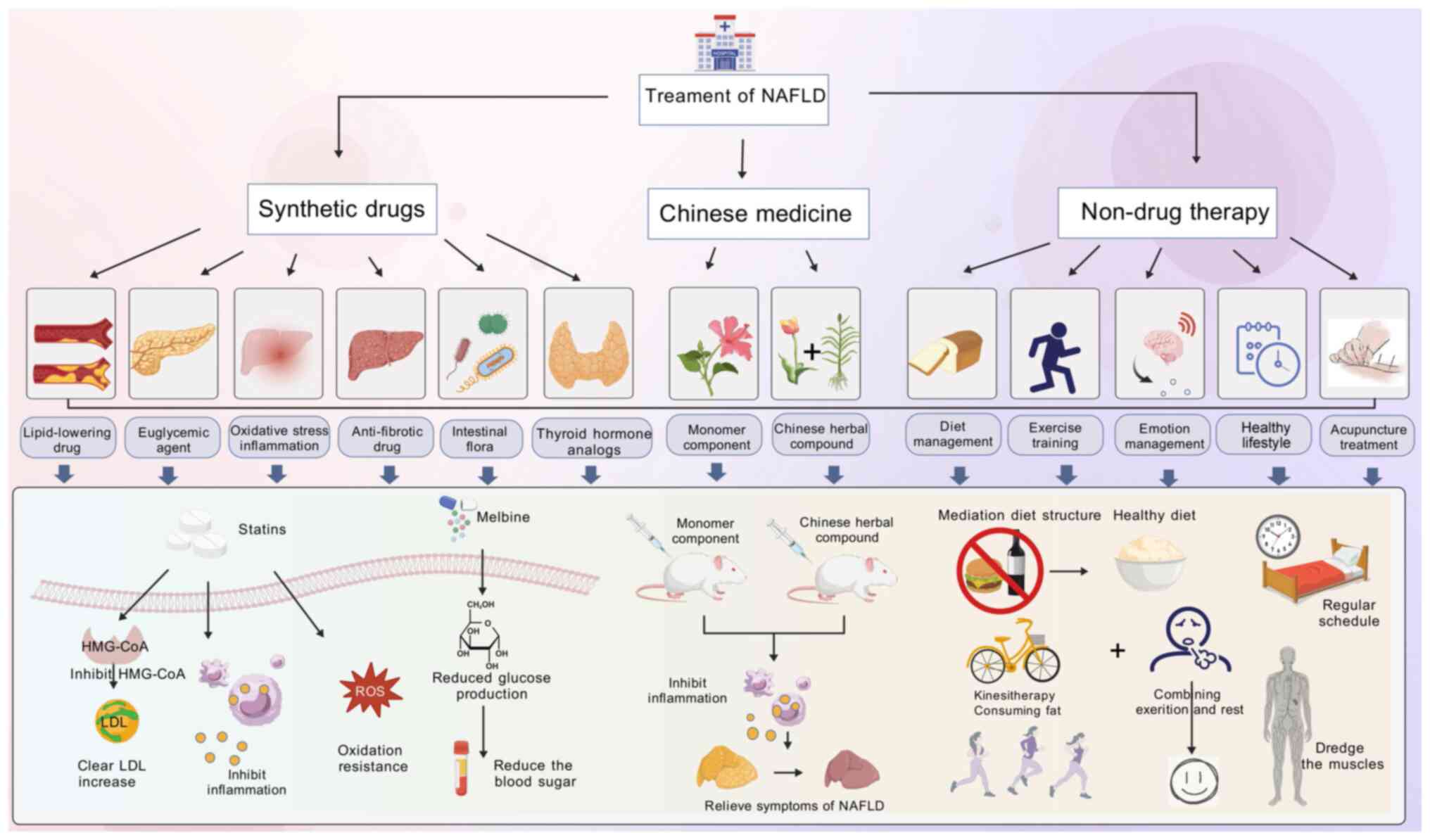
and to reduce hepatic fat deposition in patients (234). Currently, the main clinical
drugs for MASLD are lipid-lowering drugs, insulin sensitizers,
anti-fibrotic and thyroid hormone drugs. Moreover, inhibition or
reduction of microRNA activity by targeted drugs can alleviate the
condition, which provides a new idea for the proposal of MASH
typing and the development of individualized treatment modalities
(235,236). The precise treatment of
different subtypes of MASH patients with targeted drugs is also the
development trend of future drug research (Fig. 5). The drugs for the targets in
the treatment of MASLD disease are shown in Table V.
Lipid metabolism disorder is the main clinical
characteristic of MASLD patients and it is an important factor in
the development of MASLD to hepatitis and cirrhosis, so
lipid-lowering has become an important method of treatment for
MASLD patients. At present, the best lipid-lowering effect of drugs
is from statins, their mechanisms are mainly through the inhibition
of hydroxy methylglutaryl coenzyme A (HMG-CoA), thereby blocking
the synthesis of hepatic cholesterol and causing hepatic
compensatory LDL receptor synthesis, so that the plasma LDL and LDL
clearance increases. At the same time, statins can also exert
anti-oxidant and anti-inflammatory effects (237). However, there are more problems
in the use of statins, such as producing hepatotoxicity and muscle
toxicity (238). In recent
years, it has been found that inhibition of proprotein
convertase-subtilisin-kexin type 9 (PCSK9) can markedly reduce LDL
levels. Another experimental study found that the combination of
statins with PCSK9 inhibitors was more effective than statins alone
(239). In addition, ezetimibe
and PCSK9 inhibitors (IIb, C) can be used in combination in
statin-intolerant patients (240).
IR is a key link in the pathogenesis of MASLD,
insulin sensitizers can effectively target the 'one strike' and
increase insulin sensitivity to improve IR. Thus, enhancing the
sensitivity of effector organs to insulin has become an important
way to treat MASLD. Metformin, glucagon-like peptide-1 (GLP-1),
thiazolidinediones and other drugs are currently more popular.
Metformin contains two guanidinium groups in its
structure and is a common oral metformin hypoglycemic drug in
clinical practice. It lowers blood glucose by decreasing glucose
production in the liver, increasing glucose metabolism and thus
improving IR and has a certain effect on weight loss and regulation
of lipid metabolism (241).
However, metformin is generally not used alone in the treatment of
MASLD (242). Some studies have
shown that metformin can markedly reduce the levels of ghrelin,
insulin and C-peptide in MASLD patients, but the improvement of
liver fibrosis and liver inflammation is not significant (243,244). Although metformin is not the
first choice for the treatment of MASLD, it is effective in
improving body weight, lipids and glucose metabolism in patients
with MASLD or MASLD combined with T2DM (245).
GLP-1 is a well-established target in the field of
diabetes. Liraglutide is a GLP-1 agonist used for the treatment of
type 2 diabetes (1.2 to 1.8 mg/day) and obesity (3 mg/day). In
addition to acting on the pancreas, GLP-1 can improve peripheral
insulin sensitivity, participate in the physiological regulation of
blood glucose, increase hepatic glucose uptake and glycogen
synthesis, delay gastric emptying and reduce appetite and reduce
the occurrence of atherosclerosis (246). Several GLP-1 drugs have been
studied in clinical trials for their ability to promote insulin
secretion, induce β-cell proliferation, inhibit postprandial
glycogen release and delay gastric emptying. It has been shown that
GLP-1 receptor agonists can enhance insulin sensitivity in effector
organs, promote fatty acid oxidation, reduce lipogenesis and
improve glucose metabolism after binding to the receptor (247).
Polyene phosphatidylcholine has anti-oxidant,
anti-inflammatory properties, reduces hepatocyte damage and
apoptosis and can effectively target the pathological symptoms
caused by MASLD and its combination with metformin drugs for the
treatment of patients with MASLD as well as MASH is markedly more
effective than monotherapy (248). Obeticholic acid (OCA), as a
nuclear transcription factor FXR agonist, has good application in
the treatment of MASLD by affecting the gene expression of various
metabolic and cellular damage pathways, such as lipid metabolism,
IR and oxidative stress (249).
Pioglitazones are first-generation insulin
sensitizers, which can specifically bind and activate PPAR-γ,
improve IR, participate in glucose and lipid metabolism and inhibit
the expression of LP and the expression of TNF-α, so as to reduce
the hepatic lipid deposition and inhibit liver inflammation and
fibrosis formation (250).
Sumida and Yoneda (251) found
that pioglitazone not only improved IR but also improved hepatic
glucose-lipid metabolism, steatosis and inflammatory necrosis in
patients with MASLD by activating PPAR-γ. The American Academy of
Liver Diseases recommended pioglitazone in 2017 guidelines for the
diagnosis and treatment of MASLD (252). Pioglitazone is not widely
available because of safety concerns, including congestive heart
failure, bone fractures in women and the possibility that
pioglitazone may lead to an increased risk of bladder cancer
(253). The second-generation
insulin sensitizer MSDC-0602K did not show the side effects
associated with the first-generation insulin sensitizers. According
to the report of the latest phase IIb 52-week double-blind study
(254), MSDC-0602K markedly
reduced fasting glucose, insulin, glycosylated hemoglobin and
markers of liver injury without dose-limiting side effects.
However, it did not show a significant effect on the liver
histology of the biopsy technique used. The information gained from
this trial may provide a pre-basis for future study.
In the disease process of MASLD, increased
oxidative stress and defective anti-oxidant defense mechanisms
promote liver injury as well as disease progression to MASH
(255). Therefore, drugs with
anti-oxidant activity can be used in the treatment of MASLD.
Vitamin E is present in the phospholipid bilayer of cell membranes
and helps to prevent oxidative damage caused by free radicals
(256). The randomized
controlled trial of PIVENS selected non-diabetic and non-cirrhotic
MASH patients, conducted a 2-year trial of vitamin E (800 IU/d),
pioglitazone and placebo and showed that vitamin E had significant
histological improvements and that 36% of patients treated with
vitamin E had improved steatosis, inflammation and remission of
MASH (257). This suggested
that vitamin E is favorable for the treatment of MASLD and MASH
(258). However, long-term use
of vitamin E may increase the risk of prostate cancer and
hemorrhagic shock, so vitamin E should not be used for long-term
treatment of MASH and MASLD (259). Another study found that the
active ingredient in silymarin could improve liver fibrosis and
reverse MASLD by reducing oxidative stress and inflammation
(260). Although silymarin did
not meet the primary endpoint in MASH patients, it markedly
improved liver fibrosis compared with placebo (260).
ZSP1601 is a pan-phosphodiesterase inhibitor, which
is the first small molecule innovative drug to obtain clinical
trial approval for the treatment of MASH in China. Phase Ia
clinical trials were conducted in healthy populations and ZSP1601
had a peak time of 1.5-2.5 h and a half-life of 6.34-8.64 h. The
drug was well tolerated when compared with the placebo group
(261). Its safety and
pharmacokinetic profile supports further efficacy assessment in
MASH patients. In the Ib/IIa clinical trial, ZSP601 was shown to
have a favorable safety and tolerability profile in MASH patients,
with a pharmacokinetic profile consistent with that of healthy
subjects. After receiving 28 days of treatment, different dose
groups of ZSP601 showed significant improvements in metabolism,
hepatic fat content, inflammation and fibrosis biomarker indices
(262), supporting the
evaluation of its long-term safety and efficacy in a larger sample
size of MASH patients.
Apoptosis is closely related to the formation of
hepatic fibrosis, hepatocyte apoptosis releases excessive DNA
fragments, which stimulates hepatic stellate cell activation and
promotes the formation of fibrosis. In the liver, TNF mediates a
number of biological reactions, reduces oxidative stress and fights
against fibrosis and it is a potential therapeutic drug for MASLD.
Pentoxifylline has been suggested as a potential therapeutic agent
for MASH because of its ability to reduce oxidative stress
production and its anti-fibrotic effects. Researchers treated
patients with biopsy-proven MASH with either placebo or
pentoxifylline 400 mg/day for 1 year in a small RDBPCT clinical
trial (263). The results
showed an improvement in histologic features in 38.5% of the
subjects in the pentoxifylline treatment group (13.8% in the
control group, P=0.036).
Apoptosis signal-regulating kinase 1 (ASK1) is a
kinase in the MAP3 kinase family that activates the p38/JNK pathway
downstream of TNF-α, leading to apoptosis and fibrosis. Studies in
mice have shown that ASK-1 promotes TNF-α-mediated IR and steatosis
(263), whereas inhibition of
ASK1 ameliorates diet-induced steatosis and fibrosis (264). GS-4997 is an oral ASK1
inhibitor and a randomized, double-blind, open phase II clinical
trial in patients with MASH and fibrosis is currently underway to
evaluate the efficacy of 24 weeks of treatment with GS-4997 or
GS-4997 and simtuzumab. ASK-1 inhibitors have been shown to improve
inflammation and fibrosis in animal models of MASH, but
Selonsertib's phase III trial does not meet its primary endpoint
(265).
C-C motif chemokine receptor 2/5 (CCR2/5) are
inflammatory chemokine receptors that are highly expressed in MASH.
Chemokines induce cell migration in the direction of increased
chemokine concentration and exert their biological effects by
interacting with G-protein-linked transmembrane receptors. CCR2 on
the surface of macrophages promotes inflammation,
neovascularization and activation of hepatic stellate cells,
exacerbating hepatic fibrosis, whereas CCR5 is mainly expressed by
hepatic stellate cells, causing hepatic fibrosis (266). Inhibition of the activity of
such receptors can reduce the inflammatory response in the liver
and prevent or slow down liver fibrosis. Cenicriviroc (CVC) is an
orally potent dual inhibitor of CCR2/5, which has been shown to
ameliorate the progression of hepatic fibrosis in MASH mice
(267). A phase IIb clinical
trial with a sample of MASH patients showed that the anti-fibrotic
results of CVC in the second year were consistent with those of the
first year and remained significant in the two-year treatment
group, demonstrating good tolerability and efficacy (268). CVC was in a phase III clinical
study (AURORA, NCT03028740) aimed at evaluating its efficacy and
safety in adult MASH patients with hepatic fibrosis (269). However, the AURORA phase III
trial was forced to be terminated prematurely due to the failure to
meet the objectives of its first phase results.
Elafibranor is an agonist of PPAR-α and PPAR-δ.
Studies have shown that elafibranor has the effect of improving
insulin sensitivity, balancing blood glucose, regulating lipid
metabolism and reducing inflammatory response (270). Ratziu et al (271) conducted an international,
multicenter, randomized, double-blind, placebo-controlled study to
evaluate the safety and efficacy of elafibranor in patients with
MASH. MASH patients without cirrhosis were randomized in a 1:1:1
ratio to receive elafibranor 80, 120 mg, or placebo for 52 weeks.
Liver biopsies, as well as clinical and laboratory evaluations,
were performed on patients every 2 months. The results of the
available studies suggest that elafibranor 120 mg/day for 1 year in
moderate-to-severe patients may alleviate MASH fibrosis.
The degree of hepatic fibrosis is used to determine
the progression of MASLD and most targeted clinical trials end in
the improvement of fibrosis. In addition to acting on the
pathogenesis of MASLD, some anti-fibrotic drugs have great
potential. It has been shown that activation of HSCs can exacerbate
the degree of hepatic fibrosis (272). Galactose lectin-3 (Gal-3)
protein is closely associated with inflammation and liver fibrosis.
Inhibiting Gal-3 can markedly reduce the symptoms of liver fibrosis
in animals and clinical trials have also shown that it has an
improved therapeutic effect on subjects with MASH and bridging
fibrosis (273). Pirfenidone is
a TGF-β inhibitor, clinically used in the treatment of idiopathic
pulmonary fibrosis and another study found that it markedly
improved the pathological features of liver fibrosis in animals
(273). Belapectin (GR-MD-02)
is a Gal-3 inhibitor developed by Galectin Therapeutics. Gal-3
protein is involved in a variety of inflammatory and fibrotic
processes. Animal studies have demonstrated that Belapectin reduces
fibrosis. Clinical trials have also demonstrated that it is well
tolerated in subjects with MASH with bridging fibrosis and has
beneficial prevention and treatment of esophageal varices in
patients with MASH liver cirrhosis (273).
Dysbiosis of intestinal flora is closely associated
with liver disease and cancer (274). Thus, manipulation of the
microbiome through diet, probiotics, prebiotics and other
medications is a viable line of research for the treatment of liver
diseases, including MASLD. It has been found that prebiotics can
reduce the accumulation of TG in the liver by decreasing the
production of fat and decreasing the expression of genes such as
FAS (275). In addition,
probiotics can repair leaky gut and delay the progression of NAFL
to MASH by modulating nuclear receptor expression and improving IR
in liver and adipose tissue (276). In a clinical study by
Malaguarnera et al (277), Bifidobacterium bifidum
was found to markedly improve lipids, IR index, liver tissue
inflammation and liver fibrosis in patients with MASH. Wang et
al (278) found that
Bifidobacterium trifidum tablets markedly reduced the levels
of lipids, inflammatory factors, LPS and lipocalin in a rat model
of MASH. Triclosan is a widely used antibiotic that ameliorates the
ecological dysbiosis of the gut microbiome associated with MASLD by
inhibiting pathogenic gram-negative bacteria. Solithromycin is a
new-generation macrolide antibiotic clinically used to treat
bacterial infections. In a mouse model of MASH induced by both diet
and STZ, solithromycin was shown to ameliorate hepatocellular
ballooning and inflammation, reduce blood glucose levels and
downregulate hepatic gluconeogenic enzyme expression (279). Based on preclinical results, a
Phase II open clinical trial study is currently underway to
evaluate whether 13 weeks of solithromycin treatment in
non-cirrhotic MASH patients can improve liver histologic
characteristics.
Thyroid hormone receptor (THR-β) is highly
expressed in hepatocytes and plays a key role in regulating the
impaired metabolic pathway of MASH (280). The degree of hepatic
hypothyroidism is higher in patients with MASH (281). This suggests that hepatic
hypothyroidism may contribute to the development of MASH to some
extent. Moreover, drugs targeting this condition are currently
approved for MASH treatment.
Resmetirom is an oral, liver-targeted THR agonist,
which is 28-fold more selective for THR-β than triiodothyronine for
THR-α (282). In MASH,
Resmetirom's selectivity for THR-β contributes to hepatic-mediated
thyroid hormone metabolism while avoiding the adverse effects of
excess thyroid hormones in the heart and bone mediated through
THR-α. Resmetirom and other thyroid hormone analogs reduce hepatic
TG, inflammatory factors, fibrosis markers and ALT levels and
reduce hepatic steatosis lipid peroxidation (283). In a multicenter, randomized,
double-blind, placebo-controlled Phase III clinical trial of
MAESTRO-MASH, the proportions of patients who achieved the primary
endpoint of MASH symptom resolution without worsening of hepatic
fibrosis at 52 weeks were 25.9 and 29.9% in the Resmetirom 80 and
100 mg dosage groups, respectively, compared with 9.7% in the
placebo group (P<0.001). In addition, there were significant
reductions in several lipid and lipoprotein metrics, including the
key secondary endpoint of LDL-C (284). This trial was the first phase
III clinical trial to achieve the two primary endpoints proposed by
the FDA in patients with MASH. Accordingly, based on these key
clinical data, the FDA approved the drug on March 15, 2024, for the
treatment of patients with non-cirrhotic MASH with moderate to
advanced liver fibrosis. This is the first MASH treatment approved
by the FDA and represents a landmark breakthrough in the field.
VK2809 is another THR-β selective agonist with
specificity for liver tissue and good therapeutic potential. It
belongs to a family of prodrugs that cleave and release potent
thyroxine analogs in vivo. A randomized, double-blind,
multicenter and placebo-controlled phase II study was conducted to
evaluate the efficacy, safety and tolerability of VK2809 in
reducing LDL-C and liver fat content in patients with primary
hypercholesterolemia and MASLD (285). Patients were dosed for 12 weeks
and subsequently discontinued for 4 weeks. Results showed a
statistically significant 53.8% median reduction in liver fat in
patients using 5 mg of VK2809 daily, 88% of cases in patients using
VK2809 showed ≥30% reduction in liver fat at 12 weeks. In all dose
groups, VK2809 had a favorable safety and tolerability profile,
with no adverse reactions reported.
Medicinal plants have important advantages in
preventing and improving MASLD, so treatment of MASLD by medicinal
plants is a potential therapeutic means (286). Medicinal plants have a history
of several hundred years in the prevention and treatment of liver
diseases. Compared with chemical pharmaceuticals, medicinal plants
have a holistic concept and the idea of diagnosis and treatment,
which is the most significant and basic feature of medicinal plants
and shows its advantages in the treatment of MASLD (287). The treatment of MASLD by
medicinal plants focuses on the holistic theory of liver
preservation, which manifests itself in a variety of mechanistic
forms, including anti-oxidative stress, lipid metabolism
regulation, anti-inflammation, anti-fibrosis and intestinal
microbiota regulation (288).
It can be seen that the treatment of MASLD by medicinal plants is
more individualized and comprehensive, which is in line with the
characteristics of MASLD (Fig.
5).
Single medicinal plants have multi-component,
multi-target and multi-dimensional effects in anti-MASLD. Exploring
the monomers of medicinal plants for the treatment of MASLD studies
have found that natural compounds such as polysaccharides,
terpenoids, glycosides, alkaloids, flavonoids, phenols and other
natural compounds have a wide range of biological activities. By
regulating various signaling pathways, they can exert comprehensive
benefits such as inhibiting inflammatory response, improving lipid
metabolism, reducing IR, then effectively alleviating the symptoms
of MASLD. A summary of some studies on the prevention and treatment
of MASLD by medicinal plants is shown in Table VI.
Based on evidence-based treatment, medicinal plants
combine different chemical components for specific etiology and
pathogenesis and exert the advantages of multi-targeting and
synergistic effects in the prevention and treatment of MASLD. The
mechanisms are summarized in the following Table VII.
Non-pharmacological treatments for MASLD are mainly
life-related initiatives. In general, weight control, improved
dietary patterns and lifestyle modifications can prevent the
development of metabolic syndrome and MASLD (374). Proper aerobic exercise with
intermittent high-intensity exercise can effectively reduce excess
body fat. Hannah and Harrison (375) found that MASLD patients' weight
loss of 3-5% slowed down the process of fatty liver lesions, weight
loss of 5-7% may result in reversal of fibrosis. Obese and diabetic
patients are more likely to plan their diets reasonably, adjust
their daily routines and reduce their calorie intake to prevent
MASLD at source (Fig. 5).
Dietary management should follow the principles of
nutritional balance, limiting energy intake and adjusting dietary
structure. Firstly, to supplement dietary fiber and minerals: Eat
more food rich in low calorie, high protein and high fiber, mix
coarse and fine grains and choose more vegetables, fruits, fungi
and seaweed to ensure sufficient fiber intake and maintain
intestinal microbial homeostasis. Secondly, to supplement vitamins
and appropriate amounts of micronutrients: eat more food rich in
vitamins and micronutrients selenium (purple cabbage, beans,
seafood and seaweed) can accelerate the decomposition of lipid
peroxidation and prevent liver fibrosis (376). Reduce the intake of
carbohydrates: Eat less high sugar, high fat, high cholesterol,
spicy, fried and salty food. In order to reduce the degree of
obesity and promote the oxidation and decomposition of fatty acids,
reduce the content of FFA and the burden of fat accumulation on the
liver (377). In addition,
fasting therapy: Patients only drink a moderate amount of water and
eat low-calorie food for a certain period of time, plus a moderate
amount of exercise to prevent and control MASLD, usually not more
than 10 days. After the fasting period, the use of food from soft
and easy to digest food gradually over to ordinary meals. For
patients with fatty liver caused by excessive weight loss, food
containing unsaturated fatty acids can be given to increase satiety
and nutrition according to the patient's own situation and their
fat intake should not exceed 30% (378). In other aspects: Develop good
dietary habits, eat three meals a day regularly and quantitatively,
avoid overeating diet, regular breakfast, reduce the number of fast
food and dinner, drink more tea and coffee.
Exercise training therapy is an effective means to
promote the recovery of MASLD patients, which has advantages of
being green and inexpensive. Aerobic exercise can improve the rate
of fat oxidation, increase fat consumption and reduce the
accumulation of liver fat (379). At present, with the social and
economic development and the increase of pressure at work, people
are physically and mentally exhausted. It is easy to occur a
variety of chronic diseases due to less exercise training or
insufficient continuity, so it is more important to guide the MASLD
patients to engage actively in exercise training. Li et al
(380) used exercise training
to intervene in MASLD, 3 times a week, each time lasting 3-4 times,
lasting 40-60 min and the continuous intervention for 24 weeks,
which could effectively improve the lipid metabolism level of
patients with mild, moderate and severe MASLD, reduce blood glucose
and improve the function of the liver.
Anxiety, depression and other negative emotions
will increase the risk of MASLD (381). Therefore, patients should
always maintain a stable state of mind, guard against arrogance and
impatience, combine work and rest and learn to apply exercise,
music, muscle relaxation in daily life and other methods to
alleviate their own negative emotions, relax the body and mind and
ultimately to achieve the effect of liver detoxification and
restoration of liver function.
Circadian rhythm disorders can cause MASLD, so
patients should develop good living habits, including go to bed
early, get up early, not stay up late, regular personal work and
rest. At the same time, regular checks of blood glucose, blood
lipids, blood pressure and abdominal ultrasound, in order to timely
detection of the occurrence of MASLD and its risk factors (381).
As a complex metabolic disease involving multiple
liver injuries, the incidence of MASLD is not only increasing year
by year, but also tends affect younger and younger individuals, so
its prevention and treatment should not be postponed. The
pathogenesis of MASLD is complex and progressive, the liver
histopathological diagnosis of MASLD is difficult to achieve in
humans and suitable experimental models must be established to
study MASLD, thus posing great challenges to its prevention and
drug development. The present study reviewed the complex
pathogenesis of MASLD, including IR, lipotoxicity, immune system
disorders inducing inflammatory responses, intestinal flora
disorders and genetic factors, as well as the study of Chinese
medicine on the pathogenesis of MASLD (Fig. 6). MASLD arises from the
interconnection and influence of multiple organs and systems, which
can not only act individually, but also interact and synergize with
each other to promote the development of MASLD and the specific
triggering mechanisms still need to be explored at a deeper
level.
In recent years, the study of the molecular
mechanism of MASLD has attracted much attention. The notch
signaling pathway is a highly conserved transmembrane signaling
pathway. It activates signal transduction through receptor-ligand
interactions, which affected gene expression. The Notch signaling
pathway plays a key role in hepatic lipid metabolism disorders,
inflammatory responses and liver fibrosis (382). adenosine
5'-monophosphate-activated protein kinase (AMPK), as a core hub of
energy metabolism, plays a crucial role in MASLD by regulating
fatty acid synthesis, oxidation, autophagy and inflammation.
Researches showed that various monomers of medicinal plants by
inhibiting key factors of lipid synthesis (SREBP-1c, FAS and ACC),
promoting fatty acid oxidation (PPARα and CPT-1), improving insulin
resistance, enhancing autophagy (ULK1 and LC3) and inhibiting
inflammation (NF-κB) and oxidative stress (Nrf2) (383). A large amount of basic
researches shows that the NF-κB pathway is a crucial pathway for
mediating inflammatory and oxidative stress responses and also
plays an important role in the occurrence and development of MASLD.
When the internal environment of the body changed, the levels of
inflammatory factors in the body increases markedly. The NF-κB
pathway is activated by activating the expression of inflammation
and immune related receptors on the surface of liver cell
membranes. The NF-κB pathway regulates various factors upstream and
downstream, further mediating the inflammatory response, oxidative
stress response, liver fibrosis, cell apoptosis, autophagy and
pyroptosis of liver tissue to promote the process of MASLD
(384). Chitosan markedly
improves symptoms of MASLD by inhibiting lipid production,
regulating inflammatory responses, alleviating oxidative stress,
improving insulin resistance and regulating gut microbiota through
multiple mechanisms. The specific mechanisms included that it
inhibits the expression of transcription factors, activates the
AMPK signaling pathway to promote fatty acid oxidation and inhibits
the activity of signaling pathways such as PI3K/AKT/mTOR (385).
Through the continuous efforts of researchers at
home and abroad, the study of experimental models of MASLD disease
(including animal models and cellular models) has made great
progress. Ideal disease models should be similar to human
pathogenesis, simple, inexpensive, fast modelling, low animal
mortality, high replication rate, good reproducibility and easy to
use. In the process of practical application, researchers should
understand the species differences between experimental animals and
human beings, the formation mechanism of MASLD model and
pathological changes are very different from those of human beings
when they suffer from the disease and they need to select
appropriate models according to specific research purposes and
needs and consider the complications of the MASLD model. The TCM
syndrome models, guided by TCM theory and based on the basic
principle of evidence-based treatment, has obvious advantages in
the treatment of fatty liver, but the formation principle, method
and judgement index of the model are difficult to be standardized
and need to be further verified (Fig. 6). Future research should
establish a unified standard for the models and on the basis of
improving the existing models, more efforts should be devoted to
exploring new modelling methods, so that the experimental models
can be more closely related to the characteristics of human MASLD,
with a view to carrying out more in-depth research on the disease,
elucidating its pathogenesis and an improved chance of preventing
and treating MASLD.
Due to the complex pathogenesis of MASLD, there is
no specific clinical drug and the use of a single treatment is
limited. In the light of the current research results,
individualized dietary control and lifestyle changes are the basic
treatment for MASLD. A healthy lifestyle with good dietary habits,
active treatment of the primary disease and selection of
appropriate medication as an adjuvant therapy are conducive to
intervening and controlling the development of MASLD and preventing
further deterioration of the disease. Currently, effective
therapeutic agents for MASLD are focused on multiple potential
targets, with multi-target, multi-pathway therapeutic measures and
targeted agents for key aspects of the target genes or metabolic
pathways will also be applied in the clinic. However, various drugs
are still in the research stage and in the future, clinical
attention should not only be paid to the efficacy and safety of
such drugs, but also need to further evaluate the effects of
combining drugs with different mechanisms, so as to ultimately
provide patients with a reasonable individualized medication plan
(Fig. 6). In addition to
synthetic drugs, natural medicines (TCM, Chinese medicine
compounds), are characterized by multiple components and multiple
targets of action, so the development of new natural medicines with
multiple targets of action, maintaining a healthy composition of
intestinal flora and promoting energy metabolism of the body, play
a therapeutic role in MASLD from another angle.
Not applicable.
KL was responsible for data curation and writing
the original draft. CC, LW and ZS were responsible for formal
analysis and methodology. JL, MA and QL were responsible for
conceptualization and investigation. HH, QM, XW and RW were
responsible for supervision, funding acquisition, writing,
reviewing and editing. Data authentication is not applicable. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by National Natural Science
Foundation of China (grant nos 82360682 and 82360825), Major
Science and Technology Research Projects of Nanchang City [grant
no. Hongkezi(2023)137-2], Training Project of Ganpo Juncai Support
Plan for High level and High skilled Leading Talent in 2024 (grant
no. 20240003188), 'Chunhui Plan' Collaborative Research Project of
Ministry of Education of the People's Republic of China (grant nos.
H ZKY20220385 and 202200200), Jiangxi Provincial Natural Science
Foundation (grant nos. 20242BAB26172, 20224BAB206104 and
20224BAB206112) and Jiangxi University of Chinese Medicine Science
and Technology Innovation Team Development Program (grant no.
CXTD-22002).
|
1
|
Ludwig J, Viggiano TR, McGill DB and Oh
BJ: Nonalcoholic steatohepatitis: Mayo clinic experiences with a
hitherto unnamed disease. Mayo Clin Proc. 55:434–438. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong W, Jiang H, Li Y, Lv L, Gong Y, Li B,
Wang H and Zeng H: Interpretable machine learning analysis of
immunoinflammatory biomarkers for predicting CHD among NAFLD
patients. Cardiovasc Diabetol. 24:2632025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Charu V, Liang JW, Mannalithara A, Kwong
A, Tian L and Kim WR: Benchmarking clinical risk prediction
algorithms with ensemble machine learning for the noninvasive
diagnosis of liver fibrosis in NAFLD. Hepatology. 80:11842024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gawrieh S, Vilar-Gomez E, Wilson LA, Pike
F, Kleiner DE, Neuschwander-Tetri BA, Diehl AM, Dasarathy S,
Kowdley KV, Hameed B, et al: Increases and decreases in liver
stiffness measurement are independently associated with the risk of
liver-related events in NAFLD. J Hepatol. 81:600–608. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marjot T: The endothelium as the
gatekeeper of insulin's action on metabolic tissues: Implications
for MASLD and MASH. J Hepatol. 83:808–810. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nadeau KJ, Mayer-Davis EJ, Gubitosi-Klug
R, Zeitler PS, Kahn SE and Dabelea D; SEARCH TODAY, RISE and
DISCOVERY study groups: Youth-onset type 2 diabetes: What we've
learned from key youth-onset type 2 diabetes studies, what we still
don't know, and why it is important. Diabetes Care. 48:1136–1149.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
The Lancet Gastroenterology Hepatology:
Redefining non-alcoholic fatty liver disease: What's in a name?
Lancet Gastroenterol Hepatol. 5:4192020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lazarus JV, Newsome PN, Francque SM,
Kanwal F, Terrault NA and Rinella ME: Reply: A multi-society Delphi
consensus statement on new fatty liver disease nomenclature.
Hepatology. 79:E93–E94. 2024. View Article : Google Scholar
|
|
9
|
Zhang F, Zhang X, Lv B, Sun R, Zhao T and
He N: Changes in epidemiology and treatment outcomes after the
academic renaming from NAFLD to MAFLD/MASLD. Chin Hepatol.
7:900–903. 2025.In Chinese.
|
|
10
|
Bae JC: No More NAFLD: The term is now
MASLD. Endocrinol Metab (Seoul). 39:92–94. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drygalski K: Pharmacological treatment of
MASLD: Contemporary treatment and future perspectives. Int J Mol
Sci. 26:65182025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Zhang Z, Jiang X, Li L, Hu T, Wang B
and Deng Z: Meta-analysis and data mining of Chinese herbal
medicines for the treatment of metabolic dysfunction-associated
steatotic liver disease. Acad J Guangzhou Med Univ. 53:17–26.
2025.In Chinese.
|
|
13
|
Younossi ZM, Golabi P, Paik JM, Henry A,
Dongen CV and Henry L: The global epidemiology of nonalcoholic
fatty liver disease (NAFLD) and nonalcoholic steatohepatitis
(NASH): A systematic review. Hepatology. 77:1335–1347. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX,
Zhang P, She ZG, Zhu L, Cai J and Li H: Unexpected rapid increase
in the burden of NAFLD in China from 2008 to 2018: A systematic
review and meta-analysis. Hepatology. 70:1119–1133. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benedict M and Zhang X: Non-alcoholic
fatty liver disease: An expanded review. World J Gastroenterol.
9:715–732. 2017.
|
|
16
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-Meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016. View Article : Google Scholar
|
|
17
|
Fang YL, Chen H, Wang CL and Liang L:
Pathogenesis of non-alcoholic fatty liver disease in children and
adolescence: from 'two hit theory' to 'multiple hit model'. World J
Gastroenterol. 24:2974–2983. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X and Gao H: Research progress of
non-alcoholic fatty liver disease. J Hubei Univ Sci Technol (Med
Sci). 33:364–368. 2019.In Chinese.
|
|
19
|
Tilg H and Moschen AR: Evolution of
inflammation in nonalcoholic fatty liver disease: The multiple
parallel hits hypothesis. Hepatology. 52:1836–1846. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buzzetti E, Pinzani M and Tsochatzis EA:
The multiple-hit pathogenesis of non-alcoholic fatty liver disease
(NAFLD). Metabolism. 65:1038–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mariana MV and Cortez-Pinto H:
Non-alcoholic fatty liver disease: What the clinician needs to
know. World J Gastroenterol. 20:12956–12980. 2014. View Article : Google Scholar
|
|
22
|
Fang C, Cai X, Hayashi S, Hao S, Sakiyama
H, Wang X, Yang Q, Akira S, Nishiguchi S, Fujiwara N, et al:
Caffeine-stimulated muscle IL-6 mediates alleviation of
non-alcoholic fatty liver disease. Biochim Biophys Acta Mol Cell
Biol Lipids. 1864:271–280. 2019. View Article : Google Scholar
|
|
23
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: Activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thanapirom K and Tsochatzis EA:
Non-alcoholic fatty liver disease (NAFLD) and the quest for
effective treatments. Hepatobiliary Surg Nutr. 8:77–79. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilkinson AL, Qurashi M and Shetty S: The
role of sinusoidal endothelial cells in the axis of inflammation
and cancer within the liver. Front Physiol. 11:9902020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furuta K, Guo Q, Hirsova P and Ibrahim SH:
Emerging roles of liver sinusoidal endothelial cells in
nonalcoholic steatohepatitis. Biology (Basel). 9:3952020.PubMed/NCBI
|
|
27
|
Nasiri-Ansari N, Androutsakos T, Flessa
CM, Kyrou I, Siasos G, Randeva HS, Kassi E and Papavassiliou AG:
Endothelial cell dysfunction and nonalcoholic fatty liver disease
(NAFLD): A concise review. Cells. 11:25112022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen G, Cai X and Lu L: Role of hepatic
sinusoidal endothelial cells in nonalcoholic steatohepatitis. Chin
Hepatol. 29:998–1002. 2024.In Chinese.
|
|
29
|
Hammoutene A and Rautou PE: Role of liver
sinusoidal endothelial cells in non-alcoholic fatty liver disease.
Hepatology. 70:1278–1291. 2019. View Article : Google Scholar
|
|
30
|
Liu J, Ren CQ, Feng YL and He JR:
Relationship between liver fibrosis and insulin resistance in
patients with type 2 diabetes and nonalcoholic fatty liver disease.
Chin J Clin Res. 34:443–448. 2021.In Chinese.
|
|
31
|
Wang Y, Xu D, Xu L and Wu Y: Peripheral
blood TLR4, NF-κB mRNA in patients with type 2 diabetes mellitus
complicated with non-alcoholic fatty liver disease and their
correlations with insulin resistance. Lab Med Clin. 19:925–929.
2022.In Chinese.
|
|
32
|
Dashti N, Williams DL and Alaupovic P:
Effects of oleate and insulin on the production rates and cellular
mRNA concentrations of apolipoproteins in HepG2 cells. J Lipid Res.
30:1365–1373. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Ni X and Hua J: Effect of hepatocyte
fatty degeneration induced by free fatty acid on macrophage
polarization. J Clin Hepatol. 37:385–389. 2021.In Chinese.
|
|
34
|
Zhang HB and Wang Y: Research progress of
non-alcoholic fatty liver disease. Hainan Med J. 28:1651–1653.
2017.In Chinese.
|
|
35
|
Ye Y and Yu J: Adiponectin level, its gene
polymorphism and nonalcoholic fatty liver disease. Basic Clin Med.
34:1285–1288. 2014.In Chinese.
|
|
36
|
Zou WS, Chen J and Zhang H: Expression and
significance of osteopontin, leptin, visfatin and adiponectin in
bile and gall-bladder mucosa. China J Mod Med. 29:30–35. 2019.In
Chinese.
|
|
37
|
Shah SV and Fonseca VA: Iron and diabetes
revisited. Diabetes Care. 34:1676–1677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhan X: Research on the relationship
between the iron metabolism parameters level and TCM syndrome types
in nonalcoholic fatty liver disease. Fujian Univ Tradit Chin Med.
2020.In Chinese.
|
|
39
|
Nelson JE, Wilson L, Brunt EM, Yeh MM,
Kleiner DE, Unalp-Arida A and Kowdley KV; Nonalcoholic
Steatohepatitis Clinical Research Network: Relationship between the
pattern of hepatic iron deposition and histological severity in
nonalcoholic fatty liver disease. Hepatology. 53:448–457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baumeister SE, Völzke H, Marschall P, John
U, Schmidt CO, Flessa S and Alte D: Impact of fatty liver disease
on health care utilization and costs in a general population: A
5-year observation. Gastroenterology. 134:85–94. 2008. View Article : Google Scholar
|
|
41
|
Pagano C, Soardo G, Esposito W, Fallo F,
Basan L, Donnini D, Federspil G, Sechi LA and Vettor R: Plasma
adiponectin is decreased in nonalcoholic fatty liver disease. Eur J
Endocrinol. 152:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cohen ED and Fisher Edward A: Lipoprotein
metabolism, dyslipidemia, and nonalcoholic fatty liver disease.
Semin Liver Dis. 33:380–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang YY, Liang FX, Lu W, Song YJ, Ren JF,
Zhou YD, Huang XX and Yang SR: Effects of electroacupuncture on
insulin sensitivity and ediponectin/resistin gene expression in
adipose tissue of obese insulin resistant rats. J Basic Chin Med.
27:1444–1450. 2021.In Chinese.
|
|
44
|
Xu MX, Wang M and Yang WW: Gold-quercetin
nanoparticles prevent metabolic endotoxemia-induced kidney injury
by regulating TLR4/NF-kappaB signaling and Nrf2 pathway in high fat
diet fed mice. Int J Nanomedicine. 12:327–345. 2017. View Article : Google Scholar :
|
|
45
|
Mu H, Zhang Z, Liang C and Liu N: Study on
the effect and its mechanism of carvedilol on leptin-induced
activation and proliferation of human hepatic stellate cells. China
Pharm. 28:2620–2624. 2017.In Chinese.
|
|
46
|
Begriche K, Massart J, Robin MA, Bonnet F
and Fromenty B: Mitochondrial adaptations and dysfunctions in
nonalcoholic fatty liver disease. Hepatology. 58:1497–1507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scott FL, Stec B, Pop C, Dobaczewska MK,
Lee JJ, Monosov E, Robinson H, Salvesen GS, Schwarzenbacher R and
Riedl SJ: The Fas-FADD death domain complex structure unravels
signalling by receptor clustering. Nature. 457:1019–1022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arroyave-Ospina JC, Wu Z, Geng Y and
Moshage H: Role of oxidative stress in the pathogenesis of
non-alcoholic fatty liver disease: Implications for prevention and
therapy. Antioxidants (Basel). 10:1742021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dong S, Liu P and Sun MY: Role of
'two-hit' in non-alcoholic fatty liver disease. J Clin Hepatol.
28:551–555. 2012.In Chinese.
|
|
50
|
Liu Y: Oxidative stress and hepatic
protection. Chin J Gastroenterol Hepatol. 20:594–597. 2011.In
Chinese.
|
|
51
|
Lamkanfi M, Sarkar A, Vande Walle L,
Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD and Dixit
VM: Inflammasome-dependent release of the alarmin HMGB1 in
endotoxemia. Immunology. 185:4385–4392. 2010.
|
|
52
|
Mridha AR, Haczeyni F, Yeh MM, Haigh WG,
Ioannou GN, Barn V, Ajamieh H, Adams L, Hamdorf JM, Teoh NC and
Farrell GC: TLR9 is up-regulated in human and murine NASH: Pivotal
role in inflammatory recruitment and cell survival. Clin Sci
(Lond). 131:2145–2159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sharifnia T, Antoun J, Verriere TG, Suarez
G, Wattacheril J, Wilson KT, Peek RM Jr, Abumrad NN and Flynn CR:
Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest
Liver Physiol. 309:G270–G278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rocha DM, Caldas AP, Oliveira LL, Bressan
J and Hermsdorff HH: Saturated fatty acids trigger TLR4-mediated
inflammatory response. Atherosclerosis. 244:211–215. 2016.
View Article : Google Scholar
|
|
55
|
Glass CK and Olefsky JM: Inflammation and
lipid signaling in the etiology of insulin resistance. Cell Metab.
15:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Seimon TA, Nadolski MJ, Liao XH, Magallon
J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL,
Tsimikas S, et al: Atherogenic lipids and lipo proteins trigger
CD36-TLR2-dependent apoptosis in macrophages un dergoing
endoplasmic reticulum stress. Cell Metab. 12:467–482. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Miura K and Ohnishi H: Role of gut
microbiota and toll-like receptors in nonalcoholic fatty liver
disease. World J Gastroenterol. 20:7381–7391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ashraf NU and Sheikh TA: Endoplasmic
reticulum stress and oxidative stress in the pathogenesis of
non-alcoholic fatty liver disease. Free Radic Res. 49:1405–1418.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Amir M and Czaja MJ: Autophagy in
nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol.
5:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gäbele E, Dostert K, Hofmann C, Wiest R,
Schölmerich J, Hellerbrand C and Obermeier F: DSS induced colitis
increases portal LPS levels and enhances hepatic inflammation and
fibrogenesis in experimental NASH. Hepatology. 55:1391–1399. 2011.
View Article : Google Scholar
|
|
61
|
Simonen P, Kotronen A, Hallikainen M,
Sevastianova K, Makkonen J, Hakkarainen A, Lundbom N, Miettinen TA,
Gylling H and Yki-Järvinen H: Cholesterol synthesis is increased
and absorption decreased in non-alcoholic fatty liver disease
independent of obesity. Hepatology. 54:153–159. 2011. View Article : Google Scholar
|
|
62
|
Enjoji M, Yasutake K, Kohjima M and
Nakamuta M: Nutrition and nonalcoholic fatty liver disease: The
significance of cholesterol. Int J Hepatol. 2012:9258072012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Subramanian S, Goodspeed L, Wang S, Kim J,
Zeng LX, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O'Brien KD, et
al: Dietary cholesterol exacerbates hepatic steatosis and
inflammation in obese LDL receptor deficient mice. J Lipid Res.
52:1626–1635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ersoy BA, Maner-Smith KM, Li Y, Alpertunga
I and Cohen DE: Thioesterase-mediated control of cellular calcium
homeostasis enables hepatic ER stress. J Clin Invest. 128:141–156.
2018. View Article : Google Scholar :
|
|
65
|
Ghemrawi R, Battaglia-Hsu F and Arnold C:
Endoplasmic reticulum stress in metabolic disorders. Cells.
7:632018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Henkel AS: Unfolded protein response
sensors in hepatic lipid metabolism and nonalcoholic fatty liver
disease. Semin Liver Dis. 38:320–332. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
DeZwaan-McCabe D, Sheldon RD, Gorecki MC,
Guo DF, Gansemer ER, Kaufman RJ, Rahmouni K, Gillum MP, Taylor EB,
Teesch LM and Rutkowski DT: ER Stress inhibits liver fatty acid
oxidation while unmitigated stress leads to anorexia-induced
lipolysis and both liver and kidney steatosis. Cell Rep.
19:1794–1806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Maillo C, Martín J, Sebastián D,
Hernández-Alvarez M, García-Rocha M, Reina O, Zorzano A, Fernandez
M and Méndez R: Circadian- and UPR-dependent control of CPEB4
mediates a translational response to counteract hepatic steatosis
under ER stress. Nat Cell Biol. 19:94–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Y, Huang B, Jiang X, Chen W, Zhang J,
Wei Y, Chen Y, Lian M, Bian Z, Miao Q, et al: Mucosal-associated
invariant T cells improve nonalcoholic fatty liver disease through
regulating macrophage polarization. Front Immunol. 9:19942018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ma J, Zhou Q and Li H: Gut Microbiota and
nonalcoholic fatty liver disease: Insights on mechanisms and
therapy. Nutrients. 9:11242017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Harte AL, da Silva NF, Creely SJ, McGee
KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS,
Sharada HM, et al: Elevated endotoxin levels in non-alcoholic fatty
liver disease. J Inflammation (Lond). 7:152010. View Article : Google Scholar
|
|
73
|
Ley RE, Turnbaugh PJ, Klein S and Gordon
JI: Microbial ecology: Human gut microbes associated with obesity.
Nature. 444:1022–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Janssen AWF, Houben T, Katiraei S, Dijk W,
Boutens L, van der Bolt N, Wang Z, Brown JM, Hazen SL, Mandard S,
et al: Modulation of the gut microbiota impacts nonalcoholic fatty
liver disease: A potential role for bile acids. J Lipid Res.
58:1399–1416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Le Roy T, Llopis M, Lepage P, Bruneau A,
Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et
al: Intestinal microbiota determines development of non-alcoholic
fatty liver disease in mice. Gut. 62:1787–1794. 2013. View Article : Google Scholar
|
|
76
|
Hu H, Lin A, Kong M, Yao X, Yin M, Xia H,
Ma J and Liu H: Intestinal microbiome and NAFLD: Molecular insights
and therapeutic perspectives. World J Gastroenterol. 20:142–158.
2020. View Article : Google Scholar
|
|
77
|
Rennert C, Heil T, Schicht G, Stilkerich
A, Seidemann L, Kegel-Hübner V, Seehofer D and Damm G: Prolonged
lipid accumulation in cultured primary human hepatocytes rather
leads to ER stress than oxidative stress. Int J Mol Sci.
21:70972020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wesolowski SR, Kasmi KC, Jonscher KR and
Friedman JE: Developmental origins of NAFLD: A womb with a clue.
Nat Rev Gastroenterol Hepatol. 14:81–96. 2017. View Article : Google Scholar :
|
|
79
|
Arab JP, Karpen SJ, Dawson PA, Arrese M
and Trauner M: Bile acids and nonalcoholic fatty liver disease:
Molecular insights and therapeutic perspectives. Hepatology.
65:350–362. 2017. View Article : Google Scholar
|
|
80
|
Jiang C, Xie C, Li F, Zhang L, Nichols RG,
Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, et al: Intestinal
farnesoid X receptor signaling promotes nonalcoholic fatty liver
disease. J Clin Invest. 125:386–402. 2015. View Article : Google Scholar :
|
|
81
|
Thomas C, Gioiello A, Noriega L, Strehle
A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski
M, et al: TGR5 mediated bile acid sensing controls glucose
homeostasis. Cell Metab. 10:167–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhou T, Zheng Y, Liang H and Tang J:
Progress of bile acid metabolism in chronic liver disease. J Integr
Tradit Chin West Med Hepatol. 30:173–175. 2020.In Chinese.
|
|
83
|
Watanabe M, Houten SM, Mataki C,
Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O,
Kodama T, et al: Bile acids induce energy expenditure by promoting
intracellular thyroid hormone activation. Nature. 439:484–489.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang F, Wang YY, Zhang D, Zhao XR and Liu
K: Research on curative effects of regulating bile acid metabolism
and intestinal flora in treatment of nonalcoholic fatty liver
disease. China Med Pharm. 9:36–40. 452019.In Chinese.
|
|
85
|
Jiang LY and Xiao XH: Research progress on
correlation between gut microbiota and non-alcoholic fatty liver
disease. J Clin Pathol Res. 36:2060–2065. 2016.
|
|
86
|
Carr RM and Reid AE: FXR agonists as
therapeutic agents for non-alcoholic fatty liver disease. Curr
Atheroscler Rep. 17:5002015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu K, Xu SY, Xu B, Chen D and Li N: The
protective role of early autophagy in nonalcoholic fatty liver.
Beijing Med J. 33:947–950. 2011.In Chinese.
|
|
88
|
Wang XL, Xiong JR, Zhao QM, Jiang W and Li
P: The dual role of autophagy in disease. Prog Mod Biomed.
14:4997–5000. 2014.In Chinese.
|
|
89
|
Yang L, Li P, Fu S, Calay ES and
Hotamisligil GS: Defective hepatic autophagy in obesity promotes ER
stress and causes insulin resistance. Cell Metab. 11:467–478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ma DW, Ha J, Yoon KS, Kang I, Choi TG and
Kim SS: Innate immune system in the pathogenesis of non-alcoholic
fatty liver disease. Nutrients. 15:20682023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kazankov K, Jørgensen SMD, Thomsen KL,
Møller HJ, Vilstrup H, George J, Schuppan D and Grønbæk H: The role
of macrophages in nonalcoholic fatty liver disease and nonalcoholic
steatohepatitis. Nat Rev Gastroenterol Hepatol. 16:145–159. 2019.
View Article : Google Scholar
|
|
92
|
Tilg H, Effenberger M and Adolph TE: A
role for il-1 inhibitors in the treatment of non-alcoholic fatty
liver disease (NAFLD)? Expert Opin Investig Drugs. 29:1032020.
View Article : Google Scholar
|
|
93
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: Remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Garcia-Ruiz C, Mato JM, Vance D, Kaplowitz
N and Fernández-Checa JC: Acid sphingomyelinase-ceramide system in
steatohepatitis: A novel target regulating multiple pathways. J
Hepatol. 62:2192015. View Article : Google Scholar
|
|
95
|
Fantuzzi G: Adipose tissue, adipokines,
and inflammation. J Allergy Clin Immunol. 115:911–919; quiz 920.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tong Q, Wang XL, Li SB, Yang GL, Jin S,
Gao ZY and Liu XB: Combined detection of il-6 and il-8 is
beneficial to the diagnosis of early-stage esophageal squamous cell
cancer: A preliminary study based on the screening of serum markers
using protein chips. OncoTargets Ther. 11:5777–5787. 2018.
View Article : Google Scholar
|
|
97
|
Tikhomirova AS, Kislyakov VA, Baykova IE
and Nikitin IG: Clinical-morphological parallels of the PNPLA3 gene
polymorphism in patients with nonalcoholic fatty liver disease. Ter
Arkh. 90:85–88. 2018.
|
|
98
|
Dai G, Liu P, Li X, Zhou X and He S:
Association between PNPLA3 rs738409 polymorphism and nonalcoholic
fatty liver disease (NAFLD) susceptibility and severity: a
meta-analysis. Medicine (Baltimore). 98:e143242019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Dongiovanni P, Petta S, Maglio C,
Fracanzani AL, Pipitone R, Mozzi E, Motta BM, Kaminska D, Rametta
R, Grimaudo S, et al: Transmembrane 6 superfamily member 2 gene
variant disentangles nonalcoholic steatohepatitis from
cardiovascular disease. Hepatology. 61:506–514. 2015. View Article : Google Scholar
|
|
100
|
Wu S, Zheng B, Liu T, Zhu Z, Gu W and Liu
Q: 17beta-hydroxysteroid dehydrogenase 3 deficiency due to novel
compound heterozygous variants of HSD17B3 gene in a sib pair.
Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 38:787–790. 2021.In Chinese.
PubMed/NCBI
|
|
101
|
Dai LL, Li SD, Ma YC, Tang JR, Lv JY,
Zhang YQ, Miao YL, Ma YQ, Li CM, Chu YY, et al: MicroRNA-30b
regulates insulin sensitivity by targeting SERCA2b in non-alcoholic
fatty liver disease. Liver Int. 39:1504–1513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yu J, Peng J, Luan Z, Zheng F and Su W:
MicroRNAs as a novel tool in the diagnosis of liver lipid
dysregulation and fatty liver disease. Molecules. 24:2302019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Dongiovanni P, Meroni M, Longo M, Fargion
S and Fracanzani AL: miRNA signature in NAFLD: A turning point for
a non-invasive diagnosis. Int J Mol Sci. 19:39662018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang M, Sun W, Zhou M and Tang Y:
MicroRNA-27a regulates hepatic lipid metabolism and alleviates
NAFLD via repressing FAS and SCD1. Sci Rep. 7:144932017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Szabo G and Csak T: Role of microRNAs in
NAFLD/NASH. Dig Dis Sci. 61:1314–1324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
López-Riera M, Conde I, Quintas G, Pedrola
L, Zaragoza A, Perez-Rojas J, Salcedo M, Benlloch S, Castell JV and
Jover R: Non-invasive prediction of NAFLD severity: A
comprehensive, independent validation of previously postulated
serum microRNA biomarkers. Sci Rep. 8:106062018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Guo Y, Xiong Y, Sheng Q, Zhao S,
Wattacheril J and Flynn CR: A micro-RNA expression signature for
human NAFLD progression. J Gastroenterol. 51:1022–1030. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Luo ZL, Tang LJ, Wang T, Dai RW, Ren JD,
Cheng L, Xiang K and Tian FZ: Effects of treatment with hydrogen
sulfide on methionine-choline deficient diet-induced non-alcoholic
steatohepatitis in rats. J Gastroenterol Hepatol. 29:215–222. 2014.
View Article : Google Scholar
|
|
109
|
Wu D, Zheng N, Qi K, Cheng H, Sun Z, Gao
B, Zhang Y, Pang W, Huangfu C, Ji S, et al: Exogenous hydrogen
sulfide mitigates the fatty liver in obese mice through improving
lipid metabolism and antioxidant potential. Med Gas Res. 5:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Salehi-Sahlabadi A, Sadat S, Beigrezaei S,
Pourmasomi M, Feizi A, Ghiasvand R, Hadi A, Clark CCT and
Miraghajani M: Dietary patterns and risk of non-alcoholic fatty
liver disease. BMC Gastroenterol. 21:412021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu H and Ye H: Research progress on the
relationship between sleep, circadian rhythm, intestinal flora and
non-alcoholic fatty liver disease. Mod Pract Med. 31:565–568.
2019.In Chinese.
|
|
112
|
Wei YT, Lee PY, Lin CY, Chen HJ, Lin CC,
Wu JS, Chang YF, Wu CL and Guo HR: Non-alcoholic fatty liver
disease among patients with sleep disorders: A nationwide study of
Taiwan. BMC Gastroenterol. 20:322020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Marin-Alejandre BA, Abete I, Cantero I,
Riezu-Boj JI, Milagro FI, Monreal JI, Elorz M, Herrero JI,
Benito-Boillos A, Quiroga J, et al: Association between sleep
disturbances and liver status in obese subjects with nonalcoholic
fatty liver disease: A comparison with healthy controls. Nutrients.
11:3222019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ai Y, Yang X, Pan X and Ye J: Role of
circadian clock genes in the development and progression of
nonalcoholic fatty liver disease. J Clin Hepatol. 35:2327–2330.
2019.In Chinese.
|
|
115
|
Reinke H and Asher G: Circadian clock
control of liver metabolic functions. Gastroenterology.
150:573–580. 2016. View Article : Google Scholar
|
|
116
|
Wen Y: Relationship between social mental
factors and non-alcoholic fatty liver disease: A case-control
study. China J Health Psychol. 26:565–568. 2018.In Chinese.
|
|
117
|
Cho IY, Chang Y, Sung E, Kang JH, Wild SH,
Byrne CD, Shin H and Ryu S: Depression and increased risk of
non-alcoholic fatty liver disease in individuals with obesity.
Epidemiol Psychiatr Sci. 30:e232021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Youssef NA, Abdelmalek MF, Binks M, Guy
CD, Omenetti A, Smith AD, Diehl AM and Suzuki A: Associations of
depression, anxiety and antidepressants with histological severity
of nonalcoholic fatty liver disease. Liver Int. 33:1062–1070. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Peluso I, Manafikhi H, Reggi R and Palmery
M: Effects of red wine on postprandial stress: Potential
implication in non-alcoholic fatty liver disease development. Eur J
Clin Nutr. 54:497–507. 2015. View Article : Google Scholar
|
|
120
|
Yu D, Shu XO, Xiang YB, Li H, Yang G, Gao
YT, Zheng W and Zhang X: Higher dietary choline intake is
associated with lower risk of nonalcoholic fatty liver in
normal-weight Chinese women. J Nutr. 144:2034–2040. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kado A, Inoue Y, Moriya M, Tsutsumi K,
Ikeuchi T, Okushin K, Yotsuyanagi K, Koike H and Fujishiro M:
Triglyceride level and soft drink consumption predict nonalcoholic
fatty liver disease in nonobese male adolescents. Hepatol Res.
53:497–510. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Khazaei Y, Dehghanseresht N, Ebrahimi
Mousavi S, Nazari M, Salamat S, Asbaghi O and Mansoori A:
Association between protein intake from different animal and plant
origins and the risk of non-alcoholic fatty liver disease: A
case-control study. Clin Nutr Res. 12:29–39. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lanaspa MA, Kuwabara M, Andres-Hernando A,
Li N, Cicerchi C, Jensen T, Orlicky DJ, Roncal-Jimenez CA, Ishimoto
T, Nakagawa T, et al: High salt intake causes leptin resistance and
obesity in mice by stimulating endogenous fructose production and
metabolism. Proc Natl Acad Sci USA. 115:3138–3143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Liang YF, Kuang HY, Qin W, Zhu WB, Liu NJ,
Zuo J and Han S: Correlation between salt intake and non-alcoholic
fatty liver disease in type 2 diabetes mellitus patients in
northern China. Chin J Mult Organ Dis Elderly. 18:765–768. 2019.In
Chinese.
|
|
125
|
Chen S, Teoh NC, Chitturi S and Farrell
GC: Coffee and non-alcoholic fatty liver disease: Brewing evidence
for hepatoprotection? J Gastroenterol Hepatol. 29:435–441. 2014.
View Article : Google Scholar
|
|
126
|
Sinha RA, Farah BL, Singh BK, Siddique MM,
Li Y, Wu YJ, Ilkayeva OR, Gooding J, Ching J, Zhou J, et al:
Caffeine stimulates hepatic lipid metabolism by the
autophagy-lysosomal pathway in mice. Hepatology. 59:1366–1380.
2014. View Article : Google Scholar
|
|
127
|
Chen B, Sun L, Zeng G, Shen Z, Wang K, Yin
L, Xu F, Wang P, Ding Y, Nie Q, et al: Gut bacteria alleviate
smoking-related NASH by degrading gut nicotine. Nature.
610:562–568. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jung HS, Chang Y, Kwon MJ, Sung E, Yun KE,
Cho YK, Shin H and Ryu S: Smoking and the risk of non-alcoholic
fatty liver disease: A cohort study. Am J Gastroenterol.
114:453–463. 2019. View Article : Google Scholar
|
|
129
|
Ma H, Zhao J and Xu J: The correlation
between heavy metal ions in blood and metabolic
dysfunction-associated steatotic liver disease from 1999 to 2018
based on NHANES data. Front Public Health. 12:15129012025.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Salman M, Kamel MA, El-Nabi SEH, Ismail
AHA, Ullah S, Al-Ghamdi A, Hathout HMR and El-Garawani IM: The
regulation of HBP1, SIRT1, and SREBP-1c genes and the related
microRNAs in non-alcoholic fatty liver rats: The association with
the folic acid anti-steatosis. PLoS One. 17:e02654552022.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhou Q, Xu W, Wang L and Duan Y:
Polychlorinated biphenyl 118 induced nonalcoholic fatty liver
disease in rats. J Nanjing Med Univ (Nat Sci). 39:659–663. 2019.In
Chinese.
|
|
132
|
Stephen Robert JM, Peddha MS and
Srivastava AK: Effect of silymarin and quercetin in a miniaturized
scaffold in wistar rats against non-alcoholic fatty liver disease.
ACS Omega. 6:20735–20745. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhang M, Yuan Y, Wang Q, Li X, Men J and
Lin M: The Chinese medicine Chai Hu Li Zhong Tang protects against
non-alcoholic fatty liver disease by activating AMPKα. Biosci Rep.
38:BSR201806442018. View Article : Google Scholar
|
|
134
|
Lin M, Deng G, Ou Y, Chen Z, Huang J, Kang
M and Li J: Establishment of non-alcoholic fatty liver disease
model in young rats induced by high-fat diet. J Med Theor Pract.
32:2321–2323. 23262019.In Chinese.
|
|
135
|
Jin Y, Xing W, Lyu A, Zhang J, Yang W, Xu
Y, Wang Q and Li J: Comparison of CYP enzyme activities in rat
models of nonalcoholic fatty liver established by different
methods. Chin J Comp Med. 28:75–83. 2018.In Chinese.
|
|
136
|
Chen XQ, Xu LS, Li DF and Deng WP: Effects
of glucagon-like peptide-1 analog on CDAA diet-induced models of
nonalcoholic fatty liver fibrosis in rats. J Evid-Based Med.
17:163–170. 2017.In Chinese.
|
|
137
|
Yan D, Li SY and Wei YY: Study on
establishment of animal model with diabetes complicated fatty liver
induced by streptozocin and nicotinamide. Chin J Clin Pharmacol.
31:1846–1848. 2015.In Chinese.
|
|
138
|
Li X and Li Y: Discussion on nonalcoholic
fatty liver disease animal model. Chin Community Doctors. 33:5–8.
2017.In Chinese.
|
|
139
|
Yong HY, Larrouy-Maumus G, Zloh M, Smyth
R, Ataya R, Benton CM and Munday MR: Early detection of metabolic
changes in drug-induced steatosis using metabolomics approache. RSC
Adv. 10:41047–41057. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Che X and Han J: Effect of FGF21 on
TLR4/p38MAPK signaling pathway in nonalcoholic fatty liver diseases
of rats. J Med Res. 47:130–135. 2018.In Chinese.
|
|
141
|
Hakkak R, Spray B, Børsheim E and
Korourian S: Diet containing soy protein concentrate with low and
high isoflavones for 9 weeks protects against non-alcoholic fatty
liver steatosis using obese zucker rats. Front Nutr. 9:9135712022.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Matsumoto Y, Fujita S, Yamagishi A, Shirai
T, Maeda Y, Suzuki T, Kobayashi KI, Inoue J and Yamamoto Y: Brown
rice inhibits development of nonalcoholic fatty liver disease in
obese zucker (fa/fa) rats by increasing lipid oxidation via
activation of retinoic acid synthesis. J Nutr. 151:2705–2713. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xia QS, Gao Y, Wen-Bin W, Wu F, Dong H, Xu
LJ, Fang K, Hu ML, Yuan F, Lu FE and Gong J: Ban-xia-xie-xin-tang
ameliorates hepatic steatosis by regulating cidea and cidec
expression in HFD-fed mice. Phytomedicine. 105:1543512022.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Shi Y, Laba PC, Zhang DY, Weng SQ, Liu XY
and Wang HQ: Effects of bifidobacteria on high-fat diet-induced
non-alcoholic fatty liver disease in C57BL/6 mice. Chin J Clin Med.
29:473–480. 2022.In Chinese.
|
|
145
|
Yu H, Yi X, Gao X, Ji J, Liu Z, Xia G, Li
C, Zhang X and Shen X: Tilapia-head chondroitin sulfate protects
against nonalcoholic fatty liver disease via modulating the
gut-liver axis in High-Fat-Diet-Fed C57BL/6 mice. Foods.
11:9222022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
De Minicis S, Agostinelli L, Rychlicki C,
Sorice GP, Saccomanno S, Candelaresi C, Giaccari A, Trozzi L,
Pierantonelli I, Mingarelli E, et al: HCC development is associated
to peripheral insulin resistance in a mouse model of NASH. PLoS
One. 9:e00971362014. View Article : Google Scholar
|
|
147
|
Li XC, Zhang XY, Xu S, Li H and Zhang YH:
Establishment of a non-alcoholic fatty liver model in C57BL/6J
mice. In: Proceedings of the Symposium on Veterinary Toxicology and
Feed Toxicology and the 4th National Congress of the Veterinary
Toxicology Committee of the Chinese Society of Toxicology; Beijing.
pp. 94–95. 2013, In Chinese.
|
|
148
|
Feng Z, Pang L, Chen S, Wei J, Huang Q,
Wang Y and Lin X: The protective effect of Melissa officinalis on
dexamethasone-induced nonalcoholic fatty liver in C57BL/6J mice. J
Chin Med Mater. 44:961–965. 2021.In Chinese.
|
|
149
|
Asgharpour A, Cazanave SC, Pacana T,
Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK,
Mirshahi F, et al: A diet-induced animal model of non-alcoholic
fatty liver disease and hepatocellular cancer. J Hepatol.
65:579–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ma H, Zhu G, Zhang X, Yang R, Xie Y, Liu X
and Wang B: An establishment method of a mouse model of
non-alcoholic fatty liver disease. J Anhui Agric Sci. 49:80–84.
2021.In Chinese.
|
|
151
|
Jahn D, Dorbath D, Kircher S, Nier A,
Bergheim I, Lenaerts K, Hermanns HM and Geier A: Beneficial effects
of vitamin d treatment in an obese mouse model of non-alcoholic
steatohepatitis. Nutrients. 11:772019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang JJ, Fang HL, Li CW, Chen FC, Tang WJ,
Jiang XY, Wu HY and Chen DL: Establishment of non-alcoholic fatty
liver model in mice. J Clin Rehabilit Tissue Eng Res. 15:4395–4399.
2011.In Chinese.
|
|
153
|
Liu T, Tan F, Long X, Pan Y, Mu J, Zhou X,
Yi R and Zhao X: Improvement effect of lotus leaf flavonoids on
carbon tetrachloride-induced liver injury in mice. Biomedicines.
8:412020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Malloy VL, Perrone CE, Mattocks DA, Ables
GP, Caliendo NS, Orentreich DS and Orentreich N: Methionine
restriction prevents the progression of hepatic steatosis in
leptin-deficient obese mice. Metabolism. 62:1651–1661. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Son YJ, Jung DS, Shin JM, Kim M, Yoo G and
Nho CW: Yellow loosestrife (lysimachia vulgaris var. davurica)
ameliorates liver fibrosis in db/db mice with methionine and
choline-deficient diet-induced nonalcoholic steatohepatitis. BMC
Complement Med and Ther. 21:442021. View Article : Google Scholar
|
|
156
|
Yu W, Zhang J, Liu Y, Liu F, Shao H and
Han G: Improvement effect of mussel polysaccharide α-d-glucan on
non-alcoholic fatty liver apolipoprotein E knockout mice induced by
high fat diet. Sci Technol Food Ind. 43:369–376. 2022.In
Chinese.
|
|
157
|
Song L, Tang SQ, Tong JW, Cai P, Liu JX,
Ren XJ and Huang W: Study on the mechanism of Xuefu Zhuyu decoction
in preventing and treating NAFLD. Chin J Integr Tradit West Med.
40:1103–1106. 2020.In Chinese.
|
|
158
|
Yang P, Wang Y, Tang W, Sun W, Ma Y, Lin
S, Jing J, Jiang L, Shi H, Song Z and Yu L: Western diet induces
severe nonalcoholic steatohepatitis, ductular reaction, and hepatic
fibrosis in liver CGI-58 knockout mice. Sci Rep. 10:47012020.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Piguet AC, Saran U, Simillion C, Keller I,
Terracciano L, Reeves HL and Dufour JF: Regular exercise decreases
liver tumors development in hepatocyte-specific PTEN-deficient mice
independently of steatosis. J Hepatol. 62:1296–1303. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Semba T, Nishimura M, Nishimura S, Ohara
O, Ishige T, Ohno S, Nonaka K, Sogawa K, Satoh M, Sawai S, et al:
The FLS (fatty liver Shionogi) mouse reveals local expressions of
lipocalin-2, CXCL1 and CXCL9 in the liver with non-alcoholic
steatohepatitis. BMC Gastroenterol. 13:1202013. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Li JZ, Huang Y, Karaman R, Ivanova PT,
Brown HA, Roddy T, Castro-Perez J, Cohen JC and Hobbs HH: Chronic
overexpression of PNPLA3I148M in mouse liver causes hepatic
steatosis. J Clin Invest. 122:4130–4144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yin HQ, Kim M, Kim JH, Kong G, Lee MO,
Kang KS, Yoon BI, Kim HL and Lee BH: Hepatic gene expression
profiling and lipid homeostasis in mice exposed to steatogenic
drug, tetracycline. Toxicol Sci. 94:206–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Li Z, Jin H, Oh SY and Ji GE: Anti-obese
effects of two lactobacilli and two bifidobacteria on ICR mice fed
on a high fat diet. Biochem Biophys Res Commun. 480:222–227. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Yu Z, He Y, Luo W and Li L: Establishment
of AKT gene-mediated non-alcoholic fatty liver models in mice. Acta
Med Univ Sci Technol Huazhong. 45:170–175. 2016.In Chinese.
|
|
165
|
López-Lemus UA, Garza-Guajardo R,
Barboza-Quintana O, Rodríguez-Hernandez A, García-Rivera A,
Madrigal-Pérez VM, Guzmán-Esquivel J, García-Labastida LE,
Soriano-Hernández AD, Martínez-Fierro ML, et al: Association
between nonalcoholic fatty liver disease and severe male
reproductive organ impairment (germinal epithelial loss): study on
a mouse model and on human patients. Am J Mens Health. 12:639–648.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Musolino V, Gliozzi M, Scarano F, Bosco F,
Scicchitano M, Nucera S, Carresi C, Ruga S, Zito MC, Maiuolo J, et
al: Bergamot polyphenols improve dyslipidemia and
pathophysiological features in a mouse model of non-alcoholic fatty
liver disease. Sci Rep. 10:25652020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Chow JD, Jones ME, Prelle K, Simpson ER
and Boon WC: A selective estrogen receptor α agonist ameliorates
hepatic steatosis in the male aromatase knockout mouse. J
Endocrinol. 210:323–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Komatsu G, Nonomura T, Sasaki M, Ishida Y,
Arai S and Miyazaki T: AIM-deficient mouse fed a high-trans-fat,
high-cholesterol diet: A new animal model for nonalcoholic fatty
liver disease. Exp Anim. 68:147–158. 2019. View Article : Google Scholar
|
|
169
|
Nguyen TTP, Kim DY, Lee YG, Lee YS, Truong
XT, Lee JH, Song DK, Kwon TK, Park SH, Jung CH, et al: SREBP-1c
impairs ULK1 sulfhydration-mediated autophagic flux to promote
hepatic steatosis in high-fat-diet-fed mice. Mol Cell.
81:3820–3832.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Deguise MO, Pileggi C, De Repentigny Y,
Beauvais A, Tierney A, Chehade L, Michaud J, Llavero-Hurtado M,
Lamont D, Atrih A, et al: SMN depleted mice offer a robust and
rapid onset model of nonalcoholic fatty liver disease. Cell Mol
Gastroenterol Hepatol. 12:354–377.e3. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Cano A, Buque X, Martínez-Uña M,
Aurrekoetxea I, Menor A, García-Rodríguez JL, Lu SC,
Martínez-Chantar ML, Mato JM, Ochoa B and Aspichueta P: Methionine
adenosyltransferase 1A gene deletion disrupts hepatic very
low-density lipoprotein assembly in mice. Hepatology. 54:1975–1986.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Heintz MM, Kumar R, Rutledge MM and
Baldwin WS: Cyp2b-nullmale mice are susceptible to diet-induced
obesity and perturbations in lipid homeostasis. J Nutr Biochem.
70:125–137. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Edmunds LR, Xie B, Mills AM, Huckestein
BR, Undamatla R, Murali A, Pangburn MM, Martin J, Sipula I, Kaufman
BA, et al: Liver-specific Prkn knockout mice are more susceptible
to diet-induced hepatic steatosis and insulin resistance. Mol
Metab. 41:1010512020. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yang W, Yan X, Chen R, Xin X, Ge S, Zhao
Y, Yan X and Zhang J: Smad4 deficiency in hepatocytes attenuates
NAFLD progression via inhibition of lipogenesis and macrophage
polarization. Cell Death Dis. 16:582025. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Yang YQ, Feng J, Bai X, Shen ZM, Liu XW,
Gao J, Xie JY, Hu JH and Gao C: Establishment of a Microtus fortis
model of non-alcoholic fatty liver. Acta Lab Anim Sci Sin.
21:34–38. 2013.In Chinese.
|
|
176
|
Yu Z, Wang H, Gao G, Ke L, Zhou J, Rao P
and Yu C: Effects of freshwater clam soup nanoparticles on
non-alcoholic fatty liver disease of Meriones unguieulataus. J Chin
Inst Food Sci Technol. 21:116–120. 2021.In Chinese.
|
|
177
|
Bhathena J, Kulamarva A, Martoni C,
Urbanska AM, Malhotra M, Paul A and Prakash S: Diet-induced
metabolic hamster model of nonalcoholic fatty liver disease.
Diabetes Metab Syndr Obes. 4:195–203. 2011.PubMed/NCBI
|
|
178
|
Cui CX, Deng JN, Li Y, Liu YY, Fan JY, Mu
HN, Sun HY, Wang YH and Han JY: Silibinin capsules improves high
fat diet-induced nonalcoholic fatty liver disease in hamsters
through modifying hepatic de novo lipogenesis and fatty acid
oxidation. J Ethnopharmacol. 208:24–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zhao CZ, Jiang W, Zhu YY, Wang CZ, Zhong
WH, Wu G, Chen J, Zhu MN, Wu QL, Du XL, et al: Highland barley
monascus purpureus went extract ameliorates high-fat,
high-fructose, high-cholesterol diet induced nonalcoholic fatty
liver disease by regulating lipid metabolism in golden hamsters. J
Ethnopharmacol. 286:1149222022. View Article : Google Scholar
|
|
180
|
Skat-Rørdam J, Pedersen K, Skovsted GF,
Gregersen I, Vangsgaard S, Ipsen DH, Latta M, Lykkesfeldt J and
Tveden-Nyborg P: Vitamin C deficiency may delay diet-induced NASH
regression in the guinea pig. Antioxidants (Basel). 11:692021.
View Article : Google Scholar
|
|
181
|
Jin M, Zheng L, Wei Y, Cheng J, Zhang D,
Yan S, Qin H, Wang Q, Ci X and Feng H: Enterobacter cloacae
aggravates metabolic disease by inducing inflammation and lipid
accumulation. Environ Toxicol Pharmacol. 90:1038192022. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Ipsen DH, SkatRørdam J, Tsamouri MM, Latta
M, Lykkesfeldt J and TvedenNyborg P: Molecular drivers of
non-alcoholic steatohepatitis are sustained in mild-to-late
fibrosis progression in a guinea pig model. Mol Genet Genomics.
294:649–661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Pedersen K, Ipsen DH, Skat-Rrdam J,
Lykkesfeldt J and Tveden-Nyborg P: Dietary long-chain fatty acids
accelerate metabolic dysfunction in guinea pigs with non-alcoholic
steatohepatitis. Nutrients. 15:24452023. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Wang Y, Zhang P, Su X, Yu Q, Chen Y, Guan
H, Liu E and Fan J: Establishment of a novel nonalcoholic fatty
liver disease model using cholesterol-fed rabbits with reference to
the potential role of endoplasmic reticulum stress. Mol Med Rep.
18:2898–2904. 2018.PubMed/NCBI
|
|
185
|
Sturzeneker MCS, de Noronha L, Olandoski
M, Wendling LU and Precoma DB: Ramipril significantly attenuates
the development of non-alcoholic steatohepatitis in hyperlipidaemic
rabbits. Am J Cardiovasc Dis. 9:8–17. 2019.PubMed/NCBI
|
|
186
|
Nguyen TN, Podkowa AS, Tam AY, Arnold EC,
Miller RJ, Park TH, Do MN and Oelze ML: Characterizing fatty liver
in vivo in rabbits, using quantitative ultrasound. Ultrasound Med
Biol. 45:2049–2062. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Zheng W, Liu YL, Shang HB, Zhang Y, Ma DW,
Hou N, Wang J, Sun XT, Peng Y, Pan L, et al: Characterization of
spontaneously-developed non-alcoholic fatty liver disease in aged
rhesus monkeys. Diabetol Metab Syndr. 10:682018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Cydylo MA, Davis AT and Kavanagh K: Fatty
liver promotes fibrosis in monkeys consuming high fructose. Obesity
(Silver Spring). 25:290–293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Kramer JA, Grindley J, Crowell AM, Makaron
L, Kohli R, Kirby M, Mansfield KG and Wachtman LM: The common
marmoset as a model for the study of nonalcoholic fatty liver
disease and nonalcoholic steatohepatitis. Vet Pathol. 52:404–413.
2015. View Article : Google Scholar
|
|
190
|
Hamid H, Zhang JY, Li WX, Liu C, Li ML,
Zhao LH, Ji C and Ma QG: Interactions between the cecal microbiota
and non-alcoholic steatohepatitis using laying hens as the model.
Poult Sci. 98:2509–2521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhu L, Liao R, Huang J, Yan H, Xiao C,
Yang Y, Wang H and Yang C: The miR-216/miR-217 Cluster regulates
lipid metabolism in laying hens with fatty liver syndrome via
PPAR/SREBP signaling pathway. Front Vet Sci. 9:9138412022.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Tian WH, Wang Z, Yue YX, Li H, Li ZJ, Han
RL, Tian YD, Kang XT and Liu XJ: Mir-34a-5p increases hepatic
triglycerides and total cholesterol levels by regulating acsl1
protein expression in laying hens. Int J Mol Sci. 20:44202019.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Song J, Shi X, Li X, Liang Q, Zeng L, Li
G, Yan Y, Xu G and Zheng J: Associations of the T329S polymorphism
in flavin-containing monooxygenase 3 with atherosclerosis and fatty
liver syndrome in 90-week-old hens. Front Vet Sci. 9:8686022022.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Delgado ME, Cárdenas BI, Farran N and
Fernandez M: Metabolic reprogramming of liver fibrosis. Cells.
10:36042021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Mazza G, Al-Akkad W and Rombouts K:
Engineering in vitro models of hepatofibrogenesis. Adv Drug Deliv
Rev. 121:147–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Soret PA, Magusto J, Housset C and
Gautheron J: In vitro and in vivo models of non-alcoholic fatty
liver disease: A critical appraisal. J Clin Med. 10:362020.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Zhong Y, Yu JS, Wang X, Binas B and Yoo
HH: Chemical-based primary human hepatocyte monolayer culture for
the study of drug metabolism and hepato-toxicity: comparison with
the spheroid model. FASEB J. 35:e213792021. View Article : Google Scholar
|
|
198
|
Kanasaki A, Iida T, Murao K, Shirouchi B
and Sato M: D-Allulose enhances uptake of HDL-cholesterol into
rat's primary hepatocyte via SR-B1. Cytotechnology. 72:295–301.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Ng IC, Zhang L, Shen NNYY, Soong YT, Ng
CW, Koh PKS, Zhou Y and Yu H: Isolation of primary rat hepatocytes
with multiparameter perfusion control. J Vis Exp. e622892021.
|
|
200
|
Xie Y, Yao J, Jin W, Ren L and Li X:
Induction and maturation of hepatocyte-like cells in vitro: Focus
on technological advances and challenges. Front Cell Dev Biol.
9:7659802021. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Oseini AM, Cole BK, Issa D, Feaver RE and
Sanyal AJ: Translating scientific discovery: The need for
preclinical models of nonalcoholic steatohepatitis. Hepatol Int.
12:6–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zhao S, Rong C, Zhang S, Liu Y and Chen J:
Protective effects of polysaccharides of Auricularia auricula
polysaccharide on ethanol-induced liver injury. Jiangsu Agric Sci.
45:142–144. 2017.In Chinese.
|
|
203
|
Yu QQ, Meng MR, Liu YY, Cheng YX and Chen
Y: Preventive effect and underlying mechanism of dihydrocumin on
NAFLD in oleic acid-treated HepG2 cells. Chin Tradit Herb Drugs.
49:1092–1099. 2018.In Chinese.
|
|
204
|
Mann DA: Human induced pluripotent stem
cell-derived hepatocytes for toxicology testing. Expert Opin Drug
Metab Toxicol. 11:1–5. 2015. View Article : Google Scholar
|
|
205
|
Chen Y and Ma K: NLRC4 inflammasome
activation regulated by TNF-α promotes inflammatory responses in
nonalcoholic fatty liver disease. Biochem Biophys Res Commun.
511:524–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Giraudi PJ, Becerra VJB, Marina V,
Chavez-Tapiaa NC, Tiribelli C and Rosso N: The importance of the
interaction between hepatocyte and hepatic stellate cells in
fibrogenesis induced by fatty accumulation. Exp Mol Pathol.
98:85–92. 2015. View Article : Google Scholar
|
|
207
|
Feaver RE, Cole BK, Lawson MJ, Hoang SA,
Marukian S, Blackman BR, Figler RA, Sanyal AJ, Wamhoff BR and Dash
A: Development of an in vitro human liver system for interrogating
nonalcoholic steatohepatitis. JCI Insight. 1:e909542016. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Salerno S, Campana C, Morelli S, Drioli E
and De Bartolo L: Human hepatocytes and endothelial cells in
organotypic membrane systems. Biomaterials. 32:8848–8859. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Ebrahimkhani MR, Neiman JA, Raredon MS,
Hughes DJ and Griffith LG: Bioreactor technologies to support liver
function in vitro. Adv Drug Deliv Rev. 69:132–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kamei KI, Yoshioka M, Terada S, Tokunaga Y
and Chen Y: Three-dimensional cultured liver-on-a-Chip with mature
hepatocyte-like cells derived from human pluripotent stem cells.
Biomed Microdevices. 21:732019. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Kostrzewski T, Maraver P, Ouro-Gnao L,
Levi A, Snow S, Miedzik A, Rombouts K and Hughes D: A
microphysiological system for studying nonalcoholic
steatohepatitis. Hepatol Commun. 4:77–91. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Kostrzewski T, Cornforth T, Snow SA,
Ouro-Gnao L, Rowe C, Large EM and Hughes DJ: Three-dimensional
perfused human in vitro model of non-alcoholic fatty liver disease.
World J Gastroenterol. 23:204–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Gaskell H, Sharma P, Colley HE, Murdoch C,
Williams DP and Webb SD: Characterization of a functional C3A liver
spheroid model. Toxicol Res (Camb). 5:1053–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Rose S, Ezan F, Cuvellier M, Bruyère A,
Legagneux V, Langouët S and Baffet G: Generation of proliferating
human adult hepatocytes using optimized 3D culture conditions. Sci
Rep. 11:5152021. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Vorrink SU, Zhou Y, Ingelman-Sundberg M
and Lauschke VM: Prediction of drug-induced hepatotoxicity using
long-term stable primary hepatic 3D spheroid cultures in chemically
defined conditions. Toxicol Sci. 163:655–665. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Messner S, Fredriksson L, Lauschke VM,
Roessger K, Escher C, Bober M, Kelm JM, Ingelman-Sundberg M and
Moritz W: Transcriptomic, proteomic, and functional long-term
characterization of multicellular three-dimensional human liver
microtissues. Appl In Vitro Toxicol. 4:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Rubiano A, Indapurkar A, Yokosawa R,
Miedzik A, Rosenzweig B, Arefin A, Moulin CM, Dame K, Hartman N,
Volpe DA, et al: Characterizing the reproducibility in using a
liver microphysiological system for assaying drug toxicity,
metabolism, and accumulation. Clin Transl Sci. 14:1049–1061. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Hu H, Gehart H, Artegiani B,
López-Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa
Lopes SM, Begthel H, Korving J, et al: Long-term expansion of
functional mouse and human hepatocytes as 3D organoids. Cell.
175:1591–1606. e192018. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Guan Y, Xu D, Garfin PM, Ehmer U, Hurwitz
M, Enns G, Michie S, Wu M, Zheng M, Nishimura T, et al: Human
hepatic organoids for the analysis of human genetic diseases. J
Clin Invest Insight. 2:e949542017.
|
|
220
|
Wang Y, Wang H, Deng P, Tao T, Liu H and
Wu S, Chen W and Wu S: Modeling human nonalcoholic fatty liver
disease (NAFLD) with an organoids-on-a-chip system. ACS Biomater
Sci Eng. 6:5734–5743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Fernandes R, Luo X, Tsao CY, Payne GF,
Ghodssi R, Rubloffde GW and Bentley WE: Biological nanofactories
facilitate spatially selective capture and manipulation of quorum
sensing bacteria in a bioMEMS device. Lab Chip. 10:1128–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Kang D, Hong G, An S, Jang I, Yun WS, Shim
JH and Jin S: Bioprinting of multiscaled hepatic lobules within a
highly vascularized construct. Small. 16:e19055052020. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Mazzocchi A, Devarasetty M, Huntwork R,
Soker S and Skardal A: Optimization of collagen type I-hyaluronan
hybrid bioink for 3D bioprinted liver microenvironments.
Biofabrication. 11:0150032018. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Do A, Zahrawi F and Mehal WZ: Therapeutic
landscape of metabolic dysfunction-associated steatohepatitis
(MASH). Nat Rev Drug Discov. 24:171–189. 2025. View Article : Google Scholar
|
|
225
|
Dai Q, Ain Q, Seth N, Rooney M and
Zipprich A: Liver sinusoidal endothelial cells: Friend or foe in
metabolic dysfunction-associated steatotic liver disease/metabolic
dysfunction-associated steatohepatitis. Dig Liver Dis. 57:493–503.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Kuchay MS, Choudhary NS and Ramos-Molina
B: Pathophysiological underpinnings of metabolic
dysfunction-associated steatotic liver disease. Am J Physiol Cell
Physiol. 328:C1637–C1666. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Sun HS, Nie CC, Li P, Liu Y and Gao L:
Pharmacological treatment of non-alcoholic fatty liver disease in
rats induced by high-fat diet combined with chronic stress. Chin J
Gerontol. 40:177–181. 2020.In Chinese.
|
|
228
|
Cheng F, Ma C, Wang X, Zhai C, Wang G, Xu
X, Mu J, Li C, Wang Z, Zhang X, et al: Effect of traditional
Chinese medicine formula sinisan on chronic restraint
stress-induced nonalcoholic fatty liver disease: A rat study. BMC
Complement Altern Med. 17:2032017. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Cardoso A, Ferreira SC, Araújo MA, Mendes
R, do Carmo LO, Moreira L, Ferreira L, Couto CA, Reis IA, Anastácio
LR and Faria LC: Association between body composition, eating
behavior, dietary intake, and metabolic dysfunction-associated
steatotic liver disease in liver transplant recipients. Clin Nutr.
52:154–161. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Zhong Q, Zhou R, Huang YN, Huang RD, Li
FR, Chen HW, Wei YF, Liu K, Cao BF, Liao KY, et al: Frailty and
risk of metabolic dysfunction-associated steatotic liver disease
and other chronic liver diseases. J Hepatol. 82:427–437. 2025.
View Article : Google Scholar
|
|
231
|
Kineman RD, Del Rio-Moreno M and Waxman
DJ: Liver-specific actions of GH and IGF1 that protect against
MASLD. Nat Rev Endocrinol. 21:105–117. 2025. View Article : Google Scholar
|
|
232
|
Liu M, Zhang H, Niu M and Shen Y:
Protective effects of decoction for strengthening spleen, tonifying
qi, activating blood in rats with type 2 diabetes and non-alcoholic
fatty liver disease. Mod J Integr Tradit Chin West Med.
31:3246–3251. 32682022.In Chinese.
|
|
233
|
Shi H: Effect of HYRG capsule on glucose
and lipid metabolism-related enzymes oxidiation products in rats
with qi and blood stasis type nonalcohlic fatty liver disease.
Lanzhou: Gansu Univ Chin Med; 2019, In Chinese.
|
|
234
|
Chitturi S, Wong VW, Chan WK, Wong GL,
Wong SK, Sollano J, Ni YH, Liu CJ, Lin YC, Lesmana LA, et al: The
Asia pacific working party on non-alcoholic fatty liver disease
guidelines 2017-Part 2: Management and special groups. J
Gastroenterol Hepatol. 33:86–98. 2018. View Article : Google Scholar
|
|
235
|
Gjorgjieva M, Sobolewski C, Dolicka D,
Correia de Sousa M and Foti M: MiRNAs and NAFLD: From
pathophysiology to therapy. Gut. 68:2065–2079. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Hanin G, Yayon N, Tzur Y, Haviv R, Bennett
ER, Udi S, Krishnamoorthy YR, Kotsiliti E, Zangen R, Efron B, et
al: MiRNA-132 induces hepatic steatosis and hyperlipidaemia by
synergistic multitarget suppression. Gut. 67:1124–1134. 2018.
View Article : Google Scholar
|
|
237
|
Huang TT, Zhou YR, Zhang XJ and Liu SS:
Advances in the treatment of nonalcoholic fatty liver disease. Med
Rev. 21:1406–1409. 2015.In Chinese.
|
|
238
|
Liu JL and Li SH: Efficacy and safety of
evolocumab and alirocumab in reducing lipids and cardiovascular
events. J Evid-Based Med. 15:334–338. 2015.In Chinese.
|
|
239
|
Steffens D, Bramlage P, Scheeff C, Kasner
M, Hassanein A, Friebel J and Rauch-Kröhnert U: PCSK9 inhibitors
and cardiovascular outcomes. Expert Opin Biol Ther. 20:35–47. 2020.
View Article : Google Scholar
|
|
240
|
Mach F, Baigent C, Catapano AL, Koskinas
KC, Casula M, Badimon L, Packard CJ, Bayes-Genis A, Cikes M,
Fruchart GC, et al: 2019 ESC/EAS guidelines for the management of
dyslipidaemias: lipid modification to reduce cardiovascular risk.
Eur Heart J. 41:111–188. 2020. View Article : Google Scholar
|
|
241
|
Yerevanian A and Soukas AA: Metformin:
Mechanisms in human obesity and weight loss. Curr Obes Rep.
8:156–164. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Cui H and Zhang X: Occurrence and clinical
management of nonalcoholic fatty liver disease in obesity patients:
A literature review. J Pediatr Endocrinol Metab. 33:579–584. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Adorini L, Pruzanski M and Shapiro D:
Farnesoid X receptor targeting to treat nonalcoholic
steatohepatitis. Drug Discov Today. 17:988–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Mudaliar S, Henry RR, Sanyal AJ, Morrow L,
Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe
E, et al: Efficacy and safety of the farnesoid X receptor agonist
obeticholic acid in patients with type 2 diabetes and nonalcoholic
fatty liver disease. Gastroenterology. 145:574–582.e1. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Sundelin EIO, Gormsen LC, Heebøll S,
Vendelbo MH, Jakobsen S, Munk OL, Feddersen S, Brøsen K,
Hamilton-Dutoit SJ, Pedersen SB, et al: Hepatic exposure of
metformin in patients with non-alcoholic fatty liver disease. Br J
Pharmacol. 85:1761–1770. 2019.
|
|
246
|
Knudsen LB and Lau J: The discovery and
development of liraglutide and semaglutide. Front Endocrinol
(Lausanne). 10:1552019. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
López-Vicario C, González-Périz A, Rius B,
Morán-Salvador E, García-Alonso V, Lozano JJ, Bataller R, Cofán M,
Kang JX, Arroyo V, et al: Molecular interplay between Δ5/Δ6
desaturases and long-chain fatty acids in the pathogenesis of
non-alcoholic steatohepatitis. Gut. 63:344–355. 2014. View Article : Google Scholar
|
|
248
|
Liu BB and Xu KS: Drug therapy for
non-alcoholic fatty liver disease. Chin J Pract Intern Med.
39:214–217. 2019.In Chinese.
|
|
249
|
Abenavoli L, Falalyeyeva T, Boccuto L,
Tsyryuk O and Kobyliak N: Obeticholic acid: A new era in the
treatment of nonalcoholic fatty liver disease. Pharmaceuticals.
11:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Bell DSH and Jerkins T: In praise of
pioglitazone: An economically efficacious therapy for type 2
diabetes and other manifestations of the metabolic syndrome.
Diabetes Obes Metab. 25:3093–3102. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Sumida Y and Yoneda M: Current and future
pharmacological therapies for NAFLD/NASH. J Gastroenterol.
53:362–376. 2018. View Article : Google Scholar :
|
|
252
|
Chen F, Fei X, Wang J, Shen Z, Liu W, Shao
Z, Chen Y and Zhu Z: Effects of Qi-Ho expectorant formula on serum
leptin and lipocalin in patients with non-alcoholic fatty liver
disease. Lishizhen Med Mater Med Res. 26:843–844. 2015.In
Chinese.
|
|
253
|
Lewis JD, Habel LA, Quesenberry CP, Strom
BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn
DJ, et al: Pioglitazone use and risk of bladder cancer and other
common cancers in persons with diabetes. JAMA. 314:265–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Harrison SA, Alkhouri N, Davison BA,
Sanyal A, Edwards C, Colca JR, Lee BH, Loomba R, Cusi K, Kolterman
O, et al: Insulin sensitizer MSDC-0602K in non-alcoholic
steatohepatitis: A randomized, double blind, placebo-controlled
phase IIb study. J Hepatol. 72:613–626. 2020. View Article : Google Scholar
|
|
255
|
Chen Z, Tian R, She Z, Cai J and Li H:
Role of oxidative stress in the pathogenesis of nonalcoholic fatty
liver disease. Free Radic Biol Med. 152:116–141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Nagashimada M and Ota T: Role of vitamin E
in nonalcoholic fatty liver disease. IUBMB Life. 71:516–522. 2019.
View Article : Google Scholar
|
|
257
|
Sanyal AJ, Chalasani N, Kowdley KV,
McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE,
Tonascia J, Unalp A, et al: Pioglitazone, vitamin E, or placebo for
nonalcoholic steatohepatitis. N Engl J Med. 362:1675–1685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Lavine JE: Vitamin E treatment of
nonalcoholic steatohepatitis in children: A pilot study. J Pediatr.
136:734–738. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Liu YL and Zhang QZ: An excerpt of the
Asia-Pacific Working Party on nonalcoholic fatty liver disease
guidelines 2017. J Clin Hepatol. 33:2278–2282. 2017.In Chinese.
|
|
260
|
Wah Kheong C, Nik Mustapha NR and Mahadeva
S: A randomized trial of silymarin for the treatment of
nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol.
15:1940–1949.e8. 2017. View Article : Google Scholar
|
|
261
|
Zhu X, Wu M, Wang H, Li H, Lin J, Peng Y,
Hu Y, Li C and Ding Y: Safety, tolerability, and pharmacokinetics
of the novel pan-phosphodiesterase inhibitor ZSP1601 in healthy
subjects: A double-blinded, placebo-controlled first-in-human
single-dose and multiple-dose escalation and food effect study.
Expert Opin Investig Drugs. 30:579–589. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Hu Y, Li H, Zhang H, Chen X, Chen J, Xu Z,
You H, Dong R, Peng Y, Li J, et al: ZSP1601, a novel
pan-phosphodiesterase inhibitor for the treatment of NAFLD, A
randomized, placebo-controlled phase Ib/IIa trial. Nat Commun.
14:64092023. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Xiang M, Wang PX, Wang AB, Zhang XJ, Zhang
Y, Zhang P, Mei FH, Chen MH and Li H: Targeting hepatic TRAF1-ASK1
signaling to improve inflammation, insulin resistance, and hepatic
steatosis. J Hepatol. 64:1365–1377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Budas G, Karnik S, Jonnson T, Shafizadeh
T, Watkins S, Breckenridge D and Tumas D: Reduction of liver
steatosis and fibrosis with an ASK1 inhibitor in a murine model of
NASH is accompanied by improvements in cholesterol, bile acid and
lipid metabolism. J Hepatol. 64:S0702016. View Article : Google Scholar
|
|
265
|
Harrison SA, Wong VW, Okanoue T, Bzowej N,
Vuppalanchi R, Younes Z, Kohli A, Sarin S, Caldwell SH, Alkhouri N,
et al: Selonsertib for patients with bridging fibrosis or
compensated cirrhosis due to NASH: Results from randomized phase
III STELLAR trials. J Hepatol. 73:26–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Lefere S, Devisscher L and Tacke F:
Targeting CCR2/5 in the treatment of nonalcoholic steatohepatitis
(NASH) and fibrosis: opportunities and challenges. Expert Opin
Investig Drugs. 29:89–92. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Kruger AJ, Fuchs BC, Masia R, Holmes JA,
Salloum S, Sojoodi M, Ferreira DS, Rutledge SM, Caravan P,
Alatrakchi N, et al: Prolonged cenicriviroc therapy reduces hepatic
fibrosis despite steatohepatitis in a diet-induced mouse model of
nonalcoholic steatohepatitis. Hepatol Commun. 2:529–545. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Ratziu V, Sanyal A, Harrison SA, Wong VW,
Francque S, Goodman Z, Aithal GP, Kowdley KV, Seyedkazemi S,
Fischer L, et al: Cenicriviroc treatment for adults with
nonalcoholic steatohepatitis and fibrosis: Final analysis of the
phase 2b centaur study. Hepatology. 72:892–905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Anstee QM, Neuschwander-Tetri BA, Wong VW,
Abdelmalek MF, Younossi ZM, Yuan J, Pecoraro ML, Seyedkazemi S,
Fischer L, Bedossa P, et al: Cenicriviroc for the treatment of
liver fibrosis in adults with nonalcoholic steatohepatitis: Aurora
phase 3 study design. Contemp Clin Trials. 89:1059222020.
View Article : Google Scholar
|
|
270
|
Pawlak M, Lefebvre P and Staels B:
Molecular mechanism of PPARα action and its impact on lipid
metabolism, inflammation and fibrosis in non-alcoholic fatty liver
disease. J Hepatol. 62:720–733. 2015. View Article : Google Scholar
|
|
271
|
Ratziu V, Harrison SA, Francque S, Bedossa
P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M,
Caldwell S, et al: Elafibranor, an agonist of the peroxisome
proliferator-activated receptor-α and-δ, induces resolution of
nonalcoholic steatohepatitis without fibrosis worsening.
Gastroenterology. 150:1147–1159.e5. 2016. View Article : Google Scholar
|
|
272
|
Wang J, Sun F, Yang JJ and Lu C: Effect of
DNMT3A regulation of Drp1 on activated proliferation and migration
capacity of hepatic stellate cells. Chin Pharmacol Bull.
38:1542–1547. 2022.In Chinese.
|
|
273
|
Chalasani N, Abdelmalek MF, Garcia-Tsao G,
Vuppalanchi R, Alkhouri N, Rinella M, Noureddin M, Pyko M, Shiffman
M, Sanyal A, et al: Effects of belapectin, an inhibitor of
Galectin-3, in patients with nonalcoholic steatohepatitis with
cirrhosis and portal hypertension. Gastroenterology.
158:1334–1345.e5. 2020. View Article : Google Scholar
|
|
274
|
Bi Z, Li G, Chen G, Yang Z, Long F, Luo L,
Yang B and Tang D: Exploring the correlation between intestinal
flora dysbiosis and hepatocellular carcinoma based on the theory of
righteousness and evil in Chinese medicine. Mod J Integr Tradit
Chin West Med. 31:1229–1234. 2022.In Chinese.
|
|
275
|
Said I, Ahad H and Said A: Gut microbiome
in non-alcoholic fatty liver disease associated hepatocellular
carcinoma: Current knowledge and potential for therapeutics. World
J Gastrointest Oncol. 14:947–958. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Liu Q, Liu Y, Li F, Gu Z, Liu M, Shao T,
Zhang L, Zhou G, Pan C, He L, et al: Probiotic culture supernatant
improves metabolic function through FGF21-adiponectin pathway in
mice. J Nutr Biochem. 75:1082562020. View Article : Google Scholar
|
|
277
|
Malaguarnera M, Vacante M, Antic T,
Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G,
Mistretta A, Li Volti G and Galvano F: Bifidobacterium longum with
fructo-oligosaccharides in patients with nonalcoholic
steatohepatitis. Dig Dis Sci. 57:545–553. 2012. View Article : Google Scholar
|
|
278
|
Wang H, Chen Q, Li H and Zhang Z:
Adiponectin alleviates LPS-induced inflammatory response of
microglia through A20/NLRP3/caspase-1 pathway. J Xi'an Jiaotong
Univ (Med Sci). 42:529–533. 2021.In Chinese.
|
|
279
|
Fernandes P, Hashiguchi T, Fujii M and
Yoneyama H: Anti-NASH effects of solithromycin in NASH-HCC mouse
model. Gastroenterology. 146:145–146. 2014. View Article : Google Scholar
|
|
280
|
Kannt A, Wohlfart P, Madsen AN, Veidal SS,
Feigh M and Schmoll D: Activation of thyroid hormone receptor-β
improved disease activity and metabolism independent of body weight
in a mouse model of non-alcoholic steatohepatitis and fibrosis. Br
J Pharmacol. 178:2412–2423. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Mavromati M and Jornayvaz FR:
Hypothyroidism-associated dyslipidemia: Potential molecular
mechanisms leading to NAFLD. Int J Mol Sci. 22:127972021.
View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Hu L, Gu Y, Liang J, Ning M, Yang J, Zhang
Y, Qu H, Yang Y, Leng Y and Zhou B: Discovery of highly potent and
selective thyroid hormone receptor β agonists for the treatment of
nonalcoholic steatohepatitis. J Med Chem. 66:3284–3300. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Karim G and Bansal MB: Resmetirom: An
orally administered, small-molecule, liver-directed, β-selective
THR agonist for the treatment of non-alcoholic fatty liver disease
and non-alcoholic steatohepatitis. Touch Rev Endocrinol. 19:60–70.
2023.
|
|
284
|
Harrison SA, Bedossa P, Guy CD,
Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW,
Rinella ME, et al: A phase 3, randomized, controlled trial of
resmetirom in NASH with liver fibrosis. N Engl J Med. 390:497–509.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Loomba R, Neutel J, Mohseni R, Bernard D,
Severance R, Dao M, Saini S, Margaritescu C, Homer K, Tran B, et
al: VK2809, a novel liver-directed thyroid receptor beta agonist,
significantly reduces liver fat with both low and high doses in
patients with non-alcoholic fatty liver disease: A phase 2
randomized, placebo-controlled trial. J Hepatology. 70:e150–e151.
2019. View Article : Google Scholar
|
|
286
|
Leng YR, Zhang MH, Luo JG and Zhang H:
Pathogenesis of NASH and promising natural products. Chin J Nat
Med. 19:12–27. 2021.PubMed/NCBI
|
|
287
|
Chen M, Xie Y, Gong S, Wang Y, Yu H, Zhou
T, Huang F, Guo X, Zhang H, Huang R, et al: Traditional Chinese
medicine in the treatment of nonalcoholic steatohepatitis.
Pharmacol Res. 172:1058492021. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Dai X, Feng J, Chen Y, Huang S, Shi X, Liu
X and Sun Y: Traditional Chinese medicine in nonalcoholic fatty
liver disease: molecular insights and therapeutic perspectives.
Chin Med. 16:682021. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Deng Y, Liu H, Wu X, Cheng L, He M, Wang T
and Ye L: Research progress on the hepatoprotective effects of
polydatin. Chin J Comp Med. 31:136–140. 2021.In Chinese.
|
|
290
|
Pan L, Lv B, Jiang X, Wang T, Ma X, Chang
N, Wang X and Gao X: Identification of NF-κB inhibitors following
shenfu injection and bioactivity-integrated UPLC/Q-TOF-MS and
screening for related anti-inflammatory targets in vitro and in
silico. J Ethnopharmacol. 19:658–667. 2016.
|
|
291
|
Li L, Wang YL, Qin HY, Qu YY, Yang TS,
Wang ZY and Xie JR: Research progress on berberine in treatment of
nonalcoholic fatty liver disease by regulating gut-liver axis. Chin
Tradit Herb Drugs. 52:1501–1509. 2021.In Chinese.
|
|
292
|
Liu YL, Zhang QZ, Wang YR, Fu LN, Han JS,
Zhang J and Wang BM: Astragaloside IV Improves high-fat
diet-induced hepatic steatosis in nonalcoholic fatty liver disease
rats by regulating inflammatory factors level via TLR4/NF-κB
signaling pathway. Front Pharmacol. 11:6050642020. View Article : Google Scholar
|
|
293
|
Zhao ZM, Wu JZ, Yao Z, Li YJ, Hou W, Chen
WH and Shi AH: Effects of Cassia glycosides on the rats with
non-alcoholic fatty liver disease through Toll-like receptor 4 and
nuclear factor-κB. Chin J Clin Pharmacol. 35:2863–2867. 2019.In
Chinese.
|
|
294
|
Paudel P, Jung HA and Choi JS:
Anthraquinone and naphthopyrone glycosides from cassia obtusifolia
seeds mediate hepatoprotection via Nrf2-mediated HO-1 activation
and MAPK modulation. Arch Pharm Res. 41:677–689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Zhang Y, Cheng S, Zhou F, Zhang L, Su Z,
Zhao D, Chen Y and Jia Y: Effects of geniposide on inflammation and
oxidative stress of ApoE knockout mice with atherosclerosis and
none-alcoholic fatty liver disease. Tradit Chin Drug Res Clin
Pharmacol. 26:581–586. 2015.In Chinese.
|
|
296
|
Lee JH, Jung IR, Choi SE, Lee SM, Lee SJ,
Han SJ, Kim HJ, Kim DJ, Lee KW and Kang Y: Toxicity generated
through inhibition of pyruvate carboxylase and carnitine palmitoyl
transferase-1 is similar to high glucose/palmitate-induced
glucolipotoxicity in INS-1 beta cells. Mol Cell Endocrinol.
383:48–59. 2014. View Article : Google Scholar
|
|
297
|
Xu QM, Gao Y, Li ZM, Wei RM, Ma XH, Jin L
and Zhang KF: The effect of gentiopicroside on TLR-4/NF-κB and
AMPK/Nrf2 in non-alcoholic fatty liver disease. Nat Prod Res Dev.
32:1652–1658. 2020.In Chinese.
|
|
298
|
Molteni M, Gemma S and Rossetti C: The
role of toll-like receptor 4 in infectious and noninfectious
inflammation. Mediators Inflamm. 2016:69789362016. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
Wang QB, Xu FP, Wei CX, Peng J and Dong
XD: Research progress on free radicals in the human body. Zhonghua
Liu Xing Bing Xue Za Zhi. 37:1175–1182. 2016.In Chinese. PubMed/NCBI
|
|
300
|
Huang Z, Wei Y, Wang Z, Lai PL, Zeng BH,
Xu JJ and Yang YL: Effects of total glucosides paeony on TLR4 and
JNK protein expression in inflammatory pathway of NAFLD rats. J
Hunan Normal Univ (Med Sci). 15:23–27. 2018.In Chinese.
|
|
301
|
Shen S, Wang K, Zhi Y, Shen W and Huang L:
Gypenosides improves nonalcoholic fatty liver disease induced by
high-fat diet induced through regulating LPS/TLR4 signaling
pathway. Cell Cycle. 19:3042–3053. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Li X, Fang C, Xu Y, Xu B and Zheng X:
Effect of hesperidin on CYP2E1 expression in nonalcoholic fatty
liver rats induced by high fructose diet. Mod J Integr Tradit Chin
West Med. 29:2635–2639. 2020.In Chinese.
|
|
303
|
Park S, Choi Y, Um SJ, Yoon SK and Park T:
Oleuropein attenuates hepatic steatosis induced by high-fat diet in
mice. J Hepatol. 54:984–993. 2011. View Article : Google Scholar
|
|
304
|
Chen M, Liu G, Fang Z, Gao W, Song Y, Lei
L, Du X and Li X: Buddleoside alleviates nonalcoholic
steatohepatitis by targeting the AMPK-TFEB signaling pathway.
Autophagy. 21:1316–1334. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Yin Y, Liu H, Zheng Z, Lu R and Jiang Z:
Genistein can ameliorate hepatic inflammatory reaction in
nonalcoholic steatohepatitis rats. Biomed Pharmacother.
111:1290–1296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Liu X, Sun R, Li Z, Lv P, Sun X, Olson MA
and Gong Y: Luteolin alleviates non-alcoholic fatty liver disease
in rats via restoration of intestinal mucosal barrier damage and
microbiota imbalance involving in gut-liver axis. Arch Biochem
Biophys. 711:1090192021. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
Kou X, Hong M, Pan F, Huang X, Meng Q,
Zhang Y and Ke Q: Inhibitory effects of nobiletin-mediated
interfacial instability of bile salt emulsified oil droplets on
lipid digestion. Food Chem. 444:1387512024. View Article : Google Scholar : PubMed/NCBI
|
|
308
|
He TT, Qian YL and Chen WM: Study on
constitution classification of TCM for 260 nonalcoholic fatty liver
disease patients. Inner Mong J Tradit Chin Med. 37:1–2. 2018.In
Chinese.
|
|
309
|
Pan YT, Xu FY, Yu XZ and Shang WB:
Research progress on therapeutic targets of active components in
Chinese herbs for treatment of nonalcoholic fatty liver disease.
Zhongguo Zhong Yao Za Zhi. 42:1109–1112. 2017.In Chinese.
PubMed/NCBI
|
|
310
|
Liang ZQ, Bai JH, Zhao RH, Liu YH, Li LM
and Lin QX: Effect of ginkgo flavone on nonalcoholic fatty liver
disease in mice and the correlation analysis on NF-κB in the action
mechanism. Nat Prod Res Dev. 28:277–282. 2016.In Chinese.
|
|
311
|
Huang L, Ding W, Wang MQ, Wang ZG, Chen
HH, Chen W, Yang Q, Lu TN, Yang Q and He JM: Tanshinone IIA
ameliorates non-alcoholic fatty liver disease through targeting
peroxisome proliferator-activated receptor gamma and toll-like
receptor 4. J Int Med Res. 47:5239–5255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Zheng Y, Wang J, Wang J, Xie H and Zhao T:
Effect of curcumol on the fenestrae of liver sinusoidal endothelial
cells based on NF-κB signaling pathway. Evid Based Complement
Alternat Med. 2020:85906382020. View Article : Google Scholar
|
|
313
|
Han LP, Sun B, Li CJ, Xie Y and Chen LM:
Effect of celastrol on toll-like receptor 4-mediated inflammatory
response in free fatty acid-induced HepG2 cells. Int J Mol Med.
42:2053–2061. 2018.PubMed/NCBI
|
|
314
|
Ye Z, Zhu K, He Y, Chen L and Zhang J:
Mechanism of asiatic acid improving nonalcoholic fatty liver
disease by regulating TLR4/NF-κB/NLRP3 signaling pathway. China
Food Addit. 35:201–207. 2024.In Chinese.
|
|
315
|
Malekinejad H, Zeynali-Moghaddam S,
Rezaei-Golmisheh A, Alenabi A, Malekinejad F, Alizadeh A and
Shafie-Irannejad V: Lupeol attenuated the NAFLD and PCOS-induced
metabolic, oxidative, hormonal, histopathological, and molecular
injuries in mice. Res Pharm Sci. 18:551–565. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Brusotti G, Montanari R, Capelli D,
Cattaneo G, Laghezza A, Tortorella P, Loiodice F, Peiretti F,
Bonardo B, Paiardini A, et al: Betulinic acid is a PPARγ antagonist
that improves glucose uptake, promotes osteogenesis and inhibits
adipogenesis. Sci Rep. 7:57772017. View Article : Google Scholar
|
|
317
|
Hou L and Xiao Y: Study on the
distribution of Chinese medicine evidence elements in non-alcoholic
fatty liver disease. Mod J Integr Tradit Chin West Med.
24:2116–2118. 2015.In Chinese.
|
|
318
|
Zhang L, Zhao Y, Li Z, Guo D and Fu S:
Correlative study between ultrasonic echo intensity parameters and
Traditional Chinese Medicine syndromes of fatty liver. Chin J
Ultrasound Med. 28:55–58. 2012.In Chinese.
|
|
319
|
He F, Zhang X and Wen X: Effects of
crataegolic acid on inflammatory response and oxidative stress in
non-alcoholic fatty liver disease model mice induced by high-fat
diet. China Pharm. 30:901–905. 2019.In Chinese.
|
|
320
|
Zhou X, Ren Q, Wang B, Fang G, Ling Y and
Li X: Alisol A 24-Acetate Isolated from the alismatis rhizoma
improves hepatic lipid deposition in hyperlipidemic mice by
ABCA1/ABCG1 pathway. J Nanosci Nanotechnol. 19:5496–5502. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
321
|
Ho C, Gao Y, Zheng D, Liu Y, Shan S, Fang
B, Zhao Y, Song D, Zhang Y and Li Q: Alisol A attenuates
high-fat-diet-induced obesity and metabolic disorders via the
AMPK/ACC/SREBP1c pathway. J Cell Mol Med. 23:5108–5118. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Li X, Chen XX, Xu Y, Xu XB, Wu WF, Zhao Q
and Hu JN: Construction of Glycogen-Based Nanoparticles loaded with
resveratrol for the alleviation of high-fat diet-induced
nonalcoholic fatty liver disease. Biomacromolecules. 23:409–423.
2022. View Article : Google Scholar
|
|
323
|
Feng D, Zou J, Su D, Mai H, Zhang S, Li P
and Zheng X: Curcumin prevents high-fat diet-induced hepatic
steatosis in ApoE−/− mice by improving intestinal
barrier function and reducing endotoxin and liver TLR4/NF-κB
inflammation. Nutr Metab (Lond). 16:792019. View Article : Google Scholar
|
|
324
|
Zhang M, Zhang Y, Tang L, Gong Z, Han L
and Wang D: Gastrodin inhibits non-alcoholic fatty liver disease
via mediating SREBP1c signaling pathway. Chin J Exp Tradit Med
Formul. 30:70–77. 2024.In Chinese.
|
|
325
|
Xiao J, Zhang R, Zhou Q, Liu L, Huang F,
Deng Y, Ma Y, Wei Z, Tang X and Zhang M: Lychee (litchi chinensis
sonn.) pulp phenolic extract provides protection against alcoholic
liver injury in mice by alleviating intestinal microbiota
dysbiosis, intestinal barrier dysfunction and liver inflammation. J
Agric Food Chem. 65:9675–9684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
326
|
Aithal GP, Thomas JA, Kaye PV, Lawson A,
Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L and Webber
J: Randomized, placebo-controlled trial of pioglitazone in
nondiabetic subjects with nonalcoholic steatohepatitis.
Gastroenterology. 135:1176–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
327
|
Castellino G, Nikolic D, Magán-Fernández
A, Malfa GA, Chianetta R, Patti AM, Amato A, Montalto G, Toth PP,
Banach M, Cicero AFG and Rizzo M: Altilix® supplement containing
chlorogenic acid and luteolin improved hepatic and cardiometabolic
parameters in subjects with metabolic syndrome: A 6 month
randomized, double-blind, placebo-controlled study. Nutrients.
11:25802019. View Article : Google Scholar
|
|
328
|
Tan Y, Kim J, Cheng J, Ong M, Lao WG, Jin
XL, Lin YG, Xiao L, Zhu XQ and Qu XQ: Green tea polyphenols
ameliorate non-alcoholic fatty liver disease through upregulating
AMPK activation in high fat fed Zucker fatty rats. World J
Gastroenterol. 23:3805–3814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
329
|
Chen X, Ren Y, Kong W and Wang Y: Effect
of salvianolic acid B on oxidative stress in a cell model of
nonalcoholic fatty liver disease. J Clin Hepatol. 34:2175–2181.
2018.In Chinese.
|
|
330
|
Zhang X, Li T, Liu S, Wu Q, Lin Z, Pan Y
and Alitongbieke G: Mechanism of berberine improving non-alcoholic
fatty liver induced by high-fat diet in mice. Chin J Bioprocess
Eng. 19:657–661. 2021.In Chinese.
|
|
331
|
Kim R, Kim SB, Cho EH, Park SH, Park SB,
Hong SK and Chae G: CD44 expression in patients with combined
hepatocellular cholangiocarcinoma. Ann Surg Treat Res. 89:9–16.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
332
|
Sun T, Xue M, Yang J, Pei Z, Zhang N, Qin
K and Liang H: Metabolic regulation mechanism of fucoidan via
intestinal microecology in diseases. J Sci Food Agric.
101:4456–4463. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
333
|
Cani PD and Delzenne NM: The role of the
gut microbiota in energy metabolism and metabolic disease. Curr
Pharm Des. 15:1546–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
334
|
Zhang DD, Zhang JG, Wang YZ, Liu Y, Liu GL
and Li XY: Per-Arnt-Sim kinase (PASK): An emerging regulator of
mammalian glucose and lipid metabolism. Nutrients. 7:7437–7450.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
335
|
Rahban M, Zolghadri S, Salehi N, Ahmad F,
Haertlé T, Rezaei-Ghaleh N, Sawyer L and Saboury AA: Thermal
stability enhancement: fundamental concepts of protein engineering
strategies to manipulate the flexible structure. Int J Biol
Macromol. 214:642–644. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
336
|
Zhong M, Yan Y, Yuan H, A R, Xu G, Cai F,
Yang Y, Wang Y and Zhang W: Astragalus mongholicus polysaccharides
ameliorate hepatic lipid accumulation and inflammation as well as
modulate gut microbiota in NAFLD rats. Food Funct. 13:7287–7301.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
337
|
Wang L, Li W, Li Y, Chen G, Zhao L, Li W,
Wang S, Wang C, Feng Y and Zhang Y: Dried tangerine peel
polysaccharide (DTPP) alleviates hepatic steatosis by suppressing
TLR4/MD-2-mediated inflammation and endoplasmic reticulum stress.
Bioorg Chem. 147:1073692024. View Article : Google Scholar : PubMed/NCBI
|
|
338
|
Yu J, Fan Y, Yang Y, He D, Song B and Yao
Z: Influence of dendrobium nobile polysaccharide on the expression
of TLR4 and HO-1 in rats with nonalcoholic fatty liver disease.
West J Tradit Chin Med. 34:25–29. 2021.In Chinese.
|
|
339
|
Liu HP, Zhang RJ, Cao HK, Gao Y and Wang
YW: Study on effects of Polygala fallax polysaccharide on
nonalcoholic fatty liver in rats by PPAR-γ and TLR-4/NF-κB
signaling pathway. J Chin Med Mater. 47:1247–1252. 2024.In
Chinese.
|
|
340
|
Zhang K and Jin L: Protective effect and
mechanism of dicliptera chinensis polysaccharides on non-alcoholic
fatty liver disease in rats based on PPAR-γ and TLR-4/NF-κB
signaling pathway. Pharmacol Clin Chin Mater Med. 35:103–107.
2019.In Chinese.
|
|
341
|
Zhao H, Gao X, Liu Z, Zhang L, Fang X, Sun
J, Zhang Z and Sun Y: Sodium alginate prevents non-alcoholic fatty
liver disease by modulating the gut-liver axis in high-fat diet-fed
rats. Nutrients. 14:48462022. View Article : Google Scholar : PubMed/NCBI
|
|
342
|
Liu X, Su J, Shi Y, Guo Y, Suheryani I,
Zhao S, Deng Y, Meng W, Chen Y, Sun L and Dai R: Herbal Formula,
Baogan Yihao (BGYH), prevented dimethylnitrosamine(DMN)-indu ced
liver injury in rats. Drug Dev Res. 78:155–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
343
|
Xie R, Lin D, Yang L and Li F: Protective
effect and mechanism of zingerone on rats with nonalcoholic fatty
liver disease. J Shenyang Pharm Univ. 39:684–689. 2022.In
Chinese.
|
|
344
|
Lv YL, Liu CC, Liu H, Xu KY, Qiao L and
Bao JF: Effects of rhein on TLR4 signaling pathway in NAFLD rats.
Chin J Health Lab Tec. 28:2580–2584. 2018.In Chinese.
|
|
345
|
Wei J, Zhen YZ, Cui J, He FL, Shen T, Hu
G, Ren XH and Lin YJ: Rhein lysinate decreases inflammation and
adipose infiltration in KK/HlJ diabetic mice with nonalcoholic
fatty liver disease. Arch Pharm Res. 39:960–969. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
346
|
Dong X, Fu J, Yin X, Cao S, Li X, Lin L,
Huyiligeqi and Ni J: Emodin: a review of its pharmacology, toxicity
and pharmacokinetics. Phytother Res. 30:1207–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
347
|
Ye J, Zheng J, Tian X, Xu B, Yuan F, Wang
B, Yang Z and Huang F: Fucoxanthin attenuates free fatty
acid-induced nonalcoholic fatty liver disease by regulating lipid
metabolism/oxidative stress/inflammation via the AMPK/Nrf2/TLR4
signaling pathway. Mar Drugs. 20:2252022. View Article : Google Scholar : PubMed/NCBI
|
|
348
|
Borstlap WAA, Musters GD, Stassen LPS, van
Westreenen HL, Hess D, van Dieren S, Festen S, van der Zaag EJ,
Tanis PJ and Bemelman WA: Vacuum-assisted early transanal closure
of leaking low colorectal anastomoses: The CLEAN study. Surg
Endosc. 32:315–327. 2018. View Article : Google Scholar :
|
|
349
|
Yu S, Jiang J, Li Q, Liu X, Wang Z, Yang L
and Ding L: Schisantherin a alleviates non-alcoholic fatty liver
disease by restoring intestinal barrier function. Front Cell Infect
Microbiol. 12:8550082022. View Article : Google Scholar : PubMed/NCBI
|
|
350
|
Gu L, Zhang Y, Zhang S, Zhao H, Wang Y,
Kan D, Zhang Y, Guo L, Lv J, Hao Q, et al: Coix lacryma-jobi seed
oil reduces fat accumulation in nonalcoholic fatty liver disease by
inhibiting the activation of the p-AMPK/SePP1/apoER2 pathway. J
Oleo Sci. 70:685–696. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
351
|
Pan MX, Zheng CY, Deng YJ, Tang KR, Nie H,
Xie JQ, Liu DD, Tu GF, Yang QH and Zhang YP: Hepatic protective
effects of shenling baizhu powder, a herbal compound, against
inflammatory damage via TLR4/NLRP3 signalling pathway in rats with
nonalcoholic fatty liver disease. J Integr Med. 19:428–438. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
352
|
Du N, Yang J, Xu D, Zhang S and Shi Z:
Fuzi Lizhong decoction modulates bile acid metabolism in
nonalcoholic fatty liver disease viathe FXR-FGF15 signaling
pathway. Med J West China. 36:1576–1581. 2024.In Chinese.
|
|
353
|
Liu H, Xu J, Li H, Zhang L and Xu P:
Network pharmacology-based investigation to explore the effect and
mechanism of erchen decoction against the nonalcoholic fatty liver
disease. Anat Rec (Hoboken). 304:2605–2619. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
354
|
Zhang Y, Zhou G, Chen Z, Guan W, Zhang J,
Bi M, Wang F, You X, Liao Y, Zheng S, et al: Si-Wu-Tang alleviates
nonalcoholic fatty liver disease via blocking tlr4-jnk and
caspase-8-gsdmd signaling pathways. Evid Based Complement Alternat
Med. 2020:87864242020. View Article : Google Scholar : PubMed/NCBI
|
|
355
|
Su L, Lin J, Liu X, Ye X and Chen J:
Clinical study on Xiaoyao San prescription combined with silymarin
capsules for non-alcoholic fatty liver disease with syndrome of
liver depression and spleen deficiency. New Chin Med. 56:42–46.
2024.In Chinese.
|
|
356
|
Feng W, Su A, Huang X and Wang C:
Mechanism of Huangqisan regulating autophagy by AMPK/mTOR signaling
pathway against hepatic steatosis. Chin J Exp Tradit Med Formul.
29:21–30. 2023.In Chinese.
|
|
357
|
Xia M, Zhang Z, Shen H and Kang J: Study
on clinical regression of non-alcoholic fatty liver disease by
nourishing yin and softening liver method. J New Chin Med.
48:52–54. 2016.In Chinese.
|
|
358
|
Lu S, Lu S, Chen Y, Shen T and Li Y:
Qinghua formula for the treatment of non-alcoholic fatty liver
disease with phlegm internal obstruction type: A clinical study.
World Sci Technol - Mod Tradit Chin Med. 21:1759–1765. 2019.In
Chinese.
|
|
359
|
Xie WN, Peng HB and Li Y, Huang LP, Chen
SX, Wu YF and Li Y: Liver with liver stagnation and spleen
deficiency syndrome and intestinal microflora. Chin J Exp Tradit
Med Formul. 27:129–137. 2021.In Chinese.
|
|
360
|
Xu L, Fu J, Fang F, Chen LZ and Zhuang GF:
Effect of modfied Yinchen Wuling San in treating non-alcoholic
fatty liver disease with moisture and heat implication and on
intestinal microflora. Chin J Exp Tradit Med Formul. 25:127–132.
2019.In Chinese.
|
|
361
|
Zhan YH, Wang XH and Wang F: Regulatory
effect of modified Banxia Xiexintang on insulin resistance of
patients with nonalcoholic fatty liver. Chin J Exp Tradit Med
Formul. 27:117–122. 2021.In Chinese.
|
|
362
|
Wan Y, Zhang Z, Song G and Ouyang J:
Clinical effect of Yishen Tiaogan recipe combined with probiotics
on nonalcoholic fatty liver disease. Chin Arch Tradit Chin Med.
38:10–13. 2020.In Chinese.
|
|
363
|
Chen L, Huang Q, Liu S and Lu Z: Clinical
observation of Wuling powder combined with polyene
phosphatidylcholine on patients with nonalcoholic fatty liver
disease. Chin Arch Tradit Chin Med. 38:190–193. 2020.In
Chinese.
|
|
364
|
Mian F and Yang R: Study on the effects of
activating blood and eliminating lipids formula on TNF-α, TGF and
FFA in patients with non-alcoholic fatty liver disease. World
Latest Med Inf. 18:136–137. 2018.In Chinese.
|
|
365
|
Zhang Y, Ni Y, Zhang J, Li J and Liu H:
Effectiveness of spleen-strengthening and fat-eliminating formula
in treating patients with type 2 diabetes mellitus combined with
nonalcoholic fatty liver disease and its effect on HMGB1 and TLR4
levels. Lishizhen Med Mater Med Res. 35:261–264. 2024.In
Chinese.
|
|
366
|
Wang S, Ning M, Wang Y, Yang X and Li M:
Study on the therapeutic effect and mechanism of
anemarrhenae-hawthorn drink on non-alcoholic fatty liver in rats.
Anhui Med Pharm J. 27:1927–1932. 2023.In Chinese.
|
|
367
|
Wang S, Xu WX, Shen MY, Li C, La XJ, Li
JA, Qi YJ, Wang XP and Wu Z: Effect of Tongping Zhigan formula on
liver tissue inflammation and TLR4/MyD88/NF-κB pathway in
non-alcoholic fatty liver mice. J Hebei Tradit Chin Med Pharmacol.
39:1–6. 2024.In Chinese.
|
|
368
|
Ding Y, Ou X, Li C, Li X and Lian S: An
investigation of the mechanism of Qinggan Jiangzhuo particles in
the treatment of nonalcoholic fatty liver disease based on the
TLR4/NF-κB signaling pathway. Jilin J Chin Med. 41:1489–1492.
2021.In Chinese.
|
|
369
|
Chen Z, Xiang S, Yang M, Xia F and Zhou B:
Study on the molecular mechanism of liver-protecting and
lipid-cleansing tablets for the treatment of non-alcoholic fatty
liver disease based on network pharmacology. J Chin Med Mater.
43:1462–1468. 2020.In Chinese.
|
|
370
|
Ye M, Xue J, Li F, Yang Y, He J and Tang
Y: Study on regulation and mechanism of Huazhi Fugan granules on
TLR4/NF-κB pathway and fatty iron death in non-alcoholic fatty
liver. Mod J Integr Tradit Chin West Med. 30:3307–3312. 2021.In
Chinese.
|
|
371
|
Guo HH, Shen HR, Zhang HJ, Wang LL, Han YX
and Jiang JD: Dengzhan shengmai inhibits nonalcoholic fatty liver
disease via regulating intestinal microenvironment. Acta Pharm Sin.
57:3524–3534. 2022.In Chinese.
|
|
372
|
Liao Y, Chi X, Yang Y, Wu Y, Zhan Y and
Song Y: Effects of Zhibitai on the expressions of ERK and TLRs in
liver tissues of NAFLD rats. Pharmacol Clin Chin Mater Med.
35:130–134. 2019.In Chinese.
|
|
373
|
Yang JF, Xue JB, Tao YY, Huang K, Lu J and
Liu CH: Mechanism of Kuhuang Granules in treating non-alcoholic
fatty liver disease based on network pharmacology and experimental
validation. J Integr Tradit Chin West Med Hepatol. 32:58–62.
2022.In Chinese.
|
|
374
|
Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah
JA and Kanaley JA: Energy-matched moderate and high intensity
exercise training improves nonalcoholic fatty liver disease risk
independent of changes in body mass or abdominal
adiposity-a-randomized trial. Metabolism. 78:128–140. 2018.
View Article : Google Scholar
|
|
375
|
Hannah WN Jr and Harrison SA: Lifestyle
and dietary interventions in the management of nonalcoholic fatty
liver disease. Dig Dis Sci. 61:1365–1374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
376
|
Fogacci F, Rizzoli E, Giovannini M, Bove
M, D'Addato S, Borghi C and Cicero AFG: Effect of dietary
supplementation with eufortyn colesterolo plus on serum lipids,
endothelial reactivity, indexes of non-alcoholic fatty liver
disease and systemic inflammation in healthy subjects with
polygenic hypercholesterolemia: The ANEMONE study. Nutrients.
14:20992022. View Article : Google Scholar
|
|
377
|
Fatemeh H, Abdollah H, Bizhan H,
Seyed-Saeed S and Kambiz AA: An energy-restricted high-protein diet
supplemented with beta-cryptoxanthin alleviated oxidative stress
and inflammation in nonalcoholic fatty liver disease: A randomized
controlled trial. Nutr Res. 73:15–26. 2020. View Article : Google Scholar
|
|
378
|
Yang M, Xue J, Li F, Dong L and Fu Q:
Effect of Chinese medicine starvation-free fasting therapy on
glucolipid metabolism and liver fat deposition in patients with
nonalcoholic fatty liver disease. China Med Herald. 17:135–138.
2020.In Chinese.
|
|
379
|
Yang R, Wan L, Zhu H and Peng Y: The
effect of 12 week-maximum fat oxidation intensity (FATmax) exercise
on microvascular function in obese patients with nonalcoholic fatty
liver disease and its mechanism. Gen Physiol Biophys. 42:251–262.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
380
|
Li H, Sun P, Chen Y and Jia H: Research on
the intervention of NAFLD by Baduanjin. J Chengdu Sport Univ.
44:79–83. 902018.In Chinese.
|
|
381
|
Vahedi H, Atefi M, Entezari MH and
Hassanzadeh A: The effect of sesame oil consumption compared to
sunflower oil on lipid profile, blood pressure, and anthropometric
indices in women with non-alcoholic fatty liver disease: A
randomized double-blind controlled trial. Trials. 23:5512022.
View Article : Google Scholar : PubMed/NCBI
|
|
382
|
Guo W, Li Z, Anagnostopoulos G, Kong WT,
Zhang S, Chakarov S, Shin A, Qian J, Zhu Y, Bai W, et al: Notch
signaling regulates macrophage-mediated inflammation in metabolic
dysfunction-associated steatotic liver disease. Immunity.
57:2310–2327.e6. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
383
|
Palomer X, Wang JR, Escalona C, Wu S,
Wahli W and Vázquez-Carrera M: Targeting AMPK as a potential
treatment for hepatic fibrosis in MASLD. Trends Pharmacol Sci.
46:551–566. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
384
|
Nóvoa E, da Silva Lima N, Gonzalez-Rellan
MJ, Chantada-Vazquez MDP, Verheij J, Rodriguez A, Esquinas-Roman
EM, Fondevila MF, Koning M, Fernandez U, et al: Mitochondrial
antiviral signaling protein enhances MASLD progression through the
ERK/TNFα/NFκβ pathway. Hepatology. 81:1535–1552. 2025. View Article : Google Scholar
|
|
385
|
Yu Y, Wang Q, Huang X and Li Z: GA
receptor targeted chitosan oligosaccharide polymer nanoparticles
improve non-alcoholic fatty liver disease by inhibiting
ferroptosis. Int J Biol Macromol. 278:1347792024. View Article : Google Scholar : PubMed/NCBI
|