Introduction
Cognitive impairment encompasses a continuum of
conditions characterized by deficits in memory, attention,
executive function, language and visuospatial abilities, ranging
from mild cognitive impairment (MCI) to more severe forms such as
dementia, including Alzheimer's disease (AD), vascular dementia and
other neurodegenerative disorders (1). These cognitive deficits
significantly compromise individual autonomy, reduce quality of
life and impose growing burdens on caregivers and healthcare
systems (2). As the global
population ages, the prevalence of cognitive disorders is projected
to rise dramatically, amplifying their public health and
socio-economic impact. Despite advances in pharmacological and
behavioral interventions, current treatment options remain limited
in efficacy, particularly for dementia, underscoring the urgent
need to identify modifiable risk factors and implement preventive
strategies at earlier stages of disease progression.
One such modifiable factor that has gained
increasing attention is diet-particularly the role of high-fat
diets (HFDs) in the development and progression of cognitive
dysfunction. An HFD is typically defined as one in which total
energy intake from fats exceeds 35-40%, with a disproportionate
enrichment in saturated fatty acids (SFAs) and trans fats, often
coupled with insufficient intake of carbohydrates, essential amino
acids, dietary fiber and micronutrients (3). Over the past several decades, the
global spread of Western dietary patterns-marked by increased
consumption of processed foods, sugary beverages and high-fat fast
food-has contributed to the widespread adoption of HFDs across both
developed and developing nations (4). As a result, the incidence of
metabolic disorders linked to high-fat consumption, such as
obesity, type 2 diabetes, dyslipidemia and non-alcoholic fatty
liver disease, has escalated to epidemic proportions (5-7).
Emerging research suggests that chronic exposure to
HFDs may not only impair systemic metabolic homeostasis but also
exert deleterious effects on brain health (3,8,9).
Epidemiological studies have demonstrated that individuals with
high dietary fat intake, particularly SFAs, have a significantly
increased risk of developing MCI and dementia (10,11). For instance, longitudinal cohort
studies have revealed that older adults adhering to diets rich in
saturated fats experience accelerated cognitive decline and exhibit
higher incidences of AD compared to those following diets high in
polyunsaturated or monounsaturated fats (12). Conversely, adherence to dietary
patterns rich in unsaturated fatty acids-such as the Mediterranean
or Dietary Approaches to Stop Hypertension (DASH) diets-has been
associated with reduced cognitive decline and improved neurological
outcomes, supporting the notion that the type and quality of
dietary fat play a critical role in modulating cognitive health
(13,14).
HFD-induced cognitive impairment is driven by a
multifactorial network of neuropathological processes, including
neuroinflammation, oxidative stress, mitochondrial dysfunction,
insulin resistance and gut microbiota dysbiosis (15). These mechanisms collectively
disrupt synaptic plasticity, impair neurogenesis and compromise
neuronal survival, particularly in hippocampal regions critical for
learning and memory. Individual susceptibility-such as advanced
age, female sex and genetic predispositions like the apolipoprotein
E ε4 (APOE-ε4) allele-can amplify these deleterious effects.
Given the rising global prevalence of both HFD
consumption and dementia-related disorders, elucidating the
mechanistic links between diet and neurodegeneration has become a
pressing public health priority. Importantly, the modifiable nature
of diet offers a compelling avenue for early intervention. Emerging
strategies-including adherence to Mediterranean or DASH dietary
patterns, regular physical exercise, insulin-sensitizing or
anti-inflammatory pharmacotherapy, and microbiota-targeted
interventions such as probiotics-show promise in mitigating
HFD-associated cognitive decline. A more comprehensive
understanding of how dietary fats interact with neuroimmune,
metabolic and microbial pathways may pave the way for personalized,
mechanism-based prevention and treatment strategies aimed at
preserving cognitive function across the lifespan.
Association between HFD and cognitive
function
Epidemiological and experimental evidence
of cognitive impairment induced by HFDs
A substantial body of epidemiological research has
identified a significant association between chronic HFD
consumption and increased risk of cognitive decline, MCI and
dementia (Table I) (8,16-21). Prospective cohort studies in
diverse populations have consistently shown that long-term
adherence to HFD-particularly those rich in SFAs-is linked to
poorer cognitive outcomes and a higher incidence of AD (10,11). For instance, a longitudinal study
involving ~6,000 older adults reported that individuals in the
highest quartile of SFA intake experienced markedly faster
cognitive decline and a >2-fold increased risk of developing AD
compared to those in the lowest quartile (22,23). Similar trends of accelerated
cognitive deterioration have been observed in other
population-based studies, further supporting the link between high
SFA intake and impaired cognitive outcomes (8,24). Complementary findings from animal
studies further substantiate this association: Rodents exposed to
chronic SFA-enriched diets exhibit impairments in spatial learning
and memory, along with pathological features characteristic of AD,
such as enhanced β-amyloid deposition and tau hyperphosphorylation
(25). Early manifestations of
cognitive impairment in HFD models include disrupted circadian
rhythms and diminished semantic fluency, with clinical symptoms
predominantly affecting memory, executive function, learning
ability and other early cognitive domains associated with
neurodegeneration. Experimental evidence indicates that HFD impairs
spatial memory, as reflected by increased escape latency in the
Morris water maze, indicative of hippocampal dysfunction (26). In humans, elevated serum free
fatty acids (FFAs) are associated with impaired delayed recall,
particularly in individuals with reduced hippocampal volume
(27,28). High intake of SFAs has been
consistently linked to memory decline in older adults. A
longitudinal study involving >2,500 elderly participants found
that those with the highest SFA intake had more than double the
risk of developing AD compared to those with the lowest intake
(24). A meta-analysis of four
independent cohorts revealed that individuals in the highest SFA
quartile had a 39% higher risk of AD and a 105% higher risk of
all-cause dementia compared to the lowest quartile (29). Dose-response analysis indicated
that each additional 4 grams per day of SFA intake were associated
with a 15% increased risk of AD. Furthermore, excessive SFA intake
has been linked to accelerated episodic and prospective memory
loss, particularly in older women, who exhibited a 40% slower
recall rate and prolonged task completion times in
neuropsychological assessments such as the Trail Making Test
(30).
 | Table IEffects of HFD on cognitive function:
Evidence from animal and human studies. |
Table I
Effects of HFD on cognitive function:
Evidence from animal and human studies.
| Subjects | Study design | Diet used | Main findings | (Refs.) |
|---|
| 6-month-old male
C57BL/6 mice (aged model) | 1, 2 and 4-month
randomized controlled feeding (HFD vs. normal diet) | HFD: 60 kcal% fat;
normal diet: 10 kcal% fat | ↓ Object place
recognition; ↑ Tau hyperphosphorylation, microglial activation,
IL-6 in hippocampus | (16) |
| Transgenic AD mice
(tauopathy model) | 30-week HFD feeding
starting at 2 months of age | HFD: 23.5% fat;
Control diet: 4.8% fat | ↑ Depression-like
behavior; ↓ cognition; ↑ Tau pathology and metabolic disorders | (17) |
| Juvenile/adolescent
rodents (mice and rats) | Various durations
(short to long term) HFD feeding | High-fat/high-sugar
diets (varied fat content) | ↓ Spatial and
relational memory; altered neurotransmission; ↓ neurogenesis; ↑
hippocampal inflammation | (18) |
| Control and triple
transgenic Alzheimer's (3xTg-AD; triple-transgenic) mice | Long-term HFD vs.
normal diet, with chronic intranasal insulin intervention | HFD vs. normal diet
(exact fat % not specified) | ↓ Cognition; ↑
brain insulin resistance; ↓ brain mass and dentate gyrus size;
intranasal insulin improved outcomes | (19) |
| Humans (review of
multiple studies) and rodents | Review of acute and
chronic exposure studies | High-energy diets
high in fat and/or sugar (varied fat types) | ↑ Memory impairment
before weight gain; ↑ inflammation; ↓ BDNF; potential benefits from
omega-3 and curcumin supplementation | (20) |
| Healthy male adults
(22±1 years) (n=16) | Randomized
crossover design; 5 days of high-fat, low-carbohydrate diet vs.
standard diet, 2-week washout; cardiac and cognitive
assessments | High-fat (75% of
calories), low-carbohydrate diet for 5 days | ↑ Plasma free fatty
acids; ↓ cardiac PCr/ATP; ↓ attention, speed and mood | (21) |
Beyond memory, executive dysfunction is another
hallmark of HFD-induced impairment. Clinically, this manifests as
reduced cognitive flexibility and impaired decision-making, while
in rodent models, this has been demonstrated by increased error
rates in reversal learning tasks, attributed to disrupted synaptic
plasticity in the prefrontal cortex (PFC). Impulse control deficits
are also evident, as shown by decreased inhibition performance in
Go/No-Go tasks, potentially linked to downregulation of striatal
dopamine D2 receptors (31).
Learning processes are likewise compromised: Mice on
an HFD exhibit reduced exploration of novel objects, suggesting
deficits in novelty recognition, and extinction of conditioned fear
memory is delayed, likely due to amygdala-PFC circuit disruptions
that contribute to anxiety-related cognitive rigidity. These
impairments are associated with altered expression of synaptic
proteins such as synaptophysin and postsynaptic density protein 95
(PSD-95), as well as reduced levels of bone-derived neurotrophic
factor (BDNF), all of which are critical for synaptic plasticity
(32). Chronic HFD has been
shown to downregulate these proteins in the hippocampus and cortex,
impairing neuronal communication and long-term potentiation (LTP)
(33).
Taken together, current epidemiological and
experimental evidence suggest that long-term HFD, particularly
SFA-rich diets, exert a detrimental effect on multiple cognitive
domains, including memory, executive function and learning.
However, it is important to acknowledge the inherent limitations of
observational studies, including potential residual confounding and
lack of causal inference. Therefore, further well-designed
randomized controlled trials (RCTs) are necessary to validate these
associations and inform evidence-based dietary guidelines for
cognitive health promotion.
Modifiers of HFD-induced cognitive
impairment
The adverse cognitive effects of HFDs do not occur
in isolation but are significantly modulated by individual-level
factors such as age, sex, and genetic predisposition.
Age is a well-established modifier of HFD-induced
cognitive outcomes. Numerous studies have shown that older
individuals are more susceptible to the detrimental neurological
effects of chronic HFD exposure compared to younger populations.
Aging is associated with increased vulnerability to HFD-induced
neuroinflammation, oxidative stress and insulin resistance-key
mechanisms contributing to accelerated cognitive decline and
heightened dementia risk (34,35). Animal studies corroborate these
findings, demonstrating that aged rodents exhibit more severe
cognitive impairments and neuropathological changes in response to
prolonged HFD intake than younger counterparts (36-38). Epidemiological data in humans
further support these age-related interactions, indicating more
pronounced cognitive deficits among older adults consuming an
SFA-rich diet (8,39).
Sex differences also influence HFD-related cognitive
vulnerability. Both clinical and preclinical evidence suggests that
females may be more sensitive to cognitive impairments associated
with high dietary fat intake. Population-based studies report
stronger associations between HFD and cognitive decline in women
than in men (40). Similarly,
animal models show enhanced diet-induced insulin resistance,
neuroinflammation and hippocampal dysfunction in female subjects,
potentially due to hormonal fluctuations and the loss of
estrogen-mediated neuroprotection with ageing (26,41,42).
Genetic predisposition further modulates the impact
of HFD on cognitive function. The APOE-ε4 allele-an established
genetic risk factor for AD-has been shown to amplify the
neurocognitive consequences of chronic HFD exposure. Carriers of
the APOE-ε4 allele demonstrate faster cognitive decline and greater
vulnerability to HFD-related neurodegeneration than non-carriers
(43,44). Additionally, polymorphisms in
genes involved in inflammatory and metabolic pathways may
exacerbate HFD-induced disruptions in lipid metabolism, neuroimmune
responses and cerebral insulin signaling, thereby potentiating
cognitive dysfunction (45,46).
In summary, the cognitive impact of HFDs is strongly
modulated by host-specific factors such as age, sex and genetic
background. These interactions underscore the multifaceted nature
of HFD-induced cognitive impairment. A comprehensive understanding
of how HFD synergizes with individual risk factors is essential for
developing effective strategies to mitigate cognitive decline.
Potential mechanisms
HFDs, particularly those rich in SFAs, have
consistently been associated with adverse cognitive outcomes.
Evidence from both preclinical models and clinical studies
indicates that excessive SFA intake induces a cascade of
deleterious pathophysiological processes, including
microglia-mediated neuroinflammation, increased oxidative stress,
impaired insulin signaling, gut microbiota dysbiosis and disruption
of the blood-brain barrier (BBB) integrity. These interrelated
mechanisms converge to impair neuronal function and accelerate
cognitive decline (47-50).
Neuroinflammation
Neuroinflammation is recognized as a central
mechanism through which HFDs contribute to cognitive impairment.
Chronic HFD consumption activates microglia-the principal immune
effector cells in the central nervous system (CNS)-prompting a
shift from a homeostatic surveillance state toward a
pro-inflammatory M1 phenotype (46,51). Under physiological conditions,
microglia play key roles in maintaining synaptic plasticity,
clearing cellular debris, and supporting adult neurogenesis.
However, diets rich in SFAs-such as palmitic acid, lauric acid and
stearic acid-trigger aberrant microglial activation. SFAs bind to
Toll-like receptor 4 (TLR4) on microglial surfaces, activating
MyD88-dependent signaling pathways and serving as potent inducers
of nuclear factor κB and c-Jun N-terminal kinase signaling
cascades. These pathways, together with endoplasmic reticulum
stress responses, initiate and amplify pro-inflammatory signaling
(52). Consequently, activated
microglia release large quantities of inflammatory cytokines
(TNF-α, IL-1β, IL-6, C-C motif chemokine ligand 2) and reactive
oxygen species (ROS), leading to synaptic loss, neuronal injury,
and impaired hippocampal function (53,54). Furthermore, SFAs may act
synergistically with advanced glycation end products derived from
processed lipids to exacerbate neuroinflammation. These cytokines
amplify local neuroinflammatory cascades and directly disrupt
neuronal integrity by impairing synaptic transmission, reducing
synaptic plasticity, and promoting neuronal apoptosis.
Notably, HFD-induced neuroinflammation can occur
within days of dietary exposure, preceding systemic metabolic
disturbances. This inflammatory response often begins in the
mediobasal hypothalamus (MBH)-a region highly sensitive to
circulating lipid signals due to its specialized median eminence
BBB and tanycyte-mediated transport. HFDs also promote the
recruitment of bone marrow-derived myeloid cells to the MBH and
hippocampus, where they differentiate into microglia-like
pro-inflammatory cells, reinforcing the inflammatory milieu and
enhancing M1 polarization (55).
Importantly, this neuroinflammatory response occurs independently
of body weight gain, suggesting a direct impact of dietary fat on
central immune activation. Prolonged HFD exposure further
propagates glial activation to other brain regions, including the
hippocampus and prefrontal cortex, contributing to spatial memory
deficits and depression-like behaviors (16).
Prolonged HFD exposure further propagates glial
activation to other brain regions, including the hippocampus and
prefrontal cortex, contributing to spatial memory deficits and
depression-like behaviors (56-58). For instance, Sobesky et al
(59) demonstrated that mice
maintained on an HFD exhibited marked microglial activation in the
hippocampus, elevated IL-1β expression and significant spatial
memory deficits in the Morris water maze. Similarly, Spencer et
al (60) reported that
prolonged consumption of SFAs in rodents increased microglial
activation and TNF-α expression, resulting in decreased synaptic
density and impaired hippocampal-dependent learning tasks. Notably,
pharmacological inhibition of microglial activation attenuated
these cognitive impairments, highlighting a causal role for
neuroinflammation (16,51,61).
Mitochondrial dysfunction and oxidative
stress
Mitochondrial dysfunction is a pivotal mechanism by
which HFD consumption contributes to cognitive impairment, often
accompanied by energy dysregulation and enhanced oxidative stress
(Fig. 1). HFD induces a
disruption of mitochondrial dynamics, favoring pathological
fragmentation. Fatty acids enter cells via fatty acid transport
proteins and are directed toward either mitochondrial β-oxidation
(for 2-18 carbon chains) or peroxisomal metabolism (for ≥26 carbon
chains via ATP binding cassette transporters). In mitochondria,
fatty acid oxidation is carnitine-dependent and contributes to ATP
production. However, chronic exposure to high levels of saturated
SFAs impairs mitochondrial dynamics. Specifically, SFA-rich diets
markedly upregulate dynamin-related protein 1 (Drp1), particularly
its phosphorylation at Ser616, promoting Drp1 translocation from
the cytosol to the outer mitochondrial membrane, where it
oligomerizes to drive mitochondrial fission. Concurrently, the
expression of fusion-related proteins-including mitofusins 1/2 and
optic atrophy 1-is significantly reduced, impairing mitochondrial
fusion capacity. This imbalance leads to mitochondrial network
fragmentation, structural disruption and functional decline.
Notably, such metabolic impairment extends beyond the central
nervous system. Bone marrow-derived monocytes exposed to an HFD
exhibit similar mitochondrial fragmentation, lactate accumulation
and bioenergetic inefficiency, suggesting a systemic mitochondrial
reprogramming that may amplify the neuroenergetic imbalance through
peripheral-to-central metabolic cross-talk (62,63).
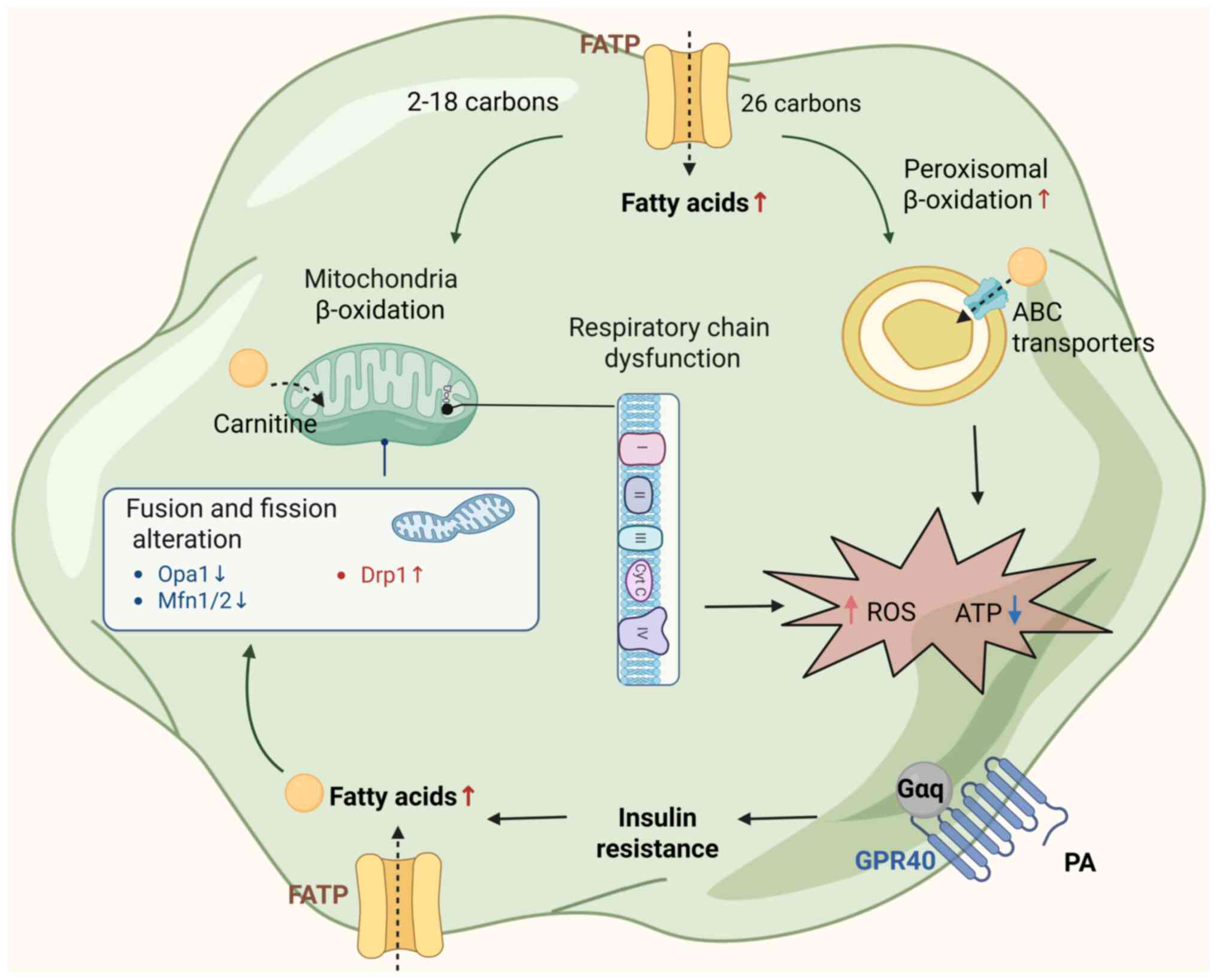 | Figure 1Mechanism of mitochondrial
dysfunction induced by HFD. HFD increases circulating FFAs, which
are taken up via FATPs and directed to mitochondrial and
peroxisomal β-oxidation. Insulin resistance promotes excessive FFA
influx, overwhelming mitochondrial capacity. Impaired mitochondrial
dynamics (↓Opa1, ↓Mfn1/2, ↑Drp1) and respiratory chain dysfunction
lead to reduced ATP production and elevated ROS levels. These
alterations contribute to cellular energy failure and oxidative
stress, exacerbating cognitive decline. ABC, ATP binding cassette;
HFD, high-fat diet; FATPs, fatty acid transport proteins; FFAs,
free fatty acids; ROS, reactive oxygen species; ATP, adenosine
triphosphate; OPA1, optic atrophy protein 1; Mfn1/2, mitofusin 1/2;
Drp1, dynamin-related protein 1; Cyt c, cytochrome c; PA,
phosphatidic acid; GPR, G protein-coupled receptor; Gαq, guanine
nucleotide-binding protein α q subunit. |
Furthermore, excessive SFAs stimulate mitochondrial
fatty acid β-oxidation, enhancing electron flux through complexes I
and III of the electron transport chain. Additionally, peroxisomal
β-oxidation does not generate ATP directly. Instead, it transfers
electrons directly to molecular oxygen. This process increases
electron leakage, leading to overproduction of ROS, including
superoxide anion (O2−), hydrogen peroxide
(H2O2) and hydroxyl radical (·OH) (9,64-67). These ROS species damage
mitochondrial DNA, respiratory enzymes and phospholipid membranes,
further exacerbating mitochondrial dysfunction (68-70). Excess ROS accumulation causes
oxidative damage via lipid peroxidation, protein oxidation and DNA
fragmentation. Lipid peroxidation products, such as malondialdehyde
(MDA) and 4-hydroxynonenal (4-HNE), are particularly toxic to
neurons. Elevated levels of MDA and 4-HNE have been detected in the
hippocampus and cortex of HFD-fed animals and are closely
associated with impairments in spatial memory, working memory and
executive function (71,72). For instance, Liu et al
(73) demonstrated that
long-term HFD exposure in mice led to significantly increased
hippocampal MDA levels, reduced antioxidant enzyme activities
[e.g., superoxide dismutase (SOD) and glutathione peroxidase], and
worsened spatial learning in the Morris water maze.
Insulin resistance
Insulin signaling is essential for maintaining
neuronal glucose metabolism, mitochondrial bioenergetics, synaptic
plasticity and neuronal survival-all of which are fundamental to
cognitive function. In the CNS, insulin binds to its receptor,
triggering the phosphorylation of insulin receptor substrates
(IRS), which in turn activates the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) pathway (45,74,75). This cascade not only promotes
neuronal glucose uptake via glucose transporters (GLUT), i.e.,
GLUT3 and GLUT4, and supports mitochondrial function, but also
plays key roles in neurogenesis, synaptic remodeling and cell
viability.
HFD exposure impairs both peripheral and central
insulin signaling, contributing to systemic insulin resistance.
Peripherally, an HFD may diminish insulin's anti-lipolytic
function, resulting in excessive FFA release from adipose tissue.
These circulating FFAs accumulate in metabolic organs-including
liver, skeletal muscle, brain and astrocytes-overloading oxidative
pathways and increasing mitochondrial and peroxisomal β-oxidation
(Fig. 1). Notably, palmitic acid
(a major SFA in HFDs) reduces GLUT1 expression at the BBB, thereby
limiting glucose delivery to the brain, diminished glucose uptake
and compromised neuronal energy homeostasis (76-78).
In the CNS, insulin resistance particularly affects
the hippocampus and prefrontal cortex-regions crucial for memory
encoding and executive function (36,45,79). Dysfunctional insulin signaling
reduces Akt activation and downregulates BDNF, contributing to
synaptic degeneration, reduced dendritic spine density and impaired
LTP, which are key substrates of cognitive performance (80,81). For instance, Arnold et al
(48) reported that HFD-fed mice
showed reduced insulin receptor activity, lower Akt phosphorylation
and impaired hippocampal synaptic plasticity, as well as spatial
learning and memory deficits. Similarly, Kothari et al
(36) demonstrated that chronic
HFD exposure suppressed BDNF expression and reduced dendritic
complexity in the hippocampus, correlating with cognitive decline.
Furthermore, HFD-induced insulin resistance may promote AD-like
neuropathology via multiple mechanisms. It activates downstream
kinases such as glycogen synthase kinase-3β, inducing pathological
tau hyperphosphorylation at Ser396/404 and facilitating
neurofibrillary tangle formation. It also suppresses
insulin-degrading enzyme, reducing Aβ clearance and accelerating
amyloid plaque accumulation. Additionally, impaired insulin
signaling inhibits the proliferation and differentiation of neural
progenitor cells, thereby limiting adult hippocampal neurogenesis
and reducing cognitive flexibility and plasticity (82).
Gut microbiota dysbiosis
The gut microbiota-comprising trillions of bacteria,
archaea, viruses and fungi-plays a pivotal role in regulating host
metabolism, immune responses and CNS function via the gut-brain
axis (83-85) (Fig. 2). Human studies have shown that
individuals consuming Western-style high-fat diets HFDs exhibit
distinct gut microbial profiles associated with impaired memory and
executive function (86). These
profiles are typically characterized by a reduction in beneficial
genera such as Bifidobacterium and Lactobacillus, and
an overrepresentation of pro-inflammatory taxa including members of
the Firmicutes, Proteobacteria and
Desulfobacterota phyla (87-90).
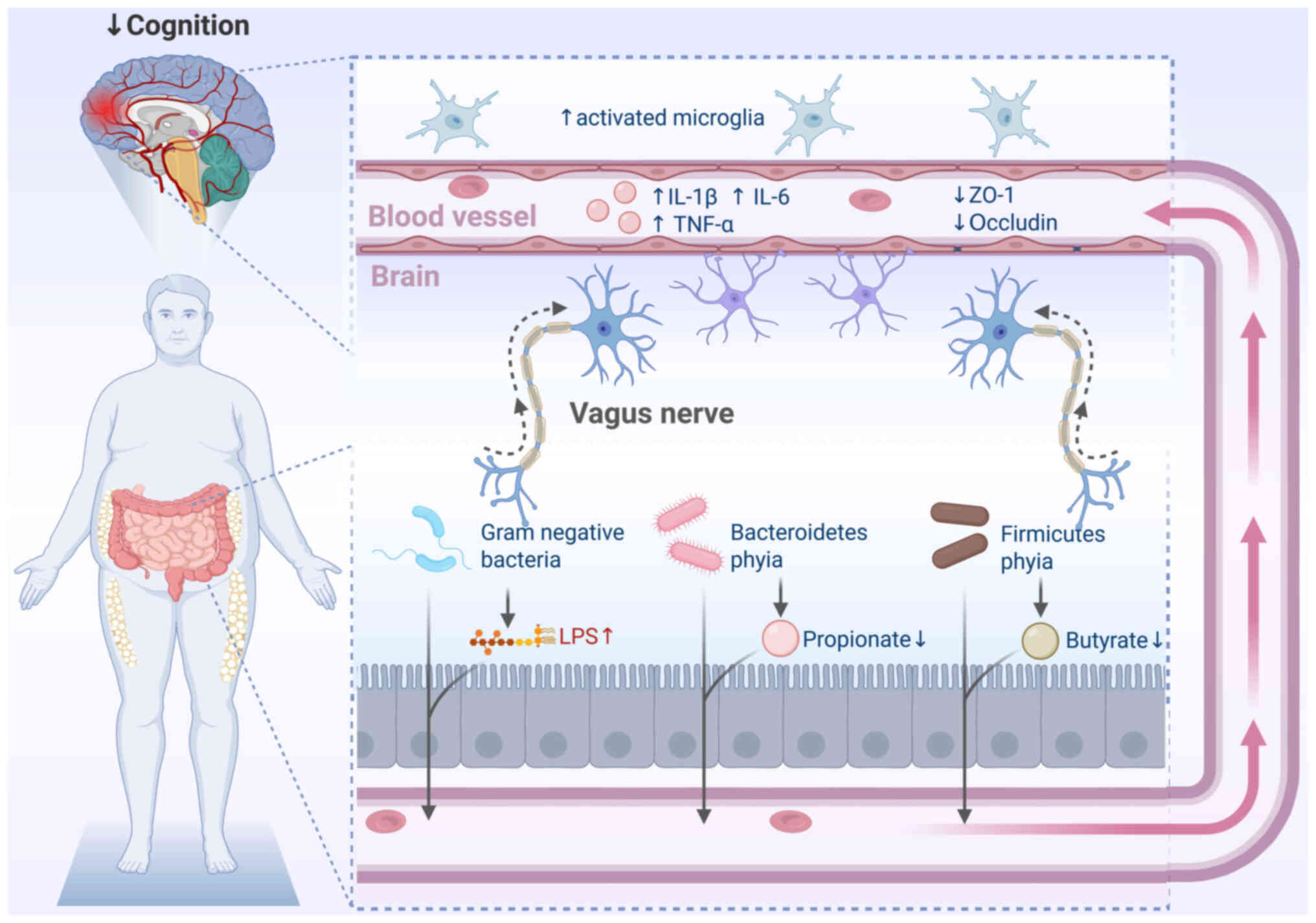 | Figure 2Gut-brain axis disruption in
HFD-induced cognitive impairment. HFD induces gut microbiota
dysbiosis, characterized by an increased
Firmicutes-to-Bacteroidetes ratio and elevated levels of
Gram-negative Proteobacteria. This leads to elevated LPS and
reduced SCFAs, such as propionate and butyrate. These changes
compromise intestinal barrier integrity, allowing microbial
products to enter circulation and activate neuroinflammatory
pathways via the vagus nerve or systemic cytokines. Consequent
microglial activation, elevated IL-1β, IL-6 and TNF-α, and reduced
tight junction proteins (ZO-1, occludin), contribute to blood-brain
barrier disruption and cognitive decline. HFD, high-fat diet; LPS,
lipopolysaccharides; SCFAs, short-chain fatty acids; IL,
interleukin; TNF, tumor necrosis factor; ZO-1, zonula
occludens-1. |
Animal models have validated these associations:
Fecal microbiota transplantation from HFD-fed mice into germ-free
recipients reproduces spatial learning deficits and
neuroinflammation (91). By
contrast, microbiota-targeted interventions-including probiotics
(Bifidobacterium, Lactobacillus), prebiotics (e.g.,
inulin, fructooligosaccharides) and polyphenol-rich diets-have
demonstrated the ability to restore gut microbial homeostasis,
enhance short-chain fatty acid (SCFA) production, suppress systemic
inflammation and improve cognitive outcomes in HFD-fed animal
(92,93).
HFD-induced dysbiosis alters gut-brain axis
communication through multiple pathways, including neural (e.g.,
vagus nerve), humoral, and immune mechanisms (94). One of the most significant
consequences is the dysregulation of microbial metabolite
production. HFD significantly reduces SCFAs such as acetate,
propionate, and butyrate-metabolites essential for maintaining gut
barrier integrity, regulating immune responses, and exerting
anti-inflammatory and neuroprotective functions. Concurrently, HFD
increases the production of harmful metabolites such as
lipopolysaccharide (LPS), trimethylamine-N-oxide and secondary bile
acids (86,95,96). Depletion of SCFAs impairs the
expression of antimicrobial peptides, particularly regenerating
islet-derived protein 3γ, further weakening the gut epithelial
barrier and increasing intestinal permeability. This allows
microbial products like LPS to enter systemic circulation, where
they activate TLR4 and trigger inflammatory cascades that elevate
circulating pro-inflammatory cytokines such as TNF-α, IL-1β and
IL-6 (97,98). These cytokines subsequently
exacerbate BBB permeability, promote microglial activation and
initiate CNS neuroinflammation (99).
Other potential mechanisms
In addition to inflammation, oxidative stress,
insulin resistance, and gut dysbiosis, HFD consumption contributes
to cognitive deterioration through several additional mechanisms,
including BBB disruption, epigenetic modifications, and
neurotransmitter imbalance.
One critical pathway involves the breakdown of BBB
integrity. Chronic HFD exposure significantly increases BBB
permeability, as evidenced by enhanced Evans blue dye extravasation
and increased infiltration of peripheral immune cells into the
brain parenchyma-findings closely correlated with impaired spatial
memory performance (100). This
compromised barrier allows peripheral inflammatory mediators, ROS
and LPS to enter the CNS, thereby promoting microglial activation,
neuroinflammation and cerebral Aβ deposition. Studies have shown
that saturated fat-enriched diets exacerbate BBB disruption and
facilitate plasma-derived Aβ accumulation in the brain, aggravating
AD-like neuropathology (101).
Additionally, oxidized cholesterol produced during high-temperature
cooking, along with excess SFAs, directly damages cerebrovascular
endothelial cells, reduces cerebral perfusion and promotes the
development of white matter hyperintensities-radiological markers
strongly linked to executive dysfunction and cognitive decline
(102).
Epigenetic dysregulation is another significant
contributor. Chronic HFD intake has been shown to reduce histone
acetylation and increase DNA methylation at the promoter region of
BDNF, leading to reduced BDNF expression. This downregulation
impairs synaptic plasticity and neurogenesis-both essential for
learning and memory (103). HFD
also disrupts the expression of core circadian clock genes such as
Bmal1 and Clock, causing neuronal metabolic
desynchronization and cognitive dysfunction. These epigenetic
changes further perpetuate neuroinflammation and oxidative stress,
creating a maladaptive neurochemical environment that exacerbates
cognitive decline (104).
HFD also induces widespread alterations in
neurotransmitter homeostasis. Animal studies have reported elevated
dopamine release alongside disruptions in multiple neurotransmitter
systems, including glutamate, acetylcholine, γ-aminobutyric acid,
serotonin and dopamine. Impaired glutamate reuptake results in
extracellular glutamate accumulation, contributing to
excitotoxicity, neuronal injury and impaired synaptic plasticity
(105). Cholinergic
dysfunction, characterized by reduced synthesis and release of
acetylcholine, impairs attention and learning capabilities
(106). Furthermore, an HFD
decreases the expression of neurotensin, a neuropeptide involved in
dopaminergic reward regulation, further disrupting reward pathways
and contributing to compulsive overeating, metabolic dysregulation
and cognitive deficits.
Intervention strategies
Given the multifactorial mechanisms underlying
HFD-induced cognitive impairment, a variety of intervention
strategies have been explored to improve cognitive function by
targeting different pathological pathways (Table II) (22,88,93,107-120).
 | Table IINutritional and therapeutic
strategies to combat cognitive decline. |
Table II
Nutritional and therapeutic
strategies to combat cognitive decline.
| Intervention
type | Main
intake/substances or strains | Key components and
mechanisms | Cognitive
improvement and research findings | (Refs.) |
|---|
| MIND diet | Leafy greens (e.g.,
spinach, kale)
Berries (e.g., blueberries, strawberries)
Nuts
Fish
Whole grains
Poultry
Legumes
Olive oil | ↑ Antioxidants
(vitamins E, C, polyphenols, lavonoids)
↑ Omega-3 fatty acids
↑ Dietary fiber
↓ Inflammation
Promotes antioxidant and anti-inflammatory protection of brain
cells | ↓ Alzheimer's
disease risk (up to 53%)
↓ Cognitive decline rate (equivalent to being cognitively '7.5
years younger')
↑ Memory, executive function and language abilities | (22,107,108) |
| Mediterranean
diet | Fresh fruits and
vegetables
Whole grains
Fish and seafood
Olive oil (main fat source)
Nuts
Moderate red wine
Legumes | ↑ Monounsaturated
fatty acids (olive oil)
↑ Omega-3 fatty acids
↑ Antioxidants (vitamins E, C, polyphenols)
↓ Inflammation and oxidative stress
Improves cerebrovascular health | ↓ Mild cognitive
impairment and dementia risk
↑ Memory and language function
↑ Brain vascular health
↓ Neuroinflammation and oxidative stress | (109,110) |
| Antidiabetic
drugs | Metformin | Metformin: ↑ AMPK
activity, improves energy metabolism, modulates gut microbiota, ↓
inflammation and oxidative stress | ↓ Cognitive decline
rate and significantly reduced dementia risk
↑ Memory and language functions | (111,112) |
| Intranasal
insulin | Intranasal insulin:
↑ Brain insulin receptor activation, improves neuronal glucose
uptake and synaptic plasticity | | (113,114) |
| Anti-inflammatory
drugs | Various
anti-inflammatory agents (research ongoing) | ↓ Release of
pro-inflammatory cytokines in the brain
↓ Oxidative stress and neuronal damage
↓ Neuroinflammation | ↓ Rate of cognitive
decline, especially in inflammation-related cognitive
impairment
Still under clinical research | (115,116) |
| Probiotics | Lactobacillus
rhamnosus | Modulates gut
microbiota balance
↓ Pro-inflammatory cytokines,
↓ neuroinflammation
Influences neurotransmitters (dopamine, serotonin) and brain
signaling | ↓ Beta-amyloid
deposition
↑ Spatial memory
↑ Attention and language learning abilities (clinical trials) | (93,117,118) |
| Bifidobacterium
longum | ↑ Gut barrier
function
↑ Short-chain fatty acid production, anti-inflammatory
effects
Regulates neuroendocrine and immune responses | ↑ Cognitive
function (especially combined with omega-3
supplementation)
-↑ Mood and sleep quality, indirectly promoting cognitive
health | (88,119,120) |
Dietary interventions
Dietary modification is among the most accessible
and effective strategies for mitigating cognitive impairment
associated with chronic HFD exposure. Nutritional patterns
emphasizing balanced macronutrient intake-such as the Mediterranean
diet and DASH diet-have been consistently linked to reduced risks
of cognitive decline and dementia (121).
The Mediterranean diet is characterized by high
consumption of fruits, vegetables, whole grains, legumes, fish,
nuts and olive oil, with moderate intake of dairy products and red
wine. Epidemiological and interventional studies have demonstrated
significant cognitive benefits associated with this dietary pattern
(122). For instance, the
large-scale PREDIMED RCT reported that older adults adhering to a
Mediterranean diet supplemented with either extra-virgin olive oil
or mixed nuts exhibited substantial improvements in memory and
executive function compared to those following a low-fat control
diet (123). Similarly, the
DASH diet-emphasizing fruits, vegetables, lean protein sources such
as skinless poultry, fish and legumes, and low-fat dairy products
while limiting saturated fat intake-has been shown to benefit
cognitive function (108,124,125). Older adults adhering to the
DASH diet demonstrate superior performance on executive function
and memory tasks relative to control groups (108).
These neuroprotective effects are largely attributed
to high intake of polyunsaturated fatty acids (PUFAs), particularly
eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which
enhance neuronal membrane fluidity, reduce neuroinflammation,
promote neurogenesis and support synaptic plasticity (109,126-128). Observational studies have
consistently linked greater omega-3 PUFA consumption, particularly
from fatty fish and nuts, with improved cognitive outcomes and a
reduced risk of dementia. These findings are corroborated by
clinical trials demonstrating that omega-3 supplementation improves
memory and executive performance in older adults (129-131). Likewise, monounsaturated fatty
acids, such as oleic acid-abundant in olive oil-have shown
cognitive benefits by reducing oxidative stress, improving brain
insulin sensitivity and enhancing cerebrovascular function
(123).
Meal timing also plays a pivotal role in metabolic
regulation and cognitive health. Time-restricted eating, which
confines caloric intake to defined daily windows, has been shown to
restore circadian rhythmicity by normalizing clock gene expression
[e.g., brain and muscle ARNT-like 1 (Bmal1)] and reducing
hippocampal oxidative stress in HFD-fed models (132,133). Additionally, dietary
supplementation with antioxidants-including vitamins E and C, and
polyphenols, such as resveratrol, quercetin and curcumin-has
demonstrated efficacy in attenuating oxidative stress, preserving
mitochondrial function and improving cognitive outcomes.
Resveratrol, in particular, enhances mitochondrial biogenesis,
lowers oxidative burden and maintains synaptic integrity in
HFD-exposed rodents, ultimately improving learning and memory
performance (134). Similarly,
curcumin has been shown to reduce lipid peroxidation, normalize
antioxidant enzyme activity and mitigate memory deficits in
diet-induced obesity models (135).
Importantly, dietary interventions achieve greater
efficacy when combined with structured physical exercise. Evidence
from both preclinical and clinical studies suggests that
synergistic effects arise from this integration: While diet
optimizes nutrient intake, reduces oxidative stress and restores
metabolic balance, exercise further enhances neurotrophic support,
insulin sensitivity and cerebral blood flow (136,137). For instance, overweight
individuals adhering to Mediterranean or DASH dietary patterns
alongside regular aerobic training exhibited more pronounced
improvements in executive function and memory performance compared
to those receiving either intervention alone (138).
Exercise interventions
Regular physical exercise represents a potent
intervention to counteract cognitive decline associated with
chronic HFD exposure, particularly in the context of HFD-induced
insulin resistance (139,140).
In preclinical studies, voluntary aerobic exercise
markedly increases BDNF expression and promotes hippocampal
neurogenesis, thereby reversing HFD-induced deficits in memory
performance (141). Exercise
also upregulates other neurotrophic mediators, including nerve
growth factor and insulin-like growth factor-1, which collectively
enhance neuronal survival, dendritic branching and synaptogenesis
(142). For instance, rats
undergoing aerobic training after chronic HFD exposure exhibited
increased expression of synaptic proteins, such as synaptophysin
and PSD-95, correlating with improved spatial learning and memory
performance (143).
These animal findings are mirrored in clinical
studies. In older adults, aerobic exercise has been shown to
increase hippocampal volume, elevate serum BDNF levels and enhance
spatial memory, supporting the translational relevance of animal
data (139). Furthermore,
overweight or obese individuals engaging in structured aerobic
training-particularly when combined with dietary
modifications-exhibited significant improvements in executive
function, processing speed and attentional control when compared to
sedentary controls (144).
From a metabolic perspective, aerobic exercise
improves systemic and cerebral glucose homeostasis by upregulating
GLUT4 expression in skeletal muscle and brain tissues (145). In HFD-fed rodents, treadmill
exercise restored hippocampal insulin signaling pathways, including
IRS-1 phosphorylation and PI3K/Akt activity, thereby reversing
HFD-induced cognitive decline (146). Exercise also exerts potent
anti-inflammatory effects in the CNS. It reduces the expression of
pro-inflammatory cytokines such as IL-1β and TNF-α, and attenuates
microglial activation within the hippocampus and cortex. For
instance, in AD models, treadmill training ameliorated memory
deficits by downregulating IL-1β and phosphodiesterase-5 expression
and restoring phosphorylated cAMP response element-binding protein
signaling (140,147). Similarly, voluntary wheel
running reversed HFD-induced microglial activation and rescued
memory consolidation processes (148). In addition, regular physical
activity combats oxidative stress by enhancing the activity of
endogenous antioxidant enzymes-including SOD, catalase and
glutathione peroxidase-while preserving mitochondrial integrity and
reducing ROS accumulation in brain tissue (149).
Microbiota-targeted interventions
Microbiota-targeted therapies-including probiotics,
prebiotics and next-generation bacterial strains-have emerged as
promising strategies to modulate the gut-brain axis and ameliorate
cognitive impairment associated with HFD exposure.
Probiotics, defined as live beneficial
microorganisms (e.g., Lactobacillus and
Bifidobacterium species), and prebiotics, which are
non-digestible fibers such as inulin and fructooligosaccharides
(FOS) that selectively promote the growth of commensal microbes,
have demonstrated consistent neuroprotective effects in preclinical
models. For example, oral administration of Lactobacillus
plantarum in HFD-fed mice restored gut microbial diversity,
reduced systemic and hippocampal inflammation, upregulated synaptic
proteins such as synaptophysin and PSD-95, and reversed deficits in
learning and memory (117).
Similarly, supplementation with Bifidobacterium-containing
probiotic formulations normalized microbial composition, enhanced
hippocampal BDNF expression and alleviated HFD-induced spatial
memory deficits and anxiety-like behaviors (150).
Clinical trials further support the cognitive
benefits of microbiota modulation. In an RCT, elderly individuals
with MCI receiving a multispecies probiotic mixture (L.
acidophilus, L. casei, B. bifidum) exhibited significant
improvements in cognitive performance, reduced circulating
proinflammatory cytokines and enhanced antioxidant enzyme activity
compared to placebo (151).
Similarly, prebiotic interventions with inulin or FOS have been
shown to increase the abundance of SCFA-producing bacteria,
strengthen intestinal barrier integrity, suppress systemic
inflammation and subsequently improve cognitive outcomes in
populations with metabolic syndrome or cognitive decline.
Pharmacological interventions
Pharmacological interventions targeting HFD-induced
cognitive impairment primarily aim to modulate neuroinflammation,
oxidative stress, insulin resistance, and disrupted neuroplasticity
signaling. A range of agents-including anti-inflammatory drugs,
insulin sensitizers, and neurotrophic modulators-have demonstrated
cognitive benefits in both preclinical and clinical studies.
Anti-inflammatory agents such as nonsteroidal
anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors
have shown promise in preclinical models. For instance, celecoxib
effectively attenuates microglial activation, reduces
pro-inflammatory cytokines including IL-1β and TNF-α, and improves
hippocampal-dependent memory performance in HFD-fed rodents
(102,152). Minocycline, a tetracycline
derivative with central anti-inflammatory and neuroprotective
properties, similarly reduces hippocampal inflammation, preserves
synaptic architecture, and enhances spatial learning and memory
(153,154).
Insulin sensitizers, including metformin and
thiazolidinediones (e.g., rosiglitazone, pioglitazone), exert
neuroprotective effects by restoring central insulin signaling,
reducing neuroinflammation and mitigating oxidative stress
(155). Metformin improves
hippocampal IRS phosphorylation and downstream PI3K/Akt signaling,
enhancing synaptic plasticity and memory function in metabolic
dysfunction models (156,157). Peroxisome
proliferator-activated receptor γ agonists further promote adult
neurogenesis and synaptic remodeling, contributing to cognitive
improvements in HFD-fed animals (158). In addition, glucagon-like
peptide-1 receptor agonists (e.g., liraglutide, exenatide)
represent a novel class of therapeutics with dual metabolic and
neuroprotective actions (159).
These agents enhance insulin signaling in the brain, reduce
hippocampal inflammation and oxidative stress, stimulate
neurogenesis, and improve learning and memory performance in
HFD-exposed rodents. Clinical trials in obese and diabetic patients
have also demonstrated cognitive benefits, suggesting translational
potential for diet-associated neurocognitive disorders (160,161). Neuroplasticity-targeted
therapies offer additional avenues. Pharmacological agents that
enhance BDNF signaling-such as selective serotonin reuptake
inhibitors (SSRIs), PDE inhibitors and histone deacetylase
inhibitors-have been shown to restore synaptic plasticity and
cognitive function. For instance, fluoxetine, a commonly used SSRI,
upregulates hippocampal BDNF expression and reverses cognitive
deficits in HFD-fed rodents (162,163).
Limitations and future perspectives
Limitations
Despite substantial evidence linking chronic HFD
intake to cognitive impairment, several limitations remain in
current research, which hinder the establishment of definitive
causal relationships (26,55).
A major limitation lies in the predominant reliance
on observational epidemiological studies and preclinical animal
models. While these approaches have elucidated critical mechanistic
insights, their applicability to human physiology remains limited
due to species-specific metabolic, neuroimmune and behavioral
differences (164). Notably,
numerous rodent studies utilize acute or supra-physiological doses
of dietary fat, which fail to recapitulate the chronic, moderate
exposures and complex eating patterns typical of human dietary
habits-including meal timing, social eating behaviors and food
processing. Furthermore, substantial heterogeneity across
studies-including variations in dietary fat composition (e.g.,
saturated vs. unsaturated), intervention durations, cognitive
assessment tools and demographic characteristics complicates
cross-study comparisons and undermines the generalizability of
findings (165).
The bidirectional relationship between metabolic
dysfunction and gut microbiota dysbiosis poses another unresolved
challenge. It remains elusive whether microbial alterations serve
as a causal antecedent or secondary consequence of HFD-induced
metabolic changes (16).
Similarly, while microglial activation is frequently implicated in
cognitive decline, conflicting data persist. For instance,
inhibition of microglial activation via Triggering Receptor
Expressed on Myeloid Cells 2 (TREM2) deletion has been shown to
mitigate cognitive deficits in HFD-fed mice, suggesting a
pathogenic role (166).
Conversely, other models of neuroinflammation do not consistently
produce cognitive impairments, indicating that additional
mechanisms-such as BBB disruption, mitochondrial dysfunction and
oxidative stress-likely contribute to cognitive decline in a
context-dependent manner (167).
Genetic and epigenetic heterogeneity in human
populations adds further complexity. Polymorphisms in genes such as
TLR4, or variations in neuroimmune and metabolic regulatory
pathways, may modulate individual vulnerability to HFD-related
cognitive impairment and influence response to microbiota-targeted
interventions (168).
Furthermore, neuromodulatory strategies-such as vagus nerve
stimulation-while efficacious in preclinical settings, have shown
inconsistent cognitive benefits in human trials, underscoring the
challenges of translational fidelity (169).
Future perspectives
Given the multifaceted and interconnected mechanisms
by which chronic HFD contributes to cognitive decline, future
research must adopt a more integrative and mechanistically grounded
approach to uncover effective preventive and therapeutic
strategies. While preclinical studies have yielded extensive
insights, their translational relevance remains limited (121,170,171). Robust clinical validation
through well-powered, longitudinal RCTs is urgently needed to
establish causal links between dietary fat subtypes and cognitive
outcomes, particularly across genetically and metabolically diverse
populations. Such trials should incorporate long-term exposure
assessments and harmonized cognitive endpoints to improve
comparability and external validity (172).
At the mechanistic level, the field is poised to
benefit from emerging tools such as single-cell multi-omics,
spatial transcriptomics and metabolic flux analysis. These
approaches allow for high-resolution dissection of how HFD alters
brain homeostasis at cellular and molecular levels-uncovering how
neuronal, glial, immune and vascular cell types interact in
spatially distinct and temporally dynamic ways (173). Integrating these datasets may
clarify unresolved questions, such as whether microbiota dysbiosis
precedes metabolic dysfunction or merely reflects it, and how the
gut-brain axis mediates diet-induced neuroinflammation and synaptic
degeneration.
Importantly, the pronounced heterogeneity in
individual responses to HFD highlights the necessity of
personalized interventions. Moving beyond 'one-size-fits-all'
recommendations, future strategies should be tailored to individual
characteristics such as APOE genotype, insulin sensitivity,
microbiome enterotype and sex-specific vulnerabilities. Precision
nutrition-guided by validated biomarkers such as plasma ceramides
or microbial metabolites-holds particular promise for early risk
stratification and targeted intervention (174). Furthermore, there is growing
recognition that single-modality interventions are unlikely to
fully reverse or prevent HFD-induced cognitive dysfunction. Future
studies should prioritize multimodal strategies that combine
dietary modification, structured physical activity, pharmacologic
agents targeting neuroinflammation or insulin resistance,
microbiota-targeted therapies (e.g., Akkermansia muciniphila
supplementation) and neuromodulation techniques (e.g., transcranial
direct current stimulation). Such integrative approaches are better
positioned to tackle the complex neurobiological cascades
underlying metabolic-cognitive deterioration.
Conclusions
HFD consumption accelerates cognitive decline
through converging mechanisms such as neuroinflammation, oxidative
stress, insulin resistance, gut dysbiosis and synaptic dysfunction.
These effects are further modulated by genetic and demographic
factors, including APOE genotype, age and sex. Current evidence
supports the efficacy of integrative interventions-such as
Mediterranean-style diets, physical exercise, metabolic modulators
and microbiota-targeted therapies-in mitigating HFD-induced
neurodegeneration. Future research should focus on validating these
strategies in diverse populations via large-scale trials and
leveraging multi-omics technologies to guide precision
prevention.
Availability of data and materials
Not applicable.
Authors' contributions
MY was involved in the conceptualization of the
study, drafted the manuscript, writing-review & editing and
visualization. FW contributed to the conceptualization of the study
and writing-review & editing. FX, XH and HW were responsible
for writing-review & editing. MY and FW performed the
literature search and selection. LZ and HZ contributed to
writing-review & editing acquired funding and supervised the
study. All authors have read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 82505742 and 82274663); the Hubei
Provincial Administration of Traditional Chinese Medicine Research
Fund (grant no. ZY2025M053); the Joint Foundation Project of Hubei
Provincial Natural Science Foundation (grant no. 2025AFD527) and
Jianghan University Fundamental Research Program (grant no.
2024JCYJ13).
References
|
1
|
Roberts R and Knopman DS: Classification
and epidemiology of MCI. Clin Geriatr Med. 29:753–772. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wimo A, Guerchet M, Ali GC, Wu YT, Prina
AM, Winblad B, Jönsson L, Liu Z and Prince M: The worldwide costs
of dementia 2015 and comparisons with 2010. Alzheimers Dement.
13:1–7. 2017. View Article : Google Scholar
|
|
3
|
Yi W, Chen F, Yuan M, Wang C, Wang S, Wen
J, Zou Q, Pu Y and Cai Z: High-fat diet induces cognitive
impairment through repression of SIRT1/AMPK-mediated autophagy. Exp
Neurol. 371:1145912024. View Article : Google Scholar
|
|
4
|
GBD 2019 Dementia Forecasting
Collaborators: Estimation of the global prevalence of dementia in
2019 and forecasted prevalence in 2050: An analysis for the global
burden of disease study 2019. Lancet Public Health. 7:e105–e125.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frazier K, Kambal A, Zale EA, Pierre JF,
Hubert N, Miyoshi S, Miyoshi J, Ringus DL, Harris D, Yang K, et al:
High-fat diet disrupts REG3γ and gut microbial rhythms promoting
metabolic dysfunction. Cell Host Microbe. 30:809–823. 2022.
View Article : Google Scholar
|
|
6
|
Wang X, Song R, Clinchamps M and Dutheil
F: New insights into high-fat diet with chronic diseases.
Nutrients. 15:40312023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones-Muhammad M and Warrington JP: When
high-fat diet plus hypertension does not equal vascular
dysfunction. Am J Physiol Heart Circ Physiol. 321:H128–H130. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao GY, Li M, Han L, Tayie F, Yao SS,
Huang Z, Ai P, Liu YZ, Hu YH and Xu B: Dietary fat intake and
cognitive function among older populations: A systematic review and
meta-analysis. J Prev Alzheimers Dis. 6:204–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song M, Bai Y and Song F: High-fat diet
and neuroinflammation: The role of mitochondria. Pharmacol Res.
212:1076152025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roberts RO, Roberts LA, Geda YE, Cha RH,
Pankratz VS, O'Connor HM, Knopman DS and Petersen RC: Relative
intake of macronutrients impacts risk of mild cognitive impairment
or dementia. J Alzheimers Dis. 32:329–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valentin-Escalera J, Leclerc M and Calon
F: High-Fat diets in animal models of Alzheimer's disease: How can
eating too much fat increase Alzheimer's disease Risk? J Alzheimers
Dis. 97:977–1005. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoscheidt S, Sanderlin AH, Baker LD, Jung
Y, Lockhart S, Kellar D, Whitlow CT, Hanson AJ, Friedman S,
Register T, et al: Mediterranean and western diet effects on
Alzheimer's disease biomarkers, cerebral perfusion, and cognition
in mid-life: A randomized trial. Alzheimers Dement. 18:457–468.
2022. View Article : Google Scholar
|
|
13
|
Yanai H: Effects of N-3 polyunsaturated
fatty acids on dementia. J Clin Med Res. 9:1–9. 2017. View Article : Google Scholar
|
|
14
|
Román GC, Jackson RE, Gadhia R, Román AN
and Reis J: Mediterranean diet: The role of long-chain ω-3 fatty
acids in fish; polyphenols in fruits, vegetables, cereals, coffee,
tea, cacao and wine; probiotics and vitamins in prevention of
stroke, age-related cognitive decline, and Alzheimer disease. Rev
Neurol (Paris). 175:724–741. 2019. View Article : Google Scholar
|
|
15
|
Di Meco A and Praticò D: Early-life
exposure to high-fat diet influences brain health in aging mice.
Aging Cell. 18:e130402019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Paula GC, Brunetta HS, Engel DF, Gaspar
JM, Velloso LA, Engblom D, de Oliveira J and de Bem AF: Hippocampal
function is impaired by a short-term high-fat diet in mice:
Increased blood-brain barrier permeability and neuroinflammation as
triggering events. Front Neurosci. 15:7341582021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong J, Deng I, Kelliny S, Lin L,
Bobrovskaya L and Zhou X: Long term high fat diet induces metabolic
disorders and aggravates behavioral disorders and cognitive
deficits in MAPT P301L transgenic mice. Metab Brain Dis.
37:1941–1957. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boitard C, Cavaroc A, Sauvant J, Aubert A,
Castanon N, Layé S and Ferreira G: Impairment of
hippocampal-dependent memory induced by juvenile high-fat diet
intake is associated with enhanced hippocampal inflammation in
rats. Brain Behav Immun. 40:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanguinetti E, Guzzardi MA, Panetta D,
Tripodi M, De Sena V, Quaglierini M, Burchielli S, Salvadori PA and
Iozzo P: Combined effect of fatty diet and cognitive decline on
brain metabolism, food intake, body weight, and counteraction by
intranasal insulin therapy in 3xTg mice. Front Cell Neurosci.
13:1882019. View Article : Google Scholar
|
|
20
|
Attuquayefio T, Stevenson RJ, Boakes RA,
Oaten MJ, Yeomans MR, Mahmut M and Francis HM: A high-fat
high-sugar diet predicts poorer hippocampal-related memory and a
reduced ability to suppress wanting under satiety. J Exp Psychol
Anim Learn Cogn. 42:415–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holloway CJ, Cochlin LE, Emmanuel Y,
Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight
NS, Saxby BK, et al: A high-fat diet impairs cardiac high-energy
phosphate metabolism and cognitive function in healthy human
subjects. Am J Clin Nutr. 93:748–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris MC, Tangney CC, Wang Y, Sacks FM,
Barnes LL, Bennett DA and Aggarwal NT: MIND diet slows cognitive
decline with aging. Alzheimers Dement. 11:1015–1022. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morris MC, Evans DA, Bienias JL, Tangney
CC, Bennett DA, Aggarwal N, Schneider J and Wilson RS: Dietary fats
and the risk of incident Alzheimer disease. Arch Neurol.
60:194–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morris MC, Evans DA, Bienias JL, Tangney
CC and Wilson RS: Dietary fat intake and 6-year cognitive change in
an older biracial community population. Neurology. 62:1573–1579.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galloway S, Takechi R, Nesbit M,
Pallebage-Gamarallage MM, Lam V and Mamo JCL: The differential
effects of fatty acids on enterocytic abundance of amyloid-beta.
Lipids Health Dis. 18:2092019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Evans AK, Saw NL, Woods CE, Vidano LM,
Blumenfeld SE, Lam RK, Chu EK, Reading C and Shamloo M: Impact of
high-fat diet on cognitive behavior and central and systemic
inflammation with aging and sex differences in mice. Brain Behav
Immun. 118:334–354. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boraxbekk CJ, Stomby A, Ryberg M, Lindahl
B, Larsson C, Nyberg L and Olsson T: diet-induced weight loss
alters functional brain responses during an episodic memory task.
Obes Facts. 8:261–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao X, Yang C, Jia X, Yu Z, Wang C, Zhao
J, Chen Y, Xie B, Zhuang H, Sun C, et al: High-fat diet consumption
promotes adolescent neurobehavioral abnormalities and hippocampal
structural alterations via microglial overactivation accompanied by
an elevated serum free fatty acid concentration. Brain Behav Immun.
119:236–250. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ruan Y, Tang J, Guo X, Li K and Li D:
Dietary fat intake and risk of Alzheimer's disease and dementia: A
meta-analysis of cohort studies. Curr Alzheimer Res. 15:869–876.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You SC, Geschwind MD, Sha SJ, Apple A,
Satris G, Wood KA, Johnson ET, Gooblar J, Feuerstein JS, Finkbeiner
S, et al: Executive functions in premanifest Huntington's disease.
Mov Disord. 29:405–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klaus K, Vaht M, Pennington K and Harro J:
Interactive effects of DRD2 rs6277 polymorphism, environment and
sex on impulsivity in a population-representative study. Behav
Brain Res. 403:1131312021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nieto-Estévez V, Defterali Ç and Vicario
C: Distinct effects of BDNF and NT-3 on the dendrites and
presynaptic boutons of developing olfactory bulb GABAergic
interneurons in vitro. Cell Mol Neurobiol. 42:1399–1417. 2022.
View Article : Google Scholar
|
|
33
|
McLean FH, Grant C, Morris AC, Horgan GW,
Polanski AJ, Allan K, Campbell FM, Langston RF and Williams LM:
Rapid and reversible impairment of episodic memory by a high-fat
diet in mice. Sci Rep. 8:119762018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Peng Y, Deng W, Xiang Q, Zhang W
and Liu M: Association between dietary inflammatory index and
cognitive impairment among American elderly: A cross-sectional
study. Front Aging Neurosci. 16:13718732024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles
CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T,
Galvan V, Ballabh P, et al: Obesity in aging exacerbates
neuroinflammation, dysregulating synaptic function-related genes
and altering eicosanoid synthesis in the mouse hippocampus:
Potential role in impaired synaptic plasticity and cognitive
decline. J Gerontol A Biol Sci Med Sci. 74:290–298. 2019.
View Article : Google Scholar :
|
|
36
|
Kothari V, Luo Y, Tornabene T, O'Neill AM,
Greene MW, Geetha T and Babu JR: High fat diet induces brain
insulin resistance and cognitive impairment in mice. Biochim
Biophys Acta Mol Basis Dis. 1863:499–508. 2017. View Article : Google Scholar
|
|
37
|
Julien C, Tremblay C, Phivilay A,
Berthiaume L, Emond V, Julien P and Calon F: High-fat diet
aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse
model. Neurobiol Aging. 31:1516–1531. 2010. View Article : Google Scholar
|
|
38
|
Henn RE, Elzinga SE, Glass E, Parent R,
Guo K, Allouch AM, Mendelson FE, Hayes J, Webber-Davis I, Murphy
GG, et al: Obesity-induced neuroinflammation and cognitive
impairment in young adult versus middle-aged mice. Immun Ageing.
19:672022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morris MC, Evans DA, Tangney CC, Bienias
JL, Schneider JA, Wilson RS and Scherr PA: Dietary copper and high
saturated and trans fat intakes associated with cognitive decline.
Arch Neurol. 63:1085–1088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chatterjee S, Peters SA, Woodward M,
Arango SM, Batty GD, Beckett N, Beiser A, Borenstein AR, Crane PK,
Haan M, et al: Type 2 diabetes as a risk factor for dementia in
women compared with men: A pooled analysis of 2.3 million people
comprising more than 100,000 cases of dementia. Diabetes Care.
39:300–307. 2016. View Article : Google Scholar :
|
|
41
|
Sanz-Martos AB, Roca M, Plaza A, Merino B,
Ruiz-Gayo M and Olmo ND: Long-term saturated fat-enriched diets
impair hippocampal learning and memory processes in a sex-dependent
manner. Neuropharmacology. 259:1101082024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Muscat SM, Butler MJ, Mackey-Alfonso SE
and Barrientos RM: Young adult and aged female rats are vulnerable
to amygdala-dependent, but not hippocampus-dependent, memory
impairment following short-term high-fat diet. Brain Res Bull.
195:145–156. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Armstrong NM, An Y, Beason-Held L, Doshi
J, Erus G, Ferrucci L, Davatzikos C and Resnick SM: Predictors of
neurodegeneration differ between cognitively normal and
subsequently impaired older adults. Neurobiol Aging. 75:178–186.
2019. View Article : Google Scholar :
|
|
44
|
Laitinen MH, Ngandu T, Rovio S, Helkala
EL, Uusitalo U, Viitanen M, Nissinen A, Tuomilehto J, Soininen H
and Kivipelto M: Fat intake at midlife and risk of dementia and
Alzheimer's disease: A population-based study. Dement Geriatr Cogn
Disord. 22:2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kleinridders A, Ferris HA, Cai W and Kahn
CR: Insulin action in brain regulates systemic metabolism and brain
function. Diabetes. 63:2232–2243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martiskainen H, Helisalmi S, Viswanathan
J, Kurki M, Hall A, Herukka SK, Sarajärvi T, Natunen T, Kurkinen
KM, Huovinen J, et al: Effects of Alzheimer's disease-associated
risk loci on cerebrospinal fluid biomarkers and disease
progression: A polygenic risk score approach. J Alzheimers Dis.
43:565–573. 2015. View Article : Google Scholar
|
|
47
|
Fan L, Borenstein AR, Wang S, Nho K, Zhu
X, Wen W, Huang X, Mortimer JA, Shrubsole MJ and Dai Q; Alzheimer's
Disease Neuroimaging Initiative: Associations of circulating
saturated long-chain fatty acids with risk of mild cognitive
impairment and Alzheimer's disease in the Alzheimer's disease
neuroimaging initiative (ADNI) cohort. EBioMedicine. 97:1048182023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arnold SE, Lucki I, Brookshire BR, Carlson
GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF and Kim
SF: High fat diet produces brain insulin resistance,
synaptodendritic abnormalities and altered behavior in mice.
Neurobiol Dis. 67:79–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li C, Shi L, Wang Y, Peng C, Wu L, Zhang Y
and Du Z: High-fat diet exacerbates lead-induced blood-brain
barrier disruption by disrupting tight junction integrity. Environ
Toxicol. 36:1412–1421. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Meza-Miranda ER, Camargo A, Rangel-Zuñiga
OA, Delgado-Lista J, Garcia-Rios A, Perez-Martinez P, Tasset-Cuevas
I, Tunez I, Tinahones FJ, Perez-Jimenez F and Lopez-Miranda J:
Postprandial oxidative stress is modulated by dietary fat in
adipose tissue from elderly people. Age (Dordr). 36:507–517. 2014.
View Article : Google Scholar
|
|
51
|
Valdearcos M, Douglass JD, Robblee MM,
Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres
BA, Thaler JP and Koliwad SK: Microglial inflammatory signaling
orchestrates the hypothalamic immune response to dietary excess and
mediates obesity susceptibility. Cell Metab. 26:185–197. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yanguas-Casás N, Crespo-Castrillo A, de
Ceballos ML, Chowen JA, Azcoitia I, Arevalo MA and Garcia-Segura
LM: Sex differences in the phagocytic and migratory activity of
microglia and their impairment by palmitic acid. Glia. 66:522–537.
2018. View Article : Google Scholar
|
|
53
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar
|
|
54
|
Ransohoff RM: How neuroinflammation
contributes to neurodegeneration. Science. 353:777–783. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liang Z, Gong X, Ye R, Zhao Y, Yu J, Zhao
Y and Bao J: Long-Term high-fat diet consumption induces cognitive
decline accompanied by tau hyper-phosphorylation and microglial
activation in aging. Nutrients. 15:2502023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kipnis J, Cohen H, Cardon M, Ziv Y and
Schwartz M: T cell deficiency leads to cognitive dysfunction:
Implications for therapeutic vaccination for schizophrenia and
other psychiatric conditions. Proc Natl Acad Sci USA.
101:8180–8185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yirmiya R and Goshen I: Immune modulation
of learning, memory, neural plasticity and neurogenesis. Brain
Behav Immun. 25:181–213. 2011. View Article : Google Scholar
|
|
58
|
Toyama K, Koibuchi N, Hasegawa Y, Uekawa
K, Yasuda O, Sueta D, Nakagawa T, Ma M, Kusaka H, Lin B, et al:
ASK1 is involved in cognitive impairment caused by long-term
high-fat diet feeding in mice. Sci Rep. 5:108442015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sobesky JL, Barrientos RM, De May HS,
Thompson BM, Weber MD, Watkins LR and Maier SF: High-fat diet
consumption disrupts memory and primes elevations in hippocampal
IL-1β, an effect that can be prevented with dietary reversal or
IL-1 receptor antagonism. Brain Behav Immun. 42:22–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Spencer SJ, Korosi A, Layé S, Shukitt-Hale
B and Barrientos RM: Food for thought: how nutrition impacts
cognition and emotion. NPJ Sci Food. 1:72017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Crupi R, Marino A and Cuzzocrea S: n-3
fatty acids: Role in neurogenesis and neuroplasticity. Curr Med
Chem. 20:2953–2963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Butler MJ, Mackey-Alfonso SE, Massa N,
Baskin KK and Barrientos RM: Dietary fatty acids differentially
impact phagocytosis, inflammatory gene expression, and
mitochondrial respiration in microglial and neuronal cell models.
Front Cell Neurosci. 17:12272412023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Alkan I, Altunkaynak BZ, Gültekin Gİ and
Bayçu C: Hippocampal neural cell loss in high-fat diet-induced
obese rats-exploring the protein networks, ultrastructure,
biochemical and bioinformatical markers. J Chem Neuroanat.
114:1019472021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lin MT and Beal MF: Mitochondrial
dysfunction and oxidative stress in neurodegenerative diseases.
Nature. 443:787–795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kelty TJ, Taylor CL, Wieschhaus NE, Thorne
PK, Amin AR, Mueller CM, Olver TD, Tharp DL, Emter CA, Caulk AW and
Rector RS: Western diet-induced obesity results in brain
mitochondrial dysfunction in female Ossabaw swine. Front Mol
Neurosci. 16:13208792023. View Article : Google Scholar :
|
|
66
|
Grel H, Woznica D, Ratajczak K, Kalwarczyk
E, Anchimowicz J, Switlik W, Olejnik P, Zielonka P, Stobiecka M and
Jakiela S: Mitochondrial dynamics in neurodegenerative diseases:
Unraveling the role of fusion and fission processes. Int J Mol Sci.
24:130332023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Madreiter-Sokolowski CT, Hiden U, Krstic
J, Panzitt K, Wagner M, Enzinger C, Khalil M, Abdellatif M, Malle
E, Madl T, et al: Targeting organ-specific mitochondrial
dysfunction to improve biological aging. Pharmacol Ther.
262:1087102024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Haack F, Lemcke H, Ewald R, Rharass T and
Uhrmacher AM: Spatio-temporal model of endogenous ROS and
raft-dependent WNT/beta-catenin signaling driving cell fate
commitment in human neural progenitor cells. PLoS Comput Biol.
11:e10041062015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ghosh P, Fontanella RA, Scisciola L,
Pesapane A, Taktaz F, Franzese M, Puocci A, Ceriello A,
Prattichizzo F, Rizzo MR, et al: Targeting redox imbalance in
neurodegeneration: Characterizing the role of GLP-1 receptor
agonists. Theranostics. 13:4872–4884. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bórquez DA, Urrutia PJ, Wilson C, van
Zundert B, Núñez MT and González-Billault C: Dissecting the role of
redox signaling in neuronal development. J Neurochem. 137:506–517.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Morrison CD, Pistell PJ, Ingram DK,
Johnson WD, Liu Y, Fernandez-Kim SO, White CL and Purpera MN: High
fat diet increases hippocampal oxidative stress and cognitive
impairment in aged mice: Implications for decreased Nrf2 signaling.
J Neurochem. 114:1581–1589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Iqbal G and Ahmed T: Co-exposure of metals
and high fat diet causes aging like neuropathological changes in
non-aged mice brain. Brain Res Bull. 147:148–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Y, Fu X, Lan N, Li S, Zhang J, Wang S,
Li C, Shang Y, Huang T and Zhang L: Luteolin protects against high
fat diet-induced cognitive deficits in obesity mice. Behav Brain
Res. 267:178–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Könner AC, Janoschek R, Plum L, Jordan SD,
Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al: Insulin
action in AgRP-expressing neurons is required for suppression of
hepatic glucose production. Cell Metab. 5:438–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang CC, Lee CC and Hsu KS: An
investigation into signal transduction mechanisms involved in
insulin-induced long-term depression in the CA1 region of the
hippocampus. J Neurochem. 89:217–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pearson-Leary J and McNay EC: Novel roles
for the insulin-regulated glucose transporter-4 in hippocampally
dependent memory. J Neurosci. 36:11851–11864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Steen E, Terry BM, Rivera EJ, Cannon JL,
Neely TR, Tavares R, Xu XJ, Wands JR and de la Monte SM: Impaired
insulin and insulin-like growth factor expression and signaling
mechanisms in Alzheimer's disease-is this type 3 diabetes? J
Alzheimers Dis. 7:63–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Denver P, Gault VA and McClean PL:
Sustained high-fat diet modulates inflammation, insulin signalling
and cognition in mice and a modified xenin peptide ameliorates
neuropathology in a chronic high-fat model. Diabetes Obes Metab.
20:1166–1175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Arnold SE, Arvanitakis Z, Macauley-Rambach
SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S, Buettner C,
Stoeckel LE, et al: Brain insulin resistance in type 2 diabetes and
Alzheimer disease: Concepts and conundrums. Nat Rev Neurol.
14:168–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ehtewish H, Arredouani A and El-Agnaf O:
Diagnostic, prognostic, and mechanistic biomarkers of diabetes
mellitus-associated cognitive decline. Int J Mol Sci. 23:61442022.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang H, Liang JL, Wu QY, Li JX, Liu Y, Wu
LW, Huang JL, Wu XW, Wang MH and Chen N: Swimming suppresses
cognitive decline of HFD-induced obese mice through reversing
hippocampal inflammation, insulin resistance, and BDNF level.
Nutrients. 14:24322022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bomfim TR, Forny-Germano L, Sathler LB,
Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo
HM, McClean P, et al: An anti-diabetes agent protects the mouse
brain from defective insulin signaling caused by Alzheimer's
disease-associated Aβ oligomers. J Clin Invest. 122:1339–1353.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Socała K, Doboszewska U, Szopa A, Serefko
A, Włodarczyk M, Zielińska A, Poleszak E, Fichna J and Wlaź P: The
role of microbiota-gut-brain axis in neuropsychiatric and
neurological disorders. Pharmacol Res. 172:1058402021. View Article : Google Scholar
|
|
84
|
Sharon G, Sampson TR, Geschwind DH and
Mazmanian SK: The central nervous system and the gut microbiome.
Cell. 167:915–932. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Carabotti M, Scirocco A, Maselli MA and
Severi C: The gut-brain axis: Interactions between enteric
microbiota, central and enteric nervous systems. Ann Gastroenterol.
28:203–209. 2015.PubMed/NCBI
|
|
86
|
Fasina OB, Wang J, Mo J, Osada H, Ohno H,
Pan W, Xiang L and Qi J: Gastrodin from gastrodia elata enhances
cognitive function and neuroprotection of AD mice via the
regulation of gut microbiota composition and inhibition of neuron
inflammation. Front Pharmacol. 13:8142712022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Schneeberger M, Everard A, Gómez-Valadés
AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M and Cani
PD: Akkermansia muciniphila inversely correlates with the onset of
inflammation, altered adipose tissue metabolism and metabolic
disorders during obesity in mice. Sci Rep. 5:166432015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang B, Qiu J, Qu Z, Xiao R, Wang L, Tian
P, Zhang H, Chen W and Wang G: Bifidobacterium adolescentis
FJSSZ23M10 modulates gut microbiota and metabolism to alleviate
obesity through strain-specific genomic features. Food Funct.
16:2415–2431. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey
FE, Knight R and Gordon JI: The effect of diet on the human gut
microbiome: A metagenomic analysis in humanized gnotobiotic mice.
Sci Transl Med. 1:6ra142009. View Article : Google Scholar
|
|
90
|
Wan Y, Wang F, Yuan J, Li J, Jiang D,
Zhang J, Li H, Wang R, Tang J, Huang T, et al: Effects of dietary
fat on gut microbiota and faecal metabolites, and their
relationship with cardiometabolic risk factors: A 6-month
randomised controlled-feeding trial. Gut. 68:1417–1429. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bruce-Keller AJ, Salbaum JM, Luo M,
Blanchard E IV, Taylor CM, Welsh DA and Berthoud HR: Obese-type gut
microbiota induce neurobehavioral changes in the absence of
obesity. Biol Psychiatry. 77:607–615. 2015. View Article : Google Scholar :
|
|
92
|
Zheng M, Ye H, Yang X, Shen L, Dang X, Liu
X, Gong Y, Wu Q, Wang L, Ge X, et al: Probiotic Clostridium
butyricum ameliorates cognitive impairment in obesity via the
microbiota-gut-brain axis. Brain Behav Immun. 115:565–587. 2024.
View Article : Google Scholar
|
|
93
|
Krüger JF, Hillesheim E, Pereira ACSN,
Camargo CQ and Rabito EI: Probiotics for dementia: A systematic
review and meta-analysis of randomized controlled trials. Nutr Rev.
79:160–170. 2021. View Article : Google Scholar
|
|
94
|
Fung TC, Olson CA and Hsiao EY:
Interactions between the microbiota, immune and nervous systems in
health and disease. Nat Neurosci. 20:145–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Visekruna A and Luu M: The role of
short-chain fatty acids and bile acids in intestinal and liver
function, inflammation, and carcinogenesis. Front Cell Dev Biol.
9:7032182021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lin H, An Y, Hao F, Wang Y and Tang H:
Correlations of fecal metabonomic and microbiomic changes induced
by high-fat diet in the pre-obesity state. Sci Rep. 6:216182016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Koh A, De Vadder F, Kovatcheva-Datchary P
and Bäckhed F: From dietary fiber to host physiology: Short-chain
fatty acids as key bacterial metabolites. Cell. 165:1332–1345.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Stilling RM, van de Wouw M, Clarke G,
Stanton C, Dinan TG and Cryan JF: The neuropharmacology of
butyrate: The bread and butter of the microbiota-gut-brain axis?
Neurochem Int. 99:110–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Stephenson J, Nutma E, van der Valk P and
Amor S: Inflammation in CNS neurodegenerative diseases. Immunology.
154:204–219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hargrave SL, Davidson TL, Zheng W and
Kinzig KP: Western diets induce blood-brain barrier leakage and
alter spatial strategies in rats. Behav Neurosci. 130:123–135.
2016. View Article : Google Scholar :
|
|
101
|
Takechi R, Galloway S,
Pallebage-Gamarallage MM, Lam V, Dhaliwal SS and Mamo JC: Probucol
prevents blood-brain barrier dysfunction in wild-type mice induced
by saturated fat or cholesterol feeding. Clin Exp Pharmacol
Physiol. 40:45–52. 2013. View Article : Google Scholar
|
|
102
|
Tan BL and Norhaizan ME: Effect of
high-fat diets on oxidative stress, cellular inflammatory response
and cognitive function. Nutrients. 11:25792019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Heyward FD, Walton RG, Carle MS, Coleman
MA, Garvey WT and Sweatt JD: Adult mice maintained on a high-fat
diet exhibit object location memory deficits and reduced
hippocampal SIRT1 gene expression. Neurobiol Learn Mem. 98:25–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Finnerup NB, Kuner R and Jensen TS:
Neuropathic pain: From mechanisms to treatment. Physiol Rev.
101:259–301. 2021. View Article : Google Scholar
|
|
105
|
Hardingham GE and Bading H: Synaptic
versus extrasynaptic NMDA receptor signalling: Implications for
neurodegenerative disorders. Nat Rev Neurosci. 11:682–696. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Martinelli I, Tayebati SK, Roy P, Di
Bonaventura MV, Moruzzi M, Cifani C, Amenta F and Tomassoni D:
Obesity-Related brain cholinergic system impairment in
high-fat-diet-fed rats. Nutrients. 14:12432022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Morris MC, Tangney CC, Wang Y, Sacks FM,
Bennett DA and Aggarwal NT: MIND diet associated with reduced
incidence of Alzheimer's disease. Alzheimers Dement. 11:1007–1014.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Barnes LL, Dhana K, Liu X, Carey VJ,
Ventrelle J, Johnson K, Hollings CS, Bishop L, Laranjo N, Stubbs
BJ, et al: Trial of the MIND diet for prevention of cognitive
decline in older persons. N Engl J Med. 389:602–611. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Franco GA, Interdonato L, Cordaro M,
Cuzzocrea S and Di Paola R: Bioactive compounds of the
mediterranean diet as nutritional support to fight
neurodegenerative disease. Int J Mol Sci. 24:73182023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Martínez-Lapiscina EH, Clavero P, Toledo
E, Estruch R, Salas-Salvadó J, San Julián B, Sanchez-Tainta A, Ros
E, Valls-Pedret C and Martinez-Gonzalez MÁ: Mediterranean diet
improves cognition: The PREDIMED-NAVARRA randomised trial. J Neurol
Neurosurg Psychiatry. 84:1318–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ng TP, Feng L, Yap KB, Lee TS, Tan CH and
Winblad B: Long-term metformin usage and cognitive function among
older adults with diabetes. J Alzheimers Dis. 41:61–68. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhu X, Shen J, Feng S, Huang C, Wang H,
Huo F and Liu H: Akkermansia muciniphila, which is enriched in the
gut microbiota by metformin, improves cognitive function in aged
mice by reducing the proinflammatory cytokine interleukin-6.
Microbiome. 11:1202023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lochhead JJ, Kellohen KL, Ronaldson PT and
Davis TP: Distribution of insulin in trigeminal nerve and brain
after intranasal administration. Sci Rep. 9:26212019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Stranahan AM, Norman ED, Lee K, Cutler RG,
Telljohann RS, Egan JM and Mattson MP: Diet-induced insulin
resistance impairs hippocampal synaptic plasticity and cognition in
middle-aged rats. Hippocampus. 18:1085–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kaelber S, Pantcheva P and Borlongan CV:
Drug- and cell-based therapies for targeting neuroinflammation in
traumatic brain injury. Neural Regen Res. 11:1575–1576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fielder E, Tweedy C, Wilson C, Oakley F,
LeBeau FEN, Passos JF, Mann DA, von Zglinicki T and Jurk D:
Anti-inflammatory treatment rescues memory deficits during aging in
nfkb1−/− mice. Aging Cell. 19:e131882020. View Article : Google Scholar
|
|
117
|
Wang QJ, Shen YE, Wang X, Fu S, Zhang X,
Zhang YN and Wang RT: Concomitant memantine and Lactobacillus
plantarum treatment attenuates cognitive impairments in APP/PS1
mice. Aging (Albany NY). 12:628–649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li X, Zheng P, Cao W, Cao Y, She X, Yang
H, Ma K, Wu F, Gao X, Fu Y, et al: Lactobacillus rhamnosus GG
ameliorates noise-induced cognitive deficits and systemic
inflammation in rats by modulating the gut-brain axis. Front Cell
Infect Microbiol. 13:10673672023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Dong J, Ping L, Cao T, Sun L, Liu D, Wang
S, Huo G and Li B: Immunomodulatory effects of the Bifidobacterium
longum BL-10 on lipopolysaccharide-induced intestinal mucosal
immune injury. Front Immunol. 13:9477552022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mandelbaum N, Zhang L, Carasso S, Ziv T,
Lifshiz-Simon S, Davidovich I, Luz I, Berinstein E, Gefen T, Cooks
T, et al: Extracellular vesicles of the Gram-positive gut symbiont
Bifidobacterium longum induce immune-modulatory, anti-inflammatory
effects. NPJ Biofilms Microbiomes. 9:302023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Smith PJ and Blumenthal JA: Dietary
factors and cognitive decline. J Prev Alzheimers Dis. 3:53–64.
2016.PubMed/NCBI
|
|
122
|
Petersson SD and Philippou E:
Mediterranean diet, cognitive function, and dementia: A systematic
review of the evidence. Adv Nutr. 7:889–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Valls-Pedret C, Sala-Vila A, Serra-Mir M,
Corella D, de la Torre R, Martínez-González MÁ, Martínez-Lapiscina
EH, Fitó M, Pérez-Heras A, Salas-Salvadó J, et al: Mediterranean
diet and age-related cognitive decline: A randomized clinical
trial. JAMA Intern Med. 175:1094–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Liu X, Morris MC, Dhana K, Ventrelle J,
Johnson K, Bishop L, Hollings CS, Boulin A, Laranjo N, Stubbs BJ,
et al: Mediterranean-DASH Intervention for Neurodegenerative Delay
(MIND) study: Rationale, design and baseline characteristics of a
randomized control trial of the MIND diet on cognitive decline.
Contemp Clin Trials. 102:1062702021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Jiwani R, Robbins R, Neri A, Renero J,
Lopez E and Serra MC: Effect of dietary intake through whole foods
on cognitive function: Review of randomized controlled trials. Curr
Nutr Rep. 11:146–160. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Bazinet RP and Layé S: Polyunsaturated
fatty acids and their metabolites in brain function and disease.
Nat Rev Neurosci. 15:771–785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Dyall SC: Long-chain omega-3 fatty acids
and the brain: A review of the independent and shared effects of
EPA, DPA and DHA. Front Aging Neurosci. 7:522015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Calon F and Cole G: Neuroprotective action
of omega-3 polyunsaturated fatty acids against neurodegenerative
diseases: Evidence from animal studies. Prostaglandins Leukot
Essent Fatty Acids. 77:287–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Külzow N, Witte AV, Kerti L, Grittner U,
Schuchardt JP, Hahn A and Flöel A: Impact of omega-3 fatty acid
supplementation on memory functions in healthy older adults. J
Alzheimers Dis. 51:713–725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Danthiir V, Hosking DE, Nettelbeck T,
Vincent AD, Wilson C, O'Callaghan N, Calvaresi E, Clifton P and
Wittert GA: An 18-mo randomized, double-blind, placebo-controlled
trial of DHA-rich fish oil to prevent age-related cognitive decline
in cognitively normal older adults. Am J Clin Nutr. 107:754–762.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Power R, Nolan JM, Prado-Cabrero A, Roche
W, Coen R, Power T and Mulcahy R: Omega-3 fatty acid, carotenoid
and vitamin E supplementation improves working memory in older
adults: A randomised clinical trial. Clin Nutr. 41:405–414. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Engin A: Misalignment of circadian rhythms
in diet-induced obesity. Adv Exp Med Biol. 1460:27–71. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Altaha B, Heddes M, Pilorz V, Niu Y,
Gorbunova E, Gigl M, Kleigrewe K, Oster H, Haller D and Kiessling
S: Genetic and environmental circadian disruption induce weight
gain through changes in the gut microbiome. Mol Metab.
66:1016282022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Su X, Li Q, Yang M, Zhang W, Liu X, Ba Y,
Deng Q, Zhang Y, Han L and Huang H: Resveratrol protects against a
high-fat diet-induced neuroinflammation by suppressing
mitochondrial fission via targeting SIRT1/PGC-1α. Exp Neurol.
380:1148992024. View Article : Google Scholar
|
|
135
|
Pivari F, Mingione A, Piazzini G,
Ceccarani C, Ottaviano E, Brasacchio C, Dei Cas M, Vischi M,
Cozzolino MG, Fogagnolo P, et al: Curcumin supplementation
(Meriva®) modulates inflammation, lipid peroxidation and
gut microbiota composition in chronic kidney disease. Nutrients.
14:2312022. View Article : Google Scholar
|
|
136
|
Alghadir A, Gabr SA and Iqbal A: Enhancing
cognitive performance and mitigating dyslipidemia: The impact of
moderate aerobic training on sedentary older adults. BMC Geriatr.
24:6782024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Blumenthal JA, Smith PJ, Mabe S,
Hinderliter A, Lin PH, Liao L, Welsh-Bohmer KA, Browndyke JN, Kraus
WE, Doraiswamy PM, et al: Lifestyle and neurocognition in older
adults with cognitive impairments: A randomized trial. Neurology.
92:e212–e223. 2019. View Article : Google Scholar :
|
|
138
|
Saslow LR, Jones LM, Sen A, Wolfson JA,
Diez HL, O'Brien A, Leung CW, Bayandorian H, Daubenmier J, Missel
AL and Richardson C: Comparing very low-carbohydrate vs DASH diets
for overweight or obese adults with hypertension and prediabetes or
type 2 diabetes: A randomized trial. Ann Fam Med. 21:256–263. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Erickson KI, Voss MW, Prakash RS, Basak C,
Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, et al:
Exercise training increases size of hippocampus and improves
memory. Proc Natl Acad Sci USA. 108:3017–3022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sabouri M, Kordi M, Shabkhiz F,
Taghibeikzadehbadr P and Geramian ZS: Moderate treadmill exercise
improves spatial learning and memory deficits possibly via changing
PDE-5, IL-1 β and pCREB expression. Exp Gerontol. 139:1110562020.
View Article : Google Scholar
|
|
141
|
Noble EE, Mavanji V, Little MR, Billington
CJ, Kotz CM and Wang C: Exercise reduces diet-induced cognitive
decline and increases hippocampal brain-derived neurotrophic factor
in CA3 neurons. Neurobiol Learn Mem. 114:40–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
De la Rosa A, Olaso-Gonzalez G,
Arc-Chagnaud C, Millan F, Salvador-Pascual A, García-Lucerga C,
Blasco-Lafarga C, Garcia-Dominguez E, Carretero A, Correas A, et
al: Physical exercise in the prevention and treatment of
Alzheimer's disease. J Sport Health Sci. 9:394–404. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Chang YS, Lin CL, Lee CW, Lin HC, Wu YT
and Shih YH: Exercise normalized the hippocampal renin-angiotensin
system and restored spatial memory function, neurogenesis, and
blood-brain barrier permeability in the 2K1C-hypertensive mouse.
Int J Mol Sci. 23:55312022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Smith PJ, Blumenthal JA, Babyak MA,
Craighead L, Welsh-Bohmer KA, Browndyke JN, Strauman TA and
Sherwood A: Effects of the dietary approaches to stop hypertension
diet, exercise, and caloric restriction on neurocognition in
overweight adults with high blood pressure. Hypertension.
55:1331–1338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cartee GD, Hepple RT, Bamman MM and
Zierath JR: Exercise promotes healthy aging of skeletal muscle.
Cell Metab. 23:1034–1047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kang EB and Cho JY: Effects of treadmill
exercise on brain insulin signaling and β-amyloid in
intracerebroventricular streptozotocin induced-memory impairment in
rats. J Exerc Nutrition Biochem. 18:89–96. 2014. View Article : Google Scholar
|
|
147
|
Gleeson M, Bishop NC, Stensel DJ, Lindley
MR, Mastana SS and Nimmo MA: The anti-inflammatory effects of
exercise: Mechanisms and implications for the prevention and
treatment of disease. Nat Rev Immunol. 11:607–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Spencer SJ, D'Angelo H, Soch A, Watkins
LR, Maier SF and Barrientos RM: High-fat diet and aging interact to
produce neuroinflammation and impair hippocampal- and
amygdalar-dependent memory. Neurobiol Aging. 58:88–100. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Ionescu-Tucker A and Cotman CW: Emerging
roles of oxidative stress in brain aging and Alzheimer's disease.
Neurobiol Aging. 107:86–95. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hadizadeh M, Hamidi GA and Salami M:
Probiotic supplementation improves the cognitive function and the
anxiety-like behaviors in the stressed rats. Iran J Basic Med Sci.
22:506–514. 2019.PubMed/NCBI
|
|
151
|
Akbari E, Asemi Z, Kakhaki RD, Bahmani F,
Kouchaki E, Tamtaji OR, Hamidi GA and Salami M: Effect of probiotic
supplementation on cognitive function and metabolic status in
alzheimer's disease: A randomized, double-blind and controlled
trial. Front Aging Neurosci. 8:2562016. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Small GW, Siddarth P, Silverman DH, Ercoli
LM, Miller KJ, Lavretsky H, Bookheimer SY, Huang SC, Barrio JR and
Phelps ME: Cognitive and cerebral metabolic effects of celecoxib
versus placebo in people with age-related memory loss: Randomized
controlled study. Am J Geriatr Psychiatry. 16:999–1009. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kohman RA, Bhattacharya TK, Kilby C, Bucko
P and Rhodes JS: Effects of minocycline on spatial learning,
hippocampal neurogenesis and microglia in aged and adult mice.
Behav Brain Res. 242:17–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Leigh SJ, Kaakoush NO, Westbrook RF and
Morris MJ: Minocycline-induced microbiome alterations predict
cafeteria diet-induced spatial recognition memory impairments in
rats. Transl Psychiatry. 10:922020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Craft S and Watson GS: Insulin and
neurodegenerative disease: Shared and specific mechanisms. Lancet
Neurol. 3:169–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ismail TR, Yap CG, Naidu R and Pamidi N:
Environmental enrichment and metformin improve metabolic functions,
hippocampal neuron survival, and hippocampal-dependent memory in
high-fat/high-sucrose diet-induced type 2 diabetic rats. Biology
(Basel). 12:169–178. 2023.
|
|
157
|
Ameen O, Samaka RM and Abo-Elsoud RAA:
Metformin alleviates neurocognitive impairment in aging via
activation of AMPK/BDNF/PI3K pathway. Sci Rep. 12:170842022.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
d'Angelo M, Castelli V, Catanesi M,
Antonosante A, Dominguez-Benot R, Ippoliti R, Benedetti E and
Cimini A: PPARγ and cognitive performance. Int J Mol Sci.
20:50682019. View Article : Google Scholar
|
|
159
|
Chen Z, Deng X, Shi C, Jing H, Tian Y,
Zhong J, Chen G, Xu Y, Luo Y and Zhu Y: GLP-1R-positive neurons in
the lateral septum mediate the anorectic and weight-lowering
effects of liraglutide in mice. J Clin Invest. 134:e1782392024.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Delanogare E, Bullich S, Barbosa LADS,
Barros WM, Braga SP, Kraus SI, Kasprowicz JN, Dos Santos GJ, Guiard
BP and Moreira ELG: Metformin improves neurobehavioral impairments
of streptozotocin-treated and western diet-fed mice: Beyond
glucose-lowering effects. Fundam Clin Pharmacol. 37:94–106. 2023.
View Article : Google Scholar
|
|
161
|
Lennox R, Porter DW, Flatt PR, Holscher C,
Irwin N and Gault VA: Comparison of the independent and combined
effects of sub-chronic therapy with metformin and a stable GLP-1
receptor agonist on cognitive function, hippocampal synaptic
plasticity and metabolic control in high-fat fed mice.
Neuropharmacology. 86:22–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Leal G, Comprido D and Duarte CB:
BDNF-induced local protein synthesis and synaptic plasticity.
Neuropharmacology. 76:639–656. 2014. View Article : Google Scholar
|
|
163
|
Zuo X, Zhu Z, Liu M, Zhao Q, Li X, Zhao X
and Feng X: Fluoxetine ameliorates cognitive deficits in high-fat
diet mice by regulating BDNF expression. ACS Chem Neurosci.
15:4229–4240. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Davidson TL, Jones S, Roy M and Stevenson
RJ: the cognitive control of eating and body weight: It's more than
what you 'Think'. Front Psychol. 10:622019. View Article : Google Scholar
|
|
165
|
Maki KC, Slavin JL, Rains TM and
Kris-Etherton PM: Limitations of observational evidence:
Implications for evidence-based dietary recommendations. Adv Nutr.
5:7–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wu M, Liao M, Huang R, Chen C, Tian T,
Wang H, Li J, Li J, Sun Y, Wu C, et al: Hippocampal overexpression
of TREM2 ameliorates high fat diet induced cognitive impairment and
modulates phenotypic polarization of the microglia. Genes Dis.
9:401–414. 2022. View Article : Google Scholar :
|
|
167
|
Song T, Song X, Zhu C, Patrick R, Skurla
M, Santangelo I, Green M, Harper D, Ren B, Forester BP, et al:
Mitochondrial dysfunction, oxidative stress, neuroinflammation, and
metabolic alterations in the progression of Alzheimer's disease: A
meta-analysis of in vivo magnetic resonance spectroscopy studies.
Ageing Res Rev. 72:1015032021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Mozhui K, Lu AT, Li CZ, Haghani A,
Sandoval-Sierra JV, Wu Y, Williams RW and Horvath S: Genetic loci
and metabolic states associated with murine epigenetic aging.
Elife. 11:e752442022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Kamoga R, Rukundo GZ, Kalungi S,
Obungoloch J, Obua C and Ihunwo A: Vagus nerve stimulation in
dementia: A scoping review of clinical and pre-clinical studies.
AIMS Neurosci. 11:398–420. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Amelianchik A, Sweetland-Martin L and
Norris EH: The effect of dietary fat consumption on Alzheimer's
disease pathogenesis in mouse models. Transl Psychiatry.
12:2932022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Elzinga SE, Guo K, Turfah A, Henn RE,
Webber-Davis IF, Hayes JM, Pacut CM, Teener SJ, Carter AD, Rigan
DM, et al: Metabolic stress and age drive inflammation and
cognitive decline in mice and humans. Alzheimers Dement.
21:e700602025. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Townsend RF, Logan D, O'Neill RF, Prinelli
F, Woodside JV and McEvoy CT: Whole dietary patterns, cognitive
decline and cognitive disorders: A systematic review of prospective
and intervention studies. Nutrients. 15:3332023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Yako H, Niimi N, Takaku S and Sango K:
Advantages of omics approaches for elucidating metabolic changes in
diabetic peripheral neuropathy. Front Endocrinol. 14:12084412023.
View Article : Google Scholar
|
|
174
|
Zhang R, Zhang M and Wang P: The intricate
interplay between dietary habits and cognitive function: Insights
from the gut-brain axis. Front Nutr. 12:15393552025. View Article : Google Scholar : PubMed/NCBI
|
















