Introduction
Epigenetics constitutes an important part of the
immunoregulatory mechanisms within the human body. The
posttranslational modification of histones represents a notable
epigenetic mechanism that influences DNA structure and function,
primarily encompassing mechanisms such as methylation, acetylation,
phosphorylation and ubiquitination. Among them, histone acetylation
is a commonly and extensively studied epigenetic regulatory
mechanism. It carries out a pivotal role in modulating chromatin
activity and subsequent gene transcription (1). The process of histone acetylation
is reversible and is meticulously regulated by a series of enzymes.
For instance, histones undergo acetylation under the catalytic
influence of histone acetyltransferases (HATs) families. However,
this acetylation can be reversed and removed by histone
deacetylases (HDACs), leading to the inhibition of gene
transcription. As their name implies, HDACs are responsible for
removing acetyl groups from histones, thereby influencing the
expression of DNA-encoded genes that are associated with these
histone molecules (2). Moreover,
an abundance of evidence suggests that HDACs can also deacetylate
non-histone proteins, which consequently exerts influence on
cellular physiological processes (3). Given their effects on the cellular
functions, HDACs carry out a pivotal role in regulating both innate
and adaptive immune pathways. Consequently, HDACs are widely
recognized as key inflammatory and immune regulators. Numerous HDAC
inhibitors (HDACis) have been developed as therapeutic agents, with
cancer currently being their main clinical indication. Promising
clinical progress is being made regarding their therapeutic effects
on inflammatory diseases, neurodegenerative diseases, HIV infection
and other conditions (4,5). As aforementioned, HDAC-mediated
deacetylation holds great importance for gene transcriptional
activity and can serve as an effective target for regulating the
immune response of the body.
The gut microbiota constitutes a highly intricate
community of microorganisms that colonize in the human intestinal
tract. It facilitates the digestion and metabolism of nutrients, as
well as promoting the maintenance of host immune homeostasis. This
beneficial effect can be largely attributed to the key molecular
signals delivered by a 'healthy' microbiome. These signals can stem
from microbial surface antigens or active metabolites, and they
hold importance for the proper development and function of the
immune system (6,7). A previous study revealed that
microbial signals are capable of calibrating the transcriptional
programs of host cells through epigenetic modifications, which in
turn regulate both innate and adaptive immune responses. Notably,
metabolites originating from the microbiota, acting as key
epigenetic substrates and enzyme regulators, have the potential to
promote crosstalk between the microbiota and the host by inducing
histone modifications (8).
Short-chain fatty acids (SCFAs) are metabolic products generated by
gut microbes and are recognized as pivotal mediators in the
interplay between the microbiota and the immune system.
Importantly, SCFAs can exert immunomodulatory effects at both local
intestinal and systemic levels, primarily relying on evolutionarily
conserved processes involving the G protein-coupled receptor (GPCR)
signaling and the HDAC inhibition (9). Specifically, SCFAs can function as
the HDACi, targeting the epigenome by inducing chromatin remodeling
that subsequently influences cellular processes and functions.
Typically, the HDAC inhibition targeted by SCFAs fosters a
tolerogenic or anti-inflammatory cellular phenotype, which carries
out a key role in maintaining immune homeostasis. It is
well-established that the anti-inflammatory effects of SCFAs,
grounded in HDAC inhibition, encompass not only epithelial cells
but also a diverse range of immune cells, including peripheral
blood monocytes, neutrophils, dendritic cells (DCs), macrophages
and T cells (6,10,11). Collectively, these findings
substantiate the key notion that SCFA metabolites can act as
epigenetic regulators, thereby enabling the gut microbiota to
modulate host immune responses.
In recent years, the multifaceted HDAC inhibition
exhibited by SCFAs has been increasingly elucidated. SCFAs not only
increase the epithelial barrier function in the gut but also shape
the host immune system, thereby promoting both intestinal and
systemic immune homeostasis. Given these findings, the present
systematic review discusses, at both cellular and systemic levels,
the regulatory effects of SCFAs on host immunity mediated by HDAC
inhibition. The present review was conducted in accordance with the
PRISMA guidelines (12).
Comprehensive searches were carried out across Medline (https://www.nlm.nih.gov/medline/), PubMed
(https://pubmed.ncbi.nlm.nih.gov/),
Google Scholar (https://scholar.google.com/), Web of Science (URL:
https://www.webofscience.com/) and other
databases (https://www.sciencedirect.com/) from inception until
September 30, 2025. The search strategy utilized the full terms
'short-chain fatty acids' (abbreviated as 'SCFAs') and 'histone
deacetylases' (abbreviated as 'HDAC'), along with other core terms
such as 'acetate', 'propionate', 'butyrate' and 'acetylation'.
These were combined with modifiers, including 'gut microbiota',
'immune cells', 'T/B cells', 'immune diseases' and
'anti-bacterial/anti-inflammatory effects', using Boolean operators
('AND', 'OR') to refine the search. Examples of search strings are
as follows: ('Short-chain fatty acids' OR 'SCFAs') AND 'gut
microbiota', ('short-chain fatty acids' OR 'SCFAs') AND 'histone
deacetylases' OR 'HDAC' OR 'acetylation') AND ('anti-bacterial
effects' OR 'anti-inflammatory effects') and ('acetate' OR
'propionate' OR 'butyrate') AND ('histone deacetylases' OR 'HDAC'
OR 'acetylation') AND ('immune cells' OR 'T/B cells' OR 'immune
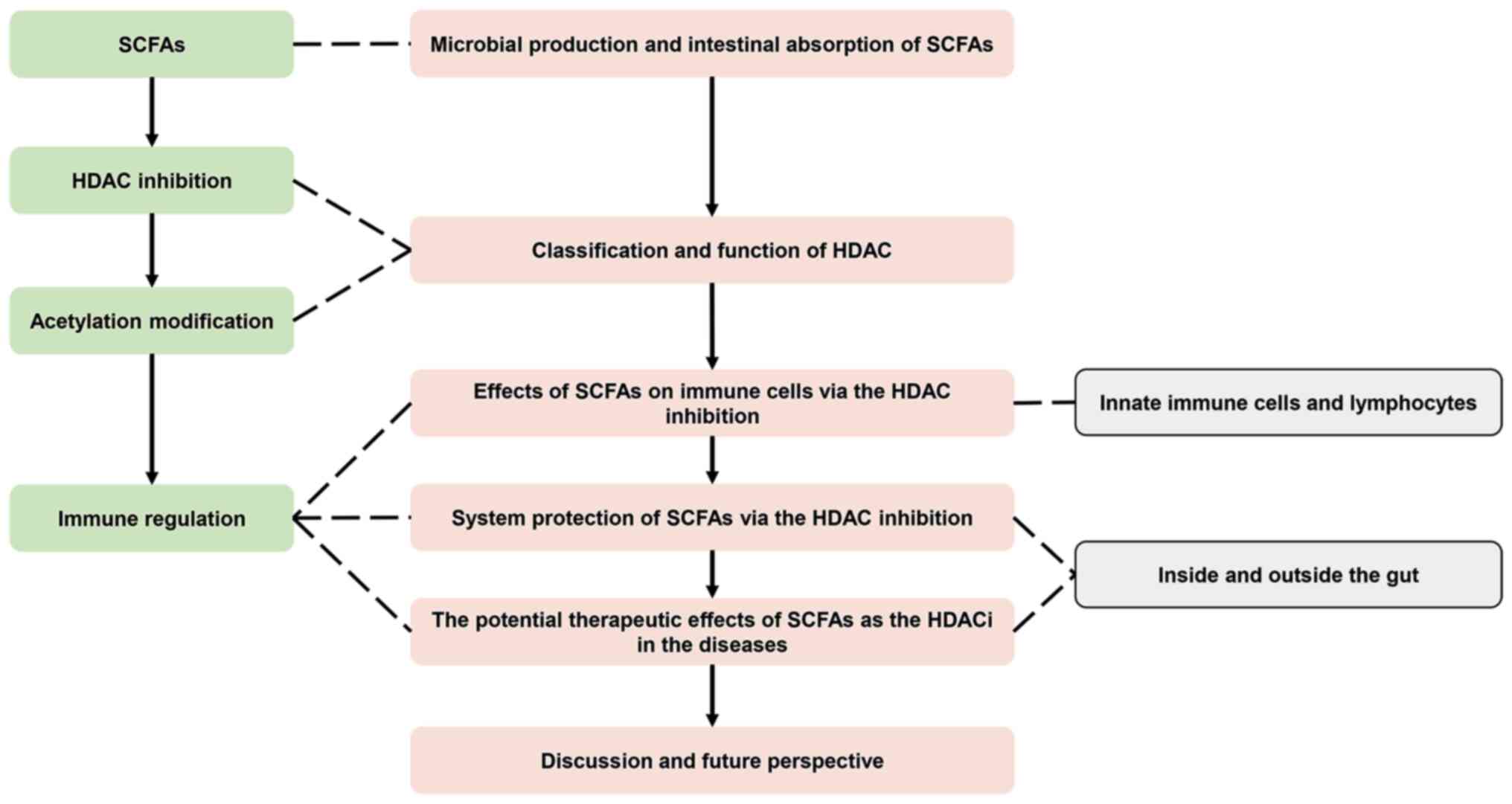
diseases'). According to these, the present review begins by
providing an overview of the microbial production and intestinal
absorption of SCFAs, accompanied by a detailed description of the
classification and function of HDACs. Furthermore, it offers a
comprehensive summary of the direct effects exerted by SCFAs on
immune cells (innate immune cells and lymphocytes) through HDAC
inhibition, as well as the systemic protective roles they carry out
both within and outside the gut. Finally, the present review delves
into the therapeutic potential of SCFAs as HDACi in ameliorating
various immune-related diseases and discusses the research
prospects. The outline of the present review is depicted in
Fig. 1.
Microbial synthesis and intestinal
absorption of SCFAs
Indigestible carbohydrates, such as dietary fibers,
serve as the key sources of SCFAs (13). Given that the human gut lacks
specific metabolic enzymes, carbohydrates require fermentation by
intestinal microorganisms to be hydrolyzed and subsequently
converted into SCFAs. The gut microbial population, which ranges
from 1013 to 1014 in number, encodes the vast
majority of carbohydrate-active enzymes that complete the metabolic
conversion of carbohydrates into SCFAs (13). SCFAs encompass acetate (C2),
propionate (C3), butyrate (C4), valerate (C5), caproate (C6), as
well as other branched SCFAs such as iso-valeric acid and
iso-butyric acid. Notably, acetate, propionate and butyrate
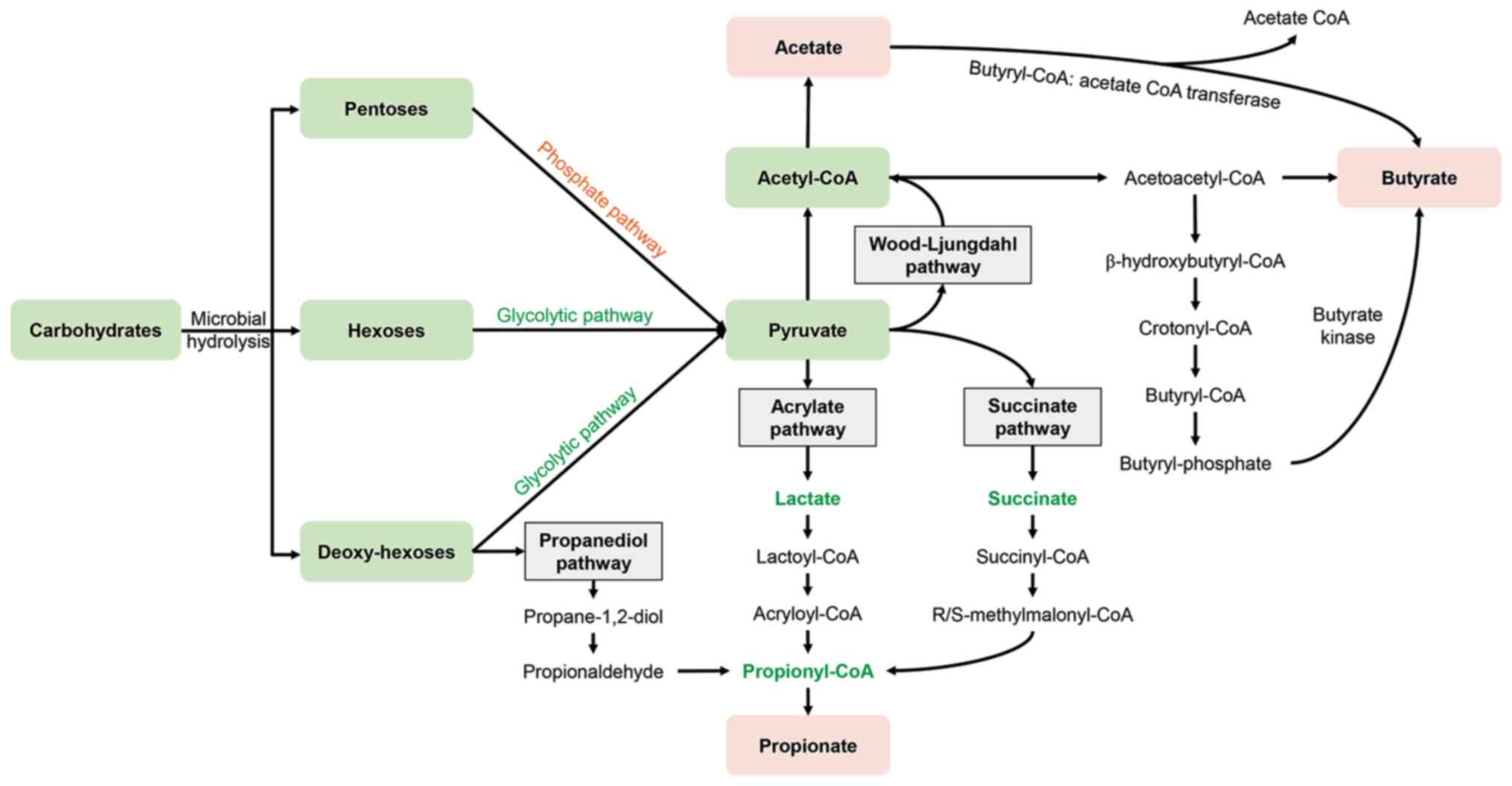
constitute the majority (90-95%) of intestinal SCFAs (14). The synthesis of these SCFAs
occurs through distinct pathways, yet all can trace their origins
back to the common precursor, pyruvate. Pyruvate is generated
through the glycolytic pathway of (deoxy-) hexoses and the pentose
phosphate pathway, following the microbial hydrolysis of
carbohydrates. Gut microbiota can produce acetate by metabolizing
pyruvate through either the acetyl-CoA pathway or the
Wood-Ljungdahl pathway. The biosynthesis of propionate proceeds via
three pathways: The acrylate pathway, the succinate pathway and the
propanediol pathway. Butyrate synthesis is facilitated by the key
enzymes such as butyrate kinase or butyryl-CoA: Acetate CoA
transferase (15,16) (Fig. 2). Acetate serves as the primary
net product of the majority of intestinal bacteria, while the
synthesis of propionate and butyrate demonstrates distinct,
species-specific characteristics. For example, bacteria that
generate propionate through the succinate pathway predominantly
belong to the phyla Bacteroidetes and Negativicutes
(within the Firmicutes phylum) (15). The propanediol pathway, which is
responsible for metabolizing deoxy-hexoses such as fucose and
rhamnose, is notably more prevalent among members of the
Lachnospiraceae family. Furthermore, Coprococcus
catus, a genus within Lachnospiraceae, has the ability
to increase propionate production via the acrylate pathway
(17). Numerous microbes
originating from the Firmicutes phylum, including
Faecalibacterium prausnitzii, Eubacterium biforme and
Eubacterium rectale, are capable of generating butyrate
through the butyryl-CoA: Acetate CoA-transferase pathway. By
contrast, a limited species of Firmicutes, such as
Coprococcus eutactus and Subdoligranulum variabile,
employ butyrate kinase for butyrate synthesis (18).
The types and quantities of SCFAs are determined by
the factors such as the type of digested substrate and the
composition of the microbiota. The uncontroversial finding is that
the relative molar ratio of the three main SCFAs-acetate,
propionate and butyrate-in the intestine and feces is ~60:20:20%
(19). SCFAs generated by
microbes can be absorbed by the colonic epithelium and subsequently
enter the bloodstream. The main transmembrane transport mechanisms
involved in this process include passive diffusion, GPCRs and
carrier transportation. Carrier proteins responsible for mediating
the epithelial transport of SCFAs comprise H+-coupled
monocarboxylate transporters (MCTs) and Na+-coupled sodium-coupled
MCTs (SMCTs). The expression of these transporters is modulated by
physiological conditions as well as disease states. MCT1 is
expressed on the apical membrane, while both MCT1 and MCT4 are
expressed on the basolateral membrane of colonic epithelium. These
transporters facilitate the influx of SCFAs from the lumen and
their efflux into the bloodstream. Additionally, SMCT1 and 2 are
exclusively expressed on the apical membrane of colonic epithelial
cells, where they mediate the influx of SCFAs from the lumen
(20,21). After being absorbed, different
SCFAs exhibit distinct distribution patterns within the body. For
example, the majority of butyrate serves as the primary energy
source for the intestinal mucosal epithelium. Propionate can cross
the intestinal epithelium and once absorbed, it undergoes
metabolism in the liver and participates in gluconeogenesis.
Conversely, acetate can enter the blood to exert its effects in the
peripheral circulation and finally be metabolized by peripheral
tissues (22).
SCFAs carry out a key role in maintaining host
health and influencing disease progression. On the one hand, SCFAs
can regulate the expression of relevant genes to protect intestinal
epithelial cells (IECs), as well as promote energy homeostasis and
host metabolism. SCFAs can also affect the activity and function of
innate immune cells, including macrophages, neutrophils and DCs,
along with T and B cells. Through these actions, they effectively
regulate the immune system and inflammatory responses (23). SCFAs are able to modulate immune
and inflammatory responses through two main mechanisms: Activating
GPCRs (specifically GPR41, GPR43 and GPR109A) and inhibiting HDACs.
However, it is important to emphasize that SCFAs display both
pro-inflammatory and anti-inflammatory effects. These dual effects
are likely associated with their local concentration and the
activity of the receptors they bind to (23,24).
Therefore, SCFAs constitute a vital class of
metabolites that are efficiently synthesized by the gut microbiota
and subsequently absorbed and utilized by the intestinal tract. The
absorbed SCFAs can regulate energy metabolism, as well as immune
and inflammatory responses, thus carrying out a pivotal role in
maintaining both intestinal and host homeostasis (23,24).
Classification and function of HDACs
HDACs exhibit evolutionary conservation in
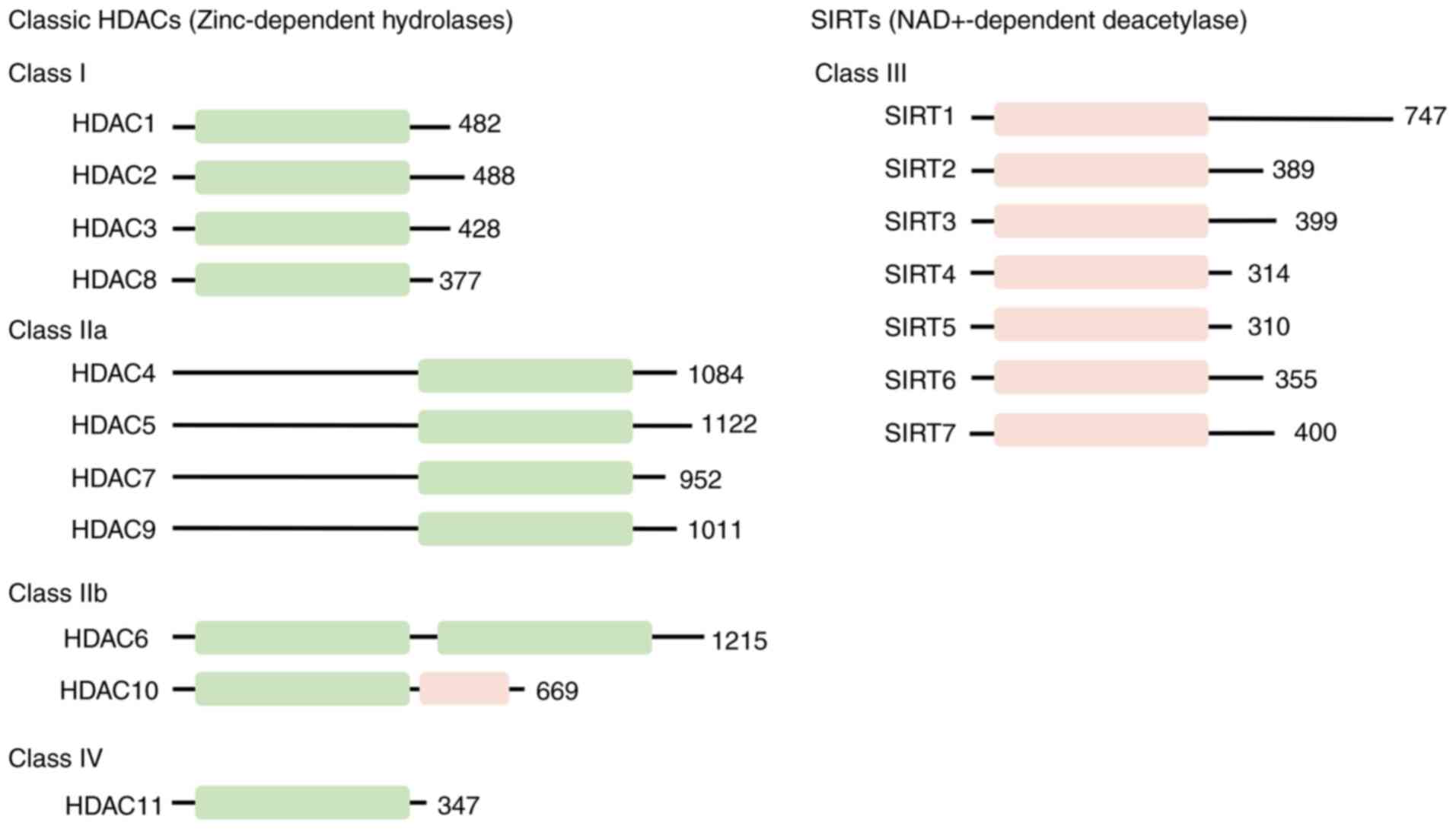
organisms. Based on differences in sequence similarity and
enzymatic mechanisms, HDACs can be categorized into four distinct
classes: Class I Rpd3-like proteins, including HDAC1, 2, 3 and 8;
class II Hda1-like proteins, including HDAC4, 5, 6, 7, 9 and 10;
class III Sir2-like proteins, namely sirtuins (SIRTs)1-7, which are
structurally distinct from class I or II HDACs; and the unique
class IV protein, HDAC11 (Fig.
3) (25,26). Class I HDACs are detectable
within the nucleus and demonstrate ubiquitous expression and share
sequence similarity with the yeast Rpd3 protein. Conversely, class
II HDACs display sequence similarity to the yeast Hda1 protein and
carry out tissue-specific roles. Class II HDACs can be further
subdivided into class IIa (HDAC4, 5, 7 and 9) and class IIb (HDAC6
and 10). Class IIa HDACs possess relatively low activity and are
capable of shuttling between the cytosol and nucleus, whereas class
IIb HDACs prefer to act on non-histone proteins and are primarily
located in the cytosol (25,26). The class IV protein HDAC11 shares
sequence similarity with both class I and II proteins. Furthermore,
class I, II and IV all belong to the family of zinc-dependent
hydrolases and are collectively known as classical HDACs. By
contrast, class III HDACs mainly comprise NAD+-dependent SIRTs1-7,
which share sequence similarity with the Sir2 protein (27,28). Except for HDAC11, the
physiological roles of other HDACs have been extensively
investigated. For example, class I and II HDACs can modulate a
variety of cellular functions, including cell proliferation,
differentiation and development. They are also implicated in
cellular inflammation, immune responses and even cancer progression
(29-31). SRITs are associated with a wide
range of cellular processes, such as cell survival, cell cycle
progression, apoptosis, DNA repair and cellular metabolism.
Dysregulation of their enzymatic activity is associated with the
pathogenesis of tumors, metabolic disorders, infectious diseases
and neurodegenerative conditions (28).
Epigenetic alterations that cause human diseases are
emerging as ideal candidates for therapeutic interventions. The
deacetylation activity of HDACs, which is intricately associated
with various cellular processes, positions them as potentially
effective targets for treating human diseases (32). At present, HDACis constitute a
category of natural or synthetic compounds that exhibit potent
epigenetic regulatory capabilities. These inhibitors can disrupt
HDAC function and induce histone acetylation to modulate the
expression of genes. Importantly, HDACis exhibit pleiotropic
effects at both the cellular and systemic levels and are widely
acknowledged for their extensive therapeutic potential in
inflammatory diseases and cancer (32). Notably, microbial SCFAs
themselves act as the representative HDACis. The extensive HDAC
inhibitory activity of SCFAs across various cell types, as well as
their crucial roles in immunoregulation, are being systematically
investigated. Consequently, the present review focused on and
summarized the immunomodulatory effects of SCFAs as HDACis,
encompassing their effects from immune cells to the systemic
level.
Effects of SCFAs on immune cells via HDAC
inhibition
SCFAs are vital microbial metabolites that notably
influence the intricate interplay between microbes and the host
immune system. HDAC inhibition stands as the principal mechanism by
which SCFAs regulate gene acetylation modifications in numerous
immune cells. Importantly, SCFAs can serve as potent inhibitors of
class I/II HDACs (33). In this
section, a comprehensive review of the regulatory effects of SCFAs
on immune cell activities and functions via HDAC inhibition was
conducted (Fig. 4). The present
review focuses on the inhibitory effects of microbial SCFAs on
class I and II HDACs in innate immune cells, as well as their
effects on the associated acetylation modifications (such as
histones H3 and H4). These immune cells mainly include macrophages,
DCs, neutrophils, mast cells (MCs) and natural killer (NK) cells,
along with T/B lymphocytes (Fig.
4). This highlights the pivotal regulatory role of SCFAs, which
is mediated through HDAC inhibition, at the immune cell level.
Thus, this allows for a deeper understanding of the direct
contribution of microbial SCFAs to the host immune system from the
perspective of epigenetic regulation.
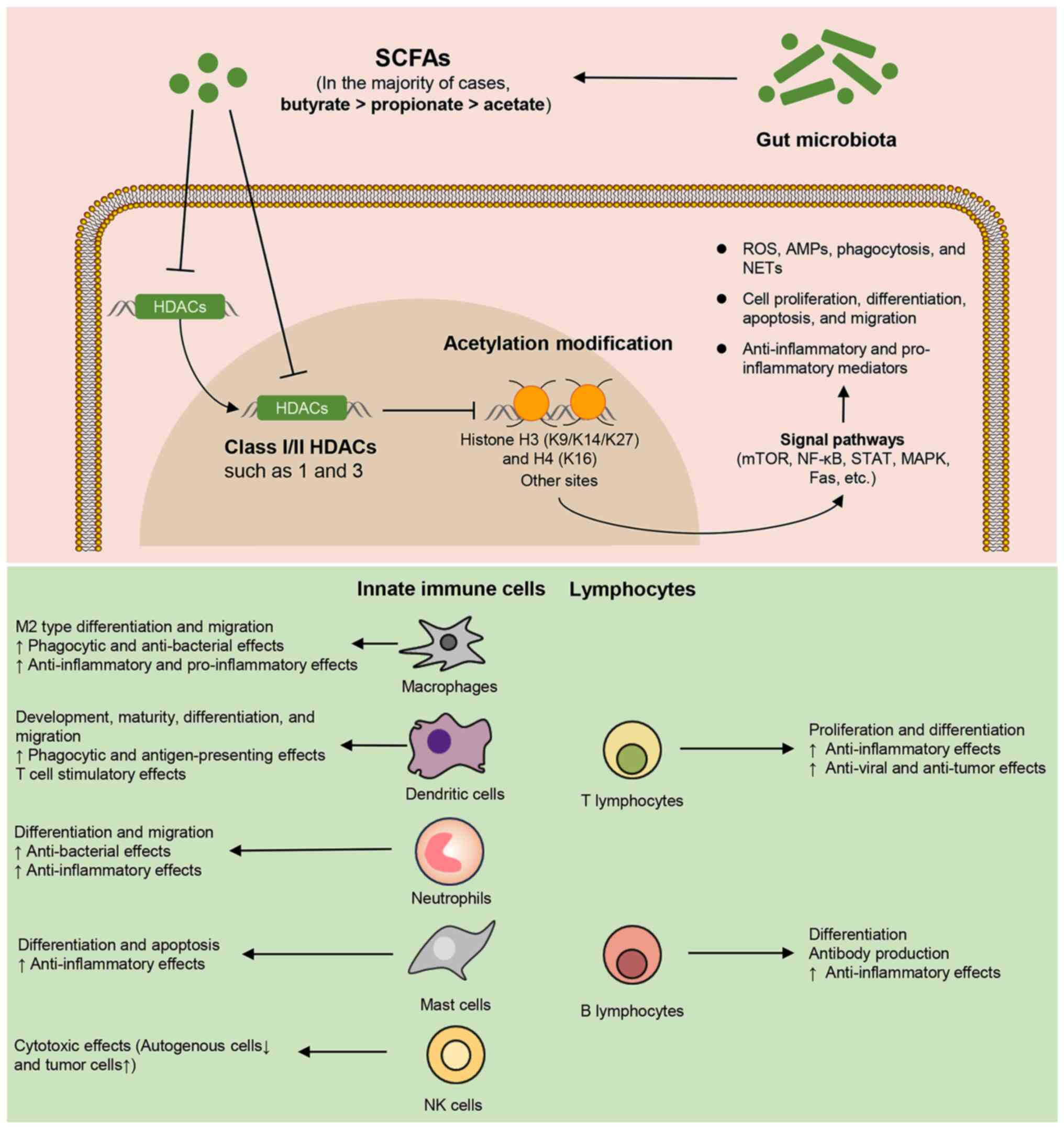 | Figure 4Regulatory effects of microbial SCFAs
on immune cell activities and function via HDAC inhibition. SCFAs,
primarily consisting of acetate, propionate and butyrate, are
capable of promoting acetylation modifications at histones H3
(K9/K14/K27), H4 (K16) and other sites by inhibiting class I/II
HDACs. In the majority of cases, the intensity of HDAC inhibition
by SCFAs is in the order of butyrate > propionate > acetate.
The HDACi effect of SCFAs enables them to modulate the expression
of various genes and signaling pathways, ultimately influencing the
activities and functions of immune cells. SCFAs, short chain fatty
acids; HDACs, histone deacetylases; HDACi, histone deacetylase
inhibitor; ROS, reactive oxygen species; AMPs, antimicrobial
peptides; NETs, neutrophil extracellular traps; M2, macrophage
subset 2. |
Innate immune cells
Macrophages
Macrophages are extensively distributed throughout
the body and carry out a pivotal role in innate immunity, primarily
by eliminating bacteria, regulating inflammation and promoting
tissue repair (34). In
particular, the tissue-resident macrophages exhibit direct
activities aimed at eliminating invading bacteria and increasing
the defenses of the body. Butyrate emerges as a representative SCFA
that modulates the anti-bacterial functions of macrophages. Its
underlying mechanism is considered to be associated with HDAC
inhibition rather than GPCR activities. For example, SCFAs can
enhance the anti-bacterial activity of macrophages differentiated
in vitro. Notably, 1 mM butyrate exhibits a stronger effect
compared with 1 mM propionate, while acetate at the same dosage
fails to demonstrate such an activity. The potent anti-bacterial
function induced by butyrate stems from glycolysis and mTOR
inhibition, which does not require GPCRs but instead depends on
HDAC3 inhibition (34). Butyrate
(given orally at 150 mM for 21 days or used in vitro at 1 mM
for 24 h) can enhance the anti-bacterial activity of macrophages by
promoting the production of reactive oxygen species (ROS) after
infection, markedly improving leptospirosis in hamsters (35). However, acetate and propionate do
not have this effect. Mechanistically, butyrate promotes ROS
production through MCT-mediated HDAC3 inhibition (35). In addition to phagocytosis and
anti-bacterial actions, butyrate at 2 mM for 48 h can also restrict
the alternative activation of macrophages induced by IL-4,
including inhibiting the activity of regulatory T (Treg) cells and
reducing their production of IL-17A. The effects of butyrate can be
replicated by HDACis such as trichostatin A (TSA) and valproic acid
(VPA) and this replication occurs independently of GPCR signaling
(36). Furthermore, the
hydroxylated derivative of butyrate, 4-hydroxybutyrate, can also
promote the production of antimicrobial peptides (AMPs) in mouse
bone marrow-derived macrophages (BMDMs) in an HDAC
inhibition-dependent manner, thereby enhancing the endogenous
anti-bacterial activity of the immune system. Specifically,
butyrate within the concentration range of 0.5-12 mM, when treated
for 24 h, can markedly increase the expression levels of
cathelicidin LL-37 (37).
The response of activated macrophages to
inflammation is determined by distinct cellular subsets: The
pro-inflammatory M1 phenotype and the anti-inflammatory M2
phenotype. M1 macrophages are induced by inflammatory stimuli such
as IFN-γ and lipopolysaccharide (LPS) and they participate in
inflammatory and immune responses by secreting pro-inflammatory
cytokines and presenting antigens. M2 macrophages, on the other
hand, can be activated by cytokines such as IL4 and IL13, and
mainly secrete anti-inflammatory cytokines such as IL-10 and TGF-β,
carrying out a key role in inflammation resolution and immune
modulation (38). In the
majority of cases, SCFAs, primarily butyrate, can attenuate the
release of pro-inflammatory mediators from activated macrophages
through inhibition of HDAC, thus exerting anti-inflammatory
effects. For instance, when compared with acetate and propionate,
butyrate at 1 mM for 24 h demonstrates a more potent reduction in
the levels of pro-inflammatory mediators including NO, IL-6 and
IL-12p40 released by LPS-induced BMDMs. It also inhibits the
transcription of IL-6, Nos2, IL-12a and IL-12b in colonic lamina
propria (LP) macrophages both in vitro and in vivo,
thereby producing anti-inflammatory effects. The anti-inflammatory
effects of butyrate are independent of GPCRs, and instead increase
the acetylation levels of histone 3 lysine 9 (H3K9) at the promoter
regions of IL-6, Nos2 and IL-12b in cells via HDAC inhibition
(39). In staphylococcal
lipoprotein (Sa.LPP)-induced macrophage inflammation, butyrate and
propionate (but not acetate) at 3 mM for 21 h can markedly suppress
the NF-κB, IFN-β/STAT1 and HDAC pathways, leading to a reduction in
cellular NO release. Similarly, butyrate exerts a stronger effect
when compared with propionate in inducing H3K9 acetylation.
Additionally, the HDAC inhibitor TSA also decreases NO production
induced by Sa.LPP. These findings imply that HDAC inhibition,
rather than GPCR signaling, serves as the key molecular mechanism
by which butyrate and propionate inhibit NO release from
macrophages induced by Sa.LPP (40). Furthermore, butyrate can also
inhibit inflammatory responses by regulating other macrophage
activities, with its underlying mechanisms being associated with
HDAC inhibition (41,42). For instance, macrophage migration
is associated with tissue inflammation and immune responses.
Treatment with 2 mM butyrate for 1 h can inhibit the migratory
activity of RAW264.7 cells and rat peritoneal macrophages triggered
by LPS. This effect is mediated by inhibiting the activities of
iNOS, steroid receptor coactivator and focal adhesion kinase,
similar to the effects of the HDACi TSA (41). In addition to restricting
inflammatory macrophages, butyrate exerts anti-inflammatory effects
by promoting the polarization of M2 macrophages, both in
vitro at a concentration of 50 μg/ml for 24 h and in
vivo at 150 mM for 11 days. Furthermore, it enhances the
migratory and wound-healing capabilities of M2-BMDMs. Butyrate
primarily enhances the STAT6 signaling pathway through its HDAC
inhibitory activity, which involves HDAC1 inhibition and H3K9
acetylation. This action facilitates polarization of BMDMs toward
the anti-inflammatory M2 phenotype, thereby alleviating
inflammatory bowel disease (IBD) (42). Collectively, the evidence
underscores the anti-inflammatory activities of SCFAs, especially
butyrate, in macrophages through HDAC inhibition. These activities
include inhibiting the release of inflammatory mediators,
regulating cell migration and promoting M2-type cell polarization.
Such mechanisms hold therapeutic potential for treating
inflammatory diseases.
SCFAs exhibit anti-inflammatory properties under
homeostatic conditions, whereas they can trigger pro-inflammatory
programs in the presence of pathogen- and danger-associated
molecular patterns. For example, butyrate and propionate has been
have been shown to activate the NLRP3 inflammasome and subsequent
IL-1β release in LPS-induced macrophages through HDAC inhibition.
Butyrate, at a concentration of 1 mM, can notably promote IL-1β
release and at 4 mM, it can induce the maximal release of IL-1β
starting from 6 h of stimulation. Mechanistically, the inhibitory
effect of butyrate on HDAC promotes the acetylation of H3K27,
blocks the transcription of FLICE-like inhibitory protein and
IL-10, thereby activating the NLRP3 inflammasome without inducing
pyroptosis (43). Park et
al (44) demonstrated that
sodium butyrate is capable of upregulating the gene expression of
COX-2 in RAW264.7 macrophages that are stimulated by LPS. This
upregulation occurs via MAPK-dependent increases in the
phosphorylation and acetylation of histone H3 at the COX-2 promoter
region. COX-2 is a pivotal enzyme implicated in inflammatory
responses and is rapidly activated in macrophages induced by LPS.
When 5 mM butyrate is co-administered with 10 ng/ml LPS, it elicits
the most notable induction of COX-2 promoter activity. By contrast,
butyrate at this concentration alone exerts no discernible effect
on the promoter activity of COX-2. These research findings
collectively imply that SCFAs do not invariably exert
anti-inflammatory effects.
DCs
DCs serve as professional phagocytes and
antigen-presenting cells, carrying out a pivotal role in initiating
T-cell activation and adaptive immune responses. DCs mainly
comprise two major types, namely immature DCs (iDCs) and mature DCs
(mDCs). iDCs can capture antigens and, upon immune stimulation,
transform into mDCs, thereby activating their antigen-presentation
and T-cell stimulation functions (45). SCFAs are involved in regulating
the development, maturation, differentiation, migration and
antigen-presentation functions of DCs. Research suggests that some
of these regulatory mechanisms are associated with HDAC inhibition.
For example, the SCFAs butyrate and propionate both at 0.5 mM for 6
days can inhibit the development of DCs from bone marrow precursor
cells induced by granulocyte-macrophage colony-stimulating factor
(GM-CSF), while having no impact on granulocyte development.
Mechanistically, these SCFAs are transported into cells via the
transporter (Slc5a8) and inhibit the expression of PU.1 and RelB
through their ability to inhibit HDAC (histone H4 acetylation).
Acetate is also a substrate for Slc5a8, but it is not an HDACi and
does not influence DC development. This suggests that HDAC
inhibition carries out a vital role in DC development (46).
The SCFA butyrate can induce DCs to remain in an
immature state and impede their maturation. Research has revealed
that butyrate at 1 mM for 24 h can markedly downregulate the
expression of surface markers on mDCs. Additionally, it enhances
the intracellular endocytic capacity of these cells, diminishes
their T-cell stimulatory ability, promotes the production of IL-10
and inhibits the production of IL-12 and IFN-γ. These findings
collectively underscore the specific immunosuppressive
characteristics of butyrate (47). However, the regulatory effect of
butyrate on the phenotypic differentiation of certain DCs may not
always be associated with HDAC inhibition. For instance, butyrate,
when applied at a concentration of 200 μM for 5 d, does not
hinder DC differentiation induced by IL-4 and GM-CSF, whereas it
suppresses the acquisition of CD1a that is triggered by cytokine
and TLR2 agonist stimulation. Nevertheless, this regulatory effect
on CD1a acquisition is not closely associated with the HDAC
inhibitory activity of butyrate (48). Upon activation, iDCs transform
into mDCs, which can migrate to secondary lymphoid organs and
initiate cellular immunity. However, butyrate (200 nM; 24 h) and
other HDACi, including TSA and scriptaid, demonstrate effective
inhibitory effects on the migration of iDCs stimulated by
macrophage inflammatory protein-1α, as well as on the chemotaxis of
mDCs. The actions of these HDACi involve a reduction in the surface
expression of C-C chemokine receptor type 1 on iDCs and C-X-C
chemokine receptor type 4 on mDCs. Additionally, they inhibit the
phosphorylation of MAPKs, specifically p38, ERK1/2 and JNK
(49,50). The uptake of foreign antigens by
DCs and their subsequent presentation to T cells can be affected by
their dendritic activity. A study has revealed that SCFAs, such as
butyric acid (1 mM) and valeric acid (10 mM) applied for 24 h, can
promote dendritic elongation by inhibiting HDACs rather than
relying on GPCRs, activating the SFK/PI3K/Rho signaling pathway and
inducing actin polymerization. These effects ultimately lead to an
enhanced capacity for antigen uptake and presentation in DCs.
Notably, the HDACi TSA also replicates the dendrite-promoting
effects of SCFAs (51).
DCs carry out a pivotal role in activating T-cell
immune responses due to their remarkable capacity for antigen
uptake. Among the SCFAs, butyrate stands out as a representative
HDACi that can influence the regulation of DC functions in T-cell
proliferation and differentiation (52-54). In the majority of cases, butyrate
exhibits immunosuppressive properties in T-cell responses
stimulated by DCs. For example, butyrate can alter the
developmental trajectory of conventional DCs (cDCs). When
administered at 0.5 mM for 4 days, butyrate selectively inhibits
the cDC2 lineage and their ability to drive CD4+ T cell
proliferation. However, it promotes an increase in the
differentiation of Foxp3+ Treg cells, thereby contributing to the
intestinal tissue homeostasis. These effects of butyrate are
independent of GPCR activity and are instead attributed to the
inhibition of HDAC3 (52).
Butyrate at 2 mM for 48 h has the ability to induce the production
of retinaldehyde dehydrogenase 1 (RALDH1), an enzyme that is
responsible for retinoic acid synthesis in DCs. Consequently, it
inhibits the maturation and metabolic reprogramming of LPS-induced
human monocyte-derived DCs and promotes their polarization towards
IL-10-producing type 1 Treg cells. The effects of butyrate rely on
RALDH activity, which is driven by the combined action of HDAC
inhibition and GPR109A signaling, thereby inducing a tolerogenic DC
phenotype (53). Additionally,
butyrate promotes the production of the pro-inflammatory cytokine
IL-17 by T cells, an effect that is associated with the release of
cytokine IL-23 by activated DCs. Berndt et al (54) by using LPS to induce mouse bone
marrow-derived DCs, discovered that 1 mM butyrate for 18 h could
considerably inhibit LPS-induced DC maturation and downregulate
IL-12 production, while concurrently increasing IL-23 production.
The upregulation of the mRNA subunit IL-23p19 at the
pre-translational level is consistent with the effect of HDACi on
epigenetic modifications of gene expression. The researchers
observed that butyrate treatment increased the production of IL-17
and IL-10 by T cells co-cultured with activated DCs, but had no
such effect on IFN-γ, IL-4 or IL-13. The in vivo therapeutic
efficacy of butyrate is dependent on the route of administration.
Specifically, oral administration exacerbates colitis, whereas
systemic administration improves it. These findings collectively
suggest that butyrate induces the production of DC IL-23 and the
T-cell pro-inflammatory cytokine IL-17, thereby modulating
chemically induced colitis (54).
Neutrophils
Neutrophils represent a key type of immune cell that
defends against various pathogens, with their anti-bacterial
mechanism of releasing neutrophil extracellular traps (NETs).
Research has revealed that SCFAs at physiological concentrations
can regulate NET formation in neutrophils. For instance, SCFAs
(acetate, propionate and butyrate) at the colonic concentrations
are capable of inducing NET formation in neutrophils in
vitro. When tested at concentrations of SCFAs present in human
peripheral blood, only acetate at concentrations of 100 μM
(fasting) and 700 μM (postprandial) can considerably induce
NET formation. This effect of SCFAs is partially mediated by
FFA2R/GPR43 (55). Furthermore,
the mechanism by which acetate acting as a HDACi to regulate NET
release has also been revealed (56). For example, acetate (at a
concentration of 10 mM for 24 h) can considerably enhance the
acetylation of histone Ace-H3, H3K9ace and H3K14ace, thereby
promoting NETosis in neutrophil-like HL-60 cells, which is a
specific form of cell death in which neutrophils release NETs
(56).
The accumulation and activation of neutrophils at
inflamed sites are associated with the mechanisms of tissue
inflammation. The SCFAs, primarily butyrate, exert notable
anti-inflammatory effects on activated neutrophils, and this
mechanism is associated with HDAC inhibition (57-60). For instance, 1.6 mM butyrate can
markedly reduce the production of pro-inflammatory mediators,
namely TNF-α, CINC-2αβ and NO, in both in vitro and
ex-vivo neutrophils stimulated by LPS. Notably, propionate
exhibits a relatively weaker effect compared with butyrate. It can
exert notable inhibitory effects on the release of pro-inflammatory
mediators from neutrophils when its concentration reaches 12 mM.
Their anti-inflammatory properties are associated with the
inhibition of HDAC activity and NF-κB activation (57). Furthermore, the anti-inflammatory
effects of butyrate on peripheral neutrophils have been
well-validated in both intestinal inflammation models and patients
with enteritis. For example, oral administration of 200 mM butyrate
can markedly alleviate dextran sodium sulfate (DSS)-induced colitis
in mice by inhibiting the neutrophil-derived production of
pro-inflammatory cytokines, chemokines, calprotectins and NET
formation. In addition, 0.5 mM butyrate can suppress the migration
and NET formation of neutrophils isolated from patients with IBD,
which encompasses Crohn's disease and ulcerative colitis.
Mechanistically, the influence of butyrate on the production of
pro-inflammatory mediators is mediated in an HDACi-dependent manner
rather than through GPCR signaling (58). In general, a reduction in
neutrophil apoptosis is associated with persistent inflammation and
may trigger local and systemic inflammatory responses.
Consequently, regulating neutrophil apoptosis is a key event for
resolving inflammation. Additionally, the HDACi SCFAs may be
important microbial metabolites for regulating neutrophil
apoptosis. For example, butyrate and propionate, each at 4 mM for
20 h, can notably induce apoptosis in both inactivated and
TNF-α/LPS-activated neutrophils. Their mechanisms are independent
of GPCRs and MAPKs. Their mechanisms may be related to their HDAC
inhibitory activity, which promotes the acetylation of histone H3
and controls the mRNA expression of associated apoptotic protein a1
(59). Butyrate can induce a
reduction in the proliferation and an elevation in the apoptosis of
CD34+ progenitor cells, and quantitatively and qualitatively impair
terminal neutrophil differentiation. This effect of butyrate is
accompanied by the increased acetylation of histones 3 and 4,
specifically at the H3K9 and H4K16 sites. This observation is
consistent with the action exhibited by the HDACi, VPA (60). Taken together, all the findings
suggest that butyrate can act as a potent HDACi to suppress
neutrophil function and promote its apoptosis, ultimately leading
to an effective suppression of tissue inflammation caused by
neutrophils.
MCs
Activation of MCs carries out a pivotal role in
inflammation and allergic reactions. SCFAs, owing to their HDACi
activity, can effectively suppress the activity and function of
MCs. Among SCFAs, butyrate and propionate, rather than acetate,
inhibit the activation of primary human or mouse MCs via both IgE-
and non-IgE-mediated degranulation in a concentration-dependent
manner. These effects are associated with HDAC inhibition and are
independent of the stimulation of SCFA receptors GPR41, GPR43 or
peroxisome proliferator-activated receptor. Epigenomic analysis
reveals that 5 mM butyrate not only triggers a redistribution of
global H3K27 acetylation levels, but also induces a notable
reduction in acetylation at the promoter regions of genes including
the tyrosine kinases BTK, SYK and LAT. These kinases are key
transducers in the FcεRI signaling pathway that mediates MC
activation (61). Additionally,
the HDACi butyrate can inhibit the proliferation and induce
apoptosis in P815 mastocytoma cells. It also suppresses
FcεRI-dependent cytokine production in murine primary bone
marrow-derived mast cells (BMMCs). Mechanistically, butyrate at 2
mM for 12 h notably enhances H3K9 acetylation in both P815 and
BMMCs, while reducing the FcεRI-dependent mRNA expression of TNF-α
and IL-6 in BMMCs. This effect is similarly observed with TSA, a
well-known HDACi (62). A study
conducted by Gudneppanavar et al (62) found that butyrate (5 mM, 24 h)
can act as an HDACi to downregulate the growth factor stem cell
factor (SCF) receptor KIT and its associated phosphorylation,
thereby notably attenuating SCF-mediated proliferation and
pro-inflammatory cytokine secretion in BMMCs. Mechanistically,
butyrate primarily affects MC function through the inhibition of
HDAC1/3. These findings suggest that butyrate can weaken MC
function and its inflammatory response by epigenetically modifying
histones and downregulating the SCF/KIT/p38/Erk signaling axis
(63). Similarly, the
proliferative activities of three transformed human MC
lines-HMC-1.1, HMC-1.2 and LAD2-are also sensitive to the
inhibitory effects of the HDACi butyrate. Butyrate (0-1,000
μM) considerably reduces the expression of c-KIT mRNA and
KIT protein in these three cell lines in a dose-dependent manner.
The effects of butyrate are associated with cell cycle arrest and a
moderate increase in histamine content, tryptase expression and
granularity (64).
NK cells
NK cells are of importance in anti-viral defense,
anti-tumor responses and immune regulation. They are capable of
directly eliminating virus-infected cells and cancer cells, as well
as regulating the activities of other immune cells through cytokine
secretion. The SCFA butyrate (250 μM, 24 h) can inhibit HDAC
activity on human NK cells and influence the epigenetic landscape,
phenotype and regulatory functions of NK cells. Specifically,
butyrate induces the generation of CD69+ NK cells while
simultaneously reducing the proportion of CD56bright NK
cells. The inhibition of CD56bright NK cells by butyrate
can weaken the attack of NK cells on autologous CD4+ T cells
(65). In addition, similar to
other HDACi, butyrate (5 mM) can enhance the sensitivity of DAOY
and PC3 tumor cells to the cytotoxic effects of IL-2-activated
peripheral blood mononuclear cells (PBMCs). NK cells serve as the
primary effector cells involved in the lysis of tumor cells.
Butyrate also increases the tumor surface expression of ligands for
activating NK receptors, namely NKG2D and DNAM-1. As a result, it
enhances the susceptibility of tumor cells to the cytotoxic effects
of NK cells (66).
Lymphocytes
T lymphocytes
T lymphocytes are the primary effector cells in
cellular immunity, mediating inflammatory and immune responses
through the production of various cytokines. There is a wide
variety of T cells, including CD4+ T cells, CD8+ T cells, γδ T
cells and innate lymphoid cells (ILCs). CD4+ T cells are further
subdivided into subsets such as Th1, Th2, Th17 and Treg (67). Under physiological conditions,
SCFAs have a regulatory role in inhibiting T cell activation,
thereby mitigating tissue inflammatory responses. For instance,
within intestinal tissues, butyrate (0.0625-0.5 mM) for 4 days is
more effective than other SCFAs in reducing the activity of in
vitro human LP CD4+ T cells and the production of inflammatory
cytokines such as IFN-γ and IL-17. The anti-inflammatory effects of
butyrate are mediated by both histone acetylation and GPR43
signaling (68). Additionally,
butyrate (used in vivo at 200 mM or in vitro at 0.5
mM) has a broad stimulatory effect on IL-22 production by CD4+ T
cells and ILCs in mouse spleens, mesenteric lymph nodes, LP and
human peripheral blood. The anti-inflammatory effect of butyrate
resulting from this is associated with GPR41 and HDAC inhibition
(69). Similar to other HDACi,
butyrate (1.1 mM) can induce T cell anergy, a state of
antigen-specific proliferative unresponsiveness in CD4+ T cells
exposed to antigen. The effect of butyrate in inducing
proliferative unresponsiveness in Th1 cells does not rely on
general histone hyperacetylation but rather on the secondary effect
of histone acetylation, namely the upregulation of p21(Cip1)
(70). Furthermore, the
anti-inflammatory effects of butyrate include inhibiting
IFN-γ-mediated inflammation in colonic epithelial cells and
inducing T cell apoptosis to eliminate inflammatory sources.
Mechanistic analysis indicated that high concentrations of butyrate
(>3 mM) inhibit the activation and proliferation of murine
splenic CD4+ and CD8+ T cells in vitro and suppresses
IFN-γ-induced STAT1 activation and iNOS upregulation in human
colonic epithelial cells. Importantly, butyrate can induce
hyperacetylation of the Fas promoter and upregulation of Fas by
inhibiting HDAC1 activity, thereby inducing apoptosis in CD4+ and
CD8+ T cells. This leads to the inhibition of T cell activation and
the elimination of the inflammatory source in the colon (71).
However, SCFAs also possess a regulatory role in
enhancing the T cell immune activity, particularly in cellular
immunity against tumors and viruses. Research has revealed that a
relatively high concentration of butyrate (1 mM) can maintain or
even increase the ability of T cell receptor (TCR)-transduced CD4+
and CD8+ T cells to release cytokines in response to antigens.
Mechanistically, HDAC inhibition by butyrate can enhance the
transgenic expression of T cells in vitro and their
anti-tumor function. This indicates that the HDACi butyrate can
augment the tumor-responsive effect of functionally TCR-transduced
T cells (72). SCFAs,
specifically butyrate and pentanoate, can enhance the anti-tumor
activity of two CD8+ T cell subsets, cytotoxic T lymphocytes and
chimeric antigen receptor T cells, through metabolic and epigenetic
reprogramming. In particular, they can promote the function of mTOR
as a central cellular metabolic sensor and inhibit class I HDAC
activity. Consequently, these CD8+ T cells exhibit increased
production of effector molecules such as CD25, IFN-γ and TNF-α
(73). In addition, 1 mM
butyrate can also promote the production of granzyme B and IFN-γ in
Tc17 cells, a subset of CD8+ T cells. The increased IFN-γ
expression induced by butyrate is mediated through its HDAC
inhibitory activity rather than through GPR41 and GPR43 signaling
pathways. Moreover, a relatively high concentration of acetate (25
mM) can also increase the production of IFN-γ in CD8+ T
lymphocytes, but its underlying mechanism relies on cellular
metabolism and mTOR activity (74). Acetate also has a regulatory role
in increasing the susceptibility of T cells to HIV-1. Alterations
in the fecal microbiota and intestinal epithelial damage associated
with HIV-1 infection are likely to causing microbial translocation,
thereby exacerbating disease progression and virus-related
comorbidities. Notably, the physiological concentration of acetate,
that is 20 mM, is capable of enhancing viral production in primary
human CD4+ T cells. It also promotes the integration of HIV-1 DNA
into the host genome by impairing class I/II HDAC activity. This
suggests that acetate can increase the susceptibility of CD4+ T
cells to productive HIV-1 infection and may influence the
progression of HIV-1-mediated diseases (75). These findings indicate that SCFAs
acting as HDACi may hold importance for adoptive immunotherapy
against cancer and anti-viral immunity.
Depending on distinct cytokine environments, SCFAs
can regulate the differentiation process of naïve T cells into
effector cells and Treg cells. For instance, acetate (10 mM),
propionate (1 mM) and butyrate (0.5 mM) can induce the
differentiation of naïve T cells into Th1 and Th17 cells under
polarizing conditions: Th17 (IL-1β, IL-6, IL-21, IL-23, TGF-β1,
anti-IL-4 and anti-IFN-γ) or Th1 (IL-12, IL-2 and anti-IL-4). They
can also induce the generation of Treg cells such as IL-10+ T cells
and Foxp3+ T cells. This effect of SCFAs on T cell differentiation
is independent of GPCR receptor signals (GPR41 or GPR43) but
depends on HDAC inhibitory activity. Specifically, the inhibition
of HDAC by SCFAs increases the acetylation of p70 S6 kinase
(P70S6K) and the phosphorylation of rS6, which subsequently drives
T cell differentiation through the mTOR pathway to promote either
immunity or immune tolerance (76). However, SCFAs are also capable of
exerting distinct regulatory effects on T cell differentiation. A
study conducted by Chen et al (77) revealed that 0.5 mM butyrate can
promote the expression of IFN-γ and T-bet to facilitate Th1 cell
differentiation while inhibiting the expression of IL-17, Rorα and
Rorγt to suppress Th17 cell differentiation. Notably, under both
Th1 and Th17 conditions, butyrate can promote the production of
IL-10. This, in consequence, diminishes the capacity of T cells to
induce colitis, and this effect partially depends on the role of
Blimp1. Mechanistically, butyrate promotes Th1 differentiation (but
not Th17) by inhibiting HDACs, and this effect is independent of
GPR43 signaling. Kespohl et al (78) discovered that butyrate at low
concentrations (0.1-0.5 mM) can promote the differentiation of
Foxp3+ Tregs under TGF-β1 conditions. By contrast, when the
concentration reaches 1 mM, butyrate induces the expression of
Th1-associated genes, namely T-bet and IFN-γ, in T cells. These
dual effects are mediated by the SCFA-induced HDAC inhibition (H3
acetylation) and are independent of the FFA2/GPR43 and FFA3/GPR41
receptors, as well as the Slc5a8 transporter of SCFAs.
Additionally, butyrate elevates the expression levels of T-bet and
IFN-γ in the colon, exacerbating acute colitis in germ-free (GF)
mice. These findings suggest that butyrate requires TGF-β1 to
mediate the differentiation of anti-inflammatory Foxp3+ Tregs and
can promote inflammatory Th1-related factors at high
concentrations, with both effects being associated with HDAC
inhibition (78).
Among these T cell subsets, the balance between Th17
and Treg cells is a key factor in determining tissue immune
homeostasis and can be regulated by SCFAs (79). Studies (80,81) have revealed that butyrate
produced by Faecalibacterium prausnitzii, rather than other
metabolites, can ameliorate colitis in a DSS-induced mouse model.
Mechanistically, butyrate (used in vitro at 0.62 mM or in
vivo at 100 mg/kg) inhibits HDAC3 and c-Myc-related metabolism
in T cells, reducing Th17 differentiation. It also promotes the
expression of Foxp3 by inhibiting HDAC1 and blocks the
IL-6/STAT3/IL-17 downstream pathway. Through these mechanisms,
butyrate helps maintain Th17/Treg balance in the gut and exerts
potent anti-inflammatory effect (80,81). Butyrate at 1 mM for 5 days is
also capable of mediating the K16 acetylation of histone H4, thus
upregulating RorγT expression in differentiated Th17 cells while
downregulating it in CD4+ T cells under Th17 differentiation
conditions. This indicates that butyrate can primarily participate
in the epigenetic regulation of RorγT in Th17 cells through HDAC
inhibition and is related to specific stages of T cell
differentiation (82). More
importantly, microbiota-derived butyrate can also promote the gene
expression and protein acetylation of Foxp3 to facilitate the
differentiation of peripheral Treg cells. Butyrate (500 μM)
exerts additional regulatory effects by reducing the expression of
pro-inflammatory cytokines in DCs. It achieves this through the
acetylation of histone H3, which indirectly promotes the induction
of Treg cells. Similarly, propionate exerts HDAC inhibitory
activity, enabling it to promote the differentiation of peripheral
Treg cells. By contrast, acetate does not possess this activity
(83). The aforementioned
findings demonstrate that SCFAs can regulate the differentiation
and function of T cell subsets based on their HDAC inhibitory
activity.
B lymphocytes
SCFAs have the capacity to modulate B cell
differentiation, antibody responses and antibody-driven
autoimmunity. Notably, propionate and butyrate, primarily at low
doses (30 and 20 mM, respectively), can affect B cell function by
moderately enhancing class-switch DNA recombination (CSR). However,
at higher doses (propionate, 150 mM and butyrate, 140 mM), they
lead to a reduction in the expression of activation-induced
cytidine deaminase (AID) and Blimp1, as well as a decrease in CSR,
somatic hypermutation and plasma cell (PC) differentiation. These
effects stem from the direct regulatory role of SCFAs as the HDACi
on the intrinsic epigenetic of B cells (84). For instance, by exerting HDAC
inhibitory activity, SCFAs upregulate microRNAs that specifically
target Aicda and Prdm1 mRNAs. As a result, the expression levels of
Aicda and Prdm1 (which respectively encode AID and Blimp1) within B
cells are diminished (84).
Furthermore, as a potent immunosuppressive agent,
butyrate can drive the differentiation of regulatory B cells
(Bregs) and enhance their anti-inflammatory functions. B10 cells
represent a subset of Bregs that can produce the anti-inflammatory
cytokine IL-10. SCFAs (0.5 mM) for 48 h can promote the generation
of mouse and human B10 cells in vitro. Additionally, an
increase of B10 cells by 150 mM butyrate has been observed in both
healthy mice and mice with DSS-induced colitis. The effects of
SCFAs are dependent on HDAC inhibitory activity and are independent
of GPCRs. Butyrate, similar to other HDACi, can activate the p38
MAPK signaling pathway to facilitate B10 cell generation (85). Moreover, butyrate at 0.5 mM for
24 h possesses the ability to regulate the differentiation and
anti-inflammatory functions of IL-10+IgM+ PCs. Mechanistically, the
inhibition of HDAC3 represents a potential pathway through which
butyrate induces the differentiation of IL-10+IgM+ PCs and the
expression of IL-10 (86).
Collectively, these findings demonstrate that SCFAs exhibit
regulatory activity on B cell differentiation and function based on
HDAC inhibition.
System protection of SCFAs via HDAC
inhibition
In addition to immune cells, SCFAs also contribute
to the regulation of tissue-resident cells, thereby influencing the
host's immune equilibrium. Histone acetylation modifications are
widely recognized as being associated with key cellular processes
under both physiological and pathological conditions. In this
section, the relevant findings regarding the host-protective
effects (categorized into intestinal and extra-intestinal effects)
of microbial SCFAs based on HDAC inhibition are summarized. These
highlight that SCFAs regulate the activity and function of
intestinal immune cells and IECs through their HDAC inhibitory
activity, thereby modulating the local gut immune homeostasis. In
particular, SCFAs, mainly acetate, propionate and butyrate, exert
intestinal epithelial protective effects by inhibiting class I and
class II HDACs (2, 3 and 8) and promoting acetylation modifications
of histones H3/H4 and other sites. These protective effects
encompass, but are not limited to, the proliferation and apoptosis,
anti-bacterial effect, barrier function, absorption and metabolic
function, inflammation and immune responses of IECs (Fig. 5). Similarly, the HDAC inhibitory
activity and acetylation-modified effects of these SCFAs contribute
to tissue protection in extra-intestinal organs such as the liver,
kidney, nerves and blood vessels. Mechanistically, SCFAs can
inhibit class I and II HDACs (1, 2, 3, 4 and 8) and promote
acetylation modifications of histone H3/H4 and other sites. This
influences a wide range of activities and functions of various cell
types, including hepatocytes and hepatic stellate cells (HSCs) in
the liver, renal macrophages and epithelial cells in the kidney,
neuronal cells, microglia and astrocytes in the nervous system, and
vascular endothelial cells (Fig.
6). The aforementioned findings underscore the systemic
protection of SCFAs based on HDAC inhibition, which are associated
with the functional regulation of multiple tissue-resident cells
both inside and outside the gut.
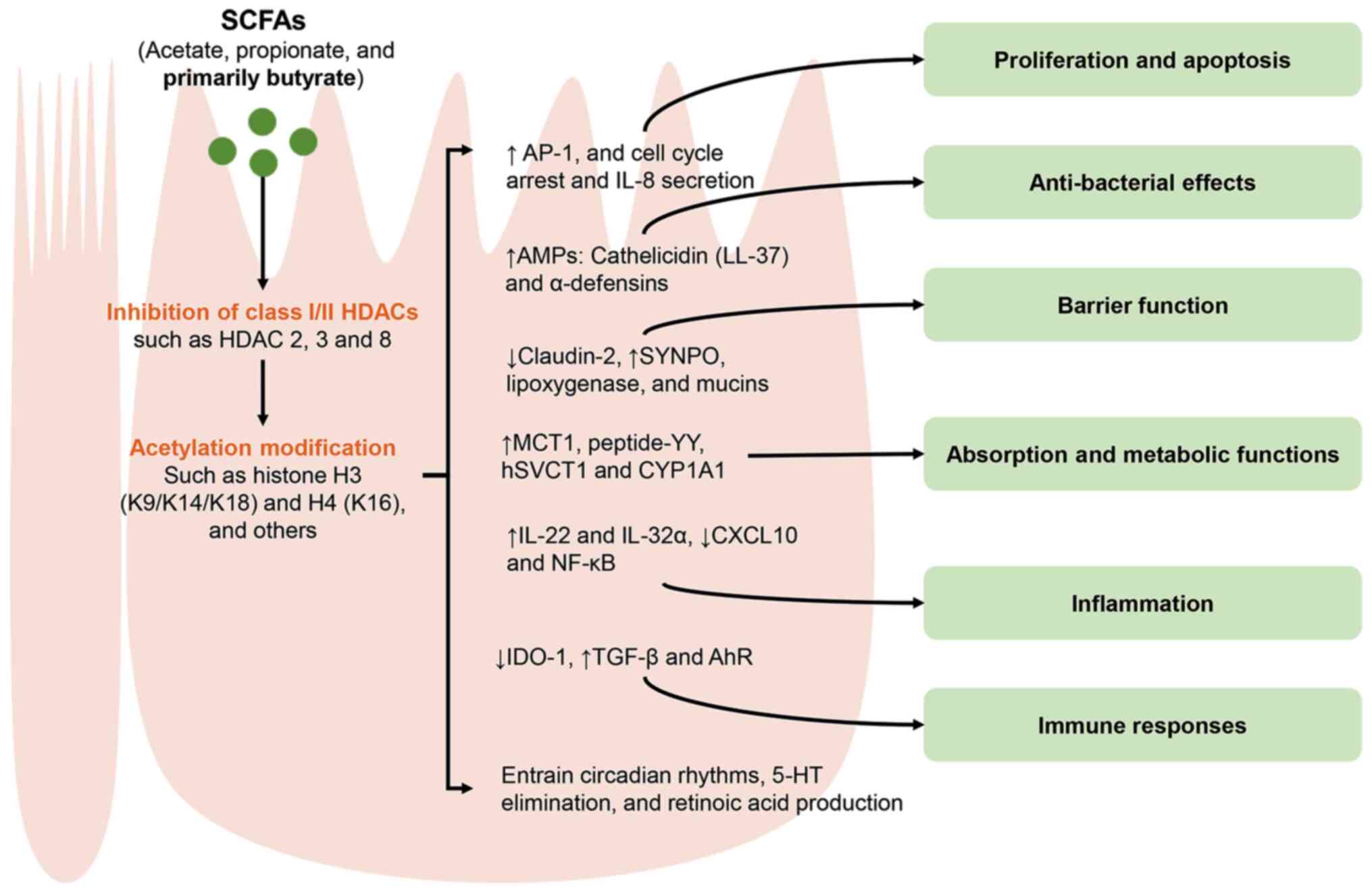 | Figure 5Regulatory effects of SCFAs on the
activities and function of IECs through HDAC inhibition. SCFAs,
such as acetate, propionate and primarily butyrate, can extensively
modulate various cellular signaling pathways by inhibiting class
I/II HDACs (2, 3 and 8) and promoting acetylation modifications of
histones H3 (K9/K14/K18), H4 (K16) and other sites. Consequently,
these actions have a notable impact on the activities and function
of IECs. IECs, intestinal epithelial cells; SCFAs, short chain
fatty acids; HDACs, histone deacetylases; AP-1, activator
protein-1; AMPs, antimicrobial peptides; MCT, monocarboxylate
transporters; SYNPO, synaptopodin; hSVCT, human sodium-dependent
vitamin C transporters; CYP1A1, cytochrome P450 1A1; CXCL10, C-X-C
motif chemokine ligand 10; IDO-1, indolamine 2,3-dioxygenase 1;
AhR, aryl hydrocarbon receptor; 5-HT,
5-hydroxytryptamine/serotonin. |
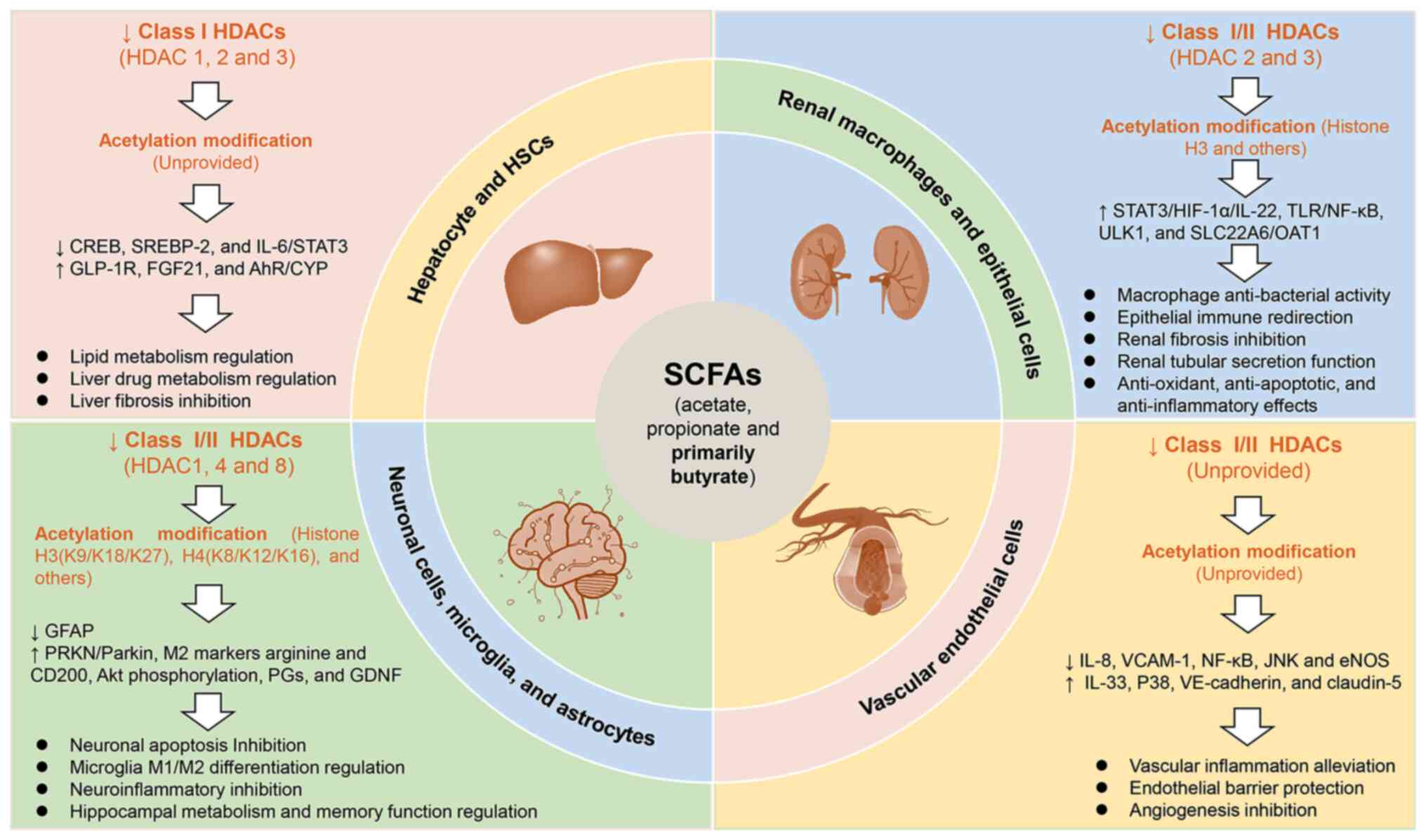 | Figure 6The extraintestinal protective
effects of SCFAs based on HDAC inhibition. The SCFAs, namely
acetate, propionate and primarily butyrate, exert immunoprotective
effects on extra-intestinal organs, including the liver, kidney,
nerves and blood vessels. These effects are achieved through the
mechanism of HDAC inhibition. Mechanistically, SCFAs are capable of
inhibiting the activities of class I and II HDACs (1, 2, 3, 4 and
8), while simultaneously regulating acetylation modifications at
histone H3 (K9/K18/K27), H4 (K8/K12/K16) and other sites. These
actions modulate the activities and functions of different
tissue-resident cells, ultimately contributing to the systemic
protection of SCFAs. SCFAs, short chain fatty acids; HDAC, histone
deacetylase; CREB, cyclic adenosine monophosphate response element
binding protein; SREBP, sterol regulatory element-binding
transcription factor; GLP-1R, glucagon-like peptide-1 receptor;
FGF21, fibroblast growth factor 21; AhR, aryl hydrocarbon receptor;
CYP, cytochromes P450; GFAP, glial fibrillary acidic protein; PRKN,
parkin RBR E3 ubiquitin protein ligase; M1, macrophage subset 1;
M2, macrophage subset 2; PGs, prostaglandins; GDNF, glial cell line
derived neurotrophic factor; HSCs, hepatic stellate cellsHIF-1α,
hypoxia inducible factor-1α; TLR, toll-like receptor; ULK1, unc-51
like autophagy activating kinase 1; SLC22A6, solute carrier family
22 member 6; OAT1, organic anion transporter-1; VCAM-1, vascular
cell adhesion molecule-1; eNOS, endothelial nitric oxide
synthase. |
Inside the gut
Immune cells
SCFAs can alleviate intestinal immune cell
inflammation through HDAC inhibition and acetylation modification.
For instance, research has revealed that butyrate is more effective
than acetate and propionate in markedly reducing LPS-induced
expression of pro-inflammatory mediators in both BMDMs and colonic
LP macrophages in vitro and in vivo. This
anti-inflammatory effect of butyrate is independent of GPCRs and
instead occurs through HDAC inhibition, which increases H3K9
acetylation at the promoter regions of cellular inflammatory genes
(39). In addition, under
colitis conditions, butyrate can improve anemia and reduce TNF-α
production by colonic macrophages. It does so by promoting
ferroportin (FPN)-dependent iron export from macrophages, thus
regulating iron homeostasis. The FPN-inducing effect of butyrate (1
mM in vitro) relies on its inhibitory activity against HDAC
at the Slc40a1 promoter (87).
Compared with other SCFAs, butyrate (0.0625-0.5 mM) administered
for 4 days more effectively reduces the activation, proliferation
and production of inflammatory cytokines (such as IFN-γ and IL-17)
in human intestinal LP CD4+ T cells in vitro. The effects of
butyrate can be attributed to its promotion of histone acetylation
in both unstimulated and TCR-stimulated CD4+ T cells, as well as
through GPR43 signaling (68).
Butyrate (used in vivo at 200 mM or in vitro at 0.5
mM) can also promote IL-22 production by intestinal and circulating
CD4+ T cells and ILCs via GPR41 and HDAC inhibition, thereby
suppressing intestinal infection and inflammation. Mechanistically,
butyrate upregulates the expression of AhR and HIF-1α, which are
differentially regulated by mTOR and STAT3, leading to increased
IL-22 levels in CD4+ T cells under Th1 conditions. Importantly,
butyrate also increases the accessibility of HIF-1α binding sites
at the IL-22 promoter in CD4+ T cells through histone modification
(69). γδT cells are
tissue-resident T cells that carry out innate immune functions and
can produce the pro-inflammatory cytokine IL-17, participating in
host defense and the regulation of tissue inflammation. A previous
study has revealed that the SCFA propionate (200 mM in vivo
or 1 mM in vitro) can directly act on intestinal γδT cell
populations and inhibit IL-17 production, with its mechanism of
action being associated with HDAC inhibition. Therefore, the impact
of propionate on γδT cell activity may provide a potential pathway
to prevent chronic intestinal inflammation and cancer progression
(88).
IECs
The regulatory roles of microbial SCFAs in the
activities and function of IECs through HDAC inhibition are
currently under extensive investigation (89-119). These effects of SCFAs are
multifaceted, encompassing but not limited to cellular activities
such as proliferation, differentiation and anti-bacterial action,
as well as cellular functions including absorption, metabolism,
inflammation and immunity.
First and foremost, SCFAs can regulate the
proliferative activity of IECs via HDAC inhibition or histone
acetylation. For instance, 2 mM butyrate can activate the AP-1
response in colonic epithelial cells by inhibiting HDACs. AP-1 is a
transcription factor that carries out a key role in determining
cell proliferation, differentiation, transformation, cell migration
and apoptosis (89). A recent
study has demonstrated that butyrate at 150 mM in vivo or 1
mM in vitro can also restrict the differentiation and
proliferation of intestinal tuft cells by inhibiting HDAC3, serving
as an important product of the microbiota in calibrating intestinal
type 2 immunity (90). Another
SCFA, propionate, can promote the spreading and polarization of
epithelial cells by inhibiting HDACs and activating GPR43 and
STAT3, thereby enhancing the renewal rate and persistence of the
intestinal epithelium (91). It
is important to emphasize that, by contrast, butyrate exerts an
inhibitory effect on the cellular activity of cancerous IECs or can
induce apoptosis. For example, Mariadason et al (92) revealed that 2 mM butyrate
markedly triggers activities such as cell cycle arrest, apoptosis,
IL-8 secretion, in undifferentiated colon Caco-2 cells, and
consistently, while consistently and selectively inducing high
levels of histone hyperacetylation. Previously, it was reported
that butyrate could have contradictory effects on the activity of
normal colonic epithelial cells and cancer cells. For instance,
when exposed to the HDACi butyrate at a concentration of 1-2 mM,
colon cancer cell lines exhibit a notable increase in alkaline
phosphatase activities, urokinase receptor expression and IL-8
secretion. By contrast, butyrate inhibits alkaline phosphatase
activities in the primary normal cells but markedly suppresses
urokinase receptor and IL-8 secretion (93). Another example is that 5 mM
butyrate can inhibit cell proliferation and stimulate cell
differentiation in the colon cancer cell line HT-29. The effects of
butyrate in this cell line stem from the induction of cyclin D3 and
p21, as well as its role as an HDACi in promoting histone H4
hyperacetylation (94).
AMPs are potent defensive molecules released by IECs
that participate in innate immunity, carrying out a key role in
maintaining mucosal homeostasis (95). Current research has yielded
substantial evidence indicating that HDACs affect the expression of
relevant defense genes. In particular, the regulatory function of
microbial SCFAs as HDACi in modulating the anti-bacterial activity
of IECs, mediated by AMPs, is being gradually elucidated. For
example, Fischer et al (95) has indicated that inhibiting HDAC
activity can enhance AMP production from IECs upon bacterial
challenge, without increasing the expression of inflammatory
cytokines. Cathelicidin (LL-37) is a major AMP within the
intestinal non-specific innate immune system. The SCFA butyrate
(2-4 mM) can induce the expression of LL-37 in a time-dependent
manner in gastrointestinal cancer cells that lack LL-37. The
induction of LL-37 by butyrate is due to the parallel acetylation
of histone H4 and non-histone HMGN2, and is associated with the
suppression of MEK-ERK signaling pathway (96). α-defensins, also known as
cryptdins, are the primary bactericidal AMP molecules produced by
intestinal Paneth cells and their activation requires MMP7. SCFAs,
including butyrate, propionate and acetate, induce the expression
of α-defensins and MMP7 in Paneth cells in vitro. Histone
deacetylation (specifically HDAC8 inhibition) and STAT3 might be
involved in the butyrate (1-2 mM)-mediated induction of α-defensin,
contributing to the restoration of the intestinal anti-microbial
barrier function (97). However,
not all regulation of AMP genes by SCFAs is determined by
inhibiting HDAC activity. For instance, the induction of host
defense peptides, namely pBD3 and pEP2C, by butyrate in porcine
IECs (IPEC J2) is associated with the expression of TLR2 and EGFR,
rather than HDAC inhibition (98).
HDAC inhibition carries out a key role in how SCFAs
maintain the barrier function of IECs. Representative SCFAs, namely
butyrate, propionate and acetate, reduce the activation of
epithelial Caco-2 cells induced by inflammatory cytokines such as
TNF-α, IFN-γ and IL-1β. In particular, butyrate (0-8 mM) exerts a
pronounced dose-dependent preventive effect on inflammation and
inflammation-induced barrier disruption in both
inflammation-induced Caco-2 cells and co-culture models of
PBMCs/Caco-2 cells. Additionally, butyrate can regulate the release
of inflammatory cytokines from activated PBMCs and immune cell
phenotypes. Meanwhile, the HDACi can replicate the epithelial
barrier-protective effect of butyrate (99). Notably, the mechanisms by which
SCFAs, mainly butyrate, enhance IEC barrier function by inhibiting
HDAC expression are currently being elucidated (100-103). For example, one study showed
that butyrate (5 or 10 mM) can suppress the expression of
claudin-2, a permeability-promoting tight-junction protein, in IECs
in an IL-10RA-dependent manner. This suppression is achieved
through the combined actions of HDAC inhibition (H3K9 acetylation)
and STAT3 activation (100).
The actin-binding protein synaptopodin (SYNPO) is localized within
intestinal epithelial tight junctions (TJs) and F-actin stress
fibers, carrying out a key role in maintaining barrier integrity
and cell motility. The mechanism underlying the relationship
between butyrate (5 mM) and epithelial barrier restoration likely
involves the induction of SYNPO expression through HDAC inhibition
in both epithelial cell lines and murine colonic enteroids
(101). Furthermore, 1 mM
butyrate can reduce TJ permeability in intestinal monolayers by
activating lipoxygenase and the HDACi TSA mimics this effect of
butyrate (102). When butyrate
(2 mM for 24 h) serves as an energy source for the human polarized
colonic goblet cell line HT29-Cl.16E, it promotes the expression of
mucin (MUC) genes that protect the epithelial barrier. It is
noteworthy that the HDAC inhibitory action of butyrate can
participate in regulating the expression of MUC3 gene, yet it does
not affect the expression of the MUC2, MUC5AC and MUC5B genes
(103).
SCFAs can regulate the absorption and metabolic
function of IECs through HDAC inhibition. Indeed, the
representative SCFA, butyrate, serves as the primary energy source
for colonic cells. However, the absorption of butyrate is not
actually secondary to its inhibition of HDACs. A study has
demonstrated that butyrate at 5 mM for 24 h activates the MCT1
promoter via the NF-κB pathway rather than through HDAC, leading to
an enhanced absorption of SCFAs in Caco-2 cells (104). Consequently, as the effective
HDACi, SCFAs carry out a more notable role in regulating the
absorption and metabolic behaviors of nutrients or other exogenous
substances in the IECs. For instance, when propionate and butyrate
are each administered at 2 mM for 24 h, they considerably increase
the expression of peptide-YY (PYY) in both human intestinal cell
lines and mouse primary cells. PYY is an important hormone secreted
by heterogeneous enteroendocrine cells that is involved in food
intake and insulin secretion. This effect is primarily attributed
to the HDAC-inhibitory activity of SCFAs and the partial role of
FFA2, also known as GPR43 (105). Butyrate can also mediate
epigenetic regulation of the sodium-dependent vitamin C
transporters (such as hSVCT1) by inhibiting HDAC isoforms 2 and 3,
thereby regulating the absorption of vitamin C in the intestinal
epithelium (106).
Nevertheless, butyrate also has the ability to regulate the
metabolism of cytotoxic substances in epithelial cells. Sodium
butyrate (0.5 mM) can induce the acetylation of histone H3 (at
Lys14) and histone H4 (at Lys16) in colonic epithelial cells. This
action triggers the expression of cytochrome P450 1A1 (CYP1A1), a
metabolic enzyme for dietary carcinogens such as benzo[a]pyrene
(BaP) (107).
The regulatory role of SCFAs in epithelial
inflammation can also be partially attributed to the inhibition of
HDAC. Specifically, SCFAs can directly modulate the cytokine
releasing of IECs through inhibiting HDAC activity. For instance,
butyrate at 3 mM for 16 h can markedly enhance the signaling of the
protective cytokine IL-22 in human epithelial Caco-2 and DLD1
cells. Independently of IL-22R1 regulation, butyrate can increase
the accessibility of STAT3 at affected gene loci, and this
mechanism is associated with HDAC inhibition (108). When administered at the same
concentration of 10 mM for 12 h, butyrate, rather than acetate and
propionate, can stimulate the expression of the cytotoxic
pro-inflammatory cytokine IL-32α in IEC lines, namely HT-29, SW480
and T84. Additionally, butyrate can enhance IL-1β-induced
expression of IL-32α mRNA. This effect is not associated with the
activity of PI3K, a mechanism involved in IL-32α expression.
Instead, it predominantly depends more heavily on the inhibition of
HDACs (109). When compared
with acetate and propionate, 2 mM butyrate emerges as the most
potent inhibitor in preventing the release of CXCL10 from the IEC
HT-29 cells activated by IFN-γ and IFN-γ+TNF-α. This action
contributes to reducing epithelial inflammation, maintaining immune
homeostasis and upholding the integrity of the gut barrier.
Butyrate also inhibits the protein expression of IRF9 and
phosphorylated JAK2, along with the mRNA expression of CXCL10,
SOCS1, JAK2 and IRF9 in cells. By inhibiting HDAC activity,
butyrate prevents the epithelial release of CXCL10, with an effect
comparable with that of the well-known HDACi such as TSA (110). The impact of butyrate on the
colonic inflammatory response is partly due to its regulation on
NF-κB activation based on HDAC inhibition. Exposure of HT-29 cells
to 4 mM butyrate for 18 h eliminates their constitutive NF-κB p50
dimer activity. Another notable finding is that butyrate
selectively regulates NF-κB activation. It can markedly suppress
the activation of NF-κB triggered by TNF-α and phorbol ester, while
enhancing the NF-κB activation induced by IL-1β. The regulatory
effect of butyrate on NF-κB may be partially attributed to its
ability to inhibit HDACs, as the HDACi TSA exerts a similar effect
(111).
SCFAs, primarily butyrate, also possess regulatory
effects on the immune activity of IECs, which can be influenced by
the HDAC inhibitory activity. For instance, butyrate (0.5-8 mM; 24
h) can modulate the expression of the immune effector indolamine
2,3-dioxygenase 1 (IDO-1) in both human primary IECs and IEC cell
lines. Independent of the transcriptional mechanisms involving
GPCRs (GPR41, GPR43 and GPR109a), butyrate downregulates IDO-1
expression through a dual mechanism: A reduction in STAT1 levels
and its HDAC inhibitory property (112). The SCFA butyrate also regulates
host T-cell responses by interacting with IECs to improve the
intestinal mucosal immune system. One notable effect is the
enhancement of the accumulation of anti-inflammatory Treg cells in
the gut. In vitro studies have demonstrated that butyrate
can induce the TGF-β production in primary IEC cells or cell lines,
promoting the expression of Foxp3 and the anti-inflammatory
cytokine IL-10 under Treg conditions. This inductive effect of
butyrate is independent of GPCRs and their associated signaling
pathways but relies on HDAC inhibition mediated by SP1 (113,114). A study has shown that SCFAs
(acetate, propionate and butyrate) and AhR play similar protective
roles in intestinal inflammation and Treg cell induction. In
particular, 5 mM butyrate markedly increases the expression of
AhR-responsive genes (such as Cyp1a1/CYP1A1) in mouse colon cells
YAMC and human colon cells Caco-2. The HDACi, including
panobinostat and vorinostat, exhibit effects similar to those of
SCFAs. They enhance AhR ligand-mediated induction and subsequent
histone acetylation (115).
Modoux et al (116) have
uncovered that butyrate is not an AhR ligand but acts as an HDACi
to remodel chromatin. By synergizing with known ligands, butyrate
increases the recruitment of AhR to the promoters of target genes,
thereby enhancing AhR activation.
Moreover, when SCFAs act as HDACi, they can also
influence intestinal epithelial homeostasis through alternative
pathways (117-119). For instance, previous research
has disclosed that SCFAs (primarily 1 mM butyrate) produced by gut
microbiota can entrain intestinal epithelial circadian rhythms via
an HDACi-dependent mechanism. This finding deepens the
comprehension of how the microbial and circadian rhythm networks
jointly regulate intestinal epithelial homeostasis (117). Both treatment with the HDACi
butyrate (5 mM) and TSA (1 μM) for 24 h can reduce the
expression of the serotonin (5-HT) transporter (SERT) in Caco-2
cells. Consequently, this reduction promotes the clearance of
extracellular 5-HT. Reduced SERT expression and the subsequent high
levels of 5-HT are associated with various intestinal diseases,
such as inflammation or intestinal infections. The mechanism by
which butyrate and TSA downregulate intestinal SERT involves the
inhibition of HDAC2 and an increased binding of acetylated histone
H3 or H4 to the human SERT (hSERT) promoter 1 (hSERTp1) (118). Moreover, the SCFA butyrate at 2
mM for 24 h has the ability to stimulate the production of retinoic
acid in the IEC cell line through HDAC3 inhibition (resulting in
H3K18 acetylation), thereby promoting mucosal homeostasis. This is
attributed to the fact that IECs play a key role in upholding
mucosal immune homeostasis, through the production of retinoic acid
(119).
Outside the gut
Hepatic protection
Current evidence indicates that among SCFAs,
butyrate primarily exerts regulatory effects on the activity and
function of hepatocytes through HDAC inhibition. The HDACi action
of butyrate is mainly associated with the metabolic functions of
the liver, with a particular emphasis on lipid metabolism. For
instance, Zheng et al (120) discovered that butyrate (at 2 mM
in vitro and 5% butyrate by weight in vivo) can
suppress lipogenic genes and activate genes related to lipid
oxidation in hepatocytes. As a result, it ameliorates hepatic
steatosis and abnormal lipid metabolism in mice fed with high-fat
and fiber-deficient diets. The underlying molecular mechanism of
butyrate has been elucidated as the activation of the liver
GPR41/43-mediated CaMKII-CREB signaling pathway and the inhibition
of the HDAC1-CREB signaling pathway. Glucagon-like peptide-1
(GLP-1) is a protein involved in regulating metabolic processes and
has a beneficial effect on non-alcoholic fatty liver disease and
steatohepatitis. The study has shown that butyrate can reverse the
decline in hepatic GLP-1 caused by a high-fat diet and alleviate
hepatic steatosis. From a mechanistic perspective, butyrate (5 mM)
for 24 h enhances the expression of GLP-1R in liver HepG2 cells by
inhibiting HDAC2, rather than through the activity of GPR43/GPR109a
(121). In addition, butyrate
(500 mg/kg body weight and 0.5 mM, respectively) can induce the
gene expression of FGF21 in the liver and HepG2 cells by inhibiting
HDAC3. FGF21 is known to stimulate fatty acid oxidation and ketone
body production (122). Similar
to the HDACi TSA, 5 mM butyrate can also effectively reduce the
cholesterol content in HepG2 cells, suggesting that HDAC inhibition
is its underlying mechanism of action. Importantly, the potential
mechanisms by which these HDACi regulate hepatic cholesterol
metabolism are also associated with impaired SREBP-2 signaling
(123). Butyrate also exerts
regulatory effects on hepatic drug metabolism. Cytochromes P450
(CYPs) are key enzymes in liver drug metabolism. A previous study
has shown that butyrate (1-5 mM) can activate the AhR receptor and
increase the expression of its target gene CYP in the liver cell
line HepG2-C3 and primary human hepatocytes. The activation of
hepatic AhR by butyrate may be attributed to the epigenetic
regulatory effect of HDAC inhibition (124). The activation of HSCs is
closely associated with the development of liver fibrosis. Notably,
HDACs are key regulators of HSC activation. Zhao et al
(125) revealed that the HDACi
butyrate can inhibit the activity of liver stellate cells LX2 by
inhibiting HDAC2 and the subsequent activation of the IL-6/STAT3
pathway. The in vivo study has also demonstrated that
butyrate (0.3 mg/g) can alleviate liver fibrosis in bile duct
ligation mice and improve the intestinal microenvironment by
inhibiting the HDAC2/IL-6/STAT3 pathway.
Renal protection
SCFAs are also recognized as a key class of renal
protectants. Leveraging their HDAC inhibitory activity, SCFAs can
markedly contribute to the bactericidal activity of head kidney
macrophages (HKMs). Research conducted by Zhang et al
(126) has shown that the SCFA
butyrate (10 mM; 24 h) can activate the STAT3/HIF-1α/IL-22
signaling pathway through HDAC3 inhibition and GPCR action, thereby
inducing HKM autophagy that aids in pathogen clearance. Further
evidence suggests that SCFAs exert renoprotective effects by
modulating the activity and function of renal epithelial cells
through HDAC inhibition. As effective HDACi, treatment with
butyrate (10 mM), propionate (10 mM) and TSA (2 μM) each for
30 min can all result in a rapid increase in histone acetylation in
renal-derived epithelial cells 293 and enhance NF-κB activation
induced by TLR agonists. This indicates that SCFAs can rapidly
alter the epigenetic state of epithelial cells, resulting in the
redirection of epithelial immune responses (10). SCFAs propionate and butyrate
administered orally at 300 mg/kg/day for 8 weeks can trigger
autophagy in renal tubular cells and alleviate renal fibrosis in
diabetic mice. They achieve this by inhibiting HDAC2, which
promotes histone H3 acetylation in the ULK1 promoter and
facilitates the transcription of downstream genes (127). When applied at a concentration
of 1 mM each for 24 h, butyrate and propionate can notably increase
the activity of the organic anion transporter-1 by upregulating the
SLC22A6 gene, thereby regulating the secretory function of renal
tubular cells and maintaining kidney health. It has been shown that
SCFAs operate independently of GPCR activation and instead exert
their effects by inhibiting the expression of class II HDAC genes
(128). Importantly, the
renoprotective effects of butyrate, a representative HDACi, on
epithelial cells extend far beyond these mechanisms. For instance,
butyrate (1 mM) can act as an anti-oxidant and inhibit apoptosis in
high glucose-induced normal rat kidney tubular epithelial (NRK-52E)
cells, with its mechanism of action related to HDAC2 inhibition
(129). Moreover, butyrate (400
mg/kg, i.p.) can ameliorate proteinuria and alleviate
glomerulosclerosis and tissue inflammation by maintaining podocytes
in the glomerular basement membrane through epigenetic and
GPR109a-mediated mechanisms. The protective effects of butyrate are
associated with an increase in podocyte-related proteins and the
restoration of acetylation and methylation at their gene promoter
sites (130).
Neuroprotection
SCFAs are considered highly promising
neuroprotective and anti-inflammatory agents, playing a key role in
the activity and function of neuronal cells as well as glial cells,
including as microglia and astrocytes. Importantly, SCFAs may act
as neuroprotective agents due to their HDAC inhibitory properties
(131-140).
Primarily, butyrate, which serves as a major
component of SCFAs, exerts direct protective effects on neuronal
cells through HDAC inhibition and subsequent histone acetylation.
Research has revealed that metabolic disturbances in SCFAs, mainly
characterized by low levels of butyrate, are associated with an
increase in neuronal apoptosis. This phenomenon occurs as a result
of the elevated expression of HDAC4 in the rat hippocampus and the
consequent alteration of H4K8ac, H4K12ac and H4K16ac. This finding
underscores the notable neuroprotective potential of butyrate, as
it can inhibit the HDAC4-mediated neuronal apoptosis pathway via
the gut-brain axis (131).
Excessive mitochondrial accumulation in diabetes represents one of
the leading causes of cognitive impairment, as it induces neuronal
apoptosis. A study conducted by Cho et al (132) revealed that butyrate (500
μM; 30 min) can ameliorate high glucose-induced neuronal
mitophagy dysfunction and neuronal apoptosis by restoring
PRKN/Parkin expression levels. This positions butyrate as an
important active component in protecting against diabetes-related
cognitive impairment. Mechanistically, butyrate primarily restores
the PRKN-mediated neuronal mitophagy function by inhibiting the
RELA-HDAC8 complex.
Microglia represent a distinct type of glial cells
within the nervous system, endowed with phagocytic capabilities.
Their abnormal activation is associated with inflammation in the
central nervous system. Microglia manifest two distinct phenotypes:
The M1 phenotype predominantly produces pro-inflammatory mediators,
whereas the M2 phenotype releases anti-inflammatory factors that
are involved in immunosuppression and neuroprotection. SCFAs can
directly modulate the activity and function of microglia, which are
the innate immune cells in the brain, thereby alleviating
neuroinflammation. For instance, propionate and butyrate given
orally at 300 mg/kg each for 7 days can reduce the activation of
microglia and the levels of pro-inflammatory cytokines, as well as
inhibit HDAC1 expression levels and promote the H3K9 acetylation in
the brains of GF rats (133).
Notably, butyrate emerges as a key SCFA in regulating microglial
activity. A study has shown that butyrate (0.1 mM; 1 and 10 mM) can
polarize the M1 phenotype of microglia towards the M2 phenotype
after oxygen and glucose deprivation. Acting as an HDACi, butyrate
suppresses the expression of pro-inflammatory genes CD86 and IL1-β
in microglia, and increases the expression of anti-inflammatory M2
markers, arginase and CD200 (134). Butyrate at a concentration of
1.2 mM, along with other HDACi, can markedly induce apoptosis and
attenuate the pro-inflammatory response in microglia activated by
LPS. This process is accompanied by a loss of mitochondrial
membrane potential and hyperacetylation of histone H3 (135). The study by Wang et al
(136) confirmed the
anti-inflammatory effect of butyrate in LPS-induced microglia from
the perspective of microglial process elongation. They discovered
that 5 mM butyrate could promote Akt phosphorylation and subsequent
functional elongation of microglial processes, thereby alleviating
neuroinflammation, an effect associated with HDAC inhibition
(136). However, low
concentrations of butyrate (0.01-1 mM) can markedly enhance the
inflammatory response of LPS-induced microglia. The effects of
butyrate include promoting the release of prostaglandins (PGs) such
as PGE2, PGD2 and 8-iso-PGF2α, as well as the release and gene
expression of pro-inflammatory cytokines, without affecting the
activation of MAPKs p38, ERK1/2 and JNK signaling pathways.
Importantly, the promoting effect of butyrate on PGs release from
activated microglia is associated with histone acetylation at the
H3-K18 site (137).
SCFAs, primarily butyrate, exhibit neuroprotective
activity in astrocytes by exerting HDAC inhibitory effects.
Research by Wu et al (138) revealed that the
astrocyte-protective role of butyrate, mediated by HDAC inhibition,
could serve as a potential therapeutic target for neurodegenerative
diseases such as Parkinson's disease. This is attributed to the
fact that butyrate (1.2 mM), similar to other HDACi such as VPA and
TSA, protects dopaminergic neurons in neuron-glia cultures and
upregulates the expression of astrocyte-derived neurotrophic
factors, glial cell line derived neurotrophic factor (GDNF) and
brain-derived neurotrophic factor. The effects of butyrate rely, at
least partially, on HDAC inhibition, which increases GDNF promoter
activity and induces hyperacetylation of histone H3 associated with
the GDNF promoter. Furthermore, the HDAC inhibitory effect of
butyrate is pivotal for preserving energy metabolism and memory
function in hippocampal astrocytes. However, a maternal
high-fructose diet can disrupt butyrate signaling and subsequent
HDAC4-mediated epigenetic changes, leading to reduced mitochondrial
function in the hippocampus (139). Nevertheless, the HDAC
inhibitory effect of butyrate can occasionally restrict astrocyte
activity to sustain normal neural function. For instance, butyrate
(5 mM; 24 h) diminishes the expression of glial fibrillary acidic
protein (GFAP), the main intermediate filament in astrocytes, in
both primary human astrocytes and astrocytoma cells through HDAC
inhibition (H3K27 acetylation). Conversely, dysregulation of gene
expression of histone acetylation can lead to GFAP aggregation,
which is a hallmark of human diseases such as Alexander's disease
(140).
Vascular protection
SCFAs primarily exhibit vascular protective
activity by ameliorating endothelial inflammation through the
inhibition of HDACs. For instance, research conducted by Li et
al (141) revealed that
SCFAs are capable of suppressing the endothelial inflammatory
responses induced by LPS or TNF-α, as well as the overexpression of
vascular cell adhesion molecule-1. The underlying mechanisms are
associated with the activation of GPR41/43 or the inhibition of
HDACs in human umbilical vein endothelial cells (HUVECs).
Specifically, the activation of GPR41/43 mediates the effects of
acetate on IL-6 and IL-8 production, along with the effects of
propionate and butyrate on IL-6 production. Additionally, HDAC
inhibition mediates the impacts of propionate (0.3 mM) and butyrate
(0.1 mM) for 12 h on IL-8 production, VCAM-1 expression, and the
adhesion of PBMC to an endothelial monolayer. Furthermore, the
researchers also validated the anti-inflammatory mechanisms of
propionate and butyrate in TNF-α-induced endothelial activation. It
was discovered that the HDACs/IL-33/NF-κB pathway mediates their
inhibitory activity on endothelial IL-8 production. By contrast,
the HDACs/MAPK signaling pathway, rather than IL-33, is involved in
their inhibitory effect on VCAM-1 expression (142). These findings contribute to the
understanding of the endothelial-protective effects of SCFAs based
on HDAC inhibition.
Besides the aforementioned effects, SCFAs confer
other forms of vascular protection through HDAC inhibition,
including endothelial barrier protection and angiogenesis
inhibition. For example, acetate, propionate and butyrate have all
been found to effectively reduce paracellular permeability, thereby
protecting the endothelial barrier. Propionate and butyrate at low
concentrations of 1 and 0.5 mM, respectively, can decrease
paracellular permeability in HUVECs without inducing cell damage,
whereas acetate reduces paracellular permeability in a
concentration-dependent manner. Notably, the HDACi TSA at lower
concentrations also reduce paracellular permeability, suggesting
the potential for HDAC inhibition in mediating SCFA-induced
endothelial barrier protection (143). Butyrate, in particular, carries
out a pivotal role in maintaining endothelial integrity and renal
metabolic homeostasis. It can reduce the proliferation of human
glomerular microvascular endothelial cells (hgMVECs), strengthen
the endothelial barrier by increasing the expression of VE-cadherin
and claudin-5, and promote mitochondrial biogenesis. Additionally,
butyrate mitigates the LPS-induced increase in oxygen consumption
to protect mitochondrial function in hgMVECs (144). Endothelial NOS (eNOS)-derived
NO carries out a role in the vasorelaxation and angiogenesis
signaling of endothelial cells. Class I and II HDACi can
effectively suppress angiogenesis stimulated by hypoxia or vascular
endothelial growth factor. Butyrate (10 μM), along with
other HDACi such as TSA (1 μM) and MS-275 (10 μM),
can reduce the expression of both eNOS protein and mRNA in HUVECs
(145).
The potential therapeutic effects of SCFAs
as HDACi in diseases
At present, epigenetics has emerged as a promising
field for elucidating the pathogenesis of human diseases.
Epigenetic regulation does not alter the genomic sequence but can
affect gene expression, cellular function and susceptibility to
diseases. Importantly, epigenetic modifications are intricate and
reversible, depending on multiple variable factors. Consequently,
epigenetic therapies hold great promise as innovative treatment
modalities for human diseases (146,147). Acetylation modification by HDAC
is a common form of epigenetic regulation. HDACs are responsible
for the deacetylation of histones and non-histone proteins, serving
as key regulators of gene transcription and cellular functions.
Over the years, HDACs have been recognized as key therapeutic
targets for a wide range of human diseases, including tissue
fibrosis, autoimmune/inflammatory diseases, metabolic disorders and
neurological diseases (148).
Currently, HDACi that block the activity of specific or a range of
HDACs have become potential epigenetic regulators, carrying out a
key role in the treatment of human diseases. Notably, several HDACi
approved by the US Food and Drug Administration, including
belinostat, panobinostat, romidepsin, suberoylanilide hydroxamic
acid (SAHA) and VPA. These inhibitors exhibit remarkable
anti-inflammatory properties that contribute to the improvement of
various inflammatory diseases (149). Furthermore, certain HDACi, such
as VPA, SAHA and TSA, also possess neuroprotective effects and have
demonstrated considerable therapeutic efficacy in neurological
diseases (150). The
aforementioned findings suggest that these HDACi may possess
therapeutic potential in maintaining host immune homeostasis across
various diseases through epigenetic regulation.
It is widely acknowledged that SCFAs are vital
microbial metabolites that represent a class of potential HDACi to
markedly impact human health. As previously discussed, acetate,
propionate and butyrate, which are key components of SCFAs, can act
as HDACi to exert immunomodulatory effects at both the immune cells
and systemic levels. In particular, the HDAC-inhibitory activity of
SCFAs can induce immunomodulatory and tissue-protective effects not
only within the local intestinal milieu but also in
extra-intestinal organs, such as the liver, kidneys, nerves and
blood vessels. The aforementioned findings suggest that SCFAs hold
great promise as effective HDACi for maintaining host immune
homeostasis. To date, the therapeutic potential of SCFAs through
HDAC inhibition in various immune-related diseases, including both
intestinal and extraintestinal conditions, is being extensively
investigated. Therefore, this section aims to provide a concise
summary of the relevant findings, which are systematically compiled
in the Table I (80,81,151-155), Table II (120,121,125,156-158), Table III (127,129,159-161), Table IV (132,162-165), Table V (166-168), and Table VI (35,169-175).
 | Table IPotential therapeutic effects of
SCFAs as HDACis in intestinal diseases. |
Table I
Potential therapeutic effects of
SCFAs as HDACis in intestinal diseases.
| Groups | Disease models | Therapeutic
effects | Associated
cells | Associated
acetylation | Associated
HDACs | (Refs.) |
|---|
| Butyrate | DSS-induced
colitis | Enhance intestinal
epithelial barrier function and ameliorate intestinal
inflammation | IECs | Histone
acetylation | Inhibit the
HDAC8/NF-κB pathway to enhance Slc26a3 expression | (151) |
| DSS-induced
colitis | Ameliorate
intestinal inflammation | IECs and
Macrophages | Histone H3
acetylation | Unprovided | (152) |
| DSS-induced
colitis | Ameliorate
intestinal inflammation | Neutrophils | Histone H3
acetylation | Inhibit HDAC9 | (153) |
| DSS- and
TNBS-induced colitis | Maintain Th17/Treg
balance and ameliorate intestinal inflammation | T cells (Th17/Treg
cells) |
Acetylation-promoted degradation of
c-Myc | Inhibit HDAC1 and
3 | (80,81) |
| Propionate | DSS-induced
colitis | Modulate the
function of macrophages and ameliorate intestinal inflammation | Macrophages | Histone
acetylation | Inhibit HDAC1 and
2 | (154,155) |
 | Table IIPotential therapeutic effects of
SCFAs as HDACi in liver diseases. |
Table II
Potential therapeutic effects of
SCFAs as HDACi in liver diseases.
| Authors, year | Groups | Disease models | Therapeutic
effects | Associated
cells | Associated
acetylation | Associated
HDACs | (Refs.) |
|---|
| Zhang et al,
2024 | Butyrate | Alcoholic liver
disease (ALD) | Regulate macrophage
polarization and suppresses hepatic inflammation | Macrophages | Unprovided | Inhibit
HDAC1/miR-155 axis | (156) |
| Zhao et al,
2025 | | Biliary
atresia-induced liver fibrosis | Alleviate liver
fibrosis | HSCs | Unprovided | Inhibit
HDAC2/IL-6/STAT3 pathway | (125) |
| Zhou et al,
2018 | | High-fat
diet-induced non-alcoholic steatohepatitis | Alleviate hepatic
steatosis | Hepatocytes | Unprovided | Inhibit HDAC2 to
increase hepatic GLP-1R expression | (121) |
| Zheng et al,
2023 | | High-fat and
fiber-deficient diet-induced Non-alcoholic fatty liver disease | Attenuate hepatic
steatosis and improve hepatic lipid metabolism | Hepatocytes | Unprovided | Regulate
GPR41/43-CaMKII/HDAC1-CREB pathway | (120) |
| Sun et al,
2014 | | Ischemia
reperfusion-induced liver injury | Attenuate hepatic
injury and inflammation | Unprovided | Histone H3
acetylation | Unprovided | (157) |
| Olaniyi and Amusa,
2020 | Acetate |
Streptozotocin-nicotinamide-induced
diabetes mellitus | Alleviate hepatic
lipid dysregulation and its accompanied injury | Unprovided | Unprovided | Inhibit HDACs | (158) |
 | Table IIIPotential therapeutic effects of
SCFAs as HDACi in kidney diseases. |
Table III
Potential therapeutic effects of
SCFAs as HDACi in kidney diseases.
| Authors, year | Groups | Disease models | Therapeutic
effects | Associated
cells | Associated
acetylation | Associated
HDACs | (Refs.) |
|---|
| Liu et al,
2021 | Acetate,
propionateor butyrate | Folic acid-induced
acute kidney injury and subsequent chronic kidney injury | Attenuate both
acute and chronic kidney inflammation and reduce fibrosis | Unprovided | Unprovided | Inhibit HDAC3, 4, 5
(butyrate), 7 and 10 | (159) |
| Ma and Wang,
2022 | Propionate or
butyrate | STZ-induced
diabetes mellitus | Enhance autophagy
of renal tubular cells and attenuate renal fibrosis | Renal tubule
cells | Histone H3
acetylation | Inhibit HDAC2 to
increase ULK1 expression | (127) |
| Du et al,
2020 | Butyrate | Diabetes mellitus
(db/db) | Relieve renal
damage and renal tubular cell apoptosis | Renal tubular
cells | Unprovided | Inhibit HDAC2 | (129) |
| Khan and Jena,
2014 | | STZ-induced
diabetes mellitus | Ameliorate the
fibrogenesis, apoptosis and DNA damage in the kidney | Unprovided | Unprovided | Inhibit HDAC | (160) |
| Al-Harbi et
al, 2018 | Acetate | Sepsis-induced
AKI | Attenuate oxidative
stress in T cells | T cells | Unprovided | Inhibit HDAC to
suppress NOX2/ROS signaling | (161) |
 | Table IVPotential therapeutic effects of
SCFAs as HDACi in nervous diseases. |
Table IV
Potential therapeutic effects of
SCFAs as HDACi in nervous diseases.
| Authors, year | Groups | Disease models | Therapeutic
effects | Associated
cells | Associated
acetylation | Associated
HDACs | (Refs.) |
|---|
| Li et al,
2022 | Propionate and/or
butyrate | Chronic
postsurgical pain-related cognition dysfunction | Alleviate
pain-induced cognitive deficits | Unprovided | H4K12 and lysine
acetylation | Inhibit HDAC and
increase ACCS2 | (162) |
| Patnala et
al, 2017 | Butyrate | Ischemic
stroke | Mitigate
microglia-mediated neuroinflammation | Microglia | H3K9
acetylation | Inhibit HDAC | (163) |
| Sharma et
al, 2015 | | 6-OHDA-induced
Parkinson's disease | Improve behavioral
abnormalities and attenuate oxidative stress and
neuroinflammation | Unprovided | Histone H3
acetylation | Unprovided | (164) |
| Cho et al,
2024 | | STZ-induced
diabetes mellitus | Ameliorate neuronal
mitophagy | Neurons | Histone H3
acetylation | Inhibit RELA-HDAC8
complexes to restore PRKN expression | (132) |
| Soliman et
al, 2012 | Acetate | LPS-induced
neuroinflammation | Attenuate
neuroglial activation and neuroinflammation | Neuroglia | Histone H3K9, H4K8
and H4K16 acetylation | Inhibit HDAC7 | (165) |
 | Table VPotential therapeutic effects of
SCFAs as HDACi in vascular diseases. |
Table V
Potential therapeutic effects of
SCFAs as HDACi in vascular diseases.
| Authors, year | Groups | Disease models | Therapeutic
effects | Associated
cells | Associated
acetylation | Associated
HDACs | (Refs.) |
|---|
| Moleón et
al, 2023 | Acetate or
butyrate | TLR7-induced
systemic lupus erythematosus | Prevent vascular
dysfunction and elevated blood pressure and mitigate vascular
oxidative stress | Unprovided | Unprovided | Inhibit HDAC3 | (166) |
| Ma et al,
2023 | Butyrate | High-fat diet
(HFD)-induced atherosclerosis | Regulate
macrophages polarization and ameliorate atherosclerotic
inflammation | Macrophages | Unprovided | Inhibit HDAC3 | (167) |
| Karoor et
al, 2021 | | Hypoxia-induced
pulmonary hypertension | Attenuate pulmonary
vascular remodeling and inflammation | Microvascular
endothelial cells | Histone H3
acetylation | Unprovided | (168) |
 | Table VIPotential therapeutic effects of
SCFAs as HDACi in other diseases. |
Table VI
Potential therapeutic effects of
SCFAs as HDACi in other diseases.
| Authors, year | Groups | Disease models | Therapeutic
effects | Associated
cells | Associated
acetylation | Associated
HDACs | (Refs.) |
|---|
| Chen et al,
2024 | SCFAs | HFD-induced
gestational diabetes mellitus | Elevate lipid
transportation | IECs | H3K27
acetylation | Inhibit HDAC3 to
reduce CD36 expression | (169) |
| Chen et al,
2024 | Butyrate | Acute
leptospirosis | Promote the
bactericidal activity of macrophages | Macrophages | Unprovided | Inhibit HDAC3 to
promote ROS production | (35) |
| Jiang et al,
2024 | | Chronic obstructive
pulmonary disease | Suppress
ILC2-dependent airway inflammation | ILC2 cells | NFIL3 promoter
acetylation | Unprovided | (170) |
| Whitt et al,
2018 | | HFD-induced
obesity | Reduce body weight
and improve metabolic profile | IECs | Unprovided | Inhibit HDAC3 | (171) |
| Islam et al,
2022 | | Ovalbumin-induced
allergic asthma | Ameliorate airway
inflammation and fibrosis | Unprovided | Histone H3K9
acetylation | Inhibit HDAC1 | (172) |
| Olaniyi et
al, 2024 | Acetate | Letrozole-induced
polycystic ovarian syndrome | Reverse cardiac
energy depletion and attendant cardiomorbidities | Unprovided | Unprovided | Inhibit
HDAC2/mTOR | (173) |
| Olaniyi et
al, 2020 | | STZ-NA-induced
diabetes mellitus | Rectify cardiac
metabolic disturbance | Unprovided | Unprovided | Inhibit HDAC | (174) |
| Du et al,
2022 | Propionate | Experimental
autoimmune prostatitis | Ameliorate the
prostatic inflammation and restore Th17/Treg imbalance | T cells (Th17/Treg
cells) | Unprovided | Inhibit HDAC6 | (175) |
A review of these findings reveals that acetate,
propionate and butyrate display a wide spectrum of therapeutic
activities against diseases associated with, but not limited to,
the intestine, liver, kidney, nerves and blood vessels (Fig. 7). These SCFAs are capable of
effectively inhibiting class I and II HDACs while promoting histone
acetylation. Consequently, they facilitate disease remission by
influencing downstream genes such as NF-κB, miR-155 and GLP-1R.
This thus leads to the favorable outcomes such as inflammation
resolution, reduced tissue damage and metabolic regulation.
Importantly, the regulatory effects of SCFAs, especially butyrate,
as HDACi can be directly observed in certain immune and non-immune
cells. For example, butyrate can act on IECs, macrophages,
neutrophils and T cells to inhibit HDACs and promote histone
acetylation, thereby regulating gene transcription and alleviating
colonic inflammation. Additionally, butyrate suppresses the
activity of HDAC1/2 in hepatocytes, HSCs and macrophages, thereby
resulting in its anti-inflammatory, anti-fibrotic and lipid
metabolism regulatory effects in the liver. These findings indicate
that SCFAs, when functioning as HDACi, are equipped with intricate
regulatory mechanisms. They directly inhibit HDAC activity and
promote histone acetylation to regulate the transcriptional
activity of downstream genes, ultimately modulating the activity
and function of immune and non-immune cells. These findings further
elucidate that the considerable HDAC-inhibitory activity of SCFAs
thus generated promotes their potential as therapeutic agents,
contributing to the maintenance of immune balance in various
diseases.
Discussion and future perspective
In recent years, epigenetic pathways have emerged
as a key means by which gut microbiota communicates with the host
immune system (8). Specifically,
microbial metabolites SCFAs are regarded as key epigenetic
regulators that induce local and systemic immunomodulatory
activities through HDAC inhibition and associated histone
acetylation. The present review provides valuable insights into the
role of SCFAs as HDACi in the host immune system, highlighting
their potential functions in immune cell and systemic protection.
It is well-established that SCFAs exhibit targeted inhibitory
effects on class I and II HDACs. Subsequently, they affect the
tissue immune homeostasis both inside and outside the gut by
regulating cellular immune activities. A substantial portion of
these effects can be attributed to the direct regulatory actions of
SCFAs on various immune cells. The present review initially
concentrates on elucidating how SCFAs, through HDAC inhibition,
regulate the cellular activities and functions of innate immune
cells, including macrophages, DCs, neutrophils, MCs and NK cells,
as well as T/B lymphocytes. These discoveries aid in the
understanding, at the immune cell level, of the molecular pathways
by which SCFAs promote histone acetylation modification and
regulate host immunity via HDAC inhibition. Building on this, the
present review proceeds to summarize the protective effects of
SCFAs in both the intestine and extra-intestinal organs, thereby
emphasizing the systemic immunomodulatory activities of SCFAs based
on HDAC inhibition. Collectively, these aspects contribute to a
profound understanding, from the perspective of HDAC inhibition, of
the important contribution of SCFAs as microbial epigenetic
regulators to the host immune balance.
Epigenetic modifications wield a substantial
influence over chromatin alterations and gene expression. These
modifications are reversible and subject to intervention by
environmental factors. However, aberrant epigenetic modifications
frequently lead to dysregulated gene expression, which is
associated with the onset and progression of various human
diseases. In particular, abnormal acetylation modifications
mediated by HDACs are considered a key mechanism underlying
numerous human diseases, including inflammatory diseases,
neurological disorders, metabolic diseases and beyond (146,148,176). As previously discussed,
microbial SCFAs are potent HDACi that possess both cellular and
systemic immunomodulatory activities. Consequently, the present
review also revisited the therapeutic activities of SCFAs based on
HDAC inhibition in immune-related diseases both inside and outside
the intestine. These findings suggest that SCFAs promote the
inhibitory activities of class I and II HDACs and associated
histone acetylation in various disease models, including those of
the intestine, liver, kidney, nerves and blood vessels. This
enables them to exert a wide range of therapeutic effects,
encompassing anti-inflammatory, anti-fibrotic, vascular-protective
and neuro-protective effects (151-175). These aspects underscore the
potential of SCFAs as epigenetic therapeutic agents by acting as
HDACi.
Revisiting the aforementioned content, it becomes
readily apparent that the majority of current research focuses on
the SCFAs acetate, propionate and butyrate, with the latter
seemingly exhibiting a stronger HDAC-inhibiting effect compared
with the former two. For instance, butyrate proves to be more
efficacious than acetate and propionate in considerably reducing
the activation and pro-inflammatory mediator levels of different
immune cells, such as macrophages, neutrophils and MCs through
HDACi-dependent pathways (39,57,61). These findings have also indicated
that butyrate has a relatively broad inhibitory activity against
class I and II HDACs and promotes histone acetylation, both at the
levels of immune cells and systemic protection. Moreover, butyrate
exhibits extensive HDAC-inhibiting activity in the treatment of
various diseases both inside and outside the gut. This implies that
butyrate may be a representative HDACi among SCFAs, carrying out a
key role in regulating the host immune system and treating
diseases. However, the mechanism by which butyrate, acting as an
HDACi, mediates acetylation modifications to influence immune
homeostasis both inside and outside the gut still lacks in-depth
exploration. This is primarily because to the best of our
knowledge, the potential mechanisms underlying the regulation of
site-specific histone acetylation by butyrate and its connection to
gene activity remain largely unknown. In addition to histones,
HDACs can also regulate the acetylation of other substrates,
including lysine residues and transcription factors, ultimately
affecting gene transcription outcomes (177). Therefore, butyrate may attain
broad regulation of gene expression by targeting multiple sites to
modulate histone acetylation. A large number of high-throughput
sequencing technologies, such as single cell and spatial omics
(including epigenomics, transcriptomics, proteomics and
metabolomics) technologies, may enable more precise dynamic
observation and correlation analysis of the regulatory mechanisms
of butyrate in both temporal and spatial dimensions (178,179). The majority of evidence
supporting butyrate as a potent HDACi primarily stems from the
aforementioned findings such as animal and in vitro
research, yet it has yet to provide reliable validation in human
patients. This poses a challenge to the advancement of its
epigenetic therapy. Human pharmacokinetic data indicate that orally
administered butyrate is rapidly absorbed in the small intestine,
rather than in the colon where the gut microbiota resides.
Consequently, the pharmacological activity of oral butyrate may
differ from, or even be limited compared with, that of butyrate
originating from the colon. Exploiting an appropriate delivery
system can markedly leverage the pharmacokinetics of butyrate and
optimize its action mechanisms, thereby accelerating its
development and clinical application (180). For instance, Gong et al
(181 developed and implemented a synthetic living bacteria to
deliver butyrate to the intestine, elucidating the key role of gut
butyrate in preventing colitis and maintaining intestinal mucosal
homeostasis through the inhibition of colonic HDAC3.
Moreover, it is important to note that propionate
and acetate also exhibit specific HDAC-inhibiting effects. For
example, propionate can reduce IL-17 production by intestinal γδT
cell populations based on HDAC inhibition, thereby promoting the
alleviation of intestinal inflammation (88). Among the SCFAs at human
peripheral blood concentrations, only acetate can markedly induce
the formation of NETs and its mechanism of action is related to
both GPR43 and histone acetylation (55,56). These may be associated with the
actual physiological concentrations of SCFAs. The colon generates
500-600 mM of SCFAs per day, with acetate, propionate and butyrate
constituting the predominant portion (182). However, merely a minor
proportion of SCFAs in the colon can be absorbed into the systemic
circulation. The concentrations of acetate, propionate and butyrate
in plasma are detected as 25-250 μM, 1.4-13.4 μM and
0.5-14.2 μM, respectively (182). Additionally, the tissue uptake
capacity can also affect the in vivo concentrations of
SCFAs. For instance, within human brain tissues, the average
concentration of butyrate stands at 17.0 pmol/mg, while the average
concentration of propionate is 18.8 pmol/mg (182). Colonic administration of
butyrate (100 mM; 60 ml) increases the release of butyrate in the
human gut and its uptake by the liver, yet it does not affect the
splanchnic butyrate release. This indicates that the liver
metabolism can prevent an increase in systemic butyrate
concentrations, further demonstrating that this dose of colonic
SCFA administration is safe (183). Consequently, when exploring the
systemic effects of SCFAs, the influence of their physiological
concentrations cannot be disregarded. However, at present, the
majority of preclinical studies have not given due attention to
this issue. If deemed necessary, it is advisable to conduct
investigations into the acetylation modification mechanism of
microbial SCFAs as HDACi from the perspective of actual
physiological concentrations. This approach will facilitate a more
in-depth understanding of the immunomodulatory activities exhibited
by different SCFAs. To sum up, systematically and thoroughly
elucidating the acetylation mechanism by which SCFAs act as HDACi
presents an exciting challenge. Achieving this will enable the
attainment of a clearer understanding of the epigenetic regulatory
activity of microbial SCFAs in the host immune system and provide
novel insights for the treatment of human diseases.
Conclusion
In conclusion, the present review primarily
elucidates the immune-regulatory roles of microbial metabolites,
SCFAs, when acting as the HDACi. This is because SCFAs can
extensively inhibit the activities of class I and II HDACs and
promote histone acetylation, directly modulating the activation and
functions of various immune cells, such as macrophages, DCs,
neutrophils, MCs and NK cells. In parallel, a wealth of evidence
indicates that through their HDAC-inhibiting properties, SCFAs can
exert a variety of systemic protective effects. These effects
extend beyond the local gut to encompass extra-intestinal organs,
such as the liver, kidney, nerves and blood vessels. Given their
HDAC inhibitory effects, SCFAs may serve as potential epigenetic
regulators and carry out a key role in the treatment of multiple
immune-related diseases within both the intestine and
extra-intestinal regions. Among the SCFAs, butyrate emerges as a
representative HDACi. However, the acetylation mechanism associated
with the HDACi activity of SCFAs still requires in-depth
investigation. This is essential to advance the understanding of
their epigenetic regulatory activities in the host immune system
and to facilitate the development of therapeutic approaches for
various diseases.
Availability of data and materials
Not applicable.
Authors' contributions
JW is responsible for the investigation, writing
and funding of the present review. QZ, SZ, JL, XF and BH
contributed to the investigation and technical graphics. YH and XA
supported the funding and revised the present review. All authors
read and approved the final manuscript. Data authentication not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science Foundation of Sichuan
Province (grant nos. 2024NSFSC1840 and 2025ZNSFSC1820), National
Natural Science Foundation of China (grant no. 82004058), Research
and Development Project of Affiliated Hospital of North Sichuan
Medical College (grant no. 2023-2GC007) and Sichuan Provincial
Hospital Association Young Pharmacists Research Fund Project (grant
no. 22014).
References
|
1
|
Millán-Zambrano G, Burton A, Bannister AJ
and Schneider R: Histone post-translational modifications-cause and
consequence of genome function. Nat Rev Genet. 23:563–580. 2022.
View Article : Google Scholar
|
|
2
|
Zhao S, Zhang X and Li H: Beyond histone
acetylation-writing and erasing histone acylations. Curr Opin
Struct Biol. 53:169–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shvedunova M and Akhtar A: Modulation of
cellular processes by histone and Non-histone protein acetylation.
Nat Rev Mol Cell Biol. 23:329–349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shakespear MR, Halili MA, Irvine KM,
Fairlie DP and Sweet MJ: Histone deacetylases as regulators of
inflammation and immunity. Trends Immunol. 32:335–343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bondarev AD, Attwood MM, Jonsson J,
Chubarev VN, Tarasov VV and Schiöth HB: Recent developments of HDAC
inhibitors: Emerging indications and novel molecules. Br J Clin
Pharmacol. 87:4577–4597. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rooks MG and Garrett WS: Gut microbiota,
metabolites and host immunity. Nat Rev Immunol. 16:341–352. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, He M, Yang M and Ai X: Gut
microbiota as a key regulator of intestinal mucosal immunity. Life
Sci. 345:1226122024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woo V and Alenghat T: Epigenetic
regulation by gut microbiota. Gut Microbes. 14:20224072022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mann ER, Lam YK and Uhlig HH: Short-chain
fatty acids: Linking diet, the microbiome and immunity. Nat Rev
Immunol. 24:577–595. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin MY, de Zoete MR, van Putten JP and
Strijbis K: Redirection of epithelial immune responses by
Short-Chain fatty acids through inhibition of histone deacetylases.
Front Immunol. 6:5542015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Licciardi PV, Ververis K and Karagiannis
TC: Histone deacetylase inhibition and dietary Short-chain Fatty
acids. ISRN Allergy. 2011:8696472011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wardman JF, Bains RK, Rahfeld P and
Withers SG: Carbohydrate-active enzymes (CAZymes) in the gut
microbiome. Nat Rev Microbiol. 20:542–556. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki M, Suaini NHA, Afghani J, Heye KN,
O'Mahony L, Venter C, Lauener R, Frei R and Roduit C: Systematic
review of the association between Short-chain fatty acids and
allergic diseases. Allergy. 79:1789–1811. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deleu S, Machiels K, Raes J, Verbeke K and
Vermeire S: Short chain fatty acids and its producing organisms: An
overlooked therapy for IBD? EBioMedicine. 66:1032932021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Portincasa P, Bonfrate L, Vacca M, De
Angelis M, Farella I, Lanza E, Khalil M, Wang DQ, Sperandio M and
Di Ciaula A: Gut microbiota and short chain fatty acids:
Implications in glucose homeostasis. Int J Mol Sci. 23:11052022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reichardt N, Duncan SH, Young P, Belenguer
A, McWilliam Leitch C, Scott KP, Flint HJ and Louis P: Phylogenetic
distribution of three pathways for propionate production within the
human gut microbiota. ISME J. 8:1323–1335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Louis P and Flint HJ: Formation of
propionate and butyrate by the human colonic microbiota. Environ
Microbiol. 19:29–41. 2017. View Article : Google Scholar
|
|
19
|
Mahdi T, Desmons A, Krasniqi P, Lacorte
JM, Kapel N, Lamazière A, Fourati S and Eguether T: Effect of stool
sampling on a routine clinical method for the quantification of six
short chain fatty acids in stool using gas Chromatography-mass
spectrometry. Microorganisms. 12:8282024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sivaprakasam S, Bhutia YD, Yang S and
Ganapathy V: Short-chain fatty acid transporters: Role in colonic
homeostasis. Compr Physiol. 8:299–314. 2017. View Article : Google Scholar
|
|
21
|
Wang LY, He LH, Xu LJ and Li SB:
Short-chain fatty acids: Bridges between diet, gut microbiota, and
health. J Gastroenterol Hepatol. 39:1728–1736. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morrison DJ and Preston T: Formation of
short chain fatty acids by the gut microbiota and their impact on
human metabolism. Gut Microbes. 7:189–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao Y, Cai X, Fei W, Ye Y, Zhao M and
Zheng C: The role of Short-chain fatty acids in immunity,
inflammation and metabolism. Crit Rev Food Sci Nutr. 62:1–12. 2022.
View Article : Google Scholar
|
|
24
|
Li M, van Esch B, Wagenaar GTM, Garssen J,
Folkerts G and Henricks PAJ: Pro- and anti-inflammatory effects of
short chain fatty acids on immune and endothelial cells. Eur J
Pharmacol. 831:52–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seto E and Yoshida M: Erasers of histone
acetylation: The histone deacetylase enzymes. Cold Spring Harb
Perspect Biol. 6:a0187132014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bassett SA and Barnett MP: The role of
dietary histone deacetylases (HDACs) inhibitors in health and
disease. Nutrients. 6:4273–4301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kutil Z, Novakova Z, Meleshin M, Mikesova
J, Schutkowski M and Barinka C: Histone deacetylase 11 is a
Fatty-acid deacylase. ACS Chem Biol. 13:685–693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schiedel M, Robaa D, Rumpf T, Sippl W and
Jung M: The current state of NAD+ -Dependent histone deacetylases
(Sirtuins) as novel therapeutic targets. Med Res Rev. 38:147–200.
2018. View Article : Google Scholar
|
|
29
|
Reichert N, Choukrallah MA and Matthias P:
Multiple roles of class I HDACs in proliferation, differentiation,
and development. Cell Mol Life Sci. 69:2173–2187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parra M: Class IIa HDACs-new insights into
their functions in physiology and pathology. FEBS J. 282:1736–1744.
2015. View Article : Google Scholar
|
|
31
|
Witt O, Deubzer HE, Milde T and Oehme I:
HDAC family: What are the cancer relevant targets? Cancer Lett.
277:8–21. 2009. View Article : Google Scholar
|
|
32
|
Hull EE, Montgomery MR and Leyva KJ: HDAC
Inhibitors as epigenetic regulators of the immune system: Impacts
on cancer therapy and inflammatory diseases. Biomed Res Int.
2016:87972062016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grabiec AM and Potempa J: Epigenetic
regulation in bacterial infections: Targeting histone deacetylases.
Crit Rev Microbiol. 44:336–350. 2018. View Article : Google Scholar :
|
|
34
|
Schulthess J, Pandey S, Capitani M,
Rue-Albrecht KC, Arnold I, Franchini F, Chomka A, Ilott NE,
Johnston DGW, Pires E, et al: The short Chain fatty acid butyrate
imprints an antimicrobial program in macrophages. Immunity.
50:432–445.e437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Xie X, Sun N, Liu X, Liu J, Zhang
W and Cao Y: Gut microbiota-derived butyrate improved acute
leptospirosis in hamster via promoting macrophage ROS mediated by
HDAC3 inhibition. mBio. 15:e01906242024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernando MR, Saxena A, Reyes JL and McKay
DM: Butyrate enhances antibacterial effects while suppressing other
features of alternative activation in IL-4-induced macrophages. Am
J Physiol Gastrointest Liver Physiol. 310:G822–G831. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pineda Molina C, Hussey GS, Eriksson J,
Shulock MA, Cárdenas Bonilla LL, Giglio RM, Gandhi RM, Sicari BM,
Wang D, Londono R, et al: 4-Hydroxybutyrate promotes endogenous
antimicrobial peptide expression in macrophages. Tissue Eng Part A.
25:693–706. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Strizova Z, Benesova I, Bartolini R,
Novysedlak R, Cecrdlova E, Foley LK and Striz I: M1/M2 macrophages
and their overlaps-myth or reality? Clin Sci (Lond). 137:1067–1093.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang PV, Hao L, Offermanns S and
Medzhitov R: The microbial metabolite butyrate regulates intestinal
macrophage function via histone deacetylase inhibition. Proc Natl
Acad Sci USA. 111:2247–2252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park JW, Kim HY, Kim MG, Jeong S, Yun CH
and Han SH: Short-chain fatty acids inhibit staphylococcal
Lipoprotein-induced nitric oxide production in murine macrophages.
Immune Netw. 19:e92019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maa MC, Chang MY, Hsieh MY, Chen YJ, Yang
CJ, Chen ZC, Li YK, Yen CK, Wu RR and Leu TH: Butyrate reduced
lipopolysaccharide-mediated macrophage migration by suppression of
Src enhancement and focal adhesion kinase activity. J Nutr Biochem.
21:1186–1192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji J, Shu D, Zheng M, Wang J, Luo C, Wang
Y, Guo F, Zou X, Lv X, Li Y, et al: Microbial metabolite butyrate
facilitates M2 macrophage polarization and function. Sci Rep.
6:248382016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang W, Dernst A, Martin B, Lorenzi L,
Cadefau-Fabregat M, Phulphagar K, Wagener A, Budden C, Stair N,
Wagner T, et al: Butyrate and propionate are microbial danger
signals that activate the NLRP3 inflammasome in human macrophages
upon TLR stimulation. Cell Rep. 43:1147362024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park GY, Joo M, Pedchenko T, Blackwell TS
and Christman JW: Regulation of macrophage cyclooxygenase-2 gene
expression by modifications of histone H3. Am J Physiol Lung Cell
Mol Physiol. 286:L956–L962. 2004. View Article : Google Scholar
|
|
45
|
Cougoule C, Lastrucci C, Guiet R, Mascarau
R, Meunier E, Lugo-Villarino G, Neyrolles O, Poincloux R and
Maridonneau-Parini I: Podosomes, but not the maturation status,
determine the protease-Dependent 3D migration in human dendritic
cells. Front Immunol. 9:8462018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Singh N, Thangaraju M, Prasad PD, Martin
PM, Lambert NA, Boettger T, Offermanns S and Ganapathy V: Blockade
of dendritic cell development by bacterial fermentation products
butyrate and propionate through a transporter (Slc5a8)-dependent
inhibition of histone deacetylases. J Biol Chem. 285:27601–27608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu L, Li L, Min J, Wang J, Wu H, Zeng Y,
Chen S and Chu Z: Butyrate interferes with the differentiation and
function of human monocyte-derived dendritic cells. Cell Immunol.
277:66–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nascimento CR, Freire-de-Lima CG, da Silva
de Oliveira A, Rumjanek FD and Rumjanek VM: The short chain fatty
acid sodium butyrate regulates the induction of CD1a in developing
dendritic cells. Immunobiology. 216:275–284. 2011. View Article : Google Scholar
|
|
49
|
Kim YH and Lee JK: Histone deacetylase
inhibitors suppress immature dendritic cell's migration by
regulating CC chemokine receptor 1 expression. Cell Immunol.
316:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim YH, Han SB and Lee JK: Histone
deacetylase inhibitors suppress CXCR4-mediated dendritic cell
migration by regulation of maturation process. Cell Immunol.
284:139–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Inamoto T, Furuta K, Han C, Uneme M, Kano
T, Ishikawa K and Kaito C: Short-chain fatty acids stimulate
dendrite elongation in dendritic cells by inhibiting histone
deacetylase. FEBS J. 290:5794–5810. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Andrusaite A, Lewis J, Frede A, Farthing
A, Kästele V, Montgomery J, Mowat A, Mann E and Milling S:
Microbiota-derived butyrate inhibits cDC development via HDAC
inhibition, diminishing their ability to prime T cells. Mucosal
Immunol. 17:1199–1211. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kaisar MMM, Pelgrom LR, van der Ham AJ,
Yazdanbakhsh M and Everts B: Butyrate conditions human dendritic
cells to prime type 1 Regulatory T cells via both histone
deacetylase inhibition and G Protein-coupled receptor 109A
signaling. Front Immunol. 8:14292017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Berndt BE, Zhang M, Owyang SY, Cole TS,
Wang TW, Luther J, Veniaminova NA, Merchant JL, Chen CC, Huffnagle
GB, et al: Butyrate increases IL-23 production by stimulated
dendritic cells. Am J Physiol Gastrointest Liver Physiol.
303:G1384–G1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Íñiguez-Gutiérrez L, Godínez-Méndez LA,
Fafutis-Morris M, Padilla-Arellano JR, Corona-Rivera A,
Bueno-Topete MR, Rojas-Rejón ÓA and Delgado-Rizo V: Physiological
concentrations of short-chain fatty acids induce the formation of
neutrophil extracellular traps in vitro. Int J Immunopathol
Pharmacol. 34:20587384209589492020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yasuda H, Takishita Y, Morita A, Tsutsumi
T, Nakagawa N and Sato EF: Sodium acetate enhances neutrophil
extracellular trap formation via histone acetylation pathway in
Neutrophil-like HL-60 Cells. Int J Mol Sci. 25:87572024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vinolo MA, Rodrigues HG, Hatanaka E, Sato
FT, Sampaio SC and Curi R: Suppressive effect of short-chain fatty
acids on production of proinflammatory mediators by neutrophils. J
Nutr Biochem. 22:849–855. 2011. View Article : Google Scholar
|
|
58
|
Li G, Lin J, Zhang C, Gao H, Lu H, Gao X,
Zhu R, Li Z, Li M and Liu Z: Microbiota metabolite butyrate
constrains neutrophil functions and ameliorates mucosal
inflammation in inflammatory bowel disease. Gut Microbes.
13:19682572021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Aoyama M, Kotani J and Usami M: Butyrate
and propionate induced activated or non-activated neutrophil
apoptosis via HDAC inhibitor activity but without activating
GPR-41/GPR-43 pathways. Nutrition. 26:653–661. 2010. View Article : Google Scholar
|
|
60
|
Bartels M, Geest CR, Bierings M,
Buitenhuis M and Coffer PJ: Histone deacetylase inhibition
modulates cell fate decisions during myeloid differentiation.
Haematologica. 95:1052–1060. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Folkerts J, Redegeld F, Folkerts G,
Blokhuis B, van den Berg MPM, de Bruijn MJW, van IWFJ, Junt T, Tam
SY, Galli SJ, et al: Butyrate inhibits human mast cell activation
via epigenetic regulation of FcεRI-mediated signaling. Allergy.
75:1966–1978. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang H, Du M, Yang Q and Zhu MJ: Butyrate
suppresses murine mast cell proliferation and cytokine production
through inhibiting histone deacetylase. J Nutr Biochem. 27:299–306.
2016. View Article : Google Scholar
|
|
63
|
Gudneppanavar R, Sabu Kattuman EE, Teegala
LR, Southard E, Tummala R, Joe B, Thodeti CK and Paruchuri S:
Epigenetic histone modification by butyrate downregulates KIT and
attenuates mast cell function. J Cell Mol Med. 27:2983–2994. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
MacDonald CA, Qian H, Pundir P and Kulka
M: Sodium butyrate supresses malignant human mast cell
proliferation, downregulates expression of KIT and promotes
differentiation. Front Allergy. 4:11097172023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Carlini F, Squillario M, Casella V, Capaia
M, Lusi V, Bagnara D, Colombo M, Palmeri S, Ivaldi F, Loiacono F,
et al: Butyrate enhances CD56bright NK cell-driven killing of
activated T cells and modulates NK cell chromatin accessibility.
Genes Immun. 26:342–351. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Schmudde M, Braun A, Pende D, Sonnemann J,
Klier U, Beck JF, Moretta L and Bröker BM: Histone deacetylase
inhibitors sensitize tumour cells for cytotoxic effects of natural
killer cells. Cancer Lett. 272:110–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dong C: Cytokine regulation and function
in T cells. Annu Rev Immunol. 39:51–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kibbie JJ, Dillon SM, Thompson TA, Purba
CM, McCarter MD and Wilson CC: Butyrate directly decreases human
gut lamina propria CD4 T cell function through histone deacetylase
(HDAC) inhibition and GPR43 signaling. Immunobiology.
226:1521262021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang W, Yu T, Huang X, Bilotta AJ, Xu L,
Lu Y, Sun J, Pan F, Zhou J, Zhang W, et al: Intestinal
microbiota-derived short-chain fatty acids regulation of immune
cell IL-22 production and gut immunity. Nat Commun. 11:44572020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dagtas AS, Edens RE and Gilbert KM:
Histone deacetylase inhibitor uses p21(Cip1) to maintain anergy in
CD4+ T cells. Int Immunopharmacol. 9:1289–1297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zimmerman MA, Singh N, Martin PM,
Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH
and Liu K: Butyrate suppresses colonic inflammation through
HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T
cells. Am J Physiol Gastrointest Liver Physiol. 302:G1405–G415.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Moore TV, Scurti GM, DeJong M, Wang SY,
Dalheim AV, Wagner CR, Hutchens KA, Speiser JJ, Godellas CV,
Fountain C, et al: HDAC inhibition prevents transgene expression
downregulation and loss-of-function in T-cell-receptor-transduced T
cells. Mol Ther Oncolytics. 20:352–363. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Luu M, Riester Z, Baldrich A, Reichardt N,
Yuille S, Busetti A, Klein M, Wempe A, Leister H, Raifer H, et al:
Microbial short-chain fatty acids modulate CD8+ T cell responses
and improve adoptive immunotherapy for cancer. Nat Commun.
12:40772021. View Article : Google Scholar :
|
|
74
|
Luu M, Weigand K, Wedi F, Breidenbend C,
Leister H, Pautz S, Adhikary T and Visekruna A: Regulation of the
effector function of CD8+ T cells by gut microbiota-derived
metabolite butyrate. Sci Rep. 8:144302018. View Article : Google Scholar :
|
|
75
|
Bolduc JF, Hany L, Barat C, Ouellet M and
Tremblay MJ: Epigenetic metabolite acetate inhibits class I/II
histone deacetylases, promotes histone acetylation, and increases
HIV-1 integration in CD4+ T cells. J Virol. 91:e01943–e01916. 2017.
View Article : Google Scholar :
|
|
76
|
Park J, Kim M, Kang SG, Jannasch AH,
Cooper B, Patterson J and Kim CH: Short-chain fatty acids induce
both effector and regulatory T cells by suppression of histone
deacetylases and regulation of the mTOR-S6K pathway. Mucosal
Immunol. 8:80–93. 2015. View Article : Google Scholar
|
|
77
|
Chen L, Sun M, Wu W, Yang W, Huang X, Xiao
Y, Ma C, Xu L, Yao S, Liu Z and Cong Y: Microbiota metabolite
butyrate differentially regulates Th1 and Th17 cells'
Differentiation and function in induction of colitis. Inflamm Bowel
Dis. 25:1450–1461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kespohl M, Vachharajani N, Luu M, Harb H,
Pautz S, Wolff S, Sillner N, Walker A, Schmitt-Kopplin P, Boettger
T, et al: The microbial metabolite butyrate induces expression of
Th1-associated factors in CD4+ T cells. Front Immunol. 8:10362017.
View Article : Google Scholar :
|
|
79
|
Wang J, Hou Y, Mu L, Yang M and Ai X: Gut
microbiota contributes to the intestinal and extraintestinal immune
homeostasis by balancing Th17/Treg cells. Int Immunopharmacol.
143:1135702024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang M, Zhou L, Wang Y, Dorfman RG, Tang
D, Xu L, Pan Y, Zhou Q, Li Y, Yin Y, et al: Faecalibacterium
prausnitzii produces butyrate to decrease c-Myc-related metabolism
and Th17 differentiation by inhibiting histone deacetylase 3. Int
Immunol. 31:499–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou L, Zhang M, Wang Y, Dorfman RG, Liu
H, Yu T, Chen X, Tang D, Xu L, Yin Y, et al: Faecalibacterium
prausnitzii produces butyrate to maintain Th17/treg balance and to
ameliorate colorectal colitis by inhibiting histone deacetylase 1.
Inflamm Bowel Dis. 24:1926–1940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sałkowska A, Karaś K, Walczak-Drzewiecka
A, Dastych J and Ratajewski M: Differentiation stage-specific
effect of histone deacetylase inhibitors on the expression of RORγT
in human lymphocytes. J Leukoc Biol. 102:1487–1495. 2017.
View Article : Google Scholar
|
|
83
|
Arpaia N, Campbell C, Fan X, Dikiy S, van
der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ and
Rudensky AY: Metabolites produced by commensal bacteria promote
peripheral regulatory T-cell generation. Nature. 504:451–455. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sanchez HN, Moroney JB, Gan H, Shen T, Im
JL, Li T, Taylor JR, Zan H and Casali P: B cell-intrinsic
epigenetic modulation of antibody responses by dietary
fiber-derived short-chain fatty acids. Nat Commun. 11:602020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zou F, Qiu Y, Huang Y, Zou H, Cheng X, Niu
Q, Luo A and Sun J: Effects of short-chain fatty acids in
inhibiting HDAC and activating p38 MAPK are critical for promoting
B10 cell generation and function. Cell Death Dis. 12:5822021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Föh B, Buhre JS, Lunding HB,
Moreno-Fernandez ME, König P, Sina C, Divanovic S and Ehlers M:
Microbial metabolite butyrate promotes induction of IL-10+IgM+
plasma cells. PLoS One. 17:e02660712022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xiao P, Cai X, Zhang Z, Guo K, Ke Y, Hu Z,
Song Z, Zhao Y, Yao L, Shen M, et al: Butyrate prevents the
pathogenic Anemia-inflammation circuit by facilitating macrophage
iron export. Adv Sci (Weinh). 11:e23065712024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dupraz L, Magniez A, Rolhion N, Richard
ML, Da Costa G, Touch S, Mayeur C, Planchais J, Agus A, Danne C, et
al: Gut microbiota-derived short-chain fatty acids regulate IL-17
production by mouse and human intestinal γδ T cells. Cell Rep.
36:1093322021. View Article : Google Scholar
|
|
89
|
Nepelska M, Cultrone A, Béguet-Crespel F,
Le Roux K, Doré J, Arulampalam V and Blottière HM: Butyrate
produced by commensal bacteria potentiates phorbol esters induced
AP-1 response in human intestinal epithelial cells. PLoS One.
7:e528692012. View Article : Google Scholar
|
|
90
|
Eshleman EM, Rice T, Potter C, Waddell A,
Hashimoto-Hill S, Woo V, Field S, Engleman L, Lim HW, Schumacher
MA, et al: Microbiota-derived butyrate restricts tuft cell
differentiation via histone deacetylase 3 to modulate intestinal
type 2 immunity. Immunity. 57:319–332.e316. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bilotta AJ, Ma C, Yang W, Yu Y, Yu Y, Zhao
X, Zhou Z, Yao S, Dann SM and Cong Y: Propionate enhances cell
speed and persistence to promote intestinal epithelial turnover and
repair. Cell Mol Gastroenterol Hepatol. 11:1023–1044. 2021.
View Article : Google Scholar :
|
|
92
|
Mariadason JM, Velcich A, Wilson AJ,
Augenlicht LH and Gibson PR: Resistance to butyrate-induced cell
differentiation and apoptosis during spontaneous Caco-2 cell
differentiation. Gastroenterology. 120:889–899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gibson PR, Rosella O, Wilson AJ,
Mariadason JM, Rickard K, Byron K and Barkla DH: Colonic epithelial
cell activation and the paradoxical effects of butyrate.
Carcinogenesis. 20:539–544. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Siavoshian S, Segain JP, Kornprobst M,
Bonnet C, Cherbut C, Galmiche JP and Blottière HM: Butyrate and
trichostatin A effects on the proliferation/differentiation of
human intestinal epithelial cells: Induction of cyclin D3 and p21
expression. Gut. 46:507–514. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fischer N, Sechet E, Friedman R, Amiot A,
Sobhani I, Nigro G, Sansonetti PJ and Sperandio B: Histone
deacetylase inhibition enhances antimicrobial peptide but not
inflammatory cytokine expression upon bacterial challenge. Proc
Natl Acad Sci USA. 113:E2993–E3001. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Schauber J, Iffland K, Frisch S, Kudlich
T, Schmausser B, Eck M, Menzel T, Gostner A, Lührs H and Scheppach
W: Histone-deacetylase inhibitors induce the cathelicidin LL-37 in
gastrointestinal cells. Mol Immunol. 41:847–854. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Beisner J, Filipe Rosa L, Kaden-Volynets
V, Stolzer I, Günther C and Bischoff SC: Prebiotic inulin and
sodium butyrate attenuate Obesity-induced intestinal barrier
dysfunction by induction of antimicrobial peptides. Front Immunol.
12:6783602021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Dou X, Gao N, Lan J, Han J, Yang Y and
Shan A: TLR2/EGFR are two sensors for pBD3 and pEP2C induction by
sodium butyrate independent of HDAC inhibition. J Agric Food Chem.
68:512–522. 2020. View Article : Google Scholar
|
|
99
|
Korsten S, Vromans H, Garssen J and
Willemsen LEM: Butyrate protects barrier integrity and suppresses
immune activation in a Caco-2/PBMC Co-Culture model while HDAC
inhibition mimics butyrate in restoring Cytokine-induced barrier
disruption. Nutrients. 15:27602023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zheng L, Kelly CJ, Battista KD, Schaefer
R, Lanis JM, Alexeev EE, Wang RX, Onyiah JC, Kominsky DJ and Colgan
SP: Microbial-derived butyrate promotes epithelial barrier function
through IL-10 Receptor-dependent repression of Claudin-2. J
Immunol. 199:2976–2984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang RX, Lee JS, Campbell EL and Colgan
SP: Microbiota-derived butyrate dynamically regulates intestinal
homeostasis through regulation of actin-associated protein
synaptopodin. Proc Natl Acad Sci USA. 117:11648–11657. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ohata A, Usami M and Miyoshi M:
Short-chain fatty acids alter tight junction permeability in
intestinal monolayer cells via lipoxygenase activation. Nutrition.
21:838–847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gaudier E, Jarry A, Blottière HM, de
Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C and Hoebler
C: Butyrate specifically modulates MUC gene expression in
intestinal epithelial goblet cells deprived of glucose. Am J
Physiol Gastrointest Liver Physiol. 287:G1168–G1174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Borthakur A, Saksena S, Gill RK, Alrefai
WA, Ramaswamy K and Dudeja PK: Regulation of monocarboxylate
transporter 1 (MCT1) promoter by butyrate in human intestinal
epithelial cells: Involvement of NF-kappaB pathway. J Cell Biochem.
103:1452–1463. 2008. View Article : Google Scholar
|
|
105
|
Larraufie P, Martin-Gallausiaux C, Lapaque
N, Dore J, Gribble FM, Reimann F and Blottiere HM: SCFAs strongly
stimulate PYY production in human enteroendocrine cells. Sci Rep.
8:742018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Subramanian VS, Teafatiller T, Moradi H
and Marchant JS: Histone deacetylase inhibitors regulate vitamin C
transporter functional expression in intestinal epithelial cells. J
Nutr Biochem. 98:1088382021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zapletal O, Tylichová Z, Neča J, Kohoutek
J, Machala M, Milcová A, Pokorná M, Topinka J, Moyer MP, Hofmanová
J, et al: Butyrate alters expression of cytochrome P450 1A1 and
metabolism of benzo[a]pyrene via its histone deacetylase activity
in colon epithelial cell models. Arch Toxicol. 91:2135–2150. 2017.
View Article : Google Scholar
|
|
108
|
Bachmann M, Meissner C, Pfeilschifter J
and Mühl H: Cooperation between the bacterial-derived short-chain
fatty acid butyrate and interleukin-22 detected in human Caco2
colon epithelial/carcinoma cells. Biofactors. 43:283–292. 2017.
View Article : Google Scholar
|
|
109
|
Kobori A, Bamba S, Imaeda H, Ban H,
Tsujikawa T, Saito Y, Fujiyama Y and Andoh A: Butyrate stimulates
IL-32alpha expression in human intestinal epithelial cell lines.
World J Gastroenterol. 16:2355–2361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Korsten S, Peracic L, van Groeningen LMB,
Diks MAP, Vromans H, Garssen J and Willemsen LEM: Butyrate prevents
induction of CXCL10 and Non-canonical IRF9 expression by activated
human intestinal epithelial cells via HDAC inhibition. Int J Mol
Sci. 23:39802022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Inan MS, Rasoulpour RJ, Yin L, Hubbard AK,
Rosenberg DW and Giardina C: The luminal short-chain fatty acid
butyrate modulates NF-kappaB activity in a human colonic epithelial
cell line. Gastroenterology. 118:724–734. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Martin-Gallausiaux C, Larraufie P, Jarry
A, Béguet-Crespel F, Marinelli L, Ledue F, Reimann F, Blottière HM
and Lapaque N: Butyrate produced by commensal bacteria
Down-regulates indolamine 2,3-Dioxygenase 1 (IDO-1) expression via
a dual mechanism in human intestinal epithelial cells. Front
Immunol. 9:28382018. View Article : Google Scholar
|
|
113
|
Martin-Gallausiaux C, Béguet-Crespel F,
Marinelli L, Jamet A, Ledue F, Blottière HM and Lapaque N: Butyrate
produced by gut commensal bacteria activates TGF-beta1 expression
through the transcription factor SP1 in human intestinal epithelial
cells. Sci Rep. 8:97422018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xu L, Ma C, Huang X, Yang W, Chen L,
Bilotta AJ, Yao S and Cong Y: Microbiota metabolites short-chain
fatty acid butyrate conditions intestinal epithelial cells to
promote development of Treg cells and T cell IL-10 production. J
Immunol. 200:53.162018. View Article : Google Scholar
|
|
115
|
Jin UH, Cheng Y, Park H, Davidson LA,
Callaway ES, Chapkin RS, Jayaraman A, Asante A, Allred C, Weaver EA
and Safe S: Short chain fatty acids enhance aryl hydrocarbon (Ah)
responsiveness in mouse colonocytes and Caco-2 human colon cancer
cells. Sci Rep. 7:101632017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Modoux M, Rolhion N, Lefevre JH, Oeuvray
C, Nádvorník P, Illes P, Emond P, Parc Y, Mani S, Dvorak Z and
Sokol H: Butyrate acts through HDAC inhibition to enhance aryl
hydrocarbon receptor activation by gut microbiota-derived ligands.
Gut Microbes. 14:21056372022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Fawad JA, Luzader DH, Hanson GF, Moutinho
TJ Jr, McKinney CA, Mitchell PG, Brown-Steinke K, Kumar A, Park M,
Lee S, et al: Histone deacetylase inhibition by gut
microbe-Generated Short-Chain fatty acids entrains intestinal
epithelial circadian rhythms. Gastroenterology. 163:1377–1390.e11.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gill RK, Kumar A, Malhotra P, Maher D,
Singh V, Dudeja PK, Alrefai W and Saksena S: Regulation of
intestinal serotonin transporter expression via epigenetic
mechanisms: Role of HDAC2. Am J Physiol Cell Physiol.
304:C334–C341. 2013. View Article : Google Scholar :
|
|
119
|
Schilderink R, Verseijden C, Seppen J,
Muncan V, van den Brink GR, Lambers TT, van Tol EA and de Jonge WJ:
The SCFA butyrate stimulates the epithelial production of retinoic
acid via inhibition of epithelial HDAC. Am J Physiol Gastrointest
Liver Physiol. 310:G1138–G1146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zheng M, Yang X, Wu Q, Gong Y, Pang N, Ge
X, Nagaratnam N, Jiang P, Zhou M, Hu T, et al: Butyrate attenuates
hepatic steatosis induced by a High-fat and Fiber-deficient diet
via the hepatic GPR41/43-CaMKII/HDAC1-CREB pathway. Mol Nutr Food
Res. 67:e22005972023. View Article : Google Scholar
|
|
121
|
Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ,
Liu XL, Pan Q, Zhou H and Fan JG: Sodium butyrate reduces high-fat
diet-induced non-alcoholic steatohepatitis through upregulation of
hepatic GLP-1R expression. Exp Mol Med. 50:1–12. 2018. View Article : Google Scholar
|
|
122
|
Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J and
Jia W: Sodium butyrate stimulates expression of fibroblast growth
factor 21 in liver by inhibition of histone deacetylase 3.
Diabetes. 61:797–806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Bridgeman S, Woo HC, Newsholme P and
Mamotte C: Butyrate lowers cellular cholesterol through HDAC
Inhibition and Impaired SREBP-2 Signalling. Int J Mol Sci.
23:155062022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jourova L, Anzenbacherova E, Dostal Z,
Anzenbacher P, Briolotti P, Rigal E, Daujat-Chavanieu M and
Gerbal-Chaloin S: Butyrate, a typical product of gut microbiome,
affects function of the AhR gene, being a possible agent of
crosstalk between gut microbiome, and hepatic drug metabolism. J
Nutr Biochem. 107:1090422022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhao Y, Xu X, Liu S, Wang X, Musha J, Li
T, Ge L, Sun Y, Zhang S, Zhao L and Zhan J: Butyrate inhibits
histone deacetylase 2 expression to alleviate liver fibrosis in
biliary atresia. BMC Pediatr. 25:2862025. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang J, Wang W, Liang S, Zhou X, Rekha
RS, Gudmundsson GH, Bergman P, Ai Q, Mai K and Wan M: Butyrate
induces STAT3/HIF-1α/IL-22 signaling via GPCR and HDAC3 inhibition
to activate autophagy in head kidney macrophages from turbot
(Scophthalmus maximus L.). Fish Shellfish Immunol. 143:1092142023.
View Article : Google Scholar
|
|
127
|
Ma X and Wang Q: Short-Chain fatty acids
attenuate renal fibrosis and enhance autophagy of renal tubular
cells in diabetic mice through the HDAC2/ULK1 axis. Endocrinol
Metab (Seoul). 37:432–443. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Giordano L, Ahmed S, van der Made TK,
Masereeuw R and Mihăilă SM: Gut microbial-derived short chain fatty
acids enhance kidney proximal tubule cell secretory function.
Biomed Pharmacother. 188:1182142025. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Du Y, Tang G and Yuan W: Suppression of
HDAC2 by sodium butyrate alleviates apoptosis of kidney cells in
db/db mice and HG-induced NRK-52E cells. Int J Mol Med. 45:210–222.
2020.
|
|
130
|
Felizardo RJF, de Almeida DC, Pereira RL,
Watanabe IKM, Doimo NTS, Ribeiro WR, Cenedeze MA, Hiyane MI, Amano
MT, Braga TT, et al: Gut microbial metabolite butyrate protects
against proteinuric kidney disease through epigenetic- and
GPR109a-mediated mechanisms. FASEB J. 33:11894–11908. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Xu Y, Wei S, Zhu L, Huang C, Yang T, Wang
S, Zhang Y, Duan Y, Li X, Wang Z, et al: Low expression of the
intestinal metabolite butyric acid and the corresponding memory
pattern regulate HDAC4 to promote apoptosis in rat hippocampal
neurons. Ecotoxicol Environ Saf. 253:1146602023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Cho JH, Chae CW, Lim JR, Jung YH, Han SJ,
Yoon JH, Park JY and Han HJ: Sodium butyrate ameliorates high
glucose-suppressed neuronal mitophagy by restoring PRKN expression
via inhibiting the RELA-HDAC8 complex. Autophagy. 20:1505–1522.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Song L, Sun Q, Zheng H, Zhang Y, Wang Y,
Liu S and Duan L: Roseburia hominis alleviates neuroinflammation
via Short-Chain fatty acids through histone deacetylase inhibition.
Mol Nutr Food Res. 66:e22001642022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ziabska K, Gargas J, Sypecka J and
Ziemka-Nalecz M: The impact of the histone deacetylase inhibitor
sodium butyrate on microglial polarization after oxygen and glucose
deprivation. Pharmacol Rep. 74:909–919. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Chen PS, Wang CC, Bortner CD, Peng GS, Wu
X, Pang H, Lu RB, Gean PW, Chuang DM and Hong JS: Valproic acid and
other histone deacetylase inhibitors induce microglial apoptosis
and attenuate lipopolysaccharide-induced dopaminergic
neurotoxicity. Neuroscience. 149:203–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wang P, Zhang Y, Gong Y, Yang R, Chen Z,
Hu W, Wu Y, Gao M, Xu X, Qin Y and Huang C: Sodium butyrate
triggers a functional elongation of microglial process via
Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol Dis.
111:12–25. 2018. View Article : Google Scholar
|
|
137
|
Singh V, Bhatia HS, Kumar A, de Oliveira
AC and Fiebich BL: Histone deacetylase inhibitors valproic acid and
sodium butyrate enhance prostaglandins release in
lipopolysaccharide-activated primary microglia. Neuroscience.
265:147–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wu X, Chen PS, Dallas S, Wilson B, Block
ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, et al: Histone
deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene
transcription and protect dopaminergic neurons. Int J
Neuropsychopharmacol. 11:1123–1134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wu KLH, Liu WC, Wu CW, Fu MH, Huang HM,
Tain YL, Liang CK, Hung CY, Chen IC, Hung PL, et al: Butyrate
reduction and HDAC4 increase underlie maternal high
fructose-induced metabolic dysfunction in hippocampal astrocytes in
female rats. J Nutr Biochem. 126:1095712024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kanski R, Sneeboer MA, van Bodegraven EJ,
Sluijs JA, Kropff W, Vermunt MW, Creyghton MP, De Filippis L,
Vescovi A, Aronica E, et al: Histone acetylation in astrocytes
suppresses GFAP and stimulates a reorganization of the intermediate
filament network. J Cell Sci. 127:4368–4380. 2014.PubMed/NCBI
|
|
141
|
Li M, van Esch B, Henricks PAJ, Folkerts G
and Garssen J: The Anti-inflammatory effects of short Chain fatty
acids on Lipopolysaccharide- or tumor necrosis factor α-Stimulated
endothelial cells via activation of GPR41/43 and inhibition of
HDACs. Front Pharmacol. 9:5332018. View Article : Google Scholar
|
|
142
|
Li M, van Esch B, Henricks PAJ, Garssen J
and Folkerts G: IL-33 is involved in the Anti-inflammatory effects
of butyrate and propionate on TNFα-activated endothelial cells. Int
J Mol Sci. 22:24472021. View Article : Google Scholar
|
|
143
|
Miyoshi M, Usami M and Ohata A:
Short-chain fatty acids and trichostatin A alter tight junction
permeability in human umbilical vein endothelial cells. Nutrition.
24:1189–1198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Nicese MN, Bijkerk R, Van Zonneveld AJ,
Van den Berg BM and Rotmans JI: Sodium butyrate as key regulator of
mitochondrial function and barrier integrity of human glomerular
endothelial cells. Int J Mol Sci. 24:130902023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Rössig L, Li H, Fisslthaler B, Urbich C,
Fleming I, Förstermann U, Zeiher AM and Dimmeler S: Inhibitors of
histone deacetylation downregulate the expression of endothelial
nitric oxide synthase and compromise endothelial cell function in
vasorelaxation and angiogenesis. Circ Res. 91:837–844. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Farsetti A, Illi B and Gaetano C: How
epigenetics impacts on human diseases. Eur J Intern Med. 114:15–22.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Surace AEA and Hedrich CM: The role of
epigenetics in Autoimmune/inflammatory disease. Front Immunol.
10:15252019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Tang J, Yan H and Zhuang S: Histone
deacetylases as targets for treatment of multiple diseases. Clin
Sci (Lond). 124:651–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang SY, Zhang LY, Wen R, Yang N and
Zhang TN: Histone deacetylases and their inhibitors in inflammatory
diseases. Biomed Pharmacother. 179:1172952024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Zhang LY, Zhang SY, Wen R, Zhang TN and
Yang N: Role of histone deacetylases and their inhibitors in
neurological diseases. Pharmacol Res. 208:1074102024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Peng K, Xiao S, Xia S, Li C, Yu H and Yu
Q: Butyrate inhibits the HDAC8/NF-κB Pathway to Enhance Slc26a3
expression and improve the intestinal epithelial barrier to relieve
colitis. J Agric Food Chem. 72:24400–24416. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lee C, Kim BG, Kim JH, Chun J, Im JP and
Kim JS: Sodium butyrate inhibits the NF-kappa B signaling pathway
and histone deacetylation, and attenuates experimental colitis in
an IL-10 independent manner. Int Immunopharmacol. 51:47–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Simeoli R, Mattace Raso G, Pirozzi C, Lama
A, Santoro A, Russo R, Montero-Melendez T, Berni Canani R,
Calignano A, Perretti M and Meli R: An orally administered
butyrate-releasing derivative reduces neutrophil recruitment and
inflammation in dextran sulphate sodium-induced murine colitis. Br
J Pharmacol. 174:1484–1496. 2017. View Article : Google Scholar
|
|
154
|
Feng Z, Wang X, Kang G, Zhao J, Ye Y, Liu
L, Huang H and Cao X: P006 Engineered propionate-producing bacteria
attenuates murine colitis by modulating the immune function of
resident macrophages via histone deacetylase. J Crohn's Colitis.
17:i174–i177. 2023. View Article : Google Scholar
|
|
155
|
Kang G, Wang X, Gao M, Wang L, Feng Z,
Meng S, Wu J, Zhu Z, Gao X, Cao X and Huang H: Propionate-producing
engineered probiotics ameliorated murine ulcerative colitis by
restoring anti-inflammatory macrophage via the GPR43/HDAC1/IL-10
axis. Bioeng Transl Med. 9:e106822024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhang L, Ma Z, Zhang X, Wang J, Tian W,
Ren Y, Liu Y, Wang T, Li Y, Liu Y, et al: Butyrate alleviates
alcoholic liver disease-associated inflammation through macrophage
regulation and polarization via the HDAC1/miR-155 axis. Int
Immunopharmacol. 131:1118522024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Sun J, Wu Q, Sun H and Qiao Y: Inhibition
of histone deacetylase by butyrate protects rat liver from ischemic
reperfusion injury. Int J Mol Sci. 15:21069–21079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Olaniyi KS and Amusa OA: Sodium
acetate-mediated inhibition of histone deacetylase alleviates
hepatic lipid dysregulation and its accompanied injury in
streptozotocin-nicotinamide-induced diabetic rats. Biomed
Pharmacother. 128:1102262020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Liu Y, Li YJ, Loh YW, Singer J, Zhu W,
Macia L, Mackay CR, Wang W, Chadban SJ and Wu H: Fiber derived
microbial metabolites prevent acute kidney injury through G-Protein
coupled receptors and HDAC inhibition. Front Cell Dev Biol.
9:6486392021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Khan S and Jena G: Sodium butyrate, a HDAC
inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis,
apoptosis and DNA damage in the kidney of juvenile diabetic rats.
Food Chem Toxicol. 73:127–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Al-Harbi NO, Nadeem A, Ahmad SF, Alotaibi
MR, AlAsmari AF, Alanazi WA, Al-Harbi MM, El-Sherbeeny AM and
Ibrahim KE: Short chain fatty acid, acetate ameliorates
sepsis-induced acute kidney injury by inhibition of NADPH oxidase
signaling in T cells. Int Immunopharmacol. 58:24–31. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li Z, Sun T, He Z, Li Z, Zhang W, Wang J
and Xiang H: SCFAs ameliorate chronic postsurgical Pain-related
cognition dysfunction via the ACSS2-HDAC2 axis in rats. Mol
Neurobiol. 59:6211–6227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Patnala R, Arumugam TV, Gupta N and Dheen
ST: HDAC inhibitor sodium Butyrate-mediated epigenetic regulation
enhances neuroprotective function of microglia during ischemic
stroke. Mol Neurobiol. 54:6391–6411. 2017. View Article : Google Scholar
|
|
164
|
Sharma S, Taliyan R and Singh S:
Beneficial effects of sodium butyrate in 6-OHDA induced
neurotoxicity and behavioral abnormalities: Modulation of histone
deacetylase activity. Behav Brain Res. 291:306–314. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Soliman ML, Smith MD, Houdek HM and
Rosenberger TA: Acetate supplementation modulates brain histone
acetylation and decreases interleukin-1β expression in a rat model
of neuroinflammation. J Neuroinflammation. 9:512012. View Article : Google Scholar
|
|
166
|
Moleón J, González-Correa C, Miñano S,
Robles-Vera I, de la Visitación N, Barranco AM, Gómez-Guzmán M,
Sánchez M, Riesco P, Guerra-Hernández E, et al: Protective effect
of microbiota-derived short chain fatty acids on vascular
dysfunction in mice with systemic lupus erythematosus induced by
toll like receptor 7 activation. Pharmacol Res. 198:1069972023.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Ma H, Yang L, Liu Y, Yan R, Wang R, Zhang
P, Bai Z, Liu Y, Ren Y, Li Y, et al: Butyrate suppresses
atherosclerotic inflammation by regulating macrophages and
polarization via GPR43/HDAC-miRNAs axis in ApoE-/-mice. PLoS One.
18:e02826852023. View Article : Google Scholar
|
|
168
|
Karoor V, Strassheim D, Sullivan T, Verin
A, Umapathy NS, Dempsey EC, Frank DN, Stenmark KR and
Gerasimovskaya E: The Short-Chain fatty acid butyrate attenuates
pulmonary vascular remodeling and inflammation in Hypoxia-induced
pulmonary hypertension. Int J Mol Sci. 22:99162021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Chen H, Wang SH, Li HL, Zhou XB, Zhou LW,
Chen C, Mansell T, Novakovic B, Saffery R, Baker PN, et al: The
attenuation of gut microbiota-derived short-chain fatty acids
elevates lipid transportation through suppression of the intestinal
HDAC3-H3K27ac-PPAR-γ axis in gestational diabetes mellitus. J Nutr
Biochem. 133:1097082024. View Article : Google Scholar
|
|
170
|
Jiang M, Wang J, Li Z, Xu D, Jing J, Li F,
Ding J and Li Q: Dietary Fiber-derived microbial butyrate
suppresses ILC2-dependent airway inflammation in COPD. Mediators
Inflamm. 2024:62634472024. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Whitt J, Woo V, Lee P, Moncivaiz J,
Haberman Y, Denson L, Tso P and Alenghat T: Disruption of
epithelial HDAC3 in intestine prevents Diet-induced obesity in
mice. Gastroenterology. 155:501–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Islam R, Dash D and Singh R: Intranasal
curcumin and sodium butyrate modulates airway inflammation and
fibrosis via HDAC inhibition in allergic asthma. Cytokine.
149:1557202022. View Article : Google Scholar
|
|
173
|
Olaniyi KS, Areloegbe SE and Fiemotongha
FE: Cardiac energy depletion in a rat model of polycystic ovarian
syndrome is reversed by acetate and associated with inhibitory
effect of HDAC2/mTOR. Eur J Pharmacol. 962:1762432024. View Article : Google Scholar
|
|
174
|
Olaniyi KS, Amusa OA, Areola ED and
Olatunji LA: Suppression of HDAC by sodium acetate rectifies
cardiac metabolic disturbance in
streptozotocin-nicotinamide-induced diabetic rats. Exp Biol Med
(Maywood). 245:667–676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Du HX, Yue SY, Niu D, Liu C, Zhang LG,
Chen J, Chen Y, Guan Y, Hua XL, Li C, et al: Gut microflora
modulates Th17/Treg cell differentiation in experimental autoimmune
prostatitis via the Short-Chain fatty acid propionate. Front
Immunol. 13:9152182022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Cavalli G and Heard E: Advances in
epigenetics link genetics to the environment and disease. Nature.
571:489–499. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Narita T, Weinert BT and Choudhary C:
Functions and mechanisms of non-histone protein acetylation. Nat
Rev Mol Cell Biol. 20:156–174. 2019. View Article : Google Scholar
|
|
178
|
Lang C, Campbell KR, Ryan BJ, Carling P,
Attar M, Vowles J, Perestenko OV, Bowden R, Baig F, Kasten M, et
al: Single-cell sequencing of iPSC-dopamine neurons reconstructs
disease progression and identifies HDAC4 as a regulator of
parkinson cell phenotypes. Cell Stem Cell. 24:93–106.e6. 2019.
View Article : Google Scholar :
|
|
179
|
Sussman JH, Xu J, Amankulor N and Tan K:
Dissecting the tumor microenvironment of epigenetically driven
gliomas: Opportunities for single-cell and spatial multiomics.
Neurooncol Adv. 5:vdad1012023.PubMed/NCBI
|
|
180
|
Camargo Tavares L and Marques FZ: Clinical
trial evidence of the gut microbial metabolite butyrate in
hypertension. Hypertension. 81:2137–2139. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Gong X, Geng H, Yang Y, Zhang S, He Z, Fan
Y, Yin F, Zhang Z and Chen GQ: Metabolic engineering of commensal
bacteria for gut butyrate delivery and dissection of host-microbe
interaction. Metab Eng. 80:94–106. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Dalile B, Van Oudenhove L, Vervliet B and
Verbeke K: The role of short-chain fatty acids in
microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol.
16:461–478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
van der Beek CM, Bloemen JG, van den Broek
MA, Lenaerts K, Venema K, Buurman WA and Dejong CH: Hepatic uptake
of rectally administered butyrate prevents an increase in systemic
butyrate concentrations in humans. J Nutr. 145:2019–2024. 2015.
View Article : Google Scholar : PubMed/NCBI
|