Introduction
Temporomandibular disorders (TMDs) are a group of
clinical conditions involving the temporomandibular joint (TMJ)
and/or masticatory muscles, characterized by shared symptoms,
including joint structural abnormalities, occlusal disturbances,
muscular dysfunction and psychological factors (1). It is estimated that ~34% of the
global population is affected by TMD each year (2,3).
Although TMDs can exhibit a degree of self-limiting behavior, lack
of timely and effective early intervention often leads to delayed
recovery, progressive TMJ dysfunction, structural deformities and
even craniofacial dysmorphisms, ultimately imposing long-term
burdens on physical and psychological well-being of patients
(4). Therefore, early
intervention is critically important in halting the progression of
TMD. The pathophysiology of TMD is complex, with inflammation
playing a central role. Unlike the pronounced synovial inflammation
observed in rheumatoid arthritis (RA), TMD is typically considered
a low-grade inflammatory state (5). Nevertheless, the inflammatory
microenvironment of the synovium remains a key therapeutic target
in TMD, as with RA. Current early-stage treatments for TMDs include
physical therapy and occlusal splints, which require high patient
compliance and frequent clinical visits, burdening individuals
(6). Nonsteroidal
anti-inflammatory drugs (NSAIDs) and corticosteroids can offer
temporary pain relief during acute episodes but are unsuitable for
long-term use due to adverse side effects (7). Commonly used chondroprotective
agents, such as glucosamine, primarily target cartilage repair but
lack efficacy in directly modulating synovitis over the long term
(8,9). Thus, there is an urgent need to
develop a safe, convenient and effective pharmacological
strategy.
Quercetin (also known as Diosmetin) is a natural
flavonoid compound predominantly found in citrus fruits, legumes
and other plant-based sources (10). A growing body of recent reviews
highlights its diverse pharmacological properties, including
anti-inflammatory, anticancer and antioxidant activities (11-15). Emerging evidence suggests that
quercetin may slow subchondral bone loss by inhibiting
osteoclastogenesis, positioning it as a promising therapeutic agent
for osteoarthritis (16).
However, the effects of quercetin on the TMJ, particularly its
pharmacological role and underlying mechanisms in TMJ synovitis,
remain largely unexplored. In recent years, the advent of network
pharmacology and molecular docking techniques has provided novel
strategies for investigating the mechanisms of natural compounds
(17,18). These technologies can
systematically analyze drug targets and signaling pathways
associated with diseases to reveal their potential therapeutic
mechanisms.
Although the therapeutic efficacy of quercetin has
been preliminarily validated in various inflammatory diseases
(19), its specific role in
TMD-related synovitis, particularly whether it exerts
anti-inflammatory effects via the p38 MAPK signaling pathway, has
not been systematically investigated. The p38 MAPK signaling
pathway is known to play a critical role in regulating synovial
inflammation and cell apoptosis and has been implicated in the
pathogenesis of numerous inflammatory conditions (20-22). Exploring
whether quercetin can modulate this pathway offers important
theoretical value for understanding its mechanism of action.
Although several treatments have shown efficacy in alleviating TMD
symptoms, there remains a lack of pharmacological agents capable of
providing long-term control of synovitis by targeting specific
signaling pathways (23,24). Therefore, investigating whether
quercetin can improve the clinical manifestations of TMD synovitis
through a defined molecular mechanism, especially by modulating the
p38 MAPK signaling pathway, holds innovative potential.
The present study aimed to investigate the potential
therapeutic effects of quercetin in TMD-related synovitis, with a
particular focus on verifying whether quercetin alleviates synovial
inflammation by modulating the p38 MAPK signaling pathway, thereby
promoting synoviocyte apoptosis and inhibiting subchondral bone
destruction. From a scientific perspective, this research filled a
critical knowledge gap regarding the role of quercetin in TMD
synovitis and elucidated its underlying mechanism of action through
the regulation of the p38 MAPK signaling pathway. The findings are
expected to provide a theoretical foundation for the clinical
application of quercetin in inflammatory joint disorders.
Clinically, as a natural compound with proven safety and
bioactivity, quercetin holds marked promise. If its efficacy is
confirmed, the present study could offer a novel pharmacological
strategy for treating TMD, particularly for early-stage,
non-invasive, or adjunctive therapy. Ultimately, the outcomes of
this research may contribute to improving patient quality of life,
slowing disease progression and enhancing long-term prognosis in
TMD while promoting the clinical translation of natural products in
inflammatory disease management.
Materials and methods
Identification and intersection analysis
of potential targets of quercetin in the treatment of TMJ
synovitis
To identify potential therapeutic targets associated
with TMJ synovitis, disease-related genes were retrieved using the
keywords 'synovitis' and 'temporomandibular joint' from the
DisGeNET (https://www.disgenet.org/), GeneCards
(https://www.genecards.org/) and OMIM
(https://omim.org/) databases. Meanwhile, the potential
targets of quercetin were predicted using SwissTargetPrediction
(http://www.swisstargetprediction.ch/), SuperPred
(https://prediction.charite.de/) and
PharmMapper (http://lilab-ecust.cn/pharmmapper), with the species
restricted to Homo sapiens. An intersection analysis was
performed between the disease-related targets and the
quercetin-predicted targets. All candidate proteins were then
standardized using the UniProt database (https://www.uniprot.org/) to obtain the official human
gene names. The overlapping targets, representing potential key
quercetin targets in TMJ synovitis, were identified and visualized
using a Venn diagram generated by a bioinformatics visualization
platform(http://www.bioinformatics.com.cn/).
Construction of the protein-protein
interaction (PPI) network and identification of core targets
To further investigate the potential mechanisms by
which quercetin exerts therapeutic effects on TMJ synovitis, 95
intersecting target genes were imported into the STRING database
(https://cn.string-db.org/). The species
was set to Homo sapiens and the minimum required interaction
confidence score was set to 0.07 to construct a high-confidence PPI
network. The PPI network data (in TSV format) were downloaded and
imported into Cytoscape software (version 3.10.1; https://cytoscape.org/) for visualization and
topological analysis. The NetworkAnalyzer plugin was used to
calculate the topological parameters of each node, including degree
centrality, betweenness centrality and closeness centrality, to
comprehensively evaluate the importance of each protein within the
network. Core target identification was performed using the
CytoHubba plugin in Cytoscape. Nodes were ranked based on the
Maximal Clique Centrality (MCC) algorithm (25) and the top 10 key targets were
selected to construct a core subnet, which was then prioritized for
subsequent mechanistic studies.
Gene Ontology (GO) and Kyoto Encyclopedia
of Genes and Genomes (KEGG) signaling pathway enrichment
analysis
Functional annotation and signaling pathway
enrichment analysis were performed using the DAVID database
(https://david.ncifcrf.gov/tools.jsp).
GO enrichment analysis included three major categories: Biological
Process (BP), Molecular Function (MF) and Cellular Component (CC).
KEGG analysis was used to identify markedly enriched signaling
pathways. All GO terms and pathways were ranked based on adjusted
P-values and the false discovery rate (FDR) was applied to correct
for multiple hypothesis testing and reduce false-positive results.
The enrichment results were then imported into a bioinformatics
visualization platform (http://www.bioinformatics.com.cn/) and displayed in
the form of bar plots and bubble charts to intuitively present the
most markedly enriched GO terms and KEGG pathways.
Molecular docking validation
The three-dimensional crystal structures of target
proteins were obtained from the Protein Data Bank (PDB; https://www.rcsb.org/). PyMOL (v2.1; https://pymol.org/2/) removed impurities such as
ligands, small molecular ions and water molecules. The preprocessed
protein structures were imported into AutoDock Tools (v1.5.6;
http://autodock.scripps.edu/resources/adt), where
polar hydrogens were added, Gasteiger charges were assigned and
atom types were specified. The prepared protein structures were
saved in the .pdbqt format for subsequent docking analysis. The
chemical structure of quercetin was retrieved from the PubChem
database (https://pubchem.ncbi.nlm.nih.gov/). The molecular
geometry was optimized using Chem3D (https://www.perkinelmer.com/category/chemdraw) through
energy minimization and the structure was converted to .mol2
format. It was then imported into AutoDock Tools, where charges
were added and rotatable bonds were defined. The final ligand
structure was saved as a .pdbqt file. Docking grid boxes were
defined based on the active site residues of the target proteins,
with specified center coordinates and box dimensions. Molecular
docking was performed using AutoDock Vina and the resulting binding
conformations were ranked according to binding affinity (kcal/mol).
The conformation with the lowest binding energy was selected as the
optimal binding pose. The protein-ligand complexes were visualized
using PyMOL to display the three-dimensional binding modes. Key
interaction residues, such as hydrogen bonds and hydrophobic
interactions, were analyzed to provide a structural basis for
subsequent mechanistic investigations.
Molecular dynamics (MD) simulation
MD simulations were conducted on the protein-ligand
complexes with the highest binding affinities obtained from docking
analysis to further evaluate the binding stability and interaction
characteristics between quercetin and its target proteins. The
simulations were performed using the GROMACS software package
(v.2020; https://www.gromacs.org/). The
AMBER99SB-ILDN force field was applied for the protein components,
while the General AMBER Force Field (GAFF) was used for the
small-molecule ligand. Ligand topology files were generated using
the sobtop tool and the atomic charges were calculated using the
Restrained Electrostatic Potential method (26). The solvent environment was
constructed using the TIP3P water model (27), with a solvation box extending at
≤1.0 nm from the protein atoms in all directions. Counterions
(Na+ or Cl−) were added to neutralize the
system based on the total charge. Following system construction,
energy minimization was performed using the steepest descent
algorithm to eliminate unfavorable contacts. The system was then
subjected to an NVT ensemble (constant number of particles, volume
and temperature) for 50 psec to gradually raise the temperature to
300 K for thermal equilibration. This was followed by a 50 psec NPT
ensemble (constant number of particles, pressure and temperature)
equilibration phase. A 100 nsec production MD simulation was
subsequently carried out to assess the structural stability and
dynamic interactions of the protein-ligand complexes under
physiological conditions. The binding free energy (ΔGbinding)
between the protein and ligand was calculated using the molecular
mechanics/Poisson-Boltzmann surface area (MM/PBSA) method with the
g_mmpbsa tool in GROMACS (https://g_mmpbsa.readthedocs.io/), providing a
quantitative evaluation of complex stability.
Cell culture and treatment
The human synovial cell line SW982 was obtained from
the National Certified Cell Bank and cultured in L-15 medium
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. 11415064)
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.; cat. no. A5669401) and 1% penicillin-streptomycin
solution (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
15140122). Cells were maintained at 37°C in a humidified incubator
with 5% CO2. To establish an in vitro
inflammatory model, cells were stimulated with 10 ng/ml recombinant
human IL-1β (PeproTech, Inc.) for 24 h. Following stimulation,
cells were treated with quercetin (MedChemExpress; cat. no.
HY-N0125,) at concentrations of 20, 40 and 80 μM for an
additional 24 h. Quercetin was dissolved in DMSO and diluted to the
desired concentrations, ensuring the final DMSO concentration did
not exceed 0.1%. The experimental groups included i) untreated
control, ii) IL-1β-only model group and iii) IL-1β + quercetin
treatment groups at different concentrations. All experiments were
performed in biological replicates to ensure data reliability.
To investigate pathway-specific mechanisms, p38 MAPK
signaling pathway modulators were applied. After 24 h of IL-1β (10
ng/ml) stimulation, SW982 cells were pretreated for 30 min with
either the p38 MAPK activator anisomycin (10 μM;
MedChemExpress; cat. no. HY-18982) or the inhibitor SB203580 (10
μM, HY-10256, MedChemExpress), followed by co-treatment with
40 μM quercetin for another 24 h (28,29). Subsequent assays included cell
viability analysis Cell Counting Kit-8 (CCK-8), apoptosis detection
(Annexin V/PI flow cytometry), inflammatory cytokine quantification
enzyme-linked immunosorbent assay (ELISA) and protein expression
analysis of phosphorylated p38 MAPK (p-p38 MAPK; western blotting).
The experimental setup consisted of five groups: i) Control; ii)
IL-1β; iii) IL-1β + quercetin; iv) IL-1β + quercetin + SB203580 all
v) IL-1β + quercetin + anisomycin. All experiments were conducted
in triplicate to ensure reproducibility.
Cell proliferation assay
SW982 cells were seeded into 96-well plates at a
density of 1×104 cells per well and allowed to adhere
for 24 h. After attachment, the cells were treated with IL-1β (10
ng/ml) combined with various concentrations of quercetin (2.5, 5,
10, 20, 40, 80 and 100 μM) and incubated for 24 h. Following
treatment, 10 μl of CCK-8 reagent (Saint-Bio; cat. no.
ST1008) was added to each well and incubated at 37°C for 2 h. The
optical density (OD) at 450 nm was measured using a microplate
reader (Multiscan MK3, Thermo Fisher Scientific, USA) to assess
cell viability.
Apoptosis assay
SW982 cells were seeded in 6-well plates and treated
with quercetin at 20, 40 and 80 μM concentrations for 48 h.
Following treatment, cells from each group were harvested and
washed twice with PBS. Apoptosis was assessed using an Annexin
V-APC/7-AAD dual-staining apoptosis detection kit (Nanjing KeyGen
Biotech Co., Ltd.; cat. no. KGA1106). Then, ~1×106 cells
per group were resuspended in 500 μl of binding buffer,
followed by the addition of 5 μl Annexin V-APC and 5
μl 7-AAD. The samples were incubated in the dark at room
temperature for 15 min. Stained cells were then analyzed using a
flow cytometer (FACSCalibur, BD Biosciences), with at least 10,000
events collected per sample. Flow cytometric data were analyzed
using FlowJo software (version 10.0; BD Biosciences). Cell debris
was excluded by forward scatter and side scatter gating and
apoptotic cells were identified based on staining patterns: Early
apoptosis (Annexin V+/7-AAD−) and late
apoptosis (Annexin V+/7-AAD+). The apoptosis
rate for each treatment group was subsequently calculated. The
total apoptosis rate was calculated as the sum of the percentages
of early (Annexin V+/7-AAD−) and late
(Annexin V+/7-AAD+) apoptotic cells.
ELISA
After 24 h of treatment, the culture supernatants of
SW982 cells were collected and centrifuged at 1,000 × g for 10 min
to remove cellular debris. The clarified supernatants were reserved
for subsequent analysis. The protein levels of matrix
metalloproteinase 3 (MMP3), MMP9 and MMP13 were measured using
ELISA kits (Wuhan Boster Biological Technology, Ltd.; cat. nos.
EK0461, EK0465 and EK0486), following the manufacturer's
instructions. All samples were assayed in duplicate and standard
curves were generated to convert OD values into concentrations
(pg/ml). OD values were measured at 450 nm using a microplate
reader (Infinite F200; Tecan Group, Ltd.), with background
subtraction based on blank wells. The resulting data were used to
evaluate differences in the expression levels of inflammatory
cytokines among the various treatment groups.
Western blot analysis
Western blotting was performed to detect the
expression levels of inflammation-related signaling pathway
proteins in SW982 cells. Total protein was extracted using RIPA
lysis buffer (Beyotime Institute of Biotechnology; cat. no.
P0013B), and protein concentrations were determined using a BCA
protein assay kit (Beyotime Institute of Biotechnology). Equal
amounts of protein (20 μg per lane) were separated by 10%
SDS-PAGE and transferred onto PVDF membranes (MilliporeSigma; cat.
no. IPVH00010). Membranes were blocked with 5% BSA (MilliporeSigma)
at room temperature for 1 h. Subsequently, the membranes were
incubated overnight at 4°C with the following primary antibodies:
p-p38 MAPK (Thr180/Tyr182; cat. no. 9211) and total p38 MAPK (cat.
no. 9212) from Cell Signaling Technology, Inc.; phosphorylated
(p-)JNK, JNK, p-ERK, and ERK (cat. nos. SC7345, SC6254, SC7348, and
SC514302) from Santa Cruz Biotechnology, Inc.; IL-6, TNF-α, and
β-Tubulin (cat. nos. ab315214, ab9324, and ab307164) from Abcam.
After washing, membranes were incubated for 1 h with HRP-conjugated
goat anti-rabbit or anti-mouse secondary antibodies (cat. nos.
ab205718 and ab205719, Abcam). Protein bands were visualized using
an ECL detection reagent (Tanon Science and Technology Co., Ltd.)
and imaged with the Tanon 4600 chemiluminescence imaging system
(Tanon Science and Technology Co., Ltd.). Band intensities were
quantified using ImageJ software (NIH, version 1.8.0), and
β-Tubulin was used as the internal loading control.
Animal experiments
A total of 20 male Sprague-Dawley (SD) rats (7-8
weeks old; weighing 200-250 g) were obtained from an accredited
laboratory animal center. Specifically, all animals were purchased
from Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China), and housed under standard conditions (22±2°C,
50±10% humidity, and a 12-h light/dark cycle) with free access to
food and water. All experimental procedures were approved by the
Animal Ethics Committee of China Medical University (approval no:
kt2022112) and conducted by institutional guidelines (30). The TMJ synovitis model was
established based on a previously described method (31), with slight modifications.
Inflammation was induced by intra-articular injection of 50
μl of complete Freund's adjuvant (CFA) into both TMJ
cavities. One week after model induction, drug administration was
initiated and continued daily for 14 consecutive days. Quercetin
was dissolved in propylene glycol and administered orally via
gavage once daily. The animals were randomly divided into four
groups (n=5 per group): Control group: Bilateral injection of 50
μl saline; CFA group: Bilateral injection of 50 μl
CFA; CFA + 20D group: CFA injection followed by quercetin treatment
at 20 mg/kg/day; CFA + 40D group: CFA injection followed by
quercetin treatment at 40 mg/kg/day.
Tissue collection and processing
At the end of the treatment period (Day 28), rats
were euthanized by intraperitoneal injection of an overdose of
sodium pentobarbital. Bilateral TMJ were collected for subsequent
analyses and allocated to the following experimental
procedures:
Micro-computed tomography (Micro-CT): TMJ tissues
were fixed in 4% paraformaldehyde for 24 h at 4°C, and then
transferred to 70% ethanol for storage before scanning and
morphometric analysis.
Histological Staining: Samples were decalcified in
10% EDTA solution at room temperature for 4 weeks, followed by
paraffin embedding and sectioning.
ELISA: Synovial tissues were isolated and
immediately stored at -80°C.
Reverse transcription-quantitative (RT-q)
PCR
Total RNA was extracted from SW982 cells and rat
synovial tissues. SW982 cells were seeded at a density of
1×106 cells per well in 6-well plates prior to RNA
extraction. Samples were lysed using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.; cat. no. 15596026),
and RNA was purified using the MiniBEST Universal RNA Extraction
Kit (Takara Biotechnology Co., Ltd.; cat. no. 9767), following the
manufacturer's protocol. RNA purity and concentration were assessed
using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.), and samples with A260/A280 ratios between 1.8 and 2.0 were
used for further experiments.
For each sample, 1 μg of total RNA was
reverse-transcribed into complementary DNA (cDNA) using
PrimeScript™ RT Master Mix (Takara Biotechnology Co., Ltd.; cat.
no. RR036A), according to the manufacturer's instructions. qPCR was
carried out using the SYBR® Premix Ex Taq II (Takara
Biotechnology Co., Ltd.; cat. no. RR820A) in a 25 μl
reaction mixture containing 10 μl SYBR Green mix, 1
μl forward primer, 1 μl reverse primer, 2 μl
template cDNA, and 6 μl RNase-free water. The amplification
program was as follows: initial denaturation at 95°C for 30 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec and
annealing/extension at 60°C for 34 sec. All reactions were
performed in triplicate and each experiment was independently
repeated three times.
Relative gene expression levels were calculated
using the 2−ΔΔCq method as described by Livak and
Schmittgen (32). GAPDH was used
as the internal reference gene depending on species. The sequences
of all primers used for qPCR are listed in Tables SI (human) and SII (rat).
ELISA detection
To evaluate the expression levels of
inflammation-related cytokines and proteins involved in the MAPK
signaling pathway, ELISA assays were performed on both the culture
supernatants of SW982 cells and total protein extracts from rat
synovial tissues.
After treatment with various concentrations of
quercetin for 24 h, culture supernatants from SW982 cells were
collected and centrifuged at 1,000 × g for 10 min at 4°C to remove
cellular debris. MMP3, MMP9 and MMP13 concentrations were measured
using commercial ELISA kits Wuhan Boster Biological Technology,
Ltd.; cat. nos. EK0461, EK0465 and EK0486), following the
manufacturer's protocols. All samples were analyzed in technical
duplicates (n=2) and protein concentrations (pg/ml) were calculated
based on standard curves. Absorbance was measured at 450 nm using a
microplate reader (Infinite F200; Tecan Group, Ltd.), with
background correction applied. The results were used to analyze
changes in MMP secretion among the different treatment groups.
For synovial tissue, total protein was extracted and
analyzed using the InstantOne ELISA kits (Thermo Fisher Scientific,
Inc.; cat. no. 85-86195) to detect the phosphorylation levels of
p-ERK1/2, p-p38 MAPK and p-JNK. Equal amounts of protein were added
to each well, followed by 50 μl of antibody working solution
per well. The plates were incubated at room temperature for 1 h in
the dark. After incubation, the plates were washed three times and
substrate solution was added. The reaction was terminated and
absorbance was measured at 450 nm. The resulting data were used to
compare the activation levels of MAPK signaling pathway components
between groups.
Histological staining
Following decalcification, condylar tissues were
dehydrated using a graded ethanol series and embedded in paraffin.
Serial midsagittal sections with a thickness of 4 μm were
prepared. Hematoxylin and eosin (H&E) staining was performed at
room temperature (22-25°C), with hematoxylin staining for 8 min and
eosin staining for 2 min, to assess the degree of synovitis. The
synovial tissue sections were scored using the Gynther synovitis
grading method (33).
Safranin O-Fast Green staining was conducted at room
temperature (22-25°C), with Fast Green staining for 3 min followed
by Safranin O staining for 5 min, to evaluate the condylar
cartilage structure and proteoglycan content. The stained sections
were observed under a light microscope (Nikon Corporation).
Cartilage matrix staining intensity and structural integrity were
assessed using a previously established scoring system (34).
Immunohistochemistry (IHC) staining
IHC was performed to detect the expression of
inflammation- and signaling pathway-related proteins in synovial
tissue, including MMP13, IL-6 and p-p38 MAPK. Paraffin-embedded
tissue sections were deparaffinized, rehydrated and incubated
overnight at 4°C with the following primary antibodies: MMP13
(Abcam; cat. no. ab237604; 1:2,000), IL-6 (Abcam; cat. no. ab9324;
1:500) and p-p38 MAPK (Cell Signaling Technology, Inc.; cat. no.
4511, 1:400). The following day, color development was performed
using a DAB chromogenic substrate. Sections without primary
antibodies served as negative controls. After staining, the
relative protein expression levels in the synovial tissue were
quantified using Image Pro Plus software (version 6.0.0.260; Media
Cybernetics) by measuring the integrated optical density (IOD)
across multiple fields of view.
Dual immunofluorescence staining and
co-localization analysis
Paraffin-embedded synovial tissue sections (4
μm thick) were deparaffinized, rehydrated and subjected to
high-temperature antigen retrieval in 0.01 M citrate buffer (pH
6.0). After cooling to room temperature, sections were
permeabilized with 0.3% Triton X-100 for 10 min. All washing steps
were performed using PBS (pH 7.4). Non-specific binding sites were
blocked using 5% goat serum (Beyotime Institute of Biotechnology)
at room temperature for 1 h. Sections were then incubated overnight
at 4°C in the dark with the following combinations of primary
antibodies: p-p38 MAPK (Cell Signaling Technology, Inc.; cat. no.
4511, 1:400) + IL-6 (Abcam; cat. no. ab9324; 1:500), or p-p38 MAPK
+ MMP13 (Abcam; cat. no. ab237604; 1:500). The next day, Alexa
Fluor® 488-conjugated goat anti-mouse IgG (Thermo Fisher
Scientific, Inc.; cat. no. A11008) and Alexa Fluor®
594-conjugated goat anti-rabbit IgG (cat. no. A11012) secondary
antibodies were applied at a dilution of 1:500 for 1 h at room
temperature in the dark. Nuclei were counterstained with DAPI
(Thermo Fisher Scientific) at room temperature for 5 min. After
mounting, images were acquired using a confocal laser scanning
microscope (Nikon A1; Nikon Corporation) at 400× magnification
under consistent exposure settings. Co-localization and
fluorescence intensity analysis were performed using ImageJ
software (NIH, version 1.8.0). The fluorescence intensity of
overlapping regions in the red and green channels was quantified
and expressed as relative fluorescence intensity (Relative
fluorescence intensity, A.U.) for subsequent statistical
comparisons.
Micro-CT and 3D reconstruction
To evaluate the protective effect of quercetin on
condylar bone structure, bilateral TMJ samples were collected from
rats and subjected to bone morphometric analysis using a Micro-CT
system. The scanning parameters were set as follows: 80 kV voltage
and 56 μA current. The acquired raw data were processed and
reconstructed in three dimensions using CTAn and CTVox software
(Bruker Corporation). A cylindrical region of interest (ROI) with a
diameter of 0.86 mm and a height of 0.765 mm was selected beneath
the interface between the anterior condylar cartilage and the
subchondral bone. This ROI was used for quantitative analysis of
trabecular bone microarchitecture. The analyzed parameters included
Bone Volume/Tissue Volume (BV/TV): the ratio of bone volume to
total tissue volume; Trabecular Thickness (Tb.Th); Trabecular
Number (Tb.N); Trabecular Separation (Tb.Sp). All parameters were
automatically calculated according to the standard protocols of the
Micro-CT system. The results were used to compare changes in
trabecular bone structure among the different treatment groups.
Statistical analysis
All experimental data were presented as the mean ±
standard deviation (mean ± SD). Prior to intergroup comparisons,
data were tested for normality using the Shapiro-Wilk test and for
homogeneity of variance using Levene's test to ensure the
assumptions for parametric analysis were met. For comparisons
between two independent groups, two-tailed independent sample
t-tests were conducted. For comparisons among multiple groups,
one-way analysis of variance (ANOVA) was performed, followed by
post hoc multiple comparisons using Tukey's HSD or Bonferroni
correction to control for false-positive results. Statistical
analyses and graphical plotting were conducted using GraphPad Prism
6.0 software (Dotmatics). P<0.05 was considered to indicate a
statistically significant difference. Significance levels were
indicated as: *P<0.05, **P<0.01 and
***P<0.001.
Results
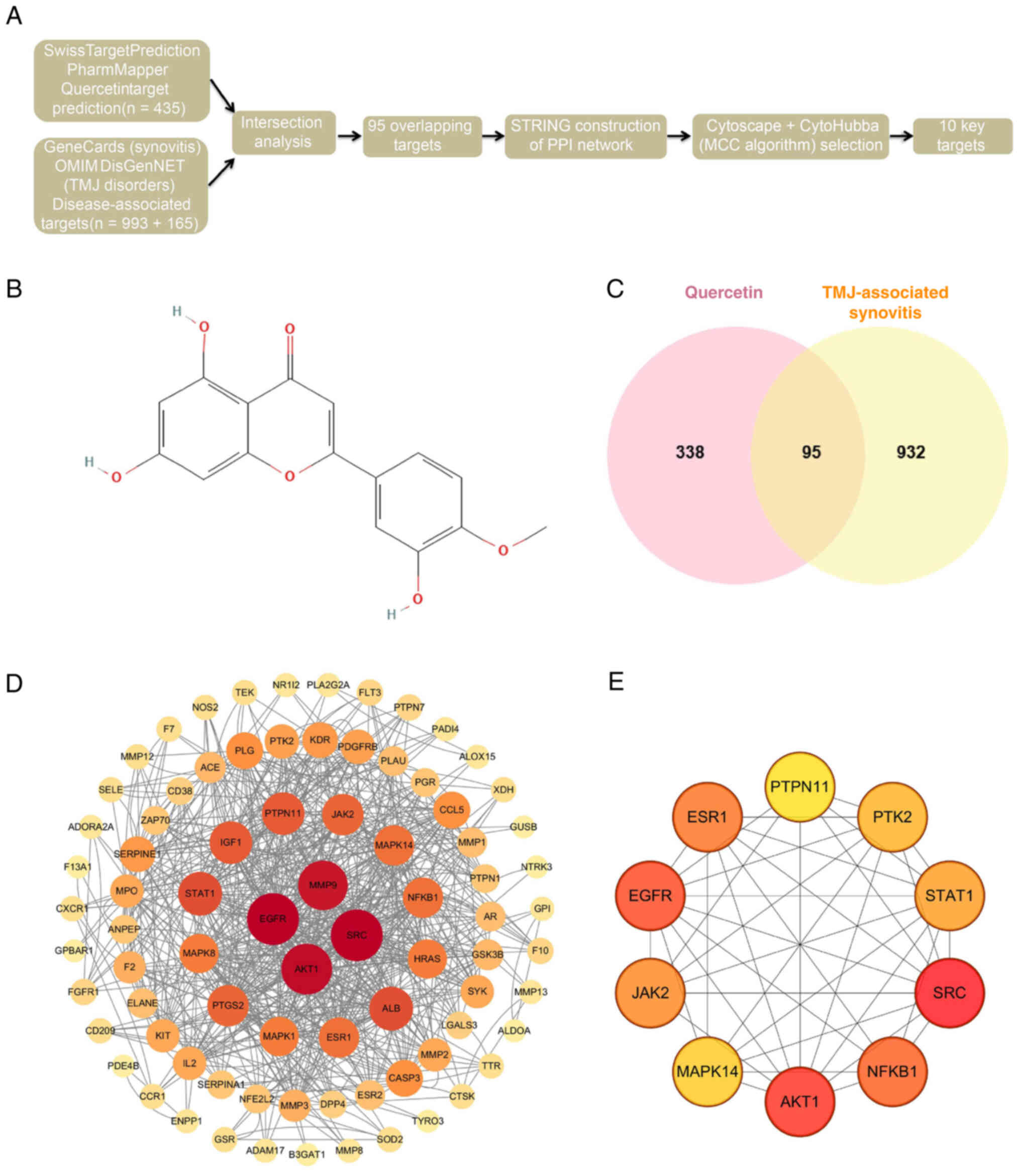
Bioinformatics reveals that quercetin may
alleviate TMJ synovitis by targeting core genes in the MAPK
signaling pathway
To systematically explore the potential mechanisms
by which quercetin alleviates TMJ synovitis, a network
pharmacology-based approach was employed to integrate data from
multiple databases (Fig. 1A).
The 2D chemical structure of quercetin was retrieved from the
PubChem database (Fig. 1B). 435
potential quercetin targets were predicted using
SwissTargetPrediction, SuperPred and PharmMapper Server. Meanwhile,
993 synovitis-related targets and 165 TMJ disorder-related targets
were collected from GeneCards, OMIM and DisGeNET databases. An
intersection analysis identified 95 overlapping genes potentially
involved in disease modulation (Fig.
1C). To further investigate the underlying mechanism, these 95
intersecting targets were imported into the STRING database to
construct a PPI network, which was then visualized using Cytoscape
software (v3.10.1; Fig. 1D).
After removing disconnected nodes, the reconstructed network
included 83 nodes and 321 edges, indicating high interconnectivity
among targets. The network exhibited a median degree centrality of
15.47 and a betweenness centrality of 141.59, suggesting that
several nodes exert strong regulatory influence. Using the
CytoHubba plugin and the MCC algorithm, the top 10 hub genes were
identified, including MAPK14, MMP13 and IL6 (Fig. 1E). These genes displayed high
connectivity within the PPI network and were markedly enriched in
GO and KEGG pathway analyses, indicating their potential role as
key targets through which quercetin exerts its anti-inflammatory
effects.
GO and KEGG enrichment analyses reveal
that quercetin targets may alleviate synovitis via regulation of
the IL-17 and MAPK signaling pathways
GO and KEGG enrichment analyses were conducted on
the 95 intersecting candidate targets to gain deeper insights into
the functional characteristics of potential of quercetin targets.
GO enrichment covered three major categories: BP, MF and CC
(Fig. 2A). In the BP category,
the target genes were markedly enriched in terms such as 'positive
regulation of cell migration', 'activation of phosphatidylinositol
3-kinase signaling', and 'proteolysis'. For MF, significant
enrichments included 'serine-type endopeptidase activity',
'tyrosine kinase activity', and 'enzyme binding'. In the CC
category, the targets were predominantly localized to the
'extracellular region,' 'membrane structure', and 'exosomes'.
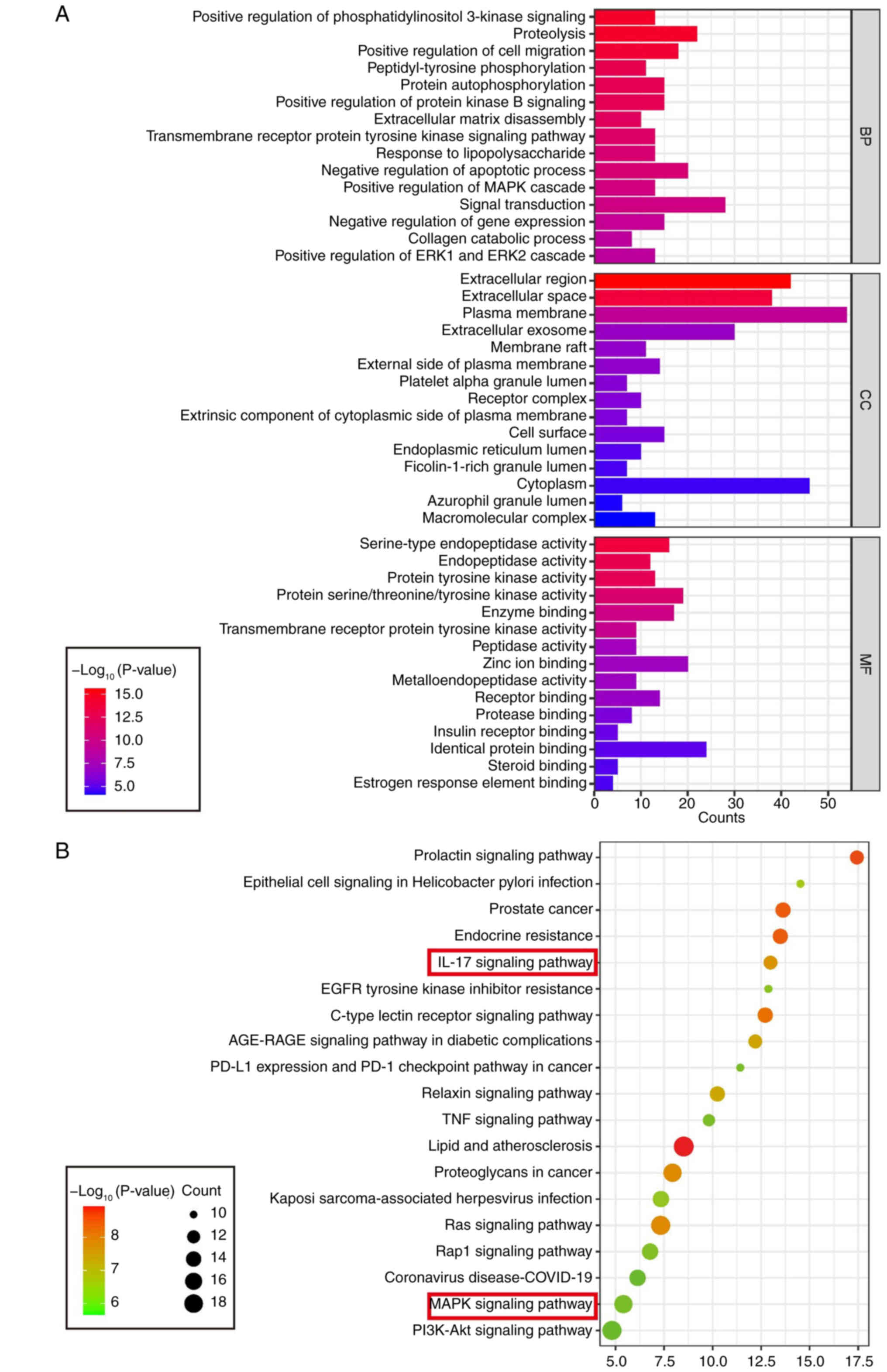 | Figure 2Identifying key signaling pathways
and MF enrichment patterns associated with quercetin in synovitis.
(A) GO enrichment analysis of potential of quercetin targets,
showing the top 15 markedly enriched terms across three categories:
BP, MF and CC; (B) KEGG pathway enrichment analysis of the
predicted targets, displaying the top 15 markedly enriched
signaling pathways. Notably, the IL-17, MAPK, TNF and PI3K-Akt
pathways were among the most markedly enriched, suggesting that
quercetin may exert anti-synovitis effects by modulating these
inflammation-related pathways. GO, Gene Ontology; BP, Biological
Process; MF, Molecular Function; CC, Cellular Component; KEGG,
Kyoto Encyclopedia of Genes and Genomes. |
KEGG pathway enrichment analysis further identified
several highly significant inflammation-related signaling pathways,
including the IL-17 signaling pathway, MAPK signaling pathway, TNF
signaling pathway and PI3K-Akt signaling pathway (Fig. 2B). Integrating these findings
with the PPI network topology analysis, key hub targets such as
MAPK14, MMP13 and IL6 were primarily enriched in the IL-17 and MAPK
pathways. These results suggest that quercetin may exert its
therapeutic effects on TMJ synovitis by regulating these critical
inflammatory signaling pathways.
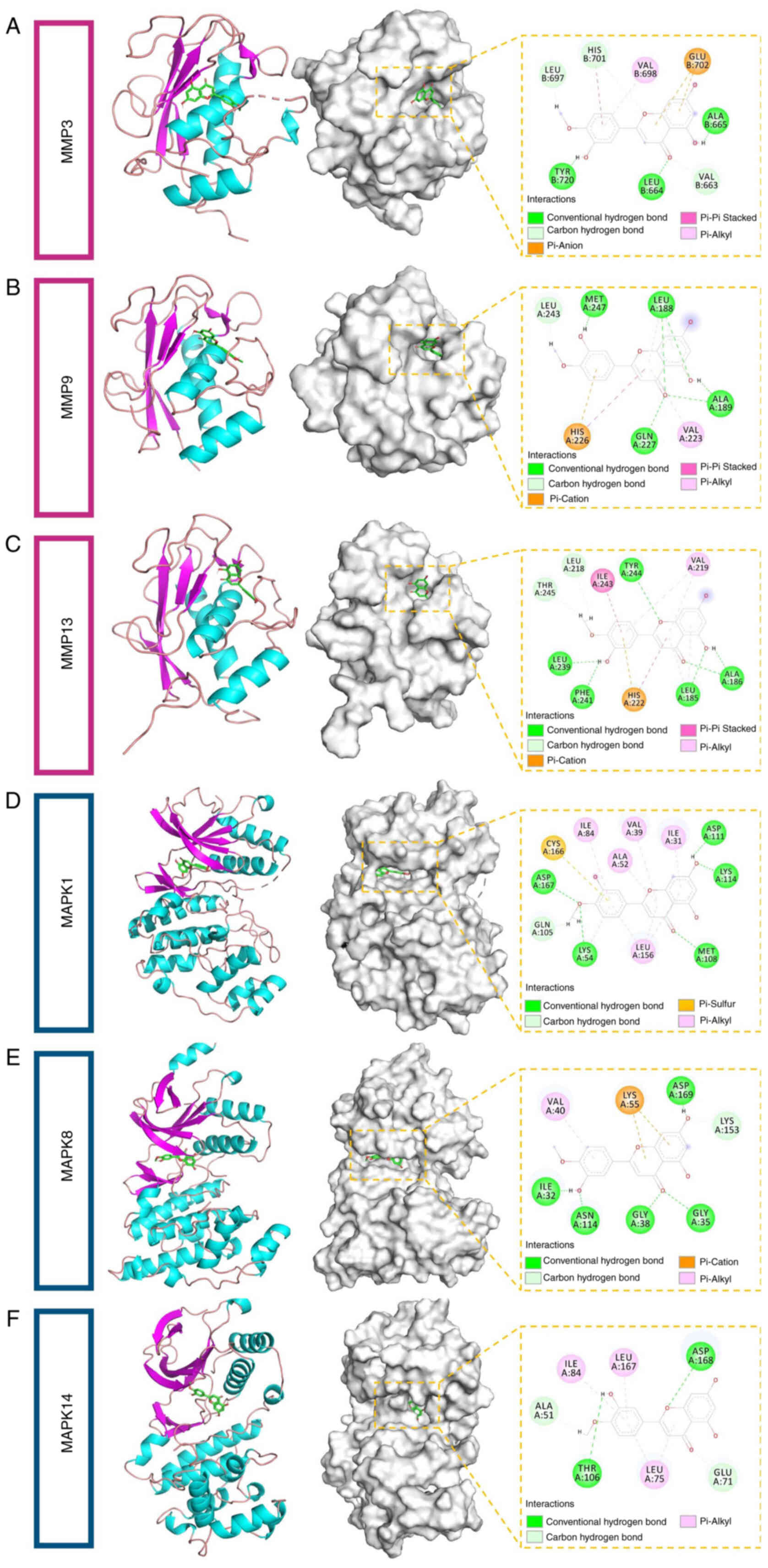
Molecular docking confirms stable binding
of quercetin to key targets in the IL-17 and MAPK signaling
pathways
To further validate the biological relevance of the
GO and KEGG enrichment results, molecular docking analyses were
performed using key proteins enriched in the IL-17 and MAPK
signaling pathways, aiming to assess the structural binding
affinity of quercetin to these core targets. A total of 23
representative proteins were selected as docking candidates,
including GSK3B, MMP13, MMP1, CASP3, MMP3, PTGS2, MMP9, S100A9,
MAPK1, MAPK8, MAPK14, NFKB1, PDGFRB, FLT3, IGF1, EGFR, KIT, KDR,
AKT1, TEK, PTPN7, HARS and FGFR1. These targets encompass protein
kinases, transcription factors and inflammatory effectors.
Molecular docking was performed using AutoDock Vina and quercetin
demonstrated favorable binding affinities across multiple targets,
with binding energies below −7 kcal/mol. The strongest binding
affinities were observed with MMP3, MMP9, MMP13, MAPK1, MAPK8 and
MAPK14 (Table I), suggesting
that these proteins may serve as direct binding targets of
quercetin. Further structural analysis using PyMOL revealed key
interactions between quercetin and these proteins, including
hydrogen bonds and hydrophobic interactions, confirming the
specificity and stability of the binding conformations (Fig. 3A-F). These findings provide
structural evidence supporting the hypothesis that quercetin
mitigates inflammation by inhibiting MMP activity and MAPK
phosphorylation, thereby laying a theoretical foundation for
subsequent in vitro and in vivo validation
experiments.
 | Table IThe docking results for protein with
diosmetin. |
Table I
The docking results for protein with
diosmetin.
| Target | Compound | Docking score
(kcal/mol) |
|---|
| MMP3 | Diosmetin | −6.45 |
| MMP9 | Diosmetin | −7.12 |
| MMP13 | Diosmetin | −7.12 |
| MAPK1 | Diosmetin | −7.30 |
| MAPK8 | Diosmetin | −7.30 |
| MAPK14 | Diosmetin | −8.30 |
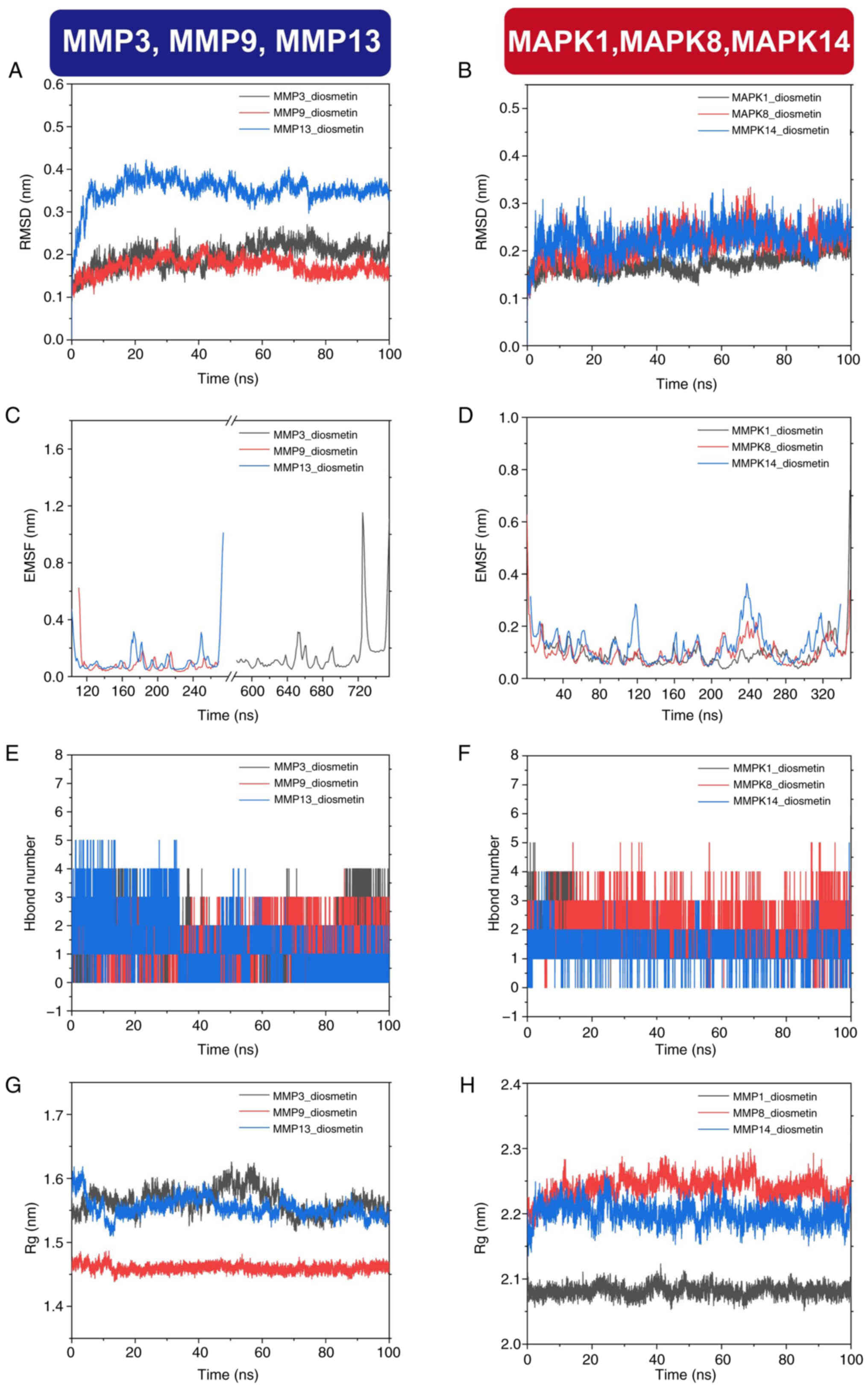
MD simulations confirm stable binding of
quercetin to MAPK and MMP targets
To further validate the molecular docking results
and assess the dynamic stability of quercetin-target interactions,
100-nsec MD simulations were performed on the six protein-ligand
complexes with the lowest binding energies. The selected proteins,
MMP3, MMP9, MMP13, MAPK1, MAPK8 and MAPK14, are core targets within
the IL-17 and MAPK signaling pathways. Root-mean-square deviation
(RMSD) analysis was used to evaluate the overall structural
stability of the complexes. The RMSD values for all complexes
remained within a reasonable range, indicating that the
protein-ligand conformations were dynamically stable throughout the
simulation period. Notably, the MMP9-quercetin complex exhibited an
average RMSD of <2 Å and reached dynamic equilibrium ~25 nsec,
reflecting excellent protein stability (Fig. 4A). Similarly, the MMP3-quercetin
and MMP13-quercetin complexes maintained average RMSD values below
3 Å and 3.5 Å, respectively and reached relatively stable states
after 20 nsec (Fig. 4A). For the
MAPK complexes, the average RMSD values of MAPK1, MAPK8 and MAPK14
were below 2.5 Å, with stable conformations achieved after ~40 nsec
(Fig. 4B). The RMSD analysis
reflects the average deviation of the protein structures from their
initial conformations over time, serving as a quantitative measure
of system stability.
To assess the flexibility of amino acid residues
during binding, root-mean-square fluctuation (RMSF) analysis was
performed. The results revealed that most residues exhibited only
minor fluctuations, with slight variations mainly localized to
hinge regions or protein surface loops. All six protein-ligand
complexes maintained structurally stable conformations throughout
the simulation, indicating that quercetin binding did not induce
significant conformational disruptions (Fig. 4C and D). The number of hydrogen
bonds formed between quercetin and each target protein was
monitored to further evaluate the interaction dynamics during the
simulation. The number of hydrogen bonds remained relatively stable
across the simulation period, ranging from 0-5 per complex
(Fig. 4E and F). These hydrogen
bonds served as key non-covalent forces contributing to the
structural stability of the complexes.
The radius of gyration (Rg) was analyzed to measure
each protein's compactness. Results showed that all proteins
remained tightly folded throughout the 100 nsec simulation, with
MMP9 and MAPK1 displaying the least fluctuation in Rg values,
further supporting their classification as highly stable binding
targets. Specifically, the Rg values of MMP9 and MMP13 fluctuated
within the 1.51-1.62 nm range, while MAPK8 and MAPK14 ranged
between 2.14 and 2.28 nm (Fig. 4G
and H).
Binding free energies were calculated using the
MM/PBSA method. As shown in Table
II, all six protein-ligand complexes exhibited negative binding
energies, indicating favorable thermodynamic stability. The binding
affinity was primarily driven by van der Waals interactions and
electrostatic forces, while solvent-accessible surface area (SASA)
and polar solvation energy made smaller contributions.
 | Table IIThe binding energy by MMGBSA
(KJ/mol). |
Table II
The binding energy by MMGBSA
(KJ/mol).
| Systems | EVDW | EELE | EGB | ESA | Gbinding
energy |
|---|
| MMP3-Diosmeti, |
−108.058±11.266 | −52.647±9.564 | 12.887±11.881 | −3.174±0.658 |
−150.992±15.419 |
| MMP9-Diosmetin | −94.776±15.567 | −41.137±11.778 | 17.653±9.008 | −3.084±0.184 | −121.344±5.838 |
|
MMP13-Diosmetin | −28.588±6.169 | −28.588±6.169 | 19.406±5.331 | −121.344±5.838 |
−130.609±14.595 |
|
MAPK1-Diosmetin |
−127.628±16.138 | −67.43±9.475 | 132.512±18.163 | −14.56±0.618 | −77.106±12.674 |
|
MAPK8-Diosmetin | −138.591±8.773 | −53.998±16.495 | 142.141±15.198 | −15.028±0.662 | −65.475±9.43 |
|
MAPK14-Diosmetin |
−136.477±13.193 | −46.145±12.439 | 141.91±18.201 | −16.068±0.773 | −56.78±12.6 |
In summary, MD simulations verified that quercetin
can form stable complexes with multiple targets (particularly MAPK
and MMP family members), providing the structural and energetic
foundation for subsequent in vitro cellular experiments and
animal studies to validate its anti-inflammatory mechanisms.
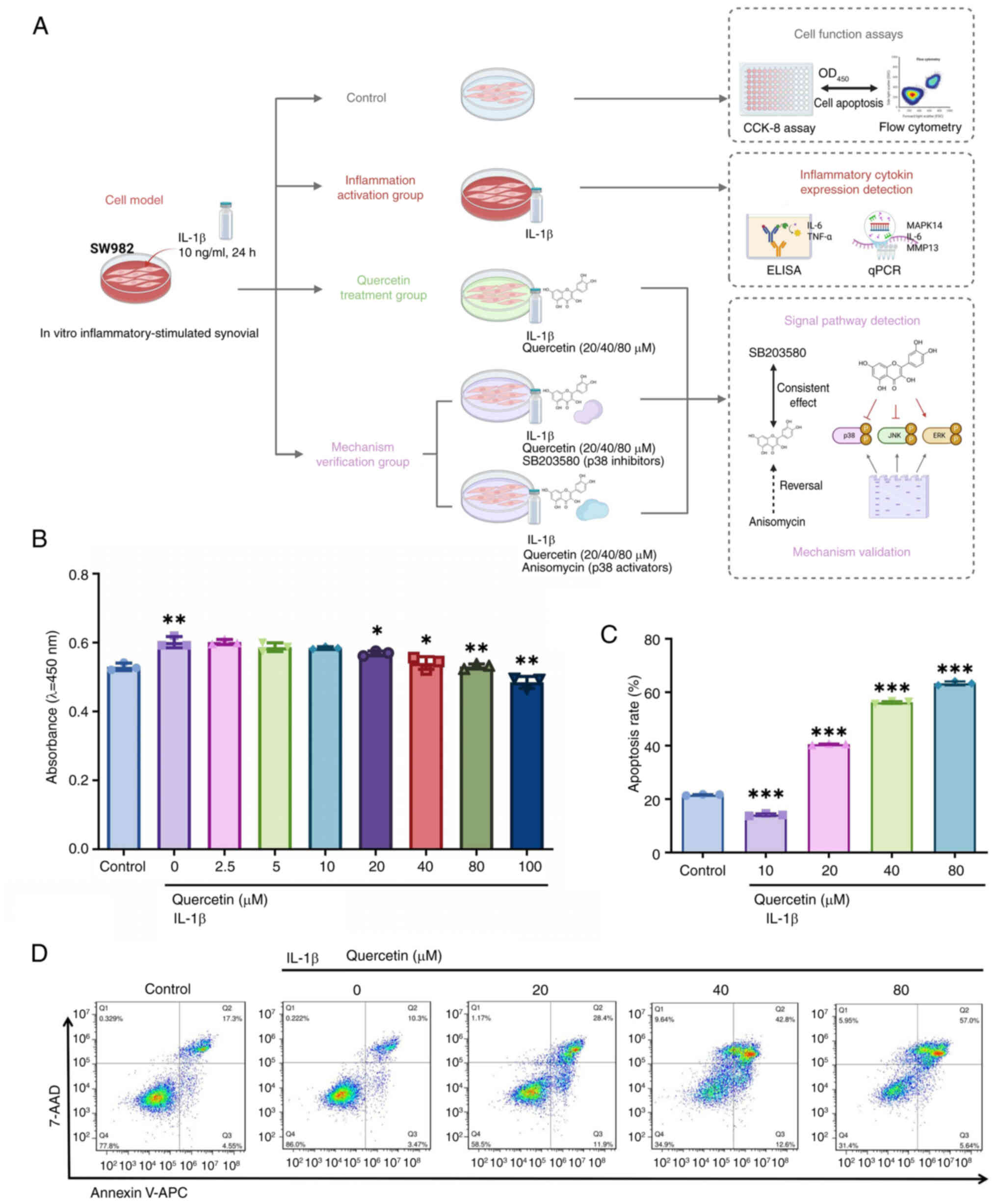
Quercetin reverses inflammatory-induced
synoviocyte dysfunction by promoting apoptosis and inhibiting
prolifera- tion
Previous molecular docking and dynamics simulations
suggested that quercetin can stably bind to key targets such as
MMPsec and MAPKs, indicating its potential to modulate signaling
pathways associated with inflammation and cell proliferation. To
further verify its biological effects at the cellular level, the
present study investigated the regulatory role of quercetin in an
in vitro inflammatory model of synoviocytes (Fig. 5A). An inflammatory state was
established in SW982 cells by IL-1β (10 ng/ml) stimulation,
followed by treatment with various concentrations of quercetin
(0-100 μM). CCK-8 assay results showed that quercetin
markedly inhibited IL-1β-induced abnormal proliferation in a
dose-dependent manner within the range of 20-100 μM
(Fig. 5B). Based on these
findings, 20, 40 and 80 μM were selected for subsequent
experiments. Flow cytometry analysis further demonstrated that
quercetin treatment markedly increased the apoptosis rate of
synoviocytes, showing a clear dose-dependent trend (Fig. 5C and 5D).
These results indicate that quercetin exhibits
strong molecular binding to inflammation, and proliferation-related
targets. It effectively suppresses inflammatory-induced synoviocyte
overproliferation and promotes apoptosis at the cellular level.
These findings provide a solid foundation for subsequent functional
validation of its effects on MMP secretion and MAPK signaling
pathway activity.
Quercetin attenuates synovial
inflammation by regulating MMP expression and inhibiting MAPK
signaling pathway phosphorylation
To further elucidate the anti-inflammatory mechanism
of quercetin in vitro, its regulatory effects on MMPsec and
inflammatory cytokines were examined. ELISA results showed that
quercetin markedly reduced the secretion levels of MMP3, MMP9 and
MMP13 under IL-1β stimulation, suggesting its potential role in
protecting cartilage by inhibiting extracellular matrix degradation
in synoviocytes (Fig. 6A).
Additionally, western blot analysis revealed a dose-dependent
decrease in the protein expression of inflammatory cytokines TNF-α,
IL-1β and IL-6 following quercetin treatment (Fig. 6B), further supporting its
anti-inflammatory effects. To determine whether these effects are
mediated via the MAPK signaling pathway, the phosphorylation levels
of key MAPK family members p38, ERK1/2 and JNK were assessed. The
results demonstrated that quercetin markedly inhibited the
phosphorylation of p38 MAPK and JNK, while having no significant
effect on p-ERK1/2 levels (Fig.
6C-E), indicating that its anti-inflammatory effects were
primarily mediated through the p38 MAPK and JNK signaling pathways.
Together with previous findings from molecular docking and
enrichment analyses, these in vitro results confirmed that
quercetin alleviated synovial inflammation through coordinated
regulation of MMP expression and inhibition of MAPK pathway
activation, laying a mechanistic foundation for its therapeutic
potential in synovitis.
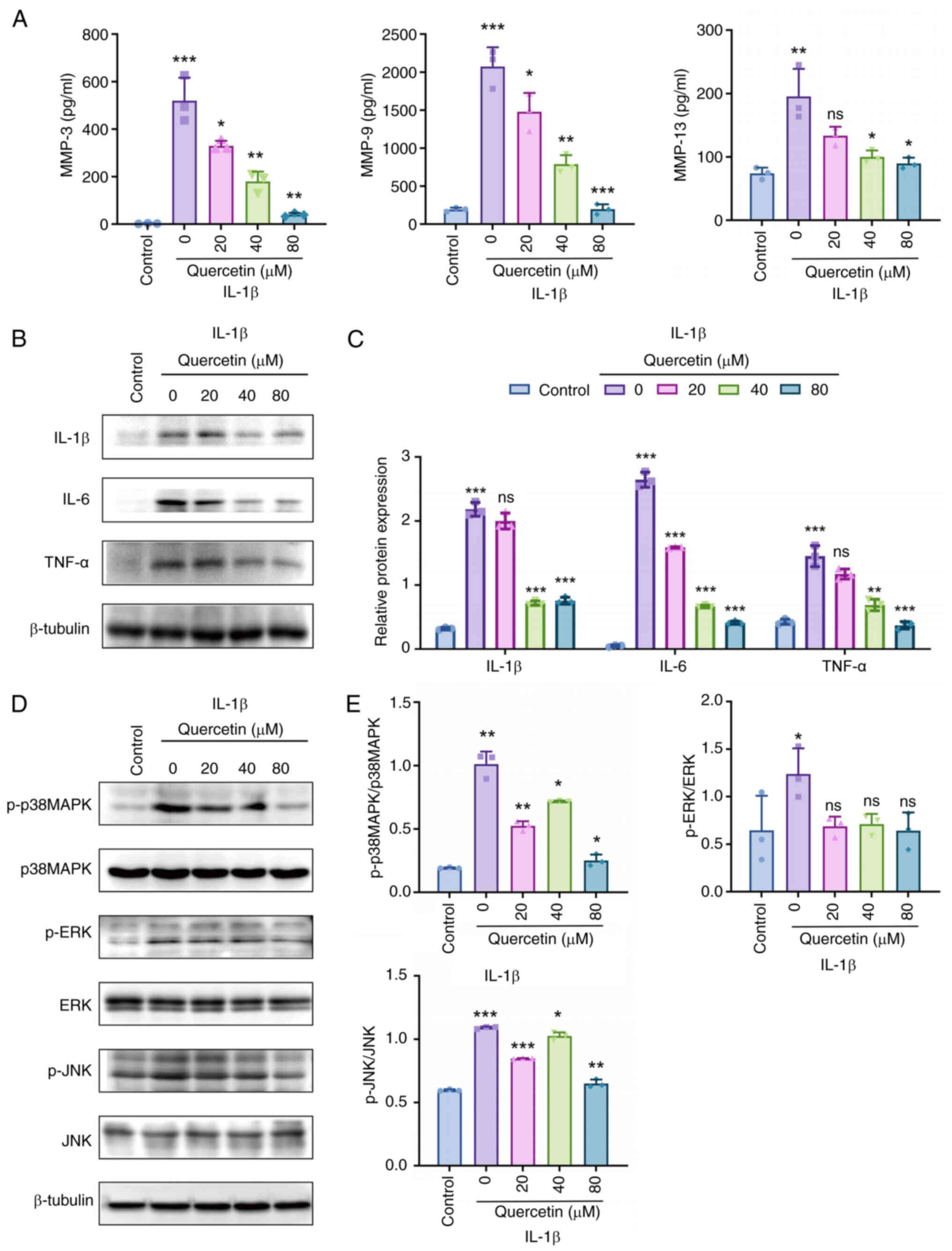 | Figure 6Multi-level validation of
quercetin-mediated regulation of inflammatory cytokines and MMPs
via inhibition of the p38 MAPK signaling pathway. (A) ELISA
analysis showing the effects of different concentrations of
quercetin on IL-1β-induced secretion of MMP3, MMP9 and MMP13 in
SW982 cells; (B) Western blot analysis of TNF-α, IL-1β and IL-6
protein expression levels, with β-tubulin as the internal control;
(C and D) Western blot analysis of phosphorylation levels of key
MAPK signaling molecules: p38, ERK1/2 and JNK; (E) Quantitative
analysis of phosphorylated proteins, showing that quercetin
markedly inhibited the expression of p-p38 and p-JNK, with no
significant effect on p-ERK1/2. All experiments were independently
repeated three times (n=3) and data are presented as mean ± SD.
Statistical comparisons were performed using one-way ANOVA.
Significance levels compared with the model group or 0 μM
group are indicated as: *P<0.05,
**P<0.01, ***P<0.001; ns:
insignificant. MMPs, matrix metalloproteinases; ELISA,
enzyme-linked immunosorbent assay; p-, phosphorylated. |
Quercetin ameliorates synovitis and
cartilage degradation via suppressing p38 MAPK-mediated
inflammatory signaling
Multiple histological and molecular techniques,
including H&E staining, Safranin O-Fast Green staining,
immunofluorescence co-localization and qPCR analysis, were employed
to assess quercetin's protective effects on synovial inflammation
and cartilage degeneration at both tissue and molecular levels.
H&E staining results revealed that the synovial
tissues of the control group exhibited normal histoarchitecture
with minimal inflammation. By contrast, the CFA model group showed
pronounced synovial hyperplasia and extensive infiltration of
inflammatory cells. Quercetin treatment markedly reduced
inflammatory cell infiltration and improved the integrity of the
synovial structure (Fig. 7A).
Safranin O-Fast Green staining further demonstrated a marked loss
of proteoglycans in the cartilage of CFA-treated rats, as indicated
by the diminished red staining. The red staining area was notably
restored in the quercetin-treated group, suggesting that quercetin
exerts a chondroprotective effect (Fig. 7B).
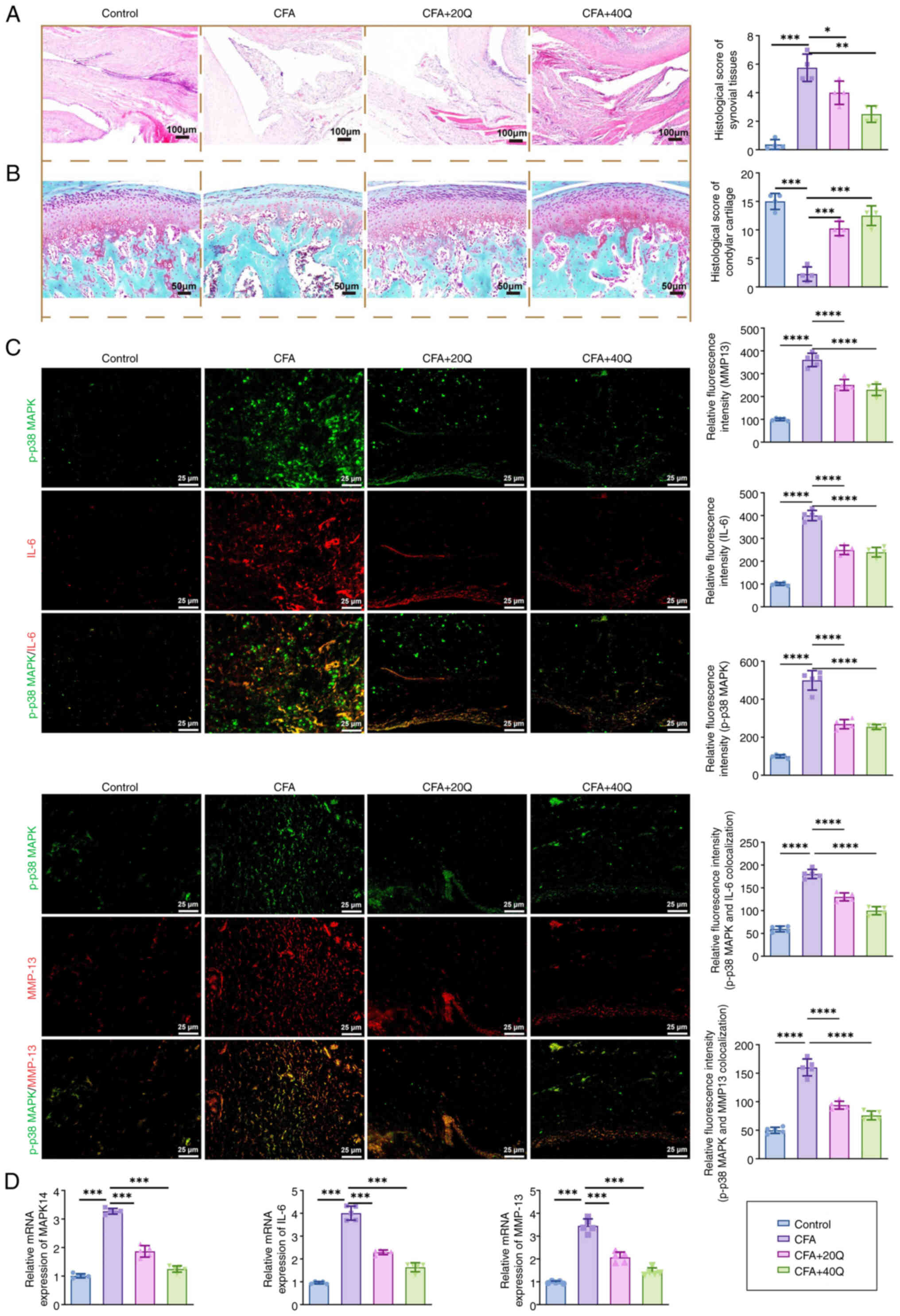 | Figure 7Multi-level analysis validates
quercetin's therapeutic effects on synovitis and its regulatory
role in inflammation-related signaling pathways. (A) H&E
staining assessing changes in synovial structure and inflammatory
cell infiltration. (B) Safranin O-Fast Green staining showing
alterations in proteoglycan distribution in condylar cartilage. (C)
Dual immunofluorescence staining analyzing the co-localization of
p-p38 MAPK (green) with IL-6 (red) and MMP13 (red) in synovial
tissue. (D) qPCR analysis of key gene expression levels, including
MAPK14, MAPKAPK2, DUSP1, IL-6, TNF-α and MMP13, in SW982 cells and
synovial tissues. All histological experiments were conducted with
five animals per group (n=5) and molecular experiments were
independently repeated three times (n=3). Data are presented as
mean ± SD. Statistical comparisons were performed using one-way
ANOVA. Significance levels: *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001; ns, insignificant. H&E, hematoxylin
and eosin; MMP, matrix metalloproteinase; qPCR, quantitative
PCR. |
To further evaluate the molecular mechanisms
underlying the anti-inflammatory effects of quercetin, dual
immunofluorescence staining was performed to assess the
co-localization of p-p38 MAPK with downstream inflammatory
cytokines in synovial tissue. As shown in Fig. 7C, in the CFA-induced synovitis
model, p-p38 MAPK was highly co-localized with IL-6 and MMP-13 in
synoviocytes, with co-expression fluorescence intensities of
182.3±15.6 and 165.2±13.2, respectively. Following quercetin
treatment, the co-expression intensity of p-p38 MAPK + IL-6 was
significantly reduced to 122.4±12.9 in the 20 mg/kg group and
91.7±10.4 in the 40 mg/kg group (*P<0.05 and
**P<0.01). Similarly, the co-expression of p-p38 MAPK
+ MMP-13 decreased to 113.5±11.7 and 85.6±9.8 at the respective
doses (*P<0.05 and **P<0.01),
indicating that quercetin effectively suppresses the
co-localization of phosphorylated p38 with key inflammatory
mediators in synovial tissue.
At the transcriptional level, qPCR analysis
(Fig. 7D) revealed that
quercetin significantly downregulated the mRNA expression of
MAPK14, IL-6 and MMP13. Specifically, MAPK14 expression decreased
from 3.27±0.25 in the CFA group to 1.38±0.16 in the 40 mg/kg
quercetin group (***P<0.001). Similarly, IL-6
expression was reduced from 4.01±0.33 to 1.72±0.21 and MMP13 from
3.62±0.27 to 1.45±0.19. The combined results from both tissue
localization and gene expression analyses conclusively demonstrated
that quercetin mediated its anti-inflammatory and chondroprotective
effects through coordinated transcriptional and signaling
regulation, specifically by inhibiting p38 MAPK pathway activation
and consequently reducing downstream expression of inflammatory
cytokines and MMPs.
In summary, quercetin alleviated synovial
inflammation and subchondral bone destruction by suppressing the
activation of the p38 MAPK signaling pathway and transcriptionally
regulating its downstream targets, including pro-inflammatory
cytokines and MMPs, in synovial tissue.
Quercetin attenuates subchondral condylar
bone destruc- tion via the p38 MAPK signaling pathway in a rat
model
To further confirm whether quercetin exerts its
therapeutic effects through the p38 MAPK signaling pathway, a
series of in vivo mechanistic validations was conducted.
InstantOne ELISA results (Fig.
8A) showed that the expression levels of p-p38 MAPK and p-JNK
were markedly elevated in the synovial tissues of the CFA group. By
contrast, quercetin treatment markedly reduced p-p38 MAPK
expression. IHC analysis further supported these findings (Fig. 8B), demonstrating that the
expression of MMP13 and IL-6 was markedly increased in the CFA
group, while quercetin treatment effectively suppressed their
levels in synovial tissues. Moreover, Micro-CT analysis (Fig. 8C-E) revealed severe subchondral
bone destruction in the condylar region of CFA-treated rats,
characterized by a decrease in BV/TV and Tb.N, along with an
increase in Tb.Sp. Quercetin administration markedly improved all
these structural parameters (Fig.
8F and 8G), indicating its
protective effect on subchondral bone.
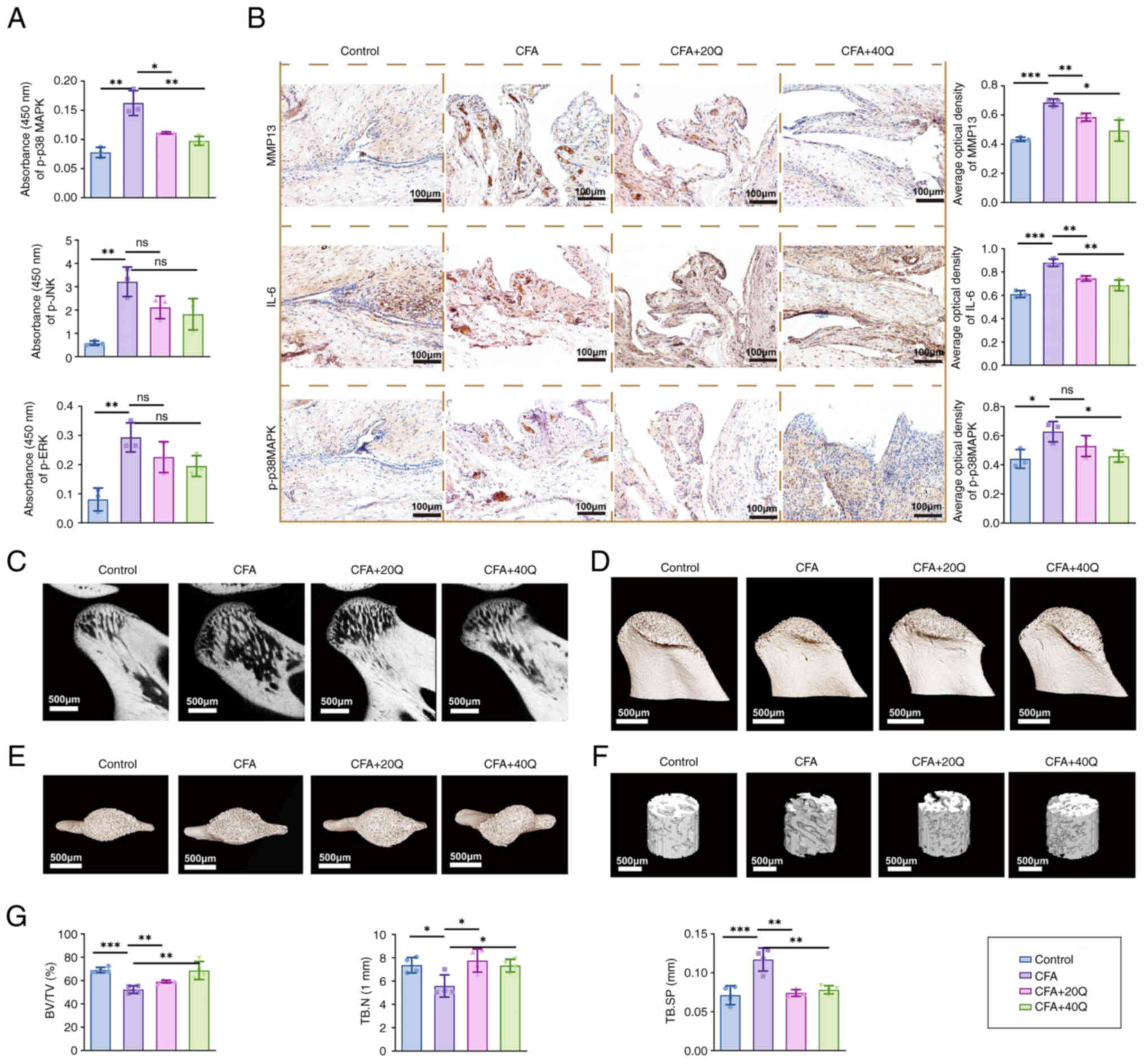 | Figure 8Quercetin alleviates inflammatory
cytokine expression and mitigates bone destruction by modulating
the p38 MAPK signaling pathway. (A) InstantOne ELISA analysis of
p-p38 MAPK, p-JNK and p-ERK levels in synovial tissue. (B) IHC
staining showing expression changes of MMP13, IL-6 and p-p38 MAPK
in synovial tissue. (C) Evaluation of subchondral bone destruction
using Micro-CT sagittal slices, (D and E) 3D reconstructions and
(F) cylindrical ROI of rat condylar bone. (G) Quantitative analysis
of BV/TV, Tb.N and Tb.Sp. Scale bar, 500 μm. Comparisons
were made against the CFA group. Each group included five rats
(n=5) and data are presented as mean ± SD. Statistical analysis was
performed using one-way ANOVA; *P<0.05,
**P<0.01, ***P<0.001. ELISA,
enzyme-linked immunosorbent assay; p-, phosphorylated; IHC,
immunohistochemistry; MMP, matrix metalloproteinase; Micro-CT,
micro-computed tomography; ROI, region of interest; BV/TV, bone
volume/tissue volume; Tb.N, trabecular number; Tb.Sp, trabecular
separation; CFA, Complete Freund's Adjuvant. |
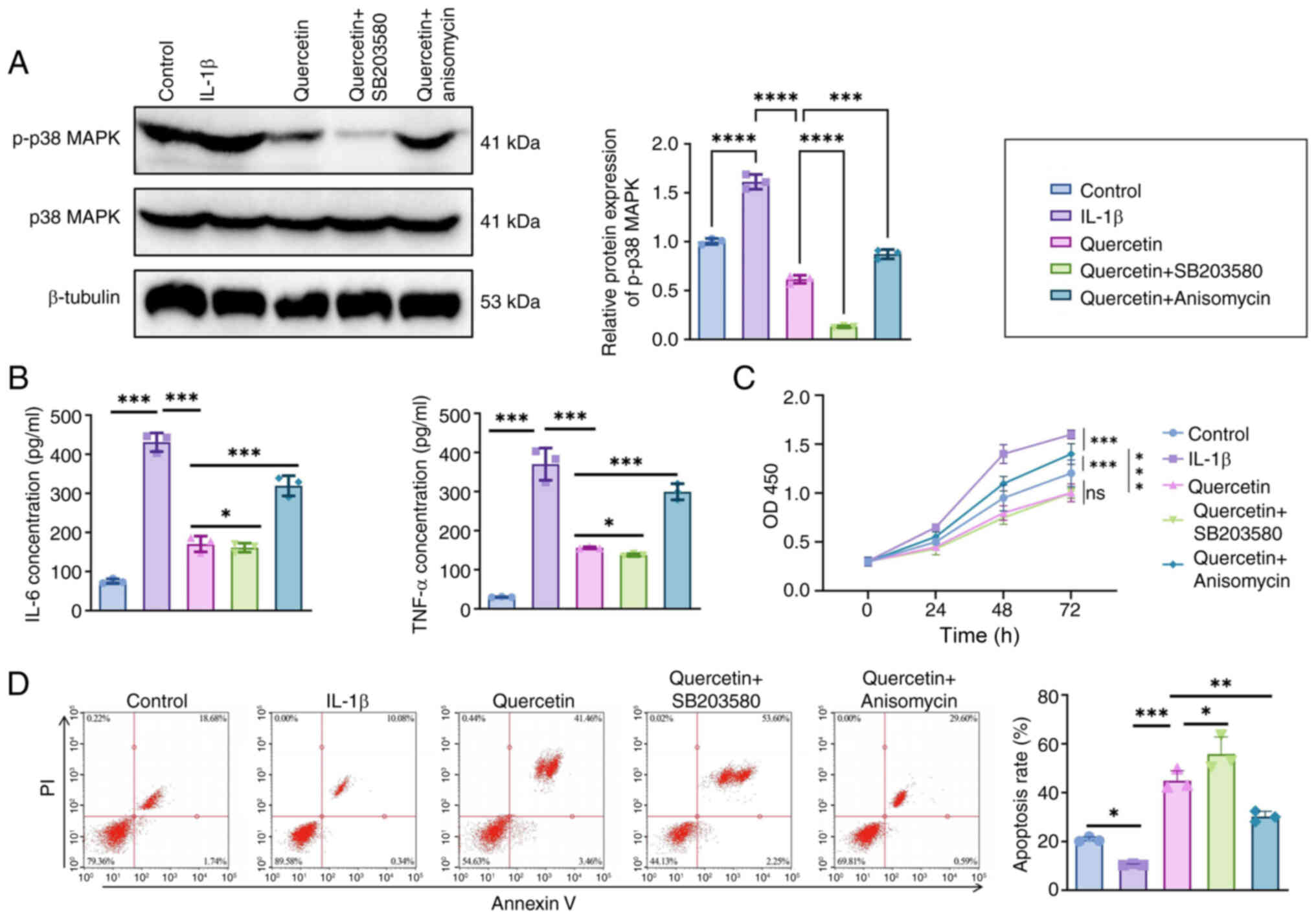
p38 MAPK agonist/inhibitor experiments
confirm the signaling pathway dependency of the anti-inflammatory
effect of quercetin
To further verify whether the anti-inflammatory
mechanism of quercetin depends on the p38 MAPK signaling pathway,
controlled experiments were conducted using both the pathway
agonist anisomycin and inhibitor SB203580 in an IL-1β-stimulated
SW982 synovial cell model.
As shown by western blot analysis (Fig. 9A), IL-1β stimulation markedly
increased the expression level of p-p38 MAPK to 1.00±0.09 (relative
grayscale value). Treatment with quercetin markedly reduced this
level to 0.42±0.06 (***P<0.001). The effect of
SB203580 was comparable to that of quercetin, reducing p-p38 MAPK
to 0.38±0.05 (not significant vs. quercetin), indicating similar
inhibitory effects. By contrast, co-treatment with the pathway
agonist anisomycin partially reversed quercetin's effect, raising
p-p38 MAPK levels to 0.83±0.07 (**P<0.01 vs.
quercetin group). These results suggest that the anti-inflammatory
effect of quercetin is, at least in part, mediated through
inhibition of the p38 MAPK signaling pathway.
ELISA results (Fig.
9B) demonstrated that IL-1β stimulation markedly increased the
secretion of IL-6 and TNF-α, with levels reaching 425.6±28.4 pg/ml
and 372.8±24.1 pg/ml, respectively. Quercetin treatment markedly
reduced these cytokine levels to 174.2±19.3 pg/ml for IL-6 and
151.6±17.7 pg/ml for TNF-α (***P<0.001). Comparable
effects were observed in the SB203580 group (IL-6: 166.5±16.4
pg/ml; TNF-α: 147.3±14.9 pg/ml; ns vs. quercetin group). However,
co-treatment with the p38 MAPK agonist anisomycin led to a partial
reversal, increasing IL-6 and TNF-α levels to 314.7±21.2 pg/ml and
296.5±18.3 pg/ml, respectively (**P<0.01 vs.
quercetin group).
Regarding cell function, CCK-8 assay results
(Fig. 9C) showed that IL-1β
stimulation markedly enhanced synoviocyte proliferation, with an
OD450 value of 1.62±0.07. Quercetin treatment effectively
suppressed proliferation (1.09±0.06; ***P<0.001) and
a similar reduction was observed in the SB203580 group (1.05±0.04;
ns vs. quercetin group). Anisomycin partially reversed this
inhibitory effect, increasing the OD450 value to 1.41±0.05
(**P<0.01).
Flow cytometry analysis using Annexin V/PI staining
(Fig. 9D) revealed that IL-1β
stimulation markedly reduced the apoptosis rate to 7.8%±1.2%.
Quercetin treatment markedly increased apoptosis to 22.6%±2.1%
(***P<0.001), while the SB203580 group showed a
comparable increase (24.3%±1.9%). Following combined treatment with
anisomycin, the apoptosis rate decreased to 12.4%±1.7%
(***P<0.01 vs. quercetin group).
Taken together, these agonist/inhibitor control
experiments clearly demonstrate that quercetin's anti-inflammatory,
pro-apoptotic and antiproliferative effects are highly dependent on
inhibiting the p38 MAPK signaling pathway, reinforcing the
specificity of its underlying mechanism.
Discussion
The present study is the first, to the best of the
authors' knowledge, to reveal the potential mechanism by which
quercetin alleviates TMJ synovitis by inhibiting the p38 MAPK
signaling pathway, thereby regulating synoviocyte inflammation and
apoptosis. Quercetin markedly suppressed abnormal proliferation of
synovial cells, promoted apoptosis and effectively reduced the
secretion of inflammatory cytokines such as IL-6 and TNF-α. These
findings not only provided new evidence for the anti-inflammatory
effects of quercetin but also offered theoretical support for the
critical role of the p38 MAPK signaling pathway in TMD-associated
synovitis. Unlike previous studies, the present study integrated
multiple experimental approaches, including molecular docking and
MD simulation, to comprehensively elucidate the mechanistic action
of quercetin via the p38 MAPK pathway. Compared with conventional
therapies, quercetin, as a natural compound, offers broad
biological activity with fewer side effects, highlighting its
promising potential for clinical application in treating TMD
synovitis.
Compared with existing studies, the innovation of
this research lies in its first systematic elucidation of the
mechanism by which quercetin acts on TMD-associated synovitis,
particularly its potential to modulate inflammation and apoptosis
via the p38 MAPK signaling pathway (11). Previous studies have primarily
focused on the effects of quercetin in RA and osteoarthritis, while
research specifically addressing TMD synovitis remains limited.
Quercetin has been demonstrated to exert anti-inflammatory effects
by regulating multiple signaling pathways, such as MAPK and NF-κB
(16). However, through the
integration of network pharmacology and experimental validation,
The present study was the first, to the best of the authors'
knowledge, to propose that quercetin may alleviate TMD synovitis by
inhibiting the p38 MAPK signaling pathway, thereby filling a
critical gap in the current literature. Unlike conventional
anti-inflammatory drugs such as NSAIDs, quercetin alleviates
inflammation, modulates immune responses and protects joint
integrity. These multifunctional properties highlight its broad
therapeutic potential.
An unexpected finding in the present study was that
quercetin markedly promotes apoptosis in synoviocytes, providing
new insight into its potential application in treating
TMD-associated synovitis. Consistent with previous reports on the
anti-inflammatory effects of quercetin (35), the present results suggested that
the pro-apoptotic effect on synovial cells may represent an
additional mechanism by which quercetin alleviates inflammation in
TMD synovitis. However, compared with other studies reporting
varying effects of quercetin on cell proliferation under different
conditions (36), the present
study observed that quercetin inhibited synoviocyte proliferation
and induced apoptosis at relatively low concentrations. This
discrepancy may be attributed to differences in experimental
conditions, including the concentration of quercetin used and the
specific cell types involved. These findings warrant further
investigation.
As a natural compound, quercetin possesses low
toxicity and a wide range of biological activities, making its
therapeutic potential in TMD-associated synovitis particularly
noteworthy. Compared with conventional pharmacological treatments,
quercetin offers distinct advantages in reducing inflammation,
promoting synoviocyte apoptosis and facilitating the repair of
subchondral bone structures (37,38). The present study demonstrated
that quercetin alleviated synovitis and improved joint function by
inhibiting the p38 MAPK signaling pathway. These findings suggested
that quercetin may be an effective adjunctive therapy for TMD
synovitis, especially for long-term management. Its natural origin
and low risk of adverse effects make it an ideal candidate for
chronic treatment in patients with TMD. In the future, quercetin
may also be combined with other anti-inflammatory agents to enhance
therapeutic efficacy while reducing long-term drug dependence in
patients, offering a promising strategy for integrated,
low-toxicity management of TMD-related joint inflammation.
Although the present study established a relatively
comprehensive mechanistic framework across multiple levels, several
limitations should be acknowledged. First, SW982 cells were used as
the sole representative of synoviocytes, without including primary
synovial cells or different synovial subtypes under varying
inflammatory conditions. Second, while the study highlighted the
role of the p38 MAPK signaling pathway, it did not further explore
the potential crosstalk with other key inflammatory pathways, such
as JNK or NF-κB. Additionally, the long-term effects of quercetin
in chronic TMD models have yet to be evaluated. Lastly, systematic
pharmacokinetic and toxicological assessments were not conducted in
the present study and future research is needed to address these
aspects to ensure quercetin's clinical safety and
applicability.
Future research may further explore the interplay
between signaling pathways, particularly the synergistic regulatory
mechanisms between the p38 MAPK signaling pathway and other
inflammation-related pathways. To enhance the physiological
relevance of in vitro experiments, primary synoviocytes or
3D organoid models should be considered. Additionally, establishing
chronic TMD animal models would allow for evaluating quercetin's
long-term therapeutic efficacy and potential to prevent disease
recurrence. Further studies should also focus on drug delivery
strategies by developing quercetin-based targeted delivery systems,
such as microspheres or nanocarriers, to improve its
bioavailability. Finally, comprehensive safety assessments and
preclinical pharmacodynamic evaluations are essential to support
future clinical translation and provide a solid foundation for
clinical trials.
The present study is the first, to the best of the
authors' knowledge, to elucidate the potential mechanism by which
quercetin alleviates TMD-associated synovitis by inhibiting the p38
MAPK signaling pathway, thereby regulating synoviocyte inflammation
and apoptosis. As a natural compound, quercetin offers a novel
therapeutic strategy for TMD due to its anti-inflammatory
properties and low toxicity profile. Although certain limitations
exist in the present study, quercetin's multiple biological
activities and favorable safety characteristics highlight its
promise as a candidate drug for treating TMD synovitis. Future
research should further validate its clinical efficacy and
investigate its potential in combination therapies, promoting its
broader application in TMD and other inflammation-related
disorders.
The present study systematically integrated network
pharmacology, molecular simulations, in vitro cellular
assays and in vivo animal models to elucidate, for the first
time, to the best of the authors' knowledge, the mechanism by which
quercetin alleviates TMJ synovitis. The results demonstrated that
quercetin exerted its effects by targeting the p38 MAPK signaling
pathway, markedly reducing its phosphorylation level. This
inhibition led to the downregulation of inflammatory cytokines such
as IL-6, TNF-α and MMPs, thereby attenuating synovial inflammation,
promoting synoviocyte apoptosis and protecting the subchondral bone
structure. The functional verification using a p38 MAPK pathway
agonist and inhibitor confirms that these effects depend highly on
p38 MAPK activity. The present study uncovered the multi-target
anti-inflammatory mechanism of quercetin and provides experimental
evidence supporting the role of the p38 MAPK signaling pathway in
TMD-related synovitis.
Supplementary Data
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
XY and YL conceived and designed the study. MC, YG
and XX performed the experiments. YG, XX and XY analyzed the data.
MC and XX wrote the manuscript. XY and YL confirm the authenticity
of all the raw data. All authors reviewed and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Ethics Committee of China Medical University (approval no.
kt2022112) and conducted by institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ANOVA
|
analysis of variance
|
|
BP
|
Biological Process
|
|
BV/TV
|
bone volume/tissue volume
|
|
CC
|
Cellular Component
|
|
CCK-8
|
Cell Counting Kit-8
|
|
cDNA
|
complementary DNA
|
|
CFA
|
Complete Freund's Adjuvant
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
GO
|
Gene Ontology
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
MCC
|
maximal clique centrality
|
|
MD
|
Molecular Dynamics
|
|
MF
|
Molecular Function
|
|
Micro-CT
|
micro-computed tomography
|
|
MM/PBSA
|
molecular mechanics/Poisson-Boltzmann
surface area
|
|
MMP
|
matrix metalloproteinase
|
|
mean ± SD
|
mean ± standard deviation
|
|
NSAIDs
|
nonsteroidal anti-inflammatory
drugs
|
|
OD
|
optical density
|
|
p-ERK
|
phosphorylated ERK
|
|
p-JNK
|
phosphorylated JNK
|
|
p-p38 MAPK
|
phosphorylated p38 MAPK
|
|
PPI
|
protein-protein interaction
|
|
qPCR
|
quantitative PCR
|
|
RA
|
rheumatoid arthritis
|
|
Rg
|
radius of gyration
|
|
RMSD
|
root-mean-square deviation
|
|
RMSF
|
root-mean-square fluctuation
|
|
ROI
|
region of interest
|
|
SD
|
Sprague-Dawley
|
|
Tb.N
|
trabecular number
|
|
Tb.Sp
|
trabecular separation
|
|
TMDs
|
temporomandibular disorders
|
|
TMJ
|
temporomandibular joint
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82370987).
References
|
1
|
Kapos FP, Exposto FG, Oyarzo JF and Durham
J: Temporomandibular disorders: A review of current concepts in
aetiology, diagnosis and management. Oral Surg. 13:321–334. 2020.
View Article : Google Scholar
|
|
2
|
Zieliński G, Pająk-Zielińska B and Ginszt
M: A meta-analysis of the global prevalence of temporomandibular
disorders. J Clin Med. 13:13652024. View Article : Google Scholar
|
|
3
|
Zieliński G: Quo vadis temporomandibular
disorders? By 2050, the global prevalence of TMD may approach 44%.
J Clin Med. 14:44142025. View Article : Google Scholar
|
|
4
|
Li DTS and Leung YY: Temporomandibular
disorders: Current concepts and controversies in diagnosis and
management. Diagnostics (Basel). 11:4592021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang XD, Zhang JN, Gan YH and Zhou YH:
Current understanding of pathogenesis and treatment of TMJ
osteoarthritis. J Dent Res. 94:666–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gil-Martinez A, Paris-Alemany A,
López-de-Uralde-Villanueva I and La Touche R: Management of pain in
patients with temporomandibular disorder (TMD): challenges and
solutions. J Pain Res. 11:571–587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andre A, Kang J and Dym H: Pharmacologic
treatment for temporomandibular and temporomandibular joint
disorders. Oral Maxillofac Surg Clin North Am. 34:49–59. 2022.
View Article : Google Scholar
|
|
8
|
Tamer TM: Hyaluronan and synovial joint:
Function, distribution and healing. Interdiscip Toxicol. 6:111–125.
2013. View Article : Google Scholar
|
|
9
|
Jerosch J: Effects of glucosamine and
chondroitin sulfate on cartilage metabolism in OA: Outlook on other
nutrient partners especially omega-3 fatty acids. Int J Rheumatol.
2011:1–17. 2011. View Article : Google Scholar
|
|
10
|
Wujec M and Feldo M: Can we improve
diosmetin activity? The state-of-the-art and promising research
directions. Molecules. 28:79102023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Z, Liu K, Liang C, Wen L, Wu J, Liu X
and Li X: Diosmetin as a promising natural therapeutic agent: In
vivo, in vitro mechanisms, and clinical studies. Phytother Res.
38:3660–3694. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aghababaei F and Hadidi M: Recent advances
in potential health benefits of quercetin. Pharmaceuticals (Basel).
16:10202023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang G, Wang Y, Yao L, Gu W, Zhao S, Shen
Z, Lin Z, Liu W and Yan T: Pharmacological activity of quercetin:
An updated review. Evid Based Complement Alternat Med. 2022:1–12.
2022. View Article : Google Scholar
|
|
14
|
Silva-Pinto PA, de Pontes JTC,
Aguilar-Morón B, Canales CSC, Pavan FR and Roque-Borda CA:
Phytochemical insights into flavonoids in cancer: Mechanisms,
therapeutic potential, and the case of quercetin. Heliyon.
11:e426822025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shabir I, Kumar Pandey V, Shams R, Dar AH,
Dash KK, Khan SA, Bashir I, Jeevarathinam G, Rusu AV, Esatbeyoglu T
and Pandiselvam R: Promising bioactive properties of quercetin for
potential food applications and health benefits: A review. Front
Nutr. 9:9997522022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding H, Ding H, Mu P, Lu X and Xu Z:
Diosmetin inhibits subchondral bone loss and indirectly protects
cartilage in a surgically-induced osteoarthritis mouse model. Chem
Biol Interact. 370:1103112023. View Article : Google Scholar
|
|
17
|
Zhao L, Zhang H, Li N, Chen J, Xu H, Wang
Y and Liang Q: Network pharmacology, a promising approach to reveal
the pharmacology mechanism of Chinese medicine formula. J
Ethnopharmacol. 309:1163062023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang P, Zhang D, Zhou W, Wang L, Wang B,
Zhang T and Li S: Network pharmacology: Towards the artificial
intelligence-based precision traditional Chinese medicine. Brief
Bioinform. 25:bbad5182023. View Article : Google Scholar
|
|
19
|
Bhoi A, Dwivedi SD, Singh D, Keshavkant S
and Singh MR: Mechanistic prospective and pharmacological
attributes of quercetin in attenuation of different types of
arthritis. 3 Biotech. 13:3622023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung
GH, Yoo BC and Cho JY: Functional roles of p38 mitogen-activated
protein kinase in macrophage-mediated inflammatory responses.
Mediator Inflamm. 2014:1–13. 2014.
|
|
21
|
Kim AL, Labasi JM, Zhu Y, Tang X, McClure
K, Gabel CA, Athar M and Bickers DR: Role of p38 MAPK in
UVB-induced inflammatory responses in the skin of SKH-1 hairless
mice. J Invest Dermatol. 124:1318–1325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar
|
|
23
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19:182017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartels YL, van Lent PLEM, van der Kraan
PM, Blom AB, Bonger KM and van den Bosch MHJ: Inhibition of TLR4
signalling to dampen joint inflammation in osteoarthritis.
Rheumatology (Oxford). 63:608–618. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: CytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 4:S4–S11. 2014.
|
|
26
|
Bayly CI, Cieplak P, Cornell W and Kollman
PA: A well-behaved electrostatic potential based method using
charge restraints for deriving atomic charges: The RESP model. J
Phys Chem. 97:10269–10280. 1993. View Article : Google Scholar
|
|
27
|
Jorgensen WL, Chandrasekhar J, Madura JD,
Impey RW and Klein ML: Comparison of simple potential functions for
simulating liquid water. J Chem Phys. 79:926–935. 1983. View Article : Google Scholar
|
|
28
|
Zakrzewska M, Opalinski L, Haugsten EM,
Otlewski J and Wiedlocha A: Crosstalk between p38 and Erk 1/2 in
downregulation of FGF1-induced signaling. IJMS. 20:18262019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mei S, Gu H, Ward A, Yang X, Guo H, He K,
Liu Z and Cao W: p38 Mitogen-activated protein kinase (MAPK)
promotes cholesterol ester accumulation in macrophages through
inhibition of macroautophagy. J Biol Chem. 287:11761–11768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
National Research Council (US); Committee
for the Update of the Guide for the Care Use of Laboratory Animals:
Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press (US); Washington, DC: 2011
|
|
31
|
Hu S, Li H, Jiang H, Liu X, Ke J and Long
X: Macrophage activation in synovitis and osteoarthritis of
temporomandibular joint and its relationship with the progression
of synovitis and bone remodeling. Am J Pathol. 194:296–306. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Gynther GW, Dijkgraaf LC, Reinholt FP,
Holmlund AB, Liem RS and de Bont LG: Synovial inflammation in
arthroscopically obtained biopsy specimens from the
temporomandibular joint: A review of the literature and a proposed
histologic grading system. J Oral Maxillofac Surg. 56:1281–1286.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng M, Yi X and Zhou Q: Overexpression
of HIF-1alpha in bone marrow mesenchymal stem cells promote the
repair of mandibular condylar osteochondral defect in a rabbit
model. J Oral and Maxillofacial Surgery. 79:345.e1–345.e15. 2021.
View Article : Google Scholar
|
|
35
|
Chen Y, Wang Y, Liu M, Zhou B and Yang G:
Diosmetin exhibits anti-proliferative and anti-inflammatory effects
on TNF-α-stimulated human rheumatoid arthritis fibroblast-like
synoviocytes through regulating the Akt and NF-κB signaling
pathways. Phytotherapy Research. 34:1310–1319. 2019. View Article : Google Scholar
|
|
36
|
Guo G and Dong J: Diosmetin attenuates
oxidative stress-induced damage to lens epithelial cells via the
mitogen-activated protein kinase (MAPK) pathway. Bioengineered.
13:11072–11081. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Yao J, Han C, Yang J, Chaudhry MT,
Wang S, Liu H and Yin Y: Quercetin, inflammation and immunity.
Nutrients. 8:1672016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng S, Gao N, Zhang Z, Chen G, Budhraja
A, Ke Z, Son YO, Wang X, Luo J and Shi X: Quercetin induces
tumor-selective apoptosis through downregulation of Mcl-1 and
activation of bax. Clin Cancer Res. 16:5679–5691. 2010. View Article : Google Scholar : PubMed/NCBI
|