Introduction
Diabetes mellitus (DM) is a common chronic metabolic
disorder with multifactorial etiology (1). It is characterized by elevated
blood glucose levels or hyperglycemia, which results from either
insufficient insulin production due to β-cell dysfunction or
impaired insulin action secondary to insulin resistance (2). In recent years, the incidence and
prevalence of DM have risen sharply due to changes in lifestyle,
including prolonged physical inactivity, sedentary behavior and
excessive intake of high-fat and high-sugar (HFHS) diets. According
to the latest epidemiological data from the International Diabetes
Federation, >425 million people world-wide are currently
affected by DM, a figure expected to rise to 629 million by 2045
(3), underscoring the growing
threat of DM to global health.
Diabetic nephropathy (DN) is one of the most common
complications of DM, affecting ~40% of patients with DM throughout
disease progression (4). DN is
primarily attributed to chronic hyperglycemia-induced microvascular
damage in the kidneys, which leads to impaired renal perfusion and
subsequently triggers inflammation, oxidative stress,
glomerulosclerosis and tubulointerstitial fibrosis, ultimately
resulting in progressive and irreversible renal dysfunction. As DN
progresses, patients may develop symptoms such as edema,
hypertension and proteinuria; in advanced stages, some individuals
require dialysis to maintain internal homeostasis, which severely
compromises both life expectancy and quality of life (5). Diabetic osteoporosis (DOP) is
another common complication of DM, with a complex pathogenesis
involving multiple pathophysiological mechanisms, including
diminished insulin-mediated inhibition of bone resorption, enhanced
bone destruction due to chronic inflammation and local accumulation
of advanced glycation end-products in bone tissue (6). The principal complication
associated with DOP is fragility fractures. Clinical investigations
have shown that individuals with DM are 1.38-1.7 times more likely
to sustain proximal femoral fractures and 2.03 times more likely to
experience vertebral fractures than healthy individuals (7-9).
Although fractures associated with DOP are not directly fatal, they
markedly impair the self-care ability of patients with DM, imposing
a substantial burden on both patients and their families. More
importantly, there appears to be a potential link between DN and
DOP, two major complications of DM. A study by Xia et al
(10) demonstrated that patients
with DN are at markedly higher risk of developing DOP compared with
those with DM without DN. Conversely, a case-control study by Yan
et al (11) identified
osteoporosis (OS) as an independent risk factor for DN among
patients with DM. These findings indicate that treatment strategies
for DM must consider both kidney and bone status to prevent the
reciprocal exacerbation between DN and DOP, a key step toward
improving clinical outcomes and extending patient lifespan.
Akebia saponin D (ASD), a natural small-molecule
bioactive compound isolated from Dipsacus asper, exhibits
multiple pharmacological properties, including anti-inflammatory,
antioxidant and anti-apoptotic effects (12). Previous cellular and animal
studies have demonstrated the therapeutic potential of ASD in
diseases such as bronchial asthma, cerebral hemorrhage and hepatic
steatosis (13-15). However, the efficacy of ASD in
treating DM complications remains largely unexplored.
Semaglutide, a long-acting glucagon-like peptide-1
(GLP-1) receptor agonist, has been widely used in clinical practice
for the management of type 2 DM (T2DM) (16). Specifically, Semaglutide
facilitates glycemic control and weight reduction in patients with
T2DM by enhancing insulin secretion, suppressing glucagon release,
delaying gastric emptying and improving insulin sensitivity
(17). However, the therapeutic
potential and underlying mechanisms of Semaglutide in DN and DOP
remain inadequately elucidated.
Accordingly, the present study intended to construct
a dual-complication DM rat model characterized by both DN and DOP
using high-fat feeding and repeated low-dose streptozotocin (STZ)
injections. ASD, selected for its documented anti-inflammatory,
antioxidant and anti-apoptotic properties and its potential
benefits in metabolic disorders and Semaglutide were then
administered to the animals to comprehensively assess their
combined efficacy in improving DN and DOP, as well as to explore
the potential mechanisms involved. It was hypothesized that ASD and
Semaglutide may exert synergistic protective effects against DN and
DOP by modulating the Klotho-p53 signaling axis. The outcomes of
this research may provide a theoretical basis for the combined use
of ASD and Semaglutide and contribute novel perspectives on the
management of DM complications.
Materials and methods
Experimental animals
A total of 42 male Sprague-Dawley rats (SPF grade;
180-220 g; 8 weeks old) were provided by Guangdong Medical
Laboratory Animal Center [Production License No. SCXK (Yue)
2022-0002]. Only male rats were used in order to avoid the
potential variability associated with the estrous cycle in females,
which could influence glucose metabolism, bone turnover and renal
function (18,19). This approach was chosen to
minimize hormonal fluctuations and ensure experimental consistency.
Rats were housed under controlled conditions (20-26°C; 30-70%
humidity; 12 h light/dark cycle) with free access to food and
water. Ethical approval for the present study was obtained from the
Animal Welfare and Ethics Committee of Guangdong Medical Laboratory
Animal Center (approval no. D202505-13).
Reagents, virus construction and animal
grouping
STZ, ASD and Semaglutide were obtained from
MedChemExpress and the chemical structures of ASD and Semaglutide
are shown in Fig. 1A and B. STZ
(30 mg/kg) was freshly prepared in 0.1 M sodium citrate buffer (pH
4.5) and administered intraperitoneally once daily for two
consecutive days. ASD (90 mg/kg/day) was dissolved in DMSO (Beijing
Solarbio Science & Technology Co., Ltd.) and diluted with PBS
(Beijing Solarbio Science & Technology Co., Ltd.; pH 7.4) for
oral gavage, with a final volume of 10 ml/kg. Semaglutide (120
μg/kg/day, subcutaneous injection, 12 weeks) was dissolved
in PBS(pH 7.4) and administered subcutaneously at a dose volume of
1 ml/kg (20). The dosage was
selected based on previous clinical and preclinical studies
demonstrating safe and effective exposure (21,22). A formal dose-response trial was
not conducted in the present study. All doses were adjusted
according to body surface area to ensure pharmacological
equivalence (23). To silence
Klotho expression, a recombinant adeno-associated virus (AAV)
encoding either Klotho-specific short hairpin (sh)RNA (sh-Klotho)
or non-targeting control shRNA (shNC) was synthesized by Shanghai
GenePharma Co., Ltd. Viral particles were prepared using standard
protocols and purified by polyethylene glycol precipitation and
ultracentrifugation and administered via tail vein injection (200
μl/injection) every three days.
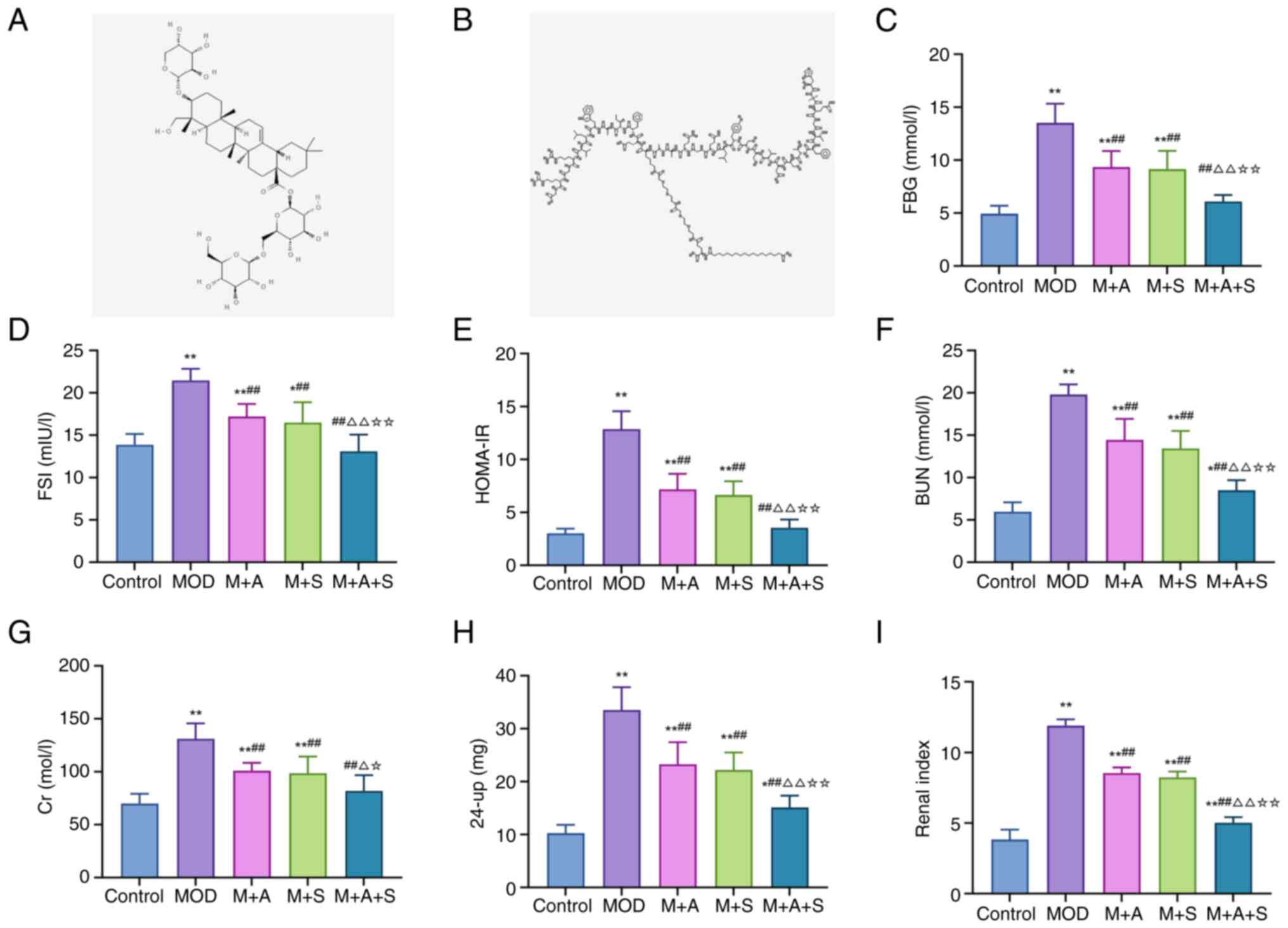 | Figure 1Chemical structures of ASD and
Semaglutide and their effects on glucose metabolism and renal
function in diabetic rats. Two-dimensional structures of (A) ASD
and (B) Semaglutide were retrieved from PubChem. Enzyme-linked
immunosorbent assay analysis of (C) FBG, (D) FSI and (E) HOMA-IR.
(F-I) Biochemical analysis of BUN, Cr, 24-up and renal index.
*P<0.05, **P<0.01 vs. Control;
##P<0.01 vs. MOD; ΔP<0.05,
ΔΔP<0.01 vs. M + A; ☆P<0.05,
☆☆P<0.01 vs. M + S. ASD, akebia saponin D; FBG,
fasting blood glucose; FSI, fasting serum insulin; HOMA-IR,
homeostatic model assessment of insulin resistance; BUN, blood urea
nitrogen; Cr, creatinine; 24-up, 24-h urinary protein. |
Following one week of acclimatization, the rats were
randomly assigned into seven groups (n=6 per group). During the
modeling process, two rats in the MOD group and one rat in the M +
S group died and one rat in the MOD group did not meet the diabetic
criteria (fasting blood glucose <7.8 mmol/l). Thus, the final
numbers of rats analyzed were: Control (n=6), MOD (n=3), M + A
(n=6), M + S (n=5), M + A + S (n=6), M + A + S + shNC (n=6) and M +
A + S + sh-Klotho (n=6). A T2DM model was established based on
previously validated methods (24-26), involving high-fat high-sucrose
(HFHS) feeding for 8 weeks followed by two consecutive low-dose
intraperitoneal injections of streptozotocin (STZ; 30 mg/kg). This
combination is widely recognized as a reliable method to induce
T2DM-like phenotypes, as it produces insulin resistance with
partial β-cell dysfunction rather than complete β-cell destruction
typical of type 1 diabetes (T1DM). Rats with fasting blood glucose
(FBG) >7.8 mmol/l and reduced insulin sensitivity were
considered successfully modeled. The experimental groups were as
follows: i) Control: Standard diet + vehicle (sodium citrate
buffer, i.p.) + saline via oral gavage for 12 weeks. ii) Model
(MOD): HFHS diet + STZ + saline via oral gavage for 12 weeks. iii)
MOD + ASD (M + A): MOD model + ASD via oral gavage for 12 weeks.
iv) MOD + Semaglutide (M + S): MOD model + Semaglutide via
subcutaneous injection for 12 weeks. v) MOD + ASD + Semaglutide (M
+ A + S): Combined ASD (oral gavage) and Semaglutide (subcutaneous)
for 12 weeks. vi) M + A + S + shNC: Same as group 5, plus tail vein
injection of AAV-shNC every three days. vii) M + A + S + sh-Klotho:
Same as group 5, plus tail vein injection of AAV-shKlotho every
three days. The ASD dose used in the present study was referenced
from the research by Yu et al (27), while the Semaglutide dose was
based on the clinically recommended safe and effective dosage
(21,22).
Sample collection and processing
After the final treatment, rats were placed in
PhenoMaster metabolic cages (TSE Systeme GmbH) for 24 h, with free
access to water but no food. Urine was collected, centrifuged
(1,000 × g; 5 min; 4°C) and supernatant was analyzed for protein
concentration using a BCA kit (Shanghai Lianshui Biotechnology Co.,
Ltd.). Results were recorded as 24-h urinary protein (24 up)
levels. The following morning, rats were anesthetized and
sacrificed by intraperitoneal injection of sodium pentobarbital at
a dose of 150 mg/kg. Body and kidney weights were measured to
calculate the renal index (kidney weight/body weight). Blood
samples were collected via abdominal aorta, centrifuged (1,500 × g;
5 min; 4°C) and serum stored at −80°C. Both kidneys and femurs were
harvested under sterile conditions, cleared of soft tissues and
stored at −80°C for further analysis.
Biochemical analysis
The femoral tissues were defatted, crushed and
acid-digested. Serum, urine and femur extracts were analyzed for
blood urea nitrogen (BUN), creatinine (Cr), calcium
(Ca2+) and phosphate (PO43−)
levels using a HITACHI-7170S automatic biochemical analyzer
(Hitachi High-Technologies Corporation). Serum levels of
glycometabolic and bone turnover markers were measured using
commercial enzyme-linked immunosorbent assay (ELISA) kits.
Glycometabolic indicators included fasting blood glucose (FBG; cat.
no. E02G0462; Shanghai BlueGene Biotech), fasting serum insulin
(FSI; cat. no. mlE2721; Shanghai Enzyme-linked Biotechnology Co.,
Ltd.). The homeostatic model assessment of insulin resistance
(HOMA-IR), which was calculated using the formula: HOMA-IR=(FBG ×
FSI)/22.5. This calculation was used to indirectly evaluate insulin
sensitivity in the diabetic rat model. Bone turnover markers were
quantified using rat ELISA kits from Shanghai Enzyme-linked
Biotechnology Co., Ltd: alkaline phosphatase (ALP; cat. no.
ml107021), procollagen type I N-terminal propeptide (PINP; cat. no.
ml038224-1), C-terminal telopeptide of type I collagen (CTX-I; cat.
no. ml003410) and tartrate-resistant acid phosphatase (TRAP; cat.
no. ml059485). Each assay was performed in triplicate.
Histological analysis of kidney and femur
tissues
After fixation in 4% paraformaldehyde solution for
24 h at 4°C, kidney and femur tissues were embedded in paraffin and
sectioned into 4-μm slices using a microtome (Leica
Biosystems). Kidney sections were made in the coronal plane and
femur sections along the longitudinal axis. Sections were
deparaffinized in xylene and rehydrated through graded ethanol
solutions (28). Hematoxylin and
eosin (H&E) staining was performed to assess renal and femoral
structural integrity. The glomerulosclerosis index (GSI) and renal
tubular interstitial injury index (RTI) were calculated according
to previous studies (29). In
femoral sections, osteoblasts and osteocytes were quantified.
Additional staining included periodic acid-Schiff (PAS) for
glomerular basement membrane injury: 0.5% periodic acid for 10 min
at room temperature (RT), Schiff reagent 15 min, two sulfite washes
(5 min each), and hematoxylin counterstain 2 min, followed by
dehydration, clearing, and mounting. Masson's trichrome was
performed for renal fibrosis evaluation: Bouin's solution 56°C, 30
min, Weigert's iron hematoxylin 10 min, Biebrich scarlet-acid
fuchsin 10 min, phosphomolybdic/phosphotungstic acid 10 min,
aniline blue 10 min, and 1% acetic acid 2 min, followed by
dehydration, clearing and mounting. Images were acquired an Olympus
BX53 microscope (Olympus Corporation) and quantified using ImageJ
v1.53 (NIH) based on three randomly selected fields per
section.
Micro-computed tomography (micro-CT)
analysis
Femoral microarchitecture was assessed by SkyScan
1276 micro-CT (Bruker Corporation). Scanning parameters included
300 μA tube current, 75 kV tube voltage, 75 msec exposure
time, 0.7° rotation step and 12 μm voxel resolution,
averaging 2 frames per step. Images were reconstructed with NRecon
(version 1.7.4.6; Bruker Corporation) software and the region of
interest was defined as a 2.4 mm volume of trabecular bone starting
1 mm proximal to the distal growth plate. Quantitative analysis
using CTAn (version 1.20.3.0; ROI) software included measurements
of bone mineral density (BMD), bone mineral content (BMC), bone
volume/tissue volume (BV/TV), bone surface/bone volume (BS/BV),
trabecular separation (Tb.Sp), trabecular thickness (Tb.Th),
trabecular number (Tb.N) and structure model index (SMI).
Three-point bending test
Femoral biomechanical properties were evaluated
using an RGWF4005 electronic universal testing machine (Shenzhen
Reger Instrument Co. Ltd.) after micro-CT scanning. During testing,
the distal and proximal ends of the femur were fixed on two support
points spaced 10 mm apart and a vertical downward force was applied
to the midshaft of the femur until fracture occurred. The ultimate
load, bending strength and Young's modulus were recorded to
evaluate mechanical integrity.
Immunofluorescence staining
Paraffin-embedded kidney and femur sections (4
μm; prepared as aforementioned) were baked at 60°C for 30-60
min before staining. Paraffin sections were subjected to
heat-induced antigen retrieval in 0.01 M sodium citrate buffer (pH
6.0) at 95°C, followed by blocking with 3% BSA (MilliporeSigma) for
1 h. Sections were incubated overnight at 4°C with primary
antibodies anti-Klotho (1:200; cat. no. PA5-21078), anti-p53
(1:200; cat. no. MA5-12453) and anti-γ-H2AX (1:200; cat. no.
MA5-33062), all from Invitrogen (Thermo Fisher Scientific, Inc.).
After primary incubation, slides were washed in PBST (3×5 min),
incubated with fluorophore-conjugated secondary antibodies for 1 h
at RT, and washed again in PBST (3×5 min). Nuclei were
counterstained with DAPI (1 μg/ml, 5 min, RT; protected from
light), rinsed in PBS, and mounted with anti-fade medium. Images
were captured from three random fields on an Olympus fluorescence
microscope (Olympus Corporation) using 10×, 20×, and 40× objectives
(identical acquisition settings across groups), and fluorescence
intensities were quantified using ImageJ v1.53 (NIH).
Western blot analysis
Total protein was extracted from bilateral residual
kidney and femur tissues using RIPA lysis buffer (Cell Signaling
Technology, Inc.) supplemented with protease and phosphatase
inhibitors. Protein concentrations were determined using a BCA
protein assay kit (Beyotime Biotechnology) and 30 μg protein
per lane was loaded. Equal amounts of protein were separated by 10%
SDS-PAGE (Bio-Rad Laboratories, Inc.), transferred to
polyvinylidene fluoride (PVDF) membranes (Wuhan Servicebio
Technology Co., Ltd.) and blocked in 5% non-fat milk for 1 h at
room temperature. Membranes were then incubated overnight at 4°C
with the following primary antibodies (all from Invitrogen; Thermo
Fisher Scientific, Inc.): TGF-β1 (cat. no. MA5-16949; 1:1,000),
α-smooth muscle actin (α-SMA; cat. no. 14-9760-82; 1:1,000),
Collagen I (cat. no. PA1-26204; 1:1,000), Collagen IV (cat. no.
PA5-104508; 1:1,000), osteoprotegerin (OPG; cat. no. MA5-15715;
1:1,000), osteocalcin (OCN; cat. no. MA1-20786; 1:1,000), receptor
activator of nuclear factor κ-b ligand (RANKL; cat. no. MA1-41161;
1:1,000), Runt-related transcription factor 2 (RUNX2; cat. no.
PA5-82787; 1:1,000), Klotho (cat. no. MA5-32784, 1:1,000),
γ-Histone H2AX (γ-H2AX; cat. no. MA5-33062, 1:1,000),
ataxia-telangiectasia mutated protein (ATM; cat. no. MA1-23152;
1:1,000), phosphorylated (p-)ATM (cat. no. MA1-2020; 1:1,000), p53
(vMA5-12557; 1:1,000), p21 (cat. no. MA5-14949, 1:1,000) and GAPDH
(vMA5-15738; 1:5,000). After TBST washes, HRP-conjugated secondary
antibodies (cat. nos. 32460 and 62-6520; 1:10,000; Thermo Fisher
Scientific, Inc.) were applied for 1 h at room temperature. Bands
were visualized using an ECL chemiluminescent substrate
(SuperSignal West Pico PLUS, Thermo Fisher Scientific, Inc.) and
imaged on a Tanon 5200 system (Tanon Science and Technology Co.,
Ltd.). Densitometry was performed using ImageJ v1.53 (NIH), and
target signals were normalized to GAPDH. Each sample was analyzed
in triplicate.
Bioinformatics analysis
Transcriptomic datasets related to DN and OS were
obtained from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) (GSE30528 and
GSE35958). Data were downloaded using GEOquery (v2.70.0;
Bioconductor; https://bioconductor.org/packages/GEOquery) in R
(v4.3.2; The R Foundation; https://www.r-project.org/), normalized and
batch-corrected with limma (v3.58.1; Bioconductor; https://bioconductor.org/packages/limma)
and differentially expressed genes (DEGs) were identified using
DESeq2. Targets of ASD and Semaglutide were retrieved from ChEMBL
(https://www.ebi.ac.uk/chembl),
intersected with DEGs related to DN and OS. Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses
were conducted via ClusterProfiler to explore biological functions
and pathways. Protein structures were downloaded from the Protein
Data Bank (https://www.rcsb.org/) and compound
structures from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Docking was
performed in AutoDock Vina (The Scripps Research Institute) and
visualized using PyMOL v2.5.4 (https://www.pymol.org/).
Statistical analysis
Statistical analyses were performed using SPSS 24.0
(IBM Corp.). The Shapiro-Wilk test and Levene's test were applied
to assess normality and homogeneity of variances. If the data met
these assumptions, one-way ANOVA followed by Tukey's post hoc test
was conducted. Data were presented as mean ± standard deviation
(SD). P<0.05 was considered to indicate a statistically
significant difference.
Results
ASD and Semaglutide improve glucose
metabolism and renal function in diabetic rats
Abnormal glucose metabolism and impaired renal
filtration and barrier functions are key features of DM and DN. To
evaluate the pharmacological effects of ASD and Semaglutide, the
present study first assessed metabolic and renal indicators.
ELISA results showed that the levels of FBG, FSI and
HOMA-IR in the MOD group were markedly higher than those in the
Control group. However, treatment with ASD or Semaglutide alone (M
+ A/M + S group) reduced these markers and the combined therapy (M
+ A + S group) led to the most significant improvement (Fig. 1C-E). Similarly, biochemical
analysis revealed that the levels of BUN, Cr, 24-up and renal index
were markedly increased in the MOD group. All parameters were
markedly decreased following treatment, with the M + A + S group
showing superior renoprotection (Fig. 1F-I). These findings suggested
that ASD and Semaglutide together improve glucose homeostasis and
attenuate renal dysfunction in diabetic rats, with combination
therapy outperforming monotherapy. Although body weight after 6
weeks of HFHS feeding was not recorded in the present study,
previous reports using the same modeling protocol have consistently
shown an initial increase in body weight during HFHS feeding
followed by a moderate decline after STZ injection, reflecting
typical T2DM progression rather than T1DM (25).
ASD and Semaglutide alleviate renal
pathological damage in diabetic rats
Kidney injury in DN involves both functional
impairment and structural abnormalities. To assess the
histopathological improvements following treatment, H&E, PAS
and Masson staining were performed.
H&E staining (Fig. 2A) revealed pronounced glomerular
hypertrophy, basement membrane thickening, mesangial expansion and
inflammatory infiltration in the MOD group. The two monotherapies
partly alleviated these lesions, while the M + A + S group
demonstrated the most comprehensive improvement, as reflected by
markedly reduced GSI and RTI scores (Fig. 2B and C). PAS staining (Fig. 2D and E) showed marked glomerular
and tubular basement membrane thickening in the MOD group, along
with prominent PAS-positive deposits. Treatment reversed these
changes, with the M + A + S group achieving the greatest reduction
in PAS-positive area. Masson staining (Fig. 2F and G) showed extensive collagen
deposition in MOD rats, which was markedly reduced after treatment.
The collagen area percentage was lowest in the M + A + S group,
indicating superior antifibrotic efficacy. At the molecular level,
western blot analysis (Fig. 2H and
I) confirmed significant upregulation of TGF-β1, α-SMA,
Collagen I and Collagen IV in the MOD group. These profibrotic
markers were markedly downregulated by combination therapy, with
levels approaching those of the Control group.
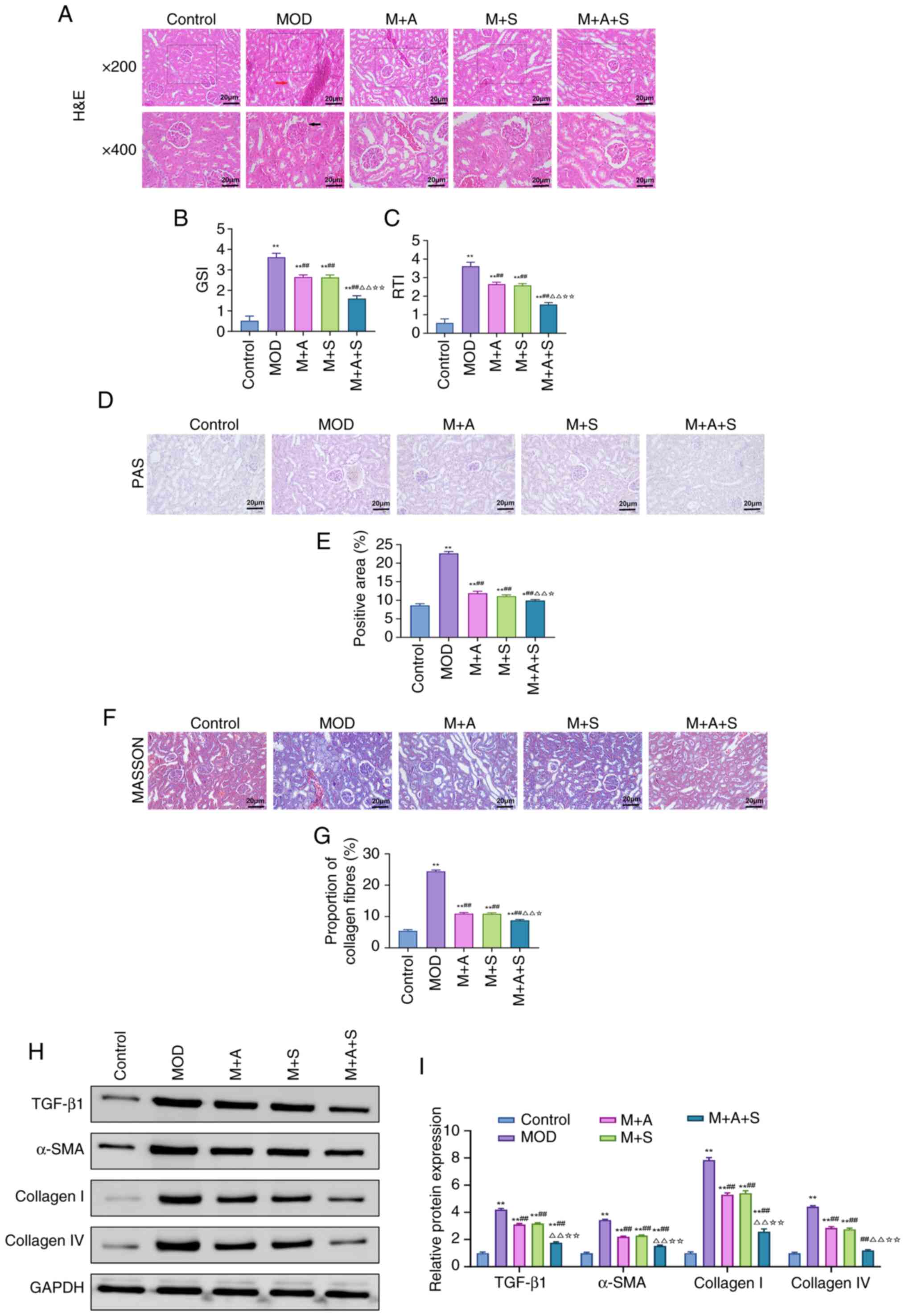 | Figure 2ASD and Semaglutide alleviate renal
pathological damage in diabetic rats. (A-C) H&E staining was
used to observe kidney structure (magnification, ×200 and ×400) and
perform GSI and RTI scoring. (D) PAS staining and (E)
quantification of PAS-positive area. (F) Masson staining and (G)
quantification of collagen deposition. (H) Western blot analysis of
TGF-β1, α-SMA, Collagen I and Collagen IV. (I) Quantification of
protein expression normalized to GAPDH. *P<0.05,
**P<0.01 vs. Control; ##P<0.01 vs. MOD;
ΔP<0.05, ΔΔP<0.01 vs. M + A;
☆P<0.05, ☆☆P<0.01 vs. M + S. ASD,
akebia saponin D; H&E, hematoxylin and eosin; GSI,
glomerulosclerosis index; RTI, renal tubular interstitial injury
index; PAS, periodic acid-Schiff; α-SMA, α-smooth muscle actin. |
Collectively, these findings demonstrated that ASD
and Semaglutide synergistically attenuate renal injury, reduce
basement membrane thickening and suppress renal fibrosis, with the
combination therapy yielding superior outcomes compared with
monotherapy.
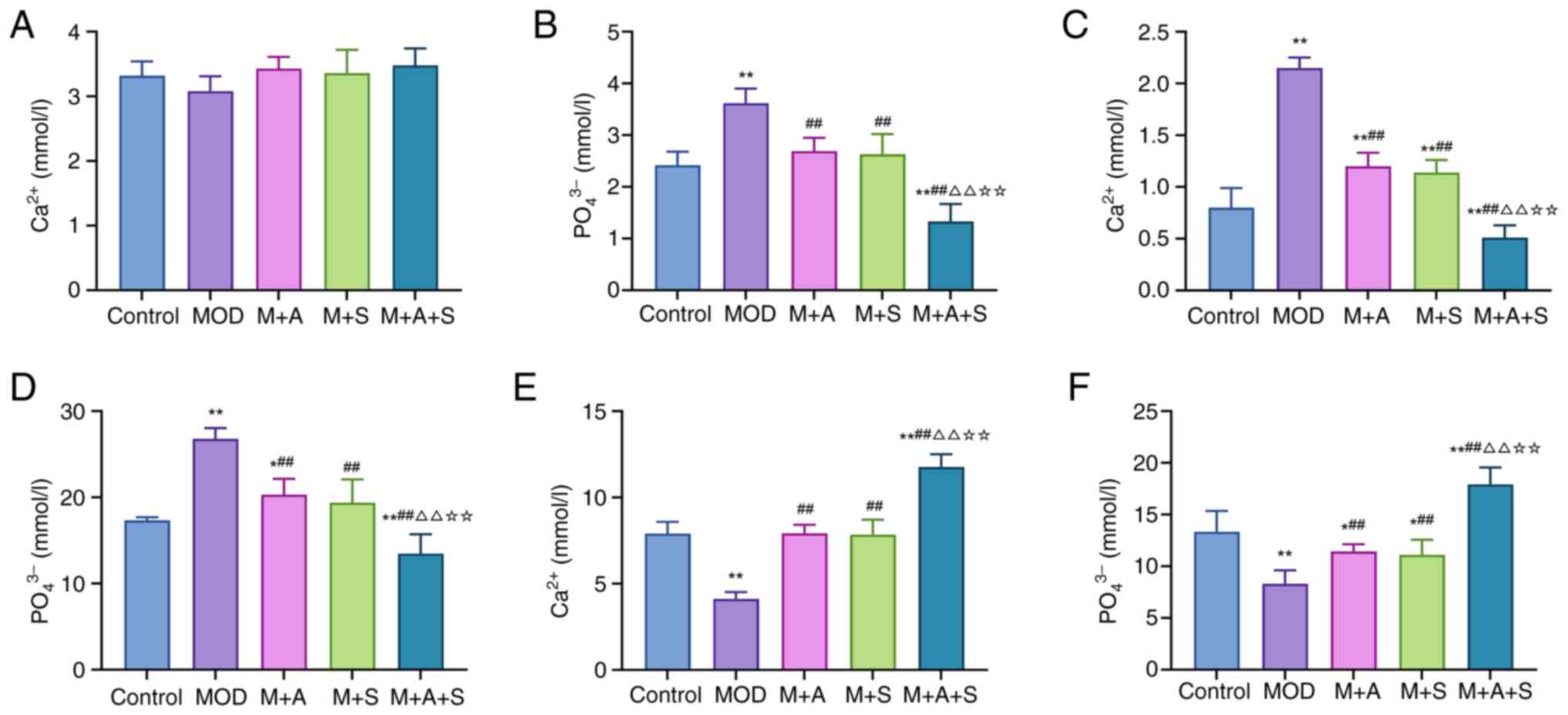
ASD and Semaglutide improve calcium and
phosphate metabolism in diabetic rats
Disrupted calcium and phosphate metabolism is a
common feature of internal environment disturbance in patients with
DM and may play an important role in DM-related renal injury and OS
(30). Therefore, the levels of
Ca2+ and PO43− in serum, urine and
femur were measured in the present study to evaluate the
therapeutic effects of ASD and Semaglutide. As shown in Fig. 3A-F, the MOD group exhibited
markedly elevated serum PO43− (Fig. 3B), increased urinary excretion of
Ca2+ and PO43− (Fig. 3C and D) and reduced femoral
Ca2+ and PO43− content (Fig. 3E and F), indicating systemic
calcium-phosphate imbalance. Treatment with either ASD or
Semaglutide partly restored these parameters, while the combined
treatment (M + A + S) achieved the most substantial correction,
including normalization of serum phosphate, reduction in renal loss
and restoration of bone mineral content. In summary, these findings
suggested that ASD and Semaglutide synergistically restore mineral
homeostasis in diabetic rats, with combination therapy
demonstrating superior efficacy.
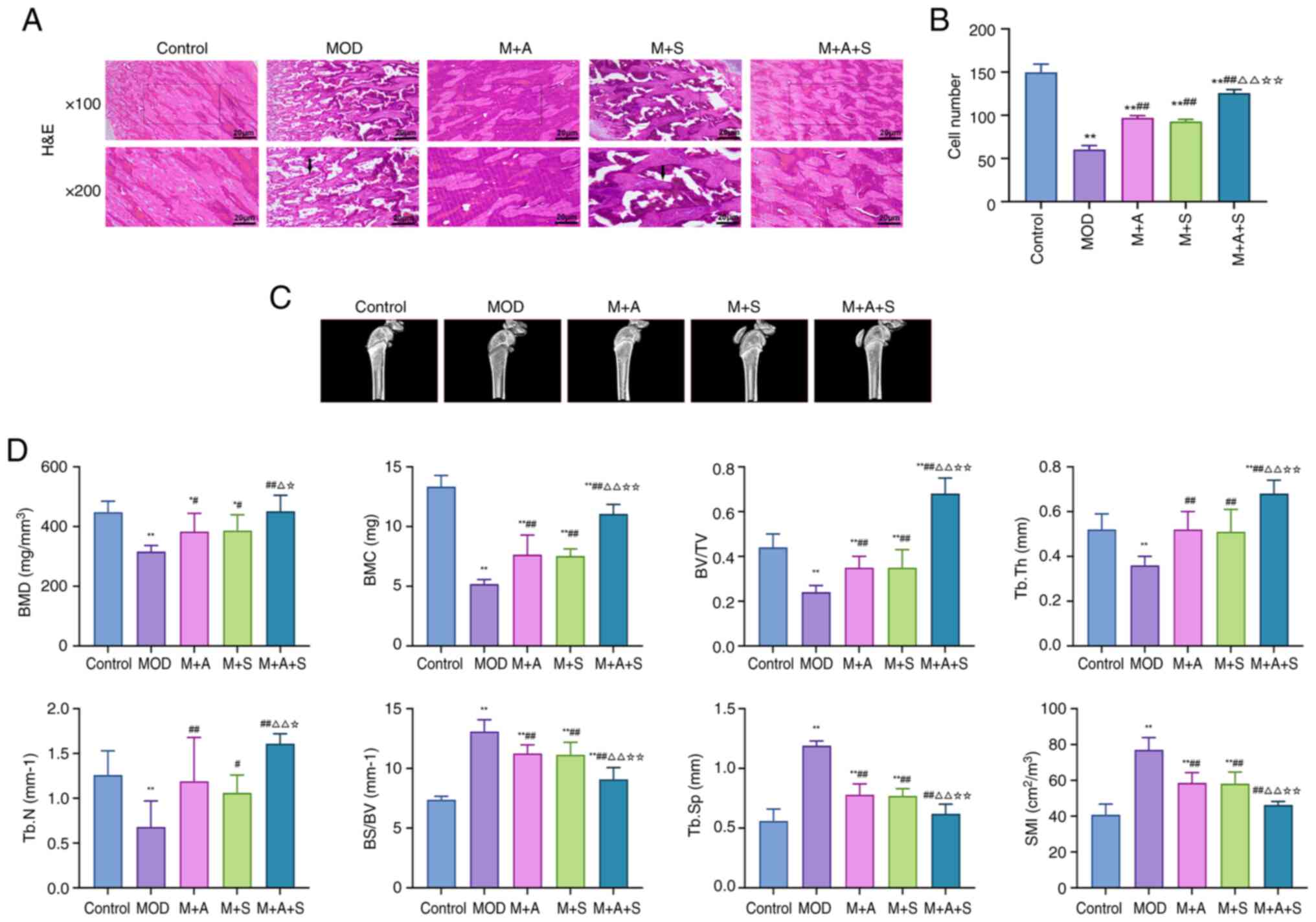
ASD and Semaglutide alleviate diabetic
osteoporosis-related femoral damage
DOP is characterized by reduced trabecular bone,
decreased bone density and disrupted bone structure (31). To evaluate structural changes,
H&E staining and micro-CT were performed.
H&E staining (Fig. 4A) revealed sparse, thinned and
disorganized trabeculae in MOD rats. Treatment with ASD or
Semaglutide partially improved trabecular integrity, while the
combined group (M + A + S) showed the most substantial recovery, as
confirmed by markedly higher osteocyte counts (Fig. 4B). Micro-CT analysis (Fig. 4C and D) demonstrated that MOD
rats had markedly lower BMD, BMC, BV/TV, Tb.Th and Tb.N,
accompanied by elevated BS/BV, Tb.Sp and SMI. These changes reflect
weakened and porous bone architecture. All parameters improved
following treatment and the M + A + S group exhibited the most
pronounced normalization, indicating a synergistic effect of ASD
and Semaglutide in preventing DOP-related skeletal
deterioration.
ASD and Semaglutide improve bone strength
and restore osteogenic-resorptive balance in diabetic rats
DOP is associated with both reduced bone mechanical
strength and dysregulated bone metabolism, increasing fracture risk
in patients with DM (32,33).
In the present study, the effects of ASD and Semaglutide on femoral
bone strength, metabolism and remodeling were comprehensively
evaluated.
Three-point bending tests revealed that MOD rats
exhibited markedly reduced ultimate load, bending strength and
elastic modulus, indicating biomechanical fragility. ASD and
Semaglutide both improved these parameters, while the combination
therapy (M + A + S) led to the most substantial enhancements,
suggesting superior restorative effects (Fig. 5A-C).
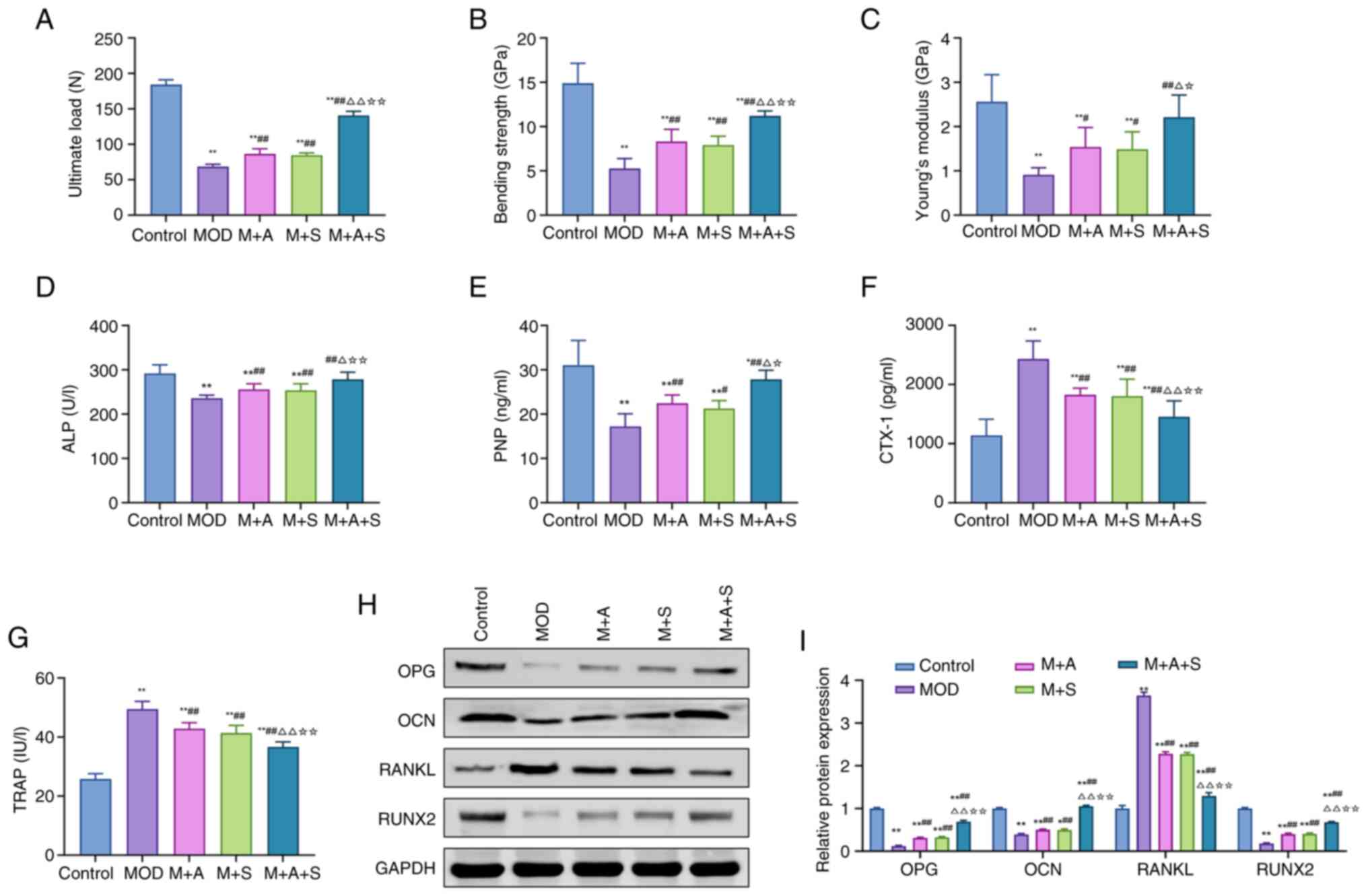 | Figure 5Effects of ASD and Semaglutide on
bone metabolism and remodeling in diabetic rats. (A-C) Three-point
bending tests measuring ultimate load, bending strength and Young's
modulus of the femur. The serum levels of (D) ALP, (E) PINP, (F)
CTX-1 and (G) TRAP were measured using ELISA. (H) Western blot and
(I) quantification of femoral protein expression (OPG, OCN, RANKL
and RUNX2). *P<0.05, **P<0.01 vs.
Control; #P<0.05, ##P<0.01 vs. MOD;
ΔP<0.05, ΔΔP<0.01 vs. M + A;
☆P<0.05, ☆☆P<0.01 vs. M + S. ASD,
akebia saponin D; ALP, alkaline phosphatase; PINP, procollagen type
I N-terminal propeptide; CTX-1, C-terminal telopeptide of type I
collagen; TRAP, tartrate-resistant acid phosphatase; OPG,
osteoprotegerin; OCN, osteocalcin; RANKL, receptor activator of
nuclear factor κ-b ligand; RUNX2, Runt-related transcription factor
2. |
To further explore underlying mechanisms, serum and
tissue markers related to bone formation and resorption were
assessed. As shown in Fig. 5D-G,
MOD rats displayed decreased levels of ALP and PINP and increased
levels of CTX-I and TRAP. These changes indicate suppressed
osteogenesis and enhanced bone resorption. Treatment with ASD or
Semaglutide partially reversed these abnormalities, with M + A + S
showing the most marked correction. At the protein level (Fig. 5H and I), MOD rats exhibited
reduced expression of OPG, OCN and RUNX2, along with upregulation
of RANKL, a key stimulator of osteoclastogenesis. ASD and
Semaglutide effectively normalized these markers, with combination
therapy demonstrating the most pronounced modulation.
Together, these results indicated that the combined
administration of ASD and Semaglutide enhances both bone mechanical
integrity and metabolic homeostasis in DOP, probably by promoting
osteogenesis and inhibiting bone resorption and achieves greater
therapeutic efficacy than either agent alone.
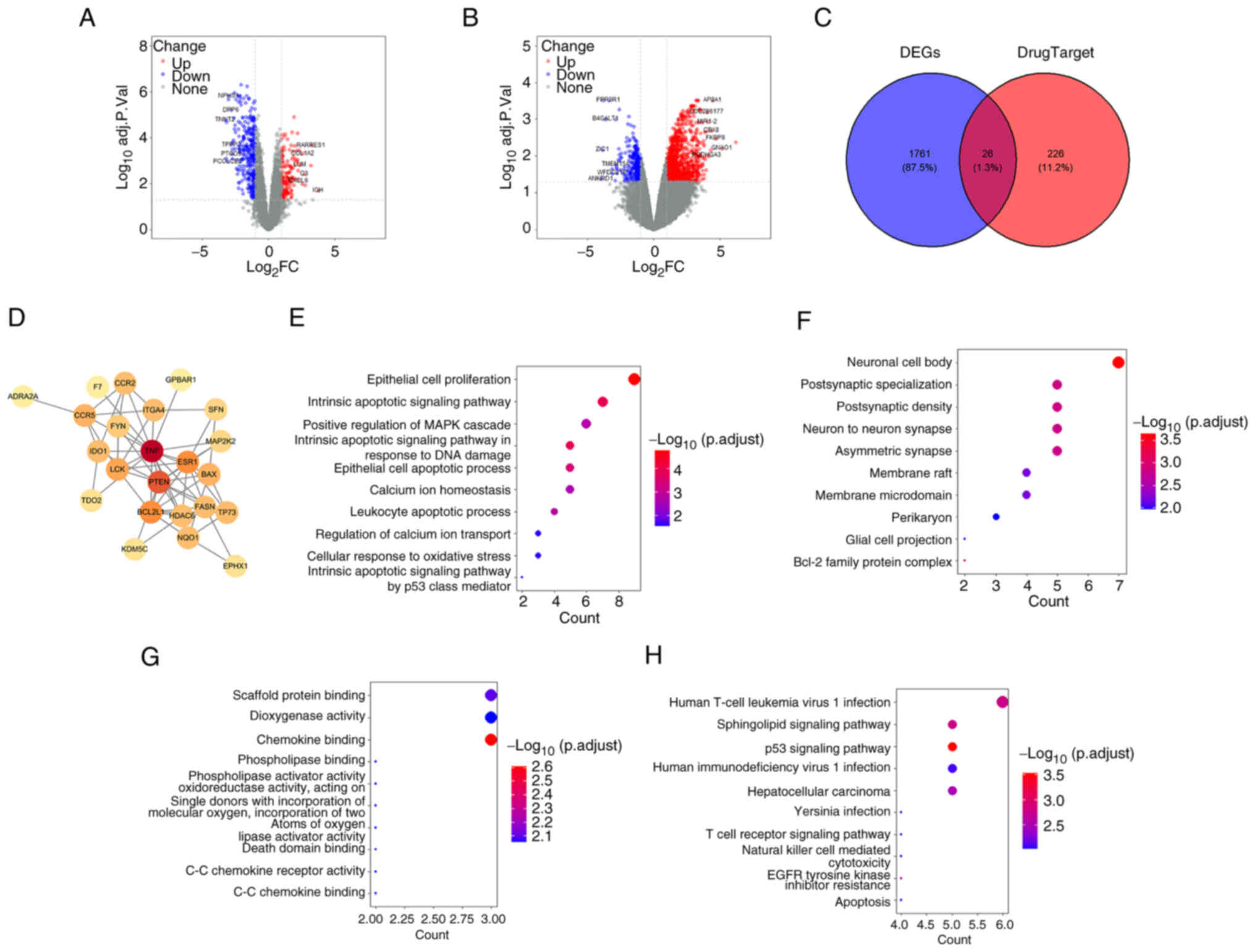
Network pharmacology analysis of ASD and
Semaglutide
After demonstrating the effectiveness of ASD and
Semaglutide in treating DN and DOP, the present study used network
pharmacology methods to further investigate their potential
molecular targets and mechanisms. Through transcriptomic data
analysis and KEGG enrichment analysis, the key molecules and
signaling pathways were identified.
The transcriptomic datasets for DN (GSE30528) and OS
(GSE35958) were both obtained from the GEO database. As shown in
Fig. 6A and B, after
differential expression analysis (adj. P<0.05;
|log2FC| >1), 1,787 disease-related genes were
identified. Drug-related targets of ASD and Semaglutide (n=252)
were obtained from ChEMBL. Their intersection yielded 26
overlapping genes (Fig. 6C),
which were used to construct a protein-protein interaction network
(Fig. 6D), with TNF, PTEN and
ESR1 emerging as central hub genes. GO enrichment analysis showed
that these targets were involved in apoptotic signaling, oxidative
stress response, MAPK cascade and calcium ion homeostasis (Fig. 6E-G). Cellular localization
included neuronal compartments and membrane microdomains, while
molecular functions encompassed chemokine binding, oxidoreductase
and death domain activity. KEGG pathway analysis (Fig. 6H) revealed enrichment in p53
signaling, sphingolipid metabolism, T-cell receptor and
apoptosis-related pathways, suggesting potential involvement of
these signaling cascades in the pharmacological actions of ASD and
Semaglutide.
These findings implied that ASD and Semaglutide may
exert therapeutic effects via shared molecular targets involved in
inflammation, apoptosis and cellular stress responses, with the p53
pathway emerging as a potential mechanistic link.
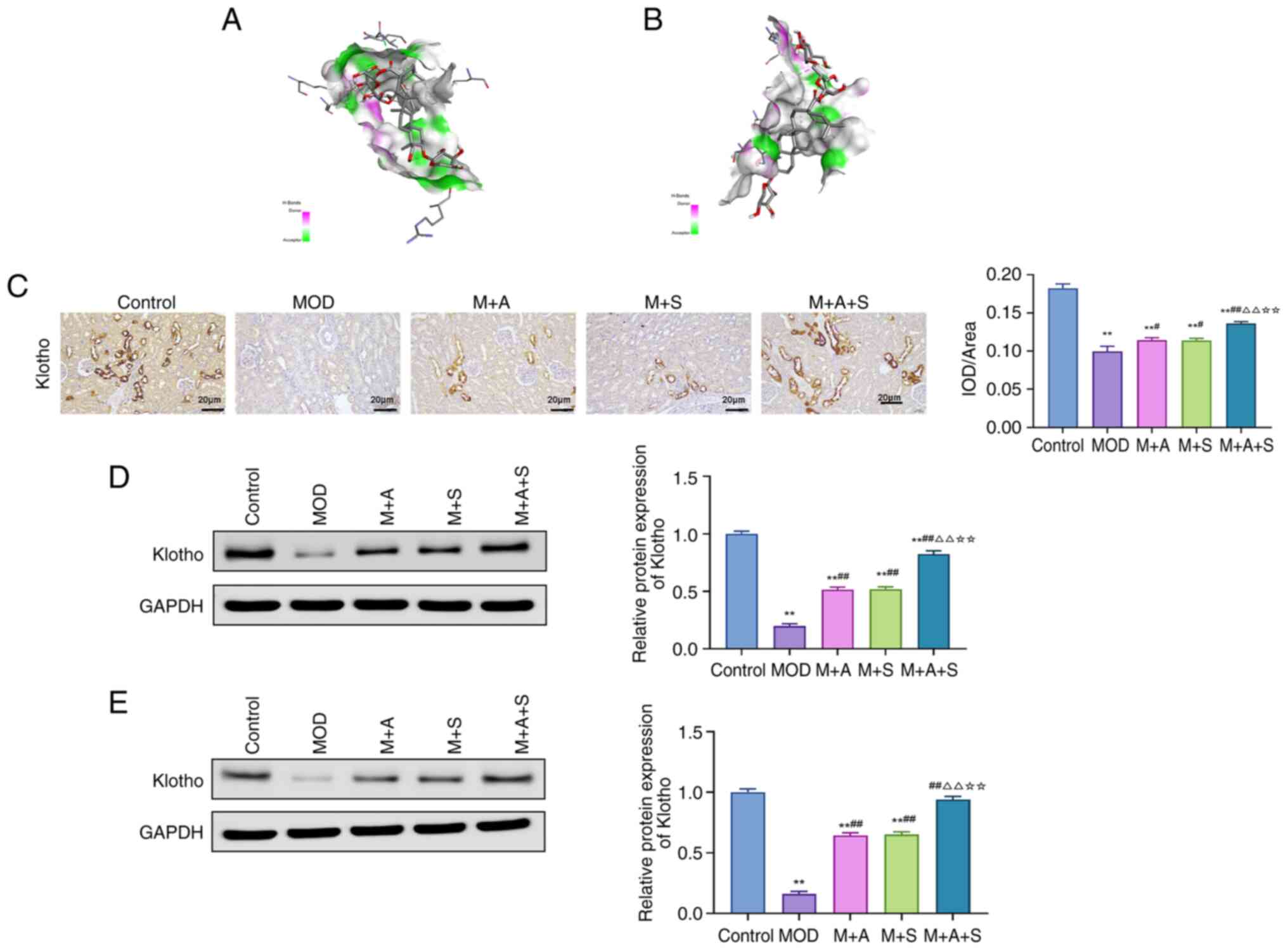
Molecular docking and expression analysis
of Klotho in diabetic rats
Given the emerging role of Klotho in diabetic
complications and its potential interaction with the p53 pathway
(34), the present study
explored whether ASD could directly bind to Klotho or p53 proteins
using molecular docking. The binding energy of ASD with Klotho
was-9.5 kcal/mol, while that with p53 was-7.6 kcal/mol, indicating
a stronger affinity for Klotho (Fig.
7A and B).
Immunofluorescence and western blotting results
showed that the Klotho protein level was markedly reduced in the
kidney and femur tissues of rats in the MOD group, while treatment
with ASD or Semaglutide markedly increased its expression, with the
most pronounced effect observed in the M + A + S group (Fig. 7C-E).
These results implied that ASD may modulate the p53
pathway by targeting Klotho protein, thereby playing a role in the
prevention of DN and DOP.
ASD and Semaglutide inhibit the p53
pathway and DNA damage in the kidney and femur tissues of diabetic
rats
Abnormal activation of the p53 signaling pathway can
lead to DNA damage, thereby exacerbating tissue injury (35). Therefore, the present study
further investigated whether ASD and Semaglutide alleviated
DN-related renal damage and DOP-related bone damage by inhibiting
the p53 signaling pathway.
Immunofluorescence results (Fig. 8A and B) revealed markedly
elevated levels of p53 and the DNA damage marker γ-H2AX in MOD
rats. Treatment with ASD or Semaglutide reduced their expression,
with the most pronounced suppression observed in the M + A + S
group. Consistently, western blot analysis (Fig. 8C-F) showed upregulation of key
proteins involved in DNA damage signaling, including ATM, p-ATM,
p53, p21 and γ-H2AX, in MOD rats. All these markers were
downregulated following ASD or Semaglutide treatment and
combination therapy yielded the strongest inhibitory effect. These
results suggested that combined treatment with ASD and Semaglutide
could suppress the excessive activation of the p53 signaling
pathway to reduce DNA damage, thereby exerting a protective effect
in DN and DOP.
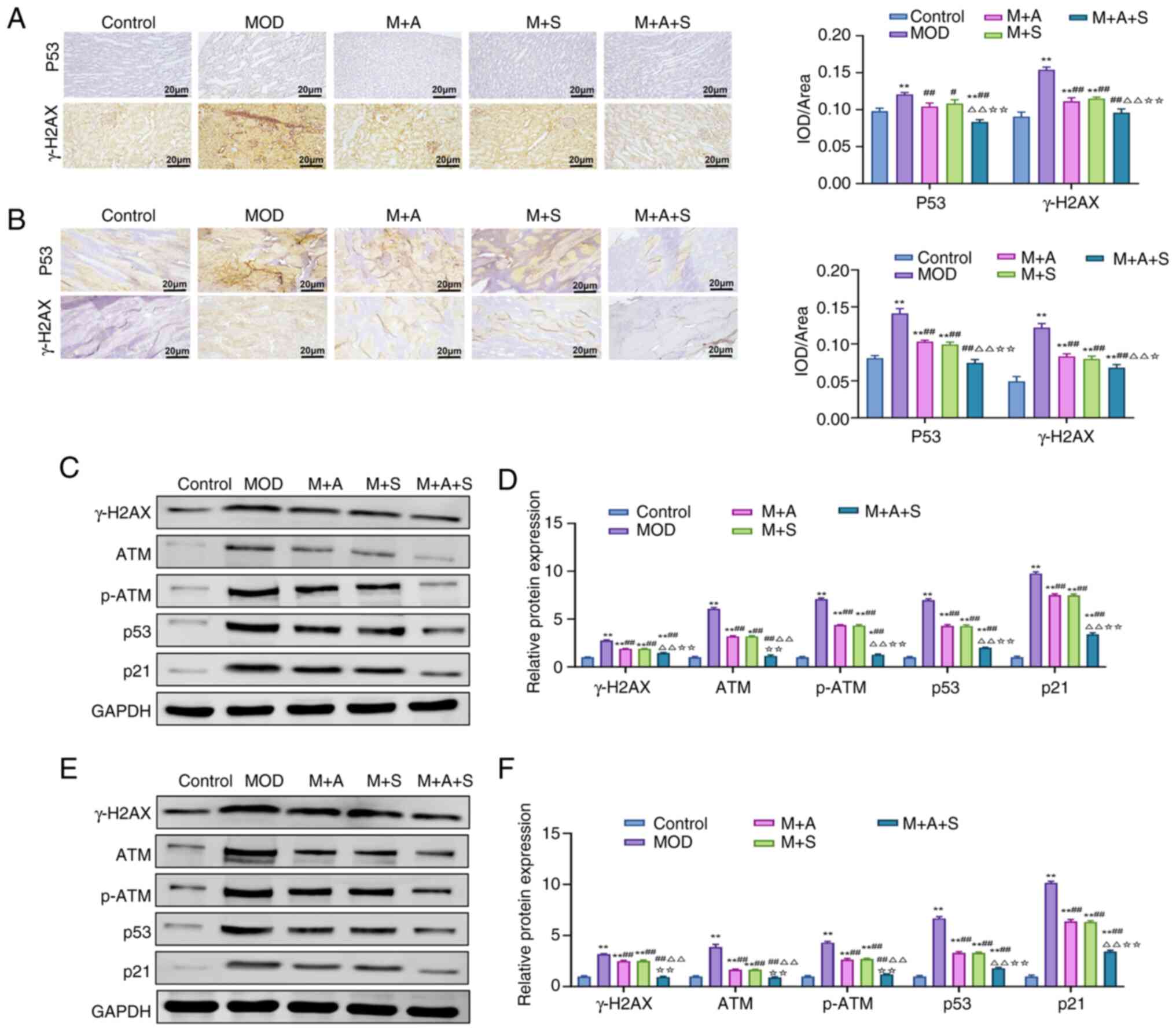 | Figure 8ASD and Semaglutide inhibit the p53
pathway and DNA damage in the kidney and femur tissues of diabetic
rats. Immunohistochemical staining of p53 and γ-H2AX in (A) kidney
and (B) femur tissues, respectively. (C-F) Western blotting and
quantification analysis of DNA damage-related proteins, including
γ-H2AX, ATM, p-ATM, p53 and p21, in (C and D) kidney and (E and F)
femur tissues. *P<0.05, **P<0.01 vs.
Control; #P<0.05, ##P<0.01 vs. MOD;
ΔΔP<0.01 vs. M + A; ☆P<0.05,
☆☆P<0.01 vs. M + S. ASD, akebia saponin D; p-,
phosphorylated; ATM, ATM, ataxia-telangiectasia mutated
protein. |
Knockdown of Klotho reverses the
therapeutic effects of ASD and Semaglutide in diabetic nephropathy
and diabetic osteoporosis
To further confirm whether Klotho served as a key
target for the protective effects of ASD and Semaglutide, the
present study applied AAV to knock down Klotho and observed its
effect on the therapeutic outcomes.
Western blotting results confirmed successful Klotho
knockdown in both kidney and femur tissues in M + A + S + sh-Klotho
group (Figs. 9A and 10A). Functionally, renal injury
indicators, including BUN, Cr, 24-up and renal index, were markedly
elevated in the MOD group and attenuated by M + A + S treatment.
However, these improvements were partially or fully reversed upon
Klotho knockdown (Fig. 9B-E).
Histopathological analysis (Fig.
9F-H) demonstrated that glomerular damage, basement membrane
thickening and fibrosis, improved in the M + A + S group, were
exacerbated again in the M + A + S + sh-Klotho group.
Correspondingly, TGF-β1, α-SMA, Collagen I and IV expression levels
were re-elevated (Fig. 9I and
J), confirming that the anti-fibrotic effects of the therapy
depended on Klotho expression.
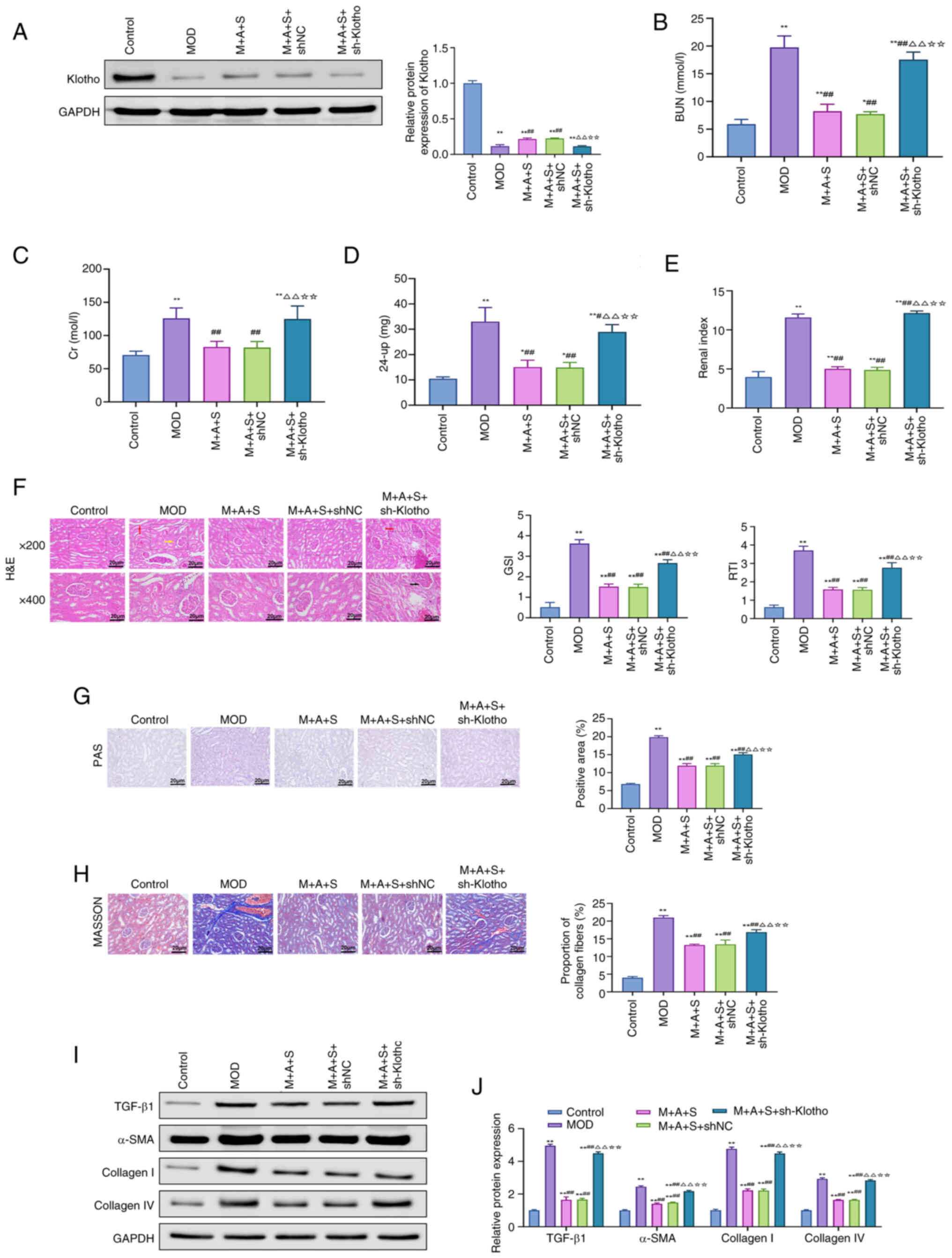 | Figure 9Klotho knockdown abrogates the
renoprotective effects of ASD and Semaglutide. (A) Western blot
analysis of Klotho protein levels in rat kidney tissues. (B-E)
Serum levels of (B) BUN, (C) Cr, (D) 24-up and (E) kidney index.
(F) H&E, (G) PAS and (H) Masson staining of renal tissue, with
corresponding quantification of GSI, RTI, PAS-positive area and
fibrosis index. (I) Western blotting and (J) quantification of
renal TGF-β1, α-SMA, Collagen I and Collagen IV protein levels.
*P<0.05, **P<0.01 vs. Control;
##P<0.01 vs. MOD; ΔΔP<0.01 vs. M + A +
S; ☆☆P<0.01 vs. M + A + S + shNC. ASD, akebia saponin
D; BUN Cr, creatinine; 24-up, 24-h urinary protein; H&E,
hematoxylin and eosin; PAS, periodic acid-Schiff; GSI,
glomerulosclerosis index; RTI, renal tubular interstitial injury
index; α-SMA, α-smooth muscle actin; sh, short hairpin. |
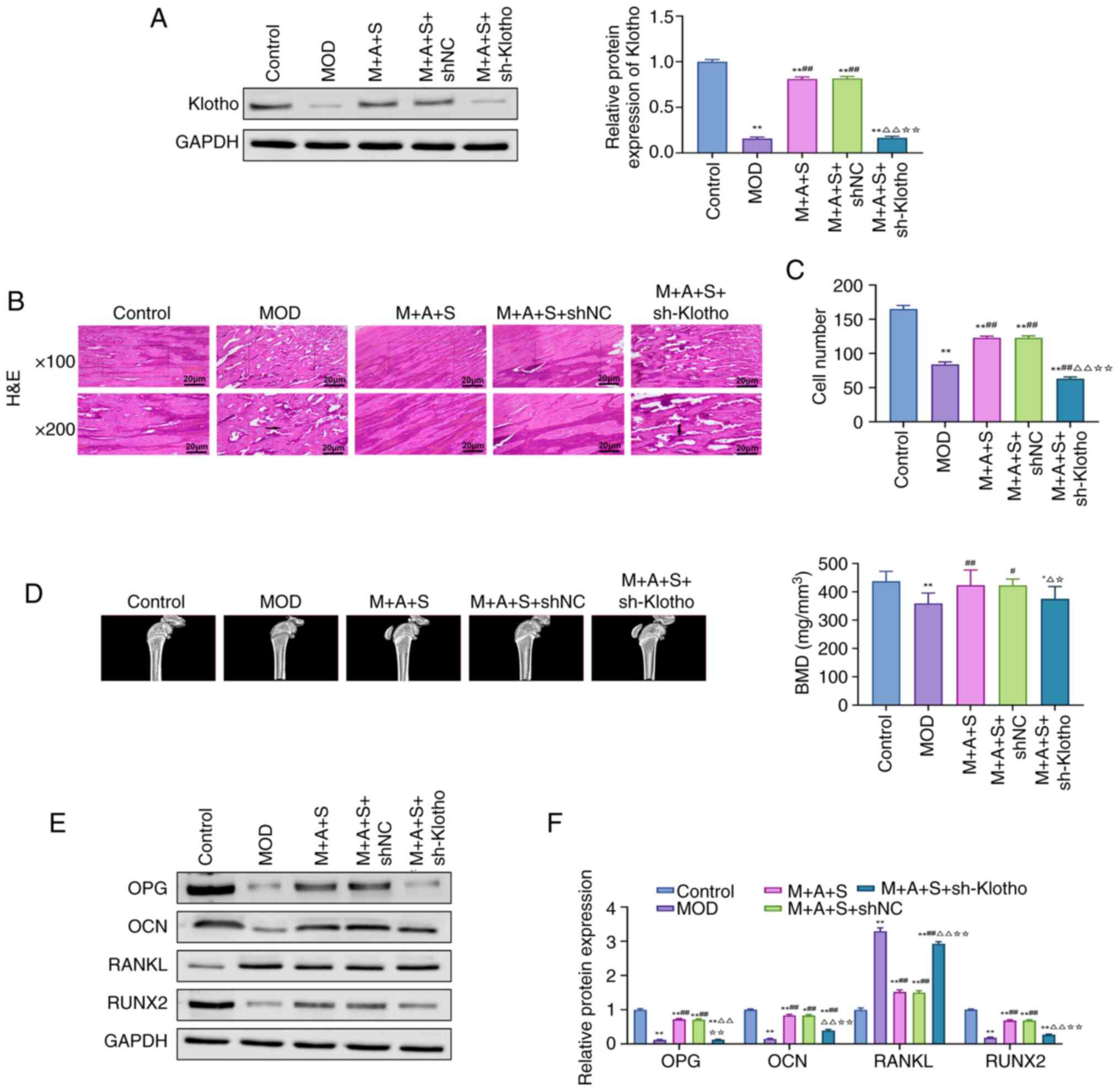 | Figure 10Knockdown of Klotho reverses the
therapeutic effects of ASD and Semaglutide in the treatment of
diabetic osteoporosis. (A) Western blot analysis of Klotho protein
levels in rat femur tissue. (B and C) H&E staining to assess
histological changes in rat femur tissues and cell counting of
osteocytes and osteoblasts. (D) Micro-CT measurement of BMD in rat
femur. (E and F) Western blot and quantification analysis of bone
metabolic markers (OPG, OCN, RANKL and RUNX2).
*P<0.05, **P<0.01 vs. Control;
#P<0.05, ##P<0.01 vs. MOD;
ΔP<0.05, ΔΔP<0.01 vs. M + A + S;
☆P<0.05, ☆☆P<0.01 vs. M + A + S + shNC.
ASD, akebia saponin D; H&E, hematoxylin and eosin; Micro-CT,
micro-computed tomography; OPG, osteoprotegerin; OCN, osteocalcin;
RANKL, receptor activator of nuclear factor κ-b ligand; RUNX2,
Runt-related transcription factor 2. |
In terms of the femur, trabecular structure
improvements observed in the M + A + S group were abolished in
sh-Klotho rats, with reduced osteoblast/osteocyte numbers and
increased marrow cavity (Fig. 10B
and C). Micro-CT analysis revealed that BMD improvements were
also lost following Klotho knockdown (Fig. 10D). Moreover, the osteoanabolic
proteins OPG, OCN and RUNX2 decreased again, while RANKL increased,
indicating a shift back toward osteoclastic activation (Fig. 10 E and F).
Collectively, these results demonstrated that the
therapeutic effects of ASD and Semaglutide on DN and DOP are
Klotho-dependent, confirming Klotho as a critical mechanistic
target.
Discussion
The present study indicated that co-administration
of ASD and Semaglutide exerted significant therapeutic effects on
both DN and DOP, potentially through modulation of the Klotho-p53
signaling axis. To the to the best of the authors' knowledge, the
present study was the first to confirm the effect of ASD and
Semaglutide combination therapy in DM complications and to
elucidate the specific signaling pathways involved. Clinically, the
present study provides a novel dual-target strategy for the
comprehensive prevention and management of DM-associated
complications. Since patients with DM often face both renal damage
and osteoporosis, the combined therapy of ASD and Semaglutide may
represent a highly valuable therapeutic strategy, potentially
reducing damage to the kidney and skeletal systems while
controlling blood glucose, which is of great importance for
improving patient quality of life and prolonging lifespan.
The first challenge of the present study lies in how
to establish an animal model that accurately reflects the
characteristics of patients with T2DM with both DN and DOP in the
real world. Although a high-fat, high-sugar diet combined with STZ
injection is widely recognized as the primary method for
constructing a DM animal model, the optimal STZ protocol remains a
subject of debate (36). A
single high-dose injection is generally regarded as mimicking T1DM,
whereas multiple low-dose injections, when combined with dietary
manipulation, are more often employed to simulate the progressive
insulin resistance and partial β-cell dysfunction characteristic of
T2DM (33). The present study
therefore adopted the latter approach, seeking to more closely
approximate the clinical features of T2DM and its associated
complications. Specifically, both single high-dose STZ injection
and multiple low-dose STZ injections have been used in different
studies. However, the mainstream view is that a single high-dose
STZ injection (typically 50-75 mg/kg) directly destroys pancreatic
β-cells, leading to absolute insulin deficiency and rapidly
inducing hyperglycemia, simulating T1DM. By contrast, multiple
low-dose STZ injections (typically 20-40 mg/kg per injection,
sustained for more than 4 weeks) gradually destroy pancreatic
β-cells and simulate the chronic process of insulin resistance and
β-cell dysfunction, improved mimicking the pathological features of
T2DM (37). Therefore, the
present study adopted a high-fat, high-sugar diet combined with
repeated low-dose STZ injections to attempt to establish a T2DM rat
model. Comparing the MOD group rats with the Control group rats, it
was found that the MOD group rats exhibited increased fasting blood
glucose, elevated HOMA-IR, abnormal renal function indicators,
destruction of kidney tissue structure, damage to femoral
trabecular microstructure and reduced femoral biomechanical
properties, thereby confirming that the MOD group rats exhibited
both the kidney damage characteristics of DN and the bone damage
characteristics of DOP. This provides a solid foundation for
subsequent research.
The present study systematically evaluated the
therapeutic synergy of ASD and Semaglutide from multiple
perspectives. When examining the effects of the combined treatment
of ASD and Semaglutide in alleviating DN kidney injury, the present
study addressed three aspects: Serum and urine markers, kidney
tissue structure and the degree of kidney fibrosis. First, it was
found that after receiving ASD and Semaglutide combination therapy,
the serum metabolic waste products BUN and Cr levels were markedly
reduced in T2DM rats, indicating a recovery in kidney filtration
function; 24-up levels decreased, suggesting an improvement in
glomerular barrier function; and the kidney index decreased,
indicating alleviated kidney enlargement and congestion. Secondly,
H&E staining visually showed reduced damage to the glomeruli
and renal tubules; PAS staining showed decreased local deposition
of glycation end products that directly damage the kidneys; and
Masson staining reflected the inhibition of the kidney fibrosis
process. Furthermore, combination therapy markedly downregulated
TGF-β1, a key pro-fibrotic cytokine, as well as the major fibrotic
matrix components α-SMA, Collagen I and Collagen IV (38), further confirming a significant
improvement in kidney fibrosis. Lu et al (39) found that ASD slows DN progression
by activating the NRF2/HO-1 pathway and inhibiting the NF-κB
pathway and multiple studies have confirmed the anti-DN effects of
Semaglutide (40,41), which are consistent with the
findings of the present study.
When elucidating the effects of ASD combined with
Semaglutide in alleviating DOP bone tissue damage, the present
study also provided evidence from multiple perspectives. First,
H&E staining clearly showed that the trabecular structure was
markedly restored in T2DM rats following combined treatment.
Second, micro-CT results indicated that the combined therapy
markedly increased the trabecular health indicators BMD, BMC,
BV/TV, Tb.Th and Tb.N, while markedly decreasing the trabecular
rarity indicators BS/BV, Tb.Sp and SMI, suggesting an improvement
in trabecular structure. Furthermore, three-point bending tests
demonstrated that the combined treatment markedly enhanced the
mechanical strength and stability of T2DM rat bones. Last,
following combined treatment, osteogenesis-related biomarkers such
as ALP, PINP, OPG, OCN and RUNX2 were markedly elevated, while bone
resorption-related markers, including CTX-1, TRAP and RANKL, were
markedly decreased (42),
further confirming the osteogenic and anti-osteoporotic effects of
ASD and Semaglutide. Previous studies have shown that Semaglutide
improves bone metabolism in patients with DM (43,44), which is consistent with our
findings. However, limited research has explored whether ASD can
improve bone quality, making this one of the significant
innovations of our study.
Importantly, to clarify the interdependency of renal
and skeletal protection, the present study investigated whether ASD
and Semaglutide synergistically attenuated the progression of both
DM-related complications and further examined calcium and
phosphorus metabolism in T2DM rats. Previous studies have shown
that dysregulation of calcium-phosphorus metabolism is a key factor
in the mutual aggravation of DN and DOP. Specifically, during DN
progression, tubular damage often leads to phosphate retention and
hyperphosphatemia, which suppresses osteoblast activity and calcium
absorption, ultimately exacerbating osteoporosis. Conversely, as
bone tissue serves as a reservoir for calcium and phosphate,
disturbances in bone metabolism can result in excessive phosphate
release, increasing the burden on renal excretion and accelerating
renal dysfunction and DN progression (45,46). The present study showed that
combined ASD and Semaglutide treatment markedly reduced serum
phosphate levels, decreased urinary calcium and phosphate excretion
and enhanced bone mineralization, thereby alleviating
calcium-phosphorus metabolic disorders and playing a critical role
in the simultaneous protection of kidney and bone health.
Upon confirming the dual efficacy of ASD and
Semaglutide in ameliorating DN and DOP, the present study further
investigated the potential molecular mechanisms, with a focus on
the Klotho-p53 signaling axis. Klotho was initially identified as
an anti-aging protein highly expressed in renal distal tubules and
the choroid plexus of the brain (47). Subsequent studies revealed that
soluble Klotho is widely present in urine, blood and cerebrospinal
fluid and participates in multiple physiological processes by
regulating various intracellular signaling pathways, including
insulin/IGF-1, p53 and Wnt (48,49). Aberrant Klotho expression is now
recognized as a hallmark of DM (50,51). Nie et al (52) reported markedly lower serum
Klotho levels in patients with DM compared with healthy controls,
with levels declining further as disease duration increased.
Similarly, Kim et al (53) found that serum Klotho may serve
as an early predictor of DN risk. Among the proteins regulated by
Klotho, p53 is particularly relevant to DM. While p53 normally
maintains oxidative balance by upregulating antioxidant genes, its
role in DM is largely deleterious, contributing to disease
progression (54). p53 can
induce β-cell DNA damage, promote β-cell apoptosis, inhibit glucose
transport and glycolysis, promote gluconeogenesis and downregulate
insulin receptor expression, all contributing to insulin resistance
and DM progression (55). Based
on this, it was hypothesized that upregulation of Klotho to
suppress p53 signaling may represent a key mechanism underlying the
therapeutic effects of ASD and Semaglutide. To test this, the
present study first conducted a bioinformatics analysis. Among
1,787 DN/DOP-related targets and 246 ASD/Semaglutide-related
targets, multiple Klotho-p53 axis-associated molecules were
enriched, with KEGG pathway analysis highlighting the p53 pathway.
Furthermore, molecular docking showed strong interactions between
ASD and both Klotho and p53, with low binding energies. In
vivo, combined ASD and Semaglutide therapy markedly increased
Klotho protein levels and reduced p53 expression in renal and
femoral tissues, while Klotho knockdown attenuated their protective
effects, supporting the hypothesis that the Klotho-p53 axis
mediates their therapeutic action.
Nevertheless, the optimal dosing strategy and
long-term safety of this combination therapy require further
evaluation. Specifically, the present study did not investigate
dose-response relationships or potential adverse effects associated
with prolonged administration of ASD and Semaglutide. This
limitation restricts the immediate clinical application of the
therapy and future studies should therefore focus on optimizing
dosing regimens, monitoring toxicity profiles and conducting
long-term safety assessments in both preclinical and clinical
settings. In addition, several limitations of the present study
should be acknowledged. First, the research was conducted in a rat
model of T2DM, which may not fully replicate the complexity of
human diabetic nephropathy and osteoporosis and therefore the
translational relevance should be interpreted with caution. Second,
the sample size was relatively modest, which may limit the
statistical power to detect subtle effects. Third, although the
present study focused on the Klotho-p53 signaling axis, other
molecular pathways could also contribute to the observed
therapeutic benefits and further mechanistic exploration is
warranted. Finally, the present study did not include long-term
follow-up or clinical validation and thus the efficacy and safety
of ASD combined with Semaglutide need to be confirmed in larger
preclinical studies and clinical trials.
In summary, the present study demonstrates that the
natural small-molecule compound ASD and the long-acting GLP-1
receptor agonist Semaglutide exert a significant synergistic effect
in the treatment of DN and DOP, with superior efficacy compared
with either agent alone. Mechanistically, their combined action in
mitigating DM-related complications is likely mediated through
modulation of the Klotho-p53 signaling axis. These findings offer
new insights into the prevention and treatment of DM-associated
complications.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QZ was responsible for conceptualization,
investigation, formal analysis and writing the original draft. DW
was responsible for methodology, data curation, validation and
visualization. HXJ was responsible for investigation, resources and
data curation. DW and HXJ confirm the authenticity of all the raw
data. ZJZ was responsible for investigation, software and formal
analysis. LYL was responsible for methodology, histological
analysis and western blotting. LNL was responsible for
investigation, immunofluorescence and micro-CT analysis. LW was
responsible for software, bioinformatics analysis, molecular
docking, writing, reviewing and editing. YMX was responsible for
conceptualization, supervision, project administration, writing the
original draft, writing, reviewing and editing. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Animal Welfare and Ethics Committee of Guangdong Medical
Laboratory Animal Center (approval no. D202505-13).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Yaoming Xue ORCID: 0000-0003-1356-4780
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82270862) and the Guangdong Basic
and Applied Basic Research Foundation (grant no.
2024A1515220024).
References
|
1
|
Yang T, Qi F, Guo F, Shao M, Song Y, Ren
G, Linlin Z, Qin G and Zhao Y: An update on chronic complications
of diabetes mellitus: From molecular mechanisms to therapeutic
strategies with a focus on metabolic memory. Mol Med. 30:712024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lovic D, Piperidou A, Zografou I, Grassos
H, Pittaras A and Manolis A: The growing epidemic of diabetes
mellitus. Curr Vasc Pharmacol. 18:104–109. 2020. View Article : Google Scholar
|
|
3
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International Diabetes Federation Diabetes Atlas, 9th edition.
Diabetes Res Clin Pract. 157:1078432019. View Article : Google Scholar
|
|
4
|
Magee C, Grieve DJ, Watson CJ and Brazil
DP: Diabetic nephropathy: A tangled web to unweave. Cardiovasc
Drugs Ther. 31:579–592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thipsawat S: Early detection of diabetic
nephropathy in patient with type 2 diabetes mellitus: A review of
the literature. Diab Vasc Dis Res. 18:147916412110588562021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao K, Jiao Y, Xing L, Zhang F and Tian F:
The role of wnt signaling in diabetes-induced osteoporosis.
Diabetol Metab Syndr. 15:842023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vestergaard P: Discrepancies in bone
mineral density and fracture risk in patients with type 1 and type
2 diabetes-a meta-analysis. Osteoporos Int. 18:427–444. 2007.
View Article : Google Scholar
|
|
8
|
Janghorbani M, Van Dam RM, Willett WC and
Hu FB: Systematic review of type 1 and type 2 diabetes mellitus and
risk of fracture. Am J Epidemiol. 166:495–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, You W, Jing Z, Wang R, Fu Z and
Wang Y: Increased risk of vertebral fracture in patients with
diabetes: A meta-analysis of cohort studies. Int Orthop.
40:1299–1307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia J, Zhong Y, Huang G, Chen Y, Shi H and
Zhang Z: The relationship between insulin resistance and
osteoporosis in elderly male type 2 diabetes mellitus and diabetic
nephropathy. Ann Endocrinol (Paris). 73:546–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan P, Xu Y, Zhang Z, Zhu J, Miao Y, Gao C
and Wan Q: Association of circulating Omentin-1 with Osteoporosis
in a Chinese Type 2 diabetic population. Mediators Inflamm.
2020:93897202020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang S, Hu T, Liu H, Lv YL, Zhang W, Li H,
Xuan L, Gong LL and Liu LH: Akebia saponin D ameliorates metabolic
syndrome (MetS) via remodeling gut microbiota and attenuating
intestinal barrier injury. Biomed Pharmacother. 138:1114412021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xuan L, Yang S, Ren L, Liu H, Zhang W, Sun
Y, Xu B, Gong L and Liu L: Akebia saponin D attenuates allergic
airway inflammation through AMPK activation. J Nat Med. 78:393–402.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu L, Ye L, Chen Y, Deng C, Zhang X, Chang
J, Feng M, Wei J, Bao X and Wang R: Integrating network
pharmacology and transcriptomic omics reveals that akebia saponin D
attenuates neutrophil extracellular Traps-induced neuroinflammation
via NTSR1/PKAc/PAD4 pathway after intracerebral hemorrhage. FASEB
J. 38:e233942024. View Article : Google Scholar
|
|
15
|
Gong LL, Yang S, Zhang W, Han FF, Lv YL,
Wan ZR, Liu H, Jia YJ, Xuan LL and Liu LH: Akebia saponin D
alleviates hepatic steatosis through BNip3 induced mitophagy. J
Pharmacol Sci. 136:189–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kristensen SL, Rorth R, Jhund PS, Docherty
KF, Sattar N, Preiss D, Køber L, Petrie MC and McMurray JJV:
Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor
agonists in patients with type 2 diabetes: A systematic review and
meta-analysis of cardiovascular outcome trials. Lancet Diabetes
Endocrinol. 7:776–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan
JY and Yuan CS: Comparative effectiveness of GLP-1 receptor
agonists on glycaemic control, body weight, and lipid profile for
type 2 diabetes: Systematic review and network meta-analysis. BMJ.
384:e0764102024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beery AK and Zucker I: Sex bias in
neuroscience and biomedical research. Neurosci Biobehav Rev.
35:565–572. 2011. View Article : Google Scholar
|
|
19
|
Mauvais-Jarvis F: Sex differences in
metabolic homeostasis, diabetes, and obesity. Biol Sex Differ.
6:142015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du J, Zhu M, Li H, Liang G, Li Y and Feng
S: Metformin attenuates cardiac remodeling in mice through the
Nrf2/Keap1 signaling pathway. Exp Ther Med. 20:838–845. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Neil PM, Birkenfeld AL, McGowan B,
Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M
and Wilding JPH: Efficacy and safety of semaglutide compared with
liraglutide and placebo for weight loss in patients with obesity: A
randomised, Double-blind, placebo and active controlled,
dose-ranging, phase 2 trial. Lancet. 392:637–649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davies M, Faerch L, Jeppesen OK,
Pakseresht A, Pedersen SD, Perreault L, Rosenstock J, Shimomura I,
Viljoen A, Wadden TA, et al: Semaglutide 2.4 mg once a week in
adults with overweight or obesity, and type 2 diabetes (STEP 2): A
randomised, double-blind, double-dummy, placebo-controlled, phase 3
trial. Lancet. 397:971–984. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nair AB and Jacob S: A simple practice
guide for dose conversion between animals and human. J Basic Clin
Pharm. 7:27–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang KC, Chuang PY, Yang TY, Tsai YH, Li
YY and Chang SF: Diabetic rats induced using a High-fat diet and
Low-dose streptozotocin treatment exhibit gut microbiota dysbiosis
and osteoporotic bone pathologies. Nutrients. 16:12202024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu MM, Dong R, Hua Z, Lv NN, Ma Y, Huang
GC, Cheng J and Xu HY: Therapeutic potential of liuwei dihuang pill
against KDM7A and Wnt/β-catenin signaling pathway in diabetic
nephropathy-related osteoporosis. Biosci Rep. 40:BSR202017782020.
View Article : Google Scholar
|
|
27
|
Yu X, Wang LN, Du QM, Ma L, Chen L, You R,
Liu L, Ling JJ, Yang ZL and Ji H: Akebia Saponin D attenuates
amyloid β-induced cognitive deficits and inflammatory response in
rats: Involvement of Akt/NF-κB pathway. Behav Brain Res.
235:200–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cardiff RD, Miller CH and Munn RJ: Manual
hematoxylin and eosin staining of mouse tissue sections. Cold
Spring Harb Protoc. 2014:655–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu LY, Yun S, Tang HT and Xu ZX: Huangkui
capsule in combination with metformin ameliorates diabetic
nephropathy via the Klotho/TGF-beta1/p38MAPK signaling pathway. J
Ethnopharmacol. 281:1135482021. View Article : Google Scholar
|
|
30
|
Yaroslavceva MV, Bondarenko ON, El-Taravi
YA, Magerramova ST, Pigarova EA, Ulyanova IN and Galstyan GR:
Etiopathogenetic features of bone metabolism in patients with
diabetes mellitus and Charcot foot. Probl Endokrinol (Mosk).
70:57–64. 2024.In Russian. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma R, Zhu R, Wang L, Guo Y, Liu C, Liu H,
Liu F, Li H, Li Y, Fu M and Zhang D: Diabetic osteoporosis: A
review of its traditional Chinese medicinal use and clinical and
preclinical research. Evid Based Complement Alternat Med.
2016:32183132016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen F, Wang P, Dai F, Zhang Q, Ying R, Ai
L and Chen Y: Correlation between blood glucose fluctuations and
osteoporosis in type 2 diabetes mellitus. Int J Endocrinol.
2025:88894202025. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishtaya GA, Anabtawi YM, Zyoud SH and
Sweileh WM: Osteoporosis knowledge and beliefs in diabetic
patients: A cross sectional study from Palestine. BMC Musculoskelet
Disord. 19:432018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prud'homme GJ and Wang Q:
Anti-Inflammatory role of the klotho protein and relevance to
aging. Cells. 13:14132024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marei HE, Althani A, Afifi N, Hasan A,
Caceci T, Pozzoli G, Morrione A, Giordano A and Cenciarelli C: p53
signaling in cancer progression and therapy. Cancer Cell Int.
21:7032021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goyal SN, Reddy NM, Patil KR, Nakhate KT,
Ojha S, Patil CR and Agrawal YO: Challenges and issues with
streptozotocin-induced diabetes-A clinically relevant animal model
to understand the diabetes pathogenesis and evaluate therapeutics.
Chem Biol Interact. 244:49–63. 2016. View Article : Google Scholar
|
|
37
|
Rais N, Ved A, Ahmad R, Parveen K, Gautam
GK, Bari DG, Shukla KS, Gaur R and Singh AP: Model of
Streptozotocin-nicotinamide induced type 2 diabetes: A comparative
review. Curr Diabetes Rev. 18:e1711211980012022. View Article : Google Scholar
|
|
38
|
Klinkhammer BM and Boor P: Kidney
fibrosis: Emerging diagnostic and therapeutic strategies. Mol
Aspects Med. 93:1012062023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu C, Fan G and Wang D: Akebia Saponin D
ameliorated kidney injury and exerted anti-inflammatory and
anti-apoptotic effects in diabetic nephropathy by activation of
NRF2/HO-1 and inhibition of NF-KB pathway. Int Immunopharmacol.
84:1064672020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moellmann J, Klinkhammer BM, Onstein J,
Stöhr R, Jankowski V, Jankowski J, Lebherz C, Tacke F, Marx N, Boor
P and Lehrke M: Glucagon-Like peptide 1 and its cleavage products
are renoprotective in murine diabetic nephropathy. Diabetes.
67:2410–2419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Veneti S and Tziomalos K: Is there a role
for glucagon-like peptide-1 receptor agonists in the management of
diabetic nephropathy? World J Diabetes. 11:370–373. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fernandez-Villabrille S, Martin-Carro B,
Martin-Virgala J, Rodríguez-Santamaria MDM, Baena-Huerta F,
Muñoz-Castañeda JR, Fernández-Martín JL, Alonso-Montes C,
Naves-Díaz M, Carrillo-López N and Panizo S: Novel biomarkers of
bone metabolism. Nutrients. 16:6052024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onoviran OF, Li D, Toombs Smith S and Raji
MA: Effects of glucagon-like peptide 1 receptor agonists on
comorbidities in older patients with diabetes mellitus. Ther Adv
Chronic Dis. 10:20406223198626912019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herrou J, Mabilleau G, Lecerf JM, Thomas
T, Biver E and Paccou J: Narrative review of effects of
Glucagon-like Peptide-1 receptor agonists on bone health in people
living with obesity. Calcif Tissue Int. 114:86–97. 2024. View Article : Google Scholar
|
|
45
|
Sun M, Wu X, Yu Y, Wang L, Xie D, Zhang Z,
Chen L, Lu A, Zhang G and Li F: Disorders of calcium and phosphorus
metabolism and the Proteomics/Metabolomics-Based research. Front
Cell Dev Biol. 8:5761102020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Winiarska A, Filipska I, Knysak M and
Stompor T: Dietary phosphorus as a marker of mineral metabolism and
progression of diabetic kidney disease. Nutrients. 13:7892021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Buchanan S, Combet E, Stenvinkel P and
Shiels PG: Klotho, Aging, and the failing kidney. Front Endocrinol
(Lausanne). 11:5602020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hosseini L, Babaie S, Shahabi P, Fekri K,
Shafiee-Kandjani AR, Mafikandi V, Maghsoumi-Norouzabad L and
Abolhasanpour N: Klotho: Molecular mechanisms and emerging
therapeutics in central nervous system diseases. Mol Biol Rep.
51:9132024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kanbay M, Copur S, Ozbek L, Mutlu A, Cejka
D, Ciceri P, Cozzolino M and Haarhaus ML: Klotho: A potential
therapeutic target in aging and neurodegeneration beyond chronic
kidney disease-a comprehensive review from the ERA CKD-MBD working
group. Clin Kidney J. 17:sfad2762024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang A, Zhang Y, Wu L, Lin Y, Lv L, Zhao
L, Xu B, Huang Y and Li M: Klotho's impact on diabetic nephropathy
and its emerging connection to diabetic retinopathy. Front
Endocrinol (Lausanne). 14:11801692023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hejrati A, Zabihi T, Riazi S and Sarv F:
Klotho: A possible diagnostic biomarker and therapeutic target in
diabetes complications. Int J Diabetes Developing Countries. 1–16.
2025.
|
|
52
|
Nie F, Wu D, Du H, Yang X, Yang M, Pang X
and Xu Y: Serum klotho protein levels and their correlations with
the progression of type 2 diabetes mellitus. J Diabetes
Complications. 31:94–98. 2017. View Article : Google Scholar
|
|
53
|
Kim SS, Song SH, Kim IJ, Lee EY, Lee SM,
Chung CH, Kwak IS, Lee EK and Kim YK: Decreased plasma alpha-Klotho
predict progression of nephropathy with type 2 diabetic patients. J
Diabetes Complications. 30:887–892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lahalle A, Lacroix M, De Blasio C, Cisse
MY, Linares LK and Le Cam L: The p53 Pathway and Metabolism: The
tree that hides the forest. Cancers (Basel). 13:1332021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kung CP and Murphy ME: The role of the p53
tumor suppressor in metabolism and diabetes. J Endocrinol.
231:R61–R75. 2016. View Article : Google Scholar : PubMed/NCBI
|