Introduction
Intrauterine growth restriction (IUGR), also known
as fetal growth restriction, is defined as a condition in which a
fetus is small for gestational age (SGA) and falls below the 10th
percentile for gestational age and sex. (1). In 2020, one in five newborns
worldwide (23.4 million) were SGA, a condition linked to ~20% of
term stillbirths (2). Maternal
nutrition and stress during pregnancy, such as high-altitude
pregnancy, smoking and preeclampsia, can lead to adverse effects
(3,4). Among these, antenatal
hypoxia-induced placental insufficiency has been identified as a
primary risk factor for stillbirths, neonatal deaths and perinatal
morbidity associated with IUGR (5-7).
Antioxidant supplementation has been attempted to treat IUGR, but
has not achieved success (8,9).
Therefore, it remains crucial to discover new and efficacious
treatments for IUGR.
The 16-amino acid mitochondria-derived peptide,
MOTS-c, is synthesized by encoding a compact open reading frame
located within the genomic sequence of the mitochondrial 12S
ribosomal RNA gene (10). It has
been reported that MOTS-c induces transcription of antioxidant
genes and enhances cellular resistance to oxidative stress injury
(11). Low plasma levels of
MOTS-c are associated with endothelial mitochondrial dysfunction in
patients with obesity and coronary artery disease (12,13). MOTS-c serves key roles in the
onset and development of cardiovascular diseases, aging and
age-related diseases (14,15). In addition, supplementation with
MOTS-c inhibits oxidative stress in rotenone-induced neuron
degeneration (13), diabetic
cardiomyopathy (16) and
diabetic nephropathy (17).
However, the role of MOTS-c in hypoxia-induced IUGR remains
unclear.
The placenta possesses a diverse range of
antioxidant defense systems that effectively mitigate the
accumulation of reactive oxygen species (ROS). Among these systems,
the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway
serves a pivotal role in governing oxidative stress-associated
disorders (18). Nrf2 is
typically bound to Kelch-like ECH-associated protein 1 (KEAP1) in
the cytoplasm, but dissociates upon exposure to ROS and moves into
the nucleus where it activates genes associated with antioxidant
defense mechanisms (19).
Furthermore, the KEAP1-Nrf2 pathway is also activated by aerobic
exercise or MOTS-c (20). We
previously demonstrated that MOTS-c could directly increase
synthesis of Nrf2 independent of protein degradation and promote
Nrf2 nucleus translocation (21,22). During pregnancy, Nrf2 protects
the fetus from adverse oxidative stress conditions by ensuring
proper placental function (23).
Evidence suggests that Nrf2 deficiency leads to reduced fetal
weight and placental volume (24). However, it remains unclear
whether MOTS-c regulates oxidative stress in IUGR via activation of
the Nrf2-mediated anti-oxidative pathway. The present study aimed
to investigate whether MOTS-c alleviates hypoxia-induced placental
restriction and IUGR by activating the Nrf2 signaling pathway.
Materials and methods
Animals
A total of 69 C57BL/6 wild-type mice (46 female and
23 male), aged 6-8 weeks, and weighing 18-20 g, were sourced from
the Jiangnan University Laboratory Animal Center (Wuxi, China).
Nrf2 knockout (KO) mice were obtained from the Model Animal
Research Institute of Nanjing (Nanjing, China). These mice were
housed in a specific pathogen-free facility environment, which
maintained an ambient humidity of 50% and a temperature range of
22-25°C. The mice had free access to food and water and were
subjected to a 12-h light-dark cycle managed by an automated light
control system. All animal experiments were approved by the Animal
Experimentation Ethics Committee of Jiangnan University [approval
nos. 2 0220930m1080501(383) and 20231115m1080530(555)]. All animal
experiments were performed in accordance with guidelines
established by the International Association for the Assessment and
Accreditation of Laboratory Animal Care (25) and the relevant laws of the
National Research Council's Guide for the Care and Use of
Laboratory Animals.
IUGR mouse model establishment and sample
collection
Females were paired with males (with a total of 23
pairs) overnight for mating purposes, and the day of observation of
a vaginal plug was designated as gestational day (GD) 0. The IUGR
mouse model was established as previously reported (26,27) and as similarly described in our
previous studies (28,29). Briefly, 17 pregnant mice were
housed under hypoxic conditions (FiO2=0.105) from GD 11
to GD 17.5, which refers to IUGR group. The control group of
pregnant mice were housed under normoxic conditions
(FiO2=0.21) throughout pregnancy. To determine the
protective effects of MOTS-c, randomly selected control and IUGR
pregnant mice received intraperitoneal injections of 5 mg/kg MOTS-c
once a day from GD 11 to 17.5 (10,30,31), which refers to the MOTS-c (n=6)
and IUGR + MOTS-c groups (n=5), respectively. Meanwhile, control
and IUGR mice were injected with an equivalent volume of
physiological saline from GD 11 to 17.5, which refers to the normal
group (n=6) and IUGR group (n=6) respectively. No suitable drug is
presently available for use as a positive control in the treatment
of hypoxia-induced IUGR (8,9),
therefore, no positive control group was included.
To explore the protective mechanisms of MOTS-c,
pregnant C57BL/6 mice and Nrf2 knockout (KO) mice were randomly
divided into six groups: Normal (n=6), Nrf2−/− (n=4),
IUGR (n=6), IUGR + MOTS-c (n=5), Nrf2−/− + IUGR (n=4),
and Nrf2−/− + IUGR + MOTS-c (n=5). MOTS-c (5 mg/kg) was
intraperitoneally injected daily from GD 11 to 17.5, with an
equivalent volume of physiological saline injected as the loading
control. On GD 17.5, each pregnant mouse was terminally
anesthetized using pentobarbital sodium (50 mg/kg,
intraperitoneally) as previously reported (32,33). The maternal blood was collected
according to our previous study (34). Briefly, the mice were fixed in a
supine position. After making a sternal incision, blood was
collected by inserting a 26-G needle vertically through the second
intercostal space into the heart, using a vacuum tube system. The
blood was incubated at room temperature for 30 min, centrifuged at
105.45 × g for 10 min at 4°C and then the separated serum was used
for the study. Finally, the mice were then euthanized via cervical
dislocation, and death was confirmed by the absence of a heartbeat
and cessation of breathing. After euthanizing the dams, caesarean
section surgery was performed via hysterectomy as according to a
previous study (35). Briefly,
the abdomen was sterilized with 70% ethanol and an abdominal
incision was made to retrieve the uterus, which was transferred
onto a sterile gauze over a heating pad. Finally, the pups were
extracted by incising the uterine wall and applying gentle
pressure. Fetuses and placentas were then collected. The total
number of fetuses were counted, and the weight of the fetuses and
placentas were measured. The placental efficiency was calculated as
follows: Placental efficiency (%)=(fetal weight/placental weight) ×
100 (36). Fetal pups were
anesthetized by inhalation of 2% isoflurane and sacrificed via
cervical dislocation. Euthanasia was confirmed by the absence of
heartbeat under the dissecting microscope, with the tissue appears
pale. Placental tissues were collected for subsequent experiments;
all collected samples were stored at −80°C until use.
Synthesis of MOTS-c peptides
The human MOTS-c peptide was synthesized by the
Mimotopes Pty Ltd., using 9-fluorenylmethoxycarbonyl solid-phase
chemistry in accordance with standard peptide synthesis protocols
(37). The synthesized peptide
had purity >95%, as determined through high-performance liquid
chromatography by Mimotopes Pty Ltd. The amino acid sequence of
MOTS-c was as follows: Met-Arg-Tr
p-Gln-Glu-Met-Gly-Tyr-Ile-Phe-Tyr-Pro-Arg-Lys-Leu-Arg. The MOTS-c
peptide was dissolved in distilled deionized water at a
concentration of 1 mg/ml and stored at −20°C.
Histopathological staining
Placental tissues were fixed in a 4%
paraformaldehyde solution at room temperature for 48 h, and embed
in paraffin and sectioned (4-μm thick). The sections were
stained with hematoxylin and eosin (cat. no. D006-1, Nanjing
Jiancheng Bioengineering Institute). Briefly, the sections were
stained with Harris's hematoxylin (0.1%) for 6 min, differentiated
with 1% acid ethanol, rinsed and blued with tap water, following
counterstaining with eosin Y (0.5-1%) for 1 min at room
temperature. Images of placenta sections were captured using a
Panoramic MIDI scanner (3DHISTECH Ltd.; magnification, ×20) and for
each sample, ~10 images were acquired. Imaging and quantification
were performed blinded. The area of the segmented blood sinusoid
regions was quantified using the area measurement tool of the
ImageJ software version 1.53k (National Institutes of Health). Data
on the areas of blood sinusoids were statistically analyzed to
calculate the means and standard deviation. The assessment of
placental angiogenesis was performed by quantifying the sinusoidal
areas.
Immunohistochemical staining
To detect intracellular antigens, the placental
sections (4-μm thick) were deparaffinized at 65°C for 60 min
and rehydrated using gradient alcohol. Antigen retrieval was
performed using tris-EDTA buffer at 98°C for 25 min, followed by
permeabilization with 0.1% Triton X-100 in PBS for 15 min at room
temperature. To minimize non-specific binding, sections were
blocked with 5% normal goat serum (cat. no. A7906; MilliporeSigma)
for 2 h at room temperature. For HRP-based assays, endogenous
peroxidases were quenched with 3% hydrogen peroxide in methanol for
15 min before blocking. The placental sections were then incubated
with the primary antibodies against CD31 (1:100; cat. no. #77699s;
Cell Signaling Technology) and MOTS-c (1:100; cat. no.
#MOTSC-101AP; FabGennix International Inc.) overnight at 4°C. At
room temperature, the samples were co-incubated with the
corresponding fluorescence labeled secondary antibody (1:100; cat.
no. 33106ES60; Shanghai Yeasen Biotechnology Co., Ltd.) for 60 min,
and freshly prepared DAB (cat. no. #CW20695; CWBIO) staining was
then performed. Finally, sections were counterstained with
hematoxylin (cat. no. I030-1; Nanjing Jiancheng Bioengineering
Institute), dehydrated and cleared. Images were captured using
Panoramic MIDI (3D HISTECH Ltd.) and analyzed using the Panoramic
Viewer software version 2.1 (3DHISTECH Ltd.).
Enzyme linked immunosorbent assay
(ELISA)
MOTS-c content in the serum and placental tissues of
the pregnant mice was quantified using an ELISA kit (cat. no
#MM-46300M1; Jiangsu Meimian Industrial Co., Ltd.) following the
manufacturer's guidelines.
Cell culture and treatments
The immortalized human umbilical vein endothelial
cells (HUVECs) and human placental trophoblast cell (HTR-8/SVneo)
were obtained from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. These cells were cultured in
Dulbecco's Modified Eagle's Medium (DMEM; cat. no. 319-005-CL;
Wisent, Inc.) enriched with 10% fetal bovine serum (FBS; cat. no.
40130ES76; Shanghai Yeasen Biotechnology Co., Ltd.) and 1%
penicillin-streptomycin mixture (cat. no. C0222; Beyotime Institute
of Biotechnology). Incubation conditions were set to a humidified
atmosphere at 37°C and 5% CO2.
In investigating the effects of MOTS-c on
hypoxia-induced HUVECs and HTR-8/SVneo cells, the cells were
pre-treated with 10 μM MOTS-c under normoxic conditions (21%
O2) or an equivalent volume of phosphate buffer saline
(PBS), followed by modeling under hypoxic conditions for 48 h; the
use of 1% O2 is a well-established model in the in
vitro study of hypoxia-related diseases, and has been
demonstrated to cause dysregulation of angiogenesis and
proliferation in HUVECs as previously described (38). To mimic the abnormal angiogenesis
under hypoxia exposure, HUVECs were exposed to an oxygen
concentration of FiO2=0.01 for 48 h. The procedure for
each experimental approach was standardized to ensure consistent
operational steps and treatment conditions across three replicates.
HUVECs and HTR-8/SVneo cells used were confined to those within the
3rd to 5th passages for experimental consistency and reliability.
The experiment was independently repeated for three times.
To determine the role of Nrf2 in hypoxia-stimulated
HUVECs, ML385 (cat. no. HY-100523; MedChemExpress), a specific Nrf2
inhibitor was used as previously reported (39). Briefly, HUVECs were stimulated
with ML358 (5 μM) for 1 h, followed by administration with
MOTS-c (10 μM) for 2 h and exposed to hypoxia (1%
O2) for an additional 48 h.
Optical Microscopy
For morphological assessment, HUVECs were examined
under a phase-contrast microscope equipped with a digital camera
(DP11, Olympus), and images were documented.
Cell viability assay
Cell viability was evaluated by the Cell Counting
Kit-8 (CCK-8) assay (cat. no. C0037; Beyotime Institute of
Biotechnology). Cells were seeded at a density of 1×104
cells/well in 96-well plates. After rinsing with PBS, the cells
were exposed to CCK-8 solution (1:10 dilution). The cells were
incubated at 37°C for 2 h, and then the absorbance were measured at
450 nm using a microplate reader (BioTek; Agilent Technologies,
Inc.).
Nrf2 overexpression in HUVECs
The Nrf2 overexpression plasmid was constructed and
obtained from OBiO Technology (Shanghai) Corp., Ltd. Briefly, after
reaching 80% confluence, the cells were transfected with pcDNA3.1
plasmid or Nrf2 overexpression plasmids (1 μg/ml) using a
transfection reagent (cat. no. C10511-05; Guangzhou RiboBio Co.,
Ltd.) and cultured at 37°C and 5% CO2. After 24 h, the
medium was changed and exposed to normoxic (21% O2) or
hypoxic (1% O2) conditions for 48 h. Cells were then
harvested for downstream analysis, with Nrf2 overexpression
verification performed by reverse transcription-quantitative PCR
(RT-qPCR).
Nrf2 siRNA transfection in HUVECs
Nrf2 siRNA was obtained from KeyGEN BioTECH.
Briefly, after reaching 80% confluence, HUVECs cells were
transfected with control siRNA (20 μM) or Nrf2 siRNA (20
μM) using a transfection reagent (cat. no. C10511-05;
Guangzhou RiboBio Co., Ltd.) according to the manufacturer's
instructions. After 12 h, the medium was changed and the cells were
cultured for 36 h at 37°C and 5% CO2. Cells were then
harvested for downstream analysis, with Nrf2 knockdown verification
performed by RT-qPCR. The siRNA sequences used were as follows:
Nrf2 sense (S), 5'-GCAGCAAACAAGAGAUGGCAAdTdT-3' and anti-sense
(AS), 5'-UUGCCAUCUCUUGUUUGCUGCdTdT-3'; and negative control siRNA
(vector-siRNA) S, 5'-UUCUCCGAACGUGUCACGUdTdT-3' and AS,
5'-ACGUGACACGUUCGGAGAAdTdT-3'.
ROS measurements
The ROS production in HUVECs and HTR-8/SVneo cells
were assessed using 2',7'-dichlorodihydro fluorescein diacetate
(DCFH-DA) diacetyldichlorofluorescein staining solution (1:1,000;
cat. no. E004-1-1; Nanjing Jiancheng Bioengineering Institute) in
the dark at 37°C for 15 min, and analyzed with a Zeiss fluorescence
microscope (Zeiss AG). The mitochondrial ROS levels were assessed
using MitoSOX (cat. no. #40778ES50; Shanghai Yeasen Biotechnology
Co., Ltd.). The cells were treated with a MitoSOX working solution
(5 mM) at 37°C for 20 min. Then, the ROS fluorescence intensity was
visualized using a confocal microscope (Zeiss AG).
Immunofluorescence staining
For Nrf2 immunofluorescence staining in HUVECs,
cells were fixed with 100% methanol for 15 min at −20°C, followed
by permeabilization with 0.1% Triton X-100 for 10 min and blocked
with 1% bovine serum albumin (BSA) (cat. no. HY-D0842;
MedChemExpress) for 60 min at room temperature. The cells were then
incubated with Nrf2 antibody (cat. no. #ab62352; Abcam) at 1/100
dilution overnight at 4°C followed by a further incubation with
Alexa 488-conjugated secondary antibody (1:200; cat. no. 33103ES60;
Shanghai Yeasen Biotechnology Co., Ltd.) at room temperature for 1
h. The Nrf2 antibody specificity was validated by Nrf2 siRNA
knockdown (Fig. S1). The nuclei
were labelled with DAPI solution (1 mg/ml; cat. no. #P0131;
Beyotime Institute of Biotechnology) for 10 min at room
temperature. Images were captured using a confocal microscope.
For Ki-67 and Nrf2 immunofluorescence staining in
placental tissues, the sections were permeabilized with 0.1% Triton
X-100 for 10 min and blocked with 5% BSA (cat. no. HY-D0842;
MedChemExpress) for 2 h at room temperature. The samples were
incubated with primary antibodies anti-Ki-67 (1:100; cat. no.
#9129; Cell Signaling Technology, Inc.) and anti-Nrf2 (1:100; cat.
no. #62352; Abcam) overnight at 4°C, followed by incubation with
secondary antibodies conjugated to Alexa Fluor 488 (cat. no.
#33103ES60; 1:200; Shanghai Yeasen Biotechnology Co., Ltd.) or
Alexa Fluor 594 (cat. no. #33112ES60; 1:200; Shanghai Yeasen
Biotechnology Co., Ltd.). Subsequently, the nuclei were stained
with DAPI (1 μg/ml; cat. no. HY-D1738; MedChemExpress) for 5
min at room temperature. Images were acquired using a Zeiss Axio
Imager 2 fluorescent microscope (Carl Zeiss AG). The number of
immunoreactivity positive cells was quantified as the percentage of
total cells. All samples were blinded during imaging and
quantification.
Tube formation assay
Matrigel (50 μl; cat. no. #082704; Shanghai
Nova Pharmaceutical Technology Co., Ltd) was added to each well of
96-well plates and allowed to solidify in a cell incubator at 37°C
with 5% CO2 for 60 min. Next, 3×104 HUVECs in
100 μl of DMEM containing 5% fetal bovine serum (cat. no.
40130ES76; Shanghai Yeasen Biotechnology Co., Ltd.) and 1%
penicillin-streptomycin (cat. no. C0222; Beyotime Institute of
Biotechnology). were added to each well. After 6 h of incubation,
tubular structures were examined and visualized using a Zeiss Axio
Imager 2 fluorescent microscope (Carl Zeiss AG), and the tube
lengths were quantified using ImageJ software version 1.53k
(National Institutes of Health). The angiogenic capability was
quantified by measuring the number of tubes and the mean mesh size
per field from three randomly selected fields per well.
L-lactate dehydrogenase (LDH), superoxide
dismutase activity (SOD) and malondialdehyde (MDA) content
LDH concentration, SOD activity and the MDA content
were quantified in the serum of pregnant mice and HUVECs using kits
from Nanjing Jiancheng Bioengineering Institute (LDH kit, cat. no.
A020-2; SOD kit, cat. no. A001-3-2: MDA kit, cat. no. A003-1-2)
following the manufacturer's protocols.
Determination of mitochondrial membrane
potential (MMP)
The MMP was assessed using JC-1 dye (Beyotime
Institute of Biotechnology). Briefly, JC-1 dye added to the cell
suspension to achieve a final dye concentration of 10 μM.
The cells were incubated with the JC-1 dye mixture at 37°C for 15
min to allow the dye to enter the cells and accumulate in the
mitochondria. JC monomers (488 nm) and JC aggregates (570 nm) were
visualized using a fluorescence microscope (Olympus Corporation),
and images were acquired from a random selection of 3-4 fields per
well.
Western blotting
Placentas and HUVEC cells were lysed using RIPA
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
containing with protease and phosphatase inhibitors (cat. no.
P1045; Beyotime Institute of Biotechnology). Nuclear and
cytoplasmic extracts from placental tissues were prepared using a
Nuclear Extraction Kit (cat. no. W037-1-1; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions. The protein concentrations were determined according
to the manufacturer instructions using a BCA Protein Assay kit
(cat. no. P0009; Beyotime Institute of Biotechnology).
Subsequently, the lysates were separated on 8 and 10% SDS-PAGE gels
and transferred onto polyvinylidene difluoride membranes (cat. no.
P2938; MilliporeSigma; Merck KGaA). Following a 2 h blocking step
at room temperature with 5% BSA (cat. no. HY-D0842; MedChemExpress)
in Tris-buffered saline, the membranes were incubated overnight at
4°C with primary antibodies targeting CD31 (1:1,000; cat. no.
77699s; Cell Signaling Technology, Inc.), vascular endothelial
growth factor receptor 2 (VEGFR2; 1:1,000; cat. no. 26415-1-AP;
Proteintech), vascular endothelial growth factor A (VEGFA; 1:1,000;
cat. no. 66828-1-Ig; Proteintech), Nrf2 (1:1,000; cat. no. 62352;
Abcam) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
1:5,000; cat. no. 263962; Abcam). After washing, the membranes were
incubated with secondary antibodies for 2 h at room temperature
(Goat Anti-Mouse IgG, 1:5,000; cat. no. CW0102S; Goat Anti-Rabbit
IgG, 1:5,000; cat. no. CW0103S; Jiangsu CoWin Biotech Co., Ltd.)
corresponding to the primary antibody source. Protein bands were
visualized using a ChemiDocTM XRS Plus luminescence image analyzer
(Bio-Rad Laboratories, Inc.) with an ECL system (MilliporeSigma;
Merck KGaA), and quantified using ImageJ software version 1.53k
(National Institutes of Health).
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Total RNA from the placental tissue was extracted
using TRIzol Reagent (cat. no. R401; Vazyme Biotech Co., Ltd.) and
then reverse-transcribed into complementary DNA using a PrimeScript
RT Reagent Kit (cat. no. R323; Vazyme Biotech Co., Ltd.) according
to the manufacturer's protocol. Target mRNA amplification was
measured using SYBR Premix Ex Taq™ (cat. no. 11201ES08; Shanghai
Yeasen Biotechnology Co., Ltd.) with a LightCycler® 480
detection PCR system (Roche Diagnostics). The PCR amplification
protocol consisted of an initial denaturation step at 95°C for 5
min, followed by 40 cycles of denaturation at 95°C for 10 sec,
annealing at 55°C for 20 sec and extension at 72°C for 20 sec.
Quantification was performed employing the 2−ΔΔCq method
(40), with GAPDH serving as the
reference gene. The primer sequences are provided in Table I.
 | Table IPrimers for reverse-transcription
quantitative PCR analysis. |
Table I
Primers for reverse-transcription
quantitative PCR analysis.
A, Mouse primers
|
|---|
| Gene | Sequence
(5'-3') |
|---|
| Pgf | F:
AGTGGAAGTGGTGCCTTTCAA |
| R:
GTGAGACACCTCATCAGGGTA |
| Fatp4 | F:
ACTGTTCTCCAAGCTAGTGCT |
| R:
GATGAAGACCCGGATGAAACG |
| Glut1 | F:
TCAAACATGGAACCACCGCTA |
| R:
AAGAGGCCGACAGAGAAGGAA |
| Snat2 | F:
GGGACATAAGGCGTATGGTCT |
| R:
GGTAGCTTGACATAGCCCCAA |
| Igf2 | F:
CGTGGCATCGTGGAAGAGT |
| R:
ACGTCCCTCTCGGACTTGG |
| Nrf2 | F:
TCTTGGAGTAAGTCGAGAAGTGT |
| R:
GTTGAAACTGAGCGAAAAAGGC |
| Gapdh | F:
AGGTCGGTGTGAACGGATTTG |
| R:
GGGGTCGTTGATGGCAACA |
|
| B, Human
primers |
|
| Gene | Sequence
(5'-3') |
|
| VEGFA | F:
AGGGCAGAATCATCACGAAGT |
| R:
AGGGTCTCGATTGGATGGCA |
| VEGFR2 | F:
GGCCCAATAATCAGAGTGGCA |
| R:
CCAGTGTCATTTCCGATCACTTT |
| CD31 | F:
CCAAGGTGGGATCGTGAGG |
| R:
TCGGAAGGATAAAACGCGGTC |
| GAPDH | F:
GGAGCGAGATCCCTCCAAAAT |
| R:
GGCTGTTGTCATACTTCTCATGG |
Statistical analyses
Statistical data are depicted as means ± standard
deviation. The D'Agostino-Pearson test and Shapiro-Wilk test were
used for normality tests. For quantitative data obeying normal
distribution, comparisons between two groups were performed using
Student's unpaired two-tailed t-test. To compare among ≥3 groups,
the Brown-Forsythe analysis was used to test for homogeneity of
variance, if the variance was homogeneous, the Tukey's test of
one-way analysis of variance was used. If variances were not
homogeneous, Dunnett's T3 test was used to test for differences
between multiple groups. The association between different targets
was analyzed using Pearson's correlation test. Analyses were
performed using the GraphPad Prism software (version 8.0.2;
Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
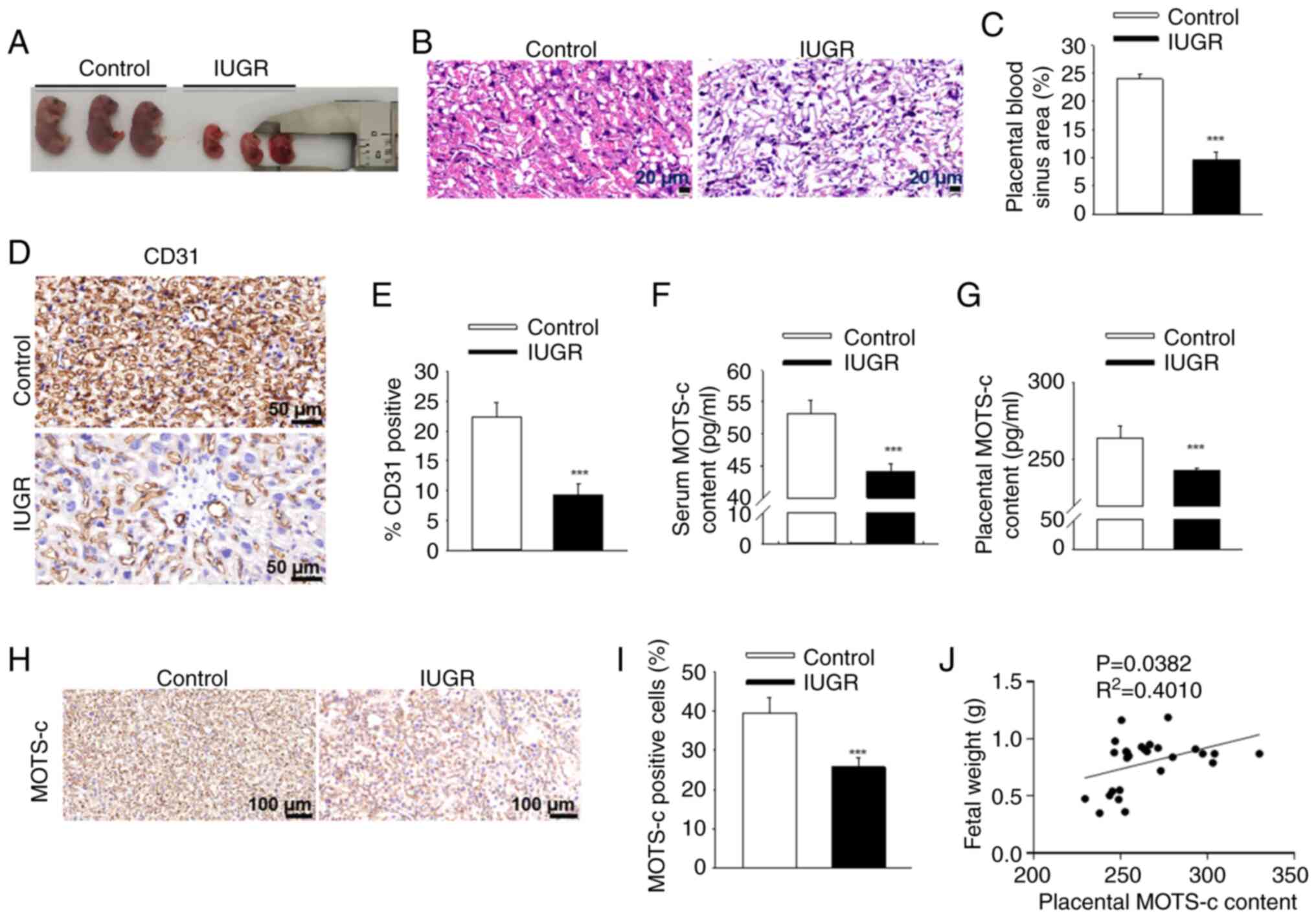
Decreased levels of MOTS-c in the serum
and placenta of pregnant mice is associated with reduced fetal
weight and lower placental vascular density
Exposure to hypoxia during pregnancy led to
significant fetal growth restriction (Fig. 1A), evidenced by reduced fetal
body weight and diminished placental efficiency (the
fetal-to-placental weight ratio; Table II). Additionally, the blood
sinus areas within the placenta were significantly reduced in IUGR
mice (Fig. 1B and C). The
expression levels of CD31, a vascular marker, were significantly
lower in the placenta of IUGR mice compared with that of the
Controlgroup (Fig. 1D and E).
These findings suggested a reduction in placental vascular density
following antenatal maternal hypoxia exposure. MOTS-c content was
significantly reduced in both maternal serum and placenta after
hypoxic exposure (Fig. 1F and
G), further validated by immunohistochemistry of placental
tissues, with 34.8% reduction of MOTS-c positive cells in IUGR mice
when compared with the control group (Fig. 1H and I). Furthermore, placental
MOTS-c levels exhibited a positive correlation with fetal mouse
weight (R2=0.4010; P=0.0382; Fig. 1J). These data suggested that the
decreased fetal weight and placental vascular density resulting
from antenatal maternal hypoxia may be associated with reduced
MOTS-c content.
 | Table IIDescriptive statistics of dams and
fetuses exposed to hypoxia. |
Table II
Descriptive statistics of dams and
fetuses exposed to hypoxia.
| Characteristic | Normal | IUGR | P-value |
|---|
| Maternal body
weight, g | 31.9±1.1 | 29.6±1.3a | 0.0087 |
| Litter size | 7.6±1.4 | 7.4±1.6 | 0.8626 |
| Litter parameters,
n | 53 | 52 | |
| Fetal weight
(g) | 0.90±0.085 | 0.49±0.075b | <0.0001 |
| Crown-rump length
(mm) | 8.6±0.4 | 8.0±0.4 | 0.0333 |
| Placenta, n | 53 | 52 | |
| Placenta weight
(g) | 0.0772±0.0102 | 0.0836±0.00790 | 0.2954 |
| Fetal/weight
ratio | 11.8±2.0 | 6.0±1.2b | 0.0001 |
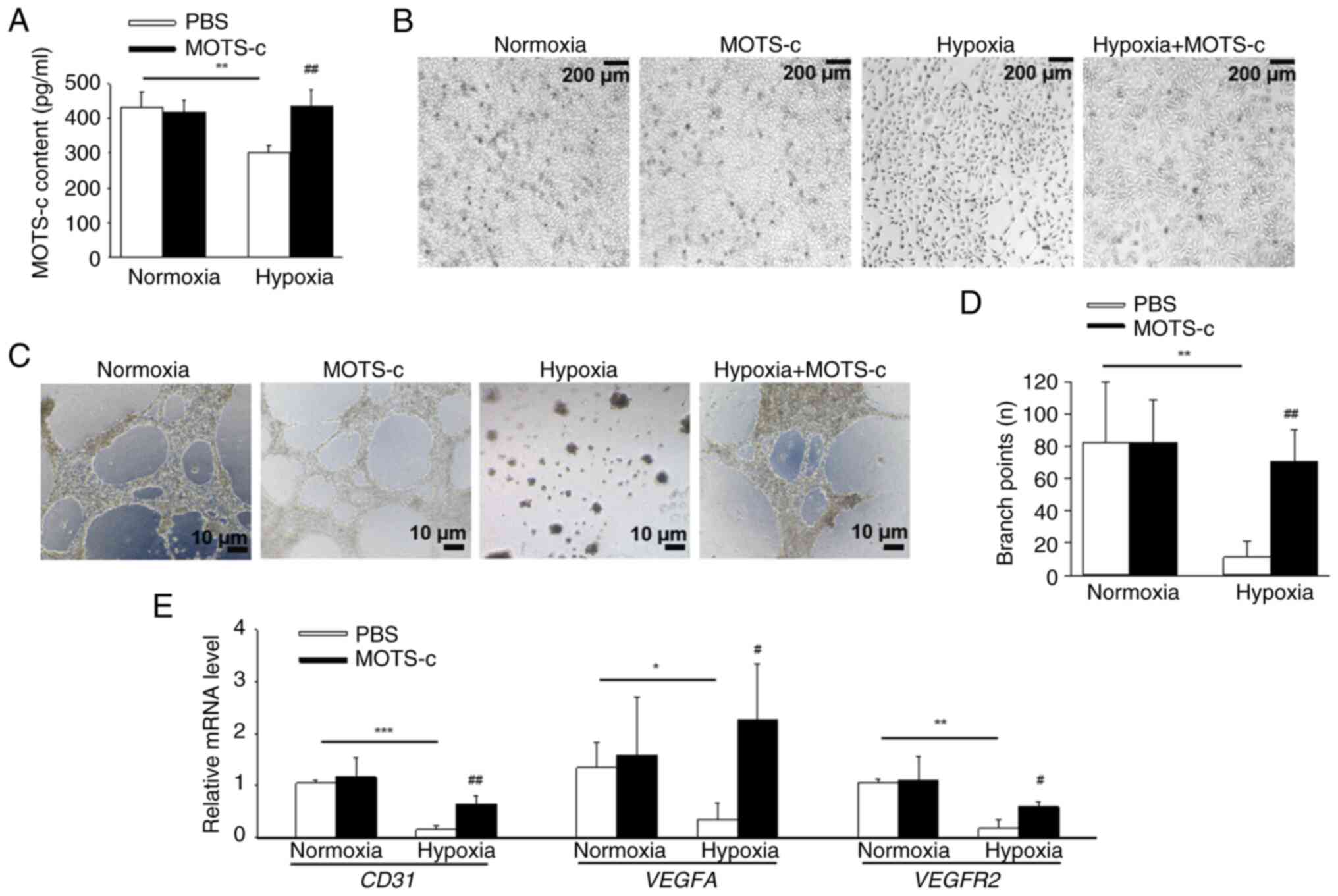
MOTS-c promotes placental angiogenesis
and increased fetal weight in IUGR mice
To assess the potential benefits of MOTS-c
supplementation on fetal growth, synthetic MOTS-c peptide was
administered intraperitoneally. The timeline of this experimental
study is schematically presented in Fig. 2A. This intervention resulted in a
significant increase in maternal serum and placental MOTS-c
content, as showed in Fig. S2A-D. When compared with that of the
normal group, the MOTS-c group mice showed no significant
differences in fetal growth, as indicated by normal size,
morphology and fetal weight (Fig.
S3A and B), fetal weight of the MOTS-c group remained normal
for up to 4 weeks after birth (Fig.
S3C). Therefore, subsequent investigations focused solely on
the effects of MOTS-c on IUGR mice. MOTS-c administration
significantly improved fetal development, with increased fetal size
and fetal weight compared with that of the IUGR group (Fig. 2B and C). Furthermore, the ratio
of fetal to placental weight was significantly increased following
MOTS-c administration in the IUGR mice compared with the untreated
IUGR mice (Fig. 2D). In
addition, MOTS-c administration significantly improved blood sinus
areas (Fig. 2E and F) and
upregulated CD31 expression in the placenta in the IUGR + MOTS-c
group compared with that of the IUGR group (Fig. 2G and H). These findings suggested
beneficial effects of MOTS-c treatment against hypoxia-induced poor
placental vascular density and subsequent fetal development. As the
VEGF pathway is recognized as a major regulatory mechanism
governing developmental angiogenesis (41), the expression levels of VEGFA and
VEGFR2 were investigated. The protein expressions levels of VEGFA
and VEGFR2 were decreased in placental tissues following hypoxic
exposure in the IUGR group, while in comparison, MOTS-c treatment
significantly upregulated the VEGF pathway in the IUGR + MOTS-c
group (Fig. 2I). These results
suggested that MOTS-c improved hypoxia-induced IUGR by promoting
placental angiogenesis.
 | Figure 2MOTS-c administration protects
against hypoxia-induced fetal growth restriction. (A) Schematic
timeline of the experimental setup. (B) Morphology of fetal mice on
GD17.5. (C) Mean fetal weights within each litter. (D) Placental
efficiency, which represents the ratio of fetal to placenta weight.
(E) Representative images of H&E staining of placental tissues.
Scale bar, 20 μm. (F) Quantification of placental blood
sinus area. (G) Representative IHC images CD31. Scale bar, 50
μm. (H) Quantification of CD31 positive area. (I) Western
blotting analysis of CD31, VEGFA and VEGFR2 protein expression in
placenta. Data are expressed as the mean ± SD. Normal, n=6; IUGR,
n=6; IUGR + MOTS-c, n=5. **P<0.01 vs. normal,
***P<0.001 vs. normal; #P<0.05 vs.
IUGR; ##P<0.01 vs. IUGR; ###P<0.001 vs.
IUGR. IUGR, intrauterine growth restriction; GD, gestational day;
VEGFA, vascular endothelial growth factor A; VEGFR2, VEGF receptor
2. |
Increased cell proliferation and
upregulation of mRNA expression of nutrient transporter genes in
placental tissues are associated with MOTS-c treatment
To investigate the role of MOTS-c on cell
proliferation in placenta subjected to IUGR, placental tissues of
the normal, IUGR and IUGR + MOTS-c group mice were stained with
Ki-67, a marker for cell proliferation, and the number of positive
cells was quantified. The concentration of Ki-67-positive cells
were significantly decreased in hypoxia-exposed placental tissues
compared with that in the normal group, which was subsequently
reversed by MOTS-c administration (Fig. 3A and B). Furthermore, the mRNA
expression levels of placental growth factor (Pgf),
sodium-dependent neutral amino acid transporter-2 (Snat2),
glucose transporter type 1 (Glut1), insulin-like growth
factor 2 (Igf2) and fatty acid transporter 4 (Fatp4)
in placental tissues were assessed. Hypoxia exposure resulted in
the downregulation of placental growth factors (Pgf and
Igf2), as well as placental nutrient transport-related genes
(Glut1, Fatp4 and Snat2) in the IUGR group;
MOTS-c treatment in the IUGR + MOTS-c group significantly increased
the mRNA expression levels when compared with that in the IUGR
group (Fig. 3C). These findings
suggested that MOTS-c treatment exerted a beneficial effect on
placental proliferation defects in hypoxic environments.
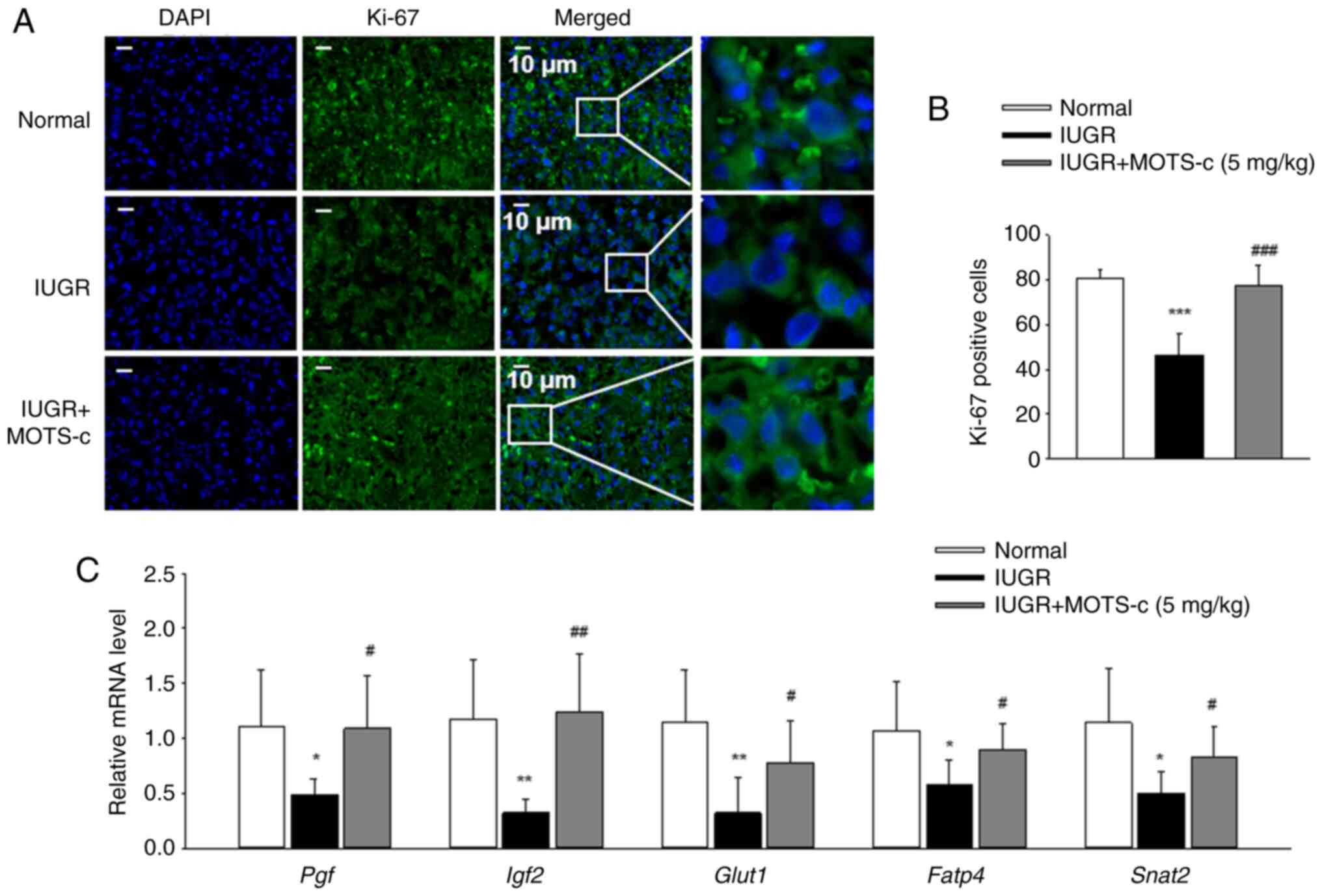 | Figure 3Administration of MOTS-c improves
placental injury. (A) Representative immunofluorescence images of
Ki-67 in placental tissues. Scale bar, 10 μm. (B)
Qualification of Ki-67 positive cells. (C) Relative mRNA expression
levels of Pgf, Igf2, Glut1, Fatp4 and
Snat2 in placenta. Data are expressed as the mean ± SD.
Normal, n=6; IUGR, n=6; IUGR + MOTS-c, n=5. *P<0.05
vs. normal, **P<0.01 vs. normal,
***P<0.001 vs. normal; #P<0.05 vs.
IUGR; ##P<0.01 vs. IUGR; ###P<0.001 vs.
IUGR. IUGR, intrauterine growth restriction; Pgf, placental growth
factor; Igf2, insulin-like growth factor 2; Glut1, glucose
transporter type 1; Fatp4, fatty acid transporter 4; Snat2,
sodium-dependent neutral amino acid transporter-2. |
MOTS-c mitigates hypoxia-mediated
oxidative stress in the placenta tissue
MOTS-c administration effectively mitigated
oxidative stress-induced damage in placental tissues, as evidenced
by a significant reduction in MDA levels and an increased SOD
activity in the IUGR + MOTS-c group compared with that of the IUGR
group (Fig. 4A and B). Nrf2,
which upregulates the expressions of numerous antioxidant genes,
serves a key role in inhibiting oxidative stress in the placenta
(42). Maternal hypoxia exposure
resulted in significantly decreased Nrf2 mRNA expression levels in
the placenta of the IUGR group, while MOTS-c treatment restored
Nrf2 mRNA expression levels in the IUGR + MOTS-c group (Fig. 4C). Furthermore, total, nuclear
and cytoplasmic Nrf2 protein expression levels were also examined.
Hypoxia restricted Nrf2 nuclear translocation, while MOTS-c
significantly increased nuclear Nrf2 expression in placental
tissues (Fig. 4D-G). The
accumulation and nuclear translocation of Nrf2 was also detected by
immunofluorescence, which demonstrated that in the IUGR + MOTS-c
group, MOTS-c treatment promoted Nrf2 nuclear translocation
compared with that in the IUGR group (Fig. 4H and I). In addition, MOTS-c
treatment was associated with increased mRNA expression levels of
heme oxygenase 1 and NAD(P)H quinone dehydrogenase 1, which are
known Nrf2 downstream targeted anti-oxidative genes (Fig. 4J). These results suggest that
MOTS-c activate Nrf2-mediated antioxidant pathway.
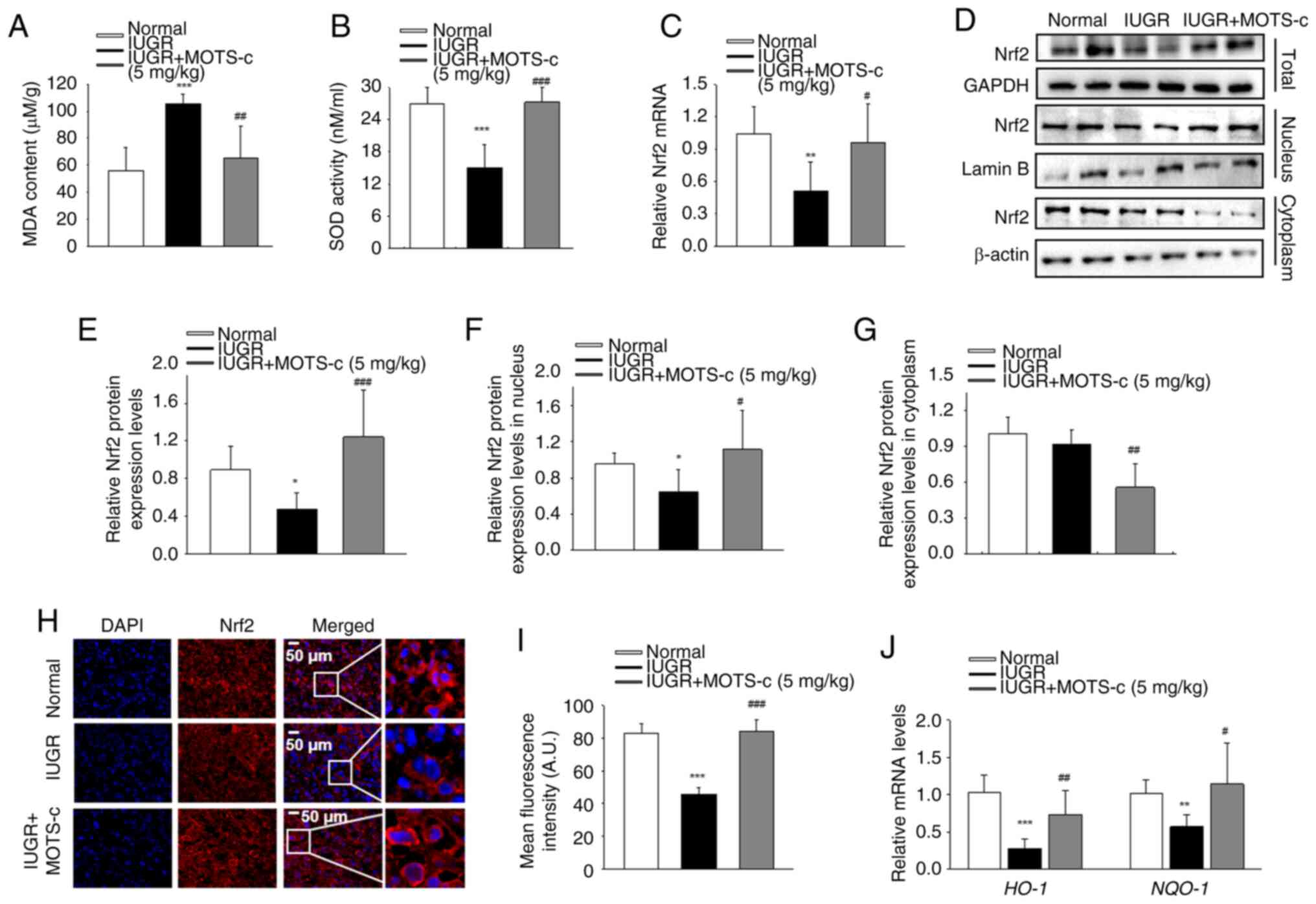 | Figure 4MOTS-c mitigates hypoxia-mediated
oxidative stress in placenta. (A) MDA content in placenta. (B) SOD
activity in placenta. (C) Nrf2 mRNA expression levels in placenta.
(D) Representative western blotting images. (E) Total Nrf2
expression in placental tissues. (F) Nuclear Nrf2 expression in
placental tissues. (G) Nrf2 expression in cytoplasm of placental
tissues. (H) Representative immunofluorescence images and (I)
quantification of Nrf2 in placenta. Scale bar, 50 μm. (J)
Relative mRNA expression levels of HO-1 and NQO-1. Data are
expressed as the mean ± SD. Normal, n=6; IUGR, n=6; IUGR + MOTS-c,
n=5. *P<0.05 vs. normal, **P<0.01 vs.
normal, ***P<0.001 vs. normal; #P<0.05
vs. IUGR; ##P<0.01 vs. IUGR; ###P<0.001
vs. IUGR. IUGR, intrauterine growth restriction; SOD, superoxide
dismutase; MDA, malondialdehyde; Nrf2, nuclear factor erythroid
2-related factor 2; HO-1, heme oxygenase 1; NQO-1, NAD(P)H quinone
dehydrogenase 1. |
MOTS-c treatment attenuates
hypoxia-induced injury in HUVEC cells
To elucidate the effects of MOTS-c on HUVECs, MOTS-c
(10 μM) was used for subsequent in vitro assays,
according to the CCK-8 results (Fig. S4A), since MOTS-c (20 and 40
μM) resulted in significantly decreased cell viability. It
was demonstrated that MOTS-c content significantly decreased after
hypoxic stimuli compared with HUVECs in normoxic conditions, which
was consistent with the in vivo results. Exogenous MOTS-c
(10 μM) significantly increased the MOTS-c content in
hypoxia-induced HUVECs compared with that in the hypoxia group.
(Fig. 5A). MOTS-c administration
restored hypoxia-induced abnormal morphology in HUVECs (Fig. 5B) and significantly increased
tube formation of HUVECs (Fig. 5C
and D) compared with that in the hypoxia group. Furthermore,
MOTS-c treatment increased mRNA expression levels of CD31,
VEGFA and VEGFR2 under hypoxic conditions in HUVECs
compared with that in the hypoxia group (Fig. 5E). In addition, MOTS-c treatment
promoted proliferation in hypoxia-exposed HUVECs, as evidenced by
the significantly increased levels of Ki-67 positive cells under
MOTS-c treatment compared with the control group (Fig. S4B and C). These results
suggested that MOTS-c mitigated hypoxia-induced injury and promoted
angiogenesis and proliferation in HUVECs.
It was further investigated whether MOTS-c functions
in other cell types in the placenta; placental trophoblast cells
are critically important for pregnancy and embryonic development
(43). Thus, human placental
trophoblast cells (HTR-8/SVneo) was also examined. MOTS-c treatment
significantly increased cell viability reduced cell injury and
enhanced the mRNA levels of nutrient transporter genes, including
Pgf, Igf2, Glut1, Fatp4 and
Snat2 (Fig. S5A-C).
Furthermore, MOTS-c treatment significantly reduced ROS production
in hypoxia-stimulated HTR-8/SVneo cells compared with that of the
PBS treated HTR-8/SVneo cells under hypoxic conditions (Fig. S5D and E). These results
indicated that the beneficial effects of MOTS-c against hypoxia was
not limited to HUVECs.
MOTS-c treatment increased Nrf2 nuclear
translocation and attenuated mitochondrial injury in HUVECs
The antioxidant effect of MOTS-c treatment was also
examined in HUVECs. Intracellular ROS production was assessed using
DCFH-DA staining, which demonstrated that MOTS-c treatment
significantly suppressed the hypoxia-induced ROS generation
compared with that of PBS-treated hypoxic cells (Fig. 6A). Furthermore, MOTS-c treatment
under hypoxic conditions resulted in significantly reduced MDA
content and restored SOD activity in HUVECs compared with that of
PBS-treated hypoxic cells (Fig. 6B
and C). Additionally, mitochondrial ROS levels were evaluated
using MitoSOX staining, which similarly demonstrated that MOTS-c
treatment significantly inhibited hypoxia-induced mitochondrial ROS
generation compared with that of PBS-treated hypoxic cells
(Fig. 6D). Concurrently, MMP
levels was assessed using JC-1 staining, which demonstrated that
MOTS-c significantly reduced MMP levels in hypoxic HUVECs compared
with untreated cells (Fig. 6E).
Consistent with the present in vivo findings, MOTS-c
treatment promoted Nrf2 nuclear translocation in hypoxia-induced
HUVECs (Fig. 6F and G). These
findings suggested that MOTS-c exerted its protective effects, at
least in part, through enhanced Nrf2 activity and by mitigating
mitochondrial injury in hypoxia-induced HUVECs.
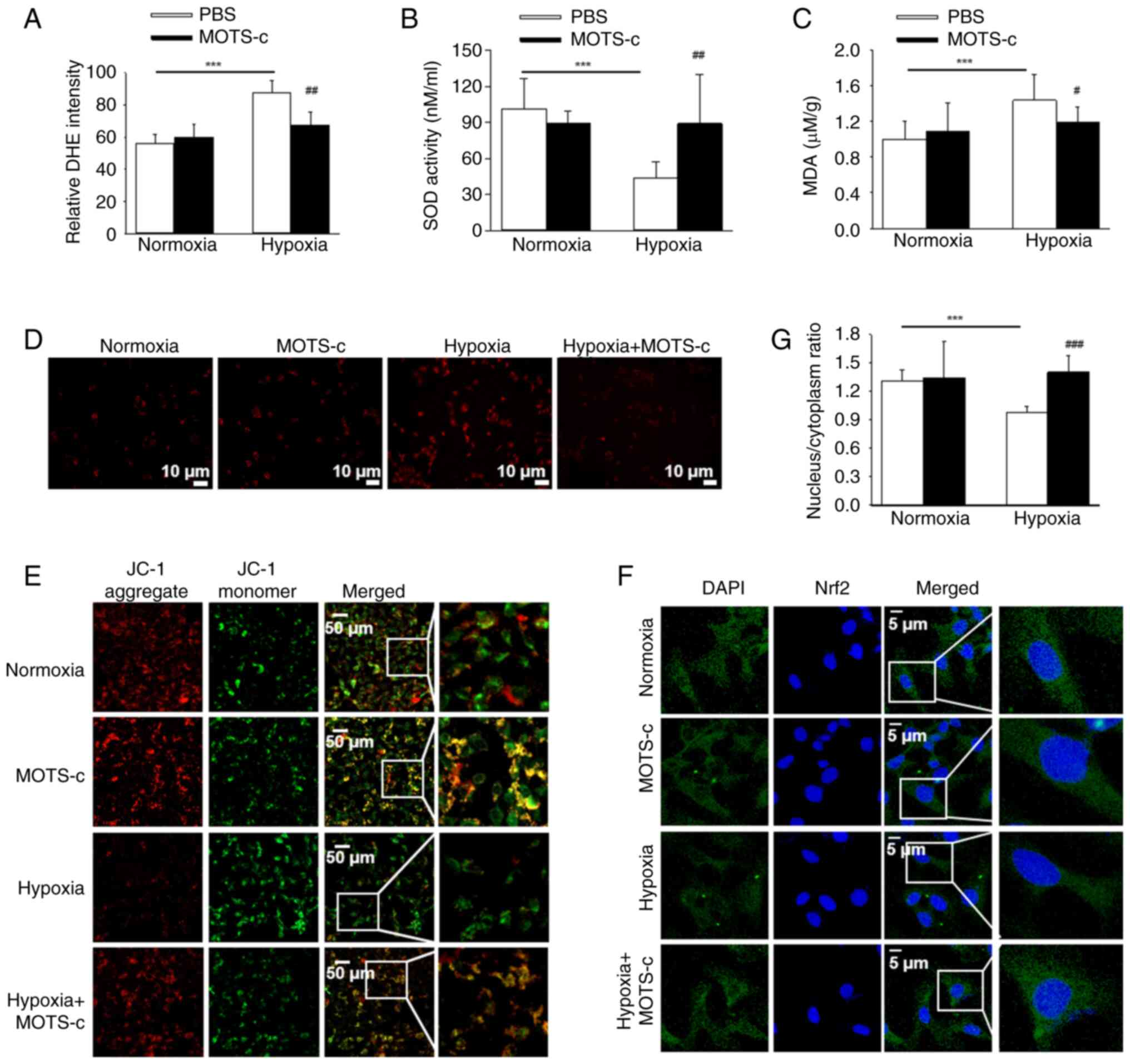 | Figure 6MOTS-c exposure attenuates oxidative
stress in HUVECs. (A) Relative intensity of DCFH-DA staining. (B)
Cellular SOD activity. (C) Cellular MDA content. (D) Representative
images of MitoSOX staining. Scale bar, 10 μm. (E)
Representative images of JC-1 staining. Scale bar, 50 μm.
(F) Representative immunofluorescence images of Nrf2 in HUVECs.
Scale bar, 5 μm. (G) Relative nuclear to cytoplasm
fluorescence ratio. Results are representative of three independent
experiments. Data are expressed as the mean ± SD, n=4.
***P<0.001, #P<0.05 vs. PBS under
hypoxic conditions, ##P<0.01 vs. PBS under hypoxic
conditions; ###P<0.001 vs. PBS under hypoxic
conditions. SOD, superoxide dismutase; MDA, malondialdehyde;
DCFH-DA,2'7'-dichlorodihydro fluorescein diacetate ; Nrf2, nuclear
factor erythroid 2-related factor 2. |
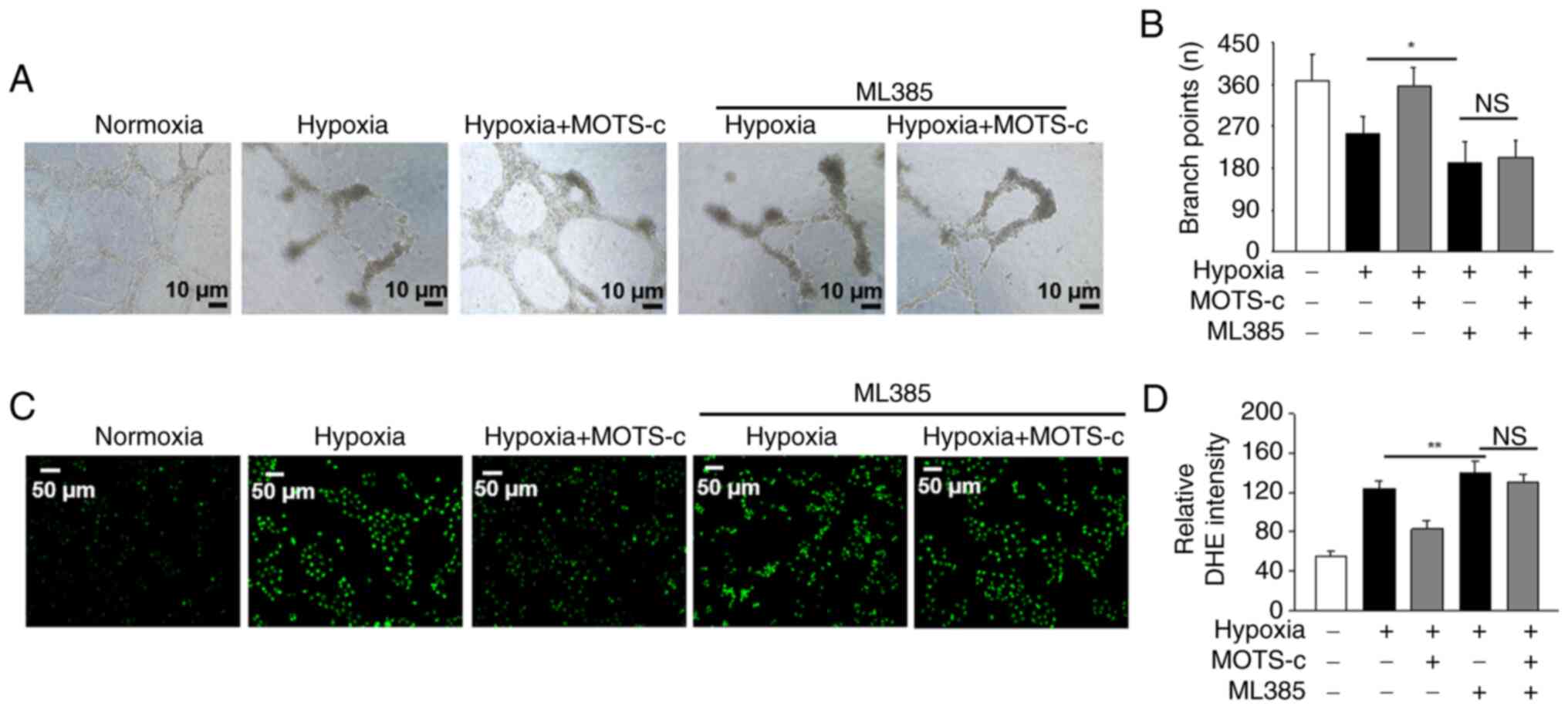
Nrf2 inhibitor ML385 offsets the
protective effect of MOTS-c against hypoxia in HUVECs
To investigate whether Nrf2 serves a role in the
protective effects of MOTS-c against hypoxia in HUVECs, ML385, a
specific Nrf2 inhibitor was used (39). It was demonstrated that
pretreatment with ML385 under hypoxic conditions inhibited tube
formation compared with the hypoxia group, implying the
significance of Nrf2. MOTS-c treatment, upon co-administration of
ML385 in hypoxia-induced HUVECs, failed to promote tube formation
(Fig. 7A and B). Additionally,
pretreatment with ML385 under hypoxic conditions increased ROS
production compared with the hypoxia group, and MOTS-c did not
reduce the generation of ROS in ML385-pretreated HUVECs under
hypoxic stimuli (Fig. 7C and D).
These findings suggested that the protective effect of MOTS-c
against hypoxia-induced HUVECs injury in HUVECs may partially rely
on Nrf2 activation.
Nrf2 overexpression fails to enhance the
beneficial effect of MOTS-c on hypoxia-stimulated HUVECs
In addition to Nrf2 inhibition, the effects of Nrf2
overexpression on hypoxia-exposed HUVECs was also determined. Under
hypoxic conditions, Nrf2 overexpression significantly increased
Nrf2 mRNA expression levels in HUVECs compared with control cells,
which indicated the efficiency of the Nrf2 transfection (Fig. 8A). Nrf2 overexpression
significantly enhanced cell viability under hypoxic conditions.
However, there was no significant difference between the Nrf OE and
MOTS-c + Nrf2 OE groups under hypoxic stimuli (Fig. 8B). In addition, Nrf2
overexpression markedly reduced ROS production under hypoxic
conditions, and Nrf2 overexpression failed to further inhibit ROS
generation in MOTS-c treated HUVECs under hypoxic conditions
(Fig. 8C and D).
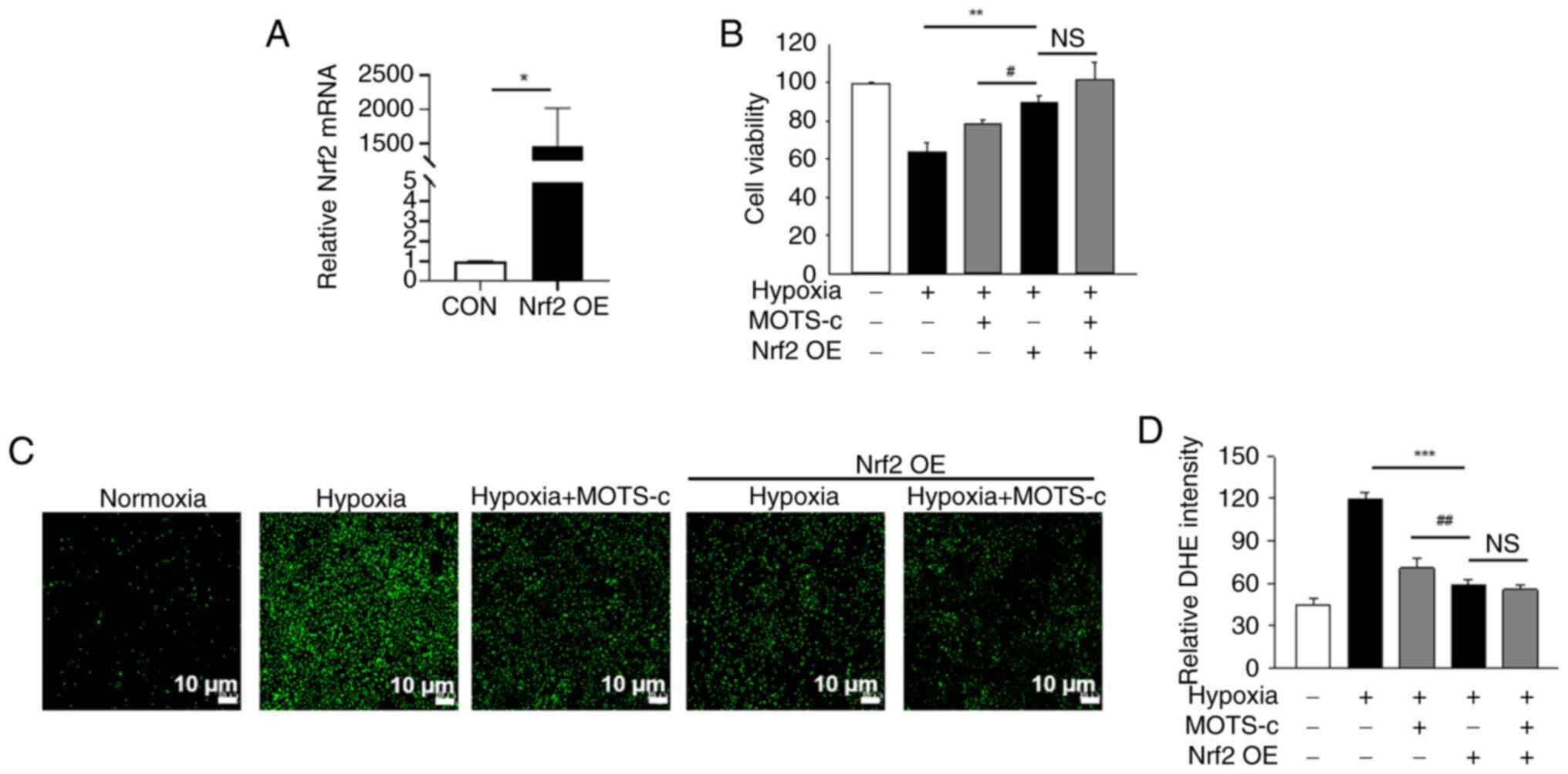 | Figure 8Nrf2 overexpression does not enhance
the beneficial effects of MOTS-c on hypoxia-stimulated HUVECs. (A)
Relative Nrf2 mRNA expression levels. (B) Cell viability. (C).
Representative images of DCFH-DA staining and (D) quantification of
cellular ROS. Scale bar, 10 μm. Data are expressed as the
mean ± SD. *P<0.05, **P<0.01,
***P<0.001, #P<0.05,
##P<0.01. NS, not significant; Nrf2, nuclear factor
erythroid 2-related factor 2; ROS, reactive oxygen species; OE,
overexpression; DCFH-DA, 2',7'-dichlorodihydro fluorescein
diacetate. |
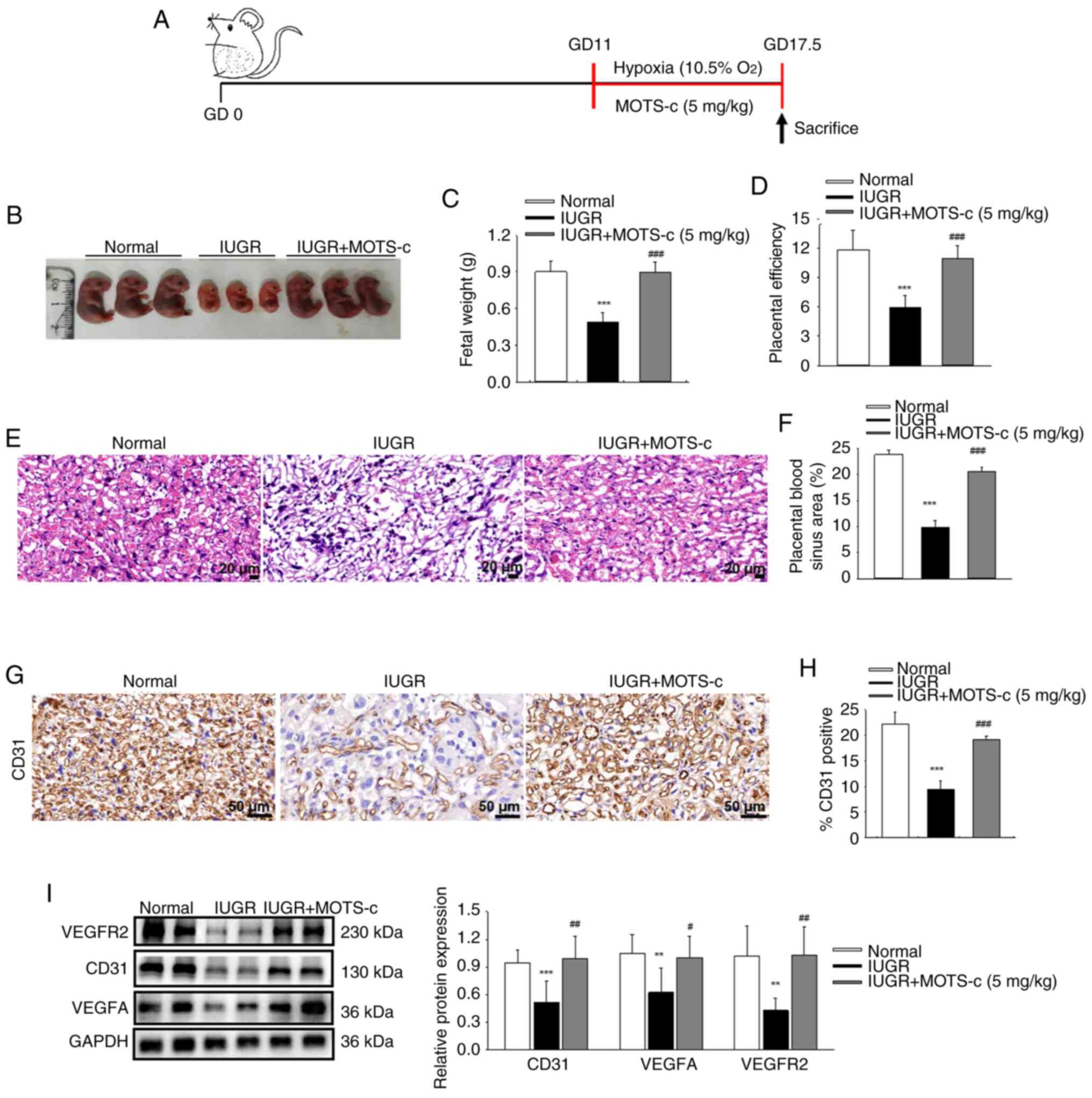
MOTS-c protects against hypoxia-induced
placental insufficiency in an Nrf2-dependent manner
To further investigate the role of Nrf2 in the
protective effects of MOTS-c against hypoxia-induced IUGR and
placental insufficiency, Nrf2−/− pregnant mice were
exposed to hypoxic conditions for further investigation. Placental
tissues from Nrf2−/− mice exhibited no Nrf2 protein
expression (Fig. S6A and B).
MOTS-c treatment did not affect fetal growth restriction in Nrf2
deficiency mice, as evidenced by unchanged fetal size, morphology
and the fetal to placenta weight ratio in Nrf2−/−
pregnant mice when compared with the hypoxia-treated
Nrf2−/− pregnant mice (Fig. 9A and B). Notably, MOTS-c
administration failed to enhance the blood sinus areas (Fig. 9C and D) and did not upregulate
the protein expressions of CD31, VEGFA and VEGFR2 in
Nrf2−/− mice (Fig.
9E). Additionally, MOTS-c supplementation did not impact the
mRNA expressions of placental growth factors, including Pgf
and Igf2 in Nrf2−/− mice. However, MOTS-c
upregulated the expression levels of placental nutrient
transport-related genes, such as Glut1, Fatp4 and
Snat2, in Nrf2−/− mice (Fig. 9F). This suggested that the
beneficial effects of MOTS-c on placental nutrient
transport-related genes might be independent of Nrf2.
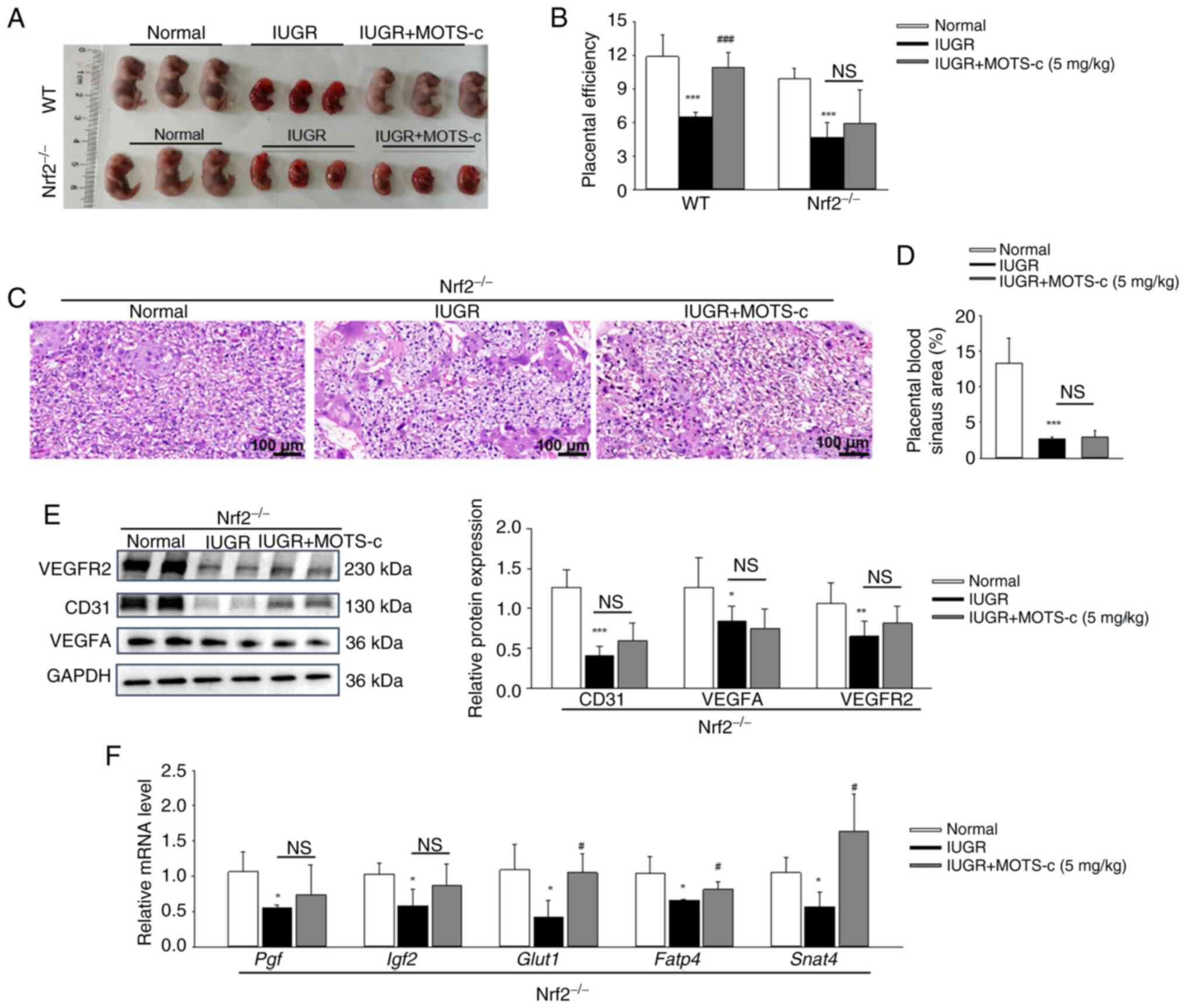 | Figure 9MOTS-c protects against
hypoxia-induced placental insufficiency in an Nrf2-dependent
manner. (A) Morphology of fetal mice on GD17.5 in WT and Nrf2 KO
mice. (B) Placental efficiency, which represents the ratio of fetal
to placenta weight. (C) Representative images of H&E staining
of placental tissues. Scale bar, 100 μm. (D) Quantification
of the placental blood sinus area. (E) Western blotting analysis of
CD31, VEGFA and VEGFR2 protein expression levels in placenta. (F)
Relative mRNA expression levels of Pgf, Igf2,
Glut1, Fatp4 and Snat2 in placenta. Data are
expressed as mean ± SD. n=4-6. *P<0.05 vs. normal,
**P<0.01 vs. normal, ***P<0.001 vs.
normal, #P<0.05 vs. IUGR, ###P<0.001
vs. IUGR.. NS, not significant; IUGR, intrauterine growth
restriction; GD, gestational day; Pgf, placental growth factor;
Nrf2, nuclear factor erythroid 2-related factor 2; Igf2,
insulin-like growth factor 2; Glut1, glucose transporter type 1;
Fatp4, fatty acid transporter 4; Snat2, sodium-dependent neutral
amino acid transporter-2; VEGFA, vascular endothelial growth factor
A; VEGFR2, VEGF receptor 2. |
Discussion
IUGR is characterized by a fetus that is small for
gestational age. A study of 600,000 stillbirths (≥22 weeks) from 12
countries reported that being small for gestational age contributed
to ~20% of term stillbirths (2).
However, there is currently no effective treatment for IUGR. The
present study investigated the effects of MOTS-c peptides on
hypoxia-induced IUGR, and demonstrated that MOTS-c administration
significantly mitigated hypoxia-induced IUGR, promoted placental
angiogenesis, suppressed oxidative stress and ameliorated placental
injury both in vivo and in vitro. Furthermore, the
protective effects of MOTS-c were shown to be Nrf2-dependent, as
the protective effect of MOTS-c were abolished not only in Nrf2
inhibitor treated HUVECs, but also in Nrf2 KO mice. To the best of
our knowledge, the present study is the first to demonstrate that
MOTS-c treatment could attenuate hypoxia-induced IUGR, and thus
suggest a new strategy for treating hypoxia-related neonatal
disease.
Prenatal hypoxia, a common consequence of
complicated pregnancies, serves a key role in triggering IUGR
(44,45). In such cases, the placenta fails
to adequately supply oxygen and nutrients to the developing fetus,
resulting in low birth weight (LBW) (7). Normal development of the placental
vascular tree is essential for both proper placental growth and
nutrient delivery from mother to fetus. Studies have reported
impaired angiogenesis in placental tissues of infants with LBW
(46,47), which has been identified as an
important pathophysiologic contributor to IUGR. VEGFA and its
receptor VEGFR2 are vital for blood vessel development and their
reduction has been observed in LBW placentas (48). Higher expression of the NADPH
oxidase 2 and lower vessel density were found in LBW placentas, as
NADPH oxidase 2 inhibited VEGFA-mediated placental angiogenesis
(49). Dietary supplementation
with adenosine has been shown to enhance placental angiogenesis and
reduce the incidence of IUGR in piglets (50). Therefore, improving placental
angiogenesis appears crucial for alleviating IUGR. Consistent with
this notion, the present study demonstrated that MOTS-c treatment
accelerated the formation of placental blood vessels in IUGR by
increasing blood sinus areas and upregulating angiogenesis-related
markers CD31 and VEGF. In addition, increased Ki-67 positive cells
in both placental tissues and hypoxia-exposed HUVECs were observed.
Ki-67 is widely considered a marker of proliferation, which is
mainly expressed during the active phases of the cell cycle:
G1, S, G2 and mitosis (51). However, low amounts of Ki-67 may
also be detected in quiescent cells (52). The present study found positive
Ki-67 signals peripheral to DAPI staining in placental tissues,
while the Ki-67 signal was totally located in the nucleus of
HUVECs. The observed extra-nuclear Ki-67 immunofluorescence signal
in tissues is a well-documented phenomenon as reported in previous
studies (53,54). It was considered that the
apparent peripheral signal potentially reflected different cell
type and cell cycle stages. Furthermore, MOTS-c was injected
intraperitoneally from GD 11 to 17.5, and beneficial effects of
MOTS-c administration on maintaining placenta angiogenesis and
proliferation, attenuating placental insufficiency, and
subsequently alleviating IUGR were observed. The timing of the
experiment was aligned with prior work, in which MOTS-c was
administered concurrently with the induction of the disease model
(10,30,31,55).
The placenta is highly susceptible to oxidative
stress, which in turn triggers placental vascular dysfunction and
insufficiency (56). Oxidative
stress in the placenta is a major contributor to the development of
IUGR. Elevated levels of MDA and decreased catalase activity have
been observed in plasma and placental tissues of pregnancies with
IUGR (57). Therefore, restoring
redox homeostasis through antioxidant therapy would be an effective
treatment for these pregnancy complications (58). However, a previous clinical trial
showed limited success in using antioxidant therapy for hypoxic
pregnancy (59,60). Therefore, further investigation
into alternative treatment options for IUGR is warranted. The
present study found that that administration of MOTS-c reduced MDA
content and increased SOD activity in placental tissues. The in
vitro experiments demonstrated a decrease in ROS generation
after MOTS-c treatment in hypoxia-induced HUVECs, which indicated
the anti-oxidative stress effects of MOTS-c. Dysfunctional
mitochondria are another source of ROS overproduction in
individuals with IUGR (61). The
present study demonstrated that MOTS-c treatment reduced MMP levels
and inhibited mitochondria ROS generation, which suggested a
protective role of MOTS-c on mitochondrial function. These results
indicated that MOTS-c ameliorated mitochondrial dysfunction and
restored antioxidant enzymes, thereby reducing ROS generation and
alleviated placental insufficiency in IUGR.
The transcription factor Nrf2 serves a crucial role
in coordinating the cellular antioxidant defense system, regulating
the expression of >100 genes involved in oxidative stress
responses and detoxification processes (62,63). The activation of Nrf2 is
suggested to be a compensatory mechanism that safeguards the fetus
against oxidative damage. A previous study reported that in both
human and rat IUGR groups, placentas had lower Nrf2 expression
levels compared with that of the control group placentas (64). Similarly, the downregulation of
Nrf2 was found in placental tissues from patients with eclampsia
(65). The present study
similarly demonstrated downregulated expression of Nrf2, especially
the reduced nuclear Nrf2 expression in hypoxia-induced maternal
placenta compared with the normal group of mice, and that MOTS-c
administration increased Nrf2 nuclear accumulation compared with
the IUGR group. Cytoplasmic and nuclear Nrf2 protein expressions
were verified through immunoblotting, and Nrf2 immunofluorescence
was performed. However, there remains a limitation regarding the
quantitative analyses of Nrf2 distribution (nuclear/cytoplasmic
ratio) in placenta tissue in the present study. The detection of
Nrf2 subcellular localization is important as nuclear translocation
of Nrf2 directly reflect its activation status (19), thus further research regarding
Nrf2 subcellular localization, particularly precise quantification
is warranted. Additionally, increased transcriptional activity of
Nrf2 under MOTS-c administration was demonstrated in the present
study. However, it was assessed at only one time point, and the
relevant pharmacokinetics of Nrf2 activation over time following
MOTS-c administration is important to optimize dosing regimen to
maintain efficacy of MOTS-c. For example, to investigate the
optimal Nrf2 activity time after MOTS-c treatment, future work
could establish the Nrf2 signaling pathway activation reporting
system using secreted Gaussia luciferase (Gluc) as the reporter
gene, thus the activity of Gluc could be monitored constantly in
MOTS-c-treated cells. Furthermore, a reporter protein
complementation imaging assay could be established to screen and
observe Nrf2 nuclear translocation in MOTS-c-treated cells and
living animals in the future.
The protective effects of MOTS-c were abolished
when the Nrf2 inhibitor ML385 and Nrf2 KO mice were used in the
present study. By contrast, Nrf2 overexpression failed to enhance
the beneficial effects of MOTS-c on cell viability and ROS
generation in hypoxia-stimulated HUVECs, in which there was no
difference between the Hypoxia + Nrf2 OE and Hypoxia + MOTS-c +
Nrf2 OE groups. Notably, under hypoxic conditions, Nrf2
overexpression (Hypoxia + Nrf2 OE group) demonstrated more
pronounced cytoprotective effects (increased cell viability and
decreased ROS production) compared with that of MOTS-c-treated
cells (Hypoxia + MOTS-c group). This result further highlighted the
central regulatory role of Nrf2 against oxidative stress damage,
which overall suggested that the protective role of MOTS-c against
hypoxia-induced abnormal placental injury and fetal growth is at
least partially dependent on Nrf2. However, the specific mechanism
how MOTS-c regulates Nrf2 has not been fully elucidated until now.
Previous studies from the present research group have demonstrated
that MOTS-c could directly increase synthesis of Nrf2 independent
of protein degradation and promote Nrf2 nuclear translocation
(21,22), and demonstrated nuclear
accumulation of Nrf2 under MOTS-c administration in the IUGR model.
Future research should focus on elucidating the regulatory
mechanism of MOTS-c on Nrf2 and investigating the impact of MOTS-c
presence or absence on the interaction between Nrf2 and KEAP1 in
response to hypoxia or other stress conditions. Notably, the mRNA
expression levels of nutrient transporters (Glut1,
Fatp4 and Snat4) were still upregulated under MOTS-c
administration in Nrf2 KO mice in the present study. It has been
reported that MOTS-c induces glucose uptake and GLUT4 translocation
in mitochondrial fusion dependent way, and that MOTS-c functionally
prevents nutrient metabolism disorders, including insulin
resistance, obesity and bone metabolism (66). These studies suggest that MOTS-c
can function on nutrient transporters in other non-Nrf2-dependent
pathways, such as targeting the mitochondrion. Our previous study
found altered glucose and lipid metabolic levels in IUGR offspring
in vivo (28); whether
MOTS-c treatment alters actual amino acid, glucose or fatty acid
levels in fetal serum and functions in regulating glucose or fatty
acid in the offspring could be investigated in future research.
The present study has several clinical
implications. First, significantly decreased maternal serum MOTS-c
content following hypoxic exposure was observed, which suggested
that plasma MOTS content could potentially serve as a novel marker
for diagnosing IUGR. Second, the in vivo and in vitro
experiments demonstrated that administration of MOTS-c effectively
attenuated hypoxia-induced IUGR, which suggested that MOTS-c
peptides may offer a novel therapeutic approach for treating
hypoxia-related neonatal disease, including IUGR.
Several limitations of the present study should be
acknowledged. First, a single dose of MOTS-c (5 mg/kg) was used in
the present study from GD11 to GD17.5; however, future work should
include a dose-response experiment from GD11 to GD17.5 or at later
stages in pregnancy to determine the optimal therapeutic
concentration, administration time and potential toxicity of
MOTS-c. Second, further randomized clinical trials are needed to
confirm the endogenous levels of MOTS-c in human placentas or
maternal serum from IUGR pregnancies to further the clinical
understanding of MOTS-c in IUGR. Currently, the MOTS-c ELISA kit is
most commonly used to determine MOTS-c content in body fluids, such
as serum. However, this detection system present challenges and can
potentially lead to strongly differing results (67). The previous study has established
an LC/MS-based method for MOTS-c quantification; therefore, serum
or amniotic fluid MOTS-c content may be measured in human IUGR
pregnancies using LC/MS in future (67). Furthermore, future studies should
focus on the long-term development of main organs such as lungs,
heart and brain in offspring mice following MOTS-c administration
in an IUGR mouse model.
In conclusion, MOTS-c treatment effectively
improved placental insufficiency in hypoxia-induced IUGR by
activating the Nrf2-mediated anti-oxidant pathway. The present
provided insights for developing MOTS-c as a therapeutic strategy
for fetal diseases associated with hypoxic pregnancy, including
IUGR.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DC, HMZ and QFP performed study conceptualization.
HMZ and XLS managed project administration and data collection.
SPL, SCL and ZXX conducted investigation, provided resources,
developed software and performed validation. YXW and JFH curated
data. DC, XLS, YXW, JFH and QFP acquired funding. DC, QFP and JFH
contributed to writing. DC and HMZ confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental procedures were approved by the
Experimental Animal Care and Use Committee of Jiangnan University
[approval nos. 20220930m1080501(383) and 20231115m1080530(555)] and
conducted according to the Guide for the Care and Use of Laboratory
Animal published by the US National Institutes of Health (8th
edition).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
GD
|
gestational day
|
|
HUVEC
|
human umbilic vein endothelial
cells
|
|
IUGR
|
intrauterine growth restriction
|
|
MDA
|
malondialdehyde
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
|
VEGFA
|
vascular endothelial growth factor
A
|
|
VEGFR2
|
VEGF receptor 2
|
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82171704 and 82100018), Natural
Science Foundation of Jiangsu Province (grant no. BE2023684),
Natural Science Foundation of Shandong Province (grant no.
ZR2021MH333), Fundamental Research Funds for the Central
Universities (grant no. JUSRP121062), Translational medicine
Project of Wuxi Commission of Health (grant no. ZH202101), Wuxi
Taihu Talent Training Project (Double hundred Medical Youth
Professionals Program; grant no. HB2020054) and the Clinical
research and translational medicine program of the Affiliated
Hospital of Jiangnan University (grant no. LCYJ202239).
References
|
1
|
Ahmadzadeh E, Polglase GR, Stojanovska V,
Herlenius E, Walker DW, Miller SL and Allison BJ: Does fetal growth
restriction induce neuropathology within the developing brainstem?
J Physiol. 601:4667–4689. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawn JE, Ohuma EO, Bradley E, Idueta LS,
Hazel E, Okwaraji YB, Erchick DJ, Yargawa J, Katz J, Lee ACC, et
al: Small babies, big risks: Global estimates of prevalence and
mortality for vulnerable newborns to accelerate change and improve
counting. Lancet. 401:1707–1719. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jardine J, Walker K, Gurol-Urganci I,
Webster K, Muller P, Hawdon J, Khalil A, Harris T and van der
Meulen J; National Maternity Perinatal Audit Project Team: Adverse
pregnancy outcomes attributable to socioeconomic and ethnic
inequalities in England: A national cohort study. Lancet.
398:1905–1912. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reynolds LP, Borowicz PP, Caton JS, Crouse
MS, Dahlen CR and Ward AK: Developmental programming of fetal
growth and development. Vet Clin North Am Food Anim Pract.
35:229–247. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hendrix MLE, Bons JA, Alers NO,
Severens-Rijvers CAH, Spaanderman MEA and Al-Nasiry S: Maternal
vascular malformation in the placenta is an indicator for fetal
growth restriction irrespective of neonatal birthweight. Placenta.
87:8–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zur RL, Kingdom JC, Parks WT and Hobson
SR: The placental basis of fetal growth restriction. Obstet Gynecol
Clin North Am. 47:81–98. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Agostin M, Di Sipio Morgia C, Vento G
and Nobile S: Long-term implications of fetal growth restriction.
World J Clin Cases. 11:2855–2863. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez M, Robbins ME, Revhaug C and
Saugstad OD: Oxygen radical disease in the newborn, revisited:
Oxidative stress and disease in the newborn period. Free Radic Biol
Med. 142:61–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joo EH, Kim YR, Kim N, Jung JE, Han SH and
Cho HY: Effect of endogenic and exogenic oxidative stress triggers
on adverse pregnancy outcomes: Preeclampsia, fetal growth
restriction, gestational diabetes mellitus and preterm birth. Int J
Mol Sci. 22:101222021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee C, Zeng J, Drew BG, Sallam T,
Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R
and Cohen P: The mitochondrial-derived peptide MOTS-c promotes
metabolic homeostasis and reduces obesity and insulin resistance.
Cell Metab. 21:443–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim KH, Son JM, Benayoun BA and Lee C: The
mitochondrial-encoded peptide MOTS-c translocates to the nucleus to
regulate nuclear gene expression in response to metabolic stress.
Cell Metab. 28:516–524.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen C, Wang J, Feng M, Peng J, Du X, Chu
H and Chen X: The mitochondrial-derived peptide MOTS-c attenuates
oxidative stress injury and the inflammatory response of H9c2 cells
through the Nrf2/ARE and NF-κB pathways. Cardiovasc Eng Technol.
13:651–661. 2022. View Article : Google Scholar
|
|
13
|
Xiao J, Zhang Q, Shan Y, Ye F, Zhang X,
Cheng J, Wang X, Zhao Y, Dan G, Chen M and Sai Y: The
mitochondrial-derived peptide (MOTS-c) interacted with Nrf2 to
defend the antioxidant system to protect dopaminergic neurons
against rotenone exposure. Mol Neurobiol. 60:5915–5930. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SJ, Miller B, Kumagai H, Silverstein
AR, Flores M and Yen K: Mitochondrial-derived peptides in aging and
age-related diseases. Geroscience. 43:1113–1121. 2021. View Article : Google Scholar :
|
|
15
|
Li Y, Li Z, Ren Y, Lei Y, Yang S, Shi Y,
Peng H, Yang W, Guo T, Yu Y and Xiong Y: Mitochondrial-derived
peptides in cardiovascular disease: Novel insights and therapeutic
opportunities. J Adv Res. 64:99–115. 2024. View Article : Google Scholar :
|
|
16
|
Wu N, Shen C, Wang J, Chen X and Zhong P:
MOTS-c peptide attenuated diabetic cardiomyopathy in STZ-induced
type 1 diabetic mouse model. Cardiovasc Drugs Ther. 39:491–498.
2025. View Article : Google Scholar
|
|
17
|
Yang M, Chen W, He L, Wang X, Liu D, Xiao
L and Sun L: The role of mitokines in diabetic nephropathy. Curr
Med Chem. 32:1276–1287. 2025. View Article : Google Scholar
|
|
18
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K and Hatayama I: An
Nrf2/small Maf heterodimer mediates the induction of phase II
detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ulasov AV, Rosenkranz AA, Georgiev GP and
Sobolev AS: Nrf2/Keap1/ARE signaling: Towards specific regulation.
Life Sci. 291:1201112022. View Article : Google Scholar
|
|
20
|
Fasipe B, Li S and Laher I: Exercise and
vascular function in sedentary lifestyles in humans. Pflugers Arch.
475:845–856. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang DD, Xu B, Sun JJ, Sui M, Li SP, Chen
YJ, Zhang YL, Wu JB, Teng SY, Pang QF and Hu CX: MOTS-c mimics
remote ischemic preconditioning in protecting against lung
ischemia-reperfusion injury by alleviating endothelial barrier
dysfunction. Free Radic Biol Med. 229:127–138. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Huang J, Zhang Y, Jiang F, Li S,
He S, Sun J, Chen D, Tong Y, Pang Q and Wu Y: The
mitochondrial-derived peptide MOTS-c alleviates radiation
pneumonitis via an Nrf2-dependent mechanism. Antioxidants (Basel).
13:6132024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamberto F, Peral-Sanchez I, Muenthaisong
S, Zana M, Willaime-Morawek S and Dinnyés A: Environmental
alterations during embryonic development: Studying the impact of
stressors on pluripotent stem cell-derived cardiomyocytes. Genes
(Basel). 12:15642021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kweider N, Huppertz B, Rath W, Lambertz J,
Caspers R, ElMoursi M, Pecks U, Kadyrov M, Fragoulis A, Pufe T and
Wruck CJ: The effects of Nrf2 deletion on placental morphology and
exchange capacity in the mouse. J Matern Fetal Neonatal Med.
30:2068–2073. 2017. View Article : Google Scholar
|
|
25
|
The American Association for:
Accreditation of Laboratory Animal Care. JAMA. 207:17071969.
View Article : Google Scholar
|
|
26
|
Wang KCW, Larcombe AN, Berry LJ, Morton
JS, Davidge ST, James AL and Noble PB: Foetal growth restriction in
mice modifies postnatal airway responsiveness in an age and
sex-dependent manner. Clin Sci (Lond). 132:273–284. 2018.
View Article : Google Scholar
|
|
27
|
Kalotas JO, Wang CJ, Noble PB and Wang
KCW: Intrauterine growth restriction promotes postnatal airway
hyperresponsiveness independent of allergic disease. Front Med
(Lausanne). 8:6743242021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Wang YY, Li SP, Zhao HM, Jiang FJ,
Wu YX, Tong Y and Pang QF: Maternal propionate supplementation
ameliorates glucose and lipid metabolic disturbance in
hypoxia-induced fetal growth restriction. Food Funct.
13:10724–10736. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen D, Man LY, Wang YY, Zhu WY, Zhao HM,
Li SP, Zhang YL, Li SC, Wu YX, Ling-Ai and Pang QF: Nrf2 deficiency
exacerbated pulmonary pyroptosis in maternal hypoxia-induced
intrauterine growth restriction offspring mice. Reprod Toxicol.
129:1086712024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu H, Tang S, Xue C, Liu Y, Wang J, Zhang
W, Luo W and Chen J: Mitochondrial-derived peptide MOTS-c increases
adipose thermogenic activation to promote cold adaptation. Int J
Mol Sci. 20:24562019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang M, Su Q, Duan Y, Fu Y, Liang M, Pan
Y, Yuan J, Wang M, Pang X, Ma J, et al: The role of MOTS-c-mediated
antioxidant defense in aerobic exercise alleviating diabetic
myocardial injury. Sci Rep. 13:197812023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, LoBue A, Heuser SK, Li J, Engelhardt
E, Papapetropoulos A, Patel HH, Lilley E, Ferdinandy P, Schulz R
and Cortese-Krott MM: Best practices for blood collection and
anaesthesia in mice: Selection, application and reporting. Br J
Pharmacol. 182:2337–2353. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohamed AS, Hosney M, Bassiony H,
Hassanein SS, Soliman AM, Fahmy SR and Gaafar K: Sodium
pentobarbital dosages for exsanguination affect biochemical,
molecular and histological measurements in rats. Sci Rep.
10:3782020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen D, Qiu YB, Gao ZQ, Wu YX, Wan BB, Liu
G, Chen JL, Zhou Q, Yu RQ and Pang QF: Sodium propionate attenuates
the lipopolysaccharide-induced epithelial-mesenchymal transition
via the PI3K/Akt/mTOR signaling pathway. J Agric Food Chem.
68:6554–6563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Y, Liu C, Li R, Zheng M, Feng B, Gao
J, Long X, Li L, Li S, Zuo X and Li Y: Lactobacillus-derived
indole-3-lactic acid ameliorates colitis in cesarean-born offspring
via activation of aryl hydrocarbon receptor. iScience.
26:1082792023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Wu Q, Wei J, Hu J and Zheng S:
Effects of aspirin, vitamin D3, and progesterone on pregnancy
outcomes in an autoimmune recurrent spontaneous abortion model.
Braz J Med Biol Res. 54:e95702021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coin I, Beyermann M and Bienert M:
Solid-phase peptide synthesis: From standard procedures to the
synthesis of difficult sequences. Nat Protoc. 2:3247–3256. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia Y, Wang Q, Liang M and Huang K: KPNA2
promotes angiogenesis by regulating STAT3 phosphorylation. J Transl
Med. 20:6272022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Gao ZQ, Wang YY, Wan BB, Liu G,
Chen JL, Wu YX, Zhou Q, Jiang SY, Yu RQ, et al: Sodium propionate
enhances Nrf2-mediated protective defense against oxidative stress
and inflammation in lipopolysaccharide-induced neonatal mice. J
Inflamm Res. 14:803–816. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
41
|
Pérez-Gutiérrez L and Ferrara N: Biology
and therapeutic targeting of vascular endothelial growth factor A.
Nat Rev Mol Cell Biol. 24:816–834. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vornic I, Buciu V, Furau CG, Gaje PN,
Ceausu RA, Dumitru CS, Barb AC, Novacescu D, Cumpanas AA, Latcu SC,
et al: Oxidative stress and placental pathogenesis: A contemporary
overview of potential biomarkers and emerging therapeutics. Int J
Mol Sci. 25:121952024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Knöfler M, Haider S, Saleh L, Pollheimer
J, Gamage TKJB and James J: Human placenta and trophoblast
development: Key molecular mechanisms and model systems. Cell Mol
Life Sci. 76:3479–3496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murray AJ: Oxygen delivery and
fetal-placental growth: Beyond a question of supply and demand?
Placenta. 33(Suppl 2): e16–e22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ducsay CA, Goyal R, Pearce WJ, Wilson S,
Hu XQ and Zhang L: Gestational hypoxia and developmental
plasticity. Physiol Rev. 98:1241–1334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su EJ, Xin H, Yin P, Dyson M, Coon J,
Farrow KN, Mestan KK and Ernst LM: Impaired fetoplacental
angiogenesis in growth-restricted fetuses with abnormal umbilical
artery doppler velocimetry is mediated by aryl hydrocarbon receptor
nuclear translocator (ARNT). J Clin Endocrinol Metab. 100:E30–E40.
2015. View Article : Google Scholar :
|
|
47
|
Carr DJ, David AL, Aitken RP, Milne JS,
Borowicz PP, Wallace JM and Redmer DA: Placental vascularity and
markers of angiogenesis in relation to prenatal growth status in
overnourished adolescent ewes. Placenta. 46:79–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu C, Yang Y, Deng M, Yang L, Shu G, Jiang
Q, Zhang S, Li X, Yin Y, Tan C and Wu G: Placentae for low birth
weight piglets are vulnerable to oxidative stress, mitochondrial
dysfunction, and impaired angiogenesis. Oxid Med Cell Longev.
2020:87154122020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu C, Wu Z, Huang Z, Hao X, Wang S, Deng
J, Yin Y and Tan C: Nox2 impairs VEGF-A-induced angiogenesis in
placenta via mitochondrial ROS-STAT3 pathway. Redox Biol.
45:1020512021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu Z, Nie J, Wu D, Huang S, Chen J, Liang
H, Hao X, Feng L, Luo H and Tan C: Dietary adenosine
supplementation improves placental angiogenesis in IUGR piglets by
up-regulating adenosine A2a receptor. Anim Nutr. 13:282–288. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lanng MB, Møller CB, Andersen ASH,
Pálsdóttir AA, Røge R, Østergaard LR and Jørgensen AS: Quality
assessment of Ki67 staining using cell line proliferation index and
stain intensity features. Cytometry A. 95:381–388. 2019. View Article : Google Scholar
|
|
52
|
Bullwinkel J, Baron-Lühr B, Lüdemann A,
Wohlenberg C, Gerdes J and Scholzen T: Ki-67 protein is associated
with ribosomal RNA transcription in quiescent and proliferating
cells. J Cell Physiol. 206:624–635. 2006. View Article : Google Scholar
|
|
53
|
Li C, Xiao N, Song W, Lam AHC, Liu F, Cui
X, Ye Z, Chen Y, Ren P, Cai J, et al: Chronic lung inflammation and
CK14+ basal cell proliferation induce persistent
alveolar-bronchiolization in SARS-CoV-2-infected hamsters.
EBioMedicine. 108:1053632024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Devi K, Tomar MS, Barsain M, Shrivastava A
and Moharana B: Regeneration capability of neonatal lung-derived
decellularized extracellular matrix in an emphysema model. J
Control Release. 372:234–250. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xinqiang Y, Quan C, Yuanyuan J and Hanmei
X: Protective effect of MOTS-c on acute lung injury induced by
lipopolysaccharide in mice. Int Immunopharmacol. 80:1061742020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu F, Tian FJ and Lin Y: Oxidative stress
in placenta: Health and diseases. Biomed Res Int. 2015:2932712015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Biri A, Bozkurt N, Turp A, Kavutcu M,
Himmetoglu O and Durak I: Role of oxidative stress in intrauterine
growth restriction. Gynecol Obstet Invest. 64:187–192. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hu XQ and Zhang L: Hypoxia and
Mitochondrial dysfunction in pregnancy complications. Antioxidants
(Basel). 10:4052021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kaandorp JJ, Benders MJNL, Schuit E,
Rademaker CM, Oudijk MA, Porath MM, Oetomo SB, Wouters MG, van
Elburg RM, Franssen MT, et al: Maternal allopurinol administration
during suspected fetal hypoxia: A novel neuroprotective
intervention? A multicentre randomised placebo controlled trial.
Arch Dis Child Fetal Neonatal Ed. 100:F216–F223. 2015. View Article : Google Scholar
|
|
60
|
Klumper J, Kaandorp JJ, Schuit E,
Groenendaal F, Koopman-Esseboom C, Mulder EJH, Van Bel F, Benders
MJNL, Mol BWJ, van Elburg RM, et al: Behavioral and
neurodevelopmental outcome of children after maternal allopurinol
administration during suspected fetal hypoxia: 5-year follow up of
the ALLO-trial. PLoS One. 13:e02010632018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rashid CS, Bansal A and Simmons RA:
Oxidative stress, intrauterine growth restriction, and
developmental programming of type 2 diabetes. Physiology
(Bethesda). 33:348–359. 2018.PubMed/NCBI
|
|
62
|
Shaw P and Chattopadhyay A: Nrf2-ARE
signaling in cellular protection: Mechanism of action and the
regulatory mechanisms. J Cell Physiol. 235:3119–3130. 2020.
View Article : Google Scholar
|
|
63
|
Zhou H, Wang Y, You Q and Jiang Z: Recent
progress in the development of small molecule Nrf2 activators: A
patent review (2017-present). Expert Opin Ther Pat. 30:209–225.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Acar N, Soylu H, Edizer I, Ozbey O, Er H,
Akkoyunlu G, Gemici B and Ustunel I: Expression of nuclear factor
erythroid 2-related factor 2 (Nrf2) and peroxiredoxin 6 (Prdx6)
proteins in healthy and pathologic placentas of human and rat. Acta
Histochem. 116:1289–1300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen S, Yin Q, Hu H, Chen Q, Huang Q and
Zhong M: AOPPs induce HTR-8/SVneo cell apoptosis by downregulating
the Nrf-2/ARE/HO-1 anti-oxidative pathway: Potential implications
for preeclampsia. Placenta. 112:1–8. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao Y, Wei X, Wei P, Lu H, Zhong L, Tan J,
Liu H and Liu Z: MOTS-c functionally prevents metabolic disorders.
Metabolites. 13:1252023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Knoop A, Thomas A and Thevis M:
Development of a mass spectrometry based detection method for the
mitochondrion-derived peptide MOTS-c in plasma samples for doping
control purposes. Rapid Commun Mass Spectrom. 33:371–380. 2019.
View Article : Google Scholar
|