Glioblastoma (GBM) stands as the most common
malignant primary brain tumor type affecting the adult central
nervous system (CNS), comprising 48% of all malignant CNS tumors
and 57% of gliomas. The isocitrate dehydrogenase (IDH) wild-type
subtype of GBM is designated as grade IV by the World Health
Organization due to its aggressive behavior (1-3).
Current GBM treatment typically involves a multifaceted approach,
including surgery, radiotherapy and chemotherapy, supported by
innovative physical therapies and targeted drugs. During surgical
procedures, gross total resection (GTR) employs advanced techniques
such as 5-aminolevulinic acid (5-ALA) fluorescence guidance and
intraoperative desorption electrospray ionization mass spectrometry
to precisely locate tumor boundaries (4-6).
Notably, the biological traits of GBM profoundly impact surgical
outcomes, with IDH-mutant tumors being more responsive to GTR due
to their reduced aggressiveness (7). In radiotherapy, the addition of
temozolomide (TMZ) to conventional radiation therapy notably
increases patient survival compared with single-modality treatment
(8). For elderly patients
(>70 years old), hypofractionated radiotherapy is used to
minimize toxicity (9).
Chemotherapy, predominantly with TMZ, operates by inducing
O6-guanine methylation, thereby causing DNA damage. A
phase III clinical trial combining TMZ with lomustine has shown
potential for extending patient lifespan (10,11). Innovative physical therapies such
as tumor-treating fields exhibit antitumor activity by causing
neuronal depolarization and disrupting microtubule formation during
cell division, specifically targeting rapidly proliferating tumor
cells (12,13). Although their combination with
TMZ can increase the median overall survival (mOS), these therapies
are associated with a higher rate of systemic side effects
(14). Targeted therapies,
including bevacizumab (BEV), enhance progression-free survival
(PFS), but do not improve OS and may increase adverse reactions
(15,16).
The pathological hallmarks of GBM are manifested in
three distinct aspects. Firstly, there is the widespread
infiltrative growth of tumor cells, which spread along nerve fiber
bundles and vascular spaces. Secondly, radially arranged
pseudopalisading structures develop around necrotic cores. Lastly,
a triad of vascular abnormalities is observed, including
pathological angiogenesis, abnormal endothelial cell proliferation
and intravascular thrombosis (17,18). Clinical studies have shown that
patients with GBM often exhibit a hypercoagulable state, which is
strongly linked to the aggressiveness of the tumor (19-22). The matrix metalloproteinase (MMP)
family plays a key role in this process (23). MMPs are zinc-dependent
endopeptidases classified into subfamilies based on their substrate
specificity and structural features, including collagenases (MMP-1,
-8 and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3 and
-10), and membrane-bound MMPs (MMP-14, -16 and -17) (24,25). These enzymes critically regulate
tumor invasion and metastasis by mediating epithelial-mesenchymal
transition (EMT), degrading extracellular matrix (ECM) components
and promoting tumor angiogenesis (26-28). In GBM, the expression of several
MMPs, particularly MMP-2 and MMP-9, is significantly increased, and
their enhanced activity correlates with invasive tumor growth and
blood-brain barrier (BBB) disruption, highlighting the crucial role
of MMPs in disease progression (29,30).
In recent years, notable advancements have occurred
in mechanistic studies on MMPs during the invasion process of GBM
(31-33). However, two major obstacles must
be addressed before their effective clinical targeting. Firstly,
the BBB, with its complex interplay of active transport systems
(including uptake and efflux proteins) and metabolic enzymes, poses
a formidable biological barrier. This barrier effectively hinders
small molecules from reaching the brain, thus considerably
diminishing drug accumulation efficiency (34). Emerging drug delivery approaches,
utilizing nano-drug delivery systems such as liposomes (35), nanoparticles (NPs) (36) and hydrogel carriers (37), offer promise for enhancing the
targeted accumulation of antitumor agents in the brain.
Additionally, the multifactorial mechanisms underlying TMZ
resistance involve not only DNA damage repair mediated by
O6-methylguanine-DNA methyltransferase (MGMT)
overexpression and drug efflux transporter activation (38,39), but also mismatch repair defects
(40), glioma stem cell (GSC)
self-renewal (41) and aberrant
cell signaling pathways (42).
Breakthroughs in synthesizing natural medicines and novel compounds
have presented innovative strategies to tackle drug resistance
(43,44), thereby expanding GBM treatment
options and paving the way for targeted therapies.
The present review comprehensively examines the
regulatory mechanisms of the MMP family in GBM invasion, focusing
on recent developments. It further explores recent progress in
therapeutic approaches, including natural bioactive compounds,
small molecules and nanotechnology-driven combinations. The aim of
the present study is to establish a theoretical foundation and
guide treatment innovations for GBM.
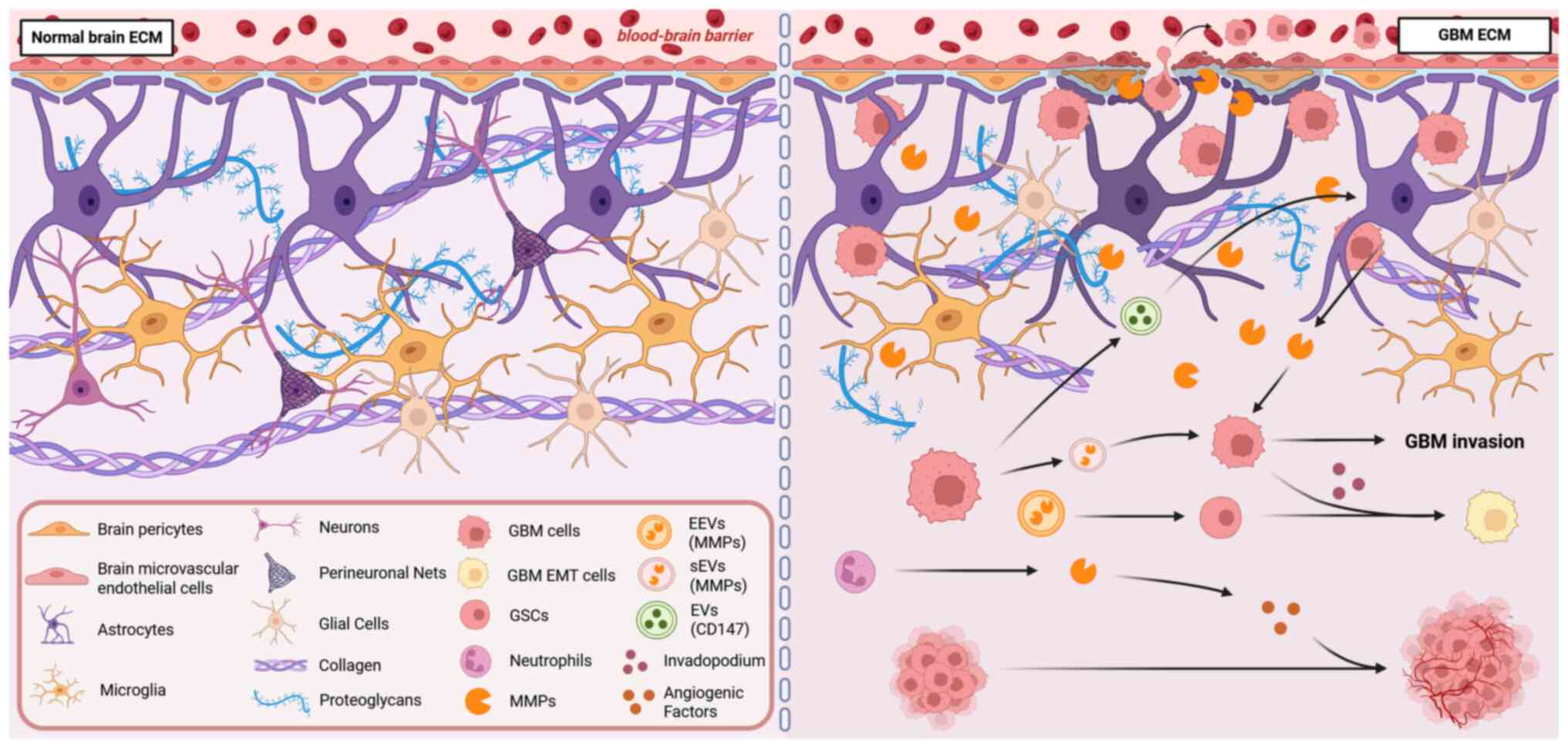
The ECM is crucial in GBM malignant invasion, a
process mediated by MMPs. In the brain, the ECM preserves the
homeostasis of the neural microenvironment via specific structures
and functions of the basilar membrane, interstitial matrix and
perineuronal nets (PNNs) (45).
However, GBM disrupts this balance by degrading the ECM and
triggering pro-invasive signals such as EMT. This occurs through
the abnormal overexpression of MMPs, including MMP-2, MMP-9 and
MMP-14, which stimulate the development of invasive pseudopodia in
tumor cells and aid in the spread of intercellular vesicles
(46-49) (Fig. 1).
The ECM in the brain plays a dual role in GBM
invasion. On one hand, GBM directly degrades ECM components by
upregulating MMPs. On the other hand, it creates a microenvironment
that promotes invasion by forming invasive pseudopodia and
releasing extracellular vesicles carrying MMPs. Specifically, the
ECM can be classified into three types based on location: i) The
basement membrane, which is located around blood vessels
(neurovascular unit), and consists of components such as collagen
and laminin, which help maintain the stability of the blood
vessel-neural interface; ii) the interstitial matrix, which is
distributed in the interstitial space between neurons and glial
cells, and forms a loose network with hyaluronic acid and
proteoglycans to support intercellular material exchange; and iii)
the PNN, which directly surrounds neuronal cell bodies and
dendrites, and is composed of a hyaluronic acid scaffold and
chondroitin sulfate proteoglycans (such as aggrecan), forming a
dense structure whose formation relies on neuronal activity
(45). In GBM, contrary to the
widespread notion that most models involve membrane-type (MT)-MMPs
activating progelatinase A, previous research has revealed that
MT-MMPs are predominantly produced by GBM cells and play a direct
role in their migration (46).
Notably, MMP-17 and MMP-25 exhibit particularly pronounced effects
in this process (46). During
GBM invasion, a marked elevation in MMP-9 levels leads to the
breakdown of the ECM. This degradation is accompanied by increased
prolidase activity, which releases metabolites such as proline.
Simultaneously, the production of proline within GBM cells serves
to further augment their invasive capabilities (47). Additional research has shown
that, from a cellular structural perspective, GBM cells have the
ability to form invasive pseudopodia equipped with matrix-degrading
functions. These cells also secrete small extracellular vesicles
(sEVs) enriched in MMP-2. These sEVs not only exhibit a close
association with pseudopod activity but can also be internalized by
adjacent GBM cells. This internalization significantly enhances the
invasive potential of the recipient cells by transferring highly
invasive pseudopod activity (48). Furthermore, vesicles released by
GBM cells can stimulate astrocytes to secrete MMP-9, thereby
further facilitating the invasion of GBM (49).
In addition, the ECM modulates tumor angiogenesis
via a dual mechanism. Firstly, it interacts with cell receptors,
activating signaling pathways such as MAPK, and thereby enhancing
endothelial cell proliferation, migration and survival (50,51). Secondly, ECM remodeling,
facilitated by proteases such as MMPs and fibrinolytic enzymes,
releases angiogenic factors, including vascular endothelial growth
factor (VEGF), transforming growth factor-β (TGF-β) and fibroblast
growth factor-2, further influencing angiogenesis (52,53). In GBM, a distinctive pathological
trait emerges where MMPs degrade the ECM, paving the way for new
tumor angiogenesis, while potentially altering the function and
structure of preexisting blood vessels (23). Notably, tumor-infiltrating
neutrophils elevate the expression of VEGFA through the secretion
of MMP-9, introducing an additional layer of angiogenesis
regulation (54). Furthermore,
previous bioinformatics analysis has uncovered a significant
association between elevated MMP-14 expression and several
angiogenesis-related signaling pathways, such as Visfatin, VEGF and
TGF-β, as well as the endothelial-mesenchymal transition process
(55). Previous research has
indicated that MMP-14 can stimulate angiogenesis in GBM (56).
EMT modulates the tumor microenvironment (TME)
through multiple mechanisms, thereby enhancing the invasiveness of
GBM. EMT augments the aggressiveness of tumor cells by inducing the
formation of actin-rich invadopodia, which are dynamic adhesive
structures capable of locally releasing MMP-mediated proteolytic
enzymes at cell-ECM contact zones, thus facilitating cellular
invasion (57). Within the TME,
endothelial cell-derived vesicles activate the NF-κB signaling
pathway within GBM stem cells (GSCs) by delivering MMPs, inducing
the transformation of pro-neural cells into a mesenchymal phenotype
and promoting the shift towards an invasive phenotype (58). Previous research has revealed an
interaction between MMP-14 and TGF-β receptor signaling in GBM.
These two factors induce the programmed activation of EMT through
the stimulation of Snail transcription factors. This synergistic
action of proteases and growth factors ultimately leads to a highly
invasive tumor phenotype (59).
MMPs are not only key molecules mediating ECM
degradation and driving tumor invasion in GBM, but their expression
and activity levels constitute critical biomarkers reflecting the
invasive potential of GBM. In GBM tissues, particularly at the
tumor invasion front and in neovascularization areas, MMP
expression is significantly higher than in normal brain tissue.
Concurrently, MMP-2 and MMP-9 expression levels have been found to
be further elevated in recurrent GBM tissues, closely correlating
with malignant biological behaviors such as tumor invasion,
dissemination and recurrence (60). Furthermore, MMPs are important in
bodily fluid tests. A prospective study showed that serum MMP-9
concentrations were significantly elevated in patients with GBM
(n=66) and correlated with tumor activity status. Serum MMP-9
levels in patients without radiological lesions were significantly
lower than in those with active disease (P=0.0002), suggesting its
potential as a serum marker for disease monitoring, although MMP-9
showed no significant correlation with OS (61). However, a subsequent larger
prospective study (n=192) challenged this view. That study, through
systematic analysis of serum samples, found that, although MMP-9 is
highly expressed in GBM tissue, its serum level did not correlate
significantly with radiological disease status (P=0.33), indicating
that circulating MMP-9 cannot reliably reflect local GBM
progression. While a longitudinal increase in serum MMP-9 showed a
weak correlation with shorter survival [hazard ratio (HR)=1.1 per
doubling; P=0.04], multivariate analysis revealed that it was not
an independent prognostic factor (P=0.11). These results further
suggest the limited clinical value of serum MMP-9 as a dynamic
monitoring or independent prognostic biomarker for GBM (62). Notably, compared with serum,
continuous monitoring in patients with recurrent GBM (n=4)
undergoing a specific biochemotherapy regimen (irinotecan,
thalidomide and doxycycline) revealed that MMP-9 levels in the
cerebrospinal fluid (CSF) significantly and continuously increased
over the treatment period (P=0.001), and this elevation preceded
magnetic resonance imaging (MRI) detection of tumor progression
signs, suggesting that CSF-derived MMP-9 could serve as an early
biomarker for GBM recurrence or progression (63).
MMPs as biomarkers can also assess treatment
response to GBM drugs. A previous prospective-retrospective
dual-cohort analysis found that, in patients with recurrent GBM,
the group with high baseline plasma MMP-2 levels, when subjected to
the anti-angiogenic drug BEV, exhibited a significantly improved
objective response rate (80 vs. 17.6%), median PFS (7.1 vs. 4.2
months) and mOS (12.8 vs. 5.9 months) compared with the low-level
group. Crucially, this predictive value was only evident in the BEV
treatment group and disappeared in the cytotoxic drug-only
treatment group, suggesting MMP-2 is a predictive biomarker
specific to BEV efficacy (64).
In addition, a retrospective analysis of the large phase III
AVAglio trial (NCT00943826) confirmed that baseline plasma MMP-9
levels could predict survival benefit from BEV in patients with
newly diagnosed GBM. Patients in the low MMP-9 group had a
significantly prolonged OS by 5.2 months (HR=0.51; P=0.0009),
whereas the high MMP-9 group showed no significant benefit
(54). Additionally,
multi-cohort transcriptome analysis revealed that low tumor tissue
MMP-9 mRNA expression was not only associated with longer OS
(P=0.0012) and PFS (P=0.0066), but also significantly predicted the
degree of survival benefit that patients received from standard TMZ
chemoradiotherapy, whereas the high MMP-9 group had limited
benefit, suggesting that tissue MMP-9 expression is a potential
predictive biomarker of TMZ efficacy (65).
In summary, the localized expression of MMPs in
tissues, their dynamic changes in bodily fluids and their value as
predictors of treatment response provide crucial molecular basis
for assessing GBM invasiveness, predicting patient prognosis,
real-time monitoring of disease status and guiding individualized
treatment strategies. Although the value of serum MMP-9 as an
independent monitoring and prognostic biomarker has been questioned
by large-sample studies, highlighting the need for careful
consideration of source specificity in its clinical application,
the overall central role of MMPs as core biomarkers in disease
progression and treatment prediction remains solid. Given the core
driving role of MMPs in the pathological process of GBM and their
biomarker value, untangling their complex regulatory networks is an
indispensable foundation for developing novel and effective
targeted intervention strategies.
Multiple signaling pathways, including PI3K/AKT,
MAPK, TGF-β and Wnt/β-catenin, play a crucial role in regulating
the expression and activity of MMP-2, MMP-9 and other MMPs
(66-69). Non-coding RNAs also participate
in the regulation of MMP expression (70,71). Additionally, epigenetic
mechanisms contribute to the modulation of MMP expression (72). At the same time, metabolic
reprogramming and alterations in the TME collectively form multiple
factors influencing MMP function (33,73-75). This diverse range of mechanisms
lays a theoretical groundwork for deciphering the invasive
processes of GBM, and pinpoints prospective targets for therapeutic
intervention (Table SI).
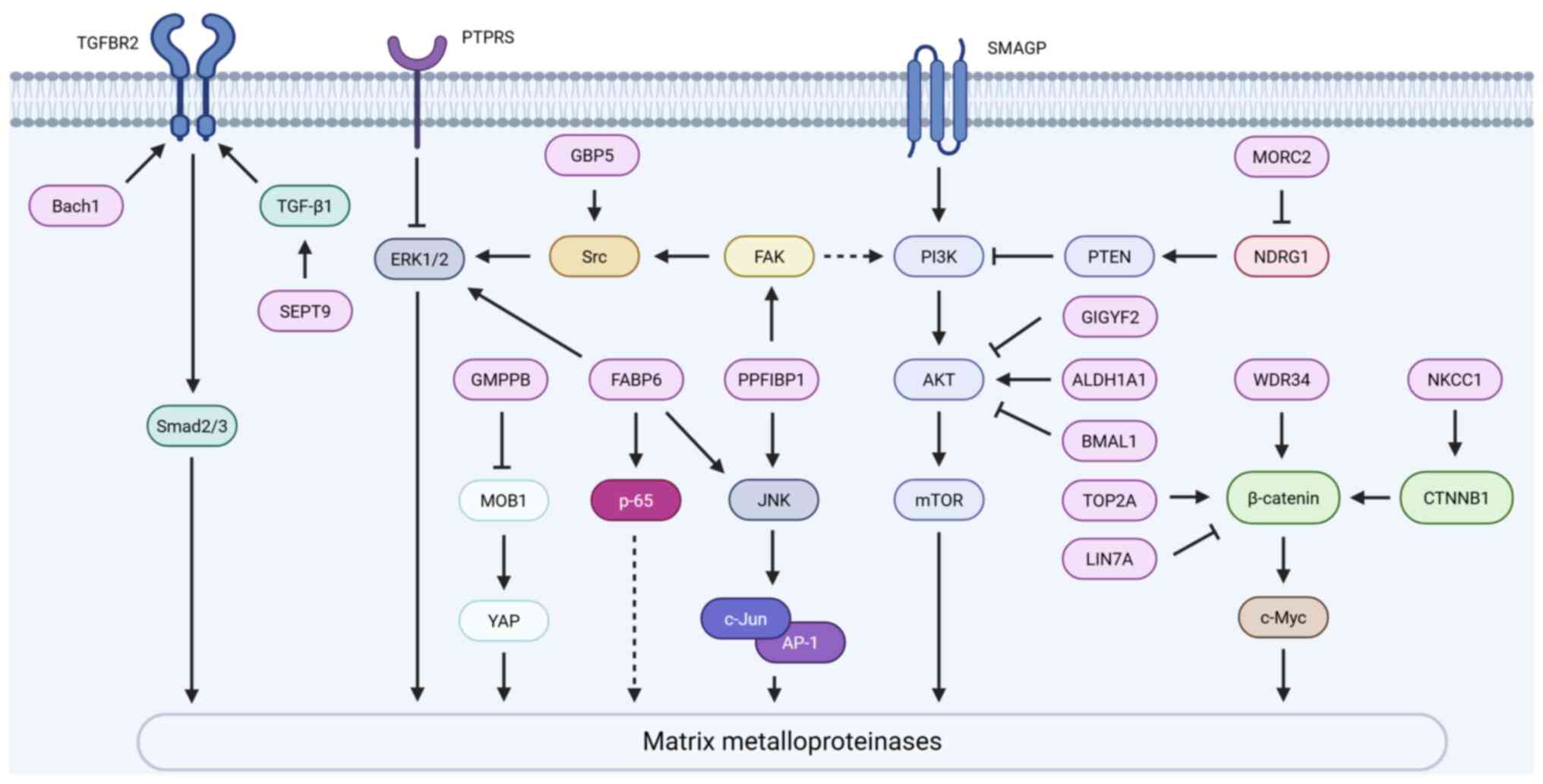
Aberrant expression of MMPs in GBM is controlled by
several signaling pathways that considerably interact (Fig. 2). First, the PI3K/AKT signaling
pathway is permanently activated and plays a critical role in GBM
(76), significantly driving the
upregulation of MMP-2 and MMP-9 expression. Aldehyde dehydrogenase
(ALDH)1A1 activates this pathway by specifically enhancing the
phosphorylation of the AKT protein at the Ser473 and Thr308
residues, thereby promoting the expression of MMP-2 and MMP-9, and
driving the invasiveness of GBM cells (77). This effect can be synergistically
amplified by small transmembrane glycoprotein (78). Notably, the micro-orchidism
family CW-type zinc finger protein 2 (MORC2), which exhibits high
expression in GBM cells, binds to and inhibits the transcription of
N-Myc downstream regulated gene 1 (NDRG1), leading to the
downregulation of phosphatase and tensin homolog (PTEN) expression.
This subsequently relieves the inhibition of the PI3K/AKT signaling
pathway, ultimately inducing the upregulation of MMP-2 and MMP-9,
and enhancing tumor invasion and migration. Conversely, knocking
down MORC2 or overexpressing NDRG1 can reverse this signaling
pathway and inhibit tumor progression (66). It is noteworthy that GRB10
interacting GYF protein 2 and brain and muscle ARNT-like protein 1
inhibit AKT phosphorylation, thereby downregulating MMP-9
expression and impairing the invasive capacity of GBM cells,
untangling the complexity of PI3K/AKT pathway regulation of MMPs
(79,80). WD repeat domain 34 in GBM
inhibits PTEN while activating both the PI3K/AKT and Wnt/β-catenin
pathways, thus significantly increasing the levels of
phosphorylated (p)-AKT, nuclear β-catenin and c-Myc, and
consequently upregulating MMP-2 and MMP-9 expression and promoting
cell invasion (81).
Regarding the Wnt/β-catenin pathway, DNA
topoisomerase IIα directly binds to the β-catenin promoter to
enhance its transcription, thus promoting β-catenin protein
expression and its nuclear accumulation, alongside a significant
increase in MMP-2 and MMP-9 mRNA levels and enzymatic activity
(67,82). By contrast, Lin-7 homolog A
inhibits the nuclear translocation of β-catenin, thereby
suppressing the expression of MMP-2 and MMP-9 as well as their
pro-invasive functions (83).
The activation of this pathway is also associated with the
sodium-potassium-chloride cotransporter 1 (NKCC1). High expression
of NKCC1 in GBM (its expression level significantly correlates with
tumor grade, P<0.05), when knocked down, leads to reduced
protein levels of the key Wnt/β-catenin pathway effector β-catenin,
which is accompanied by downregulation of MMP-2 and MMP-9, thus
significantly impairing tumor cell invasion. This indicates that
NKCC1 regulates MMP expression via the Wnt/β-catenin signaling
pathway, thereby affecting cell invasion (84).
The TGF-β signaling pathway is also an important
mechanism for regulating MMPs. Overexpression of broad-complex,
tramtrack, and Bric-à-brac domain and cap 'N' collar homolog 1
significantly upregulates TGF-β receptor 2 (TGFBR2) and its
downstream mothers against decapentaplegic homolog (Smad)2/3
protein levels, and enhances MMP-2 protein expression and secretion
activity (68). Further research
revealed that Septin 9 (SEPT9) was highly expressed in GBM tissues,
and positively correlated with TGF-β1. Knocking down SEPT9 led to a
significant reduction in MMP-9 protein expression, thereby
inhibiting GBM cell invasion. Previous in vivo experiments
further confirmed that targeted inhibition of SEPT9 effectively
reduced lung metastasis in GBM, thus highlighting the crucial role
of SEPT9 in promoting GBM distant metastasis by upregulating MMP-9
(85).
In addition to the aforementioned key pathways, the
MAPK (including the ERK and JNK branches) and NF-κB signaling
pathways are also involved in the regulation of MMPs (69,86). Receptor-type tyrosine-protein
phosphatase S expression is downregulated in GBM tissues, and its
loss leads to increased phosphorylation levels of ERK1/2,
subsequently upregulating the transcription and protein expression
of MMP-2 and MMP-3, thus promoting cell invasion (87). Fatty acid-binding protein 6
(FABP6) was shown to be highly expressed in GBM tissues; its
knockdown not only led to significant downregulation of MMP-2, but
also reduced the activation levels of p-ERK, p-JNK and p-p65
(NF-κB), ultimately inhibiting tumor cell invasion. This suggests
that FABP6 may influence MMP-2 and consequently GBM invasion by
regulating the ERK/JNK/NF-κB signaling axis (69). PPFIA binding protein 1 expression
was revealed to be positively associated with GBM progression. It
enhanced the phosphorylation levels of FAK (Y397), Src (Y416), JNK
and c-Jun, significantly upregulated MMP-2 expression, and enhanced
tumor infiltration within the brain parenchyma (86,88-90). Human guanylate-binding protein 5
was demonstrated to enhance MMP-3 expression activity by promoting
the phosphorylation of Src and ERK1/2 (91). Furthermore, GDP-mannose
pyrophosphorylase B was shown to be highly expressed in GBM, and
its knockdown activated the Hippo signaling pathway, and promoted
the phosphorylation of Mps one binder kinase activator-like 1 and
Yes-associated protein, thereby inhibiting MMP-3 expression and
impairing cell invasion ability (32).
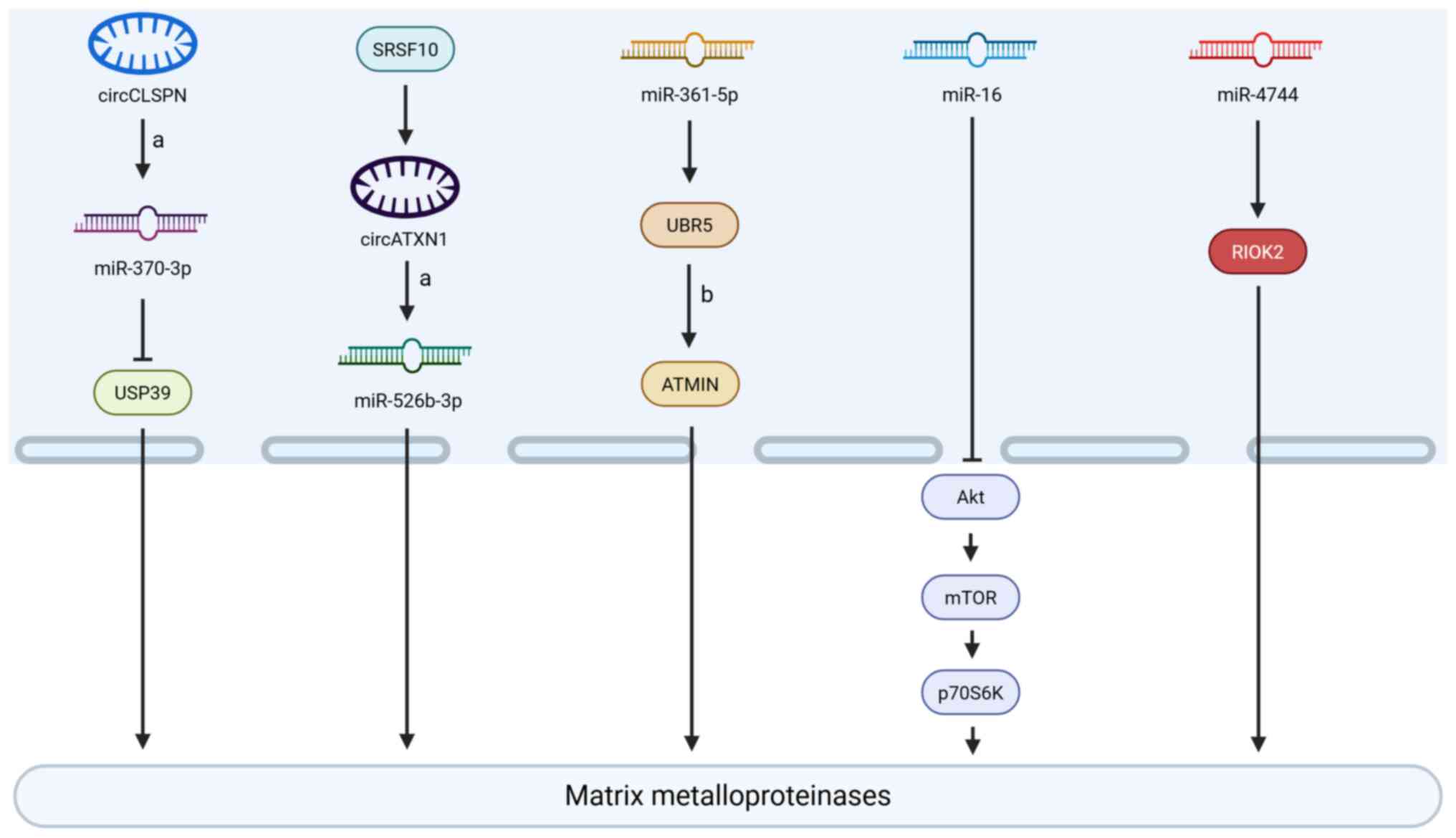
Non-coding RNAs modulate the invasive capacity of
GBM cells by interactively regulating the expression levels of
MMP-2 and MMP-9 (Fig. 3). Among
them, an endogenous circular (circ) RNA derived from exons 11 to 14
of the CLSPN gene (Homo sapiens_circ_0011591, circCLSPN) was
shown to function as a competitive endogenous RNA by sequestering
microRNA (miRNA or miR)-370-3p, thus releasing ubiquitin-specific
peptidase 39 from miRNA-mediated repression, and resulting in
marked upregulation of MMP-2 and MMP-9 expression (70,92). Concurrently, circATXN1, mediated
by serine/arginine-rich splicing factor 10, promoted MMP-2
expression, and enhanced the invasive potential of GBM cells by
binding to miR-526b-3p and blocking its inhibitory effect on
downstream target genes (93).
Both miR-361-5p and miR-16, which are significantly downregulated
in GBM, have been demonstrated to possess tumor
invasion-suppressive potential. Overexpression of miR-361-5p was
shown to directly target the ubiquitin protein ligase E3 component
N-recognin 5 (UBR5), thus inhibiting the UBR5-mediated
ubiquitination and degradation of ATM-interacting protein (ATMIN),
thereby stabilizing ATMIN protein and downregulating MMP-2
expression, ultimately blocking GBM cell migration and invasion
(71). In addition, miR-16
overexpression was demonstrated to suppress the phosphorylation of
key nodes in the PI3K/AKT/mTOR signaling pathway [namely, AKT
(Ser473), mTOR (Ser2448) and p70S6K (Thr389)], leading to
downregulation of MMP-2 and MMP-9 expression, and thereby
inhibiting GBM cell migration and invasion (94). Additionally, right open reading
frame kinase 2 (RIOK2), a member of the RIO kinase family that is
highly expressed in GBM, was shown to enhance cell migration and
invasion by upregulating MMP-2 and MMP-9. By contrast, miR-4744
could directly target and suppress RIOK2 expression, effectively
reversing its pro-invasive effects (95).
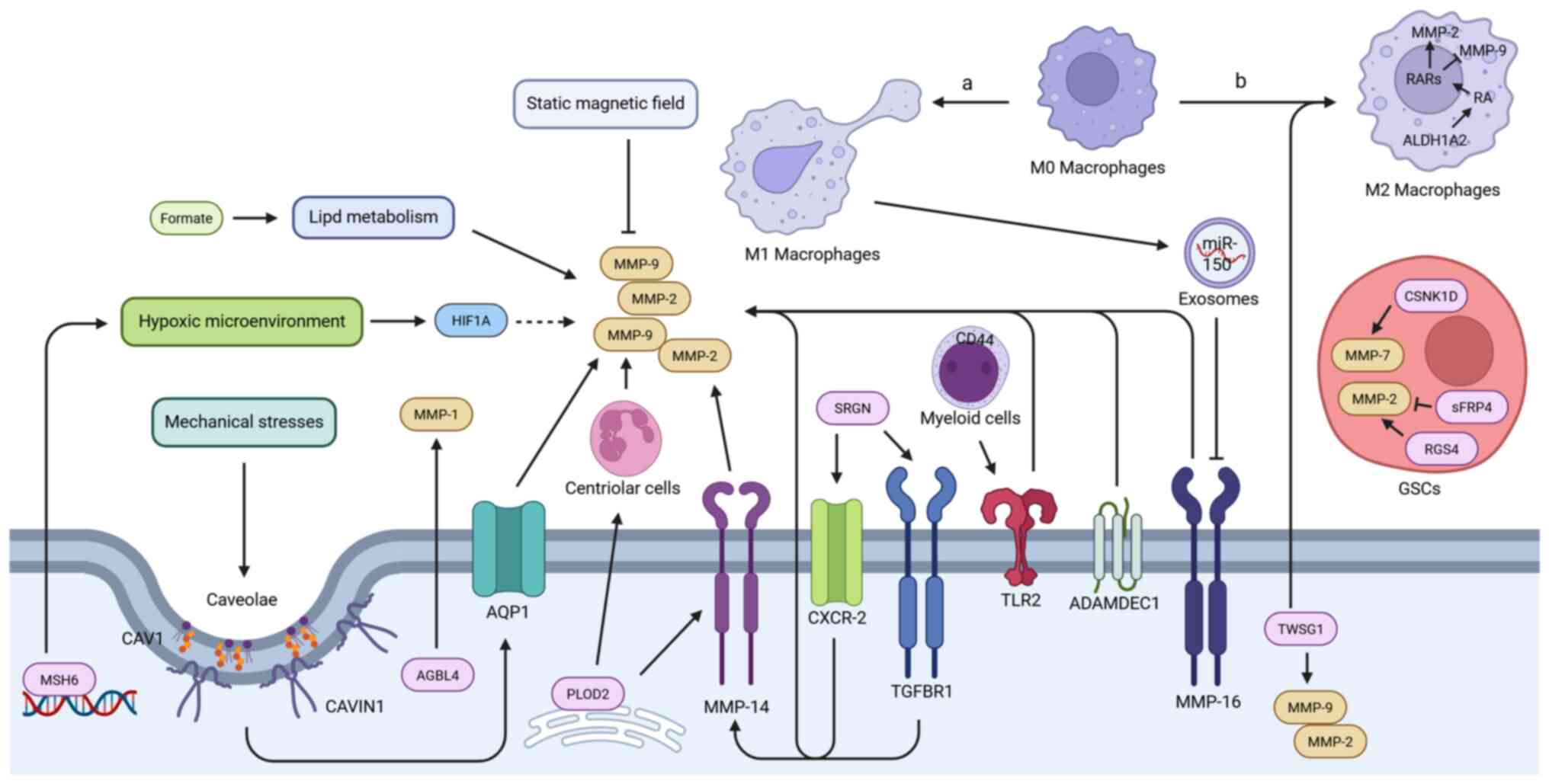
GBM invasion involves complex interactions within
the TME that modulate the expression and activity of MMPs. This
occurs through metabolic reprogramming, physical stimuli and immune
modulation (Fig. 4).
Metabolically, formate treatment was shown to significantly
activate the expression of MMP-2 and MMP-9 in GBM cells, and
enhanced their invasive capacity by reprogramming lipid metabolism.
(U-13C)glucose/glutamine stable isotope tracing and
lipidomics analysis confirmed that formate drives fatty acid
synthesis and cytosolic lipid accumulation. Fatty acid synthase
inhibitor could block this metabolic reprogramming process, and
effectively suppress formate-induced MMP-2 expression and invasion
(73,96). Concurrently, serglycin enhanced
the expression of MMP-9 and MMP-14 by activating the TGFBR1 and
C-X-C motif chemokine receptor 2 (CXCR-2) signaling axes,
contributing to the pro-invasive effect in GBM (97).
Physical environmental factors are also crucial.
Mechanical stress stimuli such as high osmotic pressure (440
mOsmol/kg) or hydrostatic pressure (30 mmHg) can significantly
upregulate the mRNA expression and secretion levels of MMP-2 and
MMP-9 in GBM cells, as well as enhance their invasive ability
(74). Previous research
indicated that such pressure stimuli upregulated the expression of
caveolin-1 (CAV1) and caveolae-associated protein 1 (CAVIN1),
promote caveolae formation, and thereby induce MMP-2 and MMP-9
expression and invasion. This process may be related to
CAV1/CAVIN1-mediated induction of aquaporin-1 (AQP1). The Cancer
Genome Atlas analysis shows that co-high expression of CAV1/AQP1
predicts poor prognosis (98,99). Notably, AQP1 has been
demonstrated to directly mediate pro-MMP-9 activation (100). Conversely, static magnetic
field (1,000±100 Gs) treatment was shown to significantly
downregulate MMP-2 protein expression in GBM cells. When combined
with TGF-β1, the inhibitory effect on MMP-2 and the accompanying
reduction in invasive capacity were more pronounced (101). A hypoxic microenvironment,
which is a hallmark feature of GBM, influences MMP regulation. The
high expression of MutS protein homolog 6 in GBM exacerbated the
accumulation of hypoxia-inducible factor 1-α (HIF1α) protein
induced by hypoxia, thereby significantly enhancing the invasive
capacity mediated by MMP-2 and MMP-9 (102). Furthermore, twisted
gastrulation BMP signaling modulator 1 was revealed to be highly
expressed in GBM. Its knockdown not only significantly inhibited
tumor cell migration and invasion, and downregulated MMP-2 and
MMP-9, but also remodeled the phenotype of tumor-associated
macrophages, reducing M2-type markers [such as CD206, arginase 1,
interleukin (IL)-10 and TGF-β] and upregulating M1-type markers
(such as inducible nitic oxide synthase and IL-1β) in co-cultured
THP-1 cells (103-106).
Intrinsic factors within tumor cells are also
important for regulating MMPs in the microenvironment. ATP/GTP
binding protein like 4 (AGBL4), which is highly expressed in
recurrent GBM tissues, has been shown to drive tumor migration by
specifically upregulating MMP-1 expression; in vivo
experiments further revealed that inhibiting AGBL4 delays
intracranial tumor progression and prolongs the survival of
tumor-bearing animals, an effect that can be partially reversed by
high MMP-1 expression (31).
ADAM-like Decysin 1 expression level in GBM was shown to be
significantly associated with tumor malignancy and poor prognosis,
and it promoted tumor cell invasion by upregulating MMP-2
expression (107).
Notably, the stemness and invasiveness of GSCs in
the microenvironment are regulated by specific proteins and MMPs.
Elevated expression of casein kinase 1D (CSNK1D) in GBM tissues was
demonstrated to promote the upregulation of GSC stemness markers
[CD133 and SRY-box transcription factor 2 (SOX2)] as well as MMP2
and MMP-7 expression upon its overexpression, enhancing cell
invasion. By contrast, knockdown of CSNK1D inhibited stemness and
invasion, and prolonged survival in model mice (75). Similarly, regulator of G protein
signaling 4 knock out also significantly downregulated MMP2
expression in GSCs and inhibited invasion (108). Conversely, secreted frizzled
related protein 4 utilized its N-terminal cysteine-rich domain and
C-terminal cysteine-rich domain to effectively inhibit MMP-2
activity in GSCs, suggesting a potential negative regulatory
mechanism (109).
The regulation of MMP expression by myeloid cells in
the microenvironment also affects tumor invasion, with macrophages
of different polarization states exhibiting distinct functions. M1
macrophages were shown to directly inhibit MMP-16 expression in
tumor cells by releasing exosome-encapsulated miR-150 (33). Given that MMP-16 could upregulate
MMP-2, this inhibition indirectly reduced MMP-2 expression levels
(110). By contrast, M2
macrophages rely on retinoic acid (RA) produced by ALDH1A2
catalysis to selectively upregulate MMP-2 while downregulating
MMP-9 expression, but this regulation does not directly affect the
protease activity of the tumor cells themselves (111). It is noteworthy that the
regulation of MMPs by myeloid cells is influenced by tumor cells.
On one hand, lysyl hydroxylase 2 expressed by GBM cells not only
promoted the release of active MMP-2 by tumor cells via
upregulating MMP-14, but also regulated secreted factors to
activate neutrophils to release MMP-9, thereby synergistically
increasing microenvironmental MMP levels and enhancing invasion
(112). On the other hand, GBM
cells could induce wild-type myeloid cells to significantly
upregulate MMP-9 mRNA expression upon Toll-like receptor 2 (TLR2)
agonist stimulation. This response was shown to be markedly
attenuated in CD44-deficient myeloid cells, which also lost their
ability to promote GBM cell invasion in Boyden chamber co-culture
models, confirming that CD44 is a key molecule mediating the
pro-invasive function of myeloid cells, partly through MMP-9
(113).
From natural products to small-molecule compounds,
both can effectively regulate the expression and activity of MMP-2,
MMP-9, MMP-1, MMP-3 and MMP-14 (114-118). Concurrently, the development of
combination therapy strategies and nanomaterial-based drug delivery
systems has provided new approaches for precise and controllable
drug release, thereby further enhancing therapeutic efficacy
(119-121). These diverse and multi-target
intervention techniques collectively present expansive
opportunities for halting the progression of GBM.
Natural active products modulate MMPs through
multi-target mechanisms, presenting potential avenues for cancer
treatment. Curcumin and its derivatives, as well as the
structurally related natural sesquiterpene Zerumbone, have been
demonstrated to effectively inhibit the invasion and migration of
GBM cells by targeting and suppressing the expression and activity
of MMP-2 and MMP-9. In an ex vivo stress model induced by
norepinephrine (NE), curcumin significantly downregulated the
expression and secretion of CD147 and its downstream effector
molecules MMP-2 and MMP-9 by inhibiting ERK1/2 phosphorylation,
thereby blocking NE-mediated GBM cell invasion (114). Concurrently,
bisdemethoxycurcumin (BDMC) was shown to act synergistically on the
PI3K/AKT, MAPK/ERK and NF-κB signaling pathways. After treating GBM
cells with BDMC for 48 h, the protein levels of key molecules,
including PI3K, p-AKT, MEK, p-ERK1/2, NF-κB, and the downstream
MMP-2 and MMP-9, were significantly downregulated, which was
accompanied by the inhibition of GBM cell migration and invasion
(122). Notably, the natural
compound Zerumbone also exhibited significant anti-invasive
effects, inhibiting the migration and invasion of GBM cells in a
concentration- and time-dependent manner. It downregulated the
total protein levels of ERK1/2 and AKT, thereby cooperatively
inhibiting the mRNA expression, protein content and enzymatic
activity of MMP-2 and MMP-9, consequently blocking GBM cell
invasion (123). Similarly, the
turmeric extract Curzerene significantly reduced MMP-9 levels in
glioma cells by inhibiting glutathione S-transferase A4 expression
and the phosphorylation of molecules of the mTOR/p70S6K signaling
axis, thus effectively suppressing GBM migration and invasion both
ex vivo and in a nude mouse xenograft model (124). Furthermore, the photodynamic
effect of curcumin provides a novel approach for inhibiting tumor
invasion. Upon activation by 430-nm blue light irradiation for 5-10
min, curcumin induced a significant increase in intracellular
reactive oxygen species (ROS) levels in GBM cells. Flow cytometry
and immunofluorescence results confirmed that elevated ROS was
accompanied by downregulation of MMP-2 and MMP-9 expression,
indicating that blue light-activated curcumin could inhibit MMP-2
and MMP-9 via the ROS pathway, thereby attenuating the invasive
potential of GBM cells (125).
Concurrently, various natural compounds effectively
inhibit GBM invasion by influencing MMP expression or activity.
Isocucurbitacin B inhibited GBM cell proliferation, migration and
invasion in a concentration- and time-dependent manner. It reduced
the mRNA and protein expression of MMP-2 and MMP-9 by
downregulating the total protein levels and phosphorylation of
PI3K, AKT and MAPK1/3, thereby blocking GBM invasiveness (126). Also acting on the MAPK
signaling pathway, Coriolus versicolor and its active
molecule, the methyl ester of 9-KODE (AM), significantly inhibited
tumor necrosis factor (TNF)-α-induced p38 MAPK phosphorylation, and
dose-dependently reduced MMP-3 mRNA and protein levels. Invasion
assays further confirmed that AM directly impaired the invasive
capacity of GBM cells (115).
The myrrh resin extract Guggulsterone (GS) significantly reduced
the expression of MMP-2 and MMP-9 in GBM cells by activating dual
degradation pathways involving the proteasome and lysosome, thereby
inhibiting their migration and invasion. This mechanism was
experimentally verified. Both the proteasome inhibitor MG132 and
the lysosome inhibitor NH4Cl effectively reversed the
GS-induced downregulation of MMP-2 and MMP-9 and the associated
inhibition of invasion. An orthotopic xenograft model further
demonstrated that GS reduced intratumoral MMP-2 levels and
prolonged the survival of tumor-bearing mice (127). The natural sesquiterpene
lactone compound Alantolactone, extracted from the roots of
Inula helenium, specifically inhibited the activity of
Lin-11, Isl-1, and Mec-3 kinase (LIMK), inducing the
dephosphorylation and activation of its key substrate, cofilin.
This subsequently increased the ratio of monomeric actin (G-actin)
to filamentous actin (F-actin), ultimately leading to significant
downregulation of MMP-2 and MMP-9 expression, thereby effectively
inhibiting the migration and invasion of GBM cells (128). Targeting the secretion process
of MMPs, the compound erythrose from rhubarb root extract inhibited
the extracellular secretion of neuroleukin by blocking its binding
to the gp78 receptor, thereby significantly downregulating the
expression levels of MMP-1 and MMP-9 in GSCs and ultimately
inhibiting their ex vivo invasive capacity (116). Furthermore, kaempferol extract
and its biotransformation product (KPF-ABR) also exhibited
significant anti-invasive effects. Both significantly downregulated
MMP-9 expression and inhibited the tumor promoter
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced activation of
cell migration. Notably, KPF-ABR, enriched with kaempferol
aglycone, was particularly effective, notably blocking the
TPA-stimulated upregulation of MMP-9 and inhibiting neurosphere
formation by GSCs (129). Other
natural products have also been shown to exert anti-invasive
effects by inhibiting MMPs, including Bacoside A and Diosgenin,
which effectively inhibited the invasive ability of GBM cells by
downregulating MMP-2 and MMP-9 (130,131).
Beyond plant-derived components, fungi, animals and
metabolites also demonstrate potential for targeting MMPs to
inhibit GBM invasion. The fungus-derived compound
10,11-dehydrocurvularin significantly downregulated MMP-2 levels by
inhibiting PI3K-p85 expression and blocking AKT phosphorylation,
thereby suppressing GBM invasion (43). Antrodia camphorate, also a
fungus, and the quinone derivative coenzyme Q (CoQ)0 from its
fermentation broth, inhibited the invasive ability of GBM cells by
downregulating the expression levels of MMP-2 and MMP-9 (132). By contrast, the structurally
similar CoQ10 exerted its anti-invasive effect by directly
inhibiting the enzymatic activity of MMP-2 and MMP-9 (133). Additionally, the animal-derived
component bee venom and the metabolite Urolithin B have been
reported to inhibit the enzymatic activity of MMP-2 and MMP-9,
thereby reducing GBM invasiveness (134,135). Notably, melatonin, an important
endogenous hormone, has also been confirmed to effectively inhibit
the invasive capacity of GBM tumor spheroids by suppressing the
mRNA and protein expression levels of MMP-9 (136).
In summary, this section systematically described
how natural active ingredients, including curcuminoids, fungal and
animal metabolites, significantly inhibit the invasive ability of
GBM by targeting key signaling pathways and proteins such as
PI3K/AKT, MAPK and NF-κB (Table
SII), thereby regulating MMP gene expression, protein secretion
and enzymatic activity. This provides a solid theoretical
foundation and candidate strategies for developing innovative
therapies against GBM metastasis.
Various small molecule compounds regulate the
expression and activity of MMPs through different molecular
mechanisms, thus inhibiting the invasive behavior of GBM. Core
signaling pathways serve as key regulatory points. For instance,
Chrysomycin A downregulated the expression of β-catenin and its
downstream target proteins c-Myc and cyclin D1 by inhibiting the
phosphorylation levels of AKT and GSK-3β, thereby regulating and
reducing MMP-2 protein expression, ultimately suppressing the
migration and invasion capabilities of GBM cells (137). The cell-penetrating peptide
trans-activator of transcription (Tat)-nuclear translocation signal
(NTS) inhibited NF-κB phosphorylation by specifically blocking the
nuclear translocation of annexin-A1, consequently downregulating
the expression and activity of MMP-2 and MMP-9 (117). The farnesoid X receptor agonist
GW4064 downregulated MMP-2 activity by inhibiting protein kinase C
alpha (PKCα) phosphorylation, an effect reversible by the PKCα
agonist phorbol 12-myristate 13-acetate (138). Concurrently, the specific
p53-Snail binding inhibitor GN25 also affected the invasion process
by reducing MMP-2 expression (139). Furthermore, metabolism
regulation is involved; PPARγ agonists (such as pioglitazone and
rosiglitazone) and the PPARα agonist WY-14643 were shown to inhibit
MMP2 by upregulating the regulatory factor X1, effectively
suppressing tumor invasion in in vivo models (140). Notably, ion channel function
has also been demonstrated to be associated with MMP regulation;
for example, the hERG channel agonist NS1643 reduced MMP-9 protein
levels (141). In terms of
epigenetic regulation, the histone deacetylase inhibitor
suberoylanilide hydroxamic acid (SAHA) significantly inhibited
MMP14 transcription in GBM cells by reducing histone H3 lysine 27
acetylation levels, and this downregulation has been demonstrated
as a key mechanism for SAHA-mediated radiosensitization (118). Non-coding RNA networks are also
crucial. The sevoflurane derivative Sev downregulated MMP-2 and
MMP-9 through the circRELN/miR-1290/RORA and miR-27b/VEGF signaling
pathways and by direct targeting by miR-34a-5p, with its
anti-invasive effects confirmed in both cellular experiments and
tumor-bearing mice (142-144). Similarly, propofol was shown to
inhibit MMP-2 and MMP-9 activity via the
circNCAPG/miR-200a-3p/RAB5A pathway (145). Viral vectors, such as the
third-generation oncolytic adenovirus TS-2021 (with E1A regulated
by Ki67/TGF-β2 UTR and carrying IL-15), was revealed to
significantly downregulate MMP3 expression by inhibiting
p-MKK4/p-JNK signaling, thereby effectively suppressing the
invasive ability of GBM cells (146).
Notably, certain compounds demonstrate significant
clinical translational potential. The novel compound NP16
(structural feature, 3,4,5-trimethoxymethyl on the N-phenyl ring)
significantly downregulates MMP-9 levels by inhibiting COX-2
expression and blocking STAT3 phosphorylation and nuclear
translocation. In a C6 orthotopic model, its tumor inhibition rate
(66.01%) surpassed that of TMZ (54.83%). It also exhibited
favorable BBB penetration capability (brain/blood concentration
ratio, 0.38) and significantly prolonged the survival of
tumor-bearing rats (147). A
class of Pt(IV) complexes, designed to overcome challenges in GBM
treatment, are based on a cisplatin core with axial ligands
comprising the anthraquinone drug Rhein and a hydrophilic acetic
acid ligand, achieving dual-drug synergistic effects via linkers of
different lengths. These complexes exhibit superior synergistic
effects compared with single agents in inhibiting MMP-2 and MMP-9
expression and cell migration, and remain active under hypoxic
conditions. Computational simulations predict enhanced BBB
penetration capability, offering a novel direction for combination
therapy (148).
In summary, this section not only untangled the
diverse molecular mechanisms by which small molecules target the
regulation of MMPs to inhibit GBM invasion (involving key signaling
pathways, metabolism, non-coding RNAs and ion channels), but also
highlighted the considerable clinical translational potential of
certain compounds [such as NP16 and novel Pt(IV) complexes] with
excellent BBB penetration and in vitro/in vivo activity in
overcoming GBM invasiveness (Table
SIII).
In recent years, various design strategies for
nanomaterial-based drug delivery systems targeting MMPs have
emerged in the field of GBM therapy. Researchers have developed
intelligent delivery platforms that specifically respond to
different MMP subtypes. For instance, dual-sensitive NPs rapidly
dissociate in the TME with high MMP-9 expression, releasing the
loaded doxorubicin while simultaneously forming nanogels. This
process significantly enhanced drug penetration and retention
within the tumor core, effectively inhibited GBM spheroids and
tumor volume, and substantially prolonged the survival time of mice
(119). Similarly, leveraging
MMP subtype responsiveness, D@MLL nanocarriers can be transported
across the BBB by monocytes driven by low-dose radiotherapy-induced
high C-C motif chemokine ligand 2 expression. Subsequently,
doxorubicin is rapidly released in areas of high MMP-2 activity,
triggering immunogenic cell death, as evidenced by significantly
upregulated calreticulin expression and high mobility group box 1
release. Concurrently, this promotes the polarization of
tumor-associated macrophages towards the M1 phenotype and activates
CD8+ T cells, thereby synergistically inhibiting GBM
progression and extending survival (149). Furthermore, nano-delivery
systems can synergize with radiotherapy to enhance efficacy. For
example, Au@DTDTPA(Gd) NPs combined with X-ray radiotherapy
significantly inhibit the invasive capacity of escape cells from
GBM spheroids, and attenuate their invasiveness and stem cell-like
characteristics (such as SOX2 downregulation) by reducing MMP-2
secretion and activity and inducing mitotic catastrophe (150). Additionally, fMbat NPs achieve
BBB-crossing capability via transferrin receptor-mediated
transport, effectively delivering batimastat to the GBM TME and
potently inhibiting MMP-2 activity in GBM cells (151). Dual-targeting liposomes
(co-loaded with daunorubicin and rofecoxib) and
carboxymethyl-stevioside-modified magnetic NPs can simultaneously
inhibit the expression and function of MMP-2 and MMP-9, effectively
suppressing GBM invasion (35,152). Notably, CuO NPs not only
significantly downregulate the expression of proteins such as
MMP-9, EphA2 and YKL-40 in the hippocampus and cells of GBM model
rats to restrict tumor invasion, but also demonstrate the potential
to improve spatial recognition and memory abilities in model rats
(36).
In summary, the aforementioned nanodrug delivery
systems, by achieving specific responses to MMP-rich
microenvironmental stimuli, controlled drug release and multiple
mechanisms of action (such as enhancing the permeability and
retention effect, triggering immune responses, synergizing with
radiotherapy, inhibiting invasion and crossing the BBB), provide
powerful novel strategies for precisely targeting and modulating
MMP-related key pathological processes in GBM (Table SIV).
In pharmacological interventions targeting MMPs,
combination therapy strategies have significantly enhanced
antitumor efficacy through multi-target synergistic effects. Among
these, the combination of chemotherapeutic agents with other
treatment modalities has demonstrated substantial potential. TMZ,
as a foundational chemotherapeutic drug, was shown to synergize
with photodynamic therapy in downregulating the expression of
MMP-2, HIF-1α and glucose transporter 1, thereby inhibiting glucose
uptake and ATP production. This effectively blocked tumor invasion
and energy supply, significantly suppressed tumor growth, and
prolonged the survival of tumor-bearing mice, with effects superior
to monotherapy (120). Natural
products and their derivatives can also synergize with TMZ. The
combination of TMZ and Chuanxiong essential oil (CEO) was
demonstrated to reverse drug resistance and inhibit GBM cell
invasion ex vivo by suppressing MMP-9 expression.
Furthermore, the combination of ligustilide, a key component of
CEO, with TMZ enhanced tumor suppression effects and TMZ
sensitivity in animal models (153). The combination of TMZ with
4-methylumbelliferone was revealed to inhibit invasion by reducing
MMP-2 activity and enhanced the drug sensitivity of cells resistant
to TMZ and vincristine (154).
The combined application of cordycepin and doxorubicin has also
been confirmed to inhibit MMP-9-mediated invasion at the cellular
level (155).
miRNA replacement therapy combined with
chemotherapeutic drugs has also demonstrated significant
synergistic potential. A previous study found that the combined
application of overexpressed miR-181a and carmustine effectively
curbs the invasive capacity of GBM cells by targeting and
inhibiting MMP-2 expression (121). It is noteworthy that the
proteasome inhibitor bortezomib alone can significantly
downregulate MMP-2 and MMP-9 expression and inhibit invasion. When
combined with the polo-like kinase 4 (PLK4) inhibitor CFI-400945,
it synergistically enhanced the downregulation of MMP-2 and MMP-9
as well as the inhibition of invasion. Conversely, overexpression
of PLK4 reversed this effect and attenuated the efficacy of
bortezomib. The core mechanism involves the synergistic activation
of PTEN expression and the inhibition of the phosphorylation and
expression of proteins of the PI3K/AKT/mTOR pathway, ultimately
leading to the downregulation of MMP-2 and MMP-9 expression
(156). Combined therapy with
TNF-related apoptosis-inducing ligand and celastrol inhibited GBM
cell invasion by upregulating GSK-3β, reducing the transcription
and protein levels of β-catenin and its downstream targets c-Myc,
cyclin D1 and MMP-2 (157).
Similarly, ex vivo experiments with aprepitant combined with
5-ALA demonstrated effective restriction of GBM cell invasion
through inhibition of MMP-2 and MMP-9 activity (158).
In summary, the above section has systematically
elaborated on strategies involving chemotherapy combined with
physical/natural therapies, miRNA replacement therapy, targeted
drug combinations and signaling pathway inhibitors (Table SV) to synergistically regulate
MMPs and their associated signaling networks (such as the
PI3K/AKT/mTOR and Wnt/β-catenin pathways). These strategies not
only significantly inhibit tumor invasion and overcome drug
resistance, but also effectively suppress tumor growth and prolong
survival, thereby establishing a solid mechanistic foundation and
providing direction for the development of more efficient,
multi-targeted anti-GBM treatment plans.
Although intervention strategies targeting MMPs have
demonstrated therapeutic potential in preclinical studies, their
clinical translation faces important challenges. These challenges
primarily stem from the inability of existing model systems to
adequately recapitulate the complex pathobiology of GBM, resulting
in a gap between preclinical data and outcomes from human
trials.
The limitations of tumor models represent the
primary obstacle. Long-term cultured GBM cell lines (such as
U-251MG) undergo significant genomic drift. Characteristic
chromosomal abnormalities (including deletions in 18q11-23 and
amplifications in 4q12) emerge in subclones, leading to aberrant
activation of key tyrosine kinase receptors such as PDGFRα. These
genetic alterations drive cells to acquire enhanced proliferative,
clonogenic and invasive capacities, causing their biological
characteristics to deviate markedly from the original tumor
features (159). More
importantly, GBM exhibits heterogeneity at both histological and
molecular levels. The TME harbors diverse cell populations
(including GSCs, differentiated tumor cells, necrotic areas and
aberrant vasculature), which display significant differences in
proliferation kinetics, invasive properties and therapeutic
sensitivity (160).
Concurrently, complex genomic variations (including mutations, copy
number alterations and epigenetic modifications) and distinct
molecular subtypes (such as proneural, classical and mesenchymal)
collectively result in differential signaling pathway activation
states and varied responses to MMP-targeted therapies (161,162). Existing models struggle to
replicate this complexity, introducing systematic bias into
efficacy evaluations. The results may not encompass all tumor cell
subpopulations, and predictions of potential adverse effects are
often inadequate. This has been corroborated by a phase II clinical
trial, where the combination of TMZ with the broad-spectrum MMP
inhibitor marimastat improved the 6-month PFS rate in patients with
recurrent GBM; however, 47% of patients experienced dose-limiting
joint toxicity (163), further
underscoring the fundamental limitations of preclinical models in
assessing toxicity risks.
The complex pathological structure of the BBB
constitutes the second major obstacle. In the GBM microenvironment,
aberrantly upregulated MMP activity and an imbalance with their
natural inhibitor, tissue inhibitor of metalloproteinases-1, lead
to degradation and loss of agrin, a key component of the
perivascular basal lamina. This subsequently disrupts the
structural integrity of astrocytic end-feet, causing BBB leakage in
the tumor core (164). However,
at the invasive front, the BBB structure remains relatively intact
and harbors active efflux pump systems, such as P-glycoprotein and
breast cancer resistance protein, creating a spatially
heterogeneous drug delivery barrier (165,166). This unique pathological
structure results in highly uneven intratumoral distribution of
therapeutic agents, making it difficult for targeted drugs to reach
effective concentrations in the invasive regions (167,168). Furthermore, the physicochemical
properties of the drugs themselves (such as lipophilicity) are
directly related to their ability to penetrate the intact BBB and
resist efflux pumps. Highly lipophilic compounds tend to accumulate
in peripheral tissues, potentially causing systemic toxicity, while
polar molecules often fail to effectively reach central targets
(169). However, existing in
vitro BBB models cannot mimic these dynamic pathological
changes and spatial heterogeneity, leading to fundamental
limitations in predicting drug permeability and the therapeutic
window.
MMPs occupy a central position in the invasion
process of GBM, primarily through three mechanisms: i) Facilitating
EMT via ECM degradation; ii) remodeling the vascular niche; and
iii) thereby fueling tumor malignancy. The expression of MMPs is
modulated by a network of signaling pathways, including PI3K/AKT,
MAPK and TGF-β, as well as transcription factors such as Snail and
activator protein 1, and non-coding RNAs. Notably, physicochemical
factors within the tumor niche also exert a marked influence on the
enzymatic activity of MMPs.
Therapeutic strategies targeting MMPs show
considerable promise. Several reported small-molecule compounds
exhibit excellent BBB penetration capability and superior ex
vivo inhibitory efficacy compared with TMZ. Nanodelivery
systems can synergistically address the dual challenges of BBB
penetration and targeted delivery. By enabling drug release
specifically in response to MMPs, these systems hold potential for
circumventing the joint toxicity associated with the broad-spectrum
inhibitor marimastat, which arises from the non-selective
inhibition of MMPs involved in joint formation (170,171). Concurrently, in cell therapy,
chlorotoxin-targeted chimeric antigen receptor (CAR) T cells
(NCT04214392), administered via intracavitary tumor injection in
patients with recurrent GBM, have shown promising preliminary
clinical data. These cells demonstrated persistence within the
tumor cavity and a favorable safety profile (absence of systemic
inflammation or anti-CAR antibody responses, and no dose-limiting
toxicities), and achieved transient disease stabilization in 3 out
of 4 patients (172), providing
initial evidence for MMP-targeted therapies.
Previous research on the side effects of MMP-related
drug interventions have revealed that TMZ can upregulate MMP-9,
enhancing tumor invasiveness (173). Although the combination of
radiotherapy and TMZ reduces GBM cell viability, it enhances the
invasive capacity of GBM cells by inducing the secretion of sEVs
carrying MMP-2 and upregulating thrombospondin-1 in invasive
pseudopodia (48,174). It is noteworthy that excessive
fluoride accumulation can significantly enhance GBM invasiveness by
activating the expression of MMP-2 and MMP-9 (175). Furthermore, physical therapies
carry potential risks; surgical thermal injury markedly increases
MMP-9 activity by activating astrocytes, thereby exacerbating the
malignant progression of GBM (176). These phenomena reveal that
single interventions may trigger compensatory invasive mechanisms,
highlighting the complexity of precisely regulating the MMP
network.
Due to the intricate MMP regulatory network and
bottlenecks in clinical translation, interdisciplinary
technological collaboration is essential. To mitigate issues of
genetic drift in GBM cell lines, prioritizing the use of
low-passage cells is crucial for ensuring experimental
reproducibility. Artificial intelligence (AI) is increasingly
becoming a key driver for overcoming clinical translation hurdles.
AI algorithms, such as convolutional neural networks and vision
transformers, can efficiently capture disease features from MRI
images, significantly improving GBM classification accuracy and
reducing scan times. Integrated with high-throughput omics data
(genomics and transcriptomics), AI can more precisely delineate GBM
molecular subtypes, laying the groundwork for targeted drug
therapies (177-180). The application of AI has
further expanded to surgical planning [including predicting
interstitial thermal therapy ablation areas to optimize prognosis
(181)], drug development [such
as predicting drug responses and mechanisms (182)] and precision medicine
[including developing personalized treatment plans or predicting
therapeutic efficacy (183)].
Simultaneously, developing physiologically relevant ex vivo
BBB models is a core component for translating preclinical research
to the clinic. Microfluidic technology has successfully established
three-dimensional co-culture BBB models incorporating human brain
vascular pericytes, astrocytes and endothelial cells. These models
exhibit physiologically relevant structure, selective permeability,
reversibility, and effectively simulate GBM-induced vascular
remodeling and drug delivery barriers (184). Three-dimensional (3D) printing
technologies, such as two-photon lithography, have also been
employed to construct controllable brain TME models, achieving
tri-culture of endothelial cells, astrocytes and glioma spheroids
to form a functional BBB (185). Notably, 3D gradient hydrogel
models, by mimicking the dynamic changes in matrix stiffness within
the GBM microenvironment, have revealed a dose-dependent
association between mechanical stress stimulation and the
regulation of MMP activity (186,187), offering novel perspectives for
understanding the mechanical mechanisms of BBB disruption.
In summary, although interventions targeting MMPs
show potential in GBM treatment, their complex biological
functions, potential pro-invasive effects and challenges in
clinical translation remain core issues to be resolved. Future
research, through multidisciplinary integration and the close
combination of basic mechanistic exploration, innovative technology
application and clinical translation studies, may transform
strategies such as targeting MMPs into clinical regimens that
improve survival outcomes for patients with GBM.
Not applicable.
BZ conceived and designed the review, as well as
wrote, reviewed, and edited the manuscript. YH performed data
extraction and synthesis, provided original insights and
interpretations, and critically revised the manuscript. HZ acquired
funding, supervised the project, designed the review scope, and
conducted expert analysis. All authors read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
We express sincere gratitude to Dr Jinpeng Hu
(Department of Neurosurgery, The First Hospital of China Medical
University, Shenyang, P.R. China) for his expert guidance in
manuscript revision, figure preparation, and table design.
The present research was supported by the Chen Xiao-Ping
Foundation for the Development of Science and Technology of Hubei
Province (grant no. CXPJJH123003-058) and the General Program of
Joint Natural Science Foundation of Liaoning Provincial Science and
Technology Plan (grant no. 2025-MSLH-480).
|
1
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2016-2020. Neuro Oncol. 25(12
Suppl 2): iv1–iv99. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao D, Yan C, Li D, Xi T, Liu X, Zhu D,
Huang G, Xu J, He Z, Wu A, et al: National brain tumour registry of
China (NBTRC) statistical report of primary brain tumours diagnosed
in china in years 2019-2020. Lancet Reg Health West Pac.
34:1007152023.PubMed/NCBI
|
|
3
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barone DG, Lawrie TA and Hart MG: Image
guided surgery for the resection of brain tumours. Cochrane
Database Syst Rev. 2014:CD0096852014.PubMed/NCBI
|
|
5
|
Pirro V, Alfaro CM, Jarmusch AK, Hattab
EM, Cohen-Gadol AA and Cooks RG: Intraoperative assessment of tumor
margins during glioma resection by desorption electrospray
ionization-mass spectrometry. Proc Natl Acad Sci USA.
114:6700–6705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Boer E, Harlaar NJ, Taruttis A,
Nagengast WB, Rosenthal EL, Ntziachristos V and Van Dam GM: Optical
innovations in surgery. Br J Surg. 102:e56–e72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hervey-Jumper SL and Berger MS: Maximizing
safe resection of low- and high-grade glioma. J Neuro-Oncol.
130:269–282. 2016. View Article : Google Scholar
|
|
8
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perry JR, Laperriere N, O'Callaghan CJ,
Brandes AA, Menten J, Phillips C, Fay M, Nishikawa R, Cairncross
JG, Roa W, et al: Short-course radiation plus temozolomide in
elderly patients with glioblastoma. N Engl J Med. 376:1027–1037.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez-Camacho A, Flores-Vázquez JG,
Moscardini-Martelli J, Torres-Ríos JA, Olmos-Guzmán A, Ortiz-Arce
CS, Cid-Sánchez DR, Pérez SR, Macías-González MDS,
Hernández-Sánchez LC, et al: Glioblastoma treatment:
State-of-the-art and future perspectives. Int J Mol Sci.
23:72072022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weller M and Le Rhun E: How did lomustine
become standard of care in recurrent glioblastoma? Cancer Treat
Rev. 87:1020292020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirson ED, Dbalý V, Tovaryš F, Vymazal J,
Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S,
Gurvich Z, Schneiderman R, et al: Alternating electric fields
arrest cell proliferation in animal tumor models and human brain
tumors. Proc Natl Acad Sci USA. 104:10152–10157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giladi M, Schneiderman RS, Voloshin T,
Porat Y, Munster M, Blat R, Sherbo S, Bomzon Z, Urman N, Itzhaki A,
et al: Mitotic spindle disruption by alternating electric fields
leads to improper chromosome segregation and mitotic catastrophe in
cancer cells. Sci Rep. 5:180462015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Rhun E, Preusser M, Roth P, Reardon DA,
van den Bent M, Wen P, Reifenberger G and Weller M: Molecular
targeted therapy of glioblastoma. Cancer Treat Rev. 80:1018962019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Navone SE, Guarnaccia L, Locatelli M,
Rampini P, Caroli M, La Verde N, Gaudino C, Bettinardi N, Riboni L,
Marfia G and Campanella R: Significance and prognostic value of the
coagulation profile in patients with glioblastoma: Implications for
personalized therapy. World Neurosurg. 121:e621–e629. 2019.
View Article : Google Scholar
|
|
18
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Streiff MB, Ye X, Kickler TS, Desideri S,
Jani J, Fisher J and Grossman SA: A prospective multicenter study
of venous thromboembolism in patients with newly-diagnosed
high-grade glioma: Hazard rate and risk factors. J Neurooncol.
124:299–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Fei W, Shi Y, Ma W, Jiao W, Tao F,
Zhu J, Wang Y and Feng X: Venous thromboembolism risk factors in
pediatric patients with high-grade glioma: A multicenter
retrospective study. Front Pediatr. 13:15952232025. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marras LC, Geerts WH and Perry JR: The
risk of venous thromboembolism is increased throughout the course
of malignant glioma: An evidence-based review. Cancer. 89:640–646.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semrad TJ, O'Donnell R, Wun T, Chew H,
Harvey D, Zhou H and White RH: Epidemiology of venous
thromboembolism in 9489 patients with malignant glioma. J
Neurosurg. 106:601–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brat DJ, Castellano-Sanchez AA, Hunter SB,
Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B and Van Meir EG:
Pseudopalisades in glioblastoma are hypoxic, express extracellular
matrix proteases, and are formed by an actively migrating cell
population. Cancer Res. 64:920–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gross J and Lapiere CM: Collagenolytic
activity in amphibian tissues: A tissue culture assay. Proc Natl
Acad Sci USA. 48:1014–1022. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quintero-Fabián S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9:13702019. View Article : Google Scholar
|
|
27
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
28
|
Niland S, Riscanevo AX and Eble JA: Matrix
metalloproteinases shape the tumor microenvironment in cancer
progression. Int J Mol Sci. 23:1462021. View Article : Google Scholar
|
|
29
|
Dibdiakova K, Majercikova Z, Galanda T,
Richterova R, Kolarovszki B, Racay P and Hatok J: Relationship
between the expression of matrix metalloproteinases and their
tissue inhibitors in patients with brain tumors. Int J Mol Sci.
25:28582024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wolburg H, Noell S, Fallier-Becker P, Mack
AF and Wolburg-Buchholz K: The disturbed blood-brain barrier in
human glioblastoma. Mol Aspects Med. 33:579–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Cheng L, Su Y, Qian Z, Wang Z,
Chen C, Li R, Zhang A, He J, Mao J, et al: AGBL4 promotes malignant
progression of glioblastoma via modulation of MMP-1 and
inflammatory pathways. Front Immunol. 15:14201822024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang ZL, Abdallah AS, Shen GX, Suarez M,
Feng P, Yu YJ, Wang Y, Zheng SH, Hu YJ, Xiao X, et al: Silencing
GMPPB inhibits the proliferation and invasion of GBM via hippo/MMP3
pathways. Int J Mol Sci. 24:147072023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan P, Wang J, Liu H, Liu X, Fu R and Feng
J: M1 macrophage-derived exosomes containing miR-150 inhibit glioma
progression by targeting MMP16. Cell Signal. 108:1107312023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hitchcock SA: Blood-brain barrier
permeability considerations for CNS-targeted compound library
design. Curr Opin Chem Biol. 12:318–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie HJ, Zhan-Dui N, Zhao J, Er-Bu AGA,
Zhen P, ZhuoMa D and Sang T: Evaluation of nanoscaled dual
targeting drug-loaded liposomes on inhibiting vasculogenic mimicry
channels of brain glioma. Artif Cells Nanomed Biotechnol.
49:595–604. 2021. View Article : Google Scholar
|
|
36
|
Tian S, Xu J, Qiao X, Zhang X, Zhang S,
Zhang Y, Xu C, Wang H and Fang C: CuO nanoparticles for glioma
treatment in vitro and in vivo. Sci Rep. 14:232292024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castro-Ribeiro ML, Castro VIB, Vieira De
Castro J, Pires RA, Reis RL, Costa BM, Ferreira H and Neves NM: The
potential of the fibronectin inhibitor arg-gly-asp-ser in the
development of therapies for glioblastoma. Int J Mol Sci.
25:49102024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang WB, Chuang JY, Ko CY, Chang WC and
Hsu TI: Dehydroepiandrosterone induces temozolomide resistance
through modulating phosphorylation and acetylation of Sp1 in
glioblastoma. Mol Neurobiol. 56:2301–2313. 2019. View Article : Google Scholar
|
|
39
|
Munoz JL, Bliss SA, Greco SJ, Ramkissoon
SH, Ligon KL and Rameshwar P: Delivery of functional anti-miR-9 by
mesenchymal stem cell-derived exosomes to glioblastoma multiforme
cells conferred chemosensitivity. Mol Ther Nucleic Acids.
2:e1262013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McFaline-Figueroa JL, Braun CJ, Stanciu M,
Nagel ZD, Mazzucato P, Sangaraju D, Cerniauskas E, Barford K,
Vargas A, Chen Y, et al: Minor changes in expression of the
mismatch repair protein MSH2 exert a major impact on glioblastoma
response to temozolomide. Cancer Res. 75:3127–3138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song H and Park KH: Regulation and
function of SOX9 during cartilage development and regeneration.
Semin Cancer Biol. 67(Pt 1): 12–23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo XR, Wu MY, Dai LJ, Huang Y, Shan MY,
Ma SN, Wang J, Peng H, Ding Y, Zhang QF, et al: Nuclear
FAM289-galectin-1 interaction controls FAM289-mediated tumor
promotion in malignant glioma. J Exp Clin Cancer Res. 38:3942019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan H, Fu Z, Lin P, Gu Y, Cao J and Li Y:
Inhibition of human glioblastoma multiforme cells by
10,11-dehydrocurvularin through the MMP-2 and PI3K/AKT signaling
pathways. Eur J Pharmacol. 936:1753482022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yuan Z, Yang Z, Li W, Wu A, Su Z, Jiang B
and Ganesan S: Triphlorethol-a attenuates U251 human glioma cancer
cell proliferation and ameliorates apoptosis through JAK2/STAT3 and
p38 MAPK/ERK signaling pathways. J Biochem Mol Toxicol.
36:e231382022. View Article : Google Scholar
|
|
45
|
De Jong JM, Broekaart DWM, Bongaarts A,
Mühlebner A, Mills JD, Van Vliet EA and Aronica E: Altered
extracellular matrix as an alternative risk factor for
epileptogenicity in brain tumors. Biomedicines. 10:24752022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thome I, Lacle R, Voß A, Bortolussi G,
Pantazis G, Schmidt A, Conrad C, Jacob R, Timmesfeld N, Bartsch JW
and Pagenstecher A: Neoplastic cells are the major source of
MT-MMPs in IDH1-mutant glioma, thus enhancing tumor-cell intrinsic
brain infiltration. Cancers (Basel). 12:24562020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sawicka MM, Sawicki K, Jadeszko M,
Bielawska K, Supruniuk E, Reszeć J, Prokop-Bielenia I, Polityńska
B, Jadeszko M, Rybaczek M, et al: Proline metabolism in WHO G4
gliomas is altered as compared to unaffected brain tissue. Cancers
(Basel). 16:4562024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Whitehead CA, Fang H, Su H, Morokoff AP,
Kaye AH, Hanssen E, Nowell CJ, Drummond KJ, Greening DW, Vella LJ,
et al: Small extracellular vesicles promote invadopodia activity in
glioblastoma cells in a therapy-dependent manner. Cell Oncol
(Dordr). 46:909–931. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Colangelo NW and Azzam EI: Extracellular
vesicles originating from glioblastoma cells increase
metalloproteinase release by astrocytes: The role of CD147
(EMMPRIN) and ionizing radiation. Cell Commun Signaling. 18:212020.
View Article : Google Scholar
|
|
50
|
Perruzzi CA, De Fougerolles AR,
Koteliansky VE, Whelan MC, Westlin WF and Senger DR: Functional
overlap and cooperativity among αv and β1 integrin subfamilies
during skin angiogenesis. J Invest Dermatol. 120:1100–1109. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Whelan MC and Senger DR: Collagen I
initiates endothelial cell morphogenesis by inducing actin
polymerization through suppression of cyclic AMP and protein kinase
A. J Biol Chem. 278:327–334. 2003. View Article : Google Scholar
|
|
52
|
Davis S, Aldrich TH, Jones PF, Acheson A,
Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre
PC and Yancopoulos GD: Isolation of angiopoietin-1, a ligand for
the TIE2 receptor, by secretion-trap expression cloning. Cell.
87:1161–1169. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiguet-Jiglaire C, Boissonneau S,
Denicolai E, Hein V, Lasseur R, Garcia J, Romain S, Appay R,
Graillon T, Mason W, et al: Plasmatic MMP9 released from
tumor-infiltrating neutrophils is predictive for bevacizumab
efficacy in glioblastoma patients: An AVAglio ancillary study. Acta
Neuropathol Commun. 10:12022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo W, Quan Q, Xu Z, Lei J and Peng R:
Bioinformatics analysis of MMP14+ myeloid cells affecting
endothelial-mesenchymal transformation and immune microenvironment
in glioma. Heliyon. 10:e268592024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yi B, Li H, Cai H, Lou X, Yu M and Li Z:
LOXL1-AS1 communicating with TIAR modulates vasculogenic mimicry in
glioma via regulation of the miR-374b-5p/MMP14 axis. J Cell Mol
Med. 26:475–490. 2022. View Article : Google Scholar
|
|
57
|
Kryczka J, Papiewska-Pajak I, Kowalska MA
and Boncela J: Cathepsin B is upregulated and mediates ECM
degradation in colon adenocarcinoma HT29 cells overexpressing
snail. Cells. 8:2032019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Adnani L, Kassouf J, Meehan B, Spinelli C,
Tawil N, Nakano I and Rak J: Angiocrine extracellular vesicles
impose mesenchymal reprogramming upon proneural glioma stem cells.
Nat Commun. 13:54942022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Djediai S, Gonzalez Suarez N, El
Cheikh-Hussein L, Rodriguez Torres S, Gresseau L, Dhayne S,
Joly-Lopez Z and Annabi B: MT1-MMP cooperates with TGF-β
receptor-mediated signaling to trigger SNAIL and induce
epithelial-to-mesenchymal-like transition in U87 glioblastoma
cells. Int J Mol Sci. 22:130062021. View Article : Google Scholar
|
|
60
|
Komatsu K, Nakanishi Y, Nemoto N, Hori T,
Sawada T and Kobayashi M: Expression and quantitative analysis of
matrix metalloproteinase-2 and-9 in human gliomas. Brain Tumor
Pathol. 21:105–112. 2004. View Article : Google Scholar
|
|
61
|
Hormigo A, Gu B, Karimi S, Riedel E,
Panageas KS, Edgar MA, Tanwar MK, Rao JS, Fleisher M, DeAngelis LM
and Holland EC: YKL-40 and matrix metalloproteinase-9 as potential
serum biomarkers for patients with high-grade gliomas. Clin Cancer
Res. 12:5698–5704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Iwamoto FM, Hottinger AF, Karimi S, Riedel
E, Dantis J, Jahdi M, Panageas KS, Lassman AB, Abrey LE, Fleisher
M, et al: Longitudinal prospective study of matrix
metalloproteinase-9 as a serum marker in gliomas. J Neuro-oncol.
105:607–612. 2011. View Article : Google Scholar
|
|
63
|
Wong ET, Alsop D, Lee D, Tam A, Barron L,
Bloom J, Gautam S and Wu JK: Cerebrospinal fluid matrix
metalloproteinase-9 increases during treatment of recurrent
malignant gliomas. Cerebrospinal Fluid Res. 5:12008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tabouret E, Boudouresque F, Barrie M,
Matta M, Boucard C, Loundou A, Carpentier A, Sanson M, Metellus P,
Figarella-Branger D, et al: Association of matrix metalloproteinase
2 plasma level with response and survival in patients treated with
bevacizumab for recurrent high-grade glioma. Neuro Oncol.
16:392–399. 2014. View Article : Google Scholar :
|
|
65
|
Li Q, Chen B, Cai J, Sun Y, Wang G, Li Y,
Li R, Feng Y, Han B, Li J, et al: Comparative analysis of matrix
metalloproteinase family members reveals that MMP9 predicts
survival and response to temozolomide in patients with primary
glioblastoma. PLoS One. 11:e01518152016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang J, Yang Y, Dong Y and Liu C:
Microrchidia family CW-type zinc finger 2 promotes the
proliferation, invasion, migration and epithelial-mesenchymal
transition of glioma by regulating PTEN/PI3K/AKT signaling via
binding to N-myc downstream regulated gene 1 promoter. Int J Mol
Med. 49:162022. View Article : Google Scholar :
|
|
67
|
Liu Y, Ma J, Song JS, Zhou HY, Li JH, Luo
C, Geng X and Zhao HX: DNA topoisomerase II alpha promotes the
metastatic characteristics of glioma cells by transcriptionally
activating β-catenin. Bioengineered. 13:2207–2216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cong Z, Yuan F, Wang H, Cai X, Zhu J, Tang
T, Zhang L, Han Y and Ma C: BTB domain and CNC homolog 1 promotes
glioma invasion mainly through regulating extracellular matrix and
increases ferroptosis sensitivity. Biochim Biophys Acta Mol Basis
Dis. 1868:1665542022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pai FC, Huang HW, Tsai YL, Tsai WC, Cheng
YC, Chang HH and Chen Y: Inhibition of FABP6 reduces tumor cell
invasion and angiogenesis through the decrease in MMP-2 and VEGF in
human glioblastoma cells. Cells. 10:27822021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hu T, Lei D, Zhou J and Zhang B: circRNA
derived from CLSPN (circCLSPN) is an oncogene in human glioblastoma
multiforme by regulating cell growth, migration and invasion via
ceRNA pathway. J Biosci. 46:662021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jia J, Ouyang Z, Wang M, Ma W, Liu M,
Zhang M and Yu M: MicroRNA-361-5p slows down gliomas development
through regulating UBR5 to elevate ATMIN protein expression. Cell
Death Dis. 12:7462021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Amini J, Zafarjafarzadeh N, Ghahramanlu S,
Mohammadalizadeh O, Mozaffari E, Bibak B and Sanadgol N: Role of
circular RNA MMP9 in glioblastoma progression: from interaction
with hnRNPC and hnRNPA1 to affecting the expression of BIRC5 by
sequestering miR -149. J Mol Recognit. 38:e31092025. View Article : Google Scholar
|
|
73
|
Delbrouck C, Kiweler N, Chen O, Pozdeev
VI, Haase L, Neises L, Oudin A, Fouquier d'Hérouël A, Shen R,
Schlicker L, et al: Formate promotes invasion and metastasis in
reliance on lipid metabolism. Cell Rep. 42:1130342023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pu W, Qiu J, Riggins GJ and Parat MO:
Matrix protease production, epithelial-to-mesenchymal transition
marker expression and invasion of glioblastoma cells in response to
osmotic or hydrostatic pressure. Sci Rep. 10:26342020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu Y, He W, Guo Y, Qu S, Yao F, Liu J,
Chai J, Yang Y, Xu T, Liu Y, et al: CSNK1D is associated with
stemness and invasiveness in glioblastoma. Pathol Res Pract.
240:1541872022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huang YK, Wang TM, Chen CY, Li CY, Wang
SC, Irshad K, Pan Y and Chang KC: The role of ALDH1A1 in
glioblastoma proliferation and invasion. Chem Biol Interact.
402:1112022024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ni H, Ji D, Huang Z and Li J: SMAGP
knockdown inhibits the malignant phenotypes of glioblastoma cells
by inactivating the PI3K/akt pathway. Arch Biochem Biophys.
695:1086282020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yang W, Yuan Q, Zhang S, Zuo M, Li T, Li
J, Zhou X, Li M, Feng W, Xia X, et al: Elevated GIGYF2 expression
suppresses tumor migration and enhances sensitivity to temozolomide
in malignant glioma. Cancer Gene Ther. 29:750–757. 2022. View Article : Google Scholar
|
|
80
|
Gwon DH, Lee WY, Shin N, Kim SI, Jeong K,
Lee WH, Kim DW, Hong J and Lee SY: BMAL1 suppresses proliferation,
migration, and invasion of U87MG cells by downregulating cyclin B1,
phospho-AKT, and metalloproteinase-9. Int J Mol Sci. 21:23522020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zuo J, Liu C, Ni H and Yu Z: WDR34 affects
PI3K/akt and wnt/β-catenin pathways to regulates malignant
biological behaviors of glioma cells. J Neurooncol. 156:281–293.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sun L, Hui AM, Su Q, Vortmeyer A,
Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey
R, et al: Neuronal and glioma-derived stem cell factor induces
angiogenesis within the brain. Cancer Cell. 9:287–300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lyu X, Shi Y, Wang D, Cao X, Guo J, Huang
G, Zhou L, Zhang M and Dong Z: Impact of LIN7A silencing on U87
cell invasion and its clinical significance in glioblastoma. Sci
Rep. 15:72122025. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun H, Long S, Wu B, Liu J and Li G: NKCC1
involvement in the epithelial-to-mesenchymal transition is a
prognostic biomarker in gliomas. PeerJ. 8:e87872020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang G, Feng W and Wu J: Down-regulation
of SEPT9 inhibits glioma progression through suppressing
TGF-β-induced epithelial-mesenchymal transition (EMT). Biomed
Pharmacother. 125:1097682020. View Article : Google Scholar
|
|
86
|
Dong C, Li X, Yang J, Yuan D, Zhou Y,
Zhang Y, Shi G, Zhang R, Liu J, Fu P and Sun M: PPFIBP1 induces
glioma cell migration and invasion through FAK/src/JNK signaling
pathway. Cell Death Dis. 12:8272021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Y, Chang L, Huang P, Cao M, Hong R,
Zhao X, He X and Xu L: Loss of PTPRS elicits potent metastatic
capability and resistance to temozolomide in glioblastoma. Mol
Carcinog. 63:1235–1247. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Phillips HS, Kharbanda S, Chen R, Forrest
WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et
al: Molecular subclasses of high-grade glioma predict prognosis,
delineate a pattern of disease progression, and resemble stages in
neurogenesis. Cancer Cell. 9:157–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Costa BM, Smith JS, Chen Y, Chen J,
Phillips HS, Aldape KD, Zardo G, Nigro J, James CD, Fridlyand J, et
al: Reversing HOXA9 oncogene activation by PI3K inhibition:
Epigenetic mechanism and prognostic significance in human
glioblastoma. Cancer Res. 70:453–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lin K, Taylor JR Jr, Wu TD, Gutierrez J,
Elliott JM, Vernes JM, Koeppen H, Phillips HS, de Sauvage FJ and
Meng YG: TMEFF2 is a PDGF-AA binding protein with
methylation-associated gene silencing in multiple cancer types
including glioma. PLoS One. 6:e186082011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yu X, Jin J, Zheng Y, Zhu H, Xu H, Ma J,
Lan Q, Zhuang Z, Chen CC and Li M: GBP5 drives malignancy of
glioblastoma via the src/ERK1/2/MMP3 pathway. Cell Death Dis.
12:2032021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang R, Zhang S, Chen X, Li N, Li J, Jia
R, Pan Y and Liang H: EIF4A3-induced circular RNA MMP9 (circMMP9)
acts as a sponge of miR-124 and promotes glioblastoma multiforme
cell tumorigenesis. Mol Cancer. 17:1662018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu X, Shen S, Zhu L, Su R, Zheng J, Ruan
X, Shao L, Wang D, Yang C and Liu Y: SRSF10 inhibits biogenesis of
circ-ATXN1 to regulate glioma angiogenesis via miR-526b-3p/MMP2
pathway. J Exp Clin Cancer Res. 39:1212020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yang Y and Zhao F: MicroRNA-16 inhibits
the growth and metastasis of human glioma cells via modulation of
PI3K/AKT/mTOR signalling pathway. Arch Med Sci. 20:839–846. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Song Y, Li C, Jin L, Xing J, Sha Z, Zhang
T, Ji D, Yu R and Gao S: RIOK2 is negatively regulated by miR-4744
and promotes glioma cell migration/invasion through
epithelial-mesenchymal transition. J Cell Mol Med. 24:4494–4509.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Meiser J, Schuster A, Pietzke M, Vande
Voorde J, Athineos D, Oizel K, Burgos-Barragan G, Wit N, Dhayade S,
Morton JP, et al: Increased formate overflow is a hallmark of
oxidative cancer. Nat Commun. 9:13682018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Manou D, Golfinopoulou MA, Alharbi SND,
Alghamdi HA, Alzahrani FM and Theocharis AD: The expression of
serglycin is required for active transforming growth factor β
receptor I tumorigenic signaling in glioblastoma cells and
paracrine activation of stromal fibroblasts via CXCR-2.
Biomolecules. 14:4612024. View Article : Google Scholar
|
|
98
|
Pu W, Qiu J, Nassar ZD, Shaw PN, McMahon
KA, Ferguson C, Parton RG, Riggins GJ, Harris JM and Parat MO: A
role for caveola-forming proteins caveolin-1 and CAVIN1 in the
pro-invasive response of glioblastoma to osmotic and hydrostatic
pressure. J Cell Mol Med. 24:3724–3738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pu W, Nassar ZD, Khabbazi S, Xie N,
McMahon KA, Parton RG, Riggins GJ, Harris JM and Parat MO:
Correlation of the invasive potential of glioblastoma and
expression of caveola-forming proteins caveolin-1 and CAVIN1. J
Neurooncol. 143:207–220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Oishi M, Munesue S, Harashima A, Nakada M,
Yamamoto Y and Hayashi Y: Aquaporin 1 elicits cell motility and
coordinates vascular bed formation by downregulating thrombospondin
type-1 domain-containing 7A in glioblastoma. Cancer Med.
9:3904–3917. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sun Z, Zhao W, Fei X, He B, Shi L, Zhang Z
and Cai S: Static magnetic field inhibits epithelial mesenchymal
transition and metastasis of glioma. Sci Rep. 15:124302025.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shi Y, Jiang J, Cui Y, Chen Y, Dong T, An
H and Liu P: MSH6 aggravates the hypoxic microenvironment via
regulating HIF1A to promote the metastasis of glioblastoma
multiforme. DNA Cell Biol. 40:93–100. 2021. View Article : Google Scholar
|
|
103
|
Feng K, Ye G, Wang H, Li S, Wen X and Chen
M: Research on the mechanism of TWSG1 in the malignant progression
of glioma cells and tumor-associated macrophage infiltration. J
Neuropathol Exp Neurol. 83:843–852. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hodgson JG, Yeh RF, Ray A, Wang NJ,
Smirnov I, Yu M, Hariono S, Silber J, Feiler HS, Gray JW, et al:
Comparative analyses of gene copy number and mRNA expression in
glioblastoma multiforme tumors and xenografts. Neuro Oncol.
11:477–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Korde LA, Lusa L, McShane L, Lebowitz PF,
Lukes L, Camphausen K, Parker JS, Swain SM, Hunter K and Zujewski
JA: Gene expression pathway analysis to predict response to
neoadjuvant docetaxel and capecitabine for breast cancer. Breast
Cancer Res Treat. 119:685–699. 2010. View Article : Google Scholar
|
|
106
|
Griesinger AM, Birks DK, Donson AM, Amani
V, Hoffman LM, Waziri A, Wang M, Handler MH and Foreman NK:
Characterization of distinct immunophenotypes across pediatric
brain tumor types. J Immunol. 191:4880–4888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Qi H, Wang P, Sun H, Li X, Hao X, Tian W,
Yu L, Tang J, Dong J and Wang H: ADAMDEC1 accelerates GBM
progression via activation of the MMP2-related pathway. Front
Oncol. 12:9450252022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Guda MR, Velpula KK, Asuthkar S, Cain CP
and Tsung AJ: Targeting RGS4 ablates glioblastoma proliferation.
Int J Mol Sci. 21:33002020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yasmin IA, Mohana Sundaram S, Banerjee A,
Varier L, Dharmarajan A and Warrier S: Netrin-like domain of sFRP4,
a wnt antagonist inhibits stemness, metastatic and invasive
properties by specifically blocking MMP-2 in cancer stem cells from
human glioma cell line U87MG. Exp Cell Res. 409:1129122021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Georgescu MM, Islam MZ, Li Y, Traylor J
and Nanda A: Novel targetable FGFR2 and FGFR3 alterations in
glioblastoma associate with aggressive phenotype and distinct gene
expression programs. Acta Neuropathol Commun. 9:692021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sanders S, Herpai DM, Rodriguez A, Huang
Y, Chou J, Hsu FC, Seals D, Mott R, Miller LD and Debinski W: The
presence and potential role of ALDH1A2 in the glioblastoma
microenvironment. Cells. 10:24852021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kreße N, Schröder H, Stein KP, Wilkens L,
Mawrin C, Sandalcioglu IE and Dumitru CA: PLOD2 is a prognostic
marker in glioblastoma that modulates the immune microenvironment
and tumor progression. Int J Mol Sci. 23:60372022. View Article : Google Scholar
|
|
113
|
Ivanova EL, Costa B, Eisemann T, Lohr S,
Boskovic P, Eichwald V, Meckler J, Jugold M, Orian-Rousseau V,
Peterziel H and Angel P: CD44 expressed by myeloid cells promotes
glioma invasion. Front Oncol. 12:9697872022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang P, Hao X, Li X, Yan Y, Tian W, Xiao
L, Wang Z and Dong J: Curcumin inhibits adverse psychological
stress-induced proliferation and invasion of glioma cells via
down-regulating the ERK/MAPK pathway. J Cell Mol Med. 25:7190–7203.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yang CL, Chik SC, Lau AS and Chan GC:
Coriolus versicolor and its bioactive molecule are potential
immunomodulators against cancer cell metastasis via inactivation of
MAPK pathway. J Ethnopharmacol. 301:1157902023. View Article : Google Scholar
|
|
116
|
Gallardo-Pérez JC, Trejo-Solís MC,
Robledo-Cadena DX, López-Marure R, Agredano-Moreno LT,
Jimenez-García LF and Sánchez-Lozada LG: Erythrose inhibits the
progression to invasiveness and reverts drug resistance of cancer
stem cells of glioblastoma. Med Oncol. 40:1042023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Luo Z, Liu L, Li X, Chen W and Lu Z:
Tat-NTS suppresses the proliferation, migration and invasion of
glioblastoma cells by inhibiting annexin-A1 nuclear translocation.
Cell Mol Neurobiol. 42:2715–2725. 2022. View Article : Google Scholar
|
|
118
|
Zhou Y, Liu H, Zheng W, Chen Q, Hu S, Pan
Y, Bai Y, Zhang J and Shao C: MMP14 contributes to HDAC
inhibition-induced radiosensitization of glioblastoma. Int J Mol
Sci. 22:104032021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Dosta P, Dion MZ, Prado M, Hurtado P,
Riojas-Javelly CJ, Cryer AM, Soria Y, Andrews Interiano N,
Muñoz-Taboada G and Artzi N: Matrix metalloproteinase- and
pH-sensitive nanoparticle system enhances drug retention and
penetration in glioblastoma. ACS Nano. 18:14145–14160. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li Y, Wang D, Zhang Z, Wang Y, Zhang Z,
Liang Z, Liu F and Chen L: Photodynamic therapy enhances the
cytotoxicity of temozolomide against glioblastoma via reprogramming
anaerobic glycolysis. Photodiagn Photodyn Ther. 42:1033422023.
View Article : Google Scholar
|
|
121
|
Rezaei T, Hejazi M, Mansoori B, Mohammadi
A, Amini M, Mosafer J, Rezaei S, Mokhtarzadeh A and Baradaran B:
microRNA-181a mediates the chemo-sensitivity of glioblastoma to
carmustine and regulates cell proliferation, migration, and
apoptosis. Eur J Pharmacol. 888:1734832020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chen CJ, Shang HS, Huang YL, Tien N, Chen
YL, Hsu SY, Wu RS, Tang CL, Lien JC, Lee MH, et al:
Bisdemethoxycurcumin suppresses human brain glioblastoma multiforme
GBM 8401 cell migration and invasion via affecting NF-κB and MMP-2
and MMP-9 signaling pathway in vitro. Environ Toxicol.
37:2388–2397. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Jalili-Nik M, Afshari AR, Sabri H, Bibak
B, Mollazadeh H and Sahebkar A: Zerumbone, a ginger sesquiterpene,
inhibits migration, invasion, and metastatic behavior of human
malignant glioblastoma multiforme in vitro. BioFactors. 47:729–739.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Cheng B, Hong X, Wang L, Cao Y, Qin D,
Zhou H and Gao D: Curzerene suppresses progression of human
glioblastoma through inhibition of glutathione S-transferase A4.
CNS Neurosci Ther. 28:690–702. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Alkahtani S, Sal-Johani N, Alarifi S and
Afzal M: Cytotoxicity mechanisms of blue-light-activated curcumin
in T98G cell line: Inducing apoptosis through ROS-dependent
downregulation of MMP pathways. Int J Mol Sci. 24:38422023.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Han M, An J, Li S, Fan H, Wang L, Du Q, Du
J, Yang Y, Song Y and Peng F: Isocucurbitacin B inhibits glioma
growth through PI3K/AKT pathways and increases glioma sensitivity
to TMZ by inhibiting hsa-mir-1286a. Cancer Drug Resist.
7:162024.PubMed/NCBI
|
|
127
|
Yang JF, Chen TM, Chang HH, Tsai YL, Tsai
WC, Huang WY, Lo CH, Lin CS, Shen PC and Chen Y: Guggulsterone
inhibits migration and invasion through proteasomal and lysosomal
degradation in human glioblastoma cells. Eur J Pharmacol.
938:1754112023. View Article : Google Scholar
|
|
128
|
Wang X, Zou S, Ren T, Zhao LJ, Yu LF, Li
XY, Yan X and Zhang LJ: Alantolactone suppresses the metastatic
phenotype and induces the apoptosis of glioblastoma cells by
targeting LIMK kinase activity and activating the cofilin/G-actin
signaling cascade. Int J Mol Med. 47:682021. View Article : Google Scholar :
|
|
129
|
Dos Santos JS, Suzan AJ, Bonafé GA,
Fernandes AMAP, Longato GB, Antônio MA, Carvalho PO and Ortega MM:
Kaempferol and biomodified kaempferol from sophora japonica extract
as potential sources of anti-cancer polyphenolics against high
grade glioma cell lines. Int J Mol Sci. 24:107162023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Khathayer F and Ray SK: Diosgenin as a
novel alternative therapy for inhibition of growth, invasion, and
angiogenesis abilities of different glioblastoma cell lines.
Neurochem Res. 45:2336–2351. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu HY, Ji YL, Du H, Chen SH, Wang DP and
Lv QL: Bacoside a inhibits the growth of glioma by promoting
apoptosis and autophagy in U251 and U87 cells. Naunyn Schmiedebergs
Arch Pharmacol. 397:2105–2120. 2024. View Article : Google Scholar
|
|
132
|
Yang HL, Chang YH, Pandey S, Bhat AA,
Vadivalagan C, Lin KY and Hseu YC: Antrodia camphorataand coenzyme
Q0, a novel quinone derivative of antrodia camphorata, impede
HIF-1α and epithelial-mesenchymal transition/metastasis in human
glioblastoma cells. Environ Toxicol. 38:1548–1564. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Frontiñán-Rubio J, Llanos-González E,
García-Carpintero S, Peinado JR, Ballesteros-Yáñez I, Rayo MV, de
la Fuente J, Pérez-García VM, Perez-Romasanta LA, Malumbres M, et
al: CoQ10 reduces glioblastoma growth and infiltration through
proteome remodeling and inhibition of angiogenesis and
inflammation. Cell Oncol (Dordr). 46:65–77. 2023. View Article : Google Scholar
|
|
134
|
Małek A, Strzemski M, Kapka-Skrzypczak L
and Kurzepa J: Anticancer activity of melittin-containing bee venom
fraction against glioblastoma cells in vitro. Int J Mol Sci.
26:23762025. View Article : Google Scholar :
|
|
135
|
Eidizade F, Soukhtanloo M, Zhiani R,
Mehrzad J and Mirzavi F: Inhibition of glioblastoma proliferation,
invasion, and migration by urolithin B through inducing G0/G1
arrest and targeting MMP-2/-9 expression and activity. BioFactors.
49:379–389. 2023. View Article : Google Scholar
|
|
136
|
Doğanlar O, Doğanlar ZB, Delen E and Doğan
A: The role of melatonin in angio-miR-associated inhibition of
tumorigenesis and invasion in human glioblastoma tumour spheroids.
Tissue Cell. 73:1016172021. View Article : Google Scholar
|
|
137
|
Liu DN, Liu M, Zhang SS, Shang YF, Song
FH, Zhang HW, Du GH and Wang YH: Chrysomycin a inhibits the
proliferation, migration and invasion of U251 and U87-MG
glioblastoma cells to exert its anti-cancer effects. Molecules.
27:61482022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lin YH, Chen TM, Lai CR, Tsai YL, Tsai WC
and Chen Y: GW4064 inhibits migration and invasion in human
glioblastoma multiforme through the downregulation of PKCα. Eur J
Pharmacol. 991:1773292025. View Article : Google Scholar
|
|
139
|
Wen ZH, Chang L, Yang SN, Yu CL, Tung FY,
Kuo HM, Lu IC, Wu CY, Shih PC, Chen WF and Chen NF: The
anti-angiogenic and anti-vasculogenic mimicry effects of GN25 in
endothelial and glioma cells. Biochim Biophys Acta Mol Cell Res.
1871:1197992024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Shan W, Zuo K and Zuo Z: Hypoglycemic
agents increase regulatory factor X1 to inhibit cancer cell
behaviour in human glioblastoma cells. J Cell Mol Med.
28:e702602024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Benn KW, Yuan PH, Chong HK, Stylii SS,
Luwor RB and French CR: hERG channel agonist NS1643 strongly
inhibits invasive astrocytoma cell line SMA-560. PLoS One.
19:e03094382024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhao H, Xing F, Yuan J, Li Z and Zhang W:
Sevoflurane inhibits migration and invasion of glioma cells via
regulating miR-34a-5p/MMP-2 axis. Life Sci. 256:1178972020.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kang X, Li H and Zhang Z: Sevoflurane
blocks glioma malignant development by upregulating circRELN
through circRELN-mediated miR-1290/RORA axis. BMC Anesthesiol.
21:2132021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhan X, Lei C and Yang L: Sevoflurane
inhibits cell proliferation and migration of glioma by targeting
the miR-27b/VEGF axis. Mol Med Rep. 23:4082021. View Article : Google Scholar :
|
|
145
|
Zhang L, Chen H, Tian C and Zheng D:
Propofol represses cell growth and metastasis by modulating the
circular RNA non-SMC condensin I complex subunit
G/MicroRNA-200a-3p/RAB5A axis in glioma. World Neurosurg.
153:e46–e58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wang J, Zhang J, Zhang Q, Zhang W, Zhang
Q, Jin G and Liu F: TS-2021, a third-generation oncolytic
adenovirus that carried Ki67 promoter, TGF-β2 5'UTR, and IL−15
against experimental glioblastoma. J Med Virol. 96:e293352024.
View Article : Google Scholar
|
|
147
|
Fan X, Li J, Long L, Shi T, Liu D, Tan W,
Zhang H, Wu X, Lei X and Wang Z: Design, synthesis and biological
evaluation of N-anthraniloyl tryptamine derivatives as pleiotropic
molecules for the therapy of malignant glioma. Eur J Med Chem.
222:1135642021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Gabano E, Gariboldi MB, Caron G, Ermondi
G, Marras E, Vallaro M and Ravera M: Application of the
anthraquinone drug rhein as an axial ligand in bifunctional pt(iv)
complexes to obtain antiproliferative agents against human
glioblastoma cells. Dalton Trans. 51:6014–6026. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kuang J, Rao ZY, Zheng DW, Kuang D, Huang
QX, Pan T, Li H, Zeng X and Zhang XZ: Nanoparticles hitchhike on
monocytes for glioblastoma treatment after low-dose radiotherapy.
ACS Nano. 17:13333–13347. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Durand M, Chateau A, Jubréaux J, Devy J,
Paquot H, Laurent G, Bazzi R, Roux S, Richet N, Reinhard-Ruch A, et
al: Radiosensitization with gadolinium chelate-coated gold
nanoparticles prevents aggressiveness and invasiveness in
glioblastoma. Int J Nanomedicine. 18:243–261. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Horta M, Soares P, Sarmento B, Leite
Pereira C and Lima RT: Nanostructured lipid carriers for enhanced
batimastat delivery across the blood-brain barrier: An in vitro
study for glioblastoma treatment. Drug Deliv Transl Res.
15:2794–2813. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Gupta R and Sharma D:
(carboxymethyl-stevioside)-coated magnetic dots for enhanced
magnetic hyperthermia and improved glioblastoma treatment. Colloids
Surf, B. 205:1118702021. View Article : Google Scholar
|
|
153
|
Ke G, Hu P, Xiong H, Zhang J, Xu H, Xiao
C, Liu Y, Cao M and Zheng Q: Enhancing temozolomide efficacy in
GBM: The synergistic role of chuanxiong rhizoma essential oil.
Phytomedicine. 140:1565752025. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Pibuel MA, Poodts D, Sias SA, Byrne A,
Hajos SE, Franco PG and Lompardía SL: 4-methylumbelliferone
enhances the effects of chemotherapy on both temozolomide-sensitive
and resistant glioblastoma cells. Sci Rep. 13:93562023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Chen J, Zhuang YD, Zhang Q, Liu S, Zhuang
BB, Wang CH and Liang RS: Exploring the mechanism of cordycepin
combined with doxorubicin in treating glioblastoma based on network
pharmacology and biological verification. PeerJ. 10:e129422022.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Wang J, Ren D, Sun Y, Xu C, Wang C, Cheng
R, Wang L, Jia G, Ren J, Ma J, et al: Inhibition of PLK4 might
enhance the anti-tumour effect of bortezomib on glioblastoma via
PTEN/PI3K/AKT/mTOR signalling pathway. J Cell Mol Med.
24:3931–3947. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Qin JJ, Niu MD, Cha Z, Geng QH, Li YL, Ren
CG, Molloy DP and Yu HR: TRAIL and celastrol combinational
treatment suppresses proliferation, migration, and invasion of
human glioblastoma cells via targeting wnt/β-catenin signaling
pathway. Chin J Integr Med. 30:322–329. 2024. View Article : Google Scholar
|
|
158
|
Ebrahimi S, Mirzavi F, Hashemy SI,
Khaleghi Ghadiri M, Stummer W and Gorji A: The in vitro anti-cancer
synergy of neurokinin-1 receptor antagonist, aprepitant, and
5-aminolevulinic acid in glioblastoma. Biofactors. 49:900–911.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Torsvik A, Stieber D, Enger PØ,
Golebiewska A, Molven A, Svendsen A, Westermark B, Niclou SP, Olsen
TK, Chekenya Enger M and Bjerkvig R: U-251 revisited: Genetic drift
and phenotypic consequences of long-term cultures of glioblastoma
cells. Cancer Med. 3:812–824. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2011-2015. Neuro Oncol. 20(suppl_4): iv1–iv86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Gritsch S, Batchelor TT and Gonzalez
Castro LN: Diagnostic, therapeutic, and prognostic implications of
the 2021 world health organization classification of tumors of the
central nervous system. Cancer. 128:47–58. 2022. View Article : Google Scholar
|
|
162
|
Satgunaseelan L, Sy J, Shivalingam B, Sim
HW, Alexander KL and Buckland ME: Prognostic and predictive
biomarkers in central nervous system tumours: The molecular state
of play. Pathology. 56:158–169. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Groves MD, Puduvalli VK, Hess KR, Jaeckle
KA, Peterson P, Yung WK and Levin VA: Phase II trial of
temozolomide plus the matrix metalloproteinase inhibitor,
marimastat, in recurrent and progressive glioblastoma multiforme. J
Clin Oncol. 20:1383–1388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wolburg H, Wolburg-Buchholz K,
Fallier-Becker P, Noell S and Mack AF: Structure and functions of
aquaporin-4-based orthogonal arrays of particles. Int Rev Cell Mol
Biol. 287:1–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Endicott JA and Ling V: The biochemistry
of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem.
58:137–171. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Van Tellingen O, Yetkin-Arik B, De Gooijer
MC, Wesseling P, Wurdinger T and De Vries HE: Overcoming the
blood-brain tumor barrier for effective glioblastoma treatment.
Drug Resist Updat. 19:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Parrish KE, Sarkaria JN and Elmquist WF:
Improving drug delivery to primary and metastatic brain tumors:
Strategies to overcome the blood-brain barrier. Clin Pharmacol
Ther. 97:336–346. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
168
|
Oberoi RK, Parrish KE, Sio TT, Mittapalli
RK, Elmquist WF and Sarkaria JN: Strategies to improve delivery of
anticancer drugs across the blood-brain barrier to treat
glioblastoma. Neuro Oncol. 18:27–36. 2016. View Article : Google Scholar
|
|
169
|
Wu D, Chen Q, Chen X, Han F, Chen Z and
Wang Y: The blood-brain barrier: Structure, regulation and drug
delivery. Signal Transduct Target Ther. 8:2172023. View Article : Google Scholar
|
|
170
|
Holmbeck K, Bianco P, Caterina J, Yamada
S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I,
et al: MT1-MMP-deficient mice develop dwarfism, osteopenia,
arthritis, and connective tissue disease due to inadequate collagen
turnover. Cell. 99:81–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Martignetti JA, Aqeel AA, Sewairi WA,
Boumah CE, Kambouris M, Mayouf SA, Sheth KV, Eid WA, Dowling O,
Harris J, et al: Mutation of the matrix metalloproteinase 2 gene
(MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat
Genet. 28:261–265. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
172
|
Barish ME, Aftabizadeh M, Hibbard J,
Blanchard MS, Ostberg JR, Wagner JR, Manchanda M, Paul J, Stiller
T, Aguilar B, et al: Chlorotoxin-directed CAR T cell therapy for
recurrent glioblastoma: Interim clinical experience demonstrating
feasibility and safety. Cell Rep Med. 6:1023022025. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Thanh HD, Lee S, Nguyen TT, Huu TN, Ahn
EJ, Cho SH, Kim MS, Moon KS and Jung C: Temozolomide promotes
matrix metalloproteinase 9 expression through p38 MAPK and JNK
pathways in glioblastoma cells. Sci Rep. 14:143412024. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Whitehead CA, Morokoff AP, Kaye AH,
Drummond KJ, Mantamadiotis T and Stylli SS: Invadopodia associated
thrombospondin-1 contributes to a post-therapy pro-invasive
response in glioblastoma cells. Exp Cell Res. 431:1137432023.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Żwierełło W, Maruszewska A,
Skórka-Majewicz M, Wszołek A and Gutowska I: Is fluoride
blameless?-the influence of fluorine compounds on the invasiveness
of the human glioma-like cell line U-87. Int J Mol Sci.
25:127732024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Huang W, Li J, Geng X, Li S, Zou Y, Li Y,
Jing C and Yu H: The reactive astrocytes after surgical brain
injury potentiates the migration, invasion, and angiogenesis of C6
glioma. World Neurosurg. 168:e595–e606. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ahmed MM, Hossain MM, Islam MR, Ali MS,
Nafi AAN, Ahmed MF, Ahmed KM, Miah MS, Rahman MM, Niu M and Islam
MK: Brain tumor detection and classification in MRI using hybrid
ViT and GRU model with explainable AI in Southern Bangladesh. Sci
Rep. 14:227972024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Rastogi A, Brugnara G, Foltyn-Dumitru M,
Mahmutoglu MA, Preetha CJ, Kobler E, Pflüger I, Schell M,
Deike-Hofmann K, Kessler T, et al: Deep-learning-based
reconstruction of undersampled MRI to reduce scan times: A
multicentre, retrospective, cohort study. Lancet Oncol. 25:400–410.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Lu CH, Wei ST, Liu JJ, Chang YJ, Lin YF,
Yu CS and Chang SL: Recognition of a novel gene signature for human
glioblastoma. Int J Mol Sci. 23:41572022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Shi J: Machine learning and bioinformatics
approaches for classification and clinical detection of bevacizumab
responsive glioblastoma subtypes based on miRNA expression. Sci
Rep. 12:86852022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Rivera CA, Bhatia S, Uppalapati V, Berke
CN, Merenzon MA, Daggubati LC, Levy AS, Shah AH, Komotar RJ and
Ivan ME: Leveraging machine learning for preoperative prediction of
supramaximal ablation in laser interstitial thermal therapy for
brain tumors. Neurosurg Focus. 57:E62024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kuenzi BM, Park J, Fong SH, Sanchez KS,
Lee J, Kreisberg JF, Ma J and Ideker T: Predicting drug response
and synergy using a deep learning model of human cancer cells.
Cancer Cell. 38:672–684.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Marques L, Costa B, Pereira M, Silva A,
Santos J, Saldanha L, Silva I, Magalhães P, Schmidt S and Vale N:
Advancing precision medicine: A review of innovative in silico
approaches for drug development, clinical pharmacology and
personalized healthcare. Pharmaceutics. 16:3322024. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Seo S, Nah S, Lee K, Choi N and Kim HN:
Triculture model of in vitro BBB and its application to study
BBB-associated chemosensitivity and drug delivery in glioblastoma.
Adv Funct Mater. 32:21068602022. View Article : Google Scholar
|
|
185
|
Tricinci O, De Pasquale D, Marino A,
Battaglini M, Pucci C and Ciofani G: A 3D biohybrid real-scale
model of the brain cancer microenvironment for advanced in vitro
testing. Adv Mater Technol. 5:20005402020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Zhu D, Trinh P, Li J, Grant GA and Yang F:
Gradient hydrogels for screening stiffness effects on
patient-derived glioblastoma xenograft cellfates in 3D. J Biomed
Mater Res A. 109:1027–1035. 2021. View Article : Google Scholar
|
|
187
|
Wang C, Sinha S, Jiang X, Murphy L, Fitch
S, Wilson C, Grant G and Yang F: Matrix stiffness modulates
patient-derived glioblastoma cell fates in three-dimensional
hydrogels. Tissue Eng Part A. 27:390–401. 2021. View Article : Google Scholar :
|