Osteoporosis, a prevalent metabolic bone disease
with notable medical and socioeconomic implications, is
characterized by low bone mass and microarchitectural
deterioration, leading to weakened bone strength and an increased
risk of fragility fractures (1).
According to the National Health and Nutrition Examination Survey
in the United States, ~20% of postmenopausal women have been
diagnosed with osteoporosis (2).
As the global population ages, this prevalence is expected to
increase in the coming years (3). For individuals with osteoporosis,
the lifetime risk of fractures can reach up to 40%; from the
perspective of patients, fractures, along with the subsequent loss
of mobility and independence, often result in a substantial decline
in quality of life. Moreover, osteoporotic fractures, particularly
of the hip and spine, are associated with a 12-month excess
mortality rate of up to 20%, primarily due to hospitalization,
which increases the risk of complications such as pneumonia or
thromboembolic events from prolonged immobility, further
compounding the public health burden (4). The pathophysiology of osteoporosis
is marked by an imbalance between bone resorption and formation,
which is influenced by factors such as aging, hormonal changes,
nutritional deficiencies and genetics (5,6).
Notably, the interaction between osteoblasts and osteoclasts is
crucial for maintaining bone homeostasis. Osteoblasts express and
secrete receptor activator of NF-κB ligand (RANKL), which promotes
the differentiation of osteoclast precursors into mature
osteoclasts. Conversely, when bone resorption disrupts the bone
structure, osteoclasts release insulin-like growth factors (IGFs),
which stimulate osteogenic differentiation (7). However, dysregulated bone
remodeling negatively affects bone mass. A reduction in osteoblast
activity, responsible for producing the calcified organic
extracellular matrix, leads to increased bone resorption.
Simultaneously, osteoclasts degrade the extracellular matrix and
the cumulative effect of these processes can result in bone loss
(8). Understanding the
underlying mechanisms of bone remodeling and the factors
influencing bone mass is critical for developing effective
strategies to prevent and treat osteoporosis.
Branched-chain amino acids (BCAAs), including
leucine, isoleucine and valine, are essential amino acids primarily
known for their role in muscle protein synthesis (9). In addition to their function in
skeletal muscle, BCAAs have been linked to the regulation of
metabolic disorders such as diabetes, cardiovascular diseases and
cancer (10). They may also
influence aging and longevity in animal models (11). Recent research has identified a
range of molecules involved in maintaining bone homeostasis, with
BCAAs emerging as potential modulators within this regulatory
network (12).
The present review summarizes the interactions
between bone metabolism and BCAA metabolism, focusing on the causes
and consequences of metabolic abnormalities. It also explores the
relationship between bone metabolism and osteoporosis, highlighting
how dysregulated BCAA metabolism may contribute to osteoporosis.
Finally, the potential of BCAAs as a treatment for osteoporosis is
discussed, offering novel insights into their role in bone health.
In conclusion, the current review may enhance understanding of the
mechanisms by which BCAAs contribute to the pathogenesis of
osteoporosis. It not only provides dietary recommendations but also
emphasizes the potential for BCAA-based interventions in the
management of osteoporosis, paving the way for novel therapeutic
approaches to combat this disease.
Bone is a dynamic, metabolically active tissue and
an essential organ system in higher vertebrates, serving as the
primary structural component of the skeleton. It is a specialized
connective tissue composed of osteocytes, osteoblasts, bone-lining
cells and osteoclasts. The bone matrix consists of ~60% inorganic
mineral phase (primarily semi-crystalline, carbonated
hydroxyapatite), 30% organic phase, which includes type I collagen
fibrils and non-collagenous proteins, and 10% water (13). Additionally, small leucine-rich
proteoglycans, a key component of non-collagenous proteins, are
present in the bone matrix (14).
Bone performs four critical functions: i) Providing
structural support and anchorage for muscles, ii) protecting vital
organs such as the brain and bone marrow, iii) supporting
hematopoiesis (15-17), and iv) serving as a metabolic
reservoir for calcium and phosphate. These physiological functions
rely on the tightly regulated processes of bone modeling and
remodeling to maintain skeletal homeostasis (18).
Bone remodeling involves the resorption of old or
damaged bone by osteoclasts, followed by the formation of new bone
by osteoblasts (19). In
contrast to bone modeling, remodeling is a coupled, spatially and
temporally coordinated process that does not alter bone shape
(20). It is a tightly regulated
physiological process and dysfunction in any component of this
system can disrupt bone metabolism, contributing to conditions such
as osteoporosis (21).
Bone metabolism is a complex process precisely
coordinated by osteoclasts, osteoblasts and osteocytes. Osteoclasts
originate from mononuclear precursors of the monocyte/macrophage
lineage, and their differentiation and activation depend on two
critical signals: Macrophage colony-stimulating factor (22) and RANKL (23,24), as substantiated by Nakahama et
al (25). The binding of
RANKL to receptor activator of NF-κB (RANK) on osteoclast
precursors initiates osteoclastogenesis (26). Mature, functional osteoclasts
form a ruffled border mediated by Src kinase (27,28) and secrete cathepsin K to degrade
the bone matrix, accomplishing bone resorption (29,30). Gelb et al (29) demonstrated that osteoclasts from
human patients with cathepsin K gene mutations fail to efficiently
degrade the bone organic matrix, providing direct clinical evidence
linking cathepsin K to osteoclastic resorption.
Osteoblasts are derived from various sources,
including bone marrow stromal cells and bone-lining cells (31). During bone resorption, factors
released from the bone matrix, such as transforming growth factor-β
(TGF-β) and IGF-1, recruit osteoblast precursors to the bone
surface (32). Osteoblast
differentiation is primarily regulated by the canonical
Wnt/β-catenin signaling pathway. Upon activation of this pathway,
β-catenin is stabilized and translocates into the nucleus, where it
binds to LEF/TCF transcription factors. This interaction activates
the expression of osteogenic genes such as Runx2, promoting the
differentiation of osteoblasts from progenitor cells into mature
cells (33). Mature osteoblasts
then secrete type I collagen and other components, forming osteoid
and facilitating its mineralization to build new bone. After
completing their task, most osteoblasts undergo apoptosis, while
some differentiate into osteocytes or revert to quiescent
bone-lining cells (34,35).
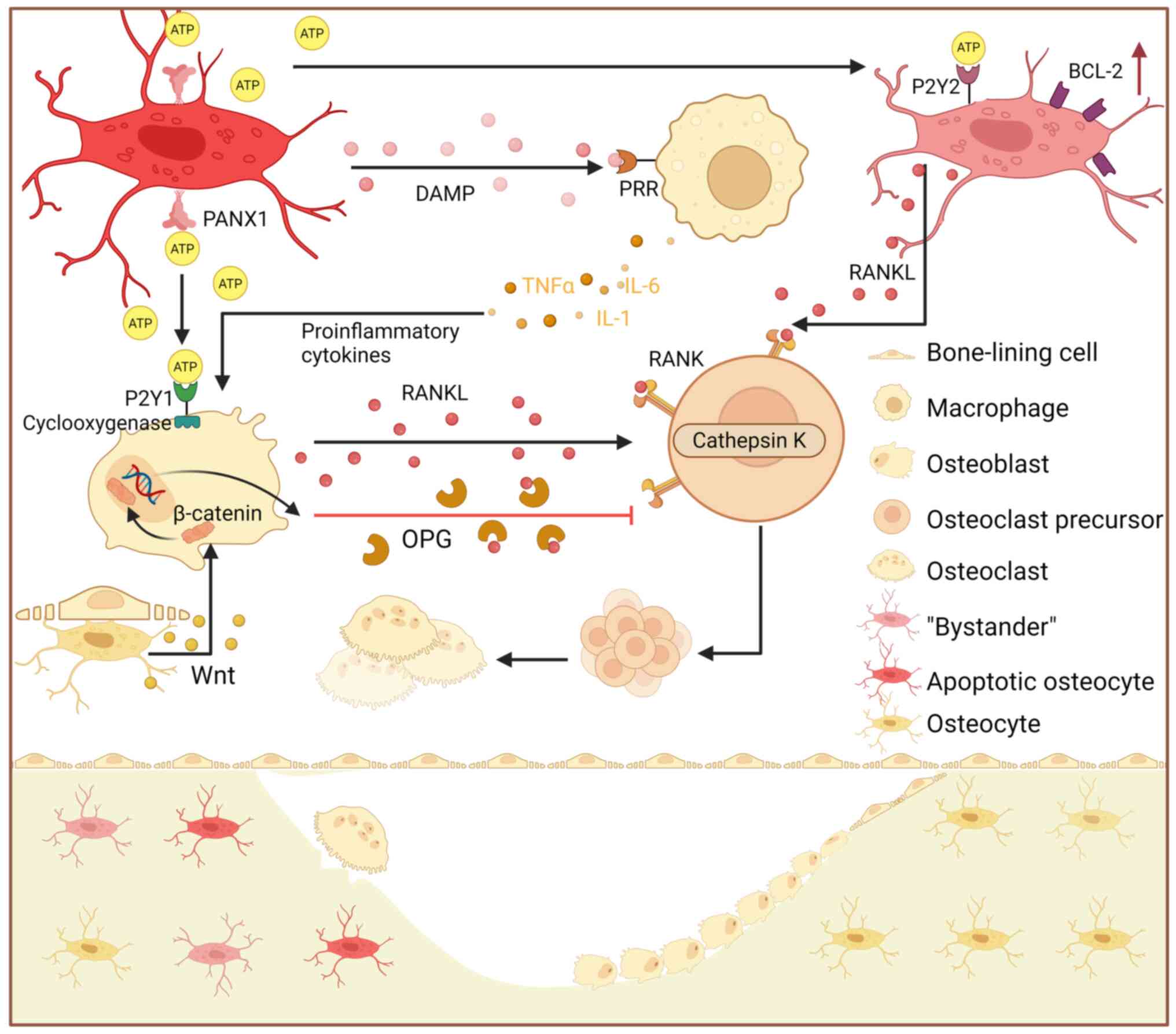
Osteocytes, embedded within the mineralized matrix,
serve as key regulators of bone homeostasis (36). By contrast, necrotic osteocytes
release damage-associated molecular patterns (37), which bind to pattern recognition
receptors (38), initiating the
production of proinflammatory cytokines, such as TNFα, IL-1 and
IL-6 (39). These cytokines
indirectly amplify RANKL signaling (40) by increasing RANKL expression in
osteoblasts (41). Osteocyte
apoptosis is a critical trigger for bone remodeling (37), as these cells recruit osteoclasts
to microcracks, initiating targeted bone resorption and maintaining
dynamic bone tissue homeostasis (42). Kringelbach et al (41) demonstrated that apoptosis in the
MLO-Y4 osteocyte cell line induces the release of adenosine
triphosphate (ATP) via the activation of Pannexin 1 (PANX1)
channels. A similar phenomenon has been observed in mice treated
with a P2X7 receptor (P2X7R) inhibitor, highlighting the role of
P2X7R as a coactivator of PANX1 (43). The released ATP subsequently
stimulates RANKL expression through a signaling cascade involving
the purinergic receptor P2Y (P2Y)1 receptor and cyclooxygenase
activity (44-46). ATP released from apoptotic
osteocytes likely binds to P2Y2 receptors on osteocytes in the
penumbra regions, known as 'bystander' osteocytes (47); this binding may also trigger
RANKL production and release (43).
The termination of bone remodeling involves multiple
steps, including the secretion of osteoprotegerin (OPG) by mature
osteoblasts and/or osteocytes, which may serve as a key inhibitory
signal (48). Additionally,
upregulation of the anti-apoptotic protein BCL-2 in 'bystander'
osteocytes near the damage site helps protect viable osteocytes
from osteoclastogenic stimuli originating from adjacent apoptotic
cells (Fig. 1) (49,50).
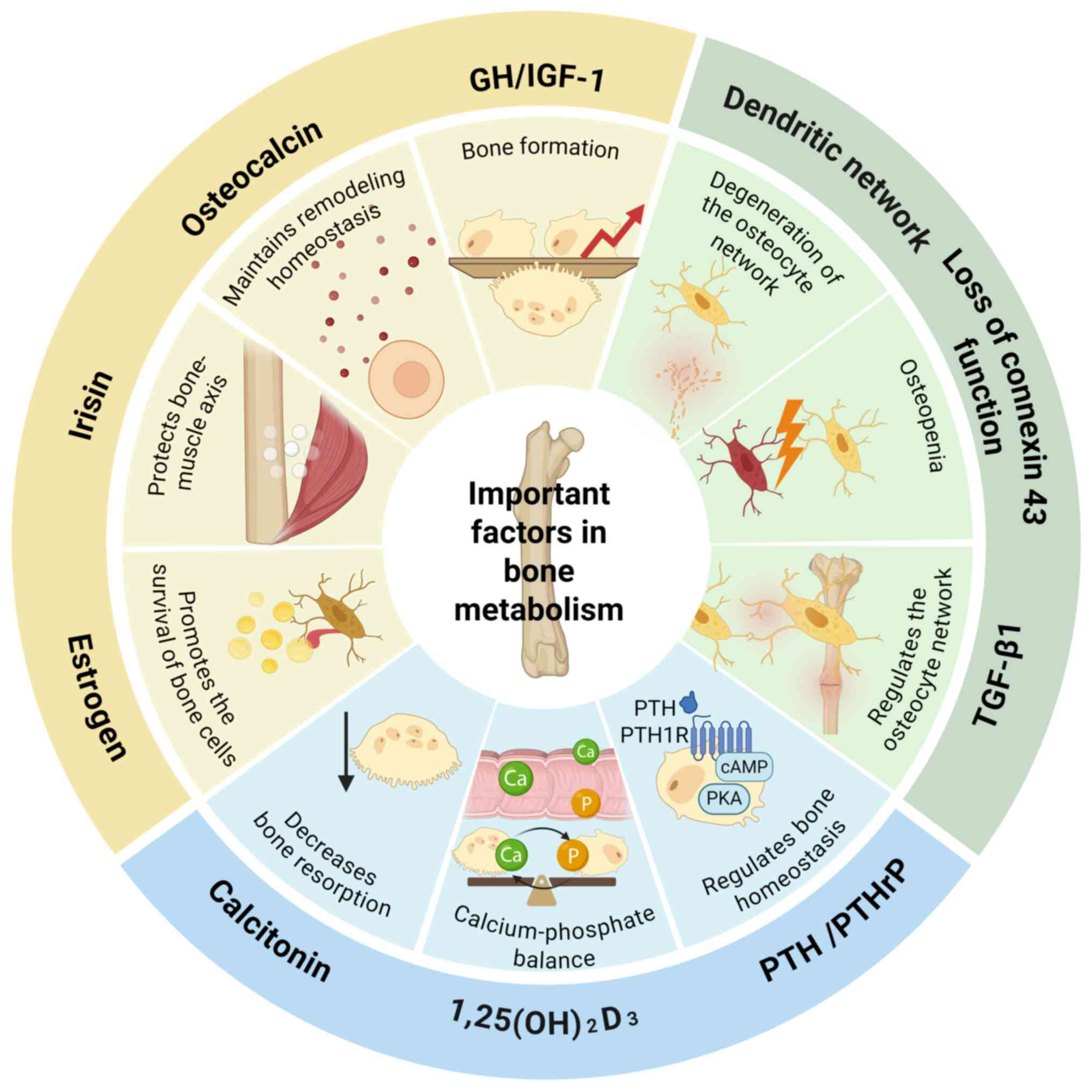
Bone metabolism is regulated by both local
intercellular signaling among bone cells and a range of systemic
factors. Calcium and phosphate regulators maintain mineral
homeostasis, which is crucial for bone mineralization.
1,25(OH)2D3 enhances intestinal calcium and
phosphate absorption directly (51), while also modulating osteoblast
and osteoclast activity (52-54). Concurrently, parathyroid hormone
(PTH) and PTH-related peptide regulate calcium-phosphate
homeostasis in bone and the kidneys by activating the cyclic
adenosine monophosphate-protein kinase A pathway through the PTH1
receptor (55,56). Elevated phosphate levels, in
turn, stimulate the secretion of fibroblast growth factor 23, which
suppresses 1,25(OH)2D3 synthesis and promotes
renal phosphate excretion, creating a critical feedback loop
(57,58). Calcitonin acts antagonistically
by directly inhibiting bone resorption to lower blood calcium
levels (59,60), thereby ensuring precise
regulation of calcium homeostasis.
Osteocyte communication and the integrity of the
network are central to the mechanosensing and signaling system of
bone. Connexin 43 (Cx43) underpins intercellular communication
among osteocytes, and its loss of function directly impairs bone
mass acquisition and maintenance (61-63). Similarly, the integrity of the
osteocyte dendritic network is essential, with age-related declines
in network function directly associated with cortical bone
deterioration (64).
Additionally, TGF-β1 signaling is a key intrinsic regulator of this
network, preserving bone mechanical integrity by modulating
dendritic length and the expression of genes such as sclerostin and
MMPs (65). These factors,
particularly in aging and metabolic diseases (including diabetes
and obesity), interact with the broader bone metabolism regulatory
network (66).
The balance between bone formation and resorption
directly determines the net outcome of bone remodeling. For
example, growth hormone, by stimulating IGF-1 expression, increases
bone turnover with a net anabolic effect, leading to modest bone
mass gain (67,68). Estrogen promotes osteocyte
survival via semaphorin 3A (69), fluid flow shear stress (70), Wnt/β-catenin signaling (71) and Cx43 expression (72), helping to counteract age-related
bone loss. The myokine irisin also serves a protective role in the
bone-muscle axis by reducing osteocyte apoptosis (73). Additionally, vitamin K-dependent
osteocalcin, secreted by osteoblasts, connects bone metabolism to
systemic energy metabolism, broadening the regulation of bone
homeostasis (Fig. 2) (74).
Bone metabolic imbalance is a complex condition
arising from a variety of physiological and pathological factors. A
primary underlying mechanism is the disruption of the dynamic
balance between osteoclastic bone resorption and osteoblastic bone
formation. This dysregulation can lead to skeletal disorders such
as osteoporosis, which is characterized by reduced bone mass and
increased susceptibility to fractures. Contributing factors include
hormonal imbalances, inadequate nutrition and genetic
predispositions (75).
Hormonal changes, particularly those involving
estrogen and PTH, serve a central role in bone metabolism. Estrogen
deficiency, commonly observed in postmenopausal women, accelerates
bone resorption, thereby promoting osteoporosis (76). Similarly, parathyroid gland
disorders disrupt calcium and phosphate metabolism, further
exacerbating bone loss (77).
The skeleton also functions as an endocrine organ; hormones such as
osteocalcin regulate both energy metabolism and bone homeostasis,
highlighting the close link between skeletal and metabolic health
(78).
Nutritional factors markedly influence bone health.
Adequate calcium and vitamin D intake is essential for maintaining
bone density, with deficiencies in these nutrients leading to
impaired bone mineralization and an increased fracture risk
(79). Additionally, while high
protein intake exerts a bone-anabolic effect, this benefit can be
counteracted by a high dietary acid load, ultimately promoting bone
loss (80). The role of vitamin
K, particularly in relation to osteocalcin, is also important;
vitamin K is involved in the carboxylation of osteocalcin, a
process essential for bone mineralization and metabolic regulation
(74).
Genetic factors and metabolic disorders predispose
individuals to bone metabolism disorders. Conditions such as
hypophosphatasia and lysosomal storage disease result in skeletal
abnormalities and heightened osteoporosis risk. These genetic
disorders often cause imbalances in calcium and phosphate
metabolism, which are critical for bone health (81). Additionally, abnormalities in
purine metabolism have been identified as a notable factor in bone
remodeling, contributing to osteoporosis in various high-risk
populations (82).
Environmental and lifestyle factors, such as
physical inactivity, smoking and excessive alcohol consumption, are
well-established negative influences on bone health. These factors
increase oxidative stress and inflammation, both of which are
detrimental to bone remodeling (83-85). The accumulation of advanced
oxidation protein products has been shown to contribute to bone-fat
imbalance during skeletal aging, highlighting the impact of
oxidative stress on bone metabolism (Table I) (86-88).
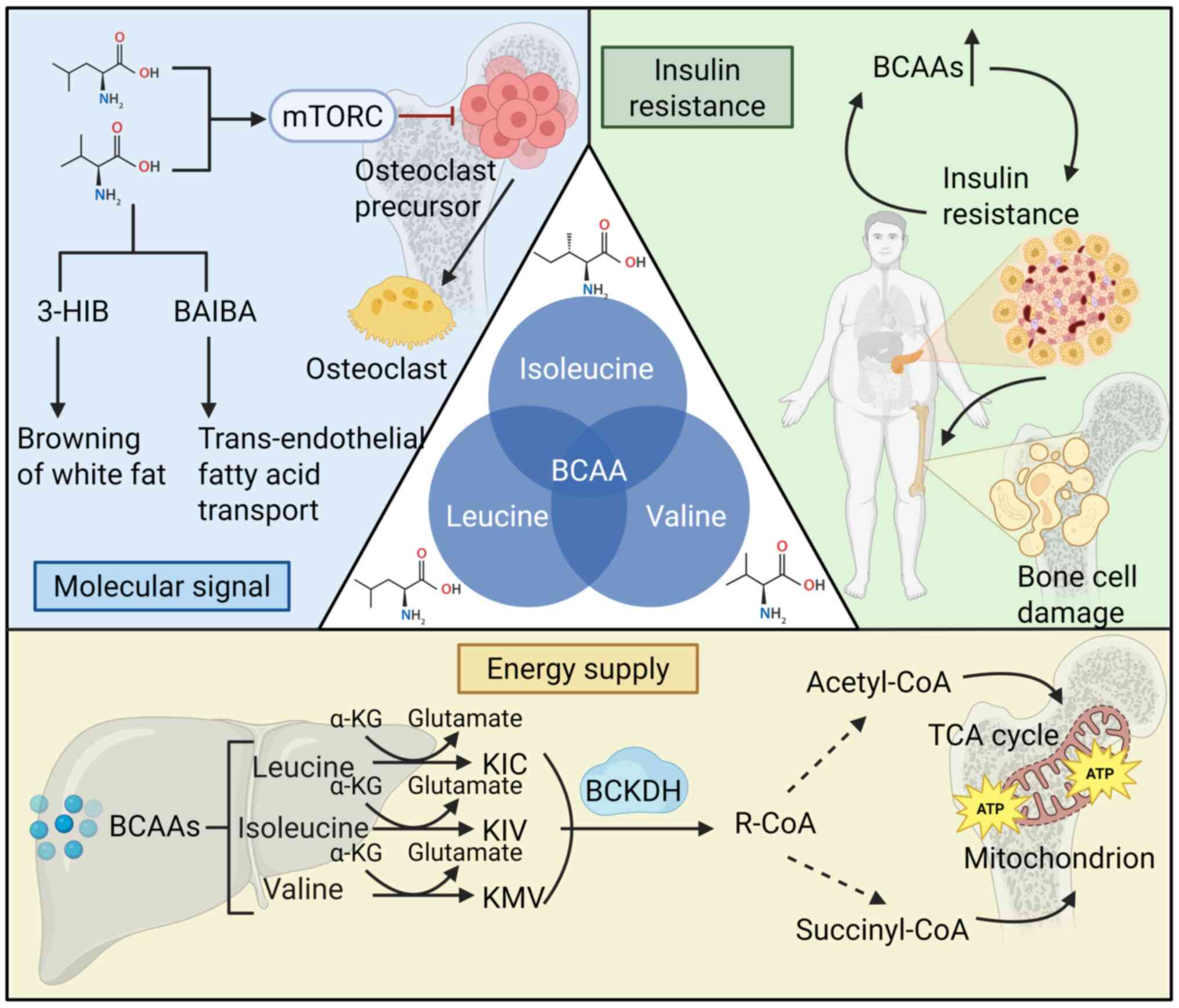
BCAAs, comprising leucine, isoleucine and valine,
are essential amino acids that must be sourced from the diet, as
they cannot be synthesized endogenously in animals (89). Despite being classified together
as BCAAs, leucine, isoleucine and valine exhibit distinct
structural, metabolic and functional characteristics, which
underlie their intricate regulation of bone metabolism (90). Leucine, with its highly
hydrophobic isobutyl side chain, is the most hydrophobic of the
three, a property essential for its function as a signaling
molecule. This hydrophobicity facilitates the role of leucine in
activating downstream targets, establishing its dominant role in
signal transduction (91).
Beyond serving as fundamental components for protein
synthesis, BCAAs are key regulators of cellular metabolism, energy
production and signal transduction (92). After ingestion, BCAAs undergo
enzymatic transformation across various tissues, with the highest
concentrations observed in skeletal muscle, brown adipose tissue,
liver, kidney and heart (93).
Enzymes such as BCAA transaminase (BCAT) and branched-chain
α-ketoacid dehydrogenase (BCKDH) catalyze the production of
important metabolites, including branched-chain α-ketoacids
(BCKAs), acetyl-CoA and succinyl-CoA, which contribute to processes
such as the tricarboxylic acid cycle and fatty acid metabolism
(Fig. 3) (94). The distinct metabolic pathways of
the three BCAAs suggest that they may exert differential effects on
bone metabolism by regulating cellular energy status, metabolite
pools and signal transduction pathways.
As critical nutrient signals, BCAAs modulate
cellular metabolism and energy production. They activate anabolic
pathways, particularly the mechanistic target of rapamycin (mTOR)
signaling pathway, which promotes protein synthesis in skeletal
muscle and enhances immune cell proliferation and function,
including T cells and natural killer cells (95). Additionally, emerging evidence
has highlighted a 'muscle-bone axis', where skeletal muscle and
bone tissue interact bidirectionally (96). Immune cells, including osteoclast
precursors and macrophages, also serve pivotal roles in bone
resorption and remodeling (97).
By influencing both the musculoskeletal and immune systems, BCAAs
likely exert indirect yet notable effects on bone metabolism and
homeostasis. Further research is needed to elucidate the direct
regulatory roles of BCAAs in bone metabolism, particularly through
pathways involving cellular metabolism and energy balance.
BCAAs and their metabolic derivatives function as
molecular signals in bone metabolism. Both leucine (98) and valine (99) activate the mTOR complex 1
(mTORC1) pathway. The biochemical pathways of BCAA metabolism are
well-characterized, and preclinical studies have indicated that
inhibition of mTOR suppresses osteoclast differentiation and
survival, potentially impairing bone resorption (100-103). These findings are further
supported by the BOLERO-2 trial, where everolimus, a clinically
used mTOR inhibitor, demonstrated beneficial effects on bone in
patients with advanced breast cancer (104). Metabolic derivatives of valine,
such as β-aminoisobutyric acid (BAIBA) and 3-hydroxyisobutyric acid
(3-HIB), act as myo-osseous metabolic messengers, regulating bone
homeostasis through distinct signaling pathways. BAIBA, regulated
by peroxisome proliferator-activated receptor (PPAR)γ
coactivator-1α (PGC-1α), activates the PPARα pathway, inducing
white adipose tissue browning, improving insulin sensitivity and
potentially enhancing osteogenesis while inhibiting
osteoclastogenesis (105).
Conversely, 3-HIB promotes trans-endothelial fatty acid transport
via FATP3/4, leading to lipotoxicity and insulin resistance, which
may disrupt bone homeostasis (106). Further research is needed to
better define the direct roles of BCAAs in bone metabolism through
these signal transduction pathways, supported by additional
well-designed clinical studies.
Metabolomic analyses from large randomized
controlled trials have established circulating BCAA levels as key
indicators of metabolic health. These levels, beyond body mass
index, predict the risk of insulin resistance, cardiovascular
diseases and diabetes, and reveal ethnic differences in
obesity-associated metabolic disturbances (107,108). A Mendelian randomization study
further indicated that elevated BCAA levels are linked to an
increased risk of type 2 diabetes (109). Collectively, these findings
suggest that dysregulated BCAA metabolism contributes to the
pathogenesis of insulin resistance, obesity and diabetes (110,111). Moreover, insulin resistance may
promote BCAA accumulation (112,113), creating a self-perpetuating
cycle. Insulin resistance, frequently accompanied by altered BCAA
metabolism, adversely affects bone health by impairing osteoblast
function and inhibiting bone formation (Fig. 3) (114).
Disorders in BCAA metabolism contribute to a complex
pathological network that impacts bone metabolism. This network
involves both direct regulation of bone cells (osteoblasts and
osteoclasts) (115), and
indirect effects mediated by systemic metabolic disturbances,
ultimately disrupting bone homeostasis (114,116).
A key mechanism through which BCAA metabolism
directly influences bone metabolism is its role in osteoclast
maturation. Studies have shown that inhibition of BCAT1, a key
enzyme in BCAA metabolism, effectively reduces osteoclast
maturation (12,117). BCAT1 catalyzes the conversion
of BCAAs to their metabolites (BCKAs), which fuel cellular energy
production (90) and sustain the
expression of essential osteoclastogenic factors (such as NFATc1
and ATP6v0d2) (118), as well
as the regulation of fusion-related genes (including DCSTAMP and
CD9) (119,120). These processes collectively
underlie its critical role in the later stages of osteoclast
differentiation. In vivo animal experiments have further
confirmed that BCAT1 is central to the osteoclast multinucleation
regulatory network. Its deletion markedly inhibits osteoclast
activity, increases bone mass and improves bone strength. These
results establish a strong link between abnormal BCAA metabolism
and bone metabolic disorders mediated by osteoclasts (117).
In contrast to osteoclasts, the impact of BCAA
metabolism on osteoblasts is more complex, highlighting the adverse
effects of abnormal BCAA metabolism on bone health. Singha et
al (121) demonstrated that
rapamycin, an mTOR inhibitor, can effectively suppress osteoblast
proliferation and differentiation by reducing RUNX2 protein
expression, decreasing alkaline phosphatase activity and inhibiting
matrix mineralization, supporting the pro-osteogenic role of mTORC1
in osteogenesis. However, dysregulated BCAA metabolism, such as
chronic overaccumulation or insufficient breakdown products, may
disrupt the mTORC1 pathway (122). Prolonged activation of mTORC1
can induce 'metabolic stress' in osteoblasts, characterized by
G1 phase cell cycle arrest and reduced proliferative
capacity, ultimately impairing bone metabolism (123). These findings suggest that
BCAAs regulate osteoblast differentiation to a certain extent via
the mTOR signaling pathway; however, its translational
applicability in humans remains to be confirmed through rigorously
designed clinical investigations.
High glutamine levels further inhibit the
mTORC2/Akt-S473/RUNX2 axis by activating mTORC1, which leads to the
ubiquitin-mediated degradation of RUNX2, thereby suppressing
osteogenic differentiation (124). These findings indicate that the
regulatory role of mTORC1 signaling in osteoblast differentiation
may be context-dependent, necessitating additional in vitro
experiments or clinical studies to confirm these results.
As aforementioned, dysregulated BCAA metabolism is
linked to various metabolic disorders, including obesity and type 2
diabetes, both of which are well-established risk factors for
osteoporosis and other bone-related conditions (125). In patients with obesity and
type 2 diabetes, elevated BCAA levels are strongly associated with
insulin resistance. BCAAs activate the mTORC1 signaling pathway,
which decouples insulin signal transduction, thereby exacerbating
insulin resistance (114,126). Furthermore, intermediate
products of BCAA metabolism, such as BCKAs, inhibit insulin signal
transduction, further aggravating metabolic disorders (127). These metabolic disruptions not
only affect systemic metabolism but also impair bone homeostasis by
disrupting energy metabolism and signal transduction in osteocytes
(115,128).
Additionally, BCAAs influence lipid metabolism, and
disruptions in this pathway can lead to altered lipid profiles that
may negatively affect bone density and strength. Impaired BCAA
catabolism has been linked to lipid accumulation, which can
adversely impact bone tissue (129,130). BCAA metabolic disorders may
worsen the complex pathological network of bone metabolism by
modulating oxidative stress and inflammatory responses. Elevated
BCAA and fatty acid levels induce mitochondrial dysfunction,
increase oxidative stress, and activate inflammatory signaling
pathways, all of which can contribute to bone metabolic diseases,
such as osteoporosis, by impairing osteocyte function and
disrupting the bone microenvironment (131,132). Furthermore, BCAA metabolic
disorders may indirectly influence bone metabolism by interfering
with lipid metabolism (133).
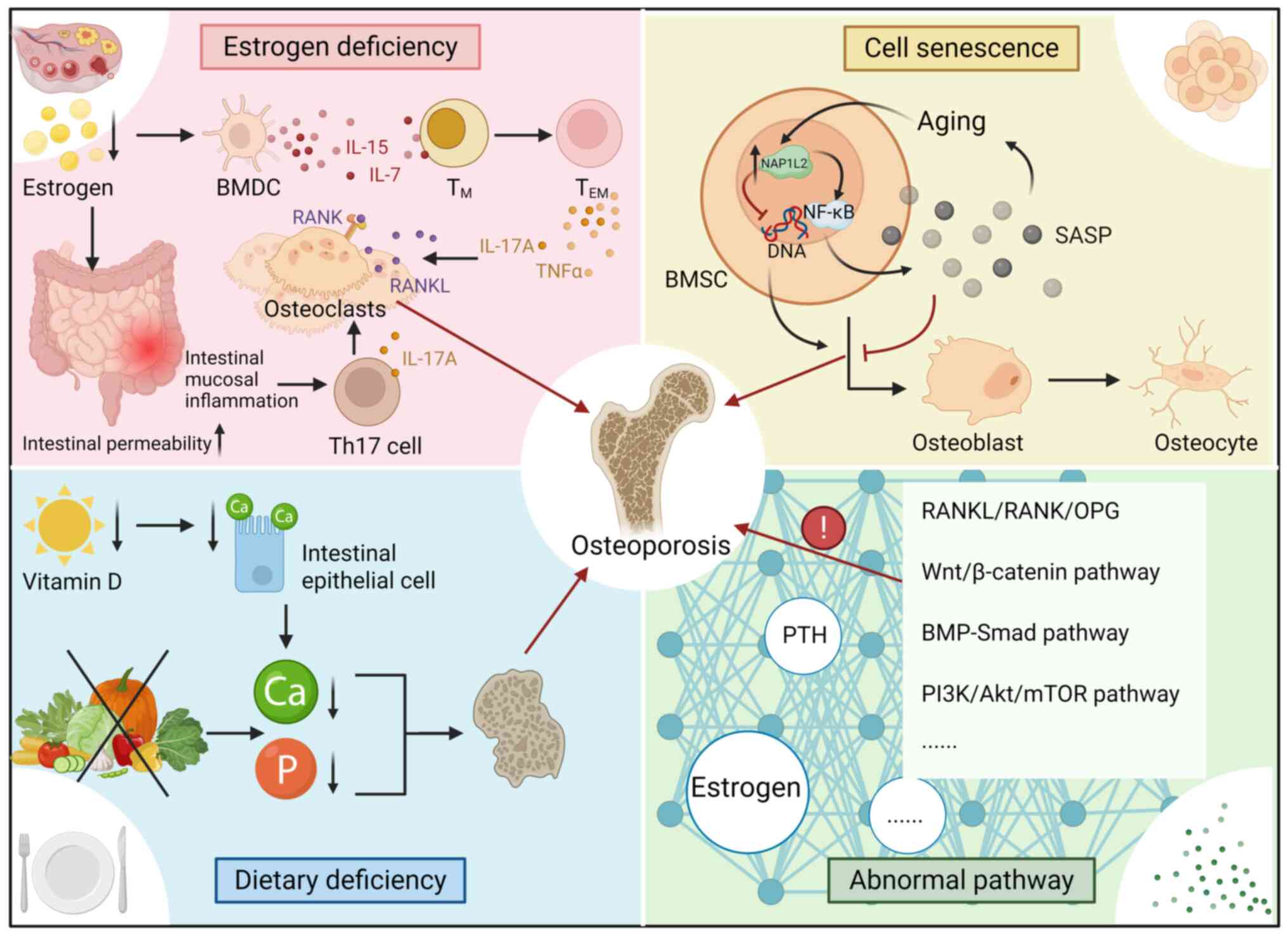
Osteoporosis is a prevalent metabolic bone disorder
characterized by generalized bone mass loss and deterioration of
bone microarchitecture, which increases bone fragility and the risk
of fragility fractures (135).
Notably, osteoporosis also elevates the likelihood of secondary
complications, such as pneumonia or thromboembolic events,
particularly due to immobilization following a fracture (4). Rather than a singular condition,
osteoporosis encompasses a spectrum of distinct pathological
states, typically classified as primary or secondary. Primary
osteoporosis includes two main subtypes: Type I, or postmenopausal
osteoporosis, which predominantly affects women after menopause due
to estrogen deficiency, and type II, also known as age-related or
senile osteoporosis, which is associated with aging in both sexes.
By contrast, secondary osteoporosis arises from various underlying
medical conditions, reduced physical activity or the adverse
effects of medical treatments (136).
The primary pathological features of osteoporosis
include decreased bone mass, disrupted bone microarchitecture and
an imbalance in bone remodeling, where bone resorption outpaces
bone formation, leading to a net loss of bone tissue (135).
Type I osteoporosis primarily results from estrogen
deficiency, which accelerates bone resorption by increasing the
RANKL/OPG ratio and enhancing osteoclast activity (137). Several bone metabolism pathways
are implicated in this process: i) Estrogen deficiency prolongs the
lifespan of bone marrow dendritic cells and increases the secretion
of IL-7 and IL-15, which promote the conversion of memory T
(TM) cells into effector TM cells. The
subsequent release of TNFα and IL-17A acts synergistically with
RANKL to stimulate osteoclastogenesis and enhance osteoclast
activity. ii) Estrogen deficiency also increases intestinal
permeability and triggers inflammation in the intestinal mucosa,
resulting in elevated production of T helper 17 (Th17) cells.
IL-17A produced by Th17 cells further enhances osteoclast
differentiation and bone resorption. Additionally, metabolites from
gut microbiota, such as butyric acid, polyamines and other
short-chain fatty acids, may indirectly promote osteoclast
activity; however, the exact mechanisms remain to be fully
elucidated (138).
Vitamin D deficiency impairs calcium absorption, a
condition further exacerbated by inadequate dietary calcium intake.
This disruption in calcium homeostasis leads to increased bone
resorption and heightened bone remodeling. In a large European
cohort study of 8,532 postmenopausal women with insufficient
vitamin D levels, 97% were found to have osteoporosis (139). Another study revealed that 82%
of individuals with osteoporosis had calcium intakes below the
recommended daily amount of 1,000 mg (140). Moreover, phosphorus deficiency,
a common marker of poor nutritional status in elderly patients, is
associated with an elevated risk of fractures (141). Thus, deficiencies in vitamin D,
calcium and phosphorus further increase fracture risk in elderly
women with osteoporosis.
During the progression of osteoporosis in the
elderly, both cellular senescence and epigenetic regulatory
pathways serve key roles in modulating bone metabolism. As aging
progresses, bone marrow mesenchymal stem cells (BMSCs) undergo
senescence, accompanied by an upregulation of nucleosome assembly
protein 1 like 2 (NAP1L2) expression. NAP1L2 contributes to bone
loss through two primary mechanisms. First, it activates the NF-κB
signaling pathway, leading to increased secretion of
senescence-associated factors, such as IL-6 and IL-8. These factors
exacerbate cellular senescence and impair the osteogenic
differentiation of BMSCs, thus negatively impacting bone formation.
Second, as a histone chaperone, NAP1L2 recruits sirtuin 1 to
deacetylate H3K14ac at the promoters of key osteogenic genes,
including Runx2, Sp7 and Bglap, causing their transcriptional
repression. This epigenetic modification further inhibits
osteogenic differentiation, reduces bone formation, and accelerates
the development of osteoporosis (142).
Abnormal cellular functions also contribute to
osteoporosis pathogenesis: i) Inhibition of osteoblast
differentiation and function, which is associated with
downregulation of the BMP/Smad (143) and Wnt/β-catenin (144) pathways, thus preventing MSCs
from differentiating into osteoblasts; ii) overactivation of
osteoclasts, bone matrix degradation is accelerated due to
lysosomal acidification abnormalities, such as increased V-ATPase
activity (145) and elevated
cathepsin K activity (146);
and iii) dysfunction in the osteocyte network, where osteocyte
apoptosis and the accumulation of microdamage impair mechanosignal
perception, inhibiting bone repair (147). Furthermore, senescence or
mechanical stimulation reduces the upregulation of sclerostin,
which antagonizes the Wnt pathway and inhibits bone formation
(148).
Dysregulated molecular signaling pathways also
contribute to osteoporosis, particularly those involved in the
RANKL/RANK/OPG system, the Wnt/β-catenin pathway, the BMP-Smad
pathway and the PI3K/Akt/mTOR pathway. Additionally, hormonal and
cytokine networks, particularly those involving PTH and estrogen,
are disrupted, with well-established roles in bone metabolism
(Table II; Fig. 4).
BCAA catabolism primarily occurs in skeletal muscle,
where they are broken down into their respective ketoacids and
further metabolized. This process is essential for maintaining
muscle function and overall metabolic balance. Impaired BCAA
catabolism can lead to elevated circulating levels of BCAAs and
their ketoacids, which are associated with metabolic disturbances
that may contribute to the development of osteoporosis. For
example, increased BCAA levels can disrupt insulin signaling
pathways, potentially resulting in insulin resistance, a recognized
risk factor for osteoporosis (149).
Moreover, the regulation of key enzymes in BCAA
metabolism, such as BCAT and BCKDH, is crucial for maintaining
metabolic homeostasis. Inhibition of BCAT1 has been shown to impair
osteoclast differentiation, suggesting its potential as a
therapeutic target to halt the progression of osteoporosis
(12). Emerging evidence has
indicated that the expression of these metabolic enzymes is
influenced by factors such as physical activity and transcriptional
coactivators, including PGC-1α. In skeletal muscle, PGC-1α enhances
the expression of BCAA-metabolizing enzymes, facilitating BCAA
degradation and potentially alleviating the detrimental effects of
their accumulation (150).
As the incidence of osteoporotic events continues to
increase, the management of osteoporosis has increasingly focused
on long-term strategies. Consequently, identifying safe, accessible
and effective nutritional interventions to maintain skeletal health
has become a key area of clinical research. BCAAs, due to their
widespread presence in daily diets and ease of adherence, have
attracted growing interest for their potential effects on bone
metabolism, suggesting translational and clinical intervention
value.
Cellular and animal studies have shown that
exogenous BCAAs can modulate bone metabolism through the activation
of signaling pathways, such as mTOR. This regulation occurs through
two concurrent effects: Promoting osteogenic differentiation and
inhibiting excessive osteoclast activation (151-154). Additionally, clinical
observational studies have identified a positive association
between higher BCAA levels and the preservation of bone mineral
density (BMD) (151). These
findings suggest that BCAA supplementation may offer therapeutic
potential in delaying osteoporosis progression. Similarly, Carbone
et al (155) and Urano
et al (156) have
indicated that higher BCAA levels are associated with better BMD.
Together, these studies imply that elevated BCAA levels may be
associated with improved bone health and a reduced risk of
osteoporosis.
However, these findings do not establish a causal
relationship. Residual confounding factors, such as total dietary
protein intake, combination with other substances or physical
activity, may influence both BCAA levels and bone health.
Additionally, the relationship between BCAAs and bone health has
been reported to vary across different disease stages or metabolic
conditions (116). Therefore,
well-designed clinical studies are critical, and further research
involving additional human data is necessary.
Among the BCAAs, leucine is recognized as a critical
amino acid for muscle function (157-159). BCAAs can effectively prevent
and improve sarcopenia, as well as reduce fat infiltration in
skeletal muscles, thus preserving muscle mass and function
(160). A clinical intervention
study conducted by Wang et al (161) demonstrated that leucine
supplementation enhances muscle performance by improving
cytoskeletal dynamics.
In elderly individuals and post-surgical
rehabilitation populations, the combination of BCAA supplementation
and exercise has proven effective in enhancing muscle strength,
which is critical for restoring mobility and providing mechanical
stimulation to the skeleton (167,168). Although the direct efficacy of
BCAA supplementation in treating osteoporosis in humans requires
further validation, it remains a promising approach and warrants
targeted clinical research.
BCAT1 catalyzes the transfer of the amine group from
BCAAs to α-ketoglutarate, generating glutamate and α-ketoacids,
which are subsequently oxidized to provide cellular energy.
Therefore, BCAT1 is crucial for preserving BCAA homeostasis and
ensuring their continued availability for energy production. In a
mouse model, Go et al (12) observed that a 400 μM BCAA
solution effectively promoted osteoclast differentiation, whereas a
higher concentration (800 μM) inhibited it, suggesting a
negative feedback mechanism for BCAAs. Additionally, Huynh et
al reported (169) that
mTORC1, a key nutrient sensor known to be activated by BCAAs
(98), inhibits osteoclast
differentiation through calcineurin and NFATc1 signaling. BCAA
levels are regulated not only by BCATs but also by their primary
transporter, LAT1. Ozaki et al demonstrated that deleting
the gene encoding LAT1 (Slc7a5) specifically in osteoclasts
resulted in osteoclast activation and bone loss (170). Furthermore, Pereira et
al (117) validated the
functional role of the macrophage multinucleation network (MMnet),
a trans-regulated gene co-expression network enriched for
osteoclast-related genes, in regulating osteoclast multinucleation,
resorption, bone mass and strength. These findings suggest that
factors such as BCAT1, mTORC1, Slc7a5 and MMnet may serve as
potential therapeutic targets to inhibit osteoclast formation,
offering a strategy for osteoporosis treatment.
A recent study indicated that elevated BCAA levels
are associated with insulin resistance and an increased risk of
metabolic syndrome (171). In
addition, a longitudinal study conducted between 2016 and 2022 with
3,090 Brazilian participants revealed a positive association
between total BCAA intake (specifically >16 g) and the risk of
obesity (172). These findings,
along with the results from other experiments, are summarized in
Table III. Additionally, in
populations with BCAA metabolism disorders, such as patients with
diabetes mellitus and liver cirrhosis, abnormal BCAA levels may
impact bone metabolism (173).
Therefore, the potential benefits of regulating BCAAs must be
evaluated in conjunction with the metabolic health status of the
individual.
The accumulation of adipose tissue within the bone
marrow space can induce an inflammatory state (176), which in turn promotes bone
resorption and impairs the differentiation of MSCs and
hematopoietic stem cells (177). Furthermore, plant-based foods,
rich in dietary fiber and polyphenols, can enhance energy
expenditure through thermogenesis and regulation of lipid
metabolism, thereby helping reduce fat accumulation (178). Increasing the proportion of
healthy plant-based foods in the diet, which may reduce bone marrow
adipose tissue or modulate the function of bone marrow adipocytes,
could serve as a potential target for improving bone quality and
has important implications for the development of novel therapeutic
strategies (179).
BCAA has attracted increasing attention due to its
diverse biological activities and therapeutic potential. The
present review focused on its effects in bone metabolism and
osteoporosis. Notably, BCAA regulates the muscular and immune
systems by activating the mTOR pathway, thereby influencing bone
function. It also acts as a molecular signal to exert direct or
indirect effects on bone metabolism and bone homeostasis. BCAA
metabolic disorders contribute to a complex pathological network
that involves not only the direct regulation of osteoblasts and
osteoclasts, but also indirect effects mediated by systemic
metabolic abnormalities. Collectively, these factors disrupt bone
homeostasis and may ultimately lead to osteoporosis.
Evidence from animal and human studies have
suggested that BCAA intake may be associated with increased BMD,
supporting its potential as a nutritional target for maintaining
bone health and preventing osteoporosis; however, most current
evidence is derived from in vitro and animal experiments,
which are limited by interspecies metabolic differences and dose
conversion issues, restricting clinical translation (180,181). Moreover, high-quality
population-based studies and prospective intervention trials remain
scarce. Existing studies often suffer from poor reproducibility due
to small sample sizes and inconsistent intervention methods
(182,183). The specific mechanisms of BCAA
action within the bone microenvironment have not been fully
elucidated, and the effects of individual metabolic differences and
disease states on BCAA metabolism require further investigation.
Future work should explore the molecular mechanisms of BCAA in
greater depth. Multicenter, large-sample, long-term randomized
controlled trials are needed to determine the long-term effects of
different BCAA doses and ratios on BMD and bone turnover markers
(155). Integration of advanced
technologies, such as metabolomics, may promote the development of
precise nutritional intervention strategies.
As research on the modulation of bone metabolism by
BCAAs and their role in osteoporosis treatment continues to grow,
these studies will further enhance the understanding and improve
strategies targeting BCAA metabolic processes in disease therapy.
The current review not only provides a reference for further
development and investigation of the impact of BCAA metabolism on
osteoporosis progression, but also serves as a guide for regulating
BCAA metabolism to achieve precision treatment of osteoporosis.
Furthermore, these targeted therapeutic strategies may offer
innovative treatment options for bone metabolism-related
diseases.
Not applicable.
QX, HZ and RY drafted and revised the manuscript.
YZ and FL contributed to the literature search, evidence
extraction, thematic organization, critical interpretation of the
reviewed studies, and the preparation and refinement of the
figures. BC contributed to the literature search, screening,
evidence evaluation, and assisted in organizing and interpreting
the included studies. XC conceived the review, supervised the
overall academic direction and critically revised the manuscript.
Data authentication is not applicable. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present review was supported by the National Natural Science
Foundation of China Incubation Project (grant no. Institute-level:
2023YNFY12009); the National College Students' Innovative
Entrepreneurial Training Plan Program (grant no. 202410403067); the
Innovation and Entrepreneurship Training Program for College
Students in Jiangxi Province (grant no. S202410403035); the
Doctoral Start-up Fund (grant no. B3477); and the Nanchang
University Second Affiliated Hospital In-Hospital Project (grant
no. 2023efy002).
|
1
|
Li H, Xiao Z, Quarles LD and Li W:
Osteoporosis: Mechanism, molecular target and current status on
drug development. Curr Med Chem. 28:1489–1507. 2021. View Article : Google Scholar
|
|
2
|
Zhang X, Wang Z, Zhang D, Ye D, Zhou Y,
Qin J and Zhang Y: The prevalence and treatment rate trends of
osteoporosis in postmenopausal women. PLoS One. 18:e02902892023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Yu W, Yin X, Cui L, Tang S, Jiang
N, Cui L, Zhao N, Lin Q, Chen L, et al: Prevalence of osteoporosis
and fracture in China: The China osteoporosis prevalence study.
JAMA Netw Open. 4:e21211062021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Center JR, Nguyen TV, Schneider D,
Sambrook PN and Eisman JA: Mortality after all major types of
osteoporotic fracture in men and women: An observational study.
Lancet. 353:878–882. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao M, Gao W, Papadimitriou JM, Zhang C,
Gao J and Zheng M: Exosomes-the enigmatic regulators of bone
homeostasis. Bone Res. 6:362018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Li Q, Peng X, Ji P, Zhang Y, Jin
J, Yuan Z, Jiang J, Tian G, Cai M, et al: Targeting
chaperone-mediated autophagy to regulate osteoclast activity as a
therapeutic strategy for osteoporosis. Mater Today Bio.
35:1023112025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harper AE, Miller RH and Block KP:
Branched-chain amino acid metabolism. Annu Rev Nutr. 4:409–454.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mansoori S, Ho MY, Ng KK and Cheng KK:
Branched-chain amino acid metabolism: Pathophysiological mechanism
and therapeutic intervention in metabolic diseases. Obes Rev.
26:e138562025. View Article : Google Scholar
|
|
11
|
Trautman ME, Richardson NE and Lamming DW:
Protein restriction and branched-chain amino acid restriction
promote geroprotective shifts in metabolism. Aging Cell.
21:e136262022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Go M, Shin E, Jang SY, Nam M, Hwang GS and
Lee SY: BCAT1 promotes osteoclast maturation by regulating
branched-chain amino acid metabolism. Exp Mol Med. 54:825–833.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weiner S and Traub W: Bone structure: From
angstroms to microns. FASEB J. 6:879–885. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hua R, Han Y, Ni Q, Fajardo RJ, Iozzo RV,
Ahmed R, Nyman JS, Wang X and Jiang JX: Pivotal roles of biglycan
and decorin in regulating bone mass, water retention, and bone
toughness. Bone Res. 13:22025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calvi LM, Adams GB, Weibrecht KW, Weber
JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P,
Bringhurst FR, et al: Osteoblastic cells regulate the
haematopoietic stem cell niche. Nature. 425:841–846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Niu C, Ye L, Huang H, He X, Tong
WG, Ross J, Haug J, Johnson T, Feng JQ, et al: Identification of
the haematopoietic stem cell niche and control of the niche size.
Nature. 425:836–841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taichman RS and Emerson SG: Human
osteoblasts support hematopoiesis through the production of
granulocyte colony-stimulating factor. J Exp Med. 179:1677–1682.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bolamperti S, Villa I and Rubinacci A:
Bone remodeling: an operational process ensuring survival and bone
mechanical competence. Bone Res. 10:482022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hattner R, Epker BN and Frost HM:
Suggested sequential mode of control of changes in cell behaviour
in adult bone remodelling. Nature. 206:489–490. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barak MM: Bone modeling or bone
remodeling: That is the question. Am J Phys Anthropol. 172:153–155.
2020. View Article : Google Scholar
|
|
21
|
Yan C, Zhang P, Qin Q, Jiang K, Luo Y,
Xiang C, He J, Chen L, Jiang D, Cui W and Li Y: 3D-printed bone
regeneration scaffolds modulate bone metabolic homeostasis through
vascularization for osteoporotic bone defects. Biomaterials.
311:1226992024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Udagawa N, Takahashi N, Akatsu T, Tanaka
H, Sasaki T, Nishihara T, Koga T, Martin TJ and Suda T: Origin of
osteoclasts: mature monocytes and macrophages are capable of
differentiating into osteoclasts under a suitable microenvironment
prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci
USA. 87:7260–7264. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakahama KI, Hidaka S, Goto K, Tada M, Doi
T, Nakamura H, Akiyama M and Shinohara M: Visualization and
quantification of RANK-RANKL binding for application to disease
investigations and drug discovery. Bone. 195:1174732025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holliday LS, Patel SS and Rody WJ Jr:
RANKL and RANK in extracellular vesicles: Surprising new players in
bone remodeling. Extracell Vesicles Circ Nucl Acids. 2:18–28.
2021.PubMed/NCBI
|
|
27
|
Soriano P, Montgomery C, Geske R and
Bradley A: Targeted disruption of the c-src proto-oncogene leads to
osteopetrosis in mice. Cell. 64:693–702. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boyce BF, Yoneda T, Lowe C, Soriano P and
Mundy GR: Requirement of pp60c-src expression for osteoclasts to
form ruffled borders and resorb bone in mice. J Clin Invest.
90:1622–1627. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gelb BD, Shi GP, Chapman HA and Desnick
RJ: Pycnodysostosis, a lysosomal disease caused by cathepsin K
deficiency. Science. 273:1236–1238. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saftig P, Hunziker E, Everts V, Jones S,
Boyde A, Wehmeyer O, Suter A and von Figura K: Functions of
cathepsin K in bone resorption. Lessons from cathepsin K deficient
mice. Adv Exp Med Biol. 477:293–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizoguchi T and Ono N: The diverse origin
of bone-forming osteoblasts. J Bone Miner Res. 36:1432–1447. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z,
Zhao L, Nagy TR, Peng X, Hu J, et al: TGF-beta1-induced migration
of bone mesenchymal stem cells couples bone resorption with
formation. Nat Med. 15:757–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bodine PV and Komm BS: Wnt signaling and
osteoblastogenesis. Rev Endocr Metab Disord. 7:33–39. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eriksen EF: Cellular mechanisms of bone
remodeling. Rev Endocr Metab Disord. 11:219–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong AR, Kim K, Lee JY, Yang JY, Kim JH,
Shin CS and Kim SW: Transformation of mature osteoblasts into bone
lining cells and RNA sequencing-based transcriptome profiling of
mouse bone during mechanical unloading. Endocrinol Metab (Seoul).
35:456–469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JS and Wein MN: Pathways controlling
formation and maintenance of the osteocyte dendrite network. Curr
Osteoporos Rep. 20:493–504. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Komori T: Cell death in chondrocytes,
osteoblasts, and osteocytes. Int J Mol Sci. 17:20452016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Messmer D, Yang H, Telusma G, Knoll F, Li
J, Messmer B, Tracey KJ and Chiorazzi N: High mobility group box
protein 1: An endogenous signal for dendritic cell maturation and
Th1 polarization. J Immunol. 173:307–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Giannoni P, Marini C, Cutrona G, Matis S,
Capra MC, Puglisi F, Luzzi P, Pigozzi S, Gaggero G, Neri A, et al:
Chronic lymphocytic leukemia cells impair osteoblastogenesis and
promote osteoclastogenesis: Role of TNFα, IL-6 and IL-11 cytokines.
Haematologica. 106:2598–2612. 2021. View Article : Google Scholar
|
|
41
|
Kringelbach TM, Aslan D, Novak I, Schwarz
P and Jørgensen NR: UTP-induced ATP release is a fine-tuned
signalling pathway in osteocytes. Purinergic Signal. 10:337–347.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Verborgt O, Gibson GJ and Schaffler MB:
Loss of osteocyte integrity in association with microdamage and
bone remodeling after fatigue in vivo. J Bone Miner Res. 15:60–67.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheung WY, Fritton JC, Morgan SA,
Seref-Ferlengez Z, Basta-Pljakic J, Thi MM, Suadicani SO, Spray DC,
Majeska RJ and Schaffler MB: Pannexin-1 and P2X7-receptor are
required for apoptotic osteocytes in fatigued bone to trigger RANKL
production in neighboring bystander osteocytes. J Bone Miner Res.
31:890–899. 2016. View Article : Google Scholar
|
|
44
|
Luckprom P, Wongkhantee S, Yongchaitrakul
T and Pavasant P: Adenosine triphosphate stimulates RANKL
expression through P2Y1 receptor-cyclo-oxygenase-dependent pathway
in human periodontal ligament cells. J Periodontal Res. 45:404–411.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Buckley KA, Hipskind RA, Gartland A,
Bowler WB and Gallagher JA: Adenosine triphosphate stimulates human
osteoclast activity via upregulation of osteoblast-expressed
receptor activator of nuclear factor-kappa B ligand. Bone.
31:582–590. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gallagher JA: ATP P2 receptors and
regulation of bone effector cells. J Musculoskelet Neuronal
Interact. 4:125–127. 2004.PubMed/NCBI
|
|
47
|
Cusato K, Bosco A, Rozental R, Guimarães
CA, Reese BE, Linden R and Spray DC: Gap junctions mediate
bystander cell death in developing retina. J Neurosci.
23:6413–6422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cawley KM, Bustamante-Gomez NC, Guha AG,
MacLeod RS, Xiong J, Gubrij I, Liu Y, Mulkey R, Palmieri M,
Thostenson JD, et al: Local production of osteoprotegerin by
osteoblasts suppresses bone resorption. Cell Rep. 32:1080522020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kennedy OD, Laudier DM, Majeska RJ, Sun HB
and Schaffler MB: Osteocyte apoptosis is required for production of
osteoclastogenic signals following bone fatigue in vivo. Bone.
64:132–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Verborgt O, Tatton NA, Majeska RJ and
Schaffler MB: Spatial distribution of Bax and Bcl-2 in osteocytes
after bone fatigue: complementary roles in bone remodeling
regulation? J Bone Miner Res. 17:907–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lieben L, Carmeliet G and Masuyama R:
Calcemic actions of vitamin D: Effects on the intestine, kidney and
bone. Best Pract Res Clin Endocrinol Metab. 25:561–572. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zarei A, Morovat A, Javaid K and Brown CP:
Vitamin D receptor expression in human bone tissue and
dose-dependent activation in resorbing osteoclasts. Bone Res.
4:160302016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lips P and van Schoor NM: The effect of
vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol
Metab. 25:585–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bikle DD: Vitamin D and bone. Curr
Osteoporos Rep. 10:151–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Arnaud CD Jr, Tenenhouse AM and Rasmussen
H: Parathyroid hormone. Annu Rev Physiol. 29:349–372. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Martin TJ, Sims NA and Seeman E:
Physiological and pharmacological roles of PTH and PTHrP in bone
using their shared receptor, PTH1R. Endocr Rev. 42:383–406. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Quarles LD: Skeletal secretion of FGF-23
regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol.
8:276–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jüppner H: Phosphate and FGF-23. Kidney
Int. 79121:S24–S27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Naot D, Musson DS and Cornish J: The
activity of peptides of the calcitonin family in bone. Physiol Rev.
99:781–805. 2019. View Article : Google Scholar
|
|
60
|
Xu J, Wang J, Chen X, Li Y, Mi J and Qin
L: The effects of calcitonin gene-related peptide on bone
homeostasis and regeneration. Curr Osteoporos Rep. 18:621–632.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lecanda F, Warlow PM, Sheikh S, Furlan F,
Steinberg TH and Civitelli R: Connexin43 deficiency causes delayed
ossification, craniofacial abnormalities, and osteoblast
dysfunction. J Cell Biol. 151:931–944. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chung DJ, Castro CH, Watkins M, Stains JP,
Chung MY, Szejnfeld VL, Willecke K, Theis M and Civitelli R: Low
peak bone mass and attenuated anabolic response to parathyroid
hormone in mice with an osteoblast-specific deletion of connexin43.
J Cell Sci. 119(Pt 20): 4187–4198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Y, Paul EM, Sathyendra V, Davison A,
Sharkey N, Bronson S, Srinivasan S, Gross TS and Donahue HJ:
Enhanced osteoclastic resorption and responsiveness to mechanical
load in gap junction deficient bone. PLoS One. 6:e235162011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tiede-Lewis LM, Xie Y, Hulbert MA, Campos
R, Dallas MR, Dusevich V, Bonewald LF and Dallas SL: Degeneration
of the osteocyte network in the C57BL/6 mouse model of aging. Aging
(Albany NY). 9:2190–2208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dole NS, Mazur CM, Acevedo C, Lopez JP,
Monteiro DA, Fowler TW, Gludovatz B, Walsh F, Regan JN, Messina S,
et al: Osteocyte-Intrinsic TGF-β signaling regulates bone quality
through perilacunar/canalicular remodeling. Cell Rep. 21:2585–2596.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kreis NN, Friemel A, Ritter A, Hentrich
AE, Siebelitz E, Louwen F and Yuan J: In-depth analysis of
obesity-associated changes in adipose tissue-derived mesenchymal
stromal/stem cells and primary cilia function. Commun Biol.
8:14622025. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Olney RC: Regulation of bone mass by
growth hormone. Med Pediatr Oncol. 41:228–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Iglesias L, Yeh JK, Castro-Magana M and
Aloia JF: Effects of growth hormone on bone modeling and remodeling
in hypophysectomized young female rats: A bone histomorphometric
study. J Bone Miner Metab. 29:159–167. 2011. View Article : Google Scholar
|
|
69
|
Hayashi M, Nakashima T, Yoshimura N,
Okamoto K, Tanaka S and Takayanagi H: Autoregulation of osteocyte
Sema3A orchestrates estrogen action and counteracts bone aging.
Cell Metab. 29:627–637.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sharma D, Larriera AI, Palacio-Mancheno
PE, Gatti V, Fritton JC, Bromage TG, Cardoso L, Doty SB and Fritton
SP: The effects of estrogen deficiency on cortical bone
microporosity and mineralization. Bone. 110:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jackson E, Lara-Castillo N, Akhter MP,
Dallas M, Scott JM, Ganesh T and Johnson ML: Osteocyte
Wnt/β-catenin pathway activation upon mechanical loading is altered
in ovariectomized mice. Bone Rep. 15:1011292021. View Article : Google Scholar
|
|
72
|
Ma L, Hua R, Tian Y, Cheng H, Fajardo RJ,
Pearson JJ, Guda T, Shropshire DB, Gu S and Jiang JX: Connexin 43
hemichannels protect bone loss during estrogen deficiency. Bone
Res. 7:112019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
He Z, Li H, Han X, Zhou F, Du J, Yang Y,
Xu Q, Zhang S, Zhang S, Zhao N, et al: Irisin inhibits osteocyte
apoptosis by activating the Erk signaling pathway in vitro and
attenuates ALCT-induced osteoarthritis in mice. Bone.
141:1155732020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Al-Suhaimi EA and Al-Jafary MA: Endocrine
roles of vitamin K-dependent-osteocalcin in the relation between
bone metabolism and metabolic disorders. Rev Endocr Metab Disord.
21:117–125. 2020. View Article : Google Scholar
|
|
75
|
Yang D, Gong G, Song J, Chen J, Wang S, Li
J and Wang G: Ferroptosis-mediated osteoclast-osteoblast crosstalk:
Signaling pathways governing bone remodeling in osteoporosis. J
Orthop Surg Res. 20:8882025. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Baek KH, Chung YS, Koh JM, Kim IJ, Kim KM,
Min YK, Park KD, Dinavahi R, Maddox J, Yang W, et al: Romosozumab
in postmenopausal Korean women with osteoporosis: A Randomized,
double-blind, placebo-controlled efficacy and safety study.
Endocrinol Metab (Seoul). 36:60–69. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kasiske BL, Kumar R, Kimmel PL, Pesavento
TE, Kalil RS, Kraus ES, Rabb H, Posselt AM, Anderson-Haag TL,
Steffes MW, et al: Abnormalities in biomarkers of mineral and bone
metabolism in kidney donors. Kidney Int. 90:861–868. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schwetz V, Pieber T and Obermayer-Pietsch
B: The endocrine role of the skeleton: Background and clinical
evidence. Eur J Endocrinol. 166:959–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kärkkäinen M, Tuppurainen M, Salovaara K,
Sandini L, Rikkonen T, Sirola J, Honkanen R, Jurvelin J, Alhava E
and Kröger H: Effect of calcium and vitamin D supplementation on
bone mineral density in women aged 65-71 years: A 3-year randomized
population-based trial (OSTPRE-FPS). Osteoporos Int. 21:2047–2055.
2010. View Article : Google Scholar
|
|
80
|
Remer T, Krupp D and Shi L: Dietary
protein's and dietary acid load's influence on bone health. Crit
Rev Food Sci Nutr. 54:1140–1150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Langeveld M and Hollak CEM: Bone health in
patients with inborn errors of metabolism. Rev Endocr Metab Disord.
19:81–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yang K, Li J and Tao L: Purine metabolism
in the development of osteoporosis. Biomed Pharmacother.
155:1137842022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Iantomasi T, Romagnoli C, Palmini G,
Donati S, Falsetti I, Miglietta F, Aurilia C, Marini F, Giusti F
and Brandi ML: Oxidative stress and inflammation in osteoporosis:
Molecular mechanisms involved and the relationship with microRNAs.
Int J Mol Sci. 24:37722023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Johnson JT, Hussain MA, Cherian KE, Kapoor
N and Paul TV: Chronic alcohol consumption and its impact on bone
and metabolic health - A narrative review. Indian J Endocrinol
Metab. 26:206–212. 2022.PubMed/NCBI
|
|
85
|
Ehnert S, Aspera-Werz RH, Ihle C, Trost M,
Zirn B, Flesch I, Schröter S, Relja B and Nussler AK: Smoking
dependent alterations in bone formation and inflammation represent
major risk factors for complications following total joint
arthroplasty. J Clin Med. 8:4062019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Huang YS, Gao JW, Ao RF, Liu XY, Wu DZ,
Huang JL, Tu C, Zhuang JS, Zhu SY and Zhong ZM: Accumulation of
advanced oxidation protein products aggravates bone-fat imbalance
during skeletal aging. J Orthop Translat. 51:24–36. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Schröder K: NADPH oxidases in bone
homeostasis and osteoporosis. Free Radic Biol Med. 132:67–72. 2019.
View Article : Google Scholar
|
|
88
|
Jiang N, Liu J, Guan C, Ma C, An J and
Tang X: Thioredoxin-interacting protein: A new therapeutic target
in bone metabolism disorders? Front Immunol. 13:9551282022.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Le Couteur DG, Solon-Biet SM, Cogger VC,
Ribeiro R, de Cabo R, Raubenheimer D, Cooney GJ and Simpson SJ:
Branched chain amino acids, aging and age-related health. Ageing
Res Rev. 64:1011982020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Brosnan JT and Brosnan ME: Branched-chain
amino acids: Enzyme and substrate regulation. J Nutr. 136(1 Suppl):
207S–211S. 2006. View Article : Google Scholar
|
|
91
|
Han JM, Jeong SJ, Park MC, Kim G, Kwon NH,
Kim HK, Ha SH, Ryu SH and Kim S: Leucyl-tRNA synthetase is an
intracellular leucine sensor for the mTORC1-signaling pathway.
Cell. 149:410–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang S, Zeng X, Ren M, Mao X and Qiao S:
Novel metabolic and physiological functions of branched chain amino
acids: A review. J Anim Sci Biotechnol. 8:102017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Neinast MD, Jang C, Hui S, Murashige DS,
Chu Q, Morscher RJ, Li X, Zhan L, White E, Anthony TG, et al:
Quantitative analysis of the whole-body metabolic fate of
branched-chain amino acids. Cell Metab. 29:417–429.e4. 2019.
View Article : Google Scholar
|
|
94
|
Wang W, Liu Z, Liu L, Han T, Yang X and
Sun C: Genetic predisposition to impaired metabolism of the
branched chain amino acids, dietary intakes, and risk of type 2
diabetes. Genes Nutr. 16:202021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bonvini A, Coqueiro AY, Tirapegui J,
Calder PC and Rogero MM: Immunomodulatory role of branched-chain
amino acids. Nutr Rev. 76:840–856. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Deng AF, Wang FX, Wang SC, Zhang YZ, Bai L
and Su JC: Bone-organ axes: Bidirectional crosstalk. Mil Med Res.
11:372024.PubMed/NCBI
|
|
97
|
Hasegawa T, Kikuta J, Sudo T, Matsuura Y,
Matsui T, Simmons S, Ebina K, Hirao M, Okuzaki D, Yoshida Y, et al:
Identification of a novel arthritis-associated osteoclast precursor
macrophage regulated by FoxM1. Nat Immunol. 20:1631–1643. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Anthony JC, Yoshizawa F, Anthony TG, Vary
TC, Jefferson LS and Kimball SR: Leucine stimulates translation
initiation in skeletal muscle of postabsorptive rats via a
rapamycin-sensitive pathway. J Nutr. 130:2413–2419. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang M, Zhang X, Ding Y, Yang L, Ren W,
Gao Y, Yao K, Zhou Y and Shao W: The effect of valine on the
synthesis of α-Casein in MAC-T cells and the expression and
phosphorylation of genes related to the mTOR signaling pathway. Int
J Mol Sci. 26:31792025. View Article : Google Scholar
|
|
100
|
Glantschnig H, Fisher JE, Wesolowski G,
Rodan GA and Reszka AA: M-CSF, TNFalpha and RANK ligand promote
osteoclast survival by signaling through mTOR/S6 kinase. Cell Death
Differ. 10:1165–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kneissel M, Luong-Nguyen NH, Baptist M,
Cortesi R, Zumstein-Mecker S, Kossida S, O'Reilly T, Lane H and
Susa M: Everolimus suppresses cancellous bone loss, bone
resorption, and cathepsin K expression by osteoclasts. Bone.
35:1144–1156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ory B, Moriceau G, Redini F and Heymann D:
mTOR inhibitors (rapamycin and its derivatives) and nitrogen
containing bisphosphonates: Bi-functional compounds for the
treatment of bone tumours. Curr Med Chem. 14:1381–1387. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Benslimane-Ahmim Z, Heymann D, Dizier B,
Lokajczyk A, Brion R, Laurendeau I, Bièche I, Smadja DM,
Galy-Fauroux I, Colliec-Jouault S, et al: Osteoprotegerin, a new
actor in vasculogenesis, stimulates endothelial colony-forming
cells properties. J Thromb Haemost. 9:834–843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Gnant M, Baselga J, Rugo HS, Noguchi S,
Burris HA, Piccart M, Hortobagyi GN, Eakle J, Mukai H, Iwata H, et
al: Effect of everolimus on bone marker levels and progressive
disease in bone in BOLERO-2. J Natl Cancer Inst. 105:654–663. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Roberts LD, Boström P, O'Sullivan JF,
Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S,
Georgiadi A, et al: β-Aminoisobutyric acid induces browning of
white fat and hepatic β-oxidation and is inversely correlated with
cardiometabolic risk factors. Cell Metab. 19:96–108. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jang C, Oh SF, Wada S, Rowe GC, Liu L,
Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, et al: A
branched-chain amino acid metabolite drives vascular fatty acid
transport and causes insulin resistance. Nat Med. 22:421–426. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Batch BC, Shah SH, Newgard CB, Turer CB,
Haynes C, Bain JR, Muehlbauer M, Patel MJ, Stevens RD, Appel LJ, et
al: Branched chain amino acids are novel biomarkers for
discrimination of metabolic wellness. Metabolism. 62:961–969. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Connelly MA, Wolak-Dinsmore J and Dullaart
RPF: Branched chain amino acids are associated with insulin
resistance independent of leptin and adiponectin in subjects with
varying degrees of glucose tolerance. Metab Syndr Relat Disord.
15:183–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lotta LA, Scott RA, Sharp SJ, Burgess S,
Luan J, Tillin T, Schmidt AF, Imamura F, Stewart ID, Perry JR, et
al: Genetic predisposition to an impaired metabolism of the
branched-chain amino acids and risk of type 2 diabetes: A mendelian
randomisation analysis. PLoS Med. 13:e10021792016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Felig P, Marliss E and Cahill GF Jr:
Plasma amino acid levels and insulin secretion in obesity. N Engl J
Med. 281:811–816. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Newgard CB, An J, Bain JR, Muehlbauer MJ,
Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al:
A branched-chain amino acid-related metabolic signature that
differentiates obese and lean humans and contributes to insulin
resistance. Cell Metab. 9:311–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang Q, Holmes MV, Davey Smith G and
Ala-Korpela M: Genetic support for a causal role of insulin
resistance on circulating branched-chain amino acids and
inflammation. Diabetes Care. 40:1779–1786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mahendran Y, Jonsson A, Have CT, Allin KH,
Witte DR, Jørgensen ME, Grarup N, Pedersen O, Kilpeläinen TO and
Hansen T: Genetic evidence of a causal effect of insulin resistance
on branched-chain amino acid levels. Diabetologia. 60:873–878.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lynch CJ and Adams SH: Branched-chain
amino acids in metabolic signalling and insulin resistance. Nat Rev
Endocrinol. 10:723–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liang Z, Su Z, Yang H, Zheng J, Wu X,
Huang M, Duan L, Chen S, Wei B, Fan X and Lin S: Branched-chain
amino acids in bone health: From molecular mechanisms to
therapeutic potential. Biomed Pharmacother. 192:1186452025.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhao H, Zhang F, Sun D, Wang X, Zhang X,
Zhang J, Yan F, Huang C, Xie H, Lin C, et al: Branched-Chain amino
acids exacerbate obesity-related hepatic glucose and lipid
metabolic disorders via attenuating Akt2 signaling. Diabetes.
69:1164–1177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Pereira M, Ko JH, Logan J, Protheroe H,
Kim KB, Tan ALM, Croucher PI, Park KS, Rotival M, Petretto E, et
al: A trans-eQTL network regulates osteoclast multinucleation and
bone mass. Elife. 9:e555492020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kim JH and Kim N: Regulation of NFATc1 in
osteoclast differentiation. J Bone Metab. 21:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K,
Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K,
et al: DC-STAMP is essential for cell-cell fusion in osteoclasts
and foreign body giant cells. J Exp Med. 2005. 202:345–351. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ishii M, Iwai K, Koike M, Ohshima S,
Kudo-Tanaka E, Ishii T, Mima T, Katada Y, Miyatake K, Uchiyama Y
and Saeki Y: RANKL-induced expression of tetraspanin CD9 in lipid
raft membrane microdomain is essential for cell fusion during
osteoclastogenesis. J Bone Miner Res. 21:965–976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Singha UK, Jiang Y, Yu S, Luo M, Lu Y,
Zhang J and Xiao G: Rapamycin inhibits osteoblast proliferation and
differentiation in MC3T3-E1 cells and primary mouse bone marrow
stromal cells. J Cell Biochem. 103:434–446. 2008. View Article : Google Scholar
|
|
122
|
Han HS, Ahn E, Park ES, Huh T, Choi S,
Kwon Y, Choi BH, Lee J, Choi YH, Jeong YL, et al: Impaired BCAA
catabolism in adipose tissues promotes age-associated metabolic
derangement. Nat Aging. 3:982–1000. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chen A, Jin J, Cheng S, Liu Z, Yang C,
Chen Q, Liang W, Li K, Kang D, Ouyang Z, et al: mTORC1 induces
plasma membrane depolarization and promotes preosteoblast
senescence by regulating the sodium channel Scn1a. Bone Res.
10:252022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gayatri MB, Gajula NN, Chava S and Reddy
ABM: High glutamine suppresses osteogenesis through mTORC1-mediated
inhibition of the mTORC2/AKT-473/RUNX2 axis. Cell Death Discov.
8:2772022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Arany Z and Neinast M: Branched chain
amino acids in metabolic disease. Curr Diab Rep. 18:762018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yoon MS: The emerging role of
branched-chain amino acids in insulin resistance and metabolism.
Nutrients. 8:4052016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu
Y, Chen M, Wynn RM, Wang J, Wang J, et al: Targeting BCAA
Catabolism to Treat Obesity-Associated Insulin Resistance.
Diabetes. 68:1730–1746. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhou C, Zhang J, Liu Q, Guo Y, Li M, Tao
J, Peng S, Li R, Deng X, Zhang G and Liu H: Role of amino acid
metabolism in osteoporosis: Effects on the bone microenvironment
and treatment strategies (Review). Mol Med Rep. 32:2122025.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lerin C, Goldfine AB, Boes T, Liu M, Kasif
S, Dreyfuss JM, De Sousa-Coelho AL, Daher G, Manoli I, Sysol JR, et
al: Defects in muscle branched-chain amino acid oxidation
contribute to impaired lipid metabolism. Mol Metab. 5:926–936.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
White PJ, Lapworth AL, An J, Wang L,
McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain
JR, et al: Branched-chain amino acid restriction in Zucker-fatty
rats improves muscle insulin sensitivity by enhancing efficiency of
fatty acid oxidation and acyl-glycine export. Mol Metab. 5:538–551.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Jian H, Xu Q, Wang X, Liu Y, Miao S, Li Y,
Mou T, Dong X and Zou X: Amino acid and fatty acid metabolism
disorders trigger oxidative stress and inflammatory response in
excessive dietary valine-induced NAFLD of laying hens. Front Nutr.
9:8497672022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ye Z, Wang S, Zhang C and Zhao Y:
Coordinated modulation of energy metabolism and inflammation by
branched-chain amino acids and fatty acids. Front Endocrinol
(Lausanne). 11:6172020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Surugihalli C, Muralidaran V, Ryan CE,
Patel K, Zhao D and Sunny NE: Branched-chain amino acids alter
cellular redox to induce lipid oxidation and reduce de novo
lipogenesis in the liver. Am J Physiol Endocrinol Metab.
324:E299–E313. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lin S, Miao Y, Zheng X, Dong Y, Yang Q,
Yang Q, Du S, Xu J, Zhou S and Yuan T: ANGPTL4 negatively regulates
the progression of osteosarcoma by remodeling branched-chain amino
acid metabolism. Cell Death Discov. 8:2252022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Riggs BL, Wahner HW, Seeman E, Offord KP,
Dunn WL, Mazess RB, Johnson KA and Melton LJ III: Changes in bone
mineral density of the proximal femur and spine with aging.
Differences between the postmenopausal and senile osteoporosis
syndromes. J Clin Invest. 70:716–723. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang LT, Chen LR and Chen KH:
Hormone-related and drug-induced osteoporosis: A cellular and
molecular overview. Int J Mol Sci. 24:58142023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wu D, Cline-Smith A, Shashkova E, Perla A,
Katyal A and Aurora R: T-cell mediated inflammation in
postmenopausal osteoporosis. Front Immunol. 12:6875512021.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Bruyère O, Malaise O, Neuprez A, Collette
J and Reginster JY: Prevalence of vitamin D inadequacy in European
postmenopausal women. Curr Med Res Opin. 23:1939–1944. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lee YH, Lim YW, Ling PS, Tan YY, Cheong M
and Lam KS: Inadequate dietary calcium intake in elderly patients
with hip fractures. Singapore Med J. 48:1117–1121. 2007.PubMed/NCBI
|
|
141
|
Nieves JW: Osteoporosis: The role of
micronutrients. Am J Clin Nutr. 81:1232S–1239S. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Hu M, Xing L, Zhang L, Liu F, Wang S, Xie
Y, Wang J, Jiang H, Guo J, Li X, et al: NAP1L2 drives mesenchymal
stem cell senescence and suppresses osteogenic differentiation.
Aging Cell. 21:e135512022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xue Y, Hu S, Chen C, He J, Sun J, Jin Y,
Zhang Y, Zhu G, Shi Q and Rui Y: Myokine Irisin promotes
osteogenesis by activating BMP/SMAD signaling via αV integrin and
regulates bone mass in mice. Int J Biol Sci. 18:572–584. 2022.
View Article : Google Scholar :
|
|
144
|
Mi B, Yan C, Xue H, Chen L, Panayi AC, Hu
L, Hu Y, Cao F, Sun Y, Zhou W, et al: Inhibition of circulating
miR-194-5p reverses osteoporosis through Wnt5a/β-catenin-dependent
induction of osteogenic differentiation. Mol Ther Nucleic Acids.
21:814–823. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Futai M, Sun-Wada GH, Wada Y, Matsumoto N
and Nakanishi-Matsui M: Vacuolar-type ATPase: A proton pump to
lysosomal trafficking. Proc Jpn Acad Ser B Phys Biol Sci.
95:261–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Shen S, Si M, Zeng C, Liu EK, Chen Y,
Vacher J, Zhao H, Mohan S and Xing W: Leucine repeat rich kinase 1
controls osteoclast activity by managing lysosomal trafficking and
secretion. Biology (Basel). 12:5112023.PubMed/NCBI
|
|
147
|
Li MCM, Chow SKH, Wong RMY, Qin L and
Cheung WH: The role of osteocytes-specific molecular mechanism in
regulation of mechanotransduction - A systematic review. J Orthop
Translat. 29:1–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Vasiliadis ES, Evangelopoulos DS, Kaspiris
A, Benetos IS, Vlachos C and Pneumaticos SG: The role of sclerostin
in bone diseases. J Clin Med. 11:8062022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Mann G, Mora S, Madu G and Adegoke OAJ:
Branched-chain amino acids: Catabolism in skeletal muscle and
implications for muscle and whole-body metabolism. Front Physiol.
12:7028262021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hatazawa Y, Tadaishi M, Nagaike Y, Morita
A, Ogawa Y, Ezaki O, Takai-Igarashi T, Kitaura Y, Shimomura Y,
Kamei Y and Miura S: PGC-1α-mediated branched-chain amino acid
metabolism in the skeletal muscle. PLoS One. 9:e910062014.
View Article : Google Scholar
|
|
151
|
Lv Z, Shi W and Zhang Q: Role of essential
amino acids in age-induced bone loss. Int J Mol Sci. 23:112812022.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Takegahara N, Kim H and Choi Y: Unraveling
the intricacies of osteoclast differentiation and maturation:
Insight into novel therapeutic strategies for bone-destructive
diseases. Exp Mol Med. 56:264–272. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liang Y, Cardoso FF, Parys C, Cardoso FC
and Loor JJ: Branched-Chain amino acid supplementation alters the
abundance of mechanistic target of rapamycin and insulin signaling
proteins in subcutaneous adipose explants from lactating holstein
cows. Animals (Basel). 11:27142021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kim JM, Yang YS, Hong J, Chaugule S, Chun
H, van der Meulen MCH, Xu R, Greenblatt MB and Shim JH: Biphasic
regulation of osteoblast development via the ERK MAPK-mTOR pathway.
Elife. 11:e780692022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Carbone L, Bůžková P, Fink HA, Robbins JA,
Barzilay JI, Elam RE, Isales C, Connelly MA and Mukamal KJ: Plasma
levels of branched chain amino acids, incident hip fractures, and
bone mineral density of the hip and spine. J Clin Endocrinol Metab.
108:e1358–e1364. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Urano T, Kuroda T, Uenishi K and Shiraki
M: Serum branched-chain amino acid levels are associated with
fracture risk in Japanese women. Geriatr Gerontol Int. 24:603–608.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Plotkin DL, Delcastillo K, Van Every DW,
Tipton KD, Aragon AA and Schoenfeld BJ: Isolated leucine and
branched-chain amino acid supplementation for enhancing muscular
strength and hypertrophy: A narrative review. Int J Sport Nutr
Exerc Metab. 31:292–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Cereda E, Pisati R, Rondanelli M and
Caccialanza R: Whey protein, leucine- and vitamin-D-enriched oral
nutritional supplementation for the treatment of sarcopenia.
Nutrients. 14:15242022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ely IA, Phillips BE, Smith K, Wilkinson
DJ, Piasecki M, Breen L, Larsen MS and Atherton PJ: A focus on
leucine in the nutritional regulation of human skeletal muscle
metabolism in ageing, exercise and unloading states. Clin Nutr.
42:1849–1865. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kitajima Y, Takahashi H, Akiyama T,
Murayama K, Iwane S, Kuwashiro T, Tanaka K, Kawazoe S, Ono N,
Eguchi T, et al: Supplementation with branched-chain amino acids
ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat
accumulation in the skeletal muscles of patients with liver
cirrhosis. J Gastroenterol. 53:427–437. 2018. View Article : Google Scholar
|
|
161
|
Wang S, Guo W and Dong R: Unraveling the
transcriptomic effects of leucine supplementation on muscle growth
and performance in basketball athletes. PLoS One. 20:e03166032025.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Dong Y, Yuan H, Ma G and Cao H:
Bone-muscle crosstalk under physiological and pathological
conditions. Cell Mol Life Sci. 81:3102024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Watanabe-Takano H, Ochi H, Chiba A, Matsuo
A, Kanai Y, Fukuhara S, Ito N, Sako K, Miyazaki T, Tainaka K, et
al: Mechanical load regulates bone growth via periosteal osteocrin.
Cell Rep. 36:1093802021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Klein-Nulend J, van Oers RF, Bakker AD and
Bacabac RG: Nitric oxide signaling in mechanical adaptation of
bone. Osteoporos Int. 25:1427–1437. 2014.
|
|
165
|
Holmes D: Bone: Irisin boosts bone mass.
Nat Rev Endocrinol. 11:6892015.
|
|
166
|
Sheng R, Cao M, Song M, Wang M, Zhang Y,
Shi L, Xie T, Li Y, Wang J and Rui Y: Muscle-bone crosstalk via
endocrine signals and potential targets for osteosarcopenia-related
fracture. J Orthop Translat. 43:36–46. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhang L, Guo Q, Duan Y, Wang W, Yang Y and
Yin Y, Gong S, Han M, Li F and Yin Y: Potential nutritional
healthy-aging strategy: Enhanced protein metabolism by balancing
branched-chain amino acids in a finishing pig model. Food Funct.
13:6217–6232. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ikeda T, Matsunaga Y, Kanbara M, Kamono A,
Masuda T, Watanabe M, Nakanishi R and Jinno T: Effect of exercise
therapy combined with branched-chain amino acid supplementation on
muscle strength in elderly women after total hip arthroplasty: A
randomized controlled trial. Asia Pac J Clin Nutr. 28:720–726.
2019.PubMed/NCBI
|
|
169
|
Huynh H and Wan Y: mTORC1 impedes
osteoclast differentiation via calcineurin and NFATc1. Commun Biol.
1:292018. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ozaki K, Yamada T, Horie T, Ishizaki A,
Hiraiwa M, Iezaki T, Park G, Fukasawa K, Kamada H, Tokumura K, et
al: The L-type amino acid transporter LAT1 inhibits
osteoclastogenesis and maintains bone homeostasis through the
mTORC1 pathway. Sci Signal. 12:eaaw39212019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Rivera CN, Watne RM, Wommack AJ and
Vaughan RA: The effect of insulin resistance on extracellular BCAA
accumulation and SLC25A44 expression in a myotube model of skeletal
muscle insulin resistance. Amino Acids. 55:1701–1705. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
da Silva FMO, Pimenta AM, Juvanhol LL,
Hermsdorff HHM and Bressan J: Obesity incidence according to
branched-chain amino acid intake and plant-based diet index among
Brazilian adults: A six-year follow-up of the CUME study.
Nutrients. 17:2272025. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Holeček M: The role of skeletal muscle in
the pathogenesis of altered concentrations of branched-chain amino
acids (valine, leucine, and isoleucine) in liver cirrhosis,
diabetes, and other diseases. Physiol Res. 70:293–305. 2021.
View Article : Google Scholar
|
|
174
|
You Y, Leng S, Shi J, Yang H, Chang M, Ma
Q, Zhang D, Sun H, Wang L, Gao Z, et al: Integration of
bone-targeted delivery and crosstalk modulation of liver-bone axis
for improved osteoporosis therapy. ACS Nano. 19:23955–23968. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zuo X, Zhao R, Wu M, Wang Y, Wang S, Tang
K, Wang Y, Chen J, Yan X, Cao Y and Li T: Multi-omic profiling of
sarcopenia identifies disrupted branched-chain amino acid
catabolism as a causal mechanism and therapeutic target. Nat Aging.
5:419–436. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Tilg H and Moschen AR: Adipocytokines:
Mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Olson OC, Quail DF and Joyce JA: Obesity
and the tumor microenvironment. Science. 358:1130–1131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Jarvis SE, Nguyen M and Malik VS:
Association between adherence to plant-based dietary patterns and
obesity risk: A systematic review of prospective cohort studies.
Appl Physiol Nutr Metab. 47:1115–1133. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Singh L, Tyagi S, Myers D and Duque G:
Good, bad, or ugly: The biological roles of bone marrow fat. Curr
Osteoporos Rep. 16:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Bo T and Fujii J: Primary roles of
branched chain amino acids (BCAAs) and their metabolism in
physiology and metabolic disorders. Molecules. 30:562024.
View Article : Google Scholar
|
|
181
|
Habibi M, Shili CN, Sutton J, Goodarzi P
and Pezeshki A: Dietary branched-chain amino acids modulate the
dynamics of calcium absorption and reabsorption in
protein-restricted pigs. J Anim Sci Biotechnol. 13:152022.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Salem A, Ben Maaoui K, Jahrami H,
AlMarzooqi MA, Boukhris O, Messai B, Clark CCT, Glenn JM, Ghazzaoui
HA, Bragazzi NL, et al: Attenuating muscle damage biomarkers and
muscle soreness after an exercise-induced muscle damage with
branched-chain amino acid (BCAA) supplementation: A systematic
review and meta-analysis with meta-regression. Sports Med Open.
10:422024. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Choi BH, Hyun S and Koo SH: The role of
BCAA metabolism in metabolic health and disease. Exp Mol Med.
56:1552–1559. 2024. View Article : Google Scholar : PubMed/NCBI
|