Introduction
With the widespread use of nuclear energy technology
across various fields, the likelihood of humans being exposed to
low-dose ionizing radiation has significantly increased, and the
resulting health issues have garnered attention. Different tissues
in the human body exhibit varying levels of tolerance to radiation,
with the eye lens being particularly sensitive. A study by Neriishi
et al (1) on
postoperative cataract cases among atomic bomb survivors revealed a
significant dose-response relationship between radiation exposure
and the incidence of postoperative cataracts. For each 1 Gy
increase in radiation dose, the ratio of postoperative cataract
incidence (OR) rose by 1.39, with an estimated dose threshold of
0.1 Gy (1). In a nearly 20-year
prospective cohort study of radiation technicians in the United
States, the risk of cataract was also elevated in the group with
the highest occupational exposure to ionizing radiation (average
dose 60 mGy) compared with the lowest group (average dose 5 mGy)
(2). Radiation-induced cataract
is no longer regarded as a typical tissue response with a clearly
defined threshold for relatively high doses.
Epidemiological studies have found that chronic
occupational radiation exposure significantly increases the risk of
posterior subcapsular, cortical and nuclear cataracts, especially
posterior subcapsular cataracts, with a higher risk observed in
women than in men (3). Following
exposure to low-dose ionizing radiation, the lens fails to develop
normally, leading to slow DNA damage and repair within lens
epithelial cells (LECs) (4), as
well as abnormal proliferation and differentiation (5), abnormal degradation of cell
organelles (6), and proteomic
and liposomal changes (7),
ultimately resulting in lens opacity and cataract formation. A
previous study demonstrated that low-dose ionizing radiation can
enhance the proliferation and migration of HLE-B3 cells, and
upregulates the protein levels of β-catenin, cyclin D1 and c-Myc,
which is linked to the activation of the Wnt/β-catenin signaling
pathway (8). Vigneux et
al (9) irradiated HLE-B3
cells with X-rays, which also revealed a clear radiation damage
response. The proliferation and migration of HLE-B3 cells
significantly decreased after 0.2 Gy irradiation, with
proliferation peaking at 7 days post-irradiation (9). By contrast, the specific reaction
of SRA01/04 cells to radiation damage has not been reported in
detail. SRA01/04 cells and HLE B3 cells are both Human lens
epithelial cell lines. The SRA01/04 cell line is widely used in
studying pathogenesis of cataracts, such as the oxidative stress
model, autophagy regulation and lens fibrosis. According to the
literature, whole-genome expression analysis of HLE-B3 and SRA01/04
cells were performed by Illumina Human HT12 Expression Bead Chip
microarray. The results showed that both cell lines significantly
expressed several genes related to lens biology or cataract,
including PAX6, ZEB2, PVRL3, SPARC, COL4A1 and SLC16A12 (10). As another human lens epithelial
cell line, SRA01/04 cells may exhibit different biological
characteristics under the same experimental conditions.
Importantly, based on prior studies, the detailed molecular
mechanism by which the Wnt/β-catenin signaling pathway induces lens
opacity in response to low-dose ionizing radiation urgently
requires further investigation.
A preliminary gene chip analysis results showed that
the expression of the HMGB1 gene underwent significant changes in
SRA01/04 cells after 0.2 Gy of γ-radiation (11). The expression of HMGB1 is closely
related to cell survival, proliferation and migration (12). Shu et al (13) found that increased expression of
HMGB1 in chondrocytes induces phosphorylation of GSK-3β and
upregulates β-catenin expression, thereby promoting chondrocyte
apoptosis and cartilage matrix degradation. Wang et al
(14) found that elevated
expression of HMGB1 promotes the proliferation and migration of
lung cancer cells, and this oncogenic behavior is mediated through
the activation of the Wnt/β-catenin signaling pathway. Chen et
al (15) also reported that
elevated expression of HMGB1 in human bronchial epithelial cells
leads to the phosphorylation and inactivation of GSK3β through the
PI3K/AKT signaling pathway, which results in the accumulation of
β-catenin in the cytoplasm and its subsequent translocation to the
nucleus for expression, thereby inducing the occurrence of
epithelial-mesenchymal transition (EMT). Therefore, it was
hypothesized that the increase of HMGB1 expression in LECs induced
by low-dose ionizing radiation can activate the Wnt/β-catenin
signaling pathway, thus promoting the proliferation and migration
of LECs and inducing lens opacification.
To address the knowledge gaps in understanding the
damaging effects of SRA01/04 cells under low-dose ionizing
radiation and the molecular mechanisms regulated by the
Wnt/β-catenin signaling pathway, SRA01/04 cells were infected with
lentivirus to inhibit HMGB1 expression, and the regulatory
relationship between HMGB1 expression and the Wnt/β-catenin
signaling pathway in these cells following low-dose ionizing
radiation exposure. The present study was specifically designed to
address two fundamental objectives: First, to delineate the
mechanistic relationship between low-dose ionizing
radiation-induced alterations in proliferative and migratory
capacities of SRA01/04 cells and concurrent Wnt/β-catenin signaling
activation. Second, to systematically characterize the molecular
circuitry governing radiation-triggered Wnt/β-catenin pathway
activation in human LECs, with particular emphasis on identifying
key regulatory nodes within this pathological cascade.
Materials and methods
SRA01/04 cell line
SRA01/04 cells are adherent cells obtained from the
American Type Culture Collection and are derived from normal LECs
of infants with retinopathy of prematurity. The cell line was
established by transfecting these cells with a plasmid vector
carrying the Simian Virus 40 large T antigen (TagSV40) (16). Cells were authenticated by STR
profiling and verified to be mycoplasma-free.
Cell irradiation
In the present study, low doses were set at 0.05,
0.075, 0.1 and 0.2 Gy, while high doses were at 0.5 and 2 Gy,
serving as controls. The irradiation methods were as follows: The
SRA01/04 cells were irradiated with 0.05, 0.075, 0.1, 0.2 and 0.5
Gy gamma rays using a 137Cs radiation source at a dose
rate of 9.73 mGy/min, conducted at the National Secondary Standard
Dosimetry Laboratory of the Radiation Safety Institute of the
Chinese Center for Disease Control and Prevention. A ferrous
sulfate dosimeter was utilized for radiation source calibration to
ensure accurate dosing. SRA01/04 cells were also irradiated with 2
Gy γ-rays from a 60Co radioactive source at a dose rate
of 1 Gy/min at the Beijing Irradiation Center. The distance from
the sample to the cobalt source was 68 cm, and the dose was
calibrated by physical measurement using an ionization chamber,
with a dose calibration uncertainty of 0.1%.
Cell proliferation assay
For this assay, SRA01/04 cells (6,000 cells/well)
were seeded in 100 μl of medium in 96-well plates, with six
wells seeded for each group. On the second day after adherent
growth, the cells were randomly assigned to the 0-2 Gy irradiation
groups. A total of 10 μl of Cell Counting Kit-8 (CCK-8)
reagent (Dojindo Laboratories, Inc.) were added to each well at
various times post-irradiation, and the plates were incubated for 1
h at 37°C. Absorbance values were measured at 450 nm using a
microplate reader once color development was adequate. This
experiment was independently repeated three times. Cell viability
was determined using the following formula: Cell
viability=(irradiated group optical density (OD) value-blank group
OD value)/(0 Gy group OD value-blank group OD value).
Gap closure assay
SRA01/04 cells in favorable growth condition were
harvested, and 50 μl of the cell suspension at a density of
2×105 cells/ml was seeded into each well of an Ibidi
culture insert, placed within a culture dish. A total of 1 ml of
medium with 10% FBS was added to the dish surrounding each insert,
with three replicate wells for each group (17). The following day, after the cells
had adhered, they were irradiated with γ-rays at doses of 0, 0.05,
0.075, 0.1, 0.2, 0.5 and 2 Gy. Immediately after irradiation, the
inserts were gently removed using sterile tweezers to create
uniform cell-free wounds (scratches). The dishes were then returned
to the incubator.
Cell migration into the gap area was monitored at 8,
24, 48 and 72 h using an inverted microscope, and images were
captured. Closure of the gap at each time point was measured using
ImageJ 1.54 software (National Institutes of Health). The gap
closure rate was calculated as follows: The Gap Closure Rate
(%)=[(Initial Gap Width-Gap Width at Time t)/Initial Gap Width]
×100%. In total, 3 fields of view were analyzed for each dosage and
time point, and an average gap closure rate was calculated.
Transwell migration assay
The healthy SRA01/04 cells were inoculated into T-25
culture flasks and subjected to γ-ray irradiation at various doses:
0, 0.05, 0.075, 0.1, 0.2, 0.5 and 2 Gy. At 8 h, 48 h and 7 days
post-irradiation, the cells were resuspended at a density of
4×104 cells/ml. A total of 200 μl of the
suspension was added to the upper chamber of Transwell inserts
(5-μm pore size). The lower chamber received 600 μl
of medium supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) as a chemoattractant. After the cells adhered to
the chamber surface, the medium with 10% FBS in the chambers was
replaced with serum-free medium. The cells were allowed to migrate
in a 37°C incubator for 8 h. The medium was discarded, and the
Transwell inserts were washed twice with phosphate-buffered saline
(PBS). Non-migratory cells on the upper surface were wiped off,
while migratory cells on the underside of the filter were fixed
with 4% paraformaldehyde at room temperature for 10-30 min,
immersed in anhydrous methanol for 20 min, and stained with Giemsa.
All migratory cells on the entire membrane surface were counted
under a light microscope. This experiment was independently
repeated three times.
Clone formation experiment
Following the Transwell migration assay, SRA01/04
cells that had migrated to the lower chamber after γ-ray
irradiation at various doses were harvested. These cells were
trypsinized, resuspended, and seeded into 6-well plates at a
density of 1,000 cells per well (three replicate wells per group).
Cells were cultured for 7-10 days, with medium changes every 3
days, to allow clonogenic colony formation. Upon observation of
clearly defined colonies (cell clusters larger than 50 μm in
diameter), cultures were terminated. Cells were gently washed once
with 1X PBS, fixed with anhydrous methanol for 30 min, and stained
with Giemsa for 30 min. After air-drying at room temperature, cell
clones were manually counted and images were captured.
Mouse irradiation and Giemsa staining of
posterior lens capsule
Six-week-old Specific pathogen free (SPF) C57BL/6J
mice (15 males weighing 20±2 g and 15 females weighing 18±2 g) were
ordered from Beijing Vital River Laboratory Animal Technology Co.,
Ltd. [License no.: (SCXK (Beijing) 2021-0006], raised in the animal
room of the Institute of Occupational Health and Poisoning Control,
Chinese Center for Disease Control and Prevention. Each cage
contained 6 mice housed under 12/12-h light-dark cycle. The
temperature was kept at ~22°C and humidity at 40-60%. Sterile
distilled water and SPF-grade feed were provided ad libitum.
The mice were checked daily for weight, health and behavior.
C57BL/6J mice (three males and three females per
group) were subjected to whole-body γ-ray irradiation at doses of
0, 0.05, 0.1, 0.2 and 2 Gy. For the 0.05, 0.1 and 0.2 Gy
irradiations, a 137Cs radiation source was used with a
dose rate of 9.73 mGy/min. For the 2 Gy irradiation, a
60Co radiation source was employed with a dose rate of 1
Gy/min.
Six months after irradiation at different doses,
mice were euthanized by an intraperitoneal injection of an overdose
of 1% pentobarbital sodium (150 mg/kg), death was confirmed by the
absence of a heartbeat and respiratory arrest, and their eyeballs
were enucleated. The lenses were dissected out, with the lens
capsules separated. The posterior lens capsules of the mice were
spread flat on glass slides with the inner side facing up, and
Giemsa staining solution was directly applied, allowing it to stain
for 20-30 min. The stained posterior capsules were then examined
under an inverted microscope for the presence of stained cells.
All animal experimental procedures were approved by
the Experimental Animal Welfare and Ethics Committee of the
National Institute for Radiological Protection, Chinese Center for
Disease Control and Prevention [approval no. (2022)005; Beijing
China].
Western blot assay
To analyze protein expression in SRA01/04 cells,
cell lysates were first prepared and mixed with 5X loading buffer,
followed by boiling at 100°C for 10 min to denature the proteins.
The BCA method was used for protein quantification. The denatured
protein samples (30 μg per lane) were then subjected to
electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels
to separate the proteins based on their molecular weights. After
electrophoresis, the separated proteins were transferred onto
nitrocellulose membranes. The membranes were blocked with 5%
skimmed milk in Tris-buffered saline containing 0.1% Tween 20
(TBST) for 1 h at room temperature to prevent non-specific binding
of antibodies. A variety of primary antibodies, including rabbit
anti-β-catenin (1:1,000; cat. no. 8480; Cell Signaling Technology,
Inc.), rabbit anti-cyclin D1 (1:200; cat. no. ab16663; Abcam),
rabbit anti-c-Myc (1:1,000; cat. no. 18583; Cell Signaling
Technology, Inc.), rabbit anti-HMGB1 (1:10,000; cat. no. ab79823;
Abcam), rabbit anti-Phospho-β-catenin (1:1,000; cat. no. 9561; Cell
Signaling Technology, Inc.), and mouse anti-GAPDH (1:1,000; cat.
no. TA-08; ZSGB-BIO), were added and incubated overnight at 4°C to
allow specific binding to their target proteins. After incubation,
the membranes were washed three times with TBST and then incubated
with anti-mouse secondary antibodies (1:4,000; cat. no. ZB-5305;
ZSGB-BIO) or anti-rabbit secondary antibodies (1:3,000; cat. no.
ZB-5301; ZSGB-BIO) for 1 h. Immunoreactive bands were detected
using the SuperSignal West Pico PLUS chemiluminescent substrate
(Thermo Fisher Scientific, Inc.). The membranes were developed and
imaged on the ChemiDoc XRC+ chemiluminescence imaging system
(Bio-Rad Laboratories, Inc.). The gray value of the bands was
measured using Image Lab (Bio-Rad Laboratories, Inc.). All protein
expression experiments were independently repeated three times.
Immunofluorescence microscopy
analyses
A cell suspension of 2×105 cells/ml was
inoculated onto sterile coverslips in a 24-well plate. The
following day, after the cells had adhered and proliferated, they
were exposed to 0, 0.05, 0.075, 0.1, 0.2, 0.5 and 2 Gy of
γ-radiation. After irradiation, the cells were fixed with 4%
paraformaldehyde at room temperature for 10 min. The coverslips
were then carefully oriented with the cell-containing side up, and
PBS containing 0.2% Triton X-100 was added to permeabilize the
cells for 10 min at room temperature. Next, to block non-specific
antibody binding, the coverslips were treated with PBS containing
3% BSA (Beijing Solarbio Science & Technology Co., Ltd.) and
incubated on a slow shaker at room temperature for 1 h. The primary
antibody rabbit anti-β-catenin (1:80; cat. no. 8480; Cell Signaling
Technology, Inc.) was added to the samples. After incubation, the
coverslips were washed three times with PBS and then incubated with
the secondary antibodies: Anti-rabbit IgG (H+L), F(ab')2
Fragment (Alexa Fluor® 594 Conjugate) (1:600; cat. no.
8890; Cell Signaling Technology, Inc.) for 1 h.
VECTASHIELD® Antifade Mounting Medium with DAPI (Vector
Laboratories, Inc.) was added to stain the nuclei for 10 min in the
dark at room temperature. After washing with 1X PBS, the coverslips
were mounted. The cellular localization of β-catenin was observed
and photographed using a laser confocal microscope (LSM700; Zeiss
GmbH).
shRNA lentivirus vector construction
The construction of the shRNA lentiviral vector was
completed by Shanghai GeneChem Co., Ltd. The lentiviral vector
skeleton was GV493 (hU6-MCS-CBh-gcGFP-IRES-puromycin). A total of
three candidate interference sequences were designed for the
knockdown of the HMGB1 gene in cells, as detailed in Table I. Negative control (NC) cell
lines were constructed from lentiviral vectors with a control
sequence (TTCTCCGAACGTGTCACGT) inserted at the same site.
 | Table IInterference sequence. |
Table I
Interference sequence.
| Sequence name | Interference
sequence (5'-3') |
|---|
| sh1-HMGB1 |
GATGCAGCTTATACGAAATAA |
| sh2-HMGB1 |
TCGGGAGGAGCATAAGAAGAA |
| sh3-HMGB1 |
TTCCTCTTCTGCTCTGAGTAT |
The shRNA expression vector was co-transfected with
two packaging plasmids, pHelper 1.0 and pHelper 2.0, into 293T
cells using the transfection reagent. Lentiviral particles were
harvested from the culture supernatant of 293T packaging cells at
48 h post-transfection. The collected supernatant was pooled and
clarified by centrifugation at 4,000 × g for 10 min at 4°C,
followed by filtration through a 0.45-μm membrane. The viral
particles were concentrated via ultracentrifugation at 25,000 rpm
for 2 h at 4°C. The resulting viral pellet was resuspended in a
sterile PBS solution, aliquoted, and stored at −80°C.
Well-grown SRA01/04 cells were seeded into 6-well
plates. The following day, after cell attachment, they were
transduced with lentivirus at a multiplicity of infection (MOI) of
10. The viral supernatant was replaced with fresh complete medium
after 16-24 h of incubation, and the cells were cultured for an
additional 48 h. Transduction efficiency was confirmed to exceed
80% by assessing the intensity of green fluorescent protein (GFP)
expression under a fluorescence microscope. At 72 h
post-transduction, the medium was replaced with a selection medium
containing 2 μg/ml puromycin to select for stably transduced
cells. After 1 week of selection, stable knock-down cell lines were
established. Subsequently, the cells were expanded in medium with a
reduced puromycin concentration of 0.67 μg/ml. All
experiments were conducted following one week of this expansion
phase.
Statistical analyses
SPSS 27.0 software (IBM Corp.) was used for
statistical analysis of data. GraphPad Prism 9 software (Dotmatics)
was used for plotting. Experimental data were expressed as the mean
± standard deviation (SD). Independent sample t-tests were used for
comparisons between two groups, while one-way ANOVA followed by
Fisher's LSD post hoc test or the Kruskal-Wallis H test followed by
Dunn's post hoc test was applied for comparisons among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of ionizing radiation on cell
proliferation and migration
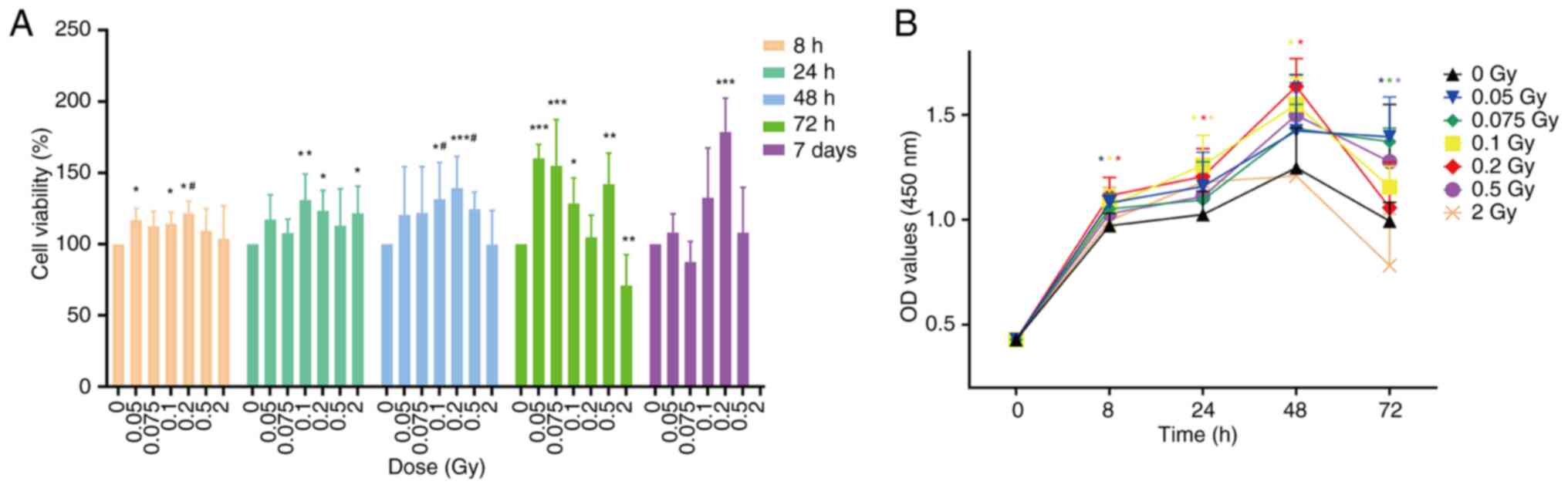
Cell viability was determined by CCK-8 assay. At 8 h
after irradiation with 0.05, 0.1 and 0.2 Gy, cell viability was
significantly enhanced compared with the non-irradiated group
(P<0.05). By 24 h post-irradiation, significant enhancement in
viability was observed in cells treated with 0.1, 0.2, and 2 Gy,
with statistical significance at P<0.05 or P<0.01. At 48 h
post-irradiation, cell viability in the low-dose groups and the 0.5
Gy group was enhanced compared with the non-irradiated group, with
the most significant increase observed after 0.2 Gy irradiation
(P<0.001). However, cell viability began to decline in the 2 Gy
group, showing no significant difference when compared with the
non-irradiated group (P>0.05). At 72 h post-irradiation, cell
viability in the low-dose groups and the 0.5 Gy group remained
higher than that of the non-irradiated group, while cell viability
in the 2 Gy group was significantly reduced compared with the
non-irradiated group (P<0.01) (Fig. 1A). Additionally, in Fig. 1B it is also illustrated that the
OD values of all groups gradually increased from 0 to 48 h
post-irradiation. At 72 h, although the OD values of the low-dose
and 0.5 Gy groups showed a slight decrease, they remained higher
than the non-irradiated group. Overall, from 0 to 72 h
post-irradiation, the OD values of the low-dose and 0.5 Gy groups
were higher than those of the non-irradiated group, indicating
increased cell proliferation. By contrast, the OD value of the 2 Gy
group was lower than the non-irradiated group at 72 h
post-irradiation, indicating reduced proliferative capacity. These
changes suggest that low-dose ionizing radiation (0.05-0.2 Gy)
promotes the proliferation of SRA01/04 cells, particularly after
irradiation with 0.1 or 0.2 Gy, where significant enhancements in
proliferation capacity were observed between 8 and 48 h
(P<0.05). Conversely, 2 Gy irradiation weakened cell
proliferation capacity.
To exclude the potential stimulation effect of
low-dose ionizing radiation on early-stage cell proliferation, the
CCK-8 method was utilized to evaluate cell viability 7 days
post-irradiation with various doses. As depicted in Fig. 1, the cell viability of the 0.2 Gy
group was significantly increased compared with the non-irradiated
group (P<0.001), while the 0.1 Gy group showed a non-significant
increase (P>0.05).
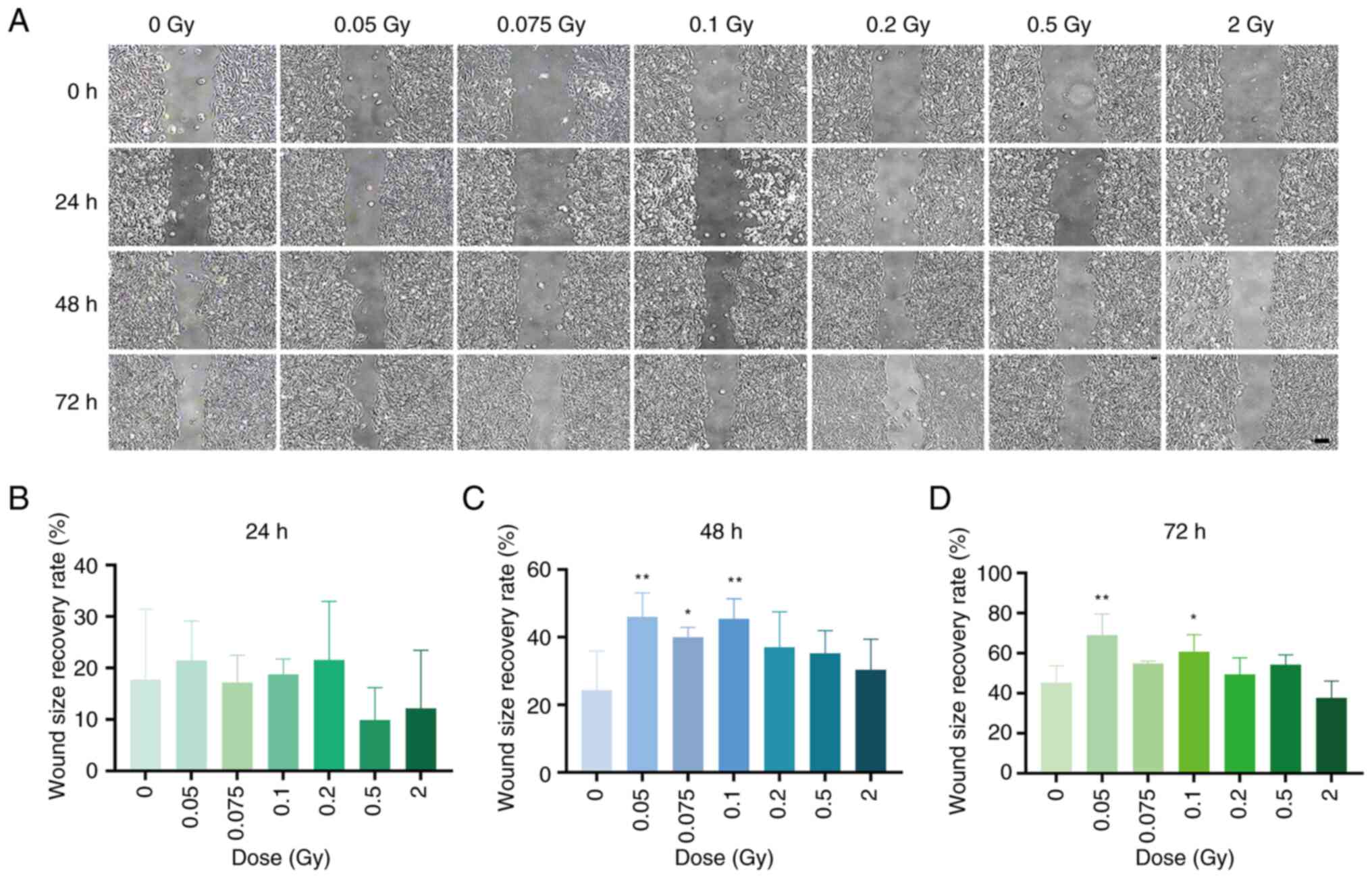
The effects of various doses of γ-ray irradiation on
SRA01/04 cell migration were evaluated using the gap closure and
Transwell migration assay. The gap closure assay indicated that at
48 h post-irradiation with different doses of γ-rays, the gap
closure ability of the 0.05, 0.075 and 0.1 Gy groups was
significantly enhanced compared with the non-irradiated group
(P<0.05 or 0.01). At 72 h post-irradiation, the gap closure
ability of the 0.05 and 0.1 Gy groups continued to show
significantly increased(P<0.05 or P<0.01), whereas the 2 Gy
group displayed a non-significant decrease in gap closure ability
(P>0.05) (Fig. 2A and B). The
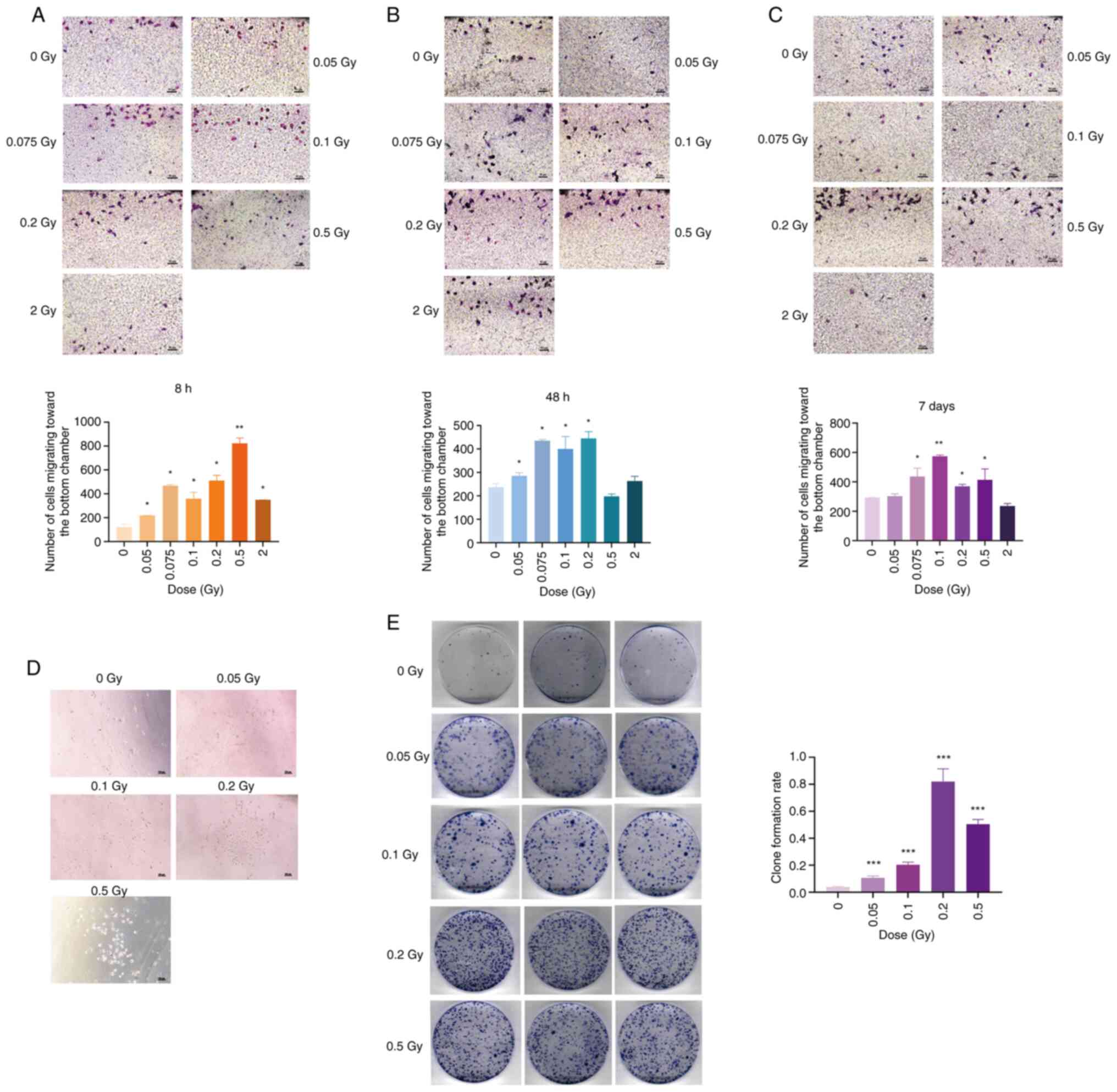
Transwell migration assay revealed that at 8 h after irradiation,
all irradiated groups demonstrated a significantly higher number of
migratory cells than the unirradiated group (P<0.05 or
P<0.01) (Fig. 3A). At 48 h
post-irradiation, low-dose irradiated cells exhibited significantly
increased migration compared with the non-irradiated group
(P<0.05), while no significant difference was observed in the
0.5 and 2 Gy groups (P>0.05) (Fig. 3B). After 7 days of irradiation
with 0.075, 0.1, 0.2 and 0.5 Gy, the number of migratory cells
remained significantly higher than in the non-irradiated group
(P<0.05 or P<0.01), whereas 2 Gy irradiation resulted in a
non-significant decrease in migration ability (P>0.05) (Fig. 3C). Collectively, these findings
suggest that low-dose ionizing radiation (0.05-0.2 Gy) promotes
SRA01/04 cell migration.
Furthermore, in the Transwell migration assay
conducted 7 days after irradiation, it was observed that some
SRA01/04 cells not only migrated to the bottom of the chamber but
also took up residence within the lower pores, continuing to
proliferate along the walls (Fig.
3D). It has been reported that cultured SRA01/04 cells are not
sensitive to serum and can survive in a medium containing 1% serum.
When serum concentration is restored, these cells can revert to
normal proliferation patterns (18). The adherent cells at each dose
point were collected, and the proliferation ability of the cells
that had migrated into the wells 7 days after irradiation was
assessed using a colony formation assay. Results presented in
Fig. 3E indicated that 7 days
after SRA01/04 cells were irradiated with 0.05, 0.1, 0.2 and 0.5
Gy, the number of clone colonies formed by the migratory cells was
significantly higher than in the non-irradiated group (P<0.001).
This suggests that low-dose ionizing radiation can promote the
proliferation of migratory SRA01/04 cells.
Effect of ionizing radiation on the
migration of mouse LECs
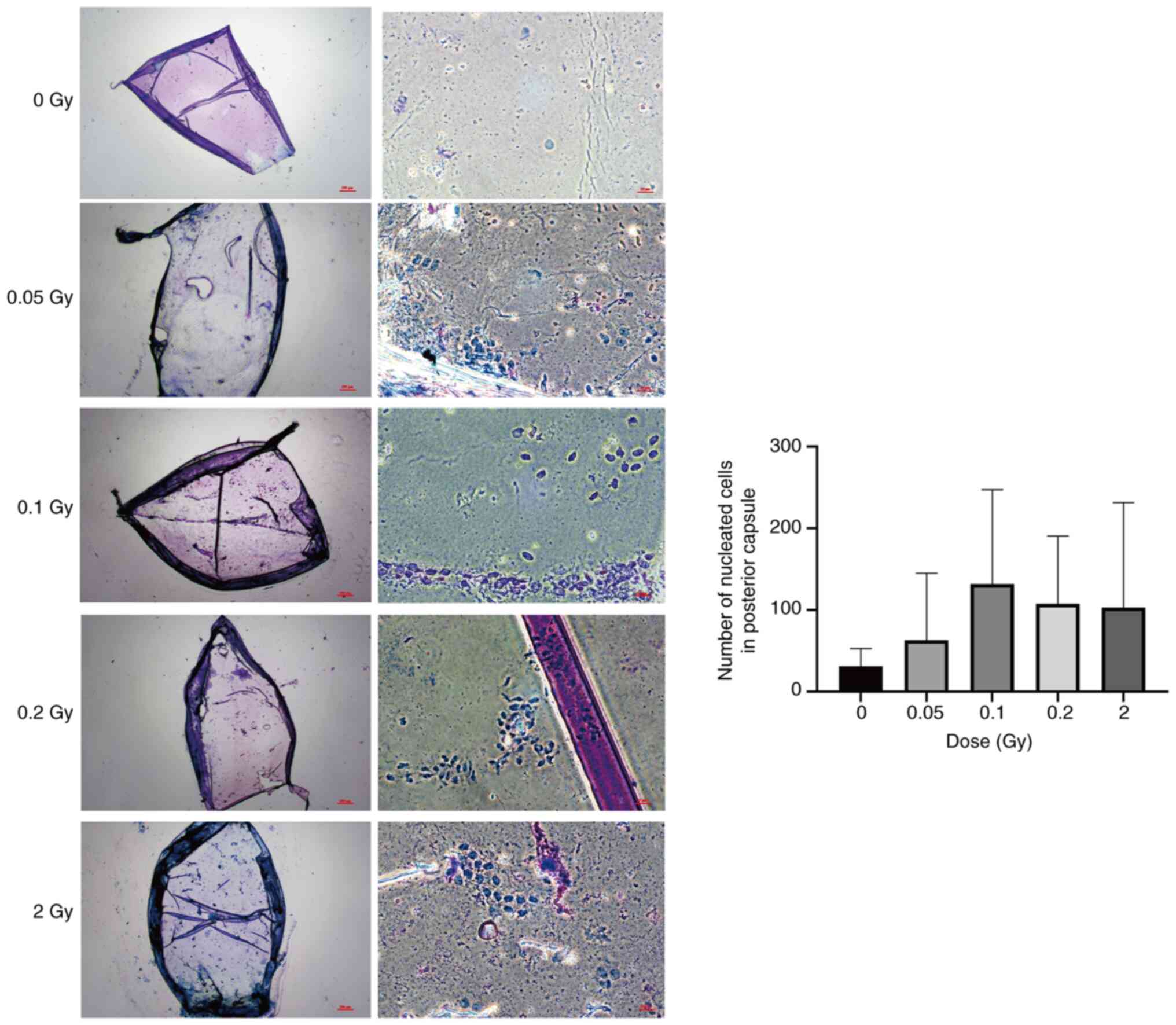
In a previous study by the authors, it was reported
that in the third month following exposure to low dose ionizing
radiation, scattered and randomly distributed migrating cells with
regular nuclei were observed within the posterior lens capsule of
mice (8). In the current study,
both the radiation dose and observation time were escalated. At 6
months after irradiation with low doses (0.05-0.2 Gy) and 2 Gy,
scattered nucleated cells with regular nuclear morphology were
still observed in the posterior capsule of all irradiated groups
(Fig. 4). After 0.1 Gy
irradiation, the highest number of nucleated cells in the posterior
capsule was observed, consistent with our previous findings.
However, at six months post-irradiation, the lens aspect ratio
distortion described by Markiewicz et al (19) was not observed in either the
low-dose or 2 Gy groups. Additionally, no lens opacity was
detected.
Effect of low-dose ionizing radiation on
β-catenin and its related protein expression in SRA01/04 cells
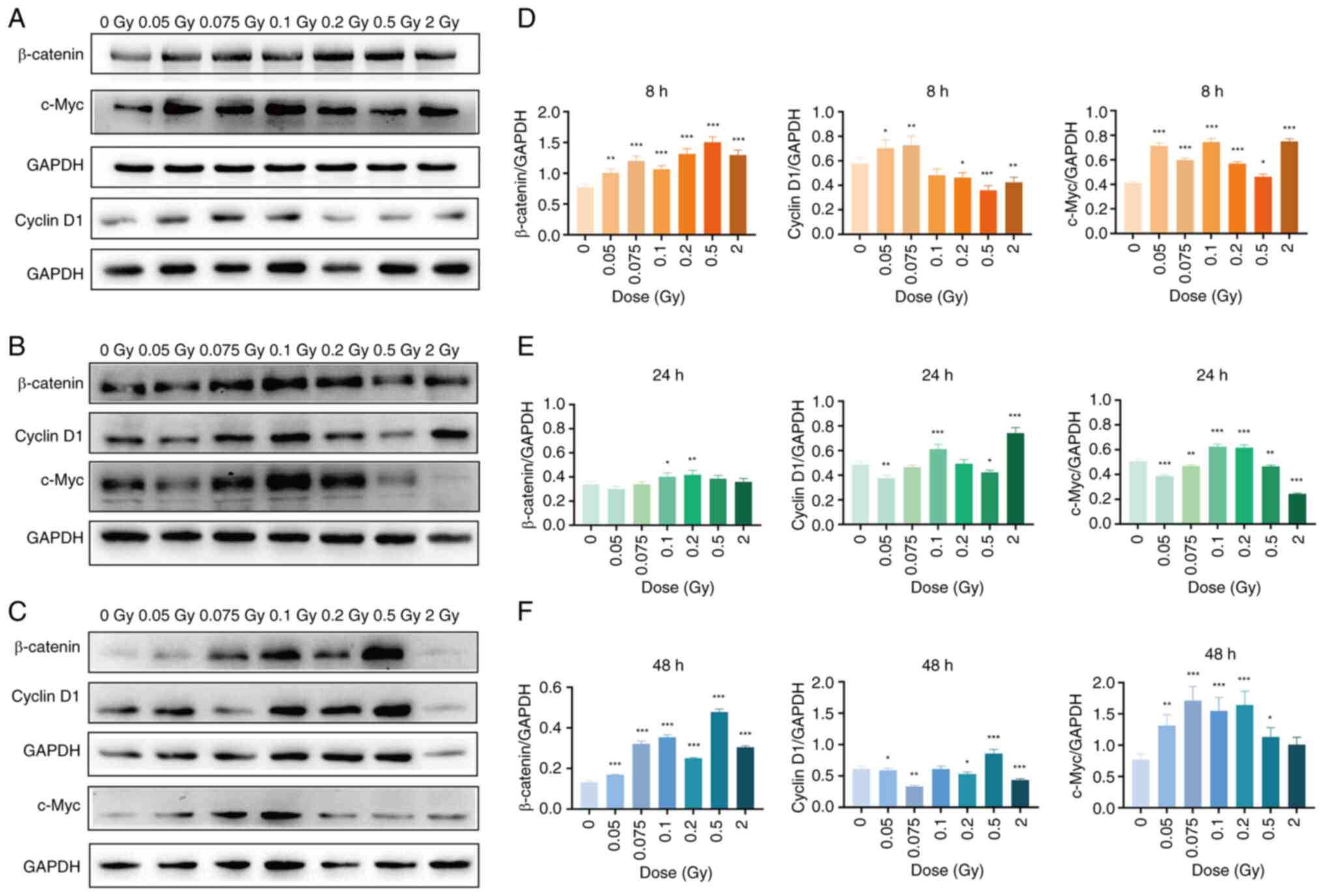
As shown in Fig.
5, the expression levels of β-catenin and c-Myc in the
irradiated group were significantly higher than those in the
non-irradiated group at 8 h after exposure to different doses of
γ-rays (P<0.05, P<0.01 or P<0.001). Meanwhile, cyclin D1
expression was also significantly elevated in the 0.05 and 0.075 Gy
groups relative to the non-irradiated group (P<0.05 or
P<0.01) (Fig. 5A). At 24 h
post-irradiation, β-catenin and c-Myc expression remained
significantly higher in the 0.1 and 0.2 Gy groups (P<0.05,
P<0.01 or P<0.001), while c-Myc expression in the 2 Gy group
significantly decreased (P<0.001) compared with the
non-irradiated group. Moreover, the cyclin D1 expression level was
significantly higher in the 0.1 and 2 Gy groups (P<0.001)
compared with the non-irradiated group (Fig. 5B). At 48 h, low-dose (0.05-0.2
Gy) and 0.5 Gy irradiation continued to induce significant
upregulation of β-catenin and c-Myc compared with the
non-irradiated group (P<0.05, P<0.01 or P<0.001). Cyclin
D1 expression increased significantly compared with the
non-irradiated group 48 h after 0.5 Gy irradiation (P<0.001) but
was significantly lower after 2 Gy irradiation compared with the
non-irradiated group (P<0.001) (Fig. 5C). These results indicated that
low-dose ionizing radiation can differentially upregulate the
protein expressions of β-catenin, cyclin D1 and c-Myc in SRA01/04
cells.
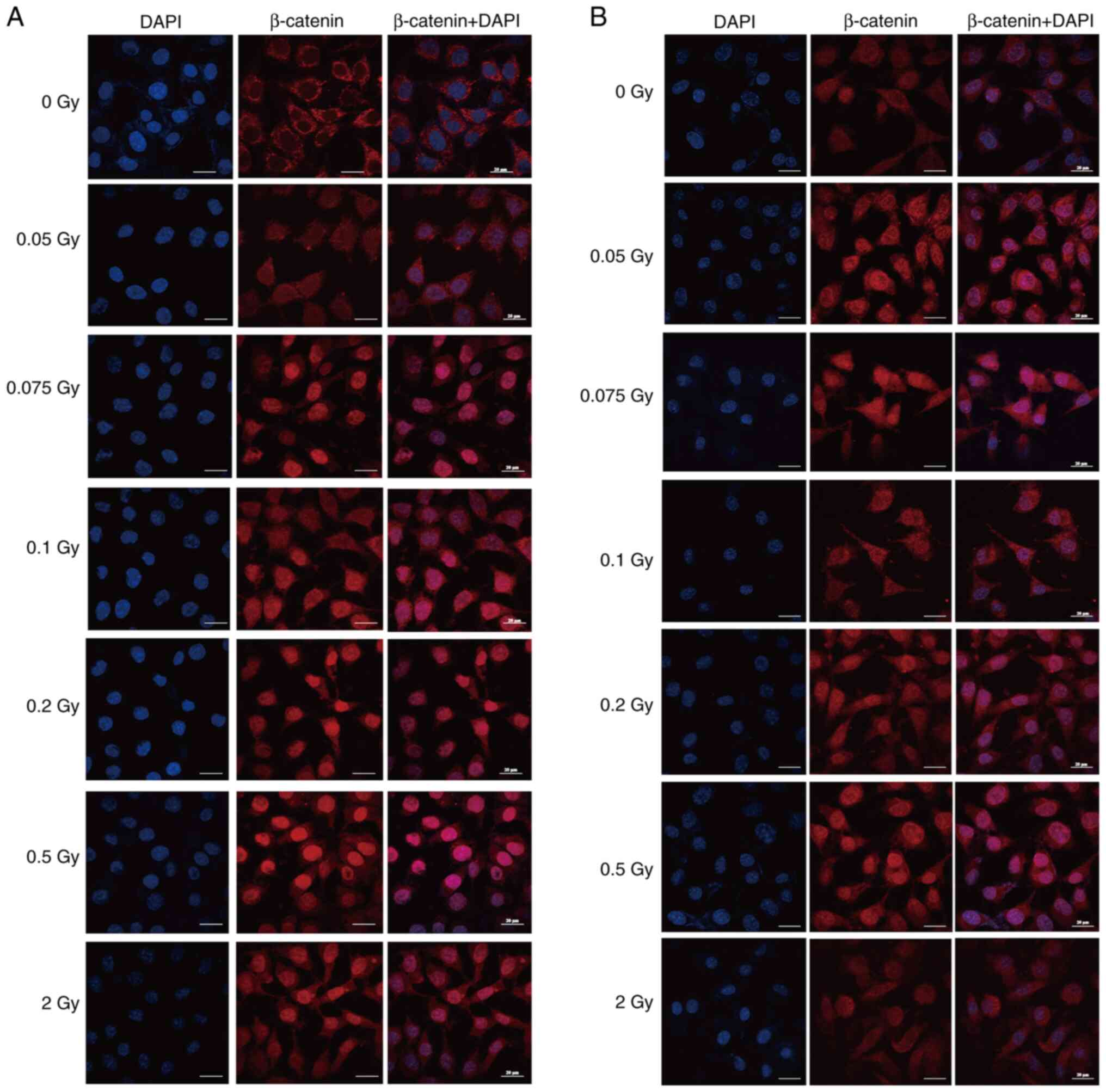
To elucidate the impact of γ-ray irradiation on the
expression and localization of β-catenin in SRA01/04 cells,
immunofluorescence assays were conducted with cells exposed to a
spectrum of γ-ray doses. As shown in Fig. 6A and B, β-catenin was mainly
localized in the cell membrane and cytoplasm or uniformly expressed
on the cell membrane in the non-irradiated group. At 24 and 48 h
after low-dose (0.05-0.2 Gy) and 0.5 Gy irradiation, a distinct
translocation of β-catenin into the nucleus was observed with
notable accumulation within the nucleus. After 2 Gy irradiation,
β-catenin was also significantly expressed in the nucleus at 24 h,
while the localization of β-catenin did not significantly change
compared with the non-irradiated group at 48 h. These results
suggested that low-dose ionizing radiation may activate the
Wnt/β-catenin signaling pathway in SRA01/04 cells, which is
associated with abnormal cell proliferation and migration.
Effect of HMGB1 on the expression and
localization of β-catenin after low-dose γ-irradiation
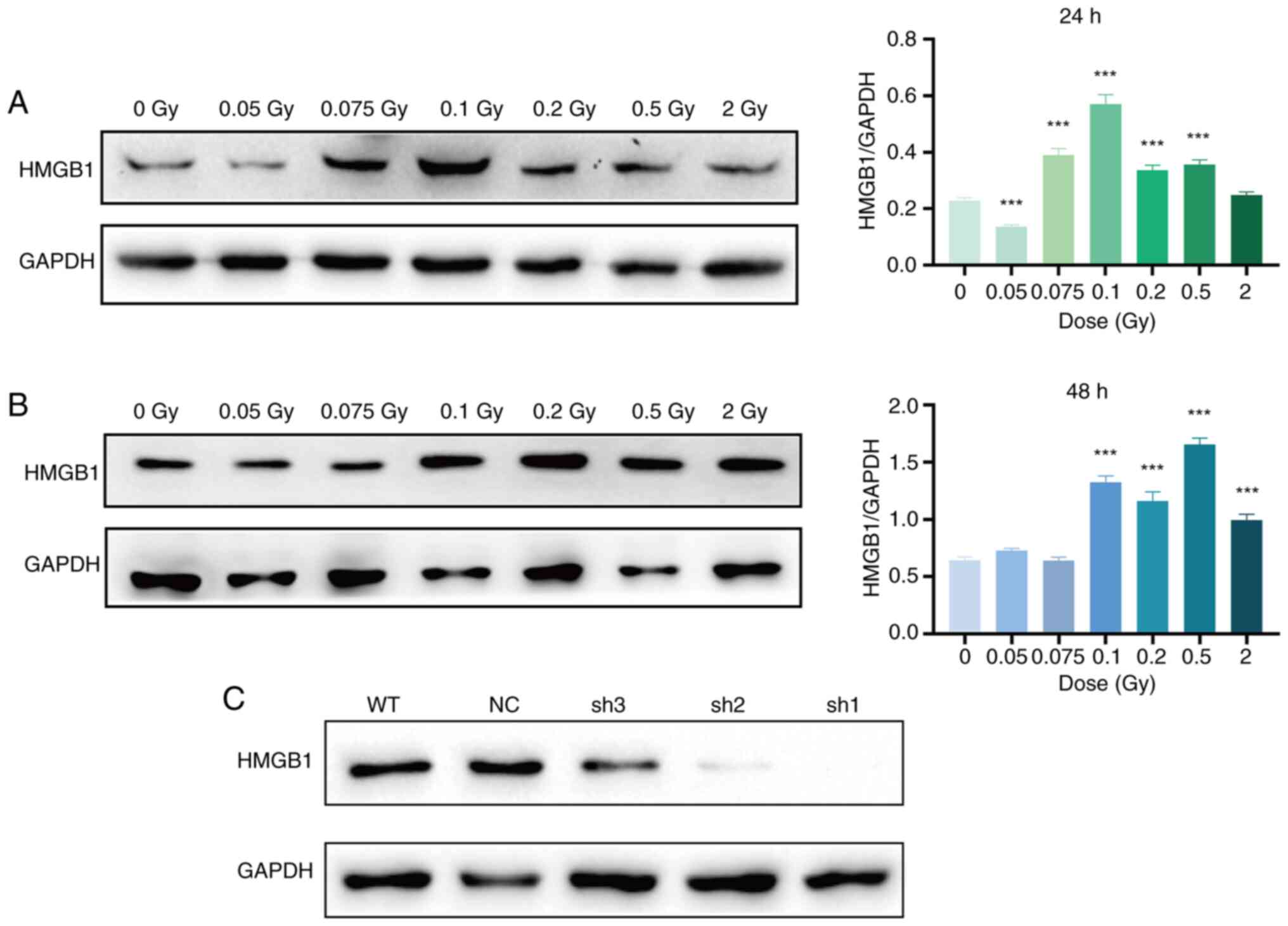
In SRA01/04 cells, HMGB1 expression significantly
increased at 24 h after 0.075, 0.1, 0.2 and 0.5 Gy irradiation and
at 48 h after 0.1, 0.2, 0.5 and 2 Gy irradiation compared with the
non-irradiated group (P<0.001) (Fig. 7A and B). Subsequently, the cells
were infected with lentivirus, and the knockout effects of three
different interfering sequences targeting HMGB1 were evaluated by
western blot analysis. Both sh1 and sh2 interfering sequences
markedly reduced HMGB1 protein expression, with the sh1 sequence
emerging as the most effective in HMGB1 suppression. The sh1
sequence was selected for subsequent experiments (Fig. 7C). Leveraging this foundation,
the intricate relationship between HMGB1 expression in LECs after
exposure to low-dose ionizing radiation and the activation of the
Wnt/β-catenin signaling pathway were explored.
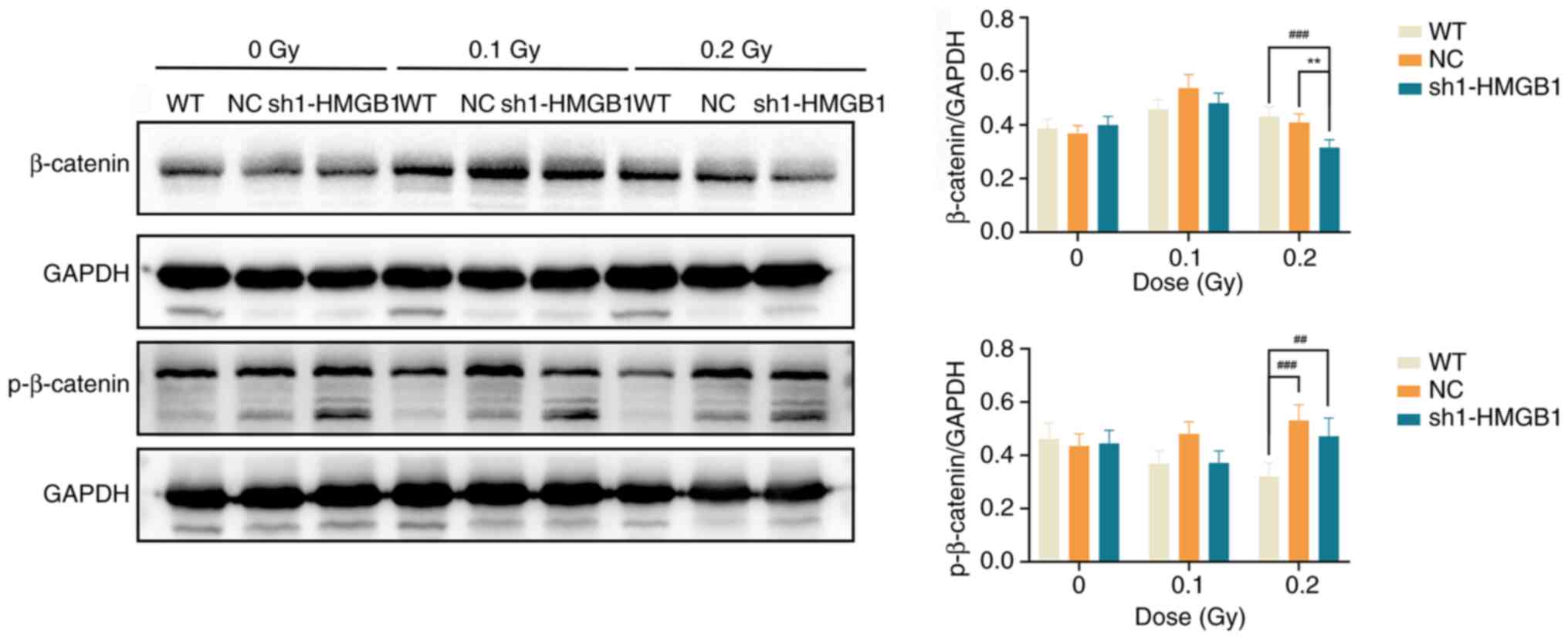
Previous studies have established that nuclear
translocation of β-catenin is a key step in Wnt signaling, while
its phosphorylation is the primary mechanism controlling the
pathway's 'on' and 'off' states, phosphorylation of β-catenin at
Ser33, Ser37 and Thr41 can prevent its nuclear translocation and
the activation of target gene transcription (20,21). Western blot analysis was used to
assess the expression of total and phosphorylated β-catenin
(p-β-catenin) in HMGB1-knockout cells. The results showed that in
the non-irradiated groups, there were no significant differences in
total β-catenin or p-β-catenin among the different cell groups
(P>0.05). However, at 48 h following exposure to low-dose
radiation (particularly at 0.2 Gy), the knockdown group exhibited a
significantly lower level of total β-catenin compared with the
wild-type (WT) and NC groups (P<0.01 or P<0.001); conversely,
the level of p-β-catenin was significantly higher in the knockdown
group than in the WT group (P<0.01), suggesting an inhibition of
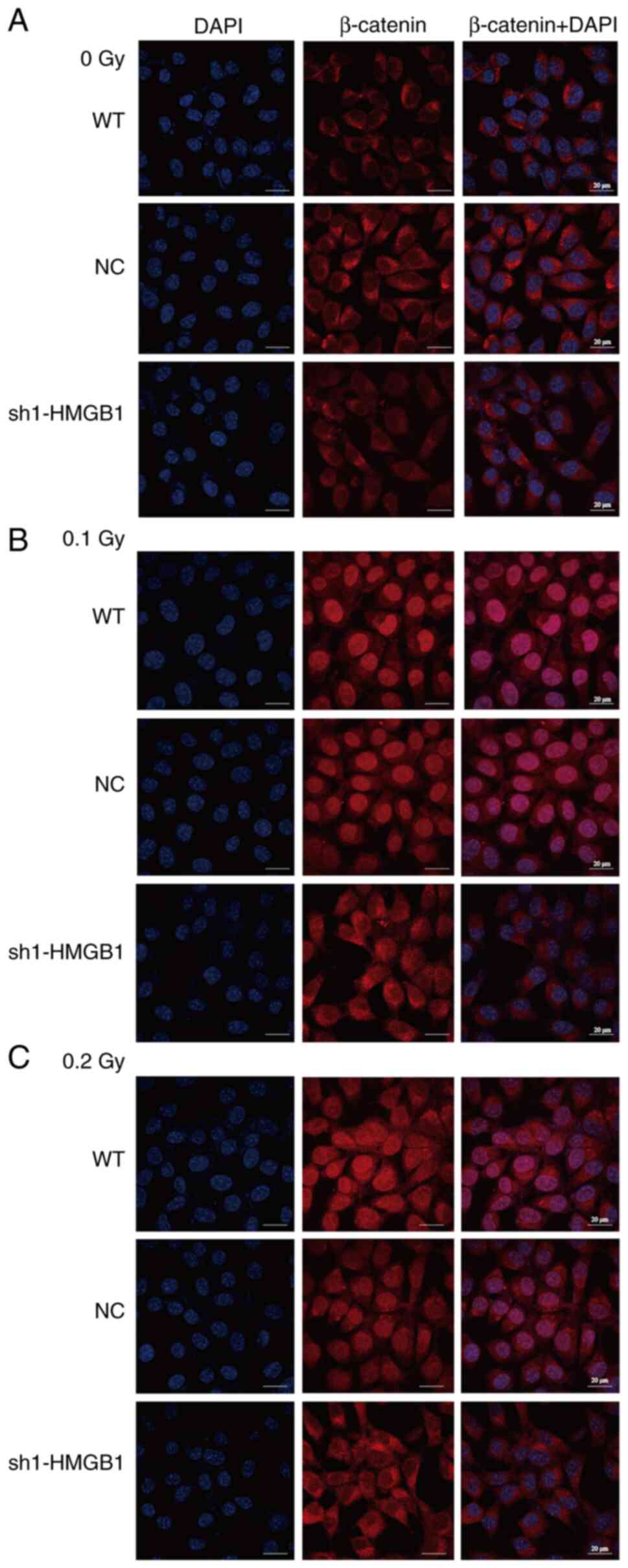
the Wnt/β-catenin signaling pathway after HMGB1-knockdown (Fig. 8). Using confocal laser scanning
microscopy, the localization of β-catenin was examined in
HMGB1-knockdown cells exposed to various low doses of γ-radiation.
The findings of the present study indicated that in unirradiated
cells, β-catenin expression was predominantly confined to the cell
membrane and cytoplasm in the WT, NC and HMGB1-knockdown groups,
suggesting that the Wnt/β-catenin signaling pathway remained in
inactivated state (Fig. 9A).
Nevertheless, at 48 h post-exposure to 0.1 and 0.2 Gy radiation,
Wnt/β-catenin signaling pathway significantly activated, marked by
the expression of β-catenin translocated into the nucleus in the WT
and NC groups (Fig. 9B and C).
By contrast, in the HMGB1-knockdown group, β-catenin remained
predominantly expressed in the cell membrane and cytoplasm,
exhibiting minimal nuclear expression and no detectable nuclear
translocation (Fig. 9B and C).
This observation suggests that low-dose ionizing radiation cannot
fully activate the intracellular Wnt/β-catenin signaling pathway in
HMGB1-knockdown cells.
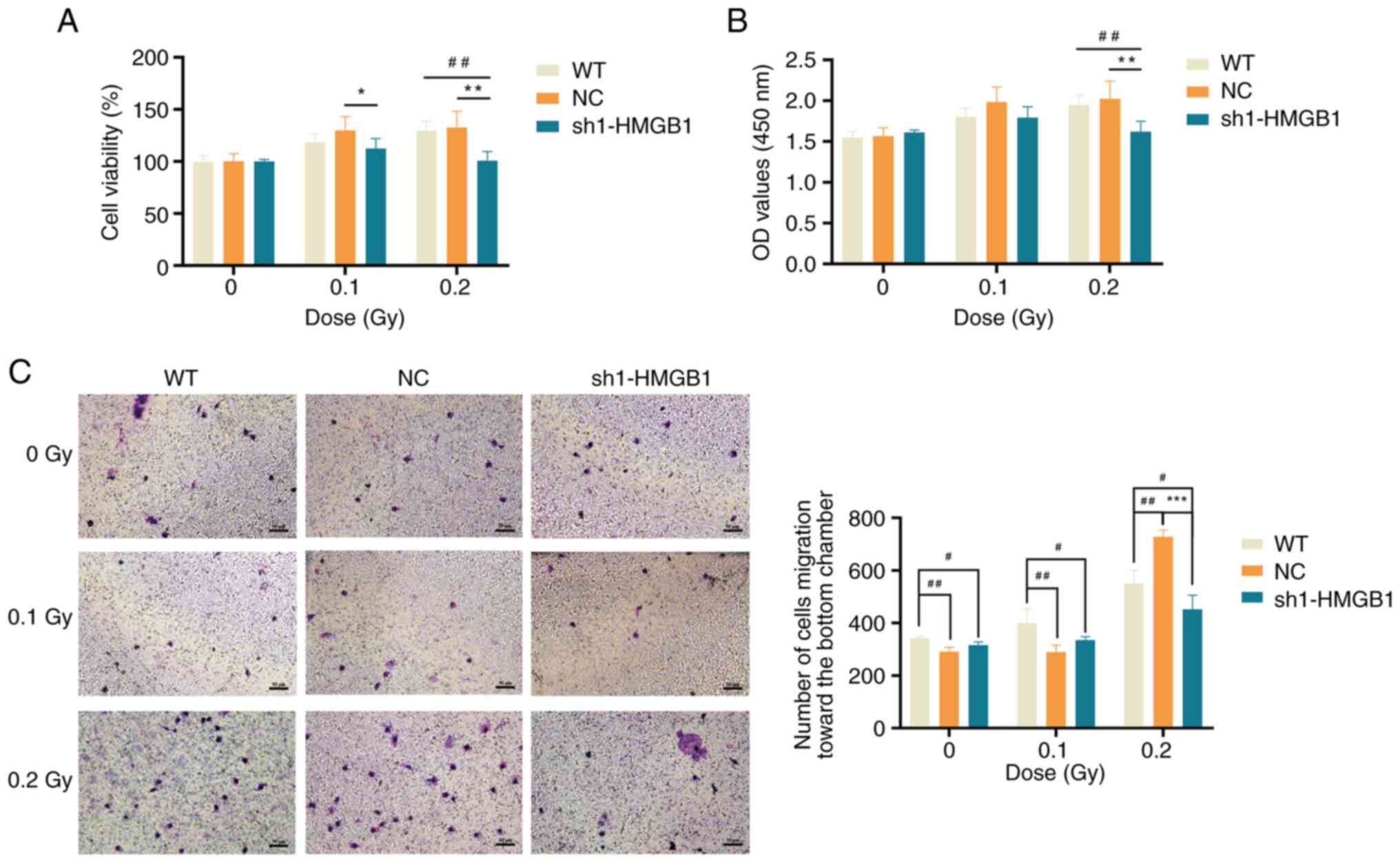
Impact of HMGB1 on cell proliferation and
migration after low-dose γ-ray irradiation
To investigate the role of HMGB1 in abnormal
proliferation and migration of LECs induced by low-dose ionizing
radiation, CCK-8 and Transwell migration assays were conducted to
assess the proliferation and migration abilities of HMGB1-knockdown
cells at 48 h after 0.1 and 0.2 Gy irradiation. The results of
CCK-8 assay indicated that, without radiation, there was no
significant difference in OD values between the knockdown group and
the NC or WT groups (P>0.05) (Fig. 10A and B), suggesting that cell
proliferation was not significantly influenced by HMGB1 knockdown.
However, at 48 h after 0.1 Gy irradiation, the cell viability of
the knockdown group was significantly diminished compared with the
NC group (P<0.05) and also demonstrated a reduction compared
with the WT group, with lower OD values than both NC and WT groups
(Fig. 10A and B). Similarly, at
48 h after 0.2 Gy irradiation, both cell viability and OD values of
the knockdown group were significantly lower than those of the NC
and WT groups (P<0.01) (Fig. 10A
and B). These findings indicated that the proliferative ability
of the knockdown group is weakened compared with the WT and NC
groups after the same low-dose γ-ray exposure.
The results of the Transwell migration assays showed
that at 48 h whether the cells were unirradiated or exposed to 0.1
Gy irradiation, the WT group exhibited a significantly stronger
cell migration ability compared with both the NC group and the
knockdown group (P<0.05 or P<0.01) (Fig. 10C). This suggested that HMGB1
knockdown diminishes cell migration ability, although it is also
possible that the lentivirus itself exerts an influence on the
migration of SRA01/04 cells. At 48 h after 0.2 Gy irradiation, the
cell migration ability of the knockdown group was significantly
weaker than the NC group and the WT group (P<0.05 or P<0.001)
(Fig. 10C). These findings
indicated that the migration ability of the knockdown group is
weakened compared with the WT groups after the same low-dose γ-ray
exposure.
Discussion
The induction of cataracts by ionizing radiation is
a complex process likely associated with multiple factors such as
DNA damage, oxidative stress and telomere abnormalities (22). Although significant strides have
been made in research, the mechanism of opacification in the lens
posterior capsule due to low-dose ionizing radiation remains far
from fully elucidated.
The present study investigated the potential
mechanism by which low-dose ionizing radiation induces abnormal
proliferation and migration of LECs, leading to lens opacity. It
was initially identified that low-dose ionizing radiation enhanced
HMGB1 expression in SRA01/04 cells. Furthermore, the impact of
HMGB1 expression on the proliferation and migration of LECs
following exposure to low-dose ionizing radiation may be associated
with the activation of the Wnt/β-catenin signaling pathway.
It has been previously reported that low-dose
ionizing radiation can promote abnormal proliferation and migration
of HLE-B3 cells. The HLE-B3 cell line is an immortalized human lens
epithelial cell line that maintains proliferation ability, stable
epithelial morphology in long-term culture and stable
differentiation ability (23).
However, a single cell line may not fully replicate the
physiological and pathological properties of normal LECs, as these
cells may exhibit genetic and phenotypic variations compared with
the actual cells. Genetic background plays an important role in
low-dose ionizing radiation-induced lens opacity. For instance, Gao
et al (24) found in a
case-control study of individuals from the Yangjiang
high-background radiation area that those carrying the allele of
ATM rs189037 and the C allele of TP53 rs1042522 have an increased
risk of radiation-induced lens opacity. To improve the
comprehensiveness of the study, another common human lens
epithelial cell line, SRA01/04, was selected. During continuous
culture, SRA01/04 cells maintain their characteristics as
epithelial cells and do not differentiate into lens fiber cells.
The cell proliferation experiment showed that both cell viability
and the number of live cells increased after irradiating SRA01/04
cells with 0.05-0.5 Gy γ-rays at 8-72 h. Cell viability remained
elevated at 7 days after low-dose irradiation, indicating a lack of
significant dose-response relationship. This further supports the
idea that low-dose ionizing radiation can promote proliferation in
LECs. However, exposure to a higher dose of 2 Gy resulted in a
temporary increase in cell proliferative capacity at 24 h
post-irradiation. At 72 h and beyond after 2 Gy irradiation, the
cell proliferative capacity diminished or showed no significant
difference compared with the unirradiated group. In a study by Chen
et al (25) on the
effects of neutron radiation on rat lenses, high-dose ionizing
radiation significantly induced apoptosis in LECs, while the
low-dose radiation group exhibited less apoptosis. The heightened
sensitivity of lens cells to radiation is not necessarily linked to
ionizing radiation-induced cell death.
Additionally, results from the migration assay
revealed an increase in the number of cells migrating to the bottom
of the Transwell chamber at different time points after low-dose
ionizing radiation. This was accompanied by an expansion of the gap
closure area, indicating that low-dose ionizing radiation enhances
the migratory ability of SRA01/04 cells. A colony formation assay
demonstrated that low-dose ionizing radiation significantly
promoted the proliferation of migratory SRA01/04 cells. It was
observed that SRA01/04 cells continued to grow well and maintained
their proliferation ability after migrating from serum-free medium
to a medium containing 10% FBS following low-dose radiation
exposure. The irradiated group exhibited a significantly higher
number of colony-forming units compared with the non-irradiated
group. Low-dose ionizing radiation also markedly enhanced the
proliferation of migratory cells. Meanwhile, the effects of 0.05,
0.1, 0.2 and 2 Gy on the proliferation and density of mouse lens
cells over an extended period were assessed, discovering scattered
nucleated cells in the posterior capsule of the lens after
irradiation. Nucleated cells were also detected in the posterior
lens capsule of unirradiated mice, potentially related to a
heightened risk of spontaneous cataract development in middle-aged
mice. It was hypothesized that extended exposure to low-dose
radiation may lead to changes in lens shape and visual impairment
over time, which necessitates confirmation through population
epidemiological studies.
Precise spatiotemporal regulation of canonical Wnt
signaling is crucial for eye development. In a lens injury model
simulating cataract surgery, researchers detected Wnt signaling
activation in residual LECs within 12 h post-surgery, indicating an
upregulation during the development of posterior capsular
opacification (26). Numerous
studies have implicated the Wnt/β-catenin signaling pathway in the
proliferation, migration and EMT of HLE-B3 cells (8,27). It was found that the
Wnt/β-catenin signaling pathway also plays an important role in the
proliferation and migration of SRA01/04 cells induced by low-dose
ionizing radiation. The protein expressions of β-catenin, cyclin D1
and c-Myc in SRA01/04 cells were increased to varying degrees after
different doses of irradiation at various times. At certain time
points, the expression of these three proteins exhibited a
double-phase reaction; that is, the expression level increased with
the dose after low-dose irradiation, usually peaking at 0.1 Gy, and
could increase again after higher doses. Similar observations were
reported by Chauhan et al (28). Whole-genome sequencing was
performed on HLE cells 20 h post-exposure to X-ray irradiation at
doses of 0, 0.01, 0.05, 0.25, 0.5, 2 and 5 Gy. Some genes
demonstrated a nonlinear biphasic response in terms of fold change
at low doses (<0.5 Gy), reaching a peak at 0.25 Gy. At doses
greater than 0.5 Gy, the fold change of these genes gradually
increased. Low-dose ionizing radiation significantly reduced the
expression of β-catenin protein on the cell membrane and increased
the nuclear translocation of β-catenin, suggesting that the
proliferation and migration of SRA01/04 cells promoted by low-dose
ionizing radiation were related to the activation of the
Wnt/β-catenin signaling pathway. The molecular mechanism by which
low-dose ionizing radiation activates the Wnt/β-catenin signaling
pathway inducing lens opacity will be explored.
HMGB1, one of the most abundant non-histone nuclear
proteins, is normally located in the nucleus and plays a role in
regulating gene transcription, DNA damage repair and chromosome
structure stability (29). It
can also be released passively by dead or damaged cells or actively
secreted by activated immune cells into the extracellular
environment, triggering an inflammatory response (30). Increased expression and secretion
of HMGB1 are associated with tumor proliferation, invasion and
migration in various cancers, and HMGB1 can serve as a potential
biomarker to identify tumors (31). In the present study, HMGB1
expression in SRA01/04 cells was inhibited using lentiviral
infection technology. Following 0.2 Gy γ-ray irradiation, total
β-catenin expression decreased in the knockdown cells compared with
WT cells, while phosphorylated β-catenin expression increased.
Immunofluorescence analysis further revealed that after low-dose
γ-ray irradiation, β-catenin no longer translocated to the nucleus
in HMGB1-knockdown cells. Genetic ablation of HMGB1 abolished
radiation-induced β-catenin nuclear translocation, suggesting that
HMGB1 may be involved in regulating the Wnt/β-catenin signaling
pathway activated by low-dose ionizing radiation. Previous studies
also have reported that the Wnt/β-catenin signaling pathway is
regulated by HMGB1. Wang et al (32) found that miR-665 deactivates the
Wnt/β-catenin signaling pathway in retinoblastoma by directly
targeting HMGB1, thereby inhibiting tumor development. Zhou et
al (33) showed that
administering exogenous HMGB1 to rats with myocardial infarction
improved cardiac function via Wnt/β-catenin signaling pathway
activation. In the cell proliferation and migration experiments of
the present study, HMGB1-knockdown cells subjected to 0.1 or 0.2 Gy
γ-ray irradiation exhibited significantly reduced proliferation and
migration capabilities compared with the WT group irradiated with
the same dose, resulting in 77% reduction in proliferation rate and
82% suppression of migratory activity. In addition, the
proliferation and migration abilities of knockdown cells after
low-dose irradiation were still enhanced compared with those of
unirradiated knockdown cells, indicating that low-dose ionizing
radiation can promote the proliferation and migration of HMGB1
knockdown cells, but the promoting effect was weaker than that of
WT cells. Based on in vitro research results, future study
by the authors will use lens-specific HMGB1-conditional knockout
mice to clarify the role of the HMGB1/Wnt-β-catenin signaling axis
in mediating low-dose ionizing radiation-induced ocular lens
opacity.
In summary, the preliminary findings of the present
study indicated that low-dose γ-ray irradiation upregulates HMGB1
expression in SRA01/04 cells, which may promote cell proliferation
and migration by activating the Wnt/β-catenin signaling pathway.
The activation of the Wnt/β-catenin signaling pathway appears to be
partially dependent on HMGB1 expression. Future investigations are
needed to further explore the interactions and molecular mechanisms
between the two in regulating the functional abnormalities of LECs
induced by low-dose ionizing radiation. The present study unravels
a novel molecular target for lens opacity induced by low-dose
ionizing radiation, aiding in early intervention and treatment of
radiation-induced cataracts.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PW and MT conceived and designed the study. PW and
CP conducted experimental research and acquired data. PW, CP and LF
performed data analysis, interpretation and visualization. DY, YH
and YL prepared the experimental animal model and examined tissue
staining. All authors contributed to drafting the manuscript or
revising the manuscript. All authors read and approved the final
version of the manuscript and confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present study was approved [approval no.
(2022)005] by the Experimental Animal Welfare and Ethics Committee
of the National Institute for Radiological Protection, Chinese
Center for Disease Control and Prevention (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Neriishi K, Nakashima E, Minamoto A,
Fujiwara S, Akahoshi M, Mishima HK, Kitaoka T and Shore RE:
Postoperative cataract cases among atomic bomb survivors: Radiation
dose response and threshold. Radiat Res. 168:404–408. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chodick G, Bekiroglu N, Hauptmann M,
Alexander BH, Freedman DM, Doody MM, Cheung LC, Simon SL, Weinstock
RM, Bouville A and Sigurdson AJ: Risk of cataract after exposure to
low doses of ionizing radiation: A 20-year prospective cohort study
among US radiologic technologists. Am J Epidemiol. 168:620–631.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azizova TV, Hamada N, Grigoryeva ES and
Bragin EV: Risk of various types of cataracts in a cohort of Mayak
workers following chronic occupational exposure to ionizing
radiation. Eur J Epidemiol. 33:1193–1204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmadi M, Barnard S, Ainsbury E and Kadhim
M: Early responses to low-dose ionizing radiation in cellular lens
epithelial models. Radiat Res. 197:78–91. 2022.
|
|
5
|
Barnard S, Uwineza A, Kalligeraki A,
McCarron R, Kruse F, Ainsbury EA and Quinlan RA: Lens epithelial
cell proliferation in response to ionizing radiation. Radiat Res.
197:92–99. 2022.
|
|
6
|
Siddam AD, Gautier-Courteille C,
Perez-Campos L, Anand D, Kakrana A, Dang CA, Legagneux V, Méreau A,
Viet J, Gross JM, et al: The RNA-binding protein Celf1
post-transcriptionally regulates p27Kip1 and Dnase2b to control
fiber cell nuclear degradation in lens development. PLoS Genet.
14:e10072782018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar B and Reilly MA: The development,
growth, and regeneration of the crystalline lens: A review. Curr
Eye Res. 45:313–326. 2020. View Article : Google Scholar
|
|
8
|
Wang P, Li YW, Lu X, Liu Y, Tian XL, Gao
L, Liu QJ, Fan L and Tian M: Low-dose ionizing radiation: Effects
on the proliferation and migration of lens epithelial cells via
activation of the Wnt/β-catenin pathway. Mutat Res Genet Toxicol
Environ Mutagen. 888:5036372023. View Article : Google Scholar
|
|
9
|
Vigneux G, Pirkkanen J, Laframboise T,
Prescott H, Tharmalingam S and Thome C: Radiation-induced
alterations in proliferation, migration, and adhesion in lens
epithelial cells and implications for cataract development.
Bioengineering (Basel). 9:292022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Terrell AM, Anand D and Lachke SA:
Molecular characterization of human lens epithelial cell lines
HLE-B3 and SRA01/04 and their utility to model lens biology. Invest
Ophthalmol Vis Sci. 56:40112015.
|
|
11
|
Wang P, Fan L, Lu X, Gao L, Liu Q and Tian
M: Identification of differential mRNA expression profiles in lens
epithelial cells induced by low-dose ionizing radiation. Radiat
Prot. 43:175–185. 2023.In Chinese.
|
|
12
|
Li PF, Borgia F, Custurone P, Vaccaro M,
Pioggia G and Gangemi S: Role of HMGB1 in cutaneous melanoma: State
of the art. Int J Mol Sci. 23:39272022.
|
|
13
|
Shu Z, Miao X, Tang T, Zhan P, Zeng L and
Jiang Y: The GSK-3β/β-catenin signaling pathway is involved in
HMGB1-induced chondrocyte apoptosis and cartilage matrix
degradation. Int J Mol Med. 45:769–778. 2020.PubMed/NCBI
|
|
14
|
Wang XH, Zhang SY, Shi M and Xu XP: HMGB1
promotes the proliferation and metastasis of lung cancer by
activating the Wnt/β-catenin pathway. Technol Cancer Res Treat.
19:15330338209480542020. View Article : Google Scholar
|
|
15
|
Chen YC, Statt S, Wu R, Chang HT, Liao JW,
Wang CN, Shyu WC and Lee CC: High mobility group box 1-induced
epithelial mesenchymal transition in human airway epithelial cells.
Sci Rep. 6:188152016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weatherbee B, Barton JR, Siddam AD, Anand
D and Lachke SA: Molecular characterization of the human lens
epithelium-derived cell line SRA01/04. Exp Eye Res. 188:1077872019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin M, Gao D, Wang R, Sik A and Liu K:
Possible involvement of TGF-β-SMAD-mediated epithelial-mesenchymal
transition in pro-metastatic property of PAX6. Oncol Rep.
44:555–564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oharazawa H, Ibaraki N, Lin LR and Reddy
VN: The effects of extracellular matrix on cell attachment,
proliferation and migration in a human lens epithelial cell line.
Exp Eye Res. 69:603–610. 1999. View Article : Google Scholar
|
|
19
|
Markiewicz E, Barnard S, Haines J, Coster
M, van Geel O, Wu W, Richards S, Ainsbury E, Rothkamm K, Bouffler S
and Quinlan RA: Nonlinear ionizing radiation-induced changes in eye
lens cell proliferation, cyclin D1 expression and lens shape. Open
Biol. 5:1500112015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim W, Kim M and Jho EH: Wnt/β-catenin
signalling: From plasma membrane to nucleus. Biochem J. 450:9–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shah K and Kazi JU:
Phosphorylation-dependent regulation of WNT/beta-catenin signaling.
Front Oncol. 12:8587822022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamada N: Ionizing radiation sensitivity
of the ocular lens and its dose rate dependence. Int J Radiat Biol.
93:1024–1034. 2017. View Article : Google Scholar
|
|
23
|
Andley UP, Rhim JS, Chylack LJ Jr and
Fleming TP: Propagation and immortalization of human lens
epithelial cells in culture. Invest Ophthalmol Vis Sci.
35:3094–3102. 1994.PubMed/NCBI
|
|
24
|
Gao Y, Su YP, Li XL, Lei SJ, Chen HF, Cui
SY, Zhang SF, Zou JM, Liu QJ and Sun QF: ATM and TP53 polymorphisms
modified susceptibility to radiation-induced lens opacity in
natural high background radiation area, China. Int J Radiat Biol.
98:1235–1242. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Feng J, Liu J, Zhou H, Luo H, Xue
C and Gao W: Effects of neutron radiation on Nrf2-regulated
antioxidant defense systems in rat lens. Exp Ther Med. 21:3342021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Mahesh P, Wang Y, Novo SG, Shihan
MH, Hayward-Piatkovskyi B and Duncan MK: Spatiotemporal dynamics of
canonical Wnt signaling during embryonic eye development and
posterior capsular opacification (PCO). Exp Eye Res. 175:148–158.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao XL, Song H, Chen Z and Tang X: Wnt3a
promotes epithelial-mesenchymal transition, migration, and
proliferation of lens epithelial cells. Mol Vis. 18:1983–1990.
2012.PubMed/NCBI
|
|
28
|
Chauhan V, Rowan-Carroll A, Gagné R, Kuo
B, Williams A and Yauk CL: The use of in vitro transcriptional data
to identify thresholds of effects in a human lens epithelial
cell-line exposed to ionizing radiation. Int J Radiat Biol.
95:156–169. 2019. View Article : Google Scholar
|
|
29
|
Kianian F, Kadkhodaee M, Sadeghipour HR,
Karimian SM and Seifi B: An overview of high-mobility group box 1,
a potent pro-inflammatory cytokine in asthma. J Basic Clin Physiol
Pharmacol. 31:2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ulloa L and Messmer D: High-mobility group
box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev.
17:189–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Jiang Z, Yan J and Ying S: HMGB1
as a potential biomarker and therapeutic target for malignant
mesothelioma. Dis Markers. 2019:41831572019.PubMed/NCBI
|
|
32
|
Wang S, Du S, Lv Y, Zhang F and Wang W:
MicroRNA-665 inhibits the oncogenicity of retinoblastoma by
directly targeting high-mobility group box 1 and inactivating the
Wnt/β-catenin pathway. Cancer Manag Res. 11:3111–3123. 2019.
View Article : Google Scholar :
|
|
33
|
Zhou X, Hu X, Xie J, Xu C, Xu W and Jiang
H: Exogenous high-mobility group box 1 protein injection improves
cardiac function after myocardial infarction: Involvement of Wnt
signaling activation. J Biomed Biotechnol. 2012:7438792012.
View Article : Google Scholar : PubMed/NCBI
|