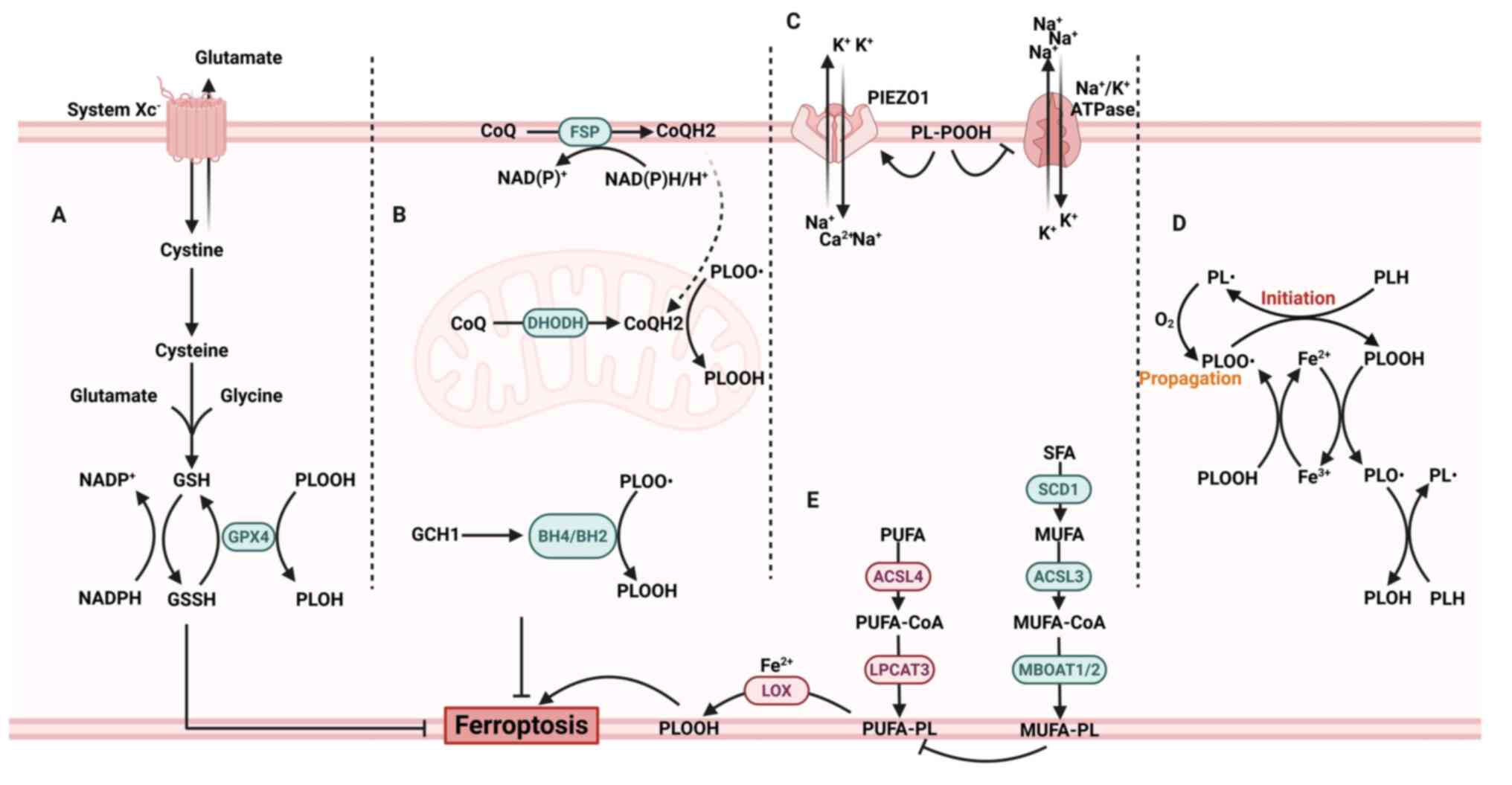
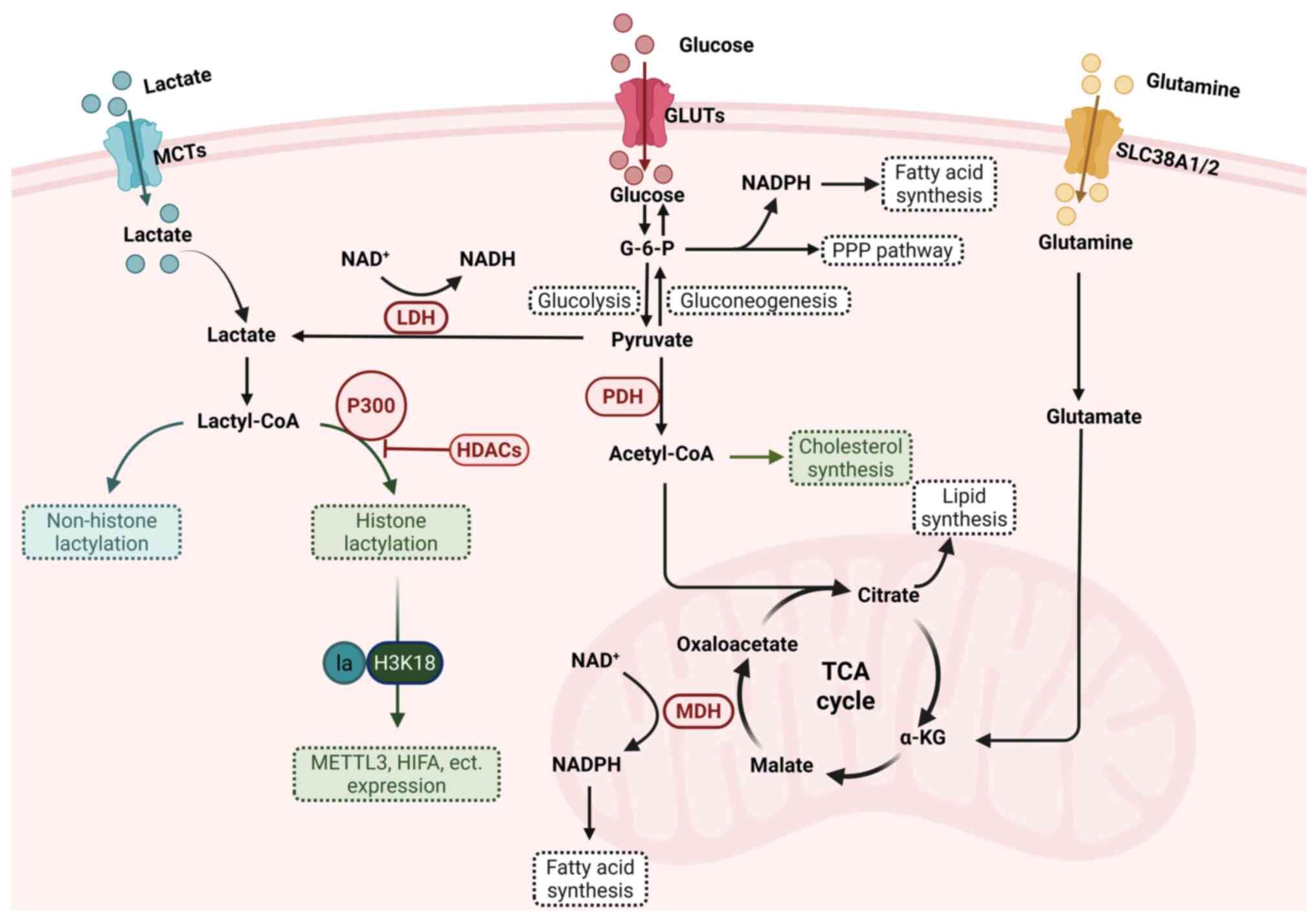
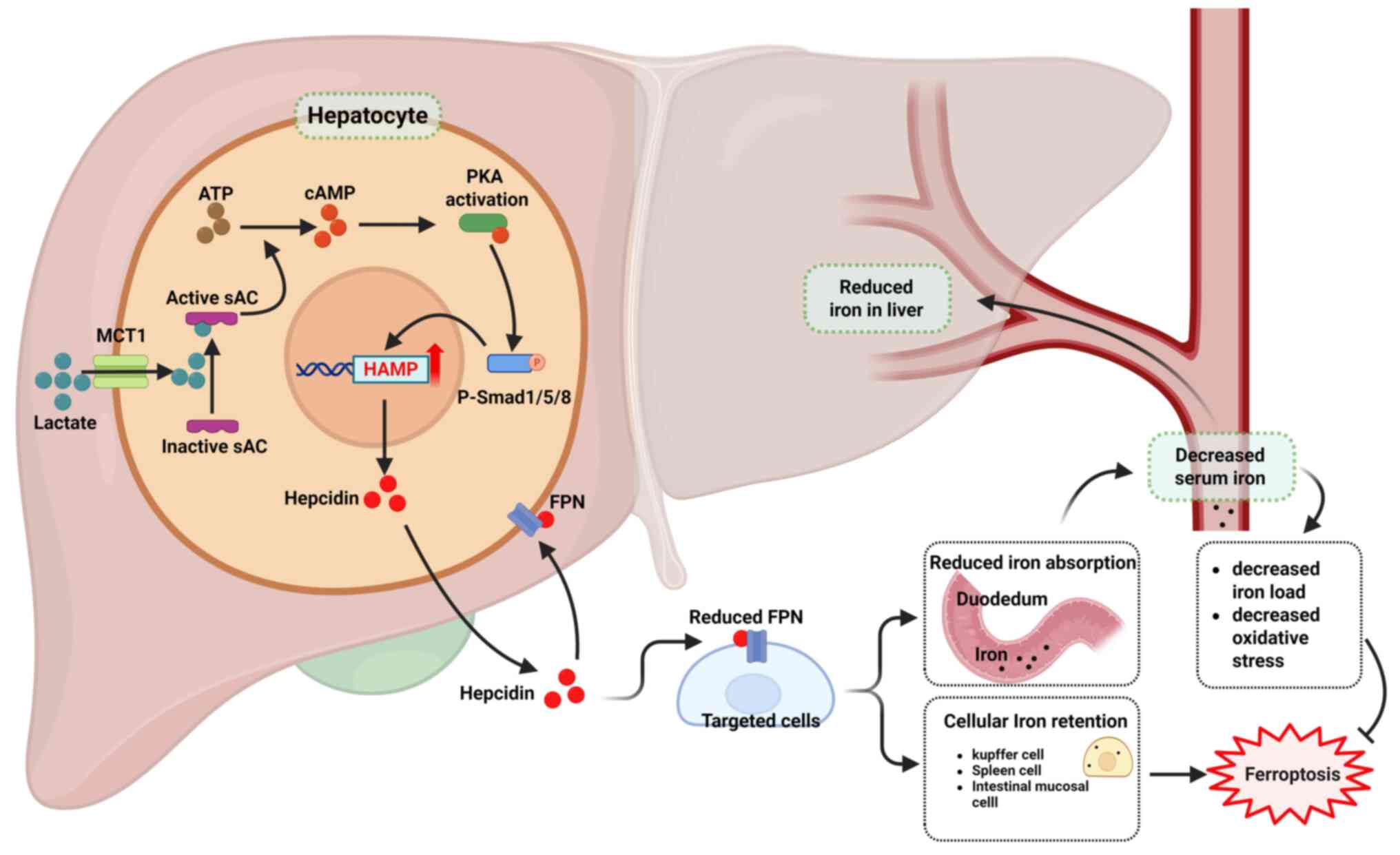
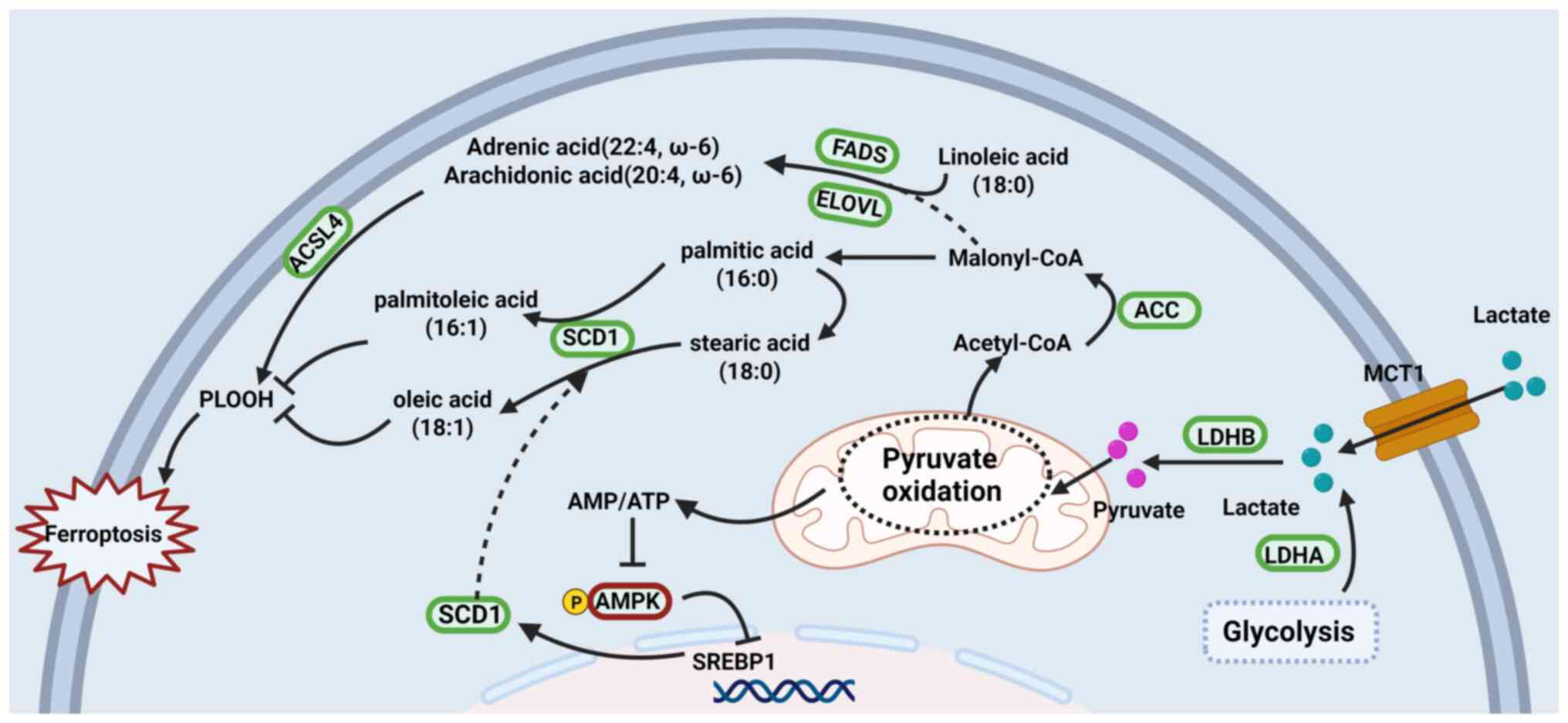
|
1
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dixon SJ and Olzmann JA: The cell biology
of ferroptosis. Nat Rev Mol Cell Biol. 25:424–442. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mishima E, Nakamura T, Doll S, Proneth B,
Fedorova M, Pratt DA, Friedmann Angeli JP, Dixon SJ, Wahida A and
Conrad M: Recommendations for robust and reproducible research on
ferroptosis. Nat Rev Mol Cell Biol. 26:615–630. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Q, Meng Y, Li D, Yao L, Le J, Liu Y,
Sun Y, Zeng F, Chen X and Deng G: Ferroptosis in cancer: From
molecular mechanisms to therapeutic strategies. Signal Transduct
Target Ther. 9:552024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brooks GA: The science and translation of
lactate shuttle theory. Cell Metab. 27:757–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Felmlee MA, Jones RS, Rodriguez-Cruz V,
Follman KE and Morris ME: Monocarboxylate transporters (SLC16):
Function, regulation, and role in health and disease. Pharmacol
Rev. 72:466–485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown TP and Ganapathy V: Lactate/GPR81
signaling and proton motive force in cancer: Role in angiogenesis,
immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther.
206:1074512020. View Article : Google Scholar
|
|
9
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hua S, Jeong HN, Dimapasoc LM, Kang I, Han
C, Choi JS, Lebrilla CB and An HJ: Isomer-specific LC/MS and
LC/MS/MS profiling of the mouse serum N-glycome revealing a number
of novel sialylated N-glycans. Anal Chem. 85:4636–4643. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Sun L, Gao P and Hu H: Lactylation
in cancer: Current understanding and challenges. Cancer Cell.
42:1803–1807. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu YQ, Yang Q and He GW:
Post-translational acylation of proteins in cardiac hypertrophy.
Nat Rev Cardiol. 22:944–960. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Conrad M and Pratt DA: The chemical basis
of ferroptosis. Nat Chem Biol. 15:1137–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Badgley MA, Kremer DM, Maurer HC,
DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J,
Firl CEM, et al: Cysteine depletion induces pancreatic tumor
ferroptosis in mice. Science. 368:85–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang D, Liang W, Huo D, Wang H, Wang Y,
Cong C, Zhang C, Yan S, Gao M, Su X, et al: SPY1 inhibits neuronal
ferroptosis in amyotrophic lateral sclerosis by reducing lipid
peroxidation through regulation of GCH1 and TFR1. Cell Death
Differ. 30:369–382. 2023. View Article : Google Scholar :
|
|
21
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kappler A, Bryce C, Mansor M, Lueder U,
Byrne JM and Swanner ED: An evolving view on biogeochemical cycling
of iron. Nat Rev Microbiol. 19:360–374. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cañeque T, Baron L, Müller S, Carmona A,
Colombeau L, Versini A, Solier S, Gaillet C, Sindikubwabo F,
Sampaio JL, et al: Activation of lysosomal iron triggers
ferroptosis in cancer. Nature. 642:492–500. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Magtanong L, Ko PJ and Dixon SJ: Emerging
roles for lipids in non-apoptotic cell death. Cell Death Differ.
23:1099–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwörer S, Vardhana SA and Thompson CB:
Cancer metabolism drives a stromal regenerative response. Cell
Metab. 29:576–591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Won W, Bhalla M, Lee JH and Lee CJ:
Astrocytes as key regulators of neural signaling in health and
disease. Annu Rev Neurosci. 48:251–276. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Certo M, Tsai CH, Pucino V, Ho PC and
Mauro C: Lactate modulation of immune responses in inflammatory
versus tumour microenvironments. Nat Rev Immunol. 21:151–161. 2021.
View Article : Google Scholar
|
|
28
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y and Patti GJ: The Warburg effect: A
signature of mitochondrial overload. Trends Cell Biol.
33:1014–1020. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Zhan L, Yanxiang Guo J, et al:
Glucose feeds the TCA cycle via circulating lactate. Nature.
551:115–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Faubert B, Li KY, Cai L, Hensley CT, Kim
J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al:
Lactate metabolism in human lung tumors. Cell. 171:358–371.e9.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Huang Y, Yang J, Zhou FQ, Zhao L
and Zhou H: Pyruvate is a prospective alkalizer to correct hypoxic
lactic acidosis. Mil Med Res. 5:132018.PubMed/NCBI
|
|
34
|
DeBerardinis RJ, Mancuso A, Daikhin E,
Nissim I, Yudkoff M, Wehrli S and Thompson CB: Beyond aerobic
glycolysis: Transformed cells can engage in glutamine metabolism
that exceeds the requirement for protein and nucleotide synthesis.
Proc Natl Acad Sci USA. 104:19345–19350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ivashkiv LB: The hypoxia-lactate axis
tempers inflammation. Nat Rev Immunol. 20:85–86. 2020. View Article : Google Scholar :
|
|
37
|
Taylor CT and Scholz CC: The effect of HIF
on metabolism and immunity. Nat Rev Nephrol. 18:573–587. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang GL and Semenza GL: General
involvement of hypoxia-inducible factor 1 in transcriptional
response to hypoxia. Proc Natl Acad Sci USA. 90:4304–4308. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Silagi ES, Schipani E, Shapiro IM and
Risbud MV: The role of HIF proteins in maintaining the metabolic
health of the intervertebral disc. Nat Rev Rheumatol. 17:426–439.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iyer NV, Kotch LE, Agani F, Leung SW,
Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY
and Semenza GL: Cellular and developmental control of O2
homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev.
12:149–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seagroves TN, Ryan HE, Lu H, Wouters BG,
Knapp M, Thibault P, Laderoute K and Johnson RS: Transcription
factor HIF-1 is a necessary mediator of the pasteur effect in
mammalian cells. Mol Cell Biol. 21:3436–3444. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
downregulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Claps G, Faouzi S, Quidville V, Chehade F,
Shen S, Vagner S and Robert C: The multiple roles of LDH in cancer.
Nat Rev Clin Oncol. 19:749–762. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ždralević M, Brand A, Di Ianni L, Dettmer
K, Reinders J, Singer K, Peter K, Schnell A, Bruss C, Decking SM,
et al: Double genetic disruption of lactate dehydrogenases A and B
is required to ablate the 'Warburg effect' restricting tumor growth
to oxidative metabolism. J Biol Chem. 293:15947–15961. 2018.
View Article : Google Scholar
|
|
46
|
Kim EY, Chung TW, Han CW, Park SY, Park
KH, Jang SB and Ha KT: A novel lactate dehydrogenase inhibitor,
1-(Phenylseleno)-4-(Trifluoromethyl) benzene, suppresses tumor
growth through apoptotic cell death. Sci Rep. 9:39692019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park JS, Saeed K, Jo MH, Kim MW, Lee HJ,
Park CB, Lee G and Kim MO: LDHB deficiency promotes mitochondrial
dysfunction mediated oxidative stress and neurodegeneration in
adult mouse brain. Antioxidants (Basel). 11:2612022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin M, Li S, Liu M, Zhu W, Chen Y, Qiu W,
Li Q, Li Y, Chen J, Zhou Y, et al: GUCY1A1-LDHA axis suppresses
ferroptosis in cardiac ischemia-reperfusion injury. Circ Res.
137:986–1005. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao L, Deng H, Zhang J, Zamboni N, Yang
H, Gao Y, Yang Z, Xu D, Zhong H, van Geest G, et al: Lactate
dehydrogenase B noncanonically promotes ferroptosis defense in
KRAS-driven lung cancer. Cell Death Differ. 32:632–645. 2025.
View Article : Google Scholar :
|
|
50
|
Doherty JR, Yang C, Scott KE, Cameron MD,
Fallahi M, Li W, Hall MA, Amelio AL, Mishra JK, Li F, et al:
Blocking lactate export by inhibiting the Myc target MCT1 disables
glycolysis and glutathione synthesis. Cancer Res. 74:908–920. 2014.
View Article : Google Scholar :
|
|
51
|
Wang N, Wang W, Wang X, Mang G, Chen J,
Yan X, Tong Z, Yang Q, Wang M, Chen L, et al: Histone lactylation
boosts reparative gene activation post-myocardial infarction. Circ
Res. 131:893–908. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ahmed K, Tunaru S, Tang C, Müller M, Gille
A, Sassmann A, Hanson J and Offermanns S: An autocrine lactate loop
mediates insulin-dependent inhibition of lipolysis through GPR81.
Cell Metab. 11:311–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roland CL, Arumugam T, Deng D, Liu SH,
Philip B, Gomez S, Burns WR, Ramachandran V, Wang H,
Cruz-Monserrate Z and Logsdon CD: Cell surface lactate receptor
GPR81 is crucial for cancer cell survival. Cancer Res.
74:5301–5310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee YJ, Shin KJ, Park SA, Park KS, Park S,
Heo K, Seo YK, Noh DY, Ryu SH and Suh PG: G-protein-coupled
receptor 81 promotes a malignant phenotype in breast cancer through
angiogenic factor secretion. Oncotarget. 7:70898–70911. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu
B, Yang M, Cao W, Wang L and Wu Z: Tumor cell-derived lactate
induces TAZ-dependent upregulation of PD-L1 through GPR81 in human
lung cancer cells. Oncogene. 36:5829–5839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Luo M, Zhu J, Ren J, Tong Y, Wang L, Ma S
and Wang J: Lactate increases tumor malignancy by promoting tumor
small extracellular vesicles production via the
GPR81-cAMP-PKA-HIF-1α axis. Front Oncol. 12:10365432022. View Article : Google Scholar
|
|
57
|
Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z,
Cai K, Zhao Y and Luo Z: HCAR1/MCT1 regulates tumor ferroptosis
through the lactate-mediated AMPK-SCD1 activity and its therapeutic
implications. Cell Rep. 33:1084872020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang P, Li H, Sun M, Guo X, Liao Y, Hu M,
Ye P and Liu R: Zinc deficiency drives ferroptosis resistance by
lactate production in esophageal squamous cell carcinoma. Free
Radic Biol Med. 213:512–522. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Benjamin D, Robay D, Hindupur SK, Pohlmann
J, Colombi M, El-Shemerly MY, Maira SM, Moroni C, Lane HA and Hall
MN: Dual inhibition of the lactate transporters MCT1 and MCT4 Is
synthetic lethal with metformin due to NAD+ depletion in cancer
cells. Cell Rep. 25:3047–3058.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang S, Liu W, Ganz T and Liu S:
Exploring the relationship between hyperlactatemia and anemia.
Trends Endocrinol Metab. 35:300–307. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sim M, Garvican-Lewis LA, Cox GR, Govus A,
McKay AKA, Stellingwerff T and Peeling P: Iron considerations for
the athlete: A narrative review. Eur J Appl Physiol. 119:1463–1478.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nemeth E, Tuttle MS, Powelson J, Vaughn
MB, Donovan A, Ward DM, Ganz T and Kaplan J: Hepcidin regulates
cellular iron efflux by binding to ferroportin and inducing its
internalization. Science. 306:2090–2093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu W, Zhang S, Li Q, Wu Y, Jia X, Feng W,
Li Z, Shi Y, Hou Q, Ma J, et al: Lactate modulates iron metabolism
by binding soluble adenylyl cyclase. Cell Metab. 35:1597–1612.e6.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu W, Wu Y, Wei H, Ma J, Feng W, Yang Q,
Zhang S, Ganz T and Liu S: Lactate administration improves
laboratory parameters in murine models of iron overload. Blood.
143:1045–1049. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang J, Wang Y, Fan M, Guan Y, Zhang W,
Huang F, Zhang Z, Li X, Yuan B, Liu W, et al: Reactive oxygen
species regulation by NCF1 governs ferroptosis susceptibility of
Kupffer cells to MASH. Cell Metab. 36:1745–1763.e6. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Davaanyam D, Lee H, Seol SI, Oh SA, Kim SW
and Lee JK: HMGB1 induces hepcidin upregulation in astrocytes and
causes an acute iron surge and subsequent ferroptosis in the
postischemic brain. Exp Mol Med. 55:2402–2416. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Davaanyam D, Seol SI, Oh SA, Lee H and Lee
JK: Hepatocyte activation and liver injury following cerebral
ischemia promote HMGB1-mediated hepcidin upregulation in
hepatocytes and regulation of systemic iron levels. Exp Mol Med.
56:2171–2183. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gao M, Monian P, Quadri N, Ramasamy R and
Jiang X: Glutaminolysis and transferrin regulate ferroptosis. Mol
Cell. 59:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh
HJ III, Kang R and Tang D: Autophagy promotes ferroptosis by
degradation of ferritin. Autophagy. 12:1425–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li A, Gong Z, Long Y, Li Y, Liu C, Lu X,
Li Q, He X, Lu H, Wu K, et al: Lactylation of LSD1 is an acquired
epigenetic vulnerability of BRAFi/MEKi-resistant melanoma. Dev
Cell. 60:1974–1990.e11. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang K, Guo L, Li X, Hu Y and Luo N:
Cancer-associated fibroblasts promote doxorubicin resistance in
triple-negative breast cancer through enhancing ZFP64 histone
lactylation to regulate ferroptosis. J Transl Med. 23:2472025.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang L, Wang X, Che W, Zhou S and Feng Y:
METTL3 silenced inhibited the ferroptosis development via
regulating the TFRC levels in the intracerebral hemorrhage
progression. Brain Res. 1811:1483732023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gong F, Zheng X, Xu W, Xie R, Liu W, Pei
L, Zhong M, Shi W, Qu H, Mao E, et al: H3K14la drives endothelial
dysfunction in sepsis-induced ARDS by promoting
SLC40A1/transferrin-mediated ferroptosis. MedComm. 6:e700492025.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Martinez-Outschoorn UE, Peiris-Pagés M,
Pestell RG, Sotgia F and Lisanti MP: Cancer metabolism: A
therapeutic perspective. Nat Rev Clin Oncol. 14:11–31. 2017.
View Article : Google Scholar
|
|
75
|
Zheng J and Conrad M: Ferroptosis: When
metabolism meets cell death. Physiol Rev. 105:651–706. 2025.
View Article : Google Scholar
|
|
76
|
de Kivit S, Mensink M, Kostidis S, Derks
RJE, Zaal EA, Heijink M, Verleng LJ, de Vries E, Schrama E,
Blomberg N, et al: Immune suppression by human thymus-derived
effector Tregs relies on glucose/lactate-fueled fatty acid
synthesis. Cell Rep. 43:1146812024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li C, Dong X, Du W, Shi X, Chen K, Zhang W
and Gao M: LKB1-AMPK axis negatively regulates ferroptosis by
inhibiting fatty acid synthesis. Signal Transduct Target Ther.
5:1872020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Song X, Liu J, Kuang F, Chen X, Zeh HJ
III, Kang R, Kroemer G, Xie Y and Tang D: PDK4 dictates metabolic
resistance to ferroptosis by suppressing pyruvate oxidation and
fatty acid synthesis. Cell Rep. 34:1087672021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sondermeijer BM, Battjes S, van Dijk TH,
Ackermans MT, Serlie MJ, Nieuwdorp M, Groen AK, Dallinga-Thie GM
and Stroes ES: Lactate increases hepatic secretion of
VLDL-triglycerides in humans. Atherosclerosis. 228:443–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fan Z, Ye M, Liu D, Zhou W, Zeng T, He S
and Li Y: Lactate drives the ESM1-SCD1 axis to inhibit the
antitumor CD8+ T-cell response by activating the
Wnt/β-catenin pathway in ovarian cancer cells and inducing
cisplatin resistance. Int Immunopharmacol. 137:1124612024.
View Article : Google Scholar
|
|
82
|
Wu D, Spencer CB, Ortoga L, Zhang H and
Miao C: Histone lactylation-regulated METTL3 promotes ferroptosis
via m6A-modification on ACSL4 in sepsis-associated lung injury.
Redox Biol. 74:1031942024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pope LE and Dixon SJ: Regulation of
ferroptosis by lipid metabolism. Trends Cell Biol. 33:1077–1087.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tilton WM, Seaman C, Carriero D and
Piomelli S: Regulation of glycolysis in the erythrocyte: Role of
the lactate/pyruvate and NAD/NADH ratios. J Lab Clin Med.
118:146–152. 1991.PubMed/NCBI
|
|
85
|
Quinn WJ III, Jiao J, TeSlaa T, Stadanlick
J, Wang Z, Wang L, Akimova T, Angelin A, Schäfer PM, Cully MD, et
al: Lactate limits T cell proliferation via the NAD(H) redox state.
Cell Rep. 33:1085002020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Luengo A, Li Z, Gui DY, Sullivan LB,
Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, et al:
Increased demand for NAD+ relative to ATP drives aerobic
glycolysis. Mol Cell. 81:691–707.e6. 2021. View Article : Google Scholar
|
|
87
|
Corkey BE and Deeney JT: The redox
communication network as a regulator of metabolism. Front Physiol.
11:5677962020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yang S and Lian G: ROS and diseases: Role
in metabolism and energy supply. Mol Cell Biochem. 467:1–12. 2020.
View Article : Google Scholar :
|
|
89
|
Young A, Oldford C and Mailloux RJ:
Lactate dehydrogenase supports lactate oxidation in mitochondria
isolated from different mouse tissues. Redox Biol. 28:1013392020.
View Article : Google Scholar
|
|
90
|
Bauzá-Thorbrügge M, Peris E, Zamani S,
Micallef P, Paul A, Bartesaghi S, Benrick A and Wernstedt Asterholm
I: NRF2 is essential for adaptative browning of white adipocytes.
Redox Biol. 68:1029512023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jia L, Liao M, Mou A, Zheng Q, Yang W, Yu
Z, Cui Y, Xia X, Qin Y, Chen M and Xiao B: Rheb-regulated
mitochondrial pyruvate metabolism of Schwann cells linked to axon
stability. Dev Cell. 56:2980–2994.e6. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rydström J: Mitochondrial NADPH,
transhydrogenase and disease. Biochim Biophys Acta. 1757:721–726.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin W, Lu X, Yang H, Huang L, Huang W,
Tang Y, Liu S, Wang H and Zhang Y: Metabolic heterogeneity protects
metastatic mucosal melanomas cells from ferroptosis. Int J Mol Med.
50:1242022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ying M, You D, Zhu X, Cai L, Zeng S and Hu
X: Lactate and glutamine support NADPH generation in cancer cells
under glucose deprived conditions. Redox Biol. 46:1020652021.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang W, Guo C, Jiang K, Ying M and Hu X:
Quantification of lactate from various metabolic pathways and
quantification issues of lactate isotopologues and isotopmers. Sci
Rep. 7:84892017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang YF, Wang G, Ding L, Bai ZR, Leng Y,
Tian JW, Zhang JZ, Li YQ, Ahmad, Qin YH, et al: Lactate-upregulated
NADPH-dependent NOX4 expression via HCAR1/PI3K pathway contributes
to ROS-induced osteoarthritis chondrocyte damage. Redox Biol.
67:1028672023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Taylor JP and Tse HM: The role of NADPH
oxidases in infectious and inflammatory diseases. Redox Biol.
48:1021592021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sun F, He Y, Yang Z, Xu G, Wang R, Juan Z
and Sun X: Propofol pretreatment inhibits ferroptosis and
alleviates myocardial ischemia-reperfusion injury through the
SLC16A13-AMPK-GPX4 pathway. Biomed Pharmacother. 179:1173452024.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cheng F, Dou J, Yang Y, Sun S, Chen R,
Zhang Z, Wei H, Li J and Wu Z: Drug-induced lactate confers
ferroptosis resistance via p38-SGK1-NEDD4L-dependent upregulation
of GPX4 in NSCLC cells. Cell Death Discov. 9:1652023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tauffenberger A, Fiumelli H, Almustafa S
and Magistretti PJ: Lactate and pyruvate promote oxidative stress
resistance through hormetic ROS signaling. Cell Death Dis.
10:6532019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zou GP, Wang T, Xiao JX, Wang XY, Jiang
LP, Tou FF, Chen ZP, Qu XH and Han XJ: Lactate protects against
oxidative stress-induced retinal degeneration by activating
autophagy. Free Radic Biol Med. 194:209–219. 2023. View Article : Google Scholar
|
|
102
|
Ma M, Zhang Y, Pu K and Tang W:
Nanomaterial-enabled metabolic reprogramming strategies for
boosting antitumor immunity. Chem Soc Rev. 54:653–714. 2025.
View Article : Google Scholar
|
|
103
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Besnier E, Coquerel D, Kouadri G, Clavier
T, Favory R, Duburcq T, Lesur O, Bekri S, Richard V, Mulder P and
Tamion F: Hypertonic sodium lactate improves microcirculation,
cardiac function, and inflammation in a rat model of sepsis. Crit
Care. 24:3542020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zeng M, Niu Y, Huang J and Deng L:
Advances in neutrophil extracellular traps and ferroptosis in
sepsis-induced cardiomyopathy. Front Immunol. 16:15903132025.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang T, Chen L, Kueth G, Shao E, Wang X,
Ha T, Williams DL, Li C, Fan M and Yang K: Lactate's impact on
immune cells in sepsis: Unraveling the complex interplay. Front
Immunol. 15:14834002024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Irizarry-Caro RA, McDaniel MM, Overcast
GR, Jain VG, Troutman TD and Pasare C: TLR signaling adapter BCAP
regulates inflammatory to reparatory macrophage transition by
promoting histone lactylation. Proc Natl Acad Sci USA.
117:30628–30638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hu Y, He Z, Li Z, Wang Y, Wu N, Sun H,
Zhou Z, Hu Q and Cong X: Lactylation: The novel histone
modification influence on gene expression, protein function, and
disease. Clin Epigenetics. 16:722024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Su F, Xie K, He X, Orbegozo D, Hosokawa K,
Post EH, Donadello K, Taccone FS, Creteur J and Vincent JL: The
harmful effects of hypertonic sodium lactate administration in
hyperdynamic septic shock. Shock. 46:663–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Tang R, Xu J, Zhang B, Liu J, Liang C, Hua
J, Meng Q, Yu X and Shi S: Ferroptosis, necroptosis, and pyroptosis
in anticancer immunity. J Hematol Oncol. 13:1102020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Fang Y, Liu W, Tang Z, Ji X, Zhou Y, Song
S, Tian M, Tao C, Huang R, Zhu G, et al: Monocarboxylate
transporter 4 inhibition potentiates hepatocellular carcinoma
immunotherapy through enhancing T cell infiltration and immune
attack. Hepatology. 77:109–123. 2023. View Article : Google Scholar
|
|
112
|
Hagihara H, Shoji H, Otabi H, Toyoda A,
Katoh K, Namihira M and Miyakawa T: Protein lactylation induced by
neural excitation. Cell Rep. 37:1098202021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sun K, Shi Y, Yan C, Wang S, Han L, Li F,
Xu X, Wang Y, Sun J, Kang Z and Shi J: Glycolysis-derived lactate
induces ACSL4 expression and lactylation to activate ferroptosis
during intervertebral disc degeneration. Adv Sci (Weinh).
12:e24161492025. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang T, Huang X, Feng S and Shao H:
Lactate-dependent HIF1A transcriptional activation exacerbates
severe acute pancreatitis through the ACSL4/LPCAT3/ALOX15 pathway
induced ferroptosis. J Cell Biochem. 126:e306872025. View Article : Google Scholar
|
|
115
|
Wu Q, You L, Nepovimova E, Heger Z, Wu W,
Kuca K and Adam V: Hypoxia-inducible factors: Master regulators of
hypoxic tumor immune escape. J Hematol Oncol. 15:772022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Deng J, Li Y, Yin L, Liu S, Li Y, Liao W,
Mu L, Luo X and Qin J: Histone lactylation enhances GCLC expression
and thus promotes chemoresistance of colorectal cancer stem cells
through inhibiting ferroptosis. Cell Death Dis. 16:1932025.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Huang J, Xie H, Li J, Huang X, Cai Y, Yang
R, Yang D, Bao W, Zhou Y, Li T and Lu Q: Histone lactylation drives
liver cancer metastasis by facilitating NSF1-mediated ferroptosis
resistance after microwave ablation. Redox Biol. 81:1035532025.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yuan J, Yang M, Wu Z, Wu J, Zheng K, Wang
J, Zeng Q, Chen M, Lv T, Shi Y, et al: The lactate-primed KAT8-PCK2
axis exacerbates hepatic ferroptosis during ischemia/reperfusion
injury by reprogramming OXSM-dependent mitochondrial fatty acid
synthesis. Adv Sci (Weinh). 12:e24141412025. View Article : Google Scholar
|
|
119
|
An X, He J, Xie P, Li C, Xia M, Guo D, Bi
B, Wu G, Xu J, Yu W and Ren Z: The effect of tau K677 lactylation
on ferritinophagy and ferroptosis in Alzheimer's disease. Free
Radic Biol Med. 224:685–706. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
She H, Hu Y, Zhao G, Du Y, Wu Y, Chen W,
Li Y, Wang Y, Tan L, Zhou Y, et al: Dexmedetomidine ameliorates
myocardial ischemia-reperfusion injury by inhibiting MDH2
lactylation via regulating metabolic reprogramming. Adv Sci
(Weinh). 11:e24094992024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Niu K, Chen Z, Li M, Ma G, Deng Y, Zhang
J, Wei D, Wang J and Zhao Y: NSUN2 lactylation drives cancer cell
resistance to ferroptosis through enhancing GCLC-dependent
glutathione synthesis. Redox Biol. 79:1034792025. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yu Y, Huang X, Liang C and Zhang P:
Evodiamine impairs HIF1A histone lactylation to inhibit
Sema3A-mediated angiogenesis and PD-L1 by inducing ferroptosis in
prostate cancer. Eur J Pharmacol. 957:1760072023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xiong J, Ge X, Pan D, Zhu Y, Zhou Y, Gao
Y, Wang H, Wang X, Gu Y, Ye W, et al: Metabolic reprogramming in
astrocytes prevents neuronal death through a UCHL1/PFKFB3/H4K8la
positive feedback loop. Cell Death Differ. 32:1214–1230. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Deng H, Zhao L, Ge H, Gao Y, Fu Y, Lin Y,
Masoodi M, Losmanova T, Medová M, Ott J, et al: Ubiquinol-mediated
suppression of mitochondria-associated ferroptosis is a targetable
function of lactate dehydrogenase B in cancer. Nat Commun.
16:25972025. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Moreira JD, Hamraz M, Abolhassani M, Bigan
E, Pérès S, Paulevé L, Nogueira ML, Steyaert JM and Schwartz L: The
redox status of cancer cells supports mechanisms behind the warburg
effect. Metabolites. 6:332016. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Halestrap AP and Wilson MC: The
monocarboxylate transporter family-role and regulation. IUBMB Life.
64:109–119. 2012. View Article : Google Scholar
|
|
127
|
Singh M, Afonso J, Sharma D, Gupta R and
Kumar V, Rani R, Baltazar F and Kumar V: Targeting monocarboxylate
transporters (MCTs) in cancer: How close are we to the clinics?
Semin Cancer Biol. 90:1–14. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hosonuma M and Yoshimura K: Association
between pH regulation of the tumor microenvironment and
immunological state. Front Oncol. 13:11755632023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang X, Zhao Y, Hu Y, Fei Y, Zhao Y, Xue
C, Cai K, Li M and Luo Z: Activatable biomineralized nanoplatform
remodels the intracellular environment of multidrug-resistant
tumors for enhanced ferroptosis/apoptosis therapy. Small.
17:e21022692021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jackson VN and Halestrap AP: The kinetics,
substrate, and inhibitor specificity of the monocarboxylate
(lactate) transporter of rat liver cells determined using the
fluorescent intracellular pH indicator,
2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem.
271:861–868. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Bozzo L, Puyal J and Chatton JY: Lactate
modulates the activity of primary cortical neurons through a
receptor-mediated pathway. PLoS One. 8:e717212013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang Z, Su W, Wei X, Qu S, Zhao D, Zhou J,
Wang Y, Guan Q, Qin C, Xiang J, et al: HIF-1α drives resistance to
ferroptosis in solid tumors by promoting lactate production and
activating SLC1A1. Cell Rep. 42:1129452023. View Article : Google Scholar
|
|
133
|
Bae C, Sachs F and Gottlieb PA:
Protonation of the human PIEZO1 ion channel stabilizes
inactivation. J Biol Chem. 290:5167–5173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Jin C, Zhang DP, Lin Z, Lin YZ, Shi YF,
Dong XY, Jin MQ, Song FQ, Du ST, Feng YZ, et al: Piezo1-mediated
ferroptosis delays wound healing in aging mice by regulating the
transcriptional activity of SLC7A11 through activating
transcription factor 3. Research (Wash D C). 8:07182025.PubMed/NCBI
|
|
135
|
Koppula P, Zhang Y, Zhuang L and Gan B:
Amino acid transporter SLC7A11/xCT at the crossroads of regulating
redox homeostasis and nutrient dependency of cancer. Cancer Commun
(Lond). 38:122018.PubMed/NCBI
|
|
136
|
Hu K, Li K, Lv J, Feng J, Chen J, Wu H,
Cheng F, Jiang W, Wang J, Pei H, et al: Suppression of the
SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant
lung adenocarcinoma. J Clin Invest. 130:1752–1766. 2020. View Article : Google Scholar :
|
|
137
|
Harris IS, Treloar AE, Inoue S, Sasaki M,
Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA,
et al: Glutathione and thioredoxin antioxidant pathways synergize
to drive cancer initiation and progression. Cancer Cell.
27:211–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhang L, Hobeika CS, Khabibullin D, Yu D,
Filippakis H, Alchoueiry M, Tang Y, Lam HC, Tsvetkov P, Georgiou G,
et al: Hypersensitivity to ferroptosis in chromophobe RCC is
mediated by a glutathione metabolic dependency and cystine import
via solute carrier family 7 member 11. Proc Natl Acad Sci USA.
119:e21228401192022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Koppula P, Lei G, Zhang Y, Yan Y, Mao C,
Kondiparthi L, Shi J, Liu X, Horbath A, Das M, et al: A targetable
CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1
inactive lung cancers. Nat Commun. 13:22062022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Rojo de la Vega M, Chapman E and Zhang DD:
NRF2 and the hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Rawat SG, Tiwari RK, Jaiswara PK, Gupta
VK, Sonker P, Vishvakarma NK, Kumar S, Pathak C, Gautam V and Kumar
A: Phosphodiesterase 5 inhibitor sildenafil potentiates the
antitumor activity of cisplatin by ROS-mediated apoptosis: A role
of deregulated glucose metabolism. Apoptosis. 27:606–618. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Kobayashi M, Narumi K, Furugen A and Iseki
K: Transport function, regulation, and biology of human
monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4). Pharmacol
Ther. 226:1078622021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ding Y, Chen X, Liu C, Ge W, Wang Q, Hao
X, Wang M, Chen Y and Zhang Q: Identification of a small molecule
as inducer of ferroptosis and apoptosis through ubiquitination of
GPX4 in triple negative breast cancer cells. J Hematol Oncol.
14:192021. View Article : Google Scholar
|
|
144
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhou L, Mo Y, Zhang H, Zhang M, Xu J and
Liang S: Role of AMPK-regulated autophagy in retinal pigment
epithelial cell homeostasis: A review. Medicine (Baltimore).
103:e389082024. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chen X, Yu C, Kang R, Kroemer G and Tang
D: Cellular degradation systems in ferroptosis. Cell Death Differ.
28:1135–1148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gao M, Monian P, Pan Q, Zhang W, Xiang J
and Jiang X: Ferroptosis is an autophagic cell death process. Cell
Res. 26:1021–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zhou B, Liu J, Kang R, Klionsky DJ,
Kroemer G and Tang D: Ferroptosis is a type of autophagy-dependent
cell death. Semin Cancer Biol. 66:89–100. 2020. View Article : Google Scholar
|
|
149
|
Yang M, Chen P, Liu J, Zhu S, Kroemer G,
Klionsky DJ, Lotze MT, Zeh HJ, Kang R and Tang D: Clockophagy is a
novel selective autophagy process favoring ferroptosis. Sci Adv.
5:eaaw22382019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Chen J, Zhu Y, Wu C and Shi J: Engineering
lactate-modulating nanomedicines for cancer therapy. Chem Soc Rev.
52:973–1000. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Annoni F, Peluso L, Gouvêa Bogossian E,
Creteur J, Zanier ER and Taccone FS: Brain protection after anoxic
brain injury: Is lactate supplementation helpful? Cells.
10:17142021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Fei Y and Ding Y: The role of ferroptosis
in neurodegenerative diseases. Front Cell Neurosci. 18:14759342024.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Pan RY, He L, Zhang J, Liu X, Liao Y, Gao
J, Liao Y, Yan Y, Li Q, Zhou X, et al: Positive feedback regulation
of microglial glucose metabolism by histone H4 lysine 12
lactylation in Alzheimer's disease. Cell Metab. 34:634–648.e6.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Devos D, Labreuche J, Rascol O, Corvol JC,
Duhamel A, Guyon Delannoy P, Poewe W, Compta Y, Pavese N, Růžička
E, et al: Trial of deferiprone in Parkinson's disease. N Engl J
Med. 387:2045–2055. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Chen L, Shen Q, Liu Y, Zhang Y, Sun L, Ma
X, Song N and Xie J: Homeostasis and metabolism of iron and other
metal ions in neurodegenerative diseases. Signal Transduct Target
Ther. 10:312025. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Berthet C, Lei H, Thevenet J, Gruetter R,
Magistretti PJ and Hirt L: Neuroprotective role of lactate after
cerebral ischemia. J Cereb Blood Flow Metab. 29:1780–1789. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Cerina M, Levers M, Keller JM and Frega M:
Neuroprotective role of lactate in a human in vitro model of the
ischemic penumbra. Sci Rep. 14:79732024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Xiong XY, Pan XR, Luo XX, Wang YF, Zhang
XX, Yang SH, Zhong ZQ, Liu C, Chen Q, Wang PF, et al:
Astrocyte-derived lactate aggravates brain injury of ischemic
stroke in mice by promoting the formation of protein lactylation.
Theranostics. 14:4297–4317. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Cai M, Wang H, Song H, Yang R, Wang L, Xue
X, Sun W and Hu J: Lactate Is answerable for brain function and
treating brain diseases: Energy substrates and signal molecule.
Front Nutr. 9:8009012022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Liu Y, Hu P, Cheng H, Xu F and Ye Y: The
impact of glycolysis on ischemic stroke: From molecular mechanisms
to clinical applications. Front Neurol. 16:15143942025. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Cler M, Perez-Amodio S, Valls-Lacalle L,
Martinez E, Barba I, Ganse GFS, Engel E and Rodriguez-Sinovas A:
Abstract 4146509: L-lactic acid reduces infarct size after ischemia
in isolated mouse hearts through acidosis, MCT1-mediated uptake,
and metabolic reprogramming. Circulation. 150(Suppl1):
A41465092024. View Article : Google Scholar
|
|
162
|
Berthet C, Castillo X, Magistretti PJ and
Hirt L: New evidence of neuroprotection by lactate after transient
focal cerebral ischaemia: Extended benefit after
intracerebroventricular injection and efficacy of intravenous
administration. Cerebrovasc Dis. 34:329–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Nalos M, Kholodniak E, Smith L, Orde S,
Ting I, Slama M, Seppelt I, McLean AS and Huang S: The comparative
effects of 3% saline and 0.5M sodium lactate on cardiac function: A
randomised, crossover study in volunteers. Crit Care Resusc.
20:124–130. 2018.PubMed/NCBI
|
|
164
|
Nalos M, Leverve X, Huang S, Weisbrodt L,
Parkin R, Seppelt I, Ting I and Mclean A: Half-molar sodium lactate
infusion improves cardiac performance in acute heart failure: A
pilot randomised controlled clinical trial. Crit Care. 18:R482014.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Koyama T: Lactated Ringer's solution for
preventing myocardial reperfusion injury. Int J Cardiol Heart Vasc.
15:1–8. 2017.PubMed/NCBI
|
|
166
|
Nolt B, Tu F, Wang X, Ha T, Winter R,
Williams DL and Li C: Lactate and immunosuppression in sepsis.
Shock. 49:120–125. 2018. View Article : Google Scholar :
|
|
167
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Sonpavde G, Matveev V, Burke JM, Caton JR,
Fleming MT, Hutson TE, Galsky MD, Berry WR, Karlov P, Holmlund JT,
et al: Randomized phase II trial of docetaxel plus prednisone in
combination with placebo or AT-101, an oral small molecule Bcl-2
family antagonist, as first-line therapy for metastatic
castration-resistant prostate cancer. Ann Oncol. 23:1803–1808.
2012. View Article : Google Scholar
|
|
169
|
Wu J, Gu X, Zhang J, Mi Z, He Z, Dong Y,
Ge W, Ghimire K, Rong P, Wang W and Ma X: 4-OI protects MIN6 cells
from oxidative stress injury by reducing LDHA-mediated ROS
generation. Biomolecules. 12:12362022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhao Z, Han F, Yang S, Wu J and Zhan W:
Oxamate-mediated inhibition of lactate dehydrogenase induces
protective autophagy in gastric cancer cells: Involvement of the
Akt-mTOR signaling pathway. Cancer Lett. 358:17–26. 2015.
View Article : Google Scholar
|
|
171
|
Farabegoli F, Vettraino M, Manerba M,
Fiume L, Roberti M and Di Stefano G: Galloflavin, a new lactate
dehydrogenase inhibitor, induces the death of human breast cancer
cells with different glycolytic attitude by affecting distinct
signaling pathways. Eur J Pharm Sci. 47:729–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Granchi C, Roy S, Giacomelli C, Macchia M,
Tuccinardi T, Martinelli A, Lanza M, Betti L, Giannaccini G,
Lucacchini A, et al: Discovery of N-hydroxyindole-based inhibitors
of human lactate dehydrogenase isoform A (LDH-A) as starvation
agents against cancer cells. J Med Chem. 54:1599–1612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Laganá G, Barreca D, Calderaro A and
Bellocco E: Lactate dehydrogenase inhibition: Biochemical relevance
and therapeutical potential. Curr Med Chem. 26:3242–3252. 2019.
View Article : Google Scholar
|
|
174
|
Ippolito L, Morandi A, Giannoni E and
Chiarugi P: Lactate: A metabolic driver in the tumour landscape.
Trends Biochem Sci. 44:153–166. 2019. View Article : Google Scholar
|
|
175
|
Raez LE, Papadopoulos K, Ricart AD,
Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ,
Tolba K, Langmuir VK, et al: A phase I dose-escalation trial of
2-deoxy-D-glucose alone or combined with docetaxel in patients with
advanced solid tumors. Cancer Chemother Pharmacol. 71:523–530.
2013. View Article : Google Scholar
|
|
176
|
Sutendra G and Michelakis ED: Pyruvate
dehydrogenase kinase as a novel therapeutic target in oncology.
Front Oncol. 3:382013. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Zhang Y, Du X, He Z, Gao S, Ye L, Ji J,
Yang X and Zhai G: A vanadium-based nanoplatform synergizing
ferroptotic-like therapy with glucose metabolism intervention for
enhanced cancer cell death and antitumor immunity. ACS Nano.
17:11537–11556. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Li F, Zhu P, Zheng B, Lu Z, Fang C, Fu Y
and Li X: A customized biohybrid presenting cascade responses to
tumor microenvironment. Adv Mater. 36:e24049012024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Deng X, Zhu Y, Dai Z, Liu Q, Song Z, Liu
T, Huang Y and Chen H: A bimetallic nanomodulator to reverse
immunosuppression via sonodynamic-ferroptosis and lactate
metabolism modulation. Small. 20:e24045802024. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Nancolas B, Guo L, Zhou R, Nath K, Nelson
DS, Leeper DB, Blair IA, Glickson JD and Halestrap AP: The
anti-tumour agent lonidamine is a potent inhibitor of the
mitochondrial pyruvate carrier and plasma membrane monocarboxylate
transporters. Biochem J. 473:929–936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Halford S, Veal GJ, Wedge SR, Payne GS,
Bacon CM, Sloan P, Dragoni I, Heinzmann K, Potter S, Salisbury BM,
et al: A phase I dose-escalation study of AZD3965, an oral
monocarboxylate transporter 1 inhibitor, in patients with advanced
cancer. Clin Cancer Res. 29:1429–1439. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Puri S and Juvale K: Monocarboxylate
transporter 1 and 4 inhibitors as potential therapeutics for
treating solid tumours: A review with structure-activity
relationship insights. Eur J Med Chem. 199:1123932020. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Khan A, Valli E, Lam H, Scott DA, Murray
J, Hanssen KM, Eden G, Gamble LD, Pandher R, Flemming CL, et al:
Targeting metabolic activity in high-risk neuroblastoma through
Monocarboxylate transporter 1 (MCT1) inhibition. Oncogene.
39:3555–3570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Kirk P, Wilson MC, Heddle C, Brown MH,
Barclay AN and Halestrap AP: CD147 is tightly associated with
lactate transporters MCT1 and MCT4 and facilitates their cell
surface expression. EMBO J. 19:3896–3904. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhu J, Cai H, Xu C, Wang W, Song X, Li B,
Shen Y and Dong X: Acidity-responsive nanoreactors destructed
'Warburg effect' for toxic-acidosis and starvation synergistic
therapy. Small. 19:e23040582023. View Article : Google Scholar
|
|
186
|
Kobayashi M, Otsuka Y, Itagaki S, Hirano T
and Iseki K: Inhibitory effects of statins on human monocarboxylate
transporter 4. Int J Pharm. 317:19–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Chen ZX, Liu MD, Guo DK, Zou MZ, Wang SB,
Cheng H, Zhong Z and Zhang XZ: A MSN-based tumor-targeted
nanoplatform to interfere with lactate metabolism to induce tumor
cell acidosis for tumor suppression and anti-metastasis. Nanoscale.
12:2966–2972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Minhas PS, Jones JR, Latif-Hernandez A,
Sugiura Y, Durairaj AS, Wang Q, Mhatre SD, Uenaka T, Crapser J,
Conley T, et al: Restoring hippocampal glucose metabolism rescues
cognition across Alzheimer's disease pathologies. Science.
385:eabm61312024. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zeng Z, Huang Q, Mao L, Wu J, An S, Chen Z
and Zhang W: The pyruvate dehydrogenase complex in sepsis:
Metabolic regulation and targeted therapy. Front Nutr.
8:7831642021. View Article : Google Scholar :
|
|
190
|
Ryoo SM and Kim WY: Clinical applications
of lactate testing in patients with sepsis and septic shock. J
Emerg Crit Care Med. 2:142018. View Article : Google Scholar
|
|
191
|
Wei Y, Zhuang J, Li J, Wang Z, Wang J,
Zhang X and Leng J: Lactate trajectories and outcomes in patients
with sepsis in the intensive care unit: Group-based trajectory
modeling. Front Public Health. 13:16102202025. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Liu S, Yang T, Jiang Q, Zhang L, Shi X,
Liu X and Li X: Lactate and lactylation in sepsis: A comprehensive
review. J Inflamm Res. 17:4405–4417. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Visker JR, Cluntun AA, Velasco-Silva JN,
Eberhardt DR, Cedeño-Rosario L, Shankar TS, Hamouche R, Ling J,
Kwak H, Hillas JY, et al: Enhancing mitochondrial pyruvate
metabolism ameliorates ischemic reperfusion injury in the heart.
JCI Insight. 9:e1809062024. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Fei M, Zhang H, Meng F, An G, Tang J, Tong
J, Xiong L, Liu Q and Li C: Enhanced lactate accumulation
upregulates PD-L1 expression to delay neutrophil apoptosis in
sepsis. VIEW. 5:202300532024. View Article : Google Scholar
|
|
195
|
Suzuki A, Stern SA, Bozdagi O, Huntley GW,
Walker RH, Magistretti PJ and Alberini CM: Astrocyte-neuron lactate
transport is required for long-term memory formation. Cell.
144:810–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Lottes RG, Newton DA, Spyropoulos DD and
Baatz JE: Lactate as substrate for mitochondrial respiration in
alveolar epithelial type II cells. Am J Physiol Lung Cell Mol
Physiol. 308:L953–L961. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Brown CW, Amante JJ, Goel HL and Mercurio
AM: The α6β4 integrin promotes resistance to ferroptosis. J Cell
Biol. 216:4287–4297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Pucino V, Certo M, Bulusu V, Cucchi D,
Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et
al: Lactate buildup at the site of chronic inflammation promotes
disease by inducing CD4+ T cell metabolic rewiring. Cell
Metab. 30:1055–1074.e8. 2019. View Article : Google Scholar
|
|
199
|
Lei P, Walker T and Ayton S:
Neuroferroptosis in health and diseases. Nat Rev Neurosci.
26:497–511. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zheng X, Boyer L, Jin M, Mertens J, Kim Y,
Ma L, Ma L, Hamm M, Gage FH and Hunter T: Metabolic reprogramming
during neuronal differentiation from aerobic glycolysis to neuronal
oxidative phosphorylation. Elife. 5:e133742016. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Bittar PG, Charnay Y, Pellerin L, Bouras C
and Magistretti PJ: Selective distribution of lactate dehydrogenase
isoenzymes in neurons and astrocytes of human brain. J Cereb Blood
Flow Metab. 16:1079–1089. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Wu N, Wei X, Yu S, Yang L and Zhang X:
Lactate in ferroptosis regulation: A new perspective on tumor
progression and therapy. Pharmacol Res. 218:1078412025. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Kennedy L, Glesaaen ER, Palibrk V, Pannone
M, Wang W, Al-Jabri A, Suganthan R, Meyer N, Austbø ML, Lin X, et
al: Lactate receptor HCAR1 regulates neurogenesis and microglia
activation after neonatal hypoxia-ischemia. Elife. 11:e764512022.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Hu L, Huang S, Chen G, Li B, Li T, Lin M,
Huang Y, Xiao Z, Shuai X and Su Z: Nanodrugs incorporating LDHA
siRNA inhibit M2-like polarization of TAMs and amplify autophagy to
assist oxaliplatin chemotherapy against colorectal cancer. ACS Appl
Mater Interfaces. 14:31625–31633. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Lu H, Liang B, Hu A, Zhou H, Jia C, Aji A,
Chen Q, Ma Y, Cui W, Jiang L and Dong J: Engineered biomimetic
cancer cell membrane nanosystems trigger gas-immunometabolic
therapy for spinal-metastasized tumors. Adv Mater. 37:e24126552025.
View Article : Google Scholar
|