|
1
|
Miao C, Huang Y, Zhang C, Wang X, Wang B,
Zhou X, Song Y, Wu P, Chen ZS and Feng Y: Post-translational
modifications in drug resistance. Drug Resist Updat. 78:1011732025.
View Article : Google Scholar
|
|
2
|
Hirano A, Fu YH and Ptáček LJ: The
intricate dance of post-translational modifications in the rhythm
of life. Nat Struct Mol Biol. 23:1053–1060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tharuka MDN, Courelli AS and Chen Y:
Immune regulation by the SUMO family. Nat Rev Immunol. 25:608–620.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhong Q, Xiao X, Qiu Y, Xu Z, Chen C,
Chong B, Zhao X, Hai S, Li S, An Z and Dai L: Protein
posttranslational modifications in health and diseases: Functions,
regulatory mechanisms, and therapeutic implications. MedComm.
4:e2612023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Hu J, Wu S, Fleishman JS, Li Y, Xu
Y, Zou W, Wang J, Feng Y, Chen J and Wang H: Targeting epigenetic
and posttranslational modifications regulating ferroptosis for the
treatment of diseases. Signal Transduct Target Ther. 8:4492023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
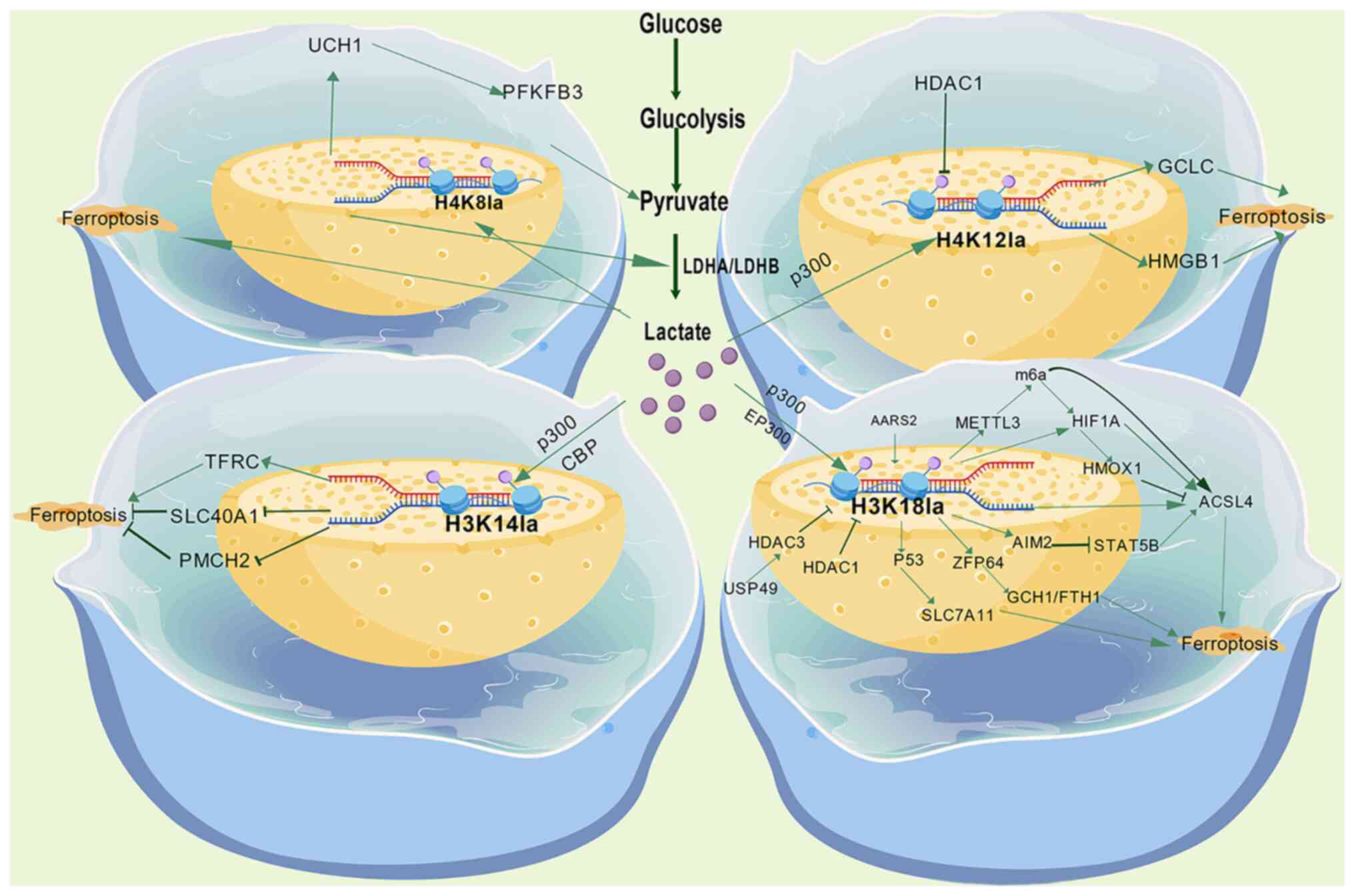
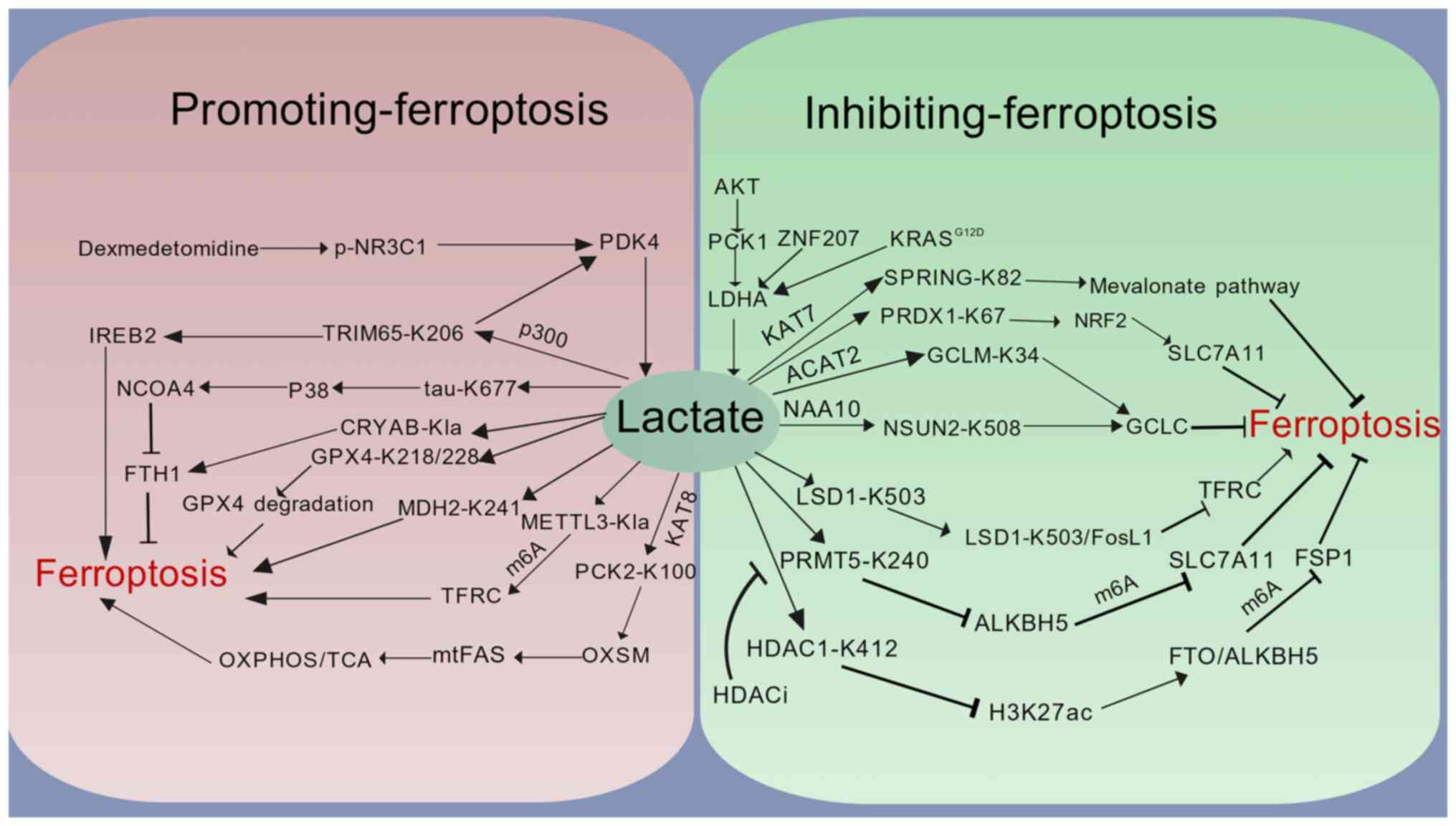
Brooks GA: The science and translation of
lactate shuttle theory. Cell Metab. 27:757–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan, Yanxiang Guo J, et
al: Glucose feeds the TCA cycle via circulating lactate. Nature.
551:115–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brooks GA, Osmond AD, Arevalo JA, Duong
JJ, Curl CC, Moreno-Santillan DD and Leija RG: Lactate as a myokine
and exerkine: drivers and signals of physiology and metabolism. J
Appl Physiol (1985). 134:529–548. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao S, Chai H, Tao T, Zhang L, Yang X, Li
X, Yi Z, Wang Y, An J, Wen G, et al: Role of lactate and lactate
metabolism in liver diseases (Review). Int J Mol Med. 54:592024.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Sun L, Gao P and Hu H: Lactylation
in cancer: Current understanding and challenges. Cancer Cell.
42:1803–1807. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Huang Z, Chen Y, Tian H, Chai P,
Shen Y, Yao Y, Xu S, Ge S and Jia R: Lactate and lactylation in
cancer. Signal Transduct Target Ther. 10:382025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Huang L, Gu Y, Cang W, Sun P and
Xiang Y: Lactate-lactylation hands between metabolic reprogramming
and immunosuppression. Int J Mol Sci. 23:119432022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Zhao F and Qu Y: Lactylation: A
novel post-translational modification with clinical implications in
CNS Diseases. Biomolecules. 14:11752024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li R, Yang Y, Wang H, Zhang T, Duan F, Wu
K, Yang S, Xu K, Jiang X and Sun X: Lactate and lactylation in the
brain: Current progress and perspectives. Cell Mol Neurobiol.
43:2541–2555. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng X and Du J: Histone and non-histone
lactylation: Molecular mechanisms, biological functions, diseases,
and therapeutic targets. Mol Biomed. 6:382025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Certo M, Llibre A, Lee W and Mauro C:
Understanding lactate sensing and signalling. Trends Endocrinol
Metab. 33:722–735. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shang S, Liu J and Hua F: Protein
acylation: Mechanisms, biological functions and therapeutic
targets. Signal Transduct Target Ther. 7:3962022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan Z, Liu Z, Zhang N, Wei W, Cheng K, Sun
H and Hao Q: Identification of SIRT3 as an eraser of H4K16la.
iScience. 26:1077572023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreno-Yruela C, Zhang D, Wei W, Bæk M,
Liu W, Gao J, Danková D, Nielsen AL, Bolding JE, Yang L, et al:
Class I histone deacetylases (HDAC1-3) are histone lysine
delactylases. Sci Adv. 8:eabi66962022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Huang X, Yang Y, Sun Y, Zhao Y,
Zhang Z, Qiu D, Wu Y, Wu G and Lei L: Dux activates
metabolism-lactylation-MET network during early iPSC reprogramming
with Brg1 as the histone lactylation reader. Nucleic Acids Res.
52:5529–5548. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: an iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng J and Conrad M: Ferroptosis: When
metabolism meets cell death. Physiol Rev. 105:651–706. 2025.
View Article : Google Scholar
|
|
26
|
Wang Z, Wu C, Yin D and Do K: Ferroptosis:
Mechanism and role in diabetes-related cardiovascular diseases.
Cardiovasc Diabetol. 24:602025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao L, Zhou X, Xie F and Zhang L, Yan H,
Huang J, Zhang C, Zhou F, Chen J and Zhang L: Ferroptosis in cancer
and cancer immunotherapy. Cancer Commun (Lond). 42:88–116. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar :
|
|
29
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar
|
|
31
|
Alves F, Lane D, Nguyen TPM, Bush AI and
Ayton S: In defence of ferroptosis. Signal Transduct Target Ther.
10:22025. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar :
|
|
33
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG and
Gao LC: System Xc -/GSH/GPX4 axis: An important antioxidant system
for the ferroptosis in drug-resistant solid tumor therapy. Front
Pharmacol. 13:9102922022. View Article : Google Scholar
|
|
35
|
Du Y and Guo Z: Recent progress in
ferroptosis: Inducers and inhibitors. Cell Death Discov. 8:5012022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pal D and Sandur SK: Novel role of
peroxiredoxin 6 in ferroptosis: Bridging selenium transport with
enzymatic functions. Free Radic Biol Med. 238:611–620. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ru Q, Li Y, Chen L, Wu Y, Min J and Wang
F: Iron homeostasis and ferroptosis in human diseases: mechanisms
and therapeutic prospects. Signal Transduct Target Ther. 9:2712024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Zou L, Li X, Guo L, Hu B, Ye H
and Liu Y: SLC40A1 in iron metabolism, ferroptosis, and disease: A
review. WIREs Mech Dis. 16:e16442024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng D, Liu J, Piao H, Zhu Z, Wei R and
Liu K: ROS-triggered endothelial cell death mechanisms: Focus on
pyroptosis, parthanatos, and ferroptosis. Front Immunol.
13:10392412022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2022. View Article : Google Scholar
|
|
41
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pope LE and Dixon SJ: Regulation of
ferroptosis by lipid metabolism. Trends Cell Biol. 33:1077–1087.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tilton WM, Seaman C, Carriero D and
Piomelli S: Regulation of glycolysis in the erythrocyte: role of
the lactate/pyruvate and NAD/NADH ratios. J Lab Clin Med.
118:146–152. 1991.PubMed/NCBI
|
|
45
|
Ying W: NAD+/NADH and NADP+/NADPH in
cellular functions and cell death: Regulation and biological
consequences. Antioxid Redox Signal. 10:179–206. 2008. View Article : Google Scholar
|
|
46
|
Pucino V, Certo M, Bulusu V, Cucchi D,
Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et
al: Lactate buildup at the site of chronic inflammation promotes
disease by inducing CD4+ T cell metabolic rewiring. Cell Metab.
30:1055–1074.e8. 2019. View Article : Google Scholar
|
|
47
|
Reddy A, Winther S, Tran N, Xiao H, Jakob
J, Garrity R, Smith A, Ordonez M, Laznik-Bogoslavski D, Rothstein
JD, et al: Monocarboxylate transporters facilitate succinate uptake
into brown adipocytes. Nat Metab. 6:567–577. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding K, Liu C, Li L, Yang M, Jiang N, Luo
S and Sun L: Acyl-CoA synthase ACSL4: An essential target in
ferroptosis and fatty acid metabolism. Chin Med J (Engl).
136:2521–2537. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lan H, Gao Y, Zhao Z, Mei Z and Wang F:
Ferroptosis: Redox imbalance and hematological tumorigenesis. Front
Oncol. 12:8346812022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen J, Liu Z, Yue Z, Tan Q, Yin H, Wang
H, Chen Z, Zhu Y and Zheng J: EP300-mediated H3K18la regulation of
METTL3 promotes macrophage ferroptosis and atherosclerosis through
the m6A modification of SLC7A11. Biochim Biophys Acta Gen Subj.
1869:1308382025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deng J, Li Y, Yin L, Liu S, Li Y, Liao W,
Mu L, Luo X and Qin J: Histone lactylation enhances GCLC expression
and thus promotes chemoresistance of colorectal cancer stem cells
through inhibiting ferroptosis. Cell Death Dis. 16:1932025.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gong F, Zheng X, Xu W, Xie R, Liu W, Pei
L, Zhong M, Shi W, Qu H, Mao E, et al: H3K14la drives endothelial
dysfunction in sepsis-induced ARDS by promoting
SLC40A1/transferrin-mediated ferroptosis. MedComm. 6:e700492025.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu J, Xiong Y, Zhang Y, Liang H, Cheng K,
Lu Y, Cai G, Wu Y, Fan Y, Chen X, et al: Simvastatin overcomes the
pPCK1-pLDHA-SPRINGlac axis-mediated ferroptosis and
chemo-immunotherapy resistance in AKT-hyperactivated intrahepatic
cholangiocarcinoma. Cancer Commun (Lond). 45:1038–1071. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen Y, Yan Q, Ruan S, Cui J, Li Z, Zhang
Z, Yang J, Fang J, Liu S, Huang S, et al: GCLM lactylation mediated
by ACAT2 promotes ferroptosis resistance in
KRASG12D-mutant cancer. Cell Rep. 44:1157742025.
View Article : Google Scholar
|
|
55
|
Niu K, Chen Z, Li M, Ma G, Deng Y, Zhang
J, Wei D, Wang J and Zhao Y: NSUN2 lactylation drives cancer cell
resistance to ferroptosis through enhancing GCLC-dependent
glutathione synthesis. Redox Biol. 79:1034792025. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Z, Su W, Zhang Q, Niu L, Feng B,
Zhang Y, Huang F, He J, Zhou Q, Zhou X, et al: Lactylation of HDAC1
confers resistance to ferroptosis in colorectal cancer. Adv Sci
(Weinh). 12:e24088452025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu J, Li Y, Ma R, Chen Y, Wang J, Zhang
L, Wang B, Zhang Z, Huang L, Zhang H, et al: Cold atmospheric
plasma drives USP49/HDAC3 axis mediated ferroptosis as a novel
therapeutic strategy in endometrial cancer via reinforcing
lactylation dependent p53 expression. J Transl Med. 23:4422025.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang G, Liu X, Li Y, Li L, Xiang J, Liang
Z, Jiang M and Yang S: TRIM65 as a key regulator of ferroptosis and
glycolysis in lactate-driven renal tubular injury and diabetic
kidney disease. Cell Rep. 44:1160912025. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu Z, Zhou Y, Li M, Xiao Z, Zhao Z and Li
Y: Dimeric PKM2 induces ferroptosis from intestinal
ischemia/reperfusion in mice by histone H4 lysine 12
lactylation-mediated HMGB1 transcription activation through the
lactic acid/p300 axis. Biochim Biophys Acta Mol Basis Dis.
1871:1679982025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang K, Guo L, Li X, Hu Y and Luo N:
Cancer-associated fibroblasts promote doxorubicin resistance in
triple-negative breast cancer through enhancing ZFP64 histone
lactylation to regulate ferroptosis. J Transl Med. 23:2472025.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lai WKM and Pugh BF: Understanding
nucleosome dynamics and their links to gene expression and DNA
replication. Nat Rev Mol Cell Biol. 18:548–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Millán-Zambrano G, Burton A, Bannister AJ
and Schneider R: Histone post-translational modifications-cause and
consequence of genome function. Nat Rev Genet. 23:563–580. 2022.
View Article : Google Scholar
|
|
63
|
Wu D, Spencer CB, Ortoga L, Zhang H and
Miao C: Histone lactylation-regulated METTL3 promotes ferroptosis
via m6A-modification on ACSL4 in sepsis-associated lung injury.
Redox Biol. 74:1031942024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liang Z, Liu J, Gou Y, Wang H, Li Z, Cao
Y, Zhang H, Bai R and Zhang Z: Elevated histone lactylation
mediates ferroptosis resistance in endometriosis through the
METTL3-Regulated HIF1A/HMOX1 signaling pathway. Adv Sci (Weinh).
12:e082202025. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu S, Liu M, Wang X and Wang S: The
histone lactylation of AIM2 influences the suppression of
ferroptosis by ACSL4 through STAT5B and promotes the progression of
lung cancer. FASEB J. 39:e703082025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhu J, Wu X, Mu M, Zhang Q and Zhao X:
TEC-mediated tRF-31R9J regulates histone lactylation and
acetylation by HDAC1 to suppress hepatocyte ferroptosis and improve
non-alcoholic steatohepatitis. Clin Epigenetics. 17:92025.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang J, Xie H, Li J, Huang X, Cai Y, Yang
R, Yang D, Bao W, Zhou Y, Li T and Lu Q: Histone lactylation drives
liver cancer metastasis by facilitating NSF1-mediated ferroptosis
resistance after microwave ablation. Redox Biol. 81:1035532025.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xiong J, Ge X, Pan D, Zhu Y, Zhou Y, Gao
Y, Wang H, Wang X, Gu Y, Ye W, et al: Metabolic reprogramming in
astrocytes prevents neuronal death through a UCHL1/PFKFB3/H4K8la
positive feedback loop. Cell Death Differ. 32:1214–1230. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu J, Tang D and Kang R: Targeting GPX4
in ferroptosis and cancer: chemical strategies and challenges.
Trends Pharmacol Sci. 45:666–670. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Y, Yue Q, Song X, Du W and Liu R:
Hypoxia/reoxygenation-induced glycolysis mediates myocardial
ischemia-reperfusion injury through promoting the lactylation of
GPX4. J Cardiovasc Transl Res. 18:762–774. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Di Sanzo M, Quaresima B, Biamonte F,
Palmieri C and Faniello MC: FTH1 pseudogenes in cancer and cell
metabolism. Cells. 9:25542020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Muhoberac BB and Vidal R: Iron, ferritin,
Hereditary ferritinopathy, and neurodegeneration. Front Neurosci.
13:11952019. View Article : Google Scholar
|
|
73
|
An X, He J, Xie P, Li C, Xia M, Guo D, Bi
B, Wu G, Xu J, Yu W and Ren Z: The effect of tau K677 lactylation
on ferritinophagy and ferroptosis in Alzheimer's disease. Free
Radic Biol Med. 224:685–706. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tian B, Li X, Li W, Shi Z, He X, Wang S,
Zhu X, Shi N, Li Y, Wan P and Zhu C: CRYAB suppresses ferroptosis
and promotes osteogenic differentiation of human bone marrow stem
cells via binding and stabilizing FTH1. Aging (Albany NY).
16:8965–8979. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yuan J, Yang M, Wu Z, Wu J, Zheng K, Wang
J, Zeng Q, Chen M, Lv T, Shi Y, et al: The Lactate-Primed KAT8-PCK2
axis exacerbates hepatic ferroptosis during ischemia/reperfusion
injury by reprogramming OXSM-Dependent mitochondrial fatty acid
synthesis. Adv Sci (Weinh). 12:e24141412025. View Article : Google Scholar
|
|
76
|
She H, Hu Y, Zhao G, Du Y, Wu Y, Chen W,
Li Y, Wang Y, Tan L, Zhou Y, et al: Dexmedetomidine ameliorates
myocardial ischemia-reperfusion injury by inhibiting MDH2
lactylation via regulating metabolic reprogramming. Adv Sci
(Weinh). 11:e24094992024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang L, Wang X, Che W, Zhou S and Feng Y:
METTL3 silenced inhibited the ferroptosis development via
regulating the TFRC levels in the Intracerebral hemorrhage
progression. Brain Res. 1811:1483732023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qu S, Feng B, Xing M, Qiu Y, Ma L, Yang Z,
Ji Y, Huang F, Wang Y, Zhou J, et al: PRMT5 K240lac confers
ferroptosis resistance via ALKBH5/SLC7A11 axis in colorectal
cancer. Oncogene. 44:2814–2830. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li A, Gong Z, Long Y, Li Y, Liu C, Lu X,
Li Q, He X, Lu H, Wu K, et al: Lactylation of LSD1 is an acquired
epigenetic vulnerability of BRAFi/MEKi-resistant melanoma. Dev
Cell. 60:1974–1990.e11. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Deng L, He S, Guo N, Tian W, Zhang W and
Luo L: Molecular mechanisms of ferroptosis and relevance to
inflammation. Inflamm Res. 72:281–299. 2023. View Article : Google Scholar
|
|
81
|
Chen Y, Fang ZM, Yi X, Wei X and Jiang DS:
The interaction between ferroptosis and inflammatory signaling
pathways. Cell Death Dis. 14:2052023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fang Y, Li Z, Yang L, Li W, Wang Y, Kong
Z, Miao J, Chen Y, Bian Y and Zeng L: Emerging roles of lactate in
acute and chronic inflammation. Cell Commun Signal. 22:2762024.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Qiao X, Yin J, Zheng Z, Li L and Feng X:
Endothelial cell dynamics in sepsis-induced acute lung injury and
acute respiratory distress syndrome: pathogenesis and therapeutic
implications. Cell Commun Signal. 22:2412024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li W, Li D, Chen Y, Abudou H, Wang H, Cai
J, Wang Y, Liu Z, Liu Y and Fan H: Classic signaling pathways in
alveolar injury and repair involved in sepsis-induced ALI/ARDS: New
research progress and prospect. Dis Markers.
2022:63623442022.PubMed/NCBI
|
|
85
|
Zhang T, Huang X, Feng S and Shao H:
Lactate-Dependent HIF1A transcriptional activation exacerbates
severe acute pancreatitis through the ACSL4/LPCAT3/ALOX15 pathway
induced ferroptosis. J Cell Biochem. 126:e306872025. View Article : Google Scholar
|
|
86
|
Han X, Bao J, Ni J, Li B, Song P, Wan R,
Wang X, Hu G and Chen C: Qing Xia Jie Yi Formula granules
alleviated acute pancreatitis through inhibition of M1 macrophage
polarization by suppressing glycolysis. J Ethnopharmacol.
325:1177502024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang Y, Wu S, Li Q, Sun H and Wang H:
Pharmacological inhibition of ferroptosis as a therapeutic target
for neurodegenerative diseases and strokes. Adv Sci (Weinh).
10:e23003252023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sheng W, Liao S, Wang D, Liu P and Zeng H:
The role of ferroptosis in osteoarthritis: Progress and prospects.
Biochem Biophys Res Commun. 733:1506832024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang J, Chen T and Gao F: Mechanism and
application prospect of ferroptosis inhibitors in improving
osteoporosis. Front Endocrinol (Lausanne). 15:14926102024.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, Zhao CY, Zhou Z, Li CC and Wang
Q: The effect of lactate dehydrogenase B and its mediated histone
lactylation on chondrocyte ferroptosis during osteoarthritis. J
Orthop Surg. 20:4932025. View Article : Google Scholar
|
|
91
|
Sun K, Shi Y, Yan C, Wang S, Han L, Li F,
Xu X, Wang Y, Sun J, Kang Z and Shi J: Glycolysis-derived lactate
induces ACSL4 Expression and lactylation to activate ferroptosis
during intervertebral disc degeneration. Adv Sci (Weinh).
12:e24161492025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li X, Ma N, Xu J, Zhang Y, Yang P, Su X,
Xing Y, An N, Yang F, Zhang G, et al: Targeting ferroptosis:
pathological mechanism and treatment of ischemia-reperfusion
injury. Oxid Med Cell Longev. 2021:15879222021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dong W, Huang SX, Qin ML and Pan Z:
Mitochondrial alanyl-tRNA synthetase 2 mediates histone lactylation
to promote ferroptosis in intestinal ischemia-reperfusion injury.
World J Gastrointest Surg. 17:1067772025. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun T, Zhang JN, Lan T, Shi L, Hu L, Yan
L, Wei C, Hei L, Wu W, Luo Z, et al: H3K14 lactylation exacerbates
neuronal ferroptosis by inhibiting calcium efflux following
intracerebral hemorrhagic stroke. Cell Death Dis. 16:5532025.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Apostolova P and Pearce EL: Lactic acid
and lactate: Revisiting the physiological roles in the tumor
microenvironment. Trends Immunol. 43:969–977. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sun Q, Wu J, Zhu G, Li T, Zhu X, Ni B, Xu
B, Ma X and Li J: Lactate-related metabolic reprogramming and
immune regulation in colorectal cancer. Front Endocrinol.
13:10899182023. View Article : Google Scholar
|
|
97
|
Zhang W, Xia M, Li J, Liu G, Sun Y, Chen X
and Zhong J: Warburg effect and lactylation in cancer: Mechanisms
for chemoresistance. Mol Med. 31:1462025. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liao M, Yao D, Wu L, Luo C, Wang Z, Zhang
J and Liu B: Targeting the Warburg effect: A revisited perspective
from molecular mechanisms to traditional and innovative therapeutic
strategies in cancer. Acta Pharm Sin B. 14:953–1008. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Boedtkjer E and Pedersen SF: The acidic
tumor microenvironment as a driver of cancer. Annu Rev Physiol.
82:103–126. 2020. View Article : Google Scholar
|
|
100
|
Wang T, Ye Z, Li Z, Jing DS, Fan GX, Liu
MQ, Zhuo QF, Ji SR, Yu XJ, Xu XW and Qin Y: Lactate-induced protein
lactylation: A bridge between epigenetics and metabolic
reprogramming in cancer. Cell Prolif. 56:e134782023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sun Y, Wang H, Cui Z, Yu T, Song Y, Gao H,
Tang R, Wang X, Li B, Li W and Wang Z: Lactylation in cancer
progression and drug resistance. Drug Resist Updat. 81:1012482025.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yang T, Zhang S, Nie K, Cheng C, Peng X,
Huo J and Zhang Y: ZNF207-driven PRDX1 lactylation and NRF2
activation in regorafenib resistance and ferroptosis evasion. Drug
Resist Updat. 82:1012742025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yu Y, Huang X, Liang C and Zhang P:
Evodiamine impairs HIF1A histone lactylation to inhibit
Sema3A-mediated angiogenesis and PD-L1 by inducing ferroptosis in
prostate cancer. Eur J Pharmacol. 957:1760072023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tong Q, Huang C, Tong Q and Zhang Z:
Histone lactylation: A new frontier in laryngeal cancer research
(Review). Oncol Lett. 30:4212025. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bao C, Ma Q, Ying X, Wang F, Hou Y, Wang
D, Zhu L, Huang J and He C: Histone lactylation in macrophage
biology and disease: From plasticity regulation to therapeutic
implications. EBioMedicine. 111:1055022025. View Article : Google Scholar :
|
|
106
|
Bao Q, Wan N, He Z, Cao J, Yuan W, Hao H
and Ye H: Subcellular proteomic mapping of lysine lactylation. J Am
Soc Mass Spectrom. 35:3221–3232. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhao W, Zhang J, Chen K, Yuan J, Zhai L
and Tan M: Mass spectrometry-based characterization of histone
post-translational modification. Curr Opin Chem Biol.
88:1026222025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sun Y, Chen Y and Peng T: A bioorthogonal
chemical reporter for the detection and identification of protein
lactylation. Chem Sci. 13:6019–6027. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang X, Liu Y, Rekowski MJ and Wang N:
Lactylation of tau in human Alzheimer's disease brains. Alzheimers
Dement. 21:e144812025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chu X, Di C, Chang P, Li L, Feng Z, Xiao
S, Yan X, Xu X, Li H, Qi R, et al: Lactylated Histone H3K18 as a
potential biomarker for the diagnosis and predicting the severity
of septic shock. Front Immunol. 12:7866662022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nuñez R, Sidlowski PFW, Steen EA,
Wynia-Smith SL, Sprague DJ, Keyes RF and Smith BC: The TRIM33
bromodomain recognizes histone lysine lactylation. ACS Chem Biol.
19:2418–2428. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang Y, Fan J, Meng X, Shu Q, Wu Y, Chu
GC, Ji R, Ye Y, Wu X, Shi J, et al: Development of nucleus-targeted
histone-tail-based photoaffinity probes to profile the epigenetic
interactome in native cells. Nat Commun. 16:4152025. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Crabb S, Plummer R, Greystoke A, Carter L,
Pacey S, Walter H, Coyle VM, Knurowski T, Clegg K, Ashby F, et al:
560TiP a phase I/IIa study to evaluate the safety and efficacy of
CCS1477, a first in clinic inhibitor of p300/CBP, as monotherapy in
patients with selected molecular alterations. Ann Oncol.
32:S6172021. View Article : Google Scholar
|
|
114
|
Nicosia L, Spencer GJ, Brooks N, Ciceri F,
Wiseman DH, Pegg N, West W, Knurowski T, Frese K, Clegg K, et al:
Therapeutic targeting of EP300/CBP by bromodomain inhibition in
hematologic malignancies. Cancer Cell. 41:2136–2153.e13. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Armstrong AJ, Gordon MS, Reimers MA,
Sedkov A, Lipford K, Snavely-Merhaut J, Kumar S, Guichard SM and
Shore N: The courage study: A first-in-human phase 1 study of the
CBP/p300 inhibitor FT-7051 in men with metastatic
castration-resistant prostate cancer. J Clin Oncol. 39:TPS50852021.
View Article : Google Scholar
|
|
116
|
Marks PA and Breslow R: Dimethyl sulfoxide
to vorinostat: Development of this histone deacetylase inhibitor as
an anti-cancer drug. Nat Biotechnol. 25:84–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Campbell P and Thomas CM: Belinostat for
the treatment of relapsed or refractory peripheral T-cell lymphoma.
J Oncol Pharm Pract. 23:143–147. 2017. View Article : Google Scholar
|
|
118
|
Tzogani K, van Hennik P, Walsh I, De
Graeff P, Folin A, Sjöberg J, Salmonson T, Bergh J, Laane E, Ludwig
H, et al: EMA review of panobinostat (farydak) for the treatment of
adult patients with relapsed and/or refractory multiple myeloma.
Oncologist. 23:631–636. 2018. View Article : Google Scholar :
|
|
119
|
Lu X, Ning Z, Li Z, Cao H and Wang X:
Development of chidamide for peripheral T-cell lymphoma, the first
orphan drug approved in China. Intractable Rare Dis Res. 5:185–191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yardley DA, Ismail-Khan RR, Melichar B,
Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee
MJ and Trepel JB: Randomized phase II, double-blind,
placebo-controlled study of exemestane with or without entinostat
in postmenopausal women with locally recurrent or metastatic
estrogen receptor-positive breast cancer progressing on treatment
with a nonsteroidal aromatase inhibitor. J Clin Oncol.
31:2128–2135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui
S, Wang S, Ouyang Q, Yin Y, Geng C, et al: Tucidinostat plus
exemestane for postmenopausal patients with advanced, hormone
receptor-positive breast cancer (ACE): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:806–815. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kitir B, Maolanon AR, Ohm RG, Colaço AR,
Fristrup P, Madsen AS and Olsen CA: Chemical editing of macrocyclic
natural products and kinetic profiling reveal slow, tight-binding
histone deacetylase inhibitors with picomolar affinities.
Biochemistry. 56:5134–5146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Whitehead L, Dobler MR, Radetich B, Zhu Y,
Atadja PW, Claiborne T, Grob JE, McRiner A, Pancost MR, Patnaik A,
et al: Human HDAC isoform selectivity achieved via exploitation of
the acetate release channel with structurally unique small molecule
inhibitors. Bioorg Med Chem. 19:4626–4634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang Y, Stowe RL, Pinello CE, Tian G,
Madoux F, Li D, Zhao LY, Li JL, Wang Y, Wang Y, et al:
Identification of histone deacetylase inhibitors with
benzoylhydrazide scaffold that selectively inhibit class I histone
deacetylases. Chem Biol. 22:273–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Rosás-Umbert M, Ruiz-Riol M, Fernández MA,
Marszalek M, Coll P, Manzardo C, Cedeño S, Miró JM, Clotet B, Hanke
T, et al: In vivo effects of romidepsin on T-cell activation,
apoptosis and function in the BCN02 HIV-1 kick&kill clinical
trial. Front Immunol. 11:4182020. View Article : Google Scholar
|
|
126
|
Sandoná M, Consalvi S, Tucciarone L, Puri
PL and Saccone V: HDAC inhibitors for muscular dystrophies:
Progress and prospects. Expert Opin Orphan Drugs. 4:125–127. 2016.
View Article : Google Scholar
|
|
127
|
Wang X, Shen X, Xu Y, Xu S, Xia F, Zhu B,
Liu Y, Wang W, Wu H and Wang F: The etiological changes of
acetylation in peripheral nerve injury-induced neuropathic
hypersensitivity. Mol Pain. 14:17448069187984082018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bhatt AN, Shenoy S, Munjal S, Chinnadurai
V, Agarwal A, Vinoth Kumar A, Shanavas A, Kanwar R and Chandna S:
2-deoxy-d-glucose as an adjunct to standard of care in the medical
management of COVID-19: A proof-of-concept and dose-ranging
randomised phase II clinical trial. BMC Infect Dis. 22:6692022.
View Article : Google Scholar
|
|
129
|
Sharma D, Singh M and Rani R: Role of LDH
in tumor glycolysis: Regulation of LDHA by small molecules for
cancer therapeutics. Semin Cancer Biol. 87:184–195. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Halford SER, Walter H, McKay P, Townsend
W, Linton K, Heinzmann K, Dragoni I, Brotherton L, Veal G, Siskos
A, et al: Phase I expansion study of the first-in-class
monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients
with diffuse large B-cell lymphoma (DLBCL) and burkitt lymphoma
(BL). J Clin Oncol. 39:31152021. View Article : Google Scholar
|
|
131
|
Halford SER, Jones P, Wedge S, Hirschberg
S, Katugampola S, Veal G, Payne G, Bacon C, Potter S, Griffin M, et
al: A first-in-human first-in-class (FIC) trial of the
monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients
with advanced solid tumours. J Clin Oncol. 35:25162017. View Article : Google Scholar
|
|
132
|
Halford S, Veal GJ, Wedge SR, Payne GS,
Bacon CM, Sloan P, Dragoni I, Heinzmann K, Potter S, Salisbury BM,
et al: A phase I dose-escalation study of AZD3965, an oral
monocarboxylate transporter 1 inhibitor, in patients with advanced
cancer. Clin Cancer Res. 29:1429–1439. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Singh M, Afonso J, Sharma D, Gupta R and
Kumar V, Rani R, Baltazar F and Kumar V: Targeting monocarboxylate
transporters (MCTs) in cancer: How close are we to the clinics?
Semin Cancer Biol. 90:1–14. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang W, Shan G, Bi G, Hu Z, Yi Y, Zeng D,
Lin Z and Zhan C: Lactylation and regulated cell death. Biochim
Biophys Acta Mol Cell Res. 1872:1199272025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gong T, Wang QD, Loughran PA, Li YH, Scott
MJ, Billiar TR, Liu YT and Fan J: Mechanism of lactic
acidemia-promoted pulmonary endothelial cells death in sepsis: role
for CIRP-ZBP1-PANoptosis pathway. Mil Med Res. 11:712024.PubMed/NCBI
|
|
136
|
Xu H, Li L, Wang S, Wang Z, Qu L, Wang C
and Xu K: Royal jelly acid suppresses hepatocellular carcinoma
tumorigenicity by inhibiting H3 histone lactylation at H3K9la and
H3K14la sites. Phytomedicine Int J Phytother Phytopharm.
118:1549402023.
|
|
137
|
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L,
Xu Y, He J, Lan J, Ou Q, et al: Tumor-derived lactate promotes
resistance to bevacizumab treatment by facilitating autophagy
enhancer protein RUBCNL expression through histone H3 lysine 18
lactylation (H3K18la) in colorectal cancer. Autophagy. 20:114–130.
2024. View Article : Google Scholar :
|
|
138
|
Sun Y, Chen Y, Xu Y, Zhang Y, Lu M, Li M,
Zhou L and Peng T: Genetic encoding of ε-N-L-lactyllysine for
detecting delactylase activity in living cells. Chem Commun (Camb).
58:8544–8547. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Jiang S, Li H, Zhang L, Mu W, Zhang Y,
Chen T, Wu J, Tang H, Zheng S, Liu Y, et al: Generic diagramming
platform (GDP): A comprehensive database of high-quality biomedical
graphics. Nucleic Acids Res. 53(D1): D1670–D1676. 2025. View Article : Google Scholar :
|