Post-translational modifications (PTMs) increase
proteomic functional diversity by covalently adding functional
groups, regulating the proteolytic cleavage of subunits or the
degradation of entire proteins. These include glycosylation,
ubiquitination, small ubiquitin-like modification, acetylation,
phosphorylation and palmitoylation, affecting nearly all aspects of
cell biology and pathology (1-5).
Lactate, once considered a waste product of glucose metabolism, has
been revealed in recent years as not only a key circulating energy
substrate, but also as a signaling molecule. Notably, it profoundly
regulates gene expression and cell fate through a novel PTM, lysine
lactylation (Kla) (6-10). In 2019, Zhang et al
(11) first reported histone Kla
in Nature, inaugurating a new era in lactate biology research. Kla
has rapidly emerged as a research hotspot in life sciences, with
its mechanisms and pathological significance providing
transformative insights in fields such as cancer, immunology and
neurological diseases (11-16). Kla involves the transfer of a
lactyl group to the amino group of lysine residues, a process
regulated by a series of enzymes and metabolites, including
lactate, 'writer' enzymes, 'reader' proteins and 'eraser' enzymes
(17). Lactate accumulation is
the core trigger for Kla, with its level being positively
associated with the degree of Kla modification (18,19). Under the action of 'writers',
L-lactyl-CoA derived from lactate is transferred to proteins,
initiating Kla. Erasers remove L-lactyl-CoA from proteins to
terminate Kla, while 'reader' proteins recognize Kla and transduce
signals to downstream targets (20-23).
The concept of ferroptosis was first proposed in
2012, referring to a unique form of programmed cell death triggered
by iron-dependent lipid peroxidation pathways. It participates in
multiple physiological and pathological processes and is prevalent
in various diseases (24-28).
In recent years, significant progress has been made in
understanding the mechanisms of ferroptosis, including iron
homeostasis imbalance, lipid peroxidation and the disruption of
antioxidant systems (29-33).
Furthermore, numerous factors regulate cellular sensitivity to
ferroptosis under pathological conditions, thereby influencing
disease progression (27).
System xCT is an antiporter, with solute carrier family (SLC)7A11
as a key component, responsible for transporting cysteine and
glutamate. It increases cellular cysteine uptake, which is
converted to glutathione (GSH) under the action of thioredoxin
reductase 1. Glutathione (GSH) peroxidase 4 (GPX4) relies on GSH as
a substrate to enhance its activity, promoting the conversion of
phospholipid hydroperoxides (PL-OOH) to lipid alcohols. Thus,
system xCT prevents ferroptosis by reducing PL-OOH accumulation.
Erastin and RAS-selective lethal 3 (RSL3, a ferroptosis inducer),
as inhibitors of system xCT and GPX4, respectively, promote
ferroptosis (34-36). An imbalance in iron homeostasis
is another classical pathway in ferroptosis, primarily regulated by
a network involving transferrin receptor 1 (TFR1), iron regulatory
protein (IRP)1 and IRP2, affecting cellular iron uptake, storage
and release (30,37,38). Reactive oxygen species (ROS)
generation and phospholipid peroxidation require numerous metabolic
enzymes, with iron acting as their catalyst and essential element.
Iron-dependent Fenton reactions rapidly amplify PL-OOH and generate
various reactive radicals, inducing ferroptosis in cancer cells
(39-41). Unrestricted lipid peroxidation is
a hallmark of ferroptosis. Acyl-CoA synthetase long-chain family
member 4 (ACSL4) is a key enzyme converting polyunsaturated fatty
acids (PUFAs) to phospholipids (PUFA-PEs). PUFA-PEs promote
intracellular lipid peroxide accumulation under the action of
various enzymes. Phospholipids rich in PUFAs in cell membranes and
phospholipid peroxidation are considered the direct executors of
ferroptosis (42,43).
Kla was initially discovered as a process modifying
histone lysine residues, thereby altering binding to target gene
promoters and activating transcription to exert biological effects.
Subsequently, it expanded to non-histone lactylation. In
ferroptosis, lactylation primarily involves Kla on histones H3 and
H4, binding to promoter regions of target genes to alter
transcriptional activity. On the other hand, Kla can directly
modify non-histone lysine residues to mediate ferroptosis,
demonstrating the multifaceted nature of Kla in regulating
ferroptosis. Furthermore, Kla is subject to a complex interplay of
upstream signals. A key determinant involves the participating
lactyltransferases ('writers') and delactylases.
EP300/p300/CREB-binding protein (CBP) are well-established
'writers' of histone lactylation (50-52), while other transferases such as
lysine acetyltransferase (KAT), acetyl-CoA acetyltransferase 2
(ACAT2) and N-alpha-acetyltransferase 10 (NAA10) are emerging as
modifiers of non-histone substrates (53-55). By contrast, histone deacetylase 1
(HDAC1) and HDAC3 have been identified as possessing delactylase
activity (56,57). Their substrate specificity is
influenced by expression patterns, spatial localization and
interactions with specific factors in different cellular
microenvironments. Additionally, metabolic status serves as a
fundamental upstream signal. Lactate accumulation can directly
drive lactyltransferase-catalyzed lactylation (55,58,59). Local pH in microenvironments,
such as tumors, can also influence enzyme kinetics and substrate
accessibility (60). Finally,
crosstalk with other PTMs creates a regulatory network; for
instance, prior acetylation or phosphorylation at or near lysine
residues may competitively inhibit or cooperatively promote
subsequent lactylation (56).
This intricate network, comprising writers, metabolic signals and
competitive PTMs, ultimately determines whether lactylation at
specific sites on given proteins promotes or suppresses ferroptosis
under particular pathophysiological conditions.
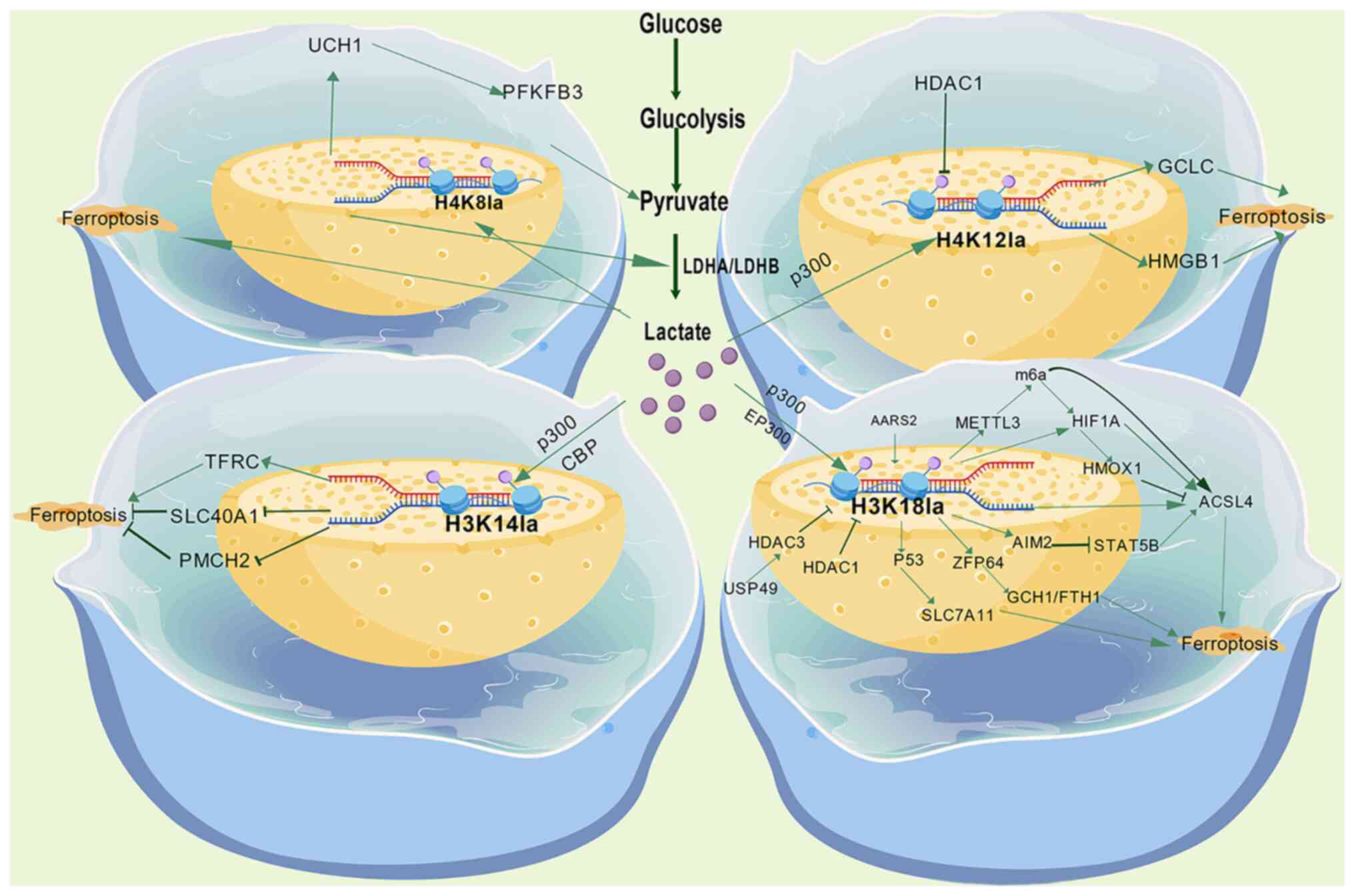
The nucleosome, consisting of a core histone octamer
(two each of H2A, H2B, H3 and H4) and wrapped DNA, is the
fundamental unit of chromatin. Typically, PTMs are key mechanisms
dynamically regulating chromatin structure and DNA templating
processes such as gene transcription (61,62). Lactylation modifications at
histone H3 lysine 18 (H3K18la), lysine 14 (H3K14la) and histone H4
lysine 8 (H4K8la), and lysine 12 (H4K12la) alter histone function
by the covalent addition of a lactyl group, mediating epigenetic
reprogramming to regulate ferroptosis. The effect is dependent on
the modification site, target gene and disease microenvironment
(Fig. 1).
H3K18la is the most extensively studied histone
lactylation site in ferroptosis. One of its core regulatory targets
is the key fatty acid metabolism enzyme, ACSL4; elevated ACSL4
protein levels can induce transient mitochondrial activation, ROS
accumulation and lipid peroxidation, ultimately driving
ferroptosis. Notably, H3K18la plays context-dependent dual roles in
regulating ACSL4. However, despite differing regulatory directions
in different diseases, the outcome consistently promotes disease
progression. In inflammatory diseases, lactate accumulation
upregulates ACSL4 via H3K18la, promoting ferroptosis-mediated
disease progression. The mechanisms involved include H3K18la
enrichment in promoter regions, which enhances hypoxia-inducible
factor (HIF)1A transcriptional activity, thereby transcriptionally
activating ACSL4. Additionally, H3K18la can directly bind the ACSL4
gene promoter region to increase transcription (53,54). Simultaneously, in sepsis-mediated
lung injury, H3K18la upregulates methyltransferase-like 3 (METTL3)
by enhancing its promoter activity, increasing ACSL4 mRNA stability
via N6-methyladenosine (m6A) RNA methylation
modification and reader protein YTH domain-containing protein 1
dependency, leading to an elevated protein expression of ACSL4
(63). Conversely, in cancer,
H3K18la suppresses ACSL4 expression, enhancing ferroptosis
resistance and promoting disease progression. In endometriosis,
H3K18la binds the promoter of methyltransferase METTL3, promoting
its expression, which subsequently stabilizes HIF1A mRNA via m6ARNA
methylation and upregulates heme oxygenase 1 (HMOX1), ultimately
downregulating ACSL4 (64).
H3K18la binding to the promoter region significantly enhances
absent in melanoma 2 (AIM2) transcription, downregulating ACSL4
expression by promoting ubiquitin-mediated degradation of
transcription factor STAT5B. This leads to decreased cellular lipid
peroxidation, reduced GSH depletion and limited ferrous iron
(Fe2+) accumulation, ultimately inhibiting ferroptosis
(65). Furthermore, H3K18la can
influence ferroptosis by regulating other key genes. H3K18la
binding promotes transcription of zinc finger protein (ZFP)64,
which directly binds the promoter regions of ferroptosis-related
genes GTP cyclohydrolase 1 (GCH1) and ferritin heavy chain 1
(FTH1), promoting their transcription. GCH1 reduces ROS
accumulation by inhibiting lipid peroxidation, while FTH1 reduces
iron-dependent lipid peroxidation triggered by sequestering
intracellular free Fe2; both synergistically inhibit
ferroptosis (60). The reduced
expression of H3K18la specifically decreases ferroptosis driver
genes [activating transcription factor (ATF3), ATF4 and ChaC
glutathione specific gamma-glutamylcyclotransferase 1 (CHAC1)],
thereby inhibiting ferroptosis (66). H3K18la enrichment at the promoter
region of the key iron-sulfur cluster synthesis enzyme NSF1
cysteine desulfurase (NFS1) enhances its transcription. NFS1
inhibits lipid peroxidation and ferroptosis by maintaining iron ion
homeostasis (67).
H3K14la represents lactylation at another H3 site,
K14, and is a clear signal promoting ferroptosis. H3K14la
transcriptionally activates transferrin receptor (TFRC) and
suppresses ferroportin SLC40A1 expression by binding the promoter
regions of ferroptosis-related genes, leading to intracellular iron
overload, lipid peroxidation and mitochondrial damage, ultimately
triggering endothelial cell ferroptosis (52).
H4K8la represents lactylation at histone H4 K8. Its
core mechanism involves tight coupling with glycolysis.
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), a
key glycolytic rate-limiting enzyme, is stabilized by
deubiquitinase ubiquitin C-terminal hydrolase L1 (UCHL1), cleaving
its K48-linked ubiquitin chains, promoting lactate production.
Increased lactate levels further induce H4K8la modification,
forming a positive feedback loop that activates UCHL1 and
glycolytic-related genes [e.g., LDHA, pyruvate kinase M2 (PKM), MYC
and Yes-associated protein 1], amplifying the inhibition of
ferroptosis (68).
H4K12la, catalyzed by lactyltransferase p300 at
histone H4 lysine 12, is enriched at the glutamate-cysteine ligase
catalytic subunit (GCLC) promoter region, enhancing glutathione
synthesis, inhibiting lipid peroxide accumulation and ultimately
blocking the ferroptosis pathway (51).
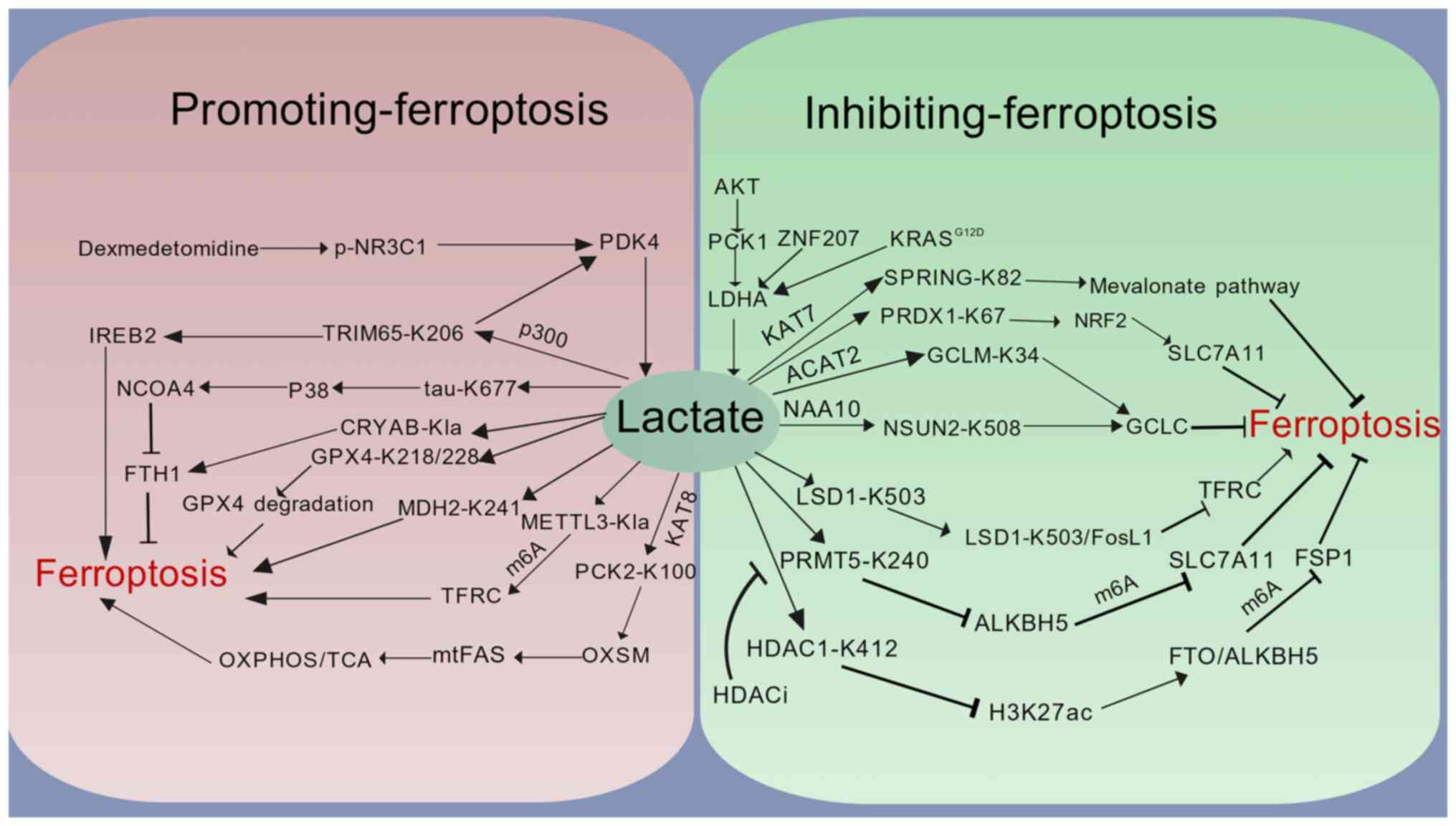
Beyond histones, Kla precisely regulates cell fate
by directly modifying key ferroptosis enzymes. This regulation
operates through three core mechanisms: By directly targeting key
ferroptosis effectors, remodeling cellular metabolic pathways and
reprogramming the epigenetic landscape, collectively constituting a
dynamic hub for ferroptosis regulation in response to metabolic
stress (Fig. 2).
At the level of the direct targeting of key
molecules, lactylation acts directly on core ferroptosis proteins,
altering their stability or functional activity to regulate
cellular antioxidant capacity and iron metabolism, thereby
influencing ferroptosis progression. GPX4 is a key inhibitor of
oxidative stress-induced ferroptosis. Lactate-driven lactylation at
lysines 218 and 228 (K218/K228) of GPX4 significantly reduces its
protein stability, impairing its ability to clear lipid peroxides
and enhancing ferroptosis sensitivity (69,70). FTH1 alters intracellular iron
homeostasis by influencing iron storage and release, and iron
imbalance is a major trigger for ferroptosis (71,72). Tau protein lactylation at K677
upregulates nuclear receptor coactivator 4 (NCOA4) expression by
activating p38 phosphorylation signaling, subsequently lowering
FTH1 levels and inducing ferroptosis (73). Conversely, alpha-crystallin B
chain (CRYAB), via lactylation, directly binds and stabilizes the
FTH1 protein to enhance ferroptosis resistance (74).
Metabolic reprogramming constitutes the second major
mechanism by which lactylation regulates ferroptosis, driving lipid
peroxidation by intervening in mitochondrial energy metabolism and
lipid synthesis pathways. The lactylation of the mitochondrial
phosphoenolpyruvate carboxykinase 2 (PCK2) protein at K100
competitively inhibits E3 ubiquitin ligase Parkin-mediated
ubiquitination and degradation of mitochondrial 3-oxoacyl-ACP
synthase (OXSM), the rate-limiting enzyme of mitochondrial fatty
acid synthesis (mtFAS). This promotes mtFAS pathway metabolic
reprogramming, exacerbating ferroptosis (75). The inhibition of lactylation at
K241 of malate dehydrogenase 2 (MDH2) maintains MDH2 catalytic
activity, sustaining the tricarboxylic acid (TCA) cycle and
blocking ferroptosis (76).
Lactylation at K82 of sterol regulatory element-binding protein
(SREBP) pathway regulator in Golgi (SPRING) protein activates the
SREBP2-driven mevalonate pathway, ultimately conferring ferroptosis
resistance to cancer cells through enhanced antioxidant capacity
(53).
Epigenetic reprogramming forms the third pillar of
lactylation-mediated ferroptosis regulation. It dynamically affects
the transcription and mRNA stability of ferroptosis-related genes
by modifying epigenetic regulators, such as RNA methylation enzymes
and histone modifiers. In the epitranscriptome, m6A methylation
bidirectionally regulates ferroptosis by increasing mRNA stability.
On the one hand, lactylation of the m6A writer, METTL3, increases
m6A methylation on TFRC mRNA, promoting TFRC mRNA stability,
accelerating cellular iron uptake and inducing ferroptosis
(77). De-lactylation at K412 of
HDAC1 activates the transcription of the m6A erasers, fat mass and
obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5),
suppressing m6A modification on ferroptosis suppressor protein 1
(FSP1) mRNA and accelerating its degradation, thereby enhancing
ferroptosis sensitivity (56).
Conversely, lactylation at K240 of protein arginine
methyltransferase 5 inhibits the transcription of the m6A eraser
ALKBH5, leading to increased m6A modification and enhanced
stability of solute carrier family 7 member 11 (SLC7A11) mRNA,
conferring resistance to ferroptosis (78). m5C methylation is another way to
regulate mRNA stability. Lactylation at K508 of NOP2/Sun RNA
methyltransferase 2 (NSUN2) catalyzes m5C formation, maintaining
GCLC mRNA stability, promoting GCLC-dependent glutathione synthesis
and avoiding ferroptosis in cancer cells within the acidic tumor
microenvironment (55).
Furthermore, in epigenomics, lactylation can regulate gene
transcription through key proteins beyond histones. For instance,
lactylation at K503 of lysine-specific demethylase 1 (LSD1)
enhances the formation of a stable complex with transcription
factor FosL1, specifically enriching at the ferroportin receptor
TFRC promoter region to suppress TFRC transcription, reduce iron
uptake and enhance ferroptosis resistance (79).
As an emerging metabolic-epigenetic regulatory
mechanism, Kla plays a core role in reshaping cell fate by
targeting ferroptosis in inflammatory injury, neurodegenerative
diseases, cancer and I/R injury, altering disease outcomes through
actions on key targets. This section integrates the latest research
progress on Kla-mediated ferroptosis in diseases, discusses the
underlying pathological mechanisms and explores translational
therapeutic potential (Table
I).
Lactylation drives pro-ferroptotic pathways
promoting disease progression. Previous studies have suggested that
the inhibition of ferroptosis holds broad prospects for the
prevention and treatment of inflammatory diseases. Lactate, the end
product of glycolysis, accumulates in the extracellular
environment, and the resulting acidosis is a hallmark of
inflammatory diseases. Lactate in the microenvironment promotes
inflammatory disease progression via Kla-mediated ferroptosis,
rendering the inhibition of glycolysis and ferroptosis a promising
therapeutic approach (7,80-82). Sepsis is a major cause of
mortality in infectious diseases, and among organs susceptible to
its harmful effects, the lungs are most frequently affected
(83,84). Research indicates that
lactylation-mediated ferroptosis promotes sepsis-associated lung
injury. In sepsis-associated acute respiratory distress syndrome,
H3K14la in pulmonary vascular endothelial cells transcriptionally
activates TFRC and suppresses ferroportin SLC40A1, leading to iron
overload and mitochondrial-damaging ferroptosis, promoting disease
progression. Oxamate, an LDHA inhibitor reducing lactate
production, lowers H3K14la modification levels, subsequently
downregulating TFRC transcription, reducing iron uptake and
alleviating endothelial cell ferroptosis (52). Sepsis-induced lung injury further
reveals the core role of the H3K18la-METTL3-ACSL4 axis, inducing
mitochondrial fatty acid oxidation-dependent ferroptosis; the
METTL3 inhibitor, STM2457, effectively blocks this pathway
(63). Severe acute pancreatitis
(SAP) and non-alcoholic steatohepatitis (NASH) are non-infectious
inflammatory diseases. In SAP, aberrant glycolysis causes lactate
accumulation, enhancing HIF1A promoter activity via histone H3K18
lactylation, which transcriptionally activates the
ACSL4/lysophosphatidylcholine acyltransferase 3/arachidonate
lipoxygenase pathway, inducing pancreatic acinar cell ferroptosis
and ultimately exacerbating organ damage. As previously
demonstrated, combining a glycolysis inhibitor with a ferroptosis
inducer (Ferrostatin-1) significantly alleviates organ damage
(85). Previous research has
also confirmed that Qing Xia Jie Yi Formula granules alleviate
acute pancreatitis by inhibiting the glycolysis pathway (86). In NASH, irisin (TEC) recruits
HDAC1 via tRF-31R9J to clear H3K18la, suppressing ferroptosis
driver genes such as ATF3/CHAC1, protecting hepatocytes from
ferroptosis and providing a novel intervention target for metabolic
liver disease (66). These
findings establish the 'lactylation-pro-ferroptosis gene
activation' axis as a hub in the inflammatory cascade. The
inhibition of glycolysis and lactylation may thus be a strategy for
the treatment of infectious and inflammatory diseases.
Ferroptosis is a critical factor in degenerative
diseases and its pharmacological inhibition is a therapeutic target
for these conditions (87-89). Lactylation is a novel mechanism
regulating ferroptosis, and modulating this process provides new
hope for treatment. In degenerative diseases, one disease-promoting
mechanism involves lactylation-mediated iron overload to trigger
ferroptosis by regulating key genes in iron homeostasis. Research
on Alzheimer's disease has found that tau protein de-lactylation
(K677R mutation) inhibits p38 phosphorylation, downregulates NCOA4
and reduces ferritinophagy-dependent free iron release, thereby
alleviating neuronal ferroptosis and improving cognitive function
(73). Breakthrough research in
osteoporosis (OP) revealed significantly downregulated CRYAB/FTH1
expression in patients with OP. CRYAB de-lactylation promotes
ferroptosis in bone marrow-derived mesenchymal stem cells and
inhibits osteogenic differentiation (74). These studies highlight the impact
of lactylation on cell survival and disease progression via iron
homeostasis regulation. Promoting ACSL4 expression via lactylation
is another key mechanism regulating ferroptosis in degenerative
diseases. LDHB promotes H3K18la and ACSL4 expression by increasing
microenvironment lactylation levels, inducing chondrocyte
ferroptosis and further promoting osteoarthritis development;
inhibiting glycolysis alleviates osteoarthritis (90). During inflammation-induced human
intervertebral disc degeneration, the shift from aerobic to
anaerobic metabolism produces lactate that not only promotes
H3K18la and ACSL4 transcription, but also increases ACSL4
lactylation at K412. Concurrently, lactate reduces the expression
of sirtuin 3 (a Kla eraser), suppressing the clearance of ACSL4
lactylation. These factors increase cellular ferroptosis and
promote disease progression (91).
Ferroptosis is recognized as a cause of reperfusion
injury and organ failure, and the development of ferroptosis
modulators provides novel opportunities for the treatment of I/R
injury (92). Lactate
accumulation is a hallmark of ischemia, and lactate-driven
ferroptosis is often a key factor in disease progression. The
inhibition of lactylation-mediated ferroptosis has been shown to
exert positive effects in various studies on diseases. Research
indicates that lactate accumulation upregulates METTL3 expression
via EP300-mediated H3K18la, subsequently accelerating SLC7A11 mRNA
degradation in an m6A modification- and YTH N6-methyladenosine RNA
binding protein F2-dependent manner, ultimately inducing macrophage
ferroptosis and promoting coronary heart disease progression
(50). In myocardial I/R,
enhanced glycolysis and lactate accumulation promote the
lactylation of GPX4 at K218 and K228, destabilizing GPX4 and
enhancing cardiomyocyte sensitivity to ferroptosis, exacerbating
injury (70). Dexmedetomidine
provides a novel cardioprotective strategy by inhibiting lactate
production, blocking MDH2 K241 lactylation, restoring TCA cycle
function and upregulating GPX4 (76). In intestinal ischemia,
mitochondrial alanyl-tRNA synthetase 2 epigenetically upregulates
ACSL4 transcriptional activity by enhancing H3K18la, driving lipid
peroxidation and ferroptosis to worsen intestinal injury (93). In mice with intestinal I/R injury
and intestinal epithelial cells subjected to hypoxia-reoxygenation,
the nuclear translocation of pyruvate kinase M2 (PKM2) dimers
promotes the expression of glycolytic key enzymes (GLUT1/enolase
1/LDHA) and lactate accumulation. Lactate drives histone modifier
p300 to specifically catalyze H4K12la, enriching at the promoter
region to significantly enhance high-mobility group box 1 (HMGB1)
transcription. This disrupts the ferroptosis regulatory balance
(ACSL4↑/GPX4↓), promoting intestinal damage. Targeting PKM2
dimerization (ML265) or HMGB1 (Gly-cyrrhizin) blocks this pathway
and alleviates tissue injury (59). Research on liver I/R has revealed
that lactate activates PCK2 K100 lactylation, stabilizing
mitochondrial fatty acid synthase OXSM, and promoting lipid
peroxidation and ferroptosis. KAT8 inhibitors or adeno-associated
virus-short hairpin RNA targeting PCK2 significantly reduce liver
injury (75). Intracerebral
hemorrhage causes lactate accumulation and an acidic
microenvironment within neurons. Lactate, mediated by histone
acetyltransferases p300/CBP, specifically promotes lactylation at
the H3K14 site. H3K14la functions as an epigenetic mark, directly
inhibiting the transcription of the calcium pump plasma membrane
Ca2+ ATPase 2 gene, hindering intracellular calcium
efflux, causing calcium overload and exacerbating neuronal
ferroptosis. The elevated lactate microenvironment induces the
lactylation of METTL3 protein, increasing m6A methylation on TFRC
mRNA, accelerating cellular iron uptake, leading to Fe2+
accumulation, elevated ROS and malondialdehyde levels, and
ultimately inducing ferroptosis (77,94). In diabetic nephropathy, a
high-lactate microenvironment inhibits the E3 ubiquitin ligase
activity of tripartite motif-containing protein 65 (TRIM65) via
p300-mediated lactylation at K206, preventing the degradation of
its two key substrates, glycolytic kinase pyruvate dehydrogenase
kinase 4 and iron regulatory protein iron-responsive
element-binding protein 2. This promotes glycolytic flux and
induces iron overload and lipid peroxidation, activating
ferroptosis. Targeting the TRIM65-K206 lactylation site or reducing
lactate production via sodium-glucose cotransporter 2 inhibitors
restores the dual inhibition of ferroptosis and glycolysis by
TRIM65 (58). However, lactate
accumulation is not always detrimental to tissue injury. In spinal
cord injury, deubiquitinase UCHL1 stabilizes the key glycolytic
enzyme PFKFB3 by cleaving its K48-linked ubiquitin chains,
enhancing glycolysis and lactate production in astrocytes. The
produced lactate serves as an energy substrate for neurons and
inhibits neuronal ferroptosis. Furthermore, elevated lactate levels
induce H4K8la in astrocytes, promoting UCHL1 and glycolytic gene
(e.g., PKM and LDHB) transcription, forming a positive feedback
loop that amplifies the protective effect (62).
Under both hypoxic and aerobic conditions, tumor
cells prefer glycolysis over oxidative phosphorylation as their
primary energy source to meet rapid growth and proliferation
demands, resulting in high glycolytic flux, massive lactate
production and an acidic tumor microenvironment (94-98). Lactate accumulation in the tumor
microenvironment promotes tumor cell invasion, metastasis and drug
resistance by facilitating lactylation (12,79,99-101). Lactylation regulates key
ferroptosis molecules through diverse targets, serving not only as
a survival strategy for tumor cells to adapt to the
microenvironment, but also as a crucial mechanism to evade
therapeutic pressure.
Lactate is considered the initiator of
lactylation-regulated ferroptosis. Therefore, reducing lactate
production by inhibiting key glycolytic enzymes can suppress tumor
growth. Aberrant glycolysis in lung cancer cells causes lactate
accumulation, inducing histone H3K18 lactylation at the AIM2 gene
promoter region, significantly enhancing AIM2 transcription. This
promotes the ubiquitin-mediated degradation of the transcription
factor STAT5B, downregulating its downstream target gene ACSL4 and
ultimately inhibiting ferroptosis. The natural compound Shikonin
can target and inhibit PKM2 (a key glycolytic enzyme) and AIM2
expression, reversing histone lactylation-mediated AIM2 activation
(65). Cancer-associated
fibroblasts enhance histone H3K18 lactylation by secreting lactate,
activating ZFP64, which promotes GCH1 and FTH1 transcription. GCH1
reduces ROS accumulation by inhibiting lipid peroxidation, and FTH1
reduces iron-dependent lipid peroxidation triggered by sequestering
free Fe2+; both synergistically inhibit ferroptosis,
enhancing tumor cell drug resistance. The lactate production
inhibitor Oxamate can reverse this resistant phenotype (60). ZNF207 increases lactate levels by
upregulating LDHA expression, inducing lactylation at lysine 67
(K67 lactylation) of antioxidant protein peroxiredoxin 1 (PRDX1).
Lactylated PRDX1 binds the transcription factor nuclear factor
erythroid 2-related factor 2 (NRF2) and promotes its nuclear
translocation, activating the NRF2 antioxidant pathway and
upregulating ferroptosis inhibitor genes, such as SLC7A11, GPX4 and
HMOX1, ultimately causing drug resistance in hepatocellular
carcinoma (HCC) cells. Knockdown of ZNF207, blocking PRDX1
lactylation at K67, or inhibition of NRF2 activity, restore
regorafenib sensitivity and induce ferroptosis (102).
The inhibition of the lactylation of key ferroptosis
proteins is considered an effective anti-cancer strategy, as it can
promote ferroptosis and suppress tumor growth. Lactate maintains
GCLC mRNA stability via the NAA10-mediated lactylation of NSUN2
K508, evading cancer cell ferroptosis in the acidic tumor
microenvironment (55). The
re-activation of glycolysis causes lactate re-accumulation,
inducing lactylation at lysine 503 of LSD1, blocking
TRIM21-mediated LSD1 degradation and altering its genomic
localization. The inhibition of H3K4me2 modification suppresses
TFRC transcription, reducing cellular iron uptake, inhibiting
ferroptosis and promoting the survival of resistant cells.
Inhibition of LSD1 activates ferroptosis and effectively suppresses
tumor growth (79). HDAC
inhibitors reduce the lactylation of HDAC1 at K412, which in turn
activates the transcription of the m6A demethylases, FTO and ALKBH5
via H3K27ac. This reduces m6A modification on ferroptosis
suppressor protein FSP1 mRNA, accelerating its degradation and
ultimately enhancing lipid peroxide accumulation and sensitivity to
ferroptosis. Vorinostat (SAHA) and Trichostatin A (TSA) both
significantly reduce HDAC1 K412 lactylation and inhibit tumor
growth (56). As previously
demonstrated, following incomplete microwave ablation, a sublethal
temperature zone forms in the residual tumor area, triggering
enhanced glycolysis and lactate accumulation in HCC cells,
specifically upregulating H3K18la. This enhances NFS1
transcription, suppressing lipid peroxidation and ferroptosis by
maintaining iron ion homeostasis, thereby enhancing the metastatic
capacity of HCC cells. NFS1 deletion combined with oxaliplatin
(OXA) synergistically inhibits HCC metastasis and overcomes
resistance to OXA (67).
Evodiamine inhibits monocarboxylate transporter 4 (MCT4) function
to block lactate signaling, directly reducing H3K18la to restore
Semaphorin 3A transcription and suppress programmed cell death
ligand 1. It also downregulates GPX4, inducing ferroptosis, and
ultimately inhibiting tumor growth (103).
Lactate enhances the resistance of tumor cells to
ferroptosis through lactyltransferase-mediated lactylation.
Therefore, the inhibition of lactyltransferase activity to promote
ferroptosis is a potential strategy for cancer therapy. Driven by
AKT hyperactivation, phosphorylated PCK1 interacts with LDHA,
enhancing glycolytic activity and lactate production, promoting the
KAT7-mediated lactylation of SPRING at K82. This subsequently
activates the SREBP2-driven mevalonate pathway, promoting
resistance to ferroptosis (53).
Lactyltransferase p300 catalyzes H4K12la, which transcriptionally
activates GCLC, enhancing glutathione synthesis, inhibiting lipid
peroxide accumulation and ultimately blocking the ferroptosis
pathway. The inhibition of p300, LDHA or GCLC enhances sensitivity
to chemotherapy (51). KRAS G12D
mutation enhances LDHA phosphorylation via the MEK/ERK pathway,
promoting glycolysis and lactate generation. This induces the
ACAT2-mediated lactylation of glutamate-cysteine ligase modifier
subunit (GCLM) at K34, elevating GCL activity, increasing GSH
synthesis and conferring ferroptosis resistance to KRASG 12D-mutant
cancer cells. Inhibiting ACAT2 or mutating GCLM-K34R restores the
sensitivity of cancer cells to ferroptosis inducers (e.g.,
erastin/RSL3) and significantly suppresses tumor growth (54).
As an emerging epigenetic regulatory mechanism, the
precise detection of lactylation is crucial for elucidating its
functions in physiological and pathological processes. The
detection of lactylation primarily relies on mass spectrometry and
specific antibody-based detection. Mass spectrometry, particularly
liquid chromatography-tandem mass spectrometry, serves as the core
method, enabling the highly sensitive identification of specific
sites and quantification of modification levels. This method
involves digesting protein samples with proteases to generate a
mixture of peptides. These peptides are then separated by liquid
chromatography and are subjected to precise mass detection and
sequence analysis via mass spectrometry. This allows for the
unbiased identification of specific lactylation sites and their
absolute or relative quantification. However, challenges include
potential misidentification due to the similar chemical properties
of lactylation and acetylation, and the need for higher-resolution
instruments due to low modification abundance (11,100,104-107). Specific antibody-based
detection utilizes antibodies targeting specific modification
sites. Techniques such as western blot analysis for quantification,
immunofluorescence for observing intranuclear distribution and
co-immunoprecipitation for exploring protein interactions enable
the in situ and intuitive observation of the subcellular
localization and tissue distribution characteristics of
lactylation. This approach provides advantages, such as operational
simplicity and high sensitivity; however, it is associated with
risks of non-specific binding and the limited availability of
commercial antibodies (108-112). The comprehensive application of
these methods provides key technical support for elucidating the
role of lactylation in tumorigenesis and development, laying the
foundation for its clinical translation.
The intricate interplay between lactylation and
ferroptosis has positioned the Kla-ferroptosis axis as an
attractive novel therapeutic frontier across a broad spectrum of
human diseases. Although basic research continues to uncover the
enzymatic regulation, site-specific functions and context-dependent
outcomes of this axis, its translational potential demands focused
exploration. Looking ahead, clinical development targeting this
axis may proceed through the following strategies: First, directly
targeting lactylation regulatory mechanisms holds significant
promise. Developing inhibitors of specific lactyltransferases
('writers') or activators of delactylases ('erasers') could enable
precise control over pro-ferroptotic or anti-ferroptotic
lactylation events (17,20). For example, p300/CBP inhibitors
(e.g., SGC-CBP30, A-485) and KAT8 inhibitors (KAT8-IN-1/MC4033) may
disrupt lactylation that drives disease progression (51,75). Conversely, deacetylase inhibitors
(SAHA, TSA and TEC) can modulate lactylation and sensitize cells to
ferroptosis, highlighting the therapeutic feasibility of targeting
'erasers' (56,66). Second, regulating lactate
metabolism provides a broader, yet effective strategy with which to
indirectly modulate the entire Kla-ferroptosis axis (7,101). Inhibiting key glycolytic
enzymes (Oxamate, 2-DG, Shikonin and Simvastatin) or disrupting
lactate transport via monocarboxylate transporters (MCTs), e.g.,
using Syrosingopine to inhibit MCT4, can effectively deplete
lactate, the fundamental driver of lactylation (60,65,103). This approach is particularly
attractive in tumors and inflammatory microenvironments
characterized by a high glycolytic flux (12,82).
To date, to the best of our knowledge, no clinical
studies have been designed with 'direct regulation of protein
lactylation' as the primary endpoint. However, some drugs targeting
lactylation-regulating enzymes and lactate metabolism-related
proteins have entered clinical trials. Among them, the most
advanced is CCS1477 (inobrodib). It is a highly effective p300/CBP
bromodomain inhibitor. Its phase I/IIa clinical trials in advanced
solid tumors and hematological malignancies have been initiated
(NCT04068597) (113,114). In patients with metastatic
castration-resistant prostate cancer (mCRPC), CCS1477 monotherapy
was shown to reduce the expression of key oncogenic protein MYC and
proliferation marker Ki-67 in tumor tissues (113). In trials for
relapsed/refractory acute myeloid leukemia and multiple myeloma,
the drug demonstrated the ability to induce leukemia cell
differentiation and significantly reduce myeloma-related
serum/urine biomarkers. Notably, in the hematological malignancy
cohort, about one-third of heavily pretreated patients responded to
CCS1477 monotherapy. Some of these patients had progression-free
survival exceeding 12 months (114). Another potent p300/CBP
bromodomain inhibitor, FT-7051, has also shown early clinical
progress. Early results from an open-label phase I trial in
patients with mCRPC indicated that its monotherapy achieved the
effective exposure threshold predicted by
pharmacokinetic/pharmacodynamic models. However, safety concerns
exist. Approximately 80% of patients experienced mild to moderate
treatment-related adverse events, including hyperglycemia (115). NEO2734 (EP31670) is a dual
bromodomain inhibitor targeting both p300/CBP and bromodomain and
extra-terminal motif proteins. Due to its broader mechanism, it has
entered a phase I trial. This trial aims to treat patients with
advanced cancer, including those with metastatic nuclear protein in
testis midline carcinoma and refractory mCRPC (NCT05488548); the
results are not yet available. Furthermore, drug development
targeting HDAC has advanced significantly. Currently, five HDAC
inhibitors are approved for clinical use in hematological
malignancies. These include Vorinostat and Romidepsin for cutaneous
T-cell lymphoma, Belinostat for peripheral T-cell lymphoma,
Panobinostat for relapsed/refractory multiple myeloma and
Tucidinostat for peripheral T-cell lymphoma (116-119). In solid tumors, HDAC inhibitor
monotherapy shows limited efficacy. However, combination strategies
with other agents hold promise. For example, the selective HDAC
inhibitors Tucidinostat and Entinostat combined with endocrine
therapy (e.g., Exemestane) improved progression-free survival and
the objective response rate in hormone receptor-positive breast
cancer trials (120,121). Combining HDAC inhibitors with
immune checkpoint inhibitors also demonstrated synergistic
anti-tumor activity in various solid tumors, such as melanoma and
colorectal cancer (122,123).
Challenges and failures exist in this field. Some trial results did
not meet primary endpoints. For instance, Mocetinostat monotherapy
was ineffective in urothelial carcinoma. Entinostat combined with
Atezolizumab did not significantly prolong progression-free
survival in advanced triple-negative breast cancer (124). Additionally, HDAC inhibitor
applications are expanding into non-oncological areas. Romidepsin
is being explored for HIV latency reversal (125), and Givinostat and Ricolinostat
show potential in clinical studies for Duchenne muscular dystrophy
and diabetic neuropathic pain, respectively (126,127). Overall, further development of
HDAC inhibitors in solid tumors still faces toxicity issues. Future
efforts should focus on optimizing combination strategies and
developing more subtype-selective inhibitors to enhance their
therapeutic value.
Research on lactate metabolism-related proteins
remains limited. Glycolysis is the primary source of lactate.
2-deoxy-D-glucose (2-DG) is a broad-spectrum glycolysis inhibitor.
Intranasal administration of 3.5% 2-DG was safe and well-tolerated
in healthy volunteers. Systemic exposure was almost negligible.
Local nasal concentrations reached effective anti-viral levels
in vitro (NCT05314933) (128). LDHA is a key enzyme for lactate
production in glycolysis. Its inhibitors show extensive
pre-clinical evidence across multiple cancers. They can inhibit
glycolysis, reduce lactate and acidification, suppress invasion and
metastasis, and enhance chemo/radiotherapy sensitivity. However,
most findings are still at the pre-clinical stage (129). For lactate transmembrane
transport inhibition, only one MCT-targeting drug, AZD3965 (an MCT1
inhibitor), has entered clinical trials. It aims to evaluate safety
and preliminary efficacy in patients with advanced cancer
(NCT01791595). Preliminary results indicate manageable safety
(130-132). Specific MCT4 inhibitors are
mostly in pre-clinical development. However, dual inhibition or
combination with LDH/GLUT inhibitors appears more promising. This
approach may sustainably reduce lactate supply, improve the acidic
microenvironment and restore sensitivity to ferroptosis (133).
In summary, clinical interventions directly
regulating lactylation are still emerging. However, drugs targeting
its key nodes have established a considerable clinical and
translational foundation. Based on metabolic resistance mechanisms
and solid tumor treatment experience, it may be recommended to
prioritize combination therapies that integrate
metabolic-epigenetic-ferroptosis signaling. This should be
accompanied by biomarker stratification and optimized
administration strategies, such as intranasal delivery for high
lesion exposure and low systemic exposure. These steps will help
accelerate the clinical translation of the Kla-ferroptosis
axis.
Notably, the cross-regulatory mechanisms between
lactylation and other programmed cell death pathways are
increasingly becoming a focus of research. Beyond ferroptosis, Kla
may modulate processes such as necroptosis, apoptosis and autophagy
by modifying key proteins, forming a complex network governing cell
fate decisions (134). For
instance, preliminary studies suggest that lactate accumulation can
influence the activity of receptor interacting serine/threonine
kinase 3, a core necroptosis protein, via lactylation, thereby
modulating cell death patterns in inflammatory diseases (135). In tumor models, Kla may promote
cell survival and resistance to therapy by inhibiting apoptotic
signaling pathways (e.g., BCL-2 family proteins) or enhancing the
stability of autophagy-related proteins (e.g., light chain 3)
(136,137). Such cross-regulation not only
reveals the central role of metabolic-epigenetic networks in
multiple death pathways, but also provides new insight for
developing combination therapies targeting lactylation. Future
studies are required to systematically elucidate the site-specific
functions of Kla in necroptosis, apoptosis and autophagy, and
clarify the dynamic interactions among these pathways to more
comprehensively evaluate the therapeutic potential of targeting
Kla.
However, the field still faces multiple challenges:
Structural similarities between lactylation-regulating enzymes and
other acyltransferases/deacetylases necessitate highly specific
drugs to avoid off-target effects on PTMs such as acetylation; the
dual role of the Kla-ferroptosis axis in diseases (e.g., promoting
injury in sepsis while exerting protection in spinal cord injury)
calls for cell- and microenvironment-specific targeted delivery
systems; notably, dynamic detection technologies for lactylation
remain underdeveloped, requiring novel in vivo imaging
probes to facilitate patient stratification and treatment
monitoring (108,112,138). Further studies are thus
required to focus on designing highly specific modulators,
constructing intelligent delivery systems and validating dynamic
lactylation biomarkers. This may broaden the understanding of cell
fate regulation mechanisms and may provide novel paradigms for the
treatment of cancer, as well as inflammatory, degenerative and
ischemic diseases.
Not applicable.
All authors contributed to the study conception and
design. Material preparation, data collection and analysis were
performed by ZL, YZ and SZ. The first draft of the manuscript was
written by ZL and KW. All authors commented on previous versions of
the manuscript. Data authentication is not applicable. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
No funding was received.
|
1
|
Miao C, Huang Y, Zhang C, Wang X, Wang B,
Zhou X, Song Y, Wu P, Chen ZS and Feng Y: Post-translational
modifications in drug resistance. Drug Resist Updat. 78:1011732025.
View Article : Google Scholar
|
|
2
|
Hirano A, Fu YH and Ptáček LJ: The
intricate dance of post-translational modifications in the rhythm
of life. Nat Struct Mol Biol. 23:1053–1060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tharuka MDN, Courelli AS and Chen Y:
Immune regulation by the SUMO family. Nat Rev Immunol. 25:608–620.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhong Q, Xiao X, Qiu Y, Xu Z, Chen C,
Chong B, Zhao X, Hai S, Li S, An Z and Dai L: Protein
posttranslational modifications in health and diseases: Functions,
regulatory mechanisms, and therapeutic implications. MedComm.
4:e2612023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Hu J, Wu S, Fleishman JS, Li Y, Xu
Y, Zou W, Wang J, Feng Y, Chen J and Wang H: Targeting epigenetic
and posttranslational modifications regulating ferroptosis for the
treatment of diseases. Signal Transduct Target Ther. 8:4492023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rabinowitz JD and Enerbäck S: Lactate: The
ugly duckling of energy metabolism. Nat Metab. 2:566–571. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brooks GA: The science and translation of
lactate shuttle theory. Cell Metab. 27:757–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan, Yanxiang Guo J, et
al: Glucose feeds the TCA cycle via circulating lactate. Nature.
551:115–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brooks GA, Osmond AD, Arevalo JA, Duong
JJ, Curl CC, Moreno-Santillan DD and Leija RG: Lactate as a myokine
and exerkine: drivers and signals of physiology and metabolism. J
Appl Physiol (1985). 134:529–548. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao S, Chai H, Tao T, Zhang L, Yang X, Li
X, Yi Z, Wang Y, An J, Wen G, et al: Role of lactate and lactate
metabolism in liver diseases (Review). Int J Mol Med. 54:592024.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Sun L, Gao P and Hu H: Lactylation
in cancer: Current understanding and challenges. Cancer Cell.
42:1803–1807. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Huang Z, Chen Y, Tian H, Chai P,
Shen Y, Yao Y, Xu S, Ge S and Jia R: Lactate and lactylation in
cancer. Signal Transduct Target Ther. 10:382025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Huang L, Gu Y, Cang W, Sun P and
Xiang Y: Lactate-lactylation hands between metabolic reprogramming
and immunosuppression. Int J Mol Sci. 23:119432022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Zhao F and Qu Y: Lactylation: A
novel post-translational modification with clinical implications in
CNS Diseases. Biomolecules. 14:11752024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li R, Yang Y, Wang H, Zhang T, Duan F, Wu
K, Yang S, Xu K, Jiang X and Sun X: Lactate and lactylation in the
brain: Current progress and perspectives. Cell Mol Neurobiol.
43:2541–2555. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng X and Du J: Histone and non-histone
lactylation: Molecular mechanisms, biological functions, diseases,
and therapeutic targets. Mol Biomed. 6:382025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Certo M, Llibre A, Lee W and Mauro C:
Understanding lactate sensing and signalling. Trends Endocrinol
Metab. 33:722–735. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shang S, Liu J and Hua F: Protein
acylation: Mechanisms, biological functions and therapeutic
targets. Signal Transduct Target Ther. 7:3962022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan Z, Liu Z, Zhang N, Wei W, Cheng K, Sun
H and Hao Q: Identification of SIRT3 as an eraser of H4K16la.
iScience. 26:1077572023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreno-Yruela C, Zhang D, Wei W, Bæk M,
Liu W, Gao J, Danková D, Nielsen AL, Bolding JE, Yang L, et al:
Class I histone deacetylases (HDAC1-3) are histone lysine
delactylases. Sci Adv. 8:eabi66962022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Huang X, Yang Y, Sun Y, Zhao Y,
Zhang Z, Qiu D, Wu Y, Wu G and Lei L: Dux activates
metabolism-lactylation-MET network during early iPSC reprogramming
with Brg1 as the histone lactylation reader. Nucleic Acids Res.
52:5529–5548. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: an iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng J and Conrad M: Ferroptosis: When
metabolism meets cell death. Physiol Rev. 105:651–706. 2025.
View Article : Google Scholar
|
|
26
|
Wang Z, Wu C, Yin D and Do K: Ferroptosis:
Mechanism and role in diabetes-related cardiovascular diseases.
Cardiovasc Diabetol. 24:602025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao L, Zhou X, Xie F and Zhang L, Yan H,
Huang J, Zhang C, Zhou F, Chen J and Zhang L: Ferroptosis in cancer
and cancer immunotherapy. Cancer Commun (Lond). 42:88–116. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar :
|
|
29
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar
|
|
31
|
Alves F, Lane D, Nguyen TPM, Bush AI and
Ayton S: In defence of ferroptosis. Signal Transduct Target Ther.
10:22025. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar :
|
|
33
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG and
Gao LC: System Xc -/GSH/GPX4 axis: An important antioxidant system
for the ferroptosis in drug-resistant solid tumor therapy. Front
Pharmacol. 13:9102922022. View Article : Google Scholar
|
|
35
|
Du Y and Guo Z: Recent progress in
ferroptosis: Inducers and inhibitors. Cell Death Discov. 8:5012022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pal D and Sandur SK: Novel role of
peroxiredoxin 6 in ferroptosis: Bridging selenium transport with
enzymatic functions. Free Radic Biol Med. 238:611–620. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ru Q, Li Y, Chen L, Wu Y, Min J and Wang
F: Iron homeostasis and ferroptosis in human diseases: mechanisms
and therapeutic prospects. Signal Transduct Target Ther. 9:2712024.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Zou L, Li X, Guo L, Hu B, Ye H
and Liu Y: SLC40A1 in iron metabolism, ferroptosis, and disease: A
review. WIREs Mech Dis. 16:e16442024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng D, Liu J, Piao H, Zhu Z, Wei R and
Liu K: ROS-triggered endothelial cell death mechanisms: Focus on
pyroptosis, parthanatos, and ferroptosis. Front Immunol.
13:10392412022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2022. View Article : Google Scholar
|
|
41
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pope LE and Dixon SJ: Regulation of
ferroptosis by lipid metabolism. Trends Cell Biol. 33:1077–1087.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tilton WM, Seaman C, Carriero D and
Piomelli S: Regulation of glycolysis in the erythrocyte: role of
the lactate/pyruvate and NAD/NADH ratios. J Lab Clin Med.
118:146–152. 1991.PubMed/NCBI
|
|
45
|
Ying W: NAD+/NADH and NADP+/NADPH in
cellular functions and cell death: Regulation and biological
consequences. Antioxid Redox Signal. 10:179–206. 2008. View Article : Google Scholar
|
|
46
|
Pucino V, Certo M, Bulusu V, Cucchi D,
Goldmann K, Pontarini E, Haas R, Smith J, Headland SE, Blighe K, et
al: Lactate buildup at the site of chronic inflammation promotes
disease by inducing CD4+ T cell metabolic rewiring. Cell Metab.
30:1055–1074.e8. 2019. View Article : Google Scholar
|
|
47
|
Reddy A, Winther S, Tran N, Xiao H, Jakob
J, Garrity R, Smith A, Ordonez M, Laznik-Bogoslavski D, Rothstein
JD, et al: Monocarboxylate transporters facilitate succinate uptake
into brown adipocytes. Nat Metab. 6:567–577. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding K, Liu C, Li L, Yang M, Jiang N, Luo
S and Sun L: Acyl-CoA synthase ACSL4: An essential target in
ferroptosis and fatty acid metabolism. Chin Med J (Engl).
136:2521–2537. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lan H, Gao Y, Zhao Z, Mei Z and Wang F:
Ferroptosis: Redox imbalance and hematological tumorigenesis. Front
Oncol. 12:8346812022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen J, Liu Z, Yue Z, Tan Q, Yin H, Wang
H, Chen Z, Zhu Y and Zheng J: EP300-mediated H3K18la regulation of
METTL3 promotes macrophage ferroptosis and atherosclerosis through
the m6A modification of SLC7A11. Biochim Biophys Acta Gen Subj.
1869:1308382025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deng J, Li Y, Yin L, Liu S, Li Y, Liao W,
Mu L, Luo X and Qin J: Histone lactylation enhances GCLC expression
and thus promotes chemoresistance of colorectal cancer stem cells
through inhibiting ferroptosis. Cell Death Dis. 16:1932025.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gong F, Zheng X, Xu W, Xie R, Liu W, Pei
L, Zhong M, Shi W, Qu H, Mao E, et al: H3K14la drives endothelial
dysfunction in sepsis-induced ARDS by promoting
SLC40A1/transferrin-mediated ferroptosis. MedComm. 6:e700492025.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu J, Xiong Y, Zhang Y, Liang H, Cheng K,
Lu Y, Cai G, Wu Y, Fan Y, Chen X, et al: Simvastatin overcomes the
pPCK1-pLDHA-SPRINGlac axis-mediated ferroptosis and
chemo-immunotherapy resistance in AKT-hyperactivated intrahepatic
cholangiocarcinoma. Cancer Commun (Lond). 45:1038–1071. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen Y, Yan Q, Ruan S, Cui J, Li Z, Zhang
Z, Yang J, Fang J, Liu S, Huang S, et al: GCLM lactylation mediated
by ACAT2 promotes ferroptosis resistance in
KRASG12D-mutant cancer. Cell Rep. 44:1157742025.
View Article : Google Scholar
|
|
55
|
Niu K, Chen Z, Li M, Ma G, Deng Y, Zhang
J, Wei D, Wang J and Zhao Y: NSUN2 lactylation drives cancer cell
resistance to ferroptosis through enhancing GCLC-dependent
glutathione synthesis. Redox Biol. 79:1034792025. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Z, Su W, Zhang Q, Niu L, Feng B,
Zhang Y, Huang F, He J, Zhou Q, Zhou X, et al: Lactylation of HDAC1
confers resistance to ferroptosis in colorectal cancer. Adv Sci
(Weinh). 12:e24088452025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu J, Li Y, Ma R, Chen Y, Wang J, Zhang
L, Wang B, Zhang Z, Huang L, Zhang H, et al: Cold atmospheric
plasma drives USP49/HDAC3 axis mediated ferroptosis as a novel
therapeutic strategy in endometrial cancer via reinforcing
lactylation dependent p53 expression. J Transl Med. 23:4422025.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang G, Liu X, Li Y, Li L, Xiang J, Liang
Z, Jiang M and Yang S: TRIM65 as a key regulator of ferroptosis and
glycolysis in lactate-driven renal tubular injury and diabetic
kidney disease. Cell Rep. 44:1160912025. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu Z, Zhou Y, Li M, Xiao Z, Zhao Z and Li
Y: Dimeric PKM2 induces ferroptosis from intestinal
ischemia/reperfusion in mice by histone H4 lysine 12
lactylation-mediated HMGB1 transcription activation through the
lactic acid/p300 axis. Biochim Biophys Acta Mol Basis Dis.
1871:1679982025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang K, Guo L, Li X, Hu Y and Luo N:
Cancer-associated fibroblasts promote doxorubicin resistance in
triple-negative breast cancer through enhancing ZFP64 histone
lactylation to regulate ferroptosis. J Transl Med. 23:2472025.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lai WKM and Pugh BF: Understanding
nucleosome dynamics and their links to gene expression and DNA
replication. Nat Rev Mol Cell Biol. 18:548–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Millán-Zambrano G, Burton A, Bannister AJ
and Schneider R: Histone post-translational modifications-cause and
consequence of genome function. Nat Rev Genet. 23:563–580. 2022.
View Article : Google Scholar
|
|
63
|
Wu D, Spencer CB, Ortoga L, Zhang H and
Miao C: Histone lactylation-regulated METTL3 promotes ferroptosis
via m6A-modification on ACSL4 in sepsis-associated lung injury.
Redox Biol. 74:1031942024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liang Z, Liu J, Gou Y, Wang H, Li Z, Cao
Y, Zhang H, Bai R and Zhang Z: Elevated histone lactylation
mediates ferroptosis resistance in endometriosis through the
METTL3-Regulated HIF1A/HMOX1 signaling pathway. Adv Sci (Weinh).
12:e082202025. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu S, Liu M, Wang X and Wang S: The
histone lactylation of AIM2 influences the suppression of
ferroptosis by ACSL4 through STAT5B and promotes the progression of
lung cancer. FASEB J. 39:e703082025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhu J, Wu X, Mu M, Zhang Q and Zhao X:
TEC-mediated tRF-31R9J regulates histone lactylation and
acetylation by HDAC1 to suppress hepatocyte ferroptosis and improve
non-alcoholic steatohepatitis. Clin Epigenetics. 17:92025.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang J, Xie H, Li J, Huang X, Cai Y, Yang
R, Yang D, Bao W, Zhou Y, Li T and Lu Q: Histone lactylation drives
liver cancer metastasis by facilitating NSF1-mediated ferroptosis
resistance after microwave ablation. Redox Biol. 81:1035532025.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xiong J, Ge X, Pan D, Zhu Y, Zhou Y, Gao
Y, Wang H, Wang X, Gu Y, Ye W, et al: Metabolic reprogramming in
astrocytes prevents neuronal death through a UCHL1/PFKFB3/H4K8la
positive feedback loop. Cell Death Differ. 32:1214–1230. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu J, Tang D and Kang R: Targeting GPX4
in ferroptosis and cancer: chemical strategies and challenges.
Trends Pharmacol Sci. 45:666–670. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Y, Yue Q, Song X, Du W and Liu R:
Hypoxia/reoxygenation-induced glycolysis mediates myocardial
ischemia-reperfusion injury through promoting the lactylation of
GPX4. J Cardiovasc Transl Res. 18:762–774. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Di Sanzo M, Quaresima B, Biamonte F,
Palmieri C and Faniello MC: FTH1 pseudogenes in cancer and cell
metabolism. Cells. 9:25542020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Muhoberac BB and Vidal R: Iron, ferritin,
Hereditary ferritinopathy, and neurodegeneration. Front Neurosci.
13:11952019. View Article : Google Scholar
|
|
73
|
An X, He J, Xie P, Li C, Xia M, Guo D, Bi
B, Wu G, Xu J, Yu W and Ren Z: The effect of tau K677 lactylation
on ferritinophagy and ferroptosis in Alzheimer's disease. Free
Radic Biol Med. 224:685–706. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tian B, Li X, Li W, Shi Z, He X, Wang S,
Zhu X, Shi N, Li Y, Wan P and Zhu C: CRYAB suppresses ferroptosis
and promotes osteogenic differentiation of human bone marrow stem
cells via binding and stabilizing FTH1. Aging (Albany NY).
16:8965–8979. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yuan J, Yang M, Wu Z, Wu J, Zheng K, Wang
J, Zeng Q, Chen M, Lv T, Shi Y, et al: The Lactate-Primed KAT8-PCK2
axis exacerbates hepatic ferroptosis during ischemia/reperfusion
injury by reprogramming OXSM-Dependent mitochondrial fatty acid
synthesis. Adv Sci (Weinh). 12:e24141412025. View Article : Google Scholar
|
|
76
|
She H, Hu Y, Zhao G, Du Y, Wu Y, Chen W,
Li Y, Wang Y, Tan L, Zhou Y, et al: Dexmedetomidine ameliorates
myocardial ischemia-reperfusion injury by inhibiting MDH2
lactylation via regulating metabolic reprogramming. Adv Sci
(Weinh). 11:e24094992024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang L, Wang X, Che W, Zhou S and Feng Y:
METTL3 silenced inhibited the ferroptosis development via
regulating the TFRC levels in the Intracerebral hemorrhage
progression. Brain Res. 1811:1483732023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Qu S, Feng B, Xing M, Qiu Y, Ma L, Yang Z,
Ji Y, Huang F, Wang Y, Zhou J, et al: PRMT5 K240lac confers
ferroptosis resistance via ALKBH5/SLC7A11 axis in colorectal
cancer. Oncogene. 44:2814–2830. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li A, Gong Z, Long Y, Li Y, Liu C, Lu X,
Li Q, He X, Lu H, Wu K, et al: Lactylation of LSD1 is an acquired
epigenetic vulnerability of BRAFi/MEKi-resistant melanoma. Dev
Cell. 60:1974–1990.e11. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Deng L, He S, Guo N, Tian W, Zhang W and
Luo L: Molecular mechanisms of ferroptosis and relevance to
inflammation. Inflamm Res. 72:281–299. 2023. View Article : Google Scholar
|
|
81
|
Chen Y, Fang ZM, Yi X, Wei X and Jiang DS:
The interaction between ferroptosis and inflammatory signaling
pathways. Cell Death Dis. 14:2052023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fang Y, Li Z, Yang L, Li W, Wang Y, Kong
Z, Miao J, Chen Y, Bian Y and Zeng L: Emerging roles of lactate in
acute and chronic inflammation. Cell Commun Signal. 22:2762024.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Qiao X, Yin J, Zheng Z, Li L and Feng X:
Endothelial cell dynamics in sepsis-induced acute lung injury and
acute respiratory distress syndrome: pathogenesis and therapeutic
implications. Cell Commun Signal. 22:2412024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li W, Li D, Chen Y, Abudou H, Wang H, Cai
J, Wang Y, Liu Z, Liu Y and Fan H: Classic signaling pathways in
alveolar injury and repair involved in sepsis-induced ALI/ARDS: New
research progress and prospect. Dis Markers.
2022:63623442022.PubMed/NCBI
|
|
85
|
Zhang T, Huang X, Feng S and Shao H:
Lactate-Dependent HIF1A transcriptional activation exacerbates
severe acute pancreatitis through the ACSL4/LPCAT3/ALOX15 pathway
induced ferroptosis. J Cell Biochem. 126:e306872025. View Article : Google Scholar
|
|
86
|
Han X, Bao J, Ni J, Li B, Song P, Wan R,
Wang X, Hu G and Chen C: Qing Xia Jie Yi Formula granules
alleviated acute pancreatitis through inhibition of M1 macrophage
polarization by suppressing glycolysis. J Ethnopharmacol.
325:1177502024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang Y, Wu S, Li Q, Sun H and Wang H:
Pharmacological inhibition of ferroptosis as a therapeutic target
for neurodegenerative diseases and strokes. Adv Sci (Weinh).
10:e23003252023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sheng W, Liao S, Wang D, Liu P and Zeng H:
The role of ferroptosis in osteoarthritis: Progress and prospects.
Biochem Biophys Res Commun. 733:1506832024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang J, Chen T and Gao F: Mechanism and
application prospect of ferroptosis inhibitors in improving
osteoporosis. Front Endocrinol (Lausanne). 15:14926102024.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang Y, Zhao CY, Zhou Z, Li CC and Wang
Q: The effect of lactate dehydrogenase B and its mediated histone
lactylation on chondrocyte ferroptosis during osteoarthritis. J
Orthop Surg. 20:4932025. View Article : Google Scholar
|
|
91
|
Sun K, Shi Y, Yan C, Wang S, Han L, Li F,
Xu X, Wang Y, Sun J, Kang Z and Shi J: Glycolysis-derived lactate
induces ACSL4 Expression and lactylation to activate ferroptosis
during intervertebral disc degeneration. Adv Sci (Weinh).
12:e24161492025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li X, Ma N, Xu J, Zhang Y, Yang P, Su X,
Xing Y, An N, Yang F, Zhang G, et al: Targeting ferroptosis:
pathological mechanism and treatment of ischemia-reperfusion
injury. Oxid Med Cell Longev. 2021:15879222021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Dong W, Huang SX, Qin ML and Pan Z:
Mitochondrial alanyl-tRNA synthetase 2 mediates histone lactylation
to promote ferroptosis in intestinal ischemia-reperfusion injury.
World J Gastrointest Surg. 17:1067772025. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun T, Zhang JN, Lan T, Shi L, Hu L, Yan
L, Wei C, Hei L, Wu W, Luo Z, et al: H3K14 lactylation exacerbates
neuronal ferroptosis by inhibiting calcium efflux following
intracerebral hemorrhagic stroke. Cell Death Dis. 16:5532025.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Apostolova P and Pearce EL: Lactic acid
and lactate: Revisiting the physiological roles in the tumor
microenvironment. Trends Immunol. 43:969–977. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sun Q, Wu J, Zhu G, Li T, Zhu X, Ni B, Xu
B, Ma X and Li J: Lactate-related metabolic reprogramming and
immune regulation in colorectal cancer. Front Endocrinol.
13:10899182023. View Article : Google Scholar
|
|
97
|
Zhang W, Xia M, Li J, Liu G, Sun Y, Chen X
and Zhong J: Warburg effect and lactylation in cancer: Mechanisms
for chemoresistance. Mol Med. 31:1462025. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liao M, Yao D, Wu L, Luo C, Wang Z, Zhang
J and Liu B: Targeting the Warburg effect: A revisited perspective
from molecular mechanisms to traditional and innovative therapeutic
strategies in cancer. Acta Pharm Sin B. 14:953–1008. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Boedtkjer E and Pedersen SF: The acidic
tumor microenvironment as a driver of cancer. Annu Rev Physiol.
82:103–126. 2020. View Article : Google Scholar
|
|
100
|
Wang T, Ye Z, Li Z, Jing DS, Fan GX, Liu
MQ, Zhuo QF, Ji SR, Yu XJ, Xu XW and Qin Y: Lactate-induced protein
lactylation: A bridge between epigenetics and metabolic
reprogramming in cancer. Cell Prolif. 56:e134782023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sun Y, Wang H, Cui Z, Yu T, Song Y, Gao H,
Tang R, Wang X, Li B, Li W and Wang Z: Lactylation in cancer
progression and drug resistance. Drug Resist Updat. 81:1012482025.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yang T, Zhang S, Nie K, Cheng C, Peng X,
Huo J and Zhang Y: ZNF207-driven PRDX1 lactylation and NRF2
activation in regorafenib resistance and ferroptosis evasion. Drug
Resist Updat. 82:1012742025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yu Y, Huang X, Liang C and Zhang P:
Evodiamine impairs HIF1A histone lactylation to inhibit
Sema3A-mediated angiogenesis and PD-L1 by inducing ferroptosis in
prostate cancer. Eur J Pharmacol. 957:1760072023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tong Q, Huang C, Tong Q and Zhang Z:
Histone lactylation: A new frontier in laryngeal cancer research
(Review). Oncol Lett. 30:4212025. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bao C, Ma Q, Ying X, Wang F, Hou Y, Wang
D, Zhu L, Huang J and He C: Histone lactylation in macrophage
biology and disease: From plasticity regulation to therapeutic
implications. EBioMedicine. 111:1055022025. View Article : Google Scholar :
|
|
106
|
Bao Q, Wan N, He Z, Cao J, Yuan W, Hao H
and Ye H: Subcellular proteomic mapping of lysine lactylation. J Am
Soc Mass Spectrom. 35:3221–3232. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhao W, Zhang J, Chen K, Yuan J, Zhai L
and Tan M: Mass spectrometry-based characterization of histone
post-translational modification. Curr Opin Chem Biol.
88:1026222025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sun Y, Chen Y and Peng T: A bioorthogonal
chemical reporter for the detection and identification of protein
lactylation. Chem Sci. 13:6019–6027. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang X, Liu Y, Rekowski MJ and Wang N:
Lactylation of tau in human Alzheimer's disease brains. Alzheimers
Dement. 21:e144812025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chu X, Di C, Chang P, Li L, Feng Z, Xiao
S, Yan X, Xu X, Li H, Qi R, et al: Lactylated Histone H3K18 as a
potential biomarker for the diagnosis and predicting the severity
of septic shock. Front Immunol. 12:7866662022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nuñez R, Sidlowski PFW, Steen EA,
Wynia-Smith SL, Sprague DJ, Keyes RF and Smith BC: The TRIM33
bromodomain recognizes histone lysine lactylation. ACS Chem Biol.
19:2418–2428. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang Y, Fan J, Meng X, Shu Q, Wu Y, Chu
GC, Ji R, Ye Y, Wu X, Shi J, et al: Development of nucleus-targeted
histone-tail-based photoaffinity probes to profile the epigenetic
interactome in native cells. Nat Commun. 16:4152025. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Crabb S, Plummer R, Greystoke A, Carter L,
Pacey S, Walter H, Coyle VM, Knurowski T, Clegg K, Ashby F, et al:
560TiP a phase I/IIa study to evaluate the safety and efficacy of
CCS1477, a first in clinic inhibitor of p300/CBP, as monotherapy in
patients with selected molecular alterations. Ann Oncol.
32:S6172021. View Article : Google Scholar
|
|
114
|
Nicosia L, Spencer GJ, Brooks N, Ciceri F,
Wiseman DH, Pegg N, West W, Knurowski T, Frese K, Clegg K, et al:
Therapeutic targeting of EP300/CBP by bromodomain inhibition in
hematologic malignancies. Cancer Cell. 41:2136–2153.e13. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Armstrong AJ, Gordon MS, Reimers MA,
Sedkov A, Lipford K, Snavely-Merhaut J, Kumar S, Guichard SM and
Shore N: The courage study: A first-in-human phase 1 study of the
CBP/p300 inhibitor FT-7051 in men with metastatic
castration-resistant prostate cancer. J Clin Oncol. 39:TPS50852021.
View Article : Google Scholar
|
|
116
|
Marks PA and Breslow R: Dimethyl sulfoxide
to vorinostat: Development of this histone deacetylase inhibitor as
an anti-cancer drug. Nat Biotechnol. 25:84–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Campbell P and Thomas CM: Belinostat for
the treatment of relapsed or refractory peripheral T-cell lymphoma.
J Oncol Pharm Pract. 23:143–147. 2017. View Article : Google Scholar
|
|
118
|
Tzogani K, van Hennik P, Walsh I, De
Graeff P, Folin A, Sjöberg J, Salmonson T, Bergh J, Laane E, Ludwig
H, et al: EMA review of panobinostat (farydak) for the treatment of
adult patients with relapsed and/or refractory multiple myeloma.
Oncologist. 23:631–636. 2018. View Article : Google Scholar :
|
|
119
|
Lu X, Ning Z, Li Z, Cao H and Wang X:
Development of chidamide for peripheral T-cell lymphoma, the first
orphan drug approved in China. Intractable Rare Dis Res. 5:185–191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yardley DA, Ismail-Khan RR, Melichar B,
Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee
MJ and Trepel JB: Randomized phase II, double-blind,
placebo-controlled study of exemestane with or without entinostat
in postmenopausal women with locally recurrent or metastatic
estrogen receptor-positive breast cancer progressing on treatment
with a nonsteroidal aromatase inhibitor. J Clin Oncol.
31:2128–2135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui
S, Wang S, Ouyang Q, Yin Y, Geng C, et al: Tucidinostat plus
exemestane for postmenopausal patients with advanced, hormone
receptor-positive breast cancer (ACE): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:806–815. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Kitir B, Maolanon AR, Ohm RG, Colaço AR,
Fristrup P, Madsen AS and Olsen CA: Chemical editing of macrocyclic
natural products and kinetic profiling reveal slow, tight-binding
histone deacetylase inhibitors with picomolar affinities.
Biochemistry. 56:5134–5146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Whitehead L, Dobler MR, Radetich B, Zhu Y,
Atadja PW, Claiborne T, Grob JE, McRiner A, Pancost MR, Patnaik A,
et al: Human HDAC isoform selectivity achieved via exploitation of
the acetate release channel with structurally unique small molecule
inhibitors. Bioorg Med Chem. 19:4626–4634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang Y, Stowe RL, Pinello CE, Tian G,
Madoux F, Li D, Zhao LY, Li JL, Wang Y, Wang Y, et al:
Identification of histone deacetylase inhibitors with
benzoylhydrazide scaffold that selectively inhibit class I histone
deacetylases. Chem Biol. 22:273–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Rosás-Umbert M, Ruiz-Riol M, Fernández MA,
Marszalek M, Coll P, Manzardo C, Cedeño S, Miró JM, Clotet B, Hanke
T, et al: In vivo effects of romidepsin on T-cell activation,
apoptosis and function in the BCN02 HIV-1 kick&kill clinical
trial. Front Immunol. 11:4182020. View Article : Google Scholar
|
|
126
|
Sandoná M, Consalvi S, Tucciarone L, Puri
PL and Saccone V: HDAC inhibitors for muscular dystrophies:
Progress and prospects. Expert Opin Orphan Drugs. 4:125–127. 2016.
View Article : Google Scholar
|
|
127
|
Wang X, Shen X, Xu Y, Xu S, Xia F, Zhu B,
Liu Y, Wang W, Wu H and Wang F: The etiological changes of
acetylation in peripheral nerve injury-induced neuropathic
hypersensitivity. Mol Pain. 14:17448069187984082018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bhatt AN, Shenoy S, Munjal S, Chinnadurai
V, Agarwal A, Vinoth Kumar A, Shanavas A, Kanwar R and Chandna S:
2-deoxy-d-glucose as an adjunct to standard of care in the medical
management of COVID-19: A proof-of-concept and dose-ranging
randomised phase II clinical trial. BMC Infect Dis. 22:6692022.
View Article : Google Scholar
|
|
129
|
Sharma D, Singh M and Rani R: Role of LDH
in tumor glycolysis: Regulation of LDHA by small molecules for
cancer therapeutics. Semin Cancer Biol. 87:184–195. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Halford SER, Walter H, McKay P, Townsend
W, Linton K, Heinzmann K, Dragoni I, Brotherton L, Veal G, Siskos
A, et al: Phase I expansion study of the first-in-class
monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients
with diffuse large B-cell lymphoma (DLBCL) and burkitt lymphoma
(BL). J Clin Oncol. 39:31152021. View Article : Google Scholar
|
|
131
|
Halford SER, Jones P, Wedge S, Hirschberg
S, Katugampola S, Veal G, Payne G, Bacon C, Potter S, Griffin M, et
al: A first-in-human first-in-class (FIC) trial of the
monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients
with advanced solid tumours. J Clin Oncol. 35:25162017. View Article : Google Scholar
|
|
132
|
Halford S, Veal GJ, Wedge SR, Payne GS,
Bacon CM, Sloan P, Dragoni I, Heinzmann K, Potter S, Salisbury BM,
et al: A phase I dose-escalation study of AZD3965, an oral
monocarboxylate transporter 1 inhibitor, in patients with advanced
cancer. Clin Cancer Res. 29:1429–1439. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Singh M, Afonso J, Sharma D, Gupta R and
Kumar V, Rani R, Baltazar F and Kumar V: Targeting monocarboxylate
transporters (MCTs) in cancer: How close are we to the clinics?
Semin Cancer Biol. 90:1–14. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang W, Shan G, Bi G, Hu Z, Yi Y, Zeng D,
Lin Z and Zhan C: Lactylation and regulated cell death. Biochim
Biophys Acta Mol Cell Res. 1872:1199272025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gong T, Wang QD, Loughran PA, Li YH, Scott
MJ, Billiar TR, Liu YT and Fan J: Mechanism of lactic
acidemia-promoted pulmonary endothelial cells death in sepsis: role
for CIRP-ZBP1-PANoptosis pathway. Mil Med Res. 11:712024.PubMed/NCBI
|
|
136
|
Xu H, Li L, Wang S, Wang Z, Qu L, Wang C
and Xu K: Royal jelly acid suppresses hepatocellular carcinoma
tumorigenicity by inhibiting H3 histone lactylation at H3K9la and
H3K14la sites. Phytomedicine Int J Phytother Phytopharm.
118:1549402023.
|
|
137
|
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L,
Xu Y, He J, Lan J, Ou Q, et al: Tumor-derived lactate promotes
resistance to bevacizumab treatment by facilitating autophagy
enhancer protein RUBCNL expression through histone H3 lysine 18
lactylation (H3K18la) in colorectal cancer. Autophagy. 20:114–130.
2024. View Article : Google Scholar :
|
|
138
|
Sun Y, Chen Y, Xu Y, Zhang Y, Lu M, Li M,
Zhou L and Peng T: Genetic encoding of ε-N-L-lactyllysine for
detecting delactylase activity in living cells. Chem Commun (Camb).
58:8544–8547. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Jiang S, Li H, Zhang L, Mu W, Zhang Y,
Chen T, Wu J, Tang H, Zheng S, Liu Y, et al: Generic diagramming
platform (GDP): A comprehensive database of high-quality biomedical
graphics. Nucleic Acids Res. 53(D1): D1670–D1676. 2025. View Article : Google Scholar :
|