Atherosclerosis is a chronic inflammatory disease
characterized by endothelial dysfunction, lipid accumulation and
plaque formation within arterial walls, notably contributing to
cardiovascular morbidity and mortality on a global scale (1). According to the 2024 Heart Disease
and Stroke Statistics Update of American Heart Association,
cardiovascular diseases, for which atherosclerosis is the
underlying pathology in most cases, remain the leading cause of
death globally (2). Traditional
therapeutic approaches, such as the use of statins, have proven
effective in reducing cholesterol levels and decreasing the
incidence of cardiovascular events (3). However, these treatments are
frequently associated with adverse effects and may not be suitable
for all patients. Consequently, there is an urgent demand for
alternative therapeutic strategies that can effectively manage
atherosclerosis while minimizing side effects. Recent advancements
in nanotechnology have highlighted the potential of nanomaterials
to carry out a pivotal role in the treatment and prevention of
atherosclerosis (4).
Nanomaterials exhibit unique properties, including high surface
area, modifiability and targeted delivery capabilities, rendering
them particularly advantageous for drug delivery and the modulation
of cellular functions. Furthermore, exercise has been shown to
improve vascular function through various mechanisms, including
enhanced endothelial nitric oxide (NO) production and reduced
oxidative stress (5,6). Combining the therapeutic potential
of nanomaterials with the beneficial effects of exercise presents a
novel approach to enhance vascular health and combat
atherosclerosis.
Previous research suggests that nanomaterials can
modulate the inflammatory response in vascular cells, thereby
mitigating the progression of atherosclerosis (7-9).
Certain nanoparticles, for example, have been shown to suppress the
expression of pro-inflammatory cytokines and facilitate the
resolution of inflammation in endothelial cells (ECs) (9,10). Nanomaterials can potentially halt
or reverse atherosclerosis by targeting inflammatory pathways
(11). Combining these with
exercise, which releases factors such as NO to improve endothelial
function (12), may enhance
their therapeutic effects (13).
Exercise could also increase the delivery and effectiveness of
nanomaterial treatments in the vascular system.
Previous research has highlighted the importance of
understanding the interactions between nanomaterials and the
vascular microenvironment (14,15). The distinctive properties of
nanomaterials considerably affect their behavior within biological
systems, encompassing aspects such as biocompatibility,
biodistribution and pharmacokinetics (16). Consequently, an in-depth
examination of these interactions is imperative for optimizing the
design of nanomaterials specifically engineered for vascular
applications.
The present review explores how combining
nanomaterials with physical exercise can impact atherosclerosis. It
examines numerous studies on using nanomaterials to improve
endothelial function, reduce inflammation and aid lipid clearance
in atherosclerotic lesions. Additionally, it examines how exercise
enhances the effectiveness of nanomaterial therapies and the
biological mechanisms involved. Ultimately, integrating
nanomaterials with physical exercise presents a promising strategy
for improved vascular health and reduced cardiovascular disease
burden. Future research should focus on understanding these
synergistic effects and exploring clinical applications for
managing atherosclerosis.
Nanomaterials have emerged as promising agents for
the protection of vascular ECs by reducing oxidative stress and
inflammation, and enhance the release of NO (17,18). For example, gold nanoparticles
have been demonstrated to activate the nuclear factor erythroid
2-related factor 2 signaling pathway (19), which reduces reactive oxygen
species (ROS) production. This activation markedly reduces
oxidative stress in ECs, preserving their function and integrity
(19). Moreover, silica
nanoparticles carrying anti-inflammatory drugs effectively inhibit
the NF-κB pathway, reducing endothelial inflammation (20). This is key as chronic
inflammation carries out a key role in endothelial dysfunction and
the advancement of atherosclerosis (20). Additionally, carbon-based
nanomaterials can mimic endothelial nitric oxide synthase (eNOS)
activity, promoting the release of NO, which is key for
vasodilation (21). This
increase in NO improves vascular tone and contributes to the
protective effects on ECs, highlighting their potential as
treatments for vascular diseases.
Nanomaterials carry out a key role in influencing
VSMC phenotypes by inhibiting pathological proliferation and
maintaining a contractile phenotype (22). Polymer-based nanoparticles are
used to deliver miR-145, which regulates the transcription factor
Krüppel-like factor (KLF) 4, key for VSMC phenotype modulation
(23). By modulating the
expression of KLF4, these nanoparticles effectively inhibit the
pathological VSMCs proliferation, a key characteristic of
atherosclerosis (24,25). Furthermore, magnetic
nanoparticles activate the RhoA/ROCK pathway, thereby promoting the
maintenance of a contractile phenotype in VSMCs. This mechanical
activation is essential for preventing the phenotypic transition
from a contractile to a synthetic state, a process frequently
associated with vascular remodeling and the development of
atherosclerotic plaques (26).
Additionally, nanofibrous scaffolds provide mechanical cues that
influence the remodeling of the extracellular matrix, consequently
affecting collagen secretion and the overall vascular architecture
(27).
Optimization of nanomaterial-based targeted delivery
systems is essential for improving their therapeutic efficacy in
vascular applications. Surface modification techniques, such as
polyethylene glycol-ylation, have been utilized to extend the
circulation time of nanoparticles within the bloodstream (28), thereby enhancing their
bioavailability and reducing premature clearance by the immune
system. Moreover, the incorporation of targeting ligands, such as
RGD peptides, can substantially increase the specificity of these
nanomaterials for diseased vascular sites, facilitating more
effective treatment of conditions such as atherosclerosis (29). Stimuli-responsive systems such as
pH-sensitive nanocarriers allow localized drug release in the
acidic environments of atherosclerotic plaques, increasing drug
efficacy and reducing side effects (30). Combining therapies, such as
photothermal treatment with drug delivery, enables precise control
over treatment timing and location, optimizing outcomes in vascular
diseases (31). These strategies
enhance the role of nanomaterials in vascular health therapy.
Exercise applies mechanical forces to the vascular
endothelium, mainly through blood flow-induced shear stress, which
activates signaling pathways key for endothelial function (32). This includes the activation of
eNOS, leading to increased NO production, essential for vascular
relaxation and homeostasis (33). Shear stress also boosts KLF2
expression, protecting ECs by regulating inflammation and vascular
tone (34). Additionally,
exercise-induced blood flow influences the cell cycle of vascular
wall cells, promoting their proliferation and migration, key for
vascular remodeling and repair (35). Shear stress activates
mechanotransduction pathways, such as the integrin-FAK-Akt
signaling cascade, which are important for ECs to adapt to
mechanical stimuli and preserve vascular integrity (36,37).
Physical exercise triggers metabolic changes in
various tissues, including the vascular system, by activating AMPK
and Peroxisome proliferator-activated receptor γ coactivator 1-α
(PGC-1α), which increase mitochondrial function and biogenesis
(38,39). This enhances energy production
and oxidative capacity in ECs, improving vascular function.
Exercise also facilitates lipid metabolism by delivering liver X
receptor agonists through nanocarriers, regulating cholesterol and
inflammation in vascular tissues (40,41). Increased autophagic flux,
supported by exercise and mTOR-inhibiting nanomedicines (42), helps degrade damaged organelles
and proteins (43,44), enhancing cellular health and
vascular healing.
Physical exercise triggers changes in the vascular
microenvironment, affecting circulating factors that improve
vascular function (45). A key
element is irisin, a myokine released during exercise, which
increases vascular permeability and aids drug delivery, especially
in nanomedicine. Moreover, exercise also redistributes immune
cells, notably stabilizing atherosclerotic plaques through the
activation of Ly6C^low monocytes (46). This alteration in immune cell
dynamics carries out a key role in promoting vascular health and
stability. Additionally, exercise-regulated sympathetic nervous
system activity can enhance the distribution and effectiveness of
therapeutic agents, including nanomedicines, in targeted vascular
tissues (47). These adaptations
underscore the role of exercise in improving vascular health and
function.
Exercise and nanomaterials work together at the
molecular level to combat atherosclerosis through several
mechanisms (51). A key
mechanism is epigenetic regulation, where exercise boosts the
demethylation effects of histone deacetylase inhibitors delivered
by nanocarriers, enhancing their therapeutic impact on vascular
health (52,53). Additionally, exercise-induced
exosomes and exosome-like nanoparticles interact to strengthen
cellular signaling pathways vital for cardiovascular disease
prevention and treatment (54).
Furthermore, the timing of exercise can affect the efficacy of
pH-sensitive nanomedicines, indicating that exercise-related
biological rhythms can influence nanomaterial therapy outcomes
(55). These molecular insights
underscore the complex interplay between exercise and
nanotechnology in the treatment of atherosclerosis, emphasizing the
need for a tailored approach that considers both physical activity
and nanomaterial properties.
Advanced imaging technologies, such as cutting-edge
imaging technologies, have markedly validated the combined effects
of exercise and nanomaterials. PET/MRI has been key in tracking
nanoparticle distribution during exercise, offering insights into
treatment dynamics by visualizing their accumulation in
atherosclerotic lesions (56).
Furthermore, super-resolution microscopy has precisely observed
nanomedicine deposition on plaque fibrous caps after exercise,
suggesting enhanced targeted delivery (57). Additionally, Raman spectroscopy
effectively monitors biochemical changes in plaques post-exercise
and nanomaterial treatment (58,59). Collectively, these advanced
imaging techniques not only validate the synergistic effects
observed in preclinical models but also pave the way for future
clinical applications, highlighting the potential of integrating
exercise with nanomedicine to mitigate atherosclerosis.
Numerous therapeutic drugs can slow atherosclerosis
by enhancing SMC function. Several therapeutic agents include short
hairpin RNA, small interfering (si)RNAs, natural extracts and
chemicals (60-63). However, the application of these
therapeutic agents for the treatment of metabolic disorders is
unfortunately limited by pharmacological issues, including drug
instability, rapid degradation and poor specificity (64).
Nanomedicine has attracted widespread attention due
to its advantage in drug delivery (65). As theragnostic agents with high
molecular specificity, nanoparticles have been shown to serve as
drug delivery carriers to facilitate the diagnosis and treatment of
diseases. Due to their dimensions <100 nm (ASTM International,
2006), the advantages of nanoparticles include i) efficient
delivery of drug insoluble or poorly soluble in water, ii) targeted
drug delivery in a tissue/cell type -specific pattern, iii)
controlled absorption and sustained drug release, iv) delivery of
drugs to intracellular sites of action or to the soma, v) high
binding ability and capture efficiency due to a relatively high
surface to volume ratio, vi) delivery of two or more drugs for
combination therapy due to the nano-sizes, vii) visualization of
drug sites by combining therapeutic agents with imaging modalities;
viii) protect the therapeutic agents and increase their stability,
viiii) immune-evading and tumor-targeting capabilities and x) great
biocompatibility and high drug loading efficiency in vivo
drug efficacy (66-70). Nanomedicine has incomparable
advantages over ordinary drugs. It has high solubility, can greatly
enhance the absorption and bioavailability of oral drugs, can make
the drug pass through the blood-brain barrier to act on the central
nervous system, can penetrate the epidermis to enhance the
absorption of the preparation and can also enhance the drug
targeting. Therefore, nanoparticles have a wide application in the
field of biomedicine due to their aforementioned medical
characteristics.
Despite its advantages, nanomedicine has several
drawbacks, such as toxicity, genotoxicity and carcinogenicity.
Given their small size, nanoparticles can circulate in the body and
accumulate in tissues, posing health risks. Metal nanoparticles
such as titanium dioxide, gold, silver and platinum can harm human
health. For example, 21 nm titanium dioxide nanoparticles can cause
neuroinflammation, brain and spleen injury, heart damage, and lung
toxicity (71,72). Gold nanoparticles have been found
in urine and blood 3 months after exposure (73), and platinum nanoparticles
accumulate in the liver and spleen, causing liver and kidney
toxicity (74). To date, the
advantages of using nanoparticles over traditional medical
strategies seem to outweigh their disadvantages. Nanoparticles in
medicine must be designed carefully and validated through a series
of experiments to ensure their safety.
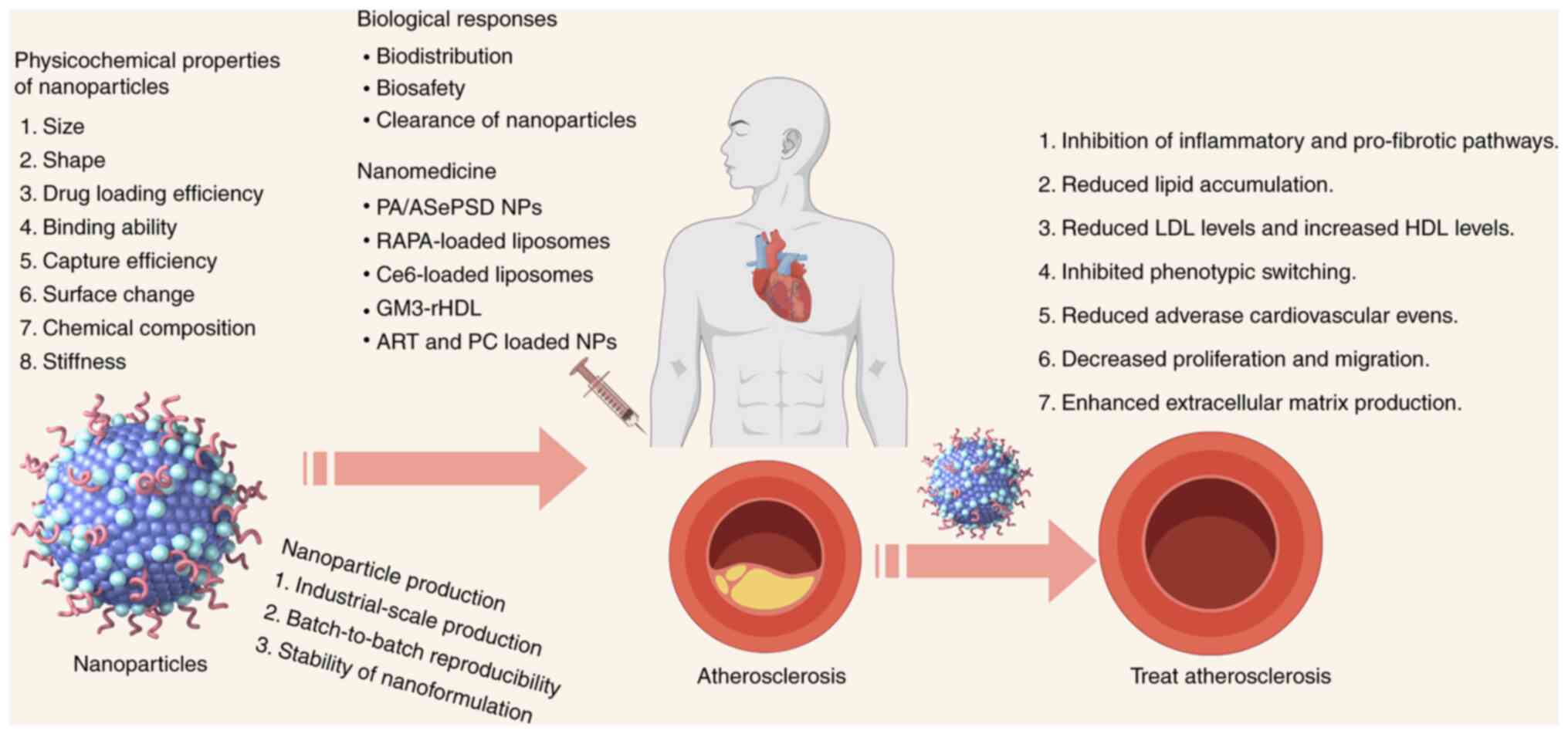
Nanomedicine, using nanomaterials such as
nanoparticles, is rapidly advancing in treating atherosclerosis
(Fig. 1). Nanotechnology
markedly impacts healthcare, including targeted nanotherapeutics
(75), medical imaging (76), diagnostics (77), vaccines (78) and regenerative medicine. Previous
studies show nanoparticles can slow vascular disease by targeting
vascular cells (79-110) (Table I). Due to its importance, several
reviews cover the role of nanomedicine in treating diseases such as
cancer, atherosclerosis and diabetes (77,111,112). Therefore, it is important to
increase awareness of the benefits of pharmaceutical nanotechnology
and existing treatments for atherosclerosis (Fig. 1).
Imbalanced SMC proliferation can cause pathological
angiogenesis, leading to plaque growth and rupture in coronary
arteries, potentially resulting in cardiovascular mortality.
Accurate ultrasound detection of nanoparticles on atherosclerotic
plaque neovascularization can aid in early diagnosis of unstable
plaques. Super-paramagnetic iron oxide particles (SPIONs)
conjugated with annexin A5 are more effective than non-targeted
SPIONs in identifying atherosclerotic lesions in rabbits (113). Additionally, synthetic high
density lipoprotein nanoparticles with Apo A1 (APOA1) and triphenyl
phosphonium cations can detect mitochondrial membrane potential
collapse and apoptotic cells (114). The mTOR pathway is involved in
VSMC and pulmonary arterial SMCs (PASMCs) proliferation in
pulmonary arterial hypertension (115,116), and administration of mTOR siRNA
nanomedicines can markedly inhibit PASMC proliferation after
hypoxia (115). Nanoparticles
notably attenuate oxidized low-density lipoprotein-induced SMC
proliferation and thrombosis through downregulating the expression
of scavenger receptor and key proinflammatory markers, implying the
promise of nanomedicine for next-generation cardiovascular
therapeutics (117). Therefore,
nanomedicines carrying drugs may be promising therapeutics for
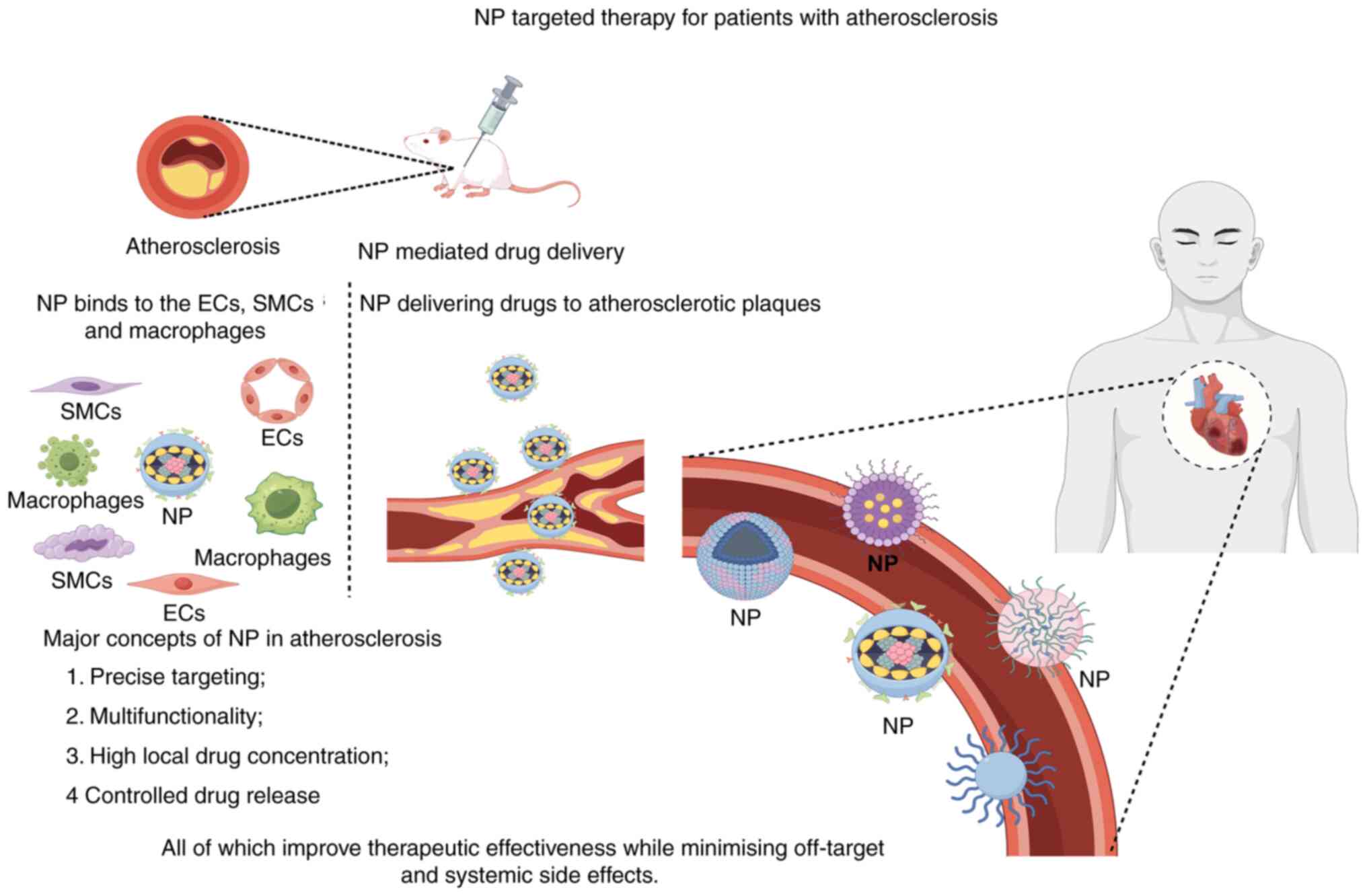
diseases associated with the proliferation of SMCs (Fig. 2).
Nanomedicine offers an effective approach to
treating atherosclerosis by improving SMC dysfunction and
inhibiting cell proliferation (118). In early atherosclerosis, SMCs
increase adhesion molecules and pro-inflammatory cytokines. A
nano-delivery system, including 17-βE loaded nano-emulsions,
notably reduced the expression of these molecules (119), highlighting its therapeutic
potential. Additionally, miR-146a-5p nanomedicines, which target
inflammation via the NF-κB pathway, reduced neointimal hyperplasia
in rat arteries post-revascularization (120). Thus, nanomedicines can
effectively deliver drugs to injury sites, showing promise against
neointimal hyperplasia.
Atherosclerosis is a chronic disease marked by lipid
deposition and inflammation in arterial walls (121), leading to plaque growth and
potential rupture, which can cause myocardial infarction (MI). The
severity of the disease is influenced by plaque size and
composition, affecting stability. Unstable plaques may rupture
under altered blood flow, blocking capillaries and causing
cardiovascular events such as MI, stroke and peripheral artery
disease (122). Endothelial
injury exacerbates atherosclerosis by promoting platelet
aggregation, coagulation and SMC proliferation (123), making the endothelium vital in
plaque development, stability and rupture.
Previous studies indicate that nanoparticles can
deliver drugs to ECs for diagnosing or treating atherosclerotic
diseases (79-110,124,125) (Table I; Fig. 2). Ultrasound imaging of plaque
neovascularization can improve early detection of unstable plaques.
VEGFR-2 targeting antibody nano-microbubbles are more effective
than blank ones in detecting plaques in rats (126). IL-10, an inflammation
regulator, suppresses pro-inflammatory responses in atherosclerosis
by inhibiting NF-κB activation (127). Branched poly (β-amino ester)
nanoparticles with IL-10 plasmid DNA can slow atherosclerosis
progression (128). The
nanoparticle-VHPKQHRGGSGC peptide drug delivery system targets ECs,
enhancing the anti-inflammatory effects of IL-10 on atherosclerotic
plaques (128). Triptolide
could inhibit inflammatory responses and angiogenesis in ECs
(129), which might improve the
symptoms and delay the progression of atherosclerosis. APRPG
(Ala-Pro-Arg-Pro-Gly) peptide-modified nanoliposomes, a novel
sustained-release drug delivery system targeting ECs, enhances
inhibitory effects of triptolide on laser-induced
neovascularization (130).
Nanomedicine not only inhibits angiogenesis but also enhances
endothelial function to treat atherosclerosis. In primary ECs,
amorphous selenium quantum dots improved endothelium-dependent
relaxation and accelerated wound healing, reducing atherosclerotic
plaque in aortic arteries (131). Encapsulating cherry extract
from Prunus avium L. in nanoparticles protected HUVECs from
oxidative stress and decreased ROS production (132). Molybdenum disulfide
nanoparticles (MoS2 NPs) show promise as a multifunctional
therapeutic, reversing hydrogen peroxide-induced endothelial
senescence by enhancing autophagy (133). Additionally, mRNA-p5RHH
nanoparticles can be assembled into nuclease-resistant
nanoparticles (134).
Cyclin-dependent kinase inhibitor p27Kip1 nanoparticles
target endothelial denudation areas, promoting vessel
reendothelialization and reducing neointima formation after injury
(135). Mesoporous silica
nanoparticles with CD9 antibodies deliver rosuvastatin to senescent
ECs and macrophages, slowing atherosclerosis progression in
apoE−/− mice (136).
These EC-targeted nanotherapies are promising for atherosclerosis
and other cardiovascular diseases. For instance, silica-coated
magnetic iron oxide nanoparticles can label endothelial progenitor
cells (EPCs) from male rats, forming magnetized EPCs (137). Transplanting these EPCs
improves cardiac function, reduces infarction size and decreases
myocardial cell apoptosis, enhancing myocardial infarction
treatment (138).
Invasive therapy technology, such as NO-producing
vascular stents, offers a novel therapeutic strategy for treating
arterial stenosis. A study by Yang et al (139) demonstrated that stents
functionalized with 3,3-diselenodipropionic acid and
S-nitrosothiols implanted into vessels via nanomaterials can
produce NO, promote EC growth, reduce platelet activation and VSMC
activity (Fig. 2). This
nanocoating technique creates an endothelium-like environment,
presenting a novel treatment strategy for cardiovascular
diseases.
Atherosclerosis is an inflammatory disease of the
artery walls involving immune cells such as macrophages, which are
key in its progression (77,140). Targeting macrophage-related
processes offers potential for diagnostics and therapies. Early in
atherogenesis, arterial ECs recruit macrophages via
chemokine-receptor interactions and adhesion molecules such as
intercellular adhesion molecule 1 and vascular cell adhesion
molecule 1 (141). This
recruitment system is redundant, allowing for the inhibition of
plaque formation by targeting multiple adhesion molecules (141). Additionally, vascular
macrophages contribute to atherosclerosis through the expression of
the olfactory receptor Olfr2 and related signaling molecules
(142). Aberrant activation and
polarization of arterial macrophages affect apoptosis and
efferocytosis, associate with lipid accumulation and the
inflammatory response in macrophages to the development of
atherosclerotic lesions (143).
Indeed, suppression of macrophages reduced atherosclerosis in mice
(144), emphasizing the
importance of detecting subclinical disease in humans for
monocyte-directed treatment.
Nanotechnology could enhance atherosclerosis
treatment by developing new diagnostic and therapeutic methods to
lower cardiovascular disease risk. Gao et al (145) introduced a biomimetic drug
delivery system using ROS-responsive nanoparticles coated with
macrophage membranes, which block proinflammatory cytokines to
reduce inflammation. This approach, along with pharmacotherapy,
increases atherosclerosis treatment efficacy, indicating that
macrophage membrane-coated systems are well-suited for inflammatory
diseases (145).
Rapamycin-loaded nanoparticles using activated macrophage membrane
proteins have shown promise by inhibiting macrophage proliferation
and reducing inflammation and plaque changes (146). Long-term use of PLGA
nanoparticles enhances lysosomal degradation, reduces macrophage
apoptosis, necrotic core formation and cytotoxic protein
aggregates, while increasing fibrous cap formation (147), suggesting they can alleviate
macrophage lysosomal dysfunction in atherosclerosis. RNA
interference (RNAi) with nanoparticles effectively inhibited key
adhesion molecules, reducing macrophage recruitment to
atherosclerotic lesions (141).
Thus, nanomedicine can improve atherosclerosis by targeting
macrophages (Fig. 2). However,
nanoparticles can also worsen vascular disease; for example,
inhaled silica nanoparticles can exacerbate lesions and increase
pro-inflammatory M1 macrophages (148).
Exercise-induced acute and adaptive physiological
changes may notably improve the in vivo microenvironment for
nano-drugs, thereby enhancing their efficacy (149). This synergistic effect can be
attributed to several mechanistic pathways: i) Hemodynamics and
vascular permeability: Exercise increases cardiac output and tissue
blood flow, potentially facilitating the delivery and accumulation
of nanoparticles in target tissues such as the heart or skeletal
muscle, ii) metabolic and immune reprogramming: Exercise-induced
metabolic alterations, such as reduced insulin resistance and
immunomodulation, including an increase in anti-inflammatory M2
macrophages, may enhance the susceptibility of target cells to
therapeutic agents such as nucleic acids and small molecules, while
also mitigating inflammatory responses that could degrade
nanoparticles, iii) cellular uptake: The upregulation of specific
cell surface receptors, such as certain integrins, during exercise,
when aligned with the targeting ligands on nanoparticles, could
markedly enhance cellular internalization. Consequently, exercise
should not be viewed merely as an adjuvant therapy but rather as a
'bio-enhancer' that actively modulates the host environment to
optimize pharmacokinetics.
Despite promising advancements in preclinical
research and the application of nanomaterials, challenges remain
that could hinder progress. A primary challenge lies in the ethical
implications associated with ongoing clinical trials, especially
with fast-evolving science and issues such as COVID-19 (150). Ensuring the relevance and value
of clinical trials as new information emerges is key for
maintaining public trust and scientific integrity. Furthermore, the
integration of nanomaterials into clinical practice presents
notable obstacles, including concerns regarding toxicity,
immunogenicity and regulatory approval processes (151). However, these challenges also
present opportunities for innovation and interdisciplinary
collaboration. By fostering partnerships among researchers,
clinicians and regulatory bodies, the field can develop robust
frameworks that address these issues while advancing scientific
knowledge and improving patient outcomes. Future research should
focus on overcoming these barriers to fully harness the potential
of emerging technologies in personalized medicine and preclinical
research.
Nanoparticles have several advantages over
traditional therapeutic agents, including the ability to target
specific tissues or cells, controlled drug release and reduced
toxicity. Nonetheless, there are important limitations and
challenges in cardiovascular nanomedicine that should be noted
(79-110,152,153) (Table I). Future advancements in
nanoparticle-based therapies for the diagnosis and treatment of
arteriosclerosis are anticipated, with ongoing development and
refinement in this field (4,7,77). These nanoparticles have the
potential to inhibit pro-atherogenic processes in macrophages,
VSMCs and ECs, thereby enhancing the resolution of inflammation and
stabilizing plaques. Furthermore, this work addresses the enduring
challenge of translating nanomaterials into clinical applications
by summarizing current obstacles and suggesting avenues for
innovation and enhancement in nanomaterial design. This may involve
the creation of novel and more efficacious therapeutic agents, the
development of improved targeting strategies to ensure precise
delivery of nanoparticles to plaque sites, and the integration of
nanoparticle-based therapies with other treatment modalities, such
as surgical interventions or stenting.
The utilization of nanoparticles coated with natural
membranes derived from cells or extracellular vesicles for targeted
drug delivery to the skin has been explored (154). Recent advancements in imaging
techniques have enabled scientists to observe and comprehend the
interactions of nanoparticles with biological systems at an
ultrastructural level (155).
Techniques such as cryo-electron microscopy, super-resolution
microscopy and advanced spectroscopy are pivotal for elucidating
the safety, biodistribution, and fundamental mechanisms of action
of nanomedicines. Interfacial self-assembly nanoarrays pertain to
spontaneously organized nanostructures at interfaces, which depend
on the intrinsic properties of the materials involved, including
surface energy, molecular structure and interactions (156). Moreover, plasmonic alloys have
been shown to enhance metabolic fingerprints, facilitating the
rapid diagnosis and classification of MI (157). This advancement holds the
potential to reduce the duration of emergency department visits and
improve MI treatment outcomes. Thus, nanomedicine is transforming
from a simple 'drug deliveryman' to a 'comprehensive medical
platform' that can intelligently interact with life systems,
analyze diseases at the molecular level, and achieve precise
diagnosis and efficient treatment integration.
It is imperative to critically evaluate the
heterogeneous findings on nanomaterials. Some studies highlight
their benefits, while other studies raise concerns about their
biocompatibility and long-term implications (158,159). A balanced approach is needed to
weigh positive results against potential risks, guiding the
development of standardized clinical protocols. Future research
should prioritize elucidating the mechanisms that govern the
interactions between nanomaterials and cardiovascular cells,
alongside optimizing nanomaterial design for improved efficacy and
safety. There is an urgent need for rigorously designed clinical
trials to evaluate the long-term impacts of nanomaterials in the
treatment of atherosclerosis. Additionally, interdisciplinary
collaborations among materials scientists, biologists and
clinicians will be key to advancing this field.
The present review highlights the considerable
potential of the nano-platform for applications such as
tumor-targeted therapy; however, there are considerable challenges
associated with its clinical translation, particularly in relation
to long-term in vivo safety and biodistribution. Due to
their material composition and size, the nanoparticles are likely
to undergo sequestration and clearance predominantly in the liver
and spleen. The potential risks of long-term toxicity,
immunogenicity and off-target accumulation necessitate
comprehensive evaluation in future studies, particularly through
systematic long-term animal experiments. Addressing these safety
concerns is essential for the advancement of this platform toward
clinical application and will constitute a primary focus of
forthcoming research.
The present review posits a key perspective:
Nanomaterials and exercise should not be viewed as independent
interventions but rather as synergistic agents that converge on
common molecular pathways, thereby offering a transformative
approach to cardiovascular therapy. This integration propels the
field forward in two notable ways. Firstly, on a mechanistic level,
it introduces a novel concept of 'bidirectional regulation', for
example, in the context of inflammatory pathways, exercise promotes
an anti-inflammatory state by modulating myokines such as IL-6,
which increases IL-10 and decreases TNF-α levels (160). Concurrently, nanomaterials can
be engineered to deliver anti-inflammatory agents (161), such as siRNA targeting TNF-α,
directly to gut microbiota for ulcerative colitis therapy (162). This dual approach effectively
remodels the inflammatory microenvironment through both 'systemic
modulation' and 'localized precision strike' strategies. Secondly,
at a translational level, it introduces an innovative paradigm of
'preconditioning combined with therapy'. Exercise acts as a safe
and cost-effective systemic 'preconditioning' strategy that
optimizes the overall physiological environment by enhancing
vascular function and reducing oxidative stress (163). This creates a less favorable
environment for the subsequent administration of nanotherapeutics.
This integrated approach not only has the potential to enhance the
efficacy of monotherapies but also addresses the multi-pathway
dysregulation characteristic of complex diseases, representing a
key direction for the future integration of precision medicine and
lifestyle interventions.
Not applicable.
QZ, GLZ, WS and LHY conceived the structure of the
manuscript; QZ and LY drafted the initial manuscript; QZ, GZ, WS,
JC and LHY revised and edited the manuscript. All authors
contributed to the preparation of this manuscript. All the authors
approved the final manuscript. Data authentication not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther. 7:1312022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin SS, Aday AW, Almarzooq ZI, Anderson
CAM, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ,
Boehme AK, et al: 2024 Heart disease and stroke statistics: A
report of us and global data from the American Heart Association.
Circulation. 149:e347–e913. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oveisgharan S, Yu L, Barnes LL, Agrawal S,
Schneider JA, Bennett DA and Buchman AS: Association of statins
with cerebral atherosclerosis and incident parkinsonism in older
adults. Neurology. 98:e1976–e1984. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng J, Huang H, Chen Y and Wu R:
Nanomedicine for diagnosis and treatment of atherosclerosis. Adv
Sci (Weinh). 10:e23042942023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powers SK, Deminice R, Ozdemir M,
Yoshihara T, Bomkamp MP and Hyatt H: Exercise-induced oxidative
stress: Friend or foe? J Sport Health Sci. 9:415–425. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arazi H, Eghbali E and Suzuki K: Creatine
supplementation, physical exercise and oxidative stress markers: A
review of the mechanisms and effectiveness. Nutrients. 13:8692021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Zhang X, Millican R, Sherwood J,
Martin S, Jo H, Yoon YS, Brott BC and Jun HW: Recent advances in
nanomaterials for therapy and diagnosis for atherosclerosis. Adv
Drug Deliv Rev. 170:142–199. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Z, Chen W, Hu K, Luo Y, Zeng W, He X,
Li T, Ouyang J, Li Y, Xie L, et al: Resolvin D1 delivery to
lesional macrophages using antioxidative black phosphorus
nanosheets for atherosclerosis treatment. Nat Nanotechnol.
19:1386–1398. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang J, Li T, Xiong X, Yang Q, Su Z, Zheng
M and Chen Q: Colchicine delivered by a novel nanoparticle platform
alleviates atherosclerosis by targeted inhibition of NF-κB/NLRP3
pathways in inflammatory endothelial cells. J Nanobiotechnology.
21:4602023. View Article : Google Scholar
|
|
10
|
Zhou L, Tang S, Li F, Wu Y, Li S, Cui L,
Luo J, Yang L, Ren Z, Zhang J, et al: Ceria nanoparticles
prophylactic used for renal ischemia-reperfusion injury treatment
by attenuating oxidative stress and inflammatory response.
Biomaterials. 287:1216862022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao M, Tang M, Ho W, Teng Y, Chen Q, Bu L,
Xu X and Zhang XQ: Modulating plaque inflammation via targeted mRNA
nanoparticles for the treatment of atherosclerosis. ACS Nano.
17:17721–17739. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi Y, Wang S, Luo Y, Huang W, Chen L,
Zhang Y, Liang X, Tang J, Zhang Y, Zhang L, et al: Exercise-induced
Nitric oxide contributes to spatial memory and hippocampal
capillaries in rats. Int J Sports Med. 41:951–961. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lima MR, Moreira BJ, Bertuzzi R and
Lima-Silva AE: Could nanotechnology improve exercise performance?
Evidence from animal studies. Braz J Med Biol Res. 57:e133602024.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang-Bishop L, Kimmel BR, Ngwa VM, Madden
MZ, Baljon JJ, Florian DC, Hanna A, Pastora LE, Sheehy TL,
Kwiatkowski AJ, et al: STING-activating nanoparticles normalize the
vascular-immune interface to potentiate cancer immunotherapy. Sci
Immunol. 8:eadd11532023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Zhao Z, Luo Y, Ning T, Liu P, Chen
Q, Chu Y, Guo Q, Zhang Y, Zhou W, et al: Macrophage-disguised
manganese dioxide nanoparticles for neuroprotection by reducing
oxidative stress and modulating inflammatory microenvironment in
acute ischemic stroke. Adv Sci (Weinh). 8:e21015262021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Donskyi IS, Tang W, Deng S, Liu
D, Zhang S, Zhao Q and Xing B: Biological effects of black
phosphorus nanomaterials on mammalian cells and animals. Angew Chem
Int Ed Engl. 62:e2022133362023. View Article : Google Scholar :
|
|
17
|
Luo L, Zhang H, Zhang S, Luo C, Kan X, Lv
J, Zhao P, Tian Z and Li C: Extracellular vesicle-derived silk
fibroin nanoparticles loaded with MFGE8 accelerate skin ulcer
healing by targeting the vascular endothelial cells. J
Nanobiotechnology. 21:4552023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Germande O, Baudrimont M, Beaufils F,
Freund-Michel V, Ducret T, Quignard JF, Errera MH, Lacomme S,
Gontier E, Mornet S, et al: NiONPs-induced alteration in calcium
signaling and mitochondrial function in pulmonary artery
endothelial cells involves oxidative stress and TRPV4 channels
disruption. Nanotoxicology. 16:29–51. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serra MF, Cotias AC, Pimentel AS, Arantes
ACS, Pires ALA, Lanzetti M, Hickmann JM, Barreto E, Carvalho VF,
Silva PMRE, et al: Gold nanoparticles inhibit steroid-insensitive
asthma in mice preserving histone deacetylase 2 and NRF2 pathways.
Antioxidants (Basel). 11:16592022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arnst J, Jing Z, Cohen C, Ha SW,
Viggeswarapu M and Beck GR: Bioactive silica nanoparticles target
autophagy, NF-κB, and MAPK pathways to inhibit osteoclastogenesis.
Biomaterials. 301:1222382023. View Article : Google Scholar
|
|
21
|
Kabirian F, Baatsen P, Smet M, Shavandi A,
Mela P and Heying R: Carbon nanotubes as a nitric oxide
nano-reservoir improved the controlled release profile in 3D
printed biodegradable vascular grafts. Sci Rep. 13:46622023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keyoumu Y, Huo Q, Cheng L, Ma H, Zhang M,
Ma Y and Ma X: The detailed biological investigations about
combined effects of novel polyphenolic and photo-plasmonic
nanoparticles loaded graphene nanosheets on coronary endothelial
cells and isolated rat aortic rings. J Photochem Photobiol B.
202:1116662019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang K, Sun C, Li H, Liu X, Deng J, Chen
S, Zeng L, Chen J, Liu X, Kuang J, et al: N6-methyladenosine-driven
miR-143/145-KLF4 circuit orchestrates the phenotypic switch of
pulmonary artery smooth muscle cells. Cell Mol Life Sci.
81:2562024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park C, Baek KI and Jo H: Saving KLF2/4
from γ-protocadherin to reduce vascular inflammation and
atherosclerosis. Nat Cardiovasc Res. 3:1021–1023. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Zhang K, Li T, Maruf A, Qin X, Luo
L, Zhong Y, Qiu J, McGinty S, Pontrelli G, et al: Macrophage
membrane functionalized biomimetic nanoparticles for targeted
anti-atherosclerosis applications. Theranostics. 11:164–180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang W, Lu C, Chu F, Bu K, Ma H, Wang Q,
Jiao Z, Wang S, Yang X, Gao Y, et al: Fluoride-induced hypertension
by regulating RhoA/ROCK pathway and phenotypic transformation of
vascular smooth muscle cells: In vitro and in vivo evidence.
Ecotoxicol Environ Saf. 281:1166812024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hao D, Swindell HS, Ramasubramanian L, Liu
R, Lam KS, Farmer DL and Wang A: Extracellular matrix mimicking
nanofibrous scaffolds modified with mesenchymal stem cell-derived
extracellular vesicles for improved vascularization. Front Bioeng
Biotechnol. 8:6332020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hatakeyama H, Akita H and Harashima H: A
multifunctional envelope type nano device (MEND) for gene delivery
to tumours based on the EPR effect: A strategy for overcoming the
PEG dilemma. Adv Drug Deliv Rev. 63:152–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitagawa T, Kosuge H, Uchida M, Iida Y,
Dalman RL, Douglas T and McConnell MV: RGD targeting of human
ferritin iron oxide nanoparticles enhances in vivo MRI of vascular
inflammation and angiogenesis in experimental carotid disease and
abdominal aortic aneurysm. J Magn Reson Imaging. 45:1144–1153.
2017. View Article : Google Scholar :
|
|
30
|
Liu S, Zhao Y, Shen M, Hao Y, Wu X, Yao Y,
Li Y and Yang Q: Hyaluronic acid targeted and pH-responsive
multifunctional nanoparticles for chemo-photothermal synergistic
therapy of atherosclerosis. J Mater Chem B. 10:562–570. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Li F, Wang Y, Li W, Wu J, Hu X, Tang
T and Liu X: Multiple delivery strategies of nanocarriers for
myocardial ischemia-reperfusion injury: Current strategies and
future prospective. Drug Deliv. 31:22985142024. View Article : Google Scholar :
|
|
32
|
Walsh D, Kostyunina DS, Blake A, Boylan J
and McLoughlin P: Shear stress-induced restoration of pulmonary
microvascular endothelial barrier function following ischemia
reperfusion injury requires VEGFR2 signaling. Am J Physiol Lung
Cell Mol Physiol. 328:L389–L404. 2025. View Article : Google Scholar
|
|
33
|
Martelli A, Abate F, Roggia M, Benedetti
G, Caradonna E, Calderone V, Tenore GC, Cosconati S, Novellino E
and Stornaiuolo M: Trimethylamine N-Oxide (TMAO) acts as inhibitor
of endothelial nitric oxide synthase (eNOS) and Hampers NO
production and acetylcholine-mediated vasorelaxation in rat aortas.
Antioxidants (Basel). 14:5172025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Min L, Zhong F, Gu L, Lee K and He JC:
Krüppel-like factor 2 is an endoprotective transcription factor in
diabetic kidney disease. Am J Physiol Cell Physiol. 327:C477–C486.
2024. View Article : Google Scholar
|
|
35
|
Yang Q, Chen S, Wang X, Yang X, Chen L,
Huang T, Zheng Y, Zheng X, Wu X, Sun Y and Wu J: Exercise mitigates
endothelial pyroptosis and atherosclerosis by downregulating NEAT1
through N6-methyladenosine modifications. Arterioscler Thromb Vasc
Biol. 43:910–926. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanaka K, Chen M, Prendergast A, Zhuang Z,
Nasiri A, Joshi D, Hintzen J, Chung M, Kumar A, Mani A, et al:
Latrophilin-2 mediates fluid shear stress mechanotransduction at
endothelial junctions. EMBO J. 43:3175–3191. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Russell-Puleri S, Dela Paz NG, Adams D,
Chattopadhyay M, Cancel L, Ebong E, Orr AW, Frangos JA and Tarbell
JM: Fluid shear stress induces upregulation of COX-2 and PGI2
release in endothelial cells via a pathway involving PECAM-1, PI3K,
FAK, and p38. Am J Physiol Heart Circ Physiol. 312:H485–H500. 2017.
View Article : Google Scholar :
|
|
38
|
Palabiyik AA and Palabiyik E:
Pharmacological approaches to enhance mitochondrial biogenesis:
Focus on PGC-1Α, AMPK, and SIRT1 in cellular health. Mol Biol Rep.
52:2702025. View Article : Google Scholar
|
|
39
|
Zhu C, Zhang Z, Zhu Y, Du Y, Han C, Zhao
Q, Li Q, Hou J, Zhang J, He W and Qin Y: Study on the role of
Dihuang Yinzi in regulating the AMPK/SIRT1/PGC-1α pathway to
promote mitochondrial biogenesis and improve Alzheimer's disease. J
Ethnopharmacol. 337:1188592025. View Article : Google Scholar
|
|
40
|
Pinto PR, Rocco DDFM, Okuda LS,
Machado-Lima A, Castilho G, da Silva KS, Gomes DJ, Pinto Rde S,
Iborra RT, Ferreira Gda S, et al: Aerobic exercise training
enhances the in vivo cholesterol trafficking from macrophages to
the liver independently of changes in the expression of genes
involved in lipid flux in macrophages and aorta. Lipids Health Dis.
14:1092015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
da Silva Pereira JA, de Souza GP,
Virgilio-da-Silva JV, Prodonoff JS, de Castro G, Pimentel LF,
Mousovich-Neto F, Davanzo GG, Aguiar CF, Breda CNS, et al: LXR
regulation of adipose tissue inflammation during obesity is
associated with dysregulated macrophage function. Obesity (Silver
Spring). 33:78–90. 2025. View Article : Google Scholar
|
|
42
|
Chiang CF, Wang ZZ, Hsu YH, Miaw SC and
Lin WL: Exercise improves the outcome of anticancer treatment with
ultrasound-hyperthermia-enhanced nanochemotherapy and autophagy
inhibitor. PLoS One. 18:e02883802023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao Y, Ying X, Pang X, Lin Y, Shen J,
Zhao Y, Shen W, Yang Y, Hong Z, Wu W, et al: Exercise-induced Sesn2
mediates autophagic flux to alleviate neural damage after ischemic
stroke in mice. Exp Neurol. 386:1151742025. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao Y, Hong Z, Lin Y, Shen W, Yang Y, Zuo
Z and Hu X: Exercise pretreatment alleviates neuroinflammation and
oxidative stress by TFEB-mediated autophagic flux in mice with
ischemic stroke. Exp Neurol. 364:1143802023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tarumi T, Tomoto T, Sugawara J and Zhang
R: Aerobic exercise training for the aging brain: Effective dosing
and vascular mechanism. Exerc Sport Sci Rev. 53:31–40. 2025.
View Article : Google Scholar
|
|
46
|
Herbin O, Regelmann AG, Ramkhelawon B,
Weinstein EG, Moore KJ and Alexandropoulos K: Monocyte adhesion and
plaque recruitment during atherosclerosis development is regulated
by the adapter protein Chat-H/SHEP1. Arterioscler Thromb Vasc Biol.
36:1791–1801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Raine LB, McDonald K, Shigeta TT, Hsieh
SS, Hunt J, Chiarlitti NA, Lim M, Gebhardt K, Collins N, De Lisio
M, et al: Sympathetic nervous system and exercise affects cognition
in youth (SNEACY): Study protocol for a randomized crossover trial.
Trials. 22:1542021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dash R, Yadav M, Biswal J, Chandra A, Goel
VK, Sharma T, Prusty SK and Mohapatra S: Modeling of chitosan
modified PLGA atorvastatin-curcumin conjugate (AT-CU)
nanoparticles, overcoming the barriers associated with PLGA: An
approach for better management of atherosclerosis. Int J Pharm.
640:1230092023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Janssen SLJE, van Everdingen WM, Saalmink
WBJ, Lamers SK, Vroemen WHM, Denessen EJS, Berge K, Bekers O,
Hopman MTE, Brink M, et al: Relationship between exercise-induced
cardiac troponin elevations and occult coronary atherosclerosis in
Middle-aged athletes. J Am Coll Cardiol. 85:2370–2382. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yan Q, Yan X, Yang X, Li S and Song J: The
use of PET/MRI in radiotherapy. Insights Imaging. 15:632024.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ficker ES, Maranhão RC, Chacra APM, Neves
VC, Negrão CE, Martins VC and Vinagre CG: Exercise training
accelerates the removal from plasma of LDL-like nanoemulsion in
moderately hypercholesterolemic subjects. Atherosclerosis.
212:230–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shanmugam G, Rakshit S and Sarkar K: HDAC
inhibitors: Targets for tumor therapy, immune modulation and lung
diseases. Transl Oncol. 16:1013122022. View Article : Google Scholar
|
|
53
|
Kitahara M, Inoue T, Mani H, Takamatsu Y,
Ikegami R, Tohyama H and Maejima H: Exercise and pharmacological
inhibition of histone deacetylase improves cognitive function
accompanied by an increase of gene expressions crucial for neuronal
plasticity in the hippocampus. Neurosci Lett. 749:1357492021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lai Z, Liang J, Zhang J, Mao Y, Zheng X,
Shen X, Lin W and Xu G: Exosomes as a delivery tool of
exercise-induced beneficial factors for the prevention and
treatment of cardiovascular disease: A systematic review and
meta-analysis. Front Physiol. 14:11900952023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sharma AK, Kumar A, Sahu M, Sharma G,
Datusalia AK and Rajput SK: Exercise preconditioning and low dose
copper nanoparticles exhibits cardioprotection through targeting
GSK-3β phosphorylation in ischemia/reperfusion induced myocardial
infarction. Microvasc Res. 120:59–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Watkins LE, Goyal A, Gatti AA and Kogan F:
Imaging of joint response to exercise with MRI and PET. Skeletal
Radiol. 52:2159–2183. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Coelho S, Baek J, Walsh J, Gooding JJ and
Gaus K: 3D active stabilization for single-molecule imaging. Nat
Protoc. 16:497–515. 2021. View Article : Google Scholar
|
|
58
|
Karageorgou MA, Bouziotis P, Stiliaris E
and Stamopoulos D: Radiolabeled iron oxide nanoparticles as dual
modality contrast agents in SPECT/MRI and PET/MRI. Nanomaterials
(Basel). 13:5032023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bouziotis P, Psimadas D, Tsotakos T,
Stamopoulos D and Tsoukalas C: Radiolabeled iron oxide
nanoparticles as dual-modality SPECT/MRI and PET/MRI agents. Curr
Top Med Chem. 12:2694–2702. 2012. View Article : Google Scholar
|
|
60
|
Zhang X, Xie K, Zhou H, Wu Y, Li C, Liu Y,
Liu Z, Xu Q, Liu S, Xiao D and Tao Y: Role of non-coding RNAs and
RNA modifiers in cancer therapy resistance. Mol Cancer. 19:472020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang HN, Xiang JZ, Qi Z and Du M: Plant
extracts in prevention of obesity. Crit Rev Food Sci Nutr.
62:2221–2234. 2022. View Article : Google Scholar
|
|
62
|
Raguram A, Banskota S and Liu DR:
Therapeutic in vivo delivery of gene editing agents. Cell.
185:2806–2827. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Marini I, Uzun G, Jamal K and Bakchoul T:
Treatment of drug-induced immune thrombocytopenias. Haematologica.
107:1264–1277. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Peñaherrera-Pazmiño AB, Criollo M and
Gonzalez-Pastor R: Phytochemical nanoencapsulation and
microfluidics drive gene and tumor microenvironment modulation.
Front Pharmacol. 16:16947522025. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Takedatsu H, Mitsuyama K and Torimura T:
Nanomedicine and drug delivery strategies for treatment of
inflammatory bowel disease. World J Gastroenterol. 21:11343–11352.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cordeiro RA, Santo D, Farinha D, Serra A,
Faneca H and Coelho JFJ: High transfection efficiency promoted by
tailor-made cationic tri-block copolymer-based nanoparticles. Acta
Biomater. 47:113–123. 2017. View Article : Google Scholar
|
|
67
|
Li Z, Li G, Xu J, Li C, Han S, Zhang C, Wu
P, Lin Y, Wang C, Zhang J, et al: Hydrogel transformed from
nanoparticles for prevention of tissue injury and treatment of
inflammatory diseases. Adv Mater. 34:e21091782022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sun R, Wang X, Nie Y, Hu A, Liu H, Zhang
K, Zhang L, Wu Q, Li K, Liu C, et al: Targeted trapping of
endogenous endothelial progenitor cells for myocardial ischemic
injury repair through neutrophil-mediated SPIO
nanoparticle-conjugated CD34 antibody delivery and imaging. Acta
Biomater. 146:421–433. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang Y, Malcolm DW and Benoit DSW:
Controlled and sustained delivery of siRNA/NPs from hydrogels
expedites bone fracture healing. Biomaterials. 139:127–138. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jing L, Qu H, Wu D, Zhu C, Yang Y, Jin X,
Zheng J, Shi X, Yan X and Wang Y: Platelet-camouflaged
nanococktail: Simultaneous inhibition of drug-resistant tumor
growth and metastasis via a cancer cells and tumor vasculature
dual-targeting strategy. Theranostics. 8:2683–2695. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hou J, Wang L, Wang C, Zhang S, Liu H, Li
S and Wang X: Toxicity and mechanisms of action of titanium dioxide
nanoparticles in living organisms. J Environ Sci (China). 75:40–53.
2019. View Article : Google Scholar
|
|
72
|
Haghighat F, Kim Y, Sourinejad I, Yu IJ
and Johari SA: Titanium dioxide nanoparticles affect the toxicity
of silver nanoparticles in common carp (Cyprinus carpio).
Chemosphere. 262:1278052021. View Article : Google Scholar
|
|
73
|
Miller MR, Raftis JB, Langrish JP, McLean
SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere
AJF, et al: Inhaled nanoparticles accumulate at sites of vascular
disease. ACS Nano. 11:4542–4552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Czubacka E and Czerczak S: Are platinum
nanoparticles safe to human health? Med Pr. 70:487–495. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sun D, Chen J, Wang Y, Ji H, Peng R, Jin L
and Wu W: Advances in refunctionalization of erythrocyte-based
nanomedicine for enhancing cancer-targeted drug delivery.
Theranostics. 9:6885–6900. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li P, Wang D, Hu J and Yang X: The role of
imaging in targeted delivery of nanomedicine for cancer therapy.
Adv Drug Deliv Rev. 189:1144472022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen W, Schilperoort M, Cao Y, Shi J,
Tabas I and Tao W: Macrophage-targeted nanomedicine for the
diagnosis and treatment of atherosclerosis. Nat Rev Cardiol.
19:228–249. 2022. View Article : Google Scholar
|
|
78
|
Patra S, Singh M, Wasnik K, Pareek D,
Gupta PS, Mukherjee S and Paik P: Polymeric nanoparticle based
diagnosis and nanomedicine for treatment and development of
vaccines for cerebral malaria: A review on recent advancement. ACS
Appl Bio Mater. 4:7342–7365. 2021. View Article : Google Scholar
|
|
79
|
Choi KA, Kim JH, Ryu K and Kaushik N:
Current nanomedicine for targeted vascular disease treatment:
Trends and perspectives. Int J Mol Sci. 23:123972022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Aili T, Zong JB, Zhou YF, Liu YX, Yang XL,
Hu B and Wu JH: Recent advances of self-assembled nanoparticles in
the diagnosis and treatment of atherosclerosis. Theranostics.
14:7505–7533. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hossaini Nasr S, Rashidijahanabad Z,
Ramadan S, Kauffman N, Parameswaran N, Zinn KR, Qian C, Arora R,
Agnew D and Huang X: Effective atherosclerotic plaque inflammation
inhibition with targeted drug delivery by hyaluronan conjugated
atorvastatin nanoparticles. Nanoscale. 12:9541–9556. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu X, Lin C, Zhong W, Yuan Z, Yan P and
Guan S: Effective attenuation of arteriosclerosis following
lymphatic-targeted delivery of hyaluronic acid-decorated rapamycin
liposomes. Int J Nanomedicine. 18:4403–4419. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu H, She P, Ma B, Zhao Z, Li G and Wang
Y: ROS responsive nanoparticles loaded with lipid-specific AIEgen
for atherosclerosis-targeted diagnosis and bifunctional therapy.
Biomaterials. 288:1217342022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Karami Z, Mehrzad J, Akrami M and
Hosseinkhani S: Anti-inflammation-based treatment of
atherosclerosis using Gliclazide-loaded biomimetic nanoghosts. Sci
Rep. 13:138802023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Groner J, Tognazzi M, Walter M,
Fleischmann D, Mietzner R, Ziegler CE, Goepferich AM and Breunig M:
Encapsulation of pioglitazone into Polymer-nanoparticles for
potential treatment of atherosclerotic diseases. ACS Appl Bio
Mater. 6:2111–2121. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zou L, Zhang Y, Cheraga N, Abodunrin OD,
Qu KY, Qiao L, Ma YQ, Chen LJ and Huang NP: Chlorin e6 (Ce6)-loaded
plaque-specific liposome with enhanced photodynamic therapy effect
for atherosclerosis treatment. Talanta. 265:1247722023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhu L, Li H, Li J, Zhong Y, Wu S, Yan M,
Ni S, Zhang K, Wang G, Qu K, et al: Biomimetic nanoparticles to
enhance the reverse cholesterol transport for selectively
inhibiting development into foam cell in atherosclerosis. J
Nanobiotechnology. 21:3072023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xu Z, Wu Z, Huang S, Ye K, Jiang Y, Liu J,
Liu J, Lu X and Li B: A metal-organic framework-based
immunomodulatory nanoplatform for anti-atherosclerosis treatment. J
Control Release. 354:615–625. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ma Q, Wu S, Yang L, Wei Y, He C, Wang W,
Zhao Y, Wang Z, Yang S, Shi D, et al: Hyaluronic Acid-guided
cerasome nano-agents for simultaneous imaging and treatment of
advanced atherosclerosis. Adv Sci (Weinh). 10:e22024162022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xu H, She P, Zhao Z, Ma B, Li G and Wang
Y: Duplex responsive nanoplatform with cascade targeting for
atherosclerosis photoacoustic diagnosis and multichannel
combination therapy. Adv Mater. 35:e23004392023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huang X, Zhang Y, Zhang W, Qin C, Zhu Y,
Fang Y, Wang Y, Tang C and Cao F: Osteopontin-targeted and
PPARδ-agonist-loaded nanoparticles efficiently reduce
atherosclerosis in apolipoprotein E−/− mice. ACS Omega.
7:28767–28778. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sheng J, Zu Z, Zhang Y, Zhu H, Qi J, Zheng
T, Tian Y and Zhang L: Targeted therapy of atherosclerosis by
zeolitic imidazolate framework-8 nanoparticles loaded with losartan
potassium via simultaneous lipid-scavenging and anti-inflammation.
J Mater Chem B. 10:5925–5937. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Boersma B, Möller K, Wehl L, Puddinu V,
Huard A, Fauteux-Daniel S, Bourquin C, Palmer G and Bein T:
Inhibition of IL-1β release from macrophages targeted with
necrosulfonamide-loaded porous nanoparticles. J Control Release.
351:989–1002. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhou H, You P, Liu H, Fan J, Tong C, Yang
A, Jiang Y and Liu B: Artemisinin and Procyanidins loaded
multifunctional nanocomplexes alleviate atherosclerosis via
simultaneously modulating lipid influx and cholesterol efflux. J
Control Release. 341:828–843. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mehta S, Bongcaron V, Nguyen TK, Jirwanka
Y, Maluenda A, Walsh APG, Palasubramaniam J, Hulett MD, Srivastava
R, Bobik A, et al: An Ultrasound-responsive theranostic
Cyclodextrin-loaded nanoparticle for multimodal imaging and therapy
for atherosclerosis. Small. 18:e22009672022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cheraga N, Ye Z, Xu MJ, Zou L, Sun NC,
Hang Y, Shan CJ, Yang ZZ, Chen LJ and Huang NP: Targeted therapy of
atherosclerosis by pH-sensitive hyaluronic acid nanoparticles
co-delivering all-trans retinal and rapamycin. Nanoscale.
14:8709–8726. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Song K, Tang Z, Song Z, Meng S, Yang X,
Guo H, Zhu Y and Wang X: Hyaluronic Acid-functionalized mesoporous
silica nanoparticles loading simvastatin for targeted therapy of
atherosclerosis. Pharmaceutics. 14:12652022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sun W, Xu Y, Yao Y, Yue J, Wu Z, Li H,
Shen G, Liao Y, Wang H and Zhou W: Self-oxygenation mesoporous
MnO2 nanoparticles with ultra-high drug loading capacity
for targeted arteriosclerosis therapy. J Nanobiotechnology.
20:882022. View Article : Google Scholar
|
|
99
|
De Negri Atanasio G, Ferrari PF, Baião A,
Perego P, Sarmento B, Palombo D and Campardelli R: Bevacizumab
encapsulation into PLGA nanoparticles functionalized with
immunouteroglobin-1 as an innovative delivery system for
atherosclerosis. Int J Biol Macromol. 221:1618–1630. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
du Toit LC, Hulisani Demana P and Essop
Choonara Y: A nano-enabled biotinylated anti-LDL theranostic system
to modulate systemic LDL cholesterol. Int J Pharm. 628:1222582022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Deuringer B, Härdtner C, Krebs K, Thomann
R, Holzer M, Hilgendorf I and Süss R: Everolimus-loaded
reconstituted high-density lipoprotein prepared by a novel dual
centrifugation approach for Anti-atherosclerotic therapy. Int J
Nanomedicine. 17:5081–5097. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wei B, Li Y, Ao M, Shao W, Wang K, Rong T,
Zhou Y and Chen Y: Ganglioside GM3-functionalized reconstituted
high-density lipoprotein (GM3-rHDL) as a novel nanocarrier enhances
antiatherosclerotic efficacy of statins in apoE−/−
C57BL/6 mice. Pharmaceutics. 14:25342022. View Article : Google Scholar
|
|
103
|
Jebari-Benslaiman S, Uribe KB,
Benito-Vicente A, Galicia-Garcia U, Larrea-Sebal A, Santin I,
Alloza I, Vandenbroeck K, Ostolaza H and Martín C: Boosting
cholesterol efflux from foam cells by sequential administration of
rHDL to deliver MicroRNA and to remove cholesterol in a triple-cell
2D atherosclerosis model. Small. 18:e21059152022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang Z, Zhuang J, Sun D, Ding Q, Zheng H,
Li H, Zhang X, Du Y, Ma T and Meng Q: Netrin-1 monoclonal
antibody-functionalized nanoparticle loaded with metformin prevents
the progression of abdominal aortic aneurysms. Int J Nanomedicine.
18:627–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen X, Wu Y, Li R, Li C, Xu L, Qiao W and
Dong N: Galactose-modified nanoparticles for delivery of microRNA
to mitigate the progress of abdominal aortic aneurysms via
regulating macrophage polarization. Nanomedicine. 44:1025642022.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dhital S, Rice CD and Vyavahare NR:
Reversal of elastase-induced abdominal aortic aneurysm following
the delivery of nanoparticle-based pentagalloyl glucose (PGG) is
associated with reduced inflammatory and immune markers. Eur J
Pharmacol. 910:1744872021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fukuhara N, Honda Y, Ukita N, Matsui M,
Miura Y and Hoshina K: Efficient suppression of abdominal aortic
aneurysm expansion in rats through systemic administration of
statin-loaded nanomedicine. Int J Mol Sci. 21:87022020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Cheng J, Zhang R, Li C, Tao H, Dou Y, Wang
Y, Hu H and Zhang J: A Targeting nanotherapy for abdominal aortic
aneurysms. J Am Coll Cardiol. 72:2591–2605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Nosoudi N, Chowdhury A, Siclari S,
Karamched S, Parasaram V, Parrish J, Gerard P and Vyavahare N:
Reversal of vascular calcification and aneurysms in a rat model
using dual targeted therapy with EDTA- and PGG-loaded
nanoparticles. Theranostics. 6:1975–1987. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Boada C, Zinger A, Tsao C, Zhao P,
Martinez JO, Hartman K, Naoi T, Sukhoveshin R, Sushnitha M,
Molinaro R, et al: Rapamycin-loaded biomimetic nanoparticles
reverse vascular inflammation. Circ Res. 126:25–37. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bhatia SN, Chen X, Dobrovolskaia MA and
Lammers T: Cancer nanomedicine. Nat Rev Cancer. 22:550–556. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Luo XM, Yan C and Feng YM: Nanomedicine
for the treatment of diabetes-associated cardiovascular diseases
and fibrosis. Adv Drug Deliv Rev. 172:234–248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
van Tilborg GAF, Vucic E, Strijkers GJ,
Cormode DP, Mani V, Skajaa T, Reutelingsperger CP, Fayad ZA, Mulder
WJ and Nicolay K: Annexin A5-functionalized bimodal nanoparticles
for MRI and fluorescence imaging of atherosclerotic plaques.
Bioconjug Chem. 21:1794–1803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Marrache S and Dhar S: Biodegradable
synthetic high-density lipoprotein nanoparticles for
atherosclerosis. Proc Natl Acad Sci USA. 110:9445–9450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang H, Gong Y, Wang Z, Jiang L, Chen R,
Fan X, Zhu H, Han L, Li X, Xiao J and Kong X: Apelin inhibits the
proliferation and migration of rat PASMCs via the activation of
PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia.
J Cell Mol Med. 18:542–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zheng H, Zhai W, Zhong C, Hong Q, Li H,
Rui B, Zhu X, Que D, Feng L, Yu B, et al: Nkx2-3 induces autophagy
inhibiting proliferation and migration of vascular smooth muscle
cells via AMPK/mTOR signaling pathway. J Cell Physiol.
236:7342–7355. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chan JW, Lewis DR, Petersen LK, Moghe PV
and Uhrich KE: Amphiphilic macromolecule nanoassemblies suppress
smooth muscle cell proliferation and platelet adhesion.
Biomaterials. 84:219–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Anghelache M, Voicu G, Deleanu M, Turtoi
M, Safciuc F, Anton R, Boteanu D, Fenyo IM, Manduteanu I,
Simionescu M and Calin M: Biomimetic nanocarriers of Pro-resolving
lipid mediators for resolution of inflammation in atherosclerosis.
Adv Healthc Mater. 13:e23022382024. View Article : Google Scholar
|
|
119
|
Deshpande D, Kethireddy S, Janero DR and
Amiji MM: Therapeutic efficacy of an ω-3-fatty acid-containing 17-β
estradiol Nano-delivery system against experimental
atherosclerosis. PLoS One. 11:e01473372016. View Article : Google Scholar
|
|
120
|
Sano M, Akagi D, Naito M, Hoshina K,
Miyata K, Kataoka K and Ishihara S: Systemic single administration
of anti-inflammatory microRNA 146a-5p loaded in polymeric
nanomedicines with active targetability attenuates neointimal
hyperplasia by controlling inflammation in injured arteries in a
rat model. FASEB J. 36:e224862022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Basatemur GL, Jørgensen HF, Clarke MCH,
Bennett MR and Mallat Z: Vascular smooth muscle cells in
atherosclerosis. Nat Rev Cardiol. 16:727–744. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Bentzon JF, Otsuka F, Virmani R and Falk
E: Mechanisms of plaque formation and rupture. Circ Res.
114:1852–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Halcox JPJ, Schenke WH, Zalos G,
Mincemoyer R, Prasad A, Waclawiw MA, Nour KR and Quyyumi AA:
Prognostic value of coronary vascular endothelial dysfunction.
Circulation. 106:653–658. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hou L, Zhang M, Liu L, Zhong Q, Xie M and
Zhao G: Therapeutic applications of nanomedicine in metabolic
diseases by targeting the endothelium. QJM. 116:493–501. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Deng Y, Deng X, Li Y, Tian J, Wu M, Tang
J, Liang X, Yang X, He X, Liu Y, et al: Size-adjustable
nanoparticles co-target macrophages and endothelial cells for
enhanced atherosclerosis therapy. Colloids Surf B Biointerfaces.
255:1149522025. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang Y, Feng Y, Yang X, Wang W and Wang Y:
Diagnosis of atherosclerotic plaques using vascular endothelial
growth factor receptor-2 targeting antibody Nano-microbubble as
ultrasound contrast agent. Comput Math Methods Med.
2022:65245922022.PubMed/NCBI
|
|
127
|
Bu T, Li Z, Hou Y, Sun W, Zhang R, Zhao L,
Wei M, Yang G and Yuan L: Exosome-mediated delivery of
inflammation-responsive mRNA for controlled atherosclerosis
treatment. Theranostics. 11:9988–10000. 2021. View Article : Google Scholar :
|
|
128
|
Distasio N, Dierick F, Ebrahimian T,
Tabrizian M and Lehoux S: Design and development of branched
Poly(β-aminoester) nanoparticles for Interleukin-10 gene delivery
in a mouse model of atherosclerosis. Acta Biomater. 143:356–371.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Song C, Wang Y, Cui L, Yan F and Shen S:
Triptolide attenuates lipopolysaccharide-induced inflammatory
responses in human endothelial cells: Involvement of NF-κB pathway.
BMC Complement Altern Med. 19:1982019. View Article : Google Scholar
|
|
130
|
Lai K, Li Y, Gong Y, Li L, Huang C, Xu F,
Zhong X and Jin C: Triptolide-nanoliposome-APRPG, a novel
sustained-release drug delivery system targeting vascular
endothelial cells, enhances the inhibitory effects of triptolide on
laser-induced choroidal neovascularization. Biomed Pharmacother.
131:1107372020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhu ML, Wang G, Wang H, Guo YM, Song P, Xu
J, Li P, Wang S and Yang L: Amorphous nano-selenium quantum dots
improve endothelial dysfunction in rats and prevent atherosclerosis
in mice through Na/H exchanger 1 inhibition. Vascul Pharmacol.
115:26–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Beconcini D, Fabiano A, Zambito Y, Berni
R, Santoni T, Piras AM and Di Stefano R: Chitosan-based
nanoparticles containing cherry Extract from Prunus avium L. to
improve the resistance of endothelial cells to oxidative stress.
Nutrients. 10:15982018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ke S, Lai Y, Zhou T, Li L, Wang Y, Ren L
and Ye S: Molybdenum disulfide nanoparticles resist oxidative
Stress-mediated impairment of autophagic flux and mitigate
endothelial cell senescence and angiogenic dysfunctions. ACS
Biomater Sci Eng. 4:663–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yan H, Hu Y, Lyu Y, Akk A, Hirbe AC,
Wickline SA, Pan H, Roberson EDO and Pham CTN: Augmented expression
of superoxide dismutase 2 mitigates progression and rupture of
experimental abdominal aortic aneurysm. Theranostics. 15:4016–4032.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lockhart JH, VanWye J, Banerjee R,
Wickline SA, Pan H and Totary-Jain H: Self-assembled miRNA-switch
nanoparticles target denuded regions and prevent restenosis. Mol
Ther. 29:1744–1757. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Pham LM, Kim EC, Ou W, Phung CD, Nguyen
TT, Pham TT, Poudel K, Gautam M, Nguyen HT, Jeong JH, et al:
Targeting and clearance of senescent foamy macrophages and
senescent endothelial cells by antibody-functionalized mesoporous
silica nanoparticles for alleviating aorta atherosclerosis.
Biomaterials. 269:1206772021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhang BF, Jiang H, Chen J, Hu Q, Yang S
and Liu XP: Silica-coated magnetic nanoparticles labeled
endothelial progenitor cells alleviate ischemic myocardial injury
and improve long-term cardiac function with magnetic field guidance
in rats with myocardial infarction. J Cell Physiol.
234:18544–18559. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yu B, Li H, Zhang Z, Chen P, Wang L, Fan
X, Ning X, Pan Y, Zhou F, Hu X, et al: Extracellular vesicles
engineering by silicates-activated endothelial progenitor cells for
myocardial infarction treatment in male mice. Nat Commun.
14:20942023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang Z, Yang Y, Xiong K, Li X, Qi P, Tu Q,
Jing F, Weng Y, Wang J and Huang N: Nitric oxide producing coating
mimicking endothelium function for multifunctional vascular stents.
Biomaterials. 63:80–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Huang SF, Zhao G, Peng XF and Ye WC: The
pathogenic role of long Non-coding RNA H19 in atherosclerosis the
miR-146a-5p/ANGPTL4 pathway. Front Cardiovasc Med. 8:7701632021.
View Article : Google Scholar
|
|
141
|
Sager HB, Dutta P, Dahlman JE, Hulsmans M,
Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K,
et al: RNAi targeting multiple cell adhesion molecules reduces
immune cell recruitment and vascular inflammation after myocardial
infarction. Sci Transl Med. 8:342ra3802016. View Article : Google Scholar
|
|
142
|
Orecchioni M, Kobiyama K, Winkels H,
Ghosheh Y, McArdle S, Mikulski Z, Kiosses WB, Fan Z, Wen L, Jung Y,
et al: Olfactory receptor 2 in vascular macrophages drives
atherosclerosis by NLRP3-dependent IL-1 production. Science.
375:214–221. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Seneviratne AN, Edsfeldt A, Cole JE,
Kassiteridi C, Swart M, Park I, Green P, Khoyratty T, Saliba D,
Goddard ME, et al: Interferon regulatory factor 5 controls necrotic
core formation in atherosclerotic lesions by impairing
efferocytosis. Circulation. 136:1140–1154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Stoneman V, Braganza D, Figg N, Mercer J,
Lang R, Goddard M and Bennett M: Monocyte/macrophage suppression in
CD11b diphtheria toxin receptor transgenic mice differentially
affects atherogenesis and established plaques. Circ Res.
100:884–893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gao C, Huang Q, Liu C, Kwong CHT, Yue L,
Wan JB, Lee SMY and Wang R: Treatment of atherosclerosis by
macrophage-biomimetic nanoparticles via targeted pharmacotherapy
and sequestration of proinflammatory cytokines. Nat Commun.
11:26222020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Boada C, Zinger A, Tsao C, Zhao P,
Martinez JO, Hartman K, Naoi T, Sukhoveshin R, Sushnitha M,
Molinaro R, et al: Rapamycin-loaded biomimetic nanoparticles
reverse vascular inflammation. Circ Res. 126:25–37. 2020.
View Article : Google Scholar
|
|
147
|
Zhang X, Misra SK, Moitra P, Zhang X,
Jeong SJ, Stitham J, Rodriguez-Velez A, Park A, Yeh YS, Gillanders
WE, et al: Use of acidic nanoparticles to rescue macrophage
lysosomal dysfunction in atherosclerosis. Autophagy. 19:886–903.
2023. View Article : Google Scholar :
|
|
148
|
Stachyra K, Wiśniewska A, Kiepura A, Kuś
K, Rolski F, Czepiel K, Chmura Ł, Majka G, Surmiak M, Polaczek J,
et al: Inhaled silica nanoparticles exacerbate atherosclerosis
through skewing macrophage polarization towards M1 phenotype.
Ecotoxicol Environ Saf. 230:1131122021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Izquierdo M, Merchant RA, Morley JE, Anker
SD, Aprahamian I, Arai H, Aubertin-Leheudre M, Bernabei R, Cadore
EL, Cesari M, et al: International exercise recommendations in
older adults (ICFSR): Expert elines. J Nutr Health Aging.
25:824–853. 2021. View Article : Google Scholar
|
|
150
|
Bierer BE, Zarin DA and Gelinas L:
Deprioritization of ongoing clinical trials. Ethics Hum Res.
45:27–33. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang J, Ding Y, Chong K, Cui M, Cao Z,
Tang C, Tian Z, Hu Y, Zhao Y and Jiang S: Recent advances in lipid
nanoparticles and their safety concerns for mRNA delivery. Vaccines
(Basel). 12:11482024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Jiang Y, Zhou Y, Li Z and Guo L:
Nanomedicine in cardiovascular and cerebrovascular diseases:
Targeted nanozyme therapies and their clinical potential and
current challenges. J Nanobiotechnology. 23:5432025. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang J, Zhao W, Zhang Z, Liu X, Xie T,
Wang L, Xue Y and Zhang Y: A Journey of challenges and victories: A
bibliometric worldview of nanomedicine since the 21st Century. Adv
Mater. 36:e23089152024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Macário-Soares A, Sousa-Oliveira I,
Correia M, Pires PC, Sharma A, Kumar Jha N, Zare EN, Veiga F, Gowda
BHJ, Borzacchiello A, et al: Cell membrane and extracellular
vesicle membrane-coated nanoparticles: An envisaged approach for
the management of skin conditions. View. 5:202400432024. View Article : Google Scholar
|
|
155
|
Ghosh A, Gupta A, Jena S, Kirti A,
Choudhury A, Saha U, Sinha A, Kumari S, Kujawska M, Kaushik A and
Verma SK: Advances in posterity of visualization in paradigm of
nano-level ultra-structures for nano-bio interaction studies. View.
6:202400422025. View Article : Google Scholar
|
|
156
|
Li Y, Liang D, Wang R, Yang S, Liu W, Sang
Q, Pu J, Wang Y and Qian K: Interfacial Self-assembly
nanostructures: Constructions and applications. Small.
20:e24053182024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zhang Z, Li Y, Wang R, Yang S, Li P, Zhao
K, Gu Y, Meng K, Li J, Pu J, et al: Plasmonic alloys enhance
metabolic fingerprints for rapid diagnosis and classification of
myocardial infarction. Nano Today. 62:1027022025. View Article : Google Scholar
|
|
158
|
Nel AE, Mädler L, Velegol D, Xia T, Hoek
EM, Somasundaran P, Klaessig F, Castranova V and Thompson M:
Understanding biophysicochemical interactions at the nano-bio
interface. Nat Mater. 8:543–557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang Q, Zhang J, Song J, Liu Y, Ren X and
Zhao Y: Protein-based nanomedicine for therapeutic benefits of
cancer. ACS Nano. 15:8001–8038. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Luo B, Xiang D, Ji X, Chen X, Li R, Zhang
S, Meng Y, Nieman DC and Chen P: The anti-inflammatory effects of
exercise on autoimmune diseases: A 20-year systematic review. J
Sport Health Sci. 13:353–367. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S,
Liu C, Barnes S, Grizzle W, Miller D and Zhang HG: A novel
nanoparticle drug delivery system: The anti-inflammatory activity
of curcumin is enhanced when encapsulated in exosomes. Mol Ther.
18:1606–1614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Cui C, Du M, Zhao Y, Tang J, Liu M, Min G,
Chen R, Zhang Q, Sun Z and Weng H: Functional Ginger-derived
extracellular vesicles-coated ZIF-8 containing TNF-α siRNA for
ulcerative colitis therapy by modulating gut microbiota. ACS Appl
Mater Interfaces. 16:53460–53473. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang E, Rossman MJ, Groot HJ, Garten RS,
Jarrett CL, Helgerud J, Hoff J and Richardson RS: Leg vascular
function with advancing age in men: The impact of physical activity
and endurance exercise training. J Appl Physiol (1985).
139:473–481. 2025. View Article : Google Scholar : PubMed/NCBI
|