Introduction
Pain, recognized as one of humanity's most
fundamental physiological and psychological experiences, has been
designated by the international medical community as the 'fifth
vital sign' following blood pressure, temperature, respiration, and
pulse (1). With global aging
populations and increasing burdens of chronic diseases, the
prevalence of pain has risen steadily and severely compromised
patients' quality of life (2).
This highlights the urgent need for innovative analytical
approaches. To address this, the present study used the integration
of multi-pathway analysis to establish a systematic analytical
framework, aiming to advance precision diagnosis and treatment of
pain.
Definition of pain
In medical science, pain is defined as a complex
feeling that extends beyond mere physiological tissue injury. It
involves intricate interactions among psychological, emotional and
social factors (3). The
International Association for the Study of Pain revised its
definition in 2020, emphasizing that pain is 'an unpleasant sensory
and emotional experience associated with actual or potential tissue
damage, or described in terms of such damage' (4). This definition transcends
traditional biological perspectives, underscoring the subjectivity
and variability of pain. Individual perceptions of pain are
influenced by multiple factors, including physiological status,
psychological cognition, cultural background, and social
environment (5).
Classification of pain
Modern medicine classifies pain into three
categories (6): i) Nociceptive
pain: Arising from direct or potential tissue injury. Nociceptive
pain is generally unrelated to nerve distribution but is associated
with pathological damage causing the pain, such as degenerative
changes, trauma, muscle spasm, or visceral disorders (7-10). ii) Neuropathic pain: Resulting
from peripheral or central nervous system lesions. It is typically
linked to abnormal sensory perception caused by damage to nerves
responsible for pain signal transduction and transmission (11). Clinically, neuropathic pain often
manifests as spontaneous, persistent severe pain (12). iii) Central sensitization-induced
pain: Caused by central nervous system sensitization in the absence
of overt tissue damage or neurological lesions (13).
Clinical evaluation, management and
medical significance
The clinical evaluation and management of pain must
incorporate both objective and subjective dimensions. Quantitative
tools such as the visual analogue scale (VAS) or numeric rating
scale (NRS) are commonly used to quantify pain assessment, while
multidimensional instruments such as the McGill pain questionnaire
(MPQ) further analyze pain characteristics, emotional experiences
and effects on quality of life (14). For neuropathic pain, definitive
diagnosis requires combined neuroelectrophysiological testing.
Therapeutic strategies emphasize concurrent use of pharmacological
and non-pharmacological interventions (15). For chronic pain,
multidisciplinary collaboration models have become mainstream,
aiming to restore functional capacity through comprehensive
medical, psychological and social support interventions rather than
solely pursuing 'pain-free' outcomes (16). The medical significance of pain
lies not only in its role as a pathological warning signal but also
in its status as a critical global public health issue. The
resulting socioeconomic burdens and diminished quality of life
demand systematic solutions (17).
Research objectives and innovations
The present review aimed to achieve three
interrelated and clinically oriented goals, while addressing the
limitations of traditional reviews and opening up new perspectives
for pain research. However, existing research has mostly focused on
analyzing analgesic mechanisms through a single pathway (18-20). To overcome this limitation, the
present review integrated multi-channel analysis methods and
established a systematic analysis framework. The present review
innovatively combined research methods such as in-depth analysis of
molecular mechanisms, optimization of transformation models and
interdisciplinary technology integration. In summary, the aims and
innovations of these summaries and analyses are to bridge the gap
between basic research and clinical practice, providing theoretical
support for developing personalized pain management strategies and
constructing a precise pain diagnosis and treatment system, to
accelerate the transformation and application of mechanism oriented
therapies and ultimately to promote pain management research into a
new era of high-efficiency, low toxicity and personalized
treatment.
Methods
To comprehensively review the research progress on
models, mechanisms, therapies and prospect of pain, the present
review conducted a search in existing scientific databases using
the terms 'pain model', 'pain mechanism', 'pain therapy', 'pain
prospect'. Relevant literature on pain was obtained from both
online and offline databases, covering the period from 1987-2025,
totaling 334 references. Online databases included Elsevier
(https://www.elsever.com/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://www.webof-science.com/), Google
Scholar (https://scholar.google.com/), Wiley
(https://www.wiley.com/), EMBASE (https://www.embase.com/), SpringerLink (https://link.springer.com/), Cochrane Library
(https://clinicaltrials.gov/) and China
national knowledge infrastructure (CNKI) (https://www.cnki.net/). Other related references were
sourced from pharmacopoeias and other articles. To ensure a
comprehensive review, the literature search strategy was designed
to encompass both modern scientific research and historical
scholarly works. For official pharmacopoeias (such as Chinese
Pharmacopoeia) and articles from non-mainstream journals, specific
inclusion criteria were established. These sources were included
based on their recognized historical importance, foundational role
in theory development, or provision of unique data not available in
peer-reviewed journals. Figs.
1-6 were drawn by BioGDP
(https://biogdp.com/) in this paper.
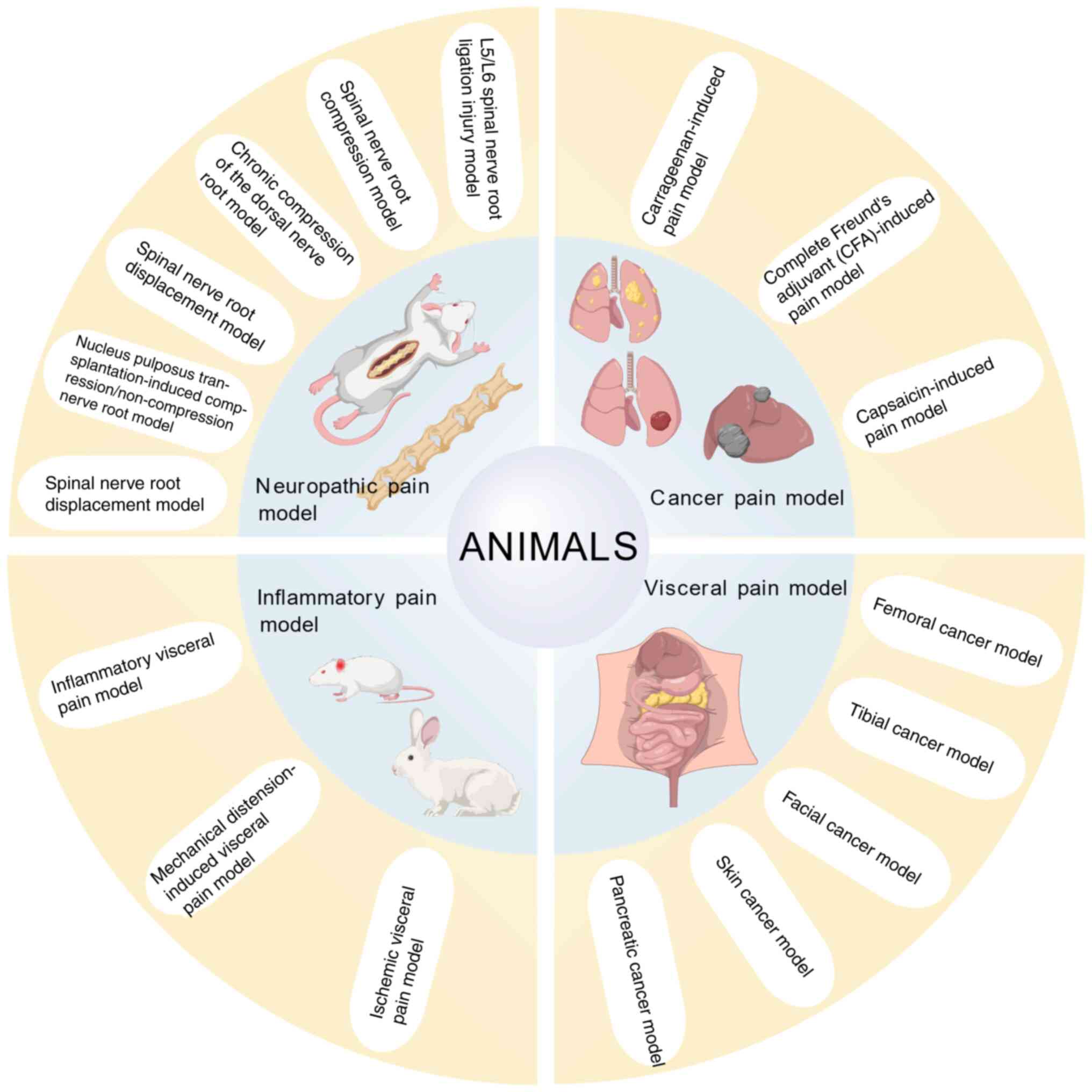
Models of pain
The selection of experimental animals for pain
models demonstrates remarkable diversity (Data S1). Primate species
exhibit high anatomical and physiological similarity to humans,
enabling superior simulation of human disease progression and
serving as ideal candidates for chronic pain modeling. Notably,
their advanced nervous systems, including complex cortical pain
processing pathways and homologous pain-related brain regions (such
as the anterior cingulate cortex and insula), allow for the study
of subjective pain-like experiences (such as pain-related anxiety
or cognitive modulation of pain) that are difficult to replicate in
lower-order species; this unique advantage makes primates
invaluable for validating the translational potential of novel
analgesics targeting central pain mechanisms, especially for
chronic conditions such as post-herpetic neuralgia where human
cognitive and emotional factors strongly influence pain outcomes.
However, their demanding husbandry conditions, limited
availability, high costs and ethical constraints have restricted
their widespread adoption. Successful pain studies have been
conducted using large mammals such as pigs, dogs and cats (21), though their adoption is limited
by substantial practical and ethical challenges, further limiting
their scalability. Rodents are the most widely used models due to
their physiological similarity to humans, low cost, ease of
handling and scalability, enabling reliable mechanistic and
translational studies (22).
Additionally, zebrafish, fruit flies and nematodes (23,24) demonstrate significant promise in
pain genetics and molecular mechanism studies. Their
well-characterized genome sequences, controllable genetic
manipulations, simple body architectures and short life cycles
offer unique advantages for investigating pain-related cellular and
molecular pathways, warranting broader application in this
field.
Since experimental animals cannot express pain
directly as can humans, specific assessment methods are needed to
indirectly judge their pain status. Clinical evaluation of pain in
patients relies on methods such as medical interviews, physical
examinations and standardized questionnaire assessments. However,
experimental animals cannot verbally communicate discomfort during
nociceptive stimulation, so pain assessment for them mainly falls
into two categories: Naturalistic readouts based on spontaneous
behaviors and stimulus-evoked readouts via calibrated
interventions.
Spontaneous behaviors
Spontaneous behaviors refer to unprovoked behavioral
manifestations that reflect animals' inherent responses to pain.
These include foot withdrawal reflexes, excessive grooming,
scratching, vocalization, reduced exploratory activity, increased
immobility and changes in facial expression. In pathological pain
states, animals further exhibit hyperalgesic symptoms
(characterized by enhanced responsiveness to stimuli and reduced
reaction thresholds), where mild provocation can induce spontaneous
defensive behaviors such as foot withdrawal, licking, or abdominal
retraction.
Stimulus-evoked readouts
Unlike naturalistic readouts, stimulus-evoked
readouts require active induction of behavioral alterations using
specialized equipment, instruments, or reagents with calibrated
stimuli. This approach converts pain perception into numerically
representable outcomes, enabling indirect objective assessment of
pain intensity and sensitivity. Common stimulus-evoked assessment
methods for pain sensitivity in animals include mechanical,
thermal, and cold hyperalgesia tests, as well as specialized
detection protocols. i) Mechanical hyperalgesia assessment:
Mechanical pain thresholds are determined using Von Frey filaments
applied to the plantar or abdominal regions, with the threshold
defined as the minimum stimulus intensity eliciting a rapid
withdrawal reflex (25). ii)
Thermal hyperalgesia assessment: Thermal pain thresholds are
assessed by irradiating the animal's paw with a radiant heat source
and recording the latency to withdrawal (26). Cold hyperalgesia assessment: For
cold hyperalgesia evaluation, animals are placed on a 4°C thermal
plate, and withdrawal responses (foot withdrawal, licking, jumping)
are measured by latency or frequency within a specified time frame
(27). iii) Specialized
detection method: The abdominal withdrawal reflex (AWR) experiment
evaluates visceral sensitivity and pain thresholds through gradual
pressure inflation of the rectum. Metrics such as the pressure
required to elicit abdominal muscle contractions and arching of the
spine, or the number of contractions observed under fixed pressure
over time, are recorded to assess changes in visceral
nociception.
The classification of pain models is mainly based on
three core dimensions: Etiological source, pathological mechanism
characteristics and technology construction. Integrating
longitudinal disease evolution, horizontal mechanistic interactions
and cross-validated assessments, the four pain models (neuropathic
pain, cancer-induced pain, visceral pain and inflammatory pain
animal models) constitute a systematic framework. This approach
maintains the distinctiveness of each model while collectively
illuminating the dynamic pain network, offering a stratified and
multi-faceted perspective for exploring mechanisms and devising
treatments.
The present study systematically reviewed the
preparation methods and experimental cycles of commonly used pain
models across four major categories: neuropathic pain model,
cancer-induced pain model, visceral pain model and inflammatory
pain animal model. The present review summarized the evaluation
criteria along with their advantages and limitations. The novelty
of the present review lies in its emphasis on the hierarchical,
multi-dimensional framework for pain model classification and
analysis. Unlike typical pain model reviews that predominantly
focus on individual model types, the present review highlighted the
established framework, integrating etiological sources,
pathological mechanisms and construction technologies, enabling
effective cross-validation across different pain types. By
constructing such an interconnected analytical framework, the
present review goes beyond the scope of traditional reviews that
merely catalog models and their parameters; it provided a
translational research tool to bridge gaps between different pain
model systems, guiding researchers to design more comprehensive
experiments and promoting the development of targeted analgesic
strategies applicable to multiple pain conditions. To visually
demonstrate the classification framework and representative
examples of these models, schematic diagrams are specifically
illustrated in Fig. 1.
Neuropathic pain model
Neuropathic pain arises from damage or dysfunction
of the peripheral or central somatosensory nervous system, commonly
occurring secondary to trauma, ischemia, metabolic disorders, or
toxin exposure (28). Modern
medical research has identified three primary mechanisms underlying
radicular neuropathic pain: Mechanical compression, neural root
inflammatory response and neurohumoral response. Establishing an
idealized animal model is crucial for studying these mechanisms, as
it serves as an excellent experimental platform for exploring
various clinical treatment strategies, testing novel drugs and
conducting fundamental experimental research. In the process of
researching neuropathic pain models, there are also some research
bottlenecks: i) Ethical concerns (severity of pain induction). ii)
Lack of full human translation (rodent models cannot capture
subjective aspects of pain). The present review systematically
summarized the current modeling methods, technical features and
methodological advantages and limitations of mainstream neuropathic
pain models (Table I), providing
a structured reference framework for researchers to screen models
based on experimental objectives. In Table I, SD rats were mainly selected as
experimental animals and the indicator was mechanical pain
threshold.
 | Table ICharacteristics of different types of
neuropathic pain models. |
Table I
Characteristics of different types of
neuropathic pain models.
| Authors, year | Type | Animal | Method | Time | Indicators | Advantages | Disadvantages | (Refs.) |
|---|
| Bennett et
al, 1988; Zhang et al, 2019 | Sciatic nerve
chronic constriction injury pain model | ICR mice | Sciatic nerve
ligation | 14 days | Spontaneous pain,
mechanical pain threshold, thermal pain threshold, cold pain
threshold | Simple operation,
stable pain sensitivity | Variable ligation
tightness causing inconsistent injury severity | (45,46) |
| Kim et al,
1992 | SNL pain model | SD rats | L5/L6 SNL | 16 weeks | Mechanical pain
threshold, thermal pain threshold | Consistent ligation
position and degree | Relatively complex
procedure, severe trauma with high infection risk | (29) |
| Zhu et al,
2018 | SNL pain model | ICR mice | L5 spinal nerve
ligation | 17 days | Mechanical pain
threshold, thermal pain threshold | Consistent ligation
position and degree | Relatively complex
procedure, severe trauma with high infection risk | (47) |
| Meng et al,
2020 | Spinal cord
injury | SD rats | Weight-drop impact
on central spinal cord | 28 days | Mechanical pain
threshold | Cost-effective
implementation, clinically relevant pathogenesis | Difficult impact
localization, potential rebound-induced secondary injury | (48) |
| Shih et al,
2017 | CPSP pain
model | SD rats | Collagenase
injection into thalamic ventral posterolateral nucleus | 4 weeks | Mechanical pain
threshold, thermal pain threshold | High hemorrhage
stability, high success rate, low mortality | Chronic minor
vascular leakage, Minimal hematoma mass effect | (49) |
| Lu et al,
2018 | CPSP pain
model | SD rats | Autologous blood
injection into thalamic ventral posterolateral nucleus | 35 days | Mechanical pain
threshold | Clinically
consistent intracerebral hemorrhage progression | Needle track blood
reflux impairing hematoma formatio | (50) |
| Li et al,
2019 | Infraorbital nerve
chronic constriction injury pain model | SD rats | Infraorbital nerve
ligation | 35 days | Mechanical pain
threshold | Simplified
procedure mimicking clinical trigeminal compression-induced
allodynia | Technically
demanding with significant tissue damage | (51) |
| Cao et al,
2013 | Infraorbital nerve
transection pain model | SD rats | Partial transection
of lateral infraorbital nerve | 35 days | Mechanical pain
threshold | Consistent injury
severity, prolonged pain sensitivity duration | Variable ligation
tightness causing inconsistent injury severity | (52) |
L5/L6 spinal nerve root ligation (SNL)
injury model
The SNL injury model was first established by Kim
and Chung (29). This model
employed Sprague-Dawley (SD) rats as experimental subjects. The
modeling procedure involved: ligation of the L5 and L6 spinal
nerves following exposure via a dorsal surgical approach. The SNL
model induces reliable neuropathic pain phenotypes, including
mechanical hyperalgesia and allodynia, which closely mirror
clinical radicular pain (30,31).
Subsequent studies by Huang et al (32) compared the effects of preserving
compared with resecting paravertebral muscle groups on neuropathic
pain, muscle damage and inflammatory markers. Their findings
confirmed that SNL models retaining paravertebral muscles
effectively reduce postoperative muscle injury and inflammatory
responses, thereby enhancing reproducibility and clinical
relevance. This model's strengths include precise nerve root
segment localization, facilitating nerve root-specific studies, and
controllable surgical trauma, meeting animal ethics requirements.
However, technical challenges of SNL models persist: standardized
ligation procedures directly affect reproducibility, as minor
deviations in parameters such as ligature tension, fixation
position, or surgical technique may induce inter-individual
variability, representing the primary technical bottleneck for this
model. Furthermore, the model inherently induces moderate to severe
neuropathic pain, raising ethical concerns regarding animal
welfare. Its primary translational limitation lies in the
fundamental species differences in pain processing; rodent models
cannot capture the multidimensional subjective experience of human
chronic neuropathic pain. Compared with chronic compression models,
the SNL model offers superior technical reproducibility due to its
straightforward surgical approach but demonstrates lower
pathophysiological fidelity in simulating the gradual progression
of clinical degenerative disorders. While highly suited for
studying acute nerve injury mechanisms and screening analgesic
efficacy, its utility for evaluating long-term therapeutic
interventions may be limited by the static nature of
ligation-induced injury.
Spinal nerve root compression model
The chronic spinal nerve root compression model
(CSNRC) was first established by Wang et al (33). This model used SD rats as
experimental subjects. Experimental validation confirms that
mechanical compression in this model induces axonal demyelination
of the nerve root and activates interleukin-1β (IL-1β) and tumor
necrosis factor-α (TNF-α) inflammatory cytokine cascades mediated
by spinal dorsal horn microglia, ultimately triggering classic
neuropathic pain phenotypes such as mechanical allodynia and
spontaneous pain (34). These
pathological characteristics exhibit significant correlation with
clinical radicular pain caused by lumbar spinal stenosis and
intervertebral disc herniation, establishing this model as a
reliable platform for investigating chronic compressive nerve
injury. The CSNRC model demonstrates significant technical
advancements over traditional nerve ligation approaches.
Furthermore, the model enables quantifiable stratification of
neural injury severity through precise adjustments to the
compression device thickness (0.3-0.5 mm). Additionally, its
reversible compression design provides a dynamic investigative
platform for studying decompression therapeutics. Postoperative
material displacement may introduce experimental deviations,
indicating that procedural stability optimization remains a
critical challenge requiring resolution.
Ethical considerations must be noted due to the
persistent pain state induced by chronic compression. More
importantly, while the model replicates structural compression, it
cannot model the complex psychosocial factors that markedly
influence chronic pain perception and disability in human patients.
In contrast to ligation models that create acute axonal injury,
compression models such as CSNRC provide superior
pathophysiological fidelity for degenerative conditions by
mimicking the progressive nature of clinical nerve root
compression. They exhibit clinical relevance for spinal stenosis
research and offer unique advantages for testing decompression
therapies and chronic drug interventions. However, this enhanced
fidelity comes at the cost of technical reproducibility, as the
requirement for microscopic precision in compression device
placement introduces greater procedural variability compared with
ligation techniques. The progressive nature of compression in the
CSNRC model may offer improved predictive validity for long-term
drug efficacy against chronic neuropathic pain compared with acute
injury models. However, its ability to forecast clinical outcomes
remains constrained by the same fundamental limitations of rodent
models: simplified neuroanatomy, lack of comorbid conditions, and
an inability to assess the effect of therapy on pain-related
quality of life and affect.
Chronic compression of dorsal root
ganglia model (CCDRG)
The CCDRG model was optimized by Zhang et al
(35). This model involved
implanting customized stainless steel rods into the L5
intervertebral foramen to induce sustained compression of dorsal
root ganglia. Experimental evidence confirms that the CCDRG model
triggers abnormal neuronal discharges in compressed dorsal root
ganglia, leading to mechanical hyperalgesia and spontaneous pain
behaviors. Its pathological mechanisms involve increased neuronal
excitability, inflammatory factor release and glial cell
activation, demonstrating significant correlation with clinically
observed radicular pain caused by intervertebral foramen stenosis
(36). The advantage of this
model is its minimally invasive foraminal approach, which achieves
ganglia-specific compression without spinal canal opening, thereby
reducing surgical infection risks while preserving neural pathway
integrity. A study has shown that compared with traditional nerve
root exposure models, the CCDRG model increases postoperative
survival rates by ~30% and more accurately simulates the chronic
compressive pathology of intervertebral disc herniation (35). However, anatomical differences in
the intervertebral foramen of different sizes of rats require
custom steel rod sizes, resulting in higher experimental costs and
potential implant displacement-key technical bottlenecks that can
affect the stability of experimental data. From an ethical
standpoint, the chronic nature of the compression involves
prolonged animal suffering. A fundamental limitation for clinical
translation is the model's inability to account for the top-down
neuromodulatory and psychological components that are integral to
the human chronic pain experience.
Spinal nerve root displacement model
The spinal nerve root displacement model was
developed by Finskas et al (37) and employs biomechanical
stimulation of nerve roots through intervertebral disc puncture.
Some studies demonstrate that mechanical traction in this model
induces abnormal neuronal discharges in dorsal root ganglia,
leading to ipsilateral hindpaw mechanical hyperalgesia and motor
dysfunction, with pain characteristics closely mimicking the
pathological progression of nerve root edema secondary to clinical
intervertebral disc herniation (38). Mechanistic investigations reveal
a positive correlation between puncture depth and disc degeneration
rate, with radicular pain arising from dual mechanisms: Enhanced
mechanical stimulation due to reduced intervertebral space height
and peripheral sensitization triggered by elevated pro-inflammatory
cytokines (IL-6, TNF-α) in dorsal root ganglia. This combined
structural injury and inflammatory activation makes the model an
ideal platform for studying dynamic nerve root compression
mechanisms. Compared with traditional static compression models,
its advantages lie in simulating the pathophysiological process of
post-herniation nerve root displacement, retaining vascular supply
system integrity, and exhibiting pronounced laterality-specific
pain behavioral indices. However, precise control of the puncture
angle and bony fixation stability of the puncture needle are
critical technical factors ensuring experimental reproducibility.
Ethical oversight is crucial given the invasive disc puncture
procedure. The model's translational relevance is constrained by
the inherent inability of rodent models to replicate the human
subjective pain experience and the complex brain-level changes
associated with chronic pain.
Nucleus pulposus transplantation
model
The autologous nucleus pulposus transplantation
model, co-developed by Shamji et al (39) and Kim et al (40), focuses on exploiting the chemical
irritative effects of autologous disc tissue. This model employs SD
rats as experimental subjects. Experimental validation confirms
that transplanted nucleus pulposus tissue directly activates
transient receptor potential vanilloid (TRPV) 1 channels in dorsal
root ganglia by releasing inflammatory mediators such as IL-6 and
prostaglandin E2 (PGE2), inducing classic neuropathic pain
manifestations (41). Cho et
al (42) demonstrated
through model modifications that even without mechanical
compression, chemical stimulation from nucleus pulposus tissue
alone can activate satellite glial cells in dorsal root ganglia,
upregulate the nuclear factor kappa B (NF-κB) signaling pathway and
ultimately induce inflammatory radicular pain. These pathological
features closely resemble non-compressive radicular pain in
clinical disc herniation patients, providing a critical
experimental platform for studying chemically mediated radicular
pain mechanisms. The model reduces immune rejection by using
autologous tissue. It also simulates mechanical compression and
chemical stimulation, and includes stable, quantifiable behavioral
indicators of pain. However, individual differences in the
absorption rate of the nucleus pulposus may affect the duration of
the inflammatory response, so preoperative magnetic resonance
imaging (MRI) is needed to assess the severity of disc degeneration
to enhance experimental consistency. The model's limitation for
translational research stems from the rodent nervous system's
simplified processing of nociception compared with humans, failing
to incorporate the supraspinal and psychosocial determinants of
chronic pain.
Spinal nerve root chemical stimulation
model
Zhao et al (43) established the spinal nerve root
chemical stimulation model, which uses formaldehyde to simulate
non-compressive radicular pain by mimicking chemical inflammatory
mediators. Experimental evidence confirmed that
formaldehyde-induced chemical stimulation activated transient
receptor potential ankyrin (TRPA)1 channels in dorsal root ganglia,
triggering neuropeptide release (CGRP, substance P) and resulting
in persistent mechanical hyperalgesia with behavioral
characteristics highly analogous to clinical discogenic radiculitis
(44). This model transcends
traditional mechanical compression frameworks by elucidating
inflammation-mediated radicular pain mechanisms via controlling
chemical stimulation. Compared with autologous nucleus pulposus
transplantation models, its advantages includes eliminating
mechanical compression variables to focus on chemical
pathogenicity, strong dose-response controllability of formaldehyde
concentration and minimal surgical trauma with high procedural
standardization. However, technical challenges persist: Different
chemical stimulants may activate divergent pain signaling pathways,
requiring multi-group controlled experiments to validate specific
inflammatory mediators' nociceptive roles, a critical bottleneck
for broader model application. The core translational limitation
shared by all rodent models is their inability to emulate the
complex, subjective and emotionally charged nature of human chronic
pain, which is profoundly influenced by higher-order brain
functions and psychosocial context. Contemporary neuropathic pain
modeling has evolved from singular mechanical compression to
multidimensional pathological mechanism simulations, with various
models demonstrating marked specificity and complementarity in pain
mechanism research. The L5/L6 ligation model provides an ideal
platform for investigating acute nerve injury mechanisms through
precise nerve root localization, while chronic compression models
(CSNRC, CCDRG) more closely approximate the progressive
pathological processes of clinical degenerative disorders. Recently
developed dynamic compression models (spinal nerve root
displacement) and chemical stimulation models (nucleus pulposus
transplantation/formaldehyde stimulation) have transcended
traditional mechanical injury paradigms, offering novel
perspectives for deciphering inflammation-mechanics interactions in
radicular pain pathogenesis (45-47). Current model systems still face
critical bottlenecks between mechanistic fidelity and clinical
translatability. On one hand, significant neuroanatomical
disparities between rodents and humans, such as nerve root vascular
supply patterns and spatial distribution of dorsal root ganglia,
may compromise pathological translation of mechanical stimuli. On
the other hand, overreliance on reflexive nociceptive behavioral
metrics in model evaluation fails to capture the
affective-cognitive dimensions of chronic pain.
A critical and universal limitation is the inherent
inability of models to replicate the human subjective pain
experience, which is shaped by complex cortical processing,
psychological state and social factors. All models involve
ethically significant pain induction, necessitating rigorous
justification and mitigation of suffering. The selection between
ligation and compression models involves fundamental trade-offs:
While ligation offers technical simplicity and reproducibility for
acute injury studies, compression models provide superior
pathophysiological fidelity for chronic degenerative conditions
(48-50). Similarly, chemical stimulation
models excel in isolating inflammatory mechanisms but lack the
mechanical component central to a number of clinical conditions.
The optimal model choice therefore depends on the specific research
objectives, whether prioritizing mechanistic isolation, clinical
relevance, therapeutic testing applicability, or technical
practicality.
A paramount consideration that underpins all model
selection is their documented track record in predicting clinical
efficacy. The high failure rate of neuropathic pain drugs
transitioning from robust preclinical results to successful human
trials highlights a pervasive translational crisis. This disconnect
is multifactorial, stemming from over-reliance on reflexive pain
measures in animals that do not capture the multidimensional human
pain experience, fundamental species differences in drug metabolism
and pharmacokinetics, and the absence of common comorbidities (such
as anxiety or depression) in animal models that markedly modulate
drug response in patients (51,52). Therefore, while animal models
remain indispensable for mechanistic insight and initial screening,
researchers must interpret preclinical efficacy data with caution.
Future model development must prioritize the integration of outcome
measures that bridge this translational gap, such as non-reflexive
pain behaviors, functional outcomes, and if possible, measures of
affective pain components. Furthermore, employing a battery of
models that capture different aspects of the human condition,
rather than relying on a single model, may provide a more realistic
and predictive preclinical assessment of therapeutic potential.
Future model development should prioritize constructing
large-animal chronic compression models to enhance anatomical
comparability, integrating advanced behavioral assessments such as
functional neuroimaging and conditioning place aversion and
engineering transgenic animals for spatiotemporally controlling
activation of specific pain pathways.
Cancer-related pain model
Cancer-related pain is caused by malignant tumors
themselves, metastatic lesions, or anti-tumor therapies (53), commonly observed in a number of
patients with advanced-stage cancers. Bone cancer pain results from
bone metastasis in various advanced malignancies, which is a
primary pain inducer. Injecting controlled quantities of tumor
cells into different anatomical sites enables the development of
multiple cancer pain models, including facial, cutaneous and
visceral cancer pain. Rodent bone cancer pain models currently
represent the most widely used chronic cancer pain paradigms. Rats,
owing to their larger body mass and skeletal structure, permit
controllable ranges for tumor cell injection volumes, making them
prevalent in bone cancer pain model preparation. Based on tumor
type and modeling site, the following systematically summarized and
compared construction methods, characteristics and methodological
advantages/limitations of prevalent cancer pain models, providing
researchers with a reference framework for model selection aligned
with experimental objectives (Table
II).
 | Table IICharacteristics of different types of
cancer pain models. |
Table II
Characteristics of different types of
cancer pain models.
| Authors, year | Type | Animal | Method | Time | Indicators | Advantages | Disadvantages | (Refs.) |
|---|
| Schwei et
al, 1999 | Femoral cancer pain
model | B6C3-Fe-a/a,
C3H/HeJ mice | Injection of
fibrosarcoma cells into distal femoral marrow cavity | 21 days | Spontaneous pain,
mechanical pain threshold | High stability,
mass reproducibility possible | Technically
demanding, requires precise tumor cell concentration control | (72) |
| Wang et al,
2019 | Tibial cancer pain
model | SD rats | Injection of Walker
256 cells into tibial marrow cavity | 21 days | Spontaneous pain,
mechanical pain threshold | High viability and
invasiveness of Walker 256 cells | High in vivo
culture costs, prolonged experimental cycles | (73) |
| Kopruszinski et
al, 2018 | Facial cancer pain
model | Wistar rats | Injection of Walker
256 cell suspension into right vibrissal pad | 6 days | Spontaneous pain,
mechanical pain threshold, thermal pain threshold | High viability and
invasiveness of Walker 256 cells | High in vivo
culture costs, prolonged experimental cycles | (58) |
| Huang et al,
2019 | Cutaneous cancer
pain model l | SD rats | Subcutaneous
injection of Walker 256 cells into hindpaw plantar region | 17 days | Mechanical pain
threshold, thermal pain threshold | High viability and
invasiveness of Walker 256 cells | High in vivo
culture costs, prolonged experimental cycles | (74) |
| Wang et al,
2017 | Pancreatic cancer
pain model | BALB/c-nu mice | Orthotopic
implantation of SW1990 cells into pancreas | 14-28 days | Spontaneous pain,
mechanical pain threshold | High stability,
mass reproducibility possible | Technically
demanding, requires precise tumor cell concentration control | (75) |
Femoral cancer pain model
The femoral cancer model replicates clinical
pathological features of metastatic bone pain through orthotopic
tumor cell inoculation into rodent femoral marrow cavities. A study
indicated that model construction required consideration of three
key factors: Animal strain, tumor cell type and inoculation
technique (54). Critical
femoral cancer model replication technologies involved precise cell
inoculation and systematic validation. Surgical procedures require
anesthesia-assisted exposure of the femoral intercondylar fossa,
followed by slow marrow cavity infusion of cell suspensions using
26G microinjectors, with bone wax sealing post-injection to prevent
cell leakage. Pain behavioral assessments employ dynamic
monitoring: Von Frey filament testing reveals 40-60% reductions in
mechanical withdrawal thresholds post-modeling; tail compression
tests show markedly elevated pressure sensitivity correlating
positively with radiographic bone destruction severity.
Radiographic verification identifies periosteal thickening at 14
days and pathological fractures at 21 days via X-ray, while
micro-CT quantifies bone microstructure parameters to establish
structural-pain correlations (55). Histopathological analyses
confirms tumor cell infiltration and osteoclast activation, with
molecular assays demonstrating upregulated spinal dorsal horn glial
fibrillary acidic protein and IL-1β expression, indicating
neuroinflammatory involvement in pain maintenance.
Tibial cancer pain model
The tibial cancer pain model was served as a
critical tool for investigating cancer-induced bone pain mechanisms
and therapeutic strategies. The most prevalent model involves
intratibial marrow cavity injection of breast carcinoma cells,
effectively mimicking human metastatic the pathological features
and pain behaviors of bone cancer. The core mechanisms of the model
involves tumor cell-induced osteolysis and neuroinflammation
(56,57). The model also facilitates
analgesic intervention evaluation: Electroacupuncture attenuated
pain via autophagy-mediated NLRP3 suppression or NRG1/ErbB2
signaling modulation of glial activation, while opioids
paradoxically promoted tumor angiogenesis through μ opioid
receptor (MOR) upregulation. Despite standardized operability and
clear pathology, strict control of inoculation parameters and
postoperative infection prevention remain essential for model
stability and reproducibility.
Facial cancer pain model
In preclinical facial cancer pain research, the
Walker-256 tumor cell-induced rat model has become predominant
(58). Derived from rat mammary
carcinoma and maintained via intraperitoneal passage, these cells
form facial tumors demonstrating local tissue infiltration, neural
compression and inflammatory mediator release, closely resembling
clinical head/neck cancer pain mechanisms. Key evaluation metrics
included thermal hyperalgesia, mechanical allodynia and spontaneous
face-grooming behaviors (59).
Conditioned place preference testing quantifies pain relief
motivation through analgesic-paired environment preference, while
comorbid anxiety-like behaviors facilitates the study of
pain-emotion interactions (60).
The model has validated analgesics (morphine, bosentan and
pregabalin) and revealed peripheral endothelin receptor-trigeminal
sensitization linkages (61).
Despite its usefulness, interspecies differences limit
translational relevance, prompting recent attempts to engraft human
carcinoma cells in immunocompromised hosts, though applications
remained exploratory. Overall, the Walker-256 facial cancer model,
with standardized protocols, phenotype clarity and clinical pain
mimicry, remain indispensable for mechanistic and therapeutic
investigation.
Cutaneous cancer pain model
Malignant melanoma, a highly aggressive and lethal
form of skin cancer, exhibits extremely high mortality rates as
most patients are diagnosed at advanced stages (62). Although pain is not a primary
clinical symptom, 7% of patients experience pain, with >50% of
metastatic melanoma cases requiring palliative care and morphine
treatment due to neuropathic pain components (63). However, current models
predominantly target limb skin, lacking successful constructions
for other anatomical regions. Furthermore, a toe cancer model
developed ulceration and hemorrhage 14 days post-inoculation
(64), precluding oral herbal
analgesic evaluation while remaining valuable for injectable drug
mechanism studies. The plantar skin cancer model has emerged as a
novel paradigm for cancer pain following bone cancer and
chemotherapy models. Despite its limited variety, it provides
critical platforms for mechanistic and therapeutic exploration. Xie
and Wang (65) established a
validated toe skin tumor model through murine left hindpaw plantar
tumor cell injections, demonstrating pain behavior alterations.
Intrathecal amiloride administration elevated thermal pain
thresholds, alleviated thermal hypersensitivity and suppressed
spinal dorsal horn acid-sensing ion channel 3 protein expression,
offering novel clinical therapeutic insights. Tabata et al
(66) constructed melanoma pain
models via hindlimb subcutaneous melanoma cell inoculation,
revealing that tropomyosin receptor kinase A (TrkA) inhibitory
peptides reduced paw edema and melanoma-induced pain through TrkA
receptor modulation.
Pancreatic cancer pain model
The establishment of pancreatic cancer pain model is
fundamental for investigating pathogenesis and therapeutic
strategies. Current models include chemically induced, transplanted
tumor, genetically engineered, organoid and diabetes-associated
composite paradigms (67).
Ectopic grafts facilitate tumor monitoring but poorly replicate
tumor microenvironments, whereas orthotopic injections improve
emulated clinical pathology despite surgical complexity (68). Patient-derived xenograft model
preserved tumor heterogeneity by engrafting human tumors into
immunocompromised mice, though host stromal cell replacement occurs
during passaging (69).
Genetically engineered models simulate pancreatic carcinogenesis
through clustered regularly interspaced short palindromic
repeats-associated protein 9-mediated mutations, providing
human-like tumor progression for mechanistic/targeted therapy
studies despite high costs and technical demands. Organoid models
reconstructed 3D tumor architectures from patient tissues, enable
orthotopic transplantation to recapitulate malignant transformation
with high fidelity and personalized potential, though
standardization and microenvironmental completeness required
refinement (67-69). Diabetes-associated composite
models combine high-fat diets with streptozotocin-induced type 2
diabetes, followed by luciferase-tagged pancreatic cancer cell
implantation. Integrated with bioluminescence imaging, these models
illuminated metabolic-tumor interactions by dynamically visualizing
hyperglycemia-driven tumor growth (70). Modern cancer pain models have
transcended traditional tumor transplantation methods, establishing
a three-dimensional framework focused on bone-derived pain, soft
tissue invasion pain and neuroinvasive pain (71). In bone metastasis models, lower
limb bone injection systems successfully replicate clinical
intermittent severe pain and mechanical hyperalgesia by simulating
osteolytic processes, while spinal metastasis models complete the
research toolkit for vertebral metastatic pain. Notably, the
neuroinvasive triple-negative breast cancer model, established via
MDA-MB-231 cell injection into nerve bundles, achieved breakthrough
precision in neuropathic cancer pain simulation, with mechanical
allodynia phenotypes persisting one month longer than conventional
models (72,73). However, current bone cancer
evaluation systems overly focus on osteoclast activity indicators,
inadequately reflecting astrocyte-CXCL12/CXCR4 signaling-mediated
pain sensitization within tumor microenvironments. Additionally,
existing pain assessment criteria fail to distinguish neural
mechanisms between resting spontaneous pain and movement-associated
pain (74,75).
Visceral pain model
Appropriate experimental visceral pain models are
prerequisites for mechanistic investigations. Advances in
anatomical and neurobiological research have enabled the
development of multiple visceral pain models, laying foundations
for basic/clinical studies. Effective models must simulate clinical
pathophysiological features while ensuring reproducibility,
operability and cost-effectiveness. Visceral pain exhibited unique
characteristics due to sparse sensory neuron distribution and
complex convergent nociceptive pathways: i) Diffuse localization
with ambiguous pain origins, ii) referred pain at distant somatic
sites, iii) organ-specific pain susceptibility linked to nociceptor
distribution, iv) autonomic/motor reflexes and v) non-correlation
with visceral damage (76).
Visceral pain exhibits characteristics of ambiguous localization,
referred pain and non-fully injury-associated features, owing to
sparse sensory neuron distribution, peripheral cross-innervation of
nociceptive afferent fibers, and complex multichannel nociceptive
pathways (77). The construction
of visceral pain model must meet requirements for clinical
pathophysiological similarity, high reproducibility and operational
feasibility. Current models are categorized into inflammatory,
mechanical distension, ischemic and electrical stimulation types
based on modeling stimuli (78).
The present review systematically summarized and compared the
construction methods, characteristics and advantages/limitations of
major visceral pain models to guide researchers in selecting
appropriate models based on experimental objectives (Table III).
 | Table IIICharacteristics of different types of
visceral pain models. |
Table III
Characteristics of different types of
visceral pain models.
| Authors, year | Type | Animal | Method | Time | Indicators | Advantages | Disadvantages | (Refs.) |
|---|
| Wang et al,
2021 | Capsaicin-induced
inflammatory pain model | C57BL/6J mice | Gastric gavage of
capsaicin | 7 days | Spontaneous
pain | Simple operation
without surgery/injection | High individual
variability requiring strict dose control; mild inflammatory marker
changes | (101) |
| Yao et al,
2020 | Stress pain
model | SD rats | Senna leaf extract
gavage + physical stress | 14 days | Colorectal
distension and abdominal withdrawal | Mimics IBS under
stress conditions | Divergence from
multifactorial IBS etiology; stability needs improvement | (102) |
| Felice et
al, 2014 | Neonatal maternal
separation pain model | SD rats | Maternal separation
rearing | 10 days | Colorectal
distension and abdominal withdrawal | Non-invasive, high
operability | Requires neonatal
pups; long modeling cycle; low success rate | (103) |
| Wu et al,
2018 | Colitis pain
model | SD rats | TNBS enema | 4 weeks | Colorectal
distension and abdominal withdrawal | Direct colonic
action with minimal systemic effects | Long modeling
cycle; high mortality if improperly operated | (104) |
| Liu et al,
2020 | Pancreatitis pain
model | SD rats | DBTC tail vein
injection | 7 days | Spontaneous pain,
Mechanical pain threshold | Simple
implementation | Significant
systemic impacts; uncontrolled ethanol intake in drinking
water | (105) |
| Furuta et
al, 2018 | Interstitial
cystitis pain model | F344 rats | Hydrochloric acid
bladder perfusion | 2 weeks | Spontaneous
pain | Direct bladder
action with minimal systemic effects | Urethral
catheterization-induced trauma affecting behavioral
performance | (106) |
| Denget al,
2024 | Acetic acid
writhing pain model | C57/BL6 mice | Intraperitoneal
acetic acid injection | 21 days | Spontaneous
pain | Rapid behavioral
responses, high reproducibility | Uncontrolled
diffusion rate/direction of acetic acid | (107) |
Inflammatory visceral pain model
The inflammatory visceral pain model simulates pain
through chemical or biological stimulation-induced local
inflammation (79,80). An acute pancreatitis model
employed retrograde sodium taurocholate injection into the
pancreatic duct or subcapsular punctures to replicate biliary
pancreatitis pathology but suffered from high surgical trauma and
mortality (81,82). Sigmoid colon pain model induced
localized inflammation using 5% formaldehyde submucosal injection,
eliciting abdominal licking and arching within 45 min, with
self-limiting inflammation facilitating self-controlled drug
studies (83,84). In ulcerative colitis model,
dextran sulfate sodium (DSS) drinking ad libitum or
2,4,6-trinitrobenzenesulfonic acid (TNBS)/ethanol rectal
administration induced bloody stools and mucosal damage, with TNBS
offering superior reproducibility despite interindividual DSS
intake variability (85-87). A neonatal colorectal inflammation
model established chronic visceral hypersensitivity through dilute
acetic acid enemas in pups, mirroring irritable bowel syndrome
pathophysiology by single-injury induction (88). A bladder pain model used
intravesical mustard oil or acrolein to provoke dysuria, though
murine urethral injury risks exist. A cyclophosphamide-induced
acrolein metabolite model improved mimicking of natural bladder
mucosa irritation mechanisms (89-91).
Mechanical distension pain model
Mechanical distension model replicated organ
expansion or obstruction via physical stimuli. The gastroduodenal
distension model triggered teeth grinding and back arching by
inflating intragastric balloon catheters, though invasive surgery
restricted application (92).
Colorectal distension (CRD) involved anal balloon inflation to
induce abdominal contractions and pelvic lifts, with inflammatory
priming enhancing pain sensitivity, albeit with intestinal
perforation risks at excessive pressures (93-94). Neonatal repetitive CRD in pups
induced persistent visceral hypersensitivity in adulthood, serving
as a tool for chronic pain mechanism research (95).
Ischemic visceral pain model
An ischemic visceral pain model simulated myocardial
ischemia via coronary artery occlusion, yet vascular anatomical
variations caused significant behavioral heterogeneity, limiting
its use to qualitative studies (96). An electrical stimulation model
applied controlled parameters to visceral nerves to evoke pain but
lacked physiological relevance (97). A specialized maternal separation
model induced gut hypersensitivity and anxiety-like behaviors in
neonatally separated rodents through hypothalamic-pituitary-adrenal
(HPA) axis dysregulation and intestinal barrier disruption, making
them ideal for irritable bowel syndrome research (98-100). In summary, inflammatory models
excels in drug screening despite low specificity, mechanical
distension models improve approximate physiological stimuli and
maternal separation models facilitated chronic pain mechanism
exploration. Future advances require multimodal assessment systems
integrating imaging and molecular biomarkers to enhance
translational value.
Inflammatory-driven, ischemia-induced and functional
disorder paradigms have become mainstream modeling frameworks in
visceral pain research. In gut-brain axis dysfunction studies,
programmed colorectal distension techniques have established
gold-standard evaluation systems by quantifying viscerosomatic pain
correlation indices (101).
Innovative mechano-chemical pancreatic stimulation models (35 mmHg
sustained pressure combined with dynamic pH monitoring) precisely
replicate the synergistic effects of mechanical stress and acidic
microenvironments in pancreatitis pain. The research highlighted
the bladder chemo-optogenetic platform and successfully constructed
reversible neuromodulation frameworks for visceral pain pathways
(102,103). Current model systems face dual
bottlenecks in mechanistic resolution: Intestinal mechanical
stimulation models struggle to specifically regulate enteric
glial-TRPA1 ion channel signaling networks, while chronic
pancreatic injury models exhibit 48-72 h temporal discrepancies
between peripheral stellate cell activation and central
sensitization markers (104).
Technological advances such as Sox10-CreERT2-mediated conditional
gene editing of enteric glial subsets, development of bidirectional
gut-brain modulation models and deployment of miniaturized
multimodal biosensor arrays enabling synchronized autonomic rhythm
and visceral pain threshold tracking (sampling frequency ≥100 Hz)
will propel visceral pain research toward precision modulation and
systemic integration (105).
Inflammatory pain model
Inflammatory pain arises from tissue injury or
infection-triggered inflammatory responses, commonly observed in
arthritis, postoperative inflammation, or infectious diseases.
Inflammatory mediators (prostaglandins, bradykinin and cytokines)
activate peripheral nociceptors, inducing pain sensitization and
central nervous system remodeling. Clinical manifestations included
redness, swelling, heat and dysfunction, with characteristic
'protective' features and sensitivity to nonsteroidal
anti-inflammatory drugs (NSAIDs)/glucocorticoids (106). While inflammatory and visceral
pain overlap in mechanisms, they differ fundamentally: Visceral
pain exhibits diffuse localization and referral, whereas
inflammatory pain is typically well-localized and
injury-associated. The inflammatory pain model simulates
acute/chronic inflammatory states via local injections of specific
irritants into skin, plantar surfaces, muscles, or joints (107). These models induce acute
inflammation through neutrophil chemotaxis and sustained pain via
macrophage infiltration, with pain modulation mechanisms closely
linked to opioid peptide analgesia (108). Table IV systematically summarized and
compared the construction methods, characteristics, and
advantages/limitations of major inflammatory pain models to guide
researchers in selecting appropriate models based on experimental
objectives.
 | Table IVCharacteristics of different types of
inflammatory pain models. |
Table IV
Characteristics of different types of
inflammatory pain models.
| Authors, year | Type | Animal | Method | Time | Indicators | Advantages | Disadvantages | (Refs.) |
|---|
| Melo-Carrillo et
al, 2013 | Dural
neuroinflammation pain model | Wistar rats | Inflammatory
stimulant injection into dura mater via cranial drilling | 16 days | Spontaneous
pain | Closely mimics
clinical manifestations | Technically complex
with significant animal trauma | (112) |
| Burgos-Vega et
al, 2019 | Dural
neuroinflammation pain model | ICR mice | Inflammatory
stimulant injection into dura mater via cranial sutures | 7 days | Spontaneous
pain | Minimal animal
injury | Requires high
precision, suitable only for mice | (113) |
| Sufka et al,
2016 |
Nitroglycerin-induced pain model | SD rats | Intraperitoneal
injection of nitroglycerin | 2 weeks | Spontaneous
pain | Simple,
cost-effective, anesthesia-free | Systemic effects
limit intracranial vascular specificity | (114) |
| Philpott et
al, 2020 | Intra-articular
injection pain model | Wistar rats | Sodium iodoacetate
injection into knee joint cavity | 14 days | Mechanical pain
threshold | Rapid
implementation with low animal stress | Dose-dependent
effects difficult to control | (115) |
| Shi et al,
2020 | Joint
immobilization pain model | New Zealand
rabbits | 6-week left
hindlimb knee extension fixation | 6 weeks | Mechanical pain
threshold | Non-invasive joint
protection | Fixation device
detachment risks experimental validity | (116) |
| Katri et al,
2019 | Meniscus resection
pain model | Lewis rats | Medial collateral
ligament transection with meniscectomy | 44 days | Mechanical pain
threshold, cold pain threshold | Severe/persistent
osteoarthritis induction | Surgical
variability affects injury severity consistency | (117) |
| He et al,
2024 | CFA-induced
arthritis pain model | SD rats | Complete Freund's
Adjuvant (CFA) injection into right hindpaw plantar region | 42 days | Spontaneous
pain | Gold standard for
rheumatoid arthritis research | Prolonged modeling
period; systemic inflammation affects model stability | (118) |
| Bai et al,
2025 | Collagen-induced
arthritis pain model | SD rats | Bovine type II
collagen emulsified with Incomplete Freund's Adjuvant (IFA) | 28 days | Spontaneous
pain | Mimics human RA
autoimmune mechanisms | Collagen
purity/emulsion quality affects success; multi-dose
immunization | (119) |
| Koo et al,
2002 | Ankle joint
overloading pain model | SD rats | Mechanical ankle
overextension with 180° inversion | 7 days | Spontaneous
pain | No chemical
inducers required | Short pain duration
requiring rapid assessment; operator-dependent | (120) |
| Wu et al,
2023 | Carrageenan-induced
Inflammation pain model | SD rats | 1% carrageenan
injection into left hindpaw plantar region | 7 days | Spontaneous
pain | Rapid inflammatory
response | Transient
hypersensitivity; unsuitable for chronic studies | (121) |
Carrageenan-induced pain model
Local carrageenan injection triggers biphasic
inflammation: Acute neutrophil-dominant phase (24-48 h) transitions
to chronic monocyte/macrophage phase over two weeks. The model
induces thermal/mechanical hypersensitivity at injection sites and
secondary hyperalgesia in remote areas, pathologically resembling
rheumatoid arthritis (109).
Complete Freund's adjuvant (CFA)
model
CFA injection into tails or joint cavities elicits
stronger persistent inflammation than carrageenan. The CFA model
exhibits injection site swelling, 60-70% reduced hindlimb
weight-bearing, and 40% decreased wheel-running activity. Previous
studies confirmed electroacupuncture combined with spinal orphanin
FQ receptor antagonists modulates such pain, validating acupuncture
analgesia in chronic inflammation (110,111).
Capsaicin-induced pain model
Local capsaicin injection activates TRPV1 channels,
provoking neurogenic inflammation. The model features 50% thermal
hyperalgesia and 80% mechanical allodynia around injection site
with secondary mechanical hypersensitivity distally (112). Transient C-fiber
depolarization-induced hypoalgesia at injection cores provides
unique insights into spatiotemporal pain conduction dynamics
(109). The classic CFA chronic
inflammatory model establishes the temporal correlation between
macrophage M1 polarization phenotypes and mechanical hyperalgesia
through dynamic TNF-α/IL-1β expression profiling (28 days)
(113). The IL-23 local
sensitization model pioneered a γδT cell-IL-17A signaling
axis-driven neural sensitization purification system, overcoming
traditional adjuvant model heterogeneity (114). The TLR4/ATP bimodal stimulation
model achieves molecular-level spatiotemporal synchronization of
NLRP3 inflammasome activation and neuropeptide (CGRP, substance P)
release via two-photon intravital imaging. Cross-species neutrophil
metabolic cycle displays impaired biological validity in chronic
inflammatory microenvironment reconstruction (rodents <8 h vs.
humans 5-90 days) (115-117).
A peripheral innervation model exhibits clinical phenotype
disconnects, as synovial C-fiber density (<25%) inadequately
explains rheumatoid pain persistence. The lactate-ASIC3 signaling
axis in pain regulation lacks standardized paradigms from metabolic
reprogramming perspectives. Emerging solutions include dorsal root
ganglion organoid-chip models co-cultured with polarized
macrophages, microelectrode arrays for neuro-immune interaction
kinetics, optogenetic delivery systems (TRPV1-liposome carriers)
for submillimeter IL-6 control in joints and humanized chemokine
models (IL8-CXCR1 transgenics) to establish cross-species pain
translation metrics, collectively advancing inflammatory pain
research toward personalized precision medicine (118,119).
Other pain models
Somatic pain can be simulated using the formalin
model (97). Injection of 1%
formalin into the dorsal surface of the right hindpaw in rodents
induces biphasic pain responses: Phase I (immediate pain lasting
for10 min post-injection) and Phase II (delayed pain commencing
15-20 min post-injection and persisting 1 h). Phase II, which is
commonly used in experimental studies, involves distinct mechanisms
from Phase I. NSAIDs effectively attenuate Phase II pain but fail
to modulate phase I responses. Species-specific behavioral
differences are observed: Rats exhibit paw withdrawal, whereas mice
displayed licking behaviors. Phase II pain is now recognized as
peripherally mediated inflammatory pain associated with central
sensitization, making this model valuable for investigating tissue
injury-induced hyperalgesia mechanisms (120).
Pain behavioral assessments evaluate nociceptive
thresholds by applying acute noxious stimuli to elicit withdrawal
reflexes. These methods simulate physiological acute pain but lack
pathological validity, precluding standalone model development.
They are primarily employed to verify threshold changes in existing
pain models (121).
Mechanical nociceptive response testing
predominantly utilized the Von Frey test (122). Originally developed by
Maximilian Von Frey in the 19th century, this method applies
calibrated filaments to the plantar surface in ascending force
order to determine mechanical withdrawal thresholds. Alternatively,
fixed-force filaments delivered repetitive stimuli to quantify
withdrawal frequency/duration. Chaplan et al (25) subsequently optimized threshold
determination protocols. Current practice favors electronic Von
Frey systems for standardized force application. Colorectal
distension via transanal balloon inflation simulates visceral pain,
with pain thresholds assessed through AWR scoring.
However, behavioral interpretation introduce
subjectivity. Thermal/cold nociception assessments are widely
adopted in rodents. The tail-flick test measures heat avoidance
latency by applying thermal stimuli to the tail (123). The hot plate test eliminates
restraint-induced stress by observing escape behaviors on a heated
surface. The Hargreaves test quantifies hindpaw withdrawal latency
to radiant heat (124). Cold
sensitivity evaluation substituted hot water with ice baths in
tail-flick paradigms. Acetone droplet application induces cold
allodynia in hypersensitive rats but elicits nonspecific responses
(mechanical/chemical irritation) in mice (125). Semiconductor-cooled plates
dominate cold pain studies, minimizing ambient temperature
artifacts while retaining mechanical stimulation limitations
(126). Recent advances in
rodent pain assessment methodologies continue to refine precision
and translational relevance.
Targets of pain
The convergence of structural biology and
computational pharmacology has shifted to pain targets from
single-receptor antagonism to multidimensional regulatory systems.
Traditional targets such as MOR employ allosteric modulation
strategies that stabilize receptor-G protein-biased conformations,
addressing the analgesia-addiction dissociation challenge.
Discoveries at neuroimmune interfaces drive the paradigm shift from
neuron-centric to tripartite neuron-glial-immune network
modulation. This section systematically elaborated conformational
control of classical membrane receptors, ion channel
subtype-specific drug design breakthroughs, mechanistic insights
into neuroimmune interface targets and epigenetic modulation
strategies, establishing a theoretical framework for
multidimensional analgesic development.
Ionotropic channel receptors
Voltage-gated ion channels
Voltage-gated sodium channel
Voltage-gated sodium channels, transmembrane protein
complexes comprising an α-subunit and β-subunits, are classified
into nine subtypes (Nav1.1-1.9) based on α-subunit variations.
These channels mediate action potential generation/conduction and
are critically involved in neuropathic pain pathogenesis.
Specifically, Nav1.3, Nav1.7, Nav1.8, and Nav1.9 play pivotal roles
in nociceptive signaling. Nav1.3 is predominantly expressed in
embryonic/neonatal central nervous system (CNS) with minimal adult
expression, but its upregulation following peripheral/central nerve
injury is associated with neuropathic pain. miR-30b downregulation
under neuropathic conditions reduces SCN3A mRNA inhibition,
elevating Nav1.3 expression to enhance neuronal excitability
(127). Nav1.7 is predominantly
expressed in small C-fiber nociceptors of dorsal root ganglia
(DRG), mediating action potential generation in an endogenous
opioid-independent manner. Its unique slow activation/inactivation
kinetics enabled ramp current generation, lowering action potential
thresholds and amplifying subthreshold depolarizations to
critically regulate pain transduction (128,129). Nav1.8, a tetrodotoxin-resistant
sodium channel enriched in trigeminal ganglia and DRG nociceptors,
represents a high-selectivity analgesic target (130). Co-expressed with Nav1.7/1.8 in
small DRG neurons, Nav1.9 participates in familial episodic pain
syndromes and GM-CSF-induces hyperalgesia through Jak2-Stat3
pathway co-activation (131).
Pharmacological blockade of Nav1.7/1.8 channels reduces ectopic
discharges and elevates peripheral firing thresholds to alleviate
pain (132), with
Nav1.7-selective inhibitors emerging as promising next-generation
analgesics through therapeutic index optimization (133).
Voltage-gated Ca2+ channel
Voltage-gated Ca2+ channel regulates
cytosolic Ca2+ levels to influence cellular excitability
and signaling, with N-type and T-type subtypes implicated in pain
pathophysiology. The N-type blocker ziconotide, approved by the
U.S. Food and Drug Administration (FDA) for intrathecal
administration in severe chronic pain, exhibits potent analgesia
limited by narrow safety margins. T-type channels in
central/peripheral neurons mediated somatic/visceral pain
transduction, making their modulators potential analgesics
(134).
Voltage-gated K+ channel
Potassium voltage-gated channel subfamily KQT member
4 (KCNQ) validates analgesic targets, as evidenced by retigabine's
efficacy in chronic inflammatory/neuropathic pain models (135). Genetic studies of inherited
erythromelalgia identified KCNQ2-encoded Kv7.2 channels as
peripheral determinants of pain susceptibility (136), suggesting intrinsic analgesic
mechanisms beyond conventional modulation (137). Flupirtine, a retigabine analog
targeting Kv7.2, has been clinically used since 1984 despite
adverse effects. Cryo-EM structures of apo-state human Kv7.2 and
ligand-bound complexes with retigabine/ztz240 provided molecular
blueprints for improved KCNQ agonist design (138). While numerous pharmaceutical
efforts focused on KCNQ openers, only retigabine and flupirtine
have reached clinical application, underscoring the need for
optimized subtype-selective agents.
Mechanosensitive ion channel-TREK-1
As a key member of the K2P potassium channel family,
TREK-1 is regulated by G protein-coupled receptors (GPCRs) and
enriched in small sensory neurons (139). TREK-1 knockout mice exhibit
enhanced thermal sensitivity and mechanical allodynia, with
diminished osmotic pain responses particularly in PGE2-sensitized
models (140), establishing
TREK-1 as a promising multitarget analgesic candidate.
Ligand-gated ion channels
Acid-sensing ion channel (ASIC)
ASIC, a proton (H+)-activated cation
channel expressed in both peripheral and central nervous systems,
plays critical roles in pain signal transmission and modulation.
The ASIC family comprises ASIC1-ASIC4 subtypes, with ASIC1
predominating in spinal neurons and brain regions such as the
cerebral cortex and hippocampus (141). Duan et al (142) demonstrated that intrathecal
administration of ASIC1a-specific inhibitors or antisense
oligonucleotides in CFA-induced inflammatory rats markedly reduced
thermal/mechanical hyperalgesia via ASIC1a downregulation.
Stauntonia PTS extract inhibited ASIC currents and downregulated
ASIC3 protein expression, exhibiting analgesic effects in animal
models. Key active compounds YF-33 and YF-49 show dose-dependent
ASIC current suppression and potent analgesia (143). Paeoniflorin alleviates pain by
inhibiting ASIC-mediated H+-activated currents through
adenosine A1 receptor interaction, shortening Phase II pain
duration in formalin tests (144). Dexmedetomidine
concentration-dependently inhibited ASIC electrophysiological
activity via α2-adrenergic receptors (α2-ARs), mitigating
acid-induced nociceptive behaviors through peripheral α2A-Ars
(145). These findings
highlighted diverse pharmacological strategies targeting ASICs for
pain management.
α4B2 nicotinic acetylcholine receptor
The α4β2 nicotinic acetylcholine receptor (nAChR),
a ligand-gated ion channel widely expressed in the CNS/PNS,
modulates pain signaling through acetylcholine, dopamine,
γ-aminobutyric acid (GABA) and norepinephrine regulation. Its
agonists/partial agonists exhibit analgesic efficacy in
neuropathic/inflammatory pain models, reversible by nAChR
antagonists (146). However,
novel α4β2 nAChR-targeted analgesics remained preclinical,
necessitating further development.
N-methyl-D-aspartate (NMDA) receptor
NMDA receptor, an ionotropic glutamate receptor
abundant in spinal dorsal horn neurons, is a validated mediator of
pathological pain and hyperalgesia across formalin, CFA,
carrageenan and neuropathic models. Studies have revealed NMDA
receptor expression on spinal keratinocytes and involvement in
astrocyte activation during sciatic nerve injury-induced
neuropathic pain. Li et al (147) demonstrated NMDA receptor
participation in spinal microglial activation and neuroactive
substance release during acute peripheral inflammatory pain,
expanding their therapeutic potential.
Purinergic receptor
ATP-gated P2X receptor facilitates K+
efflux and Na+/Ca2+ influx through seven
subtypes (P2X1-7) expressed on neurons, immune cells and cancer
cells. P2X3 mediates acute pain in sensory neurons, P2X4
neuropathic pain in glia and P2X7 inflammatory pain in immune cells
(148). Emerging evidence
positions purinergic signaling via P2X receptors as a dual
therapeutic target for cancer progression and pain. Franceschini
and Adinolfi (148) proposed
combining standard chemotherapeutics with P2X2/3/4/7 antagonists to
simultaneously address tumor growth and cancer-related pain.
Capsaicin receptor
The transient receptor potential (TRP) channel
constitutes a family of cellular channel proteins initially
discovered in Drosophila photoreceptors, where TRP mutations
abolished sustained light-induced responses while permitting
transient voltage currents. TRP channels are expressed across
multiple tissues, with their activation via diverse stimuli
triggering cation influx to modulate cellular states. The TRP
family comprises six subfamilies: Canonical (TRPC), melastatin
(TRPM), vanilloid (TRPV), ankyrin (TRPA), polycystin (TRPP) and
mucolipin (TRPML) (149). Among
them, TRPV1, TRPA1 and TRPM8 play pivotal roles in analgesic
mechanisms. TRPV1 channel, recognized as a critical regulator of
nociception, exhibits sensitivity to thermal and chemical stimuli.
Activation induces inflammatory mediator/neurotransmitter release
from nociceptive nerve terminals to generate pain signals,
positioning TRPV1 antagonists as validated analgesic agents
(150). TRPA1 channel,
activated by reactive oxygen species and cold temperatures,
represents emerging therapeutic targets through their involvement
in mechanical/allodynic pain pathways (151). TRPM8 channel, cold-sensitive
thermoreceptors, paradoxically mediate analgesia when they activate
their agonists in neuropathic pain management (152,153). Preclinical studies confirm
TRPV1's therapeutic relevance across cancer, neuropathic,
postoperative and musculoskeletal pain models. Its selective
expression in primary nociceptors underpinned targeted intervention
strategies. Emerging approaches include potassium current enhancers
to suppress nociceptive signaling and Cav2.2/Cav3.2 calcium channel
blockade, demonstrating complementary analgesic potential (154).
GPCRs
Opioid receptor
Opioid receptors, a class of GPCRs, include
multiple subtypes such as central MOR, δ-opioid receptor (DOR),
κ-opioid receptor (KOR) and peripheral Mrg opioid receptor, all of
which modulated pain signaling (155). Despite severe adverse effects
on the central and enteric nervous systems, opioids remained the
gold standard for acute pain management. MOR, expressed in both
central and peripheral nervous systems (Fig. 2), is clinically targeted by
agonists for analgesia. The δ-opioid receptor agonist SNC80 exerts
dose-dependent analgesic and anti-depressant effects in rodents and
suppresses acid-induced writhing responses as well as chemically
evoked nociceptive behaviors via systemic administration. While
κ-opioid receptor agonists are widely used in preclinical pain
studies, their clinical analgesic efficacy in humans remains
suboptimal (156). As a
specialized opioid receptor subtype, Mrg receptor constitutes a
novel GPCR family selectively expressed in small-to-medium neurons
of trigeminal and DRG in mammals. The murine Mrg family comprises
50 members categorized into four classes (A-D), whereas humans
possessed seven Mrg receptors (MrgX1-7). MrgC receptor activation
enhances morphine's analgesic potency and duration through MOR
G-protein modulation. Wang et al (157) demonstrated that intrathecal
administration of the selective MrgC agonist BAM8-22 (3 nmol)
potentiated morphine analgesia and induced a leftward shift in
morphine's dose-response curve.
Metabotropic glutamate receptor
Metabotropic glutamate receptors (mGluRs), members
of GPCR family, encompass eight distinct subtypes. These receptors
act as modulators of neuronal excitability and synaptic
transmission, expressed at both presynaptic and postsynaptic sites
in the nervous system. Their activation produces either analgesic
or pain-sensitizing effects, depending on their anatomical location
and downstream signaling cascades. Early studies demonstrated that
mGluRs played a role in pain transmission. For instance, the
competitive mGluR antagonist L-AP3 inhibited the activation of
spinal dorsal horn neurons induced by repeated topical application
of mustard oil, suggesting that persistent pain caused by such
applications can be suppressed by blocking mGluR-mediated signaling
pathways (158). Additionally,
systemic administration of the non-competitive mGlu5 antagonist
MPEP reversed mechanical hyperalgesia induced by inflammatory
mediators such as CFA and carrageenan. Oral administration of MPEP
exhibited gastrointestinal protective effects superior to those of
NSAIDs such as indomethacin and diclofenac sodium. Furthermore,
peripheral administration of MPEP markedly reduced CFA-induced
inflammatory hyperalgesia, highlighting the critical role of
peripheral mGlu5 expression in pain modulation (158). These preclinical findings
collectively established mGluRs as promising targets for developing
novel analgesics to treat chronic inflammatory and neuropathic
pain. Future studies in animals and humans will further elucidate
the therapeutic potential of mGluRs in chronic pain management.
Adenosine receptor
Adenosine receptors, extracellular GPCRs, comprise
four subtypes (A1R, A2AR, A2BR and A3R) and participate in
physiological, non-physiological and pathological processes as part
of endogenous signaling pathways. Extensive research indicates that
systemic administration of A1R agonists alleviates pain in
preclinical models of inflammatory and neuropathic pain, with early
reports proposing A1R agonists as potential analgesics. A2AR is
expressed on peripheral inflammatory and immune cells, regulating
anti-inflammatory responses and serving as a therapeutic target in
inflammatory and immune disorders. Similarly, A2BR is expressed on
peripheral inflammatory and immune cells and its activation
enhances pro-inflammatory activities, though its role in pain
remains poorly characterized. A3R is expressed in multiple organs
and peripheral tissues, including cells involved in inflammatory
responses. Intrathecal injection of the selective A3R agonist
IB-MECA produces analgesia, while the selective A3R antagonist MRS
1220 blocks adenosine-induced analgesia following intrathecal
administration (159). These
discoveries highlight adenosine receptors as valuable targets for
novel analgesic development.
Cannabinoid receptor
Cannabis has been used for pain relief for over
four centuries, but its analgesic mechanisms have remained unclear
until the discovery of cannabinoid receptors. The first cannabinoid
receptor (CB1) was cloned from a cDNA library, followed by CB2,
cloned from HL-60 cells. CB1 receptor is abundantly expressed in
the CNS and sparsely in peripheral tissues, whereas CB2 receptor is
predominantly localized to immune cells, with lower expression in
microglia, DRG, spinal cord, and specific brain regions (160). In the CNS, CB1 receptor
modulated pain transmission in regions such as the spinal dorsal
horn, periaqueductal gray matter, dorsal raphe nucleus and ventral
posterolateral thalamus, mediating cannabinoid-induced analgesia.
Peripherally, cannabinoid analgesia involved both CB1 and CB2
receptors. Intrathecal administration of CB2-selective agonists
effectively attenuated pain in multiple inflammatory pain models,
while selective CB2 activation avoids CNS-related side effects
(hypomotility, catalepsy, hypothermia, cognitive impairment)
associated with CB1 modulation (134).
Glucagon-like peptide-1 receptor
The glucagon-like peptide-1 receptor (GLP-1R), a
member of GPCR family, is specifically expressed in microglia of
the spinal dorsal horn and markedly upregulated during peripheral
nerve injury. Intrathecal administration of GLP-1R agonists, such
as GLP-1 (7-36) and exenatide, reduce pain
hypersensitivity by 60-90% in models of formalin-induced pain and
peripheral neuropathy. This analgesic effect is completely
abolished by GLP-1R antagonists or genetic knockout of GLP-1R.
Furthermore, GLP-1R agonists alleviate persistent and neuropathic
pain via GLP-1R activation (161). The identification of this novel
signaling pathway in the spinal dorsal horn, implicated in diverse
pain hypersensitivities, offers a promising avenue for exploring
pain mechanisms and developing innovative analgesics.
Imidazoline I2 receptor
In 2011, Li et al (162) identified imidazoline I2
receptor as a novel target for analgesic. The I2 receptor or I2
binding site referred to multiple proteins with high affinity for
(3H)-idazoxan and (3H)-2-BFI. It was found that I2
receptor-targeting drugs exerted analgesic effects in rat models of
inflammatory and neuropathic pain, particularly in chronic pain
(163). Early research found
that selective I2 receptor ligands alone lacked significant
analgesia but potentiated the analgesic effects of opioids in acute
pain models (163). Ongoing
investigations into I2 receptor will provide critical insights for
developing next-generation analgesic.
Enzymes
P38 mitogen-activated protein kinase (MAPK)
The p38 MAPK signaling system serves as a central
regulatory network for cellular responses to environmental
stressors, playing pivotal roles in maintaining homeostasis,
mediating stress responses and modulating inflammatory processes
(164). This pathway integrates
diverse stressors and ligand signals through apoptosis-associated
receptors or GPCR, triggering intricate intracellular cascade
reactions. The signaling cascade involves multi-level regulatory
mechanisms including small GTPase activation and adaptor protein
interactions, ultimately activating kinase family members at the
MAP3K level (165). The p38
MAPK family comprises 20 distinct MAP3K-related kinases encoded by
separate genes, which phosphorylated substrates by recognizing
Ser-Xaa-Ala-Xaa-Ser/Thr motifs (148). Based on sequence
characteristics and inhibitor sensitivity, these kinases are
categorized into p38α/β and p38γ/δ subgroups (166). Activated p38 MAPK regulates
cellular functions by phosphorylating downstream effectors such as
MAPK activating protein kinases or directly modulating key factors
including phospholipase A2 and heat shock proteins and activating
transcription factor 2, ETS transcription factor 1, C/EBP
homologous protein and myocyte enhancer factor 2C (Fig. 3) (167). Pathologically, aberrant p38
MAPK activation in neural tissues promotes release of
pro-inflammatory mediators such as TNF-α and IL-1β (168). These cytokines enhance
nociceptive sensitization, incision length and abnormal discharges
through ion channel modulation and synaptic plasticity alterations.
Sustained pathway activation induces cascading amplification of
pain signals, forming the pathological basis of chronic pain
(164). NSAIDs and botanical
extracts exert analgesic effects via p38 MAPK modulation (169). For instance, the traditional
Chinese medicine An-Gong-Niu-Huang-Wan suppresses COX-2, IL-1β,
TNF-α, PGE2 and NO expression by inhibiting p38 MAPK and
phosphoinositide 3-kinase/protein kinase B signaling (170). Daphnetin, a natural coumarin
derivative, demonstrates potent spinal p38 MAPK inhibition in
neuropathic pain models, offering novel therapeutic insights
(171). These findings
elucidate pharmacological mechanisms of traditional remedies while
advancing targeted drug development.
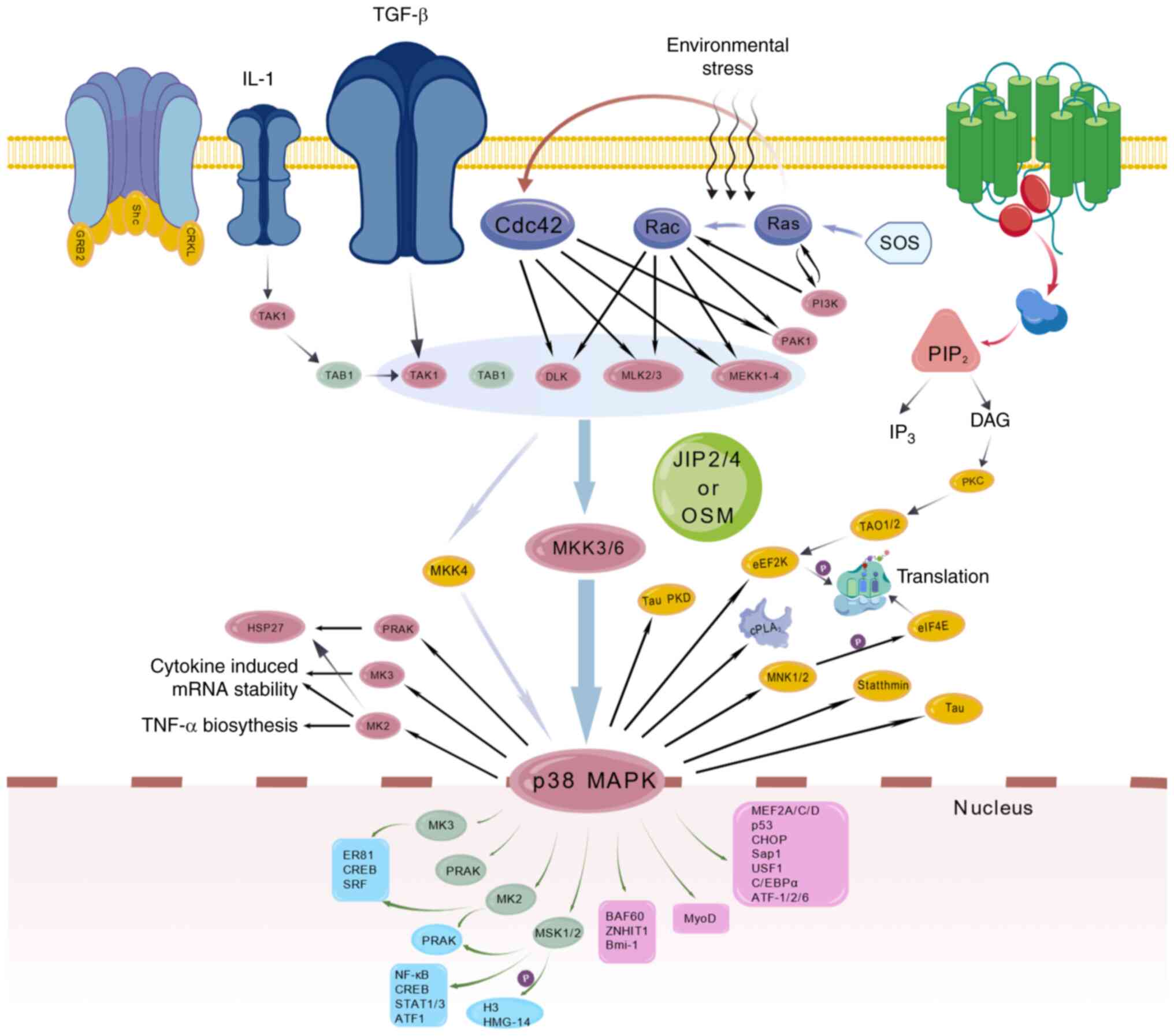 | Figure 3Signaling pathway of P38 MAPK.
ATF-1/2/6, activating transcription factor 1/2/6; BAF60,
brahma-associated factor 60; BMI-1, B-cell specific moloney murine
leukemia virus integration site 1; C/EBPα, CCAAT/enhancer-binding
protein α; CHOP, C/EBP homologous protein; Cdc42, cell division
cycle 42; CREB, cAMP response element-binding protein; DAG,
diacylglycerol; DLK, dual-leucine zipper-bearing kKinase; eEF2K,
eukaryotic elongation factor 2 kinase; elF4E, eukaryotic initiation
factor 4E; ER81, ETS-related protein 81; GAB2, GRB2-associated
binding protein 2; GSKL, glycogen synthase kinase-like; H3, histone
3; HSP27, heat shock protein 27; HMG-14, high mobility group
protein 14; IL-1, interleukin-1; IP3, inositol
trisphosphate; JIP2/4, JNK-interacting protein 2/4; MAPK,
mitogen-activated protein kinase; MEF2A/C/D, myocyte enhancer
factor 2A/C/D; MEKK1-4, mitogen-activated protein kinase kinase
kinase 1-4; MKK3/6, mitogen-activated protein kinase kinase 3/6;
MKK4, mitogen-activated protein kinase kinase 4; MLK2/3, mixed
lineage kinase 2/3; MK2, mitogen-activated protein kinase-activated
protein kinase 2; MK3, mitogen-activated protein kinase-activated
protein kinase 3; MNK1/2, MAP kinase interacting serine/threonine
kinase 1/2; MyoD, myoblast determination protein; NF-κB, nuclear
factor kappa-light-chain-enhancer of activated B cells; OSM,
oncostatin M; PAK1, p21-activated kinase 1; PIP2,
phosphatidylinositol 4,5-bisphosphate; PI3K, phosphatidylinositol
3-kinase; PKC, protein kinase C; PLA2, phospholipase
A2; PRAK, p38-regulated/activated protein kinase; SAP1,
serum response factor accessory protein 1; SOS, son of sevenless;
SRF, serum response factor; STAT1/3, signal transducer and
activator of transcription 1/3; TAB1, TGF-β activated kinase 1
binding protein 1; TAK1, TGF-β activated kinase 1; TAO1/2, thousand
and one amino acid kinase 1/2; TGF-β, transforming growth factor-β;
TNF-α, tumor necrosis factor-α; USF1, upstream stimulatory factor
1; ZNHIT1, zinc finger HIT domain-containing protein 1. |
Cyclooxygenase (COX)
COX is a rate-limiting enzyme that synthesizes
prostaglandins from arachidonic acid. It exists as two classical
isoforms, COX-1 and COX-2, which regulate diverse physiological and
pathological processes. Lu et al (172) demonstrated that cerebral COX-1
modulated late-stage neuropathic pain following sciatic nerve
injury, while COX-2 governed early-phase pain initiation. Zhou
et al (173) identified
COX-2 as a downstream effector in ephrinBs-EphBs-mediated spinal
pain signaling. The COX-3, a novel cyclooxygenase family member,
expands therapeutic targets for analgesic research (174).
Nitric oxide synthase (NOS)
NOS comprises three isoforms: Neuronal, inducible
and endothelial. NO generated through these enzymes exerts
multifaceted biological effects in pain pathophysiology.
Intrathecal NO precursors/donors exacerbated thermal hyperalgesia
and tactile allodynia in neuropathic rats, reversible by NOS
inhibitors (174). Kuboyama
et al (175) established
the critical role of NOS/NO signaling in spinal microglial
activation and nerve injury, inducing tactile allodynia using
knockout mice and providing mechanistic insights into neuropathic
pain.
Other targets
Canonical NF-κB
The NF-κB signaling pathway bifurcates into
canonical and non-canonical branches. The canonical pathway,
activated by pro-inflammatory cytokines (TNF-α and IL-1β) through
IL-1 receptor, Toll-like receptors and TNF receptor, initiated via
TNF receptor (TNFR) 1 trimerization and subsequent recruitment of
TNF receptor-associated death domain/receptor-interacting protein
1. This triggers TNF receptor-associated factor 2/5 and
cIAP1/2-mediated ubiquitination, assembling LUBAC-TAK1-NEMO/IKK
complexes. Linear ubiquitination of the NF-κB essential modulator
activates the inhibitor of nuclear factor κB kinase, which in turn
phosphorylates IκBα, enabling NF-κB dimer (RelA/p50) nuclear
translocation and inflammatory gene transcription (Fig. 4) (176,177). NF-κB's five family members
(RelA/p65, RelB, c-Rel, p50/p105 and p52/p100) crucially regulated
pain hypersensitivity through cytokine amplification. In gouty
arthritis models, astragaloside IV suppresses NF-κB-mediated
IL-2/IL-6/TNF-α expression to alleviate monosodium urate-induced
inflammation (178). Migraine
studies demonstrated traditional Chinese medicine's inhibition of
NF-κB ameliorated vascular dysfunction and central sensitization
(179). Acupuncture modulated
this pathway in rheumatoid arthritis to achieve anti-inflammatory
and immunoregulatory analgesia (180). Pharmacological interventions
targeting NF-κB included dezocine, a κ-opioid receptor modulator
that attenuated remifentanil-induced hyperalgesia via
TLR4/NF-κB/TRPA1 axis inhibition (181). Metformin alleviated
radiation-induced dermatitis pain by suppressing p38
MAPK/NF-κB-driven IL-1β/IL-6/TNF-α overproduction (182).
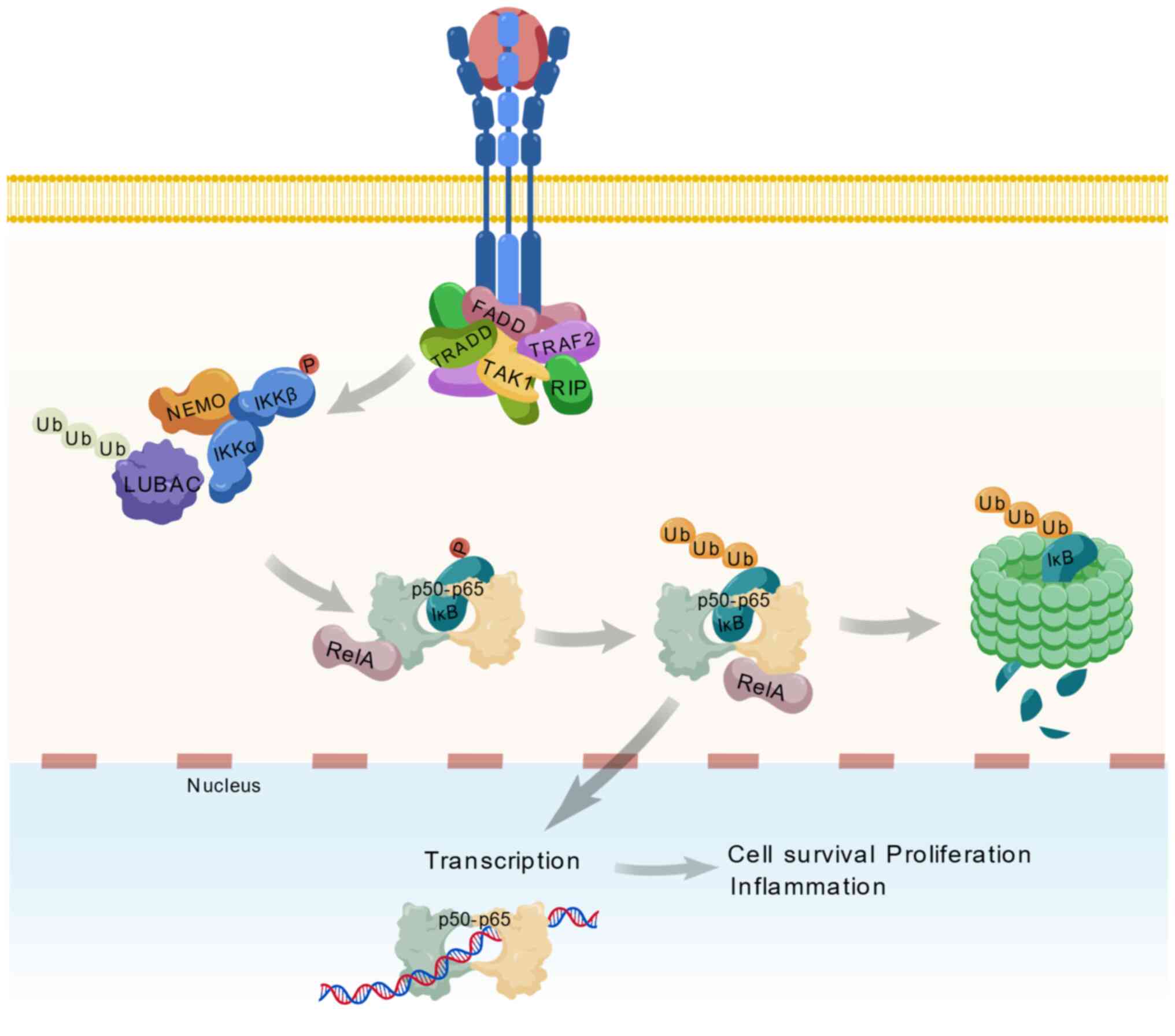 | Figure 4Signaling pathway of NF-κB. FADD,
fas-associated protein with death domain; IκB, inhibitor of κ-B;
IKKα, IκB kinase α; IKKβ, IκB kinase β; LUBAC, linear ubiquitin
chain assembly complex; NEMO, NF-κB essential modulator; NF-κB,
nuclear factor κB; p50, NF-κB subunit p50; p65, NF-κB subunit p65;
RIP, receptor-interacting protein; TAK1, transforming growth
factor-β-activated kinase 1; TRADD, TNF receptor-associated death
domain; TRAF2, TNF receptor-associated factor 2; Ub, ubiquitin. |
GABA receptor
GABA receptor expressed by inhibitory interneurons
in spinal dorsal horn laminae I-III regulate pain transmission
through GABA type A receptor (GABAA) and GABA type B receptor
(GABAB) activation in primary afferents and dorsal horn neurons
(183). GABAA receptors,
predominantly localized in DRG, induce neuronal depolarization to
block nociceptive signaling. However, their widespread CNS
distribution caused benzodiazepine-related side effects (sedation,
motor impairment and addiction), limiting clinical utility in
chronic pain. Most CNS GABAA receptors are
benzodiazepine-sensitive, composed of 2α, 2β and 1γ2 subunits, with
benzodiazepine binding sites formed by γ2 and α1/α2/α3/α5 subunits.
Knock-in mouse models revealed that benzodiazepine-induced
sedation/amnesia/convulsion relate to α1 subunits, while analgesia
involved α2 > α3 > α5 subunits. Selective activation of
spinal α2/α3 subunits of the GABA receptor minimizes adverse
effects and thus represents a novel analgesic strategy (184). Studies on sinomenine
demonstrated that its analgesic effect is completely reversed by
bicuculline, a GABAA receptor antagonist, indicating modulation via
the GABAA receptor (185).
GABAB receptors modulated peptidergic primary afferents and dorsal
horn neurons. The agonist baclofen alleviated chronic pain, spinal
injury, and trigeminal neuralgia. Early evidence showed
systemic/intrathecal baclofen produces analgesia in acute/chronic
pain models (186).
TNF-α receptor (TNFR)
TNF-α, a proinflammatory cytokine released during
tissue injury, mediates chronic neuropathic pain via TNFR1/TNFR2.
Post-nerve injury, TNF-α upregulation in affected nerves enhances
Aδ fiber firing through TNFR1/2 activation, while TNFR-neutralizing
antibodies attenuated CCI-induced pain (187). TNFR1-knockout mice lacked
thermal hyperalgesia but reduced mechanical/cold pain, whereas
TNFR2-knockouts exhibited weak mechanical/cold sensitivity
(188), confirming different
roles in neuropathic pain pathogenesis. Current research mainly
focuses on four-dimensional target innovations: i) Membrane
receptor conformational dynamics, ii) ion channel micro-domain
targeting, iii) neuroimmune interface modulation and iv) epigenetic
regulation. These advances established multidimensional
intervention systems from molecular conformations to epigenetic
memory. However, some researches were constrained by three major
bottlenecks: The inability of single-target modulation to block
multi-pathway compensatory effects in pain signaling, low
translational efficiency due to species-specific differences in
preclinical models and the dual challenges of
blood-brain/blood-nerve barrier penetration for
neuroimmune-targeted drugs. Future investigations should focus on
breakthroughs in multitarget coordination technologies and
organoid-chip validation platforms. A prioritized recommendation
involves exploring the mechanosensitive channel properties of
astrocytic gap junction protein Cx43 to develop mechanoresponsive
analgesics, potentially providing novel therapeutic avenues for
tissue fibrosis comorbid with chronic pain.
Treatments of pain
Drug therapy
Opioids
Opioids exert analgesic effects through opioid
receptors in the central and peripheral nervous systems. The
endogenous opioid system, one of the most extensively studied
innate pain modulatory systems, established opioids as the gold
standard for moderate-to-severe pain management (189). This system comprised four
7-transmembrane GPCRs: MOR, KOR, DOR and NOP. Among them, MOR, a
GPCR, mediates analgesia via G protein pathway activation that
inhibits neuronal excitation and promotes hyperpolarization
(190). Current opioid research
prioritizes developing low-adverse-effect agents through strategies
including biased agonists, multifunctional ligands, allosteric
modulators (191) and MOR
splice variant-targeting drugs (192). Anatomically, MOR is distributed
extensively across pain pathways-peripheral nociceptors, spinal
dorsal horn, thalamic nuclei, periaqueductal gray (PAG) and rostral
ventromedial medulla-forming multidimensional regulatory networks
integrating cognitive-emotional components in
cortical/hippocampal/coeruleus regions (193). Comparatively, KOR/DOR/NOP
receptors exhibit lower expression and weaker analgesia despite
overlapping distributions. Their localization in respiratory
centers underlie MOR-mediated respiratory depression (194). In reward circuits, MOR
activation in ventral tegmental area-nucleus accumbens dopaminergic
projections disinhibited GABAergic neurons to enhance dopamine
release and addiction potential (195). Molecularly, opioid receptors
signal through inhibitory G protein and β-arrestin pathways. Gi/o
activation inhibits adenylate cyclase (AC) to reduce cyclic
adenosine monophosphate (cAMP) while modulating ion channels: Gβγ
subunits suppress N-type Ca2+ channels and activate G
protein-coupled inwardly rectifying potassium channel (GPCIRPC) to
decrease neuronal excitability (196). In PAG, MOR analgesia arises
from disinhibition of glutamatergic descending pathways via
GABAergic interneuron suppression (197). A study revised classical views
by demonstrating G protein-mediated respiratory inhibition via
GPCIRPC-induced hyperpolarization in β-arrestin knockout models
(198). Oligomerization
receptor critically modulated functionality. μ/δ
heterodimers altered ligand affinity/surface density to modify
analgesia (199), while
μ/NOP heteromers attenuated signaling (200). Post-activation phosphorylation
recruited β-arrestins for desensitization/internalization,
contributing to acute tolerance. Chronic tolerance involved
compensatory upregulation of cAMP/Ca2+/protein kinase C
pathways and transcriptional plasticity (163,195). To address the opioid crisis,
novel strategies focused on biased agonists like TRV130 that
preferentially activated G protein over β-arrestin pathways,
reducing respiratory depression/nausea (201-203). PZM21 has reduced side effects,
though lower potency, compared with morphine (204). Further optimization seeks
enhanced G protein/β-arrestin signaling bias to maximize
therapeutic indices.
Non-opioid analgesics
Non-opioid analgesics are commonly used as
adjunctive therapy for moderate-to-severe cancer pain, enhancing
analgesia while reducing opioid-related adverse effects such as
constipation, nausea, vomiting, excessive sedation and respiratory
depression. These agents primarily included NSAIDs and
acetaminophen (APAP), suitable for mild-to-moderate pain. NSAIDs
alleviate inflammatory pain by inhibiting COX to suppress
prostaglandin synthesis, whereas the mechanism of APAP remains
incompletely understood, potentially involving central inhibition
and prostaglandin synthesis blockade. Limitations included
gastrointestinal bleeding, cardiovascular risks, and
hepatotoxicity.
APAP
APAP, an acetanilide-class antipyretic-analgesic,
is the most widely used over-the-counter analgesic for fever and
mild-to-moderate pain management. A study suggested that APAP acted
via central COX-3 inhibition to reduce cerebral PGE2 production
(205). APAP serves as
first-line therapy for mild cancer pain and is formulated with
opioids as second-line agents. Rapid oral absorption and hepatic
metabolism characterize APAP, while hepatotoxic intermediates
necessitate dosage control; this is especially important in
polypharmacy. The recommended maximum daily dose is ≤2000 mg/d,
especially for NSAID-contraindicated patients.
NSAIDs
NSAIDs, fundamental in pain management, exert
antipyretic, analgesic, anti-inflammatory effects via prostaglandin
synthesis inhibition. Prostaglandins mediate bone resorption in
metastatic lesions, making NSAIDs effective for bone metastasis
pain. Traditional NSAIDs exhibited frequent side effects, while
selective COX-2 inhibitors reduced gastrointestinal toxicity,
platelet inhibition and acute kidney injury risks. NSAID-induced
renal medullary prostaglandin suppression impairs opioid metabolite
clearance, potentially causing drowsiness, confusion, or myoclonus.
Renal function monitoring was critical during NSAID-opioid
coadministration.
Bisphosphonates
Bone metastases, common in advanced breast, lung,
prostate cancers, and multiple myeloma, requires prompt
intervention. Bisphosphonates (pamidronate, ibandronate and
zoledronic acid) inhibit osteoclast-mediated bone destruction,
promote remineralization and reduce skeletal-related events (SREs)
such as pathological fractures (206). Despite efficacy, osteonecrosis
of the jaw occurs ~0.01% of users after months of therapy. SREs
(bone pain, fractures and spinal compression) severely impair
quality of life and indicated disease progression. While
bisphosphonates reduce SRE incidence, 50% patients remain
unresponsive (207). Emerging
research highlights the RANKL/osteoprotegerin system as a central
pathway regulating osteoclast differentiation and bone metabolism
(208).
Denosumab
Denosumab, a monoclonal antibody targeting RANKL,
suppresses osteoclast activation, reduces bone resorption, and
increases bone density (209).
Compared with zoledronic acid, denosumab delays first SRE onset,
reduces recurrence risk and improves controlled bone metastasis
progression in breast, prostate and solid tumors (210). Advantages included renal safety
without monitoring requirements. However, hypocalcemia and jaw
necrosis are notable risks. Clinical decisions must balance
efficacy, cost, and patient-specific factors.
Caffeine
Caffeine, a xanthine alkaloid and central
stimulant, counteracted opioid-induced sedation per recent
guidelines (211). While
enhancing NSAID analgesia in non-cancer pain, its role in cancer
pain remains under investigation. A double-blind RCT in 41 advanced
cancer patients showed intravenous caffeine (200 mg/d) reduced NRS
scores by 0.833 (95% CI: 0.601-1.066) vs. 0.350 (95% CI:
0.168-0.532) with placebo (P=0.038), though clinical significance
was marginal (NRS improvement <1). Further trials were needed to
validate utility (212).
Glucocorticoids
Glucocorticoids suppress proinflammatory
cytokines/chemokines implicated in neuroglial activation and cancer
pain (213). Recommended for
postoperative pain adjuncts (214), they alleviate tumor-related
edema (215). In a multicenter
phase III trial, methylprednisolone (16 mg/d) improved fatigue,
appetite and satisfaction in advanced cancer patients despite
limited direct analgesia (216). Anti-inflammatory and anti-edema
mechanisms supported further exploration in cancer pain.
Ketamine
Ketamine, an NMDA receptor antagonist and opioid
receptor modulator, provided central/peripheral analgesia monoamine
reuptake inhibition and ion channel
(Na+/K+/Ca2+) modulation (217). Combined with epidural or
subcutaneous morphine, low-dose ketamine enhanced analgesia
duration and efficacy in refractory cancer pain, though peripheral
mechanisms remained unclear (218). Ketamine is positioned as a
feasible option for severe cases, with controllable adverse
reactions (nausea, sedation), making it an important alternative
for refractory cancer pain. Optimal dosing routes require further
study.
Platelet-activating factor (PAF)
receptor
PAF mediates neuropathic pain, with intrathecal PAF
inducing thermal hyperalgesia and mechanical allodynia reversible
by antagonists (CV-3988, TCV-309 and WEB2086) (219,220). In murine osteolytic cancer pain
models, PAF synthase/LPCAT2 upregulation is associated with
spontaneous/evoked pain behaviors. Intrathecal PAF antagonists or
siRNA-mediated receptor knockdown alleviated pain (221). Synergy with morphine enhances
analgesia while reducing constipation, suggesting PAF antagonists
as novel adjuncts for intractable cancer pain.
Adjuvant analgesics
Antidepressants
Chronic pain induces both physical suffering and
psychological distress, particularly depression, which markedly
hinders cancer pain rehabilitation. Antidepressants play critical
roles in neuropathic and cancer pain management by inhibiting
monoamine reuptake to elevate synaptic neurotransmitter levels.
Common agents included tricyclic anti-depressants (TCAs), selective
serotonin reuptake inhibitors (fluoxetine, sertraline) and
serotonin-norepinephrine reuptake inhibitors (venlafaxine,
duloxetine). Adverse effects (constipation, urinary retention)
necessitate cautious dosing initiation and gradual titration.
Sedating TCAs benefited patients with anxiety or sleep disorders,
typically administered at night. Abrupt discontinuation risks
withdrawal symptoms, requiring tapered cessation.
Antiepileptics
Neuropathic pain affects 1/3 cancer patients due to
tumor compression, infiltration, or treatment-related neural
injury. Pregabalin and gabapentin, first-line agents for
neuropathic pain, block voltage-gated calcium channels to reduce
neurotransmitter release. Pregabalin offers advantages over
gabapentin in dosing frequency, efficacy and tolerability, though
dizziness and peripheral edema remain common (222). It is worth mentioning that a
number of neuropathic pain drugs fail in clinical trials despite
strong preclinical results. Therefore, the successful launch of
each drug must undergo preclinical and clinical trials. Only when
the results of pre- and post-clinical studies are effective and
consistent, can it be successfully marketed.
Topical agents
Lidocaine alleviates cancer-related cutaneous
allodynia via rapid tissue penetration and with minimal systemic
effects, localized irritation being the primary adverse event
(223). Cannabinoid nasal
sprays [δ9-Tetrahydrocannabinol (THC) 2.5 mg/l, Cannabidio (CBD)
2.7 mg/100 ml] demonstrate analgesic, antiemetic and
appetite-stimulating effects at 1-4 sprays/day, despite regulatory
controversies (224).
Cannabinoids
Cannabinoids exert analgesia via presynaptic
receptor activation, though abuse potential limits clinical
adoption. Approved formulations such as Sativex (1:1 THC/CBD)
reduce cancer pain while improving nausea and appetite, supported
by clinical evidence (225).
Surgical and interventional
therapies
Neuroablative technique
Radiofrequency ablation (RFA) achieves durable
analgesia in chronic low back pain by targeting lumbar dorsal rami
or basivertebral nerves, with 5-year efficacy in modic
change-related pain (226,227). In oncology, RFA combined with
TACE improves 1-year survival (90.74%) and immune indices
(CD3+/CD4+/CD8+) in hepatocellular carcinoma (228). For refractory headaches, RFA
provides 182-day pain relief in 89.3% of patients with <1%
complication rates (229).
Spinal cord stimulation (SCS), as a neuromodulation technique,
effectively intervenes in the pathological processes of various
refractory neuropathic pain conditions by delivering
specific-frequency electrical pulses to the dorsal columns via
epidural electrodes. Its mechanisms involve multi-level regulation:
At the spinal segmental level, SCS activates large-diameter fibers
to inhibit nociceptive neuronal activity in the dorsal horn while
modulating central sensitization through descending inhibitory
pathways (230); animal studies
further demonstrate its capacity to suppress TRPV1 receptor and
CCL2 chemokine expression, thereby reducing neuroinflammation and
improving post-spinal injury hyperalgesia (231). Clinically, SCS exhibits broad
indications and significant efficacy, achieving >50% pain relief
in complex regional pain syndrome of the lower extremities with
sustained autonomic function and quality-of-life improvements
during long-term follow-up. For postherpetic neuralgia, SCS reduces
VAS scores from 9.4-2.6 within two weeks postoperatively, with
86.9% of patients achieving substantial symptom alleviation
(232). SCS also excels in
complex cases, such as failed back surgery syndrome with spinal
cord injury, where personalized programming enable complete pain
resolution and restoration of lower limb motor function (233). Technological advancements,
including high-frequency (10 kHz) and burst stimulation modes,
expanded SCS applications, notably improving painful neuropathy and
promoting ulcer healing in diabetic peripheral neuropathy. Despite
risks of electrode migration (2.5-9.0%) and infection, standardized
protocols and closed-loop systems enhance safety. Future research
must elucidate SCS's neuroplasticity modulation mechanisms and
optimize therapeutic strategies for peripheral neuropathy through
multicenter clinical evidence (234).
Minimally invasive surgery
Percutaneous transforaminal endoscopic discectomy
currently addresses lumbar stenosis, recurrent disc herniation and
cauda equina syndrome and enables 92.3% of patients to ambulate
within 72 h postoperatively (235). Prone positioning and visual
trephine systems reduce operative time by 32 min (236), while electrophysiological
monitoring enhances bladder function recovery (68.4%) (237). Biomechanically guided
rehabilitation lowers 1-year recurrence from 12.7-4.3% (238).
Intrathecal drug delivery systems
Intrathecal drug delivery systems (IDDS) serve as a
pivotal interventional approach for advanced cancer pain and
chronic refractory pain, achieving decoupling of analgesic efficacy
from systemic toxicity through precise spinal cord-targeted drug
delivery. Studies indicated that 55% of cancer patients experience
moderate-to-severe pain during the terminal phase, while 33-40% of
survivors develop chronic pain, with 25-77% exhibiting inadequate
response to World Health Organization (WHO) three-step oral
pharmacotherapy (239). IDDS
delivers spinal opioids (morphine, hydromorphone) and adjuvants
(bupivacaine, clonidine) at 1/300 oral equivalents, reducing
systemic toxicity (constipation: 42-9%) (240). In pancreatic cancer, IDDS
decreases NRS from 7.5-3.2 at 3 months with 82% morphine sparing
(241). Patient selection
criteria include survival >3 months, eastern cooperative
oncology group ≤2 and positive trial dose (242). Programmable pumps enable
real-time titration, cutting breakthrough pain frequency by 58% and
tolerance incidence by 17% (243,244). Table V systematically summarized and
compared the core parameters and clinical efficacy of
interventional techniques, providing a reference for researchers.
Recent advances in SCS have evolved from traditional frequency
modulation to multi-mode closed-loop systems. These systems use
implantable nanoelectrode arrays to decode the cluster-like firing
patterns of neurons with a wide dynamic range of back angles in
real time, thereby achieving dynamic topological blocking of the
notional signaling pathways (245-248). The clinical application of
magnetothermal neuromodulation was an innovation and the
superparamagnetic Fe3O4 nanoparticle was
precisely delivered to the dorsal root ganglion to produce a local
thermal effect of 42°C via an alternating magnetic field (50 kHz/30
mT) to reversibly inhibit the activity of the Nav1.8 channel. This
energy-selective neuromodulation bypassed the irreversible tissue
damage inherent in conventional radiofrequency ablation (249-251). An ongoing problem in current
intervention systems is the trade-off between mechanical accuracy
and biocompatibility; silicon carbide microelectrodes achieve 5
μm resolution nerve signal acquisition, but chronic
implant-induced glial scarring result in 30% signal attenuation per
year. As interventional techniques evolve towards editing the
emotional dimension of pain, an ethical threshold assessment system
was established to balance neuroenhancement and human integrity.
The neurophenomenological evaluation framework was integrated into
clinical trials of novel interventions and the first-person pain
experience report was combined with the dynamic entropy analysis of
brain networks to establish a multidimensional human effectiveness
evaluation standard (252-255).
 | Table VCharacteristics of interventional
therapy for pain. |
Table V
Characteristics of interventional
therapy for pain.
| Authors, year | Method | Combination | Indications | Time | Effects | (Refs.) |
|---|
| Zhang et al,
2025 | RFA | Oral Qianghuo
Shengshi decoction | Cervical
radiculopathy | 1 month | VAS; PRI; PPI; MPQ;
Serum TNF-α, CRP, IL-6, CGRP levels | (245) |
| Wang et al,
2025 | RFA | Oral Celecoxib | Cervicogenic
headache | 1 month | Headache duration
(h/week); headache frequency (episodes/week); VAS; cervical range
of motion; Pittsburgh Sleep Quality Index (PSQI); hemodynamic
parameters | (246) |
| Zhang et al,
2025 | RFA | Herbal enema | Primary liver
cancer | 7 days | Pain assessment;
traditional Chinese medicine syndrome score; immune function; serum
growth factor levels | (247) |
| Zhou et al,
2024 | Nerve Block | SCS | postherpetic
neuralgia (PHN) | 3 weeks | Pain assessment;
sleep quality; clinical efficacy | (248) |
| Han et al,
2023 | SCS | Ozone trigger point
injection | Neuropathic
pain | 2 weeks | Clinical efficacy;
mechanical pain threshold; pain intensity; inflammatory
factors | (249) |
| Wang et al,
2022 | PTED | Red Light
irradiation; oral Chinese herbal medicine | Lumbar disc
herniation | 14 days | VAS; Oswestry
Disability Index (ODI); modified MacNab Criteria; adverse
events/complications | (250) |
| Peng et al,
2022 | PTED | Oral Qizhu
decoction (Astragalus-Atractylodes combination) | Lumbar disc
herniation | 7 days | Clinical efficacy
rate; Japanese Orthopaedic Association score (JOA); ODI; VAS | (251) |
| Liu et al,
2021 | PTED | Tenghuang Jianjia
capsule (osteoarthritis formula) | Lumbar disc
herniation | 3 months | VAS; ODI | (252) |
| Sun et al,
2024 | IDDS | Online pain
management | Advanced cancer
pain | 3 months | NRS; Self-Rating
Anxiety Scale (SAS); Self-Rating Depression Scale (SDS); Cancer
Pain Self-Efficacy Scale; Quality of Life Scale for Cancer Patients
(QLQ-C30) | (253) |
| Qiao et al,
2020 | IDDS | Intravenous drug
injection | Acute neuromyelitis
optica spectrum disorders (NMOSD) | 4 weeks | Expanded Disability
Status Scale; Activities of Daily Living (ADL) scale; Recurrence
rate | (254) |
| Li et al,
2016 | PIPI | - | Refractory upper
thoracic cancer pain | 6 months | VAS; Quality of
Life (SF-36); Self-Rating Depression Scale (SDS); Side effects | (255) |
Physical therapy
Physical modality therapy
Transcutaneous electrical nerve stimulation (TENS)
achieves analgesia and functional improvement through peripheral
neuromodulation. As a non-invasive electrical stimulation
technique, TENS alleviates pain by modulating peripheral nerve
conduction and central pain signal integration. In knee
osteoarthritis (KOA) treatment, TENS combined with isokinetic
eccentric training markedly reduced VAS scores and Western Ontario
and McMaster Universities Osteoarthritis Index (WOMAC) indices
while enhancing quadriceps strength and quality of life (256). Its mechanism involved
suppressing hyperexcitability of dorsal horn neurons and improving
local blood circulation. For lumbar disc herniation, transcutaneous
auricular vagus nerve stimulation outperformed conventional Chinese
medicine in pain relief (VAS) and lumbar function (JOA) by
regulating cholinergic anti-inflammatory pathways (257), highlighting the unique value of
vagal stimulation in inflammatory pain management. In obstetrics,
TENS applied during trial labor effectively alleviated first-stage
labor pain, shortened delivery time, and reduced cesarean rates
(258), with high-frequency
stimulation activating Aβ fibers to produce segmental analgesia.
For primary dysmenorrhea, TENS combined with Sanyinjiao moxibustion
prolonged analgesia duration to 8-12 h, achieving 88.57% overall
efficacy (259), demonstrating
synergy between traditional therapies and modern
electrophysiological techniques. TENS intervention at spinal T10-S2
levels for postpartum low back pain not only reduced pain scores
but also enhanced lumbar muscle strength and corrected
fear-avoidance beliefs, achieving bio-psychological dual
improvement (260) and
indicating TENS's multidimensional potential in chronic pain
management. Ultrasound and laser therapies promote tissue repair
via biophysical effects. Ultrasound improves local
microenvironments through mechanical vibration and thermal effects.
In chronic pelvic pain, ultrasound combined with herbal penetration
increases efficacy to 95.12%, as pulsed intensity enhanced
transdermal drug absorption and pelvic floor muscle metabolism
(261). For heel pain,
ultrasound combined with semiconductor laser therapy achieved 92.9%
effectiveness through synergistic mechanisms: Ultrasound promotes
calcaneal periosteal microcirculation reconstruction, while laser
inhibits local substance P release (262). In non-specific low back pain,
focused ultrasound activated deep fascial tension receptors,
reducing VAS scores by 4.2 points, markedly outperforming
traditional massage (263).
Notably, ultrasound parameters required individualized adjustment:
Pulsed stimulation improved walking distance in KOA but had limited
effects on joint stiffness (264), necessitating combined exercise
therapy to enhance mobility. Transcranial magnetic stimulation
(TMS) mitigated neuropathic pain via central neural remodeling.
High-frequency repetitive TMS (rTMS) targeting the primary motor
cortex modulated thalamocortical-limbic circuits, showing efficacy
in post-stroke central pain. A 10 Hz protocol reduced VAS scores by
3.2 points and improved HAMD depression scores, probably by
restoring dorsolateral prefrontal cortex-periaqueductal gray
(DLPFC-PAG) inhibitory pathways (265,266). In post-spinal cord injury
neuropathic pain, intermittent θ burst stimulation (50 Hz) combined
with motor training lowered resting motor threshold by 13%,
enhancing corticospinal tract efficiency and lower limb
coordination (267).
Stimulation target selection affected outcomes: Dorsal attention
network θ-wave synchronization strengthened default mode network
(DMN) inhibition of pain signals, supporting multi-regional
neuromodulation strategies (268). For chronic pain with mood
disorders, 20 Hz rTMS targeting DLPFC increased SF-36 quality of
life scores by 28 points, correlating with elevated serum
brain-derived neurotrophic factor levels (r=0.67) (269), suggesting neurotrophic pathway
involvement in pain-emotion comorbidity regulation.
Thermotherapy
Thermotherapy demonstrates multi-target mechanisms
in rehabilitation through precise local thermal regulation.
Previous research showed thermal stimulation promoted blood flow,
modulated inflammatory factors and enhanced immune responses. For
chronic pelvic inflammatory disease, Luo and Li (270) found electroacupuncture combined
with infrared therapy markedly reduced pain scores (P<0.05) and
increased CD4+/CD8+ ratios, indicating immune enhancement through
improved pelvic microcirculation. Similarly, Gan et al
(271) reported 94.29% symptom
improvement in patients with epigastric pain using
Pi-Wei-Pei-Yuan-Powder, containing Codonopsis, Angelica and
Zanthoxylum, acupoint application and infrared irradiation, with
minimal gastrointestinal side effects. In cancer pain management,
thermotherapy exhibited synergistic effects. Ou et al
(272) combined acupoint
application of cancer pain ointment with whole-body infrared
hyperthermia, achieving 92.5% pain control efficacy and improved
Karnofsky performance status scores (P<0.001), with higher
1-year survival rates (P<0.05). This approach modulated tumor
microenvironments and inhibited vascular endothelial growth factor
expression by ameliorating peritumoral hypoxia, reducing opioid
dependence (272). In
hepatocellular carcinoma pain, Liao et al (273) tailored herbal compresses and
irradiation intensity to pain severity, integrating psychological
and dietary support into a 'clinician-nurse-patient' tripartite
model. Infrared irradiation (40-45°C in pelvic inflammation)
enhanced drug absorption and metabolic activity, balancing efficacy
and safety (270). Future
studies should explore thermotherapy combined with biologics or
gene therapy for chronic and oncological rehabilitation.
Traditional external therapies
Traditional external therapies integrate meridian
theory and herbal pharmacology, combining transdermal drug delivery
and acupoint stimulation. Clinical applications include herbal
patches, infrared thermotherapy, and their combinations for
epigastric pain, cancer pain, and arthralgia. For example,
Pi-Wei-Pei-Yuan-Powder applied with infrared irradiation expanded
skin pores for enhanced absorption, alleviating epigastric
distension and acid reflux with 14.29% higher efficacy compared
with standard care (271).
Similar approaches extended to hepatocellular carcinoma pain:
Xie-Tong-Gao applied to liver regions with infrared irradiation
achieved 46.6% complete remission and 36% faster onset compared
with oral tramadol (273).
Wu-Tou San with infrared therapy for arthralgia reduced knee
stiffness by 47% at 12 weeks via transdermal aconitine absorption
(274). In cancer pain,
external therapies reduced opioid reliance. Guangzhou University of
Chinese Medicine's cancer pain ointment whole-body hyperthermia
achieved 92.5% pain control and 12.7% higher 1-year survival than
WHO step-three therapy (272).
Yangjiang Hospital's Sihuang San with deep thermotherapy reduced
breakthrough pain episodes by 22% through microcirculation
enhancement and prostaglandin inhibition (275). These methods avoided first-pass
metabolism and exploited infrared-induced capillary permeability
(40-42°C) for deep tissue penetration. Safety profiles favor
external therapies: Pi-Wei-Pei-Yuan-Powder caused only 5% skin
erythema (271) and cancer pain
ointment showed no hepatorenal toxicity (272). Overall, traditional external
therapies offer simple, convenient, effective and economical
solutions, particularly in minimizing systemic side effects and
improving compliance. rTMS achieves targeted intervention in the
affective dimension of pain by modulating anterior cingulate
cortex-insula functional connectivity through θ-burst stimulation
protocols, while photobiomodulation therapy enhances photonic
energy conversion efficiency via gold nanorod-mediated surface
plasmon resonance effects, specifically activating the
mitochondrial Complex IV electron transport chain. A transformative
breakthrough lies in wearable closed-loop ultrasound systems, which
dynamically dissolve calprotectin deposits at myofascial trigger
points through real-time monitoring of shear wave propagation
speeds (276). Building on
current advancements, interdisciplinary strategies could
prioritize: Developing multi-physics collaborative intervention
systems, constructing nano-catalytic sonodynamic technologies by
combining ultrasound with bismuth sulfide nanozymes to generate
reactive oxygen species in situ for clearing inflammatory
microenvironments in dorsal root ganglia and engineering
intelligent wearable flexible electronics integrating skin
impedance monitoring with adaptive TENS parameters for real-time
closed-loop control of peripheral sensitization (277). Notably, mechanosensitive
miRNA-targeted delivery systems warrant exploration, exploiting
low-frequency ultrasound to activate the piezoelectric response of
nanocarriers for epigenetic editing of pain-related genes. Table VI systematically summarized and
compared the core parameters and clinical efficacy of physical
therapy, providing a reference for researchers.
 | Table VICharacteristics of physical therapy
for pain. |
Table VI
Characteristics of physical therapy
for pain.
| Authors, year | Method | Combination | Indications | Time | Effects | (Refs.) |
|---|
| Zhu et al,
2025 | TENS | Isokinetic
eccentric training | KOA | 4 weeks | VAS score, WOMAC
Index, quadriceps strength (Biodex Test), GQOLI-74 quality of
life | (256) |
| Bi et al,
2025 | rTMS | Conventional
rehabilitation | CPSP | 4 weeks | VAS score, HAMA
Anxiety Scale, HAMD Depression Scale, SRSS Sleep Quality Score | (265) |
| Chen et al,
2025 | TAVNS | Multimodal
analgesia | Post-TKA | 14 days | NRS score, pain
threshold, SAS anxiety score, complication rate | (276) |
| Yang et al,
2024 | Ultrasound | TENS | Chronic pelvic | 4 weeks | VAS score, WOMAC
pain/function scores, 6-min walk distance, ambulation capacity | (264) |
| Guan et al,
2009 | Ultrasound +
semiconductor laser | Physical
modality | Heel | 20 days | Pain relief rate
(cured/effective/improved), heel spur imaging changes | (262) |
| Feng et al,
2024 | TENS | Moxibustion | Primary
dysmenorrhea | 3 weeks | VAS score,
analgesic onset/duration, adverse reaction rate | (259) |
| Pang et al,
2024 | Deep
thermotherapy | Herbal external
application | Hepatocellular
carcinoma | 7 days | NRS score, quality
of life (QOL-LC), liver/kidney function, breakthrough pain
episodes | (275) |
| Mao et al,
2014 | Deep
thermotherapy | Acupoint
application | Post-lung
surgery | 7 days | VAS score,
recurrence rate, quality of life, meridian qi-blood flow
improvement | (277) |
| Gunn et al,
2011 | Infrared
irradiation |
Electroacupuncture | Painful obstruction
syndrome | 12 weeks | VAS score, immune
function (CD4+/CD8+), hemorheology (blood viscosity) | (27) |
| Men et al,
2024 | Infrared
irradiation | Herbal patch
application | Epigastric pain due
to spleen-stomach deficiency-cold | 2 weeks | VAS score, clinical
efficacy (cured/improved), adverse reactions (skin burns) | (274) |
| Ou et al,
2017 | Infrared
irradiation | Infrared
irradiation | KOA | 3 weeks | VAS score, knee
range of motion (Melle Score), complication rate | (272) |
Psychological interventions
Cognitive behavioral therapy
(CBT)
CBT has been validated as a critical psychological
intervention for chronic low back pain (CLBP) management through
systematic reviews and randomized controlled trials (RCTs). A
meta-analysis of 22 RCTs demonstrated that CBT markedly improved
pain intensity, functional disability, fear-avoidance beliefs and
self-efficacy post-intervention, with sustained effects over a
3-month follow-up. Notably, when combined with other active
therapies, CBT showed superior pain and disability reduction
compared with physical therapy alone (P<0.05), suggesting
synergistic benefits of multimodal interventions (278). Innovative delivery formats,
such as the 2-h empowered relief program, exhibited non-inferiority
to standard 8-session CBT in reducing catastrophic pain, intensity
and functional limitations, with effects persisting for 3 months
(279). This high-efficacy,
brief protocol reduces treatment costs and enhances accessibility
in resource-limited settings. Comparative studies revealed
differential efficacy profiles between CBT and mindfulness-based
stress reduction. CBT demonstrated superior long-term functional
maintenance at 52 weeks (P<0.05), potentially due to its
structured cognitive restructuring and behavioral activation
strategies (280). However, the
effect of CBT on depressive symptoms remains controversial: A
systematic review of 10 RCTs found only three studies showing
significant depression score improvements with CBT-physical therapy
combinations, while one high-quality trial reported greater
depression reduction in the physical therapy group (281). These inconsistencies underscore
the need for standardized depression screening and outcome
assessment protocols. Subacute low back pain (SALBP) research
highlights critical knowledge gaps. A systematic review of 20 years
literature identified only six relevant RCTs, three of which
conflated SALBP with chronic pain (282). Heterogeneity in SALBP
definitions (7-12 weeks) and intervention protocols (2-9 sessions),
along with limited validation in USA healthcare systems, impede
general application. For instance, Hadley et al's cluster
RCT (283) found no significant
efficacy for primary care-delivered CBT, probably due to fidelity
issues and insufficient provider training. These limitations
necessitated rigorous multicenter studies to define optimal
intervention timing and dosing for preventing chronic pain.
Technological innovations such as internet-based CBT (iCBT) and
telephone CBT theoretically enhanced accessibility but lacked
robust evidence in CLBP. Existing studies predominantly focused on
European healthcare systems, with limited exploration of cultural
adaptations (283). Cognitive
functional therapy, a CBT variant employing a four-stage model,
demonstrated functional improvements at 3-year follow-up, so
head-to-head comparisons with classic CBT are warranted (281).
Biofeedback and neurofeedback
Biofeedback and neurofeedback technologies offer
non-invasive neuromodulation for chronic pain and psychosomatic
disorders by enabling real-time self-regulation of autonomic and
central neural activity (284).
In fibromyalgia (FM), EEG neurofeedback targeting sensorimotor
rhythm (SMR; 12-15 Hz) improved anxiety, depression and pain
intensity, probably via thalamocortical circuit desensitization
(285). Goldway et al's
(285) limbic system
neurofeedback, using fMRI-guided EEG models to modulate amygdala
activity, extended REM latency (P<0.05) and improved sleep
quality in FM patients, with 3-year follow-up showing greater pain
reduction compared with sham (P<0.05), suggesting delayed
analgesia through HPA axis normalization post-autonomic rebalancing
(286). Heart rate variability
(HRV) biofeedback demonstrates a special value in cancer survival.
The RCT of Burch et al (287) reported HRV coherence
(0.37-0.84; P=0.01) after 4-6 weeks of training, alongside
significant sleep (d=1.19) and daytime function (d=0.86)
improvements, mediated by vagal tone enhancement via respiratory
sinus arrhythmia. Pediatric functional abdominal pain studies
replicated these effects, with 63.6% achieving full remission and
>50% pain reduction in residual cases, implying gut-brain axis
modulation (288).
Neurofeedback-CBT synergism showed promise in multiple sclerosis
pain. The EEG neurofeedback-enhanced hypnotic analgesia of Jensen
et al (289), augmenting
prefrontal θ (4-8 Hz) to boost default mode-executive network
coupling, outperformed relaxation in pain relief, suggesting
θ-driven neuroplasticity amplifies hypnotic suggestibility. Hassett
et al (286) observed
immediate HRV improvements but delayed blood pressure variability
and symptom relief (3 months) in FM, possibly due to gradual
baroreflex sensitivity adaptation. Current limitations include
small sample sizes, short follow-ups, protocol heterogeneity and
inadequate sham controls (285). Future directions should
integrate multimodal feedback and machine learning for personalized
parameter optimization. In conclusion, biofeedback and
neurofeedback enabled mechanism-driven neuromodulation, offering
symptom relief and neuroplastic disease modification, thus
advancing non-pharmacological strategies for chronic psychosomatic
conditions.
Complementary and alternative
therapies
In the realm of complementary and alternative
medicine, innovative therapeutic modalities have demonstrated
unique clinical efficacy through rigorous research. Balanced
acupuncture, a modernized adaptation of traditional Chinese
medicine, exhibited significant advantages in treating
cervicobrachial and lumbocrural pain. RCTs confirmed that balanced
acupuncture reduced pain scores and recurrence rates by modulating
inflammatory mediator absorption, releasing adhesions, and
activating endogenous analgesic systems. This holistic approach
showed 89.7% clinical efficacy in degenerative conditions such as
cervical spondylosis and lumbar disc herniation, with shorter
treatment cycles than conventional acupuncture (290). When combined with herbal
formulations such as Yi-Qi-Shu-Jin-Decoction, balanced acupuncture
synergistically improved Constant-Murley shoulder scores and
achieved 91% enhancement in Japanese Orthopedic Association (JOA)
assessment scores (291).
Chiropractic manipulation demonstrated value in spinal disorders.
For postpartum lumbosacral pain, sacroiliac joint semi-dislocation
correction via American chiropractic techniques combined with
Governor Vessel massage reduced VAS scores and ODI (292). In spine-related epigastric
pain, chiropractic intervention shortened pain relief duration and
lowers Hamilton anxiety scale scores compared with traditional
massage (293). Further studies
revealed chiropractic care improved neurological function in lumbar
disc herniation, increasing straight-leg raise angles and reducing
ODI (294). Herbal external
therapies remodeled postoperative management. Evodia paste combined
with herbal enemas reduced pethidine use by 63% and maintained
postoperative VAS scores below 3.0 within 12 h in pelvic surgery.
This transdermal system, stabilized blood drug concentrations for
6-8 h while regulating PGE2 (-54%) and serotonin levels (-38%)
(295). For chronic pain,
Shen-Tong-Zhu-Yu-Decoction with tuina massage increased lumbar
flexion by 25° and elevated JOA by 31 points in low back pain
(296).
Physical agent therapies have achieved
breakthroughs in osteoarticular diseases. Low-intensity pulsed
ultrasound (LIPUS) for KOA WOMAC elevated Lysholm scores by 29
points and reduced synovial IL-1β (-62%) and TNF-α (-58%) after 8
weeks (297). In angina
pectoris, LIPUS normalized cardiac autonomic function (LF/HF ratio
normalization: 81%), reducing angina frequency by 67% with 92%
lower weekly costs compared with PCI (298). Minimally invasive interventions
combined with traditional methods redefine pain management.
Percutaneous transforaminal endoscopic surgery with epidural
steroid injections shortened hospitalization to 3.2 days and
achieved 73% ODI improvement at 1 week postoperatively. Targeted
compound betamethasone injections elevated perineural drug
concentrations 8-fold, inhibiting fibrosis (76% scar area reduction
on MRI) and reducing long-term recurrence risk by 43% (299). Evidence-based studies
standardized complementary therapies. For fibromyalgia, acupuncture
combined with chiropractic care reduced MPQ scores by 58%,
sustaining efficacy for 9.2 months (300). In geriatric chronic pain,
acupuncture with meridian-based tuina increased lumbar flexion by
18° and lowered PGE2 (-64%) (290). Current trends show
precision-driven standardization: AI-based acupuncture point
localization (±1.2 mm), real-time force monitoring in chiropractic
manipulation (293), and
multi-omics studies revealing balanced acupuncture modulates 287
differentially expressed genes, primarily in TLR4/NF-κB and
PI3K-Akt pathways (290).
3D-printed orthoses achieved 98% biomechanical adaptability
(294), heralding a precision
medicine era in complementary therapies.
The current pain management paradigm integrates
multimodal synergies under the biopsychosocial model.
Pharmacologically, NSAIDs remained first-line via COX-mediated
prostaglandin inhibition, while epigenetic drugs such as HDAC
inhibitors enabled durable analgesia through pain-gene methylation.
Opioid therapy has shifted toward precision μ-receptor
targeting, with pharmacogenomics reducing morphine-equivalent
dosing errors by 37.2% (P<0.01). Technological innovations
included high-intensity focused ultrasound for 0.5 mm-precise deep
tissue heating via HSP70 activation, and closed-loop spinal cord
stimulation (CL-SCS) systems that adjusted parameters via real-time
β-wave detection, achieving 68.4% 6-month pain relief (95% CI:
62.1-74.7) in neuropathic pain. Digital therapeutics such as
virtual reality exposure therapy reprograms prefrontal-amygdala
circuits and reduces catastrophic pain by 19.3% in fibromyalgia.
Mechanistic advances in complementary therapies reveal that
acupuncture analgesia extends beyond endorphin release to
astrocytic connexin 43 (Cx43) networks. Functional near-infrared
spectroscopy confirmed Zu-San-Li stimulation induced θ-band power
spectral changes in the DMN (P=0.032). Persistent challenges
include: i) Low AUC values in pain response prediction due to
heterogeneity; ii) absent frameworks for quantifying multi-target
drug synergism; iii) incomplete causal chains across
molecular-cellular-system effects. Future research must prioritize
biomechanical microenvironment omics-based decision trees to
overcome these barriers.
Prospects for analgesic drugs
The development of analgesic drugs represents a
critical global health priority, currently evolving beyond
traditional paradigms toward precision, personalization and
multidimensional integration. First, pathophysiological research
continues to deepen the understanding of pain biology, providing
theoretical foundations for target discovery. Second, the precise
identification of individual pain heterogeneity drives a paradigm
shift from 'population treatment' to 'stratified intervention'.
Cross-disciplinary integration of neuroscience, artificial
intelligence and pharmacology catalyzes disruptive technological
pathways. Concurrently, exploration of comorbid mechanisms linking
pain with psychiatric disorders and metabolic dysregulation opens
possibilities for 'multi-effect' strategies in complex chronic
pain. Finally, the modernization of traditional Chinese medicine's
holistic philosophy and natural medicinal resources infuses novel
analgesic molecular libraries with ancestral wisdom. These six
interconnected directions collectively outline a multidimensional
convergence framework for future analgesic development.
Pathophysiology-driven analgesic
drugs
Pathophysiology-driven analgesic drugs are emerging
as the pivotal breakthroughs in pain therapeutics. Traditional
analgesics largely relied on phenotypic screening, with target
identification lagging behind clinical efficacy validation,
resulting in ambiguous mechanisms and significant side effects.
Recent advances in molecular biology and neuroscience have
established mechanism-guided target discovery as the mainstream
strategy. For instance, in-depth studies of the calcitonin
gene-related peptide (CGRP) pathway in migraine pathogenesis
directly enabled the development of CGRP receptor monoclonal
antibodies. By precisely blocking CGRP-receptor interactions, these
agents effectively inhibited trigeminovascular activation,
representing the first migraine prophylactics designed via
pathophysiological insights (301,302). This case exemplified the
translational 'mechanism-to-target-to-drug' pipeline.
Technologically, three core systems dominate this field (Fig. 5): i) Neural circuit-specific
modulation tools, including chemogenetics, optogenetics, and in
vivo calcium imaging, enable spatiotemporally precise
manipulation of pain-related brain regions, elucidating
neuroanatomical bases of pain signaling (303,304), ii) molecular screening
innovations, such as single-cell transcriptomics, reveal gene
expression profiles of specific dorsal root ganglion neuron
subtypes, identifying druggable ion channel targets such as TRPV1
and Nav1.7 (305,306), ii) public genomic databases
facilitate rapid cross-species comparison of pain-related gene
expression, markedly enhancing target prioritization (307). However, despite standardized
mechanism-to-drug workflows, clinical translation failure rates are
>60%, probably due to rodent models inadequately replicating
human chronic pain's affective-cognitive components and
interspecies target protein conformational differences (308).
Opioid modification and novel strategies
for non-opioid receptors
Opioids remain the most effective drugs for
moderate to severe pain. Since the WHO established the three-step
analgesic ladder for cancer pain, opioid-based regimens have become
globally pivotal. However, severe adverse effects, respiratory
depression, addiction/withdrawal risks and societal crises, are the
critical challenges in analgesic research (309,311). In the late 19th century,
structural modifications of morphine yielded derivatives such as
heroin and fentanyl (312).
Recent decades have focused on molecular-level target separation:
Biased agonists (TRV130, YZJ-4729 and SHR8554) enhance analgesia by
activating MORs while inhibiting β-arrestin2 negative signaling
(313), multifunctional ligands
(cholecystokinin) mitigate adverse effects via concurrent
modulation of intracellular cascades (314). These findings suggest
μ-receptor downstream pathway-specific agonists could yield
novel opioids with reducing toxicity. Multifunctional ligands
targeting both opioid and non-opioid systems showed clinical
promise. Drugs such as tapentadol and tramadol, combining
μ-receptor agonism with norepinephrine/serotonin reuptake
inhibition, exemplify this approach (315). The evidence suggests
receptor-system dissociation will be a key strategy for
next-generation opioids (316).
Beyond opioid receptor dependence, non-opioid mechanisms are
gaining traction to circumvent addiction and side effects. The NIH
prioritized funding for such research, with increased USA
governmental investment (316).
It was identified that novel non-opioid circuits mediate analgesia
(317). There were two
strategies dominated this field: i) Advanced neurobiological
techniques (single-cell sequencing, molecular profiling) to
identify non-opioid analgesic circuits and druggable targets, ii)
repurposing known non-opioid substances by targeting their
mechanisms for new drug development (317-319). With sustained funding and
robust strategies, non-opioid receptor-dependent analgesics hold
immense potential (Fig. 6).
Innovation in personalized analgesic
concepts
Current pain research is transitioning from
'universal treatment' to 'personalized precision intervention',
driven by deepened understanding of pain phenotypic heterogeneity.
Traditional analgesics exhibit clinical response rates <50%,
with some patients experiencing hyperalgesia aggravation,
highlighting the critical influence of individual genetic
backgrounds and neuroplasticity (309). Genome-wide association studies
in neuropathic pain have identified polymorphisms in sodium
voltage-gated channel α subunit 9 and catechol-O-methyltransferase
correlating with pregabalin efficacy, providing molecular rationale
for genotype-guided dosing (310,311). Core technologies enabling
personalized analgesia include: i) Pain biomarker screening
(191), ii) dynamic
pharmacodynamic models integrating metabolomics and
pharmacokinetics for dose optimization (312) and iii) smart drug delivery
systems such as microneedle-integrated transdermal patches
regulating release rates via local IL-6/TNF-α sensing (203). However, some challenges still
exist: Immature algorithms for multidimensional pain assessment,
insufficient trial cohorts for rare genetic variants and
cost-resource disparities in precision medicine (313). Multi-center cohorts and
AI-assisted diagnostics are essential to overcome these
barriers.
Interdisciplinary perspectives on pain
mechanisms and analgesic drugs
Cross-disciplinary integration is revolutionizing
pain research through novel analytical frameworks. While
traditional pharmacology focuses on single targets, convergent
neuroscience-bioinformatics-materials science strategies enable
multidimensional analysis of pain-emotion comorbidity networks.
AI-assisted drug design predicts Nav1.8 allosteric modulator
conformations to accelerate lead compound discovery (320), nanodelivery systems using
pH-responsive hydrogels to concentrate gabapentin in spinal dorsal
horn inflammatory micro-environments (3x local concentration; 40%
systemic toxicity reduction) (314), photoacoustic imaging combined
with gene editing, visualizing real-time microglia-neuron synaptic
interactions in chronic pain models to reveal P2X4-mediated
neuroimmune mechanisms (315,316) and organ-on-chip models
co-culturing dorsal root ganglia and spinal tissues to mimic
neuropathic pain signaling for high-throughput screening (317). However, interdisciplinary
progress will face data standardization hurdles and ethical
controversies, necessitating cross-sector collaboration in the
future (318).
Comorbidity mechanism-based analgesic
drugs
Chronic pain patients frequently present with
comorbidities-anxiety, depression, cognitive impairment and sleep
disorders that complicate diagnosis and treatment (321-324). Preclinical studies reveal that
chronic inflammatory pain induced both somatic hypersensitivity and
depressive-like behaviors, forming a pain-depression comorbid state
with distinct sensory and affective neural mechanisms (325). Neuroimaging and molecular
studies demonstrate overlapping brain regions (ventral tegmental
area, nucleus accumbens and prefrontal cortex) (326-328) and signaling molecules
(brain-derived neurotrophic factor) (329,330) mediating both pain and emotion.
This mechanistic overlap underpins the therapeutic potential of
multitarget drugs as evidenced by antidepressants (gabapentin,
pregabalin) alleviating chronic pain (331). Future research must prioritize
comorbidity-specific biomarkers and combination therapies targeting
multifactorial pathophysiology.
TCM-based analgesic strategy
development
TCM claims that pain arises from 'obstruction
causing pain' and 'malnourishment causing pain', with etiologies
including what TCM terms qi stagnation, blood stasis, cold
coagulation, blood deficiency and fluid depletion. Clinically, TCM
formulations, alone or combined with Western drugs, exhibited
favorable efficacy and safety, though mechanistic clarity remains
limited (332). Modern studies
revealed TCM components exerted analgesia via multiple pathways:
Enhancing central catecholamines, activating opioid receptors,
suppressing GABA receptor expression and boosting endogenous
analgesic release. Acupuncture, widely used for migraines, visceral
pain and musculoskeletal pain, mediates analgesia through opioid
peptides, glutamate, serotonin, and cholecystokinin signaling
(333,334). Integrating advanced
neurotechnologies to decipher TCM mechanisms and develop targeted
therapies will represent a pivotal future direction in the
future.
Conclusion
In summary, pain is a complex condition involving
multifaceted mechanisms and current treatments remain limited with
substantial clinical challenges. The present review discussed key
advances in pain research, highlighting the transition from
symptomatic management to integrated mechanistic approaches, with
an emphasis on enhancing clinical relevance and patient care.
First, the pain model was systematically validated.
By evaluating animal pain models, the advantages and limitations of
their translational applications can be clarified, providing a
standardized and more accurate validation platform for preclinical
drug testing and target screening.
Second, the molecular targets for pain relief have
been identified. By integrating ion channels, GPCRs and
inflammation related enzymes, the pathogenesis of pain is
elucidated, with a focus on novel targets with clinical application
potential, directly solving the problem of the lack of precise
molecular mechanism basis for current analgesics.
Finally, a multimodal treatment strategy has been
integrated by analyzing the synergistic effects between drug
therapy, interventional therapy and non-drug intervention,
comprehensively summarizing the current research status of
treatment strategies and constructing a pain management framework
directly applicable to clinical practice.
In the future, research and treatment of pain
should focus on the following aspects: i) Precision medicine
leveraging multi-omics biomarkers and AI, ii) cross-disciplinary
technologies (optogenetics, nanorobotics), iii) comorbidity
mechanisms for multitarget analgesics and iv) TCM modernization
through evidence-based formulations and global standardization.
Supplementary Data
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article and in the
'Data S1' supplementary file.
Authors' contributions
CC and XW were responsible for data curation and
writing the original draft. KL and LW were responsible for formal
analysis and methodology. MA and QL were responsible for
conceptualization and investigation. HH, QM, RW, XW and WL were
responsible for supervision, funding acquisition, writing,
reviewing and editing. Data authentication is not applicable. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AWR
|
abdominal withdrawal reflex
|
|
cAMP
|
cyclic adenosine monophosphate
|
|
CBT
|
cognitive behavioral therapy
|
|
CSNRC
|
chronic spinal nerve root
compression
|
|
DSS
|
dextran sulfate sodium
|
|
IDDS
|
intrathecal drug delivery system
|
|
MPQ
|
McGill pain questionnaire
|
|
NOP
|
nociceptin opioid peptide
|
|
NOS
|
nitric oxide synthase
|
|
NRS
|
numeric rating scale
|
|
NSAIDs
|
nonsteroidal anti-inflammatory
drugs
|
|
ODI
|
Oswestry disability index
|
|
SCS
|
spinal cord stimulation
|
|
SNL
|
spinal nerve ligation
|
|
TENS
|
transcutaneous electrical nerve
stimulation
|
|
TMS
|
transcranial magnetic stimulation
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TRP
|
transient receptor potenti
|
|
TRPM
|
transient receptor potential
melastatin
|
|
TRPP
|
transient receptor potential
polycystin
|
Acknowledgements
Not applicable.
Funding
The present review was supported by National Natural Science
Foundation of China (grant nos. 82360682 and 82360825), Major
Science and Technology Research Projects of Nanchang City [grant
no. Hongkezi (2023)137-2], Training Project of Ganpo Juncai Support
Plan for High level and High skilled Leading Talent in 2024 (grant
no. 20240003188), Chunhui Plan 'Collaborative Research Project of
Ministry of Education of the People's Republic of China' (grant
nos. HZKY20220385 and 202200200), Jiangxi Provincial Natural
Science Foundation (grant nos. 20242BAB26172, 20224BAB206104 and
20224BAB206112), Jiangxi University of Chinese Medicine Science and
Technology Innovation Team Development Program (grant no.
CXTD-22002) and Sustainable Utilization of Chinese Material Medical
Resources (grant no. 2024SSY07082).
References
|
1
|
Rogers MP and Kuo PC: Pain as the fifth
vital sign. J Am Coll Surg. 231:601–602. 2020. View Article : Google Scholar
|
|
2
|
Carroll CP and Brandow AM: Chronic pain:
Prevalence and management. Hematol Oncol Clin North Am.
36:1151–1165. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ushida T: Chronic pain:
Definition/conception/classification of pain. Brain Nerve.
75:201–205. 2023.In Japanese. PubMed/NCBI
|
|
4
|
Raja SN, Carr DB, Cohen M, Finnerup NB,
Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, et al:
The revised international association for the study of pain
definition of pain: Concepts, challenges, and compromises. Pain.
161:1976–1982. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peters ML: Emotional and cognitive
influences on pain experience. Mod Trends Pharmacopsychiatry.
30:138–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen SP, Vase L and Hooten WM: Chronic
pain: An update on burden, best practices, and new advances.
Lancet. 397:2082–2097. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel EA and Perloff MD: Radicular pain
syndromes: Cervical, lumbar, and spinal stenosis. Semin Neurol.
38:634–639. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashburn MA and Fine PG: Persistent pain
following trauma. Mil Med. 154:86–89. 2020. View Article : Google Scholar
|
|
9
|
Roicke H, Köhler W, Baum P and Krasselt M:
Non-inflammatory muscle pain. Dtsch Med Wochenschr. 145:887–894.
2020.In German. PubMed/NCBI
|
|
10
|
Ford AC, Vanner S, Kashyap PC and Nasser
Y: Chronic visceral pain: new peripheral mechanistic insights and
resulting treatments. Gastroenterology. 166:976–994. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Finnerup NB, Kuner R and Jensen TS:
Neuropathic pain: From mechanisms to treatment. Physiol Rev.
101:259–301. 2021. View Article : Google Scholar
|
|
12
|
Erpelding N and Borsook D: Capturing brain
metrics of neuropathic pain using nuclear magnetic resonance. Pain
Manag. 3:395–409. 2013. View Article : Google Scholar
|
|
13
|
Scheuren PS, Rosner J, Curt A and Hubli M:
Pain-autonomic interaction: A surrogate marker of central
sensitization. Eur J Pain. 24:2015–2026. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hawker GA, Mian S, Kendzerska T and French
M: Measures of adult pain: Visual analog scale for pain (VAS pain),
numeric rating scale for pain (NRS pain), McGill pain questionnaire
(MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain
grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS),
and measure of intermittent and constant osteoarthritis pain
(ICOAP). Arthritis Care Res (Hoboken). 63(Suppl 11): S240–S252.
2011. View Article : Google Scholar
|
|
15
|
Weigl M, Letzel J and Angst F: Prognostic
factors for the improvement of pain and disability following
multidisciplinary rehabilitation in patients with chronic neck
pain. BMC Musculoskelet Disord. 22:3302021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rutter-Locher Z, Kirkham BW, Bannister K,
Bennett DL, Buckley CD, Taams LS and Denk F: An interdisciplinary
perspective on peripheral drivers of pain in rheumatoid arthritis.
Nat Rev Rheumatol. 20:671–682. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Todd A, McNamara CL, Balaj M, Huijts T,
Akhter N, Thomson K, Kasim A, Eikemo TA and Bambra C: The European
epidemic: Pain prevalence and socioeconomic inequalities in pain
across 19 European countries. Eur J Pain. 23:1425–1436. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito A and Yoshimura M: Mechanisms of the
analgesic effect of calcitonin on chronic pain by alteration of
receptor or channel expression. Mol Pain. 13:17448069177203162017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bannister K, Sachau J, Baron R and
Dickenson AH: Neuropathic pain: Mechanism-based therapeutics. Annu
Rev Pharmacol Toxicol. 60:257–274. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang R, Han L, Gao Q, Chen D, Wang Y,
Zhang X, Yu X, Zhang Y, Li Z and Bai C: Progress on active
analgesic components and mechanisms of commonly used traditional
Chinese medicines: A comprehensive review. J Pharm Pharm Sci.
21:437–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boscan P, Monnet E, Mama K, Twedt DC,
Congdon J, Eickhoff JC and Steffey EP: A dog model to study ovary,
ovarian ligament and visceral pain. Vet Anaesth Analg. 38:260–266.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mogil JS: Animal models of pain: Progress
and challenges. Nat Rev Neurosci. 10:283–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Milinkeviciute G, Gentile C and Neely GG:
Drosophila as a tool for studying the conserved genetics of pain.
Clin Genet. 82:359–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao WD, Volgin AD, Alpyshov ET, Friend AJ,
Strekalova TV, de Abreu MS, Collins C, Amstislavskaya TG, Demin KA
and Kalueff AV: Opioid neurobiology, neurogenetics and
neuropharmacology in zebrafish. Neuroscience. 404:218–232. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gunn A, Bobeck EN, Weber C and Morgan MM:
The influence of non-nociceptive factors on hot-plate latency in
rats. J Pain. 12:222–227. 2011. View Article : Google Scholar
|
|
28
|
Wang JS, Chen B, Li MY, Zhao X and Guo Y:
Animal models and analgesia mechanism analysis commonly used of
acupuncture analgesia. Liaoning J Tradit Chin Med. 44:435–438.
2017.In Chinese.
|
|
29
|
Ho Kim S and Mo Chung J: An experimental
model for peripheral neuropathy produced by segmental spinal nerve
ligation in the rat. Pain. 50:355–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang JS and Li BF: Choice and
establishment of mirror-image pain model of selective spinal nerve
ligation in rats. Med Innov China. 11:30–32. 2014.In Chinese.
|
|
31
|
Chung JM, Kim HK and Chung KS: Segmental
spinal nerve ligation model of neuropathic pain. Methods Mol Med.
99:35–45. 2004.PubMed/NCBI
|
|
32
|
Huang YG, Zhang Q, Wu H and Zhang CQ: A
comparison of surgical invasions for spinal nerve ligation with or
without paraspinal muscle removal in a rat neuropathic pain model.
Biomed Res Int. 2016:67412952016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang YJ, Wan C, Shen PZ, Wang HB, Shi YY
and Shi Q: A model study of experimental lumbar nerve root
compression. Chin J Tradit Med Traumatol Orthop. 7:9–12. 1999.In
Chinese.
|
|
34
|
Xue F, Wei Y, Chen Y, Wang Y and Gao L: A
rat model for chronic spinal nerve root compression. Eur Spine J.
23:435–446. 2014. View Article : Google Scholar :
|
|
35
|
Zhang Y, Zhao D, Li X, Gao B, Sun C, Zhou
S, Ma Y, Chen X and Xu D: The Wnt/β-Catenin pathway regulated
cytokines for pathological neuropathic pain in chronic compression
of dorsal root ganglion model. Neural Plast. 2021:66801922021.
View Article : Google Scholar
|
|
36
|
Lin ZG, Jiang SC, Cheng YB, Song PF and
Fang M: Experimental study of Tuina on DRG neurons P2X3 receptor of
lumbar disc herniation rats. Chin Arch Tradit Chin Med.
35:2475–2479. 2017.In Chinese.
|
|
37
|
Finskas O, Blixt A, Fujioka Y and Olmarker
K: New, clinically more relevant model for nerve root injury in the
rat. Spine (Phila Pa 1976). 38:1744–1748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai A, Moon A, Purmessur D, Skovrlj B,
Laudier DM, Winkelstein BA, Cho SK, Hecht AC and Iatridis JC:
Annular puncture with tumor necrosis factor-alpha injection
enhances painful behavior with disc degeneration in vivo. Spine J.
16:420–431. 2016. View Article : Google Scholar :
|
|
39
|
Shamji MF, Allen KD, So S, Jing L, Adams
SB Jr, Schuh R, Huebner J, Kraus VB, Friedman AH, Setton LA and
Richardson WJ: Gait abnormalities and inflammatory cytokines in an
autologous nucleus pulposus model of radiculopathy. Spine (Phila Pa
1976). 34:648–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SJ, Park SM, Cho YW, Jung YJ, Lee DG,
Jang SH, Park HW, Hwang SJ and Ahn SH: Changes in expression of
mRNA for interleukin-8 and effects of interleukin-8 receptor
inhibitor in the spinal dorsal horn in a rat model of lumbar disc
herniation. Spine (Phila Pa 1976). 36:2139–2146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang JJ, Song W, Luo WY, Wei M, Sun LB,
Zou XN and Liao WM: Autologous nucleus pulposus transplantation to
lumbar 5 dorsal root ganglion after epineurium dissection in rats:
A modified model of non-compressive lumbar herniation. Chin Med J
(Engl). 124:2009–2014. 2011.PubMed/NCBI
|
|
42
|
Cho HK, Ahn SH, Kim SY, Choi MJ, Hwang SJ
and Cho YW: Changes in the expressions of Iba1 and calcitonin
gene-related peptide in adjacent lumbar spinal segments after
lumbar disc herniation in a rat model. J Korean Med Sci.
30:1902–1910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao CP, Zhu ML, Wang TT, Liu XL and Liu
CL: A chemical radiculitis model in the rat: Establishment and
evaluation. Chin J Tissue Eng Res. 23:1030–1034. 2019.In
Chinese.
|
|
44
|
Zhang JJ, Wei M, Lai YR, Sun LB and Liao
WM: Non-compressive effect on ultra-structural changes in the
dorsal root ganglion following autograft of nucleus pulposus in
rats. Chin J Nerv Ment Dis. 35:280–284. 2009.In Chinese.
|
|
45
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang G, Liu N, Zhu C, Ma L, Yang J, Du J,
Zhang W, Sun T, Niu J and Yu J: Antinociceptive effect of
isoorientin against neuropathic pain induced by the chronic
constriction injury of the sciatic nerve in mice. Int
Immunopharmacol. 75:1057532019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu CY, Xu QH, Mao ZY and Lin N:
Application of three artemisinin derivatives in neuropathic pain:
Evaluating co-curation of nociceptive and emotional syndromes in
spinal cord ligation mice. Zhongguo Zhong Yao Za Zhi. 43:3058–3063.
2018.In Chinese. PubMed/NCBI
|
|
48
|
Meng K and Wang Y: Effect of BMP7 on
neuropathic pain in rats with spinal cord injury. Chin J Pain Med.
26:174–179. 2020.In Chinese.
|
|
49
|
Shih HC, Kuan YH and Shyu BC: Targeting
brain-derived neurotrophic factor in the medial thalamus for the
treatment of central poststroke pain in a rodent model. Pain.
158:1302–1313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu HF, Xu CY, Zhang L, Gan L, Chen C, Yan
MY, Guo XN, Fang Q, Xu GY, Zhang YB, et al: A new central
post-stroke pain rat model: Autologous blood injected thalamic
hemorrhage involved increased expression of P2X4 receptor. Neurosci
Lett. 687:124–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Y, Jiao H, Ren W and Ren F: TRESK
alleviates trigeminal neuralgia induced by infraorbital nerve
chronic constriction injury in rats. Mol Pain.
15:17448069198825112019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cao Y, Wang H, Chiang CY, Dostrovsky JO
and Sessle BJ: Pregabalin suppresses nociceptive behavior and
central sensitization in a rat trigeminal neuropathic pain model. J
Pain. 14:193–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bennett MI, Kaasa S, Barke A, Korwisi B,
Rief W and Treede RD: IASP Taskforce for the Classification of
Chronic Pain: The IASP classification of chronic pain for ICD-11:
Chronic cancer-related pain. Pain. 160:38–44. 2019. View Article : Google Scholar
|
|
54
|
Luger NM, Mach DB, Sevcik MA and Mantyh
PW: Bone cancer pain: From model to mechanism to therapy. J Pain
Symptom Manage. 29(5 Suppl): S32–4S6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Currie GL, Delaney A, Bennett MI,
Dickenson AH, Egan KJ, Vesterinen HM, Sena ES, Macleod MR, Colvin
LA and Fallon MT: Animal models of bone cancer pain: systematic
review and meta-analyses. Pain. 54:917–926. 2013. View Article : Google Scholar
|
|
56
|
He XX: Study on the mechanism of
autophagy-NLRP3 inflammasome pathway in the relief of bone cancer
pain by electroacupuncture. China Three Gorges University; 2023, In
Chinese.
|
|
57
|
Fan SD: Role of NRG1/ErbB2 signal pathway
in electroacupunture treating cancer pain. Shanghai Univ Tradit
Chin Med; 2020, In Chinese.
|
|
58
|
Kopruszinski CM, Dos Reis RC, Gambeta E,
Acco A, Rae GA, King T and Chichorro JG: Blockade of endothelin
receptors reduces tumor-induced ongoing pain and evoked
hypersensitivity in a rat model of facial carcinoma induced pain.
Eur J Pharmacol. 818:132–140. 2018. View Article : Google Scholar
|
|
59
|
Kopruszinski CM, Dos Reis RC, Rae GA and
Chichorro JG: Blockade of peripheral endothelin receptors abolishes
heat hyperalgesia and spontaneous nociceptive behavior in a rat
model of facial cancer. Arch Oral Biol. 97:231–237. 2019.
View Article : Google Scholar
|
|
60
|
Gambeta E, Kopruszinski CM, Reis RC,
Zanoveli JM and Chichorro JG: Evaluation of heat hyperalgesia and
anxiety like-behaviors in a rat model of orofacial cancer. Neurosci
Lett. 619:100–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gambeta E, Kopruszinski CM, Reis RC,
Zanoveli JM and Chichorro JG: Facial pain and anxiety-like behavior
are reduced by pregabalin in a model of facial carcinoma in rats.
Neuropharmacology. 125:263–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo TT, Wumaier TA, Hu JJ and Yang LL:
Analysis of the prognosis of cutaneous melanoma and its influencing
factors. Oncol Prog. 22:141–145. 2024.In Chinese.
|
|
63
|
Olbrich K, Costard L, Moser CV, Syhr KM,
King-Himmelreich TS, Wolters MC, Schmidtko A, Geisslinger G and
Niederberger E: Cleavage of SNAP-25 ameliorates cancer pain in a
mouse model of melanoma. Eur J Pain. 21:101–111. 2017. View Article : Google Scholar
|
|
64
|
Wang H, Shao DH and Ma P: Analgesic effect
of gastrodin on metastasizing in cancer-induced pain mouse model. J
Jiangsu Univ (Med Ed). 25:195–198. 2015.In Chinese.
|
|
65
|
Xie R and Wang H: Effect of intrathecal
amiloride on the ASIC-3 expression and pain behavior in mice skin
cancer pain. Chin Clin Oncol. 22:588–591. 2017.In Chinese.
|
|
66
|
Tabata M, Murata E, Ueda K, Kato-Kogoe N,
Kuroda Y and Hirose M: Effects of TrkA inhibitory peptide on
cancer-induced pain in a mouse melanoma model. J Anesth.
26:545–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yan ZL, Xu XF, Xin JQ and Zhang H:
Comparison and selection of animal models of pancreatic cancer. J
Clin Hepatol. 38:2908–2912. 2022.In Chinese.
|
|
68
|
Mallya K, Gautam SK, Aithal A, Batra SK
and Jain M: Modeling pancreatic cancer in mice for experimental
therapeutics. Biochim Biophys Acta Rev Cancer. 1876:1885542021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu D and Yang F: Current state and future
perspectives of biological models in pancreatic cancer. Lab Anim
Sci. 40:76–82. 2023.In Chinese.
|
|
70
|
Hu RP, Shang LF, Wang HJ, Che HX, Wang ML,
Yang H, Jin YY, Zhang FF and Zhang JL: Mechanism of effect of
rosiglitazone on pancreatic cancer in diabetic mice based on impact
of PPARγ on glucose transport and metabolism. Chin Pharmacol Bull.
40:1325–1334. 2024.In Chinese.
|
|
71
|
Xu Y, Huang X, Tang Z, Li R and Qin W:
Establishment and in vivo imaging observation of a nude mouse model
of type 2 diabetes mellitus and pancreatic cancer. J Clin Hepatol.
40:1231–1239. 2024.In Chinese.
|
|
72
|
Schwei MJ, Honore P, Rogers SD,
Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR and Mantyh
PW: Neurochemical and cellular reorganization of the spinal cord in
a murine model of bone cancer pain. J Neurosci. 19:10886–10897.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang W, Jiang Q, Wu J, Tang W and Xu M:
Upregulation of bone morphogenetic protein 2 (Bmp2) in dorsal root
ganglion in a rat model of bone cancer pain. Mol Pain.
15:17448069188242502019. View Article : Google Scholar :
|
|
74
|
Huang HQ, Liu YP, Wu X and He XM:
Establishment of a rat model of skin cancer pain. J Zhengzhou Univ
(Med Sci). 54:236–240. 2019.In Chinese.
|
|
75
|
Wang L, Xu H, Ge Y, Zhu H, Yu D, Yu W and
Lu Z: Establishment of a murine pancreatic cancer pain model and
microarray analysis of pain associated genes in the spinal cord
dorsal horn. Mol Med Rep. 16:4429–4436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Grundy L, Erickson A and Brierley SM:
Visceral pain. Annu Rev Physiol. 81:261–284. 2019. View Article : Google Scholar
|
|
77
|
Ayoub R, Jarrar Q, Ali D, Moshawih S,
Jarrar Y, Hakim M and Zakaria Z: Synthesis of mefenamic acid with
pronounced anti-nociceptive effects and a proposed activity on
GABA, opioid and glutamate receptors. Eur J Pharm Sci.
163:1058652021. View Article : Google Scholar
|
|
78
|
Vasincu IM, Apotrosoaei M, Constantin S,
Butnaru M, Vereștiuc L, Lupușoru CE, Buron F, Routier S, Lupașcu D,
Taușer RG and Profire L: New ibuprofen derivatives with
thiazolidine-4-one scaffold with improved pharmaco-toxicological
profile. BMC Pharmacol Toxicol. 22:102021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu X, Zhang Q, Han M and Du J:
Intrapericardial capsaicin and bradykinin induce different
cardiac-somatic and cardiovascular reflexes in rats. Auton
Neurosci. 198:28–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
McDermott DA, Meller ST, Gebhart GF and
Gutterman DD: Use of an indwelling catheter for examining
cardiovascular responses to pericardial administration of
bradykinin in rat. Cardiovasc Res. 30:39–46. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cen Y, Liu C, Li X, Yan Z, Kuang M, Su Y,
Pan X, Qin R, Liu X, Zheng J and Zhou H: Artesunate ameliorates
severe acute pancreatitis (SAP) in rats by inhibiting expression of
pro-inflammatory cytokines and Toll-like receptor 4. Int
Immunopharmacol. 38:252–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yang JJ, Zhang D and Chen JY: Advances in
animal models of acute pancreatitis. Med J Chin PLA. 44:984–990.
2019.In Chinese.
|
|
83
|
Wang YT and Lv GW: A model of formalin
induced acute visceral inflammatory pain. Chin J Appl Physiol.
15:372–376. 1999.In Chinese.
|
|
84
|
Ness TJ and Gebhart GF: Characterization
of neuronal responses to noxious visceral and somatic stimuli in
the medial lumbosacral spinal cord of the rat. J Neurophysiol.
57:1867–1892. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wirtz S, Popp V, Kindermann M, Gerlach K,
Weigmann B, Fichtner-Feigl S and Neurath MF: Chemically induced
mouse models of acute and chronic intestinal inflammation. Nat
Protoc. 12:1295–1309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lu Y, Lin H, Zhang J, Wei J, Sun J and Han
L: Sijunzi Decoction attenuates 2, 4, 6-trinitrobenzene sulfonic
acid (TNBS)-induced colitis in rats and ameliorates TNBS-induced
claudin-2 damage via NF-κB pathway in Caco2 cells. BMC Complement
Altern Med. 17:352017. View Article : Google Scholar
|
|
87
|
Mazor Y, Engelmayer N, Nashashibi H,
Rottenfußer L, Lev S and Binshtok AM: Attenuation of
colitis-induced visceral hypersensitivity and pain by selective
silencing of TRPV1-expressing fibers in rat colon. Inflamm Bowel
Dis. 30:1843–1851. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Weng RX, Chen W, Tang JN, Sun Q, Li M, Xu
X, Zhang PA, Zhang Y, Hu CY and Xu GY: Targeting spinal TRAF6
expression attenuates chronic visceral pain in adult rats with
neonatal colonic inflammation. Mol Pain. 16:17448069209180592020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ryu CM, Yu HY, Lee HY, Shin JH, Lee S, Ju
H, Paulson B, Lee S, Kim S, Lim J, et al: Longitudinal intravital
imaging of transplanted mesenchymal stem cells elucidates their
functional integration and therapeutic potency in an animal model
of interstitial cystitis/bladder pain syndrome. Theranostics.
8:5610–5624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
El-Hamamsy D: Bladder wall injection of
mesenchymal stem cells ameliorates bladder inflammation,
overactivity and nociception in a chemically induced interstitial
cystitis-like rat model. Int Urogynecol J. 30:845–846. 2019.
View Article : Google Scholar
|
|
91
|
Song PH, Chun SY, Chung JW, Kim YY, Lee
HJ, Lee JN, Ha YS, Yoo ES, Kwon TG, Kim J, et al: Comparison of 5
different rat models to establish a standard animal model for
research into interstitial cystitis. Int Neurourol J. 21:163–170.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sakurai J, Obata K, Ozaki N, Tokunaga A,
Kobayashi K, Yamanaka H, Dai Y, Kondo T, Miyoshi K, Sugiura Y, et
al: Activation of extracellular signal-regulated protein kinase in
sensory neurons after noxious gastric distention and its
involvement in acute visceral pain in rats. Gastroenterology.
134:1094–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Julia V, Mezzasalma T and Buéno L:
Influence of bradykinin in gastrointestinal disorders and visceral
pain induced by acute or chronic inflammation in rats. Dig Dis Sci.
40:1913–1921. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
İlkaya F, Bilge SS, Bozkurt A, Baş DB,
Erdal A, Çiftçioğlu E and Kesim Y: The antinociceptive effect of
intravenous imipramine in colorectal distension-induced visceral
pain in rats: The role of serotonergic and noradrenergic receptors.
Pharmacol Biochem Behav. 122:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Al-Chaer ED, Kawasaki M and Pasricha PJ: A
new model of chronic visceral hypersensitivity in adult rats
induced by colon irritation during postnatal development.
Gastroenterology. 119:1276–1285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
An ZY, Tian JF, Zhao X, Zhang MD, Zhang
LJ, Yang XY, Liu LB, Chen LY and Song XT: PET evaluation of
myocardial perfusion function after percutaneous coronary
intervention in patients with chronic total occlusion: A systematic
review and meta-analysis. Scand Cardiovasc J. 58:23021742024.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang WW, Lei J and You HJ: Research
progress of experimental animal models of visceral pain. Prog
Biochem Biophys. 49:858–866. 2022.In Chinese.
|
|
98
|
Hu S, Xiao Y, Zhu L, Li L, Hu CY, Jiang X
and Xu GY: Neonatal maternal deprivation sensitizes voltage-gated
sodium channel currents in colon-specific dorsal root ganglion
neurons in rats. Am J Physiol Gastrointest Liver Physiol.
304:G311–G321. 2013. View Article : Google Scholar
|
|
99
|
Barreau F, Ferrier L, Fioramonti J and
Bueno L: New insights in the etiology and pathophysiology of
irritable bowel syndrome: Contribution of neonatal stress models.
Pediatr Res. 62:240–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bondar NP, Lepeshko AA and Reshetnikov VV:
Effects of early-life stress on social and anxiety-like behaviors
in adult mice: Sex-specific effects. Behav Neurol.
2018:15389312018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang C, Sun DF, Sun LJ, Wang YL, Hu LH, Ye
WC, Fang ZJ and Deng Q: The intervention of shrimp head enzymatic
hydrolysate on capsaicin-induced systemic low-grade inflammation
and the structure and function of gut microbiota in mice. Chin J
Microecol. 33:1–9. 2021.In Chinese.
|
|
102
|
Yao JP, Zhao Y, Chen Y, Chen LP, Feng XM,
Li Y and Zhou SY: Effect of electroacupuncture on intestinal
epithelial mucosal barrier function in rats with
diarrhea-predominant irritable bowel syndrome. Zhen Ci Yan Jiu.
45:357–362. 2020.In Chinese. PubMed/NCBI
|
|
103
|
Felice VD, Gibney SM, Gosselin RD, Dinan
TG, O'Mahony SM and Cryan JF: Differential activation of the
prefrontal cortex and amygdala following psychological stress and
colorectal distension in the maternally separated rat.
Neuroscience. 267:252–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wu J, Wang H, Wang JX, Luo YF, Yang Y and
Gong XX: Effects of Dajianzhong decoction on serum IL-6, TNF-α and
IRAK-4 mRNA expression in colon mucosa of chronic inflammatory
visceral pain model rats. J Tradit Chin Med. 59:1592–1596. 2018.In
Chinese.
|
|
105
|
Liu Q, Ko CY, Zheng C, Ye L, Liu B, Gao H,
Huang D and Chou D: Decreased glutamatergic synaptic strength in
the periaqueductal gray contributes to maintenance of visceral pain
in male rats with experimental pancreatitis. Neuroscience.
428:60–69. 2020. View Article : Google Scholar
|
|
106
|
Furuta A, Yamamoto T, Igarashi T, Suzuki
Y, Egawa S and Yoshimura N: Bladder wall injection of mesenchymal
stem cells ameliorates bladder inflammation, overactivity, and
nociception in a chemically induced interstitial cystitis-like rat
model. Int Urogynecol J. 29:1615–1622. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Deng W, Zou H, Qian L, de Souza SC, Chen Q
and Cao S: Stauntonia chinensis injection relieves neuropathic pain
by increasing the expression of PSD-95 and reducing the
proliferation of phagocytic microglia. Ibrain. 10:3–18. 2023.
View Article : Google Scholar
|
|
108
|
Kandhare AD, Raygude KS, Ghosh P, Ghule AE
and Bodhankar SL: Therapeutic role of curcumin in prevention of
biochemical and behavioral aberration induced by alcoholic
neuropathy in laboratory animals. Neurosci Lett. 511:18–22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu YR, Lian KX and Gu XL: Research
progress in mouse models of inflammatory diseases. Chin J Comp Med.
32:120–126. 2022.In Chinese.
|
|
110
|
Ghosh S, Wise LE, Chen Y, Gujjar R,
Mahadevan A, Cravatt BF and Lichtman AH: The monoacylglycerol
lipase inhibitor JZL184 suppresses inflammatory pain in the mouse
carrageenan model. Life Sci. 92:498–505. 2013. View Article : Google Scholar :
|
|
111
|
Wang WJ, Lu J, Huang YR, Niu CS, Ma Q, Hao
HW, Li LM, Wang JR and Tu Y: Influences of electroacupuncture on
content of hypothalamus ENK and spinal OFQ in rats with chronic
inflammatory pain. J Beijing Univ Tradit Chin Med. 33:196–199.
2010.
|
|
112
|
Melo-Carrillo A and Lopez-Avila A: A
chronic animal model of migraine, induced by repeated meningeal
nociception, characterized by a behavioral and pharmacological
approach. Cephalalgia. 33:1096–1105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Burgos-Vega CC, Quigley LD, Trevisan Dos
Santos G, Yan F, Asiedu M, Jacobs B, Motina M, Safdar N, Yousuf H,
Avona A, et al: Non-invasive dural stimulation in mice: A novel
preclinical model of migraine. Cephalalgia. 39:123–134. 2019.
View Article : Google Scholar :
|
|
114
|
Sufka KJ, Staszko SM, Johnson AP, Davis
ME, Davis RE and Smitherman TA: Clinically relevant behavioral
endpoints in a recurrent nitroglycerin migraine model in rats. J
Headache Pain. 17:402016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Philpott HT and McDougall JJ: Combatting
joint pain and inflammation by dual inhibition of monoacylglycerol
lipase and cyclooxygenase-2 in a rat model of osteoarthritis.
Arthritis Res Ther. 22:92020. View Article : Google Scholar :
|
|
116
|
Shi X, Yu W, Wang T, Battulga O, Wang C,
Shu Q, Yang X, Liu C and Guo C: Electroacupuncture alleviates
cartilage degradation: Improvement in cartilage biomechanics via
pain relief and potentiation of muscle function in a rabbit model
of knee osteoarthritis. Biomed Pharmacother. 123:1097242020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Katri A, Dąbrowska A, Löfvall H, Ding M,
Karsdal MA, Andreassen KV, Thudium CS and Henriksen K: Combining
naproxen and a dual amylin and calcitonin receptor agonist improves
pain and structural outcomes in the collagen-induced arthritis rat
model. Arthritis Res Ther. 21:682019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
He JY, Qin SY, Zheng JQ, Luo S, Xiong ZY
and Yu QQ: Therapeutic effect of total triterpenoids of Chaenomeles
speciosa combined with indomethacin on rheumatoid arthritis induced
by Freund's complete adjuvant in rats. Pharmacol Clin Chin Mater
Med. 40:42–50. 2024.In Chinese.
|
|
119
|
Bai Q, Li HL, Yang JJ, Cheng WG, Lv CH,
Bai SS, Wang ZD, Jin FM and Wang HD: Effect of Yiqi Juanbi formula
on synovial inflammation and the TLR4/MAPKs/NF-κB signaling pathway
in collagen-induced arthritis rats. Chin Tradit Patent Med.
47:590–595. 2025.In Chinese.
|
|
120
|
Koo ST, Park YI, Lim KS, Chung K and Chung
JM: Acupuncture analgesia in a new rat model of ankle sprain pain.
Pain. 99:423–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wu D, Nan LT, Li SS, Yao TY and Yu SQ:
AMPK-mediated analgesic mechanism in rats with carrageenan-induced
inflammatory pain. J Youjiang Med Univ Nationalities. 45:435–438.
2023.In Chinese.
|
|
122
|
Deuis JR, Dvorakova LS and Vetter I:
Methods used to evaluate pain behaviors in rodents. Front Mol
Neurosci. 10:2482017. View Article : Google Scholar
|
|
123
|
Mizoguchi H, Fukumoto K, Sakamoto G, Jin
S, Toyama A, Wang T, Suzumura A and Sato J: Maternal separation as
a risk factor for aggravation of neuropathic pain in later life in
mice. Behav Brain Res. 359:942–949. 2019. View Article : Google Scholar
|
|
124
|
Muralidharan A, Sotocinal SG, Austin JS
and Mogil JS: The influence of aging and duration of nerve injury
on the antiallodynic efficacy of analgesics in laboratory mice.
Pain Rep. 5:e8242020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Schwartz ES and Gebhart GF: Visceral pain.
Curr Top Behav Neurosci. 20:171–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Peng H and Huang D: Advances in pain
assessment methods in rodent models. Chin J Pain Med. 20:505–508.
2014.In Chinese.
|
|
127
|
Su S, Shao J, Zhao Q, Ren X, Cai W, Li L,
Bai Q, Chen X, Xu B, Wang J, et al: MiR-30b attenuates neuropathic
pain by regulating voltage-gated sodium channel Nav1.3 in rats.
Front Mol Neurosci. 10:1262017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gomez K, Stratton HJ, Duran P, Loya S,
Tang C, Calderon-Rivera A, François-Moutal L, Khanna M, Madura CL,
Luo S, et al: Identification and targeting of a unique NaV1.7
domain driving chronic pain. Proc Natl Acad Sci USA.
120:e22178001202023. View Article : Google Scholar :
|
|
129
|
Shen T and Wang DM: Sodium channel NaV1. 7
and neuropathic pain. Chin J Biochem Mol Biol. 38:725–735. 2022.In
Chinese.
|
|
130
|
Zhou X, Ma T, Yang L, Peng S, Li L, Wang
Z, Xiao Z, Zhang Q, Wang L, Huang Y, et al: Spider venom-derived
peptide induces hyperalgesia in Nav1.7 knockout mice by activating
Nav1.9 channels. Nat Commun. 11:22932020. View Article : Google Scholar :
|
|
131
|
Strickland IT, Martindale JC, Woodhams PL,
Reeve AJ, Chessell IP and McQueen DS: Changes in the expression of
NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root
ganglia innervating the rat knee joint in a model of chronic
inflammatory joint pain. Eur J Pain. 12:564–572. 2008. View Article : Google Scholar
|
|
132
|
Kingwell K: Nav1.7 withholds its pain
potential. Nat Rev Drug Discov. Apr 8–2019.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cardoso FC, Castro J, Grundy L, Schober G,
Garcia-Caraballo S, Zhao T, Herzig V, King GF, Brierley SM and
Lewis RJ: A spider-venom peptide with multitarget activity on
sodium and calcium channels alleviates chronic visceral pain in a
model of irritable bowel syndrome. Pain. 162:569–581. 2021.
View Article : Google Scholar
|
|
134
|
Wolkerstorfer A, Handler N and Buschmann
H: New approaches to treating pain. Bioorg Med Chem Lett.
26:1103–1119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang HR, Hu SW, Zhang S, Song Y, Wang XY,
Wang L, Li YY, Yu YM, Liu H, Liu D, et al: KCNQ Channels in the
mesolimbic reward circuit regulate nociception in chronic pain in
mice. Neurosci Bull. 37:597–610. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Nestler EJ and Waxman SG: Resilience to
stress and resilience to pain: Lessons from molecular neurobiology
and genetics. Trends Mol Med. 26:924–935. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Han Y, Zhai XJ, Chen DD, Zhou Y, Zhou DY,
Zhang WX, Ji R, Li QZ, Gao YH, Cao JL and Zhang H: Recent advances
in the neurobiology of susceptibility and resilience to pain. Chin
J Pain Med. 28:571–581. 2022.In Chinese.
|
|
138
|
Li T, Wu K, Yue Z, Wang Y, Zhang F and
Shen H: Structural basis for the modulation of human KCNQ4 by
small-molecule drugs. Mol Cell. 81:25–37.e4. 2021. View Article : Google Scholar
|
|
139
|
Tsantoulas C and McMahon SB: Opening paths
to novel analgesics: The role of potassium channels in chronic
pain. Trends Neurosci. 37:146–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Alloui A, Zimmermann K, Mamet J, Duprat F,
Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, et
al: TREK-1, a K+ channel involved in polymodal pain perception.
EMBO J. 25:2368–2376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhou RP and Chen FH: Research progress on
role of acid-sensing ion channels in rheumatoid arthritis. Chin
Pharmacol Bull. 31:315–318. 2015.In Chinese.
|
|
142
|
Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu
L, Chen J and Xu TL: Upregulation of acid-sensing ion channel
ASIC1a in spinal dorsal horn neurons contributes to inflammatory
pain hypersensitivity. J Neurosci. 27:11139–11148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ma MJ: Study on the analgesic and
antipruritic mechanisms of saponins from Stauntonia chinensis and
its active components by modulating acid-sensing ion channels.
South-Central Univ Nationalities; 2021, In Chinese.
|
|
144
|
Xiong Z, Xie JX, Zhu KB, Zhang YT and Yang
R: Anti-nociceptive effects of paeoniflorin in formalin-induced
pain by regulating acid-sensing ion channels. Herald Med.
38:1403–1407. 2019.In Chinese.
|
|
145
|
Su SS, Liu J, Lin S and Wang DL: Research
progress on the application of ketamine combined with
dexmedetomidine. Med Innov China. 22:184–188. 2025.In Chinese.
|
|
146
|
Nirogi R, Goura V, Abraham R and Jayarajan
P: α4β2* neuronal nicotinic receptor ligands (agonist, partial
agonist and positive allosteric modulators) as therapeutic
prospects for pain. Eur J Pharmacol. 712:22–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Li L, Wu Y, Bai Z, Hu Y and Li W: Blockade
of NMDA receptors decreased spinal microglia activation in bee
venom induced acute inflammatory pain in rats. Neurol Res.
39:271–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Franceschini A and Adinolfi E: P2X
receptors: New players in cancer pain. World J Biol Chem.
5:4292014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Souza Monteiro de Araujo D, Nassini R,
Geppetti P and De Logu F: TRPA1 as a therapeutic target for
nociceptive pain. Expert Opin Ther Targets. 24:997–1008. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wei J, Su W, Zhao Y, Wei Z, Hua Y, Xue P,
Zhu X, Chen Y and Chen G: Maresin 1 promotes nerve regeneration and
alleviates neuropathic pain after nerve injury. J
Neuroinflammation. 19:322022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mahmoud O, Soares GB and Yosipovitch G:
Transient receptor potential channels and itch. Int J Mol Sci.
24:4202022. View Article : Google Scholar
|
|
152
|
Iftinca M, Defaye M and Altier C:
TRPV1-targeted drugs in development for human pain conditions.
Drugs. 81:7–27. 2021. View Article : Google Scholar
|
|
153
|
Zhang Y, Gao ZB, Xin XM and Zheng YM:
Research progress on the pharmacological activity of lappaconitine.
Chin Bull Life Sci. 33:1089–1095. 2021.In Chinese.
|
|
154
|
Cai S, Gomez K, Moutal A and Khanna R:
Targeting T-type/CaV3.2 channels for chronic pain. Transl Res.
234:20–30. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yi SP, Chen ZM, Zhang JH and Zhang KJ:
Research progress of interactions among different opioid receptor
subtypes. Chin Pharmacol Bull. 28:1493–1496. 2012.In Chinese.
|
|
156
|
Stein C: Opioids, sensory systems and
chronic pain. Eur J Pharmacol. 716:179–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wang D, Chen T, Zhou X, Couture R and Hong
Y: Activation of Mas oncogene-related gene (Mrg) C receptors
enhances morphine-induced analgesia through modulation of coupling
of μ-opioid receptor to Gi-protein in rat spinal dorsal horn.
Neuroscience. 253:455–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Montana MC and Gereau RW: Metabotropic
glutamate receptors as targets for analgesia: Antagonism,
activation, and allosteric modulation. Curr Pharm Biotechnol.
12:1681–1688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Sawynok J: Adenosine receptor targets for
pain. Neuroscience. 338:1–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Chiou LC, Hu SS and Ho YC: Targeting the
cannabinoid system for pain relief? Acta Anaesthesiol Taiwan.
51:161–170. 2013. View Article : Google Scholar
|
|
161
|
Gong N, Fan H, Ma AN, Xiao Q and Wang YX:
Geniposide and its iridoid analogs exhibit antinociception by
acting at the spinal GLP-1 receptors. Neuropharmacology. 84:31–45.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li JX, Zhang Y and Winter JC:
Morphine-induced antinociception in the rat: Supra-additive
interactions with-additive interactions with imidazoline
I2 receptor ligands. Eur J Pharmacol. 669:59–65. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Thorn DA, Siemian JN, Zhang Y and Li JX:
Anti-hyperalgesic effects of imidazoline I2 receptor ligands in a
rat model of inflammatory pain: Interactions with oxycodone.
Psychopharmacology (Berl). 232:3309–3318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Falcicchia C, Tozzi F, Arancio O,
Watterson DM and Origlia N: Involvement of p38 MAPK in synaptic
function and dysfunction. Int J Mol Sci. 21:56242020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Melamed Kadosh D, Beenstock J, Engelberg D
and Admon A: Differential modulation of the phosphoproteome by the
MAP kinases isoforms p38α and p38β. Int J Mol Sci. 24:124422023.
View Article : Google Scholar
|
|
166
|
Dong N, Li X, Xue C, Zhang L, Wang C, Xu X
and Shan A: Astragalus polysaccharides alleviates LPS-induced
inflammation via the NF-κB/MAPK signaling pathway. J Cell Physiol.
235:5525–5540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Liu BP, Zhang YP, Yang ZY, Liu MJ, Zhang
C, Zhao YT and Cai S: ω-3 DPA protected neurons from
neuroinflammation by balancing microglia M1/M2 polarizations
through inhibiting NF-κB/MAPK p38 signaling and activating
neuron-BDNF-PI3K/AKT pathways. Mar Drugs. 19:5872021. View Article : Google Scholar
|
|
168
|
Li FS and Weng JK: Demystifying
traditional herbal medicine with modern approach. Nat Plants.
3:171092017. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Huang Y, Zhang D, Li ZY, Yang YT, Wu LJ,
Zhang J, Zhi FY, Li XY, Shi Z, Hong J and Ma XP: Moxibustion eases
chronic inflammatory visceral pain in rats via MAPK signaling
pathway in the spinal cord. J Pain Res. 12:2999–3012. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Jimi E, Huang F and Nakatomi C: NF-κB
signaling regulates physiological and pathological chondrogenesis.
Int J Mol Sci. 20:62752019. View Article : Google Scholar
|
|
171
|
Liang W, Zhang T, Zhang M, Gao J, Huang R,
Huang X, Chen J, Cheng L, Zhang L, Huang Z, et al: Daphnetin
ameliorates neuropathic pain via regulation of microglial responses
and glycerophospholipid metabolism in the spinal cord.
Pharmaceuticals (Basel). 17:7892024. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Lu ZH, Xiong XY, Lin GC, Meng JR and Mei
QB: Change of COX-1/2 expression in brain after spared nerve
injury-induced neuropathic pain and analgesic effects of COX
inhibitors with different selectivity. Chin J Neuroanat. 22:27–32.
2006.In Chinese.
|
|
173
|
Zhou XL, Wang Y, Zhang CJ, Yu LN, Cao JL
and Yan M: COX-2 is required for the modulation of spinal
nociceptive information related to ephrinB/EphB signalling. Eur J
Pain. 19:1277–1287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Chandrasekharan NV, Dai H, Roos KL,
Evanson NK, Tomsik J, Elton TS and Simmons DL: COX-3, a
cyclooxygenase-1 variant inhibited by acetaminophen and other
analgesic/antipyretic drugs: Cloning, structure, and expression.
Proc Natl Acad Sci USA. 99:13926–13931. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kuboyama K, Tsuda M, Tsutsui M, Toyohara
Y, Tozaki-Saitoh H, Shimokawa H, Yanagihara N and Inoue K: Reduced
spinal microglial activation and neuropathic pain after nerve
injury in mice lacking all three nitric oxide synthases. Mol Pain.
7:502011. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wu Y, Yang Y, Wang L, Chen Y, Han X, Sun
L, Chen H and Chen Q: Effect of bifidobacterium on osteoclasts:
TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front
Endocrinol (Lausanne). 14:11092962023. View Article : Google Scholar
|
|
177
|
Verma S, Dutta A, Dahiya A and Kalra N:
Quercetin-3-Rutinoside alleviates radiation-induced lung
inflammation and fibrosis via regulation of NF-κB/TGF-β1 signaling.
Phytomedicine. 99:1540042022. View Article : Google Scholar
|
|
178
|
Sun Y and Wang HX: Astragaloside IV
alleviates inflammatory response in pulmonary hypertension rats
through the NF-KB/NLRP3 signaling pathway. Chin Tradit Patent Med.
45:578–582. 2023.In Chinese.
|
|
179
|
Sun YL, Zhao JY, Qin YS and Ren GH:
Research progress on Traditional Chinese Medicine for migraine
treatment based on signaling pathways. J Pract Tradit Chin Intern
Med. 38:88–91. 2024.In Chinese.
|
|
180
|
Wang D, Chen B, Tang Y, Liu HQ, Li J and
Yang SL: Acupuncture and moxibustion regulation of NF - κB
signaling pathway in the treatment of rheumatoid arthritis. Chin J
Ethnomed Ethnopharm. 33:68–71. 2024.In Chinese.
|
|
181
|
Chen QZ, Huang YJ and Gu CM: Dezocine
alleviates remifentanil-induced hyperalgesia via targeting the
Toll-like receptor 4/nuclear factor kappa-B pathway. Hebei Med J.
46:181–185. 1912024.In Chinese.
|
|
182
|
Cao J, Liu H, An Q and Han F: Metformin
alleviates pathologic pain in mice with radiation dermatitis by
inhibiting p38MAPK/NF-κB signaling pathway. Nan Fang Yi Ke Da Xue
Xue Bao. 43:1815–1820. 2023.PubMed/NCBI
|
|
183
|
Benke D: GABA(B) receptors and pain. Curr
Top Behav Neurosci. 52:213–239. 2022. View Article : Google Scholar
|
|
184
|
Zeilhofer HU, Mohler H and Di Lio A:
GABAergic analgesia: New insights from mutant mice and
subtype-selective agonists. Trends Pharmacol Sci. 30:397–402. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhu Q, Sun Y, Zhu J, Fang T, Zhang W and
Li JX: Antinociceptive effects of sinomenine in a rat model of
neuropathic pain. Sci Rep. 4:72702014. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Bowery NG: GABAB receptor: A site of
therapeutic benefit. Curr Opin Pharmacol. 6:37–43. 2006. View Article : Google Scholar
|
|
187
|
Zeng XY, Zhang Q, Wang J, Yu J, Han SP and
Wang JY: Distinct role of tumor necrosis factor receptor subtypes 1
and 2 in the red nucleus in the development of neuropathic pain.
Neurosci Lett. 569:43–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Vogel C, Stallforth S and Sommer C:
Altered pain behavior and regeneration after nerve injury in TNF
receptor deficient mice. J Peripher Nerv Syst. 11:294–303. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wang Y, Zhuang Y, DiBerto JF, Zhou XE,
Schmitz GP, Yuan Q, Jain MK, Liu W, Melcher K, Jiang Y, et al:
Structures of the entire human opioid receptor family. Cell.
186:413–427.e17. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Siuda ER, Carr R III, Rominger DH and
Violin JD: Biased mu-opioid receptor ligands: A promising new
generation of pain therapeutics. Curr Opin Pharmacol. 32:77–84.
2017. View Article : Google Scholar
|
|
191
|
Obeng S, Hiranita T, Leon F, McMahon LR
and McCurdy CR: Novel approaches, drug candidates, and targets in
pain drug discovery. J Med Chem. 64:6523–6548. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Abrimian A, Kraft T and Pan YX: Endogenous
opioid peptides and alternatively spliced mu opioid receptor seven
transmembrane carboxyl-terminal variants. Int J Mol Sci.
22:37792021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Paul AK, Smith CM, Rahmatullah M,
Nissapatorn V, Wilairatana P, Spetea M, Gueven N and Dietis N:
Opioid analgesia and opioid-induced adverse effects: A review.
Pharmaceuticals (Basel). 14:10912021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Baldo BA and Rose MA: Mechanisms of
opioid-induced respiratory depression. Arch Toxicol. 96:2247–2260.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Dang VC and Christie MJ: Mechanisms of
rapid opioid receptor desensitization, resensitization and
tolerance in brain neurons. Br J Pharmacol. 165:1704–1716. 2012.
View Article : Google Scholar :
|
|
196
|
Faouzi A, Varga BR and Majumdar S: Biased
opioid ligands. Molecules. 25:42572022. View Article : Google Scholar
|
|
197
|
Bagley EE and Ingram SL: Endogenous opioid
peptides in the descending pain modulatory circuit.
Neuropharmacology. 173:1081312020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Kliewer A, Gillis A, Hill R, Schmiedel F,
Bailey C, Kelly E, Henderson G, Christie MJ and Schulz S:
Morphine-induced respiratory depression is independent of
beta-arrestin2 signalling. Br J Pharmacol. 177:2923–2931. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Fujita W: The possible role of MOPr-DOPr
heteromers and its regulatory protein RTP4 at sensory neurons in
relation to pain perception. Front Cell Neurosci. 14:6093622020.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Schröder W, Lambert DG, Ko MC and Koch T:
Functional plasticity of the N/OFQ-NOP receptor system determines
analgesic properties of NOP receptor agonists. Br J Pharmacol.
171:3777–3800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Williams JT, Ingram SL, Henderson G,
Chavkin C, Von Zastrow M, Schulz S, Koch T, Evans CJ and Christie
MJ: Regulation of μ-opioid receptors: desensitization,
phosphorylation, internalization, and tolerance. Pharmacol Rev.
65:223–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Violin JD, Crombie AL, Soergel DG and Lark
MW: Biased ligands at G-protein-coupled receptors: promise and
progress. Trends Pharmacol Sci. 35:308–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Soergel DG, Subach RA, Burnham N, Lark MW,
James IE, Sadler BM, Skobieranda F, Violin JD and Webster LR:
Biased agonism of the μ-opioid receptor by TRV130 increases
analgesia and reduces on-target adverse effects versus morphine: A
randomized, double-blind, placebo-controlled, crossover study in
healthy volunteers. Pain. 155:1829–1835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Manglik A, Lin H, Aryal DK, McCorvy JD,
Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, et al:
Structure-based discovery of opioid analgesics with reduced side
effects. Nature. 537:185–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Botting R and Ayoub SS: COX-3 and the
mechanism of action of paracetamol/acetaminophen. Prostaglandins
Leukot Essent Fatty Acids. 72:85–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Kautio AL, Haanpää M, Kautiainen H, Kalso
E and Saarto T: Burden of chemotherapy-induced neuropathy-a
cross-sectional study. Support Care Cancer. 19:1991–1996. 2011.
|
|
207
|
Van Poznak CH, Temin S, Yee GC, Janjan NA,
Barlow WE, Biermann JS, Bosserman LD, Geoghegan C, Hillner BE,
Theriault RL, et al: American society of clinical oncology
executive summary of the clinical practice guideline update on the
role of bone-modifying agents in metastatic breast cancer. J Clin
Oncol. 29:1221–1227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Findlay DM and Haynes DR: Mechanisms of
bone loss in rheumatoid arthritis. Mod Rheumatol. 15:232–240. 2005.
View Article : Google Scholar
|
|
209
|
Jones DH, Nakashima T, Sanchez OH,
Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R,
Hojilla CV, et al: Regulation of cancer cell migration and bone
metastasis by RANKL. Nature. 440:692–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Lipton A, Fizazi K, Stopeck AT, Henry DH,
Brown JE, Yardley DR, Richardson GE, Siena S, Maroto P, Clemens M,
et al: Superiority of denosumab to zoledronic acid for prevention
of skeletal-related events: A combined analysis of 3 pivotal,
randomised, phase 3 trials. Eur J Cancer. 48:3082–3092. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Committee of Cancer Overall Evaluation,
China Anti-Cancer Association: Expert consensus on integrated
prevention and treatment of opioid-related adverse drug reactions
(2024 edition). Chin J Clin Oncol. 51:757–763. 2024.In Chinese.
|
|
212
|
Suh SY, Choi YS, Oh SC, Kim YS, Cho K, Bae
WK, Lee JH, Seo AR and Ahn HY: Caffeine as an adjuvant therapy to
opioids in cancer pain: A randomized, double-blind,
placebo-controlled trial. J Pain Symptom Manage. 46:474–482. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Zhang JM and An J: Cytokines,
inflammation, and pain. Int Anesthesiol Clin. 45:27–37. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Salerno A and Hermann R: Efficacy and
safety of steroid use for postoperative pain relief. Update and
review of the medical literature. J Bone Joint Surg Am.
88:1361–1372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Ryan R, Booth S and Price S:
Corticosteroid-use in primary and secondary brain tumour patients:
A review. J Neurooncol. 106:449–459. 2012. View Article : Google Scholar
|
|
216
|
Paulsen Ø, Klepstad P, Rosland JH, Aass N,
Albert E, Fayers P and Kaasa S: Efficacy of methylprednisolone on
pain, fatigue, and appetite loss in patients with advanced cancer
using opioids: A randomized, placebo-controlled, double-blind
trial. J Clin Oncol. 32:3221–3228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Mihaljević S, Pavlović M, Reiner K and
Ćaćić M: Therapeutic mechanisms of ketamine. Psychiatr Danub.
32:325–333. 2020. View Article : Google Scholar
|
|
218
|
Chang M, Hu D, Chen FQ, Shi F, Yu Y, Wang
X, Fu SO and Xie P: Efficacy and immunomodulatory effects of
low-dose ketamine as an adjunct to morphine in patient-controlled
analgesia for advanced cancer pain. Chin J Pain Med. 21:152–155.
2015.In Chinese.
|
|
219
|
Morita K, Kitayama T, Morioka N and Dohi
T: Glycinergic mediation of tactile allodynia induced by
platelet-activating factor (PAF) through glutamate-NO-cyclic GMP
signalling in spinal cord in mice. Pain. 138:525–536. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Hasegawa S, Kohro Y, Shiratori M, Ishii S,
Shimizu T, Tsuda M and Inoue K: Role of PAF receptor in
proinflammatory cytokine expression in the dorsal root ganglion and
tactile allodynia in a rodent model of neuropathic pain. PLoS One.
5:e104672010. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Morita K, Shiraishi S, Motoyama N,
Kitayama T, Kanematsu T, Uezono Y and Dohi T: Palliation of bone
cancer pain by antagonists of platelet-activating factor receptors.
PLoS One. 9:e917462014. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
García de Paredes ML, Del Moral González
F, Martínez Del Prado P, Martí Ciriquián JL, Enrech Francés S, Cobo
Dols M, Esteban González E, Ortega Granados AL, Majem Tarruella M,
Cumplido Burón JD, et al: First evidence of oncologic neuropathic
pain prevalence after screening 8615 cancer patients. Results of
the On study. Ann Oncol. 22:924–930. 2011. View Article : Google Scholar
|
|
223
|
Fleming JA and O'Connor BD: Use of
lidocaine patches for neuropathic pain in a comprehensive cancer
centre. Pain Res Manag. 14:381–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Portenoy RK, Ganae-Motan ED, Allende S,
Yanagihara R, Shaiova L, Weinstein S, McQuade R, Wright S and
Fallon MT: Nabiximols for opioid-treated cancer patients with
poorly-controlled chronic pain: A randomized, placebo-controlled,
graded-dose trial. J Pain. 13:438–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Colloca L, Ludman T, Bouhassira D, Baron
R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N,
Finnerup NB, et al: Neuropathic pain. Nat Rev Dis Primers.
3:170022017. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Ye H, Song GP, Wang SJ, Shen XG, Qin LY
and Long SL: Clinical efficacy of radiofrequency ablation in the
treatment of lumbar spine nerve posterior branch entrapment
syndrome. Guangdong Med J. 46:228–232. 2025.In Chinese.
|
|
227
|
Li JZ, Yan WP, Qiang TL, Liu JP, Li JK and
Mao JW: Advances in research on intravertebral basivertebral nerve
radiofrequency ablation for treatment of chronic low back pain due
to Modic changes. J Tradit Chin Orthop Traumatol. 36:55–58.
622024.In Chinese.
|
|
228
|
Han S, Xiao B, Hou SY and Sun JJ: Effect
of DSA-based radiofrequency ablation and TACE interventional
surgery on the short-term efficacy and complications of
hepatocellular carcinoma. Pract J Cancer. 40:147–150. 1552025.In
Chinese.
|
|
229
|
Abd-Elsayed A, Nguyen S and Fiala K:
Radiofrequency ablation for treating headache. Curr Pain Headache
Rep. 23:182019. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Horsch S and Claeys L: Epidural spinal
cord stimulation in the treatment of severe peripheral arterial
occlusive disease. Ann Vasc Surg. 8:468–474. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Yin J, Sun SS, Cai M, Liu HJ and Jin Y:
Analgesic mechanism study of neuropathic pain relieved by early
spinal cord stimulation in rats with spinal cord injury. Chin J
Pain Med. 30:494–500. 2024.In Chinese.
|
|
232
|
Sivaramakrishnan A, Solomon JM and
Manikandan N: Comparison of transcutaneous electrical nerve
stimulation (TENS) and functional electrical stimulation (FES) for
spasticity in spinal cord injury - a pilot randomized cross-over
trial. J Spinal Cord Med. 41:397–406. 2018. View Article : Google Scholar :
|
|
233
|
Li T and Chen J: Spinal cord stimulation
for functional restoration in spinal cord injury: A narrative
review. Cureus. 17:e786102025.PubMed/NCBI
|
|
234
|
Zong YF, Dong TY and Bao M: Progress on
spinal cord stimulation in treatment of peripheral neuropathy. Chin
J Contemp Neurol Neurosurg. 25:72–77. 2025.In Chinese.
|
|
235
|
Qi DB and Zhao SB: Treatment of lumbar
disc herniation complicated with cauda equina symptoms by
percutaneous transforaminal endoscopic discectomy. J Clin Orthop.
28:34–37. 2025.In Chinese.
|
|
236
|
He Y, Li ZL, Jia T, Ren DW and Xi TP: The
effect of percutaneous transforaminal endoscopic discectomy in the
treatment of lumbar disc herniation. J Clin Orthop. 27:780–784.
2024.In Chinese.
|
|
237
|
Zhang B, Zhu HH, Dong H, Wang K, Xu LZ, Li
YW and Mei W: Clinical efficacy of percutaneous endoscopic
transforaminal technique for recurrent lumbar disc herniation. Chin
J Min Inv Surg. 23:813–817. 2023.In Chinese.
|
|
238
|
Ji T, Chen SL, Li N, Cao M and Cui W:
Effect of percutaneous transforaminal endoscopic discectomy on pain
and serum biomarkers (CPK/Myoglobin) in patients with lumbar disc
herniation: A mechanistic and clinical correlation study. J
Cervicodynia Lumbodynia. 44:827–830. 2023.In Chinese.
|
|
239
|
Miscov R, Gulisano HA and Bjarkam CR:
Treatment of patients with chronic malignant pain with intrathecal
morphine. Ugeskr Laeger. 186:082305412024.In Danish.
|
|
240
|
Rosen SM, Bromberg TA, Padda G, Barsa J,
Dunbar E, Dwarakanath G, Navalgund Y, Jaffe T, Yearwood TL, Creamer
M and Deer T: Intrathecal administration of Infumorph® vs
compounded morphine for treatment of intractable pain using the
Prometra® programmable pump. Pain Med. 14:865–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Sun C, Wang YT, Dai YJ, Liu ZH, Yang J,
Cheng ZQ, Dong DS, Wang CF, Zhao GL, Lu GJ, et al: Programmable
pump for intrathecal morphine delivery to cisterna magna: clinical
implications in novel management of refractory pain above middle
thoracic vertebrae level utilizing a prospective trial protocol and
review. Anesth Pain Med. 11:e1158732021. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
De Andrés J, Rubio-Haro R, De
Andres-Serrano C, Asensio-Samper JM and Fabregat-Cid G: Intrathecal
drug delivery. Methods Mol Biol. 2059:75–108. 2020. View Article : Google Scholar
|
|
243
|
The polyanalgesic consensus conference
(PACC): Recommendations on intrathecal drug infusion systems best
practices and guidelines. Neuromodulation. 20:405–406. 2017.
View Article : Google Scholar
|
|
244
|
Penn RD: Intrathecal medication delivery.
Neurosurg Clin N Am. 14:381–387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Zhang HF, Guo XQ and Wang QJ: Qianghuo
Shengshi decoction combined with radiofrequency ablation for
cervical radiculopathy with wind-cold obstruction syndrome: A
randomized controlled trial on pain relief and functional recovery.
TCM Res. 38:42–46. 2025.In Chinese.
|
|
246
|
Wang J and Wang YT: Efficacy of celecoxib
combined with radiofrequency ablation of posterior branch of
cervical spinal nerve in treatment for cervicogenic headache and
its effect on cervical hemodynamics, pain and sleep quality. China
Med Eng. 33:72–76. 2025.In Chinese.
|
|
247
|
Zhang SC: Clinical observation of
percutaneous radiofrequency ablation of hepatocellular carcinoma
combined with Traditional Chinese Medicine enema. Guide China Med.
23:154–156. 2025.In Chinese.
|
|
248
|
Zhou XQ, Jiang CY, Fang JL and Li G:
Effect of nerve block combined with spinal cord electrical
stimulation on sleep quality and pain degree of patients with
postherpetic neuralgia. J Clin Exp Med. 23:659–662. 2024.In
Chinese.
|
|
249
|
Han J: Effect of temporary spinal cord
stimulation combined with ozone injection on mechanical pain
thresholds and inflammatory factors in patients with neuropathic
pain. Pract Clin J Integr Tradit Chin West Med. 23:5–8. 2023.In
Chinese.
|
|
250
|
Wang Z, Jia BQ, Zhang XG, Li YZ, Wang SF
and Yao Y: The therapeutic effect of red light irradiation plus
oral TCM medicine and TESSYS on lumbar disc herniation. Clin J Chin
Med. 14:110–113. 2022.In Chinese.
|
|
251
|
Peng LP, Liu LH, Gu ZC, Jiang DF, Yin ZY
and Li ZY: Clinical efficacy of Qizhu Yin combined with
percutaneous transforaminal endoscopic discectomy in the treatment
of lumbar disc herniation. North Pharm. 19:90–92. 2022.In
Chinese.
|
|
252
|
Liu HP and Dong XH: Clinical effect of
percutaneous foraminoscopy combined with Tenghuang Jiangu Capsule
in the treatment of lumbar intervertebral disc herniation patients.
Lab Med Clin. 18:2981–2983. 2021.In Chinese.
|
|
253
|
Sun LH, Tang Q, Gu GL, Fan XS and Yuan M:
Therapeutic effect of intrathecal drug delivery system combined
with pain management on middle- and late-stage homebound severe
cancer pain patients. J Guizhou Med Univ. 49:1353–1359. 2024.In
Chinese.
|
|
254
|
Qiao YY, Liu B and Lu Y: Clinical efficacy
of conventional intravenous and intrathecal drug therapies in acute
neuromyelitis optica spectrum disorders. Chin Gen Pract.
23:1513–1516. 15222020.In Chinese.
|
|
255
|
Li JJ, Zhao Y, Gu CM and Liu J: Research
progress of intrathecal analgesia. China Contin Med Educ. 8:60–61.
2016.In Chinese.
|
|
256
|
Zhu L and Zhao XT: The effect of
percutaneous nerve electrical stimulation combined with isokinetic
centrifugal training on pain severity and muscle strength in
patients with knee osteoarthritis. Proceed Clin Med. 34:109–112.
2025.In Chinese.
|
|
257
|
Ren HX, Xin ZJ, Ji WS, Wang N and Meng XZ:
Clinical efficacy of transcutaneous auricular vagus nerve
stimulation in treatment of lumbar disc herniation. Med J Chin PAP.
35:740–744. 2024.In Chinese.
|
|
258
|
Gu YY, Xu FP, Li ZZ, Zhang GY and Gu CY:
Meta-analysis on transcutaneous electrical nerve stimulation (TENS)
for pain relief among women undergoing vaginal trial of labor. Chin
J Women Child Health. 15:63–72. 2024.In Chinese.
|
|
259
|
Feng YH, Feng YL and Zheng CS: Sanyinjiao
(SP6) moxibustion combined with transcutaneous electrical nerve
stimulation in the treatment of primary dysmenorrhea. Chin Med Mod
Distance Educ China. 22:124–126. 2024.In Chinese.
|
|
260
|
Nie XM, Jin F and Shi JL: Efficacy of
transcutaneous spinal cord stimulation in alleviating
pregnancy-related postpartum low back pain: A prospective clinical
study. Med Forum. 28(4-6): 212024.In Chinese.
|
|
261
|
Liu GH, Cai YJ, Wei MX, Guan LP, Zhao XJ
and Liu XM: Efficacy of self-developed Yanwu formula combined with
ultrasound-mediated drug delivery in treating chronic pelvic pain
with dampness-heat and blood stasis syndrome: a randomized
controlled trial. Chin J Clin Rational Drug Use. 16:95–97. 2023.In
Chinese.
|
|
262
|
Guan CY and Sun ZC: Clinical efficacy of
combined ultrasound and low-level laser therapy in the management
of chronic heel pain: A randomized controlled trial. Chin J
Convalescent Med. 18:6242009.In Chinese.
|
|
263
|
Luo FZ: Curative effect analysis of
ultrasound on non-specific low back pain. Smart Healthcare.
6:47–48. 2020.In Chinese.
|
|
264
|
Yang H, Cai JH, Zhou C, Zhang SJ, Xiao T,
Hu BC, Ai JF and Li Z: Effects of ultrasound combined with
transcutaneous electrical nerve stimulation on knee joint pain and
function in patients with knee osteoarthritis. Chin J Rehabil Med.
39:1174–1179. 2024.In Chinese.
|
|
265
|
Bi YL, Zhai HW, Zhang JL and Wang SY:
Observation on the efficacy of high-frequency repetitive
transcranial magnetic stimulation at different frequencies in
treating central post-stroke pain. Chin J Rehabil. 40:32–35.
2025.In Chinese.
|
|
266
|
Zhang YW, Wang H, Gao SN, Sun XX, Chen C,
Shen YY and Ding L: Efficacy of repetitive transcranial magnetic
stimulation in relieving pain and quality of life in patients with
acute central poststroke pain. Pract Geriatr. 38:1250–1254. 2024.In
Chinese.
|
|
267
|
Wang J, Song JY, Xu B, Lyu HZ, Zhao XB,
Gong HR and Li YX: Effects of repetitive transcranial magnetic
stimulation combined with exercise training on patients with
incomplete spinal cord injuries. J Trauma Surg. 26:936–940. 2024.In
Chinese.
|
|
268
|
Sun YJ, Zhang JF, Yan L, Fan SY, Qian YL,
Cong S, Wang Y and Yu T: Mechanism of transcranial magnetic
stimulation therapy for neuropathic pain. Rehabilitation Med.
34:625–632. 2024.In Chinese. View Article : Google Scholar
|
|
269
|
Xie Y, Pan J, Chen J, Zhang D and Jin S:
Acupuncture combined with repeated transcranial magnetic
stimulation for upper limb motor function after stroke: A
systematic review and meta-analysis. Neurorehabilitation.
53:423–438. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Luo WW and Li TL: Observation of the
clinical efficacy of electro-acupuncture combined with infrared ray
in the treatment of chronic pelvic pain caused by pelvic
inflammatory diseases. Guiding J Tradit Chin Med Pharmacol.
29:110–113. 2023.In Chinese.
|
|
271
|
Gan PQ, Wang YY, Pan Q and Shi FF: Nursing
and effection of Piwei Peiyuansan combined with infrared lamp
irradiation in treating 35 patients with epigastric pain. Anhui
Med. 20:1412–1414. 2016.In Chinese.
|
|
272
|
Ou JW, Wang XP, Zhang XT and Sun XS:
Effect of Aitong Gao acupoint application combined with whole-body
infrared hyperthermia on cancer-related pain. Guangdong Med J.
38:1761–1763. 2017.In Chinese.
|
|
273
|
Liao TH, Hu LM, Yu ZS, Huang CJ, Liu JB
and Mo CM: Clinical efficacy of Xietong Gao external application
combined with infrared irradiation in alleviating hepatocellular
carcinoma-related pain: A case series of 40 patients. J Pract
Tradit Chin Med. 28:1048–1049. 2012.In Chinese.
|
|
274
|
Men JR, Gao SF, Yin JF, Lei YW and Hu YF:
Efficacy of external application of traditional Chinese medicine
combined with far infrared irradiation and dialectical dietary
intervention in patients with lumbar disc herniation. Hebei J TCM.
46:1863–1866. 2024.In Chinese.
|
|
275
|
Pang ZH, Qin TM and Ruan MJ: Clinical
efficacy of combined deep hyperthermia and Sihuang powder external
application in managing intermediate-advanced primary liver cancer
with cancer-related pain. Mod Med Health Res. 8:102–105. 2024.In
Chinese.
|
|
276
|
Chen XH and Yan Y: Clinical study on
transcutaneous auricular vagus nerve stimulation combined with
multimodal analgesia for rehabilitation after total knee
arthroplasty. Chin J Convalescent Med. 34:22–26. 2025.In
Chinese.
|
|
277
|
Mao M, Sun YH and Peng YG: Clinical
research of external application of 'Aitongxiao̓ combined with
thermal therapy in releasing moderate to severe pain due to cancer.
Shanghai J Tradit Chin Med. 48:44–47. 2014.In Chinese.
|
|
278
|
Yang J, Lo WAL, Zheng F, Cheng X, Yu Q and
Wang C: Evaluation of cognitive behavioral therapy on improving
pain, fear avoidance, and self-efficacy in patients with chronic
low back pain: A systematic review and meta-analysis. Pain Res
Manag. 2022:42761752022. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Darnall BD, Roy A, Chen AL, Ziadni MS,
Keane RT, You DS, Slater K, Poupore-King H, Mackey I, Kao MC, et
al: Comparison of a single-session pain management skills
intervention with a single-session health education intervention
and 8 sessions of cognitive behavioral therapy in adults with
chronic low back pain: A randomized clinical trial. JAMA Netw Open.
4:e21134012021. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Cherkin DC, Sherman KJ, Balderson BH, Cook
AJ, Anderson ML, Hawkes RJ, Hansen KE and Turner JA: Effect of
mindfulness-based stress reduction vs cognitive behavioral therapy
or usual care on back pain and functional limitations in adults
with chronic low back pain: A Randomized clinical trial. JAMA.
315:1240–1249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Hajihasani A, Rouhani M, Salavati M,
Hedayati R and Kahlaee AH: The influence of cognitive behavioral
therapy on pain, quality of life, and depression in patients
receiving physical therapy for chronic low back pain: A systematic
review. PM R. 11:167–176. 2019. View Article : Google Scholar
|
|
282
|
Mariano TY, Urman RD, Hutchison CA,
Jamison RN and Edwards RR: Cognitive behavioral therapy (CBT) for
subacute low back pain: A systematic review. Curr Pain Headache
Rep. 22:152018. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Hadley G and Novitch MB: CBT and CFT for
chronic pain. Curr Pain Headache Rep. 25:352021. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Torres CB, Barona EJG, Molina MG, Sánchez
MEG and Manso JMM: A systematic review of EEG neurofeedback in
fibromyalgia to treat psychological variables, chronic pain and
general health. Eur Arch Psychiatry Clin Neurosci. 274:981–999.
2024. View Article : Google Scholar :
|
|
285
|
Goldway N, Ablin J, Lubin O, Zamir Y,
Keynan JN, Or-Borichev A, Cavazza M, Charles F, Intrator N, Brill
S, et al: Volitional limbic neuromodulation exerts a beneficial
clinical effect on Fibromyalgia. NeuroImage. 186:758–770. 2019.
View Article : Google Scholar
|
|
286
|
Hassett AL, Radvanski DC, Vaschillo EG,
Vaschillo B, Sigal LH, Karavidas MK, Buyske S and Lehrer PM: A
pilot study of the efficacy of heart rate variability (HRV)
biofeedback in patients with fibromyalgia. Appl Psychophysiol
Biofeedback. 32:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Burch JB, Ginsberg JP, McLain AC, Franco
R, Stokes S, Susko K, Hendry W, Crowley E, Christ A, Hanna J, et
al: Symptom management among cancer survivors: randomized pilot
intervention trial of heart rate variability biofeedback. Appl
Psychophysiol Biofeedback. 45:99–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Stern MJ, Guiles RAF and Gevirtz R: HRV
biofeedback for pediatric irritable bowel syndrome and functional
abdominal pain: A clinical replication series. Appl Psychophysiol
Biofeedback. 39:287–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Jensen MP, Gianas A, George HR, Sherlin
LH, Kraft GH and Ehde D: Use of neurofeedback to enhance response
to hypnotic analgesia in individuals with multiple sclerosis. Int J
Clin Exp Hypn. 64:1–23. 2016. View Article : Google Scholar
|
|
290
|
Zhao X: Clinical observation on
acupuncture combined with meridian-based tuina in treating elderly
patients with neck, shoulder, lower back, and leg pain. Inner
Mongolia J Tradit Chin Med. 43:134–135. 1682024.In Chinese.
|
|
291
|
Su L, Li X and Yu P: Effect of Yiqi Shujin
decoction combined with meridian massage on TCM symptoms and
functional recovery in patients with neck, shoulder, low back and
leg pain. J Med Inform. 37:130–134. 2024.In Chinese.
|
|
292
|
Gausel AM, Dalen I, Kjærmann I, Malmqvist
S, Andersen K, Larsen JP and Økland I: Adding chiropractic
treatment to individual rehabilitation for persistent pelvic girdle
pain 3 to 6 months after delivery: A pilot randomized trial. J
Manipulative Physiol Ther. 42:601–607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Duan JQ, Chen ZX, Hu P, Zhang XL, Zhang W
and Wang Y: Effect of chiropractic therapy for spinal epigastric
pain. Chin J Convalescent Med. 33:80–84. 2024.In Chinese.
|
|
294
|
Chen B, Dai GW, Tian XY, Chen ZR, Wang LK
and Yang XK: The clinical effect of chiropractic therapy in the
treatment of 30 cases of spinal calcaneodynia. Chin Community
Doctors. 30:96–97. 2014.In Chinese.
|
|
295
|
Wei YH, Xu M and Chen XF: Study for the
effect of Traditional Chinese medicine enema combined with fructus
Evodiae Rutecarpae on preemptive analgesia after pelvic operation.
J Nurses Train. 31:772–774. 2016.In Chinese.
|
|
296
|
Xiong ZL: Clinical observation on massage
combined with Shentong Zhuyu decoction in treating Qi Stagnation
and blood stasis lumbago. Chin Med Mod Distance Educ China.
18:82–84. 2024.In Chinese.
|
|
297
|
Song S, Liu Y, Zhang BX, Teng C and Zhao
SS: Clinical efficacy observation of low intensity pulse ultrasound
combined with quadriceps femoris muscle strength intensive training
in the treatment of patients with knee osteoarthritis. Prog Mod
Biomed. 20:2668–2671. 2020.In Chinese.
|
|
298
|
Chen C, Zhang JP, Peng J, Cao AH, Chen XM,
Zhang C and Shen L: Efficacy of low-intensity pulsed ultrasound
therapy on heart rate variability in elderly patients with coronary
heart disease. Chin J Med Front (Electron Version). 15:44–49.
2023.In Chinese.
|
|
299
|
Liu L, Wu JY, Zhang CL and Zhu BL:
Percutaneous transforaminal endoscopic discectomy for treatment of
lumbar disc herniation under epidural anesthesia. J Clin Orthop.
27:636–639. 2024.In Chinese.
|
|
300
|
Chen YD: Clinical research of acupuncture,
chiropractic therapy combined with amitriptyline in the treatment
of fibromyalgia syndrome. China Pract Med. 10:24–25. 2015.In
Chinese.
|
|
301
|
Russo AF and Hay DL: CGRP physiology,
pharmacology, and therapeutic targets: Migraine and beyond. Physiol
Rev. 103:1565–1644. 2023. View Article : Google Scholar :
|
|
302
|
Markham A: Erenumab: First global
approval. Drugs. 78:1157–1161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Zhou W, Ye C, Wang H, Mao Y, Zhang W, Liu
A, Yang CL, Li T, Hayashi L, Zhao W, et al: Sound induces analgesia
through corticothalamic circuits. Science. 377:198–204. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Gu X, Zhang YZ, O'Malley JJ, De Preter CC,
Penzo M and Hoon MA: Neurons in the caudal ventrolateral medulla
mediate descending pain control. Nat Neurosci. 26:594–605. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Grandi FC, Baskar R, Smeriglio P,
Murkherjee S, Indelli PF, Amanatullah DF, Goodman S, Chu C, Bendall
S and Bhutani N: Single-cell mass cytometry reveals cross-talk
between inflammation-dampening and inflammation-amplifying cells in
osteoarthritic cartilage. Sci Adv. 6:eaay53522020. View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Li X, Lin S, Lin Y, Su Y, Wang C, Huang L,
Zhao J and Tian G: The analgesic mechanism of Xi Shao Formula
research on pain based on metabolomics. J Tradit Chin Med Sci.
10:448–460. 2023.
|
|
307
|
Schneeberger M, Brice NL, Pellegrino K,
Parolari L, Shaked JT, Page KJ, Marchildon F, Barrows DW, Carroll
TS, Topilko T, et al: Pharmacological targeting of glutamatergic
neurons within the brainstem for weight reduction. Nat Metab.
4:1495–1513. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Woolf CJ: Capturing novel non-opioid pain
targets. Biol Psychiatry. 87:74–81. 2020. View Article : Google Scholar
|
|
309
|
Eisenstein M: Treading the tightrope of
opioid restrictions. Nature. 573:S13–S15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
310
|
DeWeerdt S: Tracing the US opioid crisis
to its roots. Nature. 573:S10–S12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Stein C: Pain inhibition by opioids-new
concepts. Anaesthesist. 68:97–103. 2019.In German. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Stanley TH: The fentanyl story. J Pain.
15:1215–1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Ni Y, Gao H, Ouyang W, Yang G, Cheng M and
Ding L: Pharmacokinetics, metabolite profiling, safety and
tolerability of YZJ-4729 tartrate, a novel G protein-biased
μ-opioid receptor agonist, in healthy Chinese subjects. Front
Pharmacol. 14:12953192024. View Article : Google Scholar
|
|
314
|
Hou Y, Zou G, Wang X, Guo H, Ma X, Cheng
X, Xie Z, Zuo X, Xia J, Mao H, et al: Coordinated activity of a
central pathway drives associative opioid analgesic tolerance. Sci
Adv. 9:eabo56272023. View Article : Google Scholar : PubMed/NCBI
|
|
315
|
Dart RC, Cicero TJ, Surratt HL, Rosenblum
A, Bartelson BB and Adams EH: Assessment of the abuse of tapentadol
immediate release: the first 24 months. J Opioid Manag. 8:395–402.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
316
|
Grosser T, Woolf CJ and FitzGerald GA:
Time for nonaddictive relief of pain. Science. 355:1026–1027. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
317
|
Zhang G, Cui M, Ji R, Zou S, Song L, Fan
B, Yang L, Wang D, Hu S, Zhang X, et al: Neural and molecular
investigation into the paraventricular thalamic-nucleus accumbens
circuit for pain sensation and non-opioid analgesia. Pharmacol Res.
191:1067762023. View Article : Google Scholar : PubMed/NCBI
|
|
318
|
Ai L, Han Y, Ji R, Zhou DY, Zhang WX, Xie
A, Zhai XJ, Cao JL and Zhang HX: Research progress in pain
treatment and analgesic targets. Chin J Pain Med. 29:484–494.
2023.In Chinese.
|
|
319
|
Neubert MJ, Kincaid W and Heinricher MM:
Nociceptive facilitating neurons in the rostral ventromedial
medulla. Pain. 110:158–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
320
|
Shi R, Chai Y, Feng H, Xie L, Zhang L,
Zhong T, Chen J, Yan P, Zhu B, Zhao J and Zhou C: Study of the mass
balance, biotransformation and safety of [14C] SHR8554, a novel
μ-opioid receptor injection, in healthy Chinese subjects. Front
Pharmacol. 14:12311022023. View Article : Google Scholar
|
|
321
|
Wang D, Pan X, Zhou Y, Wu Z, Ren K, Liu H,
Huang C, Yu Y, He T, Zhang X, et al: Lateral septum-lateral
hypothalamus circuit dysfunction in comorbid pain and anxiety. Mol
Psychiatry. 28:1090–1100. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
322
|
Ji YW, Shen ZL, Zhang X, Zhang K, Jia T,
Xu X, Geng H, Han Y, Yin C, Yang JJ, et al: Plasticity in ventral
pallidal cholinergic neuron-derived circuits contributes to
comorbid chronic pain-like and depression-like behaviour in male
mice. Nat Commun. 14:21822023. View Article : Google Scholar : PubMed/NCBI
|
|
323
|
Zhang Y, Feng J, Ou C, Zhou X and Liao Y:
AQP4 mitigates chronic neuropathic pain-induced cognitive
impairment in mice. Behav Brain Res. 440:1142822023. View Article : Google Scholar : PubMed/NCBI
|
|
324
|
Sun H, Li Z, Qiu Z, Shen Y, Guo Q, Hu SW,
Ding HL, An S and Cao JL: A common neuronal ensemble in nucleus
accumbens regulates pain-like behaviour and sleep. Nat Commun.
14:47002023. View Article : Google Scholar : PubMed/NCBI
|
|
325
|
Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian
Y, Mao Y, Wang L, Xie W, Zhong H, et al: A neural circuit for
comorbid depressive symptoms in chronic pain. Nat Neurosci.
22:1649–1658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
326
|
Zhang CK, Wang P, Ji YY, Zhao JS, Gu JX,
Yan XX, Fan HW, Zhang MM, Qiao Y, Liu XD, et al: Potentiation of
the lateral habenula-ventral tegmental area pathway underlines the
susceptibility to depression in mice with chronic pain. Sci China
Life Sci. 67:67–82. 2024. View Article : Google Scholar
|
|
327
|
Dong Y, Li Y, Xiang X, Xiao ZC, Hu J, Li
Y, Li H and Hu H: Stress relief as a natural resilience mechanism
against depression-like behaviors. Neuron. 111:3789–3801.e6. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
328
|
Dai W, Huang S, Luo Y, Cheng X, Xia P,
Yang M, Zhao P, Zhang Y, Lin WJ and Ye X: Sex-specific
transcriptomic signatures in brain regions critical for neuropathic
pain-induced depression. Front Mol Neurosci. 15:8869162022.
View Article : Google Scholar : PubMed/NCBI
|
|
329
|
Nguyen VT, Hill B, Sims N, Heck A, Negron
M, Lusk C and Galindo CL: Brain-derived neurotrophic factor rs6265
(Val66Met) single nucleotide polymorphism as a master modifier of
human pathophysiology. Neural Regen Res. 18:102–106. 2023.
View Article : Google Scholar
|
|
330
|
Wang CS, Kavalali ET and Monteggia LM:
BDNF signaling in context: From synaptic regulation to psychiatric
disorders. Cell. 185:62–76. 2022. View Article : Google Scholar :
|
|
331
|
Martínez T, Mariscal G, de la Rubia Ortí
JE and Barrios C: Efficacy and safety of pregabalin and gabapentin
in spinal stenosis: A systematic review and meta-analysis. Front
Pharmacol. 14:12494782023. View Article : Google Scholar : PubMed/NCBI
|
|
332
|
Pu ZH, Peng C, Xie XF, Luo M, Zhu H, Feng
R and Xiong L: Alkaloids from the rhizomes of Ligusticum striatum
exert anti-migraine effects through regulating 5-HT1B
receptor and c-Jun. J Ethnopharmacol. 237:39–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
333
|
Pang LN, Chen XM, Lan YY, Huang QL, Yu XM,
Qi L and Wang ZF: Research progress of acupuncture analgesia based
on autonomic nerve regulation pathway. Acupunct Herbal Med.
3:285–295. 2023. View Article : Google Scholar
|
|
334
|
Zhao ZQ: Neural mechanism underlying
acupuncture analgesia. Prog Neurobiol. 85:355–375. 2008. View Article : Google Scholar : PubMed/NCBI
|