|
1
|
Tournadre A, Vial G, Capel F, Soubrier M
and Boirie Y: Sarcopenia. Joint Bone Spine. 86:309–314. 2019.
View Article : Google Scholar
|
|
2
|
Larsson L, Degens H, Li M, Salviati L, Lee
YI, Thompson W, Kirkland JL and Sandri M: Sarcopenia: Aging-related
loss of muscle mass and function. Physiol Rev. 99:427–511. 2019.
View Article : Google Scholar :
|
|
3
|
Cruz-Jentoft AJ and Sayer AA: Sarcopenia.
Lancet. 393:2636–2646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrocelli JJ, Mahmassani ZS, Fix DK,
Montgomery JA, Reidy PT, McKenzie AI, de Hart NM, Ferrara PJ,
Kelley JJ, Eshima H, et al: Metformin and leucine increase
satellite cells and collagen remodeling during disuse and recovery
in aged muscle. FASEB J. 35:e218622021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HJ, Jung DW and Williams DR: Age is
just a number: Progress and obstacles in the discovery of new
candidate drugs for sarcopenia. Cells. 12:26082023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huo F, Liu Q and Liu H: Contribution of
muscle satellite cells to sarcopenia. Front Physiol. 13:8927492022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt M, Schüler SC, Hüttner SS, von
Eyss B and von Maltzahn J: Adult stem cells at work: Regenerating
skeletal muscle. Cell Mol Life Sci. 76:2559–2570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haroon M, Boers HE, Bakker AD, Bloks NGC,
Hoogaars WMH, Giordani L, Musters RJP, Deldicque L, Koppo K, Le
Grand F, et al: Reduced growth rate of aged muscle stem cells is
associated with impaired mechanosensitivity. Aging (Albany NY).
14:28–53. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qazi TH, Duda GN, Ort MJ, Perka C,
Geissler S and Winkler T: Cell therapy to improve regeneration of
skeletal muscle injuries. J Cachexia Sarcopenia Muscle. 10:501–516.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Judson RN and Rossi FMV: Towards stem cell
therapies for skeletal muscle repair. NPJ Regen Med. 5:102020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du H, Shih CH, Wosczyna MN, Mueller AA,
Cho J, Aggarwal A, Rando TA and Feldman BJ: Macrophage-released
ADAMTS1 promotes muscle stem cell activation. Nat Commun.
8:6692017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mourikis P, Sambasivan R, Castel D,
Rocheteau P, Bizzarro V and Tajbakhsh S: A critical requirement for
notch signaling in maintenance of the quiescent skeletal muscle
stem cell state. Stem Cells. 30:243–252. 2012. View Article : Google Scholar
|
|
13
|
Lee SH, Kim SY, Gwon YG, Lee C, Cho IH,
Kim TW and Choi BK: Recombinant ADAMTS1 promotes muscle cell
differentiation and alleviates muscle atrophy by repressing NOTCH1.
BMB Rep. 57:539–545. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tarum J, Degens H, Turner MD, Stewart C,
Sale C and Santos L: Modelling skeletal muscle ageing and repair in
vitro. J Tissue Eng Regen Med. 2023:98022352023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pawlikowski B, Betta ND, Antwine T and
Olwin BB: Skeletal muscle stem cell self-renewal and
differentiation kinetics revealed by EdU lineage tracing during
regeneration. bioRxiv. https://doi.org/10.1101/627851.
|
|
16
|
Al Shoyaib A, Archie SR and Karamyan VT:
Intraperitoneal route of drug administration: Should it be used in
experimental animal studies? Pharm Res. 37:122019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ibraheem D, Elaissari A and Fessi H:
Administration strategies for proteins and peptides. Int J Pharm.
477:578–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeshita H, Yamamoto K, Nozato S, Inagaki
T, Tsuchimochi H, Shirai M, Yamamoto R, Imaizumi Y, Hongyo K,
Yokoyama S, et al: Modified forelimb grip strength test detects
aging-associated physiological decline in skeletal muscle function
in male mice. Sci Rep. 7:423232017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghaibour K, Rizk J, Ebel C, Ye T, Philipps
M, Schreiber V, Metzger D and Duteil D: An efficient protocol for
CUT&RUN analysis of FACS-isolated mouse satellite cells. J Vis
Exp. 197:e652152023.
|
|
20
|
Gilda JE and Gomes AV: Stain-Free total
protein staining is a superior loading control to β-actin for
Western blots. Anal Biochem. 440:186–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fortes MA, Marzuca-Nassr GN, Vitzel KF, da
Justa Pinheiro CH, Newsholme P and Curi R: Housekeeping proteins:
How useful are they in skeletal muscle diabetes studies and muscle
hypertrophy models? Anal Biochem. 504:38–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paul RG, Hennebry AS, Elston MS, Conaglen
JV and McMahon CD: Regulation of murine skeletal muscle growth by
STAT5B is age- and sex-specific. Skelet Muscle. 9:192019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang R, Kumar B, Doud EH, Mosley AL,
Alexander MS, Kunkel LM and Nakshatri H: Skeletal muscle-specific
overexpression of miR-486 limits mammary tumor-induced skeletal
muscle functional limitations. Mol Ther Nucleic Acids. 28:231–248.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Forcina L, Cosentino M and Musarò A:
Mechanisms regulating muscle regeneration: Insights into the
interrelated and time-dependent phases of tissue healing. Cells.
9:12972020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choo HJ, Canner JP, Vest KE, Thompson Z
and Pavlath GK: A tale of two niches: Differential functions for
VCAM-1 in satellite cells under basal and injured conditions. Am J
Physiol Cell Physiol. 313:C392–C404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gustafsson T and Ulfhake B: Aging skeletal
muscles: What are the mechanisms of age-related loss of strength
and muscle mass, and can we impede its development and progression?
Int J Mol Sci. 25:109322024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xing HY, Liu N and Zhou MW: Satellite cell
proliferation and myofiber cross-section area increase after
electrical stimulation following sciatic nerve crush injury in
rADAMTS-1. Chin Med J (Engl). 133:1952–1960. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meng J, Lv Z, Chen X, Sun C, Jin C, Ding K
and Chen C: LBP1C-2 from Lycium barbarum maintains skeletal muscle
satellite cell pool by interaction with FGFR1. iScience.
26:1065732023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agarwal M, Sharma A, Kumar P, Kumar A,
Bharadwaj A, Saini M, Kardon G and Mathew SJ: Myosin heavy
chain-embryonic regulates skeletal muscle differentiation during
mammalian development. Development. 147:dev1845072020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Xiao Y, Zheng Y, Yang L and Wang
D: An anti-ADAMTS1 treatment relieved muscle dysfunction and
fibrosis in dystrophic mice. Life Sci. 281:1197562021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mierzejewski B, Grabowska I, Michalska Z,
Zdunczyk K, Zareba F, Irhashava A, Chrzaszcz M, Patrycy M,
Streminska W, Janczyk-Ilach K, et al: SDF-1 and NOTCH signaling in
myogenic cell differentiation: The role of miRNA10a, 425, and 5100.
Stem Cell Res Ther. 14:2042023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buas MF and Kadesch T: Regulation of
skeletal myogenesis by Notch. Exp Cell Res. 316:3028–3033. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo D, de Morree A, Boutet S, Quach N,
Natu V, Rustagi A and Rando TA: Deltex2 represses MyoD expression
and inhibits myogenic differentiation by acting as a negative
regulator of Jmjd1c. Proc Natl Acad Sci USA. 114:E3071–E3080. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isesele PO and Mazurak VC: Regulation of
skeletal muscle satellite cell differentiation by Omega-3
polyunsaturated fatty acids: A critical review. Front Physiol.
12:6820912021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adhikari A, Kim W and Davie J: Myogenin is
required for assembly of the transcription machinery on muscle
genes during skeletal muscle differentiation. PLoS One.
16:e02456182021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Faralli H and Dilworth FJ: Turning on
myogenin in muscle: A paradigm for understanding mechanisms of
tissue-specific gene expression. Comp Funct Genomics.
2012:8363742012. View Article : Google Scholar :
|
|
38
|
Owens J, Moreira K and Bain G:
Characterization of primary human skeletal muscle cells from
multiple commercial sources. In Vitro Cell Dev Biol Anim.
49:695–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding R, Horie M, Nagasaka S, Ohsumi S,
Shimizu K, Honda H, Nagamori E, Fujita H and Kawamoto T: Effect of
cell-extracellular matrix interaction on myogenic characteristics
and artificial skeletal muscle tissue. J Biosci Bioeng. 130:98–105.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sanvee GM, Bouitbir J and Krähenbühl S:
C2C12 myoblasts are more sensitive to the toxic effects of
simvastatin than myotubes and show impaired proliferation and
myotube formation. Biochem Pharmacol. 190:1146492021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Henderson CA, Gomez CG, Novak SM, Mi-Mi L
and Gregorio CC: Overview of the muscle cytoskeleton. Compr
Physiol. 7:891–944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lehka L and Rędowicz MJ: Mechanisms
regulating myoblast fusion: A multilevel interplay. Semin Cell Dev
Biol. 104:81–92. 2020. View Article : Google Scholar
|
|
43
|
Lazure F, Blackburn DM, Corchado AH,
Sahinyan K, Karam N, Sharanek A, Nguyen D, Lepper C, Najafabadi HS,
Perkins TJ, et al: Myf6/MRF4 is a myogenic niche regulator required
for the maintenance of the muscle stem cell pool. EMBO Rep.
21:e494992020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Krampert M, Kuenzle S, Thai SN, Lee N,
Iruela-Arispe ML and Werner S: ADAMTS1 proteinase is up-regulated
in wounded skin and regulates migration of fibroblasts and
endothelial cells. J Biol Chem. 280:23844–23852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dungan CM, Murach KA, Zdunek CJ, Tang ZJ,
Nolt GL, Brightwell CR, Hettinger Z, Englund DA, Liu Z, Fry CS, et
al: Deletion of SA β-Gal+ cells using senolytics improves muscle
regeneration in old mice. Aging Cell. 21:e135282022. View Article : Google Scholar
|
|
46
|
Always SE, Paez HG, Pitzer CR, Ferrandi
PJ, Khan MM, Mohamed JS, Carson JA and Deschenes MR: Mitochondria
transplant therapy improves regeneration and restoration of injured
skeletal muscle. J Cachexia Sarcopenia Muscle. 14:493–507. 2023.
View Article : Google Scholar
|
|
47
|
Kim JW, Manickam R, Sinha P, Xuan W, Huang
J, Awad K, Brotto M and Tipparaju SM: P7C3 ameliorates barium
chloride-induced skeletal muscle injury activating transcriptomic
and epigenetic modulation of myogenic regulatory factors. J Cell
Physiol. 239:e313462024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang YX and Rudnicki MA: Sataellite cells,
the engines of muscle repair. Nat Rev Mol Cell Biol. 13:127–133.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kerr HL, Krumm K, Anderson B, Christiani
A, Strait L, Li T, Irwin B, Jiang S, Rybachok A, Chen A, et al:
Mouse sarcopenia model reveals sex-and age-specific differences in
phenotypic and molecular characteristics. J Clin Invest.
134:e1728902024. View Article : Google Scholar
|
|
50
|
Owen AM and Fry CS: Decoding the decline:
Unveiling drivers of sarcopenia. J Clin Invest. 134:e1833022024.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tao L, Huang W, Li Z, Wang W, Lei X, Chen
J, Song X, Lu F, Fan S and Zhang L: Transcriptome analysis of
differentially expressed genes and molecular pathways involved in
C2C12 cells myogenic differentiation. Mol Biotechnol. 67:3640–3655.
2025. View Article : Google Scholar
|















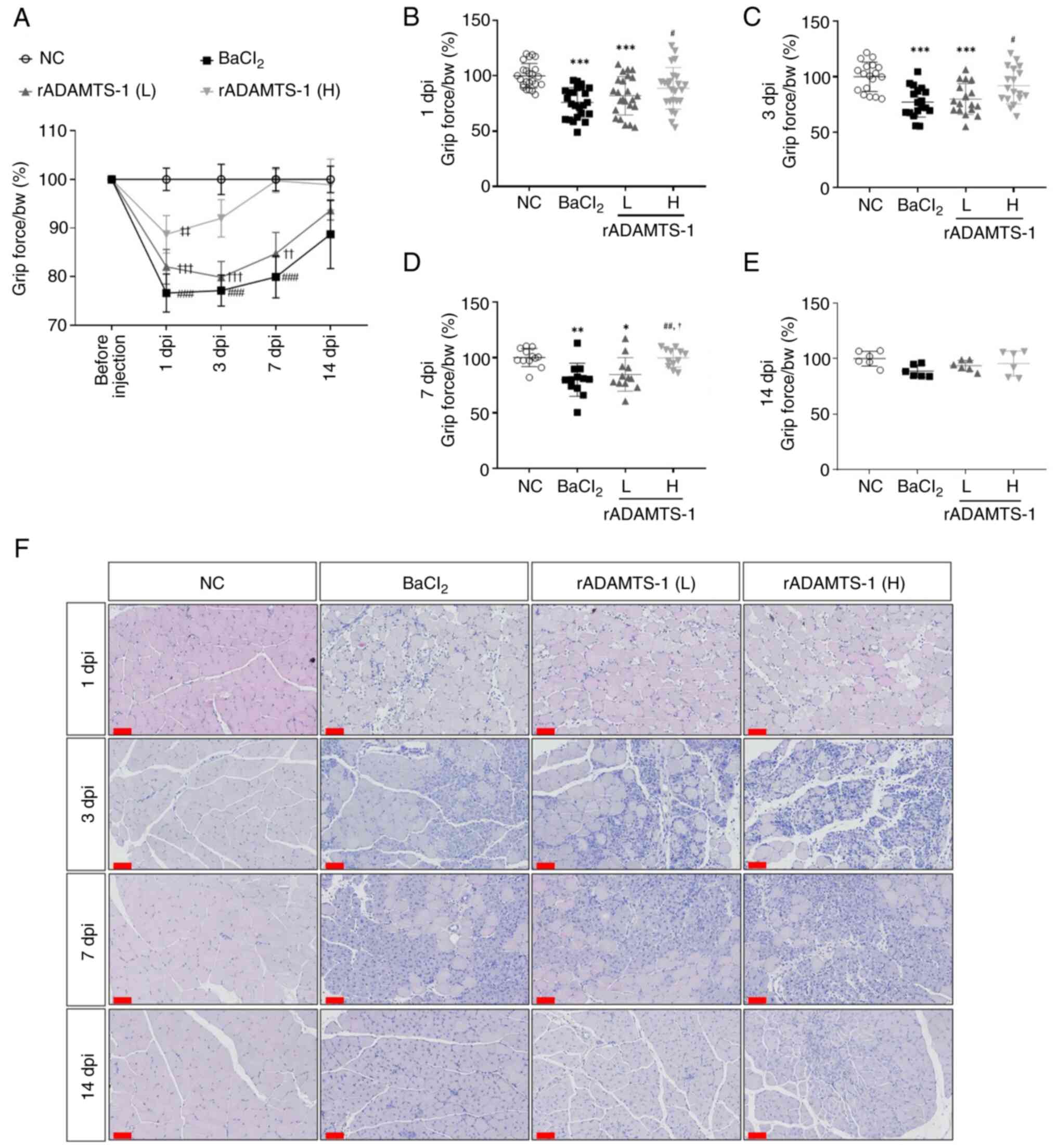
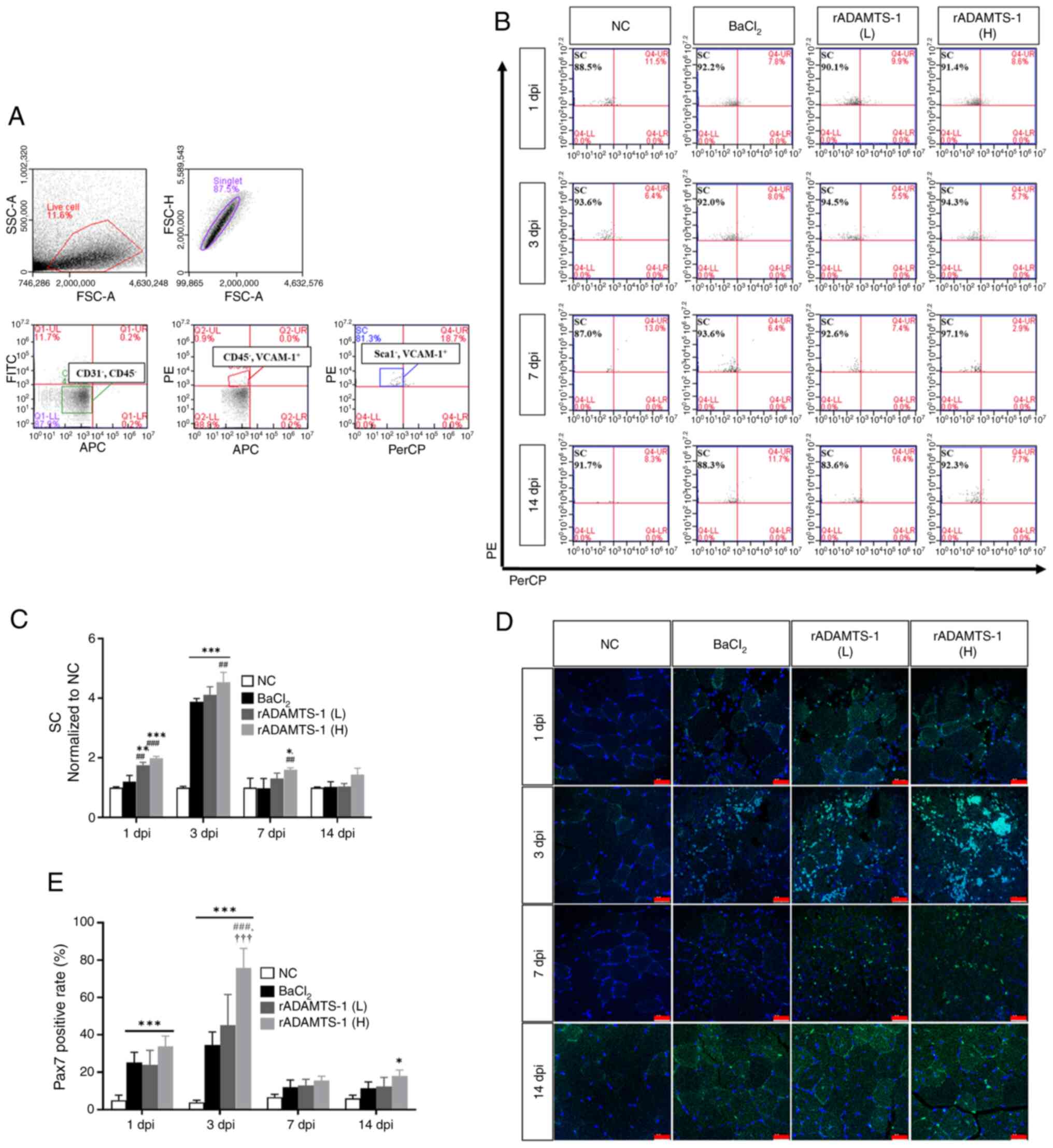
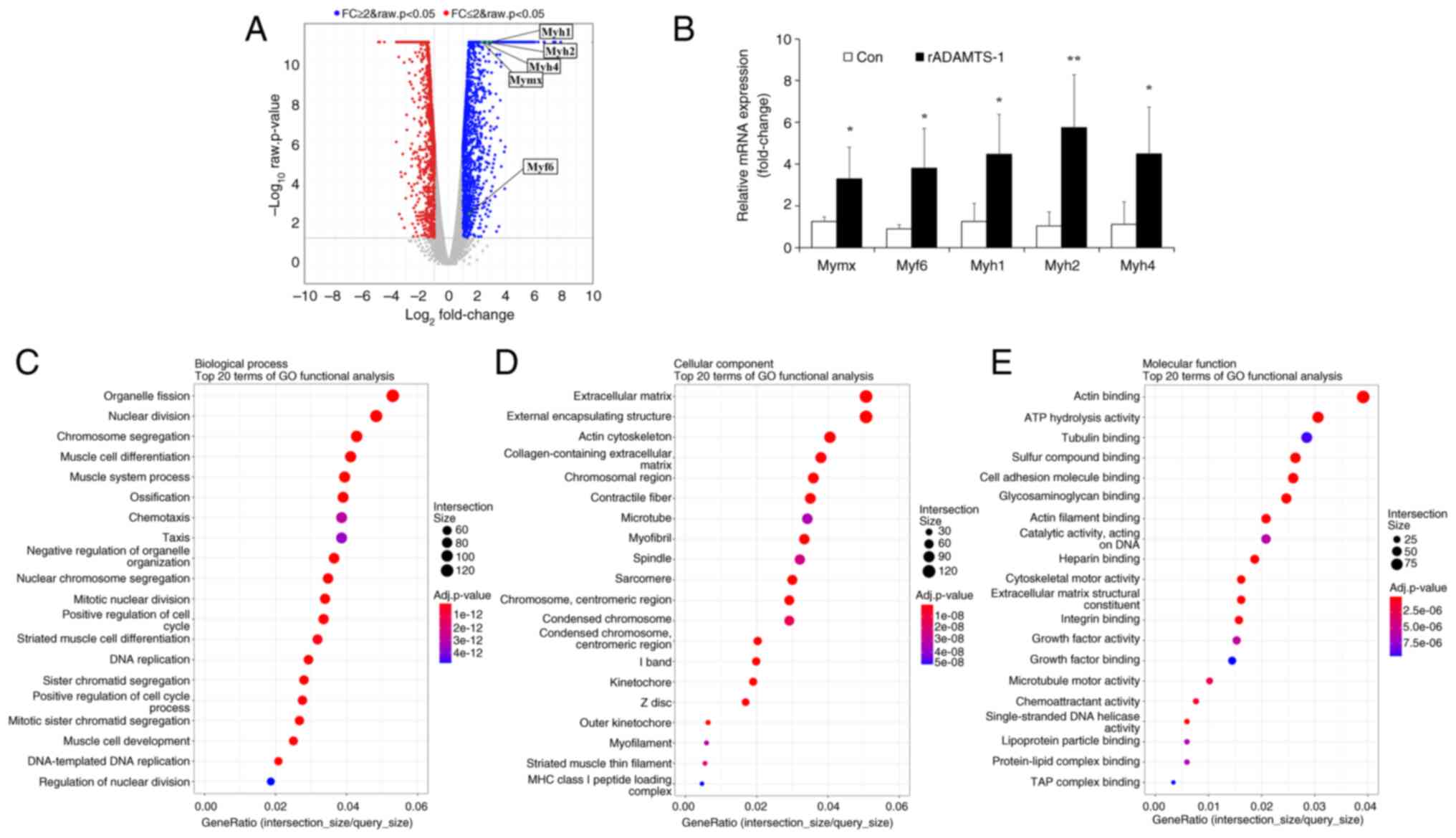
![Effects of rADAMTS-1 on the
expression of ADAMTS-1-associated markers in
BaCl2-injured TA muscle. Groups included NC,
BaCl2, rADAMTS-1 treatment groups (L), and (H). Daily
intraperitoneal injections of rADAMTS-1 were initiated immediately
after injury and continued until the designated time point for
analysis. (A) Temporal expression patterns of ADAMTS-1, NICD, and
Hes-1 in the NC and BaCl2 groups at 1, 3, 7, and 14 days
post-injury (dpi). Comparative expression levels of (B) ADAMTS-1,
(C) NICD, and (D) Hes-1 among treatment groups [NC,
BaCl2, rADAMTS-1 (L), and rADAMTS-1 (H)] at each time
point. Mice received daily intraperitoneal injections of rADAMTS-1
until the designated dpi. Each data point represents an individual
biological replicate; error bars indicate standard deviation.
Statistical significance in panels (A) was determined using one-way
analysis of variance, followed by Tukey's honestly significant
difference post hoc test, as indicated as follows:
**P<0.01 and ***P<0.001 vs. 1 dpi;
##P<0.01 and ###P<0.001 vs. 3 dpi. For
panels (B-D), statistical comparisons were made using one-way
analysis of variance with Tukey's post hoc test:
*P<0.05, **P<0.01 and
***P<0.001 vs. NC; #P<0.05
##P<0.01 and ###P<0.001 vs.
BaCl2; †P<0.05, ††P<0.01 and
†††P<0.001 vs. rADAMTS-1 (L). ADAMTS-1, a disintegrin
and metalloproteinase with thrombospondin motifs 1; rADAMTS-1,
recombinant ADAMTS-1; BaCl2, barium chloride; TA,
tibialis anterior; NC, non-injured control; rADAMTS-1 (L),
rADAMTS-1 at 5 mg/kg; rADAMTS-1 (H), rADAMTS-1 at 10 mg/kg; NICD,
Notch intracellular domain; Hes-1, hairy and enhancer of split-1;
dpi, days post-injury.](/article_images/ijmm/57/2/ijmm-57-02-05718-g02.jpg)