Introduction
Fibrosis is defined as the overabundant deposition
of the extracellular matrix (ECM), which includes collagen and
fibronectin (FN), leading to the accumulation of substantial
fibrous connective tissue in areas of inflammatory infiltration or
within and around damaged tissues (1). It is a pathological condition that
eventually triggers severe scar hyperplasia; organ dysfunction,
such as heart failure, idiopathic pulmonary fibrosis (IPF),
end-stage renal disease (ESRD), end-stage liver disease and even
death (2). If epithelial or
endothelial regeneration capacity cannot be synchronized with
recurrent damage during cell infection, exposure to toxic factors,
or autoimmunity, or if other uncertain factors continuously induce
epithelial or endothelial damage, then the corresponding
mesenchymal cells are activated and a considerable amount of ECM is
deposited; this phenomenon is also the mechanism of fibrotic
reaction in most organs (3).
Organ fibrosis is a dynamic pathological process. In contrast to
irreversible scar tissue formation, it is highly plastic and can
reversely change the course of fibrosis by targeting key effector
cells or signaling (4).
Epidemiological data have shown that fibrosis-related health events
pose a great threat to the health of a quarter of the population
worldwide and that the annual incidence of major fibrosis-related
diseases is close to 1 in 20 (5). Nintedanib and pirfenidone are
prescription drugs approved by a number of developed and developing
countries for intervening in fibrosis in specific organs and are
available to patients with IPF. Thus far, however, drugs that
specifically target the fibrosis of the liver, kidney, or heart
have yet to be approved in the medical market. In addition,
although nintedanib has been approved for the medical management of
systemic sclerosis (SSc)-related interstitial lung disease and
progressive fibrosing interstitial lung disorders, marked advances
are needed regarding the development of fibrotic drugs, especially
highly effective drugs that can specifically act on affected
organs, thereby achieving reversible intervention in organ fibrosis
diseases (6).
Multiple mechanisms determine the progression of
fibrotic diseases and traditional Chinese medicine (TCM) can exert
multicomponent, multitarget and multipathway therapeutic benefits.
Medicinal resources, including Chinese herbal compounds, materials,
extracts and formulas and their combinations may improve fibrotic
diseases comprehensively with reduced pharmacological toxicity
under the cumulative effects of the aforementioned therapeutic
characteristics (7,8). Chinese herbal medicine has a
protective effect against multiorgan fibrosis. This effect mainly
manifests by improving myocardial perfusion and hemodynamic
function, repairing ultrastructural damage in cardiac tissue,
inhibiting cell apoptosis and cardiac remodeling and alleviating
cardiac dysfunction (8). Active
products derived from single herbs and TCM formulas effectively
intervene in pulmonary fibrosis (PF) by targeting inflammation,
oxidative stress and fibrotic effector molecules (7). Furthermore, a main mechanism
through which Chinese herbal medicines alleviate renal fibrosis is
the regulation of growth factors and fibrotic effector cytokines
and the activation of related signaling pathways. These activities
further improve the renal inflammatory microenvironment and inhibit
reactive oxygen species (ROS) generation and renal cell
proliferation (9). Given that
multiple signaling molecules are involved in the development of
liver fibrosis, treatment strategies for liver fibrosis are no
longer limited to a single targeted inflammatory response and the
multitarget therapeutic effects of Chinese herbal medicine may meet
the needs of the multimodal alleviation of liver fibrosis (10). Intervention modes targeting
metabolic mechanisms may become a key strategy to delay fibrosis.
The widespread application of network pharmacology is conducive to
constructing the affiliation between the main active ingredients in
herbal medicines and metabolic targets, implying that the medical
value of Chinese herbal medicine is receiving increasing attention
(5,11). The present review is based on
databases, such as PubMed, Web of Science, Elsevier and
ScienceDirect and SpringerLink. 'Astragaloside IV (AS-IV)',
'fibrosis', 'pharmacokinetics', 'toxicity', 'bioavailability', and
other keywords were used for retrieval, with relevant case reports,
conference abstracts and duplicate studies being excluded. The
present review mainly focuses on AS-IV, the natural saponin
compound purified from Astragalus membranaceus Bunge
(Astragalus), named Huangqi in Chinese and summarizes
and discusses its metabolic regulatory mechanisms and key drug
targets in alleviating fibrotic diseases from 63 basic experimental
literatures (Fig. 1). It is
hoped that the present review will provide additional theoretical
support for the intervention of tissue fibroses by TCM or a
combination of traditional Chinese and Western medicine and it
calls for optimizing the bioavailability of natural products to
improve the effectiveness of drug-targeted organ therapy.
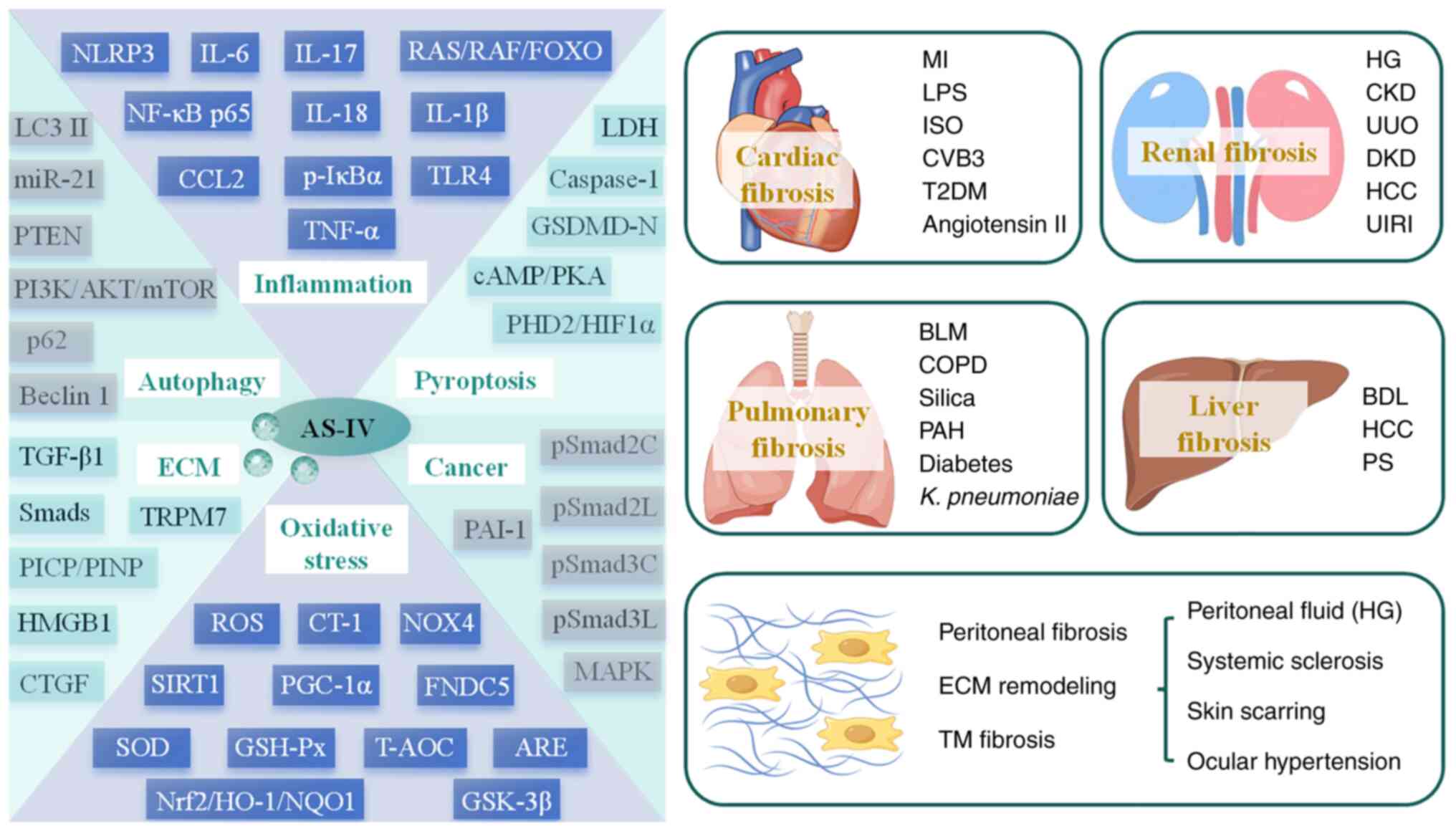 | Figure 1A general overview of AS-IV
regulation of fibrotic diseases. AKT, protein kinase B; ARE,
antioxidant response element; AS-IV, astragaloside IV; BDL, bile
duct ligation; BLM, bleomycin; cAMP, cyclic adenosine
monophosphate; CCL2, C-C motif chemokine ligand 2; CKD, chronic
kidney disease; COPD, chronic obstructive pulmonary disease; CT-1,
cardiotrophin-1; CTGF, connective tissue growth factor; CVB3,
coxsackievirus B3; DKD, diabetic kidney disease; ECM, extracellular
matrix; FNDC5, fibronectin type III domain-containing protein 5;
FOXO, forkhead box O; GSDMD-N, gasdermin D N-terminal domain;
GSH-Px, glutathione peroxidase; GSK-3β, glycogen synthase
kinase-3β; HCC, hepatocellular carcinoma; HG, high glucose; HIF1α,
hypoxia-inducible factor 1α; HMGB1, high mobility group box-1;
HO-1, heme oxygenase-1; IL-6, interleukin-6; ISO, isoproterenol;
IκBα, inhibitor of NF-kappa B α; K. pneumoniae,
Klebsiella pneumoniae; LC3 II, light chain 3 II; LDH,
lactate dehydrogenase; LPS, lipopolysaccharide; MAPK,
mitogen-activated protein kinase; MI, myocardial infarction;
miR-21, microRNA-21; mTOR, mammalian target of rapamycin; NF-κB,
nuclear factor-kappa B; NLRP3, nucleotide-binding oligomerization
domain-like receptor thermal protein domain associated protein 3;
NOX4, NADPH oxidase 4; NQO1, NAD(P)H:quinone oxidoreductase 1;
Nrf2, nuclear factor erythroid 2-related factor 2; PAH, pulmonary
artery hypertension; PAI-1, plasminogen activator inhibitor-1;
PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1
α; PHD2, prolyl-4-hydroxylase 2; PI3K,
phosphatidylinositol-3-kinase; PICP, procollagen type I
carboxy-terminal propeptide; PINP, procollagen type I N-terminal
propeptide; PKA, protein kinase A; PS, porcine-serum; pSmad3C,
COOH-terminal phosphorylation of Smad3; pSmad3L, phosphorylation of
the linker region of Smad3; PTEN, phosphatase and tensin homolog;
RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma; ROS,
reactive oxygen species; SIRT1, sirtuin 1; SOD, superoxide
dismutase; T2DM, type 2 diabetes mellitus; T-AOC, total antioxidant
capacity; TGF-β1, transforming growth factor-β1; TLR4, toll-like
receptor 4; TM, trabecular meshwork; TNF-α, tumor necrosis
factor-α; TRPM7, transient receptor potential cation channel,
subfamily M, member 7; UIRI, unilateral ischemia-reperfusion
injury; UUO, unilateral ureteral obstruction. |
Overview of AS-IV
Biological characteristics of AS-IV
Astragalus, a member of the leguminous
family, is a perennial herb that is mostly produced in temperate
and subtropical regions (12).
It has been used as medicine for >2,000 years (13). More than 200 compounds, such as
flavonoids, polysaccharides, triterpene saponins and certain trace
elements, are found in Astragalus (14,15). Furthermore, in addition to being
a type of TCM with tonic properties, Astragalus contains
various antifibrotic pharmacological ingredients, including
calycosin, AS-IV, polysaccharides and formononetin (16). Astragalus encompasses
>2,000 species and different Astragalus species often
have varying pharmacological components. This situation leads to
variances in pharmacokinetics in mice administered
Astragalus from different sources (16,17). Current pharmacokinetic research
on Astragalus is limited because of seasonal diversity,
growth location and planting years (16).
Astragalosides include various components, such as
astragalosides I, II and IV. Among these natural compounds,
high-purity AS-IV extracted from Astragalus has the
strongest pharmacological activity (18). It can also be used as a
representative marker to quantify the quality of Astragalus
(19). Astragaloside undergoes
mutual transformation during oral and intestinal digestion. This
transformation is mainly reflected in the changes in AS-IV content.
After the acetyl group is hydrolyzed, astragaloside II can undergo
biotransformation, resulting in a corresponding increase in the
amount of AS-IV during oral digestion. AS-IV content is stable
during gastric digestion. During intestinal digestion, the contents
of the three family members of astragaloside increase, indicating
that other cycloartane-type triterpenoid saponins in
Astragalus have been transformed into AS-IV. Moreover, this
phenomenon reveals that transacetylation is involved in the
quantitative change in astragaloside during digestion (20).
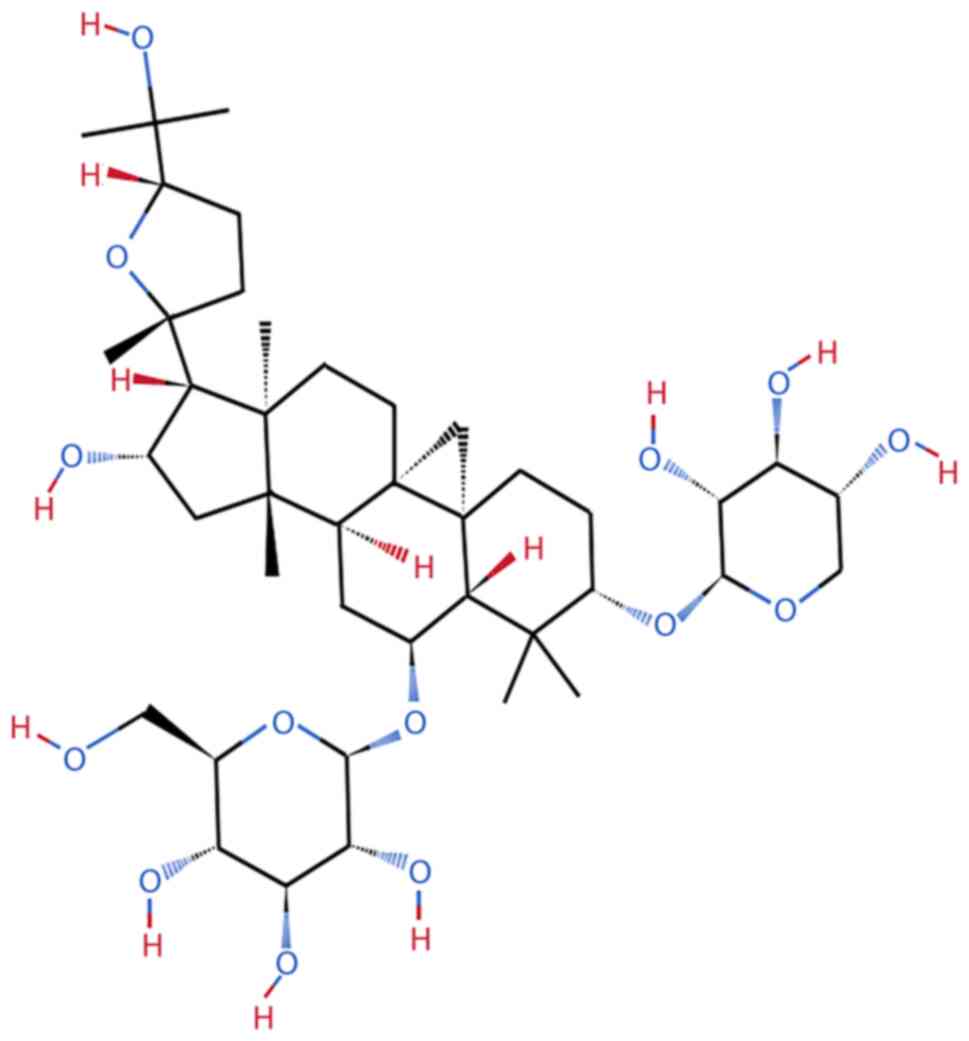
AS-IV is a highly polar lanolin alcohol-shaped
tetracyclic triterpenoid saponin; it has poor hydrophilicity, the
molecular formula of C41H68O14 and
molecular weight of 784.97 Da and is easily soluble in ethanol,
methanol, or acetone (21,22) (Fig. 2). The extraction and separation
of AS-IV through ultrafiltration, high-speed centrifugation and
ultrasonic extraction can reduce the effect of the low water
solubility of AS-IV and yield high contents of AS-IV with a
shortened production cycle and reduced loss. This also indicates
that the innovation of drug extraction and separation technology
facilitates the gradual identification of various saponin
components of Astragalus via gas chromatography-mass
spectrometry and high-performance liquid chromatography. AS-IV,
which has been approved as a medicine and food homologous bioactive
product in China, has become increasingly popular in the clinical
and food markets (23-25). It demonstrates considerable
pharmacological benefits in improving hypertension (26), diabetes (27), infectious diseases (28), viral infections (29), ischemia-reperfusion injury
(30) and anxiety (31), as well as anti-inflammation
(32), antioxidative stress
(33), antiperspirant and
antidiarrheal (34) effects.
Cycloastragenol (CA) is an aglycone and metabolite of AS-IV. The
use of AS-IV and CA in the pharmaceutical market as telomerase
activators indicates that AS-IV could also potentially be a viable
antiaging medication (24).
Toxicity of AS-IV
The median lethal dose of Astragalus after
acute oral administration to rats was found to exceed 250 g/kg body
weight and no toxic reactions occurred after the oral
administration of Astragalus for 90 days at a dose of 15
g/kg body weight (16).
Furthermore, no adverse reactions were observed after the
intraperitoneal injection or intravenous administration of extracts
containing Astragalus saponins and polysaccharides to rats
and beagle dogs for three months. The safe dose ranges for rats and
beagle dogs are 5.7-39.9 and 2.85-19.95 g/kg, respectively, which
are 70 or 35 times that for humans (0.57 g/kg for an average weight
of 70 kg) (35). AS-IV does not
cause obvious symptoms or organ toxicity when used in clinical
treatment and the oral administration of the conventional doses of
AS-IV has no side effects on liver and kidney function (36). Notably, in Sprague-Dawley rats
and New Zealand rabbits, the intravenous administration of 0.5-1.0
mg/kg AS-IV during pregnancy can produce certain toxic effects on
the mother and fetus but does not cause fetal malformations
(37). Further studies have
shown that in rats, the administration of 1.0 mg/kg AS-IV for up to
four weeks during gestation can induce fur formation, eye opening
and neurodevelopmental lag in newborn rats but does not affect the
cognitive function of newborn rats (38). Furthermore, the intravenous
administration of 0.25-1.0 mg/kg AS-IV is detrimental to the
reproductive ability of rats. Administering AS-IV at a dosage of
1.0 mg/kg/day to treat cardiovascular disease (CVD) during
pregnancy may also induce side effects (37,38). Therefore, although AS-IV poses
almost no toxicity risk to the general population, attention should
be paid to the safe dose range of AS-IV during pregnancy and AS-IV
should be taken with caution.
Compared with the oral administration of single
drugs, the intravenous administration of AS-IV preparations often
exhibits more remarkable bioavailability. In a test population,
after the intravenous injection of 200-500 ml of astragaloside
containing AS-IV for one week, only mild symptoms, such as
transient episodes of elevated total bilirubin and rashes, appeared
and subsided spontaneously (39). In addition, various organs or
tissues of the body show good tolerance to astragaloside injection,
with the highest tolerated dose reaching 600 ml. Even if
astragaloside is intravenously infused at a rate of up to 4 ml/min,
no remarkable adverse reactions in humans occurred (39).
Antifibrotic effects of AS-IV
Cardiac fibrosis
The occurrence of cardiac fibrosis is closely
related to various CVDs, including chronic heart failure, atrial
fibrillation, cardiac remodeling after acute myocardial infarction
(MI), valvular heart disease, hypertension and ischemic injury
(40). Between 60-70% of human
heart cells are in the form of fibroblasts, which
over-differentiate into myofibroblasts when the heart is stimulated
by proinflammatory factors or ROS. These myofibroblasts are a major
source of ECM deposited in the cardiac interstitium. They can
further induce cardiac contractile and diastolic dysfunction and
trigger pathological cardiac remodeling (41,42). ECM in the cardiac interstitium is
mainly composed of type I collagen (collagen I). Myocardial
fibrosis (MF) may lead to electrical heterogeneity and destroy
cardiac ejection function. Severe cardiac fibrosis even predisposes
to irreversible cardiovascular lesions, such as excessive
arrhythmias and sudden cardiac death (43). Given that cardiovascular events
have gradually become the main cause of global mortality and that
fatal events related to CVDs once accounted for >40% of deaths
in China, active intervention focusing on cardiac fibrosis has
become a key strategy for promoting the development of human health
(44). Anticoagulation,
antiplatelet, thrombolysis and myocardial reperfusion strategies
are widely used to reduce the area of MI and are the main medical
means to relieve MI. However, they are often accompanied with side
effects. For example, they indirectly lead to the aggravation of
the extent of myocardial injury. Therefore, the application of
high-efficiency drugs with low myocardial toxicity can help achieve
ideal prognostic effects. Among these drugs, AS-IV is a derivative
of Chinese herbal medicine that not only lacks organ toxicity when
administered at regular doses but can also achieve considerable
therapeutic benefits in anticardiac fibrosis.
Inflammation
The nucleotide-binding oligomerization domain-like
receptor thermal protein domain associated protein 3 (NLRP3)
inflammasome acts as a multiprotein complex that concurrently
facilitates the onset of inflammation and cardiac fibrosis
(45). The activated NLRP3
inflammasome not only maintains myocardial interstitial
inflammatory infiltration, but its induced proinflammatory factors,
such as interleukin (IL)-18 and IL-1β, also activate profibrotic
growth factors and promote the deposition of collagen and
development of a profibrotic population of fibroblasts (46). AS-IV administration markedly
inhibits MF induced by isoproterenol (ISO) treatment. This effect
may be associated with a decrease in the expression of caspase-1
and IL-18 in cardiac tissue caused by AS-IV through the targeted
inhibition of NLRP3 inflammasome activation (41). In addition, ventricular
remodeling phenomena, such as myocardial hypertrophy, fibrosis and
cardiomyocyte apoptosis, are closely related to the development of
end-stage CVDs. The C-C motif chemokine ligand 2 (CCL2) gene can
regulate the expression of inflammatory factors, such as the
proinflammatory factor IL-6, enriched in the nuclear factor-κ B
(NF-κB) signaling pathway. Therefore, CCL2 is defined as a
mechanistic target of AS-IV for antilipopolysaccharide-induced
inflammation in H9C2 cardiomyoblasts. This relationship can be
attributed to the ability of CCL2 inactivation to regulate
negatively the lipopolysaccharide (LPS)-activated NF-κB signaling
pathway and the high expression of its target genes IL-17 and tumor
necrosis factor-α (TNF-α). Moreover, CCL2 may be an effector
molecule for MF and the overexpression of fibrosis-related genes
(proliferating cell nuclear antigen, collagen I and collagen III)
induced by LPS is favorably connected with CCL2 activity. AS-IV can
also serve as a potential CCL2 inhibitor to improve LPS-induced
myocardial remodeling. Furthermore, AS-IV blocks NF-κB from
entering the cytosol and hinders the inactivated inhibitor of NF-κB
(IκB) to drive LPS-induced NF-κB p65 overexpression, thereby
effectively inhibiting cardiac hypertrophy and the fibrosis of
LPS-induced H9C2 cells (47).
Oxidative stress
Evidence indicate that cardiotrophin-1 (CT-1), as a
key mediator of cardiac remodeling, is involved in cardiac
fibroblast (CF) and vascular smooth muscle cell proliferation,
collagen I deposition and ECM synthesis to a certain extent, with
CT-1 being closely related to cardiomyocyte survival and cardiac
hypertrophy (48,49). Notably, driving CT-1
overexpression is related to the release of ROS and treatment with
AS-IV and the ROS inhibitor N-acetylcysteine targets the
production of ROS in CFs, inhibits ISO-induced CF proliferation and
CT-1 overexpression and reduces collagen I deposition in
cardiomyocytes. These effects indicate that inhibiting the driving
effect of ROS on CT-1 expression is a potential mechanism of the
anti-MF effect of AS-IV. However, whether mitochondrial NADPH
oxidase 4, mitochondrial superoxide dismutase and mitochondrial
catalase contribute to the negative regulation of oxidative stress
in myocardial tissue by AS-IV remains inconclusive (50). ISO is a typical inducer of
cardiac fibrosis and its profibrotic mechanism is intimately linked
to the generation of ROS and activation of c-Jun N-terminal kinase
(JNK), p38 mitogen-activated protein kinase (MAPK) and
extracellular signal-regulated kinase (ERK) (51,52). Notably, Dai et al
(53) found that AS-IV can
prevent profibrotic MAPK family members from undergoing
phosphorylation and that the ROS inhibitor N-acetylcysteine
mimics AS-IV's suppressive effect on MAPK activation. That is,
their study finally demonstrated that AS-IV may exert an
anticardiac fibrotic benefit by downregulating oxidative
stress-mediated MAPK phosphorylation through the targeted
inhibition of ISO-induced ROS production. Fibronectin type III
domain-containing protein 5 (FNDC5) is highly active in the brain
and heart and crosstalk has been found between the sirtuin 1
(SIRT1)/peroxisome proliferator-activated receptor γ coactivator 1
α (PGC-1α)/FNDC5 signaling cascade and improvement in diabetic MF
(54). Cong et al found
that in mice, AS-IV activates the SIRT1/PGC-1α/FNDC5 signaling
pathway, therefore effectively inhibiting atrial fibrosis, atrial
fibrillation and oxidative stress induced by angiotensin II
(55).
Lipid metabolism
Although the heart is not a typical organ for lipid
storage, a pathological increase in serum lipid levels causes fatty
acids to enter myocardial cells and some fatty acids are forced to
be stored in myocardial cells in the form of triglycerides. When
lipid deposition reaches a certain extent, the production of
lipid-toxic intermediates, such as ceramides, diacylglycerols and
ROS, become an important mechanism mediating myocardial
inflammation and fibrosis in patients with diabetes and obesity
(56). AS-IV not only helps
alleviate cardiac lipid deposition by regulating plasma
triglyceride, total cholesterol, high-density lipoprotein and
myocardial free fatty acid (FFA) contents but also inhibits type 2
diabetes mellitus (T2DM)-related myocardial damage induced by
increased serum creatine kinase isoenzyme and lactate dehydrogenase
(LDH) levels and suppresses the driving effect of inflammatory
infiltration on MF. AS-IV mainly exerts these effects by targeting
the downregulation of TNF-α expression and alleviating the
aggravating effect of TNF-α-activated inflammatory cytokines,
including IL-1β and IL-6, on MF. It thereby simultaneously inhibits
the proinflammatory and pro-MF effects of TNF-α because TNF-α not
only originates from the heart but can also be targeted to change
the pathophysiology of the heart (57). Therefore, AS-IV can inhibit
inflammatory infiltration and fibrosis-induced myocardial damage.
This inhibitory effect may be associated with mitigating the toxic
damage of lipid deposition on the myocardium (57).
Pyroptosis
Pyroptosis is a form of programmed cell death that
is intimately associated with the initiation of proinflammatory
responses mediated by caspase-1 (58). However, the cleavage of
pro-caspase-1 into active caspase-1 can be inhibited through the
targeted inhibition of NLRP3 inflammasome activation, further
blocking the transformation of the cytokine precursors pro-IL-1β
and pro-IL-18 into bioactive IL-1β and IL-18, respectively.
Therefore, this process is beneficial to relieve cardiac
dysfunction caused by cardiomyocyte pyroptosis and inflammatory
infiltration after MI. Zhang et al (59) clarified the upstream and
downstream relationships between oxidative stress and cardiomyocyte
pyroptosis. That is, AS-IV can inhibit MI-induced ROS release,
thereby alleviating NLRP3 inflammasome activation and cardiomyocyte
pyroptosis. This effect is achieved mainly through the suppression
of the levels of NLRP3, cleaved caspase-1, cleaved IL-1β and
cleaved IL-18 and the pyroptosis-related protein gasdermin D
N-terminal domain (GSDMD-N) induced by MI. Furthermore, α-smooth
muscle actin (α-SMA) and FN overexpression, as well as collagen I
and III deposition, can be suppressed by AS-IV. AS-IV also
decreases the number of apoptotic cardiomyocytes and compensatory
hypertrophy of cardiomyocytes to improve cardiac function. AS-IV
has been found to downregulate the ROS/caspase-1/GSDMD signaling
pathway, thereby attenuating MF and cardiac remodeling induced by
MI. This mechanism has also been demonstrated at the cellular
level. That is, AS-IV may exert an anti-inflammatory effect by
inhibiting macrophage pyroptosis in vitro (59).
Aging
The senescence-associated secretory phenotype (SASP)
continuously drives inflammatory infiltration and ECM synthesis and
progressively aggravates collagen deposition and fibrosis by
inducing the release of transforming growth factor-β1 (TGF-β1),
matrix metalloproteinases (MMPs) and the inflammatory cytokines
TNF-α and IL-1β. Therefore, selectively regulating the release of
SASP components effectively interferes with MF (60,61). Shi et al (62) detected a high correlation between
cellular senescence and p53 signaling pathway-related genes and
proteins through transcriptomics and proteomics analysis and found
that the activity of the aging-related indicators p16, p21 and p53
of this pathway are markedly inhibited by AS-IV treatment in the
ISO-induced MF group. Given that the senescence of CFs and release
of SASP are triggered by oxidative stress and SASP secreted by
senescent cells also promotes the oxidative stress effect again,
these phenomena form a vicious cycle and induce a progressive
exacerbation of the degree of MF. Therefore, the mechanism by which
AS-IV inhibits MF may be attributed to regulatory effects on
oxidative stress and the release of aging markers (62-64).
Collagen deposition
Coxsackievirus B3 (CVB3) not only serves as a human
pathogen that induces acute and chronic viral myocarditis in
adolescents but is also an important inducer of dilated
cardiomyopathy (DCM) with MF (65). The synthesis and degradation of
collagen I in cardiomyocytes depends on serum procollagen type I
N-terminal propeptide (PINP) and procollagen type I
carboxy-terminal propeptide (PICP) concentrations. AS-IV inhibits
collagen deposition and TGF-β1 expression in the cardiomyocytes of
a DCM model after CVB3 infection. Notably, although collagens I and
III are substantially deposited in patients with DCM, only the
content of collagen I in the myocardial tissue of DCM is markedly
decreased by AS-IV. This effect is consistent with the
downregulation of the PICP:PINP ratio and serum PICP concentration,
both of which represent the excessive synthesis of collagen I in
the myocardium. It also indirectly proves that AS-IV can exert
considerable resistance against fibrosis induced by ISO and CVB3
regardless of whether cardiac fibrosis is induced by the
β-adrenergic receptor or not (65).
Gut microbiota
Various gut microbiota and fecal metabolites
establish metabolic communication with the host or the intestinal
microenvironment, thus forming metabolic crosstalk with cardiac
fibrosis (66). In mammals,
intestinal Akkermansia abundance plays a vital part in
negatively regulating the development of metabolic syndrome and
CVDs, such as atherosclerosis. In addition, intestinal
Akkermansia can intervene in cardiovascular events by
regulating the levels of fecal metabolites. Fecal metabolites, such
as hydroxyprolyl-leucine, valyl-isoleucine and
nepsilon-acetyl-L-lysine, are positively associated with the
abundance of Akkermansia. Akkermansia negatively
regulates phenylacetylglycine content. This phenomenon may be
related to the increased risk of macrovascular disease because of
the upregulation of phenylalanine, which is the hydrolysis product
of phenylacetylglycine, in plasma (67,68). Studies have found that AS-IV can
lessen the severity of ISO-induced cardiac fibrosis by increasing
the abundance of the gut microorganism Akkermansia, reducing
phenylalanine levels and increasing leucine and lysine levels
(68).
TRPM7
Endogenous microRNAs (miRNAs/miRs) are a perennial
research hotspot in the field of fibrotic disorders because of
their involvement in regulating the Smad3-mediated TGF-β/Smads
pathway. Transient receptor potential cation channel, subfamily M,
member 7 (TRPM7) participates in inducing the proliferation and
differentiation of fibroblasts and promoting ECM synthesis and
collagen deposition. Among miRNAs, miR-135a is an upstream mediator
that regulates the expression of the profibrotic factor TRPM7.
AS-IV may alleviate cardiac fibrosis through the targeted
intervention of miR-135a expression (42). Wei et al (42) revealed that AS-IV can inhibit the
downregulation of miR-135a expression in ISO-treated rats and
promote the negative regulation of TRPM7 by miR-135a and the
inhibition of TRPM7 activity further promotes the inactivation of
the TGF-β/Smads pathway. Furthermore, AS-IV treatment leads to a
reduction in the expression levels of TGF-β1 and phosphorylated
(p-)Smad3 while promoting the activation of Smad7 and intervention
in rat neonatal CFs also shows a similar antifibrotic effect,
indicating that the key mechanism for AS-IV to alleviate cardiac
fibrosis may be the regulation of the miR-135a-TRPM7-TGF-β/Smads
axis. Notably, TRPM7 channels can also regulate Ca2+
input in fibroblasts lacking voltage-gated calcium channels.
Therefore, the mechanism through which AS-IV exerts its anti-MF
effect may also involve the suppression of TRPM7 channel activity
in CFs under hypoxic conditions, as well as downregulation in the
ion-channel currents of fibroblasts (43) (Fig. 3).
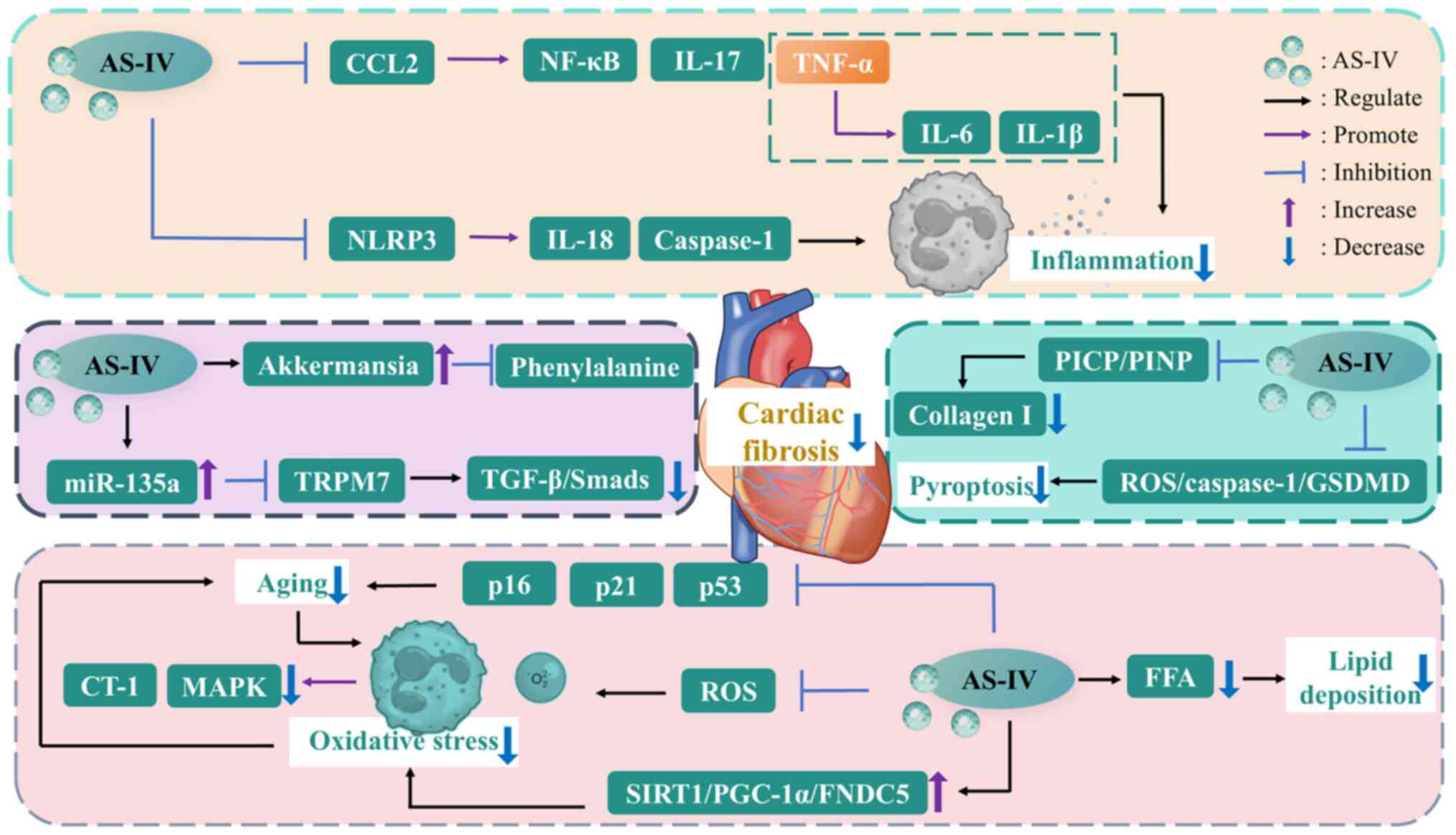 | Figure 3The mechanisms of AS-IV against
cardiac fibrosis. AS-IV, astragaloside IV; CCL2, C-C motif
chemokine ligand 2; collagen I, type I collagen; CT-1,
cardiotrophin-1; FFA, free fatty acid; FNDC5, fibronectin type III
domain-containing protein 5; GSDMD, gasdermin D; IL-17,
interleukin-17; MAPK, mitogen-activated protein kinase; NF-κB,
nuclear factor-kappa B; NLRP3, nucleotide-binding oligomerization
domain-like receptor thermal protein domain associated protein 3;
PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1
α; PICP, procollagen type I carboxy-terminal propeptide; PINP,
procollagen type I N-terminal propeptide; ROS, reactive oxygen
species; SIRT1, sirtuin 1; TGF-β, transforming growth factor-β;
TNF-α, tumor necrosis factor-α; TRPM7, transient receptor potential
cation channel, subfamily M, member 7. |
Notably, most of the existing studies on the
anticardiac fibrosis effect of AS-IV are based on in vitro
and in vivo models with remarkable differences in
pathophysiology from human cardiac fibrosis. Clinical research in
this area is limited and the role of AS-IV in ischemic or
nonischemic cardiac fibrosis remains unclear. Furthermore, although
AS-IV is relatively safe, whether its coadministration with other
drugs for CVD treatment leads to drug interactions and whether the
risks of such drug interactions increase the liver and kidney
burden of patients with other comorbidities and trigger potential
toxicity are unknown. Further studies, by incorporating the
detection of indicators, such as ejection fraction and cardiac
systolic or diastolic function, enrich the research value of
AS-IV's anticardiac fibrosis effect. In addition, no research data
on the targeting and accumulation of AS-IV in cardiac tissue exist.
Therefore, evaluating the effective anticardiac fibrosis
therapeutic dose, administration frequency and treatment course of
AS-IV is difficult and the universality and clinical applicability
of research results still need verification.
Pulmonary fibrosis
Diverse heterogeneous interstitial lung diseases can
eventually develop into PF (69). PF can lead to the deposition of
scar tissue in the lung parenchyma, impaired gas exchange function
and even respiratory failure. IPF is also considered a chronic,
fatal interstitial pneumonia with complex causes (70). Smoking, dust inhalation, drug
use, gastroesophageal reflux, genetic factors, viral infection and
autoimmunity are listed as clear PF-related risk factors (71). In the early stage of fibrosis,
the lungs are dominated by chronic inflammation, tissue edema and
congestion. This stage is followed by damage to alveolar epithelial
cells; the massive migration and proliferation of mesenchymal
cells; and the degradation of ECM, including collagen, laminin and
tenascin-C. The continuously deposited ECM promotes scar tissue
hyperplasia, eventually causing a progressive decline in lung
function (72). The typical
pathological features of PF include the persistent inflammatory
responses and structural remodeling of the airways triggered by the
gradual decline in lung function (73). Although glucocorticoids are
mainly used to treat PF, they cannot achieve the expected clinical
efficacy because of their certain side effects on the body
(74). Pirfenidone and
nintedanib, which target the inhibition of inflammasome and
tyrosine kinase activities, respectively, have been approved for
use in the global medical market and are mainly employed to improve
symptoms of dyspnea and lung function decline related to IPF
(75). Although the application
of nanomaterials in the development of new antifibrotic drugs has
begun to receive focus, paying attention to natural Chinese herbal
resources against PF remains necessary (76).
Inflammation and oxidative stress
Exposing the body to silica dust particles for a
long time can induce the diffuse nodular fibrosis of the lungs and
chronic pulmonary inflammation involving alveolar macrophages,
alveolar epithelial cells, fibroblasts, lymphocytes and other
mediators. This condition, which is a typical pathological
manifestation of silicosis, further promotes lung function decline,
dyspnea and even death (77). In
addition, the long-term stimulation of alveolar macrophages by
silica particles drives the release of ROS and oxidative stress can
further exacerbate inflammatory effects in the feed-forward cycle.
AS-IV inhibits silicosis-related inflammation and oxidative stress
by downregulating the expression of inflammatory factors, such as
TNF-α, IL-1β and IL-6 and upregulating the activities of the
antioxidant enzymes SOD and glutathione peroxidase (GSH-Px). This
effect may stem from the ability of AS-IV to block the TGF-β1/Smads
signaling pathway, thereby alleviating silicosis-related fibrosis
(77). In summary, AS-IV
negatively regulates fibroblast fibrosis, as primarily indicated by
the remarkable promotion of the dephosphorylation of Smad2 and
Smad3 in fibroblasts and restoration of Smad7 activity, indicating
that AS-IV mediates positive feedback inhibition and the negative
feedback enhancement of the TGF-β1/Smads signaling pathway
(77,78). As aforementioned, NLRP3 is a
multiprotein complex involved in fibrosis-related inflammatory
responses. Hou et al (79) found that AS-IV administration
could promote the inactivation of NLRP3 and the fibrosis-related
effector proteins collagen I, collagen II and α-SMA in
TGF-β-induced PF, thereby alleviating epithelial-mesenchymal
transition (EMT). Furthermore, AS-IV dose-dependently inhibits
pulmonary inflammatory infiltration and fibrosis induced by
bleomycin (BLM) injection. This effect is associated with the
downregulation of total cell, neutrophil, macrophage and lymphocyte
counts in bronchoalveolar lavage fluid targeted by AS-IV (80). AS-IV can also inhibit increased
levels of malondialdehyde (MDA) and ROS, as well as promotes the
activity of the endogenous antioxidant indicator SOD and the total
antioxidant capacity (T-AOC), thereby exerting considerable
pharmacological benefits in alleviating BLM-induced PF-related
oxidative stress (80).
Klebsiella pneumoniae (K. pneumoniae),
a Gram-negative capsulated bacterium, is known to induce pneumonia
(81). Li et al (82) found that AS-IV administration can
target the downregulation of p-Smad2/Smad2, p-Smad3/Smad3 and
p-IκBα/IκBα levels induced by K. pneumoniae. This finding
suggests that the mechanism by which AS-IV downregulates the NF-κB
inflammatory signaling pathway may be attributed to the negative
regulation of the TGF-β1/Smad pathway. However, although the study
demonstrated AS-IV's mediating function on the Smad and NF-κB
pathways, it did not explore whether the quantity of lung
colony-forming units during K. pneumoniae infection changed
markedly with AS-IV intervention, suggesting that the effect of
AS-IV on bacterial load in lung tissue is unclear (82). AS-IV and ligustrazine extracted
from Astragalus and Ligusticum chuanxiong,
respectively, are natural Chinese herbal components with
antifibrotic activity (83,84). However, in view of their poor
hydrophilicity, the introduction of nanoparticles is beneficial to
improve their penetration into the mucosa. Therefore, AS-IV and LIG
have been coloaded onto inhalable nanoparticles (AS_LIG@PPGC NPs)
to achieve the maximum intervention for PF through noninvasive
pulmonary inhalation therapy (85). NPs are not easily degraded after
being inhaled by the body, can target lung tissue for more than 24
h and are heavily enriched in the right lower lobe and left lung.
In addition, they are diffusely distributed in lung tissue,
demonstrating that the use of polyethylene
glycol-polylactic-co-glycolic acid NPs (PPGC NPs) can improve the
ability of a single agent to target tissue (85). Zheng et al (85) found that the administration of
AS_LIG@ PPGC NPs inhibits the overexpression of the myofibroblast
marker α-SMA and fibrotic effector factor TGF-β1. Moreover, it
reduces the deposition of the ECM component collagen 1A1 and the
release of the inflammatory cytokines TNF-α, IL-6 and IL-1β. In
addition, the authors highlighted the therapeutic benefits of AS-IV
in mitigating the levels of pulmonary inflammation and fibrosis
from the perspective of integrated traditional Chinese and Western
medicine. These effects are mainly attributed to the ability of
AS-IV to regulate the release of NOX4-derived ROS negatively and
hinder ROS to drive the proinflammatory and profibrotic effects of
the activated NLRP3 inflammasome (85). Targeting the reduction of ROS
production can also prevent p38 MAPK from being activated and the
excessive release of ROS further aggravates the upregulation of
NOX4 activity. AS-IV's negative regulatory effect on the
NOX4/NLRP3/p38 MAPK axis is complicated by the closed-loop chain
formed among NOX4, ROS, the NLRP3 inflammasome and p38 MAPK.
Applying AS_LIG@PPGC NPs to intervene in PF can further compensate
for the limitations of treatment with a single TCM preparation
(85).
Pyroptosis
The progressive pulmonary artery structural
remodeling exhibited by pulmonary artery hypertension (PAH) induces
a surge in right ventricular pressure. Hypoxia is a key inducer of
pulmonary artery remodeling. The ability of mammalian cells to
adapt to hypoxic signal stimulation relies on the promotion of
oxygen transport and body metabolism by hypoxia-inducible factor 1α
(HIF1α) (86,87). Under normoxic conditions, HIF1α
hydroxylation and degradation depend on the catalytic effect of
prolyl-4-hydroxylase 2 (PHD2) and the downregulation of PHD2
activity is positively associated with pulmonary vascular
remodeling and PAH (88,89). Drugs, such as endothelin receptor
blockers and prostacyclin, are insufficient to decrease the 20-30%
mortality rate of patients with PAH. Xi et al (87) focused on natural herbal medicine
to treat PAH-related fibrosis and found that AS-IV can inhibit the
activity of the NLRP3 inflammasome. The authors also discovered
that AS-IV can diminish the release of the proinflammatory
cytokines IL-1β and IL-18 in the lung tissue of PAH rats; interfere
with the upregulation of MMPs/tissue inhibitor of metalloprotease 4
and the deposition of FN and collagen I induced by PAH and
negatively regulate the expression of the pyroptosis-related
markers GSDMD-N and cleaved caspase-1 under hypoxic conditions and
the activity of LDH in pulmonary artery smooth muscle cells
(PASMCs). Furthermore, AS-IV promotes an increase in PHD2
expression and concurrently suppresses the expression of HIF1α. In
short, NLRP3-mediated pyroptosis and fibrosis in PASMCs can be
markedly inhibited by AS-IV targeting PHD2/HIF1α signaling.
Aging
In network pharmacology analysis, Yuan et al
(90) selected PF-related
signaling pathways through the Kyoto Encyclopedia of Genes and
Genomes pathway enrichment analysis and used cytohubba to analyze
the protein-protein interaction network associated with AS-IV and
its PF targets. Given that the authors found that the top 10 key
target proteins were markedly enriched in the cellular senescence
pathways, they further used molecular docking to verify that the
cellular senescence marker proteins p53, p21 and p16 are potential
binding targets of AS-IV. By focusing on the mechanism of
PF-related cell aging, they found that AS-IV further reduces
oxidative stress-induced senescence by limiting ROS generation in
the BLM-induced PF mouse model and alveolar epithelial (A549)
cells. The metabolic imbalance in senescent cells induces
pathological changes in protein expression and SASP release.
However, AS-IV not only enhances the expression of the epithelial
protein marker E-cadherin while blocking the synthesis of the
mesenchymal protein marker vimentin but also suppresses the
expression levels of the aging markers p53, p21 and p16. Therefore,
alleviating EMT and cellular senescence is an anti-PF mechanism of
AS-IV (90).
Collagen deposition
High mobility group box-1 (HMGB1), in addition to
inducing inflammatory reactions, is an effector molecule related to
PF. This function is related to its promotion of the
transdifferentiation of fibroblasts and induction of EMT and ECM
protein deposition (91).
However, the extent of ECM protein deposition depends on the
activity of cells that synthesize ECM. Such activity can be
reflected by the expression of the myofibroblast marker α-SMA. ECM
proteins contain a variety of collagen components. Given that
hydroxyproline (HYP) is a primary ingredient of collagen, its
content may be positively associated with the progression of PF
(92). Li et al (92) revealed that AS-IV can target the
downregulation of α-SMA, HMGB1, HYP and collagen III in lung tissue
and further inhibit the synthesis of ECM components, including
laminin and hyaluronic acid. This effect is mediated by promoting
the inactivation of HMGB1, thereby effectively alleviating
BLM-induced PF.
Non-coding RNAs (lncRNAs)
Zinc finger E-box binding homeobox 1 (ZEB1) is
involved in the regulation of the transcription factor networks
linked to EMT and is a key inducer of PF (93). lncRNAs mediate the regulation of
miRNAs, among which miR-200c, a member of the miR-200 family, is
closely related to fibrotic effects, such as EMT, cell invasion and
proliferation (94,95). Through dual-luciferase reporter
gene experiments and bioinformatics, Guan et al (70) found that miR-200c has a
consistent binding site with lncRNA-activated by transforming
growth factor β (lncRNA-ATB) and that the downstream of miR-200c
can regulate the expression of ZEB1. These findings are consistent
with the fact that in patients with silicosis, overexpressed
lncRNA-ATB can interact with miR-200c and target ZEB1 expression
upregulation, thus driving EMT occurrence (70,96). However, in A549 cells used as an
IPF-associated EMT model, AS-IV was found to inhibit lncRNA-ATB by
acting as a competitive endogenous RNA to mediate the sponging of
miR-200c in IPF to target the expression of ZEB1. That is, AS-IV
negatively regulates the level of lncRNA-ATB, promotes the
overexpression of miR-200c and further downregulates the nuclear
level of ZEB1 in TGFβ1-induced EMT model cells, indicating that the
lncRNA-ATB/miR-200c/ZEB1 regulatory axis is a target of AS-IV to
alleviate IPF (70). Circular
RNAs, a member of the endogenous non-coding RNA family, can combine
with miRNA and serve as miRNA sponges to target changes in the
biological functions of downstream genes (97). circ_0008898 overexpression is
positively associated with PF progression. Zhu et al
(98) discovered that
TGF-β1-induced circ_0008898 overexpression in human fetal lung
fibroblast 1 (HFL1) cells could be negatively regulated by AS-IV
(98,99). TGF-β1 can also induce a reduction
in miR-211-5p expression in HFL1 cells. That is, miR-211-5p may
serve as a potential binding target of circ_0008898 given that
miRNAs can target changes in mRNA expression and a rise in
miR-211-5p expression is strongly connected to the inactivation of
HMGB1 in HFL1 cells. HMGB1 has been further confirmed to be a
downstream regulator of miR-211-5p through bioinformatics
prediction and experimental validation (98). Notably, HMGB1 overexpression in
TGF-β1-treated HFL1 cells has a crucial role in driving the
advancement of PF. In summary, AS-IV may rely on the
circ_0008898/miR-211-5p/HMGB1 axis as a key signaling target to
inhibit PF progression in TGF-β1-treated HFL1 cells (98).
The application of Danggui Buxue Decoction (DBT) as
an antifibrotic supplement mainly relies on the pharmacological
activities of AS-IV and ferulic acid (FA) (100). In addition, miRNA can pair with
mRNA and mediate changes in protein expression through the
transcription pathway. miR-29 can inhibit collagen fiber deposition
by targeting various cytokines. For example, the 3'-UTR of the
target gene TGF-β1 is one of its main sites of action. Tong et
al (101) found that on the
basis of the antifibrotic properties of miR-29, AS-IV + FA
(AF) can use miR-29b as a drug target to induce Smad3
dephosphorylation further and alleviate oxidative stress and PF by
triggering nuclear factor erythroid 2-related factor 2 (Nrf2)
activation and interfering with TGF-β1/Smad3 signaling. This
finding also indirectly shows that the antifibrotic effect of
supplementation with combinations of Chinese herbal medicine
preparations may be improved than that of supplementation with
single drugs. Autophagy deficiency is a key cause of IPF. Its
profibrotic mechanism mainly stems from fibrotic effects, such as
inducing fibroblast transdifferentiation and ECM deposition
(102). According to Li et
al (103), AS-IV could
prevent TGF-β1-treated A549 cells from showing a reduction in light
chain 3B fluorescence signal intensity, indicating that AS-IV may
function as an autophagy agonist. Although some studies have found
that TGF-β1 treatment may motivate fibroblasts to overexpress
miR-21 and overactivated miR-21 further exacerbates the induction
effect of TGF-β1 on PF, AS-IV can inhibit the pathological
upregulation of miR-21 expression. This effect is mainly attributed
to the fact that the antifibrotic effect of AS-IV is reversibly
mediated by miR-21 agonists (103,104). Bioinformatics analysis provides
evidence that phosphatase and tensin homolog (PTEN) is one of the
binding targets of miR-21 (103). Given that PTEN, a molecular
inhibitor of phosphatidylinositol-3-kinase (PI3K), can negatively
regulate PI3K/protein kinase B (AKT)/mammalian target of rapamycin
(mTOR) signaling through downstream effects and mTOR is an effector
molecule that drives autophagy blockage, in-depth research has
confirmed that AS-IV can negatively regulate PI3K/AKT/mTOR
signaling by targeting the upregulation of PTEN activity, thereby
promoting the inhibitory effect of autophagy on IPF (103,105,106).
PM2.5 is a fine particulate matter involved in the
formation of haze. PM2.5 suspended in the air can adsorb
microorganisms and directly enter alveoli, markedly downregulating
the activity of the anti-inflammatory factor miR-362-3p in alveolar
epithelial cells and promoting the overexpression of the downstream
target runt-related transcription factor 1 (RUNX1) of miR-362-3p,
in which RUNX1 induces the transdifferentiation of fibroblasts into
myofibroblasts, driving the occurrence of respiratory diseases,
such as PF (107,108). AS-IV targets the overexpression
of miR-362-3p and negatively regulates the activity of its
downstream transcription factor RUNX1 in rat lung tissue and
alveolar epithelial cells to alleviate the inhibitory effect of
PM2.5 on the proliferation of rat alveolar epithelial cells L2 and
its driving effect on LDH release. In addition, AS-IV suppresses
PM2.5-induced alveolar wall thickening and collagen III deposition
in lung tissue. Furthermore, it reverses the apoptosis rate of rat
lung tissue and L2 cells; the secretion of the proinflammatory
factors TNF-α, IL-1β and IL-6; and the overactivation of cleaved
caspase-3, p-p65 and α-SMA, thereby inhibiting PM2.5-driven PF
(108). Therefore, AS-IV may be
a natural therapeutic agent for alleviating air pollution-related
PF by regulating the miR-362-3p/RUNX1 signaling cascade.
Forkhead box O3a (FOXO3a)
FOXO3a is extensively expressed in the majority of
bodily tissues and cells. TGF-β1 is an upstream factor that
activates the PI3K/AKT signaling pathway and promotes AKT
phosphorylation, thereby markedly downregulating the activity of
FOXO transcription factors. FOXO3a inactivation is an important
factor for promoting fibroblast collagen deposition and α-SMA
overexpression, which further induces the occurrence of IPF
(109,110). However, AS-IV regulates the
inactivation of the PI3K/AKT pathway by targeting TGF-β1, further
blocking the hyperphosphorylation and inactivation of FOXO3a,
downregulating EMT and the expression of α-SMA and increasing the
levels of the epithelial cell marker E-cadherin, thus resulting in
considerable resistance to BLM-induced IPF (84). Smoking has been confirmed to a
key risk factor for inducing PF. Chronic obstructive pulmonary
disease (COPD) is often triggered by excessive smoking and
accompanied with persistent respiratory inflammatory infiltration,
which further increases the risk of PF (111). Given that rat sarcoma (RAS) can
regulate the downstream expression of the fibrotic effector
molecule FOXO3a, RAS activity and EMT development have been
demonstrated to be markedly positively associated (112). Through multiple protein and
small-molecule interaction experiments and amino acid site mutation
approaches, Zhang et al (113) found that RAS is a binding
target of AS-IV. AS-IV can inhibit RAS expression to induce the
dephosphorylation of c-Raf338 and FOXO3a. Therefore, by
targeting the expression of the non-Smad signaling pathway
RAS/rapidly accelerated fibrosarcoma (RAF)/FOXO signaling cascade
and its downstream inflammatory factors TNF-α and IL-1β, AS-IV
markedly inhibits the transdifferentiation of fibroblasts and
alleviates LPS and cigarette smoke treatment-induced COPD-related
EMT progression.
Advanced glycation end-product
(AGE)-receptor for AGE (RAGE)
Patients with T2DM can be complicated with diabetic
PF (114). In view of the
epidemiological data showing that T2DM and its complications have
become the cause of death of ~6.7 million individuals, focusing on
the application of Bu Yang Huan Wu Decoction, which has the effect
of reducing blood sugar and blood lipids, in PF intervention
(115) is essential. Elevated
blood glucose concentration induces the nonenzymatic glycosylation
of proteins, such as collagen and elastin, thereby promoting the
production of AGEs. RAGE, which is found to be widely expressed in
lung tissue, can act as an AGE inhibitor (115). Through network pharmacology
analysis, Guo et al (115) discovered that regulating the
AGE-RAGE signaling cascade and the gene expression of its
downstream effector molecules is the key mechanism through which
bioactive ingredients, such as AS-IV, in Bu Yang Huan Wu Decoction
exert anti-PF effects. Hydrogen bonding is a key parameter
indicating the binding degree of a protein to a ligand. Evidence
from molecular docking and dynamics simulations supports that the
complex system formed by the downstream target gene IL-6 of the
AGE-RAGE signaling pathway and AS-IV has certain stability likely
because IL-6-AS-IV binds to an average of three hydrogen bonds.
This phenomenon indicates that the binding affinity between the two
is strong and also implies that AS-IV can target AGE-RAGE signaling
and negatively regulate the induction of collagen deposition and
ECM synthesis in lung tissue by IL-6, the downstream fibrotic
effector molecule of the AGE-RAGE signaling pathway. That is, AS-IV
can markedly inhibit high glucose (HG)-induced PF (Fig. 4).
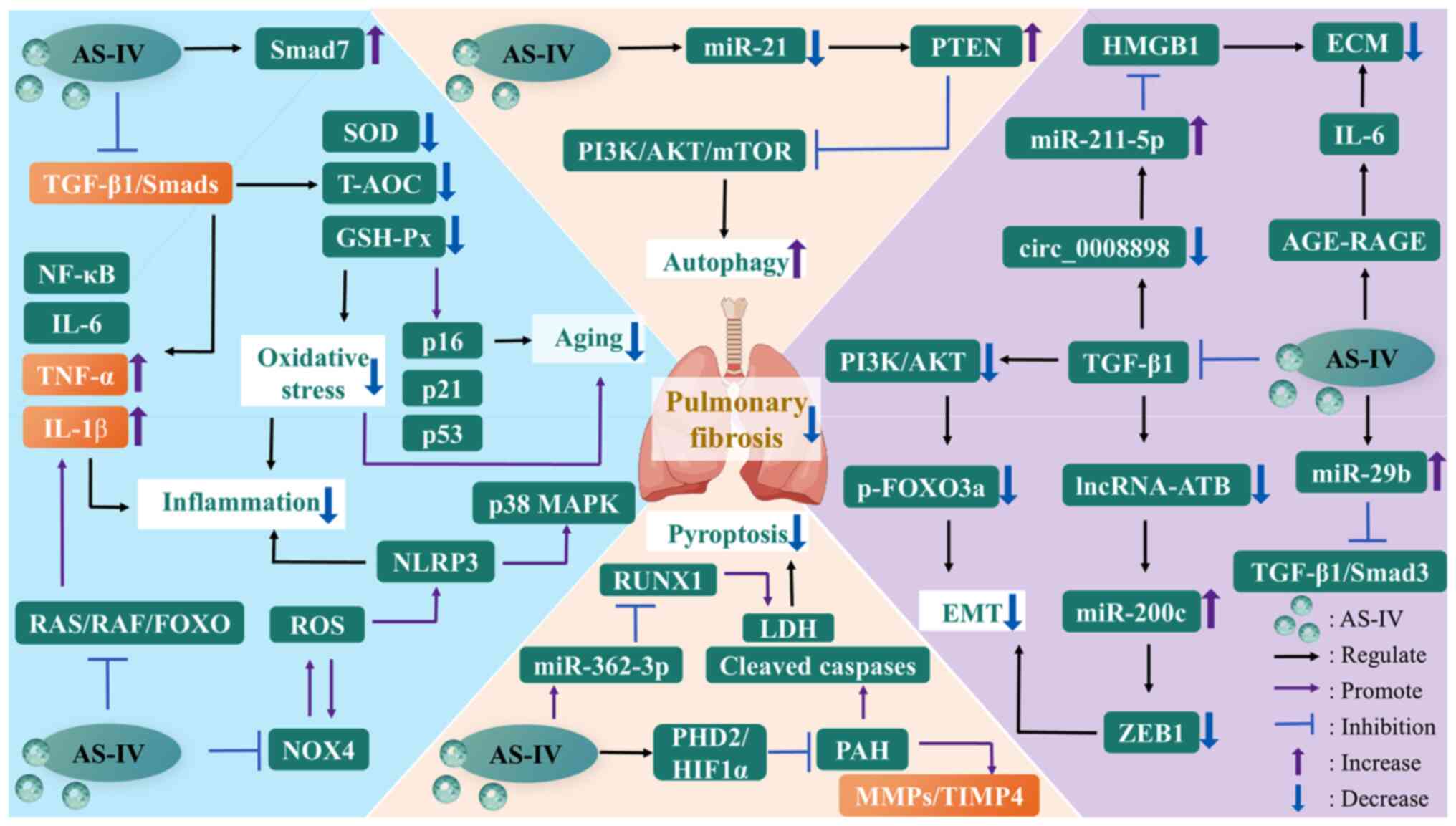 | Figure 4The mechanisms of AS-IV against
pulmonary fibrosis. AGE, advanced glycation end-product; AKT,
protein kinase B; AS-IV, astragaloside IV; ECM, extracellular
matrix; EMT, epithelial-mesenchymal transition; FOXO, forkhead box
O; GSH-Px, glutathione peroxidase; HIF1α, hypoxia-inducible factor
1α; HMGB1, high mobility group box-1; IL-1β, interleukin-1β; LDH,
lactate dehydrogenase; lncRNA-ATB, long non-coding RNA activated by
transforming growth factor β; MAPK, mitogen-activated protein
kinase; miR-21, microRNA-21; MMP, matrix metalloproteinase; mTOR,
mammalian target of rapamycin; NF-κB, nuclear factor-kappa B;
NLRP3, nucleotide-binding oligomerization domain-like receptor
thermal protein domain associated protein 3; NOX4, NADPH oxidase 4;
PAH, pulmonary artery hypertension; PHD2, prolyl-4-hydroxylase 2;
PI3K, phosphatidylinositol-3-kinase; PTEN, phosphatase and tensin
homolog; RAF, rapidly accelerated fibrosarcoma; RAGE, receptor for
AGE; RAS, rat sarcoma; ROS, reactive oxygen species; RUNX1,
runt-related transcription factor 1; SOD, superoxide dismutase;
T-AOC, total antioxidant capacity; TGF-β1, transforming growth
factor-β1; TIMP4, tissue inhibitor of metalloprotease 4; TNF-α,
tumor necrosis factor-α; ZEB1, zinc finger E-box binding homeobox
1. |
PF is a heterogeneous disease and the therapeutic
effect of AS-IV may vary across different subtypes of PF. In
addition, animal models usually simulate acute or subacute PF,
whereas PF in humans is a progressive condition. Therefore, the
long-term intervention effect of AS-IV still needs to be verified
in human clinical studies. The triggers of human PF, such as PF
induced by drugs, connective tissue diseases, or inhaled dust, are
difficult to replicate fully through animal experiments. Moreover,
the evidence from tests encompassing pulmonary function indicators,
including vital capacity and diffusion capacity, remains
insufficient. Not only that, the comparative analysis of the
therapeutic effects between AS-IV and anti-PF drugs, such as
pirfenidone and nintedanib and the dose-response relationship
between the dosage and anti-PF effect of AS-IV remain unclear.
Finally, the oral bioavailability of AS-IV is limited. Additional
data are required to confirm whether approaches, such as aerosol
inhalation and dosage form optimization, can increase the
concentration of AS-IV in lung tissues, ensure its accumulation in
damaged lung tissues and enable its effective interaction with lung
cells.
Renal fibrosis
The main characteristics of chronic kidney disease
(CKD) include progressive renal tissue structural lesions and
impaired renal excretory function, which may be due to functional
nephron defects caused by diverse causative variables, including
diabetes, interstitial nephritis, glomerulonephritis, obstructive
nephropathy and polycystic kidney disease (116). CKD affects >10% of the
overall population worldwide and in the United States alone, ~14%
of adults are affected by pathological factors related to CKD
(117,118). Among these factors, renal
fibrosis is often a unified pathological manifestation of
progressive CKD regardless of the progression of CKD. If the
prognosis is inappropriate, then end-stage renal failure is often
the final outcome (119). The
transdifferentiation of intrinsic renal cells, such as mesangial
cells (MCs), epithelial cells, endothelial cells, or fibroblasts,
into myofibroblasts is a key mechanism for inducing renal fibrosis.
Various cytokines that maintain the homeostasis of ECM deposition
and degradation and proinflammatory mediators are also involved in
the pathogenesis of renal fibrosis (120). In addition, the excessive
deposition of fibrous connective tissue has adverse effects on the
physiological function of renal tubules and glomerular filtration
rate; this phenomenon also causes the tubulointerstitial space and
glomerulus to become key sites for frequent fibrosis (121,122). Although dialysis and kidney
transplantation are currently commonly used to intervene in ESRD,
renal replacement therapy may induce some uncertain risk factors,
necessitating intervening in CKD as early as possible to avoid its
progressive deterioration into ESRD (123). Given that whether fibrosis is a
cause or a pathological manifestation of CKD is unclear and
intervening in the progression of CKD by directly targeting the
treatment of renal fibrosis requires further in-depth research,
conservative antifibrotic treatments, such as the use of
well-tolerated Chinese herbal medicines, may achieve the negative
regulation of various kidney diseases in their initial phases
(124).
Diabetic kidney disease-induced renal
fibrosis
Lipid metabolism
Diabetic kidney disease (DKD) can cause glomerular
ultrastructural alterations by inducing the deposition of ECM
proteins, such as FN and DKD, lacking effective prognosis may cause
the induction of ESRD. This situation can explain why ~1/3 of
individuals with diabetes develop irreversible end-stage renal
failure (125). Human
glomerular mesangial cells (HMCs) can participate in the occurrence
of DKD-related fibrosis via the uptake of FFAs flowing into the
kidney. The uptake of medium- and long-chain FFAs mainly depends on
the transport activity of cluster of differentiation 36 (CD36)
protein, which is widely expressed in renal glomeruli and involved
in the induction effect of FFAs on HMC fibrosis to a certain
extent. AS-IV can negatively regulate the expression of FN and type
IV collagen α 1 and the TGF-β1/Smad2/3 signaling pathway in the
palmitic acid (PA)-treated HMC fibrosis model. It can also inhibit
the activation of CD36 expression in a high-fat environment induced
by PA. Given that NOX4-derived ROS play a vital role in regulating
the progression of DKD and NOX4 can cross-talk with the
TGF-β1/Smad2/3 pathway and become a fibrotic effector molecule,
NOX4 may be the key target for the inhibition of DKD-related renal
fibrosis (125). Nath et
al (126) also verified the
profibrotic effect of CD36. That is, the uptake of PA by CD36
induces the TGF-β1/Smad2/3 signaling cascade and mediates the
occurrence of EMT in hepatocellular carcinoma (HCC). This situation
indicates that NOX4 and TGF-β1 overexpression may be attributed to
the regulatory effects of upstream CD36 activation. However, AS-IV
markedly reduces the expression of CD36, NOX4 and TGF-β1 in
PA-treated HMCs (125).
Inflammation and oxidative stress
In the diabetic phase, renal cells exhibit high
glucose uptake. However, other cell populations, such as glomerular
MCs, are unable to regulate the decrease in glucose transport
rates. This inability induces an imbalance in intracellular glucose
homeostasis and promotes cytosolic and mitochondrial ROS release
(127). In addition,
ROS-mediated oxidative stress induces the activation of the
TGF-β/Smads signaling pathway, which in turn regulates the fibrosis
of renal tubular epithelial cells (RTECs). Du et al
(127) found that the combined
administration of ginsenoside Rg1 (G-Rg1) and AS-IV is more
effective than monotherapy in interfering with TGF-β1/Smads
signaling mainly because G-Rg1 has strong pharmacological activity
in the negative regulation of TGF-β1 expression, whereas Smad7
activity targeted by AS-IV is remarkably upregulated. In the
treatment of DKD, AS-IV acts as an enhancer of the antioxidative
stress effect of G-Rg1. The main manifestation of this effect is
that after coadministration, the levels of catalase, GSH-Px and
T-AOC maximally increase, whereas MDA shows reduced activity
(127). In addition, abnormally
elevated blood glucose concentrations can drive ROS release and
oxidative stress. AGEs are protein derivatives that can mediate
proinflammatory responses and oxidative stress. They all have an
intimate connection with the occurrence of DKD. Zhang et al
(128) found that in DKD rats,
AS-IV administration can reduce the excessive synthesis of AGEs and
the overexpression of inflammatory factors. Moreover, AS-IV
treatment can reduce the excessive synthesis of collagen IV in the
basement membrane and the excessive deposition of FN in the renal
mesangium and interstitium. The authors also verified the
activation effect of the excessive release of ROS on cytokines and
transcription factors. This phenomenon is not only a reason for the
induction of the massive deposition of ECM in the kidney area but
also aggravates renal fibrosis complicated by DKD and the
development of end-stage renal failure (129). Furthermore, transcriptomic
analysis has shown that the inflammation-related NLR signaling
pathway is a pharmacological target of AS-IV and can potentially
participate in the way that AS-IV ameliorates renal fibrosis
complicated by DKD in rats (128).
mTORC1/p70S6K
mTOR complex 1 (mTORC1) can regulate the
phosphorylation of ribosomal protein S6 kinase β-1 (p70S6K)
downstream, thus participating in cell proliferation or growth to
some extent. However, the development of DKD is intimately
associated with pathologically driven mTORC1/p70S6K signaling,
which may further induce DKD complicated by RTEC
transdifferentiation (130).
Chen et al (131) found
that in human proximal tubular epithelial (HK-2) cells treated with
different concentrations of HG, AS-IV can target the upregulation
of the epithelial cell marker E-cadherin and negatively regulate
α-SMA. Moreover, AS-IV downregulates the phosphorylation of mTOR
and its downstream molecule p70S6K. That is, it has an inhibitory
effect on the signal transduction of the mTORC1/p70S6K pathway. In
addition, a negative correlation exists between zinc finger
transcription factors (Snail, Slug, Twist and ZEB1) and E-cadherin
expression. Nevertheless, the mTORC1/p70S6K pathway is also highly
associated with the activities of the transcription factors Snail
and Twist (130). AS-IV can
target the inactivation of HG-induced Snail and Twist proteins and
that the ECM protein components FN and collagen IV are
downregulated when AS-IV intervenes in DKD (131). In summary, AS-IV further
negatively regulates renal tubular EMT induced by HG concentration
by inhibiting the overactivation of the mTORC1/p70S6K signaling
pathway and downregulates the expression of the transcription
factors Snail and Twist in HK-2 cells, simulating DKD in
vitro (131).
miRNA
Proteinuria is mainly used as a clinical diagnostic
criterion of DKD, a microvascular complication of diabetes and is
often accompanied with renal fibrosis. Highly differentiated
podocytes participate in forming the outermost layer of defense for
glomerular filtration. Adverse stress reactions may affect the
differentiation structure of podocytes, further causing
macromolecular plasma proteins to penetrate the damaged filtration
barrier directly and flow into the urinary filtrate, thus
aggravating the occurrence of renal fibrosis (132). MCs are a type of glomerular
cells. Their excessive activation also induces the enhanced
expression of α-SMA and promotes the deposition of large amounts of
ECM proteins, thereby participating in the formation of DKD-related
renal fibrosis (133). The
progression of mammalian kidney disease is known to be markedly
associated with the expression of miR-21, which transcriptionally
suppresses the expression of downstream target genes (134). However, AS-IV
concentration-dependently decreases the levels of miR-21 and the
mesenchymal marker α-SMA in HG-induced podocytes and MCs and the
level of the epithelial marker nephrin in podocytes is positively
associated with the concentration of AS-IV administration (135). In addition, podocyte damage and
MC proliferation are inextricably linked to the involvement of the
Wnt/β-catenin and TGF-β1/Smads signaling cascades. However, AS-IV
markedly lowers the upregulated β-catenin, TGF-β1 and p-Smad3
levels in podocytes and MCs with miR-21 overexpression and promotes
the recovery of Smad7 activity. In summary, AS-IV impedes the
advancement of DKD-related renal fibrosis and maintains highly
differentiated podocyte morphology and MC stability by negatively
regulating the levels of highly activated miR-21 and inhibiting the
driving effect of miR-21 on the downstream Wnt/β-catenin and
TGF-β1/Smads signaling cascades. These functions provide new
insights into the AS-IV targeted treatment of glomerular-related
diseases (135).
Autophagy
As a deacetylase, SIRT1 plays a key role in
mediating renal dysfunction, such as podocyte injury (136). In addition, during HG-induced
podocyte apoptosis, activated NF-κB can serve as a signaling
molecule that inhibits autophagy by targeting LC3 II
downregulation. However, according to Wang et al (137), AS-IV further impedes podocyte
EMT by reversing the negative effect of HG treatment on the
activity of the autophagy markers Beclin1 and LC3 II. By contrast,
SIRT1 activator helps enhance this phenomenon, indicating that
AS-IV activates SIRT1 to deacetylate the NF-κB p65 subunit and may
execute pharmacological activity as an autophagy agonist. At the
same time, N-cadherin and α-SMA expression by AS-IV in podocytes
treated with different HG concentrations are reversed under the
mediation of the autophagy inhibitor 3-methyladenine, the
activation of E-cadherin and nephrin levels and the inhibition of
TGF-β. This effect further demonstrates that AS-IV can serve as a
pharmacological autophagy inducer in DKD, thereby protecting
glomerular structure and alleviating the HG environment that drives
podocyte EMT (137). Moreover,
Wang et al (138) found
that AS-IV pretreatment concentration-dependently suppresses α-SMA,
FN and collagen IV expression in MCs induced by HG and the
prohibitive effect of AS-IV on the activation and proliferation of
MCs induced by HG is still related to autophagy activation mediated
by SIRT1-NF-κB signaling transduction.
RAF/MEK/ERK
The pathological upregulation of plasma C-X3-C
motif ligand 1 (CX3CL1) concentrations in adult patients with CKD
is often a key risk variable associated with the occurrence of CVD
and diabetes (139).
Furthermore, EMT is intricately linked to the activation of the
RAF/mitogen-activated protein kinase (MEK)/ERK signaling cascade
(140). Hu et al
(141) revealed that AS-IV
administration can negatively regulate the expression of the
mesenchymal marker vimentin and myofibroblast marker α-SMA in db/db
mice and HK-2 cells treated with HG; partially inhibit the
overexpression of CX3CL1 and p-c-Raf/c-Raf, p-MEK/MEK and
p-ERK/ERK; and upregulate the activity of the epithelial cell
marker E-cadherin (141). In
summary, AS-IV further inhibits the induction of EMT by the
RAF/MEK/ERK signaling cascade by targeting the downregulation of
CX3CL1 expression, thereby effectively alleviating the occurrence
of EMT in DKD.
TGF-β1/UUO treatment-induced renal
fibrosis
Inflammation
Renal function repair is facilitated by the
synergistic action of fibroblasts, RTECs, endothelial cells, renal
interstitial lymphocytes and macrophages that are involved in the
inflammatory response and the preservation of ECM homeostasis when
the kidney is under adverse stress (142). During repair, if the synthesis
and degradation of ECM are out of balance and a large amount of
collagen fibers are deposited, then the activation and
proliferation of fibroblasts is induced and intrinsic renal cells
are replaced. These processes constitute the occurrence of renal
fibrosis. According to Zhou et al (143), AS-IV could inhibit the
inflammatory infiltration induced by macrophages and lymphocytes in
unilateral ureteral obstruction (UUO)-treated mice and upregulate
IκBα expression. By contrast, in LPS-treated RTECs in vitro,
AS-IV could negatively regulate Toll-like receptor 4/NF-кB
signaling. This finding shows that AS-IV's anti-inflammatory action
on RTECs and inflammatory cells may be one of its potential
pharmacological mechanisms to impede the course of renal fibrosis
and CKD.
Autophagy
EMT is associated with the G2/M cycle
and partial RTEC lesions induce G2/M arrest and
participate in the occurrence of EMT under the mediation of
fibrotic effector cells. Furthermore, the mitochondrial
physiological function is markedly positively associated with the
activity of acetaldehyde dehydrogenase 2 (ALDH2). ALDH2 can target
the inhibition of autophagic activity to mitigate the functional
damage caused by LPS to the heart (144). Li et al (123) found that AS-IV can reverse the
upregulation of the autophagy indicators ATG7, Beclin1 and LC3
II/LC3 I in an adenine-treated CKD rat model and a TGF-β1-induced
in vitro HK-2 fibrosis model and drive p62 accumulation by
inhibiting autophagy. The inhibitory effects of AS-IV on
CKD-induced autophagy could be explained by the activation of the
AKT/mTOR signaling cascade and upregulation of ALDH2 activity. In
addition, following AS-IV administration, the activity of the
mediators involved in G2/M cell cycle arrest, including
p21, p53, p-p53 and p-histone H3, markedly decreased, indicating
that AS-IV can target CKD-related G2/M cell cycle
change. AS-IV induces the expression of the epithelial cell marker
E-cadherin to be upregulated and that of renal mesenchymal cell
markers to be downregulated; these effects further illustrate its
inhibitory effect on EMT (123).
TGF-β/Smad
The TGF-β receptor activates the phosphorylation of
the downstream fibrotic effector proteins Smad2 and Smad3, which
form a complex with Smad4 and are transported into the nucleus.
Smad family members, including Smad7, an inducer that promotes
TGF-β1 receptor degradation and Smad2/3 inactivation, are mostly
involved in the transcription of target genes. However, TGF-β1 has
two sides: In addition to being an inducer driving fibrotic and
anti-inflammatory responses, its inactivation may also mean
exacerbating proinflammatory effects, suggesting that antifibrotic
intervention that directly targets TGF-β may trigger other unknown
risks (145). Wang et al
(146) reported that the
expression of the myofibroblast marker a-SMA and the formation of
fibrous connective tissue in a UUO-treated rat model were
negatively regulated by AS-IV intervention. In addition, AS-IV has
been shown in vitro to promote the inactivation of fibrotic
effector connective tissue growth factor (CTGF) in rat renal
fibroblasts (NRK-49F) treated with TGF-β1, thereby inhibiting its
downstream induction of ECM synthesis and further resisting renal
fibrosis. An in vitro experiment has also shown that AS-IV
can activate the dephosphorylation of Smad2 or Smad3. Silencing the
expression of the Smad7 gene in NRK-49F cells via the small
interfering RNA technique further demonstrated that AS-IV
alleviates renal fibrosis associated with obstructive nephropathy
because of its targeted effect on the upregulation of Smad7
activity. In summary, AS-IV's antitubulointerstitial fibrosis
effect and its targeted inactivation of the TGF-β/Smad signaling
pathway are markedly positively associated (146).
PI3K/AKT/mTOR
Renal interstitial fibrosis often occurs when
various CKDs progress to advanced stages (147). Gap junctions promote
intercellular communication by mediating intercellular material
transport and signal transmission. Connexin43 (Cx43) may crosstalk
with the occurrence of fibrotic diseases (148). In addition, the inactivated
PI3K/AKT signaling cascade can mitigate TGF-β1-induced EMT and
highly activated mTOR is markedly positively associated with the
occurrence of EMT (147,149).
Lian et al (147) found
that the inactivated AKT/mTOR pathway may be an integral mediating
factor in the mechanism through which Cx43 negatively regulates EMT
in TGF-β1-treated RTECs. That is, by inhibiting the TGF-β1-induced
downregulation of Cx43 protein levels, AS-IV can interfere with
PI3K/AKT/mTOR signaling, attenuate the expression of the
mesenchymal marker α-SMA and vimentin and further prevent
TGF-β1-induced RTEC EMT. This phenomenon suggests that AS-IV may be
an effective drug for preventing EMT in RTECs and renal
interstitial fibrosis.
PI3K/AKT/GSK-3β and Wnt/β-catenin
TGF-β1 not only targets the Smad transcriptional
activator family but also regulates non-Smad-dependent signaling
pathways, such as the PI3K/AKT signaling cascade, which contributes
to fibroblast transdifferentiation and fibrosis. Network
pharmacology analysis showed that AS-IV and renal fibrosis share
multiple targets and Kyoto Encyclopedia of Genes and Genomes
pathway enrichment analysis found that the PI3K/AKT signaling
pathway has intersectionality with these shared targets, among
which AKT1 and glycogen synthase kinase-3β (GSK-3β) are markedly
enriched in the PI3K/AKT signaling cascade (150). Further in vitro cell
experiments revealed that AS-IV treatment inhibits the
TGF-β1-induced upregulation of AKT phosphorylation in rat renal
tubular epithelial (NRK-52E) and NRK-49F cells in a
concentration-dependent manner, thereby promoting its induction of
GSK-3β dephosphorylation (150). In addition, the
dephosphorylation of GSK-3β can induce β-catenin degradation,
thereby negatively regulating the progression of EMT. Consequently,
the mechanism by which AS-IV can prevent TGF-β1 from activating
β-catenin is linked to AS-IV targeting the inactivation of the
AKT/GSK-3β signaling pathway, which alleviates EMT and further
inhibits β-catenin to promote Wnt signaling pathway activation
(150). Notably, disheveled
(Dsh or Dvl) proteins often promote the dephosphorylation of
β-catenin, thereby interfering with its degradation mainly because
activated Dsh proteins drive the separation of GSK-3β from the
degradation complex, thereby promoting Wnt/β-catenin signaling and
targeting the elevation of downstream gene expression. According to
Wang et al (151), AS-IV
suppresses the overexpression of Wnt and Frizzled genes in a
UUO-induced renal interstitial fibrosis rat model, with the
activity of Dsh proteins in the cytoplasm and β-catenin in the
nucleus also being downregulated after AS-IV intervention. In
addition, stable RTEC structure depends on activated E-cadherin and
E-cadherin is inversely associated with the nuclear level of
β-catenin. Furthermore, the majority of the Wnt/β-catenin signaling
pathway's downstream target genes, such as Snail, Twist, lymphoid
enhancer binding factor-1 and Jagged1, are involved in the
development of EMT. In the rat model of UUO-induced renal
interstitial fibrosis, AS-IV can downregulate the nuclear levels of
the aforementioned target proteins that are engaged in the
Wnt/β-catenin signaling cascade downstream. To summarize, a key
mechanism by which AS-IV decelerates renal interstitial fibrosis
may be attributed to the partial inhibition or blockage of the
Wnt/β-catenin signaling pathway (151).
MAPK and NF-κB
The fibrotic effector TGF-β regulates the
occurrence of fibrosis during CKD and can participate in the
induction of MAPK family members relying on noncanonical signaling
pathways. Che et al (152) found that AS-IV substantially
suppresses the overexpression of the cell proliferation marker
proliferating cell nuclear antigen, the myofibroblast
differentiation marker α-SMA, collagen I and FN in TGF-β1-treated
renal fibroblasts. It also negatively regulates the activities of
p-IκBα and the MAPK family members ERK1/2, p38 MAPK and JNK and the
nuclear level of the NF-κB p65 subunit in TGF-β1-induced renal
fibroblasts. Comparison revealed that the efficacy of MAPK and
NF-κB inhibitors in regulating EMT is similar to that of AS-IV,
further confirming that the suppression of the MAPK and NF-κB
signaling pathways is a key mechanism through which AS-IV improves
CKD and blocks fibroblast transdifferentiation (152). In addition, tubular cell
apoptosis is involved in renal fibrosis (153). ERK, p38 MAPK and JNK are three
major family members of the MAPK pathway that mediate apoptosis and
inflammation. Targeting p38 MAPK inactivation is another key
strategy for remitting renal structural atrophy and fibrosis
induced by renal artery stenosis (154). Xu et al (155) found that the UUO-induced
apoptosis of obstructed RTECs in vivo and TGF-β1-induced
HK-2 cell apoptosis in vitro can be alleviated after AS-IV
intervention and the level of activated caspase-3 is markedly
inhibited. In addition, AS-IV promotes the dephosphorylation of the
p38 MAPK and JNK pathways in obstructed kidneys and TGF-β1-treated
HK-2 cells; negatively regulates serum creatinine and blood urea
nitrogen levels, which are indicators of renal function injury, and
the expression levels of FN and α-SMA, which are indicators of
renal tubulointerstitial fibrosis. These effects provide
pharmacological evidence for targeting AS-IV to improve renal
tubular cell survival and alleviate renal fibrosis associated with
obstructive nephropathy (155).
Moreover, inhibiting the transdifferentiation of kidney resident
cells, such as fibroblasts, pericytes, tubular cells and
endothelial cells, into myofibroblasts can negatively regulate
tubulointerstitial fibrosis (156). Meng et al (156) focused on the negative
regulatory mechanism of TCM compounds on fibrosis induced by UUO
and, through mass spectrometry, identified AS-IV and FA as the main
bioactive components in Astragalus and Radix Angelica
sinensis extracts, respectively. The authors found that
AF can downregulate the expression of serum creatinine, FN,
α-SMA and TGF-β1 in UUO-induced obstructed kidneys; inhibit
interstitial fibroblast activation; and upregulate the production
of nitric oxide, which is a key mediator that negatively regulates
renal injury (157).
Furthermore, in TGF-β1-treated NRK-49F cells in vitro,
AF mediates the downregulation of α-SMA and FN expression
and in IL-1β-treated HK-2 cells in vitro, AF targets
an increase in the dephosphorylation level of JNK. However, the
phosphorylation levels of ERK and p38, two other members of the
MAPK signaling pathway family, are not markedly affected by the
interference effect of AF. These results suggest that
AF administered on the basis of TCM compatibility can reduce
tubulointerstitial fibrosis to a certain extent (156).
cAMP/PKA
The specific receptor G-protein-coupled receptor 14
of urotensin II (UII) is an upstream molecule that regulates cyclic
adenosine monophosphate/protein kinase A (cAMP/PKA) signaling. The
activated cAMP/PKA signaling cascade further induces the downstream
fibrotic effector molecule TGF-β to be overexpressed and promotes
RTECs to acquire a mesenchymal phenotype, which triggers renal
fibrosis (158). The
phosphorylation of PKA is closely associated with inflammation and
pyroptosis mediated by the NLRP3 inflammasome (159). However, according to Zhang
et al (160), AS-IV
administration could downregulate the UUO-induced renal
fibrosis-related indicators cAMP and UII. Moreover, after in
vitro UII intervention, the activity of PKA and expression of
the pyroptosis-related indicators NLRP3, caspase-1 and GSDMD-N in
NRK-52E cells were markedly inhibited by AS-IV. In addition, by
suppressing the expression of α-SMA and FN and inducing the
blockage of EMT, AS-IV can prevent UII-treated NRK-52E cells from
losing epithelial cell morphology and acquiring the mesenchymal
phenotype. In summary, AS-IV can target the downregulation of
cAMP/PKA pathway activity and inhibit its induction of RTEC
pyroptosis and EMT, thereby alleviating renal fibrosis (160).
TGF-β1/ZEB2
TGF-β treatment or HG induction is markedly
associated with the upregulation of miR-192 activity (161). However, AS-IV administration
can dose-dependently suppress the expression of miR-192 in HK-2
cells treated with TGF-β1 (162). In addition, ZEB2 is widely
expressed in the kidney and miR-192 inactivates ZEB2 in HK-2 cells
through downstream regulation. Notably, the negative regulatory
effects of AS-IV on α-SMA, vimentin and collagen I are blocked
under the mediation of miR-192 mimics and further enhanced by
intervention with miR-192 inhibitors. This effect suggests that a
potential mechanism of the antirenal fibrosis effect of AS-IV may
be to target the inactivation of the TGF-β1/ZEB2 signaling pathway
(162,163).
Liver cancer-induced renal
fibrosis
The occurrence of liver cancer is often accompanied
with an increase in the concentration of proinflammatory factors in
the blood. This effect can easily lead to pathological
communication, such as hepatorenal syndrome, between the liver and
other organs, thereby further reducing the survival period
(164,165). TGF-β receptor type 1 is a
negative regulatory mediator of liver fibrosis primarily because of
its capacity to drive the COOH-terminal phosphorylation of Smad3
(pSmad3C) and upregulation of p21 transcriptional activity
(166). When JNK is activated
in an inflammatory state, it further induces the phosphorylation of
the linker region of Smad3 (pSmad3L), thereby targeting downstream
plasminogen activator inhibitor-1 (PAI-1) activation and
participating in fibrosis (167). This situation indicates that
the phosphorylation of different sites of Smad3 is an important
factor in inducing the completely opposite roles of pSmad3L/PAI-1
and pSmad3C/p21 signaling cascades in regulating fibrosis. However,
Wang et al (165)
revealed that AS-IV activates the pSmad3C/p21 pathway and
simultaneously antagonizes pSmad3L/PAI-1 signaling, which in turn
negatively regulates TGF-β1-induced α-SMA expression in HK-2 cells.
In addition, after oxidative damage occurs in the kidney, AS-IV
administration can regulate the Nrf2/heme oxygenase-1 (HO-1)
pathway to strengthen the expression of pNrf2 and HO-1 further and
maintain high Nrf2 activity. However, after Nrf2 is silenced, the
regulation of the pSmad3L/PAI-1 and pSmad3C/p21 signaling pathways
by AS-IV becomes imbalanced. Moreover, the kidneys of knockout mice
exhibit collagen fiber deposition, implying that renal fibrosis
also develops as liver fibrosis progresses to HCC. The regulatory
effect of Nrf2 on pSmad3C/3L is a potential pharmacological
mechanism of AS-IV in alleviating renal fibrosis complicated by
liver cancer (165) (Fig. 5).
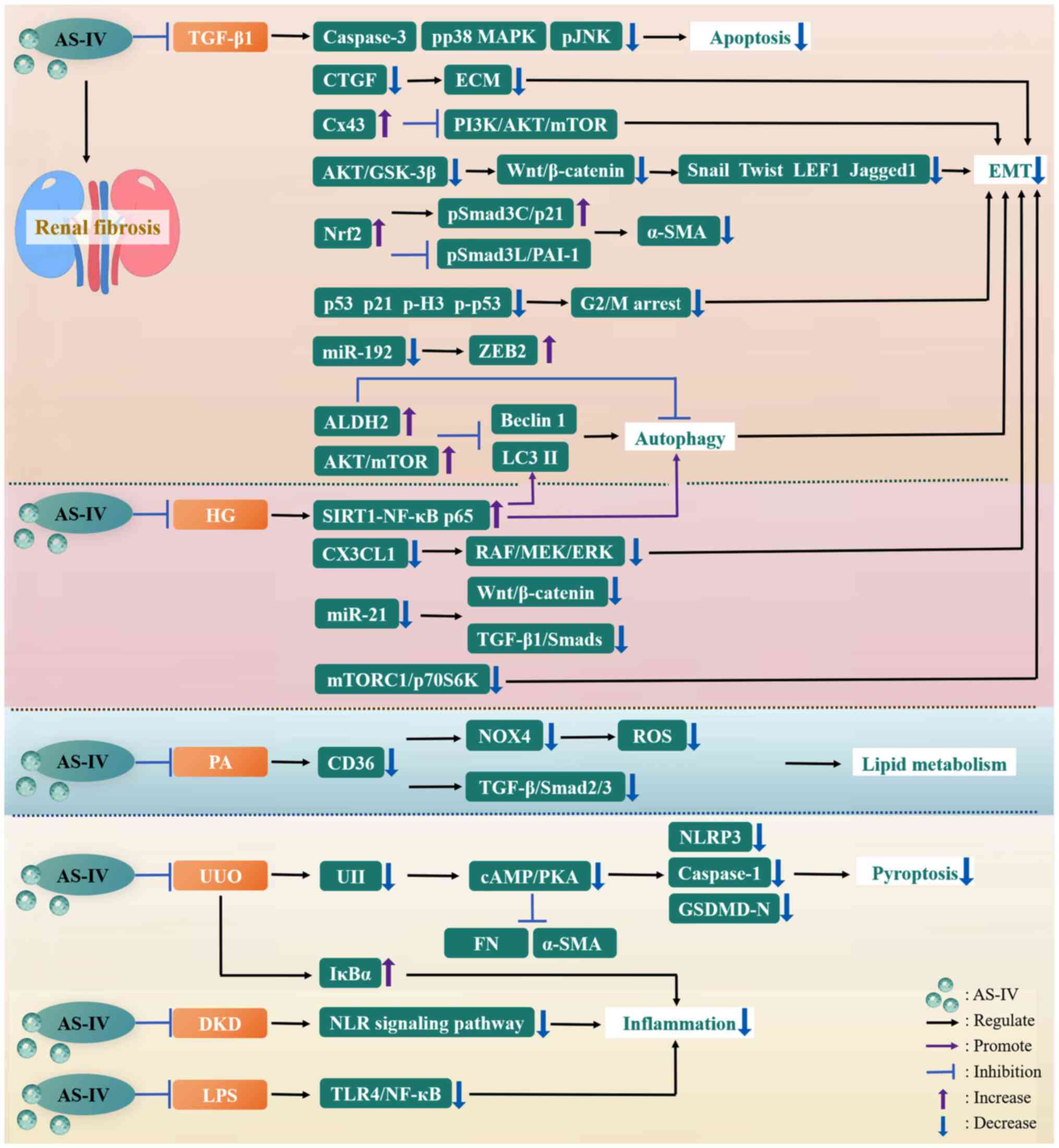 | Figure 5The mechanisms of AS-IV against renal
fibrosis. AKT, protein kinase B; ALDH2, acetaldehyde dehydrogenase
2; AS-IV, astragaloside IV; cAMP, cyclic adenosine monophosphate;
CD36, cluster of differentiation 36; CTGF, connective tissue growth
factor; CX3CL1, C-X3-C motif ligand 1; Cx43, connexin43; DKD,
diabetic kidney disease; ECM, extracellular matrix; EMT,
epithelial-mesenchymal transition; ERK, extracellular
signal-regulated kinase; FN, fibronectin; GSDMD-N, gasdermin D
N-terminal domain; GSK-3β, glycogen synthase kinase-3β; HG, high
glucose; IκBα, inhibitor of NF-kappa B α; JNK, c-Jun N-terminal
kinase; LC3 II, light chain 3 II; LEF1, lymphoid enhancer binding
factor-1; LPS, lipopolysaccharide; MAPK, mitogen-activated protein
kinase; MEK, mitogen-activated protein kinase kinase; miR-192,
microRNA-192; mTOR, mammalian target of rapamycin; mTORC1, mTOR
complex 1; NF-κB, nuclear factor-kappa B; NLR, nucleotide-binding
oligomerization domain-like receptor; NLRP3, nucleotide-binding
oligomerization domain-like receptor thermal protein domain
associated protein 3; NOX4, NADPH oxidase 4; Nrf2, nuclear factor
erythroid 2-related factor 2; p70S6K, ribosomal protein S6 kinase
β-1; PA, palmitic acid; PAI-1, plasminogen activator inhibitor-1;
p-H3, phosphorylated histone H3; PI3K,
phosphatidylinositol-3-kinase; PKA, protein kinase A; pSmad3C,
COOH-terminal phosphorylation of Smad3; pSmad3L, phosphorylation of
the linker region of Smad3; RAF, rapidly accelerated fibrosarcoma;
ROS, reactive oxygen species; RTEC, renal tubular epithelial cell;
SIRT1, sirtuin 1; TGF-β1, transforming growth factor-β1; TLR4,
toll-like receptor 4; UII, Urotensin II; UUO, unilateral ureteral
obstruction; ZEB2, zinc finger E-box binding homeobox 2; α-SMA,
α-smooth muscle actin. |
Notably, renal fibrosis induced in animal models
cannot easily replicate the chronic progressive nature of human
diseases and does not adequately reflect the progressive changes in
clinical renal function indicators, such as estimated glomerular
filtration rate and urinary protein. In addition, the recruitment
and differentiation of endogenous renal stem cells and mesenchymal
stem cells play a crucial role in regulating renal injury repair
(168,169). Whether the antifibrotic
mechanism of AS-IV involves targeting the proliferation and
differentiation of renal stem cells remains to be investigated.
Moreover, the low oral bioavailability of AS-IV may limit its
enrichment in the renal cortex and medulla. Whether the end
products of AS-IV after in vivo metabolism are also involved in the
derivation of AS-IV's antirenal fibrosis activity requires a
comprehensive mechanism integration analysis. Finally, human
nephrons exhibit unpredictable regeneration and repair effects and
the sole reliance on animal experiments cannot systematically
define the dynamic effect of AS-IV on nephron remodeling during the
multistage course of renal fibrosis (170).
Liver fibrosis
Viral hepatitis, cholestatic liver disease,
alcoholic liver disease, nonalcoholic steatohepatitis, nonalcoholic
fatty liver disease and other liver diseases can easily cause
repeated damage to the liver, repair response imbalance and
persistent inflammatory infiltration. Scar tissue and regenerative
nodules may replace parts of liver tissue, resulting in liver
fibrosis. As liver function gradually deteriorates and fibrosis
develops, highly lethal cirrhosis may be induced and prognosis
becomes increasingly challenging (171). A key contributing factor to
liver fibrosis is chronic inflammation, which drives hepatic
stellate cell (HSC) activation through the recruitment of
inflammatory cells, such as monocytes and macrophages. Among these
cells, macrophages further regulate the transdifferentiation of
stellate cells into myofibroblasts by inducing fibrogenic cytokines
and TGF-β overexpression, and the increase in the number of
myofibroblasts can promote the massive deposition of ECM (172-174). Therefore, the key mechanism
targets for intervening in liver fibrosis mainly include inhibiting
adverse stress, thus negatively regulating inflammatory responses,
HSC activation, and ECM deposition (172). In addition to TGF-β,
NLRP3-caspase-1, the Wnt/β-catenin signaling cascade, and
platelet-derived growth factor are important mediators involved in
HSC activation and fibrosis progression (175-177).
Liver fibrosis is a pathological process that is
dynamic and reversible. These characteristics suggest that the
occurrence of liver fibrosis linked to chronic liver disease may be
prevented through active interventions or targeting the reversal of
fibrosis to basal levels (178). At present, no specific
antihepatic fibrotic strategies exist, with antiviral therapy and
the induction of the elimination of harmful factors being often
used as conservative treatments to alleviate liver fibrosis.
However, for patients with advanced fibrosis, especially those that
progress to cirrhosis or are accompanied with some uncontrollable
clinical symptoms, liver transplantation is ultimately the only
option (10). Given that tissue
or organ fibrosis tends to progress slowly and nonlinearly in the
body and can last for years to decades, developing highly effective
antifibrotic agents to prevent liver fibrosis from evolving into
cirrhosis is crucial (179).
Studies have confirmed that the use of TCM has considerable
efficacy in fighting liver fibrosis (10). This section mainly focuses on the
pharmacological mechanism and feasibility of AS-IV against liver
fibrosis.
Liver cancer-related fibrosis
Primary liver cancer induced by tumor lesions
related to hepatocytes or intrahepatic bile duct cells is highly
related to the occurrence of liver fibrosis and preventing liver
fibrosis from developing can decelerate the progression of liver
cancer (167). The occurrence
of liver fibrosis and cancer is intimately associated with the
phosphorylation of different sites of Smad2/3, including pSmad3C,
which transmits a tumor-suppressive TGF-β1 signal; pSmad2C;
pSmad2L; and pSmad3L (166).
Among these sites, pSmad3L can negatively regulate the C-terminal
phosphorylation of Smad3C, thereby promoting the obstruction of
pSmad3C signaling transduction and exacerbating liver cancer
lesions (166). Zhang et
al (167) found that in
mice, fibrous connective tissue is present in the liver tissue at
12 weeks after diethylnitrosamine
(DEN)/tetrachloromethane/ethanol-induced primary liver cancer.
However, AS-IV markedly downregulates α-SMA expression, especially
in areas where inflammatory cells are infiltrating and the
extensive production of collagen fibers is reversed by AS-IV
administration. In addition, AS-IV promotes the downregulation of
the incidence and multiplicity of HCC nodules and induces the
inactivation of the liver cancer marker α-fetoprotein. This effect
indicates that AS-IV not only has antifibrotic activity but also
may participate in the negative regulation of primary liver cancer.
In primary liver cancer mouse models and HSC-T6 cells induced by
TGF-β1 in vitro, AS-IV can promote the overexpression of pSmad3C,
pNrf2, HO-1 and NAD(P)H:quinone oxidoreductase 1 (NQO1) and target
the levels of TGF-β1, pSmad3L, pSmad2C and pSmad2L for
downregulation (167). In
primary liver cancer models in vivo and TGF-β1-induced human
HCC (HepG2) cells in vitro, AS-IV can promote Nrf2/HO-1/NQO1
signaling transduction and upregulate the antioxidant activity of
pNrf2 and its downstream proteins to alleviate the progression of
liver fibrosis and primary liver cancer (167,180). Moreover, pSmad3C, pSmad3L and
pNrf2 may have crosstalk in vivo. Given that TGF-β1 is
upstream of pSmad3C/pSmad3L, the further stimulation of HSC-T6 and
HepG2 with TGF-β1 can induce Nrf2 inactivation. This phenomenon may
also imply that TGF-β1 is a negative regulator of Nrf2. However,
additional research is still required to validate the
upstream-downstream relationship of these signaling molecules
(167). In summary, AS-IV,
through its pharmacological activity against liver fibrosis,
simultaneously inhibits the antagonism between pSmad3L and pSmad3C
signaling, regulates the Nrf2/HO-1 signaling pathway and
upregulates pNrf2 activity, thereby alleviating primary liver
cancer (167).
Astragalus and Salvia miltiorrhiza
Bunge (Lamiaceae) are often employed as TCM compounds. The main
natural active ingredients derived from Astragalus and S.
miltiorrhiza constitute Compound Astragalus and S.
miltiorrhiza Extract (CASE), which includes AS-IV (181). The MAPK pathway can mediate
TGF-β/Smad signaling transduction in tumors and participate in the
induction of TGF-β-related oncogenic effects. The TGF-β signaling
molecule can target downstream PAI-1 gene overexpression, thereby
further promoting TGF-β signaling to drive tumor lesions.
Furthermore, the development of liver fibrosis is markedly
associated with the activation of the PAI-1 gene. Boye et al
(181) found that TGF-β1 can
stimulate the activation of ERK1, JNK1/2 and pp38 in HSCs and HepG2
cells in vitro. Although CASE pretreatment can inhibit the
overexpression of pERK and pJNK, CASE may be a potentiator of
TGF-β1-induced pp38 activation. Notably, in in vivo studies,
CASE can markedly inhibit the activation of three family members of
the MAPK pathway in a DEN-induced rat HCC model. Furthermore, CASE
pretreatment can negatively regulate the stimulatory effect of
TGF-β1 on the levels of p-Smad2C and p-Smad2L and the
cancer-inducing factor p-Smad3L in HSCs and HepG2 cells. By
contrast, under the interference of exogenous TGF-β1 stimulation,
CASE can further promote the negative regulatory effect of p-Smad3C
phosphorylation on tumorigenesis and inhibit Smad4 nuclear
translocation. This regulatory effect is highly important in HepG2
cells. In addition, CASE pretreatment can inhibit PAI-1
overexpression in DEN-induced HCC models and TGF-β1-induced HSCs
and HepG2 cells. In summary, CASE promotes cancer inhibitory signal
output by activating the MAPK-dependent linker dephosphorylation of
Smad2/3, further targeting the downregulation of PAI-1 expression,
thereby inhibiting MAPK to regulate cancer-inducing signals related
to TGF-β/Smad. This activity indicates that TCM compounds added
with AS-IV can modulate the MAPK pathway and TGF-β/Smad signaling
transduction in multiple targets, thereby achieving dual targeted
benefits in the treatment of liver cancer and fibrosis (181).
Bile duct ligation-related
fibrosis
Bile duct ligation (BDL) induces biliary epithelial
cell damage and compensatory proliferation and serves as an inducer
of cholestatic hepatic fibrosis by activating HSCs (182). Considering that multi-drug
combinations can simultaneously intervene in multiple targets, they
can be applied in the treatment of liver fibrosis under the
regulation of multiple mechanisms. Additionally, plants themselves
can derive certain antioxidant substances, which help mitigate the
damage to plant structures caused by the excessive release of ROS
during photosynthesis. This situation is also applicable to TCM
(183,184). Zhao et al (184) found that AF can markedly
inhibit α-SMA overexpression and FN deposition in the livers of
rats treated with BDL and HSC activation and collagen I and III
synthesis are also markedly inhibited by coadministration. In
addition, the antifibrotic effect of AF is at least
partially attributed to the improvement of oxidative stress by
AS-IV. That is, by activating the endogenous antioxidant Nrf2,
AS-IV upregulates the contents of glutathione (GSH) and SOD in the
livers of rats treated with BDL and reduces MDA levels (184). Furthermore, GSK-3β is an
inducer of Nrf2 degradation and AS-IV can activate the
phosphorylation of hepatic GSK-3β at Ser9 (inactive form) in the
BDL rat model. That is, AS-IV can inhibit excessive ROS release by
targeting GSK-3β/Nrf2 signaling pathway activation (184). In conclusion, although the
administration of AS-IV or FA alone can also undeniably improve
BDL-induced liver fibrosis to a certain extent, the combined
application of natural active products derived from Chinese herbal
medicines is effective in inhibiting the activation of HSCs. This
effect may be mediated by the synergistic mechanism of the
upregulation of the antioxidant activity of Nrf2 targeted by AS-IV
and the negative regulation of TGF-β signaling transduction by FA
(184).
Fibrosis related to in vitro cultured
activated HSCs
Compound TCM supplements often have greater
pharmacological activity than single drugs. DBT, which is composed
of Astragalus and Radix Angelica sinensis, is an
effective TCM formula with a long history. Dong et al
(185) found that the
synergistic administration of AF extracted from DBT can
markedly inhibit HSC activation and reduce ROS generation by
activating Nrf2. These effects are mainly related to the regulation
of the activation of the Nrf2/antioxidant response element pathway
by AS-IV. Furthermore, the combined administration of AF
negatively regulates the TGF-β1/Smad pathway. This action is mainly
attributed to the suppression of TGF-β1 signaling transduction and
the inactivation of Smad4 expression by FA. TGF-β can activate p38
MAPK downstream in HSCs by inducing ROS generation, thereby jointly
mediating the regulatory effect of activated p38 MAPK on collagen
fiber synthesis. AS-IV can promote p38 MAPK dephosphorylation and
is further enhanced under the regulation of FA (185). Moreover, the simultaneous
inactivation of Nrf2 and blocking of TGF-β signaling can completely
reverse the inhibitory effect of AF on p38 MAPK
phosphorylation. That is, the participation of p38 MAPK in the
downstream of this signal transduction pathway has been
demonstrated again. In summary, the aforementioned study further
confirmed that the therapeutic effect of the synergistic
application of antioxidants and TGF-β inhibitors on liver fibrosis
is greater than that of single drug supplementation (185). Markedly, the nonenzymatic
antioxidant GSH can serve as a second messenger in HSCs to mediate
TGF-β1 signaling transduction, despite oxidative stress being
considered as a third messenger (186,187). Oxidative stress is a key factor
in inducing HSC activation. Li et al (187) revealed that AS-IV can markedly
inhibit ROS release from activated HSCs and mediate the
downregulation of lipid hydroperoxide content (188). In activated HSCs, AS-IV may
promote activated Nrf2 to drive GSH synthesis by targeting Nrf2.
However, after Nrf2 is blocked, the decline in ROS and collagen I
content caused by AS-IV is reversed (187). In addition, activated p38 MAPK
can mediate collagen synthesis in HSCs. When activated HSCs are
treated with p38 MAPK kinase inhibitors, ROS, lipid hydroperoxide,
GSH production and Nrf2 activity are unaffected. That is, in HSCs,
Nrf2 may promote the GSH-mediated inactivation of p38 MAPK by
targeting increased GSH synthesis, thereby further inhibiting the
inducible effect of this kinase on collagen synthesis (187). Therefore, one possible
explanation for AS-IV's suppression of HSC activation is its
targeted effect on the upregulation of GSH content and Nrf2
activity.
Porcine-serum (PS)-related
fibrosis
The strong immune rejection reaction of receptors
to serum with an allogeneic origin can be complicated by liver
fibrosis. This phenomenon is the main mechanism by which PS induces
immune hepatic fibrosis in rats. This model differs from
traditional postnecrotic liver fibrosis in that it can well
simulate the clinical characteristics and pathological processes of
human liver diseases and cause little damage to hepatocytes
(189,190). Liu et al (190) revealed that in a rat model
treated with PS, AS-IV may prevent TGF-β1 from being overexpressed
in liver tissue and serum. Moreover, AS-IV can interfere with the
influence of TGF-β1 stimulation on the incorporation of
[3H]proline in HSCs, as demonstrated by the decreased
incorporation amount and inhibition of TGF-β1-induced proliferation
and collagen synthesis in HSCs, thereby alleviating PS-induced
immune hepatic fibrosis (Fig.
6).
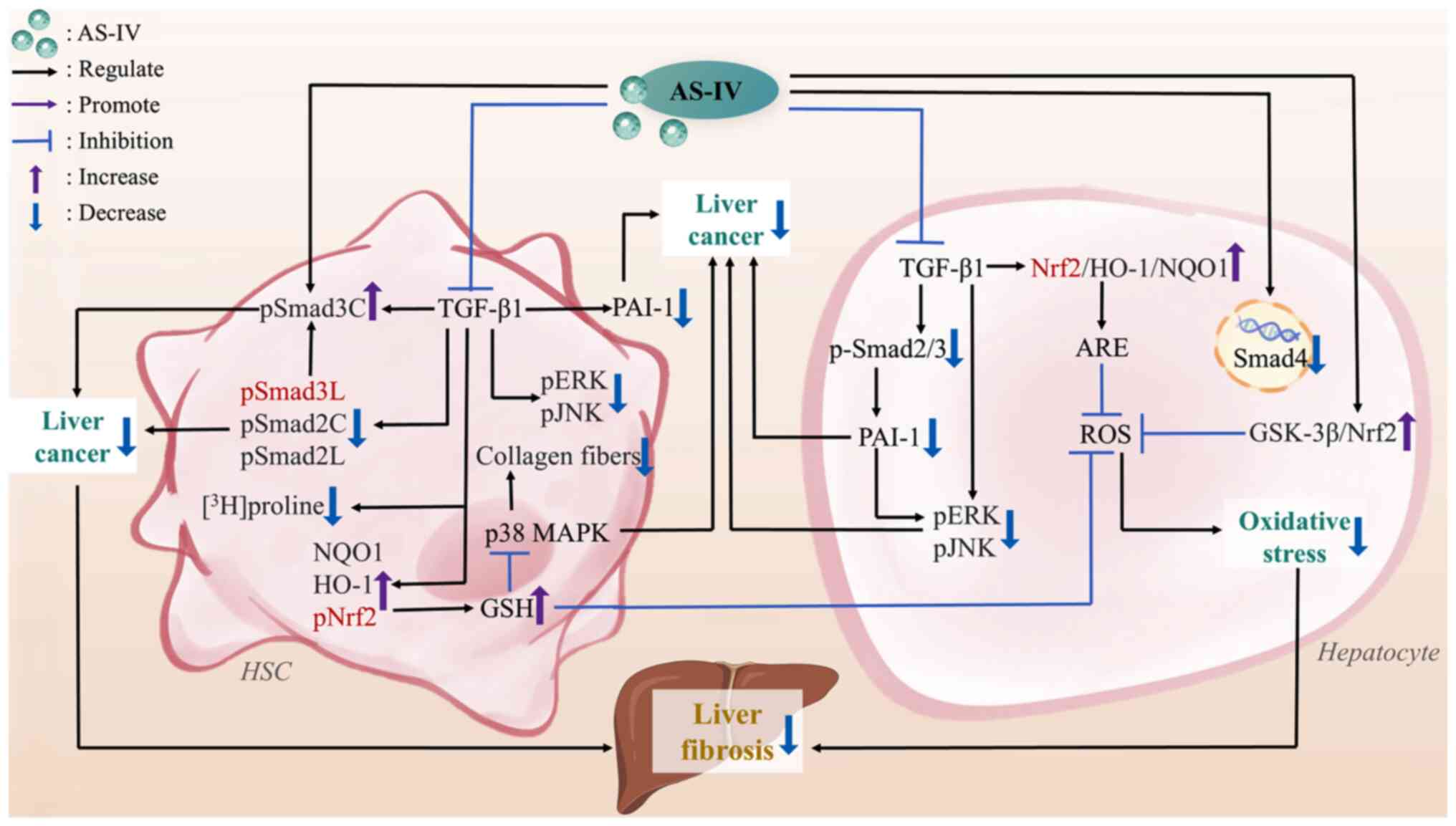 | Figure 6The mechanisms of AS-IV against liver
fibrosis. ARE, antioxidant response element; AS-IV, astragaloside
IV; GSH, glutathione; GSK-3β, glycogen synthase kinase-3β; HO-1,
heme oxygenase-1; HSC, hepatic stellate cell; MAPK,
mitogen-activated protein kinase; NQO1, NAD(P)H:quinone
oxidoreductase 1; PAI-1, plasminogen activator inhibitor-1; pERK,
phosphorylated extracellular signal-regulated kinase; pJNK,
phosphorylated c-Jun N-terminal kinase; pNrf2, phosphorylated
nuclear factor erythroid 2-related factor 2; pSmad3C, COOH-terminal
phosphorylation of Smad3; pSmad3L, phosphorylation of the linker
region of Smad3; ROS, reactive oxygen species; TGF-β1, transforming
growth factor-β1. |
Notably, in animal models, the induction pattern of
liver fibrosis often relies on high-dose interventions, primarily
causing acute or subacute liver injury. By contrast, the pathogenic
process of liver fibrosis in humans follows a chronic low-dose
pattern. Consequently, substantial differences may exist between
humans and animal models in terms of the mechanisms by which AS-IV
regulates cellular stress and acts on signaling pathways.
Furthermore, the effect of AS-IV on regulating the activation of
HSCs may be associated with the heterogeneity of HSCs. The subset
differences of HSCs might lead to the diverse induction effects of
AS-IV on the differentiation direction of HSCs, thereby influencing
the progression of liver fibrosis. In basic experiments, fibrosis
intervention is often conducted via the intraperitoneal injection
of high concentrations of AS-IV. The dose used is considerably
higher than the conventional oral dose, making realistically
simulating clinical effects difficult. Therefore, the further
introduction of liver organoids, liver chips and humanized models
can enhance the reliability of AS-IV in the prevention and
treatment of liver fibrosis.
Other tissue fibrosis
Peritoneal fibrosis
PGC-1α/ROS/apoptosis
Treatment with peritoneal dialysis (PD) is common
for patients with end-stage renal failure. However, the long-term
contact of acidic ions and high glucose concentrations in the
dialysate with the peritoneum activates peritoneal intrinsic cells.
Peritoneal mesothelial cells (PMCs) are induced to undergo
hyperglycosylation and mesothelial-mesenchymal transition (MMT),
leading to the decline in peritoneal barrier function and
occurrence of peritoneal fibrosis and destroying the ideal PD
efficacy (191-194). Mitochondrion-mediated redox
metabolism imbalance promotes apoptosis that is directly related to
peritoneal fibrosis. Xie et al (191) found that AS-IV can reduce the
thickness of peritoneal tissue in rats and promote the
overexpression of the mitochondrial synthesis-related protein
PGC-1α and mitochondrial genes. Moreover, the authors discovered
that AS-IV can downregulate the ubiquitination of PGC-1α; increase
the half-life of PGC-1α; and, by negatively regulating the
expression of cleaved-caspase-3, α-SMA and p-smad2/3, also execute
considerable antiapoptotic and antifibrotic activities. In summary,
under mediation by the PGC-1α/ROS/apoptosis signaling pathway,
AS-IV promotes mitochondrial biosynthesis, as well as the
upregulation of mitochondrial membrane potential, in the peritoneal
tissue of peritoneal fibrosis rats by activating its
pharmacological target PGC-1α. Furthermore, it reduces the
excessive accumulation of ROS in the mitochondrial membrane,
thereby inhibiting PMC apoptosis and alleviating peritoneal
fibrosis progression (191).
miR-204-5p/Foxc1/β-catenin
miRNAs can mediate intercellular signaling
transduction. Exosomes are responsible for transporting miRNAs.
Exosomes carrying miRNAs circulate in the body and are taken up by
different cells, thereby targeting functional changes in recipient
cells (192,195,196). Shan et al (192) found that after LPS activates
primary rat peritoneal macrophages and THP-1 monocyte cells, it
induces the inflammatory infiltration of the abdominal cavity and
promotes MMT. AS-IV pretreatment can alleviate the upregulation of
vimentin and α-SMA and the downregulation of E-cadherin induced by
inflammatory macrophages can also be reversed under AS-IV
mediation. In addition, primary PMCs (RPMCs) uptake LPS-stimulated
macrophage-derived exosomes. This phenomenon can further drive the
occurrence of the MMT of RPMCs. However, AS-IV pretreatment can
inhibit the promoting effect of exosomes on MMT. Furthermore, the
identification of exosomal miRNA profiles revealed that AS-IV can
activate miR-204-5p carried by inflammatory macrophage-derived
exosomes. miR-204-5p has anti-inflammatory and antifibrotic
activities and can negatively regulate the expression of forkhead
box C1 (Foxc1). Foxc1 has been reported to drive MMT progression by
activating β-catenin in fibroblasts (197). Therefore, the
miR-204-5p/Foxc1/β-catenin axis may be a key signaling cascade in
regulating the MMT of RPMCs. AS-IV can further inhibit the
activating effect of downstream Foxc1 on β-catenin by targeting
miR-204-5p overexpression, thereby alleviating peritoneal fibrosis
and abdominal cavity inflammation associated with HG dialysis.
Inflammatory macrophage-derived exosomes are also a key mediator
for the regulation of signaling transduction between macrophages
and RPMCs and alleviation of PD-induced peritoneal fibrosis by
AS-IV (192).
SSc-related fibrosis
SSc, a heterogeneous, autoimmune connective tissue
disease, is induced by the massive proliferation of fibroblasts and
excessive ECM synthesis, which causes scar tissue to replace the
physiological structure of blood vessels, skin and internal organs.
Local skin tissue lesions and organ fibrosis eventually occur. Qi
et al (198)
demonstrated that AS-IV can reduce the deposition of collagen I and
FN in SSc fibroblasts, as well as inhibiting highly activated
p-Smad3 and the nuclear localization of p-Smad3 in SSc fibroblasts.
Furthermore, AS-IV induces the blockage of TGF-β/Smad3 signaling
transduction and further regulates the inactivation of the
profibrotic mediator Smad3/friend leukemia virus integration 1
downstream of TGF-β, thereby alleviating SSc-related fibrosis.
Skin scarring and fibrosis
Skin wound treatment advocates rapid tissue repair
while minimizing scarring. Notably, the activation of CTGF by
TGF-β1 is a key factor in regulating scar tissue hyperplasia.
According to Chen et al (199), AS-IV could further enhance
wound re-epithelialization during the proliferative phase of wound
healing. This effect is mainly attributed to the remarkable
induction of keratinocyte migration and proliferation. In addition,
AS-IV exerts a scar-inhibiting effect similar to that of TGF-β
antibodies by reducing TGF-β1 secretion. Most of the collagen in
skin wounds is scarred and has lost its original highly organized
structure. However, AS-IV can improve the tensile strength of
healed skin (199). Moreover,
scar tissue is often dominated by collagen I, whereas collagen III
is lost. AS-IV can also maintain a low level of collagen I/III
content, promote the content of collagen III related to epidermal
repair and restore the elasticity of damaged skin. In short, AS-IV
promotes skin wound repair and inhibits scar hyperplasia. These
effects are related to inducing wound re-epithelialization,
negatively regulating TGF-β1 secretion, regulating collagen I/III
homeostasis and remodeling ECM (199).
Sodium alginate-gelatin (GT) hydrogel incorporated
with AS-IV can improve skin wound repair and collagen synthesis and
regulate serum TGF-β1 levels and skin tensile strength recovery.
Intervening with a drug carrier that is compatible with AS-IV may
be necessary in consideration of the frequency of medication. Solid
lipid nanoparticles (SLNs) attracted the attention of Chen et
al (200) because of their
effectiveness in acting on epidermal tissue (201). By administering AS-IV-loaded
SLN-enriched gel, the synthesis of collagen I can be induced,
thereby promoting the physiological structure of the repaired
epidermal wound to closely resemble that of the skin. In addition,
AS-IV-based SLN-gel can regulate collagen fiber arrangement and
collagen deposition and reduce scar formation. In summary,
AS-IV-based SLN-gel, mediated by the caveolae endocytosis pathway,
can induce keratinocyte proliferation and migration and promote the
uptake of AS-IV by fibroblasts. The latter phenomenon is consistent
with the ability of AS-IV-based SLN-gel to release drugs
continuously and slowly and prolong the time of drug action on
epidermal tissue repair (200).
About 2 million new cases of burns are reported
every year worldwide. Covering with wound dressings often
intervenes in the healing of local burn wounds, which are as severe
skin traumas (202).
Electrospun nanofibers can be used as structural mimics of ECM;
although biocompatible silk fibroin (SF) scaffolds provide a
supportive environment for cell adhesion and proliferation or
tissue repair, their degradation is slow and may require further
processing improvements (202).
GT is not only beneficial for compensating for the disadvantages of
SF scaffolds in degradation but is also a hydrolysis product of
collagen. Therefore, Shan et al (202) found that the combined
processing of GT-added SF scaffolds and nanofibrous wound dressings
may be beneficial for the pharmacological activity of AS-IV in
promoting healing and antiscarring. This benefit is mainly
reflected in the ability of AS-IV-loaded SF/GT nanofibrous dressing
(25/75) to release drugs quickly within 12 h, with their sustained
release time reaching 12-24 h. AS-IV-loaded SF/GT nanofibrous
dressing can promote postinjury vascular regeneration by
upregulating the activity of vascular endothelial growth factors
and regulate the relative regular arrangement of collagen fibers to
inhibit the formation of scar tissue during burn healing.
AS-IV-loaded SF/GT nanofibrous dressing (25/75) can increase the
collagen I content of the healed skin and restore normal skin
tissue structure. In addition, the swelling rate of AS-IV-loaded
SF/GT nanofibrous dressing (25/75) is markedly increased. This
effect is beneficial for absorbing excess exudate from local burned
skin tissue and preventing bacterial infections. In summary,
applying nanofibrous dressings with the optimal SF:GT ratio of
25:75 to heal burn wounds and successfully loading the insoluble
drug AS-IV can improve the biological properties of functional burn
wound dressings and promote cell proliferation and angiogenesis,
thus inhibiting scar hyperplasia (202).
Trabecular meshwork fibrosis
Of patients with glaucoma, ~74% present with
primary open-angle glaucoma (POAG) (203). The pathogenesis of POAG can be
summarized as follows: endoplasmic reticulum (ER) stress promotes
the excessive deposition of ECM in the trabecular meshwork (TM) by
interfering with ECM protein processing, thereby inducing TM
fibrosis. TM fibrosis further regulates the increase in aqueous
humor (AH) outflow resistance. The latter becomes a key cause of
increased intraocular pressure (IOP), which becomes a risk factor
that ultimately leads to the occurrence of POAG. The overexpression
of TGF-β2 in AH and TM is also an inducing factor for POAG
(204). Kasetti et al
(203) showed that AS-IV can
markedly suppress TGF-β2-induced EMT and ER stress in TM cells. In
addition, MMPs are involved in regulating AH outflow, IOP and ECM
remodeling in the TM. AS-IV can negatively regulate TGF-β2-induced
MMP9 inactivation and further enhance the compensatory upregulation
of MMP2 expression by TGF-β2 (203). Moreover, ocular AS-IV treatment
reverses TGF-β2-induced ocular hypertension and NF-κB
overexpression in mice. The downregulation of pNF-κB may activate
the endogenous TGF-β inhibitor, thereby blocking TGF-β2 signaling
transduction and reducing TM fibrosis. In summary, AS-IV markedly
alleviates TGF-β2-induced POAG-related ocular hypertension by
inhibiting TM cell fibrosis and TM tissue ER stress and
upregulating TM cell MMP activity (Table I) (203).
 | Table ISummary of antifibrotic effects and
underlying mechanisms of AS-IV. |
Table I
Summary of antifibrotic effects and
underlying mechanisms of AS-IV.
| Authors, year |
Fibroticdisease | Dosages | Models | In
vivo/in vitro | Effects and related
mechanisms | (Refs.) |
|---|
| Wan et al,
2018 | Cardiac
fibrosis | 100/200 mg/kg AS-IV
and 31.25/62.5 mg/kg CA, i.g.; 31.25 μg/ml CA. | ISO-induced cardiac
fibrosis in mice; primary rat CFs. | In vivo and
in vitro | Demonstrating a
protective effect against cardiac fibrosis by blocking the NLRP3
inflammasome pathway. | (41) |
| Wei et al,
2020 | | 10 mg/kg, p.o.; 10
μM. | ISO-induced cardiac
fibrosis in rats; CFs of neonatal rats. | In vivo and
in vitro | Targeting the
miR-135a-TRPM7-TGF-β/Smads pathway to prevent cardiac
fibrosis. | (42) |
| Lu et al,
2017 | | 5 mg/kg, 10 mg/kg,
i.p.; 1, 10 μM. | ISO-induced cardiac
fibrosis in rats; neonatal rat CFs, TRPM7 knockdown NIH-3T3
cells. | In vivo and
in vitro | Suppressing TRPM7
expression to prevent hypoxia-induced MF. | (43) |
| Chai et al,
2024 | | 5, 10, 50
μM. | LPS-induced cardiac
hypertrophy and fibrosis in H9C2 cardiomyocytes. | In
vitro | Suppressing CCL2
expression and downregulating NF-κB signaling pathway in a
CCL2-dependent manner. | (47) |
| Jia et al,
2017 | | 80 mg/kg, i.g.; 100
μM. | ISO-induced cardiac
fibrosis in rats; primary CFs. | In vivo and
in vitro | Inhibiting collagen
synthesis and CF proliferation by blocking ROS-mediated CT-1
upregulation. | (50) |
| Dai et al,
2017 | | 100 μM. | ISO-induced CF
proliferation. | In
vitro | Reducing
ROS-mediated MAPK signaling activation, which in turn prevents
ISO-induced CF proliferation and cardiac fibrosis. | (53) |
| Cong et al,
202 | | 80 mg/kg, i.p. | Ang II-induced
atrial fibrosis and atrial fibrillation in mice. | In vivo | Atrial fibrillation
and atrial fibrosis brought on by Ang II are prevented by
upregulating the SIRT1/PGC-1α/FNDC5 pathway. | (55) |
| Wang et al,
2020 | | 80 mg/kg, i.g. | STZ and HFD
treatments-induced T2DM in rats. | In vivo | Enhancing
myocardial lipid metabolism, which in turn reduces MF and
inflammation in T2DM rats. | (57) |
| Zhang et al,
2022 | | 40 mg/kg, i.g.; 100
μM. | Ligation of the LAD
coronary artery-induced MI in mice; BMDMs. | In vivo and
in vitro | MI-induced cardiac
remodeling and MF are lessened by inhibiting the
ROS/caspase-1/GSDMD signaling pathway. | (59) |
| Shi et al,
2024 | | 100, 200 mg/kg,
p.o.; 20, 40, 80 μM. | ISO-induced cardiac
fibrosis in mice; H9C2 cells. | In vivo and
in vitro | Suppressing
oxidative stress and SASPs, modulating the p53 signaling pathway to
reduce ISO-induced MF. | (62) |
| Chen et al,
2011 | | AS-IV-containing
drinking water (300 mg/l); 20 μg/ml. | CVB3-induced DCM;
primary cardiomyocytes and fibroblasts of neonatal rats. | In vivo and
in vitro | Reducing MF in DCM
brought on by CVB3 by inhibiting TGF-β1-Smad signaling. | (65) |
| Du et al,
2022 | | 100 mg/kg,
p.o. | ISO-induced cardiac
fibrosis in mice. | In vivo | Elevating
Akkermansia abundance, which influences phenylalanine metabolism to
alleviate cardiac fibrosis. | (68) |
| Guan et al,
2024 | Pulmonary
fibrosis | 5 ml/kg, i.g.; 100
μg/ml. | BLM-induced PF in
rats; A549 cells. | In vivo and
in vitro | Regulating the
lncRNA-ATB/miR-200c/ZEB1 signaling pathway to suppress the EMT
process. | (70) |
| Li et al,
2021 | | 20 mg/kg, i.p. | Silica-induced PF
in rats. | In vivo | Inhibiting the
TGF-β1/Smad signaling cascade, inflammation and oxidative stress to
alleviate PF. | (77) |
| Li et al,
2019 | | 20 mg/kg, i.p.;
7et al, 20, 60 μg/ml. | Silica-induced rat
model; silicosis fibroblasts. | In vivo and
in vitro | Demonstrating the
effect of anti-silicosis fibrosis by preventing Smad3 from being
continuously phosphorylated. | (78) |
| Hou et al,
2021 | | 7, 20, 60
μg/ml. | TGF-β-treated A549
cells. | In
vitro | Preventing EMT and
fibrosis by inhibiting the expression of the NLRP3 protein. | (79) |
| Yu et al,
2016 | | 10, 20, 50 mg/kg,
i.p. | BLM-induced PF in
rats. | In vivo | Alleviating PF by
inhibiting BLM-induced inflammation and oxidative stress. | (80) |
| Li et al,
2023 | | 5, 10, 20 mg/kg,
i.g. | K.
pneumoniae-treated rats. | In vivo | Alleviating the
inflammation by inhibiting the TGF-β1/Smad pathway. | (82) |
| Qian et al,
2018 | | 20 mg/kg, i.g.; 100
μg/ml. | BLM-induced PF in
rats; A549 cells. | In vivo and
in vitro | Preventing FOXO3a
downregulation brought on by TGF-β1/PI3K/AKT to alleviate EMT in
BLM-induced PF. | (84) |
| Zheng et al,
2024 | | AS_LIG@PPGC NPs
(AS-IV: 25 mg/kg, LIG: 50 mg/kg), inhalation. | BLM-induced PF in
mice; the murine fibroblast line L-929. | In vivo and
in vitro | Demonstrating the
antifibrotic properties by blocking the NOX4-ROS-p38 MAPK and
NOX4-NLRP3 signaling pathways. | (85) |
| Xi et al,
2023 | | 30 mg/kg, i.p.; 20
μM. | MCT-induced PAH in
rats; PASMCs. | In vivo and
in vitro | Modulating the
PHD2/HIF1α signaling pathway to inhibit NLRP3-mediated pyroptosis
and fibrosis in PASMCs. | (87) |
| Yuan et al,
2023 | | 50, 100 mg/kg;
12.5, 25, 50 μM. | BLM-induced PF in
mice; . A549 cells | In vivo and
in vitro | Alleviating PF
through the inhibition of cellular senescence and EMT. | (90) |
| Li et al,
2017 | | 10 mg/kg, i.g. | BLM-induced PF in
rats. | In vivo | Inhibiting the
release of HMGB1 and the deposition of ECM to exert the
antifibrotic property. | (92) |
| Zhu et al,
2024 | | 20 mg/kg, i.p.; 25,
50, 75 μg/ml. | BLM-induced PF in
rats; HFL1 cells. | In vivo and
in vitro | Impeding PF process
by regulating the circ_0008898/miR-211-5p/HMGB1 axis. | (98) |
| Tong et al,
2021 | | FA (24 mg/kg) +
AS-IV (40.8 mg/kg). | BLM-induced PF in
mice. | In vivo | Mitigating PF by
lowering oxidative stress and blocking the TGF-β1/Smad3 signaling
pathway through miR-29b regulation. | (101) |
| Li et al,
2024 | | 40 mg/kg, i.g.;
0-50 μmol/l. | BLM-induced IPF in
mice; A549 cells. | In vivo and
in vitro | Suppressing IPF by
triggering autophagy via the PTEN/PI3K/AKT/mTOR pathway mediated by
miR-21. | (103) |
| Tian et al,
2024 | | 100 mg/kg, i.p.; 50
μM. | PM2.5-induced PF in
rats; rat alveolar epithelial cells L2. | In vivo and
in vitro | Inhibiting
PM2.5-induced PF by regulating the miR-362-3p/RUNX1 pathway. | (108) |
| Zhang et al,
2023 | | 2 mg/kg, i.p.; 10
μM. | LPS or/and CS to
simulate PF in COPD of mice; BEAS-2B cells, N-HLF cells. | In vivo and
in vitro | Demonstrating the
effect of anti-PF in COPD by downregulating the RAS/RAF/FOXO
signaling pathway. | (113) |
| Guo et al,
2024 | | Bu Yang Huan Wu
Decoction: 0.99, 1.98, 3.97 mg/kg. | HFD, STZ and BLM
treatments-induced diabetic PF model in rats. | In vivo | Targeting the
AGE-RAGE signaling pathway to suppress IL-6-induced PF. | (115) |
| Li et al,
2023 | Renal fibrosis | 40, 80 mg/kg; 10,
50, 100, 150 μM. | Adenine-induced CKD
model in rats; HK-2 cells. | In vivo and
in vitro | Regulating
AKT/mTOR-mediated autophagy by enhancing ALDH2 expression, which
alleviates renal fibrosis. | (123) |
| Su et al,
2019 | | 20, 40, 80 mg/kg/d,
i.g.; 20, 40, 80 μM. | HFD and STZ
treatments-induced DKD in rats; HMCs. | In vivo and
in vitro | Inhibition of
oxidative stress and fibrosis caused by PA via suppressing CD36
expression. | (125) |
| Du et al,
2018 | | G-Rg1: 50 mg/kg,
i.g.; AS-IV: 16 mg/kg, i.g. | STZ-induced DKD in
rats. | In vivo | Preventing DKD by
lowering oxidative stress and blocking the TGF-β1/Smads signaling
cascade. | (127) |
| Zhang et al,
2020 | | 80 mg/kg, i.g. | HFD and STZ
treatments-induced DKD in rats. | In vivo | The downregulation
of NLR signaling by AS-IV may be linked to the inhibition of renal
fibrosis and inflammation. | (128) |
| Chen et al,
2018 | | 50, 100, 200
μg/ml. | HG-treated HK-2
cells. | In
vitro | Mitigating renal
tubular EMT by suppressing the mTORC1/p70S6K signaling
pathway. | (131) |
| Wang et al,
2018 | | 40 mg/kg, i.g.;
12.5, 25, 50 100 μM. | HFD-induced DKD
model in KK-Ay mice; MCs, primary mouse podocytes. | In vivo and
in vitro | Suppressing
podocyte dedifferentiation and MC activation caused by miR-21
overexpression. | (135) |
| Wang et al,
2019 | | 40 mg/kg, i.g.; 25,
50, 100 μmol/l. | HFD-induced DKD in
KK-Ay mice; immortalized mouse podocyte cell line. | In vivo and
in vitro | Attenuating the EMT
of podocytes by modulating the SIRT1-NF-κB pathway and autophagy
activation. | (137) |
| Wang et al,
2018 | | 40 mg/kg, i.g.; 25,
50, 100 μM. | HFD-induced DKD in
KK-Ay mice; mouse glomerular MC line. | In vivo and
in vitro | Regulating the
SIRT1-NF-κB pathway and promoting autophagy to inhibit renal
fibrosis and MC activation. | (138) |
| Hu et al,
2022 | | 10, 20, 40 mg/kg,
i.g.; 40 μM. | T2DM model in mice;
HK-2 cells. | In vivo and
in vitro | Down-regulating the
RAF/MEK/ERK pathway by suppressing CX3CL1 expression, which
alleviates the EMT. | (141) |
| Zhou et al,
2017 | | 20, 40 mg/kg, i.g.;
10, 20 μM. | UUO-induced renal
fibrosis in mice; HK-2 cells. | In vivo and
in vitro | Suppressing
inflammation through the TLR4/NF-κB signaling pathway to alleviate
renal fibrosis. | (143) |
| Wang et al,
2014 | | 3.3, 10, 33 mg/kg;
2, 5, 10, 20, 40 μmol/l. | UUO-induced renal
fibrosis in rats; NRK-49F cells. | In vivo and
in vitro | The suppression of
fibrosis is linked to the activation of Smad7, which in turn
inhibits TGF-β/Smad signaling. | (146) |
| Lian et al,
2021 | | 10, 40, 80
μg/ml. | EMT model of
NRK-52E cells induced by TGF-β1. | In
vitro | Decreasing the
phosphorylation of AKT and mTOR and increasing Cx43 expression in
RTECs to inhibit EMT. | (147) |
| Yu et al,
2022 | | 80 mg/kg, i.g.; 10,
20, 40 μM. | UIRI-induced renal
fibrosis in rats; NRK-52E and NRK-49F cells. | In vivo and
in vitro | Mitigating renal
fibrosis through the regulation of the AKT1/GSK-3β pathway. | (150) |
| Wang et al,
2014 | | 3.3, 10, 33 mg/kg,
i.g. | UUO-induced renal
interstitial fibrosis in rats. | In vivo | Lowering the
expression of proteins involved in the Wnt/β-catenin signaling
pathway to alleviate renal interstitial fibrosis. | (151) |
| Che et al,
2015 | | 10, 50, 100
μg/ml. | TGF-β1-induced
mouse renal fibroblasts. | In
vitro | Regulating the MAPK
and NF-κB signaling pathways to alleviate renal interstitial
fibrosis. | (152) |
| Xu et al,
2014 | | 20 mg/kg, i.p.; 50,
100, 200 μg/ml. | UUO-induced renal
tubulointerstitial fibrosis in mice; HK-2 cells. | In vivo and
in vitro | Alleviating renal
fibrosis via blocking the phosphorylation of p38 and JNK MAPKs and
apoptosis. | (155) |
| Meng et al,
2011 | | AF-L/H (10.8
+ 4.8/20.4 + 12 mg/kg), i.g.; AF (24 + 43
μmol·l−1). | UUO-induced renal
tubulointerstitial fibrosis in rats; NRK-49F and HK-2 cells. | In vivo and
in vitro | Suppressing the
tubular EMT and fibroblast activation and promoting NO generation
to mitigate fibrosis. | (156) |
| Zhang et al,
2024 | | 40 mg/kg, i.g.; 15
μg/ml. | UUO-induced renal
fibrosis in rats; NRK-52E cells. | In vivo and
in vitro | Preventing
pyroptosis and EMT by blocking the cAMP/PKA signaling pathway. | (160) |
| Cao et al,
2019 | | 20 mg/kg, i.g.;
7.5, 15, 30 mg/ml. | UUO-induced renal
fibrosis model in mice; HK-2 cells. | In vivo and
in vitro | Suppressing renal
fibrosis, at least partly through the inhibition of the TGF-β1/ZEB2
signaling. | (162) |
| Wang et al,
2024 | | 40 mg/kg, i.g.; 10,
20, 40 μM. | DCC
treatment-induced model of liver fibrosis to HCC in
Nrf2−/− mice; HK-2 cells. | In vivo and
in vitro | Inhibiting renal
fibrosis from hepatocarcinogenesis via the regulation of the
Nrf2/HO-1 and pSmad3C/3L signaling pathways. | (165) |
| Zhang et al,
2021 | Liver fibrosis | 20, 40, 80 mg/kg,
i.g.; 5, 10, 20 μM. | DCC-induced liver
fibrosis and liver cancer in mice; HSC-T6 and HepG2 cells. | In vivo and
in vitro | Preventing primary
liver cancer by inhibiting the development of fibrosis and
modulating pSmad3C/3L and Nrf2/HO-1 pathways. | (167) |
| Boye et al,
2015 | | CASE (60, 120, 240
mg/kg), i.g.; 20, 40, 80 μg/ml. | DEN-induced HCC in
rats; HSCs, HepG2 cells. | In vivo and
in vitro | HCC and liver
fibrosis are alleviated by suppressing PAI-1 expression and
modulating the MAPK-regulated TGF-β/Smad signaling. | (181) |
| Zhao et al,
2020 | | FA (10.8 mg/kg) and
AS-IV (4.8 mg/kg); i.g. | BDL-induced liver
fibrosis in rats. | In vivo | HSCs are
deactivated by promoting the Nrf2 pathway and inhibiting the TGF-β
pathway. | (184) |
| Dong et al,
2017 | | 4 μM FA
and/or 2 μM AS-IV. | HSC-T6 cells. | In
vitro | Suppressing HSC
activation by inhibiting p38 MAPK phosphorylation through Nrf2/ARE
pathway activation and TGF-β1/Smad pathway blocking. | (185) |
| Li et al,
2013 | | 3, 10, 30, 100
μM. | HSCs. | In
vitro | Suppressing HSC
activation by activating Nrf2 and downregulating p38 MAPK
activity. | (187) |
| Liu et al,
2009 | | 2.0, 4.0 mg/kg,
i.g.; 0, 1.5, 3, 6, 12, 24 mg/l. | PS-induced liver
fibrosis in rats; primary cultured HSCs. | In vivo and
in vitro | Down-regulating
TGF-β1 and suppressing the activation of HSCs to alleviate liver
fibrosis. | (190) |
| Xie et al,
2023 | Peritoneal
fibrosis | 20, 40 mg/kg, i.p.;
10, 20, 30 μg. | HG peritoneal
fluid-induced peritoneal fibrosis in rats; PMCs of rats. | In vivo and
in vitro | Regulating the
signaling cascade linked to mitochondrial biosynthesis, ROS and
apoptosis to suppress peritoneal fibrosis. | (191) |
| Shan et al,
2024 | | 5 or 10 mg/kg,
i.p.; 150 μM. | HG peritoneal
fluid-induced peritoneal fibrosis in rats; RMacs, RPMCs. | In vivo and
in vitro | Mitigating the
fibrosis caused by macrophage-derived exosomes in PD via the
miR-204-5p/Foxc1 pathway. | (192) |
| Qi et al,
2014 | Systemic
sclerosis-related fibrosis | 2, 4 mg/kg, i.g.;
1, 3, 10, 30 μM. | BLM-induced skin
fibrosis mice model; dermal fibroblasts from normal and SSc
samples. | In vivo and
in vitro | Mitigating fibrosis
by suppressing the TGF-β-Smad3 axis in SSc. | (198) |
| Chen et al,
2012 | Skin scarring and
fibrosis | The concentration
of 0.5%; 12.5-200 μmol/l. | The full-thickness
skin excision wounds are made in rats; HSFs and HaCaT keratinocyte
cell line. | In vivo and
in vitro | Lowering the levels
of collagen I/III and TGF-β1 secretion to inhibit the scar
complications of wound healing. | (199) |
| Chen et al,
2013 | | 0.5 mg/2 d; 50, 100
μmol/l. | The rat full-skin
excision model, skin irritation test in New Zealand rabbits; HSFs,
keratinocytes. | In vivo and
in vitro | Promoting the
proliferation and migration of keratinocytes and the drug uptake on
fibroblasts. | (200) |
| Peng et al,
2012 | | 0.5 mg
AS-IV-containing sodium alginate-GT hydrogel. | The rat skin
excision model. | In vivo | Regulating skin
regeneration and serum TGF-β1 levels, improving collagen synthesis
and skin tensile strength. | (201) |
| Shan et al,
2015 | | 0.5 mg/2 d; the
AS-IV-loaded SF/GT nanofibrous dressing (25/75). | The
partial-thickness burn wound model in rats; keratinocytes, human
fibroblast cells. | In vivo and
in vitro | Inducing
pro-angiogenesis and cell proliferation and preventing scar
complications in wounds by improving collagen organization. | (202) |
| Kasetti et
al, 2021 | Trabecular meshwork
fibrosis | AS-IV topical
ocular eye drops, 1 mM, twice a day; 50, 100 μM. | TGF-β2-induced
ocular hypertension in mice; human primary TM cells. | In vivo and
in vitro | Lowering IOP via
suppressing the TGF-β2-induced fibrotic lesions and ER stress in TM
cells. | (203) |
Clinical translation of AS-IV
Although the antifibrotic effect of AS-IV has been
confirmed in various animal or cell models, preclinical animal
experiments generally have limitations, such as methodological
problems, high bias risk and low reproducibility, as well as
differences in pathophysiology across different tissues (205). This situation indicates that
further cross-species studies, such as large-sample, double-blind
and randomized human clinical trials, remain necessary. The
signaling pathways and molecular targets regulating fibrotic
diseases are intricate and given that humans are complex organisms,
the further in-depth integration of network regulatory
relationships will promote the clinical translation of AS-IV.
In addition, most current safety evaluations of
AS-IV are based on systemic administration models, with no toxicity
dose testing specifically targeting the liver (206). In model organisms, the
administration dose of AS-IV for antifibrotic purposes show large
variations. Therefore, clarifying the reference range of safe and
effective doses will ensure the reproducibility of the antifibrotic
therapeutic outcomes of AS-IV. Given its activating effect on the
immune system, AS-IV has been confirmed to potentially induce the
development of autoimmune diseases, virtually increasing the
challenge of quantifying its toxicity (207). Furthermore, in rats, AS-IV may
trigger an increase in nerve conduction velocity and mechanical
withdrawal threshold (207).
This concurrent neurotoxicity implies that the adverse reactions of
AS-IV administration in human clinical practice may need to be
further and extensively defined through numerous toxicological
experiments. However, AS-IV administered at conventional biological
doses is generally considered safe and has been widely developed as
a bioactive component in various TCM preparations, such as
Shen-Qi-Jiang-Tang and Zhen-Qi-Fu-Zheng granules and Qi-Shen-Yi-Qi
pills (27).
Although AS-IV has strong lipophilicity
(theoretically calculated lipid/water partition coefficient of
2.0), it has a high molecular weight, low hydrophilicity, low
permeability on the intestinal mucosa and undergoes paracellular
transport in the form of passive transport, resulting in low
absorption fraction of AS-IV in various target organs or tissues of
the body (208,209). Moreover, AS-IV has poor in
vivo target specificity that limits its bioavailability.
Nevertheless, evidence has shown that copper silicate nanoparticles
modified with polyethylene glycol and loaded with AS-IV exhibit
considerable clinical efficacy in improving osteoarthritis
(210). Conductive bioadhesive
hydrogels that enable the sustained release of AS-IV have
advantages in clinical translation for mediating cardiac repair
(211). Moreover, biomimetic
nanodelivery systems loaded with AS-IV have application value in
sepsis treatment (212).
Meanwhile liposomes loaded with AS-IV are valuable in breast cancer
immunotherapy (213). All these
drug delivery systems provide sufficient reference evidence for
optimizing the organ-targeting properties of AS-IV in antifibrotic
therapy. Not only that, chitosan and sodium deoxycholate have the
biological activity of promoting the opening of tight junctions
between cells and are considered enhancers of the paracellular
transport pathway. Therefore, the synergistic administration of
chitosan and sodium deoxycholate can enhance the permeability
coefficient of AS-IV through intestinal mucosa and enhance its
pharmacodynamic absorption index (209). Notably, the lipid solubility
and cell membrane permeability of CA are superior to those of
AS-IV. Therefore, the application of biotransformation technology
and Bacillus LG-502 to promote the conversion of AS-IV into
CA, as well as the synergistic hydrolysis of AS-IV using heat- and
sugar-resistant enzymes purified from Dictyoglomus
thermophiles to improve the conversion rate of AS-IV, enable
the convenient enhancement of AS-IV's antifibrotic therapeutic
efficacy without relying on structural modification or the
introduction of novel formulations (214).
Due to the diversity of modification sites and the
uniqueness of the backbone structure, natural triterpenoids have
become a research hotspot in numerous fields (21). Among them, AS-IV is also a
tetracyclic triterpene saponin derived from natural herbs and the
side chain of the A-ring at the C-2 and C-3 positions and the
D-ring are the main modification sites. AS-IV mainly depends on the
introduction of hydroxyl, ester and nitrogen-containing groups for
modification (21). However, the
high biological activity of AS-IV dictates that it has a diverse
set of active binding sites, but this also indirectly leads to a
severe test of how to invent the highly soluble derivatives of
AS-IV by introducing hydrophilic groups or polar hydroxyl groups
(215). Astragalosidic acid
(LS-102) is a water-soluble derivative synthesized through AS-IV.
Its relative bioavailability is twice that of the parent compound.
Compared with AS-IV, the maximum plasma concentration of the
derivative and the area under the plasma concentration-time curve
are markedly increased and the permeability through the intestinal
mucosa and intestinal absorption index are improved. In addition,
the half-life of LS-102 is markedly shorter than that of AS-IV,
which means that LS-102 is not prone to excessive accumulation of
drug toxicity in the body and can be rapidly metabolized. This can
be confirmed in the toxicity experiments of acute administration of
high doses of LS-102, even when LS-102 is administered at a
single-dose of up to 5,000 mg/kg body weight, no adverse reactions
or even death are observed in mice (215). Therefore, given that the
solubility of LS-102 has been markedly improved compared to the
precursor compound AS-IV, it can be employed as a novel synthetic
drug containing carboxylic acid to treat metabolic diseases such as
obesity through oral administration (21,215). Besides the development of
LS-102 to improve the oral bioavailability of AS-IV, AS-IV-loaded
nanomicelles, as a product of novel formulation technology, act as
a crucial reference in advancing the clinical translation of AS-IV
(27). In short, chemical group
or site modification, development of derivative compounds, combined
drug administration and application of green nanomaterials are
beneficial to improving the low hydrophilicity of AS-IV. In the
future, attention should be paid to the improvement effect of these
new improved strategies on the bioavailability of AS-IV (16). Finally, differences in the
sources of Astragalus (in terms of species, purity and
quality) and extraction technologies can lead to variations in the
antifibrotic effect of AS-IV. This situation indicates that the
selection of high-quality Astragalus is an important
prerequisite for ensuring the clinical antifibrotic efficacy of
AS-IV.
Conclusions and perspectives
The antifibrotic activity of AS-IV, a natural
compound extracted from Astragalus, is affirmed. AS-IV can
regulate the abundance of gut microbiota, senescence of cells and
ion-channel currents of fibroblasts and inhibit oxidative stress,
inflammation, pyroptosis and lipotoxicity, thereby alleviating
collagen deposition in myocardial tissue. Moreover, AS-IV regulates
the TGF-β1/Smads, PHD2/HIF1α and AGE-RAGE signaling axes and
diverse noncoding RNAs and aging markers. It also maintains FOXO3a
activity, inhibits autophagy impairment and ECM synthesis. It can
be combined with nanotechnology for pulmonary inhalation therapy,
thereby demonstrating good bioactivity in alleviating PF. AS-IV
targets the expression of CD36, NOX4, TGF-β1 and MAPK and regulates
the Wnt/β-catenin, mTORC1/p70S6K, pSmad3C/p21, pSmad3L/PAI-1 and
PI3K/AKT/mTOR signaling pathways, thereby further inhibiting HMC
fibrosis, renal fibrosis complicated by DKD or liver cancer,
obstructive nephropathy-related fibrosis and the EMT of RTECs.
AS-IV relies on its anticancer, antioxidative and immune-regulating
activities to exert multitarget benefits in alleviating liver
fibrosis, which is closely related to the activation of Nrf2,
inhibition of MAPK phosphorylation and TGF-β1 overexpression and
regulation of pSmad3C/pSmad3L toward outputting tumor-suppressive
signals. Furthermore, AS-IV can inhibit EMT and PMC apoptosis by
targeting mitochondrial biosynthesis. It can also mitigate
peritoneal fibrosis mediated by macrophage-derived exosomes
carrying miRNAs. Finally, AS-IV has the effects of antiscarring,
promoting skin wound repair and alleviating SSc and glaucoma. These
effects are related to its ability to regulate collagen homeostasis
and wound re-epithelialization and inhibit TGF-β signaling
transduction and TM fibrosis.
Studies have mainly explored the regulatory effects
of AS-IV on fibrosis from the perspectives of experimental animal
models at the cellular, molecular and genetic levels, with most
only focusing on single drug components and large-sample
placebo-controlled, randomized and standardized double-blind
experimental data regarding TCM treatments awaiting further
investigation (10,14). Therefore, further combining
numerous clinical trials to confirm through convincing population
studies that AS-IV is applicable not only to antifibrotic treatment
in basic experimental research is imperative. Moreover, prospective
cohort studies involving the antifibrotic effects of AS-IV are
lacking. This situation indicates that further long-term follow-up
experiments are still needed to define the effective treatment
period of AS-IV. Therefore, introducing drug development
technologies, such as computer-aided drug design, high-throughput
screening, systems modeling and simulation to obtain derivatives of
AS-IV with increased bioavailability and conducting targeted
validation or further adopting biocompatible drug carriers, such as
nanoparticles, liposomes and micelles encapsulating AS-IV, may be
required to achieve its efficient delivery. Although some studies
have already employed high-sensitivity bioinformatics analysis
techniques to verify the potential binding targets of AS-IV for
antifibrosis effects, relying solely on acute or subacute toxicity
tests on animal models cannot directly reflect the
dose-time-pharmacological/toxicological effects of AS-IV in humans.
This limitation may become a main obstacle to the conversion of
AS-IV into a novel clinical drug. The application of reverse
pharmacokinetics may be a key strategy to improve the conversion
potential of AS-IV and research focusing on the fibrotic
microtissue model also strives to simulate the fibrotic
microenvironment in the human body. Such an approach weakens doubts
about the antifibrotic feasibility of AS-IV because of the
metabolic differences between humans and experimental animals.
Notably, although natural products are mostly bioactive substances
produced by plants to resist microbial and other exogenous
interference, their extractable medicinal content is low. In
addition, compared with chemical compounds, natural products have
more diverse structures and complex biological activities. These
characteristics increase the difficulty of their subsequent total
or semisynthesis. Therefore, optimizing lead compounds on the basis
of the structure-activity relationship analysis of AS-IV and the
innovation of plant tissue culture techniques may help further
screen small-molecule derivatives with enhanced pharmacological
activity. Finally, multiple studies have shown that AS-IV has
potential protective advantages against diverse fibrotic diseases
and its targeted pathways involved have overlaps. These overlaps
include regulating the phosphorylation of MAPK and signal
transduction of p53, TGF-β/Smads, PI3K/AKT/mTOR and other pathways.
However, current research has not verified whether AS-IV can
simultaneously inhibit multiorgan fibrosis by acting on a specific
signaling molecule or cascade. Therefore, this topic may be worth
further in-depth research in the future to enhance the multitarget,
multibenefit antifibrosis activity of AS-IV and its TCM
compounds.
Availability of data and materials
Not applicable.
Authors' contributions
MW was responsible for conceptualization, data
curation, writing and preparation of original draft. KL was
responsible for data curation, writing, reviewing, editing and
validation. JW was responsible for writing, reviewing, editing and
validation. QZ writing, reviewing, editing and data curation. XM
was responsible for writing, reviewing, editing and investigation.
WD was responsible for writing, reviewing, editing and validation.
HG was responsible for writing, reviewing, editing and
investigation. XD was responsible for writing, reviewing, editing
and validation. WW: was responsible for supervision, project
administration, writing, reviewing and editing. WX was responsible
for funding acquisition, project administration, supervision,
writing, reviewing and editing. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AGEs
|
advanced glycation end-products
|
|
AKT
|
protein kinase B
|
|
AS-IV
|
astragaloside IV
|
|
BDL
|
bile duct ligation
|
|
CA
|
cycloastragenol
|
|
DKD
|
diabetic kidney disease
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FNDC5
|
fibronectin type III
domain-containing protein 5
|
|
GSDMD-N
|
gasdermin D N-terminal domain
|
|
HCC
|
hepatocellular carcinoma
|
|
IPF
|
idiopathic pulmonary fibrosis
|
|
LEF1
|
lymphoid enhancer binding
factor-1
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MCs
|
mesangial cells
|
|
MMPs
|
matrix metalloproteinases
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
PF
|
pulmonary fibrosis
|
|
POAG
|
primary open-angle glaucoma
|
|
RTECs
|
renal tubular epithelial cells
|
|
T2DM
|
type 2 diabetes mellitus
|
|
TGF-β1
|
transforming growth factor-β1
|
|
TM
|
trabecular meshwork
|
|
UUO
|
unilateral ureteral obstruction
|
|
ZEB1
|
zinc finger E-box binding homeobox
1
|
|
α-SMA
|
α-smooth muscle actin
|
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the National Natural Science
Foundation of China (grant no. 32371185), the Shanghai Science and
Technology Plan Project (grant no. 23010504200), the Key Laboratory
of Exercise and Health Sciences of Ministry of Education (Shanghai
University of Sport; grant no. 2025KF002), the Research and
Innovation Grant for Graduate Students (Shanghai University of
Sport; grant no. YJSCX-2024-019), the Shanghai Oriental Talents
Program (Youth Project) and the Shanghai Key Lab of Human
Performance (Shanghai University of Sport; grant no.
11DZ2261100).
References
|
1
|
Zhang M and Zhang S: T cells in fibrosis
and fibrotic diseases. Front Immunol. 11:11422020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei L, Liu L, Bai M, Ning X and Sun S:
CircRNAs: Versatile players and new targets in organ fibrosis. Cell
Commun Signal. 21:902023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horowitz JC and Thannickal VJ: Mechanisms
for the resolution of organ fibrosis. Physiology (Bethesda).
34:43–55. 2019.
|
|
4
|
Hohn J, Tan W, Carver A, Barrett H and
Carver W: Roles of exosomes in cardiac fibroblast activation and
fibrosis. Cells. 10:29332021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao X, Kwan JYY, Yip K, Liu PP and Liu
FF: Targeting metabolic dysregulation for fibrosis therapy. Nat Rev
Drug Discov. 19:57–75. 2020. View Article : Google Scholar
|
|
6
|
Henderson NC, Rieder F and Wynn TA:
Fibrosis: From mechanisms to medicines. Nature. 587:555–566. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hao Y: Chinese medicine as a therapeutic
option for pulmonary fibrosis: Clinical efficacies and underlying
mechanisms. Journal of Ethnopharmacology. 2024. View Article : Google Scholar
|
|
8
|
Li X, Li L, Lei W, Chua HZ, Li Z, Huang X,
Wang Q, Li N and Zhang H: Traditional Chinese medicine as a
therapeutic option for cardiac fibrosis: Pharmacology and
mechanisms. Biomed Pharmacother. 142:1119792021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen YL, Wang SJ, Rahman K, Zhang LJ and
Zhang H: Chinese herbal formulas and renal fibrosis: An overview.
Curr Pharm Des. 24:2774–2781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Lu Y, Nian M, Sheng Q, Zhang C, Han
C, Dou X and Ding Y: Therapeutic potential and mechanism of Chinese
herbal medicines in treating fibrotic liver disease. Chin J Natl
Med. 21:643–657. 2023.
|
|
11
|
Li X, Liu Z, Liao J, Chen Q, Lu X and Fan
X: Network pharmacology approaches for research of traditional
Chinese medicines. Chin J Natl Med. 21:323–332. 2023.
|
|
12
|
Xie J, Xiong J, Ding LS, Chen L, Zhou H,
Liu L, Zhang ZF, Hu XM, Luo P and Qing LS: A efficient method to
identify cardioprotective components of Astragali Radix using a
combination of molecularly imprinted polymers-based knockout
extract and activity evaluation. J Chromatogr A. 1576:10–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bi Y, Bao H, Zhang C, Yao R and Li M:
Quality control of radix astragali (The Root of Astragalus
membranaceus var. mongholicus) along its value chains. Front
Pharmacol. 11:5623762020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong F, Qu R, Li Y, Lv Y and Dai J:
Astragalus mongholicus: A review of its anti-fibrosis properties.
Front Pharmacol. 13:9765612022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan H, Zheng X and Li M: The effects of
astragalus membranaceus active extracts on autophagy-related
diseases. Int J Mol Sci. 20:19042019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Chai Y, Xiao G, Liu Y, Xie X, Xiao
W, Zhou P, Ma W, Zhang C and Li L: Astragalus and its formulas as a
therapeutic option for fibrotic diseases: Pharmacology and
mechanisms. Front Pharmacol. 13:10403502022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M, Lan L, Li G and Sun G:
Multi-dimensional profiles combined with antioxidant activity and
fingerprint-efficacy relationship to analyze the quality of
Astragali Radix from different sources. Food Chemi. 461:1408482024.
View Article : Google Scholar
|
|
18
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A research
review on the pharmacological effects. Adv Pharmacol. 87:89–112.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi Y, Shi X, Zhao M, Ma S and Zhang Y:
Pharmacological potential of astragali radix for the treatment of
kidney diseases. Phytomedicine. 123:1551962024. View Article : Google Scholar
|
|
20
|
Song SS, Wang RY, Li ZH, Yang Y, Wang TT,
Qing LS and Luo P: Role of simulated in vitro gastrointestinal
digestion on biotransformation and bioactivity of astragalosides
from Radix Astragali. J Pharm Biomed Anal. 231:1154142023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang Y, Chen B, Liang D, Quan X, Gu R,
Meng Z, Gan H, Wu Z, Sun Y, Liu S and Dou G: Pharmacological
effects of astragaloside IV: A review. Molecules. 28:61182023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan YQ, Chen HW and Li J: Astragaloside
IV: An effective drug for the treatment of cardiovascular diseases.
Drug Des Devel Ther. 14:3731–3746. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li L, Hou X, Xu R, Liu C and Tu M:
Research review on the pharmacological effects of astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar
|
|
25
|
Li K, Cui LJ, Cao YX, Li SY, Shi LX, Qin
XM and Du YG: UHPLC Q-exactive MS-based serum metabolomics to
explore the effect mechanisms of immunological activity of
astragalus polysaccharides with different molecular weights. Front
Pharmacol. 11:5956922020. View Article : Google Scholar
|
|
26
|
Li M, Han B, Zhao H, Xu C, Xu D,
Sieniawska E, Lin X and Kai G: Biological active ingredients of
Astragali Radix and its mechanisms in treating cardiovascular and
cerebrovascular diseases. Phytomedicine. 98:1539182022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Zhang Y, Luo Y, Meng X, Pan G, Zhang
H, Li Y and Zhang B: The molecular basis of the anti-inflammatory
property of astragaloside IV for the treatment of diabetes and its
complications. Drug Des Devel Ther. 17:771–790. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Hu J, Chen X, Lei X, Feng H, Wan F
and Tan L: Traditional Chinese medicine monomers: Novel strategy
for endogenous neural stem cells activation after stroke. Front
Cell Neurosci. 15:6281152021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Indu P, Arunagirinathan N, Rameshkumar MR,
Sangeetha K, Divyadarshini A and Rajarajan S: Antiviral activity of
astragaloside II, astragaloside III and astragaloside IV compounds
against dengue virus: Computational docking and in vitro studies.
Microb Pathog. 152:1045632021. View Article : Google Scholar
|
|
30
|
Kang X, Su S, Hong W, Geng W and Tang H:
Research progress on the ability of astragaloside IV to protect the
brain against ischemia-reperfusion injury. Front Neurosci.
15:7559022021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun L, Han R, Guo F, Chen H, Wang W, Chen
Z, Liu W, Sun X and Gao C: Antagonistic effects of IL-17 and
astragaloside IV on cortical neurogenesis and cognitive behavior
after stroke in adult mice through Akt/GSK-3β pathway. Cell Death
Discov. 6:742020. View Article : Google Scholar
|
|
32
|
Li R, Shi C, Wei C, Wang C, Du H, Hong Q
and Chen X: Fufang shenhua tablet, astragali radix and its active
component astragaloside IV: Research progress on anti-inflammatory
and immunomodulatory mechanisms in the kidney. Front Pharmacol.
14:11316352023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng P, Yang R, Jiang F, Guo J, Lu X, Yang
T and He Q: Molecular mechanism of astragaloside IV in improving
endothelial dysfunction of cardiovascular diseases mediated by
oxidative stress. Oxid Med Cell Longev. 2021:14812362021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Zheng S, Brinckmann JA, Fu J, Zeng
R, Huang L and Chen S: Chemical and genetic diversity of Astragalus
mongholicus grown in different eco-climatic regions. PLoS One.
12:e01847912017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu SY, Ouyang HT, Yang JY, Huang XL, Yang
T, Duan JP, Cheng JP, Chen YX, Yang YJ and Qiong P: Subchronic
toxicity studies of Radix Astragali extract in rats and dogs. J
Ethnopharmacol. 21:352–355. 2007. View Article : Google Scholar
|
|
36
|
Zang Y, Wan J, Zhang Z, Huang S, Liu X and
Zhang W: An updated role of astragaloside IV in heart failure.
Biomed Pharmacother. 126:1100122020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiangbo Z, Xuying W, Yuping Z, Xili M,
Yiwen Z and Tianbao Z: Effect of astragaloside IV on the
embryo-fetal development of Sprague-Dawley rats and New Zealand
White rabbits. J Appl Toxicol. 29:381–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xuying W, Jiangbo Z, Yuping Z, Xili M,
Yiwen Z, Tianbao Z and Weidong Z: Effect of astragaloside IV on the
general and peripartum reproductive toxicity in sprague-dawley
rats. Int J Toxicol. 29:505–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu M, Yin J, Xie L, Zhang J, Zou C, Zou J,
Liu F, Ju W and Li P: Pharmacokinetics and tolerance of toal
astragalosides after intravenous infusion of astragalosides
injection in healthy Chinese volunteers. Phytomedicine.
20:1105–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Frangogiannis NG: Cardiac fibrosis.
Cardiovasc Res. 117:1450–1488. 2021. View Article : Google Scholar :
|
|
41
|
Wan Y, Xu L, Wang Y, Tuerdi N, Ye M and Qi
R: Preventive effects of astragaloside IV and its active sapogenin
cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3
inflammasome. Eur J Pharmacol. 833:545–554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei Y, Wu Y, Feng K, Zhao Y, Tao R, Xu H
and Tang Y: Astragaloside IV inhibits cardiac fibrosis via
miR-135a-TRPM7-TGF-β/Smads pathway. J Ethnopharmacol.
249:1124042020. View Article : Google Scholar
|
|
43
|
Lu J, Wang Q, Zhou Y, Lu XC, Liu YH, Wu Y,
Guo Q, Ma YT and Tang YQ: AstragalosideⅣ against cardiac fibrosis
by inhibiting TRPM7 channel. Phytomedicine. 30:10–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu J, Qi J, Yin P, Liu Y, You J, Lin L,
Zhou M and Wang L: Cardiovascular disease mortality-China, 2019.
China CDC Wkly. 3:323–326. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Qu H, Yang T, Kong X and Zhou H:
Regulation and functions of NLRP3 inflammasome in cardiac fibrosis:
Current knowledge and clinical significance. Biomed Pharmacother.
143:1122192021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kawaguchi M, Takahashi M, Hata T, Kashima
Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J,
et al: Inflammasome activation of cardiac fibroblasts is essential
for myocardial ischemia/reperfusion injury. Circulation.
123:594–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chai C, Sun Y, Chi R, Yang H, Yang B and
Li B: Astragaloside IV alleviates LPS-induced cardiomyocyte
hypertrophy and collagen expression associated with CCL2-mediated
activation of NF-κB signaling pathway. Biochem Biophys Res Commun.
693:1493672024. View Article : Google Scholar
|
|
48
|
Feng Y, Ye D, Wang Z, Pan H, Lu X, Wang M,
Xu Y, Yu J, Zhang J, Zhao M, et al: The role of interleukin-6
family members in cardiovascular diseases. Front Cardiovasc Med.
9:8188902022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ushakov A, Ivanchenko V and Gagarina A:
Regulation of myocardial extracellular matrix dynamic changes in
myocardial infarction and postinfarct remodeling. Curr Cardiol Rev.
16:11–24. 2020. View Article : Google Scholar :
|
|
50
|
Jia G, Leng B, Wang H and Dai H:
Inhibition of cardiotrophin-1 overexpression is involved in the
anti-fibrotic effect of Astrogaloside IV. Mol Med Rep.
16:8365–8370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cui Y, Wu J, Wang Y, Li D, Zhang F, Jin X,
Li M, Zhang J and Liu Z: Protective effects of ginsenoside F2 on
isoproterenol-induced myocardial infarction by activating the
Nrf2/HO-1 and PI3K/Akt signaling pathways. Phytomedicine.
129:1556372024. View Article : Google Scholar
|
|
52
|
Zhao T, Kee HJ, Bai L, Kim MK, Kee SJ and
Jeong MH: Selective HDAC8 inhibition attenuates
isoproterenol-induced cardiac hypertrophy and fibrosis via p38 MAPK
pathway. Front Pharmacol. 12:6777572021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dai H, Jia G, Lu M, Liang C, Wang Y and
Wang H: Astragaloside IV inhibits isoprenaline-induced cardiac
fibrosis by targeting the reactive oxygen species/mitogen-activated
protein kinase signaling axis. Mol Med Rep. 15:1765–1770. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo Y, Zhou F, Fan J, Wu T, Jia S, Li J
and Chen N: Swimming alleviates myocardial fibrosis of type II
diabetic rats through activating miR-34a-mediated
SIRT1/PGC-1α/FNDC5 signal pathway. PLoS One. 19:e03101362024.
View Article : Google Scholar
|
|
55
|
Cong X, Zhu X, Zhang X and Ning Z:
Astragaloside IV inhibits angiotensin II-induced atrial fibrosis
and atrial fibrillation by SIRT1/PGC-1α/FNDC5 pathway. Heliyon.
10:e309842024. View Article : Google Scholar
|
|
56
|
Nakamura K, Miyoshi T, Yoshida M, Akagi S,
Saito Y, Ejiri K, Matsuo N, Ichikawa K, Iwasaki K, Naito T, et al:
Pathophysiology and treatment of diabetic cardiomyopathy and heart
failure in patients with diabetes mellitus. Int J Mol Sci.
23:35872022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Z, Zhu Y, Zhang Y, Zhang J, Ji T and
Li W and Li W: Protective effects of AS-IV on diabetic
cardiomyopathy by improving myocardial lipid metabolism in rat
models of T2DM. Biomed Pharmacother. 127:1100812020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vasudevan SO, Behl B and Rathinam VA:
Pyroptosis-induced inflammation and tissue damage. Semin Immunol.
69:1017812024. View Article : Google Scholar
|
|
59
|
Zhang X, Qu H, Yang T, Liu Q and Zhou H:
Astragaloside IV attenuate MI-induced myocardial fibrosis and
cardiac remodeling by inhibiting ROS/caspase-1/GSDMD signaling
pathway. Cell Cycle. 21:2309–2322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Birch J and Gil J: Senescence and the
SASP: Many therapeutic avenues. Genes Dev. 34:1565–1576. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Campbell RA, Docherty MH, Ferenbach DA and
Mylonas KJ: The role of ageing and parenchymal senescence on
macrophage function and fibrosis. Front Immunol. 12:7007902021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi L, Deng J, He J, Zhu F, Jin Y, Zhang
X, Ren Y and Du X: Integrative transcriptomics and proteomics
analysis reveal the protection of Astragaloside IV against
myocardial fibrosis by regulating senescence. Eur J Pharmacol.
975:1766322024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ok CY, Park S, Jang HO, Takata T, Lee OH,
Bae MK and Bae SK: FK866 Protects human dental pulp cells against
oxidative stress-induced cellular senescence. Antioxidants (Basel).
10:2712021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Purnomo Y, Piccart Y, Coenen T, Prihadi JS
and Lijnen PJ: Oxidative stress and transforming growth
factor-1-induced cardiac fibrosis. Cardiovasc Hematol Disord Drug
Targets. 13:165–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang
CB, Yang Y, Zou Y, Ge J, Chen R and Chen H: Astragaloside IV
attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in
coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol.
658:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu H, Yang F and Bao Z: Gut microbiota and
myocardial fibrosis. Eur J Pharmacol. 5:1753552023. View Article : Google Scholar
|
|
67
|
Czibik G, Mezdari Z, Altintas DM, Bréhat
J, Pini M, d'Humières T, Delmont T, Radu C, Breau M, Liang H, et
al: Dysregulated phenylalanine catabolism plays a key role in the
trajectory of cardiac aging. Circulation. 144:559–574. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Du XQ, Shi LP, Chen ZW, Hu JY, Zuo B,
Xiong Y and Cao WF: Astragaloside IV ameliorates
isoprenaline-induced cardiac fibrosis in mice via modulating gut
microbiota and fecal metabolites. Front Cell Infect Microbiol.
12:8361502022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huang WJ and Tang XX: Virus infection
induced pulmonary fibrosis. J Transl Med. 19:4962021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Guan Y, Zhang J, Cai X, Cai Y, Song Z,
Huang Y, Qian W, Pan Z and Zhang X: Astragaloside IV inhibits
epithelial-mesenchymal transition and pulmonary fibrosis via
lncRNA-ATB/miR-200c/ZEB1 signaling pathway. Gene. 897:1480402024.
View Article : Google Scholar
|
|
71
|
Sgalla G, Iovene B, Calvello M, Ori M,
Varone F and Richeldi L: Idiopathic pulmonary fibrosis:
Pathogenesis and management. Respir Res. 19:322018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li LC and Kan LD: Traditional Chinese
medicine for pulmonary fibrosis therapy: Progress and future
prospects. J Ethnopharmacol. 198:45–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Savin IA, Zenkova MA and Sen'kova AV:
Bronchial asthma, airway remodeling and lung fibrosis as successive
steps of one process. Int J Mol Sci. 24:160422023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wiertz IA, Wuyts WA, van Moorsel CHM,
Vorselaars ADM, Van Es HW, Van Oosterhout MFM and Grutters JC:
Unfavourable outcome of glucocorticoid treatment in suspected
idiopathic pulmonary fibrosis. Respirology. 23:311–317. 2018.
View Article : Google Scholar
|
|
75
|
Raghu G, Remy-Jardin M, Richeldi L,
Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM,
Martinez FJ, et al: Idiopathic pulmonary fibrosis (an Update) and
progressive pulmonary fibrosis in adults: An official
ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care
Med. 205:e18–e47. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bai X, Zhao G, Chen Q, Li Z, Gao M, Ho W,
Xu X and Zhang XQ: Inhaled siRNA nanoparticles targeting IL11
inhibit lung fibrosis and improve pulmonary function post-bleomycin
challenge. Sci Adv. 8:eabn71622022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li N, Wu K, Feng F, Wang L, Zhou X and
Wang W: Astragaloside IV alleviates silica-induced pulmonary
fibrosis via inactivation of the TGF-β1/Smad2/3 signaling pathway.
Int J Mol Med. 47:162021. View Article : Google Scholar
|
|
78
|
Li N, Feng F, Wu K, Zhang H, Zhang W and
Wang W: Inhibitory effects of astragaloside IV on silica-induced
pulmonary fibrosis via inactivating TGF-β1/Smad3 signaling. Biomed
Pharmacother. 119:1093872019. View Article : Google Scholar
|
|
79
|
Hou Y, Zhen Y, Xue Q and Wang W:
Astragaloside IV attenuates TGF-β-mediated epithelial-mesenchymal
transition of pulmonary fibrosis via suppressing NLRP3 expression
in vitro. Pharmazie. 76:97–102. 2021.PubMed/NCBI
|
|
80
|
Yu WN, Sun LF and Yang H: Inhibitory
effects of astragaloside IV on bleomycin-induced pulmonary fibrosis
in rats via attenuation of oxidative stress and inflammation.
Inflammation. 39:1835–1841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang G, Zhao G, Chao X, Xie L and Wang H:
The characteristic of virulence, biofilm and antibiotic resistance
of klebsiella pneumoniae. Int J Environ Res Public Health.
17:62782020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li L, Guan J, Lin R, Wang F, Ma H, Mao C,
Guo X, Qu Z and Guan R: Astragaloside IV alleviates lung
inflammation in Klebsiella pneumonia rats by suppressing
TGF-β1/Smad pathway. Braz J Med Biol Res. 56:e122032023. View Article : Google Scholar
|
|
83
|
Gao L, Bai Y, Liang C, Han T, Liu Y, Zhou
J, Guo J, Wu J and Hu D: Celastrol-Ligustrazine compound proven to
be a novel drug candidate for idiopathic pulmonary fibrosis by
intervening in the TGF-β1 mediated pathways-an experimental in
vitro and vivo study. Mol Divers. 29:3957–3973. 2024. View Article : Google Scholar
|
|
84
|
Qian W, Cai X, Qian Q, Zhang W and Wang D:
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal
transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med.
22:4354–4365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zheng M, Liu K, Li L, Feng C and Wu G:
Traditional Chinese medicine inspired dual-drugs loaded inhalable
nano-therapeutics alleviated idiopathic pulmonary fibrosis by
targeting early inflammation and late fibrosis. J Nanobiotechnol.
22:142024. View Article : Google Scholar
|
|
86
|
Catrina SB and Zheng X: Hypoxia and
hypoxia-inducible factors in diabetes and its complications.
Diabetologia. 64:709–716. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xi J, Ma Y, Liu D and Li R: Astragaloside
IV restrains pyroptosis and fibrotic development of pulmonary
artery smooth muscle cells to ameliorate pulmonary artery
hypertension through the PHD2/HIF1α signaling pathway. BMC Pulm
Med. 23:3862023. View Article : Google Scholar
|
|
88
|
Elamaa H, Kaakinen M, Nätynki M, Szabo Z,
Ronkainen VP, Äijälä V, Mäki JM, Kerkelä R, Myllyharju J and Eklund
L: PHD2 deletion in endothelial or arterial smooth muscle cells
reveals vascular cell type-specific responses in pulmonary
hypertension and fibrosis. Angiogenesis. 25:259–274. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li X, Zhang M, Ling C, Sha H, Zou G and
Liang H: Molecular characterization and response of prolyl
hydroxylase domain (PHD) Genes to hypoxia stress in
hypophthalmichthys molitrix. Animals (Basel). 12:1312022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yuan S, Zuo B, Zhou SC, Wang M, Tan KY,
Chen ZW and Cao WF: Integrating network pharmacology and
experimental validation to explore the pharmacological mechanism of
astragaloside IV in treating bleomycin-induced pulmonary fibrosis.
Drug Des Devel Ther. 17:1289–1302. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ma J, Xu Y, Li W, Zhou Y, Wang D, Yang M,
Wang B and Chen W: High-mobility group box 1 promotes
epithelial-to-mesenchymal transition in crystalline silica induced
pulmonary inflammation and fibrosis. Toxicol Lett. 330:134–143.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li LC, Xu L, Hu Y, Cui WJ, Cui WH, Zhou WC
and Kan LD: Astragaloside IV improves bleomycin-induced pulmonary
fibrosis in rats by attenuating extracellular matrix deposition.
Front Pharmacol. 8:5132017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jin H, Park SY, Lee JE, Park H, Jeong M,
Lee H, Cho J and Lee YS: GTSE1-driven ZEB1 stabilization promotes
pulmonary fibrosis through the epithelial-to-mesenchymal
transition. Mol Ther. 32:4138–4157. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220:e2020090452021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Volpe MC, Ciucci G, Zandomenego G, Vuerich
R, Ring NAR, Vodret S, Salton F, Marchesan P, Braga L, Marcuzzo T,
et al: Flt1 produced by lung endothelial cells impairs ATII cell
transdifferentiation and repair in pulmonary fibrosis. Cell Death
Dis. 14:4372023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu Y, Li Y, Xu Q, Yao W, Wu Q, Yuan J,
Yan W, Xu T, Ji X and Ni C: Long non-coding RNA-ATB promotes EMT
during silica-induced pulmonary fibrosis by competitively binding
miR-200c. Biochim Biophys Acta Mol Basis Dis. 1864:420–431. 2018.
View Article : Google Scholar
|
|
97
|
Guan Y, Ma J and Song W: Identification of
circRNA-miRNA-mRNA regulatory network in gastric cancer by analysis
of microarray data. Cancer Cell Int. 19:1832019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhu M, Zhao L, Zhang X and Zhao R:
Astragaloside IV restrains pulmonary fibrosis progression via the
circ_0008898/miR-211-5p/HMGB1 axis. Chem Biol Drug Des.
103:e145082024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gan W, Song W, Gao Y, Zheng X, Wang F,
Zhang Z, Zen K, Liang H and Yan X: Exosomal circRNAs in the plasma
serve as novel biomarkers for IPF diagnosis and progression
prediction. J Transl Med. 22:2642024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ye Z, Xian W, Ling G, Zihang LI, Jingtian
Z, Wenkai W, Liang Z and Mei X: Efficacy of Danggui Buxue decoction
on diabetic nephropathy-induced renal fibrosis in rats and possible
mechanism. J Tradit Chin Med. 43:507–513. 2023.PubMed/NCBI
|
|
101
|
Tong J, Wu Z, Wang Y, Hao Q, Liu H, Cao F
and Jiao Y: Astragaloside IV synergizing with ferulic acid
ameliorates pulmonary fibrosis by TGF-β1/Smad3 signaling. Evid
Based Complement Alternat Med. 2021:1–9. 2021.
|
|
102
|
Yue YL, Zhang MY, Liu JY, Fang LJ and Qu
YQ: The role of autophagy in idiopathic pulmonary fibrosis: From
mechanisms to therapies. Ther Adv Respir Dis.
16:1753466622114092022. View Article : Google Scholar
|
|
103
|
Li T, Gao X, Jia R, Sun Y, Ding Y, Wang F
and Wang Y: Astragaloside IV inhibits idiopathic pulmonary fibrosis
through activation ofautophagy by miR-21-mediated
PTEN/PI3K/AKT/mTOR pathway. Cell Mol Biol (Noisy-le-grand).
70:128–136. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mo Y, Zhang Y, Wan R, Jiang M, Xu Y and
Zhang Q: miR-21 mediates nickel nanoparticle-induced pulmonary
injury and fibrosis. Nanotoxicology. 14:1175–1197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Deleyto-Seldas N and Efeyan A: The
mTOR-autophagy axis and the control of metabolism. Front Cell Dev
Biol. 9:6557312021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yehia L, Keel E and Eng C: The clinical
spectrum of PTEN mutations. Annu Rev Med. 71:103–116. 2020.
View Article : Google Scholar
|
|
107
|
Dubey S, Dubey PK, Umeshappa CS, Ghebre YT
and Krishnamurthy P: Inhibition of RUNX1 blocks the differentiation
of lung fibroblasts to myofibroblasts. J Cell Physiol.
237:2169–2182. 2023. View Article : Google Scholar
|
|
108
|
Tian H, Zhang Y, Li W, Xie G, Wu J and Liu
J: Astragaloside IV inhibits lung injury and fibrosis induced by
PM2.5 by targeting RUNX1 through miR-362-3p. Mol Biotechnol.
67:4167–4177. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Al-Tamari HM, Dabral S, Schmall A, Sarvari
P, Ruppert C, Paik J, DePinho RA, Grimminger F, Eickelberg O,
Guenther A, et al: FoxO3 an important player in fibrogenesis and
therapeutic target for idiopathic pulmonary fibrosis. EMBO Mol Med.
10:276–293. 2018. View Article : Google Scholar :
|
|
110
|
Kurebayashi Y, Baba Y, Minowa A, Nadya NA,
Azuma M, Yoshimura A, Koyasu S and Nagai S: TGF-β-induced
phosphorylation of Akt and Foxo transcription factors negatively
regulates induced regulatory T cell differentiation. Biochem
Biophys Res Commun. 480:114–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cui H, Liu X, Zhang J, Zhang K, Yao D,
Dong S, Feng S, Yang L, Li Y, Wang H, et al: Rhodiola rosea L.
Attenuates cigarette smoke and lipopolysaccharide-induced COPD in
rats via inflammation inhibition and antioxidant and antifibrosis
pathways. Evid Based Complement Alternat Med. 2:61031582021.
|
|
112
|
Su J, Morgani SM, David CJ, Wang Q, Er EE,
Huang YH, Basnet H, Zou Y, Shu W, Soni RK, et al: TGF-β
orchestrates fibrogenic and developmental EMTs via the RAS effector
RREB1. Nature. 577:566–571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang M, Wang W, Liu K, Jia C, Hou Y and
Bai G: Astragaloside IV protects against lung injury and pulmonary
fibrosis in COPD by targeting GTP-GDP domain of RAS and
downregulating the RAS/RAF/FoxO signaling pathway. Phytomedicine.
120:1550662023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mzimela N, Dimba N, Sosibo A and Khathi A:
Evaluating the impact of type 2 diabetes mellitus on pulmonary
vascular function and the development of pulmonary fibrosis. Front
Endocrinol (Lausanne). 10:14314052024. View Article : Google Scholar
|
|
115
|
Guo J, Zhang Y, Zhou R, Hao Y, Wu X, Li G
and Du Q: Deciphering the molecular mechanism of Bu Yang Huan Wu
Decoction in interference with diabetic pulmonary fibrosis via
regulating oxidative stress and lipid metabolism disorder. J Pharm
Biomed Anal. 243:1160612024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kalantar-Zadeh K, Jafar TH, Nitsch D,
Neuen BL and Perkovic V: Chronic kidney disease. Lancet.
398:786–802. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Johnson HN and Prasad-Reddy L: Updates in
chronic kidney disease. J Pharm Pract. 37:1380–1390. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kovesdy CP: Epidemiology of chronic kidney
disease: An update 2022. Kidney Int Suppl (2011). 12:7–11. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu Y, Kors L, Butter LM, Stokman G,
Claessen N, Zuurbier CJ, Girardin SE, Leemans JC, Florquin S,
Tammaro A, et al: NLRX1 prevents M2 macrophage polarization and
excessive renal fibrosis in chronic obstructive nephropathy. Cells.
13:232024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang L, Wang X, He S, Zhang F and Li Y:
Gypenosides suppress fibrosis of the renal NRK-49F cells by
targeting miR-378a-5p through the PI3K/AKT signaling pathway. J
Ethnopharmacol. 311:1164662023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Fogo AB and Harris RC: Crosstalk between
glomeruli and tubules. Nat Rev Nephrol. 21:189–199. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Patera F, Gatticchi L, Cellini B,
Chiasserini D and Reboldi G: Kidney Fibrosis and oxidative stress:
From molecular pathways to new pharmacological opportunities.
Biomolecules. 14:1372024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li D, Liu Y, Zhan Q, Zeng Y, Peng Z, He Q,
Tan Q, Cao W, Wang S and Wang J: Astragaloside IV blunts
epithelial-mesenchymal transition and G2/M arrest to alleviate
renal fibrosis via regulating ALDH2-mediated autophagy. Cells.
12:17772023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ruiz-Ortega M, Lamas S and Ortiz A:
Antifibrotic agents for the management of CKD: A review. Am J
Kidney Dis. 80:251–263. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Su Y, Chen Q, Ma K, Ju Y, Ji T, Wang Z and
Li W and Li W: Astragaloside IV inhibits palmitate-mediated
oxidative stress and fibrosis in human glomerular mesangial cells
via downregulation of CD36 expression. Pharmacol Rep. 71:319–329.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Nath A, Li I, Roberts LR and Chan C:
Elevated free fatty acid uptake via CD36 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma. Sci
Rep. 5:147522015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Du N, Xu Z, Gao M, Liu P, Sun B and Cao X:
Combination of Ginsenoside Rg1 and Astragaloside IV reduces
oxidative stress and inhibits TGF-β1/Smads signaling
cascade on renal fibrosis in rats with diabetic nephropathy. Drug
Des Devel Ther. 12:3517–3524. 2018. View Article : Google Scholar :
|
|
128
|
Zhang Y, Tao C, Xuan C, Jiang J and Cao W:
Transcriptomic analysis reveals the protection of astragaloside IV
against diabetic nephropathy by modulating inflammation. Oxid Med
Cell Longev. 2020:1–17. 2020.
|
|
129
|
Wang S, Qin S, Cai B, Zhan J and Chen Q:
Promising therapeutic mechanism for Chinese herbal medicine in
ameliorating renal fibrosis in diabetic nephropathy. Front
Endocrinol (Lausanne). 14:9326492023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lu Q, Ji XJ, Zhou YX, Yao XQ, Liu YQ,
Zhang F and Yin XX: Quercetin inhibits the mTORC1/p70S6K
signaling-mediated renal tubular epithelial-mesenchymal transition
and renal fibrosis in diabetic nephropathy. Pharmacol Res.
99:237–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chen X, Yang Y, Liu C, Chen Z and Wang D:
Astragaloside IV ameliorates high glucose-induced renal tubular
epithelial-mesenchymal transition by blocking mTORC1/p70S6K
signaling in HK-2 cells. Int J Mol Med. 43:709–716. 2018.
|
|
132
|
Herman-Edelstein M, Thomas MC,
Thallas-Bonke V, Saleem M, Cooper ME and Kantharidis P: A model for
diabetic podocytopathy. Diabetes. 60:1779–1788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Qiu Y, Kang N, Wang X, Yao Y, Cui J, Zhang
X and Zheng L: Loss of Farnesoid X receptor (FXR) accelerates
dysregulated glucose and renal injury in db/db mice. PeerJ.
11:e161552023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fujii R, Yamada H, Munetsuna E, Yamazaki
M, Ohashi K, Ishikawa H, Maeda K, Hagiwara C, Ando Y, Hashimoto S,
et al: Associations of circulating MicroRNAs (miR-17, miR-21, and
miR-150) and chronic kidney disease in a Japanese Population. J
Epidemiol. 30:177–182. 2020. View Article : Google Scholar :
|
|
135
|
Wang X, Gao Y, Tian N, Zou D, Shi Y and
Zhang N: Astragaloside IV improves renal function and fibrosis via
inhibition of miR-21-induced podocyte dedifferentiation and
mesangial cell activation in diabetic mice. Drug Des Devel Ther.
12:2431–2442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Teh YM, Mualif SA and Lim SK: A
comprehensive insight into autophagy and its potential signaling
pathways as a therapeutic target in podocyte injury. Int J Biochem
Cell Biol. 143:1061532022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang X, Gao Y, Tian N, Wang T, Shi Y, Xu J
and Wu B: Astragaloside IV inhibits glucose-induced
epithelial-mesenchymal transition of podocytes through autophagy
enhancement via the SIRT-NF-κB p65 axis. Sci Rep. 9:3232019.
View Article : Google Scholar
|
|
138
|
Wang X, Gao Y, Tian N, Zhu Z, Wang T, Xu
J, Wu B and Zhang N: Astragaloside IV represses high
glucose-induced mesangial cells activation by enhancing autophagy
via SIRT1 deacetylation of NF-κB P65 subunit. Drug Des Devel Ther.
12:2971–2980. 2018. View Article : Google Scholar :
|
|
139
|
Shah R, Matthews GJ, Shah RY, McLaughlin
C, Chen J, Wolman M, Master SR, Chai B, Xie D, Rader DJ, et al:
Serum fractalkine (CX3CL1) and cardiovascular outcomes and
diabetes: Findings from the chronic renal insufficiency cohort
(CRIC) study. Am J Kidney Dis. 66:266–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang C, Ren C, Hu Q, Shen X, Wang M, Yang
Z, Xu E, Wang X, Li Z, Yu H, et al: Histidine-rich calcium binding
protein promotes gastric cancer cell proliferation, migration,
invasion and epithelial-mesenchymal transition through Raf/MEK/ERK
signaling. J Cancer. 13:1073–1085. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Hu Y, Tang W, Liu W, Hu Z and Pan C:
Astragaloside IV alleviates renal tubular epithelial-mesenchymal
transition via CX3CL1-RAF/MEK/ERK signaling pathway in diabetic
kidney disease. Drug Des Devel Ther. 16:1605–1620. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yang Y, Song M, Liu Y, Liu H, Sun L, Peng
Y, Liu F, Venkatachalam MA and Dong Z: Renoprotective approaches
and strategies in acute kidney injury. Pharmacol Ther. 163:58–73.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhou X, Sun X, Gong X, Yang Y, Chen C,
Shan G and Yao Q: Astragaloside IV from Astragalus membranaceus
ameliorates renal interstitial fibrosis by inhibiting inflammation
via TLR4/NF-кB in vivo and in vitro. Int Immunopharmacol. 42:18–24.
2017. View Article : Google Scholar
|
|
144
|
Pang J, Peng H, Wang S, Xu X, Xu F, Wang
Q, Chen Y, Barton LA, Chen Y, Zhang Y and Ren J: Mitochondrial
ALDH2 protects against lipopolysaccharide-induced myocardial
contractile dysfunction by suppression of ER stress and autophagy.
Biochim Biophys Acta Mol Basis Dis. 1865:1627–1641. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu F: Transforming growth factor-beta1 in
diabetic kidney disease. Front Cell Dev Biol. 8:1872020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH,
Zhang XM, Peng W and Cai H: Astragaloside IV inhibits renal
tubulointerstitial fibrosis by blocking TGF-β/Smad signaling
pathway in vivo and in vitro. Exp Biol Med (Maywood).
239:1310–1324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Lian Y, Li C, Li J, Xie Y, Liu Q, Wu M,
Shi W and Meng L: Astragaloside IV attenuated TGF-β1-induced
epithelial-mesenchymal transition of renal tubular epithelial cells
via connexin43 and Akt/mTOR signaling pathway. Tissue Cell.
77:1018312021. View Article : Google Scholar
|
|
148
|
Sun X, Huang K, Haiming X, Lin Z, Yang Y,
Zhang M, Liu P and Huang H: Connexin 43 prevents the progression of
diabetic renal tubulointerstitial fibrosis by regulating the
SIRT1-HIF-1α signaling pathway. Clin Sci (Lond). 134:1573–1592.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yuan R, Fan Q, Liang X, Han S, He J, Wang
QQ, Gao H, Feng Y and Yang S: Cucurbitacin B inhibits
TGF-β1-induced epithelial-mesenchymal transition (EMT) in NSCLC
through regulating ROS and PI3K/Akt/mTOR pathways. Chin Med.
17:242022. View Article : Google Scholar
|
|
150
|
Yu X, Xiao Q, Yu X, Cheng Y, Lin H and
Xiang Z: A network pharmacology-based study on the mechanism of
astragaloside IV alleviating renal fibrosis through the AKT1/GSK-3β
pathway. J Ethnopharmacol. 297:1155352022. View Article : Google Scholar
|
|
151
|
Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH,
Zhang XM and Peng W: Astragaloside IV inhibits the up-regulation of
Wnt/β-catenin signaling in rats with unilateral ureteral
obstruction. Cell Physiol Biochem. 33:1316–1328. 2014. View Article : Google Scholar
|
|
152
|
Che X, Wang Q, Xie Y, Xu W, Shao X, Mou S
and Ni Z: Astragaloside IV suppresses transforming growth factor-β1
induced fibrosis of cultured mouse renal fibroblasts via inhibition
of the MAPK and NF-κB signaling pathways. Biochem Biophys Res
Commun. 464:1260–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ma T, Li H, Liu H, Peng Y, Lin T, Deng Z,
Jia N, Chen Z and Wang P: Neat1 promotes acute kidney injury to
chronic kidney disease by facilitating tubular epithelial cells
apoptosis via sequestering miR-129-5p. Mol Ther. 30:3313–3332.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wang D, Warner GM, Yin P, Knudsen BE,
Cheng J, Butters KA, Lien KR, Gray CE, Garovic VD, Lerman LO, et
al: Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in
a murine renal artery stenosis model. Am J Physiol Renal Physiol.
304:F938–F947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Meng L, Tang J, Wang Y, Zhao JR, Shang MY,
Zhang M, Liu SY, Qu L, Cai SQ and Li XM: Astragaloside IV
synergizes with ferulic acid to inhibit renal tubulointerstitial
fibrosis in rats with obstructive nephropathy. Br J Pharmacol.
162:1805–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Huang A, Palmer LS, Hom D, Valderrama E
and Trachtman H: The role of nitric oxide in obstructive
nephropathy. J Urol. 163:1276–1281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Deb DK, Bao R and Li YC: Critical role of
the cAMP-PKA pathway in hyperglycemia-induced epigenetic activation
of fibrogenic program in the kidney. Faseb J. 31:2065–2075. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pan H, Lin Y, Dou J, Fu Z, Yao Y, Ye S,
Zhang S, Wang N, Liu A, Li X, et al: Wedelolactone facilitates
Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to
block inflammasome activation and pyroptosis. Cell Prolif.
53:e128682020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhang L, Liu W, Li S, Wang J, Sun D, Li H,
Zhang Z, Hu Y and Fang J: Astragaloside IV alleviates renal
fibrosis by inhibiting renal tubular epithelial cell pyroptosis
induced by urotensin II through regulating the cAMP/PKA signaling
pathway. PLoS One. 19:e03043652024. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Keyhanmanesh R, Hamidian G, Lotfi H,
Zavari Z, Seyfollahzadeh M, Ghadiri A, Ahmadi M, Bahari F and Bavil
FM: Troxerutin affects nephropathy signaling events in the kidney
of type-1 diabetic male rats. Avicenna J Phytomed. 12:109–115.
2022.PubMed/NCBI
|
|
162
|
Cao Y, Zhang L, Wang Y, Fan Q and Cong Y:
Astragaloside IV attenuates renal fibrosis through repressing
epithelial-to-mesenchymal transition by inhibiting microRNA-192
expression: In vivo and in vitro studies. Am J Transl Res.
11:5029–5038. 2019.PubMed/NCBI
|
|
163
|
Kato M, Zhang J, Wang M, Lanting L, Yuan
H, Rossi JJ and Natarajan R: MicroRNA-192 in diabetic kidney
glomeruli and its function in TGF-beta-induced collagen expression
via inhibition of E-box repressors. Proc Natl Acad Sci USA.
104:3432–3437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Csak T and Bernstein D: Hepatorenal
syndrome. Clin Liver Dis. 26:165–179. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wang Q, Xu J, Li M, Chen Y, Xu Y, Li L,
Gong Y and Yang Y: Nrf2 knockout attenuates the astragaloside IV
therapeutic effect on kidney fibrosis from liver cancer by
regulating pSmad3C/3L pathways. Naunyn Schmiedebergs Arch
Pharmacol. 397:1687–1700. 2024. View Article : Google Scholar
|
|
166
|
Gong Y, Li D, Li L, Yang J, Ding H, Zhang
C, Wen G, Wu C, Fang Z, Hou S and Yang Y: Smad3 C-terminal
phosphorylation site mutation attenuates the hepatoprotective
effect of salvianolic acid B against hepatocarcinogenesis. Food
Chem Toxicol. 147:1119122021. View Article : Google Scholar
|
|
167
|
Zhang C, Li L, Hou S, Shi Z, Xu W, Wang Q,
He Y, Gong Y, Fang Z and Yang Y: Astragaloside IV inhibits
hepatocellular carcinoma by continually suppressing the development
of fibrosis and regulating pSmad3C/3L and Nrf2/HO-1 pathways. J
Ethnopharmacol. 279:1143502021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Liang R, Yan D, Zhang X, Chen X, Zhang W
and Jia H: Kidney Mesenchymal stem cells alleviate
cisplatin-induced kidney injury and apoptosis in rats. Tissue Cell.
80:1019982023. View Article : Google Scholar
|
|
169
|
Wang Y, Luo P and Wuren T: Narrative
review of mesenchymal stem cell therapy in renal diseases:
Mechanisms, clinical applications, and future directions. Stem
Cells Int. 2024:86582462024. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Raming R, Cordasic N, Kirchner P, Ekici
AB, Fahlbusch FB, Woelfle J, Hilgers KF, Hartner A and
Menendez-Castro C: Neonatal nephron loss during active
nephrogenesis results in altered expression of renal developmental
genes and markers of kidney injury. Physiol Genomics. 53:509–517.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Berumen J, Baglieri J, Kisseleva T and
Mekeel K: Liver fibrosis: Pathophysiology and clinical
implications. WIREs Mech Dis. 13:e14992021. View Article : Google Scholar
|
|
172
|
Altamirano-Barrera A, Barranco-Fragoso B
and Méndez-Sánchez N: Management strategies for liver fibrosis. Ann
Hepatol. 16:48–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Cinar R, Iyer MR, Liu Z, Cao Z, Jourdan T,
Erdelyi K, Godlewski G, Szanda G, Liu J, Park JK, et al: Hybrid
inhibitor of peripheral cannabinoid-1 receptors and inducible
nitric oxide synthase mitigates liver fibrosis. JCI Insight.
1:e873362016. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Lee YA, Wallace MC and Friedman SL:
Pathobiology of liver fibrosis: A translational success story. Gut.
64:830–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Wu J, Zhang M, Xia S, Han P, Zhao K, Peng
K, Zhou W, Tian D, Liao J and Liu J: Hepatic HRC induces hepatocyte
pyroptosis and HSCs activation via NLRP3/caspase-1 pathway. J Mol
Med (Berl). 100:1787–1799. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
El-Ashmawy NE, Al-Ashmawy GM, Fakher HE
and Khedr NF: The role of WNT/β-catenin signaling pathway and
glutamine metabolism in the pathogenesis of CCl4-induced liver
fibrosis: Repositioning of niclosamide and concerns about lithium.
Cytokine. 136:1552502020. View Article : Google Scholar
|
|
177
|
Xu XY, Geng Y, Xu HX, Ren Y, Liu DY and
Mao Y: Antrodia camphorata-derived antrodin C inhibits liver
fibrosis by blocking TGF-beta and PDGF signaling pathways. Front
Mol Biosci. 9:8355082022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Caligiuri A, Gentilini A, Pastore M, Gitto
S and Marra F: Cellular and molecular mechanisms underlying liver
fibrosis regression. Cells. 10:27592021. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Schuppan D: Liver fibrosis: Common
mechanisms and antifibrotic therapies. Clin Res Hepatol
Gastroenterol. 39:S51–S59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Mohs A, Otto T, Schneider KM, Peltzer M,
Boekschoten M, Holland CH, Hudert CA, Kalveram L, Wiegand S,
Saez-Rodriguez J, et al: Hepatocyte-specific NRF2 activation
controls fibrogenesis and carcinogenesis in steatohepatitis. J
Hepatol. 74:638–648. 2021. View Article : Google Scholar
|
|
181
|
Boye A, Wu C, Jiang Y, Wang J, Wu J, Yang
X and Yang Y: Compound Astragalus and Salvia miltiorrhiza extracts
modulate MAPK-regulated TGF-β/Smad signaling in hepatocellular
carcinoma by multi-target mechanism. J Ethnopharmacol. 169:219–228.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Wang Z, Li Q, Xiang M, Zhang F, Wei D, Wen
Z and Zhou Y: Astragaloside alleviates hepatic fibrosis function
via par2 signaling pathway in diabetic rats. Cell Physiol Biochem.
41:1156–1166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Matkowski A, Jamiołkowska-Kozlowska W and
Nawrot I: Chinese medicinal herbs as source of antioxidant
compounds-where tradition meets the future. Curr Med Chem.
20:984–1004. 2013.
|
|
184
|
Zhao XM, Zhang J, Liang YN and Niu YC:
Astragaloside IV synergizes with ferulic acid to alleviate hepatic
fibrosis in bile duct-ligated cirrhotic rats. Dig Dis Sci.
65:2925–2936. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Dong H, Guo H, Liang Y, Wang X and Niu Y:
Astragaloside IV synergizes with ferulic acid to suppress hepatic
stellate cells activation in vitro. Free Radic Res. 51:167–178.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Bleser PJD, Xu G, Rombouts K, Rogiers V
and Geerts A: Glutathione levels discriminate between oxidative
stress and transforming growth factor-beta signaling in activated
rat hepatic stellate cells. J Biol Chem. 26:33881–33887. 1999.
View Article : Google Scholar
|
|
187
|
Li X, Wang X, Han C, Wang X, Xing G, Zhou
L, Li G and Niu Y: Astragaloside IV suppresses collagen production
of activated hepatic stellate cells via oxidative stress-mediated
p38 MAPK pathway. Free Radic Biol Med. 60:168–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Dhar D, Baglieri J, Kisseleva T and
Brenner DA: Mechanisms of liver fibrosis and its role in liver
cancer. Exp Biol Med (Maywood). 245:96–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Baba Y, Saeki K, Onodera T and Doi K:
Serological and immunohistochemical studies on
porcine-serum-induced hepatic fibrosis in rats. Exp Mol Pathol.
79:229–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Liu H, Wei W, Sun W and Li X: Protective
effects of astragaloside IV on porcine-serum-induced hepatic
fibrosis in rats and in vitro effects on hepatic stellate cells. J
Ethnopharmacol. 122:502–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Xie M, Xia B, Xiao L, Yang D, Li Z, Wang
H, Wang X, Zhang X and Peng Q: Astragaloside IV ameliorates
peritoneal fibrosis by promoting PGC-1α to reduce apoptosis in
vitro and in vivo. J Cell Mol Med. 27:2945–2955. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Shan Y, Yu M, Dai H, Zhu X, Wang F, You Y,
Cao H, Sheng L, Zhao J, Tang L, et al: The role of
macrophage-derived exosomes in reversing peritoneal fibrosis:
Insights from Astragaloside IV. Phytomedicine. 129:1556832024.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Davies SJ, Bryan J, Phillips L and Russell
GI: Longitudinal changes in peritoneal kinetics: The effects of
peritoneal dialysis and peritonitis. Nephrol Dial Transplant.
11:498–506. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Ditsawanon P and Aramwit P: Preserving the
peritoneal membrane in long-term peritoneal dialysis patients. J
Clin Pharm Ther. 40:508–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Ghafouri-Fard S, Shoorei H, Dong P,
Poornajaf Y, Hussen BM, Taheri M and Dilmaghani NA: Emerging
functions and clinical applications of exosomal microRNAs in
diseases. Noncoding RNA Res. 8:350–362. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Wang J, Wang Y, Zhang H, Gao W, Lu M, Liu
W, Li Y and Yin Z: Forkhead box C1 promotes the pathology of
osteoarthritis by upregulating β-catenin in synovial fibroblasts.
FEBS J. 287:3065–3087. 2020. View Article : Google Scholar
|
|
198
|
Qi Q, Mao Y, Yi J, Li D, Zhu K and Cha X:
Anti-Fibrotic effects of astragaloside IV in systemic sclerosis.
Cell Physiol Biochem. 34:2105–2116. 2014. View Article : Google Scholar
|
|
199
|
Chen X, Peng LH, Li N, Li QM, Li P, Fung
KP, Leung PC and Gao JQ: The healing and anti-scar effects of
astragaloside IV on the wound repair in vitro and in vivo. J
Ethnopharmacol. 139:721–727. 2012. View Article : Google Scholar
|
|
200
|
Chen X, Peng LH, Shan YH, Li N, Wei W, Yu
L, Li QM, Liang WQ and Gao JQ: Astragaloside IV-loaded
nanoparticle-enriched hydrogel induces wound healing and anti-scar
activity through topical delivery. Int J Pharm. 447:171–181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Peng LH, Chen X, Chen L, Li N, Liang WQ
and Gao JQ: Topical astragaloside IV-releasing hydrogel improves
healing of skin wounds in vivo. Biol Pharm Bull. 35:881–888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Shan YH, Peng LH, Liu X, Chen X, Xiong J
and Gao JQ: Silk fibroin/gelatin electrospun nanofibrous dressing
functionalized with astragaloside IV induces healing and anti-scar
effects on burn wound. Int J Pharm. 479:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Kasetti RB, Maddineni P, Kodati B,
Nagarajan B and Yacoub S: Astragaloside IV attenuates ocular
hypertension in a mouse model of TGFβ2 induced primary open angle
glaucoma. Int J Mol Sci. 22:125082021. View Article : Google Scholar
|
|
204
|
Ashok A, Chaudhary S, Kritikos AE, Kang
MH, McDonald D, Rhee DJ and Singh N: TGFβ2-hepcidin feed-forward
loop in the trabecular meshwork implicates iron in glaucomatous
pathology. Invest Ophthalmol Vis Sci. 61:242020. View Article : Google Scholar
|
|
205
|
Zhou XT, Zou JJ, Ao C, Gong DY, Chen X and
Ma YR: Renal protective effects of astragaloside IV, in diabetes
mellitus kidney damage animal models: A systematic review,
meta-analysis. Pharmacol Res. 160:1051922020. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Ren Y, Liang H, Xie M and Zhang M: Natural
plant medications for the treatment of retinal diseases: The
blood-retinal barrier as a clue. Phytomedicine. 130:1555682024.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Zaman Q, Zhang D, Reddy OS, Wong WT and
Lai WF: Roles and mechanisms of astragaloside IV in combating
neuronal aging. Aging Dis. 13:18452022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Gu Y, Wang G, Pan G, Fawcett JP, Jiye A
and Sun J: Transport and bioavailability studies of astragaloside
IV, an active ingredient in radix astragali. Basic Clin Pharmacol
Toxicol. 95:295–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Huang CR, Wang GJ, Wu XL, Li H, Xie HT, Lv
H and Sun JG: Absorption enhancement study of astragaloside IV
based on its transport mechanism in Caco-2 cells. Eur J Drug Metab
Pharmacokinet. 31:5–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Yang J, Jiang H, Wu C, Lin Y, Tan G, Zhan
J, Han L, Zhu Y, Shang P, Liu L and Liu H: Copper silicate
nanoparticle-mediated delivery of astragaloside-IV for
osteoarthritis treatment by remodeling the articular cartilage
microenvironment. J Control Release. 381:1135832025. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Li X, Zhang X, Qi Y, Jin W, Wen Z, Zhao Y,
Li X, Yao X, Shen Z, Zhang F, et al: Conductive bioadhesive
hydrogel with controlled astragaloside IV release for
ferroptosis-mediated cardiac repair. J Control Release.
384:1138742025. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Wu S, Zhou M, Zhou H, Han L and Liu H:
Astragaloside IV-loaded biomimetic nanoparticles target IκBα to
regulate neutrophil extracellular trap formation for sepsis
therapy. J Nanobiotechnol. 23:1552025. View Article : Google Scholar
|
|
213
|
Wei L, Wang H, Ye X, Yue J, Guo H, Mao D,
Li X, Sun Y, Liu C, Liu Y and Chen Y: Oxymatrine and astragaloside
IV co-loaded liposomes: Scale-up purposes and their enhancement of
anti-PD-1 efficacy against breast cancer. Mater Today Bio.
32:1016342025. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Yu S, Peng W, Qiu F and Zhang G: Research
progress of astragaloside IV in the treatment of atopic diseases.
Biomed Pharmacother. 156:1139892022. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Qing LS, Chen TB, Sun WX, Chen L, Luo P,
Zhang ZF and Ding LS: Pharmacokinetics comparison, intestinal
absorption and acute toxicity assessment of a novel water-soluble
astragaloside IV derivative (Astragalosidic Acid, LS-102). Eur J
Drug Metab Pharmacokinet. 44:251–259. 2019. View Article : Google Scholar
|