Introduction
Of the DNA in the genome ~ 95% is noncoding DNA
among which are microRNAs (miRNAs/miRs), small non-coding RNA
sequences (18-25 nucleotides), able to regulate the expression of
one or more mRNAs (1,2). miRNAs were discovered in 1993 by
Victor Ambros and colleagues in Caenorhabditis elegans,
recently awarded 2024 Nobel Prize in Medicine for this discovery
(3). These small non-coding
sequences regulate gene expression at the post-transcriptional
level (4). Despite constituting
only 1% of the human genome, miRNAs influence up to 50% of genes
(5), playing essential roles in
development, cell proliferation, and apoptosis.
miRNAs regulate protein translation by targeting
their complementary 3' untranslated regions (3'UTRs) mRNA and by
repressing translation or degrading target mRNA. The specificity of
miRNA-mRNA interactions is crucial for determining the regulatory
outcome, which can vary from fine-tuning gene expression to more
profound effects on cellular phenotypes. Each miRNA could regulate
more than one mRNA involved in different biological processes
including proliferation (6),
cell survival (7), inflammation
(8), angiogenesis (9), oxidative stress (10) and immune response (11) among others. Some diseases are
affected by miRNA dysregulation. A tissue-specific expression
profile is needed to decipher the function and mechanism of
individual miRNAs (12).
The miRNAs are transcribed as long primary miRNa
(pri-miRNAby RNA pol II) (4).
The pri-miRNA are cleaved by Drosha-DGCR8 generating the precursor
(pre-miRNA). The pre-miRNA is transported to the cytoplasm by the
Ran-GTP dependent Exportin-5 (13). Once in the cytoplasm, the Dicer
enzyme processes the pre-miRNA, generating a mature miRNA as an
18-25 nucleotide RNA duplex. The mature miRNA is incorporated into
the RNA-induced silencing complex assembled by Dicer to promote the
post-transcriptional and mRNA target degradation (14).
In addition to its eminent intracellular role, in
2007, it was discovered that miRNAs can travel in extracellular
vesicles (EVs), such as exosomes, facilitating intercellular
communication (15,16). Additionally, they can travel
outside of these nanospheres, although they are more predisposed to
degradation by enzymes. Therefore, miRNAs are invaluable tools in
probing different aspects of a disease such as diagnosis,
progression, classification and treatment. In fact, a number of
clinical fields are now focused on the study of miRNA for diagnosis
or treatment purposes (17-19).
Angiogenesis is a multi-step process of new blood
vessel formation from existing vasculature. The angiogenesis
process can be summarized in four key steps: i) Matrix degradation
with the activation of proteases able to digest matrix components;
ii) migration of endothelial cells (EC); iii) proliferation of EC
to supply new cells for the tube formation and, iv) EC survival,
maturation and stabilization (20). Physiological angiogenesis is the
normal process that occurs during growth, development and tissue
repair. It is tightly regulated to ensure proper nutrient and
oxygen supply to the tissue. The imbalance between pro- and
anti-angiogenic factors leads to uncontrolled vasculature
over-growth named pathological angiogenesis. Pathological
angiogenesis is often associated with diseases such as cancer,
diabetic retinopathy (DR), and chronic inflammation (21). In case of tissue damage, a
temporary or permanent imbalance occurs. miRNAs are one of the
mechanisms that can regulate this balance through the
post-transcriptional regulation of pro- and anti-angiogenic
genes.
The study of miRNA can be useful in a number of
ways, from their use as biomarkers to their potential interest as
therapeutic targets for different diseases. miR-205 is one of the
most notable miRNAs, demonstrated in several studies and it is
involved in the regulation of different processes such as
angiogenesis, epithelial-mesenchymal transition and cell
proliferation, all related to the progression of diseases such as
cancer or DR. The aim of the present review is to delve into the
role of miR-205 in regulating angiogenesis as well as to analyze
the potential of its therapeutic window.
In short, miR-205 emerges as a critical factor,
distinguished by its bifunctional activity that is strictly
dependent on the biological context. This duality positions miR-205
as a master regulator of the angiogenic switch in both health and
disease. On the one hand, miR-205 exerts a pronounced
anti-angiogenic effect by repressing the expression of key
pro-vascular factors such as vascular endothelial growth factor
(VEGF) (22) and angiopoietin-2
(ANG-2) (23), acting as a
protective mechanism in hypervascular pathologies including
diabetic retinopathy, thyroid cancer, and psoriasis. On the other
hand, in conditions requiring vascular recanalization or favoring
malignancy, miR-205 adopts a pro-angiogenic role by inhibiting
tumor suppressors and anti-angiogenic molecules such as phosphatase
and tensin homolog (PTEN) (24)
and the long non-coding RNA HITT (25), thereby activating survival and
proliferation pathways, most notably the PI3K/Akt cascade.
Furthermore, the therapeutic significance of miR-205 is reinforced
by its ability to be encapsulated and transferred through EVs
(26), enabling the paracrine
modulation of angiogenesis at a distance, a mechanism with profound
implications for tumor progression and for the development of
innovative strategies in vascular regenerative medicine.
Characteristics of miR-205
Genomic location and conservation
miR-205 is a highly conserved miRNA in a number of
species. In the human genome, miR-205 is located on locus 1q32.2
within a gene annotated as miR-205 host gene (MIR205HG) and
is composed of a highly conserved structure. More specifically,
miR-205 resides between the second and third exons of LOC642587
(27). The MIR205HG gene
also acts as a noncoding RNA called LEADR/MIR205HG and is the host
gene for miR-205.
Physiological expression and
developmental roles
This miRNA is physiologically expressed in different
epithelia as cornea, breast, esophagus, thymus and bladder and is
one of the most highly expressed miRNAs in skin (28). Additionally, miR-205 is key in
development because it is involved in embryogenesis, especially in
epithelial morphogenesis, promoting endoderm and ectoderm
differentiation (29,30). In early embryonic stages, miR-205
is expressed in trophoblasts cell-linage regulating placental
development (31). miR-205 is
involved in the early lacrimal gland (32) and cornea development (33). Genetic loss of miR-205 causes
perinatal lethality due to an altered pathway in the epidermis
inhibiting stem cell self-renewal leading to skin defects (28,34,35).
Regulation of miR-205 expression
Precise regulation of miR-205 expression is
essential for development, onset and progression of different
alterations. Epigenetic modifications, such as CpG DNA methylation,
are mechanisms for gene expression regulation which may be key
miR-205 modulators. Specifically, mature miR-205 expression is
inversely associated to DNA methylation. miR-205 under expression
is related to high hypermethylation and chromatin changes leading
to epithelial-mesenchymal transition (EMT) and cell migration
(36,37). miR-205-5p transcription is
commonly repressed via miR-205 locus hypermethylation and then,
over-activates miR-205 targets. This fact has been associated with
poor prognosis in cancer (38,39). Moreover, there are different
transcription factors for miR-205, such as the p53 family, closely
involved in cell cycle and apoptosis (40). P53 stimulates miR-205 expression
levels promoting cell proliferation and EMT in cancer (41). Hypoxia inducible factor 1 α
(HIF-1α) is a member of the p53 family. This is a transcriptional
factor that represses miR-205 expression inducing EMT, angiogenesis
and tumor aggressiveness (42,43). Sp1 is a transcriptional factor
for miR-205 upregulation (44)
in contrast to Twist1 (45).
Beyond DNA methylation, histone modifications add a second layer of
epigenetic control. Repressive marks such as H3K27me3 and H3K9me2
are associated with the silencing of MIR205HG, while activating
marks such as H3K4me3 and histone acetylation (H3K9ac and H3K27ac)
promotes an open and transcriptionally active chromatin state
(37,38). These chromatin changes interact
with transcription factors such as p53, Sp1 and HIF-1α integrating
cellular stress and environmental signals into the regulation of
miR-205 (40-44). Finally, long noncoding RNAs
(lncRNAs) regulate miRNAs availability by sponging them. Increasing
evidence has indicated that lncRNAs play important roles in human
disorders, however, the functional implications have only been
studied in a few of them. One of the most important lncRNAs related
to miR-205 is MALAT1. It has been observed that under hyperglycemic
conditions, MALAT1 act as a sponge for miR-205, thereby releasing
its target ZEB1 from inhibition, which in turn represses E-cadherin
expression and consequently induces EMT (46). In osteosarcoma, it has been
observed that MALAT1 is upregulated and acts as a suppressor of
miR-205, thereby releasing SMAD4 from miR-205-mediated inhibition
and promoting cell proliferation through activation of TGF-β/SMAD4
signaling pathway (47).
Similarly, MALAT1 sponges miR-205, inhibiting its function in
cerebral ischemia and thereby leading to PTEN overexpression, which
in turn modulates the PI3K/Akt signaling pathway and promotes
apoptosis (48). There are other
lncRNAs, such as LINC00673 (49), SNHG5 (50), GAS5 (51) and ZEB1-AS1 (52) capable of sequestering miR-205 and
promoting the malignancy of tumor. Notably, it has been observed
that lncRNA SNHG5 functions as a competing endogenous RNA (ceRNA)
for miR-205-5p, suppressing its activity and increasing ABR
expression, which in turn activates the RAF/MEK/ERK pathway and
contributes to imatinib resistance in chronic myeloid leukemia
(50). In line with these
findings, lncRNA GAS5 has also been shown to suppress cervical
cancer tumorigenesis by downregulating miR-196a and miR-205,
thereby restoring the expression of their targets FOXO and PTEN,
and consequently inhibiting the PI3K/Akt pathway, which leads to
reduced cell proliferation and invasion (51). Finally, ZEB1-AS1 has been shown
to regulate colorectal cancer progression through the miR-205/YAP1
axis, acting as a sponge for miR-205 and thereby increasing YAP1
expression, which promotes cell proliferation, migration and EMT
via activation of Hippo signaling pathway (52). CircRNAs such as circ_0003520 have
also been shown to act as sponges for miR-205-5p (53). Together, these findings highlight
the pivotal role of lncRNAs as upstream regulators of miR-205,
modulating their availability across diverse pathological contexts.
By acting as molecular sponges, these lncRNAs fine-tune
miR-205-mediated repression of key targets such as ZEB1, SMAD4,
PTEN, ABR and YAP1, ultimately influencing major signaling
pathways, including PI3K/Akt, TGF-β/SMAD, RAF/MEK/ERK, and Hippo,
that govern cell proliferation, apoptosis, migration, and EMT
(Fig. 1).
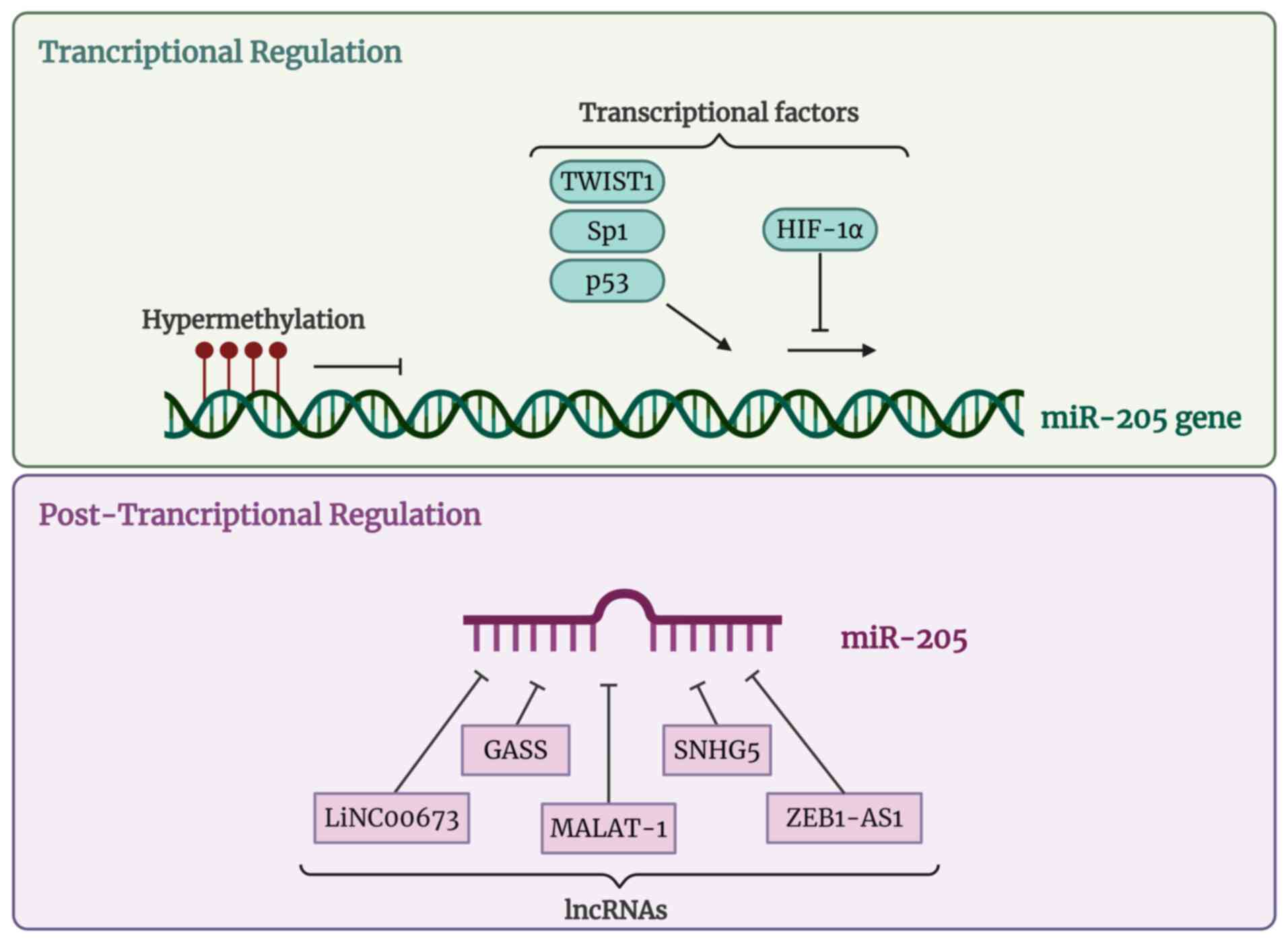 | Figure 1miR-205 transcriptional and
posttranscriptional regulation. Created in https://BioRender.com. miR, microRNA; TWIST1,
Twist-related protein 1; Sp1, Specifiticy protein 1; p53, Tumor
protein p53; HIF-1α, Hypoxia-inducible factor 1-alpha; lncRNA, long
non-coding RNA; LINC00673, Long intergenic non-protein coding RNA
673; GAS5, Growth arrest-specific 5; MALAT-1, Metastasis-associated
lung adenocarcinoma transcript 1; SNHG5, Small nucleolar RNA host
gene 5; ZEB1-AS1, Zing finger E-box binding homebox 1 antisense RNA
1. |
Context-dependent functions
The basic mechanisms of miRNAs regulation involve
mRNA translation blocked upon base complementarity binding to the
3'UTR region. In this regard, miR-205 plays opposing roles
depending on cellular contexts, cell types and target gene. In the
context of cancer, where miR-205 has been most studied, it is
paradoxically considered pro or anti-oncogenic. In thyroid cancer,
miR-205 was demonstrated to act as a tumor suppressor by repressing
the expression of CCNB2 (54).
In breast cancer it targets Küppel-like factor 12 (KLF12) (55) and angiomotin (AMOT) (56) reducing cell invasion. Focusing on
angiogenesis, it similarly demonstrates contradictory functions,
which will be discussed in further detail.
miR-205 in angiogenesis
Angiogenesis is essential in physiological processes
to provide oxygen and nutrients to tissues for their maintenance
and repair. However, angiogenesis can be associated with the onset
or progression of pathological processes. Angiogenesis is guided by
complex pathways that primarily affect EC, the main cells involved,
which undergo several steps for the formation of blood vessels:
Matrix degradation, migration, proliferation and survival (20). The angiogenesis switch depends on
the resultant molecular balance between pro-angiogenic and
anti-angiogenic factors (57).
Among the pro-angiogenic factors that act directly on EC, VEGF
(58) and fibroblast growth
factor (FGF) (59) stand out.
VEGFA (a member of VEGF family) binds to VEGF receptor 2 (VEGFR2)
inducing dimerization and transautophosphorylation of cell
cytoplasmatic tyrosine residues, activating EC migration, survival
and vascular permeability through the PI3K/Akt pathway (60). Therefore, the p38
mitogen-activated protein kinase (MAPK) is activated after
VEGFA-VEGFR2 interaction and promotes actin remodeling, cell
migration and stress response (61). FGF plays a crucial role in
angiogenesis and works synergically with VEGF inducing EC
proliferation, migration and increase vascular permeability via
PI3K/Akt and MAPK (62,63). Also, TGFβ in EC can signal via
ALK1 which leads to phosphorylation of R-Smad1/5/8 with the
consequent activation of cell migration, proliferation and
angiogenesis (64,65). Studies confirm that some
pro-angiogenic pathways involved Janus kinases/Signal Transducer
and Activators of Transcription (JAK/STAT) which are stimulated by
VEGFA-VEGFR2 interaction (66,67). This interaction induces the JAK
activation, which phosphorylates STAT proteins, acting as a
transcriptional factor of proangiogenic genes such as VEGF
(68) (Fig. 2).
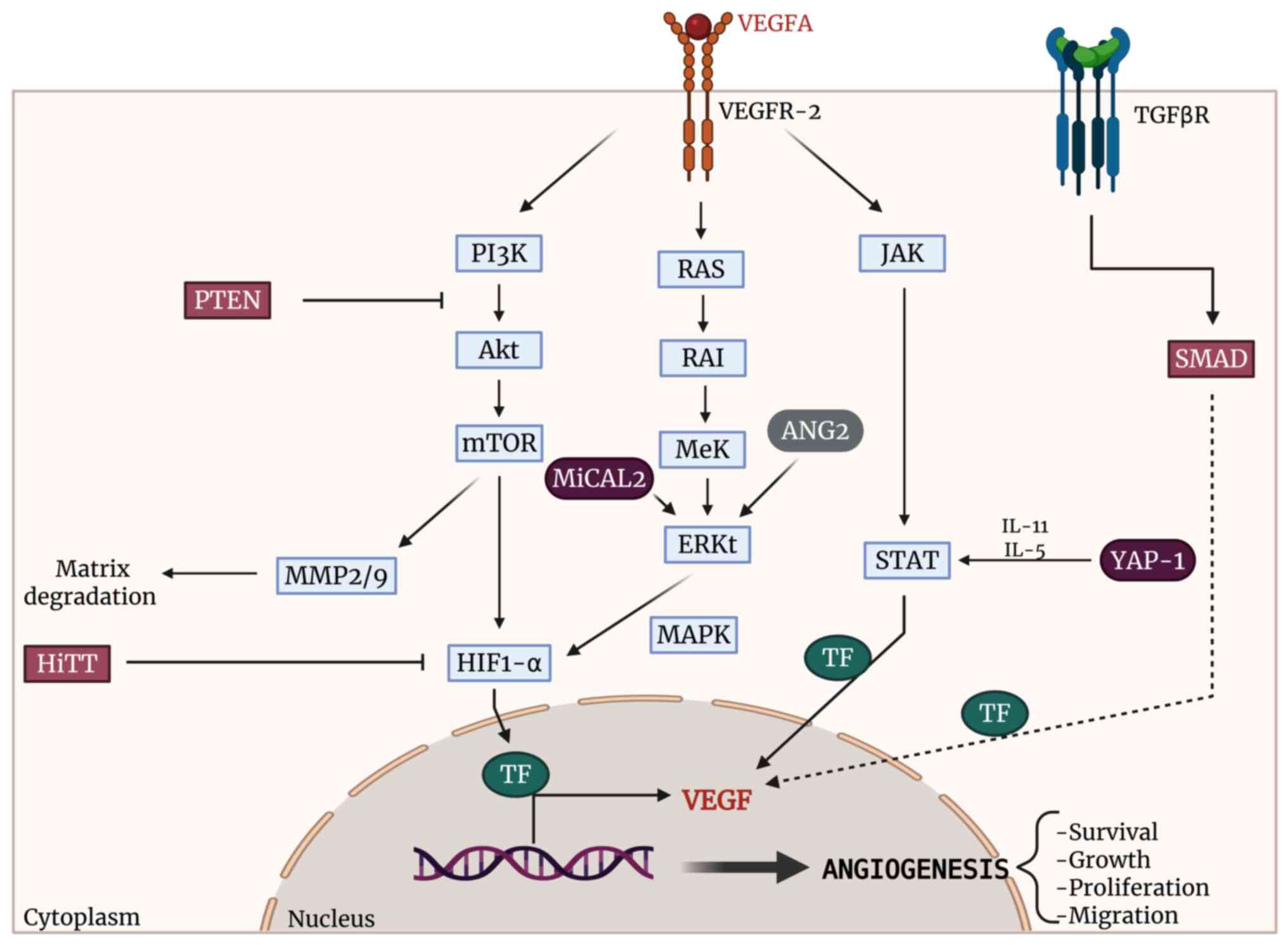 | Figure 2Representation of the main regulatory
pathways of angiogenesis and the targets of miR-205. The direct
targets of miR-205 are highlighted in purple. Created in https://BioRender.com. miR, microRNA; VEGFA, vascular
endothelial growth factor A; VEGFR-2, vascular endothelial growth
factor receptor 2; TGFβR, transforming growth factor β receptor;
PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; mTOR,
mechanistic target of rapamycin; RAS, rat sarcoma viral oncogene
homolog; RAI, Ras-associated/activated inhibitor; MEK,
mitogen-activated protein kinase kinase; ERK, extracellular
signal-regulated kinase; MAPK, mitogen-activated protein kinase;
JAK, Janus kinase; STAT, signal transducer and activator of
transcription; PTEN, phosphatase and tensin homolog; HIF-1α,
hypoxia-inducible factor 1-alpha; TF, transcription factor; MiCAL2,
microtubule-associated monooxygenase, calponin and LIM
domain-containing 2; HiTT, HIF-1α inhibitor at the transcriptional
level (lncRNA); YAP-1, yes-associated protein 1; SMAD, SMAD
signaling proteins; ANG2, angiopoietin-2; MMP2/9, matrix
metalloproteinases 2 and 9; IL-11, interleukin-11; IL-5,
interleukin-5; VEGF, vascular endothelial growth factor. |
Some of the factors involved in the aforementioned
pathways, are regulated by miR-205. miR-205 plays a significant
role in the regulation of vascular processes, which, depending on
the context including cell type, stimulation and regulated target,
can be considered pro-angiogenic or anti-angiogenic (Table I and Fig. 3). The present review aimed to
elucidate its dual role as a regulator of angiogenic processes.
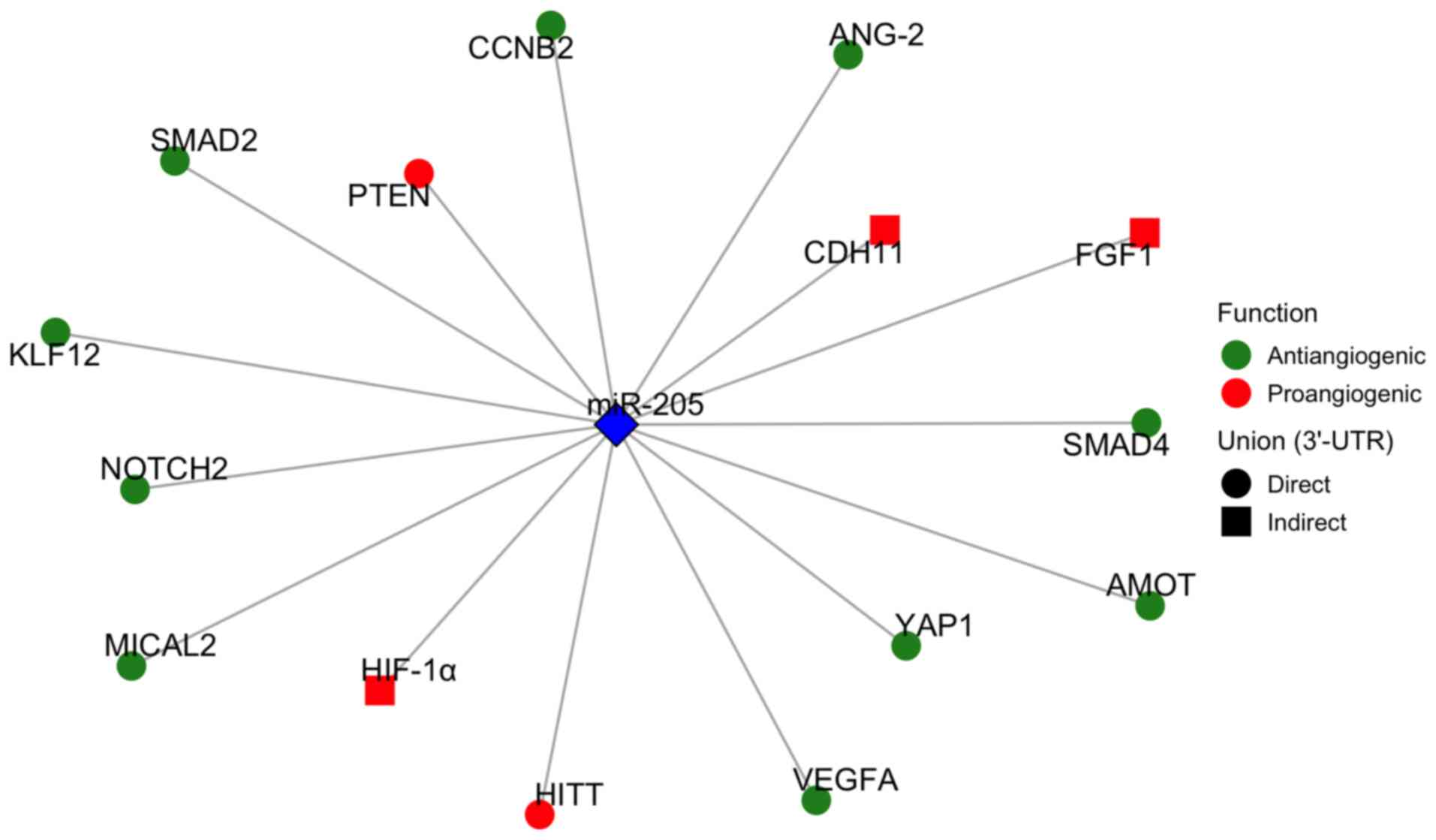 | Figure 3Representation of the 3'UTR targets
of miR-205. Targets acting as anti-angiogenic factors are shown in
green, and pro-angiogenic ones in red. Direct targets, defined by
miR-205 binding to the 3'UTR region, are depicted as circles, while
indirect targets of miR-205 are depicted as squares. miR-205,
microRNA-205; ANG-2, angiopoietin-2; AMOT, angiomotin; CCNB2,
cyclin B2; CDH11, cadherin-11; FGF1, fibroblast growth factor 1;
HIF-1α, hypoxia-inducible factor 1-alpha; HiTT, HIF-1α inhibitor at
the transcriptional level; KLF12, Krüppel-like factor 12; MiCAL2,
microtubule-associated monooxygenase, calponin and LIM
domain-containing 2; NOTCH2, neurogenic locus notch homolog protein
2; PTEN, phosphatase and tensin homolog; SMAD2, mothers against
decapentaplegic homolog 2; SMAD4, mothers against decapentaplegic
homolog 4; VEGFA, vascular endothelial growth factor A; YAP1,
yes-associated protein 1; 3'-UTR, 3'-untranslated region. |
 | Table ImiR-205 targets and related
angiogenic pathways. |
Table I
miR-205 targets and related
angiogenic pathways.
| Authors, year | Role | miR-205
targets | Related
pathway | Pathology | (Refs.) |
|---|
| Xue et al,
2020 |
Anti-angiogenic | VEGFA and
ANG-2 | MAPK | Psoriasis | (23) |
| Zhu et al,
2019 | | VEGF | VEGF | Diabetic foot | (79) |
| Tan et al,
2021 | | VEGFA | VEGFA | High glucose
conditions | (69) |
| Oltra et al,
2020 | | VEGFA | VEGF | Retinal epithelial
cell disorder | (22) |
| Gao et al,
2020 | | VEGFA | VEGFA | Cerebral Ischemic
stroke | (80) |
| Salajegheh et
al, 2015 | | VEGFA | VEGF | Thyroid cancer | (75) |
| Vosgha et
al, 2018 | | VEGFA | VEGFA | Thyroid cancer | (76) |
| Zhang et al,
2021 | | VEGFA and FGF1 | ERK1/2 | Gastric cancer | (77) |
| Ouyang et
al, 2020 | | VEGF and CDH11 | - | Osteogenesis | (82) |
| Tabruyn et
al, 2013 | | SMAD1/SMAD4 | TGF-β pathway | Hereditary
hemorrhagic telangiectasia | (65) |
| Tao et al,
2019 | | MICAL2 | ERK1/2 | Pulmonary arterial
hypertension | (99) |
| Jiang et al,
2021 | | NOTCH2 | VEGF | Mandibular
distraction osteogenesis | (103) |
| Du et al,
2017 | | YAP1 | STAT3 (IL-11 and
IL-15) | Breast cancer | (72) |
| Sun et al,
2019 | Pro-angiogenic | PTEN | PI3K/AKT | Thrombosis | (24) |
| Yao et al,
2019 | | PTEN | PI3K/AKT | Gastric cancer | (74) |
| Cai et al,
2013 | | PTEN | AKT/FOCO3A and
AKT/mTOR | Lung cancer | (73) |
| Wang et al,
2020 | | HITT | HIF-1α | Colon cancer | (25) |
The present review performed an enrichment analysis
that revealed that miR-205-associated genes are markedly
over-represented in several canonical signaling pathways implicated
in angiogenesis, vascular remodeling and diabetic complications
(Fig. 4) (24,40,41,65,69-74). Among the most enriched pathways
were the Hippo signaling pathway, AGE-RAGE signaling in diabetic
complications (69,70), PI3K-Akt (24,73,74), TGF-β (65,71), and p53/EGFR-related cancer
pathways (40,41,72), as well as processes linked to
cell cycle control, cellular senescence and FoxO signaling
(73). These findings suggest
that miR-205-5p exerts a broad regulatory influence across
interconnected angiogenic and stress-response pathways. Notably,
the Hippo/YAP1 and PI3K-Akt/PTEN axes, both central regulators of
endothelial proliferation and migration, were among the top
enriched categories. In addition, the identification of the
AGE-RAGE signaling pathway underscores the potential role of
miR-205-5p in hyperglycemia-induced vascular dysfunction (69,70).
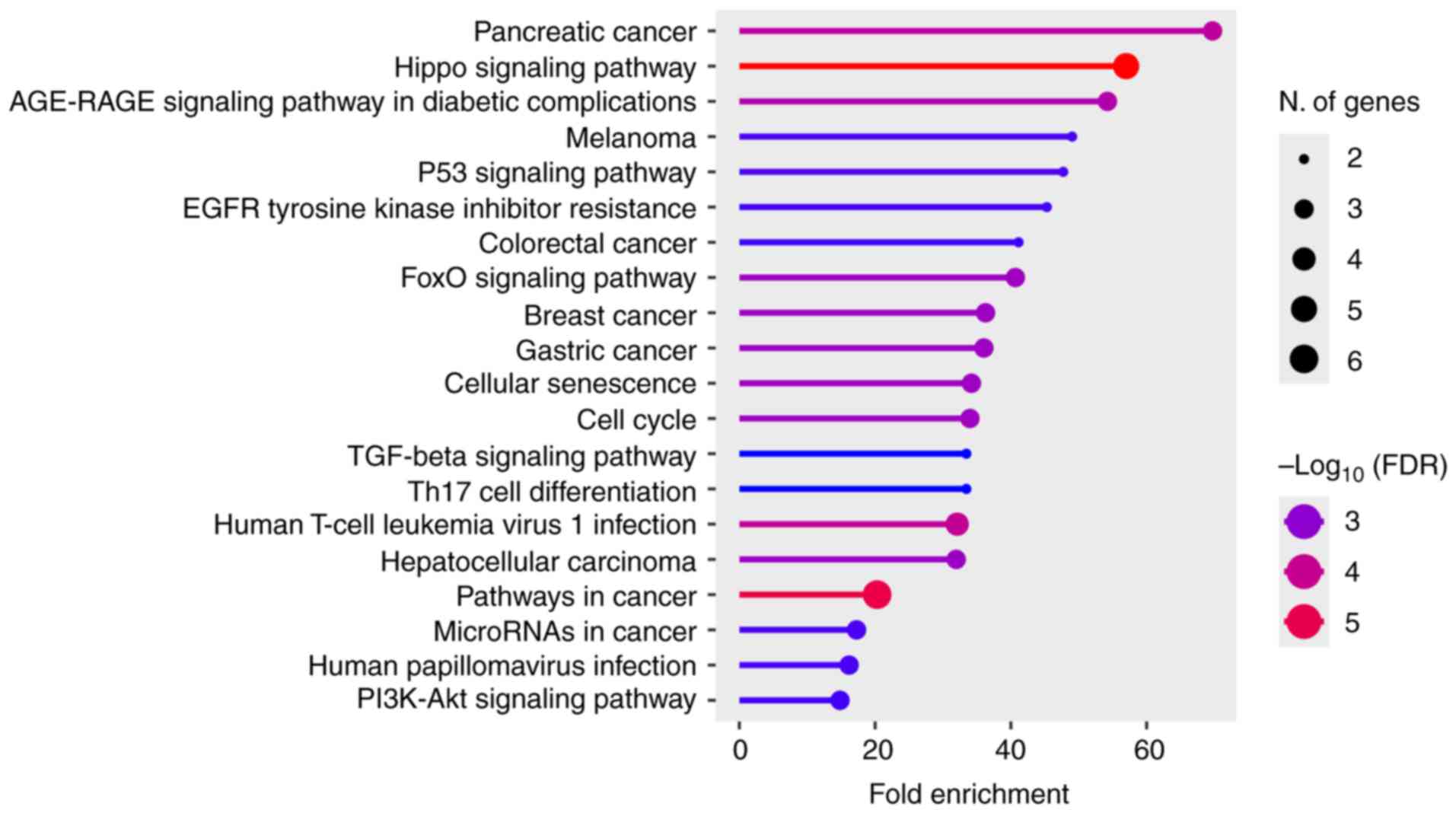 | Figure 4KEGG pathway enrichment of
miR-205-regulated genes. Dots represent enriched pathways; x-axis:
Fold Enrichment; dot size: number of genes; dot color:
-log10(FDR). The top pathways (Hippo/YAP1,
PI3K-Akt/PTEN, TGF-β/SMAD, p53/EGFR resistance, AGE-RAGE in
diabetic complications) highlight canonical mechanisms by which
miR-205 can exert anti- or pro-angiogenic effects depending on the
targeted nodes and context. KEGG Kyoto Encyclopedia of Genes and
Genomes; FDR, false discovery rate; miR-205, microRNA-205;
PI3K-Akt, phosphoinositide 3-kinase-protein kinase B; TGF-β,
transforming growth factor-β; EGFR, epidermal growth factor
receptor; HPV, human papillomavirus; AGE-RAGE, advanced glycation
end products-receptor for advanced glycation end products. |
miR-205 as anti-angiogenic regulator
One of the most studied implications of miR-205 is
its regulation of angiogenesis, where it is mostly considered to
play an anti-angiogenic role, making miR-205 a protective agent
against a number of conditions involving blood vessel formation,
such as cancer and ocular diseases, among which DR and macular
degeneration are conspicuous (22,23,69,70,75-78). This anti-angiogenic role is due
to the post-transcriptional regulation of pro-angiogenic genes
(22,69,77). The following section explains how
miR-205 regulates angiogenesis depending on the targeted gene.
miR-205 and VEGF
VEGF, as already mentioned, is one of the most
studied and relevant pro-angiogenic factors; it activates the
migration, survival and vascular permeability of EC, thereby
inducing angiogenesis (59-61). Different studies have shown that
miR-205 has affinity, through base complementarity, with the 3'UTR
region of mRNA that encodes for VEGF (22,69,77). Thus, VEGF is one of the most
studied angiogenic factors due to its involvement in various
diseases. In this regard, the present study will focus on the
VEGF-miR-205 relationship. One of the diseases in which the
regulatory role of miR-205-VEGF has been studied is psoriasis
(23). Psoriasis is
characterized by excessive neovascularization in dermis and
epidermal hyperproliferation, in fact angiogenic factors such as
VEGF and ANG-2 are elevated in plasma from patients. Xue et
al (23) observed a negative
correlation between the expression of miR-205, VEGF and ANG-2 in
skin samples. After the subcutaneous injection of a miR-205
mimetic, the authors observed a decrease in inflammation and tissue
damage. These improvements were due to the overexpression of
miR-205, which reduced the levels of VEGF and ANG-2, thus blocking
the MAPK pathway. Therefore, the authors concluded that miR-205
administration reduces the severity of psoriasis in mice.
On the protective role of miR-205, different authors
agree that the state of hyperglycemia modifies the expression
levels of miR-205, specifically decreasing them. It is hypothesized
that MALAT1 sponges miR-205, facilitating the transcription of mRNA
encoding VEGF (69,79). This regulatory mechanism can be
considered protective or not, depending on the alteration. Under
hyperglycemic conditions, an increase in proliferation, migration
and endothelial tube formation is observed, which are
pathophysiological processes associated with conditions such as DR,
due to overexpression of miR-205 (69). However, in diabetic foot ulcer
proliferation, migration and tube formation processes are
considered essential for optimal recovery. So, both studies
highlight the importance of MALAT1 and miR-205 in the regulation of
VEGF and angiogenesis, but from opposing perspectives: One focuses
on the positive therapeutic effects for diabetic foot due to the
underexpression of miR-205, while the other addresses how reducing
MALAT1 and increasing miR-205, can alleviate pathological
angiogenesis induced by high glucose levels. In any case, miR-205
is considered to have anti-angiogenic capacity. In a similar
context, under conditions of oxidative stress, such as
hyperglycemia, the expression levels of miR-205 are also found to
be reduced, while VEGF levels are elevated in retinal pigment
epithelium cells (RPE) (22,70). This suggests that miR-205 acts as
a regulatory agent of processes such as migration, proliferation
and cellular apoptosis, emphasizing its potential as an
anti-angiogenic factor in proliferative ocular diseases such as DR
and age-related macular degeneration. Building on the
MALAT1-miR-205-VEGF relationship, Gao et al (80) determined that MALAT1 serves as a
protective agent for the angiogenic function of human brain
microvascular endothelial cells under oxygen-glucose
deprivation/reoxygenation conditions. The authors' findings align
with the role of MALAT1 as a ceRNA for miR-205, sequestering it and
thereby enhancing VEGF expression. This interaction protects and
promotes angiogenesis, highlighting its potential protective role
against ischemic injury.
The role of VEGF-miR-205 relationship has also been
studied in cancer, one of the most extensively researched fields in
every aspect. Thyroid cancer is a well-studied cancer type
(75,76). Authors agree on the role of
miR-205 as a tumor suppressor in thyroid cancer. Notably, the
authors observed that miR-205 overexpression reduced angiogenesis
by decreasing VEGF expression. Additionally, miR-205 inhibited EMT,
invasion, migration and tumor growth. In gastric cancer, the
miR-205-VEGF axis has also been studied, revealing that miR-205 is
underexpressed in gastric cancer tissues (77). In renal cell carcinoma, miR-205
exerts a protective role by regulating VEGF expression, while
metformin, a common antidiabetic drug, modulates miR-205 levels
(81). These findings
demonstrate the protective effect of metformin through its
regulatory action on miR-205. Its overexpression markedly reduces
cancer cell proliferation and angiogenesis in vivo. miR-205
achieves this effect by regulating not only VEGF but also FGF1 via
ERK pathway, both of which are critical factors in the formation of
new blood vessels. Therefore, the miR-205-VEGF axis in cancer is
considered a tumor suppressor due to its inhibitory role in
angiogenesis. Consistent with the regulatory function of miR-205 on
VEGF, it has been observed that during osteogenesis, the biological
process responsible for bone tissue formation and closely linked to
vascular development, miR-205 expression levels were low, while
VEGF expression was elevated (82). These expression levels are
attributed to the lncRNA ENST00000563492, which acts as a ceRNA,
sequestering miR-205. This sequestration increases VEGF expression,
promoting angiogenesis during bone formation and facilitating bone
healing.
Hereditary hemorrhagic telangiectasia (HHT) is a
genetically heterogeneous disorder characterized by abnormal
vascular structures. In this disease elevated VEGF plasma levels
are considered HHT biological markers. By contrast, miR-205 in
plasma levels are reduced (83).
Although the authors of the present study do not mention the
specific VEGF/miR-205 relationship or inter-regulation, their
findings align with previously discussed studies: Low miR-205
levels lead to increased VEGF, which, in some cases results in
abnormal vessel formation.
In summary, considering the regulatory pair
miR-205/VEGF, the presence of miR-205 is regarded as protective, as
it reduces VEGF levels and consequently angiogenesis in ischemia,
cancer, psoriasis, or hyperglycemia conditions. However, in cases
such as diabetic foot or osteogenesis, it is the absence of miR-205
that is deemed protective, as it promotes blood vessel formation
due to increased VEGF levels. Regardless, all the mentioned studies
agree on the regulatory potential of the miR-205/VEGF axis in
angiogenic processes, with miR-205 being recognized as
anti-angiogenic.
miR-205 and angioprotein (ANG) 2
ANG-1 and ANG-2 are another type of cell growth
factors having an angiogenic activity after binding its tyrosine
kinase receptor Tie-2 which is expressed primarily on EC (84). ANG-2 is considered a miR-205
target (23), although its
angiogenic role remains to be clarified. Some authors consider that
ANG-2 may inhibit angiogenesis by promoting the shedding of
pericytes (85) while others
consider ANG-2 as a VEGF enhancer promoting angiogenesis (86). Apart from the previously
mentioned miR-205/VEGF regulation, Xue et al (23) observed that ANG-2 was
overexpressed in psoriasis patients' plasma while miR-205 was
underexpressed. The authors concluded that miR-205 acts as a
negative regulator of ANG-2, decreasing angiogenesis and cell
proliferation in the context of psoriasis following the
subcutaneous injection of the miRNA. Abouaitah et al
(87) observed that both miR-205
and ANG-2 are involved in the anticancer effects of a drug delivery
system based on mesoporous silica nanoparticles (MSNs) against
colon cancer. This strategy is widely used to enhance drug delivery
and efficacy. They found that MSN administration markedly inhibited
the expression of both miR-205 and ANG-2. These results do not
align with those reported by other researchers. Although the
authors do not specifically address the miR-205/ANG-2 relationship,
their findings suggest that, in this experimental cancer cell model
and conditions, MSNs-miR-205 treatment does not regulate ANG-2
expression. However, despite not discussing this regulation, they
highlight the protective role of miR-205 in suppressing migration,
invasion, and EMT. Similar observations were made in HHT, where
both miR-205 and ANG-2 levels were found to be low in patients'
plasma (83). Under these
conditions, miR-205 cannot be considered an ANG-2 regulator.
Therefore, further studies are needed to clarify the regulation of
ANG-2 by miR-205.
miR-205 and SMAD
SMAD proteins act as crucial transcription factors
in the regulation of angiogenesis following the activation of TGFβ
pathway in EC. TGFβ signaling begins when TGFβ binds to its surface
receptors, leading to the phosphorylation of SMAD1/5 by activin
receptor-like kinase-1. These phosphorylated SMADs then form a
complex with SMAD4, which translocate to the nucleus to regulate
the transcription of specific genes involved in key processes such
as migration and proliferation, essential for angiogenesis
(88,89). Once again, in HHT, Tabruyn et
al (65) established that
miR-205 was downregulated, affecting the TFGβ signaling pathway in
ECs. Specifically, the authors observed that miR-205 negatively
regulates the SMAD1 and SMAD4 genes. The reduction in miR-205
expression leads to an increase in SMAD1 and SMAD4 activity,
shifting the TGFβ balance toward a pro-angiogenic state, thereby
promoting migration, proliferation and tube formation in ECs. In
this context, miR-205 acts as an anti-angiogenic factor by
downregulating the TGFβ pathway through the regulation of SMAD1 and
SMAD4 (65). Similar results
were observed in an in vivo cellular model of human lung
adenocarcinoma, where miR-205 represses SMAD4 expression by
directly binding to 3'UTR region of its mRNA. This leads to a
reduction in TGFβ signaling, thereby decreasing cancer cell
invasion and migration, key processes for angiogenesis (71). Other authors state that in
glioma, miR-205 can regulate the expression of SMAD2 (90), another member of the SMAD
transcription factor family and a critical protein in tumor
proliferation regulation (91).
Duan et al (90) report
that miR-205 is underexpressed in glioma cells, leading to
increased SMAD2 expression, which promotes TGFβ signaling and
contributes to glioma cell proliferation and migration, thereby
enhancing tumor malignancy. Moreover, studies confirm these results
concretely in hyperplastic scars, characterized by excessive growth
of fibrous tissue, where miR-205 negatively regulates SMAD2
(65,71,90). The authors observed that miR-205
levels were reduced, while SMAD2 levels were elevated. The
restoration of miR-205 was able to suppress cell proliferation and
promote apoptosis, in addition to inhibiting the expression of key
extracellular matrix components, such as collagen I and II
(92). Although the authors'
results do not specifically address angiogenesis, they align with
previous findings underlining the protective role of miR-205 in
suppressing cell proliferation and migration; both essential
processes for blood vessel formation.
miR-205 and molecule interacting with
CasL 2 (MICAL2)
MICAL2 is an enzyme that catalyzes actin
oxidation-reduction destabilizing F-actin in cytoskeletal dynamics
(93). MICAL2 is required to
mediate Semaphorin3A-NRP2 response to VEGFR1 in EC, being
Semaphorin3A-NRP2 essential in the modulation of cell proliferation
and migration (94).
Furthermore, VEGF promotes the assembly of p130Cas interactome that
contains, among others, the MICAL2 protein. This interactome is
responsible for driving chemotactic signaling and angiogenic
properties of ECs (95).
Different authors have linked MICAL2 overexpression in EC to
chemoresistance and increased mortality in various types of cancer,
including breast (96), bladder
(97) and gastric cancer
(93). One of the key processes
influencing tumor progression is neoangiogenesis. Specifically,
Barravecchia et al (98)
observed that MICAL2 is expressed in ECs of pathological
neoangiogenic capillaries but not in normal capillaries.
Consequently, MICAL2 inhibition reduces EC viability and functional
performance by impairing their ability to respond to VEGF
stimulation, the authors point to MICAL2 as a possible new target
for anti-angiogenic therapy. Tao et al (99) were the first and only ones to
establish that the 3'UTR region of MICAL2 is a target of miR-205.
The authors observed that in pulmonary arterial smooth muscle cells
affected by pulmonary arterial hypertension, miR-205 is
downregulated, while MICAL2 expression levels are elevated,
promoting pulmonary arterial smooth muscle cell proliferation
through the ERK-1/2 pathway. Therefore, the authors further propose
miR-205 as an antiangiogenic agent, as it reduces MICAL2
expression, inhibits ERK-1/2 pathway activation, and consequently
diminishes the cellular response to VEGF signaling.
miR-205 and NOTCH2
The Notch pathway is a highly conserved
intercellular signaling system activated by the interaction of
transmembrane ligands with Notch receptors (Notch1-4). Ligand
binding induces cleavage of the Notch receptors and subsequent
nuclear translocation of the Notch intracellular domains binding to
multiple DNA-binding proteins. Notch, at initial angiogenesis
stages, is suppressed to allow EC to proliferate in response to
VEGF stimulation and its expression is subsequently upregulated
when EC stop proliferating and the vessels begin to stabilize
(100,101). Therefore, the Notch signaling
pathway is considered a negative regulator of angiogenesis in most
physiological contexts (101).
However, in the skeletal system, the role of Notch signaling
pathway is the opposite, being pro-angiogenic (102). There are few studies linking
miR-205 to NOTCH2. Jiang et al (103) identified NOTCH2 as a direct
target of miR-205 in the context of mandibular distraction
osteogenesis, a surgical procedure used for controlled bone
regeneration following fractures. In this setting, the NOTCH2
pathway plays a pro-angiogenic role. The authors' findings
demonstrated that miR-205 regulates osteogenesis by modulating
NOTCH2 expression, a key factor in bone angiogenesis. Inhibiting
miR-205 markedly enhanced angiogenesis, whereas its overexpression
had the opposite effect. Moreover, transduction with a lentiviral
miR-205 inhibitor successfully restored angiogenic activity,
accelerated bone regeneration and promoted local remodeling. Once
again, miR-205 emerges as an anti-angiogenic factor, which, in this
case, is considered detrimental to osteogenesis. Further studies
are needed to clarify the relationship between miR-205/NOTCH and
angiogenic processes, as well as their potential use as therapeutic
targets.
miR-205 and Yes-associated protein 1
(YAP1)
YAP1 is a key transcriptional coactivator in the
Hippo signaling pathway, which is involved in cell proliferation,
migration and cancer invasion. Overexpression of YAP1 has been
observed in several carcinomas, including lung, prostate, and colon
cancer, among others, promoting tumor growth, proliferation and
metastasis (104). Several
studies have demonstrated that YAP1 is a target of miR-205 in
different types of cancer, such as thyroid cancer (105), breast cancer (72), colon cancer (52) and gastric cancer (106). All these studies agree on the
role of miR-205 as a tumor suppressor by inhibiting YAP1. However,
YAP1 is widely recognized as an oncogene. Notably, YAP1 is not only
regulated by miR-205 but also indirectly by ZEB1-AS1, a lncRNA that
stimulates its expression on colorectal cancer tissues by acting as
a post-transcriptional regulator of miR-205 (52). Although multiple studies have
confirmed the miR-205/YAP1 axis in the regulation of cell
proliferation and migration (52,72,105,106), few have explored its connection
to angiogenic processes. Du et al (72) demonstrated that the miR-205/YAP1
axis promotes angiogenesis in breast cancer through the STAT3
pathway, independently of VEGF. In cancer-associated fibroblasts
(CAFs) from breast cancer stroma, miR-205 is downregulated,
suggesting a regulatory role. The authors' findings showed that
restoring miR-205 expression attenuates angiogenic processes.
Specifically, miR-205/YAP1 signaling activates normal breast
fibroblasts, converting them into CAFs, which in turn promote tube
formation by EC (HUVECs). However, this YAP1-driven increase in
angiogenesis was not mediated by VEGF. Instead, it enhanced the
expression of IL-11 and IL-5, maintaining the angiogenic
environment even in the presence of VEGF-neutralizing drugs. More
specifically, IL-11 and IL-5 activated the STAT3 pathway, promoting
angiogenesis. In this context, miR-205 emerges as a potential
anti-angiogenic agent, whose overexpression could reduce blood
vessel formation. There are additional studies bringing to light
the relationship between YAP1 and angiogenic processes. For
instance, in DR, YAP1 was found to play an essential role (107). MALAT1, a lncRNA that also
regulates miR-205, negatively regulates miR-200b-3a under
high-glucose conditions. The downregulation of miR-200b-3p led to
the de-repression of YAP1, increasing its expression and
pro-angiogenic activity. Although this study did not specifically
investigate the involvement of miR-205, it suggested that the
regulation of YAP1 via MALAT1 may not be limited to miR-200b-3p but
could also involve miR-205.
miR-205 as pro-angiogenic regulator
Although most studies examining miR-205 and its
implication in angiogenesis consider it to be anti-angiogenic,
there are some that document the opposite. miR-205 acts as a
pro-angiogenic by blocking the transcription of factors with
anti-angiogenic capacity, among which PTEN and HITT are
prominent.
miR-205 and PTEN
As aforementioned, miR-205 exhibits a dual pro- and
anti-angiogenic profile, potentially acting as either protective or
an inducer of pathological processes. PTEN is a widely studied
target of miR-205 due to base complementarity. In fact, different
studies demonstrate that the sequence of miR-205 is complementary
to 3'UTR of PTEN (24). One of
the contexts in which the regulation role of miR-205/PTEN has been
studied is in deep vein thrombosis (DVT). DVT refers to the
formation of blood clots, inducing blood flow disorders and
complications such as pulmonary embolism with fatal consequences
(108). Angiogenesis is
essential for thrombus recanalization and DVT resolution (109). Sun et al (24) demonstrated that the intravenous
administration of miR-205 mimics reduced thrombus size and weight,
increasing recanalization and DVT resolution. This effect was due
to the enhanced migration and angiogenic capacity and suppressed
apoptosis of EC. This pro-angiogenic effect of miR-205 was due to
the regulation of PTEN, a negative regulator of PI3K/Akt pathway.
The overexpression of miR-205 under thrombosis conditions represses
the expression of PTEN, stimulating the PI3K/Akt pathway, which as
a result induces, among others, the expression of MMP2. MMPs are
considered important proteases for matrix degradation, activated by
pro-angiogenic factors, necessary for EC migration (110). Therefore, the blockade of PTEN
in ECs by miR-205 promotes the activation of migration and cell
survival and reduces apoptosis through the PI3K/Akt pathway,
suggesting miR-205 as a protective agent against thrombosis
(24). However, other studies
show the pro-angiogenic role of miR-205/PTEN axis but with
potential pathological implications, especially in cancer contexts.
In different tumor processes AKT signaling is constitutively
activated in part because of PTEN loss of function (111,112). Lung cancer cell lines show an
upregulation of miR-205 expression repressing PTEN expression
activating AKT/FOXO3a and AKT/mTOR signaling pathways accelerating
tumor cell proliferation and blood vessel formation, both in
vivo and in vitro (73). Therefore, in tissues from
patients with gastric cancer, it was observed that the expression
of miR-205 was higher compared with healthy tissue, while the
expression of PTEN was inversely proportional to that of miR-205.
In Yao et al (74), in
vitro analyses were performed in gastric cancer cell lines,
showing that the administration of miR-205 inhibitors increased
apoptosis and reduce cell proliferation and migration by PTEN
overexpression (74). In
endometrial cancer, the regulation of PTEN by miR-205 is also
observed, suggesting miR-205 as a biomarker for endometrial cancer;
however, its functional implication in angiogenic processes was not
studied (113). These studies
concluded that miR-205 can be considered an oncogene by reducing
PTEN expression, promoting cell migration, proliferation,
angiogenesis and mitochondrial damage contributing to the malignant
phenotype of tumor processes.
miR-205 and HITT
The HIF-1α inhibitor at translational level (HITT)
is a lncRNA whose expression is typically reduced in cancer and is
associated with an advanced stage of the disease (25). As aforementioned, HIF-1α acts as
a transcriptional factor for genes involved in angiogenesis,
metastasis and EMT among others. One of the well-established HIF-1α
targets is VEGF, promoting angiogenesis, allowing the cells to
adapt to the hypoxic environment characteristic of tumor processes
(43,114). HITT inhibits HIF-1α translation
by blocking YB-1 (a transcriptional factor for HIF-1α), increasing
tumor growth and angiogenesis processes (115). Indeed, high HIF-1 levels and
low HTT levels are associated with poor cancer prognosis since they
increase metastatic potential and angiogenesis (116). Wang et al (25) demonstrated, for the first time,
the implication of miR-205 in HIF-1α/HITT axis in colorectal and
cervical cancers. The authors determined that HITT is a direct
target of miR-205, but at the same time, the miR-205 expression is
also dependent on HIF-1α under hypoxic conditions. Specifically,
the authors showed that under normoxic conditions, HITT does not
undergo HIF-1α/miR-205-mediated destabilization. Under these
conditions, HITT binds to YB-1, preventing YB-1 from associating
with the 5'-UTR region of HIF-1α, thereby inhibiting its
translation and reducing HIF-1α levels. However, under hypoxic
conditions, HITT levels are regulated by miR-205 (in a
HIF-1α-dependent manner). Elevated miR-205 levels are induced by
HIF-1α acting as a transcription factor for miR-205. In hypoxia,
miR-205 destabilizes HITT, reducing its inhibitory effect on HIF-1α
transcription, which promotes angiogenesis and tumor growth.
Therefore, miR-205 is considered a pro-angiogenic and oncogenic
agent by regulating the HITT/HIF-1α axis.
miR-205 in extracellular vesicles as
regulators of angiogenesis
EVs and exosomes are spherical lipid membranes
released by different cell types containing proteins, lipids and
nucleic acids, such as miRNAs. Among the miRNAs contained in EVs,
miR-205 has been observed (26).
EVs play crucial role in modulating biological responses,
especially in the development, growth and maturation of blood
vessels. An increasing number of studies emphasize the marked
potential of EVs to revolutionize drug delivery systems (80). Compared with traditional methods,
EVs, particularly, exosomes, offer significant advantages,
including low immunogenicity, exceptional biocompatibility and high
biosafety. They can efficiently cross challenging barriers, such as
the blood-brain barrier, while enhancing the stability of
encapsulated nucleic acid drugs and facilitating their transport
through biological barriers (77,117). EVs have shown great promise as
drug delivery systems, particularly in small interfering RNA
delivery for pancreatic cancer treatment, with encouraging results
in mice leading to ongoing clinical trials (118-120). Research emphasizes that EVs
play an active role by influencing key stages of vascular
development through different mechanisms. In fact, among the
functional proteins present in EVs, VEGF emerges as a key mediator
of angiogenesis (121). Their
well-documented involvement in these processes has sparked growing
interest in their potential therapeutic applications for
regenerative medicine and angiogenesis-related diseases (Table II) (121,122)
 | Table IImiR-205-EVs targets and related
angiogenic pathways. |
Table II
miR-205-EVs targets and related
angiogenic pathways.
| Authors, year | Role | miR-205
targets | Related
pathway | Pathology | (Refs.) |
|---|
| Zhang et al,
2025 |
Anti-angiogenic | VEGFA and
ANG-2 | VEGF | Ocular
neovascularization | (78) |
| Liu et al,
2021 | | | | Diabetic foot | (127) |
| Zhuang et
al, 2022 | | | | Oral squamous
carcinoma | (128) |
| Yang et al,
2022 | Pro-angiogenic | DSC-2 | EGRF/ERK | Nasopharyngeal
carcinoma | (124) |
| He et al,
2019 | | PTEN | PI3K/AKT | Ovarian cancer | (125) |
One of the clinical applications that promises to
have therapeutic potential effects as a regulator of angiogenesis
is the use of EVs containing miR-205. EVs carrying miR-205 can
promote EC proliferation and migration, facilitating angiogenesis.
Moreover, the injection of exosomes loaded with miR-205, obtained
from adipose-derived stem cells, reduces cardiac fibrosis and
decreases cardiomyocyte apoptosis in myocardial infarction animal
model (123). In line with
previous findings, elevated levels of miR-205 in serum
nasopharyngeal carcinoma subjects, have been associated with
disease progression and poorer survival outcomes. This association
was attributed to the regulation of desmocollin-2 (DSC2) by
miR-205. miR-205 modulates DSC2, promoting EGFR/ERK pathway and the
expression of MMP2/MMP9, thereby enhancing angiogenesis and
metastasis (124). In ovarian
cancer, it was found that the overexpression of miR-205 was
associated with metastatic progression. This miRNA could reach EC
from ovarian cancer cells through EVs, promoting angiogenesis both
in vivo and in vitro. In this case, miR-205-EVs
induce angiogenesis by regulating PTEN, thereby stimulating
the PI3K/AKT pathway and enhancing angiogenesis (125,126). In these contexts, miR-205-EVs
exhibits a pro-angiogenic role.
As reviewed in the present review, miR-205 directly
targets VEGF and ANG-2, two key drivers of
neovascularization. Research by Zhang et al (78) explored the use of EVs as delivery
vehicles for miR-205. Concretely, in an ocular neovascularization
mouse model the authors observed a significant reduction of both
neovascularization and vascular leakage by miR-205 administration
negatively regulating VEGF and ANG-2. On the other hand, it was
observed that miR-205, along with miR-195, within EVs derived from
wound fluid of diabetic foot ulcers, was able to inhibit
angiogenesis by reducing VEGF expression, negatively affecting
wound healing in patients with diabetic foot ulcers (127). These authors suggested that
both miR-205 and miR-195 could be potential therapeutic targets to
improve wound healing in diabetic foot ulcers. In the same
direction, Zhuang et al (128) observed that the anti-diabetic
drug phenformin upregulated the expression levels of miRNAs in EVs
derived from oral squamous carcinoma cells. Among the altered
miRNAs, the authors identified miR-1246 and miR-205, highlighting
their role in blocking angiogenic processes in EC due to VEGF
repression. These authors proposed phenformin as a useful tool to
modify the tumor microenvironment by reducing carcinoma growth
through changes in miRNA encapsulated in EVs. In vitro
assays using RPE cells demonstrated that miR-205 EVs-cargo was
lower under hyperglycemic conditions. Increasing the number of
miR-205 copies in EVs by using mimetics inhibited EC migration and
tube formation (26). Given
these findings, miR-205 emerges as a potent anti-angiogenic
regulator that holds promise as a therapeutic option. Its delivery
via EVs represents an innovative and effective strategy for
treating conditions such as DR and cancer.
miR-205, angiogenesis and ocular
disorders
miR-205-5p plays a crucial role in regulating gene
expression in different tissues, including the eye. In ocular
biology, it is involved in several essential processes,
particularly in the development and maintenance of the cornea.
Studies have shown that miR-205 is expressed in corneal epithelial
cells and plays a role in their differentiation and function
(33,129,130). Dysregulation of this miRNA has
been linked to corneal diseases such as keratoconus, highlighting
its importance in maintaining corneal homeostasis (131). In addition, miR-205 modulates
lacrimal gland development (32,132). Beyond the cornea, miR-205 has
also been studied in relation to retinal biology. Research
indicates its involvement in retinal development and function,
including the regulation of genes critical for photoreceptor
survival and function (133).
Angiogenesis is a prime sign of corneal and retinal
diseases including, DR, age related macular degeneration (AMD),
neovascular glaucoma and corneal neovascularization. Although
ocular angiogenesis is essential for tissue repair and maintenance,
its dysregulation can lead to severe pathologies. Currently, the
available treatments for these ocular disorders focus on blocking
VEGF through use of anti-VEGF antibodies (ranibizumab and
bevacizumab) (134). Although
these drugs can control the disease and slow the progression of
blindness in some patients, they are not completely effective,
making their overall success questionable. miRNAs have been
extensively studied as potential candidates for new therapeutic
strategies. Given their regulatory role, their use as biomarkers or
therapeutic tools in cancer and pulmonary diseases is already a
reality (18,19). Focusing on the relationship
between miR-205, angiogenesis and ocular disorders, some studies
have been conducted, with most of them agreeing on the
anti-angiogenic role of miR-205 in the ocular context.
Understanding the precise mechanisms by which mR-205 influences
these conditions could provide insights into new therapeutic
approaches for treating retinal disorders.
In patients with uveitis, an inflammatory disease
of the vascular layer of the eye (uvea), lower levels of miR-205
were detected compared with control patients (18). Even though the authors did not
establish a direct connection with vascular processes, it is well
known that the acute or chronic inflammation caused by uveitis can
induce the production of VEGF, IL-6 and TNF-β, all of which are
factors capable of promoting angiogenesis (135,136). Additionally, one of the
potential complications of uveitis that can lead to vision loss is
macular edema (137). The
absence of miR-205 in these patients could trigger an increase in
VEGF, one of the targets of miR-205, enhancing vascular processes
and highlighting the anti-angiogenic role of miR-205. However, in
patients with primary pterygium, a benign fibrovascular lesion of
the conjunctiva, miR-205 expression levels showed no significant
differences between patients and controls, reducing its relevance
in this disease (138). This
suggests that the anti-angiogenic role of miR-205 is
context-dependent, varying according to the specific cellular
environment and disease.
As previously mentioned, among the ocular diseases
that lead to blindness due to the formation of aberrant blood
vessels, AMD stands out. One of the miRNAs found to be
downregulated in the serum of patients with wet AMD was miR-205.
Low expression levels of this miRNA were associated with increased
disease severity, although no direct correlation was observed
between miR-205 expression and mortality. However, the mortality of
patients was indeed associated with the number of anti-VEGF
injections received; a higher number of injections associated with
lower survival rates, pointing out the need to develop new
therapies (139). Nevertheless,
miR-205 could still be considered a potential biomarker for
AMD.
Several in vivo studies have focused on the
role of miR-205 in angiogenesis processes, using RPE cells as a
model. This layer is a key component of the blood-retina barrier,
playing a crucial role between the bloodstream and retinal
photoreceptors. Due to its functions in light regulation, metabolic
rate and photoreceptor renewal, the RPE is constantly exposed to
oxidative stress, a major risk factor for the progression of AMD
and RD. Oltra et al (22)
used an RPE cell model exposed to high doses of
H2O2 to stimulate the oxidative stress
conditions that RPE experience. Under these stress conditions,
miR-205 expression levels were low, while VEGF levels were high,
indicating a negative correlation between them. The authors
observed that after administering miR-205 mimics, tube formation
was markedly reduced. The study concluded that under normal
conditions, miR-205 inhibits VEGF and suppresses blood vessel
formation by blocking PI3K/Akt pathway, underscoring the
anti-angiogenic role of miR-205.
Using the same cellular model but exposed to high
glucose concentrations to simulate the DR model, members of the
same research group reaffirmed the role of miR-205 in regulating
angiogenesis and cell migration through VEGF (70). Additionally, Ybarra et al
(70) were the first to
administer a miR-205 mimic via intravitreal injections in diabetic
mice, observing a significant reduction in VEGF levels. This
reduction could be linked to the improvement of aberrant vascular
processes characteristic of RD. Based on these findings, the
authors proposed miR-205 as a potential therapeutic agent against
angiogenesis through intravitreal injections. Other researchers
have included the lncRNA MALAT1 in the miR-205/VEGF axis,
indicating that under hyperglycemic conditions, high MALAT1 levels
reduce miR-205 expression, which in turn increases VEGF expression
and consequently promotes angiogenesis (69). In any case, there is a consensus
on the therapeutic potential of the miR-205/VEGF axis for
pathological angiogenesis-related conditions.
In addition, using the RPE model exposed to high
glucose levels, it was observed that miR-205, this time encapsuled
within EVs, induced changes in recipient cells that internalized
these EVs. Specifically, EVs released under control conditions
contained a higher number of miR-205 copies. After loading EVs with
synthetic miR-205 copies, tube formation and cell migration in
vitro were markedly reduced (26). These findings not only confirm
the anti-angiogenic role of miR-205 in RPE but also highlight its
potential therapeutic use when encapsulated within EVs. In fact,
Zhang et al (78)
administered EVs loaded with sufficient miR-205 copies via
intravitreal injections in a mouse model of retinal and choroidal
neovascularization. These EVs effectively strengthened the
endothelial barrier, reduced vascular leakage and, most
importantly, markedly inhibited the formation of abnormal blood
vessels (78). In this case, the
therapeutic effect of miR-205 is thought to be mediated not only by
the negative regulation of VEGF but also by the downregulation of
ANG-2.
Therefore, different studies agree on the
usefulness of miR-205 as a therapeutic strategy against the
formation of aberrant blood vessels in ocular diseases.
Furthermore, miR-205 has potential implications as a biomarker in
ocular diseases. Its expression levels in ocular tissues or
biofluids could serve as indicators of disease progression or
response to treatment. This biomarker and therapeutical potential
underscore the importance of further research into miR-205 in
ophthalmology, aiming to harness its diagnostic and therapeutic
benefits for improving eye health outcomes.
Integrative view: Explaining the dual role
of miR-205 in angiogenesis
The seemingly contradictory effect of miR-205 on
angiogenesis is increasingly understood as context-dependent,
influenced by a wide range of biological variables rather than
representing true inconsistencies. Several mechanisms may explain
this dual behavior, including cell-specific targets, disease stare,
tissue type, and microenvironmental cues, such as glucose
concentration, oxygen levels, or inflammatory mediators.
Importantly, the primary determinant of the
angiogenic role of miR-205 lies in its molecular 3'UTR target
(Fig. 3). When miR-205 represses
pro-angiogenic factors such as VEGFA (22), SMAD1/4 (65), ANG-2 (23), or MICAL2 (99), it exerts anti-angiogenic effects
by downregulating key signaling cascades that promote endothelial
proliferation and migration. This pattern has been observed in
diseases such as diabetic retinopathy, psoriasis, and thyroid
carcinoma. As shown by the enrichment analysis (Fig. 4), among the most notable pathways
were the TGF-β and Hippo/YAP-1 signaling pathways, both associated
with some of the previously mentioned anti-angiogenic targets
(SMAD1/4 and YAP1, respectively) (65,72). These findings further support the
anti-angiogenic role of miR-205 in this context.
Conversely, pro-angiogenic functions of miR-205 are
generally associated with its repression of anti-angiogenic or
tumor-suppressor genes, such as PTEN and HITT (25,73,74) (Fig. 3), which leads to the activation
of pathways such as PI3K/AKT or HIF-1α. Specifically, the PI3K/Akt
signaling pathway was among those represented in the enrichment
analysis (Fig. 4). These effects
have been documented in ovarian cancer, nasopharyngeal carcinoma
and myocardial infarction, where angiogenesis is enhanced as part
of pathological or compensatory tissue remodeling.
Studies illustrate how the same miRNA can exert
opposing effects in different tissues. For instance, inhibiting
miR-205 in diabetic foot ulcers (79) enhances VEGF expression and
promotes wound healing, while in the diabetic retina (70), the inhibition of miR-205 leads to
increased VEGF levels and the formation of aberrant blood vessels
that drive disease progression. Similarly, in gastric cancer,
miR-205 has been shown to act as an anti-angiogenic factor by
targeting VEGF in some contexts (77) and as a pro-angiogenic regulator
by repressing PTEN in others (74). These findings reinforce that the
functional outcome of miR-205 is primarily determined by the target
it regulates, rather than the miRNA sequence itself.
In summary, the dual behavior of miR-205 is not
contradictory but rather reflects a highly dynamic and
target-dependent regulatory network, modulated by tissue type,
disease context and environmental conditions. Recognizing this
complexity is crucial for developing targeted and safe therapeutic
strategies that leverage the angiogenic potential of miR-205.
Preclinical and clinical evidence on
miR-205
Emerging evidence supports the potential of
miR-205, particularly its levels in circulating EVs, as a biomarker
of angiogeneic activity and tumor aggressiveness in several
cancers. For instance, elevated levels of exosomal mir-205 in
peripheral blood have been associated with increased risk of
metastasis in patients with ovarian cancer (125). A comprehensive meta-analysis
evaluating miR-205 in non-small cell lung cancer patients
identified it as a promising biomarker for disease detection. The
diagnostic accuracy of miR-205 was high, with a pooled area under
the ROC curve (AUC) of 0.90 (95% CI: 0.87-0.92), suggesting strong
potential for both ruling in and ruling out the disease. In
clinical terms, a positive test result for miR-205 markedly
increases the post-test probability of lung cancer diagnosis (from
25-57% in hypothetical screening scenarios) (140). Moreover, in gastric cancer,
circulating mir-205 has been shown to serve as a predictive
biomarker, with higher baseline levels correlating with improved
progression-free and overall survival in patients treated with
Ramucirumab-Paclitaxel (141).
The clinical association between high miR-205 expression and
improved prognosis aligns with its canonical anti-angiogenic
mechanism; particularly its ability to suppress VEGFA and inhibit
the PI3K/AKT signaling pathway. In primary tumor environments,
where vascular control is critical, elevated miR-205 levels may
reflect a less angiogenic and less proliferative phenotype, acting
predominantly as a tumor and angiogenesis suppressor.
From a preclinical standpoint, one of the main
challenges in miR-205-based nanotherapies lies in achieving
targeted delivery to ensure the desired angiogeneic effect.
Delivery systems must be specifically designed to direct miR-205
mimic toward proliferation pathological endothelial cells (to
suppress VEGFA) or tumor cells (to inhibit proliferation or
migration), while avoiding unintended delivery to metastatic
niches, where miR-205 may promote angiogenesis through activation
EGFR/ERK pathways (70). Surface
engineering strategies, such as biomimetic coating or targeting
peptides, are being developed to improve tissue specificity and
minimize off-target activation of pro-metastatic routes. These
approaches are crucial for translating miR-205-based therapies into
safe and effective clinical interventions.
Although no clinical trials specifically targeting
miR-205 have been published to date, the feasibility of miRNA-based
pro-angiogenic therapies is supported by data from other
candidates. A notable example is miR-92a, a well-characterized
anti-angiogenic miRNA whose inhibition has shown significant
benefits in preclinical models of ischemia and diabetic wound
healing. In particular, the synthetic inhibitor MRG-110 (an
antagomir targeting miR-92a) was shown to enhance angiogenesis and
accelerate tissue repair, outperforming pro-angiogenic growth
factors used as positive controls (142). MRG-110 advanced to Phase I
clinical trials, where it was proven to be safe and capable of
improving perfusion and new blood vessel formation in human
volunteers, as evidenced by increased CD31 markers and perfusion
imaging (143). These findings
provide mechanistic proof-of-concept for therapeutic angiogenesis
through miRNA modulation.
By analogy, miR-205-based strategies may follow a
similar trajectory toward clinical application; provided that its
dual angiogenic behavior is properly controlled through context-
and tissue-specific delivery systems.
Concluding remarks
The present review underscored the dual role of
miR-205 in angiogenesis, functioning as both pro-angiogenic and
anti-angiogenic factors depending on the cellular context. Its
ability to regulate kay pathways, such as VEGF and PTEN among
others, position it as a potential therapeutic target for various
diseases. Additionally, EVs serve as an effective delivery system
for miR-205, opening new possibilities for targeted treatments.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization, MO and JB, Investigation, JB and
JS, Writing, review and editing MO, MM, MY, MP, CC, CG, CM, JS and
JB. Data authentication is not applicable. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
MARIA OLTRA, ORCID: 000-0001-6397-2702. MIRIAM
MARTÍNEZ-SANTOS, ORCID:0000-0002-9049-6174 MARIA YBARRA, ORCID:
0000-0002-5938-7209 MARIA PIRES, ORCID: 0000-0001-5461-3433 CLARA
GOMIS-COLOMA, ORCID: 0000-0003-3335-5767 CRISTINA MEDINA-TRILLO,
ORCID:0000-0003-3972-0964 FRANCISCO SANCHO, ORCID:
0000-0001-5409-5760 JORGE BARCIA, ORCID: 0000-0002-3660-7977.
Acknowledgments
Not applicable.
Funding
The present review received support from Agencia Estatal de
Investigación (grant no. PID2020-117875GB-I0), Instituto de Salud
Carlos III (ISCIII; grant no. PI21/00083) and European Union
research fund HORIZON MSCA (grant no. 2021-DN-01_RETORNA
101073316).
References
|
1
|
Bhaskaran M and Mohan M: MicroRNAs:
History, biogenesis, and their evolving role in animal development
and disease. Vet Pathol. 51:759–774. 2014. View Article : Google Scholar :
|
|
2
|
Kumar S, Vijayan M, Bhatti JS and Reddy
PH: MicroRNAs as peripheral biomarkers in aging and age-related
diseases. Prog Mol Biol Transl Sci. 146:47–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee RC, Feinbaum RL and Ambros V: The C.
Elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of MicroRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Dong F, Reinach PS, He D, Zhao X,
Chen X, Hu DN and Yan D: MicroRNA-182 suppresses HGF/SF-Induced
increases in retinal pigment epithelial cell proliferation and
migration through targeting c-Met. PLoS One. 11:e01676842016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tasharrofi N, Kouhkan F, Soleimani M,
Soheili ZS, Kabiri M, Mahmoudi Saber M and Dorkoosh FA: Survival
improvement in human retinal pigment epithelial cells via fas
receptor targeting by MiR-374a. J Cell Biochem. 118:4854–4861.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao Y, Zhou Q, Ma J, Zhao Y and Wang S:
MiR-146a Is upregulated during retinal pigment epithelium
(RPE)/Choroid aging in mice and represses IL-6 and VEGF-A
expression in RPE cells. J Clin Exp Ophthalmol. 7:5622016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Q, Gallagher R, Ufret-Vincenty R, Li
X, Olson EN and Wang S: Regulation of angiogenesis and choroidal
neovascularization by members of MicroRNA-23~27~24 Clusters. Proc
Natl Acad Sci USA. 108:8287–8292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin H, Qian J, Castillo AC, Long B, Keyes
KT, Chen G and Ye Y: Effect of MiR-23 on oxidant-induced injury in
human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci.
52:6308–6314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li K, Du Y, Jiang BL and He JF: Increased
MicroRNA-155 and Decreased MicroRNA-146a may promote ocular
inflammation and proliferation in Graves' ophthalmopathy. Med Sci
Monit. 20:639–643. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ying SY, Chang DC and Lin SL: The
MicroRNA. Methods Mol Biol. 1733:1–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi R, Qin Y, Macara IG and Cullen BR:
Exportin-5 mediates the nuclear export of Pre-MicroRNAs and short
hairpin RNAs. Genes Dev. 17:3011–3016. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Djuranovic S, Nahvi A and Green R:
miRNA-Mediated gene silencing by translational repression followed
by mRNA deadenylation and decay. Science. 336:237–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turchinovich A, Tonevitsky AG and
Burwinkel B: Extracellular MiRNA: A collision of two paradigms.
Trends Biochem Sci. 41:883–892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guiot J, Struman I, Louis E, Louis R,
Malaise M and Njock MS: Exosomal miRNAs in lung diseases: From
biologic function to therapeutic targets. J Clin Med. 8:13452019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ingenito F, Roscigno G, Affnito A, Nuzzo
S, Scognamiglio I, Quintavalle C and Condorelli G: The role of
Exo-miRNAs in cancer: A focus on therapeutic and diagnostic
applications. Int J Mol Sci. 20:46872019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naeli P, Yousefi F, Ghasemi Y,
Savardashtaki A and Mirzaei H: The role of MicroRNAs in lung
cancer: Implications for diagnosis and therapy. Curr Mol Med.
20:90–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vishwakarma S and Kaur I: Molecular
mediators and regulators of retinal angiogenesis. Semin Ophthalmol.
38:124–133. 2023. View Article : Google Scholar
|
|
21
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oltra M, Vidal-Gil L, Maisto R,
Sancho-Pelluz J and Barcia JM: Oxidative stress-induced
angiogenesis is mediated by miR-205-5p. J Cell Mol Med.
24:1428–1436. 2020. View Article : Google Scholar
|
|
23
|
Xue Y, Liu Y, Bian X, Zhang Y, Li Y, Zhang
Q and Yin M: miR-205-5p inhibits psoriasis-associated proliferation
and angiogenesis: Wnt/β-catenin and mitogen-activated protein
kinase signaling pathway are involved. J Dermatol. 47:882–892.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun LL, Xiao L, Du XL, Hong L, Li CL, Jiao
J, Li WD and Li XQ: MiR-205 promotes endothelial progenitor cell
angiogenesis and deep vein thrombosis recanalization and resolution
by targeting PTEN to regulate Akt/autophagy pathway and MMP2
expression. J Cell Mol Med. 23:8493–8504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Li L, Zhao K, Lin Q, Li H, Xue X,
Ge W, He H, Liu D, Xie H, et al: A novel LncRNA HITT forms a
regulatory loop with HIF-1α to modulate angiogenesis and tumor
growth. Cell Death Differ. 27:1431–1446. 2020. View Article : Google Scholar
|
|
26
|
Martínez-Santos M, Ybarra M, Oltra M,
Muriach M, Romero FJ, Pires ME, Sancho-Pelluz J and Barcia JM: Role
of exosomal miR-205-5p cargo in angiogenesis and cell migration.
Int J Mol Sci. 25:9342024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vosgha H, Salajegheh A, Smith RA and Lam
AK: The important roles of miR-205 in normal physiology, cancers
and as a potential therapeutic target. Curr Cancer Drug Targets.
14:621–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang D, Zhang Z, O'Loughlin E, Wang L, Fan
X, Lai EC and Yi R: MicroRNA-205 controls neonatal expansion of
skin stem cells by modulating the PI(3)K pathway. Nat Cell Biol.
15:1153–1163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnell DK, Kaur S, Stanislaw S, Konieczka
JH, Yatskievych TA and Antin PB: MicroRNA expression during chick
embryo development. Dev Dyn. 235:3156–3165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shingara J, Keiger K, Shelton J,
Laosinchai-Wolf W, Powers P, Conrad R, Brown D and Labourier E: An
optimized isolation and labeling platform for accurate microRNA
expression profiling. RNA. 11:1461–1470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mouillet JF, Chu T, Nelson DM, Mishima T
and Sadovsky Y: MiR-205 silences MED1 in hypoxic primary human
trophoblasts. FASEB J. 24:2030–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Farmer DT, Finley JK, Chen FY,
Tarifeño-Saldivia E, McNamara NA, Knox SM and McManus MT: MiR-205
is a critical regulator of lacrimal gland development. Dev Biol.
427:12–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu J, Peng H, Ruan Q, Fatima A, Getsios S
and Lavker RM: MicroRNA-205 promotes keratinocyte migration via the
lipid phosphatase SHIP2. FASEB J. 24:3950–3959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Teta M, Choi YS, Okegbe T, Wong G, Tam OH,
Chong MM, Seykora JT, Nagy A, Littman DR, Andl T and Millar SE:
Inducible deletion of epidermal dicer and drosha reveals multiple
functions for miRNAs in postnatal skin. Development. 139:1405–1416.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Farmer DT, Shariat N, Park CY, Liu HJ,
Mavropoulos A and McManus MT: Partially penetrant postnatal
lethality of an epithelial specific MicroRNA in a mouse knockout.
PLoS One. 8:e766342013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tellez CS, Juri DE, Do K, Bernauer AM,
Thomas CL, Damiani LA, Tessema M, Leng S and Belinsky SA: Tumor and
stem cell biology EMT and stem cell-like properties associated with
miR-205 and miR-200 epigenetic silencing are early manifestations
during carcinogen-induced transformation of human lung epithelial
cells. Cancer Res. 71:3087–3097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JY, Park MK, Park JH, Lee HJ, Shin DH,
Kang Y, Lee CH and Kong G: Loss of the polycomb protein Mel-18
enhances the epithelial-mesenchymal transition by ZEB1 and ZEB2
expression through the downregulation of MiR-205 in breast cancer.
Oncogene. 33:1325–1335. 2014. View Article : Google Scholar
|
|
38
|
Ferrari E and Gandellini P: Unveiling the
ups and downs of miR-205 in physiology and cancer: Transcriptional
and post-transcriptional mechanisms. Cell Death Dis. 11:9802020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hulf T, Sibbritt T, Wiklund ED, Patterson
K, Song JZ, Stirzaker C, Qu W, Nair S, Horvath LG, Armstrong NJ, et
al: Epigenetic-induced repression of microRNA-205 is associated
with MED1 activation and a poorer prognosis in localized prostate
cancer. Oncogene. 32:2891–2899. 2013. View Article : Google Scholar
|
|
40
|
Shaw PH: The Role of P53 in cell cycle
regulation. Pathol Res Pract. 192:669–675. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Piovan C, Palmieri D, Di Leva G, Braccioli
L, Casalini P, Nuovo G, Tortoreto M, Sasso M, Plantamura I, Triulzi
T, et al: Oncosuppressive role of P53-Induced miR-205 in triple
negative breast cancer. Mol Oncol. 6:458–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gandellini P, Giannoni E, Casamichele A,
Taddei ML, Callari M, Piovan C, Valdagni R, Pierotti MA, Zaffaroni
N and Chiarugi P: MiR-205 hinders the malignant interplay between
prostate cancer cells and associated fibroblasts. Antioxid Redox
Signal. 20:1045–1059. 2014. View Article : Google Scholar :
|
|
43
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan F, Mao H, Bu F, Tong X, Li J, Zhang S,
Liu X, Wang L, Wu L, Chen R, et al: Sp1-Mediated transcriptional
activation of miR-205 promotes radioresistance in esophageal
squamous cell carcinoma. Oncotarget. 8:5735–5752. 2017. View Article : Google Scholar :
|
|
45
|
Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt
L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS,
Borre M, et al: Coordinated epigenetic repression of the miR-200
family and miR-205 in invasive bladder cancer. Int J Cancer.
128:1327–1334. 2011. View Article : Google Scholar
|
|
46
|
Zhang L, Hung GC, Meng S, Evans R and Xu
J: LncRNA MALAT1 regulates hyperglycemia induced EMT in
keratinocyte via miR-205. Noncoding RNA. 9:142023.PubMed/NCBI
|
|
47
|
Li Q, Pan X, Wang X, Jiao X, Zheng J, Li Z
and Huo Y: Long noncoding RNA MALAT1 promotes cell proliferation
through suppressing miR-205 and promoting SMAD4 expression in
osteosarcoma. Oncotarget. 8:106648–106660. 2017. View Article : Google Scholar :
|
|
48
|
Gao Q and Wang Y: Long noncoding RNA
MALAT1 regulates apoptosis in ischemic stroke by sponging
miR-205-3p and modulating PTEN expression. Am J Transl Res.
12:2738–2748. 2020.PubMed/NCBI
|
|
49
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA Linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He B, Bai Y, Kang W, Zhang X and Jiang X:
LncRNA SNHG5 regulates imatinib resistance in chronic myeloid
leukemia via acting as a CeRNA against MiR-205-5p. Am J Cancer Res.
7:1704–1713. 2017.PubMed/NCBI
|
|
51
|
Yang W, Hong L, Xu X, Wang Q, Huang J and
Jiang L: LncRNA GAS5 suppresses the tumorigenesis of cervical
cancer by downregulating miR-196a and miR-205. Tumour Biol.
39:10104283177113152017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jin Z and Chen B: LncRNA ZEB1-AS1
regulates colorectal cancer cells by MiR-205/YAP1 axis. Open Med
(Wars). 15:175–184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang X, Zeng X, Xia N, Xie X and Long Y:
Circ_0003520/MiR-205-5p/CUL4B axis drives the progression of clear
cell renal carcinoma. J Biochem Mol Toxicol. 39:e702632025.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang X, Zhang H, Jiao K, Zhao C, Liu H,
Meng Q, Wang Z, Feng C and Li Y: Effect of miR-205 on proliferation
and migration of thyroid cancer cells by targeting CCNB2 and the
mechanism. Oncol Lett. 19:2568–2574. 2020.PubMed/NCBI
|
|
55
|
Guan B, Li Q, Shen L, Rao Q, Wang Y, Zhu
Y, Zhou XJ and Li XH: MicroRNA-205 directly targets krüppel-like
factor 12 and is involved in invasion and apoptosis in basal-like
breast carcinoma. Int J Oncol. 49:720–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang H and Fan Q: MicroRNA-205 inhibits
the proliferation and invasion of breast cancer by regulating AMOT
expression. Oncol Rep. 34:2163–2170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dudley AC and Griffioen AW: Pathological
angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis.
26:313–347. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gerhardt H, Golding M, Fruttiger M,
Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C,
Alitalo K, Shima D and Betsholtz C: VEGF guides angiogenic
sprouting utilizing endothelial tip cell filopodia. J Cell Biol.
161:1163–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marech I, Leporini C, Ammendola M,
Porcelli M, Gadaleta CD, Russo E, De Sarro G and Ranieri G:
Classical and non-classical proangiogenic factors as a target of
antiangiogenic therapy in tumor microenvironment. Cancer Lett.
380:216–226. 2016. View Article : Google Scholar
|
|
60
|
Roskoski R Jr: Vascular endothelial growth
factor (VEGF) signaling in tumor progression. Crit Rev Oncol
Hematol. 62:179–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lamalice L, Houle F and Huot J:
Phosphorylation of Tyr 1214 within VEGFR-2 triggers the recruitment
of nck and activation of fyn leading to SAPK2/P38 activation and
endothelial cell migration in response to VEGF. J Biol Chem.
281:34009–34020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Laddha AP and Kulkarni YA: VEGF and FGF-2:
Promising targets for the treatment of respiratory disorders.
Respir Med. 156:33–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu Y, Liu Y, Deng J, Li W and Nie X:
Fibroblast growth factor in diabetic foot ulcer: Progress and
therapeutic prospects. Front Endocrinol (Lausanne). 12:7448682021.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Goumans MJ, Valdimarsdottir G, Itoh S,
Rosendahl A, Sideras P and Ten Dijke P: Balancing the activation
state of the endothelium via two distinct TGF-Beta type I
receptors. EMBO J. 21:1743–1753. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tabruyn SP, Hansen S, Ojeda-Fernández ML,
Bovy N, Zarrabeitia R, Recio-Poveda L, Bernabéu C, Martial JA,
Botella LM and Struman I: MiR-205 is downregulated in hereditary
hemorrhagic telangiectasia and impairs TGF-Beta signaling pathways
in endothelial cells. Angiogenesis. 16:877–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Di Benedetto P, Ruscitti P, Berardicurti
O, Panzera N, Grazia N, Di Vito Nolfi M, Di Francesco B, Navarini
L, Maurizi A, Rucci N, et al: Blocking Jak/STAT signalling using
tofacitinib inhibits angiogenesis in experimental arthritis.
Arthritis Res Ther. 23:2132021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang X, Song Y, Wu Y, Dong Y, Lai L,
Zhang J, Lu B, Dai F, He L, Liu M and Yi Z: Indirubin inhibits
tumor growth by antitumor angiogenesis via blocking VEGFR2-Mediated
JAK/STAT3 signaling in endothelial cell. Int J Cancer.
129:2502–2511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gong W, Wang L, Yao JC, Ajani JA, Wei D,
Aldape KD, Xie K, Sawaya R and Huang S: Expression of activated
signal transducer and activator of transcription 3 predicts
expression of vascular endothelial growth factor in and angiogenic
phenotype of human gastric cancer. Clin Cancer Res. 11:1386–1393.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tan A, Li T, Ruan L, Yang J, Luo Y, Li L
and Wu X: Knockdown of Malat1 alleviates high-glucose-induced
angiogenesis through regulating miR-205-5p/VEGF-A axis. Exp Eye
Res. 207:1085852021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ybarra M, Martínez-Santos M, Oltra M,
Muriach M, Pires ME, Ceresoni C, Sancho-Pelluz J and Barcia JM:
MiR-205-5p modulates high glucose-induced VEGFA levels in diabetic
mice and ARPE-19 cells. Antioxidants (Basel). 14:2182025.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zeng Y, Zhu J, Shen D, Qin H, Lei Z, Li W,
Huang JA and Liu Z: Repression of Smad4 by MiR-205 moderates
TGF-β-Induced epithelial-mesenchymal transition in A549 cell lines.
Int J Oncol. 49:700–708. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Du YE, Tu G, Yang G, Li G, Yang D, Lang L,
Xi L, Sun K, Chen Y, Shu K, et al: MiR-205/YAP1 in activated
fibroblasts of breast tumor promotes VEGF-independent angiogenesis
through STAT3 signaling. Theranostics. 7:3972–3988. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cai J, Fang L, Huang Y, Li R, Yuan J, Yang
Y, Zhu X, Chen B, Wu J and Li M: MiR-205 Targets PTEN and PHLPP2 to
augment AKT signaling and drive malignant phenotypes in non-small
cell lung cancer. Cancer Res. 73:5402–5415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yao L, Shi W and Gu J: Micro-RNA 205-5p is
involved in the progression of gastric cancer and targets
phosphatase and tensin homolog (PTEN) in SGC-7901 human gastric
cancer cells. Med Sci Monit. 25:6367–6377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Salajegheh A, Vosgha H, Md Rahman A, Amin
M, Smith RA and Lam AK: Modulatory role of miR-205 in angiogenesis
and progression of thyroid cancer. J Mol Endocrinol. 55:183–196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Vosgha H, Ariana A, Smith RA and Lam AK:
miR-205 targets angiogenesis and EMT concurrently in anaplastic
thyroid carcinoma. Endocr Relat Cancer. 25:323–337. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang J, Zhang J, Pang X, Chen Z, Zhang Z,
Lei L, Xu H, Wen L, Zhu J, Jiang Y, et al: MiR-205-5p suppresses
angiogenesis in gastric cancer by downregulating the expression of
VEGFA and FGF1. Exp Cell Res. 404:1125792021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang HY, Zhang QY, Liu Q, Feng SG, Ma Y,
Wang FS, Zhu Y, Yao J and Yan B: Exosome-loading miR-205: A
two-pronged approach to ocular neovascularization therapy. J
Nanobiotechnology. 23:362025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhu L, Zhong Q, Yang T and Xiao X:
Improved therapeutic effects on diabetic foot by human mesenchymal
stem cells expressing MALAT1 as a sponge for microRNA-205-5p. Aging
(Albany NY). 11:12236–12245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gao C, Zhang CC, Yang HX and Hao YN:
MALAT1 protected the angiogenesis function of human brain
microvascular endothelial cells (HBMECs) under oxygen glucose
deprivation/re-oxygenation (OGD/R) challenge by interacting with
MiR-205-5p/VEGFA pathway. Neuroscience. 435:135–145. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Krebs M, Kotlyar MJ, Fahl J, Janaki Raman
S, Röhrig F, Marquardt A, Kübler H, Kneitz B, Schulze A and
Kalogirou C: Metformin regulates the miR-205/VEGFA axis in renal
cell carcinoma cells: Exploring a clinical synergism with tyrosine
kinase inhibitors. Urol Int. 108:49–59. 2024. View Article : Google Scholar
|
|
82
|
Ouyang Z, Tan T, Zhang X, Wan J, Zhou Y,
Jiang G, Yang D and Liu T: LncRNA ENST00000563492 promoting the
osteogenesis-angiogenesis coupling process in bone mesenchymal stem
cells (BMSCs) by functions as a ceRNA for miR-205-5p. Cell Death
Dis. 11:4862020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Botella LM, Albiñana V, Ojeda-Fernandez L,
Recio-Poveda L and Bernabéu C: Research on potential biomarkers in
hereditary hemorrhagic telangiectasia. Front Genet. 6:1152015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ribatti D: Napoleone ferrara and the saga
of vascular endothelial growth factor. Endothelium. 15:1–8. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hammes HP, Lin J, Wagner P, Feng Y, Vom
Hagen F, Krzizok T, Renner O, Breier G, Brownlee M and Deutsch U:
Angiopoietin-2 causes pericyte dropout in the normal retina:
Evidence for involvement in diabetic retinopathy. Diabetes.
53:1104–1110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Biel NM and Siemann DW: Targeting the
angiopoietin-2/Tie-2 axis in conjunction with VEGF signal
interference. Cancer Lett. 380:525–533. 2016. View Article : Google Scholar
|
|
87
|
Abouaitah K, Hassan HA, Swiderska-Sroda A,
Gohar L, Shaker OG, Wojnarowicz J, Opalinska A, Smalc-Koziorowska
J, Gierlotka S and Lojkowski W: Targeted nano-drug delivery of
colchicine against colon cancer cells by means of mesoporous silica
nanoparticles. Cancers (Basel). 12:1442020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Guo X, Niu Y, Han W, Han X, Chen Q, Tian
S, Zhu Y, Bai D and Li K: The ALK1-Smad1/5-ID1 pathway participates
in tumour angiogenesis induced by low-dose photodynamic therapy.
Int J Oncol. 62:552023. View Article : Google Scholar :
|
|
89
|
Seystahl K, Tritschler I, Szabo E,
Tabatabai G and Weller M: Differential regulation of TGF-β-Induced,
ALK-5-Mediated VEGF release by SMAD2/3 versus SMAD1/5/8 signaling
in glioblastoma. Neuro Oncol. 17:254–265. 2015. View Article : Google Scholar
|
|
90
|
Duan Y and Chen Q: TGF-Β1 Regulating
MiR-205/MiR-195 expression affects the TGF-β signal pathway by
respectively targeting SMAD2/SMAD7. Oncol Rep. 36:1837–1844. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Seoane J, Le HV, Shen L, Anderson SA and
Massagué J: Integration of smad and forkhead pathways in the
control of neuroepithelial and glioblastoma cell proliferation.
Cell. 117:211–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Qi J, Liu Y, Hu K, Zhang Y, Wu Y and Zhang
X: MicroRNA-205-5p regulates extracellular matrix production in
hyperplastic scars by targeting Smad2. Exp Ther Med. 17:2284–2290.
2019.PubMed/NCBI
|
|
93
|
Mariotti S, Barravecchia I, Vindigni C,
Pucci A, Balsamo M, Libro R, Senchenko V, Dmitriev A, Jacchetti E,
Cecchini M, et al: MICAL2 is a novel human cancer gene controlling
mesenchymal to epithelial transition involved in cancer growth and
invasion. Oncotarget. 7:1808–1825. 2016. View Article : Google Scholar :
|
|
94
|
Hou ST, Nilchi L, Li X, Gangaraju S, Jiang
SX, Aylsworth A, Monette R and Slinn J: Semaphorin3A elevates
vascular permeability and contributes to cerebral ischemia-induced
brain damage. Sci Rep. 5:78902015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Evans IM, Kennedy SA, Paliashvili K,
Santra T, Yamaji M, Lovering RC, Britton G, Frankel P, Kolch W and
Zachary IC: Vascular endothelial growth factor (VEGF) promotes
assembly of the P130Cas interactome to drive endothelial
chemotactic signaling and angiogenesis. Mol Cell Proteomics.
16:168–180. 2017. View Article : Google Scholar :
|
|
96
|
Wang Y, Deng W, Zhang Y, Sun S, Zhao S,
Chen Y, Zhao X, Liu L and Du J: MICAL2 promotes breast cancer cell
migration by maintaining epidermal growth factor receptor (EGFR)
stability and EGFR/P38 signalling activation. Acta Physiol (Oxf).
2222018.
|
|
97
|
Ho JR, Chapeaublanc E, Kirkwood L, Nicolle
R, Benhamou S, Lebret T, Allory Y, Southgate J, Radvanyi F and Goud
B: Deregulation of Rab and Rab effector genes in bladder cancer.
PLoS One. 2012(7): e394692012. View Article : Google Scholar
|
|
98
|
Barravecchia I, Mariotti S, Pucci A,
Scebba F, De Cesari C, Bicciato S, Tagliafico E, Tenedini E,
Vindigni C, Cecchini M, et al: MICAL2 is expressed in cancer
associated neo-angiogenic capillary endothelia and it is required
for endothelial cell viability, motility and VEGF response. Biochim
Biophys Acta Mol Basis Dis. 1865:2111–2124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tao W, Sun W, Zhu H and Zhang J:
MiR-205-5p suppresses pulmonary vascular smooth muscle cell
proliferation by targeting MICAL2-mediated Erk1/2 signaling.
Microvasc Res. 124:43–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Taylor KL, Henderson AM and Hughes CC:
Notch activation during endothelial cell network formation in vitro
targets the basic HLH Transcription factor HESR-1 and downregulates
VEGFR-2/KDR expression. Microvasc Res. 64:372–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ho RX, Meyer RD, Chandler KB, Ersoy E,
Park M, Bondzie PA, Rehimi N, Xu H, Costello CE and Rahimi N:
MINAR1 Is a Notch2-binding protein that inhibits angiogenesis and
breast cancer growth. J Mol Cell Biol. 10:195–204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ramasamy SK, Kusumbe AP, Wang L and Adams
RH: Endothelial notch activity promotes angiogenesis and
osteogenesis in bone. Nature. 507:376–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jiang W, Zhu P, Zhang T, Liao F, Yu Y, Liu
Y, Shen H, Zhao Z, Huang X and Zhou N: MicroRNA-205 mediates
endothelial progenitor functions in distraction osteogenesis by
targeting the transcription regulator NOTCH2. Stem Cell Res Ther.
12:1012021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li D, Wang Q, Li N and Zhang S: miR-205
targets YAP1 and inhibits proliferation and invasion in thyroid
cancer cells. Mol Med Rep. 18:1674–1681. 2018.PubMed/NCBI
|
|
106
|
Xian XS, Wang YT and Jiang XM: Propofol
inhibits proliferation and invasion of stomach cancer cells by
regulating Mir-205/Yap1 axis. Cancer Manag Res. 12:10771–10779.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Han N, Tian W, Yu N and Yu L: YAP1 Is
required for the angiogenesis in retinal microvascular endothelial
cells via the inhibition of MALAT1-mediated miR-200b-3p in high
glucose-induced diabetic retinopathy. J Cell Physiol.
235:1309–1320. 2020. View Article : Google Scholar
|
|
108
|
Giordano NJ, Jansson PS, Young MN, Hagan
KA and Kabrhel C: Epidemiology, pathophysiology, stratification,
and natural history of pulmonary embolism. Tech Vasc Interv Radiol.
20:135–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li WD and Li XQ: Endothelial progenitor
cells accelerate the resolution of deep vein thrombosis. Vascul
Pharmacol. 83:10–16. 2016. View Article : Google Scholar
|
|
110
|
Li WD, Hu N, Lei FR, Wei S, Rong JJ,
Zhuang H and Li XQ: Autophagy inhibits endothelial progenitor cells
migration via the regulation of MMP2, MMP9 and UPA under normoxia
condition. Biochem Biophys Res Commun. 466:376–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yanagi S, Kishimoto H, Kawahara K, Sasaki
T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, et al:
Pten controls lung morphogenesis, bronchioalveolar stem cells, and
onset of lung adenocarcinomas in mice. J Clin Invest.
117:2929–2940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Karaayvaz M, Zhang C, Liang S, Shroyer KR
and Ju J: Prognostic significance of miR-205 in endometrial cancer.
PLoS One. 7:e351582012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Unwith S, Zhao H, Hennah L and Ma D: The
potential role of HIF on tumour progression and dissemination. Int
J Cancer. 136:2491–2503. 2015. View Article : Google Scholar
|
|
115
|
El-Naggar AM, Veinotte CJ, Cheng H,
Grunewald TG, Negri GL, Somasekharan SP, Corkery DP, Tirode F,
Mathers J, Khan D, et al: Translational activation of HIF1α by YB-1
promotes sarcoma metastasis. Cancer Cell. 27:682–697. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Auyeung KK and Ko JK: Angiogenesis and
oxidative stress in metastatic tumor progression: pathogenesis and
novel therapeutic approach of colon cancer. Curr Pharm Des.
23:3952–3961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Heidarzadeh M, Gürsoy-Özdemir Y, Kaya M,
Eslami Abriz A, Zarebkohan A, Rahbarghazi R and Sokullu E: Exosomal
Delivery of Therapeutic Modulators through the Blood-Brain Barrier;
Promise and Pitfalls. Cell Biosci. 11:1422021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chen Y, Kleeff J and Sunami Y: Pancreatic
cancer cell- and cancer-associated fibroblast-derived exosomes in
disease progression, metastasis, and therapy. Discov Oncol.
15:2532024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kamerkar S, Lebleu VS, Sugimoto H, Yang S,
Ruivo CF, Melo SA, Lee JJ and Kalluri R: Exosomes facilitate
therapeutic targeting of oncogenic KRAS in pancreatic cancer.
Nature. 546:498–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mendt M, Kamerkar S, Sugimoto H, McAndrews
KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, et al:
Generation and testing of clinical-grade exosomes for pancreatic
cancer. JCI Insight. 3:e992632018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ateeq M, Broadwin M, Sellke FW and Abid
MR: Extracellular vesicles' role in angiogenesis and altering
angiogenic signaling. Med Sci (Basel). 12:42024.PubMed/NCBI
|
|
122
|
Todorova D, Simoncini S, Lacroix R,
Sabatier F and Dignat-George F: Extracellular vesicles in
angiogenesis. Circ Res. 120:1658–1673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang T, Li T, Niu X, Hu L, Cheng J, Guo D,
Ren H, Zhao R, Ji Z, Liu P, et al: ADSC-derived exosomes attenuate
myocardial infarction injury by promoting MiR-205-Mediated cardiac
angiogenesis. Biol Direct. 18:62023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yang W, Tan S, Yang L, Chen X, Yang R,
Oyang L, Lin J, Xia L, Wu N, Han Y, et al: Exosomal MiR-205-5p
enhances angiogenesis and nasopharyngeal carcinoma metastasis by
targeting desmocollin-2. Mol Ther Oncolytics. 24:612–623. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang
J and Wu X: Ovarian cancer cell-secreted exosomal miR-205 promotes
metastasis by inducing angiogenesis. Theranostics. 9:8206–8220.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Qu Q, Liu L, Wang L, Cui Y, Liu C, Jing X
and Xu X: Exosomes derived from hypoxic mesenchymal stem cells
restore ovarian function by enhancing angiogenesis. Stem Cell Res
Ther. 15:4962024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Liu J, Wang J, Fu W, Wang X, Chen H, Wu X,
Lao G, Wu Y, Hu M, Yang C, et al: MiR-195-5p and MiR-205-5p in
extracellular vesicles isolated from diabetic foot ulcer wound
fluid decrease angiogenesis by inhibiting VEGFA expression. Aging
(Albany NY). 13:19805–19821. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhuang D, Wang S, Liu G, Liu P, Deng H,
Sun J, Liu C, Leng X, Zhang Q, Bai F, et al: Phenformin suppresses
angiogenesis through the regulation of exosomal microRNA-1246 and
MicroRNA-205 levels derived from oral squamous cell carcinoma
cells. Front Oncol. 12:9434772022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lin D, Halilovic A, Yue P, Bellner L, Wang
K, Wang L and Zhang C: Inhibition of miR-205 impairs the
wound-healing process in human corneal epithelial cells by
targeting KIR4.1 (KCNJ10). Invest Ophthalmol Vis Sci. 54:6167–6178.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Fu JY, Yu XF, Wang HQ, Lan JW, Shao WQ and
Huo YN: MiR-205-3p protects human corneal epithelial cells from
ultraviolet damage by inhibiting autophagy via targeting TLR4/NF-κB
signaling. Eur Rev Med Pharmacol Sci. 12:6494–6504. 2020.
|
|
131
|
Hughes AE, Bradley DT, Campbell M, Lechner
J, Dash DP, Simpson DA and Willoughby CE: Mutation altering the
miR-184 seed region causes familial keratoconus with cataract. Am J
Hum Genet. 89:628–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Asal M, Koçak G, Sarı V, Reçber T, Nemutlu
E, Utine CA and Güven S: Development of lacrimal gland organoids
from iPSC derived multizonal ocular cells. Front Cell Dev Biol.
10:10588462023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Urbán P, Pöstyéni E, Czuni L, Herczeg R,
Fekete C, Gábriel R and Kovács-Valasek A: miRNA profiling of
developing rat retina in the first three postnatal weeks. Cell Mol
Neurobiol. 43:2963–2974. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
CATT Research Group; Martin DF, Maguire
MG, Ying GS, Grunwald JE, Fine SL and Jaffe GJ: Ranibizumab and
bevacizumab for neovascular age-related macular degeneration. N
Engl J Med. 364:1897–1908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Fine HF, Baffi J, Reed GF, Csaky KG and
Nussenblatt RB: Aqueous humor and plasma vascular endothelial
growth factor in uveitis-associated cystoid macular edema. Am J
Ophthalmol. 132:794–796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kozak I, Shoughy SS and Stone DU:
Intravitreal antiangiogenic therapy of uveitic macular edema: A
review. J Ocul Pharmacol Ther. 33:235–239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Massa H, Pipis SY, Adewoyin T, Vergados A,
Patra S and Panos GD: Macular edema associated with non-infectious
uveitis: Pathophysiology, etiology, prevalence, impact and
management challenges. Clin Ophthalmol. 13:1761–1777. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Içme G, Yilmaz A, Dinç E, Görür A, Fidanci
ŞB and Tamer L: Assessment of miR-182, miR-183, miR-184, and
miR-221 expressions in primary pterygium and comparison with the
normal conjunctiva. Eye Contact Lens. 45:208–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Blasiak J, Watala C, Tuuminen R, Kivinen
N, Koskela A, Uusitalo-Järvinen H, Tuulonen A, Winiarczyk M,
Mackiewicz J, Zmorzyński S, et al: Expression of VEGFA-regulating
MiRNAs and mortality in wet AMD. J Cell Mol Med. 23:8464–8471.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Li JH, Sun SS, Li N, Lv P, Xie SY and Wang
PY: MiR-205 as a promising biomarker in the diagnosis and prognosis
of lung cancer. Oncotarget. 8:91938–91949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Piccinno E, Schirizzi A, Scalavino V, De
Leonardis G, Donghia R, Fantasia A, Ricci AD, Lotesoriere C,
Giannelli G, Serino G and D'Alessandro R: Circulating miR-23b-3p,
miR-30e-3p, and miR-205-5p as novel predictive biomarkers for
ramucirumab-paclitaxel therapy outcomes in advanced gastric cancer.
Int J Mol Sci. 25:134982024. View Article : Google Scholar
|
|
142
|
Gallant-Behm CL, Piper J, Dickinson BA,
Dalby CM, Pestano LA and Jackson AL: A synthetic microRNA-92a
inhibitor (MRG-110) accelerates angiogenesis and wound healing in
diabetic and nondiabetic wounds. Wound Repair Regen. 26:311–323.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Viridian Therapeutics Inc.: MiRagen
announces new clinical data showing MRG-110 positively impacted
tissue repair and new blood vessel growth. https://investors.viridiantherapeutics.com/news/news-details/2019/miRagen-Announces-New-Clinical-Data-Showing-MRG-110-Positively-Impacted-Tissue-Repair-and-New-Blood-Vessel-Growth-10-16-2019/default.aspx.
Accessed October 13, 2025
|


















